- 1Perlmutter Cancer Center, Langone Medical Center, New York University, New York, NY, United States
- 2New York University Winthrop Hospital, Mineola, NY, United States
Stereotactic body radiotherapy (SBRT) is an increasingly used radiation modality for the treatment of both localized and metastatic prostate cancer. Substantial data suggests that prostate cancer may be more sensitive to higher doses of radiation per fraction due to its low α/β ratio. This increased sensitivity raises important questions as to how SBRT should be combined with systemic therapy for clinically significant prostate cancer, including whether androgen deprivation therapy retains its beneficial effects when combined with SBRT. Furthermore, pre-clinical and clinical data suggest pronounced immunomodulatory effects of SBRT, including observed improvements in T cell priming and trafficking. These data support investigational strategies combining SBRT with immunotherapy. Here we aim to review the data for the use of SBRT in both the local and metastatic disease settings as well as ongoing translational and clinical research examining combinations with ADT, immunotherapy and other targeted agents.
The Shift to Hypofractionated Radiation Therapy in Prostate Cancer
Prostate cancer is the second most common cancer diagnosis in men (1). Seventy seven percent of the men diagnosed will present with localized disease (2). For these men, treatment options include active surveillance, surgery or radiation with or without androgen deprivation therapy (ADT) (3). Choice of therapy is influenced by risk stratification based on prostate specific antigen (PSA), clinical stage, Gleason grade group, disease burden on biopsy, PSA density and imaging (4). For men electing radiation, conventional radiotherapy consisting of 40 to 45 treatments over 8 to 9 weeks was the previous standard. Multiple trials have shown that 4 to 6 weeks of moderately hypofractionated RT is not inferior to conventional fractionation with respect to oncologic outcomes. However, data beyond 5 years are not yet available (5–8). A large meta-analysis confirmed similar therapeutic outcomes, albeit with a small increase in acute gastrointestinal (GI) toxicity in patients receiving moderate hypofractionation (9). However, a more recent report on quality of life (QOL) assessment for patients receiving hypofractionated vs. conventional radiotherapy as part of a phase 3 trial showed no difference in any of the QOL domains between treatment arms (10). Based on the results of these prospective studies, a 2018 consensus expert panel recommended moderate hypofractionation as a standard option for patients presenting with localized disease who are to be treated with EBRT (11). Other than the advantages of shorter treatment schedules, some reports have also shown a health care cost benefit to the moderately hypofractionated schedules when compared to the cost of conventional fractionation (12). With improvements in technology, further research has evaluated even shorter courses of radiation for both localized and metastatic disease. Stereotactic body radiotherapy is a form of external beam radiotherapy that administers higher doses of radiation per fraction of therapy. This allows for high doses of radiation to be administered in a short time; usually 5 days of treatment or less.
Radiobiology Of SBRT In Prostate Cancer
Traditional External Beam Radio Therapy (EBRT) for prostate cancer is delivered in fractions of 1.8 to 2.0 Gy over 8 to 9 weeks. This fractionation leads to high doses of radiation to the tumor while allowing time for normal tissues to recover via sublethal damage repair. However, significant data suggests that prostate cancer may be more sensitive to higher doses of radiation per fraction due to its low α / β ratio. The α / β ratio or repair capacity, describes the tumor response to changes in fractionation. A high α / β ratio reflects a small sensitivity to changes in fractionation whereas a low value reflects a large sensitivity to changes in fractionation (13). An initial report estimated prostate cancer α/β value as 1.5 Gy, which supports hypofractionation (13). A more recent meta-analysis of 8 fractionation trials including 7,946 patients estimated an α/β ratio of 1.2 Gy consistent with previous findings (14). The combination of a low α/ β ratio with the potential for better control with higher doses supports shorter fractionation treatment courses.
SBRT For Localized Disease
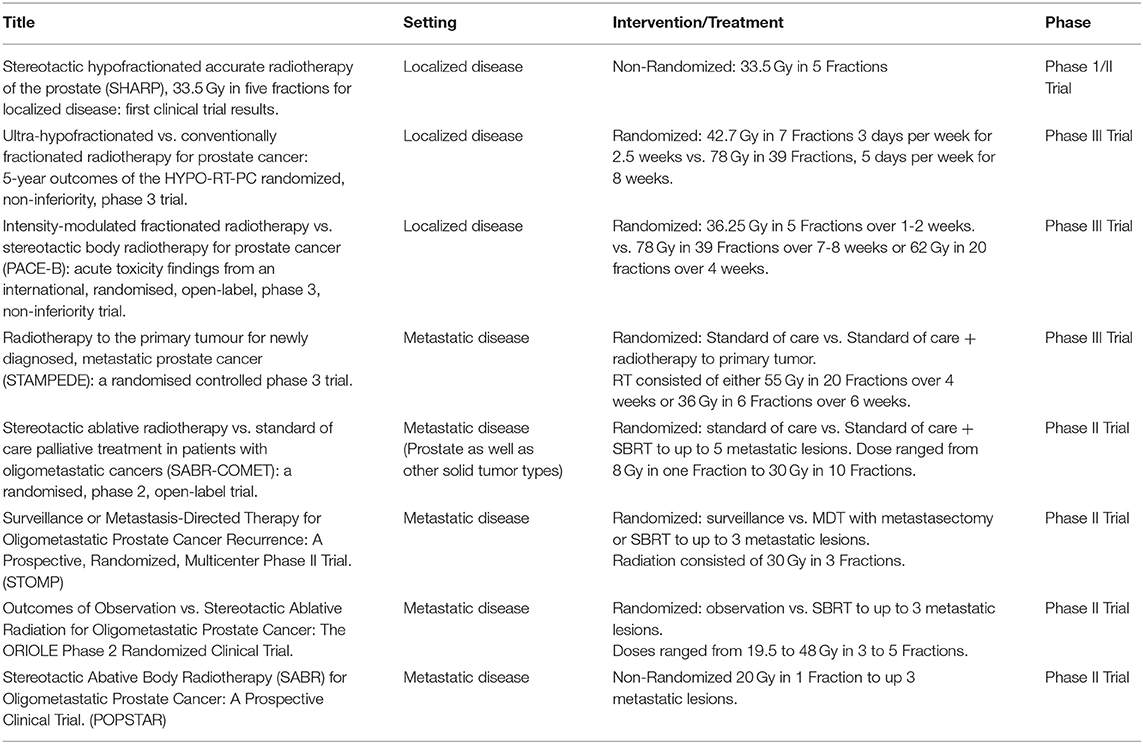
