- 1Department of Radiation Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China
- 2State Key Laboratory of Oncology in South China, Guangzhou, China
- 3Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
- 4Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China
- 5Department of Radiation Oncology, Hui Zhou Municipal Centre Hospital, Huizhou, China
Purpose: To assess the impact of comorbidity on treatment outcomes in patients with locally recurrent nasopharyngeal carcinoma (lrNPC) using intensity-modulated radiotherapy (IMRT) and to develop a nomogram that combines prognostic factors to predict clinical outcome and guide individual treatment.
Methods: This was a retrospective analysis of patients with lrNPC who were reirradiated with IMRT between 2003 and 2014. Comorbidity was evaluated by Adult Comorbidity Evaluation-27 grading (ACE-27). The significant prognostic factors (P < 0.05) by multivariate analysis using the Cox regression model were adopted into the nomogram model. Harrell concordance index (C-index) calibration curves were applied to assess this model.
Results: Between 2003 and 2014, 469 lrNPC patients treated in our institution were enrolled. Significant comorbidity (moderate or severe grade) was present in 17.1% of patients by ACE-27. Patients with no or mild comorbidity had a 5-year overall survival (OS) rate of 36.2 versus 20.0% among those with comorbidity of moderate or severe grade (P < 0.0001). The chemotherapy used was not significantly different in patients with lrNPC (P > 0.05). For the rT3–4 patients, the 5-year OS rate in the chemotherapy + radiation therapy (RT) group was 30.0 versus 16.7% for RT only (P = 0.005). The rT3–4 patients with no or mild comorbidity were associated with a higher 5-year OS rate in the chemotherapy + RT group than in the RT only group (32.1 and 17.1%, respectively; P=0.003). However, for the rT3–4 patients with a comorbidity (moderate or severe grade), the 5-year OS rate in the chemotherapy + RT group vs. RT alone was not significantly different (15.7 vs. 15.0%, respectively; p > 0.05). Eight independent prognostic factors identified from multivariable analysis were fitted into a nomogram, including comorbidity. The C-index of the nomogram was 0.715. The area under curves (AUCs) for the prediction of 1-, 3-, and 5-year overall survival were 0.770, 0.764, and 0.780, respectively.
Conclusion: Comorbidity is among eight important prognostic factors for patients undergoing reirradiation. We developed a nomogram for lrNPC patients to predict the probability of death after reirradiation and guide individualized management.
Introduction
Nasopharyngeal carcinoma (NPC) is a type of head and neck cancer. Globally, there were 129,079 new cases of NPC and 72,987 deaths reported worldwide in 2018 (1). Nevertheless, the geographical distribution is extremely unbalanced, and it is considered to be epidemic in East and Southeast Asia, and North Africa, and approximately >70% of new cases are in East and Southeast Asia (1, 2). Due to the concealed location and radiosensitivity of nasopharyngeal carcinoma, radiation therapy (RT) is the first-line treatment modality for primary NPC patients (3).
With the advancement of radiotherapy technology, the diagnostic imaging of nasopharyngeal carcinoma and the improvement of combined chemotherapy, the treatment effect and disease control rate have been improved significantly (4). Nevertheless, approximately 5–10% of patients experience locally recurrent nasopharyngeal carcinoma (lrNPC) after intensity-modulated radiotherapy (IMRT) (5–8). In patients with local recurrence, a repeat course of RT or nasopharyngectomy presents the only treatment options; however, to date, nasopharyngectomy is still challenging owing to its small space and previously irradiated anatomic space, which is often considered when feasible, or reirradiation for patients not eligible for surgery (9, 10). The emergence of IMRT has helped to overcome the technical limitations of conventional two- and three-dimensional RT and improved the therapeutic ratio of salvage RT; nevertheless, severe RT-related toxic reactions occur frequently and account for a large portion of mortality after IMRT treatment (up to 50%) (11, 12).
Hence, it has become evident that risk appropriately stratified a priori is warranted if individualized management is to be pursued for patients with lrNPC to avoid overtreatment. Sun et al. and Li et al. investigated robust prognostic models for risk stratification in lrNPC (13, 14). Nevertheless, Sun et al. only considered diabetes mellitus and hypertension among comorbidities, and Li et al. did not consider comorbidity as a prognostic factor. To address this issue, more accurate and comprehensive models are needed to identify individual risks by combining patient characteristics to assist with treatment recommendations in this patient subgroup with different risks. The ACE-27 is a modified Kaplan-Feinstein Index that assesses the severity of 27 different items related to cancer. A number of reports documenting the grading have shown reliability and validity in head and neck cancers as a cause of death, which have also been linked to predicting survival in head and neck cancers (15–18). In this study, we aimed to construct a nomogram that includes independent predictors. Moreover, we incorporated the ACE-27 into the nomogram to help clinicians predict patient survival outcomes and identify optimal candidates for local treatment with IMRT.
Materials and Methods
Patient Characteristics
A retrospective review of case records for patients with lrNPC who were reirradiated using a full-course IMRT technique was conducted at Sun Yat-sen University Cancer Center (SYSUCC) from January 2003 to December 2014. The inclusion criteria were as follows: a) pathology confirmed or evidence of local recurrence by at least one imaging study with a consistent clinical process, b) no evidence of distant metastasis, and c) using the IMRT technique for treatment. The exclusion criteria were a) pregnancy or lactation and b) secondary malignancy. The Clinical Research Ethics Committee of Sun Yat-sen University Cancer Center approved this study.
Diagnosis and Comorbidity Assessment
Patients had undergone pretreatment evaluation comprising a complete medical history, nasopharynx and neck magnetic resonance imaging (MRI) or computed tomography (CT), chest X-ray or CT, abdominal CT or ultrasonography, CT whole-body bone scan single photon emission, or 18F-fluorodeoxyglucose (18F-FDG) PET/CT. ACE-27 was performed for comorbid disease severity at diagnosis, which includes the assessment of 27 elements from twelve different organ systems. The ACE-27 grades comorbidities into one of four scores: none 0), mild 1), moderate 2), or severe 3). The total score for each patient’s comorbidities is based on the highest-ranked single disease. When two or more moderate diseases occur in different organ systems, the total comorbidity score is considered severe (18–20).
Clinical Treatment
A similar IMRT planning protocol was used as previously described (11, 21), and the total dosage for reirradiation therapy was 50–70 Gy at 1.80–2.50 Gy/fraction, five times a week on workdays, delivered by the IMRT technique. In this study, most patients received intravenous chemotherapy every 3 weeks. Treatment regimens included concomitant chemoradiotherapy plus induction chemotherapy (CCRT + IC), CCRT plus adjuvant chemotherapy (CCRT + AC), CCRT, RT, IC + RT, and RT + AC.
