- 1Department of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha, China
- 2Institute of Clinical Pharmacy, Central South University, Changsha, China
- 3Department of Pharmacy, Provincial Clinical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou, China
- 4Department of Pharmacy, Xiangya Hospital, Central South University, Changsha, China
- 5Hypertension Laboratory, Provincial Clinical College of Fujian Medical University, Fujian Provincial Cardiovascular Disease Institute, Fujian Provincial Hospital, Fuzhou, China
The impact of pharmacogenetic variants of cytochrome P450 2C19 (CYP2C19) on clopidogrel-mediated effects on platelet inhibition, inflammatory response and endothelial function, as well as risk of major adverse cardiovascular events (MACE), in coronary heart patients undergoing percutaneous coronary intervention (PCI) was investigated. To this end, we assessed the residual platelet aggregation rate (RPA), maximal aggregation rate (MAR) and plasma levels of sCD40L, sP-selectin, MMP-9, sVCAM-1 and sE-selectin after 24 h of PCI in 559 patients treated with clopidogrel and followed up for 1 year for evidence of MACE. CYP2C19 *2 and *3 variants were identified using a clopidogrel-sensitive gene detection kit. Our results showed higher RPA and MAR as well as increased sE-selectin, sCD40L, sP-selectin, MMP-9, and sVCAM-1 levels in CYP2C19 intermediate metabolizer (IM, CYP2C19*1/*2, or *1/*3), poor metabolizer (PM, CYP2C19*2/*2, *2/*3, or *3/*3) and combined IM+PM groups, relative to those in extensive metabolizers (EM, CYP2C19*1/*1). In total, 519 patients completed 1 year of follow-up, among which 69 (13.3%) experienced MACE. The risk of MACE in CYP2C19 IM+PM patients was 2.664 times higher than that in CYP2C19 EM patients (OR = 2.664 (1.397–5.193), P = 0.004). The data suggest that CYP2C19*2 and *3 variants modulate the drug efficacy of clopidogrel in coronary heart patients undergoing PCI and further enhance the risk of MACE. Accordingly, CYP2C19 pharmacogenetic profiling may be beneficial for coronary heart patients undergoing PCI to predict the efficacy of treatment with clopidogrel. We propose that IM and PM patients should benefit from treatment with higher clopidogrel doses to improve efficacy and reduce the incidence of MACE.
Introduction
Owing to dietary and lifestyle changes, the number of coronary heart disease (CHD) patients worldwide has increased over the years. Percutaneous coronary intervention (PCI) is one of the most significant developments in the treatment of CHD. However, stent thrombosis (ST) is a major complication affecting the efficacy of PCI. Currently, anti-platelet drugs, such as clopidogrel and aspirin, are used to prevent the occurrence of ST after PCI (Stone et al., 2013). Post-operative joint use of clopidogrel and aspirin antiplatelet therapy has been shown to reduce ST, death, myocardial infarction and other major adverse cardiovascular events (MACE), and has rapidly become standard treatment (Steinhubl et al., 2002; Han et al., 2015). However, a number of patients taking conventional doses of clopidogrel continue to experience severe coronary ischemic events (Müller et al., 2003; Stone et al., 2013). The rate of clopidogrel resistance is reported to range from 4.2 to 27.8% (Järemo et al., 2002; Serebruany et al., 2005), implying individual differences in the antiplatelet efficacy of clopidogrel.
Several factors affect the antiplatelet activity of clopidogrel, including inter-patient compliance, age, diabetes, smoking, and drug interactions (Sabaté et al., 2015). Although the efficacy of clopidogrel is linked to several enzymes, transporters and acceptors, such as CYP2B6, CYP3A4, CYP3A5, ABCB1, PON1, and P2Y12, the most important factors suggested to be associated with low response to the drug are CYP2C19*2 and CYP2C19*3 variants (Nagashima et al., 2013; Jiang et al., 2015; Sabaté et al., 2015; Ma et al., 2016). The impact of CYP2C19 variants on risk of MACE is controversial (Mega et al., 2009, 2010b; Bauer et al., 2011; Han et al., 2015). A systematic review and meta-analysis by Bauer et al. (2011) revealed no substantial or consistent influence of CYP2C19 variants on the clinical efficacy of clopidogrel and risk of MACE. In contrast, the groups of Mega and Han reported significantly increased risk of MACE in association with CYP2C19*2 and *3 variants (Mega et al., 2010b; Han et al., 2015; Khalil et al., 2016).
Clopidogrel, an irreversible inhibitor of the adenosine diphosphate (ADP) platelet receptor, P2Y12, is widely used to prevent thromboembolic events in patients (Wei et al., 2015). The platelet function test is usually employed to detect the antiplatelet efficacy of clopidogrel. ADP-induced optical turbidity has been the “gold standard” to evaluate the efficacy of clopidogrel for several decades (Steinhubl et al., 2002). Clopidogrel is reported to block the platelet ADP receptor to inhibit platelet aggregation, which reduces the expression and release of plasma-soluble CD40 ligand (sCD40L) and P-selectin, from platelets, preventing further platelet activation (Li et al., 2015; Obradovic et al., 2015). SCD40L is mainly generated by cleavage of CD40L from the surface of activated platelets, and is therefore considered a platelet activation marker (Gremmel et al., 2015; Li et al., 2015). P selectin is mainly located in α-particles of platelets and stick tubular bodies (Weibel-Palade) of endothelial cells (Kaufmann et al., 2013). During platelet activation and endothelial damage, the protein is rapidly expressed on the cell surface, thereby mediating leukocyte, platelet, and endothelial cell adhesion (Kaufmann et al., 2013).
