- 1Division of Oncology, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 2Genitourinary Medical Oncology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 3Anschutz Cancer Center – GU Oncology Department, University of Colorado, Aurora, CO, United States
- 4University Hospitals Seidman Cancer Center, Case Western Reserve University, Cleveland, OH, United States
- 5Genitourinary Oncology, Banner MD Anderson Cancer Center, Gilbert, AZ, United States
- 6Department of Genitourinary Oncology, Moffitt Cancer Center, Tampa, FL, United States
- 7Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Cisplatin-based chemotherapy has been the standard of care for patients with locally advanced or metastatic urothelial cancer (la/mUC). Enfortumab vedotin, an antibody-drug conjugate directed to Nectin-4, and pembrolizumab, an immune checkpoint inhibitor, are two therapies that have individually provided a survival benefit in patients with la/mUC. The combination regimen of enfortumab vedotin plus pembrolizumab was evaluated in EV-302 (KEYNOTE-A39; NCT0422385), a phase 3 study that showed statistically significant and clinically meaningful improvement in overall survival, progression-free survival, and a key secondary endpoint of overall response rate versus chemotherapy. Based on these results and those from the EV-103 (KEYNOTE-869; NCT03288545) Dose Escalation cohort, Cohort A, and Cohort K, enfortumab vedotin plus pembrolizumab was granted approval from the US Food and Drug Administration for the treatment of adults with la/mUC. While guidelines and recommendations for the management of adverse events (AEs) have been developed for immune checkpoint inhibitor monotherapy and enfortumab vedotin monotherapy, additional guidance is needed for managing AEs that occur with enfortumab vedotin plus pembrolizumab. As monotherapies, enfortumab vedotin and pembrolizumab are both associated with some of the AEs observed with the combination, such as skin reactions, pneumonitis, and diarrhea, which may confound the attribution of the AE to a specific agent and thereby complicate clinical management. In this manuscript, we aim to provide recommendations for best practice for patient care and the management of AEs of clinical interest for patients with la/mUC receiving enfortumab vedotin plus pembrolizumab, including skin reactions, peripheral neuropathy, hyperglycemia, and pneumonitis. These recommendations were developed based on published guidelines, expert opinions, and the clinical experience of the authors, which include oncologist, advanced practice provider, nursing, and pharmacy perspectives. In addition, guidance on patient education and communication is provided. With vigilant monitoring, early detection, and prompt intervention of treatment-emergent AEs based on recommended approaches described herein, it is the authors’ experience that most AEs can be managed with supportive therapy and dose modification/interruptions, allowing patients to continue treatment.
1 Introduction
The incidence of urothelial cancer is increasing worldwide and patients with locally advanced or metastatic urothelial cancer (la/mUC) have a particularly poor prognosis (1–4) and a low 5-year survival rate (5). Although cisplatin-based chemotherapy has been the standard treatment of la/mUC for decades (6, 7), most patients will progress within 7 months, and long-term survival remains poor (median overall survival [OS] of ~16–19 months) (8–10); moreover, up to half of patients are ineligible for cisplatin (9, 10). For cisplatin-ineligible patients, a carboplatin-based regimen may be used; however, this regimen is associated with a lower median OS (~11–13 months) (10–13) and tolerability remains poor. Although avelumab maintenance has been shown to improve OS in patients who had not progressed following first-line platinum-containing chemotherapy (7, 14), emerging real-world evidence suggests that approximately half of patients treated with platinum-based chemotherapy are not eligible to receive avelumab maintenance, primarily due to disease progression or death (15).
For those patients ineligible for any platinum-based therapy, immune checkpoint inhibitor (ICI) monotherapy represents a viable treatment option (7); however, objective responses only occur in approximately 20–30% of patients unselected for programmed cell death-ligand 1 (PD-L1) expression (16, 17).
Enfortumab vedotin (EV) and pembrolizumab (Pembro) are two therapies that have individually shown OS benefit in patients with la/mUC (18–21). EV is an antibody-drug conjugate (ADC) directed to Nectin-4 and consists of a monoclonal antibody attached to the microtubule-disrupting agent monomethyl auristatin E (MMAE) via a protease cleavable linker (22), while Pembro is an ICI targeting programmed cell death protein 1 (PD-1) (23). Preclinical data have shown that EV induces immunogenic cell death, and that the combination of EV and a PD-1 inhibitor may enhance antitumor activity compared to each agent alone due to their distinct and complementary engagement of the immune system (24–26).
The safety and efficacy of EV in combination with Pembro (EV + Pembro) has been studied in a phase 1b/2, open-label, multi-cohort study (EV-103/KEYNOTE-869; NCT03288545) (25, 26) and an open-label, randomized phase 3 study (EV-302/KEYNOTE-A39; NCT04223856) (10). In both studies, patients received EV 1.25 mg/kg as a 30-minute intravenous (IV) infusion on days 1 and 8 of a 21-day cycle, followed by an IV infusion of Pembro 200 mg on day 1 of a 21-day cycle. In EV-103, a total of 121 cisplatin-ineligible patients were enrolled across three cohorts: Dose Escalation Cohort (n = 5), Cohort A (n = 40), and Cohort K (n = 76); the primary objectives were safety and overall response rate (ORR) (25, 26). EV + Pembro demonstrated an ORR of 68% (95% confidence interval [CI]: 58.7–76.0) with a generally manageable safety profile (22). In EV-302, a total of 886 patients with previously untreated la/mUC were randomized, with dual primary endpoints of OS and progression-free survival (PFS). EV + Pembro demonstrated statistically significant improvements in OS and PFS versus platinum-based chemotherapy in patients with previously untreated la/mUC, nearly doubling both median OS (31.5 months versus 16.1 months; P<0.00001) and median PFS (12.5 months versus 6.3 months; P<0.00001). EV + Pembro also significantly increased ORR over platinum-based chemotherapy (67.7% [29.1% complete response] versus 44.4% [12.5% complete response]; P<0.00001). Results were consistent regardless of cisplatin-eligibility, the presence of liver metastases, and PD-L1 expression (10), and the safety profile was consistent with that seen in EV-103 (10, 25–27). Based on the results from EV-302, in December 2023 the US Food and Drug Administration approved EV + Pembro for the treatment of adults with la/mUC (28) and was subsequently, added to the National Comprehensive Cancer Network as a preferred treatment option regardless of cisplatin eligibility (7).
Although recommendations have been developed for the management of treatment-emergent AEs from ICI monotherapy (29–32) and EV monotherapy (33, 34), limited guidance is available for managing AEs that occur with EV + Pembro. In particular, EV and Pembro both contribute to some of the AEs observed, which may confound the timely attribution and clinical management of the AE.
Here, we aim to provide best practice for the oncology team on patient care and management of AEs for patients with la/mUC receiving EV + Pembro with the intent of improving the patient experience and outcomes with this novel combination. These recommendations are based on published guidelines, expert opinion, and clinical experience from a diverse group of healthcare providers (HCPs).
2 Safety of EV + Pembro in EV-302
In EV-302, the median duration of exposure was 7 months (range: 0.3 to 31.9 months; median of 9 cycles) for EV and 8.5 months (range: 0.3 to 28.5 months; median of 11 cycles) for Pembro. Patients received EV + Pembro (or at least one agent in the case one was discontinued) for a median of 9.4 months (range, 0.3 to 31.9 months; median of 12 cycles) (10, 22, 23). The most common treatment-emergent AEs for EV + Pembro included rash (68%), peripheral neuropathy (67%), and fatigue (51%) (Table 1) (22). The most common grade 3–4 treatment-emergent AEs were rash (15%), peripheral neuropathy (8%), and fatigue (6%) (22). Fatal AEs occurred in 3.9% of patients; 0.9% were considered related to treatment with EV + Pembro (one patient each with multiple organ dysfunction syndrome, immune-mediated lung disease, diarrhea, and asthenia) (10, 22). Thirty-five percent of patients experienced an AE that led to permanent discontinuation of EV, while 73% and 42% of patients experienced an AE that led to dose interruption and dose reduction of EV, respectively (22). Twenty-seven percent of patients experienced an AE that led to permanent discontinuation of Pembro and 61% of patients experienced an AE that led to dose interruption of Pembro (23). Dose modifications due to AEs of clinical interest that occurred in patients who received EV + Pembro in EV-302 are shown in Supplementary Table 1.
