- 1Faculty of Chinese Medicine and State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, Macau, China
- 2National Traditional Chinese Medicine Clinical Research Base of the Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Luzhou, China
- 3The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Luzhou, China
- 4Zhuhai MUST Science and Technology Research Institute, Zhuhai, China
Background and purpose: Buyang Huanwu decoction (BYHWD) is widely used in the treatment of ischemic stroke in the recovery period, and many clinical trials have been reported, but its clinical efficacy and safety have not been fully evaluated. In this study, we conducted a systematic review and meta-analysis to evaluate the clinical efficacy and safety of BYHWD in the recovery period.
Materials and methods: Eight databases, including CNKI, Wanfang Database, VIP Database, China Biomedical Literature Database, PubMed, Cochrane Library, EMBASE, and Web of Science, were searched from the establishment of the database to 13 April 2022. We selected all eligible randomized controlled trials of BYHWD in the treatment of ischemic stroke during the recovery period. Systematic review and meta-analysis were conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines. The National Institutes of Health Stroke Score (NIHSS) was the primary outcome, and the Chinese Stroke Scale (CSS), activities of daily living (ADL), and adverse drug reaction (ADR) were the secondary outcomes.
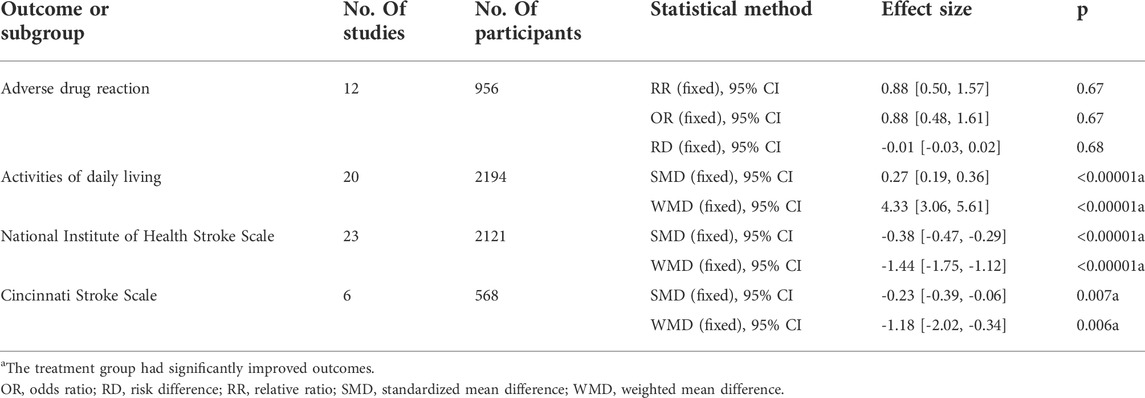
Results: A total of 39 randomized controlled trials were included, and 3,683 patients in the recovery period of ischemic stroke were recruited. Compared with conventional treatment alone, BYHWD combined with conventional treatment significantly decreased the NIHSS score (MD = -1.44, 95% CI: 1.75, -1.12, p < 0.00001), the CSS score (MD = -1.18, 95% CI: 2.02, -0.34, p = 0.006), improved the ADL (MD = 4.33, 95% CI: 3.06, 5.61, p < 0.00001), and did not increase the adverse reactions of patients (OR = 0.88, 95% CI: 0.48, 1.61, p = 0.67).
Conclusion: BYHWD is an effective and safe therapy for the recovery of ischemic stroke. To further determine the efficacy and safety of BYHWD in the treatment of ischemic stroke in the recovery period, more high-quality, multicenter, and prospective RCTs are needed.
1 Introduction
Ischemic stroke is a cerebrovascular disease caused by cerebral ischemia and hypoxia due to cerebral blood supply disorder, resulting in necrosis, softening, and the formation of infarction (Feske, 2021). Ischemic stroke is divided into the acute phase, recovery phase, and sequelae phase. The recovery period refers to 2 weeks to 6 months after the onset of the disease. This period is the key period for the recovery of patients, and it is also an important part of clinical treatment (Belova and Bogdanov, 2021). Patients with ischemic stroke are prone to neurological dysfunction, slow recovery, and many complications, which seriously affect their physical and mental health (Stinear et al., 2020). Active and effective treatments for stroke patients during the recovery period can significantly improve their daily living ability. Besides rehabilitation, antiplatelet aggregation and anticoagulant therapy are commonly used western medicine treatments for these patients, but they easily cause drug resistance and adverse reactions (Boulanger et al., 2018). In recent years, traditional Chinese medicine (TCM) has shown a good effect on ischemic stroke in the recovery period (Guo et al., 2020; Liu, et al., 2021).
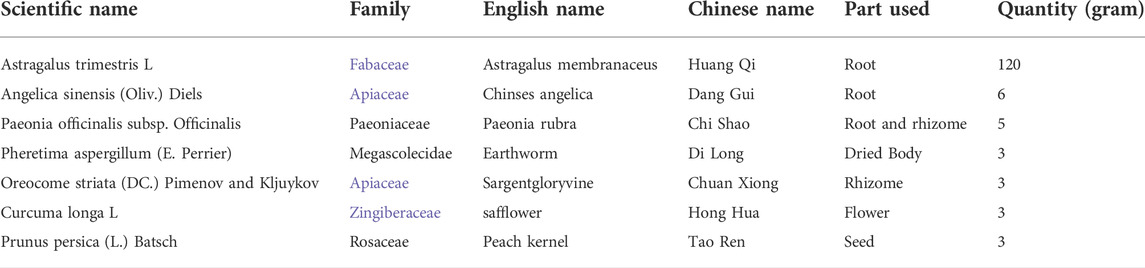
Ischemic stroke belongs to the category of “stroke” in TCM. It is mostly caused by deficiency of Qi and blood, lack of nourishment for the brain, block of phlegm and blood stasis, obstruction of brain and collaterals, deficiency of liver and kidney or hyperactivity of liver yang, and disturbance of wind and yang, which in turn causes hemiplegia, skewed tongue, hemianopia, aphasia and other symptoms (Zhao and Zhao, 2021). The main pathogenesis of ischemic stroke is characterized by “wind, fire, phlegm, Qi and blood stasis”. Wang Qingren pioneered the theory of “Qi deficiency and blood stasis” and believed that “the loss of vitality is its source” and “if vitality is deficient, it will not reach the blood vessels. Once the blood vessels have no gas, the blood will stop and become stasis” (Wang, 1999). It should be treated by supplementing qi, activating blood circulation, and removing blood stasis (Zhai et al., 2022). BYHWD is a classical prescription for regulating Qi and blood, removing phlegm and blood stasis in TCM (Shao et al., 2022). It is composed of Astragalus trimestris L [Fabaceae, the dried root of Astragalus trimestris L]; Angelica sinensis (Oliv.) Diels [Apiaceae, the dried root of Angelica sinensis (Oliv.) Diels]; Paeonia officinalis subsp. Officinalis [Paeoniaceae, the dried root and rhizome of Paeonia officinalis subsp. Officinalis]; Pheretima aspergillum (E. Perrier) [Megascolecidae, the dried body of Pheretima aspergillum (E. Perrier)]; Oreocome striata (DC.) Pimenov and Kljuykov [Apiaceae, the dried rhizome of Pheretima aspergillum (E. Perrier) ]; Oreocome striata (DC.) Pimenov and Kljuykov]; Curcuma longa L [Zingiberaceae, the dried flower of Curcuma longa L]; Prunus persica (L.) Batsch [the dried seed of Prunus persica (L.) Batsch], according to the ratio 120: 6: 5: 3: 3: 3: 3. In a previous study, an HPLC-DAD-ELSD method was developed for simultaneous determination of 12 bioactive compounds in BYHWD, including calycosin-O-β-D-glucoside ononin, calycosin, astragaloside IV and astragaloside I from Radix Astragalis; tetramethylpyrazine, ferulic acid and Z-ligustilide from Radix Angelicae Sinensis and Rhizoma Ligustici Chuanxiong; hydroxysafflor yellow A and kaempferol from Flos Carthami; paeoniflorin from Radix paeoniae Rubra; and amygdalin from Semen persicae. (Liu et al., 2010). In another study, Wang et al. found that hydroxysafflor yellow A, astragaloside IV, ferulic acid, ligustrazine, Z-ligustilide, and linoleic acid were considered to be bioactive compounds of BYHWD (Wang et al., 2021). Kaempferol, Quercetin, Mairin, Jaranol, Hederagenin and AstragalosideIV are the compounds of Astragalus trimestris L; Baicalein, Quercetagetin,Beta-carotene, and Baicalin are the compounds of Paeonia officinalis subsp. Officinalis and Curcuma longa L; Ferulic acid and Cis-ligustilide are the compounds of Angelica sinensis (Oliv.) Diels; Ligustrazine and Z-ligustilide are the compounds of Oreocome striata (DC.) Pimenov and Kljuykov; Sitosterol alpha1 and Folinic acid are the compounds of Prunus persica (L.) Batsch; Arachidonic acid and Dihydrocapsaicin are the compounds of Pheretima aspergillum (E. Perrier) (Table 1).
