- 1Department of Medical Genetics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Department of Pharmacology, Tabriz University of Medical Sciences, Tabriz, Iran
- 3Department of Anatomical Sciences, Faculty of Medicine, Birjand University of Medical Sciences, Birjand, Iran
- 4Department of Anatomical Sciences, School of Medicine, Gonabad University of Medical Sciences, Gonabad, Iran
- 5Skull Base Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 6Critical Care Quality improvement Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Apoptosis is a coordinated cellular process that occurs in several physiological situations. Dysregulation of apoptosis has been documented in numerous pathological situations, particularly cancer. Non-coding RNAs regulate apoptosis via different mechanisms. Lung cancer is among neoplastic conditions in which the role of non-coding RNAs in the regulation of apoptosis has been investigated. Non-coding RNAs that regulate apoptosis in lung cancer have functional interactions with PI3K/Akt, PTEN, GSK-3β, NF-κB, Bcl-2, Bax, p53, mTOR and other important cancer-related pathways. Globally, over-expression of apoptosis-blocking non-coding RNAs has been associated with poor prognosis of patients, while apoptosis-promoting ones have the opposite effect. In the current paper, we describe the impact of lncRNAs and miRNAs on cell apoptosis in lung cancer.
Introduction
Apoptosis is a well-organized and coordinated cellular process that happens in several physiological situations. Aberrant regulation of apoptosis has also been documented in numerous pathological situations, particularly cancer. In fact, cancer is one of the circumstances where this process is reduced, leading to evolution of malignant cells that will not perish. Apoptosis is regulated by a complex mechanism involving numerous pathways. Deficiencies in apoptotic pathways lead to malignant transformation of cells, enhancement of metastasis and induction of resistance to chemotherapy/radiotherapy. Meanwhile, apoptosis has been considered as a target of several anticancer modalities (1). Both intracellular and extracellular stimuli can regulate apoptosis. This process is described by morphological alterations in the cells including fragmentation and condensation of the nuclear compartment, permeabilization of the outer membrane of mitochondria, membrane blebbing, cell shrinkage and finally formation of apoptotic bodies (2). Two extrinsic and intrinsic pathways are involved in the induction of cell apoptosis. While the extrinsic pathway is stimulated by death receptors, namely Fas, TNF receptors and TRAILs, the intrinsic pathway is initiated by DNA damage, energy starvation and hypoxia, which can dephosphorylate and cleave pro-apoptotic proteins, resulting in their recruitment in the mitochondria (3). Both pro-apoptotic and anti-apoptotic members of the Bcl-2 family proteins regulate intrinsic apoptotic pathway (4).
Recent studies have shown that non-coding RNAs (ncRNAs) have an important regulatory role on induction of apoptosis. In fact, regulation of cell apoptosis is the main route of function of many of these transcripts in the carcinogenic events (5). This group of transcripts has several types, two of them i.e. long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) have attained more attention in cancer biology. LncRNAs have typically sizes more than 200 nucleotides and are transcribed by RNA polymerase II, except for few cases do not harbor open reading frame and translation-termination region, yet, lncRNAs can be spliced, 5’-capped and get polyadenylated tails. Their specific three-dimensional conformation permits them to interact with several classes of biomolecules including proteins, DNA or RNA. These interactions are framed through base pairing or construction of network (6). LncRNAs partake in regulation of gene expression, differentiation of cells and alteration of chromatin structure (6).
miRNAs have been shown to regulate expression of a high proportion of human genes. They mainly target 3’ UTR of genes to suppress their expression or degrade the corresponding RNAs. Several aspects of cell functioning including apoptosis is regulated by miRNAs (7). Figure 1 illustrates that aberrant expression of various ncRNAs could contribute in modulation of the mitochondrial pathway of apoptosis in the context of lung cancer.
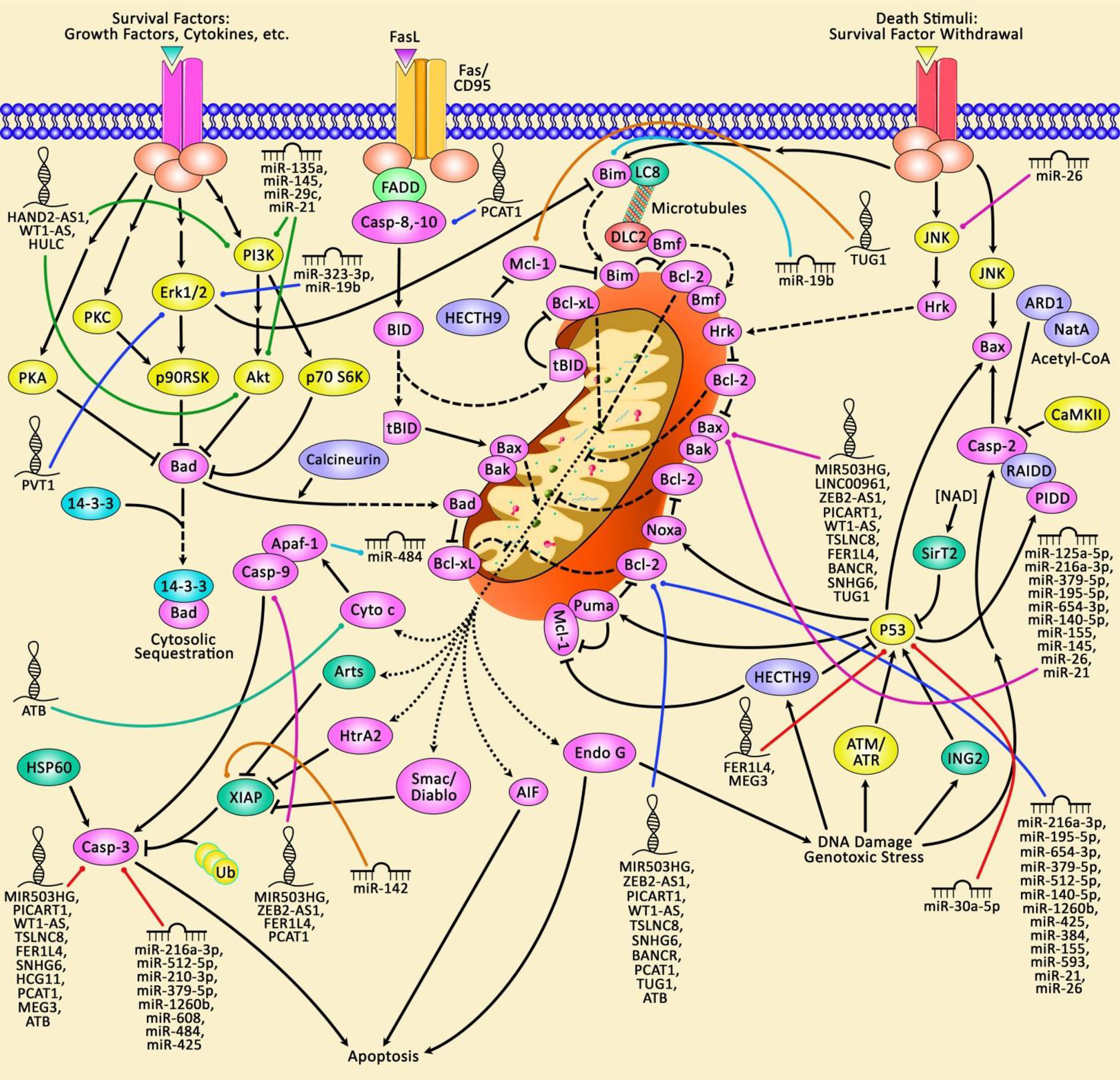
Figure 1 A schematic representation of the role of non-coding RNAs in triggering the mitochondrial pathway of apoptosis in human lung cancer. The Bcl-2 family of proteins could play an effective role in modulating apoptosis via regulating mitochondrial cascade. The anti-apoptotic proteins Bcl-2 and Bcl-xL are located in the exterior part of mitochondrial wall and can suppress cytochrome c release. The pro-apoptotic Bcl-2 proteins Bax, Bad, Bim, and Bid could be located in the cytosol but may be transferred to mitochondria following induction of death signaling pathway, where they could elevate the release of cytochrome c (8, 9). The mitochondrial cascade of apoptosis could be considered as the most commonly deregulated form of cell death in a variety of human cancers. Furthermore, aberrant expression of various non-coding RNAs could have a crucial part in dysregulating the mitochondrial pathway of apoptosis in lung cancer.