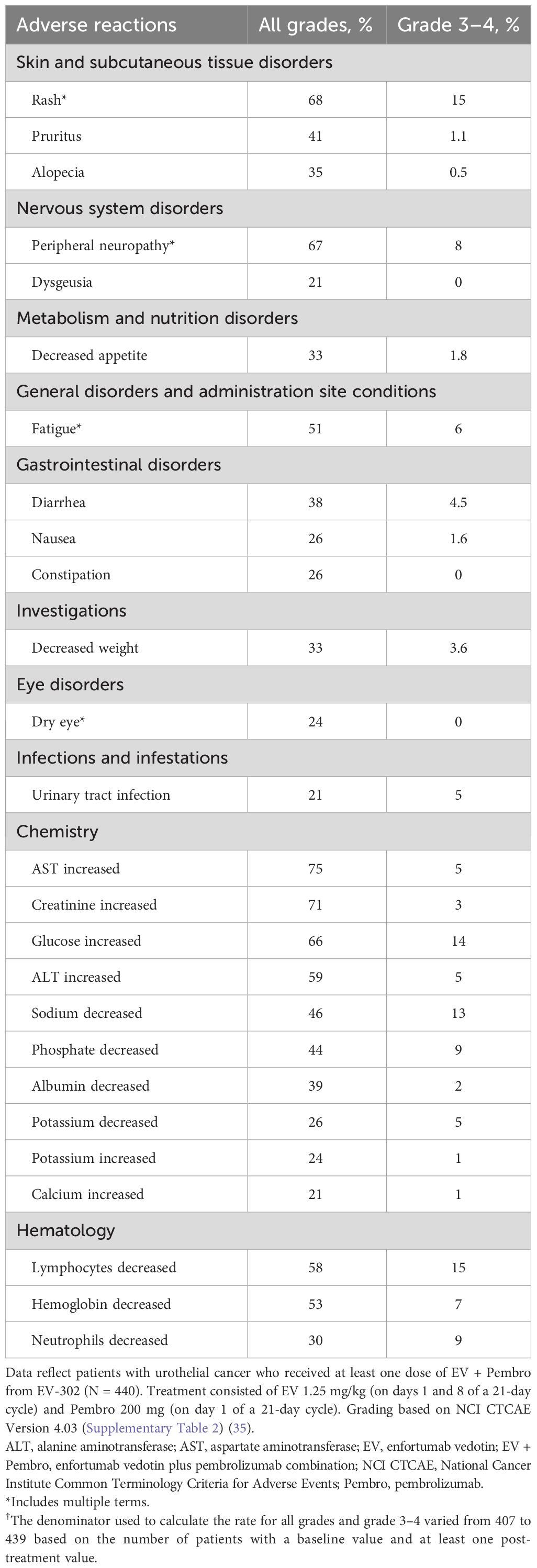
Table 1 Adverse reactions and selected laboratory abnormalities ≥20% (all grades) in patients treated with EV + Pembro in EV-302/KEYNOTE-A39 (22, 23).
3 General management of AEs with EV + Pembro
In the authors’ experience, most AEs associated with the use of EV + Pembro may be managed or mitigated via early recognition of signs or symptoms and prompt medical intervention and/or use of dose modification(s). Education on AEs associated with the use of EV + Pembro, close monitoring, and collaboration with the patient and their caregivers, as well as involvement of a multidisciplinary healthcare team, are important to aid in prompt intervention and management.
Clinicians should also be mindful of existing AE management guidelines and clinical recommendations for both EV and Pembro as monotherapies, which include the use of dose modifications. As with many combination regimens, dose modifications may include dose holds (cycle delays, skipped doses), discontinuation of one or both drugs, or dose reductions of EV (dose reductions are not recommended for Pembro) to help manage treatment-emergent AEs. EV and Pembro both contribute to some of the AEs observed, such as skin reactions, pneumonitis, and diarrhea, which may make AE attribution and management more complex (22, 23). In the EV-302 and EV-103 trials, the study protocol provided recommended dose modifications to help manage treatment-emergent AEs (10, 25, 26). As AE attribution to one agent or the other may be difficult to assess with the combination, based on authors’ clinical experience, when AE attribution is not possible or is not yet known, an appropriate dose modification should be applied to both agents. When AE attribution is possible, recommended dose modifications should be applied to the related agent as clinically appropriate (dose modifications for EV and dose holds for Pembro). Additional details are included in the Supplementary Appendix, including EV dose modifications for EV-associated AEs (Supplementary Table 3), EV dose reduction schedule and dose re-escalation recommendations (Supplementary Table 4), and Pembro dose modifications for Pembro-associated AEs (Supplementary Table 5). Critically, in all cases where patients experience rapid onset, severe clinical presentation (grade ≥3), and/or worsening symptoms despite mitigation strategies, both drugs should be withheld until appropriate clinical assessments can be completed, the patient has received appropriate supportive care, and the AE has improved to grade ≤1.
ICIs such as Pembro are associated with immune-related AEs (irAEs) caused by off-target activation of the immune system that can involve any organ or tissue, further highlighting the importance of close monitoring for potential irAEs in patients receiving EV + Pembro (23, 30–32). Involvement of the skin, gastrointestinal tract, lungs, endocrine system, and musculoskeletal systems are relatively common with irAEs, while cardiovascular, hematologic, renal, neurologic, and ophthalmologic irAEs occur less frequently though may be more severe (30, 36). In many cases, irAEs can be managed with dose interruptions and/or supportive therapy, which may include the use of corticosteroids and/or immunosuppressants (37). With Pembro, dose reduction is not recommended, and treatment should be withheld until the AE has improved (Supplementary Table 5; Supplementary Figure 1) (23). Although irAEs can occur early in treatment, onset may be delayed and duration is prolonged (31, 32, 37, 38). Conversely, many AEs associated with EV typically arise within the first few weeks after initiation, although some cases can present months later (22).
4 AEs of clinical interest for EV and/or Pembro
4.1 Skin reactions
Skin reactions occur with EV and Pembro monotherapies (22, 23, 34), and were shown to occur more frequently with the combination therapy (22). In the pooled safety population of 564 patients who received EV + Pembro in EV-302 and EV-103, skin reactions (all grades) occurred in 70% of patients (22). The majority of the skin reactions that occurred with combination therapy were maculopapular rash, macular rash, and papular rash. Grade 3–4 skin reactions occurred in 17% of patients (grade 3: 16%; grade 4: 1%). A fatal reaction of bullous dermatitis occurred in one patient (0.2%) (22). The median time of onset for grade 3–4 skin reactions was 1.7 months (range, 0.1 to 17.2 months); notably, events occurred as early as the first cycle (Figure 1) (22). Of the patients who experienced a skin reaction and had data regarding resolution (N = 391), 59% had complete resolution. Of the patients with an ongoing skin reaction, 27% (43/159) were grade ≥2 at last follow-up (22). At a median follow-up of 4 years in the EV-103 Dose Escalation Cohort/Cohort A (n = 45), 90% of patients experiencing a skin reaction had improvement or resolution of symptoms at the last follow-up (Supplementary Table 6) (27).
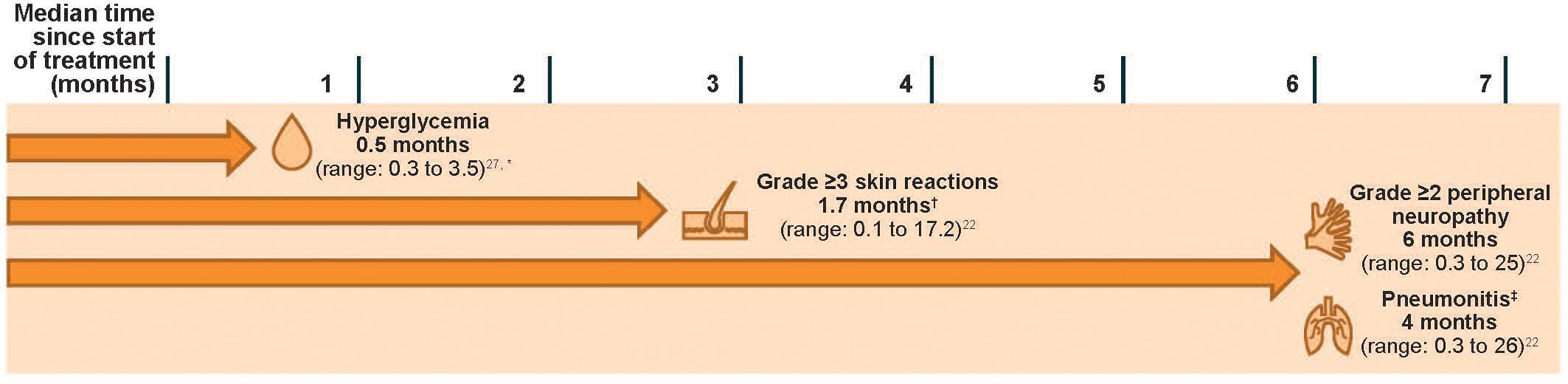
Figure 1 Median times to onset of select AEs in patients with la/mUC treated with EV + Pembro (N = 564). An AE may occur at any timepoint. Data reflect patients with urothelial cancer who received at least one dose of EV + Pembro from EV-302 and EV-103 (N = 564). Treatment consisted of EV 1.25 mg/kg (on days 1 and 8 of a 21-day cycle) and Pembro 200 mg (on day 1 of a 21-day cycle). Grading based on NCI CTCAE Version 4.03 (Supplementary Table 2) (35). AE, adverse event; EV, enfortumab vedotin; EV + Pembro, enfortumab vedotin plus pembrolizumab combination; la/mUC, locally advanced or metastatic urothelial cancer; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; Pembro, pembrolizumab. *Time to first onset for Dose Escalation Cohort/Cohort A (N = 45). Median onset for Cohort K was 0.53 months (N = 76) (26). †Skin reactions of any grade may occur as early as the first cycle. ‡The EV US prescribing information uses the term “pneumonitis/interstitial lung disease” (22).
