- 1Division of Hematology and Medical Oncology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 2Plastic and Reconstructive Surgery Division, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 3Division of Endocrinology, Diabetes and Bone Diseases, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Introduction: Lymphedema is a major public health issue for many women undergoing breast cancer treatment. Although weight loss has been reported to be beneficial in the treatment of lymphedema, no studies to date have examined the use of GLP-1RAs for the treatment of secondary lymphedema. This case report describes a patient who experienced significant resolution of her breast cancer-related lymphedema after initiation of a GLP-1RA for weight loss.
Main symptoms and/or important clinical findings: Nine months postoperatively the patient developed arm swelling and disability. While on adjuvant chemo and hormonal therapy, her weight increased dramatically and peaked 4 years later. Corresponding to her weight gain was significant worsening of her symptoms.
The main diagnoses, therapeutic interventions, and outcomes: Due to adjuvant cancer-related weight gain and inability to lose weight with diet and exercise, she was referred for evaluation and diagnosed with lymphedema. The patient started treatment with a Glucagon-like peptide 1 receptor agonist and lost 24% of her body weight over the next 13 months. The improvement in her lymphedema mirrored her weight loss. Her limb volume difference dropped from 10.3% down to 3.4% and she no longer required a compression garment. Her imaging demonstrated return of lymphatic pumping and she experienced a significant improvement in quality of life, assessed by a validated lymphedema-specific patient reported outcome (PROM). She remains on hormonal therapy, no longer needs compression and is back to regular exercise without impairment.
Conclusions: GLP-1 RAs provide a potential medical option for many patients struggling with weight gain and lymphedema. We have observed by all objective measures a significant reduction in lymphedema and the elimination of compression in the case presented as a direct result of GLP-1 RA. This may also reduce a patient’s BMI to the point where they become a good candidate for lymphovenous bypass or vascularized lymph node transplant when indicated.
Introduction
Lymphedema is a major public health issue for many women undergoing breast cancer treatment. One in three women undergoing axillary lymph node dissection and radiotherapy will develop this incurable and disabling disease (1–3). Swelling, cellulitis, and disability are typical features of this condition. The standard treatment is lifelong compression and physiotherapy, but even the most compliant patients often experience disease progression. If the results of conservative management are limited, surgery is a worthwhile consideration in the appropriate patient with reported efficacy in the literature (4–8). However, not every patient is a surgical candidate. Currently there is no medical therapy that has been widely adopted for lymphedema and there is no cure.
As far back as 1957 studies have shown that obesity can increase the risk of secondary lymphedema (9, 10). This is particularly relevant for patients undergoing breast cancer treatment where chemotherapy and endocrine therapies often result in significant weight gain (11–13). Consequently, a significant increase in body mass index (BMI) can induce or exacerbate lymphedema. Patients frequently struggle with both weight gain and lymphedema along with metabolic derangements secondary to breast cancer therapies.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs) were originally brought to market as treatments for diabetes, and were noted to induce weight loss (14). In more recent times liraglutide, semaglutide and tirzepatide have been approved for chronic weight management in adults with obesity or who are overweight with at least one weight-related condition (such as high blood pressure, type 2 diabetes, or high cholesterol) (15).
Although weight loss has been reported to be beneficial in the treatment of lymphedema, no studies to date have examined the use of GLP-1RAs for the treatment of secondary lymphedema. This case report describes a patient who experienced significant resolution of her breast cancer-related lymphedema after initiation of a GLP-1RA for weight loss.
Case
This is a 44-year-old woman who presented in 2017 for evaluation of breast cancer-related lymphedema due to arm swelling. She had a history of poorly differentiated stage IIA invasive ductal breast carcinoma with right axillary lymph node involvement. The tumor was estrogen receptor positive and human epidermal growth factor receptor 2 positive. The patient underwent neoadjuvant chemotherapy, followed by right mastectomy and axillary lymph node dissection and adjuvant radiation therapy, chemotherapy and hormonal therapy.