In the current paper, we describe the impact of lncRNAs and miRNAs on cell apoptosis in lung cancer.
miRNAs and Apoptosis in Lung Cancer
Suppression of PI3K/AKT pathway in EGFR mutant lung cancer cells has led to dysregulation of 17 miRNAs among them have been members of the miR-17~ 92 cluster. These miRNAs function in a coordinated manner to increase the activity of the EGFR cascade. Suppression of miR-19b expression in EGFR mutant lung cancer cells has led to re phosphorylation of ERK, AKT and STAT and effector proteins. Consistently, it has resulted in enhancement of apoptosis, while reduction of cell cycle progression, colony formation and migration. Administration of gefitinib along with miR-19b antagonism has decreased migration and colony formation in a synergistic manner implying the cooperation between EGFR and miR-19b in the regulation of oncogenesis. PPP2R5E and BCL2L11 have been recognized as main targets of miR-19b, through their inhibition, miR-19b regulates cell proliferation and resistance to apoptosis, respectively (10). miR-21 is another miRNA that regulates apoptosis of lung cancer cells via influencing the PI3K/Akt/NF-κB signaling pathway. Inhibition of miR-21 has enhanced apoptosis via this route. ASPP2 has been recognized as the target of miR-21 in NSCLC cells. miR-21 silencing has also inhibited migration, invasion, and epithelial-mesenchymal transition (EMT). Besides, miR-21 inhibition has stimulated cell apoptosis through caspase dependent route. Taken together, miR-21 silencing can induce cell apoptosis via reducing activity of the PI3K/Akt/NF-κB signaling (11). miR-24 is another oncogenic miRNA which is up-regulated in lung cancer tissues, particularly in high grade and large-sized tumors. Consistently, higher expression of miR-21 predicts lower overall survival (OS) of patients. Functionally, miR-24 enhances the viability, proliferation and cell cycle transition, while inhibiting cell apoptosis through binding with MAPK7 (12). miR-26 is a down-regulated miRNA in lung cancer cells. Forded over-expression of miR-26 induces cell apoptosis and enhances activity of caspase-3 and caspase-9. On the other hand, miR-26 silencing has increased levels of LC3 protein and the autophagy-associated genes in lung cancer cells. Besides, miR-26 has been shown to influence apoptosis and autophagy through suppressing expression of TGF-β in a JNK dependent route. Besides, miR-26 has been reported to affect the endoplasmic reticulum stress (ERS) signaling pathway (13). Figure 2 represents the role of several ncRNAs in regulating autophagy cascade in human lung cancer.
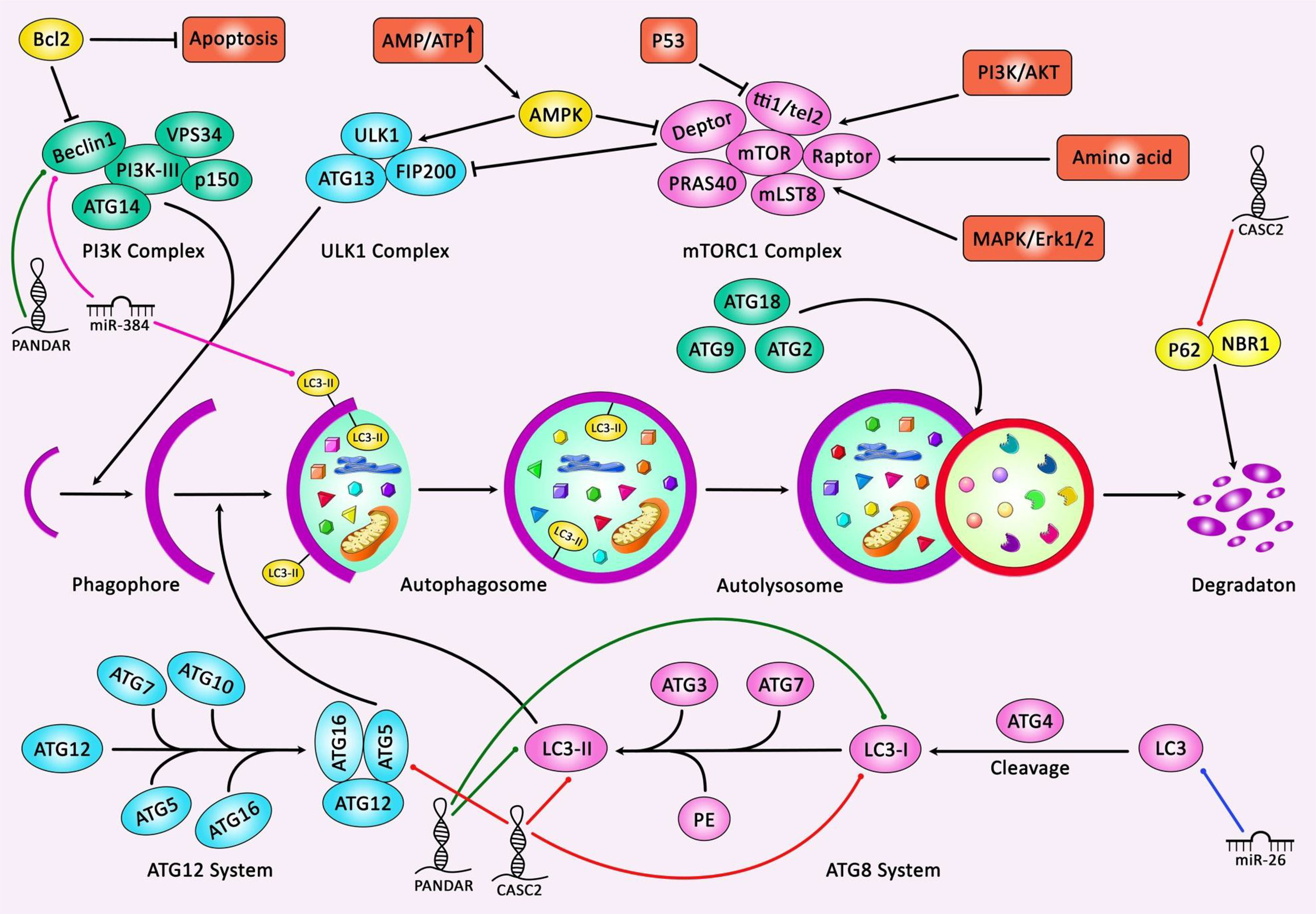
Figure 2 A schematic summary of the role of various non-coding RNAs in modulating the process of autophagy in human lung cancer. Several non-coding RNAs affect lung cancer progression through modulating autophagy and apoptosis cascades in human lung cancer cells. As an illustration, overexpression of lncRNA PANDAR as a tumor suppressor via directly targeting Beclin-1, LC3-I and LC3-II could activate both autophagy and apoptosis cascades, and thereby suppressing progression of lung cancer (14). In addition, lncRNA CASC2 could suppress autophagy and enhance apoptosis pathway in non-small cell lung cancer cells through modulating the miR-214/TRIM16 axis. Moreover, p62 expression level was significantly elevated but Atg-5 expression and the ratio of LC3-II/LC3-I were considerably reduced in the CASC2-overexpressing cells (15).
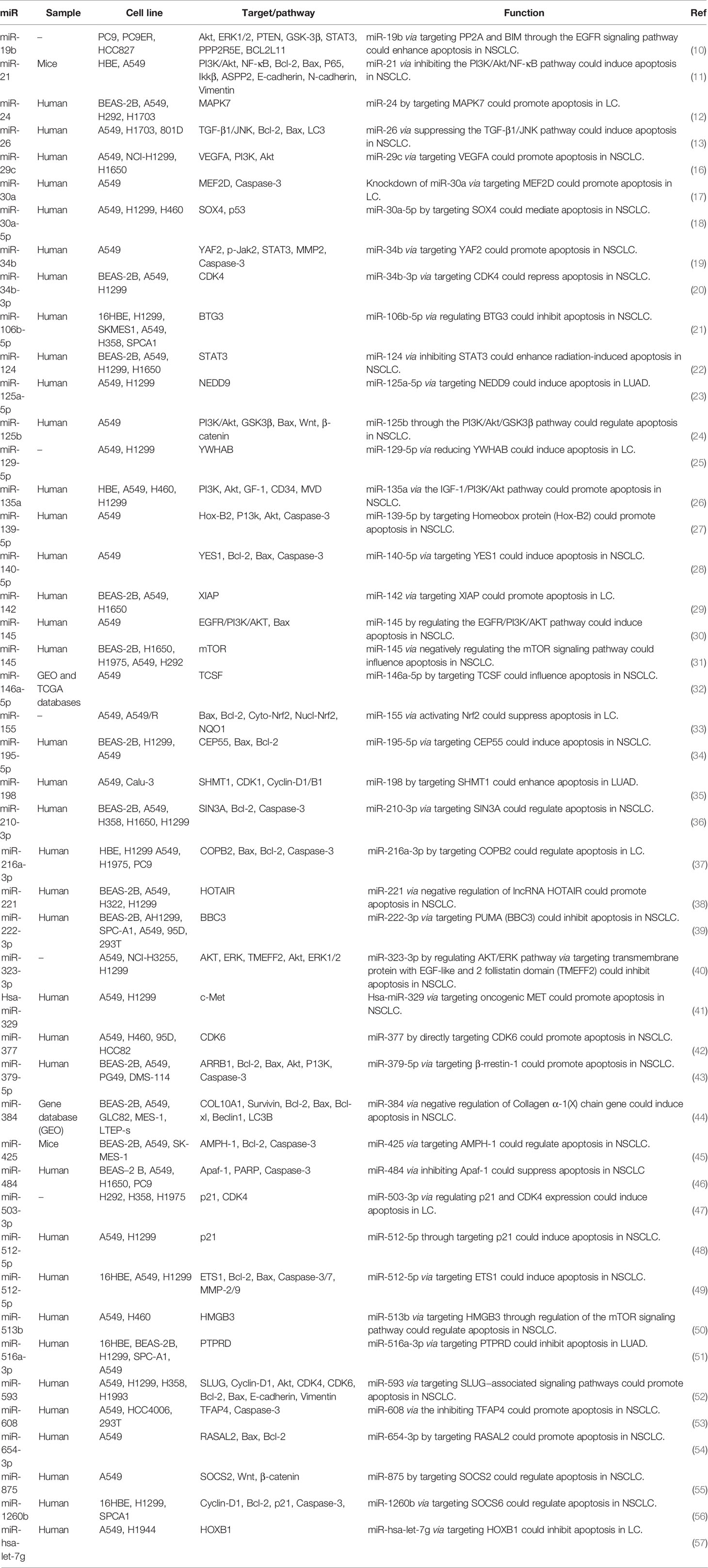
Table 1 shows the list of miRNAs that regulate apoptosis in lung cancer.
