- 1Faculty of Engineering, Science and Research Branch, Islamic Azad University, Tehran, Iran
- 2Department of Computer Engineering, Ferdowsi University of Mashhad, Mashhad, Iran
- 3Faculty of Electrical and Computer Engineering, Semnan University, Semnan, Iran
- 4Neurological Disorders Research Center, Qatar Biomedical Research Institute, Hamad Bin Khalifa University, Qatar Foundation, Doha, Qatar
- 5Department of Medical Engineering, Mashhad Branch, Islamic Azad University, Mashhad, Iran
- 6Data Science and Computational Intelligence Institute, University of Granada, Granada, Spain
- 7Institute for Intelligent Systems Research and Innovation (IISRI), Deakin University, Geelong, VIC, Australia
- 8Faculty of Engineering and IT, University of Technology Sydney (UTS), Ultimo, NSW, Australia
- 9Faculty of Medicine, Institute of Biomedicine, University of Turku, Turku, Finland
- 10Department of Computer Science, College of Engineering, Effat University, Jeddah, Saudi Arabia
- 11Ngee Ann Polytechnic, Singapore, Singapore
- 12Department of Biomedical Informatics and Medical Engineering, Asia University, Taichung, Taiwan
- 13Department of Biomedical Engineering, School of Science and Technology, Singapore University of Social Sciences, Singapore, Singapore
Autism spectrum disorder (ASD) is a brain condition characterized by diverse signs and symptoms that appear in early childhood. ASD is also associated with communication deficits and repetitive behavior in affected individuals. Various ASD detection methods have been developed, including neuroimaging modalities and psychological tests. Among these methods, magnetic resonance imaging (MRI) imaging modalities are of paramount importance to physicians. Clinicians rely on MRI modalities to diagnose ASD accurately. The MRI modalities are non-invasive methods that include functional (fMRI) and structural (sMRI) neuroimaging methods. However, diagnosing ASD with fMRI and sMRI for specialists is often laborious and time-consuming; therefore, several computer-aided design systems (CADS) based on artificial intelligence (AI) have been developed to assist specialist physicians. Conventional machine learning (ML) and deep learning (DL) are the most popular schemes of AI used for diagnosing ASD. This study aims to review the automated detection of ASD using AI. We review several CADS that have been developed using ML techniques for the automated diagnosis of ASD using MRI modalities. There has been very limited work on the use of DL techniques to develop automated diagnostic models for ASD. A summary of the studies developed using DL is provided in the Supplementary Appendix. Then, the challenges encountered during the automated diagnosis of ASD using MRI and AI techniques are described in detail. Additionally, a graphical comparison of studies using ML and DL to diagnose ASD automatically is discussed. We suggest future approaches to detecting ASDs using AI techniques and MRI neuroimaging.
Introduction
A complex intricate network of millions of neurons is responsible for monitoring and controlling each part of the human body and brain (Sparks et al., 2002; Brieber et al., 2007; Ecker et al., 2015). These networks consist of many neurons that need to be directly interconnected and synchronized (Sato et al., 2012; Hernandez et al., 2015). It has been suggested that certain disorders in the human body arise when brain networks are incorrectly connected to manage a specific activity (Gautam and Sharma, 2020; Noor et al., 2020; Khodatars et al., 2021; Loh et al., 2022). Disorders of this type can be classified into three groups based on their psychological or neural characteristics and threaten the health of many individuals across the globe. Autism spectrum disorder (ASD) (Yang et al., 2022), schizophrenia (Sadeghi et al., 2022), attention deficit hyperactivity disorder (ADHD) (Bakhtyari and Mirzaei, 2022), epilepsy (Shoeibi et al., 2021a), Parkinson’s disease (Sahu et al., 2022), and bipolar disorder (BD) (Highland and Zhou, 2022) are some of the most known neurodevelopmental disorders.
Autism spectrum disorder is a neurodevelopmental disorder that manifests in childhood and causes a variety of challenges to individuals (Ecker et al., 2015). Those with ASD have difficulties with verbal and non-verbal communication, cognitive skills, social behavior, and entertaining activities (Aghdam et al., 2019; Ahmed et al., 2020a,b). ASD begins in the early stages of embryonic development, according to research results. Autism is thought to be caused by specific signal patterns in the cortex, abnormalities in the immune system, growth hormone fluctuations, and abnormalities in the neural mirror system in the embryonic stage (Chen et al., 2022; Jayanthy and Din, 2022). The overall ASD prevalence is one in 44 children aged 8 years, and ASD is around 4 times as prevalent among boys as among girls (Rakić et al., 2020; Maenner et al., 2021). In addition to lifelong social and adaptive disorders, one of the major consequences of autism is its negative impact on quality of life (Choi, 2017; Brown et al., 2018; Bengs et al., 2020; Byeon et al., 2020; D’Souza et al., 2020; Cao et al., 2021; Chen Y. et al., 2021; Chen H. et al., 2021; Chu et al., 2022). Therefore, early diagnosis and treatment of ASD are paramount for improving the quality of life of ASD children and their families (Kasari and Smith, 2013).
According to the DSM-3, mental health professionals originally divided autism into five categories, including Asperger’s syndrome, Rett syndrome, childhood disintegrative disorder (CDD), autistic disorder, and Pervasive developmental disorder-not otherwise specified (PDD-NOS) (Volkmar et al., 1992; Matson et al., 2009). Using this method, physicians observed the symptoms of autistic individuals and compared their observations to those in the DSM-3 to diagnose the specific type of autism (Volkmar et al., 1986, 1992; Matson et al., 2009). In 2013, the DSM-5 was published, making significant changes to the categorization of autism (Volkmar and Mcpartland, 2014). DSM-5 categorizes autism severity into three levels, and more information is given in Volkmar and Mcpartland (2014). According to DSM-5, the lower the severity level of autism, the less support the child requires. Autism individuals with the second and third severity levels show moderate to severe symptoms and therefore require more frequent support. Although the DSM-5 provides explanations of the autism spectrum, these explanations are incomplete and do not provide guidance on the specific support that autistic children may require. In addition, some individuals simply do not fall into any of these categories, and ASD can change and intensify over time (Kim et al., 2014; Volkmar and Mcpartland, 2014).
Early diagnosis of ASD is of utmost importance for specialist physicians (Akhavan Aghdam et al., 2018; Anirudh and Thiagarajan, 2019; Arya et al., 2020; Al-Hiyali et al., 2021; Almuqhim and Saeed, 2021; Bayram et al., 2021). Hereafter, clinical screening methods for diagnosing ASD are introduced, including autism diagnostic interview-revised (ADI-R), childhood autism rating scale (CARS), social responsiveness scale, autism diagnostic observation schedule (ADOS), and Joseph picture self-concept scale (Thabtah and Peebles, 2019). Clinical screening methods have been proven effective in diagnosing ASD and are of great interest to specialist physicians. Additionally, these methods assist in treating and preventing the development of ASD in the early stages (Thabtah and Peebles, 2019). As well as their many advantages, the mentioned methods always pose challenges for specialists (Thabtah and Peebles, 2019). These procedures involve long questionnaires, so they are very time-consuming and require different specialist physicians to analyze the questionnaire, which has led to many criticisms of clinical screening methods.
Additionally, some ASD diagnosis tools have been provided by neurologists and psychologists, including autism spectrum quotient (AQ), a modified checklist for autism in toddlers (M-CHAT), and a childhood Asperger syndrome test (CAST) (Thabtah and Peebles, 2019). Various items in these tools can be used to diagnose different types of autism; however, these methods face different challenges in the diagnosis of ASD (Thabtah and Peebles, 2019). These tools, for example, are not considered definitive screening methods for diagnosing ASD. Because, in most cases, ASD is diagnosed by them without the presence of a specialist physician (Thabtah and Peebles, 2019). However, some of these methods do not meet DSM-5 requirements (Thabtah and Peebles, 2019). Due to this, it is necessary to provide tools that are compatible with DSM-5.
Neuroimaging techniques are one group of methods used for diagnosing neurological and mental disorders such as ASD. These methods comprise structural and functional neuroimaging modalities, which are of special interest to physicians, particularly in diagnosing various brain disorders (Shoeibi et al., 2021b,2022c). The fMRI is one of the major functional neuroimaging methods that records data in a non-invasive manner. fMRI has a high spatial resolution, making it an excellent method for examining functional connectivity in the brain. fMRI data is classified into two categories: T-fMRI and rs-fMRI. Furthermore, fMRI data are composed of a 4-dimensional tensor, which permits the 3D volume of the brain to be segmented into smaller areas, and the activity of each area is recorded for a predetermined time period. Although fMRI has provided satisfactory results in diagnosing a variety of brain disorders, these techniques are costly and too sensitive to motion artifacts (Ghassemi et al., 2020; Shoeibi et al., 2022b).
Structural and DTI have been used to examine brain anatomy and the interaction between brain regions, respectively. The structural neuroimaging modalities offer the advantage of cost-effectiveness and the availability of imaging protocols in most treatment facilities (Ghassemi et al., 2020). Physicians use sMRI modalities to diagnose autism in autistic individuals using (i) geometric features and (ii) volumetric features, which physicians have used to demonstrate that autistic people demonstrate superior brain development in comparison to normal people (Brambilla et al., 2003; Siewertsen et al., 2015; Zürcher et al., 2015; Zhang and Roeyers, 2019). Hazlett et al. (2005) studied the brain structure of 51 autistic children and 25 normal children (1.5–3 years of age). Their findings indicated that the Cerebellum white matter volume of autistic children was 2–4 times greater than that of normal children.
Although MRIs offer many advantages, MRI artifacts reduce the accuracy with which clinicians are able to diagnose autism. Additionally, ASD individuals’ MRI data is recorded with multiple slices and different protocols. Consequently, it takes considerable time to examine all MRI slices accurately, and clinicians should be highly precise. The fatigue of the physician may lead to an incorrect diagnosis of ASD in many cases. In addition, MRI data is problematic because most physicians are inexperienced in interpreting these images and may find diagnosing ASD in its early stages difficult.
Numerous treatment methods have also been provided for ASD patients so far, some of which are listed here. Transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) are two non-invasive methods to diagnose and treat various neurological and mental disorders such as ASD (Khodatars et al., 2021). Using them, the areas of the brain where ASD occurs are selected by specialist physicians. Electrical pulses are then applied to these areas to treat or control ASD (Khodatars et al., 2021). Additionally, some researchers have provided rehabilitation systems based on AI techniques to treat ASD. For example, Cai et al. (2013) presented a virtual reality (VR) system for treating ASD. They proposed a VR program for people with ASD to interact with dolphins in their work. This tool enables people with ASD to virtually be at the pool as dolphin trainers, aiming to help people with ASD learn different types of non-verbal communication through hand movements with virtual dolphins.
To improve the accuracy of ASD diagnosis, AI techniques can be used. The use of AI in diagnosing various diseases has been studied (Nogay and Adeli, 2020; Ahmadi-Dastgerdi et al., 2021; Shoeibi et al., 2022a). Several studies have demonstrated that AI techniques, along with MRI neuroimaging modalities, increase the accuracy of ASD diagnosis (Nogay and Adeli, 2020; Ahmadi-Dastgerdi et al., 2021). An increasing number of studies have been conducted on detecting ASD using ML and DL methods. Researchers first demonstrated that ASD could be diagnosed from ML using MRI neuroimaging technologies (Shoeibi et al., 2022a). Based on ML algorithms, feature extraction, dimension reduction, and classification algorithms in CADS are selected through trial and error (Parikh et al., 2019; Alizadehsani et al., 2021). Choosing an appropriate algorithm for each CADS section can be challenging without adequate knowledge of AI (Mohammadpoor et al., 2016; Parikh et al., 2019; Alizadehsani et al., 2021; Wang et al., 2021a,c). Furthermore, ML techniques are not suitable for small data sets (Ghassemi et al., 2021). Therefore, these methods do not contribute to developing software for detecting ASDs using MRI neuroimaging modalities.
Various studies are being conducted to diagnose various diseases and disorders by using these methods to overcome the challenges inherent in ML techniques (Noor et al., 2019; Al-Shoukry et al., 2020; Altinkaya et al., 2020; Yao et al., 2020). For example, in contrast to ML methods, DL uses deep layers for feature extraction and classification and requires fewer implementation steps in diagnosing ASD (Goodfellow et al., 2016). Furthermore, DL-based CADS can be more efficient and accurate with large input data.
An overview of studies relating to the detection of ASD using MRI neuroimaging methods is presented in this comprehensive systematic review. The first step was to systematically review all publications on ASD detection using MRI modalities and ML techniques. A recent review by the authors of this review discussed the use of different neuroimaging modalities and DL architectures to detect ASD (Khodatars et al., 2021). Supplementary Appendix A presents a review paper describing ASD detection in different neuroimaging modalities using DL techniques to compare ML and DL studies.
The following sections describe the following. Section 2 is a search Strategy based on PRISMA guidelines. Section 3 reviews the review papers in AI techniques for ASD diagnosis. Section 4 describes the CADS based on AI to detect ASD from MRI neuroimaging images. Section 5 presents a comparison between ML and DL studies to ASD detection using MRI modalities. Section 6 examines the most critical challenges for detecting ASD using AI methods. Future directions and conclusion sections are presented in sections 7 and 8, respectively.
Search strategy based on PRISMA guideline
The PRISMA protocol was used to select and review papers in this study (Sadeghi et al., 2022). Papers on the diagnosis of ASD by MRI modalities and AI models (ML and DL) published from 2016 to 2022 were included in this study. In this review paper, various citation databases, including IEEE, Wiley, Frontiers, ScienceDirect, SpringerLink, ACM, and ArXiv were used to search for papers in the field of ASD detection. Furthermore, Google Scholar has been used to search for the article entirety. Here are the keywords, including “ASD classification,” “Feature extraction,” “fMRI,” “sMRI,” and “Autism Spectrum Disorder,” which were used to search for articles relating to the diagnosis of ASD using ML algorithms. To search for articles related to DL, the keywords used were “Autism Spectrum Disorder,” “ASD,” fMRI,” “sMRI,” and “Deep Learning.”
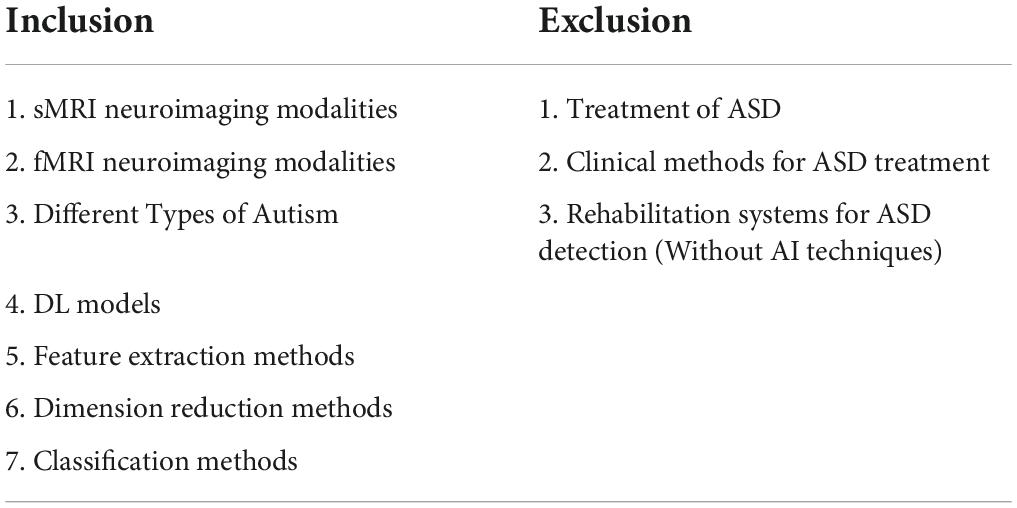
As stated above, papers were selected and reviewed based on the PRISMA protocol at three different levels. In the first level, 34 out of 316 downloaded papers were eliminated as they were out of the scope of this study. Then, 28 papers were also excluded as they did not use MRI datasets in the ASD diagnosis, followed by excluding further 21 papers due to no use of AI techniques. Therefore, 233 papers were finally selected and used in this review paper. Figure 1 shows the selection procedure of papers based on the PRISMA protocol on three levels. The key criteria for the inclusion and exclusion of papers on the ASD diagnosis based on the PRISMA protocol are shown in Table 1.
Artificial intelligence techniques for autism spectrum disorder diagnosis
For researchers in cognitive sciences, autism is a well-recognized neurodevelopmental disorder with a high prevalence in recent years. Challenges in the ASD diagnosis for physicians have resulted in extensive studies on this brain disorder. Scholars in AI, and cognitive sciences seek to develop a real diagnostic tool for ASD using various AI techniques. Accordingly, extensive studies have focused on ASD diagnosis using neuroimaging modalities and AI techniques, outlined in this section by reviewing articles in the field of ASD diagnosis using these techniques.
Pagnozzi et al. (2018) reviewed 123 articles on ASD diagnosis using sMRI modalities and reported further developments in some brain areas of ASD individuals than those of HC. They also explained that ASD caused changes in the structure of patients’ brains, including increased volume of frontal and temporal lobes, increased thickness of the frontal cortex, and increased cerebrospinal fluid volume. This study assists scholars in applying AI techniques in ASD diagnosis from sMRI modalities in future studies.
Nogay and Adeli (2020) published a review article on ASD diagnosis using brain imaging and ML techniques. They reviewed studies on ASD diagnosis for sMRI, fMRI and combined data using ML techniques and found a higher accuracy of ASD diagnosis at younger ages. They hope to develop a practical ASD diagnostic tool based on ML techniques from MRI modalities.
In another study, Xu et al. (Shoeibi et al., 2022a) reported methods and tools associated with ASD diagnosis from MRI data based on ML techniques. Initially, they introduced the most important ML-based algorithms, including feature extraction, feature selection and reduction, training and test models, and evaluation parameters.
Parlett-Pellerit et al. (Ahmadi-Dastgerdi et al., 2021) reviewed studies on unsupervised ML techniques for ASD diagnosis. In this study, various clinical data and medical imaging data were discussed for ASD diagnosis using unsupervised ML techniques.
The most important feature selection and classification algorithms for ASD diagnosis were studied in Rahman et al. (2020) paper. Their input data comprises various psychological tests such as ADOS and MRI modalities. They claimed that this study could assist scholars in developing future studies on ADS diagnosis.
A review article on the diagnosis of ASD and ADHD using AI techniques was published by Eslami et al. (2021a). They discussed DL and ML-based studies on ASD and ADHD diagnosis from MRI modalities and the most important AI techniques (DL and ML). In the ML section, the authors presented the most important feature extraction techniques, such as effective dynamic connectivity, and outlined various popular DL techniques.
Khodatars et al. (2021) presented a review paper on ASD diagnosis and rehabilitation using DL techniques. They initially introduced the public neuroimaging modalities datasets, such as MRI, pre-processing techniques, and DL models, an ASD diagnosis. Then, they summarized the studies conducted in this field in a table. They also discussed studies in the field of autism rehabilitation using DL techniques.
In this section, the most important review papers on ASD diagnosis from various data and AI techniques were discussed. In our study, ASD diagnosis papers using MRI data and various AI techniques (ML and DL) were reviewed. This paper reports all ASD diagnosis articles from 2010 to 2022. Also, the most important challenges and future works for diagnosing ASD from MRI modalities are presented. To the best of our knowledge, no similar review article has been provided, and our review article has outstanding innovations.
Computer-aided design systems for aided design systems diagnosis by magnetic resonance imaging neuroimaging modalities
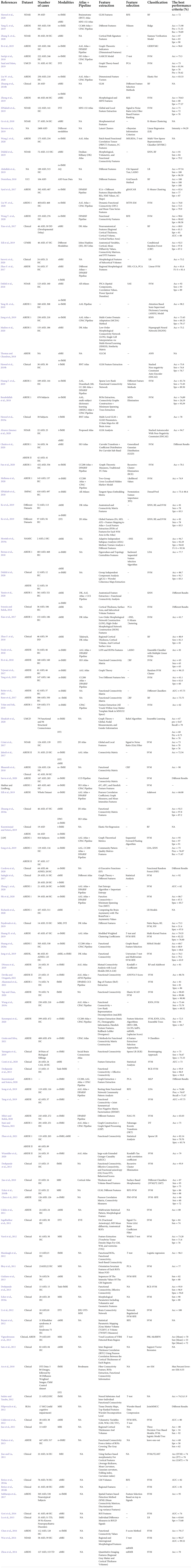
The application of CADS based on AI techniques is presented in this section and illustrated in Figure 2. The steps involved in CADS using ML for ASD detection are outlined in Figure 2. As shown in Figure 2, CADS input consists of datasets containing MRI modalities. Standard preprocessing (low-level) methods for MRI neuroimaging modalities were demonstrated as a second step. Next, we will discuss these preprocessing methods for MRI neuroimaging modalities. The third step involves feature extraction. Feature reduction or selection techniques (dimension reduction) are considered to be the fourth step in the CADS based on ML. The final step involves the use of classification algorithms. The only difference between DL-based and ML-based CADS is the feature extraction to the classification step. This procedure is carried out in deep layers in CADS based on DL. This enables the extraction of features from MRI data without the user’s intervention. Moreover, in CADS based on DL, diagnostics of ASD may be possible in case there are more input data, allowing the development of actual software for the detection of ASD. The details of ASD detection from MRI neuroimaging modalities using DL architectures are given in Supplementary Appendix (A). Here we present the details of CADS based on ML and some of the most important algorithms in each section.