Multiple retrospective and prospective trials have evaluated the role of SBRT in patients with localized prostate cancer (see Table 1). SHARP was the first prospective clinical trial evaluating 33.5 Gy in 5 fractions. In this study of 40 patients with a median follow up of 41 months, SBRT achieved a 90% rate of biochemical progression free survival (bPFS) at 48 months and no late grade 3 or higher toxicity (15). Multiple other trials have shown similar toxicity and efficacy to conventional radiation. In a recently published systematic review and meta-analysis including over 6,000 patients with a median follow up of 39 months across all patients, SBRT led to 5 and 7 year bRFS rates of 95.3 and 93.7%, respectively (16). By two years after SBRT, both urinary and bowel toxicity returned to baseline. Of note, there was a significant association between increasing dose of SBRT and improved biochemical control at the cost of worse late grade 3 or higher urinary toxicity. ADT was used in only 15% of the patients, which precludes meaningful analysis of its impact on outcomes. As such, the role of ADT in the context of SBRT is evolving.
Of these trials, HYPO-RT-PC was the first randomized trial comparing ultra-hypofractionated radiation with conventionally fractionated radiation in men with intermediate to high risk prostate cancer (17). This phase 3, non-inferiority trial randomized patients to receive 42.7 Gy in seven fractions or 78 Gy in 39 fractions; no ADT was allowed. In total, 1,200 patients were randomized into both treatment arms with most participants consisting of patients with intermediate risk disease and only 11% with high risk disease. Ultra-hypofractionation was found to be non-inferior to conventional fractionation. Failure free survival at 5 years was 84% in both groups. The ultra-hypofractionated schedule was weakly associated with worse urinary toxicity at the end of radiotherapy compared to standard fractionation, 28 vs. 23%, respectively (P = 0.057). There were no significant differences in grade 2 or worse urinary or bowel late toxicity between the two groups with the exception of increased urinary toxicity in the hypofractionated group at 1 year (6 vs. 2 %) in the conventional fractionation group (P = 0.0037). In the PACE-B trial, 874 men were randomly assigned to conventionally fractionated (78 Gy in 39 fractions) or moderately hypofractionated radiotherapy (62 Gy in 20 fractions) vs. SBRT (36.25 Gy in 5 fractions) (18). Grade 2 or higher GI or GU toxicity did not differ between the groups. In this initial acute toxicity report, there was no evidence that shortening radiotherapy courses increased acute toxicity, which contrasted with the increased acute toxicity observed in HYPO-RT-PC trial. The authors propose that the higher toxicity observed in HYPO-RT-PC might be secondary to differences in toxicity assessment, radiation dose or conformal radiotherapy technique (18).
Based on the above evidence, SBRT can now be considered as a reasonable alternative to conventionally fractionated regimens and is included as a treatment option for patients with low and intermediate risk disease in recently updated ASTRO/ASCO/AUA guidelines (19). Of note, while prospective trials have used different SBRT treatment schedules, delivery of SBRT on consecutive days is not recommended given the reported increased risk of late GI and GU toxicity with this schedule (19). In addition, PATRIOT, a phase II trial which randomized 152 patients to receive 5 fractions of SBRT for a total of 40 Gy in either a weekly or every other day schedule reported less acute GI and GU toxicity with the weekly schedule (20). With these new guidelines a shift in the treatment of patients with localized disease has been noted. A recent study showed that the proportion of patients receiving SBRT increased from 0.9% in 2004 to 19.5% in 2015. Moderate hypofractionation also increased in use but to a lesser extent with 2.7% receiving moderate hypofractionation in 2004 and 4.7% in 2015. On the other hand, the use of conventional fractionation dramatically decreased (14,699 patients treated with conventional fractionation in 2009 decreasing to 1,492 in 2011) (21). In this report, the most used fractionation schedule was 36.25 Gy in 5 fractions. While preliminary data have demonstrated promising outcomes for patients treated with high risk disease, the use of SBRT remains non-standard for this patient population and should be utilized in the setting of a clinical trial (22).
SBRT For Metastatic Prostate Cancer
Metastatic prostate cancer remains incurable and accounts for over 30,000 deaths in the United States (US) each year (2). ADT is the backbone of therapy for patients with metastatic disease. Multiple recent clinical trials have demonstrated life-prolonging benefits of abiraterone acetate, enzalutamide, apalutamide, and docetaxel when added to ADT for patients with hormone-sensitive and castration-resistant metastatic prostate cancer (23–25). The toxicities of ADT and novel hormonal agents—decreased bone density, increased risk of cardiovascular events, sexual dysfunction and neuropsychiatric symptoms—support the need for integrating metastasis-directed therapies into the treatment paradigm (26, 27). The success of SBRT in palliating painful metastatic bone lesions and its favorable toxicity profile led to increasing interest in SBRT for patients with limited metastatic disease with the goal of either sparing patients the toxicity from systemic therapies or augmenting the effects of existing systemic therapy without leading to undue toxicity (see Table 1).