Many previous studies have shown that BYHWD has a good therapeutic effect on ischemic stroke. Based on network pharmacology, Wang K et al. found that the active ingredients of Buyang Huanwu Decoction in the treatment of ischemic stroke are baicalein β- Carotene, baicalin, kaempferol, etc. (Wang K, 2021). Cai GX et al. studied the effects of BYHWD on neurological function, quality of life, and serum vascular endothelial growth factor (VEGF) in convalescent patients with cerebral infarction in a randomized controlled trial (RCT), showing that BYHWD can improve the neurological function and quality of life of convalescent patients with cerebral infarction and increase serum VEGF (Cai and Liu, 2010). Jin C et al. conducted a meta-analysis to evaluate the role of BYHWD in poststroke fatigue patients. The results showed that BYHWD could improve the fatigue severity scale score and the total clinical effective rate (Jin et al., 2021). In recent years, BYHWD has been widely used in the treatment of ischemic stroke in the recovery period, and a large number of clinical trials have described its efficacy and safety, but no rigorous clinical research can provide reliable clinical evidence. The sample size of these trials is generally not large, and it is difficult to convince the public that BYHWD has a significant effect in the treatment of ischemic stroke in the recovery period based on the results of small sample data, which limits the use and promotion of BYHWD to a certain extent. In addition, no systematic review or meta-analysis has focused on the clinical efficacy and safety of BYHWD in the recovery period of ischemic stroke. Therefore, in this study, we conducted a systematic review and meta-analysis to evaluate the clinical efficacy and safety of BYHWD in the recovery period of ischemic stroke.
2 Methods
We conducted this systematic review and meta-analysis in accordance with the PRISMA (preferred Reporting Item for Systematic Reviews and Meta-Analyses) guidelines (Liberati et al., 2009).
2.1 Search strategy
Two independent reviewers (Wang and Ren) searched CNKI, Wanfang Database, VIP Database, China Biomedical Literature Database, PubMed, Cochrane Library, EMBASE, and Web of Science. The last search date was 13 April 2022. The search terms used were (“Apoplexy” OR “Stroke” OR “Cerebral Infarction” OR “Brain Infarction” OR “Ischemic Stroke” OR “Ischemic Apoplexy” OR “Cerebrovascular accident” AND “Buyang Huanwu Decoction” AND “random” OR “randomized controlled trial” OR “controlled clinical trial” OR (RCT) OR (RCT) OR (RCTs). No restrictions were imposed on language or publication status.
2.2 Type of study
2.2.1 Inclusion criteria
1) Type of study: A randomized controlled trial using integrated traditional Chinese and Western medicine in the recovery period of ischemic stroke. The languages are limited to Chinese and English. 2) Research subjects: patients were diagnosed with ischemic stroke in the recovery period (2 weeks to 6 months after onset). 3) Interventions: The control group received conventional treatment (including controlling blood pressure, improving microcirculation, expanding cerebral vessels, using neurotrophic agents and physical therapy, etc. The experimental group was given BYHWD on the basis of conventional treatment. 4) Outcomes: Studies including the National Institute of Health Stroke Scale (NIHSS), Chinese Stroke Scale (CSS), Activities of daily living (ADL), and Adverse drug reaction (ADR).
2.2.2 Exclusion criteria
1) Nonrandomized controlled trials or studies that do not indicate the type of study. 2) The included literature can only extract part of the original data, which makes the data impossible to extract. 3) Animal experiments, literature reviews, conference papers. 4) Outcomes studies that did not include NIHSS, CSS, ADL, and ADR. 5) Studies where interventions did not meet the requirements.
2.3 Participant characteristics
Age, sex, and race were not the limiting conditions for the inclusion criteria. As long as the ischemic stroke patients in the recovery period who met the above criteria were considered to meet the inclusion criteria.
2.4 Interventions
This study selected all RCTs comparing BYHWD with conventional methods in the treatment of ischemic stroke in the recovery period, regardless of language, publication status, or blinding method. Nonrandomized trials, reviews, case reports, and animal studies were excluded. The experimental group used both BYHWD and CT, and the control group used CT alone for comparison. Conventional treatment was the same in both groups.
2.5 Outcome measures
The NIHSS was the primary outcome measure, and CSS, ADL, and ADR were the secondary outcomes.
NIHSS (National Institute of Health Stroke Scale) score, which is a quantitative indicator of the severity of AIS disease, is often used as a surrogate endpoint in clinical research and stratifies patients according to the NIHSS score to guide clinical decision-making (Yamal, 2021). It is divided into 11 items, including consciousness, gaze, visual field, facial paralysis, upper limb movement, lower limb movement, ataxia, sensation, language, dysarthria, and neglect, with a score of 0–42. The lower the score, the better the neurological function. The Chinese Stroke Scale (CSS) is based on the standard evaluation of clinical efficacy revised by the fourth national Cerebrovascular Disease Conference (The Fourth National Academic Conference on cerebrovascular disease, 1996). Efficacy standards: A sensory test is performed on the big toe. Symptom score: yes = 1, no = 0; reflex score: none = 2, diminished = 1L, normal = 0; sensory test score: abnormal = 1, normal = 0. Among them, six points are from symptoms, eight points are from the reflexes of both lower extremities, and five points are from the sensation of the thumbs. The total score is added up, from normal = 0 points to the highest score of 19 points. The patient’s ability to do daily living (ADL) was assessed by the Basel index, with a total score of 0–100. A score <40 points indicated that the patient had severe activity disorder; 41–60 points, indicated that the patient needed help to complete daily activities. 60 points meant that the patient needed some help to complete daily activities. The higher the score, the stronger the ADL (Strini et al., 2020). Adverse drug reaction (ADR) mainly referred to gastrointestinal reactions after taking drugs, such as nausea and retching. The internal consistency reliability of NIHSS, CSS, and ADL was high, and the three scales had common validity, but the predictive validity of CSS and ADL was not as comprehensive as NIHSS (Wang et al., 1999; Wu, 2007; Tao 2009).
2.6 Data extraction
Two researchers (Wang and ren) independently screened the literature in strict accordance with the inclusion and exclusion criteria. First, the literature was initially screened by reading the title and abstract and then further screened by reading the full text. In case of disagreement, a third party (Wu and Zhang) judged, and finally decided to include or exclude through discussion. Then, two researchers (Li and Bai) independently extracted and included relevant research data, including title, author, year, country, diagnosis method of ischemic stroke, the sample size of each group, age, sex, treatment method, treatment time, outcome indicators and evaluation methods, and main research results.
2.7 Methodological quality assessment
The authenticity of the RCTs was assessed by two investigators (Guo and Bai) according to the Cochrane Handbook, and the risk of bias in the literature was assessed according to the Cochrane Risk of Bias Tool. In case of disagreement, a third party (Zhang and Yang) was consulted. The risk of bias was assessed using seven criteria, including random sequence generation, concealed assignment, blinding of participants and personnel, incomplete outcome data, selective reporting, and other biases. The risk of bias was classified into three categories: “low” (+), “high” (-), and “unclear” (?). The Jadad scale quality score was used to evaluate the methodological quality of the literature, with one to two points for low quality and three to five points for high quality.
2.8 Data synthesis and analysis
Revman 5.4 software was used for meta-analysis (Copenhagen: the Nordic Cochrane Centre, the Cochrane Collaboration, 2014). Pooled effect size: Count data and measurement data were analyzed by odds ratio (OR), relative risk (RR), mean difference (MD), or standard mean difference (SMD). Heterogeneity analysis: I2 was used to assess the heterogeneity of the studies. When I2 < 50%, p > 0.1, it indicated that the heterogeneity was not significant, and a fixed effect model was used; when I2 ≥ 50%, p ≤ 0.1, it indicated that the heterogeneity was substantial, and the source of heterogeneity needed to be analyzed. If there was substantial heterogeneity, a random effect model was used (Cumpston and Li, 2019; Wang et al., 2019; Chen H. et al., 2020; Wu et al., 2020); if the heterogeneity still existed, the source of heterogeneity was analyzed from both methodological and clinical aspects, and subgroup analysis was used.
2.9 Risk of bias across trials
Funnel plots and Egger’s test were carried out to examine the potential bias in the included trials when the number of RCTs was ≥10 (Egger et al., 1997; Wu et al., 2020).
2.10 Quality of evidence
Two independent reviewers (Wang and Ren) used the GRADE (grading of recommendations, assessment, development, and evaluations) method (Guyatt et al., 2008) to evaluate the risk of bias in each included trial. If there was disagreement on the downgrade or upgrade evaluation, it was evaluated and decided by the third party (Wu and Zhang). Evidence evaluation adopts four grades of “high”, “medium”, “low” and “extremely low”.