Magnetic resonance imaging neuroimaging autism spectrum disorder datasets
Various MRI modalities datasets for ASD diagnosis are available to researchers, including UCI (Last Access 19/07/2022a), NDAR (Last access 19/07/2022b), AGRE (Last access 19/07/2022d), NIMH Repository and Genomics Resource, n.d. (Last access 19/07/2022f), Gene Expression Omnibus [GEO], n.d. (Last access 19/07/2022e), SSC (Last access 19/07/2022h), Simons VIP SFARI (Last access 19/07/2022g), and autism brain imaging data exchange (ABIDE/) (Khodatars et al., 2021). Table 2 and Supplementary Table 1 summarize studies of ASD diagnosis using ML and DL techniques. As can be seen, the ABIDE database has a special place in research. ABIDE is recognized as the most complete and freely available database of MRI modalities for the automatic diagnosis of ASD (Khodatars et al., 2021). This dataset has two parts, ABIDE 1 and ABIDE-II, containing sMRI data, rs-fMRI data, and phenotypic data. 1112 datasets are involved in ABIDE I, and 1114 datasets are included in ABIDE II (Khodatars et al., 2021). ABIDE 1 also contains preprocessed data from MRI modalities for research, known as the preprocessed connectomes project (PCP) (Khodatars et al., 2021). Additionally, other available datasets, such as NDAR, UCI, and NRGR, have been used in ASD diagnostic. The results show that these datasets have been able to achieve satisfactory results. The datasets used for each study are summarized in Table 2 and Supplementary Table 1.
Preprocessing techniques for functional and structural modalities
Preprocessing techniques are needed to help CADS to achieve promising results. The sMRI and fMRI neuroimaging modalities have implemented fixed preprocessing steps using different software packages. The most common software packages are brain extraction tools (BET) (Soltaninejad et al., 2014), FMRIB software libraries (FSL), statistical parametric mapping (SPM), and FreeSurfer (Khodatars et al., 2021). The following is the standard preprocessing steps for fMRI and sMRI neuroimaging modalities. Some of them are common for both fMRI and sMRI modalities, so we will introduce them in the fMRI-related section. Figure 3 shows the standard fMRI and sMRI techniques. Also, the pipeline preprocessing techniques for ABIDE datasets will be introduced in another section.

Figure 3. Standard preprocessing methods for MRI neuroimaging modalities: (A) preprocessing for fMRI data, (B) preprocessing for sMRI data.
The standard Preprocessing is a necessary step in fMRI, and if preprocessing is not carried out properly, it will lead to reduced performance of automated diagnosis of ASD. This step aims to extract regions suspected of having ASD and examine the function of brain neurons in those regions. The preprocessing steps of fMRI include delineation of the brain region, removal of the first few volumes, slice timing correction, inhomogeneity correction, motion correction, intensity normalization, temporal filtering, spatial smoothing, and ultimately registration standard atlas (Khodatars et al., 2021). As mentioned earlier, this step is usually carried out by a toolbox, including BET (Khodatars et al., 2021), FSL (Khodatars et al., 2021), SPM (Anand and Sahambi, 2010; Khodatars et al., 2021), FreeSurfer (Lee and Xue, 2017; Khodatars et al., 2021), etc. In reference (Khodatars et al., 2021), the details for standard preprocessing steps of fMRI modalities are elaborately explained.
The preprocessing of sMRI data extracts helps physicians examine regions with suspected ASD more accurately. Besides, low-level sMRI preprocessing methods help AI-based CADS to process important information. This process increases the accuracy and efficiency of diagnosis of ASD CADS. The most important standard sMRI covers intensity standardization, de-oblique, re-orientation, Denoising, and segmentation (Khodatars et al., 2021). In reference (Khodatars et al., 2021), each step of standard preprocessing for sMRI modalities is explained.
Pipeline methods
The pipelines are a preprocessed version of ABIDE data using standard preprocessing procedures, which researchers can use to avoid the problems of variations in the output between different studies as a result of preprocessing. The most popular ABIDE pipelines include neuroimaging analysis kit (NIAK), data processing assistant for rs- fMRI (DPARSF), the configurable pipeline for the analysis of connectomes (CPAC), and connectome computation system (CCS) (Khodatars et al., 2021).
Feature extraction
Representing data that allows ML algorithms to reason about them is the key to any related research. If this is not done, high performance cannot be achieved. Most commonly used feature extraction schemes for fMRI and sMRI are statistical, texture, morphological, non-linear, graph, functional connectivity, and structural connectivity schemes.
Statistical features
Autism spectrum disorder is typically detected with MRI modalities using statistical features, the most straightforward group of features. Despite their simplicity, these features are usually informative and can also serve as a benchmark for evaluating other methods of feature extraction as well. Additionally, the process of extracting these features is not time-consuming in comparison to other methods. However, these methods do not reveal non-linear or subtle patterns in data. Using statistical features for ASD diagnosis, Dekhil et al. (2019) extracted various statistical features from MRI data and then applied KNN and SVM algorithms in the classification step. The authors reported 81% accuracy.
Texture features
As a group of features, spatial patterns form an indispensable group, possibly the most important group, since the cognitive system of the human is mostly dependent on them. Gray-level co-occurrence matrix (GLCM) (Jafarpour et al., 2012) feature extraction is one of the most widely used methods in various research studies (Thomas and Chandran, 2018) among various textures-based features. Haweel et al. (2020) presented an ASD diagnostic method based on MRI data. Texture features and the RFE technique were used in the feature extraction and feature selection steps. Then, the authors used the RF technique for classifying features and reached an accuracy of 72%. In another study, scholars used various methods, such as Haralick, in the feature extraction step from sMRI data. Then, the authors tested different feature selection methods and reached an accuracy of 72.5%.
Morphological features
Morphological operation is an important feature extraction technique frequently used in image processing (Usha and Perumal, 2019). In these methods, features are extracted from the appearance and shape of the image. Morphological operation is often used to process binary images, but they can also be used for gray and color-level images (Gupta et al., 2019). Morphological features are also commonly used for diagnosing brain diseases from sMRI modalities. Zheng et al. (2019) proposed the idea of ASD diagnosis using morphological features. After feature extraction, RFE and SVM were tested for feature selection and classification, respectively. An accuracy of 78.63% was obtained.
Non-linear features
A non-linear characteristic of biological data is emphasized when considering non-linear features. The performance of CADS for ASD is significantly enhanced through the use of these features (Anand and Sahambi, 2010). In reference (Mellema et al., 2020), non-linear-based features of likelihood are used to detect autism using MRI neuroimaging methods. Entropies are one of the most important non-linear methods that are widely used to extract features from signals and brain images (Georgiadis et al., 2008). Functional imaging modalities are non-linear and chaotic, which has led researchers to use entropy-based non-linear features to diagnose brain disorders (Saritha et al., 2013; Wang et al., 2022). Zhang L. et al. (2020) introduced a novel ASD diagnostic method using fMRI data and a new entropy method. This study initially used fast entropy for feature extraction from preprocessed fMRI data. Then, they used the SVM algorithm for feature classification and obtained satisfactory results.
Graph features
This group of features is highly relevant to the analysis of MRI data. Graph-based features are derived first by shaping the data into a graph, and then, from the constructed graph, local and global features are extracted (Lee and Xue, 2017). Researchers have explored graph features to diagnose ASD using fMRI data in many studies. Bi et al. (2019) employed rs-fMRI from the ABIDE database for ASD diagnosis using graph and genetic-evolutionary random SVM cluster (GERSVMC) for feature extraction and classification steps, respectively, and obtained an accuracy of 62%. Saad and Islam (2019) presented an ASD diagnostic method based on graph features in another study. After feature extraction via the graph method, PCA and SVM techniques were used for feature reduction and classification, which resulted in an accuracy of 75% for ASD diagnosis.
Connectivity matrix
In order to process sMRI and fMRI neuroimaging images, feature extraction methods based on connectivity matrix methods are typically employed (Zeng et al., 2018; Yeh et al., 2021). Such features provide information about the brain’s structure and function. The functional connectivity matrix (FCM) (Zhou and Wang, 2007; Yan and Zhang, 2015) and structural connectivity matrix (SCM) (Yang et al., 2016; Ma et al., 2019) are the measures employed for fMRI and sMRI modalities, respectively. Connectivity features are mostly used in diagnosing brain disorders. Table 2 and Supplementary Table 1 outline studies on ASD diagnosis from MRI modalities using various AI techniques. Table 2 shows that connectivity methods are most frequently used for feature extraction from MRI modalities. Liu W. et al. (2020) used dynamic functional connectivity (DFC) in the feature extraction step of rs-fMRI data. The feature selection step was also conducted by the MTFS-EM method. Finally, they used the SVM method for classification and obtained an accuracy of 76.84%. In another study, Mathur and Lindberg, utilized DFC and static functional connectivity (SFC) in the feature extraction step. Then, the SVM was tested for connectivity-based classification of features. Authors could finally obtain satisfactory results for ASD diagnosis using connectivity features.
Feature reduction/selection methods
It has been shown that increasing the number of extracted features can help algorithms to represent data in a more meaningful and robust way; however, the curse of dimensionality (Fodor, 2002) may cause it to backfire and reduce performance. Several methods for reducing dimensionality and selecting features have been proposed to prevent this from occurring. In addition, these methods are widely used to increase the performance of CADS for detecting autism spectrum disorders. Several methods have previously been used in research papers, including principal component analysis (PCA) (Wold et al., 1987), recursive feature elimination (RFE) (Yan and Zhang, 2015), T-test (Zhou and Wang, 2007), autoencoder (AE) (Yang et al., 2016), conditional random forest (CRF) (Ma et al., 2019), Chi-squared (Ye and Yang, 2010), and least absolute shrinkage and selection operator (LASSO) (Muthukrishnan and Rohini, 2016). The following is a brief description of these methods.
Principal component analysis
Principal component analysis is arguably the most common dimensionality reduction method (Wold et al., 1987). It works by finding and representing data by the principal components, i.e., the vectors that preserve the most data variance. One of the benefits of PCA is its ability to find a minimal number of features required to preserve a specified variance ratio (Wold et al., 1987). Principal component analysis (PCA) is one of the most popular feature reduction techniques in medical applications and has also been used in ASD diagnosis research (Zhao F. et al., 2017; Soussia and Rekik, 2018; Saad and Islam, 2019; Kazeminejad and Sotero, 2020).
Recursive feature elimination
Recursive feature elimination is more of a wrapper-type algorithm, meaning that it is applied to a classification algorithm to find the best subset of features. As the name explains, this algorithm works by eliminating features one by one to reach the optimal number. First, a classification algorithm is trained on the dataset, ranking feature importance’s. The least important feature is then eliminated and repeated until the number of remaining features matches the desired number (Yan and Zhang, 2015). Haweel et al. (2020) proposed a novel ASD diagnostic method using the GLM feature extraction technique. After feature extraction from MRI data, the RFE technique was used for feature reduction. The RF method was also tested in the classification step with an accuracy of 72%.
T-test
To find the best set of features, T-test calculates a score for each feature, then ranks them based on that score and picks the top features as selected ones. The score shows whether the values of a feature for a class are significantly different from those for another class by calculating the mean and standard division (STD) of each feature in each class (Zhou and Wang, 2007). A new ASD diagnostic method from MRI data was introduced by Sartipi et al. (2018). First, the graph technique was used for feature extraction from sMRI modalities. Then, they applied the T-test and SVM algorithms for the feature selection and classification steps and acquired an accuracy of 75%.
Chi-squared
Chi-Square is suitable when the features are categorical, and the target variable is also categorical, such as classification. Chi-Squared measures the degree of association between two variables; thus, features that connect with the targets can be picked (Ye and Yang, 2010). When the features are numerical, we can use a T-test, or Chi-Square can be used for the numerical variable by discretizing them (Ye and Yang, 2010). In reference (Dekhil et al., 2019). The authors proposed a new ASD diagnostic method using various ML techniques from MRI data. Various methods were used for feature extraction. Then, the Chi-squared method was tested for the feature selection step. Next, the LR classification algorithm was applied, which resulted in a promising performance.
Least absolute shrinkage and selection operator
Least absolute shrinkage and selection operator is mainly a regression method; however, this algorithm can also be used for feature selection (Muthukrishnan and Rohini, 2016). Notably, linear regression with L1 regularization is called Lasso. After training, the lasso assigns a weight to each feature for the regression (Muthukrishnan and Rohini, 2016). Using these weights, there are two methods to pick the best features, first, pick the K highest valued weights; second, pick all the weights which have a value higher than a specified threshold (Muthukrishnan and Rohini, 2016). Fredo et al. (2018) proposed a new ASD diagnostic method based on Hons and Lon features. Their paper used LASSO and SVM methods for feature selection and classification. They reported an accuracy of 81%.
Classification methods
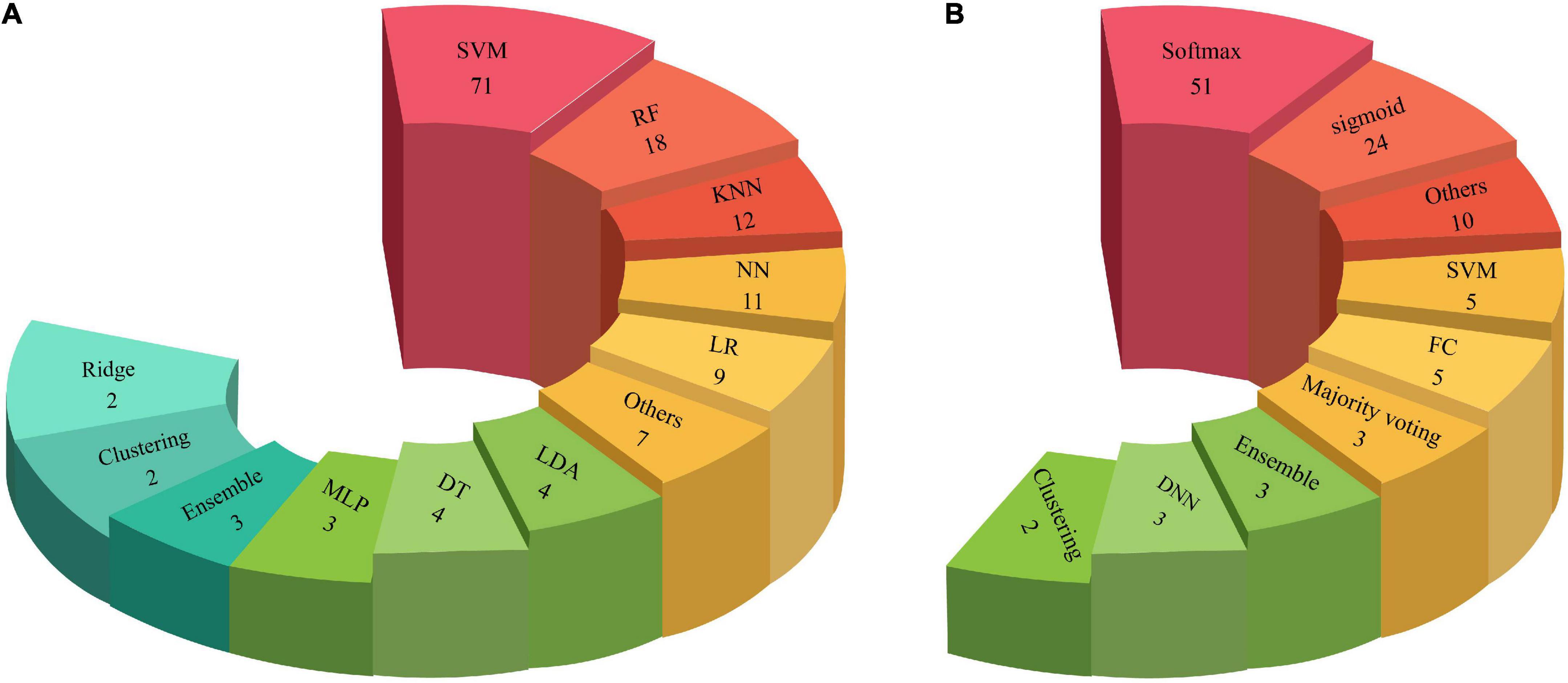
This section discusses the various classification algorithms used in CADS for ASD. As mentioned earlier, classification is the last step in a CADS based on ML methods. Support vector machine (SVM) (Noble, 2006; Suthaharan, 2016), linear discriminant analysis (LDA) (Zhang et al., 2007), k-nearest neighbor (KNN) (Liao and Vemuri, 2002), and random forest (RF) (Oshiro et al., 2012) are arguably among the most popular methods used in CADS created for ASD. Table 2 and Supplementary Table 1 show the classification algorithms used for ASD detection. A summary of classification algorithms used for automated detection of ASD are presented below.
Support vector machine
Support vector machines are among the oldest classification and has been widely used in many applications (Noble, 2006; Suthaharan, 2016). SVM tries to find the best hyperplane to separate data points; however, it only needs the dot product between every two data points (Noble, 2006; Suthaharan, 2016). Consequently, to transform data into another space, only a function that gives the dot product of two points in that space would suffice; this is also named kernel trick and is used widely in other fields. Using an appropriate kernel, SVM can usually yield high classification performances (Noble, 2006; Suthaharan, 2016).
Random forest
Random forests are an ensemble learning-based method proposed to make the decision trees robust to outliers (Oshiro et al., 2012). The basic idea is to train many trees and determine the final output based on voting among their outputs. To make the final results robust, each tree is trained only on a fraction of the data, and also each tree sees a fraction of all features. The picked ratio for both of these is the square root of the available number.
Linear discriminant analysis
Used as a tool for dimension reduction, classification, and data visualization (Zhang et al., 2007). It is simple and robust and yields interpretable classification results (Zhang et al., 2007). It works by dividing the data space into K disjoint regions that represent all the classes; then, in the testing phase, the label is determined by finding the region in which the data belongs. LDA can be used as the first benchmarking baseline before other, more complicated ones are employed for real-world classification problems (Zhang et al., 2007).
K-nearest neighbor
This classifier is among the simplest yet efficient algorithms; its main idea is to assign the label of each data point based on the label of those closest (Liao and Vemuri, 2002). Consequently, there is no training phase; however, for each test subject, the distance to all training points must be calculated, which scales with the size of the dataset; thus, this method is not applicable to enormous datasets. After finding the closest points, the final label is determined using a voting scheme (Liao and Vemuri, 2002).
Challenges in detecting autism spectrum disorder with magnetic resonance imaging neuroimaging modalities and artificial intelligence techniques
This section introduces the challenges facing ASD detection from MRI neuroimaging modalities and AI techniques. The challenges mentioned in this section cover dataset limitations, lack of access to multimodal datasets, AI techniques, and suitable hardware resources. They are briefly described below.
Unavailable magnetic resonance imaging neuroimaging datasets with different autism spectrum disorder patient
All datasets available involve two classes of ASD and control fMRI or sMRI modalities (Heinsfeld et al., 2018; El-Gazzar et al., 2019; Felouat and Oukid-Khouas, 2020). However, there are different types of ASD, and this poses a serious obstacle for researchers in AI wishing to develop systems that can detect different types of disorders. Datasets with different types of ASD can help pave the way for accurate diagnosis of various types of ASD.
Unavailable multi-modalities datasets for autism spectrum disorder diagnosis
In medical research, specialists have shown that neuroimaging multimodalities can effectively improve diagnosis of brain disorders. Neuroimaging modality fusion is one of the newest methods for diagnosing brain disorders such as ASD (Jones et al., 2011), SZ (Bora et al., 2011), and ADHD (Sibley et al., 2022). Physicians usually use MRI data with other neuroimaging modalities to diagnose brain disorders. To diagnose neurological and mental disorders, fMRI-MEG (Kober et al., 1993), MRI-PET (Loeffelbein et al., 2012), and EEG-fMRI (Valdes-Sosa et al., 2009) are the most important multimodalities. Unfortunately, the neuroimaging multimodalities datasets are not available for studies on ASD diagnosis. Such datasets might lead to practical and interesting studies in ASD diagnosis.
Challenges in artificial intelligence algorithms in diagnosing autism spectrum disorder
Computer-aided design systems based on ML algorithms are highly time-consuming and complex to design. However, if the appropriate algorithms are selected, it can accurately diagnose ASD (Iglesias et al., 2017; Khosla et al., 2019; Hiremath et al., 2020; Leming et al., 2020, 2021). DL methods automatically perform the steps from feature extraction to classification. By using intelligent feature extraction, DL eliminates the need for supervision on features, which may reduce the performance of a CADS based on DL compared to ML. Therefore, when ML methods are combined with DL, promising results can be obtained in CADS for the diagnosis of ASD.
Challenges in hardware’s
The lack of access to appropriate hardware resources is another problem encountered by researchers in the field of automated ASD detection. ASD detection datasets that are available publicly, such as ABIDE, have a lot of data; this poses many challenges for storing and processing these datasets on ordinary computers. In contrast, research in CAD implementation on cloud servers has not been seriously conducted to eliminate hardware resource problems. As a result, cloud servers are not yet extensively used for data storage and processing. Recently, some DL models called deep compact CNN models have been introduced to be implemented on hardware systems with limited resources (Zhang Z. et al., 2020). Deep compact-size CNN models require fewer hardware sources than other CNN methods (Tian et al., 2018; Wong et al., 2019). Some deep compact-size CNN methods include FBNetV3 (Srinivas et al., 2019), MobileNet (Michele et al., 2019), and TinyNet (Wu et al., 2018).
