- 1College of Resources, Sichuan Agricultural University, Chengdu, China
- 2Haisco Pharmaceutical Group Comp., Ltd., Chengdu, China
- 3Department of Cardiovascular Surgery, Xijing Hospital, Medical University of the Air Force, Xi’an, China
- 4School of Medicine, Northwest University, Xi’an, China
Introduction
In this year, more than 70,000 mpox cases in non-endemic countries around the world have been reported, most of which were in American and Europe. Monkeypox virus (MPXV) is mainly transmitted by direct contact, including close contacts with skin lesions, respiratory secretions, or contaminated items of infected patients or animals (Bunge et al., 2022; Perez Duque et al., 2022). MPXV infection outbreak usually has a central point, and the original patient should have travelled to epidemic areas or have a clear history of exposure to infectious sources (such as some animals; Bunge et al., 2022). However, the current outbreak occurs in several non-endemic countries simultaneously, and the most reported cases have neither contacted with wild animals directly nor been to the endemic countries in Africa (Perez Duque et al., 2022; Saied et al., 2022). Moreover, after the Corona Virus Disease 2019 (COVID-19), people’s social distance increases, and the probability of contact transmission was decreased. It is difficult to explain the current mpox epidemic with the common transmission pathways (Saied et al., 2022).
In our previous study (Yuan et al., 2022), through cluster analysis of MPXV based on relative synonymous codon usage (RSCU) bias, we concluded that the current mpox outbreak in American and Europe may have at least three origins: Sudan 2005—Nigeria 2017 cluster, Sierra Leone 2004 cluster, and Libya 1970 cluster. The geographical distribution of viral clusters was in cross, implying that they were multi-originated and the transmission paths might be very complex (Yuan et al., 2022).
Before this year, mpox was not listed as a sexually transmitted disease (STD). For the current outbreak, most mpox patients were gay, bisexual, and other men who have sex with men (MSM) with sex tourisms (Thornhill et al., 2022a; Thornhill et al., 2022b; Patel et al., 2022). However, for a contagious STD, the median incubation period was only about 7–9 days (Thornhill et al., 2022a; Guzzetta et al., 2022; Miura et al., 2022; Ward et al., 2022), which may be too short to cause a large-scale transmission (the incubation period of HIV was about 10 years; Román-Montoya et al., 2013). The unexpected and sudden appearance of MPXV concurrently in several non-endemic areas indicates that there may be some unnoticed transmission in some unknown duration of time followed by recent amplifier events (Alakunle and Okeke, 2022).
High ratio of mpox-HIV co-infection
A large number of mpox patients had concomitant HIV infection with a ratio of 42.2% (78/185; Català et al., 2022), 35.9% (70/195; Patel et al., 2022) or 41.3% (218/528; Thornhill et al., 2022a) respectively. Although most mpox patients were MSM (Thornhill et al., 2022a; Thornhill et al., 2022b; Patel et al., 2022), the ratios of mpox-HIV co-infection were much higher than the usual percentage of HIV diagnoses in MSM (<2%; Rao et al., 2016).
We noticed that only 8% of the patients showed detectable HIV viral loads (Català et al., 2022). Other two reports also demonstrated that 78.6% (55/70; Patel et al., 2022) or 97.4% (185/190; Thornhill et al., 2022a) patients with mpox-HIV co-infection had low HIV viral loads (<200 copies/mL). All these data suggested that HIV-positive population in mpox patients showed very good HIV control. Therefore, they were individuals living with HIV infection (but not HIV clinics with symptoms) and more likely to have high-risk sexual behaviors.
