- 1Department of Hematology and Oncology, Jiujiang University Affiliated Hospital, Jiujiang, China
- 2Department of Oncology, The First Affiliated Hospital of Ningbo University, Ningbo, China
1 Introduction
Multiple myeloma (MM) is the second most common hematologic cancer, with an estimated 35,730 new cases and 12,590 deaths annually in the United States (Siegel et al., 2023). With the introduction of new drugs, the number of drug combinations for MM treatment is constantly increasing. The 5-year overall survival (OS) rate for newly diagnosed multiple myeloma (NDMM) is 48.5% (Rajkumar, 2022). The National Comprehensive Cancer Network guideline (Ver. 2024.1) recommends triplet regimens [such as bortezomib + lenalidomide + dexamethasone (VRd) and daratumumab + lenalidomide + dexamethasone (DRd)] as the initial treatment for MM. Evidence has shown that for NDMM, triplet regimens achieve better efficacy than two-drug regimens (Derman et al., 2022; Yang et al., 2022). Currently, the triplet regimen has become the standard first-line therapy for NDMM. Recent clinical studies have explored the efficacy of quadruplet regimens in treating patients with NDMM (Table 1). In addition to studying the three categories of agents, namely, immunomodulatory drug, proteasome inhibitor, and steroid, efforts have also been devoted to study the anti-CD38 (daratumumab and isatuximab) and anti-SLAMF7 antibodies (elotuzumab). This opens a new possibility for analyzing the efficacy of quadruplet therapy as an alternative first-line treatment for NDMM. Based on concomitant cytogenetic abnormalities, NDMM patients can be classified into standard-risk (SR) and high-risk (HiR) subgroups (Rajkumar, 2022). This study aims to answer the following question: Is the quadruplet regimen better than the triplet regimen for treating patients with NDMM?
2 Controversy about the use of quadruplet regimens for treating SR NDMM patients
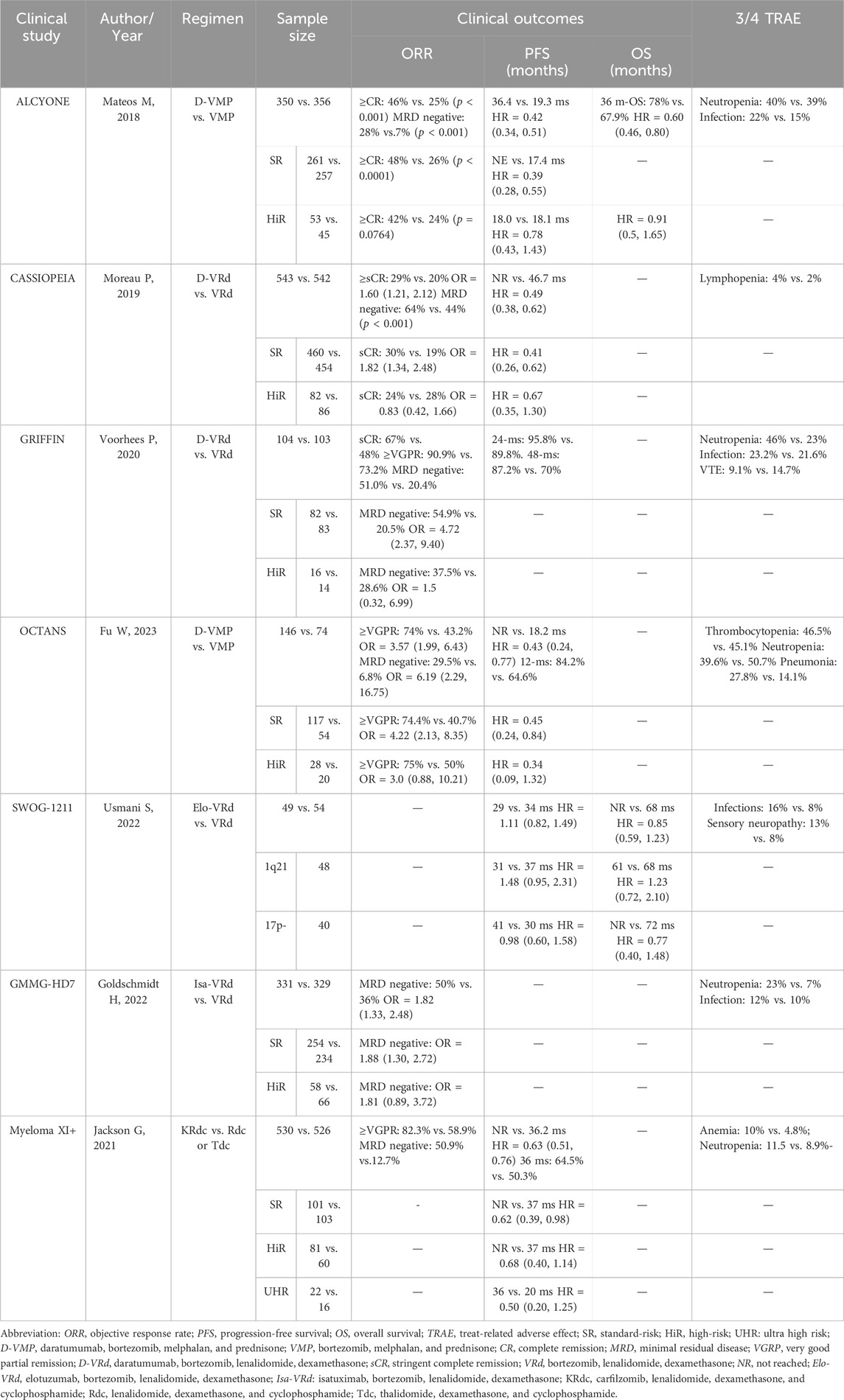
Approximately 80% of NDMM patients belong to the SR subgroup, with a median OS ranging from 8 to 10 years (Goel et al., 2022). Recent studies have explored the efficacy of quadruplet regimens containing anti-CD38 antibodies in treating NDMM patients. As shown in Table 1, except for the SWOG-1211 study (Usmani et al., 2022), the other six prospective clinical studies, namely, ALCYONE (Mateos et al., 2020), CASSIOPEIA (Moreau et al., 2021), GRIFFIN (Voorhees et al., 2023), OCTANS (Fu et al., 2023), GMMG-HD7 (Goldschmidt et al., 2022), and Myeloma XI+ (Jackson et al., 2021), revealed that for SR NDMM patients, quadruplet regimens achieved better overall response rates (ORRs), longer progression-free survival (PFS), and higher rates of minimal residual disease (MRD) negativity than corresponding triplet regimens. Of note, the status of MRD represents the depth of post-therapeutic remission and serves as an independent prognostic factor for NDMM patients (San-Miguel et al., 2022). It seems logical that increased MRD negativity by quadruplet regimens containing anti-CD38 antibodies will result in prolonged OS of SR NDMM patients. Therefore, it is rational to use quadruplet regimens containing anti-CD38 antibodies for SR NDMM patients due to their favorable efficacy compared with that of the triplet regimen (VRd).
