- 1Laboratory of Molecular Pharmacology, Department of Pharmacology, School of Pharmacy, Southwest Medical University, Luzhou, China
- 2Department of Public Health, Chengdu Medical College, Chengdu, China
- 3Department of Anesthesiology, Gulinxian People’s Hospital of Sichuan Province, Luzhou, China
- 4Department of Digestive Surgery, School of Chinese Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong
- 5Department of Orthopedics, Shanghai Institute of Traumatology and Orthopaedics, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
- 6Department of Art, Art College, Southwest Minzu University, Chengdu, China
- 7Department of Neuroscience, Beijing Institute of Basic Medical Sciences, Beijing, China
The prevalence of pancreatic cancer is sharply increasing recently, which significantly increases the economic burden of the population. At present, the primary treatment of resectable pancreatic cancer is surgical resection, followed by chemotherapy with or without radiation. However, the recurrence rates remain high even after R0 resection. This treatment strategy does not distinguish undetected metastatic disease, and it is prone to postoperative complications. Neoadjuvant therapies, including neoadjuvant chemotherapy and radiotherapy, is being increasingly utilized in borderline resectable as well as resectable pancreatic cancer. This review summarized and discussed clinical trials of neoadjuvant therapy for pancreatic cancer, comparing resection rates, outcome measures, and adverse reactions between neoadjuvant chemotherapy and neoadjuvant chemoradiotherapy.
Highlights
Surgery is the only potential cure for pancreatic cancer, but the survival duration of patients did not improve significantly. Pancreatic cancer has an obvious tendency to metastasize, and R0 resection is difficult to achieve. Neoadjuvant therapy is widely used, ranging from resectable pancreatic cancer, borderline resectable pancreatic cancer, and locally advanced pancreatic cancer. There are many options in neoadjuvant therapy, such as chemotherapy, radiotherapy, and chemoradiotherapy. Unfortunately, the choice of neoadjuvant treatment for pancreatic cancer remains controversial.
Introduction
Pancreatic cancer is one of the most common malignancies of the digestive tract, and also one of the worst prognoses, with a 5-year survival rate of only 6% (1). Based on the GLOBOCAN 2020 estimates, pancreatic cancer has ranked the seventh most common cancer in the world counting 495,773 new cases and causing 466,003 deaths (4.7% of all deaths caused by cancer) in 2020 (2). In addition, the incidence and mortality of pancreatic cancer increased with age, and it is most common in men (3). The monthly medical expenses of pancreatic cancer patients are 15 times more than that of non-pancreatic cancer patients. Therefore, it is important to plan potential new therapies to manage and control patient costs (4).
Neoadjuvant Therapy (Nat)
Frontline treatments for pancreatic cancer include surgical treatment, chemotherapy, radiation therapy, biological therapy, etc. Radical surgery is complicated and may cause more complications. Surgical treatment is local treatment, as usually the cancer tissue cannot be removed completely and it is easy to recur and metastasize. Radiation and chemotherapy use the powerful external radiation or toxic drugs to kill tumor cells in the body, unfortunately the normal cells (including immune cells) are also killed, this may induce a low immunity. Biological therapy inhibits or eliminates tumor growth by increasing the resistance of the immune system of the body to tumor cells; however, the efficiency of gene transduction is low, has poor specificity, and the efficacy of late tumors is limited (5).
Any preoperative treatment of resectable tumors, as well as treatments that may lead to surgery in the case of tumor response, are considered “neoadjuvant therapy (NAT)” (6). Unlike adjuvant therapy, NAT methods may allow the assessment of tumor response in vivo and improve compliance (7). The tolerance of NAT is better than that of adjuvant therapy, which can reduce the incidence of complications of pancreatic surgery. One of the most promising advantages of NAT for pancreatic cancer is that by converting the initial marginal or locally unresectable tumors into resectable tumors, it is possible to increase the number of surgical candidates. In addition, those who are converted to candidates for surgery have similar survival rates to those with initially resectable tumors (8). NAT contains neoadjuvant chemotherapy (NAC) and neoadjuvant chemo-radiotherapy (NACRT). NAC (with or without radiation therapy) is often used to reduce the staging of marginally resectable tumors and locally advanced tumors. The current evidence is mainly retrospective; however, it disclosed that NAT can increase the R0 resection rate and significantly increase the overall survival (9). Compared with NACRT, NAC appears to be equally effective in transforming the unresectable nature of resectable diseases, and it is also more effective in systemic tumor progression and overall survival (6).
Neoadjuvant radiotherapy with or without chemotherapy has a better survival than upfront surgery with or without adjuvant therapy among patients with a resectable pancreatic cancer (10). If surgery is the basis of treatment, providing pathologically negative margin (R0) resection is currently the only way to achieve the best cure rate (11). Macroscopic (R2) and microscopic (R1) marginal infiltration have similar survival trends with locally advanced or metastatic disease (12). Traditionally, R0 represents no cancer at the margins, while R1 represents microscopic disease at the margin, and R2 is representative of gross disease at the margins (seen by naked eye); see Table 1. For borderline resectable pancreatic cancer (BRPC), NAT could maximize the potential for an R0 resection and avoid R1/R2 resections (13). If an initially unresectable is converted to operable after NAT, microscopically complete resection has been performed (14). Resectable is the cornerstone of treatment. The ultimate goal is R0 resection. Unfortunately, even for early resectable performance, the R0 resection rate is not ideal. Therefore, it is suitable for auxiliary or neoadjuvant integrated treatment (8).
Validation Method
We collected raw data from references, which we searched from the PUBMED with “pancreatic cancer” and “neoadjuvant” as the query terms. The article types were screened as clinical trials. A total of 93 clinical trials found since October 2020. Moreover, 202 articles were searched from (https://clinicaltrials.gov/). In total, there were 295 articles included in the study. Unresectable pancreatic cancer and irrelevant literature were further excluded. Finally, 37 clinical trials on NAT of pancreatic cancer were included in this study. The 37 records were divided into the following two tables on the basis of the type of adjuvant therapy. For a detailed reference screen plot, see Figure 1.