Discussion
This paper presents and compares the research about automated ASD detection with MRI neuroimaging modalities and AI methods. First, this section comprehensively compares the conducted studies on ASD detection using ML and DL techniques. In subsection one, the number of studies conducted annually in ASD detection from MRI neuroimaging modalities using different ML and DL techniques is presented. In subsection two, the MRI datasets employed in studies on the automated diagnosis of ASD using ML and DL techniques are compared. In subsection three, the number of MRI studies conducted annually on ASD detection from MRI neuroimaging modalities is discussed. The employed atlas in ML and DL studies for ASD detection is introduced in subsection four. Finally, section five discusses MRI pipeline techniques in the diagnosis of ASD research using ML and DL methods. Ultimately, different classification algorithms for ML and DL-based diagnosis of ASD are compared.
Comparison between the numbers of papers published each year for machine learning and deep learning research
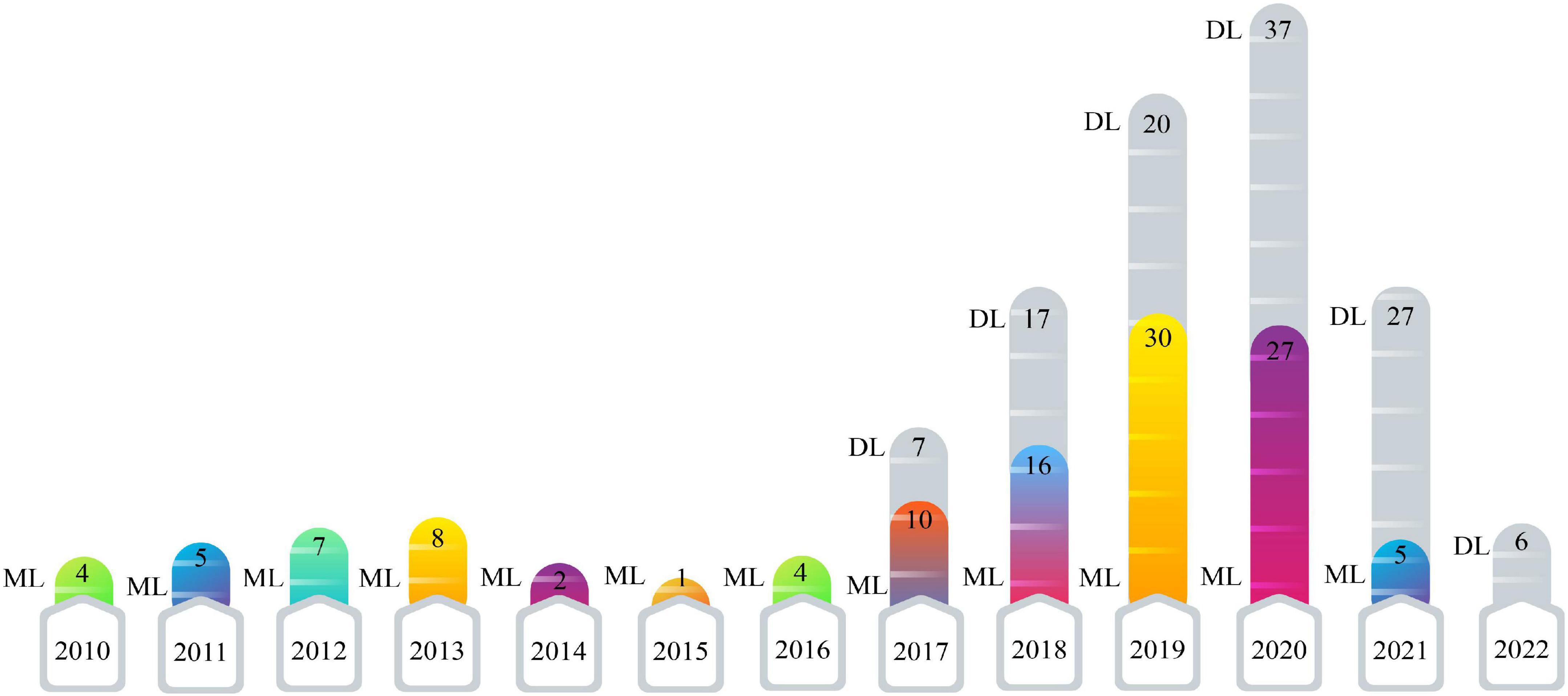
This section presents the number of published papers annually on ASD detection using AI techniques. Studies on the ASD detection from MRI modalities and ML and DL techniques began in 2017. Table 2 represents the papers on ASD detection in MRI neuroimaging modalities using ML methods. In addition, articles in ASD detection in MRI neuroimaging modalities using DL techniques are introduced in Supplementary Appendix A. Figure 4 illustrates the number of papers published annually on ML and DL techniques for ASD detection.
As demonstrated in recent years, researchers’ interest in using DL architectures has significantly grown compared to ML techniques. According to Figure 4, DL models are used more in studies on the automated diagnosis of ASD with MRI modalities than ML models. Therefore, implementing CADS based on DL techniques is promising for developing applied software for ASD detection with MRI neuroimaging modalities in the future. For automated diagnosis of ASD with MRI modalities, various datasets are proposed in ABIDE. Besides, various toolboxes are available for the implementation of different DL models. These reasons are the foundation for many studies on the automated diagnosis of ASD using DL models.
Comparison between the numbers of datasets used in the machine learning and deep learning research
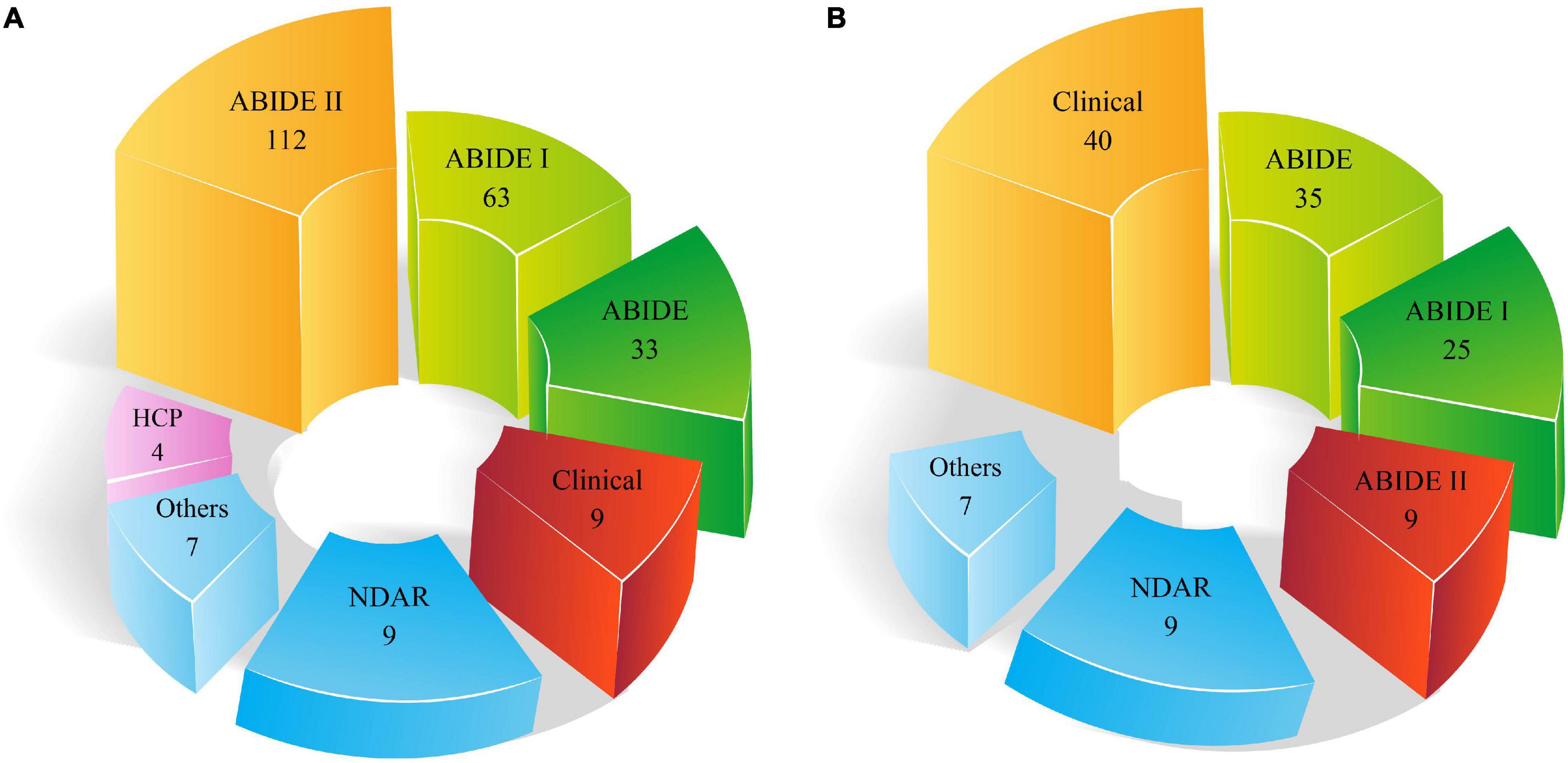
As stated in the neuroimaging modalities section, limited datasets are accessible. ABIDE is the most important dataset available in this field, which includes two datasets, ABIDE I and ABIDE II. Figure 5 demonstrates the types of datasets employed in the automated ASD diagnostic research using DL and ML techniques.
It can be noted from Figures 5A,B that a greater number of ABIDE datasets are employed in studies on the automated diagnosis of ASD. The major reason for the wide use of this dataset in various studies on the automated diagnosis of ASD is the availability of many subjects and different MRI modalities.
Comparison between the numbers of neuroimaging modalities used in the machine learning and deep learning research
The different structural and functional MRI neuroimaging modalities and ML and DL methods play an essential role in automated ASD detection. Table 2, reports studies on automated ASD detection using ML techniques and different MRI neuroimaging modalities have been presented. Moreover, Supplementary Table 1 discusses ASD detection using DL techniques. Figures 6A,B describes the annual research carried out to detect automated ASD using sMRI and fMRI neuroimaging modalities.
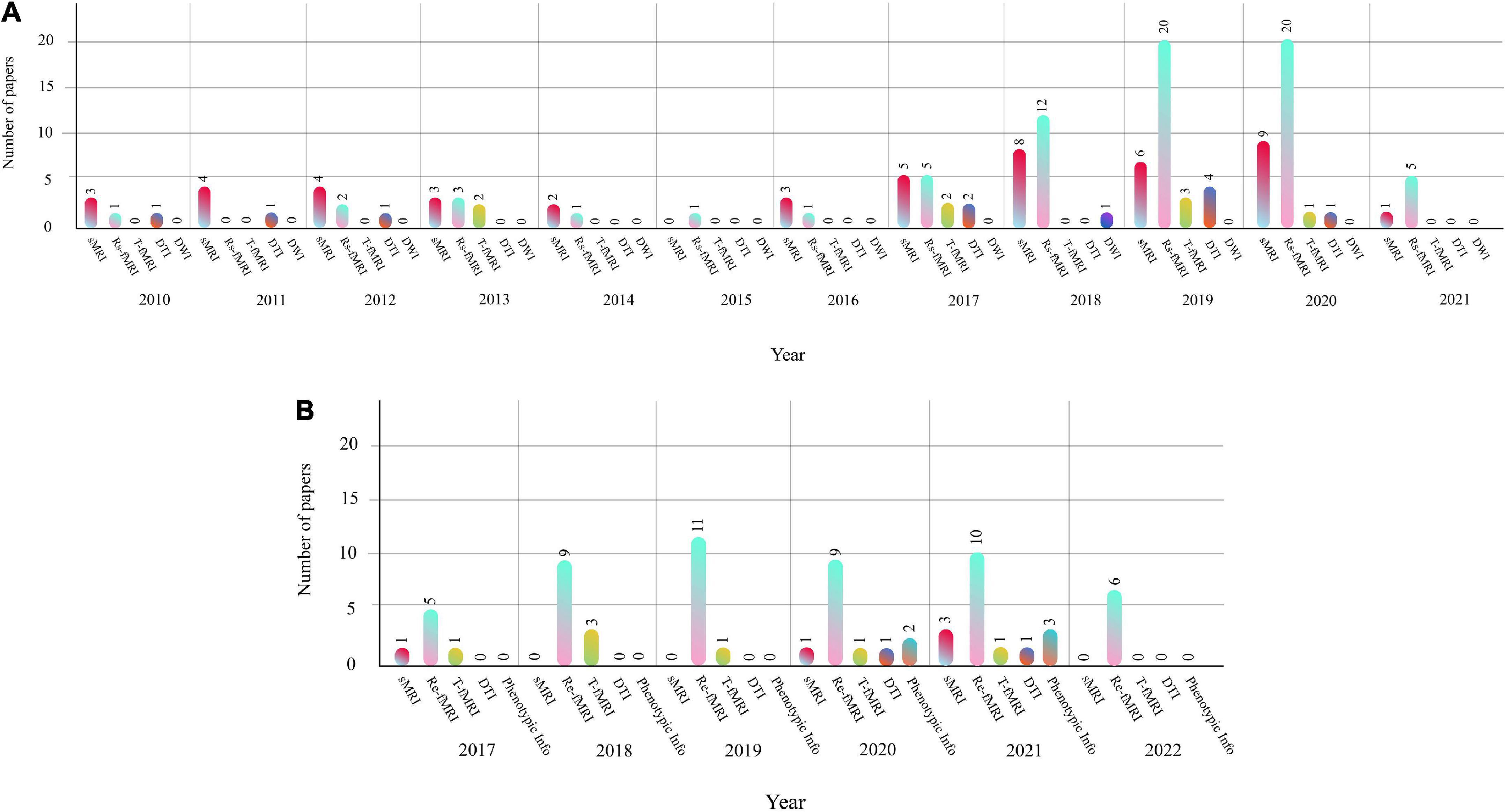
Figure 6. (A) Shows the number of MRI neuroimaging modalities used in the CADS based on ML methods. (B) Shows the number of MRI neuroimaging modalities used in the CADS based on DL methods.
As shown in Figures 6A,B, the rs-fMRI modalities are most used in studies on ASD detection using ML and DL methods. As mentioned earlier, ASD is a neurological disorder that negatively affects brain function. Accordingly, researchers have used rs-fMRI modalities most widely in studies on ASD detection using AI methods.
Comparison between the numbers of atlases used in the machine learning and deep learning research
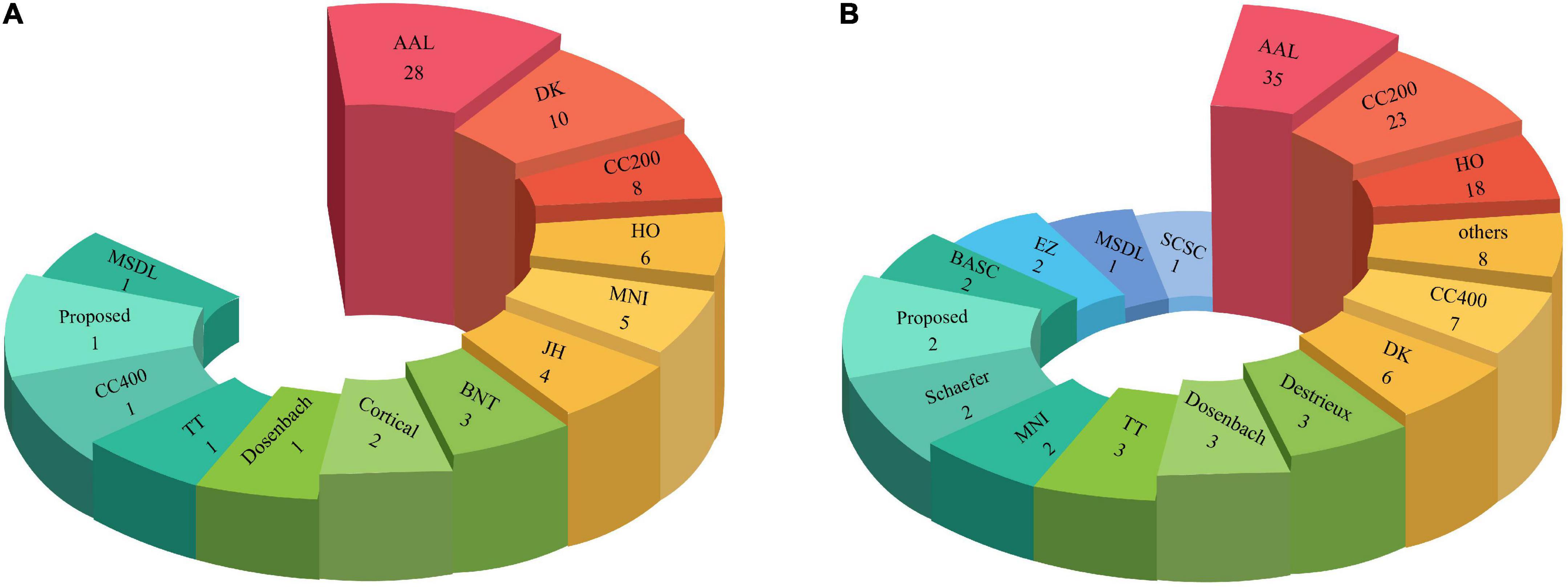
In another part of Table 2 and Supplementary Table 1, the types of Atlases for MRI neuroimaging modalities have been provided. Atlases are considered an important preprocessing step discussed in part of this section. The number of atlases employed in ML and DL research are described in Figure 7.
As shown in Figures 7A,B, the AAL atlas is most used in studies for ASD detection in MRI neuroimaging modalities using AI methods.
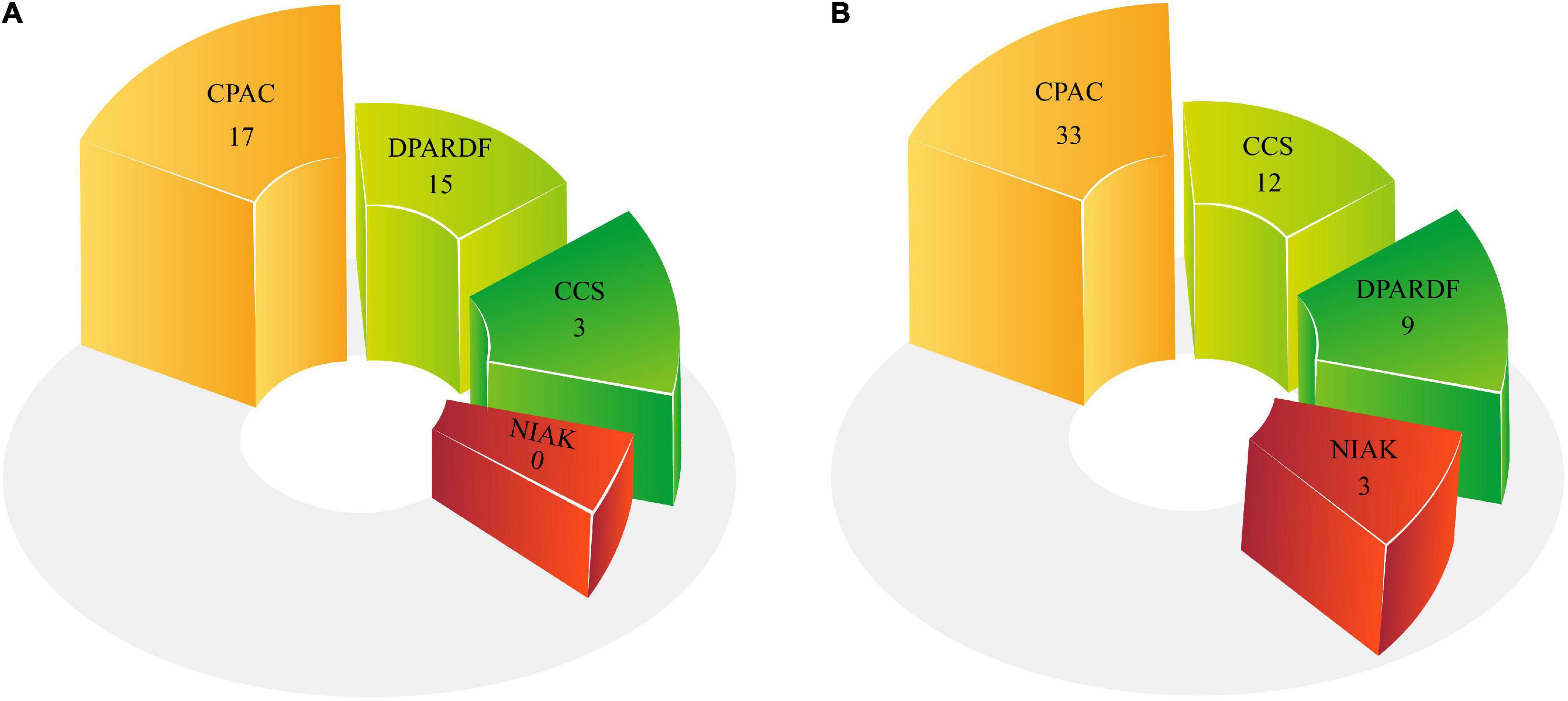
Comparison between the numbers of pipelines used in the machine learning and deep learning research
Pipelines play a significant role in preprocessing of MRI modalities. The pipelines employed in ASD data preprocessing are presented in Table 2 and Supplementary Table 1. The number of pipelines utilized in DL and ML research is shown in Figure 8. The results of the studies reveal that the CPAC pipeline is the most widely used.
Comparison between the numbers of classification methods in the machine learning and deep learning research
Classification is the last step of CADS with ML or DL methods. So far, various classification methods have been proposed in ML and DL, presented in Table 2 and Supplementary Table 1. The types of classification algorithms applied in CADS using DL and ML are depicted in Figure 9. As shown in this Figures 9A,B, it may be noted that the Softmax method is most used in DL architectures. In addition, SVM is the most widely applied in ML methods compared to other classification methods.