However, as shown in Table 1, the data on OS of the five studies are not yet available, except for the favorable OS benefit in the ALCYONE study (Mateos et al., 2020). On the contrary, the SWOG-1211 study reported no survival advantage from the quadruplet regimen of Elo-VRd over the VRd regimen (Usmani et al., 2022). Furthermore, an issue that cannot be ignored is the incremental cost-effectiveness ratio (ICER). According to a recent investigation, daratumumab + bortezomib + melphalan + predisone (D-VMP) vs. bortezomib + melphalan + predisone (VMP) has a 90.8% probability of being cost-effective at the $150,000/quality-adjusted life year willingness-to-pay threshold (Zeng et al., 2021). Compared with VMP, D-VMP may exceed the commonly accepted values of ICER in patients with NDMM in China. Thus, it is necessary to consider the cost-effectiveness of the quadruplet regimen for SR NDMM patients, especially in developing countries.
3 Controversy about the use of quadruplet regimens for treating HiR NDMM patients
Nearly 20% of patients with NDMM belong to the HiR subgroup, with features including del (17p), t (4:14), t (14:16), t (14; 20), TP53 mutation, R-ISS stage III, gain (1q) (identified using cytogenetic/fluorescence in situ hybridization analysis), high plasma cell S-phase, and HiR signature of gene expression profiling. This group also contains an ultra-high risk (UHR, i.e., double/triple-hit) subgroup. Compared with the SR NDMM subgroup, the HiR NDMM subgroup has a predicted OS of less than 3 years (Zamagni et al., 2022). According to the meta-analysis by Giri et al. (2020), incorporating daratumumab into primary regimens may improve PFS [pooled hazard ratio (HR) = 0.67, 95% confidence interval (CI): 0.47–0.95] in HiR NDMM patients. However, in the studies of COSSIPEIA and ALCYONE (Table 1), statistically significant benefits were not yet seen with the addition of daratumumab as a fourth drug to a triple-drug regimen in newly-diagnosed HiRMM. The MAIA study compared the efficacy of regimens DRd and Rd (HR = 0.53, 95% CI: 0.43–0.66, p < 0.001; HR = 0.68, 95% CI: 0.53–0.86, p = 0.0013) (Facon et al., 2021; Facon et al., 2023). Furthermore, the small sample size (only 317 in total) might have reduced the statistical power of the meta-analysis in cases of HiR NDMM. According to the results above, Mohyuddin et al. (2021) held that it is prudent to routinely use a daratumumab-based regimen for HiR NDMM patients.
