- 1Burjeel Medical City, Burjeel Holding, Abu Dhabi, United Arab Emirates
- 2Gulf Medical University, Ajman, United Arab Emirates
- 3Emirates Oncology Society, Dubai, United Arab Emirates
- 4College of Medicine, University of Sharjah, Sharjah, United Arab Emirates
- 5Gulf Cancer Society, Alsafa, Kuwait
- 6Almoosa Specialist Hospital Cancer Center, Al Ahsa, Saudi Arabia
- 7American Hospital, Dubai, United Arab Emirates
- 8National Center for Cancer Care & Research, Doha, Qatar
- 9Kuwait Cancer Control Centre, Kuwait, Kuwait
- 10Consultant Oncologist and Acting Head of Oncology Dubai Hospital, DHA, Dubai, United Arab Emirates
- 11King Khalid University Hospital, Riyadh, Saudi Arabia
- 12Cleveland Clinic, Abu Dhabi, United Arab Emirates
- 13Dr. Sulaiman Al Habib Medical Center, Riyadh, Saudi Arabia
BReast CAncer (BRCA)1 and BRCA2 gene pathogenic variants account for most hereditary breast cancers (BC). Identification of BRCA mutations can significantly influence both prognosis and treatment outcomes. Furthermore, it enables the identification of individuals who are at heightened risk of developing BC due to inherited genetic mutations. Many developing countries rely on western guidelines for BRCA testing and BC management; however, there exist wide disparities in the prevalence of risk factors, availability of medical resources, and practice patterns. Guidelines tailored to specific regions can help mitigate healthcare variations, promote consistency in treatment, and aid healthcare providers in identifying effective therapies for improving patient outcomes. Hence, oncologists from the Gulf Cooperation Council (GCC) congregated virtually in March 2023 and reviewed existing data on the epidemiology of BC, BRCA mutations, practices and challenges associated with BRCA testing and management of BRCA mutated early-stage BC in the GCC region. They also provided insights on the real-world diagnostic and treatment practices and challenges in the GCC region in the BRCA-mutated early-stage BC domain and suggested some variations to international guidelines to aid their uptake in this region.
1 Introduction
Breast cancer (BC) is the most commonly diagnosed cancer and the leading cause of cancer death among women in the Gulf Cooperation Council (GCC) region, with an age-standardized incidence rate of 34.4 per 100 000 and a mortality rate of 10.6 per 100 000 in 2020 (Supplementary Table S1) (1). In most GCC countries, the incidence of BC has increased over time among women (2). Hereditary factors are responsible for around 10% to 30% of BC cases (3) and 16% of these hereditary cases are related to germline mutations in BReast CAncer gene (BRCA)1 and BRCA2 genes (4). Other factors such as early age menarche, later age at menopause, shorter breastfeeding periods, use of oral contraceptives or hormonal therapy, dense breasts, and older age are found to be associated with increased risk of BC (5, 6).
Compared to the Western population, BCs have diverse clinical, pathological and molecular features including early onset, higher tumor grade, higher human epidermal growth factor receptor (HER)2 amplification rate, more aggressive subtypes and a lower rate of luminal subtype, in the GCC population (7–9). Evidence suggests that approximately 46.2% to 54% of BC patients are diagnosed at advanced disease stage (7, 8, 10–12), 23.3% to 28% are diagnosed with localized tumors while ≤2% with in-situ carcinoma (8, 10). In the GCC region, the vast majority of BC cases (82.1% to 93%) have invasive ductal carcinoma (IDC) (7, 8), and 19.2% to 29.5% have HER2 overexpression (7, 8, 12), while 14.3% to 26.9% have triple-negative BC (TNBC) (7, 8). The average age of patients at the presentation of BC is at least a decade younger in the GCC population compared to the Western population (<48 years vs 60 years) (7, 13).
Outcomes in BC depend primarily on timely diagnosis and access to appropriate treatment. Patients who are diagnosed at the early stages (stage 0, I, II) tend to have higher overall survival (OS) rates than people diagnosed with stage III or IV BC (14); the 5-year survival rate reported for women with stage I BC was found to be 99% and the same for patients with stage II BC was 86% (14). The 5-year survival rate in the GCC region ranges between 63% and 89%, with the highest 5-year survival rate being reported in the United Arab Emirates (UAE) and the least being reported in Bahrain (15–17). The cumulative risk for developing BC by age 70 years was 65% for BRCA1 carriers and 45% for BRCA2 carriers (18). Identification of BRCA mutation in a woman diagnosed with BC may have an impact on both prognosis and treatment (19)—especially it influences the extent of surgery such as the choice of breast-conserving surgery (BCS) or contralateral mastectomy, also predicts the effectiveness of platinum-based chemotherapy (20) and poly (ADP-ribose) polymerase (PARP) inhibitors (21). Moreover, it facilitates the identification of individuals who are at high risk of BC due to hereditary genetic mutations. This knowledge can help with making decisions regarding risk-reducing measures such as enhanced surveillance, prophylactic surgery, and chemoprevention. Although most developing countries lean on western guidelines for the management of BC, there are wide differences in the prevalence of risk factors, availability of medical resources, and practice patterns. Region-specific guidelines can curb healthcare variations, drive consistency in delivery, and help healthcare providers navigate effective therapies for improving patient outcomes. Hence, this expert opinion paper intends to provide data on the epidemiology of BC, BRCA mutations, practices, and challenges associated with BRCA testing in the GCC region. It will also provide recommendations for the BRCA testing and management of BRCA-mutated early-stage BC.
2 Methodology
A multidisciplinary panel of 9 oncologists, experts in BC, from 4 different GCC countries (Kuwait [n=1], Kingdom Saudi Arabia (KSA) [n=3], Qatar [n=1], and UAE [n=4]) congregated virtually in March 2023 to discuss gaps observed in the clinical practice and treatment goals in patients with BRCA mutated early-stage BC in GCC region. The aim was to gain insights into the evolving treatment paradigm in germline BRCA-mutated early-stage BC. The panel discussed the available data on disease burden, BRCA mutations (BRCAm), BRCA testing, and management practices along with associated challenges specific to their region. They provided strategic as well as implementable recommendations to enhance BRCA testing in early-stage BC in the GCC region. Additionally, members of the panel also provided recommendations for developing a treatment algorithm for BRCA-mutated early-stage BC. We present an expert opinion manuscript with recommendations for the BRCA testing and management of BRCA mutated early-stage BC in GCC, based on the published literature and expert clinical opinion. All the experts critically reviewed, revised, and approved the manuscript draft.
3 Germline BRCA1 or BRCA2 mutations and their implication on the prognosis and management of BC
Germline mutations in BRCA1/2 are found in 3% to 4% of all women with BC, including 10% to 20% of those with TNBC (22). The cumulative risk of developing BC by age 80 years is 72% (95% confidence interval [CI], 65% to 79%) and 69% (95% CI, 61% to 77%) in those harboring BRCA1 and BRCA2 mutation (23), respectively compared to 13% risk in the general population (24).
Patients with BRCA-mutated BC have distinct tumor characteristics, often characterized by a higher tumor-grade (25) with special immunophenotypic features (25–27), poorly differentiated infiltrating ductal carcinomas and a more aggressive phenotype (often triple negative/invasive ductal carcinomas) (25, 26), compared with the sporadic population (28, 29). In addition, patients who harbor BRCA1/2 mutations are more frequently diagnosed with BC at an early age (BRCA1 at 35 years and BRCA2 at 40 years) compared with those with sporadic disease (54 years) (25).
A meta-analysis demonstrated a significantly higher risk for ipsilateral breast recurrence (IBR) in BCRA1/2 mutation carriers compared to non-carriers following BCS at a median follow-up ≥7 years (30). Contralateral BC is more often observed in BRCA-mutated BC than in sporadic BC (31). Several studies demonstrated that BRCA-mutated BC has worse OS (32) and BC-specific survival (BCSS) than sporadic/BRCA-negative cases (33).
Emerging research on BC demonstrates that BRCA status predicts sensitivity to platinum-based chemotherapy (20), and PARP inhibitors (21), owing to the ability of these drugs to inhibit deoxyribonucleic acid (DNA) repair pathways. Evaluation of BRCA1/2 mutational status (33) in patients with BC helps to potentially expand treatment options, implement prevention strategies, and improve survival outcomes (34).
4 Epidemiology and prevalence of BRCA mutations in GCC
Except for Kuwait, the prevalence of a germline BRCAm in GCC countries ranges from 10% to 12% in unselected BC patient populations (35–38). In Kuwait germline BRCA1 mutation prevalence rate was reported as 21% (39). However, this finding in Kuwait could be due to a small sample size and selection bias; it should not be considered robust enough to affect the clinical practice. BRCA mutation prevalence is higher in patients with a family history of BC diagnosed at a young age or a family history of ovarian cancer (40).
5 Management of BRCA-mutated early-stage breast cancer
5.1 Role of MDT and genetic counselor
5.1.1 Role of MDT
Multidisciplinary teams (MDT) play a critical role in the early management of BC. The MDT approach is recommended by many international guidelines (41, 42).
MDT approach presents a significant impact on patient management (43). Patients discussed at MDT meetings are more likely to receive more accurate as well as complete pre-operative staging, and neo-adjuvant/adjuvant treatment (44). MDT care can intercept 98.8% of all medication errors, thereby improving the quality of care (45). In women with early BC, it has the potential to improve quality of life, reduce mortality, and reduce healthcare costs (46). Studies have reported that patients who are managed by MDTs have improved survival outcomes (47) and the relative risk of recurrence (hazard ratio: 0.84; 95% CI, 0.70 to 0.99) and death (hazard ratio: 0.89; 95% CI, 0.82 to 0.96) was significantly decreased compared to those who are not (48).
5.1.2 Role of genetic counselor
A genetic counselor plays a crucial role in early BC management. Genetic counseling before genetic testing is endorsed by many international guidelines (41, 42). Genetic counseling has been shown to improve patient outcomes with positive downstream effects as patients are more equipped to share the results of genetic tests with extended families (49). Some key aspects of their role in BC management are illustrated in Supplementary Figure S1.
5.2 Diagnostic work up
Breast cancer is commonly diagnosed either through screening or a symptom (e.g., pain or a palpable mass) that prompts a diagnostic examination. Mammography (bilateral) is the standard diagnostic modality for diagnosing BC (41). However, false-negative mammography results are often observed in some cases. Studies reported that false-negative mammography results are associated with factors such as higher breast tissue density, the presence of BRCA1/2 mutations and, both of which may be more prevalent in younger women (50). In such cases (high-risk patients), augmenting mammography with ultrasound can uncover additional cases of mammographically hidden cancers; the use of magnetic resonance imaging (MRI) is optional. The sensitivity of ultrasound screening appeared to be similar to that of mammography in a population with a high-risk of BC (41, 51, 52). Breast MRI may be used for staging evaluation to define the extent of cancer, in the adjuvant or neo-adjuvant settings to detect the presence of multifocal or multi-centric cancer in the ipsilateral breast, or as screening of the contralateral BC at the time of initial diagnosis (41).
