- 1State Key Laboratory of Biomembrane and Membrane Biotechnology and Peking-Tsinghua Center for Life Sciences and PKU-IDG/McGovern Institute for Brain Research, Institute of Molecular Medicine, Peking University, Beijing, China
- 2Department of Neurosurgery, Affiliated Hospital of The Air Force Institute of Aeromedicine, Beijing, China
- 3Department of Anesthesiology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Like neurons, astrocytes are abundant in the central nervous system. They contact all types of cells in the brain, communicate with them, and modulate their activity by releasing gliotransmitters, including glutamate, ATP, etc. (Newman, 2003; Halassa and Haydon, 2010; Hamilton and Attwell, 2010; Volterra et al., 2014; Verkhratsky and Nedergaard, 2018), although the role of astrocytes in neurotransmission is still debated (Nedergaard and Verkhratsky, 2012; Fiacco and McCarthy, 2018).
ATP is considered to be a powerful extracellular messenger in both the peripheral and central nervous systems (Edwards et al., 1992; Fields and Stevens, 2000; Burnstock, 2007; Verkhratsky et al., 2009). Via activating multiple receptors in glia and neurons, ATP signaling participates in many important functions including cell development and synaptic plasticity (Evans et al., 1992; Newman, 2003; Zhang et al., 2003; Agresti et al., 2005; Abbracchio et al., 2009; Butt, 2011; Wurm et al., 2011; Verkhratsky et al., 2016). However, unlike the mechanism of neurotransmission via quantal exocytosis (Katz, 1959, 1969; Augustine and Neher, 1992; Neher, 1998; Sudhof, 2004; Pankratov et al., 2006, 2007; Sudhof and Rothman, 2009), the mechanisms by which ATP is released remain controversial. Because ATP is easily hydrolyzed, monitoring its real-time release is a challenge.
Early Studies of Non-Quantal ATP Release From Astrocytes
Early studies usually applied indirect methods such as dye-uptake, ATP analog labeling, and luciferase-luciferin system tests to indirectly detect ATP release (Duan et al., 2003). Based on these studies, ATP was thought to be released through specific channels, such as connexin/pannexin hemichannels, “maxi-anion” channels, and P2X7 receptor channels (Figure 1A, Stout et al., 2002; Duan et al., 2003; Bao et al., 2004; Suadicani et al., 2006; Kang et al., 2008; Liu et al., 2008; Iglesias et al., 2009; Bennett et al., 2012). These studies used indirect measurements based on measuring dyes or currents through channels that can pass molecules larger than ATP.
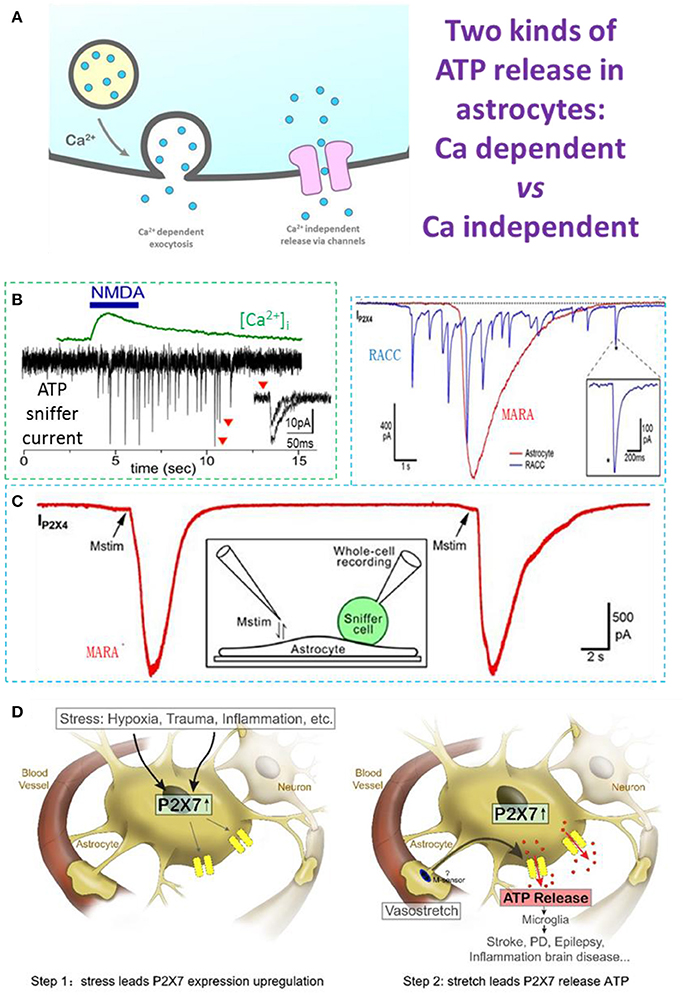
Figure 1. Ca2+-dependent and Ca2+-independent ATP release in astrocytes. (A) Two types of ATP release: Ca2+-dependent quantal release and Ca2+-independent non-quantal release. In the Ca2+-dependent pathway, secretory vesicles with packaged ATP are trafficked to the plasma membrane where they dock and fuse with it on arrival of a stimulus; this typically depends on an increase of cytosolic Ca2+. In the Ca2+-independent pathway, ATP is released through channels expressed on the astrocyte plasma membrane, such as the swelling-induced anion channel, connexin hemichannels activated by lower