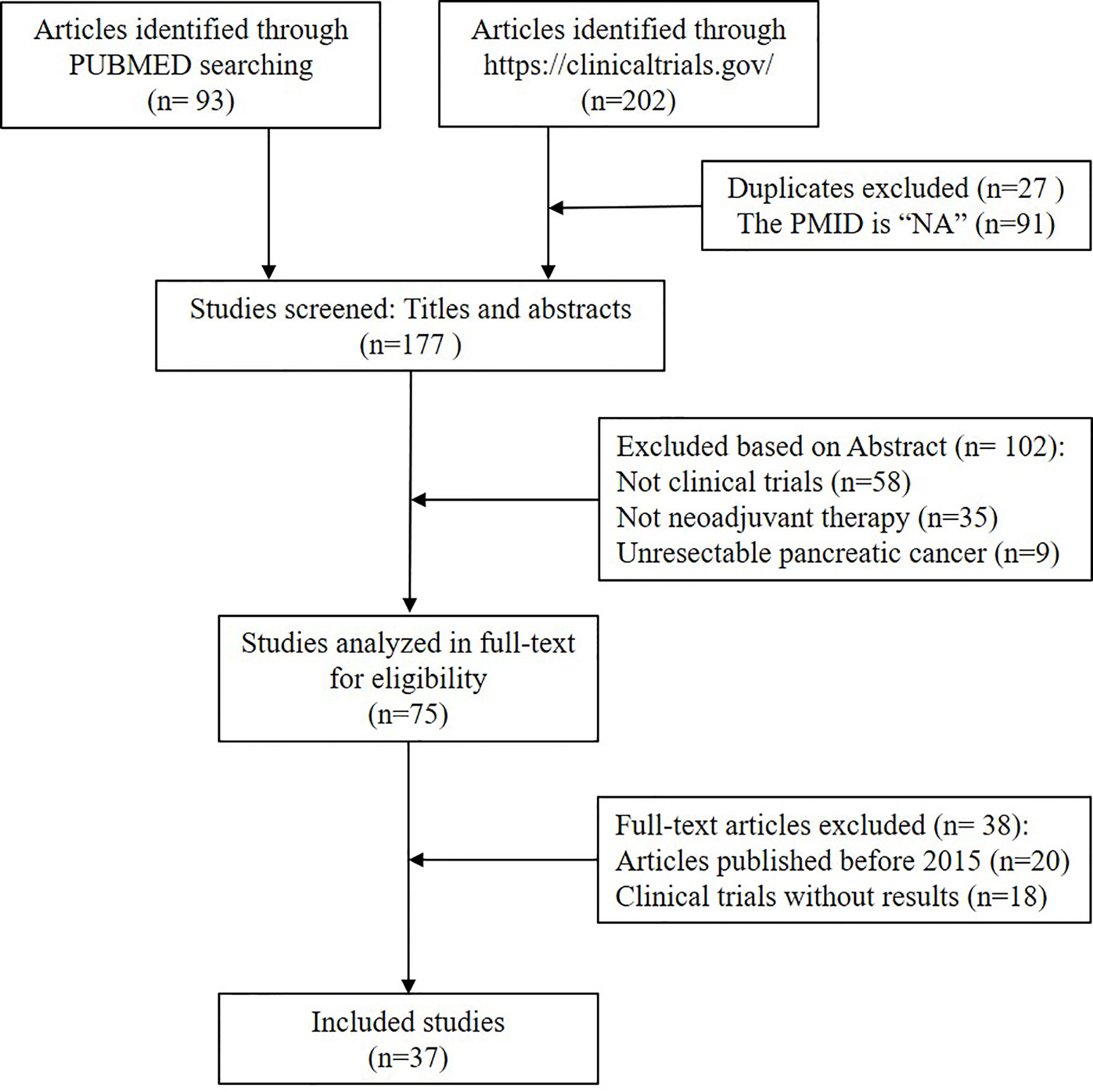
Figure 1 Flowchart of the included studies. Publications were retrieved by searching the following databases: PubMed (n = 93) and (https://clinicaltrials.gov/) (n = 202), with a total of 295 publications. The search strategy included keywords related to pancreatic cancer and neoadjuvant. All citations were screened to identify relevant studies, firstly, duplicate and unavailable PMID studies were excluded (27 duplicate and 91 unavailable PMID). Secondly, by the title and abstract (n = 102) and, thirdly, by full text screening (n = 38). A total of 258 publications were excluded. Finally, 37 were eligible for assessment by full paper.
Results
The details of the following study were extracted: first author, year of publication, interventions, study population, percentage of R0 resection after NAT, and outcome [overall survival (OS), progression-free survival (PFS), grade 3 or 4 adverse events of neutropenia, leukopenia, thrombocytopenia, and anemia].
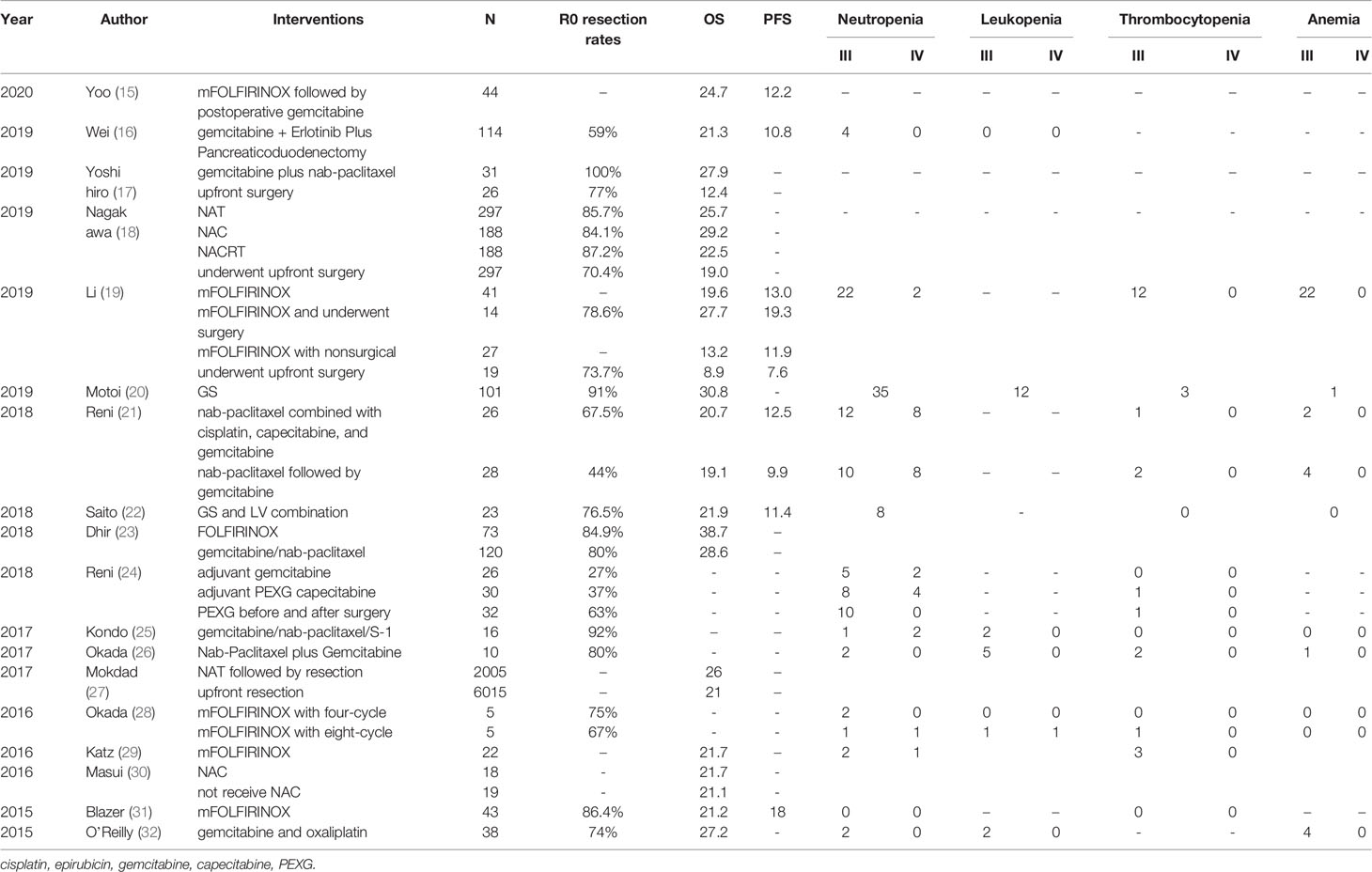
In total, there were 18 clinical trials on patients with pancreatic cancer receiving NAC before surgery, see Table 2. These clinical trials recruited 9,938 patients with a resectable pancreatic cancer. The average OS was 22.87 months, and the PFS was 12.66 months. The average R0 resection rates were 73%. In detail, Mashaal et al. performed pancreaticoduodenectomy after NAT with 5-fluorouracil, leucovorin, oxaliplatin, irinotecan (FOLFIRINOX) for pancreatic ductal adenocarcinoma (PDAC) patients in 2018, the highest OS obtained was 38.7 months. The R0 resection rate was also relatively high at 84.9% (23). In 2019, Xiang et al. evaluated the effect of the modified FOLFIRINOX (mFOLFIRINOX) regimen in patients with locally advanced pancreatic cancer (LAPC) in China, they found that patients who received mFOLFIRINOX and underwent surgery had the highest PFS of 19.3 months and the higher OS of 27.7 months (19). Similarly, Marlo et al. also performed mFOLFIRINOX on patients with BRPC and LAPC. The median PFS was 18 months