- 1Department of Pharmacology, School of Basic Medical Sciences, Peking University Health Science Center, Beijing, China
- 2Beijing Key Laboratory of Drug Dependence, National Institute on Drug Dependence, Peking University, Beijing, China
- 3Peking-Tsinghua Center for Life Sciences and PKU-IDG/McGovern Institute for Brain Research, Peking University, Beijing, China
- 4Peking University Sixth Hospital/Peking University Institute of Mental Health, Peking University, Beijing, China
- 5National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), Beijing, China
Methamphetamine abuse has become a serious public health problem. However, effective treatment for methamphetamine addiction remains elusive, especially considering its high rate of relapse after treatment. A conditioned stimulus (CS) memory retrieval–extinction procedure has been demonstrated to decrease reinstatement of cocaine, heroin, and alcohol seeking in rats, and to reduce cue-induced cravings in heroin and nicotine addicts. The goal of the present study is to explore the effect of the CS memory retrieval–extinction procedure on methamphetamine seeking in rats and the underlying mechanisms. We found that daily retrieval of methamphetamine-associated memories 1 h before extinction sessions decreased subsequent drug priming-induced reinstatement, spontaneous recovery, and renewal of methamphetamine seeking. We also found that retrieval of methamphetamine-associated memories induced neuronal activation in the basolateral amygdala (BLA), while presenting extinction within the time window of reconsolidation abolished the neuronal activation in BLA. These results indicate that the CS memory retrieval–extinction procedure could prevent reconsolidation of methamphetamine memory traces in BLA and subsequent methamphetamine craving and relapse.
Introduction
Methamphetamine addiction remains a significant public health concern worldwide, leading to devastating personal and social consequences. Frequent use of methamphetamine has been associated with severe neurotoxic effects and neurocognitive impairment (Ernst et al., 2000; Berman et al., 2008; Hart et al., 2012; Dean et al., 2013). Nevertheless, there has been no medication approved by the FDA for the treatment of methamphetamine addiction so far. Furthermore, psychosocial interventions, such as cognitive behavioral therapy, are considered to be cost- and time-intensive and with relatively poor outcomes given the high rates of relapse among methamphetamine addicts (Rawson et al., 2004; Shearer, 2007). Therefore, it is of great importance to develop more effective interventions to treat methamphetamine addiction and to prevent relapse.
Drug addiction has been considered to be a kind of aberrant reward memory (Kauer and Malenka, 2007). Associations between drug (unconditioned stimulus, US) and drug-related cues (conditioned stimulus, CS) play an important role in drug addiction and relapse (Stewart et al., 1984). In both animal and human studies, continuous exposure to drug-associated cues has been suggested to induce extinction and thus suppress drug craving and relapse. This effect has laid the theoretical foundations for exposure therapy to treat drug addiction in the clinic (Marlatt, 1990). However, the efficacy of exposure therapy is not persistent. Relapse can be induced by exposure to the drugs of abuse (reinstatement), drug-associated contexts (renewal), or the extension of withdrawal time after extinction (spontaneous recovery) (Bouton, 2002; Conklin and Tiffany, 2002).
After acquisition and stabilization, a consolidated memory can enter a labile state within a specific time window after reactivation and thus susceptible to enhancement or impairment, a process defined as reconsolidation (Nader et al., 2000; Alberini, 2005; Sorg, 2012; Reichelt and Lee, 2013). A number of studies have shown that drug-paired CS retrieval followed by pharmacological intervention could disrupt the reconsolidation of drug memories and inhibit drug conditioned place preference (CPP) and operant drug seeking and relapse (Li et al., 2010; Sanchez et al., 2010). However, most compounds used in these studies are not approved for human use except for the beta-adrenoceptor antagonist propranolol (Fricks-Gleason and Marshall, 2008; Wouda et al., 2010), and this may be an obstacle to successful translation to clinical applications.
In recent years, a CS memory retrieval–extinction procedure has been proposed as a nonpharmacological alternative to prevent reemergence of fear in rats and humans (Monfils et al., 2009; Schiller et al., 2010). When applying this CS memory retrieval–extinction procedure to drug addiction, we found that it can decrease drug-priming-induced reinstatement, renewal, and spontaneous recovery of drug (morphine, heroin, and cocaine) seeking in rats as well as cue-induced craving in heroin addicts (Xue et al., 2012). Consistent with our results, the CS memory retrieval–extinction procedure was also effective for reinstatement of morphine CPP and alcohol seeking in rats and cue-induced craving in smokers (Ma et al., 2012; Millan et al., 2013; Germeroth et al., 2017). However, the mechanisms underlying the inhibitory effect of the procedure on drug seeking have not yet been fully understood.
A large body of evidence indicates that the amygdala plays a pivotal role in retrieval, extinction, and reconsolidation of fear and drug memory (Nader et al., 2000; Lee et al., 2006; Milton et al., 2008a; Li et al., 2010; Luo et al., 2013; Xue et al., 2014), which suggested that amygdala may be implicated in the effects of CS memory retrieval–extinction. In addition, some studies have suggested that dephosphorylation of glutamate receptor 1 (GluA1), trafficking of calcium-permeable α-amino-3-hydroxyl-5-methyl-4 isoxazole-propionate receptors (CP-AMPARs), activation of L-type voltage-gated calcium channels, and the expression of immediate early gene, zinc-finger 268 protein (Zif268), and Arc may underlie the effects of the retrieval–extinction procedure (Monfils et al., 2009; Clem and Huganir, 2010; Flavell et al., 2011; Tedesco et al., 2014; Lee et al., 2016). However, few studies showed the neural substrate for the inhibitory effect of CS memory retrieval–extinction on drug seeking. Our previous study revealed that the CS memory retrieval–extinction procedure with a 10-min but not 6-h interval amplified the decrease in PKMζ expression in the basolateral amygdala (BLA) induced by extinction training (Xue et al., 2012). In the present study, we sought to identify whether the effects of the CS memory retrieval–extinction procedure can be extended to methamphetamine seeking and relapse, and we also explored the distinct neural activation patterns in the amygdala during different retrieval–extinction manipulations.
Materials and Methods
Subjects
Male Sprague–Dawley rats, weighing 260–280 g, were purchased from the Vital River Company. The rats were housed five per cage before the experiments and were individually housed after the surgery. The rats were maintained under controlled temperature (23 ± 2°C) and humidity (50 ± 5%) with free access to chow and water and were kept on a reverse 12-h light/dark cycle. The behavioral experiments were conducted during the dark phase of the cycle. The experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Biomedical Ethics Committee of animal use and protection of Peking University.
Surgery
Rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.). Silastic catheters were inserted into the right jugular vein with the tip terminating at the opening of the right atrium based on our previous studies (Xue et al., 2012; Luo et al., 2015). All rats were allowed to recover from the surgery for 5–7 days.