Severe cutaneous adverse reactions such as Stevens–Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) have occurred with both EV and Pembro as monotherapies (22, 23). Therefore, it is important to educate patients about this potential risk and closely monitor patients for the emergence of any skin reaction, as SJS/TEN can be fatal. Patients should be instructed to immediately report the signs and symptoms of potential SJS/TEN, which include desquamating rash associated with malaise, fever ≥100.4°F (≥38°C), mucosal involvement (ocular, oral, genital), or dermatodynia (skin pain) (33).
4.1.1 Management
Risk factors and recommendations for the prevention, monitoring, and management of treatment-emergent skin reactions are shown in Figure 2. Although the presentation of EV-associated and Pembro-associated skin reactions can be similar, those associated with EV most commonly present in intertriginous, flexural, acral, and truncal areas, with skin that is often fragile, thin, and friable. Regular application of topical emollients, moisturizers, or barrier-protecting agents in these areas may be effective prophylaxis (33). EV-associated skin reactions can occur as early as the first cycle of treatment with EV (33) but may occur later. Conversely, Pembro-associated rashes often do not affect skin integrity, with maculopapular rashes and pruritus being the most frequent types associated with ICIs (30, 31). Skin reactions associated with ICIs may also occur within the first cycle (37), but in the authors’ collective experience these frequently occur later than those associated with EV. Clinical experience also shows that skin reactions that persist or worsen despite withholding both drugs are most likely immune-mediated, while EV-associated skin reactions may respond to dose holds more quickly as they are hypothesized to be due to direct cytotoxicity as a result of Nectin-4 expression in the skin (33, 39). Where attribution is unclear, dose holds of both agents should be considered. A thorough clinical exam, including inspection of the mucosa, and a biopsy can assist with diagnosing the type of skin reaction, including when reactions are refractory to both topical steroids and dose modifications, as well as identifying severe cutaneous adverse reactions such as SJS/TEN (33, 37, 39). Specialist referral is strongly recommended for skin reactions where diagnosis is unclear, those assessed as grade 3 or worse, those with associated blistering, or in instances where skin reactions are not responsive to topical steroids and/or dose modifications (22, 25, 26, 30).
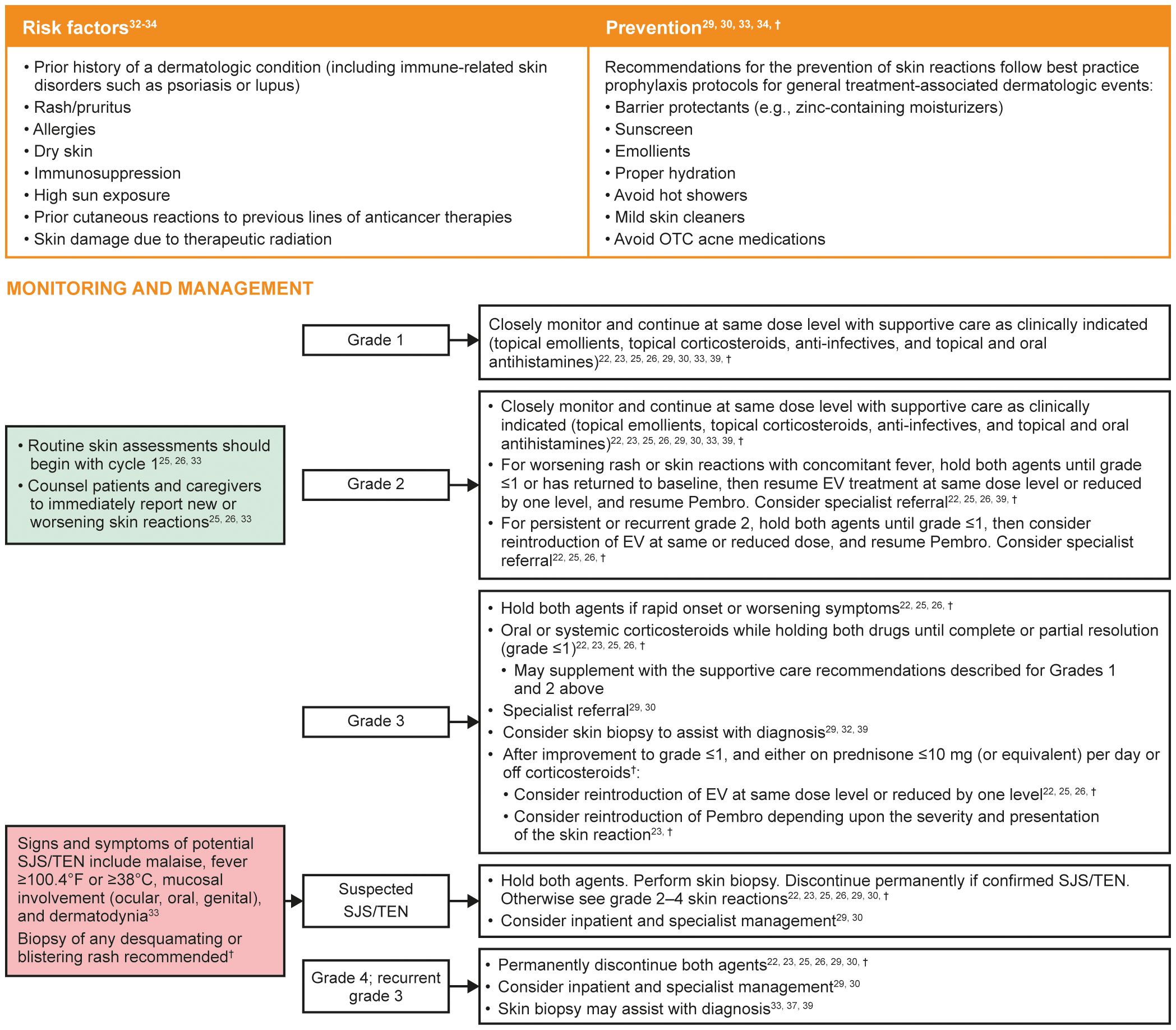
Figure 2 Risk factors and recommendations for the prevention, monitoring, and management of treatment-emergent skin reactions. Grading based on NCI CTCAE Version 4.03 (Supplementary Table 2) (35). EV, enfortumab vedotin; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; OTC, over the counter; Pembro, pembrolizumab; SJS, Stevens–Johnson syndrome; TEN, toxic epidermal necrolysis. †Recommendations based on clinical experience.
Topical emollients, topical corticosteroids, and antihistamines are often adequate to treat mild to moderate non-exfoliative rashes (22, 23). Patients should be monitored closely for signs of improvement or worsening. Due to its more rapid elimination compared with Pembro (22, 23), reintroduction of EV may be considered at the same dose or reduced dose level with close monitoring for recurrence depending on the severity and presentation of the skin reaction. Restarting Pembro at its recommended dose at the 3-week dosing interval (23) can be considered depending upon the severity and presentation of the skin reaction. In cases of rapid onset or worsening symptoms, both therapies must be withheld and oral or systemic corticosteroids should be given until the skin reaction improves to grade ≤1 or resolution occurs. Reintroduction of either drug may be considered once patients only require low-dose steroids (≤10 mg prednisone or equivalent daily) or are off steroids to avoid masking a “flare up” skin event. If SJS/TEN is suspected, both therapies must be withheld immediately, and specialist consultation should be considered to confirm the diagnosis. If SJS/TEN is confirmed, both EV and Pembro should be permanently discontinued (22, 23, 33).
4.2 Peripheral neuropathy
Peripheral neuropathy is an anticipated AE associated with MMAE-containing ADCs (19, 40), and immune-mediated neuropathies have been known to occur rarely with Pembro. In the pooled safety set of patients treated with EV + Pembro, peripheral neuropathy was the second most common AE, occurring in 67% of patients (grade 3: 7%) and was the most frequent reason for EV discontinuation (22, 23). Onset of grade ≥2 peripheral neuropathy generally occurred later in the treatment course, with a median time of onset of 6 months (range, 0.3 to 25 months; Figure 1). Of the patients who experienced neuropathy and had data regarding resolution (N = 373), 13% had complete resolution, and 87% of patients had residual neuropathy at last follow-up. Of the patients with residual neuropathy at last evaluation, 45% (146/326) had grade ≥2 neuropathy (22). At a median follow-up of 4 years, nearly 70% of patients who had treatment-related peripheral neuropathy in EV-103 Dose Escalation/Cohort A had improvement or resolution of their symptoms at their last follow-up (Supplementary Table 6) (25, 27). The median time to resolution of any-grade peripheral neuropathy was 5.2 months (interquartile range, 3.5 to 8.6 months) (25).