3 Results
3.1 Study identification and selection
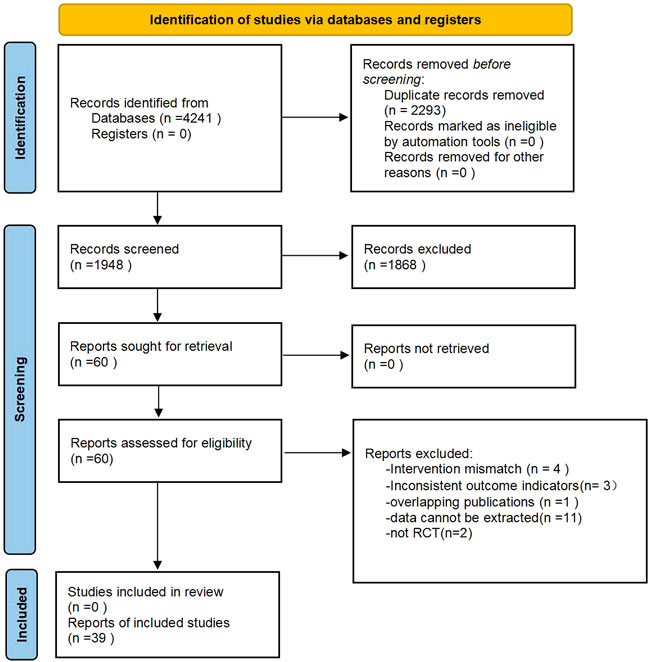
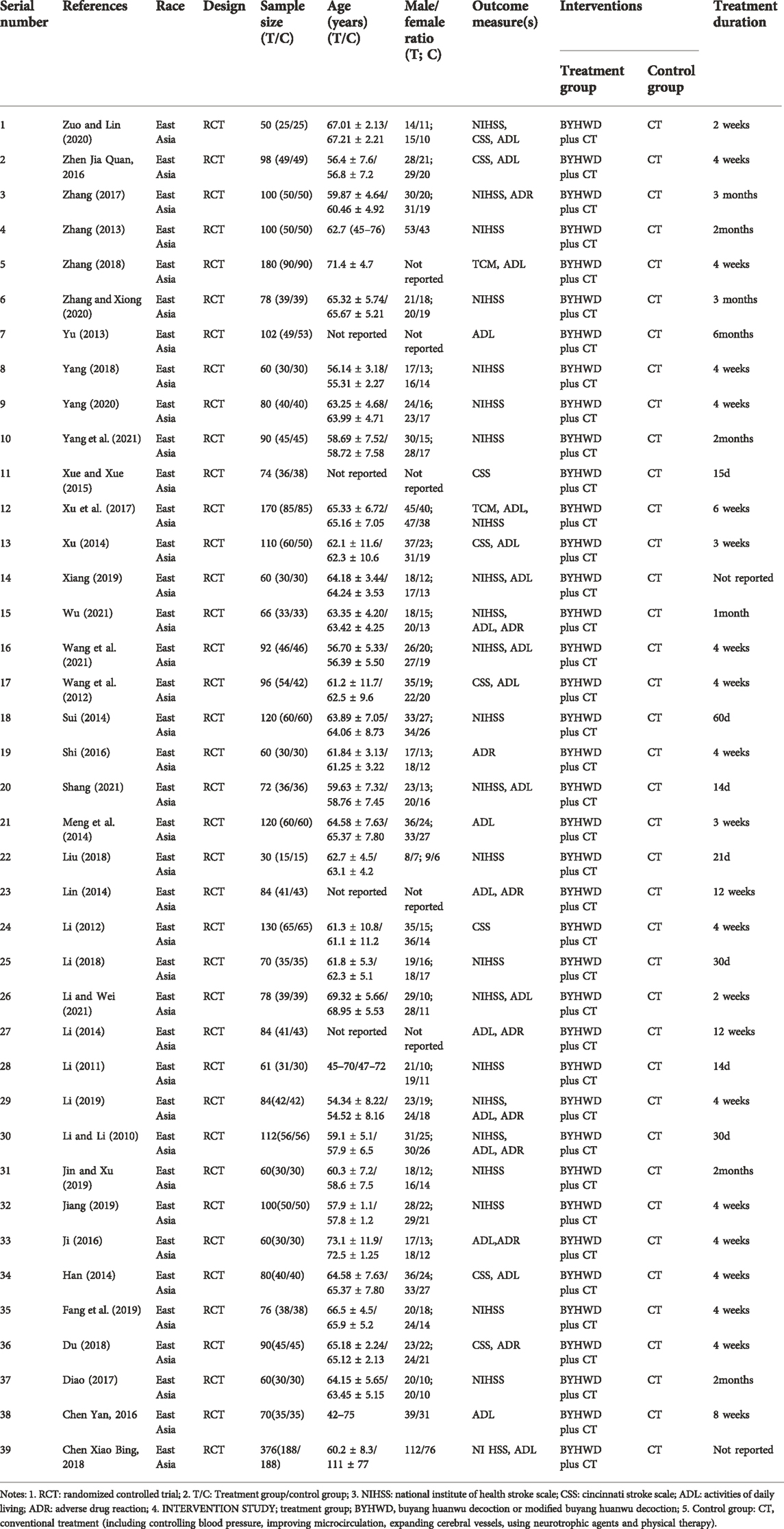
After searching major databases, a total of 4,241 articles were retrieved. A total of 2294 duplicates were excluded, and 1948 remained; 1868 were excluded after reading the title and abstract, and 60 remained; 21 were excluded after reading the full text, and 39 were finally included in the study. Figure 1 shows the general flow of the study selection process. Table 2 summarizes the general characteristics of the 39 studies.
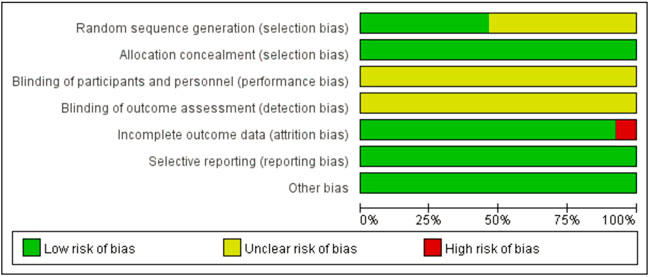
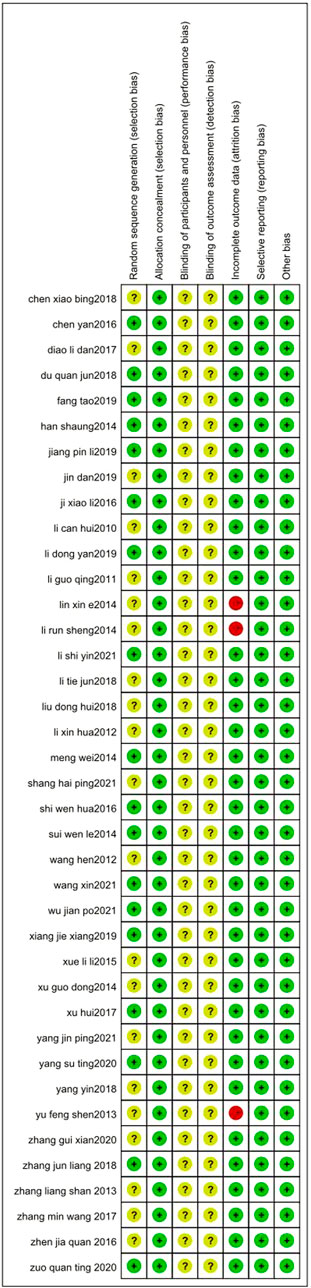
3.2 Risk of bias
The risks of bias in the trials are shown in Table 3 and Figure 2, and Figure 3. All 39 trials included in this study explicitly used random sequence generation, of which 18 described the randomization methods in detail (random number table method for 16 trials: Liang et al., 2021; Yang 2022; Zuo and Lin, 2020; Zhang, 2018; Xu et al.,2017; Xiang, 2019; Wu, 2021; Wang et al., 2021; Sui, 2014; Shi, 2016; Li and Wei, 2021; Li, 2019; Jiang, 2019; Ji, 2016; Han, 2014; Fang et al., 2019; Chen and Cao, 2016; random alphabet method for one trial: (Meng et al., 2014); random envelope method for one trial: (Du, 2018). The other 21 articles described the use of randomization but did not provide detailed information on the methods of randomization.
