- 1Department of Hematology, The First Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong, China
- 2Department of Laboratory Medicine, The First Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong, China
In acute promyelocytic leukemia (APL), hemorrhage, particularly intracranial hemorrhage, is the most common cause of early death. A central venous catheter (CVC) may provide a greater guarantee of safety and comfort to APL patients. However, CVCs have seldom been attempted in APL patients during induction therapy because of concerns about increasing the risk of hemorrhagic complications after this invasive procedure. To evaluate the hemorrhagic risk after CVC placement in APL patients during induction therapy, we retrospectively analyzed 95 newly diagnosed patients with APL from January 2010 to December 2022. Among these patients, 39 patients in the CVC group and 56 patients in the non-CVC group were included. Laboratory and clinical parameters of the two groups were collected and compared. There were no significant differences in median platelet, fibrinogen (Fbg), D-dimer, prothrombin time (PT), white blood count (WBC) and hemoglobin (Hb) between the CVC and non-CVC groups on the first day of the visit (day 0) and the following days (day 4, day 7, day 11, day 14, day 18 and day 21) (p = 0.382, p = 0.805, p = 0.456, p = 0.902, p = 0.901 and p = 0.097, respectively). The consumption of transfused platelets and Fbg was not significantly different between the CVC group and non-CVC group (5.0 vs. 4.5 units, p = 0.34, and 6.8 vs. 6.0, p = 0.36, respectively). The last day of platelet and Fbg transfusion was also not significantly different (21 vs. 19, p = 0.238 and 7.5 vs. 8.5, p = 0.684, respectively). The incidences of total hemorrhagic events and hemorrhagic death were lower in the CVC group than in the non-CVC group (17.9% vs. 37.5%, p = 0.04 and 0% vs. 16.1%, p = 0.01, respectively). The 30-day survival rate was not significantly different (92.3% vs. 82.1%, respectively, p = 0.145) for the CVC group compared with the non-CVC group. Our study suggested that CVCs did not increase the hemorrhagic risk in APL patients during induction therapy and that a CVC should be considered in this type of clinical situation.
Introduction
A high cure rate above 90% of APL patients with treatment that combines all-trans retinoic acid (ATRA), arsenic compounds and anthracycline-based chemotherapy has been demonstrated by several groups (1–3). Nevertheless, death within the first 30 days after diagnosis remains the most common cause of treatment failure (4). Hemorrhage, particularly intracranial hemorrhage, is the most common cause of early death (5, 6). The coagulation disorder in APL results from the combination of disseminated intravascular coagulation (DIC), fibrinolysis, proteolysis, thrombocytopenia, and platelet dysfunction (7).
CVCs provide a greater guarantee of safety and comfort during chemotherapy to cancer patients because many chemotherapies are infused through an intravenous access and may damage peripheral blood vessels (8), and repeated venipuncture is an unpleasant experience for patients (9). However, CVC insertion has seldom been attempted in APL patients during induction therapy because of concerns about increasing the risk of hemorrhagic complications after this invasive procedure, leading to guidelines such as those from an expert panel of the European LeukemiaNet that do not recommend the routine use of CVCs (10–12). Thus, few studies have attempted CVCs in APL patients during induction therapy. Our institution had inserted CVCs in some patients with APL, and more than ten years of data were available for this study. The aim of this retrospective study was to evaluate the hemorrhagic risk after CVC in APL patients during induction therapy by comparing the laboratory and clinical parameters between the CVC and non-CVC groups.
Patients and methods
Patients
All patients ≥14 years of age with newly diagnosed APL at the First Affiliated Hospital of Shantou University Medical College between January 2010 and December 2022 were included in this retrospective, single-center data analysis. This study was approved by the First Affiliated Hospital of Shantou University Medical College Ethics Committee and conducted in accordance with the Declaration of Helsinki.
The APL diagnosis was based on morphology and immunophenotyping and confirmed by the presence of t (15; 17) translocation by karyotyping, immunofluorescence in situ hybridization or a real-time polymerase chain reaction for the PML/RARα transcript on bone marrow samples. During the study period, 121 patients were enrolled in our center. Among those, the following patients were excluded: 9 patients who were <14 years old, 11 patients transferred to other hospitals or refused to receive treatment, and 6 patients who relapsed or were not newly diagnosed. In the current analysis, a total of 95 hospitalized patients were included.
Based on the use of a CVC and the coagulopathies that resolved within 14 days in most patients (13), the patients were divided into two groups: the CVC group (CVC inserted ≤14 days from admission) and the non-CVC group (CVC not inserted or inserted beyond 14 days from admission). There were 39 and 56 patients in the CVC and non-CVC groups, respectively. Furthermore, when we analyzed the dynamic change in hemostatic variables, 13 early death cases were excluded to minimize interference as much as possible.
Based on the induction regimens, patients were divided into four groups: the all-trans retinoic acid (ATRA) with arsenic trioxide (ATO) group, ATRA with chemotherapy group, ATRA and ATO with chemotherapy group, and ATRA monotherapy group. To overcome coagulopathy, platelet transfusions were given to maintain a platelet count of more than 30 × 109/L until resolution of the coagulopathy. Once the patient’s coagulopathy was under control, platelet transfusions were only given for patients with hemorrhagic manifestations or when the platelet count dropped below 20 × 109/L. Fbg, cryoprecipitate and/or fresh-frozen plasma was transfused to maintain Fbg >150 mg/dl. Heparin prophylaxis was not recommended, except for the treatment of thrombotic events. One unit of cryoprecipitate or 100 ml of plasma was converted to 0.25 g of Fbg in favor of the subsequent statistical analysis.
CVC insertion and management
Decisions related to directing APL patients to position the CVCs were entirely made by the treating physician mainly for chemotherapy, monitoring hemodynamics and transfusion. PICCs were used when the duration of systemic anticancer therapy was 3 months or longer or there were no adequate peripheral veins. Centrally inserted central catheters (CICCs) were used when critically APL patients required hemodynamic monitoring and transfusion for a treatment duration of 2–3 weeks.
