- 1Tulane National Primate Research Center, Tulane University, Covington, LA, United States
- 2Biomedical Sciences Training Program, Tulane University School of Medicine, New Orleans, LA, United States
- 3Tulane Brain Institute, New Orleans, LA, United States
- 4Department of Microbiology and Immunology, Tulane University School of Medicine, New Orleans, LA, United States
- 5Louisiana Cancer Research Center, New Orleans, LA, United States
- 6Tulane Center for Aging, New Orleans, LA, United States
Introduction: Despite effective combination antiretroviral therapy (cART), chronic neuroinflammation and glial dysfunction continues to be an important yet understudied issue with people living with HIV (PLWH). The endocannabinoid system is increasingly recognized as a potential therapeutic target for modulating neuroimmune environments, given its role in regulating synaptic plasticity, immune responses, and neuroinflammatory cascades. However, the extent to which cannabinoids influence HIV-associated neuroinflammation remains unclear.
Methods: This study investigates the impact of Δ9-tetrahydrocannabinol (THC) on astrocyte growth characteristics, viability, and senescence-associated cytokine release following exposure to Tat protein using primary mixed glial cultures derived from rhesus macaques. Real-time impedance-based cellular integrity assessments were conducted using the xCELLigence system, while morphological analyses and cytokine quantification were performed using phase-contrast microscopy and multiplex immunoassays.
Results: Treatment of SIV-infected macaques with THC protected the astrocytes from virus-induced senescence. Further, THC facilitated a rapid recovery from Tat-induced decline in astrocyte adhesion, suggesting a compensatory effect. THC promoted glial process elongation and morphological complexity, indicative of a shift toward a neuroprotective phenotype. Furthermore, THC significantly reduced inflammatory cytokine secretion, including TNF-α, IL-6, and IL-1β, in an apparently dose-dependent manner.
Conclusions: These findings suggest that THC may modulate neuroinflammation in PLWH by promoting astrocytic survival, suppressing inflammatory cytokine secretion, and enhancing neurotrophic signaling. However, prolonged exposure to high-dose THC may negatively impact glial survival. The results underscore the complexity of cannabinoid signaling in the CNS and highlight the potential of cannabinoid-based interventions to mitigate HIV-associated neuroinflammation.
1 Introduction
1.1 HIV-associated brain injury: persistent neuroinflammation despite viral suppression
The advent of combination antiretroviral therapy (cART) has greatly improved the life expectancy of people living with HIV (PLWH), yet the prevalence of HIV-Associated Brain Injury (HABI) has remained around 50%. HABI encompasses a spectrum of cognitive impairments resulting from HIV infection and associated neuroinflammation. Unlike prior classifications focused solely on neurocognitive decline, HABI accounts for the broader spectrum of brain injuries influenced by HIV, including neuroinflammatory cascades, glial dysfunction, and vascular contributions to cognitive impairment (Nightingale et al., 2023). Proposed mechanisms of HABI include chronic neuroinflammation driven by HIV viral reservoirs, persistent immune activation despite viral suppression, and endothelial dysfunction contributing to blood-brain barrier (BBB) impairment (Van Zandt and MacLean, 2023). Infected microglia and astrocytes serve as key mediators of neuroinflammation, releasing proinflammatory cytokines and extracellular vesicles (EVs) that propagate neural damage (Horn and MacLean, 2021). Additionally, the molecular and cellular mechanisms underlying chronic inflammation in glia are not well understood, limiting the development of targeted therapeutics. The persistence of HABI in virally suppressed individuals underscores the challenge of addressing HIV-induced neuroinflammation. This may be indicative of an earlier onset of otherwise normal brain aging processes or highlight a need for tools more specifically designed to assess HIV-induced cognitive changes.
1.2 The endocannabinoid system in HIV
Cannabis and cannabinoids are the most documented drug of abuse (DoA) in PLWH (Shiau et al., 2017). Both prescribed and self-administered, they are used for alleviation of symptoms associated with disease progression such as neuropathic pain management and appetite stimulation. The primary active components of Cannabis sativa, Δ9-tetrahydracannabinol (Δ9-THC) and cannabidiol (CBD), bind to endogenous cannabinoid receptors (endoCBRs) with varied affinity. The endocannabinoid system is comprized of endoCBRs (CB1 and CB2), their interacting lipids, endogenous cannabinoids, and the metabolic enzymes responsible for their formation and degradation (Piomelli, 2003).
Endogenous cannabinoid receptors act as presynaptic modulators of inhibitory and excitatory neurotransmitters within the CNS and beyond (Alger, 2002; Wilson and Nicoll, 2001; Pandey et al., 2009). Found in neurons and endothelial cells, CB1 is one of the most abundant G-protein-coupled receptors (GPCR) expressed in the brain (Howlett and Abood, 2017). Activation of CB1Rs in neurons reduces presynaptic GABA release, eliminates GABAergic inhibitory control of postsynaptic neurons, and excites these postsynaptic neurons through dis-inhibition (Alger, 2002). Through the binding of macrophage/microglia/astrocyte-expressed CB2, a cascade of GPCR events exerts immunomodulatory effect on target cells by inhibiting cytokine and presynaptic neurotransmitter release that ultimately alters neighboring extracellular environments. EndoCBs act through CB2 to inhibit IL-12 and IL-23 production in microglia and enhance IL-10 production by NF-κB suppression, highlighting the importance of both downregulating proinflammatory pathways and stimulating anti-inflammatory cytokine release (Correa et al., 2009, 2010).
1.3 Cannabinoid modulation of HIV neuroinflammation
The expression and function of endocannabinoid receptors is altered upon HIV infection even in the absence of exogenous cannabinoid intake (Yadav-Samudrala et al., 2024; Smyth and Collister, 2025). In cases of HIV encephalitis, an upregulation of CB1 was observed in neurons and microglia as well as an upregulation of CB2 in microglia, perivascular macrophages, and astrocytes leading to higher cell activation frequency (Cosenza-Nashat et al., 2011). PET imaging in virally suppressed patients demonstrates that the number of activated glia in the CNS of PLWH is inversely correlated with cognitive performance in virally suppressed patients, supporting this proinflammatory hypothesis (Rubin et al., 2018). Putative mechanisms behind this feed-forward loop of inflammation include prolonged cART toxicity, persistent viral reservoirs within the CNS, and damage associated with the BBB (Starr et al., 2021).
Just as HIV infection alters the endocannabinoid system, cannabinoids have been shown to alter the dynamics of HIV infection, as cannabis exposure correlates with a lower incidence of HABI (Watson et al., 2020, 2023). Cannabis-using PLWH display equal or lower viral load and lower circulating HIV nucleic acid concentrations than non-users (Thames et al., 2016; Bredt et al., 2002; Chaillon et al., 2020). Cannabis use is also linked to increased CD4+ and CD8+ T-cell counts (Thames et al., 2016; Keen et al., 2019), fewer markers of immune activation (Chaillon et al., 2020; Manuzak et al., 2018), and decreased levels of inflammatory cytokines (Watson et al., 2021; Kumar et al., 2019).
We hypothesized that SIV infection would induce senescence and dysfunction in glial cells, but that these may be inhibited by cannabinoid treatment. Our experiments examined the extent to which treatment of macaques with THC could reverse senescence in astrocytes induced by SIV infection. Further, we sought to determine if THC could reverse this senescence when applied to senescent cultures or reduce the release of pro-inflammatory cytokines. Our novel findings are important for future translational studies in animal models and serve as early insights to the prevention of HABI, and glial contributions to the inflammatory environment within brain in the context of SIV infection managed by long-term ART and how this is affected by cannabinoid administration.
2 Materials and methods
2.1 Ethical considerations
All procedures involving rhesus macaques were approved by the Tulane Institutional Animal Care and Use Committee (IACUC-protocol 3581) and adhered to the standards set by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academies Press, Washington, DC). The use of SIV Tat protein was conducted in accordance with NIH guidelines for biosafety and biosecurity.
2.2 Cell culture
For these studies, cell cultures were obtained from three populations of macaques (n = 3 for each group): SIV-negative, SIV-infected on ART, and SIV-infected treated with THC (0.32 mg/kg). Primary mixed glial cultures were derived from the frontal lobes of rhesus macaques (Macaca mulatta) following necropsy. Using previously established protocols (Guillemin et al., 1997; MacLean et al., 2002, 2004a,b; Renner et al., 2013), meninges were carefully removed, and cortical tissue was finely diced with sterile scalpels. The resulting tissue fragments were enzymatically digested with 0.25% trypsin (Invitrogen, Carlsbad, CA) and DNAse (4 U/ml, Worthington, Lakewood, NJ) at 37 °C for 60 min, followed by trituration and filtration through 110 μm pore filters (Sigma). The cell-rich slurry was subjected to three rounds of centrifugation at 1,000 rpm, washed, and resuspended in M199 medium supplemented with 0.7 mM sodium bicarbonate and 5% fetal bovine serum. Cells were initially cultured in T-25 flasks, with media replenished at 24 h.
