- 1Key Laboratory of Xinjiang Phytomedicine Resource and Utilization, Ministry of Education, Pharmacy School of Shihezi University, Xinjiang, China
- 2School of Pharmaceutical Sciences, South-Central University for Nationalities, Wuhan, China
Alhagi sparsifolia Shap. (Kokyantak) is a ethnic medicine used in the Uyghur traditional medicine system for the treatment of colds, rheumatic pains, diarrhea, stomach pains, headaches, and toothaches, in addition to being an important local source of nectar and high-quality forage grass, and playing a crucial role in improving the ecological environment. Currently, approximately 178 chemical constituents have been identified from A. sparsifolia, including flavonoids, alkaloids, phenolic acids, and 19 polysaccharides. Pharmacological studies have already confirmed that A. sparsifolia has antioxidant, anti-tumor, anti-neuroinflammatory effects, hepatoprotective effects, renoprotective effects and immune regulation. Toxicological tests and quality control studies reveal the safety and nontoxicity of A. sparsifolia. Therefore, this paper systematically summarizes the traditional uses, botany, phytochemistry, pharmacology, quality control and toxicology of A. sparsifolia, in order to provide a beneficial reference of its further research.
Introduction
The Uyghur system of medicine shares its source with ancient Greek-Arab medicine, one of the three traditional medicines in the world and dating back to more than 2,500 years in Xinjiang, China. The Uyghur system has been widely used in a clinical setting and is based on unique clinical theories. It continues to play an important and non-negligible role in preventing and curing diseases and maintaining public health. Uyghur medicine originated in the Western Regions during the ancient Neolithic period in Hotan (known as Yutian in ancient times) (Maituoheti et al., 2017). Ancient Uyghur physicians believed in Shamanism. They engaged in divination and demon removal and also used prayer and medicine to cure diseases, which formed the prototype of Uyghur medicine. Around the 5th century BC, the ancient ancestors of Uyghurs had advanced surgical techniques and methods of bone grafting (State Administration of Traditional Chinese Herbal Editorial Board, 2005). With the opening of the Silk Road and the deepening of cultural exchanges between China and the West, the Uyghurs absorbed the essence of traditional Chinese, Arabic, Persian, and Indian medicine to establish the Uyghur system of medicine, which had unique characteristics (Wang, 1994; Liu et al., 2016; Zhang, 2018). The humoral theory is the core of the theory of Uyghur medicine, which is gradually formed on the basis of the four major material theories and temperament theory (Kalbinur and Zhang, 2021). Uyghur medical humoral theory believes that hilits (humors) are produced on the basis of four major substances, namely, fire, air, water, and soil, and the four mijazs (temperamental qualities) namely, dry, hot, wet, and cold (Guan and Zhu, 1995; Aili, 1998a; Aili, 1998b). Different humors have different mijazs (Hamulati, 2003; Liu J. B. et al., 2014). The four mijazs, Sapra (bilious humor), kan (blood humor), belhem (mucus humor), sawda (black bile humor) coordinate with each other, maintaining a state of relative dynamic equilibrium to achieve normal physiological function and good health. Uyghur drugs are the “life code” for Uyghurs for longevity (Wang and Jiahan, 2011). Based on the natural temperament of people, Uyghur medicine classifies medicines from plant, animal, and mineral origins into eight medicinal properties, namely, wet, hot, dry, cold, wet-hot, wet-cold, dry-hot, and dry-cold; and nine medicinal tastes, namely, pungent, sweet, bitter, light, hot, sour, salty, astringent, strong, and oily. A combination pattern of medicinal properties-medicinal flavors-organ properties was established and the laws of dispensing Uyghur medicine prescriptions were elaborated (He, 2016). Uyghur medicine is trusted and affirmed by patients because it utilizes unique botanical drugs and formulas and is associated with a rapid onset of action and efficacy.
A. sparsifolia (syn. A. kirghizorum var. sparsifolia Shap. and A. maurorum subsp. sparsifolium (Shap.) Yakovlev) (Figure 1), belonging to the Fabaceae family is one such plant that is widely used in China (Inaturalist, 2021; Plant Photo Bank of China, 2021; Royal Botanic Garden Edinburgh, 2021; The Plant List, 2021). It is a typical species in arid and semi-arid desert regions and an important source of nectar and high-quality forage grass in the Tarim and Turpan basins (Sun, 1989; Ma, 1993). A. sparsifolia is mainly used in traditional Uyghur medicine to alleviate physical fatigue and treat colds, rheumatic pains, diarrhea, stomach pain, headaches, and toothaches and is called Kokyantak in the Uyghur language (Li et al., 1996). It is currently included in the Standard of Uyghur Medicinal Materials in the Xinjiang Autonomous Region (Xinjiang Medical Products Administration, 2010). The sugary secretion from its stem and leaves constitutes an important ethnomedicine called Tarangabin, which is effective in the treatment of abdominal pain, diarrhea, and dysentery. It has been included in the “Pharmaceutical Standards of the Ministry of Health of the People’s Republic of China” (Uyghur Medicines) (Chinese Pharmacopoeia Commission, 1998).

FIGURE 1. The figure shows the habitat (A), flowers (B) and plant specimen (C) of A. sparsifolia ((A): http://ppbc.iplant.cn/tu/5783326, (B): https://www.inaturalist.org/photos/43301374, (C): http://data.rbge.org.uk/herb/E00364493).
To date, 178 chemical constituents, including flavonoids, alkaloids, and phenolic acids, and 19 polysaccharide fragments have been identified from A. sparsifolia. Modern pharmacological studies reveal that the isolated components and crude extracts exhibit varied pharmacological activities including antioxidant, antitumor, anti-neuroinflammatory, hepatoprotective, and renoprotective effects. However, sufficient links between these pharmacological activities and the traditional uses of A. sparsifolia have not yet been established. Moreover, the bioactivities of only a few monomers have been studied. Besides, although its long-term efficacy has been demonstrated in its use as ethnic medicine, comprehensive reviews with respect to its safety and quality control are lacking. Therefore, in this review, we have summarized and analyzed, for the first time, existing studies (from 1985 to 2021) on the botany, traditional uses, phytochemistry, pharmacology, quality control, and toxicology of A. sparsifolia. Our review indicates that the research prospect for A. sparsifolia is very broad and worthy of further investigation.
Material and Methods
The available information on A. sparsifolia was collected from scientific databases and cover from 1985 up to 2021. Information on A. sparsifolia was obtained from published materials, including monographs on medicinal plants, ancient and modern recorded classics, pharmacopoeias, Standard of Uyghur Medicinal Materials in Xinjiang Uyghur Autonomous region of China and electronic databases, such as Web of Science, Science Direct, Springer, Scifinder, X-MOL, PubMed, CNKI, Wanfang DATA, Google Scholar, Baidu Scholar, Flora of China (FOR). The search terms used for this review included “Alhagi sparsifolia Shap.“, “A. kirghizorum var. sparsifolia Shap.,” and “A. maurorum subsp. sparsifolium (Shap.) Yakovlev” all of which are accepted names and synonyms, “Saccharum alhagi,” “botanical characterization,” “flavonoid compounds,” “ethnomedicinal uses,” “quality standard,” “pharmacology,” and “toxicology.” Language restrictions were not applied during the search.
Botanical Description, Geographic Distribution, and Taxonomy
Botanical Description
A. sparsifolia is a deciduous shrub unique to the arid desert region of Xinjiang and is one of the “three treasures” of the Gobi Desert in preventing land desertification, resisting wind and sand erosion, and improving saline soil (Liu, 1985; Zhang et al., 2002; Arndt et al., 2004; Thomas et al., 2008; Jiang, 2017). There are seven species of the Alhagi genus worldwide, of which three are distributed in China and one is A. sparsifolia in the Flora of China (Zhang et al., 2008; Jin et al., 2014). In regions of high temperatures and low precipitation, the injured stems and leaves of A. sparsifolia secrete Tarangabin (Li et al., 1996; Wang, 2003; Mikeremu et al., 2016).
A. sparsifolia is a semi-shrub approximately 25–40 cm tall with an upright, glabrous stem having thin stripes. The leaves are alternate and ovate, obovate, or rounded ovoid, measuring approximately 8–15 mm long and 5–10 mm wide. The apex is round with short, hard tips, and the base is cuneate, entire, and glabrous with a short petiole. The flower is racemose, axillary, and 8–10 mm long. The rachis transforms into hard, sharp thorns that are 2–3 times as long as the leaves. The thorns of annual branches have 3–6 (or 3–8) flowers, but the older ones do not. The bract is subulate and up to 1 mm long, whereas the pedicel is 1–3-mm long. The calyx is campanulate, 4–5-mm long, and pubescent. The calyx teeth are triangular or subulate-triangular and one-third to one-fourth the length of the calyx tube. The corolla is reddish purple with a standard oblong-ovate shape and is 8–9-mm long, with an obtuse or truncated apex. The base is cuneate with a short petiole, the wing is oblong and three-quarters the length of the standard, and the carina is about the same length as the standard. The ovary and legume are linear and almost glabrous (Flora of China Editorial Committee, 1998). The harvest time of the various medicinal parts of A. sparsifolia differ. Flowers and leaves are collected in early summer (april to early May), Tarangabin is collected during midsummer (June to August), the seeds are collected in autumn (July to September), and the whole grass or aboveground parts are picked during the growing period of the year (Wang, 2003).
Geographic Distribution
A. sparsifolia is distributed in Central and East Asia, mainly in China, Kazakhstan, Uzbekistan, Turkmenistan, Kyrgyzstan, and Tajikistan (GBIF, 2021; Nishanbaev et al., 2016) (Figure 2). The official website, Flora of China, states that A. sparsifolia plants are mainly found in Inner Mongolia, Gansu and Qinghai provinces, Xinjiang Autonomous Region, China (Flora of China Editorial Committee, 1998). The latest MaxEnt model simulations predict that the suitable habitats of A. sparsifolia will decrease due to the climate change scenarios of RCP 2.6 and 8.5 on the whole, indicating that the abundance of this species will show a downward trend in the future (Yang et al., 2017).
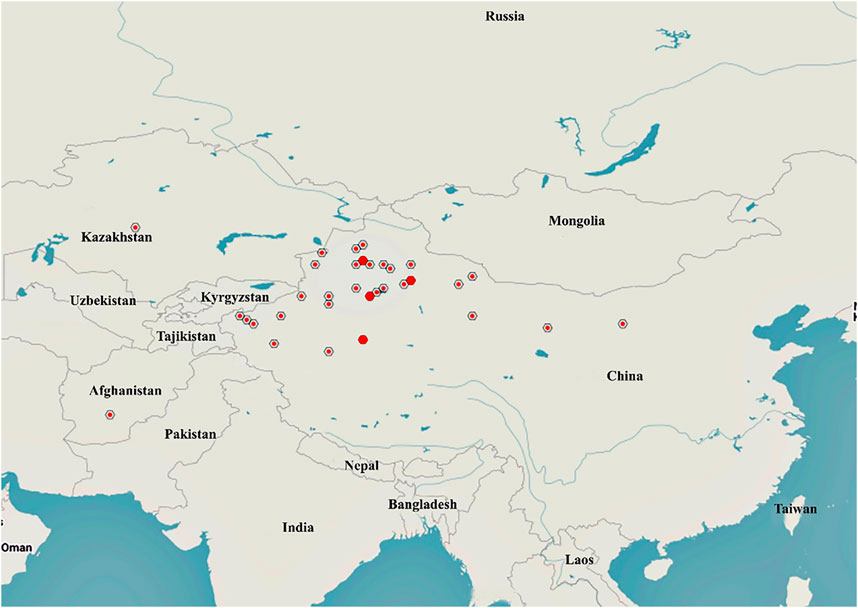
FIGURE 2. Distribution of A. sparsifolia in different countries and regions (https://www.gbif.org/species/2945088).
Taxonomy
A. sparsifolia belongs to the Fabaceae family, which consists of over 24,505 species belonging to 946 genera, including Glycine, Glycyrrhiza, Desmanthus, Lupinus, Medicago, Ormosia, and Styphnolobium. Among them, the genus Alhagi includes eight species, namely, A. graecorum Boiss., A. canescens (Regel) B. Keller & Shap., A. kirghisorum Schrenk, A. maurorum Medik., A. nepalensis (D.Don) Shap., A. pseudalhagi (M. Bieb.) Desv. ex B. Keller & Shap., A. sparsifolia Shap., A. sparsifolium (Shap.) Shap. (http://www.worldfloraonline.org/taxon/wfo-0000198672/).