Apoptosis-related miRNAs have been shown to influence survival of lung cancer patients. For instance, expression of miR-21 predicts lower OS of patients with NSCLC (12). Moreover, over-expression of miR-125b has been associated with poor prognosis in NSCLC (24).
LncRNAs and Apoptosis in Lung Cancer
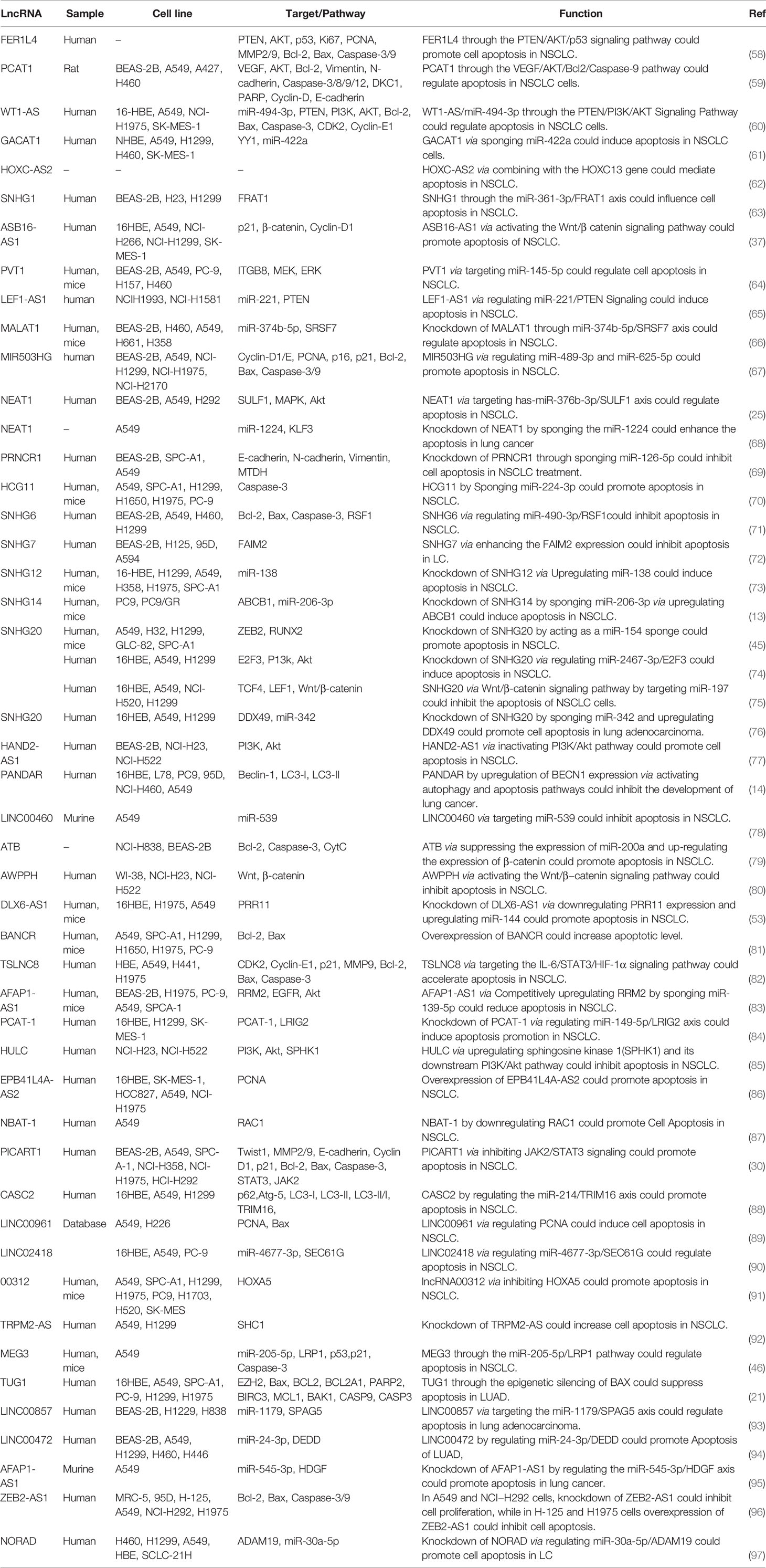
Expression of FER1L4 has been remarkably decreased in plasma and tissue samples of patients with NSCLC as well as related cell lines. Forced over-expression of this lncRNA has reduced cell proliferation, migratory aptitude and invasiveness. FER1L4 has been shown to up-regulate PTEN and p53 expressions, suppress AKT phosphorylation expression, therefore enhancing the fraction of apoptotic cells. Functionally, these effects are mediated through the PTEN/AKT/p53 pathway (58). On the other hand, expression of PCAT1 has been increased in NSCLC tissues and cell lines. In vitro studies have shown that PCAT1 stimulates cell proliferation and invasion while suppressing cell apoptosis. In addition, PCAT1 has been shown to interact with the RNA-binding protein DKC1. PCAT1 and DKC1 exert synergistic effects in NSCLC. They enhance activity of VEGF/AKT/Bcl-2/caspase9 pathway in these cells (59). WT1-AS is a down-regulated lncRNA in NSCLC cell lines which is shown to sponge miR-494-3p. Up-regulation of WT1-AS has increased apoptosis of lung cancer cells and attenuated progression of NSCLC through up-regulation of PTEN and subsequent inactivation of PI3K/AKT pathway (60). GACAT1 is another regulator of apoptosis which has been found to be up-regulated in NSCLC tissues in association with poor survival of patients. Functionally, GACAT1 enhances proliferation and cell cycle progression and inhibits apoptosis through sponging miR-422a and increasing expression of YY1 transcription factor (61). HOXC-AS2 is another up-regulated in NSCLC samples which increases proliferation, migration, and EMT, while suppressing apoptosis. HOXC13 has been identified as functional target of HOXC-AS2. Notably, HOXC-AS2 and HOXC13 can enhance expression of each other (62). Expression of SNHG1 has been found to be increased in NSCLC parallel with up-regulation of FRAT1. SNHG1 knock down has suppressed proliferation, increased cell apoptosis and precluded migration and invasiveness of these cells. Mechanistically, SNHG1 sponges miR-361-3p and to release FRAT1 from inhibitory effects of this miRNA (63). Table 2 shows the role of lncRNAs in regulation of apoptosis in lung cancer.
