- 1Division of Pulmonary and Critical Care Medicine, Wenzhou Medical University Affiliated Taizhou Hospital, Linhai, Zhejiang, China
- 2Department of Pulmonary and Critical Care Medicine, Regional Medical Center for National Institute of Respiratory Diseases, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 3Department of mental health, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China
Objective: The risk of venous thromboembolism in patients with mental illness has been insufficiently addressed. This study aimed to assess the correlation between hyperhomocysteinemia and venous thromboembolism prevalence among this population.
Methods: Patients with a diagnosis of mental illness and concurrent venous thromboembolism, admitted to Sir Run Run Shaw Hospital at Zhejiang University School of Medicine between January 2014 and December 2021, were included in the venous thromboembolism group. The control group, approximately twice the size, comprised individuals with mental illness but without venous thromboembolism. Basic clinical data were gathered for both cohorts.
Results: In psychiatric patients, elevated D-dimer levels(OR=5.60,95% CI 3.28–10.00), hyperhomocysteinemia (OR=2.37,95% CI 1.10–5.14), and hyperprolactinemia(OR= 2.68,95% CI 1.12–6.42)were significant risk factors for venous thromboembolism. According to further subgroup analyses, hyperhomocysteinemia is a significant risk factor associated with pulmonary embolism, with an OR of 5.08 (95% CI 1.20–21.48). An interaction effect between gender and homocysteine level was found, with a p-interaction of 0.022. A subsequent analysis confirmed the association between hyperhomocysteinemia and venous thromboembolism in female psychiatric patients, with an OR of 3.34 (95% CI 1.68–6.65), indicating that hyperhomocysteinemia is a significant risk factor for venous thromboembolism in women.
Conclusion: Patients with psychiatric disorders were found to have an elevated risk of venous thromboembolism, which was associated with increased levels of D-dimer, hyperprolactinemia, and hyperhomocysteinemia. A strong correlation between hyperhomocysteinemia and pulmonary embolism was identified in patients with mental illnesses. Furthermore, the study revealed that female psychiatric patients with hyperhomocysteinemia constituted a high-risk group for venous thromboembolism. This finding holds significant clinical implications, suggesting that early preventative measures could be implemented for this high-risk population to reduce the incidence of thromboembolic events during hospitalization for psychiatric patients.
Introduction
Venous thromboembolism (VTE) is a common thromboembolic disease in the population, mainly including Deep Venous Thrombosis (DVT) and Pulmonary Thromboembolism (PTE) (1). The incidence of VTE is high all over the world, some studies had found that the annual incidence of VTE could be as high as 2 ‰ (1), A study found that the hospitalization rate of venous thromboembolism in China increased about five times between 2007 and 2016, and the mortality rate of VTE was seen to rise with age over time (2). Due to the high morbidity and mortality of VTE, it has become a public social health problem.
Patients with mental illness often suffer from social withdrawal and reduced physical activity due to their illness, and most patients with obvious psychiatric symptoms need to take antipsychotic drugs to control clinical symptoms, all of which can lead to blood hypercoagulation and promote the formation of venous thrombosis (3, 4). Previous research (5, 6) has found that the overall prevalence of venous thrombosis in hospitalized patients with mental illness was as high as 1–2%. However, concerning the incidence of venous thromboembolism (VTE) in individuals with mental disorders, the current body of research is scant, and no consensus on incidence rates has been established. Consequently, to ascertain the actual incidence rates of VTE among this patient population, future studies necessitate a more comprehensive and profound investigation. Nevertheless, the fact that mental patients are easy to be complicated with VTE has not attracted enough attention in clinics, which often leads to missed diagnosis and adverse clinical outcomes.
Homocysteine is an amino acid that relies on the vitamin B6 and vitamin B12 pathways for synthesis during methionine metabolism. Homocysteine can cause oxidative stress and promote vascular inflammation through the activation of various signaling pathways, which in turn causes vascular damage (7) and induces thrombus formation. It has been found that the increase of plasma homocysteine concentration is a risk factor for many cardiovascular diseases, which significantly increases the risk of atherosclerosis and contributes to the formation of arterial thrombosis (7–9). Additional research revealed that hyperhomocysteinemia is a stand-alone risk factor for VTE (10), However, studies on the correlation between homocysteine and VTE were mostly retrospective analyses (11, 12).
Several studies have found that the concentration of serum homocysteine in patients with mental disorders such as schizophrenia, depression and Alzheimer’s disease was higher than that in the general population (13–15). Additionally, hyperhomocysteinemia may contribute to the onset and progression of certain mental illnesses.
At present, the attention caused by mental illness patients with VTE is still low, and there are few studies on the risk factors of mental illness patients with VTE. Referring to the previous literature, it is found that the incidence of HHcy in mental illness patients is much higher than that of the general population. It is speculated that HHcy may be a related risk factor for mental illness patients with VTE. Therefore, this paper intends to study the correlation between HHcy and VTE in patients with mental illness.
