- 1Department of Medical Oncology, The Affiliated Cancer Hospital of Nanjing Medical University & Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research, Nanjing, Jiangsu, China
- 2Huaian Hospital of Huaian City, Huaian Cancer Hospital, Huaian, Jiangsu, China
- 3Department of Oncology, Province Geriatric Hospital, Nanjing, Jiangsu, China
Metastatic triple-negative breast cancer (mTNBC) has the worst prognosis among breast cancer subtypes. Immune checkpoint inhibitors (ICIs) plus chemotherapy have promising survival benefits. Herein, we report a 51-year-old woman whose metastatic lesions were diagnosed as triple-negative subtype and who received tislelizumab plus eribulin treatment and achieved excellent efficacy. To our knowledge, this study is the first attempt to present tislelizumab in combination with eribulin for mTNBC treatment. New treatments resulting in prolonged survival and durable clinical responses would benefit mTNBC patients. Then, we summarize the possible influencing factors of the interaction between tislelizumab and eribulin.
Introduction
Breast cancer (BC) is the most prevalent tumor and the main cause of women’s deaths worldwide (1). Triple-negative breast cancer (TNBC), accounting for 15%–20% of all BC types, is defined as the absence of estrogen receptor (ER), progestogen receptor (PR), and human epidermal growth factor receptor 2 (HER2) (2) and has the worst prognosis (3). Because TNBC is insensitive to endocrine therapy and anti-HER2 therapy, till now, chemotherapy is still the standard treatment as the guidelines highly recommend.
In the past 10 years, immune checkpoint inhibitors (ICIs) achieved huge advances in cancer immunotherapy. Tislelizumab as a PD-1 inhibitor has a large overlap between the binding surface of PD-1 and PD-L1 and an extremely low dissociation rate compared to PD-1 (4, 5). This indicates that tislelizumab has higher targeting affinity and efficacy.
Eribulin mesylate (eribulin) is a microtubule dynamics inhibitor. Preclinical studies showed that eribulin could promote mitosis, reverse epithelial-to-mesenchymal transition, and induce vascular remodeling (6–10). Compared with other chemotherapy, eribulin significantly prolonged the median overall survival (OS) in the metastatic TNBC (mTNBC) subgroup (11, 12).
This case report presents an excellent response from a patient with mTNBC who received tislelizumab plus eribulin after the failure of first-line treatment.
Case presentation
A 51-year-old female patient presented to our cancer center with the complaint of a mass at the left breast in the upper outer quadrant since October 2018 (Figure 1). On June 17, 2019, this patient received left breast-conserving surgery and lymph node dissection in our hospital. Postoperative pathology test showed non-specific Grade II infiltrating ductal carcinoma, tumor size of 1 * 0.8 * 0.8 cm, and axillary lymph node metastasis (2/9). Immunohistochemical examination results showed ER (50%+), PR (30%+), HER2 (−), and Ki-67 (40%+) (Figures 2A, B). The patient was diagnosed with left HR+HER2− BC (pT1bN1aM0, stage IIA). Then, the patient received eight cycles of AC-T treatment, local radiotherapy, and endocrine therapy.
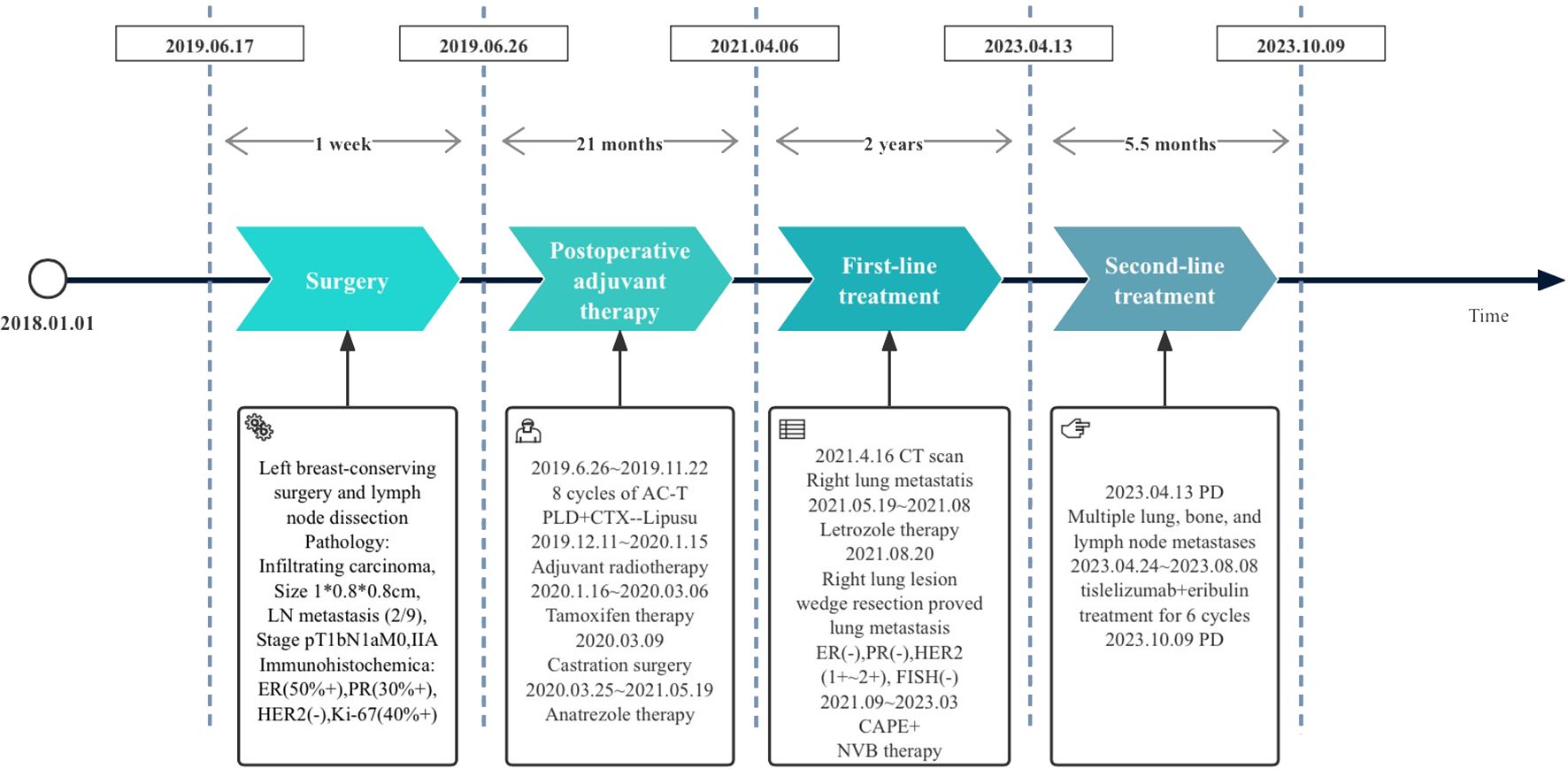
Figure 1 Treatment flowchart: PLD, pegylated liposomal doxorubicin; CTX, cyclophosphamide; Lipusu, paclitaxel liposome; CAPE, capecitabine; NVB, vinorelbine; PD, disease progression.