Intravenous Methamphetamine Self-administration Training
The procedure for methamphetamine self-administration was based on previous studies (Caprioli et al., 2015; Venniro et al., 2017). The operant chambers (AniLab Software & Instruments, Ningbo, China) had two nosepoke operandi located 5 cm above the bottom of the chambers. Nosepokes in the active operandum resulted in methamphetamine infusions and a 5-s tone-light cue. Nosepokes in the inactive operandum cannot lead to methamphetamine infusions or tone-light cues but were also recorded. The rats were trained to self-administer methamphetamine (0.10 mg/kg/infusion) during three 1-h sessions (separated by 5-min off periods) over 14 days. The self-administration training started at the beginning of the dark cycle and was performed under a fixed-ratio one (FR1) 40-s timeout-reinforcement schedule. Sessions began with the presentation of a houselight that remained on for the duration of the session. To prevent overdose, the number of methamphetamine infusions was limited to 15 per hour. After the self-administration training, rats were divided into different groups with matched methamphetamine intake during training phase.
CS Memory Retrieval
The CS memory retrieval manipulation was based on our previous studies (Xue et al., 2012; Luo et al., 2015). The rats were given 15-min daily sessions during which nosepoke responses led to the 5-s tone-light cue but not methamphetamine infusions. The 180-min daily extinction sessions began 1 h or 6 h after the CS retrieval manipulation.
Extinction
During the extinction sessions (195 min for the “no retrieval + extinction” group and 180 min for the “CS retrieval + extinction” group), the conditions were identical to that during training, with the exception that nosepoke responses led to the 5-s tone-light cue but not methamphetamine infusions. The rats underwent extinction training until their nosepokes on the active operandum were less than 20% of the mean nosepokes during the last 3 days of methamphetamine self-administration training for at least two consecutive days.
Test for Drug Seeking
Once nosepokes on the active operandum was successfully extinguished according to the criteria described above, test for methamphetamine seeking began. The testing conditions were identical to that of training phase except for the fact that active nosepokes did not lead to methamphetamine infusions. The test session started with the presentation of the houselight that remained on throughout the test. Nosepoke responding during the test led to contingent presentations of the 5-s tone-light cue that had previously been paired with methamphetamine infusions. During the drug-priming-induced reinstatement tests, the rats were given an intraperitoneal injection of methamphetamine (1 mg/kg) immediately before the sessions began. The dose of priming was based on previous studies (Cox et al., 2013; Jing et al., 2014; Baracz et al., 2016).
Immunofluorescence Staining and Imaging Analysis
Immunofluorescence assays were performed to examine Fos expressions in brain slices based on our previous studies (Xue et al., 2017; Fang et al., 2018). Rats were anesthetized with 10% chloral hydrate and perfused transcardially with 0.01 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde (PFA). Brains were dissected and post-fixed in 4% PFA before being transferred to 30% sucrose in PB at 4°C. Then, the brains were frozen in dry ice and stored at −80°C until sectioning. Coronal sections of the amygdala were cut with a Leica cryostat at 20 μm thickness and washed with PBS three times for 5 min each before being incubated in PBS containing 0.3% Triton X-100 and 2% bovine serum albumin (BSA) for 1 h at 37°C. The sections were then incubated with rabbit antibody to Fos (1:500, #2250s, Cell Signaling Technology, Danvers, MA, USA) and mouse antibody to NeuN (1:500, #MAB377, Millipore, Burlington, MA, USA) for 24 h at 4°C. After incubation with the primary antibodies, sections were rinsed with PBS four times for 5 min each and then incubated with secondary antibodies (Alexa Fluor 488-conjugated goat anti-rabbit, 1:500, #A11034, Invitrogen; Alexa Fluor 594-conjugated donkey anti-mouse, 1:500, #A21203, Invitrogen) for 3 h at room temperature. After incubation with the secondary antibodies, the sections were rinsed four times for 5 min each, mounted, and coverslipped. Images were acquired by a fluorescence microscope (VS120, Olympus) with a 20× objective lens. The number of Fos-, NeuN-, and double-labeled cells was quantified in a blind fashion according to our previous studies (Xue et al., 2017; Fang et al., 2018). In brief, we selected at least three slices for each brain region of each rat and averaged the proportions of neurons expressing Fos on either side of the specific brain region to be the percentage of neuronal activation for each rat and measured the number of Fos-, NeuN-, and double-labeled cells using Image-Pro Plus software.
Statistical Analysis
All of the data were expressed as mean ± standard error of the mean (SEM) and analyzed using analysis of variances (ANOVAs) with appropriate between- and within-subjects factors (see the “Results” section). Shapiro–Wilk’s test was applied to check normal distribution and Levene’s test was applied to check homogeneity of variance. Tukey’s test was used to conduct post hoc analyses of significant effects when prior ANOVAs indicated significant main or interaction effects (p < 0.05). p < 0.05 was considered to be statistically significant.
Results
Experiment 1: Effect of the CS Memory Retrieval–Extinction Procedure on Reinstatement of Methamphetamine Seeking
We first assessed the effect of CS memory retrieval–extinction manipulation on drug-priming-induced reinstatement of methamphetamine seeking. The rats were trained to nose poke for intravenous methamphetamine infusion for 3 h per day for 14 days, after which they were divided into three groups (n = 8–9 per group) with equivalent methamphetamine intake for each group and treated as follows: (1) in Group 1, rats received 3.25 h extinction without CS memory retrieval (no retrieval + extinction); (2) in Group 2, rats were given a 15-min CS memory retrieval 1 h before each 3-h extinction session (retrieval + 1 h + extinction); and (3) in Group 3, rats were given CS memory retrieval 6 h before each 3-h extinction session (retrieval + 6 h + extinction). Once the rats met the extinction criterion, they underwent a priming test initiated by a non-contingent methamphetamine injection (1 mg/kg, i.p.) immediately before the test (Figure 1A).