4.2.1 Management
Risk factors and recommendations for the prevention, monitoring, and management of treatment-emergent peripheral neuropathy are shown in Figure 3. Early recognition of treatment-emergent peripheral neuropathy and prompt intervention with appropriate use of dose modifications provides the best chance for resolution and may allow the patient to remain on therapy longer. As peripheral neuropathy is a cumulative AE, clinicians should be particularly aware of the potential for symptoms to develop as the duration of treatment increases. Patients may be reluctant to report signs and symptoms of peripheral neuropathy for fear of having their treatment interrupted or discontinued; however, patients should be educated that the use of dose modifications may help avoid worsening symptoms that could impact their activities of daily living or require treatment discontinuation. Patients should be informed that peripheral neuropathy can manifest as sensory and/or motor dysfunction, and any numbness and tingling of the hands or feet or muscle weakness should be reported quickly.
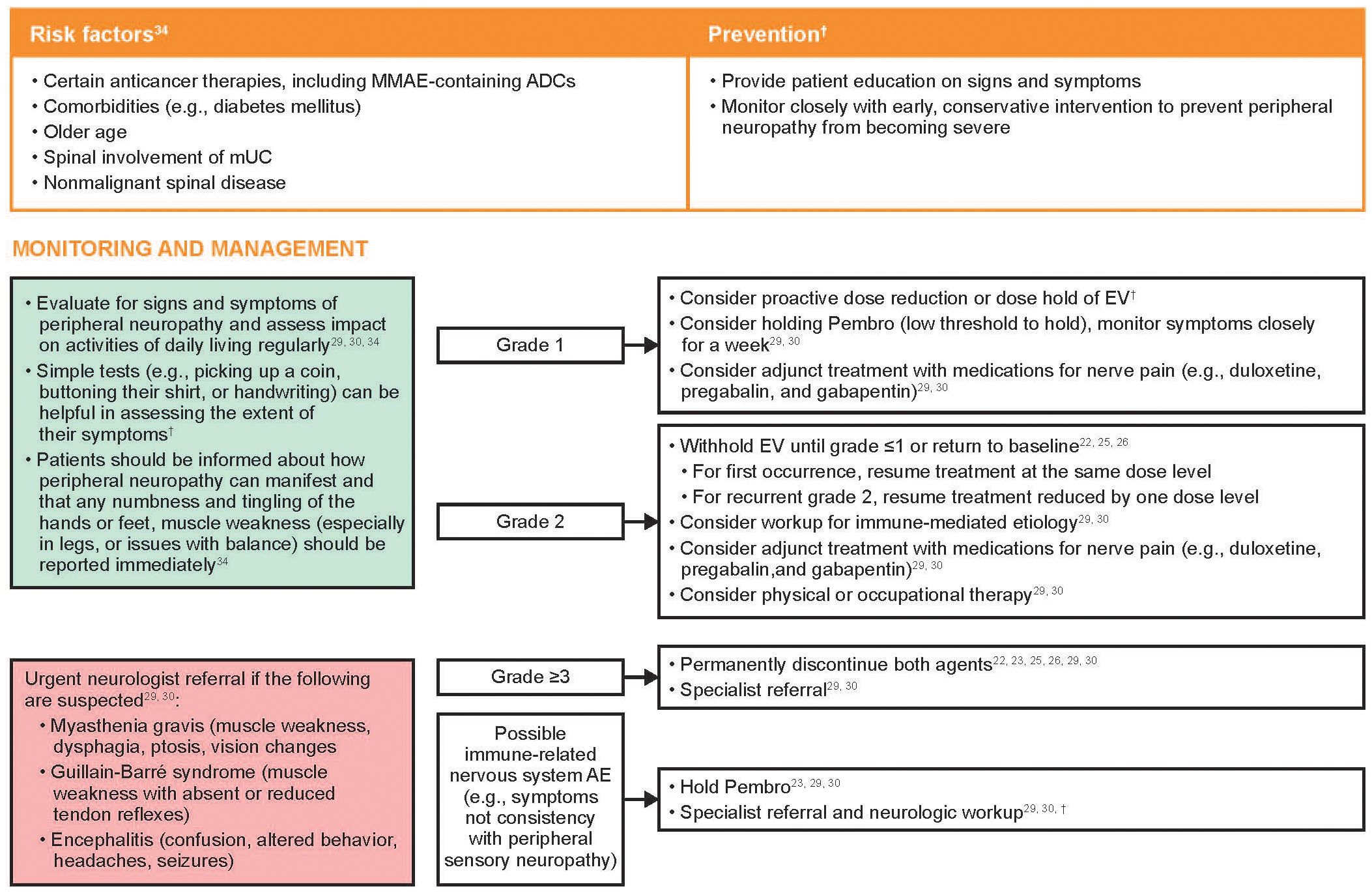
Figure 3 Risk factors and recommendations for the prevention, monitoring, and management of treatment-emergent peripheral neuropathy. Grading based on NCI CTCAE Version 4.03 (Supplementary Table 2) (35). ADC, antibody-drug conjugate; AE, adverse event; EV, enfortumab vedotin; MMAE, monomethyl auristatin E; mUC, metastatic urothelial cancer; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; Pembro, pembrolizumab. †Recommendations based on clinical experience.
Peripheral sensory neuropathy is most often attributed to EV, and generally responsive to recommended EV dose holds or dose reductions. If neurological symptoms not consistent with peripheral sensory neuropathy are observed, referral to a neurologist is recommended for further neurologic workup, which may include magnetic resonance imaging of the spine and/or brain, lumbar puncture for cerebrospinal fluid analysis, electromyography, and nerve conduction studies (41). Neurological symptoms that should prompt an urgent referral to a neurologist include muscle weakness and/or paralysis, vision changes, ptosis, dysphagia, photophobia, confusion, and speech abnormalities, as these may be signs of serious neurological conditions such as myasthenia gravis, Guillain-Barré syndrome, or encephalitis (30, 31).
Adjunct treatment with medications typically used to treat nerve pain, such as duloxetine, pregabalin, and gabapentin, may provide benefit to some patients with painful peripheral sensory neuropathy (30, 31, 41). These drugs may take several weeks to take effect and patients should be counseled that they may not provide immediate relief.
Peripheral motor neuropathy, including muscle weakness, may also occur. Thorough musculoskeletal assessments at each clinical visit should be performed, including functional evaluation of fine motor skills, gait, and balance. Interventions including physical and occupational therapy may be considered, and mechanical aids (e.g., braces) may be used to alleviate issues with loss of balance and coordination (34).
4.3 Hyperglycemia/diabetes mellitus
Hyperglycemia and diabetic ketoacidosis, including fatal events, occurred in patients treated with both EV and Pembro as monotherapies. Hyperglycemia or the development of insulin-dependent diabetes is a rare irAE of ICIs (1.8%) and is thought to be caused by the autoimmune destruction of islet cells, similar to the process seen in type 1 diabetes mellitus; remission or resolution of ICI-induced diabetes following this destruction is rare (42). In contrast, although the pathophysiology of EV-induced hyperglycemia is not well understood, it can resolve. In clinical trials with EV monotherapy, 17% of patients developed hyperglycemia of any grade, while discontinuation due to hyperglycemia was limited to <1% of patients (22). In EV-302, hyperglycemia of any grade occurred in 13.0% of patients (as compared with 14% with EV monotherapy and 0.2% with Pembro monotherapy); grade 3–4 hyperglycemia occurred in 8.9% of patients. Hyperglycemia presented at a median onset time of approximately 2 weeks with both EV as monotherapy and in combination with Pembro (Figure 1) (22, 23). In the EV-103 Dose Escalation Cohort/Cohort A (n = 45), all patients who experienced hyperglycemia had improvement or resolution of their hyperglycemia at their last follow-up, with a median time to resolution of 1.6 months (interquartile range, 0.7 to 1.6 months) (Supplementary Table 6) (25, 27). In this cohort, hyperglycemia occurred more frequently in patients with a body mass index of ≥30 kg/m2 or with baseline hyperglycemia or diabetes mellitus (25), a trend also observed in a study evaluating EV monotherapy (18, 22).