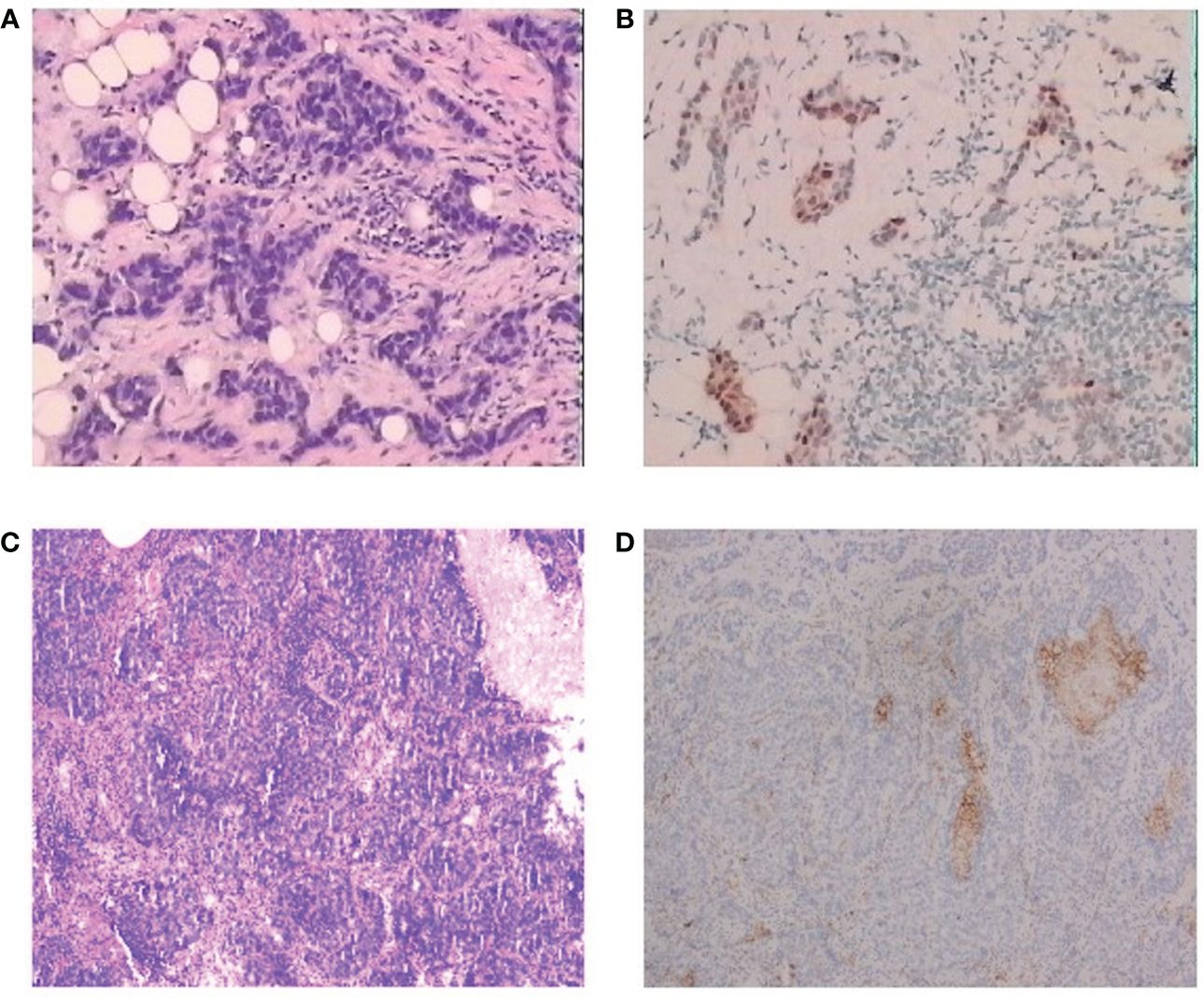
Figure 2 Pathological findings for the patient. (A) Hematoxylin and eosin staining of the tumor, diagnosed as invasive ductal carcinoma, H&E, ×100. (B) Immunohistochemistry of primary tumor, H&E, ×100. (C) Immunohistochemistry of lung metastasis, H&E, ×100. (D) Immunohistochemistry of PD-L1 of the lung metastasis, H&E, ×100.
On May 19, 2021, a right lung nodule was discovered; thus, endocrine therapy was changed to letrozole. Wedge resection of right lung lesions was performed on August 20, 2021. Postoperative pathology revealed metastatic lung lesions from breast cancer. Immunohistochemical examination results showed ER (−), PR (−), HER2 (1+~2+), and Ki-67 (60%+). Fluorescence in situ hybridization (FISH) detection showed no HER2 amplification. PD-L1 combined positive score (CPS) was 3 points (Figures 2C, D). The clinical diagnosis was mTNBC. From September 2021 to January 2022, the patient was given “capecitabine plus vinorelbine” treatment and then received capecitabine or vinorelbine maintenance therapy (Figure 3).
