- 1College of Basic Medicine and Forensic Medicine, Henan University of Science and Technology, Luoyang, Henan, China
- 2College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang, Henan, China
- 3Luoyang Vocational and Technical College, Luoyang, Henan, China
Hematologic malignancies (HMs), also referred to as hematological or blood cancers, pose significant threats to patients as they impact the blood, bone marrow, and lymphatic system. Despite significant clinical strategies using chemotherapy, radiotherapy, stem cell transplantation, targeted molecular therapy, or immunotherapy, the five-year overall survival of patients with HMs is still low. Fortunately, recent studies demonstrate that the nanodrug delivery system holds the potential to address these challenges and foster effective anti-HMs with precise treatment. In particular, cell membrane camouflaged nanodrug offers enhanced drug targeting, reduced toxicity and side effects, and/or improved immune response to HMs. This review firstly introduces the merits and demerits of clinical strategies in HMs treatment, and then summarizes the types, advantages, and disadvantages of current nanocarriers helping drug delivery in HMs treatment. Furthermore, the types, functions, and mechanisms of cell membrane fragments that help nanodrugs specifically targeted to and accumulate in HM lesions are introduced in detail. Finally, suggestions are given about their clinical translation and future designs on the surface of nanodrugs with multiple functions to improve therapeutic efficiency for cancers.
1 Introduction
Hematologic malignancies (HMs), which are also called blood cancers, often begin in the cells of the immune system or in blood-forming tissue such as the bone marrow, where stem cells develop into blood cells, affecting the bone marrow’s ability to make enough blood cells (red blood cells, white blood cells and platelets) (Greim et al., 2014). When abnormal HM cells grow uncontrollably, they can cause cancers to develop, outpacing the growth of healthy blood cells and disrupting their normal functions. According to the first detected sites, the HMs are traditionally categorized as leukemias (blood), lymphomas (lymph nodes), or myelomas (bone) (Bispo et al., 2020; Alaggio et al., 2022) (Figure 1).
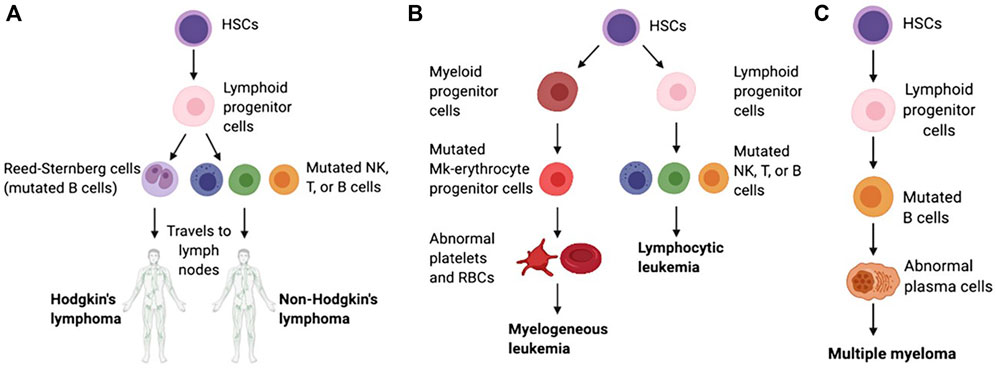
Figure 1. Schematic of the pathological differentiation of lymphoma (A), leukemia (B) and MM (C), copy from (Powsner et al., 2021) with license under CC BY 4.0.
1.1 Leukemia
Leukemia begins in early blood-forming cells in the bone marrow, the spongy tissue inside bones (Mehranfar et al., 2017). The type of leukemia depends on the type of blood cell that has become cancerous. For instance, acute lymphoblastic leukemia (ALL) is a cancer of the lymphoblasts, white blood cells that fight infection. White blood cells are the most common type of blood cell to become cancerous (lymphocytic leukemia). However, red blood cells, which carry oxygen from the lungs to the rest of the body, and platelets, which clot the blood, may also become cancerous (myelogeneous leukemia). Common leukemias include ALL, chronic lymphoblastic leukemia (CLL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), chronic myelomonocytic leukemia (CMML), chronic neutrophilic leukemia (CNL) and atypical chronic myeloid leukemia (aCML), etc (Li et al., 2014; Dao et al., 2017).
1.2 Lymphoma
Lymphoma begins in lymphocytes and white blood cells that are part of the lymphatic system, which is part of the immune system and helps fight infection and disease (McLafferty et al., 2012). Lymphoma can begin almost anywhere in the body because lymph tissue exists everywhere. The lymphomas mainly include non-Hodgkin’s lymphoma (NHL, containing T, NK, and B cell tumors) and Hodgkin’s lymphoma (HL) (Di Pietro and Good-Jacobson, 2018). Lymphomas occur in both children and adults.
1.3 Myeloma
Myeloma begins with the abnormal development of plasma cells that make antibodies that help the immune system fight infections and disease in bone marrow or soft tissue (Lee and Borrello, 2016). When there is only one tumor, the disease is referred to as plasmacytoma. When there are multiple tumors, it is known as multiple myeloma (MM). Both conditions are malignant (cancerous). Common myelomas include MM, myeloproliferative disorder (MPD), myelodysplastic syndrome (MDS), myelodysplastic/myeloproliferative disorder (MD/MPD), etc (Pati and Kundil Veetil, 2019).
The clinical strategies for treating HMs include radiotherapy (Dabaja and Spiotto, 2023), chemotherapy (Atkins and He, 2019), targeted molecular therapy (Shimada, 2019), hematopoietic stem cell transplantation (HSCT) (Sivakumar et al., 2019), immunotherapy (Kansara and Speziali, 2020), bloodless transplantation for populations who cannot receive blood products (Scharman et al., 2017) and their combinations (Wu and Singh, 2011). Radiotherapy and chemotherapy are the most traditional HMs therapeutic strategies with significant limitations owing to strong side effects (Liu et al., 2021). Targeted molecular therapy supplies higher safety and precision for HMs treatment, and some inhibitors have been approved for marketing. However, it is a pity that the low stability and off-targeting toxicity of targeted drugs limited their therapeutic effect for HMs (Huang L. et al., 2020). The HSCT program injects healthy stem cells into the blood to replace diseased bone marrow, and the new bone marrow makes the healthy cells that the body needs and helps slow or stop blood cancers (Burt et al., 2008). However, the HSCT strategy is usually unenforceable because of a need for adequately matched bone marrow (Fenwarth et al., 2021). Immunotherapy, a hot spot of clinical HMs treatment in recent years, also possess some drawbacks such as resistance to checkpoint inhibitors, causing cytokine release syndrome, etc (van de Donk and Usmani, 2018; Wei et al., 2020). Even though the 5-year survival of HMs patients has improved with various advanced clinical strategies, the average survival and cure rates are still low (Hemminki et al., 2023). Therefore, more effective methods are worth exploring to optimize HMs treatment.
The nanodrug delivery system is an excellent method to help resolve drug delivery problems in HMs treatment. The inherent physical and chemical properties of drugs including poor solubility, low stability, and no targeting ability, lead to side effects on normal tissues in the body, often limiting their therapeutic effect in clinical application (Halwani, 2022). Various nanocarriers have been developed to carry anti-HMs drugs reaching HMs lesions with focused accumulation, controlled drug release, and low side effects (Jia et al., 2023). Among nanocarriers, liposome is the U.S. Food and Drug Administration (FDA)-approved nanocarrier for drug delivery against HMs (Hani et al., 2023). The targeting accumulation of nanodrugs is a crucial factor guaranteeing drug work in HMs lesions rather than other normal tissues, and this aim is usually achieved by a decoration of targeted ligands or cell membrane camouflage on the surface of nanodrug (Deshantri et al., 2018). In this review, we will first introduce the common types of nanocarriers, and then submit in detail the application of targeting strategies for nanodrug delivery systems in HMs treatment. Finally, we will discuss the prospects and challenges of targeting decoration for nanodrug delivery systems.
2 The clinical regimens for the treatment of HMs
2.1 Radiotherapy
Radiotherapy employs high-energy beams or particles, such as X-rays, gamma rays, and charged particles, to directly damage cancer cells’ DNA or generate charged particles (free radicals) within the cells, ultimately disrupting their DNA and causing cell death (Baskar et al., 2014). Radiotherapy is generally performed with high doses targeted to a localized region to damage DNA. The advantages of radiotherapy include the complete elimination of tumors in areas where conventional surgery is not feasible, along with minimal adverse effects. The approach to radiotherapy for HMs hinges on the specific locations of cancer manifestation within the body. Radiotherapy treats HMs by eliminating cancer cells in the bloodstream, providing relief from pain or discomfort resulting from an enlarged liver, spleen, or swollen lymph nodes. Additionally, it is employed to alleviate pain stemming from bone damage caused by the proliferation of cancer cells in the bone marrow. Some specialized radiotherapy techniques for HMs include total body irradiation for leukemia patients, total skin electron beam therapy for treating mycosis fungoides (a type of T-cell lymphoma), cranio-spinal radiation for leukemia patients with central nervous system involvement, and involved site radiation therapy for treating HL, etc. However, dose-related toxicity that occurs frequently due to damage to the surrounding healthy tissue near the target site often poses a limitation. Given the widespread distribution of malignant cells in HM, radiotherapy only proves effective in a small proportion of patients.
Driven by the need to prevent long-term toxicity that elevates morbidity and mortality among long-term survivors, radiation fields and doses have been reduced (Radiation for hematologic malignancies: from cell killing to immune cell priming). Nowadays, low-dose radiation like 4 Gy is also successfully used as a curative modality for various HMs in clinical treatment (Ciammella et al., 2018; Imber et al., 2021). Low-dose radiation can activate cellular defense mechanisms that repair DNA damage, remove cells via autophagy and apoptosis that cannot be repaired, and trigger cell cycle arrest to prevent damaged cells from dividing and allowing for repair (Tharmalingam et al., 2019). In addition, it can also induce adaptive memory, protecting against future oxidative stress and enhancing the immune system (Farooque et al., 2011; Dabaja and Spiotto, 2023). The anti-tumor immune responses through low-dose radiation are ushering in a transformative era for radiotherapy in the treatment of HMs, bolstering the efficacy of immunotherapy and adoptive cell-based therapy.
2.2 Chemotherapy
Chemotherapy is a based therapeutic strategy for HMs. Because of lack of non-specifically targets, chemotherapy can affect the entire body of HMs patients, and hence can destroy metastasized cancer cells (Nygren, 2001; Chu and Sartorelli, 2018). FDA-approved chemo-drugs in HMs treatment include DNA-interactive agents such as chlormethine, bleomycin, doxorubicin, cisplatin, cyclophosphamide, daunorubicin, cytarabine, and melphalan; antimetabolites such as gemcitabine, methotrexate, fluorouracil and mercaptopurine; anti-tubulin agents like vincristine, etc (Powsner et al., 2021; Sochacka-Ćwikła et al., 2021). The time-honored first-line therapy known as the “3 + 7” regimen (daunorubicin and cytarabine) for leukemia has been a mainstay in clinical practice for over five decades, since 1973 (Li J. et al., 2023). In addition, combined utility of chemo-drugs with different mechanisms can kill more cancer cells by reducing the likelihood of cancer becoming resistant to any chemo-drug (Housman et al., 2014). However, because chemo-drugs can kill cancerous and healthy cells, which leads to patients usually experiencing side effects from treatment, hence it cannot bring about the ideal therapeutic effect (Van den Berg et al., 2017; Oun et al., 2018).
2.3 Hematopoietic stem cell transplantation (HSCT)
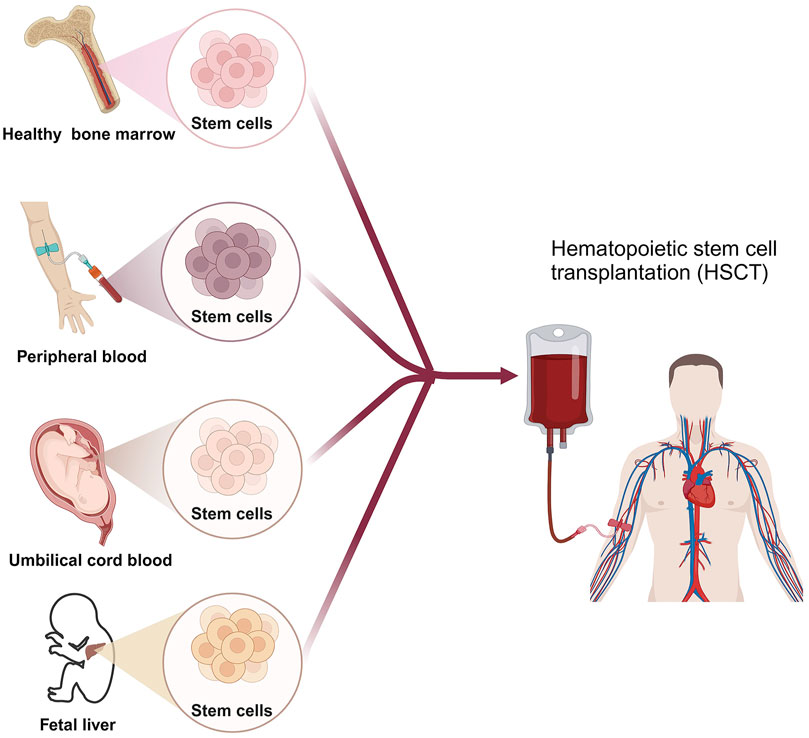
HSCT involves the intravenous infusion of hematopoietic stem cells to restore blood cell production in patients with damaged or defective bone marrow or immune systems (Chivu-Economescu and Rubach, 2017). HSCT cells can be obtained from the patient themselves (autologous transplant), another person such as a sibling or unrelated donor (allogeneic transplant), or an identical twin (syngeneic transplant) (Giralt and Bishop, 2009; Kurosawa et al., 2021). Cell sources consist of bone marrow, peripheral blood, umbilical cord blood, or rarely, fetal liver (Saulnier et al., 2005) (Figure 2). The HSCT process is mainly divided into 5 phases sequentially, including conditioning, stem cell infusion, neutropenic phase, engraftment phase, and post-engraftment period (Hatzimichael and Tuthill, 2010). More than half of autologous transplantations are conducted for the treatment of MM and NHL (Costa et al., 2015; Bhatt, 2016), while the majority of allogeneic transplants are performed for hematologic and lymphoid cancers (Majhail et al., 2015). Autologous transplantation provides rapid recovery of patient cell counts, reduces transplant-related morbidity, shortens hospitalization periods, and is cost-effective compared to allogeneic grafts. A significant advantage of an allogeneic graft is the powerful contribution of the donor’s immune system in eradicating cancer through the graft-versus-tumor effect. However, an essential barrier to HSCT in clinical application is the inability to secure suitable donors (Riezzo et al., 2017). In addition, relapse is frequently unavoidable in autologous transplantations due to the contamination of transplanted cells with cancer cells. While allogeneic HSCT circumvents relapse triggered by autologous HSCT, the diverse immune responses (graft vs. host disease) stemming from heterogeneity pose a significant obstacle to its effectiveness in treating HMs.
2.4 Targeted molecular therapy
The significant molecular heterogeneity present in HMs poses major challenges for precision medicine and the development of tailored treatment approaches. Many target sites have been developed for the treatment of HMs due to genetic or epigenetic carcinogenic mutations. Monoclonal antibodies and small selective molecules are usually used to inhibit carcinogenic mutations in HMs. Unlike chemotherapy, targeted molecular therapy has high selectivity and precision for cancer cells, thus resulting in lower toxic and side effects to healthy tissues in the body. Common types of targets mediating targeted molecular therapy in HMs are exhibited as below.
2.4.1 Protein tyrosine kinase (PTK)
PTK Mutations can result in aberrant or overexpressed PTK activity, leading to dysregulated cell signaling pathways, unchecked cell proliferation, and evasion of cell death mechanisms in various types of HMs. PTK is a family of kinases that catalyze the transfer of γ-phosphate from ATP to protein tyrosine residues. A common feature is the presence of a typical PTK domain at the carboxyl terminus, which catalyzes the phosphorylation of itself or substrates and plays an vital role in cell growth, proliferation, and differentiation (Schenk and Snaar-Jagalska, 1999; Paul and Mukhopadhyay, 2004). Most of the tyrosine kinases discovered to date are oncogene products belonging to oncogenic RNA viruses, and can also be produced by proto-oncogenes in vertebrates (Parisi et al., 2023). The PTKs are mainly divided into receptor tyrosine kinases (RTKs) and non-receptor tyrosine kinases (nRTKs) (Jiao et al., 2018). Mutations in the FMS-like tyrosine kinase-3 (FLT3) gene, particularly internal tandem duplications (ITD) and point mutations in the tyrosine kinase domain, are frequently observed in AML. These mutations dysregulate FLT3 signaling, thereby fostering cellular survival and proliferation (Kiyoi et al., 2020). Tyrosine-protein kinase KIT (c-KIT) mutations are observed in various HMs, and almost 80% of AML has KIT proto-oncogene expression (Ikeda et al., 1991). BCR-ABL tyrosine kinase mutation, typically resulting from a reciprocal translocation between chromosomes 9 and 22 to produce the BCR-ABL oncogene, is found in 90% of CML and a subset of ALL, and are more common in adults than children. This protein has constitutive tyrosine kinase activity that activates numerous downstream pathways that ultimately produces uncontrolled myeloid proliferation and leads to constitutive activation of tyrosine kinase activity, driving uncontrolled proliferation of myeloid cells (Arana-Trejo et al., 2002; Leoni and Biondi, 2015). The mutation of Bruton’s tyrosine kinase (BTK) serves as a significant contributor to B-cell lymphomas and leukemias, playing a pivotal role in their pathogenesis and progression by facilitating abnormal proliferation and survival of B-cells (Pal Singh et al., 2018). Mutations in the spleen tyrosine kinase (SYK) gene, a crucial component of B-cell receptor (BCR) signaling, have been implicated in the pathogenesis of lymphomas and leukemias. These mutations can dysregulate SYK activity, leading to aberrant proliferation, survival, and differentiation of B-cells, which play a pivotal role in the development and activation of these cells. Consequently, these alterations contribute to the development of hematological cancers (Kutsch et al., 2015). Common tyrosine kinase inhibitors (TKIs) marketed or in the progress of clinical trials in HMs treatment are shown in Table 1.
