- 1Department of Pharmaceutics, Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, China
- 2Department of Pharmaceutics, The Second Hospital of Dalian Medical University, Dalian, China
Glaucoma is one of the most common causes of blindness, thus seriously affecting people’s health and quality of life. The topical medical therapy is as the first line treatment in the management of glaucoma since it is inexpensive, convenient, effective, and safe. This review summarizes and compares extensive clinical trials on the topical medications for the treatment of glaucoma, including topical monotherapy agents, topical fixed-combination agents, topical non-fixed combination agents, and their composition, mechanism of action, efficacy, and adverse effects, which will provide reference for optimal choice of clinical medication. Fixed-combination therapeutics offer greater efficacy, reliable security, clinical compliance, and tolerance than non-fixed combination agents and monotherapy agents, which will become a prefer option for the treatment of glaucoma. Meanwhile, we also discuss new trends in the field of new fixed combinations of medications, which may better control IOP and treat glaucoma.
1 Introduction
Glaucoma is a neurodegenerative ophthalmologic disease characterized by the progressive degeneration of the retinal ganglion cells and axonal death, resulting in an irreversible blindness (Marshall et al., 2018; Garcia-Medina et al., 2020). Glaucoma is commonly related to high intraocular pressure (IOP) with a subsequent injury of the optic nerve, and eventually blindness (Goel et al., 2010). The normal physiological IOP is 5–20 mmHg, which in turn depends on the adjustment of production and absorption of the aqueous humor (AH) (Goel et al., 2010; Skrzypecki et al., 2018). The ciliary muscle produces the AH that leaves the eye by passive flows through two pathways: the conventional (trabecular meshwork, TM) and unconventional (uveoscleral) pathway (Huang et al., 2018). Normal tension glaucoma (NTG) is characterized by a IOP usually below 21 mmHg, while the primary open-angle glaucoma (POAG) has a IOP of over 22 mmHg (Razeghinejad and Lee, 2019), the latter being the main and modifiable risk factor for glaucoma and its progression (Fogagnolo and Rossetti, 2011; Jayaram, 2020). Ocular hypertension (OHT) is defined as a IOP over 21 mmHg without the damage of the optic disk and view field (Chamard et al., 2020), but it is most likely to develop into open-angle glaucoma (OAG) (Kass et al., 2021).
Currently, the treatments to cure glaucoma mainly consist of surgical intervention and topical medication therapy (Guglielmi et al., 2019; Webb, 2021). Surgical intervention is required when a patient’s visual independence is at risk (Lusthaus and Goldberg, 2019). The risks associated with the surgical procedure and the postoperative recovery are complicated with each patient and the cost is relatively expensive (Razeghinejad and Lee, 2019). Therefore, topical medication therapy with reliable effectiveness and safety is often as the preferred approach in the management of glaucoma (Conlon et al., 2017; Guglielmi et al., 2019; Webb, 2021). The classes of topical monotherapy medications to cure glaucoma include prostaglandin analogs, rho-kinase inhibitors, β-adrenergic blocking agents, α-2 adrenergic agonists, and carbonic anhydrase inhibitors, but they are often inadequate to keep IOP under control (Schehlein et al., 2017). Therefore, the combination of multiple medications is significantly necessary for an adequate control of IOP.
The lowering effect of IOP by the fixed combination and non-fixed combination medications are similar and both the combinations are superior to their constituent agents. Importantly, the fixed combination and non-fixed combination medications have reliable security with no additional new side effects comparing to monotherapy agents. Fixed combination medications have a greater convenience, compliance, and tolerance than non-fixed combination medications, since they can decrease dosing frequency and eliminate the washout effect by decreasing the number of instilled drops, the total number of bottles of medication in use, and the exposure to preservatives (Babic, 2015).
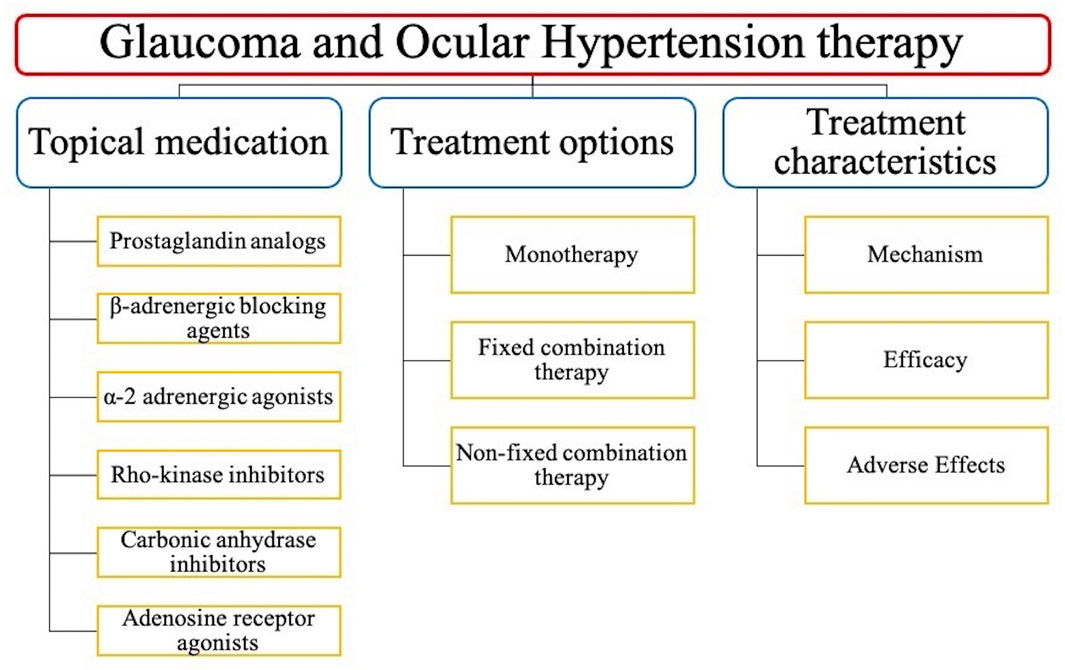
This review collects and compares extensive clinical trials on the topical anti-glaucoma medications, and summarized their mechanism of action, efficacy, adverse effects (AEs), and safety on Tables 1–6. And the structure of this review is shown on Figure 1.
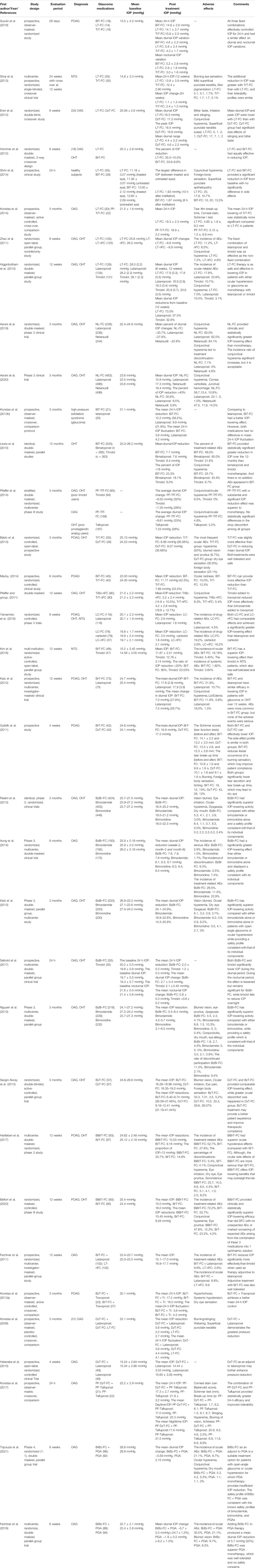
TABLE 6. A summary of clinical trials comparing different fixed combination and non-fixed combination agents.
2 Methods
This review summarizes the effectiveness and safety of topical anti-glaucoma agents based on an overall review of related literatures published. Extensive references were obtained by searching keywords: prostaglandin analogs, rho-kinase inhibitors, β-adrenergic blocking agents, α-2 adrenergic agonists, carbonic anhydrase inhibitors, fixed-combination agents, glaucoma and so on. Systematic and large-scale clinical trials, meta-analysis and review articles were the main priority; because of the lacking of related articles, small-scale experiments and related cases were referenced. The main clinical trials more elaborated were summarize on Table 6.
3 Topical Monotherapy Agents
3.1 Prostaglandin Analogs (PGAs)
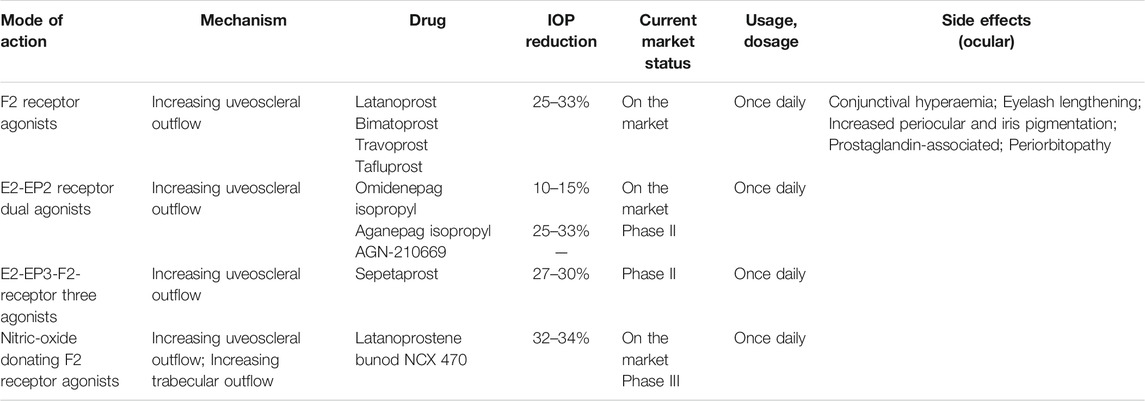
Prostaglandins (PGs) are potent biologically active metabolites of the arachidonic acid that modulate many biological responses in various tissues including the eye (Matsou and Anastasopoulos, 2018). PGAs represent the first-line medical therapy to cure POAG and OHT, since they target different PG receptors to reduce IOP (Diaconita et al., 2018; Angeli and Supuran, 2019) (Table 1).
3.1.1 Prostaglandin F2-Alpha Receptor Agonists
Prostaglandin F2 (PGF2) analogs bind to PG receptors and promote muscle relaxation and extracellular matrix remodeling in the ciliary muscle and TM, thus increasing AH outflow in both the conventional and unconventional pathways (Matsou and Anastasopoulos, 2018; Jayanetti et al., 2020). PGF2 analogs reduce IOP by 25–33% (Diaconita et al., 2018), they are widely available on the market in most countries (Matsou and Anastasopoulos, 2018), and include latanoprost (Xalatan®), bimatoprost (Lumigan®), travoprost (Travatan Z®), and tafluprost (Tapros®) (Matsou and Anastasopoulos, 2018).
PGF2 analogs exert different IOP lowering effects, as demonstrated by clinical trials and meta-analysis: bimatoprost exerts the greatest IOP lowering effect and the highest incidence of hyperemia than other PGF2 analogs (Aptel and Denis, 2011; Yu and Welge-Lussen, 2013; Takagi et al., 2018). In addition, they are well tolerated and induce mild AEs including conjunctival hyperemia, increase in the eyelash growth, as well as changes in periocular pigmentation and iris color (Aptel and Denis, 2011; Yu and Welge-Lussen, 2013).
3.1.2 Prostaglandin E2 Receptor Agonists
Prostaglandin E2 (PGE2) has high affinity for four different E-type prostaglandin (EP1-4) receptors (Matsou and Anastasopoulos, 2018). EP1-4 receptors are widely distributed in the human cornea, conjunctiva, TM, iris, ciliary body, and retina (Doucette and Walter, 2017; Matsou and Anastasopoulos, 2018). PGE2 receptor agonists increase uveoscleral outflow and induce morphological alterations in the tissues involved in the conventional pathway to decrease IOP by binding to EP1-4 receptors (Matsou and Anastasopoulos, 2018).
Prostaglandin E2 Receptor EP2 Subtype Agonists
Omidenepag Isopropyl (DE-117/G0G0H52U6K/STN-10117, Eybelis®)
Efficacy
Omidenepag isopropyl (OMDI) is a prodrug of omidenepag (Duggan, 2018) through the increase of both TM and uveoscleral outflow (Fuwa et al., 2018). The optimal dose of OMDI is 0.002% as demonstrated in the clinical trials (Aihara et al., 2019; Aihara et al., 2020a). IOP lowering effect of OMDI (0.002%) is not inferior to latanoprost (0.005%) (Aihara et al., 2019). The mean reduction of IOP after 4 weeks is 5.93 and 6.56 mmHg in the patients treated with OMDI (0.002%) and latanoprost (0.005%), respectively (Aihara et al., 2020a). In a phase 3 study, the percentage of the mean diurnal IOP reduction is 13.23% after 4 weeks of OMDI (0.002%) in patients who accepted latanoprost 0.005% with a reduction of IOP ≤15% during 8 weeks (Aihara et al., 2020b).
Safety and AEs
OMDI has better safety than latanoprost (Aihara et al., 2019). The ocular AEs induced by OMDI is mild or moderate, and the most frequently reported are conjunctival hyperemia, photophobia, and eye pain (Aihara et al., 2020a; Inoue et al., 2020a; Aihara et al., 2020b). Conjunctival hyperemia is dose-dependent (Aihara et al., 2019), and most frequently observed in small vessels (51%) in OMDI-treated eyes, and in both large and small vessels (81%) in ripasudil-treated eyes (Terao et al., 2020).
Aganepag Isopropyl (AGN-210961)
AGN-210961 has been formulated as a proprietary ophthalmic solution (Impagnatiello et al., 2019). Both AGN-210961 and bimatoprost 0.03% exert a similar IOP lowering effect at day 7 and week 4, as demonstrated by a clinical trial (NIH, 2014a). AGN-210961 do not exert any serious AEs, and the incidence of AEs in AGN-210961 group is also lower than bimatoprost group (NIH, 2014a).
AGN-210669
AGN-210669 is a sustained-release formulation of AGN-210961 (Impagnatiello et al., 2019) used in clinical trials for the treatment of POAG and OHT. But the safety and effectiveness of AGN-210669 is not superior to bimatoprost (NIH, 2013b; a). The combination of AGN-210669 (0.05%) and bimatoprost does not induce a significant change in the IOP lowering effect, and the AEs of the combination therapy also increased (NIH, 2013b; a). Therefore, AGN-210669 may not be a better medicine to control IOP.
Prostaglandin E2 Receptor EP3 Subtype Agonists
Sepetaprost (ONO-9054/DE-126)
Efficacy
The prodrug ONO-9054 is an isopropyl ester derivative of the biologically active free acid ONO-AG-367 in the treatment of glaucoma and OHT (Impagnatiello et al., 2019; Bhattaccharjee et al., 2020). ONO-9054 can rapidly be converted into ONO-AG-367 after ocular instillation and is characterized by dose-dependent systemic pharmacokinetics with rapid clearance (Suto et al., 2015; Harris et al., 2016).
A phase I clinical trial in OHT and POAG patients demonstrated that ONO-9054 reduces IOP in a dose-dependent manner and the greatest reduction is 29.6% at 9 h after the dose of ONO-9054 of 30 μg/ml on day 1 (Harris et al., 2016). In healthy volunteers, the mean reduction is 8.29 mmHg (28.2%) at 9 h after a dose of ONO-9054 of 30.0 μg/ml (Suto et al., 2015). The morning (AM) and evening (PM) dosage of 30 μg/ml ONO-9054 have similar sustained reduction in IOP, whereas the incidence of hyperemia and dryness is slightly increased in the patients subjected to the PM dose of ONO-0954 (Berlin et al., 2016). Miller Ellis, et al. observed that ONO-9054 (30 μg/ml) has a better IOP lowering effect than Xalatan once daily (Miller Ellis et al., 2017). The rate of the mean IOP reduction of ≤ −25%, ≤ −30%, and ≤ −35% in the ONO-9054-treated patients is 2.39, 2.37, and 4.85 times higher than the rate in the Xalatan-treated patients, and 2.4 times more patients achieve an IOP ≤15 mmHg than those treated with Xalatan (Miller Ellis et al., 2017).
Safety and AEs
ONO-9054 is well-tolerated and all AEs have a mild or moderate intensity (Miller Ellis et al., 2017). The AEs frequently reported are headache, anterior uveitis, vitreous detachment, conjunctival hyperemia, and blurred vision (Harris et al., 2016; Miller Ellis et al., 2017).
3.1.3 Nitric Oxide-donating Prostaglandin F2-Alpha Analog
Nitric oxide (NO)-donating prostaglandin is composed of a prostaglandin F2 analogue moiety and NO-donating moiety, thus providing an IOP-lowering effect by two independent mechanisms (Impagnatiello et al., 2019). NO is an endogenous gas working as a signaling molecule inducing TM and Schlemm’s canal cell relaxation, decreasing cell volume, enlarging cell gap, and decreasing the outflow resistance through the activation of the soluble guanylyl cyclase/cyclic guanosine monophosphate (sGC-cGMP) signaling pathway (Krauss et al., 2011; Kaufman, 2017; Cavet and DeCory, 2018; Mao et al., 2020). In addition, it decreases AH production by inhibiting the Na+, K+-ATPase pump eventually lowering IOP (Wareham et al., 2018). NO also regulates the eye blood flow and protects the optic nerve by the sGC-cGMP signaling pathway (Wareham et al., 2018; Mao et al., 2020).
Latanoprostene Bunod (Vyzulta®)
Efficacy
LBN is the first NO-donating prostaglandin F2-alpha analog on the market (Addis and Miller-Ellis, 2018; Hoy, 2018a; Mehran et al., 2020). LBN administered every evening (QD) exerted a significantly greater effect and it is safer than timolol 0.5% (BID) (Kawase et al., 2016; Liu et al., 2016; Weinreb et al., 2016; Weinreb et al., 2018). The mean IOP is significantly lower in the LBN-treated patients (17.8–18.9 mmHg) than in those treated with timolol 0.5% (19.0–19.7 mmHg) at all time points (week 2, week 6, and month 3). A total of 22.9% patients treated with LBN achieved a mean IOP ≤18 mmHg, which is significantly more than that in those treated with timolol (11.3%) (Weinreb et al., 2016).
Safety and AEs
LBN is well-tolerated with no serious AEs and system AEs. Conjunctival hyperemia, eye irritation, and eye pain are the most reported ocular AEs (Medeiros et al., 2016). Blood pressure or heart rate does not change in correlation with LBN treatment (Kawase et al., 2016; Liu et al., 2016).
NCX 470
Efficacy
NCX-470 is a novel NO-donating bimatoprost with potentially greater IOP-lowering effect than bimatoprost monotherapy (Impagnatiello et al., 2015). A phase 2 clinical trial demonstrated that NCX-470 with well-tolerated was non-inferiority to latanoprost monotherapy (NIH, 2019a). The mean IOP reduction were 7.6–9.8 mmHg and 6.3–8.8 mmHg at day 28 in NCX-470 group and latanoprost group (NIH, 2019a).
Safety and AEs
Little evidence was found of treatment-related systemic effects or drug-related serious AEs associated to NCX-470. Conjunctival hyperemia was the most frequent AE in 16.8% of patients treated with NCX-470 (0.065%) versus 6.5% of patients treated with latanoprost (NIH, 2019a).
3.2 Rho-Kinase Inhibitors
The rho-kinases are serine/threonine kinase isoforms and effectors (Schehlein and Robin, 2019). Topical rho-kinase inhibitors inhibit rho-kinase isoforms in TM, thus directly increasing AH outflow in the TM pathway and consequently reducing IOP in a significant manner (Berrino and Supuran, 2019; Moura-Coelho et al., 2019). Rho-kinase inhibitors also exert a neuroprotective effect that in turn can exert a greater positive impact on the ocular blood flow, and even an antifibrotic effect that may be helpful if conventional glaucoma surgery is needed (Tanna and Johnson, 2018) (Table 2).
3.2.1 Launched Rho-Kinase Inhibitors
Ripasudil (Glanatec®)
Efficacy
Ripasudil is the first rho-kinase inhibitor approved for the treatment of glaucoma (Berrino and Supuran, 2019). It promotes AH outflow by changing the TM cytoskeleton to reduce the outflow resistance and increasing the endothelial permeability of the Schlemm’s canal (Schehlein and Robin, 2019). Multiple clinical trials reported that ripasudil provides a great IOP lowering effect and exerts an additional reduction of IOP when used an add-on medication to PGAs and β-blockers (BBs) (Tanihara et al., 2013; Tanihara et al., 2016; Inoue and Tanihara, 2017). A phase 2 clinical study demonstrated that IOP-lowering effect of ripasudil is dose-dependent. When patients are treated with 0.1, 0.2, and 0.4% ripasudil for 8 weeks, the mean IOP reductions are 3.4 mmHg, 3.2 mmHg, and 3.5 mmHg, respectively (Tanihara et al., 2013).