Ca2+ concentrations, and ionotropic purinergic receptor channels. (B) N-methyl-D-aspartate (NMDA)-induced Ca2+-dependent quantal ATP release from a freshly-isolated astrocyte, recorded by a HEK293-P2X2 sniffer cell. (C) Typical MARA current (IP2X4) recorded with an HEK293A sniffer-cell expressing P2X4 (ATP sniffer) on an astrocyte. The astrocyte was stimulated twice and the MARA signal was reproducible. Inset, cartoon of the experimental protocol for MARA recording; Upper right: MARA vs Ca2+-dependent ATP release of burst quantal events from a rat chromaffin cell (RACC). (D) Two-step hypothesis of MARA-mediated brain diseases. Left. Step 1: Stress induces upregulation of P2X7 receptor expression in astrocytes. The stressors include hypoxia (i.e., stroke and ischemia), trauma, and CNS diseases associated with inflammation or neurodegeneration. Right. Step 2: Stretch leads to P2X7-mediated ATP release from astrocytes. The abundant ATP is an emergency signal for the neural immune system that recruits and activates microglia in the ATP “hot-spots” and promotes disease. All data adapted from Xiong et al. (2018), except B adapted from Lalo et al. (2014). All data with reproduction permission from the original publishers.
Quantal ATP Release Under Physiological Conditions
Patch-clamp recording via sniffer cells (Young and Poo, 1983; Liu et al., 2011) is a powerful tool with which to investigate the ATP release mechanism due to its high spatiotemporal resolution (Hollins and Ikeda, 1997; Pangrsic et al., 2007; Karanauskaite et al., 2009; Lalo et al., 2014; Liu et al., 2014; Lee et al., 2015). By using ATP-sniffer cells, Lalo et al. recorded quantal ATP release in astrocytes freshly isolated from mouse cortex. ATP is released by Ca2+-dependent exocytosis following the activation of metabotropic and ionotropic receptors or direct UV-uncaging. The ATP release is SNARE protein-dependent and is eliminated by pretreatment with bafilomycin, a blocker of vacuolar-type H-ATPase. The kinetics of sniffer-cell responses are consistent with the millisecond time-scale, suggesting that ATP exocytosis is from synaptic-like small vesicles (Figures 1A,B). The ATP released from astrocytes (1) activates P2X receptors in neighboring neurons to enhance excitatory signaling, and (2) down-regulates inhibitory synaptic signaling (Lalo et al., 2014).
In addition to the tiny quantal ATP release arising from Ca2+-dependent exocytosis in freshly-isolated astrocytes, an earlier report proposed that Ca2+-dependent lysosome exocytosis is responsible for quantal ATP release in cultured astrocytes (Zhang et al., 2007). They provided three lines of indirect evidence: (1) the uptake of a fluorescent ATP analog, MANT-ATP, into lysosomes; (2) the presence of ATP in biochemically-purified lysosomes; and (3) the real-time visualization of Ca2+-dependent lysosome exocytosis by total internal reflection fluorescence microscopy imaging of a false neurotransmitter (FM2-10). Although these data raised the possibility of quantal ATP release via lysosomal exocytosis, direct recording of quantal ATP release from glial lysosomes was absent.