2.3 Growth and morphology analysis
For clarity, the methods for these studies are divided into three sections:
1: Cultures from THC treated, SIV-infected animals (animals received THC),
2: Cultures from SIV-infected animals (cell cultures received THC), and
3: Cultures from naïve animals.
2.3.1 Cultures from THC treated, SIV-infected animals (animals received THC)
Primary glial cultures from SIV-infected animals that were administered THC were utilized to assess differences in cell growth and glial morphology. The macaques received THC for 4 weeks prior to SIV infection and were euthanized at 180 days post SIV infection. After 24 h in culture, the cells were gently washed and fresh media added. Media was then replaced twice weekly. Phase-contrast images were taken weekly using the 10x objective of a Zeiss Axiovert 100 microscope from 10 non-overlapping fields for each flask.
2.3.2 Cultures from SIV-infected animals (cell cultures received THC)
Primary glial cultures from SIV-infected animals on a stable regimen of ART were utilized to assess differences in cell growth and glial morphology with in vitro exposure to THC. Cultures were washed to remove debris at 24 h, and then beginning at 48 h media was collected and replaced with fresh media or media containing 5 μM, or 10 μM THC that was coded to maintain blinding for image collection and analysis. Media collections and replacements were then performed every 48 h for 14 days to ensure continued exposure to THC. Phase-contrast images were taken using the 10x objective of a Zeiss Axiovert 100 microscope from 10 non-overlapping fields for each flask prior to application of THC, at 24 h post first application, then every 72 h. Cell counts and branching complexity were quantified using FIJI ImageJ software.
2.3.3 Cultures from naïve animals
After 24 h in culture, the cells were gently washed and fresh media added. Media was then replaced twice weekly until approximately 80% confluent. Cells were subcultured into T-75 flasks or seeded onto 16-well xCELLigence E-Plates (Agilent) for real-time analysis of adhesion and proliferation. Cells were allowed to adhere for at least 24 h before experimental treatments were introduced, ensuring stable attachments and baseline impedance measurements. Morphological assessments were used to determine whether cannabinoids promoted astrocytic stellation or counteracted Tat-induced cellular activation.
2.4 xCELLigence assay: real time measures of cell adhesion, proliferation, and morphology change
Cell adhesion and proliferation was measured and plotted automatically using an xCELLigence RTCA analyzer as previously described (Renner et al., 2013; MacLean et al., 2014). Each E-Plate was equilibrated by adding 50 μL of complete media to each well and incubating for 15 min at 37 °C in 5% (CO2) before background impedance was established. 20,000 cells (from SIV-negative animals) suspended in 100 μL of complete media were applied to each well with measurements taken at 5 min intervals. Experimental conditions were performed in quadruplicate as recommended in the technical manual of the RTCA instrument. 24 h after plating, wells were treated with control media or SIV Tat (NIH HIV Reagent Program, Division of AIDS, NIAID, NIH). Following continued Tat exposure, cells were treated with 5 μM THC [The National Institute on Drug Abuse (NIDA) Drug Supply Program (DSP)]. Traces were plotted and analyzed using the installed software. Tat was added for the first time at 24 h; THC was first added to the cultures during a media change at 166 h, and again at 214 h.
2.5 Cytokine measurements
Media was collected from flasks at each media change, frozen at −80 °C and analyzed for the presence of senescence-associated secretory phenotype (SASP) markers using an eleven-plex immunoassay designed for non-human primates [Assay Genie, Ireland (Csiszar et al., 2012)]. Cytokines were measured on an LSRFortessa (BD Biosciences) and raw FCS files analyzed by Assay Genie.
To determine whether THC treatment influenced neurotrophic signaling, fibroblast growth factor basic (FGF-B) levels were measured in culture media using a single-plex immunoassay. FGF-B is a crucial mediator of synaptic plasticity and neuroprotection, and its upregulation would suggest a potential mechanism by which THC exerts its neuroprotective effects (Ray et al., 1999; Ascherl et al., 2001).
2.6 Statistical analysis
Data were analyzed using GraphPad Prism software. Comparisons between experimental conditions were conducted using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. Results were expressed as mean ± standard error of the mean (SEM), and statistical significance was defined as p < 0.05.
3 Results
3.1 THC prevents senescence in glial cultures from SIV-infected macaques
In cultures derived from research naïve animals, small cell colonies emerged between days 7 and 10, with cells adopting a spindle-like or polygonal astrocytic morphology (Figure 1). By day 14, these control cultures formed multiple areas with continuous monolayers characteristic of mature astrocyte populations, with stratification and cell stacking over time.
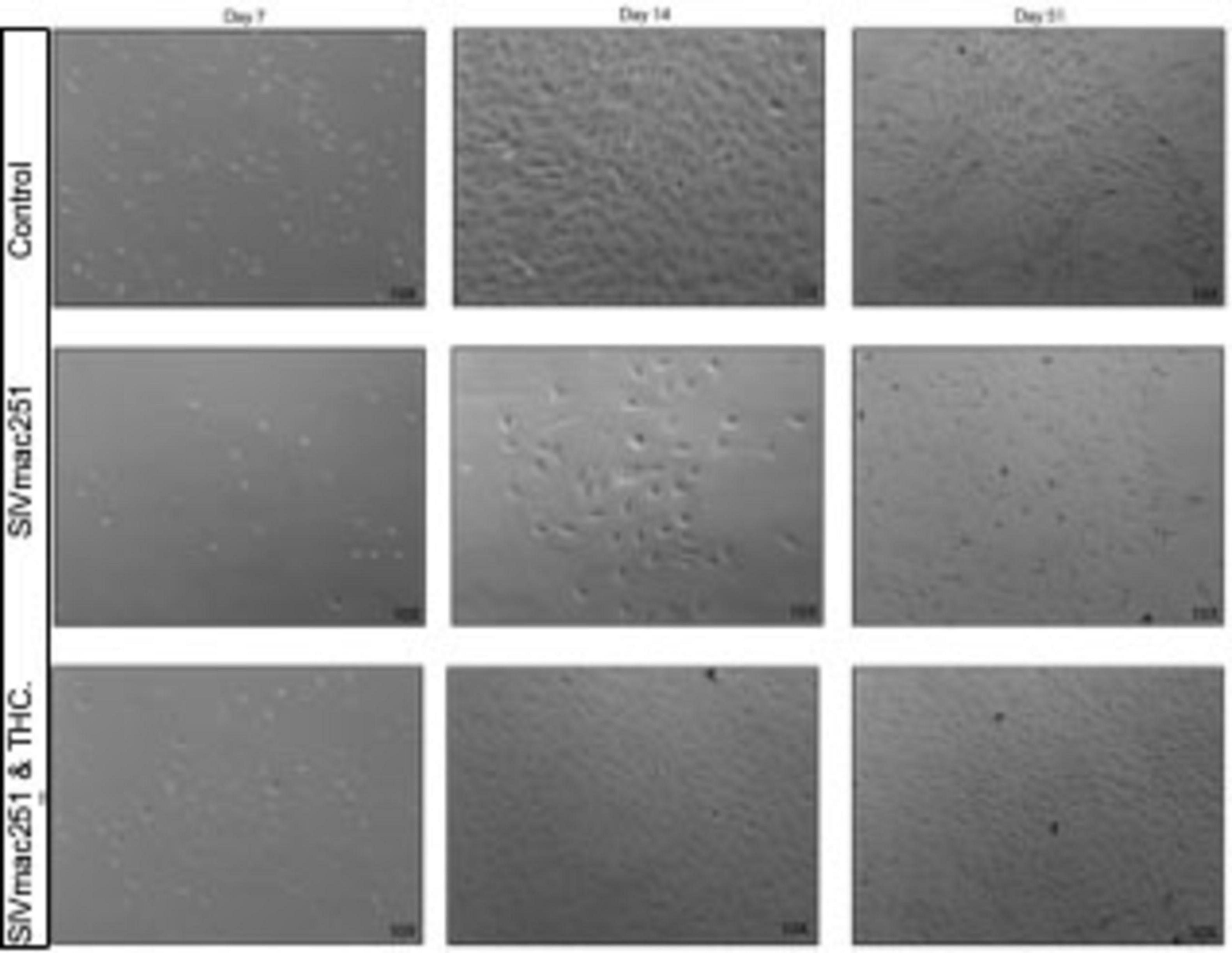
Figure 1. Δ9-tetrahydrocannabinol (THC) prevents senescence in glial cultures from simian immunodeficiency virus (SIV)-infected macaques. Representative phase-contrast microscopy images of primary mixed glial cultures derived from naïve rhesus macaques compared to SIV-infected, and SIV-infected treated with THC. Control cultures from naïve young to middle-aged macaques typically form small colonies between days 7 and 10. By day 14, cells within these colonies reach confluence, forming a uniform monolayer that continues to proliferate and stratify under healthy conditions. Glial cultures from SIV-infected animals displayed reduced cell adhesion at early time points, with fewer colonies forming by day 7. By day 14, these cultures demonstrated lower cell density and increased morphological heterogeneity, with astrocytes displaying a more reactive, hypertrophic phenotype. In contrast, cultures from SIV-infected animals that had been treated with THC while alive grew, albeit at a slightly lower rate compared with control cultures.
