- 1Laboratory of General Physiology, Department of Biology and Biotechnology “L. Spallanzani”, University of Pavia, Pavia, Italy
- 2Institute of Molecular Genetics IGM-CNR, Pavia, Italy
- 3Department of Life Sciences and Systems Biology, University of Turin, Turin, Italy
- 4Laboratory of Cellular and Molecular Physiology, Department of Medicine and Health Sciences “V. Tiberio”, University of Molise, Campobasso, Italy
The blood-brain barrier (BBB) plays a central role in maintaining the ionic milieu required for neuronal activity and in translating neuronal activity in a local elevation in cerebral blood flow (CBF). However, the molecular repertoire of the human BBB remains poorly defined. Here, we performed a systematic transcriptomic analysis of 672 genes using eight independent RNA-Seq datasets generated from the human brain endothelial cell line hCMEC/D3, the most widely used in vitro model of the human BBB. We focused on ion channels, ion transporters, G protein–coupled receptors (GPCRs), and Receptor Tyrosine Kinases (RTKs), which govern ionic homeostasis, barrier integrity, and CBF. Among the most abundantly expressed ion transporters were subunits of the mitochondrial F-type ATPase complex (F-type ATPase α subunit, F-type ATPase β subunit, F-type ATPase C subunit), reflecting the high metabolic demands of the BBB. Key regulators of intracellular Ca2+ homeostasis, including SERCA2, PMCA1/4, and SPCA1, were consistently detected, supporting efficient Ca2+ clearance across endoplasmic reticulum (ER), plasma membrane, and Golgi compartments. Our analysis of ion channels revealed a selective repertoire with prominent expression of Cl−-permeable channels (CLIC1/4, CLNS1A, VDAC1-3, VRAC) and various K+-permeable channels, including IKCa/KCa3.1, KIR2.1, KNa1.2, BKCa, KV4.1, and TREK-1. Na+-permeable channels (ENaC and NALCN), non-selective cation channels (TRP, HCN2/3), and ER- (InsP3Rs, TRICs, and putative leak channels), and lysosomes-associated (TRPML1 and TPCs) channels were also detected. Additionally, we identified transcripts for mechanosensitive channels (PIEZO1, TACAN, TMC7, TMEM63B) and gap junction proteins (Cx43, Cx45, Cx47), as well as a broad array of ionotropic and metabotropic receptors, including purinergic, adenosine, histamine, GABA, adrenergic and nicotinic receptors. Growth factor-related RTKs (FGFR, IGFR, EGFR, PDGFR, VEGFR) were consistently expressed, underscoring their role in angiogenesis, endothelial-pericyte interactions, and BBB integrity. This meta-analysis highlights the conserved expression of transporter genes across datasets, contrasted with lower and more variable expression of ion channels and receptors, suggesting that the latter may be context-dependent and dynamically regulated. These findings provide a reference framework for understanding the human BBB transportome, offering new insights into the molecular toolkit of the human BBB to support future investigations into the role of endothelial ion transport in neurological disorders.
1 Introduction
The blood-brain barrier (BBB) is a highly specialized physical barrier between the blood and the interstitial brain fluid (ISF), playing an important role in maintaining a precisely regulated microenvironment for reliable neuronal signalling (Kaplan et al., 2020) and in translating neuronal activity in a local elevation in cerebral blood flow (CBF), according to a mechanism known as neurovascular coupling (NVC) (Negri et al., 2021d; Moccia et al., 2022). The BBB is established by the association of capillary endothelial cells (cECs), pericytes, astrocytes, and neurons, that together constitute the neurovascular unit’ (NVU) (Kaplan et al., 2020). The NVU is critical for inducing and maintaining structural and functional integrity of the BBB by conferring specific features to cECs. Brain cECs lack fenestrae, show limited transcytosis, and are tightly interconnected by adherens and tight junctions. This complex organization restricts paracellular diffusion and shields the central nervous system (CNS) from blood-borne toxic compounds, thereby preserving the ionic and chemical composition of the neuronal milieu required for proper neural circuit function (Abbott et al., 2010; Kaplan et al., 2020; Benz and Liebner, 2022). Due to the high trans-endothelial resistance and the substantial lack of vesicular transport, the majority of solutes that must be exchanged between the blood and brain require a variety of mechanisms, including receptor-mediated transcytosis and active saturable transporters, such as solute carrier-mediated transport and active efflux pumps, as well as ion and water channels, ion pumps and exchangers (Abbott et al., 2010; Benz and Liebner, 2022). The movement of ions across the BBB is ensured by a rich array of ion channels and transporters for Na+, K+, Cl−, HCO3-, Ca2+ and other ions, asymmetrically distributed between the luminal and abluminal membranes of the BBB endothelium (Abbott et al., 2010). The most extensively described BBB transport mechanisms mediate the trans- or paracellular movement of hydrophilic solutes, e.g., sugars, amino and fatty acids, and xenobiotics, across brain capillaries, either into or out of the brain parenchyma, which could be targeted to improve drug delivery to the CNS (Pulgar, 2018; Rust et al., 2025). In contrast, despite their fundamental role in regulating the neural microenvironment, endothelial ion channels and transporters at the BBB are often overlooked (O'Donnell, 2014).
In addition to regulating the ionic microenvironment around central synapses and axons, the endothelial ion transport machinery is emerging as a crucial regulator of BBB function (Stoica et al., 2021; Tang et al., 2025) and NVC (Negri et al., 2021d; Moccia et al., 2022). Studies on mouse brain microvasculature showed that cECs express a variety of G-Protein Coupled Receptors (GPCRs) and ionotropic receptors as well as chemo- and mechanosensitive ion channels, which enable them to integrate neuronal (e.g., neuronal firing and synaptic activity) and blood-borne (e.g., circulating autacoids, pulsatile stretch and shear stress) signals, thereby modulating BBB permeability and cerebral blood flow (Kaplan et al., 2020; Alvarado et al., 2021; Negri et al., 2021d; Stoica et al., 2021; Moccia et al., 2022; Soda et al., 2023; Lim et al., 2024; Scarpellino et al., 2024; Tang et al., 2025). Endothelial membrane receptors primarily translate chemical and physical cues into changes in resting membrane potential (VM) and intracellular Ca2+ concentration ([Ca2+]i) (De Bock et al., 2013; Negri et al., 2021d; Stoica et al., 2021; Moccia et al., 2023b). It has been shown that mouse brain cECs may detect neuronal activity through the inward-rectifier K+ (Kir2.1) channel, which initiates and sustains the propagation of endothelial-dependent hyperpolarization (EDH) from capillaries to upstream arterioles, thereby relaxing precapillary sphincters and enhancing blood flow to downstream microvessels (Longden et al., 2017). In addition, the structural integrity and permeability of the BBB is finely modulated by synaptically released glutamate, which gates endothelial N-methyl-D-aspartate (NMDA) receptors located on the abluminal side of the capillary tube (Kim et al., 2022). Similarly, an increase in [Ca2+]i may increase the BBB permeability through the rearrangement of both adherens and tight junctions (De Bock et al., 2013; Stoica et al., 2021) and induce vasodilation at the post arteriole-capillary transitional zone by activating the endothelial nitric oxide (NO) synthase (eNOS), which mediates the production of the vasoactive gasotransmitter, NO (Longden et al., 2021). In accord, somatosensory stimulation has been shown to activate GqPCRs in mouse cECs, thereby leading to intracellular Ca2+ oscillations, NO release, and CBF increase throughout first-to third-order capillaries (Longden et al., 2021).
Emerging evidence indicates that cerebral disorders, including small vessel diseases, neurodegenerative disorders, and traumatic brain injury, impair BBB integrity and CBF regulation, by dismantling the endothelial ion signalling machinery (Peters et al., 2021; Moccia et al., 2022; Polk et al., 2023; Soda et al., 2023; Mughal et al., 2024b; Soda et al., 2024b; Rust et al., 2025). Therefore, identifying the main players in ion transport and GPCR signaling at the BBB is of fundamental importance to elucidate the physiological role of cECs within the human brain microcirculation and to design alternative therapeutic strategies for neurological and neurodegenerative disorders (Moccia et al., 2022). In the present investigation, we exploited gene expression data sets from RNA-sequencing studies on the hCMEC/D3 cell line, the most widely employed in vitro model of human BBB (Weksler et al., 2013; Helms et al., 2016; Qi et al., 2023), stored in the publicly available database databases of NCBI Sequence Read Archive (SRA) and European Nucleotide Archive (ENA). We studied the transcriptional expression of a list of 672 genes coding for ion channels, transporters, membrane ATPases, GPCRs, receptor tyrosine kinases (RTKs) based upon their documented or potential involvement in the regulation of brain cerebrovascular endothelium. We exploited a tailor-made bioinformatic tool, thus highlighting a signature expression profile with a potential physiological significance. We focus our attention on the GPCRs, RTKs, ion channels and transporters that, based on studies of mouse microcirculation, are most likely to be involved in ion transport, regulation of BBB permeability, and adaptation of CBF to metabolic neuronal demands.
2 Materials and methods
2.1 Literature search and RNA-Seq data processing
A systematic literature search was carried out to identify publicly available RNA-Seq datasets of the human brain microvascular endothelial cell line, hCMEC/D3. The NCBI Sequence Read Archive (SRA; https://www.ncbi.nlm.nih.gov/sra) was queried using the single keyword “hCMEC D3”. Studies not encompassing the whole protein-coding transcriptome (e.g., miRNA-focused studies) were discarded. Out of a total of 12 retrieved datasets, 8 were deemed suitable for our purposes and retained for subsequent analysis (see ENA and GEO accession numbers in Table 1).
To maximize comparability across datasets and minimize potential computational batch-effects due to pipeline heterogeneity, we opted to download the raw reads (in FASTQ format) for all selected studies directly from the European Nucleotide Archive (ENA; https://www.ebi.ac.uk/ena/browser) and reprocess them from scratch by using the same standardized computational pipeline. For this purpose, we developed a dedicated suite of scripts in Bash, Python, and R that combines original code with wrappers of established third-party software, thereby covering the entire process from raw reads to count tables. This suite—named x.FASTQ—is publicly available on GitHub (https://github.com/TCP-Lab/x.FASTQ) and fully documented both in the repository and in a preprint published on Preprint.org (Ruffinatti et al., 2025).
In particular, x.FASTQ was first used to retrieve sample metadata from ENA and then, based on this information, to download the FASTQ files of interest, retaining only untreated control samples and excluding all treated samples from each study. This selection resulted in 30 usable paired-end RNA-Seq runs overall (see details in Table 1). The reanalysis pipeline then proceeded as follows: adapters and low-quality bases were trimmed using BBDuk (https://archive.jgi.doe.gov/data-and-tools/software-tools/bbtools/), reads were aligned to the human reference genome (GRCh38 Ensembl Release 110 assembly) with STAR (https://github.com/alexdobin/STAR) (Dobin et al., 2013), while transcript quantification was performed with RSEM (https://github.com/deweylab/RSEM) (Li and Dewey, 2011). At each step, quality control analyses were conducted, including read quality assessment with FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/), integrated reporting with MultiQC (https://seqera.io/multiqc), and Principal Component Analysis (PCA) for the detection of potential outlier samples.
For each study, the resulting expression tables were summarized and annotated at the gene level, using Transcript Per Million (TPM) metric as a quasi-absolute expression measure (in arbitrary units) allowing comparisons of expression levels within a sample (Li and Dewey, 2011; Wagner et al., 2012). The complete set of outputs—including RSEM quantification tables, final expression matrices in TPMs, quality control reports, x.FASTQ logs from each step of the pipeline, and curated sample metadata—has been deposited in Zenodo and is freely available at the following DOI: https://doi.org/10.5281/zenodo.12729454.
We applied a TPM ≥1 threshold to define expressed genes. This choice follows common practice to exclude transcripts close to sequencing noise and to focus downstream analyses on reliably detected genes; TPM = 1 corresponds approximately to one transcript per million and is often used as a conservative cutoff that balances sensitivity and specificity for bulk RNA-seq data. To ensure that this threshold was also appropriate for our specific datasets, we examined the empirical distribution of log2 (TPM +1) values in each study. In all cases, the distribution showed a clear multimodal structure. To model it more formally, we fitted a three-component Gaussian mixture model: one component centered near zero to accommodate null (or quasi-null) expression values, a second component representing low-abundance/background transcripts, and a third component corresponding to genuinely expressed genes. We then defined the decision boundary as the intersection between the second (background) and third (expressed) Gaussian components. Notably, across studies this inferred boundary consistently fell close to TPM ≈1, thus providing independent quantitative support for the chosen cutoff. Two representative examples (for GSE76528 and GSE138309 series) are reported in Supplementary Figure S1, showing the mixture fit and the estimated boundary. We also acknowledge that some low-abundance transcripts (e.g., ion channels) can be functionally important despite low TPMs; this point is discussed in detail in the Discussion section.
2.2 Absolute expression profiling of transportome
For the downstream analysis of gene expression, we developed a custom R-based workflow called Endothelion (https://github.com/TCP-Lab/Endothelion), specifically designed to profile transportome expression in the selected hCMEC/D3 studies. Additionally, an R package named SeqLoader (https://github.com/TCP-Lab/SeqLoader) was also developed to efficiently manage the hierarchy of RNA-Seq data we collected for our meta-analysis, which consisted of multiple studies (or series), each potentially comprising multiple samples (or runs).
A comprehensive list of Genes Of Interest (GOIs) relevant to inorganic ion transport and homeostasis was assembled by integrating gene annotations from multiple sources, including the HUGO Gene Nomenclature Committee (HGNC) database (https://www.genenames.org/), the IUPHAR-DB (https://www.guidetopharmacology.org/), and Gene Ontology (https://geneontology.org/). This resulted in a curated panel of 672 GOIs, which comprised:
• all known human ion channels (436 genes, i.e., the complete channelome, including possible auxiliary or modulatory subunits),
• the entire set of aquaporins (14 genes),
• all ATPase pumps (90 genes),
• solute carriers (SLCs) specific for inorganic solutes (81 genes),
• a set of 51 receptors (GPCRs and RTKs) with roles in inorganic ion dynamics.
The complete list of GOIs is provided in Supplementary Table S1.
Expression matrices generated through the x.FASTQ pipeline (see Section 2.1) were converted to log scale, as
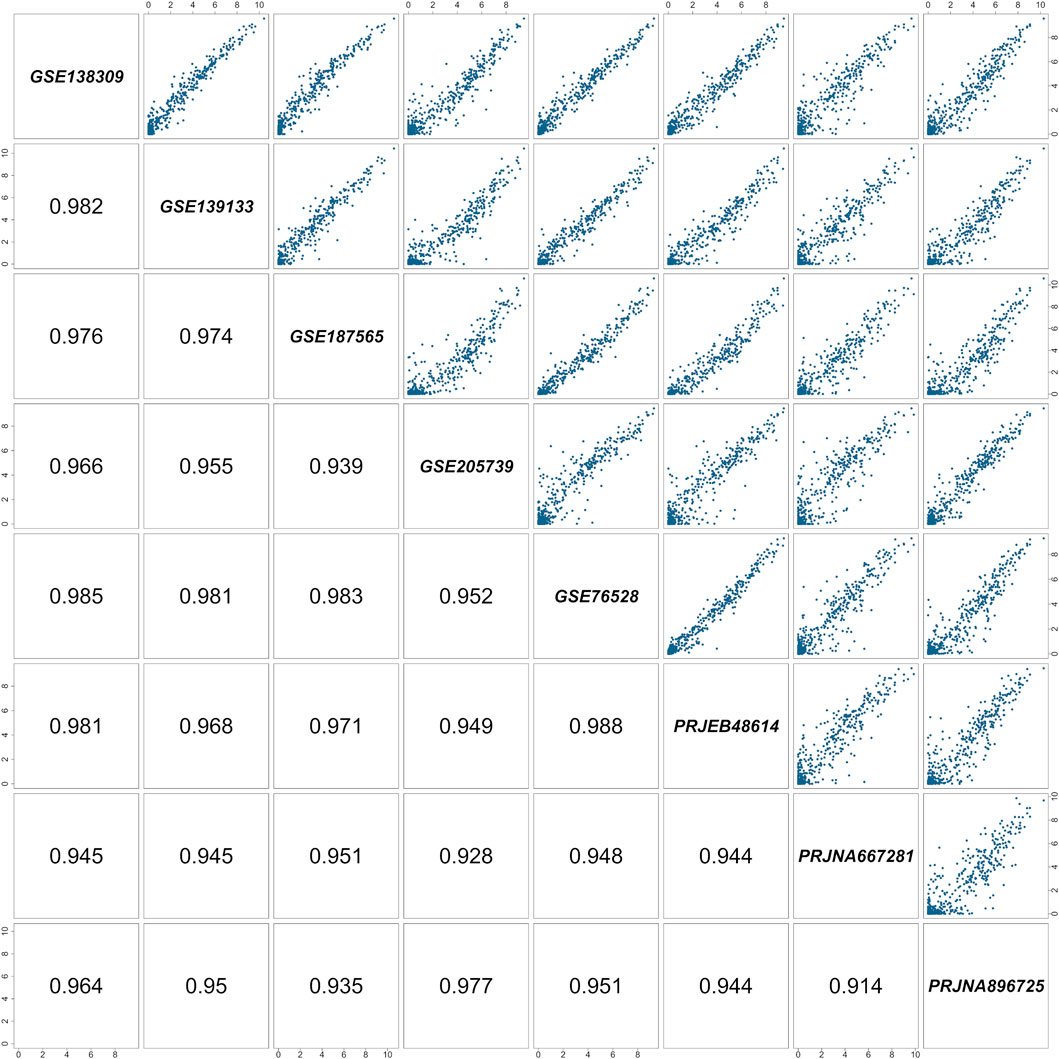
Figure 1. Pairwise correlation matrix of the RNA-Seq studies included in the meta-analysis. Each cell represents a comparison between two of the eight selected studies. Study identifiers are shown along the diagonal, pairwise scatterplots of quantile-normalized
Finally, a conventional threshold of

Figure 2. Expression levels of the top-expressed ion transporters (ATPase pumps and SLCs) in the GOI list. Genes are ordered along the y-axis by decreasing expression. The x-axis reports the mean

Figure 3. Expression levels of the top-expressed ion channels and aquaporins in the GOI list. Genes are ordered along the y-axis by decreasing expression. The x-axis reports the mean

Figure 4. Expression levels of the top-expressed membrane receptors (GPCRs and RTKs) in the GOI list. Genes are ordered along the y-axis by decreasing expression. The x-axis reports the mean
2.3 Data availability and pipeline reproducibility
All the software developed for the analyses described in this study—namely, x.FASTQ, Endothelion, and SeqLoader—is openly available through the GitHub organization page of the Turin Cell Physiology Lab (https://github.com/TCP-Lab).