Traditional Uses
A. sparsifolia was first found to be reported in “Bei shi” (AD 659) as Yang ci, whereas its medicinal value was first recorded in Tang Dynasty’s medical book, “Ben Cao Shi Yi” (AD 741) as Cao mi. In Ming Dynasty’s medical book, Compendium of Materia Medica (AD 1590), A. sparsifolia is listed as a “top grade” drug. It is a sweet, sour, and nontoxic drug usually used to treat abdominal pain, diarrhea, and dysentery. A. sparsifolia can be considered as “multiple medicinal parts in one plant,” i.e., different medicinal parts of the same plant have different pharmacological effects owing to differences in the chemical constituents and accumulation of the main components. Apart from being used routinely to treat colds and pains in various parts of the body, its leaves alleviate swelling and pain in joints; its flowers clear heat and detoxify the body; the whole plant alleviates colds and fevers, damp fever, and enteritis; and its seeds alleviate febrile dysentery and toothache (Yuan et al., 2012). Additionally, the therapeutic effects of Tarangabin depend on the route of administration. It is administered orally to treat hemorrhoids, as nasal drops to treat intractable headaches, and as eye drops to treat keratitis (Quan and Xu, 2009). As a traditional Uyghur medicine, A. sparsifolia is widely used in Uyghur medical practice in compound prescriptions with other botanical drugs. The prescription name, main composition, formulation, traditional and clinical uses, and prescription sources of A. sparsifolia are described in Table 1 (Chinese Medical Encyclopedia Committee, 2005).
Phytochemistry
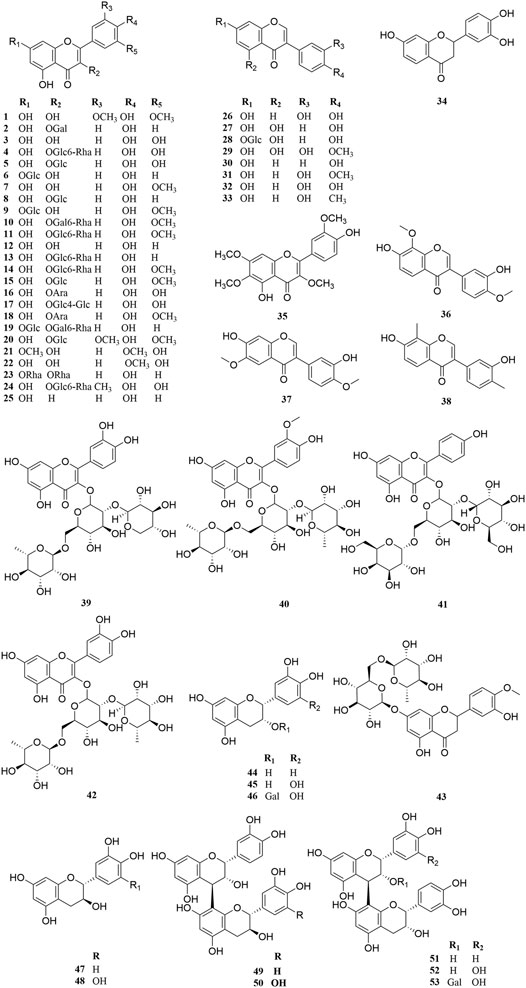
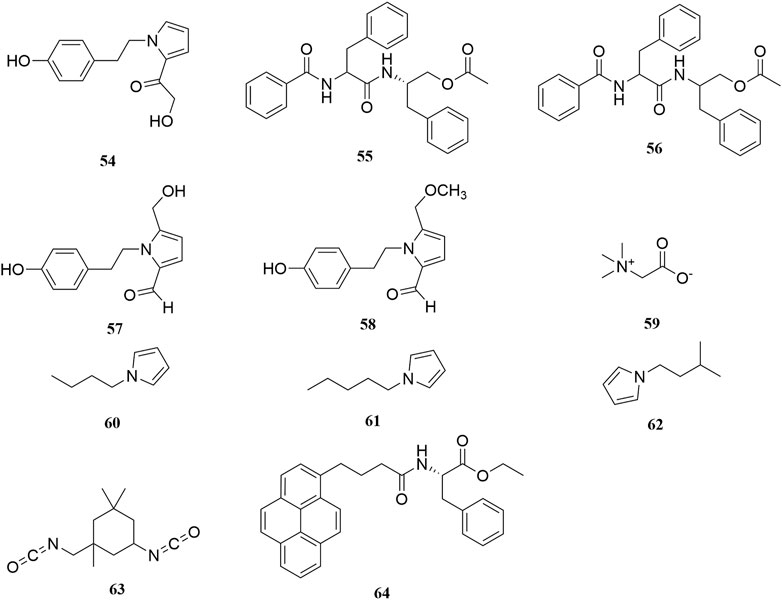
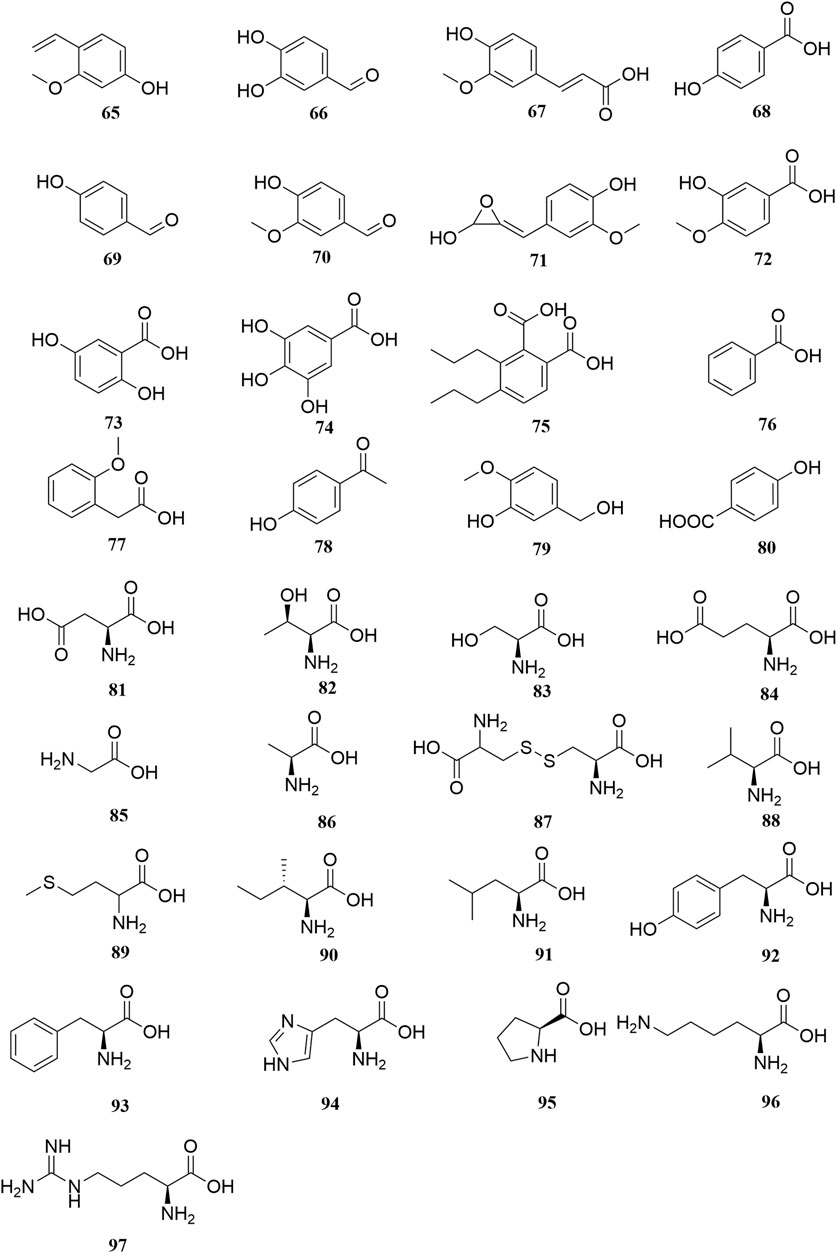
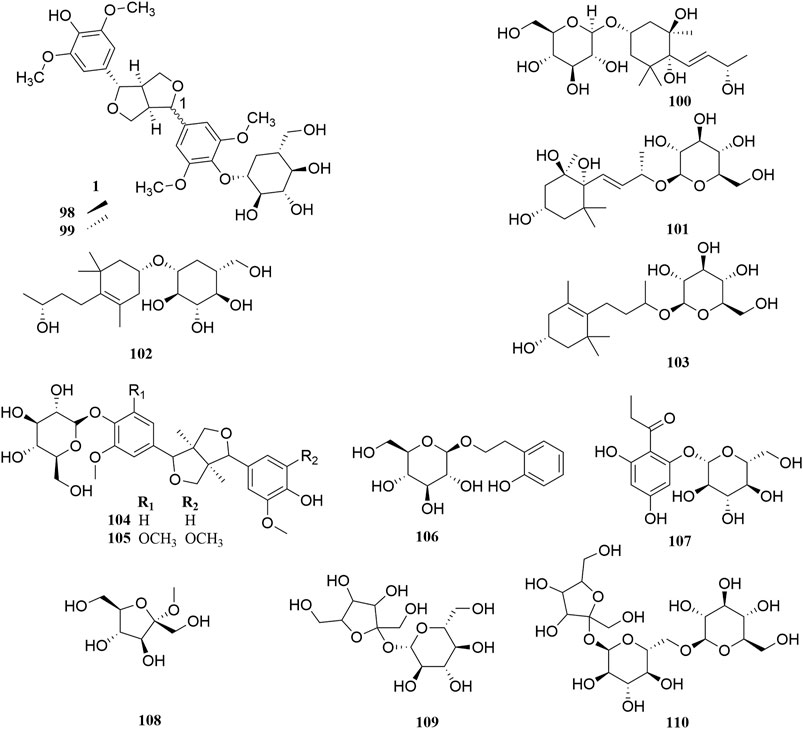
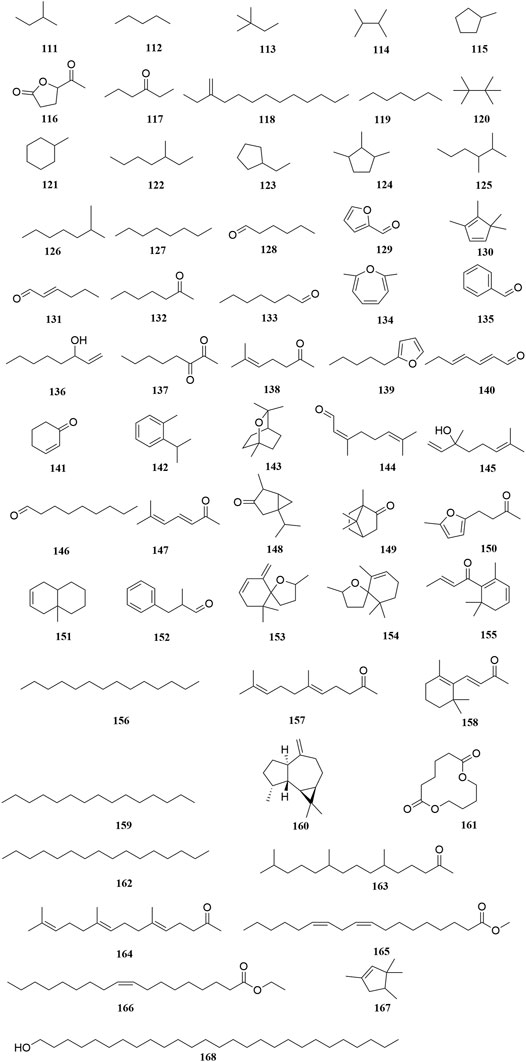
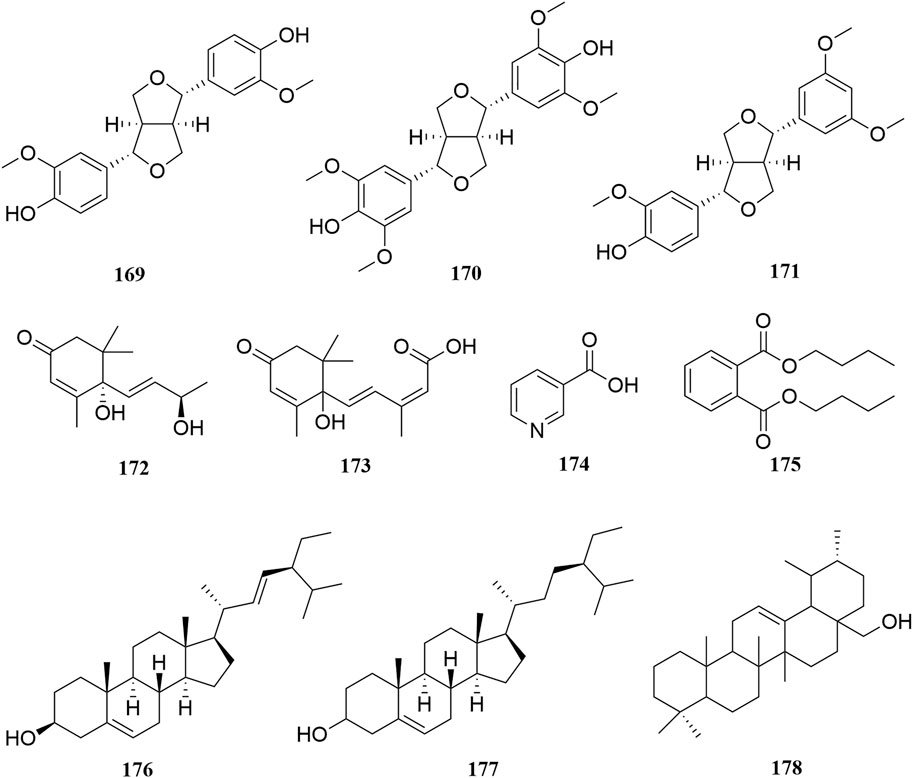
Investigation of chemical constituents from A. sparsifolia began in 1997. To date, approximately 178 chemical constituents have been identified, including flavonoids, alkaloids and phenolic acids, and 19 polysaccharides. Among them, flavonoids and polysaccharides are the predominant and characteristic constituents. The chemical constituents that have been identified are listed in Table 2 and their corresponding structures in Figures 3–8.
