- 1School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI, United States
- 2Center for Cross-Straits Cultural Development, Fujian Normal University, Fuzhou City, Fujian, China
- 3Department of Radiology, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI, United States
- 4Neuroscience Training Program, University of Wisconsin-Madison, Madison, WI, United States
- 5The Waisman Center, University of Wisconsin-Madison, Madison, WI, United States
- 6Gastroenterology and Hepatology, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI, United States
- 7Gastroenterology and Hepatology, Department of Medicine, University of Wisconsin- Madison, Madison, WI, United States
- 8Department of Psychology and Psychiatry, University of Wisconsin-Madison, Madison, WI, United States
Introduction: Crohn’s disease (CD), one of the main phenotypes of inflammatory bowel disease (IBD), can affect any part of the gastrointestinal tract. It can impact the function of gastrointestinal secretions, as well as increasing the intestinal permeability leading to an aberrant immunological response and subsequent intestinal inflammation. Studies have reported anatomical and functional brain changes in Crohn’s Disease patients (CDs), possibly due to increased inflammatory markers and microglial cells that play key roles in communicating between the brain, gut, and systemic immune system. To date, no studies have demonstrated similarities between morphological brain changes seen in IBD and brain morphometry observed in older healthy controls.
Methods: For the present study, twelve young CDs in remission (M = 26.08 years, SD = 4.9 years, 7 male) were recruited from an IBD Clinic. Data from 12 young age-matched healthy controls (HCs) (24.5 years, SD = 3.6 years, 8 male) and 12 older HCs (59 years, SD = 8 years, 8 male), previously collected for a different study under a similar MR protocol, were analyzed as controls. T1 weighted images and structural image processing techniques were used to extract surface-based brain measures, to test our hypothesis that young CDs have different brain surface morphometry than their age-matched young HCs and furthermore, appear more similar to older HCs. The phonemic verbal fluency (VF) task (the Controlled Oral Word Association Test, COWAT) (Benton, 1976) was administered to test verbal cognitive ability and executive control.
Results/Discussion: On the whole, CDs had more brain regions with differences in brain morphometry measures when compared to the young HCs as compared to the old HCs, suggesting that CD has an effect on the brain that makes it appear more similar to old HCs. Additionally, our study demonstrates this atypical brain morphometry is associated with function on a cognitive task. These results suggest that even younger CDs may be showing some evidence of structural brain changes that demonstrate increased resemblance to older HC brains rather than their similarly aged healthy counterparts.
Introduction
Crohn’s disease (CD), one of the main phenotypes of inflammatory bowel disease (IBD), can affect any part of the gastrointestinal tract (Fiorindi et al., 2022; Godala et al., 2022). It can impact the function of gastrointestinal secretions, as well as increasing the intestinal permeability leading to an aberrant immunological response and subsequent intestinal inflammation (Chichlowski and Hale, 2008). Even patients in remission experience post-inflammatory changes leading to intestinal hypersensitivity (Jacobson et al., 1995). There is evidence suggesting that the inflammatory response of IBD may affect a patient’s mental state by altering motor and sensory systems causing difficulties with cognition (Nair et al., 2016; van Langenberg et al., 2017; Sharma et al., 2021) and psychological stress precipitating mood disorders (Zhang et al., 2016; Banovic et al., 2020). The effect of IBD may also alter the brain and lead to anatomical and functional changes. Several studies have reported anatomical and functional brain changes in Crohn’s Disease patients (CDs), possibly due to increased inflammatory markers and microglial cells that play key roles in communicating between the brain, gut, and systemic immune system (Sajadinejad et al., 2012; Hou et al., 2019). It has been proposed that these systemic alterations lead to a series of changes to neuronal connections and processes resulting in anatomical or functional brain changes that impact cognitive or emotion regulation skills (Zeng et al., 2012; Nair et al., 2016; Bao et al., 2017b). These brain changes may also explain why CDs tend to have a reduced ability to regulate cognitive and emotional states than their non-CD counterparts (Thomason and Thompson, 2011; Bushnell et al., 2013; Nair et al., 2016). Additionally, anatomical and functional changes in the brain may be influenced by the comorbidities associated with CD such as chronic pain, psychological stress, anxiety, and depression (Sajadinejad et al., 2012).
There is mounting evidence suggesting that the differences observed in brain function and structure of CDs may be correlated with cognitive differences. For instance, a couple of diffusion tensor imaging (DTI) studies have identified white matter (WM) microstructural differences in CDs compared to heatlhy controls (HCs). Zikou et al. reported IBD patients (CD or ulcerative colitis) who showed decreased axial diffusivity in the right corticospinal tract (involved in motor function) and right superior longitudinal fasciculus (involved in language function) when compared to HCs (Zikou et al., 2014). Our previous DTI study identified significant alterations in WM microstructure of CDs compared to HCs in brain regions implicated in language function despite the absence of differences in a verbal fluency measure designed to assess verbal cognitive ability and executive control (Hou et al., 2020).
A meta-analysis of CDs brain imaging literature reported reduced GM volume in the medial frontal gyrus compared to that of HCs (Yeung, 2021). Bao, et al. identified cortical thickness of the left insula and orbitofrontal cortex and gray matter (GM) volumes of the right anterior cingulate cortex (ACC), dorsomedial prefrontal cortex and left insula were negatively correlated with disease duration (Bao et al., 2015). A subsequent study by Bao, et al. identified differences in GM volumes between CDs in remission with and without abdominal pain, finding lower GM volumes in the insula and ACC in CDs with pain compared to those without (Bao et al., 2017a). Other regions of cortical thickness increases, and sub-cortical volume decreases, have also been reported and correlated to pain score or disease duration (Nair et al., 2016). Zikou et al. also found brain regions of atrophy in CDs such as the bilateral fusiform and inferior temporal gyrus which are related to emotion processing (Zikou et al., 2014). A study by Thapaliya, et al., demonstrated a significant reduction in gray matter volume (GMV), white matter volume and cortical thickness in the left prefrontal gyrus and increased GMV in frontal brain regions in CDs versus HCs (Thapaliya et al., 2022). Additionally, another study found CDs with extraintestinal manifestations of the disease, but not those without such manifestations, were especially prone to cortical brain changes, suggesting that brain changes are more strongly influenced by the systemic inflammation of the disease (Thomann et al., 2016).
Our previous task-based functional magnetic resonance imaging study looking at verbal fluency of CDs in remission found that activity intensity in regions of the right hemisphere was positively correlated with disease duration. Furthermore, the study identified similar task activation patterns between young adult CDs and healthy older HCs. This suggests that young adult CD brain changes may resemble brains older healthy adults (Nair et al., 2019), perhaps due to the increase of proinflammatory cytokine exposure in both aging adults and CDs. Additionally, IBD has been associated with age-related diseases such as, Parkinson’s (Lin et al., 2016; Brudek, 2019; Zeng et al., 2022) and Alzheimer’s disease (Hillary et al., 2020; Wang et al., 2022). To date, no studies have demonstrated similarities between morphological brain changes seen in IBD and brain morphometry observed in older healthy controls.
Among many techniques, the brain cortical thickness measures using magnetic resonance imaging (MRI) have proven sensitive to examine the changes in brain structure and development with some studies having used the volumetric measurement (e.g., voxel-based morphometry) to examine CD in remission (Nair et al., 2016; Thomann et al., 2021). However, volumetric measurement has some limitations. For example, it is inadequate for investigating brain surface folding due to its lack of statistical power (Lemaitre et al., 2012; Jin et al., 2018). Other cortical surface morphometries such as the cortical thickness, fractal dimensionality (FD), gyrification, and sulcal depth also influence the volumetric results (Trost et al., 2013; Hirakawa et al., 2016).
Cortical thickness measures the distance between the points on the pial and white matter boundaries of the neocortex, in addition to measuring the gray matter morphological difference (Hirakawa et al., 2016; Seiger et al., 2018). However, cortical thickness is limited to the cortex and therefore it cannot examine non-cortical regions (Bermudez et al., 2009). Another measure, fractal dimensionality, reflects how the brain structure fits to space constraints (Yotter et al., 2011b) and is used to investigate cortical complexity of cerebral folding reported as a single numerical value (Di Ieva et al., 2014, 2015; Madan and Kensinger, 2017). Studies have demonstrated that FD is sensitive to internal shape complexity of the brain that gray matter volume and cortical thickness measures are not (Zhang et al., 2008; Madan and Kensinger, 2017; Chen et al., 2020). Gyrification examines the level of local cortical folding that relates to the integrity between subcortical and cortex circuits (Li et al., 2021). Sulcal depth, based on the Euclidean distance between the pial and outer surface (Yun et al., 2013; Li et al., 2021), is generated from the changes of gray and white matter in the cerebral cortex as well as subcortical structures, making it sensitive to the complicated folding of the cerebral surface (Im et al., 2008; Kim et al., 2008; Kochunov et al., 2008; Jin et al., 2018).