All CVCs were inserted by various specialists such as nurse practitioners. Before the puncture, an ultrasound examination is performed to assess the anatomy of the target vein and adjacent anatomic structures. After understanding the positional relationships of anatomical landmarks on the body surface, the anatomical landmark methods were used to position the catheter and when possible, ultrasound-guided methods were used to minimize bleeding complication rates. At the end of the procedure, a chest radiograph was routinely performed to determine if the catheter tip location was placed at the cavoatrial junction. Regular device checks took place according to the protocol of our institution. Insertion-site care entailed weekly changes of transparent, semi-permeable film-covered dressing.
Laboratory data
To verify an association between CVC and recovery from coagulopathy, data from day 0 to day 21 were collected for patients who survived the first 30 days. The main laboratory data were collected from the patients’ hospital files. The dynamic changes in WBC, hemoglobin (Hb) and coagulation indexes, including platelets, PT, activated partial thromboplastin time (APTT), D-dimer, and Fbg, were collected on the first day of the visit (day 0) and after induction treatment (days 4, 7, 11, 14, 18, and 21). The percentages of promyelocytes and blasts in the bone marrow and peripheral blood were determined by microscopic examinations by two experienced physicians independently. Disseminated intravascular coagulation (DIC) was defined under the criteria for the DIC scoring system released by the International Society of Thrombosis and Hemostasis (ISTH) (14). The risk stratification of APL is based on WBC and platelets: WBC of ≤10 × 109/L and platelets >40 × 109/L as low risk, WBC ≤10 × 109/L and platelets <40 × 109/L as intermediate risk, and WBC >10 × 109/L as high risk.
Follow-up of hemorrhagic and thrombotic events
To verify an association between CVC and hemorrhagic events, clinical data from day 1 to day 30 were collected. Intracranial hemorrhage had to be confirmed by computed tomography. Hematochezia required the presence of melena or hematemesis. Hematuria required the presence of gross hematuria. Pulmonary hemorrhage required the symptoms of coughing up blood. Hemorrhagic death was defined as death caused by hemorrhage. Catheter-related thrombosis (CRT), also referred to as symptomatic thrombosis, was defined as thrombosis in the vein in which the catheter was inserted and documented by screening ultrasound. An ultrasound examination for CRT was only performed in patients with CRT symptoms (pain, redness, swelling on CVC insertion area, limb edema, and dysfunction of CVC). Patients without CRT symptoms did not receive ultrasound examination for CRT. Catheter occlusion was defined as inability to withdraw blood or sluggish blood return, sluggish flow, resistance or inability to flush lumen, and inability to infuse fluid.
Thirty-day survival rates
To verify an association between CVC and early death, data from day 0 to day 30 were collected. Early death was defined as deaths attributable to any cause between the first day of visit and the following 30 days.
Statistical analysis