Future works
Lack of access to huge public datasets with various ASD disorders researchers is a big challenge. As mentioned in the introduction, autism has different types (Sparks et al., 2002), and the availability of datasets containing different types of ASD is of paramount importance for researchers. Hence, presenting MRI datasets of different types of autism disorder need to be addressed in future works. These datasets help researchers conduct more studies and compare their studies with other researchers on the automated diagnosis of ASD. As mentioned in previous sections, ABIDE is a free dataset available for researchers and consists of different cases and MRI modalities of ASD patients. But it does not have many cases of DTI modalities for the diagnosis of ASD. DTI modality is one of the popular methods in ASD detection. Publicly providing more datasets of this type of modality could increase research in the ASD diagnosis field using the DTI modality.
Another future work is to provide multimodal datasets, such as fMRI-EEG, for the diagnosis of ASD. In clinical studies (Cociu et al., 2017), it has been indicated that using multimodal neuroimaging, such as fMRI-EEG, plays a pivotal role in diagnosing ASD. In addition, providing datasets with combined modalities paves the way for new studies on the diagnosis of ASD using different AI methods.
Automated diagnosis of ASD with MRI using ML techniques can be the other future work. Various methods have been proposed for feature extraction from MRI data for the diagnosis of ASD, which are summarized in Table 2. According to Table 2, fuzzy-based feature extraction techniques have not been used in the diagnosis of ASD, and they can be introduced in future work. Fuzzy techniques are important in medical applications and allow researchers to develop software close to human logic (Chanussot et al., 1999; Davidson et al., 2001; Javed et al., 2013; Jiang et al., 2017; Meena and Agilandeeswari, 2020; Ullah et al., 2020). Hence, providing graph models based on fuzzy theory can be addressed in the future, leading to the accurate diagnosis of ASD with MRI modalities. Connectivity techniques are an essential feature extraction method for structural and functional neuroimaging modalities (Bhattacharya et al., 2006; Rowe et al., 2010; Smith et al., 2012; Gilson et al., 2018; Park et al., 2018; Zarghami and Friston, 2020). Proposing new feature extraction methods based on connectivity for structural and functional neuroimaging modalities is another field for future work. Table 2 also indicates classification algorithms. In this section, fuzzy type 1 and 2 techniques can be used for data classification as future work on the diagnosis of ASD (Melin and Castillo, 2013, 2014; de Aguiar et al., 2017). Furthermore, in the future, graph theory-based classification methods can also be used to increase the performance of the CADS for automated diagnosis of ASD (Cai et al., 2018; Wu et al., 2020).
The reliability of AI models for medical diagnosis (Balagurunathan et al., 2021; Durán and Jongsma, 2021) poses another challenge for researchers, which needs to be solved before these models are usable in real-life. There is more than one direction that contributes toward this end, such as designing test and validation protocols to ensure the validity of reported results, necessitating papers to include enough information to make results reproducible (such as protocols used in top-tier conferences such as NeurIPS) and also working on explainability and interpretability of models in addition to their performances (Afnan et al., 2021).
In Supplementary Appendix (A), different studies on the automated diagnosis of ASD using MRI modalities and DL techniques is presented. It may be noted that conducted studies have used standard DL methods to diagnose ASD. In future works, graph theory (Zhang Z. et al., 2020; Ma et al., 2021), representation learning (Hamilton et al., 2017; Zhang et al., 2018), zero-shot learning (Wang et al., 2019d), Q-learning (Jang et al., 2019), attention learning (Li et al., 2018c), and advanced models of adversarial networks (Liu and Tuzel, 2016; Creswell et al., 2018) can be used for the automated diagnosis of ASD with MRI modalities.
Feature fusion technique is a new field in diagnosing different diseases, and many studies are being conducted in this field (Antropova et al., 2017; Fan et al., 2018; Hermessi et al., 2019; Liu et al., 2021; Wang et al., 2021b; Amemiya et al., 2022). The DL features can be extracted from MRI images for automated ASD detection. Ultimately, ML and DL features can be used to obtain high performance in the automated diagnosis of ASD.
Conclusion
Autism spectrum disorder is a neurological disorder with unknown symptoms that begins in childhood and cause problems in communication, social relationships, perception processing, and repetitive behaviors. In few studies, physicians have stated that ASD usually occurs due to genetic mutations or the inability of the fetus’s brain cells to obey regular growth patterns during the first steps (Sparks et al., 2002; Brieber et al., 2007; Sato et al., 2012; Ecker et al., 2015; Hernandez et al., 2015).
Physicians use different ASD detection methods, among which different neuroimaging modalities are of paramount importance (Parisot et al., 2018; Mellema et al., 2019; Ronicko et al., 2020). Among different neuroimaging modalities, MRI-based functional and structural modalities are mostly used to diagnose ASD. sMRI and fMRI provide physicians with important information on the structure and function of the brain, respectively (Sserwadda and Rekik, 2021; Tummala, 2021). However, accurate diagnosis of ASD from sMRI and fMRI is sometimes time-consuming and challenging. Moreover, factors such as tiredness or different noises in MRI modalities may lead to clinicians’ wrong diagnosis of ASD.
For this purpose, many studies are being conducted on the automated diagnosis of ASD using AI techniques, aiming to increase the performance of automated diagnosis of ASD. In general, studies on the automated diagnosis of ASD from MRI modalities using AI cover ML and DL methods. In few papers, researchers have conducted a review study in ASD detection based on DL (Khodatars et al., 2021) and ML (Brihadiswaran et al., 2019; de Belen et al., 2020; Hosseinzadeh et al., 2021; Kollias et al., 2021; Song et al., 2021; Tawhid et al., 2021) methods with different neuroimaging modalities.
This work is a comprehensive review of studies conducted on ASD detection using AI methods in different MRI neuroimaging modalities. First, AI-based CADS for ASD detection from different MRI neuroimaging modalities was introduced. Then, the steps of the CADS based on ML algorithms for automated ASD detection in MRI neuroimaging modalities were studied. Also, in this section, papers on the automated ASD detection in MRI neuroimaging modalities using ML methods are summarized in Table 2. Previously, some authors of this study previously published a review paper about automatic ASD detection in different neuroimaging modalities using DL techniques (Khodatars et al., 2021), which is summarized in Supplementary Table 1.
The most critical challenges in ASD detection in MRI neuroimaging modalities and AI methods were presented in another section. Also, this section studied the most important challenges in the automated diagnosis of ASD using MRI modalities and AI techniques. The most important challenges in the diagnosis of ASD are the lack of access to public datasets with different MRI modalities, multimodal datasets, such as fMRI-EEG, AI algorithms, and hardware resources.
In the discussion section, first, the number of published annual papers on ASD detection using ML methods and DL techniques were discussed. Then, the number of datasets used in ML and DL studies was presented. In addition, the number of different MRI neuroimaging modalities with ML and DL methods used in annual studies in ML and DL was also indicated. Also, a comparison was made between different atlases used in MRI neuroimaging preprocessing for ASD detection. In another subsection, the number of pipelines in the preprocessing step of the MRI neuroimaging modalities for CADS based on various AI methods is also examined and compared. Finally, the number of classifier algorithms used in ML and DL studies for ASD detection was discussed.
In section 7, the future works for ASD detection in MRI neuroimaging modalities and AI methods were addressed. In this section, future works on MRI datasets for the diagnosis of ASD were first discussed. Then, future works on the diagnosis of ASD using AI techniques were addressed. Besides, future works on the automated diagnosis of ASD with MRI modalities were introduced. The final section also recommended the idea of using feature fusion for the diagnosis of ASD with MRI modalities in future works. Studies on ASD detection using AI techniques indicate that researchers will use the proposed methods in the future. The proposed methods are promising in developing real software for ASD detection using MRI modalities and help clinicians quickly diagnose ASD in the early stage.
Also, research on DL-based methods for the diagnosis of ASD has experienced significant attention in recent years. In standard mode, sMRI and fMRI data are recorded in 3D and 4D. However, in most papers, researchers have utilized 2D DL models to diagnose ASD using MRI neuroimaging modalities. Due to the high computational cost of 3D DL models for diagnosing ASD, there has been less research in this field. Providing 3D DL models based on quantization techniques reduces hardware resources and increases speed. Thus, DL models using quantization techniques (Liang et al., 2021) can be exploited to diagnose ASD in the future. Memory constraints are one of the research challenges of ASD diagnosis using MRI neuroimaging modalities. In medicine, cloud computing is one of the novel technologies to address storage and data processing issues (Chen and Ran, 2019). Using cloud computing in future work may lead to other valuable research in ASD diagnosis. In this way, MRI data is first sent to the cloud for storage. Next, the implementation of DL algorithms for the diagnosis of ASD can be carried out on their computing servers.
Author contributions
ASh, NG, PM, DS, RA, and UA contributed to conceptualization. ASh, SL, AK, JG, and ASu contributed to methodology. SL, AK, SS-A, SA, ASu, JG, RA, and UA contributed to validation. MJ, RA, DS, PM, and SA contributed to formal analysis. ASh, SS-A, SA, MK, MJ, and PM contributed to writing—original draft preparation. ASu, SS-A, SA, and MK contributed to writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding provided by the Qatar National Library.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnmol.2022.999605/full#supplementary-material
References
Abdullah, A. A., Rijal, S., and Dash, S. R. (2019). Evaluation on machine learning algorithms for classification of autism spectrum disorder (ASD). J. Phys. Conf. Ser. 1372:012052.
Afnan, M. A. M., Liu, Y., Conitzer, V., Rudin, C., Mishra, A., Savulescu, J., et al. (2021). Interpretable, not black-box, artificial intelligence should be used for embryo selection. Hum. Rep. Open 2021:hoab040.
Aghdam, M. A., Sharifi, A., and Pedram, M. M. (2019). Diagnosis of autism spectrum disorders in young children based on resting-state functional magnetic resonance imaging data using convolutional neural networks. J. Dig. Imaging 32, 899–918. doi: 10.1007/s10278-019-00196-1
AGRE (n.d.). Available Online at: https://www.autismspeaks.org/agre (accessed July 19, 2022).
Ahammed, M. S., Niu, S., Ahmed, M. R., Dong, J., Gao, X., and Chen, Y. (2021). “Bag-of-features model for ASD fMRI classification using SVM,” in Proceedings of the 2021 Asia-Pacific Conference on Communications Technology and Computer Science (ACCTCS), (Shenyang: IEEE), 52–57.
Ahmadi-Dastgerdi, N., Hosseini-Nejad, H., Amiri, H., Shoeibi, A., and Gorriz, J. M. (2021). A vector quantization-based spike compression approach dedicated to multichannel neural recording microsystems. Int. J. Neural Syst. 32:2250001. doi: 10.1142/S0129065722500010
Ahmed, M. R., Zhang, Y., Liu, Y., and Liao, H. (2020b). Single volume image generator and deep learning-based ASD classification. IEEE J. Biomed. Health Inf. 24, 3044–3054. doi: 10.1109/JBHI.2020.2998603
Ahmed, M. R., Ahammed, M. S., Niu, S., and Zhang, Y. (2020a). “Deep learning approached features for ASD classification using SVM,” in Proceedings of the 2020 IEEE International Conference on Artificial Intelligence and Information Systems (ICAIIS), (Dalian: IEEE), 287–290. doi: 10.1155/2020/1394830
Akhavan Aghdam, M., Sharifi, A., and Pedram, M. M. (2018). Combination of rs-fMRI and sMRI data to discriminate autism spectrum disorders in young children using deep belief network. J. Dig. Imaging 31, 895–903. doi: 10.1007/s10278-018-0093-8
Al-Hiyali, M. I., Yahya, N., Faye, I., Khan, Z., and Alsaih, K. (2021). “Classification of BOLD FMRI signals using wavelet transform and transfer learning for detection of autism spectrum disorder,” in Proceedings of the 2020 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), (Langkawi: IEEE), 94–98.
Alizadehsani, R., Khosravi, A., Roshanzamir, M., Abdar, M., Sarrafzadegan, N., Shafie, D., et al. (2021). Coronary artery disease detection using artificial intelligence techniques: A survey of trends, geographical differences and diagnostic features 1991–2020. Comput. Biol. Med. 128:104095. doi: 10.1016/j.compbiomed.2020.104095
Almuqhim, F., and Saeed, F. (2021). ASD-SAENet: A sparse autoencoder, and deep-neural network model for detecting autism spectrum disorder (ASD) using fMRI data. Front. Comput. Neurosci. 15:654315. doi: 10.3389/fncom.2021.654315
Al-Shoukry, S., Rassem, T. H., and Makbol, N. M. (2020). Alzheimer’s diseases detection by using deep learning algorithms: A mini-review. IEEE Access 8, 77131–77141.
Altinkaya, E., Polat, K., and Barakli, B. (2020). Detection of Alzheimer’s disease and dementia states based on deep learning from MRI images: A comprehensive review. J. Inst. Electr. Comput. 1, 39–53. doi: 10.3389/fnagi.2021.720226
Alvarez-Jimenez, C., Múnera-Garzón, N., Zuluaga, M. A., Velasco, N. F., and Romero, E. (2020). Autism spectrum disorder characterization in children by capturing local-regional brain changes in MRI. Med. Phys. 47, 119–131. doi: 10.1002/mp.13901
Amemiya, S., Takao, H., Kato, S., Yamashita, H., Sakamoto, N., and Abe, O. (2022). Feature-fusion improves MRI single-shot deep learning detection of small brain metastases. J. Neuroimaging 32, 111–119. doi: 10.1111/jon.12916
An, M., Ho, H. P., Staib, L., Pelphrey, K., and Duncan, J. (2010). “Multimodal MRI analysis of brain subnetworks in autism using multi-view EM,” in Proceedings of the 2010 Conference Record of the Forty Fourth Asilomar Conference on Signals, Systems and Computers, (Pacific Grove, CA: IEEE), 786–789.
Anand, C. S., and Sahambi, J. S. (2010). Wavelet domain non-linear filtering for MRI denoising. Magn. Reson. Imaging 28, 842–861. doi: 10.1016/j.mri.2010.03.013
Anirudh, R., and Thiagarajan, J. J. (2019). “Bootstrapping graph convolutional neural networks for autism spectrum disorder classification,” in Proceedings of the ICASSP 2019-2019 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), (Piscataway, NJ: IEEE), 3197–3201.
Antropova, N., Huynh, B. Q., and Giger, M. L. (2017). A deep feature fusion methodology for breast cancer diagnosis demonstrated on three imaging modality datasets. Med. Phys. 44, 5162–5171. doi: 10.1002/mp.12453
Arya, D., Olij, R., Gupta, D. K., El Gazzar, A., Wingen, G., Worring, M., et al. (2020). “Fusing structural and functional MRIs using graph convolutional networks for autism classification,” in Proceedings of Medical imaging with deep learning, (Birmingham: PMLR), 44–61. doi: 10.1016/j.compbiomed.2022.105823
Bakhtyari, M., and Mirzaei, S. (2022). ADHD detection using dynamic connectivity patterns of EEG data and ConvLSTM with attention framework. Biomed. Signal Process. Control 76:103708.
Balagurunathan, Y., Mitchell, R., and El Naqa, I. (2021). Requirements and reliability of AI in the medical context. Phys. Med. 83, 72–78.
Bayram, M. A., and Ýlyas, Ö, and Temurtaş, F. (2021). Deep learning methods for autism spectrum disorder diagnosis based on fMRI images. Sakarya Univ. J. Comput. Inf. Sci. 4, 142–155.
Benabdallah, F. Z., El Maliani, A. D., Lotfi, D., Jennane, R., and El Hassouni, M. (2018). “Analysis of under-connectivity in autism using the minimum spanning tree: Application on large multi-site dataset,” in Proceedings 2018 9th International Symposium on Signal, Image, Video and Communications (ISIVC), (Rabat: IEEE), 296–299.
Bengs, M., Gessert, N., and Schlaefer, A. (2020). 4d spatio-temporal deep learning with 4d fmri data for autism spectrum disorder classification. arXiv [Preprint]. doi: 10.48550/arXiv.2004.10165
Bernas, A., Aldenkamp, A. P., and Zinger, S. (2018). Wavelet coherence-based classifier: A resting-state functional MRI study on neurodynamics in adolescents with high-functioning autism. Comput. Methods Programs Biomed. 154, 143–151. doi: 10.1016/j.cmpb.2017.11.017
Bhattacharya, S., Ho, M.-H. R., and Purkayastha, S. (2006). A Bayesian approach to modeling dynamic effective connectivity with fMRI data. Neuroimage 30, 794–812.
Bhaumik, R., Pradhan, A., Das, S., and Bhaumik, D. K. (2018). Predicting autism spectrum disorder using domain-adaptive cross-site evaluation. Neuroinformatics 16, 197–205. doi: 10.1007/s12021-018-9366-0
Bi, X.-A., Liu, Y., Sun, Q., Luo, X., Tan, H., Chen, J., et al. (2019). The genetic-evolutionary random support vector machine cluster analysis in autism spectrum disorder. IEEE Access 7, 30527–30535. doi: 10.3389/fphys.2018.01646
Bi, X.-A., Wang, Y., Shu, Q., Sun, Q., and Xu, Q. (2018). Classification of autism spectrum disorder using random support vector machine cluster. Front. Genet. 9:18. doi: 10.3389/fgene.2018.00018
Bloy, L., Ingalhalikar, M., Eavani, H., Roberts, T. P., Schultz, R. T., and Verma, R. (2011). HARDI based pattern classifiers for the identification of white matter pathologies. Med. Image Comput. Comput. Assist. Interv. 14, 234–241. doi: 10.1007/978-3-642-23629-7_29
Bora, E., Fornito, A., Radua, J., Walterfang, M., Seal, M., Wood, S. J., et al. (2011). Neuroanatomical abnormalities in schizophrenia: A multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr. Res. 127, 46–57. doi: 10.1016/j.schres.2010.12.020
Brambilla, P., Hardan, A., Di Nemi, S. U., Perez, J., Soares, J. C., and Barale, F. (2003). Brain anatomy and development in autism: Review of structural MRI studies. Brain Res. Bull. 61, 557–569.
Brieber, S., Neufang, S., Bruning, N., Kamp-Becker, I., Remschmidt, H., Herpertz-Dahlmann, B., et al. (2007). Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J. Child Psychol. Psychiatry 48, 1251–1258.
Brihadiswaran, G., Haputhanthri, D., Gunathilaka, S., Meedeniya, D., and Jayarathna, S. (2019). EEG-based processing and classification methodologies for autism spectrum disorder: A review. J. Comput. Sci. 15, 1161–1183. doi: 10.1109/TBCAS.2021.3089132
Brown, C. J., Kawahara, J., and Hamarneh, G. (2018). “Connectome priors in deep neural networks to predict autism,” in 2018 IEEE 15th international symposium on biomedical imaging (ISBI 2018), (Washington, DC: IEEE), 110–113.
Bryant, D. M., Hoeft, F., Lai, S., Lackey, J., Roeltgen, D., Ross, J., et al. (2012). Sex chromosomes and the brain: A study of neuroanatomy in XYY syndrome. Dev. Med. Child Neurol. 54, 1149–1156. doi: 10.1111/j.1469-8749.2012.04418.x
Byeon, K., Kwon, J., Hong, J., and Park, H. (2020). “Artificial neural network inspired by neuroimaging connectivity: Application in autism spectrum disorder,” in 2020 IEEE International Conference on Big Data and Smart Computing (BigComp), (Busan: IEEE), 575–578.
Cai, H., Zheng, V. W., and Chang, K. C.-C. (2018). A comprehensive survey of graph embedding: Problems, techniques, and applications. IEEE Trans. Knowl. Data Eng. 30, 1616–1637.
Cai, Y., Chia, N. K., Thalmann, D., Kee, N. K., Zheng, J., and Thalmann, N. M. (2013). Design and development of a virtual dolphinarium for children with autism. IEEE Trans. Neural Syst. Rehab. Eng. 21, 208–217. doi: 10.1109/TNSRE.2013.2240700
Calderoni, S., Retico, A., Biagi, L., Tancredi, R., Muratori, F., and Tosetti, M. (2012). Female children with autism spectrum disorder: An insight from mass-univariate and pattern classification analyses. Neuroimage 59, 1013–1022. doi: 10.1016/j.neuroimage.2011.08.070
Cao, M., Yang, M., Qin, C., Zhu, X., Chen, Y., Wang, J., et al. (2021). Using DeepGCN to identify the autism spectrum disorder from multi-site resting-state data. Biomed. Signal Process. Control 70:103015.
Chaitra, N., Vijaya, P., and Deshpande, G. (2020). Diagnostic prediction of autism spectrum disorder using complex network measures in a machine learning framework. Biomed. Signal Process. Control 62:102099.
Chanussot, J., Mauris, G., and Lambert, P. (1999). Fuzzy fusion techniques for linear features detection in multitemporal SAR images. IEEE Trans. Geosci. Remote Sens. 37, 1292–1305.
Chen, C. P., Keown, C. L., and Müller, R.-A. (2013). Towards Understanding autism Risk Factors: A Classification of Brain Images with Support Vector Machines. Int. J. Semant. Comput. 7, 205–213.
Chen, H., Duan, X., Liu, F., Lu, F., Ma, X., Zhang, Y., et al. (2016). Multivariate classification of autism spectrum disorder using frequency-specific resting-state functional connectivity—a multi-center study. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 1–9. doi: 10.1016/j.pnpbp.2015.06.014
Chen, H., Zhuang, F., Xiao, L., Ma, L., Liu, H., Zhang, R., et al. (2021). AMA-GCN: Adaptive Multi-layer Aggregation Graph Convolutional Network for Disease Prediction. arXiv [Preprint]. doi: 10.48550/arXiv.2106.08732
Chen, J., and Ran, X. (2019). Deep learning with edge computing: A review. Proc. IEEE 107, 1655–1674.
