- 1National Key Laboratory for Infectious Diseases Prevention and Treatment with Traditional Chinese Medicine, Chongqing Public Health Medical Center, Chongqing, China
- 2Center for Infectious Diseases, Beijing You’an Hospital, Capital Medical University, Beijing, China
- 3School of Biomedical Engineering, Capital Medical University, Beijing, China
- 4Department of Infectious Diseases, Chongqing Public Health Medical Center, Chongqing, China
Background: The life expectancy for HIV-infected individuals has improved dramatically because of improvements in antiretroviral therapy (ART). Today, a simplified two-drug regimen enhances adherence and treatment satisfaction by reducing adverse effects. Therefore, we need more evidence to show the benefits and risks of simplified ART regimens from randomized controlled trials (RCTs). We compared the efficacy and safety of raltegravir-based simplified dual therapy (DT) and of traditional triple therapy (TT) for people living with HIV/AIDS (PLWHA).
Methods: We carried out a systematic review of RCTs. After using a combination of the key words “HIV,” “raltegravir,” and “protease inhibitor” to search the English-language electronic databases from January 1, 2004, to September 11, 2019, we pooled data across eligible studies and estimated the summary effect sizes with Review Manager (version 5.3).
Results: We included eight RCTs involving 4420 PLWHA: 2187 (49.5%) received raltegravir-based simplified DT, and 2144 (48.5%) received traditional TT. The proportion of viral suppression was 79% at 48 weeks and 74% at 96 weeks in the simplified regimen, and the proportion of viral suppression was 78% at 48 weeks and 71% at 96 weeks in the traditional TT group. Furthermore, the proportion of viral suppression in the simplified DT group was greater than that in the TT group at 24 weeks (risk ratio 1.11, 95% confidence interval 1.02-1.21; p = 0.01). The CD4 cell counts in the simplified DT group were significantly higher at 48 weeks and 96 weeks than those in the group that received the traditional TT. Regarding adverse events and mortality rates, the DT and TT groups were similar. However, there was better adherence in the DT group than in the TT group.
Conclusion: We found that the simplified regimen was noninferior to TT regimen in regard to viral suppression. Furthermore, the simplified DT regimen had a better CD4 cell count and lower adverse events than the TT regimen.
Introduction
The expansion of access to antiretroviral therapy (ART) has averted millions of deaths for those who are infected with HIV (Gilks et al., 2006; WHO, 2013b). In the current World Health Organization (WHO) guidelines, the recommended initial therapy for patients infected with HIV-1 is a combination ART that includes two nucleoside reverse-transcriptase inhibitors (NRTIs) and nonnucleoside reverse-transcriptase inhibitors (NNRTIs) or a ritonavir-boosted PI (PI/r) (WHO, 2016). When NRTIs are used in first-line or second-line therapy regimens, the tolerability, safety, and toxicity profiles are limiting. For example, the combination of tenofovir (TDF) and emtricitabine (FTC) could cause renal and bone complications (McComsey et al., 2010; Hall et al., 2011; Brown, 2013). These shortcomings have led to research on alternative combinations without NRTIs to expand treatment options. The WHO’s proposed public health approach provides treatment for millions of people in low-income and middle-income countries with weak health systems (WHO, 2010 revision. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf (accessed April 1, 2013)]. This approach emphasizes the importance of simple, effective, safe, and tolerable treatment that can be performed by trained, non-health-care workers in accordance with simple procedures. In various formulations, a simplified approach can also reduce the need for multiple drug stocks (Group et al., 2013). Some trials have found the efficacy and safety of ritonavir-boosted lopinavir (LPV/r) plus raltegravir (RAL) to be largely similar to that of LPV/r plus NRTIs (Gallien et al., 2011; Nguyen et al., 2011a; Reynes et al., 2013). RAL has been increasingly used clinically in first-line and second-line ART regimens because it is more efficacious and better tolerated than NRTIs.
RAL was launched in 2007 as a first integrase inhibitor (Croxtall and Scott, 2010; Nguyen et al., 2011b) it was efficacious and generally well tolerated in patients infected with HIV-1 (Markowitz et al., 2007; Cooper et al., 2008; Lennox et al., 2010) and could serve as the third drug in a combination ART regimen (Church, 2009; Lennox et al., 2009). HIV infection is a chronic disease, and affected patients will receive life-long therapy. In treatment-experienced patients with multidrug-resistant HIV compared with patients who receive placebo plus optimized background therapy (OBT), RAL with OBT has been shown to be well tolerated, safe, and effective in producing viral suppression in treatment-experienced patients at 16 and 24 weeks (Grinsztejn et al., 2007). PI/r plus RAL was included as an alternative regimen in the 2016 WHO treatment guidelines (WHO, 2016). The WHO-preferred PI/r drugs are atazanavir, lopinavir, and darunavir on the basis of efficacy and tolerability (WHO, 2013a). Although increasing clinical evidence suggests that RAL plus PIs/r is an effective regimen (Raffi et al., 2013) to our knowledge, no meta-analysis has quantified the efficacy and safety of RAL plus PI/r compared with the efficacy and safety of two NRTIs plus PI/r.
This study aims to systematically evaluate the efficacy and safety of RAL-based simplified dual therapy (DT) in ART-naive and ART-experienced patients. We reviewed the literature to estimate differences in viral suppression, CD4 counts, adverse events, mortality, and adherence. These results will provide evidence-based recommendations for AIDS therapy.
Methods
This review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2010), registration number CRD42017082468 (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42017082468).
Data Sources
Systematic searches included all of the literature regarding RAL published in PubMed, MEDLINE, Web of Science, and Embase from January 1, 2004, to September 11, 2019. We searched for clinical trials using the following terms: “HIV,” “integrase inhibitors,” and “protease inhibitor”; only studies published in English were considered.
Literature Inclusion and Exclusion Criteria
Only randomized controlled trials (RCTs) (n > 10) were included in the meta-analysis, and all patients had been treated with PIs/r with RAL or PIs/r with two NRTIs. The included studies incorporated treatment-experienced adults and adolescents with HIV who had failed a WHO-recommended first-line NRTI-based regimen and switched to an RAL-based simplified regimen. We excluded the following: (1) reviews, letters, case observations studies, and retrospective studies; (2) animal and in vitro experiments; (3) HIV-1 patients who were younger than 12 years old or pregnant; and (4) studies not including baseline CD4 cell counts or viral load monitoring.
Study Selection and Exclusion Processes
Two investigators (YH and XH), working independently, scanned all titles and abstracts and excluded irrelevant articles. When divergence between the two investigators occurred, YC or HW arbitrated the dispute. Two investigators assessed the eligibility of full-text papers according to the inclusion and exclusion criteria. Then, data on the article’s characteristics, interventions at baseline, HIV RNA loads, CD4 cell counts, grade 3 or 4 adverse events, adherence, mortality, and drug resistance were independently extracted from the final list of selected eligible studies. The outcomes were chosen according to the WHO guidelines (WHO, 2016) and included viral suppression, the mean change in CD4 cell counts, grade 3 or 4 adverse events, drug resistance, mortality, and adherence. Any discrepancies between the investigators were resolved through discussion, and Dr. Chen arbitrated the dispute until a consensus was reached.
Study Quality Assessment
The methodological quality of the RCTs was assessed by the Cochrane risk of bias tool; there were seven domains (Higgins et al., 2011). Study quality was recorded as “high risk,” “unclear risk,” or “low risk.” Studies meeting all criteria were considered to have a low risk of bias, whereas those meeting none of the criteria were considered to have a high risk of bias. Otherwise, studies were considered to have an unclear risk of bias.