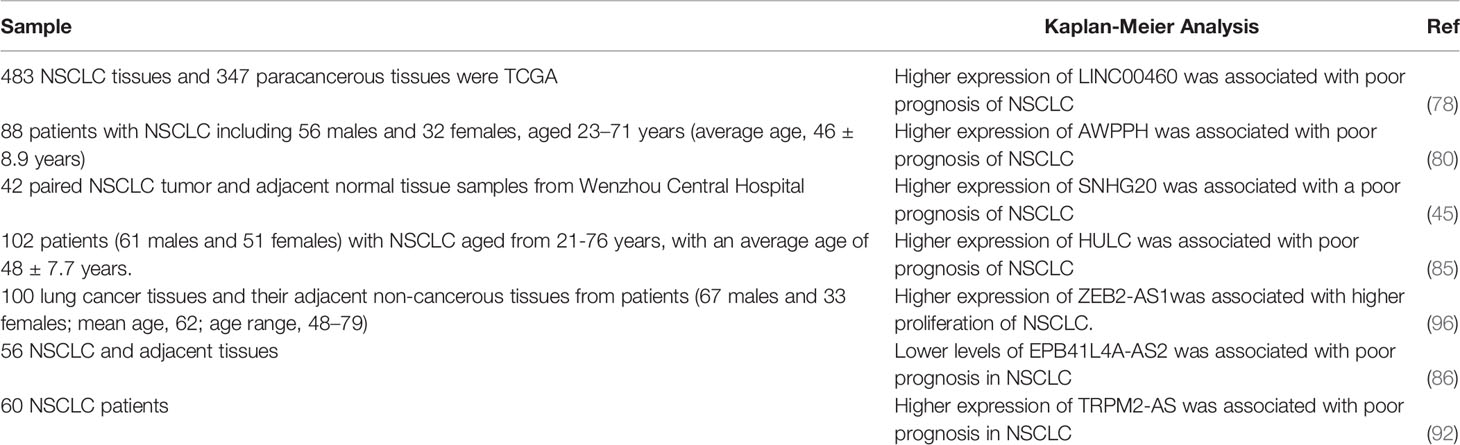
Among lncRNAs which regulate apoptosis in lung cancer cells, over-expression of LINC00460, AWAPPH, SNHG20, HULC, ZEB2-AS1 and TRPM2-AS has been associated with poor prognosis of patients, while EPB41L4A-AS2 has the opposite effect (Table 3).
ncRNAs, Cell Apoptosis and Immunotherapy
Since immunotherapy has an emerging role in the treatment of lung cancer (98), identification of the role of ncRNAs in immune regulation and response of lung cancer to immunotherapy is important. A number of apoptosis-regulating ncRNAs have essential roles in this regard. For instance, miR-155 and miR-17~ 92 are involved in differentiation regulatory T cells (Tregs) and their function (99). miR-21 and miR-26 through down-regulation of TAP1 and reduction in expression of HLA class I antigens affect response to immunotherapies (100). miR-138, miR-155, miR-34 and miR-146a have been found to affect immune checkpoints (101). MALAT1 is an lncRNA which is possibly involved in the immunotherapy resistance through induction of immunosuppressive phenotypes in stem cells (102). NEAT1 can affect response to immunotherapy through modulation of miR-155/Tim-3 (103). The exact roles of these ncRNAs in conferring resistance to immunotherapeutic approaches have not been elucidated in lung cancer; yet based on the results obtained from similar studies in other cancer types, these ncRNAs are expected to simultaneously affect apoptosis and response to immunotherapy in lung cancer.
Discussion
Cell apoptosis, as one of the major dysregulated processes in the carcinogenesis of lung cancer has been shown to be regulated by ncRNAs. In the current review, we have explained the impact of miRNAs and lncRNAs on apoptosis in lung cancer. These ncRNAs interact with PI3K/Akt, NF-κB, Wnt/β-catenin, EGFR, TGF-β and other cancer-related pathways. Therefore, they not only regulate apoptosis, but also influence other aspects of lung carcinogenesis. Figure 3 depicts the role of ncRNAs in modulating apoptosis through Wnt/β-catenin cascade in human lung cancer.
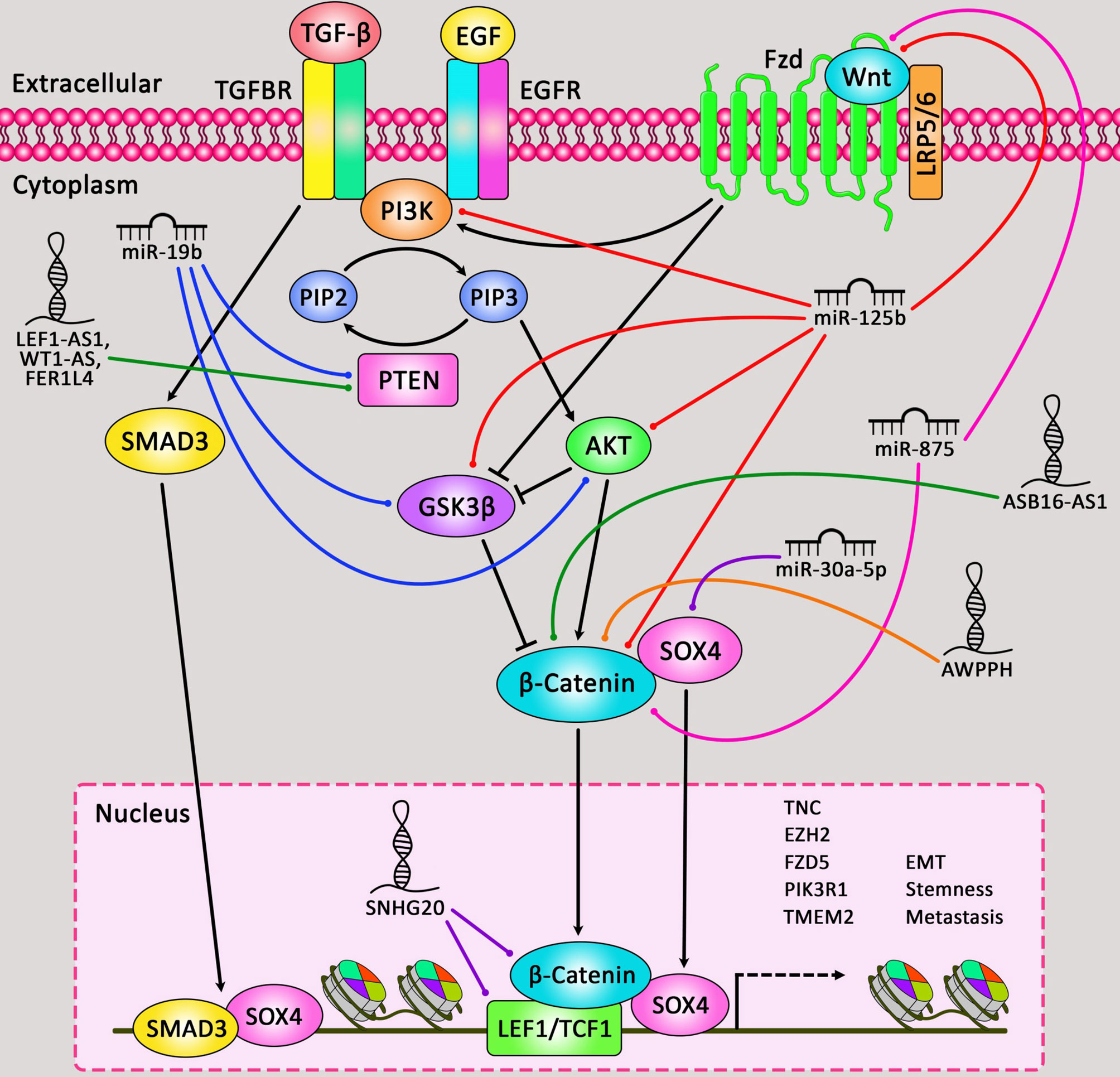
Figure 3 A schematic summary of the role of miRNAs and lncRNAs in regulating apoptosis cascade in lung cancer via Wnt/β-catenin pathway. Accumulating evidence has delineated that apoptotic cells are negative for β-catenin. This indicates that the Wnt/β-catenin signaling cascade could be inactive in apoptotic cells. Whilst, β-catenin is expressed in the membrane, cytoplasm, and nucleus of non-apoptotic epithelial cells around these apoptotic cells. Therefore, Wnt/β-catenin signaling cascade could be activated in non-apoptotic epithelial cells via apoptotic cells (104). As an illustration, downregulation of miR-125b could play an effective role in inhibiting expression of p-Akt, p-GSK3β, Wnt, and β-catenin, and could promote caspase-3 activity and Bax protein expression in human non-small cell lung cancer. Thereby, this could lead to suppressing the proliferation and triggering the apoptosis of tumor cells (24). Furthermore, another study have illustrated that upregulation of lncRNA SNHG20 could have a crucial part in elevating the proliferation and suppressing the apoptosis of NSCLC cells through targeting miR-197 via regulating the Wnt/β-catenin signaling cascade. Downregulation of this lncRNA could result in remarkable reduction of TCF and LEF1 expression in the Wnt/β-catenin pathway (75).
Manipulation of expression of apoptosis-regulating lncRNAs and miRNAs represent a strategy for combating carcinogenesis as well as resistance to chemo/radiotherapy. Some of the apoptosis-regulating miRNAs/lncRNAs have been shown to influence prognosis of lung cancer. The observed correlation between their expression and patients’ survival is due to their impact on disease progression as well as response of patients to EGFR inhibitors and chemotherapeutic agents. EMT is another important feature of lung cancer cells which is regulated by a number of apoptosis-regulating miRNAs/lncRNAs indicating the intercalation between cancer-related processes.
An acknowledged route of function of lncRNAs in the regulation of apoptosis in lung cancer is their impact on expression of miRNAs. In fact, they can sequester miRNAs and release miRNA targets from their inhibitory effects. WT1-AS/miR-494-3p, LEF1-AS1/miR-221, NEAT1/miR-1224, SNHG12/miR-138, LINC02418/miR-4677-3p, MEG3/miR-205-5p, LINC00857/miR-1179, LINC00472/miR-24-3p, AFAP1-AS1/miR-24-3p and NORAD/miR-30a-5p are examples of lncRNAs/miRNAs interactions with verified roles in the control of lung cancer cells apoptosis.
