- 1Department of Gastroenterology, Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 2Department of Neurology, The People’s Hospital of Hebei Province, Shijiazhuang, Hebei, China
- 3Department of Internal Medicine, Baoding Orthopedic Hospital/People’s Hospital of Lianchi District, Baoding, Hebei, China
In China, gastric cancer is the second most common cause of cancer-related death, after lung cancer. At present, the morbidity and mortality rates of gastric cancer are increasing, and targeted therapy for gastric cancer has become a research hotspot. Herein, we report a patient with multiple metastases from advanced gastric cancer. After identifying MET gene amplification, initial treatment induced regression of the tumor. However, in later stages, due to the overexpression or mutation of HER-2, KRAS, TP53, and other genes, the targeted drug therapy became ineffective, and the disease progressed rapidly, leading to the death of the patient.
Introduction
With changing dietary and living habits, the incidence of gastric cancer has been increasing yearly. Gastric cancer is the fifth most common malignancy worldwide (5.7%) and the third most common reason for cancer-related mortality (8.2%) (1). In China, gastric cancer is the second most common cause of cancer-related death, after lung cancer (1). The symptoms of gastric cancer are often non-specific; therefore, most patients present in the advanced stage of disease by the time they present to a doctor. Traditional palliative chemotherapy has shown limited efficacy for advanced gastric cancer whereas for gastric cancer, targeted therapy has become a research hotspot. However, due to the spatial and temporal heterogeneity of gastric cancer, the selection of drug for use as targeted therapy is difficult.
Case description
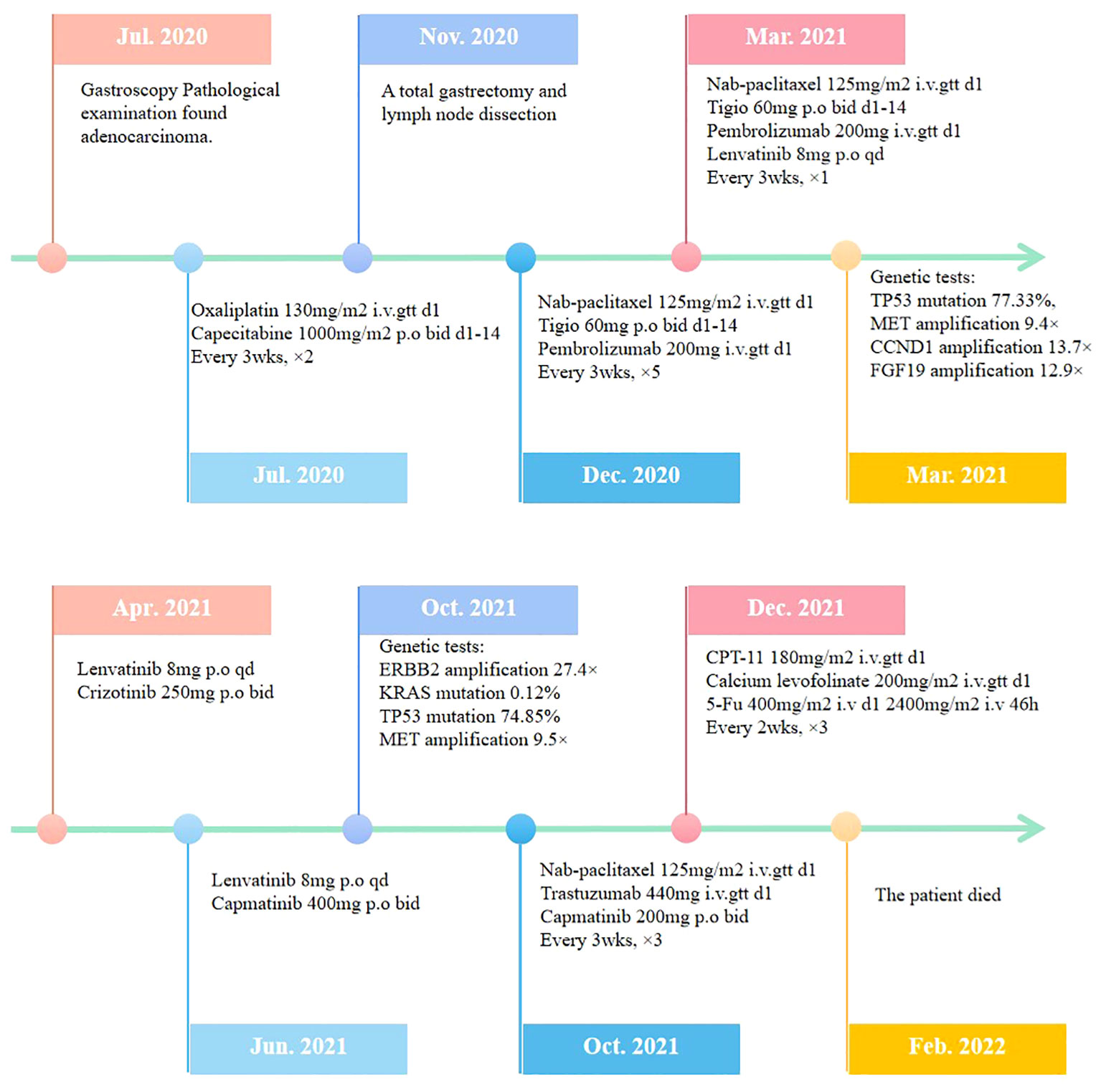
The patient was a 41-year-old male with no family history of cancer, but had a history of excessive alcohol intake and had smoked for longer than ten years. He was admitted in July 2020 with a two-week history of “choking feeling following eating.” Gastroscopy revealed ulcer-like lesions in the small curvature of the cardia and gastric body (Figure 1A). Pathological examination of a biopsy specimen identified adenocarcinoma (Figure 1B). Enhanced computed tomography (CT) suggested that the lesion was Borrmann II localized ulcer type and the maximum thickness was 3.22 cm. Laparoscopic exploration revealed gastric