Statistical Analysis
Statistical analyses were performed with RevMan 5.3 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2014). Dichotomous and continuous data are expressed as risk ratios (RRs) and mean differences (MDs), respectively, with 95% confidence intervals (95% CIs). Statistical heterogeneity was assessed by Cochrane’s Q test. If the heterogeneity test result was P ≥ 0.10 and I2 ≤ 50%, the studies were considered homogenous, and the fixed effect model was selected. In contrast, studies that were not homogeneous were assessed using a random-effects model.
Results
Characteristics of the Included Studies
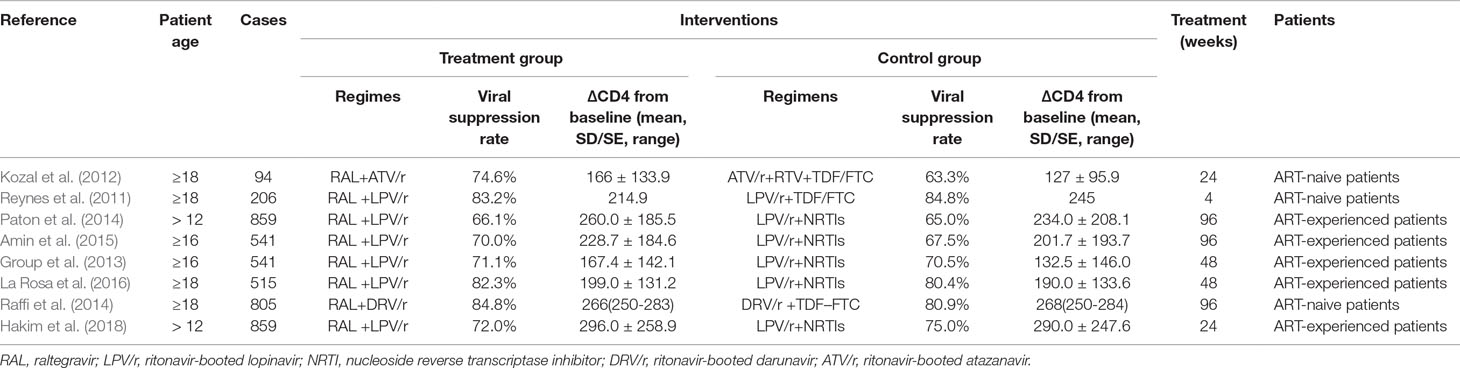
A total of 2,610 publications from four databases were identified by the initial screening; of these, Eight eligible articles were included in this meta-analysis (Reynes et al., 2011; Kozal et al., 2012; Group et al., 2013; Paton et al., 2014; Raffi et al., 2014; Amin et al., 2015; La Rosa et al., 2016; Hakim et al., 2018) (Figure 1). These trials were published from 2011 to 2018. Of these eight articles, six examined treatment with a combination of RAL and LPV/r, One examined treatment with RAL in combination with atazanavir/ritonavir (ATV/r), and one examined treated with a combination of RAL and darunavir/ritonavir (DRV/r) (Table 1). In this analysis, we included ART-naive patients and ART-experienced patients.
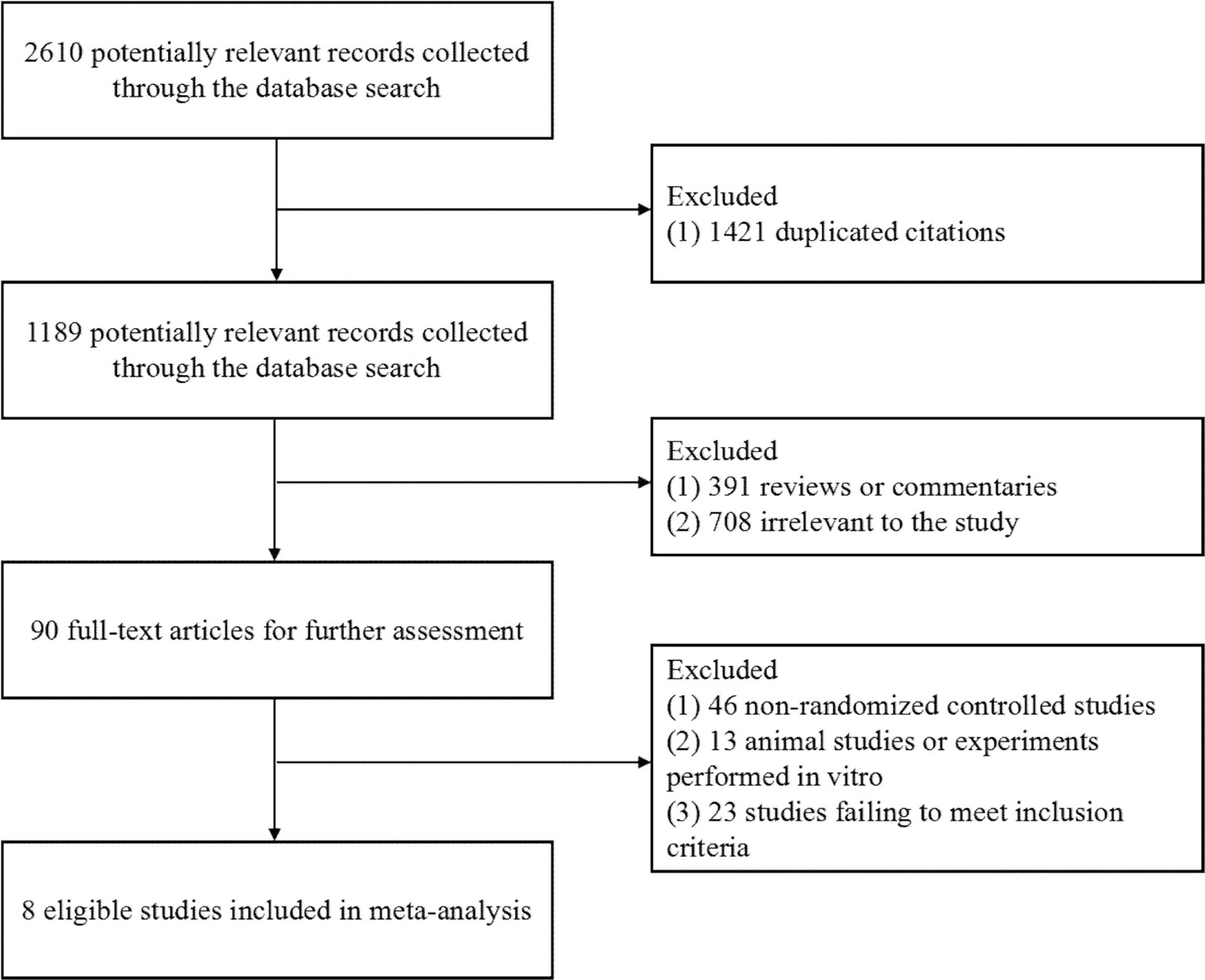
Figure 1 Flow diagram of the study selection. As shown, our initial searches yielded 2,610 records. The full texts of 1,189 articles were retrieved for detailed assessment after exclusion. Of these, 1,181 studies were subsequently excluded because they failed to meet the inclusion criteria; eight eligible studies were identified.
