- Department of Psychiatry, Medical University Pleven, Pleven, Bulgaria
Introduction: More than half of psychiatric patients have comorbid substance use disorder (dual diagnosis) and this rate, confirmed by many epidemiological studies, is substantially higher compared to general population. Combined operation of self-medication mechanisms, common etiological factors, and mutually causative influences most likely accounts for comorbidity, which, despite its clinical prevalence, remains underrepresented in psychiatric research, especially in terms of neuroimaging. The current paper attempts to review and discuss all existing methodologically sustainable structural and functional neuroimaging studies in comorbid subjects published in the last 20 years.
Methods: Performing a systematic PubMed/MEDLINE, Web of Science, and Cochrane databases search with predefined key-words and selection criteria, 43 structural and functional neuroimaging studies were analyzed.
Results: Although markedly inconsistent and confounded by a variety of sources, available data suggest that structural brain changes are slightly more pronounced, yet not qualitatively different in comorbid patients compared to non-comorbid ones. In schizophrenia (SZ) patients, somewhat greater gray matter reduction is seen in cingulate cortex, dorsolateral prefrontal and frontotemporal cortex, limbic structures (hippocampus), and basal ganglia (striatum). The magnitude of structural changes is positively correlated to duration and severity of substance use, but it is important to note that at least in the beginning of the disease, dual diagnosis subjects tend to show less brain abnormalities and better cognitive functioning than pure SZ ones suggesting lower preexisting neuropathological burden. When analysing neuroimaging findings in SZ and bipolar disorder subjects, dorsolateral prefrontal, cingular, and insular cortex emerge as common affected areas in both groups which might indicate a shared endophenotypic (i.e., transdiagnostic) disruption of brain networks involved in executive functioning, emotional processing, and social cognition, rendering affected individuals susceptible to both mental disorder and substance misuse. In patients with anxiety disorders and substance misuse, a common neuroimaging finding is reduced volume of limbic structures (n. accumbens, hippocampus and amygdala). Whether this is a neuropathological marker of common predisposition to specific behavioral symptoms and drug addiction or a result from neuroadaptation changes secondary to substance misuse is unknown. Future neuroimaging studies with larger samples, longitudinal design, and genetic subtyping are warranted to enhance current knowledge on comorbidity.
Introduction
The co-occurring mental disorder and substance use disorder (SUD), a phenomenon also referred to as comorbidity or the older term dual diagnosis (DD) (1, 2), has been consistently replicated in a number of large epidemiological studies in the last three decades (3–10). Over 50% of psychiatric inpatients have a co-occurring SUD (11), and this rate is far bigger than what is found in general population (12) and predicted by a mere coincidence model (13). On the other hand, more than half of the individuals diagnosed with SUD meet the criteria for another mental disorder, the most common ones being anxiety, mood, personality, and schizophrenia/psychotic disorders (11). Dual-diagnosis subjects impose a serious challenge because of the higher severity of medical problems, social and familial burden, and the greater incidence of relapses related to both mental disorder and SUD (14).
Despite being conceptually criticized on multiple levels (15), the invariable presence of comorbidity in everyday practice has invoked a number of explanation attempts (5, 16). They may be broadly subdivided into [1] illness-mediated theories—an index disorder causes the secondary/comorbid condition; [2] theories of common causal factors—one or more independent etiological factors increase the risk for both disorders; [3] bidirectional theories—presence of mutually reciprocal causal influences between the comorbid disorders. Most epidemiological studies indicate that in terms of occurrence, the mental disorder has a temporal priority (4, 5, 13, 17, 18), thus lending credibility to the so-called “self-medication” hypothesis (19, 20), considering SUD as a secondary result of repeating substance use in an attempt to alleviate mental disorder symptoms. It is much more likely, however, that a combination of mechanisms acts for each pattern of comorbidity in each particular patient—for example, self-medication and bidirectional mechanisms are implicated in anxiety disorders–SUD association (21, 22), while in patients with schizophrenia and SUD, common neurobiological, neurodevelopmental, and genetic causal factors are intertwined with self-medication mechanisms (20, 23, 24).
Because in the last several decades psychiatry has built diagnostic categories resting exclusively on clinical symptoms (25), most neuroimaging studies have focused on brain structure or function in patients with particular diagnosis, comparing them with healthy controls. As a result, proportionately few studies have included comorbid patients (26), and the vast majority of them focus on schizophrenia and co-occurring SUD (27). Furthermore, at least to our knowledge, there are no studies comparing groups of DD subjects with different disorders (e.g., depression vs. anxiety disorder). However, neuroimaging research suggests shared neurobiological abnormalities in phenotypically and genetically related diagnoses such as schizophrenia and bipolar disorder (BD) (28–30), and data also coalesce around the hypothesis that different psychiatric illnesses entail pertuberations along the same neural circuits (31, 32). Taking this into account, the current article aims to review and discuss reported data with some focus on the possible cross-diagnostic validity of findings.
Methods
PubMed/MEDLINE, Web of Science, and Cochrane databases were searched with the following keywords and word combinations: “Co-occurring disorders,” “Comorbidity,” “Dual diagnosis,” “Magnetic resonance imaging” (MRI) and “functional Magnetic resonance imaging (fMRi),” “Schizophrenia and substance use disorder (SUD),” “Bipolar disorder and SUD,” “Depression and SUD,” “Anxiety disorder(s) and SUD”. Besides that, in the process of analysis of the initially chosen publications, all appropriate papers indexed in the reference sections were inspected. Previous reviews focusing on similar topics were also taken into consideration as a cross-reference.
Articles were selected according to the following criteria: a) dated between January 1999 and July 2019; b) written in English; c) published in full text; d) using widely recognized and popular neuroimaging technique, e.g., MRI, PET etc.; e) performed in humans; f) including subjects meeting International Statistical Classification of Diseases,-10th Revision/Diagnostic and Statistical Manual of mental disorders, Fourth Edition (Fifth) (DSM-IV(5)) criteria for abuse of or dependence on at least one of the following substances: alcohol, cannabinoids, cocaine, hallucinogens, medicinal drugs (e.g., benzodiazepines), opioids, and stimulants (amphetamines, ecstasy).
Results
The initial search made 91 hits, of which 53 were excluded based on selection criteria. In the process of reviewing, five more relevant publications emerged from reference literature and were included. Finally, 43 studies were chosen for participation in this review, and they are summarized on Tables 1–4.
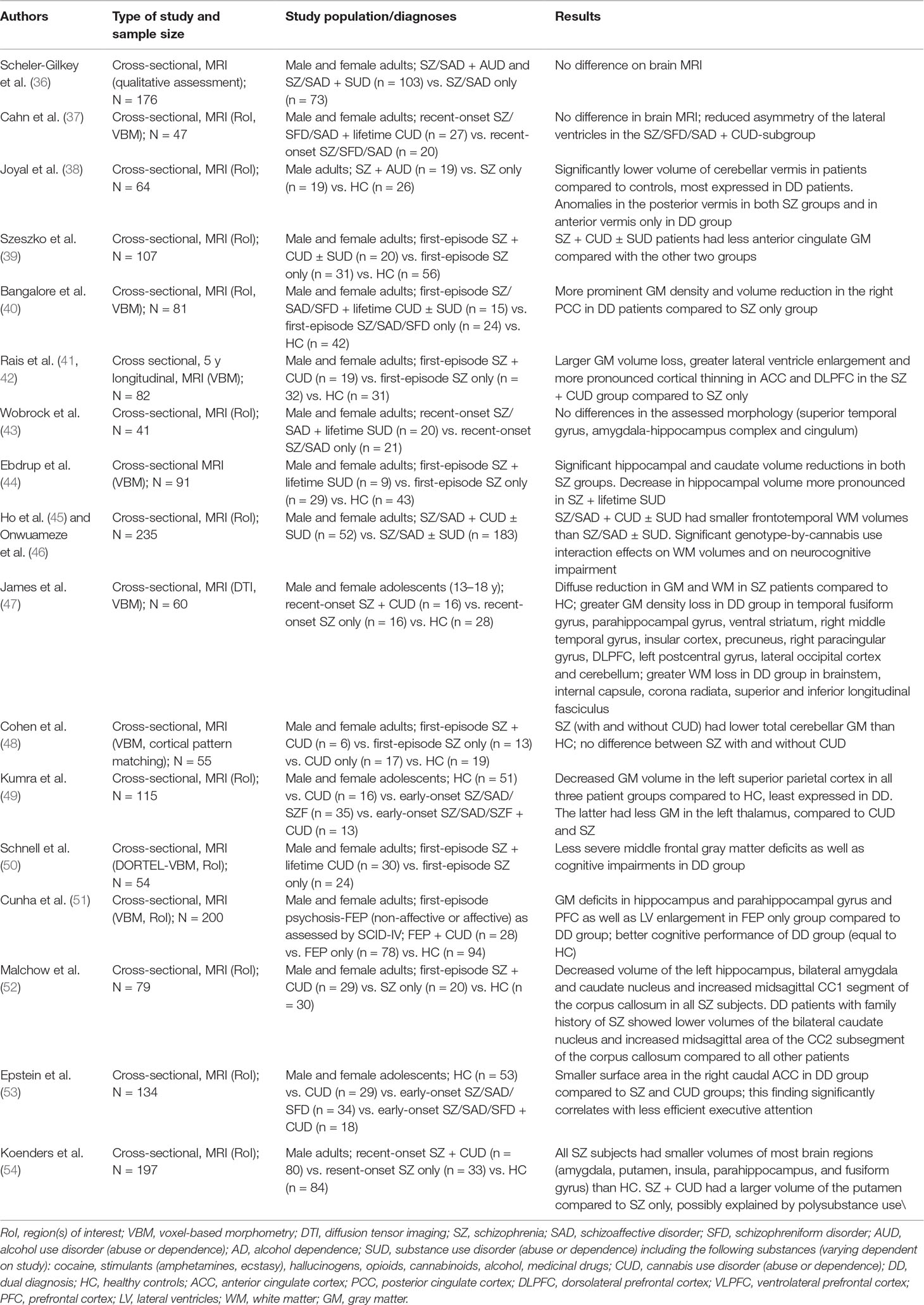
Table 1 Structural neuroimaging findings in first-episode or recent-onset schizophrenic patients with SUD (duration of symptoms <5 years).