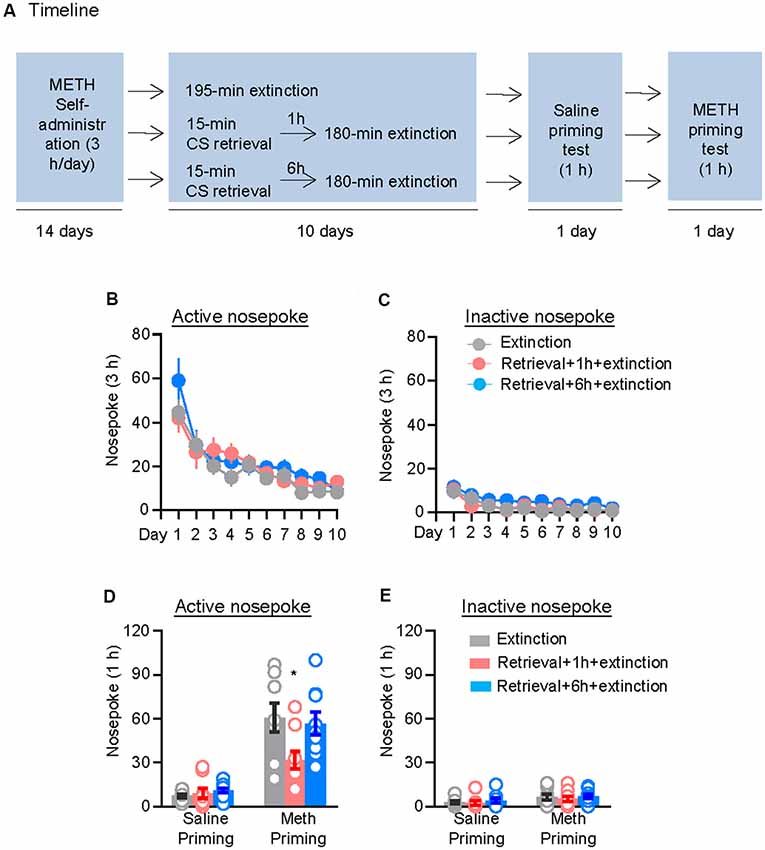
Figure 1. Memory retrieval–extinction procedure prevented drug-priming-induced reinstatement of methamphetamine seeking. (A) Experimental timeline. (B–E) Nosepokes [mean ± standard error of the mean (SEM)] on the active and inactive nosepoke operandi during the extinction sessions, the saline-priming test, and the methamphetamine (METH)-priming test. *Different from the “Extinction” group; p < 0.05; n = 8–9 rats per experimental condition.
Repeated ANOVA was used to analyze the nosepokes during extinction, with the between-subjects factor of Retrieval–extinction Strategy (no retrieval + extinction, retrieval + 1 h + extinction, and retrieval + 6 h + extinction) and the within-subjects factor of Extinction Sessions (session 1–session 10), and we found significant effect of Extinction Sessions (F(9,207) = 23.98, p < 0.01, Figure 1B) but no Retrieval–extinction Strategy × Extinction Sessions interactions (F(18,207) = 0.87, p > 0.05) on active nosepoke operandum. No group difference was observed in nosepokes on the inactive nosepoke operandum (F(18,207) = 0.49, p > 0.05, Figure 1C). The analysis of the nosepokes during reinstatement test included the between-subjects factor of Retrieval–extinction Strategy (no retrieval + extinction, retrieval + 1 h + extinction, and retrieval + 6 h + extinction) and the within-subjects factor of Test Condition (last extinction session and reinstatement test session). There were significant Retrieval–extinction Strategy × Test Condition interactions on active nosepoke operandum (F(2,23) = 3.87, p < 0.05, Figure 1D). Post hoc analysis showed that in the reinstatement test, the active nosepoke responses significantly decreased compared with the other two groups (p < 0.05). No group difference was observed in nosepokes on the inactive nosepoke operandum (F(2,23) = 0.08, p > 0.05, Figure 1E). These results indicated that exposing rats to the CS retrieval manipulations 1 h but not 6 h before the extinction sessions attenuated methamphetamine-priming-induced reinstatement of methamphetamine seeking.
Experiment 2: Effect of the CS Memory Retrieval–Extinction Procedure on Spontaneous Recovery of Methamphetamine Seeking
We used the other two groups of rats to examine the effect of the CS memory retrieval–extinction procedure on spontaneous recovery of methamphetamine seeking. After 14 consecutive days of methamphetamine self-administration training, the rats were divided into two groups (n = 9–10 per group) that were matched for their methamphetamine intake: (1) in Group 1, rats underwent 3.25-h extinction training without CS memory retrieval (no retrieval + extinction); and (2) in Group 2, rats were given CS memory retrieval 1 h before each 3-h extinction session (retrieval + 1 h + extinction). When the rats met the extinction criterion, they were housed in their homecages for 4 weeks. Then, they were tested for spontaneous recovery of the extinguished drug-seeking behavior in an extinction session in which nosepokes led to contingent delivery of the tone-light cue previously paired with methamphetamine infusions (Figure 2A).
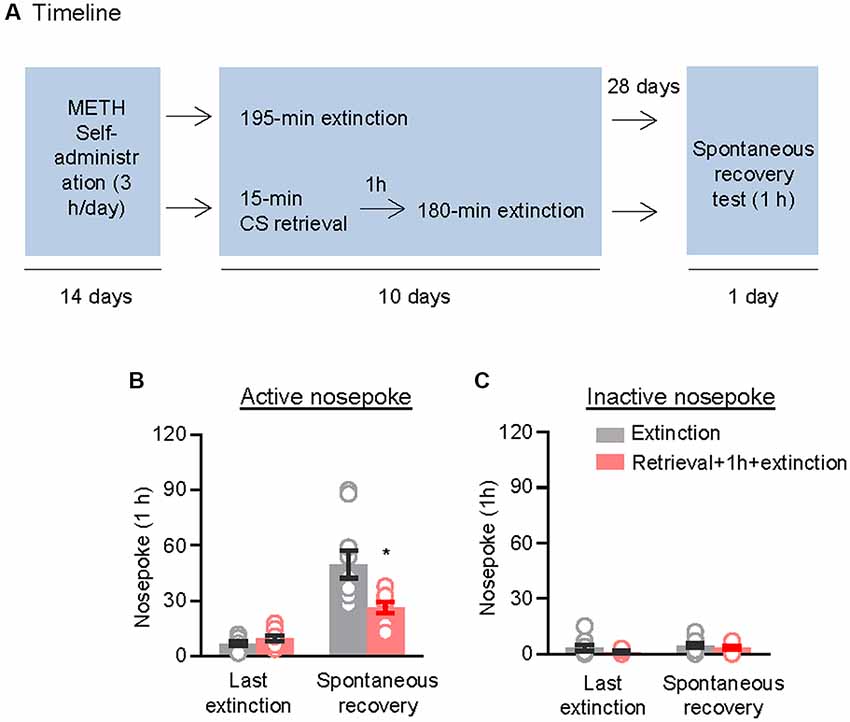
Figure 2. Memory retrieval–extinction procedure prevented spontaneous recovery of methamphetamine seeking. (A) Experimental timeline. (B,C) Number of nosepokes (mean ± SEM) on the active and inactive nosepoke operandi during the first hour of the last 3-h extinction session and the spontaneous recovery test. *Different from the “Extinction” group; p < 0.05; n = 9–10 rats per experimental condition.
Repeated ANOVA was used to analyze nosepokes during the spontaneous recovery test, with the between-subjects factor of Retrieval–extinction Strategy (no retrieval + extinction and retrieval + 1 h + extinction) and the within-subjects factor of Test Condition (last extinction session and spontaneous recovery test session). The analysis showed significant interactions between Retrieval–extinction Strategy and Test Condition (F(1,17) = 10.58, p < 0.01, Figure 2B). No group difference was observed in nosepokes on the inactive nosepoke operandum (F(1,17) = 0.30, p > 0.05, Figure 2C). The results indicated that exposing rats to the CS retrieval manipulations 1 h before the extinction sessions prevented spontaneous recovery of methamphetamine seeking.