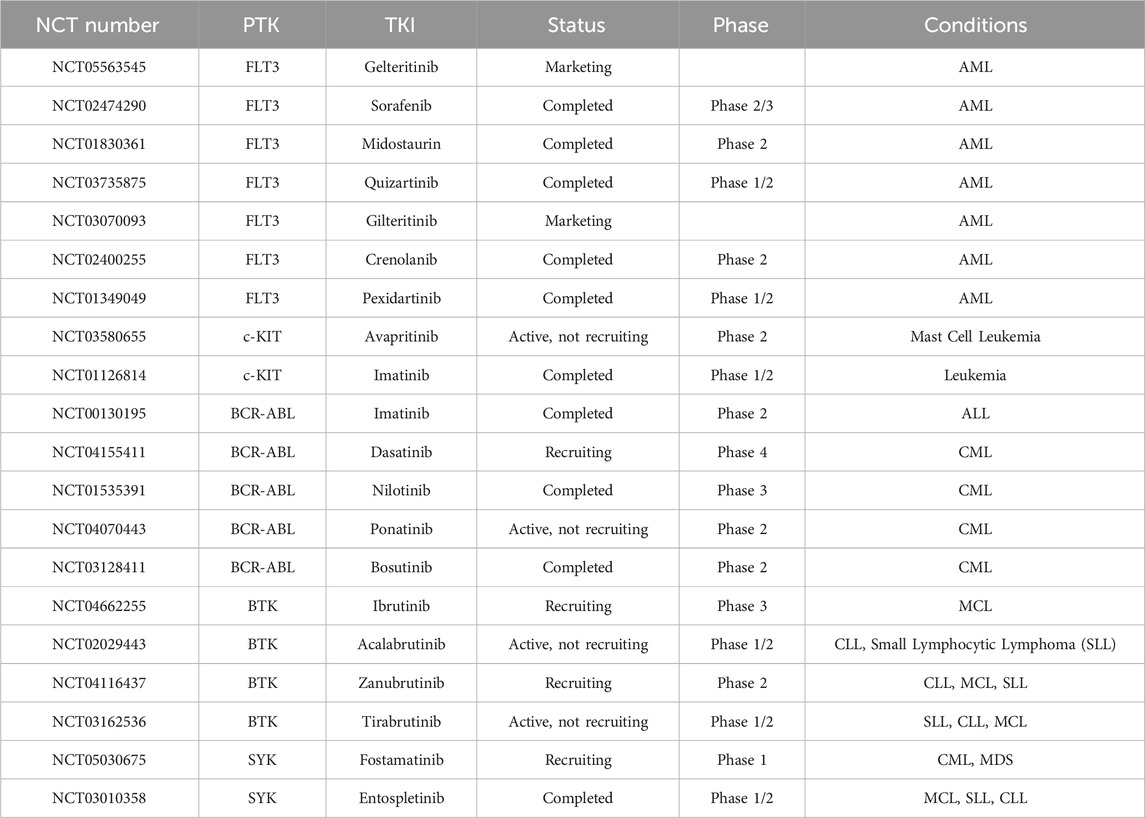
Table 1. Common TKIs in HMs treatment (from www.clinicaltrials.gov).
2.4.2 Isocitrate dehydrogenase (IDH)
IDH mutation are frequently found in certain types of leukemia, such as AML and MDS. The IDH is an essential metabolic enzyme in the Krebs Cycle and its families include IDH1 and IDH2 (Upadhyay et al., 2017). The conserved mutational hotspot of IDH1 is R132, and of IDH2 are R140 and R172. IDH mutant forms transformed α-KG into 2-hydroxyglutarate (2-HG), which induced a number of malignancies like inhibits DNA demethylases and histone demethylases (Janin et al., 2014; Mondesir et al., 2016). IDH mutation are frequently found in certain types of leukemia, such as AML and myelodysplastic syndromes (MDS). Ivosidenib (mutant IDH1 inhibitor) (Norsworthy et al., 2019) and enasidenib (mutant IDH2 inhibitor) (Fathi et al., 2020) are currently available IDH inhibitors for clinically treating AML, and ivosidenib became the first targeted drug approved to treat relapsed/refractory (R/R) MDS with IDH1 mutations in 2023.
2.4.3 B-cell lymphoma-2 (BCL-2) family
The BCL-2 family of proto-oncogenes plays a pivotal role in regulating apoptosis, the programmed cell death process, and their dysregulated expression is a hallmark of various human cancers, notably prevalent in certain lymphomas and leukemias. The BCL-2 family, including BCL-2, BCL-B, BCL-XL, BCL-W, myeloid cell leukemia sequence 1 (MCL-1) and BFL1, is a crucial regulator of apoptosis triggered by various environmental and stress signals (Cory and Adams, 2002). Anti-apoptotic protein BCL-2 is usually overexpressed in leukemias and lymphomas, leading to the occurrence and development of tumors, and limiting their response to chemotherapy (Roberts, 2020). BH3-mimetics including ABT-737 (Kuroda et al., 2006), ABT-263 (Kipps et al., 2015), and Venetoclax (Roberts et al., 2016), a class of targeted drugs that mimic the actions of BH3-only proteins binding to BCL-2 and hence inhibit BCL-2’s ability, are popular for clinical research in treating leukemias and lymphomas. MCL-1 is another important antiapoptotic protein in promoting cell survival and drug resistance in MM, AML, and NHL. Therefore, some MCL-1 inhibitors including AZD5991, S64315, AMG 176, and AMG 397, are also in clinical trials in HMs treatment.
2.4.4 Phosphatidylinositol-3-kinases (PI3Ks)
PI3Ks inhibition often mark significant progress in CLL and related malignancies. PI3Ks, a family of lipid kinases, are categorized into three main classes (I, II, and III) based on their structure and substrate specificity. Among these, Class I PI3Ks, which include p110α, p110β, p110δ, and p110γ, are most closely linked to human cancer. In 2014, a milestone was reached when idelalisib, targeting p110δ, became the first FDA-approved PI3K inhibitor. It gained approval for treating relapsed follicular lymphoma (FL), SLL, and CLL (Wei et al., 2015). Another notable PI3K inhibitor is duvelisib, an oral medication that inhibits both p110γ and p110δ. It received FDA approval in 2018 specifically for CLL patients who have undergone at least two prior therapies (Flinn et al., 2018). Ongoing clinical investigations are exploring other PI3K inhibitors targeting p110δ for CLL therapy. Prominent examples include BGB-10188, HMPL-689, parsaclisib, umbralisib, and zandelisib. Moreover, copanlisib and buparlisib are PI3K inhibitors with a broader spectrum, simultaneously targeting p110α, p110β, p110δ, and p110γ (Hus et al., 2022).
2.4.5 Epigenetic mutations
Epigenetic modifier gene mutations are common in HMs, playing a pivotal role in both the onset and advancement of cancer. As reported, some epigenetic changes such as DNA methylation-related mutations, abnormal histone deacetylase (HDAC) and bromodomain and extraterminal (BET) protein expression are recurrent in HMs (Zhao et al., 2023). Drugs targeting epigenetic changes have made clinical progress in treating HMs. Clinical DNA hypomethylating agents (HMAs) in HMs therapy mainly include azacitidine (Odenike, 2017), decitabine (Santos et al., 2010), guadecitabine (Kantarjian et al., 2017) and ASTX727 (cedazuridine/decitabine) (Abuasab et al., 2022). Common HDAC inhibitors used for clinical trials in HMs treatment include vorinostat (Hopfinger et al., 2014), belinostat (Johnston et al., 2021), panobinostat (El Omari et al., 2023), chidamide (Cao et al., 2023), and romidepsin (Bates et al., 2015). As the core member of the BET family, BRD4 regulates gene expression, and its inhibitors, such as JQ1 (Pericole et al., 2019) and AZD5153 (Rhyasen et al., 2016), are also potential clinical drugs in HMs therapy.
2.5 Immunotherapy
The continuous interaction between immune cells and cancer cells within the hematopoietic system creates an environment conducive to immune surveillance. Since malignancies originate from the same cellular sources as the immune system, cancer cells possess immunostimulatory characteristics. However, this dual nature can also lead to compromised immune responses. Significant progress has been made in cancer immunotherapy, with accelerating advancements based on diverse strategies aimed at harnessing the host immune system. In the realm of hematologic malignancies treatment, immunotherapy primarily encompasses targeted antibodies, immune checkpoint blockade (ICB), and CAR cell therapy.
2.5.1 Monoclonal antibodies
Monoclonal antibodies (mAbs) stand as a cornerstone in cancer immunotherapy. mAbs are precisely uniform IgG antibodies derived from a single B cell clone, specifically targeting unique antigenic epitopes (Weiner et al., 2009). With diverse mechanisms of action, each type of antibody engages multiple facets of immunity, orchestrating a comprehensive assault on tumor cells. mAbs can be directed against tumor-associated antigens (TAAs) and killing cancer cells through two primary pathways: i) direct induction of apoptosis via programmed cell death (PCD) (Overdijk et al., 2016); ii) immune-mediated mechanisms, notably including antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent macrophage-mediated phagocytosis, facilitated by the interaction between Fc and FcγR (Fc gamma receptor) (Tipton et al., 2015; Krejcik et al., 2016).
Rituximab, ofatumumab, obinutuzumab, and ibritumomab tiuxetan are FDA-approved mAbs that bind to CD20 antigens expressed on the surface of immune system B cells, subsequently eradicating the HMs such as CLL, diffuse large B-cell lymphoma (DLBCL), B cell NHL (B-NHL), and follicular lymphoma (FL), etc (Wierda et al., 2010; Morschhauser et al., 2013; Goede et al., 2014; Salles et al., 2017; Pierpont et al., 2018). Daratumumab and isatuximab are FDA-approved anti-CD38 mAbs for MM immunotherapy because CD38, a type II transmembrane glycoprotein, is highly expressed in MM cells (Palumbo et al., 2016; Goldschmidt et al., 2022). Michel de Weers et al. found daratumumab not only exhibited potent cytotoxicity to CD38-expressing lymphoma- and MM-derived cell lines as well as in patient MM cells by Ab-dependent cellular cytotoxicity in vitro, but also highly activated and interrupted xenograft tumor growth at low dosing in vivo (De Weers et al., 2011). In addition, other types of mAbs have been approved by FDA for treating HMs including tafasitamab (anti-CD19 mAb) for DLBCL and FL (Salles et al., 2020), alemtuzumab (anti-CD52 mAb) for peripheral T-cell lymphoma (PTCL) and CLL (Wulf et al., 2021), elotuzumab (anti-CS1 mAb) for MM (Dimopoulos et al., 2023), and milatuzumab (anti-CD74 mAb) for MM, (MCL), FL and CLL (Kaufman et al., 2008; Alinari et al., 2009; Hertlein et al., 2010; Christian et al., 2015).
2.5.2 Immune cell engagers
Some HMs treated with mAbs are often R/R. Fortunately, immune cell engagers (engineered antibodies) are designed and developed in treating R/R HMs with high efficiency in recent years (Tian et al., 2021). Immune cell engagers work by at least one arm recognizing TAAs and at least another one arm recruiting immune effector cells (CD3 for T cells and CD16a for NK cells). Therefore, immune cell engagers enable the direct targeting of immune cells to tumors, substantially mitigating resistance and serious adverse effects. Immune cell engagers are divided into bispecific antibodies (BsAbs), trispecific antibodies (TsAbs) and even tetraspecifc antibodies. Compared to BsAbs, TsAbs expand therapeutic options by introducing a third binding component. This additional moiety allows for the targeting of an extra tumor-associated antigen, enhancing specificity and preventing immune evasion. Alternatively, it can target additional costimulatory receptors on immune cells, thereby enhancing their effector functions (Liu D. et al., 2022). Until now, around 100 bispecific T cell engagers are in clinical trials, and NK cell engagers and TsAbs are currently undergoing early-stage clinical studies because of late starting (Tapia-Galisteo et al., 2023).
Blinatumomab is the first FDA-approved bispecific T cell engager, working by transiently linking CD19-positive B cells to CD3-positive T cells, resulting in induction of T-cell-mediated serial lysis of B cells and concomitant T-cell proliferation. It successfully treated Philadelphia chromosome-negative R/R B-cell precursor ALL (Zhu et al., 2016). Recent marketed T cell engagers for treating HMs include the anti-CD20×anti-CD3 mosunetuzumab for FL and epcoritamab for DLBCL, the anti-B cell maturation antigen (BCMA)×anti-CD3 teclistamab for MM, and gloftamab for DLBCL. In 2023, FDA approved BsAb talquetamab-tgvs (anti-G Protein-coupled Receptor class C group 5 member D×anti-CD3) and elranatamab-bcmm (anti-BCMA×anti-CD3) for adults with R/R MM who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. Some NK cell engagers are in clinical trials including anti-CD30×anti-CD16a AFM13 for HL and NHL and anti-CD123×anti-CD16a AFM28 for ALM, etc. Some TsAbs and tetraspecifc antibodies are also in clinical stages, such as anti-CD38×anti-CD3×anti-CD28 SAR442257 for MM and NHL, anti-CD3×anti-CD137×anti-PD-L1×anti-CD19 emfzatamab for NHL, and anti-BCMA×anti-CD16×anti-Natural Killer Group 2 member D CC-92328 × DF3001 for R/R MM, etc (www.clinicaltrials.gov).
2.5.3 Immune checkpoint blockade
Immune cells have two main mechanisms for killing tumor cells. The first is through specific signaling via immune cell receptors, and the second is through nonspecific signals. These nonspecific signals are linked to costimulatory receptors like CD28, or coinhibitory receptors like cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death-1 (PD-1) (Chen and Flies, 2013). When coinhibitory receptors are engaged, cytotoxic T cells and NK cells are suppressed, allowing tumor cells to evade the immune system. Therefore, the utility of immune checkpoint inhibitors (ICIs) to block inhibitory checkpoints can harness immune cells to effectively attack tumor cells effectively.
Many anti-CTLA-4 (ipilumumab), anti-PD-1 (pembrolizumab, nivolumab, cemiplimab), and anti-PD-L1 (atezolizumab, avelumab, durvalumab) ICB drugs are in clinical trials for the treatment of HMs (Salik et al., 2020). Nivolumab and pembrolizumab have been approved by FDA for treating HL and primary mediastinal large B-cell lymphoma, respectively (Kroll et al., 2022). As a PD-1 inhibitor, pembrolizumab has recently been used in a phase IB clinical trial with R/R MM (Ribrag et al., 2019). CTLA4-blocking antibody ipilimumab provided substantial anti-tumor activity in patients with both lymphoid and myeloid malignancies after allogeneic HSCT (Davids et al., 2015). Matthew S. Davids et al. reported a prospective clinical phase 1 trial of PD-1 blockade using nivolumab for relapsed HMs after alloHCT (Davids et al., 2020). D Liao et al. utilized anti-PD1 or anti-CTLA4 inhibitor to treat AML patients under the stage of disease remission after chemotherapy, and results demonstrated strong T cell responses against residual AML (Liao et al., 2019). Other immune checkpoint molecules on T cells, such as LAG-3, TIM-3, and TIGIT also play an important role in inactivating T cell, and inhibition of them can be a potential approach to induce T cell responses in a non-redundant manner (Anderson, 2014; Blake et al., 2016; Maruhashi et al., 2018). In addition, NK cell ICIs such as anti-NKG2D (natural-killer group 2, member D) mAb and anti-KIR mAb also showed good clinical trials in HMs patients (Vey et al., 2012; André et al., 2018). Current hinders for ICB application in HMs treatment include cytokine-release syndrome, primary resistance, and acquired resistance, etc. Finding out new immune checkpoints and inhibiting resistance may improve the above problems.