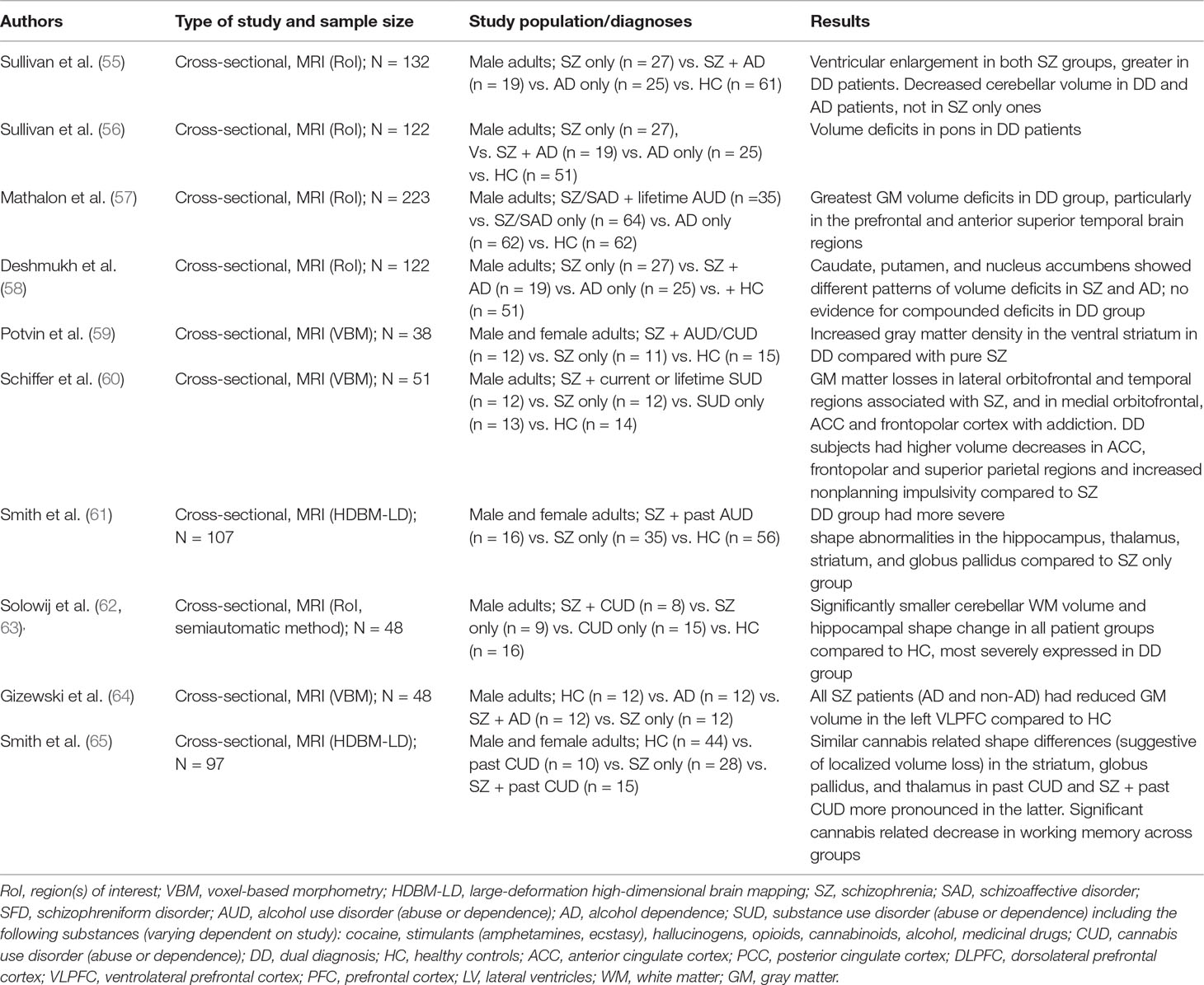
Table 2 Structural neuroimaging findings in schizophrenic patients with illness duration >5 years and comorbid SUD.
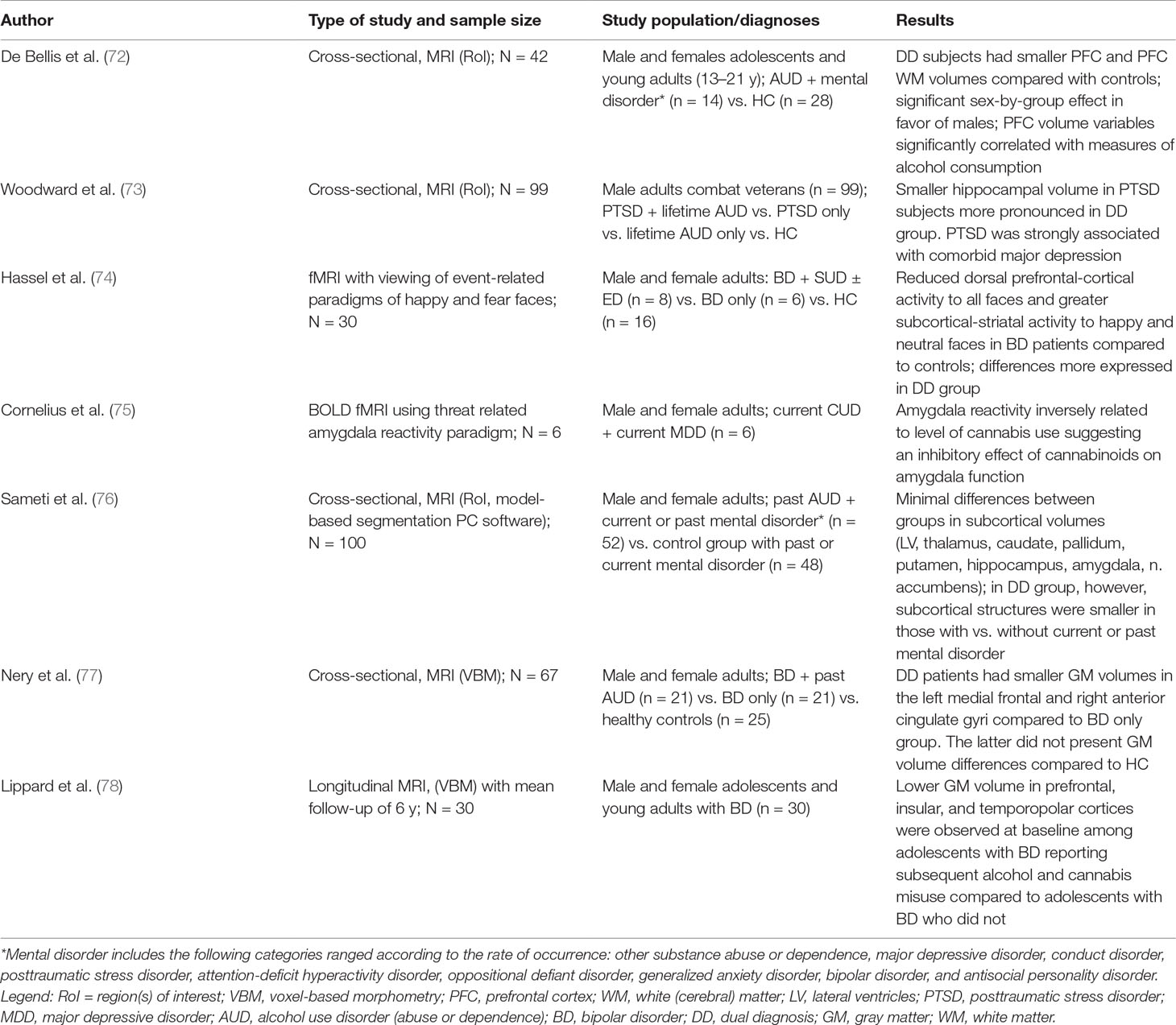
Table 4 Structural and functional neuroimaging findings in studies with nonschizophrenic patients with SUD.
