- 1Department of Hematology, Zhengzhou University People’s Hospital and Henan Provincial People’s Hospital, Zhengzhou, China
- 2Department of Hematology, Nanyang Second General Hospital, Nanyang, China
- 3Department of Hematology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 4Department of Hematology, Beijing JiShuiTan Hospital, Capital Medical University, Beijing, China
Background: DNMT3A mutations can be detected in premalignant hematopoietic stem cells and are primarily associated with clonal hematopoiesis of indeterminate potential; however, current evidence does not support assigning them to a distinct European Leukemia Net (ELN) prognostic risk stratification. CD7 is a lymphoid antigen expressed on blasts in approximately 30% of acute myeloid leukemia (AML), and its role in AML remains unclear and depends on subgroup evaluation. This study investigated the prognostic value of DNMT3A mutation combined with CD7 expression in AML.
Methods: We retrospectively analyzed the clinical data of 297 newly diagnosed non-M3 AML patients. According to the DNMT3A mutation and CD7 expression in AML cells, patients were divided into the DNMT3A-mutated/CD7-positive (CD7+), DNMT3A-mutated/CD7-negative (CD7-), DNMT3A-wild-type/CD7+, and DNMT3A-wild-type/CD7- groups.
Results: The DNMT3A-mutated/CD7+ group had lower complete remission rates and higher relapse rates. Importantly, these patients had significantly shorter overall survival (OS) and relapse-free survival (RFS). Furthermore, multivariate analysis showed that CD7+ with DNMT3A mutation was an independent risk factor for OS and RFS.
Conclusion: CD7+ with DNMT3A mutation predicts a poor prognosis in AML patients, and the immunophenotype combined with molecular genetic markers can help to further refine the current risk stratification of AML.
1 Introduction
Acute myeloid leukemia (AML) is a hematologic malignancy caused by abnormal clonal proliferation of hematopoietic stem and progenitor cells, and its clinical manifestations and prognosis are highly heterogeneous (1). Currently, the prognosis of AML patients is based mainly on cytogenetic and molecular genetic alterations (2). Although the relationship between some genetic mutations and the prognosis of AML has been clarified, the relationship between other genetic alterations and AML prognosis is not yet fully understood (3). DNA methyltransferase 3A (DNMT3A) is an epigenetic protease that, along with DNMT3B, is responsible for de novo DNA methylation (4, 5). At present, there is still some controversy about the prognostic significance of DNMT3A mutations. Most investigators contend that DNMT3A mutations are related to the poor prognosis of AML patients (6, 7). Moreover, some studies suggest that the poor prognostic value of DNMT3A mutations may be limited to specific types of AML patients (5, 8–11). However, the 2022 European Leukemia Net (ELN) guidelines state that current evidence is insufficient to classify DNMT3A mutations into a clear prognostic group (3). Therefore, more prognostic markers must be combined as a supplement to assess the prognostic value of DNMT3A mutations.
CD7 is a 40 kDa glycoprotein belonging to the immunoglobulin superfamily (12). CD7 is mainly expressed on the surface of T cells but can also be aberrantly expressed on AML blasts (13–15). In AML patients, the positive expression rate of CD7 is approximately 30% (16–18), but the prognostic significance of its expression has been inconsistently reported (17, 19–23), and further analysis and subgroup evaluation are needed.
The prognostic significance of DNMT3A mutation and CD7 expression in AML is still inconclusive, and there are currently no studies exploring the clinical significance of DNMT3A mutation combined with CD7 expression in AML patients. Therefore, we conducted a retrospective study to evaluate the prognostic value of DNMT3A mutation combined with CD7 expression in AML patients.
2 Patients and methods
2.1 Patients
In this retrospective analysis, 297 patients with de novo AML were enrolled. Samples were collected at Zhengzhou University People’s Hospital and the First Affiliated Hospital of Zhengzhou University from January 2017 to July 2021. The last date of follow-up was July 30, 2022. Patients with a history of hematologic disease, therapy-related AML or other malignancies, or acute promyelocytic leukemia (FAB-M3) were excluded. All baseline data were obtained from medical records, including age, sex, white blood cell count (WBC), hemoglobin (HB), platelet (PLT) count, lactate dehydrogenase (LDH), peripheral blood (PB) blast percentage, bone marrow (BM) blast percentage, immunophenotype, karyotype, gene mutations, and French-American-British classification (FAB) subtypes as well as the induction therapy regimen and treatment response. All patients signed an informed con-sent form at the time of treatment, and this study complied with the requirements of our institutional ethics committee (2021-140-02).
2.2 Immunophenotypic analysis
Before initial treatment, 2-3 ml of BM aspiration fluid was collected and placed in an EDTA anticoagulant tube. The Navios flow cytometer from Beckman Coulter Company in the United States was used for a 4-color immunolabeling method to detect the expression levels of CD2, CD4, CD7, CD34, CD56, CD38, CD117, CD33, CD13, HLA-DR, and others. CD45/SSC was utilized to set the gate, and the Kaluza software was employed to perform immunophenotypic analysis of BM blasts. Antigen expression was defined as positive when the amount of antigen expression was ≥20%. Representative flow cytometry plots of bone marrow blasts from AML patients in the DNMT3A-mutated/CD7-positive (CD7+) group, the DNMT3A-mutated/CD7-negative (CD7-) group, the DNMT3A-wild-type/CD7+ group, and the DNMT3A-wild-type/CD7- group are displayed in Supplementary Figure 1.
2.3 Molecular biological testing
Molecular biological examinations were performed by next-generation sequencing (NGS). Leukemic mutation genes such as FLT3-ITD, NPM1, KIT, CEBPA, DNMT3A, IDH1, IDH2, TET2, EZH2, RUNX1, ASXL1, PHF6, TP53, SF3B1, SRSF2, U2AF1, BCOR, ZRSR2, NRAS, CBL, SETBP1, ETV6 and JAK2 were analyzed.
2.4 Treatment and follow-up
Patients received standard induction chemotherapy regimens based on disease and risk stratification (24, 25), with doses slightly adjusted for each patient. The induction regimens for each group of patients are summarized in Supplementary Table 1. Induction chemotherapy regimens included IA (idarubicin 8-12 mg/m2 for 3 days, cytarabine 100-200 mg/m2 for 7 days), DA (daunorubicin 40-60 mg/m2 for 3 days, cytarabine 100-200 mg/m2 for 7 days), MA (mitoxantrone 6-8 mg/m2 for 3 days, cytarabine 100-200 mg/m2 for 7 days), or CAG+D (decitabine 20 mg/m2/d, d1-5, aclarubicin 20 mg/m2/d, d3-6, cytarabine 25 mg/m2/q12h, d7-14, and G-CSF, 300 μg/d, d1-14). Patients in complete remission (CR) continued to undergo induction chemotherapy with medium- or high-dose cytarabine, while those in partial remission (PR) or nonremission (NR) received a second cycle of induction chemotherapy or salvage therapy. Based on the availability of donors, patients with intermediate and adverse prognoses underwent hematopoietic stem cell transplantation (HSCT).