2.5.4 CAR cell therapy
CAR cell therapy stands out as one of the most promising immunotherapeutic approaches, exhibiting remarkable efficacy in the treatment of HMs and achieving significant advancements recently. Chimeric antigen receptor (CAR) cell therapies, including CAR-T cell, CAR-natural killer (CAR-NK) cell and CAR-macrophage (CAR-M) therapy, are novel forms of tumor immunotherapy (Boyiadzis et al., 2018). The CAR contains a single-chain variable fragment (scFv) that recognizes the tumor antigen at extracellular sites, a transmembrane domain derived from CD4, CD8α, CD28 or CD3ζ, none, one or more of intracellular co-stimulatory molecular such as CD28, ICOS, CD137, CD27, OX40 or/and 4-1BB, and an intracellular immune cell activation domain, such as CD3ζ chain, DAP10, DAP12 or FcεRIγ chain (Figure 3) (Khalil et al., 2016; Oberschmidt et al., 2017; Hu et al., 2018; Pfefferle and Huntington, 2020). The technology of CAR-T cell involves extracting T cells from a patient’s immune system, culturing and modifying them in vitro, equipping them with particular molecules to enable them to recognize and attack specific cancer cells, and then reintroducing the modified T cells into the patient’s body. The modified T cells become CAR-T cells, which travel throughout the body. Once these detectors receive specific signals from the surfaces of other cells, they activate the CAR-T cells and initiate an attack, eliminating the signal-carrying cells as adversaries (Han et al., 2021). Similar processes occur in CAR-NK and CAR-M (Figure 3) (Hu et al., 2018; Tariq et al., 2018; Klichinsky et al., 2020).
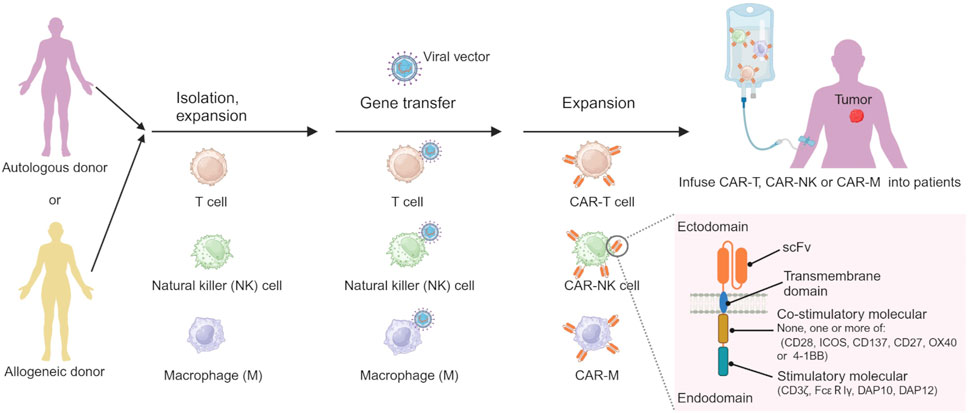
Figure 3. The processes of preparing CAR-immune cell. Created by BioRender (agreement number: TS26D15BJS).
CD19, CD20, CD22, CD33, CD56, CD70, CD79b, CD5, CD7, CD123, BCMA, and NKG2DL are the common targets of CAR cell therapy, and their application in clinical trials in HM patients, as shown in Table 2. So far, FDA-approved CAR cell medications are CAR-T cells targeting CD19, including idecabtagene vicleucel for MM, lisocabtagene maraleucel for large B-cell lymphoma, tisagenlecleucel for ALL and large B-cell lymphoma, brexucabtagene autoleucel for MCL, and axicabtagene ciloleucel for FL and large B-cell lymphoma. Despite its potential, CAR-cell therapy faces significant challenges, including neurotoxicity, cytokine release syndrome, and off-tumor toxicity. These limitations not only hinder its efficacy but also introduce unwanted side effects. Therefore, urgent research is needed to unravel the underlying molecular mechanisms and overcome these obstacles.

Table 2. CAR cell therapy against HMs in clinical trial (from www.clinicaltrials.gov).
2.6 Combined treatment
Clinical studies demonstrated that combining different clinical strategies is more effective by avoiding the pitfalls of a single treatment. The combination of mutated IDH inhibitors with induction and consolidation chemotherapy improved the overall survival rates of AML patients (Stein et al., 2021). The combination of vorinostat with chemotherapy and rituximab was available in HIV-related B-Cell NHL with high-risk features in clinical trials (Ramos et al., 2020). The combination of chemotherapy, HSCT, and tyrosine kinase inhibitors significantly improved the event-free survival rate from 20% to 80% in Philadelphia (Ph) chromosome-positive ALL (Shimada, 2019). A phase II clinical trial investigated the combination of anti-CD30 CAR-T treatment with a PD-1 inhibitor in R/R CD30-positive lymphoma. Of the 12 evaluated patients, the trial reported an impressive overall response rate of 91.7% and a complete response rate of 50%. Furthermore, 63.6% patients who got a response after CAR-T therapy sustained their response throughout the follow-up period (Sang et al., 2022). Anas Younes et al. made the antitubulin agent monomethyl auristatin E (MMAE) attach to a CD30-specific monoclonal antibody by an enzyme-cleavable linker, producing the antibody-drug conjugate (ADC) brentuximab vedotin (SGN-35) to enhance the antitumor activity of CD30-directed therapy (Younes et al., 2010). Other ADCs like gemtuzumab ozogamicin, a selective anti-CD33 antibody-calicheamicin, was approved for clinical practice (Appelbaum and Bernstein, 2017), and inotuzumab ozogamicin (INO) as ADC that consists of a humanized anti-CD22 monoclonal antibody linked to calicheamicin, was also approved for clinical practice for B-ALL patients (Kantarjian et al., 2016). Compared with single targeted therapy, the combination of BRD4 inhibitor (JQ1) and HMA (azacitidine) was more effective for inducing MDS and AML cell apoptosis (Pericole et al., 2019). FDA approved Polatuzumab Vedotin-piiq (Polivy, Palotuzumab) in combination with rituximab + cyclophosphamide, doxorubicin, and prednisone, first-line treatment of patients with diffuse large B-cell lymphoma in 2023.
3 The application of nanocarriers-mediated drug delivery
Due to the physical and chemical properties of the drugs, their clinical effectiveness is limited. Nanodrug delivery systems can overcome drug delivery problems in the body. The common types of nanocarriers for drug delivery in HMs therapy include organic, inorganic, and biological-derived nanocarriers (Figure 4) (Hani et al., 2023). Currently, the FDA-approved or clinically researched nanomedicine for treating HMs primarily employs organic nanomaterials, such as liposomes and polymer micelles. Inorganic nanomaterials, biological-derived nanomaterials, and most of organic nanomaterials for drug delivery in treatment of HMs are still in preclinical studies (Li J. et al., 2023).
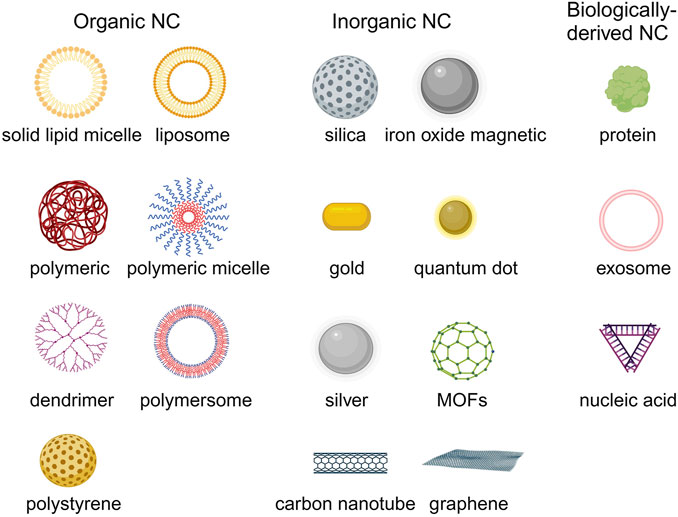
Figure 4. The common nanocarriers for drug delivery in HMs treatment. Created by BioRender (agreement number: TS26D15BJS).
3.1 Organic nanocarriers
Organic nanocarriers mainly include liposome, solid lipid micelle, polymeric nanoparticle, polymeric micelle, polymersome, polystyrene and dendrimer, etc (Kowalczuk et al., 2014; Peng et al., 2020).
3.1.1 Lipid based nanocarriers
Lipid-based nanocarriers include solid lipid micelle (SLM) and liposome. SLM is composed of a single layer of lipid molecules, and the main application of SLM is in the delivery of hydrophobic drugs by solubilizing these drugs within their lipid core (da Rocha et al., 2020). The utility of doxorubicin (DOX) and vincristine (VCR) co-encapsulated SLM to treat B-cell lymphomas had obtained good anti-cancer efficacy in lymph cancer animal model (Dong et al., 2016). Liposome is the earliest and most successful application for drug delivery in HMs treatment. Liposome compositions are phospholipids and cholesterol, which form a metastable spherical structure in an aqueous solution with a lipid bilayer that includes a membrane surrounding the internal aqueous compartment. This structure is stabilized by the cooperation of hydrophobic interactions, hydrogen bonding, van der Waals forces, and electrostatic interactions. Because liposome has hydrophobic properties in the lipid bilayer and hydrophilic properties in the aqueous compartment, it can simultaneously encapsulate hydrophobic and hydrophilic drugs. In addition, the addition of cholesterol in liposome helps stabilize the membrane bilayer and prevents leakage of drugs encapsulated in the aqueous core. There are some FDA-approved liposomal drugs for HMs treatment in the clinic, including liposomal doxorubicin (Doxil®/Caelyx®/LipoDox®) treating MM and lymphoma, liposomal daunorubicin (DaunoXome®) treating AML and non-Hodgkin lymphoma (NHL), liposomal vincristine (Marqibo) treating ALL and NHL, and liposomal cytarabine + daunorubicin (VYXEOS®) treating AML. Parts of liposomal drugs in the clinical trial stage are shown in Table 3. Compared to traditional free drugs, those liposomal drugs exhibit superior pharmacokinetic attributes, delivering more substantial clinical advantages to patients and significantly enhancing the clinical therapeutic effect (Feldman et al., 2012). In addition, the liposome can also be utilized to mimic immune cell membranes like natural killer cells by embedding functional proteins or ligands to kill HM cells (Zeinabad et al., 2023).
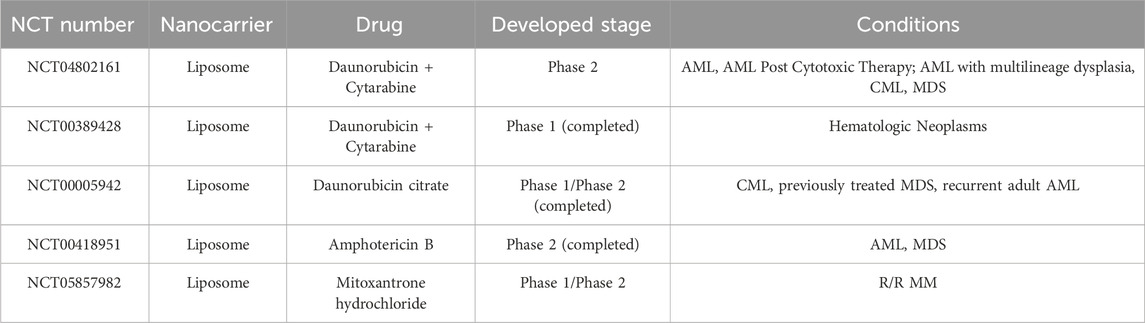
Table 3. Liposomal drugs against HMs in clinical trial (from www.clinicaltrials.gov).
3.1.2 Polymeric nanocarrier
Polymeric nanocarriers are produced from a polymeric material and are solid and colloidal particles with sizes ranging from 1 to 1,000 nm, possessing diverse structures and morphologies (Xiao et al., 2022). According to the structure difference, the types of polymeric nanocarriers are classified as polymeric nanoparticle-like, polymeric micelle, and polymersome (Kuperkar et al., 2022). Polymeric nanoparticles like poly(lactic-co-glycolic acid) are prepared by hydrophobic polymer materials in water solutions by phacoemulsification or other methods (Astete and Sabliov, 2006; Danhier et al., 2012). The behavior of amphiphilic block or graft copolymers is similar to that of conventional amphiphilic substances, and these polymers form polymeric micelles in aqueous solutions above the critical micelle concentration (Mourya et al., 2011). Polymersomes are prepared from amphiphilic polymers and are more stable than liposomes (Anajafi and Mallik, 2015). Polymersomes enable the loading of water-soluble drugs in hydrophilic inner lumen and loading lipophilic cargo in the hydrophobic part of the polymersomes’ membrane (Bleul et al., 2015). The advantages of polymeric nanocarriers include the high stability to protect drug molecules with biological activity against the environment, the high drug loading efficiency and the ability to control drug release in cancer lesions (Cano et al., 2019; Cano et al., 2020).
Pegaspargase (oncaspar) is a covalent conjugate of polyethylene glycol and asparaginase for the first-line treatment of ALL (Heo et al., 2019). Many other pegylated drugs and pegylated liposomal drugs are in clinical trials for treating HMs (NCT02526823, NCT02001818, NCT00573378). Eunji Kwak et al. used poly-L-lysine (PLL) based polymeric nanoparticle to load siBCL2 to treat non-Hodgkin’s lymphoma (NHL) (Kwak et al., 2022). Neha Mehrotra PhD et al. constructed a polylactic acid (PLA)-based block copolymeric nanocarrier for the co-delivery of navitoclax and decitabine for AML therapy (Mehrotra et al., 2023). Wen Bao et al. used daunorubicin-loaded poly(lactic-co-glycolic acid)-poly-l-lysine-polyethylene glycol (PLGA-PLL-PEG)-transferrin nanoparticles achieved effective anti-leukemia treatment in vitro and in vivo (Bao et al., 2019). Donato Cosco et al. encapsulated miR-34a into chitosan/PLGA nanoparticles against multiple myeloma in vitro and in vivo (Cosco et al., 2015). Yinan Zhong et al. applied triple-block polymer poly(ethylene glycol)-b-poly(trimethylene carbonate-co-dithiolane trimethylene carbonate)-b-spermine (PEG-P(TMC-DTC)-SP) forming polymersome to encapsulate granzyme B and achieved human multiple myeloma killing in mice model (Zhong et al., 2020).
3.2 Inorganic nanocarriers
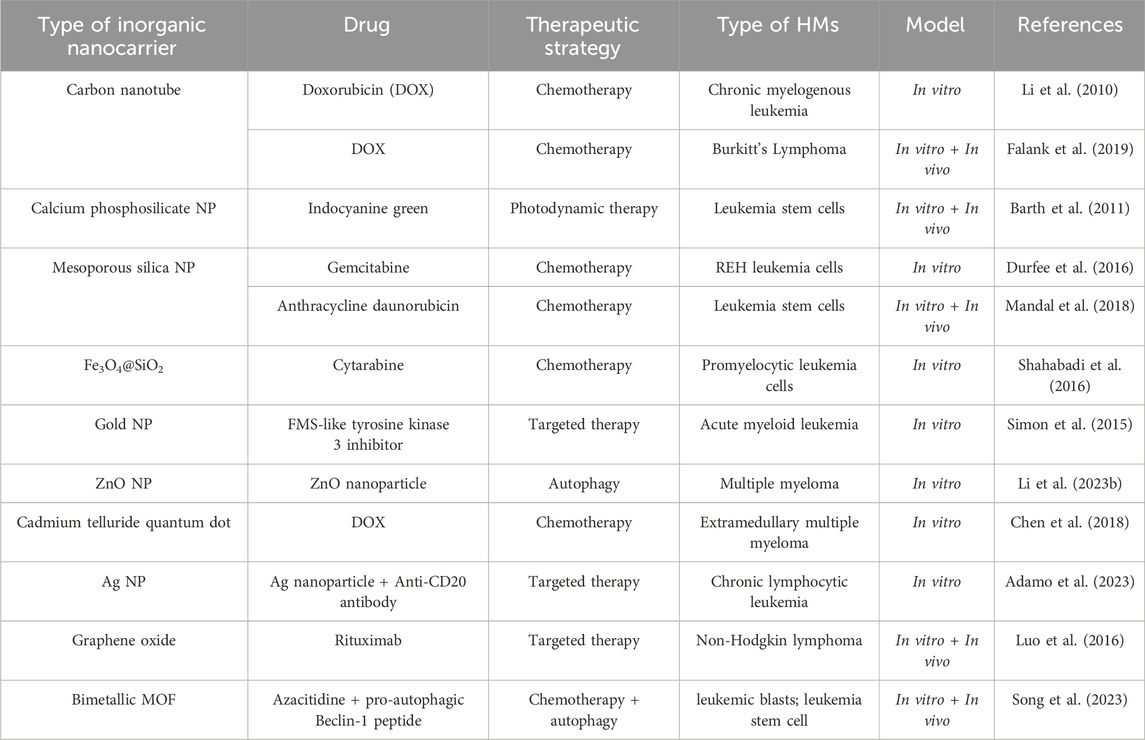
The flexible preparation options, excellent biocompatibility, adjustable size, and unique physicochemical properties make the inorganic nanomaterials popular application in imaging and drug delivery in cancer therapy (Wang et al., 2016). Furthermore, some inorganic nanoparticles can self-made medicine to kill cancer cells (Adamo et al., 2023; Li Z. et al., 2023). Inorganic nanocarriers are prepared by inorganic materials such as silica and metal materials, etc. The types of inorganic nanomaterials used for drug delivery in HMs treatment, mainly include silicon dioxide nanoparticle (NP), gold nanoparticle, silver (Ag) NP, calcium NP, carbon nanotube, quantum dot, metal-organic framework (MOF) and graphene and metal oxide (Fe3O4, ZnO, CuO), etc (Ghosn et al., 2019; Huang H. et al., 2020). Their detailed applications in preclinical HMs treatment are shown in Table 4.