Material and methods
Study population
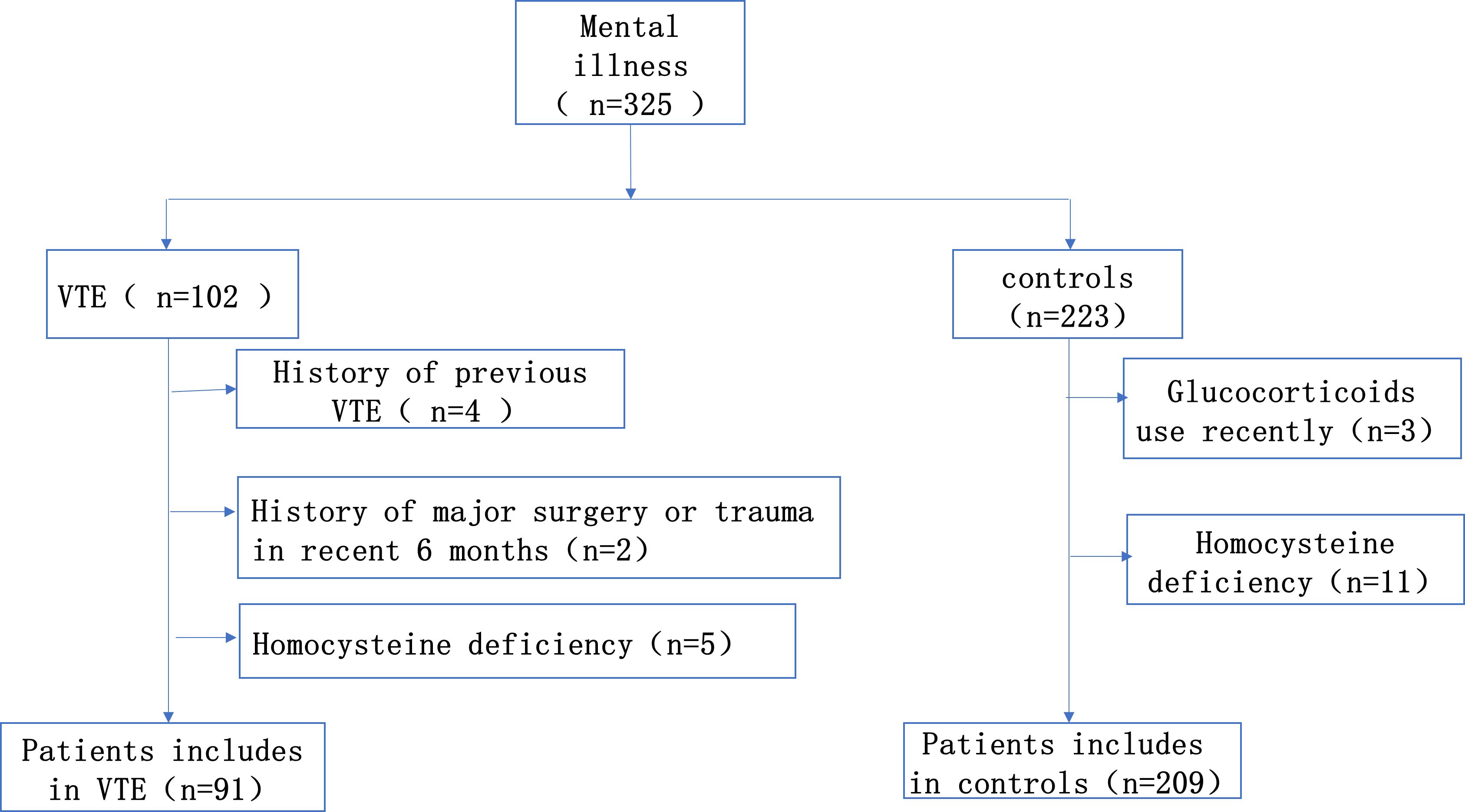
This is a single-center retrospective case-control study. From January 2014 to December 2021, we continuously enrolled 91 consecutive patients diagnosed with mental illness combined with VTE in Sir Run Run Shaw Hospital, Zhejiang University School of Medicine. Inclusion criteria for the case group: Patients with a diagnosis of both mental illness and VTE, with a diagnosis of mental illness that meets the diagnostic criteria of the International Classification of Diseases, 10th edition (ICD-10) or the American Manual of Mental Disorders and Statistics, 5th edition (DSM-V) (16). The diagnosis of deep vein thrombosis was dependent on Doppler ultrasound. And the diagnosis of pulmonary embolism was confirmed by CT pulmonary angiography. Any patients who fit one of the following descriptions were disqualified: 1. Patients diagnosed with venous thromboembolism prior to admission; 2. Patients who had taken contraceptives, folic acid, vitamin B12 and glucocorticoids within the last 6 months; 3. Patients were recently pregnant or breastfeeding; 4. Patient had a history of major surgery or trauma within the last 6 months. The appropriate subjects were selected from the psychiatric patients without VTE in the same period as the control group, the proportion was about 1:2. The study was approved by the Ethics Committee (Figure 1).
Clinical data collection and definitions
The patient’s sex, age, body mass index (BMI), smoking history, antipsychotic use, olanzapine use, antidepressant use, Complications (hypertension, diabetes, chronic kidney disease, malignant tumor, coronary heart disease, cerebral infarction) were obtained and collected from the hospital medical record system. Routine blood tests and blood biochemistry were performed upon admission to obtain the following laboratory results such as neutrophils, lymphocytes, fibrinogen, platelets, D-dimer, CRP, homocysteine, prolactin and so on. Hyperhomocysteinemia is defined as plasma homocysteine levels >15 umol/L (17). Fasting blood samples were collected on admission. Hyperprolactinemia is defined as a single plasma prolactin level above the upper limit of normal (18), the upper limit of normal prolactin for men and postmenopausal women in our hospital was set at 20 μg/L.
