- 1Department of Neurobiology and Behavior, University of California, Irvine, CA, United States
- 2Department of Neuroscience, Canadian Centre for Behavioral Neuroscience, University of Lethbridge, Lethbridge, AB, Canada
Early-life stress (ELS) and enrichment often have opposing effects on long-term cognitive abilities. Deprivation, such as institutionalized care during early childhood neurodevelopmental periods, results in lifelong working memory and recall deficits. In contrast, enrichment facilitates new learning and slows cognitive decline due to aging and neurodegenerative diseases. Similarly, in rodent models, enrichment facilitates learning whereas ELS induces prominent spatial memory deficits. Environmental enrichment (EE) and ELS can cause opposing changes in hippocampal structure (e.g., shifts in synaptic density) that largely depend on experimental conditions. However, it remains untested whether EE can rescue the behavioral disruptions caused by ELS and how this would impact the hippocampus at advanced ages. To address this, we conducted a longitudinal study on ELS mice, extensively training them on a cognitive enrichment track (ET) or an exercise alone control track (CT). After this, the mice underwent repeated memory testing followed by brain extraction for anatomical analysis of their hippocampus. We found that ET reversed spatial memory deficits at 6, 13, and 20 months and reduced the number of dentate gyrus (DG) to CA3 synapses. Surprisingly, this reduction occurred at excitatory MF synapses surrounding CA3 somas in the stratum pyramidale—a layer not typically associated with MF terminals. Collectively, these findings suggest that cognitive enrichment during early adulthood may reverse ELS-induced spatial memory deficits by adjusting synaptic connectivity between the DG and CA3.
Introduction
Severe early-life adversity, affecting close to 50% of the world’s children (Fenoglio et al., 2006), can cause both emotional and cognitive disturbances. For instance, children raised in institutionalized settings often exhibit memory deficits and poor impulse control (Pollak et al., 2010); likely contributing to their delayed language acquisition and low scholastic aptitude (Beckett et al., 2007; Eigsti et al., 2011). Evidence suggests that early childhood deprivation, such as parental neglect, has a particularly negative impact on executive function and memory (Wodarski et al., 1990; Eckenrode et al., 1993; Spratt et al., 2012) because this sensitive time period (first 2–3 years in humans and first 3–4 weeks in rodents) is critical for the maturation of brain systems necessary for learning and memory, such as the hippocampus (Malave et al., 2022; Kloc et al., 2020; Leinekugel et al., 2002). This may be why ELS during this time period can cause long-lasting deficits in declarative learning and hippocampal function. Adults who have experienced ELS have difficulty remembering episodic events and perform poorly on delayed word recall tasks (Ding and He, 2021; Ma et al., 2021; Cai et al., 2024). These individuals have smaller hippocampal volumes, especially their dentate gyrus (DG) (Humphreys et al., 2019; Youssef et al., 2019; Koyama et al., 2022; Kawamoto et al., 2023). Importantly, these findings have been replicated in rodent models designed to mimic maternal neglect (Walker et al., 2017). As adults, these ELS animals perform poorly on hippocampus-dependent spatial memory tasks such as the Morris water maze and object location memory (OLM) (Brunson et al., 2005; Cui et al., 2006; Rice et al., 2008; Ivy et al., 2010; Pollak et al., 2010; Naninck et al., 2015; Chen et al., 2016; Molet et al., 2016b; Naninck et al., 2017) consistent with human findings, these animals have smaller hippocampi and dendritic atrophy in CA3 and CA1 neurons (Brunson et al., 2005; Molet et al., 2016a; Teicher et al., 2016).
Unfortunately, there are very few non-invasive treatments for individuals suffering from the effects of ELS; however, experience-based behavioral interventions may help. For example, the Bucharest intervention project (and related studies) found that adoption of institutionalized children into more nurturing environments correlated with improved cognition and higher IQ scores (i.e., the earlier the better) (Tizard and Rees, 1974; Nelson et al., 2007; Bos et al., 2009; Almas et al., 2016). And, enriching activities later in life can also facilitate cognitive recovery (Cai et al., 2024).
In rodent models, environmental enrichment (EE) improves cognitive functions, including spatial learning, memory, and task learning (Cheng et al., 2022; Rountree-Harrison et al., 2018; Bennett et al., 2006; Williams et al., 2001; Woodcock and Richardson, 2000; Zeleznikow-Johnston et al., 2017). Consistent with this, we found that extensive cognitive enrichment on a specially designed “enrichment track” leads to dramatic improvements on a wide variety of memory tasks (Gattas et al., 2022). Mechanistically, EE promotes synaptogenesis and adult hippocampal neurogenesis (Speisman et al., 2013) increases spine count (Jung and Herms, 2014) and dendritic complexity (Connor et al., 1982), modulates synaptic signaling (Pintori et al., 2024) and enhances long-term potentiation in the hippocampus (Cortese et al., 2018; Artola et al., 2006). Additionally, it improves sensory processing and neural coding efficiency (Engineer et al., 2004; LeMessurier et al., 2019). For instance, EE improves the ability of the hippocampus to distinguish between different environments (i.e., better pattern separation), which in turn promotes better spatial learning (Bilkey et al., 2017; Ventura et al., 2024).
The DG-CA3 connection is particularly susceptible to experience-dependent plasticity (Henze et al., 2000; Urban et al., 2001). ELS and EE can have opposing effects on this such as the rate of neurogenesis in the DG and the magnitude of DG-CA3 long-term plasticity (LTP and LTD) (Alwis and Rajan, 2014; Kempermann, 2019). In contrast, both ELS and EE increase mossy fiber sprouting (Brunson et al., 2001; Galimberti et al., 2006; Bramati et al., 2023). This paradoxical finding—that both detrimental ELS and beneficial EE increase mossy fiber sprouting—highlights the need to examine not just the quantity but also the location and functional properties of these synaptic changes. This is especially interesting, considering the positive correlation between mossy fiber expansion and spatial memory performance (Schwegler et al., 1990; Ramírez-Amaya et al., 2001; Carasatorre et al., 2015). With respect to EE, however, it is important to recognize that many of these structural and physiological changes largely depend on experimental conditions such as the animal’s age and the duration of enrichment (Gogolla et al., 2009; Bednarek and Caroni, 2011); making it difficult to predict how ELA and EE would mechanistically interact.
Despite extensive research on ELS and EE individually, critical knowledge gaps remain. First, while EE has been shown to enhance cognition in normal animals, it is unknown whether cognitive enrichment can reverse established ELS-induced memory deficits, particularly across the lifespan. Second, although both ELS and EE affect hippocampal structure, the specific synaptic mechanisms underlying potential ELS-EE interactions remain unexplored. Finally, previous studies have not distinguished between the effects of cognitive enrichment versus exercise alone in the context of ELS recovery, limiting our understanding of which intervention components are most therapeutic.
We hypothesized that cognitive enrichment would reverse ELS-induced spatial memory deficits across the lifespan through specific modifications to mossy fiber synaptic connectivity between the dentate gyrus and CA3 region. To test this hypothesis, we pursued three specific objectives: (1) determine whether cognitive enrichment can reverse ELS-induced spatial memory deficits longitudinally from young adulthood through aging; (2) distinguish the effects of cognitive enrichment from exercise alone using a controlled track design; and (3) identify the underlying synaptic mechanisms by examining mossy fiber terminal density and size distribution in the hippocampus.
Using a longitudinal approach, we first trained ELS mice on our complex obstacle enrichment track (ET) (Supplementary Figures 1A, B). This 3-month protocol produces dramatic and broad long-term memory enhancements well above that of standard enrichment procedures (Gattas et al., 2022). Moreover, the incorporation of a simple ramp control track (CT) group allowed us to disambiguate the effect of exercise alone. Following this, we repeatedly tested mice on spatial and object recognition tasks across their lifespan. Finally, we assessed whether these transgenic mice (GCaMP6fs) had hippocampal structural changes using a combination of endogenous fluorescence and nanobodies. To specifically visualize MF projections and their synapses, we took advantage of the selective expression of the GCaMP6fs signal in the DG (no signal in CA3 neurons) together with excitatory synaptic markers such as PSD-95, which are primarily considered glutamatergic (Walker et al., 2001; Gutiérrez et al., 2003); however, there is some evidence that these terminals also contain GABA (Cabezas et al., 2012; Uchigashima et al., 2007). This approach allowed us to test whether cognitive enrichment could rescue ELS deficits and identify the specific synaptic adaptations underlying any behavioral recovery. Unexpectedly, we found clusters of these atypical excitatory MF synapses surrounding CA3 cell bodies whose density was regulated by the cognitive component of enrichment.
Results
Cognitive enrichment rescues the spatial memory deficits of ELS mice
First, we induced ELS using the disrupted maternal care paradigm (limited bedding and nesting method, LBN) since it causes progressive memory deficits as rodents age (Molet et al., 2016a; Rocha et al., 2021). Following this we assigned mice to either enrichment or control groups (Figure 1A). Our previous findings suggest that ET training produces broad and dramatic memory enhancements (Gattas et al., 2022). We speculated, however, that a scaled back version of the ET training protocol would more selectively benefit hippocampal circuits that are especially vulnerable to ELS (Scholz et al., 2015; Molet et al., 2016b; Hoeijmakers et al., 2018; Manno et al., 2022). Thus, we ran our enrichment protocol for three 30 min sessions per week, one quarter of our original six 1 h sessions per week.
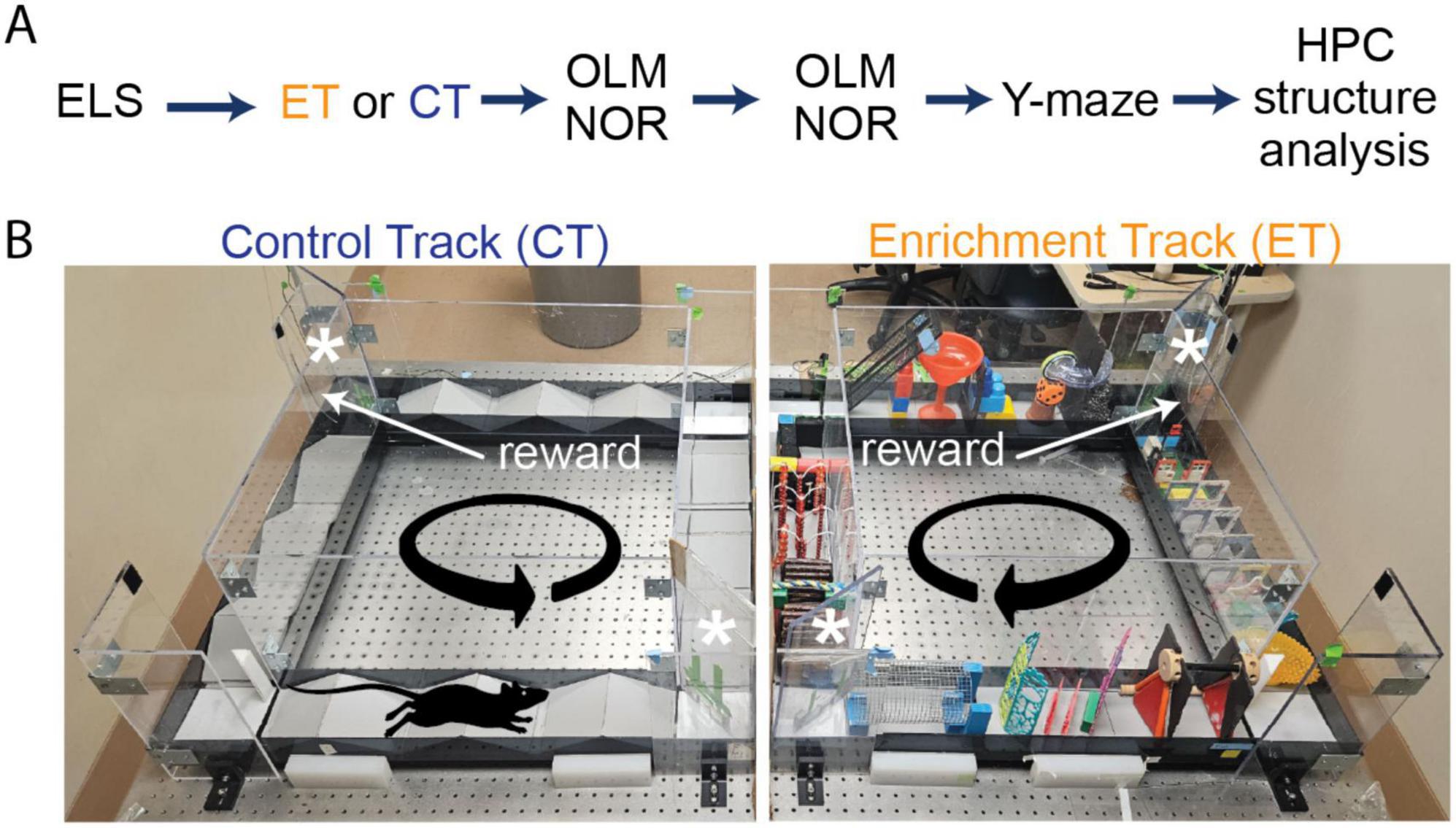
Figure 1. Experimental timeline of enrichment training, behavioral tests and structural analysis on early-life stressed (ELS) and control mice. (A) Initially, we separated ELS mice into two sex matched groups. Half of the ELS mice (four males and four females) ran on the enrichment track (ET) track (orange) while the other half (four males and four females) ran on the control track (CT) track (dark blue). Following this, both groups received longitudinal behavioral testing and hippocampal structural analysis. (B) We ran double-housed adult ELS mice on our automated side-by-side environmental enrichment (EE) setups such that one mouse ran on the control track (CT, left panel) while its cage-mate ran on the enrichment track (ET, right panel). On the ET track, mice had to navigate through multiple obstacles (obstacle configuration changed every session), whereas the CT only ran over simple ramps. We place two one-way doors (asterisks) at opposite corners of the square track to minimize backtracking. Mice received a reward (sweetened condensed milk) upon completion of each lap controlled by an automated delivery system using an overhead camera tracking system.