In contrast, cultures from SIV-infected animals exhibited a near complete lack of proliferation over the first 14 days and fail to form colonies or “islands” of cells that are apparently necessary for successful growth (unpublished observation). By day 7 (not shown), fewer cells remained adhered to the culture flasks, and colony formation was notably diminished. By day 14, these cultures displayed lower overall cell density, with hypertrophic astrocytes exhibiting enlarged somas and retracted processes, indicative of a reactive phenotype. By day 51, glia from SIV-infected animals exhibited persistent morphological abnormalities, including cellular debris, disrupted monolayer formation, and increased heterogeneity in morphology, suggesting a shift toward a neurotoxic, pro-inflammatory state.
Cultures derived from SIV-infected, THC-treated animals, by contrast, were able to adhere, form colonies, and proliferate, although at a rate slower than those from research naïve animals. It was noted that there may have been less stratification, or piling up, of the cultures as cells approached confluence.
With this context established, we next investigated the extent to which Tat protein in isolation may be responsible for changes in astrocyte adhesion, morphology, and cytokine release and if these can be reversed or inhibited with cannabinoid treatment.
3.2 Tat disrupts astrocyte adhesion and morphology
To assess the effects of Tat on astrocytes, we measured adhesion of mixed glial cultures in real time over a 40 h period following exposure to Tat (2, 3, or 5 μM). All data are baselined to vehicle-containing media, with impedance graphed as cell index (CI) using proprietary software from Agilent. The 3 μM Tat group demonstrated a downward trend, though not significant, while the low Tat (2 μM) group remained close to baseline, indicating a minimal impairment. However, 5 μM Tat induced a rapid decrease in CI, which remained low for 48 h (Figure 2A), indicating a marked reduction in cell adhesion. Replacement of the Tat-containing media with control media allowed a recovery of the CI (not shown), indicating minimal loss in viability.
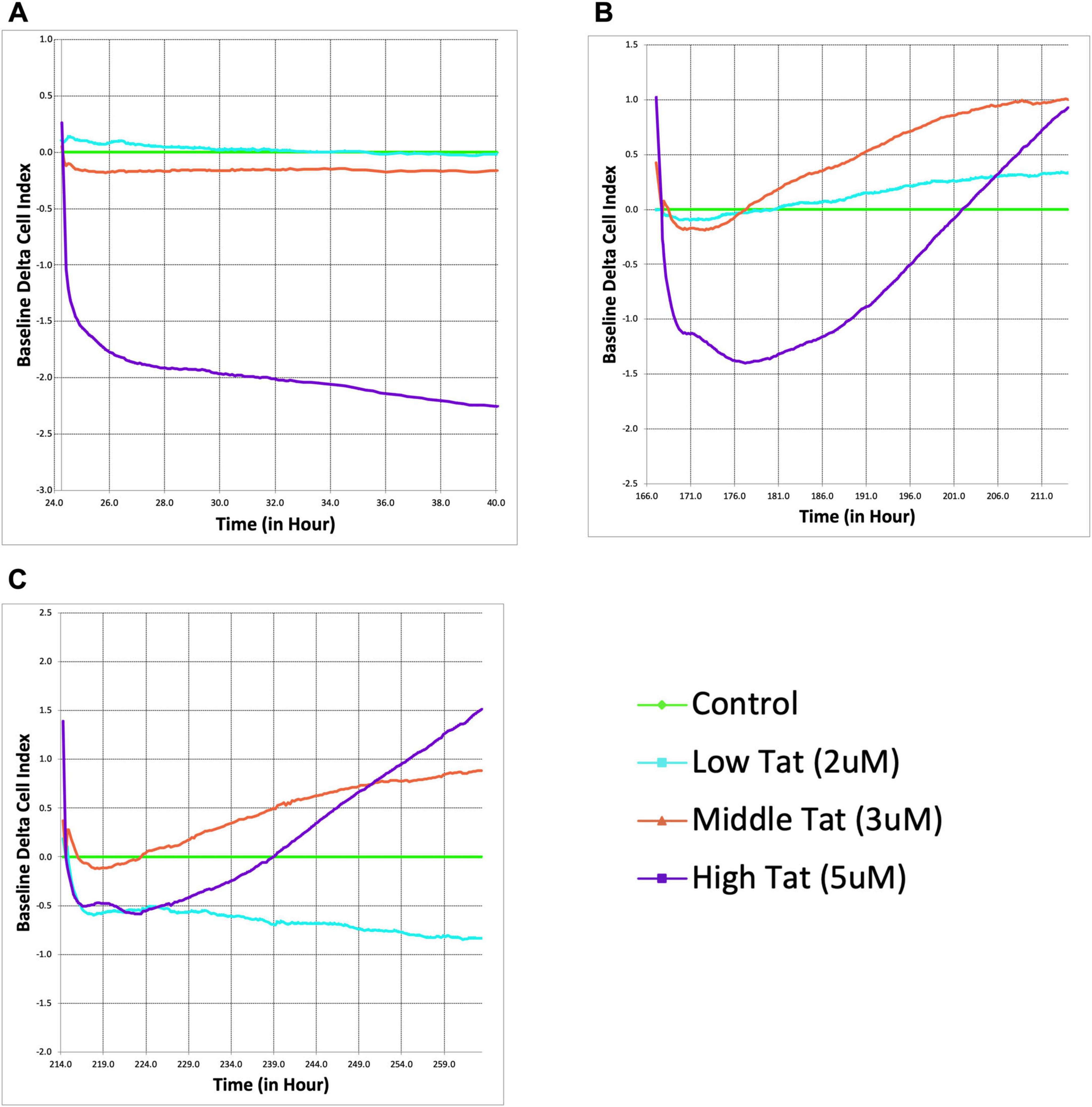
Figure 2. Δ9-tetrahydrocannabinol (THC) counteracts Tat-induced astrocyte dysfunction and enhances cellular recovery. Real-time xCELLigence assay measuring the baseline delta cell index of astrocytes exposed to Tat (2, 3, and 5 μM). Higher Tat concentrations resulted in a significant, dose-dependent decline in astrocyte adhesion and viability over time, as indicated by a decrease in cell index (A). Following THC administration (5 μM), cell index recovery was observed, particularly in the higher Tat groups (B). Indeed, the cell indices rebounded beyond the glial cultures that did not receive Tat, indicating a pronounced compensatory response. A second THC administration further enhanced astrocyte recovery, with the higher Tat groups displaying a sustained increase in cell index (C). Curiously, the cells treated with the lowest Tat concentration (2 μM) had a sustained decrease in the cell index, even in the presence of THC.
3.3 THC counteracts Tat-induced astrocyte dysfunction and enhances cellular recovery
To determine whether THC counteracts Tat-induced astrocyte dysfunction, cells were administered a single 5 μM dose of THC following 5 days of Tat exposure (Figure 2B). Surprisingly, rather than stabilizing at control levels, the 5 μM Tat group exhibited a dramatic increase in CI, far exceeding the control baseline (p = 0.0498). This suggests that THC induces an exaggerated compensatory response in stressed astrocytes, potentially through cytoskeletal remodeling, increased adhesion, and / or enhanced metabolic activity. Meanwhile, the 3 μM Tat group displayed a more moderate recovery, surpassing the control baseline (p = 0.0215), whereas cells treated with 2 μM Tat group showed only a very slight deviation from control cells.
Following a second THC administration (Figure 2C, at 214 h on the E-plate, and 190 h after first being exposed to Tat), the CI decreased to a lesser degree and responded more rapidly than previously. The 3 μM Tat treatment demonstrated an almost identical response to the first THC treatment, while the low Tat group remained relatively unchanged, or even decreased. These suggest that the impact of THC is most pronounced in astrocytes subjected to more severe Tat-induced stress.
3.4 Exogenous THC fails to reverse glial senescence in cultures from SIV-infected macaques
Given the dual role of THC in both promoting trophic support and inducing cellular stress, we examined its impact on glial survival over time. Primary mixed glia from SIV-infected macaques were cultured in control medium, or with either 5 or 10 μM THC, and viability assessed by counting the number of cells (Figure 3 and Supplemental Figure 1). Contrary to previous reports suggesting trophic effects of cannabinoids, our data reveal a progressive decline in glial cell viability with a pronounced reduction in cell count when treated with 10 μM THC, and essentially no deleterious effect at 5 μM.