Support for the latter paradigm came from the phase 3 STAMPEDE study, which demonstrated improved OS in patients with low-volume metastatic disease receiving radiation to the primary prostate tumor (28). In this trial, 2061 patients were randomly allocated to either standard of care (SOC) or SOC plus radiotherapy (55 Gy in 20 fractions or 36 Gy in 6 fractions). With a median follow up of 37 months, there was no survival advantage to radiotherapy in the general cohort. The study stratified patients by metastatic burden as defined in CHAARTED (29), in which high metastatic burden was defined as the presence of visceral metastasis or four or more bone metastases with one or more outside the vertebral bodies or pelvis. STAMPEDE demonstrated an improved OS in patients with low metastatic burden at baseline who were allocated to radiotherapy to the primary tumor. (HR 0.68, p = 0.007) (28). This data provided further support for radiation therapy as a means to prolong OS in patients with low volume metastatic prostate cancer.
Whether this approach can be improved upon through RT to all sites of disease as opposed to the primary tumor alone in patients with oligometastatic disease is currently under investigation. The oligometastatic paradigm suggests that in some patients with low volume metastatic disease, the disease is not completely disseminated, and thus ablative therapies could provide durable disease control (30). SABR-COMET was a recently reported randomized phase 2 trial of stereotactic ablative radiotherapy vs. standard palliative treatment in patients with oligometastatic cancers (31). In this study, patients with a controlled primary tumor and one to five metastatic lesions were randomized to either palliative SOC treatment alone or SOC plus SBRT to all metastatic lesions. A total of 99 patients were randomized and with a median follow up of 25 months in the control group vs. 26 months in the SABR group, the median OS reported was 28 months in the control group vs. 41 months in the SABR group. However, grade 2 or worse adverse events occurred in 9% of controls vs. 29% of those in the SABR group (P = 0.026). Treatment related deaths occurred in 3 patients in the SABR group compared to none in the control group. In this prospective study, SABR was associated with a 13-month improvement in median OS and a doubling of median PFS at the cost of an increase in toxicity and a 4.5% risk of treatment related mortality (31). Of note, out of the 99 patients included in this trial only 16 had prostate cancer, 14 of these were randomized to the radiation arm and the other 2 to the control arm. Given the limited number of prostate cancer patients in this study, a disease specific benefit cannot be proven. However, the results are encouraging for using SBRT in addition to SOC therapy in a disease agnostic manner.
The first prospective clinical trial examining the utility of metastasis directed therapy (MDT) in prostate cancer was STOMP. This was a multicenter phase 2 trial which included asymptomatic prostate cancer patients presenting with up to 3 extracranial metastatic lesions on choline PET-CT after curative intent therapy to the primary. Patients were randomized to either surveillance or MDT with either metastatectomy or SBRT to all lesions. An initial report revealed that at median follow up of 3 years ADT free survival was 13 months in the surveillance group vs. 21 months in the MDT group (32). An update of this trial including five year results was recently presented in abstract form at GU ASCO 2020 and demonstrated that 5 year ADT survival was 8% for the surveillance group vs. 34% for MDT group (HR 0.57 [80% CI: 0.38−0.84]). In addition, 5-year CRPC free survival, a secondary endpoint of the study, was 53% for the surveillance group and 76% for the MDT group (HR 0.62 [80% CI 0.35–1.09]) (33). These results further confirmed the significant difference in ADT-free survival for those patients receiving MDT.
POPSTAR was a single arm prospective trial which evaluated the feasibility and tolerability of a single fraction of stereotactic ablative radiotherapy to metastatic sites in men with prostate cancer and up to 3 metastatic lesions on sodium fluoride PET CT. A total of 33 consecutive patients received stereotactic ablative RT to a total of 50 oligometastases. Local PFS at 2 years was 97%, while distant PFS was 39%. For patients not on ADT, the 2-year freedom from ADT was 48% (34).
Lastly, ORIOLE, was a phase 2 randomized study in which patients with hormone sensitive prostate cancer and 1 to 3 asymptomatic metastases detected by conventional imaging who had not yet received ADT were randomized to SABR or observation. 54 men were randomized into the treatment arms. Progression at 6 months was observed in 19% of those receiving SABR vs. 61% of those undergoing observation (P = 0.005). No toxic effects of grade 3 or greater were observed. In addition, peripheral blood mononuclear cells (PBMC) were collected at baseline and at 90 days for deep sequencing of T-cell receptor DNA. Interestingly, differential clonotype abundance appeared more pronounced in SABR arm with significantly expanded clones when compared to observation arm (35).
Given the current body of evidence, the Italian association of radiotherapy and clinical oncology (AIRO) released a consensus statement that supported the use of SBRT as an alternative to ADT to delay systemic recurrence in patients with oligometastatic disease and up to 3 lymph node or bone metastasis (36). The use of SBRT to asymptomatic metastatic sites remains non-standard, however data from SABR-COMET, POPSTAR, ORIOLE and additional trials underway points to this as a promising area of investigation. These studies provide evidence that radiation therapy can alter the natural history of a subset of metastatic prostate cancers. Whether this mechanism proceeds through tumor debulking, direct synergy with AR-targeted therapies, or immunomodulation remains unknown. These clinical data coupled with an increasing understanding of the immunobiological effects of radiation therapy has spurred innovative clinical trials designed to address these questions.
SBRT and Tumor Immunology
A body of preclinical and clinical data suggest that radiation therapy promotes T cell priming through the release of tumor antigens and pro-inflammatory cytokines (37). Both innate and adaptive mechanisms have been highlighted as critical for the effects of high dose radiation on T cell priming. Innate effects include the positive effect on dendritic cell-mediated cross-presentation to CD8+ T cells in a type 1 interferon-dependent fashion (38). Ionizing radiation has also been shown to promote improved T cell trafficking to the tumor through upregulation of critical chemokines (39). Indeed, when combining immunotherapy and radiotherapy, an abscopal treatment response was observed in a mouse model of castrate resistant prostate cancer (40).