The mechanism underlying ST after PCI is complex. Anti-platelet drugs only act on a specific part of this complex network (Antonino et al., 2009). Chronic inflammation and endothelial damage are important steps in the pathogenesis of thrombosis after PCI (Antonino et al., 2009; Willoughby et al., 2014). Clopidogrel exerts not only anti-inflammatory activity but also protective effects on the vascular endothelium (Liu et al., 2012; Willoughby et al., 2014). The drug protects the endothelium by hindering the expression of vascular cell adhesion molecule-1 (VCAM-1) that is induced by a variety of cytokines (Yang et al., 2016). E-selectin, a cell adhesion molecule located on the vascular endothelial cell surface, is the first to be expressed in the inflammatory reaction, in turn causing aggravation of endothelial damage, enhanced inflammation, plaque rupture and thrombosis (Smadja et al., 2012). Increased levels of the adhesion molecule, gelatinase matrix metalloproteinase-9 (MMP-9), an inflammatory marker, can induce plaque rupture, resulting in thrombosis (Sternberg et al., 2016).
Considering this background, we aimed to investigate whether CYP2C19 variants are involved in (1) clopidogrel-mediated platelet effects, (2) clopidogrel-mediated inflammatory endothelial effects, and (3) risk of MACE.
Methods
Patients and Study Design
Patients were enrolled from Fujian Provincial Hospital between March 2012 and March 2014. The ethics committee of the hospital approved the study and all patients provided written informed consent. Procedures were carried out in accordance with the approved guidelines. This prospective study was designed to determine the effects of CYP2C19 variants on platelet reactivity, inflammatory and endothelial biomarkers and 1-year clinical outcomes in CHD patients undergoing PCI. Patients were eligible for study enrollment in the cases where (1) ages were higher than 18 and (2) they were diagnosed with CHD using coronary angiography and successfully treated with PCI. Exclusion criteria were as follows: (1) aspirin, clopidogrel and contrast agent allergy, (2) severe diseases, such as tumors and bleeding disorders, (3) hemodynamic instability (systolic blood pressure <90 mmHg and/or diastolic blood pressure <50 mmHg), severe ventricular dysfunction (LVEF <40%), (4) severe liver and kidney dysfunction, (5) blood disease or bleeding diathesis, platelet count <100 × 109/L and hematocrit <30%.
Platelet Function Test
After 24 h of PCI, 3 ml peripheral blood samples were collected and mixed with 3.8% trisodium citrate, and platelet aggregation was determined via the turbidimetric method in 2 h with a four-channel light transmission aggregometer (LBY-BJ4; Precil, Beijing, China). Platelet aggregation was stimulated with 5 μmol/L adenosinediphosphate (ADP). After agonist stimulation, the degree of light transmission was monitored. Results were recorded as light transmission at maximal aggregation. The platelet aggregation rate was recorded as a curve for 5 min and expressed as residual platelet aggregation rate (RPA).
After 24 h of PCI, creatinine (Cr), lipids (TC, TG, HDL-c, LDL-c), platelet count (PLT), RPA, and fibrinogen (FIB) levels were tested in the hospital laboratory and plasma levels of sCD40L, sP-selectin, MMP-9, sVCAM-1, and sE-selectin were assessed via enzyme-linked immunosorbent assay (ELISA) (HUAMEI, Wuhan, China) according to the manufacturer's instructions.
PCI and Clopidogrel Treatment
PCI was performed in accordance with the standard of care. All patients were administered 300 mg clopidogrel and 40 mg atorvastatin prior to the operation. The operator decided on the choice of anticoagulant and the use of glycoprotein IIb/IIIa inhibitor (tirofiban), as well as the type of stent. After PCI, patients were treated with 100 mg/day aspirin indefinitely and 75 mg/day clopidogrel for 1 year. We defined the success of PCI as follows: (1) achievement of complete revascularization and residual stenosis of <20% in target vessels of patients, (2) level 3 TIMI blood flow, (3) partial or complete remission of symptoms of myocardial ischemia, and (4) no serious complications during hospitalization (such as acute myocardial infarction, urgent target lesion revascularization or death).
Genotype Analysis
CYP2C19 genotypes were identified using the clopidogrel-sensitive gene detection kit (BAIO company, Shanghai, China). DNA was extracted from whole peripheral blood, followed by stepwise evaluation involving single-base primer extension, PCR amplification chip spotting, mass spectrometry, DNA microarray and analysis of data with software to obtain CYP2C19 genotypes. All steps were performed in strict accordance with the BAIO genetic analyzer manual instructions. According to the CYP2C19*1, *2, *3 genotypes, patients carrying *1/*1 were grouped as extensive metabolizers (EM), *1/*2 or *1/*3 as intermediate metabolizers (IM), and *2/*2, *2/*3 or *3/*3 as poor metabolizers (PM).
Clinical Endpoints
The clinical endpoints include the following MACE: cardiac death, myocardial infarction, and unstable angina. Cardiac death was defined as any death due to cardiovascular causes or not clearly attributable to non-cardiovascular causes. Myocardial infarction was defined as recent ischemic symptoms with ST-segment elevation or depression and abnormally elevated troponin. Unstable angina was defined as resting onset, duration greater than or equal to 20 min or more severe and longer duration of angina than before and higher frequency of onset. Diagnosis was performed during hospitalization according to serum creatine kinase levels, electrocardiogram findings and other indicators.
Statistical Analysis
Statistical analysis was carried out using SPSS software (version 11.0 for Windows; SPSS, Chicago, IL). Continuous data, presented as median and interquartile range, were compared using Kruskal-Wallis one-way analysis of variance or Mann-Whitney U test. Measurement data following normal distribution were expressed as means ± standard deviation (x ± s) and compared using t-test or univariate analysis of variance (ANOVA). Categorical variables were compared with the chi-square or Fisher exact test, as appropriate. Time-to-event outcomes were analyzed via Kaplan-Meier analysis and differences compared with the log-rank test. Logistic regression analysis was performed to assess independent predictors of MACE. P < 0.05 were considered statistically significant.