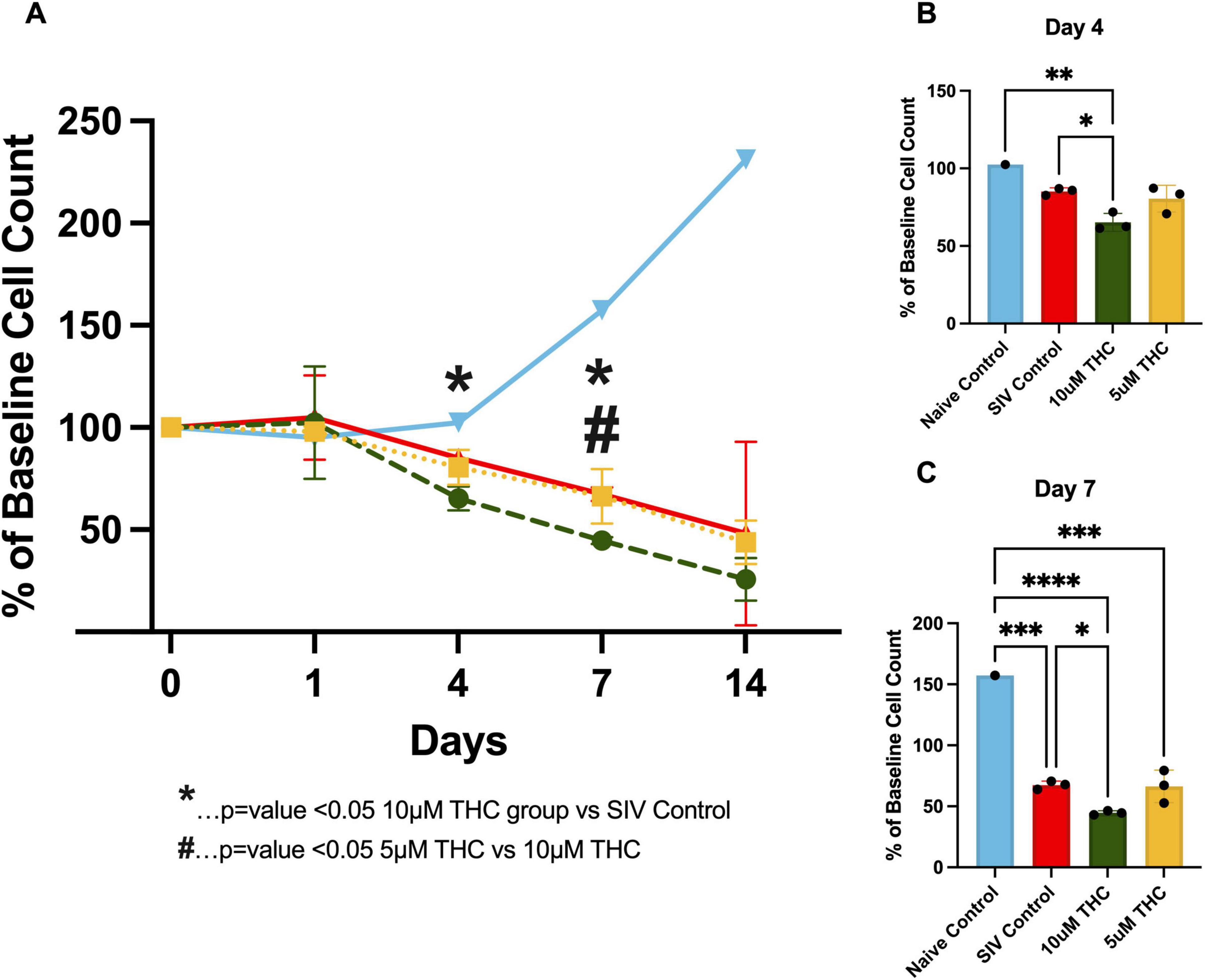
Figure 3. Exogenous Δ9-tetrahydrocannabinol (THC) fails to reverse glial senescence in cultures from simian immunodeficiency virus (SIV)-infected macaques. Quantification of astrocyte viability over initial 14 days in cultures from animals infected with SIV (A). THC exposure resulted in a progressive decline in astrocyte survival, particularly at the 10 μM concentration. By Day 4 in culture, (B) a significant reduction in cell count was observed in the 10 μM group compared to control (p < 0.05). By Day 7, (C) 10 μM THC resulted in a significantly greater decline in astrocyte viability relative to 5 μM THC and control (p < 0.05), suggesting a dose-dependent cytotoxic effect. Data are expressed as % baseline cell count ± SEM; statistical significance determined by one-way ANOVA followed by Tukey’s post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
By Day 4 (Figure 3B), 10 μM THC-treated groups showed a significant reduction in cell count compared to the control. This decline became more pronounced by Day 7 (Figure 3C), where 10 μM THC-treated cultures were significantly lower than the 5 μM and control groups. These findings suggest that while cannabinoids modulate glial function, chronic exposure to higher THC concentrations may exert detrimental effects on glial viability, highlighting the importance of dose considerations when evaluating THC’s therapeutic potential in neuroinflammatory conditions such as HIV-associated neuroinflammation.
3.5 THC enhances glial process formation and morphological complexity
To determine if this is truly fewer cells, or fewer cells relative to the untreated control, we analyzed the development of processes on the glia when treated with THC starting immediately after plating the cells (Figure 4). A clear dose-dependent enhancement in glial process formation was observed, with the 10 μM THC-treated cells exhibiting greater process length and branching complexity than the 5 μM-treated and control groups.
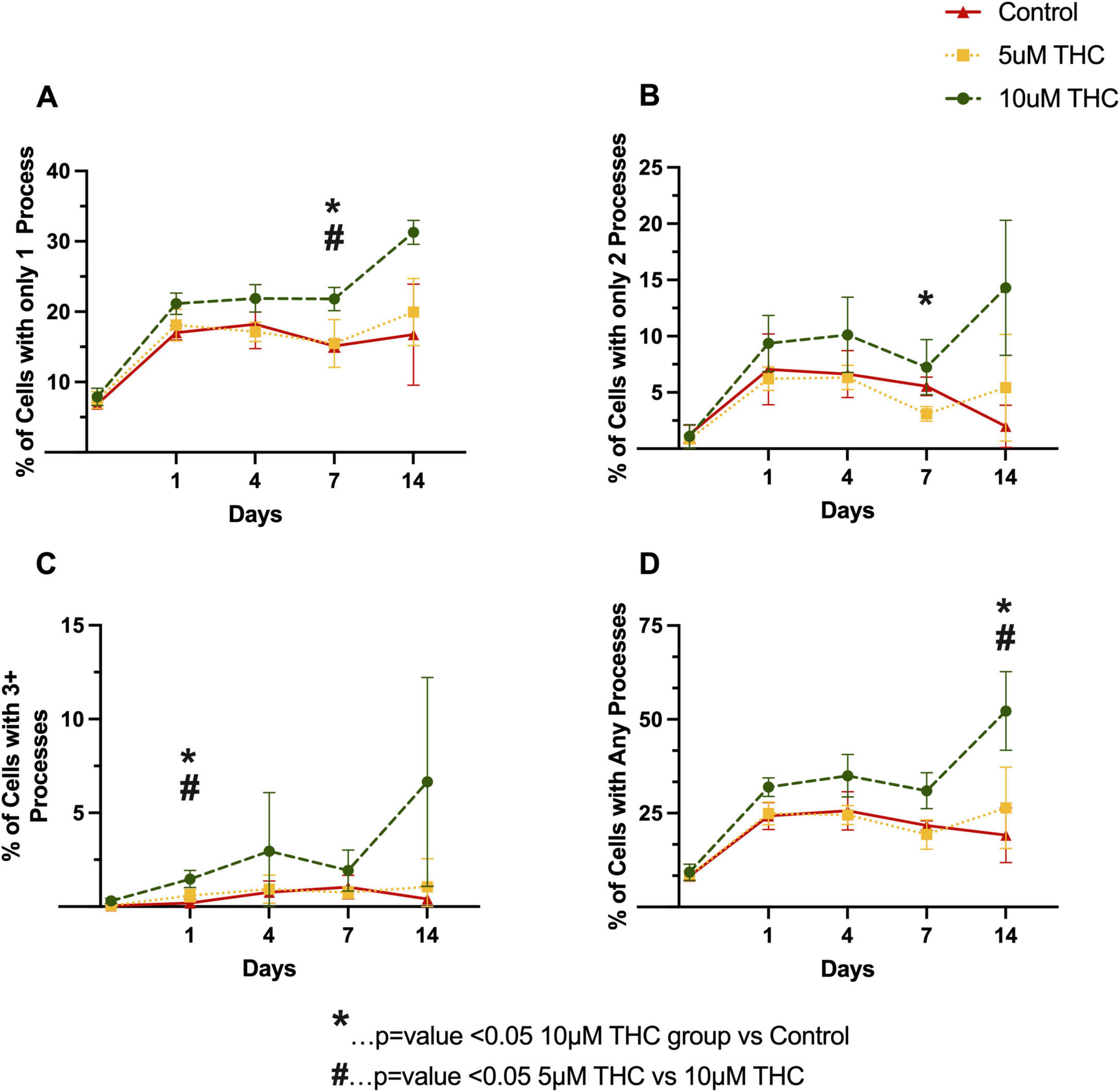
Figure 4. Δ9-tetrahydrocannabinol (THC) enhances glial process formation and morphological complexity. Quantification of glial morphology following THC exposure, assessing the percentage of cells with only one process (A), only two processes (B), three or more processes (C), and any identifiable processes (D) over the initial 14 days in culture. THC-treated astrocytes exhibited increased process formation in a dose-dependent manner, with 10 μM THC-treated cells displaying greater process elongation and branching complexity relative to controls. By Day 7 and Day 14, 10 μM THC significantly increased the percentage of cells with elongated processes compared to 5 μM THC and control (p < 0.05). Data are presented as mean ± SEM; statistical significance determined using one-way ANOVA.