adenocarcinoma stage III C (T4aN3M0). Two cycles of XELOX (oxaliplatin 150 mg d1+capecitabine 1.5 mg 2/d d1-14) was given between July 2020 and August 2020, combined with apatinib targeted therapy. Apatinib is an anti-vascular targeted drug, which was first developed, tested, and approved in China, its global efficacy and safety data are still being collected (2). In October 2020, positron emission tomography - CT (PET/CT) examination showed multiple lymph node metastasis in the para-abdominal aorta and left clavicular region. Total gastrectomy and lymph node dissection were performed in November 2020. Postoperative pathology revealed the presence of ypT0N1. Postoperative immunohistochemistry showed HER-2 (1+), MLH1 (+), MSH2 (+), PSM2 (+), MSH6 (+), EBER (-), and PD-L1 (+) CPS3. The Lauren classification indicated intestinal-type gastric cancer (IGC). One week postoperatively, enhanced CT revealed newly enlarged retroperitoneal lymph nodes (the shortest diameter of the largest lymph node was about 1.3 cm), and recrudescence was considered. After operation, AS combined with K drug (nab-paclitaxel 0.3 g d1+Tigio 60 mg 2/d d1-14+pembrolizumab 200 mg d1 q3w) regimen was used in five cycles. Evaluation of the target lesion, according to the RECIST 1.1 scoring standard, revealed enlarged stable disease (SD) in the fourth cycle (the abdominal cavity and retroperitoneal lymph nodes were increased and the largest lymph node short diameter was about 1.5 cm). To further strengthen the treatment effect, lenvatinib was added in the sixth cycle of treatment. Enhanced CT showed that the abdominal and retroperitoneal lymph nodes had increased and enlarged (the shortest diameter of the largest lymph node was about 1.6 cm) (Figure 1C). Genetic tests performed in March 2021 revealed TP53 mutation (77.33%), mesenchymal-epithelial transition factor proto-oncogene (MET) amplification (9.4x), CCND1 amplification (13.7x), and FGF19 amplification (12.9x) (Figure 2). Treatment was changed to lenvatinib 8 mg 1/d+crizotinib 250 mg 2/d. After 50 days of treatment, CA19-9 levels decreased from 427 U/mL to 17.13 U/mL, and CA72-4 reduced from ≥ 300 U/mL to 16.16 U/mL. Enhanced CT after MET targeted therapy showed that the abdominal and retroperitoneal lymph nodes had decreased than previously (Figure 1D). Because the patient had a pulsatile headache, cranial magnetic resonance imaging (MRI) performed in June 2021 showed metastatic tumor (the