Follow-Up and Endpoint
Follow-up was measured from the first day of therapy to the last follow-up or death. A range of assessments were carried out every 3 months for the first year, and then follow-up examinations were performed every 6 months thereafter until death. At each follow-up visit, routine assessments included head and neck physical examination, nasopharyngoscopy, nasopharynx and neck MRI with contrast, chest X-ray or CT, abdominal ultrasound or CT, whole-body bone scan, or (18F-FDG) PET/CT. The primary endpoint of our study was OS. OS was defined as the time from the first day of therapy to death from any cause or follow-up endpoint.
Statistical Methods
Based on patient survival status, the optimal cut-off value of the continuous variables that generated the largest χ2 value in the Mantel-Cox test assessed by X-tile software (version 3.6.3; Yale University, New Haven, CT, USA), the lrNPC patients were stratified into subgroups (22). Life-table estimation was performed according to the Kaplan-Meier method. The log-rank test was used to examine the difference in survival between groups.
Multivariable regression analyses were performed using Cox proportional hazards modeling, which was used to estimate hazard ratios (HR) and 95% confidence intervals (CI), and formed the basis for the survival prediction model. Covariates in this study included comorbidity, age, sex, hemoglobin (HB), Karnofsky performance status (KPS), and prior RT-induced grade ≥ 3 toxicity variables (late RT-induced toxicities were noted and graded according to the Radiation Therapy Oncology Group radiation morbidity scoring scheme), DNA fragmentation index (DFI), recurrent gross tumor volume (GTV), rT stage, rN stage, treatment regimen, and re-RT equivalent dose in 2-Gy fractions [EQD2]. Those variables with a 2-tailed P < 0.05 in multivariable regression analyses were considered to create a nomogram based on their contribution to the accuracy of prediction. Statistical analyses were conducted using IBM SPSS Statistics software version 25, R version 3.6.1 (R Core Team, Vienna, Austria) and GraphPad Prism version 8.
Results
Patient Characteristics, Survival, and Toxicities
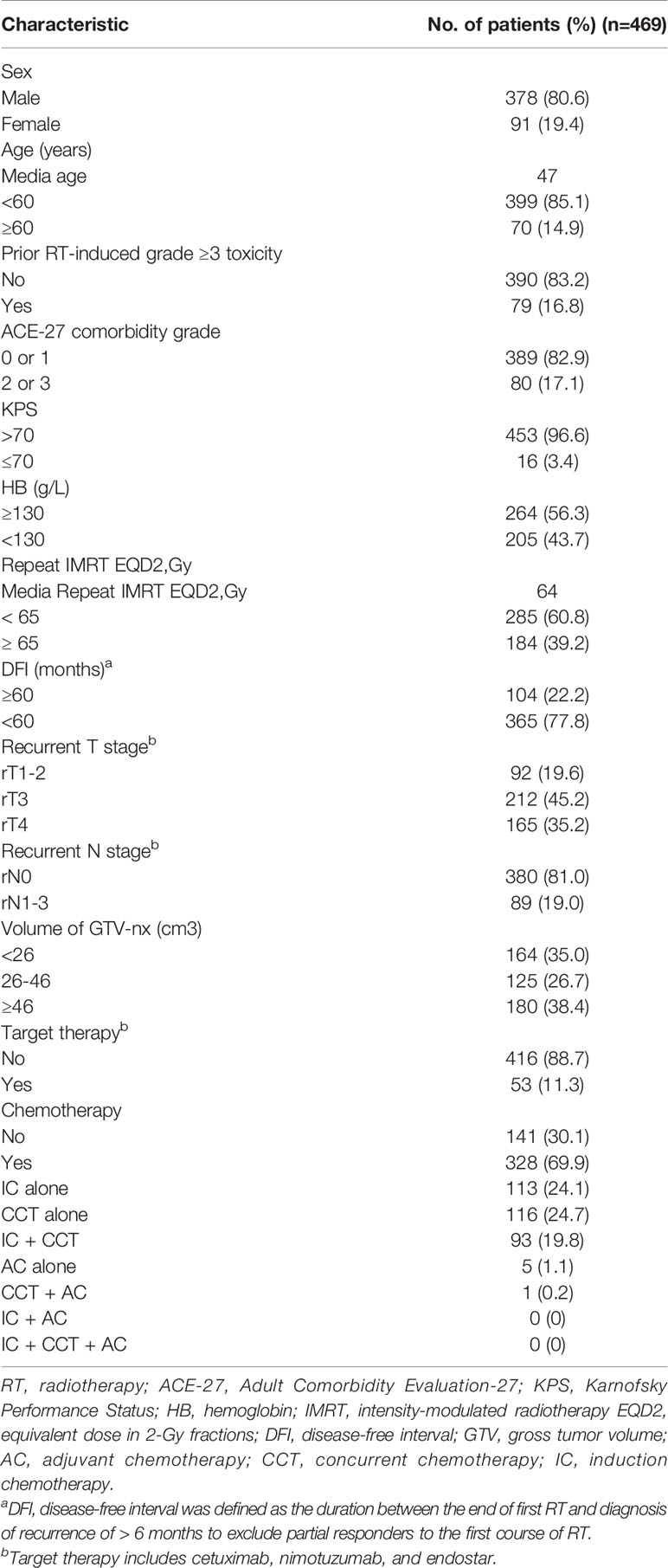
A total of 469 patients treated from January 2003 to December 2014 were retrospectively enrolled. The median age was 47 years old (range: 21–79), and 378 (80.6%) patients were male. A total of 19.6, 45.2, and 35.2% of the patients had stage rT1–2, rT3, and rT4, respectively, whereas 81.0 and 19.0% had stage rN0 and rN1–3, respectively, and 80 (17.1%) patients had prior RT-induced grade ≥ 3 toxicity. Comorbidity (moderate or severe grade) was present in 80 (17.1%) patients, and chemotherapy was delivered to 328 patients (69.9%). The median delivered EQD2 was 64 Gy [interquartile range (IQR): 61.10–67.10 Gy]. The other characteristics of the patients are detailed in Table 1.
All patients completed the IMRT treatment successfully, and the median follow-up was 36 months (range 3–193 months). The 3-year OS, locoregional relapse-free survival (LRRFS), and distant metastasis-free survival (DMFS) rates were 51.3, 70.0, and 90.0%, respectively; the 5-year OS, LRRFS, and DMFS rates were 33.4, 63.3, and 84.6%, respectively.
Treatment was not interrupted because of severe acute side effects in any patient. However, most patients experienced mild acute toxicities, including mucositis and xerostomia. Only 37 patients (7.9%) developed grade-3 acute toxicities. After reirradiation, 163 patients (34.8%) had one of the common late complications, including trismus, xerostomia, and hearing loss. A total of 141 patients (30.1%) had mucosal necrosis, 93 patients (19.8%) had temporal lobe necrosis, and 66 patients (14.1%) had cranial nerve palsy.