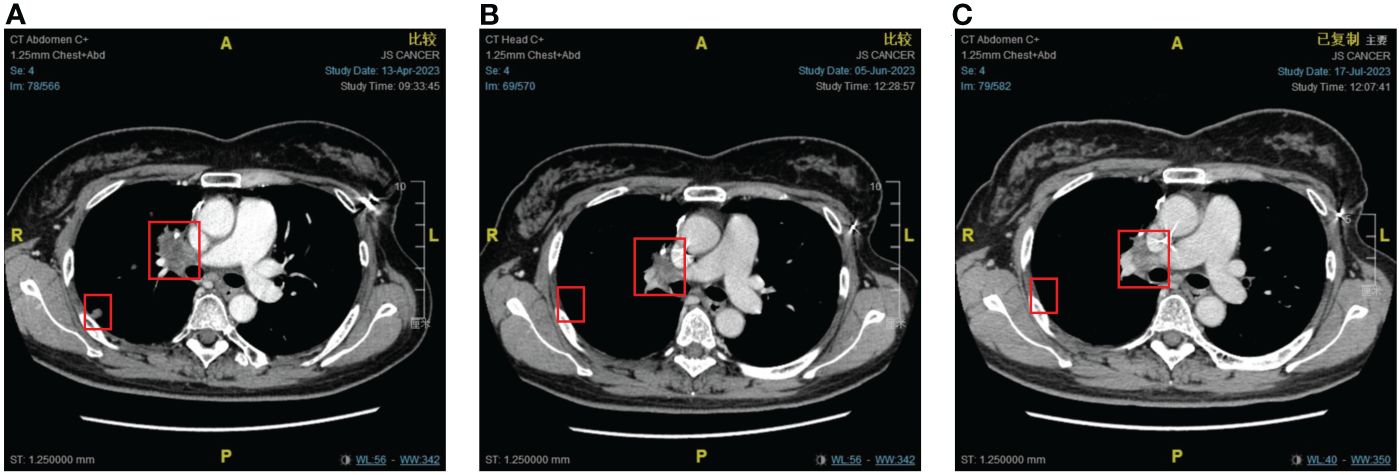
Figure 3 The response of lung metastases to immunotherapy combined with chemotherapy. (A) Before combination therapy. (B) After two cycles of treatment. (C) After four cycles of treatment.
An imaging examination on April 13, 2023, indicated progressive disease (PD) with multiple metastases all over her lungs, liver, bones, and lymph nodes. From April 24, 2023, to August 8, 2023, the patient received six cycles of “tislelizumab (200 mg d0/q3w) plus eribulin (2 mg d1,8/q3w)”. The latest CT scan was on July 17, 2023, and indicated that after four cycles of treatment, only one mass was left in the right lung (1.68 * 0.96 cm). The relief rate was more than 95% (Figures 3, 4). However, after six cycles of treatment, the patient developed new brain metastases. The progression-free survival (PFS) of this treatment was 5.5 months, and the lung lesions were almost absent.
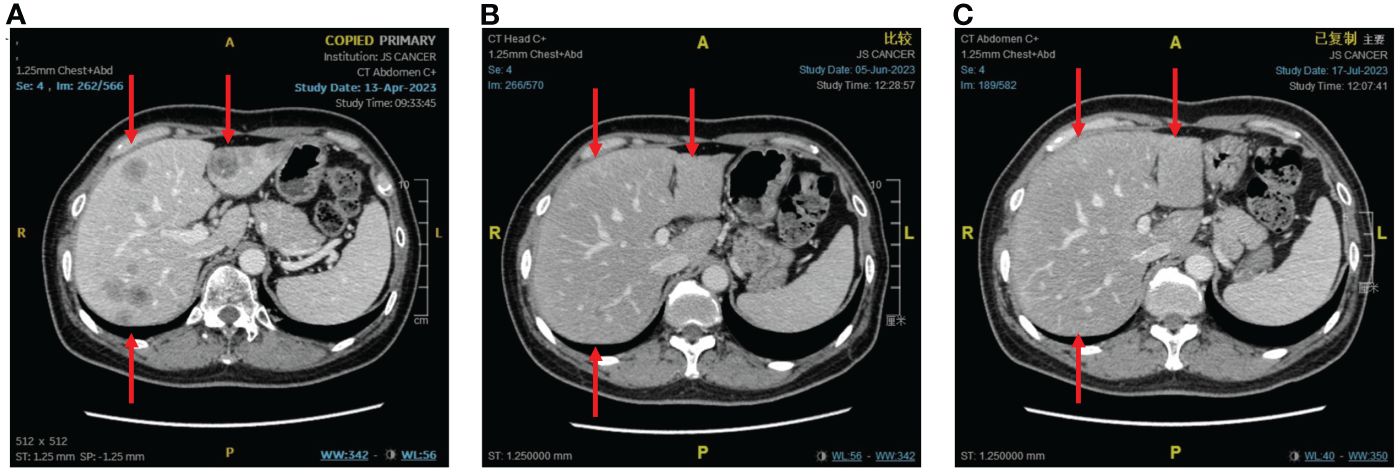
Figure 4 The response of liver metastases to immunotherapy combined with chemotherapy. (A) Before combination therapy. (B) After two cycles of treatment. (C) After four cycles of treatment.
We observed the changes in tumor biomarkers and found that with the progress of immunotherapy, the level of carbohydrate antigen 15-3 (CA15-3) significantly decreased, while the level of carbohydrate antigen 19-9 (CA19-9) achieved a certain extent increase and remained at a lower normal level. Both carbohydrate antigen 125 (CA125) and carcinoembryonic antigen (CEA) increased at first and then decreased (Figure 5). Tumor biomarkers were greatly affected by changes in the individual’s internal environment. Therefore, more clinical studies are needed to confirm whether the trend of changes in this case is a universal phenomenon and whether it has specific guiding significance for the selection of treatment plans and efficacy.