Statistical analysis
Statistical analysis was conducted using SPSS 26.0 and R 4.2.2 software. Categorical data were presented as frequencies and analyzed by chi-squared test or Fisher’s exact test as appropriate. Continuous data were expressed as mean ± SD or median with interquartile range according to the normality of the distributions. To test the differences between the groups, t-test was applied to continuous data that conform to a regular distribution, the Mann–Whitney U-test was used for continuous data that did not conform to a regular distribution. The concentration of plasma homocysteine was analyzed by categorical variables with 15 umol/L as the boundary. Logistic regressions were used to assess the relationship between homocysteine and VTE. On the basis of univariate analysis followed by Multivariate logistic regression analysis (including independent variables with P values <0.1 and sex) to explore the independent risk factors for VTE in patients with mental illness. We then explored the effect of hyperhomocysteinemia on VTE according to the VTE subtype typing. It has been shown that there is a difference between elevated plasma homocysteine levels and the occurrence of VTE in different genders (19, 20). Then the interaction between gender and Hcy is analyzed by R4.2.2 software, the p value of the interaction is calculated, and the bar chart of the interaction between Hcy and gender is drawn by using the cat_plot function in R package to visualize, and then stratified analysis is carried out according to gender. Lastly, we computed the prediction probability of combined D-dimer, homocysteine and prolactin by logistic regression model, and plotted the predictive probability value and ROC curve of VTE to evaluate the predictive performance of combined three indicators in patients with mental illness complicated with VTE. In this study, the definition of P < 0.05 is statistically significant.
Results
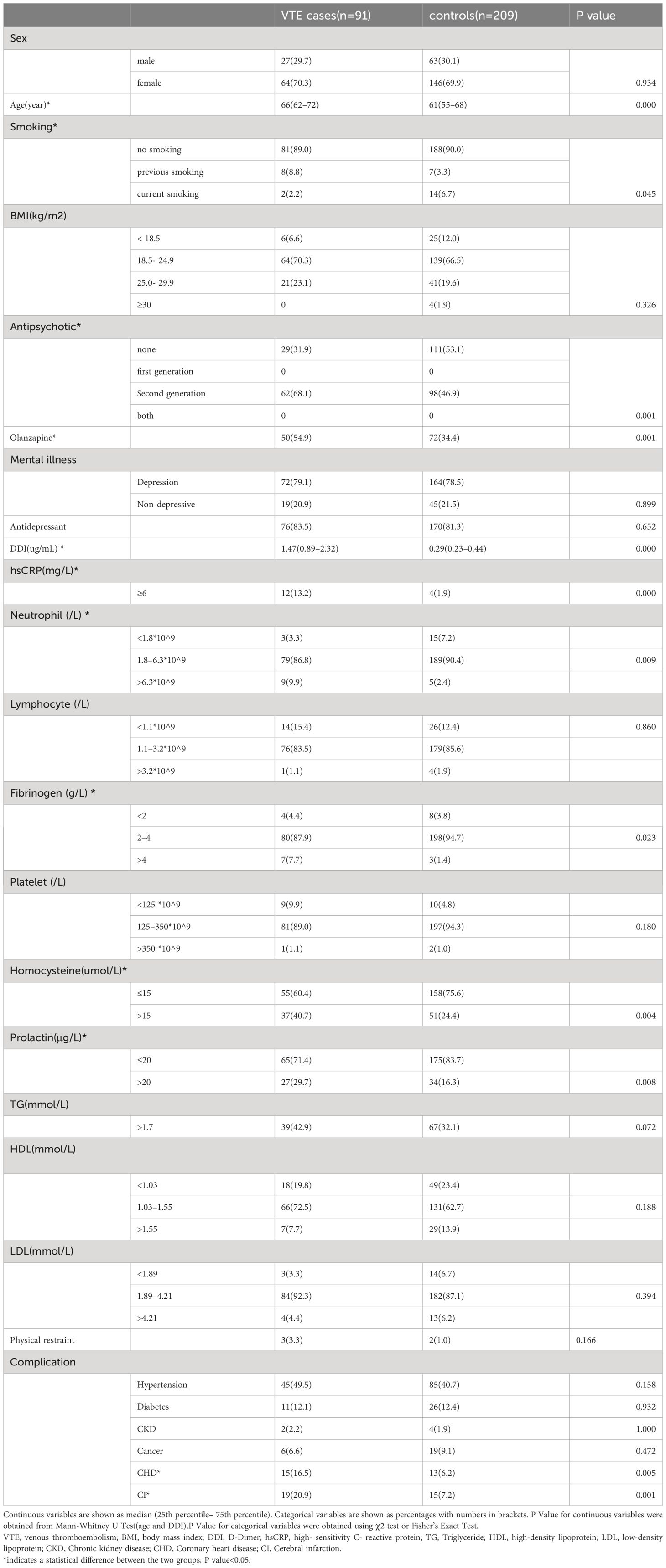
As shown in Table 1, A total of 91 patients were included in VTE group, 27 (29.7%) males and 64 (70.3%) females, with a mean age of 66 years. The control group included a total of 209 cases, 63 (30.1%) males and 146 (69.9) females, with a mean age of 61 (55–68) years. There were no significant differences in BMI, Antidepressant, Lymphocyte, Platelet, TG, LDL, HDL and Physical restraint between the 2 groups. However, age, smoking history, antipsychotic use, olanzapine use, DDI, hsCRP, neutrophils, fibrinogen, homocysteine and prolactin were found to be statistically significant in the cases and control groups (p < 0.05).