2.5 Definitions of treatment response and survival time
CR was defined as <5% blasts in BM, no blasts with Auer bodies, no blasts in peripheral blood, no extramedullary leukemia, neutrophil count ≥1.0×109/L, and PLT count ≥100× 109/L. CR with incomplete hematologic recovery (CRi) was defined as meeting all CR criteria, except residual neutropenia (<1.0×109/L) or thrombocytopenia (<100×109/L). PR was defined as the proportion of BM blasts between 5% and 25%, which should be at least 50% lower than before therapy, and the blood cell count must be within the range of CR. NR was defined as failure to achieve CR, CRi or PR after chemotherapy. The primary study endpoints were overall survival (OS), measured as the time from diagnosis to the date of death from any cause or the date of the last follow-up, and relapse-free survival (RFS), measured as the time from the date of CR or CRi to the date of hematologic relapse, death from any cause, or the date of the last follow-up (3).
2.6 Statistical analysis
The comparisons between groups were made using Kruskal-Wallis rank-sum test and one-way ANOVA for continuous variables, which are presented as medians (ranges). Fisher’s exact test or the chi-square test was used to compare categorical variables. The Kaplan−Meier method was used to examine OS and RFS, and the log rank test was used to compare group differences. To examine the parameters influencing OS and RFS, univariate and multivariate Cox proportional hazards regression were performed. All statistical analyses were carried out using SPSS software version 21.0, and graphs were created using GraphPad Prism version 9.0. Two-sided p values <0.05 were considered statistically significant. Additionally, OncoPrint was utilized to identify and visualize gene mutations in AML patients.
3 Results
3.1 Clinical characteristics
This study comprised 297 patients with a median age of 48 years (range: 5-79 years), including 172 men and 125 women. In the study population, the median and range of WBC and PLT counts, Hb levels, and percentage of blasts in BM and PB were 15.88 (0.43-384.87)×109/L, 38.5 (2-579)× 109/L, 76 (35-147) g/L, 62.0% (12.4%-96.0%), and 43% (0-97%), respectively. According to the mutation of DNMT3A and the expression of CD7, the patients were divided into the following four groups: the DNMT3A-mutated/CD7+ group (n=21), the DNMT3A-mutated/CD7- group (n=44), the DNMT3A-wild-type/CD7+ group (n=104), and the DNMT3A-wild-type/CD7-group (n=128). We found that the DNMT3A-mutated/CD7+ group had significantly higher initial WBC counts than the other three groups (all p<0.05, Table 1). The age of the DNMT3A-mutated patients was significantly higher than that of the DNMT3A-wild-type patients (p=0.001). The DNMT3A-mutated patients had higher initial PLT counts and BM blast ratios (p<0.001, p=0.030). The rate of the FAB-M5 subtype was higher in DNMT3A-mutated AML patients than in DNMT3A-wild-type AML patients (35.4% vs. 15.1%, p<0.001), and the rate of the FAB-M2 subtype was lower than that in DNMT3A-wild-type patients (38.5% vs. 59.5%, p=0.003). Patients were categorized into favorable, intermediate and adverse risk groups according to the 2022 ELN stratification, with no significant differences among the four groups (p>0.05). Moreover, no differences in any other baseline characteristics were discovered among these four groups (p>0.05, Table 1).
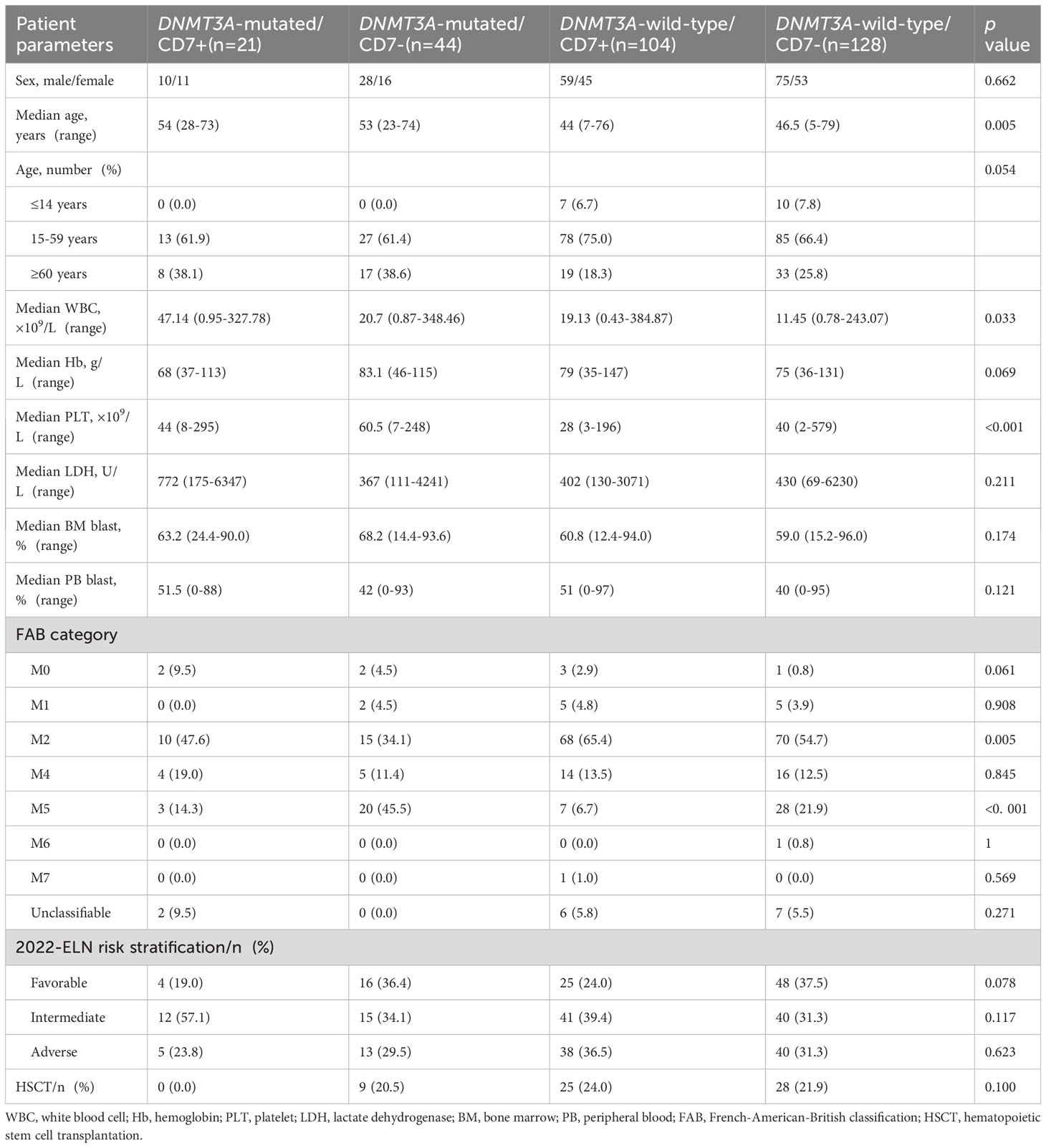
Table 1 Clinical characteristics of patients with AML classified according to the status of DNMT3A mutation and CD7 expression.