However, the dose and fractionation schedules required to achieve this immunologic priming are unclear (41). Several studies have suggested that ablative or hypofractionated radiation is associated with increased activation of antitumor CD8+ T cells when compared to conventional fractionation (42–44). For instance, a report compared the effects of a single dose of 15 Gy to a regimen of 5 fractions of 3 Gy on a murine model of melanoma and found increased numbers of IFN-γ secreting cells and increased cell lysis by CD8+ T-cells when compared to the fractionated scheme (43). Another study reported tumor rejection after a single 20 Gy dose of ablative RT in an immunocompetent murine model of B16 melanoma. Interestingly, this effect was not seen in immunodeficient mice or mice that had been depleted of CD8+ T cells (42). In this same report, immunocompetent mice treated with a fractionated scheme of 4 x 5 Gy showed a similar tumor relapse rate to that of T cell-depleted mice, suggesting that fractionated regimens may inadequately prime or mobilize tumor infiltrating lymphocytes (41). In further support of hypofractionated schemes, high dose radiation was found to reverse the immunosuppressive tumor microenvironment of CT26 and MC38 colon tumors by leading to increased CD8+ T cell infiltrate and reduced myeloid derived suppressor cells. These changes were found to be dependent on antigen presenting dendritic cells and secretion of IFN-γ. This effect was not seen with extended fractionated radiation regimens (45).
Whether SBRT-induced DNA damage leads to increased expression of neoantigens and an expanded T cell repertoire remains an open question. A recent study tested conventional fractionation (5 x 2 Gy) in combination with an anti-PD-1 antibody and reported local and systemic immune responses in mice with CT26 colon carcinoma xenografts (46). In this model, there was a high level of concordance between T-cell receptors from irradiated and non-irradiated tumors, whereas only 0.5% of T cells were unique to the irradiated tumor. This suggests that most of the reactive T cells in this model were responding to pre-existing antigens rather than neo-antigens created by radiation-induced injury. Therefore, in some circumstances, radiation may lead to increased release of tumor associated antigens (TAA) rather than directly generating new TAA. Indeed, many studies have reported enhanced priming of T cell responses after treatment with hypofractionated RT due to increased presentation of TAA to CD8+ T cells in draining lymph nodes (42, 43, 47, 48). Whether SBRT doses in certain circumstances can generate an increased number of TAAs remains an area of inquiry.
Whether these immunomodulatory effects are related to the fractionation scheme or the biological dose achieved with ablative therapy was further tested in a study with syngeneic mouse lung and melanoma tumors under the same biologically equivalent dose (BED) (49). In this study, when compared with conventional fractionation, ablative therapy suppressed recruitment of MDSCs, which in turn allowed for increased cytotoxic activity of CD8+ T cells. Treating with anti-PD-L1 antibody further improved the effects of ablative therapy on mouse survival (49). In further support of this strategy, PD-1 blockade can reverse adaptive immune resistance when combined with high dose hypofractionated therapy but not with daily fractionation (50). Lastly, in addition to its effect on the tumor immune microenvironment, other studies have shown that hypofractionated RT can alter the tumor stroma (41). For instance, a recent study developed a dynamic model that confirmed indirect tumor cell death caused by SBRT through its effect on tumor-associated endothelial cell vascular damage (51).
In addition to the above pre-clinical data a recent phase 1 clinical trial also suggested a beneficial immune effect to SBRT in combination with immunotherapy (52). In this study, patients with advanced solid tumors progressing on standard therapy received SBRT to up to four metastatic lesions followed by pembrolizumab. Pre and post SBRT biopsy specimens were analyzed in a subset of patients. In the 68 patients with imaging follow up, the objective response rate was 13.2%. Expression of interferon gamma-associated genes from post-SBRT tumor biopsy specimens significantly correlated with non-irradiated tumor response further suggesting a complementary role of SBRT in combination with immunotherapy.
Another report demonstrated that SBRT and CTLA-4 blockade with ipilimumab induced systemic anti-tumor T cells in chemo-refractory metastatic non-small cell lung cancer, a clinical context in which anti-CTLA-4 antibodies had previously failed to demonstrate efficacy alone or in combination with chemotherapy (53). In this study, increased serum interferon β after radiation and early dynamic changes in blood T cell clones were the strongest predictors of response. Whether radiotherapy can reliably induce immune responses in combination with immune checkpoint inhibition remains an area of active investigation (54). Similar prospective trials are currently addressing the potential impact of SBRT on altering the natural history of prostate cancer and will provide a rich source of correlative markers to address the effects on the TME.