Secondly, in HIV patients, some clinical characteristics of mpox might be different from non-those in non-HIV patients (Amorosa and Isaacs, 2003; Saied et al., 2022). Although in general, well-controlled HIV was not associated with severity of the symptoms, HIV-positive patients were more likely to have fevers (60% of HIV patients vs. 50% of non-HIV patients; Català et al., 2022). And the HIV-positive patients tended to show larger numbers of lesions or affected areas (Català et al., 2022). In non-HIV infected cases, the patients usually present with generalized skin rash. For the HIV infected cases, there might be the more skin lesion at genital or perinatal areas (Hammerschlag et al., 2022; Mungmunpuntipantip and Wiwanitkit, 2022). In a retrospective review of hospital records of 40 human mpox cases from Nigeria, the HIV type 1-coinfected cases showed more prolonged illness, larger lesions, and higher rates of both secondary bacterial skin infections and genital ulcers (Ogoina et al., 2020). Severe symptoms after poxvirus infections may develop in immuno-compromised individuals (Amorosa and Isaacs, 2003). So HIV-positive patients were more likely to go to the hospital, although they might seek dermatovenerologic diagnosis prior to visiting other specialists (Hammerschlag et al., 2022). A study reported that, of 20 participants admitted to hospital for clinical reasons, 15 (75.0%) had HIV co-infection (Patel et al., 2022).
The role of mild-symptomatic patients in unnoticed mpox transmission
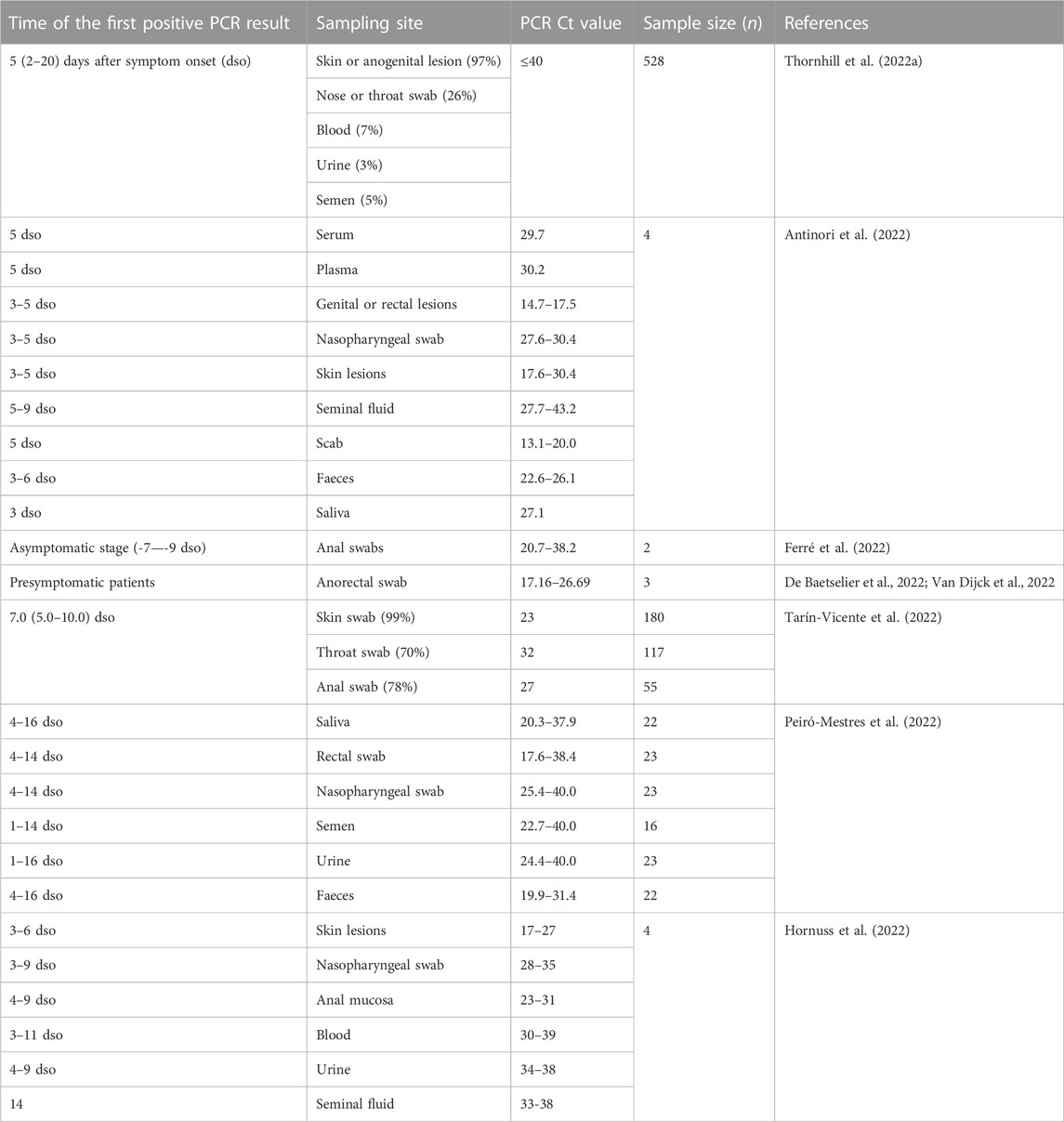
Thornhill et al. (2022a) demonstrated that the median incubation period of mpox was about 7 (3–20) days. However, longer mean incubation periods have also been reported, which were estimated to be 7.6–7.8 days (95% credible interval 6.5 to 9.9; Ward et al., 2022), 8.5 days (95% credible interval 4.2 to 17.3; Miura et al., 2022) or 9.1 days (95% credible interval 6.5 to 10.9; Guzzetta et al., 2022). The difference in incubation period may be attributed into different definition to the symptom onset. Usually, the definition of symptom onset describes the date that an individual first noticed their symptoms. However, the initial appearance after mpox virus (MPXV) infection may be just atypical (mild) genital and peri-anal rashes without severe pain (Thornhill et al., 2022a; Thornhill et al., 2022b; Patel et al., 2022; Tarín-Vicente et al., 2022). Thus, the true date of symptom onset may be earlier but not detected.
Ward et al. (2022) found that short serial intervals were more common than short incubation periods, therefore suggesting a considerable pre-symptomatic transmission. Nevertheless, the genital or rectal lesion swabs obtained from mpox patients only became positive for MPXV DNA until after 3–5 days post symptom onset (Table 1). In other words, most pre-symptomatic patients may be not infectious. The term “pre-symptomatic transmission” may be inaccurate and should be interpreted as “mild-symptomatic transmission.”