Statistical analyses were performed using SPSS 26.0. Categorical variables are presented as frequencies and were compared using the x2 test among the groups. If more than 20% of cells had less than the expected frequency of 5, Fisher’s exact test was alternatively used for analysis. Continuous variables are presented as the median (interquartile range, IQR) or mean and standard deviation and were compared using the Mann–Whitney U test and two independent samples t test for skewed data and normally distributed data, respectively. Thirty-day survival rates were analyzed using the Kaplan−Meier method and the log-rank test. A two-sided p value of ≤0.05 was considered statistically significant.
Results
Baseline characteristics
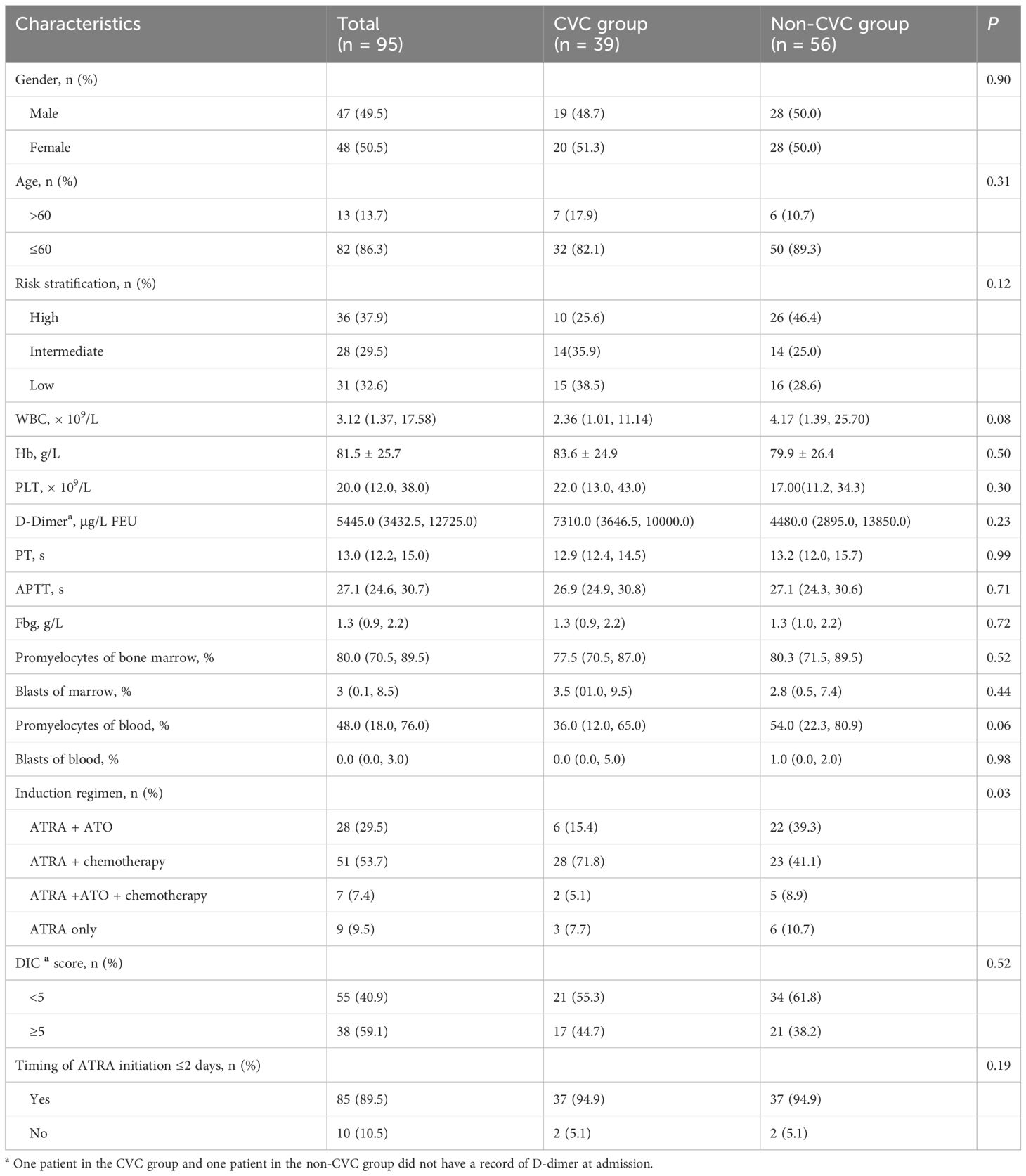
Ninety-five patients (48 females and 47 males) were included in our study. Of the total of 95 patients, 39 (41%) patients in the CVC group (36 patients with a peripherally inserted central catheter, 1 patient with an internal jugular vein catheter, 2 patients with subclavian vein catheters) and 56 (59%) patients in the non-CVC group were included in this retrospective study. Thirty-six (37.9%) patients, 28 (29.5%) and 31 (32.6%) were regarded as high-risk, intermediate-risk and low-risk individuals, respectively. Twenty-eight (29.5%) patients, 51 (53.7%) patients, 7 patients (7.3%) and 9 patients (9.5%) received ATRA with ATO, ATRA with chemotherapy, ATRA plus ATO with chemotherapy, and ATRA monotherapy, respectively. ATRA was initiated ≤2 days in 85 (89%) patients. The median WBC, platelet count, PT, APTT, Fbg and D-dimer were 3.12 × 109/L, 20 × 109/L, 13 seconds, 27.1 seconds and 1.3 g/L, 5445.0 μg/L FEU, respectively. The median percentages of promyelocytes in the bone marrow (BM) and peripheral blood (PB) were 80% and 48%, and the median percentages of blasts in BM and PB were 3% and 0%, respectively. Overall, there were no significant differences in any of the included 95 patients between the CVC group and non-CVC group regarding sex, age >60 years, risk stratification, median WBC, platelet count, PT, APTT, Fbg, D-dimer, DIC score and timing of ATRA initiation ≤2 days (p = 0.90, 0.31, 0.12, 0.08, 0.30, 0.99, 0.71, 0.72, 0.23, 0.52 and 0.19, respectively), except for the induction regimens (p = 0.03). These patient characteristics are summarized in Table 1. Moreover, when we compared the difference in the dynamic trend of coagulation data between the CVC and non-CVC groups, 13 early death cases were excluded to minimize interference as much as possible. The remaining 82 cases (36 in the CVC group and 46 in the non-CVC group) were incorporated into the following research. As summarized in Table 2, the baseline characteristics mentioned above were also not significantly different between the two groups except for the induction regimens (p = 0.01). The median day of CVC insertion was 4 (IQR: 3, 5).
CVC insertion and use
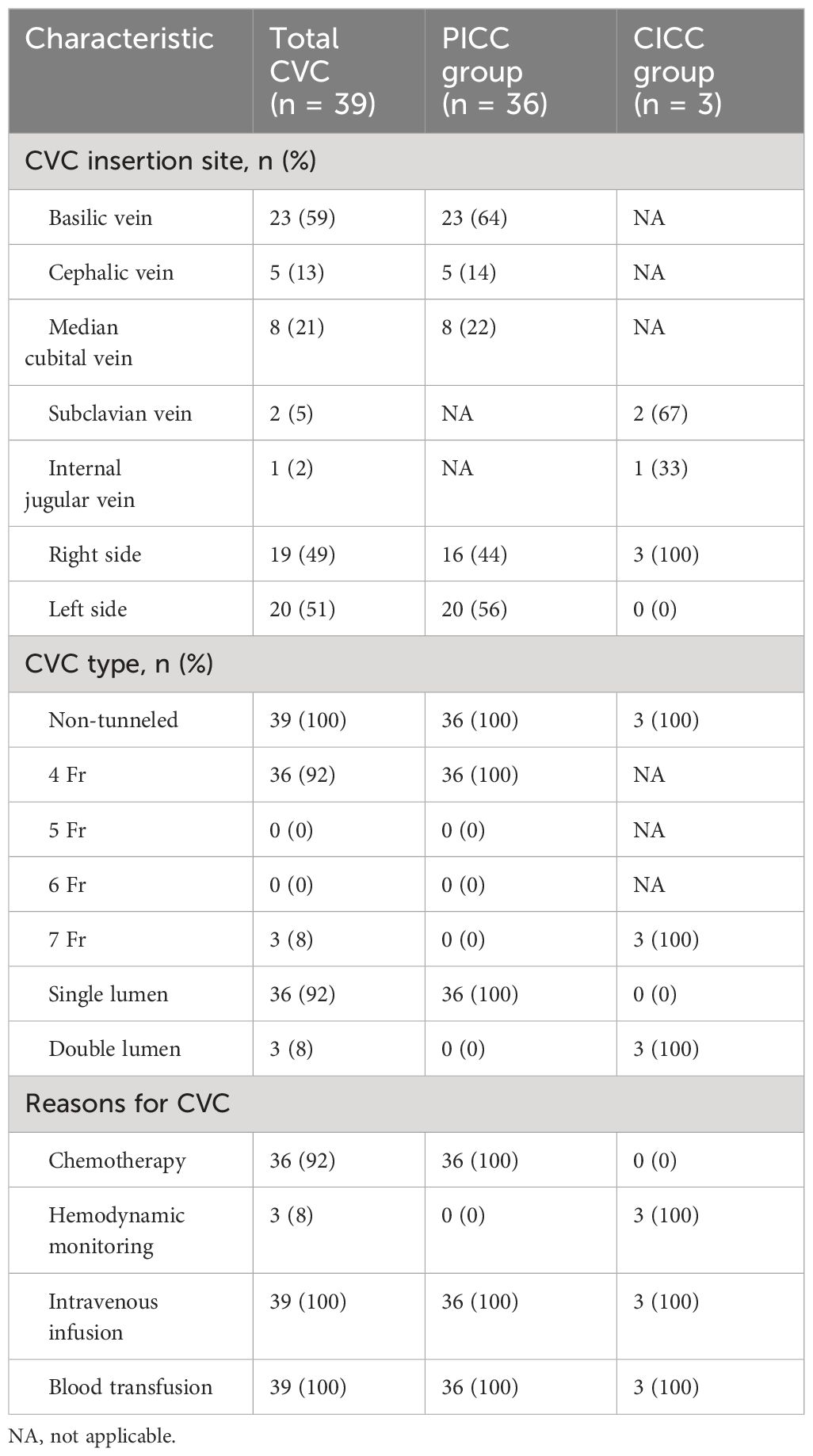
Of the 39 patients who inserted CVCs, 36 patients inserted a PICC and 3 patients inserted a CICC (1 patient with an internal jugular vein catheter, 2 patients with subclavian vein catheters). No patients received tunneled catheters. The reasons for CVC insertion were mainly as follows: chemotherapy (92%), monitoring hemodynamics (8%), blood transfusion (100%) and intravenous infusion (100%). In the PICC group, PICCs were inserted through the basilic vein in 64% of cases and all catheters inserted were 4 Fr catheters. In the CICC group, CICCs were inserted through the subclavian vein in 67% of patients and all CICCs inserted were 7 Fr catheters. Sixteen (44%) patients in the PICC group and 3 (100%) patients in the CICC group were inserted in the right arm. The characteristics and use of the catheters in both groups are summarized in Table 3.