Chen, X., Wang, Z., Zhan, Y., Cheikh, F. A., and Ullah, M. (2022). “Interpretable learning approaches in structural MRI: 3D-ResNet fused attention for autism spectrum disorder classification,” in Proceedings of Medical Imaging 2022: Computer-Aided Diagnosis, (Bellingham, WA: SPIE), 611–618.
Chen, Y., Liu, A., Fu, X., Wen, J., and Chen, X. (2021). An Invertible Dynamic Graph Convolutional Network for Multi-Center ASD Classification. Front. Neurosci. 15:828512. doi: 10.3389/fnins.2021.828512
Choi, H. (2017). Functional connectivity patterns of autism spectrum disorder identified by deep feature learning. arXiv [Preprint]. doi: 10.48550/arXiv.1707.07932
Chu, Y., Wang, G., Cao, L., Qiao, L., and Liu, M. (2022). Multi-Scale Graph Representation Learning for Autism Identification With Functional MRI. Front. Neuroinform. 15:802305. doi: 10.3389/fninf.2021.802305
Cociu, B. A., Das, S., Billeci, L., Jamal, W., Maharatna, K., Calderoni, S., et al. (2017). Multimodal functional and structural brain connectivity analysis in autism: A preliminary integrated approach with EEG, fMRI, and DTI. IEEE Trans. Cogn. Dev. Syst. 10, 213–226.
Conti, E., Retico, A., Palumbo, L., Spera, G., Bosco, P., Biagi, L., et al. (2020). Autism Spectrum Disorder and Childhood Apraxia of Speech: Early language-related hallmarks across structural MRI study. J. Pers. Med. 10:275. doi: 10.3390/jpm10040275
Cordova, M., Shada, K., Demeter, D. V., Doyle, O., Miranda-Dominguez, O., Perrone, A., et al. (2020). Heterogeneity of executive function revealed by a functional random forest approach across ADHD and ASD. Neuroimage Clin. 26:102245. doi: 10.1016/j.nicl.2020.102245
Creswell, A., White, T., Dumoulin, V., Arulkumaran, K., Sengupta, B., and Bharath, A. A. (2018). Generative adversarial networks: An overview. IEEE Signal Process. Mag. 35, 53–65.
Crimi, A., Dodero, L., Murino, V., and Sona, D. (2017). “Case-control discrimination through effective brain connectivity,” in 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), (Melbourne, VIC: IEEE), 970–973.
D’Souza, N. S., Nebel, M. B., Crocetti, D., Wymbs, N., Robinson, J., Mostofsky, S., et al. (2020). “A deep-generative hybrid model to integrate multimodal and dynamic connectivity for predicting spectrum-level deficits in autism,” in International Conference on Medical Image Computing and Computer-Assisted Intervention, (Berlin: Springer), 437–447.
Darweesh, A. N., Salem, N., and Al-Atabany, W. (2022). Classification of autism spectrum disorder using convolutional neural network. SSRN Electron. J. doi: 10.2139/ssrn.4057056
Davidson, V. J., Ryks, J., and Chu, T. (2001). Fuzzy models to predict consumer ratings for biscuits based on digital image features. IEEE Trans. Fuzzy Syst. 9, 62–67.
de Aguiar, E. P., Fernando, M. D. A., Vellasco, M. M., and Ribeiro, M. V. (2017). Set-membership type-1 fuzzy logic system applied to fault classification in a switch machine. IEEE Trans. Intell. Transp. Syst. 18, 2703–2712.
de Belen, R. A. J., Bednarz, T., Sowmya, A., and Del Favero, D. (2020). Computer vision in autism spectrum disorder research: A systematic review of published studies from 2009 to 2019. Transl. Psychiatry 10:333. doi: 10.1038/s41398-020-01015-w
Dekhil, O., Ali, M., El-Nakieb, Y., Shalaby, A., Soliman, A., Switala, A., et al. (2019). A personalized autism diagnosis CAD system using a fusion of structural MRI and resting-state functional MRI data. Front. Psychiatry 10:392. doi: 10.3389/fpsyt.2019.00392
Dekhil, O., Ali, M., Haweel, R., Elnakib, Y., Ghazal, M., Hajjdiab, H., et al. (2020). “A comprehensive framework for differentiating autism spectrum disorder from neurotypicals by fusing structural MRI and resting state functional MRI. Semin. Pediatr. Neurol. 34:100805. doi: 10.1016/j.spen.2020.100805
Dekhil, O., Hajjdiab, H., Shalaby, A., Ali, M. T., Ayinde, B., Switala, A., et al. (2018). Using resting state functional mri to build a personalized autism diagnosis system. PLoS One 13:e0206351. doi: 10.1371/journal.pone.0206351
Dekhil, O., Ismail, M., Shalaby, A., Switala, A., Elmaghraby, A., Keynton, R., et al. (2017). “A novel CAD system for autism diagnosis using structural and functional MRI,” in 2017 IEEE 14th international symposium on biomedical imaging (ISBI 2017), (Melbourne, VIC: IEEE), 995–998.
Demirhan, A. (2018). Performance of machine learning methods in determining the autism spectrum disorder cases. Mugla J. Sci. Technol. 4, 79–84.
Deshpande, G., Libero, L. E., Sreenivasan, K. R., Deshpande, H. D., and Kana, R. K. (2013). Identification of neural connectivity signatures of autism using machine learning. Front. Hum. Neurosci. 7:670. doi: 10.3389/fnhum.2013.00670
Devika, K., and Oruganti, V. R. M. (2021). “A Machine Learning Approach for Diagnosing Neurological Disorders using Longitudinal Resting-State fMRI,” in 2021 11th International Conference on Cloud Computing, Data Science & Engineering (Confluence), (Noida: IEEE), 494–499. doi: 10.1007/s10916-019-1475-2
Dolz, J., Desrosiers, C., and Ayed, I. B. (2018). 3D fully convolutional networks for subcortical segmentation in MRI: A large-scale study. Neuroimage 170, 456–470. doi: 10.1016/j.neuroimage.2017.04.039
Dominic, N., Cenggoro, T. W., Budiarto, A., and Pardamean, B. (2021). Transfer learning using inception-ResNet-v2 model to the augmented neuroimages data for autism spectrum disorder classification. Commun. Math. Biol. Neurosci. 2021:39.
DSouza, A. M., Abidin, A. Z., and Wismüller, A. (2019). “Classification of autism spectrum disorder from resting-state fMRI with mutual connectivity analysis,” in Proceedings of the Medical Imaging 2019: Biomedical Applications in Molecular, Structural, and Functional Imaging, San Diego, CA, 292–299.
DSouza, N. S., Nebel, M. B., Crocetti, D., Robinson, J., Mostofsky, S., and Venkataraman, A. (2021). M-gcn: A multimodal graph convolutional network to integrate functional and structural connectomics data to predict multidimensional phenotypic characterizations. Proc. Mach. Learn. Res. 143, 119–130.
Du, Y., Li, B., Hou, Y., and Calhoun, V. D. (2020). “A deep learning fusion model for brain disorder classification: Application to distinguishing schizophrenia and autism spectrum disorder,” in Proceedings of the 11th ACM International Conference on Bioinformatics, Computational Biology and Health Informatics, (New York, NY: ACM), 1–7. doi: 10.1145/3388440.3412478
Durán, J. M., and Jongsma, K. R. (2021). Who is afraid of black box algorithms? On the epistemological and ethical basis of trust in medical AI. J. Med. Ethics 47, 329–335.
Dvornek, N. C., Li, X., Zhuang, J., and Duncan, J. S. (2019). “Jointly discriminative and generative recurrent neural networks for learning from fMRI,” in Proceedings of the International Workshop on Machine Learning in Medical Imaging, (Berlin: Springer), 382–390.
Dvornek, N. C., Yang, D., Ventola, P., and Duncan, J. S. (2018b). “Learning generalizable recurrent neural networks from small task-fmri datasets,” in Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, eds A. Frangi, J. Schnabel, C. Davatzikos, C. Alberola-López, and G. Fichtinger (Berlin: Springer), 329–337. doi: 10.1007/978-3-030-00931-1_38
Dvornek, N. C., Ventola, P., and Duncan, J. S. (2018a). “Combining phenotypic and resting-state fMRI data for autism classification with recurrent neural networks,” in Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), (Washington, DC: IEEE), 725–728. doi: 10.1109/ISBI.2018.8363676
Dvornek, N. C., Ventola, P., Pelphrey, K. A., and Duncan, J. S. (2017). “Identifying autism from resting-state fMRI using long short-term memory networks,” in Proceedings of the 2017 International Workshop on Machine Learning in Medical Imaging, Quebec, QC, 362–370.
Ecker, C., Bookheimer, S. Y., and Murphy, D. G. (2015). Neuroimaging in autism spectrum disorder: Brain structure and function across the lifespan. Lancet Neurol. 14, 1121–1134. doi: 10.1016/S1474-4422(15)00050-2
Ecker, C., Rocha-Rego, V., Johnston, P., Mourao-Miranda, J., Marquand, A., Daly, E. M., et al. (2010b). Investigating the predictive value of whole-brain structural MR scans in autism: A pattern classification approach. Neuroimage 49, 44–56. doi: 10.1016/j.neuroimage.2009.08.024
Ecker, C., Marquand, A., Mourão-Miranda, J., Johnston, P., Daly, E. M., Brammer, M. J., et al. (2010a). Describing the brain in autism in five dimensions—magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. J. Neurosci. 30, 10612–10623. doi: 10.1523/JNEUROSCI.5413-09.2010
Eill, A., Jahedi, A., Gao, Y., Kohli, J. S., Fong, C. H., Solders, S., et al. (2019). Functional connectivities are more informative than anatomical variables in diagnostic classification of autism. Brain Connect. 9, 604–612. doi: 10.1089/brain.2019.0689
El Gazzar, A., Cerliani, L., Van Wingen, G., and Thomas, R. M. (2019). “Simple 1-D convolutional networks for resting-state fMRI based classification in autism,” in Proceedings of the 2019 International Joint Conference on Neural Networks (IJCNN), (Piscataway, NJ: IEEE), 1–6.
El-Gazzar, A., Quaak, M., Cerliani, L., Bloem, P., Wingen, G. V., and Mani Thomas, R. (2019). “A hybrid 3DCNN and 3DC-LSTM based model for 4D spatio-temporal fMRI data: an ABIDE autism classification study,” in Proceedings of the OR 2.0 Context-Aware Operating Theaters and Machine Learning in Clinical Neuroimaging, Shenzhen, 95–102.
Elnakieb, Y. A., Ali, M. T., Soliman, A., Mahmoud, A. H., Shalaby, A. M., Alghamdi, N. S., et al. (2020). Computer aided autism diagnosis using diffusion tensor imaging. IEEE Access 8, 191298–191308.
ElNakieb, Y., Ali, M. T., Dekhil, O., Khalefa, M. E., Soliman, A., Shalaby, A., et al. (2018). “Towards accurate personalized autism diagnosis using different imaging modalities: sMRI, fMRI, and DTI,” in Proceedings of the 2018 IEEE International Symposium on Signal Processing and Information Technology (ISSPIT), (Louisville, KY: IEEE), 447–452.
ElNakieb, Y., Soliman, A., Mahmoud, A., Dekhil, O., Shalaby, A., Ghazal, M., et al. (2019). “Autism spectrum disorder diagnosis framework using diffusion tensor imaging,” in Proceedings of the 2019 IEEE International Conference on Imaging Systems and Techniques (IST), (Piscataway, NJ: IEEE), 1–5.
Eslami, T., Almuqhim, F., Raiker, J. S., and Saeed, F. (2021a). Machine learning methods for diagnosing autism spectrum disorder and attention-deficit/hyperactivity disorder using functional and structural MRI: A survey. Front. Neuroinform. 14:575999. doi: 10.3389/fninf.2020.575999
Eslami, T., Raiker, J. S., and Saeed, F. (2021b). Explainable and scalable machine learning algorithms for detection of autism spectrum disorder using fMRI data. Neural Eng. Tech. Autism Spectr. Disord. 1, 39–54.
Eslami, T., and Saeed, F. (2019). “Auto-ASD-network: A technique based on deep learning and support vector machines for diagnosing autism spectrum disorder using fMRI data,” in Proceedings of the 10th ACM International Conference on Bioinformatics, Computational Biology and Health Informatics, Niagara Falls, NY, 646–651. doi: 10.1155/2020/1394830
Eslami, T., Mirjalili, V., Fong, A., Laird, A. R., and Saeed, F. (2019). ASD-DiagNet: A hybrid learning approach for detection of autism spectrum disorder using fMRI data. Front. Neuroinform. 13:70. doi: 10.3389/fninf.2019.00070
Fan, G., Chen, Y., Chen, Y., Yang, M., Wang, J., Li, C., et al. (2020). Abnormal brain regions in two-group cross-location dynamics model of autism. IEEE Access 8, 94526–94534.
Fan, Z., Sun, L., Ding, X., Huang, Y., Cai, C., and Paisley, J. (2018). “A segmentation-aware deep fusion network for compressed sensing MRI,” in Proceedings of the European Conference on Computer Vision (ECCV)), Munich, 55–70. doi: 10.1016/j.mri.2022.08.007
Felouat, H., and Oukid-Khouas, S. (2020). “Graph convolutional networks and functional connectivity for identification of autism spectrum disorder,” in Proceedings of the 2020 Second International Conference on Embedded & Distributed Systems (EDiS), (Piscataway, NJ: IEEE), 27–32.
Filipovych, R., Resnick, S. M., and Davatzikos, C. (2012). JointMMCC: Joint maximum-margin classification and clustering of imaging data. IEEE Trans. Med. Imaging 31, 1124–1140. doi: 10.1109/TMI.2012.2186977
Fodor, I. K. (2002). A survey of dimension reduction techniques. Livermore, CA: Lawrence Livermore National Lab.
Fredo, A., Jahedi, A., Reiter, M., and Müller, R.-A. (2018). Diagnostic classification of autism using resting-state fMRI data and conditional random forest. Age 12, 6–41.
Gao, J., Chen, M., Li, Y., Gao, Y., Li, Y., Cai, S., et al. (2021). Multisite autism spectrum disorder classification using convolutional neural network classifier and individual morphological brain networks. Front. Neurosci. 14:629630. doi: 10.3389/fnins.2020.629630
Gautam, R., and Sharma, M. (2020). Prevalence and diagnosis of neurological disorders using different deep learning techniques: A meta-analysis. J. Med. Syst. 44:29.
Ge, F., Chen, H., Zhang, T., Wang, X., Yuan, L., Hu, X., et al. (2018). “A novel framework for analyzing cortical folding patterns based on sulcal baselines and gyral crestlines,” in Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), (Piscataway, NJ: IEEE), 1043–1047.
Gene Expression Omnibus [GEO] (n.d.). Gene Expression Omnibus. Available online at: https://www.ncbi.nlm.nih.gov/geo (accessed July 19, 2022).
Geng, X., Yao, Q., Jiang, K., and Zhu, Y. (2020). “Deep neural generative adversarial model based on VAE+ GAN for disorder diagnosis,” in Proceedings of the 2020 International Conference on Internet of Things and Intelligent Applications (ITIA), (Piscataway, NY: IEEE), 1–7.
Georgiadis, P., Cavouras, D., Kalatzis, I., Daskalakis, A., Kagadis, G. C., Sifaki, K., et al. (2008). Improving brain tumor characterization on MRI by probabilistic neural networks and non-linear transformation of textural features. Comput. Methods Programs Biomed. 89, 24–32. doi: 10.1016/j.cmpb.2007.10.007
Ghassemi, N., Shoeibi, A., and Rouhani, M. (2020). Deep neural network with generative adversarial networks pre-training for brain tumor classification based on MR images. Biomed. Signal Process. Control 57:101678.
Ghassemi, N., Shoeibi, A., Khodatars, M., Heras, J., Rahimi, A., Zare, A., et al. (2021). Automatic diagnosis of covid-19 from ct images using cyclegan and transfer learning. arXiv [Preprint]. doi: 10.48550/arXiv.2104.11949
Gilson, M., Deco, G., Friston, K. J., Hagmann, P., Mantini, D., Betti, V., et al. (2018). Effective connectivity inferred from fMRI transition dynamics during movie viewing points to a balanced reconfiguration of cortical interactions. Neuroimage 180, 534–546. doi: 10.1016/j.neuroimage.2017.09.061
Giuliano, A., Calderoni, S., Muratori, F., Biagi, L., Tosetti, M., and Retico, A. (2013). “Multivariate analysis of structural MRI data to detect gender-related brain abnormalities in children with autism spectrum disorder,” in Proceedings of the 2013 european congress of radiology-ECR 2013, Vienna.
Gori, I., Giuliano, A., Oliva, P., Tosetti, M., Muratori, F., Calderoni, S., et al. (2016). “Processing Magnetic Resonance Image Features with One-class Support Vector Machines-Investigation of the Autism Spectrum Disorder Heterogeneity,” in Proceedings of the 2016 International Conference on Bioimaging, (Setúbal: SciTePress), 111–117.
Graña, M., and Silva, M. (2021). Impact of machine learning pipeline choices in autism prediction from functional connectivity data. Int. J. Neural Syst. 31:2150009. doi: 10.1142/S012906572150009X
Guo, X., Dominick, K. C., Minai, A. A., Li, H., Erickson, C. A., and Lu, L. J. (2017). Diagnosing autism spectrum disorder from brain resting-state functional connectivity patterns using a deep neural network with a novel feature selection method. Front. Neurosci. 11:460. doi: 10.3389/fnins.2017.00460
Gupta, D., Vij, I., and Gupta, M. (2020). Autism detection using r-fMRI: Subspace approximation and CNN based approach. Int. J. Adv. Trends Comput. Sci. Eng. 9, 1029–1036.
Gupta, N., Bhatele, P., and Khanna, P. (2019). Glioma detection on brain MRIs using texture and morphological features with ensemble learning. Biomed. Signal Process. Control 47, 115–125.
Hamilton, W. L., Ying, R., and Leskovec, J. (2017). Representation learning on graphs: Methods and applications. arXiv [Preprint]. doi: 10.48550/arXiv.1709.05584
Haweel, R., Dekhil, O., Shalaby, A., Mahmoud, A., Ghazal, M., Khalil, A., et al. (2019b). “Functional magnetic resonance imaging based framework for autism diagnosis,” in Proceedings of the 2019 Fifth International Conference on Advances in Biomedical Engineering (ICABME), (Tripoli: IEEE), 1–4.
Haweel, R., Dekhil, O., Shalaby, A., Mahmoud, A., Ghazal, M., Keynton, R., et al. (2019a). “A machine learning approach for grading autism severity levels using task-based functional MRI,” in Proceedings of the 2019 IEEE International Conference on Imaging Systems and Techniques (IST), (Abu Dhabi: IEEE), 1–5.
Haweel, R., Dekhil, O., Shalaby, A., Mahmoud, A., Ghazal, M., Khalil, A., et al. (2020). “A novel framework for grading autism severity using task-based fMRI,” in Proceedings of the 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI), (Iowa City, IA: IEEE), 1404–1407.
Haweel, R., Shalaby, A., Mahmoud, A., Seada, N., Ghoniemy, S., Ghazal, M., et al. (2021b). A robust DWT–CNN-based CAD system for early diagnosis of autism using task-based fMRI. Med. Phys. 48, 2315–2326. doi: 10.1002/mp.14692
Haweel, R., Shalaby, A., Mahmoud, A., Ghazal, M., Seada, N., Ghoniemy, S., et al. (2021a). “A Novel Dwt-Based Discriminant Features Extraction From Task-Based FMRI: An ASD diagnosis study using CNN,” in Proceedings of the 2021 IEEE 18th International Symposium on Biomedical Imaging (ISBI), (Nice: IEEE), 196–199.
Hazlett, H. C., Poe, M., Gerig, G., Smith, R. G., Provenzale, J., Ross, A., et al. (2005). Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Arch. Gen. Psychiatry 62, 1366–1376.
Heinsfeld, A. S., Franco, A. R., Craddock, R. C., Buchweitz, A., and Meneguzzi, F. (2018). Identification of autism spectrum disorder using deep learning and the ABIDE dataset. Neuroimage Clin. 17, 16–23.
Henschel, L., Conjeti, S., Estrada, S., Diers, K., Fischl, B., and Reuter, M. (2020). Fastsurfer-a fast and accurate deep learning based neuroimaging pipeline. NeuroImage 219:117012.
Hermessi, H., Mourali, O., and Zagrouba, E. (2019). Deep feature learning for soft tissue sarcoma classification in MR images via transfer learning. Expert Systems with Applications 120, 116–127.
Hernandez, L. M., Rudie, J. D., Green, S. A., Bookheimer, S., and Dapretto, M. (2015). Neural signatures of autism spectrum disorders: Insights into brain network dynamics. Neuropsychopharmacology 40, 171–189.
Highland, D., and Zhou, G. (2022). A review of detection techniques for depression and bipolar disorder. Smart Health.
Hiremath, Y., Ismail, M., Verma, R., Antunes, J., and Tiwari, P. (2020). “Combining deep and hand-crafted MRI features for identifying sex-specific differences in autism spectrum disorder versus controls,” in Proceedings of the Medical Imaging 2020: Computer-Aided Diagnosis, (Bellingham, WA: SPIE), 445–451.
Hosseinzadeh, M., Koohpayehzadeh, J., Bali, A. O., Rad, F. A., Souri, A., Mazaherinezhad, A., et al. (2021). A review on diagnostic autism spectrum disorder approaches based on the Internet of Things and Machine Learning. J. Supercomput. 77, 2590–2608.