and a higher R0 esection rate of 86.4%, and there were no adverse reactions of neutropenia and thrombocytopenia (31). What is more, Naru et al. used gemcitabine, napaclitaxel, and S-1 NAC for patients with LAPC. It had a good R0 resection rate of 92% (25). Later, Fuyuhiko et al. assessed the feasibility and survival outcomes of NAC with gemcitabine and S1 (GS) for a PDAC planned resection. This method had a considerable R0 resection rate and OS, 91% and 30.8 months, respectively (20). Moreover, in the study of Yoshihiro et al., gemcitabine combined with Nab-paclitaxel NAC for BRPC achieved the highest R0 resection rate of 100%, with a higher OS of 27.9 months (17).
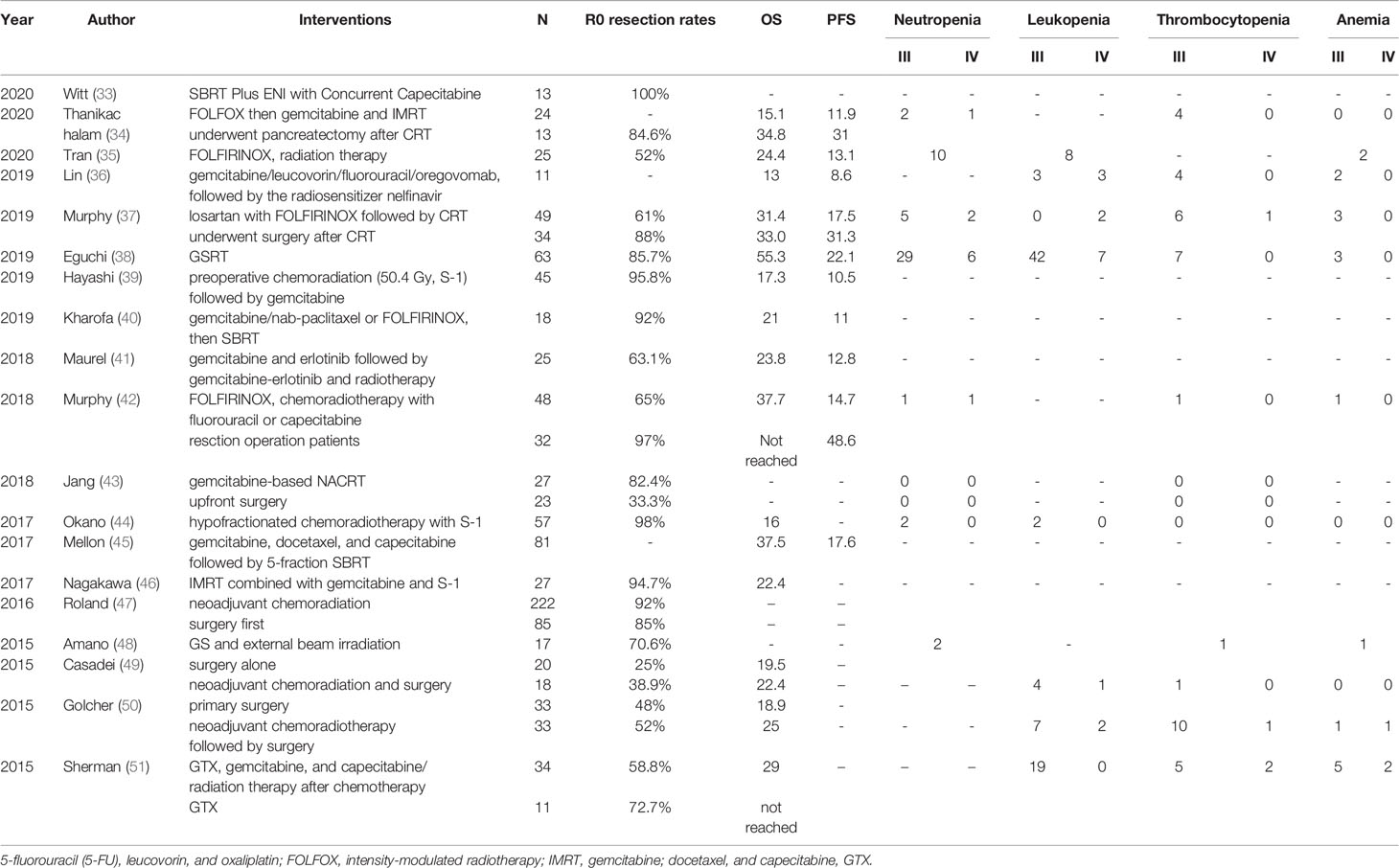
The following 20 studies clarified the results of NACRT for pancreatic cancer. From 2014 to 2020, a total of 1,030 pancreatic cancer patients were recruited, see Table 3. The average OS for these studies was 25.8 months, and the PFS was 18.4 months. For example, Hidetoshi et al. reported in 2019 that for resectable PDAC, NAT with gemcitabine and S-1, and 50.4 Gy of radiotherapy (GSRT) at the same time, the median survival time was as long as 55.3 months. However, there were 49 (total: 63) patients with adverse reactions of leukopenia in this regimen (38). Secondly, Janet et al. used FOLFIRINOX followed by individualized chemoradiotherapy (CRT) for borderline-resectable PDAC patients with fewer adverse events. The median OS was 37.7 months. Interestingly, among patients undergoing resection, the median PFS increased to 48.6 months with high R0 resection rates (92%) (42). Similarly, the study by Keiichi et al. also had high R0 resection rates (98%) with less adverse events. They used neoadjuvant S-1 with Concurrent hypofractionated radiotherapy in patients with resectable and borderline resectable PDAC. Even better, Jacob et al. used neoadjuvant (stereotactic body radiation therapy) SBRT Plus elective nodal irradiation (ENI) with concurrent capecitabine for resectable pancreatic cancer to obtain high R0 resection rates of 100% in 2020 (33).