Safety and AEs
Conjunctival hyperemia is the most frequent AE caused by ripasudil (Tanihara et al., 2013; Tanihara et al., 2016; Sakamoto et al., 2019) due to the relaxation of the smooth muscle in the blood vessels because of the inhibition of rho-kinase. However, this AE is mild, transient, and can resolve spontaneously in glaucoma patients (Sakamoto et al., 2019; Kusuhara and Nakamura, 2020). The other frequently observed AEs are blepharitis and allergic conjunctivitis (Tanihara et al., 2016).
Netarsudil (Rhopress®)
Efficacy
Netarsudil reduces the high IOP by inhibiting rho-kinase and norepinephrine transporter (Hoy, 2018b; Mehran et al., 2020). In this way, it increases TM outflow and reduces the episcleral venous pressure (Kazemi et al., 2018). Netarsudil 0.02% is well-tolerated and has a similar IOP lowering effect to timolol 0.5% twice daily (Kazemi et al., 2018; Serle et al., 2018; Khouri et al., 2019; Singh et al., 2020). Netarsudil once daily (PM) and twice daily have similar IOP lowering effect (Khouri et al., 2019). The mean IOP reduction at 3 months after netarsudil once daily and twice daily, as well as timolol twice daily is 16–21%, 22–24%, and 18–23% respectively (Serle et al., 2018).
Safety and AEs
Netarsudil causes non-serious AEs, generally mild in intensity (Kazemi et al., 2018; Serle et al., 2018; Kahook et al., 2019). The most frequent ocular AE is conjunctival hyperemia (Singh et al., 2020), followed by corneal deposits and conjunctival hemorrhage (Serle et al., 2018; Kahook et al., 2019). Other AEs include instillation site pain, blurred vision, increased lacrimation, reduction of visual acuity, eye pruritus, and erythema of the eyelid (Serle et al., 2018; Kahook et al., 2019).
3.2.2. Clinical Rho-Kinase Inhibitors
RKI-983/SNJ-1656/Y-39983
SNJ-1656, also called Y-39983 or RKI-983, promotes the regeneration of the axon in damaged retinal ganglion cells (Yang et al., 2013). However, the IOP lowering effect is not very remarkable. In a phase I clinical trial, the mean IOP reductions are 2.2, 3.8, 4.3, and 4.0 mmHg before the instillation of the eyedrops in the morning and 1.5, 5.0, 4.4 and 4.5 mmHg at 2 h after the instillation in the morning in placebo and SNJ-1656 (0.03, 0.05 or 0.1%) group (Inoue et al., 2015). AEs are mild to moderate, and conjunctival hyperemia is the most frequently reported (Inoue et al., 2015).
AMA-0076/PHP-201
AMA0076, also called PHP-201, contains carboxylic ester moieties that allow the inactivation by esterase, resulting in an increased therapeutic window (Van de Velde et al., 2014). AMA0076 with a similar IOP-lowering efficacy as Y-39983 and latanoprost was demonstrated in the white rabbit experiment, and AMA0076 was more potent in preventing the IOP elevation in the acute hypertensive model (Van de Velde et al., 2014). However, no result of AMA-0076 on clinical trials was published.
Y-27632
Y-27632 is used in the treatment of OHT since it modulates the cytoskeletal alterations in TM cells to increase the conventional outflow, and relax the ciliary muscle contraction (Chen et al., 2020; Luo et al., 2021). At present, there is no related clinical study, but glaucomatous transgenic mice experiment demonstrated that Y-27632 can significantly decreased IOP and increased outflow facility, which greatly influenced the long-term IOP-lowering effect (Chen et al., 2020).
3.3 β-adrenergic Blocking Agents
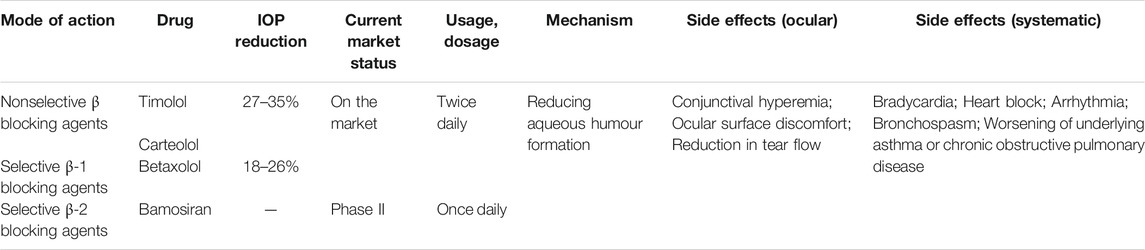
BBs actively block the β-adrenergic receptors and their pharmacological properties depend on their various effects on the adrenergic signaling pathway (Bejan-Angoulvant and Angoulvant, 2020). The ocular AEs include conjunctival hyperemia, ocular surface discomfort, reduction in tear flow, and worsening of the dryness in the eye, while the systemic AEs include bradycardia, heart block and arrhythmia, bronchospasm, and worsening of underlying asthma or chronic obstructive pulmonary disease (Dikopf et al., 2017). These AEs are caused by the direct systemic absorption of BBs after ocular administration through the nasolacrimal system or the conjunctiva, without being subjected to first pass metabolism (Morales et al., 2016; Shukla et al., 2020). Although topical BBs is not associated with excess cardiovascular mortality as revealed in a meta-analysis with large population-based studies (Pinnock et al., 2016), ophthalmic BBs are not recommended in patients with asthma, chronic obstructive pulmonary disease, bradycardia, heart block, or uncontrolled heart failure (Marshall et al., 2018) (Table 3).
3.3.1 Nonselective β-adrenergic Blocking Agents
Nonselective BBs reduce IOP by blocking β-adrenoceptor in the ciliary body and consequently decreasing AH production (Nocentini and Supuran, 2019). Timolol maleate (Timoptol®) is the first nonselective BB for the topical treatment of glaucoma and OHT (Sah and Suresh, 2017) and it reduces IOP by 27–35% (Nocentini and Supuran, 2019). Carteolol hydrochloride (Mikelan®) protects against light-induced oxidative stress in retina (Matsuo et al., 2019).
3.3.2 Selective β-1 Adrenergic Blocking Agents
Betaxolol hydrochloride (Betoptic®) is a selective β1-blocker for the topical treatment of glaucoma, exerting an IOP reduction of 18–26% less than timolol (Dikopf et al., 2017).
3.3.3 Selective β-2 Adrenergic Blocking Agents
Bamosiran (SYL040012)
Efficacy
SYL040012 targets the human β2-adrenergic receptor to inhibit its expression and consequently reduces the production of AH (Martinez et al., 2014; Jiang et al., 2021). It is also the first compound based on RNA interference (Moreno-Montanes et al., 2014). A phase I clinical trial demonstrated that no significant difference in IOP was found between the patients treated with 600 µg/eye once daily and those treated with 900 µg/eye twice daily (Moreno-Montanes et al., 2014). Patients in a phase 2 study were randomly divided to receive 80 µg (0.2%), 300 µg (0.75%), 900 µg (2.25%) SYL040012 or placebo once daily, and the results showed that 0.75% SYL040012 exerted a statistically significant reduction in IOP at day 14 (NIH, 2014c). More clinical studies are needed to explore the optimal dosing of SYL040012.
Safety and AEs
The AEs occur when SYL040012 is administered twice daily and include headache, conjunctival hyperemia, muscle spasm, unilateral stinging, bilateral itching, and difficulty in focusing. The diastolic blood pressure is significantly reduced only in patients treated with SYL0420012 at 600 µg/eye twice daily (Moreno-Montanes et al., 2014).
3.4 α-2 Adrenergic Agonists
α-adrenergic is divided into α1 and α2, which are the ones involved in the regulation of IOP. Ocular α2-adrenoreceptor induces conjunctival vasoconstriction, increases interpalpebral fissure, mydriasis, and decreases corneal oxygen tension (Cimolai, 2020). Brimonidine tartrate reduces AH production and increases the flow in TM and uveoscleral pathways (Nocentini and Supuran, 2019; Cimolai, 2020). Brimonidine tartrate (Alphagan®) is often administered as second line therapy or combined with other topical medications for the topical treatment of glaucoma and OHT (Hopf et al., 2020) resulting in an IOP reduction of 20–27% (Nocentini and Supuran, 2019; Cimolai, 2020). Brimonidine also offers a neuroprotective effect (Zhou et al., 2019; Conti et al., 2021). The significant AEs include blepharitis, blepharoconjunctivitis, conjunctivitis, conjunctival follicles, mild hyperemia, staining of the cornea, blurred vision, and foreign body sensation (Dikopf et al., 2017; Nocentini and Supuran, 2019) (Table 4).
3.5 Carbonic Anhydrase Inhibitors
Human carbonic anhydrase (hCA) is present in all living organism and it catalyzes carbon dioxide hydration that is regulating many physiological processes (Supuran et al., 2019; Supuran, 2020). hCA inhibitors exert IOP lowering effect by decreasing bicarbonate and AH secretion through the inhibition of hCA II, IV, and XII isoforms.
(Supuran et al., 2019; Ghorai et al., 2020). The first generation CAs inhibitors are sulfonamide hCA-II inhibitors including acetazolamide, methazolamide, and dichlorophenamide, which decrease IOP by 25–30% (Scozzafava and Supuran, 2014; Ghorai et al., 2020). They have been used as systemic antiglaucoma drugs for more than 50 years, but certain undesirable AEs are reported due to the inhibition of the enzymes present in the tissues than the ones in the eye (Carradori et al., 2015). The second generation of CAs inhibitors topically administered are sulfonamides, such as dorzolamide hydrochloride (Trusopt®) and brinzolamide (Azopt®), whose IOP lowering effect is comparable with the one of BBs, but less effective than PGAs (Eichhorn, 2013). Trusopt® and Azopt® are characterized by a better tolerance and less AEs compared to the first generation inhibitors, and the common reported AEs include stinging, burning or reddening of the eye, blurred vision, pruritus and bitter taste (Scozzafava and Supuran, 2014) (Table 4).
3.6 Adenosine Receptor Agonists
Adenosine is an essential component of the energy production and utilization systems of the body by providing the energetics necessary for the muscle movements, heartbeat, nerve signals, and chemical reactions (Jamwal et al., 2019). It exerts its functions by activating four adenosine receptor (AR) subtypes (A1, A2A, A2B, and A3) (Ciancetta and Jacobson, 2017). Adenosine is neuroprotective against excitotoxicity and metabolic dysfunctions that may be present in neurological and ocular diseases (Jamwal et al., 2019) (Table 4).
3.6.1 Trabodenoson (INO-8875)
Efficacy
Trabodenoson is an adenosine mimetic that selectively acts on the A1 receptor subtype (Qiu, 2021). Trabodenoson can involve in matrix metalloproteinase signaling pathway associated with glaucoma (Qiu, 2021), consequently increasing TM outflow and exerting IOP lowering effect (Li et al., 2018). Trabodenoson exerts a dose-dependent and time-dependent IOP lowering effect (Myers et al., 2016). When patients receive of trabodenoson topical administration at the dose of 50, 100, 200, or 500 μg twice daily exerts a mean IOP reduction at day 14 to 1, 1.67, 2.5, and 3.2 mmHg, respectively, and a reduction to 4.1 mmHg with the dose of 500 μg at day 28 (Myers et al., 2016).
Safety and AEs
Trabodenoson has a good safety profile (Laties et al., 2016; Myers et al., 2016). The common systematic AEs such as headache, eye pain, back pain, dermatitis, and excoriation, were more frequently observed in the placebo-treated patients than in the trabodenoson-treated patients (Laties et al., 2016). Ocular AEs are uncommon and not serious, present for not longer than 24 h, and they are self-limited, and are usually mild in intensity (Laties et al., 2016).
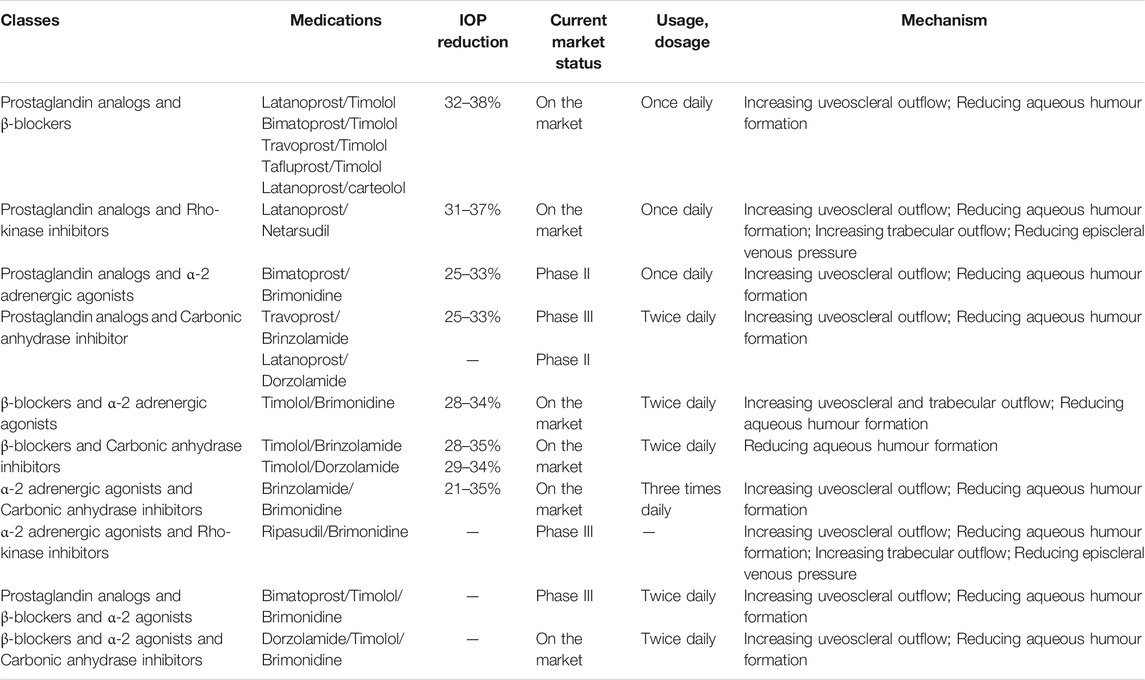
4 Topical Fixed Combination of Two Agents
4.1 Fixed Combination of Prostaglandin F2a Analogs and β-blockers
4.1.1 Fixed Combination Latanoprost and Timolol (Xalacom®)
Efficacy
LT-FC is currently one of the most widely used topical anti-glaucoma fixed-combination eye drops, which is usually chosen to cure patients with POAG and OHT who are insufficiently responsive to BBs, PGs, or other IOP lowering agents (Konstas et al., 2013c). LT-FC exerts a superior IOP lowering effect than the monotherapy using latanoprost or timolol. Mean IOP at 12 weeks is reduced to 10.2 mmHg in the LT-FC-treated patients, 8.9 mmHg in the latanoprost-treated patients and 7.2 mmHg in the timolol-treated patients and 73.5, 57.5, and 32.8% patients achieve a diurnal IOP reduction over 30% (Higginbotham et al., 2010). Zhao, et al. reported the IOP lowering effect of LT-FC versus non-fixed combination of latanoprost and timolol (LT-nFC), and their results revealed that LT-FC is noninferiority to LT-nFC regarding the mean diurnal IOP reduction after 8 weeks (8.6 versus 8.9 mmHg) (Zhao et al., 2011). Importantly, LT-FC shows a better compliance than LT-nFC (Zhao et al., 2011).
Safety and AEs
Various studies show similar AEs in patients treated with LT-FC, LT-nFC, latanoprost, and timolol monotherapies, and the most commonly reported one is conjunctival hyperemia (Higginbotham et al., 2010).
4.1.2 Fixed Combination Bimatoprost and Timolol (Ganfort®)
Efficacy
BiT-FC has present in the market of more than 30 countries and regions worldwide OHT used for the reduction of IOP in patients with POAG (Fang et al., 2015). BiT-FC significantly decreases IOP in a greater extent than PGA, or timolol monotherapy (Lewis et al., 2010; Lequeu et al., 2013). BiT-FC also has better 24 h-IOP controlling effect than latanoprost monotherapy (Konstas et al., 2013b). The rates of the mean diurnal IOP reduction over 20% are 68.1, 58.1, and 38.0% in BiT-FC, bimatoprost, and timolol-treated patients, respectively, and the rates of IOP less than 18 mmHg are 23.3, 18.1, and 8.0%, respectively (Lewis et al., 2010). The mean 24-h IOP reduction was 12.2 mmHg (39.2%) in BiT-FC group, which was better than that in latanoprost group (9.9 mmHg, 31.9%) (Konstas et al., 2013b).
Safety and AEs
No addition AEs and serious AEs are reported in BiT-FC treatment group. Although, the incidence of AEs is more in the bimatoprost-treated patients (60.0%) than in the BiT-FC- (48.0%) and timolol-treated patients (31.6%), it is acceptable. Conjunctival hyperemia is the most frequently observed AE, with an incidence of 43.4% by bimatoprost, followed by 25.7% of BiT-FC and 8.7% of timolol (Fang et al., 2015).
4.1.3 Fixed Combination of Travoprost and Timolol (DuoTrav®)
Efficacy
TrT-FC is preserved with benzalkonium chloride (BKC) and it is already introduced into the global market (Hoy et al., 2006; Konstas et al., 2012). Preservative-free (PF)-TrT-FC is one of the most recent PF agents that become available in Europe, which reduce the toxicity and improve the long-term health of the ocular surface in a better manner than TrT-FC (Denis, 2011; Konstas et al., 2012). A total of 162 patients in a prospective multicenter open-label study were treated with PGA monotherapy (travoprost, latanoprost, tafluprost, or bimatoprost) for over 3 months, then they switched to TrT-FC, and the observed reduction in IOP was 10.3 ± 12.7% (1.7 ± 3.1 mmHg), 9.4 ± 14.3% (1.6 ± 3.3 mmHg), and 10.1 ± 13.0% (1.7 ± 3.2 mmHg) after 4, 8, and 12 weeks, respectively (Nakano et al., 2015).
Safety and AEs
The most frequently observed AEs after TrT-FC treatment are ocular hyperemia, ocular discomfort, pruritus, and dryness (Hoy et al., 2006). Although, timolol has systemic side effects, no significant change from the baseline value of the mean systolic blood pressure is observed when patients switch to TrT-FC from PGA monotherapy (Nakano et al., 2015).
4.1.4 Fixed Combination of Tafluprost and Timolol (Tapcom®)
Efficacy
PF-TfT-FC was used for the reduction of IOP in adults with OAG or OHT who require a combination therapy because they are insufficiently responsive to topical monotherapy with BBs or PGs (Hoy, 2015). PF-TfT-FC has a superior IOP lowering effect compared with agents used as monotherapy (Pfeiffer et al., 2014; Kaarniranta et al., 2016; Konstas et al., 2018), which is more suitable for evening administration (Konstas et al., 2018). In a phase 1 study, the result of the mean IOP was 10.3 mmHg for PF-TfT-FC, 10.9 mmHg for PF-tafluprost, and 11.1 mmHg for PF-timolol after 8 days in healthy volunteers (Kaarniranta et al., 2016). In another clinical trial, 189 patients treated with timolol who switched to PF-TfT-FC (n = 95) or PF-timolol (n = 94), showed a reduction of IOP to 7.1–9.0 mmHg in the PF-TfT-FC-treated patients and 6.5–8.1 mmHg in the timolol-treated patients and 375 patients treated with PGA who switched to PF-TfT-FC (n = 188) or PF-tafluprost (n = 187) for 3 months showed a reduction of IOP to 8.2–9.0 mmHg in the PF-TfT-FC-treated patients and 6.8–7.4 mmHg in the PF-tafluprost-treated patients (Pfeiffer et al., 2014).
Safety and AEs
The rate of AEs is more in the PF-TfT-FC-treated patients than in those treated with the single agent (Pfeiffer et al., 2014). Patients treated with PF-timolol show the lowest incidence (15.2%) of AEs in contrast to patients treated with PF-tafluprost (36.4%) and PF-TfT-FC (48.5%). The commonly reported ocular AEs related to the treatment with PF-TfT-FC include eye pain, eye pruritus, ocular hyperemia, and photophobia (Kaarniranta et al., 2016), while hyperemia is less common in patients treated with PF-TfT-FC than in those treated with PF-latanoprost (7.1% vs 21.4%) (Konstas et al., 2018).
