- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea
- 2Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea
- 3Department of Radiation Oncology, Yonsei University College of Medicine, Seoul, South Korea
- 4Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea
Background: Sarcopenia, defined as skeletal muscle loss, has been known as a poor prognosis factor in various malignant diseases The aim of this study is to investigate the effect of sarcopenia on prognosis in patients with esophageal cancer who received concurrent chemo- and radiotherapy (CCRT).
Methods: We retrospectively collected clinical data of 287 patients with esophageal cancer who were treated by definite CCRT at Gangnam Severance and Severance hospital from August 2005 to December 2014. The cross-sectional area of muscle at the level of the third lumbar vertebra was measured using pre- and post-CCRT computed tomography images. Sarcopenia was defined as skeletal muscle index <49 cm2/m2 for men and of <31 cm2/m2 for women by Korean-specific cutoffs. Overall survival (OS) and progression free survival (PFS) were analyzed according to sarcopenia.
Results: Sarcopenia identified before CCRT did not affect OS and PFS. However, patients with post-CCRT sarcopenia showed shorter OS and PFS than patients without it (median OS: 73 months vs. 28 months; median PFS: 34 months vs. 25 months, respectively). Post-CCRT sarcopenia was an independent prognostic factor of poor OS (hazards ratio: 1.697; 95% confidence interval: 1.036–2.780; P = 0.036). In multivariate analysis, male sex (P = 0.004) and presence of CCRT-related complications, such as esophagitis or general weakness were significantly associated with post-CCRT sarcopenia (P = 0.016).
Conclusions: Sarcopenia after CCRT can be a useful predictor for long-term prognosis in patients with esophageal cancer. To control CCRT-related complications may be important to prevent skeletal muscle loss during CCRT.
Introduction
Esophageal cancer is widely known as fatal and aggressive disease (1). In the past, surgical treatment was the standard treatment for esophageal cancer. Because of the poor prognosis after surgery, a multidisciplinary approach is being made for the treatment of esophageal cancer (2, 3). Concurrent chemo- and radiotherapy (CCRT) is not only a treatment option for inoperable esophageal cancer, but also is known as prefer approach for locally advanced esophageal cancer (4, 5).
The patients with esophageal cancer mostly suffer from dysphagia and poor oral intake, and it makes deleterious effects to treatment compliance and outcomes. Therefore, it is necessary for patients with esophageal cancer to help prevent malnutrition through adequate nutritional support (6). Sarcopenia is a condition characterized by loss of muscle mass and strength, and critically associated with numerous clinical conditions such as malnutrition, endocrine disease, inflammatory disease, and malignancy (7, 8). It was originally described in the elderly people, but now usually recognized in cancer patients. The decrease in muscle mass is not always correlated with changes in body weight, so it is not always the same as the decrease in body mass index (BMI). Recent studies suggest that changes in nutritional status and body composition are associated with prognosis in cancer patients (9–12).
To assess muscle mass, various measurement methods are used. Among them, computed tomography (CT) scan showed superiority in accuracy and precision for evaluating muscle and fat mass (13). Several studies have revealed the relationship between preoperative sarcopenia and prognosis in patients with esophageal cancer (14–17).
However, in our best knowledge, there is no study of the relationship between sarcopenia and prognosis in esophageal cancer patients who received definite CCRT. Therefore, we aimed to investigate the changes in body composition before and after CCRT and the impact of pre/post-CCRT sarcopenia on the prognosis of these patients.
Materials and Methods
Patients and Clinical Characteristics
All consecutive patients who were diagnosed with esophageal cancer and treated with definite CCRT at Gangnam Severance and Severance hospital from August 2005 to December 2014 were retrospectively collected for this analysis. A total of 287 patients with pathologically proven esophageal cancer were included after dedicated electronic medical records review. Of these, 9 patients had an additional surgery after CCRT, 25 patients had overlapping of esophageal cancer with another cancer, and 55 patients did not have sufficient CT images to measure muscle mass. Thus, except for these patients, a total of 198 patients were finally included for our study.