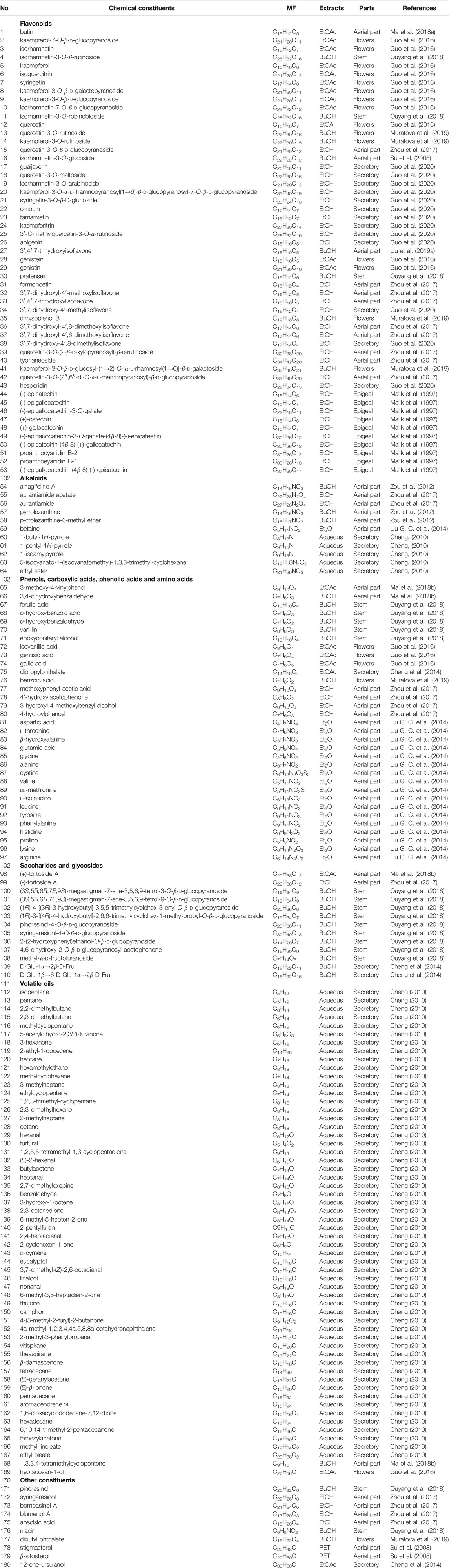
TABLE 2. Chemical components isolated and structurally identified from A. sparsifolia (MF = Molecular Formula).
Flavonoids
The Fabaceae family is rich in flavonoids, which have anti-inflammatory, antibacterial, and antitumor activity (Wang S. et al., 2019). Flavonoids have been the focus of attention in the field of drug research and development owing to their multiple biological activities and complex mechanisms (Qi and Dong, 2020). So far, over 53 flavonoids (1–53) have been reported in A. sparsifolia, including 30 flavones, 11 isoflavones, five catechins, five proanthocyanidins, and two flavanones (Guo et al., 2016; Ouyang et al., 2018; Guo et al., 2020). There are 26 flavonoid glycosides containing glucose (5–6, 8–9, 15, 20, 28), galactose (2, 46, 53), arabinose (16, 19), rhamnose (23), robinobiose (10, 19), rutinose (4, 11, 13–14, 24), sophorose (17), neohesperidose (43), and trisaccharides (39–41) (Malik et al., 1997; Su et al., 2008; Zhou et al., 2017). The parent structures of the flavonoids in A. sparsifolia are flavones, isoflavones, and catechins, all of which have phenolic hydroxyl substituents. Butin (1), the main active monomer in A. sparsifolia, can significantly inhibit the proliferation and migration of human cervical cancer Hela cells, human colon cancer HT-29 cells, human liver cancer HepG2 cells, human gastric cancer BGC823 cells, and human oral epidermoid carcinoma kB cells in vitro and was found to have synergistic anti-tumor effects in combination with 5-fluorouracil (Ma et al., 2015; Ma et al., 2018). Isorhamnetin-3-O-glucoside (2), isorhamnetin (3), and isorhamnetin-3-O-rutinoside (4) are the most abundant flavonoids and are often considered important indicators for the quality control of this plant (Xu et al., 2012; Sanawar et al., 2014). Moreover, differences in the total flavonoid content of different parts of the plant have also been reported, with the fruit and aerial parts having the highest content of total flavonoids, suggesting that different medicinal parts can be selected based on clinical use to maximize the utility of this plant (Chen et al., 2014).
Alkaloids
Alkaloids are important chemical compounds and a good research area for drug discovery. Numerous alkaloids screened from medicinal plants are known to exert antiproliferative and anticancer effects in several cancers both in vitro and in vivo (Mondal et al., 2019). To date, seven alkaloids have been isolated from this plant, including two organic amines (59, 63), six pyrrolidines (54, 57–58, 60–62), and three acylamides (55–56, 64) (Cheng, 2010; Zou et al., 2012; Liu G. C. et al., 2014; Zhou et al., 2017). Alhagifoline A (54) was the first novel compound isolated from the dried aerial parts of this plant; however, pharmacological studies related to its activity are lacking.
Phenols, Phenolic Acids, and Amino Acids
Thirty-three organic acids including phenols (65–66, 69–71, 78–79), phenolic acids (67–68, 72–77, 80), and amino acids (81–97) have been identified from A. sparsifolia (Liu G. C. et al., 2014; Cheng et al., 2014; Guo et al., 2016; Zhou et al., 2017; Ma et al., 2018b; Muratova et al., 2019). The substituents of these compounds include hydroxyl, carboxyl, methoxy, amino, and carbonyl groups. Seventeen amino acids have been isolated from the alkaline essential oils of this plant and were reported as the main components of drought resistance in this desert plant (Liu G. C. et al., 2014). The compounds 3-methoxy-4-vinylphenol (65) and 3,4-dihydroxybenzaldehyde (66) have been shown to inhibit tumor cell proliferation in the concentration range of 25–87 μM. Moreover, owing to their selectivity, these compounds may be used in the development of antitumor drugs in the future (Ma et al., 2018).
Saccharides and Glycosides
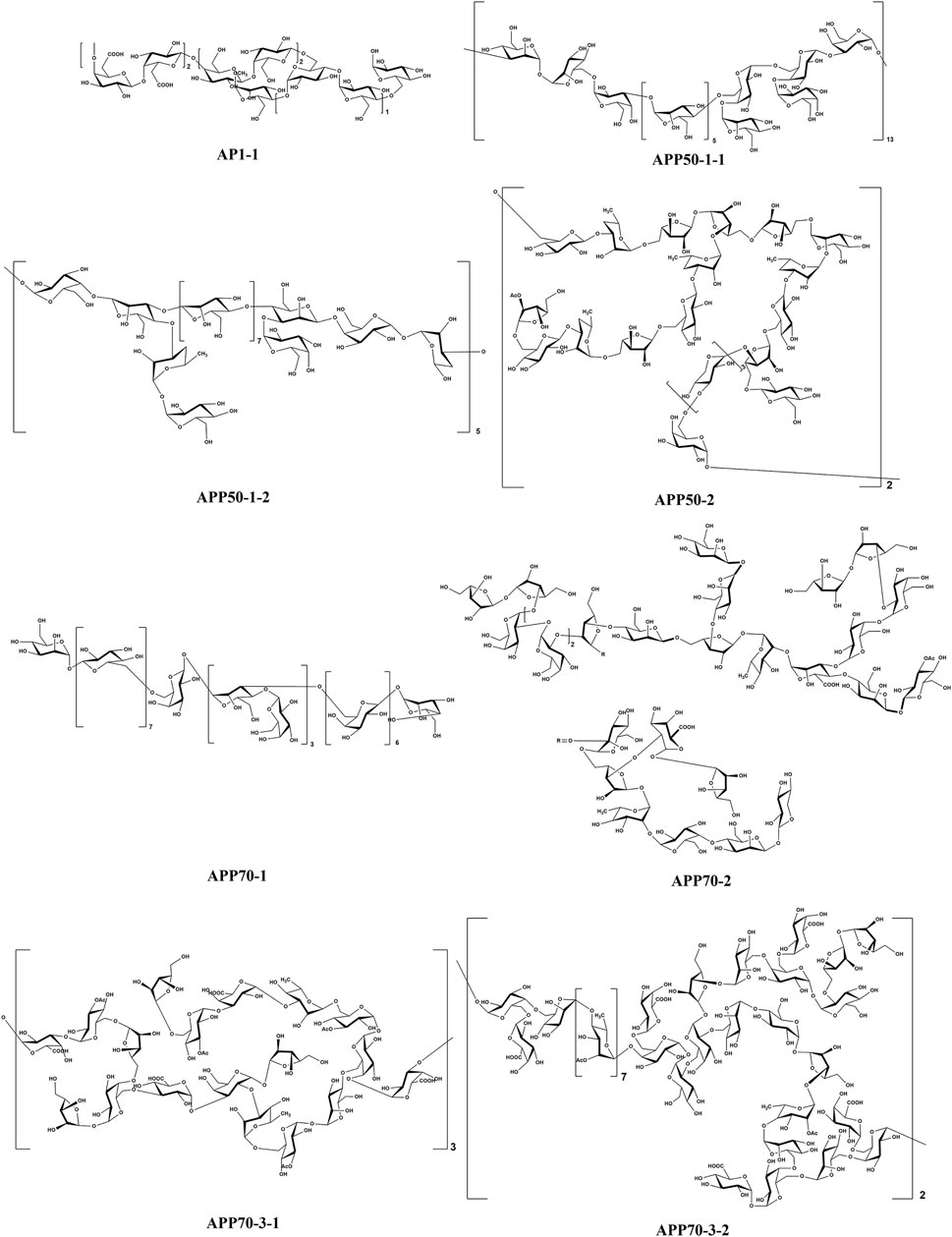
The stems of A. sparsifolia are rich in polysaccharides; thus, the isolation and purification of polysaccharides have been the emphasis of research with respect to the chemical constituents of this plant. The 19 isolated polysaccharides (AP1-1, AP1-2, AP1-3, AP1-4, AP1-5, AP2-1, AP2-2, AP2-3, AP2-4, AP2-5, SAP-1, SAP-1, SAP-3, APP50-1-1, APP50-1-2, APP50-2, APP70-1, APP70-2, APP70-3-1, APP70-3-2) contain different amounts of Rha, Ara, Xyl, Man, Glc, Gal, GlcA, and GalA (Chang et al., 2016; Jian et al., 2012; Jian et al., 2014; Li, 2020; Ma et al., 2018; Zheng, 2016; m). The possible chemical structures of seven of these polysaccharides (AP1-1, APP50-1-1, APP50-1-2, APP50-2, APP70-1, APP70-2, APP70-3-1, APP70-3-2) are summarized in Figure 9. The crude polysaccharide extract of A. sparsifolia and its monomeric components have significant antioxidant activity and can effectively scavenge free radicals in vivo. The higher the molecular weight, the stronger is the scavenging effect (Zhao et al., 2015; Ma et al., 2018). A study has reported the potential hypoglycemic effect of the crude polysaccharide of A. sparsifolia and that different doses can prevent the increase in fasting blood glucose levels in diabetic mice. The hypoglycemic mechanism may not be related to oxidative stress capacity (Zhao, 2016). In addition, two oligosaccharides (103–104) and 11 oxygen-containing glycosides (97–107) have been identified from A. sparsifolia, including alcoholic glycosides (99–102, 105, 107) and phenolic glycosides (97–98, 103–104, 106).