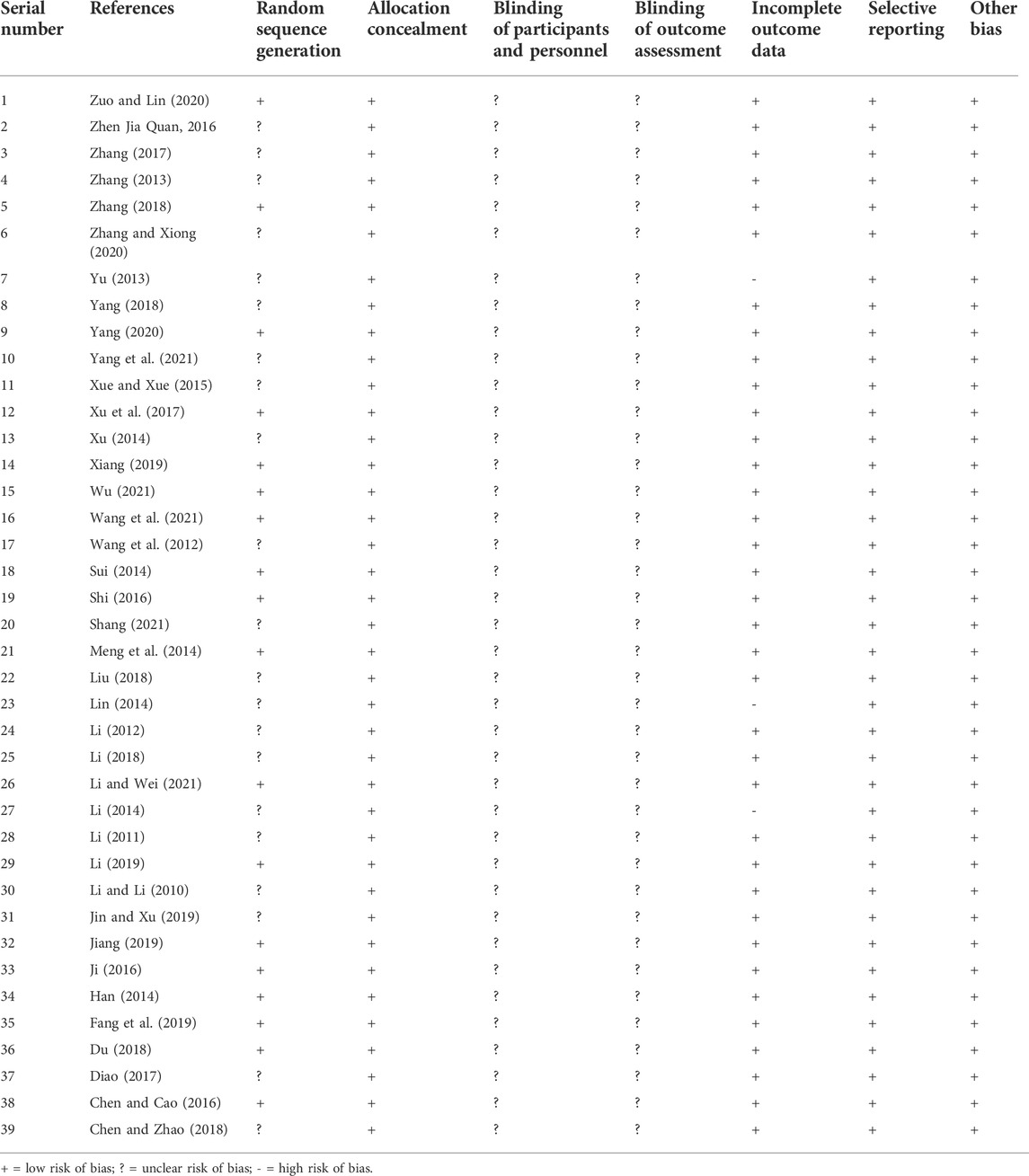
TABLE 3. The methodological quality of the included trials assessed using the Cochrane Risk of Bias Tool.
Based on the information from the included literature, all the studies performed allocation concealment. The blinding of participants or personnel and the blinding of outcome assessments were not mentioned in any of the studies. Detection bias on complete outcome data was considered low in all trials. All data were also considered to be at low risk of selective reporting and other biases.
In addition, the Jadad scale quality score is shown in Table 4. The final scores of 39 articles were all three or above, belonging to high-quality literature.
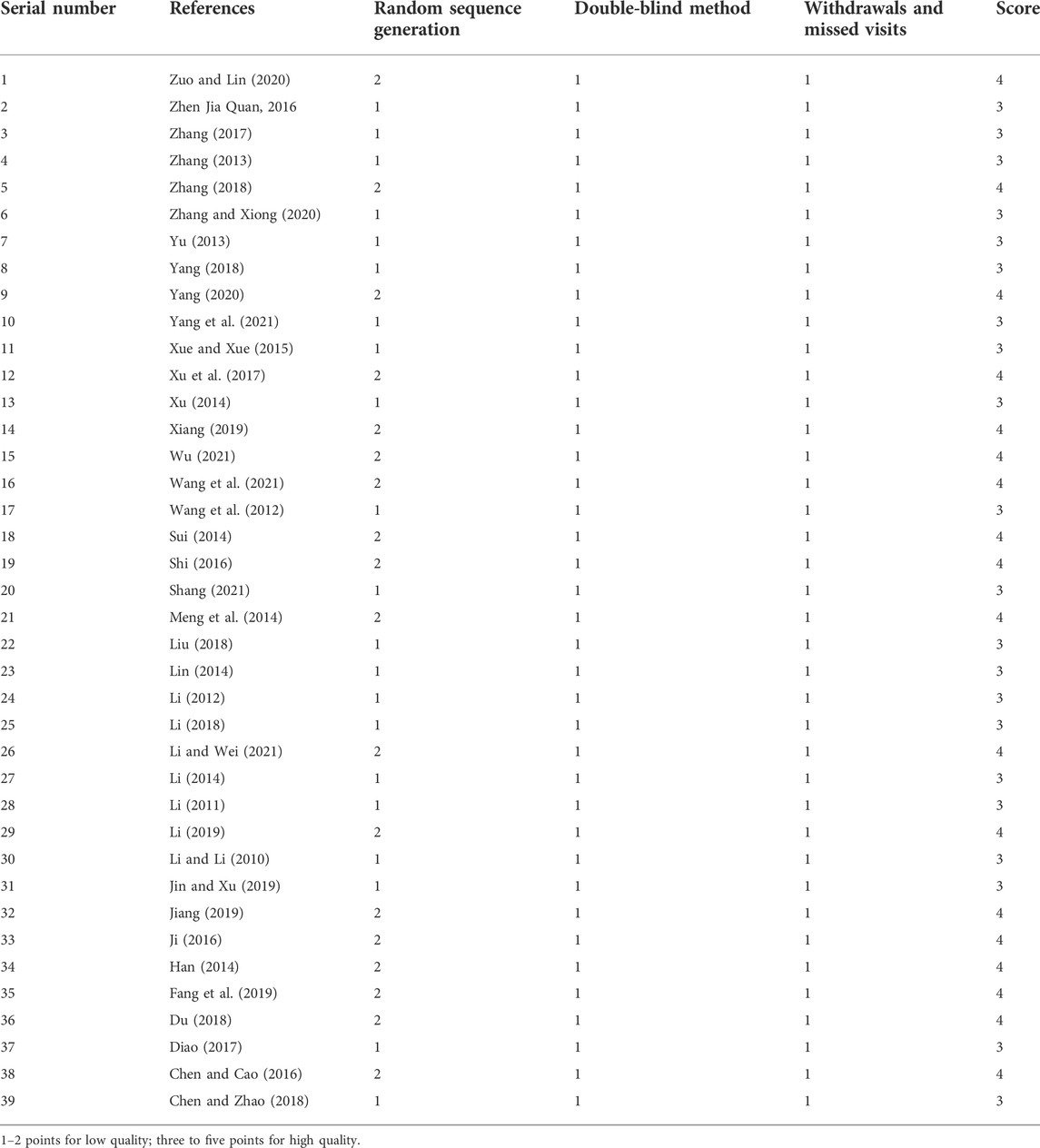
TABLE 4. The methodological quality of the included trials assessed using the Jadad scale quality score.
3.3 Outcome measures
The summary of the meta-analysis is presented in Table 5.
3.4 Primary outcome
3.4.1 NIHSS
Twenty-three articles reported the NIHSS scores of patients after BYHWD combined with conventional treatment or conventional treatment alone. Due to the results of the heterogeneity test among the studies (p = 1.00, I2 = 0%), a fixed effect model was used. The results of the meta-analysis showed that the NIHSS score of the experimental group was significantly lower than that of the control group (MD = -1.44%, 95% CI: 1.75, -1.12, p < 0.00001) (Figure 4).