The initial performance status of patients was recorded by Eastern Cooperative Oncology Group (ECOG) score. Hemoglobin, albumin and neutrophil/lymphocyte ratio (NLR) at the time of diagnosis of esophageal cancer were collected to identify patient's nutritional and inflammatory status related to prognosis (18). Tumor location, histopathologic grade, clinical stage were classified according to AJCC/UICC staging (19). Esophagogastroduodenoscopy, CT, endoscopic ultrasound, and positron emission tomography-computed tomography (PET-CT) were applied to determine the clinical stage. Patients were observed at 3 to 6 months intervals until Aug 31, 2017 or death, whichever came first. Overall survival (OS) was defined as the length of time from the date of initial diagnosis to the date of death. Progression free survival (PFS) was defined as the length of time after treatment for a cancer ended that the patient survived without any signs of symptoms of the cancer. The Institutional Review Board of Gangnam Severance Hospital waived the need for approval of this study.
Radiotherapy (RT) was performed with 3-dimentional conformal RT or intensity-modulated RT (IMRT) starting on day 1 of chemotherapy and a conventional fractionation schedule (1.8–2.0 Gy per fraction, 5 days per week) and cone-down technique were used in all patients. The gross tumor volume (GTV) was delineated using (PEC/CT) fusion on the MIM software (Cleveland, OH) or Pinnacle Radiotherapy Planning System (Phillips Medical System, Andover, MA). Endoscopic clips were also used for contouring GTV. The initial clinical target volume (CTV) included the GTV plus a margin of at least 5 cm longitudinally and 2 cm radially. The initial CTV received 30.6–50.4 Gy (median dose, 36 Gy), and at the time of cone-down, final CTV encompassed the GTV with a 2 cm margin longitudinally and radially. The total prescribed radiation dose ranged from 50.4 to 66 Gy according to the physicians' decisions. Previous study in our institution reported that higher dose (>60 Gy) yielded better outcome without increase of any toxicity and higher dose scheme has been used in our institution (20).
Two cycles of chemotherapy were administered during CCRT period. Consolidation chemotherapy was conducted between 4 and 6 weeks after the completion of CCRT. Complications associated with CCRT were defined as development of 1 or more adverse effect with common toxicity criteria grade 2 or higher during CCRT period.
Image Analysis
Because dual-energy X-ray absorptiometry (DXA) is used infrequently in routine clinical practice, we used a previously validated CT-based body composition method with scans acquired at the initial diagnosis (e.g., whole-body PET-CT or abdominal CT imaging). We also obtained post CCRT images that were taken within 6 months after the end of CCRT. The third lumbar vertebra (L3) was selected as a landmark for calculation since the cross-sectional area of tissues in this region provide an established means of estimating body composition parameters quantities in general population (21).
We obtained skeletal muscle volume and total adipose tissue volume at L3 level, and divided by CT slice thickness, respectively, to calculate skeletal muscle area and total adipose tissue area. The MIM Vista software was used to demarcate skeletal muscle, visceral fat tissue, and subcutaneous fat tissue according to predefined validated boundaries based on Hounsfield units (HUs). The following thresholds were applied: −29 to +150 HU for skeletal muscle, −150 to −50 HU for visceral fat tissue, and −190 to −30 HU for subcutaneous fat tissue. The radiation oncologist and the radiotherapy technician (Ik Jae Lee and Mi-jin Jeon) who performed these measurements was blinded to the treatment outcomes of all patients to minimize bias (Figure 1).
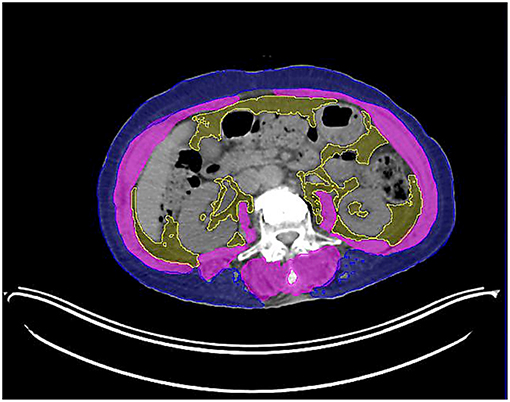
Figure 1. Axial computed tomography image of the third lumbar vertebra used for the assessment of skeletal muscle area (highlighted in pink).
Skeletal muscle index (SMI), fat-free mass (FFM), and total body fat mass (FM) were estimated as follows (21): SMI (cm2/m2) = skeletal muscle area (cm2) / height2 (m2), FFM (kg) = 0.3 * skeletal muscle area (cm2) + 6.06, FM (kg) = 0.042 * total adipose tissue area (cm2) + 11.2. In general, sarcopenia is defined as a L3 SMI of <55 cm2/m2 for men and <39 cm2/m2 for women, as proposed by international consensus of cancer cachexia (22). However, we used Korean-specific cutoff values of 49 cm2/m2 for men and 31 cm2/m2 for women based on a previous epidemiologic study using DXA and a regression equation,
to convert the CT value (9).