In the current study, we aim to build upon our previous study by using structural imaging techniques that include the cortical thickness, fractal dimensionality, gyrification, and sulcal depth (see Methods for description of each metric), to test our hypothesis that the young CDs have different brain surface morphometry than their age-matched young HCs and furthermore, appear more similar to the older HCs. Additionally, we hypothesize these structural changes will be reflected in functional outcome differences in cognitive function.
Methods
Participants
Twelve young CDs in remission (M = 26.08 years, SD = 4.9 years, 7 male) were recruited from the IBD Clinic. Data from 12 young age-matched HCs (24.5 years, SD = 3.6 years, 8 male) and 12 older HCs (59 years, SD = 8 years, 8 male), previously collected for a different study with similar MR scan protocol, were analyzed as controls. Participant characteristics are shown in Table 1. HCs had no history of substance abuse, affective, psychiatric, or neurological disorders, and were mostly right-handed (Oldfield, 1971). The participants were screened for cognitive deficits using the Mini-Mental State Examination (Folstein et al., 1975) and provided written informed consent. The protocol was reviewed and approved (#H2014–0131) by the local health sciences IRB. All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by the Institutional Review Board (IRB) of the School of Medicine and Public Health, University of Wisconsin-Madison.
Behavioral data acquisition
We administered the phonemic verbal fluency (VF) task (the Controlled Oral Word Association Test, COWAT; Benton, 1976) to test verbal cognitive ability and executive control. All CDs and HCs were tested for the VF task outside the scanner. COWAT has been extensively used in both clinical and non-clinical populations on account of its face validity (Sauzéon et al., 2011), assessment of both verbal cognitive ability and executive control (Fisk and Sharp, 2004), and high correlation with measures of attention, verbal memory, and word knowledge (Ruff et al., 1997). Participants were required to produce words beginning with the letters “F,” “A,” “S,” in three 1-min trials, respectively. A normalized VF z-score, corrected for age and education, based on the total correct responses over the 3 trials was used to quantify VF performance for each participant.
MRI data acquisition
The MRI data were acquired on a GE750 3 T MRI scanner. A whole brain high-resolution 3D T1-weighted BRAVO, IR-prepared FSPGR (Fast Spoiled Gradient Recalled Echo), MRI sequence with 156 axial slices was performed for each participant using the following parameters: TR = 8.132 ms, TE = 3.18 ms, TI = 450 ms, feld of view = 256 × 256 mm2, flip angle = 12, matrix = 256 × 256, in-plane resolution =1 × 1 mm2, slice thickness = 1.0 mm.
Cortical surface preprocessing
The Computational Anatomy Toolbox (CAT12)1, which is a plug-in software based on Statistical Parametric Mapping (SPM12)2 and integrated into MATLAB (MathWorks), was used for the T1-weighted MRI data preprocessing. The CAT12 is not only a more precise and accurate analysis of gray matter volume than the previous voxel-based morphometry plug-in toolbox in SPM (Farokhian et al., 2017; Yuksel et al., 2018), but also is fully automated for surface-based analysis (Zhuang et al., 2017). The data preprocessing with CAT12 consisted of bias-field correction, skull-stripping, and alignment to the Montreal Neurological Institute (MNI) structural template to classify gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF), as well as spatial normalization with the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) registration (1.5 mm) (Kurth et al., 2015; Zhuang et al., 2017; Yuksel et al., 2018). Subsequently, we employed a spherical harmonic method (Yotter et al., 2011a) to reparametrize the cortical surface mesh based on an algorithm that reduces area distortions (Yotter et al., 2011c) to repair any topological defects (Yotter et al., 2011a,c; Chen et al., 2020). Cortical thickness was analyzed based on the workflow specified in the study by Dahnke et al. (2013). This algorithm uses tissue segmentation to evaluate the WM distance and also projects the local maxima to other GM voxel. Values at the outer GM boundary in the WM distance map is projected back to the inner GM boundary to generate the GM thickness (Li et al., 2021). Following this, a central surface was created at the 50% level of the percentage position between the WM distance and GM thickness (Li et al., 2021). For the resultant central surface, a topology correction based on spherical harmonics was used to account for topological defects (Yotter et al., 2011a; Li et al., 2021). Moreover, the central surface was reparameterized into a common coordinate system through spherical mapping, and the spatial normalization was used with the DARTEL registration (Li et al., 2021). Spatially smoothing with 15 mm full width at half maximum (FWHM) Gaussian kernel was used for this analysis.
The fractal dimensionality estimates cortical fold complexity based on spherical harmonic reconstructions (Yotter et al., 2011a; Li et al., 2021) and is calculated as the slope of a logarithmic plot of surface area versus the maximum l-value, where the maximum l-value is a measure of the bandwidth of frequencies used to reconstruct the surface shape (Yotter et al., 2011b; Li et al., 2021). Smoothing with 15 mm FWHM Gaussian kernel was used for the fractal dimensionality analysis.
Based on the spherical harmonic reconstructions, the gyrification, as an indicator of cortical folding, was calculated as absolute mean curvature (Luders et al., 2006; Li et al., 2021). Mean curvature is an extrinsic surface measure, and provides information about the change in normal direction along the surface (Li et al., 2021). Smoothing with 15 mm FWHM Gaussian kernel was used for this analysis.
The sulcal depth measures the depth of sulci and is calculated as the Euclidean distance between the central surface and its convex hull based on the spherical harmonic reconstructions, then transformed with the sqrt function (Li et al., 2021). Smoothing with 15 mm FWHM Gaussian kernel was used for this analysis.
Statistical analysis
The demographic differences between the CDs and young or old HCs were analyzed by independent samples t-tests. Group comparisons of cortical thickness, fractal dimensionality, gyrification, and sulcal depth were performed using the CAT12 and analyzed via a non-parametric permutation technique. The Threshold-Free Cluster Enhancement (TFCE) was used in permutation testing with 5,000 permutations (Smith and Nichols, 2009). TFCE p < 0.05 images obtained were family-wise error corrected for multiple comparisons across space. The brain regions with cluster size at least 100 vertices (cluster size × percentage covered in the specific region produced by CAT12) were reported. The Desikan–Killiany atlas (DK40) (Desikan et al., 2006) was used to label the cortical regions and the results were visualized using the CAT12. Moreover, when group differences with detailed regions were observed in CAT12, we conducted the Pearson correlation between each surface index and VF score in each group in IBM SPSS version 23, with its threshold of family-wise error corrected p < 0.05.
Results
Behavior
A One-Way ANOVA showed that there were no significant differences between young CDs, young HCs, and old HCs on education, VF score, gender and handedness. Posthoc analysis revealed no age difference between young CDs and young HCs (p = 0.512), but there was a statistical difference between the ages of young CDs and old HCs (p = 0.000), and also between the young HCs and old HCs (p = 0.000) (see Table 1 for details).