Based on the importance of apoptotic pathways in determination of response of lung cancer patients to conventional as well as targeted therapies, identification of the impacts of lncRNAs/miRNAs on apoptosis and prior profiling of these ncRNAs in clinical samples would help in prediction of response of patients to each therapeutic regimen and design of personalized treatment strategies. The advent of high throughput sequencing strategies has facilitated conduction of this approach in the clinical settings.
Finally, the possibility of lncRNAs/miRNAs tracing in the peripheral blood of patients has opened a new opportunity for early detection of emergence of resistance to conventional or targeted therapies and modulation of therapeutic regimens to enhance the survival of affected individuals.
Author Contributions
MT and SG-F wrote the draft and revised it. HS, AA, JM, and MM collected the data, designed the tables and figures. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Wong RSY. Apoptosis in Cancer: From Pathogenesis to Treatment. J Exp Clin Cancer Res (2011) 30(1):87. doi: 10.1186/1756-9966-30-87
2. Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-Consumption: The Interplay of Autophagy and Apoptosis. Nat Rev Mol Cell Biol (2014) 15(2):81–94. doi: 10.1038/nrm3735
3. Liu G, Pei F, Yang F, Li L, Amin AD, Liu S, et al. Role of Autophagy and Apoptosis in Non-Small-Cell Lung Cancer. Int J Mol Sci (2017) 18(2):367. doi: 10.3390/ijms18020367
4. Hata AN, Engelman JA, Faber AC. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov (2015) 5(5):475–87. doi: 10.1158/2159-8290.CD-15-0011
5. Soudyab M, Iranpour M, Ghafouri-Fard S. The Role of Long Non-Coding RNAs in Breast Cancer. Arch Iranian Med (2016) 19(7):508–17.
6. Dhanoa JK, Sethi RS, Verma R, Arora JS, Mukhopadhyay CS. Long Non-Coding RNA: Its Evolutionary Relics and Biological Implications in Mammals: A Review. J Anim Sci Technol (2018) 60(1):1–10. doi: 10.1186/s40781-018-0183-7
7. Zaporozhchenko I, Rykova EY, Laktionov P. The Fundamentals of miRNA Biology: Structure, Biogenesis, and Regulatory Functions. Russian J Bioorg Chem (2020) 46(1):1–13. doi: 10.1134/S106816202001015X
8. Chalah A, Khosravi-Far R. The Mitochondrial Death Pathway. Programmed Cell Death Cancer Progression Ther (2008) 25–45. doi: 10.1007/978-1-4020-6554-5_3
9. Brenner D, Mak TW. Mitochondrial Cell Death Effectors. Curr Opin Cell Biol (2009) 21(6):871–7. doi: 10.1016/j.ceb.2009.09.004
10. Baumgartner U, Berger F, Gheinani AH, Burgener SS, Monastyrskaya K, Vassella E. miR-19b Enhances Proliferation and Apoptosis Resistance via the EGFR Signaling Pathway by Targeting PP2A and BIM in Non-Small Cell Lung Cancer. Mol Cancer (2018) 17(1):1–15. doi: 10.1186/s12943-018-0781-5
11. Zhou B, Wang D, Sun G, Mei F, Cui Y, Xu H. Effect of miR-21 on Apoptosis in Lung Cancer Cell Through Inhibiting the PI3K/Akt/NF-κb Signaling Pathway In Vitro and In Vivo. Cell Physiol Biochem (2018) 46(3):999–1008. doi: 10.1159/000488831
12. Zhou N, Yan H. MiR-24 Promotes the Proliferation and Apoptosis of Lung Carcinoma via Targeting MAPK7. Eur Rev Med Pharmacol Sci (2018) 22:6845–52. doi: 10.26355/eurrev_201810_16153
13. Wu K, Li J, Qi Y, Zhang C, Zhu D, Liu D, et al. SNHG14 Confers Gefitinib Resistance in Non-Small Cell Lung Cancer by Up-Regulating ABCB1 via Sponging miR-206-3p. Biomed Pharmacother (2019) 116:108995. doi: 10.1016/j.biopha.2019.108995
14. Zhang L, Wang Y, Xia S, Yang L, Wu D, Zhou Y, et al. Long Noncoding RNA PANDAR Inhibits the Development of Lung Cancer by Regulating Autophagy and Apoptosis Pathways. J Cancer (2020) 11(16):4783. doi: 10.7150/jca.45291
15. Lin Y, Leng Q, Zhan M, Jiang F. A Plasma Long Noncoding RNA Signature for Early Detection of Lung Cancer. Trans Oncol (2018) 11(5):1225–31. doi: 10.1016/j.tranon.2018.07.016
16. Zhan S, Wang C, Yin F. MicroRNA-29c Inhibits Proliferation and Promotes Apoptosis in Non-Small Cell Lung Cancer Cells by Targeting VEGFA. Mol Med Rep (2018) 17(5):6705–10. doi: 10.3892/mmr.2018.8678
17. Luan N, Wang Y, Liu X. Absent Expression of Mir−30a Promotes the Growth of Lung Cancer Cells by Targeting MEF2D. Oncol Lett (2018) 16(1):1173–9. doi: 10.3892/ol.2018.8719
18. Quan X, Li X, Yin Z, Ren Y, Zhou B. P53/miR-30a-5p/SOX4 Feedback Loop Mediates Cellular Proliferation, Apoptosis, and Migration of Non-Small-Cell Lung Cancer. J Cell Physiol (2019) 234(12):22884–95. doi: 10.1002/jcp.28851
19. Zhuang X, Zhao L, Guo S, Wei S, Zhai J, Zhou Q. miR-34b Inhibits the Migration/Invasion and Promotes Apoptosis of Non-Small-Cell Lung Cancer Cells by YAF2. Eur Rev Med Pharmacol Sci (2019) 23(5):2038–46. doi: 10.26355/eurrev_201903_17244
20. Feng H, Ge F, Du L, Zhang Z, Liu D. MiR-34b-3p Represses Cell Proliferation, Cell Cycle Progression and Cell Apoptosis in Non-Small-Cell Lung Cancer (NSCLC) by Targeting CDK4. J Cell Mol Med (2019) 23(8):5282–91. doi: 10.1111/jcmm.14404
21. Liu H, Zhou G, Fu X, Cui H, Pu G, Xiao Y, et al. Long Noncoding RNA TUG1 is a Diagnostic Factor in Lung Adenocarcinoma and Suppresses Apoptosis via Epigenetic Silencing of BAX. Oncotarget (2017) 8(60):101899. doi: 10.18632/oncotarget.22058
22. Wang M, Meng B, Liu Y, Yu J, Chen Q. MiR-124 Inhibits Growth and Enhances Radiation-Induced Apoptosis in Non-Small Cell Lung Cancer by Inhibiting STAT3. Cell Physiol Biochem (2017) 44(5). doi: 10.1159/000485907
23. Zheng H, Wu J, Shi J, Lu C, Wang Y, Sun Q, et al. miR-125a-5p Upregulation Suppresses the Proliferation and Induces the Cell Apoptosis of Lung Adenocarcinoma by Targeting NEDD9. Oncol Rep (2017) 38(3):1790–6. doi: 10.3892/or.2017.5812
24. Wang Y, Zhao M, Liu J, Sun Z, Ni J, Liu H. miRNA-125b Regulates Apoptosis of Human Non-Small Cell Lung Cancer via the PI3K/Akt/Gsk3β Signaling Pathway. Oncol Rep (2017) 38(3):1715–23. doi: 10.3892/or.2017.5808
25. Xu C, Du Z, Ren S, Liang X, Li H. MiR-129-5p Sensitization of Lung Cancer Cells to Etoposide-Induced Apoptosis by Reducing YWHAB. J Cancer (2020) 11(4):858. doi: 10.7150/jca.35410