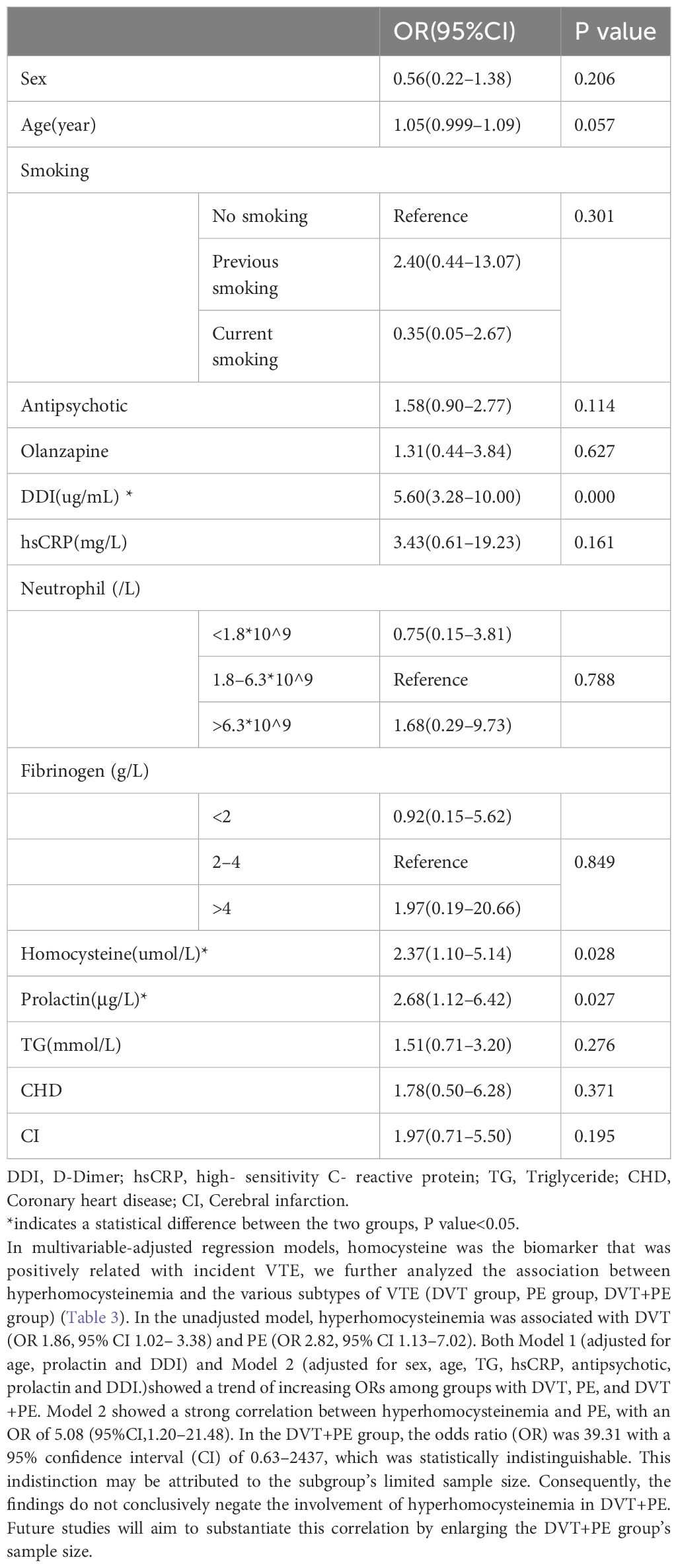
Table 2 showed the multifactorial regression analysis of the occurrence of VTE in patients with mental illness. With the occurrence of VTE event as the dependent variable, independent variables with P value<0.1 in the univariate analysis (age, smoking history, antipsychotic use, olanzapine use, D-dimer, CRP, neutrophils, fibrinogen, homocysteine, prolactin, triglycerides, coronary artery disease, cerebral infarction) and sex were included in the multifactorial regression model. The results showed that DDI (OR=5.60, 95%CI3.28–10.00), hyperhomocysteinemia (OR=2.37, 95%CI1.10–5.14) and hyperprolactinemia were statistically different between the cases and controls groups.