4.1.5 Fixed Combination of Latanoprost and Carteolol (Mikeluna®)
Efficacy
LC-FC has the same IOP lowering effect as the non-fixed combination of carteolol and latanoprost (LC-nFC) and LT-FC, and exerts a greater effect than carteolol and latanoprost monotherapy (Yamamoto et al., 2016; Inoue et al., 2018; Inoue et al., 2020b). In the study 1 of a two phase 3 clinical trial, 220 patients were treated with LC-FC (n = 113) or latanoprost (n = 116) for 8 weeks, while in the study 2, 175 patients were treated with LC-FC (n = 76), carteolol (n = 76), or LC-nFC (n = 37) for 8 weeks. The adjusted mean IOP reduction in the study 1 was 2.9 and 1.6 mmHg in the LC-FC and latanoprost-treated patients, in the study two was 3.5, 3.5, and 1.6 mmHg in LC-FC, LC-nFC, and carteolol-treated patients (Yamamoto et al., 2016). When patients with POAG or OHT were treated with LC-nFC and then switched to LC-FC, they showed an IOP of 15.0 ± 2.6, 15.1 ± 2.4, and 15.0 ± 2.4 mmHg at baseline, month 1 and 3, respectively (Inoue et al., 2018). When patients with POAG, NTG, or OHT were treated with LT-FC and then switched to LC-FC, they showed a not significantly changed mean IOP after 1 month (15.9 ± 3.1 mmHg) and 3 months (16.3 ± 3.8 mmHg) compared to the baseline IOP with LT-FC (16.1 ± 3.1 mmHg) (Inoue et al., 2020b).
Safety and AEs
The AEs related to LC-FC observed in clinical trials are mild (Yamamoto et al., 2016), and common ocular AEs include hyperemia, irritation, itching, pain, and blurred vision (Inoue et al., 2018). The incidence of ocular AEs decreases when the therapy switches from LC-nFC to LC-FC (Inoue et al., 2018), so LC-FC may provide better tolerance. The pulse rate and blood pressure do not significantly change in patients treated with LC-FC (Yamamoto et al., 2016; Inoue et al., 2018).
4.1.6 Comparison of the Fixed Combination of Prostaglandin F2a Analogs and β-blockers
When comparing the efficacy of TrT-FC with that of LT-FC, the former showed a greater IOP lowering effect (Shoji et al., 2013; Konstas et al., 2014). Mean 24-h IOP reduction was 2.6 mmHg in patients treated with PF-TrT-FC and 2.2 mmHg in those treated with LT-FC (Konstas et al., 2014). Mean reduction in IOP from the baseline (14.8 ± 3.3 mmHg) at 12 weeks is significantly greater in patients treated with TrT-FC (2.4 ± 2.3 mmHg) than in those treated with LT-FC (1.1 ± 2.3 mmHg) (Shoji et al., 2013). No significant difference in ocular or systemic AEs is observed between TrT-FC- and LT-FC-treated patients, but stinging is significantly more in patients treated with LT-FC (19%) than in those treated with latanoprost (4.8%) (Konstas et al., 2014). A comparative study between BiT-FC and LT-FC discovered a significant reduction in IOP from the baseline without any change of the anterior ocular parameters. The biggest difference in IOP between treated and untreated eyes was 1.67 mmHg (13.6%) at 8 h after the instillation of BiT-FC and 1.93 mmHg (17.8%) at 10 h after the instillation of LT-FC. Conjunctival hyperemia is the most frequent AE in BiT-FC- and LT-FC-treated patients (33.3% versus 25.0%) (Shim et al., 2014). A comparative study showed that patients treated with BiT-FC have a lower IOP than those treated with TrT-FC and the mean IOP reduction is 11.17 and 7.89 mmHg after 6 months, respectively (Macky, 2014). However, in another clinical trial in which patients whose IOP were uncontrolled on BiT-FC accepted TrT-FC showed a decrease of the mean IOP by additional 16.5% at week 8 and 69.2% patients reached the target IOP (≤18 mmHg) (Scherzer et al., 2011). The common AEs are similar in BiT-FC- and TrT-FC-treated patients and include ocular hyperemia, ocular burning, blurred vision, foreign body sensation, and allergic reaction, while stinging/burning is less severe in patients treated with TrT-FC (Scherzer et al., 2011). PF-TfT-FC is as effective as LT-FC in terms of IOP-reducing effect, and induces fewer AEs such as eye irritation and eye pain, which were significantly reduced in patients using PF-TfT-FC (Suzuki et al., 2018). Patients in a phase IV study who switched from BiT-FC to PF-TfT-FC for 12 weeks showed an IOP after the treatment with PF-TfT-FC that was clinically insignificant and statistically non-inferior compared with BiT-FC (0.34 mmHg difference). However, the related ocular AEs including lacrimation, eye pruritus, and pruritus increase (Bourne et al., 2019). In a prospective, observer-masked, randomized study including 54 patients who received BiT-FC, LT-FC, or TrT-FC, a statistically significant reduction in diurnal/nocturnal IOP was observed with all medications, such as 4.6 ± 2.3 mmHg/3.2 ± 2.8 mmHg, 5.8 ± 2.4 mmHg/2.9 ± 1.9 mmHg, and 4.3 ± 1.7 mmHg/3.0 ± 1.6 mmHg, respectively, but no significant difference was observed among the three groups (Guven Yilmaz et al., 2018).
Overall, fixed combination and non-fixed combination of PGAs and β-blockers result in a similar IOP lowering effect, and both are superior to monotherapy, but fixed-combination medicines can significantly improve patient compliance and tolerence. Comparing to eye drops containing preservatives, preserver free medicines can better decrease the incidence of ocular side effects and increase ocular comfortable. So, it is necessary to modify the eye drops to be preservative-free to improve patient compliance and tolerance.
4.2. Fixed Combination of Prostaglandin F2a Analogs and Rho-Kinase Inhibitors
4.2.1. Fixed Combination of Latanoprost and Netarsudil (Rocklatan®)
Efficacy
NL-FC is a glaucoma eye drop marketed as last and is recently approved by FDA for the treatment of OAG or OHT (Asrani et al., 2020). Multiple comparative studies observed that NL-FC had a superior IOP-lowering effect than netarsudil and latanoprost monotherapy (Asrani et al., 2019; Asrani et al., 2020). The percentage of IOP reduction observed in a phase 3 trial was 30.9–36.7% in patients treated with NL-FC compared to the 21.8–24.9% and 23.3–28.8% in patients treated with latanoprost or netarsudil monotherapy at month 3 (Asrani et al., 2019). Another phase 3 trial showed a mean diurnal IOP reduction to 7.8, 5.2, and 6.2 mmHg, respectively. NL-FC (32.3%) achieved a mean diurnal IOP ≤14 mmHg was 3 times higher than that observed after netarsudil (10.8%) and latanoprost (11.8%) monotherapy (Asrani et al., 2020).
Safety and AEs
NL-FC has a safety profile consistent with that of its individual components (Asrani et al., 2019). Conjunctival hyperemia is the most commonly reported in NL-FC group and netarsudil group (Asrani et al., 2020), which may be blood vessel smooth muscle relaxation and blood vessel dilation due to rho-kinase of calcium sensitization (Mehran et al., 2020).
4.3 Fixed Combination of Prostaglandin F2a Analogs and α-2 Agonists
4.3.1 Fixed Combination of Bimatoprost and Brimonidine
Efficacy
BiBr-FC has been evaluated in a clinical trial for the treatment of glaucoma and OHT as an ophthalmic solution in 2013. IOP lowering effect of BiBr-FC was similar to the one of bimatoprost (0.01%) monotherapy and superior to the one of brimonidine (0.2%) monotherapy (NIH, 2015b).
Safety and AEs
The incidence of AEs is more in patients treated with BiBr-FC (71.05%) than in those treated with bimatoprost (69.44%) and brimonidine tartrate (52.63%) (NIH, 2015b), and common eye disorders include conjunctival hyperemia, eye pruritus, eye discharge, blepharospasm, ocular burning, punctate keratitis, eye pain, foreign body sensation, eye irritation, and blurred vision (Netland et al., 2003; NIH, 2015b). Conjunctival hyperemia and eye pruritus show significantly higher rates in BiBr-FC- and bimatoprost-treated patients than in those treated with brimonidine, while the other AEs are similar in the 3 groups (NIH, 2015b). The similar IOP lowering effect and higher AEs may limit the development of BiBr-FC.
4.4 Fixed Combination of Prostaglandin F2a Analogs and Carbonic Anhydrase Inhibitors
4.4.1 Fixed Combination of Travoprost and Brinzolamide
Efficacy
TrBz-FC was used in phase III clinical studies for the treatment of OHT and OAG since 2014. However, both TrBz-FC and TrBz-nFC show no better IOP lowering effect than its marketed components (Travatan® solution and Azopt® suspension) at 8AM, 10AM, 12PM, 4PM, and 8PM after 6-weeks treatment (NIH, 2015a).
Safety and AEs
TrBz-FC is well-tolerated with no serious AEs and the reported AEs include ocular hyperemia, conjunctival hyperemia, eye irritation, eye pain, and dysgeusia (NIH, 2015a).
4.4.2 Fixed Combination of Latanoprost and Dorzolamide
The fixed combination of latanoprost 0.005% and dorzolamide 2% was used in phase II clinical studies at Alleanza Pharmaceuticals for the treatment of glaucoma in 2013 (NIH, 2014b). The fixed combination of latanoprost (0.0025%) and dorzolamide (2%) will be completed in 2022 (NIH, 2020).
4.5 Fixed Combination of β-blockers and α-2 Adrenergic Agonists
4.5.1 Fixed Combination of Brimonidine and Timolol (Combigan®, Aibeta®)
Efficacy
A fixed combination of brimonidine tartrate 0.2% and timolol 0.5% (Combigan®) was first launched by Canada in 2003. Another fixed combination of the ophthalmic solution composed of 0.1% brimonidine tartrate and 0.5% timolol (Aibeta®) was recently approved in Japan (Suzuki et al., 2020). Many clinical studies showed that BrT-FC had superior IOP lowering effect as well as effects in keeping it under control than their constituent agents (Spaeth et al., 2011; Kim et al., 2016), and it was noninferiority to latanoprost in reducing IOP (Katz et al., 2012). Katz, et al. found that 60.3% of patients treated with BrT-FC achieved diurnal IOP <18 mmHg compared to the 52.0% of patients treated with latanoprost after 12 weeks (Katz et al., 2012).
Safety and AEs
Tolerability and side effects were similar with the constituent parts without additional side effects. The most common local AEs of BrT-FC include eye irritation, dry eye, allergic reactions, lid erythema, lid edema, conjunctival follicles, corneal staining/erosion, and lens opacity (Katz et al., 2012; Kim et al., 2016). Systemic AEs in patients treated with BrT-FC included asthma (Kim et al., 2016).
4.6 Fixed Combination of β-blockers and Carbonic Anhydrase Inhibitors
4.6.1 Fixed Combination of Timolol and Brinzolamide (Azarga®)
Efficacy
BzT-FC was launched in several European countries in 2009. It has the lowest daily cost and best effectiveness in China compared to other treatments (Xu et al., 2020). A prospective study demonstrated that bimatoprost and BzT-FC exert similar IOP lowering effects in patients with POAG at 8 weeks (Akyol et al., 2017). Importantly, BzT-FC maybe an appropriate choice for patients after uneventful phacoemulsification cataract surgery using Viscoat and Provisc, because BzT-FC can significant prevents IOP increase during the first 24 h after surgery (Georgakopoulos et al., 2013). In a clinical trial, when patients are treated with BzT-FC immediately after surgery or do not receive any ocular hypotensive medication, IOP changes from a preoperative value of +6.7 ± 2.98, +5.3 ± 3.26, and +1.4 ± 2.46 mmHg at 6, 12, and 24 h, to a postoperative value of −0.3 ± 2.95, +0.23 ± 3.49, and −1.76 ± 2.83 mmHg (Georgakopoulos et al., 2013).
Safety and AEs
BzT-FC is well tolerated (Rossi et al., 2011), although pulse rate and systolic blood pressure significantly change (Dixit et al., 2020). The frequently observed AEs are blurred vision, eye irritation, eye pain, and foreign body sensation (Sezgin Akcay et al., 2013; Alezzandrini et al., 2014; Aihara et al., 2017).
4.6.2 Fixed Combination of Timolol and Dorzolamide (Cosopt®)
Efficacy
DzT-FC (fixed combination of Timolol 0.5% and Dorzolamide 2%) was the first IOP-lowering fixed combination approved by the US Food and Drug Administration (FDA) in the treatment of POAG and NTG (Konstas et al., 2021). PF-DzT-FC1 (preservative-free fixed combination of Timolol 0.5% and Dorzolamide 2%) and PF-DzT-FC2 (preservative-free fixed combination of Timolol 0.5% and Dorzolamide 1%) have also been launched in the market in recent year. In an open-label 2-center study, patients with NTG received DzT-FC for 12 weeks, and IOP was reduced by 21.7% at the peak drug effect and by 23.9% at 8 h after drug administration (Kim et al., 2014). DzT-FC has the same efficacy as latanoprost monotherapy in newly diagnosed NTG patients, and the difference of IOP reduction was 0.39 mmHg (Lee et al., 2016b). Visual-related quality of life and the Glaucoma Symptom Scale were significantly improved at week 8 when patients switched from DzT-FC to PF-DzT-FC1 (Abegao Pinto et al., 2014), and PF-DzT-FC1 showed a mean IOP reduction of 6.3% more than DzT-FC after 12 weeks (Renieri et al., 2010). Therefore, preservative-free DzT-FC may be a better choice than preservative DzT-FC.
Safety and AEs
DzT-FC does not exert systemic AEs and the most frequent ocular AEs are eye irritation and ocular hyperemia (Kim et al., 2014; Lee et al., 2016b; a). PF-DzT-FC1 improves local tolerability compared to DzT-FC (Renieri et al., 2010; Shedden et al., 2010).
4.6.3 Comparison of the Fixed Combination of β-blockers and Carbonic Anhydrase Inhibitors
Several clinical trials demonstrated that BzT-FC exerts a better IOP-lowering effect (Rossi et al., 2011; Sezgin Akcay et al., 2013). IOP reduction was 6.42–9.74 mmHg (26.09–37.46%) and 8.16–12.41 mmHg (31.19–41.44%), respectively (Sezgin Akcay et al., 2013). But, IOP change from the baseline at 9 AM/11 AM pooled after 8 weeks was similar between BzT-FC (3.3/3.3 mmHg) and DzT-FC (2.9/3.4 mmHg) group (Aihara et al., 2017).
The most common ocular AEs include blurred vision, eye irritation, eye pain, and foreign body sensation (Sezgin Akcay et al., 2013; Aihara et al., 2017). Patients treated with DzT-FC have a significantly higher incidence of ocular irritation (33.3% versus 7.01%), ocular pain (29.8% versus 3.5%), and foreign body sensation (28.07% versus 5.2%) than those treated with BzT-FC, whereas blurred vision was reported more in patients treated with BzT-FC than in those treated with DzT-FC (14% versus 10.5%) (Sezgin Akcay et al., 2013; Aihara et al., 2017).
Generally, both fixed-combination medications represent highly effective without clear superiority of one agent over another; however, patients show more ocular comfortable in BzT-FC group. Comparing to DzT-FC, PF-DzT-FC significantly improved effectiveness and safety, which will be equal to or better than BzT-FC.
4.7 Fixed Combination of α-2 Adrenergic Agonists and Carbonic Anhydrase Inhibitors
4.7.1 Fixed Combination of Brinzolamide and Brimonidine (Simbrinza®)
Efficacy
BzBr-FC was approved in United States and EU for the reduction of high IOP in patients with OAG and OHT, and it is the fixed-combination ophthalmic suspension to treat glaucoma available without timolol (Greig and Deeks, 2015). BzBr-FC induces a significantly lower mean IOP and exerts a more IOP reduction than either brinzolamide or brimonidine monotherapy (Katz et al., 2013; Nguyen et al., 2013; Realini et al., 2013; Aung et al., 2014). Importantly, BzBr-FC can significant lower IOP in patients after phacoemulsification cataract surgery. The mean change in postoperative IOP at 6, 12, and 24 h was −0.12, −1.12, and −1.89 mmHg in patients treated with BzBr-FC versus +3.85, +3.46, and +0.85 mmHg in patients of the control group (Georgakopoulos et al., 2020). BzBr-FC 3 times daily exerts a significantly more IOP reduction during the nocturnal period and the entire 24-h period compared to timolol 0.05% twice daily, and IOP lowering effect during the diurnal period is similar in both treatments (Seibold et al., 2017).
Safety and AEs
BzBr-FC is security with no serious AEs and other safety variables appeared (Katz et al., 2013), which also has no significant changes in visual acuity, anterior or posterior segment examination, pachymetry, and perimetry are observed (Realini et al., 2013). The most common AEs are blurred vision, dysgeusia, ocular hyperemia, dry mouth, and eye allergy (Katz et al., 2013; Realini et al., 2013; Aung et al., 2014; Weinreb et al., 2019).
4.8 Fixed-Combination of α2-Adrenoceptor Agonists and Rho-Kinase Inhibitors
4.8.1 Fixed-Concomitant Brimonidine/Ripasudil
BrRi-FC (K-232) has been used in phase III clinical trials for the treatment of POAG and OHT since 2020. At present, no new reports on this topic are available.
4.9 Comparison of Topical Fixed Combination of Two Agents
4.9.1 Comparison of the Fixed Combination of Prostaglandin F2a Analogs and Timolol With Carbonic Anhydrase Inhibitors and Timolol
Several clinical trials demonstrated that the fixed combination of prostaglandin F2a analogs and timolol has a greater IOP lowering effect than the fixed combination of carbonic anhydrase inhibitors and timolol (Eren et al., 2012; Babic et al., 2013). The mean diurnal IOP/peak IOP reduction is greater after the use of LT-FC (8.79 mmHg/7.4 mmHg) than after DzT-FC (7.79 mmHg/5.19 mmHg) at week-6 (Eren et al., 2012). The incidence of reported AEs is statistically lower after LT-FC therapy (Eren et al., 2012). A total of 86.7% of patients who received TrT-FC show a IOP reduction>25% compared with the 76.9% of patients treated with DzT-FC after 3 months (Babic et al., 2013). The most frequent ocular AEs in patients treated with TrT-FC are hyperemia (50%), blurred vision, and pruritus (6.7%), but dry eye sensation (30.8%) and foreign body sensation (23.1%) are more reported in patients treated with DzT-FC (Babic et al., 2013).
Overall, the IOP lowering effect of fixed-combination prostaglandin F2a analogs and timolol is significantly superior to fixed-combination carbonic anhydrase inhibitors and timolol, and both of them have no difference in ocular AEs. But the price of fixed-combination carbonic anhydrase inhibitors and timolol is lower, and patient can accept easily.
4.9.2 Comparison of the Fixed Combination of Prostaglandin F2a Analogs and Timolol With Prostaglandin F2a Analogs and Brimonidine
Both brimonidine tartrate (0.1%) and timolol maleate (0.5%) used as adjunctive therapies to PGAs have similar effective, and the difference is only 0.36 mmHg in IOP reduction (Mizoue et al., 2017). No unexpected AEs were reported during the study, and the highest AEs was conjunctival hyperemia (2.8%) in brimonidine-treated patients and pruritus (4%) in timolol-treated patients (Mizoue et al., 2017).
4.9.3 Comparison of the Fixed Combination of Prostaglandin F2a Analogs and Timolol With Brimonidine and Timolol
When compared directly of LT-FC, BiT-FC, and BrT-FC, no significant difference among them is observed. The reduction in IOP is 6.8 mmHg (32.1%), 6.7 ± 2.8 mmHg (30.2%), and 10.6 mmHg (42.2%) in LT-FC, BiT-FC, and BrT-FC group, respectively (Yavas et al., 2013). Hommer A, et al. also found that LT-FC and BrT-FC are effective in an equal manner in reducing IOP and they have no effect on the blood flow of the optic nerve head and the velocity of the retrobulbar flow (Hommer et al., 2012).
4.9.4 Comparison of the Fixed Combination of Carbonic Anhydrase Inhibitors and Timolol With Brimonidine and Timolol
When compared directly to BrT-FC, BzT-FC shows more effective and tolerated. Patients who switched from BrT-FC to BzT-FC show a mean IOP reduction from the baseline (on BrT-FC) to 3.6 mmHg (17.1%), and 55.3% of patients achieve an IOP <18 mmHg at week 8 (Alezzandrini et al., 2014). BzT-FC is also more effective than BrT-FC in reducing IOP after phacoemulsification surgery, and IOP value after surgery at day 3 and 5 is −1.1 ± 2.70 and −2.19 ± 2.46 mmHg in the BzT-FC-treated patients, and −1.48 ± 2.53 and −1.72 ± 2.64 mmHg in the BrT-FC-treated patients (Balsak et al., 2018).
Multiple clinical trials reported that BrT-FC is better than DzT-FC in reducing IOP, although no statistically significant difference is found (Gulkilik et al., 2011; Feke et al., 2013; Seymenoglu et al., 2015). The side-effect profile of BrT-FC is similar to that of DzT-FC. But the incidence of the burning feeling and foreign body sensation are significantly higher in patients treated with DzT-FC (43 and 28%, respectively) than in patients treated with BrT-FC (19 and 12%, respectively) (Gulkilik et al., 2011).
4.9.5 Comparison of the Fixed Combination of Carbonic Anhydrase Inhibitors and Timolol With Carbonic Anhydrase Inhibitors and Brimonidine
A clinical trial demonstrated that BzBr-FC is an effective and safe alternative β-blocker free fixed combination. A significant difference in mean morning IOP reduction is observed between DzT-FC- (7.0 ± 2.8 mmHg) and BzBr-FC-treated patients (8.4 ± 1.9 mmHg) for 12 weeks, but mean afternoon IOP reduction had no significant difference (Kozobolis et al., 2017). The common reported AEs are similar after both the two treatments (Kozobolis et al., 2017).