We then tested both groups (ELS + ET, 4M and 4F, n = 8) and (ELS + CT, 4M and 4F, n = 8) on OLM and NOR memory tests at mature (6 months), middle (13 months) and old (20 months) ages to determine if behavioral gains would last as the mice aged (Figures 1A, B). We observed a significant group level (ET vs. CT) difference in OLM [three-way ANOVA, F(1,12) = 8.80, p = 0.012] but no significant effect of age [F(1,12) = 1.69, p = 0.218] or sex [F(1,12) = 0.97, p = 0.345] (Figure 2A). In contrast to OLM and also the results of Gattas et al. (2022), we found no significant group [three-way ANOVA, F(1,12) = 0.074, p = 0.791], age [F(1,12) = 2.93, p = 0.113], or sex [F(1,12) = 0.11, p = 0.744] effect in the NOR test (Figure 2B). In the final OLM/NOR time-point (18 months), the mice, unfortunately, did not spend enough time exploring the objects to get accurate testing scores (values are the sum of investigation times for both objects). Compared to their mature and middle-age scores, these older mice spent significantly less time investigating the objects [Supplementary Figure 2B, two-way ANOVA, P < 0.0001, F(2,88) = 30.86]; likely due to habituation to the OLM/NOR setup, an age-dependent reduction in exploratory behavior, or a combination of both. To reinvigorate their exploratory behavior, we switched to a completely novel setup, the spatial Y-maze. On test day, we found that ET mice (2M &3F, n = 5) spent significantly more time exploring the novel arm than CT mice (3M &4F, n = 7) in a group comparison [two-way ANOVA, F(1,8) = 19.89, p = 0.002], with no significant effect of sex [F(1,8) = 1.81, p = 0.215], suggesting that ET produced a life-long rescue of spatial memory formation or retention (Figures 2C, D).
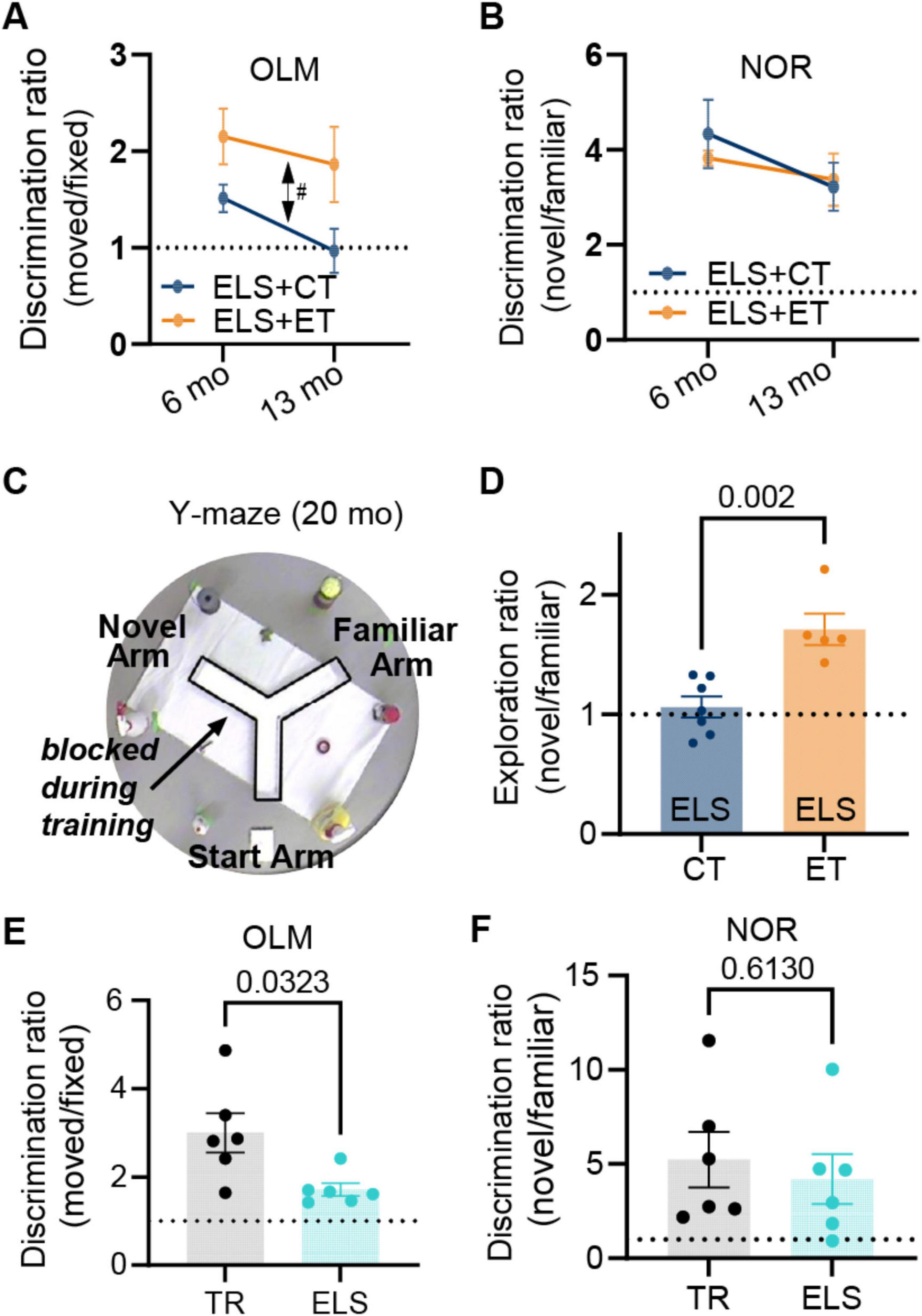
Figure 2. Enrichment track (ET) training reverses life-long spatial memory deficits caused by early-life stress (ELS). Longitudinal testing of ELS mice on object-location (OLM) and novel object recognition (NOR) at mature (6 months) and middle-age (13 months) time points revealed that. (A) The ELS + ET group (orange, n = 8 mice) had improved 24 h memory [double-sided arrow, three-way repeated measures ANOVA, F(1,12) = 8.80, p = 0.012] for OLM relative to the ELS + CT control group (dark blue, n = 8). There was no significant effect of age or sex. (B) In contrast for NOR, we found no group difference [F(1,12) = 0.074, p = 0.791] and no effect of age or sex. The same mice (n = 16) were tested on OLM and NOR. A value of 1 represents equal time with both objects (dashed line). (C) In a final (20 months) Y-maze test, the surviving mice (5M and 7F, n = 12) explored the two open arms for 5 min while the third arm remained blocked (transparent walls surrounded by distinct external cues) followed 24 h later by testing (5 min session) where mice were allowed to freely explore all three arms. (D) The ELS + ET group (orange, n = 5) spent significantly more time exploring the novel arm in comparison to the ELS + CT group (dark blue, n = 7) [two-way ANOVA, F(1,8) = 19.89, p = 0.002]. We calculated the active exploration ratios by dividing the occupancy times of the novel arm by that of the familiar arm (we excluded periods of immobility lasting longer than 1 s). (E,F) To verify that ELS alone (in the absence of EE) induced memory deficits, we tested mice on OLM and NOR using the same conditions as group 1. (E) In OLM, the TR group (black dots, n = 6 males) had significantly higher discrimination ratios (moved/fixed) compared to the ELS group (cyan dots, n = 6 males), indicating that they spent more relative time investigating the moved object (p = 0.0323, unpaired two-tailed t-test with Welch’s correction). (F) For NOR we observed that no significant difference in discrimination ratios between the same TR and ELS groups (p = 0.613, unpaired two-tailed t-test). Plots include values from individual mice [circles in (D–F)], mean [bar height and circles in (A,B)], and SEM (error bars).
Importantly, our design mitigates the exercise confound since both groups must run laps to receive rewards. In fact, the control track (CT) group, that ran over simple ramps, completed more laps than the ET group that had to navigate through complex obstacles (Supplementary Figure 1C, unpaired two-tailed t-test, p < 0.0001). Thus, additional exercise cannot account for these observed memory enhancements in the ET group.
ELS causes spatial memory deficits
Many studies have shown that ELS causes significant memory disruptions (Brunson et al., 2005; Cui et al., 2006; Rice et al., 2008; Ivy et al., 2010; Wang et al., 2011; Naninck et al., 2015, 2017; Molet et al., 2016a; Hoeijmakers et al., 2018). To confirm these findings, we tested a separate non-enriched group of male mice under either ELS or typical rearing (TR) conditions (Figure 1A, chartreuse arrow). These ELS mice and their age-matched TR controls remained under normal housing conditions until behavioral testing at mature adult ages (∼6 months). The ELS group (6 months) showed memory impairments in the OLM task (p = 0.032, two-tailed t-test, Figure 2E bottom) but not in NOR (p = 0.613, Figure 2F bottom). Taken together, our findings are consistent with other studies that ELS can cause a selective disruption of hippocampal -dependent memories (Molet et al., 2016b; Hoeijmakers et al., 2018) but also see (Ivy et al., 2010; Naninck et al., 2015).
Cognitive enrichment does not change gross hippocampal morphology
Studies have shown that enrichment and ELS can cause volumetric changes in hippocampal subfields such as the mossy fiber (MF) pathway (Brunson et al., 2005; Galimberti et al., 2006; Molet et al., 2016b; Teicher et al., 2016; Humphreys et al., 2019; Youssef et al., 2019; Bramati et al., 2023). This prompted us to examine whether these ELS GCaMP-expressing mice mice had any gross morphological changes in their hippocampal structure. We used Thy1- because our earlier pilot images revealed that their DG neurons and axons (the MF pathway) were filled with GCaMP signal. One month following the final Y-maze test (21 months of age), we extracted brains for fluorescent imaging of endogenous GCaMP and DAPI signals in the dorsal hippocampus. Importantly, this GCaMP signal was absent from CA3 neurons, indicating that the GCaMP signal in the CA3 subfield was specific to MFs (Figure 3B lower right inset). As expected, there was sparse GCaMP expression in CA1 neurons (Figure 3B top inset).