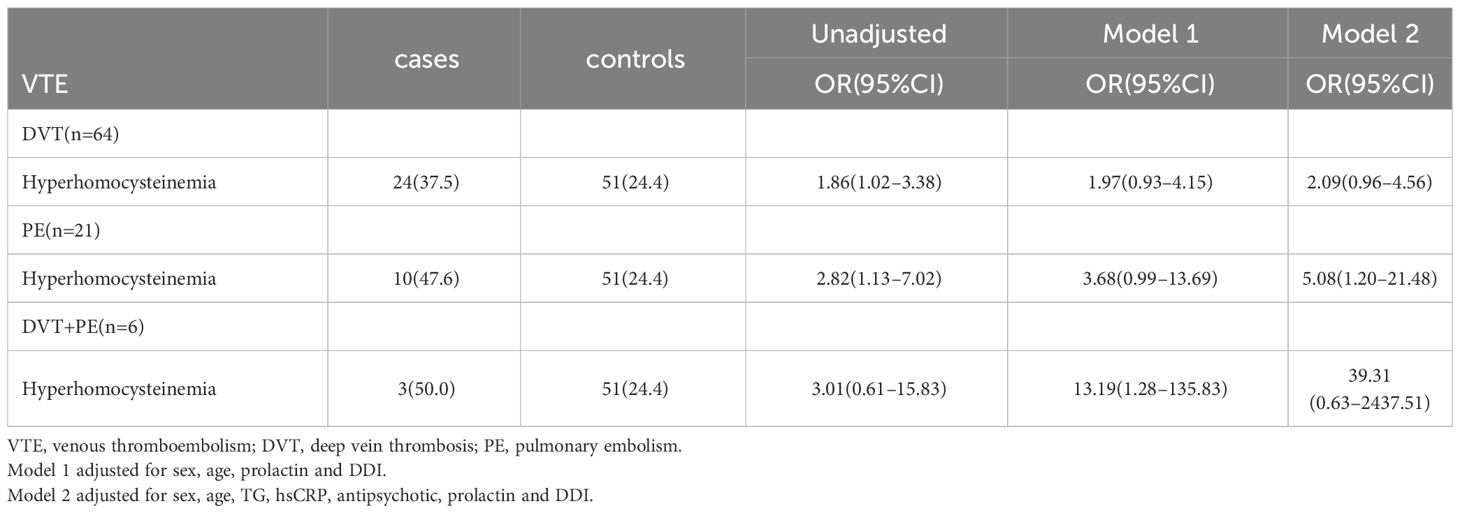
In this study, the interaction between homocysteine and sex was further analyzed, the p value of the interaction was calculated by R4.2.2 software, and the bar chart of the interaction between homocysteine and sex was drawn by using the cat_plot function in R packet, showing the interaction effects between homocysteine and sex. The y-axis denotes the VTE incidence rates. At plasma homocysteine concentrations ≤15 µmol/L, both male (0.28) and female (0.25) patients exhibited comparable rates of venous thromboembolism. Contrastingly, at levels >15 µmol/L, a pronounced divergence in incidence rates was observed between genders. Notably, the rate for female patients escalated to 0.52, a stark increase, whereas the rate for male patients remained constant at 0.31, similar to prior data. Statistical analysis revealed the p-interaction was 0.022, thereby confirming a statistically significant interaction between gender and homocysteine levels. Considering the significant interaction observed