The mild-symptomatic patients may play a key role in the early unnoticed transmission, because that the individuals may still be engaged in high-risk sexual behaviors in the first few days post symptom onset. The genital and peri-anal rashes may be rubbed raw during the sexual intercourse and the virus would be released. Then MPXV may get into the blood stream directly, if anal bleeding occurs. A case study reported a MPXV transmission to a healthcare worker through a needlestick injury, confirming a possibility of direct blood transmission (Carvalho et al., 2022).
Possible seminal transmission of MPXV
MSM are prone to have condomless sexual intercourse and leave the seminal fluid inside the body. Before this year, mpox was not known as a sexually transmitted disease. MSM usually adopt HIV pre-exposure prophylaxis (PrEP; Hodges-Mameletzis et al., 2019; Atim et al., 2020; Thornhill et al., 2022a). However, use of PrEP may be a risk factor for MPXV infection, because that MSM with PrEP do not often use condoms (Torster et al., 2022). WHO recommended PrEP since 2015 (Hodges-Mameletzis et al., 2019; Atim et al., 2020). Thus, the current correlation between sexual behaviors and MPXV infections found in this year might be explained.
The available literatures showed increasing concerns about possible seminal transmission of MPXV (Hornuss et al., 2022; Lapa et al., 2022; Noe et al., 2022; Peiró-Mestres et al., 2022; Raccagni et al., 2022; Reda et al., 2022; Reda et al., 2023). Detection of viruses in the testes is commonly secondary to viraemia because the blood–testis barrier may be liable to viruses, especially when systemic or local inflammation occurs. Viral persistence through the tract is also likely, no matter of its capability to replicate, because the testis can be an immunological-favored site for viruses (Li et al., 2012; Annandale et al., 2014; Mead et al., 2018). Interestingly, culturing MPXV was successful in two out of four patients included in two studies (Lapa et al., 2022; Noe et al., 2022), suggesting a replication competence of MPXV detected in seminal specimens.
A clinical study reported positive MPXV results in the seminal fluid obtained from mpox patients at the time closest (5–7 days) to symptoms onset with a Ct range from 27 to 30 (Antinori et al., 2022); when the symptoms may be mild. Though in a low viral load, seminal MPXV may be still contagious. Alternatively, seminal MPXV may get into the blood stream directly, if anal bleeding occurs.