Volatile Oils
To date, 58 volatile oils (111–168) from the secretions of A. sparsifolia have been separated and characterized using nuclear magnetic resonance (NMR) and gas chromatography-mass spectrometry (GC-MS) (Cheng, 2010; Guo et al., 2016; Ma et al., 2018b). The isolated compounds are mainly composed of small-molecular lipophilic compounds including monoterpenes (142–145, 148–149), a sesquiterpene (160), aliphatic hydrocarbons (111–114, 118–120, 122, 125–127, 156, 159, 162), alicyclic hydrocarbons (115, 121, 123–124, 130, 151, 167), ketones (117, 128–129, 131–133, 135, 137–138, 140–141, 144, 146–147, 150, 152, 155, 157–158, 163–164), alcohols (136, 168), ethers (134, 139, 153–154), and esters (116, 161, 165–166). The structural skeleton of the components consists of five-membered, six-membered, oxygen-containing, and other irregular ring structures. The discovery of several essential oils from A. sparsifolia has significantly enriched its chemical database.
Other Constituents
A few lignans (169–171), sterols (176–177), triterpenes (178), and other compounds with irregular chemical structures (172–175) have been isolated from A. sparsifolia. There is no common structural skeleton among these components. Compounds (169–173) were reported to have dose-dependent anti-neuroinflammatory effects.
Pharmacology
Pharmacological studies have indicated that A. sparsifolia has several pharmacological effects, including antioxidant, hepatoprotective, and renoprotective effects. Moreover, it affects the survival rate of rats in a dry and hot environment and plays a role in immune regulation and has antitumor and anti-neuroinflammatory effects. The pharmacological effects of A. sparsifolia and its monomers are summarized in Table 3 and their possible potential pharmacological mechanisms are summarized in Figure 10.
Antioxidant
A. sparsifolia supposedly exhibit the mostpotent antioxidant activities determined by TEAC assays. Phytochemical studies have shown that A. sparsifolia is rich in polysaccharide components, which are closely associated with antioxidant effect (Ma et al., 2018). This indicates that the polysaccharides from A. sparsifolia may be a rich source of natural antioxidants that may help prevent and treat diseases related to oxidative stress. Liu et al. (2021) found that the aqueous extract of A. sparsifolia stem and branch (ASSBP) demonstrated dose-dependent moderate antioxidant activity when assayed against TEAC, with serum TEAC levels of mice in the low dose group (50 mg/kg) and medium dose group (100 mg/kg) comparable to the positive control group of Lentinan (630 mg/kg) and slightly higher levels in the high dose group (200 mg/kg). The results showed that ASSBP had certain antioxidant ability and enhanced immune activity in both normal and d-galactose-induced aging mice, increased spleen and thymus indices in normal mice, increased serum SOD activity and decreased MDA content in normal mice. It is suggested that its anti-aging effect may be exerted through anti-lipid peroxidation, increasing SOD activity and decreasing MDA content.
Hepatoprotective Effects
In a study, the aqueous extracts of Tarangabin at the doses of 150, 300, 600 mg/kg had significant hepatoprotective effects at different concentrations in mice with liver injury induced by N-acetyl-para-aminophenol (APAP). It showed a significant decrease in the level of serum alanine aminotransferase (ALT) and serum aspartate aminotransferase (AST). The results of content determination showed that the polysaccharide content of the extract was as high as 69.2%, thus it is supposed that the polysaccharides have a controlling effect on APAP-induced liver injury (Aili et al., 2017). The hepatoprotective mechanism of Tarangabin has been partly attributed to the reduction of oxidative stress and inhibition of the expression of cytochrome P450 2E1 (CYP2E1) (Aili et al., 2018). In alcoholic liver disease (ALD), Tarangabin regulates the expression levels of tumor necrosis factor (TNF)-α and toll-like receptor 4 (TLR4) mRNA by acting on the lipopolysaccharide-TLR (LPS-TLR) signaling pathway, thereby improving the severity of ALD. Moreover, this plant is known to bring about the effects of reducing the gene expression of TLR4, inhibiting the release of TNF-α, preventing further signal transmission, reducing the efficiency of endotoxin signal transduction, and decreasing the production of inflammatory factors, thereby preventing hepatocyte damage. Tarangabin promotes liver tissue repair and hepatocyte regeneration and protects from liver injury (Kuerbanjiang et al., 2017; Wang X. L. et al., 2019).
In addition, A. sparsifolia significantly alleviated alcohol-induced liver injury by reducing serum ALT and AST, inhibiting MDA and hydrogen peroxide (H2O2), and increasing SOD and glutathione (GSH) level in the liver (p < 0.05). Additionally, treatment with A. sparsifolia was found to significantly reduce the expression of TNF-α and TLR4 mRNA in the brain of mice, thus accelerating alcohol metabolism and reducing oxidative stress by downregulating CYP2E1 expression (p < 0.05), which has a protective role in ALD (Kuerbanjiang et al.. 2018).
Renoprotective Effects
Tarangabin supposedly protected against high-dose gentamicin (GM)-induced acute kidney injury in mice. Although the efficacy is attributed to the organic components and Zn, Fe, Cu, Co, Ni, Mn, and K content of Tarangabin, the specific mechanism of action needs to be further elucidated (Mikeremu et al., 2013). Wumaierjiang et al. (2014) found that Tarangabin had a certain protective effect in acute renal failure caused by mercuric chloride, and the best effect was achieved when the concentration of Tarangabin was 15% (Ma et al., 2018).
Gastrointestinal Effects
A. sparsifolia and Tarangabin have a dual regulatory effect on the small intestinal motility in mice. The aqueous extract of A. sparsifolia and Tarangabin could inhibit intestinal propulsion induced by the M-choline receptor blocker, atropine sulfate, and alleviate hyperactivity of small intestinal propulsion induced by the M-choline receptor stimulant, carbamyl-B-methylcholine chloride, in a dose-dependent manner. The dual regulation effect of A. sparsifolia and Tarangabin may be attributed to the high taurine content in the plant. It has been reported that taurine has a dual regulatory effect on intracellular Ca2+. A certain dose of taurine can enhance intestinal smooth muscle contraction, but the effects of different concentrations of taurine on smooth muscle are different. Low and medium doses of taurine enhance smooth muscle contraction, whereas high doses of taurine have the opposite effect (Mikeremu et al., 2017).
In a particular study, male Sprague-Dawley (SD) rats were subjected to restraint stress and fed a high-lactose diet to establish a diarrhea-predominant model of irritable bowel syndrome (IBS-D), which was further used to evaluate the regulatory effect of A. sparsifolia extract in IBS-D. The extract was found to significantly reduce the fecal water content and abdominal wall myoelectric activity, and significantly increase the volume threshold of abdominal contractile reflex and serum nitric oxide (NO) levels. Additionally, A. sparsifolia extract was found to improve visceral hypersensitivity and decrease the myoelectric activity of the abdominal wall in IBS rats, which may be related to the decrease in the secretion and release of NO (Wei et al., 2016; Ma et al., 2018c).
In a recent study, Liu et al. (2019b) have shown that in IBS-D rats, A. sparsifolia extract can decrease the abnormally elevated levels of the gastrointestinal hormones, 5-hydroxytryptamine (5-HT), substance P (SP), and motilin (MTL); regulate water metabolism to inhibit gastrointestinal motility and delay gastric emptying; and improve abdominal distension, abdominal pain, and diarrhea, which may be the mechanism of this botanical drug in the treatment of this condition. However, the tension of the intestinal smooth muscle and related intestinal electrolyte levels were not observed in this study, and the vasoactive intestinal peptide (VIP) levels following the administration of a high dose of A. sparsifolia extract was not higher than that observed after administration of a low dose of the extract. The antagonism between various components in the extract or the effect of some components in the extract as autoinducers may be the likely causes of this abnormal phenomenon.
Affecting the Survival Rate of Rats in a Dry, Hot Environment
The incidence of summer heat strokes and heat radiation in dry and hot desert environments is increasing every year. Dong et al. (2019) found that the desert plant, A. sparsifolia, can improve the heat tolerance of rats in a dry and hot environment by delaying the increase in core body temperature, thereby improving their survival rate in dry and arid conditions.
Immune Regulation
Results from in vitro cellular assays suggested that SAP-1, SAP-2, and AP1-1 promoted the proliferation and cellular immunity of splenic lymphocytes and RAW264.7 macrophages. These components could promote the secretion of cytokines IL-1β, IL-6, IL-12, and NF-κB. The mechanism of action was via the MyD88-dependent pathway, which activated the immune response of TLR4 receptors (Han et al., 2017). Results from in vivo experiments in mice indicated that the polysaccharide extract could enhance carbon particle scavenging ability, increase serum hemolysin levels in immunosuppressed mice, and also increase serum IL-2 and IL-6 levels in cyclophosphamide-induced immunosuppressed mice (Zhao, 2016; Zhao et al., 2016; Qu, 2019). In addition, A. sparsifolia extract was found to significantly increase the number of white blood cells and lymphocytes in peripheral blood, reduce the atrophy of immune organs, improve the ability of lymphocyte transformation in cyclophosphamide-induced immunosuppression, and enhance delayed hypersensitivity in mice (Ma et al., 2017).
Anti-Tumor
The inhibitory effects of ethanol extracts from aerial part of A. sparsifolia on human gastric cancer (BGC-823), human esophageal cancer (Eca-109), human colon cancer (HT-29) and human hepatocellular carcinoma (HepG2) cells were investigated in vitro by MTT assay with IC50 of 1.62, 1.32, 1.55 and 1.45 mg/ml, respectively. To further clarify its in vivo antitumor efficacy, a CT26 mouse model of colon cancer was established. The in vivo tumor-inhibition activity was investigated by comparing the tumor-proliferation rate, growth curve, and tumor-inhibition rate in each group of mice. The results showed that only the high dose (1,000 mg/kg, intragastric × 14 qd) led to significant in vivo antitumor activity with a tumor-inhibition rate of 24.8%. Compared with that in the negative control group, serum IL-2 levels of mice that received a high dose of the extract increased significantly. Thus, the antitumor mechanism may be related to the increase in IL-2 (Ma et al., 2015).
Two active monomers, butin (1) and 3,4-dihydroxybenzaldehyde (66), extracted from the n-butanol extract of A. sparsifolia, inhibit the proliferation and migration of human cervical cancer (HeLa) cells and exert a strong inhibitory effect in vitro with synergistic antitumor effects with 5-FU. However, the related targets and signaling pathways of its antitumor effects are still unclear (Liu et al., 2019a). Using Hela cells as the target cells, the proliferation inhibition ability of each different polar extracts on tumor cells was detected by MTT assay, and butanol and ethyl acetate extracts among them were clearly shown to have certain inhibition effect on the proliferation of tumor cells. Further studies proved that butin (1), (+)-tortoside A (98), 3-methoxy-4-vinylphenol (65), 3,4-dihydroxybenzaldehyde (66), and 1,3,3,4-tetramethyl-cyclopentene (167) were the active monomers exerting antitumor effects. They also showed good inhibitory effects on HT-29, HepG2, BGC823, and KB tumor cells, exhibiting potential as antitumor agents (Ma et al., 2018b).
Anti-Neuroinflammatory Effects
Microglia cells are considered to be key innate immune cells in the central nervous system (CNS) and an important contributor to neuroinflammation. However, microglia cells are particularly sensitive to changes in their microenvironment and readily become activated in response to infection or injury. Under activated conditions, they secrete and release a mass of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interferon-γ (IFN-γ), interleukin-6 (IL-6), and free radical mediators such as nitric oxide (NO) and reactive oxygen species (ROS). Accumulation of the pro-inflammatory and neurotoxic factors might aggravate the pathogenesis of neurodegenerative diseases. The LPS-stimulated N9 cells were used to evaluate the anti-neuroinflammatory activity of 33 compounds isolated and identified from the ethanol extracts from aerial part of A. sparsifolia. Among the examined constituents, compounds 1, 3, 4, 15, 32, 36, 37, 42, 54, 65, 79, 99, 167, 169, 170, 171, 172, and 173 could considerably inhibit NO production in LPS-induced N9 microglial cells in a dose-independent manner without evident cytotoxicity at the tested concentrations. The effect of 33 compounds on the anti-neuritis activity of N9 cells by MTT assay revealed that flavonoids and lignans exhibited anti-inflammatory effects, whereas their glycosides were not as effective (compound 3 vs 40 [IC50 17.87 vs > 100], 170 vs 105 [IC50 2.68 vs > 100]). In addition, isorhamnetin (3) (IC50 17.87 μM), quercetin (4) (IC50 10.22 μM), 3′,7-dihydroxyl-4′-methoxylisoflavone (32) (IC50 17.43 μM), 3′,7-dihydroxyl-4′,6-dimethoxyl isoflavone (37) (IC50 11.21 μM), syringaresinol (170) (IC50 2.68 μM), bombasinol A (171) (IC50 7.61 μM), aurantiamide (54) (IC50 14.91 μM), and 1,3,3,4-tetramethylcyclopentene (167) (IC50 2.63 μM) showed much stronger anti-neuroinflammatory effects without obvious cytotoxicity at their effective concentration compared with the positive control, minocycline (IC50 19.89 μM). The mechanism of action may be related to the inhibition of excessive activation of microglia and thus the inhibition of the production of pro-inflammatory mediators and cytokines (Zhou et al., 2017).