Statistical Analysis
All continuous variables were reported as median and range. The chi-square and Fisher's exact tests were used to compare between various categorical variables, and Student t-test and Mann-Whitney U-test were used for non-categorical variables. Multivariate logistic regression analysis was used to identify independent risk factors for predicting post–CCRT sarcopenia. OS and PFS were estimated using the Kaplan-Meier method, and differences between curves were evaluated using the log-rank test. A Cox proportional hazards model was used to analyze risk factors that affect OS. All variables with P < 0.05 was on the univariate analysis were entered the multivariate analysis. The statistical calculations were performed using SPSS version 18.0 for Windows software (SPSS Inc., Chicago, IL, USA).
Results
Baseline Characteristics of Patients and Tumors
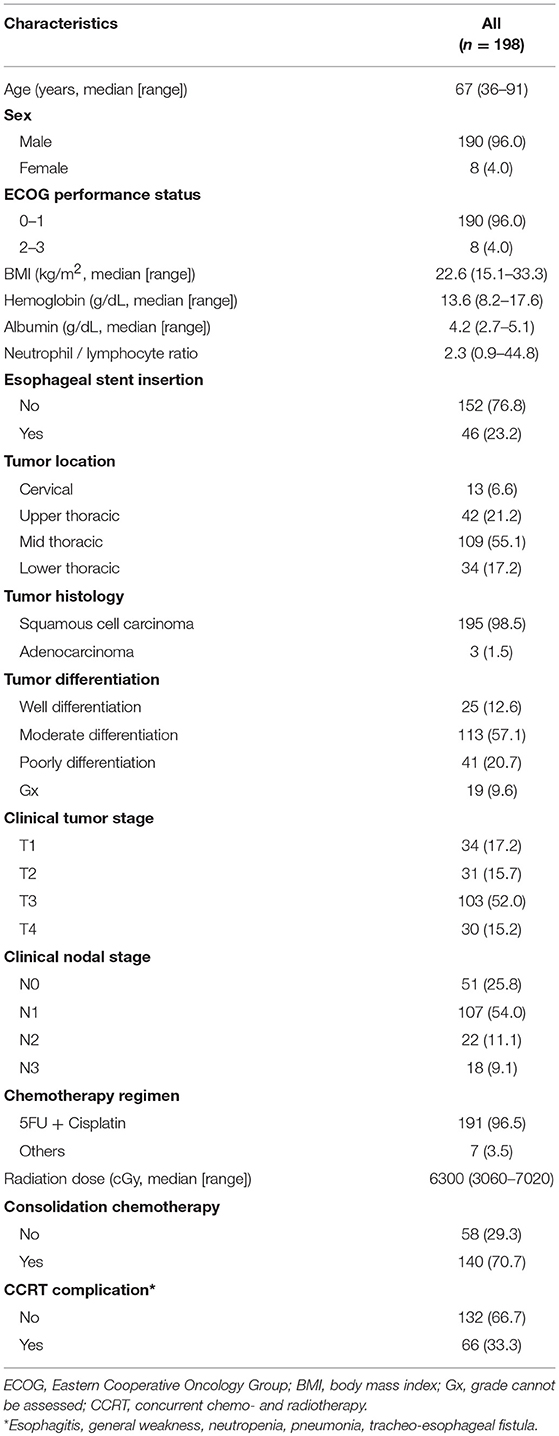
A total of 198 consecutive patients were enrolled in our study (Table 1). The median age was 67 years (range 36–91), and most of the patients were men (96%). One hundred ninety patients (96%) had good ECOG performance score (0–1). Of the 198 patients, 46 (23.2%) patients underwent esophageal stent insertion due to obstructive symptoms. More than half (55.1%) of the tumors were in the middle esophagus. The histologic type of tumor was squamous cell carcinoma in most cases (98.5%) and adenocarcinoma in 3 cases. Of all patients, 67.2% had a clinical tumor stage greater than T2, and 74.2% had a clinical nodal stage greater than N0. According to clinical TNM stage, stage I was 30 patients (15.2%), stage II was 40 patients (20.2%), stage III was 68 patients (34.3%), stage IVA was 37 patients (18.7%), and stage IVB was 23 patients (11.6%). Median total radiation dose was 6,300 cGy. There were 140 patients (70.7%) who received the consolidation chemotherapy after CCRT. We found 66 patients (33.3%) who suffered from CCRT related complications. Among them, esophagitis was the most common (n = 48). Other complications were general weakness (n = 9), neutropenia (n = 6), pneumonia (n = 2), and tracheo-esophageal fistula (n = 1).