Results
Study Population
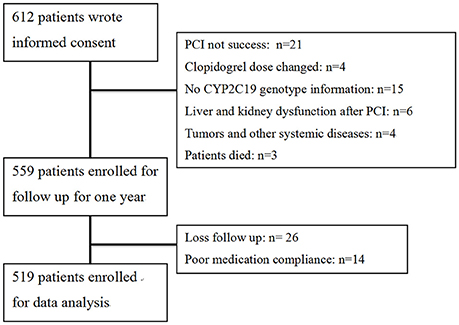
A flow diagram of the study is shown in Figure 1. A total of 612 patients provided written informed consent. Based on inclusion criteria, 559 of these patients were enrolled for 1-year follow-up. Owing to loss to follow-up and poor medication compliance, 40 patients were further excluded, leading to a final total of 519 study participants. The follow-up rate was 95.3%. Clinical characteristics of patients are presented in Table 1. According to CYP2C19 genotype, 181, 253, and 85 patients were grouped as EM, IM, and PM, respectively, for further study. Comparison of clinical characteristics among the three groups disclosed no significant differences in age, gender, body mass index (BMI), CHD risk factors (hypertension, diabetes, smoking) and previous history of myocardial infarction (P > 0.05). Levels of lipids, creatinine, platelet count and fibrinogen were comparable among the three groups (P > 0.05). Differences in usage of clinical medicines, including ACEI/ARB, β-blockers, CCB, statins, PPI, and tirofiban (P > 0.05) were not statistically significant. Moreover, left main coronary artery lesions, vascular lesions (≧3), frame lengths and bracket numbers were similar among the three groups (P > 0.05).
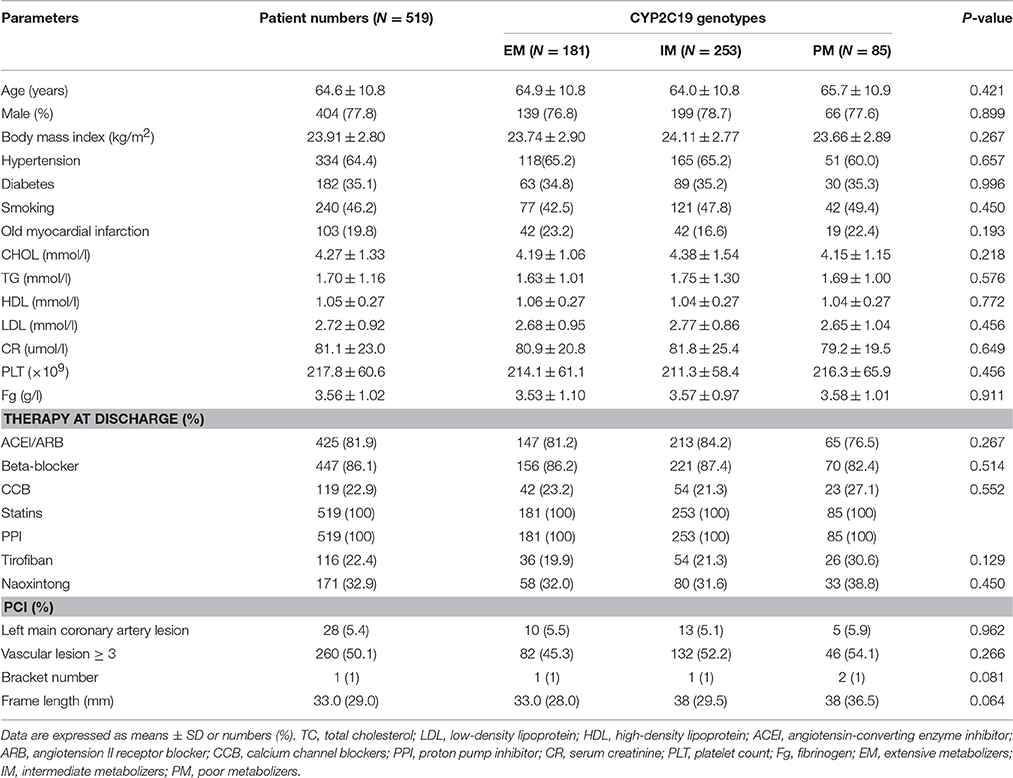
Table 1. Clinical characteristics of clopidogrel-treated patients with various genotypes of CYP2C19 after PCI.
Effects of CYP2C19 Metabolizers on the Drug Efficacy of Clopidogrel
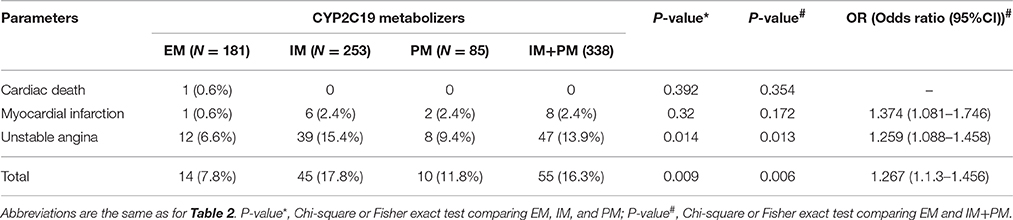
After 24 h of PCI, we measured levels of RPA, MAR, sE-selectin, sCD40L, sP-selectin, MMP-9, and sVCAM-1 in all patients. Significant differences were observed among the three groups using Kruskal-Wallis one-way analysis of variance (Table 2). All the parameters examined showed a higher trend in PM than IM patients. Comparison of these parameters in the EM and IM+PM groups revealed significant differences (Table 2, Figure 2), with higher levels in IM+PM than in EM patients (All P < 0.05).
