- 1Department of Thoracic Oncology II, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Beijing, China
- 2Department of Anesthesiology, China-Japan Friendship Hospital, Peking University China-Japan Friendship School of Clinical Medicine, Beijing, China
- 3Department of Thoracic Surgery II, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Beijing, China
Objective: This study aimed to detect circulating tumor cells (CTCs) and circulating tumor-derived endothelial cells (CTECs) in patients with advanced lung cancer, for describing the distribution characteristics of CTC and CTEC subtypes, exploring the correlation between CTC/CTEC subtypes and novel prognostic biomarkers.
Methods: A total of 52 patients with advanced lung cancer were enrolled in this study. Using the subtraction enrichment-immunofluorescence in situ hybridization (SE-iFISH) system, CTCs and CTECs derived from these patients were identified.
Results: Based on cell size, there were 49.3% small and 50.7% large CTCs, and 23.0% small and 77.0% large CTECs. Triploidy, tetraploidy, and multiploidy varied in the small and large CTCs/CTECs. Besides these three aneuploid subtypes, monoploidy was found in the small and large CTECs. Triploid and multiploid small CTCs and tetraploid large CTCs were associated with shorter overall survival (OS) in patients with advanced lung cancer. However, none of the CTECs subtypes showed a significant correlation with patient prognosis. In addition, we found strong positive correlations (P<0.0001) in the four groups including triploid small cell size CTCs and multiploid small cell size CTECs, and multiploid small cell size CTCs and monoploid small cell size CTECs. Furthermore, combined detection of the specific subtypes, including triploid small CTC and monoploid small CTEC, triploid small CTC and triploid small CTEC, and multiploid small CTC and monoploid small CTEC, were associated with poor prognosis in advanced lung cancer.
Conclusions: Aneuploid small CTCs are associated with the outcome of patients with advanced lung cancer. In particular, the combined detection of triploid small CTCs and monoploid small CTECs, triploid small CTCs and triploid small CTECs, and multiploid small CTCs and monoploid small CTECs has clinical significance for predicting prognosis in patients with advanced lung cancer.
1 Introduction
The global incidence of lung cancer is increasing, and it has become the leading cause of cancer-related deaths. Approximately 2.09 million new cases and 1.76 million deaths occur from lung cancer each year (1). However, early screening for lung cancer remains challenging, with 57% of lung cancers diagnosed when cancer metastasizes outside the lung (1–3). Despite substantial development in the oncological management of late-stage lung cancer, its prognosis remains poor. The five-year survival at all stages of non-small cell lung cancer (NSCLC) is only 19% (4, 5). The five-year survival rate of patients at different stages varies greatly: 68%-92% for stage I patients, 53%-60% for stage II patients, 13%-36% for stage III patients, and only 0%-10% for stage IV patients (6). Therefore, new strategies for prognosis assessments of advanced NSCLC are urgently needed.
To date, various biomarkers have been found to play important roles in diagnosis, relapse prediction, and drug resistance evaluation in advanced lung cancer, including circulating tumor cells (CTCs), cfDNA, extracellular matrix-associated components, soluble immunological biomarkers, miRNAs, tumor mutation burden, and genetic markers (7–13). CTCs are one of the most prominent biomarkers in the dynamic assessment of cancers and have been used by the FDA in prognostic cancer assessments since 2004. CTCs, which are considered to spread from the tumor into the peripheral blood, leading to metastasis (14), have been studied by researchers worldwide. Characterization of CTCs is considered to be closely related to emerging tumor subclones, influencing treatment response and prognosis. Recently, Kong et al. tested the genomic heterogeneity of CTC in lung and breast cancer and found CTCs genes are more similar to the metastatic tumor compared with the primary tumor (15). Lim et al. found that intratumor heterogeneity of CTCs predicted the risk of recurrence in NSCLC (16). These two studies revealed the significance of CTC heterogeneity in tumor metastasis and prognosis. Furthermore, many researchers have reported the importance of CTCs as biomarkers for prognosis, diagnosis, and drug resistance in cancer (17–22).
With the continuous progress of detection technology, various”cellular circulating tumor markers”, such as circulating tumor endothelial cells (CTECs), have been identified and require further research (17). CTECs are identified as being CD31+, where CD31+ is widely used to detect endothelial cells, whereas CTCs are identified as being CD31- (23). CTECs decrease in number after operations in esophageal and lung cancers, correlating with reductions in tumor growth (24). In addition, Lei et al. reported that the combined detection of specific CTC and CTEC heteroploid subtypes significantly helped with obtaining higher sensitivity and specificity in identifying malignant nodules in patients with early-stage NSCLC (25). As an important heterogeneity of tumor cells identified by chromosome, aneuploids are considered to play an important role in CTC and CTEC studies (26, 27). Aneuploid quantification of CTCs is a useful tool for tumor progression and metastasis, and the prediction and evaluation of therapeutic efficacy (28). For instance, Ye et al. found that triploid and small CTCs were more aggressive in liver cancer (28); while Li et al. stated that the different ploidies of chromosome 8 were closely related to both sensitivity and resistance to paclitaxel- or cisplatin-based chemotherapy in advanced gastric cancer patients (29). Lin et al. also discussed the significant versatile cellular role of aneuploid CTECs in tumor neovascularization and cancer metastasis (30). Furthermore, several studies have demonstrated that aneuploid CTCs and CTECs may exhibit a functional interplay in tumor angiogenesis, progression, metastasis, and response to therapy (31)—an important novel direction.
In this study, we enrolled patients with late‐stage lung cancer. We aimed to identify CTCs and CTECs in the peripheral blood, conduct subclass analyses of CTCs and CTECs, find a correlation between CTC and CTEC subtypes, and search for prognostic biomarkers.
2 Materials and methods
2.1 Patient enrollment and specimen collection
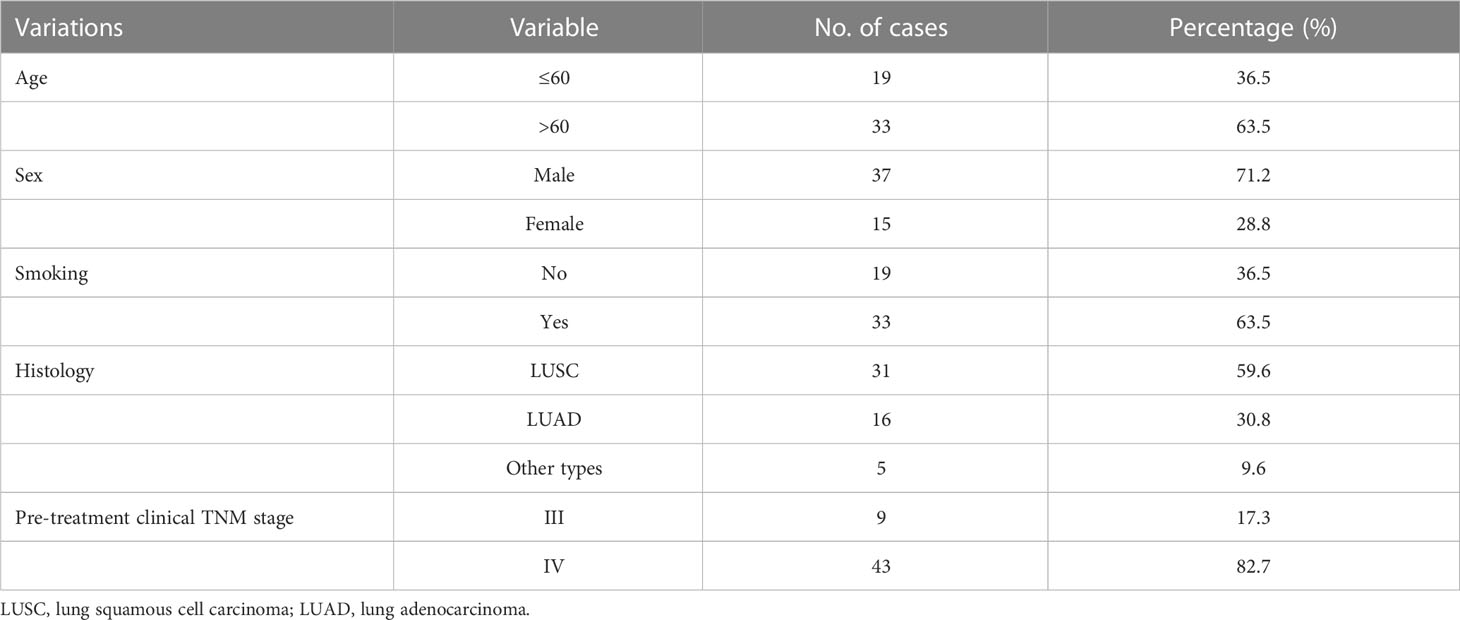
A total of 52 patients who were diagnosed with advanced lung cancer between June 2019 and October 2019, and underwent various treatments at Peking University Cancer Hospital were enrolled in this study. Eligibility criteria for patient recruitment included: (1) histological confirmation as lung cancer; (2) being considered as having stage IIIA to IV lung cancer; (3) availability of complete basic information, including age, sex, histology data, TNM stage, and follow-up data; and (4) no diagnosis of any other severe diseases. The exclusion criteria were as follows: (1) having a history of malignancies other than lung cancer within the last five years. Peripheral blood samples (7.5 mL) were collected from the 52 patients before treatment. After the blood samples were collected, they were processed within 24 hours. Each patient provided written informed consent, and this study was approved by the Institutional Ethics Committee of Peking University Cancer Hospital (IRB approval number: 2020KT65). This study was conducted in accordance with the principles of the Declaration of Helsinki.