Dynamic trend of coagulation data
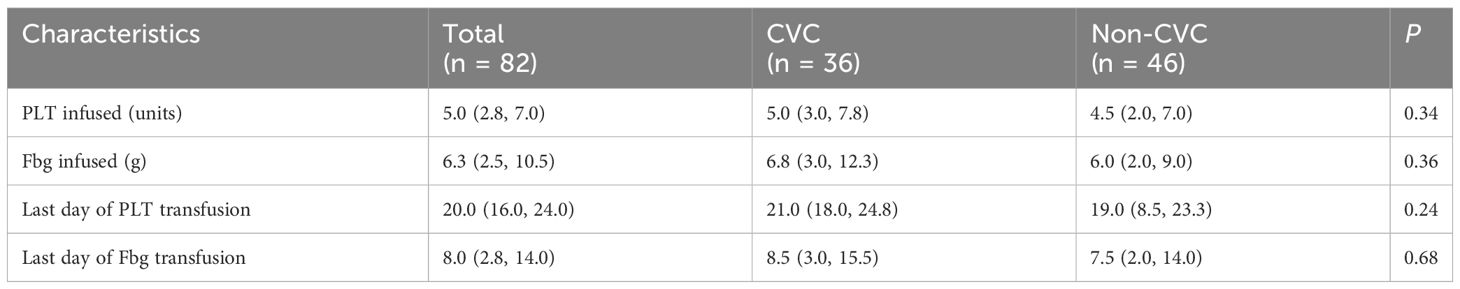
There were no significant differences in the dynamic trend of coagulation data in the 82 patients who survived the first 30 days between the CVC and non-CVC groups. The median platelet, PT, Fbg, D-dimer, WBC and Hb values at each time point (day 0, day 4, day 7, day 11, day 14, day 18 and day 21) during induction therapy showed no significant difference (p = 0.382, 0.902, 0.805, 0.456, 0.901 and 0.097, respectively), as shown in Figure 1. In addition, as summarized in Table 4, the amounts of platelets and Fbg used were also not significantly different between the two groups. The median transfusion amount of platelets was 5 units (IQR: 3.0, 7.8) in patients with CVCs and 4.5 units (IQR: 2.0, 7.0) in the non-CVC group (p = 0.34). At the same time, the median transfusion amount of human Fbg was 6.8 g (IQR: 3.0, 12.3) in the CVC group and 6.0 g (IQR: 2.0, 9.0) in the non-CVC group (p = 0.36).
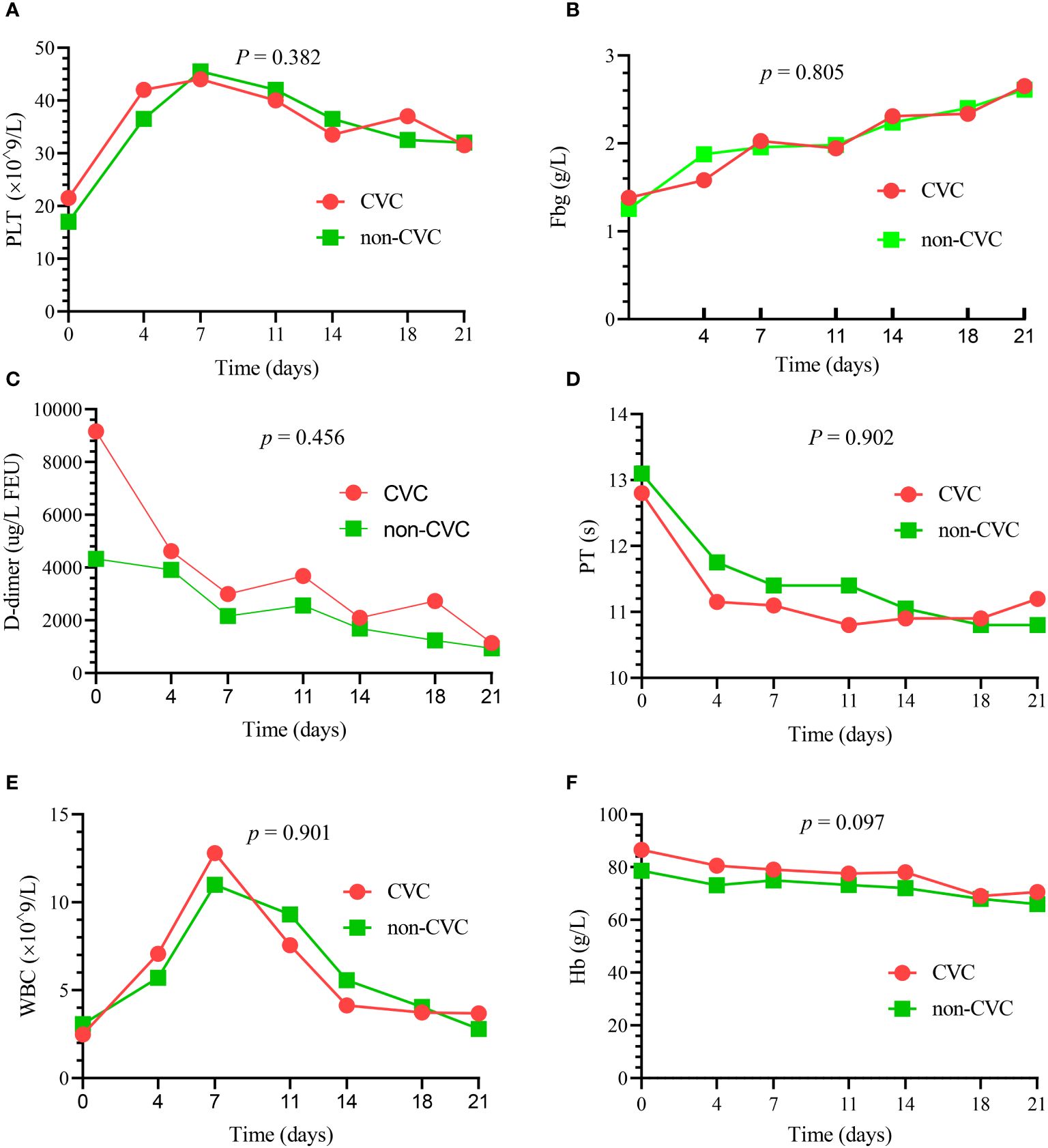
Figure 1 The dynamic changes in the principal blood parameters in 82 patients who survived the first 30 days. There were no significant differences in the median platelet (PLT) (A), median Fbg (B), median D-dimer (C), median PT (D), median WBC (E) and median Hb (F) between the CVC and non-CVC groups at seven time points (p = 0.382, 0.805, 0.456, 0.902, 0.901 and 0.097, respectively).