Schizophrenia and SUD
Schizophrenia is by far the most prevalent diagnosis in neuroimaging research on comorbidity and with regards to type of substance misuse, the majority of studies have enrolled patients with alcohol, cannabis, or multiple SUDs abuse or dependence (26, 33, 34). As for the investigational tools, all but one of the studies employ MRI (VBM, RoI, DTI) and fMRI. For perspicuity reasons, structural and functional neuroimaging studies are presented on separate tables in this review. In addition, based on the duration of illness and the age of the included population, structural neuroimaging studies for schizophrenia are subdivided into two separate tables: Table 1 for studies including adolescent and young adult subjects with first episode or recent onset of the disease (up to 5 years) and Table 2 for subjects with chronic schizophrenia lasting more than 5 years. This distinction was made for two reasons: first, this time interval was used in studies that include follow-up of patients with first-episode psychosis (41, 42). Second, studies of first-episode or recent-onset schizophrenia often include minimally treated or medication-naive subjects, which allows for better discrimination between structural changes imposed by substance use and those related to long-term antipsychotic treatment (35).
Other Mental Disorders
Only a few studies have included comorbid subjects with diagnosis other than schizophrenia (Table 3), and these are predominantly mood disorders (BD and major depression), anxiety and stress-related disorders, and also conditions typically occurring in childhood or adolescence. In terms of visualization method, five of the studies use structural MRI, and two are fMRI studies.
Discussion
The current review tries to summarize and interprete the results of all methodologically consistent neuroimaging studies that have focused on DD patients and have been carried out over the past 20 years. Prior to discussing their findings and suggesting possible implications, several essential considerations have to be emphasized.
First, the most substantial limitations of all reviewed studies examining comorbid subjects are small sample sizes. With the exception of a few studies [e.g., (36, 45–46, 51, 54, 73)], most authors have included 8 to 25 comorbid patients in their samples with a corresponding number of controls. Furthermore, some authors have employed the same or significantly overlapping sample for several different articles (55–58, 62–63, 69) or used one sample for a structural and a functional neuroimaging study (64). As a consequence, the number of examined comorbid patients is highly insufficient to allow any definite conclusions, given the discrete size differences in compared anatomical structures. Such an inference is even truer for comorbid disorders other than schizophrenia, which are extremely underrepresented in the literature. Hence, future studies with much larger samples and more diverse diagnostic categories are warranted to confirm or reject the findings reported so far.
An additional holdback to data reliability is the low geographic, racial, and ethnocultural variety of reported studies—with only one exception of a study from Brazil (51), all the rest originate from Western Europe (37, 38, 41–47, 50, 52, 54, 60, 64, 67, 69), USA and Canada (36, 39, 40, 49, 53, 55–58, 61, 65, 66, 68, 70–78), and Australia (62, 63), with no studies from Asia, Africa, and most other parts of Europe and South America. Moreover, with regards to structural neuroimaging research in schizophrenia, there is an overall sex inequality of the examined populations with males constituting at least two-thirds of the DD subjects in more than half of the studies (36, 37, 39, 43–48, 50, 52–54, 61, 65) and representing the only studied group in the rest (38, 55–58, 62–64). While reflecting the clinicoepidemiological reality of more common SUDs in men including those with a co-occurring mental illness (79), this fact further obstructs the possibility of making definite conclusions given the existing evidence for gender influence on brain morphology in schizophrenia (80). Another restriction of the available data concerns the adolescent population with co-occurring SUD and schizophrenia. Apart from the few conducted studies involving very small patient groups with cannabis misuse only (47, 49, 53), some of the published articles do not even include patients meeting the threshold criteria for abuse and/or dependence (81, 82) and for this reason have not been taken into consideration in the present review. This fact renders any sound inferences regarding structural and functional brain changes in adolescent DD patients even more problematic than those for adults.
One very important consideration concerns diagnostic heterogeneity in the studied samples. Despite the strictly defined study protocols employed by authors striving to include “pure” DD subjects in their samples, there is still marked heterogeneity in both the substance use disorder and the co-occurring mental disorder. Some of the structural neuroimaging studies including those with largest samples have enrolled patients with polysubstance misuse (36, 39, 40, 43–46, 60), and even those focusing on a specific SUD—largely cannabis—feature subjects with additional current or past substance use disorder, most often alcohol or stimulants misuse [e.g (51, 54, 69)]. Combined drug use is an important confounding factor, which, according to some of the authors, may explain certain structural differences found in DD subjects such as striatal volume differences (54, 60). Furthermore, one of the major studies in terms of sample size compares schizophrenic patients with cannabis misuse against other substance misusing (e.g., not “clear”) schizophrenic patients (45, 46). In one big study (54), the possible confounding effects of smoking, which has been reported in association with additional volume loss in schizophrenia (83), could not be separated, and this flaw is most likely also true for all the studies with adult SZ patients, although not explicitly stated by authors. As a result, the observed differences in brain morphology between comorbid and noncomorbid subjects could not be undoubtedly ascribed to the effects of a particular substance.
Substantial sample heterogeneity also exists for the co-occurring mental disorder. In fact, nearly all studies include patients with schizoaffective disorder (SAD) along with schizophrenics (SZ) in their samples (36, 37, 40, 43, 45, 46, 49, 53, 71). This shortcoming, resulting from both the inherent vagueness of SAD diagnosis and the categorical approach endorsed by structured diagnostic interviews (84), may as well bring some potentially positive implications. Specifically, considering the low clinical utility and reliability of SAD diagnostic criteria (85), and the low temporal consistency of SAD diagnosis found in longitudinal studies (86, 87), it could be hypothesized that the reported neuroimaging findings for comorbid SAD patients also apply to bipolar patients with a co-occurring SUD. Furthermore, one study has even included DSM-IV–defined “affective psychosis” subjects, and this category is largely limited to bipolar patients (51). Of course, given the scarce neuroimaging investigation focusing solely on bipolar comorbid subjects, future studies with sufficient magnitude are needed to test the validity of this assumption.
Another major drawback that should be outlined is related to the status of substance use disorders in the studied patients—particularly whether these are lifetime diagnoses in patients currently in stable and long-term remission or active phase conditions. In this line of thought, while some studies with comorbid schizophrenia and co-occurring SUD, particularly alcohol and cannabis, have only included subjects with past abuse or dependence [e.g., (40, 59, 60, 62, 63, 69)], others have investigated patients with a short or even no prior remission [e.g., (37, 54, 58)]. This might represent a substantial confounding factor since a positive correlation between recency of substance use (especially alcohol) and greater volume deficit in some brain structures such as nucleus accumbens has been reported (57).
The last constraint discussed here concerns concomitant antipsychotic treatment, which has a definite correlation with certain structural effects on the brain (35, 88). As most of the SZ study samples have been exposed to this class of medications [e.g., Refs. (41, 42, 45, 46, 54–60, 69) and others], its effects must necessarily be taken into account when discussing the results. Moreover, some authors (45–47) have found more structural changes in SZ patients with long-term therapy such as dysmorphology and volume deficits in the thalamus and striatal and other basal ganglia, more pronounced in those treated with atypical antipsychotics. Antipsychotics-related overall decrease in total brain gray and white matter has also been reported (45, 46). Taken together, these data further limit the significance and validity of reported neuroimaging findings in DD and non-DD subjects.