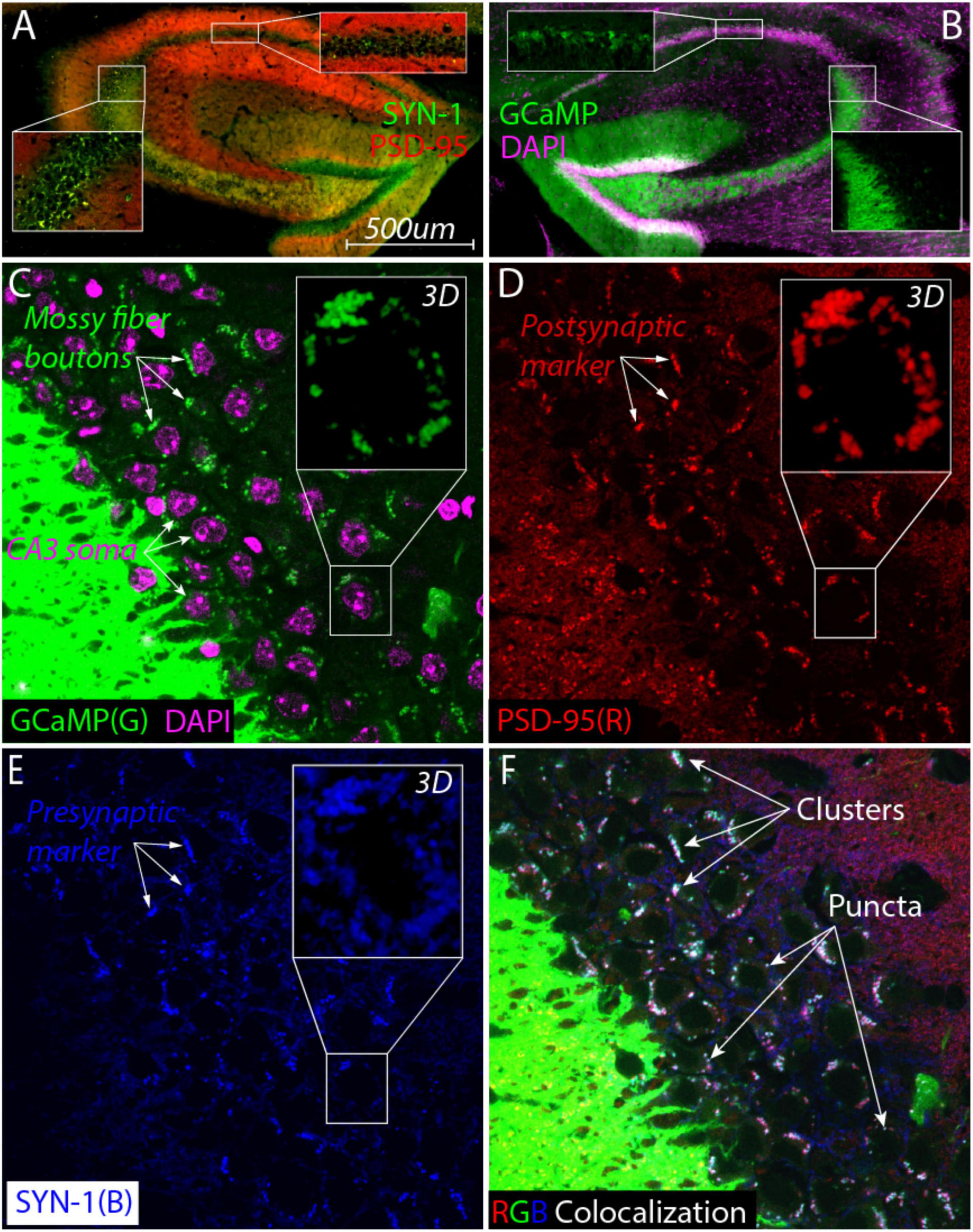
Figure 3. Large synaptic clusters containing mossy fiber boutons surround CA3 cell bodies in aged ELS mice (20 months). (A) Colocalization of postsynaptic marker αPSD-95 nanobody (red) with presynaptic marker αSynaptotagmin nanobody (SYN1, green) reveals the presence of large synaptic clusters (yellow) around CA3 soma (bottom inset) but not in the CA1 pyramidal subfield (top inset). (B) High expression of GCaMP signal (green) in the dentate gyrus cells show that mossy fibers project and appear to terminate near CA3 cell bodies (purple, DAPI). GCaMP signal is not detectable inside CA3 neurons (right inset) but it does fill a large percentage CA1 pyramidal neurons (top inset) as expected from imaging studies. (C–F) Super resolution single-plane optical section imaging of the stratum pyramidale [(C), CA3 cell bodies labeled with DAPI, purple] stained for mossy fiber boutons [(C), green], postsynaptic PSD-95 [(D), red] and presynaptic SYN-1 [(E), blue] yields extensive triple colocalization [(F), white] that consists of larger clusters and smaller individual puncta consistent with the hallmarks of MF synapses. Max Z projections of zoomed in z-stack images from a single cell body [(C–E), insets] demonstrate that these synaptic clusters surround the cell in all three dimensions.
Given the specificity of our GCaMP signal in combination with DAPI staining, we measured the areas of the GC Layer, the Hilus, and the suprapyramidal blade of the MF. After normalization to total hippocampal area (Supplementary Figure 4A), we did not detect any significant differences in these areas between ELS + ET (n = 5) and ELS + CT (n = 7) mice (Supplementary Figure 4B). The number of CA3 neurons per 0.01 mm2 was not significantly different between groups. Altogether, this suggests that ET training does not cause gross structural changes in the DG to CA3 circuit when compared to our exercise alone control group (CT).
Identification of atypical MF synapses surrounding CA3 cell bodies
To visualize synapses, we found it necessary to use nanobodies that are capable of penetrating fixed tissue (Kilisch et al., 2023) allowing us to compare presynaptic and postsynaptic markers as well as the endogenous GCaMP signal (the MF indicator) in the same slice. Immunostaining with synaptotagmin and PSD-95 revealed the presence of prominent large synaptic clusters surrounding the CA3 soma that were not as visible in other areas such as the CA1 subfield (Figure 3A top inset).
The large synaptic clusters, consistent with MF boutons, were particularly notable in the CA3 stratum pyramidale, which was unexpected. To confirm these were bona fide MF synapses, we individually visualized all three signals: mossy fiber GCaMP (Figure 3C, green), postsynaptic PSD-95 (Figure 3D, red), and presynaptic synaptotagmin (Figure 3E, blue). Around CA3 cells (purple staining in Figure 3C), we observed many clusters in the same location across all three images (white arrows in Figures 3C–E). The observed putative MF boutons colocalized with PSD-95 (Figures 3C, D) as well as VGLUT1 (Supplementary Figure 5) suggesting that they were excitatory synapses.
The triple colocalization image (overlay of all three RGB colors) revealed that many clusters were white, suggesting they were composed of all three markers (Figure 3F). These clusters varied in size, from larger clusters (top three arrows, Figure 3F) to individual puncta (bottom three arrows, Figure 3F). We performed a 2D spatial cross-correlation on these images to statistically determine the extent of colocalization. A pairwise analysis of all three combinations (PSD95-SYN1, PSD95-GCaMP, and SYN1-GCaMP) revealed significant overlap, with peaks higher than the shuffled distribution (Sup Fig. 3). This statistically significant triple colocalization of mossy fiber boutons and pre- and postsynaptic markers strongly supports the presence of excitatory MF synapses in the CA3 pyramidal layer.
Characterization of atypical MF synapses in the SP layer
Next, we examined quantitative differences in putative excitatory MF synapses between the ELS + ET and ELS + CT mice. We immunostained sections for postsynaptic PSD-95 (red) and presynaptic synaptotagmin 1 (SYN1, green) as this combination of synaptic markers had the highest overlap score (see the green line, Supplementary Figure 3). We tiled a large area of the CA3 bend to identify MF synapses (yellow clusters and white arrows) of various sizes as well as CA3 cell bodies (dark circles) (Figure 4A). Regardless of size, colocalized clusters in the stratum pyramidale (SP) region (dashed white line) of the CA3, appeared to be composed of individual puncta with an area = ∼200 pixels or 0.37 μm2 (Figure 4A).
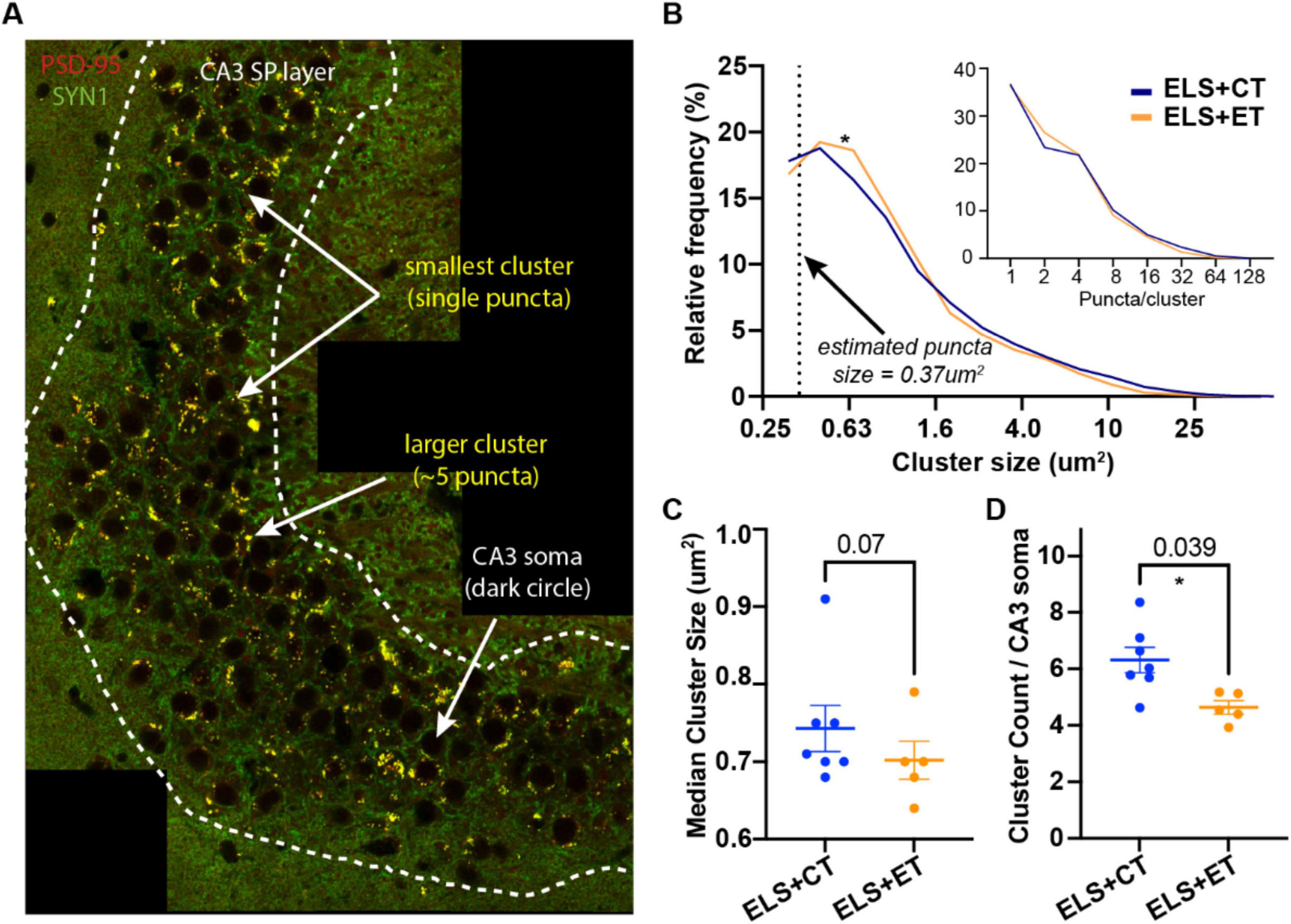
Figure 4. Enrichment track (ET) training reduces the density of putative mossy fiber (MF) synapses in the CA3. (A) A representative tiled image from an ELS + ET mouse (brain harvested following Y-maze) double-stained with PSD95 (red) and SYN1 (green) nanobodies. In the stratum pyramidale (SP) region (dashed white line) of the CA3, colocalized (yellow) clusters appear to be comprised of individual puncta (area = ∼200 pixels or 0.37 μm2). Clusters smaller than 150 pixels were removed from subsequent analysis. (B) Histogram of log-transformed cluster sizes (pooled) and estimated number of puncta/cluster (inset) identified from ELS + ET (10,497 clusters, n = 7 mice) and ELS + CT mice (5,847 clusters, n = 5 mice). Statistical analysis between the groups using rank-based (Mann Whitney, p = 0.035) and cumulative (Kolmogorov-Smirnov, p = 0.0001) methods indicate significant differences (asterisk) in their distributions, likely due to fewer puncta per cluster in ELS + ET mice (inset). Dashed line indicates the x-intercept corresponding to our estimated size of a single puncta. (C,D) A mouse-by-mouse analysis reveals that ELS + ET mice (n = 5, orange dots) have fewer clusters compared to ELS + CT controls (n = 7, dark blue dots, two-way ANOVA, F(1,8) = 6.07, p = 0.039, asterisk, (D). There was a trend for smaller median cluster sizes in the ELS + ET group (two-way ANOVA, F(1,8) = 4.36, p = 0.070, (C). Tiled sections were normalized to counts to the total number of CA3 neurons.