Stretch-Induced Ca2+-Independent ATP Release Through P2X7 Channels
It was our original goal to record quantal ATP release in cultured hippocampal astrocytes using ATP-sniffer cells. To our surprise, with three independent assays of lysosomal exocytosis, our real-time sniffer recordings clearly denied ATP release by Ca2+-dependent lysosomal exocytosis (Xiong et al., 2018). Instead, following a gentle membrane stretch, Ca2+-independent non-vesicular ATP release occurred in the cultured astrocytes (Xiong et al., 2018). In contrast to the quantal spikes of ATP release in chromaffin cells, the Mechanically-induced ATP Release from Astrocytes (MARA) displays a single spike with distinct kinetic characteristics. MARA is ~300-fold greater in release content and ~50 times longer in duration (Figure 1C, upper right inset). Mechanistically, MARA-mediated ATP release is (1) Ca2+-independent, (2) not via lysosome exocytosis, and (3) mitochondria-dependent (Xiong et al., 2018). The P2X7 receptor channel is essential for MARA-mediated ATP release, because it is profoundly inhibited by Brilliant Blue G, a selective P2X7 antagonist, as well as by RNA interference-based P2X7 knockdown (Xiong et al., 2018). Considering that the open pore of the P2X7 channel allows the permeation of cytosolic molecules of molecular weight ≤ 900 Da, P2X7 channels should be able to release ATP (507 Da) (Yan et al., 2008; Nagasawa et al., 2009), and other purines, such as AMP, ADP, or adenosine. Together, MARA occurs when mechanical stretch triggers ATP efflux through P2X7 channel pores in “activated” astrocytes expressing P2X7 receptors in culture/in vitro or hypoxia/trauma/disease in vivo (Nagasawa et al., 2009). Hypoxia might decrease mitochondrial-dependent cytosolic ATP level and partially compensate the MARA signal for the increase in P2X7 expression.
One important open question left by Xiong (Xiong et al., 2018) is the identity of the mechanical sensor by which MARA initiates mechanical-P2X7-ATP release in astrocytes. Since P2X7 itself is not mechanosensor, we hypothesize that a mechanosensor [such as piezo 1 protein (Zhao et al., 2018)] binds P2X7 and “transactivates” mechanical force to activate P2X7 [One recent example of protein-transactivation is that a voltage-sensor channel activates another binding protein of vesicle fusion-pore (Chai et al., 2017)]. Following the discovery of MARA, identification of this sensor is critical for treating possible MARA-mediated brain diseases as proposed below. In addition to P2X7 (Xiong et al., 2018), hemichannels such as connexins (Stout et al., 2002) and large conductance Cl− channel (Liu et al., 2008) have been reported to mediate the non-vesicular ATP release from astrocytes. At present it is unclear about the relative contributions to ATP release among these channel types, because the stimulations in these studies were different. These ATP-release pathways through different channels may play condition-dependent roles in astrocytes' functions.
A Hypothesis of MARA in Brain Protection and Diseases
In contrast to the Ca2+-dependent quantal ATP release in freshly-isolated astrocytes, the Ca2+-independent ATP release event (MARA) in cultured astrocytes is ~10,000 times greater (Figure 1C). P2X7 is up-regulated under ischemic conditions in vivo (Nagasawa et al., 2009), and this could contribute to the release of large amounts of ATP from cellular sources, and in the extracellular space it is quickly hydrolyzed to ADP, AMP, and adenosine, which activate their receptors and play roles in brain protection and damage (Neary et al., 2003; Choo et al., 2013; Rodrigues et al., 2015). On the other hand, the large ATP release via MARA would recruit microglia, leading to protective or pathological pathways (Dou et al., 2012). Thus, we propose that MARA could be a mechanism underlying brain diseases such as those associated with hypoxia/ischemia and trauma, as well as other neurological disorders (Parkinson's disease, Alzheimer's disease, and