As described in Table 1, in six studies, all of the subgroup analyses of HiR NDMM revealed that compared with triplet regimens, quadruplet schemes failed to yield a statistically favorable clinical outcome, including ORR, PFS, and MRD-negative rate. At this point, caution should be exercised when choosing a quadruplet regimen as the first-line treatment for HiR NDMM patients until we have OS data to justify additional adverse effects and potential long-term costs. The Myeloma XI + study found that UHR NDMM patients on the KRdc quadruplet regimen had a longer PFS than those on the Rdc or Tdc triple regimen but without any statistical difference (Jackson et al., 2021). These results strongly indicate that it is premature to recommend the use of quadruplet regimens for HiR NDMM patients.
4 Expert opinion
A network meta-analysis by Facon et al. (2022) showed that daratumumab-based regimens, including D-Rd, D-VMP, and VRd, had the highest probabilities of being more effective than Rd continuous in terms of PFS (HR: D-Rd, 0.53; D-VMP, 0.57; VRd, 0.77) and OS (HR: D-Rd, 0.68; VRd, 0.77; D-VMP, 0.78) for NDMM patients. Among them, D-Rd ranked first as the most effective treatment in terms of PFS and OS. Given the excellent efficacy of triplet regimens such as D-Rd and VRd, we recommend careful consideration when choosing a quadruplet regimen as the first-line treatment for patients with NDMM. For the SR subgroup, the use of anti-CD38 antibody-based quadruplet treatment appears to be more effective than the triplet regimen. However, cost-effectiveness should be considered, particularly in developing countries. For the HiR subgroup, based on currently available evidence, the quadruplet treatment appears to be ineffective, as no superiority in efficacy has been found compared with that of the triplet regimen.
Author contributions
JD: Conceptualization, Investigation, Writing–original draft, Writing–review and editing. SG: Data curation, Investigation, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Derman, B. A., Kansagra, A., Zonder, J., Stefka, A. T., Grinblatt, D. L., Anderson, L. D., et al. (2022). Elotuzumab and weekly carfilzomib, lenalidomide, and dexamethasone in patients with newly diagnosed multiple myeloma without transplant intent: a phase 2 measurable residual disease-adapted study. JAMA Oncol. 8 (9), 1278–1286. doi:10.1001/jamaoncol.2022.2424
Facon, T., Kumar, S. K., Plesner, T., Orlowski, R. Z., Moreau, P., Bahlis, N., et al. (2021). Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. LANCET Oncol. 22 (11), 1582–1596. doi:10.1016/S1470-2045(21)00466-6
Facon, T., Kumar, S. K., Plesner, T., Orlowski, R. Z., Moreau, P., Bahlis, N., et al. (2023). Plain language summary of the MAIA study of daratumumab plus lenalidomide and dexamethasone for the treatment of people with newly diagnosed multiple myeloma. FUTURE Oncol. 19 (13), 887–895. doi:10.2217/fon-2023-0082
Facon, T., San-Miguel, J., Dimopoulos, M. A., Mateos, M. V., Cavo, M., van Beekhuizen, S., et al. (2022). Treatment regimens for transplant-ineligible patients with newly diagnosed multiple myeloma: a systematic literature review and network meta-analysis. Adv. Ther. 39 (5), 1976–1992. doi:10.1007/s12325-022-02083-8
Fu, W., Bang, S. M., Huang, H., Kim, K., Li, W., An, G., et al. (2023). Bortezomib, melphalan, and prednisone with or without daratumumab in transplant-ineligible asian patients with newly diagnosed multiple myeloma: the phase 3 OCTANS study. Cl. Lymph. MYELOM Leuk. 23 (6), 446–455.e4. doi:10.1016/j.clml.2023.02.009
Giri, S., Grimshaw, A., Bal, S., Godby, K., Kharel, P., Djulbegovic, B., et al. (2020). Evaluation of daratumumab for the treatment of multiple myeloma in patients with high-risk cytogenetic factors: a systematic review and meta-analysis. JAMA Oncol. 6 (11), 1759–1765. doi:10.1001/jamaoncol.2020.4338
Goel, U., Usmani, S., and Kumar, S. (2022). Current approaches to management of newly diagnosed multiple myeloma. Am. J. Hematol. 97 (Suppl. 1), S3–S25. doi:10.1002/ajh.26512