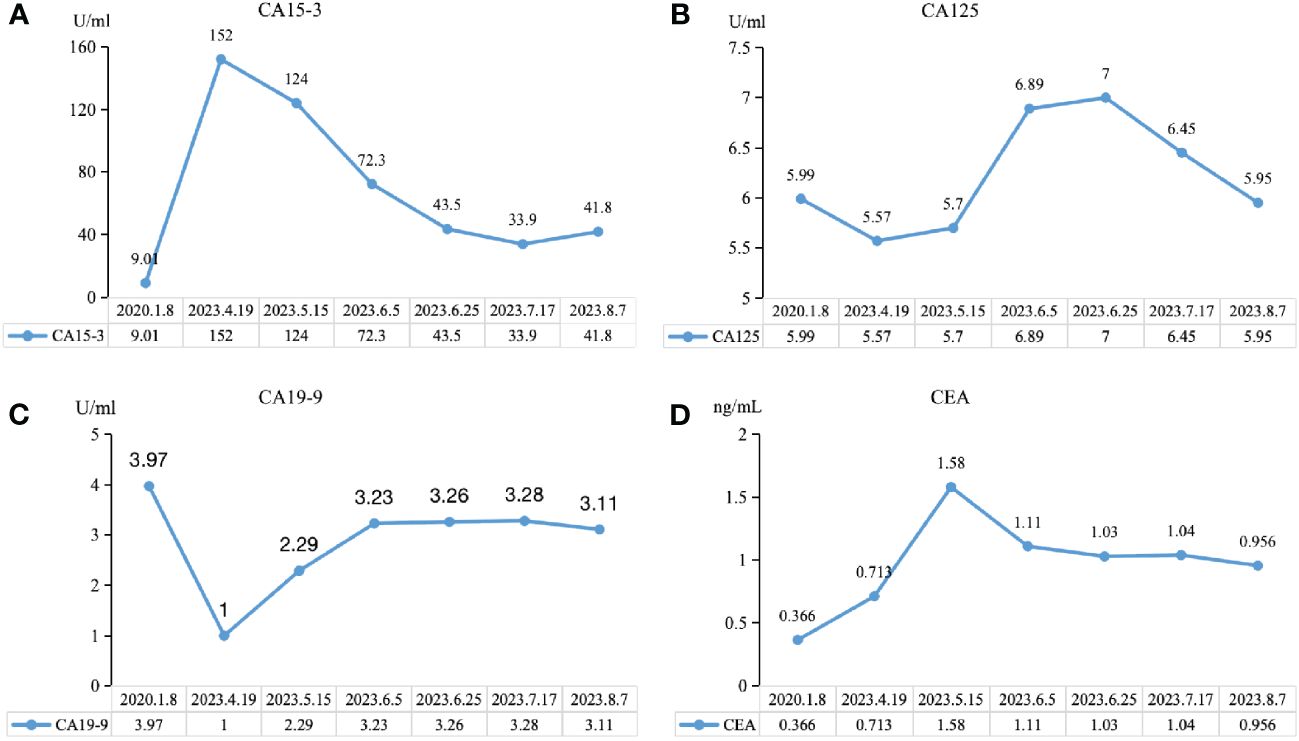
Figure 5 Trend chart of changes in tumor biomarkers before and after immunotherapy. (A) CA15-3, carbohydrate antigen 15-3. (B) CA19-9, carbohydrate antigen 19-9. (C) CA125, carbohydrate antigen 125. (D) CEA, carcinoembryonic antigen.
Discussion
In this case, after failures of chemotherapy and endocrine therapy, the patient showed an excellent response to “tislelizumab plus eribulin” treatment, suggesting that tislelizumab could be used effectively to treat mTNBC.
TNBC is insensitive to endocrine therapy and targeted therapy, and the typical treatment mode is still chemotherapy. The 5-year survival rate of patients with stage III–IV TNBC is 13%, significantly lower than that of other subtypes (13). After receiving chemotherapy, anti-HER2-targeted therapy, or endocrine comprehensive treatment, the median survival time of patients with advanced non-TNBC can be as long as 48–60 months, while the median survival time of patients with mTNBC is only 10–13 months (4, 14).
Previous studies indicated that TNBC has the highest expression of PD-L1 (approximately 40%) in both tumor cells and immune cells (2, 15–18), which can provide more targets for ICI and enhance the efficacy of immunotherapy (10, 19, 20). In the era of immune monotherapy clinical trials (21–23), only a fraction of patients could benefit from PD-1/PD-L1 inhibitor monotherapy (24). This may be due to the activities of ICIs relying on the interaction with T cells. PD-L1 binding to PD-1 may facilitate tumor immunity by suppressing cytotoxic T-cell responses (14). Higher levels of baseline T-cell infiltration are more likely to respond to ICI treatment (25, 26). In order to improve the clinical efficacy, tislelizumab is specifically engineered to minimize FcR binding on macrophages, thereby abrogating antibody-dependent cell-mediated phagocytosis (ADCP), ultimately avoiding T-cell depletion and enhancing its antitumor activity (5, 27, 28).
Tislelizumab, as a humanized immunoglobulin G4 (IgG4) PD-1 inhibitor, has a large overlap between the binding surface of PD-1 and PD-L1 and an extremely low dissociation rate compared with PD-1 (4, 5). PD-1 is one of the co-inhibitory immune checkpoint (ICP) receptors induced upon T-cell activation and widely expressed (70.3%) in tumor-infiltrating lymphocytes (TILs) (29). Of TNBC tumors, 21.9% comprise more than 50% of TILs (30). Studies of TNBC have shown a positive correlation between PD-1 expression in TILs and better clinical outcomes (31–33). The escape of tumor cells from T-cell killing was mediated by PD-1 by transducing negative signaling of effector T-cell activity by the interaction with PD-L1 (34, 35).
PD-1/PD-L1 inhibitors and chemotherapy may have synergistic effects in vivo (7, 11). Patients who received immunotherapy plus chemotherapy achieved higher OS and controllable adverse event risks (12, 20, 36). The combination of ICIs with chemotherapy has become a research hotspot to improve the clinical efficacy of ICIs.