3.3 Biologically-derived nanocarriers
Biologically-derived nanocarriers are an emerging type of drug carrier in biomedicine due to their low cost, unique physicochemical properties, biological functions, and excellent biocompatibility (Sun et al., 2021). Biologically-derived nanocarriers can be extracted from various organisms such as plants, algae, bacteria, fungi, actinomycetes, and yeast, etc (Singh et al., 2016; Karunakaran et al., 2023). The current common types of biologically-derived nanocarriers mainly include proteins, exosomes, and nucleic acids (Boyiadzis and Whiteside, 2017; Curley and Cady, 2018; Ma et al., 2021).
Proteins as the drug delivery carrier have a long history in cancer therapy (Hawkins et al., 2008; Jain et al., 2018). Paras Famta et al. applied human serum albumin (HSA) to encapsulate ibrutinib (IB-NPs), and this IB-loaded albumin nanoparticle completed significant leukemic K562 cellular killing (Famta et al., 2023). Ehsan Vafa et al. utilized the bovine serum albumin protected gold nanozymes (BSA-Au nanozymes) as a nanodrug for the treatment of acute T-type lymphoblastic leukemia (Jurkat) by production of excessive ROS and effect on the expression of anti-apoptotic genes (Bcl-2) (Vafa and Bazargan-Lari, 2021). To satisfy the demand for drug delivery, DNA, a nucleic acid material, has been designed with different shapes and sizes based on the classic Watson-Crick base-pairing for molecular self-assembly (Ma et al., 2021). Patrick D. Halley et al. successfully utilized DNA nanostructures to circumvent daunorubicin drug resistance at clinically relevant doses in a leukemia cell line model (Halley et al., 2016). The exosome is the most popular biologically-derived nanocarrier in cancer therapy in recent years (Chinnappan et al., 2020; Allegra et al., 2022). Exosomes are small (30–150 nm) membranous vesicles of endocytic origin containing cytokines, RNAs, growth factors, proteins, lipids, and metabolites, and can be produced by all cells under physiological and pathological conditions (Boyiadzis and Whiteside, 2017; Allegra et al., 2022). Qing-Hua Min et al. successfully applied exosomes mediating a horizontal transfer of drug-resistant trait in chronic myeloid leukemia cells by delivering miR-365 (Min et al., 2018). Qinhua Liu et al. used iRGD-targeted exosomes produced by engineered immature dendritic cells expressing exosomal membrane protein (Lamp2b) to load BCL6 siRNA by electroporation (Liu Q. et al., 2022). The result showed the iRGD-Exo loaded with BCL6 siRNA suppressed diffuse large B-cell lymphoma cell proliferation in vitro and inhibited tumor growth in vivo.
4 The types and applications of cell membrane camouflage on nanodrugs
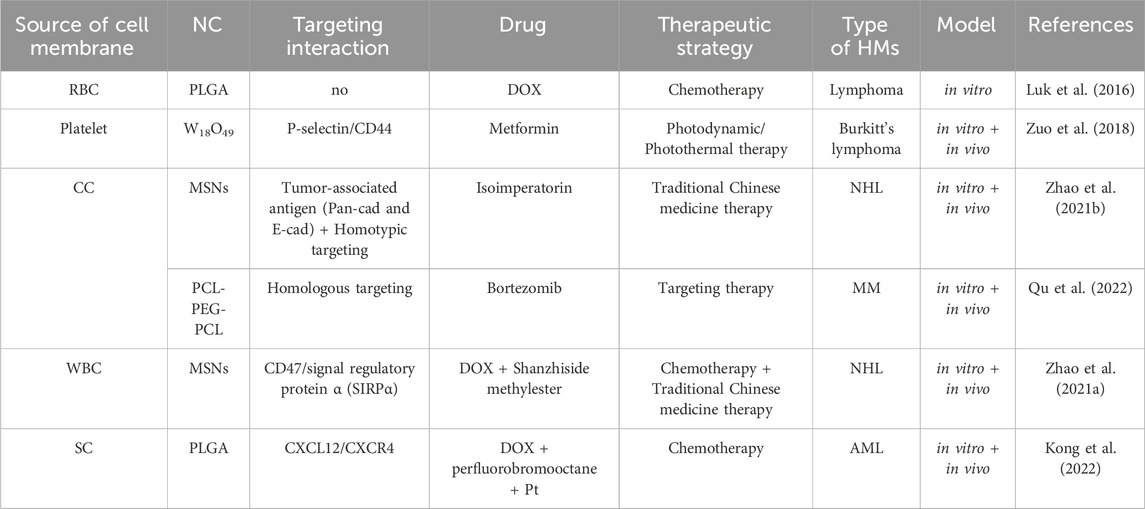
Lacking of active targeting ability weaken the delivery efficacy of the nanodrug delivery system (Wang and Thanou, 2010). Nowadays, targeting decoration using specific ligands or natural cell membrane fragments on the surface of nanodrugs can significantly increase the accumulation of nanodrugs in HM lesions, enhancing therapeutic efficiency (Ahmad et al., 2021). Better than targeting ligands using antibodies, peptides, aptamers, or small molecular, biomimetic decoration endows nanodrug with more functions, including preventing of drug leakage, target specificity and unique homing abilities to HM lesions, low toxicity and immune evasion, etc (Aryal et al., 2013; Zhang et al., 2020; Zhao et al., 2022). Common biomimetic methods include cell membrane camouflage on the surface of nanodrugs, cellular Trojan horses loading nanodrugs in intact cells and attaching nanodrugs to cell surfaces (Powsner et al., 2021). Different source cells can be used for camouflage nanodrugs, including red blood cells (RBCs), platelets, bacteria, cancer cells (CCs), stem cells (SCs), and white blood cells (WBCs), which are coated onto nanodrugs by co-extrusion, extrusion/sonication, freeze-thaw/sonication, extrusion/sonication and stirring, and more (Choi et al., 2020). In this review, we focus on a cell membrane-wrapped nanodrug delivery system, and will detailly introduce the types of various cell membrane and their mechanism and difference in application (Labernadie et al., 2017; van Manen et al., 2019; Wang et al., 2019; Zhao et al., 2021b; Harris et al., 2022; Kong et al., 2022; Wu et al., 2022; Chen et al., 2023) (Figure 5; Table 5).
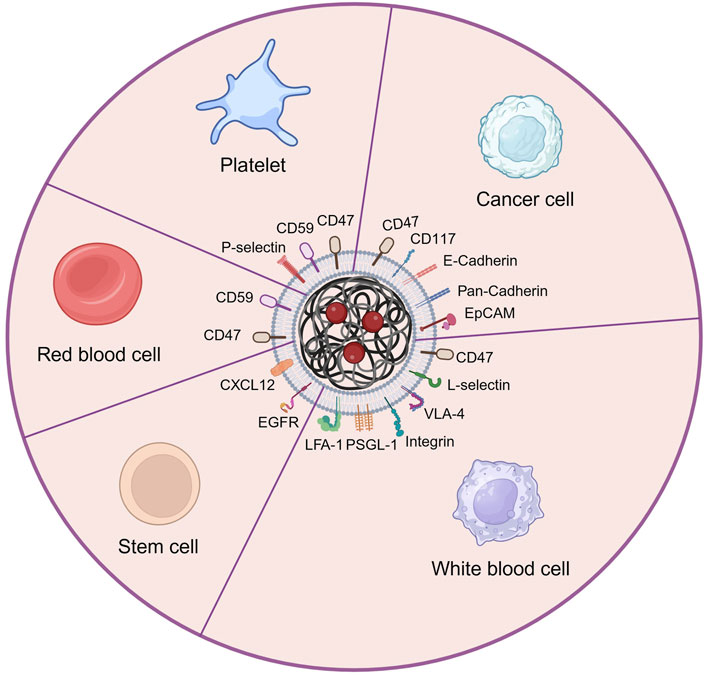
Figure 5. The main types of cell membrane and membrane proteins used for camouflaging nanodrug in HMs treatment. Created by BioRender (agreement number: NT26D16T7T).
4.1 Red blood cell membrane (RBCM)
Red blood cell (erythrocyte) membrane has been widely used for wrapping nanodrug attributed to its good biocompatibility, biodegradability, and long circulating time in the body (Rao et al., 2015; Xia et al., 2019; Nguyen et al., 2023). Liangfang Zhang’s team first developed and applied RBCM to coat nanodrug for HMs therapy. The RBCM-cloaked NPs loaded with DOX enabled reach tumor lesions through the enhanced permeability and retention (EPR) effect. Consequently, this nanodrug demonstrated superior toxicity compared to free DOX when treating AML cells (Aryal et al., 2013) and EL4 mouse lymphoma cells in vitro (Luk et al., 2016). However, lacking specific targeting ability limited the direct application of RBCM in nanodrug delivery systems (Zhang et al., 2021).
4.2 Platelet membrane (PM)
Platelet is another popular type of blood cell and chosen for extracting cell membranes to camouflage nanodugs (Zhang et al., 2018). Similar to RBCM (Zou et al., 2019), PM has an inherent immune escaping ability attributed to the CD47 and CD59 protein in PM sending “do not eat me” signals to immune cells (van Manen et al., 2019; Wang et al., 2019). Advanced to RBCM, PM has specifically cancer-targeting ability attributed to the overexpression of P-selectin, which allows PM-cloaked nanodrug to selectively bind to the CD44 receptors upregulated on the surface of cancer cells (Wang et al., 2019). Huaqin Zuo et al. built platelet-mimicking nanoparticles co-loaded with W18O49 and metformin (Met) to alleviate tumor hypoxia (PM-W18O49-Met NPs) (Zuo et al., 2018). The result exhibited that platelet membranes not only protected W18O49 from oxidation and immune evasion but also increased the accumulation of W18O49 in tumor sites via the active adhesion between platelets and lymphoma cells compared with non-platelet membrane-coated W18O49-Met NPs. Under the cooperation of W18O49 and metformin, the reactive oxygen species and heat generation significantly increased in lymphoma cells, hence inducing cellular death in vitro and tumor growth inhibition in vivo by enhanced photodynamic and photothermal therapy.
4.3 Cancer cell membrane (CCM)
The cancer cell membrane camouflage has considerable advantages in nanodrugs-mediated cancer targeting therapy because of their inherent abilities of targeting and homing to cancer lesions (Labernadie et al., 2017), or even activating immune therapeutic function to cancer (Fang et al., 2014; Guo et al., 2022; Johnson et al., 2022), expecting of prolonging blood circulation time of nanodrugs (Fang et al., 2014; Yaman et al., 2020). Qiangqiang Zhao et al. constructed OCI-LY10 cancer cell membrane (CCM) coated mesoporous silica nanoparticles (MSNs) loaded with the traditional Chinese medicine isoimperatorin (ISOIM), which was called CCM@MSNs-ISOIM (Zhao et al., 2021b). The CCM@MSNs-ISOIM not only exhibited high targeting and adhesion to OCI-LY10 cells by Ca2+-dependent proteins including Pan-Cadherin and E-Cadherin, but also significantly induced OCI-LY10 cytotoxicity in cellular level experiment and subcutaneous OCI-LY10 cell lymphoma model in nude mice. In addition, the safety study in mice blood and organs illustrated the excellent biocompatibility of CCM@MSNs-ISOIM. Jenna C. Harris et al. encapsulated DOX into PLGA nanocarriers which were coated with cytoplasmic membranes derived from human AML cells (Harris et al., 2022). Compared to DOX-loaded PEG-coated nanodrugs, DOX-loaded AML cell-coated nanodrugs exhibited significantly higher AML cell killing at the same drug concentration, which demonstrates the effective targeted accumulation effect mediated by homology targeting of CD117 from AML cell membrane. Daniel T. Johnson et al. fabricated AML cell membrane-coated and immunostimulatory adjuvant (CpG)-loaded nanoparticle (AMCNP) as the cancer vaccine to treat AML (Johnson et al., 2022). Under the double stimulation of cancer-associated antigens and immunostimulatory adjuvants, the antigen-presenting cells (APCs) were activated, finally stimulating functional leukemia-specific T cell responses to target and kill AML cells.
4.4 White blood cell membrane (WBCM)
White blood cells (leukocytes) membrane camouflaged nanodrugs are also very available in cancer therapy because they are capable of passing through blood vessels, targeting tumor sites, and avoiding immune attacks (Oroojalian et al., 2021). Better than other kinds of cell membranes, WBCM can assist nanodrugs actively across endothelial cells by the interaction of selectins (on endothelial cells) and their ligands (on WBCM) such as P-selectin glycoprotein ligand-1 and L-selectin (Si et al., 2016; Poudel et al., 2020). In addition, the expressed P-selectin glycoprotein ligand-1 (PSGL-1), L-selectin, lymphocyte function-associated antigen 1 (LFA-1), integrin, or very late antigen-4 (VLA-4) on the WBCMs increase their cell adhesion with the surface receptors of cancer cells and hence achieving active targeting function (Wu et al., 2022; Chen et al., 2023). The common WBCMs to wrap nanodrugs are usually from macrophages, neutrophils, natural killer (NK) cells, or T cells, etc (Wang et al., 2022). Neutrophils are recognized as the first responders to tissue injury and signs of chronic inflammation like tumor microenvironment. DOX and hanzhiside methylester (SM) co-loaded mesoporous silica nanoparticles (MSNs) coated by a neutrophil membrane (Nm@MSNs@(DOX/SM)) was constructed by Xianjun Ma’s research team (Zhao et al., 2021a). The in vivo anti-tumor study showed that this biomimetic nanodrug evaded immune recognition, actively accumulated in the non-Hodgkin’s lymphoma site, and released drugs in the tumor site to inhibit the inflammation of tumor microenvironment by SM and further enhanced the anti-tumor effect by DOX.
4.5 Stem cell membrane (SCM)
Stem cell membranes also have camouflaged value for nanodrugs in HMs therapy because of containing a large number of molecular recognition moieties, which play a crucial role in interactions with tumor cells, hence exhibiting a natural and high tumor affinity (Gao et al., 2016a; Gao et al., 2016b). Some bone marrow-derived steam cells such as bone marrow stromal cells (Kong et al., 2022) and leukemia stem cells (Song et al., 2023), have an inherent ability to recognize and combine with HMs, therefore their cell membrane can also be utilized for wrapping nanodrugs to target to and kill HM cells. Fei Kong et al. used bone marrow stromal cell membrane coat oxygen-carrying nanoparticle conjugated with ultra-small nanozyme (PFOB@PLGA@Pt@DOX-CM) (Kong et al., 2022). The PFOB@PLGA@Pt@DOX-CM could be actively internalized by the leukemia cells in the blood and released the loaded DOX and Pt nanozyme inside the leukemia cells to achieve synergistic antitumor efficacy. Meanwhile, the adhesive properties of the stromal cell membrane enabled the PFOB@PLGA@Pt@DOX-CM to home to the bone marrow and to kill the retained leukemia cells. Therefore, this nano-formulation possessed a synergistic anti-AML efficacy via integrating chemotherapy, nanozyme-induced cascade catalytic activity, and CXCR4 antagonism. More importantly, the bone marrow stromal cell membrane also acted as a CXCR4 antagonism to block the CXCR4/CXCL12-mediated homing of leukemia cells to the bone marrow and infiltration to other organs. Yue Song et al. applied leukemia stem cell linked with pro-autophagic Beclin-1 peptide to wrap bimetallic metal-organic framework (Mn2+/Fe3+ MOF) loading DNA hypomethylating agent (azacitidine, AZA), which is named AFMMB (Song et al., 2023). The AFMMB selectively targeted the leukemic blasts due to the homing ability of the leukemia stem cell membrane. Following internalization, AFMMB accumulated in the Golgi apparatus by binding to the Golgi-associated plant pathogenesis-related protein 1 and triggered autophagy, leading to its disintegration and release of Fe3+, Mn2+, and AZA. While AZA and Mn2+ restored the stimulator of interferon genes pathway by inhibiting DNA methylation, accumulation of Fe3+ inactivated the endogenous iron-dependent m6A demethylase, thereby increasing global m6A RNA modification and suppressing PD-L1. The dual epigenetic action enhanced the antigenicity of the AML cells by upregulating MHC-I molecules and downregulating PD-L1, resulting in enhanced T-cell-mediated immune response and further killing AML cells.