Methodological Quality of the Included Trials
These articles are all based on randomized, open-label, noninferiority, and multicenter trials. According to the Cochrane risk of bias estimation, randomized participant allocation was mentioned in all trials; six trials (Reynes et al., 2011; Group et al., 2013; Paton et al., 2014; Raffi et al., 2014; Amin et al., 2015; Hakim et al., 2018) used a specific method, whereas two trials (Kozal et al., 2012; La Rosa et al., 2016) were defined as “high risk” for using an unclear randomization method. Three trials (Group et al., 2013; Raffi et al., 2014; Amin et al., 2015) blinded the participants and investigators to the treatment, four trials (Kozal et al., 2012; Paton et al., 2014; La Rosa et al., 2016; Hakim et al., 2018) only blinded the participants to the treatment, and one trial (Reynes et al., 2011) did not mention blinding. All trials reported complete outcome data, and there was no selective reporting. Five trials (Group et al., 2013; Paton et al., 2014; Raffi et al., 2014; La Rosa et al., 2016; Hakim et al., 2018) were low risk in terms of other biases (Figure 2).
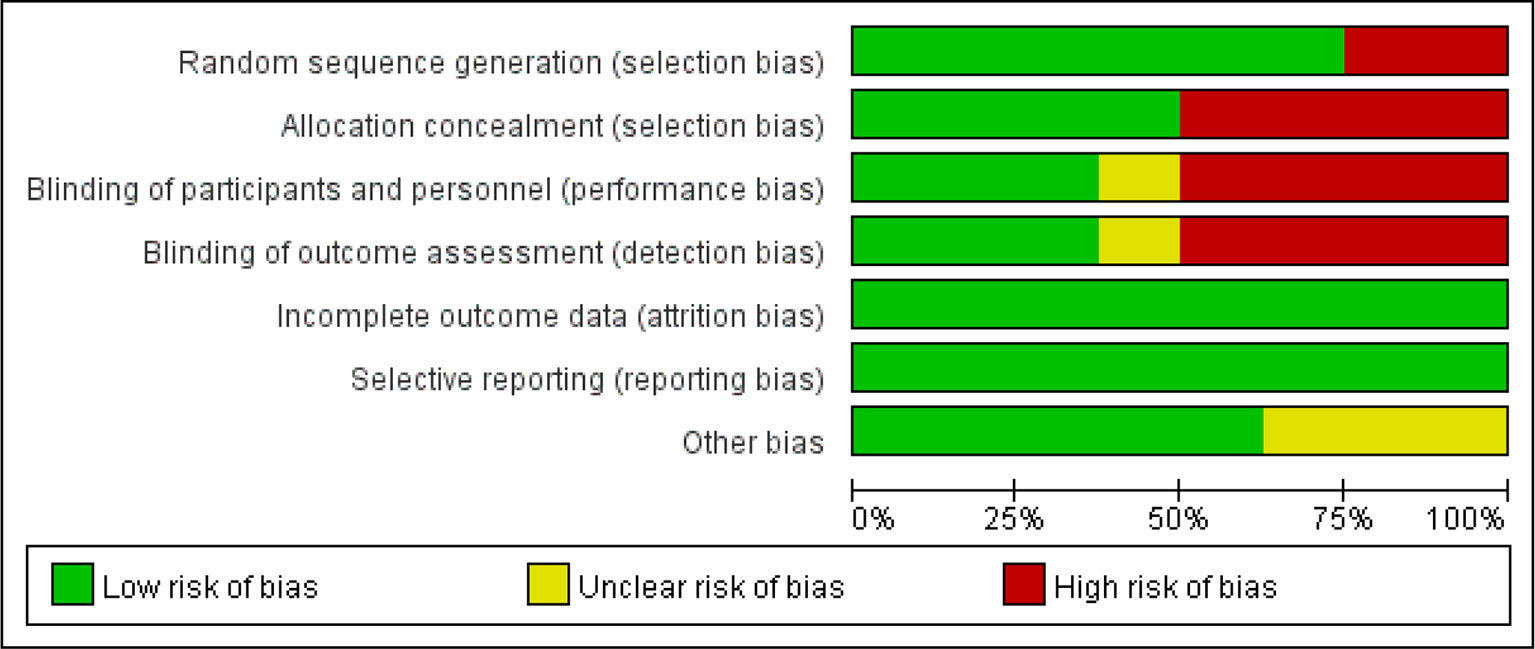
Figure 2 Quality assessment of the studies selected for systematic review. The risk of bias was used to assess the quality of the randomized controlled trials, and the majority of studies were found to be of low quality.
Outcome Measures
Plasma HIV-1 RNA Viral Load
The results of the meta-analysis in terms of the viral suppression with RAL plus PIs/r (DT group) versus PIs/r plus two NRTIs (TT group) indicated comparable effects of the different regimens (viral suppression using 50 copies per ml). Two studies (Kozal et al., 2012; Hakim et al., 2018) that included 864 participants reported outcomes and indicated a significant difference between the two regimes at 24 weeks. The effect of DT was greater than that of TT (P = 0.69, I2 = 0%) [risk ratio 1.11, 95% CI [1.02, 1.21], P = 0.01] (Figure 3). The average viral suppression rate in the TT group was 69% (95% CI: 65%–74%), whereas it was 77% (95% CI: 73%–80%) in the DT group (Table 1). At 48 weeks and 96 weeks, the efficacy in viral suppression was not different between the TT and DT groups (risk ratio 1.01, 95% CI 0.95–1.07, P = 0.73; risk ratio 1.03, 95% CI 0.98–1.08, P = 0.23, respectively) (Figure 3). The viral suppression rate in the TT group was 78% at 48 weeks and 71% (95% CI: 61%–82%) at 96 weeks, whereas the viral suppression rate in the DT group was 79% (95% CI: 71%–87%) at 48 weeks and 74% (95% CI: 61%–86%) at 96 weeks (Table 1). A funnel plot was used to express the publication bias. The plots were symmetrical, suggesting that there was no obvious publication bias.