5 Topical Non-fixed Combination of Two Agents
5.1 Non-fixed Combination of Prostaglandin F2a Analogs and α-2 Agonists
5.1.1 Non-fixed Combination of Latanoprost and Brimonidine
Efficacy
An important adjunctive (two-medicine) therapy to reduce IOP is represented by the non-fixed combination therapy of brimonidine tartrate (0.1, 0.15, 0.2%) twice daily and latanoprost (0.005%) once daily (Stewart et al., 2004). A multicenter, open-label, prospective evaluation study demonstrated that the adjunctive therapy with brimonidine purite 0.15% lead to an additional mean IOP reduction from a baseline value of 5.8 mmHg (26%) at peak drug effect after the treatment with 0.005% latanoprost and 3.3 mmHg (15%) at trough drug effect after 1 month (Mundorf et al., 2007). LBr-nFC (brimonidine 0.2% and latanoprost 0.005%) exerted a mean IOP reduction to 9.0 mmHg (33.9%) compared to DzT-FC 6.5 mmHg (25.3%) at 12 weeks in patients with pigmentary and pseudoexfoliative glaucoma (Zabriskie and Netland, 2003). Importantly, brimonidine can act as a supplement to therapy also in prevention as a neuroprotective agent (Conti et al., 2021).
Safety and AEs
No serious AEs are reported, and the most common AEs are ocular allergy (n = 2; 4.7%) and foreign body sensation (n = 2; 4.7%) in LBr-nFC-treated patients (Mundorf et al., 2007). While patients treated with LBr-nFC have a higher rate of conjunctival hyperemia than LT-FC-treated patients (Stewart et al., 2004).
5.1.2 Non-fixed Combination of Travoprost and Brimonidine
Efficacy
A comparative clinical trial showed that timolol 0.5% treatment is associated with a significantly greater reduction in IOP compared with brimonidine 0.2% when added to travoprost 0.004%. The mean IOP reduction is 3.9 mmHg for timolol and 2.3 mmHg for brimonidine and the IOP reduction is 20.2 and 13.4% on day 28, respectively (Reis et al., 2006).
Safety and AEs
No systemic AEs are reported, and slight occasional conjunctival hyperemia is the only AEs in TrBr-nFC-treated patients (Reis et al., 2006).
5.2 Non-fixed Combination of Prostaglandin F2a Analogs and Carbonic Anhydrase Inhibitors
5.2.1 Non-fixed Combination of Latanoprost and Brinzolamide
Efficacy
Several randomized clinical trials reported that the non-fixed combination of latanoprost 0.005% and brinzolamide 1% exerts a better IOP reducing effect than latanoprost monotherapy (Shoji et al., 2005; Nakamoto and Yasuda, 2007; Nakano et al., 2016). A comparative study demonstrated that LBz-nFC provides a more sustained IOP lowering effect than LT-nFC. LBz-nFC reduces both daytime and nighttime IOP, whereas LT-nFC only reduced IOP during daytime, with little effect on the nighttime value (Liu et al., 2009).
Safety and AEs
LBz-nFC represents a safe and effective treatment with no serious AEs reported during the observation period (Shoji et al., 2005). No significant difference in corneal endothelial cell density, systolic, diastolic, mean blood pressures, and pulse rates is observed before and after LBz-nFC administration (Nakamoto and Yasuda, 2007; Miura et al., 2008).
5.2.2 Non-fixed Combination of Bimatoprost and Dorzolamide
Efficacy
Non-fixed combination of 0.03% bimatoprost once in the morning and 2% dorzolamide twice daily in patients with POAG exerts an additional hypotensive effect and reduces vascular resistance in the ophthalmic artery compared to bimatoprost monotherapy (Stankiewicz et al., 2010; Stankiewicz et al., 2011). However, BiDz-nFC led to a significant IOP lowering effect only at 4:00 h time point and show a lower IOP fluctuation compared to bimatoprost (4.6 mmHg versus 6.0 mmHg) (Stankiewicz et al., 2010).
Safety and AEs
No serious AEs are reported during study and systemic hypotension is the most common AEs in patients with BiDz-nFC (Stankiewicz et al., 2010).
5.3 Non-fixed Combination of β-blockers and α2-Adrenoceptor Agonists
5.3.1 Non-fixed Combination of Betaxolol and Brimonidine
Efficacy
Non-fixed combination of 0.2% brimonidine tartrate and 0.5% betaxolol twice per day exerts statistically greater IOP reduction than their respective monotherapies (Chi et al., 2013). A comparative clinical trial revealed that the decrease IOP rate is 13.8–21.2% in patients treated with betaxolol, 19.8%–25.5% in patients treated with brimonidine and 22.2–33.2% in patients treated with BeBr-nFC after 8-weeks treatment (Chi et al., 2013).
Safety and AEs
No systemic AEs or statistical significant difference are found among patients treated with BeBr-nFC and those treated with monotherapies (Chi et al., 2013). The reported AEs include ocular foreign bodies, irritation, dizziness, headache, fatigue, and dryness of the mouth and nose (Chi et al., 2013).
5.4 Non-fixed Combination of β-blockers and Carbonic Anhydrase Inhibitors
5.4.1 Non-fixed Combination of Betaxolol and Brinzolamide
Efficacy
DzT-FC is more effective after 24 h exposure compared to the effect of the fixed combination of 0.5% betaxolol and 1% brinzolamide, and the rate of IOP reduction is 18–24% and 14–19% in DzT-FC and BeBz-nFC-treated patients, respectively (Brubaker et al., 2000).
Safety and AEs
Anticipated AEs of BeBz-nFC are consistent with the AEs after betaxolol and brinzolamide monetherapy, and the incidence of systemic AEs does not increase (Brubaker et al., 2000).
5.5 Non-fixed Combination of α-2 Agonists and Carbonic Anhydrase Inhibitors
5.5.1 Non-fixed Combination of Brimonidine and Dorzolamide
Efficacy
A randomized, double-masked study demonstrated that the non-fixed combination of dorzolamide 2% three times per day and 0.2% brimonidine tartrate two times per day exerts a greater reduction of AH flow, and BrDz-nFC has better IOP lowering effect than dorzolamide alone and brimonidine alone (Ermis et al., 2002; Tsukamoto and Larsson, 2004). The reduction in AH flow is 28.2 ± 18.0%, 19.3 ± 22.0%, and 37.2 ± 20.6% in brimonidine, dorzolamide and BrDz-nFC-treated patients, respectively and IOP reduction is 11.6 ± 10.1%, 8.5 ± 14.1%, and 17.9 ± 16.5%, respectively (Ermis et al., 2002; Tsukamoto and Larsson, 2004). Oztürk F, et al. found that non-fixed combination of timolol maleate and dorzolamide (TDz-nFC) is more effective in lowering IOP than BrDz-nFC, and the mean IOP reduction is 6.8 and 5.6 mmHg, respectively, after 1 year of treatment (Ozturk et al., 2005).
Safety and AEs
The most common ocular and systemic AEs are mild and include stinging/burning, itching, conjunctival hyperemia, irritation, dry mouth, fatigue, and bitter taste. No serious AEs are reported (Ermis et al., 2002; Ozturk et al., 2005).
5.6 Non-fixed Combination of Prostaglandin F2a Analogs and Rho-Kinase Inhibitors
5.6.1 Non-fixed Combination of Latanoprost and Ripasudil
Efficacy
A clinical trial showed that the lowering effect of IOP exerted by LRi-nFC is superior than that exerted by latanoprost monotherapy, and mean IOP reduction in LRi-nFC and latanoprost-treated patients is 2.2 and 1.8 mmHg respectively, before instillation (9 AM) and 3.2 and 1.8 mmHg respectively, after instillation (11 AM) (Tanihara et al., 2015).
Safety and AEs
No new AEs are observed when ripasudil is added to latanoprost compared to the old AEs exerted by latanoprost, and conjunctival hyperemia is the most frequent AE in patients treated with LRi-nFC and ripasudil (55.9% vs 8.7%) (Tanihara et al., 2015).
5.7 Non-fixed Combination of β-blockers and Rho-Kinase Inhibitors
5.7.1 Non-fixed Combination of Timolol and Ripasudil
Efficacy
A clinical trial demonstrated that TRi-nFC leads to a more IOP reduction than timolol monotherapy at 9 AM (before instillation) (2.4 versus 1.5 mmHg) and 11 AM (after instillation) (2.9 and 1.3 mmHg) (Tanihara et al., 2015).
Safety and AEs
No new AEs are reported when ripasudil is combined with timolol compared to the old AEs exerted by timolol, and the most common AE is conjunctival hyperemia in patients treated with TRi-nFC with 65.4% compared to 5.8% in the timolol-treated patients (Tanihara et al., 2015).
5.8 Comparison of Topical Non-fixed Combination of Two Agents
Most clinical trials demonstrated that the non-fixed combination of prostaglandin F2a analogs and carbonic anhydrase inhibitors exerts a greater IOP-lowering effect than the non-fixed combination of prostaglandin F2a analogs and alpha-2 agonists, and both of them have similar systemic and ocular AEs (Konstas et al., 2005; Feldman et al., 2007). A 3-months randomized clinical trial demonstrated that 79 patients who were treated with brinzolamide and 84 patients who were treated with brimonidine twice-daily as adjunctive therapy to travoprost have a diurnal IOP reduction to 2.8 and 2.1 mmHg, and the reduction of diurnal IOP ≥15% is 40 and 27.8% in TrBz-nFC- and TrBr-nFC-treated patients, respectively (Feldman et al., 2007). Konstas, et al. reported that the mean diurnal IOP reduction is 2.2 mmHg in LDz-nFC-treated patients versus 2.1 mmHg in LBr-nFC-treated patients (Konstas et al., 2005). Patients treated with LDz-nFC and LBr-nFC had similar ocular and systemic AEs, while a statistically more incidence of conjunctival hyperemia and bitter taste is observed in patients treated with LDz-nFC (n = 9 and 8) than in those treated with LBr-nFC (n = 3 and 0) (Konstas et al., 2005).
6 Topical Fixed Combination of Three Agents
6.1 Fixed Combination of β-blockers, α-2 Adrenergic Agonists, and Carbonic Anhydrase Inhibitors
6.1.1 Fixed Combination of Dorzolamide, Timolol, and Brimonidine (Krytantek Ofteno®)
Efficacy
TDzBr-FC has been launched in Mexico since 2007 for the treatment of glaucoma and OHT, since it decreases AH production (Nocentini and Supuran, 2019; Cimolai, 2020; Ghorai et al., 2020; Shukla et al., 2020; Supuran, 2020), and increases TM and the uveoscleral outflow (Nocentini and Supuran, 2019; Cimolai, 2020; Shukla et al., 2020). PRO-122 is a preservative-free TDzBr-FC (PF-TDzBr-FC) (NIH, 2019b).
However, several clinical studies demonstrated that IOP lowering effect of PRO-122 is not inferior to Krytantek Ofteno (NIH, 2019b). And a phase III comparative study demonstrated that the crossover between PRO-122 and TDzBr-FC does not affect IOP, and the mean IOP reduction is 0.11 mmHg difference from the baseline after 60 days (Gomez-Aguayo et al., 2018; NIH, 2019b). Furthermore, an investigator-masked, crossover study demonstrated that BiT-FC once daily exerts a greater IOP lowering effect than TDzBr-FC twice daily (Garcia-Lopez et al., 2014).
Safety and AEs
The incidence of AEs is similar between PF-TDzBr-FC and TDzBr-FC-treated patients, and ocular burning is the one with the highest incidence among the eye disorders in both groups (Gomez-Aguayo et al., 2018; NIH). The cup/disk ratio and visual field are not statistically different in BiT-FC and TDzBr-FC-treated patients (Garcia-Lopez et al., 2014).
6.2 Fixed Combination of Prostaglandin F2a Analogs, β-blockers, and α-2 Adrenergic Agonists
6.2.1 Fixed Combination of Bimatoprost, Timolol, and Brimonidine
Efficacy
Two comparative clinical studies demonstrated that TBiBr-FC has clinically and statistically significantly superior IOP lowering effects than BrT-FC (Hartleben et al., 2017; Belfort et al., 2020). A phase 3 study conducted in Mexico and Colombia revealed that the mean IOP changes from baseline are 10.03 and 9.18 mmHg, and percentage of IOP ≤13 mmHg is 33.7 and 14.8% for TBiBr-FC and BrT-FC respectively, after 12 weeks (Hartleben et al., 2017). Another phase III study conducted in Brazil demonstrated that the mean IOP reduction at 12 weeks is 10.45 mmHg in patients treated with TBiBr-FC and 8.28 mmHg in those treated with BrT-FC. A total of 28.9, 36.8, and 71.1% of patients treated with TBiBr-FC achieved levels of IOP ≤13, ≤14, and ≤16 mmHg compared to 15.7, 22.9, and 55.4% in patients treated with BrT-FC (Belfort et al., 2020). TBiBr-FC maybe a new promising fixed-combination three agents for patients whose IOP cannot be inadequate by combination two agents, but more clinical trials are needed to verify the effectiveness and safety.
Safety and AEs
The most common AEs are ocular side effects and none are serious in TBiBr-FC and BrT-FC-treated patients (Hartleben et al., 2017; Belfort et al., 2020). The most common AEs include conjunctival hyperemia, eye irritation, allergic blepharitis, allergic conjunctivitis, and dry eye. Conjunctival hyperemia, eye irritation, and eye pruritus are statistically significant in patients treated with TBiBr-FC than those treated with BrT-FC (Hartleben et al., 2017; Belfort et al., 2020).
7 Topical Non-fixed Combination of Three Agents
Topical non-fixed combinations of three agents are usually composed of a fixed combination of two agents and a monotherapy agent, and they are used in patients whose IOP cannot be well controlled by combination two agents. Importantly, there is no a safe and reliable fixed-combination three agents in widespread use today.
7.1 Non-fixed Combination of Prostaglandin F2a Analogs, β-blockers, and α-2 Adrenergic Agonists
7.1.1 Non-fixed Combination of Brimonidine, Timolol and Travoprost
Efficacy
A clinical trial demonstrated that BzT-FC plus travoprost 0.004% (TBzTr-nFC) has a greater efficacy in late afternoon and during night and better 24-h IOP control than the one exerted by TBrTr-nFC (Konstas et al., 2013a). The reduction of 24-h IOP is 3.0 mmHg (14%) exerted by TBzTr-nFC and 2.1 mmHg (10%) exerted by TBrTr-nFC. TBzTr-nFC decreases IOP better than TBrTr-nFC at all the 3 selected time points (18:00, 22:00, and 02:00), with differences ranging from 1.0 to 1.8 mmHg, and no difference is observed among the other 3 time points (06:00, 10:00, and 14:00) (Konstas et al., 2013a).
Safety and AEs
The ocular and systemic AEs are more frequently reported after the use of the non-fixed combination of three agents compared with travoprost monotherapy (Konstas et al., 2013a). Transient blurred vision occurred significantly more frequently in TBzTr-nFC-treated patients than in those treated with BzT-FC. The incidence of itchiness, hyperemia, and fatigue are more in the TBrTr-nFC-treated patients, and other AEs were similar in the two treatments (Konstas et al., 2013a).
7.1.2 Non-fixed Combination of Brimonidine, Timolol and Latanoprost
Efficacy
A 12-weeks, randomized, multicenter study demonstrated that LTBr-nFC exerts a greater IOP lowering effect than LT-nFC in patients whose IOP is not adequately controlled by latanoprost 0.005% alone (Fechtner et al., 2011). The results showed an additional IOP reduction of 8.3 mmHg (35.5%) and 6.2 mmHg (27.0%) at 10 am, the incidence of IOP less than 18 mmHg at both peak and trough measurements is 59.6% versus 42.6%, and IOP reduction over 20% is 72.3 and 57.5% in LTBr-nFC and LT-nFC-treated patients, respectively (Fechtner et al., 2011).
Safety and AEs
No statistically significant difference in overall AEs is found between patients treated with LTBr-nFC and LT-nFC, and the most common AEs are ocular allergy in the patients treated with LTBr-nFC (3.9%) and punctate keratitis in those treated with LT-nFC (2.9%) (Fechtner et al., 2011).
7.2 Non-fixed Combination of Prostaglandin F2a Analogs, β-blockers, and Carbonic Anhydrase Inhibitors
7.2.1 Non-fixed Combination of Brinzolamide, Timolol and Travoprost
Efficacy
Brinzolamide 1% twice daily plus TrT-FC (TBrTr-nFC) has a better IOP lowering effect than TrT-FC once daily (Hollo and Kothy, 2008; Goldberg et al., 2012). Goldberg I, et al. reported that brinzolamide reduces IOP by 1.1–1.5 mmHg (6–9%) when added to TrTFC, and an additional IOP reduction is observed at diurnal IOP, specifically at 08:00 and 16:00 o’clock (Goldberg et al., 2012). Another comparative study demonstrated that the average decrease of diurnal IOP is 6.2 mmHg (21.8%) after the use of travoprost, 9.3 mmHg (32.6%) after the use of TrT-FC, and 11.2 mmHg (39.3%) after the use of TBrTr-nFC (Hollo and Kothy, 2008).
Safety and AEs
No serious AEs are reported during the study (Hollo and Kothy, 2008) and common AEs including conjunctival hyperemia, eyelashes, cataract, blurring, superficial punctate keratitis, conjunctival redness, reduced visual acuity, and itching are no statistically different between patients treated with TBrTr-nFC and those treated with TrT-FC (Hollo and Kothy, 2008).
7.2.2 Non-fixed Combination of Dorzolamide, Timolol and Latanoprost
Efficacy
Most clinical studies reported that triple non-fixed combination therapy of 2% dorzolamide, 0.5% timolol, and 0.002% latanoprost has a superior IOP lowering effect (Akman et al., 2005; Lesk et al., 2008; Hatanaka et al., 2010; Konstas et al., 2017). Konstas AG, et al. found that LTDz-nFC protects IOP from fluctuation and further more IOP reduction than latanoprost, DzT-FC, and LT-FC, with a mean 24 h-IOP of 22.1 mmHg, 19.9 mmHg, 19.5 mmHg, and 16.5 mmHg, and a IOP fluctuation of 4.7 mmHg, 4.4 mmHg, 4.1 mmHg, and 3.6 mmHg in latanoprost, DzT-FC, LT-FC, and LTDz-nFC-treated patients (Konstas et al., 2017). A 4-weeks, open-label controlled clinical trial demonstrated that the mean baseline diurnal IOP is 14.44 ± 3.03 mmHg in patients treated with LTDz-nFC, which was significantly lower than that in patients treated with latanoprost monotherapy (15.60 ± 3.09 mmHg) (Hatanaka et al., 2010). Another nonrandomized interventional study revealed that the mean IOP decrease is 6.3 and 5.8 mmHg in LTDz-nFC and DzT-FC-treated patients, respectively, and the rate of IOP reduction >20% is 66.4 and 52.9% respectively, after 12 weeks (Lesk et al., 2008). Akman A, et al. found that IOP lowering effect of latanoprost 0.005% and brimonidine 0.2% as adjunctive therapies to TDz-FC is comparable (Akman et al., 2005); the mean reduction of peak/trough IOP is 5.2/3.5 mmHg after LTDz-nFC treatment and 4.6/2.9 mmHg after TBrDz-nFC treatment, and the rate of the peak/trough IOP reduction over 15% is 77.1%/40 and 77.7%/41.7%, respectively (Akman et al., 2005).
Safety and AEs
LTDz-nFC provides a safety profile consistent with that of its individual components. The majority of AEs are mild or moderate (Lesk et al., 2008), and included burning/stinging, superficial punctate keratitis, watering, itchiness, conjunctivitis hyperemia, dry eye, ocular irritation, dry mouth, foreign body sensation, and blurred vision (Konstas et al., 2008).
7.2.3 Non-fixed Combination of Dorzolamide, Timolol and Tafluprost
Efficacy
A comparative, crossover study demonstrated that PF-tafluprost 0.0015% plus DzT-FC was compared to PF-tafluprost and latanoprost monotherapies, and PF-TDzTf-nFC is statistically and clinically more effective than both the compounds used as monotherapy. The daytime/nighttime IOP is lower in PF-TDzTf-nFC-treated patients (17.0/17.6 mmHg) than in PF-tafluprost-treated patients (2.3/21.5 mmHg) and latanoprost monotherapy-treated patients (22.3/22.1 mmHg). However, PF-tafluprost (3.9 ± 1.3 mmHg) exerts a lower 24-h IOP fluctuation compared to PF-TDzTf-nFC (4.4 ± 2.3 mmHg) and latanoprost (4.6 ± 1.6 mmHg) (Konstas et al., 2017).