Changes in Body Composition Before and After Treatment
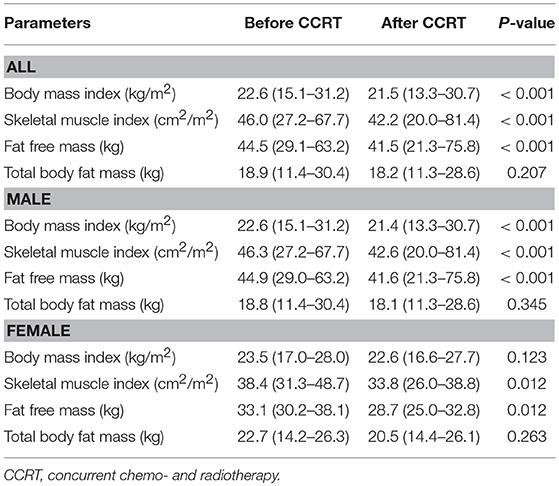
We compared BMI, SMI, FFM, and FM before and after CCRT except 25 patients who did not have post–CCRT CT image. BMI, SMI, and FFM after CCRT were statistically significantly decreased (P < 0.001). Also, we identified 25 patients with sarcopenia following CCRT. However, FM did not show any significant difference before and after CCRT. In female subgroup, there was no statistically difference in BMI before and after CCRT (Table 2).
Analysis of Risk Factors Affecting Overall Survival Rate
Patients with sarcopenia before CCRT did not showed significantly decreased OS compared to non-sarcopenic patients (Figure 2). However, patients with sarcopenia after CCRT demonstrated significantly poor OS compared to non-sarcopenic patients (median OS 45 months vs. 74 months, P = 0.007). Also, patient with sarcopenia after CCRT showed poor PFS compared to non-sarcopenia patients, but patients with sarcopenia before CCRT did not.
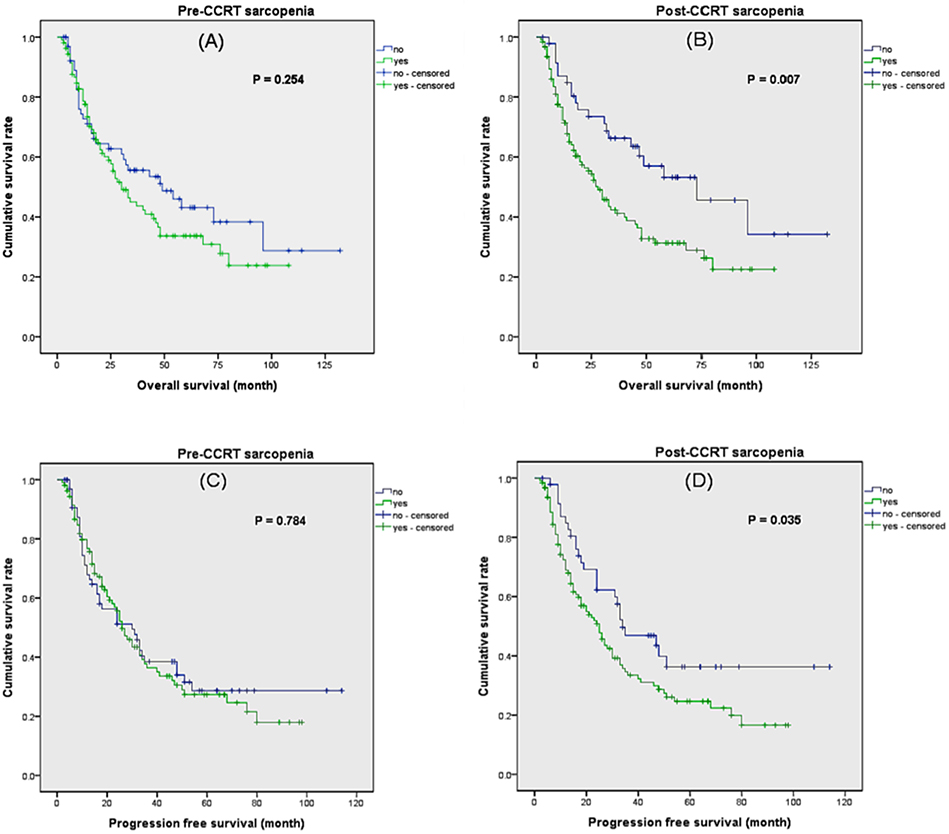
Figure 2. Kaplan–Meier analysis of overall survival and progression free survival according to sarcopenia. (A) Overall survival according to pre–CCRT sarcopenia. (B) Overall survival according to post–CCRT sarcopenia. (C) Progression free survival according to pre–CCRT sarcopenia. (D) Progression free survival according to post–CCRT sarcopenia.