cerebellar hemisphere, right occipital lobe, right centrum semiovale, and bilateral parietal lobe had multiple lesions, with a maximum diameter of 3.1 cm), which was evaluated as progressive disease (PD). Crizotinib was replaced with capmatinib, which crosses the blood-brain barrier. Head MRI after 2 months showed that the intracranial metastatic tumor was smaller than that seen in the previous film (cerebellar hemisphere, right occipital lobe, right centrum semiovale, and bilateral parietal lobe, with a maximum diameter of 2.3 cm). The lesion was evaluated as SD. After 2 months of continued oral capmatinib, CT revealed that the lesion was PD (the liver had new metastasis with a maximum diameter of 2.6 cm while the shortest diameter of the largest lymph node was 1.4 cm) (Figure 3A). No significant changes were observed in the head MRI results. Genetic analysis performed in October 2021 revealed that ERBB2 copy number was amplified (27.4x), and there was KRAS mutation (0.12%), TP53 mutation (74.85%), MET amplification (9.5x), MYC amplification (4.1x), CCND1 amplification (14.5x), and FGF19 amplification (14.3x) (Figure 2). Trastuzumab was added to the next line of treatment. The nab-paclitaxel+trastuzumab+capmatinib regimen was administered in three cycles, between October 2021 and November 2021. After one cycle, the levels of CA19-9 decreased from 2000 U/mL to 108.6 U/mL, CA72-4 showed no obvious changes, while CEA decreased from 12.51 ng/mL to 1.87 ng/mL. After two cycles, the lesion was evaluated as PD (CT showed new metastases with the shortest diameter in the mediastinal lymph nodes of 1.7 cm and in the abdominal and retroperitoneal lymph nodes of 2.4 cm, whereas, the longest diameter in the intrahepatic metastasis was 3.4 cm) (Figure 3B). MRI showed new metastasis in the thoraco-lumbar spine. Head MRI showed that the longest diameter in the bilateral cerebellar hemisphere and right parietal lobe lesions was 1.7 cm). However, the general condition of the patient was poor, with multiple metastases, multiple organ circulatory failure, disseminated intravascular coagulation (laboratory test results revealed fibrinogen of 0.88 g/L, platelets of 48,000/mL, prothrombin time of 22.1s, and D-dimer of 37 mg/L, with the indicators continuing to show deterioration), and failure to tolerate continued chemotherapy and targeted therapy. The patient died in February 2022 (Figure 4), with an overall survival of 19 months.