Asymptomatic patients might transmit the virus through seminal fluids
Asymptomatic mpox infections may be observed in both smallpox vaccinated and unvaccinated individuals (Karem et al., 2007; Guagliardo et al., 2020). Ferré et al. (2022) detected MPXV in anorectal swabs from asymptomatic MSM. Among 200 participants who were subjected to MPXV PCR tests, they reported 13 MPXV-positive participants who were initially asymptomatic (two of them showed mild symptoms 7–9 days later). However, asymptomatic patients do not develop rashes or skin lesions, where the viral loads are the highest (about 10,000 times higher than in serum; Table 1). Therefore they are believed to be of little or no epidemiologic importance. Nevertheless, Ferré et al. (2022) also found a high viral load in a patient during the asymptomatic stage with a very low Ct value of 20.7. And serology confirmed that MPXV isolated from two presymptomatic cases can be cultured (De Baetselier et al., 2022; Van Dijck et al., 2022). Whether a high viral load in seminal fluid obtained from some asymptomatic patients could be detected needs further investigations. There might be a possibility that asymptomatic patients transmit the virus through seminal fluids.
Condom, vaccines and drugs
The condom could prevent direct contact with anogenital lesions, where the viral loads are the highest (Table 1). Although the actual protection rate of condoms against mpox infection is unclear, compared with the vaccines and drugs, use of condoms may be the most effective and convenient way to control the current epidemic.
Given that in most cases, the viral load peaks after 3–5 days post symptom onset (Table 1), vaccination and/or drug treatments before this time-point may show good therapeutic effects. All highly-susceptible populations should be subjected to viral tests and priority treatments, no matter in symptomatic or asymptomatic, especially for those are too young to receive childhood smallpox vaccination, whose viral loads may be higher than unvaccinated people. However a large part of them had concomitant HIV infection (Thornhill et al., 2022a; Català et al., 2022; Patel et al., 2022). Previous studies suggested that HIV-positive individuals with CD4 cell counts of <300 cells/mm3 may develop severe complications after vaccinia virus vaccination (Amorosa and Isaacs, 2003). Thus, for those with low CD4 cell counts, the decision whether or not to vaccinate must be made within the context and circumstances of the mpox outbreak. Alternatively, the immuno-compromised people or the patients with atopic dermatitis should receive a third-generation non-replicating vaccine that was made based on modified vaccinia Ankara (MVA) (Saied et al., 2022). It is interesting to note that some MVA vaccine may be considered for post-exposure prophylaxis, ideally within 4 days of high-grade exposure (Vaughan et al., 2020).
The mainstay of clinical treatments for MPXV infections are supportive and/or symptomatic managements (Reynolds et al., 2017). Although there are a few antiviral drugs have been prescribed for mpox patients, such as Cidofovir, Brincidofovir, and Tecovirimat (Adler et al., 2022; Thornhill et al., 2022a; Rizk et al., 2022; Saied et al., 2022), no prophylactic drug has been approved. Whether some drugs could be considered in mpox pre-exposure prophylaxis needs further investigations. Besides above vaccines and drugs, Saied et al. (2022) further suggested that vaccinia immune globulin intravenous (VIGIV) or vaccine immune globulin (VIG) may be used for mpox treatments, and especially helpful to the immuno-compromised people, pregnant women, or the patients with complicated lesions.
Author contributions
SY conceived the project. S-CJ, Z-WZ, Y-FF, and X-YY performed the literature search. SY wrote the manuscript with input from S-CJ, Z-WZ, Y-FF, X-YY, Z-LL, and JH. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Sichuan Province Youth Science and Technology Innovation Team (20CXTD0062) to SY and the Applied Basic Research Program of Sichuan Province (2020YJ0410) to Z-WZ.