Hu, J., Cao, L., Li, T., Liao, B., Dong, S., and Li, P. (2020). Interpretable learning approaches in resting-state functional connectivity analysis: The case of autism spectrum disorder. Comput. Math. Methods Med. 2020:1394830. doi: 10.1155/2020/1394830
Huang, F., Elazab, A., Ouyang, L., Tan, J., Wang, T., and Lei, B. (2019). “Sparse low-rank constrained adaptive structure learning using multi-template for autism spectrum disorder diagnosis,” in Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), (Venice: IEEE), 1555–1558.
Huang, H., Liu, X., Jin, Y., Lee, S. W., Wee, C. Y., and Shen, D. (2019). Enhancing the representation of functional connectivity networks by fusing multi-view information for autism spectrum disorder diagnosis. Hum. Brain Mapp. 40, 833–854. doi: 10.1002/hbm.24415
Huang, Z.-A., Zhu, Z., Yau, C. H., and Tan, K. C. (2020b). Identifying autism spectrum disorder from resting-state fMRI using deep belief network. IEEE Trans. Neural Netw. Learn. Syst. 32, 2847–2861.
Huang, Z.-A., Liu, R., and Tan, K. C. (2020a). “Multi-Task learning for efficient diagnosis of ASD and ADHD using Resting-State fMRI data,” in Proceedings of the 2020 International Joint Conference on Neural Networks (IJCNN), (Glasgow: IEEE), 1–7.
Husna, R. N. S., Syafeeza, A., Hamid, N. A., Wong, Y., and Raihan, R. A. (2021). Functional magnetic resonance imaging for autism spectrum disorder detection using deep learning. Jurnal Teknologi 83, 45–52.
Iglesias, J. E., Lerma-Usabiaga, G., Garcia-Peraza-Herrera, L. C., Martinez, S., and Paz-Alonso, P. M. (2017). “Retrospective head motion estimation in structural brain MRI with 3D CNNs,” in Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, (Berlin: Springer), 314–322.
Ingalhalikar, M., Parker, D., Bloy, L., Roberts, T. P., and Verma, R. (2011). Diffusion based abnormality markers of pathology: Toward learned diagnostic prediction of ASD. Neuroimage 57, 918–927. doi: 10.1016/j.neuroimage.2011.05.023
Ismail, M., Barnes, G., Nitzken, M., Switala, A., Shalaby, A., Hosseini-Asl, E., et al. (2017). “A new deep-learning approach for early detection of shape variations in autism using structural MRI,” in Proceedings of the2017 IEEE International Conference on Image Processing (ICIP), (Beijing: IEEE), 1057–1061.
Itani, S., and Thanou, D. (2021). Combining anatomical and functional networks for neuropathology identification: A case study on autism spectrum disorder. Med. Image Anal. 69:101986. doi: 10.1016/j.media.2021.101986
Jafarpour, S., Sedghi, Z., and Amirani, M. C. (2012). A robust brain MRI classification with GLCM features. Int. J. Comput. Appl. 37, 1–5.
Jahedi, A., Nasamran, C. A., Faires, B., Fan, J., and Müller, R.-A. (2017). Distributed intrinsic functional connectivity patterns predict diagnostic status in large autism cohort. Brain Connect. 7, 515–525. doi: 10.1089/brain.2017.0496
Jang, B., Kim, M., Harerimana, G., and Kim, J. W. (2019). Q-learning algorithms: A comprehensive classification and applications. IEEE Access 7, 133653–133667.
Javed, U., Riaz, M. M., Ghafoor, A., and Cheema, T. A. (2013). MRI brain classification using texture features, fuzzy weighting and support vector machine. Prog. Electromagnet. Res. B 53, 73–88.
Jayanthy, A., and Din, Q. M. U. (2022). “Early detection of autism spectrum disorder using behavioral data EEG, MRI and Behavioral Data: A Review,” in Assistive Technology Intervention in Healthcare, eds S. Jain and S. Paul (Boca Raton, FL: CRC Press), 245–267.
Jha, R. R., Bhardwaj, A., Garg, D., Bhavsar, A., and Nigam, A. (2021). MHATC: Autism Spectrum Disorder identification utilizing multi-head attention encoder along with temporal consolidation modules. arXiv [Preprint]. doi: 10.48550/arXiv.2201.00404
Ji, J., and Yao, Y. (2020). Convolutional neural network with graphical Lasso to extract sparse topological features for brain disease classification. IEEE/ACM Trans. Comput. Biol. Bioinformatics 18, 2327–2338. doi: 10.1109/TCBB.2020.2989315
Ji, J., Xing, X., Yao, Y., Li, J., and Zhang, X. (2021). Convolutional kernels with an element-wise weighting mechanism for identifying abnormal brain connectivity patterns. Pattern Recogn. 109:107570.
Jiang, Q., Jin, X., Lee, S.-J., and Yao, S. (2017). A novel multi-focus image fusion method based on stationary wavelet transform and local features of fuzzy sets. IEEE Access 5, 20286–20302.
Jiang, Y., Li, Z., and Zhang, D. (2019). “Unsupervised domain adaptation for multi-center autism spectrum disorder identification,” in Proceedings of the 2019 IEEE SmartWorld, ubiquitous intelligence & computing, advanced & trusted computing, scalable computing & communications, cloud & big data computing, internet of people and smart city innovation (SmartWorld/SCALCOM/UIC/ATC/CBDCom/IOP/SCI), (Leicester: IEEE), 1608–1613.
Jiao, Y., and Lu, Z. (2011). “Predictive models for autism spectrum disorder based on multiple cortical features,” in Proceedings of the 2011 Eighth International Conference on Fuzzy Systems and Knowledge Discovery (FSKD), (Shanghai: IEEE), 1611–1615.
Jiao, Y., Chen, R., Ke, X., Cheng, L., Chu, K., Lu, Z., et al. (2011). Predictive models for subtypes of autism spectrum disorder based on single-nucleotide polymorphisms and magnetic resonance imaging. Adv. Med. Sci. 56, 334–342. doi: 10.2478/v10039-011-0042-y
Jiao, Y., Chen, R., Ke, X., Chu, K., Lu, Z., and Herskovits, E. H. (2010). Predictive models of autism spectrum disorder based on brain regional cortical thickness. Neuroimage 50, 589–599.
Jiao, Z., Li, H., and Fan, Y. (2020). “Improving diagnosis of autism spectrum disorder and disentangling its heterogeneous functional connectivity patterns using capsule networks,” in Proceedings of the 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI), (Iowa City, IA: IEEE), 1331–1334. doi: 10.1109/isbi45749.2020.9098524
Jönemo, J., Abramian, D., and Eklund, A. (2021). Evaluation of augmentation methods in classifying autism spectrum disorders from fMRI data with 3D convolutional neural networks. arXiv [Preprint]. doi: 10.48550/arXiv.2110.10489
Jones, C. R., Pickles, A., Falcaro, M., Marsden, A. J., Happé, F., Scott, S. K., et al. (2011). A multimodal approach to emotion recognition ability in autism spectrum disorders. J. Child Psychol. Psychiatry 52, 275–285.
Jung, M., Tu, Y., Park, J., Jorgenson, K., Lang, C., Song, W., et al. (2019). Surface-based shared and distinct resting functional connectivity in attention-deficit hyperactivity disorder and autism spectrum disorder. Br. J. Psychiatry 214, 339–344. doi: 10.1192/bjp.2018.248
Karampasi, A., Kakkos, I., Miloulis, S.-T., Zorzos, I., Dimitrakopoulos, G. N., Gkiatis, K., et al. (2020). “A machine learning fMRI approach in the diagnosis of autism,” in Proceedings of the 2020 IEEE International Conference on Big Data (Big Data), (Atlanta, GA: IEEE), 3628–3631.
Kasari, C., and Smith, T. (2013). Interventions in schools for children with autism spectrum disorder: Methods and recommendations. Autism 17, 254–267.
Kazeminejad, A., and Sotero, R. C. (2019). Topological properties of resting-state fMRI functional networks improve machine learning-based autism classification. Front. Neurosci. 12:1018. doi: 10.3389/fnins.2018.01018
Kazeminejad, A., and Sotero, R. C. (2020). The importance of anti-correlations in graph theory based classification of autism spectrum disorder. Front. Neurosci. 14:676. doi: 10.3389/fnins.2020.00676
Ke, F., Choi, S., Kang, Y. H., Cheon, K.-A., and Lee, S. W. (2020). Exploring the structural and strategic bases of autism spectrum disorders with deep learning. IEEE Access 8, 153341–153352.
Ke, Q., Zhang, J., Wei, W., Damaševičius, R., and Woźniak, M. (2019). Adaptive independent subspace analysis of brain magnetic resonance imaging data. IEEE Access 7, 12252–12261.
Khodatars, M., Shoeibi, A., Sadeghi, D., Ghaasemi, N., Jafari, M., Moridian, P., et al. (2021). Deep learning for neuroimaging-based diagnosis and rehabilitation of autism spectrum disorder: A review. Computers in Biology and Medicine 139, 104949. doi: 10.1016/j.compbiomed.2021.104949
Khosla, M., Jamison, K., Kuceyeski, A., and Sabuncu, M. R. (2018). “3D convolutional neural networks for classification of functional connectomes,” in Deep Learning in Medical Image Analysis and Multimodal Learning for Clinical Decision Support, (Cham: Springer), 137–145.
Khosla, M., Jamison, K., Kuceyeski, A., and Sabuncu, M. R. (2019). Ensemble learning with 3D convolutional neural networks for functional connectome-based prediction. NeuroImage 199, 651–662. doi: 10.1016/j.neuroimage.2019.06.012
Kim, Y. S., Fombonne, E., Koh, Y.-J., Kim, S.-J., Cheon, K.-A., and Leventhal, B. L. (2014). A comparison of DSM-IV pervasive developmental disorder and DSM-5 autism spectrum disorder prevalence in an epidemiologic sample. J. Am. Acad. Child Adolesc. Psychiatry 53, 500–508. doi: 10.1016/j.jaac.2013.12.021
Kober, H., Grummich, P., and Vieth, J. (1993). Precise fusion of MEG and MRI tomography using a surface fit. Biomed Eng. 38, 355–356.
Kollias, K.-F., Syriopoulou-Delli, C. K., Sarigiannidis, P., and Fragulis, G. F. (2021). The contribution of machine learning and eye-tracking technology in autism spectrum disorder research: A systematic review. Electronics 10:2982.
Kong, Y., Gao, J., Xu, Y., Pan, Y., Wang, J., and Liu, J. (2019). Classification of autism spectrum disorder by combining brain connectivity and deep neural network classifier. Neurocomputing 324, 63–68.
Lee, T.-W., and Xue, S.-W. (2017). Linking graph features of anatomical architecture to regional brain activity: A multi-modal MRI study. Neurosci. Lett. 651, 123–127. doi: 10.1016/j.neulet.2017.05.005
Leming, M. J., Baron-Cohen, S., and Suckling, J. (2021). Single-participant structural similarity matrices lead to greater accuracy in classification of participants than function in autism in MRI. Mol. Autism 12, 1–15. doi: 10.1186/s13229-021-00439-5
Leming, M., Górriz, J. M., and Suckling, J. (2020). Ensemble deep learning on large, mixed-site fMRI datasets in autism and other tasks. Int. J. Neural Syst. 30:2050012. doi: 10.1142/S0129065720500124
Li, G., Chen, M.-H., Li, G., Wu, D., Sun, Q., Shen, D., et al. (2019). “A preliminary volumetric MRI study of amygdala and hippocampal subfields in autism during infancy,” in Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), (Venice: IEEE), 1052–1056. doi: 10.1109/ISBI.2019.8759439
Li, X., Dvornek, N. C., Papademetris, X., Zhuang, J., Staib, L. H., Ventola, P., et al. (2018c). “2-channel convolutional 3D deep neural network (2CC3D) for fMRI analysis: ASD classification and feature learning,” in Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), (Washington, DC: IEEE), 1252–1255. doi: 10.1109/isbi.2018.8363798
Li, G., Liu, M., Sun, Q., Shen, D., and Wang, L. (2018a). “Early diagnosis of autism disease by multi-channel CNNs,” in Proceedings of the International Workshop on Machine Learning in Medical Imaging, (Berlin: Springer), 303–309. doi: 10.1007/978-3-030-00919-9_35
Li, H., Parikh, N. A., and He, L. (2018b). A novel transfer learning approach to enhance deep neural network classification of brain functional connectomes. Front. Neurosci. 12:491. doi: 10.3389/fnins.2018.00491
Li, X., Dvornek, N. C., Zhou, Y., Zhuang, J., Ventola, P., and Duncan, J. S. (2018d). “Efficient interpretation of deep learning models using graph structure and cooperative game theory: Application to ASD biomarker discovery,” in Proceedings of the International Conference on Information Processing in Medical Imaging, (Berlin: Springer), 718–730. doi: 10.1007/978-3-030-20351-1_56
Li, X., Dvornek, N. C., Zhuang, J., Ventola, P., and Duncan, J. S. (2018e). “Brain biomarker interpretation in ASD using deep learning and fMRI,” in Proceedings of the International conference on medical image computing and computer-assisted intervention, (Berlin: Springer), 206–214.
Li, H., Xue, Z., Ellmore, T. M., Frye, R. E., and Wong, S. T. (2012). “Identification of faulty DTI-based sub-networks in autism using network regularized SVM,” in Proceedings of the 2012 9th IEEE International Symposium on Biomedical Imaging (ISBI), (Barcelona: IEEE), 550–553.
Li, J., Wang, F., Pan, J., and Wen, Z. (2021). Identification of autism spectrum disorder with functional graph discriminative network. Front. Neurosci. 15:729937. doi: 10.3389/fnins.2021.729937
Li, R., Xian, K., Shen, C., Cao, Z., Lu, H., and Hang, L. (2019). “Deep attention-based classification network for robust depth prediction,” in Asian Conference on Computer Vision, eds C. Jawahar, H. Li, G. Mori, and K. Schindler (Berlin: Springer), 663–678.
Li, X., Hect, J., Thomason, M., and Zhu, D. (2020). “Interpreting age effects of human fetal brain from spontaneous fMRI using deep 3D convolutional neural networks,” in Proceedings of the 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI), (Piscataway, NJ: IEEE), 1424–1427.
Liang, T., Glossner, J., Wang, L., Shi, S., and Zhang, X. (2021). Pruning and quantization for deep neural network acceleration: A survey. Neurocomputing 461, 370–403.
Liao, D., and Lu, H. (2018). “Classify autism and control based on deep learning and community structure on resting-state fMRI,” in Proceedings of the 2018 Tenth International Conference on Advanced Computational Intelligence (ICACI), (Xiamen: IEEE), 289–294.
Liao, Y., and Vemuri, V. R. (2002). Use of k-nearest neighbor classifier for intrusion detection. Comput. Security 21, 439–448.
Liu, J., Sheng, Y., Lan, W., Guo, R., Wang, Y., and Wang, J. (2020). Improved ASD classification using dynamic functional connectivity and multi-task feature selection. Pattern Recogn. Lett. 138, 82–87.
Liu, M.-Y., and Tuzel, O. (2016). Coupled generative adversarial networks. Proceedings of the 30th International Conference on Neural Information Processing Systems, Barcelona.
Liu, W., Liu, M., Yang, D., Wang, M., and Tao, T. (2020). “Automatic diagnosis of autism based on functional magnetic resonance imaging and elastic net,” in Proceedings of the 2020 IEEE 5th Information Technology and Mechatronics Engineering Conference (ITOEC), (Chongqing: IEEE), 104–108.
Liu, X., Wang, J., Sun, H., Chandra, S. S., Crozier, S., and Liu, F. (2021). On the regularization of feature fusion and mapping for fast MR multi-contrast imaging via iterative networks. Magn. Reson. Imaging 77, 159–168. doi: 10.1016/j.mri.2020.12.019
Loeffelbein, D. J., Souvatzoglou, M., Wankerl, V., Martinez-Möller, A., Dinges, J., Schwaiger, M., et al. (2012). PET-MRI fusion in head-and-neck oncology: current status and implications for hybrid PET/MRI. J. Oral Maxillofac. Surgery 70, 473–483. doi: 10.1016/j.joms.2011.02.120
Loh, H. W., Ooi, C. P., Barua, P. D., Palmer, E. E., Molinari, F., and Acharya, U. (2022). Automated detection of ADHD: Current trends and future perspective. Comput. Biol. Med. 146:105525. doi: 10.1016/j.compbiomed.2022.105525
Lu, H., Liu, S., Wei, H., and Tu, J. (2020). Multi-kernel fuzzy clustering based on auto-encoder for fMRI functional network. Expert Syst. Appl. 159: 113513.
Lu, J. T., Kishida, K. T., De Asis-Cruz, J., Lohrenz, T., Treadwell-Deering, D., Beauchamp, M., et al. (2015). Single-stimulus functional MRI produces a neural individual difference measure for autism spectrum disorder. Clin. Psychol. Sci. 3, 422–432. doi: 10.1177/2167702614562042
Ma, F., Gao, F., Sun, J., Zhou, H., and Hussain, A. (2019). Weakly supervised segmentation of SAR imagery using superpixel and hierarchically adversarial CRF. Remote Sens. 11:512.
Ma, X., Wu, J., Xue, S., Yang, J., Zhou, C., Sheng, Q. Z., et al. (2021). A comprehensive survey on graph anomaly detection with deep learning. IEEE Trans. Knowl. Data Eng.
Madine, M., Rekik, I., and Werghi, N. (2020). “Diagnosing autism using T1-W MRI with multi-kernel learning and hypergraph neural network,” in Proceedings of the 2020 IEEE International Conference on Image Processing (ICIP), (Abu Dhabi: IEEE), 438–442.
Maenner, M. J., Shaw, K. A., Bakian, A. V., Bilder, D. A., Durkin, M. S., Esler, A., et al. (2021). Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill. Summ. 70, 1–16. doi: 10.15585/mmwr.mm6745a7
Mahmoud, A. M., Karamti, H., and Alrowais, F. (2020a). A two consequent multi-layers deep discriminative approach for classifying fMRI images. Int. J. Artif. Intell. Tools 29:2030001.
Mahmoud, A. M., Karamti, H., and Alrowais, F. M. (2020b). “An effective sparse autoencoders based deep learning framework for fMRI scans classification,” in Proceedings of the 22nd International Conference on Enterprise Information Systems (ICEIS 2020), Prague, 540–547.
Mathur, M., and Lindberg, T. (n.d.). Autism Spectrum Disorder classification using Machine Learning techniques on fMRI. Available online at: https://mihirmathur.com/asd_classification.pdf
Matson, M. L., Mahan, S., and Matson, J. L. (2009). Parent training: A review of methods for children with autism spectrum disorders. Research in Autism Spectrum Disorders 3, 868–875.
Meena, D., and Agilandeeswari, L. (2020). Invariant features-based fuzzy inference system for animal detection and recognition using thermal images. Int. J. Fuzzy Syst. 22, 1868–1879.
Melin, P., and Castillo, O. (2013). A review on the applications of type-2 fuzzy logic in classification and pattern recognition. Expert Syst. Appl. 40, 5413–5423.
Melin, P., and Castillo, O. (2014). A review on type-2 fuzzy logic applications in clustering, classification and pattern recognition. Appl. Soft Comput. 21, 568–577.
Mellema, C. J., Treacher, A., Nguyen, K. P., and Montillo, A. (2020). “Architectural configurations, atlas granularity and functional connectivity with diagnostic value in Autism Spectrum Disorder,” in Proceedings of the 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI), (Piscataway, NJ: IEEE), 1022–1025. doi: 10.1109/ISBI45749.2020.9098555
Mellema, C., Treacher, A., Nguyen, K., and Montillo, A. (2019). “Multiple deep learning architectures achieve superior performance diagnosing autism spectrum disorder using features previously extracted from structural and functional MRI,” in Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), (Venice: IEEE), 1891–1895. doi: 10.1109/ISBI.2019.8759193
Mhiri, I., and Rekik, I. (2020). Joint functional brain network atlas estimation and feature selection for neurological disorder diagnosis with application to autism. Med. Image Anal. 60:101596. doi: 10.1016/j.media.2019.101596
Michele, A., Colin, V., and Santika, D. D. (2019). Mobilenet convolutional neural networks and support vector machines for palmprint recognition. Procedia Comput. Sci. 157, 110–117.
Mohammadpoor, M., Shoeibi, A., and Shojaee, H. (2016). A hierarchical classification method for breast tumor detection. Iran. J. Med. Phys. 13, 261–268.
Mostafa, S., Tang, L., and Wu, F.-X. (2019a). Diagnosis of autism spectrum disorder based on eigenvalues of brain networks. IEEE Access 7, 128474–128486.
Mostafa, S., Yin, W., and Wu, F.-X. (2019b). “Autoencoder based methods for diagnosis of autism spectrum disorder,” in International Conference on Computational Advances in Bio and Medical Sciences, eds I. Mãndoiu, T. Murali, G. Narasimhan, S. Rajasekaran, P. Skums, and A. Zelikovsky (Cham: Springer), 39–51.
Mozhdefarahbakhsh, A., Chitsazian, S., Chakrabarti, P., Rao, K. J., Kateb, B., and Nami, M. (2021). “A Convolutional neural network model to differentiate attention deficit hyperactivity disorder and autism spectrum disorder based on the resting State fMRI data,” in Proceedings of the 2018 Tenth International Conference on Advanced Computational Intelligence (ICACI), (Xiamen: IEEE).