Nine months postoperatively this patient developed mild upper extremity lymphedema—classified as stage 1 based on the International Society of Lymphology (ISL) staging system. Her lymphedema was well controlled but required daily compression with a negligible limb volume difference of 1.7%. She had only mildly impaired quality of life reflected by a low impairment score of 19.1 on the validated Lymphedema Life Impact Scale (LLIS). Indocyanine green lymphangiography (ICG) demonstrated mild abnormal collateralization of lymphatic vessels and presence of lymphatic pumping and flow into the axilla. Her initial weight was 49.9 kg (BMI 19.2 kg/m2). While on adjuvant chemo and hormonal therapy, her weight increased dramatically and peaked 4 years later to 66.3 kg (16.4 kg weight gain) and a BMI of 24.9. The This weight gain resulted in significant exacerbation of her lymphedema. Her limb volume difference spiked to 10.3% and her LLIS demonstrated severe disability with an impairment score that nearly tripled to 52.9. Repeat ICG lymphangiography demonstrated severe dermal backflow and lymphatic congestion now without any lymphatic pumping and no flow into the axilla. Her clinical lymphedema stage progressed from ISL 1 (mild lymphedema) to ISL 2 (moderate lymphedema).
Due to adjuvant cancer-related weight gain and inability to lose weight with diet and exercise, she was referred to endocrinology for evaluation. She was initially started on liraglutide with limited weight response. She was then switched to semaglutide titrated to 1.7 mg weekly and lost 24% of her body weight over the next 13 months. Her weight settled in at 50.3 kg (BMI 18.8). The improvement in her lymphedema mirrored her weight loss. Her limb volume difference dropped from 10.3% down to 3.4% and she no longer required a compression garment. Her LLIS impairment score dropped to 26.5, half of what it was at her peak weight. Follow-up ICG demonstrated return of lymphatic pumping (Figures 1, 2). Her ISL stage reverted to stage 1. She no longer requires compression and is back to regular exercise without impairment or swelling at 30 months following treatment with semaglutide. There were no adverse events observed.

Figure 1 This is a 44 year-old woman with breast cancer-related lymphedema who was treated with semaglutide resulting in significantly reduced limb volume, improved patient reported outcome, and improved ICG lymphangiography. Post-treatment photo is shown at 13 months follow-up without compression.
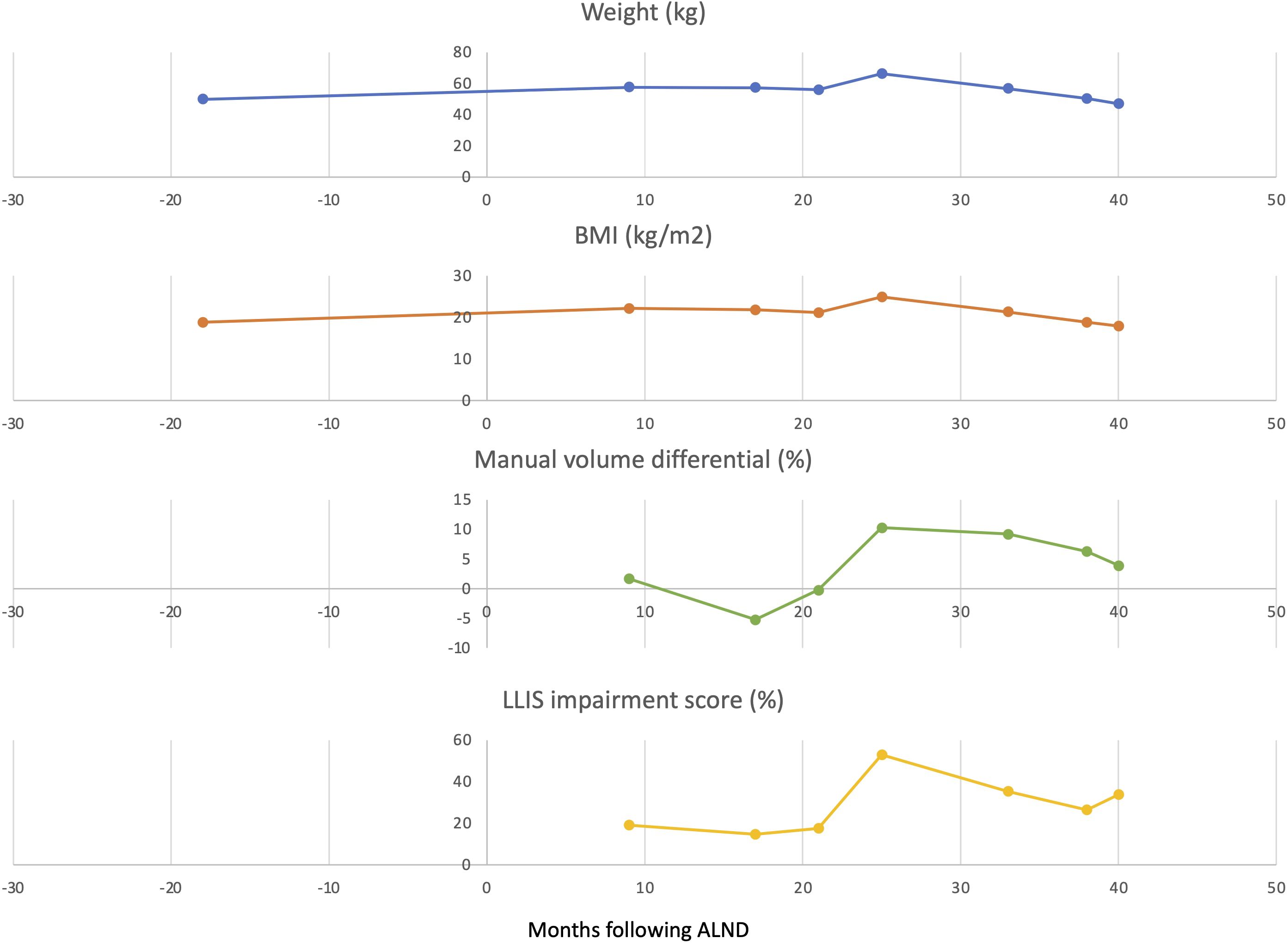
Figure 2 Weight, BMI, impairment score, and limb volume differential scores before and after treatment with GLP-1 RA. Arrows indicate the initiation of GLP-1 RA treatment.