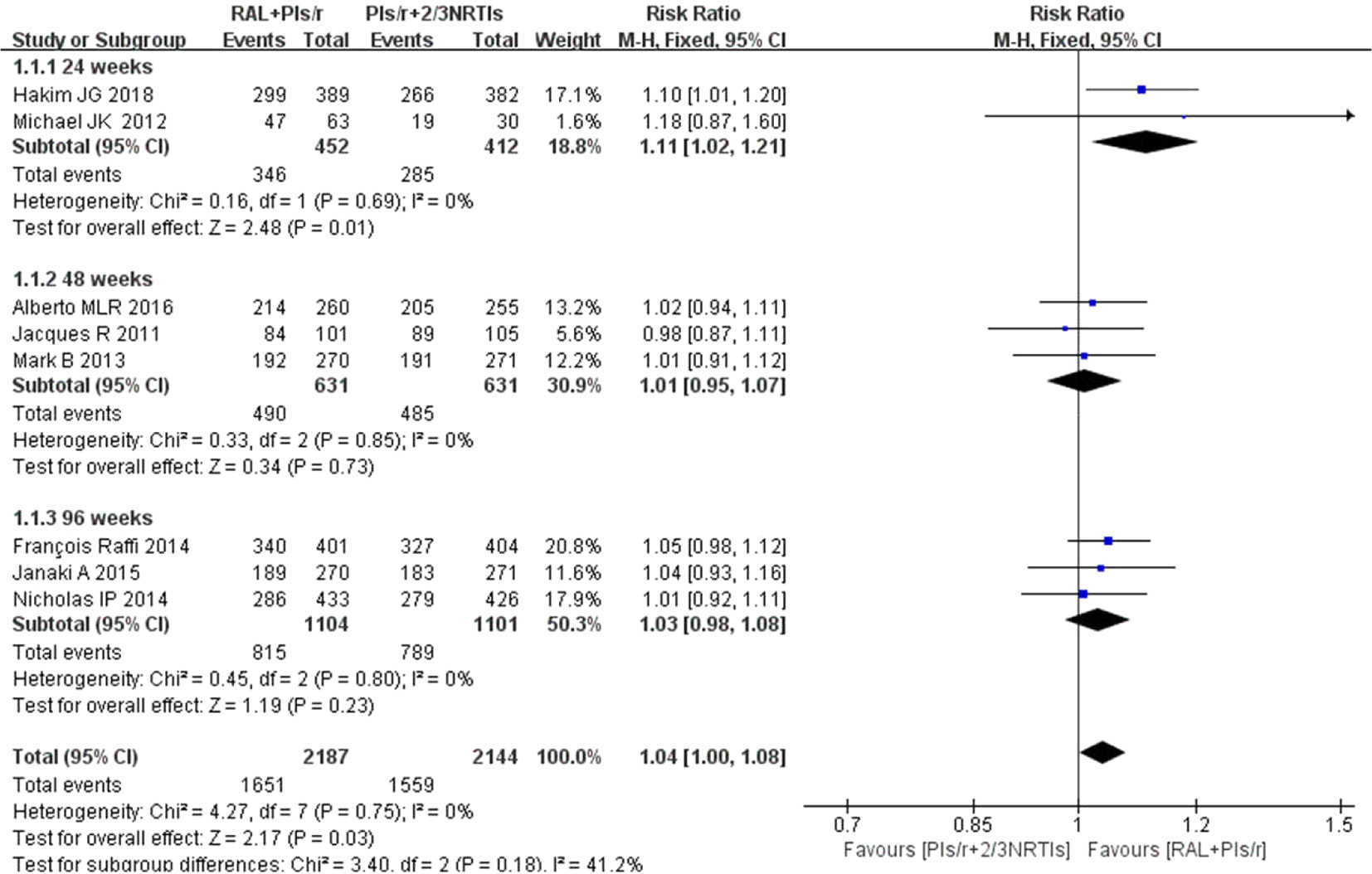
Figure 3 Forest plot comparing viral suppression with the two therapy regimens. (1) RAL = raltegravir. PIs/r = ritonavir-boosted protease inhibitor. NRTI = nucleoside or nucleotide reverse transcriptase inhibitor. (2) Study item displayed as the first author with the publication year. (3) I2 and P are the criteria of the heterogeneity test, with the —◆—pooled odds ratio, —¦— odds ratio and 95% confidence interval.
CD4 Cell Counts
Among the eight studies, five studies (Reynes et al., 2011; Group et al., 2013; Paton et al., 2014; Amin et al., 2015; La Rosa et al., 2016) contributed to the CD4 cell counts analysis. One (Reynes et al., 2011) of the five studies used the median to express the average CD4 cell counts. The findings showed that increases in mean CD4 counts were significantly higher in the DT group than in the TT group at 48 weeks and 96 weeks (mean difference 9.05, 95% CI 7.96–10.13, P < 0.01; mean difference 9.12, 95% CI 8.04–10.20, P = 0.01, respectively). This strategy showed no significant heterogeneity when the DT group was compared with the TT group at 96 weeks(P = 0.96, I2 = 0%). However, at 48 weeks, there was significant heterogeneity between the two groups (P = 0.04, I2 = 77%). In short, compared with the current standard of therapy regimen, simplified therapy showed a significant efficacy in terms of immune reconstruction (Figure 4).
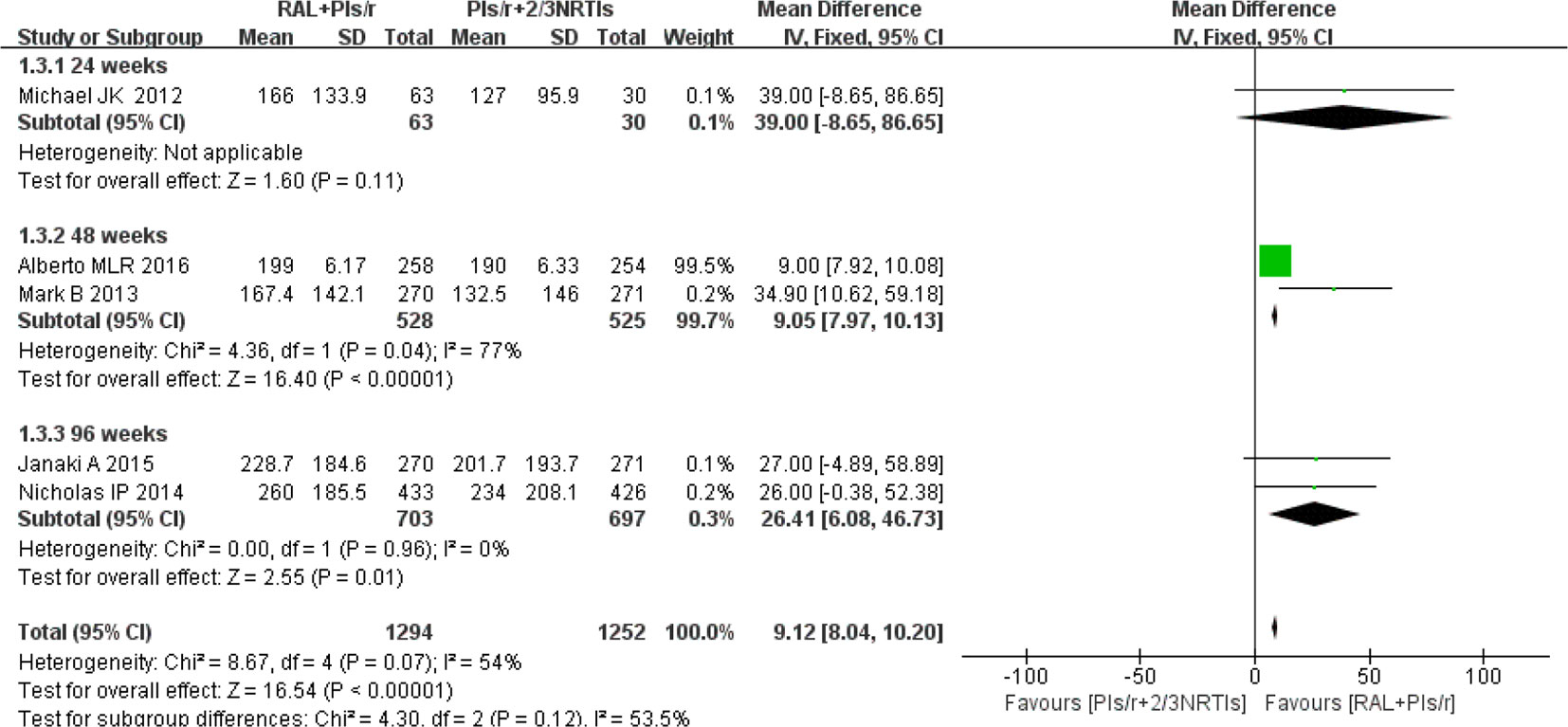
Figure 4 Forest plot comparing CD4 cell counts with simplified and traditional treatment therapy in HIV-1 patients. (1) RAL = raltegravir. PIs/r = ritonavir-boosted protease inhibitor. NRTI = nucleoside or nucleotide reverse transcriptase inhibitor. (2) Study item displayed as the first author with the publication year. (3) I2 and P are the criteria of the heterogeneity test, with the —◆—pooled odds ratio, —¦— odds ratio and 95% confidence interval.