between gender and homocysteine levels, a subgroup analysis was performed. It was found that hyperhomocysteinemia was significantly correlated with VTE in female patients with mental disorders, which was a risk factor for VTE in female patients with mental disorders, and after adjusting multiple confounding factors (age, prolactin, D-dimer, triglyceride, CRP, antipsychotics). It still has a strong correlation, OR=3.34 (95%CI=1.68–6.65). On the contrary, hyperhomocysteinemia was not associated with the occurrence of VTE in male patients with mental illness, OR=1.19 (95%CI=0.48–2.92). Figures 2, 3
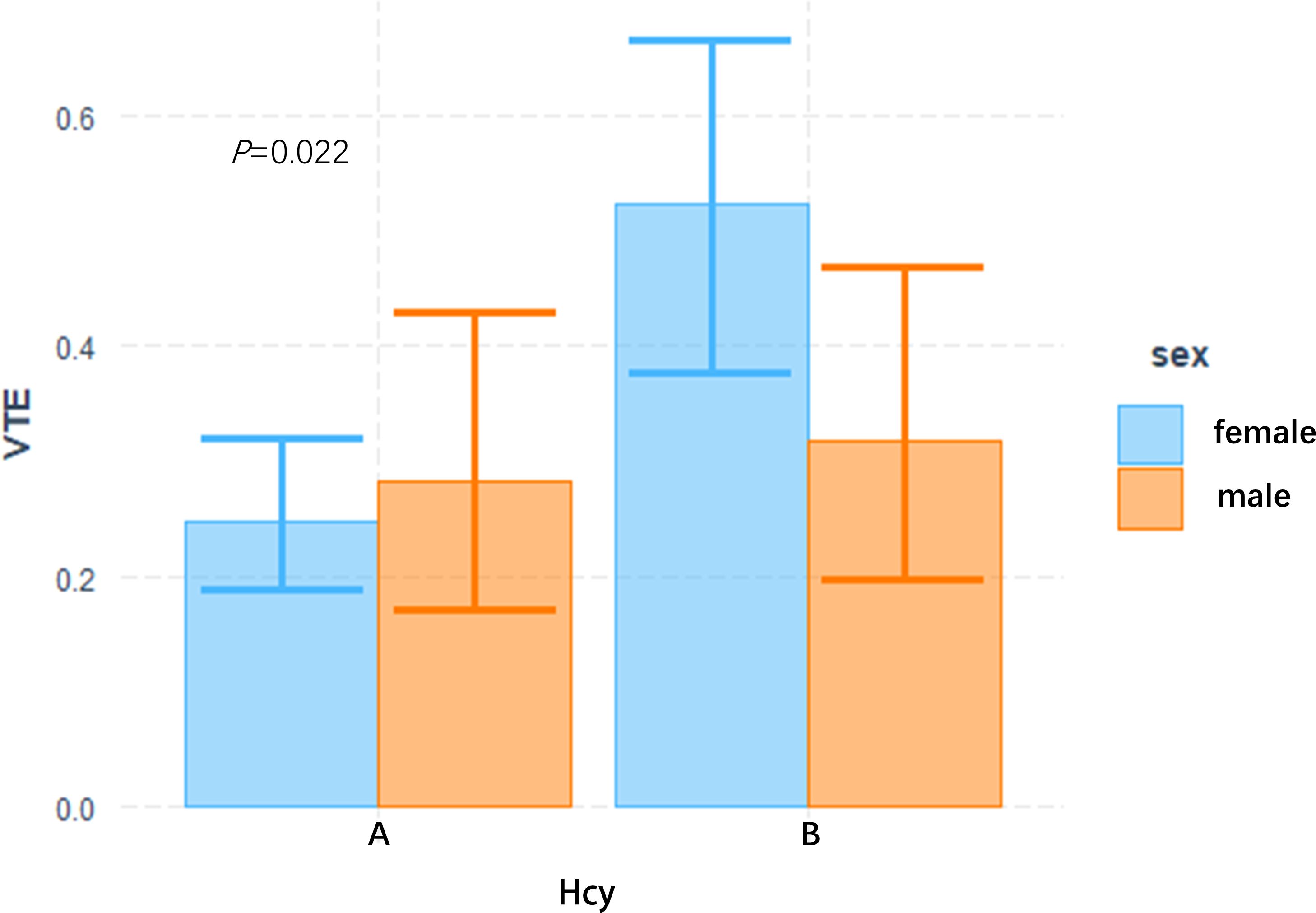
Figure 2 Interactive analysis of HCY and gender bar chart group A: plasma Hcy ≤ 15 umol/L, B group: plasma Hcy > 15 umol/L.
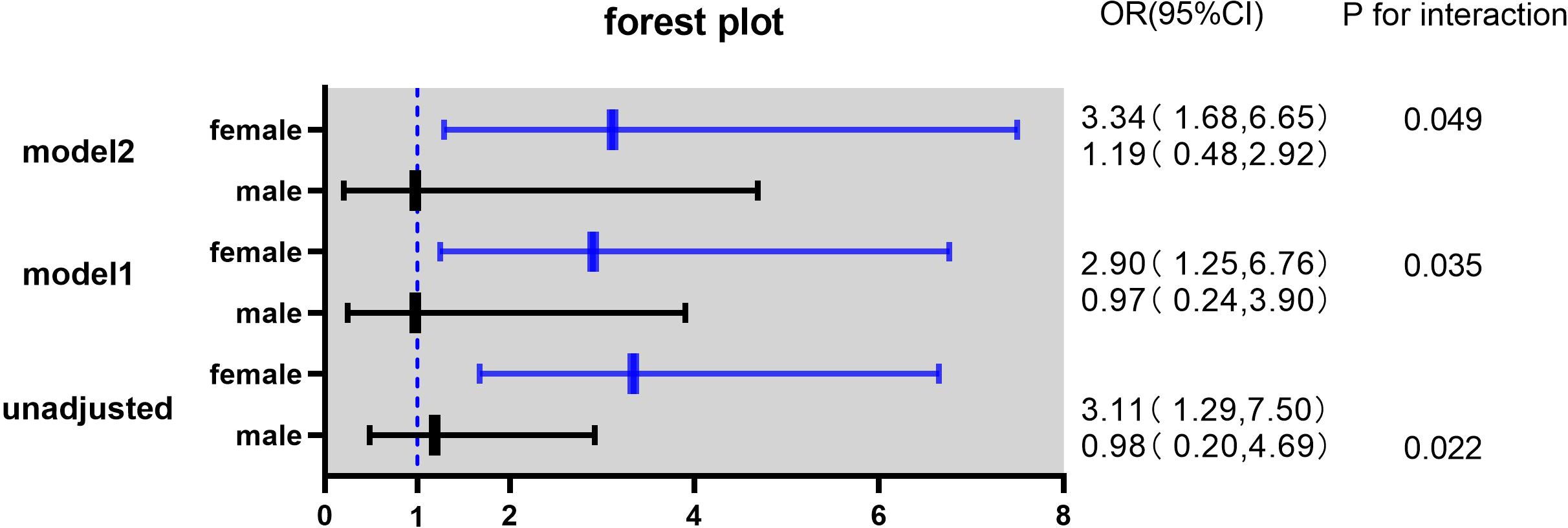
Figure 3 Odds ratios (ORs) with 95% confidence intervals (CIs) for overall venous thromboembolism according to sex.
Model 1 adjusted for age, prolactin and DDI.
Model 2 adjusted for age, TG, hsCRP, antipsychotic prolactin and DDI.