Group differences in cortical surface measures
Cortical thickness
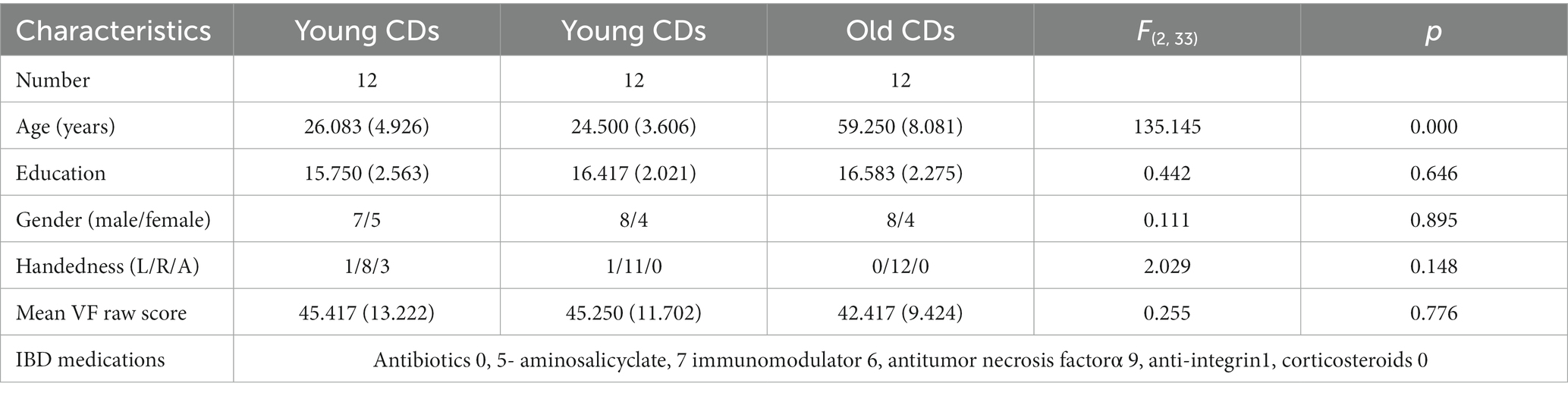
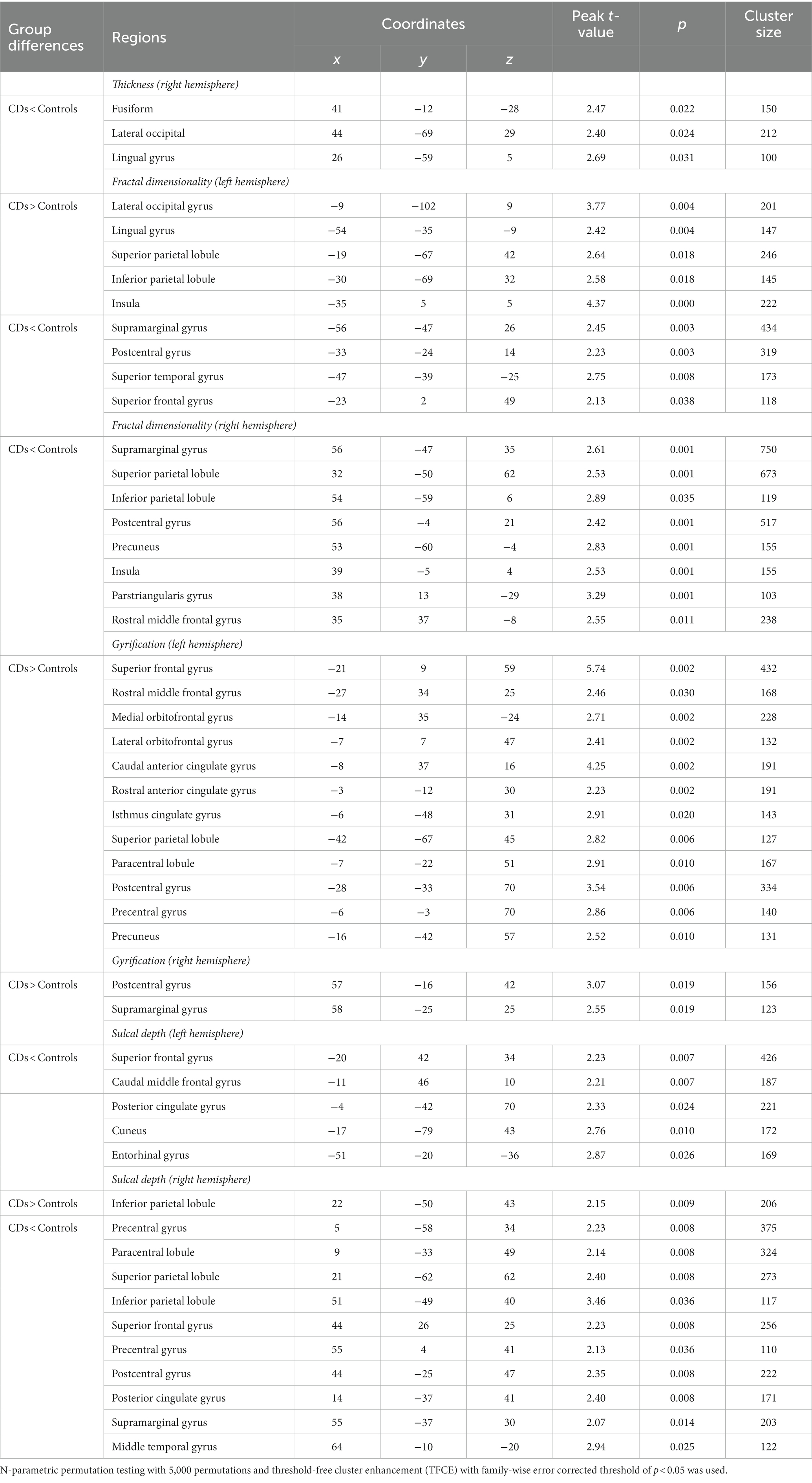
Compared to the young HCs, the young CDs demonstrated significantly decreased cortical thickness in the right fusiform, inferior occipital and lingual gyri (see Figure 1A and Table 2). However, the young CDs exhibited significantly increased cortical thickness in the right postcentral gyrus compared to the old HCs (see Figure 2A and Table 3).
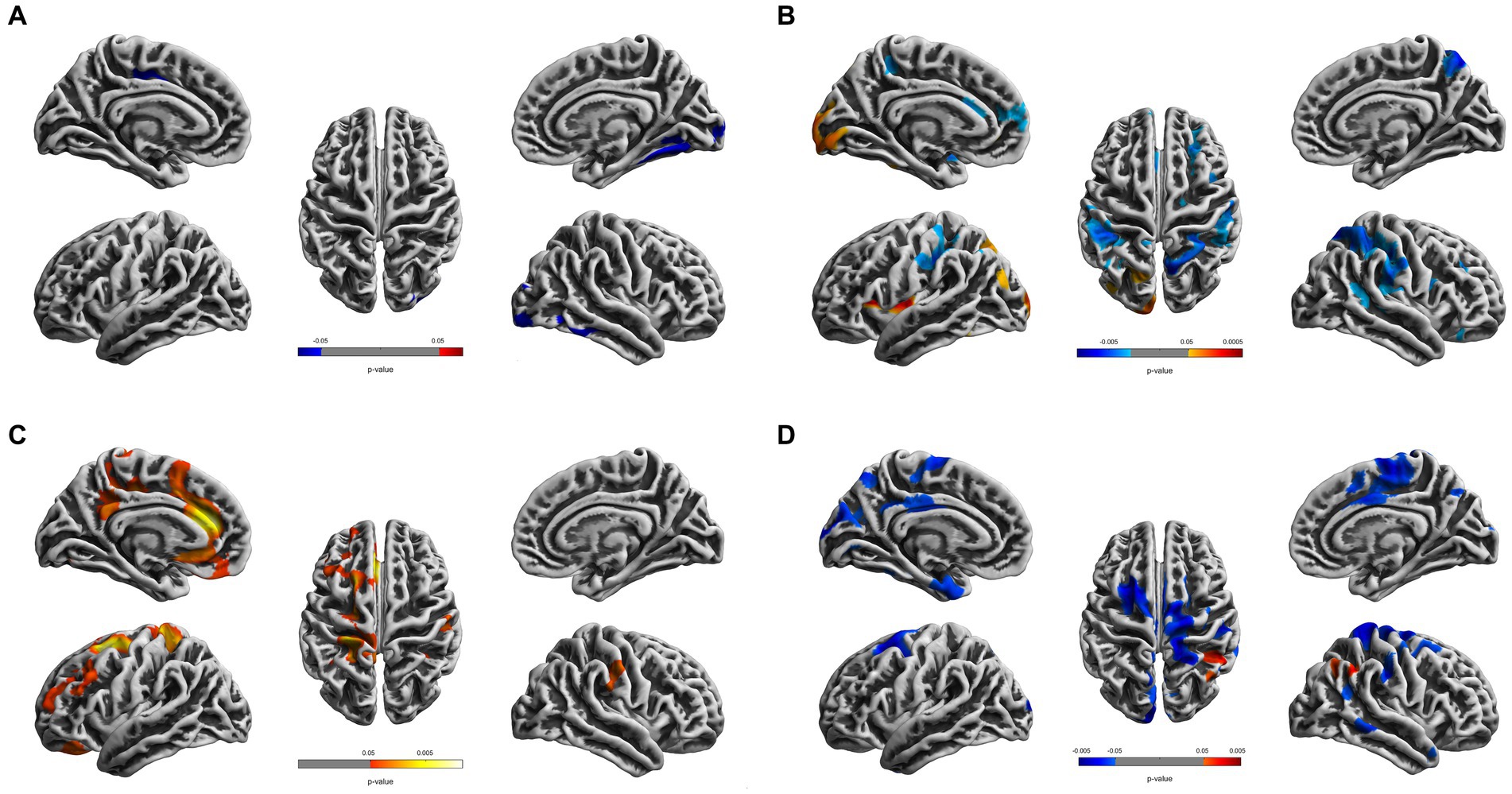
Figure 1. Cortical surface differences between young CDs and young HCs. Non-parametric permutation testing with 5,000 permutations and threshold-free cluster enhancement (TFCE) with family-wise error corrected threshold of p < 0.05 was used. Red: younger CDs increased sulcal depth compared to younger HCs. Blue: younger CDs decreased sulcal depth compared to younger HCs. (A) Cortical thickness. (B) Fractal dimensionality. (C) Gyrification (D) Sulcal depth.