2.2 Subtraction enrichment of CTC and CTEC
To identify CTCs and CTECs, we performed subtraction enrichment and immunostaining fluorescence in situ hybridization (SE-iFISH) on the samples. All the experiments were performed in accordance with the manufacturer’s instructions and investigators’ modifications. Cell enrichment was performed using the subtraction enrichment method. A 7.5mL blood sample was centrifuged at 600 × g for 5 min, and all the deposited cells were immediately loaded onto 3mL of anon-hematopoietic cell separation matrix (Cytelligen, San Diego, CA, USA). The abovementioned mixture was then centrifuged again at 400 × g for 5 min, depleting the red blood cells. Next, the abovementioned supernatants were incubated with anti-leukocyte antibody (CD45) immunomagnetic beads at 25°C for 15 min, and the separation matrix was used again, followed by centrifuging at 400 × g for 5 min. Subsequently, the solution was magnetically separated, and the magnetic beads were then removed from the supernatant. The bead-free solution was centrifuged at 500 × g for 2 min, and the cells were mixed thoroughly with 100μL of cell fixative. Finally, the cell mixture was smeared on coated CTC slides and dried overnight for subsequent iFISH processing.
2.3 iFISH
The samples thus obtained were then processed according to the manufacturer’s instructions and investigators’ modifications (Cytelligen, San Diego, CA, USA). The samples were subjected to the Vysis Centromere Probe (CEP8) Spectrum Orange (Abbott molecular, Abbott Park, Illinois,USA) for 4 hours, followed by incubation with Alexa Fluor 594-conjugated monoclonal anti-CD45 antibodies (Cytelligen, San Diego, CA, USA) and Cy5-conjugated monoclonal anti-CD31 antibodies (Cytelligen, San Diego, CA, USA) at 1: 200 dilution for 30 minutes at room temperature. Finally, 4-6-diamidino-2-phenylindole (DAPI; Life Technologies, Carlsbad,California, USA) was used to stain the nuclei. Stained cells were observed and counted under a fluorescence microscope. At least two pathologists performed CTC and CTEC counting for DAPI+, CD45-, and CD31-/+ cells, identified chromosome 8 aneuploidy under fluorescence, and conducted subclasses according to cell size and ploidy. Based on our previous study (32), CTCs or CTECs ≤5 µm in size (approximately the size of a white blood cell [WBC] or less) were considered small cell size CTCs or CTECs, whereas those>5 µm in size were considered large cell size CTCs or CTECs.
2.4 Isolation and identification of aneuploid CTCs and CTECs
CTC identification criteria were as follows (Figures 1A–F): DAPI+, CD45-, CD31-, and the identification of chromosome 8 aneuploidy. CTEC identification criteria were as follows (Figures 1G–L): DAPI+, CD45-, CD31+, and the identification of chromosome 8 aneuploidy.
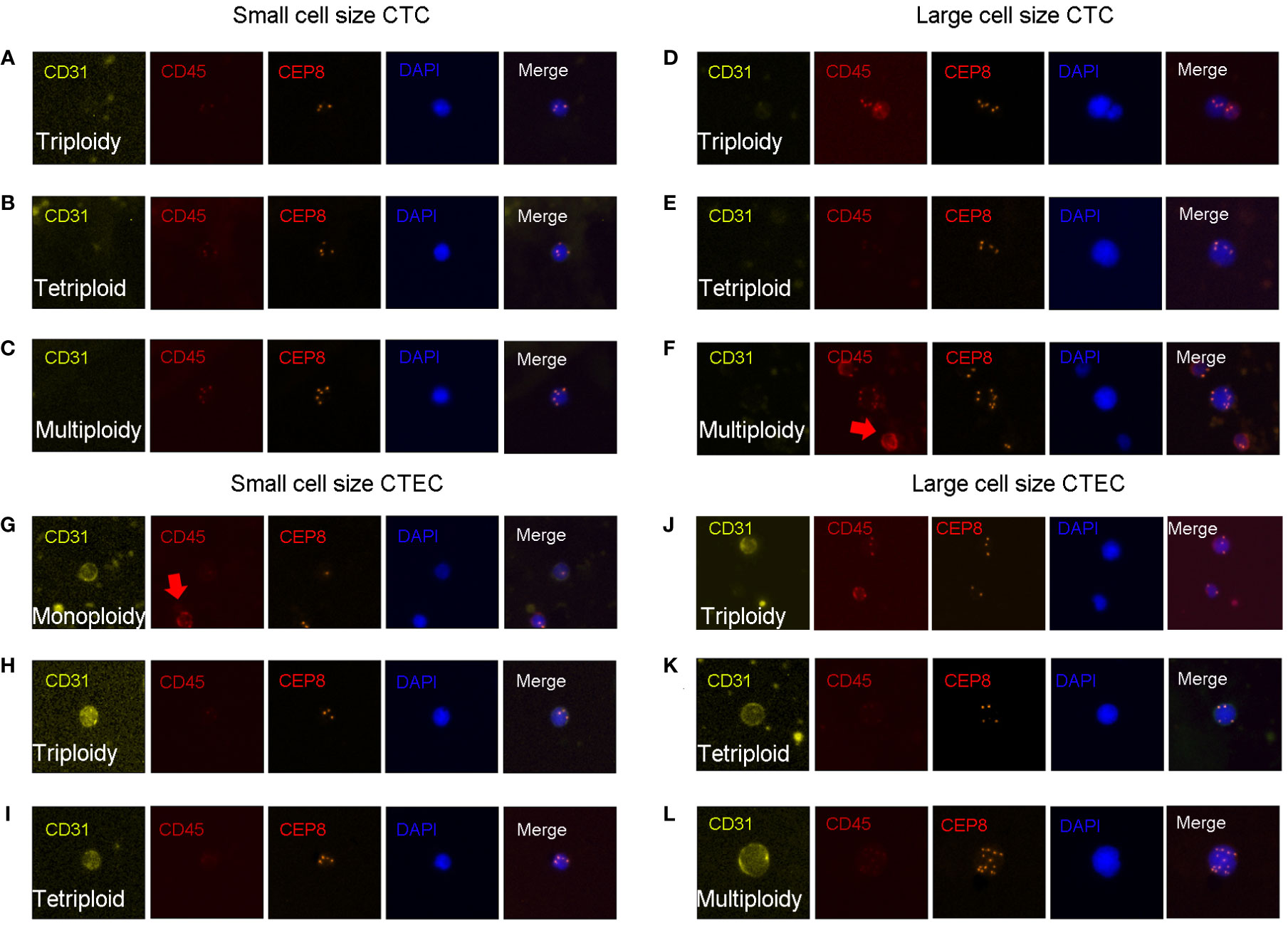
Figure 1 CTC and CTEC detection. Image representations of different sizes and ploidies of CTCs/CTECs from patients with advanced lung cancer. (A–F) CTCs are DAPI+/CD45-/CD31-/CEP8+. (G–L) CTECs are DAPI+/CD45-/CD31+/CEP8+. (A–C) Small CTCs including triploid small CTC (A), tetraploid small CTC (B), and multiploid small CTC (C). (D–F) Large CTCs including triploid large CTCs (D), tetraploid large CTCs (E), and multiploid large CTCs (F). (G–I) Small CTECs including monoploid small CTECs (G), triploid small CTECs (H), tetraploid small CTECs (I). (J–L) Large CTECs including triploid large CTECs (J), tetraploid large CTECs (K), and multiploid large CTECs (L).
With the detection of chromosomal 8 aneuploidy and cell size, we could achieve further subclassification of CTCs and CTECs. Using the general size of WBCs as the threshold, CTCs/CTECs were identified as either small (≤ 5 µm; Figures 1A–C, G-I) or large (>5 µm; Figures 1D–F, J–L). On detection of chromosomal 8 aneuploidy, CTCs were further divided into triploid (Figures A, D), tetraploid(Figures B, E), and multiploid (Figures C, F) subtypes, and CTECs were further divided into monoploid (Figure G), triploid (Figures H, J), tetraploid (Figures I, K), and multiploid (Figure L) subtypes.
2.5 Statistical analyses
All statistical analyses were performed using GraphPad Prism 7.0 and IBM SPSS Statistics software version 23.0. Correlations between CTCs and CTECs were calculated and analyzed using chi-square tests. Overall survival (OS) was defined as the duration from the treatment initiation till death. Kaplan-Meier survival plots for OS were generated based on whether CTC/CTEC numbers were more or less than the median of CTC/CTEC numbers. Log-rank tests were used to compare survival curves, and hazard ratio (HR) values were also shown at the same time. The possible significant predictors of OS were then enrolled into a multivariable Cox regression Model, identifying independent significant predictors of OS. All P values were two-sided, and P < 0.05 was defined as statistically significant.
3 Results
3.1 Distribution of CTC and CTEC subtypes in the advanced lung cancer patients
The study included 52 patients with advanced lung cancer. The characteristics of the patients are presented in Table 1. In total, 37 (71%) male and 15 (29%) female patients were included in this study, with a median age of 63 years, and an average age of 63 years (ranging from 36–78 years). For the pretreatment clinical stage, nine (17.3%) and 43 (82.7%) patients were stage III and IV, respectively.