4.3.1 Management
Risk factors and recommendations for the prevention, monitoring, and management of treatment-emergent hyperglycemia are shown in Figure 4. Other etiologies for hyperglycemia, such as infection or systemic corticosteroids, should also be considered (23, 30). If there is evidence of ketosis or insulin insufficiency and/or resistance, urgent endocrine consultation is recommended; both agents should be withheld in the setting of ketosis or grade ≥3 hyperglycemia (29, 30). The development of autoimmune diabetes may be accompanied by reduced C-peptide levels as well as the presence of GAD65 and islet cell antibodies (31, 42), which would not be present with steroid use, infection, or EV-associated hyperglycemia. EV should be withheld if non-fasting blood glucose is >250 mg/dL; once the blood glucose has improved to ≤250 mg/dL and the patient is clinically and metabolically stable, EV can be resumed at the same level. Non-fasting blood glucose should be re-tested before each EV dose (22, 26).
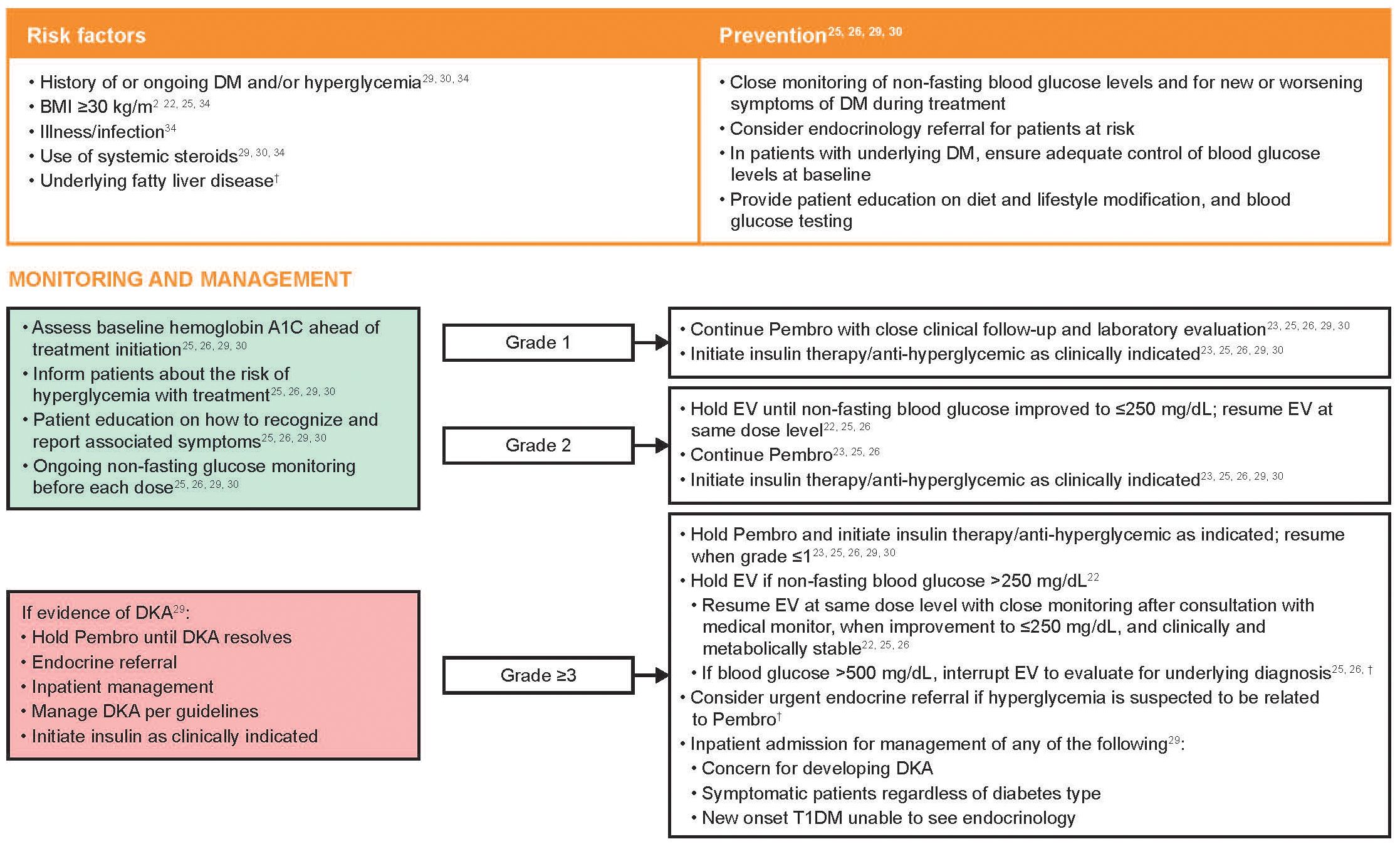
Figure 4 Risk factors and recommendations for the prevention, monitoring, and management of treatment-emergent hyperglycemia. Grading based on NCI CTCAE Version 4.03 (Supplementary Table 2) (35). BMI, body mass index; DKA, diabetic ketoacidosis; DM, diabetes mellitus; EV, enfortumab vedotin; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; Pembro, pembrolizumab; T1DM, type 1 diabetes mellitus. †Recommendations based on clinical experience.
4.4 Pneumonitis
Pneumonitis, including severe, life-threatening or fatal events, occurred in patients treated with both EV and Pembro as monotherapies (3% and 3.4%, respectively), and occurred at higher rates when given as combination therapy (22, 23). In the pooled safety population, pneumonitis occurred in 10% of patients treated with EV + Pembro (grade ≥3 in 4%; fatal in two patients [0.4%]). Median time to onset of any grade pneumonitis was 4 months (range, 0.3 to 26 months; Figure 1) (22).
4.4.1 Management
Risk factors and recommendations for the prevention, monitoring, and management of treatment-emergent pneumonitis are shown in Figure 5. In the event of symptomatic pneumonitis, both therapies should be immediately withheld. Use of corticosteroids, immunosuppressive agents, and supportive care should be provided as clinically indicated, and a referral to a pulmonary specialist should be considered. In the author’s experience, biopsy or bronchoscopy may be considered to rule out infection or other etiologies of respiratory symptoms, but the diagnostic evaluation should not significantly delay the prompt initiation of corticosteroids in a symptomatic patient. In patients with grade 2 pneumonitis, Pembro should be withheld until symptoms are partially or completely resolved (grade 1 or 0) after corticosteroid taper. EV should also be withheld until grade ≤1, at which point treatment can be resumed at the same dose level or reduced by one dose level. For patients in whom the causal agent is undetermined, once symptoms of pneumonitis resolve, EV should generally be reinitiated first along with close monitoring, followed later by Pembro. In patients with grade 3–4 pneumonitis, EV and Pembro should both be permanently discontinued. Radiographic evidence of improvement may lag weeks or months after clinical improvement is observed; therefore, radiographic improvement need not be a prerequisite for restarting treatment.
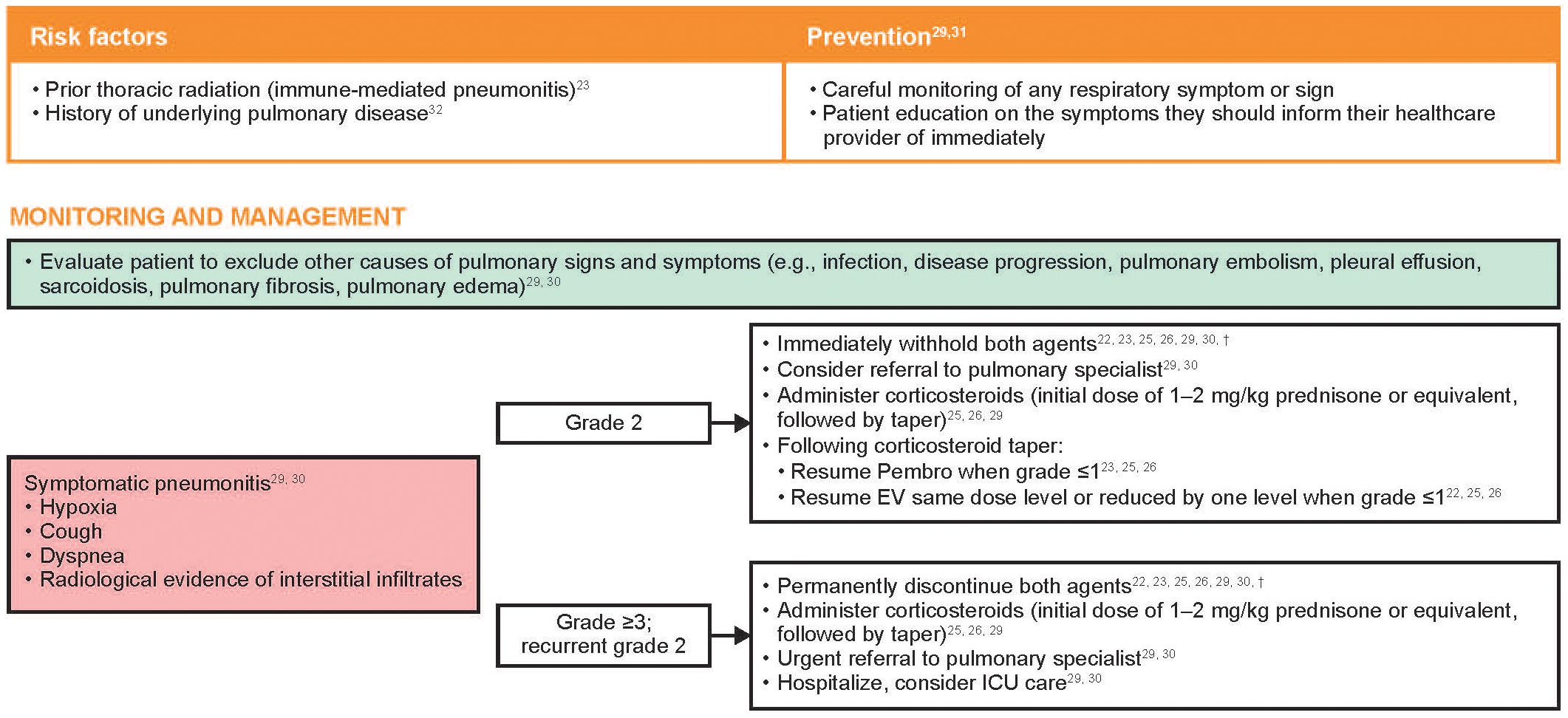
Figure 5 Risk factors and recommendations for the prevention, monitoring, and management of treatment-emergent pneumonitis*. Grading based on NCI CTCAE Version 4.03 (Supplementary Table 2) (35). EV, enfortumab vedotin; ICU, intensive care unit; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; Pembro, pembrolizumab. *The EV US prescribing information uses the term “pneumonitis/interstitial lung disease” (22). †Recommendations based on clinical experience.