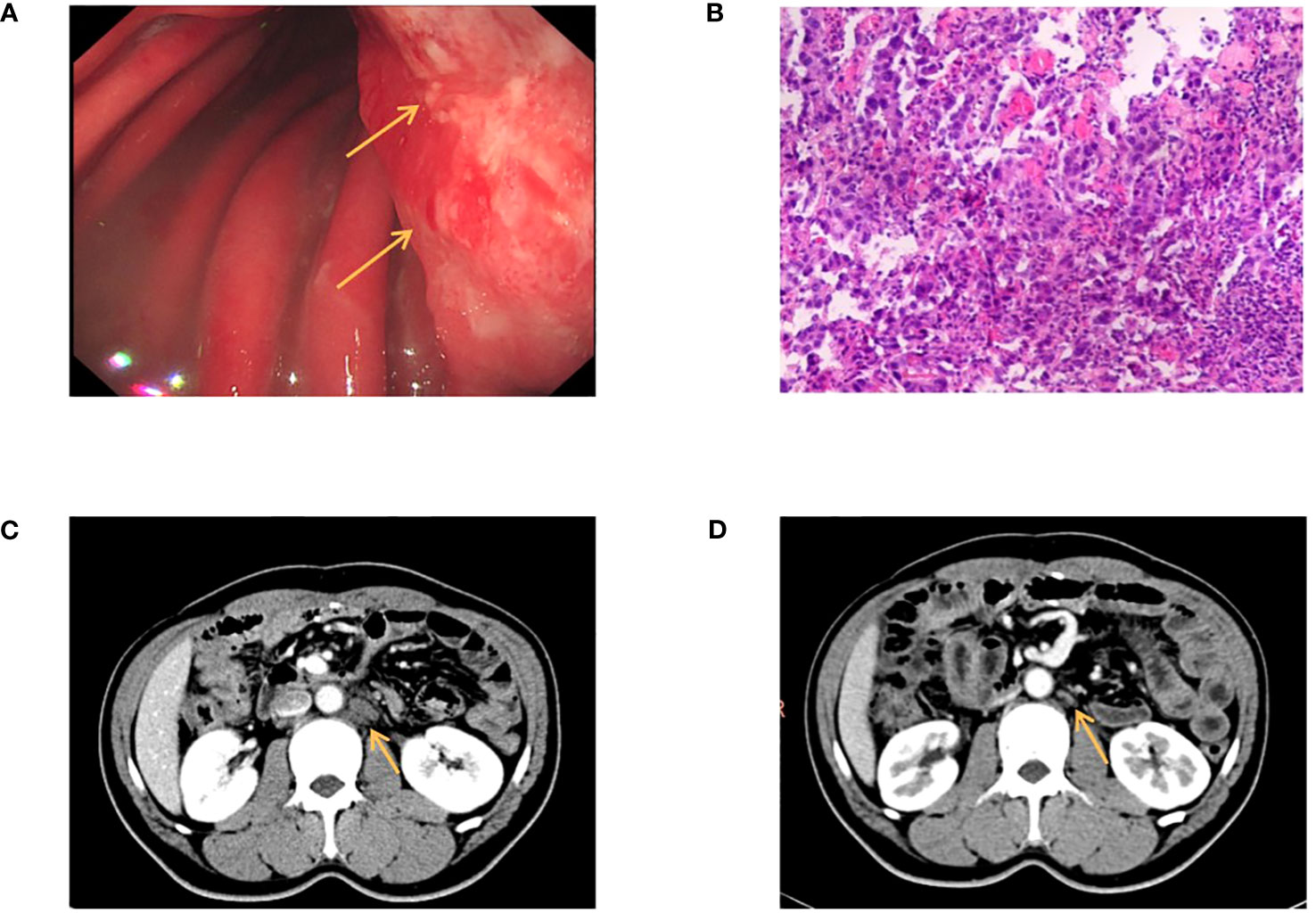
Figure 1 (A) Gastroscopy revealed ulcer-like lesions in the small curvature of cardia and gastric body. (B) Pathological examination of a biopsy specimen found adenocarcinoma. (C) Enhanced CT showed that the abdominal and retroperitoneal lymph nodes had increased and enlarged. (D) Enhanced CT after MET targeted therapy showed that the abdominal and retroperitoneal lymph nodes had decreased than anterior. The arrow in (A) indicate the locations of ulcer-like lesions in the small curvature of cardia and gastric body. The arrow (C, D) indicate the locations of enlarged retroperitoneal lymph nodes.