The Impact of Comorbidity and Chemotherapy on Survival Outcomes
By the ACE-27 grading, of the 469 lrNPC patients, 188 (40.1%) had one or more comorbidities; 108 (23.0%) patients had ACE-27 scores of 1, 65 (13.9%) had scores of 2, and 15 (3.2%) had scores of 3. Patients with no or mild comorbidity had a 5-year OS rate of 36.2 versus 20.0% among those with comorbidity of moderate or severe grade (P<0.0001; Figure 1).
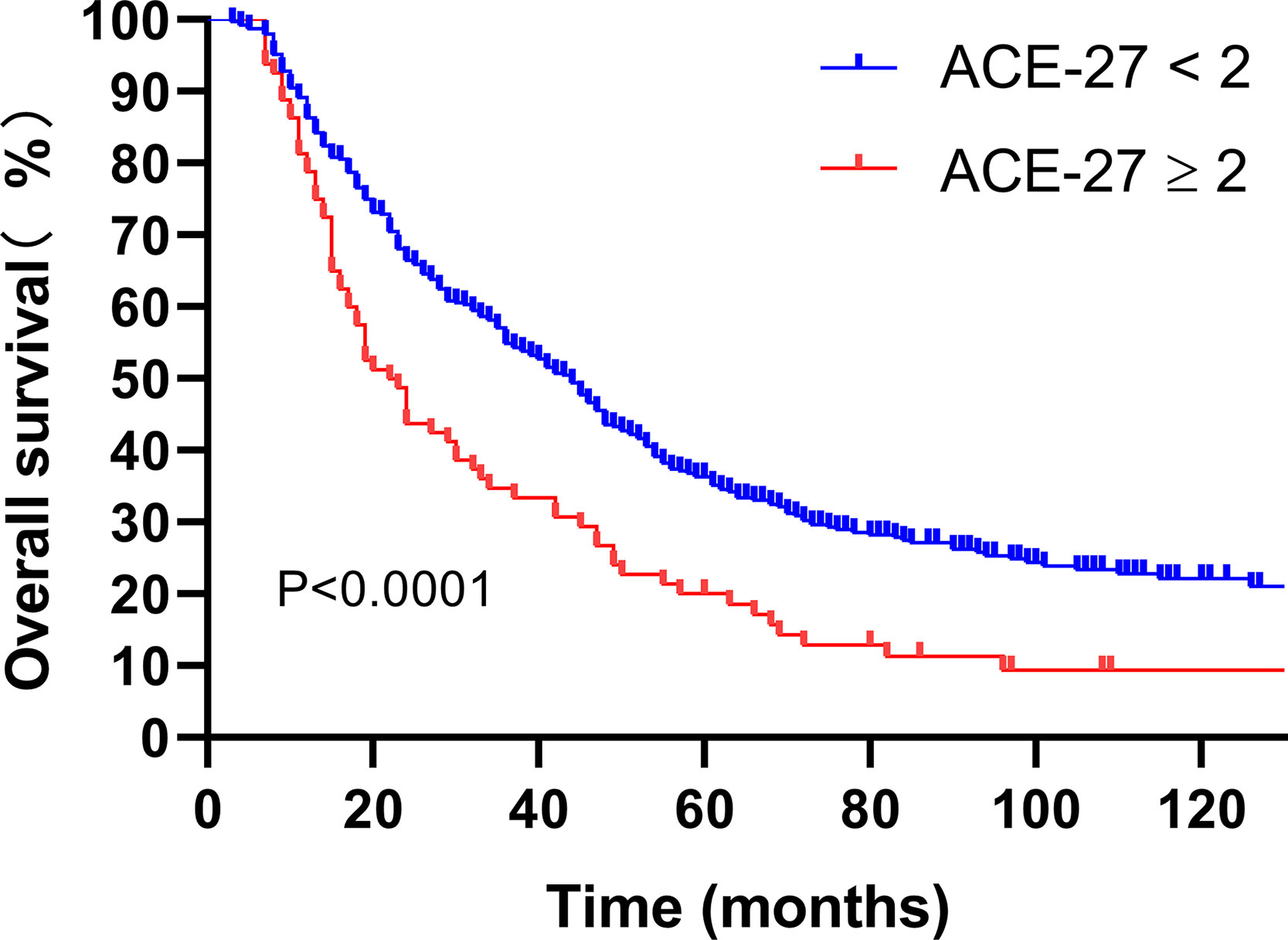
Figure 1 Kaplan-Meier analysis of overall survival is stratified by ACE-27. ACE-27, Adult Comorbidity Evaluation 27.
The prognosis of chemotherapy used was not significant in patients with lrNPC disease (P>0.05). Nevertheless, for the rT3–4 patients, the 5-year OS rate in the chemotherapy + RT group was 30.0 versus 16.7% for RT only (P=0.005). For rT3–4 patients with a comorbidity (ACE ≥ 2), the 5-year OS rate in the chemotherapy + RT group vs. for RT alone (15.7 vs. 15.0%, respectively; P>0.05) failed to confirm the positive association of chemotherapy. However, rT3–4 patients with an ACE-27 score of 0–1 had a higher 5-year OS rate in the chemotherapy + RT group than in the RT only group (32.1 and 17.1%, respectively; P=0.003). The results are presented in Supplemental Figure S1.
A total of 141/469 (30.1%) lrNPC patients only received RT, 113/469 (24.1%) received IC + RT, 93/469 (19.8%) received IC + CCRT, 116/469 (24.7%) received CCRT, and only 6/469 (1.3%) received AC, including 5/469 (1.1%) who received RT + AC and 1/469 (0.2%) who received CCRT + AC. Due to the small number of AC patients, they were excluded following survival analysis. There was no significant difference between RT only and other treatment regimens (all P>0.05). However, for the rT3–4 patients, the 5-year OS rate in the RT group was 16.7 vs. 30.4% for CCRT (P=0.011), 38.6% for CCRT + IC (P = 0.001), and 20.7% for IC + RT (P>0.05). For rT3–4 patients with a comorbidity (ACE < 2), the 5-year OS rate in the RT group was 17.1 vs. 35.2% for CCRT (P=0.003), 38.8% for CCRT + IC (P=0.002), and 22.5% for IC + RT (P>0.05). However, for rT3–4 patients with a comorbidity (ACE ≥ 2), the 5-year OS rate in the RT group was 15.0 vs. 7.2% for CCRT, 37.5% for CCRT + IC, and 14.0% for IC + RT (all P>0.05) (Supplemental Table S1 and Supplemental Figure S2). More details are shown in Figure 2.