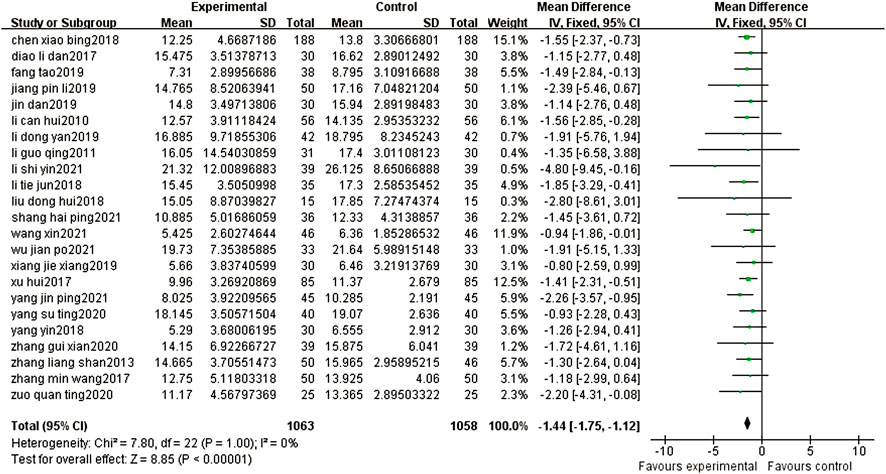
FIGURE 4. Forest plots showed that the NIHSS score of the experimental group decreased. Compared with that of the control group.
3.5 Secondary outcomes
3.5.1 CSS
Six articles reported the CSS of patients after different treatments with BYHWD plus conventional treatment or conventional treatment alone. There was no heterogeneity among the studies (p = 0.95, I2 = 0%), and a fixed effect model was used. The results of the meta-analysis showed that the CSS score of the experimental group was statistically lower than that of the control group (MD = -1.18, 95% CI: 2.02, -0.34, p = 0.006) (Figure 5).
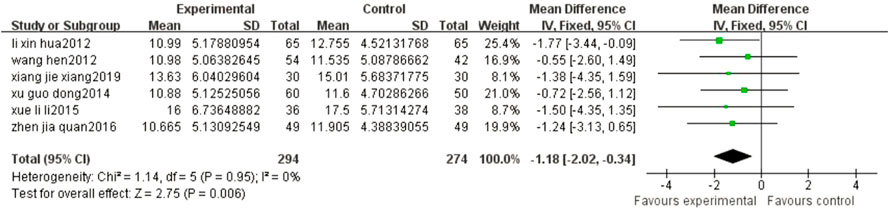
FIGURE 5. Forest plots showed that the CSS score of the experimental group decreased compared with that of the control group.
3.6 ADL
The results of the meta-analysis showed that compared with the control group, the ADL of patients in the experimental group was significantly improved (MD = 4.33, 95% CI: 3.06, 5.61, p < 0.00001) (Figure 6).
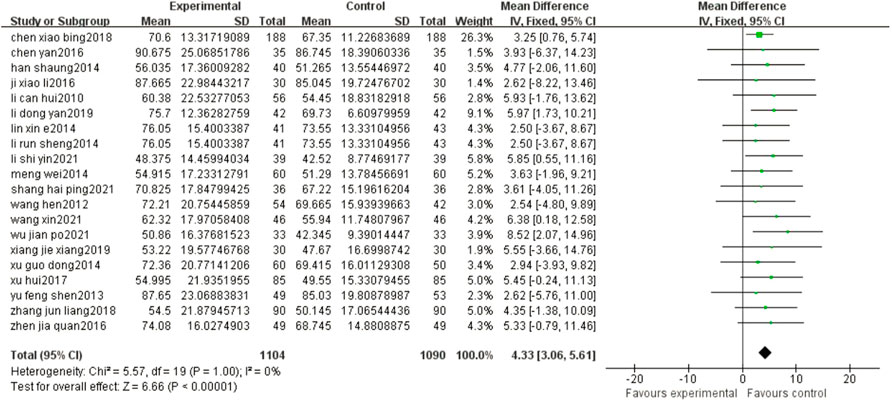
FIGURE 6. Forest plots showed that there was a significant improvement in the ADL in the experimental group compared with that of the control group.
3.8 ADR
The results of the meta-analysis showed that compared with conventional treatment alone, BYHWD plus conventional treatment did not increase the adverse reactions of patients (OR = 0.88, 95% CI: 0.48, 1.61, p = 0.67) (Figure 7).
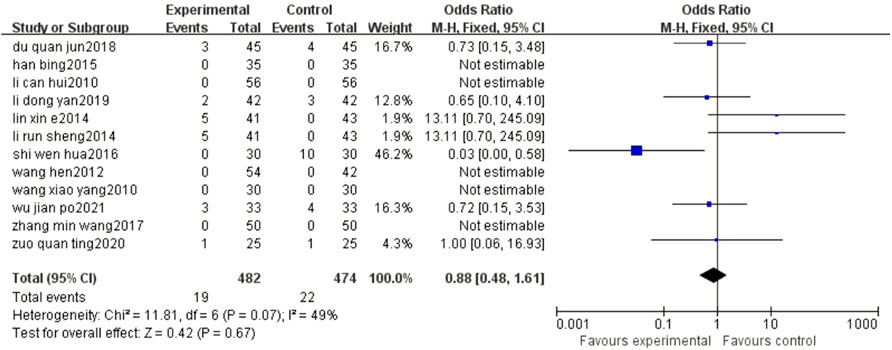
FIGURE 7. Forest plots showed that the increase in ADR in the experimental group was not obvious compared with that of the control group.
3.9 Publication bias
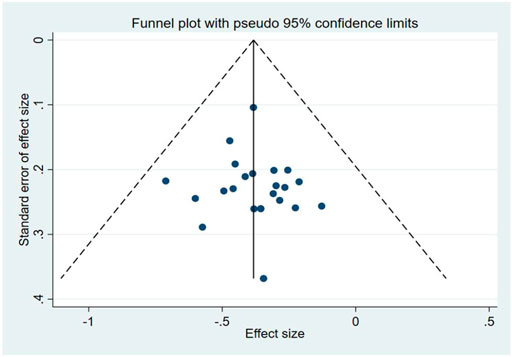
The funnel plots of the NIHSS suggested that there was a possible publication bias in small trials (Figure 8). Egger’s test also indicated there was significant publication bias (p = 0.672).
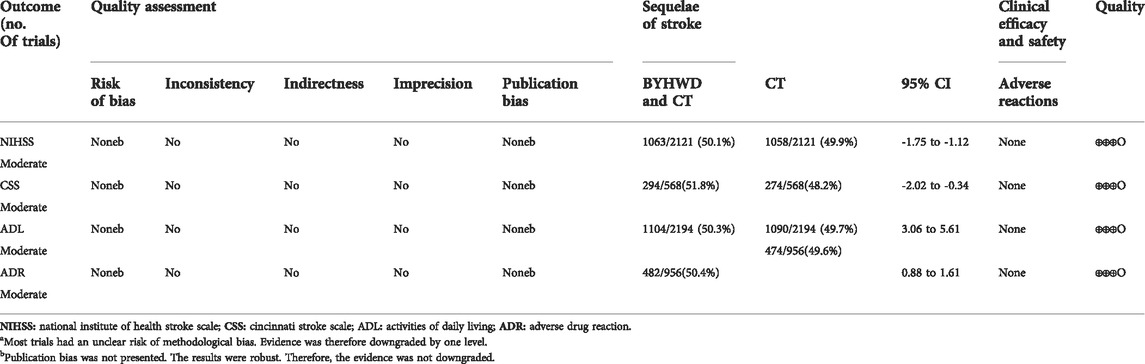
3.10 Quality of evidence
Using GRADE, we assessed the certainty of the evidence to be moderate to low for outcomes for which data were available. In all 39 trials, the quality of evidence was downgraded by one level because of the unclear risk of method bias in some trials. The total number of patients was enough for each outcome, and the statistical heterogeneity of the results was small, so the quality of evidence for these outcomes was upgraded by one level. Consequently, the quality of evidence was moderate for the NIHSS, ADL, and ADR scores (Table 6).
4 Discussion
Stroke is the main cause of disability and the second leading cause of death in the world (Paul and Candelario-Jalil, 2021). Ischemic stroke has become a global health problem that seriously threatens human life and health (Jiang et al., 2020). With the continuous development of medicine, the methods of treating ischemic stroke are also increasing, and TCM treatment has always played an important role in it. BYHWD is a classical Chinese medicine prescription for the treatment of ischemic stroke in the recovery period and has a good clinical effect on ischemic stroke in the recovery period (Liu et al., 2022). Therefore, this meta-analysis aimed to evaluate the clinical efficacy and safety of BYHWD in the recovery period of ischemic stroke patients.