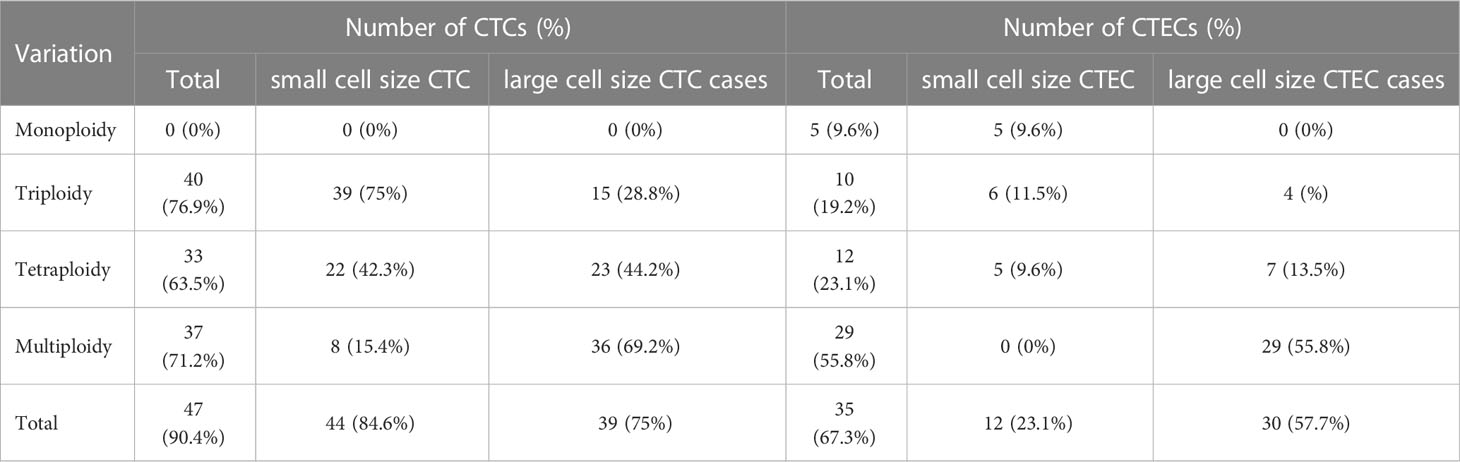
In our study of 52 patients, we found 491 CTCs in our patient cohort, including 249 (50.7%) small CTCs and 242(49.3%) large CTCs (Figure 2A). In addition, total, small, and large CTCs were detected in 90.4% (47/52), 84.6% (44/52), and 75% (39/52) of the patients, respectively (Table 2). The heteroploid features of CTCs are shown in Figures 2B–D. Triploidy accounted for the largest proportion of small CTCs (74.3%), followed by tetraploidy (20.1%) and multiploidy (5.6%); however, multiploidy accounted for the largest proportion of large CTCs (69.4%), followed by tetraploidy (15.3%) and triploidy (15.3%).
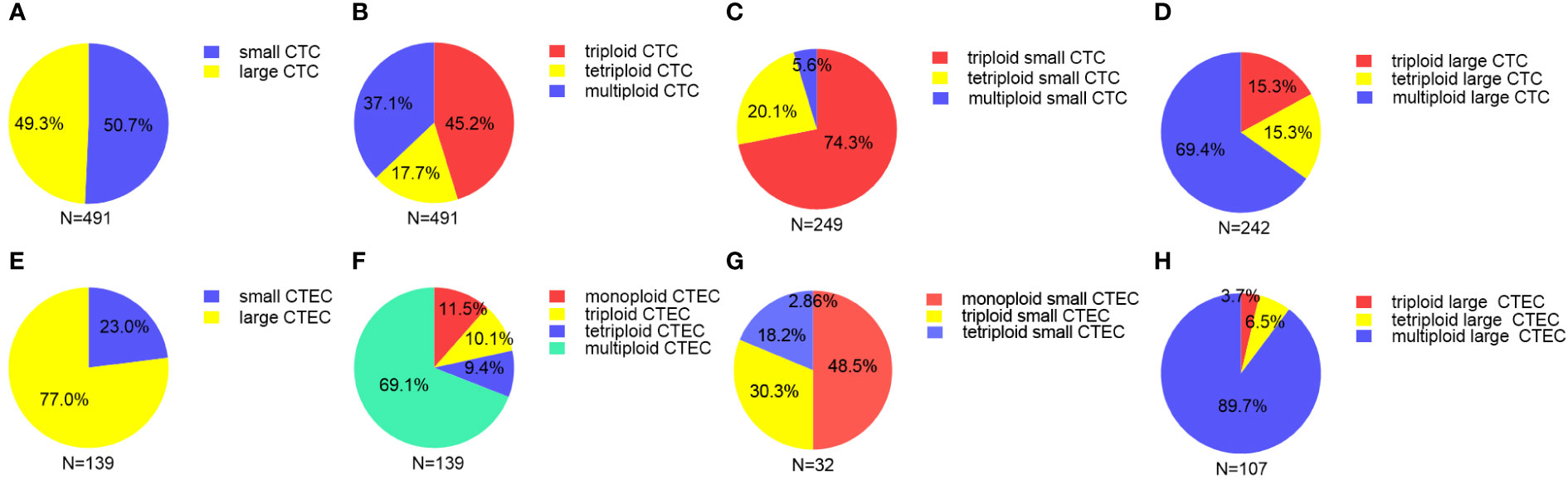
Figure 2 Proportion of different sizes and aneuploid CTCs/CTECs (A) Proportion of small and large CTCs. (B) Proportion of heteroploid subtypes of total CTCs. (C) Proportion of heteroploid subtypes of small CTCs. (D) Proportion of heteroploid subtypes of large CTCs. (E) Proportion of small and large CTECs. (F) Proportion of heteroploid subtypes of total CTECs. (G) Proportion of heteroploid subtypes of small CTECs. (H) Proportion of heteroploid subtypes of large CTECs.
As for CTECs, we found 139 CTECs in our patient cohort, including 32 (23.0%) small CTECs and 107 (77%) large CTECs (Figure 2E). Total, small, and large CTECs were detected in 67.3% (35/52), 23.1% (12/52), and 57.7% (30/52) of patients, respectively (Table 2). Monoploidy accounted for the highest proportion of small CTECs (48.5%); however, multiploidy accounted for the highest proportion of large CTECs (89.7%) (Figures 2F–H).
3.2 CTC/CTEC subtypes and OS
To further investigate the role of CTCs in prognosis, we analyzed the correlation between the heteroploid subtypes of small/large cell size CTCs and OS in patients with advanced lung cancer. Based on the median value of CTCs, we found that patients with triploid small CTCs>1, multiploid small CTCs>0, and tetraploid large CTCs>0 had shorter OS than patients with triploid small CTCs ≤ 1, multiploid small CTCs=0 and tetraploid large CTCs=0 (Figures 3A, C, E); However, patients with tetraploid small CTC>0, triploid large CTC>0, and multiploid large CTC>1 had no differences with tetraploid small CTCs=0, triploid large CTCs=0, and multiploid large CTCs ≤ 1 (Figures 3B, D, F). In summary, CTC heteroploidy subtypes that were detected using the SE-iFISH system, including triploid and multiploid small CTCs, together with tetraploid large CTCs, correlated with the prognosis of advanced lung cancer.
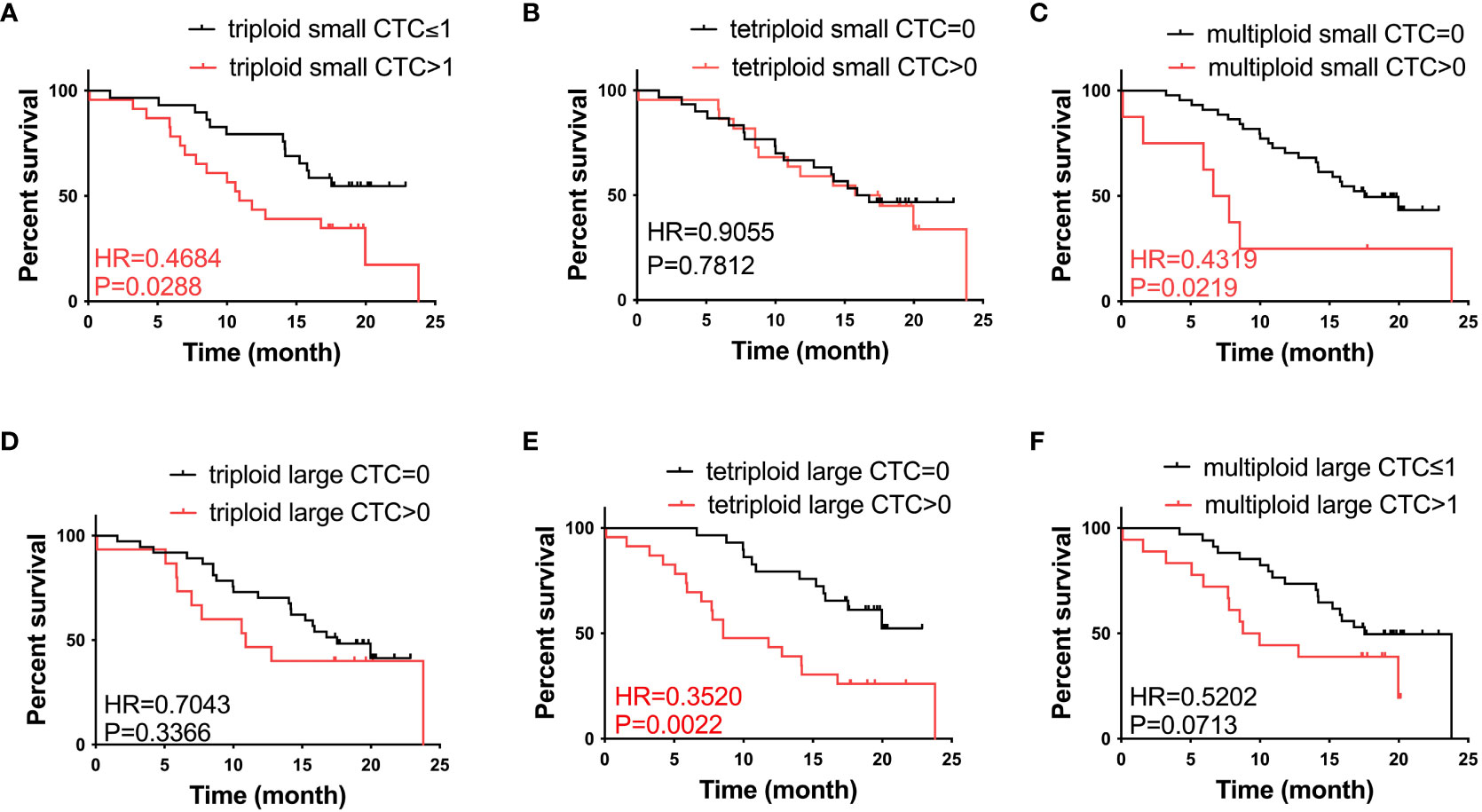
Figure 3 Survival analysis. (A) Patients with triploid small CTCs>1 had shorter OS compared to those with triploid CTCs ≤ 1 (P < 0.05). (B) Tetraploid small CTC subtypes were not correlated with OS (P > 0.05). (C) Patients with multiploid small CTCs>0 had shorter OS compared to those with multiploid CTCs=0 (P < 0.05). (D) Triploid large CTC subtype was not correlated to OS (P > 0.05). (E) Patients with tetraploid large CTCs>0 had shorter OS compared to those with tetraploid CTCs=0 (P < 0.05). (F) Multiploid small CTC subtype was not correlated to OS (P > 0.05).