We used automated counting to determine frequency and size of clusters in the tiled images (2 per mice) between ELS + ET and ELS + CT mice. In total, ELS + ET mice had 10,497 clusters (n = 7 mice) while ELS + CT mice had 5,847 clusters (n = 5 mice). Statistical analysis between the pooled groups using rank-based (Mann-Whitney, p = 0.035) and cumulative (Kolmogorov-Smirnov, p = 0.0001) methods indicated significant differences in their size distributions. ELS + ET mice (orange line) had fewer large clusters and proportionally more smaller clusters than ELS + CT controls (blue line, Figure 4B). ELS + ET mice had a corresponding shift in puncta per cluster in (Figure 4B inset); assuming that the larger clusters are composed of discrete puncta.
In a mouse-by-mouse analysis, normalized per CA3 neuron, we found that ELS + ET mice had on average 27% fewer clusters (4.75 ± 0.15, n = 5, orange dots) compared to ELS + CT controls (6.31 ± 0.63, n = 7, dark blue dots, two-way ANOVA, F(1,8) = 6.07, p = 0.039) with no significant sex differences [F(1,8) = 0.043, p = 0.841 Figure 4D). There was no significant group or sex difference in median cluster size [two-way ANOVA, group: F(1,8) = 4.36, p = 0.070; sex: F(1,8) = 0.0009, p = 0.976, Figure 4C], although there was a small trend for smaller synapses in ELS + ET mice consistent with our distribution analysis. These findings suggest that ET training reduces the number of mossy fiber (MF) synapses surrounding CA3 somas in ELS mice without significantly affecting the size of individual synaptic clusters.
Discussion
In a mouse model of ELS, we found that cognitive enrichment training during young adulthood leads to long-lasting changes in spatial memory and hippocampal structure. To our knowledge this is the first demonstration of these effects in a rodent model of maternal neglect. Compared to exercise alone controls (ELS + CT), mice that underwent enrichment training (ELS + ET) showed significant improvements in long-term OLM (24 h) memory at both mature (6 months) and middle-ages (13 months). Additionally, at 20 months, ELS + ET mice performed better memory in a final spatial Y-maze test. However, we found no group differences in NOR, a task which relies less on the hippocampus (Oliveira et al., 2010; Barker and Warburton, 2011; Cohen and Stackman, 2015). Surprisingly, our anatomical analysis of the hippocampus revealed that aged ELS mice had prominent MF-associated excitatory clusters surrounding the CA3 neurons, an area not typically associated with MF synapses. Furthermore, we found that in aged ELS mice early enrichment reduced the number of these atypical MF synapses by ∼25%.
An important caveat in our findings is that within the stratum pyramidale, we cannot distinguish between atypical excitatory mossy fiber boutons that synapse onto pyramidal cells versus those that synapse onto inhibitory interneurons (Henze et al., 2000; Urban et al., 2001). However, excitatory pyramidal neurons vastly outnumber inhibitory interneurons in the CA3 pyramidal layer, with interneurons comprising only approximately 10%–15% of the total neuronal population (Freund and Buzsáki, 1996; Klausberger and Somogyi, 2008). This numerical predominance suggests that the reduction in excitatory mossy fiber synaptic clusters we quantified likely represents connections onto pyramidal cells. Nevertheless, given that interneurons in this layer provide feed-forward inhibition that regulates CA3 excitability (Jinde et al., 2012), and that environmental enrichment has been shown to modulate inhibitory neurotransmission (Speisman et al., 2013; Cortese et al., 2018), changes in mossy fiber connectivity onto inhibitory neurons could alter CA3 pattern separation. Future studies using cell-type-specific markers or electrophysiological approaches will be necessary to determine whether cognitive enrichment and/or ELS differentially alters these synapses.
There are important similarities and differences between our behavioral observations and those of previous studies. Earlier studies found that ELS can cause wide-ranging spatial (e.g., OLM) and recognition (e.g., NOR) memory impairments (Brunson et al., 2005; Hoeijmakers et al., 2018; Ivy et al., 2010; Molet et al., 2016a; Naninck et al., 2017; Bolton et al., 2020; Short et al., 2020; Rice et al., 2008). These impairments; however, appear to occur at different rates during aging. A careful study, in rats, found that spatial memory deficits start during adolescence whereas the effects of ELS on NOR memory does not occur until older ages (Molet et al., 2016b) [however, see male mice in Naninck et al. (2015) study]. Similarly, we found that our ELS alone mice (6 months) had normal NOR memory but significant deficits in OLM, consistent with the model that the hippocampus is more susceptible to assaults from ELS than other brain regions. Interestingly our observations also suggest the ET protocol has a more selective effect on hippocampus function because between ET and CT mice we found no group differences in NOR. This finding seemingly contrasts with our initial use of the ET protocol, where we found broad memory enhancements (Gattas et al., 2022). The original study; however, used a more extensive protocol (six 1 h sessions/week versus three 30 min sessions/week) that started at an earlier age. This suggests that the abbreviated ET protocol used in this study produces a more limited effect that is biased toward improvements in hippocampal function.
Contrary to our predictions, we found no differences in MF volume between the ELS + ET and ELS + CT groups. Other studies, conducted with non-stressed mice, report that enrichment causes mossy fiber expansion beyond the SL layer (Galimberti et al., 2006; Bramati et al., 2023). Unlike our design, those studies did not control for exercise, as only the EE group had access to a running wheel. Since exercise alone contributes to mossy fiber sprouting (Toscano-Silva et al., 2010), it may be exercise, rather than cognitive enrichment, that drives the growth of mossy fibers toward the CA3 cell bodies.
Several factors could explain why these synapses remain uncharacterized, despite their previous observation (Amaral and Dent, 1981; Dailey et al., 1994; Qin et al., 2001; Banks et al., 2024). Age and ELS cause CA3 dendritic atrophy and mossy fiber sprouting (Brunson et al., 2001; Galimberti et al., 2006; Adams et al., 2010; Molet et al., 2016b), which may be an attempt by the MFs to compensate for synaptic loss, shifting synapse density toward CA3 cell bodies. Another possibility is our use of nanobodies, which are ∼10 times smaller than antibodies. This increases their ability to detect antigens in fixed tissue (Kilisch et al., 2023; Fridy et al., 2024).
How the size and function of these atypical MF synapses compare to that of MF synapses in the SL layer remains unclear. Electron microscopy 3D reconstruction studies estimate the average MF bouton’s cross-sectional area to be ∼5 μm2 (Rollenhagen et al., 2007; Rollenhagen and Lübke, 2010; Murray et al., 2020), whereas our 2D average cluster size is ∼1.5 μm2. This smaller size could be because our puncta only consist of the synaptic junction (colocalization of pre- and postsynaptic markers) portion of the larger MF structure. Future structural studies with high resolution imaging such as electron microscopy are needed to determine whether these atypical synapses are similar in size to the typical “MF” synapses in the SL layer. Nevertheless, the proximity of these atypical MF synapses to the spike initiation zone suggests a tight coupling between changes in their density and CA3 activity. We suspect that the presence of these synapses would make a significant contribution to the unusually strong property of the MF-CA3 synapses (i.e., “conditional detonator”) as originally proposed by McNaughton and Morris (1987) and theorized by Marr (1971).
The reason why our cognitively enriched mice have fewer MF synapses remains an outstanding question. One possibility is increased synaptic pruning triggered by plasticity such as repeated bouts of long-term depression (LTD) (Bastrikova et al., 2008; Becker et al., 2008; Shinoda et al., 2010). The occurrence of LTD and LTP in the hippocampus largely depends on the nature of the behavioral learning task (Hagena and Manahan-Vaughan, 2024). At MF-CA3 synapses, exposure to a simple novel context facilitates LTP, while introducing large items and rearranging them, even when familiar, facilitates LTD (Kemp and Manahan-Vaughan, 2008; Hagena and Manahan-Vaughan, 2011). Our ET includes large obstacles frequently changed and rearranged, suggesting LTP may occur initially, followed by ongoing LTD and pruning as mice continue learning in the same context.
The fact that MFs from adult-born DG neurons expand beyond the SL layer (Cole et al., 2020) raises the possibility that these atypical synapses preferentially belong to adult-born DG neurons. A recent study found that increases in the sparse activity of CA3 place cells due to enrichment requires neurogenesis (Ventura et al., 2024). EE can also rescue the survival of newborn neurons following ELS (Rule et al., 2021) and newborn neurons are more plastic (Ge et al., 2007; Massa et al., 2011). However, enrichment typically promotes growth factor signaling, enhancing DG-CA3 LTP and MF synaptogenesis (Gogolla et al., 2009; Bednarek and Caroni, 2011; Schildt et al., 2013; Cao et al., 2014). And, whether adult-born DG neurons have altered MF plasticity with CA3 remains unclear. Alterations in the fraction of newborn neurons that form atypical MF synapses could represent a mechanism by which experience and neurogenesis fine tune hippocampal activity.
Our results suggest that targeted cognitive enrichment training may be especially beneficial to individuals that have suffered from ELS. A potential therapeutic avenue is playing video action games, which significantly improves spatial reasoning and memory in humans. For instance, 3D video game players outperformed non-players on hippocampal-mediated memory functions such as mental rotation and spatial visualization tasks (Green and Bavelier, 2003; Uttal et al., 2013; Clemenson and Stark, 2015). Moreover, neuroimaging studies confirm that video game training increases gray matter in the hippocampus and prefrontal cortex (Kühn et al., 2014). Future studies, however, are needed to determine whether accessible interventions like 3D video games can mitigate the long-term cognitive and hippocampal structural deficits induced by early life adversity.
Our findings suggest a potential mechanism to restore spatial learning and hippocampal function in aged ELS mice. Studies show that age and ELS increase DG and CA3 excitability (Barnes and McNaughton, 1980; Wilson et al., 2005; Patrylo et al., 2007; Simkin et al., 2015; Villanueva-Castillo et al., 2017); changes that would increase population activity and impair the ability of the hippocampus to pattern separate (Barnes et al., 1997; Tanila et al., 1997a,1997b; Redish et al., 1998; Wilson et al., 2005; Jinde et al., 2012; Figure 5A). Our cognitive enrichment causes a 27% decrease in the number of atypical MF synapses which could counteract high excitability by reducing the number of MF synapses. The resulting increase in sparse activity would favor pattern separation and improve spatial learning, as distinct contexts would have more orthogonal CA3 and CA1 representations (McNaughton and Morris, 1987; McHugh et al., 2007; Figure 5B).