Advantages and Limitations Of NAT
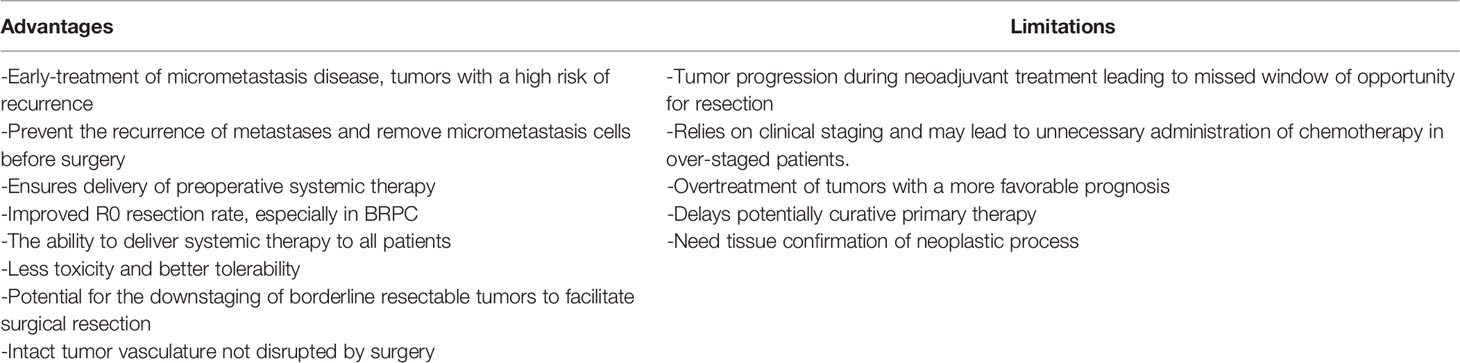
Surgical resection first, followed by systemic chemotherapy with radiotherapy or no radiotherapy, is the current recommendation for early resectable pancreatic adenocarcinoma (27). However, these diseases may not benefit from resection because this treatment strategy fails to distinguish patients with an undetected metastatic disease and aggressive disease. Recurrence rates remain high even after R0 resections. In addition, postoperative complications associated with pancreatectomy may hinder the implementation of adjuvant therapy. Early provision of NAT is considered an alternative treatment strategy. Combining it with systemic chemotherapy and concurrent radiotherapy increased the possibility of R0 resection for patients with BRPC. NAT has many benefits, including the early treatment of micrometastatic disease and high-risk recurrence tumors, etc. (17, 52). Although NAT has many advantages, it also has some limitations. During NAT, cancer may progress locally or metastasize far away, thereby jeopardizing curative surgical treatment. NAT relies on clinical staging. Insufficient staging can lead to undertreatment, and over staging can lead to the overuse of NAT (53). In addition, there is another risk of overtreatment of cancers with a poor prognosis (54), see Table 4. Some scholars pointed out that patients with an early metastatic disease who are resistant to chemotherapy can be identified by preoperative systemic treatment, and the preoperative systemic treatment ensures that more patients receive multimodal treatment (55, 56). Even if NAT has a strong effect on tumors, people are worried that NAT may have an impact on the postoperative course of the disease. In fact, some studies showed that NAT of pancreatic cancer did not increase the postoperative morbidity (57). Therefore, more effective neoadjuvant programs should be applied to patients with a resectable pancreatic cancer, such as gemcitabine plus nab-paclitaxel or mFOLFIRINOX (15).
For NAC, the FOLFIRINOX/(m)FOLFIRINOX regimen and gemcitabine plus nab-paclitaxel is a good patient selection strategy. It is now widely recognized that NAC can achieve tumor downgrading so as to increase the surgical resection rate of pancreatic cancer, and even increase the R0 resection rate (58). It has been noted that the toxicity of the neoadjuvant FOLFIRINOX was reduced after chemoradiotherapy, with a single grade 3 toxicity of less than 10% and no toxicity-related deaths (59). In addition, the effectiveness of gemcitabine against pancreatic cancer has been widely confirmed. A large number of studies proved that the effect of single-agent chemotherapy was significantly weaker than FOLFIRINOX and multi-drug combination programs such as gemcitabine plus nab-paclitaxel, so the treatment prospects of multi-drug combination programs are good (60). The GS trial showed that GS treatment was significantly higher than gemcitabine alone (61). At the same time, judging from the incidence of adverse events and the rate of surgical resection, NAC is safe and feasible (62).