We also studied the relationship between CTEC subtypes and OS in patients with advanced lung cancer. However, none of the aneuploid CTEC subtypes were significantly related to OS (Figures 4A–F).
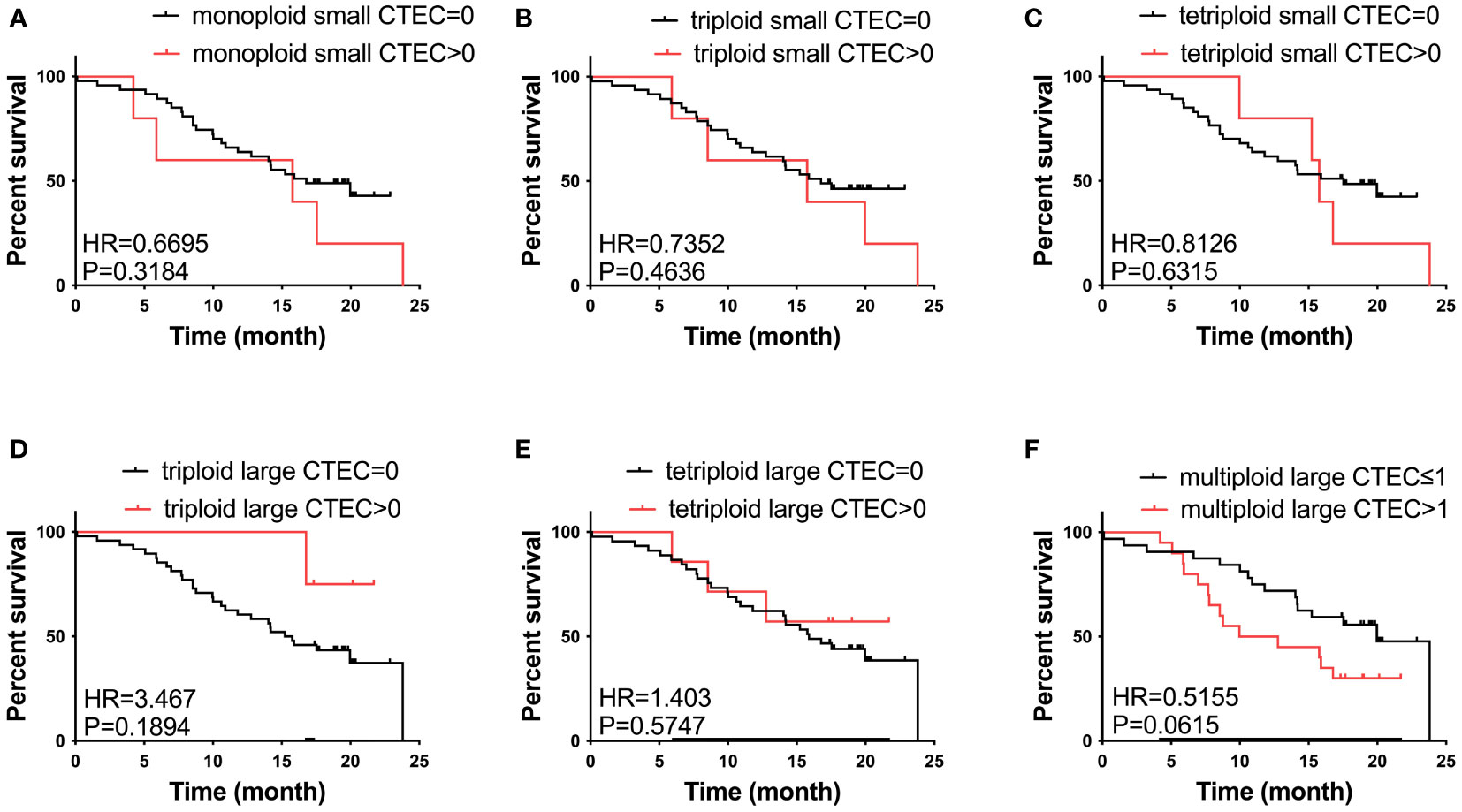
Figure 4 Survival analysis. (A–F) No CTEC subtypes were correlated to the OS of patients with advanced NSCLC (P > 0.05).
3.3 Relationships between numbers of CTC and CTEC subtypes
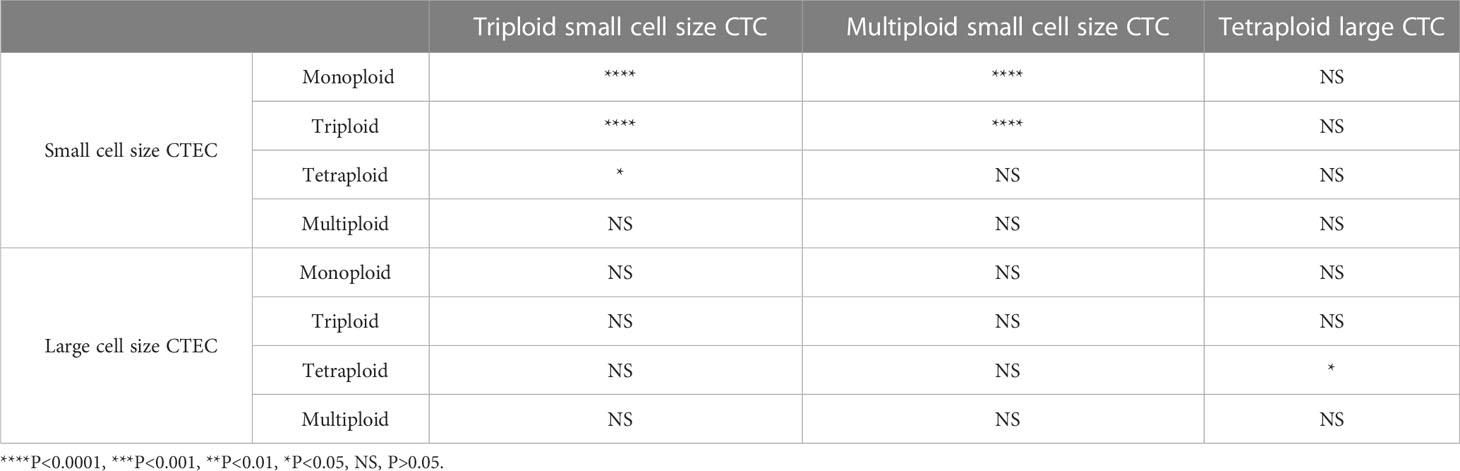
Next, we analyzed whether the CTC subtypes associated with OS (Figures 3A, C, E) were correlated with the CTEC subtypes in advanced lung cancer. As shown in Table 3, four groups, including triploid small cell size CTCs and monoploid/triploid small cell size CTECs, and multiploid small cell size CTCs and monoploid/triploid small cell size CTECs, had extremely strong positive correlations (P<0.0001). The relationships between these CTC and CTEC subtypes (P<0.0001) are described in Figure 5A–D.
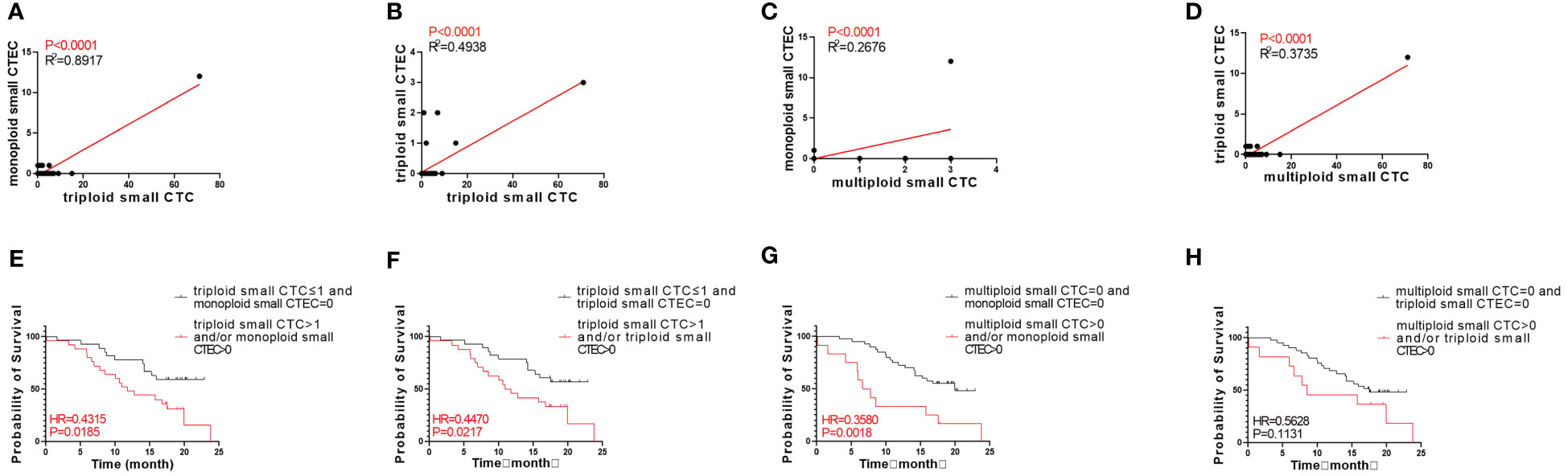
Figure 5 Correlation between CTC and CTEC subtypes, and survival of combined detection of CTC and CTEC subtypes with univariable analyses. (A–D) Correlations in four groups including triploid small CTC and monoploid small CTEC (A), triploid small CTC and triploid small CTEC (B), multiploid small CTC and monoploid small CTEC (C), and multiploid small CTC and triploid small CTEC (D). (E–H) Survival analyses in four groups including triploid small CTC≤1 and monoploid small CTEC=0 vs. triploid small CTC> 1 and/or monoploid small CTEC>0 (E), triploid small CTC≤1 and triploid small CTEC=0 vs. triploid small CTC> 1 and/or triploid small CTEC>0 (F), multiploid small CTC=0 and monoploid small CTEC=0 vs. multiploid small CTC>0 and/or monoploid small CTEC>0 (G), multiploid small CTC=0 and triploid small CTEC= 0 v s . multiploid small CTC>0 and/or triploid small CTEC> 0 (H).