Adverse Events
Regarding adverse events, we chose studies that reported grade 3 or 4 adverse events. The investigators found no significant differences in adverse events between the DT and TT groups at 96 weeks [risk ratio 0.97, 95% CI (0.80, 1.19), P = 0.80] (Figure 5). At 48 weeks, there were significantly fewer adverse events in the DT group than in the TT group (risk ratio 0.70, 95% CI [0.54, 0.91], P < 0.01).
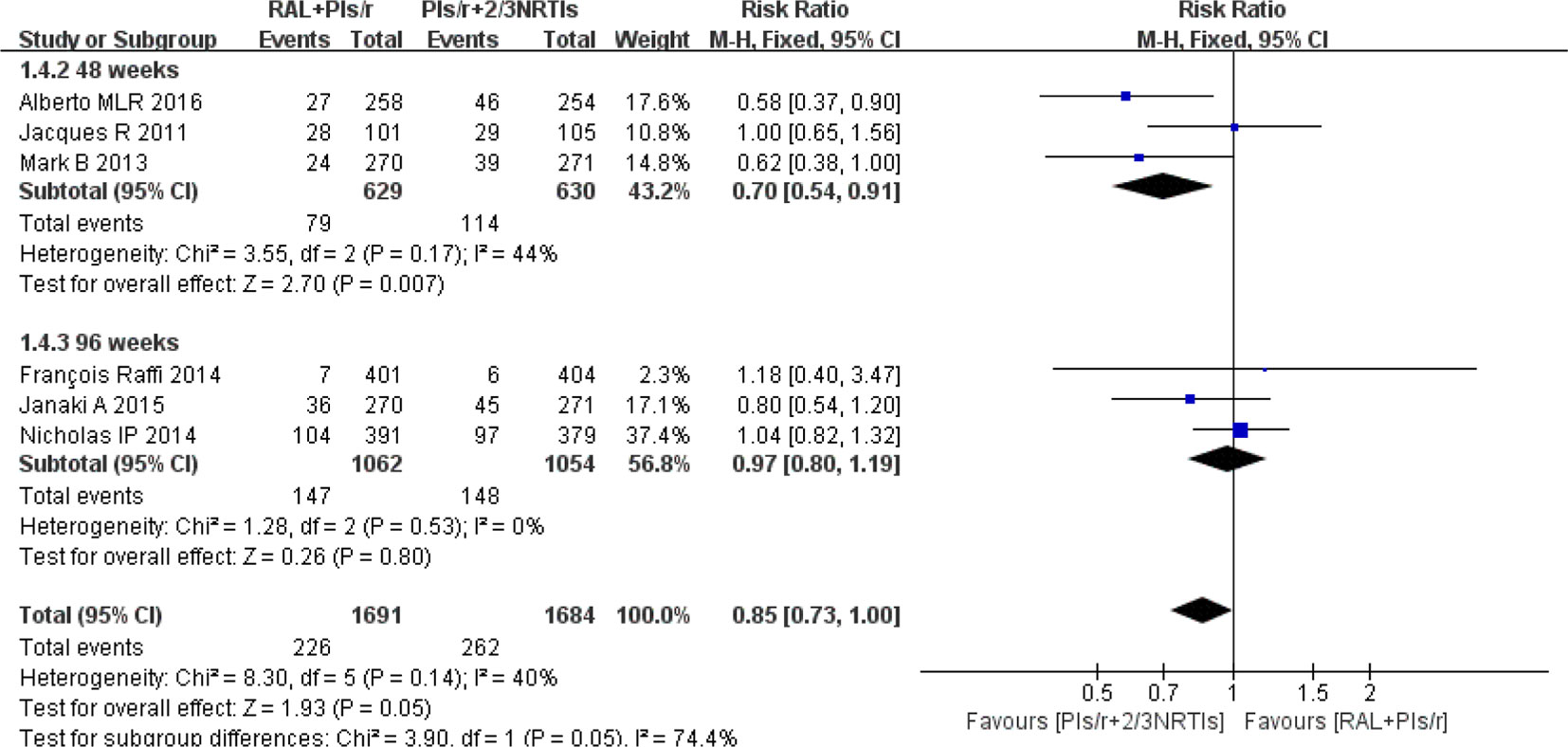
Figure 5 Forest plot comparing grade 3 or 4 adverse events with the RAL-based simplified regimen and the PIs/r-based traditional regimen in HIV-1 patients. (1) RAL = raltegravir. PIs/r = ritonavir-boosted protease inhibitor. NRTI = nucleoside or nucleotide reverse transcriptase inhibitor. (2) Study item displayed as the first author with the publication year. (3) I2 and P are the criteria of the heterogeneity test, with the —◆—pooled odds ratio, —¦— odds ratio and 95% confidence interval.
Drug Resistance
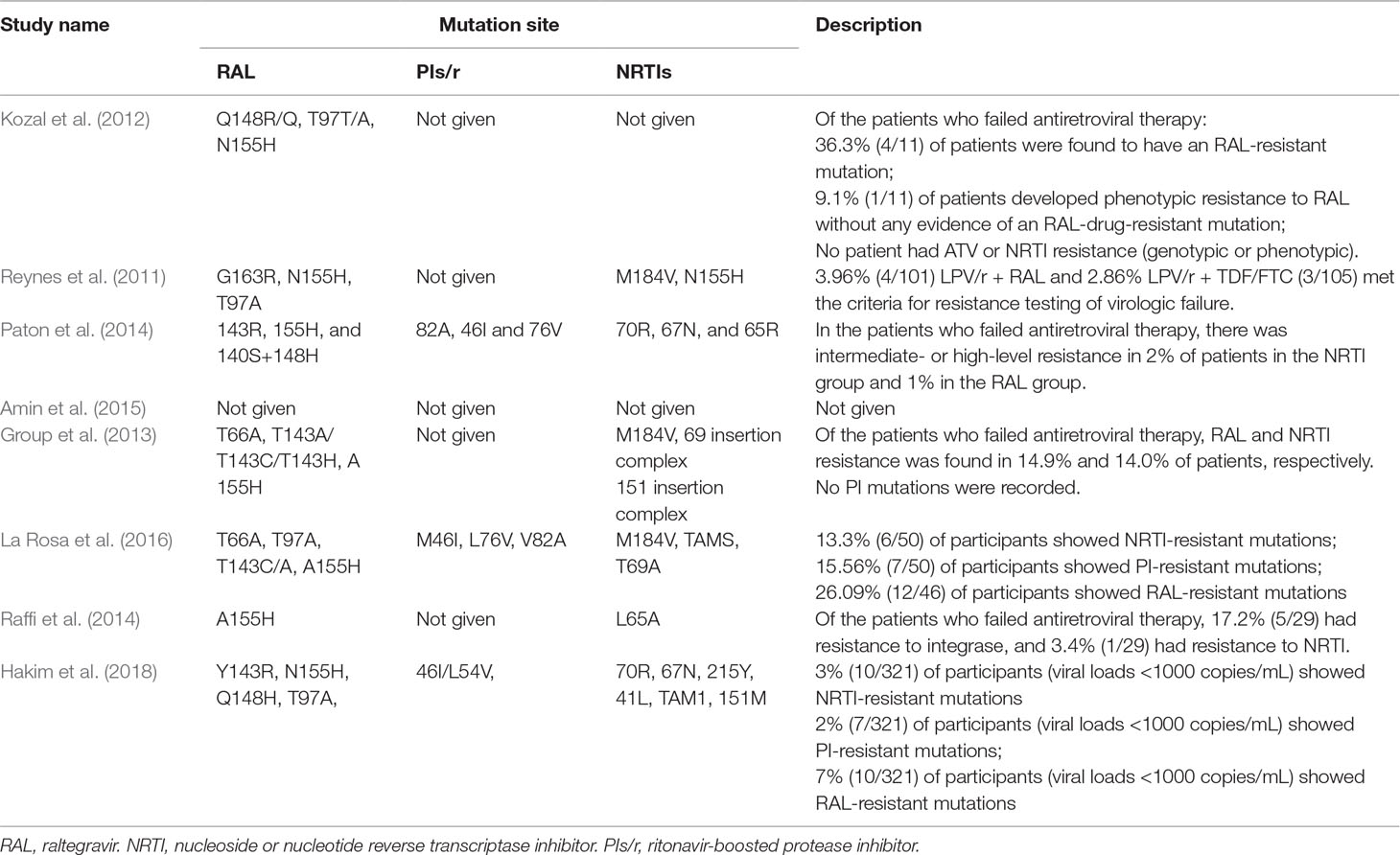
Drug resistance mutations were reported in eight studies. Seven (Reynes et al., 2011; Kozal et al., 2012; Group et al., 2013; Paton et al., 2014; Raffi et al., 2014; La Rosa et al., 2016; Hakim et al., 2018) studies reported drug resistance in patients who experienced virologic failure. Among these studies, we found that three studies reported that drug resistance mutations were associated with PIs/r in patients who failed antiretroviral therapy. Drug resistance was mainly found to occur for RAL and NRTIs. In addition, relevant mutations were found at Q148R/Q, T97T/A, 155H, 143R, and 140S+148H for RAL and at M184V, 70R, 67N, and 65R for NRTIs. Detailed information is shown in Table 2.