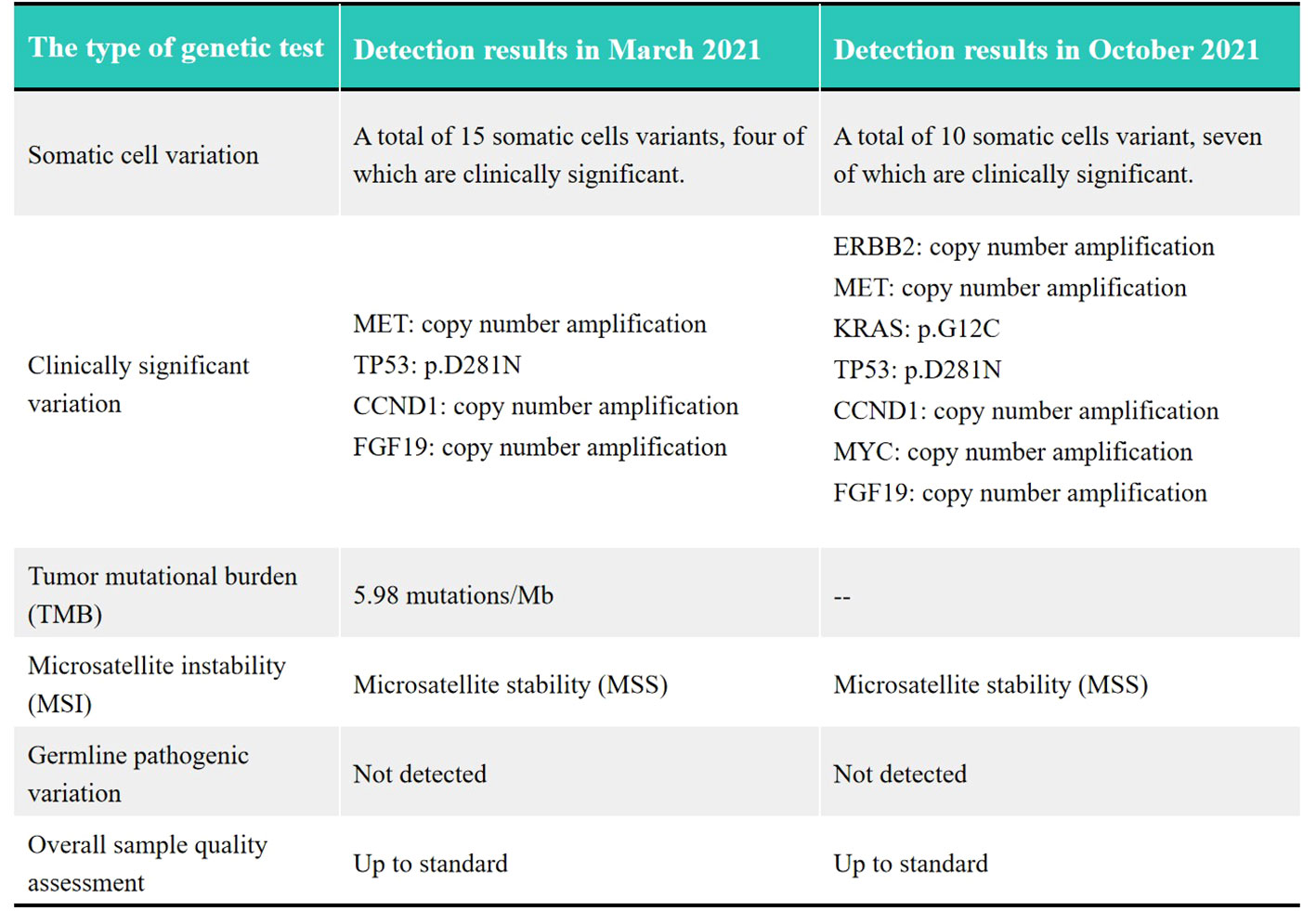
Figure 2 Comparison of genetic tests between March 2021 and October 2021. The symbol “–” means the TMB detection wasn’t performed in October 2021.

Figure 3 (A) Enhanced CT showed tha the liver had new metastasis. (B) Enhanced CT showed the maximum short diameter of abdominal and retroperitoneal lymph nodes was increased, and the maximum long diameter of intrahepatic metastasis was larged. The arrows indicate the locations of new metastases in the liver and the locations of enlarged retroperitoneal lymph nodes.
Discussion
The overall prognosis of advanced gastric cancer is poor. When the initial standard treatment effect is poor, stratified precision treatment becomes popular. In gastric cancer, the most common aberrant genes include TP53, PIK3CA, ERBB2, ERBB3, ARID1A, and KRAS, and less commonly MET, FGFR1, MYC, and CDH1 (3).