4.6 The combined application of cell membrane camouflage with ligands
To endow some cell membranes like RBCM targeting ability, or further enhance the targeting function of cell membrane camouflaged nanodrug to endothelial cells or cancer lesions, some ligands are usually embedded into cell membrane. Fangrong Zhang et al. applied CD38-targeting peptide (P) embedded RBCM wrap spherical porous hollow Trinickel monophosphide nanoparticles (Ni3P NPs) loaded with bortezomib (BTZ) to form P-R@Ni3P-BTZ nanodrug (Zhang et al., 2023). In vitro and in vivo, it proved that P-R@Ni3P-BTZ has excellent targeting ability to CD38+ MM cells and is highly effective in killing MM cells by cooperating with photothermal therapy by Ni3P and chemotherapy by BTZ. Zhen Gu’s research team constructed alendronate (Ald) targeting modification and platelet membrane-coated nanoparticulate platform for targeted delivery of BTZ at the myeloma site based on the bone microenvironment and myeloma cell sequential targeting strategy (Hu et al., 2016). In addition, tPA was decorated on the platelet membrane via biotin-streptavidin affinity to dissolve the thrombus readily and reduce the mortality of multiple myeloma patients. Ald helped tPA-Ald-PM-NP@BTZ accumulate at the bone site by chelating calcium ions rich in the bone microenvironment, decreasing the off-target effects. Furthermore, platelet membrane helped tPA-Ald-PM-NP@BTZ achieve secondary targeting from bone microenvironment to myeloma cells by specific affinity between P-Selectin on PM and CD44 receptor on myeloma cells. Finally, compared with other BTZ formulations, the tPA-Ald-PM-NP@BTZ achieved excellent anti-multiple myeloma efficacy in the multiple myeloma-bearing Nod-SCID mice and significantly prolonged the survival time of mice.
5 Conclusion and outlook
The physical and chemical properties of drugs limited their clinical application in treating HMs. Nanocarriers from different sources such as organic materials, inorganic materials, biological-derived materials, and their combination, have been largely and successfully used to resolve drug delivery problems in HMs therapy. Some liposome-based nanodrugs like Doxil® have been allowed for marketing by the FDA to patients with HMs. Even though the ERP effect allows nanodrugs to pass through broken blood vessels, lacking targeting decoration limits their active accumulation and deep penetration in tumor tissues (Subhan et al., 2021). In addition, non-targeting decorated nanodrugs are hard to recognize and combine with non-solid tumors like leukemia (Huang et al., 2019). Common targeting decoration includes modifying ligands like antibodies or cell membrane fragments on the surface of nanodrugs, or delivering nanodrugs using intact cells like immune cells by loading nanodrugs into cells or linking nanodrugs on cellular surface (Alghamdi et al., 2022).
Compared with targeting ligands, which only administrate targeting function, cell membrane camouflage not only assists nanodrugs in actively targeting to cancer sites but also enhances the biocompatibility and prolongs the blood circulation time of nanodrugs in the body, hence further improving therapeutic efficiency (Zhu et al., 2022). For HMs therapy, the main sources of cell membrane fragments are red blood cells, platelets, cancer cells, white blood cells, and stem cells. When extracting cell membrane fragments and preparing cell membrane-wrapped nanodrugs, membrane proteins are normally reserved by intelligent technologies. This is also the main reason for keeping targeting and biocompatible functions similar to intact cells (Chugh et al., 2021). Because red blood cells have no targeting ability to tumors, their membrane is usually combined with targeting ligands to co-modify nanodrugs. CCM, WBCM, and SCM have unique targeting abilities and adhesion to cancer cells because of homology targeting, immune recognition and self-updating requirements, respectively. Furthermore, some of CCM, WBCM, and SCM have the ability to activate immune responses to HMs, hence producing combined therapy with drugs. This “kill two birds with one stone” strategy that targets accumulation and immune activation mediated by cell membrane demonstrates huge advantages of cell membrane camouflage in treating HMs and other cancers.
Nowadays, even though a number of studies about (cell membrane camouflaged) nanodrug delivery system have been experimental detection in the treatment of HMs in mice models and enable significantly improve therapeutic efficiency compared with free drugs, few studies of nanodrugs are being conducted in the clinical process. Quantitative production problems, expensive expenses, and unknown long-lasting and cumulative toxicity of nanocarriers and cell membranes in the body are the main restrictions for clinical application (Xuan et al., 2023). Further laboratory and clinical trials are imperative to ensure the safety profile of nanodrugs. Additionally, hybrid cell membrane camouflage and a combination of cell membranes with ligands have been utilized for improving cancer therapeutic efficiency attributed to their versatility (Rao et al., 2020). In the future, these strategies should be explored and applied for HMs therapy so that avoiding pitfalls caused by single decoration.
In summary, with the help of various nanocarriers and biomimetic camouflage using cell membrane fragments, drugs can be successfully and highly effectively delivered into HM lesions. These studies supply more chances for the clinical application of drugs in HMs and other cancers.
Author contributions
YL: Writing–review and editing, Writing–original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization, Funding acquisition. SY: Writing–review and editing, Software, Resources, Data curation. YC: Writing–review and editing. ZH: Writing–review and editing. LF: Writing–review and editing. GL: Writing–review and editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This publication was supported by the Key Specialized Research and Development Breakthrough in Henan Province (242102310454), National Key Research and Development Program of China (2022YFE132800) and Key R&D project of Henan Province (221111310600).
Acknowledgments
The authors thank all individuals who participated in this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abuasab, T., Garcia-Manero, G., Short, N., Alvarado, Y., Issa, G. C., Islam, R., et al. (2022). Phase 2 study of ASTX727 (cedazuridine/decitabine) plus venetoclax in patients with relapsed/refractory acute myeloid leukemia (AML) or previously untreated, elderly patients with AML unfit for chemotherapy. Blood 140 (Suppl. 1), 3324–3326. doi:10.1182/blood-2022-158566
Adamo, F. M., Silva Barcelos, E. C., De Falco, F., Dorillo, E., Rompietti, C., Sorcini, D., et al. (2023). Therapeutic targeting potential of novel silver nanoparticles coated with anti-CD20 antibody against chronic lymphocytic leukemia. Cancers 15 (14), 3618. doi:10.3390/cancers15143618
Ahmad, E., Ali, A., Fatima, M. T., Kumar, A., Sumi, M. P., Sattar, R. S. A., et al. (2021). Ligand decorated biodegradable nanomedicine in the treatment of cancer. Pharmacol. Res. 167, 105544. doi:10.1016/j.phrs.2021.105544
Alaggio, R., Amador, C., Anagnostopoulos, I., Attygalle, A. D., Araujo, I. B. O., Berti, E., et al. (2022). The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia 36 (7), 1720–1748. doi:10.1038/s41375-022-01620-2
Alghamdi, M. A., Fallica, A. N., Virzì, N., Kesharwani, P., Pittalà, V., and Greish, K. (2022). The promise of nanotechnology in personalized medicine. J. Pers. Med. 12 (5), 673. doi:10.3390/jpm12050673
Alinari, L., Christian, B., Yu, B., Shin, J., Hertlein, E. K., Lapalombella, R., et al. (2009). Co-treatment with milatuzumab (Anti-CD74 mAb) and rituximab (Anti-CD20 mAb) results in the induction of mantle cell lymphoma cell death that is dependent on actin polymerization and inhibition of NF-kb. Blood 114 (22), 1694. doi:10.1182/blood.V114.22.1694.1694
Allegra, A., Petrarca, C., Di Gioacchino, M., Casciaro, M., Musolino, C., and Gangemi, S. (2022). Exosome-mediated therapeutic strategies for management of solid and hematological malignancies. Cells 11 (7), 1128. doi:10.3390/cells11071128
Anajafi, T., and Mallik, S. (2015). Polymersome-based drug-delivery strategies for cancer therapeutics. Ther. Deliv. 6 (4), 521–534. doi:10.4155/tde.14.125
Anderson, A. C. (2014). Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol. Res. 2 (5), 393–398. doi:10.1158/2326-6066.CIR-14-0039
André, P., Denis, C., Soulas, C., Bourbon-Caillet, C., Lopez, J., Arnoux, T., et al. (2018). Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 175 (7), 1731–1743. doi:10.1016/j.cell.2018.10.014
Appelbaum, F. R., and Bernstein, I. D. (2017). Gemtuzumab ozogamicin for acute myeloid leukemia. Blood 130 (22), 2373–2376. doi:10.1182/blood-2017-09-797712
Arana-Trejo, R. M., Ruíz Sánchez, E., Ignacio-Ibarra, G., Báez De La Fuente, E., Garces, O., Gómez Morales, E., et al. (2002). BCR/ABL p210, p190 and p230 fusion genes in 250 Mexican patients with chronic myeloid leukaemia (CML). Clin. Lab. Haematol. 24 (3), 145–150. doi:10.1046/j.1365-2257.2002.00413.x
Aryal, S., Hu, C.-M. J., Fang, R. H., Dehaini, D., Carpenter, C., Zhang, D.-E., et al. (2013). Erythrocyte membrane-cloaked polymeric nanoparticles for controlled drug loading and release. Nanomedicine 8 (8), 1271–1280. doi:10.2217/nnm.12.153
Astete, C. E., and Sabliov, C. M. (2006). Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 17 (3), 247–289. doi:10.1163/156856206775997322
Atkins, S., and He, F. (2019). Chemotherapy and beyond: infections in the era of old and new treatments for hematologic malignancies. Infect. Dis. Clin. 33 (2), 289–309. doi:10.1016/j.idc.2019.01.001
Bao, W., Liu, R., Xia, G., Wang, F., and Chen, B. (2019). Applications of daunorubicin-loaded PLGA-PLL-PEG-Tf nanoparticles in hematologic malignancies: an in vitro and in vivo evaluation. Drug Des. devel. Ther. 13, 1107–1115. doi:10.2147/DDDT.S195832
Barth, B. M., Altinoglu, E. I., Shanmugavelandy, S. S., Kaiser, J. M., Crespo-Gonzalez, D., Divittore, N. A., et al. (2011). Targeted indocyanine-green-loaded calcium phosphosilicate nanoparticles for in vivo photodynamic therapy of leukemia. ACS Nano 5 (7), 5325–5337. doi:10.1021/nn2005766
Baskar, R., Dai, J., Wenlong, N., Yeo, R., and Yeoh, K.-W. (2014). Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 1, 24. doi:10.3389/fmolb.2014.00024
Bates, S. E., Eisch, R., Ling, A., Rosing, D., Turner, M., Pittaluga, S., et al. (2015). Romidepsin in peripheral and cutaneous T-cell lymphoma: mechanistic implications from clinical and correlative data. Br. J. Haematol. 170 (1), 96–109. doi:10.1111/bjh.13400
Bhatt, V. R. (2016). Allogeneic stem cell transplantation for non-Hodgkin lymphoma. Curr. Hematol. Malig. Rep. 11, 196–207. doi:10.1007/s11899-016-0319-0
Bispo, J. a. B., Pinheiro, P. S., and Kobetz, E. K. (2020). Epidemiology and etiology of leukemia and lymphoma. Cold Spring Harb. Perspect. Med. 10 (6), a034819. doi:10.1101/cshperspect.a034819
Blake, S. J., Dougall, W. C., Miles, J. J., Teng, M. W., and Smyth, M. J. (2016). Molecular pathways: targeting CD96 and TIGIT for cancer immunotherapy. Clin. Cancer. Res. 22 (21), 5183–5188. doi:10.1158/1078-0432.CCR-16-0933
Bleul, R., Thiermann, R., and Maskos, M. (2015). Techniques to control polymersome size. Macromolecules 48 (20), 7396–7409. doi:10.1021/acs.macromol.5b01500
Boyiadzis, M., and Whiteside, T. (2017). The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia 31 (6), 1259–1268. doi:10.1038/leu.2017.91
Boyiadzis, M. M., Dhodapkar, M. V., Brentjens, R. J., Kochenderfer, J. N., Neelapu, S. S., Maus, M. V., et al. (2018). Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J. Immunother. Cancer 6 (1), 137. doi:10.1186/s40425-018-0460-5
Burt, R. K., Loh, Y., Pearce, W., Beohar, N., Barr, W. G., Craig, R., et al. (2008). Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA 299 (8), 925–936. doi:10.1001/jama.299.8.925
Cano, A., Ettcheto, M., Chang, J.-H., Barroso, E., Espina, M., Kühne, B. A., et al. (2019). Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer's disease mice model. J. Control. Release 301, 62–75. doi:10.1016/j.jconrel.2019.03.010
Cano, A., Sánchez-López, E., Ettcheto, M., Lopez-Machado, A., Espina, M., Souto, E. B., et al. (2020). Current advances in the development of novel polymeric nanoparticles for the treatment of neurodegenerative diseases. Nanomedicine 15 (12), 1239–1261. doi:10.2217/nnm-2019-0443
Cao, H. Y., Li, L., Xue, S. L., and Dai, H. P. (2023). Chidamide: targeting epigenetic regulation in the treatment of hematological malignancy. Hematol. Oncol. 41 (3), 301–309. doi:10.1002/hon.3088
Chen, D., Chen, B., and Yao, F. (2018). Doxorubicin-loaded PEG-CdTe quantum dots as a smart drug delivery system for extramedullary multiple myeloma treatment. Nanoscale Res. Lett. 13 (1), 373–379. doi:10.1186/s11671-018-2782-0
Chen, L., and Flies, D. B. (2013). Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 13 (4), 227–242. doi:10.1038/nri3405
Chen, Y., Zhu, M., Huang, B., Jiang, Y., and Su, J. (2023). Advances in cell membrane-coated nanoparticles and their applications for bone therapy. Biomater. Adv. 144, 213232. doi:10.1016/j.bioadv.2022.213232
Chinnappan, M., Srivastava, A., Amreddy, N., Razaq, M., Pareek, V., Ahmed, R., et al. (2020). Exosomes as drug delivery vehicle and contributor of resistance to anticancer drugs. Cancer Lett. 486, 18–28. doi:10.1016/j.canlet.2020.05.004
Chivu-Economescu, M., and Rubach, M. (2017). Hematopoietic stem cells therapies. Curr. Stem Cell Res. Ther. 12 (2), 124–133. doi:10.2174/1574888X10666151026114241
Choi, B., Park, W., Park, S.-B., Rhim, W.-K., and Han, D. K. (2020). Recent trends in cell membrane-cloaked nanoparticles for therapeutic applications. Methods 177, 2–14. doi:10.1016/j.ymeth.2019.12.004
Christian, B. A., Poi, M., Jones, J. A., Porcu, P., Maddocks, K., Flynn, J. M., et al. (2015). The combination of milatuzumab, a humanized anti-CD74 antibody, and veltuzumab, a humanized anti-CD20 antibody, demonstrates activity in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Br. J. Haematol. 169 (5), 701–710. doi:10.1111/bjh.13354
Chu, E., and Sartorelli, A. (2018). “Cancer chemotherapy,” in Basic and clinical Pharmacology. Editor B. G. Katzung (Prentice-Hall), 948–976.