Adherence and Mortality
Poor adherence is one of the important reasons for antiretroviral therapy failure, and it is also one of the important factors for drug resistance. Four studies (Group et al., 2013; Paton et al., 2014; Amin et al., 2015; Hakim et al., 2018) evaluated adherence. The investigation of adherence was mainly in the form of an adherence questionnaire. There were significant differences in adherence between the DT and TT groups [risk ratio 1.03, 95% CI (1.01, 1.06), P < 0.01]. Six studies (Group et al., 2013; Paton et al., 2014; Raffi et al., 2014; Amin et al., 2015; La Rosa et al., 2016; Hakim et al., 2018), which involved 4,117 participants, reported comparisons of the mortality between two different groups. The risk ratio of mortality in the two groups at the last visit was 0.84 (95% CI 0.63–1.12), and there were no significant differences between any regimens (P = 0.24) (Figure 6).
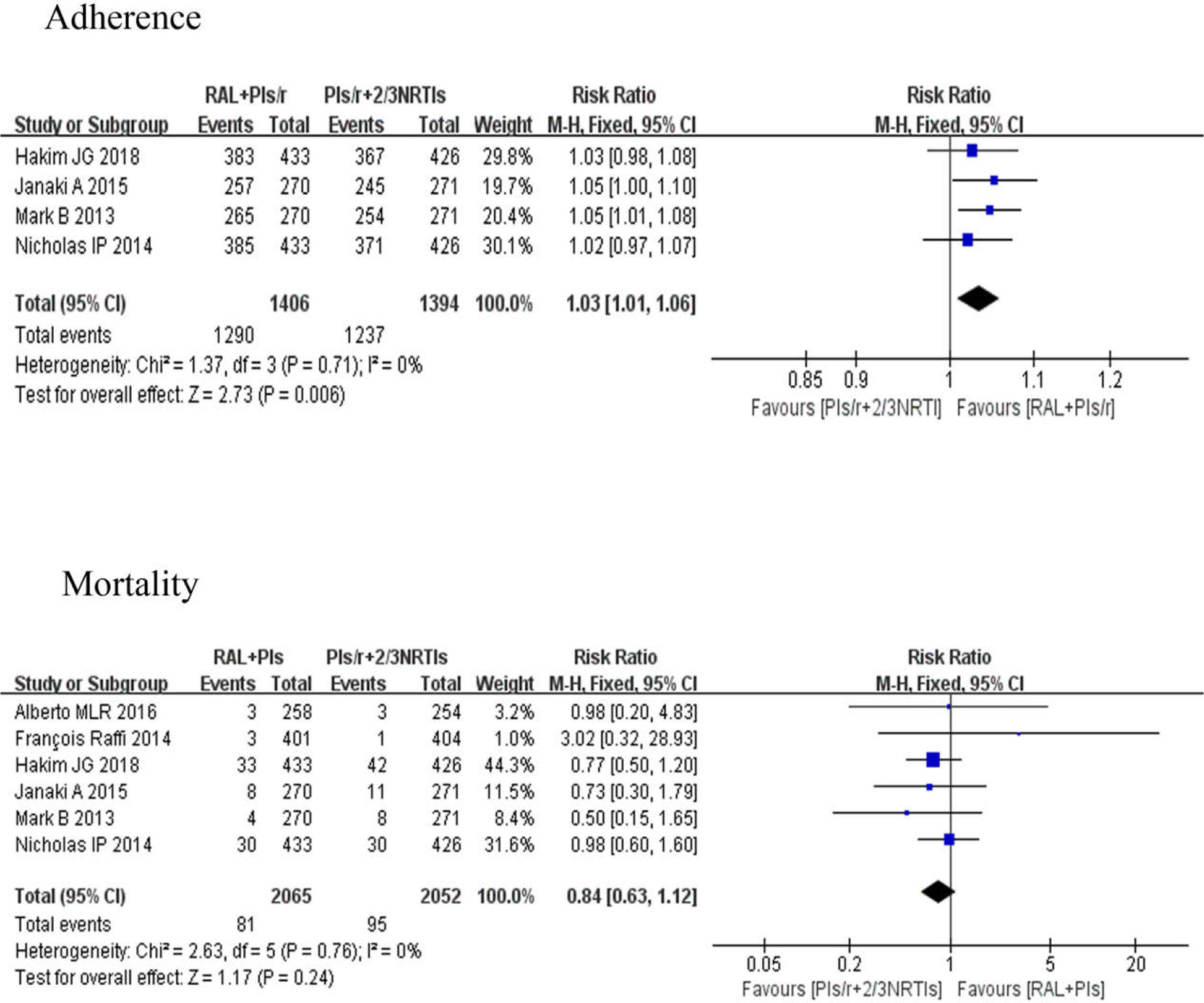
Figure 6 Forest plots comparing adherence and mortality between the two regimens. (1) RAL = raltegravir. PIs/r = ritonavir-boosted protease inhibitor. NRTI = nucleoside or nucleotide reverse transcriptase inhibitor. (2) Study item displayed as the first author with the publication year. (3) I2 and P are the criteria of the heterogeneity test, with the —◆—pooled odds ratio, —¦— odds ratio and 95% confidence interval.