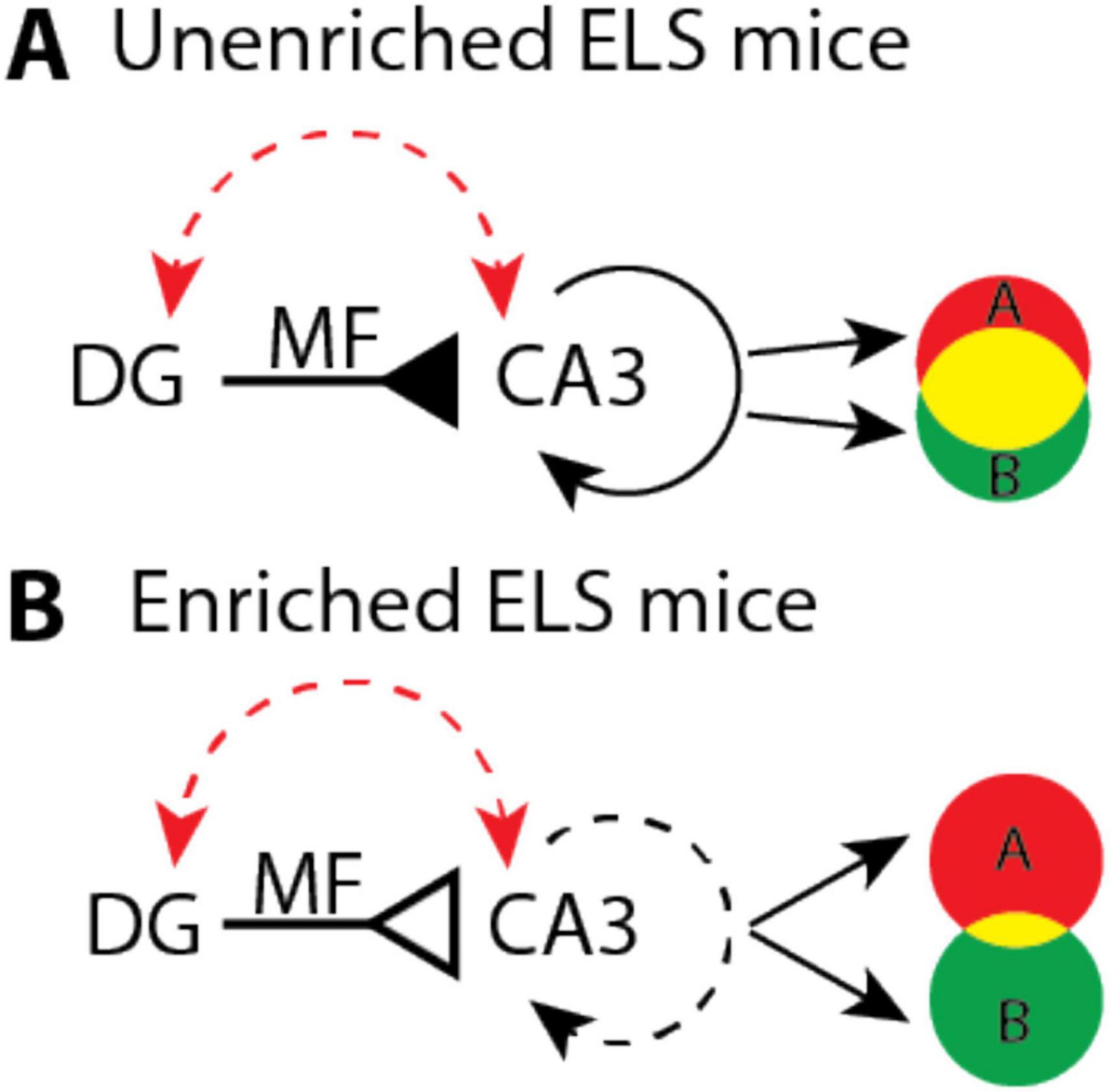
Figure 5. Hypothetical model that illustrates how enrichment could improve pattern separation in aged early-life stress (ELS) mice. (A) Age and stress (red dashed arrow) increase the excitability of dentate gyrus (DG) and CA3 which may cause mossy fiber (MF) input (solid inverted arrow) to drive dense recurrent activity (solid circular arrow). These dynamics could produce large overlaps (yellow shading) between context specific representations (A and B). (B) In enriched mice, age and stress may still lead to high levels of DG and CA3 excitability. However, a 27 percent reduction in the number of atypical MF synapses, per CA3 neuron, (open inverted arrow) could help to restore pattern separation by driving sparser recurrent activity (dashed circular arrow) that orthogonalizes (less yellow shading) context specific representations (A and B).
Materials and methods
Experimental animals
For the longitudinal study we used Thy1-GCaMP6f-GP5.17 (Jackson) mice due to the absence of reporter expression in the CA3. Upon reaching early-adulthood (2.5 months) GCaMP6f+ litter-mates were double-housed (after ELS) so that each cage had a mouse that ran ET and CT. This consisted of four cages of females (n = 8) and four cages of males (n = 8) for a total of (n = 16). Due to the longevity of this study, three males and one female mouse died of natural causes before reaching the final 20 months timepoint. After removal of the GCaMP6f+ mice, there remained six male GCamP6f– littermates (three cages). These were grouped with age typically reared (TR) age-matched C57BL/6J male mice purchased from Jackson (n = 6) for the ELS vs. TR experiment (Figures 2E, F).
Early-life stress and cognitive enrichment
The experiments conducted were in accordance with the guidelines set by the Institutional Animal Care and Use Committee (IACUC) at the University of California, Irvine. We used the ELS protocol (developed in the Baram lab) whereby mouse litters receive limited bedding and nesting (LBN) conditions from postnatal days 2–10 in their rearing cages to modify maternal behavior during early development, (Figures 1A, B). Briefly, on postnatal day 2, we replaced the normal bedding (∼6 L) with a fitted metal grate (large enough to allow dropping to collect underneath) and two nestlets so that the dam could make a rudimentary nest. On postnatal day 10, we restored the normal bedding conditions. The control group (3 L), referred to as typically reared (TR), remained in their normal cages. After reaching adulthood (3.5 months), we ran a subset of the ELS mice (N = 16) on either enrichment (ET) or control track (CT) for ten weeks (3 × 30 min sessions/week), (Gattas et al., 2022; Figure 1C). Briefly, the enrichment setup consisted of two juxtaposed square tracks: one containing obstacles, while the other had simple ramps with one-way doors located at diagonal corners (Supplementary Figures 1A, B). Initially, we trained both groups of mice (2 weeks) to run laps around the track loaded with simple ramps (12) while receiving a single milk reward dispensed from a mounted lick tube triggered at the conclusion of each lap. In the next phase, we introduced complex obstacles only to the ET group, which continued for 8 weeks (Figure 1B).
Object location memory and novel object recognition
For each time point, we conducted a set of OLM (first week) and NOR (second week) behaviors over a 2 weeks period (10 days total). The first 3 days of each week served as habituation sessions, during which animals explored empty square boxes (10 × 9 inches) for 10 min. On the training day (fourth day), mice were exposed to two identical objects for 10 min. On the test day (fifth day), one object was relocated to a new position (OLM, week 1, Figure 2A, top) or replaced with a novel object in the same position (NOR, week 2, Figure 2B, top). We used the same context box but different objects for each round of OLM and NOR to avoid object familiarity-related confounds. Boxes had unique markings on two of the walls (vertical and horizontal stripes) so that mice could easily associate the position of the objects with the box (Supplementary Figures 2A, B). All behaviors were recorded with an overhead camera using infrared emitters for low-light conditions.
Y-maze
The Y-maze setup had three identical arms (3.5 inches wide and 10.5 inches long) with transparent walls and was surrounded by distinct external cues (Figure 2C). We always placed mice in the start arm at the beginning of each session. On training day (the first exposure to the Y-maze), we barricaded the to-be novel arm (opaque blocker) so that mice could only explore the two open arms (start and familiar) for 10 min. On test day (24 h later), we removed the blocker so that mice were free to explore all three arms for 5 min. A single top-view camera captured training and testing session videos.
Behavioral analysis
The behavior videos were analyzed manually using BORIS software (Friard and Gamba, 2016) to count the duration each animal spent within 2 cm of each object (OLM/NOR) and moving inside the novel arm (spatial Y-maze). Memory performance in each behavior test was measured using discrimination ratio (DR), calculated as the ratio of time spent near novel conditions (object in a novel location for OLM or novel object for NOR or novel arm in spatial Y-maze) to time spent near familiar conditions (object in a familiar location for OLM or familiar object for NOR or familiar arm in spatial Y-maze).
Immunofluorescence staining
Following isoflurane anesthesia, mice were transcardially perfused with cold phosphate buffer solution (PBS), followed by 4% paraformaldehyde (PFA), and the extracted brain was stored in 4% PFA at 4 °C. Before slicing, brains were transferred to 30% sucrose/PBS solution and stored at 4 °C for cryoprotection. Brains were sectioned at 40 μm using a cryostat (Thermo Scientific HM525 NX) at −20 °C, and each section was stored in well plates containing cryoprotectant solution.
Anatomical targeting: Dorsal hippocampal sections were selected at approximately −1.8 mm from bregma (anterior-posterior coordinate) according to the Paxinos and Watson mouse brain atlas to visualize mossy fiber (MF) synapses in the distal CA3 region. This anatomical level was chosen to ensure regional consistency, as synaptogenesis is region-specific within hippocampal subfields, particularly in the septal versus temporal hippocampus.
Immunohistochemistry protocol: Initial attempts using traditional PSD-95 and presynaptic antibodies yielded poor results with low signal and poor colocalization. Subsequent optimization using nanobodies showed dramatic improvements. First, we incubated sections for 2 h (at room temperature) in PBS blocking buffer containing 5% normal goat serum (NGS) and 0.3% Triton X-100. Sections were then incubated with nanobodies (purchased from NanoTag Biotechnologies) that contained the appropriate combination of either anti-PSD-95 nanobody (FluoTag®-X2 anti-PSD95, Cat No: N3702-AF647-L, 2 nM, Alexa647), anti-synaptotagmin 1 nanobody (FluoTag®-X2 anti-Synaptotagmin 1, Cat No: N2302-AF568-L, 0.2 nM, AZDye568) or VGLUT1 nanobody (FluoTag®-X2 anti-VGLUT1, Cat No: N1602-AF568-L, 1 nM, AZDye568). Incubation was performed with shaking at 4 °C for 24 h in PBS containing 0.3% Triton X-100. Sections were washed four times for 10 min each alternating between PBS and TBS. At these low concentrations, fluorescent signals were more prominent in the stratum pyramidale (SP) layer compared to the stratum lucidum (SL) layer. Sections were mounted onto slides with media containing DAPI (Invitrogen SlowFade Glass Soft-set Antifade Mountant with DAPI, catalogue #S36917).
Gross morphological analysis
We took images of the entire dorsal hippocampus with a Keyence BZ-X1810 widefield fluorescence microscope with a 20x objective lens with DAPI and GCaMP filter cubes to visualize cell bodies and endogenous GCaMP6 signal. Images were loaded in Zeiss Zen software so that we could manually trace hippocampal subfields using the active contour tool. Following each outline, we took reported areas and normalized them to the total hippocampal area. To determine number of CA3 neuron we counted the number of easily identifiable large dark circles in the tiled images (described below). All tracing and counting was done by a double-blind observer.
Mossy fiber synaptic analysis
Confocal imaging: Tiled images of the distal CA3 bend were captured using an LSM 900 microscope equipped with Airyscan 2 and a 63X objective lens. PSD-95 and synaptotagmin-1 were excited using 653 and 568 nm diode lasers, respectively. Images were over-sampled (2x) to facilitate super-resolution post-processing with Zeiss’ Super Resolution Airyscan mode (2D, auto). No additional deconvolution was applied beyond the Airyscan super-resolution processing. Images were manually aligned using Imaris Stitcher, creating two composite panels per mouse (approximately 20 stitched images for each z-plane panel) selected systematically from the dorsal and ventral aspects of the CA3 bend. These panels were roughly 8,000 × 10,000 pixels (∼350 × 420 μm), 16-bit TIFF files, featuring red (PSD-95) and green (synaptotagmin-1) channels. Imaging parameters including laser power (2.3% for 640 nm and 3.0% for 561 nm), gain, and offset were kept constant across all samples to ensure quantitative comparisons. As expected, nanobody penetration was lowest in the middle of the slice so for each mouse we constructed two composite panels at 10 and 30 μm positions within the 40 μm slice. We used the same settings for non-tiled images with the following additions: laser 405 nm at 1.5% and laser 488 nm power at 1.3%. Z-stacks consisted of 15 optical sections centered at 10 μm with a 1 μm step size. Reconstructed 3D images of synapses surrounding the cell body are max Z projections of all 15 slices.