4.5 Gastrointestinal events
Gastrointestinal events occurred frequently in patients treated with EV + Pembro in the EV-302 study, including diarrhea (38%), constipation (26%), nausea (26%), and dysgeusia (21%). While most events were mild in nature, 3.6% had severe diarrhea (grade ≥3) (22, 23). In EV-103 Cohort K, patients who received EV + Pembro reported treatment-emergent diarrhea that initially worsened at week 3 but resolved at week 8 and week 24 (43).
4.5.1 Management
Risk factors and recommendations for the prevention, monitoring, and management of treatment-emergent gastrointestinal events are shown in Table 2. As diarrhea is a known AE that occurs with both EV and immunotherapy, it is important to monitor for symptoms and assess fluid and electrolyte status (22, 29). Diarrhea in conjunction with abdominal pain, rectal bleeding, mucus in the stool, and fever should prompt a referral to a gastrointestinal specialist to rule out colitis, a potentially serious irAE (29, 30); steroids can be started empirically during work-up if clinically indicated. For uncomplicated diarrhea, oral hydration, dietary modifications, and over-the-counter anti-diarrheals/anti-emetics should be utilized for symptom management. Based on clinical experience, diarrhea that transiently worsens over the first few cycles then subsequently improves is likely attributed to EV. In contrast, diarrhea that persists and/or worsens over time is most likely immune-mediated and associated with Pembro. Diarrhea that does not respond to high-dose corticosteroids may require the use of immunosuppressive treatments. Stool studies may also be performed to rule out infection and test for inflammatory markers, including stool lactoferrin and calprotectin (29).
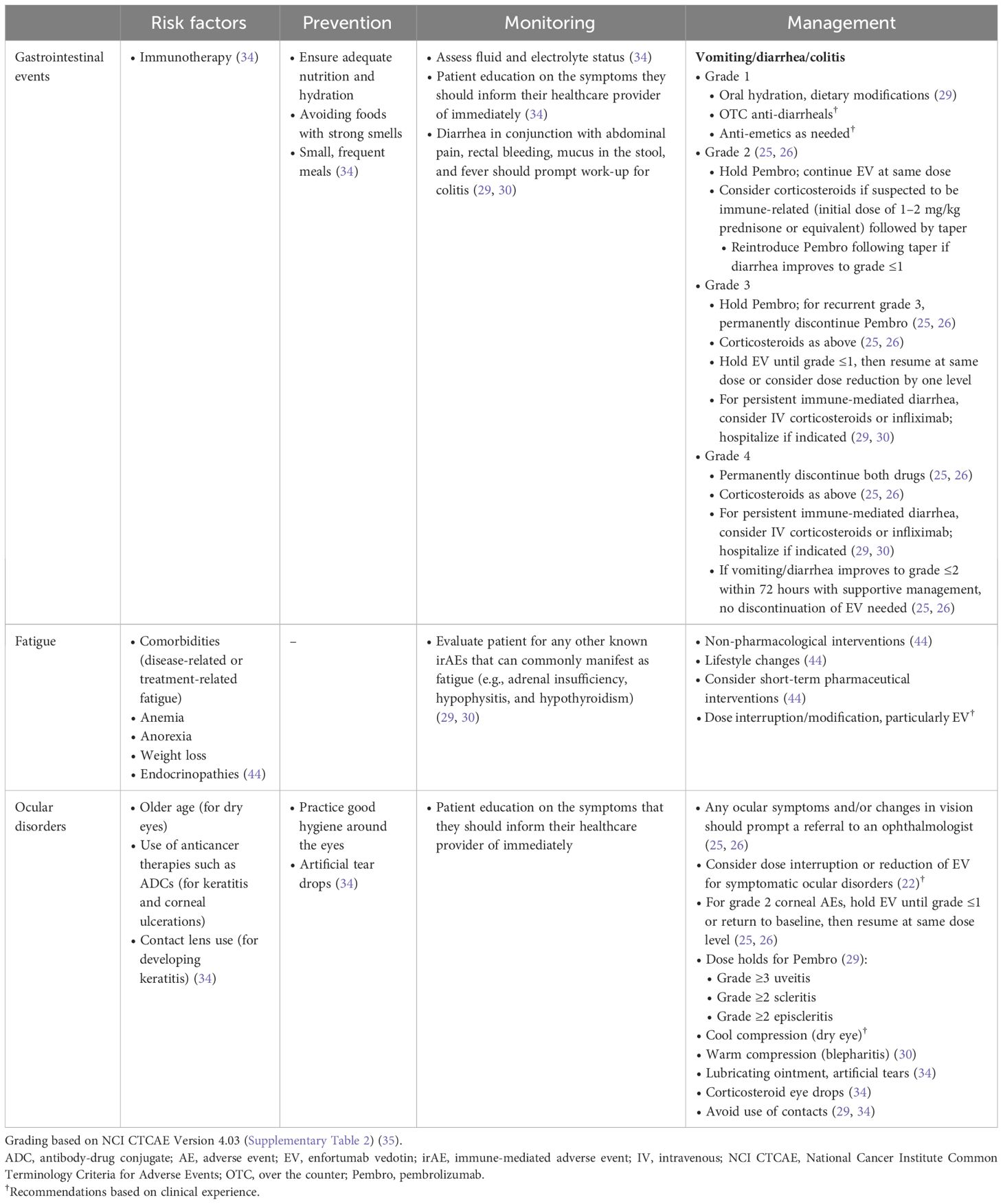
Table 2 Risk factors and recommendations for the prevention, monitoring, and management of other treatment-emergent AEs.
The recommended management of nausea and vomiting with the treatment combination is the same as for either EV or Pembro monotherapies, with prompt intervention and/or prophylaxis used to reduce the risk of complications associated with dehydration such as acute kidney failure and/or deterioration of poor pre-existing renal function.
4.6 Fatigue
Generalized fatigue is common in patients with cancer, which can be multifactorial (e.g., related to disease, treatment, anemia, anorexia, weight loss, endocrinopathy, or other factors) (44–47). In patients treated with EV + Pembro in EV-302, 51% of patients experienced fatigue, with 6% experiencing grade 3–4 fatigue (22, 23). In EV-103 Cohort K, patients who received EV + Pembro reported treatment-emergent fatigue that initially worsened at week 3 but resolved at week 8 and week 18 (43).
4.6.1 Management
Risk factors and recommendations for the prevention, monitoring, and management of treatment-emergent fatigue are shown in Table 2. Patient education and counseling are central to the effective management of fatigue (44–47). Patients should be reassured that treatment-related fatigue is not necessarily an indicator of disease progression (44). Fatigue can have a substantial impact on patients’ quality of life, therefore the approach to management should consider the patient holistically. Depending on the etiology of the fatigue, nonpharmacologic interventions may be beneficial. If diagnostic evaluation of fatigue indicates hypothyroidism and/or adrenal insufficiency as the cause (e.g., morning adrenocorticotropic hormone and corticotrophin-releasing hormone tests), suggesting an immune-mediated etiology, appropriate referral (e.g., endocrinology) and management should be initiated. In patients with grade ≥3 fatigue, EV should be withheld. EV may be reintroduced at the same dose or a reduced dose once symptoms improve to grade ≤1 (26). Consider withholding Pembro if the fatigue has not improved by the next cycle.