epilepsy) (Figure 1D). Our hypothesis is detailed as follows. The first step is MARA genesis, which is dependent on P2X7 receptor expression. The expression level of P2X7 receptors in astrocytes is up-regulated under either of the two conditions: (1) in culture, which is an extremely stressful condition lacking blood and other support for astrocytes (Narcisse et al., 2005; Bartlett et al., 2014; Burnstock, 2017; Xiong et al., 2018); and (2) in vivo, when astrocytes suffer stresses, such as hypoxia (i.e., ischemia and stroke), trauma, or some CNS diseases (for example, inflammation and/or neurodegeneration; Ballerini et al., 1996; Narcisse et al., 2005; Nagasawa et al., 2009; Burnstock, 2017). Indeed, few astrocytes express P2X7 receptors in the intact hippocampal slices under physiological conditions (Nagasawa et al., 2009; Xiong et al., 2018). As the P2X7 receptor is a key modulator of aerobic glycolysis, it has an intrinsic ability to reprogram cell metabolism to meet the needs imposed by adverse environmental conditions (Amoroso et al., 2012). The up-regulation of P2X7 receptors in astrocytes is not only a form of adaptation to stress, but is also a necessary preparation for MARA to execute its repair function in its brain region (Figure 1D, step 1). The second step is MARA activation following mechanical stimulation, which could be generated by arterioles in vivo. Astrocytes regulate cerebral blood flow to match the metabolic requirements of the brain (Gordon et al., 2008) by eliciting the vasoconstriction or vasodilation of arterioles. A tension change due to such vasoconstriction/dilation is an effective physiological mechanical stimulus (membrane stretch) for astrocytes, which are activated by deformation of their surroundings on a timescale of milliseconds (Janmey and Miller, 2011). Physiological arteriole stretch does not trigger MARA because P2X7 receptors are rare in astrocytes under normal conditions. When stresses such as hypoxia/trauma occur, the expression of astrocytic P2X7 receptors is up-regulated, and if the stress persists and exceeds a threshold, the abnormal changes in arteriole tension directly trigger MARA (Figure 1D, step 2). So, noxious stimuli up-regulate the P2X7 receptor expression in astrocytes, and this can be considered as an adaptive change in response to stress and the beginning of pathological damage, because up-regulation of P2X7 receptor expression is the prerequisite for non-quantal ATP release/MARA. When the steady-state is disturbed, MARA occurs and triggers a sustained increase in extracellular ATP, which then acts to alert the presence of a “hot spot.” This signal recruits and activates microglia, which scavenge and phagocytize injured cells and cellular debris, increase the susceptibility of neurons to damage, promote astrogliosis, and mount neuroinflammatory responses (Parpura et al., 2012; Rivera et al., 2016). In this sense, both astrocytes and microglia comprise the immune system of the CNS: each of a vast number astrocytes performs an immobile surveillance for its specific location in the brain, signaling stress, while each microglia cell acts as a mobile patrol, responding to the astrocytic “alert” signal—an unusually large increase in extracellular ATP concentration. To a certain extent, MARA from a few astrocytes recruits a few microglia to clear the site and protect the brain. However, when the stress is excessive, MARA from a large number of astrocytes may recruit too many microglia, overkill healthy tissue, and cause irreversible brain damage (disease).
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Prof. I. C. Bruce (Peking University) for helpful comments, and Xi Wu for art of Figures 1A and D. This work was supported by the National Natural Science Foundation of China (31330024, 31761133016, 21790394, 31171026, 31327901, 31521062, and 21790390), the National Key Research and Development Program of China (2016YFA0500401), and the National Basic Research Program of China (2012CB518006).