Conflict of interest
S-CJ was employed by the Haisco Pharmaceutical Group Comp., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adler, H., Gould, S., Hine, P., Snell, L. B., Wong, W., Houlihan, C. F., et al. (2022). Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 22 (8), 1153–1162. doi:10.1016/S1473-3099(22)00228-6
Alakunle, E. F., and Okeke, M. I. (2022). Monkeypox virus: A neglected zoonotic pathogen spreads globally. Nat. Rev. Microbiol. 20 (9), 507–508. doi:10.1038/s41579-022-00776-z
Amorosa, V. K., and Isaacs, S. N. (2003). Separate worlds set to collide: Smallpox, vaccinia virus vaccination, and human immunodeficiency virus and acquired immunodeficiency syndrome. Clin. Infect. Dis. 37 (3), 426–432. doi:10.1086/375823
Annandale, C. H., Holm, D. E., Ebersohn, K., and Venter, E. H. (2014). Seminal transmission of lumpy skin disease virus in heifers. Transbound. Emerg. Dis. 61 (5), 443–448. doi:10.1111/tbed.12045
Antinori, A., Mazzotta, V., Vita, S., Carletti, F., Tacconi, D., Lapini, L. E., et al. (2022). Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 27 (22), 2200421. doi:10.2807/1560-7917.ES.2022.27.22.2200421
Atim, M., Girometti, N., Hyndman, I., McOwan, A., and Whitlock, G. (2020). Post-exposure prophylaxis in the era of pre-exposure prophylaxis. HIV Med. 21 (10), 668–670. doi:10.1111/hiv.12917
Bunge, E. M., Hoet, B., Chen, L., Lienert, F., Weidenthaler, H., Baer, L. R., et al. (2022). The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 16 (2), e0010141. doi:10.1371/journal.pntd.0010141
Carvalho, L. B., Casadio, L. V. B., Polly, M., Nastri, A. C., Turdo, A. C., de Araujo Eliodoro, R. H., et al. (2022). Monkeypox virus transmission to healthcare worker through needlestick injury, Brazil. Emerg. Infect. Dis. 28 (11), 2334–2336. doi:10.3201/eid2811.221323
Català, A., Clavo Escribano, P., Riera, J., Martín-Ezquerra, G., Fernandez-Gonzalez, P., Revelles-Peñas, L., et al. (2022). Monkeypox outbreak in Spain: Clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Br. J. Dermatol. 187 (5), 765–772. doi:10.1111/bjd.21790
De Baetselier, I., Van Dijck, C., Kenyon, C., Coppens, J., Michiels, J., de Block, T., et al. (2022). Retrospective detection of asymptomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. Nat. Med. 28 (11), 2288–2292. doi:10.1038/s41591-022-02004-w
Ferré, V. M., Bachelard, A., Zaidi, M., Armand-Lefevre, L., Descamps, D., Charpentier, C., et al. (2022). Detection of monkeypox virus in anorectal swabs from asymptomatic men who have sex with men in a sexually transmitted infection screening program in Paris, France. Ann. Intern. Med. 175 (10), 1491–1492. doi:10.7326/M22-2183
Guagliardo, S. A. J., Monroe, B., Moundjoa, C., Athanase, A., Okpu, G., Burgado, J., et al. (2020). Asymptomatic orthopoxvirus circulation in humans in the wake of a monkeypox outbreak among chimpanzees in Cameroon. Am. J. Trop. Med. Hyg. 102 (1), 206–212. doi:10.4269/ajtmh.19-0467
Guzzetta, G., Mammone, A., Ferraro, F., Caraglia, A., Rapiti, A., Marziano, V., et al. (2022). Early estimates of monkeypox incubation period, generation time, and reproduction number, Italy, May-June 2022. Emerg. Infect. Dis. 28 (10), 2078–2081. doi:10.3201/eid2810.221126
Hammerschlag, Y., MacLeod, G., Papadakis, G., Adan Sanchez, A., Druce, J., Taiaroa, G., et al. (2022). Monkeypox infection presenting as genital rash, Australia, May 2022. Euro Surveill. 27 (22), 2200411. doi:10.2807/1560-7917.ES.2022.27.22.2200411
Hodges-Mameletzis, I., Fonner, V. A., Dalal, S., Mugo, N., Msimanga-Radebe, B., and Baggaley, R. (2019). Pre-exposure prophylaxis for HIV prevention in women: Current status and future directions. Drugs 79 (12), 1263–1276. doi:10.1007/s40265-019-01143-8