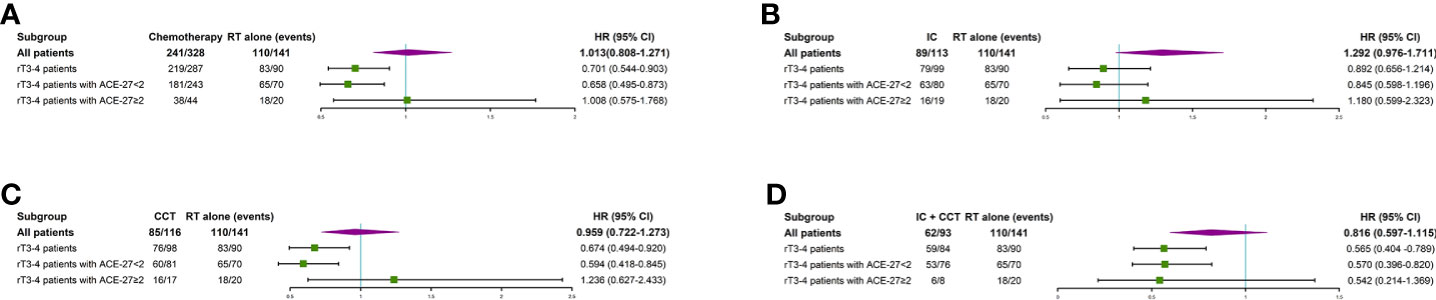
Figure 2 Subgroup analyses of overall survival for patients with radiotherapy only or chemotherapy plus radiotherapy (A), overall survival for patients with radiotherapy only or induction chemotherapy plus radiotherapy (B), overall survival for patients with radiotherapy only or concurrent chemotherapy plus radiotherapy (C), and overall survival for patients with radiotherapy only or induction chemotherapy plus concurrent chemotherapy plus radiotherapy (D). IC, induction chemotherapy; CCT, concurrent chemotherapy; CI, confidence interval; HR, hazard ratio.
Univariate Analysis and Multivariate Analysis
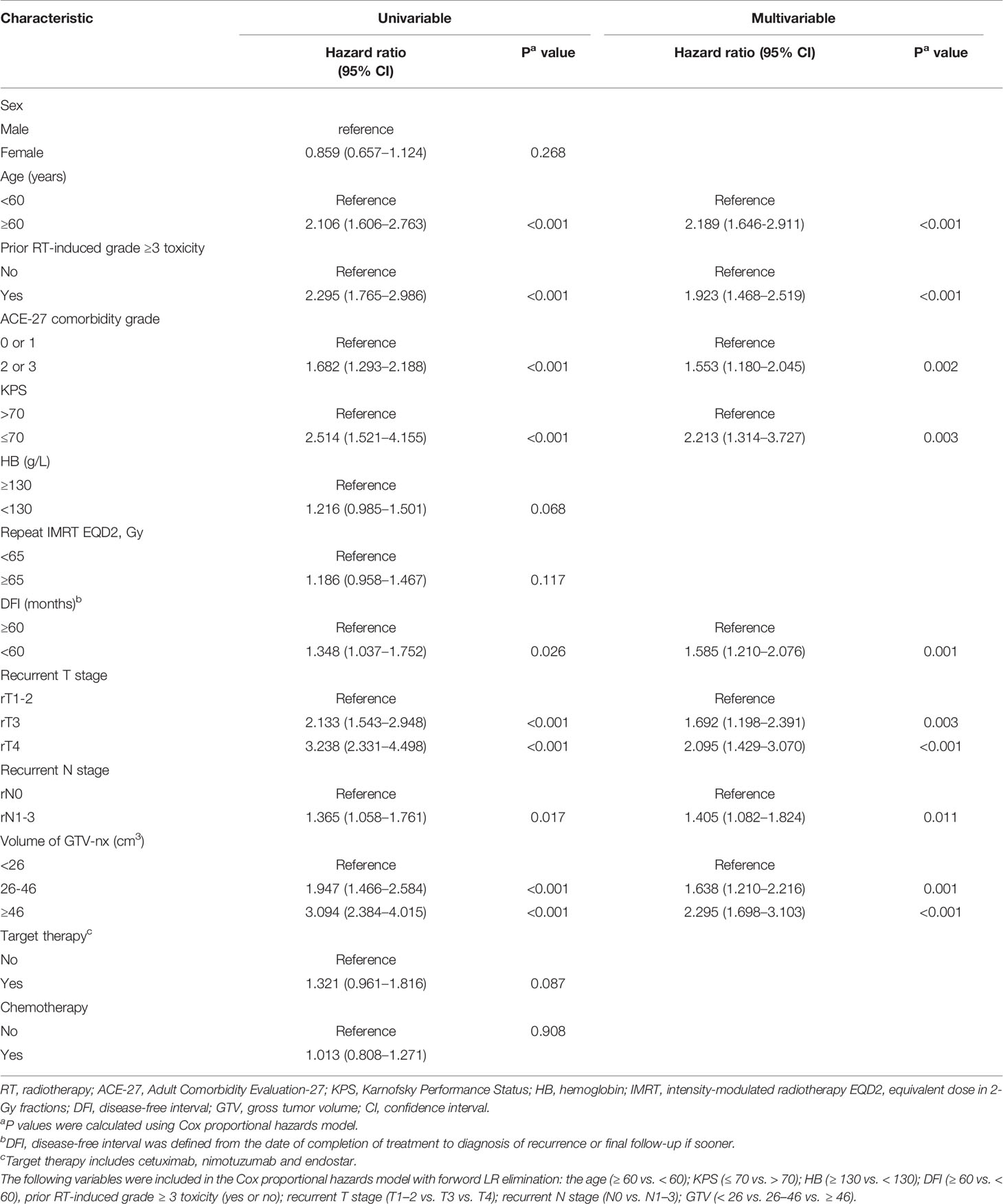
Next, it was necessary to assess the prognostic significance of continuous variables and avoid any predetermined cutoff points. The X-tile analysis identified 1 optimal cut-off point, and the age, HB, EQD2, and DFI were 59 years, 128 g/L, 64.69 Gy, and 59 months, respectively. The analysis identified two optimal GTV cut-off points, 25.65 and 46 cc. For the use of minimum P statistics by Miller-Siegmund P-value correction, the best cut-off value is obtained (22). To optimize the cutoff value for its potential acceptance and clinical application, we rounded to the nearest integer in further analysis. Age (< 60 versus ≥ 60 years); HB (≤ 130 versus > 130 g/L); EQD2 (< 65 versus ≥ 65 Gy); and DFI (< 60 versus ≥ 60 months) were investigated. Similarly, the nearest integers of 26 and 46 cc were selected. In addition, based on clinical practice, a score of 70 was the cut-off value of KPS. Thus, we performed a univariable analysis on those variables that may be potential prognostic factors (Table 2). These variables were analyzed for association with OS by using Cox proportional hazards regression model hazard ratios (HRs), and the univariable P < 0.1 was included in the multivariable analysis. From the results of univariable analysis, age, prior RT-induced grade ≥ 3 toxicity, ACE-27, GTV, KPS, DFI, rT stage, and rN stage were significant survival predictors. On multivariate analysis, we found that age (P < 0.001), prior RT-induced grade ≥ 3 toxicity (P<0.001), KPS (P=0.003), ACE-27 (P=0.002), DFI (P=0.001), rT stage (P<0.001), rN stage (P=0.011), and GTV (P<0.001) remained independent prognostic factors.