3.2 Correlation between CD7 expression and other immunophenotypes
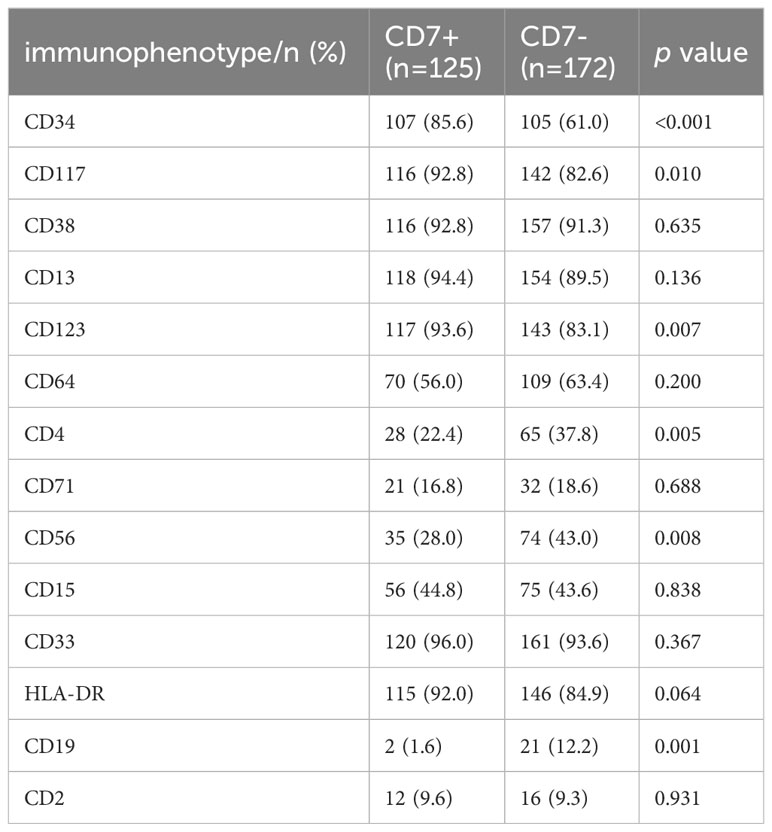
Our results showed that the positive expression proportions of CD34, CD117 and CD123 were significantly higher in the CD7+ group than in the CD7- group (p<0.05, Table 2); the positive expression proportions of CD4, CD56 and CD19 were significantly lower in the CD7+ group than in the CD7- group (p<0.05; Table 2). The differences in the proportions of CD38, HLA-DR, CD15, CD33, CD2, CD64 and CD71 expression between the two groups were compared separately, and the results were not significantly different (p>0.05, Table 2).
3.3 Correlation of DNMT3A mutation with other molecular genetics
According to the 2022 ELN genetic risk stratification, we divided AML patients into favorable (n=36), intermediate (n=242), and adverse (n=19) karyotype groups. Within the intermediate karyotype group, the CEBPA bZIP mutation rate was significantly lower in the DNMT3A-mutated group (0.0%) than in the DNMT3A-wild-type group (12.0%); the FLT3-ITD mutation rate (32.2%), NPM1 mutation rate (47.5%) and IDH1/2 mutation rate (28.8%) were significantly higher in the DNMT3A-mutated group than in the DNMT3A-wild-type group (11.5%, 13.1%, 10.9%), the differences were statistically significant (p<0.05, Table 3). However, no differences in gene mutation rates were observed between the DNMT3A-mutated group and the DNMT3A-wild-type group in the favorable and adverse karyotype groups (Supplementary Table 2). Our further analysis revealed that the DNMT3A-mutated/CD7+ group still exhibited a higher FLT3-ITD mutation rate compared with the non-DNMT3Amut/CD7+ group (Supplementary Table 3). In addition, we generated an oncoprint to display the distribution of gene mutations in AML patients (Figure 1).
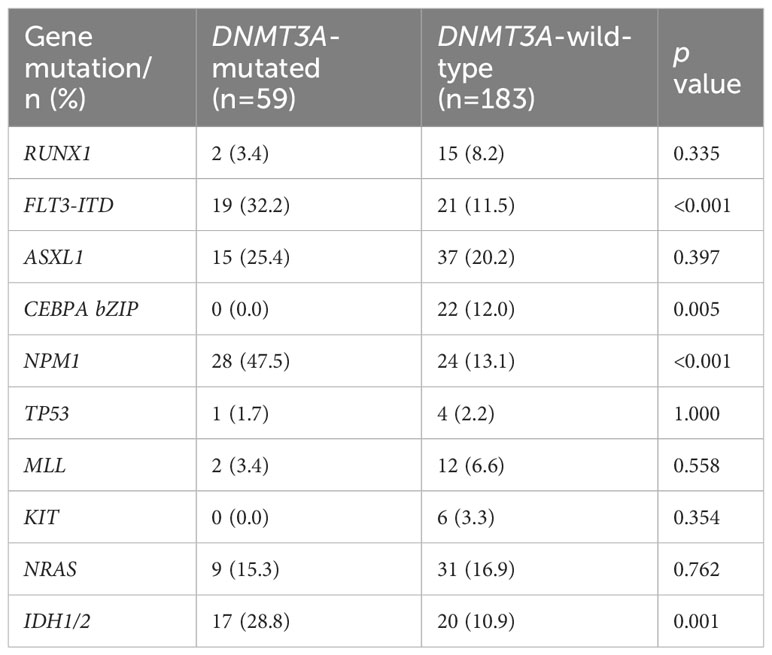
Table 3 Correlation of DNMT3A mutation status with other molecular genetic mutations in intermediate karyotype.