With all the considerations emphasized so far, the results of structural and functional neuroimaging studies in DD patients will be analyzed below as follows:
Structural and Functional Neuroimaging Findings in Schizophrenia and SUD
As mentioned earlier, most of the studies have included predominantly cannabis, alcohol, and combined misuse subjects, and there is marked inconsistency across study findings, especially for cannabis use disorders (54). Whereas some studies detect no or insignificant differences between DD and non-DD patients (37, 43, 48, 58, 64), others suggest more severe structural changes in DD compared to non-DD patients (39–42, 44-47, 52, 60–63), and finally some authors find the opposite correlation (50, 51, 54). With regard to recent-onset or first-episode schizophrenia studies (i.e., with illness duration up to 5 years), reported findings consolidate around greater volume reduction on the expense of gray matter in DD subjects, with most commonly affected areas being anterior and posterior cingulate cortex (ACC, PCC) (39–42, 53), dorsolateral prefrontal cortex (DLPFC) (41, 42, 47), hippocampus (44, 52), striatum (47, 52), and frontotemporal cortex and cerebellum (39–42, 47). In addition, less cerebral white matter in frontotemporal cortex (45, 46) as well as diffuse subcortical areas (47) has been reported. None of these findings, however, is specific, and they represent only quantitative difference as compared to pure schizophrenia. Expectedly, in some studies, the magnitude of structural disruptions is positively correlated with neurocognitive impairment (45, 46, 53). Studies with chronic patients (i.e., with illness duration >5 years) also show slightly more pronounced volume loss in DD subjects affecting prefrontal, frontotemporal, parietal, and anterior cingulate cortical areas (57, 60), hippocampus (61–63), thalamus, striatum and globus pallidus (61, 65), cerebellar gray and white matter (55, 62, 63), and pons (56). Again, no specificity of findings may be claimed as the same are often reported in literature both with regard to pure schizophrenia (89) and pure alcohol and cannabis misuse (90, 91). The most distinctive neurocognitive impairment pattern in this group of studies was reported by Schiffer et al. (2010) who found significantly greater impulsivity in DD subjects compared to pure SZ ones, while executive functioning deficits of both groups were on par.
As noted above, studies that fail to demonstrate differences between DD and non-DD groups also exist. For example, with respect to alcohol use disorders (AUDs), Gizewski et al. (2013) found similar gray matter volume decrease in the left VLPFC in long-term abstinent alcoholic and nonalcoholic SZ groups compared to healthy controls. Deshmukh et al. (2005) comparing pure chronic SZ patients versus chronic alcohol-dependent SZ ones with a varying duration of preceding abstinence demonstrated somewhat greater volume deficit in SZ in striatum (putamen) and n. accumbens with no evidence for a compounded structural deficit in DD subjects (58). In the same sample, however, other authors did demonstrate greater ventricular enlargement and cerebellar volume loss (55) and more pronounced gray matter deficits in prefrontal and superior temporal cortex (57) and pons (56) in DD group, with the latter structure not considered as directly affected by schizophrenia (26). In a mixed SUD sample of recent-onset schizophrenics, Wobrock et al. (2009) found no differences in superior temporal gyrus, amygdala-hippocampus complex, and cingulum between comorbid and noncomorbid subjects (43). In a similar group of SZ patients with and without co-occurring alcohol or cannabis use disorder, Potvin et al. (2007) also did not find significant structural brain differences between groups except for increased gray matter density in the ventral striatum for the DD group (59). Interestingly, a similar finding of increased dorsal striatum (putamen) was reported by Koenders et al. (2009) in a comparison of cannabis misuse versus noncannabis misuse schizophrenic patients (54). As deficits in other areas (limbic structures, anterior cingulate, orbitofrontal and fusiform gyrus, insula, thalamus, and caudate) were not detected, the findings of both studies could be related to striatal neuroadaptation changes emerging from repetitive drug use (54, 59). Further considering cannabis use disorders, Cohen et al. (2012) did not detect differences in comorbid and noncomorbid subjects in a first-episode schizophrenia sample in which both groups had lower total cerebellar gray matter than healthy controls (48). Similarly, investigating a group of adolescent early-onset schizophrenia subjects with and without co-occurring cannabis use disorder, Kumra et al. (2012) did not find additional volumetric deficit in DD patients compared to pure EOS and pure CUDs, while all three groups had smaller gray matter volume in the left parietal cortex than controls. DD patients had somewhat smaller left hypothalamus than pure SZ subjects, but for the left parietal cortical surface, the opposite relationship was observed, and it was the size of this area that showed significant positive association with results on a neurocognitive test for attention and working memory supporting its stronger implication in schizophrenia-related neuropathological processes (49).
Finally, studies that favor DD subjects in terms of severity of illness and associated neuroimaging findings will be discussed. In the first of them, with stringent inclusion criteria regarding schizophrenia, Schnell et al. (2012) found less severe gray matter deficits in the left DLPFC in first-episode patients with past cannabis use disorder versus pure schizophrenia ones. Moreover, this result was paired with superior cognitive performance in verbal and working memory tests in the DD group (50). Similarly, in a larger study employing less rigorous selection criteria and thus including both nonaffective- and affective-type first-episode psychosis, Cunha et al. (2012) reported milder gray matter deficits in hippocampus, parahippocampal gyrus, and prefrontal cortex; smaller lateral ventricles enlargement and better cognitive performance in patients with cannabis use disorders versus those without (51). These results are in striking controversy with data from Rais et al. (41, 42), Ho et al. (45) and Onwuameze et al. (46) reported above, which indicate both more significant gray and white matter deficits in a number of cortical structures and worse performance on neurocognitive tests in cannabis misusing SZ subjects. In fact, explanations exist for both adverse and beneficial effects on marijuana in schizophrenia population. Regarding the former, theories suggest neurotoxic effects of cannabis, which are either direct via disturbed control of the endogenous cannabinoid system on glutamate and γ-aminobutyric acid release and subsequent impairment in maturation of neural circuitries in adolescence (92) or an indirect including complex genotype-by-cannabis interactions that leads to brain morphologic changes. Supporting that, in the only study of its type, Ho et al. (45) found significant association between more severe frontotemporal white matter deficits in DD subjects and a particular genetic variant of the cannabinoid 1 receptor (CNR1). The alternative set of explanations generally regards DD patients as a specific subgroup that is intrinsically less vulnerable to schizophrenia than cannabis-naive patients, has better premorbid cognitive functions and social adjustment (93), and probably would not have developed psychotic symptoms without the effects of substance use. Such a hypothesis regarding not only marijuana, but SUDs in general, is supported by the available functional neuroimaging research. Nearly all studies of this type report better preserved functioning in areas associated with emotional processing—medial prefrontal cortex (66, 68, 70) and social cognition—ventrolateral prefrontal cortex and anterior insular cortex (64). In addition, data show that areas associated with verbal processing and attention (posterior cingulate cortex, inferior parietal lobe and precentral gyrus) and executive functioning (DLPFC) also show higher activity in DD patients (69, 70). In further support of the hypothesis that comorbid SZ subjects might represent a subgroup with less neurobiological abnormalities than noncomorbid SZ, Thompson et al. (71) in a recent [11C] raclopride study hypothesized that a hypersensitivity of D2 receptors rather than excess presynaptic dopamine release is the predominant dopaminergic alteration in comorbid subjects. However, at least some preexisting neuropathological diathesis is seemingly necessary to reach psychotic state as witnessed by the study of Uhlmann et al. (2016) showing thinner prefrontal and temporal cortical areas and decreased hippocampal volume in methamphetamine-dependent patients with psychosis versus those without (94). In fact, both sets of explanations are not necessarily mutually exclusive: as hypothesized by Cunha et al. (51), the exposure to cannabis or other substances may be a prerequisite for development of first episode of psychosis in an initially relatively “preserved” brain, but with repeating use, severe gray matter deficit occurs, which is accountable for worse clinical and cognitive presentations of dual-diagnosis patients reported in longitudinal studies.