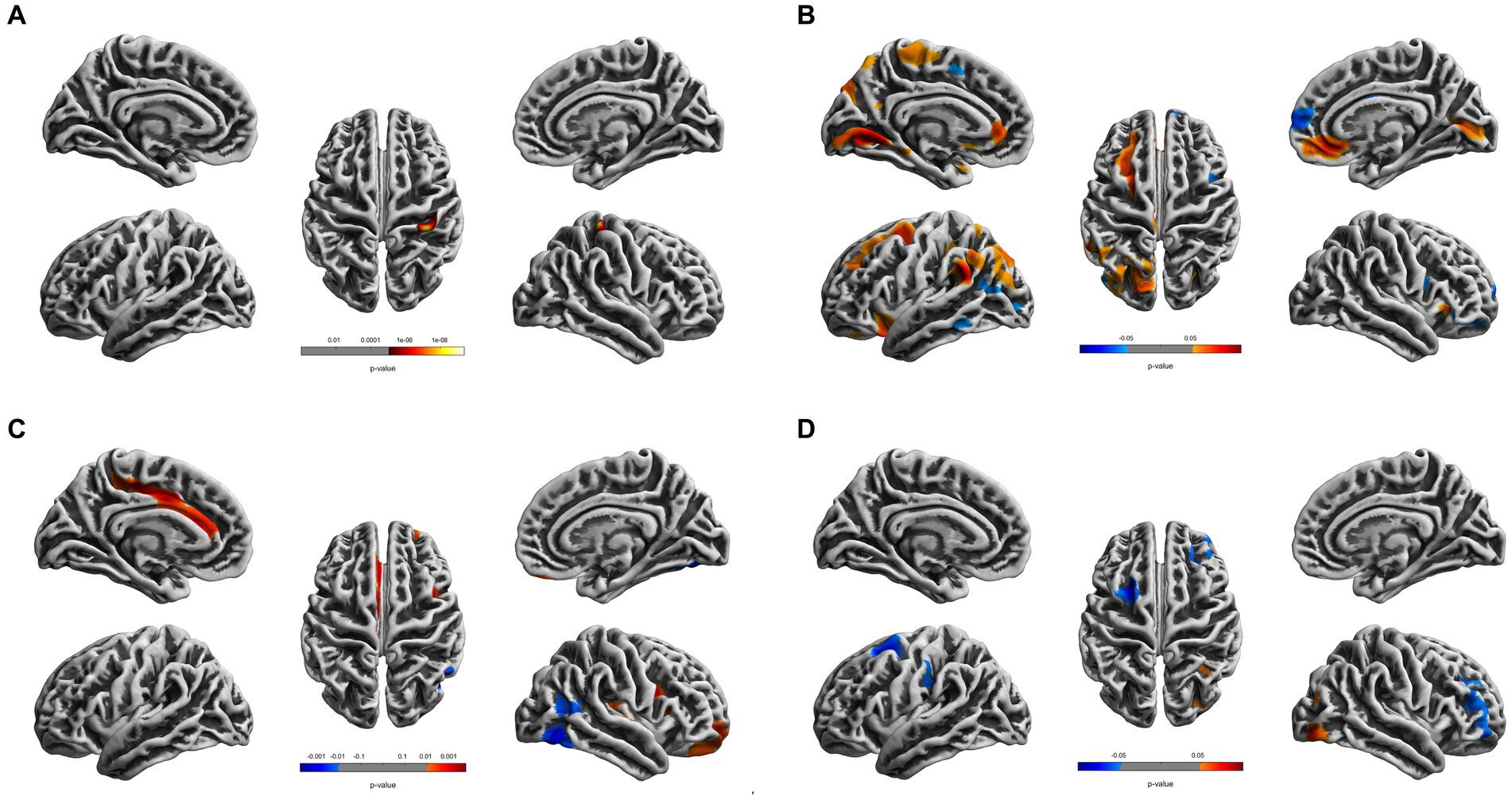
Figure 2. Cortical surface differences between younger CDs and older HCs. Non-parametric permutation testing with 5,000 permutations and threshold-free cluster enhancement (TFCE) with a family-wise error correction threshold of p < 0.05 was used. Red: younger CDs increased sulcal depth compared to older HCs. Blue: younger CDs decreased sulcal depth compared to older HCs. (A) Cortical thickness. (B) Fractal dimensionality. (C) Gyrification. (D) Sulcal depth.
Fractal dimensionality
The fractal dimensionality revealed bi-directional results. When compared to young HCs, the young CDs showed significant increases in the lateral occipital, lingual and insula gyri, as well as, the superior and inferior parietal lobules in the left hemisphere. Contrarily, significant decreases in fractal dimensionality were observed in the young CDs compared to the young HCs in the left superior temporal and superior frontal gyri, right superior and inferior parietal lobules, right precuneus, insula, parstriangularis, rostral middle frontal gyri, as well as the bilateral supramarginal and postcentral gyri (see Figure 1B and Table 2).
The fractal dimensionality also demonstrated bi-directional results between young CDs and old HCs. Compared to the old HCs, the young CDs exhibited significantly increased fractal dimensionality in the superior frontal, rostral and caudal middle frontal, lateral orbitofrontal, lingual, supramarginal and rostral anterior cingulate gyri, superior and paracentral lobules in the left hemisphere, and the right pericalcarine and medial orbitofrontal gyri in the right hemisphere. However, the young CDs also showed significantly decreased fractal dimensionality in the left inferior parietal lobule and the right superior frontal and parstriangularis gyri compared to the old HCs (see Figure 2B and Table 3).
Gyrification index
Compared to the young HCs, the young CDs illustrated significant increased gyrification in the superior frontal, rostral middle frontal, medial and lateral orbitofrontal, caudal and rostral anterior cingulate, isthmus cingulate, precentral, precuneus gyri, superior parietal, and paracentral lobules in the left hemisphere, as well as the supramarginal gyrus in the right hemisphere (see Figure 1C and Table 2).
Compared to the old HCs, the young CDs exhibited significantly increased gyrification in the posterior and caudal anterior cingulate gyri, and paracentral lobule in the left hemisphere, in the superior temporal, insula, rostral middle frontal and lateral orbitofrontal gyri in the right hemisphere, as well as significantly decreased gyrification in the right fusiform and lateral occipital gyri (see Figure 2C and Table 3).
Sulcal depth
Compared to the young HCs, the young CDs showed significantly increased sulcal depth only in the right inferior parietal lobule. They also revealed significantly decreased sulcal depth in the caudal middle frontal, cuneus, entorhinal gyri in the left hemisphere, the precentral, postcentral, supramarginal and middle temporal gyri, superior and inferior parietal lobules, and paracentral lobule in the right hemisphere, as well as the bilateral superior frontal and posterior cingulate gyri in the bilateral hemispheres (see Figure 1D and Table 2).
Compared to the old HCs, the young CDs presented with significantly increased sulcal depth only in the right lateral occipital gyrus, and exhibited significantly decreased sulcal depth in the left superior frontal and caudal middle frontal gyri, as well as the right rostral middle frontal gyrus (see Figure 2D and Table 3).
Correlation analysis
The correlation analysis was conducted to examine the relationship between the cortical morphology and VF raw score in each group. Table 4 shows that the CDs showed significant correlations between the VF score and the left supramarginal gyrus in fractal dimensionality, and the left caudal anterior cingulate, the left posterior cingulate, and precentral gyri in gyrification. The young HCs revealed significant correlations between the VF score and the left superior frontal gyrus in fractal dimensionality and the left superior frontal gyrus in gyrification.
Table 4 also illustrates the old HCs showed significant correlations between the VF score and the left lingual and supramarginal gyri in fractal dimensionality and the right lateral occipital gyrus in sulcal depth.