References
Abbracchio, M. P., Burnstock, G., Verkhratsky, A., and Zimmermann, H. (2009). Purinergic signalling in the nervous system: an overview. Trends Neurosci. 32, 19–29. doi: 10.1016/j.tins.2008.10.001
Agresti, C., Meomartini, M. E., Amadio, S., Ambrosini, E., Volonté, C., Aloisi, F., et al. (2005). ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res. Brain Res. Rev. 48, 157–165. doi: 10.1016/j.brainresrev.2004.12.005
Amoroso, F., Falzoni, S., Adinolfi, E., Ferrari, D., and Di Virgilio, F. (2012). The P2X7 receptor is a key modulator of aerobic glycolysis. Cell Death Amp. Dis. 3:e370. doi: 10.1038/cddis.2012.105
Augustine, G. J., and Neher, E. (1992). Calcium requirements for secretion in bovine chromaffin cells. J. Physiol. 450, 247–271. doi: 10.1113/jphysiol.1992.sp019126
Ballerini, P., Rathbone, M. P., Di Iorio, P., Renzetti, A., Giuliani, P., D'alimonte, I., et al. (1996). Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. Neuroreport 7, 2533–2537. doi: 10.1097/00001756-199611040-00026
Bao, L., Locovei, S., and Dahl, G. (2004). Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 572, 65–68. doi: 10.1016/j.febslet.2004.07.009
Bartlett, R., Stokes, L., and Sluyter, R. (2014). The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 66, 638–675. doi: 10.1124/pr.113.008003
Bennett, M. V., Garré, J. M., Orellana, J. A., Bukauskas, F. F., Nedergaard, M., and Saez, J. C. (2012). Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 1487, 3–15. doi: 10.1016/j.brainres.2012.08.042
Burnstock, G. (2007). Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87, 659–797. doi: 10.1152/physrev.00043.2006
Burnstock, G. (2017). Purinergic signalling and neurological diseases: an update. CNS Neurol. Disord. Drug Targets 16, 257–265. doi: 10.2174/1871527315666160922104848
Butt, A. M. (2011). ATP: a ubiquitous gliotransmitter integrating neuron-glial networks. Semin. Cell Dev. Biol. 22, 205–213. doi: 10.1016/j.semcdb.2011.02.023
Chai, Z., Wang, C., Huang, R., Wang, Y., Zhang, X., Wu, Q., et al. (2017). CaV2.2 gates calcium-independent but voltage-dependent secretion in mammalian sensory neurons. Neuron 96, 1317–1326.e1314. doi: 10.1016/j.neuron.2017.10.028
Choo, A. M., Miller, W. J., Chen, Y. C., Nibley, P., Patel, T. P., Goletiani, C., et al. (2013). Antagonism of purinergic signalling improves recovery from traumatic brain injury. Brain 136, 65–80. doi: 10.1093/brain/aws286
Dou, Y., Wu, H. J., Li, H. Q., Qin, S., Wang, Y. E., Li, J., et al. (2012). Microglial migration mediated by ATP-induced ATP release from lysosomes. Cell Res. 22, 1022–1033. doi: 10.1038/cr.2012.10
Duan, S., Anderson, C. M., Keung, E. C., Chen, Y., Chen, Y., and Swanson, R. A. (2003). P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J. Neurosci. 23, 1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003
Edwards, F. A., Gibb, A. J., and Colquhoun, D. (1992). ATP receptor-mediated synaptic currents in the central nervous system. Nature 359, 144–147. doi: 10.1038/359144a0
Evans, R. J., Derkach, V., and Surprenant, A. (1992). ATP mediates fast synaptic transmission in mammalian neurons. Nature 357, 503–505. doi: 10.1038/357503a0
Fiacco, T. A., and McCarthy, K. D. (2018). Multiple lines of evidence indicate that gliotransmission does not occur under physiological conditions. J. Neurosci. 38, 3–13. doi: 10.1523/JNEUROSCI.0016-17.2017
Fields, R. D., and Stevens, B. (2000). ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci. 23, 625–633. doi: 10.1016/S0166-2236(00)01674-X
Gordon, G. R., Choi, H. B., Rungta, R. L., Ellis-Davies, G. C., and MacVicar, B. A. (2008). Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456, 745–749. doi: 10.1038/nature07525
Halassa, M. M., and Haydon, P. G. (2010). Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 72, 335–355. doi: 10.1146/annurev-physiol-021909-135843