Because the surgical treatment of pancreatic cancer has a high risk of local recurrence, and radiotherapy is expected to improve the control of local diseases. Gemcitabine was chosen as the drug during concurrent radiotherapy due to its well-known radio sensitizing properties (63). A clinical trial evaluated the effects of gemcitabine-based NACRT. Compared with upfront surgery, patients who received gemcitabine-based NACRT showed a benefit from OS (17.1 months vs. 13.5 months) and an increased R0 resection rate (65% vs. 31%) (64). The use of full-dose GSRT for NAT of resectable PDAC uncovered the outstanding clinical efficacy and acceptable tolerability, and achieved a low local recurrence rate (38). Yuichi et al. demonstrated that IMRT combined with gemcitabine and S-1 can be used as NACRT for patients with a resectable pancreatic cancer with low gastrointestinal toxicity. IMRT can provide a more effective NACRT through powerful chemotherapy drugs (46). As a component of NAT, SBRT has a good safety and tolerability (65). The advantages of SBRT are that it reduces the treatment time and can accurately locate the target area, but the disadvantage is that it does not provide the opportunity to selectively kill tumor cells using radio sensitizing chemotherapy (35).
What is more, clarifying the tumor characterization before the surgery or chemotherapy is of great importance. For example, several scientific society including Okusaka et al. (66), Dumonceau et al. (67), Jenssen et al. (68), and Eloubeidi et al. (69) recommended to use the EUS guided tissue acquisition before surgery and neoadjuvant chemotherapy, therefore the most appropriate treatment therapy may be approached soon.
Conclusion and Prospection
NAT improved the OS and PFS time of patients with a resectable pancreatic cancer compared with upfront surgery. The combination of multidisciplinary NAT with systemic chemotherapy and concurrent radiotherapy increases the possibility of R0 resection for patients with a resectable marginal pancreatic cancer. Judging from the incidence of adverse events and the rate of surgical resection, NAC is safe and feasible. In short, NAT significantly improved the R0 resection rate and sufficient survival duration. NAC and NACRT provide oncological benefits for patients with BRPC. However, the choice of pancreatic cancer NAT regimen, drug dosage, timing of administration, and drug cycle also need further research. How to select patients who are suitable for NAT and formulate the most optimized NAT solution will be a problem that we urgently need to solve. The ultimate goal of scientists is to allow more patients with a resectable pancreatic cancer to benefit from NAT in order to improve their prognosis. NAT is one of the major advances in multidisciplinary oncology in the past few decades, which requires a multidisciplinary treatment team and the best infrastructure for complex oncology care.
Author Contributions
LY and FX wrote the paper. XW and JC collected data from the reference. YB and QL collected the data. FL drew the figure. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Chengdu Medical College Foundation (CYZ19-33), Chengdu Science and Technology Bureau focuses on research and development support plan (2019-YF09-00097-SN), the popular scientific research project of Sichuan Health Commission (20PJ171), and Sichuan undergraduate innovation and startup program funding support (S201913705080, S201913705130, S201913705059, S202013705070, S202013705075, S202013705108), and Yunnan education program (SYSX202036). National Natural Science Foundation of China (82073833) and Southwest University for Nationalities special fund for basic scientific research operations of central universities (2021NYB08).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for Locally Advanced Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis. Lancet Oncol (2016) 17:801–10. doi: 10.1016/S1470-2045(16)00172-8
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
3. Ilic M, Ilic I. Epidemiology of Pancreatic Cancer. World J Gastroenterol (2016) 22:9694–705. doi: 10.3748/wjg.v22.i44.9694
4. Soefje SA. Managing the Economic Impact of Advanced Pancreatic Cancer. Am J Manag Care (2019) 25:S11–s16.
5. Zhang X, Li M. Chapter 21 - Clinical Treatment and Progress of Biological Immunity for Pancreatic Cancer. In: Li M, Lu L, Xiao Y, Fu D, Zhang H, editors. Integrative Pancreatic Intervention Therapy, vol. 2021. Tongji University Press Co., Ltd.: Elsevier (2021). p. 497–504.
6. Heinrich S, Lang H. Neoadjuvant Therapy of Pancreatic Cancer: Definitions and Benefits. Int J Mol Sci (2017) 18:1622. doi: 10.3390/ijms18081622
7. Hooper C, Hardy-Smith P, Handlinger J. Ganglioneuritis Causing High Mortalities in Farmed Australian Abalone (Haliotis Laevigata and Haliotis Rubra). Aust Vet J (2007) 85:188–93. doi: 10.1111/j.1751-0813.2007.00155.x
8. Cellini F, Arcelli A, Simoni N, Caravatta L, Buwenge M, Calabrese A, et al. Basics and Frontiers on Pancreatic Cancer for Radiation Oncology: Target Delineation, SBRT, SIB TechniqueMRgRT, Particle Therapy, Immunotherapy and Clinical Guidelines. Cancers (Basel) (2020) 12:1729. doi: 10.3390/cancers12071729
9. Carnegie RB, Arzul I, Bushek D. Managing Marine Mollusc Diseases in the Context of Regional and International Commerce: Policy Issues and Emerging Concerns. Philos Trans R Soc Lond B Biol Sci (2016) 371:20150215. doi: 10.1098/rstb.2015.0215
10. Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG, et al. Meta-Analysis Comparing Upfront Surgery With Neoadjuvant Treatment in Patients With Resectable or Borderline Resectable Pancreatic Cancer. Br J Surg (2018) 105:946–58. doi: 10.1002/bjs.10870
11. Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative Resection is the Single Most Important Factor Determining Outcome in Patients With Pancreatic Adenocarcinoma. Br J Surg (2004) 91:586–94. doi: 10.1002/bjs.4484
12. Neoptolemos JP, Stocken DD, Dunn JA, Almond J, Beger HG, Pederzoli P, et al. Influence of Resection Margins on Survival for Patients With Pancreatic Cancer Treated by Adjuvant Chemoradiation and/or Chemotherapy in the ESPAC-1 Randomized Controlled Trial. Ann Surg (2001) 234:758–68. doi: 10.1097/00000658-200112000-00007
13. di Sebastiano P, Grottola T, di Mola FF. Borderline Resectable Pancreatic Cancer and the Role of Neoadjuvant Chemoradiotherapy. Updates Surg (2016) 68:235–9. doi: 10.1007/s13304-016-0392-x
14. Strobel O, Berens V, Hinz U, Hartwig W, Hackert T, Bergmann F, et al. Resection After Neoadjuvant Therapy for Locally Advanced, "Unresectable" Pancreatic Cancer. Surgery (2012) 152:S33–42. doi: 10.1016/j.surg.2012.05.029
15. Yoo C, Lee SS, Song KB, Jeong JH, Hyung J, Park DH, et al. Neoadjuvant Modified FOLFIRINOX Followed by Postoperative Gemcitabine in Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Study for Clinical and Biomarker Analysis. Br J Cancer (2020) 123:362–8. doi: 10.1038/s41416-020-0867-x
16. Wei AC, Ou FS, Shi Q, Carrero X, O'Reilly EM, Meyerhardt J, et al. Perioperative Gemcitabine + Erlotinib Plus Pancreaticoduodenectomy for Resectable Pancreatic Adenocarcinoma: ACOSOG Z5041 (Alliance) Phase II Trial. Ann Surg Oncol (2019) 26:4489–97. doi: 10.1245/s10434-019-07685-1
17. Miyasaka Y, Ohtsuka T, Kimura R, Matsuda R, Mori Y, Nakata K, et al. Neoadjuvant Chemotherapy With Gemcitabine Plus Nab-Paclitaxel for Borderline Resectable Pancreatic Cancer Potentially Improves Survival and Facilitates Surgery. Ann Surg Oncol (2019) 26:1528–34. doi: 10.1245/s10434-019-07309-8
18. Nagakawa Y, Sahara Y, Hosokawa Y, Murakami Y, Yamaue H, Satoi S, et al. Clinical Impact of Neoadjuvant Chemotherapy and Chemoradiotherapy in Borderline Resectable Pancreatic Cancer: Analysis of 884 Patients at Facilities Specializing in Pancreatic Surgery. Ann Surg Oncol (2019) 26:1629–36. doi: 10.1245/s10434-018-07131-8
19. Li X, Guo C, Li Q, Wei S, Zhang Q, Chen Y, et al. Association of Modified-FOLFIRINOX-Regimen-Based Neoadjuvant Therapy With Outcomes of Locally Advanced Pancreatic Cancer in Chinese Population. Oncologist (2019) 24:301–e393. doi: 10.1634/theoncologist.2018-0696
20. Motoi F, Satoi S, Honda G, Wada K, Shinchi H, Matsumoto I, et al. Unno M. A Single-Arm, Phase II Trial of Neoadjuvant Gemcitabine and S1 in Patients With Resectable and Borderline Resectable Pancreatic Adenocarcinoma: PREP-01 Study. J Gastroenterol (2019) 54:194–203. doi: 10.1007/s00535-018-1506-7
21. Reni M, Zanon S, Balzano G, Passoni P, Pircher C, Chiaravalli M, et al. A Randomised Phase 2 Trial of Nab-Paclitaxel Plus Gemcitabine With or Without Capecitabine and Cisplatin in Locally Advanced or Borderline Resectable Pancreatic Adenocarcinoma. Eur J Cancer (2018) 102:95–102. doi: 10.1016/j.ejca.2018.07.007
22. Saito K, Isayama H, Sakamoto Y, Nakai Y, Ishigaki K, Tanaka M, et al. A Phase II Trial of Gemcitabine, S-1 and LV Combination (GSL) Neoadjuvant Chemotherapy for Patients With Borderline Resectable and Locally Advanced Pancreatic Cancer. Med Oncol (2018) 35:100. doi: 10.1007/s12032-018-1158-8
23. Dhir M, Zenati MS, Hamad A, Singhi AD, Bahary N, Hogg ME, et al. FOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel for Neoadjuvant Treatment of Resectable and Borderline Resectable Pancreatic Head Adenocarcinoma. Ann Surg Oncol (2018) 25:1896–903. doi: 10.1245/s10434-018-6512-8
24. Reni M, Balzano G, Zanon S, Zerbi A, Rimassa L, Castoldi R, et al. Safety and Efficacy of Preoperative or Postoperative Chemotherapy for Resectable Pancreatic Adenocarcinoma (PACT-15): A Randomised, Open-Label, Phase 2-3 Trial. Lancet Gastroenterol Hepatol (2018) 3:413–23. doi: 10.1016/S2468-1253(18)30081-5
25. Kondo N, Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakagawa N, et al. A Phase 1 Study of Gemcitabine/Nab-Paclitaxel/S-1 (GAS) Combination Neoadjuvant Chemotherapy for Patients With Locally Advanced Pancreatic Adenocarcinoma. Cancer Chemother Pharmacol (2017) 79:775–81. doi: 10.1007/s00280-017-3274-0
26. Okada KI, Hirono S, Kawai M, Miyazawa M, Shimizu A, Kitahata Y, et al. Phase I Study of Nab-Paclitaxel Plus Gemcitabine as Neoadjuvant Therapy for Borderline Resectable Pancreatic Cancer. Anticancer Res (2017) 37:853–8. doi: 10.21873/anticanres.11389
27. Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, et al. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J Clin Oncol (2017) 35:515–22. doi: 10.1200/JCO.2016.68.5081
28. Okada K, Kawai M, Hirono S, Satoi S, Yanagimoto H, Ioka T, et al. Impact of Treatment Duration of Neoadjuvant FIRINOX in Patients With Borderline Resectable Pancreatic Cancer: A Pilot Trial. Cancer Chemother Pharmacol (2016) 78:719–26. doi: 10.1007/s00280-016-3121-8
29. Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh Rde W, Collisson E, et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg (2016) 151:e161137. doi: 10.1001/jamasurg.2016.1137
30. Masui T, Doi R, Kawaguchi Y, Sato A, Nakano K, Ito T, et al. Concurrent Gemcitabine+S-1 Neoadjuvant Chemotherapy Contributes to the Improved Survival of Patients With Small Borderline-Resectable Pancreatic Cancer Tumors. Surg Today (2016) 46:1282–9. doi: 10.1007/s00595-016-1310-z
31. Blazer M, Wu C, Goldberg RM, Phillips G, Schmidt C, Muscarella P, et al. Neoadjuvant Modified (M) FOLFIRINOX for Locally Advanced Unresectable (LAPC) and Borderline Resectable (BRPC) Adenocarcinoma of the Pancreas. Ann Surg Oncol (2015) 22:1153–9. doi: 10.1245/s10434-014-4225-1