Experiment 3: Effect of the CS Memory Retrieval–Extinction Procedure on Renewal of Methamphetamine Seeking
We further demonstrated the effect of the CS memory retrieval–extinction procedure on renewal of methamphetamine seeking under a modified ABA renewal (training in context A, extinction in context B, testing in context A) procedure based on previous studies (Xue et al., 2012; Luo et al., 2015), with two counterbalanced contexts: context A had stainless steel rod floor and gray walls, while context B had granular flat floor and walls covered in wallpaper with black and white patterns. Rats were first trained to self-administer methamphetamine in context A for 14 days. Then, they were divided into two groups (n = 8–10 per group) with matched methamphetamine intake and underwent extinction training in context B: (1) in Group 1, rats underwent 3.25-h extinction training without CS memory retrieval (no retrieval + extinction); and (2) in Group 2, rats were given CS memory retrieval 1 h before every 3-h extinction session (retrieval + 1 h + extinction). The CS memory retrieval manipulation was a 15-min exposure to context B, during which nosepokes led to presentation of discrete cues but not methamphetamine. After the rats met the extinction criterion, they underwent a renewal test in context A, during which nosepokes led to contingent delivery of tone-light cues previously paired with methamphetamine infusions but not methamphetamine (Figure 3A).
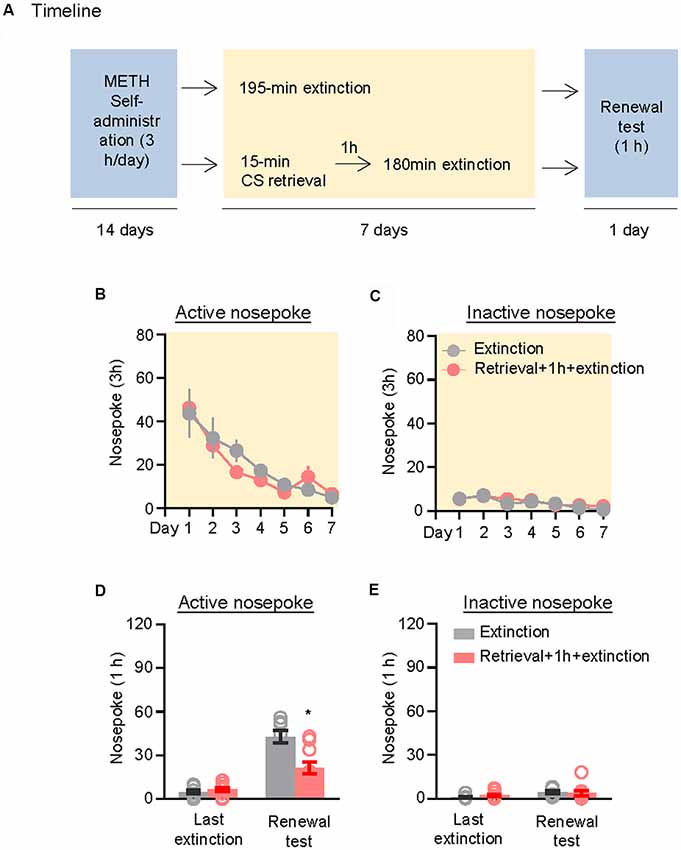
Figure 3. Memory retrieval–extinction procedure prevented renewal of methamphetamine seeking. (A) Experimental timeline. (B–E) Number of nosepokes (mean ± SEM) on the active and inactive nosepoke operandi during the first hour of the last 3-h extinction session and the renewal test. *Different from the “Extinction” group; p < 0.05; n = 8–10 rats per experimental condition.
Repeated ANOVA was used to analyze the nosepokes during extinction, with the between-subjects factor of Retrieval–extinction Strategy (no retrieval + extinction, retrieval + 1 h + extinction) and the within-subjects factor of Extinction Sessions (session 1–session 7). There was only significant effect of Extinction Sessions (F(6,96) = 21.26, p < 0.01, Figure 3B) but no Retrieval–extinction Strategy × Extinction Sessions interactions (F(6,96) = 0.78, p > 0.05) on active nosepoke operandum. No group difference was observed in nosepokes on the inactive nosepoke operandum (F(6,96) = 0.29, p > 0.05, Figure 3C). Repeated ANOVA was used to analyze the nosepokes during renewal test, including the between-subjects factor of Retrieval–extinction Strategy (no retrieval + extinction, retrieval + 1 h + extinction) and the within-subjects factor of Test Condition (last extinction session, renewal test session). There were significant Retrieval–extinction Strategy × Test Condition interactions on active nosepoke operandum (F(1,16) = 14.74, p < 0.01, Figure 3D). No group difference was observed in responding to the inactive nosepoke operandum (F(1,16) = 1.52, p > 0.05, Figure 3E). The results indicated that exposing rats to the CS retrieval manipulations 1 h before the extinction sessions prevented subsequent renewal of methamphetamine seeking.
Experiment 4: Effect of the CS Memory Retrieval–Extinction Procedure on Neuronal Activation in Amygdala
Finally, we investigated whether the inhibitory effect of the CS memory retrieval–extinction procedure on reinstatement of methamphetamine seeking was associated with neuronal activation in BLA and central amygdala (CeA), two subregions of amygdala (LeDoux, 2007). Fos was widely used as the marker of neural activations (Morgan and Curran, 1991; Xue et al., 2017; Luo et al., 2018; Venniro et al., 2018), and it has been found to mediate memory reconsolidation (Miller and Marshall, 2005). Thus, we assessed whether Fos expression was different for standard extinction, extinction within the time window of reconsolidation, and extinction out of the time window of reconsolidation. Rats were first trained to self-administer methamphetamine for 14 days. One day later, they were divided into five groups (n = 4–5 per group) with matched methamphetamine intake and perfused following different manipulations (Figure 4A): (1) in Group 1, rats did not undergo either CS memory retrieval or extinction training (no retrieval + no extinction); (2) in Group 2, rats only underwent CS memory retrieval (retrieval + no extinction); (3) in Group 3, rats underwent 3.25-h extinction training without CS memory retrieval (no memory retrieval + extinction); (4) in Group 4, rats were given CS memory retrieval 1 h before the 3-h extinction session (retrieval + 1 h + extinction); and (5) in Group 5, rats were given CS memory retrieval 6 h before the 3-h extinction session (retrieval + 6 h + extinction). The rats were perfused 1.5 h after different manipulations and their brains were removed for immunofluorescence assays to assess the co-expression of Fos and NeuN (a marker of neurons) in the BLA (Figures 4B,C) and CeA (Figure 5A). One-way ANOVA was used to analyze immunofluorescence data, revealing the significant effect of Retrieval–extinction Strategy (F(4,16) = 26.96, p < 0.01, Figure 4D). Post hoc analysis showed that, compared with the no retrieval group, both retrieval + no extinction and no retrieval + extinction groups increased the Fos expression (p < 0.01). More importantly, the CS memory retrieval–extinction manipulation with a 1-h but not 6-h interval attenuated neuronal activation in the BLA compared with the extinction group (p < 0.01). In contrast, no significant difference in Fos expression was found in the CeA of all groups (F(4,16) = 1.56, p-values > 0.05, Figure 5B), indicating that the changes in Fos expression induced by CS memory retrieval–extinction was specific to BLA.