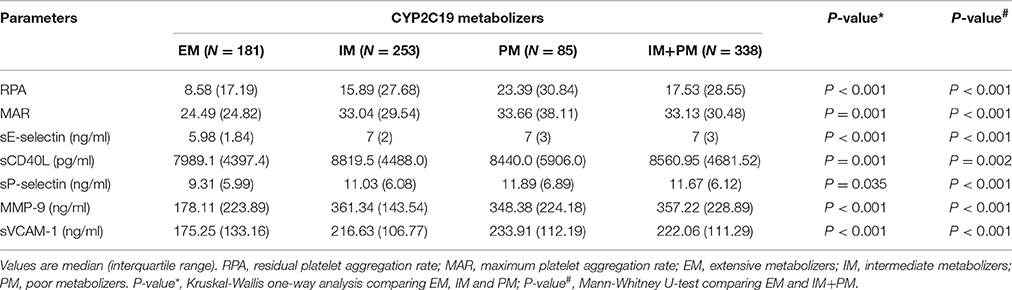
Table 2. Comparison of residual platelet reactivity, inflammatory, and endothelial biomarkers in clopidogrel-treated patients from various CYP2C19 metabolizer groups after PCI.
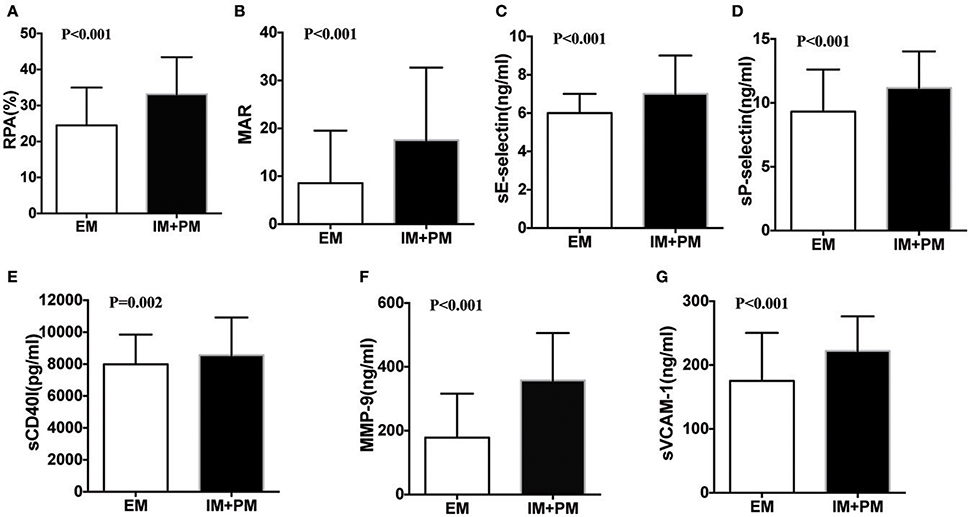
Figure 2. Comparison of residual platelet aggregation indexes in patients stratified by CYP2C19 metabolizers after PCI. (A) RPA, (B) MAR, (C) sE-selectin, (D) sP-selectin, (E) sCD40l, (F) MMP-9, and (G) sVCAM-1 between EM and IM+PM patients.
Effects of CYP2C19 Metabolizers on Clinical Outcomes
One-year follow-up was completed in 559 patients. The total clinical outcomes are shown in Figure 3. During the follow-up period, 69 patients (13.3%) experienced adverse cardiovascular events (1 cardiac death, 9 myocardial infarction and 59 unstable angina). The total frequency of MACE was different among EM, IM and PM patient groups (7.8% vs. 17.8% vs. 11.8%, P = 0.009). The frequency of unstable angina differed among EM, IM and PM patients (6.6% vs. 15.4% vs. 9.8%, P = 0.014). We observed a strong trend toward higher frequency of unstable angina and MACE in IM patients, compared to PM patients. Comparison of the total frequencies of MACE and unstable angina in EM and IM+PM groups additionally revealed significant differences (Table 3). MACE-free survival was further analyzed in patients groups stratified by CYP2C19 metabolizer status. As shown in Figure 4, CYP2C19 EM patients exhibited significantly lower rates of MACE, compared with patients in the IM, PM, and combined IM+PM groups.
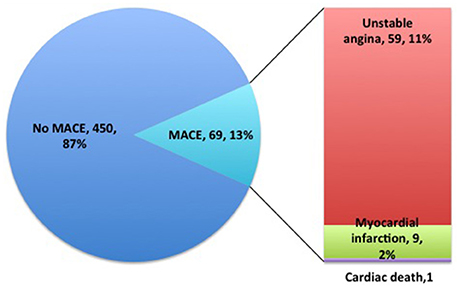
Figure 3. One-year follow-up clinical outcome distributions of clopidogrel-treated patients after PCI.
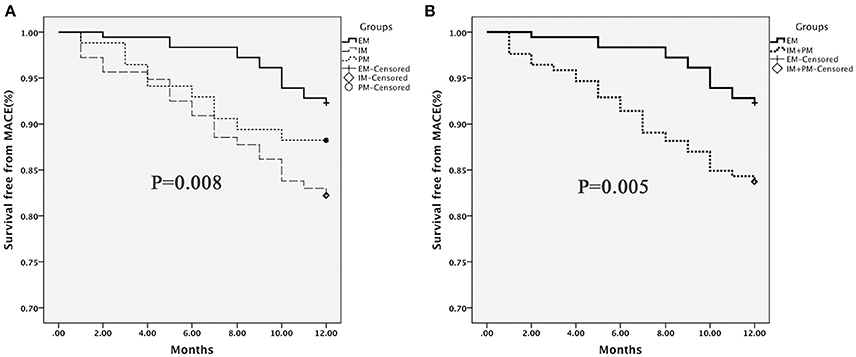
Figure 4. MACE-free survival in patients stratified by CYP2C19 metabolizer status. (A) EM vs. IM vs. PM; (B) EM vs. IM+PM. Differences were compared using the log-rank test.