Discussion
This case report describes a new potential medical treatment for lymphedema using GLP-1RAs. We had followed this patient closely with all available metrics that objectively demonstrated not only resolution of her lymphedema but also eliminated the need for compression. This was a particularly exciting finding as she is similar to many women we see with breast cancer-related lymphedema: node positive disease requiring multimodal adjuvant therapy resulting in significant weight gain leading to or exacerbating their lymphedema. Currently there are no validated medical treatments for lymphedema that have gained traction. The potential for a drug that may help many of our patients is intriguing and worthy of a formal study.
Weight gain is a common consequence of adjuvant treatment for breast cancer (11, 13, 16, 17). There is also a clear association between elevated BMI and lymphedema (9, 10, 18–24). However, the effects of weight loss on lymphedema have yielded mixed results albeit with several confounding factors (20, 25–28). Two publications by Shaw et al, demonstrated modest weight loss led to a significant reduction in limb volume (26, 27). Schmidt and colleagues did not observe an effect of weight loss on limb volume (28). However, the mean weight loss in the experimental groups were 7% to 8% loss in body weight. This may not be an adequate amount of weight loss to register a difference. In contrast, this case report observed a 24% loss in body weight. Finally, limb volume recordings are confounded by any changes in compression during the study period which could affect the volume. The most compelling evidence of a real effect of GLP-1RA in this case report is that this patient no longer required compression and her limb volume was significantly reduced. In contrast, the post-treatment compression requirements in other studies are not reported making it difficult to draw meaningful conclusions.
In this report, both the patient’s impairment score and limb volume correlated with both weight gain and weight loss. She had gained 16.4 kg (36 lbs.), causing a dramatic progression of her lymphedema: her impairment score nearly tripled, and her limb volume increased from 1.7% to greater than 10% despite compression and physiotherapy. It is worthwhile noting that this patient was not obese, nor was she diabetic. After treatment with a GLP-1RA, she had lost 24% of her body weight corresponding to a greater than 30-pound weight loss. This is far more significant weight loss than reported in prior studies which may explain the larger effect on her lymphedema.