Based on the abovementioned results, we examined whether the combined detection of CTC and CTEC subtypes is important in determining the prognosis associated with advanced lung cancer. The combined detection of triploid small CTCs and monoploid small CTECs, triploid small CTCs and triploid small CTECs, and multiploid small CTCs and monoploid small CTECs (P<0.05) could also predict prognosis in advanced lung cancer (Figures 5E–H). Patients with triploid small CTCs>1 and/or monoploid small CTECs>0 had shorter OS than did patients with triploid small CTCs ≤ 1 and monoploid small CTECs=0 (P=0.0185, Figure 5E). Patients with triploid small CTCs>1 and/or triploid small CTECs>0 had shorter OS than did patients with triploid small CTCs ≤ 1 and triploid small CTECs=0 (P=0.0217, Figure 5F). Patients with multiploid small CTCs>0 and/or monoploid small CTECs>0 had shorter overall survival than patients with multiploid small CTCs=0 and monoploid small CTECs=0 (P=0.0018, Figure 5G). However, OS of patients with multiploid small CTCs>0 and/or triploid CTECs>0 was not different from that of patients with multiploid small CTCs=0 and triploid small CTEC s=0 (P=0.1131, Figure 5H). Furthermore, we noticed that these three combined detections had a better effect in predicting prognosis, which had smaller p-values than did detecting small CTC subtypes independently (Table 4). Taking the combined detection of triploid small CTCs and monoploid small CTECs for instance, the p-value for detecting triploid small CTCs independently was 0.0288; however, the p-value for the combined detection of triploid small CTCs and monoploid small CTECs was only 0.0185, indicating better detection efficiency of the combined detection.
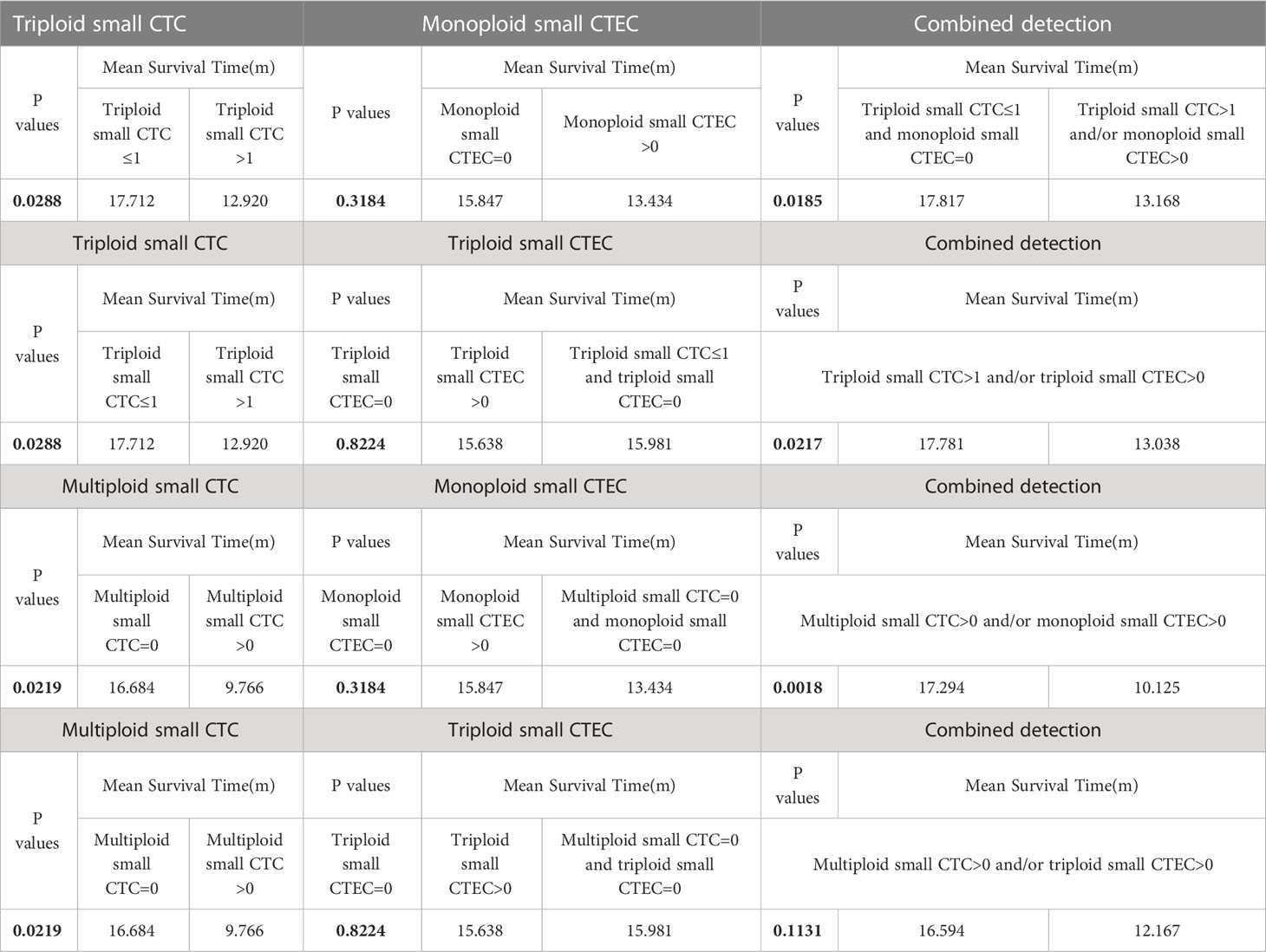
Table 4 Survival analyses of CTC subtypes, CTEC subtypes, and combinations of CTC and CTEC subtypes.
3.4 Combined detection of CTCs and CTECs by multivariable Cox regression analyses for OS
According to the results, the combined detections of three groups (Group 1: triploid small CTCs ≤ 1 and monoploid small CTECs=0 vs. triploid small CTCs>1 and/or monoploid small CTECs>0; Group 2: triploid small CTCs ≤ 1 and triploid small CTECs=0 vs. triploid small CTCs>1 and/or triploid small CTECs>0; and Group 3: multiploid small CTCs=0 and monoploid small CTECs=0 vs. multiploid small CTCs>0 and/or monoploid small CTECs>0) showed a significant difference in OS by univariable analysis. Furthermore, we analyzed these groups in OS by the multivariable Cox regression analyses, revealing that combined detection of group 1 (hazard ratio: 0.47, 95% CI: 0.225-0.981; P<0.05), group 2 (hazard ratio: 0.429, 95% CI 0.204-0.903, P<0.05), and group 3 (hazard ratio: 0.312, 95% CI: 0.144-0.676, P<0.05) were significant independent predictors for longer OS, respectively (Table 5; Figure 6).