3.6 THC suppresses senescence-associated secretory phenotype in mixed glia cultures
To determine if the glial polarization and adhesion altered secretion of pro-inflammatory and pro-senescence cytokines, we employed an 11-plex immunoassay of glial cultures derived from SIV-infected animals. Levels of IL-6 and MCP-1 were significantly reduced by treatment with either 5 or 10 μM THC by 10 days in culture (Figure 5), whereas, 10 μM THC-treated cultures had significantly lower levels of IL-1B, INF-γ, and IL-8. The other cytokines measured showed no significant alteration. The suppression of senescence-associated proinflammatory cytokine secretion is particularly relevant to HIV-associated neurocognitive disorders, as chronic neuroinflammation contributes to neuronal damage, synaptic dysfunction, and cognitive decline. These data reinforce previous findings that CB2 receptor activation dampens neuroinflammatory pathways, thereby alleviating immune activation and excitotoxic stress within the CNS.
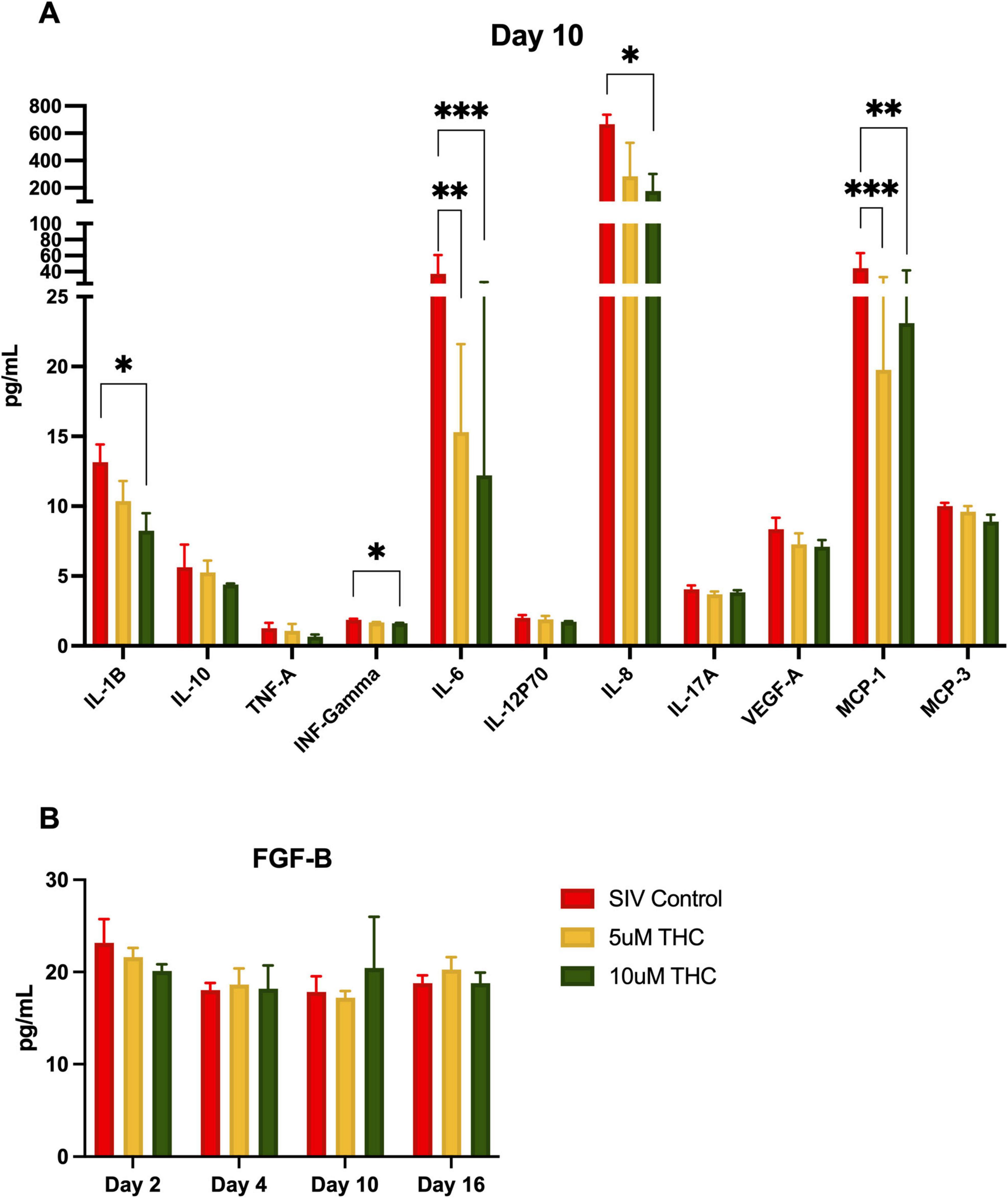
Figure 5. Δ9-tetrahydrocannabinol (THC) suppresses senescence-associated secretory phenotype in mixed glia cultures. Treatment of primary glial cultures from simian immunodeficiency virus (SIV)-infected macaques with THC altered secretion of multiple cytokines (A), with IL-1B, INF-γ, and IL-8 significantly reduced by 10 μM THC (*p < 0.05, **p < 0.01, ***p < 0.001). Both 5 μM and 10 μM THC reduced secretion of IL-6 and MCP-1. While other cytokines had reduced expression (including TNF-a, this did not reach the significance threshold). Fibroblast growth factor-basic (FGF-B) levels were quantified over time, revealing a slight, though not significant, increase in FGF-B secretion in 10 μM THC-treated astrocytes (B). Data are presented as mean ± SEM; statistical significance determined using one-way ANOVA followed by Tukey’s post hoc test.
FGF-B plays a crucial role in neuroprotection, synaptic plasticity, and glial homeostasis, and its significant upregulation in the serum of HIV-infected individuals reinforces THC’s potential anti-inflammatory effects. Therefore, we measured release of FGF-B in the same culture supernatant as above. We were somewhat surprised that there was no significant change in the level of FGF-B with either THC treatment (B), although there was a minor increase at 10 days of treatment with 10 μM THC.
4 Discussion
In this study, we provide new insights into how SIV infection and Tat protein affect glial function, morphology, and survival, with a particular focus on the potential modulatory role of cannabinoids in HIV-associated neuroinflammation. By integrating real-time impedance assessments, morphological analysis, cytokine profiling, and neurotrophic factor measurements, we demonstrate both the detrimental effects of viral neurotoxins and the complex role of THC in regulating glial responses.
Before investigating the effects of Tat and THC, we first examined how SIV infection itself alters glial proliferation and morphology (Figure 1). In agreement with previous in vivo studies demonstrating glial dysfunction and neuroinflammation in HIV/SIV infection, SIV+ glial cultures exhibited reduced adhesion, delayed colony formation, and disrupted monolayer development. By later time points, SIV+ cultures showed persistent abnormalities, including hypertrophic morphologies, with enlarged somas and retracted processes, indicating a shift toward a reactive phenotype that may exacerbate neuroinflammatory signaling. Notably, astrocytes derived from SIV+ animals that had received THC, had morphologic phenotypes more similar to those grown from naïve animals, further supporting the hypothesis that SIV infection primes glial populations for dysfunction, but that these may be inhibited by cannabinoid treatment. Studies of the molecular mechanisms underlying this are worth exploring, but are outwith the scope of this preliminary study.