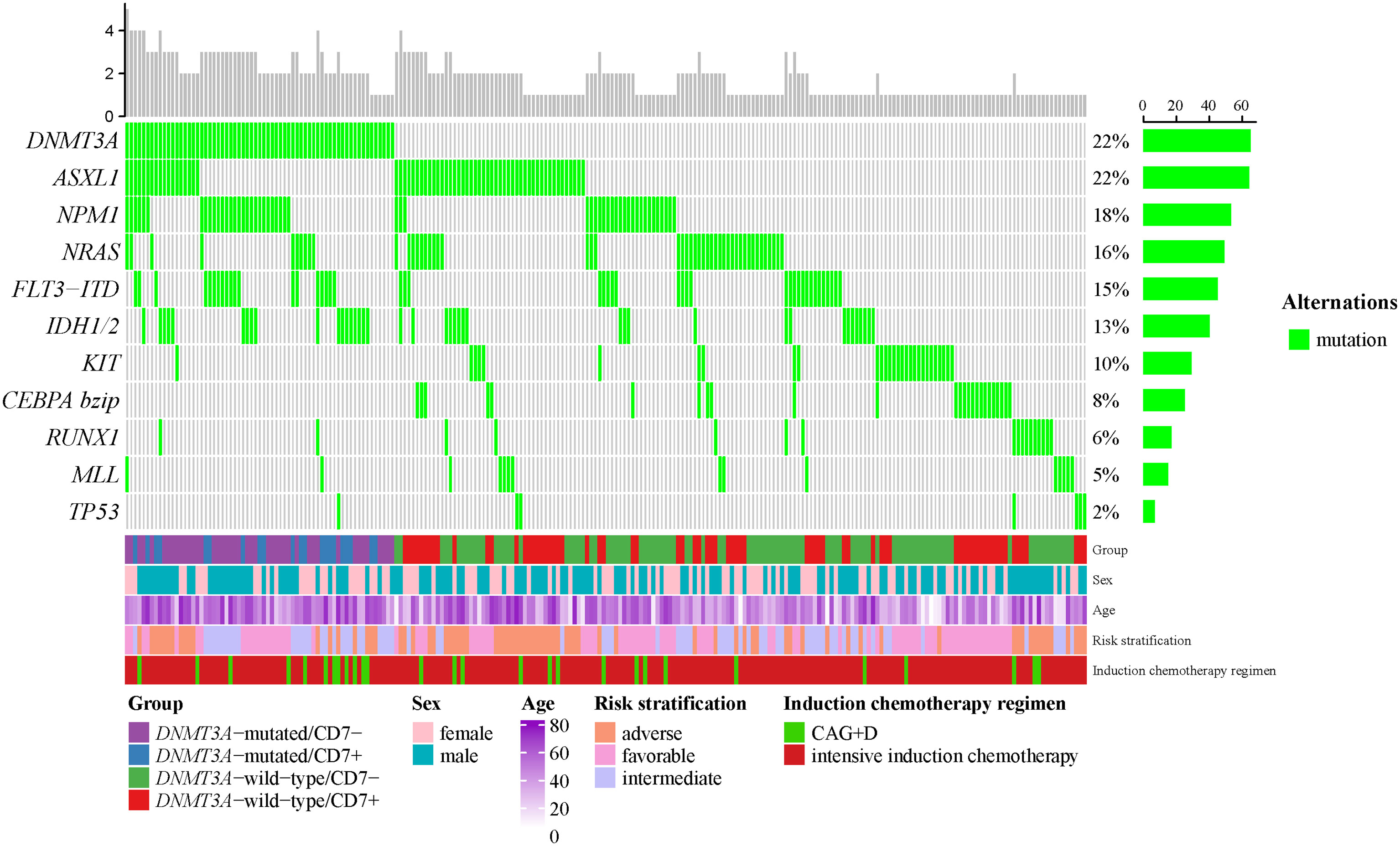
Figure 1 The OncoPrint of genes in AML. Mutations were colored as green. The upper barplot indicates the number of genetic mutation per patient, while the right barplot shows the proportion of genetic mutations per gene. The group, sex, age, 2022-ELN risk stratification and induction chemotherapy regimen were added as annotations for the patients.
3.4 Prognostic impact of DNMT3A mutation and CD7 expression on AML patients respectively
AML patients were divided into two groups based on their DNMT3A mutation status; the median OS time was 22 months for DNMT3A-mutated patients and 32 months for DNMT3A-wild-type patients, with no statistically significant difference (p>0.05, Figure 2A); similarly, the median RFS of the DNMT3A-mutated group was not significantly different from that of the DNMT3A-wild-type group (10 months vs. 22 months, p>0.05, Figure 2B). AML patients were divided into two groups according to CD7 expression; the median OS time was 24 months in the CD7+ group and 51 months in the CD7- group, and the difference in OS between the two groups was not significantly different (p>0.05, Figure 2C); the median RFS time was 20 months and 19 months in the CD7+ and CD7- groups, respectively, and the difference was also not significantly different (p>0.05, Figure 2D).
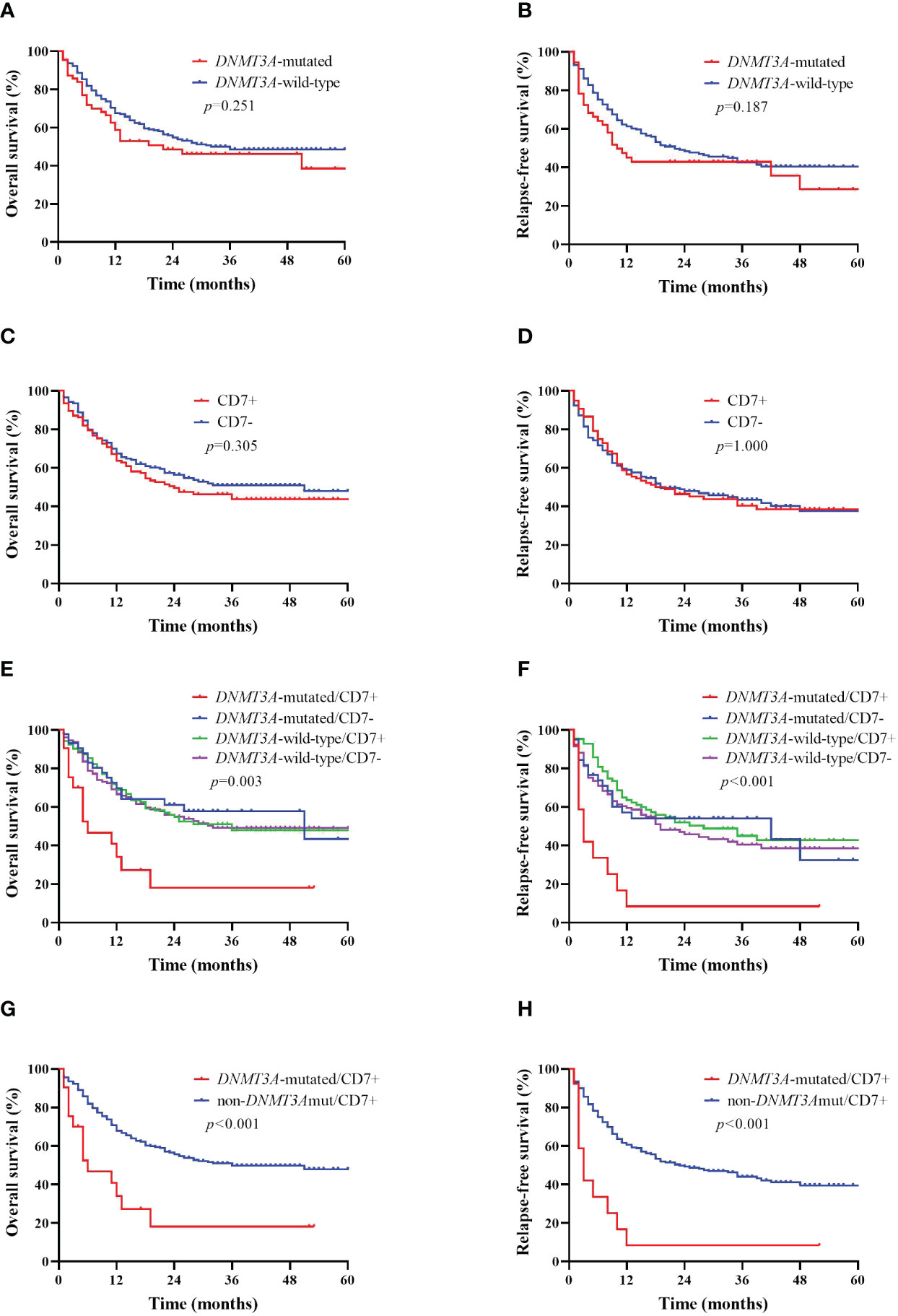
Figure 2 Kaplan–Meier curves of overall survival (OS) and relapse-free survival (RFS) in AML patients according to DNMT3A mutation and CD7 expression. (A, B) Kaplan–Meier curves of OS and RFS in DNMT3A mutation and DNMT3A wild-type AML patients. (C, D) Kaplan–Meier curves of OS and RFS in CD7+ and CD7- AML patients. (E, F) Kaplan–Meier curves of OS and RFS of AML patients in the DNMT3A-mutated/CD7+ group, DNMT3A-mutated/CD7- group, DNMT3A-wild-type/CD7+ group and the DNMT3A-wild-type/CD7- group. (G, H) Kaplan–Meier curves of OS and RFS in DNMT3A-mutated/CD7+ and non-DNMT3Amut/CD7+ AML patients.