In addition, to ensure full transparency and reproducibility, we implemented the entire analysis workflow within the Kerblam! project management open-source framework (https://www.kerblam.dev/) (Visentin et al., 2025). By design, this guarantees that all steps of the Endothelion pipeline can be reproduced in a standardized and automated manner.
Upon cloning the Endothelion repository (https://github.com/TCP-Lab/Endothelion), users can seamlessly retrieve the processed expression tables (i.e., outputs of the x.FASTQ pipeline for all included studies) directly from the corresponding Zenodo repository, just by running the command kerblam data fetch. Then, the full workflow for transportome expression analysis in hCMEC/D3 can be reproduced locally with the single command kerblam run hCMEC_D3. Similarly, the list of GOIs can be independently regenerated by executing the dedicated workflow (kerblam run make_geneset).
3 Results
3.1 Data collection and filtering of ion channels, transporters, and receptors
To maximize the number of datasets available for our meta-analysis on hCMEC/D3, we performed a systematic search in the NCBI SRA, which yielded eight suitable RNA-Seq studies (Table 1). FASTQ files corresponding exclusively to the untreated control samples (30 paired-end runs in total) were retrieved and reanalyzed using our standardized pipeline, x.FASTQ, as described in the Materials and Methods. Normalized expression matrices, expressed as
3.2 Gene expression profiling across datasets
For each GOI, the mean expression level across the
3.3 Global expression pattern and functional implications
3.3.1 Ion transporters
Among the most highly expressed ion transporter genes, several subunits of the mitochondrial F-type ATPase complex (e.g., ATP5F1A, ATP5F1B, ATP5MC1, etc.) were consistently detected across all datasets (Figure 2; Supplementary Table S2). This reflects the high metabolic demand of cECs, which rely on oxidative phosphorylation to fulfill their high ATP demand (Lai et al., 2023; Shao et al., 2025; Zou et al., 2025). Furthermore, our transcriptomic analysis revealed the expression of multiple genes involved in intracellular Ca2+ homeostasis, including ATP2A2 (SERCA2), ATP2B1 and ATP2B4 (PMCA1 and PMCA4), and ATP2C1 (SPCA1). These genes support active Ca2+ clearance across the ER, plasma membrane, and Golgi, respectively (Moccia et al., 2002b; Berra-Romani et al., 2019b; Alexander et al., 2023a). By contrast, we also detected low levels of ATP2A1 (SERCA1), which is the isoform typically associated with skeletal muscle (Periasamy and Kalyanasundaram, 2007). Similarly, genes showing consistently low expression levels across all datasets include the copper-transporters ATP7A, which is widely expressed with low tissue-specificity (Samimi et al., 2003; Niciu et al., 2007; Sudhahar et al., 2020; Ash et al., 2021), and ATP7B, which is involved in copper homeostasis in liver (Pierson et al., 2018), and the Na+/H+ exchangers (NHE) 3 and 5, typically enriched in kidney and gastrointestinal tract (Gerbino et al., 2025), and brain tissue (Diering et al., 2009), respectively. Furthermore, sodium/calcium exchanger 1 (SLC8A1), which has recently been identified at the protein level in and associated with the onset of the intracellular Ca2+ oscillations elicited by removal of extracellular Na+ (0 [Na+]o) in hCMEC/D3 cells (Brunetti et al., 2025), was also found at rather low levels across all datasets.
3.3.2 Ion channels
As showed in Figure 3 and Supplementary Table S3, our transcriptomic analysis detected several Cl−-permeable channels among the most highly expressed ion channel-related genes highly, including: the Chloride Intracellular Ion Channel family (CLIC1, CLIC4), Chloride Nucleotide-Sensitive Channel 1A (CLNS1A), Voltage-Dependent Anion-Selective Channel isoforms (VDAC1-3), Leucine-Rich Repeat-Containing 8A (LRRC8A), suggesting a key role in intracellular Cl− regulation and cell volume control, which are essential processes for maintaining BBB integrity and regulating the driving force promoting extracellular Ca2+ entry (Nilius and Droogmans, 2001; Alexander et al., 2023b; Liu et al., 2024; Tian et al., 2025).
Despite their functional relevance in mouse brain capillary ECs (Longden et al., 2016), our transcriptomic analysis revealed the low to moderate expression of several K+ channels. Notably, we found the expression of the intermediate conductance Ca2+-activated channel gene (KCNN4) (Figure 3; Supplementary Table S3), previously detected in mouse arteriolar but not capillary ECs (Longden et al., 2017), which contributes to EDH (Earley, 2011). We also found the transcript for the inwardly-rectifying KIR2.1 channel (KCNJ2), which senses the modest increase in extracellular K+ caused by neuronal activity and thereby initiates EDH (Longden et al., 2017), expressed across all datasets, while KIR6.1 (KCNJ8), which is a critical subunit for ATP-dependent K+ channels and is critical to CBF control in mouse brain microcirculation (Sancho et al., 2022), was undetectable (Figure 3; Supplementary Table S3). On the contrary, we found the transcript for KNa1.2 channels, which has never been reported in vascular endothelium previously, as well as BKCa channels, which is usually expressed in vascular smooth muscle cells (Longden et al., 2016) (Figure 3; Supplementary Table S3). Moreover, we found that hCMEC/D3 cells express the transcripts encoding for connexin Cx43, Cx45 and Cx47, which may form the inter-endothelial gap junctions that enable EDH to electrotonically spread towards the upstream arterioles (Krolak et al., 2025) (Figure 3; Supplementary Table S3). Noteworthy, we also found transcripts encoding for the voltage-gated K+ channels KV4.1 subunit and its auxiliary KChIP2 (KCNIP2) and KChIP3 (KCNIP3) subunits (Alexander et al., 2023b), and for the polymodal two-pore domain K+ channels, TREK-1 (KCNK2) and TWIK-2 (KCNK6) (Avalos Prado et al., 2022) (Figure 3; Supplementary Table S3). Interestingly, the regulatory subunits of KV1 and KV2 channels (KCNAB2 and KCNAB3) were detected, even though the transcripts of the corresponding pore-forming channels were not identified (Figure 3; Supplementary Table S4). Similarly, auxiliary subunits of voltage-gated Ca2+ channels (i.e., CACNB1, CACNB3, CACNA2D1, CACNA2D4, CACNG6, and CACNG8) were found, but not their pore-forming α subunits (Figure 3; Supplementary Table S4).
Our transcriptomic analysis showed also the low to moderate expression of different Na+-permeable channels in hCMEC/D3 cells, including the Na+ leakage channel (NALCN), which may contribute to maintain the resting VM (Soda et al., 2024a), the hyperpolarization-activated cyclic nucleotide-gated channels 2/3 (HCN2; HCN3) (Figure 3; Supplementary Table S3), which may help repolarizing the VM following EDH (Garcia and Longden, 2020), and the α-subunits of several voltage-gated Na+ channels (i.e., NaV1.5, NaV1.6 and NaV1.7 along with their ancillary β1 subunit) (Figure 3; Supplementary Tables S3, S4). Moreover, we found transcript of several non-selective cation (NSC) channels, such as the epithelial Na+ channel subunits, α and δ (α-ENaC/SCNN1A and δ-ENaC/SCNN1D), which were recently found to promote CBF recover to the baseline in the mouse brain microcirculation (Duncan et al., 2020; Lim et al., 2024), and TRPV4, already identified at protein level in hCMEC/D3 cells, where it shapes the Ca2+ response to arachidonic acid (Berra-Romani et al., 2019a) (Figure 3; Supplementary Table S3). In addition to TRPV4, we confirmed the expression of TRPV1 and TRPV2 transcripts (Luo et al., 2020) (Figure 3; Supplementary Table S3). Other members of the TRP family were identified across the datasets, including TRPC1 and TRPC4, TRPM2, TRPM4 and TRPM7 (Figure 3; Supplementary Table S3).
The mechanosensitive cation channel Piezo1 stands out among the most highly expressed genes across the datasets (Figure 3; Supplementary Table S3), in line with recent studies demonstrating its contribution to fine-tune the hemodynamic response in the mouse brain microcirculation (Lim et al., 2024). In addition, herein we revealed the presence of other putative mechanosensors at the plasma membrane, including TACAN (TMEM120A) (Niu et al., 2021; Kang and Lee, 2024), TMC7 (Zhang et al., 2024), TMEM63A and TMEM63B (Zheng et al., 2023), although the precise functions of these channels in ECs remain largely unexplored. In addition, we also identified transcripts for PKD1 and PKD2, which respectively encode polycystin-1 (TRPP1) and polycystin-2 (TRPP2), which may also play a role in vascular mechano-transduction (Solano et al., 2025).
As shown in Figure 3 and Supplementary Table S3, we also reported the expression of several intracellular Ca2+-releasing channels in hCMEC/D3 cells. Notably, we detected transcripts encoding for inositol 1,4,5-trisphosphate (InsP3) receptors (InsP3Rs), which mediate ER Ca2+ release, namely, ITP3R1/InsP3R1, ITP3R2/InsP3R2, and ITP3R3/InsP3R3. We also confirmed the expression of two-pore channels (TPCN1/TPC1 and TPCN2/TPC2) and TRPML1 (MCLON1), which were already found to promote lysosomal Ca2+ in hCMEC/D3 cells (Berra-Romani et al., 2020; Brunetti et al., 2024a). By contrast, transcripts for ryanodine receptors (RyRs) were not detected, as previously shown in hCMEC/D3 cells (Zuccolo et al., 2019). In addition, we identified expression of TMEM38A and TMEM38B (Figure 3; Supplementary Table S3), which encode the trimeric intracellular cation channels TRIC-A and TRIC-B, known to support ER Ca2+ homeostasis (Zhou et al., 2014). We also found transcripts for a number of putative ER Ca2+ leak channels, including TMBIM1, TMBIM4, TMBIM6, TMEM109 (Mitsugumin 23), PSEN1 (Presenilin 1), and TMCO1 (Tu et al., 2006; Liu, 2017; Bouron, 2020) (Figure 3; Supplementary Table S3).
Moreover, the high expression of mitochondria-associated proteins (e.g., the Growth Hormone Inducible Transmembrane protein, GHITM, and the Bax inhibitor 1, TMBIM6) reflects the elevated metabolic demands and stress response capabilities of brain cECs (Figure 3; Supplementary Table S3) (Kim et al., 2008; Oka et al., 2008; Austin et al., 2022; Patron et al., 2022). Furthermore, genes involved in ER protein trafficking (e.g., SEC61A1) are consistent with the scenario of an active intracellular signaling environment (Nishi et al., 1998; Lang et al., 2011; Venturi et al., 2011), potentially linked to tight regulation of protein synthesis.
Overall, these findings suggest that hCMEC/D3 cells express a specialized, although selective, repertoire of ion channels. Notably, several of the detected transcripts were expressed at relatively low levels, which is consistent with their primary roles in other tissues or specialized cell types. For instance, our transcriptomic analysis revealed the expression of transcripts coding for the α subunits of voltage-gated Na+ channels, such as SCN9A and SCN8A, which are predominantly expressed in neurons, or SCN5A in cardiac myocytes, and for the Ca2+-permeable channels CATSPER1/2, which are essential components of sperm-specific Ca2+ signaling (Alexander et al., 2023a).
3.3.3 Membrane receptors
We detected the transcripts of both ionotropic (i.e., P2X4, P2X5 and P2X7) (Scarpellino et al., 2022) and metabotropic (i.e., P2Y2 and P2Y11) (Moccia et al., 2001; Berra-Romani et al., 2008) purinergic receptors (Figures 3, 4; Supplementary Tables S3, S5), consistently with a previous report (Bintig et al., 2012). Similarly, we confirmed the expression of adenosine receptor-related genes (ADORA1, ADORA2A, ADORA2B), which were also previously detected and shown to modulate permeability in hCMEC/D3 cell monolayers (Bader et al., 2017). Similarly, we confirmed the expression of the transcript for histamine receptor H1 (HRH1) (Figure 4; Supplementary Table S5), a GPCR that elicits intracellular Ca2+ oscillations and NO release induced by both histamine and 0 [Na+]o in hCMEC/D3 cells (Berra-Romani et al., 2020; Brunetti et al., 2025). Interestingly, we detected the GABAAR ε subunit (GABRE) (Figure 3; Supplementary Table S3) and GABABR subunit 1 (GABBR1) (Figure 4; Supplementary Table S5), which contribute to support GABA-induced Ca2+ signals in hCMEC/D3 cells (Negri et al., 2022). Conversely. we did not detected the expression of multiple additional subunits for the ionotropic GABAA and metabotropic GABAB receptors that were previously detected at protein level in hCMEC/D3 cells (Negri et al., 2022).We also found transcripts for the nicotinic acetylcholine receptors (nAchRs) subunit α5 and β1 (CHRNA5 and CHNRB, respectively) and for CHRFAM7A, a negative regulator of the nicotinic acetylcholine receptor α7 (nAChR7), whereas the transcript for CHRNA7 was not found (Figure 4; Supplementary Table S3). On the contrary, we did not detect the transcript encoding for the M5 muscarinic acetylcholine receptor (CHRM5), which has previously been identified in hCMEC/D3 cells (Zuccolo et al., 2019). Moreover, our transcriptomic analysis showed the expression of α1-, β1- and β2- adrenergic receptors (ADRA1B; ADRB1; ADRB2) (Figure 4; Supplementary Table S5). Interestingly, we did not find transcripts for both ionotropic and metabotropic glutamate receptors, respectively the N-methyl-D-aspartate (NMDA) receptors GluN1 (GRIN1), GluN2C (GRIN2C) and GluN3B (GRIN3B), and the Group 1 metabotropic receptors (GRM1 and GRM5), which were previously detected in hCMEC/D3 cells (Negri et al., 2020; Negri et al., 2021a).
Growth factor-related RTKs are key regulators of vascular biology and play critical roles in the development, maintenance, and dynamic modulation of the BBB (Obermeier et al., 2013). Our transcriptomic analysis revealed the expression of the transcripts for FGFR-1 (fibroblast growth factor receptor 1) and FGFR-3, IGF1R (insulin-like growth factor 1 receptor), EGFR (epidermal growth factor receptor), PDGFRB (platelet-derived growth factor beta), VEGFR-1 (vascular endothelial growth factor receptor-1 or Flt-1) and VEGFR-2 (or KDR) family members (Figure 4; Supplementary Table S6). Collectively, these findings provide new insights into the repertoire of ionotropic receptors, GPCRs, and RTKs expressed in hCMEC/D3 cells, which may serve as a reference to investigate the complex signaling network that regulates the structural and functional properties of the BBB, making them potential therapeutic targets in a range of neurological diseases.
4 Discussion
In this study we investigated the expression pattern of 672 genes by exploiting datasets from eight different RNA-Seq analysis conducted on hCMEC/D3 cells. Most of the knowledge about the ion transport mechanisms and the GPCR signaling toolkit of the BBB has been generated by in vivo, ex vivo, or in vitro studies carried out on mouse microcirculation. Therefore, profiling the expression of the ion transport mechanisms, GPCRs, and RTKs of the human BBB may aid in delineating how the human brain cECs maintain the ionic milieu that sustains neuronal activity and how they sense and transduce neuronal activity and subtle alterations in the physicochemical properties of local microenvironment, such as extracellular acidosis or functional hyperemia. The hCMEC/D3 cell line represents the most widespread model of human BBB (Weksler et al., 2013; Helms et al., 2016; Qi et al., 2023). So far, the great majority of studies regarding the endothelial transport across the human BBB focused on the two major classes of drug and nutrient transporters: adenosine triphosphate binding cassette (ABC) and solute carrier (SLC) transporters (Okura et al., 2014; Higuchi et al., 2021; Kumabe et al., 2025). Therefore, we have only considered the genes encoding for ion channels, ion transporters, membrane ATPases, GPCRs, and RTKs, as they are altogether responsible for the finely tuned regulation of the major processes regulated by the BBB: ion transport, changes in BBB permeability, and CBF regulation. Furthermore, if available, we correlated functional expression data previously reported on hCMEC/D3 cells, with the transcriptional expression profile emerging from the RNA-Seq datasets. For space constraints, we focus on transporters, ion channels, GPCRs, and RTKs for which a functional role within the NVU is well understood (Garcia and Longden, 2020).