Quantitative analysis: For subsequent analysis on the tiled images, FIJI was used to crop images focusing on the pyramidal cell bodies of the SP layer. These cropped, composite images served as input for the MF synapse detection algorithm. Each channel was independently thresholded based on fluorescence intensity to create binary masks of equal size. Threshold values of 515 for PSD-95 and 350 for Synaptotagmin were selected because they captured all identifiable clusters while excluding apparent noise, with similar mask sizes observed with threshold variations within a 5% range. The masks were combined using a pixel-wise AND operation and applied to the original 2-channel image, retaining only pixel values above the respective thresholds, with all other pixels set to 0 (black). Clusters smaller than 150 pixels were removed from subsequent analysis to eliminate imaging artifacts, so only clusters with an area exceeding 75% of 0.37 μm2 (0.27 μm2) were included. Large images were processed in parallel using smaller blocks of 500 × 500 pixels, with a flood-fill algorithm applied to every fourth pixel (provided it was a non-zero value) to detect cluster masks. Visual inspections were performed on two images by manual counting to validate the automated detection, showing 97% agreement between automated and manual counts. Duplicate detections at block edges were filtered to ensure a unique set of clusters. Mossy fiber bouton density was quantified as the number of synaptic clusters per CA3 neuron within the pyramidal cell layer, normalized across the two composite panels per mouse. All image acquisition and quantitative analyses were performed by investigators blinded to experimental groups. The analysis code is freely available online at GitHub.1
Statistical analysis
Behavioral tests were analyzed using repeated measures and two-way ANOVA designs (α = 0.05). Object location memory (OLM) and novel object recognition (NOR) were analyzed using three-way repeated measures ANOVA (Age × Group × Sex) with n = 16 mice total (ELS + ET: 4M and 4F, n = 8; ELS + CT: 4M and 4F, n = 8). For OLM, only the Group main effect was statistically significant [F(1,12) = 8.80, p = 0.012], while all other main effects and interactions were non-significant: Age [F(1,12) = 1.69, p = 0.218], Sex [F(1,12) = 0.97, p = 0.345], Age × Group [F(1,12) = 0.16, p = 0.700], Age × Sex [F(1,12) = 0.081, p = 0.780], Group × Sex [F(1,12) = 0.0004, p = 0.984], and Age × Group × Sex [F(1,12) = 0.33, p = 0.579]. For NOR, no main effects or interactions reached statistical significance: Age [F(1,12) = 2.93, p = 0.113], Group [F(1,12) = 0.074, p = 0.791], Sex [F(1,12) = 0.11, p = 0.744], Age × Group [F(1,12) = 1.66, p = 0.222], Age × Sex [F(1,12) = 0.53, p = 0.479], Group × Sex [F(1,12) = 4.48, p = 0.056], and Age × Group × Sex [F(1,12) = 0.55, p = 0.472]. Object exploration time (OLM and NOR combined) was analyzed using a two-way ANOVA (Test Type × Group) with the same sample sizes. Only the Test Type main effect was statistically significant [F(2,88) = 30.86, p < 0.0001], while Group [F(1,88) = 0.886, p = 0.349] and Test Type × Group interaction [F(2,88) = 0.101, p = 0.904] were non-significant. Y-maze data were analyzed using two-way ANOVA (Group × Sex) with n = 12 mice total (ELS + ET: 2M and 3F, n = 5; ELS + CT: 3M and 4F, n = 7). Only the Group main effect was statistically significant [F(1,8) = 19.89, p = 0.002], while Sex [F(1,8) = 1.81, p = 0.215] and Group × Sex interaction [F(1,8) = 0.81, p = 0.393] were non-significant.
Synaptic analyses were conducted using two-way ANOVA (Group × Sex, α = 0.05) with n = 12 mice total (ELS + ET: 2M and 3F, n = 5; ELS + CT: 3M and 4F, n = 7). A significant Group main effect was found for cluster count [F(1,8) = 6.07, p = 0.039], with Sex [F(1,8) = 0.043, p = 0.841] and Group × Sex interaction [F(1,8) = 0.073, p = 0.794] being non-significant. For cluster size, no main effects or interactions reached statistical significance: Group [F(1,8) = 4.36, p = 0.070], Sex [F(1,8) = 0.0009, p = 0.976], and Group × Sex interaction [F(1,8) = 0.42, p = 0.535].
Gross morphological analyses were conducted using two-way ANOVA (Group × Sex, α = 0.05) with n = 12 mice total (ELS + ET: 2M and 3F, n = 5; ELS + CT: 3M and 4F, n = 7). All hippocampal morphology measures showed non-significant results including CA3 neuron density [Group: F(1,8) = 1.734, p = 0.224; Sex: F(1,8) = 0.026, p = 0.876; Group × Sex: F(1,8) = 0.012, p = 0.917], hilus area [Group: F(1,8) = 0.318, p = 0.589; Sex: F(1,8) = 0.268, p = 0.619; Group × Sex: F(1,8) = 0.117, p = 0.741], mossy fiber area [Group: F(1,8) = 0.596, p = 0.462; Sex: F(1,8) = 4.265, p = 0.073; Group × Sex: F(1,8) = 0.144, p = 0.714], and dentate gyrus area [Group: F(1,8) = 0.024, p = 0.881; Sex: F(1,8) = 4.056, p = 0.079; Group × Sex: F(1,8) = 0.098, p = 0.762]. Sex effects approached significance for both mossy fiber area (p = 0.073) and dentate gyrus area (p = 0.079).
Summary of significant findings: Cognitive enrichment training (ET) significantly improved spatial memory performance in ELS mice, as evidenced by significant Group effects in both OLM (p = 0.012) and Y-maze (p = 0.002) tasks, but not in the hippocampus-independent NOR task. At the synaptic level, ET training significantly reduced the number of atypical mossy fiber synaptic clusters surrounding CA3 cell bodies (p = 0.039), while gross hippocampal morphology remained unchanged. These findings suggest that cognitive enrichment rescues ELS-induced spatial memory deficits through selective modifications of synaptic connectivity rather than gross structural changes.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee at the University of California, Irvine. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JS: Writing – original draft. EG: Writing – review & editing. RB: Writing – review & editing. RS: Writing – review & editing. MC: Writing – review & editing. BM: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was initiated by a 1-year UCI conte seed grant to JS and EG and further supported by NIH BRAIN grants: R01 NS121764 and RF1 NS132041 to BM. This study was made possible in part through access to the Optical Biology Core Facility of the Developmental Biology Center, a shared resource supported by the Cancer Center Support Grant (CA-62203) and NIH-S10OD032327-01.
Acknowledgments
We thank Tallie Z. Baram for her advice on experimental design and members of her lab, Annabel Short and Rachael Hokenson who provided us with details about conducting and analyzing the ELS and behavioral experiments. In the McNaughton lab, we thank Mariya Vodyanyk for establishing behavioral analysis methods as well as Aida Andujo and Varleen Kaur for excellent technical assistance with mouse behavior.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2025.1646883/full#supplementary-material
Footnotes
References
Adams, M. M., Donohue, H. S., Linville, M. C., Iversen, E. A., Newton, I. G., and Brunso-Bechtold, J. K. (2010). Age-related synapse loss in hippocampal CA3 is not reversed by caloric restriction. Neuroscience 171, 373–382. doi: 10.1016/j.neuroscience.2010.09.022
Almas, A. N., Degnan, K. A., Nelson, C. A., Zeanah, C. H., and Fox, N. A. (2016). IQ at age 12 following a history of institutional care: Findings from the bucharest early intervention project. Dev. Psychol. 52, 1858–1866. doi: 10.1037/dev0000167
Alwis, D. S., and Rajan, R. (2014). Environmental enrichment and the sensory brain: The role of enrichment in remediating brain injury. Front. Syst. Neurosci. 8:156. doi: 10.3389/fnsys.2014.00156
Amaral, D. G., and Dent, J. A. (1981). Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J. Comp. Neurol. 195, 51–86. doi: 10.1002/cne.901950106
Artola, A., von Frijtag, J. C., Fermont, P. C., Gispen, W. H., Schrama, L. H., Kamal, A., et al. (2006). Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur. J. Neurosci. 23, 261–272. doi: 10.1111/j.1460-9568.2005.04552.x
Banks, E., Gutekunst, C. A., Vargish, G. A., Eaton, A., Pelkey, K. A., McBain, C. J., et al. (2024). An enhancer-AAV approach selectively targeting dentate granule cells of the mouse hippocampus. Cell Rep. Methods 4:100684. doi: 10.1016/j.crmeth.2023.100684
Barker, G. R. I., and Warburton, E. C. (2011). When is the hippocampus involved in recognition memory? J. Neurosci. 31, 10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011
Barnes, C. A., and McNaughton, B. L. (1980). Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J. Physiol. 309, 473–485. doi: 10.1113/jphysiol.1980.sp013521
Barnes, C. A., Suster, M. S., Shen, J., and McNaughton, B. L. (1997). Multistability of cognitive maps in the hippocampus of old rats. Nature 388, 272–275. doi: 10.1038/40859
Bastrikova, N., Gardner, G. A., Reece, J. M., Jeromin, A., and Dudek, S. M. (2008). Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc. Natl. Acad. Sci. U. S. A. 105, 3123–3127. doi: 10.1073/pnas.0800027105
Becker, N., Wierenga, C. J., Fonseca, R., Bonhoeffer, T., and Nägerl, U. V. (2008). LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron 60, 590–597. doi: 10.1016/j.neuron.2008.09.018
Beckett, C., Maughan, B., Rutter, M., Castle, J., Colvert, E., Groothues, C., et al. (2007). Scholastic attainment following severe early institutional deprivation: A study of children adopted from Romania. J. Abnorm. Child Psychol. 35, 1063–1073. doi: 10.1007/s10802-007-9155-y
Bednarek, E., and Caroni, P. (2011). β-Adducin is required for stable assembly of new synapses and improved memory upon environmental enrichment. Neuron 69, 1132–1146. doi: 10.1016/j.neuron.2011.02.034
Bennett, J. C., McRae, P. A., Levy, L. J., and Frick, K. M. (2006). Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiol. Learn. Mem.. 85, 139–152. doi: 10.1016/j.nlm.2005.09.003
Bilkey, D. K., Cheyne, K. R., Eckert, M. J., Lu, X., Chowdhury, S., Worley, P. F., et al. (2017). Exposure to complex environments results in more sparse representations of space in the hippocampus. Hippocampus 27, 1178–1191. doi: 10.1002/hipo.22762
Bolton, J. L., Schulmann, A., Garcia-Curran, M. M., Regev, L., Chen, Y., Kamei, N., et al. (2020). Unexpected transcriptional programs contribute to hippocampal memory deficits and neuronal stunting after early-life adversity. Cell Rep. 33:108511. doi: 10.1016/j.celrep.2020.108511
Bos, K. J., Fox, N., Zeanah, C. H., and Nelson Iii, C. A. (2009). Effects of early psychosocial deprivation on the development of memory and executive function. Front. Behav. Neurosci. 3:16. doi: 10.3389/neuro.08.016.2009
Bramati, G., Stauffer, P., Nigri, M., Wolfer, D. P., and Amrein, I. (2023). Environmental enrichment improves hippocampus-dependent spatial learning in female C57BL/6 mice in novel IntelliCage sweet reward-based behavioral tests. Front. Behav. Neurosci. 17:1256744. doi: 10.3389/fnbeh.2023.1256744
Brunson, K. L., Eghbal-Ahmadi, M., Bender, R., Chen, Y., and Baram, T. Z. (2001). Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc. Natl. Acad. Sci. U. S. A. 98, 8856–8861. doi: 10.1073/pnas.151224898
Brunson, K. L., Kramár, E., Lin, B., Chen, Y., Colgin, L. L., Yanagihara, T. K., et al. (2005). Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 25, 9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005
Cabezas, C., Irinopoulou, T., Gauvain, G., and Poncer, J. C. (2012). Presynaptic but not postsynaptic GABA signaling at unitary mossy fiber synapses. J. Neurosci. 32, 11835–11840. doi: 10.1523/JNEUROSCI.5543-11.2012
Cai, X., Bai, X., and Zhou, S. (2024). Childhood adversities and memory function in later life: The mediating role of activity participation. BMC Geriatr. 24:536. doi: 10.1186/s12877-024-05145-4