Given this pre-existing glial dysfunction in SIV+ cultures, we next investigated whether Tat exposure alone can induce these impairments and whether THC has a compensatory or neuroprotective effect. Tat exposure induced a dose-dependent decline in glial cell index, demonstrating its well-documented inflammatory/negative effects on neural cells (Cosenza-Nashat et al., 2011; Rubin et al., 2018). We were surprised to find that treatment with 5 μM THC not only reversed the Tat-induced decline in cell index but, in two of the three Tat exposure groups, led to cell index values exceeding those of untreated controls (Figure 2). These suggest that Tat and THC may interact in a concentration-dependent manner, with higher Tat exposure shifting glial cells into a state more receptive to cannabinoid-mediated neuroprotection. The ability of THC to not only prevent further decline but enhance cell index in the presence of high Tat concentrations may indicate a potential compensatory or trophic effect of cannabinoid signaling in the context of Tat-induced neuroinflammation. Interestingly, repeated THC administration produced a sustained recovery in cell index, particularly in the high Tat group, raising the possibility of a compensatory response in surviving astrocytes. While Tat is traditionally regarded as neurotoxic, these findings suggest that Tat-induced glial activation may create a therapeutic window in which cannabinoid receptor signaling could exert a compensatory effect. This was unexpected, especially considering that in glia derived from SIV-infected animals, THC not only failed to restore baseline cell counts but worsened the decline (Figure 3).
The decline in cell numbers (Figure 3) highlights the need for further investigation into whether the increased cell index reflects improved astrocyte function or a maladaptive response, such as excessive glial activation or metabolic stress. Understanding how Tat primes astrocytes for a heightened response to THC could provide valuable insight into the dynamics of neuroimmune signaling in PLWH. Future studies will need to investigate whether the increased cell index in the high Tat group reflects improved astrocyte function or represents a pathological overcompensation, such as excessive glial activation or metabolic dysregulation (League et al., 2024; Ton and Xiong, 2013; Borgmann and Ghorpade, 2015; Fan and He, 2016; Hermes et al., 2021; Cotto et al., 2019).
Further supporting THC’s role in neuroprotection or possibly a reparative astrocytic phenotype, THC modulated glial morphology, promoting stellation and increased process complexity in primary mixed glial cultures (Figure 4). Given that astrocyte morphology is closely linked to functional outcomes in neuroinflammatory conditions, these findings suggest a shift toward a neuroprotective phenotype (Renner et al., 2012, Lee et al., 2014). The changes in astrocyte morphology observed in this study are consistent with cannabinoid receptor activation attenuating glial reactivity and promoting cellular adaptations that support neuronal survival. Similar effects have been observed in models of neurodegenerative disease, where cannabinoid treatment modulates glial activation states and reduces inflammatory signaling (Henriquez et al., 2020).
CB2 receptor activation enhances anti-inflammatory cytokine production, including IL-10, while suppressing proinflammatory mediators such as IL-12 and IL-23 (Correa et al., 2010). Our study adds to this by demonstrating that THC significantly reduced the secretion of senescence-associated proinflammatory cytokines, including IFN-γ, IL-6, and IL-1β, reinforcing its potential to counteract chronic neuroinflammation (Csiszar et al., 2012). Cannabis use in HIV-positive individuals is associated with lower levels of CNS inflammation and a reduced risk of neurocognitive impairment (Watson et al., 2020, 2023). Taken together, these findings suggest that THC fosters an anti-inflammatory, neuroprotective environment by modulating glial morphology, cytokine secretion, and trophic factor production, even though it may not enhance overall glial cell survival. This aligns with prior studies indicating that CB1 and CB2 receptor activation can suppress neuroinflammatory pathways and influence glial function (Correa et al., 2009; Manuzak et al., 2018). Given that cannabinoids regulate immune activation and synaptic function, the results presented here suggest that THC exerts neuroprotective and anti-inflammatory effects to counteract key mechanisms underlying HIV pathogenesis through modulation of glial adhesion, proliferation, and cytokine secretion.
Given the persistent burden of HABI despite effective antiretroviral therapy, novel strategies targeting neuroinflammation and synaptic dysfunction are urgently needed. These current findings provide compelling evidence for cannabinoid-based therapeutics as a strategy to mitigate neuroinflammation and synaptic dysfunction in PLWH. However, they also underscore the complexity of cannabinoid signaling in the CNS, emphasizing the need for precise modulation of the endocannabinoid system to achieve optimal neuroprotective outcomes. THC’s ability to suppress inflammatory cytokines, promote glial homeostasis, and enhance neurotrophic signaling suggests that cannabinoid-based interventions may offer a viable approach to slowing HAND progression, though the impact on overall glial viability remains a critical consideration.
An important consideration is the differential effects of cannabinoid compounds. While this study focuses on Δ9-THC, cannabidiol (CBD) has also been shown to exert anti-inflammatory and neuroprotective effects without the psychoactive properties of THC (DeMarino et al., 2022; Kaddour et al., 2022). Comparative analyses of THC and CBD in modulating cognitive function, neuroinflammation, and neurodegeneration in SIV-infected macaques may provide valuable insights into the development of targeted cannabinoid therapies. Further research using in vivo models and clinical studies is needed to assess the long-term efficacy and safety of THC in preserving neurocognitive function in people living with HIV.
We note there are a number of limitations in this preliminary study, including not examining Reactive Oxygen Species, calcium signaling, and glial cells in multiple brain regions. Indeed, with hindsight, we could have performed a series of studies to directly compare senescence signaling in several brain regions and correlated this with viral load (RNA or DNA). Further, as most of the brain tissues were generously donated by collaborators at TNPRC, we had no control over the lengths of time that animals were infected with SIV or treated with THC. As noted above, further studies, especially using in vivo models, will be required to determine if there is any substantial benefit to using THC for a potential therapeutic application.
This study provides compelling evidence that THC exerts neuroprotective and anti-inflammatory effects in HIV-associated brain injury (League et al., 2024) and supports the growing interest in cannabinoid-based therapeutics for neuroinflammatory disorders (Jana et al., 2024; Möller et al., 2025; Cárdenas-Rodríguez et al., 2024; Alraddadi et al., 2025). Understanding the molecular pathways underlying this response will be critical for developing targeted cannabinoid-based interventions for HIV-associated neuroinflammation. Future research will focus on elucidating the precise molecular mechanisms underlying THC’s effects on glial function and assessing the long-term efficacy and safety of cannabinoids in PLWH.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Tulane Institutional Animal Care and Use Committee (Protocol number: 3581). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AV: Data curation, Methodology, Writing – review & editing, Investigation, Writing – original draft, Formal analysis. MH: Validation, Data curation, Methodology, Conceptualization, Writing – original draft, Investigation, Writing – review & editing, Formal analysis. TP: Writing – original draft, Methodology, Investigation, Conceptualization. SD: Conceptualization, Writing – review & editing. EF: Methodology, Investigation, Writing – review & editing. AM: Formal analysis, Funding acquisition, Visualization, Project administration, Resources, Validation, Data curation, Software, Writing – review & editing, Methodology, Supervision, Conceptualization, Writing – original draft, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. NHP studies in the MacLean Lab are supported by NIH funds: R21-MH113517, R01-HL152804, R01-NS104016, R21-MH125716, U42OD024282, U42OD010568, and the TNPRC base grant P51-OD11104. Flow Cytometry data acquisition and analysis was supported by NIH S10 OD026800. Tulane University also supports this research through the Tulane Brain Institute and the Tulane Neuroscience Program. Antiretroviral drugs were generously provided by Gilead Sciences, Inc. (TDF and FTC) and ViiV Healthcare (DTG). Simian Immunodeficiency Virus (SIV)mac239 Tat Region, ARP-12765, contributed by DAIDS/NIAID, was obtained through the NIH HIV Reagent Program, Division of AIDS, NIAID, NIH. A total of 5 mg/mL Δ9-Tetrahydrocannabinol (THC) in ethyl alcohol (DEA code 7370) was provided the National Institute on Drug Abuse (NIDA) Drug Supply Program (DSP). Fresh brain tissue from several NIH-funded studies were made available at necropsy. We are indebted to the several collaborators over the years, including Ronald Veazey, Nicholas Maness, Mahesh Mohan, and Andrew Lackner.