Routine pathologic evaluation of the primary tumor and cytology/histology of the axillary nodes, if involvement is suspected remains the most critical element in determining the prognosis of patients with BC (53). Pathological diagnosis should be based on a core needle biopsy, preferably obtained by ultrasound or stereotactic guidance (53). Additionally, the analysis of specific biomarkers, such as hormone receptors (estrogen and progesterone receptors) and HER2 are important in guiding targeted therapies (53). HER2 testing is routinely recommended in all cases of invasive BCs (41).
5.2.1 Genetic testing for BRCA mutations
The National Comprehensive Cancer Network® (NCCN®) (54), and several other professional organizations (53, 55) recommend genetic testing for patients who are at high risk for harboring a pathogenic mutation in one of the BC–predisposition genes. These organizations have developed criteria based on personal/family history and age of onset of cancer to identify patients at high risk (Table 1) (53–56, 62). The majority of these guidelines are primarily based on the probability of carrying pathogenic mutations in BRCA genes. Some of these guidelines (55) propose the use of screening tools (57–61) to identify a family history associated with an increased risk for potentially harmful mutations in BC-susceptibility genes (BRCA1 or BRCA2). Recent studies indicated that nearly 50% of women with BC with germline predisposing mutations are missed by current testing criteria (63). Furthermore, family history–based criteria have limited pertinency in patients who are adopted or unaware of the family history of cancer or have limited family structure (64). Studies on universal testing indicate that guidelines should be broadened to encompass testing of all patients diagnosed with BC (65–67). Researchers report that universal genetic testing after BC diagnosis can uncover clinically significant germline pathogenic variants that might otherwise escape detection due to narrow selection criteria as per current testing guidelines (66).
BRCA1 and BRCA2 gene mutations account for most actionable genetic BC predispositions and are increasingly used for personalized BC management and PARP inhibitors therapy of BRCA-related cancer (68). BRCA1 and BRCA2 mutations are found to be associated with a younger age of onset (25). Also, in GCC countries, the mean age at diagnosis was less than 48 years (with 69% of cases between 25-54 years) (7, 13). Thus, the experts have proposed the criteria mentioned in Box 1 for BRCA testing.
Research investigating next-generation sequencing workflows for BRCA1/2 genes in samples associated with hereditary breast and ovarian cancer has shown outstanding performance, achieving nearly 100% sensitivity and specificity, while also proving to be cost-effective when compared to single-site mutation testing specifically for these genes (69).
Diagnostic laboratories have identified numerous variants of uncertain (or unknown) significance (VUS) in the BRCA1 and BRCA2 genes, owing to their large size and the extensive screening conducted on them. One study reported a VUS frequency rate for BRCA1 and BRCA2 of 13% for 10,000 consecutive individuals (70). Studies from the GCC region reported a high rate of VUS ranging from 14.5% to 25.4% (36, 38). However, initiatives to reclassify BRCA VUS are likely to reduce this number (71). Evaluating a VUS in BRCA genes is a complex undertaking, but it is reported that VUS can be characterized by gathering evidence from databases that document well-characterized populations, and in silico assessment (71).
5.2.2 Impact of timing of genetic testing on surgical decision
Traditionally, BRCA testing is conducted after primary surgery for BC; later once testing results are available, patients identified with BRCA mutations undergo a second breast surgery/bilateral salpingo-oophorectomy for risk reduction. Recent advancements in genetic testing have significantly reduced turnaround time for BRCA1/2 mutation tests. This has allowed patients to undergo genetic testing without the need to delay treatment. Consequently, integration of test results into management decisions can now be seamlessly achieved at the time of diagnosis. This may eliminate the requirement for a second breast surgery for risk reduction, as some women diagnosed with deleterious mutations choose to concurrently undergo therapeutic surgery for the affected breast and risk-reducing surgery for the contralateral breast.
Studies indicated that genetic diagnosis before surgery has an impact on the surgical decision, choosing unilateral mastectomy or bilateral mastectomy in BRCA mutation carriers with BC (72). A study conducted on patients with unilateral BC reported that only 14.7% of patients with unknown BRCA mutation status before surgery received contralateral mastectomy in contrast to 76.4% of patients who underwent contralateral prophylactic mastectomy with BRCA mutation status known preoperatively. These data support preoperative genetic testing for BRCA mutation in patients with newly diagnosed BC to enable appropriate planning of surgical treatment decisions (73). Hence, providing genetic counseling and BRCA testing before surgical approach and developing treatment strategies for patients with a high risk of BC is important (72).
5.2.3 Current BRCA testing landscape, challenges, and expert recommendations for improving
In the GCC region, there are variations in practices for BRCA testing. Currently, few accredited loco-regional laboratories perform BRCA testing. In most countries limited number of cancer centers have a dedicated genetic counselor; counseling is often provided by the medical oncologist, with evident variations in skills and knowledge in this niche area of expertise. Financial support for BRCA testing is provided by pharmaceutical companies. In this region, very few patients are referred for BRCA testing, particularly in government hospitals because most patients neither have insurance nor are willing to pay for the test and the referral is left at the discretion of individual physicians, surgeons, oncologists, and finally patients, which causes inconsistency of BRCA testing. This has resulted in the lack of a regional integrated genetic database. In government hospitals, there are constraints concerning indications, the budget, and a long waiting list for performing screening tests. However, in private institutions, BRCA testing is being endorsed for almost all or high-risk BC patients who have insurance or are willing to pay for the tests. In some countries, genetic testing is often excluded from health insurance policies, making it difficult for patients with cancer to access this crucial service. At present, there are no region-specific genetic testing algorithms or guidelines that regulate BRCA testing in the GCC region. Currently, NCCN Guidelines® (41) are followed for recommending germline BRCA testing. Universal genome testing (wherever possible) or BRCA testing for an extended population with indications beyond those in the guidelines is advised by the majority of experts considering the treatment-related benefits of BRCA testing and it is implemented in some centers in the GCC, yet we believe there are few centers offering universal testing.
A correspondence article published in 2016 reported that in GCC, most molecular diagnostic samples were sent to Western countries for testing and analysis, and the results from a significant amount of these samples came back negative or inconclusive (74). This finding warrants an immediate need to establish accredited molecular diagnostics locally to customize the molecular genetic approaches. Real-world data have suggested a significant deficit in physician-driven referrals for BRCA testing in guideline-eligible BC patients primarily due to a lack of access and knowledge about the criteria for testing (75). Less than 60% of guideline-eligible patients with BC received BRCA testing in the United States of America (USA) and European countries (75, 76). In contrast, 97% of guideline-eligible BC patients received BRCA testing in Israel (76). Lack of knowledge among community oncologists/surgeons about the selection criteria is one of the important barriers for implementing BRCA testing in the GCC region. Expert recommendations for BRCA testing and for supportive measures for improving BRCA testing in the GCC region are present in Box 1.1 and Box 1.2.
Box 1.1. Expert recommendations for BRCA testing in the GCC region.
- Genetic counseling and testing for germline BRCA1 and BRCA2 mutations should be indicated in the following scenarios:
Individual (BC)
- Age less than or equal to 50 years
- Triple-negative BC at any age
- Bilateral BC at any age
- HR-positive BC with N2 disease at any age
- BC with Ashkenazi Jewish or Icelandic heritages
- Any patient who is eligible for adjuvant PARP inhibitor
- Male BC
Positive family history is defined as below:
- ≥1 close blood relatives with BC at age ≤ 50
- ≥1 close blood relative with male BC
- ≥1 case of a blood relative with ovarian cancer
- ≥1 case of a blood relative with pancreatic cancer
- ≥1 case of a blood relative with prostate cancer with metastatic, or high- or very-high-risk group
- ≥3 diagnoses of breast or prostate cancer (any grade) on the same side of the family including the patient with BC
- Individuals affected with BC (not meeting testing criteria listed above) or individual unaffected with BC with a first- or second-degree blood relative meeting any of the criteria listed above
BC, Breast cancer; BRCA, BReast CAncer gene; HR, Hormone receptor; PARP, poly-ADP ribose polymerase
Box 1.2. Expert recommendations for supportive measures for improving BRCA testing in the GCC region.
▪ Need refined definitive region-specific guidelines for BRCA testing
▪ Raise awareness among community oncologists/surgeons about the selection criteria for BRCA testing
▪ Increased access to testing would likely lead to more patients pursuing testing and improving rates of identification of gene carriers
▪ All institutions should have access to genetic counselors who are experienced in counseling patients with BC
▪ Regulators and stakeholders should work to make BRCA testing widely available and accessible for BC patients
▪ Systematically discuss the cases of variants of unknown significant mutations with a genetic counselor in an MDT approach
▪ For patients with VUS, genetic counselors need to follow up with the patients to check if they develop any other cancer in their family
▪ Create a database for BRCA variants and BRCA pathogenic variants and compare it with the global data
▪ There is an urgent need for extensive, well-controlled, genetic epidemiological studies to provide accurate BRCA1 and BRCA2 mutation prevalence among patients with BC in the GCC region
▪ Establish a centralized laboratory that provides free BRCA tests and delivers timely test results without discrepancies
▪ It is recommended to make BRCA testing available for all patients whenever possible. An approach to generalize BRCA testing to the general population with BC instead of restricting it to only a group of patients with BC (per the selective criteria) to provide more treatment benefits.
BC, Breast cancer; BRCA, BReast CAncer gene; GCC, Gulf Cooperation Council; MDT, multidisciplinary team; TNBC, Triple-negative breast cancer; VUS, variants of unknown significance
5.3 Treatment
Treatment of BRCA mutated early-stage BC is complex and involves the combination of local modalities, and systemic anticancer treatments delivered in diverse sequences.
5.3.1 Surgery
Breast-conserving surgery or mastectomy are the primary treatment options for patients with BRCA mutated early BC (77), similar to sporadic BC (53). The choice of surgery depends on factors such as age, tumor size, location, TNM stage, and patient preferences (77). BCS is the optimal surgical choice when the tumor is small and is localized to one part of the breast (53). Mastectomy is indicated for the patients who choose to undergo this procedure over BCS or where there is an inability to achieve negative surgical margins after multiple resections or received prior radiation to the chest wall/breast or other contraindications to radiotherapy (RT) (41). Retrospective studies that evaluated long-term outcomes in BRCA1/2 carriers have found no significant difference in RFS, breast cancer-specific survival (BCSS), or OS, between BCS and mastectomy; however, an increased risk of local recurrence was reported for BCS (78–81). Similar findings were reported in a systematic review by Co et al. (77), that compared survival outcomes and recurrence rates between BRCA mutation carriers who received BCS and those who received mastectomy. Overview of studies that evaluated BCS and mastectomy in early-stage BC patients with BRCA mutations are presented in Supplementary Table S2 (77–81). Based on the available evidence, the researchers have indicated that BCS could be a good choice for BRCA mutation carriers, as long as they receive appropriate counseling and have rigorous follow-up (77).