Structural and Functional Neuroimaging in Patients With Diagnoses Other Than Schizophrenia
Although far more limited as compared to the comorbid schizophrenia research, available data are consistent with more severe neuroimaging changes in this population. Starting with BD, two structural and one fMRI studies demonstrate certain differences between comorbid and noncomorbid subjects. In the earliest of them, Hassel et al. (2009) showed abnormal pattern of brain activation in a small group of bipolar patients (n = 14) compared with controls (74). Using an event-related fMRI paradigm with happy, neutral, and sad faces, they found reduced dorsal prefrontal-cortical activity to all faces and greater subcortical-striatal activity to happy and neutral faces in all bipolar patients. Interestingly, decrease in prefrontal activity was more pronounced in comorbid patients, and authors have hypothesized that this phenomenon may be linked to stronger difficulties in integrating socioemotional information and, subsequently, emotion regulation. Moreover, similarly decreased DLPFC activity has been reported in substance abusers in decision making and facial matching tasks (95, 96). In a subsequent cross-sectional MRI study, Nery et al. (2011) found smaller gray matter volumes in the left medial frontal and right anterior cingulate gyri in a sample of bipolar patients with long-term remission AUD compared to pure bipolar ones (77). As these frontal lobe subareas are connected with other prefrontal areas and high-order association regions (orbitofrontal cortex, temporal and parietal lobe, and subcortical structures) and insofar prefrontal cortex plays an important role in the inhibitory control of inappropriate compulsive behaviors such as addiction (97), the authors suggested that the observed gray matter deficits are a structural correlate of the impaired “top-down” inhibitory control in prefrontal brain areas that distinguishes BD-AUD patients from BD patients without AUD (77). Supporting this assumption, in a recent study with longitudinal design, Lippard at al. (2017) reported lower baseline gray matter volume in prefrontal, insular, and temporopolar cortices in those adolescents who later developed alcohol and cannabis misuse, suggesting a possible endophenotype significance of these findings (78). Interestingly, sex-based difference in structural findings was also observed in that while decreased baseline gray matter volume in DLPFC was positively correlated with substance use problems in both females and males, lower orbitofrontal cortex and insular gray matter predicted substance use problems in females, whereas in males, these were associated with lower right prefrontal cortex gray matter volume. In addition to that, greater depressive symptoms at baseline were associated with greater substance use problems at follow-up, and depressive symptoms in females in particular were related to lower insular gray matter volume. Besides having a potential structural biomarker implication, this latter finding may also aid to see the popular explanatory theory of depression-SUD association as a manifestation of shared neurobiological vulnerability (98) in a new light. Further focusing on sex differences in brain structure, De Bellis et al. (72) in a sample of adolescents and young adult patients with alcohol and polysubstance misuse and an array of comorbid psychiatric diagnoses [i.e., mood, anxiety, and stress-related disorders; attention-deficit/hyperactivity disorder (ADHD); conduct and oppositional defiant disorders; and antisocial personality disorder] found smaller cerebellar gray matter volumes only in males. However, as this finding correlated substantially with a co-occurring diagnosis of ADHD, it was not regarded as associated with substance use. Such an association was found in fact, but with decreased gray and white matter volumes in prefrontal cortex, which was present in both sexes. The authors hypothesized that this finding might be either the result of direct or indirect detrimental effects of the substances on PFC development, a neurotoxic interference of the same with its maturation, or, alternatively, a reflection of inherent vulnerability for delayed PFC maturation subsequently enhancing the risk for poorer cognitive functioning and greater impulsivity and hence onset of substance misuse (72).
Other than BD, posttraumatic stress disorder (PTSD) has been most studied in nonpsychotic spectrum dual-diagnosis population. In a large study with 99 PTSD war veterans, a significant proportion of which had also comorbid depression, Woodward et al. (2006) found that past alcohol abuse or dependence has a significant inverse correlation with hippocampal volume (73). Although in nonalcoholic PTSD subjects the size of this structure was also reduced, the magnitude of the structural change was much smaller, suggesting that lower hippocampal volume might be a structural marker of shared neurobiological or genetic vulnerability to both alcoholism and PTSD (73). Further support for the close association between stress responses, limbic structures activity, and psychoactive substances was found in fMRI study by Cornelius et al. (2010) who investigated a small group of patients with comorbid cannabis dependence and depression (75). By means of threat-related amygdala reactivity paradigm, they showed that this structure known for its leading role in physiological and behavioral responses to stress and rich in CB1 receptors (99) displays reduced reactivity consistently correlating with the level of cannabis use. Such a finding supports the self-medication explanation theory for anxiety and substance use disorders comorbidity presented earlier. Additional evidence for implication of comorbid mood and anxiety disorders in structural brain changes in AUDs was presented by Sameti et al. (2011). By means of structural MRI comparison, these authors found in long-term abstinent alcoholics (LTAAs) smaller nucleus accumbens and hippocampus volumes in those LTAA individuals with a lifetime anxiety disorder than in those without (76). In addition to reduced n. accumbens, in alcohol-misusing patients with current anxiety disorder, a trend toward smaller putamen volumes was observed. Notably, the same association of smaller hippocampus and amygdala volumes in LTAA was also detected in subjects with a lifetime externalizing disorder diagnosis (i.e., conduct disorder, defiant disorder, ADHD, and antisocial personality disorder). The authors hypothesized that both internalizing (i.e., mood and anxiety) and externalizing disorders are associated with disrupted hypothalamic-pituitary-adrenal (HPA) axis response to stress and with impaired interactions of the former with mesolimbic reward circuitry, but this deviation is a result of two opposite mechanisms—a hypersensitization of the HPA axis with subsequent neurotoxic hypercorticism in mood/anxiety disorders and an undersensitization with hypocorticism in externalizing disorders (76). As a consequence, vulnerability to abuse of drugs is increased in both groups—in an attempt to reduce negative psychological effects of stress in mood/anxiety disorders and as a way of stimulating reduced HPA reactivity in externalizing disorders (similar to thrill and adventure-seeking behavior typical for this group of subjects).
Conclusion
Definite conclusions would be substantially enhanced by future studies on comorbidity engaging much larger samples, endorsing more powerful longitudinal design, and enhanced by genetic polymorphism subtyping. Currently available data, although markedly inconsistent and confounded by a variety of sources (e.g., different study design, small and heterogenic samples, concomitant medications, smoking, etc.), support the assumption that in substance-misusing psychiatric patients structural brain changes are more pronounced, yet not qualitatively different from what is seen in noncomorbid subjects with psychotic and nonpsychotic diagnoses. In SZ patients, neuroimaging studies support the assumption for somewhat greater gray matter reduction in cingulate cortex (anterior and posterior), dorsolateral prefrontal and frontotemporal cortex, limbic structures (hippocampus), and basal ganglia (striatum). However, the magnitude of these structural changes is dependent on duration and severity of substance use, and at least in some of DD subjects, preexisting brain abnormalities are less pronounced than in pure SZ ones, which corresponds to better social and cognitive functioning and in general to lower neurodevelopmental and/or genetic pathological diathesis. As most studies on SZ also included schizoaffective diagnoses, thus probably enrolling a significant proportion of bipolar DD subjects, it is reasonable to compare their findings to what is reported by studies with “pure” bipolar patients. In doing so, the dorsolateral prefrontal, cingular, and insular cortices emerge as commonly affected areas in both SZ and BD. Taken together, these findings may implicate a shared endophenotypic (i.e., transdiagnostic) disruption of brain areas involved in executive functioning, emotional processing, and social cognition, which renders affected individuals susceptible to both mental disorder and substance misuse. Notably, gray matter loss in the anterior insula and dorsal part of the anterior cingular cortex has also been emphasized as a transdiagnostic finding in psychiatric patients in a nuber of recent studies (100, 101).
In comorbidity of anxiety and stress-related disorders (PTSD), as well as externalizing disorders with substance misuse, a common neuroimaging finding is the reduced volume of limbic structures (n. accumbens, hippocampus, amygdala). However, whether this reflects an underlying neuropathological characteristic predisposing to both specific behavioral symptoms and drug addiction or is a secondary effect of self-medication substance misuse on brain reward circuitry remains to be clarified.
Author Contributions
Data review and analysis and manuscript preparation are performed by the same author (KS).
Funding
This paper has been supported by a research grant form Medical University Pleven, Bulgaria.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer VN declared a past co-authorship with one of the authors KS to the handling Editor.