We anticipate that, although the hCMEC/D3 cell line represents the most widespread model of human BBB (Weksler et al., 2013; Helms et al., 2016; Qi et al., 2023), it presents intrinsic limitations that should be considered when interpreting transcriptomic and functional data. First, we considered transcriptomic data obtained from hCMEC/D3 cells cultured under static conditions, therefore lacking the shear stress generated by blood flow, which is a key physiological stimulus known to modulate endothelial gene expression, barrier properties, and mechanosensitive signaling pathways (Cucullo et al., 2011; DeStefano et al., 2017; Choublier et al., 2022; DeOre et al., 2022). Second, as an immortalized cell line, hCMEC/D3 cells may exhibit alterations in the differentiation state, metabolic features, and membrane transporter expression as compared to primary human brain microvascular endothelial cells (Weksler et al., 2013; Qi et al., 2023; Adams et al., 1989). Finally, this model does not recapitulate the multicellular complexity of the NVU, as it lacks supporting cell types such as astrocytes, pericytes, and neurons, which are essential for inducing and maintaining full BBB phenotype and function (Weksler et al., 2013; Qi et al., 2023).
4.1 The ion transport machinery responsible for the brain interstitial fluid (ISF) secretion
Ion and water transport across the BBB contributes to maintain the appropriate volume and ionic composition of the ISF. The ISF continuously interchanges with the cerebrospinal fluid due to a convective bulk flow movement facilitated by water channels, known as aquaporin-4 (AQP4) (Iliff et al., 2012; Mestre et al., 2018), which enable water to follow the osmotic gradients mainly determined by the flow of Na+ and Cl−. Surprisingly, no aquaporins have been identified to date in human brain ECs (Adiele et al., 2025); however, here we highlighted for the first time the expression of AQP3 and AQP5 in the hCMEC/D3 cell line (Supplementary Table S3). AQP3 and AQP5 are also able to conduct hydrogen peroxide (H2O2) and, by doing so, they regulate redox signaling (Tamma et al., 2018) and trigger H2O2-dependent endothelial Ca2+ signals (Martinotti et al., 2019). Given that astrocytic endfeet AQP4 is the main responsible for water transport in the human brain, the expression of AQP3 and AQP5 in hCMEC/D3 cells may reflect complementary, non-canonical functions of aquaporins, potentially dependent on the in-vitro cellular state. Indeed, both AQP3 and AQP5 have been previously detected in cultured vascular and lymphatic ECs (da Silva et al., 2018; Elkhider et al., 2020; Yang et al., 2024), astrocytes and neurons (Yamamoto et al., 2001). Their expression may be associated with the modulation of redox-sensitive pathways (Miller et al., 2010; Hara-Chikuma et al., 2015), support cell-volume regulation (Kida et al., 2005; Chen et al., 2011), migratory dynamics (Rose et al., 2021; Silva et al., 2022).
The mechanism of Na+ and Cl− secretion across the BBB has long been matter of intense investigation in rodent microcirculation (Mokgokong et al., 2014; O'Donnell, 2014; Kadry et al., 2020): Na+ and Cl− enter the BBB primarily via the Na+-K+-Cl− cotransporter (NKCC), which is located on the abluminal membrane of cECs, while Na+ primarily leaves via the Na+,K+-ATPase, which is located on the luminal side of brain capillaries (O'Donnell, 2014). The exit route for Cl− is unknown, but it is likely mediated by a Cl-permeable channel and a K+/Cl− cotransporter (KCC) (O'Donnell, 2014). The unbalance in these transport activities could lead to pathological outcome, as in the case of ischemic stroke, in which increased activity of BBB luminal Na+ transporters results in ‘hypersecretion’ of Na+, Cl−, and water into the brain interstitium (O'Donnell, 2014). Additional Na+-dependent secondary active transport systems, which are located in the abluminal membrane of mouse brain cECs, include: Na+/H+ exchanger (NHE), Na+/HCO3- cotransporter (NBC), Na+/Ca2+ exchanger (NCX), and Cl−/HCO3- exchanger (Mokgokong et al., 2014; O'Donnell, 2014; Kadry et al., 2020). Furthermore, the abluminal side of the BBB expresses several K+-permeable channels, e.g., the inwardly rectifying KIR2.1 and the ATP-dependent KIR6.1 channels, which mediate K+ removal from brain parenchyma (O'Donnell, 2014; Kadry et al., 2020). The plasma membrane Ca2+-ATPase (PMCA) has been detected on the luminal side and may contribute to removing excess Ca2+ from the brain ISF into the blood (Manoonkitiwongsa et al., 2000). This polarized arrangement of pumps, exchangers, and ion channels is crucial for the BBB to maintain the ionic milieu required for neuronal function and synaptic activity, to prevent shifts in the ISF pH, and regulate intracellular volume (O'Donnell, 2014; Kadry et al., 2020).
In the present investigation, we screened the hCMEC/D3 RNA-Seq datasets for the transcriptional expression of transporters, ATPases and ion channels potentially involved in the production and regulation of the ISF, as reported in Supplementary Tables S2, S3. The transcripts encoding all the components of the ion transport machinery described above are expressed in hCMEC/D3 cells, thereby suggesting that the mechanisms responsible for ISF secretion are similar to those described in mouse brain microcirculation. Intriguingly, the NKCC1 protein has been detected in hCMEC/D3 cells (Luo et al., 2018) and suggested to play a role in VM modulation (Soda et al., 2024a). Furthermore, RT-qPCR confirmed that NHE1, NHE5, and NBCn1 transcripts are expressed in hCMEC/D3 cells (Luo et al., 2018). We also detected mRNA for the voltage-gated H+ channel (HV1), which has not been reported previously before in vascular ECs. HV1 channels may be critical for intracellular pH regulation by mediating H+ efflux during biochemical reactions that acutely acidify the cytosol (Luo et al., 2021). This transcriptomic profile provides a framework for understanding the movement of ions into and out of the human brain endothelium at the BBB. Studies on mouse brain microcirculation revealed that brain disorders, such as traumatic brain injury, epilepsy, ischemia, hypoxia, and neurodegenerative disorders, may cause significant changes in the expression of the ion transport machinery at the BBB (Abbott et al., 2010; Kadry et al., 2020). Therefore, this investigation may be useful to effectively target the human NVU and thereby attenuate the alterations in the ionic composition of the ISF associated with these disorders.
4.2 Electrogenesis: resting VM, EDH and other ion channels potentially involved in the regulation of VM during agonist stimulation
The repertoire of ion channels that regulate the VM of hCMEC/D3 cells, both under resting conditions and in response to extracellular stimuli, remains largely uncharacterized (Bader et al., 2017; Berra-Romani et al., 2023). It was recently shown that hCMEC/D3 cells exhibit a depolarized resting VM (∼-16 mV), largely driven by a Na+ leakage and basal Cl− permeability (Soda et al., 2024a). Basal Na+ permeability has been repeatedly reported in microvascular ECs, which may therefore display a depolarized VM (Voets et al., 1996; Nilius and Droogmans, 2001; Moccia et al., 2002a). Our transcriptional analysis showed that hCMEC/D3 cells express the NALCN channel, which is a widely expressed Na+-selective channel in the CNS. NALCN channels mediates the Na+ leak in neurons, which results in a resting VM that is a more depolarized value than the K+ equilibrium potential (EK) (Monteil et al., 2024). This is the first evidence that the NALCN channel is expressed in hCMEC/D3 cells, where it may contribute to the background Na+ conductance that maintains the resting VM well above EK (Soda et al., 2024a). Additionally, NALCN channels could underlie the basal Na+ permeability that has been described in ECs from multiple species and vascular districts (Manabe et al., 1995; Voets et al., 1996; Park et al., 2000; Moccia et al., 2002a). In accordance with this hypothesis, the background Na+ current in vascular ECs is inhibited by extracellular Ca2+ (Manabe et al., 1995; Park et al., 2000; Moccia et al., 2002a), which is a feature of NALCN channels (Monteil et al., 2024). The basal Cl− conductance that contributes to the resting VM in ECs may be mediated by either volume-sensitive (Voets et al., 1996) or Ca2+-activated Cl− channels (CaCCs) (Moccia et al., 2002a). All the members of the LRCC8/Swell1 family, which mediate volume-regulated anion channels (VRACs) (Karakas et al., 2025), are expressed in hCMEC/D3 cells (Supplementary Table S3). However, preliminary analysis suggests that CaCCs, rather than VRACs, mediate the basal Cl− permeability in hCMEC/D3 cells (Soda et al., 2024a). TMEM16A is the most abundant CaCC isoform expressed in mouse brain microvascular ECs (Suzuki et al., 2020). However, TMEM16A is absent in hCMEC/D3 cells, which display the transcripts for TMEM16F, TMEM16G, and TMEM16H (Supplementary Table S3). In addition, they express Bestrophin 1, which primarily functions as CaCC in retinal pigment epithelium, but is not typically associated with ECs (Owji et al., 2021).
The KCNJ2 gene, which encodes for the inwardly-rectifying KIR2.1 channel (Longden et al., 2017), is also expressed in the RNA-Seq datasets analyzed in the present study. The expression of KIR2.1 channels in human cerebrovascular ECs may have therapeutic relevance as studies conducted in the mouse microcirculation suggest that they are critical mediators of NVC and represent promising targets for the treatment of neurological disorders (Negri et al., 2021d). KIR2.1 channels may represent a mechanism by which human brain cECs sense neuronal firing and signal to upstream parenchymal arterioles the increased demand for blood supply (Longden et al., 2017). Therefore, future work should assess whether they are activated by the modest increase in extracellular K+ concentration that occurs during neuronal activity, as shown in mouse capillary ECs. In this view, hCMEC/D3 cells express the KCNN4 gene, encoding the IKCa/KCa3.1 channels that have not been detected at the mouse BBB (Longden et al., 2017). IKCa/KCa3.1 channels open in response to an increase in [Ca2+]i during agonist stimulation (e.g., neurotransmitters, see below), thereby promoting endothelial-dependent hyperpolarization (EDH) (Earley, 2011). K+ efflux through IKCa/KCa3.1 channels, along with K+ released during neuronal firing, may then activate the endothelial KIR2.1 channel to boost EDH, as reported in mouse brain arterioles that highly express the KCNN4 gene (Longden and Nelson, 2015; Longden et al., 2017). EDH, in turn, spreads to upstream arterioles through inter-endothelial gap junctions, thereby promoting vasorelaxation and increasing blood supply to downstream capillaries (Longden et al., 2017). In the rodent brain microcirculation, gap junctions are primarily made by connexin (Cx) 37 (Cx37), Cx37, Cx40, and Cx45 (Avila et al., 2011; Krolak et al., 2025). Here, we found that hCMEC/D3 cells express the transcripts encoding Cx43, Cx45 and Cx47, which is a Cx isoform typically associated with lymphatic endothelium (Ferrell et al., 2010; Davis et al., 2024). Intriguingly, hCMEC/D3 cells also express HCN2/3 channels (Supplementary Table S3), which are activated by membrane hyperpolarization at voltages to around −50 mV and may help repolarizing the VM following EDH (Garcia and Longden, 2020). In agreement with transcriptomic (Garcia and Longden, 2020) and functional (Longden et al., 2017) data reported from the mouse brain BBB, hCMEC/D3 cells do not express the KCNN3 gene (Supplementary Table S3), encoding the SKCa/KCa2.3 channels.
As outlined in the next section, hCMEC/D3 cells present many NSC channels that could be activated by subtle changes in the intra- or extracellular microenvironments. When NSC channels carry a high fractional Ca2+ current and are physically coupled to SKCa channels, they may cause endothelial hyperpolarization (Mehrke et al., 1991). Alternatively, they lead to endothelial depolarization (Marchenko and Sage, 1993; Wu et al., 2000; Moccia et al., 2004; Lim et al., 2024), potentially triggering an unexpected bioelectrical response in hCMEC/D3 cells. The transcriptomic analysis of all the eight RNA-Seq databases showed that these cells express the α-subunits, which form the ion-conduction pores, of several voltage-gated Na+ channels, i.e., NaV1.5, NaV1.6, and NaV1.7 (Supplementary Table S3) along with their ancillary β1 subunit (Supplementary Table S4). NaV1.5 and NaV1.7 have previously been detected in mouse endothelial cells from the skin vasculature (Rice et al., 2015) and human umbilical vein endothelial cells (Andrikopoulos et al., 2011), in which they support vascular endothelial growth factor-induced angiogenesis. Additionally, hCMEC/D3 cells express the auxiliary subunits of both voltage-gated K+ and Ca2+ channels (Supplementary Table S4), such as β, α2δ, and γ subunits, although the pore-forming KV1 and CaV isoforms are absent. However, emerging evidence suggests that auxiliary subunits of CaV1.2 channels can engage in signaling pathways independent of their interaction with ion channels (Risher and Eroglu, 2020; Vergnol et al., 2022). For instance, the Cavβ3 subunits, which is expressed in hCMEC/D3 cells (Supplementary Table S4), regulates the BBB permeability in the mouse microcirculation (Martus et al., 2024). Conversely, hCMEC/D3 cells express the transcripts for the molecular components of the A-type K+ current, i.e., the pore forming KV4.1 subunit and its auxiliary KCHIP3 (KCNIP3) subunits (Alexander et al., 2023b). This current is likely to be inactivated at the resting VM, but could de-inactivate following agonist-induced hyperpolarization, thereby contributing to modulating the bioelectrical activity of hCMEC/D3 cells.
A number of K+ channels that have never been detected in vascular ECs could act as a brake on depolarization. Our transcriptomic analysis showed that hCMEC/D3 cells express Na+-activated K+ channels (KNa1.2 or Slo2.1), which may be activated by Na+ entry through NSC channels, and BKCa channels (Supplementary Table S3), which may be activated by both Ca2+ entry and membrane depolarization (Alexander et al., 2023b). Unlike SKCa channels, BKCa channels are generally not expressed in vascular endothelial cells; however, they are abundantly present in vascular smooth muscle cells, in which they promote vasoconstriction (Longden et al., 2016). Similarly, KNa1.2 channels have never been reported in vascular endothelium. The finding that hCMEC/D3 cells undergo hyperpolarization upon a reduction in extracellular Na+ concentration suggests that KNa1.2 channels are not activated by the basal Na+ entry (Soda et al., 2024a). Yet, they could be gated by the massive Na+ entry that occurs upon activation of NSC channels (Kaczmarek, 2013), thereby contributing to restoring the resting VM.
4.3 The intracellular Ca2+ toolkit
An increase in endothelial [Ca2+]i is critical to fine tune several functions of the BBB, including the regulation of paracellular permeability through the modulation of junctional and cytoskeletal proteins (De Bock et al., 2013), the hemodynamic response to synaptic activity (Negri et al., 2021d; Moccia et al., 2022), and sprouting angiogenesis (Lambrichts et al., 2025). Here, we investigated the expression of the multifaceted components of the Ca2+ signaling toolkit in hCMEC/D3 cells. We focused on both the Ca2+ pumps and exchangers that maintain and restore resting Ca2+ levels following agonist stimulation (Supplementary Table S2) and on the ion channels that generate intracellular Ca2+ signals with specific spatio-temporal profiles (Supplementary Table S3), thereby driving a wide variety of cellular responses (Bintig et al., 2012; Berra-Romani et al., 2020; Berra-Romani et al., 2023; Brunetti et al., 2025; Soda et al., 2025).
Our transcriptional analysis detected specific subunits of plasma membrane Ca2+ ATPase (PMCA1 and PMCA4), sarco-endoplasmic reticulum Ca2+-ATPase (SERCA2), and Na+/Ca2+ exchanger (NCX1) (Supplementary Table S2). Interestingly, we have recently demonstrated that the NCX1 protein is expressed in hCMEC/D3 cells (Brunetti et al., 2025), whereas a comprehensive RT-qPCR analysis has previously confirmed the presence of the transcripts encoding PMCA1, PMCA4 and SERCA2 (Zuccolo et al., 2019). The endothelial Ca2+ response to chemical stimulation is primarily initiated by Ca2+ mobilization from the endoplasmic reticulum (ER), the most abundant endothelial Ca2+ reservoir, and maintained by extracellular Ca2+ entry across the plasma membrane (Moccia et al., 2023a). Transcript analysis revealed the expression of several candidate ER Ca2+ leak channels in hCMEC/D3 cells (Supplementary Table S3): TMBIM2 (Lisak et al., 2015), TMBIM6 (Lisak et al., 2015), and mitsugumin 23 (Venturi et al., 2011). ER Ca2+ depletion induced by pharmacological inhibition of SERCA activity could occur through any of these pathways, while TMBIM2 and TMBIM6 may also be expressed in the Golgi apparatus (Lisak et al., 2015). Ca2+ release from ER requires agonist binding to GPCRs (mainly coupling to Gq protein), or TKRs, activating distinct isoforms of phospholipase C (i.e., respectively, PLCβ and PLCγ). PLC cleaves the membrane phospholipid phosphoinositide 4,5-bisphosphate (PIP2) into the second messengers, diacylglycerol (DAG) and InsP3, which mobilizes Ca2+ by gating InsP3Rs on the ER (Moccia et al., 2023a; Moccia et al., 2023b). InsP3Rs represent the major family of ER Ca2+-releasing channels in the endothelial lineage, as all the known InsP3R isoforms, i.e., InsP3R1, InsP3R2 and InsP3R3, are expressed in vascular ECs (Moccia et al., 2023b). Herein, we confirmed that InsP3R1, InsP3R2 and InsP3R3 are also expressed in the hCMEC/D3 cell line (Supplementary Table S3). Interestingly, a previous RT-qPCR analysis failed to detect the transcripts encoding for InsP3R1 and InsP3R2 (Zuccolo et al., 2019). In contrast, ryanodine receptors (RyRs), are unlikely to participate in Ca2+ mobilization from ER in endothelial cells (Thakore and Earley, 2019): none of the three known RyR isoforms (RYR1, RYR2, RYR3) is expressed in the hCMEC/D3 cell line (Supplementary Table S3), as previously confirmed by RT-qPCR (Zuccolo et al., 2019). In addition to ER Ca2+ release, another key component shaping endothelial Ca2+ dynamics is lysosomal Ca2+ release through TPCs, which are gated by the intracellular messenger nicotinic acid adenine dinucleotide phosphate (NAADP) and may be present in two distinct isoforms, i.e., TPC1 and TPC2 (Negri et al., 2021b). Herein, we confirmed that they are both expressed in hCMEC/D3 cells (Supplementary Table S3), as previously demonstrated by RT-qPCR (Zuccolo et al., 2019). The transcriptomic analysis confirms that agonist-induced intracellular Ca2+ release in human cerebrovascular endothelial cells is supported by ER Ca2+ release through InsP3Rs and lysosomal Ca2+ mobilization through TPCs, as shown by functional analyses (Berra-Romani et al., 2020; Negri et al., 2022). Interestingly, the dataset analysis also revealed the expression of another lysosomal Ca2+ channel, i.e., TRPML1 (Supplementary Table S3), which plays a crucial role in autophagy and endolysosomal trafficking. The endothelial role of TRPML1 is not clear yet (Negri et al., 2021b), but we have recently demonstrated that the TRPML1 protein is expressed in acidic endolysosomal vesicles, contributes to ER Ca2+ refilling and supports NO release (Brunetti et al., 2024a).