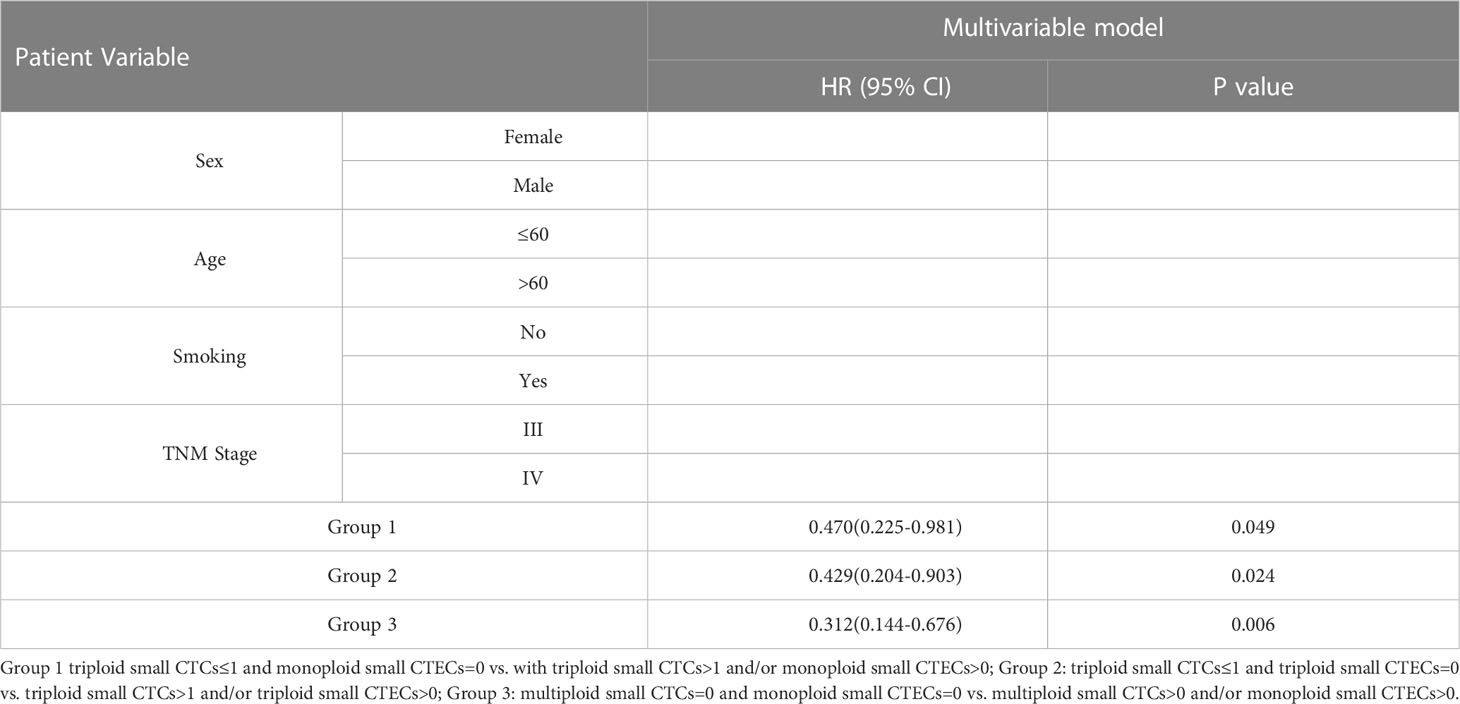
Table 5 Multivariable cox regression analyses for OS in the groups of combinational CTC and CTEC subtypes.
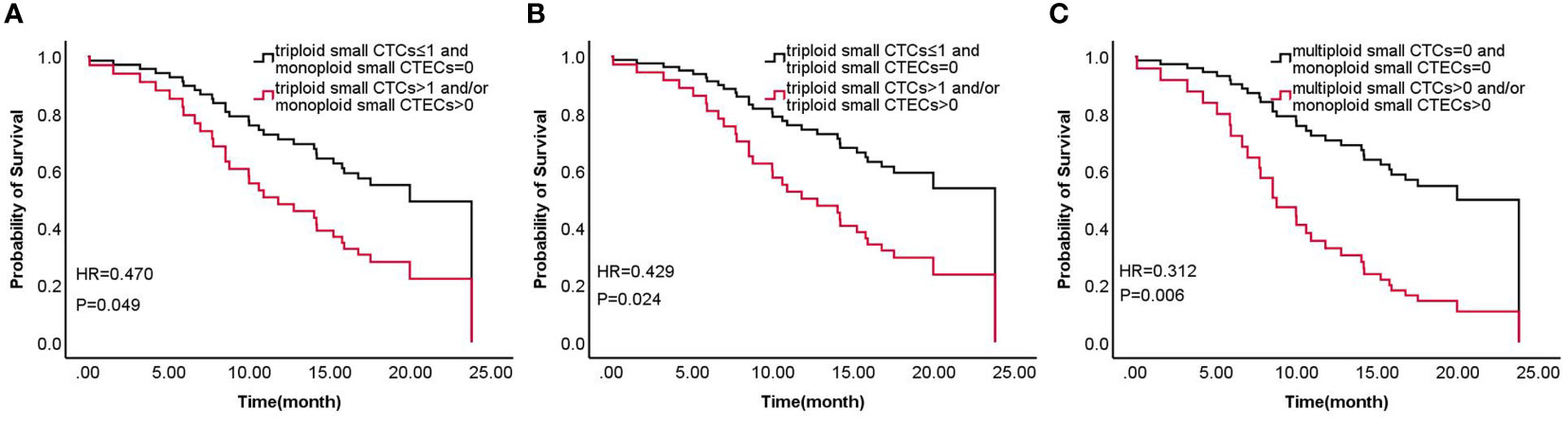
Figure 6 Survival of combined detection of CTC and CTEC subtypes multivariable cox regression analyses in three groups (A–C) including triploid small CTCs≤1 and monoploid small CTECs=0 vs. triploid small CTCs>1 and/or monoploid small CTECs>0 (A), triploid small CTCs≤ 1 and triploid small CTECs=0 vs. triploid small CTCs>1 and/or triploid small CTECs>0 (B), and multiploid small CTCs=0 and monoploid small CTECs=0 vs. multiploid small CTCs>0 and/or monoploid small CTECs>0 (C).
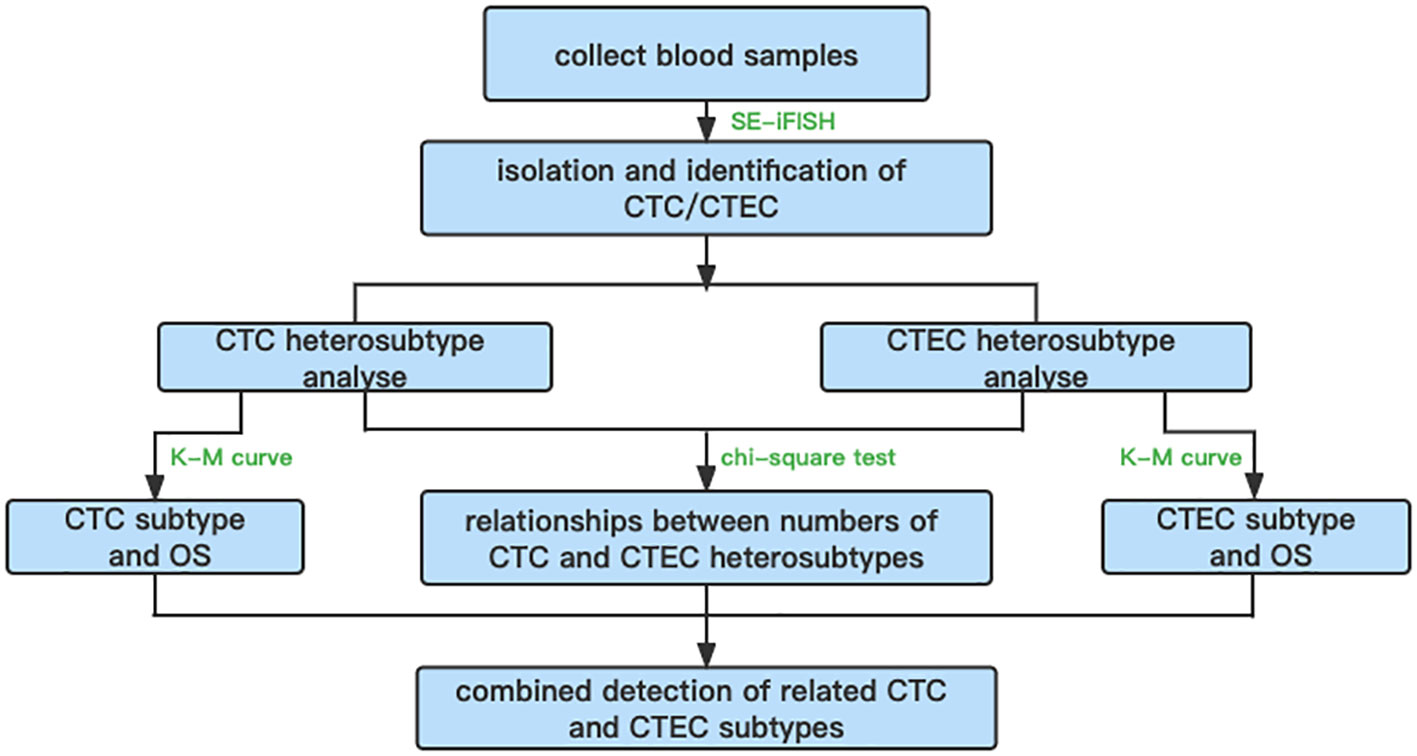
In summary, an overall flowchart of this study is shown in Figure 7.
4 Discussion
In this study, we explored the relationship between CTC and CTEC subtypes in patients with advanced lung cancer and attempted to identify prognostic biomarkers of this disease. Using the SE-iFISH method, we found that the detection rates of CTCs and CTECs were as high as 90% and 67%, respectively. Regarding the distribution of CTC and CTEC subtypes, we distinguished CTCs and CTECs in two dimensions, namely, heteroploid characteristics of chromosome 8 and cell size. Then, we further studied these CTC/CTEC subtypes and the prognosis of advanced lung cancer patients.
Hiroaki et al. showed that tumor cells undergoing epithelial-to-mesenchymal transition (EMT) were smaller in size than those without EMT characteristics (33). Wang et al. found that the majority of CTCs that were Vimentin+ (a marker for mesenchymal cells) were small in size and demonstrated different features from CTCs that were Vimentin- (34). Chen et al. found that the sizes of circulating tumor cell clusters can be used to track therapeutic resistance and prognosis in advanced gastric cancer (35). PD-L1+ aneuploid CTECs could exhibit resistance to immunotherapy in advanced NSCLC (31). In addition, the size of CTCs and clinical characteristics, such as cancer metastasis and progression (36), and post-surgical recurrence (37) were found to be relevant. These studies revealed that small cell size CTCs with more malignant behavior are associated with tumor progression and poor prognosis. Therefore, in this study, we did a subclassification of CTCs/CTECs into small cell size (≤white blood cell (WBC)) and large cell size (>WBC). We found that small CTCs accounted for 50.7% of the total CTCs, while large CTCs accounted for 49.3%; however, small CTECs accounted for only 23% of the total CTECs, and large CTECs accounted for 77% of the total CTECs.