Safety and AEs
The rates of AEs in PF-TDzTf-nFC and latanoprost-treated patients are similar, and they are were more than those in PF-tafluprost-treated patients. Burning (6.9%), stinging (20.9%), and bitter taste (11.6%) are significantly more common in patients treated with PF-TDzTf-nFC than in those treated with PF-tafluprost monotherapy (Konstas et al., 2017).
7.2.4 Comparison of Non-fixed Combination of Prostaglandin F2a Analogs, β-blockers, and Carbonic Anhydrase Inhibitors
When patients received brinzolamide 1% (2 times a day) or dorzolamide 1% (3 times a day) plus a fixed combination therapy of latanoprost and a BB, the mean IOP reduction is 1.9 and 1.8 mmHg at week 8, respectively (Tsukamoto et al., 2005). The common AEs observed in this study include ocular irritation and blurred vision. Dorzolamide causes more intense ocular irritation than brinzolamide (74% vs 16%), and no significant difference in blurred vision is observed between the two groups (52% vs 37%) (Tsukamoto et al., 2005).
Both brinzolamide and dorzolamide added to a fixed-combination latanoprost-β-blockers show a similar IOP lowering effect, but brinzolamide (2 times a day) presents a better patient compliance and ocular comfortable than dorzolamide (3 times a day), which is more meaningful for glaucoma patients.
7.3 Non-fixed Combination of Prostaglandin F2a Analogs, α-2 Adrenergic Agonists and Carbonic Anhydrase Inhibitors
7.3.1 Non-fixed Combination of Brinzolamide, Brimonidine and Travoprost
Efficacy
A phase 4 clinical trial involving 233 patients randomly treated with BzBr-FC 3 times per day plus travoprost 0.004% once daily (BzBrTr-nFC) or vehicle plus travoprost 0.004% for 6 weeks revealed that the mean diurnal IOP change from travoprost-treated baseline is significant more in BzBrTr-nFC-treated patients (5.0 mmHg, 21.9%) than in travoprost-treated patients (2.0 mmHg, 8.8%) (Feldman et al., 2016).
Safety and AEs
The incidence of AEs is higher in patients treated with BzBrTr-nFC compared to those treated with travoprost (30.8% 14.7%), and the most common AE in BzBrTr-nFC group is conjunctival hyperemia (Feldman et al., 2016).
7.3.2 Comparison of Non-fixed Combination of Prostaglandin F2a Analogs, α-2 Adrenergic Agonists, and Carbonic Anhydrase Inhibitors
Several clinical trials reported that BzBr-FC as an adjunct to PGA exerts an additional IOP lowering effect (Feldman et al., 2016; Topouzis et al., 2021). The percentage of mean IOP reduction is 24.7% in patients treated with BzBr-FC plus PGA and 8.2% in those treated with vehicle plus PGA at week 6 (Feldman et al., 2016). 60.0% of patients achieving the goal of IOP ≤18 mmHg after BzBr-FC plus PGA treatment versus 20.7% of patients treated with vehicle plus PGA (Topouzis et al., 2021).
AEs in BzBr-FC plus PGA-treated patients (23.2%) are higher than those in patients treated with PGA (4.3%) (Topouzis et al., 2021), but it’s perfectly acceptable. The highest incidence of ocular and non-ocular AEs occurring in patients treated with BzBr-FC plus PGA are ocular hyperemia (5.3%) and dry mouth (5.3%) (Topouzis et al., 2021). The other common AEs include eye irritation, eye pruritus, and ocular hyperemia (Topouzis et al., 2021).
8 Discussion and Opinion
Nowadays, multiple classes of topical eye drops are available for the treatment of glaucoma, but it is still a challenge to establish the most reasonable and optimal prescription for every patient because systematically extensive clinical studies on different classes of medications are still limited. In this review, the most recent articles on topical anti-hypertensive ophthalmic agents and their characteristics were summarized, which will provide a reference for glaucoma patient care. Monotherapy is usually used for initial treatment, which cannot usually reduce IOP to a physiologically level for long time. Therefore, combination multiple agents play a vital role for long-term control of IOP. Fixed combination and non-fixed combination of two agents results in similar IOP-lowering effect, which are also superior to monotherapy, and no significant difference in local and systematic AEs was observed among them. If combination two agents fail to reduce IOP sufficiently, the second drug can be replaced or a third medication can be added to the fixed combination, or a fixed-combination of three agents can be used. Now, fixed combination three agents, such as TDzBr-FC, cannot be widely available in various countries due to its obvious increase of AEs and no significant improvement in IOP, and TBiBr-FC is still being in clinical trial. Research and development of new, reliable, effective and safe fixed combination three agents is a trend of great importance. Netarsudil is a new launched Rho-kinase inhibitor, which provides a better neuroprotection effect superior to other eye drops. Rho-kinase inhibitors might be combined with β-blockers and be suitable for those who are not candidates for PGAs. Furthermore, another potential fixed combination including latanoprost, netarsudil and timolol, may provide better IOP lowering effect and patient compliance, lower preservative exposure and the incidence of side effects by targeting more mechanisms with once per day comparing to traditional marketed medicines.
The current review revealed that the use of a fixed combination of topical anti-hypertensive ophthalmic agents is recommended as early as possible in patients with glaucoma, to better control its development and decrease the damage of the optic nerve. Fixed combination agents not only provide a better effect than monotherapy but also result in a better convenience and tolerance, and less AEs and ocular discomfortable than non-fixed combination. Apart from the effect and safety, local availability and costs are also important factors to consider. Thus, fixed combination agents are a promising choice in the treatment of glaucoma and ocular hypertension.
9 Conclusion
Glaucoma is still one of the most common causes of irreversible vision loss, and the reduction and control of IOP are necessary to decrease the rate of visual field deterioration to treat glaucoma. Despite numerous surgical approaches and classes of IOP-lowering drugs available, glaucoma remains a challenging chronic disease. Many new emerging strategies including medicines and surgery are developed for the treatment of glaucoma by affecting different pathways, providing a new choice to patients and offering additivity and complementary to current therapeutics, although they need long-term rigorous verification of safety and efficacy. The current recent studies revealed that fixed combination therapy with reliable safety and effectiveness is significantly important for patients with glaucoma and effective on glaucoma, and it’s of great importance to develop fixed combination agents with a high effect for a better control of glaucoma.
Author Contributions
TZ and QJ designed and revised this manuscript. TW wrote the manuscript. LC polished language in English. All of the authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abegão Pinto, L., Vandewalle, E., Gerlier, L., Stalmans, I., and Cosopt, U. D. S. S. G. (2014). Improvement in Glaucoma Patient Quality of Life by Therapy Switch to Preservative-free Timolol/dorzolamide Fixed Combination. Ophthalmologica 231 (3), 166–171. doi:10.1159/000356468
Addis, V. M., and Miller-Ellis, E. (2018). Latanoprostene Bunod Ophthalmic Solution 0.024% in the Treatment of Open-Angle Glaucoma: Design, Development, and Place in Therapy. Clin. Ophthalmol. 12, 2649–2657. doi:10.2147/OPTH.S156038
Aihara, M., Adachi, M., Matsuo, H., Togano, T., Fukuchi, T., Sasaki, N., et al. (2017). Additive Effects and Safety of Fixed Combination Therapy with 1% Brinzolamide and 0.5% Timolol versus 1% Dorzolamide and 0.5% Timolol in Prostaglandin-Treated Glaucoma Patients. Acta Ophthalmol. 95 (8), e720–e726. doi:10.1111/aos.13401
Aihara, M., Lu, F., Kawata, H., Iwata, A., Liu, K., Odani-Kawabata, N., et al. (2019). Phase 2, Randomized, Dose-Finding Studies of Omidenepag Isopropyl, a Selective EP2 Agonist, in Patients with Primary Open-Angle Glaucoma or Ocular Hypertension. J. Glaucoma 28 (5), 375–385. doi:10.1097/IJG.0000000000001221
Aihara, M., Lu, F., Kawata, H., Iwata, A., Odani-Kawabata, N., and Shams, N. K. (2020a). Omidenepag Isopropyl versus Latanoprost in Primary Open-Angle Glaucoma and Ocular Hypertension: The Phase 3 AYAME Study. Am. J. Ophthalmol. 220, 53–63. doi:10.1016/j.ajo.2020.06.003
Aihara, M., Ropo, A., Lu, F., Kawata, H., Iwata, A., Odani-Kawabata, N., et al. (2020b). Intraocular Pressure-Lowering Effect of Omidenepag Isopropyl in Latanoprost Non-/low-responder Patients with Primary Open-Angle Glaucoma or Ocular Hypertension: the FUJI Study. Jpn. J. Ophthalmol. 64 (4), 398–406. doi:10.1007/s10384-020-00748-x
Akman, A., Cetinkaya, A., Akova, Y. A., and Ertan, A. (2005). Comparison of Additional Intraocular Pressure-Lowering Effects of Latanoprost vs Brimonidine in Primary Open-Angle Glaucoma Patients with Intraocular Pressure Uncontrolled by Timolol-Dorzolamide Combination. Eye (Lond) 19 (2), 145–151. doi:10.1038/sj.eye.6701428
Akyol, N., Kalkisim, A., Turk, A., Kola, M., and Imamoglu, H. I. (2017). Evaluation of the Effects on Choroidal Thickness of Bimatoprost 0.03% versus a Brinzolamide 1.0%/timolol Maleate 0.5% Fixed Combination. Cutan. Ocul. Toxicol. 36 (4), 397–403. doi:10.1080/15569527.2017.1315128
Alezzandrini, A., Hubatsch, D., and Alfaro, R. (2014). Efficacy and Tolerability of Fixed-Combination Brinzolamide/timolol in Latin American Patients with Open-Angle Glaucoma or Ocular Hypertension Previously on Brimonidine/timolol Fixed Combination. Adv. Ther. 31 (9), 975–985. doi:10.1007/s12325-014-0145-5
Angeli, A., and Supuran, C. T. (2019). Prostaglandin Receptor Agonists as Antiglaucoma Agents (A Patent Review 2013 - 2018). Expert Opin. Ther. Pat 29 (10), 793–803. doi:10.1080/13543776.2019.1661992
Aptel, F., and Denis, P. (2011). Balancing Efficacy and Tolerability of Prostaglandin Analogues and Prostaglandin-Timolol Fixed Combinations in Primary Open-Angle Glaucoma. Curr. Med. Res. Opin. 27 (10), 1949–1958. doi:10.1185/03007995.2011.613923
Asrani, S., Bacharach, J., Holland, E., McKee, H., Sheng, H., Lewis, R. A., et al. (2020). Fixed-Dose Combination of Netarsudil and Latanoprost in Ocular Hypertension and Open-Angle Glaucoma: Pooled Efficacy/Safety Analysis of Phase 3 MERCURY-1 and -2. Adv. Ther. 37 (4), 1620–1631. doi:10.1007/s12325-020-01277-2
Asrani, S., Robin, A. L., Serle, J. B., Lewis, R. A., Usner, D. W., Kopczynski, C. C., et al. (2019). Netarsudil/Latanoprost Fixed-Dose Combination for Elevated Intraocular Pressure: Three-Month Data from a Randomized Phase 3 Trial. Am. J. Ophthalmol. 207, 248–257. doi:10.1016/j.ajo.2019.06.016
Aung, T., Laganovska, G., Hernandez Paredes, T. J., Branch, J. D., Tsorbatzoglou, A., and Goldberg, I. (2014). Twice-daily Brinzolamide/brimonidine Fixed Combination versus Brinzolamide or Brimonidine in Open-Angle Glaucoma or Ocular Hypertension. Ophthalmology 121 (12), 2348–2355. doi:10.1016/j.ophtha.2014.06.022
Babic, N., Andreic, V., Miljkovic, A., Grkovic, D., and Jovanovic, P. (2013). Comparison of the Efficacy and Safety of Fixed Combination Travoprost/timolol and Dorzolamide/timolol in Patients with Primary Open-Angle Glaucoma and Ocular Hypertension. Srp Arh Celok Lek 141 (7-8), 441–446. doi:10.2298/sarh1308441b
Babic, N. (2015). Fixed Combinations of Glaucoma Medications. Srp Arh Celok Lek 143 (9-10), 626–631. doi:10.2298/sarh1510626b
Balsak, S., Kaydu, A., Erdem, S., Fuat Alakus, M., and Ozkurt, Z. G. (2018). Brimonidine-timolol versus Brinzolamide-Timolol for Treatment of Elevated Intraocular Pressure after Phacoemulsification Surgery. Int. Ophthalmol. 38 (4), 1583–1589. doi:10.1007/s10792-017-0626-z
Bejan-Angoulvant, T., and Angoulvant, D. (2020). Update on Beta Blockers in 2020. Rev. Med. Interne 41 (11), 741–747. doi:10.1016/j.revmed.2020.04.007
Belfort, R., Paula, J. S., Lopes Silva, M. J., Della Paolera, M., Kim, T., Chen, M. Y., et al. (2020). Fixed-combination Bimatoprost/Brimonidine/Timolol in Glaucoma: A Randomized, Masked, Controlled, Phase III Study Conducted in Brazil☆. Clin. Ther. 42 (2), 263–275. doi:10.1016/j.clinthera.2019.12.008
Berlin, M. S., Rowe-Rendleman, C., Ahmed, I., Ross, D. T., Fujii, A., Ouchi, T., et al. (2016). EP3/FP Dual Receptor Agonist ONO-9054 Administered Morning or Evening to Patients with Open-Angle Glaucoma or Ocular Hypertension: Results of a Randomised Crossover Study. Br. J. Ophthalmol. 100 (6), 843–847. doi:10.1136/bjophthalmol-2015-307000
Berrino, E., and Supuran, C. T. (2019). Rho-kinase Inhibitors in the Management of Glaucoma. Expert Opin. Ther. Pat 29 (10), 817–827. doi:10.1080/13543776.2019.1670812
Bhattaccharjee, S. A., Murnane, K. S., and Banga, A. K. (2020). Transdermal Delivery of Breakthrough Therapeutics for the Management of Treatment-Resistant and post-partum Depression. Int. J. Pharm. 591, 120007. doi:10.1016/j.ijpharm.2020.120007
Bourne, R. R. A., Kaarniranta, K., Lorenz, K., Traverso, C. E., Vuorinen, J., and Ropo, A. (2019). Changes in Ocular Signs and Symptoms in Patients Switching from Bimatoprost-Timolol to Tafluprost-Timolol Eye Drops: an Open-Label Phase IV Study. BMJ Open 9 (4), e024129. doi:10.1136/bmjopen-2018-024129
Brubaker, R. F., Ingram, C. J., Schoff, E. O., and Nau, C. B. (2000). Comparison of the Efficacy of Betaxolol-Brinzolamide and Timolol-Dorzolamide as Suppressors of Aqueous Humor Flow in Human Subjects. Ophthalmology 107 (2), 283–287. doi:10.1016/s0161-6420(99)00044-5
Carradori, S., Mollica, A., De Monte, C., Ganese, A., and Supuran, C. T. (2015). Nitric Oxide Donors and Selective Carbonic Anhydrase Inhibitors: a Dual Pharmacological Approach for the Treatment of Glaucoma, Cancer and Osteoporosis. Molecules 20 (4), 5667–5679. doi:10.3390/molecules20045667
Cavet, M. E., and DeCory, H. H. (2018). The Role of Nitric Oxide in the Intraocular Pressure Lowering Efficacy of Latanoprostene Bunod: Review of Nonclinical Studies. J. Ocul. Pharmacol. Ther. 34 (1-2), 52–60. doi:10.1089/jop.2016.0188
Chamard, C., Villain, M., Bron, A., Causse, A., Bentaleb, Y., Pelen, F., et al. (2020). Prevalence of Unknown Ocular Hypertension, Glaucoma Suspects, and Glaucoma in Patients Seen in an Ophthalmology Center in France. Ophthalmic Res. 63 (3), 295–301. doi:10.1159/000504717
Chen, W., Yang, X., Fang, J., Zhang, Y., Zhu, W., and Yang, X. (2020). Rho-Associated Protein Kinase Inhibitor Treatment Promotes Proliferation and Phagocytosis in Trabecular Meshwork Cells. Front. Pharmacol. 11, 302. doi:10.3389/fphar.2020.00302
Chi, W., Li, A., Wang, S., and Zhu, X. (2013). Efficacy of Combined Administration of 0.2% Brimonidine and 0.5% Betaxolol in Treatment of Primary Open Angle Glaucoma and Ocular Hypertension. Eye Sci. 28 (4), 190–194.