Univariate and Multivariate Logistic Regression Analysis of the Impact Factors of MACE after PCI
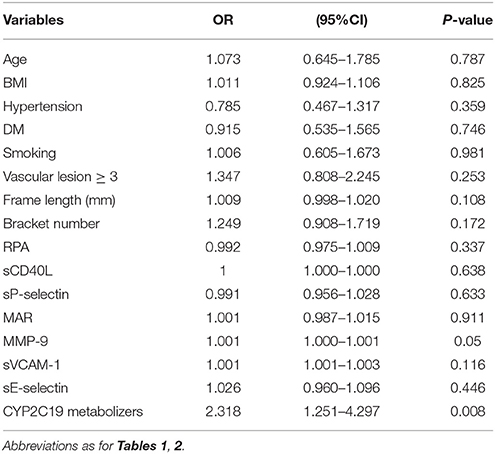
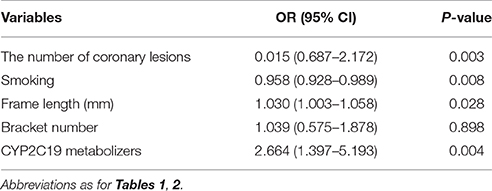
Using MACE after PCI as the dependent variable and age, BMI, CHD risk factors (hypertension, diabetes, smoking), vascular lesions ≥ 3, frame length, bracket number, levels of platelet activity indicators after PCI and CYP2C19 metabolizers (IM+PM and EM) as independent variables, we analyzed whether these relevant parameters influence the occurrence of MACE. Univariate logistic regression analysis showed significant association of CYP2C19 metabolizers with occurrence of MACE (OR = 2.318 (1.251–4.297), P = 0.008) (Table 4). Multivariate logistic regression analysis further disclosed that smoking, frame length and CYP2C19 IM+PM genotypes are significantly associated with risk of MACE (P = 0.008, P = 0.028, P = 0.004, respectively). Notably, the risk of MACE in CYP2C19 IM+PM patients was 2.664 times higher than that in CYP2C19 EM patients (OR = 2.664 (1.397–5.193), P = 0.004) (Table 5).
Discussion
The current study focused on determining whether CYP2C19 genetic variants modulate clopidogrel-mediated antiplatelet effects and risk of MACE in patients undergoing PCI. Our main findings were as follows: (1) CYP2C19*2 and *3 variants influence RPA, MAR and levels of sE-selectin, sCD40L, sP-selectin, MMP-9, and sVCAM-1 in patients following PCI. The efficacy of clopidogrel is reduced in IM+PM, compared to EM patients. (2) CYP2C19 EM patients have lower risk of MACE, compared with IM, PM, and IM+PM groups. (3) CYP2C19*2 and *3 variants are independent risk factors of MACE in patients undergoing PCI.
Several studies have consistently reported that the CYP2C19*2 polymorphism is associated with lower antiplatelet response and increases the rate of recurrent cardiovascular events in Caucasian, Korean, and Chinese patients (Brandt et al., 2007; Shuldiner et al., 2009; Hochholzer et al., 2010; Oh et al., 2012; Liu et al., 2013). Moreover, the CYP2C19 *3 variant is associated with clopidogrel-mediated antiplatelet effects, especially in Asia. Since the incidence of the CYP2C19 *3 variant A allele is less than 1% in Caucasian populations, no carriers of CYP2C19 *3 were identified among 187 Caucasian healthy subjects and 237 Caucasian patients undergoing PCI in an earlier study (Geisler et al., 2008; Shuldiner et al., 2009). Conversely, the frequency of the CYP2C19 *3 variant in several Asian population studies was significantly higher at >20% (Lee et al., 2009; Yamamoto et al., 2011). Interestingly, CYP2C19*17, another functional variant, is additionally reported to contribute to the antiplatelet effect of clopidogrel (Sibbing et al., 2010), but its frequency is relatively rare (Chen et al., 2008; Sibbing et al., 2010). In the current study, we defined EM, IM, and PM patients treated with clopidogrel according to CYP2C19*2 and *3 variant status to reflect CYP2C19 enzymatic activity more comprehensively and accurately.
To date, no large-scale clinical studies on clopidogrel resistance have been conducted, mainly due to the lack of clear and accepted definitions. Over several decades, the ADP-induced optical turbidimetric platelet aggregation assay, the traditional “gold standard” method to detect platelet function, has been employed to evaluate the antiplatelet efficacy of clopidogrel. However, in studies using this platelet function test, cutoff values for high on-treatment platelet reactivity established via receiver operating characteristic curve analysis were variable (Palmerini et al., 2014). A number of studies used other measures, such as SYNTAX score or platelet reactivity index, to investigate associations with risk of mortality, myocardial infarction and ST in patients with non–ST segment elevation acute coronary syndrome undergoing PCI (Palmerini et al., 2011, 2014). Clopidogrel blocks the platelet ADP receptor to inhibit platelet aggregation, in turn reducing expression of sCD40L and P-selectin and releasing them from platelets to prevent further platelet activation (Blake and Ridker, 2001; Steinhubl et al., 2002; Conde and Kleiman, 2003; Azar et al., 2006). Since the levels of sCD40L and sP-selectin are significantly different between the resting and activated states of platelets, they could be effectively evaluated as markers of platelet activation. These indexes can also be used for early assessment of the extent of platelet activation and anti-platelet effects to avoid the occurrence of ischemic and thrombotic events. We not only determined MAR and RPA, but also sCD40L and P-selectin levels in patients undergoing PCI after 24 h. Levels of these molecules were significantly lower in EM than IM+PM patients, supporting the conclusion that CYP2C19 metabolizers influence the anti-platelet effect of clopidogrel.
ST and in-stent restenosis involve platelet activation, inflammation and endothelial injury factor interactions. Clopidogrel has unique anti-inflammatory effects and could improve endothelial function (Ferroni et al., 2003; Heitzer et al., 2006; Husted et al., 2010; Zhang et al., 2015). Accordingly, we measured the levels of inflammation and endothelial injury factors, such as MMP-9, sVCAM-1, and sE-selectin, in patients undergoing PCI. Our results disclosed differences in MMP-9, sVCAM-1 and sE-selectin contents among CYP2C19 variant groups. Specifically, MMP-9, sVCAM-1, and sE-selectin levels were significantly higher in CYP2C19 IM and PM than EM patients, implying that the CYP2C19 variants influence the metabolic activity of clopidogrel, further affecting its pharmacological ability to suppress inflammation and improve endothelial function.