Discussion
Until recently, antiretroviral therapy has typically consisted of two reverse-transcriptase inhibitors and a PI/r or an NRTI for treatment-naive and treatment-experience people living with HIV/AIDS (PLWHA) (Boyd, 2011). For maintaining long-term suppressive therapy with the best quality of life, a relatively simple and tolerable regimen is needed (Amin et al., 2015). Drug resistance threatens the long-term efficacy of ART in both developed and developing countries and has led to the development of a new class of drugs termed integrase inhibitors (Wainberg et al., 2011; Whitney et al., 2011; Gupta et al., 2012). RAL, which suppresses the RNA replication of HIV-1 strains, appeared on the market in 2007 and has quickly become a staple of the anti-HIV-1 drug arsenal. The clinical use of RAL represents a milestone that appeared 10 years after ART was introduced to treat AIDS. A PI/r plus RAL was included as an alternative regimen in the 2016 WHO treatment guidelines (WHO, 2016). The WHO-preferred PI/r are atazanavir, lopinavir, and darunavir on the basis of efficacy and tolerability (WHO, 2013a).
This meta-analysis of eight RCTs involving 4,327 PLWHA assessed the clinical value of the effect of DT group versus TT group. All of the studies were based on patients showing a posttreatment plasma HIV-1 RNA viral load <50 copies/mL. According to the viral suppression outcome, DT group is noninferior to TT group at 48 and 96 weeks, whereas the DT regimen was superior to the TT regimen at 24 weeks. The cause of this may be the patient drug sensitivity and high adherence. For the CD4 cell counts, the DT was superior to the TT at 48 weeks and 96 weeks. This indicated that DT group was better than TT group in terms of immunological reconstitution. While there was no clinical significance, the change in CD4 cell counts was 17.6 cell/mm3 and 26.5 cell/mm3 higher in the DT group than in the TT group at 48 weeks and 96 weeks, respectively. Grade 3 or 4 adverse events resulting from drug regimens have always been a primary focus. In this study, we found no significant difference in adverse events between the DT and TT groups at 96 weeks. At 48 weeks, the DT group had fewer adverse events than the TT group (P < 0.01). The common adverse events in the DT group were fever, skin rash, neurological events, and headache (Reynes et al., 2011; Paton et al., 2014; La Rosa et al., 2016; Hakim et al., 2018). Clinicians should monitor these adverse events and treat them in real time to reduce the interruption of treatment due to adverse events. With respect to drug resistance, the relevant RAL mutation sites were mainly N155H, Q148H/K/R, G140S, Y143R, and T97A (Reynes et al., 2011; Kozal et al., 2012Group et al., 2013; Paton et al., 2014; Raffi et al., 2014; La Rosa et al., 2016; Hakim et al., 2018). A patient may develop resistance with the occurrence of any two mutations. Three studies (Paton et al., 2014; La Rosa et al., 2016; Hakim et al., 2018) mentioned PI (LPV)-resistance mutation sites, which were M46I, L76V, and V82A. In our review, we found that, despite the presence of mutations, there were few patients who failed ART due to RAL or PIs resistance. However, multidrug combinations and the pill burden of such programs will lead to poor treatment compliance issues. Our review found that RAL plus PIs/r could enhance the adherence of patients. To a great extent, reducing the number of patients with poor adherence and antiretroviral failure may prolong the life of patients. At the same time, drugs with a high genetic barrier can be used as the main treatment to avoid premature cessation due to drug resistance and untimely treatment. Although RAL exhibits rapid, efficient, and long-lasting antiretroviral activity, it is expensive and is currently not included on the list of free antiretroviral drugs in China; as such, RAL is bound to be a heavy economic burden to most AIDS patients.
Our study has some limitations. First, the number of included studies in our review was too low. The analysis of some important outcomes, including mortality and the number of and reason for discontinuations, was limited by a low number of events. Second, the varying numbers of participants brought some uncertainty to the research results. The statistical analysis will incorporate a certain deviation, resulting in result errors. Finally, the studies were from various settings, including low-income, middle-income, and high-income countries; most were from low-income countries. Although this is an external factor, we cannot ignore the internal effects of external factors.
Increasing attention has been directed toward the development of drugs for PLWHA who have failed antiretroviral therapy. This meta-analysis indicates that combinations of RAL with PIs/r not only decrease the plasma HIV-1 RNA load and enhance the CD4 cell counts but also result in fewer side effects. Additionally, there was no obvious drug resistance to these combinations. In summary, RAL combined with PIs/r could be a beneficial and safe therapeutic choice for PLWHA, and higher adherence rates and realization of immune reconstruction could improve quality of life and enhance patient preference; this strategy is worthy of clinical promotion.
Author Contributions
HW, XH, and YH conceptualized the study and developed the research protocol. YH identified articles for full-text review and extracted the data that matched the inclusion criteria. YH and HC performed the statistical analyses. All authors contributed to the writing of the manuscript. YC and XH polished and revised the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Science and Technology Major Project of China During the 13th Five-year Plan Period (2018ZX10302104001), (2017ZX10201101); the Chinese Medicine Science and Technology Project (ZY201702047), (ZY201702045); the Health and Family Planning Commission in Chongqing Medical Scientific Research Projects (2018QNXM012); the Major Project of Beijing Municipal Science and Technology Committee (D161100000416003); and the National Natural Science Foundation of China (81701984). The abstract of this article was first accepted and published as a conference abstract at The Lancet-CAMS Health Summit (Yinqiu, ; Huang et al., 2018). Thank you for the National Science and Technology Major Project of China During the 13th Five-year Plan Period (2017ZX10205501001) support of this article.
References
Amin, J., Boyd, M. A., Kumarasamy, N., Moore, C. L., Losso, M. H., Nwizu, C. A., et al. (2015). Raltegravir non-inferior to nucleoside based regimen in second-line therapy with lopinavir/ritonavir over 96 weeks: a randomised open label study for the treatment of HIV-1 infection. PLoS One 10 (2), e0118228. doi: 10.1371/journal.pone.0118228
Boyd, S. D. (2011). Management of HIV infection in treatment-naive patients: a review of the most current recommendations. Am. J. Health Syst. Pharm. 68 (11), 991–1001. doi: 10.2146/ajhp100156
Brown, T. T. (2013). Challenges in the management of osteoporosis and vitamin D deficiency in HIV infection. Top Antivir. Med. 21 (3), 115–118.