Translating The Biology of SBRT To The Clinic
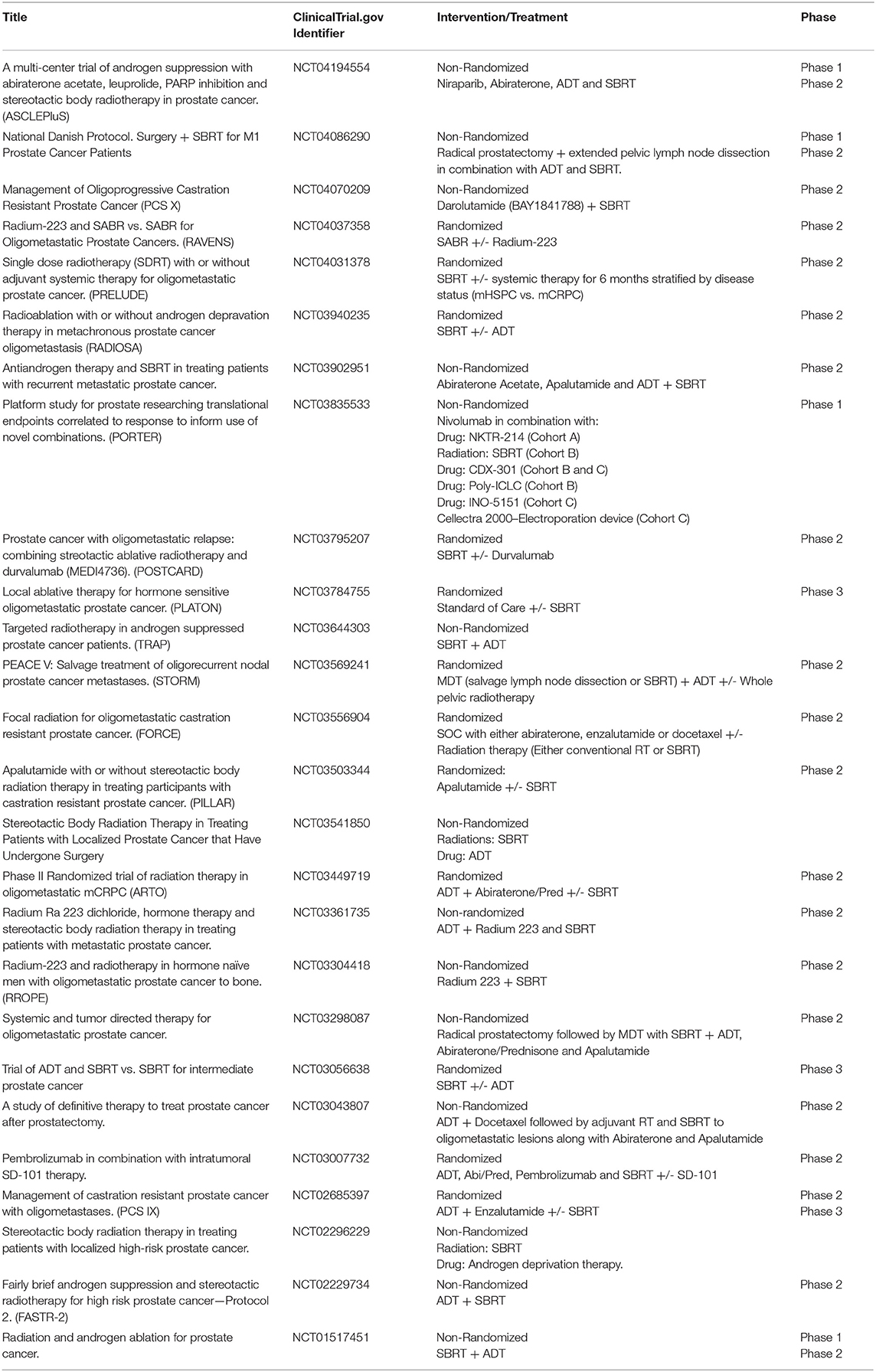
SBRT is an increasingly used treatment modality for both localized and metastatic disease and multiple ongoing trials (Table 2) are now examining ways in which to achieve superior clinical outcomes through novel combinations of SBRT with systemic therapy.
Localized Prostate Cancer
ADT can synergize with conventional RT by inducing radiosensitivity due to its effect on tumor cell DNA repair inhibition (55). However, the higher radiation doses achieved with SBRT as well as its immune priming effects may obviate the need for ADT. Alternatively, the immunostimulatory effects of ADT may synergize with SBRT. Several trials are testing whether ADT can improve outcomes in men treated with SBRT alone (NCT01517451, NCT03056638, NCT02296229, NCT02229734). Notable among these studies is NTC03056638, a randomized phase 3 trial, in which patients are randomized to SBRT (8 Gy x 5 fractions) plus placebo vs. SBRT plus 6 months of ADT in men with intermediate risk localized disease. The primary endpoint of the study is pathologic complete response on a post-treatment biopsy 24–30 months after treatment. Larger studies with an MFS endpoint will likely be needed to establish SBRT with ADT as a standard of care for unfavorable intermediate and high risk localized prostate cancer.
Metastatic Prostate Cancer (Hormone-Sensitive and CRPC)
Based on the impressive safety profile of SBRT used in the context of the STAMPEDE trial, several trials are extending these results to test the hypothesis that SBRT can eradicate MRD in oligometastatic hormone sensitive prostate cancer. Notably, RADIOSA (NCT03940235), a randomized phase II trial in patients with oligometastatic prostate cancer with a maximum of 3 lesions, aims to compare progression-free survival between SBRT to all metastatic lesions alone or in combination with 6 months of ADT. The PLATON trial (NCT03784755) will be a randomized phase 3 trial which will test if SBRT adds to standard of care (SOC) systemic therapy. Patients with newly diagnosed metastatic prostate cancer with up to 3 metastatic lesions amenable to ablative therapy (SBRT or surgery) will be treated with SOC systemic therapy plus local ablative therapy to all sites of disease vs. SOC alone. Of note, radiation to the primary if previously untreated is allowed in both arms as per STAMPEDE. Primary outcome will be failure free survival and secondary outcomes include rPFS, and OS.
Checkpoint inhibitors have had limited benefit in the treatment of patients with metastatic prostate cancer (56). The phase 3 trial of ipilimumab vs. placebo after radiotherapy (8 Gy x 1) in patients with mCRPC that had progressed after docetaxel chemotherapy failed to demonstrate improvements in OS (57). However, the trial reported signs of clinical activity with some patients showing reductions in PSA and improvement in PFS. Later, another randomized phase 3 trial compared ipilimumab vs. placebo in asymptomatic or minimally symptomatic patients with mCRPC naïve to chemotherapy (58). As with the previous trial, ipilimumab did not improve OS but again demonstrated increased PFS and PSA response rates in a subset of the patients. PD-1 blockade has had similarly disappointing results. Nivolumab failed to induce any anti-tumor responses in the first 17 patients with mCRPC treated. In a non-randomized parallel arm study of 258 patients, pembrolizumab treatment was associated with a 6% PSA response rate and 5% soft tissue tumor response rate. Whether SBRT, through its reported immunostimulatory effects, can synergize with anti-PD-(L)-1 checkpoint blockade is the subject of multiple ongoing trials.