References
1. Jacobs W, Cahill K, Gold M. Historical and conceptual issues. In: Stohler R, Röslerr W, editors. Dual diagnosis—the evolving conceptual network. Karger (2007). p. 54–64. doi: 10.1159/000085908
2. Drake RE, McLaugglin P, Pepper B, Minkoff K. Dual diagnosis of major mental illness and substance disorder: an overview. New Dir Ment Health Serv (1991) 50:3–12. doi: 10.1002/yd.23319915003
3. Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) study. JAMA (1990) 264:2511–8. doi: 10.1001/jama.1990.03450190043026
4. Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, Leaf PJ. The epidemiology of co-occurring addictive and mental disorders: implications for prevention and service utilization. Am J Orthopsychiatry (1996) 66:17–31. doi: 10.1037/h0080151
5. Merikangas KR, Mehta RL, Molnar BE, Walters EE, Swendsen JD, Aguilar-Gaziola S, et al. The comorbidity of substance use disorders with mood and anxiety disorders: results of the International Consortium in Psychiatric Epidemiology (I.C.P.E.). Addict Behav (1998) 23(6):893–907. doi: 10.1016/S0306-4603(98)00076-8
6. Grant B, Stinson F, Dawson D, Chou P, Dufour M, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. From the Laboratory of Epidemiology and Biometry, Division of Intramural Clinical and Biological Research (Drs Grant, Stinson, Dawson, and Chou and Mr Pickering), and the Office of the Director (Dr Dufour), the National Instituteon Alcohol Abuse and Alcoholism, and the Division of Epidemiology, Services, and Prevention Research, National Institute on Drug Abuse (Dr Compton), National Institutes of Health, Department of Health and Human Services, Bethesda, Md;and the Demographic Surveys’ Division, US Census Bureau, Suitland, Md (MrKaplan). 8. Arch Gen Psychiatry (2004) 61(8):807–16. doi: 10.1001/archpsyc.61.8.807
7. Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry (2005) 62(6):617–27. doi: 10.1001/archpsyc.62.6.617
8. Hintz T, Mann K. Comorbidity in alcohol use disorders: focus on mood, anxiety and personality. In: Stohler R, Röslerr W, editors. Dual diagnosis—the evolving conceptual network. Karger (2007). p. 65–91. doi: 10.1159/000085910
9. Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry (2007) 64(5):566–76. doi: 10.1001/archpsyc.64.5.566
10. Toftdahl NG, Nordentoft M, Hjorthøj C. Prevalence of substance use disorders in psychiatric patients: a nationwide Danish population-based study. Soc Psychiatry Psychiatr Epidemiol (2016) 51(1):129–40. doi: 10.1007/s00127-015-1104-4
11. Dore G. Psychiatric comorbidity. In: Latt N, Conigrave K, Saunders JB, Marshall EJ, Nutt D, editors. Oxford specialist handbooks addiction medicine. Oxford University Press (2009). p. 295–309.
12. Menezes PR, Johnson S, Thornicraft G, Marshall J, Prosser D, Bebbington P. Drug and alcohol problems among individuals with severe mental illness in South London. British. Journal of Psychiatry (1996) 168:612–9. doi: 10.1192/bjp.168.5.612
13. Kessler RC. The epidemiology of dual diagnosis. Biol Psychiatry (2004) 56:730–7. doi: 10.1016/j.biopsych.2004.06.034
14. Mueser KT, Noordsy DL, Drake RE, Fox L. Integrated treatment for dual disorders: a guide to effective practice. New York: Guilford (2003) p. 3–15.
15. van Loo HM, Romeijn JW, de Jonge P, Schoevers RA. Psychiatric comorbidity and causal disease models. Preventive Medicine (2013) 57(6):748–52. doi: 10.1016/j.ypmed.2012.10.018
16. Mueser KT, Drake RE, Turner W, McGovern M. Comorbid substance use disorders and psychiatric disorders. In: Miller WR, Carroll KM, editors. Rethinking substance abuse: what the science shows, and what we should do about it. Guilford (2006). p. 115–33.
17. Frisher M, Crome I, Macleod J, Millson D, Croft P. Substance misuse and psychiatric illness: prospective observational study using the general practice research database. J Epidemiol Community Health (2005) 59:847–50. doi: 10.1136/jech.2004.030833
18. Swendsen J, Conway KP, Degenhardt L, Gantz M, Jin R, Merikangas KR, et al. Mental disorders as risk factors for substance use, abuse and dependence: results from the 10-year follow-up of the National Comorbidity Survey. Addiction (2010) 105(6):1117–28. doi: 10.1111/j.1360-0443.2010.02902.x
19. Khantzian EJ. The self-medication hypothesis of addictive disorders. Am J Psychiatry (1985) 142:1259–64. doi: 10.1176/ajp.142.11.1259
20. Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry (1997) 4(5):231–44. doi: 10.3109/10673229709030550
21. Moggi F. Etiological theories on the relationship of mental disorders and substance use disorders. In: Stohler R, Röslerr W, editors. Dual diagnosis—the evolving conceptual network. Karger (2007). p. 1–14. doi: 10.1159/000085851
22. Kushner MG, Krueger R, Frye B, Peterson J. Epidemiological perspectives on co-occurring anxiety disorder and substance use disorder. In: Stewart SH, Conrod PJ, editors. Anxiety and substance use disorders: the vicious cycle of comorbidity. Springer (2008). p. 3–17. doi: 10.1007/978-0-387-74290-8_1
23. Volkow ND. Substance use disorders in schizophrenia—clinical implications of comorbidity. Schizophr Bull (2009) 35(3):469–72. doi: 10.1093/schbul/sbp016
24. Thoma P, Daum I. Comorbid substance use in schizophrenia: a selective overview of neurobiological and cognitive underpinnings. Psychiatry Clin Neurosci (2013) 67:367–83. doi: 10.1111/pcn.12072
25. Fischer BA. A review of American psychiatry through its diagnoses: the history and development of the Diagnostic and Statistical Manual of Mental Disorders. J Nerv Ment Dis (2012) 200(12):1022–30. doi: 10.1097/NMD.0b013e318275cf19
26. Balhara YP, Kuppili PP, Gupta R. Neurobiology of comorbid substance use disorders and psychiatric disorders: current state of evidence. J Addict Nurs (2017) 28(1):11–26. doi: 10.1097/JAN.0000000000000155
27. Adan A, Arredondo AY, Capella MM, Prat G, Forero DA, Navarro JF. Neurobiological underpinnings and modulating factors in schizophrenia spectrum disorders with a comorbid substance use disorder: a systematic review. Neurosci Biobehav Rev (2017) 75:361–77. doi: 10.1016/j.neubiorev.2017.01.038
28. Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res (2010) 117(1):1–12. doi: 10.1016/j.schres.2009.12.022
29. Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry (2013) 71(2):109–18. doi: 10.1001/jamapsychiatry.2013.3469
30. Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. (2013) 381(9875):1371–9. doi: 10.1016/S0140-6736(12)62129-1
31. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci (2011) 15(10):483–506. doi: 10.1016/j.tics.2011.08.003
32. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol (2012) 8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049
33. Walter M, Denier D, Vogel M, Lang UE. Effects on psychoactive substances in schizophrenia—findings of structural and functional neuroimaging. Curr Top Med Chem (2012) 12(21):2426–33. doi: 10.2174/1568026611212210013
34. Malchow B, Hasan A, Fusar-Poli P, Schmitt A, Falkai P, Wobrock T. Cannabis abuse and brain morphology in schizophrenia: a review of the available evidence. Eur Arch Psychiatry Clin Neurosci (2013) 263(1):3–13. doi: 10.1007/s00406-012-0346-3
35. Ho B-C, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry (2011) 68:128–37. doi: 10.1001/archgenpsychiatry.2010.199
36. Scheller-Gilkey G, Lewine RRJ, Caudle J, Brown FW. Schizophrenia, substance use and brain morphology. Schizophr Res (1999) 35:113–20. doi: 10.1016/S0920-9964(98)00096-6
37. Cahn W, Hulshoff Pol HE, Caspers E, van Haren NEM, Schnack HG, Kahn RS. Cannabis and brain morphology in recent-onset schizophrenia. Schizophr Res (2004) 67:305–7. doi: 10.1016/S0920-9964(03)00003-3
38. Joyal CC, Pennanen C, Tiihonen E, Laakso MP, Tiihonen J, Aronen HJ. MRI volumetry of the vermis and the cerebellar hemispheres in men with schizophrenia. Psychiatry Res Neuroimaging (2004) 131(2):115–24. doi: 10.1016/j.pscychresns.2003.09.003
39. Szeszko PR, Robinson DG, Sevy S, Kumra S, Rupp CI, Betensky JD, et al. Anterior cingulate greymatter deficits and cannabis use in first-episode schizophrenia. Br J Psychiatry (2007) 190:230–6. doi: 10.1192/bjp.bp.106.024521
40. Bangalore SS, Prasad KM, Montrose DM, Goradia DD, Diwadkar VA, Keshavan MS. Cannabis use and brain structural alterations in first episode schizophrenia—a region of interest, voxel based morphometric study. Schizophr Res (2008) 99:196. doi: 10.1016/j.schres.2007.11.029
41. Rais M, Cahn W, Van Haren N, Schnack H, Caspers E, Pol Hulshoff H, et al. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am J Psychiatry (2008) 165:490–6. doi: 10.1176/appi.ajp.2007.07071110
42. Rais M, van Haren NE, Cahn W, Schnack HG, Lepage C, Collins L, et al. Cannabis use and progressive cortical thickness loss in areas rich in CB1 receptors during the first five years of schizophrenia. Eur Neuropsychopharmacol (2010) 20(12):855–65. doi: 10.1016/j.euroneuro.2010.08.008