The patient in this report is similar to many patients undergoing axillary dissection, radiation, chemotherapy and hormonal therapy. The consequence of lymphedema exacerbated by weight gain from cancer treatment can be debilitating. Weight gain of this nature is often refractory to diet and exercise leaving many patients without the hope of improvement. While we routinely perform lymphatic surgery for lymphedema, many patients are not candidates for surgery because of high BMI or do not want surgical intervention. The GLP-1RAs reliably result in an average weight loss of 15% of the patient’s body weight. The newly improved glucagon-dependent insulinotropic polypeptide/GLP-1 RAs result in more than 20% weight loss in a recent clinical trial (15, 29, 30).
GLP-1RAs may be appropriate in patients with a BMI of 25 or greater and does require a rigorous prospective study to further characterize the role of GLP-1RAs in the treatment of lymphedema. It is also worth investigating pathways that GLP-1RAs may affect in the pathophysiology of lymphedema as it is unclear whether their potential effects are related to weight loss alone or interactions with known inflammatory pathways of lymphedema.
Regarding the mechanism of action, is the GLP-1RA reducing lymphedema because of the induced weight loss or is there another mechanism at play? GLP-1 receptors are widely distributed throughout the body and have multiple biological effects: suppressing appetite, reducing neuroinflammation, regulating blood lipid metabolism and reducing fat deposition (31). GLP-1 RAs also elevate adiponectin levels and reduce leptin levels which may have a direct effect on lymphedema (32, 33). Adiponectin is an adipocyte complement-related protein that exhibits anti-inflammatory properties and is inversely correlated with BMI (34–38). Leptin has pro-inflammatory properties and upregulates the secretion of inflammatory cytokines (39). Adiponectin has been implicated in adipogenesis commonly observed in patients with lymphedema (40). Lymphedema fluid collected from lymphedema patients demonstrate lower adiponectin levels and higher leptin levels compared to their own plasma (35). Interestingly, systemic administration of adiponectin in a lymphedema mouse model effectively reduced lymphedema by inducing lymphatic vessel formation (41). Given the effects of GLP-1 RAs on adiponectin and leptin, there may be beneficial effects on lymphatic function and regeneration but this is a subject for future study.
Lymphedema is a fundamentally immunologic disease characterized by lymph stasis inducing CD4+ T cell activation and T-helper 2 cell differentiation (42, 43). This results in upregulation of chemokines inducing migration of lymphocytes into the skin (44). GLP-1RAs have anti-inflammatory properties that may modulate the immune-system (45, 46). An in vitro study by Lieberman et al, demonstrated that GLP-1 RA inhibits chemokine-related migration of human CD4+ lymphocytes (47). The effect of GLP-1RAs on lymphedema’s immunological landscape has not yet been studied but it is possible that GLP-1RAs anti-inflammatory properties may mitigate symptoms of lymphedema. Specific studies assessing the potential immunologic effects of GLP-1 RAs on lymphedema are needed.
It is important to note that the Semaglutide Treatment Effect in People with Obesity (STEP1) clinical trial excluded individuals with a history of malignant neoplasms within five years of screening and therefore, there are no data from clinical trials regarding the weight loss effects, and potential risks or benefits of semaglutide in individuals with breast cancer. Numerous studies have found a positive correlation between obesity and breast cancer incidence, recurrence, and mortality, particularly in postmenopausal patients with estrogen receptor-positive breast cancer, and pre-menopausal triple negative breast cancer (TNBC) (48). Weight loss would therefore theoretically be beneficial to reduce these risks. Surprisingly, a systematic review and meta-analysis of randomized controlled trials found no difference in the risk of developing breast cancer in individuals taking GLP-1RAs compared with controls (49). The effects of GLP-1RAs on breast cancer progression has led to conflicting findings in in vitro and preclinical rodent models. In the human estrogen receptor-positive MCF7 breast cancer cell line, GLP-1RA have consistently been reported to have anti-proliferative and pro-apoptotic effects (50–53). In contrast, in the MDA-MB-231 TNBC cell line, the GLP1-RA liraglutide was found to stimulate cell growth in vitro, but exendin-4 reduced the proliferation (53–55). In vivo, liraglutide was also reported to accelerate the growth of murine 4T1 cells in a syngeneic model (55), but exendin-4 inhibited the growth of MDA-MB-231 and MDA-MB-468 TNBC xenografts (53). Therefore, the preclinical studies support a direct anti-tumor effect of GLP-1RA in hormone receptor positive breast cancers, the subtype of breast cancer with which our patient was being treated; however, more research is needed to understand the conflicting effects of these medications in other subtypes of breast cancer.