Through the CellSearch system, Krebs et al. found 21% of positive CTCs (at baseline of > 2 CTCs) in the total cases of lung cancer patients (n=101) with IIIa to IV stages lung cancer and 32% of positive CTCs in NSCLC cases with stage IV lung cancer (n=60) (38). With the CellSearch system, Juan et al. also used two CTCs as a baseline and found that 24% of patients (n=37) had positive CTCs in the NSCLC patients who received chemotherapy (39). Using the SE-iFISH method, Ge et al. found that 92% of lung cancer patients (24/26) showed positive CTCs at a baseline of one CTC (40); Ye et al. reported that 92.9% (79/85) of lung cancer patients had positive CTCs (28). In our previous study, 88% and 94% of patients with resectable NSCLC were positive for total circulating aneuploid cells (CACs) in pre- and post-surgery, respectively (32). In the present study, we found that CTCs were identified in 90.4% (47/52) of lung cancer patients with advanced stage. Since the heterogenicity and high frequency of CTCs in these lung cancers, we analyzed the role of the size and aneuploid subtypes of CTCs/CTECs in the prognosis of these patients. In our previous study on early-stage lung cancer, small CACs accounted for only 18% of the total CACs (32). Furthermore, in our study on advanced lung cancer, small CTCs accounted for 50.7% of all CTCs. In addition, we found that small CTECs only accounted for 23% of all CTECs, and mainly comprised monoploid (16/32) and triploid (10/32) CTECs.
Chromosomal instability mainly leads to chromosome aneuploidy, which is a common feature in solid tumors and the causes of aneuploidy contain kinetochore–microtubule attachments and dynamics, centrosome number, spindle assembly checkpoint, and chromosome cohesion (26, 27). Several studies clarified the relationship between subtypes of aneuploidy CTCs and clinical characteristics, diagnosis, prognosis, and drug resistance (29, 31, 32, 37, 41, 42). Herein, aneuploidy CTCs/CTECs including monoploidy, triploidy, tetraploidy, and multiploidy subtypes were also identified. Triploid and multiploid small CTCs, together with tetraploid large CTCs were found to associate with the prognosis of advanced lung cancer.
CTCs are used in the diagnosis, prognosis evaluation, efficacy evaluation, drug resistance and recurrence monitoring, and precision treatment target screening of various tumors. CTCs have shown important clinical application value and have become a hot topic in tumor research (43–45). In addition, with the continuous development of CTC detection technology, another “cellular circulating tumor marker” (that is, CTECs) has been preliminary studied (30, 31, 42). Among the endothelial cells that constitute the tumor vascular system, most are tumor-derived endothelial cells that express a high level of CD31. These endothelial cells also exhibit cytogenetic abnormalities in aneuploid chromosomes. A previous study on the CTEC karyotype illustrated that normal endothelial cells are strictly diploid, whereas tumor endothelial cells (TECs) contain multiple chromosomal aneuploidies. Tumor-derived endothelial cells, which have the dual characteristics of malignant tumors and an endothelial vascularization ability, are endothelialized cancer cells. These cells enter the circulation from blood vessels to become CTECs, and this migration may play an important role in the formation of new blood vessels in metastatic tumors, thus having important clinical significance (30, 46, 47).
SE-iFISH is a detection technology that can identify target cells from peripheral blood cells by combining the detection of tumor marker expression on the surface of tumor cells and chromosomal aneuploidy. Therefore, comprehensive co-detection of aneuploid CTCs (CD31-) and CTECs (CD31+) using SE-iFISH was performed simultaneously in our study (25). In this way, high specificity (with respect to detecting CTCs/CTECs by iFISH) was ensured by distinguishing between aneuploidy and specific markers in the target cells. Based on this SE-iFISH system, we found that some CTC subtypes (triploid and multiploid small CTCs and tetraploid large CTCs) could be biomarkers for shorter OS; however, CTEC subtypes did not exhibit this characteristic.
Lin et al. hypothesized that aneuploid CTECs and CTCs cross-talk and influence each other in the circulation, leading to cancer metastasis and progression in some way (30). Lei et al. found that the combined detection of aneuploid CTECs and CTCs could be a good biomarker for the diagnosis of early-stage lung cancer (25). Therefore, we further investigated whether CTC and CTEC subtypes were related in patients with advanced lung cancer. According to our results, Pearson correlation tests confirmed significantly positive correlations in four groups, including triploid small cell size CTCs and monoploid/triploid small cell size CTECs, and multiploid small cell size CTCs and monoploid/triploid small cell size CTECs (P<0.0001). In addition, we found that the combined detection of three pairs of related CTC and CTEC subtypes (including triploid small CTC and monoploid small CTEC, triploid small CTC and triploid small CTEC, and multiploid small CTC and monoploid small CTEC) helped predict poor OS, even better than small CTC detection alone.
Recently, Lin reported that aneuploid TECs are generated from the “cancerization of stromal endothelial cells” and “endothelialization of carcinoma cells” in the hypoxic tumor microenvironment. Both of these processes are deeply involved in hypoxia-triggered epithelial-to-mesenchymal transition (EMT) and endothelial-to-mesenchymal transition (EndoMT) (30), which might be the reason why CTECs play important roles in predicting prognosis. Furthermore, CTECs are TECs that flow into the peripheral circulation, and CTCs have been widely reported to be associated with tumor EMT, which may explain why the numbers of CTCs and CTECs were closely correlated in our study (33, 34).
Unlike single detection of CTCs, synchronous and combined detection of CTCs and CTECs plays an important role in the evaluation of tumor prognosis. In addition, the combined detection of CTCs and CTECs also has research potential in clinical applications, such as tumor diagnosis and real-time monitoring of curative effect prediction and recurrence, which may provide effective technical support for individualized, accurate diagnosis and treatment of patients. However, the role of these aneuploid malignant cells in tumor formation and metastasis requires further study.
5 Conclusion
Triploid and multiploid small CTCs are good prognostic biomarkers for advanced lung cancer. Moreover, combined detection of small CTC and small CTEC heteroploid subtypes can predict OS in advanced lung cancer, and it showed better detection efficiency than that of individualized detection alone.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the medical ethics committee of Beijing Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JZ, YH, LW, YY, and JF contributed to conception and design of the study. Medical practitioners, WH, GT, DW, YW, LD, ZZ, YY provided patients and patient data for the study. JZ, YH, LW, and JF organized the database, and JZ, YH, LW, and JF performed the statistical analysis. JZ, YH, LW, and JF supported the literature, statistical methods and the detection method. JZ, YH, LW, and JF wrote the manuscript. JZ, YH, and LW provided correlated images. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Capital’s Funds for Health Improvement and Research (grant number 2020-2-2153).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bade BC, Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med (2020) 41:1–24. doi: 10.1016/j.ccm.2019.10.001
2. Blandin Knight S, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C. Progress and prospects of early detection in lung cancer. Open Biol 7 (2017) 7:170070. doi: 10.1098/rsob.170070
3. Henley SJ, Ward EM, Scott S, Ma J, Anderson RN, Firth AU, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer (2020) 126:2225–49. doi: 10.1002/cncr.32802
4. Wu R, Yu S, Ye J, Wang Y, Zhao Z, Liu H, et al. A multicenter retrospective study on the prognosis of stage III unresectable mutant non-small cell lung cancer with tyrosine kinase inhibitors therapy. Front Oncol (2021) 11:692703. doi: 10.3389/fonc.2021.692703
5. Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol (2017) 8:1–20. doi: 10.5306/wjco.v8.i1.1
6. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol (2016) 11:39–51. S. International Association for the Study of Lung Cancer, A.B. Prognostic Factors Committee, I. Participating, S. International Association for the Study of Lung Cancer, B. Prognostic Factors Committee Advisory and I. Participating. doi: 10.1016/j.jtho.2015.09.009
7. de Wit S, Rossi E, Weber S, Tamminga M, Manicone M, Swennenhuis JF, et al. Single tube liquid biopsy for advanced non-small cell lung cancer. Int J Cancer (2019) 144:3127–37. doi: 10.1002/ijc.32056
8. Pellini B, Szymanski J, Chin RI, Jones PA, Chaudhuri AA. Liquid biopsies using circulating tumor DNA in non-small cell lung cancer. Thorac Surg Clin (2020) 30:165–77. doi: 10.1016/j.thorsurg.2020.01.005