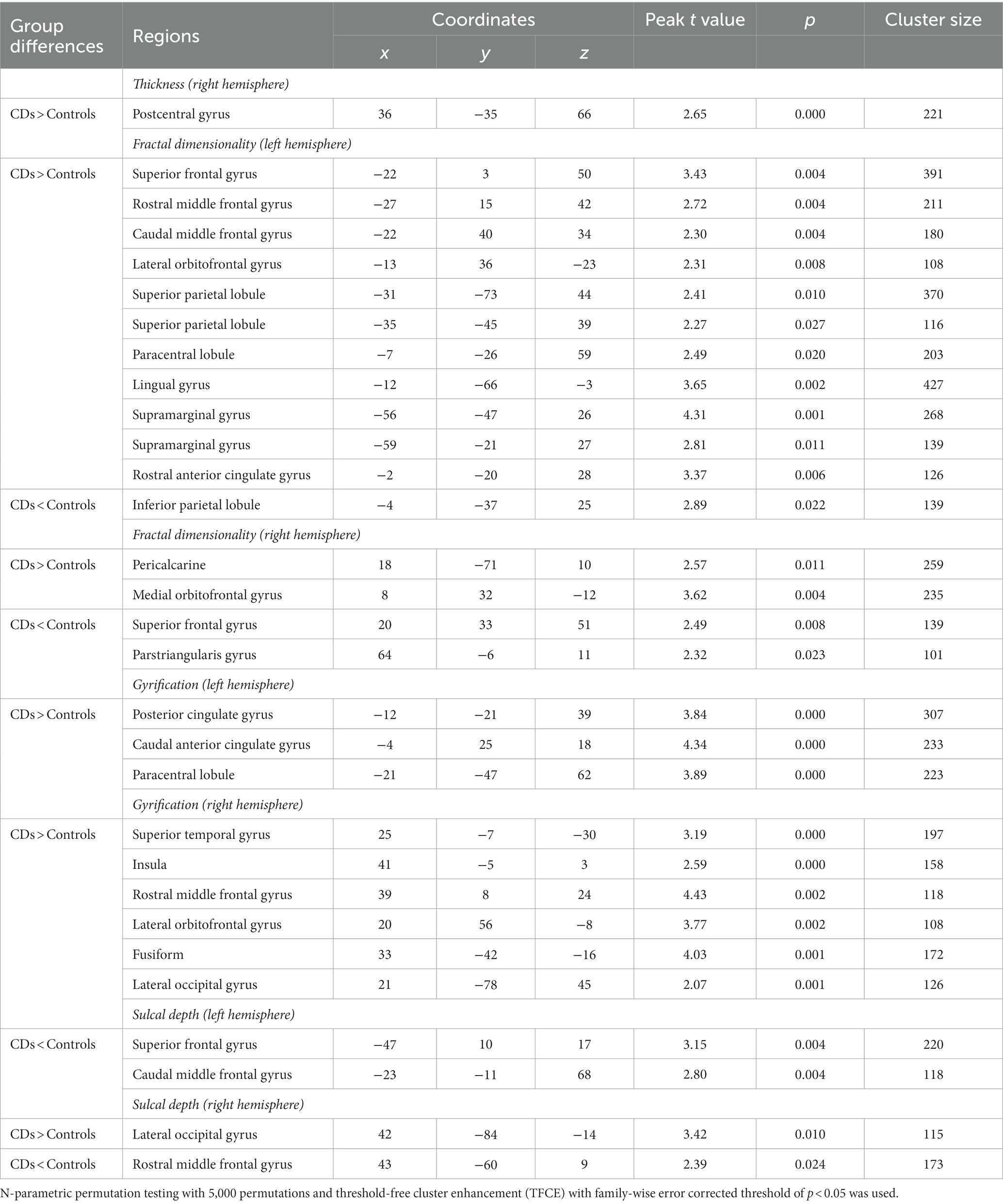
Figure 3 illustrates the correlation analysis across groups we conducted using Fisher’s r to z transformation to determine if the 3 groups had statistically significantly different relationships for a given cortical measurement, brain region, and VF score. This analysis was completed for the 8 combinations of cortical measurements and brain regions with significant correlations with VF seen in Table 4 to determine if there were dose–response effects between groups. Figure 3 demonstrated CDs had significantly different slopes for gyrification of the left precentral and caudal anterior gyri, as well as, fractal dimensionality of the left supramarginal gyrus compared to both young and old HCs. The remaining analyses were non-significant.

Figure 3. Group comparisons for all regions and cortical brain metrics with significant correlations with verbal fluency. Key: CD = Crohn’s disease patients, OldHC = old healthy controls, YoungHC = young healthy controls. p-values: N.S. = no significance, * = p < 0.05–0.01, ** = p < 0.01–0.001, *** = p < 0.001.
Discussion
The current study reported notable differences in brain morphometry between the young CDs and both young and old HCs. There were numerous findings where CDs had decreases in cortical surface measures in some regions, but also increased measures in other regions compared to both young and old HCs, suggesting that CD does not just affect the brain in one particular direction and remodeling may be occurring.
On the whole, Tables 2, 3 demonstrated that CDs had more brain regions with differences in brain morphometry measures when compared to the young HCs (54 regions with differences) as compared to the old HCs (30 regions with differences), suggesting that CD may alter brain structure making it appear more similar to old HCs. For reference, S1a – S1d and S2a – S2d are Supplementary Tables of the brain regions with non-significant differences for Tables 2, 3, respectively. Additionally, Tables 2, 3 demonstrated CDs had atypical brain morphometries compared to both young and old HCs in key regions of well-described cognitive networks, such as the default mode network (DFM) and language function pathways. The DFM, a network negatively associated with attention and associated with states of day-dreaming and mindwandering, changes as a function of age and is thought to be partially responsible for cognitive decline and memory dysfunction seen in healthy aging populations (Tsvetanov et al., 2016; Chaovalitwongse et al., 2017; Staffaroni et al., 2018). Interestingly, our study demonstrated CDs have more regions associated with the DFM (such as the posterior cingulate gyrus and inferior parietal lobule) that are significantly different from young HCs as opposed to old HCs (17 vs. 8, respectively). While our study did not set out to assess DFM function or connectivity, based on these findings it appears that CDs have structures involved in the DFM that more closely resemble older HCs. While there is evidence that suggests CD has an impact on the DFM (Thomann et al., 2017; Kornelsen et al., 2020; Skrobisz et al., 2020), further research is needed to assess the effect of CD on DFM function and connectivity and if it might resemble a form of accelerated aging. Neural pathways involved in language function (such as the supramarginal and pars triangularis gyri and the inferior parietal lobe) also appear to have brain regions in the CDs that more closely resemble old HCs compared to young HCs. In Tables 2, 3, CDs have more regions associated with language function that are significantly different from young HCs as opposed to old HCs (11 vs. 5, respectively). However, our study did not find any differences in VF performance to suggest these atypical morphometries have an impact on performance. Further discussion of VF and brain morphometry is discussed later.
These findings of atypical brain morphometries of CDs appearing more similar to old HCs is inline with our previous study that found fMRI task activation patterns during a verbal fluency task were more similar among young CDs and healthy aging older HCs than the young HCs (Nair et al., 2019). Furthermore, in the present study CDs had increased FD in the left inferior parietal lobule and decreased FD in the left supramarginal gyrus compared to young HC, whereas, the association between CDs and old HCs were reversed in these brain regions. This possibly suggests that CDs are moving toward brain morphometry that resembles older HCs. However, there are a number of other significant differences in brain region morphometries between CDs and both young and old HCs that were significantly different in the same direction (i.e., CDs < both old and young HCs or CDs > both old and young in a given brain region), making associations based on individual brain regions difficult to interpret. Perhaps a study with a larger sample size can clarify these associations to assist with interpretation. Nevertheless, it is apparent that CDs exhibit different brain morphometry compared to HCs as demonstrated by previous studies (Zikou et al., 2014; Bao et al., 2015; Nair et al., 2016; Thomann et al., 2016; Bao et al., 2017a; Yeung, 2021; Thapaliya et al., 2022).
Current literature suggests that both the innate and adaptive immune system in CD are involved in altering intestinal mucosal permeability, making bacterial translocation and systemic inflammation possible, with interactions between host inflammation and microbiota implicated in disease progression (Petagna et al., 2020). Similarly, gut barrier dysfunction has been implicated in bacterial translocation and organ failure in a variety of diseases such as Grave’s disease (Zheng et al., 2021), acute pancreatitis (Li et al., 2020), hepatic disorders (Chopyk and Grakoui, 2020), stress and mood disorders (Doney et al., 2022), and Alzheimer’s disease (Megur et al., 2020; Liu et al., 2021). In addition, gut barrier dyfunction may be a primary driver of systemic inflammation and organ failure observed in the elderly population (Deitch, 1990). Not only this, but there is evidence that elderly patients may not have an increased strength of the inflammatory response, but a more protracted response that is responsible for poorer outcomes during similar pathologic insults in an older population as compared to their younger counterparts (Fagiolo et al., 1993; Kudoh et al., 2001; Pinheiro da Silva et al., 2013; Ren et al., 2014). This protracted inflammatory response seen in older patients is not too dissimilar to the chronicity seen in CD, and one could argue the chronicity may even be more pronounced in the CD population given its lifelong recurring and remitting course. Furthermore, recent studies suggest certain inflammatory markers, such as IL18R1, demonstrate a causal relationship with both IBD and pathologies of the aging brain, such as Alzheimer’s disease (Hillary et al., 2020), further demonstrating a link between IBD and aging brain function.
To investigate the effects CD has on brain function our study had CDs and HCs complete a VF task to explore differences in cognitive function between groups. Although there were no group differences, there was an association between better performance on the VF task and the FD in the left supramarginal gyrus in both the CDs and old HCs that was not present in the young HCs group. There were no overlapping associations between brain morphometry and VF task performance between CDs and young HCs. With performance remaining the same across groups, this may suggest a shift in function compensation by the CDs that more similarly resembles that of the old HCs.