Conclusion
The possibility that a GLP-1RA may significantly improve lymphedema in patients with excess weight gain is an exciting one. Weight gain is a common consequence of breast cancer treatment often exacerbating lymphedema. GLP-1 RAs provide a potential medical option for many patients struggling with weight gain and lymphedema. We have observed by all objective measures a significant reduction in lymphedema and the elimination of compression in the case presented as a direct result of GLP-1 RA treatment. This may also reduce a patient’s BMI to the point where they become a good candidate for lymphovenous bypass or vascularized lymph node transplant when indicated. At this time, however, these conclusions are limited to speculation given the nature of a single case report. Future prospective studies are needed to quantify the efficacy and better understanding the cellular mechanisms of GLP-1 RAs on lymphedema.
Patient perspective
Following the patient’s weight gain induced by adjuvant therapy, she reported significant limb swelling that required daily use of compression garments and resulted in a substantial quality of life impairment. Following 13 months of treatment with semaglutide, she reported significant improvement in her limb volume, stopped wearing a compression garment entirely and experienced a significant quality of life improvement.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FC: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. SB: Data curation, Writing – original draft, Writing – review & editing, Conceptualization. EG: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. JD: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
JD is a paid consultant for the Stryker Corporation, has intellectual property rights with Elucida Oncology and equity interest in Welwaze Medical, LLC, and has a royalty agreement with Springer Publishers for Multimodal Management of Upper and Lower Extremity Lymphedema. EG is a paid consultant for Novartis, Flare Therapeutics, Reactive Biosciences and Seagen, and receives grant support from NIH/NCI R37CA266853 and NIH/NHLBI R38HL172261.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. (2013) 14:500–15. doi: 10.1016/s1470-2045(13)70076-7
2. Shah C, Vicini FA. Breast cancer-related arm lymphedema: incidence rates, diagnostic techniques, optimal management and risk reduction strategies. Int J Radiat Oncol Biol Phys. (2011) 81:907–14. doi: 10.1016/j.ijrobp.2011.05.043
3. Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. (2009) 16:1959–72. doi: 10.1245/s10434-009-0452-2
4. Ciudad P, Bolletta A, Kaciulyte J, Losco L, Manrique OJ, Cigna E, et al. The breast cancer-related lymphedema multidisciplinary approach: Algorithm for conservative and multimodal surgical treatment. Microsurgery. (2023) 43:427–36. doi: 10.1002/micr.30990
5. Bolletta A, Taranto G, Losco L, Elia R, Sert G, Ribuffo D, et al. Combined lymph node transfer and suction-assisted lipectomy in lymphedema treatment: a prospective study. Microsurgery. (2022) 42:433–40. doi: 10.1002/micr.30855
6. Chang DW, Dayan J, Greene AK, MacDonald JK, Masia J, Mehrara B, et al. Surgical treatment of lymphedema: A systematic review and meta-analysis of controlled trials. Results of a consensus conference. Plast Reconstr Surg. (2021) 147:975–93. doi: 10.1097/prs.0000000000007783
7. Schaverien MV, Asaad M, Selber JC, Liu J, Chen DN, Hall MS, et al. Outcomes of vascularized lymph node transplantation for treatment of lymphedema. J Am Coll Surg. (2021) 232:982–94. doi: 10.1016/j.jamcollsurg.2021.03.002
8. Brown S, Mehrara BJ, Coriddi M, McGrath L, Cavalli M, Dayan JH. A prospective study on the safety and efficacy of vascularized lymph node transplant. Ann Surg. (2022) 276:635–53. doi: 10.1097/sla.0000000000005591