To avoid the interference of transfused component blood, we provided the last day of platelet and Fbg transfusion as more convincing evidence, indicating the time of platelet recovery and coagulopathy correction. As summarized in Table 4, the time of platelet recovery and coagulopathy correction were not significantly different. Although the median time of platelet recovery in the CVC group was longer than that in the non-CVC group (21 vs. 19, respectively), it did not differ statistically (p = 0.24). Meanwhile, coagulopathy correction (last day of Fbg transfusion) was faster in the non-CVC group than in the CVC group (7.5 vs. 8.5, respectively), but these differences were not statistically significant (p = 0.68).
Hemorrhagic events
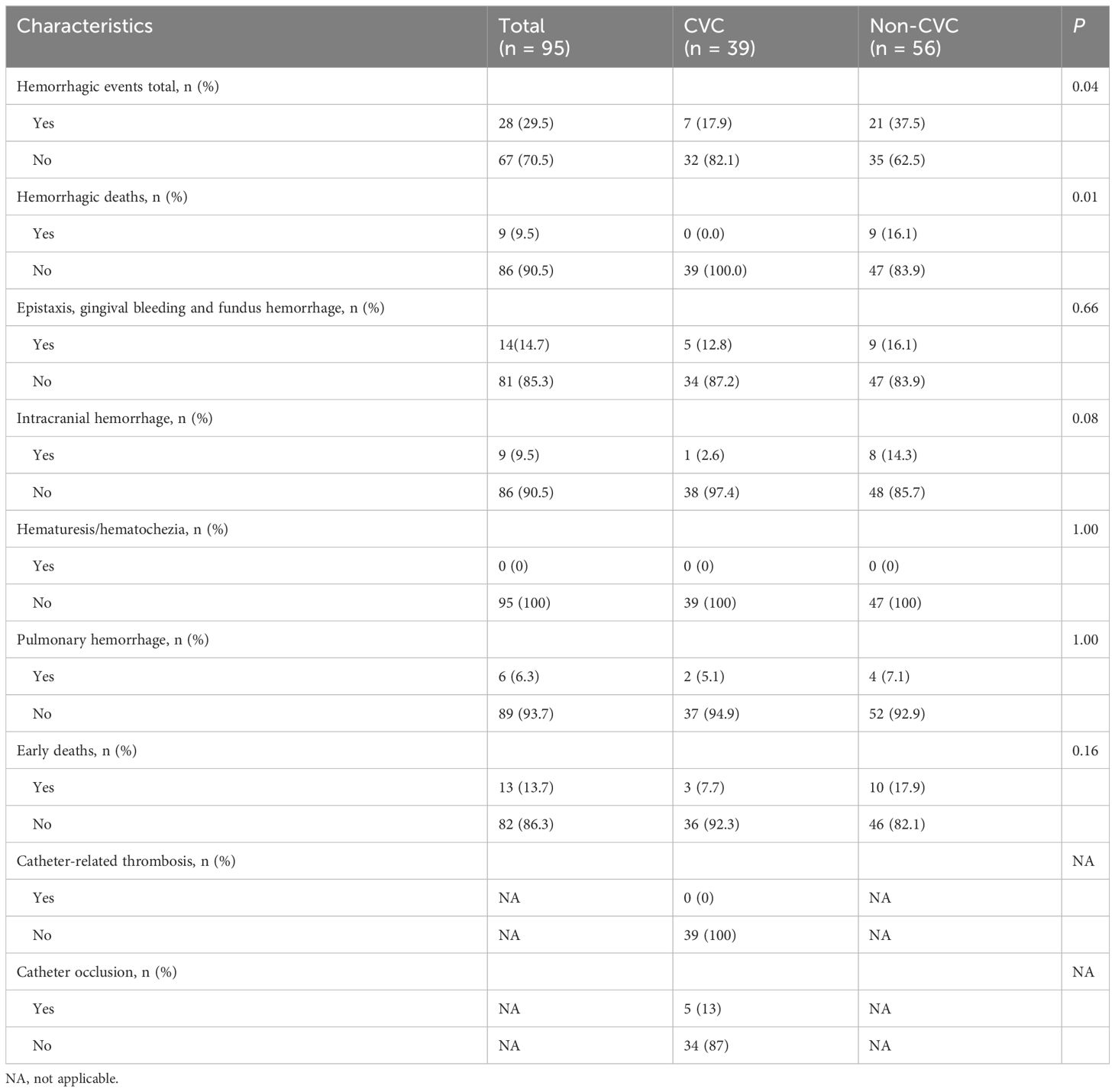
Overall, 28 (29.5%) of the 95 patients developed hemorrhagic events, and 9 (9.5%) patients developed hemorrhagic deaths. The incidences of total bleeding events and hemorrhagic deaths during induction therapy were lower in the CVC group than in the non-CVC group (7 (17.9%) vs. 21 (37.5%), p = 0.04; 0 (0.0%) vs. 9 (16.1%), p = 0.01). Specifically, 5 (12.8%) patients in the CVC group and 9 (16.1%) patients in the non-CVC group had oral, eye or nasal bleeding (p = 0.66), 2 (5.1%) patients in the CVC group and 4 (7.1%) patients in the non-CVC group experienced pulmonary hemorrhage (p = 1.00), and 1 (2.6%) patient in the CVC group and 8 (14.3%) patients in the non-CVC group experienced intracranial hemorrhage. Catheter occlusion occurred in 5 cases while no symptomatic catheter-related thrombosis was observed. These data are summarized in Table 5.