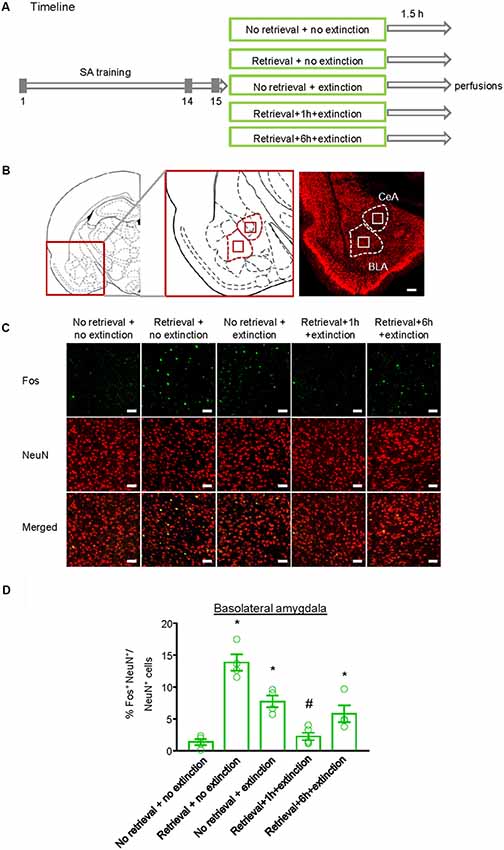
Figure 4. Effect of the conditioned stimulus (CS) retrieval–extinction procedure on neuronal activation in basolateral amygdala (BLA). (A) Experimental timeline. Rats were trained to self-administer methamphetamine during three 1-h daily sessions over 14 days. One day later, rats were divided into five experimental groups and were perfused 1.5 h after the treatment. (B) Coronal section schematic indicating the region of BLA and central amygdala (CeA) with NeuN immunofluorescence staining. Scale bar is 200 μm. (C) Representative images showing green (Fos protein), red (NeuN protein), and double-labeled neurons in the BLA in different experimental manipulations. Scale bars represent 50 μm. (D) Percentage of activated cells in the BLA in different experimental conditions. The CS memory retrieval–extinction manipulation with a 1-h but not 6-h interval attenuated neuronal activations in the BLA. n = 4–5 per experimental condition. Data are mean ± SEM of number of overlap (Fos + NeuN protein-IR/Fos-IR). *Different from the “No retrieval + no extinction” group. #Different from the “No retrieval + extinction” group, one-way analysis of variance (ANOVA), p < 0.05.
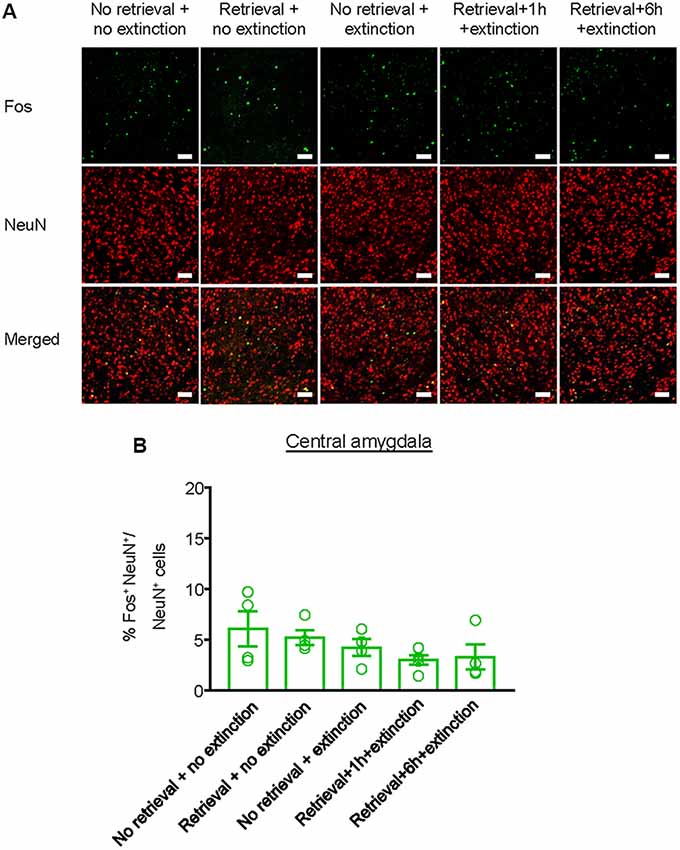
Figure 5. Effect of the memory retrieval–extinction procedure on neuronal activation in the CeA. (A) Representative images showing green (Fos protein), red (NeuN protein), and double-labeled neurons in the CeA in different experimental manipulations. Scale bars represent 50 μm. (B) Percentage of activated cells in the CeA in different experimental conditions. The memory retrieval–extinction manipulation with a 1-h interval did not affect neuronal activations in the CeA compared with the extinction group. n = 4–5 per experimental condition. Data are mean ± SEM of number of overlap (Fos + NeuN protein-IR/Fos-IR).
Discussion
In the current study, we demonstrated that the memory retrieval–extinction procedure could be an effective method to prevent methamphetamine seeking and relapse. Daily extinction training 1 h after CS retrieval significantly attenuated drug-priming-induced reinstatement of methamphetamine seeking. In addition, the memory retrieval–extinction procedure reduced relapse following 28 days of abstinence, indicating its long-lasting effect on drug addiction. In an ABA renewal model, the memory retrieval–extinction procedure also impaired the context-induced reinstatement of methamphetamine seeking. Despite the fact that the memory retrieval–extinction procedure was proved effective in preventing drug seeking and relapse in both animals and human addicts (Ma et al., 2012; Xue et al., 2012; Millan et al., 2013; Sartor and Aston-Jones, 2014; Germeroth et al., 2017), the neural mechanisms involved in this process remains largely elusive. Here, we found that presenting extinction within the time window of reconsolidation, but not presenting extinction out of the time window of reconsolidation or standard extinction training inhibited retrieval-induced neuronal activation in the BLA. Taken together, these results revealed that memory retrieval–extinction manipulations could potentially be utilized for the treatment of methamphetamine addiction, and its efficacy may be related to engagement of BLA.
The development of a memory retrieval–extinction procedure was based on the theory of reconsolidation as well as pharmacological studies targeting memory retrieval and reconsolidation process (Lee et al., 2006; Milekic et al., 2006; Milton et al., 2008b; Li et al., 2010; Sanchez et al., 2010; Wouda et al., 2010). Reconsolidation is defined as a process during which a stabilized memory turns into a labile phase induced by memory retrieval and thus can be modified (Sorg, 2012; Forcato et al., 2014; Nader, 2015; Lee et al., 2017). Increasing evidence suggested that only pharmacological or behavioral manipulations within a limited time interval after memory retrieval could disrupt memory reconsolidation, indicating the existence of a reconsolidation time window (Walker et al., 2003; Li et al., 2010; Schiller et al., 2010; Xue et al., 2012; Luo et al., 2015). In line with previous studies, we found that extinction training performed 6 h after memory retrieval failed to prevent the drug-priming-induced reinstatement of methamphetamine seeking.