Notable among the ongoing trials is POSTCARD, a randomized phase II trial that will test SBRT with or without durvalumab, a monoclonal antibody against PD-L1, in patients with oligometastatic hormone sensitive prostate cancer. Patients with up to 5 bone or lymph node metastases as seen on Ga-68-PSMA PET/CT will be included. Primary outcome will be PFS with secondary outcomes including ADT free survival, time to castrate resistance, prostate cancer specific survival, and OS. This innovative trial will provide an important dataset to assess whether PD-L1 blockade immunotherapy enhances the treatment response to SBRT.
Several trials including the FORCE trial (NCT03556904) are testing whether the addition of radiotherapy to SOC systemic therapy improves objective PFS compared to systemic therapy alone in patients with oligometastatic CRPC. In this trial, patients with up to 5 metastatic lesions will be randomized into the different treatment arms. Systemic therapy will consist of abiraterone, enzalutamide, or docetaxel in combination with ADT. Radiation will range between conventional 30 Gy in 10 fractions to SBRT with 50 Gy in 5 fractions. While this trial does not limit radiation to SBRT alone and allows for conventional radiation schedules, it will help determine whether MDT with radiation adds to the efficacy of different systemic therapies. Correlative studies on peripheral blood and on-treatment biopsies should substantially add to our understanding of the effects of various RT schedules on the TME.
The development of mCRPC is associated with multiple changes to the immune TME. A recent report showed that CRPC cells are more resistant to RT combined with ADT and demonstrate higher levels of MDSCs when compared to hormone sensitive cells (59). These immunosuppressive cells exert a regulatory effect on T cell effector cells promoting an immune permissive environment which makes a T cell response less likely. Multiple trials (NCT03835533, NCT03007732) are testing novel immunotherapeutic combinations along with SBRT with aims to bypass the immunosuppressive microenvironment of CRPC and thus stimulate T cell anti-tumor responses. Notable in this group is PORTER (NCT03835533) cohort B, an active trial testing SBRT (30−50 Gy in 1-5 fractions) in combination with Nivolumab, CDX-301 (a recombinant human Flt3L) (60) and Polyinosinic-polycytidylic acid-poly-l-lysine carboxymethylcellulose (poly-ICLC). CDX-301 has been previously shown in clinical studies to expand key subsets of myeloid and plasmacytoid dendritic cells (60) Poly-ICLC is a synthetic double stranded RNA complex that has been shown to activate toll-like receptor 3 and stimulate intratumoral immune responses in patients (61). By combining radiation with checkpoint inhibition and other novel immunotherapy agents, this and other trials aim to overcome the immunosuppressive environment of CRPC and succeed where previous checkpoint monotherapy trials had failed.
Conclusion
SBRT is an increasingly used treatment for men with early stage favorable risk prostate cancer. Its use in high risk and metastatic prostate cancer has demonstrated impressive preliminary data with respect to tolerability and oncologic outcomes. Here we have reviewed, the unique radiobiology of SBRT with respect to DNA repair, immunogenic cell death, and downstream T cell priming and the various novel clinical trials that have used SBRT in innovative ways with standard and investigational systemic treatments for prostate cancer. Analysis of specimens derived from these carefully designed studies will likely be instructive as to the biological consequences of SBRT and future opportunities for synergy with systemic treatments.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. (2019) 9:217–22. doi: 10.2991/jegh.k.191008.001
2. National Cancer Institute. SEER Cancer Stat Facts: Prostate Cancer. Available online at: https://seer.cancer.gov/statfacts/html/prost.html.
3. Bekelman JE, Rumble RB, Chen RC, Pisansky TM, Finelli A, Feifer A, et al. Clinically localized prostate cancer: ASCO clinical practice guideline endorsement of an american urological association/American society for radiation oncology/Society of urologic oncology guideline. J Clin Oncol. (2018) 2018:JCO.18.00606. doi: 10.1200/jco.18.00606
4. Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically localized prostate cancer: aUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol. (2018) 199:683–90. doi: 10.1016/j.juro.2017.11.095
5. Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 cHHiP trial. Lancet Oncol. (2016) 17:1047–60. doi: 10.1016/S1470-2045(16)30102-4
6. Incrocci L, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E, Krol S, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. (2016) 17:1061–9. doi: 10.1016/S1470-2045(16)30070-5
7. Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-Risk prostate cancer. J Clin Oncol. (2016) 34:2325–32. doi: 10.1200/jco.2016.67.0448
8. Catton CN, Lukka H, Gu CS, Martin JM, Supiot S, Chung PWM, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. (2017) 35:1884–90. doi: 10.1200/JCO.2016.71.7397
9. Datta NR, Stutz E, Rogers S, Bodis S. Conventional versus hypofractionated radiation therapy for localized or locally advanced prostate cancer: a Systematic review and meta-analysis along with therapeutic implications. Int J Radiat Oncol Biol Phys. (2017) 99:573–89. doi: 10.1016/j.ijrobp.2017.07.021