Hamilton, N. B., and Attwell, D. (2010). Do astrocytes really exocytose neurotransmitters? Nat. Rev. Neurosci. 11, 227–238. doi: 10.1038/nrn2803
Hollins, B., and Ikeda, S. R. (1997). Heterologous expression of a P2x-purinoceptor in rat chromaffin cells detects vesicular ATP release. J. Neurophysiol. 78, 3069–3076. doi: 10.1152/jn.1997.78.6.3069
Iglesias, R., Dahl, G., Qiu, F., Spray, D. C., and Scemes, E. (2009). Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J. Neurosci. 29, 7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009
Janmey, P. A., and Miller, R. T. (2011). Mechanisms of mechanical signaling in development and disease. J. Cell Sci. 124, 9–18. doi: 10.1242/jcs.071001
Kang, J., Kang, N., Lovatt, D., Torres, A., Zhao, Z., Lin, J., et al. (2008). Connexin 43 hemichannels are permeable to ATP. J. Neurosci. 28, 4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008
Karanauskaite, J., Hoppa, M. B., Braun, M., Galvanovskis, J., and Rorsman, P. (2009). Quantal ATP release in rat beta-cells by exocytosis of insulin-containing LDCVs. Pflugers Arch. 458, 389–401. doi: 10.1007/s00424-008-0610-6
Katz, B. (1959). Nature of the nerve impulse. Rev. Mod. Phys 8, 629–638. doi: 10.1103/RevModPhys.31.466
Katz, B. (1969). The release of neural transmitter substances. J. Neurol. Neurosurg. Psychiatry 32:638. doi: 10.1136/jnnp.32.6.638
Lalo, U., Palygin, O., Rasooli-Nejad, S., Andrew, J., Haydon, P. G., and Pankratov, Y. (2014). Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 12:e1001747. doi: 10.1371/journal.pbio.1001747
Lee, J., Chun, Y. E., Han, K. S., Lee, J., Woo, D. H., and Lee, C. J. (2015). Ca(2+) Entry is required for mechanical stimulation-induced ATP release from astrocyte. Exp. Neurobiol. 24, 17–23. doi: 10.5607/en.2015.24.1.17
Liu, H. T., Toychiev, A. H., Takahashi, N., Sabirov, R. Z., and Okada, Y. (2008). Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res. 18, 558–565. doi: 10.1038/cr.2008.49
Liu, T., Li, H., Gounko, N. V., Zhou, Z., Xu, A., Hong, W., et al. (2014). Detection of insulin granule exocytosis by an electrophysiology method with high temporal resolution reveals enlarged insulin granule pool in BIG3-knockout mice. Am. J. Physiol. Endocrinol. Metab. 307, E611–E618. doi: 10.1152/ajpendo.00208.2014
Liu, T., Sun, L., Xiong, Y., Shang, S., Guo, N., Teng, S., et al. (2011). Calcium triggers exocytosis from two types of organelles in a single astrocyte. J. Neurosci. 31, 10593–10601. doi: 10.1523/JNEUROSCI.6401-10.2011
Nagasawa, K., Escartin, C., and Swanson, R. A. (2009). Astrocyte cultures exhibit P2X7 receptor channel opening in the absence of exogenous ligands. Glia 57, 622–633. doi: 10.1002/glia.20791
Narcisse, L., Scemes, E., Zhao, Y., Lee, S. C., and Brosnan, C. F. (2005). The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia 49, 245–258. doi: 10.1002/glia.20110
Neary, J. T., Kang, Y., Willoughby, K. A., and Ellis, E. F. (2003). Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J. Neurosci. 23, 2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003
Nedergaard, M., and Verkhratsky, A. (2012). Artifact versus reality–how astrocytes contribute to synaptic events. Glia 60, 1013–1023. doi: 10.1002/glia.22288
Neher, E. (1998). Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 20, 389–399. doi: 10.1016/S0896-6273(00)80983-6
Newman, E. A. (2003). New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 26, 536–542. doi: 10.1016/S0166-2236(03)00237-6
Pangrsic, T., Potokar, M., Stenovec, M., Kreft, M., Fabbretti, E., Nistri, A., et al. (2007). Exocytotic release of ATP from cultured astrocytes. J. Biol. Chem. 282, 28749–28758. doi: 10.1074/jbc.M700290200
Pankratov, Y., Lalo, U., Verkhratsky, A., and North, R. A. (2006). Vesicular release of ATP at central synapses. Pflugers Arch. 452, 589–597. doi: 10.1007/s00424-006-0061-x