3.5 Prognostic impact of CD7+ with DNMT3A mutation in AML patients
The median OS time for patients in the DNMT3A-mutated/CD7+ group was 6 months, whereas that in the DNMT3A-mutated/CD7- group was 51 months (p=0.003, Figure 2E); similarly, the median OS in the DNMT3A-mutated/CD7+ group was significantly shorter than that in the DNMT3A-wild-type/CD7+ and DNMT3A-wild-type/CD7- groups (6 months vs. 36 months, p=0.001; 6 months vs. 32 months, p=0.001, Figure 2E). In addition, the median RFS was significantly shorter in the DNMT3A-mutated/CD7+ group than in the other three groups (3 months vs. 42 months, p=0.003; 3 months vs. 28 months, p<0.001; 3 months vs. 19 months, p<0.001, Figure 2F). Therefore, we reclassified AML patients into the DNMT3A-mutated/CD7+ group (n=21) and the other (non-DNMT3Amut/CD7+) group (n=276) based on whether they had concomitant DNMT3A mutation and positive CD7 expression. Comparing the OS and RFS of patients in the two groups separately, we found that the DNMT3A-mutated/CD7+ group had significantly shorter OS and RFS (p<0.001, Figures 2G, H).
3.6 Effect of CD7+ with DNMT3A mutation on the initial treatment response in AML patients
Among the entire cohort of 297 patients, CR (CR/CRi) was achieved in 233 patients (78.5%) after two cycles of induction chemotherapy.
CR was achieved in 12 patients (57.1%) in the DNMT3A-mutated/CD7+ group compared to 221 patients (80.1%) in the non-DNMT3Amut/CD7+ group, and the CR rate was significantly lower in the DNMT3A-mutated/CD7+ group than in the non-DNMT3Amut/CD7+ group (p=0.003). Of the 233 patients with CR, 97 experienced disease recurrence, including 9 (75.0%) in the DNMT3A-mutated/CD7+ group and 88 (39.8%) in the non-DNMT3Amut/CD7+ group. Patients in the DNMT3A-mutated/CD7+ group presented a higher risk of recurrence (p=0.032, Table 4). Consistent with this, we found that DNMT3A-mutated/CD7+ patients still showed a low CR rate and a high relapse rate among patients who received intensive induction therapy (Supplementary Table 4).
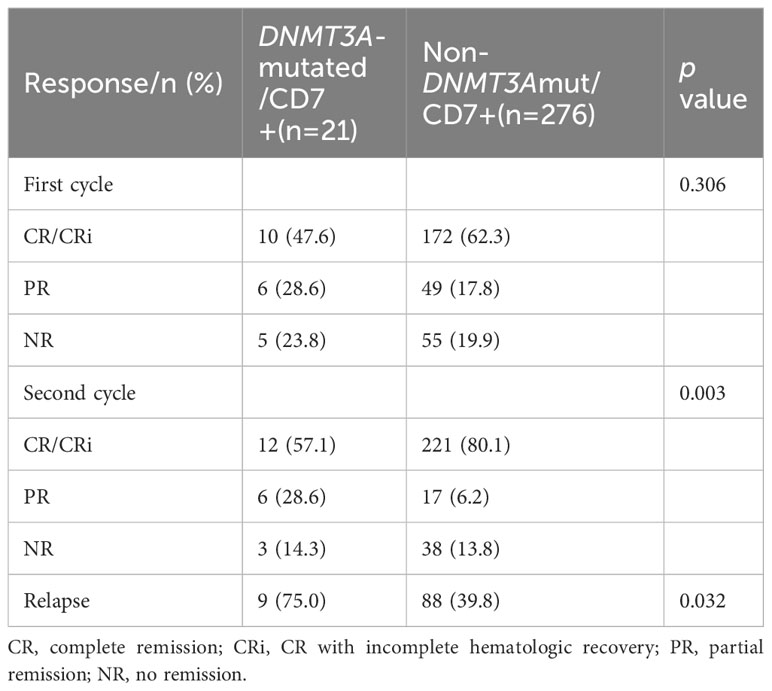
Table 4 Treatment response to the first and second cycles of induction chemotherapy between the two groups.
3.7 Univariate and multivariable analyses of clinical prognostic factors
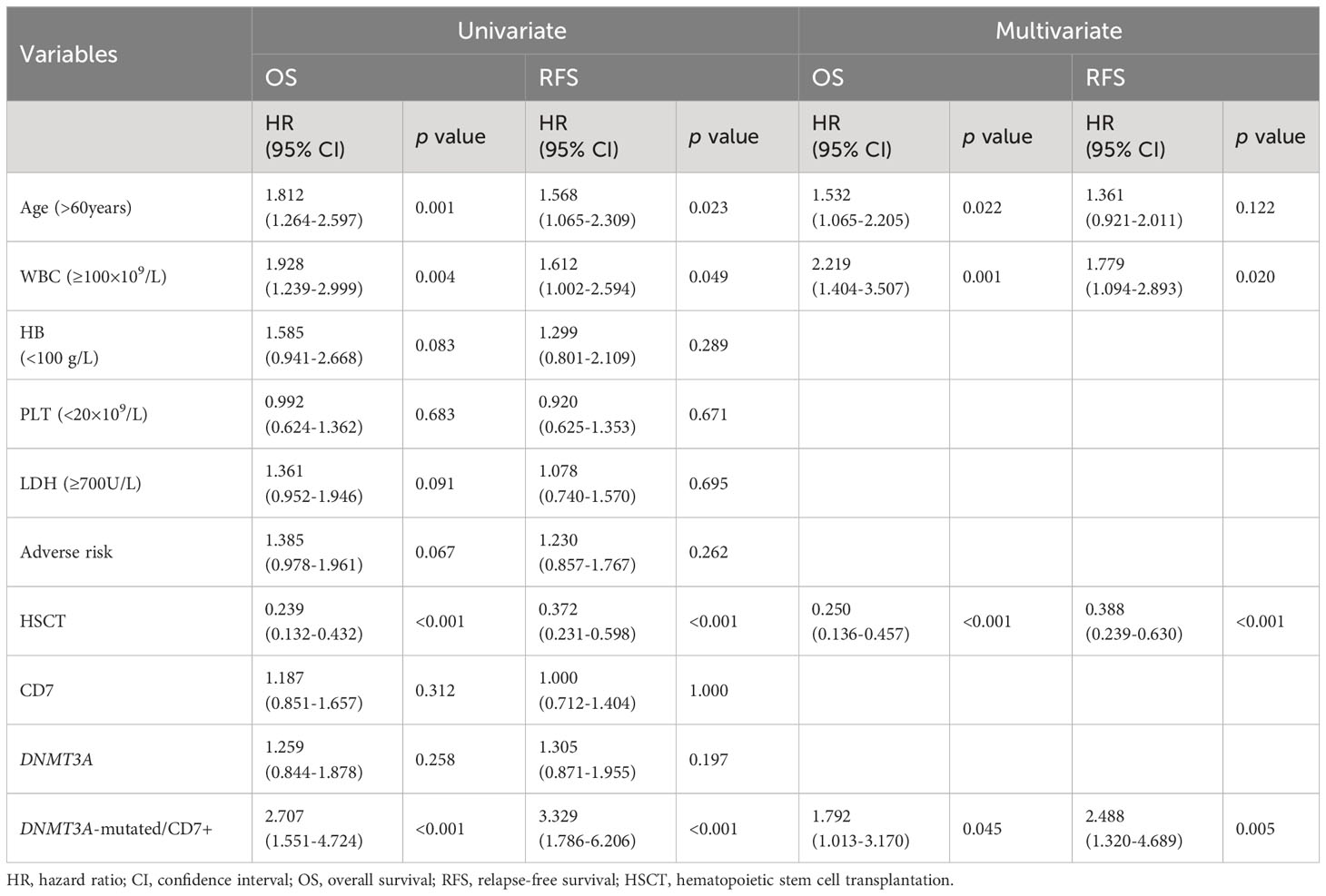
We analyzed various prognostic factors affecting OS and RFS in AML patients. Univariate Cox regression analysis showed that age >60 years, WBC count ≥100 × 109/L, no HSCT, and DNMT3A-mutated/CD7+ were risk factors for poorer OS and RFS in patients with newly diagnosed AML. Multifactorial Cox regression analysis showed that DNMT3A-mutated/CD7+ remained significantly associated with shorter OS and RFS (Table 5). In addition, we further analyzed the factors affecting prognosis in patients who did not undergo HSCT and those who received intensive induction therapy, and multifactorial Cox regression analysis still suggested that DNMT3A-mutated/CD7+ was an independent risk factor for AML patients (Supplementary Table 5).
4 Discussion
In this study, the importance of DNMT3A mutation combined with CD7 expression for the precise stratification of AML patients was evaluated. Our results showed that CD7+ AML patients with DNMT3A mutations had lower CR rates and higher relapse rates. More importantly, our data showed for the first time that OS and RFS were significantly shorter in AML patients with DNMT3A mutations combined with CD7 expression, whereas DNMT3A mutations or CD7-positive expression alone had no significant effect on prognosis.
The CD7 antigen is a T-cell-related antigen that is expressed on thymocytes, T cells, and natural killer cells as well as lymphocytes and myeloid progenitors in healthy individuals (18), whereas 30% of AML patients exhibit positive CD7 expression (16–18). There is still controversy about whether CD7+ indicates poor prognosis of AML. Our data showed that there was no significant difference in OS and RFS between CD7+ AML patients and CD7- AML patients, which was similar to that reported by Fang et al. (23), suggesting that CD7 expression may not be related to the prognosis of AML. However, Ogata et al. found differences in the prognostic impact of CD7+ in AML patients under different cytogenetic conditions (18). Therefore, the prognostic significance of CD7 positivity may need further subgroup analysis.