Other Activities
In addition to the above pharmacological effects, the isolated compounds and crude extract of A. sparsifolia showed antibacterial, antidiabetic, and growth-promoting effects. The aqueous extract exhibited good antibacterial activity against Escherichia coli and Staphylococcus aureus and had a minimum inhibitory concentration (MIC) of 62.5 mg/ml (Lei et al., 2004). A gavage of the polysaccharide (at a dose of 200 mg/kg) administered to male mice with hyperglycemia significantly suppressed fasting blood glucose levels (Zheng, 2016). Moreover, the polysaccharides were found to exert growth-promoting and hemoglobin-increasing effects in mice (Hairula et al., 2014).
Quality Control
Although Uyghur medicines have shown unique efficacy in the treatment of several diseases and gained increasing attention and recognition, there is still a big gap in the industrialization, standardization, and the mode of standardization of Uyghur medicines. First, there is the problem of poor basic research. According to statistics, there are still about 250 Uyghur drugs without defined standards among the 500 commonly used drugs. Some of the drugs for which standards are available have unclear identification of origin, phenomena of synonym or homonym, translation errors, and unverified Latin names. Secondly, the level of quality standards of plant species used in Uyghur medicine species is low, the number of standardized species is small, and the identification specificity is not strong; thus, effective quality control of Uyghur medicine poses a challenge. Thirdly, the scientific clinical research evaluation system including the clinical efficacy evaluation index and research methods of Uyghur medicine has not been established, and evaluation of the clinical efficacy of Uyghur medicine lacks a standardized, objective, and recognized index. There is a lack of in-depth research and exploration of the rationality of the composition of ethnic medicines, the scientific nature of the process, and the active components of drugs. Therefore, it is crucial to establish complete quality standards and suitable extraction methods for the quality control of A. sparsifolia.
Studies on the Quality Standards of A. sparsifolia
A. sparsifolia, as a common ethnic medicine in Uyghur medicine, has a long history of use. Presently, A. sparsifolia is not included in Pharmacopoeia of the People’s Republic of China (ChP). With continuous improvements in modern separation and identification methods, some investigators have used various methods to evaluate the chemical compounds and control the quality of A. sparsifolia. For example, Hu (2010) determined the moisture, total ash, leachate, and total flavonoid content of A. sparsifolia in Tuokexun County, Xinjiang, by referring to the identification items and standardized methods in the ChP. The specificity of isorhamnetin (3), isorhamnetin-3-O-glucoside (17), and isorhamnetin-3-O-rutinoside (12) in A. sparsifolia was determined using TLC, whereas the levels of the main chemical component, isorhamnetin-3-O-rutinoside, were determined using HPLC and found to be 0.1355%, on average. In the same year, Sanawar et al. (2012) conducted a similar study on 12 A. sparsifolia samples collected from the Turpan region of Xinjiang. In addition, they focused on describing the plant traits and determining isorhamnetin content using HPLC. The average isorhamnetin content was determined to be 0.14%. To improve the credibility and accuracy of the quality standard, the XinJiang Institute of Chinese Traditional Medica and Ethical Materia Medica conducted a more comprehensive quality standard study on samples collected from five different regions in Xinjiang by random sampling. The findings revealed that the impurities did not exceed 0.5%, moisture content did not exceed 10%, and total ash content did not exceed 12%. Moreover, unique TLC and HPLC methods for rutin were established (Xu et al., 2012). In addition, Chen et al. (2014) determined the levels of total polysaccharides and rutin in eight samples of A. sparsifolia using ultraviolet spectrophotometry (UVS) and compared its levels in different parts of the plant. The results showed that the total polysaccharide and rutin content in the fruit and aerial parts were relatively high. In another study, HPLC and similarity evaluations were used to establish the chromatographic fingerprints of 10 A. sparsifolia samples collected from different townships in Tuokexun County, Xinjiang (Sanawar et al., 2013), which revealed 11 common peaks in the fingerprints of A. sparsifolia obtained from 10 habitats. However, there were differences in the peak heights of the common peaks in A. sparsifolia obtained from different habitats, indicating differences in the levels of the primary components of botanical drugs obtained from different sources due to local climate and harvesting time. Guo et al. (2020) were the first to use UPLC-Q-TOF-MS for the qualitative analysis of flavonoids from Tarangabin. Using a web-based database and masslynx 4.1 workstation, 40 compounds were analyzed and identified, of which 22 were reported for the first time. Their study provides a scientific basis for the establishment of a compound database and quality standards. Establishment of quality standards for A. sparsifolia will accelerate its entry into the ChP and provide theoretical support and serve as a reference standard for its use in a clinical setting.
Extraction and Separation Methods
Flavonoids are the main components and active compounds in A. sparsifolia; thus, optimizing their extraction is essential for quality control and ensuring efficacy. Su et al. (2009) found that the extract (1 g: 20 ml) of A. sparsifolia collected from Tuokexun County purified with 40% ethanol (1.5 h × 3 times) could extract 1.70% of the total flavonoid based on orthogonal experiments. At a temperature of 90°C, its extracts (1 g: 20 ml) were purified with 40% ethanol (1 h × 3 times), and the extraction ratio of total flavonoids was 1.33% when A. sparsifolia samples from Urumqi, Xinjiang, were used (Shi et al., 2014). Guo et al. investigated the enrichment ability of AB-8, DM301, and D-101 macroporous resins for total flavonoids in prickly sugars, and finally selected AB-8 resin for the enrichment and purification of total flavonoids from prickly sugars. The ideal extraction process was obtained based on Box-Behnken response-surface optimization, wherein ethanol concentration was 67%, the material:liquid ratio was 1:25, extraction time was 75 min, and extraction temperature was 75°C. The average total flavonoid yield extracted from Tarangabin collected from Hotan, Xinjiang was 0.3889% (Guo et al., 2020).
Toxicology
It is well known that drugs have dual effects, namely therapeutic effects and adverse reactions. In recent years, Uyghur medicines have been widely used and the incidence of adverse reactions has increased accordingly, thereby raising serious questions regarding their safety in a clinical setting. Toxicity studies on Uyghur drugs will help provide a reference for drugs in the treatment of diseases and ensuring the safe use of drugs. Chronic toxicity studies of different doses (3.0, 1.0, 0.3 g/kg) of the total flavonoid extract from the aerial parts of A. sparsifolia in mice show that the routine hematological and biochemical indices after 13 weeks of gavage were not different compared with the control group. Furthermore, no significant differences were found between the control and drug-treated groups, and the isolated organs did not exhibit any pathological changes following drug treatment, indicating that A. sparsifolia extracts to be safe over a wide dose range. Similar results were obtained for the total alkaloid extracts from the aerial parts of A. sparsifolia (Ma et al., 2014; Zheng et al., 2014). Nevertheless, these findings constitute insufficient evidence to prove the nontoxicity and safety of A. sparsifolia. Acute toxicity experiments should be conducted to assess the safety and reliability of this drug when administered at regular doses.
Future Outlooks
In this review, we have provided a critical analysis of the botany, traditional uses, phytochemistry, pharmacology, quality control, and toxicology of A. sparsifolia, which is widely used in the traditional Uyghur system of medicine for the treatment of colds, rheumatic pains, diarrhea, stomach aches, headaches, and toothaches. Modern pharmacological studies have shown the plant components to exert antioxidant, antineuritic, antitumor, immunomodulatory, hepatoprotective, and renoprotective effects. With an improvement in extraction techniques and advancement in pharmacological research, some success has been achieved in determining the chemical composition and pharmacological effects of this plant. However, further studies are warranted for a more thorough understanding. Therefore, we have highlighted and summarized a few topics, which should be investigated further.
First, owing to a lack of basic research in the Uyghur medical system, there exist problems of inaccurate plant nomenclature, leading to misuse and confusion with respect to their use. Kurban and Vonlanthen believe that Alhagi pesudalhagi, Alhagi kirghisorum Schrenk, Alhagi maurorum Medik, and Alhagi camelorum Medik all refer to the plant (A. sparsifolia) (Kurban et al., 1998; Vonlanthen et al., 2010; Yuan et al., 2012). At present, there is only one species of A. sparsifolia in the Flora of China, and it is believed that A. pseudalhagi, A. maurorum var. Sparsifolium, and A. camelorum are synonyms for this plant. However, in the NCBI and TPL databases, A. sparsifolia Shap, A. pseudalhagi, A. camelorum Medik, and A. kirghisorum Schrenk belong to different species of the same genus. The literature search revealed that A. pesudalhagi and A. sparsifolia were used interchangeably in many studies, which also indicates that this review is based on literature that may not be very reliable. Furthermore, the safety and quality evaluation of this plant has been reported in several studies; however, the established evaluation methods are nonstandardized and incomplete due to the technical and methodological limitations at that time. Several researchers have evaluated the quality of A. sparsifolia from different origins or different harvesting periods and analyzed the similarities and differences of their chemical composition. However, one or two samples were often used to represent an appellation or even a province, and the reliability of the quality evaluation was greatly diminished by using a certain component (total flavonoids or polysaccharides) or even a single component (isorhamnetin or its glycosides) for quality evaluation. Therefore, standardized medicinal standards should be established to guide the medicinal use and ensure the quality of drug preparations when using this medicinal plant.