The MET is located on the long arm of human chromosome 7 and encodes a c-MET protein with tyrosine kinase activity. Hepatocyte growth factor (HGF) is the only ligand of c-MET (4). After HGF binds to c-MET, receptor homodimerization, autophosphorylation of carboxyl-terminal tyrosine residues, and activation of mitogen-activated protein kinase (MAPK), phosphatidylinositol 3 kinase (PI3K), and Rac1-Cdc42 signals occur (5), activating the downstream PI3K/AKT/mTOR and RAS/RAF/MEK/ERK pathways. MET abnormalities mainly include skip mutation of the MET14 exon, amplification of the MET gene, and abnormal expression of the c-MET protein (6). Among these, the overexpression of c-MET protein is the most common (50-65%) (7), followed by MET gene amplification; however, MET gene mutations rarely occur (8). MET gene amplification is usually mutually exclusive to other abnormally changed genes, and less likely to co-amplify (3.4-7%). MET amplification is usually found in IGC (9, 10). Crizotinib acts on the MET/ALK/ROS signaling pathway and is currently the most commonly used tyrosine kinase inhibitor (TKI). However, for gastric cancer with c-MET overexpression or mutation, the therapeutic effect still needs to be confirmed. In one study, tissues were collected from 262 patients with advanced gastric cancer, and cancer cells were treated with crizotinib. The findings showed that cells with a c-MET mutation and overexpression of MET protein were not sensitive to crizotinib. However, cells with amplified MET were sensitive to crizotinib (11). The patient we report also had C-met amplification and benefited from the treatment. This further demonstrates the efficacy of crizotinib in patients with MET gene amplification. Unlike crizotinib, capmatinib can cross the blood-brain barrier (12), making it the best choice for gastric cancer with MET amplification and brain metastasis.
In the late stage of disease progression, the HER-2 (ERBB2) gene was amplified, and trastuzumab was specifically administered. HER-2 activates and mediates the activation of downstream signaling pathways through the formation of heterodimers and autophosphorylation of tyrosine kinases (13). It is primarily involved in the Ras/MAPK/ERK and PI3K/Akt/mTOR pathways. When HER-2 is abnormally expressed, the downstream pathway is abnormally activated, thus promoting the proliferation, differentiation, invasion, and other abnormal activities of tumor cells. The positive rate of abnormal expression of HER-2 in gastric cancer is 7.3% - 20.2% (14). Moreover, abnormal expression of HER-2 gene is more common in IGC than in diffuse-type of gastric cancer (DGC) (15). Trastuzumab is a human IgG1 monoclonal antibody from recombinant DNA that selectively binds to the extracellular domain IV of HER-2, blocks the abnormal activation of HER-2, and then inhibits the downstream signaling pathway and tumor cell proliferation (14). The TOGA trial confirmed that chemotherapy combined with trastuzumab effectively prolonged the median overall survival time of patients with advanced gastric cancer with positive HER-2 (16).
The efficacy of trastuzumab in HER-2 positive patients has been fully confirmed; however, most of these studies are based on abnormal changes in the primary HER-2 gene. In this case, abnormal amplification of the MET gene was initially observed, but with no abnormality in the HER-2 gene. After continuous MET-targeted therapy, symptoms and examination results showed good curative effect. In later stages, the disease progressed, and new gene abnormalities were found, including HER-2, TP53, and KRAS. However, the cause of HER-2 mutations remained unexplored. Similar studies have reported the efficacy of trastuzumab combined with crizotinib in the treatment of HER-2-amplified and MET-amplified metastatic gastric cancers (17). However, unlike our case, the patient was diagnosed with multiple metastases of gastric adenocarcinoma, and HER-2 gene amplification was detected. After trastuzumab treatment, MET amplification was detected using delayed second-generation sequencing. After combining the two targeted drugs, the symptoms were temporarily relieved (performance status improved from 4 to 2). Another case report suggested that amplification of MET gene might be the driving factor for gastric cancer (4). After two months of crizotinib administration in patients with liver metastasis from advanced gastric cancer, the metastasis was significantly controlled and progression free survival (PFS) was prolonged to 20 months.