Pankratov, Y., Lalo, U., Verkhratsky, A., and North, R. A. (2007). Quantal release of ATP in mouse cortex. J. Gen. Physiol. 129, 257–265. doi: 10.1085/jgp.200609693
Parpura, V., Heneka, M. T., Montana, V., Oliet, S. H., Schousboe, A., Haydon, P. G., et al. (2012). Glial cells in (patho)physiology. J. Neurochem. 121, 4–27. doi: 10.1111/j.1471-4159.2012.07664.x
Rivera, A., Vanzulli, I., and Butt, A. M. (2016). A central role for ATP signalling in glial interactions in the CNS. Curr. Drug Targets 17, 1829–1833. doi: 10.2174/1389450117666160711154529
Rodrigues, R. J., Tomé, A. R., and Cunha, R. A. (2015). ATP as a multi-target danger signal in the brain. Front. Neurosci. 9:148. doi: 10.3389/fnins.2015.00148
Stout, C. E., Costantin, J. L., Naus, C. C., and Charles, A. C. (2002). Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 277, 10482–10488. doi: 10.1074/jbc.M109902200
Suadicani, S. O., Brosnan, C. F., and Scemes, E. (2006). P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 26, 1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006
Sudhof, T. C. (2004). The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547. doi: 10.1146/annurev.neuro.26.041002.131412
Sudhof, T. C., and Rothman, J. E. (2009). Membrane fusion: grappling with SNARE and SM proteins. Science 323, 474–477. doi: 10.1126/science.1161748
Verkhratsky, A., Krishtal, O. A., and Burnstock, G. (2009). Purinoceptors on neuroglia. Mol. Neurobiol. 39, 190–208. doi: 10.1007/s12035-009-8063-2
Verkhratsky, A., Matteoli, M., Parpura, V., Mothet, J. P., and Zorec, R. (2016). Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J. 35, 239–257. doi: 10.15252/embj.201592705
Verkhratsky, A., and Nedergaard, M. (2018). Physiology of Astroglia. Physiol. Rev. 98, 239–389. doi: 10.1152/physrev.00042.2016
Volterra, A., Liaudet, N., and Savtchouk, I. (2014). Astrocyte Ca2+ signalling: an unexpected complexity. Nat. Rev. Neurosci. 15, 327–335. doi: 10.1038/nrn3725
Wurm, A., Pannicke, T., Iandiev, I., Francke, M., Hollborn, M., Wiedemann, P., et al. (2011). Purinergic signaling involved in Muller cell function in the mammalian retina. Prog. Retin. Eye Res. 30, 324–342. doi: 10.1016/j.preteyeres.2011.06.001
Xiong, Y., Teng, S., Zheng, L., Sun, S., Li, J., Guo, N., et al. (2018). Stretch-induced Ca(2+) -independent ATP release in hippocampal astrocytes. J. Physiol. 596, 1931–1947. doi: 10.1113/JP275805
Yan, Z., Li, S., Liang, Z., Tomić, M., and Stojilkovic, S. S. (2008). The P2X7 receptor channel pore dilates under physiological ion conditions. J. Gen. Physiol. 132, 563–573. doi: 10.1085/jgp.200810059
Young, S. H., and Poo, M. M. (1983). Spontaneous release of transmitter from growth cones of embryonic neurones. Nature 305, 634–637. doi: 10.1038/305634a0
Zhang, J. M., Wang, H. K., Ye, C. Q., Ge, W., Chen, Y., Jiang, Z. L., et al. (2003). ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40, 971–982. doi: 10.1016/S0896-6273(03)00717-7
Zhang, Z., Chen, G., Zhou, W., Song, A., Xu, T., Luo, Q., et al. (2007). Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 9, 945–953. doi: 10.1038/ncb1620
Keywords: glial transmitter, ATP, astrocyte, exocytosis, P2X7, calcium, mechanical stimulation
Citation: Xiong Y, Sun S, Teng S, Jin M and Zhou Z (2018) Ca2+-Dependent and Ca2+-Independent ATP Release in Astrocytes. Front. Mol. Neurosci. 11:224. doi: 10.3389/fnmol.2018.00224
Received: 29 March 2018; Accepted: 07 June 2018;
Published: 02 July 2018.
Edited by:
Alexej Verkhratsky, University of Manchester, United KingdomReviewed by:
Yuriy Pankratov, University of Warwick, United KingdomCopyright © 2018 Xiong, Sun, Teng, Jin and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingfei Xiong, eWZ4aW9uZ0AxMzkuY29t
Mu Jin, amlubXUwMTE5QHRvbS5jb20=
Zhuan Zhou, enpob3VAcGt1LmVkdS5jbg==
†These authors have contributed equally to this work.
 Yingfei Xiong1,2*†
Yingfei Xiong1,2*† Mu Jin
Mu Jin Zhuan Zhou
Zhuan Zhou