Agonist-induced extracellular Ca2+ entry in ECs mainly occurs through the Store-Operated Ca2+ Entry (SOCE) pathway, which is mediated by the interaction between Stromal Interaction Molecule (STIM) and ORAI proteins or members of the Transient Receptor Potential Canonical (TRPC) channels, including TRPC1 and TRPC4 (Moccia et al., 2023a). Our transcriptional analysis revealed that both STIM1 and STIM2, which serve as sensors of the luminal Ca2+ concentration, are expressed in hCMEC/D3 (Supplementary Table S3), although previous RT-qPCR analysis failed to detect STIM1 transcripts (Zuccolo et al., 2019). Similarly, we confirmed the previous report that all the ORAI isoforms, i.e., ORAI1, ORAI2 and ORAI3, are expressed in human brain cECs (Supplementary Table S3). It is worth noting that the transcripts encoding for TRPC1 and TRPC4 were present in all the eight datasets analysed (Supplementary Table S3), while it was not detected by RT-PCR analysis (Zuccolo et al., 2019). However, the pharmacological profile of the SOCE machinery in hCMEC/D3 cells suggests the involvement of ORA1 channels rather than TRPC1 (Zuccolo et al., 2019; Negri et al., 2020; Negri et al., 2021a). TRPC1 may be gated by a store-independent mechanism, as it has also been shown to assemble with TRPP2 and function as a mechano-sensor in mouse cerebrovascular endothelium (Berrout et al., 2012). Notably, the transcript for TRPP2 is expressed in hCMEC/D3 cells (Supplementary Table S3).
4.4 Ionotropic receptors, GPCRs, and RTKs
It has long been known that the brain microcirculation receives extensive innervation from both local neurons, such as pyramidal neurons and γ-aminobutyric (GABA) interneurons, and subcortical afferents, such as basal forebrain, locus coeruleus, ventral tegmental area, and Raphe (Schaeffer and Iadecola, 2021). Multiple lines of evidence indicate that neuronal activity can stimulate NVC (Negri et al., 2021d; Moccia et al., 2022; Soda et al., 2024b), promote angiogenesis (Li et al., 2018; Agrud et al., 2022), and regulate BBB permeability (De Bock et al., 2013; Kaplan et al., 2020) by directly activating cECs within the NVU. Our transcriptomic analysis revealed that hCMEC/D3 cells express typical endothelial as well as neuronal ionotropic (Supplementary Table S3), GPCRs (Supplementary Table S5), and RTKs (Supplementary Table S6). We detected the presence of the transcripts for the purinergic P2X4, P2X5, and P2X7, which mediate Ca2+-permeable non-selective cation channels (Scarpellino et al., 2022), and P2Y2 and P2Y11, which are GqPCRs triggering InsP3-dependent ER Ca2+ release (Moccia et al., 2001; Berra-Romani et al., 2008). This finding is consistent with a previous report demonstrating the expression and functional role of these purinergic receptors, as well as P2Y12, in hCMEC/D3 cells (Bintig et al., 2012). Similarly, we confirmed the presence of the histamine receptor H1 (Supplementary Table S5), which elicits histamine- and 0 [Na+]o-induced intracellular Ca2+ oscillations and NO release (Berra-Romani et al., 2020; Brunetti et al., 2025), as well as of the GABAAR ε subunit and GABAB receptor subunit 1, which contribute to support GABA-induced Ca2+ signals in hCMEC/D3 cells (Negri et al., 2022). Interestingly, our transcriptomic analysis also showed the expression of the transcripts coding for α1- and α2-adrenergic receptors (Supplementary Table S5), which could be involved in the regulation of the human BBB permeability by catecholamines, as shown in the mouse microcirculation (Urayama et al., 2015). Additionally, hCMEC/D3 cells are equipped with the β1-and β2-adrenergic receptors (Supplementary Table S5), which were shown to modulate the angiogenic activity in the human brain microvascular EC (HBMEC) line (Annabi et al., 2009). Consistent with this evidence, an early study documented the presence of α1-, α2, β1-, and β2-adrenergic receptors in microvascular ECs isolated from three different regions of the human brain (Bacic et al., 1992). Our transcriptomic analysis revealed the presence of the transcript for the glycine receptor (GlyR) β subunit (Supplementary Table S3), which may promote post-ischemic angiogenesis and neurological regeneration in the mouse brain (Xu et al., 2024). We also found transcripts for several nAchRs, such as α5 and β1 (Supplementary Table S3). However, the α5-nAchR subunit only plays a modulatory role and is therefore unlikely to mediate acetylcholine-induced inward currents (Alexander et al., 2023a), whereas it could contribute to cancer growth and angiogenesis by engaging the Smad signaling pathway in a flux-independent manner (Cai et al., 2025). Similarly, the β1 subunit primarily contributes to the muscle nAchR and has never been detected in the brain (Tae and Adams, 2023). Furthermore, hCMEC/D3 cells also express Dupα7, which serves as dominant negative inhibitor of nAchR7 (Dang et al., 2015). These findings are, therefore, consistent with the report that nicotine does not trigger a detectable increase in [Ca2+]i in hCMEC/D3 cells (Zuccolo et al., 2019), but additional work is mandatory to assess the functional role of α5-nAchR, β1-nAchR, and Dupα7 at the human BBB. It is likely that these subunits signal in a flux-independent, i.e., metabotropic, manner, as already demonstrated for nAchRs (Montes de Oca Balderas, 2022) and ionotropic glutamate receptors (Brunetti et al., 2024b). Finally, our transcriptomic analysis confirmed the expression of transcripts for adenosine receptors, such as A1, A2a and A2b, which were previously detected and shown to modulate permeability in hCMEC/D3 cell monolayers (Bader et al., 2017). Moreover, the endothelial adenosine receptors could play a role in NVC, by activating KATP channels and promoting EDH (Mughal et al., 2024a). It should, however, be pointed out that we could not find a significant expression of the transcripts for several GPCRs that were previously detected functionally characterized in hCMEC/D3 cells, including M5 receptors for acetylcholine (Zuccolo et al., 2019), NMDA and Group 1 metabotropic receptors for glutamate (Negri et al., 2020; Negri et al., 2021a), and multiple additional subunits for the ionotropic GABAA and metabotropic GABAB receptors (Negri et al., 2022). The potential reasons for this discrepancy will be discussed below.
Our transcriptomic analysis identified members of the FGFR, IGFR, EGFR, PDGFR, and VEGFR families (Supplementary Table S6), a repertoire that likely reflects the multifaceted signaling environment of the brain endothelium. Several of these receptors have already been linked to BBB biology in experimental models. For example, IGF1R contributes to BBB preservation in mice (Gulej et al., 2024) and enhances endothelial stability through junctional protein upregulation (Higashi et al., 2020). PDGFR beta (PDGR-B) is essential for endothelial–pericyte communication and vascular maturation (Shen et al., 2019), while FGFR1 activation has been associated with improved barrier tightness and permeability control in vivo (Chen et al., 2020; Kriauciunaite et al., 2023). VEGF is the master regulator of angiogenesis throughout peripheral circulation (Moccia et al., 2019), including the brain microvasculature (Lange et al., 2016). VEGF regulates microvessel density and endothelial permeability at the BBB, although pathological VEGF signaling may disrupt BBB integrity and cause vascular leakage (Lange et al., 2016). The effect of VEGF on the cerebral microcirculation is primarily mediated by VEGFR-2 (also known as KDR) (Wen et al., 2023), which is abundantly expressed in all the 8 RNA-Seq datasets (Supplementary Table S6). This finding is consistent with recent reports confirming VEGFR-2 expression and signaling in the hCMEC/D3 cell line (Zhang et al., 2019; Sandoval et al., 2025). We also detected transcripts for VEGFR-1 (also known as Flt-1) (Supplementary Table S6), which has been previously described in hCMEC/D3 cells, although its functional role remains unclear (Teles et al., 2025). In the human brain microcirculation, VEGFR-1 is likely to exert protective roles against inflammatory cues or stress-induced disruption of the BBB, as reported in other cellular (Salmeri et al., 2013) and animal (Schreurs et al., 2012) models. Finally, we confirmed the expression of EGFR, which has recently been shown to regulate the permeability of hCMEC/D3 cell monolayers by recruiting the c-Jun N-terminal kinase signaling pathway (Chen et al., 2015). Collectively, the detection of this RTK subset highlights that hCMEC/D3 cells not only present barrier-related features but also possess the molecular machinery to respond to angiogenic and trophic cues, important to maintain BBB integrity.
4.5 Sensing changes in extra/intracellular environments
Brain endothelial cells must constantly adapt to shear stress, oxidative stress, and changes in the ionic composition of the ISF. Our transcriptomic analysis revealed that, to do so, they may exploit several mechano-sensitive, pH-sensitive and redox-sensitive ion channels. A local increase in CBF is critical for supplying active neurons with oxygen and nutrients (Negri et al., 2021d). The hemodynamic response to neuronal activity also causes a local increase in shear stress that can be detected by brain cECs through mechano-sensitive ion channels. Our transcriptomic analysis revealed that hCMEC/D3 cells express several mechanosensitive NSC channels detected in other EC types (Lim and Harraz, 2024), including (Supplementary Table S3): Piezo1, TRPV4, PKD1 (TRPP1), PKD2 (TRPP1), ENaC, and TRPM7. Consistent with this finding, we previously showed that TRPV4 protein is expressed and mediates robust NO release in hCMEC/D3 cells (Berra-Romani et al., 2019a). Moreover, endothelial Piezo1 channels and ENaC have recently been detected in the mouse brain microcirculation (Duncan et al., 2020; Lim et al., 2024). The available evidence suggests that these mechanosensitive NSC channels play distinct roles during NVC: TRPV4-mediated NO release may support endothelium-dependent vasodilation (Diaz-Otero et al., 2019), while Piezo1-and ENaC-mediated depolarization promotes CBF recovery to the baseline (Ashley et al., 2018; Duncan et al., 2020; Lim et al., 2024). At the current stage, it is unpredictable whether PKD1/PKD2 and TRPM7 channels mediate vasorelaxation through NO release or vasoconstriction through membrane depolarization. Intriguingly, hCMEC/D3 cells also express the TMEM120 gene, encoding the TACAN protein, which were claimed either to serve as mechano-sensitive NSC channels or to modulate Piezo and PKD2 channels (Kang and Lee, 2024). Our in silico results also showed the expression of the genes encoding TREK1 channels in human cerebrovascular endothelial cells (Supplementary Table S3). These are polymodal K+-selective channels that can integrate mechanical (e.g., membrane stretch), thermal (increase in temperature from ∼17 to ∼40 °C), and chemical (e.g., arachidonic acid, pH, GPCRs) (Avalos Prado et al., 2022). TREK1 channels may support EDH in response to either the initial phase of the hemodynamic response (when the arteriolar-capillary transitional zone undergoes a local increase in shear stress) or downstream of the GqPCRs that contribute to NVC.
In addition to being mechano-sensitive, ENaC and TRPM7 channels may play a crucial role in regulating BBB permeability, brain angiogenesis and inflammation, by detecting changes in Na+ and Mg2+ concentrations in the ISF (Zhu et al., 2018; Maier et al., 2022; Zhang et al., 2022). Particularly, hyponatremia has been reported in epilepsy and cortical spreading depression (Gankam Kengne, 2023), whereas Mg2+ deficiency has been reported in Alzheimer’s disease (Barbagallo et al., 2011) and age-related dementia (Ozturk and Cillier, 2006). Future studies are necessary to determine whether the polymodal ENaC and TRPM7 channels serve as mechano- and/or chemo-sensors in human cerebrovascular endothelial cells. The transcriptomic profile also suggests that hCMEC/D3 cells possess a repertoire of pH- and redox-sensitive ion channels, which may enable them to detect chemical changes in the extracellular milieu of the brain. Extracellular acidification may be associated with a variety of neurological disorders, including migraine, traumatic brain injury, ischemic stroke, multiple sclerosis, and epileptic seizures (Cheng et al., 2025). Studies on mouse microcirculation showed that brain microvascular ECs may detect reductions in extracellular pH through ASIC type 1 (ASIC1) (Lin et al., 2014) and TREK-1 channels (Fang et al., 2019), which exert a neuroprotective role by promoting vasodilation. In addition to TREK-1, ASIC1 channels were detected in all the five RNA-Seq datasets analyzed, suggesting that these endothelial pH-sensitive ion channels may also play a role in human brain microvasculature. Intriguingly, both ASIC1 and TREK-1 channels could contribute to promoting endothelial dysfunction upon strong and/or prolonged brain ischemia (Zhang et al., 2020; Zheng et al., 2022). Gene expression analysis showed that hCMEC/D3 cells express several redox-sensitive NSC channels (Negri et al., 2021c; Schaller et al., 2025), such as TRPM2, TRPV1, TRPV2 and TRPV4 (Supplementary Table S3). In addition to TRPV4 (Berra-Romani et al., 2019a), a recent study confirmed that hCMEC/D3 cells express a functional TRPV2 protein, while TRPV1-mediated Ca2+ entry was barely detectable (Luo et al., 2020). Consistently, our transcriptomic analysis shows that TRPV2 gene expression is significantly higher than that of TRPV1, although single-cell Ca2+ imaging will be necessary to determine whether TRPV1 activation leads to an increase in [Ca2+]i. Intriguingly, TRPM2 has been shown to be the primary mediator of ROS-dependent BBB degradation in several brain disorders (Ding et al., 2021), including ischemic stroke (Zong et al., 2024) and Alzheimer’s disease (Park et al., 2014). A preliminary investigation confirmed that the TRPM2 protein is expressed and mediates oxidative stress-induced injury in hCMEC/D3 cell monolayers (Huang et al., 2021).
4.6 The apparent discrepancy between transcriptomic and functional data
Interestingly, the expression pattern of transporter-related genes (Figure 1) markedly differs from that of ion channel genes (Figure 2). While the majority of transporters exhibit relatively high and consistent expression across datasets, ion channels tend to display lower overall expression and greater inter-sample variability. This pattern suggests a more functionally essential expression of transporters in hCMEC/D3 cells, whereas ion channels may play more context-dependent or specialized roles (see also below). Indeed, transporters are often responsible for constitutive cellular functions, such as nutrient uptake, metabolite exchange, and waste removal. These functions are critical for endothelial homeostasis and are generally preserved across endothelial subtypes and vascular districts. Additionally, the blend of endothelial transporters is critical for controlling the neuronal excitability as well as synaptic signaling. As a result, their transcript levels are typically stable and reproducible, even across datasets generated under differing experimental conditions.