The IMpassion130 trial proved that patients can benefit from atezolizumab combined with nab-paclitaxel, especially patients with PD-L1 expression (36). However, in IMpassion131, the immunotherapy subgroup obtained worse OS than the placebo subgroup (20). The conflicting results directly lead Roche to withdraw the indication of atezolizumab in mTNBC. Pembrolizumab plus chemotherapy significantly improved the PFS of mTNBC patients with PD-L1 positive score (CPS) ≥10 in the KEYNOTE-355 trial. Therefore, the 2023 Chinese Society of Clinical Oncology (CSCO) guidelines recommend PD-1 inhibitors combined with chemotherapy for mTNBC rescue treatment. In the ENHANCE1 trial, eribulin along with pembrolizumab was given to patients who had mTNBC and ≤2 prior systemic anticancer therapies in the metastatic setting. The results showed an objective response rate (ORR) of 23.4% (95% CI: 17.2–30.5), and the median OS of mTNBC patients who received “pembrolizumab or placebo plus eribulin” as first- to third-line therapy was 16.1 versus 11.1 months (3, 37). The ENHANCE1 study provides strong evidence supporting the application of eribulin combined with PD-1 inhibitors in mTNBC patients and the further clinical development of eribulin plus pembrolizumab as a potential antitumor strategy for mTNBC patients (Table 1).
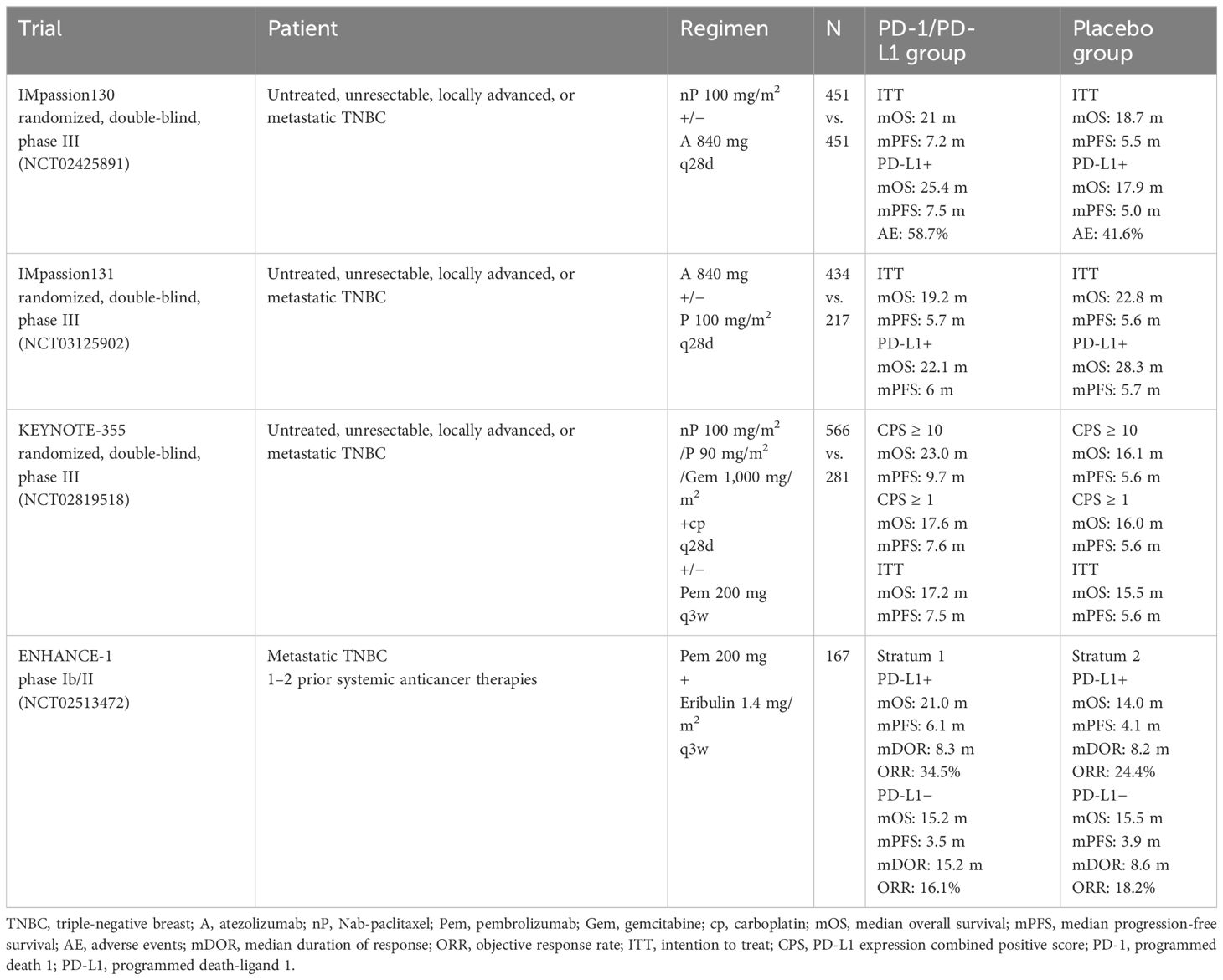
Table 1 Clinical trials of PD-1/PD-L1 checkpoint inhibitor therapy in metastatic triple-negative breast cancer.
The synergistic effect between tislelizumab and eribulin is currently only at the theoretical level, and more experiments are needed. Previous reports proved that eribulin in combination with anti-PD-1 antibody showed marked antitumor activity, while 4T1#31 tumors failed to respond to anti-PD-1 antibody. Therefore, eribulin may inhibit an immune evasion mechanism involving immune checkpoints. Eribulin has been observed to have immunomodulatory activity in vivo, and the function of inducing vascular remodeling may be the unique activity of eribulin. It was reported that eribulin induced reoxygenation by vascular remodeling to improve the hypoxic condition in mTNBC patients (10, 38). In addition, immunosuppressive factor transforming growth factorβ (TGF-β) was evaluated in terms of pSmad2 expression, which is typically associated with hypoxic conditions, and showed a decrease after eribulin treatment. TGF-β is crucial to epithelial-to-mesenchymal transition (EMT) and immune regulation, including the induction of regulatory T cells and the suppression of effector T cells. Eribulin can enhance the antitumor immune response by inhibiting the EMT and improving the immune microenvironment in breast cancer. Several retrospective studies proposed that eribulin promoted an antitumor immune response by increasing CD8+ T-cell and NK cell infiltration and inhibiting EMT (38, 39). However, CD8+ T cells prevent tumor progression by causing extracellular vesicle (EV)-mediated depletion of mesenchymal tumor within the tumor environment and stromal cells (40). The number of TILs has been reported as a predictive marker for eribulin responders (41). Eribulin activates interferon (IFN) signaling, and the activation of IFNγ signaling likely plays a key role in the increased infiltration of activated T cells and NK cells into tumors. Moreover, IFNγ also contributes to the efficacy of ICIs. Compared with those of non-responders, patients who respond to ICIs usually have higher expression scores of IFNγ-related genes (42). Therefore, activation of IFNγ signaling by eribulin could improve patients’ response to ICIs (39) (Figure 6).