Church, J. A. (2009). Maraviroc for Previously Treated Patients With R5 HIV-1 Infection. Pediatrics 124 (Supplement 2), S157.152–S158. doi: 10.1542/peds.2009-1870EEEE
Cooper, D. A., Steigbigel, R. T., Gatell, J. M., Rockstroh, J. K., Katlama, C., Yeni, P., et al. (2008). Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N. Engl. J. Med. 359 (4), 355–365. doi: 10.1056/NEJMoa0708978
Croxtall, J. D., Scott, L. J. (2010). Raltegravir: in treatment-naive patients with HIV-1 infection. Drugs 70 (5), 631–642. doi: 10.2165/11204590-000000000-00000
Gallien, S., Braun, J., Delaugerre, C., Charreau, I., Reynes, J., Jeanblanc, F., et al. (2011). Efficacy and safety of raltegravir in treatment-experienced HIV-1-infected patients switching from enfuvirtide-based regimen: 48 week results of the randomized EASIER ANRS 138 trial. J. Antimicrob. Chemother. 66 (9), 2099–2106. doi: 10.1093/jac/dkr269
Gilks, C. F., Crowley, S., Ekpini, R., Gove, S., Perriens, J., Souteyrand, Y., et al. (2006). The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet 368 (9534), 505–510. doi: 10.1016/S0140-6736(06)69158-7
Grinsztejn, B., Nguyen, B. Y., Katlama, C., Gatell, J. M., Lazzarin, A., Vittecoq, D., et al. (2007). Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet 369 (9569), 1261–1269. doi: 10.1016/S0140-6736(07)60597-2
Group, S.-L. S., Boyd, M. A., Kumarasamy, N., Moore, C. L., Nwizu, C., Losso, M. H., et al. (2013). Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study. Lancet 381 (9883), 2091–2099. doi: 10.1016/S0140-6736(13)61164-2
Gupta, R. K., Jordan, M. R., Sultan, B. J., Hill, A., Davis, D. H. J., Gregson, J., et al. (2012). Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet 380 (9849), 1250–1258. doi: 10.1016/S0140-6736(12)61038-1
Hakim, J. G., Thompson, J., Kityo, C., Hoppe, A., Kambugu, A., van Oosterhout, J. J., et al. (2018). Lopinavir plus nucleoside reverse-transcriptase inhibitors, lopinavir plus raltegravir, or lopinavir monotherapy for second-line treatment of HIV (EARNEST): 144-week follow-up results from a randomised controlled trial. Lancet Infect. Dis. 18 (1), 47–57. doi: 10.1016/S1473-3099(17)30630-8
Hall, A. M., Hendry, B. M., Nitsch, D., Connolly, J. O. (2011). Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am. J. Kidney Dis. 57 (5), 773–780. doi: 10.1053/j.ajkd.2011.01.022
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi: 10.1136/bmj.d5928
Kozal, M. J., Lupo, S., DeJesus, E., Molina, J. M., McDonald, C., Raffi, F., et al. (2012). A nucleoside- and ritonavir-sparing regimen containing atazanavir plus raltegravir in antiretroviral treatment-naive HIV-infected patients: SPARTAN study results. HIV Clin. Trials 13 (3), 119–130. doi: 10.1310/hct1303-119
La Rosa, A. M., Harrison, L. J., Taiwo, B., Wallis, C. L., Zheng, L., Kim, P., et al. (2016). Raltegravir in second-line antiretroviral therapy in resource-limited settings (SELECT): a randomised, phase 3, non-inferiority study. Lancet HIV 3 (6), e247–e258. doi: 10.1016/S2352-3018(16)30011-X
Lennox, J. L., Dejesus, E., Berger, D. S., Lazzarin, A., Pollard, R. B., Ramalho Madruga, J. V., et al. (2010). Raltegravir versus Efavirenz regimen in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J. Acquir. Immune Defic. Syndr. 55 (1), 39–48. doi: 10.1097/QAI.0b013e3181da1287
Lennox, J. L., DeJesus, E., Lazzarin, A., Pollard, R. B., Madruga, J. V., Berger, D., et al. (2009). Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 374 (9692), 796–806. doi: 10.1016/S0140-6736(09)60918-1
Markowitz, M., Nguyen, B. Y., Gotuzzo, E., Mendo, F., Ratanasuwan, W., Kovacs, C., et al. (2007). Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J. Acquir. Immune Defic. Syndr. 46 (2), 125–133. doi: 10.1097/QAI.0b013e318157131c
McComsey, G. A., Tebas, P., Shane, E., Yin, M. T., Overton, E. T., Huang, J. S., et al. (2010). Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin. Infect. Dis. 51 (8), 937–946. doi: 10.1086/656412
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., Group, P. (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8 (5), 336–341. doi: 10.1016/j.ijsu.2010.02.007
Nguyen, A., Calmy, A., Delhumeau, C., Mercier, I., Cavassini, M., Mello, A. F., et al. (2011a). A randomized cross-over study to compare raltegravir and efavirenz (SWITCH-ER study). AIDS 25 (12), 1481–1487. doi: 10.1097/QAD.0b013e328348dab0
Nguyen, B. Y., Isaacs, R. D., Teppler, H., Leavitt, R. Y., Sklar, P., Iwamoto, M., et al. (2011b). Raltegravir: the first HIV-1 integrase strand transfer inhibitor in the HIV armamentarium. Ann. N. Y. Acad. Sci. 1222, 83–89. doi: 10.1111/j.1749-6632.2011.05972.x
Paton, N. I., Kityo, C., Hoppe, A., Reid, A., Kambugu, A., Lugemwa, A., et al. (2014). Assessment of second-line antiretroviral regimen for HIV therapy in Africa. N. Engl. J. Med. 371 (3), 234–247. doi: 10.1056/NEJMoa1311274
Raffi, F., Babiker, A. G., Richert, L., Molina, J. M., George, E. C., Antinori, A., et al. (2014). Ritonavir-boosted darunavir combined with raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults infected with HIV-1: 96 week results from the NEAT001/ANRS143 randomised non-inferiority trial. Lancet 384 (9958), 1942–1951. doi: 10.1016/S0140-6736(14)61170-3
Raffi, F., Jaeger, H., Quiros-Roldan, E., Albrecht, H., Belonosova, E., Gatell, J. M., et al. (2013). Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect. Dis. 13 (11), 927–935. doi: 10.1016/S1473-3099(13)70257-3
Reynes, J., Lawal, A., Pulido, F., Soto-Malave, R., Gathe, J., Tian, M., et al. (2011). Examination of noninferiority, safety, and tolerability of lopinavir/ritonavir and raltegravir compared with lopinavir/ritonavir and tenofovir/emtricitabine in antiretroviral-naive subjects: the progress study, 48-week results. HIV Clin. Trials 12 (5), 255–267. doi: 10.1310/hct1205-255
Reynes, J., Trinh, R., Pulido, F., Soto-Malave, R., Gathe, J., Qaqish, R., et al. (2013). Lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res. Hum. Retroviruses 29 (2), 256–265. doi: 10.1089/aid.2011.0275
Wainberg, M. A., Zaharatos, G. J., Brenner, B. G. (2011). Development of antiretroviral drug resistance. N. Engl. J. Med. 365 (7), 637–646. doi: 10.1056/NEJMra1004180
Whitney, J. B., Lim, S. Y., Wainberg, M. A. (2011). Evolutionary mechanisms of retroviral persistence. AIDS Rev. 13 (4), 234–239.
WHO. (2013a). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Geneva: World Health Organization.
WHO. (2013b). Global update on HIV treatment 2013: results, impact and opportunities. Geneva: World Health Organization.
WHO. (2016). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization.
WHO. (2010) (revision. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf(accessed April 1, 2013)). Antiretroviral Therapy for HIV Infection in Adults and Adolescents. Recommendations for a public health approach. Geneva: World Health Organization.
Yinqiu, Huang Comparative efficacy and safety of raltegravir-based simplified regimen for people living with HIV infection: a systematic review and meta-analysis. (Report). Lancet. 192 (Supp. 1), S12. https://www.thelancet.com/journals/lancet/issue/vol392nonull/PIIS0140-6736(18)X0045-2
Keywords: HIV, raltegravir, protease inhibitor, efficacy and safety, simplified regimen, meta-analysis
Citation: Huang Y, Huang X, Chen H, Wu H and Chen Y (2019) Efficacy and Safety of Raltegravir-Based Dual Therapy in AIDS Patients: A Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 10:1225. doi: 10.3389/fphar.2019.01225
Received: 25 July 2019; Accepted: 23 September 2019;
Published: 17 October 2019.
Edited by:
Bernd Rosenkranz, Stellenbosch University, South AfricaReviewed by:
Jantjie Taljaard, Stellenbosch University, South AfricaDomenico Criscuolo, Italian Society of Pharmaceutical Medicine, Italy
Copyright © 2019 Huang, Huang, Chen, Wu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Wu, whdoc@sina.com; Yao-Kai Chen, yaokaichen@hotmail.com
†These authors have contributed equally to this work
 Yinqiu Huang
Yinqiu Huang Xiaojie Huang2†
Xiaojie Huang2†