Ciancetta, A., and Jacobson, K. A. (2017). Structural Probing and Molecular Modeling of the A₃ Adenosine Receptor: A Focus on Agonist Binding. Molecules 22 (3). doi:10.3390/molecules22030449
Cimolai, N. (2020). A Review of Neuropsychiatric Adverse Events from Topical Ophthalmic Brimonidine. Hum. Exp. Toxicol. 39 (10), 1279–1290. doi:10.1177/0960327120918307
Conlon, R., Saheb, H., and Ahmed, (2017). Glaucoma Treatment Trends: a Review. Can. J. Ophthalmol. 52 (1), 114–124. doi:10.1016/j.jcjo.2016.07.013
Conti, F., Romano, G. L., Eandi, C. M., Toro, M. D., Rejdak, R., Di Benedetto, G., et al. (2021). Brimonidine Is Neuroprotective in Animal Paradigm of Retinal Ganglion Cell Damage. Front. Pharmacol. 12, 705405. doi:10.3389/fphar.2021.705405
Denis, P. (2011). Travoprost/timolol Fixed Combination in the Management of Open-Angle Glaucoma: a Clinical Review. Expert Opin. Pharmacother. 12 (3), 463–471. doi:10.1517/14656566.2011.551007
Diaconita, V., Quinn, M., Jamal, D., Dishan, B., Malvankar-Mehta, M. S., and Hutnik, C. (2018). Washout Duration of Prostaglandin Analogues: A Systematic Review and Meta-Analysis. J. Ophthalmol. 2018, 3190684. doi:10.1155/2018/3190684
Dikopf, M. S., Vajaranant, T. S., and Edward, D. P. (2017). Topical Treatment of Glaucoma: Established and Emerging Pharmacology. Expert Opin. Pharmacother. 18 (9), 885–898. doi:10.1080/14656566.2017.1328498
Dixit, A., Ashish, A., and Sharma, R. (2020). A Comparative Study on Efficacy of Fixed Combination Timolol/brinzolamide versus Travoprost Monotherapy in Drug-Naïve Open-Angle Glaucoma Patients. Ther. Adv. Ophthalmol. 12, 2515841420909666. doi:10.1177/2515841420909666
Doucette, L. P., and Walter, M. A. (2017). Prostaglandins in the Eye: Function, Expression, and Roles in Glaucoma. Ophthalmic Genet. 38 (2), 108–116. doi:10.3109/13816810.2016.1164193
Duggan, S. (2018). Omidenepag Isopropyl Ophthalmic Solution 0.002%: First Global Approval. Drugs 78 (18), 1925–1929. doi:10.1007/s40265-018-1016-1
Eichhorn, M. (2013). Mode of Action, Clinical Profile and Relevance of Carbonic Anhydrase Inhibitors in Glaucoma Therapy. Klin Monbl Augenheilkd 230 (2), 146–149. doi:10.1055/s-0032-1328163
Eren, M. H., Gungel, H., Altan, C., Pasaoglu, I. B., and Sabanci, S. (2012). Comparison of Dorzolamide/timolol and Latanoprost/timolol Fixed Combinations on Diurnal Intraocular Pressure Control in Primary Open-Angle Glaucoma. J. Ocul. Pharmacol. Ther. 28 (4), 381–386. doi:10.1089/jop.2011.0105
Ermis, S. S., Ozturk, F., and Inan, U. U. (2002). The Short-Term IOP-Lowering Effect of Brimonidine 0.2% and Dorzolomide 2% Combination in Primary Open-Angle Glaucoma. Acta Ophthalmol. Scand. 80 (6), 632–634. doi:10.1034/j.1600-0420.2002.800614.x
Fang, Y., Ling, Z., and Sun, X. (2015). Fixed-combination Treatments for Intraocular Hypertension in Chinese Patients - Focus on Bimatoprost-Timolol. Drug Des. Devel Ther. 9, 2617–2625. doi:10.2147/DDDT.S80338
Fechtner, R. D., Harasymowycz, P., Nixon, D. R., Vold, S. D., Zaman, F., Williams, J. M., et al. (2011). Twelve-week, Randomized, Multicenter Study Comparing a Fixed Combination of Brimonidine-Timolol with Timolol as Therapy Adjunctive to Latanoprost. Clin. Ophthalmol. 5, 945–953. doi:10.2147/OPTH.S19999
Fechtner, R. D., Myers, J. S., Hubatsch, D. A., Budenz, D. L., and DuBiner, H. B. (2016). Ocular Hypotensive Effect of Fixed-Combination Brinzolamide/brimonidine Adjunctive to a Prostaglandin Analog: a Randomized Clinical Trial. Eye (Lond) 30 (10), 1343–1350. doi:10.1038/eye.2016.126
Feke, G. T., Rhee, D. J., Turalba, A. V., and Pasquale, L. R. (2013). Effects of Dorzolamide-Timolol and Brimonidine-Timolol on Retinal Vascular Autoregulation and Ocular Perfusion Pressure in Primary Open Angle Glaucoma. J. Ocul. Pharmacol. Ther. 29 (7), 639–645. doi:10.1089/jop.2012.0271
Feldman, R. M., Katz, G., McMenemy, M., Hubatsch, D. A., and Realini, T. (2016). A Randomized Trial of Fixed-Dose Combination Brinzolamide 1%/Brimonidine 0.2% as Adjunctive Therapy to Travoprost 0.004. Am. J. Ophthalmol. 165, 188–197. doi:10.1016/j.ajo.2016.02.026
Feldman, R. M., Tanna, A. P., Gross, R. L., Chuang, A. Z., Baker, L., Reynolds, A., et al. (2007). Comparison of the Ocular Hypotensive Efficacy of Adjunctive Brimonidine 0.15% or Brinzolamide 1% in Combination with Travoprost 0.004%. Ophthalmology 114 (7), 1248–1254. doi:10.1016/j.ophtha.2007.03.012
Fogagnolo, P., and Rossetti, L. (2011). Medical Treatment of Glaucoma: Present and Future. Expert Opin. Investig. Drugs 20 (7), 947–959. doi:10.1517/13543784.2011.579901
Fuwa, M., Toris, C. B., Fan, S., Taniguchi, T., Ichikawa, M., Odani-Kawabata, N., et al. (2018). Effects of a Novel Selective EP2 Receptor Agonist, Omidenepag Isopropyl, on Aqueous Humor Dynamics in Laser-Induced Ocular Hypertensive Monkeys. J. Ocul. Pharmacol. Ther. 34 (7), 531–537. doi:10.1089/jop.2017.0146
García-López, A., Paczka, J. A., Jiménez-Román, J., and Hartleben, C. (2014). Efficacy and Tolerability of Fixed-Combination Bimatoprost/timolol versus Fixed-Combination Dorzolamide/brimonidine/timolol in Patients with Primary Open-Angle Glaucoma or Ocular Hypertension: a Multicenter, Prospective, Crossover Study. BMC Ophthalmol. 14, 161. doi:10.1186/1471-2415-14-161
Garcia-Medina, J. J., Rubio-Velazquez, E., Lopez-Bernal, M. D., Cobo-Martinez, A., Zanon-Moreno, V., Pinazo-Duran, M. D., et al. (2020). Glaucoma and Antioxidants: Review and Update. Antioxidants (Basel) 9 (11). doi:10.3390/antiox9111031
Georgakopoulos, C. D., Kagkelaris, K., Pagoulatos, D., Plotas, P., and Makri, O. E. (2020). Brinzolamide-brimonidine Fixed Combination for the Prevention of Intraocular Pressure Elevation after Phacoemulsification. Eur. J. Ophthalmol. 30 (2), 293–298. doi:10.1177/1120672118817997
Georgakopoulos, C. D., Makri, O. E., Plotas, P., and Pharmakakis, N. (2013). Brinzolamide-timolol Fixed Combination for the Prevention of Intraocular Pressure Elevation after Phacoemulsification. Clin. Exp. Ophthalmol. 41 (7), 662–667. doi:10.1111/ceo.12092
Ghorai, S., Pulya, S., Ghosh, K., Panda, P., Ghosh, B., and Gayen, S. (2020). Structure-activity Relationship of Human Carbonic Anhydrase-II Inhibitors: Detailed Insight for Future Development as Anti-glaucoma Agents. Bioorg. Chem. 95, 103557. doi:10.1016/j.bioorg.2019.103557
Goel, M., Picciani, R. G., Lee, R. K., and Bhattacharya, S. K. (2010). Aqueous Humor Dynamics: a Review. Open Ophthalmol. J. 4, 52–59. doi:10.2174/1874364101004010052
Goldberg, I., Crowston, J. G., Jasek, M. C., Stewart, J. A., Stewart, W. C., and Group, A. S. I. (2012). Intraocular Pressure-Lowering Efficacy of Brinzolamide when Added to Travoprost/timolol Fixed Combination as Adjunctive Therapy. J. Glaucoma 21 (1), 55–59. doi:10.1097/IJG.0b013e3181fc8142
Gómez-Aguayo, F., Paczka, J. A., Leñero-Córdova, R., Jiménez-Román, J., Davila-Villarreal, J., Hartleben, C., et al. (2018). A Phase III Randomized Clinical Trial of a 0.5% Timolol + 0.2% Brimonidine + 2.0% Dorzolamide Fixed Combination, Preservative-free Ophthalmic Solution vs. 0.5% Timolol + 0.2% Brimonidine + 2.0% Dorzolamide Fixed Combination in Patients with Controlled Primary Open-Angle Glaucoma. Ophthalmol. Ther. 7 (1), 145–156. doi:10.1007/s40123-018-0128-8
Greig, S. L., and Deeks, E. D. (2015). Brinzolamide/brimonidine: a Review of its Use in Patients with Open-Angle Glaucoma or Ocular Hypertension. Drugs Aging 32 (3), 251–260. doi:10.1007/s40266-015-0250-4
Guglielmi, P., Carradori, S., Campestre, C., and Poce, G. (2019). Novel Therapies for Glaucoma: a Patent Review (2013-2019). Expert Opin. Ther. Pat 29 (10), 769–780. doi:10.1080/13543776.2019.1653279
Gulkilik, G., Oba, E., and Odabası, M. (2011). Comparison of Fixed Combinations of Dorzolamide/timolol and Brimonidine/timolol in Patients with Primary Open-Angle Glaucoma. Int. Ophthalmol. 31 (6), 447–451. doi:10.1007/s10792-011-9495-z
Guven Yilmaz, S., Degirmenci, C., Karakoyun, Y. E., Yusifov, E., and Ates, H. (2018). The Efficacy and Safety of Bimatoprost/timolol Maleate, Latanoprost/timolol Maleate, and Travoprost/timolol Maleate Fixed Combinations on 24-h IOP. Int. Ophthalmol. 38 (4), 1425–1431. doi:10.1007/s10792-017-0601-8
Harris, A., Ward, C. L., Rowe-Rendleman, C. L., Ouchi, T., Wood, A., Fujii, A., et al. (2016). Ocular Hypotensive Effect of ONO-9054, an EP3/FP Receptor Agonist: Results of a Randomized, Placebo-Controlled, Dose Escalation Study. J. Glaucoma 25 (10), e826–e833. doi:10.1097/IJG.0000000000000449
Hartleben, C., Parra, J. C., Batoosingh, A., Bernstein, P., and Goodkin, M. (2017). A Masked, Randomized, Phase 3 Comparison of Triple Fixed-Combination Bimatoprost/Brimonidine/Timolol versus Fixed-Combination Brimonidine/Timolol for Lowering Intraocular Pressure. J. Ophthalmol. 2017, 4586763. doi:10.1155/2017/4586763
Hatanaka, M., Reis, A., Sano, M. E., and Susanna, R. (2010). Additive Intraocular Pressure Reduction Effect of Fixed Combination of Maleate Timolol 0.5%/dorzolamide 2% (Cosopt) on Monotherapy with Latanoprost (Xalatan) in Patients with Elevated Intraocular Pressure: a Prospective, 4-week, Open-Label, Randomized, Controlled Clinical Trial. J. Glaucoma 19 (5), 331–335. doi:10.1097/IJG.0b013e3181b4cab4
Higginbotham, E. J., Olander, K. W., Kim, E. E., Grunden, J. W., Kwok, K. K., Tressler, C. S., et al. (2010). Fixed Combination of Latanoprost and Timolol vs Individual Components for Primary Open-Angle Glaucoma or Ocular Hypertension: a Randomized, Double-Masked Study. Arch. Ophthalmol. 128 (2), 165–172. doi:10.1001/archophthalmol.2009.384
Holló, G., and Kóthy, P. (2008). Intraocular Pressure Reduction with Travoprost/timolol Fixed Combination, with and without Adjunctive Brinzolamide, in Glaucoma. Curr. Med. Res. Opin. 24 (6), 1755–1761. doi:10.1185/03007990802159273
Hommer, A., Sperl, P., Resch, H., Popa-Cherecheanu, A., Qiao, C., Schmetterer, L., et al. (2012). A Double-Masked Randomized Crossover Study Comparing the Effect of Latanoprost/timolol and Brimonidine/timolol Fixed Combination on Intraocular Pressure and Ocular Blood Flow in Patients with Primary Open-Angle Glaucoma or Ocular Hypertension. J. Ocul. Pharmacol. Ther. 28 (6), 569–575. doi:10.1089/jop.2011.0165
Hopf, S., Mercieca, K., Pfeiffer, N., and Prokosch-Willing, V. (2020). Brimonidine-associated Uveitis - a Descriptive Case Series. BMC Ophthalmol. 20 (1), 489. doi:10.1186/s12886-020-01762-w
Hoy, S. M., Keam, S. J., and Keating, G. M. (2006). Travoprost/timolol. Drugs Aging 23 (7), 587–589. discussion 598-589. doi:10.2165/00002512-200623070-00005
Hoy, S. M. (2018a). Latanoprostene Bunod Ophthalmic Solution 0.024%: A Review in Open-Angle Glaucoma and Ocular Hypertension. Drugs 78 (7), 773–780. doi:10.1007/s40265-018-0914-6
Hoy, S. M. (2018b). Netarsudil Ophthalmic Solution 0.02%: First Global Approval. Drugs 78 (3), 389–396. doi:10.1007/s40265-018-0877-7
Hoy, S. M. (2015). Tafluprost/Timolol: A Review in Open-Angle Glaucoma or Ocular Hypertension. Drugs 75 (15), 1807–1813. doi:10.1007/s40265-015-0476-9
Huang, A. S., Francis, B. A., and Weinreb, R. N. (2018). Structural and Functional Imaging of Aqueous Humour Outflow: a Review. Clin. Exp. Ophthalmol. 46 (2), 158–168. doi:10.1111/ceo.13064
Impagnatiello, F., Bastia, E., Almirante, N., Brambilla, S., Duquesroix, B., Kothe, A. C., et al. (2019). Prostaglandin Analogues and Nitric Oxide Contribution in the Treatment of Ocular Hypertension and Glaucoma. Br. J. Pharmacol. 176 (8), 1079–1089. doi:10.1111/bph.14328
Impagnatiello, F., Toris, C. B., Batugo, M., Prasanna, G., Borghi, V., Bastia, E., et al. (2015). Intraocular Pressure-Lowering Activity of NCX 470, a Novel Nitric Oxide-Donating Bimatoprost in Preclinical Models. Invest. Ophthalmol. Vis. Sci. 56 (11), 6558–6564. doi:10.1167/iovs.15-17190
Inoue, K., Inoue, J., Kunimatsu-Sanuki, S., Nozaki, N., Shimizu, K., Ishida, K., et al. (2020a). Short-Term Efficacy and Safety of Omidenepag Isopropyl in Patients with Normal-Tension Glaucoma. Clin. Ophthalmol. 14, 2943–2949. doi:10.2147/OPTH.S271789
Inoue, K., Piao, H., Iwasa, M., Ishida, K., and Tomita, G. (2020b). Short-Term Efficacy and Safety of Switching from a Latanoprost/Timolol Fixed Combination to a Latanoprost/Carteolol Fixed Combination. Clin. Ophthalmol. 14, 1207–1214. doi:10.2147/OPTH.S240425
Inoue, K., Shiokawa, M., Iwasa, M., Ishida, K., and Tomita, G. (2018). Short-term Efficacy and Safety of a Latanoprost/Carteolol Fixed Combination Switched from Concomitant Therapy to in Patients with Primary Open-Angle Glaucoma or Ocular Hypertension. J. Glaucoma 27 (12), 1175–1180. doi:10.1097/IJG.0000000000001091
Inoue, T., and Tanihara, H. (2017). Ripasudil Hydrochloride Hydrate: Targeting Rho Kinase in the Treatment of Glaucoma. Expert Opin. Pharmacother. 18 (15), 1669–1673. doi:10.1080/14656566.2017.1378344
Inoue, T., Tanihara, H., Tokushige, H., and Araie, M. (2015). Efficacy and Safety of SNJ-1656 in Primary Open-Angle Glaucoma or Ocular Hypertension. Acta Ophthalmol. 93 (5), e393–5. doi:10.1111/aos.12641
Jamwal, S., Mittal, A., Kumar, P., Alhayani, D. M., and Al-Aboudi, A. (2019). Therapeutic Potential of Agonists and Antagonists of A1, A2a, A2b and A3 Adenosine Receptors. Curr. Pharm. Des. 25 (26), 2892–2905. doi:10.2174/1381612825666190716112319
Jayanetti, V., Sandhu, S., and Lusthaus, J. A. (2020). The Latest Drugs in Development that Reduce Intraocular Pressure in Ocular Hypertension and Glaucoma. J. Exp. Pharmacol. 12, 539–548. doi:10.2147/JEP.S281187
Jayaram, H. (2020). Intraocular Pressure Reduction in Glaucoma: Does Every mmHg Count? Taiwan J. Ophthalmol. 10 (4), 255–258. doi:10.4103/tjo.tjo_63_20
Jiang, J., Zhang, X., Tang, Y., Li, S., and Chen, J. (2021). Progress on Ocular siRNA Gene-Silencing Therapy and Drug Delivery Systems. Fundam. Clin. Pharmacol. 35 (1), 4–24. doi:10.1111/fcp.12561
Kaarniranta, K., Ikäheimo, K., Mannermaa, E., and Ropo, A. (2016). Pharmacokinetics, Efficacy, and Safety of the Preservative-free Fixed Combination of Tafluprost 0.0015% and Timolol 0.5% in Healthy Volunteers: A Phase I Comparison vs. The Corresponding Preservative-free Monotherapies. Clin. Pharmacokinet. 55 (4), 485–494. doi:10.1007/s40262-015-0331-x
Kahook, M. Y., Serle, J. B., Mah, F. S., Kim, T., Raizman, M. B., Heah, T., et al. (2019). Long-term Safety and Ocular Hypotensive Efficacy Evaluation of Netarsudil Ophthalmic Solution: Rho Kinase Elevated IOP Treatment Trial (ROCKET-2). Am. J. Ophthalmol. 200, 130–137. doi:10.1016/j.ajo.2019.01.003
Kass, M. A., Heuer, D. K., Higginbotham, E. J., Parrish, R. K., Khanna, C. L., Brandt, J. D., et al. (2021). Assessment of Cumulative Incidence and Severity of Primary Open-Angle Glaucoma Among Participants in the Ocular Hypertension Treatment Study after 20 Years of Follow-Up. Jama Ophthalmol. 139 (5), 558–566. doi:10.1001/jamaophthalmol.2021.034110.1001/jamaophthalmol.2021.3041
Katz, G., Dubiner, H., Samples, J., Vold, S., and Sall, K. (2013). Three-month Randomized Trial of Fixed-Combination Brinzolamide, 1%, and Brimonidine, 0.2%. Jama Ophthalmol. 131 (6), 724–730. doi:10.1001/jamaophthalmol.2013.188
Katz, L. J., Rauchman, S. H., Cottingham, A. J., Simmons, S. T., Williams, J. M., Schiffman, R. M., et al. (2012). Fixed-combination Brimonidine-Timolol versus Latanoprost in Glaucoma and Ocular Hypertension: a 12-week, Randomized, Comparison Study. Curr. Med. Res. Opin. 28 (5), 781–788. doi:10.1185/03007995.2012.681036
Kaufman, P. L. (2017). Latanoprostene Bunod Ophthalmic Solution 0.024% for IOP Lowering in Glaucoma and Ocular Hypertension. Expert Opin. Pharmacother. 18 (4), 433–444. doi:10.1080/14656566.2017.1293654
Kawase, K., Vittitow, J. L., Weinreb, R. N., Araie, M., and Group, J. S. (2016). Long-term Safety and Efficacy of Latanoprostene Bunod 0.024% in Japanese Subjects with Open-Angle Glaucoma or Ocular Hypertension: The JUPITER Study. Adv. Ther. 33 (9), 1612–1627. doi:10.1007/s12325-016-0385-7
Kazemi, A., McLaren, J. W., Kopczynski, C. C., Heah, T. G., Novack, G. D., and Sit, A. J. (2018). The Effects of Netarsudil Ophthalmic Solution on Aqueous Humor Dynamics in a Randomized Study in Humans. J. Ocul. Pharmacol. Ther. 34 (5), 380–386. doi:10.1089/jop.2017.0138
Khouri, A. S., Serle, J. B., Bacharach, J., Usner, D. W., Lewis, R. A., Braswell, P., et al. (2019). Once-Daily Netarsudil versus Twice-Daily Timolol in Patients with Elevated Intraocular Pressure: The Randomized Phase 3 ROCKET-4 Study. Am. J. Ophthalmol. 204, 97–104. doi:10.1016/j.ajo.2019.03.002
Kim, J. M., Kim, T. W., Kim, C. Y., Kim, H. K., and Park, K. H. (2016). Comparison of the Intraocular Pressure-Lowering Effect and Safety of Brimonidine/timolol Fixed Combination and 0.5% Timolol in normal-tension Glaucoma Patients. Jpn. J. Ophthalmol. 60 (1), 20–26. doi:10.1007/s10384-015-0420-2
Kim, T. W., Kim, M., Lee, E. J., Jeoung, J. W., and Park, K. H. (2014). Intraocular Pressure-Lowering Efficacy of Dorzolamide/timolol Fixed Combination in normal-tension Glaucoma. J. Glaucoma 23 (5), 329–332. doi:10.1097/IJG.0b013e3182741f4d
Konstas, A. G., Boboridis, K. G., Kapis, P., Marinopoulos, K., Voudouragkaki, I. C., Panayiotou, D., et al. (2017). 24-Hour Efficacy and Ocular Surface Health with Preservative-free Tafluprost Alone and in Conjunction with Preservative-free Dorzolamide/Timolol Fixed Combination in Open-Angle Glaucoma Patients Insufficiently Controlled with Preserved Latanoprost Monotherapy. Adv. Ther. 34 (1), 221–235. doi:10.1007/s12325-016-0448-9
Konstas, A. G., Holló, G., Haidich, A. B., Mikropoulos, D. G., Giannopoulos, T., Voudouragkaki, I. C., et al. (2013a). Comparison of 24-hour Intraocular Pressure Reduction Obtained with Brinzolamide/timolol or Brimonidine/timolol Fixed-Combination Adjunctive to Travoprost Therapy. J. Ocul. Pharmacol. Ther. 29 (7), 652–657. doi:10.1089/jop.2012.0195
Konstas, A. G., Holló, G., Mikropoulos, D. G., Haidich, A. B., Dimopoulos, A. T., Empeslidis, T., et al. (2013b). 24-hour Efficacy of the Bimatoprost-Timolol Fixed Combination versus Latanoprost as First Choice Therapy in Subjects with High-Pressure Exfoliation Syndrome and Glaucoma. Br. J. Ophthalmol. 97 (7), 857–861. doi:10.1136/bjophthalmol-2012-302843
Konstas, A. G., Karabatsas, C. H., Lallos, N., Georgiadis, N., Kotsimpou, A., Stewart, J. A., et al. (2005). 24-hour Intraocular Pressures with Brimonidine Purite versus Dorzolamide Added to Latanoprost in Primary Open-Angle Glaucoma Subjects. Ophthalmology 112 (4), 603–608. doi:10.1016/j.ophtha.2004.11.032
Konstas, A. G., Katsanos, A., Athanasopoulos, G. P., Voudouragkaki, I. C., Panagiotou, E. S., Pagkalidou, E., et al. (2018). Preservative-free Tafluprost/timolol Fixed Combination: Comparative 24-h Efficacy Administered Morning or Evening in Open-Angle Glaucoma Patients. Expert Opin. Pharmacother. 19 (18), 1981–1988. doi:10.1080/14656566.2018.1534958
Konstas, A. G., Mikropoulos, D., Dimopoulos, A. T., Moumtzis, G., Nelson, L. A., and Stewart, W. C. (2008). Second-line Therapy with Dorzolamide/timolol or Latanoprost/timolol Fixed Combination versus Adding Dorzolamide/timolol Fixed Combination to Latanoprost Monotherapy. Br. J. Ophthalmol. 92 (11), 1498–1502. doi:10.1136/bjo.2008.145219
Konstas, A. G., Mocan, M. C., Katsanos, A., Voudouragkaki, I. C., and Irkec, M. (2013c). Latanoprost/timolol Fixed Combination for the Treatment of Glaucoma. Expert Opin. Pharmacother. 14 (13), 1815–1827. doi:10.1517/14656566.2013.813482
Konstas, A. G., Quaranta, L., and Realini, T. (2012). Overview of the [corrected] Travoprost/timolol BAK-free Fixed Combination. Expert Opin. Pharmacother. 13 (5), 757–766. doi:10.1517/14656566.2012.662485
Konstas, A. G., Schmetterer, L., Katsanos, A., Hutnik, C. M. L., Holló, G., Quaranta, L., et al. (2021). Dorzolamide/Timolol Fixed Combination: Learning from the Past and Looking toward the Future. Adv. Ther. 38 (1), 24–51. doi:10.1007/s12325-020-01525-5
Konstas, A. G., Voudouragkaki, I. C., Boboridis, K. G., Haidich, A. B., Paschalinou, E., Giannopoulos, T., et al. (2014). 24-hour Efficacy of Travoprost/timolol BAK-free versus Latanoprost/timolol Fixed Combinations in Patients Insufficiently Controlled with Latanoprost. Adv. Ther. 31 (6), 592–603. doi:10.1007/s12325-014-0125-9
Kozobolis, V., Panos, G. D., Konstantinidis, A., and Labiris, G. (2017). Comparison of Dorzolamide/timolol vs Brinzolamide/brimonidine Fixed Combination Therapy in the Management of Primary Open-Angle Glaucoma. Eur. J. Ophthalmol. 27 (2), 160–163. doi:10.5301/ejo.5000826
Krauss, A. H., Impagnatiello, F., Toris, C. B., Gale, D. C., Prasanna, G., Borghi, V., et al. (2011). Ocular Hypotensive Activity of BOL-303259-X, a Nitric Oxide Donating Prostaglandin F2α Agonist, in Preclinical Models. Exp. Eye Res. 93 (3), 250–255. doi:10.1016/j.exer.2011.03.001
Kusuhara, S., and Nakamura, M. (2020). Ripasudil Hydrochloride Hydrate in the Treatment of Glaucoma: Safety, Efficacy, and Patient Selection. Clin. Ophthalmol. 14, 1229–1236. doi:10.2147/OPTH.S216907
Laties, A., Rich, C. C., Stoltz, R., Humbert, V., Brickman, C., McVicar, W., et al. (2016). A Randomized Phase 1 Dose Escalation Study to Evaluate Safety, Tolerability, and Pharmacokinetics of Trabodenoson in Healthy Adult Volunteers. J. Ocul. Pharmacol. Ther. 32 (8), 548–554. doi:10.1089/jop.2015.0147
Lee, N. Y., Park, H. Y., and Park, C. K. (2016a). Comparison of the Effects of Dorzolamide/Timolol Fixed Combination versus Latanoprost on Intraocular Pressure and Ocular Perfusion Pressure in Patients with Normal-Tension Glaucoma: A Randomized, Crossover Clinical Trial. PLoS One 11 (1), e0146680. doi:10.1371/journal.pone.0146680
Lee, N. Y., Park, H. Y., and Park, C. K. (2016b). Effects of a Dorzolamide/timolol Fixed Combination on Diurnal Intraocular Pressure, Heart Rate, Blood Pressure, and Ocular Perfusion Pressure in normal-tension Glaucoma. Jpn. J. Ophthalmol. 60 (5), 377–382. doi:10.1007/s10384-016-0455-z
Lequeu, I., Theuwis, K., Abegāo Pinto, L., Vandewalle, E., and Stalmans, I. (2013). Long Term IOP Lowering Efficacy of Bimatoprost/timolol Fixed Combination: a 12 Month Prospective Study. Bull. Soc. Belge Ophtalmol (322), 105–110.