9. Treves N. An evaluation of the etiological factors of lymphedema following radical mastectomy. An analysis of 1,007 cases. Cancer. (1957) 10:444–59. doi: 10.1002/(ISSN)1097-0142
10. Mehrara BJ, Greene AK. Lymphedema and obesity: is there a link? Plast Reconstr Surg. (2014) 134:154e–60e. doi: 10.1097/prs.0000000000000268
11. Raghavendra A, Sinha AK, Valle-Goffin J, Shen Y, Tripathy D, Barcenas CH. Determinants of weight gain during adjuvant endocrine therapy and association of such weight gain with recurrence in long-term breast cancer survivors. Clin Breast Cancer. (2018) 18:e7–e13. doi: 10.1016/j.clbc.2017.11.006
12. Saquib N, Flatt SW, Natarajan L, Thomson CA, Bardwell WA, Caan B, et al. Weight gain and recovery of pre-cancer weight after breast cancer treatments: evidence from the women’s healthy eating and living (WHEL) study. Breast Cancer Res Treat. (2007) 105:177–86. doi: 10.1007/s10549-006-9442-2
13. Gadéa E, Thivat E, Planchat E, Morio B, Durando X. Importance of metabolic changes induced by chemotherapy on prognosis of early-stage breast cancer patients: a review of potential mechanisms. Obes Rev. (2012) 13:368–80. doi: 10.1111/j.1467-789X.2011.00957.x
14. Inzucchi SE, McGuire DK. New drugs for the treatment of diabetes: part II: Incretin-based therapy and beyond. Circulation. (2008) 117:574–84. doi: 10.1161/circulationaha.107.735795
15. Jensterle M, Rizzo M, Haluzík M, Janež A. Efficacy of GLP-1 RA approved for weight management in patients with or without diabetes: a narrative review. Adv Ther. (2022) 39:2452–67. doi: 10.1007/s12325-022-02153-x
16. Camoriano JK, Loprinzi CL, Ingle JN, Therneau TM, Krook JE, Veeder MH. Weight change in women treated with adjuvant therapy or observed following mastectomy for node-positive breast cancer. J Clin Oncol. (1990) 8:1327–34. doi: 10.1200/JCO.1990.8.8.1327
17. Nichols HB, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Holmes MD, Bersch AJ, et al. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. (2009) 18:1403–9. doi: 10.1158/1055-9965.EPI-08-1094
18. McLaughlin SA, Staley AC, Vicini F, Thiruchelvam P, Hutchison NA, Mendez J, et al. Considerations for clinicians in the diagnosis, prevention, and treatment of breast cancer-related lymphedema: recommendations from a multidisciplinary expert ASBrS panel: part 1: definitions, assessments, education, and future directions. Ann Surg Oncol. (2017) 24:2818–26. doi: 10.1245/s10434-017-5982-4
19. Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J. (2010) 16:48–54. doi: 10.1111/tbj.2009.16.issue-1
20. Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. (2001) 92:1368–77. doi: 10.1002/(ISSN)1097-0142
21. Wilke LG, McCall LM, Posther KE, Whitworth PW, Reintgen DS, Leitch AM, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. (2006) 13:491–500. doi: 10.1245/ASO.2006.05.013
22. Werner R, McCormick B, Petrek J, Cox L, Cirrincione C, Gray J, et al. Arm edema in conservatively managed breast cancer: obesity is a major predictive factor. Radiology. (1991) 180:177–84. doi: 10.1148/radiology.180.1.2052688
23. McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. (2008) 26:5213–9. doi: 10.1200/jco.2008.16.3725
24. Rochlin DH, Barrio AV, McLaughlin S, Zee Van KJ, Woods JF, Dayan JH, et al. Feasibility and clinical utility of prediction models for breast cancer-related lymphedema incorporating racial differences in disease incidence. JAMA Surg. (2023) 158:954–64. doi: 10.1001/jamasurg.2023.2414
25. Roberts SA, Gillespie TC, Shui AM, Brunelle CL, Daniell KM, Locascio JJ, et al. Weight loss does not decrease risk of breast cancer–related arm lymphedema. Cancer. (2021) 127:3939–45. doi: 10.1002/cncr.33819