A total of 39 studies involving 3,683 patients were included in this meta-analysis, and BYHWD combined with conventional treatment and conventional treatment alone were compared in patients with ischemic stroke in the recovery period. Under normal circumstances, clinical ischemic stroke patients often leave symptoms of different degrees of neurological deficits. Improving the symptoms of this neurological deficit and improving the activities of daily living have always been the top priorities in the treatment of cerebral infarction (Xing and Bai, 2020). Therefore, in this study, the NIHSS was the primary outcome measure, and CSS, ADR, and ADL were the secondary outcomes. The above indicators were used as clinical trial observation and efficacy evaluation indicators. The National Institute of Health Stroke Scale (NIHSS) score (Yamal 2021), which is a quantitative indicator of the severity of the stroke, is often used as a surrogate endpoint in clinical research and stratifies patients according to the NIHSS score to guide clinical decision-making. Effective treatment was defined as a decrease in the NIHSS score by more than four points or complete disappearance of symptoms after treatment. The CSS score includes horizontal gaze, level of consciousness, speech, limb flexibility, and walking ability. The higher the score, the worse the patient’s condition and the worse the neurological function (Cai and Zhang, 2022). The results of this study show that, compared with conventional treatment alone, BYHWD combined with conventional treatment can reduce the NIHSS score and CSS score of patients, suggesting that BYHWD can improve the neurological function of ischemic stroke patients in the recovery period. In terms of activities of daily living, the activities of daily living in the experimental group were stronger than those in the control group. Moreover, the results of the meta-analysis indicated that compared with the control group, the treatment of the experimental group in the recovery period of ischemic stroke did not increase the adverse reactions of patients. All the results prove that BYHWD is an effective therapy to improve the recovery period of ischemic stroke, which is beneficial for relieving the patient’s condition, promoting the improvement of the patient’s neurological function, and improving the quality of life without increasing adverse reactions.
BYHWD comes from Wang Qingren’s “Yilin Correction” in the Qing Dynasty. It is a commonly used prescription for the treatment of ischemic stroke. “This prescription treats hemiplegia, crooked eyes, slurred speech, salivation at the corners of the mouth, dry stools, frequent urination, and incontinence of enuresis” (Wu, 2019). This prescription has the compatibility characteristics of “not to remove blood stasis to activate blood, but to invigorate Qi to activate blood” (Liang et al., 2021). Astragalus trimestris L is the monarch drug in BYHWT, which has the effects of nourishing vitality, promoting blood circulation, and removing blood stasis; Angelica sinensis (Oliv.) Diels is the ministerial drug of the formula, which can activate blood and nourish blood and remove blood stasis; Other botanical drugs have activities of expectorating phlegm and dredging collaterals. The combination of the above drugs can have a synergistic effect and achieve the effects of nourishing Qi and promoting blood circulation, removing blood stasis, and dredging collaterals. As a popular traditional Chinese medicine formula, BYHWD was widely used for treating ischemic diseases. However, there are few studies focused on the effects of BYHWD on neurodegenerative diseases, and the underlying molecular mechanisms are largely elusive. Li Z et al. established a neurotoxic model in PC12 cells and adopted an innovative experimental grouping method to investigate the neuroprotective effects of BYHWD on neurotoxicity induced by 6-Hydroxydopamine (6-OHDA) exposure. They found that BYHWD had neuroprotective effects against the 6-OHDA-induced neurotoxicity via Akt/GSK3β pathway based on serum pharmacology methodology. (Li et al., 2016). Another study found that BYHWD could modulate multiple signaling pathways including the Jak/Stat3/cyclin D1 signaling pathway, EGFR/PI3K/Akt/Bad/14–three to three signaling pathway, caveolin-1, and Hes1. The modulations of these cellular signaling pathways contributed to the anti-apoptotic cell death, improvement of the neural stem cell proliferation, astrogenesis, and neurogenesis in post-ischemia brains, subsequently inducing the recovery of the neurological functions in the post-ischemic brains (Chen X. et al., 2020). In addition, modern studies have shown that BYHWD can reduce cerebral infarct size and improve neurological deficits in ischemic stroke rats and attenuate neuronal damage in rats with cerebral ischemia/reperfusion (I/R) injury (Li et al., 2021); BYHWD can promote neurogenesis and angiogenesis in rats with cerebral ischemia (Zhuge et al., 2020); BYHWD can protect the integrity of the neurovascular unit and improve the permeability of the blood-brain barrier, thereby improving stroke caused by cerebral ischemia (Zheng et al., 2021). Therefore, BYHWD can effectively treat ischemic stroke and can be widely used in the clinical treatment of ischemic stroke.
Limitations of this study: 1) Although the included trials were described as “random grouping”, most of the trials did not describe specific grouping methods, blinding, allocation concealment, etc., so the possibility of selection bias cannot be ruled out; 2) Samples of most included studies were relatively small; 3) The efficacy evaluation of most studies was subject to a certain degree of subjectivity, and there was a lack of standard quantitative research; 4) Due to generally low quality of the included trials, this study can only draw very limited conclusions. There is an urgent need to improve the quality of the design and report of such studies.
5 Conclusion
Compared with conventional treatment alone, BYHWD combined with conventional treatment contributed to a significant improvement in clinical efficacy, neurological function, and activities of daily living, while it did not increase adverse reactions. Due to the limitations of this study, the quality of the included trials was generally low. In the future, more clinical trials with standardized designs, strict implementations, and large samples are needed to further verify the clinical efficacy and safety of BYHWD in the treatment of ischemic stroke in the recovery period and provide a more reliable evidence-based basis for clinical application.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RW, JR, SL, XB, and WG are equal contributors and co-first authors.
Funding
This work was financially supported by the Science and Technology Development Fund, Macau SAR (File No: 0077/2019/A2, 0040/2021/AGJ and SKL-QRCM(MUST)-2020-2022); Sichuan Academy of traditional Chinese medicine reserve candidate project (File No: Sichuan Chinese Medicine Letter (2020) No. 85).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CT, conventional treatment; NIHSS, National Institute of Health Stroke Scale; CSS, Chinese Stroke Scale; TCM, traditional Chinese medicine; ADL: activities of daily living; ADR, adverse drug reaction; RCT, randomized controlled trial; T/C, treatment group/control group.
References
Belova, A. N., Bogdanov, E. I., Zhadnov, V. A., Kamchatnov, P. R., and Kurushina, O. V. (2021). [Therapy of moderate cognitive impairment in the early recov ery period of ischemic stroke]. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova 121 (5), 33–39. doi:10.17116/jnevro202112105133
Boulanger, J. M., Lindsay, M. P., Gubitz, G., Smith, E. E., Stotts, G., Foley, N., et al. (2018). Canadian stroke best practice recommendations for acute stroke management: Prehospital, emergency department, and acute inpatient stroke care, 6th edition, update 2018. Int. J. Stroke 13 (9), 949–984. doi:10.1177/1747493018786616
Cai, G. X., and Liu, B. Y. (2010). Effect of ultra-micronized Buyang Huanwu decoction on neurological function, quality of life, and serum vascular endothelial growth factor in patients convalescent from cerebral infarction. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 22 (10), 591–594.
Cai, Z., and Zhang, L. (2022). The effect of combined treatment with ginkgo diterpene lactone meglumine and ozagrel in patients with acute cerebral infarction and its influence on CSS score[J]. Guizhou Med. 46 (02), 251–252.
Chalos, V., van der Ende, N. A. M., Mulder, M. J. H. L., Venema, E., and Dijkland, S. A. (2020). National Institutes of health stroke scale: An alternative primary outcome measure for trials of acute treatment for ischemic stroke. Stroke 51 (1), 282–290. doi:10.1161/STROKEAHA.119.026791
Chen, H., Yao, X., Li, T., Lam, C. W. K., Zhang, R., Zhang, H., et al. (2020a). Compound kushen injection combined with platinum-based chemotherapy for stage III/IV non-small cell lung cancer: A meta-analysis of 37 RCTs following the PRISMA guidelines. J. Cancer 11 (7), 1883–1898. doi:10.7150/jca.40267
Chen, X., Chen, H., He, Y., Fu, S., Liu, H., Wang, Q., et al. (2020b). Proteomics-guided study on Buyang huanwu decoction for its neuroprotective and neurogenic mechanisms for transient ischemic stroke: Involvements of EGFR/PI3K/Akt/Bad/14-3-3 and jak2/stat3/cyclin D1 signaling cascades. Mol. Neurobiol. 57 (10), 4305–4321. doi:10.1007/s12035-020-02016-y
Chen, X., and Zhao, D. (2018). Analysis of the effect of Buyang Huanwu Decoction on 188 patients with cerebral infarction in convalescence. Chin. Community Physician 34 (26), 115117. doi:10.3969/j.issn.1007-614x.2018.26071
Chen, Y., and Cao, J. (2016). Clinical observation on 35 cases of cerebral infarction in recovery period treated with combination of traditional Chinese and western medicine. Pract. Integr. Chin. West. Med. Clin. 16 (5), 16–18. doi:10.13638/j.issn.1671-4040
Cumpston, M., Li, T., Chandler, J., Welch, V. A., and Higgins, J. P. (2019). Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for systematic reviews of interventions. Cochrane Database Syst. Rev. 10, ED000142. doi:10.1002/14651858.ED000142
Diao, L. (2017). Study on clinical efficacy of Buyang Huanwu Decoction in treating 30 patients with cerebral infarction in convalescence. China CME 9 (18), 180–181. doi:10.3969/j.issn.1674-9308.2017.18.099
Du, Q. (2018). Observation on the therapeutic effect of Buyang Huanwu Decoction on stroke recovery. J. Chronic Dis. 7, 1. doi:10.16440/j.cnki.1674-8166.20180517.001
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi:10.1136/bmj.315.7109.629
Encephalopathy Emergency Collaborative Group of the State Administration of Traditional Chinese Medicine (1995). Stroke diagnosis and efficacy evaluation criteria (trial version) [M]. Beijing: China Traditional Chinese Medicine Press, 1–240.