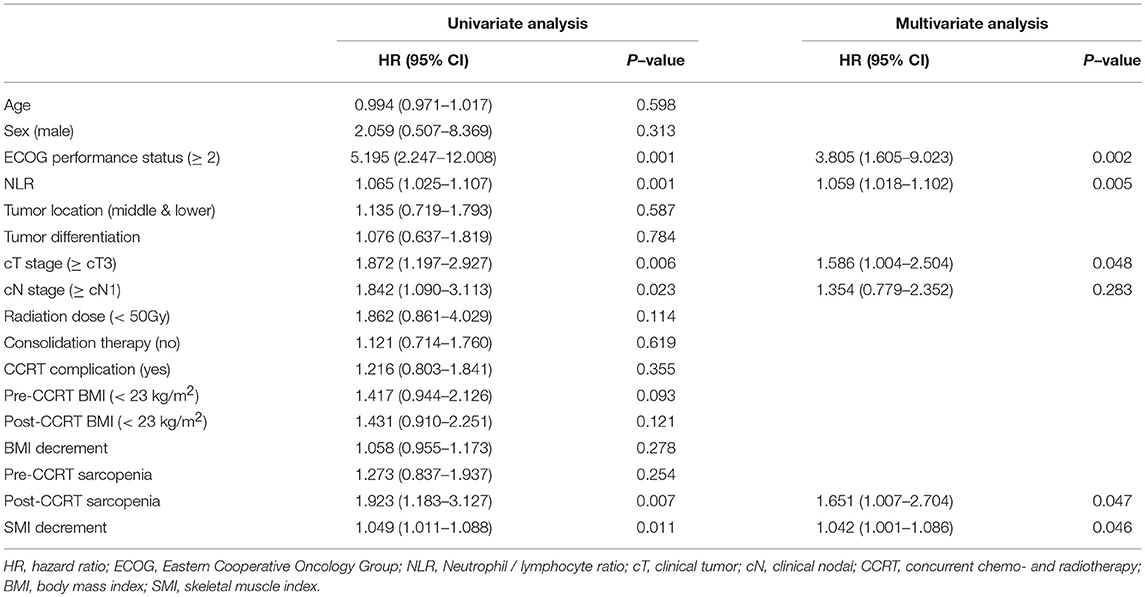
We analyzed several risk factors that might influence OS (Table 3). In univariate analysis, higher ECOG performance status (≥2), higher NLR, clinical tumor stage (≥cT3), and clinical nodal stage (≥cN1) showed significant relationship with OS. The presence of sarcopenia before CCRT did not affect OS. In contrast, patients who were diagnosed as sarcopenia after CCRT showed impaired survival. Multivariate analysis revealed that higher ECOG performance status, higher NLR, higher clinical tumor stage, and post-CCRT sarcopenia were independent risk factors for decreased OS. Furthermore, SMI decrement was associated with poor OS regardless of sarcopenia. On the other hand, tumor location, tumor differentiation, radiation dose, consolidation therapy, and CCRT complication did not show statistical correlation with OS.
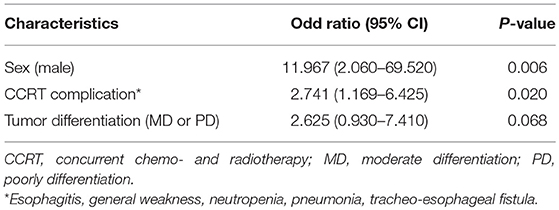
Also, we analyzed what factors affected post–CCRT sarcopenia. The relationship between the factors described in Table 1 and post–CCRT sarcopenia was analyzed. As a result, male sex, CCRT complications, and tumor differentiation (moderate and poorly differentiation) showed statistically significant relationship in univariate analysis. In multivariate analysis, male sex, and CCRT complications were significantly associated with post–CCRT sarcopenia (Table 4).