26. Shaw C, Mortimer P, Judd PA. A randomized controlled trial of weight reduction as a treatment for breast cancer-related lymphedema. Cancer. (2007) 110:1868–74. doi: 10.1002/cncr.22994
27. Shaw C, Mortimer P, Judd PA. Randomized controlled trial comparing a low-fat diet with a weight-reduction diet in breast cancer-related lymphedema. Cancer. (2007) 109:1949–56. doi: 10.1002/cncr.22638
28. Schmitz KH, Troxel AB, Dean LT, DeMichele A, Brown JC, Sturgeon K, et al. Effect of home-based exercise and weight loss programs on breast cancer–related lymphedema outcomes among overweight breast cancer survivors: the WISER survivor randomized clinical trial. JAMA Oncol. (2019) 5:1605–13. doi: 10.1001/jamaoncol.2019.2109
29. Jensen AB, Renström F, Aczél S, Folie P, Biraima-Steinemann M, Beuschlein F, et al. Efficacy of the glucagon-like peptide-1 receptor agonists liraglutide and semaglutide for the treatment of weight regain after bariatric surgery: a retrospective observational study. Obes Surg. (2023) 33:1017–25. doi: 10.1007/s11695-023-06484-8
30. Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. (2019) 7:776–85. doi: 10.1016/s2213-8587(19)30249-9
31. Zhao X, Wang M, Wen Z, Lu Z, Cui L, Fu C, et al. GLP-1 receptor agonists: beyond their pancreatic effects. Front Endocrinol (Lausanne). (2021) 12:721135. doi: 10.3389/fendo.2021.721135
32. Simental-Mendía LE, Sánchez-García A, Linden-Torres E, Simental-Mendía M. Effect of glucagon-like peptide-1 receptor agonists on circulating levels of leptin and resistin: A meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. (2021) 177:108899. doi: 10.1016/j.diabres.2021.108899
33. Simental-Mendía LE, Sánchez-García A, Linden-Torres E, Simental-Mendía M. Impact of glucagon-like peptide-1 receptor agonists on adiponectin concentrations: A meta-analysis of randomized controlled trials. Br J Clin Pharmacol. (2021) 87:4140–9. doi: 10.1111/bcp.14855
34. Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, et al. Plasma adiponectin levels in overweight and obese Asians. Obes Res. (2002) 10:1104–10. doi: 10.1038/oby.2002.150
35. Koc M, Wald M, Varaliová Z, Ondrůjová B, Čížková T, Brychta M, et al. Lymphedema alters lipolytic, lipogenic, immune and angiogenic properties of adipose tissue: a hypothesis-generating study in breast cancer survivors. Sci Rep. (2021) 11:8171. doi: 10.1038/s41598-021-87494-3
36. Coelho M, Oliveira T, Fernandes R. State of the art paper Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. (2013) 9:191–200. doi: 10.5114/aoms.2013.33181
37. Ahl S, Guenther M, Zhao S, James R, Marks J, Szabo A, et al. Adiponectin levels differentiate metabolically healthy vs unhealthy among obese and nonobese white individuals. J Clin Endocrinol Metab. (2015) 100:4172–80. doi: 10.1210/jc.2015-2765
38. Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. (2007) 380:24–30. doi: 10.1016/j.cca.2007.01.026
39. Iikuni N, Lam QL, Lu L, Matarese G, La Cava A. Leptin and inflammation. Curr Immunol Rev. (2008) 4:70–9. doi: 10.2174/157339508784325046
40. Aschen S, Zampell JC, Elhadad S, Weitman E, Andrade Brot De M, Mehrara BJ. Regulation of adipogenesis by lymphatic fluid stasis: part II. Expression of adipose differentiation genes. Plast Reconstr Surg. (2012) 129:838–47. doi: 10.1097/PRS.0b013e3182450b47
41. Shimizu Y, Shibata R, Ishii M, Ohashi K, Kambara T, Uemura Y, et al. Adiponectin-mediated modulation of lymphatic vessel formation and lymphedema. J Am Heart Assoc. (2013) 2:e000438. doi: 10.1161/jaha.113.000438
42. Ly CL, Cuzzone DA, Kataru RP, Mehrara BJ. Small numbers of CD4+ T cells can induce development of lymphedema. Plast Reconstr Surg. (2019) 143:518e–26e. doi: 10.1097/prs.0000000000005322
43. Avraham T, Zampell JC, Yan A, Elhadad S, Weitman ES, Rockson SG, et al. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. (2013) 27:1114–26. doi: 10.1096/fj.12-222695