However, Figure 3 (VF vs. FD supramarginal) demonstrates CDs have significantly different correlations for supramarginal fractal dimensionality and VF performance compared to both young and old HCs where increasing fractal dimensionality is associated with better VF for CDs, contradicting this assertion. Additionally, gyrification of both the left caudal anterior cingulate and left precentral gyrus have significantly different correlations with VF performance for CDs compared to both young and old HCs where increasing gyrification is associated with better VF. With the remaining brain regions and cortical measures for CDs, Young HCs, and old HCs that were associated with VF performance not having statistically significantly different slopes among groups, it appears as though CDs may have a different adaptation pattern for performing the VF task as compared to both old and young HCs. In fact, 6 out of the 8 comparisons in Figure 3 demonstrated a positive relationship with CDs compared to 3 out of the 8 for both healthy control groups. This seems to suggest CDs recruit more brain regions in order to perform the same VF task as compared to both young and old HCs. Its possbile these differences are a result of the varying medications CDs require to combat the disease process or a result of the disease process itself, but a causal relationship is not assessable within the constraints of the present study. Lastly, with the limited sample size of our study, it is possible that the lack of positive associations seen with VF performance in the young and old HCs is an artifact of the study and perhaps, further research with a larger study population will help elucidate more measures and regions of significance that can assist with interpretation of these findings.
Interestingly, the HAROLD (hemispheric asymmetry reduction in older adults) model of hemispheric aging was not demonstrated in the CDs VF performance, but was identified in the old HCs; with the old HCs having VF performance correlate with sulcal depth of the right lateral occipital gyrus, whereas a left lateralization of language performance is the predominant finding in both CDs and young HCs (Cabeza, 2002). Perhaps these findings are a result of CDs not having progressed as far on the bi-hemispheric pattern of aging timeline or have not had enough time to develop new brain response patterns involving this brain region. Lastly, given that studies have shown that education, employment, and income are not significantly different between patients with IBD and healthy individuals (El-Matary et al., 2017), it is possible that CD patients might adopt adaptive cognitive strategies to maintain function despite structural and functional brain changes resulting from this lifelong disease. Although the investigation of brain changes in CD patients has become an increasing focus of several recent studies to explore brain-gut interactions (Thomann et al., 2017; Peppas et al., 2021), our study demonstrates that atypical brain morphometry of CDs is more similar to old HCs and this atypical brain morphometry is associated with function on a cognitive task. These results suggest that even younger CDs may be showing some evidence of structural brain changes that demonstrate increased resemblance to older HC brains rather than their similarly aged healthy counterparts. However, the current study demonstrated that these structural brain changes did not result in similar brain response patterns on a cognitive task as compared to young or old HCs. Future longitudinal studies will be needed in order to better understand the effect CD has on brain structure and function over time and whether or not it resembles a form of accelerated aging.
The modest sample size is a limitation and the results can be substantiated with adequately powered future studies. All of our CDs were on treatments with at least one standard IBD maintenance medication; however, the number and combination of medications they were taking, as well as the classes of those medications, varied among participants. Differences in medication regimens might have influenced brain morphometry or task performance. The duration of the disease and the age at CD diagnosis also varied among patients, which could have contributed to the changes observed in their brain morphometry.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Wisconsin Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SS was responsible for funding acquisition. VP, PB-P, SS, and VN conceived and designed the experiments. VN, PB-P, and SS helped with data acquisition. JH preprocessed the data and wrote the manuscript methods and results section. BY, SS, and JH analyzed the data. BY wrote the intro and discussion of the manuscript. VN, NA, DC, PB-P, SS, and VP provided guidance for data analysis, and manuscript writing and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by NIH grant R01NS123378. The NIH core grant Waisman Center from the National Institute of Child Health and Human Development (IDDRC P50HD105353) and UWSMPH Department of radiology R&D funds are also acknowledged.
Conflict of interest
Dr. Saha is a consultant for UCB Biosciences, Inc. All the other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All authors further declare that the research was conducted in the absence of any non-financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2024.1210939/full#supplementary-material
Footnotes
References
Banovic, I., Montreuil, L., Derrey-Bunel, M., Scrima, F., Savoye, G., Beaugerie, L., et al. (2020). Toward further understanding of Crohn's disease-related fatigue: the role of depression and emotional processing. Front. Psychol. 11:703. doi: 10.3389/fpsyg.2020.00703
Bao, C. H., Liu, P., Liu, H. R., Wu, L. Y., Shi, Y., Chen, W. F., et al. (2015). Alterations in brain grey matter structures in patients with crohn's disease and their correlation with psychological distress. J. Crohns Colitis 9, 532–540. doi: 10.1093/ecco-jcc/jjv057
Bao, C., Liu, P., Shi, Y., Wu, L., Jin, X., Zeng, X., et al. (2017a). Differences in brain gray matter volume in patients with Crohn's disease with and without abdominal pain. Oncotarget 8, 93624–93632. doi: 10.18632/oncotarget.21161
Bao, C., Wang, D., Liu, P., Shi, Y., Jin, X., Wu, L., et al. (2017b). Effect of electro-acupuncture and Moxibustion on brain connectivity in patients with Crohn's disease: a resting-state fMRI study. Front. Hum. Neurosci. 11:559. doi: 10.3389/fnhum.2017.00559
Benton, A. (1976). “Multilingual aphasia examination” (2nd ed.). K. Hamsher (Iowa City: AJA Associates).
Bermudez, P., Lerch, J. P., Evans, A. C., and Zatorre, R. J. (2009). Neuroanatomical correlates of musicianship as revealed by cortical thickness and voxel-based morphometry. Cereb. Cortex 19, 1583–1596. doi: 10.1093/cercor/bhn196
Brudek, T. (2019). Inflammatory bowel diseases and Parkinson's disease. J. Parkinsons Dis. 9, S331–S344. doi: 10.3233/JPD-191729
Bushnell, M. C., Ceko, M., and Low, L. A. (2013). Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 14, 502–511. doi: 10.1038/nrn3516
Cabeza, R. (2002). Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol. Aging 17, 85–100. doi: 10.1037//0882-7974.17.1.85
Chaovalitwongse, W. A., Won, D., Seref, O., Borghesani, P., Askren, M. K., Willis, S., et al. (2017). Network optimization of functional connectivity within default mode network regions to detect cognitive decline. IEEE Trans. Neural Syst. Rehabil. Eng. 25, 1079–1089. doi: 10.1109/TNSRE.2017.2679056
Chen, J. H., Huang, N. X., Zou, T. X., and Chen, H. J. (2020). Brain cortical complexity alteration in amyotrophic lateral sclerosis: a preliminary fractal dimensionality study. Biomed. Res. Int. 2020, 1521679–1521676. doi: 10.1155/2020/1521679
Chichlowski, M., and Hale, L. P. (2008). Bacterial-mucosal interactions in inflammatory bowel disease: an alliance gone bad. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G1139–G1149. doi: 10.1152/ajpgi.90516.2008
Chopyk, D. M., and Grakoui, A. (2020). Contribution of the intestinal microbiome and gut barrier to hepatic disorders. Gastroenterology 159, 849–863. doi: 10.1053/j.gastro.2020.04.077
Dahnke, R., Yotter, R. A., and Gaser, C. (2013). Cortical thickness and central surface estimation. NeuroImage 65, 336–348. doi: 10.1016/j.neuroimage.2012.09.050