Changes in Sarcopenic Status After CCRT and Overall Survival
All study population were classified according to the timing of onset of sarcopenia (Figure 3). They were divided 3 groups: patients who were not diagnosed with sarcopenia after CCRT (group A, n = 48, median follow up = 38 months), patients who were not diagnosed with sarcopenia before CCRT, but were diagnosed with sarcopenia after CCRT (group B, n = 25, median follow up = 11 months), patients who were diagnosed with sarcopenia before and after CCRT (group C, n = 101, median follow up = 20 months). Patients who had never been diagnosed with sarcopenia (group A) had a significantly longer survival rate than group B and C (P = 0.016 and 0.024, respectively). However, patients with post–CCRT sarcopenia had no difference in survival rates regardless of pre–CCRT sarcopenia (P = 0.454). In other words, patients with post–CCRT sarcopenia showed worse overall survival than patients without post–CCRT sarcopenia. Furthermore, the presence of pre–CCRT sarcopenia did not affect the overall survival rate.
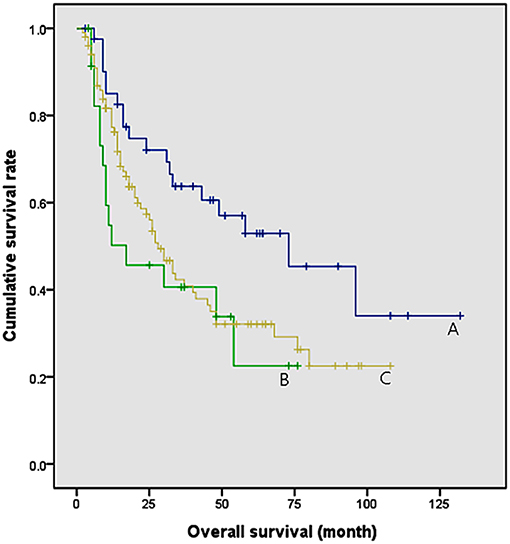
Figure 3. Comparison of survival rates according to the time of onset of sarcopenia. Group A: patients who were not diagnosed with sarcopenia after CCRT (median survival = 73 months). Group B: patients who were not diagnosed with sarcopenia before CCRT, but were diagnosed with sarcopenia after CCRT (median survival = 17 months). Group C: patients who were diagnosed with sarcopenia before and after CCRT (median survival = 28 months). Group A and B, P = 0.016; Group A and C, P = 0.024; Group B and C, P = 0.454.
Discussion
In this study, we investigated the relationship between the sarcopenia and the prognosis of patients with esophageal cancer who treated with definite CCRT. Although there have been many similar studies, most of them have been related to surgery, and studies in patients with CCRT have been lacking (14–17). In addition, our study is characterized by comparing muscle mass before and after treatment. As a result, the patient with sarcopenia before CCRT did not show significant association with overall survival, but the patient with sarcopenia after CCRT did. In other words, presence of sarcopenia after CCRT affected an unfavorable effect on the prognosis, regardless of whether it occurred before or after treatment. Also, it was found that male sex and CCRT related complications were associated with post CCRT sarcopenia.
The relationship between sarcopenia and prognosis has been known as controversial in patients with surgically treated esophageal cancer. Sheetz et al. reported that core muscle size was associated with poor OS, in patients following esophagectomy. In contrast, it was not associated with OS, in patients receiving neoadjuvant chemoradiation (6). Similar to this study, another study found that the presence of sarcopenia did not show significant correlation with a short- and long-term outcome in esophageal cancer patients after neoadjuvant chemoradiotherapy followed by esophagectomy (14). However, there were several papers that proved the relationship between sarcopenia and clinical outcome in patients after esophageal resection who underwent neoadjuvant chemoradiotherapy (15–17). Our study shows differences from previous studies in that only patients who underwent definite CCRT were included. In addition, the previous studies were mainly executed in Western countries such as Europe and the United States, but our study is characterized by research conducted in Asia where esophageal squamous cell carcinomas develop more often than adenocarcinoma.