A critical factor contributing to the low apparent transcript levels of many ion channels is that these classes of membrane proteins are often expressed at intrinsically low copy numbers (Grygorczyk et al., 1984; Beck et al., 2011; Lockwich et al., 2011; O'Leary et al., 2013; Thillaiappan et al., 2019; Gorur-Shandilya et al., 2020; Ali Shah and Ou, 2021; von Lindern et al., 2022), yet can exert substantial physiological effects due to high ligand sensitivity, steep dose–response relationships, and strong downstream amplification within signaling cascades. In accord, many ion channels operate in highly localized membrane microdomains, such as caveolae and myo-endothelial gap junctions, where even sparse protein expression is sufficient for robust functional output (Berra-Romani et al., 2013; Pires et al., 2015; Sullivan et al., 2015; Murphy and Sandow, 2019; Ottolini et al., 2020; Jackson, 2022; Moccia et al., 2023b; Huo et al., 2025). Consequently, low transcript abundance does not necessarily reflect limited functional capacity, and the presence of functional receptors or channels can be underestimated when mRNA-based quantification is used as the sole readout. Additionally, several transcripts that have been characterized molecularly and functionally in hCMEC/D3 cells, were undetectable in any of the eight public RNA-Seq datasets examined. These transcripts include several components of the Ca2+ handling machinery (Berridge et al., 2003), such as ion channels, e.g., TRPA1 (Berra-Romani et al., 2023; Soda et al., 2025), GABAA (Negri et al., 2022) and NMDA receptors (Negri et al., 2021a), and GqPCRs, e.g., P2Y12 receptors (Bintig et al., 2012), M5 muscarinic receptors (Zuccolo et al., 2019), GABAB receptors, and Group 1 metabotropic glutamate receptors (Negri et al., 2020). The mismatch between mRNA abundance and the expression levels of membrane proteins, has long been matter of intense discussion (Misquitta et al., 2006; Vogel and Marcotte, 2012; Li et al., 2020). In the case of components of the Ca2+ signaling machinery, this discrepancy likely reflects tight post-transcriptional regulation of mRNA stability (Misquitta et al., 2006; Dragoni et al., 2014; Sobradillo et al., 2014; Zuccolo et al., 2018). Transcripts with low abundance or very short half-lives are rapidly degraded after translation, leading to transient mRNA levels that are difficult to capture, especially in bulk RNA-Seq experiments, where signal averaging further reduces sensitivity to short-lived transcripts (Urich et al., 2012). The discordance between protein levels and transcript levels has already been reported for NMDA receptors (Gazzaley et al., 1996; Awobuluyi et al., 2003), P2Y12 receptors (Rauch et al., 2010; Pavlovic et al., 2020), mGluR5 (Iyo et al., 2010; Fatemi et al., 2013), and GABAA receptors (Murgas et al., 2022). Consistent with this, a recent investigation mining a publicly available RNA-Seq dataset failed to detect the transcripts for NMDARs (Garcia and Longden, 2020), which are known to be expressed and regulate the BBB permeability in the mouse microcirculation (Macrez et al., 2016; Mehra et al., 2020). Beyond biological regulation, technical and methodological factors can further contribute to apparent transcript-protein mismatches. Variations in sequencing depth, strandedness, and reference annotation all affect detection sensitivity for low-abundance or alternatively spliced transcripts. Moreover, library-preparation biases, such as the widespread use of poly(A) selection during mRNA enrichment, can distort transcript representation by preferentially capturing polyadenylated transcripts while underrepresenting non-polyadenylated or atypically-processed mRNAs (Yang et al., 2011; Wehrspaun et al., 2014; Witmer et al., 2024). In addition, when highly expressed genes dominate the sequencing read pool, low-abundance/rare mRNAs may fall beyond detection thresholds. Furthermore, if the transcripts exhibit high sequence similarity to paralogs or pseudogenes, their reads may be difficult to map uniquely to the reference genome. This can lead to them being filtered out during standard bioinformatics analysis, as reported for ribosomal proteins (Tonner et al., 2012). On the contrary, transporter genes, often expressed at moderate-to-high levels, tend to yield more robust and reproducible signals in both bulk and single-cell RNA sequencing datasets. These results highlight the importance of targeted experimental validation, such as qRT-PCR, targeted RNA capture, immunoblotting, immunofluorescence, or functional assays, to confirm transcript presence when public RNA-Seq datasets yield negative results.
5 Conclusion
Taken together, these findings suggest that ion transporter, GPCR, and RTK toolkit is a reliable and consistent readout for characterizing the endothelial transcriptome across datasets, albeit ion channel profiles may require careful interpretation and validation, particularly in the context of endothelial functional diversity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
GS: Writing – original draft, Methodology, Data curation, Investigation, Validation, Formal Analysis. VB: Investigation, Data curation, Formal Analysis, Writing – review and editing. FS: Validation, Writing – review and editing, Methodology, Investigation. LV: Methodology, Formal Analysis, Data curation, Investigation, Validation, Writing – review and editing. GB: Validation, Writing – review and editing, Resources. FR: Validation, Methodology, Conceptualization, Formal Analysis, Investigation, Writing – original draft, Data curation. FM: Conceptualization, Investigation, Data curation, Validation, Writing – original draft, Formal Analysis, Project administration.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This research has been supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006)—A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The reviewer (ST) declared a past co-authorship with the author (FM) to the handling editor at the time of review.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1733266/full#supplementary-material
References
Abbott N. J., Patabendige A. A., Dolman D. E., Yusof S. R., Begley D. J. (2010). Structure and function of the blood-brain barrier. Neurobiol. Dis. 37 (1), 13–25. doi:10.1016/j.nbd.2009.07.030
Adams D. J., Barakeh J., Laskey R., Van Breemen C. (1989). Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 3 (12), 2389–2400. doi:10.1096/fasebj.3.12.2477294
Adiele R. C., Tham M., Lucchinetti C. F., Popescu B. F. G. (2025). A systematic study of the distribution and expression of aquaporin water channels in normal adult human brain. J. Neurol. 272 (9), 574. doi:10.1007/s00415-025-13304-9
Agrud A., Subburaju S., Goel P., Ren J., Kumar A. S., Caldarone B. J., et al. (2022). Gabrb3 endothelial cell-specific knockout mice display abnormal blood flow, hypertension, and behavioral dysfunction. Sci. Rep. 12 (1), 4922. doi:10.1038/s41598-022-08806-9
Alexander S. P. H., Fabbro D., Kelly E., Mathie A. A., Peters J. A., Veale E. L., et al. (2023a). The concise guide to PHARMACOLOGY 2023/24: transporters. Br. J. Pharmacol. 180 (Suppl. 2), S374–S469. doi:10.1111/bph.16182
Alexander S. P. H., Mathie A. A., Peters J. A., Veale E. L., Striessnig J., Kelly E., et al. (2023b). The concise Guide to PHARMACOLOGY 2023/24: ion channels. Br. J. Pharmacol. 180 (Suppl. 2), S145–S222. doi:10.1111/bph.16178
Ali Shah S. M., Ou Y. Y. (2021). TRP-BERT: discrimination of transient receptor potential (TRP) channels using contextual representations from deep bidirectional transformer based on BERT. Comput. Biol. Med. 137, 104821. doi:10.1016/j.compbiomed.2021.104821
Alvarado M. G., Thakore P., Earley S. (2021). Transient receptor potential channel ankyrin 1: a unique regulator of vascular function. Cells 10 (5), 1167. doi:10.3390/cells10051167
Andrikopoulos P., Fraser S. P., Patterson L., Ahmad Z., Burcu H., Ottaviani D., et al. (2011). Angiogenic functions of voltage-gated Na+ Channels in human endothelial cells: modulation of vascular endothelial growth factor (VEGF) signaling. J. Biol. Chem. 286 (19), 16846–16860. doi:10.1074/jbc.M110.187559
Annabi B., Lachambre M. P., Plouffe K., Moumdjian R., Beliveau R. (2009). Propranolol adrenergic blockade inhibits human brain endothelial cells tubulogenesis and matrix metalloproteinase-9 secretion. Pharmacol. Res. 60 (5), 438–445. doi:10.1016/j.phrs.2009.05.005
Ash D., Sudhahar V., Youn S. W., Okur M. N., Das A., O'Bryan J. P., et al. (2021). The P-type ATPase transporter ATP7A promotes angiogenesis by limiting autophagic degradation of VEGFR2. Nat. Commun. 12 (1), 3091. doi:10.1038/s41467-021-23408-1
Ashley Z., Mugloo S., McDonald F. J., Fronius M. (2018). Epithelial Na(+) channel differentially contributes to shear stress-mediated vascular responsiveness in carotid and mesenteric arteries from mice. Am. J. Physiol. Heart Circ. Physiol. 314 (5), H1022–H1032. doi:10.1152/ajpheart.00506.2017
Austin S., Mekis R., Mohammed S. E. M., Scalise M., Wang W. A., Galluccio M., et al. (2022). TMBIM5 is the Ca(2+)/H(+) antiporter of mammalian mitochondria. EMBO Rep. 23 (12), e54978. doi:10.15252/embr.202254978
Avalos Prado P., Chassot A. A., Landra-Willm A., Sandoz G. (2022). Regulation of two-pore-domain potassium TREK channels and their involvement in pain perception and migraine. Neurosci. Lett. 773, 136494. doi:10.1016/j.neulet.2022.136494
Avila M. A., Sell S. L., Hawkins B. E., Hellmich H. L., Boone D. R., Crookshanks J. M., et al. (2011). Cerebrovascular connexin expression: effects of traumatic brain injury. J. Neurotrauma 28 (9), 1803–1811. doi:10.1089/neu.2011.1900
Awobuluyi M., Lipton S. A., Sucher N. J. (2003). Translationally distinct populations of NMDA receptor subunit NR1 mRNA in the developing rat brain. J. Neurochem. 87 (5), 1066–1075. doi:10.1046/j.1471-4159.2003.02048.x
Bacic F., McCarron R. M., Uematsu S., Spatz M. (1992). Adrenergic receptors coupled to adenylate cyclase in human cerebromicrovascular endothelium. Metab. Brain Dis. 7 (3), 125–137. doi:10.1007/BF01000158
Bader A., Bintig W., Begandt D., Klett A., Siller I. G., Gregor C., et al. (2017). Adenosine receptors regulate gap junction coupling of the human cerebral microvascular endothelial cells hCMEC/D3 by Ca(2+) influx through cyclic nucleotide-gated channels. J. Physiol. 595 (8), 2497–2517. doi:10.1113/JP273150
Barbagallo M., Belvedere M., Di Bella G., Dominguez L. J. (2011). Altered ionized magnesium levels in mild-to-moderate Alzheimer's disease. Magnes. Res. 24 (3), S115–S121. doi:10.1684/mrh.2011.0287
Beck M., Schmidt A., Malmstroem J., Claassen M., Ori A., Szymborska A., et al. (2011). The quantitative proteome of a human cell line. Mol. Syst. Biol. 7, 549. doi:10.1038/msb.2011.82
Benz F., Liebner S. (2022). Structure and function of the blood-brain barrier (BBB). Handb. Exp. Pharmacol. 273, 3–31. doi:10.1007/164_2020_404
Berra-Romani R., Raqeeb A., Avelino-Cruz J. E., Moccia F., Oldani A., Speroni F., et al. (2008). Ca2+ signaling in injured in situ endothelium of rat aorta. Cell. Calcium 44 (3), 298–309. doi:10.1016/j.ceca.2007.12.007
Berra-Romani R., Avelino-Cruz J. E., Raqeeb A., Della Corte A., Cinelli M., Montagnani S., et al. (2013). Ca(2)(+)-dependent nitric oxide release in the injured endothelium of excised rat aorta: a promising mechanism applying in vascular prosthetic devices in aging patients. BMC Surg. 13 (Suppl. 2), S40. doi:10.1186/1471-2482-13-S2-S40
Berra-Romani R., Faris P., Negri S., Botta L., Genova T., Moccia F. (2019a). Arachidonic acid evokes an increase in intracellular Ca(2+) concentration and nitric oxide production in endothelial cells from human brain microcirculation. Cells 8 (7), 689. doi:10.3390/cells8070689
Berra-Romani R., Guzman-Silva A., Vargaz-Guadarrama A., Flores-Alonso J. C., Alonso-Romero J., Trevino S., et al. (2019b). Type 2 diabetes alters intracellular Ca(2+) handling in native endothelium of excised Rat Aorta. Int. J. Mol. Sci. 21 (1), 250. doi:10.3390/ijms21010250
Berra-Romani R., Faris P., Pellavio G., Orgiu M., Negri S., Forcaia G., et al. (2020). Histamine induces intracellular Ca(2+) oscillations and nitric oxide release in endothelial cells from brain microvascular circulation. J. Cell. Physiol. 235 (2), 1515–1530. doi:10.1002/jcp.29071
Berra-Romani R., Brunetti V., Pellavio G., Soda T., Laforenza U., Scarpellino G., et al. (2023). Allyl isothiocianate induces Ca(2+) signals and nitric oxide release by inducing reactive oxygen species production in the human cerebrovascular endothelial cell line hCMEC/D3. Cells 12 (13), 1732. doi:10.3390/cells12131732
Berridge M. J., Bootman M. D., Roderick H. L. (2003). Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell. Biol. 4 (7), 517–529. doi:10.1038/nrm1155
Berrout J., Jin M., O'Neil R. G. (2012). Critical role of TRPP2 and TRPC1 channels in stretch-induced injury of blood-brain barrier endothelial cells. Brain Res. 1436, 1–12. doi:10.1016/j.brainres.2011.11.044
Bintig W., Begandt D., Schlingmann B., Gerhard L., Pangalos M., Dreyer L., et al. (2012). Purine receptors and Ca(2+) signalling in the human blood-brain barrier endothelial cell line hCMEC/D3. Purinergic Signal 8 (1), 71–80. doi:10.1007/s11302-011-9262-7
Bouron A. (2020). Transcriptomic profiling of Ca2+ transport systems during the Formation of the cerebral cortex in mice. Cells 9 (8), 1800. doi:10.3390/cells9081800
Brunetti V., Berra-Romani R., Conca F., Soda T., Biella G. R., Gerbino A., et al. (2024a). Lysosomal TRPML1 triggers global Ca(2+) signals and nitric oxide release in human cerebrovascular endothelial cells. Front. Physiol. 15, 1426783. doi:10.3389/fphys.2024.1426783
Brunetti V., Soda T., Berra-Romani R., De Sarro G., Guerra G., Scarpellino G., et al. (2024b). Two signaling modes are better than one: Flux-Independent signaling by ionotropic glutamate receptors is coming of age. Biomedicines 12 (4), 880. doi:10.3390/biomedicines12040880
Brunetti V., Berra-Romani R., Coyotl-Santiago N., Esquitin-Gonzalez Y., Chinigo G., Biella G. R., et al. (2025). Histamine 1 receptors and reverse-mode Na(+)/Ca(2+) exchanger drive extracellular Na(+)-Dependent intracellular Ca(2+) oscillations in human cerebrovascular endothelial cells. Cell. Calcium 131, 103067. doi:10.1016/j.ceca.2025.103067
Cai J., Wang J., Wang Z., Wang J., Jia Y., Ma X. (2025). Perspectives on the alpha5 nicotinic acetylcholine receptor in lung cancer progression. Front. Cell. Dev. Biol. 13, 1489958. doi:10.3389/fcell.2025.1489958
Cantu Gutierrez M. E., Hill M. C., Largoza G. E., Gillespie W. B., Martin J. F., Wythe J. D. (2025). Mapping the transcriptional and epigenetic landscape of organotypic endothelial diversity in the developing and adult mouse. Nat. Cardiovasc. Res. 4 (4), 473–495. doi:10.1038/s44161-025-00618-0
Chen Z., Zhang Z., Gu Y., Bai C. (2011). Impaired migration and cell volume regulation in aquaporin 5-deficient SPC-A1 cells. Respir. Physiol. Neurobiol. 176 (3), 110–117. doi:10.1016/j.resp.2011.02.001
Chen L., Liu W., Wang P., Xue Y., Su Q., Zeng C., et al. (2015). Endophilin-1 regulates blood-brain barrier permeability via EGFR-JNK signaling pathway. Brain Res. 1606, 44–53. doi:10.1016/j.brainres.2015.02.032