Gentile et al, suggested that young BRCA-mutated patients with small tumors may not need an up-front mastectomy (82). Although data from the Danish Breast Cancer Group, reported a reduced risk of death for risk-reducing contralateral mastectomy (RRCM) (adjusted OS hazard ratio: 0.42, p=0.01) (83). A systematic review and meta-analysis by Fayanju et al. reported that RRCM may not necessarily result in improvement of OS, despite reducing the risk of contralateral BC (84). Limited data are available on the survival impact of RRCM in BRCA mutated patients with unilateral BC. For patients who require a mastectomy, breast reconstruction (immediate or delayed) could be an option (53). The nipple-sparing mastectomy has proven to be safe in patients carrying BRCA mutations, as both a therapeutic option and in terms of risk-reduction, due to its minimal local recurrence rates compared to a modified radical mastectomy (53, 85), although more data and longer follow-up are needed.
5.3.2 Radiotherapy
A meta-analysis on randomized controlled clinical trials (RCTs) demonstrated a significant reduction in the 10-year risk of recurrence (absolute reduction 15.7%, 95% CI 13.7-17.7, 2p<0.00001) and 15-year risk of BC death (absolute reduction 3.8%, 1.6-6.0, 2p=0.00005) in patients with early BC who received whole breast irradiation after BCS compared with those who received BCS alone (86). Researchers reported that tumors harboring BRCA mutations could be sensitive to RT, because ionizing radiation has the ability to induce double standard breaks (DNBs) in DNA, and BRCA genes play a key role in repairing such DNBs (68). Multiple studies have reported that the risk of local recurrence after BCS and RT is comparable between patients with BRCA-mutated BC and those with sporadic BC (22, 87, 88). Similarly, studies have proven the equivalence in the survival of patients with BRCA mutations between BCS with RT vs mastectomy (78) or BCS vs mastectomy with RT (81). No evidence of impaired survival and toxicity related to irradiation has been observed in patients with BRCA mutations, suggesting that RT may be safe in these patients and should not be withheld (89, 90). Pierce et al (78) compared 10-year rates of IBTR/events after BCS and RT among BRCA1/2 mutation carriers and women with sporadic BC and found no statistically significant difference. An overview of studies that evaluated adjuvant RT efficacy in BC patients with BRCA mutations is presented in Supplementary Table S3 (78, 81, 87–89).
5.3.3 Chemotherapy
Chemotherapy is recommended in the vast majority of TNBC, HER2-positive BC, and in high-risk luminal-like HER2-negative BC (53). The most frequently used regimen includes taxanes and/or anthracyclines but in selected patients, cyclophosphamide/5-fluorouracil (5-FU)/methotrexate may still be used (53). The taxanes, namely paclitaxel and docetaxel, play a significant role in the therapeutic management of BC. Patients with hormone receptor (HR) negative BC carrying BRCA1 mutations exhibited less sensitivity to taxane chemotherapy than non-BRCA1 mutation carriers with HR negative BC (91). Conversely, in patients with HR-positive BC with BRCA1 and BRCA2 mutations and sporadic cases, similar sensitivities were reported with taxane therapy (91). In the Arun et al. study, BRCA1 mutation carriers showed higher pathological complete response (pCR) (46% vs 22%) compared to patients with sporadic BC, when treated with the combination of anthracycline-taxane, in neoadjuvant settings (92). Multiple studies have indicated that BRCA1/2 mutation carriers are more prone to exhibiting a favorable response to neoadjuvant anthracycline-based regimens (92–94) or single-agent cisplatin (95), as evidenced by a higher rate of achieving pCR. In the INFORM trial, neoadjuvant single-agent cisplatin did not yield superior pCR rates when compared to the combination of doxorubicin and cyclophosphamide in patients with HER2 negative early BC who carry BRCA1/2 mutations (96). In the TNT trial carboplatin demonstrated a markedly greater response in patients with BRCA mutated and TNBC as compared to docetaxel (20).
Studies that explored the utilization of platinum agents in neoadjuvant settings in patients with BRCA-mutated early-stage TNBC demonstrated high pCR rates with the addition of platinum agents to standard chemotherapy regimens (anthracycline, cyclophosphamide, taxanes) (97–99). Zang et al., reported improved recurrence‐free survival (RFS) and OS rates among BRCA1/2-mutated TNBC patients when carboplatin is added to standard anthracycline-taxane-based neoadjuvant chemotherapy (NACT) (100). On the contrary, in the GeparSixto trial and Brightness trial no additional benefit was observed in patients with BRCA-mutated TNBC with the addition of a platinum agent (carboplatin) to NACT (99, 101). An overview of studies that evaluated chemotherapy efficacy in BC patients with BRCA mutations are presented in Supplementary Table S4 (91, 92, 94, 99–101).
5.3.4 Endocrine therapy
Endocrine therapy (ET) is a common treatment option for early-stage BC. It is often used as adjuvant therapy after surgery to reduce the risk of BC recurrence, or as neoadjuvant therapy to shrink the tumor before surgery (53). Adjuvant tamoxifen (given for 5 years) showed a 31% decrease in mortality rate from BC in patients with estrogen receptor (ER)-positive BC and proved to be superior to 1 or 2 years of tamoxifen treatment (102). However, in patients with a BRCA-mutated HR-positive BC, ET exhibited a lower survival rate in comparison to their counterparts who do not possess the BRCA mutation (103). Empirical evidence indicates that tamoxifen use is associated with a reduction in contralateral BC risk among BRCA mutation carriers (104, 105), with a suggested influence on both ER-positive and -negative disease.
5.3.5 Cyclin-dependent kinase 4/6 inhibitor
In MonarchE phase III trial, abemaciclib (cyclin-dependent kinase 4 and 6 inhibitor plus ET demonstrated superior invasive DFS (iDFS) compared with ET alone (hazard ratio: 0.75; 95% CI, 0.60 to 0.93, p = 0.01), with 2-year iDFS rates of 92.2% versus 88.7%, respectively in patients with HR-positive, HER2 negative, high-risk (≥ 4 positive nodes; 1–3 nodes involved and at least one of the following: tumor size ≥5 cm, histologic grade 3, or central Ki-67 ≥20%) early BC, in adjuvant settings (106). However, information on the BRCAm status of the patients who participated in the MonarchE trial is not available. The international guidelines endorse abemaciclib for HR-positive, HER2-negative germline BRCAm carriers who have undergone surgery first and have 1–3 positive nodes (107).
5.3.6 Poly(ADP‐ribose) polymerase inhibitors
With the promising results for PARP inhibitors (Olaparib, talazoparib) in the treatment of BRCA-mutated BC in metastatic/advanced settings (108, 109), clinical trials are investigating their potential role in early-stage disease, as monotherapy or with other cytotoxic agents or with immunotherapy in neoadjuvant and adjuvant settings. Currently, the PARP inhibitor, olaparib is approved for the adjuvant treatment of adult patients with germline BRCA-mutated HER2-negative high-risk early BC who have been treated with neoadjuvant or adjuvant chemotherapy based on the results of the OlympiA trial (110, 111).
OlympiA was a double-blinded, phase III trial conducted to evaluate the safety and efficacy of adjuvant olaparib therapy versus placebo in high-risk, germline BRCA-mutated, HER2-negative early BC who received local treatment and neoadjuvant or adjuvant chemotherapy (at least 6 cycles anthracyclines or/and taxanes. OlympiA examined four patient populations considered to have HER2-negative disease at high risk of recurrence, and inclusion criteria varied based on tumor subtype and therapy setting (Table 2) (111).
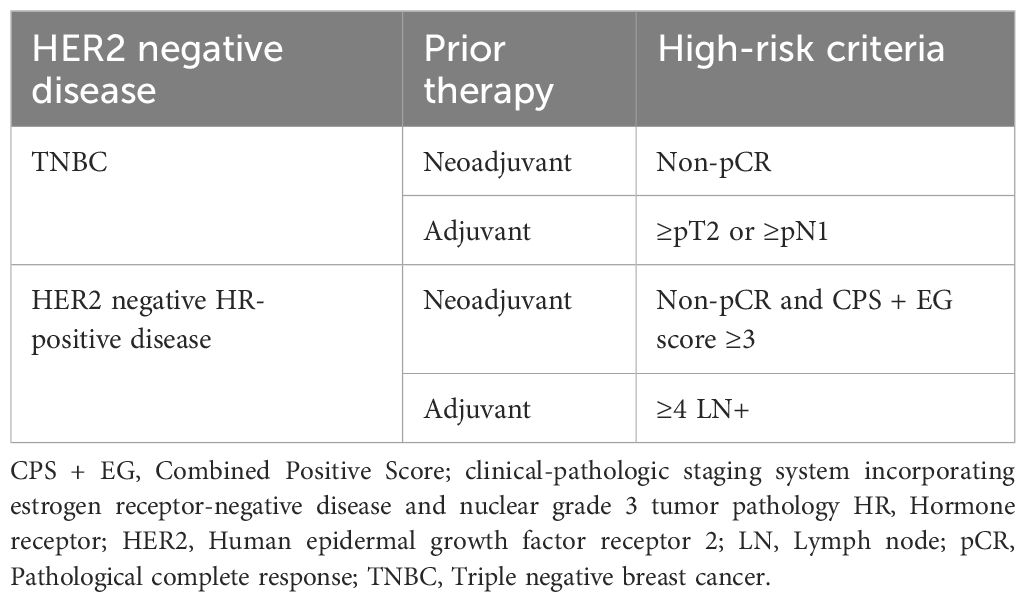
Table 2 High-risk patient populations in the OlympiA Trial (111).
At the initial interim analysis (111), a significant decrease in the disease recurrence or death was observed with the use of olaparib, successfully achieving the primary objective of the study (hazard ratio 0.58; p<0.001). In the second-interim analysis, a statistically significant and clinically meaningful improvement in OS was observed with olaparib compared with placebo (hazard ratio: 0.68; p=0.009) with an absolute improvement in 4-year OS of 3.4% (89.8% olaparib; 86.4% placebo) (112). The survival benefit of olaparib was observed irrespective of germline BRCA status, HR status, prior platinum use, and adjuvant chemotherapy or NACT (112). The international guidelines have updated their BC treatment guidelines to include treatment with the PARP inhibitor olaparib for one year after completing chemotherapy, surgery, and radiation (if used) to improve outcomes in patients with an inherited mutation in BRCA1/2 with early-stage, HER2-negative BC who have a high risk for recurrence (113).
In the phase II study, talazoparib monotherapy elicited pCR rates that were comparable to those observed with combination anthracycline and taxane-based chemotherapy regimens when used in the neoadjuvant settings in patients with BRCA1/2 positive, early HER2-negative BC (114). In the phase II single-arm NEOTALA trial, talazoparib yielded promising pCR rates in patients with BRCA mutated early BC comparable to those historically observed with combination anthracycline- and taxane-based chemotherapy regimens (115).