Murdaugh, D. L., Shinkareva, S. V., Deshpande, H. R., Wang, J., Pennick, M. R., and Kana, R. K. (2012). Differential deactivation during mentalizing and classification of autism based on default mode network connectivity. PLoS One 7:e50064. doi: 10.1371/journal.pone.0050064
Muthukrishnan, R., and Rohini, R. (2016). “LASSO: A feature selection technique in predictive modeling for machine learning,” in Proceedings of the 2016 IEEE international conference on advances in computer applications (ICACA), (Coimbatore: IEEE), 18–20.
NDAR (n.d.). Welcome to the NIMH Data Archive. Available online at: https://ndar.nih.gov (accessed July 19, 2022).
Nielsen, J. A., Zielinski, B. A., Fletcher, P. T., Alexander, A. L., Lange, N., Bigler, E. D., et al. (2013). Multisite functional connectivity MRI classification of autism: ABIDE results. Front. Hum. Neurosci. 7:599. doi: 10.3389/fnhum.2013.00599
NIMH Repository and Genomics Resource, (n.d.). Comprehensive Data + Tools to Support Your Research. Available online at: https://www.nimhgenetics.org (accessed July 19, 2022).
Niu, K., Guo, J., Pan, Y., Gao, X., Peng, X., Li, N., et al. (2020). Multichannel deep attention neural networks for the classification of autism spectrum disorder using neuroimaging and personal characteristic data. Complexity 2020:1357853. doi: 10.1155/2020/1357853
Noble, W. S. (2006). What is a support vector machine? Nat. Biotechnol. 24, 1565–1567. doi: 10.1038/nbt1206-1565
Nogay, H. S., and Adeli, H. (2020). Machine learning (ML) for the diagnosis of autism spectrum disorder (ASD) using brain imaging. Rev. Neurosci. 31, 825–841. doi: 10.1515/revneuro-2020-0043
Noor, M. B. T., Zenia, N. Z., Kaiser, M. S., Mahmud, M., and Mamun, S. A. (2019). “Detecting neurodegenerative disease from MRI: A brief review on a deep learning perspective,” in International conference on brain informatics, eds P. Liang, V. Goel, and C. Shan (Cham: Springer), 115–125. doi: 10.1007/978-3-030-37078-7_12
Noor, M. B. T., Zenia, N. Z., Kaiser, M. S., Mamun, S. A., and Mahmud, M. (2020). Application of deep learning in detecting neurological disorders from magnetic resonance images: A survey on the detection of Alzheimer’s disease, Parkinson’s disease and schizophrenia. Brain Informatics 7, 1–21. doi: 10.1186/s40708-020-00112-2
Oshiro, T. M., Perez, P. S., and Baranauskas, J. A. (2012). “How many trees in a random forest?” in International workshop on machine learning and data mining in pattern recognition, ed. P. Perner (Berlin: Springer), 154–168. doi: 10.1007/978-3-642-31537-4_13
Pagnozzi, A. M., Conti, E., Calderoni, S., Fripp, J., and Rose, S. E. (2018). A systematic review of structural MRI biomarkers in autism spectrum disorder: A machine learning perspective. Int. J. Dev. Neurosci. 71, 68–82. doi: 10.1016/j.ijdevneu.2018.08.010
Parikh, M. N., Li, H., and He, L. (2019). Enhancing diagnosis of autism with optimized machine learning models and personal characteristic data. Front. Comput. Neurosci. 13:9. doi: 10.3389/fncom.2019.00009
Parisot, S., Ktena, S. I., Ferrante, E., Lee, M., Guerrero, R., Glocker, B., et al. (2018). Disease prediction using graph convolutional networks: Application to autism spectrum disorder and Alzheimer’s disease. Med. Image Anal. 48, 117–130. doi: 10.1016/j.media.2018.06.001
Park, H.-J., Friston, K. J., Pae, C., Park, B., and Razi, A. (2018). Dynamic effective connectivity in resting state fMRI. NeuroImage 180, 594–608. doi: 10.1016/j.neuroimage.2017.11.033
Payabvash, S., Palacios, E. M., Owen, J. P., Wang, M. B., Tavassoli, T., Gerdes, M., et al. (2019). White matter connectome edge density in children with autism spectrum disorders: Potential imaging biomarkers using machine-learning models. Brain Connect. 9, 209–220. doi: 10.1089/brain.2018.0658
Pinaya, W. H., Mechelli, A., and Sato, J. R. (2019). Using deep autoencoders to identify abnormal brain structural patterns in neuropsychiatric disorders: A large-scale multi-sample study. Hum. Brain Mapp. 40, 944–954. doi: 10.1002/hbm.24423
Pominova, M., Kondrateva, E., Sharaev, M., Bernstein, A., and Burnaev, E. (2021). “Fader networks for domain adaptation on fMRI: Abide-ii study,” in Proceedings of the Thirteenth International Conference on Machine Vision, (Bellingham, WA: SPIE), 570–577. doi: 10.1117/12.2587348
Pugazhenthi, B., Senapathy, G., and Pavithra, M. (2019). “Identification of autism in MR brain images using deep learning networks,” in Proceedings of the 2019 International Conference on Smart Structures and Systems (ICSSS), (Chennai: IEEE), 1–7. doi: 10.3389/fnins.2021.756868
Rahman, M. M., Usman, O. L., Muniyandi, R. C., Sahran, S., Mohamed, S., and Razak, R. A. (2020). A Review of machine learning methods of feature selection and classification for autism spectrum disorder. Brain Sci. 10:949. doi: 10.3390/brainsci10120949
Rakić, M., Cabezas, M., Kushibar, K., Oliver, A., and Lladó, X. (2020). Improving the detection of autism spectrum disorder by combining structural and functional MRI information. Neuroimage Clin. 25:102181. doi: 10.1016/j.nicl.2020.102181
Rane, S., Jolly, E., Park, A., Jang, H., and Craddock, C. (2017). Developing predictive imaging biomarkers using whole-brain classifiers: Application to the ABIDE I dataset. Res. Ideas Outcomes 3:e12733. doi: 10.3897/rio.3.e12733
Reiter, M. A., Jahedi, A., Fredo, A., Fishman, I., Bailey, B., and Müller, R.-A. (2021). Performance of machine learning classification models of autism using resting-state fMRI is contingent on sample heterogeneity. Neural Comput. Appl. 33, 3299–3310. doi: 10.1007/s00521-020-05193-y
Retico, A., Giuliano, A., Tancredi, R., Cosenza, A., Apicella, F., Narzisi, A., et al. (2016a). The effect of gender on the neuroanatomy of children with autism spectrum disorders: A support vector machine case-control study. Mol. Autism 7, 1–20. doi: 10.1186/s13229-015-0067-3
Retico, A., Gori, I., Giuliano, A., Muratori, F., and Calderoni, S. (2016b). One-class support vector machines identify the language and default mode regions as common patterns of structural alterations in young children with autism spectrum disorders. Front. Neurosci. 10:306. doi: 10.3389/fnins.2016.00306
Richards, R., Greimel, E., Kliemann, D., Koerte, I. K., Schulte-Körne, G., Reuter, M., et al. (2020). Increased hippocampal shape asymmetry and volumetric ventricular asymmetry in autism spectrum disorder. Neuroimage Clin. 26:102207. doi: 10.1016/j.nicl.2020.102207
Ronicko, J. F. A., Thomas, J., Thangavel, P., Koneru, V., Langs, G., and Dauwels, J. (2020). Diagnostic classification of autism using resting-state fMRI data improves with full correlation functional brain connectivity compared to partial correlation. J. Neurosci. Methods 345:108884. doi: 10.1016/j.jneumeth.2020.108884
Rowe, J. B., Hughes, L. E., Barker, R. A., and Owen, A. M. (2010). Dynamic causal modelling of effective connectivity from fMRI: Are results reproducible and sensitive to Parkinson’s disease and its treatment? Neuroimage 52, 1015–1026. doi: 10.1016/j.neuroimage.2009.12.080
Saad, M., and Islam, S. M. R. (2019). “Brain connectivity network analysis and classifications from diffusion tensor imaging,” in Proceedings of the 2019 International Conference on Robotics, Electrical and Signal Processing Techniques (ICREST), (Dhaka: IEEE), 422–427. doi: 10.1109/ICREST.2019.8644080
Sadato, N., and Tanabe, H. C. (2012). “Neural substrates and inter-individual functional connectivity during mutual gaze and joint attention using dual functional MRI,” in Proceedings of the 2012 ICME International Conference on Complex Medical Engineering (CME), (Kobe: IEEE), 527–530. doi: 10.1109/ICCME.2012.6275596
Sadeghi, D., Shoeibi, A., Ghassemi, N., Moridian, P., Khadem, A., Alizadehsani, R., et al. (2022). An overview of artificial intelligence techniques for diagnosis of Schizophrenia based on magnetic resonance imaging modalities: Methods, challenges, and future works. Comput. Biol. Med. 146:105554. doi: 10.1016/j.compbiomed.2022.105554
Sadeghi, M., Khosrowabadi, R., Bakouie, F., Mahdavi, H., Eslahchi, C., and Pouretemad, H. (2017). Screening of autism based on task-free fMRI using graph theoretical approach. Psychiatry Res. Neuroimaging 263, 48–56. doi: 10.1016/j.pscychresns.2017.02.004
Sahu, L., Sharma, R., Sahu, I., Das, M., Sahu, B., and Kumar, R. (2022). Efficient detection of Parkinson’s disease using deep learning techniques over medical data. Expert Syst. 39:e12787. doi: 10.1111/exsy.12787
Sairam, K., Naren, J., Vithya, G., and Srivathsan, S. (2019). Computer aided system for autism spectrum disorder using deep learning methods. Int. J. Psychosoc. Rehabil 23, 418–425. doi: 10.1371/journal.pone.0253094
Saritha, M., Joseph, K. P., and Mathew, A. T. (2013). Classification of MRI brain images using combined wavelet entropy based spider web plots and probabilistic neural network. Pattern Recogn. Lett. 34, 2151–2156. doi: 10.1016/j.patrec.2013.08.017
Sarovic, D., Hadjikhani, N., Schneiderman, J., Lundström, S., and Gillberg, C. (2020). Autism classified by magnetic resonance imaging: A pilot study of a potential diagnostic tool. Int. J. Methods Psychiatr. Research 29, 1–18. doi: 10.1002/mpr.1846
Sartipi, S., Shayesteh, M. G., and Kalbkhani, H. (2018). “Diagnosing of autism spectrum disorder based on GARCH variance series for rs-fMRI data,” in Proceedings of the 2018 9th International Symposium on Telecommunications (IST), (Tehran: IEEE), 86–90. doi: 10.1109/ISTEL.2018.8661147
Sato, J. R., Hoexter, M. Q., De Magalhães Oliveira, P. P. Jr., Brammer, M. J., Murphy, D., Ecker, C., et al. (2013). Inter-regional cortical thickness correlations are associated with autistic symptoms: A machine-learning approach. J. Psychiatr. Res. 47, 453–459. doi: 10.1016/j.jpsychires.2012.11.017
Sato, W., Toichi, M., Uono, S., and Kochiyama, T. (2012). Impaired social brain network for processing dynamic facial expressions in autism spectrum disorders. BMC Neurosci. 13:99. doi: 10.1186/1471-2202-13-99
Savva, A. D., Karampasi, A. S., and Matsopoulos, G. K. (2020). “Deriving resting-state fMRI biomarkers for classification of autism spectrum disorder,” in Autism 360°, eds U. Das N. Papaneophytou and T. El-Kour (Amsterdam: Elsevier), 101–123. doi: 10.1016/B978-0-12-818466-0.00006-X
Sewani, H., and Kashef, R. (2020). An autoencoder-based deep learning classifier for efficient diagnosis of autism. Children 7:182. doi: 10.3390/children7100182
SFARI (n.d.). Available online at: https://www.sfari.org/funded-project/simons-variation-in-individualsproject-simons-vip (accessed July 19, 2022).
Shahamat, H., and Abadeh, M. S. (2020). Brain MRI analysis using a deep learning based evolutionary approach. Neural Netw. 126, 218–234. doi: 10.1016/j.neunet.2020.03.017
Shao, L., Fu, C., You, Y., and Fu, D. (2021). Classification of ASD based on fMRI data with deep learning. Cogn. Neurodyn. 15, 961–974. doi: 10.1007/s11571-021-09683-0
Sherkatghanad, Z., Akhondzadeh, M., Salari, S., Zomorodi-Moghadam, M., Abdar, M., Acharya, U. R., et al. (2020). Automated detection of autism spectrum disorder using a convolutional neural network. Front. Neurosci. 13:325. doi: 10.3389/fnins.2019.01325
Shi, C., Zhang, J., and Wu, X. (2020). An fMRI feature selection method based on a minimum spanning tree for identifying patients with autism. Symmetry 12:1995. doi: 10.3390/sym12121995
Shoeibi, A., Sadeghi, D., Moridian, P., Ghassemi, N., Heras, J., Alizadehsani, R., et al. (2021b). Automatic diagnosis of schizophrenia in EEG signals using CNN-LSTM models. Front. Neuroinform. 15:777977. doi: 10.3389/fninf.2021.777977
Shoeibi, A., Rezaei, M., Ghassemi, N., Namadchian, Z., Zare, A., and Gorriz, J. M. (2022c). “Automatic Diagnosis of Schizophrenia in EEG Signals Using Functional Connectivity Features and CNN-LSTM Model,” in Proceedings of the International Work-Conference on the Interplay Between Natural and Artificial Computation, (Berlin: Springer), 63–73. doi: 10.1007/978-3-031-06242-1_7
Shoeibi, A., Ghassemi, N., Khodatars, M., Moridian, P., Khosravi, A., Zare, A., et al. (2022b). Automatic diagnosis of schizophrenia and attention deficit hyperactivity disorder in rs-fMRI modality using convolutional autoencoder model and interval type-2 Fuzzy Regression. arXiv [Preprint]. doi: 10.48550/arXiv.2205.15858
Shoeibi, A., Ghassemi, N., Khodatars, M., Jafari, M., Moridian, P., Alizadehsani, R., et al. (2021a). Applications of epileptic seizures detection in neuroimaging modalities using deep learning techniques: methods, challenges, and future works. arXiv [Preprint]. doi: 10.48550/arXiv.2105.14278
Shoeibi, A., Ghassemi, N., Khodatars, M., Moridian, P., Alizadehsani, R., Zare, A., et al. (2022a). Detection of epileptic seizures on EEG signals using ANFIS classifier, autoencoders and fuzzy entropies. Biomed. Signal Process. Control 73:103417. doi: 10.1016/j.bspc.2021.103417
Shrivastava, S., Mishra, U., Singh, N., Chandra, A., and Verma, S. (2020). “Control or autism-classification using convolutional neural networks on functional MRI,” in Proceedings of the 2020 11th International Conference on Computing, Communication and Networking Technologies (ICCCNT), (Kharagpur: IEEE), 1–6. doi: 10.1109/ICCCNT49239.2020.9225506
Sibley, M. H., Arnold, L. E., Swanson, J. M., Hechtman, L. T., Kennedy, T. M., Owens, E., et al. (2022). Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. Am. J. Psychiatry 179, 142–151. doi: 10.1176/appi.ajp.2021.21010032
Siewertsen, C. M., French, E. D., and Teramoto, M. (2015). Autism spectrum disorder and pet therapy. Adv. Mind Body Med. 29, 22–25.
Smith, J. F., Pillai, A., Chen, K., and Horwitz, B. (2012). Effective connectivity modeling for fMRI: Six issues and possible solutions using linear dynamic systems. Front. Syst. Neurosci. 5:104. doi: 10.3389/fnsys.2011.00104
Soltaninejad, M., Ye, X., Yang, G., Allinson, N., and Lambrou, T. (2014). Brain tumour grading in different MRI protocols using SVM on statistical features. Egham: British Machine Vision Association.
Song, D.-Y., Topriceanu, C.-C., Ilie-Ablachim, D. C., Kinali, M., and Bisdas, S. (2021). Machine learning with neuroimaging data to identify autism spectrum disorder: A systematic review and meta-analysis. Neuroradiology 63, 2057–2072. doi: 10.1007/s00234-021-02774-z
Song, Y., Epalle, T. M., and Lu, H. (2019). Characterizing and predicting autism spectrum disorder by performing resting-state functional network community pattern analysis. Front. Hum. Neurosci. 13:203. doi: 10.3389/fnhum.2019.00203
Soussia, M., and Rekik, I. (2018). Unsupervised manifold learning using high-order morphological brain networks derived from T1-w MRI for autism diagnosis. Front. Neuroinform. 12:70. doi: 10.3389/fninf.2018.00070
Sparks, B., Friedman, S., Shaw, D., Aylward, E. H., Echelard, D., Artru, A., et al. (2002). Brain structural abnormalities in young children with autism spectrum disorder. Neurology 59, 184–192. doi: 10.1212/WNL.59.2.184
Srinivas, S. V. K., Nair, H., and Vidyasagar, V. (2019). Hardware aware neural network architectures using FbNet. arXiv [Preprint]. doi: 10.48550/arXiv.1906.07214
SSC. (n.d.). Preclinical diagnosis of magnetic resonance (MR) brain images via discrete wavelet packet transform with Tsallis entropy and generalized eigenvalue proximal support vector machine (GEPSVM). Available online at: https://www.sfari.org/resource/simons-simplex-collection
Sserwadda, A., and Rekik, I. (2021). Topology-guided cyclic brain connectivity generation using geometric deep learning. J. Neurosci. Methods 353:108988. doi: 10.1016/j.jneumeth.2020.108988
Stevens, E., Dixon, D. R., Novack, M. N., Granpeesheh, D., Smith, T., and Linstead, E. (2019). Identification and analysis of behavioral phenotypes in autism spectrum disorder via unsupervised machine learning. Int. J. Medical Inf. 129, 29–36. doi: 10.1016/j.ijmedinf.2019.05.006
Subah, F. Z., Deb, K., Dhar, P. K., and Koshiba, T. (2021). A deep learning approach to predict autism spectrum disorder using multisite resting-state fMRI. Appl. Sci. 11:3636. doi: 10.1186/s12868-016-0283-6
Subbaraju, V., Suresh, M. B., Sundaram, S., and Narasimhan, S. (2017). Identifying differences in brain activities and an accurate detection of autism spectrum disorder using resting state functional-magnetic resonance imaging: A spatial filtering approach. Med. Image Anal. 35, 375–389. doi: 10.1016/j.media.2016.08.003
Sujit, S. J., Coronado, I., Kamali, A., Narayana, P. A., and Gabr, R. E. (2019). Automated image quality evaluation of structural brain MRI using an ensemble of deep learning networks. J. Magn. Reson. Imaging 50, 1260–1267. doi: 10.1002/jmri.26693
Suthaharan, S. (2016). “Support vector machine,” in Machine learning models and algorithms for big data classification, (Berlin: Springer), 207–235. doi: 10.1007/978-1-4899-7641-3_9
Syed, M. A., Yang, Z., Hu, X. P., and Deshpande, G. (2017). Investigating brain connectomic alterations in autism using the reproducibility of independent components derived from resting state functional MRI data. Front. Neurosci. 11:459. doi: 10.3389/fnins.2017.00459
Tang, L., Mostafa, S., Liao, B., and Wu, F.-X. (2019). A network clustering based feature selection strategy for classifying autism spectrum disorder. BMC Med. Genomics 12, (Suppl. 7):153. doi: 10.1186/s12920-019-0598-0
Tang, M., Kumar, P., Chen, H., and Shrivastava, A. (2020). Deep multimodal learning for the diagnosis of autism spectrum disorder. J. Imaging 6:47. doi: 10.3390/jimaging6060047
Tawhid, M. N. A., Siuly, S., Wang, H., Whittaker, F., Wang, K., and Zhang, Y. (2021). A spectrogram image based intelligent technique for automatic detection of autism spectrum disorder from EEG. PLoS One 16:e0253094. doi: 10.1371/journal.pone.0253094
Tejwani, R., Liska, A., You, H., Reinen, J., and Das, P. (2017). Autism classification using brain functional connectivity dynamics and machine learning. arXiv [Preprint]. doi: 10.48550/arXiv.1712.08041
Thabtah, F., and Peebles, D. (2019). Early autism screening: a comprehensive review. Int. J. Environ. Res. Public Health 16:3502. doi: 10.3390/ijerph16183502
Thomas, M., and Chandran, A. (2018). “Artificial neural network for diagnosing autism spectrum disorder,” in Proceedings of the 2018 2nd International Conference on Trends in Electronics and Informatics (ICOEI), (Tirunelveli: IEEE), 930–933. doi: 10.1109/ICOEI.2018.8553781
Thomas, R. M., Gallo, S., Cerliani, L., Zhutovsky, P., El-Gazzar, A., and Van Wingen, G. (2020). Classifying autism spectrum disorder using the temporal statistics of resting-state functional MRI data with 3D convolutional neural networks. Front. Psychiatry 11:440. doi: 10.3389/fpsyt.2020.00440
Thyreau, B., and Taki, Y. (2020). Learning a cortical parcellation of the brain robust to the MRI segmentation with convolutional neural networks. Med. Image Anal. 61:101639. doi: 10.1016/j.media.2020.101639
Tian, Y., Gelernter, J., Wang, X., Chen, W., Gao, J., Zhang, Y., et al. (2018). Lane marking detection via deep convolutional neural network. Neurocomputing 280, 46–55. doi: 10.1016/j.neucom.2017.09.098
Tolan, E., and Isik, Z. (2018). “Graph theory based classification of brain connectivity network for autism spectrum disorder,” in Proceedings of the International Conference on Bioinformatics and Biomedical Engineering, (Berlin: Springer), 520–530. doi: 10.1007/978-3-319-78723-7_45
Tummala, S. (2021). “Deep learning framework using siamese neural network for diagnosis of autism from brain magnetic resonance imaging,” in Proceedings of the 2021 6th International Conference for Convergence in Technology (I2CT), (Piscataway, NJ: IEEE), 1–5. doi: 10.1002/aur.2626
UCI (n.d.). Center for Machine Learning and Intelligent Systems. Available online at: https://archive.ics.uci.edu/ml/datasets.php (Accessed July 19, 2022).