Lesk, M. R., Koulis, T., Sampalis, F., Sampalis, J. S., and Bastien, N. R. (2008). Effectiveness and Safety of Dorzolamide-Timolol Alone or Combined with Latanoprost in Open-Angle Glaucoma or Ocular Hypertension. Ann. Pharmacother. 42 (4), 498–504. doi:10.1345/aph.1K565
Lewis, R. A., Gross, R. L., Sall, K. N., Schiffman, R. M., Liu, C. C., Batoosingh, A. L., et al. (2010). The Safety and Efficacy of Bimatoprost/timolol Fixed Combination: a 1-year Double-Masked, Randomized Parallel Comparison to its Individual Components in Patients with Glaucoma or Ocular Hypertension. J. Glaucoma 19 (6), 424–426. doi:10.1097/IJG.0b013e3181bdb586
Li, G., Torrejon, K. Y., Unser, A. M., Ahmed, F., Navarro, I. D., Baumgartner, R. A., et al. (2018). Trabodenoson, an Adenosine Mimetic with A1 Receptor Selectivity Lowers Intraocular Pressure by Increasing Conventional Outflow Facility in Mice. Invest. Ophthalmol. Vis. Sci. 59 (1), 383–392. doi:10.1167/iovs.17-23212
Liu, J. H., Medeiros, F. A., Slight, J. R., and Weinreb, R. N. (2009). Comparing Diurnal and Nocturnal Effects of Brinzolamide and Timolol on Intraocular Pressure in Patients Receiving Latanoprost Monotherapy. Ophthalmology 116 (3), 449–454. doi:10.1016/j.ophtha.2008.09.054
Liu, J. H. K., Slight, J. R., Vittitow, J. L., Scassellati Sforzolini, B., and Weinreb, R. N. (2016). Efficacy of Latanoprostene Bunod 0.024% Compared with Timolol 0.5% in Lowering Intraocular Pressure over 24 hours. Am. J. Ophthalmol. 169, 249–257. doi:10.1016/j.ajo.2016.04.019
Luo, L. J., Nguyen, D. D., and Lai, J. Y. (2021). Harnessing the Tunable Cavity of Nanoceria for Enhancing Y-27632-Mediated Alleviation of Ocular Hypertension. Theranostics 11 (11), 5447–5463. doi:10.7150/thno.54525
Lusthaus, J., and Goldberg, I. (2019). Current Management of Glaucoma. Med. J. Aust. 210 (4), 180–187. doi:10.5694/mja2.50020
Macky, T. A. (2014). Bimatoprost/timolol versus Travoprost/timolol Fixed Combinations in an Egyptian Population: a Hospital-Based Prospective Randomized Study. J. Glaucoma 23 (8), 561–566. doi:10.1097/IJG.0b013e3182867be3
Mao, Y. J., Wu, J. B., Yang, Z. Q., Zhang, Y. H., and Huang, Z. J. (2020). Nitric Oxide Donating Anti-glaucoma Drugs: Advances and Prospects. Chin. J. Nat. Med. 18 (4), 275–283. doi:10.1016/S1875-5364(20)30035-2
Marshall, L. L., Hayslett, R. L., and Stevens, G. A. (2018). Therapy for Open-Angle Glaucoma. Consult Pharm. 33 (8), 432–445. doi:10.4140/TCP.n.2018.432
Martínez, T., González, M. V., Roehl, I., Wright, N., Pañeda, C., and Jiménez, A. I. (2014). In Vitro and In Vivo Efficacy of SYL040012, a Novel siRNA Compound for Treatment of Glaucoma. Mol. Ther. 22 (1), 81–91. doi:10.1038/mt.2013.216
Matsou, A., and Anastasopoulos, E. (2018). Investigational Drugs Targeting Prostaglandin Receptors for the Treatment of Glaucoma. Expert Opin. Investig. Drugs 27 (10), 777–785. doi:10.1080/13543784.2018.1526279
Matsuo, M., Kuse, Y., Takahashi, K., Kuwahara, K., Tanito, M., Kaidzu, S., et al. (2019). Carteolol Hydrochloride Reduces Visible Light-Induced Retinal Damage In Vivo and BSO/glutamate-induced Oxidative Stress In Vitro. J. Pharmacol. Sci. 139 (2), 84–90. doi:10.1016/j.jphs.2018.11.010
Medeiros, F. A., Martin, K. R., Peace, J., Scassellati Sforzolini, B., Vittitow, J. L., and Weinreb, R. N. (2016). Comparison of Latanoprostene Bunod 0.024% and Timolol Maleate 0.5% in Open-Angle Glaucoma or Ocular Hypertension: The LUNAR Study. Am. J. Ophthalmol. 168, 250–259. doi:10.1016/j.ajo.2016.05.012
Mehran, N. A., Sinha, S., and Razeghinejad, R. (2020). New Glaucoma Medications: Latanoprostene Bunod, Netarsudil, and Fixed Combination Netarsudil-Latanoprost. Eye (Lond) 34 (1), 72–88. doi:10.1038/s41433-019-0671-0
Miller Ellis, E., Berlin, M. S., Ward, C. L., Sharpe, J. A., Jamil, A., and Harris, A. (2017). Ocular Hypotensive Effect of the Novel EP3/FP Agonist ONO-9054 versus Xalatan: Results of a 28-day, Double-Masked, Randomised Study. Br. J. Ophthalmol. 101 (6), 796–800. doi:10.1136/bjophthalmol-2016-309023
Miura, K., Ito, K., Okawa, C., Sugimoto, K., Matsunaga, K., and Uji, Y. (2008). Comparison of Ocular Hypotensive Effect and Safety of Brinzolamide and Timolol Added to Latanoprost. J. Glaucoma 17 (3), 233–237. doi:10.1097/IJG.0b013e31815072fe
Mizoue, S., Nitta, K., Shirakashi, M., Nitta, A., Yamabayashi, S., Kimura, T., et al. (2017). Erratum to: Multicenter, Randomized, Investigator-Masked Study Comparing Brimonidine Tartrate 0.1% and Timolol Maleate 0.5% as Adjunctive Therapies to Prostaglandin Analogues in Normal-Tension Glaucoma. Adv. Ther. 34 (6), 2179–1448. doi:10.1007/s12325-017-0552-510.1007/s12325-017-0607-7
Morales, D. R., Dreischulte, T., Lipworth, B. J., Donnan, P. T., Jackson, C., and Guthrie, B. (2016). Respiratory Effect of Beta-Blocker Eye Drops in Asthma: Population-Based Study and Meta-Analysis of Clinical Trials. Br. J. Clin. Pharmacol. 82 (3), 814–822. doi:10.1111/bcp.13006
Moreno-Montañés, J., Sádaba, B., Ruz, V., Gómez-Guiu, A., Zarranz, J., González, M. V., et al. (2014). Phase I Clinical Trial of SYL040012, a Small Interfering RNA Targeting β-adrenergic Receptor 2, for Lowering Intraocular Pressure. Mol. Ther. 22 (1), 226–232. doi:10.1038/mt.2013.217
Moura-Coelho, N., Tavares Ferreira, J., Bruxelas, C. P., Dutra-Medeiros, M., Cunha, J. P., and Pinto Proença, R. (2019). Rho Kinase Inhibitors-A Review on the Physiology and Clinical Use in Ophthalmology. Graefes Arch. Clin. Exp. Ophthalmol. 257 (6), 1101–1117. doi:10.1007/s00417-019-04283-5
Mundorf, T., Noecker, R. J., and Earl, M. (2007). Ocular Hypotensive Efficacy of Brimonidine 0.15% as Adjunctive Therapy with Latanoprost 0.005% in Patients with Open-Angle Glaucoma or Ocular Hypertension. Adv. Ther. 24 (2), 302–309. doi:10.1007/BF02849898
Myers, J. S., Sall, K. N., DuBiner, H., Slomowitz, N., McVicar, W., Rich, C. C., et al. (2016). A Dose-Escalation Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of 2 and 4 Weeks of Twice-Daily Ocular Trabodenoson in Adults with Ocular Hypertension or Primary Open-Angle Glaucoma. J. Ocul. Pharmacol. Ther. 32 (8), 555–562. doi:10.1089/jop.2015.0148
Nakamoto, K., and Yasuda, N. (2007). Effect of Concomitant Use of Latanoprost and Brinzolamide on 24-hour Variation of IOP in normal-tension Glaucoma. J. Glaucoma 16 (4), 352–357. doi:10.1097/IJG.0b013e318033b491
Nakano, T., Inoue, R., Kimura, T., Suzumura, H., Tanino, T., Yamazaki, Y., et al. (2016). Effects of Brinzolamide, a Topical Carbonic Anhydrase Inhibitor, on Corneal Endothelial Cells. Adv. Ther. 33 (8), 1452–1459. doi:10.1007/s12325-016-0373-y
Nakano, T., Mizoue, S., Fuse, N., Iwase, A., Matsumoto, S., and Yoshikawa, K. (2015). Fixed Combination of Travoprost and Timolol Maleate Reduces Intraocular Pressure in Japanese Patients with Primary Open-Angle Glaucoma or Ocular Hypertension: A Prospective Multicenter Open-Label Study. Adv. Ther. 32 (9), 823–837. doi:10.1007/s12325-015-0246-9
Netland, P. A., Michael, M., Rosner, S. A., Katzman, B., and Macy, J. I. (2003). Brimonidine Purite and Bimatoprost Compared with Timolol and Latanoprost in Patients with Glaucoma and Ocular Hypertension. Adv. Ther. 20 (1), 20–30. doi:10.1007/BF02850116
Nguyen, Q. H., McMenemy, M. G., Realini, T., Whitson, J. T., and Goode, S. M. (2013). Phase 3 Randomized 3-month Trial with an Ongoing 3-month Safety Extension of Fixed-Combination Brinzolamide 1%/brimonidine 0.2%. J. Ocul. Pharmacol. Ther. 29 (3), 290–297. doi:10.1089/jop.2012.0235
NIH (2015a). 6-Week Proof-Of-Concept Study of Travoprost/Brinzolamide Ophthalmic Suspension in Subjects with Open-Angle Glaucoma or Ocular Hypertension. ClinicalTrials.Gov Identifier. NCT02140060. Last update: December 29, 2015. U.S. National Library of Medicine. Available at: https://clinicaltrials.gov/ct2/show/NCT02140060 (Accessed July 27, 2021).
NIH (2020). A Clinical Study to Evaluate the Efficacy and Safety of CKD-351. ClinicalTrials.Gov Identifier. NCT04448223. Last update. U.S. National Library of Medicine. Available at: https://clinicaltrials.gov/ct2/show/NCT04448223 (Accessed July 27, 2021).
NIH (2015b). A Safety and Efficacy Study of Fixed-Combination Bimatoprost and Brimonidine in Chronic Glaucoma or Ocular Hypertension. ClinicalTrials.Gov Identifier. NCT01863953. Last update: January 26, 2015. U.S. National Library of Medicine. Available at: https://clinicaltrials.gov/ct2/show/NCT01863953 (Accessed July 27, 2021).
NIH (2019a). NCX-470 vs. Latanoprost in Open-Angle Glaucoma/ocular Hypertension (Phase II). Last update: October 02, 2019. Available at: https://www.cortellis.com/drugdiscovery/entity/clinicalstudies/430486?ent=UONY7w09 (Accessed July 27, 2021).
NIH (2013a). Safety and Efficacy of AGN-210669 Ophthalmic Solution Compared with Bimatoprost Ophthalmic Solution in Patients with Open-Angle Glaucoma or Ocular Hypertension. ClinicalTrials.Gov Identifier. NCT01291108. Last update: November 6, 2013. U.S. National Library of Medicine. Available at: https://clinicaltrials.gov/ct2/show/NCT01291108 (Accessed July 27, 2021).
NIH (2014a). Safety and Efficacy of AGN-210961 Ophthalmic Solution Compared with Bimatoprost Ophthalmic Solution in Patients with Glaucoma or Ocular Hypertension. ClinicalTrials.Gov Identifier. : NCT01110499. Last update: August 5, 2014. U.S. National Library of Medicine. Available at: https://clinicaltrials.gov/ct2/show/NCT01110499 (Accessed July 27, 2021).
NIH (2013b). Safety and Efficacy of Three Formulations of AGN-210669 Ophthalmic Solution Compared with Bimatoprost Ophthalmic Solution. ClinicalTrials.Gov Identifier: NCT01001195. Last update: October 18, 2013. Available at: https://clinicaltrials.gov/ct2/show/NCT01001195 (Accessed July 27, 2021).
NIH (2019b). Safety and Tolerability of the Preservative-free Ophthalmic Solution PRO-122 Compared with Krytantek Ofteno® (PRO-122/I). ClinicalTrials.Gov Identifier. NCT03966365. Last update: December 13, 2019. U.S. National Library of Medicine. Available at: https://clinicaltrials.gov/ct2/show/NCT03966365 (Accessed July 27, 2021).
NIH (2014b). Study to Compare the Safety and Efficacy of ALZ-1101 to Latanoprost in Patients with Intraocular Pressure Inadequately Controlled by Latanoprost. ClinicalTrials.Gov Identifier. NCT01896180. Last update: September 30, 2014. U.S. National Library of Medicine. Available at: https://clinicaltrials.gov/ct2/show/NCT01896180 (Accessed July 27, 2021).
NIH (2014c). SYL-040012 in Glaucoma: The NCT01739244 Study (Phase II). ClinicalTrials.Gov Identifier. NCT01739244. Last update: May 08, 2014. U.S. National Library of Medicine. Available at: https://www.cortellis.com/drugdiscovery/entity/clinicalstudies/273312?ent=0aG2DDkm (Accessed July 27, 2021).