In the phase II ISPY-2 trial, veliparib plus carboplatin and paclitaxel showed better pathological complete response (51%, 95% CI, 36% to 66%) in early-stage TNBC, compared with paclitaxel alone (26%, 95% CI, 9% to 43%) (116). Similar pCR rates were demonstrated with the addition of veliparib plus carboplatin or carboplatin alone to NACT in the BrighTNess trial (53% vs 31%) (99). After a median follow-up of 4.5 years, event-free survival (EFS) was significantly improved for the veliparib, carboplatin, plus paclitaxel group relative to the paclitaxel alone group (hazard ratio: 0.63, p=0.02), but no difference was observed between veliparib, carboplatin, plus paclitaxel group and the carboplatin plus paclitaxel group (hazard ratio: 1.12, p= 0.62) (117). The addition of veliparib did not impact the EFS (117). Clinical trial data on oral PARP inhibitors is presented in Table 3 (111, 112, 114–117).
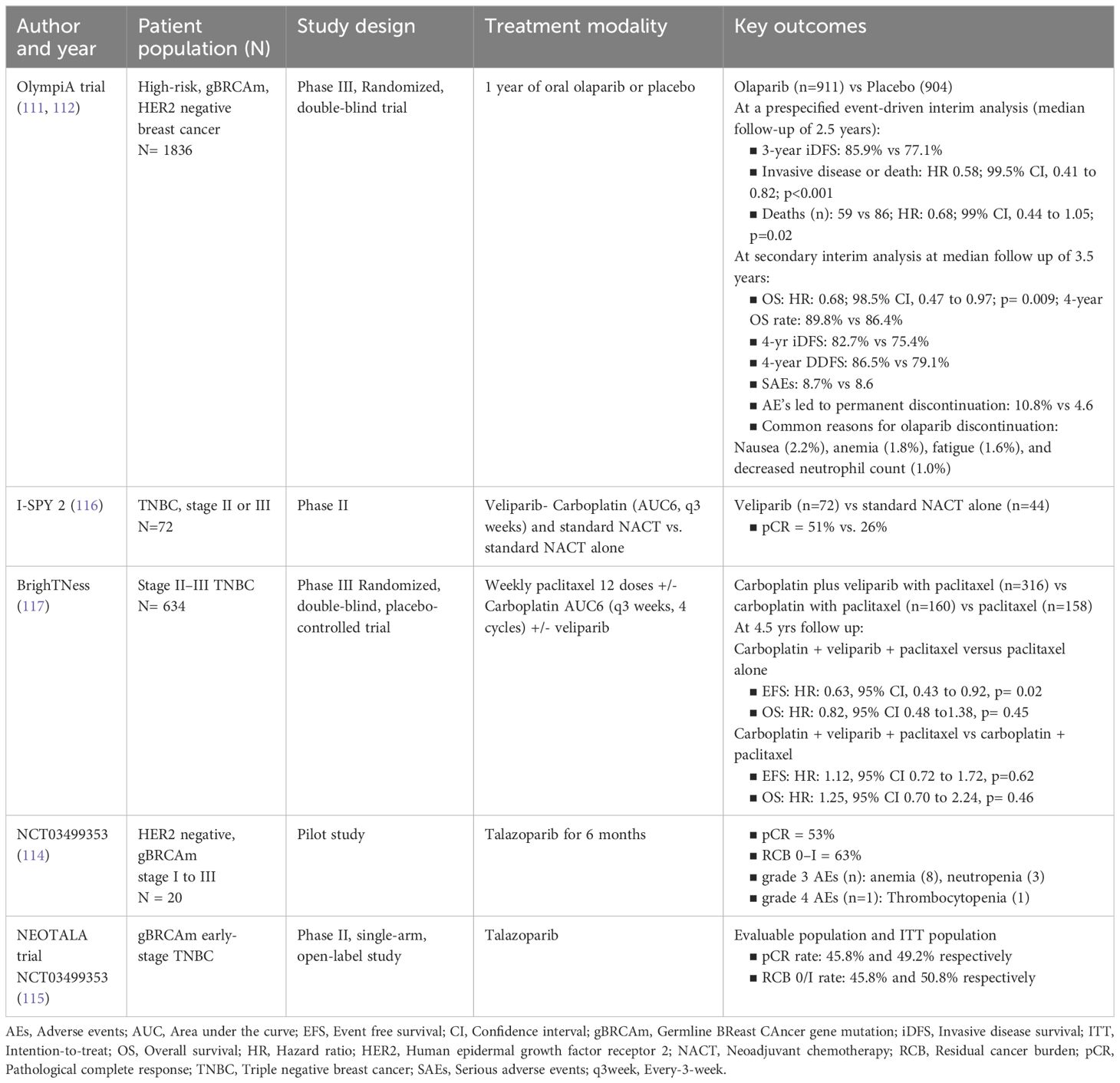
Table 3 Clinical trials of oral PARP inhibitors for early-stage breast cancer in the neoadjuvant/adjuvant settings.
5.3.7 Combination of PARP inhibitors and immunotherapy agents
Multiple clinical trials have assessed the efficacy of combining PARP inhibitors and immune checkpoint inhibitors (ICIs) in the context of metastatic cancer. These trials include MEDIOLA (118), TOPACIO (119), KEYLYNK-007 (120), and JAVELIN PARP Medley (121). The results of these trials have demonstrated a favorable toxicity profile for pembrolizumab (119, 120), durvalumab (118), and avelumab (121). The combination of PARP inhibitors and ICIs may emerge as a promising therapeutic strategy for patients harboring BRCA mutations. These tumors appear as more immunogenic due to their higher levels of tumor-infiltrating lymphocytes, higher mutational burden, and expression of immune checkpoint inhibitory molecules compared to BCs without BRCA mutations. Encouraged by results for ICIs from the metastatic setting, ICIs are now being assessed for the treatment of advanced mutated BC and early-stage BC with BRCA mutations (DORA trial, DOLAF, KEYLYNK trial, NCT03329937, NCT03150576, and NCT03499353).
In the phase II ISPY-2 trial, neoadjuvant durvalumab and olaparib exhibited superior efficacy in terms of pCR over standard NACT in HER2-negative BC, particularly in a highly sensitive subset of high-risk HR-positive, HER2-negative patients (64% vs 22%) (122). Although it remains uncertain whether the incorporation of immunotherapy alongside PARP inhibitors yields superior outcomes in comparison to single-agent PARP inhibitors, safety data obtained in the metastatic context indicate that the combination is well-tolerated. Despite the lack of empirical evidence, data extrapolation consequently advocates for the co-administration of adjuvant pembrolizumab and olaparib in high-risk patients with residual disease following chemo-immunotherapy.
6 Healthcare infrastructure in the GCC region for managing BRCA-mutated early-stage BC
In recent years, GCC countries have made significant progress in enhancing the healthcare infrastructure for BC management. Most of these countries have specialized cancer centers (King Faisal Specialist Hospital & Research Centre in KSA, Tawam Hospital Comprehensive Cancer Center in UAE, National Oncology Centre at the Royal Hospital in Oman, Kuwait Cancer Control Center in Kuwait, The Bahrain Oncology Center at King Hammad University Hospital in Bahrain) equipped with advanced technology and staffed by MDT healthcare professionals (123–128). These centers function as central points for cancer diagnosis, treatment, and survivorship care, providing patients with comprehensive and specialized services. With the exception to Oman, the healthcare infrastructure in these countries is equipped with state-of-the-art diagnostic tools like mammography, ultrasound, MRI, PET and molecular testing (123–128). In Saudi Arabia, oncology services are offered through various public institutions (126). In UAE, several general oncology care services have been initiated across the nation to enable cancer patients to access healthcare facilities closer to their homes (125). In Kuwait and Bahrain, all the general hospitals are equipped with a radiology department along with molecular imaging and nuclear medicine (123, 124). In UAE, radiotherapy facilities are located across the country and offer advanced treatment options (125).
Besides in countries like KSA, UAE and Qatar, cancer care is provided free of charge to all their citizens through health insurance and non-profit organizations to non-citizens (125, 126, 128). Such care includes laboratory tests, clinical imaging, systemic anti-cancer therapies, surgery, and radiotherapy. Bahraini citizens, on the other hand, receive free treatment at public hospitals. Most GCC nations provide an extensive array of treatment choices such as surgery, chemotherapy, radiation therapy, hormone therapy and targeted therapy guaranteeing individualized and efficient healthcare for patients (123–127).
7 Challenges in management of BRCA-mutated early-stage BC
Limited availability of specialized BC centers and oncology services in certain GCC countries can impede timely access to comprehensive care, including diagnosis, treatment, and support services. Within GCC countries, geographic disparities in healthcare infrastructure and resources may result in unequal access to BC care, especially for patients residing in remote or rural areas far from major healthcare facilities. In most GCC countries patients have access to all historically used drugs; however, there is a wide disparity in accessing novel targeted therapies such as abemaciclib olaparib, and talazoparib due to lack of approval or non-reimbursement, or high costs. For example, in UAE and Saudi Arabia, drugs like abemaciclib, olaparib are approved for BRCA mutated BC (7). However, in countries like Kuwait they are not accessible yet for management of early-stage BC. Hence, governments may need to take efforts to ensure that novel drugs are readily available to patients from these countries.
Financial barriers, such as high treatment costs, insurance limitations, and out-of-pocket expenses, may present challenges for patients, particularly non-citizens and those without sufficient insurance coverage, despite efforts to provide free or subsidized cancer care for citizens. Besides, patient factors such as adherence to treatment protocols, including medication regimens and follow-up appointments, can be difficult due to factors such as treatment-related side effects, logistical challenges, and cultural beliefs about illness and treatment. Cultural beliefs and social stigma surrounding cancer, specifically BC, can influence patient decision-making, treatment-seeking behavior, and disclosure of diagnosis. Fear, misconceptions, and cultural taboos may contribute to delayed presentation, reluctance to seek medical help, and adherence issues. Gender norms and cultural expectations regarding women’s roles and health-seeking behavior can impact access to BC care. Cultural factors related to modesty, privacy concerns, and family dynamics may affect women’s ability to access screening services, discuss symptoms, and seek timely medical care.
Thus, it is imperative to increase awareness among people toward BC, genetic testing, and advocacy for insurance coverage. Additionally providing support services to promote treatment adherence and address psychosocial needs is essential.
8 Expert panel recommendations for the management of BRCA mutated early BC in germline BRCA carriers
Expert panel recommendations for the management of early BC are presented in Box 2 and the treatment algorithms are presented in Figure 1 (129).