Chugh, V., Vijaya Krishna, K., and Pandit, A. (2021). Cell membrane-coated mimics: a methodological approach for fabrication, characterization for therapeutic applications, and challenges for clinical translation. ACS Nano 15 (11), 17080–17123. doi:10.1021/acsnano.1c03800
Ciammella, P., Luminari, S., Arcaini, L., and Filippi, A. R. (2018). Renewed interest for low-dose radiation therapy in follicular lymphomas: from biology to clinical applications. Hematol. Oncol. 36 (5), 723–732. doi:10.1002/hon.2538
Cory, S., and Adams, J. M. (2002). The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2 (9), 647–656. doi:10.1038/nrc883
Cosco, D., Cilurzo, F., Maiuolo, J., Federico, C., Di Martino, M. T., Cristiano, M. C., et al. (2015). Delivery of miR-34a by chitosan/PLGA nanoplexes for the anticancer treatment of multiple myeloma. Sci. Rep. 5 (1), 17579. doi:10.1038/srep17579
Costa, L. J., Kumar, S., Stowell, S. A., and Dermer, S. J. (2015). Mobilization and transplantation patterns of autologous hematopoietic stem cells in multiple myeloma and non-Hodgkin lymphoma. Cancer Control. 22 (1), 87–94. doi:10.1177/107327481502200111
Curley, S. M., and Cady, N. C. (2018). Biologically-derived nanomaterials for targeted therapeutic delivery to the brain. Sci. Prog. 101 (3), 273–292. doi:10.3184/003685018X15306123582346
Dabaja, B., and Spiotto, M. (2023). Radiation for hematologic malignancies: from cell killing to immune cell priming. Front. Oncol. 13, 1205836. doi:10.3389/fonc.2023.1205836
Danhier, F., Ansorena, E., Silva, J. M., Coco, R., Le Breton, A., and Préat, V. (2012). PLGA-based nanoparticles: an overview of biomedical applications. J. Control. Release 161 (2), 505–522. doi:10.1016/j.jconrel.2012.01.043
Dao, K.-H. T., Tyner, J. W., and Gotlib, J. (2017). Recent progress in chronic neutrophilic leukemia and atypical chronic myeloid leukemia. Curr. Hematol. Malig. Rep. 12 (5), 432–441. doi:10.1007/s11899-017-0413-y
Da Rocha, M. C. O., Da Silva, P. B., Radicchi, M. A., Andrade, B. Y. G., De Oliveira, J. V., Venus, T., et al. (2020). Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J. Nanobiotechnol. 18, 43–20. doi:10.1186/s12951-020-00604-7
Davids, M. S., Kim, H. T., Costello, C., Herrera, A. F., Locke, F. L., Maegawa, R. O., et al. (2020). A multicenter phase 1 study of nivolumab for relapsed hematologic malignancies after allogeneic transplantation. Blood 135 (24), 2182–2191. doi:10.1182/blood.2019004710
Davids, M. S., Kim, H. T., Costello, C. L., Mcsweeney, P. A., Liguori, R., Lukez, A., et al. (2015). A multicenter phase I/Ib study of ipilimumab for relapsed hematologic malignancies after allogeneic hematopoietic stem cell transplantation. Blood 126 (23), 860. doi:10.1182/blood.V126.23.860.860
Deshantri, A. K., Moreira, A. V., Ecker, V., Mandhane, S. N., Schiffelers, R. M., Buchner, M., et al. (2018). Nanomedicines for the treatment of hematological malignancies. J. Control. Release 287, 194–215. doi:10.1016/j.jconrel.2018.08.034
De Weers, M., Tai, Y.-T., Van Der Veer, M. S., Bakker, J. M., Vink, T., Jacobs, D. C., et al. (2011). Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J. Immunol. 186 (3), 1840–1848. doi:10.4049/jimmunol.1003032
Dimopoulos, M. A., Dytfeld, D., Grosicki, S., Moreau, P., Takezako, N., Hori, M., et al. (2023). Elotuzumab plus pomalidomide and dexamethasone for relapsed/refractory multiple myeloma: final overall survival analysis from the randomized phase II ELOQUENT-3 trial. J. Clin. Oncol. 41 (3), 568–578. doi:10.1200/jco.21.02815
Di Pietro, A., and Good-Jacobson, K. L. (2018). Disrupting the code: epigenetic dysregulation of lymphocyte function during infectious disease and lymphoma development. J. Immunol. 201 (4), 1109–1118. doi:10.4049/jimmunol.1800137
Dong, X., Wang, W., Qu, H., Han, D., Zheng, J., and Sun, G. (2016). Targeted delivery of doxorubicin and vincristine to lymph cancer: evaluation of novel nanostructured lipid carriers in vitro and in vivo. Drug Deliv. 23 (4), 1374–1378. doi:10.3109/10717544.2015.1041580
Durfee, P. N., Lin, Y.-S., Dunphy, D. R., Muniz, A. E. J., Butler, K. S., Humphrey, K. R., et al. (2016). Mesoporous silica nanoparticle-supported lipid bilayers (protocells) for active targeting and delivery to individual leukemia cells. ACS Nano 10 (9), 8325–8345. doi:10.1021/acsnano.6b02819
El Omari, N., Bakrim, S., Khalid, A., Abdalla, A. N., Almalki, W. H., Lee, L.-H., et al. (2023). Molecular mechanisms underlying the clinical efficacy of panobinostat involve Stochasticity of epigenetic signaling, sensitization to anticancer drugs, and induction of cellular cell death related to cellular stresses. Biomed. Pharmacother. 164, 114886. doi:10.1016/j.biopha.2023.114886
Falank, C., Tasset, A. W., Farrell, M., Harris, S., Everill, P., Marinkovic, M., et al. (2019). Development of medical-grade, discrete, multi-walled carbon nanotubes as drug delivery molecules to enhance the treatment of hematological malignancies. Nanomedicine 20, 102025. doi:10.1016/j.nano.2019.102025
Famta, P., Shah, S., Vambhurkar, G., Srinivasarao, D. A., Jain, N., Begum, N., et al. (2023). Quality by design endorsed fabrication of Ibrutinib-loaded human serum albumin nanoparticles for the management of leukemia. Eur. J. Pharm. Biopharm. 190, 94–106. doi:10.1016/j.ejpb.2023.07.008
Fang, R. H., Hu, C.-M. J., Luk, B. T., Gao, W., Copp, J. A., Tai, Y., et al. (2014). Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 14 (4), 2181–2188. doi:10.1021/nl500618u
Farooque, A., Mathur, R., Verma, A., Kaul, V., Bhatt, A. N., Adhikari, J. S., et al. (2011). Low-dose radiation therapy of cancer: role of immune enhancement. Expert Rev. Anticancer Ther. 11 (5), 791–802. doi:10.1586/era.10.217
Fathi, A. T., Li, S., Soiffer, R. J., Levis, M., Mims, A. S., Devine, S. M., et al. (2020). A phase I study of the IDH2 inhibitor enasidenib as maintenance therapy for IDH2-mutant myeloid neoplasms following hematopoietic cell transplantation. Blood 136, 4–5. doi:10.1182/blood-2020-140176
Feldman, E. J., Kolitz, J. E., Trang, J. M., Liboiron, B. D., Swenson, C. E., Chiarella, M. T., et al. (2012). Pharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine:daunorubicin, in patients with advanced leukemia. Leuk. Res. 36 (10), 1283–1289. doi:10.1016/j.leukres.2012.07.006
Fenwarth, L., Thomas, X., De Botton, S., Duployez, N., Bourhis, J.-H., Lesieur, A., et al. (2021). A personalized approach to guide allogeneic stem cell transplantation in younger adults with acute myeloid leukemia. Blood 137 (4), 524–532. doi:10.1182/blood.2020005524
Flinn, I. W., Hillmen, P., Montillo, M., Nagy, Z., Illés, Á., Etienne, G., et al. (2018). The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood 132 (23), 2446–2455. doi:10.1182/blood-2018-05-850461
Gao, C., Lin, Z., Jurado-Sánchez, B., Lin, X., Wu, Z., and He, Q. (2016a). Stem cell membrane-coated nanogels for highly efficient in vivo tumor targeted drug delivery. Small 12 (30), 4056–4062. doi:10.1002/smll.201600624
Gao, C., Lin, Z., Wu, Z., Lin, X., and He, Q. (2016b). Stem-cell-membrane camouflaging on near-infrared photoactivated upconversion nanoarchitectures for in vivo remote-controlled photodynamic therapy. ACS Appl. Mat. Interfaces 8 (50), 34252–34260. doi:10.1021/acsami.6b12865
Ghosn, Y., Kamareddine, M. H., Tawk, A., Elia, C., El Mahmoud, A., Terro, K., et al. (2019). Inorganic nanoparticles as drug delivery systems and their potential role in the treatment of chronic myelogenous leukaemia. Technol. Cancer Res. Treat. 18, 1533033819853241. doi:10.1177/1533033819853241
Giralt, S., and Bishop, M. R. (2009). Principles and overview of allogeneic hematopoietic stem cell transplantation. Cancer Treat. Res. 144, 1–21. doi:10.1007/978-0-387-78580-6_1
Goede, V., Fischer, K., Busch, R., Engelke, A., Eichhorst, B., Wendtner, C. M., et al. (2014). Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. New Engl. J. Med. 370 (12), 1101–1110. doi:10.1056/NEJMoa1313984
Goldschmidt, H., Mai, E. K., Bertsch, U., Fenk, R., Nievergall, E., Tichy, D., et al. (2022). Addition of isatuximab to lenalidomide, bortezomib, and dexamethasone as induction therapy for newly diagnosed, transplantation-eligible patients with multiple myeloma (GMMG-HD7): part 1 of an open-label, multicentre, randomised, active-controlled, phase 3 trial. Lancet. Haematol. 9 (11), e810–e821. doi:10.1016/s2352-3026(22)00263-0
Greim, H., Kaden, D. A., Larson, R. A., Palermo, C. M., Rice, J. M., Ross, D., et al. (2014). The bone marrow niche, stem cells, and leukemia: impact of drugs, chemicals, and the environment. Ann. N. Y. Acad. Sci. 1310 (1), 7–31. doi:10.1111/nyas.12362
Guo, Y., Wang, Z., Shi, X., and Shen, M. (2022). Engineered cancer cell membranes: an emerging agent for efficient cancer theranostics. Exploration 2 (1), 20210171. doi:10.1002/exp.20210171
Halley, P. D., Lucas, C. R., Mcwilliams, E. M., Webber, M. J., Patton, R. A., Kural, C., et al. (2016). Daunorubicin-loaded DNA origami nanostructures circumvent drug-resistance mechanisms in a leukemia model. Small 12 (3), 308–320. doi:10.1002/smll.201502118
Halwani, A. A. (2022). Development of pharmaceutical nanomedicines: from the bench to the market. Pharmaceutics 14 (1), 106. doi:10.3390/pharmaceutics14010106
Han, D., Xu, Z., Zhuang, Y., Ye, Z., and Qian, Q. (2021). Current progress in CAR-T cell therapy for hematological malignancies. J. Cancer 12 (2), 326–334. doi:10.7150/jca.48976
Hani, U., Gowda, B. J., Haider, N., Ramesh, K., Paul, K., Ashique, S., et al. (2023). Nanoparticle-based approaches for treatment of hematological malignancies: a comprehensive review. AAPS PharmSciTech 24 (8), 233. doi:10.1208/s12249-023-02670-0
Harris, J. C., Sterin, E. H., and Day, E. S. (2022). Membrane-wrapped nanoparticles for enhanced chemotherapy of acute myeloid leukemia. ACS Biomater. Sci. Eng. 8 (10), 4439–4448. doi:10.1021/acsbiomaterials.2c00832
Hatzimichael, E., and Tuthill, M. (2010). Hematopoietic stem cell transplantation. Stem Cells Cloning Adv. Appl. 3, 105–117. doi:10.2147/SCCAA.S6815
Hawkins, M. J., Soon-Shiong, P., and Desai, N. (2008). Protein nanoparticles as drug carriers in clinical medicine. Adv. Drug Del. Rev. 60 (8), 876–885. doi:10.1016/j.addr.2007.08.044
Hemminki, K., Hemminki, J., Försti, A., and Sud, A. (2023). Survival in hematological malignancies in the Nordic countries through a half century with correlation to treatment. Leukemia 37 (4), 854–863. doi:10.1038/s41375-023-01852-w
Heo, Y. A., Syed, Y. Y., and Keam, S. J. (2019). Pegaspargase: a review in acute lymphoblastic leukaemia. Drugs 79 (7), 767–777. doi:10.1007/s40265-019-01120-1
Hertlein, E., Triantafillou, G., Sass, E. J., Hessler, J. D., Zhang, X., Jarjoura, D., et al. (2010). Milatuzumab immunoliposomes induce cell death in CLL by promoting accumulation of CD74 on the surface of B cells. Blood 116 (14), 2554–2558. doi:10.1182/blood-2009-11-253203
Hopfinger, G., Nösslinger, T., Lang, A., Linkesch, W., Melchardt, T., Weiss, L., et al. (2014). Lenalidomide in combination with vorinostat and dexamethasone for the treatment of relapsed/refractory peripheral T cell lymphoma (PTCL): report of a phase I/II trial. Ann. Hematol. 93, 459–462. doi:10.1007/s00277-014-2009-0
Housman, G., Byler, S., Heerboth, S., Lapinska, K., Longacre, M., Snyder, N., et al. (2014). Drug resistance in cancer: an overview. Cancers 6 (3), 1769–1792. doi:10.3390/cancers6031769
Hu, Q., Qian, C., Sun, W., Wang, J., Chen, Z., Bomba, H. N., et al. (2016). Engineered nanoplatelets for enhanced treatment of multiple myeloma and thrombus. Adv. Mat. 28 (43), 9573–9580. doi:10.1002/adma.201603463
Hu, Y., Tian, Z.-G., and Zhang, C. (2018). Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacol. Sin. 39 (2), 167–176. doi:10.1038/aps.2017.125
Huang, H., Feng, W., Chen, Y., and Shi, J. (2020a). Inorganic nanoparticles in clinical trials and translations. Nano today 35, 100972. doi:10.1016/j.nantod.2020.100972
Huang, L., Huang, J., Huang, J., Xue, H., Liang, Z., Wu, J., et al. (2020b). Nanomedicine–a promising therapy for hematological malignancies. Biomater. Sci. 8 (9), 2376–2393. doi:10.1039/D0BM00129E
Huang, X., Lin, H., Huang, F., Xie, Y., Wong, K. H., Chen, X., et al. (2019). Targeting approaches of nanomedicines in acute myeloid leukemia. Dose-response a Publ. Int. Hormesis Soc. 17 (4), 1559325819887048. doi:10.1177/1559325819887048
Hus, I., Puła, B., and Robak, T. (2022). PI3K inhibitors for the treatment of chronic lymphocytic leukemia: current status and future perspectives. Cancers 14 (6), 1571. doi:10.3390/cancers14061571
Ikeda, H., Kanakura, Y., Tamaki, T., Kuriu, A., Kitayama, H., Ishikawa, J., et al. (1991). Expression and functional role of the proto-oncogene c-kit in acute myeloblastic leukemia cells. Blood 78 (11), 2962–2968. doi:10.1182/blood.v78.11.2962.2962
Imber, B. S., Chau, K. W., Lee, J., Lee, J., Casey, D. L., Yang, J. C., et al. (2021). Excellent response to very-low-dose radiation (4 Gy) for indolent B-cell lymphomas: is 4 Gy suitable for curable patients? Blood Adv. 5 (20), 4185–4197. doi:10.1182/bloodadvances.2021004939
Jain, A., Singh, S. K., Arya, S. K., Kundu, S. C., and Kapoor, S. (2018). Protein nanoparticles: promising platforms for drug delivery applications. ACS Biomater. Sci. Eng. 4 (12), 3939–3961. doi:10.1021/acsbiomaterials.8b01098
Janin, M., Mylonas, E., Saada, V., Micol, J.-B., Renneville, A., Quivoron, C., et al. (2014). Serum 2-hydroxyglutarate production in IDH1-and IDH2-mutated de novo acute myeloid leukemia: a study by the Acute Leukemia French Association group. J. Clin. Oncol. 32 (4), 297–305. doi:10.1200/JCO.2013.50.2047
Jia, Y., Sun, C., Chen, T., Zhu, H., Wang, T., Ye, Y., et al. (2023). Recent advance in phytonanomedicine and mineral nanomedicine delivery system of the treatment for acute myeloid leukemia. J. Nanobiotechnol. 21 (1), 240. doi:10.1186/s12951-023-01968-2
Jiao, Q., Bi, L., Ren, Y., Song, S., Wang, Q., and Wang, Y.-S. (2018). Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer 17 (1), 36–12. doi:10.1186/s12943-018-0801-5
Johnson, D. T., Zhou, J., Kroll, A. V., Fang, R. H., Yan, M., Xiao, C., et al. (2022). Acute myeloid leukemia cell membrane-coated nanoparticles for cancer vaccination immunotherapy. Leukemia 36 (4), 994–1005. doi:10.1038/s41375-021-01432-w
Johnston, P. B., Cashen, A. F., Nikolinakos, P. G., Beaven, A. W., Barta, S. K., Bhat, G., et al. (2021). Belinostat in combination with standard cyclophosphamide, doxorubicin, vincristine and prednisone as first-line treatment for patients with newly diagnosed peripheral T-cell lymphoma. Exp. Hematol. 10, 15–11. doi:10.1186/s40164-021-00203-8
Kansara, R., and Speziali, C. (2020). Immunotherapy in hematologic malignancies. Curr. Oncol. 27 (s2), S124–S131. doi:10.3747/co.27.5117
Kantarjian, H. M., Deangelo, D. J., Stelljes, M., Martinelli, G., Liedtke, M., Stock, W., et al. (2016). Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. New Engl. J. Med. 375 (8), 740–753. doi:10.1056/NEJMoa1509277
Kantarjian, H. M., Roboz, G. J., Kropf, P. L., Yee, K. W., O'connell, C. L., Tibes, R., et al. (2017). Guadecitabine (SGI-110) in treatment-naive patients with acute myeloid leukaemia: phase 2 results from a multicentre, randomised, phase 1/2 trial. Lancet Oncol. 18 (10), 1317–1326. doi:10.1016/S1470-2045(17)30576-4
Karunakaran, G., Sudha, K. G., Ali, S., and Cho, E.-B. (2023). Biosynthesis of nanoparticles from various biological sources and its biomedical applications. Molecules 28 (11), 4527. doi:10.3390/molecules28114527
Kaufman, J., Niesvizky, R., Stadtmauer, E. A., Chanan-Khan, A., Siegel, D., Horne, H., et al. (2008). First trial of humanized anti-CD74 monoclonal antibody (MAb), milatuzumab, in multiple myeloma. Blood 112 (11), 3697. doi:10.1182/blood.V112.11.3697.3697