43. Wobrock T, Sittinger H, Behrendt B, D’Amelio R, Falkai P. Comorbid substance abuse and brain morphology in recent-onset psychosis. Eur Arch Psychiatry Clin Neurosci (2009) 259(1):28–36. doi: 10.1007/s00406-008-0831-x
44. Ebdrup BH, Glenthøj B, Rasmussen H, Aggernaes B, Langkilde AR, Paulson OB, et al. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci (2010) 35(2):95–104. doi: 10.1503/jpn.090049
45. Ho BC, Wassink TH, Ziebell S, Andreasen NC. Cannabinoid receptor 1 gene polymorphisms and marijuana misuse interactions on white matter and cognitive deficits in schizophrenia. Schizophr Res (2011) 128:66–75. doi: 10.1016/j.schres.2011.02.021
46. Onwuameze OE, Nam KW, Epping EA, Wassink TH, Ziebell S, Andreasen NC, et al. MAPK14 and CNR1 gene variant interactions: effects on brain volume deficits in schizophrenia patients with marijuana misuse. Psychol Med (2013) 43(3):619–31. doi: 10.1017/S0033291712001559
47. James A, Hough M, James S, Winmill L, Burge L, Nijhawan S, et al. Greater white and grey matter changes associated with early cannabis use in adolescent-onset schizophrenia (AOS). Schizophr Res (2011) 128:91–7. doi: 10.1016/j.schres.2011.02.014
48. Cohen M, Rasser PE, Peck G, Carr VJ, Ward PB, Thompson PM, et al. Cerebellar grey-matter deficits, cannabis use and first-episode schizophrenia in adolescents and young adults. Int J Neuropsychopharmacol (2012) 15:297–307. doi: 10.1017/S146114571100068X
49. Kumra S, Robinson P, Tambyraja R, Jensen D, Schimunek C, Houri A, et al. Parietal lobe volume deficits in adolescents with schizophrenia and adolescents with cannabis use disorders. J Am Acad Child Adolesc Psychiatry (2012) 51(2):171–80. doi: 10.1016/j.jaac.2011.11.001
50. Schnell T, Kleiman A, GouzoulisMayfrank E, Daumann J, Becker B. Increased gray matter density in patients with schizophrenia and cannabis use: a voxelbased morphometric study using DARTEL. Schizophr Res (2012) 138(2–3):183187. doi: 10.1016/j.schres.2012.03.021
51. Cunha PJ, Rosa PG, Ayres Ade M, Duran FL, Santos LC, Scazufca M. Cannabis use, cognition and brain structure in first-episode psychosis. Schizophr Res (2013) 147(2–3):209–15. doi: 10.1016/j.schres.2013.04.009
52. Malchow B, Hasan A, Schneider-Axmann T, Jatzko A, Gruber O, Schmitt A, et al. Effects of cannabis and familial loading on subcortical brain volumes in first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci (2013) 263 Suppl 2:S155–68. doi: 10.1007/s00406-013-0451-y
53. Epstein KA, Kumra S. Executive attention impairment in adolescents with schizophrenia who have used cannabis. Schizophr Res (2014) 157(1–3):48–54. doi: 10.1016/j.schres.2014.04.035
54. Koenders L, Machielsen MW, van der Meer FJ, van Gasselt AC, Meijer CJ, van den Brink W, et al. Brain volume in male patients with recent onset schizophrenia with and without cannabis use disorders. J Psychiatry Neurosci (2015) 40(3):197–206. doi: 10.1503/jpn.140081
55. Sullivan EV, Deshmukh A, Desmond JE, Mathalon DH, Rosenbloom MJ, Lim KO, et al. Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Arch Gen Psychiatry (2000) 57(9):894–902. doi: 10.1001/archpsyc.57.9.894
56. Sullivan EV, Rosenbloom MJ, Serventi KL, Deshmukh A, Pfefferbaum A. The effects of alcohol dependence comorbidity and antipsychotic medication on volumes of the thalamus and pons in schizophrenia. Am J Psychiatry (2003) 160(6):1110–6. doi: 10.1176/appi.ajp.160.6.1110
57. Mathalon DH, Pfefferbaum A, Lim KO, Rosenbloom MJ, Sullivan EV. Compounded brain volume deficits in schizophrenia-alcoholism comorbidity. Arch Gen Psychiatry (2003) 60(3):245–52. doi: 10.1001/archpsyc.60.3.245
58. Deshmukh A, Rosenbloom MJ, De Rosa E, Sullivan EV, Pfefferbaum A. Regional striatal volume abnormalities in schizophrenia: effects of comorbidity for alcoholism, recency of alcoholic drinking, and antipsychotic medication type. Schizophr Res (2005) 79(2–3):189–200. doi: 10.1016/j.schres.2005.04.025
59. Potvin S, Mancini-Marïe A, Fahim C, Mensour B, Lévesque J, Karama S, et al. Increased striatal gray matter densities in patients with schizophrenia and substance use disorder: a voxel-based morphometry study. Psychiatry Res (2007) 154(3):275–9. doi: 10.1016/j.pscychresns.2006.11.009
60. Schiffer B, Müller BW, Scherbaum N, Forsting M, Wiltfang J, Leygraf N, et al. Impulsivity-related brain volume deficits in schizophrenia-addiction comorbidity. Brain (2010) 133(10):3093–103. doi: 10.1093/brain/awq153
61. Smith MJ, Wang L, Cronenwett W, Goldman MB, Mamah D, Barch DM, et al. Alcohol use disorders contribute to hippocampal and subcortical shape differences in schizophrenia. Schizophr Res (2011) 131(1–3):174–83. doi: 10.1016/j.schres.2011.05.014
62. Solowij N, Yucel M, Respondek C, Whittle S, Lindsay E, Pantelis C, et al. Cerebellar white-matter changes in cannabis users with and without schizophrenia. Psychol Med (2011) 41:2349–59. doi: 10.1017/S003329171100050X
63. Solowij N, Walterfang M, Lubman DI, Whittle S, Lorenzetti V, Styner M, et al. Alteration to hippocampal shape in cannabis users with and without schizophrenia. Schizophr Res (2013) 143(1):179–84. doi: 10.1016/j.schres.2012.10.040
64. Gizewski ER, Müller BW, Scherbaum N, Lieb B, Forsting M, Wiltfang J, et al. The impact of alcohol dependence on social brain function. Addict Biol (2013) 18(1):109–20. doi: 10.1111/j.1369-1600.2012.00437.x
65. Smith AJ, Cobia DJ, Wang L, Alpert KL, Cronenwett WJ, Goldman MB, et al. Cannabis-related working memory deficits and associated subcortical morphological differences in healthy individuals and schizophrenia subjects. Schizophr Bull (2014) 40(2):287–99. doi: 10.1093/schbul/sbt176
66. Mancini-Marïe A, Potvin S, Fahim C, Beauregard M, Mensour B, Stip E. Neural correlates of the affect regulation model in schizophrenia patients with substance use history: a functional magnetic resonance imaging study. J Clin Psychiatry (2006) 67(3):342–50. doi: 10.4088/JCP.v67n0302
67. Joyal CC, Putkonen A, Mancini-Marïe A, Hodgins S, Kononen M, Boulay L. et al. Violent persons with schizophrenia and comorbid disorders: a functional magnetic resonance imaging study. Schizophr Res (2007) 91(1-3):97–102. doi: 10.1016/j.schres.2006.12.014
68. Potvin S, Mancini-Marïe A, Fahim C, Mensour B, Stip E. Processing of social emotion in patients with schizophrenia and substance use disorder: an fMRI study. Soc Neurosci (2007) 2(2):1060116. doi: 10.1080/17470910701376787
69. Løberg EM, Nygård M, Berle JØ, Johnsen E, Kroken RA, Jørgensen HA, et al. An fMRI study of neuronal activation in schizophrenia patients with and without previous cannabis use 4:51]. Front Psychiatry (2012) 3:94. doi: 10.3389/fpsyt.2012.00094
70. Bourque J, Mendrek A, Durand M, Lakis N, Lipp O, Stip E, et al. Cannabis abuse is associated with better emotional memory in schizophrenia: a functional magnetic resonance imaging study. Psychiatry Res (2013) 214(1):24–32. doi: 10.1016/j.pscychresns.2013.05.012
71. Thompson JL, Urban N, Slifstein M, Xu X, Kegeles LS, Girgis RR, et al. Striatal dopamine release in schizophrenia comorbid with substance dependence. Mol Psychiatry (2013) 18(8):909–15. doi: 10.1038/mp.2012.109
72. De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res (2005) 29(9):1590–600. doi: 10.1097/01.alc.0000179368.87886.76
73. Woodward SH, Kaloupek DG, Streeter CC, Kimble MO, Reiss AL, Eliez S, et al. Hippocampal volume, PTSD and alcoholism in combat veterans. Am J Psychiatry (2006) 163:674–81. doi: 10.1176/appi.ajp.163.4.674
74. Hassel S, Almeida JR, Frank E, Versace A, Nau SA, Klein CR, et al. Prefrontal cortical and striatal activity to happy and fear faces in bipolar disorder is associated with comorbid substance abuse and eating disorder. J Affect Disord (2009) 118(1–3):19–27. doi: 10.1016/j.jad.2009.01.021
75. Cornelius JR, Aizenstein HJ, Hariri AR. Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addict Behav (2010) 35(6):644–6. doi: 10.1016/j.addbeh.2010.02.004
76. Sameti M, Smith S, Patenaude B, Fein G. Subcortical volumes in long-term abstinent alcoholics: associations with psychiatric comorbidity. Alcohol Clin Exp Res (2011) 35(6):1067–80. doi: 10.1111/j.1530-0277.2011.01440.x
77. Nery FG, Matsuo K, Nicoletti MA, Monkul ES, Zunta-Soares GB, Hatch JP, et al. Association between prior alcohol use disorders and decreased prefrontal gray matter volumes in bipolar I disorder patients. Neurosci Lett. (2011) 503(2):136–40. doi: 10.1016/j.neulet.2011.08.026
78. Lippard ETC, Mazure CM, Johnston JAY, Spencer L, Weathers J, Pittman B, et al. Brain circuitry associated with the development of substance use in bipolar disorder and preliminary evidence for sexual dimorphism in adolescents. J Neurosci Res (2017) 95(1–2):777–91. doi: 10.1002/jnr.23901
79. Winklbaur B, Ebner N, Sachs G, Thau K, Fischer G. Substance abuse in patients with schizophrenia. Dialogues Clin Neurosci (2006) 8(1):37–43.
80. Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry (2010) 22:41728. doi: 10.3109/09540261.2010.515205
81. Peters BD, de Haan L, Vlieger E, Majoie CB, Heeten GJ, Linszen DH. Recent-onset schizophrenia and adolescent cannabis use: MRI evidence for structural hyperconnectivity. Psychopharmacol Bull (2009) 42(2):75–88.
82. Dekker N, Schmitz N, Peters BD, van Amelsvoort TA, Linszen DH, de Haan L. Cannabis use and callosal white matter structure and integrity in recent onset schizophrenia. Psychiatry Res Neuroimaging (2010) 181(1):51–6. doi: 10.1016/j.pscychresns.2009.06.003
83. Schneider CE, White T, Hass J, Geisler D, Wallace SR, Roessner V, et al. Smoking status as a potential confounder in the study of brain structure in schizophrenia. J Psychiatr Res (2014) 50:84–91. doi: 10.1016/j.jpsychires.2013.12.004
84. Keshavan MS, Morris DW, Sweeney JA, Pearlson G, Thaker G, Seidman LJ, et al. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res (2011) 133(1-3):250–4. doi: 10.1016/j.schres.2011.09.005
85. Wilson JE, Nian H, Heckers S. The schizoaffective disorder diagnosis: a conundrum in the clinical setting. Eur Arch Psychiatry Clin Neurosci (2014) 264(1):29–34. doi: 10.1007/s00406-013-0410-7
86. Salvatore P, Baldessarini RJ, Tohen M, Khalsa HM, Sanchez-Toledo JP, Zarate CA, et al. McLean-Harvard international first-episode project: two-year stability of DSM-IV diagnoses in 500 first-episode psychotic disorder patients. J Clin Psychiatry (2009) 70:458–66. doi: 10.4088/JCP.08m04227
87. Schwartz JE, Fennig S, Tanenberg-Karant M, Carlson G, Craig T, Galambos N, et al. Congruence of diagnoses 2 years after a first-admission diagnosis of psychosis. Arch Gen Psychiatry (2000) 57:593–600. doi: 10.1001/archpsyc.57.6.593
88. Roiz-Santiañez R, Suarez-Pinilla P, Crespo-Facorro B. Brain structural effects of antipsychotic treatment in schizophrenia: a systematic review. Curr Neuropharmacol (2015) 13(4):422–34. doi: 10.2174/1570159X13666150429002536
89. Dietsche B, Kircher T, Falkenberg I. Structural brain changes in schizophrenia at different stages of the illness: a selective review of longitudinal magnetic resonance imaging studies. Aust N Z J Psychiatry (2017) 51(5):500–8. doi: 10.1177/0004867417699473
90. Beck A, Wüstenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, et al. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry (2012) 69(8):842–52. doi: 10.1001/archgenpsychiatry.2011.2026
91. Battistella G, Fornari E, Annoni JM, Chitioui H, Dao K, Fabritius M, et al. Long-term effects of cannabis on brain structure. Neuropsychopharmacology (2014) 39(9):2041–8. doi: 10.1038/npp.2014.67
92. Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol (2010) 92(3):370–85. doi: 10.1016/j.pneurobio.2010.06.010
93. Rodríguez-Sánchez JM, Ayesa-Arriola R, Mata I, Moreno-Calle T, Perez-Iglesias R, González-Blanch C, et al. Cannabis use and cognitive functioning in first episode schizophrenia patients. Schizophr Res (2010) 124(1–3):142–51. doi: 10.1016/j.schres.2010.08.017
94. Uhlmann A, Fouche JP, Koen N, Meintjes EM, Wilson D, Stein DJ. Fronto-temporal alterations and affect regulation in methamphetamine dependence with and without a history of psychosis. Psychiatry Res Neuroimaging (2016) 248:30–8. doi: 10.1016/j.pscychresns.2016.01.010
95. Ersche KD, Fletcher PC, Lewis SJ, Clark L, Stocks-Gee G, London M, et al. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (2005) 180:612–23. doi: 10.1007/s00213-005-2205-7
96. Payer DE, Lieberman MD, Monterosso JR, Xu J, Fong TW, London ED. Differences in cortical activity between methamphetamine-dependent and healthy individuals performing a facial affect matching task. Drug Alcohol Depend (2008) 93:93–102. doi: 10.1016/j.drugalcdep.2007.09.009
97. Abernathy K, Chandler LJ, Woodward JJ. Alcohol and the prefrontal cortex. Int Rev Neurobiol (2010) 91:289–320. doi: 10.1016/S0074-7742(10)91009-X
98. Quello SB, Brady KT, Sonne SC. Mood disorders and substance use disorder: a complex comorbidity. Sci Pract Perspect (2005) 3(1):13–21. doi: 10.1151/spp053113
99. Perra S, Pillolla G, Luchicchi A, Pistis M. Alcohol inhibits spontaneous activity of basolateral amygdala projection neurons in the rat: involvement of the endocannabinoid system. Alcohol Clin Exp Res (2008) 32(4):443–9. doi: 10.1111/j.1530-0277.2007.00588.x
100. Goodkind M, Eickhoff SB, Oathes DJ, Ying J, Chang A, Jones-Hagata L, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry (2015) 72(4):305–15. doi: 10.1001/jamapsychiatry.2014.2206
Keywords: neuroimaging studies, comorbidity, substance use disorders, mood disorders, anxiety disorders
Citation: Stoychev KR (2019) Neuroimaging Studies in Patients With Mental Disorder and Co-occurring Substance Use Disorder: Summary of Findings. Front. Psychiatry 10:702. doi: 10.3389/fpsyt.2019.00702
Received: 31 July 2019; Accepted: 30 August 2019;
Published: 23 October 2019.
Edited by:
Drozdstoy Stoyanov Stoyanov, Plovdiv Medical University, BulgariaReviewed by:
Vladimir Venkov Nakov, National Center of Public Health and Analyses, BulgariaDeyan Hrusafov, Medical University of Varna, Bulgaria
Rayna Noncheva Mandova, Medical University of Varna, Bulgaria
Copyright © 2019 Stoychev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaloyan Rumenov Stoychev, kaloyan_stoichev@abv.bg
 Kaloyan Rumenov Stoychev
Kaloyan Rumenov Stoychev