Establishment and Evaluation of a Nomogram Model for Overall Survival
Based on the eight independent prognostic factors, a nomogram was established for predicting the 1-, 3-, and 5-year OS for lrNPC (Figure 3). Each variable has a corresponding score according to the point scale, and we obtained the total score by calculating the score of each variable. Next, by mapping the total score on the probability scale, the OS probabilities could be estimated at the 1-, 3-, and 5-year time points (Figure 4)
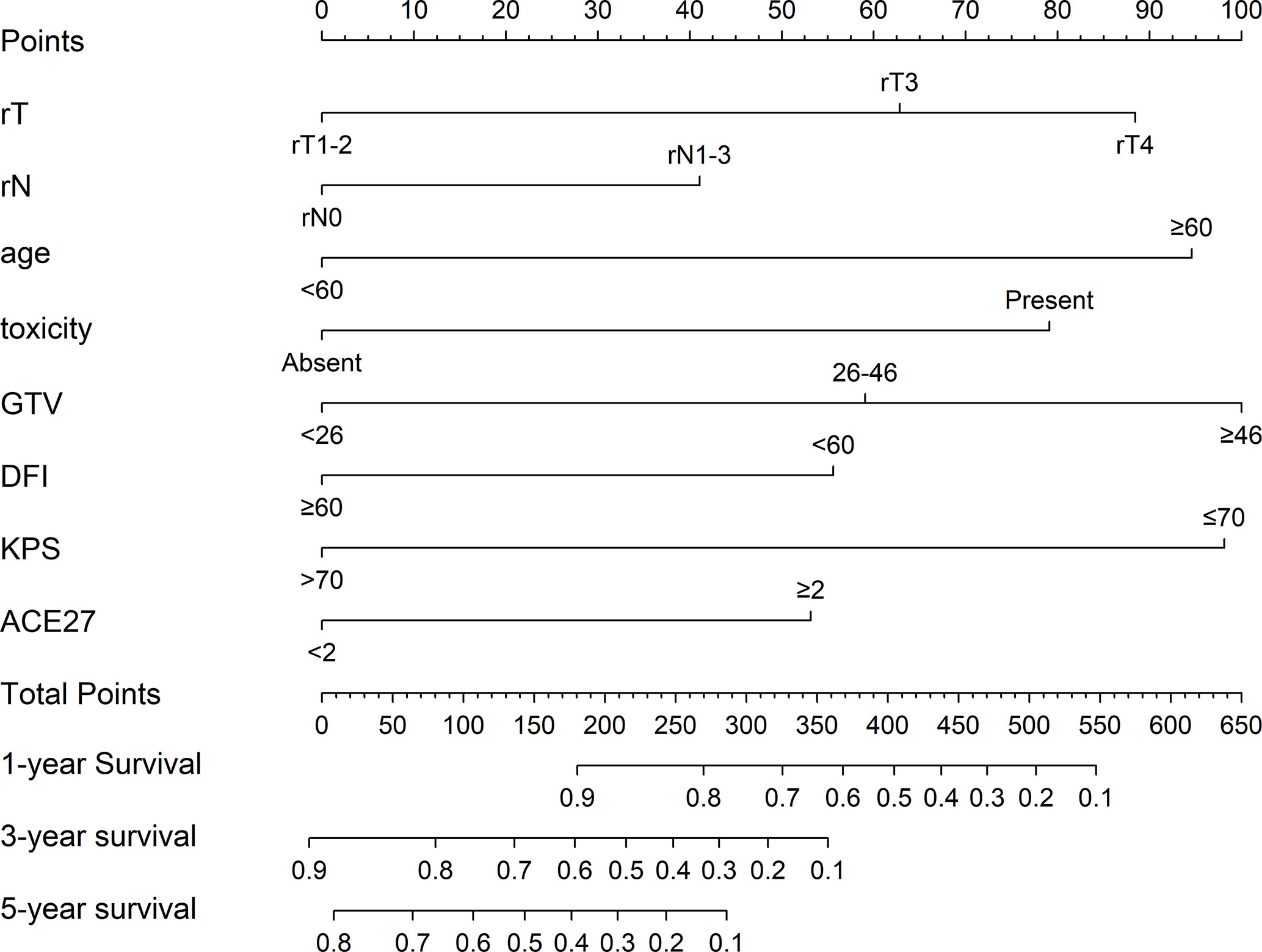
Figure 3 Prognostic nomogram for locally recurrent nasopharyngeal carcinoma (lrNPC) patients: a line was drawn straight down to predict the 1-, 3-, or 5-year overall survival. rT, recurrent T stage; rN, recurrent N stage; toxicity, prior RT-induced grade ≥ 3 toxicity; GTV, gross tumor volume; DFI, disease-free interval; KPS, Karnofsky Performance Status; ACE-27, Adult Comorbidity Evaluation-27.
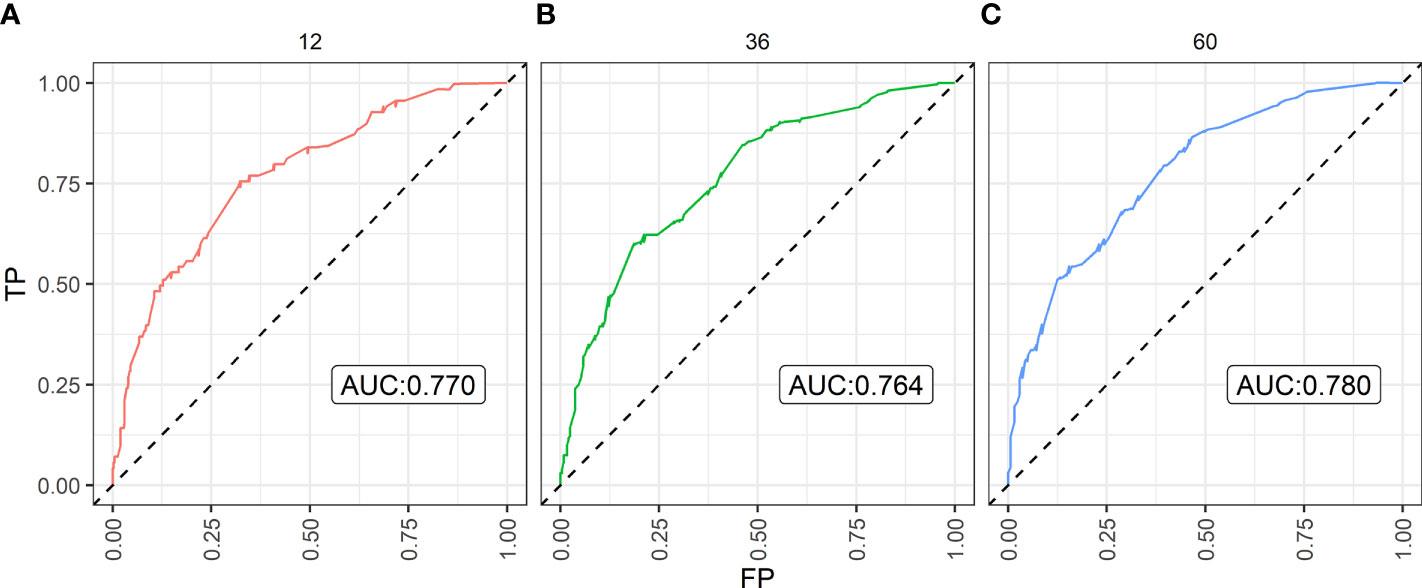
Figure 4 The Area Under Curve (AUC) of the prediction nomogram on (A) 1-year, (B) 3-year, and (C) 5-year overall survival. TP, true positive; FP, false positive.