Through the binary Logistic regression model in SPSS26.0, the prediction probability of VTE was calculated by combining the three laboratory indexes of D-dimer, homocysteine and prolactin, and then the ROC curve of prediction probability and VTE was drawn (Figure 4). The AUC area was 0.912 (95%CI:0.873–0.951), and the sensitivity and specificity of the best cutoff value was 0.934 and 0.828 respectively. It is suggested that the combination of D-dimer, homocysteine and prolactin has high diagnostic value for VTE in patients with mental disorders.
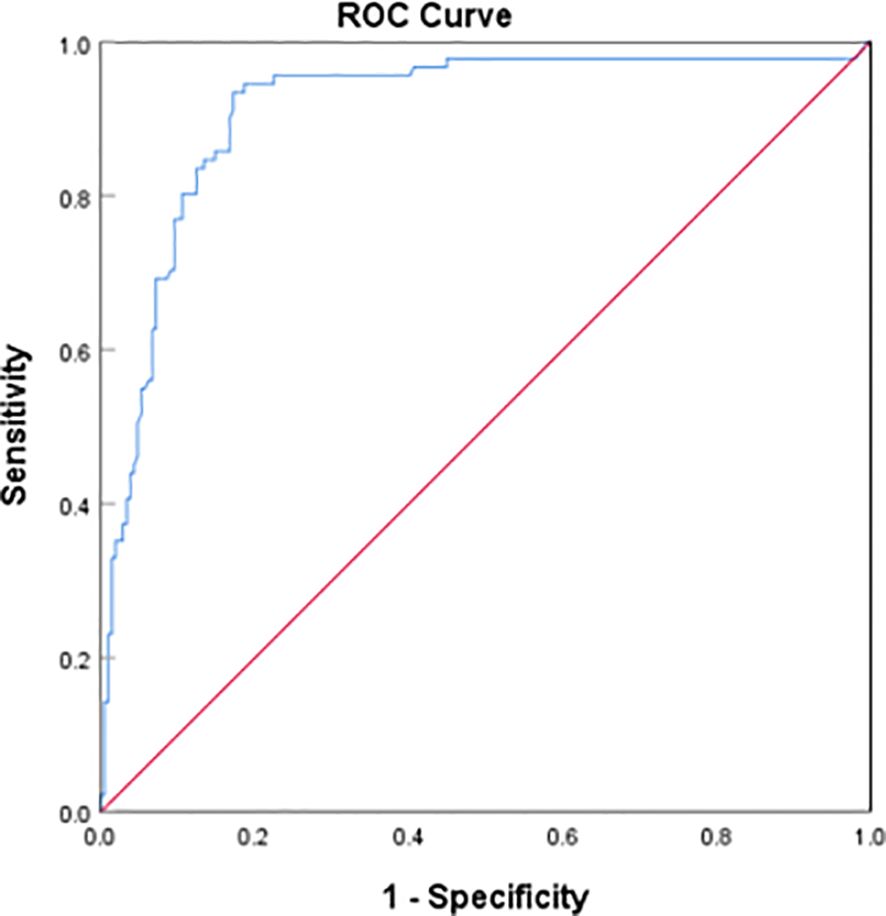
Figure 4 Receiver operating characteristic (ROC) plot of DDI+hyperhomocysteinemia + hyperprolactinemia predicting presence of VTE AUC area was 0.912 (95%CI:0.873–0.951).
Discussion
In this study, it was found that hyperhomocysteinemia, hyperprolactinemia and elevated D-dimer were the risk factors of VTE in patients with mental illness, and after adjusting for a variety of confounding factors, the above three indexes were still strongly associated with the occurrence of VTE in patients with mental illness. Subsequently, the correlation of hyperhomocysteinemia among VTE subgroups was further studied, and it was found that hyperhomocysteinemia was a risk factor for DVT and PE in patients with mental illness in the model with unadjusted confounding factors. After adjusting for confounding factors, the correlation of hyperhomocysteinemia increased in DVT group, PE group and DVT+PE group, but only the p value of PE group was < 0.05. However, due to the relatively small number of cases in the subgroup analysis, there may be some bias, which leads to the underestimation of the relationship between HHcy and DVT+PE group and DVT group, so a larger sample is needed in the future. In addition, this study also found that there was an interaction between homocysteine and gender. Hyperhomocysteinemia significantly increased the risk of VTE in female patients with mental illness, and after adjusting for confounding factors, the interaction still existed. The follow-up stratified analysis gives more accurate results, suggesting that female mental patients with hyperhomocysteinemia are at high risk for VTE. The three factors of hyperhomocysteinemia, hyperprolactinemia and D-dimer were included in the ROC curve, and the AUC area was 0.912, suggesting that it has important reference value for the diagnosis of VTE in patients with mental disorders.
In recent years, more and more researchers were concerned that patients with mental illness were prone to co-occurring VTE with atypical clinical symptoms, which were easily missed. The reduction of activity caused by mental illness itself and the use of antipsychotic drugs during treatment can greatly increase the risk of venous thrombosis in patients with mental illness (21–23). However, in terms of clinical diagnosis and treatment, the attention to mental illness complicated with VTE is still low, and there is a high rate of missed diagnosis, which can easily lead to poor prognosis of patients.
However, the risk factors for the occurrence of venous thrombosis in patients with mental illness remain unclear and may be associated with the mental illness itself and the administration of antipsychotic drugs.