32. OʼReilly EM, Perelshteyn A, Jarnagin WR, Schattner M, Gerdes H, Capanu M, et al. A Single-Arm, Nonrandomized Phase II Trial of Neoadjuvant Gemcitabine and Oxaliplatin in Patients With Resectable Pancreas Adenocarcinoma. Ann Surg (2014) 260:142–8. doi: 10.1097/SLA.0000000000000251
33. Witt JS, Kuczmarska-Haas A, Lubner M, Reeder SB, Cho SY, Minter R, et al. A Phase 1 Dose Escalation Study of Neoadjuvant SBRT Plus Elective Nodal Radiation With Concurrent Capecitabine for Resectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys (2021) 109:458–63. doi: 10.1016/j.ijrobp.2020.09.010
34. Thanikachalam K, Damarla V, Seixas T, Dobrosotskaya I, Wollner I, Kwon D, et al. Neoadjuvant Phase II Trial of Chemoradiotherapy in Patients With Resectable and Borderline Resectable Pancreatic Cancer. Am J Clin Oncol (2020) 43:435–41. doi: 10.1097/COC.0000000000000688
35. Tran NH, Sahai V, Griffith KA, Nathan H, Kaza R, Cuneo KC, et al. Phase 2 Trial of Neoadjuvant FOLFIRINOX and Intensity Modulated Radiation Therapy Concurrent With Fixed-Dose Rate-Gemcitabine in Patients With Borderline Resectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys (2020) 106:124–33. doi: 10.1016/j.ijrobp.2019.08.057
36. Lin C, Verma V, Lazenby A, Ly QP, Berim LD, Schwarz JK, et al. Phase I/II Trial of Neoadjuvant Oregovomab-Based Chemoimmunotherapy Followed by Stereotactic Body Radiotherapy and Nelfinavir For Locally Advanced Pancreatic Adenocarcinoma. Am J Clin Oncol (2019) 42:755–60. doi: 10.1097/COC.0000000000000599
37. Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, et al. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol (2019) 5:1020–7. doi: 10.1001/jamaoncol.2019.0892
38. Eguchi H, Takeda Y, Takahashi H, Nakahira S, Kashiwazaki M, Shimizu J, et al. A Prospective, Open-Label, Multicenter Phase 2 Trial of Neoadjuvant Therapy Using Full-Dose Gemcitabine and S-1 Concurrent With Radiation for Resectable Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol (2019) 26:4498–505. doi: 10.1245/s10434-019-07735-8
39. Hayashi T, Nakamura T, Kimura Y, Yoshida M, Someya M, Kawakami H, et al. Phase 2 Study of Neoadjuvant Treatment of Sequential S-1-Based Concurrent Chemoradiation Therapy Followed by Systemic Chemotherapy With Gemcitabine for Borderline Resectable Pancreatic Adenocarcinoma (HOPS-BR 01). Int J Radiat Oncol Biol Phys (2019) 105:606–17. doi: 10.1016/j.ijrobp.2019.07.004
40. Kharofa J, Mierzwa M, Olowokure O, Sussman J, Latif T, Gupta A, et al. Pattern of Marginal Local Failure in a Phase II Trial of Neoadjuvant Chemotherapy and Stereotactic Body Radiation Therapy for Resectable and Borderline Resectable Pancreas Cancer. Am J Clin Oncol (2019) 42:247–52. doi: 10.1097/COC.0000000000000518
41. Maurel J, Sánchez-Cabús S, Laquente B, Gaba L, Visa L, Fabregat J, et al. Outcomes After Neoadjuvant Treatment With Gemcitabine and Erlotinib Followed by Gemcitabine-Erlotinib and Radiotherapy for Resectable Pancreatic Cancer (GEMCAD 10-03 Trial). Cancer Chemother Pharmacol (2018) 82:935–43. doi: 10.1007/s00280-018-3682-9
42. Murphy JE, Wo JY, Ryan DP, Jiang W, Yeap BY, Drapek LC, et al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol (2018) 4:963–9. doi: 10.1001/jamaoncol.2018.0329
43. Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH, et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-Label, Multicenter Phase 2/3 Trial. Ann Surg (2018) 268:215–22. doi: 10.1097/SLA.0000000000002705
44. Okano K, Suto H, Oshima M, Maeda E, Yamamoto N, Kakinoki K, et al. A Prospective Phase II Trial of Neoadjuvant S-1 With Concurrent Hypofractionated Radiotherapy in Patients With Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol (2017) 24:2777–84. doi: 10.1245/s10434-017-5921-4
45. Mellon EA, Jin WH, Frakes JM, Centeno BA, Strom TJ, Springett GM, et al. Predictors and Survival for Pathologic Tumor Response Grade in Borderline Resectable and Locally Advanced Pancreatic Cancer Treated With Induction Chemotherapy and Neoadjuvant Stereotactic Body Radiotherapy. Acta Oncol (2017) 56:391–7. doi: 10.1080/0284186X.2016.1256497
46. Nagakawa Y, Hosokawa Y, Nakayama H, Sahara Y, Takishita C, Nakajima T, et al. A Phase II Trial of Neoadjuvant Chemoradiotherapy With Intensity-Modulated Radiotherapy Combined With Gemcitabine and S-1 for Borderline-Resectable Pancreatic Cancer With Arterial Involvement. Cancer Chemother Pharmacol (2017) 79:951–7. doi: 10.1007/s00280-017-3288-7
47. Roland CL, Yang AD, Katz MH, Chatterjee D, Wang H, Lin H, et al. Neoadjuvant Therapy is Associated With a Reduced Lymph Node Ratio in Patients With Potentially Resectable Pancreatic Cancer. Ann Surg Oncol (2015) 22:1168–75. doi: 10.1245/s10434-014-4192-6
48. Amano R, Kimura K, Nakata B, Yamazoe S, Motomura H, Yamamoto A, et al. Pancreatectomy With Major Arterial Resection After Neoadjuvant Chemoradiotherapy Gemcitabine and S-1 and Concurrent Radiotherapy for Locally Advanced Unresectable Pancreatic Cancer. Surgery (2015) 158:191–200. doi: 10.1016/j.surg.2015.02.016
49. Casadei R, Di Marco M, Ricci C, Santini D, Serra C, Calculli L, et al. Neoadjuvant Chemoradiotherapy and Surgery Versus Surgery Alone in Resectable Pancreatic Cancer: A Single-Center Prospective, Randomized, Controlled Trial Which Failed to Achieve Accrual Targets. J Gastrointest Surg (2015) 19:1802–12. doi: 10.1007/s11605-015-2890-4
50. Golcher H, Brunner TB, Witzigmann H, Marti L, Bechstein WO, Bruns C, et al. Neoadjuvant Chemoradiation Therapy With Gemcitabine/Cisplatin and Surgery Versus Immediate Surgery in Resectable Pancreatic Cancer: Results of the First Prospective Randomized Phase II Trial. Strahlenther Onkol (2015) 191:7–16. doi: 10.1007/s00066-014-0737-7