10. Bruner DW, Pugh SL, Lee WR, Hall WA, Dignam JJ, Low D, et al. Quality of life in patients with low-Risk prostate cancer treated with hypofractionated vs. conventional radiotherapy: a Phase 3 randomized clinical trial. JAMA Oncol. (2019) 5:664–70. doi: 10.1001/jamaoncol.2018.6752
11. Morgan SC, Hoffman K, Loblaw DA, Buyyounouski MK, Patton C, Barocas D, et al. Hypofractionated radiation therapy for localized prostate cancer: executive summary of an aSTRO, aSCO, and aUA evidence-Based guideline. Pract Radiat Oncol. (2018) 8:354–60. doi: 10.1016/j.prro.2018.08.002
12. Zemplenyi AT, Kalo Z, Kovacs G, Farkas R, Beothe T, Banyai D, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care (Engl). (2018) 27:12430. doi: 10.1111/ecc.12430
13. Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. (1999) 43:1095–101. doi: 10.1016/S0360-3016(98)00438-6
14. Vogelius IR, Bentzen SM. Dose response and fractionation sensitivity of prostate cancer after external beam radiation therapy: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. (2018) 100:858–65. doi: 10.1016/j.ijrobp.2017.12.011
15. Madsen BL, Hsi RA, Pham HT, Fowler JF, Esagui L, Corman J. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 gy in five fractions for localized disease: first clinical trial results. Int J Radiat Oncol Biol Phys. (2007) 67:1099–105. doi: 10.1016/j.ijrobp.2006.10.050
16. Jackson WC, Silva J, Hartman HE, Dess RT, Kishan AU, Beeler WH, et al. Stereotactic body radiation therapy for localized prostate cancer: a Systematic review and meta-Analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol Biol Phys. (2019) 104:778–89. doi: 10.1016/j.ijrobp.2019.06.1912
17. Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the hYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. (2019) 394:385–95. doi: 10.1016/S0140-6736(19)31131-6
18. Brand DH, Tree AC, Ostler P, van der Voet H, Loblaw A, Chu W, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. The Lancet Oncology. (2019) 20:1531–43. doi: 10.1016/S1470-2045(19)30569-8
19. Morgan SC, Hoffman K, Loblaw DA, Buyyounouski MK, Patton C, Barocas D, et al. Hypofractionated radiation therapy for localized prostate cancer: executive summary of an aSTRO, aSCO and aUA evidence-Based guideline. J Urol. (2019) 201:528–34. doi: 10.1097/JU.0000000000000071
20. Quon HC, Ong A, Cheung P, Chu W, Chung HT, Vesprini D, et al. Once-weekly versus every-other-day stereotactic body radiotherapy in patients with prostate cancer (PATRIOT): a phase 2 randomized trial. Radiother Oncol. (2018) 127:206–12. doi: 10.1016/j.radonc.2018.02.029
21. Malouff TD, Stross WC, Seneviratne DS, Waddle MR, May BC, Buskirk SJ, et al. Current use of stereotactic body radiation therapy for low and intermediate risk prostate cancer: a National cancer database analysis. Prostate Cancer Prostatic Dis. (2020) 23:349–55. doi: 10.1038/s41391-019-0191-9.
22. Gonzalez-Motta A, Roach M III. Stereotactic body radiation therapy (SBRT) for high-risk prostate cancer: where are we now? Pract Radiat Oncol. (2018) 8:185–202. doi: 10.1016/j.prro.2017.11.008
23. Sydes MR, Spears MR, Mason MD, Clarke NW, Dearnaley DP, de Bono JS, et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the sTAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. (2018) 29:1235–48. doi: 10.1093/annonc/mdy072
24. Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-Sensitive prostate cancer. N Engl J Med. (2019) 381:13–24. doi: 10.1056/NEJMoa1903307
25. Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-Line therapy in metastatic prostate cancer. N Engl J Med. (2019) 381:121–31. doi: 10.1056/NEJMoa1903835
26. Magee DE, Singal RK. Androgen deprivation therapy: indications, methods of utilization, side effects and their management. Can J Urol. (2020) 27:11–6.
27. Izard JP, Siemens DR. Androgen deprivation therapy and mental health: impact on depression and cognition. European urology focus. (2020). doi: 10.1016/j.euf.2019.11.010. [Epub ahead of print].
28. Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. (2018) 392:2353–66. doi: 10.1016/S0140-6736(18)32486-3
29. Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-Sensitive prostate cancer. N Engl J Med. (2015) 373:737–46. doi: 10.1056/NEJMoa1503747
30. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. (1995) 13:8–10. doi: 10.1200/JCO.1995.13.1.8
31. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. (2019) 393:2051–8. doi: 10.1016/S0140-6736(18)32487-5
32. Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-Directed therapy for oligometastatic prostate cancer recurrence: a Prospective, randomized, multicenter phase iI trial. J Clin Oncol. (2018) 36:446–53. doi: 10.1200/JCO.2017.75.4853
33. Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, Bruycker AD, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): five-year results of a randomized phase II trial. J Clin Oncol. (2020) 38:10–. doi: 10.1200/JCO.2020.38.6_suppl.10
34. Siva S, Bressel M, Murphy DG, Shaw M, Chander S, Violet J, et al. Stereotactic abative body radiotherapy (SABR) for oligometastatic prostate cancer: a Prospective clinical trial. Eur Urol. (2018) 74:455–62. doi: 10.1016/j.eururo.2018.06.004
35. Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of observation vs. stereotactic ablative radiation for oligometastatic prostate cancer: the oRIOLE phase 2 randomized clinical trial. JAMA Oncology. (2020). doi: 10.1001/jamaoncol.2020.0147. [Epub ahead of print].