Some studies have shown that DNMT3A gene mutations precede the AML stage (26, 27). Therefore, the impact of DNMT3A gene mutations on the prognosis of AML patients should be given sufficient attention. In our analysis, there was no significant difference in OS and RFS between the DNMT3A-mutated group and the DNMT3A-wild-type group. Univariate and multivariate analysis showed that DNMT3A mutation was also unrelated with OS and EFS. The present study is consistent with previous studies showing that DNMT3A mutation cannot be used as an independent prognostic marker for AML patients (28, 29). However, some studies have found that DNMT3A mutations in AML patients affect patient prognosis and are negatively associated with patient prognosis (6, 7), and this adverse prognostic effect may be limited to specific types of AML groups (8–11). Therefore, we further analyzed their prognostic impact in combination with specific immunophenotypes.
Interestingly, when we combined DNMT3A mutation with CD7-positive expression, we found that patients in the DNMT3A-mutated/CD7+ group had significantly shorter OS and RFS compared with patients in the other three groups. However, no significant differences were observed in OS and RFS among the remaining three groups. Therefore, we reclassified AML patients into DNMT3A-mutated/CD7+ and non-DNMT3Amut/CD7+ groups. Notably, we found that patients in the DNMT3A-mutated/CD7+ group had lower CR rates, higher relapse rates, and shorter OS and RFS. Multivariate regression analysis also showed that CD7+ with DNMT3A mutation was a Powerful Predictor for poor prognosis in AML patients. This result has not yet been reported in the literature.
The reason for the poorer prognosis of DNMT3A-mutated/CD7+ patients is currently unknown. We hypothesize that it is related to the following factors. First, we observed higher WBC counts at the initial diagnosis in patients with DNMT3A-mutated/CD7+, potentially explaining their poorer prognosis. In addition, our data showed that AML patients with DNMT3A mutations frequently carry NPM1 and FLT3-ITD mutations, which is consistent with previous studies (9, 11, 29, 30). Studies have shown that AML patients with mutations in all three DNMT3A/FLT3/NPM1 genes have a higher disease load and lower cumulative overall survival (31–33). Our data also showed that the DNMT3A mutation group was associated with lower CEBPA bZIP mutation rates. Increasing evidence suggests that CEBPA bZIP in-frame mutations are associated with favorable AML prognoses and have been incorporated into ELN risk stratification (3, 34–36). Therefore, we infer this is related to the poor prognosis of DNMT3A-mutated/CD7+ patients. Furthermore, we also found that the positive expression proportion of CD34, CD117, and CD123 was significantly higher in the CD7+ AML group than in the CD7- AML group, and the proportion of positive expression of CD4- was lower than in the CD7- AML group. CD34 and CD117 are precursor markers, and previous studies have shown that CD34-positive expression is associated with lower CR rates (17, 37–39). CD123 is a specific antigen on the surface of leukemia stem cells (LSCs) (40), and several studies have found that CD123 is an independent risk factor for CR and OS in AML patients (40, 41). Relevant literature shows that AML leukemia cells with CD4 expression originate from a relatively mature stage (42). Our study showed that CD7+ was associated with high expression of immature phenotype and low expression of mature phenotype. This association may contribute to the poorer clinical outcomes of AML patients with DNMT3A-mutated/CD7+. However, the underlying mechanism of this association requires further exploratory studies.
As this study was a retrospective study, there may be a small number of cases in some groups after grouping, which may have biased the data. In addition, the heterogeneity of treatment regimens may confound the results of this study. The data we collected showed no patients in the DNMT3A-mutated/CD7+ group who underwent HSCT. We consider that the reason for this phenomenon may be related to the small sample size in this subgroup analysis. Furthermore, not undergoing HSCT may also be influenced by the patient’s financial status, HLA matching, and complications. Therefore, large-scale, multicenter prospective studies are needed to further validate the prognostic value of CD7+ with DNMT3A mutation.