Deitch, E. A. (1990). The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch. Surg. 125, 403–404. doi: 10.1001/archsurg.1990.01410150125024
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Di Ieva, A., Esteban, F. J., Grizzi, F., Klonowski, W., and Martin-Landrove, M. (2015). Fractals in the neurosciences, part II: clinical applications and future perspectives. Neuroscientist 21, 30–43. doi: 10.1177/1073858413513928
Di Ieva, A., Grizzi, F., Jelinek, H., Pellionisz, A. J., and Losa, G. A. (2014). Fractals in the neurosciences, part I: general principles and basic neurosciences. Neuroscientist 20, 403–417. doi: 10.1177/1073858413513927
Doney, E., Cadoret, A., Dion-Albert, L., Lebel, M., and Menard, C. (2022). Inflammation-driven brain and gut barrier dysfunction in stress and mood disorders. Eur. J. Neurosci. 55, 2851–2894. doi: 10.1111/ejn.15239
El-Matary, W., Dufault, B., Moroz, S. P., Schellenberg, J., and Bernstein, C. N. (2017). Education, employment, income, and marital status among adults diagnosed with inflammatory bowel diseases during childhood or adolescence. Clin. Gastroenterol. Hepatol. 15, 518–524. doi: 10.1016/j.cgh.2016.09.146
Fagiolo, U., Cossarizza, A., Scala, E., Fanales-Belasio, E., Ortolani, C., Cozzi, E., et al. (1993). Increased cytokine production in mononuclear cells of healthy elderly people. Eur. J. Immunol. 23, 2375–2378. doi: 10.1002/eji.1830230950
Farokhian, F., Beheshti, I., Sone, D., and Matsuda, H. (2017). Comparing CAT12 and VBM8 for detecting brain morphological abnormalities in temporal lobe epilepsy. Front. Neurol. 8:428. doi: 10.3389/fneur.2017.00428
Fiorindi, C., Russo, E., Balocchini, L., Amedei, A., and Giudici, F. (2022). Inflammatory bowel disease and customized nutritional intervention focusing on gut microbiome balance. Nutrients 14, 4117. doi: 10.3390/nu14194117
Fisk, J. E., and Sharp, C. A. (2004). Age-related impairment in executive functioning: updating, inhibition, shifting, and access. J. Clin. Exp. Neuropsychol. 26, 874–890. doi: 10.1080/13803390490510680
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Godala, M., Gaszynska, E., Zatorski, H., and Malecka-Wojciesko, E. (2022). Dietary interventions in inflammatory bowel disease. Nutrients 14, 4261. doi: 10.3390/nu14204261
Hillary, R. F., Trejo-Banos, D., Kousathanas, A., McCartney, D. L., Harris, S. E., Stevenson, A. J., et al. (2020). Multi-method genome- and epigenome-wide studies of inflammatory protein levels in healthy older adults. Genome Med. 12:60. doi: 10.1186/s13073-020-00754-1
Hirakawa, H., Akiyoshi, J., Muronaga, M., Tanaka, Y., Ishitobi, Y., Inoue, A., et al. (2016). FKBP5 is associated with amygdala volume in the human brain and mood state: a voxel-based morphometry (VBM) study. Int. J. Psychiatry Clin. Pract. 20, 106–115. doi: 10.3109/13651501.2016.1144772
Hou, J., Dodd, K., Nair, V. A., Rajan, S., Beniwal-Patel, P., Saha, S., et al. (2020). Alterations in brain white matter microstructural properties in patients with Crohn's disease in remission. Sci. Rep. 10:2145. doi: 10.1038/s41598-020-59098-w
Hou, J., Mohanty, R., Nair, V. A., Dodd, K., Beniwal-Patel, P., Saha, S., et al. (2019). Alterations in resting-state functional connectivity in patients with Crohn's disease in remission. Sci. Rep. 9:7412. doi: 10.1038/s41598-019-43878-0
Im, K., Lee, J. M., Seo, S. W., Hyung Kim, S., Kim, S. I., and Na, D. L. (2008). Sulcal morphology changes and their relationship with cortical thickness and gyral white matter volume in mild cognitive impairment and Alzheimer's disease. NeuroImage 43, 103–113. doi: 10.1016/j.neuroimage.2008.07.016
Jacobson, K., McHugh, K., and Collins, S. M. (1995). Experimental colitis alters myenteric nerve function at inflamed and noninflamed sites in the rat. Gastroenterology 109, 718–722. doi: 10.1016/0016-5085(95)90378-x
Jin, K., Zhang, T., Shaw, M., Sachdev, P., and Cherbuin, N. (2018). Relationship between Sulcal characteristics and brain aging. Front. Aging Neurosci. 10:339. doi: 10.3389/fnagi.2018.00339
Kim, J. S., Lee, J. S., Park, M. H., Kang, H., Lee, J. J., Lee, H. J., et al. (2008). Assessment of cerebral glucose metabolism in cat deafness model: strategies for improving the voxel-based statistical analysis for animal PET studies. Mol. Imaging Biol. 10, 154–161. doi: 10.1007/s11307-008-0140-9
Kochunov, P., Thompson, P. M., Coyle, T. R., Lancaster, J. L., Kochunov, V., Royall, D., et al. (2008). Relationship among neuroimaging indices of cerebral health during normal aging. Hum. Brain Mapp. 29, 36–45. doi: 10.1002/hbm.20369
Kornelsen, J., Wilson, A., Labus, J. S., Witges, K., Mayer, E. A., and Bernstein, C. N. (2020). Brain resting-state network alterations associated with Crohn's disease. Front. Neurol. 11:48. doi: 10.3389/fneur.2020.00048
Kudoh, A., Katagai, H., Takazawa, T., and Matsuki, A. (2001). Plasma proinflammatory cytokine response to surgical stress in elderly patients. Cytokine 15, 270–273. doi: 10.1006/cyto.2001.0927
Kurth, F., Gaser, C., and Luders, E. (2015). A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM). Nat. Protoc. 10, 293–304. doi: 10.1038/nprot.2015.014
Lemaitre, H., Goldman, A. L., Sambataro, F., Verchinski, B. A., Meyer-Lindenberg, A., Weinberger, D. R., et al. (2012). Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol. Aging 33:617.e1. doi: 10.1016/j.neurobiolaging.2010.07.013
Li, X. Y., He, C., Zhu, Y., and Lu, N. H. (2020). Role of gut microbiota on intestinal barrier function in acute pancreatitis. World J. Gastroenterol. 26, 2187–2193. doi: 10.3748/wjg.v26.i18.2187
Li, Y., Wang, N., Wang, H., Lv, Y., Zou, Q., and Wang, J. (2021). Surface-based single-subject morphological brain networks: effects of morphological index, brain parcellation and similarity measure, sample size-varying stability and test-retest reliability. NeuroImage 235:118018. doi: 10.1016/j.neuroimage.2021.118018
Lin, J. C., Lin, C. S., Hsu, C. W., Lin, C. L., and Kao, C. H. (2016). Association between Parkinson's disease and inflammatory bowel disease: a Nationwide Taiwanese retrospective cohort study. Inflamm. Bowel Dis. 22, 1049–1055. doi: 10.1097/MIB.0000000000000735
Liu, S., Gao, J., Liu, K., and Zhang, H. L. (2021). Microbiota-gut-brain axis and Alzheimer's disease: implications of the blood-brain barrier as an intervention target. Mech. Ageing Dev. 199:111560. doi: 10.1016/j.mad.2021.111560
Luders, E., Thompson, P. M., Narr, K. L., Toga, A. W., Jancke, L., and Gaser, C. (2006). A curvature-based approach to estimate local gyrification on the cortical surface. NeuroImage 29, 1224–1230. doi: 10.1016/j.neuroimage.2005.08.049
Madan, C. R., and Kensinger, E. A. (2017). Age-related differences in the structural complexity of subcortical and ventricular structures. Neurobiol. Aging 50, 87–95. doi: 10.1016/j.neurobiolaging.2016.10.023
Megur, A., Baltriukienė, D., Bukelskienė, V., and Burokas, A. (2020). The microbiota-gut-brain Axis and Alzheimer's disease: Neuroinflammation is to blame? Nutrients 13, 37. doi: 10.3390/nu13010037
Nair, V. A., Beniwal-Patel, P., Mbah, I., Young, B. M., Prabhakaran, V., and Saha, S. (2016). Structural imaging changes and behavioral correlates in patients with Crohn's disease in remission. Front. Hum. Neurosci. 10:460. doi: 10.3389/fnhum.2016.00460
Nair, V. A., Dodd, K., Rajan, S., Santhanubosu, A., Beniwal-Patel, P., Saha, S., et al. (2019). A verbal fluency task-based brain activation fMRI study in patients with Crohn's disease in remission. J. Neuroimaging 29, 630–639. doi: 10.1111/jon.12634
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. doi: 10.1016/0028-3932(71)90067-4
Peppas, S., Pansieri, C., Piovani, D., Danese, S., Peyrin-Biroulet, L., Tsantes, A. G., et al. (2021). The brain-gut Axis: psychological functioning and inflammatory bowel diseases. J. Clin. Med. 10, 377. doi: 10.3390/jcm10030377
Petagna, L., Antonelli, A., Ganini, C., Bellato, V., Campanelli, M., Divizia, A., et al. (2020). Pathophysiology of Crohn's disease inflammation and recurrence. Biol. Direct 15:23. doi: 10.1186/s13062-020-00280-5
Pinheiro da Silva, F., Zampieri, F. G., Barbeiro, D. F., Barbeiro, H. V., Goulart, A. C., Torggler Filho, F., et al. (2013). Septic shock in older people: a prospective cohort study. Immun. Ageing 10:21. doi: 10.1186/1742-4933-10-21