We believe that HER-2 mutation in this case was a secondary event. Therefore, the patient responded initially to administration of trastuzumab, but the improvement was not sustained. This might be due to the overactivation of primary MET, leading to heterodimer phosphorylation and formation of heterodimers with EGFR, HER-2, HER-3, and RET receptor tyrosine kinases, affecting the normal transduction of other signaling pathways (18). The signal triggered by this heterodimer increased the resistance to EGFR or HER-2 targeted therapy. In addition, mutations in TP53, KRAS, BRAF, RET, and other genes appeared in the later stages of the disease. The mutated TP53 gene can enhance MET signaling, expression of c-MET protein, and activation of the downstream signaling pathway of MET (19). Studies have shown that in HER-2-positive metastatic gastric cancer patients, the KRAS mutation rate in drug-resistant patients is also higher than that in sensitive patients, and the activated KRAS gene can promote epithelial-mesenchymal cell transformation, promoting the transformation of gastric cancer cells into tumor stem cells, resulting in treatment resistance (20). The patient described in this paper had advanced gastric cancer, and new metastatic lesions continued to appear as the disease progressed. At present, it is considered that for this patient, MET gene is the driving gene for gastric cancer. However, because of the spatial and temporal heterogeneity of gastric cancer, it is impossible to determine the heterogeneity of the driving genes among the metastatic foci. With the occurrence of late gene overexpression and mutations, genes interact with each other, including the promoting effect of KRAS mutations on the abnormal expression of MET and abnormal activation of downstream signaling pathways. The abnormal transformation of the KRAS gene in gastric cancer cells, the enhancement of abnormal MET signal by TP53 mutation, and other changes, can create complex interactions. This leads to the unsatisfactory efficacy of gastric cancer for c-met amplification therapy compared with lung cancer.
Owing to the high heterogeneity of gastric cancer, accurate molecular classification is of great significance for its diagnosis, treatment, and prognosis. The realization of accurate molecular therapy for gastric cancer is a direction for future research, but there is still a long way to go.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ: Writing – original draft, Writing – review & editing, Data curation. XRZ: Writing – original draft, Writing – review & editing, Data curation. DG: Writing – review & editing. CZ: Writing – review & editing. YW: Writing – review & editing. YF: Writing – review & editing. SG: Writing – review & editing. JW: Funding acquisition, Supervision, Writing – review & editing. FZ: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Key Research and Development Projects of Hebei Province (Grant no. 19277736D).
Acknowledgments
We thank the patient for agreeing to release his clinical data for scientific research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Geng RX, Song L, Li J, Zhao L. The safety of apatinib for the treatment of gastric cancer. Expert Opin Drug Saf (2018) 17:1145–50. doi: 10.1080/14740338.2018.1535592
3. Ibarrola-Villava M, Llorca-Cardeñosa MJ, Tarazona N, Mongort C, Fleitas T, Perez-Fidalgo JA, et al. Deregulation of ARID1A, CDH1, cMET and PIK3CA and target-related microRNA expression in gastric cancer. Oncotarget (2015) 6:26935–45. doi: 10.18632/oncotarget.4775
4. Hou GX, Song BB. Gastric cancer patient with c-MET amplification treated with crizotinib after failed multi-line treatment: A case report and literature review. Math Biosci Eng (2019) 16:5923–30. doi: 10.3934/mbe.2019296
5. Gherardi E, Birchmeier W, Birchmeier C, Woude GV. Targeting MET in cancer: Rationale and progress. Nat Rev Cancer (2012) 12:89–103. doi: 10.1038/nrc3205