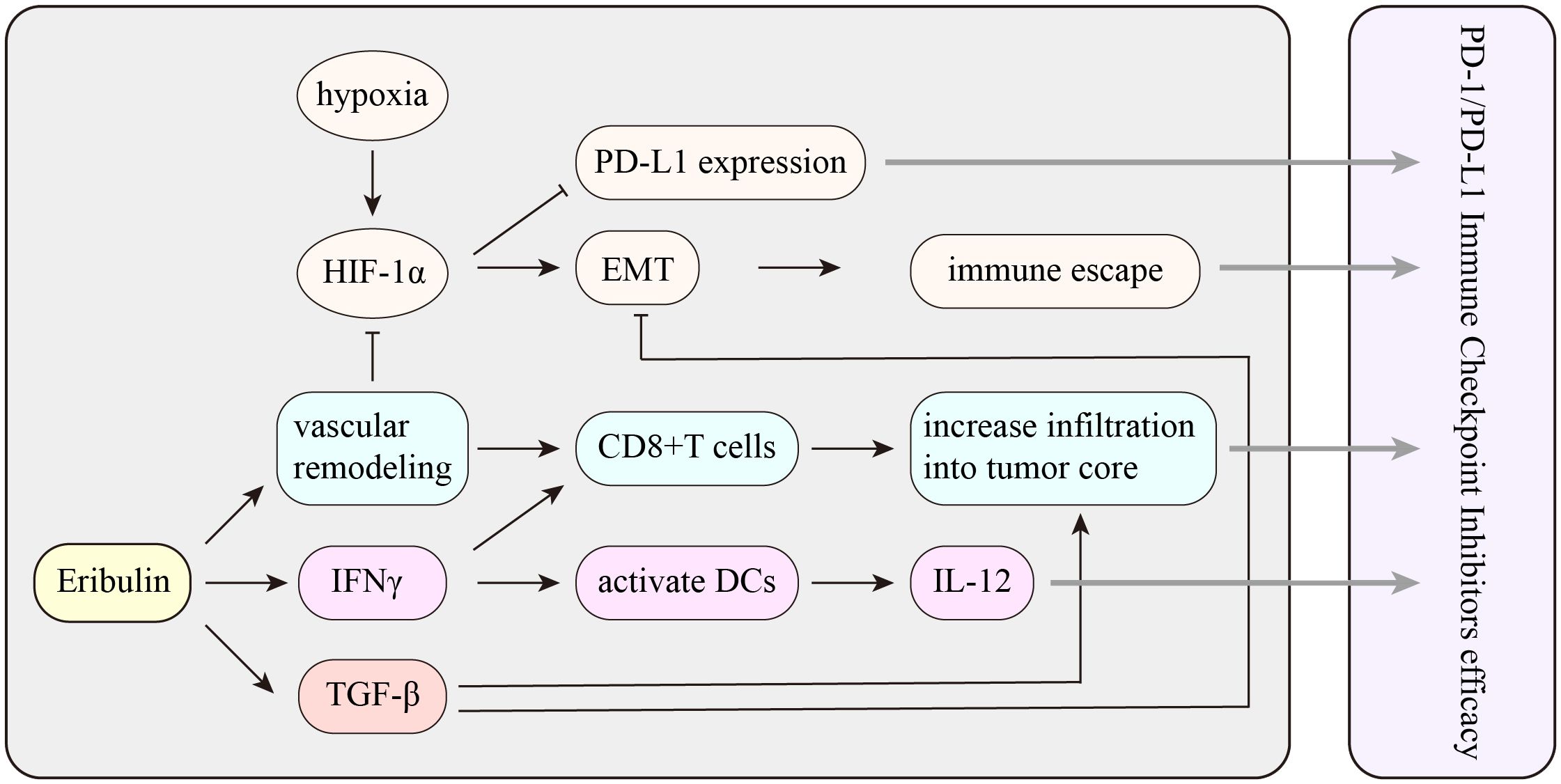
Figure 6 The potential mechanism of the interaction between eribulin and PD-1/PD-L1 immune checkpoint inhibitors. HIF-1α, hypoxia-inducible factor 1α; EMT, epithelial-to-mesenchymal transition; TGF-β, transforming growth factorβ; IFNγ, interferon γ.
Although tislelizumab has been observed to have good efficacy in several solid tumors (15, 43–45), its therapeutic efficacy in mTNBC has not been reported before. The patient reported in this case achieved great response to “tislelizumab plus eribulin” treatment, and no high grade of adverse event was observed, confirming the therapeutic potential of this regimen in mTNBC. Given that this is only a case report and the lack of long-term follow-up data, the clinical efficacy of this treatment regimen remains to be further confirmed by more clinical practice.