Considering that the memory retrieval manipulation was performed within the consolidation window of extinction memory, an alternative explanation for the effect of retrieval–extinction procedure could be that the extinction memory was facilitated, leaving the original rewarding memory less susceptible to reinstatement, spontaneous recovery, and renewal. In fact, pharmacological interventions that facilitated consolidation of extinction successfully attenuated the reinstatement, spontaneous recovery, and renewal of fear response and drug seeking (Quirk and Mueller, 2008; Malvaez et al., 2010). Nevertheless, the reduction in methamphetamine seeking and relapse does not seem to be related with enhanced extinction, considering the comparable rate of extinction among different groups.
In recent years, there has been some progress in uncovering the neural substrates of the effect of memory retrieval–extinction procedure on memories (Cahill and Milton, 2019). For example, Monfils et al. (2009) showed that a second CS presented 1 h after the initial retrieval led to dephosphorylation of GluA1 in the lateral amygdala, which may underlie the disappearance of fear reemergence in the retrieval–extinction paradigm. Consistently, Clem and Huganir (2010) found that Ser845 mutant mice did not show reduction in fear reemergence after the retrieval–extinction procedure. Moreover, they found synaptic removal of CP-AMPARs in the lateral amygdala in the retrieval–extinction procedure but not standard extinction (Clem and Huganir, 2010). All of these results suggested that the retrieval–extinction procedure triggered dephosphorylation of the GluA1Ser845 and produced depotentiation of the original memory, rather than enhancement of extinction (Monfils et al., 2009; Clem and Huganir, 2010). Tedesco et al. (2014) found an increase in the expression of zinc-finger 268 protein and phosphorylated ribosomal protein S6 in the prefrontal cortex and lateral amygdala during fear retrieval–extinction compared with extinction alone. They proposed that the effect of the retrieval–extinction process was more similar to reconsolidation updating than extinction facilitation or reconsolidation disruption (Tedesco et al., 2014). Lee et al. (2016) found fewer Arc-staining cells in the lateral amygdala during the late phase retrieval–extinction group. However, in contrast to the relatively more research focusing on the role of retrieval–extinction in fear, knowledge about the neural mechanism underlying the effect of retrieval–extinction on drug addiction remains scarce. In the current study, we examined the changes of Fos, one of the immediate early genes that is involved in processes of reconsolidation and extinction (Miller and Marshall, 2005; Siahposht-Khachaki et al., 2017, 2018), after retrieval, extinction, and retrieval–extinction procedures. We found that Fos expression increased after retrieval and extinction, consistent with previous studies (Nic Dhonnchadha et al., 2012; Xue et al., 2017). The increased activity of BLA neurons detected after extinction might indicate the extinction memory, i.e., a new inhibitory memory of CS-no reward. For the groups of “retrieval + 1 h + extinction” and “retrieval + 6 h + extinction,” the activity of BLA neurons might indicate the effects of updating the original “CS-reward” trace with a new “CS-no reward” trace within or outside the reconsolidation time window, respectively. Presenting extinction 1 h after retrieval decreased Fos expression to the level of no retrieval. It is interesting that the phenomena did not occur in the standard extinction or extinction 6 h after retrieval. These results suggest that decreased Fos expression may not be due to the differences in time between first re-exposure to the chamber and perfusion, but reflect the interaction between retrieval and extinction, i.e., interference of reconsolidation by extinction or interference of extinction by reconsolidation. The boundary conditions and interaction of reconsolidation and extinction abolished the neuronal activation and Fos expression and may weaken the memory engram of addiction memory in BLA. In addition, there was no difference among these groups in the CeA. Consistently, our previous study has shown that the memory retrieval–extinction procedure with a 10-min but not 6-h interval or standard extinction decreased PKMζ expression in the BLA (Xue et al., 2012). Together with previous studies in fear memory, these findings suggested that the retrieval–extinction procedure involves mechanisms that differ from standard extinction. Further studies combining optogenetics and electrophysiology are warranted to elucidate the causal role of BLA in the memory retrieval–extinction procedure. In addition, BLA has been considered to have intricate connections with the prefrontal cortex, hippocampus, and nucleus accumbens, as well as sensory association areas (Brog et al., 1993; McDonald, 1998; Ghashghaei and Barbas, 2002; Ghashghaei et al., 2007). Future studies should investigate how BLA interacts with other brain areas to mediate the effect of memory retrieval–extinction on drug seeking.
In summary, we showed significant inhibitory effects of the memory retrieval–extinction procedure on drug-priming-induced reinstatement, spontaneous recovery, and renewal of methamphetamine seeking in rats, suggesting that it could be a promising method for decreasing relapse in methamphetamine addicts. Furthermore, the attenuated neuronal activation in the BLA and disrupted memory reconsolidation may be associated with the favorable behavioral outcomes of the memory retrieval–extinction procedure.
Ethics Statement
All of the experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Biomedical Ethics Committee on animal use and protection of Peking University.
Author Contributions
Y-YC, L-BZ, Y-XX, and LL designed the experiments. Y-YC, L-BZ, and YL performed the experiments. Y-YC, L-BZ, and Y-XX analyzed and interpreted the data. S-QM, Y-MG, and JS commented on the manuscript. Y-YC and Y-XX wrote the manuscript.