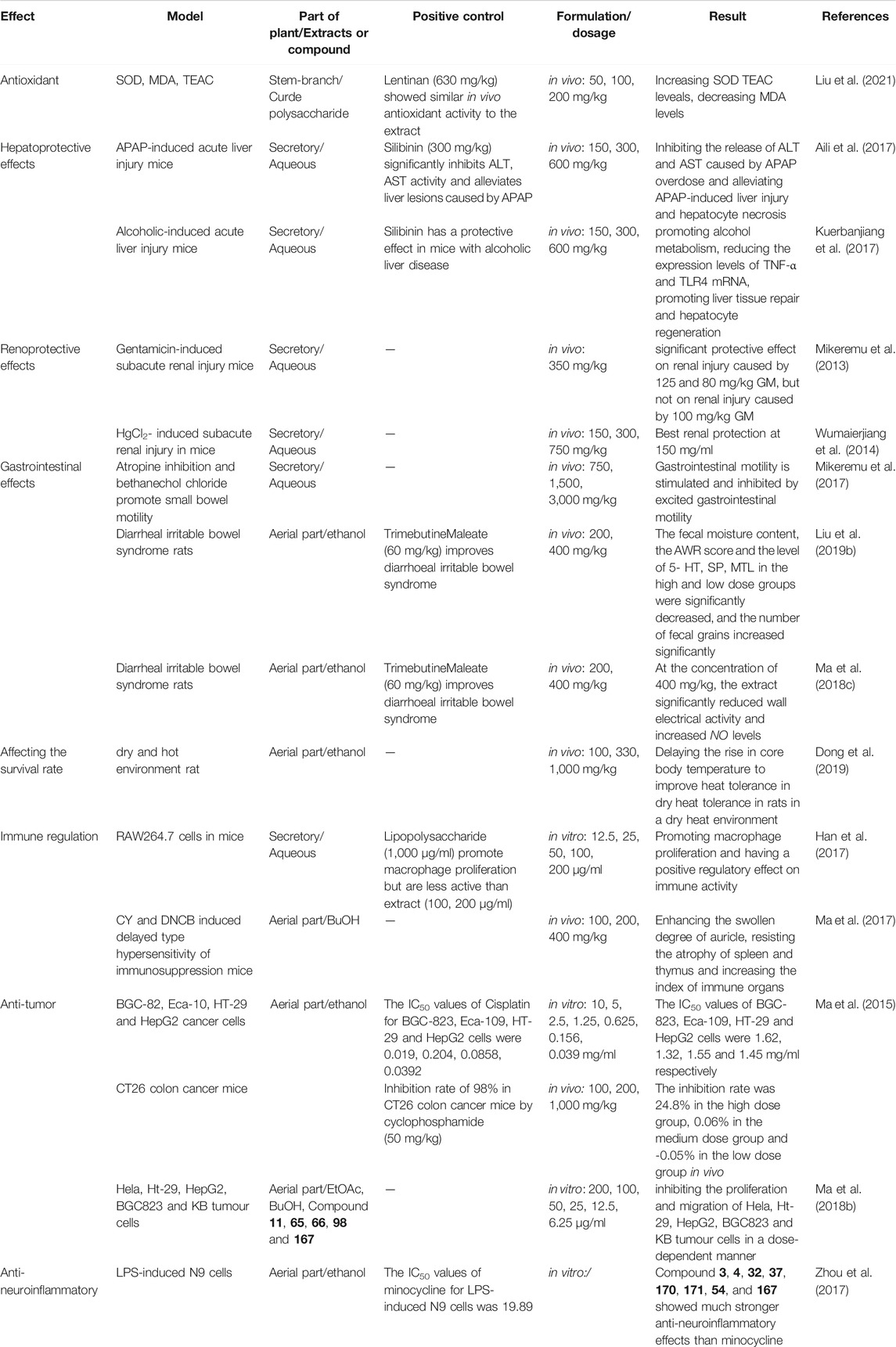
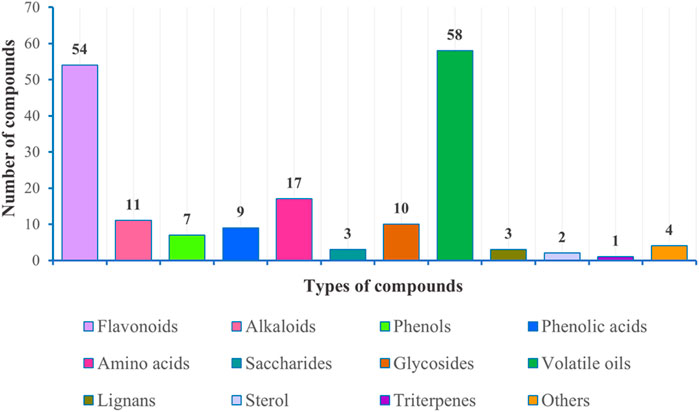
Second, we determined the extent of research conducted on the chemical composition of different medicinal parts of the plant, and the percentage (%) of active components was calculated based on the number of isolated compounds from extracts of different parts of the plant (Figure 11). To further clarify the main chemical constituents in the plant, the number of different types of compounds was statistically analyzed (Figure 12). By comparing the data, we found that the study of the chemical composition of A. sparsifolia was mainly focused on the secretory parts (43.58%), aerial parts (27.93%), flowers (12.29%), stem (10.61%), and epigeal parts (5.59%), with almost no studies on its seeds and roots. Tannins, saponins, and coumarins have been isolated from the seeds and roots of other plants from the same genus, their pharmacological activities have been demonstrated, and their presence has been confirmed using colorimetric assay; however, similar studies for A. sparsifolia have not been reported (Cheng, 2010; Srivastava et al., 2014; Muhammad et al., 2015). Therefore, the chemical constituents of the seeds and roots should be studied with the aim of isolating the active compounds and enriching the existing knowledge of the chemical constituents of A. sparsifolia. Although a wide range of pharmacological activities of various flavonoids and polysaccharides isolated from A. sparsifolia have been reported, research on the pharmacological effects and targets of alkaloids, phenols, and phenolic acids is relatively scarce, which deserve to be further explored. The compounds with the parent nucleus structure of flavone are the most abundant chemical constituents of the plant, among which Butin (1) is the material basis for the significant in vitro and in vivo antitumor effects of the plant. It is necessary and meaningful to further verify whether other compounds with the same parent nucleus structure have the same antitumor activity. Polysaccharides are considered to be biologically active components with antioxidant, hepatoprotective effects, renoprotective effects, regulation of the intestinal and immune regulation. However, polysaccharides are structurally complex and difficult to isolate, and it is challenging and promising to carry out studies on their chemical composition. Furthermore, most studies have focused on the analysis of serum biochemical parameters and cellular expression levels without the in-depth exploration of specific mechanisms and pharmacological targets, severely limiting the possibility of exploring other potential pharmacological activities and developing the active components as new drugs. Therefore, the chemical composition of A. sparsifolia should be further elucidated to better understand the pharmacological activity and specificity of each compound.
Third, Numerous studies have shown that A. sparsifolia may be a promising candidate for the treatment of cancer and alcoholic liver disease. Flavonoid and phenolic acid active compounds from A. sparsifolia exert their antitumor effects mainly through inhibition of proliferation and migration, induction of apoptosis and improvement of immune function. The hepatoprotective mechanism is attributed to the reduction of oxidative stress and inhibition of CYP2E1 expression, and improvement of ALD by acting on the LPS-TLR signaling pathway to regulate the expression levels of TNF-α and TLR4 mRNA. Nevertheless, the relevant targets and signaling pathways for the anti-tumor effects and the specific active compounds for the hepatoprotective effect are still unclear and need further investigation. Polysaccharides are a class of natural macromolecules with various pharmacological activities, and the results have shown that polysaccharides in A. sparsifolia can be used for the prevention and treatment of diseases related to aging and oxidative stress, and are a class of natural antioxidants worth developing. A. sparsifolia has also been reported to have antidiabetic, antibacterial, anti-inflammatory, immunomodulatory and gastrointestinal effects, but the studies are not comprehensive enough and its relevant pharmacological properties need to be confirmed by designing additional and more in-depth pharmacological experiments. In addition, A. sparsifolia has been used as ethnic medicine by Uyghurs for hundreds of years and numerous empirical prescriptions known for their significant therapeutic effects have emerged. Nevertheless, the relationship between traditionally reported outcomes and modern pharmacological activity has not been thoroughly investigated. Traditionally, the plant has been used in the treatment of pain in various parts of the body owing to its unique efficacy, but modern pharmacological studies have not yet identified any compounds in this plant that are analgesic in nature. A. sparsifolia belongs to class II wet-heat drugs in the Uyghur system of medicine, in which drugs exhibiting antihypertensive, anticancer, and antidiabetic effects are included; however, no scientific experiments have been designed to prove these effects. The urinary tract effect, antipyretic effect, musculoskeletal effect and cardiac effect have been already investigated in other plants of the same genus, but so far no similar activity has been reported in this plant and the studies of similar activity are the focus of future research on this plant (Tavassoli, 2020). Chronic toxicity tests reveal the safety and nontoxicity of A. sparsifolia; however, this evidence may be insufficient. Therefore, additional studies evaluating the acute toxicity, safety profile, reproductive toxicity, and genotoxicity are warranted to systemically evaluate the toxicity of the extract or its chemical constituents using various animal models. Although the entire plant or its single components have been widely used by Uyghurs, few studies have reported the pharmacokinetic parameters such as the Cmax, T1/2, area under the curve, and bioavailability of the monomers or extracts in in vivo studies. Therefore, pharmacokinetic studies to obtain relevant parameters for further clinical studies are much needed.
Lastly, other studies have also shown that A. sparsifolia exhibits cold resistance, drought resistance, salt tolerance, and wind-sand resistance, and plays an important role in land reclamation and preventing land from being eroded by wind-blown sand (Dong, 2000; Yi, 2020). It is rich in crude protein and crude fat, and these levels can reach 14.2 and 3.5%, respectively, during flowering, making it one of the most nutritious forage grasses in desert areas (Jin, 1994; Wang Y. J. et al., 2019). It is also considered a source of nectar and is known to have several advantages including its widespread distribution, long flowering period, high honey secretion, and high sugar content (Gao, 2005). Moreover, it plays a key role in improving the salinity of soils and enhancing nitrogen cycling in the ecosystem. These findings suggest that A. sparsifolia is not only a medicinal plant with potential for the treatment of diseases but is also valuable in environmental protection. However, with environmental and climatic changes, the suitability of the habitat for A. sparsifolia is decreasing and the abundance of this species shows a downward trend. Thus, A. sparsifolia reserves should be established according to local conditions. On the one hand, the establishment of a plant reserve can protect this rich medicinal resource. More importantly, this step can help effectively curb desertification of the oasis and play a role in improving and stabilizing the ecology of the region.
To summarize, A. sparsifolia is a valuable and abundant medicinal resource with promising therapeutic properties and good scope for further exploration. Going forward, more comprehensive studies on the characterization of its active ingredients, determination of its pharmacological mechanisms, and establishment of quality control and toxicity are extremely important to further validate the clinical efficacy and safety of A. sparsifolia extracts and isolated bioactive components.
Author Contributions
KP (arnebia@126.com), HT (th_pha@shzu.edu.cn) conceived and designed the review; FW (497702040@qq.cpm), XY (xzyang@mail.scuec.edu.cn) were responsible for the collection of documents; FW wrote the manuscript; XY revised the manuscript. All authors read and approved the final manuscript.
Funding
The work was financially supported by Regional collaborative innovation project of Xinjiang Uyghur Autonomous Region – The science and technology partnership program and international science and technology cooperation program of Shanghai Cooperation Organization (2020E01016), and National Natural Science Foundation of China grants (81,774,000 and 81,911,540,487).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.761811/full#supplementary-material
References
Aili, A., Jiang, Z. H., Kuerbanjiang, M., Mikeremu, S., and Zhang, X. Y. (2018). Protective Effect of Alhagi Sparsifolia against Acetaminophen-Induced Liver Injury in Mice. Trop. J. Pharm. Res. 17 (4), 641. doi:10.4314/tjpr.v17i4.11
Aili, A., Kuerbanjiang, M., Jiang, Z. H., Zhang, X. Y., and Mikeremu, S. (2017). Protective Effect of Saccharum Alhagi Against N-Acetyl-Para-Aminophenol Induced Liver Injury in Mice. Chin. Anim. Husb. Vet. Med. 44 (7), 2185–2190. doi:10.16431/j.cnki.1671-7236.2017.07.040
Aili, R. (1998a). Exploring the Basic Theories of Uyghur Medicine (Volume 1). J. Med. Pharm. Chin. Minor. 4 (2), 4–6. doi:10.16041/j.cnki.cn15-1175.1998.02.001
Aili, R. (1998b). Exploring the Basic Theories of Uyghur Medicine (Volume 2). J. Med. Pharm. Chin. Minor. 4 (2), 9–11. doi:10.16041/j.cnki.cn15-1175.1998.03.004
Arndt, S. K., Arampatsis, C., Foetzki, A., Li, X., Zeng, F., and Zhang, X. (2004). Contrasting Patterns of Leaf Solute Accumulation and Salt Adaptation in Four Phreatophytic Desert Plants in a Hyperarid Desert With Saline Groundwater. J. Arid Environments. 59, 259–270. doi:10.1016/j.jaridenv.2004.01.017
Chang, J. M., Li, G. R., Zheng, J., and Cheng, Y. F. (2016). Structural Elucidation of Polysaccharides From Saccharum Alhagi. Chin. J. New Drugs. 25 (24), 2826–2830.
Chen, L., Chen, G., and Xu, X. Q. (2014). Dynamic Changes of Rutin and Total Polysaccharides in Alhagi Sparsifolin From Different Parts. Chin. J. Exper. Tradit. Med. Form. 20 (11), 66–69. doi:10.13422/j.cnki.syfjx.2014110066
Cheng, Y. F. (2010). Fundamental Studies of Chemical Constituents of Saccharum Alhagi. Xinjiang, China: Xinjiang Medical University (Dissertation).
Cheng, Y. F., Li, G. R., and Chang, J. M. (2014). Study of Chemical Constituents of Saccharum Alhagi. Chin. Meas. Test. 40 (2), 53–55. doi:10.11857/j.issn.1674-5124.2014.02.013
Chinese Medical Encyclopedia Committee (2005). Chinese Medical Encyclopedia: Uyghur Medicine. Shanghai: Shanghai Science and Technology Publishers.
Chinese Pharmacopoeia Commission (1998). Chinese Pharmacopoeia-Uyghur Medicine Branch Volume. Xinjiang: Xinjiang Science and Technology Health Publishing House, 51.
Dong, X., Wei, H. Y., Lei, Z., Xu, Q., Song, L. Y., Wang, H. W., et al. (2019). Effect of Alhagi Extract on Survival Rate of Rats in Dry-Heat Environment. Chin. J. Comp. Med. 29 (10), 74–78. doi:10.3969/j.issn.1671-7856.2019.10.013
Dong, Z. G. (2000). Economic Value and Utilization of Alhagi Sparsifolia in Arid Desert Area. J. Tarim. Univ. Agric. Reclam. 12 (3), 58–60.
Gao, X. Y. (2005). General Situation of Bee Industry in Xinjiang. J. Bee. 25 (9), 40–41. doi:10.3969/j.issn.1003-9139.2005.09.035
GBIF (2021). Species. Available at: https://www.gbif.org/species/3039284 (Accessed March 15, 2021).
Guo, D., Xue, W. J., Zou, G. A., and Aisa, H. A. (2016). Chemical Composition of Alhagi Sparsifolia Flowers. Chem. Nat. Compd. 52 (6), 1095–1097. doi:10.1007/s10600-016-1871-5
Guo, M. X., Wu, X., and Feng, Y. F. (2020). Optimization of Extraction Process and Rapid Analysis of Total Flavonoids From Saccharum Alhagi. Tradit. Chin. Drug Res. Clin. Pharm. 31 (9), 1110–1116. doi:10.19378/j.issn.1003-9783.2020.09.016
Hairula, M., Makadesi, A., Wumaierjiang, Y., and Mikeremu, S. (2014). Preliminary Investigation on the Growth-Promoting Effect of Prickly Sugar on Mice. Xinjiang Anim. Husb. (11), 29–31+38. doi:10.3969/j.issn.1003-4889.2014.11.016
Hamulati, U. (2003). Temper and Humoral Theory and its Modern Research. Urumqi: Xinjiang Technology and Hygiene Publishing House.