Thirty-day survival analysis
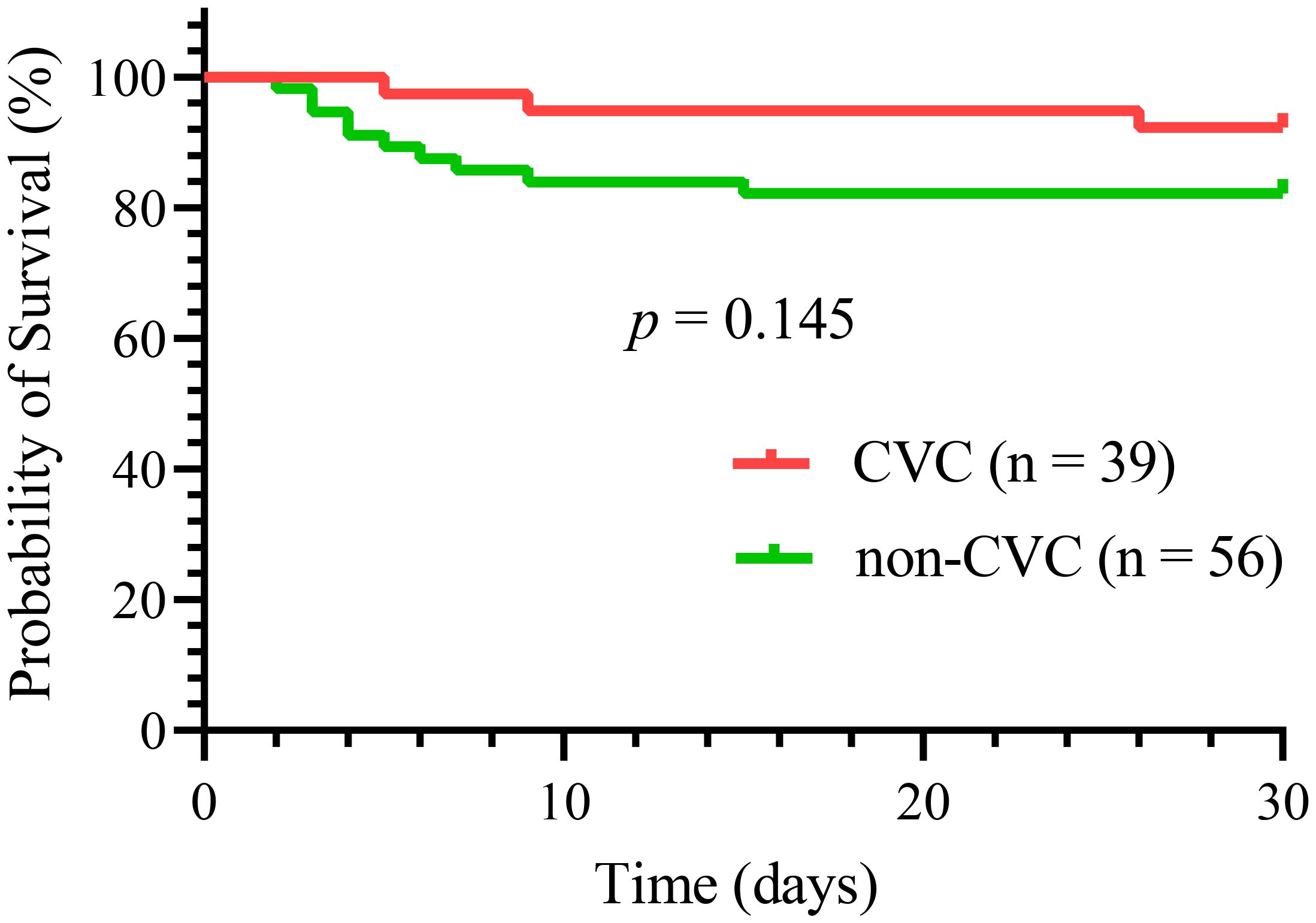
Overall, 13 (13.7%) early deaths occurred in all 95 patients in our study. Of all the early deaths, hemorrhage was the most common cause of induction death (9 patients, 69%), followed by respiratory failure (2 patients, 15%), differentiation syndrome (1 patient, 8%) and multiorgan failure, mostly likely resulting from thrombotic thrombocytopenic purpura (1 patient, 8%). Hemorrhagic mortalities were almost exclusively caused by intracranial (8 patients, 8.4%) and pulmonary hemorrhages (1 patient, 1.1%). Thirty-day survival did not differ significantly between the two groups, as shown in Figure 2 (p = 0.15). Three deaths (7.7%) occurred in the CVC group compared with ten (17.9%) in the non-CVC group. The causes of early death in the CVC group were one differentiation syndrome, one multiorgan failure, and one aspiratory failure. In the 10 patients who died in the non-CVC group at 30 days, the causes of death were eight intracranial hemorrhages, one pulmonary hemorrhage and one aspiratory failure.