Goldschmidt, H., Mai, E. K., Bertsch, U., Fenk, R., Nievergall, E., Tichy, D., et al. (2022). Addition of isatuximab to lenalidomide, bortezomib, and dexamethasone as induction therapy for newly diagnosed, transplantation-eligible patients with multiple myeloma (GMMG-HD7): part 1 of an open-label, multicentre, randomised, active-controlled, phase 3 trial. LANCET Haematol. 9 (11), e810–e821. doi:10.1016/S2352-3026(22)00263-0
Jackson, G. H., Pawlyn, C., Cairns, D. A., de Tute, R. M., Hockaday, A., Collett, C., et al. (2021). Carfilzomib, lenalidomide, dexamethasone, and cyclophosphamide (KRdc) as induction therapy for transplant-eligible, newly diagnosed multiple myeloma patients (Myeloma XI+): interim analysis of an open-label randomised controlled trial. PLOS Med. 18 (1), e1003454. doi:10.1371/journal.pmed.1003454
Mateos, M. V., Cavo, M., Blade, J., Dimopoulos, M. A., Suzuki, K., Jakubowiak, A., et al. (2020). Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. LANCET 395 (10218), 132–141. doi:10.1016/S0140-6736(19)32956-3
Mohyuddin, G. R., Abdallah, A. O., and McClune, B. (2021). Caution with routine use of daratumumab for newly diagnosed high-risk multiple myeloma. JAMA Oncol. 7 (4), 635. doi:10.1001/jamaoncol.2020.8008
Moreau, P., Hulin, C., Perrot, A., Arnulf, B., Belhadj, K., Benboubker, L., et al. (2021). Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): an open-label, randomised, phase 3 trial. LANCET Oncol. 22 (10), 1378–1390. doi:10.1016/S1470-2045(21)00428-9
Rajkumar, S. V. (2022). Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 97 (8), 1086–1107. doi:10.1002/ajh.26590
San-Miguel, J., Avet-Loiseau, H., Paiva, B., Kumar, S., Dimopoulos, M. A., Facon, T., et al. (2022). Sustained minimal residual disease negativity in newly diagnosed multiple myeloma and the impact of daratumumab in MAIA and ALCYONE. BLOOD 139 (4), 492–501. doi:10.1182/blood.2020010439
Siegel, R. L., Miller, K. D., Wagle, N. S., and Jemal, A. (2023). Cancer statistics, 2023. CA-CANCER J. Clin. 73 (1), 17–48. doi:10.3322/caac.21763
Usmani, S. Z., Hoering, A., Ailawadhi, S., Sexton, R., Lipe, B., Valent, J. N., et al. (2022). Randomized phase II trial of bortezomib, lenalidomide, dexamthasone with/without elotuzumab for newly diagnosed, high risk multiple myeloma (SWOG-1211). J. Clin. Oncol. 40 (16), 8054. doi:10.1200/jco.2022.40.16_suppl.8054
Voorhees, P. M., Sborov, D. W., Laubach, J., Kaufman, J. L., Reeves, B., Rodriguez, C., et al. (2023). Addition of daratumumab to lenalidomide, bortezomib, and dexamethasone for transplantation-eligible patients with newly diagnosed multiple myeloma (GRIFFIN): final analysis of an open-label, randomised, phase 2 trial. LANCET Haematol. 10 (10), e825–e837. doi:10.1016/S2352-3026(23)00217-X
Yang, G., Geng, C., Jian, Y., Zhou, H., and Chen, W. (2022). Triplet RVd induction for transplant-eligible newly diagnosed multiple myeloma: a systematic review and meta-analysis. Adv. Ther. 39 (8), 3799–3834. doi:10.1007/s12325-022-02195-1
Zamagni, E., Barbato, S., and Cavo, M. (2022). How I treat high-risk multiple myeloma. BLOOD 139 (19), 2889–2903. doi:10.1182/blood.2020008733
Keywords: quadruplet regimen, newly-diagnosed, multiple myeloma, standard risk, high risk
Citation: Ding J and Gong S (2024) Quadruplet regimen for newly diagnosed multiple myeloma is effective in the standard-risk subgroup but not in the high-risk subgroup. Front. Pharmacol. 15:1398879. doi: 10.3389/fphar.2024.1398879
Received: 10 March 2024; Accepted: 29 April 2024;
Published: 09 May 2024.
Edited by:
Junmin Zhang, Lanzhou University, ChinaReviewed by:
Qiang Wang, Houston Methodist Research Institute, United StatesCopyright © 2024 Ding and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianghua Ding, doctor0922@126.com
†These authors have contributed equally to this work
 Jianghua Ding
Jianghua Ding Shengping Gong2†
Shengping Gong2†