36. D'Angelillo RM, Francolini G, Ingrosso G, Ravo V, Triggiani L, Magli A, et al. Consensus statements on ablative radiotherapy for oligometastatic prostate cancer: a position paper of italian association of radiotherapy and clinical oncology (AIRO). Crit Rev Oncol Hematol. (2019) 138:24–8. doi: 10.1016/j.critrevonc.2019.03.014
37. Kalina JL, Neilson DS, Comber AP, Rauw JM, Alexander AS, Vergidis J, et al. Immune modulation by androgen deprivation and radiation therapy: implications for prostate cancer immunotherapy. Cancers (Basel). (2017) 9:13. doi: 10.3390/cancers9020013
38. Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i Interferon-Dependent innate and adaptive immunity. Cancer Res. (2011) 71:2488–96. doi: 10.1158/0008-5472.CAN-10-2820
39. Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-Induced cXCL16 release by breast cancer cells attracts effector t Cells. J Immunol. (2008) 181:3099–107. doi: 10.4049/jimmunol.181.5.3099
40. Dudzinski SO, Cameron BD, Wang J, Rathmell JC, Giorgio TD, Kirschner AN. Combination immunotherapy and radiotherapy causes an abscopal treatment response in a mouse model of castration resistant prostate cancer. J Immunother Cancer. (2019) 7:218. doi: 10.1186/s40425-019-0704-z
41. Arnold KM, Flynn NJ, Raben A, Romak L, Yu Y, Dicker AP, et al. The impact of radiation on the tumor microenvironment: effect of dose and fractionation schedules. Cancer Growth Metastasis. (2018) 11:1179064418761639. doi: 10.1177/1179064418761639
42. Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require cD8+ t cells: changing strategies for cancer treatment. Blood. (2009) 114:589–95. doi: 10.1182/blood-2009-02-206870
43. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of b16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. (2005) 174:7516–23. doi: 10.4049/jimmunol.174.12.7516
44. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. (2009) 15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265
45. Filatenkov A, Baker J, Mueller AM, Kenkel J, Ahn GO, Dutt S, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. (2015) 21:3727–39. doi: 10.1158/1078-0432.CCR-14-2824
46. Dovedi SJ, Cheadle EJ, Popple AL, Poon E, Morrow M, Stewart R, et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal t-cell populations when combined with pD-1 blockade. Clin Cancer Res. (2017) 23:5514–26. doi: 10.1158/1078-0432.CCR-16-1673
47. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. (2007) 13:1050–9. doi: 10.1038/nm1622
48. Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector cD8+ t cells via dendritic cell activation. J Immunol. (2012) 189:558–66. doi: 10.4049/jimmunol.1200563
49. Lan J, Li R, Yin LM, Deng L, Gui J, Chen BQ, et al. Targeting myeloid-derived suppressor cells and programmed death ligand 1 confers therapeutic advantage of ablative hypofractionated radiation therapy compared with conventional fractionated radiation therapy. Int J Radiat Oncol Biol Phys. (2018) 101:74–87. doi: 10.1016/j.ijrobp.2018.01.071
50. Morisada M, Clavijo PE, Moore E, Sun L, Chamberlin M, Van Waes C, et al. PD-1 blockade reverses adaptive immune resistance induced by high-dose hypofractionated but not low-dose daily fractionated radiation. Oncoimmunology. (2018) 7:e1395996. doi: 10.1080/2162402X.2017.1395996
51. Rodriguez-Barbeito P, Diaz-Botana P, Gago-Arias A, Feijoo M, Neira S, Guiu-Souto J, et al. A model of indirect cell death caused by tumor vascular damage after high-Dose radiotherapy. Cancer Res. (2019) 79:6044–53. doi: 10.1158/0008-5472.CAN-19-0181
52. Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. (2018) 36:1611–8. doi: 10.1200/JCO.2017.76.2229
53. Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to cTLA-4 blockade. Nat Med. (2018) 24:1845–51. doi: 10.1038/s41591-018-0232-2
54. Demaria S, Coleman CN, Formenti SC. Radiotherapy: changing the game in immunotherapy. Trends Cancer. (2016) 2:286–94. doi: 10.1016/j.trecan.2016.05.002
55. Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, et al. Androgen receptor signaling regulates dNA repair in prostate cancers. Cancer Discov. (2013) 3:1245–53. doi: 10.1158/2159-8290.CD-13-0172
56. Venturini NJ, Drake CG. Immunotherapy for prostate cancer. Cold Spring Harb Perspect Med. (2019) 9:5. doi: 10.1101/cshperspect.a030627
57. Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. (2014) 15:700–12. doi: 10.1016/S1470-2045(14)70189-5
58. Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-Blind, phase iII trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-Naive castration-Resistant prostate cancer. J Clin Oncol. (2017) 35:40–7. doi: 10.1200/JCO.2016.69.1584
59. Wu CT, Chen WC, Chen MF. The response of prostate cancer to androgen deprivation and irradiation due to immune modulation. Cancers (Basel). (2018) 11:1. doi: 10.3390/cancers11010020
60. Anandasabapathy N, Breton G, Hurley A, Caskey M, Trumpfheller C, Sarma P, et al. Efficacy and safety of cDX-301, recombinant human flt3L, at expanding dendritic cells and hematopoietic stem cells in healthy human volunteers. Bone Marrow Transplant. (2015) 50:924–30. doi: 10.1038/bmt.2015.74
Keywords: SBRT, prostate cancer, immunotherapy, ADT, minimal residual disease, androgen suppression therapy
Citation: Adorno Febles VR, Blacksburg S, Haas JA and Wise DR (2020) Translating the Immunobiology of SBRT to Novel Therapeutic Combinations for Advanced Prostate Cancer. Front. Oncol. 10:830. doi: 10.3389/fonc.2020.00830
Received: 02 March 2020; Accepted: 28 April 2020;
Published: 27 May 2020.
Edited by:
Walter J. Storkus, University of Pittsburgh, United StatesReviewed by:
Ryan Phillips, School of Medicine, Johns Hopkins University, United StatesMatthew Pierre Deek, Johns Hopkins Medicine, United States
Copyright © 2020 Adorno Febles, Blacksburg, Haas and Wise. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David R. Wise, david.wise@nyulangone.org
 Victor R. Adorno Febles
Victor R. Adorno Febles Seth Blacksburg
Seth Blacksburg Jonathan A. Haas
Jonathan A. Haas David R. Wise
David R. Wise