Conclusion
This case report is the first to describe that one mTNBC patient in our cancer center treated with “tislelizumab plus eribulin” after multi-line therapy achieved significant pathological remission. Tislelizumab plus eribulin seems to be a novel promising regimen in TNBC. However, eribulin may have a potential activity to enhance the efficacy of ICIs, and further studies are crucial to clarify the interactions between tislelizumab and eribulin in mTNBC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Jiangsu Cancer Hospital Affiliated with Jiangsu Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. XS: Conceptualization, Data curation, Investigation, Project administration, Writing – original draft. KZ: Data curation, Investigation, Project administration, Writing – original draft. SH: Project administration, Resources, Writing – original draft. YS: Data curation, Investigation, Writing – original draft. YJ: Data curation, Methodology, Writing – original draft. YL: Data curation, Investigation, Writing – original draft. LL: Data curation, Investigation, Writing – original draft. YC: Data curation, Investigation, Writing – original draft. FZ: Data curation, Investigation, Writing – original draft. YD: Data curation, Methodology, Writing – original draft. ZW: Data curation, Methodology, Writing – original draft. SL: Data curation, Methodology, Writing – original draft. ZQ: Conceptualization, Writing – review & editing. XX: Conceptualization, Writing – original draft. CL: Conceptualization, Writing – review & editing. BS: Conceptualization, Writing – review & editing. GZ: Conceptualization, Writing – review & editing. CC: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing. XW: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing. JF: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Science foundation item: (Y2018088, Z2021008).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: Cancer J Clin. (2023) . 73:17–48. doi: 10.3322/caac.21763
2. Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. (2016) . 13:674–90. doi: 10.1038/nrclinonc.2016.66
3. Li CH, Karantza V, Aktan G, Lala M. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review. Breast Cancer Res: BCR. (2019) . 21:143. doi: 10.1186/s13058-019-1210-4
4. Hong Y, Feng Y, Sun H, Zhang B, Wu H, Zhu Q, et al. Tislelizumab uniquely binds to the CC’ loop of PD-1 with slow-dissociated rate and complete PD-L1 blockage. FEBS Open Bio. (2021) 11(3):782–92. doi: 10.1002/2211-5463.13102
5. Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong W, et al. The binding of an anti-PD-1 antibody to FcγRι has a profound impact on its biological functions. Cancer Immunol Immunother. (2018) 67(7):1079–90. doi: 10.1007/s00262-018-2160-x
6. Niwa Y, Asano M, Nakagawa T, France D, Semba T, Funahashi Y. Antitumor activity of eribulin after fulvestrant plus CDK4/6 inhibitor in breast cancer patient-derived xenograft models. Anticancer Res. (2020) . 40:6699–712. doi: 10.21873/anticanres.14693
7. Yoshida T, Ozawa Y, Kimura T, Sato Y, Kuznetsov G, Xu S, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial–mesenchymal transition (EMT) to mesenchymal–epithelial transition (MET) states. Br J Cancer. (2014).
8. Funahashi Y, Okamoto K, Adachi Y, Semba T, Uesugi M, Ozawa Y, et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. (2014) 105:1334–42. doi: 10.1111/cas.12488
9. Dybdal-Hargreaves NF, Risinger AL, Mooberry SL. Eribulin mesylate: mechanism of action of a unique microtubule-targeting agent. Clin Cancer Res Off J Am Assoc Cancer Res. (2015) 21(11):2445–52. doi: 10.1158/1078-0432.CCR-14-3252
10. Ueda S, Saeki T, Takeuchi H, Shigekawa T, Yamane T, Kuji I, et al. In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: a comparison to bevacizumab. Br J Cancer. (2016) 114:1212–8. doi: 10.1038/bjc.2016.122
11. Twelves C, Awada A, Cortes J, Yelle L, Velikova G, Olivo MS, et al. Subgroup analyses from a phase 3, open-label, randomized study of eribulin mesylate versus capecitabine in pretreated patients with advanced or metastatic breast cancer. Breast Cancer Basic Clin Res. (2016) 10:77–84. doi: 10.4137/BCBCR.S39615
12. Twelves C, Cortes J, Vahdat L, Olivo M, He Y, Kaufman PA, et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat. (2014) 148:553–61. doi: 10.1007/s10549-014-3144-y
13. Bartsch R. ESMO 2020: highlights in breast cancer. Memo. (2021) . 14:184–7. doi: 10.1007/s12254-021-00713-5
14. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. (2002) . 99:12293–7. doi: 10.1073/pnas.192461099
15. Lu S, Wang J, Yu Y, Yu X, Wang M. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous non-small cell lung cancer (RATIONALE 304): A randomized phase 3 trial. J Thorac Oncol. (2021) 16(9):1512–22. doi: 10.1016/j.jtho.2021.05.005
16. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. (2018) . 379:2108–21. doi: 10.1056/NEJMoa1809615
17. Sikandar B, Qureshi MA, Naseem S, Khan S, Mirza T. Increased tumour infiltration of CD4+ and CD8+ T-lymphocytes in patients with triple negative breast cancer suggests susceptibility to immune therapy. Asian Pacific J Cancer Prevention: APJCP. (2017) . 18:1827–32. doi: 10.22034/apjcp.2017.18.7.1827
18. Li H, He Z, Yang Q, Wang J, Ling Y. Clinicopathologic features and prognosis of triple-negative breast cancer in China: a retrospective cohort study of patients under 35 years old. Int J Clin Exp Pathol. (2021) 14:24–33.