Khalil, D. N., Smith, E. L., Brentjens, R. J., and Wolchok, J. D. (2016). The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 13 (5), 273–290. doi:10.1038/nrclinonc.2016.25
Kipps, T. J., Eradat, H., Grosicki, S., Catalano, J., Cosolo, W., Dyagil, I. S., et al. (2015). A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk. Lymphoma 56 (10), 2826–2833. doi:10.3109/10428194.2015.1030638
Kiyoi, H., Kawashima, N., and Ishikawa, Y. (2020). FLT3 mutations in acute myeloid leukemia: therapeutic paradigm beyond inhibitor development. Cancer Sci. 111 (2), 312–322. doi:10.1111/cas.14274
Klichinsky, M., Ruella, M., Shestova, O., Lu, X. M., Best, A., Zeeman, M., et al. (2020). Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38 (8), 947–953. doi:10.1038/s41587-020-0462-y
Kong, F., He, H., Bai, H., Yang, F., Ma, M., Gu, N., et al. (2022). A biomimetic nanocomposite with enzyme-like activities and CXCR4 antagonism efficiently enhances the therapeutic efficacy of acute myeloid leukemia. Bioact. Mat. 18, 526–538. doi:10.1016/j.bioactmat.2022.03.022
Kowalczuk, A., Trzcinska, R., Trzebicka, B., Müller, A. H., Dworak, A., and Tsvetanov, C. B. (2014). Loading of polymer nanocarriers: factors, mechanisms and applications. Prog. Polym. Sci. 39 (1), 43–86. doi:10.1016/j.progpolymsci.2013.10.004
Krejcik, J., Casneuf, T., Nijhof, I. S., Verbist, B., Bald, J., Plesner, T., et al. (2016). Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 128 (3), 384–394. doi:10.1182/blood-2015-12-687749
Kroll, M. H., Rojas-Hernandez, C., and Yee, C. (2022). Hematologic complications of immune checkpoint inhibitors. Blood 139 (25), 3594–3604. doi:10.1182/blood.2020009016
Kuperkar, K., Patel, D., Atanase, L. I., and Bahadur, P. (2022). Amphiphilic block copolymers: their structures, and self-assembly to polymeric micelles and polymersomes as drug delivery vehicles. Polymers 14 (21), 4702. doi:10.3390/polym14214702
Kuroda, J., Puthalakath, H., Cragg, M. S., Kelly, P. N., Bouillet, P., Huang, D. C., et al. (2006). Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc. Natl. Acad. Sci. 103 (40), 14907–14912. doi:10.1073/pnas.0606176103
Kurosawa, S., Mizuno, S., Arai, Y., Masuko, M., Kanda, J., Kohno, K., et al. (2021). Syngeneic hematopoietic stem cell transplantation for acute myeloid leukemia: a propensity score-matched analysis. Blood Cancer J. 11 (9), 159. doi:10.1038/s41408-021-00553-w
Kutsch, N., Marks, R., Ratei, R., Held, T. K., and Schmidt-Hieber, M. (2015). Role of tyrosine kinase inhibitors in indolent and other mature B-cell neoplasms. Biomark. Insights 10 (Suppl. 3), 15–23. doi:10.4137/bmi.S22434
Kwak, E., Kim, T., Yang, K., Kim, Y. M., Han, H. S., Park, K. H., et al. (2022). Surface-functionalized polymeric siRNA nanoparticles for tunable targeting and intracellular delivery to hematologic cancer cells. Biomacromolecules 23 (6), 2255–2263. doi:10.1021/acs.biomac.1c01497
Labernadie, A., Kato, T., Brugués, A., Serra-Picamal, X., Derzsi, S., Arwert, E., et al. (2017). A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 19 (3), 224–237. doi:10.1038/ncb3478
Lee, S. J., and Borrello, I. (2016). Role of the immune response in disease progression and therapy in multiple myeloma. Cancer Res. Treat. 169, 207–225. doi:10.1007/978-3-319-40320-5_12
Leoni, V., and Biondi, A. (2015). Tyrosine kinase inhibitors in BCR-ABL positive acute lymphoblastic leukemia. Haematologica 100 (3), 295–299. doi:10.3324/haematol.2015.124016
Li, B., Gale, R. P., and Xiao, Z. (2014). Molecular genetics of chronic neutrophilic leukemia, chronic myelomonocytic leukemia and atypical chronic myeloid leukemia. J. Hematol. Oncol. 7 (1), 93–97. doi:10.1186/s13045-014-0093-1
Li, J., Wang, Q., Han, Y., Jiang, L., Lu, S., Wang, B., et al. (2023a). Development and application of nanomaterials, nanotechnology and nanomedicine for treating hematological malignancies. J. Hematol. Oncol. 16 (1), 65. doi:10.1186/s13045-023-01460-2
Li, R., Wu, R. A., Zhao, L., Wu, M., Yang, L., and Zou, H. (2010). P-glycoprotein antibody functionalized carbon nanotube overcomes the multidrug resistance of human leukemia cells. ACS Nano 4 (3), 1399–1408. doi:10.1021/nn9011225
Li, Z., Yin, X., Lyu, C., Wang, J., Liu, K., Cui, S., et al. (2023b). Zinc oxide nanoparticles trigger autophagy in the human multiple myeloma cell line RPMI8226: an in vitro study. Biol. Trace Elem. Res. 202, 913–926. doi:10.1007/s12011-023-03737-6
Liao, D., Wang, M., Liao, Y., Li, J., and Niu, T. (2019). A review of efficacy and safety of checkpoint inhibitor for the treatment of acute myeloid leukemia. Front. Pharmacol. 10, 609. doi:10.3389/fphar.2019.00609
Liu, D., Qi, X., Wei, X., Zhao, L., Wang, X., Li, S., et al. (2022a). A Novel Her2/VEGFR2/CD3 trispecific antibody with an optimal structural design showed improved T-cell-redirecting antitumor efficacy. Theranostics 12 (18), 7788–7803. doi:10.7150/thno.75037
Liu, Q., Dai, G., Wu, Y., Zhang, M., Yang, M., Wang, X., et al. (2022b). iRGD-modified exosomes-delivered BCL6 siRNA inhibit the progression of diffuse large B-cell lymphoma. Front. Oncol. 12, 822805. doi:10.3389/fonc.2022.822805
Liu, Y.-Q., Wang, X.-L., He, D.-H., and Cheng, Y.-X. (2021). Protection against chemotherapy-and radiotherapy-induced side effects: a review based on the mechanisms and therapeutic opportunities of phytochemicals. Phytomedicine 80, 153402. doi:10.1016/j.phymed.2020.153402
Luk, B. T., Fang, R. H., Hu, C.-M. J., Copp, J. A., Thamphiwatana, S., Dehaini, D., et al. (2016). Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics 6 (7), 1004–1011. doi:10.7150/thno.14471
Luo, C., Deng, Z., Li, L., Clayton, F., Chen, A. L., Wei, R., et al. (2016). Association of rituximab with graphene oxide confers direct cytotoxicity for CD20-positive lymphoma cells. Oncotarget 7 (11), 12806–12822. doi:10.18632/oncotarget.7230
Ma, W., Zhan, Y., Zhang, Y., Mao, C., Xie, X., and Lin, Y. (2021). The biological applications of DNA nanomaterials: current challenges and future directions. Signal Transduct. Target. Ther. 6 (1), 351. doi:10.1038/s41392-021-00727-9
Majhail, N. S., Farnia, S. H., Carpenter, P. A., Champlin, R. E., Crawford, S., Marks, D. I., et al. (2015). Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transpl. 21 (11), 1863–1869. doi:10.1016/j.bbmt.2015.07.032
Mandal, T., Beck, M., Kirsten, N., Lindén, M., and Buske, C. (2018). Targeting murine leukemic stem cells by antibody functionalized mesoporous silica nanoparticles. Sci. Rep. 8 (1), 989. doi:10.1038/s41598-017-18932-4
Maruhashi, T., Okazaki, I.-M., Sugiura, D., Takahashi, S., Maeda, T. K., Shimizu, K., et al. (2018). LAG-3 inhibits the activation of CD4+ T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII. Nat. Immunol. 19 (12), 1415–1426. doi:10.1038/s41590-018-0217-9
Mclafferty, E., Hendry, C., and Farley, A. (2012). The lymphatic system. Nurs. Stand. 27, 37–42. doi:10.7748/ns2012.12.27.15.37.c9482
Mehranfar, S., Zeinali, S., Hosseini, R., Akbarzadeh, A., Mohammadian, M., and Feizi, A. H. P. (2017). History of leukemia: diagnosis and treatment from beginning to now. Galen. Med. J. 6 (1). doi:10.31661/gmj.v6i1.702
Mehrotra, N., Anees, M., Tiwari, S., Kharbanda, S., and Singh, H. (2023). Polylactic acid based polymeric nanoparticle mediated co-delivery of navitoclax and decitabine for cancer therapy. Nanomedicine 47, 102627. doi:10.1016/j.nano.2022.102627
Min, Q.-H., Wang, X.-Z., Zhang, J., Chen, Q.-G., Li, S.-Q., Liu, X.-Q., et al. (2018). Exosomes derived from imatinib-resistant chronic myeloid leukemia cells mediate a horizontal transfer of drug-resistant trait by delivering miR-365. Exp. Cell Res. 362 (2), 386–393. doi:10.1016/j.yexcr.2017.12.001
Mondesir, J., Willekens, C., Touat, M., and De Botton, S. (2016). IDH1 and IDH2 mutations as novel therapeutic targets: current perspectives. J. Blood Med. 7, 171–180. doi:10.2147/JBM.S70716
Morschhauser, F., Radford, J., Van Hoof, A., Botto, B., Rohatiner, A. Z., Salles, G., et al. (2013). 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: updated results after a median follow-up of 7.3 years from the International, Randomized, Phase III First-LineIndolent trial. J. Clin. Oncol. 31 (16), 1977–1983. doi:10.1200/jco.2012.45.6400
Mourya, V., Inamdar, N., Nawale, R., and Kulthe, S. (2011). Polymeric micelles: general considerations and their applications. Indian J. Pharm. Educ. Res. 45 (2), 128–138.
Nguyen, P. H. D., Jayasinghe, M. K., Le, A. H., Peng, B., and Le, M. T. (2023). Advances in drug delivery systems based on red blood cells and their membrane-derived nanoparticles. ACS Nano 17 (6), 5187–5210. doi:10.1021/acsnano.2c11965
Norsworthy, K. J., Luo, L., Hsu, V., Gudi, R., Dorff, S. E., Przepiorka, D., et al. (2019). FDA approval summary: ivosidenib for relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase-1 mutation. Clin. Cancer. Res. 25 (11), 3205–3209. doi:10.1158/1078-0432.CCR-18-3749
Nygren, P. (2001). What is cancer chemotherapy? Acta Oncol. 40 (2-3), 166–174. doi:10.1080/02841860151116204
Oberschmidt, O., Kloess, S., and Koehl, U. (2017). Redirected primary human chimeric antigen receptor natural killer cells as an “off-the-shelf immunotherapy” for improvement in cancer treatment. Front. Immunol. 8, 654. doi:10.3389/fimmu.2017.00654
Odenike, O. (2017). Incorporating novel approaches in the management of MDS beyond conventional hypomethylating agents. Hematol. Am. Soc. Hematol. Educ. Program 2017 (1), 460–469. doi:10.1182/asheducation-2017.1.460
Oroojalian, F., Beygi, M., Baradaran, B., Mokhtarzadeh, A., and Shahbazi, M. A. (2021). Immune cell membrane-coated biomimetic nanoparticles for targeted cancer therapy. Small 17 (12), 2006484. doi:10.1002/smll.202006484
Oun, R., Moussa, Y. E., and Wheate, N. J. (2018). The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. 47 (19), 6645–6653. doi:10.1039/c8dt00838h
Overdijk, M. B., Jansen, J. H., Nederend, M., Lammerts Van Bueren, J. J., Groen, R. W., Parren, P. W., et al. (2016). The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via fcγ receptor-mediated cross-linking. J. Immunol. 197 (3), 807–813. doi:10.4049/jimmunol.1501351
Pal Singh, S., Dammeijer, F., and Hendriks, R. W. (2018). Role of Bruton's tyrosine kinase in B cells and malignancies. Mol. Cancer 17 (1), 57. doi:10.1186/s12943-018-0779-z
Palumbo, A., Chanan-Khan, A., Weisel, K., Nooka, A. K., Masszi, T., Beksac, M., et al. (2016). Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N. Engl. J. Med. 375 (8), 754–766. doi:10.1056/NEJMoa1606038
Parisi, F., Fonti, N., Millanta, F., Freer, G., Pistello, M., and Poli, A. (2023). Exploring the link between viruses and cancer in companion animals: a comprehensive and comparative analysis. Infect. Agent. Cancer 18 (1), 40. doi:10.1186/s13027-023-00518-7
Pati, H., and Kundil Veetil, K. (2019). Myelodysplastic syndrome/myeloproliferative neoplasm (MDS/MPN) overlap syndromes: molecular pathogenetic mechanisms and their implications. Indian J. Hematol. Blood Transfus. 35, 3–11. doi:10.1007/s12288-019-01084-y
Paul, M. K., and Mukhopadhyay, A. K. (2004). Tyrosine kinase–role and significance in cancer. Int. J. Med. Sci. 1 (2), 101–115. doi:10.7150/ijms.1.101
Peng, Y., Bariwal, J., Kumar, V., Tan, C., and Mahato, R. I. (2020). Organic nanocarriers for delivery and targeting of therapeutic agents for cancer treatment. Adv. Ther. 3 (2), 1900136. doi:10.1002/adtp.201900136
Pericole, F. V., Lazarini, M., De Paiva, L. B., Duarte, A. D. S. S., Vieira Ferro, K. P., Niemann, F. S., et al. (2019). BRD4 inhibition enhances azacitidine efficacy in acute myeloid leukemia and myelodysplastic syndromes. Front. Oncol. 9, 16. doi:10.3389/fonc.2019.00016
Pfefferle, A., and Huntington, N. D. (2020). You have got a fast CAR: chimeric antigen receptor NK cells in cancer therapy. Cancers 12 (3), 706. doi:10.3390/cancers12030706
Pierpont, T. M., Limper, C. B., and Richards, K. L. (2018). Past, present, and future of rituximab—the world’s first oncology monoclonal antibody therapy. Front. Oncol. 8, 163. doi:10.3389/fonc.2018.00163
Poudel, K., Banstola, A., Gautam, M., Soe, Z., Phung, C. D., Pham, L. M., et al. (2020). Macrophage-membrane-camouflaged disintegrable and excretable nanoconstruct for deep tumor penetration. ACS Appl. Mat. Interfaces 12 (51), 56767–56781. doi:10.1021/acsami.0c17235
Powsner, E. H., Harris, J. C., and Day, E. S. (2021). Biomimetic nanoparticles for the treatment of hematologic malignancies. Adv. NanoBiomed Res. 1 (4), 2000047. doi:10.1002/anbr.202000047
Qu, Y., Chu, B., Wei, X., Chen, Y., Yang, Y., Hu, D., et al. (2022). Cancer-cell-biomimetic nanoparticles for targeted therapy of multiple myeloma based on bone marrow homing. Adv. Mat. 34 (46), e2107883. doi:10.1002/adma.202107883
Ramos, J. C., Sparano, J. A., Chadburn, A., Reid, E. G., Ambinder, R. F., Siegel, E. R., et al. (2020). Impact of Myc in HIV-associated non-Hodgkin lymphomas treated with EPOCH and outcomes with vorinostat (AMC-075 trial). Blood 136 (11), 1284–1297. doi:10.1182/blood.2019003959
Rao, L., Bu, L. L., Xu, J. H., Cai, B., Yu, G. T., Yu, X., et al. (2015). Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small 11 (46), 6225–6236. doi:10.1002/smll.201502388
Rao, L., Wu, L., Liu, Z., Tian, R., Yu, G., Zhou, Z., et al. (2020). Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat. Commun. 11 (1), 4909. doi:10.1038/s41467-020-18626-y
Rhyasen, G. W., Hattersley, M. M., Yao, Y., Dulak, A., Wang, W., Petteruti, P., et al. (2016). AZD5153: a novel bivalent BET bromodomain inhibitor highly active against hematologic malignancies. Mol. Cancer Ther. 15 (11), 2563–2574. doi:10.1158/1535-7163.MCT-16-0141
Ribrag, V., Avigan, D. E., Green, D. J., Wise-Draper, T., Posada, J. G., Vij, R., et al. (2019). Phase 1b trial of pembrolizumab monotherapy for relapsed/refractory multiple myeloma: KEYNOTE-013. Br. J. Haematol. 186 (3), e41–e44. doi:10.1111/bjh.15888
Riezzo, I., Pascale, N., La Russa, R., Liso, A., Salerno, M., and Turillazzi, E. (2017). Donor selection for allogenic hemopoietic stem cell transplantation: clinical and ethical considerations. Stem Cells Int. 2017, 5250790. doi:10.1155/2017/5250790
Roberts, A. W. (2020). Therapeutic development and current uses of BCL-2 inhibition. Hematol. Am. Soc. Hematol. Educ. Program 2020 (1), 1–9. doi:10.1182/hematology.2020000154
Roberts, A. W., Davids, M. S., Pagel, J. M., Kahl, B. S., Puvvada, S. D., Gerecitano, J. F., et al. (2016). Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. New Engl. J. Med. 374 (4), 311–322. doi:10.1056/NEJMoa1513257
Salik, B., Smyth, M. J., and Nakamura, K. (2020). Targeting immune checkpoints in hematological malignancies. J. Hematol. 13 (1), 111–119. doi:10.1186/s13045-020-00947-6
Salles, G., Barrett, M., Foà, R., Maurer, J., O'brien, S., Valente, N., et al. (2017). Rituximab in B-cell hematologic malignancies: a review of 20 Years of clinical experience. Adv. Ther. 34 (10), 2232–2273. doi:10.1007/s12325-017-0612-x
Salles, G., Duell, J., González Barca, E., Tournilhac, O., Jurczak, W., Liberati, A. M., et al. (2020). Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet. Oncol. 21 (7), 978–988. doi:10.1016/s1470-2045(20)30225-4
Sang, W., Wang, X., Geng, H., Li, T., Li, D., Zhang, B., et al. (2022). Anti-PD-1 therapy enhances the efficacy of CD30-directed chimeric antigen receptor T cell therapy in patients with relapsed/refractory CD30+ lymphoma. Front. Immunol. 13, 858021. doi:10.3389/fimmu.2022.858021
Santos, F. P., Kantarjian, H., Garcia-Manero, G., Issa, J.-P., and Ravandi, F. (2010). Decitabine in the treatment of myelodysplastic syndromes. Expert Rev. Anticancer Ther. 10 (1), 9–22. doi:10.1586/era.09.164
Saulnier, N., Di Campli, C., Zocco, M. A., Di Gioacchino, G., Novi, M., and Gasbarrini, A. (2005). From stem cell to solid organ. Bone marrow, peripheral blood or umbilical cord blood as favorable source? Eur. Rev. Med. Pharmacol. Sci. 9 (6), 315–324.