44. García Nores GD, Ly CL, Cuzzone DA, Kataru RP, Hespe GE, Torrisi JS, et al. CD4(+) T cells are activated in regional lymph nodes and migrate to skin to initiate lymphedema. Nat Commun. (2018) 9:1970. doi: 10.1038/s41467-018-04418-y
45. Bendotti G, Montefusco L, Lunati ME, Usuelli V, Pastore I, Lazzaroni E, et al. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharmacol Res. (2022) 182:106320. doi: 10.1016/j.phrs.2022.106320
46. Farr OM, Tsoukas MA, Triantafyllou G, Dincer F, Filippaios A, Ko B-J, et al. Short-term administration of the GLP-1 analog liraglutide decreases circulating leptin and increases GIP levels and these changes are associated with alterations in CNS responses to food cues: A randomized, placebo-controlled, crossover study. Metabolism. (2016) 65:945–53. doi: 10.1016/j.metabol.2016.03.009
47. Liberman A, Esser M, Marx N, Burgmaier M. Glucagon-like peptide-1 (9-36) inhibits chemokine-induced migration of human CD4-positive lymphocytes. PloS One. (2013) 8:e58445. doi: 10.1371/journal.pone.0058445
48. Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. (2017) 67:378–97. doi: 10.3322/caac.21405
49. Piccoli GF, Mesquita LA, Stein C, Aziz M, Zoldan M, Degobi NAH, et al. Do GLP-1 receptor agonists increase the risk of breast cancer? A systematic review and meta-analysis. J Clin Endocrinol Metab. (2021) 106:912–21. doi: 10.1210/clinem/dgaa891
50. Alanteet AA, Attia HA, Shaheen S, Alfayez M, Alshanawani B. Anti-proliferative activity of glucagon-like peptide-1 receptor agonist on obesity-associated breast cancer: the impact on modulating adipokines' Expression in adipocytes and cancer cells. Dose Response. (2021) 19:1559325821995651. doi: 10.1177/1559325821995651
51. Alanteet A, Attia H, Alfayez M, Mahmood A, Alsaleh K, Alsanea S. Liraglutide attenuates obese-associated breast cancer cell proliferation via inhibiting PI3K/Akt/mTOR signaling pathway. Saudi Pharm J. (2024) 32:101923. doi: 10.1016/j.jsps.2023.101923
52. Zhao W, Zhang X, Zhou Z, Sun B, Gu W, Liu J, et al. Liraglutide inhibits the proliferation and promotes the apoptosis of MCF-7 human breast cancer cells through downregulation of microRNA-27a expression. Mol Med Rep. (2018) 17:5202–12. doi: 10.3892/mmr.2018.8475
53. Ligumsky H, Wolf I, Israeli S, Haimsohn M, Ferber S, Karasik A, et al. The peptide-hormone glucagon-like peptide-1 activates cAMP and inhibits growth of breast cancer cells. Breast Cancer Res Treat. (2012) 132:449–61. doi: 10.1007/s10549-011-1585-0
54. Shadboorestan A, Tarighi P, Koosha M, Faghihi H, Ghahremani MH, Montazeri H. Growth promotion and increased ATP-binding cassette transporters expression by liraglutide in triple negative breast cancer cell line MDA-MB-231. Drug Res (Stuttg). (2021) 71:307–11. doi: 10.1055/a-1345-7890
Keywords: lymphedema, cancer-related lymphedema, glucagon-like peptide 1 receptor agonists, GLP-1, GLP-1RA, breast cancer, case report
Citation: Crowley F, Brown S, Gallagher EJ and Dayan JH (2024) GLP-1 receptor agonist as an effective treatment for breast cancer-related lymphedema: a case report. Front. Oncol. 14:1392375. doi: 10.3389/fonc.2024.1392375
Received: 27 February 2024; Accepted: 02 April 2024;
Published: 18 April 2024.
Edited by:
Karuna Rasineni, University of Nebraska Medical Center, United StatesReviewed by:
Luigi Losco, University of Salerno, ItalyAsher Rajkumar Rajan, University of Nebraska Medical Center, United States
Copyright © 2024 Crowley, Brown, Gallagher and Dayan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph H. Dayan, dayanj@mskcc.org
†These authors have contributed equally to this work
 Fionnuala Crowley
Fionnuala Crowley Stav Brown
Stav Brown Emily J. Gallagher
Emily J. Gallagher Joseph H. Dayan
Joseph H. Dayan