Fang, T., Wang, G., and Zhao, J. (2019). Effect of Buyang Huanwu Decoction on serum IGF-1 level and cognitive function of patients with cerebral infarction in convalescence. Hainan Med. J. 30 (23), 3027–3029. doi:10.3969/j.issn.1003-6350.2019.23.010
Feske, S. K. (2021). Ischemic stroke. Am. J. Med. 134 (12), 1457–1464. doi:10.1016/j.amjmed.2021.07.027
Guo, Q., Yang, S., Yang, D., Zhang, N., Li, X., Chen, T., et al. (2020). Differential mRNA expression combined with network pharmacology reveals network effects of Liangxue Tongyu Prescription for acute intracerebral hemorrhagic rats. J. Ethnopharmacol. 246, 112231. doi:10.1016/j.jep.2019.112231
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). Grade: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336 (7650), 924–926. doi:10.1136/bmj.39489.470347.AD
Han, S. (2014). Effect of Buyang Huanwu Decoction on the recovery of neurological function and daily living ability of patients with cerebral infarction in the recovery period (Qi deficiency and blood stasis syndrome). Chin. J. Disabil. Med. 11, 193–194. doi:10.13214/j.cnki.cjotadm.2014.11.184
Ji, X. (2016). Clinical observation on the treatment of cerebral infarction of qi deficiency and blood stasis type with modified Buyang huanwu decoction. Dis. Monit. Control 10 (6), 458–459.
Jiang, C. T., Wu, W. F., Deng, Y. H., and Ge, J. W. (2020). Modulators of microglia activation and polarization in ischemic stroke (Review). Mol. Med. Rep. 21 (5), 2006–2018. doi:10.3892/mmr.2020.11003
Jiang, P. (2019). Quantitative evaluation and analysis of therapeutic effect of Buyang Huanwu Decoction on qi deficiency and blood stasis syndrome in stroke recovery. Health Care Guide 29, 252.
Jin, C., Kwon, S., Cho, S. Y., Park, S. U., Jung, W. S., Moon, S. K., et al. (2021). A systematic review and meta-analysis of the effects of herbal medicine Buyang huanwu tang in patients with poststroke fatigue. Evid. Based. Complement. Altern. Med. 2021, 4835488. doi:10.1155/2021/4835488
Jin, D., and Xu, Q. (2019). Study on the improvement of nerve function and quality of life of patients with cerebral infarction in the recovery period by Buyang Huanwu Decoction. Electron. J. Clin. Med. Literature 6 (A1), 174.
Li, C., and Li, Z. (2010). The effect of supplementing qi and activating blood circulation on the quality of life and curative effect of patients with cerebral infarction in the recovery period. J. Traditional Chin. Med. 16 (6), 41–43. doi:10.3969/j.issn.1672-951X.2010.06.021
Li, D. (2019). Prognostic analysis of patients with cerebral infarction in convalescence treated with Buyang Huanwu Decoction. China Pract. Med. 14 (13), 127–128. doi:10.14163/j.cnki.11-5547/r.2019.13.068
Li, G. (2011). Clinical observation on modified Buyang huanwu decoction in treating cerebral infarction in recovery period[J]. Med. Inf. late issue 24 (1), 2. doi:10.3969/j.issn.1674-7860.2014.35.033
Li, H., Peng, D., Zhang, S. J., Zhang, Y., Wang, Q., and Guan, L. (2021). Buyang Huanwu Decoction promotes neurogenesis via sirtuin 1/autophagy pathway in a cerebral ischemia model. Mol. Med. Rep. 24 (5), 791. doi:10.3892/mmr.2021.12431
Li, R. (2014). Clinical observation on Buyang huanwu decoction in the treatment of cerebral infarction in the recovery period. Inn. Mong. J. Traditional Chin. Med. 33 (17), 10–11.
Li, S., and Wei, Z. (2021). Effect of Buyang Huanwu Decoction on neurological function and serum oxLDL, MDA, SOD of stroke patients in recovery period. Guangming Tradit. Chin. Med. 36 (19), 3296–3298. doi:10.3969/j.issn.1003-8914.2021.19.032
Li, T. (2018). Clinical observation of Buyang Huanwu decoction in treating cerebral infarction in convalescence Oriental. food Ther. health care 1, 267.
Li, X. (2012). Analysis of the clinical efficacy of Buyang Huanwu Decoction in the recovery period of cerebral infarction. China Med. Eng. 20 (12), 125.
Li, Z., Wang, H., Wang, Q., and Sun, J. (2016). Buyang huanwu decoction vigorously rescues PC12 cells against 6-OHDA-induced neurotoxicity via akt/gsk3β pathway based on serum pharmacology methodology. Rejuvenation Res. 19 (6), 467–477. doi:10.1089/rej.2015.1798
Liang, R., Tang, Q., Wang, L., Yue, P., and Zhu, L. (2021). Buyang huanwu decoction combined with probiotics or prebiotics for functional recovery from stroke: A meta-analysis protocol for systematic review. Med. Baltim. 100 (51), e28371. doi:10.1097/MD.0000000000028371
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P. A., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 339, b2700. doi:10.1136/bmj.b2700
Lin, X. (2014). Buyang Huanwu Decoction in the treatment of 41 patients with cerebral infarction in the recovery period. Modern Distance Education of Chinese Med. 12 (11), 129–130. doi:10.3969/j.issn.1672-2779.2014.11.082
Liu, D. (2018). Clinical observation on Buyang huanwu decoction in treating patients with qi deficiency and blood stasis syndrome in the recovery period of cerebral infarction. Electron. J. Cardiovasc. Dis. Integr. Traditional Chin. West. Med. 6 (15), 143.
Liu, E. H., Qi, L. W., Cheng, X. L., peng, Y. B., and Li, P. (2010). Simultaneous determination of twelve bioactive constituents in Buyang Huanwu decoction by HPLC-DAD-ELSD and HPLC-TOF/MS. Biomed. Chromatogr. 24 (2), 125–131. doi:10.1002/bmc.1269
Liu, M., Pu, Y., Gu, J., He, Q., Liu, Y., Zeng, Y., et al. (2021). Evaluation of zhilong huoxue tongyu capsule in the treatment of acute cerebral infarction: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 86, 153566. doi:10.1016/j.phymed.2021.153566
Liu, W., Zhang, Y., Zhou, X., and Zeng, K. (2022). Research progress on the mechanism of Buyang Huanwu Decoction against cerebral ischemia[J]. New Chin. Med. Clin. Pharmacol. 33 (03), 411–418.
Meng, W., Li, J., and Zhang, L. (2014). Clinical observation on modified Buyang huanwu decoction in the treatment of cerebral infarction recovery (qi deficiency and blood stasis syndrome). J. Yunnan Univ. Traditional Chin. Med. 2, 74–76.
Paul, S., and Candelario-Jalil, E. (2021). Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 335, 113518. doi:10.1016/j.expneurol.2020.113518
Shang, H. (2021). Effect of Buyang huanwu decoction on cerebral infarction recovery. Big Vis. Health 9, 85.
Shao, L., She, Y., Yong, S., Chen, B., Yi, J., Li, Y., et al. (2022). An evidence-based evaluation of Buyang huanwu decoction for the treatment of the sequelae of stroke: A PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 104, 154312. doi:10.1016/j.phymed.2022.154312
Shi, W. (2016). Observation on the clinical efficacy of traditional Chinese medicine in treating patients with stroke in convalescence. Health Care Guide 32, 23.
Stinear, C. M., Lang, C. E., Zeiler, S., and Byblow, W. D. (2020). Advances and challenges in stroke rehabilitation. Lancet. Neurol. 19 (4), 348–360. doi:10.1016/S1474-4422(19)30415-6
Strini, V., Piazzetta, N., Gallo, A., and Schiavolin, R. (2020). Barthel index: Creation and validation of two cut-offs using the BRASS index. Acta Biomed. 91 (2-S), 19–26. doi:10.23750/abm.v91i2-S.9226
Sui, W. (2014). Li aiping clinical study on Buyang huanwu decoction to improve neurological function defects in the recovery period of ischemic stroke. New Chin. Med. 46 (7), 40–42. doi:10.13457/j.cnki.jncm.2014.07.19
Tao, Z. (2009). Reliability, validity and sensitivity of Chinese scale for clinical neurological deficit of stroke patients. Acad. J. Sec. Mil. Med. Univ. 30 (03), 283–285. doi:10.3724/sp.j.1008.2009.00283
The Fourth National Academic Conference on cerebrovascular disease (1996). sScoring criteria for clinical neurological deficits of stroke patients [J]. Chin. J. Neurology 29 (6), 381–382.