Scharman, C. D., Burger, D., Shatzel, J. J., Kim, E., and Deloughery, T. G. (2017). Treatment of individuals who cannot receive blood products for religious or other reasons. Am. J. Hematol. 92 (12), 1370–1381. doi:10.1002/ajh.24889
Schenk, P. W., and Snaar-Jagalska, B. E. (1999). Signal perception and transduction: the role of protein kinases. Biochim. Biophys. Acta 1449 (1), 1–24. doi:10.1016/S0167-4889(98)00178-5
Shahabadi, N., Falsafi, M., and Mansouri, K. (2016). Improving antiproliferative effect of the anticancer drug cytarabine on human promyelocytic leukemia cells by coating on Fe3O4@SiO2 nanoparticles. Colloids Surf. B. Biointerfaces 141, 213–222. doi:10.1016/j.colsurfb.2016.01.054
Shimada, A. (2019). Hematological malignancies and molecular targeting therapy. Eur. J. Pharmacol. 862, 172641. doi:10.1016/j.ejphar.2019.172641
Si, J., Shao, S., Shen, Y., and Wang, K. (2016). Macrophages as active nanocarriers for targeted early and adjuvant cancer chemotherapy. Small 12 (37), 5108–5119. doi:10.1002/smll.201601282
Simon, T., Tomuleasa, C., Bojan, A., Berindan-Neagoe, I., Boca, S., and Astilean, S. (2015). Design of FLT3 inhibitor-gold nanoparticle conjugates as potential therapeutic agents for the treatment of acute myeloid leukemia. Nanoscale Res. Lett. 10 (1), 466. doi:10.1186/s11671-015-1154-2
Singh, P., Kim, Y.-J., Zhang, D., and Yang, D.-C. (2016). Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 34 (7), 588–599. doi:10.1016/j.tibtech.2016.02.006
Sivakumar, N. A., Sukumaran, G., and Ganapathy, D. (2019). Stem-cell therapy for leukemia. Drug Invent. Today 11 (9).
Sochacka-Ćwikła, A., Mączyński, M., and Regiec, A. (2021). FDA-approved drugs for hematological malignancies—the last decade review. Cancers 14 (1), 87. doi:10.3390/cancers14010087
Song, Y., Zhang, L., Wang, Y., Han, M., Wang, Z., Wang, N., et al. (2023). A bimetallic metal-organic-framework-based biomimetic nanoplatform enhances anti-leukemia immunity via synchronizing DNA demethylation and RNA hypermethylation. Adv. Mat. 35 (16), e2210895. doi:10.1002/adma.202210895
Stein, E. M., Dinardo, C. D., Fathi, A. T., Mims, A. S., Savona, M. R., Stein, A. S., et al. (2021). Ivosidenib or enasidenib combined with intensive chemotherapy in patients with newly diagnosed AML: a phase 1 study. Blood 138, 1792–1803. doi:10.1182/blood.2020007233
Subhan, M. A., Yalamarty, S. S. K., Filipczak, N., Parveen, F., and Torchilin, V. P. (2021). Recent advances in tumor targeting via EPR effect for cancer treatment. J. Pers. Med. 11 (6), 571. doi:10.3390/jpm11060571
Sun, H., Zhang, Z., Kang, X., Dai, Q., Song, A., Hao, J., et al. (2021). Biologically-derived nanoparticles for chemo-ferroptosis combination therapy. Mat. Chem. Front. 5 (10), 3813–3822. doi:10.1039/D1QM00295C
Tapia-Galisteo, A., Álvarez-Vallina, L., and Sanz, L. (2023). Bi- and trispecific immune cell engagers for immunotherapy of hematological malignancies. J. Hematol. Oncol. 16 (1), 83. doi:10.1186/s13045-023-01482-w
Tariq, S. M., Haider, S. A., Hasan, M., Tahir, A., Khan, M., Rehan, A., et al. (2018). Chimeric antigen receptor T-cell therapy: a beacon of hope in the fight against cancer. Cureus 10 (10), e3486. doi:10.7759/cureus.3486
Tharmalingam, S., Sreetharan, S., Brooks, A. L., and Boreham, D. R. (2019). Re-evaluation of the linear no-threshold (LNT) model using new paradigms and modern molecular studies. Chem.-Biol. Interact. 301, 54–67. doi:10.1016/j.cbi.2018.11.013
Tian, Z., Liu, M., Zhang, Y., and Wang, X. (2021). Bispecific T cell engagers: an emerging therapy for management of hematologic malignancies. J. Hematol. Oncol. 14 (1), 75. doi:10.1186/s13045-021-01084-4
Tipton, T. R., Mockridge, C. I., French, R. R., Tutt, A. L., Cragg, M. S., and Beers, S. A. (2015). Anti-mouse FcγRIV antibody 9E9 also blocks FcγRIII in vivo. Blood 126 (24), 2643–2645. doi:10.1182/blood-2015-09-671339
Upadhyay, V. A., Brunner, A. M., and Fathi, A. T. (2017). Isocitrate dehydrogenase (IDH) inhibition as treatment of myeloid malignancies: progress and future directions. Pharmacol. Ther. 177, 123–128. doi:10.1016/j.pharmthera.2017.03.003
Vafa, E., and Bazargan-Lari, R. (2021). Bovine serum albumin protected gold nanozymes as a novel anti-cancer nanodrug for acute T-type lymphoblastic leukemia treatment via effect on the expression of anti-apoptotic genes. Appl. Biol. Chem. 64 (1), 86. doi:10.1186/s13765-021-00659-6
Van De Donk, N., and Usmani, S. Z. (2018). CD38 antibodies in multiple myeloma: mechanisms of action and modes of resistance. Front. Immunol. 9, 2134. doi:10.3389/fimmu.2018.02134
Van Den Berg, M., Winkels, R., De Kruif, J. T. C., Van Laarhoven, H., Visser, M., De Vries, J., et al. (2017). Weight change during chemotherapy in breast cancer patients: a meta-analysis. BMC cancer 17 (1), 259. doi:10.1186/s12885-017-3242-4
Van Manen, L., Peters, A. L., Van Der Sluijs, P. M., Nieuwland, R., Van Bruggen, R., and Juffermans, N. P. (2019). Clearance and phenotype of extracellular vesicles after red blood cell transfusion in a human endotoxemia model. Transfus. Apher. Sci. 58 (4), 508–511. doi:10.1016/j.transci.2019.05.008
Vey, N., Bourhis, J.-H., Boissel, N., Bordessoule, D., Prebet, T., Charbonnier, A., et al. (2012). A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood 120 (22), 4317–4323. doi:10.1182/blood-2012-06-437558
Wang, D., Wang, S., Zhou, Z., Bai, D., Zhang, Q., Ai, X., et al. (2022). White blood cell membrane-coated nanoparticles: recent development and medical applications. Adv. Healthc. Mat. 11 (7), 2101349. doi:10.1002/adhm.202101349
Wang, F., Li, C., Cheng, J., and Yuan, Z. (2016). Recent advances on inorganic nanoparticle-based cancer therapeutic agents. Int. J. Env. Res. Public Health 13 (12), 1182. doi:10.3390/ijerph13121182
Wang, H., Wu, J., Williams, G. R., Fan, Q., Niu, S., Wu, J., et al. (2019). Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J. Nanobiotechnol. 17, 60–16. doi:10.1186/s12951-019-0494-y
Wang, M., and Thanou, M. (2010). Targeting nanoparticles to cancer. Pharmacol. Res. 62 (2), 90–99. doi:10.1016/j.phrs.2010.03.005
Wei, J., Liu, Y., Wang, C., Zhang, Y., Tong, C., Dai, G., et al. (2020). The model of cytokine release syndrome in CAR T-cell treatment for B-cell non-Hodgkin lymphoma. Signal Transduct. Target. Ther. 5 (1), 134. doi:10.1038/s41392-020-00256-x
Wei, M., Wang, X., Song, Z., Jiao, M., Ding, J., Meng, L. H., et al. (2015). Targeting PI3Kδ: emerging therapy for chronic lymphocytic leukemia and beyond. Med. Res. Rev. 35 (4), 720–752. doi:10.1002/med.21341
Weiner, L. M., Dhodapkar, M. V., and Ferrone, S. (2009). Monoclonal antibodies for cancer immunotherapy. Lancet 373 (9668), 1033–1040. doi:10.1016/s0140-6736(09)60251-8
Wierda, W. G., Kipps, T. J., Mayer, J., Stilgenbauer, S., Williams, C. D., Hellmann, A., et al. (2010). Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J. Clin. Oncol. 28 (10), 1749–1755. doi:10.1200/JCO.2009.25.3187
Wu, S., and Singh, R. K. (2011). Resistance to chemotherapy and molecularly targeted therapies: rationale for combination therapy in malignant melanoma. Curr. Mol. Med. 11 (7), 553–563. doi:10.2174/156652411800615153
Wu, Y., Wan, S., Yang, S., Hu, H., Zhang, C., Lai, J., et al. (2022). Macrophage cell membrane-based nanoparticles: a new promising biomimetic platform for targeted delivery and treatment. J. Nanobiotechnol. 20 (1), 542. doi:10.1186/s12951-022-01746-6
Wulf, G. G., Altmann, B., Ziepert, M., D'amore, F., Held, G., Greil, R., et al. (2021). Alemtuzumab plus CHOP versus CHOP in elderly patients with peripheral T-cell lymphoma: the DSHNHL2006-1B/ACT-2 trial. Leukemia 35 (1), 143–155. doi:10.1038/s41375-020-0838-5
Xia, Q., Zhang, Y., Li, Z., Hou, X., and Feng, N. (2019). Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm. Sin. B 9 (4), 675–689. doi:10.1016/j.apsb.2019.01.011
Xiao, X., Teng, F., Shi, C., Chen, J., Wu, S., Wang, B., et al. (2022). Polymeric nanoparticles-Promising carriers for cancer therapy. Front. Bioeng. Biotechnol. 10, 1024143. doi:10.3389/fbioe.2022.1024143
Xuan, L., Ju, Z., Skonieczna, M., Zhou, P. K., and Huang, R. (2023). Nanoparticles-induced potential toxicity on human health: applications, toxicity mechanisms, and evaluation models. MedComm 4 (4), e327. doi:10.1002/mco2.327
Yaman, S., Chintapula, U., Rodriguez, E., Ramachandramoorthy, H., and Nguyen, K. T. (2020). Cell-mediated and cell membrane-coated nanoparticles for drug delivery and cancer therapy. Cancer Drug Resist 3 (4), 879–911. doi:10.20517/cdr.2020.55
Younes, A., Bartlett, N. L., Leonard, J. P., Kennedy, D. A., Lynch, C. M., Sievers, E. L., et al. (2010). Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. New Engl. J. Med. 363 (19), 1812–1821. doi:10.1056/NEJMoa1002965
Zeinabad, H. A., Yeoh, W. J., Arif, M., Lomora, M., Banz, Y., Riether, C., et al. (2023). Natural killer cell-mimic nanoparticles can actively target and kill acute myeloid leukemia cells. Biomaterials 298, 122126. doi:10.1016/j.biomaterials.2023.122126
Zhang, F., Yang, Q., Tang, S., Jiang, S., Zhao, Q., Li, J., et al. (2023). CD38-targeted and erythrocyte membrane camouflaged nanodrug delivery system for photothermal and chemotherapy in multiple myeloma. Int. J. Pharm. 643, 123241. doi:10.1016/j.ijpharm.2023.123241
Zhang, M., Du, Y., Wang, S., and Chen, B. (2020). A review of biomimetic nanoparticle drug delivery systems based on cell membranes. Drug Des. devel. Ther. 14, 5495–5503. doi:10.2147/DDDT.S282368
Zhang, Y., Liu, G., Wei, J., and Nie, G. (2018). Platelet membrane-based and tumor-associated platelettargeted drug delivery systems for cancer therapy. Front. Med. 12, 667–677. doi:10.1007/s11684-017-0583-y
Zhang, Y., Xia, Q., Wu, T., He, Z., Li, Y., Li, Z., et al. (2021). A novel multi-functionalized multicellular nanodelivery system for non-small cell lung cancer photochemotherapy. J. Nanobiotechnol. 19 (1), 245. doi:10.1186/s12951-021-00977-3
Zhao, A., Zhou, H., Yang, J., Li, M., and Niu, T. (2023). Epigenetic regulation in hematopoiesis and its implications in the targeted therapy of hematologic malignancies. Signal Transduct. target. Ther. 8 (1), 71. doi:10.1038/s41392-023-01342-6
Zhao, J., Ruan, J., Lv, G., Shan, Q., Fan, Z., Wang, H., et al. (2022). Cell membrane-based biomimetic nanosystems for advanced drug delivery in cancer therapy: a comprehensive review. Colloids Surf. B. Biointerfaces 215, 112503. doi:10.1016/j.colsurfb.2022.112503
Zhao, Q., Jiang, D., Sun, X., Mo, Q., Chen, S., Chen, W., et al. (2021a). Biomimetic nanotherapy: core–shell structured nanocomplexes based on the neutrophil membrane for targeted therapy of lymphoma. J. Nanobiotechnol. 19 (1), 179. doi:10.1186/s12951-021-00922-4
Zhao, Q., Sun, X., Wu, B., Shang, Y., Huang, X., Dong, H., et al. (2021b). Construction of homologous cancer cell membrane camouflage in a nano-drug delivery system for the treatment of lymphoma. J. Nanobiotechnol. 19, 8–19. doi:10.1186/s12951-020-00738-8
Zhong, Y., Meng, F., Zhang, W., Li, B., Van Hest, J. C., and Zhong, Z. (2020). CD44-targeted vesicles encapsulating granzyme B as artificial killer cells for potent inhibition of human multiple myeloma in mice. J. Control. Release 320, 421–430. doi:10.1016/j.jconrel.2020.02.004
Zhu, L., Zhong, Y., Wu, S., Yan, M., Cao, Y., Mou, N., et al. (2022). Cell membrane camouflaged biomimetic nanoparticles: focusing on tumor theranostics. Mater. today. Bio 14, 100228. doi:10.1016/j.mtbio.2022.100228
Zhu, M., Wu, B., Brandl, C., Johnson, J., Wolf, A., Chow, A., et al. (2016). Blinatumomab, a bispecific T-cell engager (BiTE(®)) for CD-19 targeted cancer immunotherapy: clinical Pharmacology and its implications. Clin. Pharmacokinet. 55, 1271–1288. doi:10.1007/s40262-016-0405-4
Zou, M. Z., Liu, W. L., Gao, F., Bai, X. F., Chen, H. S., Zeng, X., et al. (2019). Artificial natural killer cells for specific tumor inhibition and renegade macrophage re-education. Adv. Mat. 31 (43), 1904495. doi:10.1002/adma.201904495
Keywords: hematologic malignancies, clinical regimens, nanocarrier, nanodrug delivery system, cell membrane camouflage
Citation: Liu Y, Yu S, Chen Y, Hu Z, Fan L and Liang G (2024) The clinical regimens and cell membrane camouflaged nanodrug delivery systems in hematologic malignancies treatment. Front. Pharmacol. 15:1376955. doi: 10.3389/fphar.2024.1376955
Received: 26 January 2024; Accepted: 02 April 2024;
Published: 16 April 2024.
Edited by:
Feifei Yang, University of Jinan, ChinaCopyright © 2024 Liu, Yu, Chen, Hu, Fan and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaofeng Liang, lgfeng990448@haust.edu.cn
 Yuanyuan Liu
Yuanyuan Liu Shanwu Yu2
Shanwu Yu2 Yixiang Chen
Yixiang Chen Lingling Fan
Lingling Fan Gaofeng Liang
Gaofeng Liang