We followed up 559 patients undergoing PCI treated with clopidogrel for 1 year, among which 519 were finally enrolled. Our data revealed a higher percentage of MACE in CYP2C19 IM and PM than EM patients. Moreover, the risk of MACE in IM+PM patients was 1.267 times higher than that in EM patients. Univariate analysis consistently showed that CYP2C19 metabolizers influence the occurrence of MACE after PCI. After adjustment for age, BMI, CHD risk factors (hypertension, diabetes, smoking), vascular lesions ≥3, frame length, bracket number and levels of platelet activity indicators (RPA, MAR, sCD40L, P-selectin, MMP-9, sVCAM-1, and sE-selectin), data from multivariate analysis showed that the risk of MACE in IM+PM patients is 2.664 times higher than that in EM patients. Our results are consistent with earlier studies showing that CYP2C19 variants influence the risk of MACE in ACS or PCI patients treated with clopidogrel (Sibbing et al., 2009; Mega et al., 2010a; Wallentin et al., 2010; Jang et al., 2012). In both univariate and multivariate analyses, we did not establish platelet activity indicators or other inflammation and endothelial injury factors related to the risk of MACE. Previous studies have investigated sVCAM-1, E-selectin and other factors that influence the prognosis of acute myocardial infarction patients. The majority of subjects in our study were unstable angina patients undergoing PCI. Since these factors were examined after 24 h of PCI, the detected levels may not reflect occurrence of MACE, and further research is therefore required to establish this issue. Moreover, during the follow-up period, our study was limited to MACE data and did not include additional factors that could have influenced survival-free analysis, such as liver or kidney dysfunction.
Taken together, our results suggest that CYP2C19*2 and *3 variants influence the levels of RPA, MAR, sE-selectin, sCD40L, sP-selectin, MMP-9, and sVCAM-1 in coronary heart patients undergoing PCI and modulate the drug efficacy of clopidogrel, further increasing the risk of MACE. Based on these findings, we propose that CYP2C19 pharmacogenetic profiling may be beneficial for coronary heart patients undergoing PCI to predict whether treatment with clopidogrel is a feasible option. IM and PM patients may benefit from higher clopidigrel doses to improve efficacy and reduce the incidence of MACE.
Author Contributions
HS, JQ, and HC conceived and designed the experiments. HS, ZC, and HZ performed the experiments. JQ, QQ, and HS analyzed the data. HC and JQ contributed reagents/materials/analysis tools. HS, JQ, ST, and HC wrote the paper. All authors reviewed the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81373838, No. 81403021, and No. 81273647) and Health and Family Planning Commission of Fujian Province Medical Innovation project (No. 2014-CX-5); the National Scientific foundation of China (No. 81503166, 81603208) and the Youth Foundation of Xiangtan Hospital in Central South University (2014Q08).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Antonino, M. J., Mahla, E., Bliden, K. P., Tantry, U. S., and Gurbel, P. A. (2009). Effect of long-term clopidogrel treatment on platelet function and inflammation in patients undergoing coronary arterial stenting. Am. J. Cardiol. 103, 1546–1550. doi: 10.1016/j.amjcard.2009.01.367
Azar, R. R., Kassab, R., Zoghbi, A., Aboujaoudé, S., El-Osta, H., Ghorra, P., et al. (2006). Effects of clopidogrel on soluble CD40 ligand and on high-sensitivity C-reactive protein in patients with stable coronary artery disease. Am. Heart J. 151, 521.e1–521.e4. doi: 10.1016/j.ahj.2005.10.021
Bauer, T., Bouman, H. J., van Werkum, J. W., Ford, N. F., ten Berg, J. M., and Taubert, D. (2011). Impact of CYP2C19 variant genotypes on clinical efficacy of antiplatelet treatment with clopidogrel: systematic review and meta-analysis. BMJ 343:d4588. doi: 10.1136/bmj.d4588
Blake, G. J., and Ridker, P. M. (2001). Novel clinical markers of vascular wall inflammation. Circ. Res. 89, 763–771. doi: 10.1161/hh2101.099270
Brandt, J. T., Close, S. L., Iturria, S. J., Payne, C. D., Farid, N. A., Ernest, C. S. II, et al. (2007). Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J. Thromb. Haemost. 5, 2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x
Chen, L., Qin, S., Xie, J., Tang, J., Yang, L., Shen, W., et al. (2008). Genetic polymorphism analysis of CYP2C19 in Chinese Han populations from different geographic areas of mainland China. Pharmacogenomics 9, 691–702. doi: 10.2217/14622416.9.6.691
Conde, I. D., and Kleiman, N. S. (2003). Soluble CD40 ligand in acute coronary syndromes. N. Engl. J. Med. 348, 2575–2577. doi: 10.1056/NEJM200306193482516
Ferroni, P., Basili, S., Martini, F., Cardarello, C. M., Ceci, F., Di Franco, M., et al. (2003). Serum metalloproteinase 9 levels in patients with coronary artery disease: a novel marker of inflammation. J. Investig. Med. 51, 295–300. doi: 10.1136/jim-51-05-17
Geisler, T., Schaeffeler, E., Dippon, J., Winter, S., Buse, V., Bischofs, C., et al. (2008). CYP2C19 and nongenetic factors predict poor responsiveness to clopidogrel loading dose after coronary stent implantation. Pharmacogenomics 9, 1251–1259. doi: 10.2217/14622416.9.9.1251