26. Zhou Y, Li S, Li J, Wang D, Li Q. Effect of microRNA-135a on Cell Proliferation, Migration, Invasion, Apoptosis and Tumor Angiogenesis Through the IGF-1/PI3K/Akt Signaling Pathway in Non-Small Cell Lung Cancer. Cell Physiol Biochem (2017) 42(4):1431–46. doi: 10.1159/000479207
27. Du H, Yn B, Liu C, Zhong A, Niu Y, Tang X. Mir−139−5p Enhances Cisplatin Sensitivity in Non−Small Cell Lung Cancer Cells by Inhibiting Cell Proliferation and Promoting Apoptosis via the Targeting of Homeobox Protein Hox−B2. Mol Med Rep (2021) 23(2):1–. doi: 10.3892/mmr.2020.11743
28. Zhou W, Wang X, Yin D, Xue L, Ma Z, Wang Z, et al. Effect of Mir−140−5p on the Regulation of Proliferation and Apoptosis in NSCLC and its Underlying Mechanism. Exp Ther Med (2019) 18(2):1350–6. doi: 10.3892/etm.2019.7701
29. Wu X, Li Q, Zhang N, Li M, Li K. MiR-142 Inhibits Lung Cancer Cell Proliferation and Promotes Apoptosis by Targeting XIAP. Eur Rev Med Pharmacol Sci (2019) 23(17):7430–7. doi: 10.26355/eurrev_201909_18852
30. Zhao J, Cheng W, He X, Liu Y, Wang F, Gao Y. Long non-Coding RNA PICART1 Suppresses Proliferation and Promotes Apoptosis in Lung Cancer Cells by Inhibiting JAK2/STAT3 Signaling. Neoplasma (2018) 65(05):779–89. doi: 10.4149/neo_2018_171130N778
31. Li JC, Zheng JQ. Effect of microRNA-145 on Proliferation and Apoptosis of Human Non-Small Cell Lung Cancer A549 Cells by Regulating mTOR Signaling Pathway. J Cell Biochem (2017). doi: 10.1002/jcb.26629
32. Huang WT, He RQ, Li XJ, Ma J, Peng ZG, Zhong JC, et al. Mir−146a−5p Targets TCSF and Influences Cell Growth and Apoptosis to Repress NSCLC Progression. Oncol Rep (2019) 41(4):2226–40. doi: 10.3892/or.2019.7030
33. Gu S, Lai Y, Chen H, Liu Y, Zhang Z. miR-155 Mediates Arsenic Trioxide Resistance by Activating Nrf2 and Suppressing Apoptosis in Lung Cancer Cells. Sci Rep (2017) 7(1):1–13. doi: 10.1038/s41598-017-06061-x
34. Luo J, Pan J, Jin Y, Li M, Chen M. MiR-195-5p Inhibits Proliferation and Induces Apoptosis of Non-Small Cell Lung Cancer Cells by Targeting CEP55. OncoTarg Ther (2019) 12:11465. doi: 10.2147/OTT.S226921
35. Wu S, Zhang G, Li P, Chen S, Zhang F, Li J, et al. miR-198 Targets SHMT1 to Inhibit Cell Proliferation and Enhance Cell Apoptosis in Lung Adenocarcinoma. Tumor Biol (2016) 37(4):5193–202. doi: 10.1007/s13277-015-4369-z
36. Ren J, Li X, Dong H, Suo L, Zhang J, Zhang L, et al. Mir−210−3p Regulates the Proliferation and Apoptosis of Non−Small Cell Lung Cancer Cells by Targeting SIN3A. Exp Ther Med (2019) 18(4):2565–73. doi: 10.3892/etm.2019.7867
37. Han Y, Cai Y, Lai X, Wang Z, Wei S, Tan K, et al. lncRNA RMRP Prevents Mitochondrial Dysfunction and Cardiomyocyte Apoptosis via the miR-1-5p/Hsp70 Axis in LPS-Induced Sepsis Mice. Inflammation (2020) 43(2):605–18. doi: 10.1007/s10753-019-01141-8
38. Sun Y, Li J, Chen C. Effects of miR-221 on the Apoptosis of Non-Small Cell Lung Cancer Cells by lncRNA HOTAIR. Eur Rev Med Pharmacol Sci (2019) 23:4226–33. doi: 10.26355/eurrev_201905_17927
39. Chen W, Li X. MiR-222-3p Promotes Cell Proliferation and Inhibits Apoptosis by Targeting PUMA (BBC3) in Non-Small Cell Lung Cancer. Technol Cancer Res Treat (2020) 19. doi: 10.1177/1533033820922558
40. Fan J-m, Zheng Z-r, Zeng Y-M, Chen X-y. MiR-323-3p Targeting Transmembrane Protein With EGF-Like and 2 Follistatin Domain (TMEFF2) Inhibits Human Lung Cancer A549 Cell Apoptosis by Regulation of AKT and ERK Signaling Pathways. Med Sci Monitor: Int Med J Exp Clin Res (2020) 26:e919454–1. doi: 10.12659/MSM.919454
41. Sun C-C, Li S-J, Zhang F, Pan J-Y, Wang L, Yang C-L, et al. Hsa-miR-329 Exerts Tumor Suppressor Function Through Down-Regulation of MET in Non-Small Cell Lung Cancer. Oncotarget (2016) 7(16):21510. doi: 10.18632/oncotarget.7517
42. Zhang J, Zhao M, Xue Z, Liu Y, Wang Y. miR-377 Inhibited Tumorous Behaviors of Non-Small Cell Lung Cancer Through Directly Targeting CDK6. Eur Rev Med Pharmacol Sci (2016) 20(21):4494–9.
43. Jiang Y, Zhu P, Gao Y, Wang A. Mir−379−5p Inhibits Cell Proliferation and Promotes Cell Apoptosis in Non−Small Cell Lung Cancer by Targeting β−Arrestin−1. Mol Med Rep (2020) 22(6):4499–508. doi: 10.3892/mmr.2020.11553
44. Guo Q, Zheng M, Xu Y, Wang N, Zhao W. MiR-384 Induces Apoptosis and Autophagy of Non-Small Cell Lung Cancer Cells Through the Negative Regulation of Collagen α-1 (X) Chain Gene. Biosci Rep (2019) 39(2). doi: 10.1042/BSR20181523
45. Lingling J, Xiangao J, Guiqing H, Jichan S, Feifei S, Haiyan Z. SNHG20 Knockdown Suppresses Proliferation, Migration and Invasion, and Promotes Apoptosis in Non-Small Cell Lung Cancer Through Acting as a miR-154 Sponge. Biomed Pharmacother (2019) 112:108648. doi: 10.1016/j.biopha.2019.108648
46. Wang P, Chen D, Ma H, Li Y. Long non-Coding RNA MEG3 Regulates Proliferation and Apoptosis in Non-Small Cell Lung Cancer via the miR-205-5p/LRP1 Pathway. RSC Adv (2017) 7(78):49710–9. doi: 10.1039/C7RA08057C
47. Sun Y, Li L, Xing S, Pan Y, Shi Y, Zhang L, et al. miR-503-3p Induces Apoptosis of Lung Cancer Cells by Regulating P21 and CDK4 Expression. Cancer Biomarkers (2017) 20(4):597–608. doi: 10.3233/CBM-170585
48. Chu K, Gao G, Yang X, Ren S, Li Y, Wu H, et al. MiR-512-5p Induces Apoptosis and Inhibits Glycolysis by Targeting P21 in Non-Small Cell Lung Cancer Cells. Int J Oncol (2016) 48(2):577–86. doi: 10.3892/ijo.2015.3279
49. Cao B, Tan S, Tang H, Chen Y, Shu P. Mir−512−5p Suppresses Proliferation, Migration and Invasion, and Induces Apoptosis in Non−Small Cell Lung Cancer Cells by Targeting ETS1. Mol Med Rep (2019) 19(5):3604–14. doi: 10.3892/mmr.2019.10022
50. Wang J, Sheng Z, Cai Y. Effects of microRNA-513b on Cell Proliferation, Apoptosis, Invasion, and Migration by Targeting HMGB3 Through Regulation of mTOR Signaling Pathway in Non-Small-Cell Lung Cancer. J Cell Physiol (2019) 234(7):10934–41. doi: 10.1002/jcp.27921
51. Wu A, Yang X, Zhang B, Wang S, Li G. miR-516a-3p Promotes Proliferation, Migration, and Invasion and Inhibits Apoptosis in Lung Adenocarcinoma by Targeting PTPRD. Int J Clin Exp Pathol (2019) 12(11):4222.