Discussion
In this manuscript, we explored the safety of CVCs in APL patients during induction therapy. The results suggested that coagulopathies in the CVC group could be corrected as rapidly as that in the non-CVC group. Meanwhile, CVCs do not increase the risk of bleeding events, and the 30-day survival rate for the CVC group was comparable to that for the non-CVC group.
Our study showed that the resolution of coagulopathies was not significantly different between the CVC and non-CVC groups at different points during days 0–21 for the 82 patients surviving more than 30 days, as shown in Table 4; Figure 1. The median Fbg recovery was 8 days in our study, and this result was in agreement with the outcome of other studies (13), which suggested that Fbg was reversed within two weeks in most of the patients. Our findings also support the view that Fbg is an early and sensitive indicator of improvement in coagulopathy (15). A recent study showed that Fbg <1.0 g/L was an independent risk factor for early death (16). Therefore, the recovery of Fbg is essential for the prevention of early death. Interestingly, the recovery and consumption of Fbg were not significantly different between the CVC group and the non-CVC group. These results suggest that CVCs do not worsen the pace of Fbg recovery and increase the consumption of Fbg.
Hemorrhage is the common complications during induction therapy for APL patients. In our study, 28 (29.5%) patients developed hemorrhagic events, and 9 (9.5%) patients developed hemorrhagic deaths. The hemorrhagic death rates in our study were higher than those in both the clinical studies of LPA96 and LPA99 (5.1% and 5%, respectively) (17). These differences may result from the inclusion of patients who were not fit or too old for inclusion in clinical trials. Moreover, hemorrhagic deaths were exclusively caused by intracranial (8 patients, 88.9%) and pulmonary hemorrhagic events (1 patient, 11.1%) in our study, and these results are consistent with what de la Serna et al. reported (17) Surprisingly, the incidence of intracranial hemorrhage in the CVC group was lower than that in the non-CVC group, and the difference significantly differed (0.0% vs. 16.1%, respectively, p = 0.01). These results suggest that CVCs do not increase the risk of hemorrhagic events, especially the most common intracranial hemorrhage.
Although a significant improvement has been reported in terms of the rates of early death, there are still discrepancies regarding the early death rates among clinical, registration, or retrospective studies. In our study, the early death rate was 13.7% for all 95 included patients within the first 30 days, as shown in Table 5; Figure 2. These results are higher than a study that revealed an overall induction mortality rate of 3.2% in APL patients treated with ATRA, idarubicin, and IV arsenic trioxide (18) but lower than that in a Swedish registry study (29%) (19). The main reason for the higher early death rate in our study compared with clinical trials is probably the inclusion of patients with very early deaths, as well as of patients who were not fit or too old for inclusion in clinical trials. The PETHEMA group reported that approximately 5% of patients were considered not eligible for induction therapy due to very poor clinical conditions, mostly due to lethal or life-threatening hemorrhagic events before starting therapy (20). In addition, the early death rate was lower in the CVC group than in the non-CVC group (7.7% vs. 17.9%, respectively); however, this difference was not significant (p = 0.16). Early death was closely linked to age >60 years (17) and several blood chemistry characteristics, such as initial Fbg level and WBC (21). In our study, these characteristics were comparable, and the early death rate was not significantly different between the two groups. Of note, the induction regimens, which may impact early death, were not similar between the two groups in our study. Studies of anthracycline-free regimens using arsenic trioxide (ATO) showed less early death and fewer reported cases of hemorrhagic death (22, 23). While more patients used the arsenic trioxide (ATO)-containing regimens in the non-CVC group in our study, the early death rate was not significantly different between the two groups. Overall, these results suggest that CVCs do not increase the risk of early death.
However, our study has some limitations. First, although the risk stratification in both groups were comparable (p = 0.12), the percentage of patients with high risk was lower in the CVC group than in the non-CVC group (10% vs. 26%). Moreover, APL patients with a higher risk had a greater incidence of hemorrhage than did patients with an intermediate/low risk during induction therapy (16, 19). Therefore, due to the retrospective nature and the relatively small size of our study, this issue might still be an important confounding variable and further prospective studies are needed. Second, the reasons why some patients in the non-CVC group did not have CVCs placed were unclear because they depended entirely on the clinician’s determination. Third, many factors that could interfere with the hemostatic system in APL, such as concomitant infections, were not analyzed.
In conclusion, our study suggested that CVCs did not increase the hemorrhagic risk in APL patients during induction therapy, and we offered information that a CVC should be considered in a clinical situation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the First Affiliated Hospital of Shantou University Medical College Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin due to the retrospective nature of the study.