Chen P., Tang H., Zhang Q., Xu L., Zhou W., Hu X., et al. (2020). Basic fibroblast growth factor (bFGF) protects the blood-brain barrier by binding of FGFR1 and activating the ERK signaling pathway after intra-abdominal hypertension and traumatic brain injury. Med. Sci. Monit. 26, e922009. doi:10.12659/MSM.922009
Cheng Y., Zhai Y. J., Wang S. S., Fan Y. Y. (2025). Proton-sensitive receptors/channels: therapeutic target for ischemic stroke, focusing on their roles and mechanisms. Curr. Neuropharmacol. 23. doi:10.2174/011570159X381737250710050221
Choublier N., Taghi M., Menet M. C., Le Gall M., Bruce J., Chafey P., et al. (2022). Exposure of human cerebral microvascular endothelial cells hCMEC/D3 to laminar shear stress induces vascular protective responses. Fluids Barriers CNS 19 (1), 41. doi:10.1186/s12987-022-00344-w
Cucullo L., Hossain M., Puvenna V., Marchi N., Janigro D. (2011). The role of shear stress in blood-brain barrier endothelial physiology. BMC Neurosci. 12, 40. doi:10.1186/1471-2202-12-40
da Silva I. V., Barroso M., Moura T., Castro R., Soveral G. (2018). Endothelial aquaporins and hypomethylation: potential implications for atherosclerosis and cardiovascular disease. Int. J. Mol. Sci. 19 (1), 130. doi:10.3390/ijms19010130
Dang X., Eliceiri B. P., Baird A., Costantini T. W. (2015). CHRFAM7A: a human-specific alpha7-nicotinic acetylcholine receptor gene shows differential responsiveness of human intestinal epithelial cells to LPS. FASEB J. 29 (6), 2292–2302. doi:10.1096/fj.14-268037
Davis M. J., Castorena-Gonzalez J. A., Li M., Zawieja S. D., Simon A. M., Geng X., et al. (2024). Connexin-45 is expressed in mouse lymphatic endothelium and required for lymphatic valve function. JCI Insight 9 (16), e169931. doi:10.1172/jci.insight.169931
De Bock M., Wang N., Decrock E., Bol M., Gadicherla A. K., Culot M., et al. (2013). Endothelial calcium dynamics, connexin channels and blood-brain barrier function. Prog. Neurobiol. 108, 1–20. doi:10.1016/j.pneurobio.2013.06.001
DeOre B. J., Partyka P. P., Fan F., Galie P. A. (2022). CD44 mediates shear stress mechanotransduction in an in vitro blood-brain barrier model through small GTPases RhoA and Rac1. FASEB J. 36 (5), e22278. doi:10.1096/fj.202100822RR
DeStefano J. G., Xu Z. S., Williams A. J., Yimam N., Searson P. C. (2017). Effect of shear stress on iPSC-derived human brain microvascular endothelial cells (dhBMECs). Fluids Barriers CNS 14 (1), 20. doi:10.1186/s12987-017-0068-z
Diaz-Otero J. M., Yen T. C., Ahmad A., Laimon-Thomson E., Abolibdeh B., Kelly K., et al. (2019). Transient receptor potential vanilloid 4 channels are important regulators of parenchymal arteriole dilation and cognitive function. Microcirculation 26 (6), e12535. doi:10.1111/micc.12535
Diering G. H., Church J., Numata M. (2009). Secretory carrier membrane protein 2 regulates cell-surface targeting of brain-enriched Na+/H+ exchanger NHE5. J. Biol. Chem. 284 (20), 13892–13903. doi:10.1074/jbc.M807055200
Ding R., Yin Y. L., Jiang L. H. (2021). Reactive oxygen species-induced TRPM2-Mediated Ca(2+) signalling in endothelial cells. Antioxidants (Basel) 10 (5), 718. doi:10.3390/antiox10050718
Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., et al. (2013). STAR: ultrafast universal RNA-Seq aligner. Bioinformatics 29 (1), 15–21. doi:10.1093/bioinformatics/bts635
Dragoni S., Laforenza U., Bonetti E., Reforgiato M., Poletto V., Lodola F., et al. (2014). Enhanced expression of stim, orai, and TRPC transcripts and proteins in endothelial progenitor cells isolated from patients with primary myelofibrosis. PLoS One 9 (3), e91099. doi:10.1371/journal.pone.0091099
Duncan J. W., Younes S. T., Hildebrandt E., Ryan M. J., Granger J. P., Drummond H. A. (2020). Tumor necrosis factor-alpha impairs cerebral blood flow in pregnant rats: role of vascular beta-epithelial Na(+) channel. Am. J. Physiol. Heart Circ. Physiol. 318 (4), H1018–H1027. doi:10.1152/ajpheart.00744.2019
Earley S. (2011). Endothelium-dependent cerebral artery dilation mediated by transient receptor potential and Ca2+-activated K+ channels. J. Cardiovasc Pharmacol. 57 (2), 148–153. doi:10.1097/FJC.0b013e3181f580d9
Elkhider A., Wang B., Ouyang X., Al-Azab M., Walana W., Sun X., et al. (2020). Aquaporin 5 promotes tumor migration and angiogenesis in non-small cell lung cancer cell line H1299. Oncol. Lett. 19 (3), 1665–1672. doi:10.3892/ol.2020.11251
Fan Y. S., Wang B., Wang D., Xu X., Gao C., Li Y., et al. (2021). Atorvastatin combined with low-dose dexamethasone for vascular endothelial cell dysfunction induced by chronic subdural hematoma. Neural Regen. Res. 16 (3), 523–530. doi:10.4103/1673-5374.293152
Fang Y., Tian Y., Huang Q., Wan Y., Xu L., Wang W., et al. (2019). Deficiency of TREK-1 potassium channel exacerbates blood-brain barrier damage and neuroinflammation after intracerebral hemorrhage in mice. J. Neuroinflammation 16 (1), 96. doi:10.1186/s12974-019-1485-5
Fatemi S. H., Folsom T. D., Rooney R. J., Thuras P. D. (2013). mRNA and protein expression for novel GABAA receptors theta and rho2 are altered in schizophrenia and mood disorders; relevance to FMRP-mGluR5 signaling pathway. Transl. Psychiatry 3 (6), e271. doi:10.1038/tp.2013.46
Ferrell R. E., Baty C. J., Kimak M. A., Karlsson J. M., Lawrence E. C., Franke-Snyder M., et al. (2010). GJC2 missense mutations cause human lymphedema. Am. J. Hum. Genet. 86 (6), 943–948. doi:10.1016/j.ajhg.2010.04.010
Gankam Kengne F. (2023). Adaptation of the brain to hyponatremia and its clinical implications. J. Clin. Med. 12 (5), 1714. doi:10.3390/jcm12051714
Garcia D. C. G., Longden T. A. (2020). Ion channels in capillary endothelium. Curr. Top. Membr. 85, 261–300. doi:10.1016/bs.ctm.2020.01.005
Gazzaley A. H., Weiland N. G., McEwen B. S., Morrison J. H. (1996). Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J. Neurosci. 16 (21), 6830–6838. doi:10.1523/JNEUROSCI.16-21-06830.1996
Gerbino C., Foglietta F., Corsi D., Nardini P., Cangemi L., Benetti E., et al. (2025). Histamine regulates the activity and the expression of the Na(+)/H(+) exchanger (NHE)3 in human epithelial HK-2 cells. Inflamm. Res. 74 (1), 122. doi:10.1007/s00011-025-02095-4
Gil E., Venturini C., Stirling D., Turner C., Tezera L. B., Ercoli G., et al. (2022). Pericyte derived chemokines amplify neutrophil recruitment across the cerebrovascular endothelial barrier. Front. Immunol. 13, 935798. doi:10.3389/fimmu.2022.935798
Gorur-Shandilya S., Marder E., O'Leary T. (2020). Activity-dependent compensation of cell size is vulnerable to targeted deletion of ion channels. Sci. Rep. 10 (1), 15989. doi:10.1038/s41598-020-72977-6
Grygorczyk R., Schwarz W., Passow H. (1984). Ca2+-activated K+ channels in human red cells. Comparison of single-channel currents with ion fluxes. Biophys. J. 45 (4), 693–698. doi:10.1016/S0006-3495(84)84211-3
Gulej R., Csik B., Faakye J., Tarantini S., Shanmugarama S., Chandragiri S. S., et al. (2024). Endothelial deficiency of insulin-like growth factor-1 receptor leads to blood-brain barrier disruption and accelerated endothelial senescence in mice, mimicking aspects of the brain aging phenotype. Microcirculation 31 (2), e12840. doi:10.1111/micc.12840
Hara-Chikuma M., Satooka H., Watanabe S., Honda T., Miyachi Y., Watanabe T., et al. (2015). Aquaporin-3-mediated hydrogen peroxide transport is required for NF-kappaB signalling in keratinocytes and development of psoriasis. Nat. Commun. 6, 7454. doi:10.1038/ncomms8454
Helms H. C., Abbott N. J., Burek M., Cecchelli R., Couraud P. O., Deli M. A., et al. (2016). In vitro models of the blood-brain barrier: an overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow. Metab. 36 (5), 862–890. doi:10.1177/0271678X16630991
Higashi Y., Sukhanov S., Shai S. Y., Danchuk S., Snarski P., Li Z., et al. (2020). Endothelial deficiency of insulin-like growth factor-1 receptor reduces endothelial barrier function and promotes atherosclerosis in Apoe-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 319 (4), H730–H743. doi:10.1152/ajpheart.00064.2020
Higuchi K., Sivaprakasam S., Sennoune S. R., Ogura J., Bhutia Y. D., Rueda R., et al. (2021). A proton-coupled transport system for beta-hydroxy-beta-methylbutyrate (HMB) in blood-brain barrier endothelial cell line hCMEC/D3. Nutrients 13 (9), 3220. doi:10.3390/nu13093220
Huang J., Zhang R., Wang S., Zhang D., Leung C. K., Yang G., et al. (2021). Methamphetamine and HIV-Tat protein synergistically induce oxidative stress and blood-brain barrier damage via transient receptor potential melastatin 2 channel. Front. Pharmacol. 12, 619436. doi:10.3389/fphar.2021.619436
Huo J., Mo L., Lv X., Du Y., Yang H. (2025). Ion channel regulation in caveolae and its pathological implications. Cells 14 (9), 631. doi:10.3390/cells14090631
Iliff J. J., Wang M., Liao Y., Plogg B. A., Peng W., Gundersen G. A., et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4 (147), 147ra111. doi:10.1126/scitranslmed.3003748
Iyo A. H., Feyissa A. M., Chandran A., Austin M. C., Regunathan S., Karolewicz B. (2010). Chronic corticosterone administration down-regulates metabotropic glutamate receptor 5 protein expression in the rat hippocampus. Neuroscience 169 (4), 1567–1574. doi:10.1016/j.neuroscience.2010.06.023
Jackson W. F. (2022). Endothelial ion channels and cell-cell communication in the microcirculation. Front. Physiol. 13, 805149. doi:10.3389/fphys.2022.805149
Kaczmarek L. K. (2013). Slack, slick and sodium-activated potassium channels. ISRN Neurosci. 2013, 354262. doi:10.1155/2013/354262
Kadry H., Noorani B., Cucullo L. (2020). A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 17 (1), 69. doi:10.1186/s12987-020-00230-3
Kalari K. R., Thompson K. J., Nair A. A., Tang X., Bockol M. A., Jhawar N., et al. (2016). BBBomics-Human blood brain barrier transcriptomics hub. Front. Neurosci. 10, 71. doi:10.3389/fnins.2016.00071
Kang H., Lee C. J. (2024). Transmembrane proteins with unknown function (TMEMs) as ion channels: electrophysiological properties, structure, and pathophysiological roles. Exp. Mol. Med. 56 (4), 850–860. doi:10.1038/s12276-024-01206-1
Kaplan L., Chow B. W., Gu C. (2020). Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat. Rev. Neurosci. 21 (8), 416–432. doi:10.1038/s41583-020-0322-2
Karakas E., Strange K., Denton J. S. (2025). Recent advances in structural characterization of volume-regulated anion channels (VRACs). J. Physiol. 603 (15), 4201–4211. doi:10.1113/JP286189
Kida H., Miyoshi T., Manabe K., Takahashi N., Konno T., Ueda S., et al. (2005). Roles of aquaporin-3 water channels in volume-regulatory water flow in a human epithelial cell line. J. Membr. Biol. 208 (1), 55–64. doi:10.1007/s00232-005-0819-7
Kim H. R., Lee G. H., Ha K. C., Ahn T., Moon J. Y., Lee B. J., et al. (2008). Bax Inhibitor-1 is a pH-dependent regulator of Ca2+ channel activity in the endoplasmic reticulum. J. Biol. Chem. 283 (23), 15946–15955. doi:10.1074/jbc.M800075200
Kim K. S., Jeon M. T., Kim E. S., Lee C. H., Kim D. G. (2022). Activation of NMDA receptors in brain endothelial cells increases transcellular permeability. Fluids Barriers CNS 19 (1), 70. doi:10.1186/s12987-022-00364-6
Kriauciunaite K., Pociute A., Kausyle A., Verkhratsky A., Pivoriunas A. (2023). Basic fibroblast growth factor opens and closes the endothelial blood-brain barrier in a concentration-dependent manner. Neurochem. Res. 48 (4), 1211–1221. doi:10.1007/s11064-022-03678-x
Krolak T., Kaplan L., Navas K., Chen L., Birmingham A., Ryvkin D., et al. (2025). Brain endothelial gap junction coupling enables rapid vasodilation propagation during neurovascular coupling. Cell. 188, 5003–5019.e22. doi:10.1016/j.cell.2025.06.030
Kumabe H., Masuda T., Ito S., Furihata T., Toda A., Mogi M., et al. (2025). Proteome profile differences among human, monkey, and mouse brain microvessels and cultured brain microvascular endothelial cells. Fluids Barriers CNS 22 (1), 53. doi:10.1186/s12987-025-00650-z
Lai Y., Zhang Y., Zhou S., Xu J., Du Z., Feng Z., et al. (2023). Structure of the human ATP synthase. Mol. Cell. 83 (12), 2137–2147 e2134. doi:10.1016/j.molcel.2023.04.029
Lambrichts S. M. P., van Oostenbrugge R. J., Foulquier S. (2025). TRPV4 in cerebral small vessel disease: a key interacting partner. Vasc. Pharmacol. 159, 107492. doi:10.1016/j.vph.2025.107492
Lang S., Erdmann F., Jung M., Wagner R., Cavalie A., Zimmermann R. (2011). Sec61 complexes form ubiquitous ER Ca2+ leak channels. Channels (Austin) 5 (3), 228–235. doi:10.4161/chan.5.3.15314
Lange C., Storkebaum E., de Almodovar C. R., Dewerchin M., Carmeliet P. (2016). Vascular endothelial growth factor: a neurovascular target in neurological diseases. Nat. Rev. Neurol. 12 (8), 439–454. doi:10.1038/nrneurol.2016.88
Li B., Dewey C. N. (2011). RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinforma. 12, 323. doi:10.1186/1471-2105-12-323
Li S., Kumar T. P., Joshee S., Kirschstein T., Subburaju S., Khalili J. S., et al. (2018). Endothelial cell-derived GABA signaling modulates neuronal migration and postnatal behavior. Cell. Res. 28 (2), 221–248. doi:10.1038/cr.2017.135
Li J., Zhang Y., Yang C., Rong R. (2020). Discrepant mRNA and protein expression in immune cells. Curr. Genomics 21 (8), 560–563. doi:10.2174/1389202921999200716103758
Lim X. R., Harraz O. F. (2024). Mechanosensing by vascular endothelium. Annu. Rev. Physiol. 86, 71–97. doi:10.1146/annurev-physiol-042022-030946
Lim X. R., Abd-Alhaseeb M. M., Ippolito M., Koide M., Senatore A. J., Plante C., et al. (2024). Endothelial Piezo1 channel mediates mechano-feedback control of brain blood flow. Nat. Commun. 15 (1), 8686. doi:10.1038/s41467-024-52969-0
Lin L. H., Jin J., Nashelsky M. B., Talman W. T. (2014). Acid-sensing ion channel 1 and nitric oxide synthase are in adjacent layers in the wall of rat and human cerebral arteries. J. Chem. Neuroanat. 61-62, 161–168. doi:10.1016/j.jchemneu.2014.10.002
Lisak D. A., Schacht T., Enders V., Habicht J., Kiviluoto S., Schneider J., et al. (2015). The transmembrane bax inhibitor motif (TMBIM) containing protein family: tissue expression, intracellular localization and effects on the ER CA(2)(+)-filling state. Biochim. Biophys. Acta 1853 (9), 2104–2114. doi:10.1016/j.bbamcr.2015.03.002
Liu Q. (2017). TMBIM-Mediated Ca(2+) homeostasis and cell death. Biochim. Biophys. Acta Mol. Cell. Res. 1864 (6), 850–857. doi:10.1016/j.bbamcr.2016.12.023
Liu X., Wu Y., Zhao Y., Huang Y., Xu K., Wang J., et al. (2021). Identification of Plasmodium falciparum-specific protein PIESP2 as a novel virulence factor related to cerebral malaria. Int. J. Biol. Macromol. 177, 535–547. doi:10.1016/j.ijbiomac.2021.02.145
Liu R., Collier J. M., Abdul-Rahman N. H., Capuk O., Zhang Z., Begum G. (2024). Dysregulation of ion channels and transporters and blood-brain barrier dysfunction in Alzheimer's Disease and vascular dementia. Aging Dis. 15 (4), 1748–1770. doi:10.14336/AD.2023.1201
Lockwich T., Makusky A., Kowalak J. A., Markey S. P., Ambudkar I. S. (2011). “Proteomic analysis of TRPC channels,” in TRP channels. Editor M. X. Zhu (Boca Raton (FL)).