52. Wei F, Wang M, Li Z, Wang Y, Zhou Y. Mir−593 Inhibits Proliferation and Invasion and Promotes Apoptosis in Non−Small Cell Lung Cancer Cells by Targeting SLUG−associated Signaling Pathways. Mol Med Rep (2019) 20(6):5172–82. doi: 10.3892/mmr.2019.10776
53. Huang Y, Ni R, Wang J, Liu Y. Knockdown of lncRNA DLX6-AS1 Inhibits Cell Proliferation, Migration and Invasion While Promotes Apoptosis by Downregulating PRR11 Expression and Upregulating miR-144 in Non-Small Cell Lung Cancer. Biomed Pharmacother (2019) 109:1851–9. doi: 10.1016/j.biopha.2018.09.151
54. Xiong J, Xing S, Dong Z, Niu L, Xu Q, Li Y, et al. Mir−654−3p Suppresses Cell Viability and Promotes Apoptosis by Targeting RASAL2 in Non−Small−Cell Lung Cancer. Mol Med Rep (2021) 23(2):1–. doi: 10.3892/mmr.2020.11763
55. Tong J, Wang L, Ling X, Wang M, Cao W, Liu Y. MiR-875 Can Regulate the Proliferation and Apoptosis of Non-Small Cell Lung Cancer Cells via Targeting SOCS2. Eur Rev Med Pharmacol Sci (2019) 23(12):5235–41. doi: 10.26355/eurrev_201906_18189
56. Xia Y, Wei K, Yang F-M, Hu L-Q, Pan C-F, Pan X-L, et al. Correction: miR-1260b, Mediated by YY1, Activates KIT Signaling by Targeting SOCS6 to Regulate Cell Proliferation and Apoptosis in NSCLC. Cell Death Dis (2020) 11(4):1–3. doi: 10.1038/s41419-020-2452-x
57. Gao W, Cui H, Li Q, Zhong H, Yu J, Li P, et al. Upregulation of microRNA-218 Reduces Cardiac Microvascular Endothelial Cells Injury Induced by Coronary Artery Disease Through the Inhibition of HMGB1. J Cell Physiol (2020) 235(3):3079–95. doi: 10.1002/jcp.29214
58. Ouyang L, Yang M, Wang X, Fan J, Liu X, Zhang Y, et al. Long non−Coding RNA FER1L4 Inhibits Cell Proliferation and Promotes Cell Apoptosis via the PTEN/AKT/p53 Signaling Pathway in Lung Cancer. Oncol Rep (2021) 45(1):359–67. doi: 10.3892/or.2020.7861
59. Liu S-Y, Zhao Z-Y, Qiao Z, Li S-M, Zhang W-N. LncRNA PCAT1 Interacts With DKC1 to Regulate Proliferation, Invasion and Apoptosis in NSCLC Cells via the VEGF/AKT/Bcl2/Caspase9 Pathway. Cell Transplant (2021) 30:0963689720986071. doi: 10.1177/0963689720986071
60. Wu C, Yang J, Li R, Lin X, Wu J, Wu J. LncRNA WT1-AS/miR-494-3p Regulates Cell Proliferation, Apoptosis, Migration and Invasion via PTEN/PI3K/AKT Signaling Pathway in Non-Small Cell Lung Cancer. OncoTarg Ther (2021) 14:891–904. doi: 10.2147/OTT.S278233
61. Zhong Y, Lin H, Li Q, Liu C, Zhong L. Downregulation of Long Non−Coding RNA GACAT1 Suppresses Proliferation and Induces Apoptosis of NSCLC Cells by Sponging microRNA−422a. Int J Mol Med (2021) 47(2):659–67. doi: 10.3892/ijmm.2020.4826
62. Liu B, Li J, Li JM, Liu GY, Wang YS. HOXC-AS2 Mediates the Proliferation, Apoptosis, and Migration of Non-Small Cell Lung Cancer by Combining With HOXC13 Gene. Cell Cycle (2021) 20(2):236–46. doi: 10.1080/15384101.2020.1868161
63. Li X, Zheng H. LncRNA SNHG1 Influences Cell Proliferation, Migration, Invasion, and Apoptosis of Non-Small Cell Lung Cancer Cells via the miR-361-3p/FRAT1 Axis. Thoracic Cancer (2020) 11(2):295–304. doi: 10.1111/1759-7714.13256
64. Wei C, Zhao X, Qiu H, Ming Z, Liu K, Yan J. The Long non-Coding RNA PVT1/miR-145-5p/ITGB8 Axis Regulates Cell Proliferation, Apoptosis, Migration and Invasion in Non-Small Cell Lung Cancer Cells. Neoplasma (2020) 67(4):802–12. doi: 10.4149/neo_2020_190723N657
65. Xiang C, Zhang Y, Zhang Y, Liu C, Hou Y, Zhang Y. lncRNA LEF1-AS1 Promotes Proliferation and Induces Apoptosis of Non-Small-Cell Lung Cancer Cells by Regulating miR-221/PTEN Signaling. Cancer Manage Res (2020) 12:3845. doi: 10.2147/CMAR.S246422
66. Song J, Su Z, Shen Q. Long non-Coding RNA MALAT1 Regulates Proliferation, Apoptosis, Migration and Invasion via miR-374b-5p/SRSF7 Axis in Non-Small Cell Lung Cancer. Eur Rev Med Pharmacol Sci (2020) 24:1853–62. doi: 10.26355/eurrev_202002_20363
67. Dao R, Wudu M, Hui L, Jiang J, Xu Y, Ren H, et al. Knockdown of lncRNA MIR503HG Suppresses Proliferation and Promotes Apoptosis of Non-Small Cell Lung Cancer Cells by Regulating miR-489-3p and miR-625-5p. Pathol-Res Pract (2020) 216(3):152823. doi: 10.1016/j.prp.2020.152823
68. Yu P, Wang Y, Lv W, Kou D, Hu H, Guo S, et al. LncRNA NEAT1/miR-1224/KLF3 Contributes to Cell Proliferation, Apoptosis and Invasion in Lung Cancer. Eur Rev Med Pharmacol Sci (2019) 23(19):8403–10. doi: 10.26355/eurrev_201910_19151
69. Guo R, Hu T, Liu Y, He Y, Cao Y. Long non-Coding RNA PRNCR1 Modulates Non-Small Cell Lung Cancer Cell Proliferation, Apoptosis, Migration, Invasion, and EMT Through PRNCR1/miR-126-5p/MTDH Axis. Biosci Rep (2020) 40(7):BSR20193153. doi: 10.1042/BSR20193153
70. Wang G, Liu L, Zhang J, Huang C, Chen Y, Bai W, et al. LncRNA HCG11 Suppresses Cell Proliferation and Promotes Apoptosis via Sponging miR-224-3p in Non-Small-Cell Lung Cancer Cells. OncoTarg Ther (2020) 13:6553. doi: 10.2147/OTT.S244181
71. Dong Z, Liu H, Zhao G. Long Noncoding RNA SNHG6 Promotes Proliferation and Inhibits Apoptosis in Non-Small Cell Lung Cancer Cells by Regulating miR-490-3p/RSF1 Axis. Cancer Biother Radiopharmaceut (2020) 35(5):351–61. doi: 10.1089/cbr.2019.3120
72. She K, Huang J, Zhou H, Huang T, Chen G, He J. lncRNA-SNHG7 Promotes the Proliferation, Migration and Invasion and Inhibits Apoptosis of Lung Cancer Cells by Enhancing the FAIM2 Expression. Oncol Rep (2016) 36(5):2673–80. doi: 10.3892/or.2016.5105
73. Liu L, Chen P, Wang M, Li X, Wang J, Yin M, et al. C2c1-sgRNA Complex Structure Reveals RNA-Guided DNA Cleavage Mechanism. Mol Cell (2017) 65(2):310–22. doi: 10.1016/j.molcel.2016.11.040
74. Chen H, Tan X, Ding Y. Knockdown SNHG20 Suppresses Nonsmall Cell Lung Cancer Development by Repressing Proliferation, Migration and Invasion, and Inducing Apoptosis by Regulating miR-2467-3p/E2F3. Cancer Biother Radiopharmaceut (2020) 36(4):360–70. doi: 10.1089/cbr.2019.3430
75. Wang Z, Zhao Y, Yu Y, Liu N, Zou Q, Liang F, et al. Effects of lncRNA SNHG20 on Proliferation and Apoptosis of Non-Small Cell Lung Cancer Cells Through Wnt/β-Catenin Signaling Pathway. Eur Rev Med Pharmacol Sci (2020) 24(1):230–7. doi: 10.26355/eurrev_202001_19915
76. Wang X, Gu G, Zhu H, Lu S, Abuduwaili K, Liu C. LncRNA SNHG20 Promoted Proliferation, Invasion and Inhibited Cell Apoptosis of Lung Adenocarcinoma via Sponging miR-342 and Upregulating DDX49. Thoracic Cancer (2020) 11(12):3510–20. doi: 10.1111/1759-7714.13693
77. Gao T, Dai X, Jiang Y, He X, Yuan S, Zhao P. LncRNA HAND2-AS1 Inhibits Proliferation and Promotes Apoptosis of Non-Small Cell Lung Cancer Cells by Inactivating PI3K/Akt Pathway. Biosci Rep (2020) 40(11):BSR20201870. doi: 10.1042/BSR20201870
78. Wang H, Kang L, Qin X, Xu J, Fei J. LINC00460 Promotes Proliferation and Inhibits Apoptosis of Non-Small Cell Lung Cancer Cells Through Targeted Regulation of miR-539. Eur Rev Med Pharmacol Sci (2020) 24(12):6752–8. doi: 10.26355/eurrev_202006_21663
79. Wang T, Tang X, Liu Y. LncRNA-ATB Promotes Apoptosis of Non-Small Cell Lung Cancer Cells Through MiR-200a/β-Catenin. J BUON (2019) 24(6):2280–6.