4.7 Ocular disorders
In patients treated with EV + Pembro in EV-302, the most common ocular disorder was dry eye (24%, as compared with 40% with EV monotherapy and <1% with ICIs) and was generally mild (22, 23, 31).
4.7.1 Management
Risk factors and recommendations for the prevention, monitoring, and management of treatment-emergent ocular reactions are shown in Table 2. Recommendations for symptomatic management may include cool compresses over closed eyes for dry eye, warm compresses for conjunctivitis or blepharitis, lubricating ointment or artificial tears, and corticosteroid eye drops as clinically indicated. Referral to an ophthalmologist is recommended for diagnosis and management of ocular disorders, when feasible. Consider dose interruption or dose reduction of EV for symptomatic ocular disorders (22). Dose holds for Pembro may be indicated in some cases (such as with grade ≥3 uveitis, grade ≥2 scleritis, and grade ≥2 episcleritis (29).
5 Patient education
In the authors’ experience, patients may be more forthcoming with information on their treatment experience when they understand that dose modifications are a normal part of receiving cancer treatment and were used in the clinical trials to manage AEs. Patients may minimize or ignore what they perceive to be mild reactions or withhold information out of concern that therapy may be interrupted or discontinued by their treating physician/team. Therefore, HCPs should emphasize to their patients that prompt management of AEs via dose modifications may allow them to stay on therapy longer. It is important to encourage patients and their caregivers to report anything out of the ordinary so that the care team can determine the seriousness of the AE and whether additional care may be needed. In addition, some patients may not proactively report issues being addressed by their primary care physician or other providers, highlighting the importance of encouraging the patient to follow up with their oncology care team after other visits.
Prior to initiating treatment with EV + Pembro, it is important to manage patient expectations through clear discussions of treatment benefits and risks as related to known potential AEs. Patient education should place emphasis on AEs of clinical interest, especially those that may occur at any time, may take time to resolve (e.g., peripheral sensory neuropathy), or may not resolve. The patient information documents for EV and for Pembro from the respective US prescribing information labels should be provided to patients as a resource (22, 23). Patients should be counselled to report any new and/or worsening symptoms to their care team to determine whether prompt intervention is necessary (Supplementary Table 7). Frequent, regular engagement and communication with patients and their caregivers are critical to enable timely identification and appropriate triage of AEs and coordinate care with other healthcare providers. Every visit where EV + Pembro is administered creates an opportunity to ask about and assess AEs (Supplementary Table 7). Mobile applications that can track side effects in real time, such as the ASCO Cancer.Net mobile application, can also be valuable tools for patient follow-up and detection of potentially more serious AEs (48).
Where possible, information should be conveyed both verbally and provided in written form. Patient education fact cards can be used to remind patients of potential AEs, self-care measures, medication information, scheduling, important contact information, and when to contact the care team. When warranted, oncologists should consider connecting patients with other specialists who may be able to help manage AEs, such as dermatologists, neurologists, gastroenterologists, endocrinologists, pulmonologists, or ophthalmologists. Patient advocacy groups, such as the Bladder Cancer Advocacy Network, also have extensive resources available for patients with bladder cancer.
6 Conclusions
Education on AEs associated with the use of EV + Pembro, proactive monitoring, assessment and management of AEs may help minimize toxicity and maximize clinical benefit and patient experience with EV + Pembro. A partnership between HCPs and patients and their caregivers, frequent communication, and upfront patient education are key in identifying emerging AEs. Factors such as the onset and characteristics of AEs and certain diagnostic steps may help the oncology care team determine attribution of AEs and appropriate clinical management, including dose modifications, supportive measures, and other interventions. As demonstrated in EV-302, EV + Pembro nearly doubled both median OS and median PFS in patients with previously untreated la/mUC regardless of cisplatin-eligibility and had a safety profile consistent with that observed in EV-103. Additional data from EV-302, as well as ongoing clinical trials in muscle invasive bladder cancer (EV-303/KN-905 [NCT03924895]; EV-304/KN-B15 [NCT04700124]), are expected to provide additional insight into best practices for minimizing toxicity and maximizing patient experience and clinical benefit with this novel combination.
Author contributions
BB: Writing – original draft, Writing – review & editing. AM: Writing – original draft, Writing – review & editing. HA: Writing – original draft, Writing – review & editing. CE: Writing – original draft, Writing – review & editing. IB: Writing – original draft, Writing – review & editing. JR: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Astellas Pharma US; Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; and Seagen, which was acquired by Pfizer in December 2023.
Acknowledgments
The authors would like to thank the patients, their families, and all investigators involved in this study. Expert dermatology review was provided by Drs. Allison Gordon and Alina Markova, both of Memorial Sloan Kettering Cancer Center, New York, NY, USA. Medical writing support was provided by Cindy Cheung, MBBS (MD) of Scion, London, UK, which was supported by Seagen Inc., which was acquired by Pfizer in December 2023 and Astellas Pharma Global Development, Inc. according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M15-0288).
Conflict of interest
The authors declare the following competing interests: BB: Consulting/Advisory: Seagen; AM: Consulting/Advisory: Seagen; CE: Consulting/Advisory: Astellas; Speaker's Bureau: Pfizer; IB: Consulting/Advisory: AstraZeneca, Aveo, Janssen; Speakers Bureau: Natera, Caris Life Sciences; JR: Consulting/Advisory: Astellas; Honoraria: Gilead Sciences; Other: Moffitt Cancer Center; MM: Stock and Other Ownership Interests: Pfizer, Merck, Gilead Sciences; Consulting or Advisory Role: Loxo/Lilly; Research Funding: Merck Inst, Roche/Genentech Inst, Bristol Myers Squibb Inst, Mirati Therapeutics Inst, Incyte Inst, Seagen Inst, G1 Therapeutics Inst, Alliance Foundation Trials Inst, Alliance for Clinical Trials in Oncology Inst, Clovis Oncology Inst, Arvinas Inst, ALX Oncology Inst, Loxo Inst, Hoosier Cancer Research Network Inst; Other Relationship: Elsevier, Medscape.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study received funding from Astellas Pharma US; Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; and Seagen Inc. The funder had the following involvement with the study: study design, collection, analysis and interpretation of data, as well as data checking of information provided in the manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1326715/full#supplementary-material
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
2. Richters A, Aben KKH, Kiemeney L. The global burden of urinary bladder cancer: an update. World J Urol. (2020) 38:1895–904. doi: 10.1007/s00345-019-02984-4
3. Wong MCS, Fung FDH, Leung C, Cheung WWL, Goggins WB, Ng CF. The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Sci Rep. (2018) 8:1129. doi: 10.1038/s41598-018-19199-z
4. Shah MV, McGovern A, Hepp Z. PCN108 - targeted literature review of the burden of illness in urothelial carcinoma. Value Health. (2018) 21:S32–3. doi: 10.1016/j.jval.2018.09.191
5. National Cancer Institute. SEER Cancer Stat Facts: Bladder Cancer (2024). Available online at: https://seer.cancer.gov/statfacts/html/urinb.html (Accessed January 17, 2024).
6. Koufopoulou M, Miranda PAP, Kazmierska P, Deshpande S, Gaitonde P. Clinical evidence for the first-line treatment of advanced urothelial carcinoma: Current paradigms and emerging treatment options. Cancer Treat Rev. (2020) 89:102072. doi: 10.1016/j.ctrv.2020.102072
7. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Bladder Cancer (2024). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/bladder_blocks.pdf (Accessed February 26, 2024).
8. van der Heijden MS, Sonpavde G, Powles T, Necchi A, Burotto M, Schenker M, et al. Nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N Engl J Med. (2023) 389:1778–89. doi: 10.1056/NEJMoa2309863
9. Morgans AK, Galsky MD, Wright P, Hepp Z, Chang N, Willmon CL, et al. Real-world treatment patterns and clinical outcomes with first-line therapy in patients with locally advanced/metastatic urothelial carcinoma by cisplatin-eligibility. Urol Oncol. (2023) 41:357 e11–57.e21. doi: 10.1016/j.urolonc.2023.03.012
10. Powles T, Perez-Valderrama B, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. (2024) 390(10):875–88. doi: 10.1056/NEJMoa2312117
11. Richters A, Boormans JL, van der Heijden MS, van der Heijden AG, Meijer RP, Mehra N, et al. Overall survival of patients receiving cisplatin or carboplatin for primary metastatic urothelial carcinoma of the bladder: A contemporary Dutch nationwide cohort study. Eur Urol Focus. (2022) 8:995–1002. doi: 10.1016/j.euf.2021.08.009
12. Bilen MA, Robinson SB, Schroeder A, Peng J, Kim R, Liu FX, et al. Clinical and economic outcomes in patients with metastatic urothelial carcinoma receiving first-line systemic treatment (the IMPACT UC I Study). Oncologist. (2023) 28:790–98. doi: 10.1093/oncolo/oyad174
13. Holmsten K, Jensen NV, Mouritsen LS, Jonsson E, Mellnert C, Agerbæk M, et al. Vinflunine/gemcitabine versus carboplatin/gemcitabine as first-line treatment in cisplatin-ineligible patients with advanced urothelial carcinoma: A randomised phase II trial (VINGEM). Eur J Cancer. (2020) 127:173–82. doi: 10.1016/j.ejca.2019.08.033
14. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. (2020) 383:1218–30. doi: 10.1056/NEJMoa2002788
15. Morgans AK, Powles T, Shih V, Wright P, Hepp Z, Chang N, et al. Real-world avelumab maintenance eligibility and treatment patterns in cisplatin-ineligible patients with locally advanced or metastatic urothelial carcinoma. San Antonio, TX, USA: Academy of Managed Care Pharmacy (AMCP) (2023).
16. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. (2017) 389:67–76. doi: 10.1016/S0140-6736(16)32455-2
17. Stenehjem DD, Tran D, Nkrumah MA, Gupta S. PD1/PDL1 inhibitors for the treatment of advanced urothelial bladder cancer. Onco Targets Ther. (2018) 11:5973–89. doi: 10.2147/OTT
18. Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Durán I, Lee JL, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. (2021) 384:1125–35. doi: 10.1056/NEJMoa2035807
19. Yu EY, Petrylak DP, O'Donnell PH, Lee JL, van der Heijden MS, Loriot Y, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV−201): a multicentre, single-arm, phase 2 trial. Lancet Oncol. (2021) 22:872–82. doi: 10.1016/S1470-2045(21)00094-2
20. Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. (2017) 18:1483–92. doi: 10.1016/S1470-2045(17)30616-2
21. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. (2017) 376:1015–26. doi: 10.1056/NEJMoa1613683
22. Seagen, Astellas Pharma US Inc. PADCEV (enfortumab vedotin-ejfv) for injection (2023). Available online at: https://astellas.us/docs/PADCEV_label.pdf (Accessed January 18, 2024).
23. Merck & Co. KEYTRUDA (pembrolizumab) injection (2024). Available online at: https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf (Accessed January 17, 2024).
24. Olson D, Younan P, Liu BA, Blahnik-Fagan G, Gosink J, Snead K, et al. 1187 Enfortumab vedotin induces immunogenic cell death, elicits antitumor immune memory, and shows enhanced preclinical activity in combination with immune checkpoint inhibitors. J Immunother Cancer. (2022) 10:A1231–A31.
25. Hoimes CJ, Flaig TW, Milowsky MI, Friedlander TW, Bilen MA, Gupta S, et al. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J Clin Oncol. (2023) 41:22–31. doi: 10.1200/JCO.22.01643
26. O'Donnell PH, Milowsky MI, Petrylak DP, Hoimes CJ, Flaig TW, Mar N, et al. Enfortumab vedotin with or without pembrolizumab in cisplatin-ineligible patients with previously untreated locally advanced or metastatic urothelial cancer. J Clin Oncol. (2023) 41:4107–17. doi: 10.1200/JCO.22.02887
27. Gupta S, Rosenberg J, McKay RR, Flaig TW, Petrylak DP, Hoimes CJ, et al. Study EV-103 dose escalation/cohort a: Long-term outcome of enfortumab vedotin + pembrolizumab in first-line (1l) cisplatin-ineligible locally advanced or metastatic urothelial carcinoma (la/mUC) with nearly 4 years of follow-up. Alexandria, VA: ASCO (2023). doi: 10.1200/JCO.2023.41.16_suppl.4505
28. U.S. Food and Drug Administration. FDA approves enfortumab vedotin-ejfv with pembrolizumab for locally advanced or metastatic urothelial cancer (2023). Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-enfortumab-vedotin-ejfv-pembrolizumab-locally-advanced-or-metastatic-urothelial-cancer (Accessed February 22 2024).
29. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Management of Immunotherapy-Related Toxicities (2023). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf (Accessed May 23, 2023).
30. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. (2018) 36:1714–68. doi: 10.1200/JOP.18.00005
31. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv119–iv42. doi: 10.1093/annonc/mdx225
32. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2021-002435
33. Lacouture ME, Patel AB, Rosenberg JE, O'Donnell PH. Management of dermatologic events associated with the Nectin-4-directed antibody-drug conjugate enfortumab vedotin. Oncologist. (2022) 27:e223–e32. doi: 10.1093/oncolo/oyac001
34. Pace A, Brower B, Conway D, Leis D. Enfortumab vedotin: Nursing perspectives on the management of adverse events in patients with locally advanced or metastatic urothelial carcinoma. Clin J Oncol Nurs. (2021) 25:E1–9. doi: 10.1188/21.CJON.E1-E9
35. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) (2010). Available online at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_4.03.xlsx (Accessed July 21, 2023).
36. Wu Z, Chen Q, Qu L, Li M, Wang L, Mir MC, et al. Adverse events of immune checkpoint inhibitors therapy for urologic cancer patients in clinical trials: A collaborative systematic review and meta-analysis. Eur Urol. (2022) 81:414–25. doi: 10.1016/j.eururo.2022.01.028
37. Morgado M, Placido A, Morgado S, Roque F. Management of the adverse effects of immune checkpoint inhibitors. Vaccines (Basel). (2020) 8:575. doi: 10.3390/vaccines8040575
38. Raschi E, Gatti M, Gelsomino F, Ardizzoni A, Poluzzi E, De Ponti F. Lessons to be learnt from real-world studies on immune-related adverse events with checkpoint inhibitors: A clinical perspective from pharmacovigilance. Target Oncol. (2020) 15:449–66. doi: 10.1007/s11523-020-00738-6
39. Ingen-Housz-Oro S, Elshot YS, Segura S, Marchand A, Pouessel D, Kluger N, et al. Skin toxicity of enfortumab vedotin: Proposal of a specific management algorithm. J Eur Acad Dermatol Venereol. (2024) 38:e99–e101. doi: 10.1111/jdv.19454
40. Rosenberg JE, O'Donnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol. (2019) 37:2592–600. doi: 10.1200/JCO.19.01140
41. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. (2021) 39:4073–126. doi: 10.1200/JCO.21.01440
42. Kotwal A, Haddox C, Block M, Kudva YC. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care. (2019) 7:e000591. doi: 10.1136/bmjdrc-2018-000591
43. Milowsky M, O'Donnell PH, Hoimes CJ, Petrylak DP, Flaig TW, Moon HH, et al. Patient-reported outcomes in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer treated with enfortumabvedotinalone or in combination with pembrolizumab in the Phase 1b/2 EV-103 Cohort K study. ASCO GU (2023). doi: 10.1200/JCO.2023.41.6_suppl.439
44. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Cancer-Related Fatigue (2023). Alexandria, VA. Available online at: https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf (Accessed May 22, 2023).
45. Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. (2014) 11:597–609. doi: 10.1038/nrclinonc.2014.127
46. Escalante CP, Manzullo EF. Cancer-related fatigue: the approach and treatment. J Gen Intern Med. (2009) 24 Suppl 2:S412–6. doi: 10.1007/s11606-009-1056-z
47. Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, et al. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol. (2020) 31:713–23. doi: 10.1016/j.annonc.2020.02.016
48. Cancer.net. Managing your care (2023). Available online at: https://www.cancer.net/navigating-cancer-care/managing-your-care.
Keywords: enfortumab vedotin, pembrolizumab, urothelial cancer, anticancer therapy, adverse events, immune-related adverse events, irAE
Citation: Brower B, McCoy A, Ahmad H, Eitman C, Bowman IA, Rembisz J and Milowsky MI (2024) Managing potential adverse events during treatment with enfortumab vedotin + pembrolizumab in patients with advanced urothelial cancer. Front. Oncol. 14:1326715. doi: 10.3389/fonc.2024.1326715
Received: 23 October 2023; Accepted: 25 March 2024;
Published: 22 April 2024.
Edited by:
Umang Swami, The University of Utah, United StatesReviewed by:
Takeshi Yuasa, Japanese Foundation for Cancer Research, JapanVadim S. Koshkin, University of California, San Francisco, United States
Copyright © 2024 Brower, McCoy, Ahmad, Eitman, Bowman, Rembisz and Milowsky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew I. Milowsky, matt_milowsky@med.unc.edu
 Blaine Brower1
Blaine Brower1 Asia McCoy
Asia McCoy Hiba Ahmad
Hiba Ahmad Cheryl Eitman
Cheryl Eitman I. Alex Bowman
I. Alex Bowman Jennifer Rembisz
Jennifer Rembisz Matthew I. Milowsky
Matthew I. Milowsky