6. Drilon A, Cappuzzo F, Ou SI, Camidge DR. Targeting MET in lung cancer: Will expectations finally be MET? J Thorac Oncol (2017) 12:15–26. doi: 10.1016/j.jtho.2016.10.014
7. Janjigian YY, Tang LH, Coit DG, Kelsen DP, Francone TD, Weiser MR, et al. MET Expression and amplification in patients with localized gastric cancer. Cancer Epidemiol Biomarkers Prevent (2011) 20:1021–7. doi: 10.1158/1055-9965.EPI-10-1080
8. El Darsa H, El Sayed R, Abdel-Rahman O. MET inhibitors for the treatment of gastric cancer: What’s their potential? J Exp Pharmacol (2020) 6:349–61. doi: 10.2147/JEP.S242958
9. Lee HE, Kim MA, Lee HS, Jung EJ, Yang HK, Lee BL, et al. MET in gastric carcinomas: comparsion between protein expression and gene copy number and impact on clinical outcome. Br J Cancer (2012) 107:325–33. doi: 10.1038/bjc.2012.237
10. Liu YJ, Shen D, Yin X, Gavine P, Zhang T, Su X, et al. HER2, MET and FGFR2 oncogenic driver alterations define distinct molecular segments for targeted therapies in gastric carcinoma. Br J Cancer (2014) 110:1169–78. doi: 10.1038/bjc.2014.61
11. Kim S, Ahn JM, Bae WJ, Han JH, Lee D. Quantitation of ligand is critical for ligand-dependent MET signalling activation and determines MET-targeted therapeutic response in gastric cancer. Gastric Cancer (2021) 24:577–88. doi: 10.1007/s10120-020-01139-4
12. Wolf J, Seto T, Han J-Y, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med (2020) 383:944–57. doi: 10.1056/NEJMoa2002787
13. Tebbutt N, Pedersen MW, Johns TG. Targeting the ERBB family in cancer: Couples therapy. Nat Rev Cancer (2013) 13:663–73. doi: 10.1038/nrc3559
14. Zhu Y, Zhu X, Wei X, Tang C, Zhang W. HER2-targeted therapies in gastric cancer. Biochim Biophys Acta Rev Cancer (2021) 1876:188549. doi: 10.1016/j.bbcan.2021.188549
15. Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer (2015) 18:476–84. doi: 10.1007/s10120-014-0402-y
16. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X
17. Liu M. Combination treatment with trastuzumab and crizotinib in metastatic gastric cancer harboring Her-2 amplification and c-MET amplification: A case report. Medicine (2021) 100:1–5. doi: 10.1097/MD.0000000000027017
18. Tanizaki J, Okamoto I, Sakai K, Nakagawa K. Differential roles of trans-phosphorylated EGFR, HER2, HER3, and RET as heterodimerisation partners of MET in lung cancer with MET amplification. Br J Cancer (2011) 105:807–13. doi: 10.1038/bjc.2011.322
19. Qin S, Chan SL, Sukeepaisarnjaroen W, Han G, Choo SP, Sriuranpong V, et al. A phase II study of the efficacy and safety of the MET inhibitor capmatinib (INC280) in patients with advanced hepatocellular carcinoma. Ther Adv Med Oncol (2019) 11:1758835919889001. doi: 10.1177/1758835919889001
Keywords: gastric adenocarcinoma, MET amplification, HER-2 amplification, targeted therapy, heterogeneity
Citation: Zhang X, Zhang X, Geng D, Zhao C, Wang Y, Fan Y, Gao S, Wei J and Zhang F (2023) Targeted therapy for multiple gene mutations in multiple metastases of advanced gastric cancer: a case report. Front. Oncol. 13:1257011. doi: 10.3389/fonc.2023.1257011
Received: 11 July 2023; Accepted: 24 November 2023;
Published: 15 December 2023.
Edited by:
Arunkumar Krishnan, Levine Cancer Institute, United StatesReviewed by:
Manidhar Reddy Lekkala, University of Kansas Medical Center, United StatesXiaobin Shang, Tianjin Medical University Cancer Institute and Hospital, China
Copyright © 2023 Zhang, Zhang, Geng, Zhao, Wang, Fan, Gao, Wei and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinmei Wei, NTkyNzY1NjA4QHFxLmNvbQ==; Fengbin Zhang, emhhbmdmZW5nYmluMTk4MUAxNjMuY29t
†These authors have contributed equally to this work
 Xin Zhang
Xin Zhang Xinran Zhang1†
Xinran Zhang1† Dandan Geng
Dandan Geng Chenguang Zhao
Chenguang Zhao Yingnan Wang
Yingnan Wang Fengbin Zhang
Fengbin Zhang