51. Sherman WH, Chu K, Chabot J, Allendorf J, Schrope BA, Hecht E, et al. Neoadjuvant Gemcitabine, Docetaxel, and Capecitabine Followed by Gemcitabine and Capecitabine/Radiation Therapy and Surgery in Locally Advanced, Unresectable Pancreatic Adenocarcinoma. Cancer (2015) 121:673–80. doi: 10.1002/cncr.29112
52. Ielpo B, Caruso R, Duran H, Diaz E, Fabra I, Malavé L, et al. A Comparative Study of Neoadjuvant Treatment With Gemcitabine Plus Nab-Paclitaxel Versus Surgery First for Pancreatic Adenocarcinoma. Surg Oncol (2017) 26:402–10. doi: 10.1016/j.suronc.2017.08.003
53. Lordick F, Gockel I. Chances, Risks and Limitations of Neoadjuvant Therapy in Surgical Oncology. Innovative Surg Sci (2016) 1:3–11. doi: 10.1515/iss-2016-0004
54. Xu J, Zhan H, Li F, Hu S, Wang L. Neoadjuvant Therapy for Pancreatic Cancer: Limitations and Advances of Response Assessment (Review). Oncol Rep (2021) 45:26. doi: 10.3892/or.2021.7977
55. Labori KJ, Katz MH, Tzeng CW, Bjørnbeth BA, Cvancarova M, Edwin B, et al. Impact of Early Disease Progression and Surgical Complications on Adjuvant Chemotherapy Completion Rates and Survival in Patients Undergoing the Surgery First Approach for Resectable Pancreatic Ductal Adenocarcinoma - A Population-Based Cohort Study. Acta Oncol (2016) 55:265–77. doi: 10.3109/0284186X.2015.1068445
56. Bergquist JR, Ivanics T, Shubert CR, Habermann EB, Smoot RL, Kendrick ML, et al. Type of Resection (Whipple vs. Distal) Does Not Affect the National Failure to Provide Post-Resection Adjuvant Chemotherapy in Localized Pancreatic Cancer. Ann Surg Oncol (2017) 24:1731–8. doi: 10.1245/s10434-016-5762-6
57. Cooper AB, Parmar AD, Riall TS, Hall BL, Katz MH, Aloia TA, et al. Does the Use of Neoadjuvant Therapy for Pancreatic Adenocarcinoma Increase Postoperative Morbidity and Mortality Rates? J Gastrointest Surg (2015) 19:80–86; discussion 86-87. doi: 10.1007/s11605-014-2620-3
58. Raufi AG, Manji GA, Chabot JA, Bates SE. Neoadjuvant Treatment for Pancreatic Cancer. Semin Oncol (2019) 46:19–27. doi: 10.1053/j.seminoncol.2018.12.002
59. Marijnen CA, Nagtegaal ID, Kapiteijn E, Kranenbarg EK, Noordijk EM, van Krieken JH, et al. Radiotherapy Does Not Compensate for Positive Resection Margins in Rectal Cancer Patients: Report of a Multicenter Randomized Trial. Int J Radiat Oncol Biol Phys (2003) 55:1311–20. doi: 10.1016/S0360-3016(02)04291-8
60. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased Survival in Pancreatic Cancer With Nab-Paclitaxel Plus Gemcitabine. N Engl J Med (2013) 369:1691–703. doi: 10.1056/NEJMoa1304369
61. Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, et al. Randomized Phase III Study of Gemcitabine Plus S-1, S-1 Alone, or Gemcitabine Alone in Patients With Locally Advanced and Metastatic Pancreatic Cancer in Japan and Taiwan: GEST Study. J Clin Oncol (2013) 31:1640–8. doi: 10.1200/JCO.2012.43.3680
62. Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-Analysis of Response and Resection Percentages. PloS Med (2010) 7:e1000267. doi: 10.1371/journal.pmed.1000267
63. Morgan MA, Parsels LA, Maybaum J, Lawrence TS. Improving Gemcitabine-Mediated Radiosensitization Using Molecularly Targeted Therapy: A Review. Clin Cancer Res (2008) 14:6744–50. doi: 10.1158/1078-0432.CCR-08-1032
64. Van Tienhoven G, Versteijne E, Suker M, Groothuis KBC, Busch OR, Bonsing BA, et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer (PREOPANC-1): A Randomized, Controlled, Multicenter Phase III Trial. J Clin Oncol (2018) 36:LBA4002–LBA4002. doi: 10.1200/JCO.2018.36.18_suppl.LBA4002
65. Herman JM, Chang DT, Goodman KA, Dholakia AS, Raman SP, Hacker-Prietz A, et al. Phase 2 Multi-Institutional Trial Evaluating Gemcitabine and Stereotactic Body Radiotherapy for Patients With Locally Advanced Unresectable Pancreatic Adenocarcinoma. Cancer (2015) 121:1128–37. doi: 10.1002/cncr.29161
66. Okusaka T, Nakamura M, Yoshida M, Kitano M, Uesaka K, Ito Y, et al. Clinical Practice Guidelines for Pancreatic Cancer 2019 From the Japan Pancreas Society: A Synopsis. Pancreas (2020) 49:326–35. doi: 10.1097/MPA.0000000000001513
67. Dumonceau JM, Deprez PH, Jenssen C, Iglesias-Garcia J, Larghi A, Vanbiervliet G, et al. Indications, Results, and Clinical Impact of Endoscopic Ultrasound (EUS)-Guided Sampling in Gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated January 2017. Endoscopy (2017) 49:695–714. doi: 10.1055/s-0043-109021
68. Jenssen C, Hocke M, Fusaroli P, Gilja OH, Buscarini E, Havre RF, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part IV - EUS-Guided Interventions: General Aspects and EUS-Guided Sampling (Short Version). Ultraschall Med (2016) 37:157–69. doi: 10.1055/s-0035-1553788
Keywords: pancreatic cancer, neoadjuvant chemotherapy, neoadjuvant chemo-radiotherapy, treatment, neoadjuvant therapy
Citation: Yang L, Bai Y, Li Q, Chen J, Liu F, Weng X and Xu F (2021) Analysis of the Curative Effect of Neoadjuvant Therapy on Pancreatic Cancer. Front. Oncol. 11:695645. doi: 10.3389/fonc.2021.695645
Received: 15 April 2021; Accepted: 30 July 2021;
Published: 18 August 2021.
Edited by:
Prasanna K. Santhekadur, JSS Academy of Higher Education and Research, IndiaReviewed by:
Rinu Sharma, Guru Gobind Singh Indraprastha University, IndiaAndrea Liostti, Local Health Authority of Imola, Italy
Copyright © 2021 Yang, Bai, Li, Chen, Liu, Weng and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiechuan Weng, wengxc2000@163.com; Fan Xu, xufan@cmc.edu.cn
†These authors have contributed equally to this work
 Liqiong Yang
Liqiong Yang Yun Bai2†
Yun Bai2† Xiechuan Weng
Xiechuan Weng Fan Xu
Fan Xu