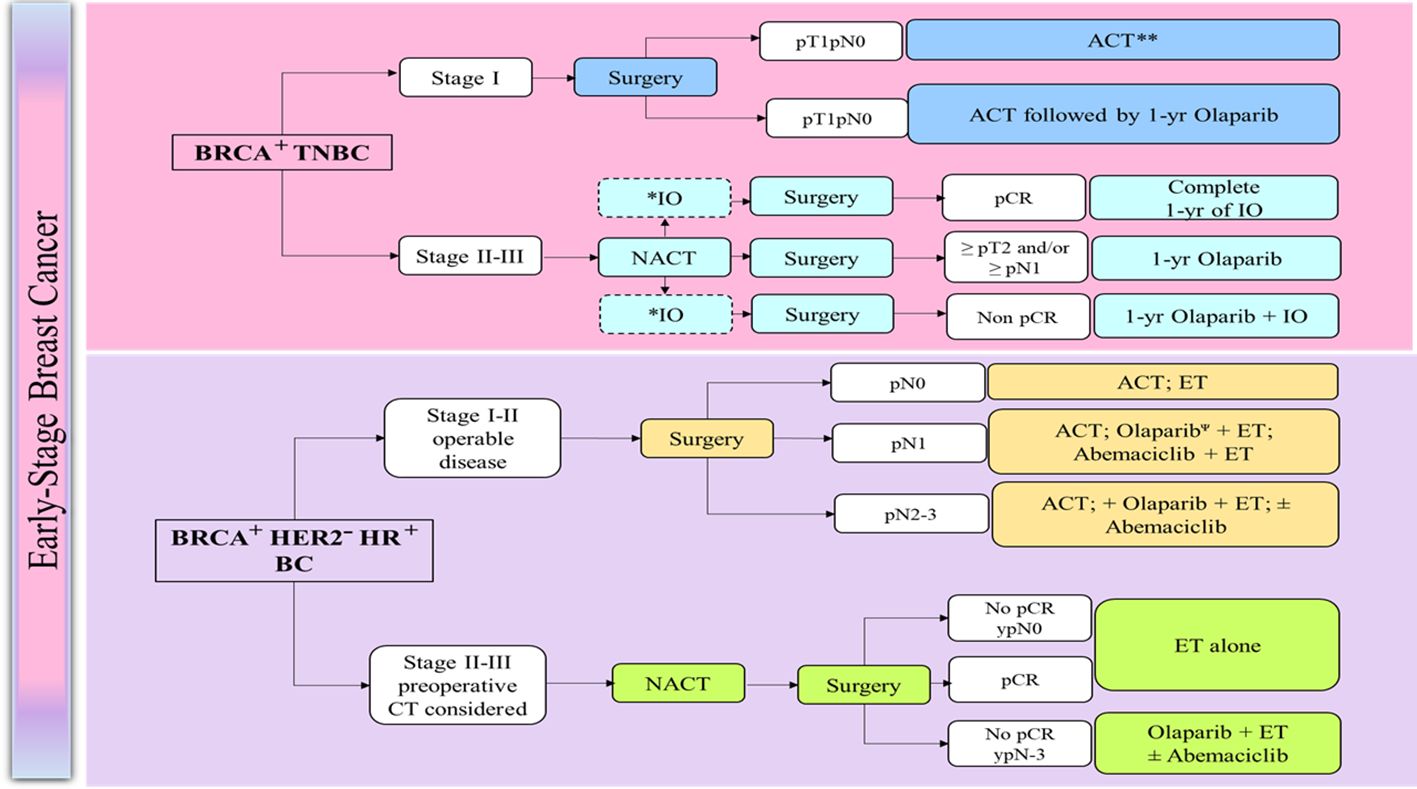
Figure 1 Management of early breast cancer in germline BRCA1/2 mutation carriers (129). ACT, Adjuvant chemotherapy; BRCA, BReast CAncer gene; CT, chemotherapy; CPS + EG, Combined Positive Score; clinical-pathologic staging system incorporating estrogen receptor-negative disease and nuclear grade 3 tumor pathology; ER, estrogen receptor; ET, endocrine therapy; HR, Harmone receptor; HER2, human epidermal growth factor receptor 2; NACT, Neoadjuvant chemotherapy; N0, node-negative; LN, Lymph node; pCR, Pathological complete response; TNBC, triple-negative breast cancer. *: PDL1 positive; **: case-by case for pT1a, Ψ: Preferred after chemotherapy, case-by-case for pT1a.
Box 2. Panel recommendations for the management of early BC in Germline BRCA carriers.
▪ BCS is the preferred local treatment option for the majority of BRCA-mutated early BC patients, with the use of oncoplastic techniques, to maintain good cosmetic outcomes in technically challenging cases, when needed.
▪ After surgical resection, careful assessment of resection margins is essential. No tumor at the inked margin is recommended for either in situ disease or invasive BCs and >2 mm for in situ disease is recommended (41).
▪ Prophylactic contralateral mastectomy should be considered for patients with a very early disease with T0, T1, or M0 with germline BRCA mutations.
▪ Breast reconstruction should be available and proposed to all women requiring mastectomy. Immediate breast reconstruction should be offered to the vast majority of patients, except for those presenting with inflammatory cancer.
▪ The optimal reconstruction technique for each patient should be discussed individually taking into account anatomic, treatment and patient-related factors and preferences.
▪ Postoperative RT is strongly recommended after BCS. Post-mastectomy RT is recommended for high-risk patients, including those with involved resection margins, involved axillary lymph nodes and T3–T4 tumors; it should also be considered in patients with 1–3 positive axillary lymph nodes.
▪ Patients fulfilling the OlympiA criteria are considered high-risk patients for recurrence.
▪ The panel recommends offering 1-year of adjuvant olaparib for patients with high-risk, early-stage HER2-negative BC with germline BRCA mutations after completion of neoadjuvant chemotherapy and local treatment, including radiation.
▪ In patients with TNBC with germline BRCA mutations and PDL1 expression who did not achieve pCR with neoadjuvant treatment, a combination of pembrolizumab with olaparib is recommended. However, there is no clinical evidence to support the recommendation.
BC, Breast cancer; BCS, Breast conservative surgery; BRCA, BReast CAncer gene; HER2, Human epidermal growth factor receptor 2; pCR, pathologic complete response; RT, Radiotherapy.
9 Conclusion
BRCA testing is an important and critical diagnostic tool in the early-stage BC care as it enables personalized treatment plans to improve patient outcomes. With continued advancements in research and technology, the role of BRCA testing in the management of BC is likely to expand further, offering new opportunities for early detection, risk reduction, and personalized care for patients with BRCA-mutated BC. With the availability of comprehensive and cost-effective genetic testing for germline BRCA1/2 mutations and the valuable information it provides for treatment options, it may be reasonable to consider BRCA testing beyond previously established selection criteria. The inclusion of olaparib as an adjuvant therapy for early BC patients with BRCA mutations marks a significant advancement in the treatment of this patient population. It is imperative for surgical/medical oncologists to consider it in the locoregional systemic management of early BC for improved patient outcomes.
Author contributions
HA-S: Conceptualization, Writing – original draft, Writing – review & editing. AA: Conceptualization, Writing – original draft, Writing – review & editing. FA: Conceptualization, Writing – original draft, Writing – review & editing. FC: Conceptualization, Writing – original draft, Writing – review & editing. ST: Conceptualization, Writing – original draft, Writing – review & editing. SHT: Conceptualization, Writing – original draft, Writing – review & editing. SK: Conceptualization, Writing – original draft, Writing – review & editing. SO: Conceptualization, Writing – original draft, Writing – review & editing. OA: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The preparation of this expert opinion manuscript and funding of the journal’s article processing charges was supported by AstraZeneca FZ LLC. The authors declare that this study received funding from AstraZeneca FZ LLC. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
The authors would like to thank Dr. Sasikala Somara and Dr. Suvarna Chavan, of Fortrea Scientific Pvt Ltd (formerly Labcorp Scientific Services & Solutions Pvt Ltd) for medical writing support in accordance with Good Publication Practice 2022 guidelines.
Conflict of interest
HA-S received support provision of study material/medical writing for present manuscript from AstraZeneca; received Research support from Roche and Merck; received speaker honoraria from Roche, Novartis, AstraZeneca, BMS, MSD, Pfizer, Eli Lilly and Gilead; received travel support from Novartis, Gilead and MSD for attending meetings; Participated in Advisory Board Meetings for Novartis, AstraZeneca, BMS, MSD, Pfizer, Eli Lilly, Menirini and Roche. AA received speaker honoraria from AstraZeneca, Pfizer, MSD, Novartis, Eli Lilly, Hikmah, Janssen, Menirini, Gilead, BMS for lectures, presentations, speakers’ bureaus, manuscript writing or educational events; received travel support from MSD and Gilead for attending meetings; Participated in Data Safety Monitoring Board or Advisory Board Meetings for AstraZeneca/Daiichi Sankyo, Pfizer, MSD, Novartis, Eli Lilly, Hikmah, Roche, Janssen, Menirini, Gilead, and BMS. FA received support provision of study material/medical writing for present manuscript from AstraZeneca; received speaker honoraria from Roche, Novartis, AstraZeneca, BMS, MSD, Pfizer, Eli Lilly and Gilead; Received travel support from Novartis, Gilead and Menirini for attending meetings; Participated in Advisory Board Meetings for Novartis, AstraZeneca, BMS, MSD, Pfizer, Eli Lilly, and Roche. FC received support provision of study material and medical writing for the present manuscript from AstraZeneca. received consulting fees from AstraZeneca for the advisory role; Received speaker honoraria from AstraZeneca; Received travel support from AstraZeneca, Novartis, Pfizer, MSD, and Eli Lilly for attending meetings. Participated in Advisory Board Meetings for AstraZeneca. ST received funding support for medical writing for the present manuscript from AstraZeneca and received speaker honoraria from Roche, Novartis, AstraZeneca, Eli Lilly, MSD, and Pfizer; received travel support from Roche, Novartis, AstraZeneca, and Pfizer for attending meetings. SK received consulting fees from Eli Lilly, Pierre Fabre, Roche, Novartis, MSD, Pfizer, IPSOS, AstraZeneca, Merck, BMS, Al BORJ Lab, EKSE, Abbott, Astellas, New Bridge, Genomic Health, and Al Hikmah Co. Received speaker honoraria from Eli Lilly, Pierre Fabre, Roche, Novartis, MSD, Pfizer, AstraZeneca, Merck, Al BORJ Lab, Abbott, Astellas, New Bridge, Genomic Health and Al Hikmah Co. Received travel support for attending meetings from Pierre Fabre, Roche, Novartis, MSD, AstraZeneca, Merck, Astellas, New Bridge, Genomic Health and Al Hikmah Co. Participated in a Data Safety Monitoring Board or Advisory Board for Roche. SO received support provision of study material and medical writing for the present manuscript from AstraZeneca; Received consulting fees from AstraZeneca for the advisory role; Received speaker honoraria from AstraZeneca; Received travel support from AstraZeneca, MSD, Novartis, and Pfizer for attending the meeting; Participated in Advisory Board for AstraZeneca. OA received support provision of study material/medical writing for the present manuscript from AstraZeneca; Received speaker honoraria from Roche, Novartis, AstraZeneca, BMS, MSD, Pfizer, Eli Lilly and Gilead; Received travel support from Novartis, Gilead and Menirini for attending meetings; Participated in Advisory Board Meetings for Novartis, Astra Zeneca, BMS, MSD, Pfizer, Eli Lilly and Roche.