19. Donoghue M, Lemery SJ, Yuan W, He K, Sridhara R, Shord S, et al. Eribulin mesylate for the treatment of patients with refractory metastatic breast cancer: use of a “Physician’s choice” Control arm in a randomized approval trial. Clin Cancer Res. (2012) 18:1496–505. doi: 10.1158/1078-0432.CCR-11-2149
20. Jamiyan T, Kuroda H, Yamaguchi R, Nakazato Y, Noda S, Onozaki M, et al. Prognostic impact of a tumor-infiltrating lymphocyte subtype in triple negative cancer of the breast. Breast Cancer (Tokyo Japan). (2020) . 27:880–92. doi: 10.1007/s12282-020-01084-1
21. Adams SS, Rugo P, Winer HS, Loirat EP, Awada D, Cescon A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. (2019) 30(3):397–404. doi: 10.1093/annonc/mdy517
22. Schoffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, Choy E, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Oncologie. (2016) 18(9/10):562–4. doi: 10.1016/S0140-6736(15)01283-0
23. Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Twelves C. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. (2011) 377:914–23. doi: 10.1016/S0140-6736(11)60070-6
24. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Cancer Rev. (2015) 14(4):847–56. doi: 10.1158/1535-7163.MCT-14-0983
25. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. (2014) 515:563. doi: 10.1038/nature14011
26. Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. (2020) 52:17–35. doi: 10.1016/j.immuni.2019.12.011
27. Chen X, Song X, Li K, Zhang T. FcγR-binding is an important functional attribute for immune checkpoint antibodies in cancer immunotherapy. Front Immunol. (2019) 10. doi: 10.3389/fimmu.2019.00292
28. Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ. FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell. (2015) 28(3):285–95. doi: 10.1016/j.ccell.2015.09.011
29. Ren X, Wu H, Lu J, Zhang Y, Luo Y, Xu Q, et al. PD1 protein expression in tumor infiltrated lymphocytes rather than PDL1 in tumor cells predicts survival in triple-negative breast cancer. Cancer Biol Ther. (2018) . 19:373–80. doi: 10.1080/15384047.2018.1423919
30. Pruneri G, Vingiani A, Bagnardi V, Rotmensz N, De Rose A, Palazzo A, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. (2016) . 27:249–56. doi: 10.1093/annonc/mdv571
31. Fernandez-Poma SM, Salas-Benito D, Lozano T, Casares N, Riezu-Boj JI, Mancheño U, et al. Expansion of tumor-infiltrating CD8(+) T cells expressing PD-1 improves the efficacy of adoptive T-cell therapy. Cancer Res. (2017) . 77:3672–84. doi: 10.1158/0008-5472.Can-17-0236
32. Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest. (2014) . 124:2246–59. doi: 10.1172/jci73639
33. Datar I, Sanmamed MF, Wang J, Henick BS, Choi J, Badri T, et al. Expression analysis and significance of PD-1, LAG-3, and TIM-3 in human non-small cell lung cancer using spatially resolved and multiparametric single-cell analysis. Clin Cancer Res. (2019) . 25:4663–73. doi: 10.1158/1078-0432.Ccr-18-4142
34. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. (2002) . 8:793–800. doi: 10.1038/nm730
35. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. (2000) . 192:1027–34. doi: 10.1084/jem.192.7.1027
36. Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2020) . 21:44–59. doi: 10.1016/s1470-2045(19)30689-8
37. Tolaney SM, Kalinsky K, Kaklamani VG, D’Adamo DR, Aktan G, Tsai ML, et al. Eribulin plus pembrolizumab in patients with metastatic triple-negative breast cancer (ENHANCE 1): A phase ib/II study. Clin Cancer Res. (2021) . 27:3061–8. doi: 10.1158/1078-0432.CCR-20-4726
38. Kashiwagi S, Asano Y, Goto W, Takada K, Takahashi K, Hatano T, et al. Mesenchymal-epithelial transition and tumor vascular remodeling in eribulin chemotherapy for breast cancer. Anticancer Res. (2018) . 38:401–10. doi: 10.21873/anticanres.12236
39. Niwa Y, Adachi K, Tabata K, Ishida R, Hotta K, Ishida T, et al. Liposome-encapsulated eribulin shows enhanced antitumor activity over eribulin for combination therapy with anti-PD-1 antibody. Mol Cancer Ther. (2023) . 22:499–510. doi: 10.1158/1535-7163.Mct-22-0475
40. Seo N, Shirakura Y, Tahara Y, Momose F, Harada N, Ikeda H, et al. Activated CD8(+) T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat Commun. (2018) . 9:435. doi: 10.1038/s41467-018-02865-1
41. Kashiwagi S, Asano Y, Goto W, Takada K, Takahashi K, Noda S, et al. Use of Tumor-infiltrating lymphocytes (TILs) to predict the treatment response to eribulin chemotherapy in breast cancer. PloS One. (2017) . 12:e0170634. doi: 10.1371/journal.pone.0170634
42. Jorgovanovic D, Song M, Wang L, Zhang Y. Roles of IFN-γ in tumor progression and regression: a review. biomark Res. (2020) . 8:49. doi: 10.1186/s40364-020-00228-x
43. Xu J, Bai Y, Xu N, Li E, Wang B, Wang J, et al. Tislelizumab plus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma and gastric/gastroesophageal junction adenocarcinoma. Clin Cancer Res. (2020) 26(17):4542–50. doi: 10.1158/1078-0432.CCR-19-3561
44. Wang J, Lu S, Yu X, Hu Y, Yang N. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non–small-cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol. (2021) 7(5):709–17. doi: 10.1001/jamaoncol.2021.0366
Keywords: metastatic triple negative breast cancer (mTNBC), immune checkpoint inhibitors (ICIS), tislelizumab, eribulin mesylate, case report
Citation: Ma Y, Shi X, Zhao K, Hu S, Shi Y, Jiang Y, Liu Y, Lu L, Chang Y, Zhou F, Dai Y, Wu Z, Li S, Qian Z, Xu X, Li C, Shen B, Zhou G, Chen C, Wang X and Feng J (2024) Optimal response to tislelizumab plus chemotherapy in metastatic triple-negative breast cancer: a case report and literature review. Front. Oncol. 14:1328844. doi: 10.3389/fonc.2024.1328844
Received: 30 October 2023; Accepted: 12 March 2024;
Published: 28 March 2024.
Edited by:
Peter Hamar, Semmelweis University, HungaryReviewed by:
Mariana Segovia, National Autonomous University of Mexico, MexicoTaobo Hu, Peking University People’s Hospital, China
Copyright © 2024 Ma, Shi, Zhao, Hu, Shi, Jiang, Liu, Lu, Chang, Zhou, Dai, Wu, Li, Qian, Xu, Li, Shen, Zhou, Chen, Wang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Chen, njmudoctor@163.com; Xiaohua Wang, wangxiaohua@jszlyy.com.cn; Jifeng Feng, jifeng_feng@163.com
 Yuxin Ma
Yuxin Ma Xinhong Shi1
Xinhong Shi1 Kun Zhao
Kun Zhao Yue Shi
Yue Shi Lin Lu
Lin Lu Yingying Dai
Yingying Dai Cheng Chen
Cheng Chen Xiaohua Wang
Xiaohua Wang Jifeng Feng
Jifeng Feng