Han, Z. J., He, J. Y., and Chang, J. M. (2017). The Influence of Polysaccharides From Saccharum Alhagi on the Immune Activity of Macrophage Cell Line RAW264.7. J. Xinjiang Med. Univ. 40 (03), 361–365. doi:10.3969/j.issn.1009-5551.2017.03.023
He, J. W. (2016). A Study on the Complementary Nature and Taste of Uyghur Medicine and the Prescription Dispensing Rules of Uyghur Medicine with Isoheterogeneous Black Bile Quality Evidence. Beijing, China: Beijing University of Chinese Medicine (Dissertation).
Hu, S. C. (2010). Study on the Chemical Constituents of Petroleum Ether Site of Senecio Densiserratus Chang and Establishment of Quality Standard of Uyghur Medicine Alhagi Sparsifolia Shap. Gansu, China: Lanzhou University (Dissertation).
Inaturalist (2021). Photos of Alhagi Sparsifolia. Available at: https://www.inaturalist.org/photos/43301374 (Accessed March 15, 2021).
Jian, L. J., Chang, J. M., Ablise, M., Li, G. R., and He, J. W. (2014). Isolation, Purification, and Structural Elucidation of Polysaccharides From Alhagi-Honey. J. Asian Nat. Prod. Res. 16 (7), 783–789. doi:10.1080/10286020.2014.898633
Jian, L. J., Li, G. R., and Chang, J. M. (2012). Determination of Monosaccharide Composition in Polysaccharide of Saccharum Alhagi by Pre-Column Derivatization-High Performance Capillary Electrophoresis. Chin. J. New Drugs. 21 (05), 575–578.
Jiang, H. (2017). The Comprehensive Evaluation of Feed Value of Alhagi Sparsifolia Shap. And Alfalfa Mix-Silage. Beijing, China: China Agricultural University (Dissertation).
Jin, Q. (1994). Alhagi Sparsifolia Schap.: A Potentially Utilizable Forage in saline Soils. Tasks Veg. Sci. 32, 285–288. doi:10.1007/978-94-011-0818-8_29
Jin, X. H., An, R., Li, Y., Qu, J. J., and Ge, J. Z. (2014). Study on the Habitat Adaptation of Alhagi Sparsifolia. Tianjin Agric. Sci. 20 (10), 14–18. doi:10.3969/j.issn.1006-6500.2014.10.004
Kalbinur, K., and Zhang, S. B. (2021). Advances in Uyghur Medical Research. Hunan J. Tradit. Chin. Med. 37 (03), 189–192. doi:10.16808/j.cnki.issn1003-7705.2021.03.064
Kuerbanjiang, M., Aili, A., Jiang, Z. H., Zhang, X. Y., and Mikeremu, S. (2017). Effects of Saccharum Alhagi on the Expression of TNF-α and TLR4 and Pathological Changes in Alcoholic Liver Disease. Prog. Vet. Med. 38 (6), 29–32.
Kuerbanjiang, M., Jiang, Z. H., Aili, A., Mikeremu, S., and Zhang, X. Y. (2018). Hepatoprotective Effect of Alhagi Sparsifolia Against Alcoholic Liver Injury in Mice. Braz. J. Pharm. Sci. 54, e17732. doi:10.1590/s2175-97902018000317732
Kurban, H., Saneoka, H., Nehira, K., Adilla, R., and Fujita, K. (1998). Effect of Salinity on Growth and Accumulation of Organic and Inorganic Solutes in the Leguminous PlantsAlhagi PseudoalhagiandVigna Radiata. Soil Sci. Plant Nutr. 44, 589–597. doi:10.1080/00380768.1998.10414482
Lei, H., Ying, L., Qi, C. N., and Jia, G. Z. (2004). Analysis of the Medicinal Properties of More Than Ten Kinds of Plants Including Astragalus in Tarim. J. Tradit. Vet. Sci. 5, 8–10.
Li, J. S., Liu, Y. M., and Cai, S. Q. (1996). Study on the Resources, Commodity Circulation and Ethnic Application of Uyghur Medical Alhagi Sparsifolia. J. Med. Pharma. Chin. Minor. 2 (2), 39–40. doi:10.16041/j.cnki.cn15-1175.1996.02.033
Li, J. X. (2020). Isolation, Purification and Structural Identification of Polysaccharides from Two Plants. Guangzhou, China: Guangdong Pharmaceutical University (Dissertation).
Liu, G. C., Luo, Y., Dong, F. J., and Ma, X. W. (2014). Study on Extraction, Composition Analysis and Drought Resistance Mechanism of Alkaline Essential Oil From Alhagi Sparsifolia. Xinjiang Farm. Res. Sci. Techno. 37 (11), 42–44. doi:10.3969/j.issn.1001-361X.2014.11.020
Liu, J. B., Yao, H., and Xiao, H. (2014). Advances in the Molecular Epidemiology of Uyghur Medical Humor. J. Xinjiang Med. Univ. 37 (6), 676–677+681.
Liu, W. X., Gao, Z., Jia, X. G., and Halmurat, U. (2016). Uyghur Medicine on Ancient and Modern Times Silk Road. Mod. Chin. Med. 18 (10), 1253–1256. doi:10.13313/j.issn.1673-4890.2016.10.003
Liu, X. S., Ma, X. L., Shi, L. L., Aleteng, T. Y., Zhang, D. P., Li, N., et al. (2019a). Separation and Purification of Alhagi Sparsifolia N-Butanol Extract Monomeric Compounds and Study on Their Effects on Human Cervical Cancer HeLa Cells. J. China. Pharm. 30 (4), 506–512. doi:10.6039/j.issn.1001-0408.2019.04.15
Liu, X. S., Wei, H. Y., Shi, L. L., Zhang, D. P., and Ma, X. L. (2019b). Effects of Alhagi Sparsifolia Shap Extract on Water Metabolism and Gastrointestinal Hormone Levels in Rats With Diarrhea- Predominant of Irritable Bowel Syndrome. Chin. J. New Drugs Clin. Remed. 38 (3), 169–173. doi:10.14109/j.cnki.xyylc.2019.03.010
Liu, Y., Li, C., Ma, H. Q., Sun, T. T., Wang, C. L., Li, Y., et al. (2021). Study on the Antioxidant Activity of Crude Polysaccharides From Alhagi Sparsifolia Shap. Stem-branch Vivo. China. Pharm. 24 (2), 354–358.
Liu, Y. X. (1985). Flora in Desertis Reipublicae Populorum Sinarum (Toumos 1). Beijing: Science Press.
Ma, C. H. (1993). The fine Pasture in the Desert and Semi-Desert Area — Alhagi Sparsifolia Shap. Chin. Herb. Sci. 4, 36.
Ma, H. Q., Liu, Y., Zhang, H., Zhu, X., Mu, J., Wang, C. L., et al. (2018). Study on Physicochemical Properties and Antioxidant Activity of Crude Polysaccharide From Alhagi Sparsifolia Shap. Stem-branch. China. Pharm. 21 (1), 48–52. doi:10.3969/j.issn.1008-049X.2018.01.011
Ma, X. L., Liu, X. S., Wei, H. Y., and Han, L. L. (2018a). Preparation of Alhagi Sparsifolia Shap Monomer Compound and Screening for Hela Cell Activity of Human Cervical Cancer. J. Xinjiang Med. Univ. 41 (4), 486–490. doi:10.3969/j.issn.1009-5551.2018.04.021
Ma, X. L., Shi, L. L., Liu, X. S., Zhang, D. P., and Wei, H. Y. (2018b). Preparation of Alhagi Sparsifolia Monomer Compound and Screening of its Anti-Tumor Effect In Vitro. Pharma. Clin. Chin. Mater. Clin. Med. 34 (4), 58–61. doi:10.13412/j.cnki.zyyl.2018.04.013
Ma, X. L., Shi, L. L., Liu, X. S., Zhang, D. P., and Wei, H. Y. (2018c). Regulatory Effect of Alhagi Sparsifolia Extract on Abdominal Wall Myoelectric Activity and Serum NO Level in Rats With Diarrheal Irritable Bowel Syndrome (IBS-D). Pharm. Clin. Chin. Mater. Med. 34 (5), 78–81. doi:10.13412/j.cnki.zyyl.2018.05.019
Ma, X. L., Wei, H. Y., Jia, X. G., Xiatiguli, A., and Xu, X. Q. (2014). The Long-Term Toxicity experiment of Alhagi Sparsifolia Shap (L) Capsule in Rats. Pharm. Clin. Chin. Mater. Med. 30 (2), 113–115. doi:10.13412/j.cnki.zyyl.2014.02.036
Ma, X. L., Wei, H. Y., Xu, X. Q., and Xiatiguli, A. (2015). Antitumor Activity of Alhagi Sparsifolia Shap (L.) Extract In Vivo and In Vitro. Nat. Prod. Res. Dev. 27 (3), 517–520. doi:10.16333/j.1001-6880.2015.03.030
Ma, X. L., Xu, J. G., Xiatiguli, A., and Wei, H. Y. (2017). Effects of Alhagi Sparsifolia Shap (L.) Extract on Delayed Type Hypersensitivity of Immunosuppression Mice. Xinjiang Med. J. 47 (10), 1103–1105.
Maituoheti, J., Abudoula, M., and Abibai, A. (2017). The Origin, Characteristics and Value of Uyghur Medicine. Chin. J. Ethnomed. Ethnopharm. 26 (2), 3–7.
Malik, A., Kuliev, Z. A., Akhmedov, U. A., Vdovin, A. D., and Abdullaev, N. D. (1997). Catechins and Proanthocyanidins ofAlhagi Sparsifolia. I. Chem. Nat. Compd. 33 (2), 174–178. doi:10.1007/bf02291536
Mikeremu, S., Hairula, M., and Yao, C. (2013). Preliminary Studies of Protective Effects of Saccharum Alhagi on Kidney Injury of Mice Induced by Gentamicin. Chin. Anim. Husb. Vet. Med. 40 (3), 230–233. doi:10.3969/j.issn.1671-7236.2013.03.053
Mikeremu, S., Jiang, Z. H., Wumaierjiang, Y., Aili, A., and Kuerbanjiang, M. (2016). Research Progress on Effective Components and Pharmacological Effects of Saccharum Alhagi in Traditional Uyghur Medicine. J. Chin. Med. Mater. 39 (10), 2397–2399. doi:10.13863/j.issn1001-4454.2016.10.059
Mikeremu, S., Kuerbanjiang, M., Hu, M. Y., Aili, A., and Yang, Y. (2017). Effects of Water Extracts of Alhagi Sparsifolia Shap and Alhagi Honey on Intestinal Propulsion in Mice. J. Food Saf. Qual. 8 (3), 890–894. doi:10.19812/j.cnki.jfsq11-5956/ts.2017.03.029
Mondal, A., Gandhi, A., Fimognari, C., Atanasov, A. G., and Bishayee, A. (2019). Alkaloids for Cancer Prevention and Therapy: Current Progress and Future Perspectives. Eur. J. Pharmacol. 858, 172472. doi:10.1016/j.ejphar.2019.172472
Muhammad, G., Hussain, M. A., Anwar, F., Ashraf, M., and Gilani, A. H. (2015). Alhagi: a Plant Genus Rich in Bioactives for Pharmaceuticals. Phytother. Res. 29 (1), 1–13. doi:10.1002/ptr.5222
Muratova, M. S., Zou, G. A., Jenis, J., and Aisa, H. A. (2019). Chemical Constituents of Alhagi Sparsifolia. Chem. Nat. Compd. 55 (5), 932–933. doi:10.1007/s10600-019-02850-0
Nishanbaev, S. Z., Bobakulov, K. M., Nigmatullaev, A. M., Sham′yanov, I. D., Okhundedaev, B. S., and Abdullaev, N. D. (2016). Volatile Compounds From the Aerial Parts of Four Alhagi Species Growing in Uzbekistan. Chem. Nat. Compd. 52 (1), 167–170. doi:10.1007/s10600-016-1582-y
Ouyang, F., Rui, D. Y., Chen, Y. J., Ju, H. P., Tao, J., Zhang, S., et al. (2018). Study on Chemical Constituents From Stem of Alhagi Sparsifolia. J. Chin. Med. Mater. 41 (1), 119–123. doi:10.13863/j.issn1001-4454.2018.01.025
Parviz Tavassoli, A., Anushiravani, M., Hoseini, S. M., Nikakhtar, Z., Naghedi Baghdar, H., Ramezani, M., et al. (2020). Phytochemistry and Therapeutic Effects of Alhagi Spp. And Tarangabin in the Traditional and Modern Medicine: a Review. J. Herbmed Pharmacol. 9 (2), 86–104. doi:10.34172/jhp.2020.13
Plant Photo Bank of China (2021). Available at: http://ppbc.iplant.cn/tu/5783326 (Accessed March 15, 2021).