Ren, X., Du, H., Li, Y., Yao, X., Huang, J., Li, Z., et al. (2014). Age-related activation of MKK/p38/NF-κB signaling pathway in lung: from mouse to human. Exp. Gerontol. 57, 29–40. doi: 10.1016/j.exger.2014.04.017
Ruff, R. M., Light, R. H., Parker, S. B., and Levin, H. S. (1997). The psychological construct of word fluency. Brain Lang. 57, 394–405. doi: 10.1006/brln.1997.1755
Sajadinejad, M. S., Asgari, K., Molavi, H., Kalantari, M., and Adibi, P. (2012). Psychological issues in inflammatory bowel disease: an overview. Gastroenterol. Res. Pract. 2012:106502. doi: 10.1155/2012/106502
Sauzéon, H., Raboutet, C., Rodrigues, J., Langevin, S., Schelstraete, M. A., Feyereisen, M., et al. (2011). Verbal knowledge as a compensation determinant of adult age differences in verbal fluency tasks over time. J. Adult Dev. 18, 144–154. doi: 10.1007/s10804-010-9107-6
Seiger, R., Ganger, S., Kranz, G. S., Hahn, A., and Lanzenberger, R. (2018). Cortical thickness estimations of free surfer and the CAT12 toolbox in patients with Alzheimer's disease and healthy controls. J. Neuroimaging 28, 515–523. doi: 10.1111/jon.12521
Sharma, N., Dhiman, S., Bodh, V., Sharma, D., Sharma, R., Sharma, S., et al. (2021). Cognitive dysfunction in ulcerative colitis patients in remission and its comparison with patients with irritable bowel syndrome and healthy controls. Indian J. Gastroenterol. 40, 169–175. doi: 10.1007/s12664-020-01122-y
Skrobisz, K., Piotrowicz, G., Naumczyk, P., Sabisz, A., Markiet, K., Rydzewska, G., et al. (2020). Imaging of morphological background in selected functional and inflammatory gastrointestinal diseases in fMRI. Front. Psych. 11:461. doi: 10.3389/fpsyt.2020.00461
Smith, S. M., and Nichols, T. E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44, 83–98. doi: 10.1016/j.neuroimage.2008.03.061
Staffaroni, A. M., Brown, J. A., Casaletto, K. B., Elahi, F. M., Deng, J., Neuhaus, J., et al. (2018). The longitudinal trajectory of default mode network connectivity in healthy older adults varies as a function of age and is associated with changes in episodic memory and processing speed. J. Neurosci. 38, 2809–2817. doi: 10.1523/JNEUROSCI.3067-17.2018
Thapaliya, G., Eldeghaidy, S., Asghar, M., McGing, J., Radford, S., Francis, S., et al. (2022). The relationship between central nervous system morphometry changes and key symptoms in Crohn's disease. Brain Imaging Behav. 17, 149–160. doi: 10.1007/s11682-022-00742-6
Thomann, A. K., Griebe, M., Thomann, P. A., Hirjak, D., Ebert, M. P., Szabo, K., et al. (2017). Intrinsic neural network dysfunction in quiescent Crohn's disease. Sci. Rep. 7:11579. doi: 10.1038/s41598-017-11792-y
Thomann, A. K., Schmitgen, M. M., Kmuche, D., Ebert, M. P., Thomann, P. A., Szabo, K., et al. (2021). Exploring joint patterns of brain structure and function in inflammatory bowel diseases using multimodal data fusion. Neurogastroenterol. Motil. 33:e14078. doi: 10.1111/nmo.14078
Thomann, A. K., Thomann, P. A., Wolf, R. C., Hirjak, D., Schmahl, C., Ebert, M. P., et al. (2016). Altered markers of brain development in Crohn's disease with Extraintestinal manifestations - a pilot study. PLoS One 11:e0163202. doi: 10.1371/journal.pone.0163202
Thomason, M. E., and Thompson, P. M. (2011). Diffusion imaging, white matter, and psychopathology. Annu. Rev. Clin. Psychol. 7, 63–85. doi: 10.1146/annurev-clinpsy-032210-104507
Trost, S., Platz, B., Usher, J., Scherk, H., Wobrock, T., Ekawardhani, S., et al. (2013). DISC1 (disrupted-in-schizophrenia 1) is associated with cortical grey matter volumes in the human brain: a voxel-based morphometry (VBM) study. J. Psychiatr. Res. 47, 188–196. doi: 10.1016/j.jpsychires.2012.10.006
Tsvetanov, K. A., Henson, R. N., Tyler, L. K., Razi, A., Geerligs, L., Ham, T. E., et al. (2016). Extrinsic and intrinsic brain network connectivity maintains cognition across the lifespan despite accelerated decay of regional brain activation. J. Neurosci. 36, 3115–3126. doi: 10.1523/JNEUROSCI.2733-15.2016
van Langenberg, D. R., Yelland, G. W., Robinson, S. R., and Gibson, P. R. (2017). Cognitive impairment in Crohn's disease is associated with systemic inflammation, symptom burden and sleep disturbance. United European Gastroenterol. J. 5, 579–587. doi: 10.1177/2050640616663397
Wang, D., Zhang, X., and Du, H. (2022). Inflammatory bowel disease: a potential pathogenic factor of Alzheimer's disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 119:110610. doi: 10.1016/j.pnpbp.2022.110610
Yeung, A. W. K. (2021). Structural and functional changes in the brain of patients with Crohn's disease: an activation likelihood estimation meta-analysis. Brain Imaging Behav. 15, 807–818. doi: 10.1007/s11682-020-00291-w
Yotter, R. A., Dahnke, R., Thompson, P. M., and Gaser, C. (2011a). Topological correction of brain surface meshes using spherical harmonics. Hum. Brain Mapp. 32, 1109–1124. doi: 10.1002/hbm.21095
Yotter, R. A., Nenadic, I., Ziegler, G., Thompson, P. M., and Gaser, C. (2011b). Local cortical surface complexity maps from spherical harmonic reconstructions. NeuroImage 56, 961–973. doi: 10.1016/j.neuroimage.2011.02.007
Yotter, R. A., Thompson, P. M., and Gaser, C. (2011c). Algorithms to improve the reparameterization of spherical mappings of brain surface meshes. J. Neuroimaging 21, e134–e147. doi: 10.1111/j.1552-6569.2010.00484.x
Yuksel, D., Engelen, J., Schuster, V., Dietsche, B., Konrad, C., Jansen, A., et al. (2018). Longitudinal brain volume changes in major depressive disorder. J. Neural Transm. (Vienna) 125, 1433–1447. doi: 10.1007/s00702-018-1919-8
Yun, H. J., Im, K., Jin-Ju, Y., Yoon, U., and Lee, J. M. (2013). Automated sulcal depth measurement on cortical surface reflecting geometrical properties of sulci. PLoS One 8:e55977. doi: 10.1371/journal.pone.0055977
Zeng, F., Qin, W., Ma, T., Sun, J., Tang, Y., Yuan, K., et al. (2012). Influence of acupuncture treatment on cerebral activity in functional dyspepsia patients and its relationship with efficacy. Am. J. Gastroenterol. 107, 1236–1247. doi: 10.1038/ajg.2012.53
Zeng, J., Wang, X., Pan, F., and Mao, Z. (2022). The relationship between Parkinson's disease and gastrointestinal diseases. Front. Aging Neurosci. 14:955919. doi: 10.3389/fnagi.2022.955919
Zhang, L., Butler, A. J., Sun, C. K., Sahgal, V., Wittenberg, G. F., and Yue, G. H. (2008). Fractal dimension assessment of brain white matter structural complexity post stroke in relation to upper-extremity motor function. Brain Res. 1228, 229–240. doi: 10.1016/j.brainres.2008.06.008
Zhang, M., Hong, L., Zhang, T., Lin, Y., Zheng, S., Zhou, X., et al. (2016). Illness perceptions and stress: mediators between disease severity and psychological well-being and quality of life among patients with Crohn's disease. Patient Prefer. Adherence 10, 2387–2396. doi: 10.2147/PPA.S118413
Zheng, D., Liao, H., Chen, S., Liu, X., Mao, C., Zhang, C., et al. (2021). Elevated levels of circulating biomarkers related to leaky gut syndrome and bacterial translocation are associated with Graves' disease. Front. Endocrinol. 12:796212. doi: 10.3389/fendo.2021.796212
Zhuang, Y., Zeng, X., Wang, B., Huang, M., Gong, H., and Zhou, F. (2017). Cortical surface thickness in the middle-aged brain with white matter Hyperintense lesions. Front. Aging Neurosci. 9:225. doi: 10.3389/fnagi.2017.00225
Keywords: Crohn’s disease, IBD, structural imaging, cognitive function, gut-brain axis, aging
Citation: Yeske B, Hou J, Chu DY, Adluru N, Nair VA, Beniwal-Patel P, Saha S and Prabhakaran V (2024) Structural brain morphometry differences and similarities between young patients with Crohn’s disease in remission and healthy young and old controls. Front. Neurosci. 18:1210939. doi: 10.3389/fnins.2024.1210939
Edited by:
Zhongming Liu, University of Michigan, United StatesReviewed by:
Gita Thapaliya, Johns Hopkins University, United StatesAndy Wai Kan Yeung, University of Hong Kong, China
Copyright © 2024 Yeske, Hou, Chu, Adluru, Nair, Beniwal-Patel, Saha and Prabhakaran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin Yeske, byeske@wisc.edu
†These authors have contributed equally to this work and share first authorship
 Benjamin Yeske
Benjamin Yeske Jiancheng Hou
Jiancheng Hou Daniel Y. Chu
Daniel Y. Chu Nagesh Adluru
Nagesh Adluru Veena A. Nair
Veena A. Nair Poonam Beniwal-Patel6
Poonam Beniwal-Patel6 Sumona Saha
Sumona Saha Vivek Prabhakaran
Vivek Prabhakaran