Nocentini, A., and Supuran, C. T. (2019). Adrenergic Agonists and Antagonists as Antiglaucoma Agents: a Literature and Patent Review (2013-2019). Expert Opin. Ther. Pat 29 (10), 805–815. doi:10.1080/13543776.2019.1665023
Oztürk, F., Ermiş, S. S., Inan, U. U., Aşagidag, A., and Yaman, S. (2005). Comparison of the Efficacy and Safety of Dorzolamide 2% when Added to Brimonidine 0.2% or Timolol Maleate 0.5% in Patients with Primary Open-Angle Glaucoma. J. Ocul. Pharmacol. Ther. 21 (1), 68–74. doi:10.1089/jop.2005.21.68
Pfeiffer, N., and group, T. (2011). Timolol versus Brinzolamide Added to Travoprost in Glaucoma or Ocular Hypertension. Graefes Arch. Clin. Exp. Ophthalmol. 249 (7), 1065–1071. doi:10.1007/s00417-011-1650-8
Pfeiffer, N., Traverso, C. E., Lorenz, K., Saarela, V., Liinamaa, J., Uusitalo, H., et al. (2014). A 6-month Study Comparing Efficacy, Safety, and Tolerability of the Preservative-free Fixed Combination of Tafluprost 0.0015% and Timolol 0.5% versus Each of its Individual Preservative-free Components. Adv. Ther. 31 (12), 1228–1246. doi:10.1007/s12325-014-0163-3
Pinnock, C., Yip, J. L., Khawaja, A. P., Luben, R., Hayat, S., Broadway, D. C., et al. (2016). Topical Beta-Blockers and Cardiovascular Mortality: Systematic Review and Meta-Analysis with Data from the EPIC-Norfolk Cohort Study. Ophthalmic Epidemiol. 23 (5), 277–284. doi:10.1080/09286586.2016.1213301
Qiu, T. G. (2021). Trabodenoson on Trabecular Meshwork Rejuvenation: a Comprehensive Review of Clinical Data. Expert Opin. Investig. Drugs 30 (3), 227–236. doi:10.1080/13543784.2021.1873276
Razeghinejad, M. R., and Lee, D. (2019). Managing normal Tension Glaucoma by Lowering the Intraocular Pressure. Surv. Ophthalmol. 64 (1), 111–116. doi:10.1016/j.survophthal.2018.07.003
Realini, T., Nguyen, Q. H., Katz, G., and DuBiner, H. (2013). Fixed-combination Brinzolamide 1%/brimonidine 0.2% vs Monotherapy with Brinzolamide or Brimonidine in Patients with Open-Angle Glaucoma or Ocular Hypertension: Results of a Pooled Analysis of Two Phase 3 Studies. Eye (Lond) 27 (7), 841–847. doi:10.1038/eye.2013.83
Reis, R., Queiroz, C. F., Santos, L. C., Avila, M. P., and Magacho, L. (2006). A Randomized, Investigator-Masked, 4-week Study Comparing Timolol Maleate 0.5%, Brinzolamide 1%, and Brimonidine Tartrate 0.2% as Adjunctive Therapies to Travoprost 0.004% in Adults with Primary Open-Angle Glaucoma or Ocular Hypertension. Clin. Ther. 28 (4), 552–559. doi:10.1016/j.clinthera.2006.04.007
Renieri, G., Führer, K., Scheithe, K., Lorenz, K., Pfeiffer, N., and Thieme, H. (2010). Efficacy and Tolerability of Preservative-free Eye Drops Containing a Fixed Combination of Dorzolamide and Timolol in Glaucoma Patients. J. Ocul. Pharmacol. Ther. 26 (6), 597–603. doi:10.1089/jop.2010.0060
Rossi, G. C., Pasinetti, G. M., Sandolo, F., Bordin, M., and Bianchi, P. E. (2011). From Dorzolamide 2%/timolol 0.5% to Brinzolamide 1%/timolol 0.5% Fixed Combination: a 6-month, Multicenter, Open-Label Tolerability Switch Study. Expert Opin. Pharmacother. 12 (16), 2425–2431. doi:10.1517/14656566.2011.589384
Sah, A. K., and Suresh, P. K. (2017). Medical Management of Glaucoma: Focus on Ophthalmologic Drug Delivery Systems of Timolol Maleate. Artif. Cell Nanomed Biotechnol 45 (3), 448–459. doi:10.3109/21691401.2016.1160917
Sakamoto, E., Ishida, W., Sumi, T., Kishimoto, T., Tada, K., Fukuda, K., et al. (2019). Evaluation of Offset of Conjunctival Hyperemia Induced by a Rho-Kinase Inhibitor; 0.4% Ripasudil Ophthalmic Solution Clinical Trial. Sci. Rep. 9 (1), 3755. doi:10.1038/s41598-019-40255-9
Schehlein, E. M., Novack, G. D., and Robin, A. L. (2017). New Classes of Glaucoma Medications. Curr. Opin. Ophthalmol. 28 (2), 161–168. doi:10.1097/ICU.0000000000000346
Schehlein, E. M., and Robin, A. L. (2019). Rho-Associated Kinase Inhibitors: Evolving Strategies in Glaucoma Treatment. Drugs 79 (10), 1031–1036. doi:10.1007/s40265-019-01130-z
Scherzer, M. L., Liehneova, I., Negrete, F. J., and Schnober, D. (2011). Travoprost 0.004%/timolol 0.5% Fixed Combination in Patients Transitioning from Fixed or Unfixed Bimatoprost 0.03%/timolol 0.5%. Adv. Ther. 28 (8), 661–670. doi:10.1007/s12325-011-0043-z
Scozzafava, A., and Supuran, C. T. (2014). Glaucoma and the Applications of Carbonic Anhydrase Inhibitors. Subcell Biochem. 75, 349–359. doi:10.1007/978-94-007-7359-2_17
Seibold, L. K., DeWitt, P. E., Kroehl, M. E., and Kahook, M. Y. (2017). The 24-Hour Effects of Brinzolamide/Brimonidine Fixed Combination and Timolol on Intraocular Pressure and Ocular Perfusion Pressure. J. Ocul. Pharmacol. Ther. 33 (3), 161–169. doi:10.1089/jop.2016.0141
Serle, J. B., Katz, L. J., McLaurin, E., Heah, T., Ramirez-Davis, N., Usner, D. W., et al. (2018). Two Phase 3 Clinical Trials Comparing the Safety and Efficacy of Netarsudil to Timolol in Patients with Elevated Intraocular Pressure: Rho Kinase Elevated IOP Treatment Trial 1 and 2 (ROCKET-1 and ROCKET-2). Am. J. Ophthalmol. 186, 116–127. doi:10.1016/j.ajo.2017.11.019
Seymenoğlu, G., Baser, E. F., Öztürk, B., and Gülhan, C. (2015). Comparison of Dorzolamide/timolol versus Brimonidine/timolol Fixed Combination Therapy in the Management of Steroid-Induced Ocular Hypertension. J. Glaucoma 24 (2), 111–116. doi:10.1097/IJG.0b013e31829d9b5c
Sezgin Akçay, B. İ., Güney, E., Bozkurt, K. T., Unlü, C., and Akçali, G. (2013). The Safety and Efficacy of Brinzolamide 1%/timolol 0.5% Fixed Combination versus Dorzolamide 2%/timolol 0.5% in Patients with Open-Angle Glaucoma or Ocular Hypertension. J. Ocul. Pharmacol. Ther. 29 (10), 882–886. doi:10.1089/jop.2013.0102
Shedden, A., Adamsons, I. A., Getson, A. J., Laurence, J. K., Lines, C. R., Hewitt, D. J., et al. (2010). Comparison of the Efficacy and Tolerability of Preservative-free and Preservative-Containing Formulations of the Dorzolamide/timolol Fixed Combination (COSOPT™) in Patients with Elevated Intraocular Pressure in a Randomized Clinical Trial. Graefes Arch. Clin. Exp. Ophthalmol. 248 (12), 1757–1764. doi:10.1007/s00417-010-1397-7
Shim, S. H., Kim, J. M., Choi, C. Y., and Kim, C. Y. (2014). Diurnal Intraocular Pressure with Bimatoprost/timolol Fixed Combination versus Latanoprost/timolol Fixed Combination in Healthy Subjects. Korean J. Ophthalmol. 28 (1), 39–48. doi:10.3341/kjo.2014.28.1.39
Shoji, N., Ogata, H., Suyama, H., Ishikawa, H., Suzuki, H., Morita, T., et al. (2005). Intraocular Pressure Lowering Effect of Brinzolamide 1.0% as Adjunctive Therapy to Latanoprost 0.005% in Patients with Open Angle Glaucoma or Ocular Hypertension: an Uncontrolled, Open-Label Study. Curr. Med. Res. Opin. 21 (4), 503–508. doi:10.1185/030079905x38222
Shoji, T., Sato, H., Mizukawa, A., Hirota, N., Enoki, T., Kojima, T., et al. (2013). Hypotensive Effect of Latanoprost/timolol versus Travoprost/timolol Fixed Combinations in NTG Patients: a Randomized, Multicenter, Crossover Clinical Trial. Invest. Ophthalmol. Vis. Sci. 54 (9), 6242–6247. doi:10.1167/iovs.13-11942
Shukla, A. G., Razeghinejad, R., and Myers, J. S. (2020). Balancing Treatments for Patients with Systemic Hypertension and Glaucoma. Expert Opin. Pharmacother. 21 (18), 2225–2230. doi:10.1080/14656566.2020.1810235
Singh, I. P., Fechtner, R. D., Myers, J. S., Kim, T., Usner, D. W., McKee, H., et al. (2020). Pooled Efficacy and Safety Profile of Netarsudil Ophthalmic Solution 0.02% in Patients with Open-Angle Glaucoma or Ocular Hypertension. J. Glaucoma 29 (10), 878–884. doi:10.1097/IJG.0000000000001634
Skrzypecki, J., Grabska-Liberek, I., Przybek, J., and Ufnal, M. (2018). A Common Humoral Background of Intraocular and Arterial Blood Pressure Dysregulation. Curr. Med. Res. Opin. 34 (3), 521–529. doi:10.1080/03007995.2017.1415203
Spaeth, G. L., Bernstein, P., Caprioli, J., and Schiffman, R. M. (2011). Control of Intraocular Pressure and Fluctuation with Fixed-Combination Brimonidine-Timolol versus Brimonidine or Timolol Monotherapy. Am. J. Ophthalmol. 151 (1), 93–e4. doi:10.1016/j.ajo.2010.07.024
Stankiewicz, A., Misiuk-Hojło, M., Grabska-Liberek, I., Romanowska-Dixon, B., Wierzbowska, J., Wasyluk, J., et al. (2011). Intraocular Pressure and Ocular Hemodynamics in Patients with Primary Open-Angle Glaucoma Treated with the Combination of Morning Dosing of Bimatoprost and Dorzolamide Hydrochloride. Acta Ophthalmol. 89 (1), e57–63. doi:10.1111/j.1755-3768.2010.02036.x
Stankiewicz, A., Wierzbowska, J., Siemiatkowska, A., Fuksinska, B., Robaszkiewicz, J., Zegadlo, A., et al. (2010). The Additive Effect of Dorzolamide Hydrochloride (Trusopt) and a Morning Dose of Bimatoprost (Lumigan) on Intraocular Pressure and Retrobulbar Blood Flow in Patients with Primary Open-Angle Glaucoma. Br. J. Ophthalmol. 94 (10), 1307–1311. doi:10.1136/bjo.2009.162859
Stewart, W. C., Stewart, J. A., Day, D. G., Sharpe, E. D., and Jenkins, J. N. (2004). Efficacy and Safety of the Latanoprost/timolol Maleate Fixed Combination vs Concomitant Brimonidine and Latanoprost Therapy. Eye (Lond) 18 (10), 990–995. doi:10.1038/sj.eye.6701375
Supuran, C. T., Altamimi, A. S. A., and Carta, F. (2019). Carbonic Anhydrase Inhibition and the Management of Glaucoma: a Literature and Patent Review 2013-2019. Expert Opin. Ther. Pat 29 (10), 781–792. doi:10.1080/13543776.2019.1679117
Supuran, C. T. (2020). An Update on Drug Interaction Considerations in the Therapeutic Use of Carbonic Anhydrase Inhibitors. Expert Opin. Drug Metab. Toxicol. 16 (4), 297–307. doi:10.1080/17425255.2020.1743679
Suto, F., Rowe-Rendleman, C. L., Ouchi, T., Jamil, A., Wood, A., and Ward, C. L. (2015). A Novel Dual Agonist of EP3 and FP Receptors for OAG and OHT: Safety, Pharmacokinetics, and Pharmacodynamics of ONO-9054 in Healthy Volunteers. Invest. Ophthalmol. Vis. Sci. 56 (13), 7963–7970. doi:10.1167/iovs.15-18166
Suzuki, G., Kunikane, E., Shinno, K., Kozai, S., Kurata, M., and Kawamura, A. (2020). Ocular and Systemic Pharmacokinetics of Brimonidine and Timolol after Topical Administration in Rabbits: Comparison between Fixed-Combination and Single Drugs. Ophthalmol. Ther. 9 (1), 115–125. doi:10.1007/s40123-020-00229-x
Suzuki, K., Otsuka, N., Otsuka, N., Hizaki, H., Hashimoto, M., and Kuwayama, Y. (2018). Tafluprost/Timolol versus Latanoprost/Timolol Study, GMulticenter, Randomized, Controlled Study Comparing Tafluprost/Timolol Fixed Combination with Latanoprost/Timolol Fixed Combination in Primary Open-Angle Glaucoma and Ocular Hypertension. Adv. Ther. 35 (6), 796–808. doi:10.1007/s12325-018-0718-9
Takagi, Y., Santo, K., Hashimoto, M., and Fukuchi, T. (2018). Ocular Hypotensive Effects of Prostaglandin Analogs in Japanese Patients with normal-tension Glaucoma: a Literature Review. Clin. Ophthalmol. 12, 1837–1844. doi:10.2147/OPTH.S166657
Tanihara, H., Inoue, T., Yamamoto, T., Kuwayama, Y., Abe, H., Araie, M., et al. (2013). Phase 2 Randomized Clinical Study of a Rho Kinase Inhibitor, K-115, in Primary Open-Angle Glaucoma and Ocular Hypertension. Am. J. Ophthalmol. 156 (4), 731–736. doi:10.1016/j.ajo.2013.05.016
Tanihara, H., Inoue, T., Yamamoto, T., Kuwayama, Y., Abe, H., Fukushima, A., et al. (2016). One-year Clinical Evaluation of 0.4% Ripasudil (K-115) in Patients with Open-Angle Glaucoma and Ocular Hypertension. Acta Ophthalmol. 94 (1), e26–34. doi:10.1111/aos.12829
Tanihara, H., Inoue, T., Yamamoto, T., Kuwayama, Y., Abe, H., Suganami, H., et al. (2015). Additive Intraocular Pressure-Lowering Effects of the Rho Kinase Inhibitor Ripasudil (K-115) Combined with Timolol or Latanoprost: A Report of 2 Randomized Clinical Trials. Jama Ophthalmol. 133 (7), 755–761. doi:10.1001/jamaophthalmol.2015.0525
Tanna, A. P., and Johnson, M. (2018). Rho Kinase Inhibitors as a Novel Treatment for Glaucoma and Ocular Hypertension. Ophthalmology 125 (11), 1741–1756. doi:10.1016/j.ophtha.2018.04.040
Terao, E., Nakakura, S., Nagata, Y., Dote, S., Tabuchi, H., and Kiuchi, Y. (2020). Evaluation of Patterns and Correlations of the Degree of Conjunctival Hyperemia Induced by Omidenepag Isopropyl 0.002% and Ripasudil 0.4. Cureus 12 (9), e10368. doi:10.7759/cureus.10368
Topouzis, F., Goldberg, I., Bell, K., Tatham, A. J., Ridolfi, A., Hubatsch, D., et al. (2021). Brinzolamide/brimonidine Fixed-Dose Combination Bid as an Adjunct to a Prostaglandin Analog for Open-Angle Glaucoma/ocular Hypertension. Eur. J. Ophthalmol. 31 (1), 103–111. doi:10.1177/1120672119878044
Tsukamoto, H., and Larsson, L. I. (2004). Aqueous Humor Flow in normal Human Eyes Treated with Brimonidine and Dorzolamide, Alone and in Combination. Arch. Ophthalmol. 122 (2), 190–193. doi:10.1001/archopht.122.2.190
Tsukamoto, H., Noma, H., Matsuyama, S., Ikeda, H., and Mishima, H. K. (2005). The Efficacy and Safety of Topical Brinzolamide and Dorzolamide when Added to the Combination Therapy of Latanoprost and a Beta-Blocker in Patients with Glaucoma. J. Ocul. Pharmacol. Ther. 21 (2), 170–173. doi:10.1089/jop.2005.21.170
Van de Velde, S., Van Bergen, T., Sijnave, D., Hollanders, K., Castermans, K., Defert, O., et al. (2014). AMA0076, a Novel, Locally Acting Rho Kinase Inhibitor, Potently Lowers Intraocular Pressure in New Zealand white Rabbits with Minimal Hyperemia. Invest. Ophthalmol. Vis. Sci. 55 (2), 1006–1016. doi:10.1167/iovs.13-13157
Wareham, L. K., Buys, E. S., and Sappington, R. M. (2018). The Nitric Oxide-Guanylate Cyclase Pathway and Glaucoma. Nitric Oxide 77, 75–87. doi:10.1016/j.niox.2018.04.010
Webb, T. E. R. (2021). A Review of Glaucoma Surgical Therapy. Vet. Ophthalmol. 24 Suppl 1 (Suppl. 1), 34–38. doi:10.1111/vop.12852
Weinreb, R. N., Bacharach, J., Fechtner, R. D., Kahook, M. Y., Wirta, D., Burmaster, S., et al. (2019). 24-Hour Intraocular Pressure Control with Fixed-Dose Combination Brinzolamide 1%/Brimonidine 0.2%: A Multicenter, Randomized Trial. Ophthalmology 126 (8), 1095–1104. doi:10.1016/j.ophtha.2018.10.040
Weinreb, R. N., Liebmann, J. M., Martin, K. R., Kaufman, P. L., and Vittitow, J. L. (2018). Latanoprostene Bunod 0.024% in Subjects with Open-Angle Glaucoma or Ocular Hypertension: Pooled Phase 3 Study Findings. J. Glaucoma 27 (1), 7–15. doi:10.1097/IJG.0000000000000831
Weinreb, R. N., Scassellati Sforzolini, B., Vittitow, J., and Liebmann, J. (2016). Latanoprostene Bunod 0.024% versus Timolol Maleate 0.5% in Subjects with Open-Angle Glaucoma or Ocular Hypertension: The APOLLO Study. Ophthalmology 123 (5), 965–973. doi:10.1016/j.ophtha.2016.01.019
Xu, C., Guo, R., Huang, D., Ji, J., and Liu, W. (2020). Daily Costs and Cost Effectiveness of Glaucoma Fixed Combinations in China. J. Ophthalmol. 2020, 2406783. doi:10.1155/2020/2406783
Yamamoto, T., Ikegami, T., Ishikawa, Y., Kikuchi, S., Opc, E. L., and Study, G. (2016). Randomized, Controlled, Phase 3 Trials of Carteolol/Latanoprost Fixed Combination in Primary Open-Angle Glaucoma or Ocular Hypertension. Am. J. Ophthalmol. 171, 35–46. doi:10.1016/j.ajo.2016.08.022
Yang, Z., Wang, J., Liu, X., Cheng, Y., Deng, L., and Zhong, Y. (2013). Y-39983 Downregulates RhoA/Rho-Associated Kinase Expression during its Promotion of Axonal Regeneration. Oncol. Rep. 29 (3), 1140–1146. doi:10.3892/or.2012.2205
Yavas, G. F., Kusbeci, T., Polat, O., Karadas, M., Ermis, S. S., and Inan, U. U. (2013). Comparison of Latanoprost, Brimonidine Tartrate, and Bimatoprost Plus Timolol Maleate in Fixed Combinations. Turk J. Med. Sci. 43 (2), 321–325. doi:10.3906/sag-1204-20
Yu, A., and Welge-Lüßen, U. (2013). Mechanisms, Clinical Profile and Role of Prostaglandin and Prostamide Analogues in Antiglaucomatous Therapy. Klin Monbl Augenheilkd 230 (2), 127–132. doi:10.1055/s-0032-1327946
Zabriskie, N., and Netland, P. A. (2003). Comparison of Brimonidine/Latanoprost and Timolol/Dorzolamide: Two Randomized, Double-Masked, Parallel Clinical trialsComparison of Brimonidine/latanoprost and Timolol/dorzolamide: Two Randomized, Double-Masked, Parallel Clinical Trials. Adv. Ther. 20 (2), 92–100. doi:10.1007/BF02850256
Zhao, J. L., Ge, J., Li, X. X., Li, Y. M., Sheng, Y. H., Sun, N. X., et al. (2011). Comparative Efficacy and Safety of the Fixed versus Unfixed Combination of Latanoprost and Timolol in Chinese Patients with Open-Angle Glaucoma or Ocular Hypertension. BMC Ophthalmol. 11, 23. doi:10.1186/1471-2415-11-23
Zhou, X., Zhang, T., and Wu, J. (2019). Brimonidine Enhances Inhibitory Postsynaptic Activity of OFF- and ON-type Retinal Ganglion Cells in a Wistar Rat Chronic Glaucoma Model. Exp. Eye Res. 189, 107833. doi:10.1016/j.exer.2019.107833
Glossary
IOP intraocular pressure
TM trabecular meshwork
NTG normal tension glaucoma
POAG primary open-angle glaucoma
AH aqueous humor
OHT ocular hypertension
AEs adverse effects
PGs prostaglandins
PGF2 prostaglandin F2
PGE2 prostaglandin E2
OMDI omidenepag isopropyl
NO nitric oxide
sGC-cGMP soluble guanylyl cyclase/cyclic guanosine monophosphate
QD every evening
LBN latanoprostene bunod
BBs β-blockers
hCA human carbonic anhydrase
AR adenosine receptor
LT-FC fixed combination Latanoprost and Timolol
BiT-FC fixed combination Bimatoprost and Timolol
TrT-FC fixed combination of Travoprost and Timolol
BKC benzalkonium chloride
PF preservative-free
TfT-FC fixed combination of Tafluprost and Timolol
LC-FC fixed combination of Latanoprost and carteolol
LC-nFC non-fixed combination of carteolol and latanoprost
NL-FC fixed combination of Latanoprost and Netarsudil
BiBr-FC fixed combination of Bimatoprost and Brimonidine
TrBz-FC fixed combination of Travoprost and Brinzolamide
LDz-FC fixed combination of Latanoprost and Dorzolamide
BrT-FC fixed combination of Brimonidine and Timolol
BzT-FC fixed combination of Timolol and Brinzolamide
DzT-FC fixed combination of Timolol and Dorzolamide
BzBr-FC fixed combination of Brinzolamide and Brimonidine
BrRi-FC fixed-concomitant of Brimonidine and Ripasudil
LBr-nFC non-fixed combination of Latanoprost and Brimonidine
TrBr-nFC non-fixed combination of Travoprost and Brimonidine
LBz-nFC non-fixed combination of Latanoprost and Brinzolamide
BiDz-nFC non-fixed combination of Bimatoprost and Dorzolamide
BeBr-nFC non-fixed combination of betaxolol and Brimonidine
BeBz-nFC non-fixed combination of betaxolol and Brinzolamide
BrDz-nFC non-fixed combination of Brimonidine and Dorzolamide
LRi-nFC non-fixed combination of Latanoprost and Ripasudil
TRi-nFC non-fixed combination of Timolol and Ripasudil
TDzBr-FC fixed combination of Dorzolamide, Timolol, and Brimonidine
TBiBr-FC fixed combination of Bimatoprost, Timolol, and Brimonidine
TBrTr-nFC non-fixed combination of Brimonidine, Timolol and Travoprost
TBzTr-nFC non-fixed combination of Brinzolamide, Timolol and Travoprost
LTBr-nFC non-fixed combination of Brimonidine, Timolol and Latanoprost
LTDz-nFC non-fixed combination of Dorzolamide, Timolol and Latanoprost
TDzTf-nFC non-fixed combination of Dorzolamide, Timolol and Tafluprost.
Keywords: glaucoma, monotherapy, fixed combination therapy, non-fixed combination therapy, intraocular pressure, adverse effects
Citation: Wang T, Cao L, Jiang Q and Zhang T (2021) Topical Medication Therapy for Glaucoma and Ocular Hypertension. Front. Pharmacol. 12:749858. doi: 10.3389/fphar.2021.749858
Received: 30 July 2021; Accepted: 16 November 2021;
Published: 01 December 2021.
Edited by:
Mario Damiano Toro, Medical University of Lublin, PolandReviewed by:
Giovanni Luca Romano, University of Catania, ItalyChiara Posarelli, Università degli Studi di Pisa, Italy
Copyright © 2021 Wang, Cao, Jiang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qikun Jiang, amlhbmdxaWt1bmxxcUAxNjMuY29t; Tianhong Zhang, emhhbmd0aDEwNThAYWxpeXVuLmNvbQ==
 Tao Wang1
Tao Wang1 Tianhong Zhang
Tianhong Zhang