Acknowledgments
We would like to thank the pathologists and staff of the Anatomic Pathology Core RRID:SCR_024606, Clinical Pathology Core RRID:SCR_024609, Confocal Microscopy and Molecular Pathology Core RRID:SCR_024613, Virus Characterization, Isolation, Production and Sequencing Core RRID:SCR_024679, and Flow Cytometry Core RRID:SCR_024611, Tulane National Primate Research Center RRID:SCR_008167. This work could not happen without the dedicated staff and veterinarians of the Division of Veterinary Medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2025.1642917/full#supplementary-material
Supplementary Figure 1 | Representative images of SIV+ mixed glial cultures treated with THC. Phase-contrast microscopy images of primary mixed glial cultures derived from SIV+ rhesus macaques following administration of 5 or 10 μM THC, compared to control conditions, over a 14 days period. Images were captured every 48 h to assess changes in cell number, density, morphology, and process formation over time. While the higher concentration of THC led to a progressive reduction in overall cell density (as observed in Figure 3), surviving glial cells exhibited enhanced stellation and process elongation: a possible shift toward a neuroprotective phenotype. This suggests that THC promotes cytoskeletal remodeling and astrocyte activation, potentially facilitating enhanced synaptic support and immune modulation despite an overall reduction in cell viability. Notably, in the 10 μM THC condition, glial cells demonstrated the most pronounced process elongation and branching complexity, possibly compensating for the decline in total cell numbers. These findings highlight the dual role of THC in modulating glial survival and function, where higher THC concentrations may induce cellular stress leading to cell loss, yet simultaneously promote a more complex and supportive astrocytic network.
References
Alger, B. E. (2002). Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog. Neurobiol. 68, 247–286. doi: 10.1016/s0301-0082(02)00080-1
Alraddadi, E. A., Aljuhani, F. F., Alsamiri, G. Y., Hafez, S. Y., Alselami, G., Almarghalani, D. A., et al. (2025). The effects of cannabinoids on ischemic stroke-associated neuroinflammation: A systematic review. J. Neuroimmune Pharmacol. 20:12. doi: 10.1007/s11481-025-10171-z
Ascherl, G., Sgadari, C., Bugarini, R., Bogner, J., Schatz, O., Ensoli, B., et al. (2001). Serum concentrations of fibroblast growth factor 2 are increased in HIV type 1-infected patients and inversely related to survival probability. AIDS Res. Hum. Retroviruses 17, 1035–1039. doi: 10.1089/088922201300343717
Borgmann, K., and Ghorpade, A. (2015). HIV-1, methamphetamine and astrocytes at neuroinflammatory Crossroads. Front. Microbiol. 6:1143. doi: 10.3389/fmicb.2015.01143
Bredt, B. M., Higuera-Alhino, D., Shade, S. B., Hebert, S. J., McCune, J. M., and Abrams, D. I. (2002). Short-term effects of cannabinoids on immune phenotype and function in HIV-1-infected patients. J. Clin. Pharmacol. 42, 82S–89S. doi: 10.1002/j.1552-4604.2002.tb06007.x
Cárdenas-Rodríguez, N., Ignacio-Mejía, I., Correa-Basurto, J., Carrasco-Vargas, H., Vargas-Hernández, M. A., Albores-Méndez, E. M., et al. (2024). Possible role of cannabis in the management of neuroinflammation in patients with Post-COVID condition. Int. J. Mol. Sci. 25:3805. doi: 10.3390/ijms25073805
Chaillon, A., Nakazawa, M., Anderson, C., Christensen-Quick, A., Ellis, R. J., Franklin, D., et al. (2020). Effect of cannabis use on human immunodeficiency virus DNA during suppressive antiretroviral therapy. Clin. Infect. Dis. 70, 140–143. doi: 10.1093/cid/ciz387
Correa, F., Docagne, F., Mestre, L., Clemente, D., Hernangómez, M., Loría, F., et al. (2009). A role for CB2 receptors in anandamide signalling pathways involved in the regulation of IL-12 and IL-23 in microglial cells. Biochem. Pharmacol. 77, 86–100. doi: 10.1016/j.bcp.2008.09.014
Correa, F., Hernangómez, M., Mestre, L., Loría, F., Spagnolo, A., Docagne, F., et al. (2010). Anandamide enhances IL-10 production in activated microglia by targeting CB(2) receptors: Roles of ERK1/2. JNK, and NF-kappaB. Glia 58, 135–147. doi: 10.1002/glia.20907
Cosenza-Nashat, M. A., Bauman, A., Zhao, M. L., Morgello, S., Suh, H. S., and Lee, S. C. (2011). Cannabinoid receptor expression in HIV encephalitis and HIV-associated neuropathologic comorbidities. Neuropathol. Appl. Neurobiol. 37, 464–483. doi: 10.1111/j.1365-2990.2011.01177.x
Cotto, B., Natarajanseenivasan, K., and Langford, D. (2019). HIV-1 infection alters energy metabolism in the brain: Contributions to HIV-associated neurocognitive disorders. Prog. Neurobiol. 181:101616. doi: 10.1016/j.pneurobio.2019.101616
Csiszar, A., Sosnowska, D., Wang, M., Lakatta, E. G., Sonntag, W. E., and Ungvari, Z. (2012). Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: Reversal by resveratrol treatment. J. Gerontol. A Biol. Sci. Med. Sci. 67, 811–820. doi: 10.1093/gerona/glr228
DeMarino, C., Cowen, M., Khatkar, P., Cotto, B., Branscome, H., Kim, Y., et al. (2022). Cannabinoids reduce extracellular vesicle release from HIV-1 infected myeloid cells and inhibit viral transcription. Cells 11:723. doi: 10.3390/cells11040723
Fan, Y., and He, J. J. (2016). HIV-1 tat promotes lysosomal exocytosis in astrocytes and contributes to astrocyte-mediated tat neurotoxicity. J. Biol. Chem. 291, 22830–22840. doi: 10.1074/jbc.M116.731836
Guillemin, G., Boussin, F. D., Croitoru, J., Franck-Duchenne, M., Le Grand, R., Lazarini, F., et al. (1997). Obtention and characterization of primary astrocyte and microglial cultures from adult monkey brains. J. Neurosci. Res. 49, 576–591. doi: 10.1002/(SICI)1097-4547(19970901)49:5<576::AID-JNR8>3.0.CO;2-8
Henriquez, J. E., Bach, A. P., Matos-Fernandez, K. M., Crawford, R. B., and Kaminski, N. E. (2020). Δ9-Tetrahydrocannabinol (THC) impairs CD8+ T cell-mediated activation of astrocytes. J. Neuroimmune Pharmacol. 15, 863–874. doi: 10.1007/s11481-020-09912-z
Hermes, D. J., Yadav-Samudrala, B. J., Xu, C., Paniccia, J. E., Meeker, R. B., Armstrong, M. L., et al. (2021). GPR18 drives FAAH inhibition-induced neuroprotection against HIV-1 Tat-induced neurodegeneration. Exp. Neurol. 341:113699. doi: 10.1016/j.expneurol.2021.113699
Horn, M. D., and MacLean, A. G. (2021). Extracellular vesicles as a means of viral immune evasion, CNS invasion, and glia-induced neurodegeneration. Front. Cell Neurosci. 15:695899. doi: 10.3389/fncel.2021.695899
Howlett, A. C., and Abood, M. E. (2017). CB1 and CB2 receptor pharmacology. Adv. Pharmacol. 80, 169–206. doi: 10.1016/bs.apha.2017.03.007
Jana, A., Nath, A., Sen, P., Kundu, S., Alghamdi, B. S., Abujamel, T. S., et al. (2024). Unraveling the endocannabinoid system: Exploring its therapeutic potential in autism spectrum disorder. Neuromol. Med. 26:20. doi: 10.1007/s12017-024-08781-6
Kaddour, H., McDew-White, M., Madeira, M. M., Tranquille, M. A., Tsirka, S. E., Mohan, M., et al. (2022). Chronic delta-9-tetrahydrocannabinol (THC) treatment counteracts SIV-induced modulation of proinflammatory microRNA cargo in basal ganglia-derived extracellular vesicles. J. Neuroinflamm. 19:225. doi: 10.1186/s12974-022-02586-9
Keen, L., Abbate, A., Blanden, G., Priddie, C., Moeller, F. G., and Rathore, M. (2019). Confirmed marijuana use and lymphocyte count in black people living with HIV. Drug Alcohol. Depend. 198, 112–115. doi: 10.1016/j.drugalcdep.2018.11.018
Kumar, V., Torben, W., Mansfield, J., Alvarez, X., Vande Stouwe, C., Li, J., et al. (2019). Cannabinoid attenuation of intestinal inflammation in chronic SIV-Infected rhesus macaques involves T cell modulation and differential expression of Micro-RNAs and pro-inflammatory genes. Front. Immunol. 10:914. doi: 10.3389/fimmu.2019.00914