Sarcopenia could be a characteristic of esophageal cancer itself or one of the complications associated with treatment. Cancer treatments such as surgery, chemotherapy, or RT are known to contribute to muscle loss by causing anorexia. Also, various circulating inflammatory mediators such as tumor necrosis factor-α and interluekin-6 have been implicated in excessive muscle proteolysis (23). In our study, we revealed that patients with sarcopenia after CCRT were associated with a poor prognosis. In contrast, the presence of sarcopenia before treatment was not associated with prognosis. CCRT complications were one of the factors when multivariate analysis of factors related to the incidence of post-CCRT sarcopenia. Although the cause of sarcopenia after CCRT is not yet established, the characteristics of esophageal cancer itself, and changes in nutritional status due to the complications of CCRT may contribute to sarcopenia. In our study, esophagitis was the most common complication after CCRT. It could cause dysphagia or odynophagia, and aggravate malnutrition for patients (24). Not only topical anesthetics and analgesics, but also dietary modification could be helpful to control the symptoms (25). Preventing the occurrence of sarcopenia by providing adequate nutritional support and proper management for complications during the CCRT period could facilitate the improvement of the patient's prognosis.
Our study has several limitations. First, we collected data retrospectively from electro medical records. It was not a prospective study, and some data were limited. We could not include 25 patients who did not undergo skeletal muscle mass assessment after CCRT, and they should be considered in the interpretation of data because the possibility of their poor prognosis. Also, toxicity surveillance was performed imperfectly due to retrospective design. Second, the definition of sarcopenia was different from that of Western countries. International consensus of cancer cachexia proposed the definition of sarcopenia as a L3 muscle index of <55 cm2/m2 for men and of <39 cm2/m2 for women (22). However, we did not use this definition because the BMI of Western and Eastern people were quite different. If western definitions are applied, the incidence of pre-CCRT sarcopenia in our study rises from 62.6 to 84.8%. We consider that the definition used in Western countries are not necessarily applicable to Korean esophageal cancer patients who generally have a smaller physique.
In conclusion, we investigated the relationship between prognosis and sarcopenia in esophageal cancer patients receiving definite CCRT, and found that patients with sarcopenia identified after CCRT were associated with a poor prognosis. That is to say, post–CCRT sarcopenia can be a reliable predictor for prognosis. This means that proper support for preventing skeletal muscle loss during CCRT may help improve the prognosis of patients with esophageal cancer. In particular, complications that occur during CCRT may impair the nutritional status of the patient, so it is important to cope with complications appropriately.
Ethics Statement
The Institutional Review Board of Gangnam Severance Hospital waived the need for approval of this study.
Author Contributions
DWM conducted the analysis and interpretation of the data, drafted the article, and revised it critically for important intellectual content. YNC made substantial contributions to the analysis and interpretation of the data, and revised it critically for important intellectual content. M-JJ made substantial contributions to the analysis and interpretation of the data. J-HK made substantial contributions to the conception and design, interpreted the data and revised it critically for important intellectual content, approved the article for publication and agreed to be accountable for all aspects of the work. IJL interpreted the data, critically revised it for important intellectual content, approved the article for publication and agreed to be accountable for all aspects of the work. YHY, JJP, DHJ, HJP, CGL, JWK, and HCJ critically revised the article for important intellectual content.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
2. Park JW, Kim JH, Choi EK, Lee SW, Yoon SM, Song SY, et al. Prognosis of esophageal cancer patients with pathologic complete response after preoperative concurrent chemoradiotherapy. Int J Rad Oncol Biol Phys. (2011) 81:691–7. doi: 10.1016/j.ijrobp.2010.06.041
3. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. (2006) 355:11–20. doi: 10.1056/NEJMoa055531
4. Kato H, Nakajima M. Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg. (2013) 61:330–5. doi: 10.1007/s11748-013-0246-0.
5. Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. (2011) 12:681–92. doi: 10.1016/s1470-2045(11)70142-5
6. Sheetz KH, Zhao L, Holcombe SA, Wang SC, Reddy RM, Lin J, et al. Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophag. (2013) 26:716–22. doi: 10.1111/dote.12020
7. Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.” Clin Nutr. (2010) 29:154–9. doi: 10.1016/j.clnu.2009.12.004
8. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Age. (2010) 39:412–23. doi: 10.1093/ageing/afq034
9. Kim EY, Kim YS, Park I, Ahn HK, Cho EK, Jeong YM. Prognostic significance of CT-determined sarcopenia in patients with small-cell lung cancer. J Thorac Oncol. (2015) 10:1795–9. doi: 10.1097/JTO.0000000000000690
10. Iritani S, Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol. (2015) 50:323–32. doi: 10.1007/s00535-014-0964-9
11. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. (2016) 57:58–67. doi: 10.1016/j.ejca.2015.12.030
12. Zheng ZF, Lu J, Zheng CH, Li P, Xie JW, Wang JB, et al. A novel prognostic scoring system based on preoperative sarcopenia predicts the long-term outcome for patients after R0 resection for gastric cancer: experiences of a high-volume center. Ann Surg Oncol. (2017) 24:1795–803. doi: 10.1245/s10434-017-5813-7
13. Cooper C, Fielding R, Visser M, van Loon LJ, Rolland Y, Orwoll E, et al. Tools in the assessment of sarcopenia. Calc Tis Int. (2013) 93:201–10. doi: 10.1007/s00223-013-9757-z
14. Grotenhuis BA, Shapiro J, van Adrichem S, de Vries M, Koek M, Wijnhoven BP, et al. Sarcopenia/muscle mass is not a prognostic factor for short- and long-term outcome after esophagectomy for cancer. World J Surg. (2016) 40:2698–704. doi: 10.1007/s00268-016-3603-1
15. Paireder M, Asari R, Kristo I, Rieder E, Tamandl D, Ba-Ssalamah A, et al. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur J Surg Oncol. (2017) 43:478–84. doi: 10.1016/j.ejso.2016.11.015
16. Yip C, Goh V, Davies A, Gossage J, Mitchell-Hay R, Hynes O, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol. (2014) 24:998–1005. doi: 10.1007/s00330-014-3110-4
17. Reisinger KW, Bosmans JW, Uittenbogaart M, Alsoumali A, Poeze M, Sosef MN, et al. Loss of skeletal muscle mass during neoadjuvant chemoradiotherapy predicts postoperative mortality in esophageal cancer surgery. Ann Surg Oncol. (2015) 22:4445–52. doi: 10.1245/s10434-015-4558-4
18. Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, et al. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysis. Ann Surg Oncol. (2016) 23:646–54. doi: 10.1245/s10434-015-4869-5
19. Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothor Surg. (2017) 6:119–30. doi: 10.21037/acs.2017.03.14
20. Kim HJ, Suh YG, Lee YC, Lee SK, Shin SK, Cho BC, et al. Dose-response relationship between radiation dose and loco-regional control in patients with stage II-III esophageal cancer treated with definitive chemoradiotherapy. Cancer Res Treat. (2017) 49:669–77. doi: 10.4143/crt.2016.354
21. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metabol. (2008) 33:997–1006. doi: 10.1139/h08-075
22. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. (2011) 12:489–95. doi: 10.1016/s1470-2045(10)70218-7
23. Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. (2015) 14:58–74. doi: 10.1038/nrd4467
24. Chowhan NM. Injurious effects of radiation on the esophagus. Am J Gastroenterol. (1990) 85:115–20.
Keywords: esophageal cancer, concurrent chemo- and radiotherapy, sarcopenia, prognosis, complication
Citation: Ma DW, Cho Y, Jeon M, Kim J-H, Lee IJ, Youn YH, Park JJ, Jung DH, Park H, Lee CG, Kim JW and Jeung HC (2019) Relationship Between Sarcopenia and Prognosis in Patient With Concurrent Chemo-Radiation Therapy for Esophageal Cancer. Front. Oncol. 9:366. doi: 10.3389/fonc.2019.00366
Received: 14 September 2018; Accepted: 18 April 2019;
Published: 08 May 2019.
Edited by:
John James Tentler, University of Colorado Denver, United StatesReviewed by:
Ali Coskun, Izmir Bozyaka Egitim ve Araştirma Hastanesi, TurkeySigne Friesland, Karolinska University Hospital, Sweden
Copyright © 2019 Ma, Cho, Jeon, Kim, Lee, Youn, Park, Jung, Park, Lee, Kim and Jeung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie-Hyun Kim, otilia94@yuhs.ac
Ik Jae Lee, ikjae412@yuhs.ac
 Dae Won Ma
Dae Won Ma Yeona Cho2,3
Yeona Cho2,3 Jie-Hyun Kim
Jie-Hyun Kim Ik Jae Lee
Ik Jae Lee Hyojin Park
Hyojin Park Chang Geol Lee
Chang Geol Lee Jun Won Kim
Jun Won Kim