Funding
This work was supported in part by the Natural Science Foundation of China (Nos. 81871046, 81722018, 81821092, and U180220091), the National Basic Research Program of China (Nos. 2015CB856400, 2015CB559200, and 2015CB553503), and Beijing Brain Project (Z171100000117014).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alberini, C. M. (2005). Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 28, 51–56. doi: 10.1016/j.tins.2004.11.001
Baracz, S. J., Everett, N. A., McGregor, I. S., and Cornish, J. L. (2016). Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats. Addict. Biol. 21, 316–325. doi: 10.1111/adb.12198
Berman, S., O’Neill, J., Fears, S., Bartzokis, G., and London, E. D. (2008). Abuse of amphetamines and structural abnormalities in the brain. Ann. N Y Acad. Sci. 1141, 195–220. doi: 10.1196/annals.1441.031
Bouton, M. E. (2002). Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol. Psychiatry 52, 976–986. doi: 10.1016/s0006-3223(02)01546-9
Brog, J. S., Salyapongse, A., Deutch, A. Y., and Zahm, D. S. (1993). The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol. 338, 255–278. doi: 10.1002/cne.903380209
Cahill, E. N., and Milton, A. L. (2019). Neurochemical and molecular mechanisms underlying the retrieval–extinction effect. Psychopharmacology 236, 111–132. doi: 10.1007/s00213-018-5121-3
Caprioli, D., Zeric, T., Thorndike, E. B., and Venniro, M. (2015). Persistent palatable food preference in rats with a history of limited and extended access to methamphetamine self-administration. Addict. Biol. 20, 913–926. doi: 10.1111/adb.12220
Clem, R. L., and Huganir, R. L. (2010). Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 330, 1108–1112. doi: 10.1126/science.1195298
Conklin, C. A., and Tiffany, S. T. (2002). Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97, 155–167. doi: 10.1046/j.1360-0443.2002.00014.x
Cox, B. M., Young, A. B., See, R. E., and Reichel, C. M. (2013). Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology 38, 2343–2353. doi: 10.1016/j.psyneuen.2013.05.005
Dean, A. C., Groman, S. M., Morales, A. M., and London, E. D. (2013). An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 38, 259–274. doi: 10.1038/npp.2012.179
Ernst, T., Chang, L., Leonido-Yee, M., and Speck, O. (2000). Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology 54, 1344–1349. doi: 10.1212/wnl.54.6.1344
Fang, Q., Li, Z., Huang, G. D., Zhang, H. H., Chen, Y. Y., Zhang, L. B., et al. (2018). Traumatic stress produces distinct activations of GABAergic and glutamatergic neurons in amygdala. Front. Neurosci. 12:387. doi: 10.3389/fnins.2018.00387
Flavell, C. R., Barber, D. J., and Lee, J. L. (2011). Behavioural memory reconsolidation of food and fear memories. Nat. Commun. 2:504. doi: 10.1038/ncomms1515
Forcato, C., Fernandez, R. S., and Pedreira, M. E. (2014). Strengthening a consolidated memory: the key role of the reconsolidation process. J. Physiol. Paris. 108, 323–333. doi: 10.1016/j.jphysparis.2014.09.001
Fricks-Gleason, A. N., and Marshall, J. F. (2008). Post-retrieval β-adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learn. Mem. 15, 643–648. doi: 10.1101/lm.1054608
Germeroth, L. J., Carpenter, M. J., Baker, N. L., Froeliger, B., LaRowe, S. D., and Saladin, M. E. (2017). Effect of a brief memory updating intervention on smoking behavior: a randomized clinical trial. JAMA Psychiatry 74, 214–223. doi: 10.1001/jamapsychiatry.2016.3148
Ghashghaei, H. T., and Barbas, H. (2002). Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 115, 1261–1279. doi: 10.1016/s0306-4522(02)00446-3
Ghashghaei, H. T., Hilgetag, C. C., and Barbas, H. (2007). Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34, 905–923. doi: 10.1016/j.neuroimage.2006.09.046
Hart, C. L., Marvin, C. B., Silver, R., and Smith, E. E. (2012). Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology 37, 586–608. doi: 10.1038/npp.2011.276
Jing, L., Zhang, Y., and Li, J. X. (2014). Effects of the trace amine associated receptor 1 agonist RO5263397 on abuse-related behavioral indices of methamphetamine in rats. Int. J. Neuropsychopharmacol. 18:pyu060. doi: 10.1093/ijnp/pyu060
Kauer, J. A., and Malenka, R. C. (2007). Synaptic plasticity and addiction. Nat. Rev. Neurosci. 8, 844–858. doi: 10.1038/nrn2234
Lee, H. J., Haberman, R. P., Roquet, R. F., and Monfils, M. H. (2016). Extinction and retrieval + extinction of conditioned fear differentially activate medial prefrontal cortex and amygdala in rats. Front. Behav. Neurosci. 9:369. doi: 10.3389/fnbeh.2015.00369
Lee, J. L., Milton, A. L., and Everitt, B. J. (2006). Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J. Neurosci. 26, 5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006
Lee, J. L. C., Nader, K., and Schiller, D. (2017). An update on memory reconsolidation updating. Trends Cogn. Sci. 21, 531–545. doi: 10.1016/j.tics.2017.04.006
Li, F. Q., Xue, Y. X., Wang, J. S., Fang, Q., Li, Y. Q., Zhu, W. L., et al. (2010). Basolateral amygdala cdk5 activity mediates consolidation and reconsolidation of memories for cocaine cues. J. Neurosci. 30, 10351–10359. doi: 10.1523/JNEUROSCI.2112-10.2010
Luo, S. X., Huang, J., Li, Q., Mohammad, H., Lee, C. Y., Krishna, K., et al. (2018). Regulation of feeding by somatostatin neurons in the tuberal nucleus. Science 361, 76–81. doi: 10.1126/science.aar4983
Luo, Y.-X., Xue, Y.-X., Liu, J.-F., Shi, H.-S., Jian, M., Han, Y., et al. (2015). A novel UCS memory retrieval–extinction procedure to inhibit relapse to drug seeking. Nat. Commun. 6:7675. doi: 10.1038/ncomms8675
Luo, Y.-X., Xue, Y.-X., Shen, H.-W., and Lu, L. (2013). Role of amygdala in drug memory. Neurobiol. Learn. Mem. 105, 159–173. doi: 10.1016/j.nlm.2013.06.017
Ma, X., Zhang, J. J., and Yu, L. C. (2012). Post-retrieval extinction training enhances or hinders the extinction of morphine-induced conditioned place preference in rats dependent on the retrieval–extinction interval. Psychopharmacology 221, 19–26. doi: 10.1007/s00213-011-2545-4
Malvaez, M., Sanchis-Segura, C., Vo, D., Lattal, K. M., and Wood, M. A. (2010). Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol. Psychiatry 67, 36–43. doi: 10.1016/j.biopsych.2009.07.032
Marlatt, G. A. (1990). Cue exposure and relapse prevention in the treatment of addictive behaviors. Addict. Behav. 15, 395–399. doi: 10.1016/0306-4603(90)90048-3
McDonald, A. J. (1998). Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 55, 257–332. doi: 10.1016/s0301-0082(98)00003-3