Cao, W., Duan, J., Wang, X., Zhong, X., Hu, Z., Huang, F., et al. (2014). Early enriched environment induces an increased conversion of proBDNF to BDNF in the adult rat’s hippocampus. Behav. Brain Res. 265, 76–83. doi: 10.1016/j.bbr.2014.02.022
Carasatorre, M., Ochoa-Alvarez, A., Velázquez-Campos, G., Lozano-Flores, C., Ramírez-Amaya, V., and Díaz-Cintra, S. Y. (2015). Hippocampal synaptic expansion induced by spatial experience in rats correlates with improved information processing in the hippocampus. PLoS One 10:e0132676. doi: 10.1371/journal.pone.0132676
Chen, Y., Molet, J., Lauterborn, J. C., Trieu, B. H., Bolton, J. L., Patterson, K. P., et al. (2016). Converging, synergistic actions of multiple stress hormones mediate enduring memory impairments after acute simultaneous stresses. J. Neurosci. 36, 11295–11307. doi: 10.1523/JNEUROSCI.2542-16.2016
Cheng, S. T., Liu, S., Ou-Yang, B., Dai, X. Y., and Cheng, L. (2022). Specific effects of characteristics of enriched environment on innovative problem solving by animals. Psychol. Sci. 33, 1097–1111. doi: 10.1177/09567976211070562
Clemenson, G. D., and Stark, C. E. (2015). Virtual environmental enrichment through video games improves hippocampal-associated memory. J. Neurosci. 35, 16116–16125. doi: 10.1523/JNEUROSCI.2580-15.2015
Cohen, S. J., and Stackman, R. W. (2015). Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 285, 105–117. doi: 10.1016/j.bbr.2014.08.002
Cole, J. D., Espinueva, D. F., Seib, D. R., Ash, A. M., Cooke, M. B., Cahill, S. P., et al. (2020). Adult-Born hippocampal neurons undergo extended development and are morphologically distinct from neonatally-born neurons. J. Neurosci. 40, 5740–5756. doi: 10.1523/JNEUROSCI.1665-19.2020
Connor, J. R., Wang, E. C., and Diamond, M. C. (1982). Increased length of terminal dendritic segments in old adult rats’ somatosensory cortex: An environmentally induced response. Exp. Neurol. 78, 466–470. doi: 10.1016/0014-4886(82)90064-4
Cortese, G. P., Olin, A., O’Riordan, K., Hullinger, R., and Burger, C. (2018). Environmental enrichment improves hippocampal function in aged rats by enhancing learning and memory, LTP, and mGluR5-Homer1c activity. Neurobiol. Aging 63, 1–11. doi: 10.1016/j.neurobiolaging.2017.11.004
Cui, M., Yang, Y., Yang, J., Zhang, J., Han, H., Ma, W., et al. (2006). Enriched environment experience overcomes the memory deficits and depressive-like behavior induced by early life stress. Neurosci. Lett. 404, 208–212. doi: 10.1016/j.neulet.2006.05.048
Dailey, M. E., Buchanan, J., Bergles, D. E., and Smith, S. J. (1994). Mossy fiber growth and synaptogenesis in rat hippocampal slices in vitro. J. Neurosci. 14(3 Pt 1), 1060–1078. doi: 10.1523/JNEUROSCI.14-03-01060.1994
Ding, R., and He, P. (2021). Associations between childhood adversities and late-life cognitive function: Potential mechanisms. Soc. Sci. Med. 291:114478. doi: 10.1016/j.socscimed.2021.114478
Eckenrode, J., Laird, M., and Doris, J. (1993). School performance and disciplinary problems among abused and neglected children. Dev. Psychol. 29, 53–62. doi: 10.1037/0012-1649.29.1.53
Eigsti, I. M., Weitzman, C., Schuh, J., de Marchena, A., and Casey, B. J. (2011). Language and cognitive outcomes in internationally adopted children. Dev. Psychopathol. 23, 629–646. doi: 10.1017/S0954579411000204
Engineer, N. D., Percaccio, C. R., Pandya, P. K., Moucha, R., Rathbun, D. L., and Kilgard, M. P. (2004). Environmental enrichment improves response strength, threshold, selectivity, and latency of auditory cortex neurons. J. Neurophysiol. 92, 73–82. doi: 10.1152/jn.00059.2004
Fenoglio, K. A., Brunson, K. L., and Baram, T. Z. (2006). Hippocampal neuroplasticity induced by early-life stress: Functional and molecular aspects. Front. Neuroendocrinol. 27:180–192. doi: 10.1016/j.yfrne.2006.02.001
Freund, T. F., and Buzsáki, G. (1996). Interneurons of the hippocampus. Hippocampus 6, 347–470. doi: 10.1002/(SICI)1098-106319966:4<347::AID-HIPO1>3.0.CO;2-I
Friard, O., and Gamba, M. (2016). BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. doi: 10.1111/2041-210X.12584
Fridy, P. C., Farrell, R. J., Molloy, K. R., Keegan, S., Wang, J., Jacobs, E. Y., et al. (2024). A new generation of nanobody research tools using improved mass spectrometry-based discovery methods. J. Biol. Chem. 300:107623. doi: 10.1016/j.jbc.2024.107623
Galimberti, I., Gogolla, N., Alberi, S., Santos, A. F., Muller, D., and Caroni, P. (2006). Long-term rearrangements of hippocampal mossy fiber terminal connectivity in the adult regulated by experience. Neuron 50, 749–763. doi: 10.1016/j.neuron.2006.04.026
Gattas, S., Collett, H. A., Huff, A. E., Creighton, S. D., Weber, S. E., Buckhalter, S. S., et al. (2022). A rodent obstacle course procedure controls delivery of enrichment and enhances complex cognitive functions. NPJ Sci. Learn. 7:21. doi: 10.1038/s41539-022-00134-x
Ge, S., Yang, C. H., Hsu, K. S., Ming, G. L., and Song, H. (2007). A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54, 559–566. doi: 10.1016/j.neuron.2007.05.002
Gogolla, N., Galimberti, I., Deguchi, Y., and Caroni, P. (2009). Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron 62, 510–525. doi: 10.1016/j.neuron.2009.04.022
Green, C. S., and Bavelier, D. (2003). Action video game modifies visual selective attention. Nature 423, 534–537. doi: 10.1038/nature01647
Gutiérrez, R., Romo-Parra, H., Maqueda, J., Vivar, C., Ramìrez, M., Morales, M. A., et al. (2003). Plasticity of the GABAergic phenotype of the “glutamatergic” granule cells of the rat dentate gyrus. J. Neurosci. 23, 5594–5598. doi: 10.1523/JNEUROSCI.23-13-05594.2003
Hagena, H., and Manahan-Vaughan, D. (2011). Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb. Cortex 21, 2442–2449. doi: 10.1093/cercor/bhq271
Hagena, H., and Manahan-Vaughan, D. (2024). Interplay of hippocampal long-term potentiation and long-term depression in enabling memory representations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 379:20230229. doi: 10.1098/rstb.2023.0229
Henze, D. A., Urban, N. N., and Barrionuevo, G. (2000). The multifarious hippocampal mossy fiber pathway: A review. Neuroscience 98, 407–427. doi: 10.1016/s0306-4522(00)00146-9
Hoeijmakers, L., Amelianchik, A., Verhaag, F., Kotah, J., Lucassen, P. J., and Korosi, A. (2018). Early-Life stress does not aggravate spatial memory or the process of hippocampal neurogenesis in adult and middle-aged APP/PS1 mice. Front. Aging Neurosci. 10:61. doi: 10.3389/fnagi.2018.00061
Humphreys, K. L., King, L. S., Sacchet, M. D., Camacho, M. C., Colich, N. L., Ordaz, S. J., et al. (2019). Evidence for a sensitive period in the effects of early life stress on hippocampal volume. Dev. Sci. 22, e12775. doi: 10.1111/desc.12775
Ivy, A. S., Rex, C. S., Chen, Y., Dubé, C., Maras, P. M., Grigoriadis, D. E., et al. (2010). Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci. 30, 13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010
Jinde, S., Zsiros, V., Jiang, Z., Nakao, K., Pickel, J., Kohno, K., et al. (2012). Hilar mossy cell degeneration causes transient dentate granule cell hyperexcitability and impaired pattern separation. Neuron 76, 1189–1200. doi: 10.1016/j.neuron.2012.10.036
Jung, C. K., and Herms, J. (2014). Structural dynamics of dendritic spines are influenced by an environmental enrichment: An in vivo imaging study. Cereb. Cortex 24, 377–384. doi: 10.1093/cercor/bhs317
Kawamoto, M., Takagishi, H., Ishihara, T., Takagi, S., Kanai, R., Sugihara, G., et al. (2023). Hippocampal volume mediates the relationship of parental rejection in childhood with social cognition in healthy adults. Sci. Rep. 13:19167. doi: 10.1038/s41598-023-46512-2
Kemp, A., and Manahan-Vaughan, D. (2008). The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb. Cortex 18, 968–977. doi: 10.1093/cercor/bhm136
Kempermann, G. (2019). Environmental enrichment, new neurons and the neurobiology of individuality. Nat. Rev. Neurosci. 20, 235–245. doi: 10.1038/s41583-019-0120-x
Kilisch, M., Gere-Becker, M., Wüstefeld, L., Bonnas, C., Crauel, A., Mechmershausen, M., et al. (2023). Simple and highly efficient detection of PSD95 using a nanobody and its recombinant heavy-chain antibody derivatives. Int. J. Mol. Sci. 24:7294. doi: 10.3390/ijms24087294
Klausberger, T., and Somogyi, P. (2008). Neuronal diversity and temporal dynamics: The unity of hippocampal circuit operations. Science 321, 53–57. doi: 10.1126/science.1149381
Kloc, M. L., Velasquez, F., Niedecker, R. W., Barry, J. M., and Holmes, G. L. (2020). Disruption of hippocampal rhythms via optogenetic stimulation during the critical period for memory development impairs spatial cognition. Brain Stimul. 13, 1535–1547. doi: 10.1016/j.brs.2020.08.011
Koyama, Y., Fujiwara, T., Murayama, H., Machida, M., Inoue, S., and Shobugawa, Y. (2022). Association between adverse childhood experiences and brain volumes among Japanese community-dwelling older people: Findings from the NEIGE study. Child Abuse Negl. 124:105456. doi: 10.1016/j.chiabu.2021.105456
Kühn, S., Gleich, T., Lorenz, R. C., Lindenberger, U., and Gallinat, J. (2014). Playing Super Mario induces structural brain plasticity: Gray matter changes resulting from training with a commercial video game. Mol. Psychiatry 19, 265–271. doi: 10.1038/mp.2013.120
Leinekugel, X., Khazipov, R., Cannon, R., Hirase, H., Ben-Ari, Y., and Buzsáki, G. (2002). Correlated bursts of activity in the neonatal hippocampus in vivo. Science 296, 2049–2052. doi: 10.1126/science.1071111
LeMessurier, A. M., Laboy-Juárez, K. J., McClain, K., Chen, S., Nguyen, T., and Feldman, D. E. (2019). Enrichment drives emergence of functional columns and improves sensory coding in the whisker map in L2/3 of mouse S1. Elife 8:e46321. doi: 10.7554/eLife.46321
Ma, J., Yang, Y., Wan, Y., Shen, C., and Qiu, P. (2021). The influence of childhood adversities on mid to late cognitive function: From the perspective of life course. PLoS One 16:e0256297. doi: 10.1371/journal.pone.0256297
Malave, L., van Dijk, M. T., and Anacker, C. (2022). Early life adversity shapes neural circuit function during sensitive postnatal developmental periods. Transl. Psychiatry 12:306. doi: 10.1038/s41398-022-02092-9
Manno, F. A. M., Kumar, R., An, Z., Khan, M. S., Su, J., Liu, J., et al. (2022). Structural and functional hippocampal correlations in environmental enrichment during the adolescent to adulthood transition in mice. Front. Syst. Neurosci. 15:807297. doi: 10.3389/fnsys.2021.807297
Marr, D. (1971). Simple memory: A theory for archicortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 262, 23–81. doi: 10.1098/rstb.1971.0078
Massa, F., Koehl, M., Wiesner, T., Grosjean, N., Revest, J. M., Piazza, P. V., et al. (2011). Conditional reduction of adult neurogenesis impairs bidirectional hippocampal synaptic plasticity. Proc. Natl. Acad. Sci. U. S. A. 108, 6644–6649. doi: 10.1073/pnas.1016928108
McHugh, T. J., Jones, M. W., Quinn, J. J., Balthasar, N., Coppari, R., Elmquist, J. K., et al. (2007). Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317, 94–99. doi: 10.1126/science.1140263
McNaughton, B. L., and Morris, R. G. M. (1987). Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 10, 408–415. doi: 10.1016/0166-2236(87)90011-7
Molet, J., Heins, K., Zhuo, X., Mei, Y. T., Regev, L., Baram, T. Z., et al. (2016a). Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl. Psychiatry 6:e702. doi: 10.1038/tp.2015.200
Molet, J., Maras, P. M., Kinney-Lang, E., Harris, N. G., Rashid, F., Ivy, A. S., et al. (2016b). MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus 26, 1618–1632. doi: 10.1002/hipo.22661
Murray, K. D., Liu, X. B., King, A. N., Luu, J. D., and Cheng, H. J. (2020). Age-Related changes in synaptic plasticity associated with mossy fiber terminal integration during adult neurogenesis. eNeuro 7:ENEURO.0030-20.2020. doi: 10.1523/ENEURO.0030-20.2020.