Gremmel, T., Frelinger, A. L. III, and Michelson, A. D. (2015). Soluble CD40 ligand in aspirin-treated patients undergoing cardiac catheterization. PLoS ONE 10:e0134599. doi: 10.1371/journal.pone.0134599
Han, Y., Lv, H. H., Liu, X., Dong, Q., Yang, X. L., Li, S. X., et al. (2015). Influence of genetic polymorphisms on clopidogrel response and clinical outcomes in patients with acute ischemic stroke cyp2c19 genotype on clopidogrel response. CNS Neurosci. Ther. 21, 692–697. doi: 10.1111/cns.12426
Heitzer, T., Rudolph, V., Schwedhelm, E., Karstens, M., Sydow, K., Ortak, M., et al. (2006). Clopidogrel improves systemic endothelial nitric oxide bioavailability in patients with coronary artery disease: evidence for antioxidant and antiinflammatory effects. Arterioscler. Thromb. Vasc. Biol. 26, 1648–1652. doi: 10.1161/01.ATV.0000225288.74170.dc
Hochholzer, W., Trenk, D., Fromm, M. F., Valina, C. M., Stratz, C., Bestehorn, H. P., et al. (2010). Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J. Am. Coll. Cardiol. 55, 2427–2434. doi: 10.1016/j.jacc.2010.02.031
Husted, S., Storey, R. F., Harrington, R. A., Emanuelsson, H., and Cannon, C. P. (2010). Changes in inflammatory biomarkers in patients treated with ticagrelor or clopidogrel. Clin. Cardiol. 33, 206–212. doi: 10.1002/clc.20732
Jang, J. S., Cho, K. I., Jin, H. Y., Seo, J. S., Yang, T. H., Kim, D. K., et al. (2012). Meta-analysis of cytochrome P450 2C19 polymorphism and risk of adverse clinical outcomes among coronary artery disease patients of different ethnic groups treated with clopidogrel. Am. J. Cardiol. 110, 502–508. doi: 10.1016/j.amjcard.2012.04.020
Järemo, P., Lindahl, T. L., Fransson, S. G., and Richter, A. (2002). Individual variations of platelet inhibition after loading doses of clopidogrel. J. Intern. Med. 252, 233–238. doi: 10.1046/j.1365-2796.2002.01027.x
Jiang, X. L., Samant, S., Lesko, L. J., and Schmidt, S. (2015). Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin. Pharmacokinet. 54, 147–166. doi: 10.1007/s40262-014-0230-6
Kaufmann, J., Wellnhofer, E., Kappert, K., Urban, D., Meyborg, H., Hauptmann, T., et al. (2013). Soluble P-selectin level correlates with acetylsalicylic acid but not with clopidogrel response in patients with stable coronary artery disease after a percutaneous coronary intervention. Coron. Artery Dis. 24, 312–320. doi: 10.1097/MCA.0b013e328360efd3
Khalil, B. M., Shahin, M. H., Solayman, M. H., Langaee, T., Schaalan, M. F., Gong, Y., et al. (2016). Genetic and nongenetic factors affecting clopidogrel response in the egyptian population. Clin. Transl. Sci. 9, 23–28. doi: 10.1111/cts.12383
Lee, J. M., Park, S., Shin, D. J., Choi, D., Shim, C. Y., Ko, Y. G., et al. (2009). Relation of genetic polymorphisms in the cytochrome P450 gene with clopidogrel resistance after drug-eluting stent implantation in Koreans. Am. J. Cardiol. 104, 46–51. doi: 10.1016/j.amjcard.2009.02.045
Li, J., Wang, Y., Lin, J., Wang, D., Wang, A., Zhao, X., et al. (2015). Soluble CD40L is a useful marker to predict future strokes in patients with minor stroke and transient ischemic attack. Stroke 46, 1990–1992. doi: 10.1161/STROKEAHA.115.008685
Liu, O., Jia, L., Liu, X., Wang, Y., Wang, X., Qin, Y., et al. (2012). Clopidogrel, a platelet P2Y12 receptor inhibitor, reduces vascular inflammation and angiotensin II induced-abdominal aortic aneurysm progression. PLoS ONE 7:e51707. doi: 10.1371/journal.pone.0051707
Liu, Y., Liu, N., Li, W., Shao, H., Zhi, H., and Li, J. (2013). Relationship of CYP2C19*2 and CYP2C19*3 gene polymorphism with clopidogrel response variability and recurrent cardiovascular events in Chinese patients undergoing percutaneous coronary intervention. Pharmacology 91, 165–172. doi: 10.1159/000346736
Ma, Y., Yang, Y., Wang, F., Moyer, M. P., Wei, Q., Zhang, P., et al. (2016). Long non-coding RNA CCAL regulates colorectal cancer progression by activating Wnt/β-catenin signalling pathway via suppression of activator protein 2α. Gut 65, 1494–1504. doi: 10.1136/gutjnl-2014-308392
Mega, J. L., Close, S. L., Wiviott, S. D., Shen, L., Hockett, R. D., Brandt, J. T., et al. (2009). Cytochrome p-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 360, 354–362. doi: 10.1056/NEJMoa0809171
Mega, J. L., Close, S. L., Wiviott, S. D., Shen, L., Walker, J. R., Simon, T., et al. (2010a). Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet 376, 1312–1319. doi: 10.1016/S0140-6736(10)61273-1
Mega, J. L., Simon, T., Collet, J. P., Anderson, J. L., Antman, E. M., Bliden, K., et al. (2010b). Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 304, 1821–1830. doi: 10.1001/jama.2010.1543
Müller, I., Besta, F., Schulz, C., Massberg, S., Schönig, A., and Gawaz, M. (2003). Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb. Haemost. 89, 783–787. doi: 10.1267/THRO03050783
Nagashima, Z., Tsukahara, K., Morita, S., Endo, T., Sugano, T., Hibi, K., et al. (2013). Platelet reactivity in the early and late phases of acute coronary syndromes according to cytochrome P450 2C19 phenotypes. J. Cardiol. 62, 158–164. doi: 10.1016/j.jjcc.2013.03.006
Obradovic, S., Djukanovic, N., Todorovic, Z., Markovic, I., Zamaklar-Trifunovic, D., Protic, D., et al. (2015). Men with lower HDL cholesterol levels have significant increment of soluble CD40 ligand and high-sensitivity CRP levels following the cessation of long-term clopidogrel therapy. J. Atheroscler. Thromb. 22, 284–292. doi: 10.5551/jat.26765