The remaining author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1358982/full#supplementary-material
References
1. Cancer today. Available online at: http://gco.iarc.fr/today/home (Accessed March 13, 2023).
2. Chaabna K, Ladumor H, Cheema S. Ecological study of breast cancer incidence among nationals and nonnationals in the Gulf Cooperation Council countries. East Mediterr Health J Rev Sante Mediterr Orient Al-Majallah Al-Sihhiyah Li-Sharq Al-Mutawassit. (2023) 29:40–8. doi: 10.26719/emhj.23.005
3. Apostolou P, Fostira F. Hereditary breast cancer: the era of new susceptibility genes. BioMed Res Int. (2013) 2013:747318. doi: 10.1155/2013/747318
4. van der Groep P, van der Wall E, van Diest PJ. Pathology of hereditary breast cancer. Cell Oncol Dordr. (2011) 34:71–88. doi: 10.1007/s13402-011-0010-3
5. Hong R, Xu B. Breast cancer: an up-to-date review and future perspectives. Cancer Commun Lond Engl. (2022) 42:913–36. doi: 10.1002/cac2.12358
6. Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer Targets Ther. (2019) 11:151–64. doi: 10.2147/BCTT.S176070
7. Al-Shamsi HO, Abdelwahed N, Al-Awadhi A, Albashir M, Abyad AM, Rafii S, et al. Breast cancer in the United Arab Emirates. JCO Glob Oncol. (2023) 9:e2200247. doi: 10.1200/GO.22.00247
8. AlShehri S, Abulkhair O a. M, Al Kushi A, Musaad S, Al Olayan A, Jazieh AR. Clinical and pathologic profile of breast cancer in a tertiary cancer center in Saudi Arabia. J Clin Oncol. (2011) 29:1603–3. doi: 10.1200/jco.2011.29.15_suppl.1603
9. Albeshan SM, Mackey MG, Hossain SZ, Alfuraih AA, Brennan PC. Breast cancer epidemiology in gulf cooperation council countries: A regional and international comparison. Clin Breast Cancer. (2018) 18:e381–92. doi: 10.1016/j.clbc.2017.07.006
10. Fadhil I, Alkuwari M, Al Tahan F, Alsaleh K, Alsaadoon D. Early detection of breast cancer in gulf cooperation council countries: case studies. J Glob Oncol. (2018) 4:50s–s. doi: 10.1200/jgo.18.64800
11. Hamadeh RR, Abulfatih NM, Fekri MA, Al-Mehza HE. Epidemiology of breast cancer among Bahraini women. Sultan Qaboos Univ Med J. (2014) 14:e176–82.
12. Mehdi I, Monem AA, Al Bahrani B, Ramadhan FA. Breast cancer molecular subtypes in Oman: correlation with age, histology, and stage distribution - analysis of 542 cases. Gulf J Oncolog. (2014) 1:38–48.
13. Najjar H, Easson A. Age at diagnosis of breast cancer in Arab nations. Int J Surg Lond Engl. (2010) 8:448–52. doi: 10.1016/j.ijsu.2010.05.012
14. Surveillance Research Program, National Cancer Institute. SEER*Explorer. Breast Cancer – SEER 5-year relative survival rates, 2012-2018, by stage at diagnosis, female, all races, all ages . Available online at: https://seer.cancer.gov/statfacts/html/breast.html (Accessed March 14, 2023).
15. Hassanipour S, Maghsoudi A, Rezaeian S, Arab-Zozani M, Mokhtari AM, Abdzadeh E, et al. Survival rate of breast cancer in eastern mediterranean region countries: A systematic review and meta-analysis. Ann Glob Health. (2019) 85:138. doi: 10.5334/aogh.2521
16. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang X-S, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet Lond Engl. (2015) 385:977–1010. doi: 10.1016/S0140-6736(14)62038-9
17. Elobaid Y, Aamir M, Grivna M, Suliman A, Attoub S, Mousa H, et al. Breast cancer survival and its prognostic factors in the United Arab Emirates: A retrospective study. PloS One. (2021) 16:e0251118. doi: 10.1371/journal.pone.0251118
18. Antoniou A, Pharoah PDP, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. (2003) 72:1117–30. doi: 10.1086/375033
19. Grindedal EM, Heramb C, Karsrud I, Ariansen SL, Mæhle L, Undlien DE, et al. Current guidelines for BRCA testing of breast cancer patients are insufficient to detect all mutation carriers. BMC Cancer. (2017) 17:438. doi: 10.1186/s12885-017-3422-2
20. Tutt A, Tovey H, Cheang MC, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. (2018) 24. doi: 10.1038/s41591-018-0009-7
21. Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. (2009) 361:123–34. doi: 10.1056/NEJMoa0900212
22. Tung NM, Boughey JC, Pierce LJ, Robson ME, Bedrosian I, Dietz JR, et al. Management of hereditary breast cancer: american society of clinical oncology, american society for radiation oncology, and society of surgical oncology guideline. J Clin Oncol. (2020) 38:2080–106. doi: 10.1200/JCO.20.00299
23. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. (2017) 317:2402–16. doi: 10.1001/jama.2017.7112
24. Street W. Breast Cancer Facts & Figures 2019-2020. Atlanta: American Cancer Society, Inc. (2019) Vol. 44.
25. Becourt S, Cohen-Haguenauer O, Ledoux F, Nguyen O, Cuvier C, Giacchetti S, et al. Comparison of clinicopathological (CP) features and outcome of breast cancers (BC) in BRCA-mutation carriers patients, with a family history without BRCA-mutation and with sporadic disease. J Clin Oncol. (2018) 36:e13522–2. doi: 10.1200/JCO.2018.36.15_suppl.e13522
26. Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. (2012) 21:134–47. doi: 10.1158/1055-9965.EPI-11-0775
27. Aleskandarany M, Caracappa D, Nolan CC, Macmillan RD, Ellis IO, Rakha EA, et al. DNA damage response markers are differentially expressed in BRCA-mutated breast cancers. Breast Cancer Res Treat. (2015) 150:81–90. doi: 10.1007/s10549-015-3306-6
28. Bordeleau L, Panchal S, Goodwin P. Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Res Treat. (2010) 119:13–24. doi: 10.1007/s10549-009-0566-z
29. Lakhani SR, Gusterson BA, Jacquemier J, Sloane JP, Anderson TJ, van de Vijver MJ, et al. The pathology of familial breast cancer: histological features of cancers in families not attributable to mutations in BRCA1 or BRCA2. Clin Cancer Res Off J Am Assoc Cancer Res. (2000) 6:782–9.
30. Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat. (2014) 144:443–55. doi: 10.1007/s10549-014-2890-1
31. Graeser MK, Engel C, Rhiem K, Gadzicki D, Bick U, Kast K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol Off J Am Soc Clin Oncol. (2009) 27:5887–92. doi: 10.1200/JCO.2008.19.9430
32. Zhu Y, Wu J, Zhang C, Sun S, Zhang J, Liu W, et al. BRCA mutations and survival in breast cancer: an updated systematic review and meta-analysis. Oncotarget. (2016) 7:70113–27. doi: 10.18632/oncotarget.12158
33. Baretta Z, Mocellin S, Goldin E, Olopade OI, Huo D. Effect of BRCA germline mutations on breast cancer prognosis: A systematic review and meta-analysis. Med (Baltimore). (2016) 95:e4975. doi: 10.1097/MD.0000000000004975
34. Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. (2010) 304:967–75. doi: 10.1001/jama.2010.1237
35. Bujassoum S, Bugrein H, Alsulaiman R, Ghazouani H. Qatar’s experience with hereditary breast and ovarian cancer and high risk clinic: A retrospective study 2013-2016. Int J Res - Granthaalayah. (2017) 5:185–96. doi: 10.29121/granthaalayah.v5.i10.2017
36. Altinoz A, Al Ameri M, Qureshi W, Boush N, Nair SC, Abdel-Aziz A. Clinicopathological characteristics of gene-positive breast cancer in the United Arab Emirates. Breast Edinb Scotl. (2020) 53:119–24. doi: 10.1016/j.breast.2020.07.005
37. Al Hannan F, Keogh MB, Taha S, Al Buainain L. Characterization of BRCA1 and BRCA2 genetic variants in a cohort of Bahraini breast cancer patients using next-generation sequencing. Mol Genet Genomic Med. (2019) 7:e00771. doi: 10.1002/mgg3.771
38. Abulkhair O, Al Balwi M, Makram O, Alsubaie L, Faris M, Shehata H, et al. Prevalence of BRCA1 and BRCA2 mutations among high-risk Saudi patients with breast cancer. J Glob Oncol. (2018) 4. doi: 10.1200/JGO.18.00066
39. Amirrad M, Al-Mulla F, Varadharaj G, John B, Saji T, Anim JT. BRCA1 gene expression in breast cancer in Kuwait: correlation with prognostic parameters. Med Princ Pract Int J Kuwait Univ Health Sci Cent. (2005) 14:67–72. doi: 10.1159/000083913
40. Kast K, Rhiem K, Wappenschmidt B, Hahnen E, Hauke J, Bluemcke B, et al. Prevalence of BRCA1/2 germline mutations in 21 401 families with breast and ovarian cancer. J Med Genet. (2016) 53:465–71. doi: 10.1136/jmedgenet-2015-103672
41. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer (2023). National Comprehensive Cancer Network, Inc (Accessed 24 October 2023).
42. Early and locally advanced breast cancer: diagnosis and management (2018). Available online at: www.nice.org.uk/guidance/ng101.