5 Conclusions
In conclusion, we found that CD7+ with DNMT3A mutation is an effective prognostic marker in AML patients and has a negative impact on the OS and RFS of these patients. Our results reveal the clinical value of immunophenotyping for the precise molecular genetic stratification of AML.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethical Committee of Henan Provincial People’s Hospital (2021-140-02). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YB: Conceptualization, Writing – original draft. XS: Investigation, Formal analysis, Writing – original draft. ML: Investigation, Writing – review & editing. XN: Resources, Writing – review & editing. WC: Resources, Writing – review & editing. JN: Writing – review & editing. XX: Writing – review & editing. YC: Writing – review & editing. KS: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was partially supported by the National Natural Science Foundation of China (No. 81971508), the Henan Provincial Science and Technology Research Project (No. 222102310101) and Henan Province Medical Science and Technology Tackling Program Joint Co-Construction Project (No. LHGJ20230023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1342998/full#supplementary-material
References
1. Kennedy VE, Smith CC. FLT3 mutations in acute myeloid leukemia: key concepts and emerging controversies. Front Oncol. (2020) 10:612880. doi: 10.3389/fonc.2020.612880
2. Horton SJ, Huntly BJ. Recent advances in acute myeloid leukemia stem cell biology. Haematologica. (2012) 97:966–74. doi: 10.3324/haematol.2011.054734
3. Dohner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. (2022) 140:1345–77. doi: 10.1182/blood.2022016867
4. Sato H, Wheat JC, Steidl U, Ito K. DNMT3A and TET2 in the pre-leukemic phase of hematopoietic disorders. Front Oncol. (2016) 6:187. doi: 10.3389/fonc.2016.00187
5. Yang L, Shen K, Zhang M, Zhang W, Cai H, Lin L, et al. Clinical features and microRNA expression patterns between AML patients with DNMT3A R882 and frameshift mutations. Front Oncol. (2019) 9:1133. doi: 10.3389/fonc.2019.01133
6. Zare-Abdollahi D, Safari S, Movafagh A, Riazi-Isfahani S, Ghadyani M, Hashemi-Gorji F, et al. A mutational and expressional analysis of DNMT3A in acute myeloid leukemia cytogenetic subgroups. Hematology. (2015) 20:397–404. doi: 10.1179/1607845415Y.0000000001
7. Hou HA, Kuo YY, Liu CY, Chou WC, Lee MC, Chen CY, et al. DNMT3A mutations in acute myeloid leukemia: stability during disease evolution and clinical implications. Blood. (2012) 119:559–68. doi: 10.1182/blood-2011-07-369934
8. Tan M, Ng IKS, Chen Z, Ban K, Ng C, Chiu L, et al. Clinical implications of DNMT3A mutations in a Southeast Asian cohort of acute myeloid leukaemia patients. J Clin Pathol. (2017) 70:669–76. doi: 10.1136/jclinpath-2016-204195
9. Loghavi S, Zuo Z, Ravandi F, Kantarjian HM, Bueso-Ramos C, Zhang L, et al. Clinical features of de novo acute myeloid leukemia with concurrent DNMT3A, FLT3 and NPM1 mutations. J Hematol Oncol. (2014) 7:74. doi: 10.1186/s13045-014-0074-4
10. Ahn JS, Kim HJ, Kim YK, Lee SS, Jung SH, Yang DH, et al. DNMT3A R882 mutation with FLT3-ITD positivity is an extremely poor prognostic factor in patients with normal-karyotype acute myeloid leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2016) 22:61–70. doi: 10.1016/j.bbmt.2015.07.030
11. Marcucci G, Metzeler KH, Schwind S, Becker H, Maharry K, Mrozek K, et al. Age-related prognostic impact of different types of DNMT3A mutations in adults with primary cytogenetically normal acute myeloid leukemia. J Clin Oncol. (2012) 30:742–50. doi: 10.1200/JCO.2011.39.2092
12. Rabinowich H, Pricop L, Herberman RB, Whiteside TL. Expression and function of CD7 molecule on human natural killer cells. J Immunol. (1994) 152:517–26. doi: 10.4049/jimmunol.152.2.517
13. Ochi K, Fuji S, Takano K, Tajima K, Ito A, Tanaka T, et al. The putative anti-leukemic effects of anti-thymocyte globulins in patients with CD7-positive acute myeloid leukemia. Bone Marrow Transplant. (2018) 53:1019–29. doi: 10.1038/s41409-018-0115-7
14. KITa K, Miwa H, Nakase K, Kawakami K, Kobayashi T, Shirakawa S, et al. Clinical importance of CD7 expression in acute myelocytic leukemia. The Japan Cooperative Group of Leukemia/Lymphoma. Blood. (1993) 81:2399–405. doi: 10.1182/blood.V81.9.2399.2399
15. Saxena A, Sheridan DP, Card RT, McPeek AM, Mewdell CC, Skinnider LF. Biologic and clinical significance of CD7 expression in acute myeloid leukemia. Am J Hematol. (1998) 58:278–84. doi: 10.1002/(sici)1096-8652(199808)58:4<278::aid-ajh5>3.0.co;2-n
16. Cruse JM, Lewis RE, Pierce S, Lam J, Tadros Y. Aberrant expression of CD7, CD56, and CD79a antigens in acute myeloid leukemias. Exp Mol Pathol. (2005) 79:39–41. doi: 10.1016/j.yexmp.2005.02.003
17. Chang H, Salma F, Yi QL, Patterson B, Brien B, Minden MD. Prognostic relevance of immunophenotyping in 379 patients with acute myeloid leukemia. Leuk Res. (2004) 28:43–8. doi: 10.1016/s0145-2126(03)00180-2
18. Ogata K, Yokose N, Shioi Y, Ishida Y, Tomiyama J, Hamaguchi H, et al. Reappraisal of the clinical significance of CD7 expression in association with cytogenetics in de novo acute myeloid leukaemia. Br J Haematol. (2001) 115:612–5. doi: 10.1046/j.1365-2141.2001.03139.x
19. Chang H, Yeung J, Brandwein J, Yi QL. CD7 expression predicts poor disease free survival and post-remission survival in patients with acute myeloid leukemia and normal karyotype. Leuk Res. (2007) 31:157–62. doi: 10.1016/j.leukres.2006.06.001
20. Lv K, Cai C, Chen J, Xu M, Wan L, Zhou M, et al. Prognostic value of lymphoid marker CD7 expression in acute myeloid leukemia patients undergoing allogeneic hematopoietic cell transplantation in first morphological complete remission. Int J Hematol. (2021) 114:464–71. doi: 10.1007/s12185-021-03182-y
21. Tiftik N, Bolaman Z, Batun S, Ayyildiz O, Isikdogan A, Kadikoylu G, et al. The importance of CD7 and CD56 antigens in acute leukaemias. Int J Clin Pract. (2004) 58:149–52. doi: 10.1111/j.1368-5031.2004.0018.x
22. Kornblau SM, Thall P, Huh YO, Estey E, Andreeff M. Analysis of CD7 expression in acute myelogenous leukemia: martingale residual plots combined with 'optimal' cutpoint analysis reveals absence of prognostic significance. Leukemia. (1995) 9:1735–41.