Uddin, L. Q., Menon, V., Young, C. B., Ryali, S., Chen, T., Khouzam, A., et al. (2011). Multivariate searchlight classification of structural magnetic resonance imaging in children and adolescents with autism. Biol. Psychiatry 70, 833–841. doi: 10.1016/j.biopsych.2011.07.014
Ullah, H., Ullah, B., Wu, L., Abdalla, F. Y., Ren, G., and Zhao, Y. (2020). Multi-modality medical images fusion based on local-features fuzzy sets and novel sum-modified-Laplacian in non-subsampled shearlet transform domain. Biomed. Signal Process. Control 57:101724. doi: 10.1016/j.bspc.2019.101724
Usha, R., and Perumal, K. (2019). SVM classification of brain images from MRI scans using morphological transformation and GLCM texture features. Int. J. Comput. Systems Eng. 5, 18–23. doi: 10.1504/IJCSYSE.2019.098415
Valdes-Sosa, P. A., Sanchez-Bornot, J. M., Sotero, R. C., Iturria-Medina, Y., Aleman-Gomez, Y., Bosch-Bayard, J., et al. (2009). Model driven EEG/fMRI fusion of brain oscillations. Hum. Brain Mapp. 30, 2701–2721. doi: 10.1002/hbm.20704
Varol, E., Gaonkar, B., Erus, G., Schultz, R., and Davatzikos, C. (2012). “Feature ranking based nested support vector machine ensemble for medical image classification,” in Proceedings of the 2012 9Th IEEE international symposium on biomedical imaging (ISBI), (Piscataway, NJ: IEEE), 146–149. doi: 10.1109/ISBI.2012.6235505
Vigneshwaran, S., Mahanand, B., Suresh, S., and Savitha, R. (2013). “Autism spectrum disorder detection using projection based learning meta-cognitive RBF network,” in Proceedings of the 2013 International Joint Conference on Neural Networks (IJCNN), (Dallas, TX: IEEE), 1–8. doi: 10.1109/IJCNN.2013.6706777
Volkmar, F. R., and Mcpartland, J. C. (2014). From Kanner to DSM-5: autism as an evolving diagnostic concept. Annu. Rev. Clin. Psychol. 10, 193–212. doi: 10.1146/annurev-clinpsy-032813-153710
Volkmar, F. R., Cicchetti, D. V., Bregman, J., and Cohen, D. J. (1992). Three diagnostic systems for autism: DSM-III, DSM-III-R, and ICD-10. J. Autism Dev. Disord. 22, 483–492.
Volkmar, F. R., Cohen, D. J., and Paul, R. (1986). An evaluation of DSM-III criteria for infantile autism. J. Am. Acad. Child Psychiatry 25, 190–197. doi: 10.1016/S0002-7138(09)60226-0
Wang, W., Zheng, V. W., Yu, H., and Miao, C. (2019d). A survey of zero-shot learning: Settings, methods, and applications. ACM Trans. Intell. Syst. Technol. 10, 1–37. doi: 10.1145/3293318
Wang, C., Xiao, Z., and Wu, J. (2019b). Functional connectivity-based classification of autism and control using SVM-RFECV on rs-fMRI data. Physica Med. 65, 99–105. doi: 10.1016/j.ejmp.2019.08.010
Wang, C., Xiao, Z., Wang, B., and Wu, J. (2019a). Identification of autism based on SVM-RFE and stacked sparse auto-encoder. IEEE Access 7, 118030–118036. doi: 10.1109/ACCESS.2019.2936639
Wang, M., Zhang, D., Huang, J., Yap, P.-T., Shen, D., and Liu, M. (2019c). Identifying autism spectrum disorder with multi-site fMRI via low-rank domain adaptation. IEEE Trans. Med. Imaging 39, 644–655. doi: 10.1109/TMI.2019.2933160
Wang, J., Zhang, L., Wang, Q., Chen, L., Shi, J., Chen, X., et al. (2020). Multi-class ASD classification based on functional connectivity and functional correlation tensor via multi-source domain adaptation and multi-view sparse representation. IEEE Trans. Med. Imaging 39, 3137–3147. doi: 10.1109/TMI.2020.2987817
Wang, L., Li, K., and Hu, X. P. (2021a). Graph convolutional network for fMRI analysis based on connectivity neighborhood. Netw. Neurosci. 5, 83–95. doi: 10.1162/netn_a_00171
Wang, S. H., Nayak, D. R., Guttery, D. S., Zhang, X., and Zhang, Y. D. (2021c). COVID-19 classification by CCSHNet with deep fusion using transfer learning and discriminant correlation analysis. Information Fusion 68, 131–148. doi: 10.1016/j.inffus.2020.11.005
Wang, S.-H., Du, J., Xu, H., Yang, D., Ye, Y., Chen, Y., et al. (2021b). Automatic discrimination of different sequences and phases of liver MRI using a dense feature fusion neural network: A preliminary study. Abdom. Radiol. 46, 4576–4587. doi: 10.1007/s00261-021-03142-4
Wang, Y., Liu, J., Xiang, Y., Wang, J., Chen, Q., and Chong, J. (2022). MAGE: Automatic diagnosis of autism spectrum disorders using multi-atlas graph convolutional networks and ensemble learning. Neurocomputing 469, 346–353. doi: 10.1016/j.neucom.2020.06.152
Wang, Y., Wang, J., Wu, F.-X., Hayrat, R., and Liu, J. (2020). AIMAFE: Autism spectrum disorder identification with multi-atlas deep feature representation and ensemble learning. J. Neurosci. Methods 343:108840. doi: 10.1016/j.jneumeth.2020.108840
Wee, C. Y., Wang, L., Shi, F., Yap, P. T., and Shen, D. (2014). Diagnosis of autism spectrum disorders using regional and interregional morphological features. Hum. Brain Mapp. 35, 3414–3430. doi: 10.1002/hbm.22411
Wen, G., Cao, P., Bao, H., Yang, W., Zheng, T., and Zaiane, O. (2022). MVS-GCN: A prior brain structure learning-guided multi-view graph convolution network for autism spectrum disorder diagnosis. Comput. Biol. Med. 142:105239. doi: 10.1016/j.compbiomed.2022.105239
Wismüller, A., Foxe, J. J., Geha, P., and Saboksayr, S. S. (2020). “Large-scale Extended Granger Causality (lsXGC) for classification of Autism Spectrum Disorder from resting-state functional MRI,” in Proceedings of the Medical Imaging 2020: Computer-Aided Diagnosis, (Bellingham, WA: SPIE), 458–465. doi: 10.1117/12.2550027
Wold, S., Esbensen, K., and Geladi, P. (1987). Principal component analysis. Chemometr. Intell. Lab. Syst. 2, 37–52. doi: 10.1016/0169-7439(87)80084-9
Wong, A., Famuori, M., Shafiee, M. J., Li, F., Chwyl, B., and Chung, J. (2019). “Yolo nano: A highly compact you only look once convolutional neural network for object detection,” in Proceedings of the 2019 Fifth Workshop on Energy Efficient Machine Learning and Cognitive Computing-NeurIPS Edition (EMC2-NIPS), (Piscataway, NJ: IEEE), 22–25. doi: 10.1109/EMC2-NIPS53020.2019.00013
Wu, W., Wu, A., and Zheng, W.-S. (2018). “Light person re-identification by multi-cue tiny net,” in Proceedings of the 2018 25th IEEE International Conference on Image Processing (ICIP), (Athens: IEEE), 1643–1647. doi: 10.1109/ICIP.2018.8451738
Wu, Z., Pan, S., Chen, F., Long, G., Zhang, C., and Philip, S. Y. (2020). A comprehensive survey on graph neural networks. IEEE Trans. Neural Netw. Learn. Syst. 32, 4–24. doi: 10.1109/TNNLS.2020.2978386
Xiao, X., Fang, H., Wu, J., Xiao, C., Xiao, T., Qian, L., et al. (2017). Diagnostic model generated by MRI-derived brain features in toddlers with autism spectrum disorder. Autism Res. 10, 620–630. doi: 10.1002/aur.1711
Xiao, Z., Wang, C., Jia, N., and Wu, J. (2018). SAE-based classification of school-aged children with autism spectrum disorders using functional magnetic resonance imaging. Multimed. Tools Appl. 77, 22809–22820. doi: 10.1007/s11042-018-5625-1
Xu, L., Geng, X., He, X., Li, J., and Yu, J. (2019). Prediction in autism by deep learning short-time spontaneous hemodynamic fluctuations. Front. Neurosci. 13:1120. doi: 10.3389/fnins.2019.01120
Xu, L., Liu, Y., Yu, J., Li, X., Yu, X., Cheng, H., et al. (2020). Characterizing autism spectrum disorder by deep learning spontaneous brain activity from functional near-infrared spectroscopy. J. Neurosci. Methods 331:108538. doi: 10.1016/j.jneumeth.2019.108538
Yamagata, B., Itahashi, T., Fujino, J., Ohta, H., Nakamura, M., Kato, N., et al. (2019). Machine learning approach to identify a resting-state functional connectivity pattern serving as an endophenotype of autism spectrum disorder. Brain Imaging Behav. 13, 1689–1698. doi: 10.1007/s11682-018-9973-2
Yan, K., and Zhang, D. (2015). Feature selection and analysis on correlated gas sensor data with recursive feature elimination. Sens. Actuat. B Chem. 212, 353–363. doi: 10.1016/j.snb.2015.02.025
Yang, M., Zhong, Q., Chen, L., Huang, F., and Lei, B. (2019). “Attention based semi-supervised dictionary learning for diagnosis of autism spectrum disorders,” in Proceedings of the 2019 IEEE International Conference on Multimedia & Expo Workshops (ICMEW), (Piscataway, NJ: IEEE), 7–12. doi: 10.1109/ICMEW.2019.00009
Yang, R., Ke, F., Liu, H., Zhou, M., and Cao, H.-M. (2021). Exploring sMRI biomarkers for diagnosis of autism spectrum disorders based on multi class activation mapping models. IEEE Access 9, 124122–124131. doi: 10.1109/ACCESS.2021.3069211
Yang, X., Islam, M. S., and Khaled, A. A. (2019). “Functional connectivity magnetic resonance imaging classification of autism spectrum disorder using the multisite ABIDE dataset,” in Proceedings of the 2019 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), (Chicago, IL: IEEE), 1–4.
Yang, X., Sarraf, S., and Zhang, N. (2018). Deep learning-based framework for Autism functional MRI image classification. J. Arkansas Acad. Sci. 72, 47–52. doi: 10.1016/j.neunet.2020.03.017
Yang, X., Schrader, P. T., and Zhang, N. (2020). A deep neural network study of the ABIDE repository on autism spectrum classification. Int. J. Adv. Comput. Sci. Appl. 11. doi: 10.3389/fnins.2018.00491
Yang, X., Zhang, N., and Schrader, P. (2022). A study of brain networks for autism spectrum disorder classification using resting-state functional connectivity. Mach. Lear. Appl. 8:100290. doi: 10.1016/j.mlwa.2022.100290
Yang, Y., Wu, Q. J., and Wang, Y. (2016). Autoencoder with invertible functions for dimension reduction and image reconstruction. IEEE Trans. Syst. Man Cybern. Syst. 48, 1065–1079. doi: 10.1109/TSMC.2016.2637279
Yao, A. D., Cheng, D. L., Pan, I., and Kitamura, F. (2020). Deep learning in neuroradiology: A systematic review of current algorithms and approaches for the new wave of imaging technology. Radiol. Artif. Intell. 2:e190026. doi: 10.1148/ryai.2020190026
Yap, S. Y., and Chan, W. H. (2020). Elastic SCAD SVM cluster for the selection of significant functional connectivity in autism spectrum disorder classification. Acad. Fundam. Comput. Res. 1.
Yassin, W., Nakatani, H., Zhu, Y., Kojima, M., Owada, K., Kuwabara, H., et al. (2020). Machine-learning classification using neuroimaging data in schizophrenia, autism, ultra-high risk and first-episode psychosis. Transl. Psychiatry 10, 1–11. doi: 10.1038/s41398-020-00965-5
Ye, Z., and Yang, J. (2010). Sliced inverse moment regression using weighted chi-squared tests for dimension reduction. J. Statis. Plann. Inference 140, 3121–3131. doi: 10.1016/j.jspi.2010.04.015
Yeh, C. H., Jones, D. K., Liang, X., Descoteaux, M., and Connelly, A. (2021). Mapping structural connectivity using diffusion MRI: Challenges and opportunities. J. Magn. Reson. Imaging 53, 1666–1682.
Yin, W., Li, L., and Wu, F.-X. (2022). A semi-supervised autoencoder for autism disease diagnosis. Neurocomputing 483, 140–147. doi: 10.1016/j.neucom.2022.02.017
Yin, W., Mostafa, S., and Wu, F.-X. (2021). Diagnosis of autism spectrum disorder based on functional brain networks with deep learning. J. Comput. Biol. 28, 146–165. doi: 10.1089/cmb.2020.0252
You, Y., Liu, H., Zhang, S., and Shao, L. (2020). “Classification of autism based on fMRI data with feature-fused convolutional neural network,” in Proceedings of the Cyberspace Data and Intelligence, and Cyber-Living, Syndrome, and Health, (Berlin: Springer), 77–88. doi: 10.1007/978-981-33-4336-8_7
Zarghami, T. S., and Friston, K. J. (2020). Dynamic effective connectivity. Neuroimage 207:116453. doi: 10.1016/j.neuroimage.2019.116453
Zeng, L.-L., Wang, H., Hu, P., Yang, B., Pu, W., Shen, H., et al. (2018). Multi-site diagnostic classification of schizophrenia using discriminant deep learning with functional connectivity MRI. EBioMedicine 30, 74–85.
Zhan, Y., Wei, J., Liang, J., Xu, X., He, R., Robbins, T. W., et al. (2021). Diagnostic classification for human autism and obsessive-compulsive disorder based on machine learning from a primate genetic model. Am. J. Psychiatry 178, 65–76. doi: 10.1176/appi.ajp.2020.19101091
Zhang, D., Yin, J., Zhu, X., and Zhang, C. (2018). Network representation learning: A survey. IEEE Trans. Big Data 6, 3–28. doi: 10.1109/TBDATA.2018.2850013
Zhang, F., and Roeyers, H. (2019). Exploring brain functions in autism spectrum disorder: A systematic review on functional near-infrared spectroscopy (fNIRS) studies. Int. J. Psychophysiol. 137, 41–53. doi: 10.1016/j.ijpsycho.2019.01.003
Zhang, F., Wei, Y., Liu, J., Wang, Y., Xi, W., and Pan, Y. (2022). Identification of Autism spectrum disorder based on a novel feature selection method and Variational Autoencoder. arXiv [Preprint]. doi: 10.48550/arXiv.2204.03654
Zhang, L., Wang, X.-H., and Li, L. (2020). Diagnosing autism spectrum disorder using brain entropy: A fast entropy method. Comput. Methods Programs Biomed. 190:105240. doi: 10.1016/j.cmpb.2019.105240
Zhang, Z., Cui, P., and Zhu, W. (2020). Deep learning on graphs: A survey. IEEE Trans. Knowl. Data Eng. 34, 249–270. doi: 10.1109/TKDE.2020.2981333
Zhang, M., Zhao, X., Zhang, W., Chaddad, A., Evans, A., and Poline, J. B. (2020). “Deep discriminative learning for autism spectrum disorder classification,” in Proceedings of the International Conference on Database and Expert Systems Applications, (Berlin: Springer), 435–443. doi: 10.1007/978-3-030-59003-1_29
Zhang, X., Ding, X., Wu, Z., Xia, J., Ni, H., Xu, X., et al. (2020). “Siamese verification framework for autism identification during infancy using cortical path signature features,” in Proceedings of the 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI), (Piscataway, NJ: IEEE), 1–4. doi: 10.1109/isbi45749.2020.9098385
Zhang, Y. D., Dong, Z., Wang, S. H., Yu, X., Yao, X., Zhou, Q., et al. (2020). Advances in multimodal data fusion in neuroimaging: Overview, challenges, and novel orientation. Inf. Fusion 64, 149–187. doi: 10.1016/j.inffus.2020.07.006
Zhang, Y., Zhou, X., Witt, R. M., Sabatini, B. L., Adjeroh, D., and Wong, S. T. (2007). Dendritic spine detection using curvilinear structure detector and LDA classifier. Neuroimage 36, 346–360. doi: 10.1016/j.neuroimage.2007.02.044
Zhao, F., Qiao, L., Shi, F., Yap, P.-T., and Shen, D. (2017). Feature fusion via hierarchical supervised local CCA for diagnosis of autism spectrum disorder. Brain Imaging Behav. 11, 1050–1060. doi: 10.1007/s11682-016-9587-5
Zhao, F., Zhang, H., Rekik, I., An, Z., and Shen, D. (2018). Diagnosis of autism spectrum disorders using multi-level high-order functional networks derived from resting-state functional MRI. Front. Hum. Neurosci. 12:184. doi: 10.3389/fnhum.2018.00184
Zhao, Y., Dai, H., Zhang, W., Ge, F., and Liu, T. (2019). “Two-stage spatial temporal deep learning framework for functional brain network modeling,” in Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), (Venice: IEEE), 1576–1580.
Zhao, Y., Dong, Q., Zhang, S., Zhang, W., Chen, H., Jiang, X., et al. (2017). Automatic recognition of fMRI-derived functional networks using 3-D convolutional neural networks. IEEE Trans. Biomed. Eng. 65, 1975–1984. doi: 10.1109/TBME.2017.2715281
Zhao, Y., Ge, F., Zhang, S., and Liu, T. (2018). “3D deep convolutional neural network revealed the value of brain network overlap in differentiating autism spectrum disorder from healthy controls,” in Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, (Berlin: Springer), 172–180. doi: 10.1007/978-3-030-00931-1_20
Zheng, W., Eilam-Stock, T., Wu, T., Spagna, A., Chen, C., Hu, B., et al. (2019). Multi-feature based network revealing the structural abnormalities in autism spectrum disorder. IEEE Trans. Affect. Comput. 12, 732–742. doi: 10.1109/TAFFC.2018.2890597
Zhou, N., and Wang, L. (2007). A modified T-test feature selection method and its application on the HapMap genotype data. Genomics Proteomics Bioinformatics 5, 242–249. doi: 10.1016/S1672-0229(08)60011-X
Zhou, Y., Yu, F., and Duong, T. (2014). Multiparametric MRI characterization and prediction in autism spectrum disorder using graph theory and machine learning. PLoS One 9:e90405. doi: 10.1371/journal.pone.0090405
Zhuang, J., Dvornek, N. C., Li, X., Yang, D., Ventola, P., and Duncan, J. S. (2018b). “Prediction of pivotal response treatment outcome with task fMRI using random forest and variable selection,” in Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), (Piscataway, NJ: IEEE), 97–100. doi: 10.1109/ISBI.2018.8363531
Zhuang, J., Dvornek, N. C., Li, X., Ventola, P., and Duncan, J. S. (2018a). “Prediction of severity and treatment outcome for ASD from fMRI,” in Proceedings of the International workshop on predictive intelligence in medicine, (Berlin: Springer), 9–17. doi: 10.1007/978-3-030-00320-3_2
Keywords: ASD diagnosis, neuroimaging, MRI modalities, machine learning, deep learning
Citation: Moridian P, Ghassemi N, Jafari M, Salloum-Asfar S, Sadeghi D, Khodatars M, Shoeibi A, Khosravi A, Ling SH, Subasi A, Alizadehsani R, Gorriz JM, Abdulla SA and Acharya UR (2022) Automatic autism spectrum disorder detection using artificial intelligence methods with MRI neuroimaging: A review. Front. Mol. Neurosci. 15:999605. doi: 10.3389/fnmol.2022.999605
Received: 21 July 2022; Accepted: 09 August 2022;
Published: 04 October 2022.
Edited by:
Dezhi Liao, University of Minnesota Twin Cities, United StatesReviewed by:
Y. Y. Cai, Nanyang Technological University, SingaporeYu-Dong Zhang, University of Leicester, United Kingdom
Copyright © 2022 Moridian, Ghassemi, Jafari, Salloum-Asfar, Sadeghi, Khodatars, Shoeibi, Khosravi, Ling, Subasi, Alizadehsani, Gorriz, Abdulla and Acharya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Afshin Shoeibi, QWZzaGluLnNob2VpYmlAZ21haWwuY29t; Sara A. Abdulla, c2FhYmR1bGxhQGhia3UuZWR1LnFh
 Parisa Moridian
Parisa Moridian Navid Ghassemi
Navid Ghassemi Mahboobeh Jafari
Mahboobeh Jafari Salam Salloum-Asfar
Salam Salloum-Asfar Delaram Sadeghi
Delaram Sadeghi Marjane Khodatars5
Marjane Khodatars5 Afshin Shoeibi
Afshin Shoeibi Abbas Khosravi
Abbas Khosravi Sai Ho Ling
Sai Ho Ling Abdulhamit Subasi
Abdulhamit Subasi Roohallah Alizadehsani
Roohallah Alizadehsani Juan M. Gorriz
Juan M. Gorriz Sara A. Abdulla
Sara A. Abdulla U. Rajendra Acharya
U. Rajendra Acharya