43. Murthy V, Nobre S, Sparber L, Schaefer S, Santoro E, McDermott J, et al. Multidisciplinary breast conference improves patient management and treatment. Surg Sci. (2014) 05:314–9. doi: 10.4236/ss.2014.57053
44. Pillay B, Wootten AC, Crowe H, Corcoran N, Tran B, Bowden P, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. (2016) 42:56–72. doi: 10.1016/j.ctrv.2015.11.007
45. Serrano-Fabiá A, Albert-Marí A, Almenar-Cubells D, Jiménez-Torres NV. Multidisciplinary system for detecting medication errors in antineoplastic chemotherapy. J Oncol Pharm Pract Off Publ Int Soc Oncol Pharm Pract. (2010) 16:105–12. doi: 10.1177/1078155209340482
46. Zorbas H, Rainbird K, Luxford K, Barraclough B, Redman S. Multidisciplinary care for women with early breast cancer in the Australian context: what does it mean? Med J Aust. (2003) 179:528–31. doi: 10.5694/j.1326-5377.2003.tb05678.x
47. Kočo L, Weekenstroo HHA, Lambregts DMJ, Sedelaar JPM, Prokop M, Fütterer JJ, et al. The effects of multidisciplinary team meetings on clinical practice for colorectal, lung, prostate and breast cancer: A systematic review. Cancers. (2021) 13:4159. doi: 10.3390/cancers13164159
48. Tsai C-H, Hsieh H-F, Lai T-W, Kung P-T, Kuo W-Y, Tsai W-C. Effect of multidisciplinary team care on the risk of recurrence in breast cancer patients: A national matched cohort study. Breast Off J Eur Soc Mastol. (2020) 53:68–76. doi: 10.1016/j.breast.2020.07.001
49. Chiang J, Ngeow J. The management of BRCA1 and BRCA2 carriers in Singapore. Chin Clin Oncol. (2020) 9:62. doi: 10.21037/cco-20-104
50. Tilanus-Linthorst M, Verhoog L, Obdeijn I-M, Bartels K, Menke-Pluymers M, Eggermont A, et al. A BRCA1/2 mutation, high breast density and prominent pushing margins of a tumor independently contribute to a frequent false-negative mammography. Int J Cancer. (2002) 102:91–5. doi: 10.1002/ijc.10666
51. Warner E, Plewes DB, Hill KA, Causer PA, Zubovits JT, Jong RA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. (2004) 292:1317–25. doi: 10.1001/jama.292.11.1317
52. Riedl CC, Ponhold L, Flöry D, Weber M, Kroiss R, Wagner T, et al. Magnetic resonance imaging of the breast improves detection of invasive cancer, preinvasive cancer, and premalignant lesions during surveillance of women at high risk for breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. (2007) 13:6144–52. doi: 10.1158/1078-0432.CCR-07-1270
53. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2019) 30:1194–220. doi: 10.1093/annonc/mdz173
54. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic V.2.2024 (2023). National Comprehensive Cancer Network, Inc (Accessed 21 October 2023).
55. Preventive Services Task Force US, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US preventive services task force recommendation statement. JAMA. (2019) 322:652–65. doi: 10.1001/jama.2019.10987
56. NICE Guidelines Committee. Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. London, UK: National Institute for Health and Care Excellence (2019).
57. Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. (2000) 58:299–308. doi: 10.1034/j.1399-0004.2000.580408.x
58. Evans DGR, Eccles DM, Rahman N, Young K, Bulman M, Amir E, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. (2004) 41:474–80. doi: 10.1136/jmg.2003.017996
59. Bellcross CA, Lemke AA, Pape LS, Tess AL, Meisner LT. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med Off J Am Coll Med Genet. (2009) 11:783–9. doi: 10.1097/GIM.0b013e3181b9b04a
60. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population-based screening. Cancer. (2006) 107:1769–76. doi: 10.1002/cncr.22202
61. Ashton-Prolla P, Giacomazzi J, Schmidt AV, Roth FL, Palmero EI, Kalakun L, et al. Development and validation of a simple questionnaire for the identification of hereditary breast cancer in primary care. BMC Cancer. (2009) 9:283. doi: 10.1186/1471-2407-9-283
62. Consensus-Guideline-on-Genetic-Testing-for-Hereditary-Breast-Cancer.pdf. Available online at: https://www.breastsurgeons.org/docs/statements/Consensus-Guideline-on-Genetic-Testing-for-Hereditary-Breast-Cancer.pdf (Accessed April 11, 2023).
63. Beitsch PD, Whitworth PW, Hughes K, Patel R, Rosen B, Compagnoni G, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol Off J Am Soc Clin Oncol. (2019) 37:453–60. doi: 10.1200/JCO.18.01631
64. Weitzel JN, Lagos VI, Cullinane CA, Gambol PJ, Culver JO, Blazer KR, et al. Limited family structure and BRCA gene mutation status in single cases of breast cancer. JAMA. (2007) 297:2587–95. doi: 10.1001/jama.297.23.2587
65. Whitworth PW, Beitsch PD, Patel R, Rosen B, Compagnoni G, Baron PL, et al. Clinical utility of universal germline genetic testing for patients with breast cancer. JAMA Netw Open. (2022) 5:e2232787. doi: 10.1001/jamanetworkopen.2022.32787
66. De Silva DL, Stafford L, Skandarajah AR, Sinclair M, Devereux L, Hogg K, et al. Universal genetic testing for women with newly diagnosed breast cancer in the context of multidisciplinary team care. Med J Aust. (2023) 218:368–73. doi: 10.5694/mja2.51906
67. Copur MS. Universal genetic testing for all breast cancer patients. Oncol Williston Park N. (2019) 33:683731.
68. Tung NM, Garber JE. BRCA1/2 testing: therapeutic implications for breast cancer management. Br J Cancer. (2018) 119:141–52. doi: 10.1038/s41416-018-0127-5
69. Feliubadaló L, Lopez-Doriga A, Castellsagué E, del Valle J, Menéndez M, Tornero E, et al. Next-generation sequencing meets genetic diagnostics: development of a comprehensive workflow for the analysis of BRCA1 and BRCA2 genes. Eur J Hum Genet. (2013) 21:864–70. doi: 10.1038/ejhg.2012.270
70. Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, Lingenfelter B, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol Off J Am Soc Clin Oncol. (2002) 20:1480–90. doi: 10.1200/JCO.2002.20.6.1480
71. Wallace AJ. New challenges for BRCA testing: a view from the diagnostic laboratory. Eur J Hum Genet. (2016) 24:S10–8. doi: 10.1038/ejhg.2016.94
72. Park S, Lee JE, Ryu JM, Kim I, Bae SY, Lee SK, et al. Genetic Diagnosis before Surgery has an Impact on Surgical Decision in BRCA Mutation Carriers with Breast Cancer. World J Surg. (2018) 42:1384–90. doi: 10.1007/s00268-017-4342-7
73. Yadav S, Reeves A, Campian S, Sufka A, Zakalik D. Preoperative genetic testing impacts surgical decision making in BRCA mutation carriers with breast cancer: a retrospective cohort analysis. Hered Cancer Clin Pract. (2017) 15:11. doi: 10.1186/s13053-017-0071-z
74. Zayed H, Ouhtit A. Accredited genetic testing in the Arab Gulf region: reinventing the wheel. J Hum Genet. (2016) 61:673–4. doi: 10.1038/jhg.2016.22
75. Vorobiof DA. Real-World data (RWD) of physician’s advice on BRCA genetic testing for ovary (OC) and breast cancer (BC) patients (pts). J Clin Oncol. (2020) 38:e19357–7. doi: 10.1200/JCO.2020.38.15_suppl.e19357
76. Lux MP, Niyazov A, Lewis K, Rider A, Arondekar B, Mahtani R. 314P Real-world (RW) multi-country study of BRCA1/2 testing in adult patients (pts) with HER2–advanced breast cancer (ABC). Ann Oncol. (2020) 31:S368–9. doi: 10.1016/j.annonc.2020.08.416
77. Co M, Liu T, Leung J, Li CH, Tse T, Wong M, et al. Breast conserving surgery for BRCA mutation carriers—A systematic review. Clin Breast Cancer. (2020) 20:e244–50. doi: 10.1016/j.clbc.2019.07.014
78. Pierce LJ, Phillips K-A, Griffith KA, Buys S, Gaffney DK, Moran MS, et al. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat. (2010) 121:389–98. doi: 10.1007/s10549-010-0894-z
79. Nilsson MP, Hartman L, Kristoffersson U, Johannsson OT, Borg Å, Henriksson K, et al. High risk of in-breast tumor recurrence after BRCA1/2-associated breast cancer. Breast Cancer Res Treat. (2014) 147:571–8. doi: 10.1007/s10549-014-3115-3
80. van den Broek AJ, Schmidt MK, van ‘t Veer LJ, Oldenburg HSA, Rutgers EJ, Russell NS, et al. Prognostic impact of breast-conserving therapy versus mastectomy of BRCA1/2 mutation carriers compared with noncarriers in a consecutive series of young breast cancer patients. Ann Surg. (2019) 270:364. doi: 10.1097/SLA.0000000000002804
81. Wan Q, Su L, Ouyang T, Li J, Wang T, Fan Z, et al. Comparison of survival after breast-conserving therapy vs mastectomy among patients with or without the BRCA1/2 variant in a large series of unselected Chinese patients with breast cancer. JAMA Netw Open. (2021) 4:e216259. doi: 10.1001/jamanetworkopen.2021.6259
82. Gentile D, Losurdo A, Sagona A, Zuradelli M, Gatzemeier W, Barbieri E, et al. Surgical management of BRCA-mutation carriers: A single institution experience. Eur J Surg Oncol. (2022) 48:1706–12. doi: 10.1016/j.ejso.2022.04.024
83. Soenderstrup IMH, Laenkholm AV, Jensen MB, Eriksen JO, Gerdes AM, Hansen TVO, et al. Clinical and molecular characterization of BRCA-associated breast cancer: results from the DBCG. Acta Oncol Stockh Swed. (2018) 57:95–101. doi: 10.1080/0284186X.2017.1398415
84. Fayanju OM, Stoll CRT, Fowler S, Colditz GA, Margenthaler JA. Contralateral prophylactic mastectomy after unilateral breast cancer: a systematic review and meta-analysis. Ann Surg. (2014) 260:1000–10. doi: 10.1097/SLA.0000000000000769
85. De La Cruz L, Moody AM, Tappy EE, Blankenship SA, Hecht EM. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: A meta-analysis and systematic review. Ann Surg Oncol. (2015) 22:3241–9. doi: 10.1245/s10434-015-4739-1
86. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomized trials. Lancet Lond Engl. (2011) 378:1707–16. doi: 10.1016/S0140-6736(11)61629-2
87. Pierce LJ, Levin AM, Rebbeck TR, Ben-David MA, Friedman E, Solin LJ, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. (2006) 24:2437–43. doi: 10.1200/JCO.2005.02.7888
88. Brekelmans CTM, Seynaeve C, Menke-Pluymers M, Brüggenwirth HT, Tilanus-Linthorst MMA, Bartels CCM, et al. Survival and prognostic factors in BRCA1-associated breast cancer. Ann Oncol Off J Eur Soc Med Oncol. (2006) 17:391–400. doi: 10.1093/annonc/mdj095