Hornuss, D., Daehne, T., Goetz, V., Mueller, M., Usadel, S., Lorz, A., et al. (2022). Transmission characteristics, replication patterns and clinical manifestations of human monkeypox virus-an in-depth analysis of four cases from Germany. Clin. Microbiol. Infect. 29, 112.e5–112.e9. doi:10.1016/j.cmi.2022.09.012
Karem, K. L., Reynolds, M., Hughes, C., Braden, Z., Nigam, P., Crotty, S., et al. (2007). Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin. Vaccine Immunol. 14 (10), 1318–1327. doi:10.1128/CVI.00148-07
Lapa, D., Carletti, F., Mazzotta, V., Matusali, G., Pinnetti, C., Meschi, S., et al. (2022). Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. Lancet Infect. Dis. 22 (9), 1267–1269. doi:10.1016/S1473-3099(22)00513-8
Li, N., Wang, T., and Han, D. (2012). Structural, cellular and molecular aspects of immune privilege in the testis. Front. Immunol. 3, 152. doi:10.3389/fimmu.2012.00152
Mead, P. S., Duggal, N. K., Hook, S. A., Delorey, M., Fischer, M., Olzenak McGuire, D., et al. (2018). Zika virus shedding in semen of symptomatic infected men. N. Engl. J. Med. 378 (15), 1377–1385. doi:10.1056/NEJMoa1711038
Miura, F., van Ewijk, C. E., Backer, J. A., Xiridou, M., Franz, E., Op de Coul, E., et al. (2022). Estimated incubation period for monkeypox cases confirmed in The Netherlands, May 2022. Euro Surveill. 27 (24), 2200448. doi:10.2807/1560-7917.ES.2022.27.24.2200448
Mungmunpuntipantip, R., and Wiwanitkit, V. (2022). Monkeypox in HIV infected cases: A summary on clinical presentation of 27 cases. Infect. Chemother. 54 (3), 549–550. doi:10.3947/ic.2022.0104
Noe, S., Zange, S., Seilmaier, M., Antwerpen, M. H., Fenzl, T., Schneider, J., et al. (2022). Clinical and virological features of first human monkeypox cases in Germany. Infection 11, 1–6. doi:10.1007/s15010-022-01874-z
Ogoina, D., Iroezindu, M., James, H. I., Oladokun, R., Yinka-Ogunleye, A., Wakama, P., et al. (2020). Clinical course and outcome of human monkeypox in Nigeria. Clin. Infect. Dis. 71 (8), e210–e214. doi:10.1093/cid/ciaa143
Patel, A., Bilinska, J., Tam, J. C. H., Da Silva Fontoura, D., Mason, C. Y., Daunt, A., et al. (2022). Clinical features and novel presentations of human monkeypox in a central london centre during the 2022 outbreak: Descriptive case series. BMJ 378, e072410. doi:10.1136/bmj-2022-072410
Peiró-Mestres, A., Fuertes, I., Camprubí-Ferrer, D., Marcos, M. Á., Vilella, A., Navarro, M., et al. (2022). Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 27 (28), 2200503. doi:10.2807/1560-7917.ES.2022.27.28.2200503
Perez Duque, M., Ribeiro, S., Martins, J. V., Casaca, P., Leite, P. P., Tavares, M., et al. (2022). Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Euro Surveill. 27 (22), 2200424. doi:10.2807/1560-7917.ES.2022.27.22.2200424
Raccagni, A. R., Candela, C., Mileto, D., Canetti, D., Bruzzesi, E., Rizzo, A., et al. (2022). Monkeypox infection among men who have sex with men: PCR testing on seminal fluids. J. Infect. 85 (5), 573–607. doi:10.1016/j.jinf.2022.07.022
Rao, S., Seth, P., Walker, T., Wang, G., Mulatu, M. S., Gilford, J., et al. (2016). HIV testing and outcomes among Hispanics/Latinos - United States, Puerto Rico, and U.S. Virgin Islands, 2014. MMWR Morb. Mortal. Wkly. Rep. 65 (40), 1099–1103. doi:10.15585/mmwr.mm6540a2
Reda, A., Abdelaal, A., Brakat, A. M., Lashin, B. I., Abouelkheir, M., Abdelazeem, B., et al. (2023). Monkeypox viral detection in semen specimens of confirmed cases: A systematic review and meta-analysis. J. Med. Virol. 95 (1), e28250. doi:10.1002/jmv.28250