80. Song Z, Du J, Zhou L, Sun B. lncRNA AWPPH Promotes Proliferation and Inhibits Apoptosis of Non−Small Cell Lung Cancer Cells by Activating the Wnt/β−Catenin Signaling Pathway. Mol Med Rep (2019) 19(5):4425–32. doi: 10.3892/mmr.2019.10089
81. Yang L, Liu G. lncRNA BANCR Suppresses Cell Viability and Invasion and Promotes Apoptosis in Non-Small-Cell Lung Cancer Cells In Vitro and In Vivo. Cancer Manage Res (2019) 11:3565. doi: 10.2147/CMAR.S194848
82. Fan H, Li J, Wang J, Hu Z. Long non-Coding RNAs (lncRNAs) Tumor-Suppressive Role of lncRNA on Chromosome 8p12 (TSLNC8) Inhibits Tumor Metastasis and Promotes Apoptosis by Regulating Interleukin 6 (IL-6)/Signal Transducer and Activator of Transcription 3 (STAT3)/hypoxia-Inducible Factor 1-Alpha (HIF-1α) Signaling Pathway in Non-Small Cell Lung Cancer. Med Sci Monitor: Int Med J Exp Clin Res (2019) 25:7624. doi: 10.12659/MSM.917565
83. Huang N, Guo W, Ren K, Li W, Jiang Y, Sun J, et al. LncRNA AFAP1-AS1 Supresses miR-139-5p and Promotes Cell Proliferation and Chemotherapy Resistance of Non-Small Cell Lung Cancer by Competitively Upregulating RRM2. Front Oncol (2019) 9:1103. doi: 10.3389/fonc.2019.01103
84. Gao L, Zeng H, Zhang T, Mao C, Wang Y, Han Z, et al. MicroRNA-21 Deficiency Attenuated Atherogenesis and Decreased Macrophage Infiltration by Targeting Dusp-8. Atherosclerosis (2019) 291:78–86. doi: 10.1016/j.atherosclerosis.2019.10.003
85. Liu L, Zhou X, Zhang J, Wang G, He J, Chen Y, et al. LncRNA HULC Promotes Non-Small Cell Lung Cancer Cell Proliferation and Inhibits the Apoptosis by Up-Regulating Sphingosine Kinase 1 (SPHK1) and its Downstream PI3K/Akt Pathway. Eur Rev Med Pharmacol Sci (2018) 22(24):8722–30. doi: 10.26355/eurrev_201812_16637
86. Shu J, Li S, Chen Y, Zhu Q, Yu X. Long non-Coding RNA EPB41L4A-AS2 Inhibited Non-Small Cell Lung Cancer Proliferation, Invasion and Promoted Cell Apoptosis. Neoplasma (2018) 65(5):664–72. doi: 10.4149/neo_2018_170713N480
87. Lei T, Lv Z, Fu J, Wang Z, Fan Z, Wang Y. LncRNA NBAT-1 is Down-Regulated in Lung Cancer and Influences Cell Proliferation, Apoptosis and Cell Cycle. Eur Rev Med Pharmacol Sci (2018) 22(7):1958–62. doi: 10.26355/eurrev_201804_14721
88. Lian Z, Lv FF, Yu J, Wang JW. The Anti-Inflammatory Effect of microRNA-383-3p Interacting With IL1R2 Against Homocysteine-Induced Endothelial Injury in Rat Coronary Arteries. J Cell Biochem (2018) 119(8):6684–94. doi: 10.1002/jcb.26854
89. Huang Z, Lei W, Tan J, Hu HB. Long Noncoding RNA LINC00961 Inhibits Cell Proliferation and Induces Cell Apoptosis in Human Non–Small Cell Lung Cancer. J Cell Biochem (2018) 119(11):9072–80. doi: 10.1002/jcb.27166
90. Han B. LncRNA LINC02418 Regulates Proliferation and Apoptosis of Non-Small Cell Lung Cancer Cells by Regulating miR-4677-3p/SEC61G. Euro Rev Med Pharma Sci (2019) 23(23):10354–62. doi: 10.26355/eurrev_201912_19673
91. Zhu Q, Lv T, Wu Y, Shi X, Liu H, Song Y. Long Non-Coding RNA 00312 Regulated by HOXA 5 Inhibits Tumour Proliferation and Promotes Apoptosis in Non-Small Cell Lung Cancer. J Cell Mol Med (2017) 21(9):2184–98. doi: 10.1111/jcmm.13142
92. Huang C, Qin Y, Liu H, Liang N, Chen Y, Ma D, et al. Downregulation of a Novel Long Noncoding RNA TRPM2-AS Promotes Apoptosis in Non–Small Cell Lung Cancer. Tumor Biol (2017) 39(2):1010428317691191. doi: 10.1177/1010428317691191
93. Wang L, Cao L, Wen C, Li J, Yu G, Liu C. LncRNA LINC00857 Regulates Lung Adenocarcinoma Progression, Apoptosis and Glycolysis by Targeting miR-1179/SPAG5 Axis. Hum Cell (2020) 33(1):195–204. doi: 10.1007/s13577-019-00296-8
94. Su C, Shi K, Cheng X, Han Y, Li Y, Yu D, et al. Long Noncoding RNA LINC00472 Inhibits Proliferation and Promotes Apoptosis of Lung Adenocarcinoma Cells via Regulating miR-24-3p/DEDD. Technol Cancer Res Treat (2018) 17:1533033818790490. doi: 10.1177/1533033818790490
95. Sun J, Min H, Yu L, Yu G, Shi Y, Sun J. The Knockdown of LncRNA AFAP1-AS1 Suppressed Cell Proliferation, Migration, and Invasion, and Promoted Apoptosis by Regulating miR-545-3p/Hepatoma-Derived Growth Factor Axis in Lung Cancer. Anti-Cancer Drugs (2020) 32(1):11–21. doi: 10.1097/CAD.0000000000001003
96. Guo Y, Hu Y, Hu M, He J, Li B. Long non-Coding RNA ZEB2-AS1 Promotes Proliferation and Inhibits Apoptosis in Human Lung Cancer Cells. Oncol Lett (2018) 15(4):5220–6. doi: 10.3892/ol.2018.7918
97. Li J, Xu X, Wei C, Liu L, Wang T. Long Noncoding RNA NORAD Regulates Lung Cancer Cell Proliferation, Apoptosis, Migration, and Invasion by the miR-30a-5p/ADAM19 Axis. Int J Clin Exp Pathol (2020) 13(1):1.
98. Naylor EC, Desani JK, Chung PK. Targeted Therapy and Immunotherapy for Lung Cancer. Surg Oncol Clinics (2016) 25(3):601–9. doi: 10.1016/j.soc.2016.02.011
99. Kunze-Schumacher H, Krueger A. The Role of MicroRNAs in Development and Function of Regulatory T Cells–Lessons for a Better Understanding of MicroRNA Biology. Front Immunol (2020) 11:2185. doi: 10.3389/fimmu.2020.02185
100. Lazaridou MF, Massa C, Handke D, Mueller A, Friedrich M, Subbarayan K, et al. Identification of microRNAs Targeting the Transporter Associated With Antigen Processing TAP1 in Melanoma. J Clin Med (2020) 9(9):2690. doi: 10.3390/jcm9092690
101. Di Martino MT, Riillo C, Scionti F, Grillone K, Polerà N, Caracciolo D, et al. miRNAs and lncRNAs as Novel Therapeutic Targets to Improve Cancer Immunotherapy. Cancers (Basel) (2021) 13(7):1587. doi: 10.3390/cancers13071587
102. Li X, Song Y, Liu F, Liu D, Miao H, Ren J, et al. Long non-Coding RNA MALAT1 Promotes Proliferation, Angiogenesis, and Immunosuppressive Properties of Mesenchymal Stem Cells by Inducing VEGF and IDO. J Cell Biochem (2017) 118(9):2780–91. doi: 10.1002/jcb.25927
103. Yan K, Fu Y, Zhu N, Wang Z, Hong JL, Li Y, et al. Repression of lncRNA NEAT1 Enhances the Antitumor Activity of CD8(+)T Cells Against Hepatocellular Carcinoma via Regulating miR-155/Tim-3. Int J Biochem Cell Biol (2019) 110:1–8. doi: 10.1016/j.biocel.2019.01.019
Keywords: lncRNA, miRNA, apoptosis, lung cancer, expression
Citation: Ghafouri-Fard S, Aghabalazade A, Shoorei H, Majidpoor J, Taheri M and Mokhtari M (2021) The Impact of lncRNAs and miRNAs on Apoptosis in Lung Cancer. Front. Oncol. 11:714795. doi: 10.3389/fonc.2021.714795
Received: 25 May 2021; Accepted: 08 July 2021;
Published: 21 July 2021.
Edited by:
Yan Gu, National Key Laboratory of Immunology, ChinaReviewed by:
Dan Qi, Baylor Scott and White Health, United StatesTupa Basuroy, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2021 Ghafouri-Fard, Aghabalazade, Shoorei, Majidpoor, Taheri and Mokhtari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Taheri, mohammad_823@yahoo.com; Majid Mokhtari, majimokh@gmail.com
 Soudeh Ghafouri-Fard
Soudeh Ghafouri-Fard Amin Aghabalazade
Amin Aghabalazade Hamed Shoorei
Hamed Shoorei Jamal Majidpoor
Jamal Majidpoor Mohammad Taheri
Mohammad Taheri Majid Mokhtari6*
Majid Mokhtari6*