89. Shanley S, McReynolds K, Ardern-Jones A, Ahern R, Fernando I, Yarnold J, et al. Late toxicity is not increased in BRCA1/BRCA2 mutation carriers undergoing breast radiotherapy in the United Kingdom. Clin Cancer Res Off J Am Assoc Cancer Res. (2006) 12:7025–32. doi: 10.1158/1078-0432.CCR-06-1244
90. Pierce LJ, Haffty BG. Radiotherapy in the treatment of hereditary breast cancer. Semin Radiat Oncol. (2011) 21:43–50. doi: 10.1016/j.semradonc.2010.08.008
91. Kriege M, Jager A, Hooning MJ, Huijskens E, Blom J, van Deurzen CHM, et al. The efficacy of taxane chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer. (2012) 118:899–907. doi: 10.1002/cncr.26351
92. Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L, et al. Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: A single-institution experience. J Clin Oncol. (2011) 29:3739–46. doi: 10.1200/JCO.2011.35.2682
93. Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol. (2010) 28:375–9. doi: 10.1200/JCO.2008.20.7019
94. Wang C, Zhang J, Wang Y, Ouyang T, Li J, Wang T, et al. Prevalence of BRCA1 mutations and responses to neoadjuvant chemotherapy among BRCA1 carriers and non-carriers with triple-negative breast cancer. Ann Oncol Off J Eur Soc Med Oncol. (2015) 26:523–8. doi: 10.1093/annonc/mdu559
95. Byrski T, Huzarski T, Dent R, Marczyk E, Jasiowka M, Gronwald J, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. (2014) 147:401–5. doi: 10.1007/s10549-014-3100-x
96. Tung N, Arun B, Hacker MR, Hofstatter E, Toppmeyer DL, Isakoff SJ, et al. TBCRC 031: randomized phase II study of neoadjuvant cisplatin versus doxorubicin-cyclophosphamide in germline BRCA carriers with HER2-negative breast cancer (the INFORM trial). J Clin Oncol Off J Am Soc Clin Oncol. (2020) 38:1539–48. doi: 10.1200/JCO.19.03292
97. Caramelo O, Silva C, Caramelo F, Frutuoso C, Pinto L, Almeida-Santos T. Efficacy of different neoadjuvant treatment regimens in BRCA-mutated triple negative breast cancer: a systematic review and meta-analysis. Hered Cancer Clin Pract. (2022) 20:34. doi: 10.1186/s13053-022-00242-0
98. von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomized phase 2 trial. Lancet Oncol. (2014) 15:747–56. doi: 10.1016/S1470-2045(14)70160-3
99. Loibl S, O’Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomized, phase 3 trial. Lancet Oncol. (2018) 19:497–509. doi: 10.1016/S1470-2045(18)30111-6
100. Zhang J, Yao L, Liu Y, Ouyang T, Li J, Wang T, et al. Impact of the addition of carboplatin to anthracycline-taxane-based neoadjuvant chemotherapy on survival in BRCA1/2-mutated triple-negative breast cancer. Int J Cancer. (2021) 148:941–9. doi: 10.1002/ijc.33234
101. Hahnen E, Lederer B, Hauke J, Loibl S, Kröber S, Schneeweiss A, et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the geparSixto randomized clinical trial. JAMA Oncol. (2017) 3:1378–85. doi: 10.1001/jamaoncol.2017.1007
102. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomized trials. Lancet. (2011) 378:771–84. doi: 10.1016/S0140-6736(11)60993-8
103. Wesolowski R, Shealy AG, Tao J, Moore HC. Differential outcomes in patients treated with endocrine therapy for early or locally advanced breast cancer based on BRCA mutation status. J Clin Oncol. (2009) 27:e22065–5. doi: 10.1200/jco.2009.27.15_suppl.e22065
104. Phillips K-A, Milne RL, Rookus MA, Daly MB, Antoniou AC, Peock S, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. (2013) 31:3091–9. doi: 10.1200/JCO.2012.47.8313
105. Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol Off J Am Soc Clin Oncol. (2004) 22:2328–35. doi: 10.1200/JCO.2004.04.033
106. Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol Off J Am Soc Clin Oncol. (2020) 38:3987–98. doi: 10.1200/JCO.20.02514
107. Desai NV, Zakalik D, Somerfield MR, Tung NM. Q and A: A new standard of care for germline BRCA1 and/or BRCA2 mutation carriers with early-stage breast cancer. JCO Oncol Pract. (2022) 18:427–9. doi: 10.1200/OP.21.00770
108. Robson M, Im S-A, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. (2017) 377:523–33. doi: 10.1056/NEJMoa1706450
109. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee K-H, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. (2018) 379:753–63. doi: 10.1056/NEJMoa1802905
110. Research C for DE and. FDA approves olaparib for adjuvant treatment of high-risk early breast cancer (2022). FDA. Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-adjuvant-treatment-high-risk-early-breast-cancer (Accessed March 20, 2023).
111. Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. (2021) 384:2394–405. doi: 10.1056/NEJMoa2105215
112. Geyer CE, Garber JE, Gelber RD, Yothers G, Taboada M, Ross L, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol Off J Eur Soc Med Oncol. (2022) 33:1250–68. doi: 10.1016/j.annonc.2022.09.159
113. Tung NM, Zakalik D, Somerfield MR. Adjuvant PARP inhibitors in patients with high-risk early-stage HER2-negative breast cancer and germline BRCA mutations: ASCO hereditary breast cancer guideline rapid recommendation update. J Clin Oncol. (2021) 39:2959–61. doi: 10.1200/JCO.21.01532
114. Litton JK, Scoggins ME, Hess KR, Adrada BE, Murthy RK, Damodaran S, et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant. J Clin Oncol Off J Am Soc Clin Oncol. (2020) 38:388–94. doi: 10.1200/JCO.19.01304
115. Litton JK, Beck JT, Jones JM, Andersen J, Blum JL, Mina LA, et al. Neoadjuvant talazoparib in patients with germline BRCA1/2 mutation-positive, early-stage triple-negative breast cancer: results of a phase II study. Oncologist. (2023) 28:845–55. doi: 10.1093/oncolo/oyad139
116. Rugo HS, Olopade OI, DeMichele A, Yau C, van ‘t Veer LJ, Buxton MB, et al. Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N Engl J Med. (2016) 375:23–34. doi: 10.1056/NEJMoa1513749
117. Geyer CE, Sikov WM, Huober J, Rugo HS, Wolmark N, O’Shaughnessy J, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann Oncol Off J Eur Soc Med Oncol. (2022) 33:384–94. doi: 10.1016/j.annonc.2022.01.009
118. Domchek SM, Postel-Vinay S, Im S-A, Park YH, Delord J-P, Italiano A, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicenter, phase 1/2, basket study. Lancet Oncol. (2020) 21:1155–64. doi: 10.1016/S1470-2045(20)30324-7
119. Vinayak S, Tolaney SM, Schwartzberg L, Mita M, McCann G, Tan AR, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. (2019) 5:1132–40. doi: 10.1001/jamaoncol.2019.1029
120. Maio M, shapira-frommer R, Yap T, Ciuleanu T, Gomez H, Hill A, et al. Abstract CT178: Olaparib plus pembrolizumab in patients with previously treated advanced solid tumors with homologous recombination repair mutation (HRRm) and/or homologous recombination deficiency (HRD): Initial results of the phase 2 KEYLYNK-007 study. Cancer Res. (2021) 81(13_Supplement):CT178. doi: 10.1158/1538-7445.AM2021-CT178
121. Yap TA, Bardia A, Dvorkin M, Galsky MD, Beck JT, Wise DR, et al. Avelumab plus talazoparib in patients with advanced solid tumors: the JAVELIN PARP medley nonrandomized controlled trial. JAMA Oncol. (2023) 9:40–50. doi: 10.1001/jamaoncol.2022.5228
122. Pusztai L, Yau C, Wolf DM, Han HS, Du L, Wallace AM, et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: Results from the adaptively randomized I-SPY2 trial. Cancer Cell. (2021) 39:989–998.e5. doi: 10.1016/j.ccell.2021.05.009
123. Pedapenki RM, Madan A. General Oncology Care in Bahrain. In: Al-Shamsi HO, Abu-Gheida IH, Iqbal F, Al-Awadhi A, editors. Cancer in the Arab World. Springer, Singapore (2022). p. 31–40. doi: 10.1007/978-981-16-7945-2_3
124. Alhuraiji A, Abdullah J, Almutairi BO, Albarrak J. General Oncology Care in Kuwait. In: Al-Shamsi HO, Abu-Gheida IH, Iqbal F, Al-Awadhi A, editors. Cancer in the Arab World. Springer, Singapore (2022). p. 99–114. doi: 10.1007/978-981-16-7945-2_7
125. Abu-Gheida IH, Nijhawan N, Al-Awadhi A, Al-Shamsi HO. General Oncology Care in the UAE. In: Al-Shamsi HO, Abu-Gheida IH, Iqbal F, Al-Awadhi A, editors. Cancer in the Arab World. Springer, Singapore (2022). p. 301–19. doi: 10.1007/978-981-16-7945-2_19
126. Abusanad A, Alghamdi M, Bakkar M, Jazieh AR. General Oncology Care in the Kingdom of Saudi arabia. In: Al-Shamsi HO, Abu-Gheida IH, Iqbal F, Al-Awadhi A, editors. Cancer in the Arab World. Springer, Singapore (2022). p. 215–33. doi: 10.1007/978-981-16-7945-2_14
127. Mehdi I, Al Farsi AA, Al Bahrani B, Al-Raisi SS. General Oncology Care in Oman. In: Al-Shamsi HO, Abu-Gheida IH, Iqbal F, Al-Awadhi A, editors. Cancer in the Arab World. Springer, Singapore (2022). p. 175–93. doi: 10.1007/978-981-16-7945-2_12
128. Al-Shamsi HO, Iqbal F. General Oncology Care in Qatar, Comoros, and Djibouti. In: Al-Shamsi HO, Abu-Gheida IH, Iqbal F, Al-Awadhi A, editors. Cancer in the Arab World. Springer, Singapore (2022). p. 339–52. doi: 10.1007/978-981-16-7945-2_21
Keywords: breast cancer, BRCA1, BRCA2, GCC, TNBC, olaparib
Citation: Al-Shamsi HO, Alwbari A, Azribi F, Calaud F, Thuruthel S, Tirmazy SHH, Kullab S, Ostomane S and Abulkhair O (2024) BRCA testing and management of BRCA-mutated early-stage breast cancer: a comprehensive statement by expert group from GCC region. Front. Oncol. 14:1358982. doi: 10.3389/fonc.2024.1358982
Received: 20 December 2023; Accepted: 28 March 2024;
Published: 25 April 2024.
Edited by:
Alberto Farolfi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Taobo Hu, Peking University People’s Hospital, ChinaValeria D’Argenio, University of Naples Federico II, Italy
Copyright © 2024 Al-Shamsi, Alwbari, Azribi, Calaud, Thuruthel, Tirmazy, Kullab, Ostomane and Abulkhair. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Omalkhair Abulkhair, Omal_danah@hotmail.com
 Humaid O. Al-Shamsi
Humaid O. Al-Shamsi Ahmed Alwbari6
Ahmed Alwbari6 Fathi Azribi
Fathi Azribi Omalkhair Abulkhair
Omalkhair Abulkhair