Author contributions
MC: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. JH: Data curation, Investigation, Writing – original draft. DZ: Data curation, Formal Analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing. FC: Data curation, Formal Analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing. YS: Data curation, Formal Analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank the medical and nurse team for their help with patient care.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Iland HJ, Collins M, Bradstock K, Supple SG, Catalano A, Hertzberg M, et al. Use of arsenic trioxide in remission induction and consolidation therapy for acute promyelocytic leukaemia in the Australasian Leukaemia and Lymphoma Group (ALLG) APML4 study: a non-randomised phase 2 trial. Lancet Haematol. (2015) 2:e357–66. doi: 10.1016/s2352-3026(15)00115-5
2. Kutny MA, Alonzo TA, Gerbing RB, Wang YC, Raimondi SC, Hirsch BA, et al. Arsenic trioxide consolidation allows anthracycline dose reduction for pediatric patients with acute promyelocytic leukemia: report from the children's oncology group phase III historically controlled trial AAML0631. J Clin Oncol. (2017) 35:3021–9. doi: 10.1200/jco.2016.71.6183
3. Yang MH, Wan WQ, Luo JS, Zheng MC, Huang K, Yang LH, et al. Multicenter randomized trial of arsenic trioxide and Realgar-Indigo naturalis formula in pediatric patients with acute promyelocytic leukemia: Interim results of the SCCLG-APL clinical study. Am J Hematol. (2018) 93:1467–73. doi: 10.1002/ajh.25271
4. Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. (2011) 118:1248–54. doi: 10.1182/blood-2011-04-346437
5. Lehmann S, Deneberg S, Antunovic P, Rangert-Derolf Å, Garelius H, Lazarevic V, et al. Early death rates remain high in high-risk APL: update from the Swedish Acute Leukemia Registry 1997-2013. Leukemia. (2017) 31:1457–9. doi: 10.1038/leu.2017.71
6. Xu F, Wang C, Yin C, Jiang X, Jiang L, Wang Z, et al. Analysis of early death in newly diagnosed acute promyelocytic leukemia patients. Med (Baltimore). (2017) 96:e9324. doi: 10.1097/md.0000000000009324
7. Hambley BC, Tomuleasa C, Ghiaur G. Coagulopathy in acute promyelocytic leukemia: can we go beyond supportive care? Front Med (Lausanne). (2021) 8:722614. doi: 10.3389/fmed.2021.722614
8. Skaff ER, Doucette S, McDiarmid S, Huebsch L, Sabloff M. Vascular access devices in leukemia: a retrospective review amongst patients treated at the Ottawa Hospital with induction chemotherapy for acute leukemia. Leuk Lymph. (2012) 53:1090–5. doi: 10.3109/10428194.2011.639879
9. Silvestri V, Nerini L, Missio G, Masini M, Faggi S, Gori A, et al. Levels of anxiety and pain during chemotherapy with peripheral versus central vascular access: an experimental evaluation. J Vasc Access. (2004) 5:147–53. doi: 10.1177/112972980400500403
10. Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. (2009) 113:1875–91. doi: 10.1182/blood-2008-04-150250
11. Sanz MA, Fenaux P, Tallman MS, Estey EH, Löwenberg B, Naoe T, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. (2019) 133:1630–43. doi: 10.1182/blood-2019-01-894980
12. Yilmaz M, Kantarjian H, Ravandi F. Acute promyelocytic leukemia current treatment algorithms. Blood Cancer J. (2021) 11:123. doi: 10.1038/s41408-021-00514-3
13. Zhu HH, Guo ZP, Jia JS, Jiang Q, Jiang H, Huang XJ. The impact of oral arsenic and all-trans-retinoic acid on coagulopathy in acute promyelocytic leukemia. Leuk Res. (2018) 65:14–9. doi: 10.1016/j.leukres.2017.11.009
14. Wada H, Matsumoto T, Hatada T. Diagnostic criteria and laboratory tests for disseminated intravascular coagulation. Expert Rev Hematol. (2012) 5:643–52. doi: 10.1586/ehm.12.57
15. Tallman MS, Altman JK. How I treat acute promyelocytic leukemia. Blood. (2009) 114:5126–35. doi: 10.1182/blood-2009-07-216457
16. Wang Y, Hou W, Li H, Tian X, Li J, Hu T, et al. Analysis of risk factors for early death in patients with acute promyelocytic leukaemia treated with arsenic trioxide. Ann Hematol. (2022) 101:1039–47. doi: 10.1007/s00277-022-04788-w
17. de la Serna J, Montesinos P, Vellenga E, Rayón C, Parody R, León A, et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. (2008) 111:3395–402. doi: 10.1182/blood-2007-07-100669
18. Iland HJ, Bradstock K, Supple SG, Catalano A, Collins M, Hertzberg M, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. (2012) 120:1570–80. doi: 10.1182/blood-2012-02-410746
19. Lehmann S, Ravn A, Carlsson L, Antunovic P, Deneberg S, Möllgård L, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia. (2011) 25:1128–34. doi: 10.1038/leu.2011.78
20. Di Bona E, Avvisati G, Castaman G, Luce Vegna M, De Sanctis V, Rodeghiero F, et al. Early haemorrhagic morbidity and mortality during remission induction with or without all-trans retinoic acid in acute promyelocytic leukaemia. Br J Haematol. (2000) 108:689–95. doi: 10.1046/j.1365-2141.2000.01936.x
21. Yanada M, Matsushita T, Asou N, Kishimoto Y, Tsuzuki M, Maeda Y, et al. Severe hemorrhagic complications during remission induction therapy for acute promyelocytic leukemia: incidence, risk factors, and influence on outcome. Eur J Haematol. (2007) 78:213–9. doi: 10.1111/j.1600-0609.2006.00803.x
22. Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. (2013) 369:111–21. doi: 10.1056/NEJMoa1300874
23. Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. (2015) 16:1295–305. doi: 10.1016/S1470-2045(15)00193-X
Keywords: central venous catheter, hemorrhage, coagulopathy, acute promyelocytic leukemia, induction therapy
Citation: Cao M, Hong J, Zhang D, Chen F and Su Y (2024) Central venous catheters do not increase the hemorrhagic risk in acute promyelocytic leukemia patients during induction therapy. Front. Oncol. 14:1332372. doi: 10.3389/fonc.2024.1332372
Received: 02 November 2023; Accepted: 25 March 2024;
Published: 12 April 2024.
Edited by:
Anna Maria Testi, Sapienza University of Rome, ItalyReviewed by:
Sezaneh Haghpanah, Shiraz University of Medical Sciences, IranAlessandro Crocoli, Bambino Gesù Children’s Hospital (IRCCS), Italy
Copyright © 2024 Cao, Hong, Zhang, Chen and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongqing Zhang, zhangdongqing1976@163.com; Feiheng Chen, 1964147450@qq.com; Yongzhong Su, suyzst@126.com
 Manxiong Cao
Manxiong Cao Jiaqiong Hong1
Jiaqiong Hong1 Dongqing Zhang
Dongqing Zhang