By bootstrap correction, the Harrell C index was 0.715 in the nomogram. The area under curves (AUCs) for the prediction of 1-, 3-, and 5-year OS were 0.770, 0.764, and 0.780, respectively. The results exhibited satisfactory accuracy for predicting the 1-, 3-, and 5-year OS for lrNPC. The calibration curves showed that the nomogram predictions were well correlated with the actual observations for 1-, 3-, and 5-year OS (Supplemental Figures S3–S5).
Discussion
In general, ACE-27 in patients with primary NPC has been reported, and it has been explained to be a major determinant of survival outcome in patients with NPC (16, 23, 24). To our knowledge, the value of ACE-27 in patients with lrNPC has not yet been analyzed, and this study is the first to describe ACE-27 in patients diagnosed with lrNPC and to assess the prognostic value of ACE-27 on the survival of patients with lrNPC. In this study, comorbidity was present in 40.1% of patients. The incidence of comorbidity in lrNPC patients is similar to that in primary NPC patients (17). However, patients with moderate and severe comorbidities were observed to have higher rates of comorbidity than primary NPC. The reason may be that the patients become susceptible to disease or the original comorbidity has been aggravated after the initial radiotherapy and chemotherapy. The patients with an ACE-27 score of 0–1 had a better survival than those patients with an ACE-27 score of 2–3. This result is similar to that previously reported in primary NPC (24). In addition, patients who had rT3–4 patients with ACE-27 scores of 0–1 who received chemotherapy had better survival than those with RT only. Nevertheless, there was no significant difference between RT only and RT + chemotherapy in patients who had rT3–4 patients with ACE-27 scores of 2–3. Our findings suggest that patients with ACE-27 scores of 2–3 cannot benefit from chemotherapy. The reason may be that those patients have a shorter median survival time, and the benefits of chemotherapy have not been observed; furthermore, comorbidity increases the risk of death from other diseases. It can explain the negative impact of rT3–4 with comorbidities (moderate or severe) on survival.
Although various chemotherapy regimens and targeted agents have been used, there is still a lack of effective clinical evidence for the use of chemotherapy in lrNPC patients (25–27). A meta-analysis did not demonstrate that the addition of chemotherapy had an impact on local failure-free survival (LFFS), DMFS, and OS (28). In our study, however, we found that rT3–4 patients who received CCRT + IC or CCRT had better survival than those who received RT only, but those who received IC + RT had no significant OS benefit compared to those who received RT alone. In particular, rT3–4 patients with a comorbidity (ACE < 2) have similar outcomes. The reason may be that concurrent chemotherapy can improve radiosensitivity, thereby improving local tumor control. Nevertheless, patients with larger GTVs often choose to have increased IC. In addition, IC related to increased associated late toxicities should also be considered. The results show that CCRT may be a preferential therapeutic regimen. It is still worth exploring whether IC can benefit lrNPC patients. Ng WT et al. reported that IC followed by CCRT and weekly cetuximab could achieve a better treatment outcome (3-year PFS and OS rates of 36 and 64%, respectively) in lrNPC (T3–T4, N0–N1, M0) patients than reported in previous studies (29). The sample size in this study is small. To obtain high-level evidence, a prospective, multicenter, phase III clinical trial should be implemented. In addition, some studies have reported that immunotherapy has acquired promising results in recurrent or metastatic nasopharyngeal carcinoma patients (30–32). Nevertheless, the studies included only a small number of lrNPC patients. The effect of immunotherapy deserves more exploration by a large-scale clinical trial of lrNPC.
Some recent studies have shown that the dose of reirradiation by IMRT is an essential prognostic factor in patients with lrNPC (11, 14, 33, 34). In a prognostic model proposed by Li et al. (14), the dose of reirradiation by IMRT (EQD2 ≥ 68 Gy) is a poor prognostic factor. It is combined with the other four factors to form a prognostic index (PI). Tian et al. demonstrated that decreasing the total dose and increasing the fraction size can achieve local control similar to that achieved with a higher dose after IMRT, and it can improve OS by reducing the incidence of severe late complications (33). However, there are small samples in this study. Another study by Ng WT et al. (34) reported that a reirradiation dose equivalent to 60 Gy (EQD2) appears to be the optimal dose for achieving the best survival outcome while balancing the probability of local control and fatal complications. Those studies indicated that 60 Gy may be an optimal dose for lrNPC patients, but these studies are based on the analysis of small samples, and the patients in the study by Ng WT reported came from multiple centers. There are great differences in treatment strategies and dose limitation standards. Moreover, in our retrospective study, EQD2 (< 65 versus ≥ 65 Gy) had no significant effect on survival; however, the incidence of mucosal necrosis in the higher EDQ2 group was higher than that in the lower EQD2 group (40.2 versus 23.5%; respectively, P<0.001) and temporal lobe necrosis (25.5 versus 16.1%; respectively, P=0.013). This means that the high reirradiation dose increases the occurrence of complications. We consider that, with dose escalation, the survival benefit is potentially offset by survival detriment because of increased late complications. A meta-analysis by Leong YH found that DFI ≥ 36 months can obtain a higher rate of LFFS, but there is no benefit in OS and DMFS (28). In our study, DFI was an important prognostic factor. The cut-off time was 60 months, and the longer DFI may allow the patient’s previously irradiated organs to recover better and reduce the degree of damage by radiotherapy.
In addition, age, rT stage, and GTV were the most important predictors of OS in previous reports (11, 14, 28). We can obtain similar results in this study. To distinguish the impact of GTV on prognosis, we obtained two cutoff values using X-tile software, and the results showed that the different GTV groups had significantly different survival prognoses. The cut-off value of hemoglobin in this study was obtained using the software X-tile and the value was 128 g/L. However, in order to facilitate the application of the nomogram in clinical practice, we selected 130 g/L as the cut-off value finally. There are great variations between the selected cut-off value of hemoglobin in the previous studies, and there is no uniform standard so far (35–37). Some reports showed that relatively low levels of hemoglobin is a poor prognostic factor for nasopharyngeal carcinoma (35, 36). However, in our study, we fail to get the similar results.