Milekic, M. H., Brown, S. D., Castellini, C., and Alberini, C. M. (2006). Persistent disruption of an established morphine conditioned place preference. J. Neurosci. 26, 3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006
Miller, C. A., and Marshall, J. F. (2005). Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 47, 873–884. doi: 10.1016/j.neuron.2005.08.006
Millan, E. Z., Milligan-Saville, J., and McNally, G. P. (2013). Memory retrieval, extinction, and reinstatement of alcohol seeking. Neurobiol. Learn. Mem. 101, 26–32. doi: 10.1016/j.nlm.2012.12.010
Milton, A. L., Lee, J. L., Butler, V. J., Gardner, R., and Everitt, B. J. (2008a). Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J. Neurosci. 28, 8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008
Milton, A. L., Lee, J. L., and Everitt, B. J. (2008b). Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on β-adrenergic receptors. Learn. Mem. 15, 88–92. doi: 10.1101/lm.825008
Monfils, M.-H., Cowansage, K. K., Klann, E., and LeDoux, J. E. (2009). Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324, 951–955. doi: 10.1126/science.1167975
Morgan, J. I., and Curran, T. (1991). Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu. Rev. Neurosci. 14, 421–451. doi: 10.1146/annurev.ne.14.030191.002225
Nader, K. (2015). Reconsolidation and the dynamic nature of memory. Cold Spring Harb. Perspect. Biol. 7:a021782. doi: 10.1101/cshperspect.a021782
Nader, K., Schafe, G. E., and Le Doux, J. E. (2000). Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726. doi: 10.1038/35021052
Nic Dhonnchadha, B. A., Lovascio, B. F., Shrestha, N., Lin, A., Leite-Morris, K. A., Man, H. Y., et al. (2012). Changes in expression of c-Fos protein following cocaine-cue extinction learning. Behav. Brain Res. 234, 100–106. doi: 10.1016/j.bbr.2012.06.010
Quirk, G. J., and Mueller, D. (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33, 56–72. doi: 10.1038/sj.npp.1301555
Rawson, R. A., Marinelli-Casey, P., Anglin, M. D., Dickow, A., Frazier, Y., Gallagher, C., et al. (2004). A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction 99, 708–717. doi: 10.1111/j.1360-0443.2004.00707.x
Reichelt, A. C., and Lee, J. L. (2013). Memory reconsolidation in aversive and appetitive settings. Front. Behav. Neurosci. 7:118. doi: 10.3389/fnbeh.2013.00118
Sanchez, H., Quinn, J. J., Torregrossa, M. M., and Taylor, J. R. (2010). Reconsolidation of a cocaine-associated stimulus requires amygdalar protein kinase A. J. Neurosci. 30, 4401–4407. doi: 10.1523/JNEUROSCI.3149-09.2010
Sartor, G. C., and Aston-Jones, G. (2014). Post-retrieval extinction attenuates cocaine memories. Neuropsychopharmacology 39, 1059–1065. doi: 10.1038/npp.2013.323
Schiller, D., Monfils, M. H., Raio, C. M., Johnson, D. C., Ledoux, J. E., and Phelps, E. A. (2010). Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463, 49–53. doi: 10.1038/nature08637
Shearer, J. (2007). Psychosocial approaches to psychostimulant dependence: a systematic review. J. Subst. Abuse Treat. 32, 41–52. doi: 10.1016/j.jsat.2006.06.012
Siahposht-Khachaki, A., Ezzatpanah, S., Razavi, Y., and Haghparast, A. (2018). NMDA receptor dependent changes in c-fos and p-CREB signaling following extinction and reinstatement of morphine place preference. Neurosci. Lett. 662, 147–151. doi: 10.1016/j.neulet.2017.10.028
Siahposht-Khachaki, A., Fatahi, Z., Yans, A., Khodagholi, F., and Haghparast, A. (2017). Involvement of AMPA/kainate glutamate receptor in the extinction and reinstatement of morphine-induced conditioned place preference: a behavioral and molecular study. Cell. Mol. Neurobiol. 37, 315–328. doi: 10.1007/s10571-016-0371-2
Sorg, B. A. (2012). Reconsolidation of drug memories. Neurosci. Biobehav. Rev. 36, 1400–1417. doi: 10.1016/j.neubiorev.2012.02.004
Stewart, J., de Wit, H., and Eikelboom, R. (1984). Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol. Rev. 91, 251–268. doi: 10.1037/0033-295x.91.2.251
Tedesco, V., Roquet, R. F., DeMis, J., Chiamulera, C., and Monfils, M. H. (2014). Extinction, applied after retrieval of auditory fear memory, selectively increases zinc-finger protein 268 and phosphorylated ribosomal protein S6 expression in prefrontal cortex and lateral amygdala. Neurobiol. Learn. Mem. 115, 78–85. doi: 10.1016/j.nlm.2014.08.015
Venniro, M., Caprioli, D., Zhang, M., Whitaker, L. R., Zhang, S., Warren, B. L., et al. (2017). The anterior insular cortex→central amygdala glutamatergic pathway is critical to relapse after contingency management. Neuron 96, 414.e8–427.e8. doi: 10.1016/j.neuron.2017.09.024
Venniro, M., Zhang, M., Caprioli, D., Hoots, J. K., Golden, S. A., Heins, C., et al. (2018). Volitional social interaction prevents drug addiction in rat models. Nat. Neurosci. 21, 1520–1529. doi: 10.1038/s41593-018-0246-6
Walker, M. P., Brakefield, T., Hobson, J. A., and Stickgold, R. (2003). Dissociable stages of human memory consolidation and reconsolidation. Nature 425, 616–620. doi: 10.1038/nature01930
Wouda, J. A., Diergaarde, L., Riga, D., van Mourik, Y., Schoffelmeer, A. N., and De Vries, T. J. (2010). Disruption of long-term alcohol-related memory reconsolidation: role of β-adrenoceptors and NMDA receptors. Front. Behav. Neurosci. 4:179. doi: 10.3389/fnbeh.2010.00179
Xue, Y.-X., Chen, Y.-Y., Zhang, L.-B., Zhang, L.-Q., Huang, G.-D., Sun, S.-C., et al. (2017). Selective inhibition of amygdala neuronal ensembles encoding nicotine-associated memories inhibits nicotine preference and relapse. Biol. Psychiatry 82, 781–793. doi: 10.1016/j.biopsych.2017.04.017
Xue, Y.-X., Luo, Y.-X., Wu, P., Shi, H. S., Xue, L.-F., Chen, C., et al. (2012). A memory retrieval–extinction procedure to prevent drug craving and relapse. Science 336, 241–245. doi: 10.1126/science.1215070
Keywords: methamphetamine, retrieval, extinction, relapse, amygdala
Citation: Chen Y-Y, Zhang L-B, Li Y, Meng S-Q, Gong Y-M, Lu L, Xue Y-X and Shi J (2019) Post-retrieval Extinction Prevents Reconsolidation of Methamphetamine Memory Traces and Subsequent Reinstatement of Methamphetamine Seeking. Front. Mol. Neurosci. 12:157. doi: 10.3389/fnmol.2019.00157
Received: 01 February 2019; Accepted: 05 June 2019;
Published: 02 July 2019.
Edited by:
Xiao-Dong Wang, Zhejiang University, ChinaReviewed by:
Yaoying Ma, Binghamton University, United StatesAmy L. Milton, University of Cambridge, United Kingdom
Copyright © 2019 Chen, Zhang, Li, Meng, Gong, Lu, Xue and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Lu, bGlubHVAYmptdS5lZHUuY24=; Yan-Xue Xue, eWFueHVleHVlQGJqbXUuZWR1LmNu; Jie Shi, c2hpamllQGJqbXUuZWR1LmNu
 Ya-Yun Chen
Ya-Yun Chen Li-Bo Zhang1,2
Li-Bo Zhang1,2 Lin Lu
Lin Lu Yan-Xue Xue
Yan-Xue Xue Jie Shi
Jie Shi