Reda, A., Sah, R., Rodriguez-Morales, A. J., and Shah, J. (2022). Viral replication and infectivity of monkeypox through semen. Lancet Infect. Dis. 22 (11), 1531–1532. doi:10.1016/S1473-3099(22)00611-9
Reynolds, M. G., McCollum, A. M., Nguete, B., Shongo Lushima, R., and Petersen, B. W. (2017). Improving the care and treatment of monkeypox patients in low-resource settings: Applying evidence from contemporary biomedical and smallpox biodefense research. Viruses 9 (12), 380. doi:10.3390/v9120380
Rizk, J. G., Lippi, G., Henry, B. M., Forthal, D. N., and Rizk, Y. (2022). Prevention and treatment of monkeypox. Drugs 82 (9), 957–963. doi:10.1007/s40265-022-01742-y
Román-Montoya, Y., Bueno-Cavanillas, A., and Lara-Porras, A. M. (2013). Evolution of HIV incubation times in AIDS patients. AIDS Care 25 (8), 1051–1061. doi:10.1080/09540121.2012.748876
Saied, A. A., Dhawan, M., Metwally, A. A., Fahrni, M. L., Choudhary, P., and Choudhary, O. P. (2022). Disease history, pathogenesis, diagnostics, and therapeutics for human monkeypox disease: A comprehensive review. Vaccines 10 (12), 2091. doi:10.3390/vaccines10122091
Tarín-Vicente, E. J., Alemany, A., Agud-Dios, M., Ubals, M., Suñer, C., Antón, A., et al. (2022). Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: A prospective observational cohort study. Lancet 400 (10353), 661–669. doi:10.1016/S0140-6736(22)01436-2
Thornhill, J. P., Barkati, S., Walmsley, S., Rockstroh, J., Antinori, A., Harrison, L. B., et al. (2022a). Monkeypox virus infection in humans across 16 countries - april-June 2022. N. Engl. J. Med. 387 (8), 679–691. doi:10.1056/NEJMoa2207323
Thornhill, J. P., Palich, R., Ghosn, J., Walmsley, S., Moschese, D., Cortes, C. P., et al. (2022b). Human monkeypox virus infection in women and non-binary individuals during the 2022 outbreaks: A global case series. Lancet 400, 1953–1965. doi:10.1016/S0140-6736(22)02187-0
Torster, L., Tegtmeyer, J., Kött, J., Christolouka, M., and Schneider, S. W. (2022). Localized monkeypox infestation in MSM on pre-exposure prophylaxis. J. Eur. Acad. Dermatol. Venereol. doi:10.1111/jdv.18539
Van Dijck, C., De Baetselier, I., Kenyon, C., Liesenborghs, L., Vercauteren, K., Van Esbroeck, M., et al. (2022). Mpox screening in high-risk populations finds no asymptomatic cases. Lancet Microbe. doi:10.1016/S2666-5247(22)00357-3
Vaughan, A., Aarons, E., Astbury, J., Brooks, T., Chand, M., Flegg, P., et al. (2020). Human-to-Human transmission of monkeypox virus, United Kingdom, october 2018. Emerg. Infect. Dis. 26 (4), 782–785. doi:10.3201/eid2604.191164
Ward, T., Christie, R., Paton, R. S., Cumming, F., and Overton, C. E. (2022). Transmission dynamics of monkeypox in the United Kingdom: Contact tracing study. BMJ 379, e073153. doi:10.1136/bmj-2022-073153
Keywords: monkeypox virus, mild-symptomatic patients, asymptomatic patients, seminal transmission, viral load
Citation: Yuan S, Jiang S-C, Zhang Z-W, Fu Y-F, Yang X-Y, Li Z-L and Hu J (2023) How and when does monkeypox (mpox) transmit: Implications for prevention and treatments. Front. Pharmacol. 13:1109928. doi: 10.3389/fphar.2022.1109928
Received: 28 November 2022; Accepted: 23 December 2022;
Published: 05 January 2023.
Edited by:
Ranjan K. Mohapatra, Government College of Engineering, Keonjhar, IndiaReviewed by:
Asmaa A. Metwally, Faculty of Veterinary Med, EgyptCopyright © 2023 Yuan, Jiang, Zhang, Fu, Yang, Li and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Yuan, roundtree318@hotmail.com
 Shu Yuan
Shu Yuan Si-Cong Jiang2
Si-Cong Jiang2 Zi-Lin Li
Zi-Lin Li