League, A. F., Yadav-Samudrala, B. J., Kolagani, R., Cline, C. A., Jacobs, I. R., Manke, J., et al. (2024). A helping HAND: Therapeutic potential of MAGL inhibition against HIV-1-associated neuroinflammation. Front. Immunol. 15:1374301. doi: 10.3389/fimmu.2024.1374301
Lee, K. M., Chiu, K. B., Renner, N. A., Sansing, H. A., Didier, P. J., and MacLean, A. G. (2014). Form follows function: Astrocyte morphology and immune dysfunction in SIV neuroAIDS. J. Neurovirol. 20, 474–484. doi: 10.1007/s13365-014-0267-1
MacLean, A. G., Orandle, M. S., MacKey, J., Williams, K. C., Alvarez, X., and Lackner, A. A. (2002). Characterization of an in vitro rhesus macaque blood-brain barrier. J. Neuroimmunol. 131, 98–103. doi: 10.1016/s0165-5728(02)00256-4
MacLean, A. G., Rasmussen, T. A., Bieniemy, D., and Lackner, A. A. (2004a). Activation of the blood-brain barrier by SIV (simian immunodeficiency virus) requires cell-associated virus and is not restricted to endothelial cell activation. Biochem. Soc. Trans. 32(Pt 5), 750–752. doi: 10.1042/BST0320750
MacLean, A. G., Rasmussen, T. A., Bieniemy, D. N., Alvarez, X., and Lackner, A. A. (2004b). SIV-induced activation of the blood-brain barrier requires cell-associated virus and is not restricted to endothelial cell activation. J. Med. Primatol. 33, 236–242. doi: 10.1111/j.1600-0684.2004.00077.x
MacLean, A. G., Walker, E., Sahu, G. K., Skowron, G., Marx, P., von Laer, D., et al. (2014). A novel real-time CTL assay to measure designer T-cell function against HIV Env(+) cells. J. Med. Primatol. 43, 341–348. doi: 10.1111/jmp.12137
Manuzak, J. A., Gott, T. M., Kirkwood, J. S., Coronado, E., Hensley-McBain, T., Miller, C., et al. (2018). Heavy cannabis use associated with reduction in activated and inflammatory immune cell frequencies in antiretroviral therapy-treated human immunodeficiency virus-infected individuals. Clin. Infect. Dis. 66, 1872–1882. doi: 10.1093/cid/cix1116
Möller, J. E. L., Schmitt, F. W., Günther, D., Stöver, A., and Bouter, Y. (2025). The synthetic cannabinoid WIN 55,212-2 attenuates cognitive and motor deficits and reduces amyloid load in 5XFAD Alzheimer mice. Pharmacol. Biochem. Behav. 247:173944. doi: 10.1016/j.pbb.2024.173944
Nightingale, S., Ances, B., Cinque, P., Dravid, A., Dreyer, A. J., Gisslén, M., et al. (2023). Cognitive impairment in people living with HIV: Consensus recommendations for a new approach. Nat. Rev. Neurol. 19, 424–433. doi: 10.1038/s41582-023-00813-2
Pandey, R., Mousawy, K., Nagarkatti, M., and Nagarkatti, P. (2009). Endocannabinoids and immune regulation. Pharmacol. Res. 60, 85–92. doi: 10.1016/j.phrs.2009.03.019
Piomelli, D. (2003). The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 4, 873–884. doi: 10.1038/nrn1247
Ray, P. E., Liu, X. H., Xu, L., and Rakusan, T. (1999). Basic fibroblast growth factor in HIV-associated hemolytic uremic syndrome. Pediatr. Nephrol. 13, 586–593. doi: 10.1007/s004670050749
Renner, N. A., Sansing, H. A., Inglis, F. M., Mehra, S., Kaushal, D., Lackner, A. A., et al. (2013). Transient acidification and subsequent proinflammatory cytokine stimulation of astrocytes induce distinct activation phenotypes. J. Cell Physiol. 228, 1284–1294. doi: 10.1002/jcp.24283
Renner, N. A., Sansing, H. A., Morici, L. A., Inglis, F. M., Lackner, A. A., and Maclean, A. G. (2012). Microglia activation by SIV-infected macrophages: alterations in morphology and cytokine secretion. J. Neurovirol. 18, 213–221. doi: 10.1007/s13365-012-0100-7
Rubin, L. H., Sacktor, N., Creighton, J., Du, Y., Endres, C. J., Pomper, M. G., et al. (2018). Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS 32, 1661–1667. doi: 10.1097/QAD.0000000000001858
Shiau, S., Arpadi, S. M., Yin, M. T., and Martins, S. S. (2017). Patterns of drug use and HIV infection among adults in a nationally representative sample. Addict. Behav. 68, 39–44. doi: 10.1016/j.addbeh.2017.01.015
Smyth, B., and Collister, D. (2025). Searching for fire amid the smoke: Will cannabinoids prove useful and safe for those with CKD? Kidney Int. Rep. 10, 988–990. doi: 10.1016/j.ekir.2025.01.031
Starr, A., Jordan-Sciutto, K. L., and Mironets, E. (2021). Confound, cause, or cure: The effect of cannabinoids on HIV-associated neurological sequelae. Viruses 13:1242. doi: 10.3390/v13071242
Thames, A. D., Mahmood, Z., Burggren, A. C., Karimian, A., and Kuhn, T. P. (2016). Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS Care 28, 628–632. doi: 10.1080/09540121.2015.1124983
Ton, H., and Xiong, H. (2013). Astrocyte dysfunctions and HIV-1 neurotoxicity. J. AIDS Clin. Res. 4:255. doi: 10.4172/2155-6113.1000255
Van Zandt, A. R., and MacLean, A. G. (2023). Advances in HIV therapeutics and cure strategies: Findings obtained through non-human primate studies. J. Neurovirol. 29, 389–399. doi: 10.1007/s13365-023-01162-y
Watson, C. W., Campbell, L. M., Sun-Suslow, N., Hong, S., Umlauf, A., Ellis, R. J., et al. (2021). Daily cannabis use is associated with lower CNS inflammation in people with HIV. J. Int. Neuropsychol. Soc. 27, 661–672. doi: 10.1017/S1355617720001447
Watson, C. W., Paolillo, E. W., Morgan, E. E., Umlauf, A., Sundermann, E. E., Ellis, R. J., et al. (2020). Cannabis exposure is associated with a lower likelihood of neurocognitive impairment in people living with HIV. J. Acquir. Immune Defic. Syndr. 83, 56–64. doi: 10.1097/QAI.0000000000002211
Watson, C. W., Sundermann, E., Helm, J., Paolillo, E. W., Hong, S., Ellis, R. J., et al. (2023). A longitudinal study of cannabis use and risk for cognitive and functional decline among older adults with HIV. AIDS Behav. 27, 3401–3413. doi: 10.1007/s10461-023-04056-6
Wilson, R. I., and Nicoll, R. A. (2001). Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410, 588–592. doi: 10.1038/35069076
Yadav-Samudrala, B. J., Ravula, H. P., Barmada, K. M., Dodson, H., Poklis, J. L., Ignatowska-Jankowska, B. M., et al. (2024). Acute effects of monoacylglycerol lipase inhibitor ABX1431 on neuronal hyperexcitability, nociception, locomotion, and the endocannabinoid system in HIV-1 tat male mice. Cannabis Cannabinoid Res. 9, 1500–1513. doi: 10.1089/can.2023.0247
Keywords: NeuroHIV, cannabinoid receptor modulation, astrocyte activation, neuroprotection, endocannabinoid signaling, HIV-associated brain injury (HABI), CB1/CB2 receptor activation, Tat neurotoxicity
Citation: Van Zandt AR, Horn MD, Peterson TA, Dickinson SY, Frost EM and MacLean AG (2025) THC reverses SIV-induced senescence in astrocytes: possible compensatory mechanism against HIV associated brain injury? Front. Cell. Neurosci. 19:1642917. doi: 10.3389/fncel.2025.1642917
Received: 10 June 2025; Accepted: 12 September 2025;
Published: 30 September 2025.
Edited by:
Walace Gomes-Leal, Federal University of Western Pará, BrazilReviewed by:
Juarez Antonio Simões Quaresma, Universidade Federal de São Paulo, BrazilLuiz Fernando Almeida Machado, Federal University of Pará, Brazil
Copyright © 2025 Van Zandt, Horn, Peterson, Dickinson, Frost and MacLean. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew G. MacLean, YW1hY2xlYW5AdHVsYW5lLmVkdQ==
†Present address: Miranda D. Horn, Department of Biological Sciences, Neuroscience Program, Ohio Wesleyan University, Delaware, OH, United States; Sarah Y. Dickinson, Department of Psychological and Brain Sciences, University of Massachusetts Amherst, Amherst, MA, United States
 Alison R. Van Zandt
Alison R. Van Zandt Miranda D. Horn
Miranda D. Horn Tiffany A. Peterson1,2
Tiffany A. Peterson1,2 Sarah Y. Dickinson
Sarah Y. Dickinson Andrew G. MacLean
Andrew G. MacLean