Longden T. A., Nelson M. T. (2015). Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation 22 (3), 183–196. doi:10.1111/micc.12190
Longden T. A., Hill-Eubanks D. C., Nelson M. T. (2016). Ion channel networks in the control of cerebral blood flow. J. Cereb. Blood Flow. Metab. 36 (3), 492–512. doi:10.1177/0271678X15616138
Longden T. A., Dabertrand F., Koide M., Gonzales A. L., Tykocki N. R., Brayden J. E., et al. (2017). Capillary K(+)-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci. 20 (5), 717–726. doi:10.1038/nn.4533
Longden T. A., Mughal A., Hennig G. W., Harraz O. F., Shui B., Lee F. K., et al. (2021). Local IP3 receptor-mediated Ca(2+) signals compound to direct blood flow in brain capillaries. Sci. Adv. 7 (30), eabh0101. doi:10.1126/sciadv.abh0101
Luo H., Gauthier M., Tan X., Landry C., Poupon J., Dehouck M. P., et al. (2018). Sodium transporters are involved in lithium influx in brain endothelial cells. Mol. Pharm. 15 (7), 2528–2538. doi:10.1021/acs.molpharmaceut.8b00018
Luo H., Saubamea B., Chasseigneaux S., Cochois V., Smirnova M., Glacial F., et al. (2020). Molecular and functional Study of transient receptor potential Vanilloid 1-4 at the rat and human blood-brain barrier reveals interspecies differences. Front. Cell. Dev. Biol. 8, 578514. doi:10.3389/fcell.2020.578514
Luo L., Song S., Ezenwukwa C. C., Jalali S., Sun B., Sun D. (2021). Ion channels and transporters in microglial function in physiology and brain diseases. Neurochem. Int. 142, 104925. doi:10.1016/j.neuint.2020.104925
Macrez R., Ortega M. C., Bardou I., Mehra A., Fournier A., Van der Pol S. M., et al. (2016). Neuroendothelial NMDA receptors as therapeutic targets in experimental autoimmune encephalomyelitis. Brain 139 (Pt 9), 2406–2419. doi:10.1093/brain/aww172
Maier J. A. M., Locatelli L., Fedele G., Cazzaniga A., Mazur A. (2022). Magnesium and the brain: a focus on neuroinflammation and neurodegeneration. Int. J. Mol. Sci. 24 (1), 223. doi:10.3390/ijms24010223
Manabe K., Takano M., Noma A. (1995). Non-selective cation current of guinea-pig endocardial endothelial cells. J. Physiol. 487 (Pt 2), 407–419. doi:10.1113/jphysiol.1995.sp020889
Manoonkitiwongsa P. S., Whitter E. F., Wareesangtip W., McMillan P. J., Nava P. B., Schultz R. L. (2000). Calcium-dependent ATPase unlike ecto-ATPase is located primarily on the luminal surface of brain endothelial cells. Histochem J. 32 (5), 313–324. doi:10.1023/a:1004093113985
Marchenko S. M., Sage S. O. (1993). Electrical properties of resting and acetylcholine-stimulated endothelium in intact rat aorta. J. Physiol. 462, 735–751. doi:10.1113/jphysiol.1993.sp019579
Martinotti S., Laforenza U., Patrone M., Moccia F., Ranzato E. (2019). Honey-Mediated wound healing: H(2)O(2) entry through AQP3 determines extracellular Ca(2+) influx. Int. J. Mol. Sci. 20 (3), 764. doi:10.3390/ijms20030764
Martus D., Williams S. K., Pichi K., Mannebach-Gotz S., Kaiser N., Wardas B., et al. (2024). Cavbeta3 contributes to the maintenance of the blood-brain barrier and alleviates symptoms of Experimental Autoimmune encephalomyelitis. Arterioscler. Thromb. Vasc. Biol. 44 (8), 1833–1851. doi:10.1161/ATVBAHA.124.321141
Mehra A., Guerit S., Macrez R., Gosselet F., Sevin E., Lebas H., et al. (2020). Non-ionotropic action of endothelial NMDA receptors on blood-brain barrier permeability via Rho/ROCK mediated phosphorylation of myosin. J. Neurosci. 40, 1778–1787. doi:10.1523/JNEUROSCI.0969-19.2019
Mehrke G., Pohl U., Daut J. (1991). Effects of vasoactive agonists on the membrane potential of cultured bovine aortic and guinea-pig coronary endothelium. J. Physiol. 439, 277–299. doi:10.1113/jphysiol.1991.sp018667
Mestre H., Hablitz L. M., Xavier A. L., Feng W., Zou W., Pu T., et al. (2018). Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7, e40070. doi:10.7554/eLife.40070
Miller E. W., Dickinson B. C., Chang C. J. (2010). Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. U. S. A. 107 (36), 15681–15686. doi:10.1073/pnas.1005776107
Misquitta C. M., Chen T., Grover A. K. (2006). Control of protein expression through mRNA stability in calcium signalling. Cell. Calcium 40 (4), 329–346. doi:10.1016/j.ceca.2006.04.004
Moccia F., Baruffi S., Spaggiari S., Coltrini D., Berra-Romani R., Signorelli S., et al. (2001). P2y1 and P2y2 receptor-operated Ca2+ signals in primary cultures of cardiac microvascular endothelial cells. Microvasc. Res. 61 (3), 240–252. doi:10.1006/mvre.2001.2306
Moccia F., Berra-Romani R., Baruffi S., Spaggiari S., Adams D. J., Taglietti V., et al. (2002a). Basal nonselective cation permeability in rat cardiac microvascular endothelial cells. Microvasc. Res. 64 (2), 187–197. doi:10.1006/mvre.2002.2430
Moccia F., Berra-Romani R., Baruffi S., Spaggiari S., Signorelli S., Castelli L., et al. (2002b). Ca2+ uptake by the endoplasmic reticulum Ca2+-ATPase in rat microvascular endothelial cells. Biochem. J. 364 (Pt 1), 235–244. doi:10.1042/bj3640235
Moccia F., Frost C., Berra-Romani R., Tanzi F., Adams D. J. (2004). Expression and function of neuronal nicotinic ACh receptors in rat microvascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 286 (2), H486–H491. doi:10.1152/ajpheart.00620.2003
Moccia F., Negri S., Shekha M., Faris P., Guerra G. (2019). Endothelial Ca(2+) signaling, angiogenesis and vasculogenesis: just what it takes to make a blood vessel. Int. J. Mol. Sci. 20 (16), 3962. doi:10.3390/ijms20163962
Moccia F., Negri S., Faris P., Angelone T. (2022). Targeting endothelial ion signalling to rescue cerebral blood flow in cerebral disorders. Vasc. Pharmacol. 145, 106997. doi:10.1016/j.vph.2022.106997
Moccia F., Brunetti V., Perna A., Guerra G., Soda T., Berra-Romani R. (2023a). The molecular heterogeneity of store-operated Ca(2+) entry in vascular endothelial cells: the different roles of Orai1 and TRPC1/TRPC4 channels in the transition from Ca(2+)-Selective to non-selective cation currents. Int. J. Mol. Sci. 24 (4), 3259. doi:10.3390/ijms24043259
Moccia F., Brunetti V., Soda T., Berra-Romani R., Scarpellino G. (2023b). Cracking the endothelial calcium (Ca(2+)) code: a matter of timing and spacing. Int. J. Mol. Sci. 24 (23), 16765. doi:10.3390/ijms242316765
Mokgokong R., Wang S., Taylor C. J., Barrand M. A., Hladky S. B. (2014). Ion transporters in brain endothelial cells that contribute to formation of brain interstitial fluid. Pflugers Arch. 466 (5), 887–901. doi:10.1007/s00424-013-1342-9
Monteil A., Guerineau N. C., Gil-Nagel A., Parra-Diaz P., Lory P., Senatore A. (2024). New insights into the physiology and pathophysiology of the atypical sodium leak channel NALCN. Physiol. Rev. 104 (1), 399–472. doi:10.1152/physrev.00014.2022
Montes de Oca Balderas P. (2022). Meeting report: flux-Independent signaling by ionotropic receptors: unforeseen roles, complexities, and challenges. J. Biol. Chem. 298 (9), 102330. doi:10.1016/j.jbc.2022.102330
Mughal A., Hennig G. W., Heppner T., Tsoukias N. M., Hill-Eubanks D., Nelson M. T. (2024a). Electrocalcium coupling in brain capillaries: rapidly traveling electrical signals ignite local calcium signals. Proc. Natl. Acad. Sci. U. S. A. 121 (51), e2415047121. doi:10.1073/pnas.2415047121
Mughal A., Sackheim A. M., Koide M., Bonson G., Ebner G., Hennig G., et al. (2024b). Pathogenic soluble tau peptide disrupts endothelial calcium signaling and vasodilation in the brain microvasculature. J. Cereb. Blood Flow. Metab. 271678X241235790, 680–688. doi:10.1177/0271678X241235790
Murgas M., Michenthaler P., Reed M. B., Gryglewski G., Lanzenberger R. (2022). Correlation of receptor density and mRNA expression patterns in the human cerebral cortex. Neuroimage 256, 119214. doi:10.1016/j.neuroimage.2022.119214
Murphy T. V., Sandow S. L. (2019). Agonist-evoked endothelial Ca(2+) signalling microdomains. Curr. Opin. Pharmacol. 45, 8–15. doi:10.1016/j.coph.2019.03.005
Negri S., Faris P., Pellavio G., Botta L., Orgiu M., Forcaia G., et al. (2020). Group 1 metabotropic glutamate receptors trigger glutamate-induced intracellular Ca(2+) signals and nitric oxide release in human brain microvascular endothelial cells. Cell. Mol. Life Sci. 77 (11), 2235–2253. doi:10.1007/s00018-019-03284-1
Negri S., Faris P., Maniezzi C., Pellavio G., Spaiardi P., Botta L., et al. (2021a). NMDA receptors elicit flux-independent intracellular Ca(2+) signals via metabotropic glutamate receptors and flux-dependent nitric oxide release in human brain microvascular endothelial cells. Cell. Calcium 99, 102454. doi:10.1016/j.ceca.2021.102454
Negri S., Faris P., Moccia F. (2021b). Endolysosomal Ca(2+) signaling in cardiovascular health and disease. Int. Rev. Cell. Mol. Biol. 363, 203–269. doi:10.1016/bs.ircmb.2021.03.001
Negri S., Faris P., Moccia F. (2021c). Reactive oxygen species and endothelial Ca(2+) signaling: brothers in arms or partners in crime? Int. J. Mol. Sci. 22 (18), 9821. doi:10.3390/ijms22189821
Negri S., Faris P., Soda T., Moccia F. (2021d). Endothelial signaling at the core of neurovascular coupling: the emerging role of endothelial inward-rectifier K(+) (kir2.1) channels and N-methyl-d-aspartate receptors in the regulation of cerebral blood flow. Int. J. Biochem. Cell. Biol. 135, 105983. doi:10.1016/j.biocel.2021.105983
Negri S., Scolari F., Vismara M., Brunetti V., Faris P., Terribile G., et al. (2022). GABA(A) and GABA(B) receptors mediate GABA-induced intracellular Ca(2+) signals in human brain Microvascular endothelial cells. Cells 11 (23), 3860. doi:10.3390/cells11233860
Niciu M. J., Ma X. M., El Meskini R., Pachter J. S., Mains R. E., Eipper B. A. (2007). Altered ATP7A expression and other compensatory responses in a murine model of Menkes disease. Neurobiol. Dis. 27 (3), 278–291. doi:10.1016/j.nbd.2007.05.004
Nilius B., Droogmans G. (2001). Ion channels and their functional role in vascular endothelium. Physiol. Rev. 81 (4), 1415–1459. doi:10.1152/physrev.2001.81.4.1415
Nishi M., Komazaki S., Iino M., Kangawa K., Takeshima H. (1998). Mitsugumin23, a novel transmembrane protein on endoplasmic reticulum and nuclear membranes. FEBS Lett. 432 (3), 191–196. doi:10.1016/s0014-5793(98)00864-3
Niu Y., Tao X., Vaisey G., Olinares P. D. B., Alwaseem H., Chait B. T., et al. (2021). Analysis of the mechanosensor channel functionality of TACAN. Elife 10, e71188. doi:10.7554/eLife.71188
O'Donnell M. E. (2014). Blood-brain barrier Na transporters in ischemic stroke. Adv. Pharmacol. 71, 113–146. doi:10.1016/bs.apha.2014.06.011
O'Leary T., Williams A. H., Caplan J. S., Marder E. (2013). Correlations in ion channel expression emerge from homeostatic tuning rules. Proc. Natl. Acad. Sci. U. S. A. 110 (28), E2645–E2654. doi:10.1073/pnas.1309966110
Obermeier B., Daneman R., Ransohoff R. M. (2013). Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 19 (12), 1584–1596. doi:10.1038/nm.3407
Oka T., Sayano T., Tamai S., Yokota S., Kato H., Fujii G., et al. (2008). Identification of a novel protein MICS1 that is involved in maintenance of mitochondrial morphology and apoptotic release of cytochrome c. Mol. Biol. Cell. 19 (6), 2597–2608. doi:10.1091/mbc.e07-12-1205
Okura T., Kato S., Deguchi Y. (2014). Functional expression of organic cation/carnitine transporter 2 (OCTN2/SLC22A5) in human brain capillary endothelial cell line hCMEC/D3, a human blood-brain barrier model. Drug Metab. Pharmacokinet. 29 (1), 69–74. doi:10.2133/dmpk.dmpk-13-rg-058
Ottolini M., Daneva Z., Chen Y. L., Cope E. L., Kasetti R. B., Zode G. S., et al. (2020). Mechanisms underlying selective coupling of endothelial Ca(2+) signals with eNOS vs. IK/SK channels in systemic and pulmonary arteries. J. Physiol. 598 (17), 3577–3596. doi:10.1113/JP279570
Owji A. P., Kittredge A., Zhang Y., Yang T. (2021). Structure and Function of the Bestrophin family of calcium-activated chloride channels. Channels (Austin) 15 (1), 604–623. doi:10.1080/19336950.2021.1981625
Ozturk S., Cillier A. E. (2006). Magnesium supplementation in the treatment of dementia patients. Med. Hypotheses 67 (5), 1223–1225. doi:10.1016/j.mehy.2006.04.047
Park S. J., Kim Y. C., Suh S. H., Rhim H., Sim J. H., Kim S. J., et al. (2000). Background nonselective cationic current and the resting membrane potential in rabbit aorta endothelial cells. Jpn. J. Physiol. 50 (6), 635–643. doi:10.2170/jjphysiol.50.635
Park L., Wang G., Moore J., Girouard H., Zhou P., Anrather J., et al. (2014). The key role of transient receptor potential melastatin-2 channels in amyloid-beta-induced neurovascular dysfunction. Nat. Commun. 5, 5318. doi:10.1038/ncomms6318
Patron M., Tarasenko D., Nolte H., Kroczek L., Ghosh M., Ohba Y., et al. (2022). Regulation of mitochondrial proteostasis by the proton gradient. EMBO J. 41 (16), e110476. doi:10.15252/embj.2021110476
Pavlovic N., Kopsida M., Gerwins P., Heindryckx F. (2020). Inhibiting P2Y12 in macrophages induces endoplasmic reticulum stress and promotes an anti-tumoral phenotype. Int. J. Mol. Sci. 21 (21), 8177. doi:10.3390/ijms21218177
Periasamy M., Kalyanasundaram A. (2007). SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve 35 (4), 430–442. doi:10.1002/mus.20745
Peters E. C., Gee M. T., Pawlowski L. N., Kath A. M., Polk F. D., Vance C. J., et al. (2021). Amyloid-beta disrupts unitary calcium entry through endothelial NMDA receptors in mouse cerebral arteries. J. Cereb. Blood Flow. Metab. 42 (1), 145–161. doi:10.1177/0271678X211039592
Pierson H., Muchenditsi A., Kim B. E., Ralle M., Zachos N., Huster D., et al. (2018). The function of ATPase copper transporter ATP7B in intestine. Gastroenterology 154 (1), 168–180 e165. doi:10.1053/j.gastro.2017.09.019
Pires P. W., Sullivan M. N., Pritchard H. A., Robinson J. J., Earley S. (2015). Unitary TRPV3 channel Ca2+ influx events elicit endothelium-dependent dilation of cerebral parenchymal arterioles. Am. J. Physiol. Heart Circ. Physiol. 309 (12), H2031–H2041. doi:10.1152/ajpheart.00140.2015
Polk F. D., Hakim M. A., Silva J. F., Behringer E. J., Pires P. W. (2023). Endothelial K(IR)2 channel dysfunction in aged cerebral parenchymal arterioles. Am. J. Physiol. Heart Circ. Physiol. 325 (6), H1360–H1372. doi:10.1152/ajpheart.00279.2023
Pulgar V. M. (2018). Transcytosis to cross the blood brain barrier, new advancements and challenges. Front. Neurosci. 12, 1019. doi:10.3389/fnins.2018.01019
Qi D., Lin H., Hu B., Wei Y. (2023). A review on in vitro model of the blood-brain barrier (BBB) based on hCMEC/D3 cells. J. Control Release 358, 78–97. doi:10.1016/j.jconrel.2023.04.020
Rauch B. H., Rosenkranz A. C., Ermler S., Bohm A., Driessen J., Fischer J. W., et al. (2010). Regulation of functionally active P2Y12 ADP receptors by thrombin in human smooth muscle cells and the presence of P2Y12 in carotid artery lesions. Arterioscler. Thromb. Vasc. Biol. 30 (12), 2434–2442. doi:10.1161/ATVBAHA.110.213702
Rice F. L., Albrecht P. J., Wymer J. P., Black J. A., Merkies I. S., Faber C. G., et al. (2015). Sodium channel Nav1.7 in vascular myocytes, endothelium, and innervating axons in human skin. Mol. Pain 11, 26. doi:10.1186/s12990-015-0024-3
Risher W. C., Eroglu C. (2020). Emerging roles for alpha2delta subunits in calcium channel function and synaptic connectivity. Curr. Opin. Neurobiol. 63, 162–169. doi:10.1016/j.conb.2020.04.007
Rose R., Kemper B., Schwab A., Schlatter E., Edemir B. (2021). Unexpected localization of AQP3 and AQP4 induced by migration of primary cultured IMCD cells. Sci. Rep. 11 (1), 11930. doi:10.1038/s41598-021-91369-y
Ruffinatti F. A., Scarpellino G., Visentin L. (2025). Bash modules for the remote analysis of RNA-Seq data. Prepr. Org. doi:10.20944/preprints202507.0213.v1
Rust R., Yin H., Achon Buil B., Sagare A. P., Kisler K. (2025). The blood-brain barrier: a help and a hindrance. Brain 148 (7), 2262–2282. doi:10.1093/brain/awaf068
Salmeri M., Motta C., Anfuso C. D., Amodeo A., Scalia M., Toscano M. A., et al. (2013). VEGF receptor-1 involvement in pericyte loss induced by Escherichia coli in an in vitro model of blood brain barrier. Cell. Microbiol. 15 (8), 1367–1384. doi:10.1111/cmi.12121
Samimi G., Varki N. M., Wilczynski S., Safaei R., Alberts D. S., Howell S. B. (2003). Increase in expression of the copper transporter ATP7A during platinum drug-based treatment is associated with poor survival in ovarian cancer patients. Clin. Cancer Res. 9 (16 Pt 1), 5853–5859.