Moreover, the present study has several limitations that deserve further discussion. First, plasma Epstein-Barr virus (EBV) DNA was regarded as an adverse prognostic biomarker in primary NPC (38). In this study, we excluded EBV DNA from the nomogram. We considered the positive rate of pretreatment plasma EBV DNA in the detection of lrNPC to be relatively low, and the prognostic significance of EBV DNA, which lacks prospective data in lrNPC, was uncertain. In addition, the method of plasma EBV DNA measurement lacked standardization, and the EBV DNA polymerase chain reaction assay is susceptible due to changes in experimental conditions (39). Second, it is a single-center retrospective study in an endemic area, and some amount of selection bias is unavoidable. Finally, our proposed models were not validated in an external cohort, and the C-indices of the nomogram showed an imperfect discrimination ability, which indicated that more factors should be considered. Nonetheless, we acknowledge these limitations of our model. The focus of this study is that it is the first large-scale study to evaluate the importance of ACE-27 and its value in predicting survival for lrNPC.
Conclusion
In summary, our study is a large-scale study of lrNPC treated with IMRT. In this study, eight prognostic factors are worth investigating before reirradiation. For patients with favorable risk factors, reirradiation should be strongly recommended because it may have better survival benefits. However, for patients with high-risk factors, palliative chemotherapy or immunotherapy should be considered.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
FH, X-WD, and C-YC provided the study concepts. R-DH, ZS, and X-HW designed the study. R-DH, ZS, and X-HW acquired the data. Y-MT, Y-LP, J-YW, and W-WX conducted the quality control of the data and algorithms. R-DH, ZS, and X-HW analyzed and interpreted the data. R-DH, ZS, and X-HW conducted the statistical analysis. R-DH, ZS, and X-HW prepared the manuscript, R-DH, ZS, and X-HW edited the manuscript. FH, X-WD, and C-YC reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (12005316), Cancer Precision Radiotherapy Spark Program of China International Medical Foundation (2019-N-11-20), and Medical Scientific Research Foundation of Guangdong Province, China (Granted No. A2016197).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.625184/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer (2019) 144(8):1941–53. doi: 10.1002/ijc.31937
3. Chen Y-P, Chan ATC, Le Q-T, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet (2019) 394(10192):64–80. doi: 10.1016/s0140-6736(19)30956-0
4. Ng WT, Lee MCH, Hung WM, Choi CW, Lee KC, Chan OSH, et al. Clinical Outcomes and Patterns of Failure After Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys (2011) 79(2):420–8. doi: 10.1016/j.ijrobp.2009.11.024
5. Setton J, Han J, Kannarunimit D, Wuu YR, Rosenberg SA, DeSelm C, et al. Long-term patterns of relapse and survival following definitive intensity-modulated radiotherapy for non-endemic nasopharyngeal carcinoma. Oral Oncol (2016) 53:67–73. doi: 10.1016/j.oraloncology.2015.11.015
6. Lee AWM, Ng WT, Chan LLK, Hung WM, Chan CCC, Sze HCK, et al. Evolution of treatment for nasopharyngeal cancer - Success and setback in the intensity-modulated radiotherapy era. Radiother Oncol (2014) 110(3):377–84. doi: 10.1016/j.radonc.2014.02.003
7. Sun XM, Su SF, Chen CY, Han F, Zhao C, Xiao WW, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: An analysis of survival and treatment toxicities. Radiother Oncol (2014) 110(3):398–403. doi: 10.1016/j.radonc.2013.10.020
8. Wang RS, Wu F, Lu HM, Wei B, Feng GS, Li GS, et al. Definitive intensity-modulated radiation therapy for nasopharyngeal carcinoma: long-term outcome of a multicenter prospective study. J Cancer Res Clin Oncol (2013) 139(1):139–45. doi: 10.1007/s00432-012-1313-0
9. Chan JYW, Tsang RKY, Wei WI. Morbidities after maxillary swing nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck-J Sci Spec Head Neck (2015) 37(4):487–92. doi: 10.1002/hed.23633
10. Chan JYW, Wei WI. Impact of resection margin status on outcome after salvage nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck-J Sci Spec Head Neck (2016) 38:E594–E9. doi: 10.1002/hed.24046
11. Han F, Zhao C, Huang SM, Lu LX, Huang Y, Deng XW, et al. Long-term Outcomes and Prognostic Factors of Re-irradiation for Locally Recurrent Nasopharyngeal Carcinoma using Intensity-modulated Radiotherapy. Clin Oncol (2012) 24(8):569–76. doi: 10.1016/j.clon.2011.11.010
12. Perri F, Scarpati GD, Caponigro F, Ionna F, Longo F, Buonopane S, et al. Management of recurrent nasopharyngeal carcinoma: current perspectives. OncoTarg Ther (2019) 12:1583–91. doi: 10.2147/ott.S188148
13. Sun XS, Liang YJ, Jia GD, Liu SL, Liu LT, Guo SS, et al. Establishment of a prognostic nomogram to identify optimal candidates for local treatment among patients with local recurrent nasopharyngeal carcinoma. Oral Oncol (2020) 106:7. doi: 10.1016/j.oraloncology.2020.104711
14. Li YQ, Tian YM, Tan SH, Liu MZ, Kusumawidjaja G, Ong EHW, et al. Prognostic Model for Stratification of Radioresistant Nasopharynx Carcinoma to Curative Salvage Radiotherapy. J Clin Oncol (2018) 36(9):891–+. doi: 10.1200/jco.2017.75.5165
15. Tanvetyanon T, Padhya T, McCaffrey J, Zhu WW, Boulware D, DeConti R, et al. Prognostic Factors for Survival After Salvage Reirradiation of Head and Neck Cancer. J Clin Oncol (2009) 27(12):1983–91. doi: 10.1200/jco.2008.20.0691
16. Sommat K, Yit NLF, Wang FQ, Lim JHC. Impact of comorbidity on tolerability and survival following curative intent intensity modulated radiotherapy in older patients with nasopharyngeal cancer. J Geriatr Oncol (2018) 9(4):352–8. doi: 10.1016/j.jgo.2018.01.006
17. Guo R, Mao YP, Chen L, Tang LL, Zhou GQ, Liu LZ, et al. Implication of comorbidity on the initiation of chemotherapy and survival outcomes in patients with locoregionally advanced nasopharyngeal carcinoma. Oncotarget (2017) 8(6):10594–601. doi: 10.18632/oncotarget.8621