23. Fang F, Zhu P, Zhang Y, Lu XZ, Dong YJ, Sun YH, et al. Clinical features and prognosis of 227 cases of acute myeloid leukemia with cross-lineage antigen expression. Zhongguo Shi Yan Xue Ye Xue Za Zhi. (2016) 24:990–7. doi: 10.7534/j.issn.1009-2137.2016.04.006
24. Leukemia & Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association. Chinese guidelines for diagnosis and treatment of adult acute myeloid leukemia (not APL) (2017). Zhonghua Xue Ye Xue Za Zhi. (2017) 38:177–82. doi: 10.3760/cma.j.issn.0253-2727.2017.03.001
25. O'Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, et al. Acute myeloid leukemia, version 3.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2017) 15:926–57. doi: 10.6004/jnccn.2017.0116
26. Walter MJ, Ding L, Shen D, Shao J, Grillot M, McLellan M, et al. Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia. (2011) 25:1153–8. doi: 10.1038/leu.2011.44
27. Abdel-Wahab O, Pardanani A, Rampal R, Lasho TL, Levine RL, Tefferi A. DNMT3A mutational analysis in primary myelofibrosis, chronic myelomonocytic leukemia and advanced phases of myeloproliferative neoplasms. Leukemia. (2011) 25:1219–20. doi: 10.1038/leu.2011.82
28. Im AP, Sehgal AR, Carroll MP, Smith BD, Tefferi A, Johnson DE, et al. DNMT3A and IDH mutations in acute myeloid leukemia and other myeloid Malignancies: associations with prognosis and potential treatment strategies. Leukemia. (2014) 28:1774–83. doi: 10.1038/leu.2014.124
29. Gaidzik VI, Schlenk RF, Paschka P, Stolzle A, Spath D, Kuendgen A, et al. Clinical impact of DNMT3A mutations in younger adult patients with acute myeloid leukemia: results of the AML Study Group (AMLSG). Blood. (2013) 121:4769–77. doi: 10.1182/blood-2012-10-461624
30. Renneville A, Boissel N, Nibourel O, Berthon C, Helevaut N, Gardin C, et al. Prognostic significance of DNA methyltransferase 3A mutations in cytogenetically normal acute myeloid leukemia: a study by the Acute Leukemia French Association. Leukemia. (2012) 26:1247–54. doi: 10.1038/leu.2011.382
31. Chen M, Zeng Z, Li X, Qin W, Cai X, Chen S, et al. Clinical features and prognostic significance of DNMT3A, FLT3, and NPM1 mutations in de novo acute myeloid leukemia patients. Int J Lab Hematol. (2023) 45(6): 899–907. doi: 10.1111/ijlh.14133
32. Elrhman H, El-Meligui YM, Elalawi SM. Prognostic impact of concurrent DNMT3A, FLT3 and NPM1 gene mutations in acute myeloid leukemia patients. Clin Lymphoma Myeloma Leuk. (2021) 21:e960–e9. doi: 10.1016/j.clml.2021.07.011
33. Qi Y, Gao SJ, Lin H, Tan YH, Liu QJ, Sun JN, et al. The clinical characteristics and prognoses of de novo acute myeloid leukemia patients with DNA methyltransferase 3A gene mutations. Zhonghua Xue Ye Xue Za Zhi. (2019) 40:227–31. doi: 10.3760/cma.j.issn.0253-2727.2019.03.012
34. Tarlock K, Lamble AJ, Wang YC, Gerbing RB, Ries RE, Loken MR, et al. CEBPA-bZip mutations are associated with favorable prognosis in de novo AML: a report from the Children's Oncology Group. Blood. (2021) 138:1137–47. doi: 10.1182/blood.2020009652
35. WaKITa S, Sakaguchi M, Oh I, Kako S, Toya T, Najima Y, et al. Prognostic impact of CEBPA bZIP domain mutation in acute myeloid leukemia. Blood Adv. (2022) 6:238–47. doi: 10.1182/bloodadvances.2021004292
36. Miyashita N, Onozawa M, Yoshida S, Kimura H, Takahashi S, Yokoyama S, et al. Prognostic impact of FLT3-ITD, NPM1 mutation and CEBPA bZIP domain mutation in cytogenetically normal acute myeloid leukemia: a Hokkaido Leukemia Net study. Int J Hematol. (2023) 118:36–46. doi: 10.1007/s12185-023-03567-1
37. Zahurak M, Karp JE, Piantadosi S, Geller RB, Burke PJ, Hurwitz CA, et al. Prognostic importance of immunophenotyping in adults with acute myelocytic leukaemia: the significance of the stem-cell glycoprotein CD34 (My10). British journal of haematology. (1990) 76(3): 340–347. doi: 10.1111/j.1365-2141.1990.tb06365.x
38. Solary E, Casasnovas RO, Campos L, Bene MC, Faure G, Maingon P, et al. Surface markers in adult acute myeloblastic leukemia: correlation of CD19+, CD34+ and CD14+/DR– phenotypes with shorter survival. Groupe d'Etude Immunologique des Leucemies (GEIL). Leukemia. (1992) 6:393–9.
39. Lauria F, Raspadori D, Ventura MA, Rondelli D, Testoni N, Tosi P, et al. The presence of lymphoid-associated antigens in adult acute myeloid leukemia is devoid of prognostic relevance. Stem cells (1995) (Dayton, Ohio) 13(4): 428–34. doi: 10.1002/stem.5530130414
40. Testa U, Riccioni R, Militi S, Coccia E, Stellacci E, Samoggia P, et al. Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood. (2002) 100:2980–8. doi: 10.1182/blood-2002-03-0852
41. Yue WQ, Tang GS, Liu M, Cheng H, Ding J, Wang T, et al. The study of expression and prognostic value of CD123 in acute myeloid leukemia bone marrow blasts. Zhonghua Xue Ye Xue Za Zhi. (2017) 38:876–82. doi: 10.3760/cma.j.issn.0253-2727.2017.10.010
Keywords: acute myeloid leukemia, CD7, Dnmt3a, immunophenotype, molecular diagnostics, prognosis
Citation: Bai Y, Sun X, Li M, Niu X, Cao W, Niu J, Xiao X, Chen Y and Sun K (2024) CD7-positive leukemic blasts with DNMT3A mutations predict poor prognosis in patients with acute myeloid leukemia. Front. Oncol. 14:1342998. doi: 10.3389/fonc.2024.1342998
Received: 22 November 2023; Accepted: 15 February 2024;
Published: 21 March 2024.
Edited by:
Carlo Finelli, Sant’Orsola-Malpighi Polyclinic, ItalyReviewed by:
Diego A. Pereira-Martins, University of Groningen, NetherlandsXiebing Bao, The First Affiliated Hospital of Soochow University, China
Copyright © 2024 Bai, Sun, Li, Niu, Cao, Niu, Xiao, Chen and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Sun, sunkai@cellscience.org
†These authors contributed equally to this work and share first authorship
 Yanliang Bai
Yanliang Bai Xiaobai Sun1†
Xiaobai Sun1† Weijie Cao
Weijie Cao Junwei Niu
Junwei Niu Yuqing Chen
Yuqing Chen Kai Sun
Kai Sun