Sancho M., Klug N. R., Mughal A., Koide M., Huerta de la Cruz S., Heppner T. J., et al. (2022). Adenosine signaling activates ATP-sensitive K(+) channels in endothelial cells and pericytes in CNS capillaries. Sci. Signal 15 (727), eabl5405. doi:10.1126/scisignal.abl5405
Sandoval H., Ibanez B., Contreras M., Troncoso F., Castro F. O., Caamano D., et al. (2025). Extracellular vesicles from Preeclampsia disrupt the blood-brain barrier by reducing CLDN5. Arterioscler. Thromb. Vasc. Biol. 45 (2), 298–311. doi:10.1161/ATVBAHA.124.321077
Scarpellino G., Genova T., Quarta E., Distasi C., Dionisi M., Fiorio Pla A., et al. (2022). P2X purinergic receptors are multisensory detectors for micro-environmental stimuli that control migration of tumoral endothelium. Cancers (Basel) 14 (11), 2743. doi:10.3390/cancers14112743
Scarpellino G., Brunetti V., Berra-Romani R., De Sarro G., Guerra G., Soda T., et al. (2024). The unexpected role of the endothelial Nitric oxide synthase at the Neurovascular unit: beyond the regulation of cerebral blood flow. Int. J. Mol. Sci. 25 (16), 9071. doi:10.3390/ijms25169071
Schaeffer S., Iadecola C. (2021). Revisiting the neurovascular unit. Nat. Neurosci. 24 (9), 1198–1209. doi:10.1038/s41593-021-00904-7
Schaller L., Kiefmann M., Gudermann T., Dietrich A. (2025). TRPV2 channels facilitate pulmonary endothelial barrier recovery after ROS-induced permeability. Redox Biol. 85, 103720. doi:10.1016/j.redox.2025.103720
Schreurs M. P., Houston E. M., May V., Cipolla M. J. (2012). The adaptation of the blood-brain barrier to vascular endothelial growth factor and placental growth factor during pregnancy. FASEB J. 26 (1), 355–362. doi:10.1096/fj.11-191916
Shao Y., Mai L., Qiao R., Liang Y., Jiao Y., Homburg J., et al. (2025). Endothelial mitochondria in the blood-brain barrier. Fluids Barriers CNS 22 (1), 88. doi:10.1186/s12987-025-00699-w
Shen J., Xu G., Zhu R., Yuan J., Ishii Y., Hamashima T., et al. (2019). PDGFR-beta restores blood-brain barrier functions in a mouse model of focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 39 (8), 1501–1515. doi:10.1177/0271678X18769515
Silva P. M., da Silva I. V., Sarmento M. J., Silva I. C., Carvalho F. A., Soveral G., et al. (2022). Aquaporin-3 and Aquaporin-5 facilitate migration and cell-cell adhesion in pancreatic cancer by modulating cell biomechanical properties. Cells 11 (8), 1308. doi:10.3390/cells11081308
Simon F., Guyot L., Garcia J., Vilchez G., Bardel C., Chenel M., et al. (2021). Impact of interleukin-6 on drug transporters and permeability in the hCMEC/D3 blood-brain barrier model. Fundam. Clin. Pharmacol. 35 (2), 397–409. doi:10.1111/fcp.12596
Sobradillo D., Hernandez-Morales M., Ubierna D., Moyer M. P., Nunez L., Villalobos C. (2014). A reciprocal shift in transient receptor potential channel 1 (TRPC1) and stromal interaction molecule 2 (STIM2) contributes to Ca2+ remodeling and cancer hallmarks in colorectal carcinoma cells. J. Biol. Chem. 289 (42), 28765–28782. doi:10.1074/jbc.M114.581678
Soda T., Brunetti V., Berra-Romani R., Moccia F. (2023). The emerging role of N-Methyl-D-Aspartate (NMDA) receptors in the cardiovascular System: physiological implications, pathological consequences, and therapeutic perspectives. Int. J. Mol. Sci. 24 (4), 3914. doi:10.3390/ijms24043914
Soda T., Negri S., Scarpellino G., Berra-Romani R., De Sarro G., Moccia F., et al. (2024a). An automated planar patch-clamp approach to measure the membrane potential and resting membrane currents in a human cerebrovascular endothelial cell line. J. Neurosci. Methods 410, 110248. doi:10.1016/j.jneumeth.2024.110248
Soda T., Pasqua T., De Sarro G., Moccia F. (2024b). Cognitive impairment and synaptic dysfunction in cardiovascular disorders: the new frontiers of the heart-brain axis. Biomedicines 12 (10), 2387. doi:10.3390/biomedicines12102387
Soda T., Brunetti V., De Sarro G., Biella G., Moccia F., Berra-Romani R., et al. (2025). Transient receptor potential ankyrin 1 (TRPA1) mediates hydrogen sulfide-induced Ca2+ entry and nitric oxide production in human cerebrovascular endothelium. Curr. Neuropharmacol. 23, 1119–1133. doi:10.2174/011570159X349872250124124612
Solano A. S., Lavanderos B., Metwally E., Earley S. (2025). Transient receptor potential channels in vascular mechanotransduction. Am. J. Hypertens. 38 (3), 151–160. doi:10.1093/ajh/hpae134
Stoica R., Rusu C. M., Staicu C. E., Burlacu A. E., Radu M., Radu B. M. (2021). Ca(2+) homeostasis in brain microvascular endothelial cells. Int. Rev. Cell. Mol. Biol. 362, 55–110. doi:10.1016/bs.ircmb.2021.01.001
Sudhahar V., Okur M. N., O'Bryan J. P., Minshall R. D., Fulton D., Ushio-Fukai M., et al. (2020). Caveolin-1 stabilizes ATP7A, a copper transporter for extracellular SOD, in vascular tissue to maintain endothelial function. Am. J. Physiol. Cell. Physiol. 319 (5), C933–C944. doi:10.1152/ajpcell.00151.2020
Sullivan M. N., Gonzales A. L., Pires P. W., Bruhl A., Leo M. D., Li W., et al. (2015). Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci. Signal 8 (358), ra2. doi:10.1126/scisignal.2005659
Suzuki T., Yasumoto M., Suzuki Y., Asai K., Imaizumi Y., Yamamura H. (2020). TMEM16A Ca(2+)-Activated Cl(-) channel regulates the proliferation and migration of Brain capillary endothelial cells. Mol. Pharmacol. 98 (1), 61–71. doi:10.1124/mol.119.118844
Tae H. S., Adams D. J. (2023). Nicotinic acetylcholine receptor subtype expression, function, and pharmacology: therapeutic potential of alpha-conotoxins. Pharmacol. Res. 191, 106747. doi:10.1016/j.phrs.2023.106747
Tamma G., Valenti G., Grossini E., Donnini S., Marino A., Marinelli R. A., et al. (2018). Aquaporin membrane channels in oxidative stress, cell signaling, and aging: recent advances and research trends. Oxid. Med. Cell. Longev. 2018, 1501847. doi:10.1155/2018/1501847
Tang Z., Li R., Guo X., Wang Z., Wu J. (2025). Regulation of blood-brain barrier integrity by brain microvascular endothelial cells in ischemic stroke: a therapeutic opportunity. Eur. J. Pharmacol. 996, 177553. doi:10.1016/j.ejphar.2025.177553
Teles R. H. G., Villarinho N. J., Yamagata A. S., Hiroki C. T., de Oliveira M. C., Tercarioli G. R., et al. (2025). Valosin-containing protein (VCP), a component of tumor-derived extracellular vesicles, impairs the barrier integrity of brain microvascular endothelial cells. BBA Adv. 7, 100130. doi:10.1016/j.bbadva.2024.100130
Thakore P., Earley S. (2019). Transient receptor potential channels and endothelial cell calcium signaling. Compr. Physiol. 9 (3), 1249–1277. doi:10.1002/cphy.c180034
Thillaiappan N. B., Chakraborty P., Hasan G., Taylor C. W. (2019). IP(3) receptors and Ca(2+) entry. Biochim. Biophys. Acta Mol. Cell. Res. 1866 (7), 1092–1100. doi:10.1016/j.bbamcr.2018.11.007
Tian Y., Wang Y., Zhao Y., Liu C., Zhang X., Zhang Y., et al. (2025). LRRC8A in endothelial cells contributes to the aberrant blood-brain barrier integrity in ischaemic stroke. Stroke Vasc. Neurol. 10, 625–636. doi:10.1136/svn-2024-003675
Tonner P., Srinivasasainagendra V., Zhang S., Zhi D. (2012). Detecting transcription of ribosomal protein pseudogenes in diverse human tissues from RNA-seq data. BMC Genomics 13, 412. doi:10.1186/1471-2164-13-412
Tu H., Nelson O., Bezprozvanny A., Wang Z., Lee S. F., Hao Y. H., et al. (2006). Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 126 (5), 981–993. doi:10.1016/j.cell.2006.06.059
Urayama A., Dohgu S., Robinson S. M., Sly W. S., Grubb J. H., Banks W. A. (2015). Alpha adrenergic induction of transport of lysosomal enzyme across the blood-brain barrier. PLoS One 10 (11), e0142347. doi:10.1371/journal.pone.0142347
Urich E., Lazic S. E., Molnos J., Wells I., Freskgard P. O. (2012). Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models. PLoS One 7 (5), e38149. doi:10.1371/journal.pone.0038149
Venturi E., Mio K., Nishi M., Ogura T., Moriya T., Pitt S. J., et al. (2011). Mitsugumin 23 forms a massive bowl-shaped assembly and cation-conducting channel. Biochemistry 50 (13), 2623–2632. doi:10.1021/bi1019447
Vergnol A., Traore M., Pietri-Rouxel F., Falcone S. (2022). New insights in CaVbeta subunits: role in the regulation of gene expression and cellular homeostasis. Front. Cell. Dev. Biol. 10, 880441. doi:10.3389/fcell.2022.880441
Visentin L., Munaron L., Ruffinatti F. A. (2025). Structuring data analysis projects in the Open science era with kerblam. F1000Res 14, 88. doi:10.12688/f1000research.157325.2
Voets T., Droogmans G., Nilius B. (1996). Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. J. Physiol. 497 (Pt 1), 95–107. doi:10.1113/jphysiol.1996.sp021752
Vogel C., Marcotte E. M. (2012). Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 13 (4), 227–232. doi:10.1038/nrg3185
von Lindern M., Egee S., Bianchi P., Kaestner L. (2022). The function of ion channels and membrane potential in red blood cells: toward a systematic analysis of the erythroid channelome. Front. Physiol. 13, 824478. doi:10.3389/fphys.2022.824478
Wagner G. P., Kin K., Lynch V. J. (2012). Measurement of mRNA abundance using RNA-Seq data: RPKM measure is inconsistent among samples. Theory Biosci. 131 (4), 281–285. doi:10.1007/s12064-012-0162-3
Wehrspaun C. C., Ponting C. P., Marques A. C. (2014). Brain-expressed 3'UTR extensions strengthen miRNA cross-talk between ion channel/transporter encoding mRNAs. Front. Genet. 5, 41. doi:10.3389/fgene.2014.00041
Weksler B., Romero I. A., Couraud P. O. (2013). The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 10 (1), 16. doi:10.1186/2045-8118-10-16
Wen L., Yan W., Zhu L., Tang C., Wang G. (2023). The role of blood flow in vessel remodeling and its regulatory mechanism during developmental angiogenesis. Cell. Mol. Life Sci. 80 (6), 162. doi:10.1007/s00018-023-04801-z
Witmer N. H., McLendon J. M., Stein C. S., Yoon J. Y., Berezhnaya E., Elrod J. W., et al. (2024). Upstream alternative polyadenylation in SCN5A produces a short transcript isoform encoding a mitochondria-localized NaV1.5 N-terminal fragment that influences cardiomyocyte respiration. bioRxiv, 2024.08.09.607406. doi:10.1101/2024.08.09.607406
Wu S., Moore T. M., Brough G. H., Whitt S. R., Chinkers M., Li M., et al. (2000). Cyclic nucleotide-gated channels mediate membrane depolarization following activation of store-operated calcium entry in endothelial cells. J. Biol. Chem. 275 (25), 18887–18896. doi:10.1074/jbc.M002795200
Wu M. L., Xie C., Li X., Sun J., Zhao J., Wang J. H. (2024). Mast cell activation triggered by SARS-CoV-2 causes inflammation in brain microvascular endothelial cells and microglia. Front. Cell. Infect. Microbiol. 14, 1358873. doi:10.3389/fcimb.2024.1358873
Xu Y., Yang Y., Yang J., Cui J., Yan J., Jiang J., et al. (2024). Glycine receptor beta subunit (GlyR-beta) promotes potential angiogenesis and neurological regeneration during early-stage recovery after cerebral ischemia stroke/reperfusion in mice. J. Integr. Neurosci. 23 (8), 145. doi:10.31083/j.jin2308145
Yamamoto N., Yoneda K., Asai K., Sobue K., Tada T., Fujita Y., et al. (2001). Alterations in the expression of the AQP family in cultured rat astrocytes during hypoxia and reoxygenation. Brain Res. Mol. Brain Res. 90 (1), 26–38. doi:10.1016/s0169-328x(01)00064-x
Yang L., Duff M. O., Graveley B. R., Carmichael G. G., Chen L. L. (2011). Genomewide characterization of non-polyadenylated RNAs. Genome Biol. 12 (2), R16. doi:10.1186/gb-2011-12-2-r16
Yang L., Wang G., Ma Y., Zhao Q., Zhao H., Wang Q., et al. (2024). TRPML1 acts as a predisposing factor in lymphedema development by regulating the subcellular localization of aquaporin-3, -5. PLoS One 19 (12), e0310653. doi:10.1371/journal.pone.0310653
Zhang Y., Ding X., Miao C., Chen J. (2019). Propofol attenuated TNF-alpha-modulated occludin expression by inhibiting Hif-1alpha/VEGF/VEGFR-2/ERK signaling pathway in hCMEC/D3 cells. BMC Anesthesiol. 19 (1), 127. doi:10.1186/s12871-019-0788-5
Zhang R. J., Yin Y. F., Xie X. J., Gu H. F. (2020). Acid-sensing ion channels: linking extracellular acidification with atherosclerosis. Clin. Chim. Acta 502, 183–190. doi:10.1016/j.cca.2019.12.027
Zhang J., Yuan H. K., Chen S., Zhang Z. R. (2022). Detrimental or beneficial: role of endothelial ENaC in vascular function. J. Cell. Physiol. 237 (1), 29–48. doi:10.1002/jcp.30505
Zhang X., Shao J., Wang C., Liu C., Hao H., Li X., et al. (2024). TMC7 functions as a suppressor of Piezo2 in primary sensory neurons blunting peripheral mechanotransduction. Cell. Rep. 43 (4), 114014. doi:10.1016/j.celrep.2024.114014
Zheng X., Yang J., Zhu Z., Fang Y., Tian Y., Xie M., et al. (2022). The two-pore domain potassium channel TREK-1 promotes blood-brain barrier breakdown and exacerbates neuronal death after focal cerebral ischemia in mice. Mol. Neurobiol. 59 (4), 2305–2327. doi:10.1007/s12035-021-02702-5
Zheng W., Rawson S., Shen Z., Tamilselvan E., Smith H. E., Halford J., et al. (2023). TMEM63 proteins function as monomeric high-threshold mechanosensitive ion channels. Neuron 111 (20), 3195–3210 e3197. doi:10.1016/j.neuron.2023.07.006
Zhou X., Lin P., Yamazaki D., Park K. H., Komazaki S., Chen S. R., et al. (2014). Trimeric intracellular cation channels and sarcoplasmic/endoplasmic reticulum calcium homeostasis. Circ. Res. 114 (4), 706–716. doi:10.1161/CIRCRESAHA.114.301816
Zhu D., Su Y., Fu B., Xu H. (2018). Magnesium reduces blood-brain barrier permeability and regulates amyloid-beta transcytosis. Mol. Neurobiol. 55 (9), 7118–7131. doi:10.1007/s12035-018-0896-0
Zong P., Feng J., Li C. X., Jellison E. R., Yue Z., Miller B., et al. (2024). Activation of endothelial TRPM2 exacerbates blood-brain barrier degradation in ischemic stroke. Cardiovasc Res. 120 (2), 188–202. doi:10.1093/cvr/cvad126
Zou W., Lv Y., Li L., Zhang S., Liang J., Wu C., et al. (2025). FOXQ1 regulates brain endothelial mitochondrial function by orchestrating calcium signaling and cristae morphology. Adv. Sci. (Weinh) 12, e03082. doi:10.1002/advs.202503082
Zuccolo E., Laforenza U., Ferulli F., Pellavio G., Scarpellino G., Tanzi M., et al. (2018). Stim and orai mediate constitutive Ca(2+) entry and control endoplasmic reticulum Ca(2+) refilling in primary cultures of colorectal carcinoma cells. Oncotarget 9 (57), 31098–31119. doi:10.18632/oncotarget.25785
Keywords: hCMEC/D3, blood-brain barrier, RNA-Seq, transporters, pumps, ion channels, G-protein coupled receptors, receptor tyrosine kinases
Citation: Scarpellino G, Brunetti V, Scolari F, Visentin L, Biella GR, Ruffinatti FA and Moccia F (2025) The ion transport, GPCR, and RTK toolkit expression in the human cerebrovascular endothelial cell line, hCMEC/D3: an Omics perspective. Front. Physiol. 16:1733266. doi: 10.3389/fphys.2025.1733266
Received: 27 October 2025; Accepted: 27 November 2025;
Published: 18 December 2025.
Edited by:
Irena Levitan, University of Illinois Chicago, United StatesReviewed by:
Erik J. Behringer, Loma Linda University, United StatesStefano Tarantini, University of Oklahoma Health Sciences Center, United States
Copyright © 2025 Scarpellino, Brunetti, Scolari, Visentin, Biella, Ruffinatti and Moccia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Moccia, ZnJhbmNlc2NvLm1vY2NpYUB1bmltb2wuaXQ=
†These authors have contributed equally to this work and share senior authorship
 Giorgia Scarpellino
Giorgia Scarpellino Valentina Brunetti
Valentina Brunetti Francesca Scolari
Francesca Scolari Luca Visentin3
Luca Visentin3 Gerardo Rosario Biella
Gerardo Rosario Biella Federico Alessandro Ruffinatti
Federico Alessandro Ruffinatti Francesco Moccia
Francesco Moccia