18. Paleri V, Wight RG, Silver CE, Haigentz M, Takes RP, Bradley PJ, et al. Comorbidity in head and neck cancer: A critical appraisal and recommendations for practice. Oral Oncol (2010) 46(10):712–9. doi: 10.1016/j.oraloncology.2010.07.008
19. Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope (2015) 125(10):2242. doi: 10.1002/lary.25278
20. Schimansky S, Lang S, Beynon R, Penfold C, Davies A, Waylen A, et al. Association between comorbidity and survival in head and neck cancer: Results from Head and Neck 5000. Head Neck (2019) 41(4):1053–62. doi: 10.1002/hed.25543
21. Tian YM, Huang WZ, Yuan X, Bai L, Zhao C, Han F. The challenge in treating locally recurrent T3-4 nasopharyngeal carcinoma: the survival benefit and severe late toxicities of re-irradiation with intensity-modulated radiotherapy. Oncotarget (2017) 8(26):43450–7. doi: 10.18632/oncotarget.15896
22. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res (2004) 10(21):7252–9. doi: 10.1158/1078-0432.Ccr-04-0713
23. Jin YN, Zhang WJ, Cai XY, Li MS, Lawrence WR, Wang SY, et al. The Characteristics and Survival Outcomes in Patients Aged 70 Years and Older with Nasopharyngeal Carcinoma in the Intensity-Modulated Radiotherapy Era. Cancer Res Treat (2019) 51(1):34–42. doi: 10.4143/crt.2017.551
24. Guo R, Chen XZ, Chen L, Jiang F, Tang LL, Mao YP, et al. Comorbidity predicts poor prognosis in nasopharyngeal carcinoma: Development and validation of a predictive score model. Radiother Oncol (2015) 114(2):249–56. doi: 10.1016/j.radonc.2014.12.002
25. Koutcher L, Lee N, Zelefsky M, Chan K, Cohen G, Pfister D, et al. Reirradiation of locally recurrent nasopharynx cancer with external beam radiotherapy with or without brachytherapy. Int J Radiat Oncol Biol Phys (2010) 76(1):130–7. doi: 10.1016/j.ijrobp.2009.01.055
26. Poon D, Yap SP, Wong ZW, Cheung YB, Leong SS, Wee J, et al. Concurrent chemoradiotherapy in locoregionally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys (2004) 59(5):1312–8. doi: 10.1016/j.ijrobp.2004.01.037
27. Chua DTT, Sham JST, Au GKH. Induction chemotherapy with cisplatin and gemcitabine followed by reirradiation for locally recurrent nasopharyngeal carcinoma. Am J Clin Oncol-Cancer Clin Trials (2005) 28(5):464–71. doi: 10.1097/01.coc.0000180389.86104.68
28. Leong YH, Soon YY, Lee KM, Wong LC, Tham IWK, Ho FCH. Long-term outcomes after reirradiation in nasopharyngeal carcinoma with intensity-modulated radiotherapy: A meta-analysis. Head Neck-J Sci Spec Head Neck (2018) 40(3):622–31. doi: 10.1002/hed.24993
29. Ng WT, Ngan RKC, Kwong DLW, Tung SY, Yuen KT, Kam MKM, et al. Prospective, Multicenter, Phase 2 Trial of Induction Chemotherapy Followed by Bio-Chemoradiotherapy for Locally Advanced Recurrent Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys (2018) 100(3):630–8. doi: 10.1016/j.ijrobp.2017.11.038
30. Hsu C, Lee SH, Ejadi S, Even C, Cohen RB, Le Tourneau C, et al. Safety and Antitumor Activity of Pembrolizumab in Patients With Programmed Death-Ligand 1-Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE-028 Study. J Clin Oncol (2017) 35(36):4050–+. doi: 10.1200/jco.2017.73.3675
31. Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J Clin Oncol (2018) 36(14):1412–+. doi: 10.1200/jco.2017.77.0388
32. Fang WF, Yang YP, Ma YX, Hong SD, Lin LZ, He XH, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol (2018) 19(10):1338–50. doi: 10.1016/s1470-2045(18)30495-9
33. Tian YM, Zhao C, Guo Y, Huang Y, Huang SM, Deng XW, et al. Effect of Total Dose and Fraction Size on Survival of Patients With Locally Recurrent Nasopharyngeal Carcinoma Treated With Intensity-Modulated Radiotherapy: A Phase 2, Single-Center, Randomized Controlled Trial. Cancer (2014) 120(22):3502–9. doi: 10.1002/cncr.28934
34. Ng WT, Lee MCH, Fung NTC, Wong ECY, Cheung AKW, Chow JCH, et al. Dose volume effects of re-irradiation for locally recurrent nasopharyngeal carcinoma. Head Neck-J Sci Spec Head Neck (2020) 42(2):180–7. doi: 10.1002/hed.25988
35. Gao J, Tao YL, Li G, Yi W, Xia YF. Involvement of difference in decrease of hemoglobin level in poor prognosis of Stage I and II nasopharyngeal carcinoma: implication in outcome of radiotherapy. Int J Radiat Oncol Biol Phys (2012) 82(4):1471–8. doi: 10.1016/j.ijrobp.2011.05.009
36. Tang LQ, Li CF, Li J, Chen WH, Chen QY, Yuan LX, et al. Establishment and Validation of Prognostic Nomograms for Endemic Nasopharyngeal Carcinoma. J Natl Cancer Inst (2016) 108(1):djv291. doi: 10.1093/jnci/djv291
37. Topkan E, Ekici NY, Ozdemir Y, Besen AA, Yildirim BA, Mertsoylu H, et al. Baseline hemoglobin <11.0 g/dL has stronger prognostic value than anemia status in nasopharynx cancers treated with chemoradiotherapy. Int J Biol Markers (2019) 34(2):139–47. doi: 10.1177/1724600818821688
38. Liu TB, Zheng ZH, Pan J, Pan LL, Chen LH. Prognostic role of plasma Epstein-Barr virus DNA load for nasopharyngeal carcinoma: a meta-analysis. Clin Invest Med (2017) 40(1):E1–E12. doi: 10.25011/cim.v40i1.28049
Keywords: comorbidity, recurrent, nasopharyngeal carcinoma, reirradiation, prognostic nomogram
Citation: Huang R-D, Sun Z, Wang X-H, Tian Y-M, Peng Y-L, Wang J-Y, Xiao W-W, Chen C-Y, Deng X-W and Han F (2021) Development of a Comorbidity-Based Nomogram to Predict Survival After Salvage Reirradiation of Locally Recurrent Nasopharyngeal Carcinoma in the Intensity-Modulated Radiotherapy Era. Front. Oncol. 10:625184. doi: 10.3389/fonc.2020.625184
Received: 02 November 2020; Accepted: 26 November 2020;
Published: 20 January 2021.
Edited by:
Nicola Silvestris, University of Bari Aldo Moro, ItalyReviewed by:
Sufang Qiu, Fujian Provincial Cancer Hospital, ChinaJun-Lin Yi, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2021 Huang, Sun, Wang, Tian, Peng, Wang, Xiao, Chen, Deng and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Han, hanfei@sysucc.org.cn; Xiao-Wu Deng, dengxw@mail.sysu.edu.cn; Chun-Yan Chen, chenchuny@sysucc.org.cn
†These authors have contributed equally to this work
 Run-Da Huang
Run-Da Huang Zhuang Sun
Zhuang Sun Xiao-Hui Wang1,2,3,4†
Xiao-Hui Wang1,2,3,4† Xiao-Wu Deng
Xiao-Wu Deng Fei Han
Fei Han