Qi, J. H., and Dong, F. X. (2020). Research Progress of the Pharmacological Action of Flavonoids. J. Beijing Union. Univ. 34 (3), 89–92. doi:10.16255/j.cnki.ldxbz.2020.03.014
Qu, Z. Z. (2019). Study on the Mechanism of Immune Activity of Saccharum Alhagi Polysaccharide on Mouse TLR4 Target. Xinjiang, China: Xinjiang Med. Univ. (Dissertation).
Quan, Z. H., and Xu, T. H. (2009). Chemical Constituents of Alhagi Sparsifolia and Its Ethnic. Jilin. J. Tradit. Chin. Med. 29 (8), 711–712. doi:10.13463/j.cnki.jlzyy.2009.08.040
Royal Botanic Garden Edinburgh (2021). Available at: http://data.rbge.org.uk/herb/E00364493 (Accessed March 15, 2021).
Sanawar, W., Alimjian, A., and Zou, G. A. (2013). Study on the Fingerprint of Alhagi Sparsifolia by High Performance Liquid Chromatography. Arid. Zone. Res. 30 (5), 873–876. doi:10.13866/j.azr.2013.05.005
Sanawar, W., Han, K. F., Amatjan, A., Zou, G. A., and Hajiakber, A. (2012). Study on the Quality Standard of Alhagi Sparsifolia. Heald. Med. 31 (8), 1074–1076. doi:10.3870/yydb.2012.08.037
Sanawar, W., Zhang, G., Zou, G. A., and Hajiakbar, A. (2014). Preparation of Isorhamnetin-3-O-β-D Rutinoside Reference Substance From Alhagi Sparsifolia. J. Xinjiang Med. Univ. 37 (1), 44–46. doi:10.3969/j.issn.1009-5551.2014.01.012
Shi, L. L., Gen, J. Z., Wei, H. Y., Jia, X. G., Xu, X. Q., and Xiatiguli, A. (2014). Optimization of Extraction Technology of Total Flavonoids From Alhagi Sparsifolia by Orthogonal Design. Xinjiang J. Tradit. Chin. Med. 32 (3), 58–60.
Srivastava, B., Sharma, H., Dey, Y. N., Wanjari, M. M., and Jadhav, A. D. (2014). Alhagi Pseudalhagi: a Review of its Phyto-Chemistry, Pharmacology, Folklore Claims and Ayurvedic Studies. Int. J. Herb. Med. 2 (2), 47–51.
State Administration of Traditional Chinese Herbal Editorial Board (2005). The Chinese Materia Medica: Uyghur Medicine Volume. Shanghai: Publishing House of Shanghai Science and Technology.
Su, X. C., Chen, L., and Aisa, H. A. (2008). Flavonoids and Sterols From Alhagi Sparsifolia. Chem. Nat. Compd. 44 (3), 365. doi:10.1007/s10600-008-9064-5
Su, X. C., Huang, Y., Zhao, Y. X., and Hajiakber, A. (2009). Studies on the Extraction Technology of Flavonoids in Alhagi Sparsifohia Shap. Lishizhen. Med. Mater. Med. Res. 20 (6), 1333–1334. doi:10.3969/j.issn.1008-0805.2009.06.018
Sun, Y. Z. (1989). Alhagi Sparsifohia Blooming and Honey Secreting and Bee colony Management. Apicul. Chin. 3, 16–17.
The Plant List (2021). Available at: http://www.theplantlist.org/(Accessed March 15, 2021).
Thomas, F. M., Foetzki, A., Gries, D., Bruelheide, H., Bruelheide, H., Li, X., et al. (2008). Regulation of the Water Status in Three Co-Occurring Phreatophytes at the Southern Fringe of the Taklamakan Desert. J. Plant Ecol. 1 (4), 227–235. doi:10.1093/jpe/rtn023
Vonlanthen, B., Zhang, X., and Bruelheide, H. (2010). Clonal Structure and Genetic Diversity of Three Desert Phreatophytes. Am. J. Bot. 97 (2), 234–242. doi:10.3732/ajb.0800329
Wang, H. Q., and Jiahan, S. B. T. (2011). Progress of Research on Factors Influencing Longevity. J. Xinjiang Med. Univ. 34 (11), 1186–1190. doi:10.3969/j.issn.1009-5551.2011.11.005
Wang, J. X. (2003). Fomes Officinalis Bres. And Alhagi Sparsifolia Shap., Which Are Commonly Used in Uyghur Medicine. Spec. Wild. Econ. Anim. Plant Res. 6 (9), 39.
Wang, S., Hu, Y., and Liu, T. (2019a). Plant Distribution and Pharmacological Activity of Flavonoids. Tradit Med. Res. 4 (5), 269–287. doi:10.53388/tmr20190824131
Wang, X. L., Liang, P. P., Zhao, M. J., Feng, J. Z., and Liu, Y. (2019b). Analysis of Nutritional Composition and Amino Acid Composition of Six Common Psammophytes in Alashan Zuoqi, Inner Mongolia. Spectrosc. Spect. Anal. 39 (1), 204–209. doi:10.3964/j.issn.1000-0593(2019)01-0204-06
Wang, Y. J., Cao, W., Li, Q. L., Zhou, Y. S., Yang, R., and Niu, X. Q. (2019c). Protective Effects of Silybum Marianum and Alhagi Sparsifolia on Acute Alcohol-Induced Liver Injury. Immunol. J. 35 (8), 665–670. doi:10.13431/j.cnki.immunol.j.20190104
Wang, X. Q. (1994). Research on the Exchange of Medicine along the Silk Road. Xijiang: Xijiang People’s Publishing House.
Wei, H. Y., Ma, X. L., Xiatiguli, A., and Xu, X. Q. (2016). Application of Alhagi Sparsifolia Extract as a Preparation Drug for the Treatment of Irritable Bowel Syndrome. China: CN201610411321.X.
Wumaierjiang, Y., Aili, A., Dillinur, , Zhang, X. H., Liu, Y. Y., Yao, G., et al. (2014). Preliminary Study on the Protective Effect of Uyghur Medicine Saccharum Alhagi on Acute Renal Injury in Mice. Anim. Husb. Vet. Med. 46, 294–295.
Xinjiang Medical Products Administration (2010). Standard of Uyghur Medicinal Materials in Xinjiang Autonomous Region. Xinjiang: Xinjiang People's Health Publishing House.
Xu, X. Q., Wei, H. Y., Shi, L. L., Xiatiguli, A., and Jia, X. G. (2012). Research on Quality Standard of Ethnic Drug Alhagi Sparsifolia Shap. Res. Pract. Chin. Med. 26 (6), 68–71. doi:10.13728/j.1673-6427.2012.06.012
Yang, X., Zheng, J. H., Mu, C., and Lin, J. (2017). Predictions of Potential Geographical Distribution of Alhagi Sparsifolia Under Climate Change. Zhongguo Zhong Yao Za Zhi. 42 (3), 450–455. doi:10.19540/j.cnki.cjcmm.20170103.004
Yi, L. J. (2020). Research Status of Physiological Characteristics and Application Value of Alhagi Sparsifolia. Grass-Feeding Livest. 205 (6), 51–54. doi:10.16863/j.cnki.1003-6377.2020.06.009
Yuan, Z. Y., Huang, P. Y., and He, X. J. (2012). Research Status and Progress on Alhagi Sparsifolia. J. Anhui Agri. 40 (18), 9674–9677. doi:10.13989/j.cnki.0517-6611.2012.18.106
Zhang, G. J., Li, N., Ni, H., and Jia, X. G. (2008). Studies on the Chemical Constituents of Alhagi. Drugs Clin. 23 (04), 157–160.
Zhang, H., Gao, S. Y., and Zheng, Q. H. (2002). Responses of NPP of Salinized Meadows to Globalchange in Hyperarid Regions. J. Arid Environ. 50 (3), 489–498. doi:10.1006/jare.2001.0863
Zhang, R. Q. (2018). Review and prospect of Silk Road Medicine Research. Chin. Med. Cult. 13 (1), 5–13. doi:10.16307/j.1673-6281.2018.01.001
Zhao, J. (2016). A Preliminary Study on Immune Activity and Mechanism of Polysaccharides from Saccharum Alhagi. Xinjiang, China: Xinjiang med. Univ. (Dissertation).
Zhao, J., Chang, J. M., Zheng, J., Cheng, Y. F., and Li, G. R. (2016). Preliminary Study on the Immune Activity of Polysaccharides From Saccharum Alhagi. Northwest. Pharm. J. 31 (02), 158–161. doi:10.3969/j.issn.1004-2407.2016.02.014
Zhao, J., Li, G. R., Zheng, J., Cheng, Y. F., and Chang, J. M. (2015). Screening of Antioxidant Activities Polysaccharides From Saccharum Alhagi. J. Xinjiang Med. Univ. 38 (12), 1479–1481. doi:10.3969/j.issn.1009-5551.2015.12.005
Zheng, J. (2016). Isolation, Purification, Structural Elucidation and Antidiabetic Activity of Polysaccharide From Saccharum Alhagi. Xinjiang, China: Xinjiang med. Univ. (Dissertation).
Zheng, X. H., Zhao, J. X., and Tao, D. Y. (2014). Study on the Toxic Effect of Alhagi Sparsifolia Alkaloids on Mice. Heilongjiang Anim. Sci. Vet. Med. (15), 188–190. doi:10.13881/j.cnki.hljxmsy.2014.0915
Zhou, D., Wei, H., Jiang, Z., Li, X., Jiao, K., Jia, X., et al. (2017). Natural Potential Neuroinflammatory Inhibitors From Alhagi Sparsifolia Shap. Bioorg. Med. Chem. Lett. 27 (4), 973–978. doi:10.1016/j.bmcl.2016.12.075
Zou, G. A., Mansur, S., Hu, S. C., Aisa, H. A., and Shakhidoyatov, K. M. (2012). Pyrrole Alkaloids From Alhagi Sparsifolia. Chem. Nat. Compd. 48 (4), 635–637. doi:10.1007/s10600-012-0330-1
Glossary
ALD alcoholic liver disease
IL-2 interleukin-2
ALT alanine aminotransfease
LPS-TLRs lipopolysaccharide-toll-like receptors
APAP N-Acetyl-para-aminophenol
MDA malondialdehyde
ASSBP A. sparsifolia stem-branch
MTL motilin
AST aspartate transaminase
NO nitric oxide
BGC-823 human gastric cancer cells
RP-HPLC reversed-phase high performance liquid chromatogra
Ca2+ calcium
SD sprague-dawley
ChP pharmacopoeia of the people’s republic of china
SOD superoxide dismutase
CY cyclophosphamide
SP substance p
CYP2E1 cytochrome p450 2e1
TEAC trolox equivalent antioxidant capacity
DNCB 2,4-dinitrochlorobenzene
TLC thin-layer chromatography
DPPH 1,1-diphenyl-2-picrylhydrazyl
TLR4 toll like receptor 4
Eca-109 human esophageal cancer cells
TNF-α tumor necrosis factor-α
GM gentamicin
UVS ultraviolet spectrophotometry
GSH glutathione
VIP vasoactive intestinal peptide
H2O2 hydrogen peroxide
•O2− superoxide anions radical
HepG2 human hepatocellular carcinoma cells
•OH− hydroxyl radicals
HPLC high performance liquid chromatography
5-FU 5-fluorouracil
HT-29 human colon cancer cells
5-HT 5-hydroxytryptamine
IBS-D irritable bowel syndrome model
MIC minimum inhibitory concentration
IC50 50% inhibitory concentration
Keywords: alhagi sparsifolia shap, traditional uses, phytochemistry, pharmacology, quality control, toxicology
Citation: Wei F, Yang X, Pang K and Tang H (2021) Traditional Uses, Chemistry, Pharmacology, Toxicology and Quality Control of Alhagi sparsifolia Shap.: A Review. Front. Pharmacol. 12:761811. doi: 10.3389/fphar.2021.761811
Received: 20 August 2021; Accepted: 28 September 2021;
Published: 14 October 2021.
Edited by:
Michael Heinrich, UCL School of Pharmacy, United KingdomReviewed by:
Taoufiq Benali, Cadi Ayyad University, MoroccoJohra Khan, Majmaah University, Saudi Arabia
Iraj Mehregan, Islamic Azad University, Iran
Copyright © 2021 Wei, Yang, Pang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kejian Pang, arnebia@126.com; Hui Tang, th_pha@shzu.edu.cn
†These authors have contributed equally to this work
 Feng Wei
Feng Wei Xinzhou Yang
Xinzhou Yang Kejian Pang1*
Kejian Pang1*