9. Mildner F, Sopper S, Amann A, Pircher A, Pall G, Kock S, et al. Systematic review: soluble immunological biomarkers in advanced non-small-cell lung cancer (NSCLC). Crit Rev Oncol Hematol (2020) 153:102948. doi: 10.1016/j.critrevonc.2020.102948
10. Hanafi AR, Jayusman AM, Alfasunu S, Sadewa AH, Pramono D, Heriyanto DS, et al. [Serum MiRNA as predictive and prognosis biomarker in advanced stage non-small cell lung cancer in Indonesia]. Zhongguo Fei Ai Za Zhi (2020) 23:321–32. doi: 10.3779/j.issn.1009-3419.2020.104.02
11. Huang D, Zhang F, Tao H, Zhang S, Ma J, Wang J, et al. Tumor mutation burden as a potential biomarker for PD-1/PD-L1 inhibition in advanced non-small cell lung cancer. Target Oncol (2020) 15:93–100. doi: 10.1007/s11523-020-00703-3
12. Wadowska K, Bil-Lula I, Trembecki L, Sliwinska-Mosson M. Genetic markers in lung cancer diagnosis: a review. Int J Mol Sci 21 (2020) 21:4569. doi: 10.3390/ijms21134569
13. Lim SB, Tan SJ, Lim WT, Lim CT. An extracellular matrix-related prognostic and predictive indicator for early-stage non-small cell lung cancer. Nat Commun (2017) 8:1734. doi: 10.1038/s41467-017-01430-6
14. Bednarz-Knoll N, Alix-Panabieres C, Pantel K. Plasticity of disseminating cancer cells in patients with epithelial malignancies. Cancer Metastasis Rev (2012) 31:673–87. doi: 10.1007/s10555-012-9370-z
15. Kong SL, Liu X, Suhaimi NM, Koh KJH, Hu M, Lee DYS, et al. Molecular characterization of circulating colorectal tumor cells defines genetic signatures for individualized cancer care. Oncotarget (2017) 8:68026–37. doi: 10.18632/oncotarget.19138
16. Lim SB, Yeo T, Lee WD, Bhagat AAS, Tan SJ, Tan DSW, et al. Addressing cellular heterogeneity in tumor and circulation for refined prognostication. Proc Natl Acad Sci U.S.A. (2019) 116:17957–62. doi: 10.1073/pnas.1907904116
17. Banko P, Lee SY, Nagygyorgy V, Zrinyi M, Chae CH, Cho DH, et al. Technologies for circulating tumor cell separation from whole blood. J Hematol Oncol (2019) 12:48. doi: 10.1186/s13045-019-0735-4
18. Goldkorn A, Tangen C, Plets M, Morrison GJ, Cunha A, Xu T, et al. Baseline circulating tumor cell count as a prognostic marker of PSA response and disease progression in metastatic castrate-sensitive prostate cancer (SWOG S1216). Clin Cancer Res (2021) 27:1967–73. doi: 10.1158/1078-0432.CCR-20-3587
19. Loreth D, Schuette M, Zinke J, Mohme M, Piffko A, Schneegans S, et al. CD74 and CD44 expression on CTCs in cancer patients with brain metastasis. Int J Mol Sci (2021) 22:6993. doi: 10.3390/ijms22136993
20. Najjar F, Alammar M, Al-Massarani G, Almalla N, Japawe A, Ikhtiar A. Circulating endothelial cells and microparticles as diagnostic and prognostic biomarkers in small-cell lung cancer. Lung Cancer (2018) 124:23–30. doi: 10.1016/j.lungcan.2018.06.033
21. Ren S, Ren XD, Guo LF, Qu XM, Shang MY, Dai XT, et al. Urine cell-free DNA as a promising biomarker for early detection of non-small cell lung cancer. J Clin Lab Anal (2020) 34:e23321. doi: 10.1002/jcla.23321
22. Ma G, Jiang Y, Liang M, Li J, Wang J, Mao X, et al. Dynamic monitoring of CD45-/CD31+/DAPI+ circulating endothelial cells aneuploid for chromosome 8 during neoadjuvant chemotherapy in locally advanced breast cancer. Ther Adv Med Oncol (2020) 12:1758835920918470. doi: 10.1177/1758835920918470
24. Mehran R, Nilsson M, Khajavi M, Du Z, Cascone T, Wu HK, et al. Tumor endothelial markers define novel subsets of cancer-specific circulating endothelial cells associated with antitumor efficacy. Cancer Res (2014) 74:2731–41. doi: 10.1158/0008-5472.CAN-13-2044
25. Lei Y, Sun N, Zhang G, Liu C, Lu Z, Huang J, et al. Combined detection of aneuploid circulating tumor-derived endothelial cells and circulating tumor cells may improve diagnosis of early stage non-small-cell lung cancer. Clin Transl Med (2020) 10:e128. doi: 10.1002/ctm2.128
26. Gordon DJ, Resio B, Pellman D. Causes and consequences of aneuploidy in cancer. Nat Rev Genet (2012) 13:189–203. doi: 10.1038/nrg3123
27. Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer (2005) 5:773–85. doi: 10.1038/nrc1714
28. Ye Z, Ding Y, Chen Z, Li Z, Ma S, Xu Z, et al. Detecting and phenotyping of aneuploid circulating tumor cells in patients with various malignancies. Cancer Biol Ther (2019) 20:546–51. doi: 10.1080/15384047.2018.1538000
29. Li Y, Zhang X, Ge S, Gao J, Gong J, Lu M, et al. Clinical significance of phenotyping and karyotyping of circulating tumor cells in patients with advanced gastric cancer. Oncotarget (2014) 5:6594–602. doi: 10.18632/oncotarget.2175
30. Lin PP. Aneuploid circulating tumor-derived endothelial cell (CTEC): a novel versatile player in tumor neovascularization and cancer metastasis. Cells (2020) 9:1539. doi: 10.3390/cells9061539
31. Zhang L, Zhang X, Liu Y, Zhang T, Wang Z, Gu M, et al. PD-L1(+) aneuploid circulating tumor endothelial cells (CTECs) exhibit resistance to the checkpoint blockade immunotherapy in advanced NSCLC patients. Cancer Lett (2020) 469:355–66. doi: 10.1016/j.canlet.2019.10.041
32. Hong Y, Si J, Zhang J, Xiong Y, Zhang J, Lin PP, et al. Small cell size circulating aneuploid cells as a biomarker of prognosis in resectable non-small cell lung cancer. Front Oncol (2021) 11:590952. doi: 10.3389/fonc.2021.590952
33. Ito H, Inoue H, Kimura S, Ohmori T, Ishikawa F, Gohda K, et al. Prognostic impact of the number of viable circulating cells with high telomerase activity in gastric cancer patients: a prospective study. Int J Oncol (2014) 45:227–34. doi: 10.3892/ijo.2014.2409
34. Wang Y, Liu Y, Zhang L, Tong L, Gao Y, Hu F, et al. Vimentin expression in circulating tumor cells (CTCs) associated with liver metastases predicts poor progression-free survival in patients with advanced lung cancer. J Cancer Res Clin Oncol (2019) 145:2911–20. doi: 10.1007/s00432-019-03040-9
35. Chen Y, Yuan J, Li Y, Li X, Yang Y, Li J, et al. Profiling heterogenous sizes of circulating tumor microemboli to track therapeutic resistance and prognosis in advanced gastric cancer. Hum Cell (2021) 34:1446–54. doi: 10.1007/s13577-021-00568-2
36. Chen JF, Ho H, Lichterman J, Lu YT, Zhang Y, Garcia MA, et al. Subclassification of prostate cancer circulating tumor cells by nuclear size reveals very small nuclear circulating tumor cells in patients with visceral metastases. Cancer (2015) 121:3240–51. doi: 10.1002/cncr.29455
37. Wang L, Li Y, Xu J, Zhang A, Wang X, Tang R, et al. Quantified postsurgical small cell size CTCs and EpCAM(+) circulating tumor stem cells with cytogenetic abnormalities in hepatocellular carcinoma patients determine cancer relapse. Cancer Lett (2018) 412:99–107. doi: 10.1016/j.canlet.2017.10.004
38. Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol (2011) 29:1556–63. doi: 10.1200/JCO.2010.28.7045
39. Juan O, Vidal J, Gisbert R, Munoz J, Macia S, Gomez-Codina J. Prognostic significance of circulating tumor cells in advanced non-small cell lung cancer patients treated with docetaxel and gemcitabine. Clin Transl Oncol (2014) 16:637–43. doi: 10.1007/s12094-013-1128-8
40. Ge F, Zhang H, Wang DD, Li L, Lin PP. Enhanced detection and comprehensive in situ phenotypic characterization of circulating and disseminated heteroploid epithelial and glioma tumor cells. Oncotarget (2015) 6:27049–64. doi: 10.18632/oncotarget.4819
41. Zhang J, Shi H, Jiang T, Liu Z, Lin PP, Chen N. Circulating tumor cells with karyotyping as a novel biomarker for diagnosis and treatment of nasopharyngeal carcinoma. BMC Cancer (2018) 18:1133. doi: 10.1186/s12885-018-5034-x
42. Cheng H, Wang S, Luan W, Ye X, Dou S, Tang Z, et al. Combined detection and subclass characteristics analysis of CTCs and CTECs by SE-iFISH in ovarian cancer. Chin J Cancer Res (2021) 33:256–70. doi: 10.21147/j.issn.1000-9604.2021.02.12
43. Hille C, Pantel K. Prostate cancer: circulating tumour cells in prostate cancer. Nat Rev Urol (2018) 15:265–6. doi: 10.1038/nrurol.2018.25
44. Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol (2012) 120:612–8. doi: 10.1097/AOG.0b013e318264f794
45. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70:7–30. doi: 10.3322/caac.21590
46. Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer (2006) 6:835–45. doi: 10.1038/nrc1971
Keywords: advanced lung cancer, circulting tumor cells, circulating tumor endothelial cells, prognosis, biomarker
Citation: Zhang J, Hong Y, Wang L, Hu W, Tian G, Wu D, Wang Y, Dai L, Zhang Z, Yang Y and Fang J (2023) Aneuploid subtypes of circulating tumor cells and circulating tumor-derived endothelial cells predict the overall survival of advanced lung cancer. Front. Oncol. 13:829054. doi: 10.3389/fonc.2023.829054
Received: 04 December 2021; Accepted: 18 April 2023;
Published: 05 May 2023.
Edited by:
Paul Takam Kamga, Université de Versailles Saint-Quentin-en-Yvelines, FranceReviewed by:
Harriet Wikman, University Medical Center Hamburg-Eppendorf, GermanySwee Jin Tan, Sysmex Asia Pacific Pte Ltd, Singapore
Copyright © 2023 Zhang, Hong, Wang, Hu, Tian, Wu, Wang, Dai, Zhang, Yang and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Fang, aiangela1982@pku.edu.cn
†These authors have contributed equally to this work and share first authorship
 Jie Zhang
Jie Zhang Yang Hong
Yang Hong Liang Wang3†
Liang Wang3† Guangming Tian
Guangming Tian Di Wu
Di Wu Yang Wang
Yang Wang Yue Yang
Yue Yang