Wang, H., Ma, Z., and Shi, Z. (2012). Observation on the therapeutic effect of Buyang Huanwu Decoction on 54 cases of cerebral infarction in the recovery period. Yunnan J. Traditional Chin. Med. 6 (6), 47–48. doi:10.3969/j.issn.1007-2349.2012.06.027
Wang, K., Lei, L., Qiao, Y., Liang, R., and Duan, J. (2021). Network pharmacology-based prediction of the active compounds and mechanism of Buyang Huanwu Decoction for ischemic stroke. Exp. Ther. Med. 22 (4), 1050. doi:10.3892/etm.2021.10484
Wang, X., Liu, Z., Sui, X., Wu, Q., Wang, J., and Xu, C. (2019). Elemene injection as adjunctive treatment to platinum-based chemotherapy in patients with stage III/IV non-small cell lung cancer: A meta-analysis following the PRISMA guidelines. phytomedicine 59, 152787. doi:10.1016/j.phymed.2018.12.010
Wang, X., Wang, Y., Liu, Z., and Qu, L. (1999). A comparative study on the reliability and validity of four stroke scales. Chin. J. Phys. Med. rehabilitation 3, 13–16.
Wang, X., Wang, R., and Xie, C. (2021). The clinical effect of Buyang Huanwu Decoction on stroke of qi deficiency and blood stasis in convalescence. J. Clin. Ration. Drug Use 14 (26), 51–53. doi:10.15887/j.cnki.13-1389/r.2021.26.018
Wu, J. (2007). Study on reliability and validity of four stroke scales. Master’s Electronic Journal. Tianjin (China): Tianjin Medical University.
Wu, J. (2021). Analysis of the clinical effect of Buyang Huanwu Decoction on patients with qi deficiency and blood stasis syndrome in the recovery period of cerebral infarction. Zhonghua Health Care 39 (17), 17–18.
Wu, Q., Yao, X., Chen, H., Liu, Z., Li, T., Fan, X., et al. (2020). Long-term aspirin use for primary cancer prevention: An updated systematic review and subgroup meta-analysis of 29 randomized clinical trials. J. Cancer 11 (21), 6460–6473. doi:10.7150/jca.49001
Wu, W. (2019). Study on the mechanism of Buyang Huanwu Decoction in the treatment of ischemic stroke [J]. Electron. J. Clin. Med. Literature 6 (17), 193. doi:10.21203/rs.3.rs-24632/v1
Xiang, J. (2019). Clinical observation on the treatment of cerebral infarction in the recovery period with modified Buyang huanwu decoction. Guangming Tradit. Chin. Med. 34 (20), 3149–3150. doi:10.3969/j.issn.1003-8914.2019.20.034
Xing, Y., and Bai, Y. (2020). A review of exercise-induced neuroplasticity in ischemic stroke: Pathology and mechanisms. Mol. Neurobiol. 57 (10), 4218–4231. doi:10.1007/s12035-020-02021-1
Xu, H., Zou, Y., Zhou, H., and Lv, J. (2017). Evaluation of therapeutic effect of Buyang Huanwu Decoction on patients with ischemic stroke in the recovery period. World J. Integr. Traditional West. Med. 12 (08), 1155–1157+1176. doi:10.13935/j.cnki.sjzx.170832
Xu, G. (2014). Observation on the therapeutic effect of Buyang Huanwu Decoction on 60 cases of cerebral infarction in the recovery perio. Med. Inf. 37, 426. doi:10.3969/j.issn.1006-1959.2014.37.668
Xue, L., and Xue, J. (2015). Clinical observation on 36 cases of cerebral infarction in recovery period treated with Buyang huanwu decoction. Pharmacol. Clin. Med. Traditional Chin. Med. 31 (2), 179–180.
Yamal, J. M. (2021). National Institutes of health stroke scale as an outcome measure for acute stroke trials. Stroke 52 (1), 142–143. doi:10.1161/STROKEAHA.120.032994
Yang, J., Wen, Y., and Zhou, H. (2021). Clinical observation of Buyang Huanwu Decoction in the treatment of cerebral infarction in convalescence. J. Pract. Chin. Med. 37 (12), 2034–2035.
Yang, S. (2020). Observation on the therapeutic effect of Buyang Huanwu Decoction on the syndrome of qi deficiency and blood stasis in the recovery period of ischemic stroke. Electron. J. Cardiovasc. Dis. Integr. Traditional Chin. West. Med. 8 (24), 164167.
Yang, Y. (2018). Observation on the application of Buyang Huanwu Decoction in the recovery period of stroke. Diet health care 5 (41), 114–115. doi:10.3969/j.issn.2095-8439.2018.41.138
Yang, Z. (2022). Based on the "brain-gut axis" to explore the research progress of traditional Chinese medicine in the treatment of ischemic stroke [J]. J. Liaoning Univ. Traditional Chin. Med. 24 (04), 130–134.
Yu, F. (2013). Analysis of clinical efficacy of Buyang Huanwu Decoction in treating cerebral infarction in convalescence. Chin. Med. Guide 22, 263–264.
Zhang, G., and Xiong, C. (2020). Observation on the effect of integrated traditional Chinese and Western medicine in treating sequelae of cerebral apoplexy of qi deficiency and blood stasis type and analysis on the influence of neurological recovery. Health Compuls. 11, 165.
Zhang, J. (2018). The improvement effect of Buyang Huanwu Decoction on the depressive state of patients with stroke in the recovery period. China Higher Medical Education, 134. 10.3969/j.issn.1002-1701.2018.10.070.
Zhang, L. (2013). Effect of buyang huanwu decoction on neurological function and quality of life of patients with cerebral infarction in recovery period. China Pharmaceutical. 22, 106. 10.3969/j.issn.1006-4931.2013.20.059.
Zhang, M. (2017). 50 cases of cerebral infarction of qi deficiency and blood stasis type in convalescence were treated with integrated traditional Chinese and Western medicine. Chin. Folk. Med. 26 (23), 111–112119.
Zheng, J. (2016). Observation on the therapeutic effect of Buyang Huanwu Decoction in the recovery period of cerebral infarction. Electron. J. Clin. Med. Literature 3 (28), 5590–55905592.
Zhao, X., and Zhou, M. (2021). Research progress of traditional Chinese medicine in the treatment of ischemic stroke[J]. Med. Rev. 27 (22), 4548–4552.
Zheng, X. Y., Zhang, Y. H., Song, W. T., Cao, H., and Liu, J. X. (2021). Effects of Buyang huanwu decoction on neurovascular units after cerebral ischemia: A review. Zhongguo Zhong Yao Za Zhi 46 (20), 5226–5232. doi:10.19540/j.cnki.cjcmm.20210610.706
Zhuge, L., Fang, Y., Jin, H., Li, L., Yang, Y., Hu, X., et al. (2020). Chinese medicine Buyang Huanwu decoction promotes neurogenesis and angiogenesis in ischemic stroke rats by upregulating miR-199a-5p expression. Zhejiang Da Xue Xue Bao Yi Xue Ban. 49 (6), 687–696. doi:10.3785/j.issn.1008-9292.2020.12.03
Keywords: Buyang Huanwu Decoction, ischemic stroke, recovery period, systematic review, meta-analysis, traditional Chinese medicine
Citation: Wang R, Ren J, Li S, Bai X, Guo W, Yang S, Wu Q and Zhang W (2022) Efficacy evaluation of Buyang Huanwu Decoction in the treatment of ischemic stroke in the recovery period: A systematic review of randomized controlled trials. Front. Pharmacol. 13:975816. doi: 10.3389/fphar.2022.975816
Received: 22 June 2022; Accepted: 22 September 2022;
Published: 14 October 2022.
Edited by:
Jiangang Shen, The University of Hong Kong, Hong Kong SAR ChinaReviewed by:
Guo-Qing Zheng, Zhejiang Chinese Medical University, ChinaZeyan Li, Shandong University, China
Copyright © 2022 Wang, Ren, Li, Bai, Guo, Yang, Wu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sijin Yang, eXNqaW1uQHNpbmEuY29t; Qibiao Wu, cWJ3dUBtdXN0LmVkdS5tbw==; Wei Zhang, eXNqaW1uQHNpbmEuY29t
†These authors have contributed equally to this work
 Raoqiong Wang
Raoqiong Wang Junhao Ren3†
Junhao Ren3† Xue Bai
Xue Bai Sijin Yang
Sijin Yang Qibiao Wu
Qibiao Wu Wei Zhang
Wei Zhang