Oh, I. Y., Park, K. W., Kang, S. H., Park, J. J., Na, S. H., Kang, H. J., et al. (2012). Association of cytochrome P450 2C19*2 polymorphism with clopidogrel response variability and cardiovascular events in Koreans treated with drug-eluting stents. Heart 98, 139–144. doi: 10.1136/hrt.2011.227272
Palmerini, T., Calabrò, P., Piscione, F., De Servi, S., Cattaneo, M., Maffeo, D., et al. (2014). Impact of gene polymorphisms, platelet reactivity, and the SYNTAX score on 1-year clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention: the GEPRESS study. JACC Cardiovasc. Interv. 7, 1117–1127. doi: 10.1016/j.jcin.2014.04.020
Palmerini, T., Genereux, P., Caixeta, A., Cristea, E., Lansky, A., Mehran, R., et al. (2011). Prognostic value of the SYNTAX score in patients with acute coronary syndromes undergoing percutaneous coronary intervention: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage StrategY) trial. J. Am. Coll. Cardiol. 57, 2389–2397. doi: 10.1016/j.jacc.2011.02.032
Sabaté, M., Brugaletta, S., Cequier, A., Iñiguez, A., Serra, A., Jimenéz-Quevedo, P., et al. (2015). Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet 387, 357–366. doi: 10.1016/S0140-6736(15)00548-6
Serebruany, V. L., Steinhubl, S. R., Berger, P. B., Malinin, A. I., Bhatt, D. L., and Topol, E. J. (2005). Variability in platelet responsiveness to clopidogrel among 544 individuals. J. Am. Coll. Cardiol. 45, 246–251. doi: 10.1016/j.jacc.2004.09.067
Shuldiner, A. R., O'Connell, J. R., Bliden, K. P., Gandhi, A., Ryan, K., Horenstein, R. B., et al. (2009). Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 302, 849–857. doi: 10.1001/jama.2009.1232
Sibbing, D., Koch, W., Gebhard, D., Schuster, T., Braun, S., Stegherr, J., et al. (2010). Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation 121, 512–518. doi: 10.1161/CIRCULATIONAHA.109.885194
Sibbing, D., Stegherr, J., Latz, W., Koch, W., Mehilli, J., Dörrler, K., et al. (2009). Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur. Heart J. 30, 916–922. doi: 10.1093/eurheartj/ehp041
Smadja, D. M., Bura, A., Szymezak, J., Blanchard, A., Remones, V., Azizi, M., et al. (2012). Effect of clopidogrel on circulating biomarkers of angiogenesis and endothelial activation. J. Cardiol. 59, 30–35. doi: 10.1016/j.jjcc.2011.09.002
Steinhubl, S. R., Berger, P. B., Mann, J. T. III, Fry, E. T., DeLago, A., Wilmer, C., et al. (2002). Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 288, 2411–2420. doi: 10.1001/jama.288.19.2411
Sternberg, Z., Chichelli, T., Sternberg, D., Sawyer, R., Ching, M., Janicke, D., et al. (2016). Relationship between inflammation and aspirin and clopidogrel antiplatelet responses in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 25, 327–334. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.001
Stone, G. W., Witzenbichler, B., Weisz, G., Rinaldi, M. J., Neumann, F. J., Metzger, D. C., et al. (2013). Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet 382, 614–623. doi: 10.1016/S0140-6736(13)61170-8
Wallentin, L., James, S., Storey, R. F., Armstrong, M., Barratt, B. J., Horrow, J., et al. (2010). Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet 376, 1320–1328. doi: 10.1016/S0140-6736(10)61274-3
Wei, Y. Q., Wang, D. G., Yang, H., and Cao, H. (2015). Cytochrome P450 CYP 2C19*2 associated with adverse 1-year cardiovascular events in patients with acute coronary syndrome. PLoS ONE 10:e0132561. doi: 10.1371/journal.pone.0132561
Willoughby, S. R., Luu, L. J., Cameron, J. D., Nelson, A. J., Schultz, C. D., Worthley, S. G., et al. (2014). Clopidogrel improves microvascular endothelial function in subjects with stable coronary artery disease. Heart Lung Circ. 23, 534–541. doi: 10.1016/j.hlc.2014.01.005
Yamamoto, K., Hokimoto, S., Chitose, T., Morita, K., Ono, T., Kaikita, K., et al. (2011). Impact of CYP2C19 polymorphism on residual platelet reactivity in patients with coronary heart disease during antiplatelet therapy. J. Cardiol. 57, 194–201. doi: 10.1016/j.jjcc.2010.10.007
Yang, H., Zhao, P., and Tian, S. (2016). Clopidogrel protects endothelium by hindering TNFα-induced VCAM-1 expression through CaMKKβ/AMPK/Nrf2 pathway. J. Diabetes Res. 2016:9128050. doi: 10.1155/2016/9128050
Keywords: CYP2C19, clopidogrel, PCI, antiplatelet therapy, metabolizers
Citation: Sun H, Qu Q, Chen Z-F, Tan S-L, Zhou H-J, Qu J and Chen H (2016) Impact of CYP2C19 Variants on Clinical Efficacy of Clopidogrel and 1-Year Clinical Outcomes in Coronary Heart Patients Undergoing Percutaneous Coronary Intervention. Front. Pharmacol. 7:453. doi: 10.3389/fphar.2016.00453
Received: 24 May 2016; Accepted: 11 November 2016;
Published: 24 November 2016.
Edited by:
Ulrich M. Zanger, Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, GermanyReviewed by:
Colin Ross, University of British Columbia, CanadaRosane Vianna-Jorge, Instituto Nacional do Câncer, Brazil
Copyright © 2016 Sun, Qu, Chen, Tan, Zhou, Qu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Qu, cXVqaWFuQGNzdS5lZHUuY24=
Hui Chen, Y2hlbmh1aXd5ZEBzaW5hLmNvbQ==
†These authors have contributed equally to this work.
 Hong Sun1,2,3
Hong Sun1,2,3 Jian Qu
Jian Qu