Naninck, E. F., Hoeijmakers, L., Kakava-Georgiadou, N., Meesters, A., Lazic, S. E., Lucassen, P. J., et al. (2015). Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus 25, 309–328. doi: 10.1002/hipo.22374
Naninck, E. F., Oosterink, J. E., Yam, K. Y., de Vries, L. P., Schierbeek, H., van Goudoever, J. B., et al. (2017). Early micronutrient supplementation protects against early stress-induced cognitive impairments. FASEB J. 31, 505–518. doi: 10.1096/fj.201600834R
Nelson, C. A., Zeanah, C. H., Fox, N. A., Marshall, P. J., Smyke, A. T., and Guthrie, D. (2007). Cognitive recovery in socially deprived young children: The bucharest early intervention project. Science 318, 1937–1940. doi: 10.1126/science.1143921
Oliveira, A. M., Hawk, J. D., Abel, T., and Havekes, R. (2010). Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn. Mem. 17, 155–160. doi: 10.1101/lm.1625310
Patrylo, P. R., Tyagi, I., Willingham, A. L., Lee, S., and Williamson, A. (2007). Dentate filter function is altered in a proepileptic fashion during aging. Epilepsia 48, 1964–1978. doi: 10.1111/j.1528-1167.2007.01139.x
Pintori, N., Piva, A., Mottarlini, F., Díaz, F. C., Maggi, C., Caffino, L., et al. (2024). Brief exposure to enriched environment rapidly shapes the glutamate synapses in the rat brain: A metaplastic fingerprint. Eur. J. Neurosci. 59, 982–995. doi: 10.1111/ejn.16279
Pollak, S. D., Nelson, C. A., Schlaak, M. F., Roeber, B. J., Wewerka, S. S., Wiik, K. L., et al. (2010). Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Dev. 81, 224–236. doi: 10.1111/j.1467-8624.2009.01391.x
Qin, L., Marrs, G. S., McKim, R., and Dailey, M. E. (2001). Hippocampal mossy fibers induce assembly and clustering of PSD95-containing postsynaptic densities independent of glutamate receptor activation. J. Comp. Neurol. 440, 284–298. doi: 10.1002/cne.1386
Ramírez-Amaya, V., Balderas, I., Sandoval, J., Escobar, M. L., and Bermúdez-Rattoni, F. (2001). Spatial long-term memory is related to mossy fiber synaptogenesis. J. Neurosci. 21, 7340–7348. doi: 10.1523/JNEUROSCI.21-18-07340.2001
Redish, A. D., McNaughton, B. L., and Barnes, C. A. (1998). Reconciling Barnes et al. (1997) and Tanila et al. (1997a,b). Hippocampus 8, 438–443. doi: 10.1002/(SICI)1098-106319988:5<438::AID-HIPO4>3.0.CO;2-Z
Rice, C. J., Sandman, C. A., Lenjavi, M. R., and Baram, T. Z. A. (2008). novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 149, 4892–4900. doi: 10.1210/en.2008-0633
Rocha, M., Wang, D., Avila-Quintero, V., Bloch, M. H., and Kaffman, A. (2021). Deficits in hippocampal-dependent memory across different rodent models of early life stress: Systematic review and meta-analysis. Transl. Psychiatry 11:231. doi: 10.1038/s41398-021-01352-4
Rollenhagen, A., and Lübke, J. H. R. (2010). The mossy fiber bouton: The “Common” or the “Unique”. Synapse? Front. Synaptic Neurosci. 2:2. doi: 10.3389/fnsyn.2010.00002
Rollenhagen, A., Sätzler, K., Rodríguez, E. P., Jonas, P., Frotscher, M., and Lübke, J. H. (2007). Structural determinants of transmission at large hippocampal mossy fiber synapses. J. Neurosci. 27, 10434–10444. doi: 10.1523/JNEUROSCI.1946-07.2007
Rountree-Harrison, D., Burton, T. J., Leamey, C. A., and Sawatari, A. (2018). Environmental enrichment expedites acquisition and improves flexibility on a temporal sequencing task in mice. Front. Behav. Neurosci. 12:51. doi: 10.3389/fnbeh.2018.00051
Rule, L., Yang, J., Watkin, H., Hall, J., and Brydges, N. M. (2021). Environmental enrichment rescues survival and function of adult-born neurons following early life stress. Mol. Psychiatry 26, 1898–1908. doi: 10.1038/s41380-020-0718-4
Schildt, S., Endres, T., Lessmann, V., and Edelmann, E. (2013). Acute and chronic interference with BDNF/TrkB-signaling impair LTP selectively at mossy fiber synapses in the CA3 region of mouse hippocampus. Neuropharmacology 71, 247–254. doi: 10.1016/j.neuropharm.2013.03.041
Scholz, J., Allemang-Grand, R., Dazai, J., and Lerch, J. P. (2015). Environmental enrichment is associated with rapid volumetric brain changes in adult mice. Neuroimage 109, 190–198. doi: 10.1016/j.neuroimage.2015.01.027
Schwegler, H., Crusio, W. E., and Brust, I. (1990). Hippocampal mossy fibers and radial-maze learning in the mouse: A correlation with spatial working memory but not with non-spatial reference memory. Neuroscience 34, 293–298. doi: 10.1016/0306-4522(90)90139-u
Shinoda, Y., Tanaka, T., Tominaga-Yoshino, K., and Ogura, A. (2010). Persistent synapse loss induced by repetitive LTD in developing rat hippocampal neurons. PLoS One 5:e10390. doi: 10.1371/journal.pone.0010390
Short, A. K., Maras, P. M., Pham, A. L., Ivy, A. S., and Baram, T. Z. (2020). Blocking CRH receptors in adults mitigates age-related memory impairments provoked by early-life adversity. Neuropsychopharmacology 45, 515–523. doi: 10.1038/s41386-019-0562-x
Simkin, D., Hattori, S., Ybarra, N., Musial, T. F., Buss, E. W., Richter, H., et al. (2015). Aging-Related hyperexcitability in CA3 pyramidal neurons is mediated by enhanced A-Type K+ channel function and expression. J. Neurosci. 35, 13206–13218. doi: 10.1523/JNEUROSCI.0193-15.2015
Speisman, R. B., Kumar, A., Rani, A., Pastoriza, J. M., Severance, J. E., Foster, T. C., et al. (2013). Environmental enrichment restores neurogenesis and rapid acquisition in aged rats. Neurobiol. Aging 34, 263–274. doi: 10.1016/j.neurobiolaging.2012.05.023
Spratt, E. G., Friedenberg, S. L., Swenson, C. C., Larosa, A., De Bellis, M. D., Macias, M. M., et al. (2012). The effects of early neglect on cognitive, language, and behavioral functioning in childhood. Psychology 3, 175–182. doi: 10.4236/psych.2012.32026
Tanila, H., Shapiro, M., Gallagher, M., and Eichenbaum, H. (1997a). Brain aging: Changes in the nature of information coding by the hippocampus. J. Neurosci. 17, 5155–5166. doi: 10.1523/JNEUROSCI.17-13-05155.1997
Tanila, H., Shapiro, M. L., and Eichenbaum, H. (1997b). Discordance of spatial representation in ensembles of hippocampal place cells. Hippocampus 7, 613–623. doi: 10.1002/(SICI)1098-106319977:6<613::AID-HIPO4>3.0.CO;2-F
Teicher, M. H., Samson, J. A., Anderson, C. M., and Ohashi, K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 17, 652–666. doi: 10.1038/nrn.2016.111
Tizard, B., and Rees, J. (1974). A comparison of the effects of adoption, restoration to the natural mother, and continued institutionalization on the cognitive development of four-year-old children. Child Dev. 45, 92–99. doi: 10.2307/1127754
Toscano-Silva, M., Gomes da Silva, S., Scorza, F. A., Bonvent, J. J., Cavalheiro, E. A., and Arida, R. M. (2010). Hippocampal mossy fiber sprouting induced by forced and voluntary physical exercise. Physiol. Behav. 101, 302–308. doi: 10.1016/j.physbeh.2010.05.012
Uchigashima, M., Fukaya, M., Watanabe, M., and Kamiya, H. (2007). Evidence against GABA release from glutamatergic mossy fiber terminals in the developing hippocampus. J. Neurosci. 27, 8088–8100. doi: 10.1523/JNEUROSCI.0702-07.2007
Urban, N. N., Henze, D. A., and Barrionuevo, G. (2001). Revisiting the role of the hippocampal mossy fiber synapse. Hippocampus 11, 408–417. doi: 10.1002/hipo.1055
Uttal, D. H., Meadow, N. G., Tipton, E., Hand, L. L., Alden, A. R., Warren, C., et al. (2013). The malleability of spatial skills: A meta-analysis of training studies. Psychol. Bull. 139, 352–402. doi: 10.1037/a0028446
Ventura, S., Duncan, S., and Ainge, J. A. (2024). Increased flexibility of CA3 memory representations following environmental enrichment. Curr. Biol. 34, 2011–2019.e7. doi: 10.1016/j.cub.2024.03.054.
Villanueva-Castillo, C., Tecuatl, C., Herrera-López, G., and Galván, E. J. (2017). Aging-related impairments of hippocampal mossy fibers synapses on CA3 pyramidal cells. Neurobiol. Aging 49, 119–137. doi: 10.1016/j.neurobiolaging.2016.09.010
Walker, C. D., Bath, K. G., Joels, M., Korosi, A., Larauche, M., Lucassen, P. J., et al. (2017). Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: Critical considerations of methodology, outcomes and translational potential. Stress 20, 421–448. doi: 10.1080/10253890.2017.1343296
Walker, M. C., Ruiz, A., and Kullmann, D. M. (2001). Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses. Neuron 29, 703–715. doi: 10.1016/s0896-6273(01)00245-8
Wang, X. D., Rammes, G., Kraev, I., Wolf, M., Liebl, C., Scharf, S. H., et al. (2011). Forebrain CRF1 modulates early-life stress-programmed cognitive deficits. J. Neurosci. 31, 13625–13634. doi: 10.1523/JNEUROSCI.2259-11.2011
Williams, B. M., Luo, Y., Ward, C., Redd, K., Gibson, R., Kuczaj, S. A., et al. (2001). Environmental enrichment: Effects on spatial memory and hippocampal CREB immunoreactivity. Physiol. Behav. 73, 649–658. doi: 10.1016/s0031-9384(01)00543-1
Wilson, I. A., Ikonen, S., Gallagher, M., Eichenbaum, H., and Tanila, H. (2005). Age-associated alterations of hippocampal place cells are subregion specific. J. Neurosci. 25, 6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005
Wodarski, J. S., Kurtz, P. D., Gaudin, J. M., and Howing, P. T. (1990). Maltreatment and the school-age child: Major academic, socioemotional, and adaptive outcomes. Soc. Work 35, 506–513. doi: 10.1093/sw/35.6.506
Woodcock, E. A., and Richardson, R. (2000). Effects of environmental enrichment on rate of contextual processing and discriminative ability in adult rats. Neurobiol. Learn. Mem. 73, 1–10. doi: 10.1006/nlme.1999.3911
Youssef, M., Atsak, P., Cardenas, J., Kosmidis, S., Leonardo, E. D., and Dranovsky, A. (2019). Early life stress delays hippocampal development and diminishes the adult stem cell pool in mice. Sci. Rep. 9:4120. doi: 10.1038/s41598-019-40868-0
Keywords: memory, synaptic plasticity, behavior, hippocampus, enrichment, early-life stress and mossy fiber
Citation: Shobe JL, Ghanbarian E, Bain R, Saxena R, Chandrasekaran M and McNaughton BL (2025) Cognitive enrichment improves spatial memory and alters hippocampal synaptic connectivity in a mouse model for early-life stress. Front. Cell. Neurosci. 19:1646883. doi: 10.3389/fncel.2025.1646883
Received: 14 June 2025; Accepted: 22 September 2025;
Published: 17 October 2025.
Edited by:
Martín Cammarota, Federal University of Rio Grande do Norte, BrazilReviewed by:
Kate Beecher, The University of Queensland, AustraliaPankaj Bhatia, Wayne State University, United States
Copyright © 2025 Shobe, Ghanbarian, Bain, Saxena, Chandrasekaran and McNaughton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justin L. Shobe, anNob2JlQHVjaS5lZHU=
†Present address: Elham Ghanbarian, Department of Neurology, University of California, Irvine, CA, United States; Rajat Saxena, Kavli Institute for Systems Neuroscience, Centre for Algorithms in the Cortex, Norwegian University of Science and Technology (NTNU), Trondheim, Norway
 Justin L. Shobe
Justin L. Shobe Elham Ghanbarian1†
Elham Ghanbarian1† Robert Bain
Robert Bain Bruce L. McNaughton
Bruce L. McNaughton