Homocysteine is an important enzyme in the regulation of methionine and cysteine metabolism. On the one hand, homocysteine levels are strongly correlated with psychiatric symptoms and patient prognosis, and they play a crucial role in the development of mental illness, particularly schizophrenia (24, 25). On the other hand, hyperhomocysteinemia is a risk factor for cardiovascular diseases, which can induce endothelial cell injury and apoptosis by inhibiting the synthesis and repair of DNA through multiple pathways (26). It can also interfere with NO metabolism of endothelial cells, lead to the imbalance of NO metabolism (27) and induce endothelial cell dysfunction. At the same time, homocysteine can directly or indirectly activate platelets, promote platelet adhesion and aggregation with a variety of blood cells and endothelial cells, and regulate the contraction of blood clots (28). In general, hyperhomocysteinemia may affect coagulation and fibrinolysis in the body from multiple pathways.
A review of previous studies shows that most studies believe that hyperhomocysteinemia is an independent risk factor for VTE and increases the risk of venous thrombosis. Multiple meta-analyses (11, 29) suggested that hyperhomocysteinemia increased the incidence of venous thrombosis in patients. The latest meta-analysis found that the increase of plasma total homocysteine level was closely related to venous thrombosis. The incidence of VTE increased by 60% for every 5 mol/L increase in plasma homocysteine(OR=1.6, 95% CI, 1.10–2.34), which might be associated with the methylenetetrahydrofolate reductase gene 677TT (MTHFR 677TT) mutation (11). MTHFR is a key enzyme in homocysteine metabolism, When its gene mutation occurs, it will lead to the weakening of related enzyme activity and the continuous accumulation of homocysteine in the blood, resulting in hyperhomocysteinemia (30). However, the above studies do not adjust the influence of common confounding factors such as age, sex, smoking history and BMI, so the conclusions may be biased. A cohort study (31) found that elevated plasma homocysteine levels were significantly associated with the future occurrence of VTE. Patients with homocysteine level ≥ 12.9 μ mol/L had a 0.3-fold increased risk compared with patients with homocysteine level ≤ 8.6 μ mol/L, with a HR of 1.31 (95%CI:1.06–1.63). Further classification of PE and DVT revealed that elevated homocysteine levels were associated with unprovoked PE (HR=2.13 (95% CI, 1.30–3.51) and unprovoked DVT (HR=1.59 (95% CI, 1.05–2.40).
However, some studies have come to the opposite conclusion. A case-control study in the Netherlands included 1,689 cases with two separate control groups, one for general population controls and the other for partner-matched controls. The case group was compared with the two groups respectively. After adjusting the common confounding factors by logistic regression, the level of homocysteine had nothing to do with VTE. Further stratification was carried out according to sex, DVT, PE, inductive VTE and non-inductive VTE. It was still found that the increase of homocysteine concentration did not increase the incidence of thrombosis in patients (32).
There is a paucity of related research in patients with mental illness complicated with VTE. A variety of mental illness can cause an increase in the concentration of plasma homocysteine in patients (13, 14). An observational study focusing on psychiatric disorders with VTE found that the most common laboratory risk factors for patients were elevated FVIII levels and hyperhomocysteinemia, with approximately 33% of patients having hyperhomocysteinemia (33). A meta-analysis (34) shows that about 30% of patients with depression have HHcy, accompanied by a decrease in folic acid levels, which is much higher than the proportion of hyperhomocysteinemia in the general population. A case-control (35) study included 93 patients with schizophrenia and 60 healthy volunteers found that patients with schizophrenia had lower concentrations of tetrahydrobiopterin and folic acid and higher levels of Hcy than healthy volunteers. Some studies have found that the prevalence of HHcy in patients with schizophrenia can reach 30–50% (13, 36). A population screening program in China, which included 110551 subjects from 31 provinces across the country, found that only about 8 per cent of patients had HHcy (37). In conclusion, the prevalence of HHcy in patients with mental illness is substantially higher than that in the general population.
Therefore, this study explored the relationship between hyperhomocysteinemia and VTE in patients with mental illness. In this study, the proportion of hyperhomocysteinemia in the case group and the control group was 40% and 24% respectively, which was much higher than that in the normal population (8%). This is consistent with the conclusion that the incidence of hyperhomocysteinemia in patients with mental illness is higher than that in the general population.
It was also found that hyperhomocysteinemia was indeed a risk factor for VTE in patients with mental illness, and it still had a high risk ratio after adjusting confounding factors. Further analysis found that there was a strong correlation between hyperhomocysteinemia and the occurrence of PE in patients with mental illness. To investigate the correlation between homocysteine levels and DVT + PE group, a larger sample study is needed in the future.
In addition, there may be gender differences in the risk of VTE in hyperhomocysteinemia. The HUNT2 study from Norwegian (38) found that the risk of VTE was approximately doubled in male patients with plasma homocysteine concentrations above the 95th percentile, OR= 2.17 (95% CI 1.20–3.91), whereas no association was observed in female patients, OR=1.00. However, the stratified analysis of Tsai et al. (12) found that hyperhomocysteinemia was strongly correlated with the incidence of VTE in younger patients (<65 years old), female, Caucasian, and non-diabetic patients.
In this paper, we examined the interaction between homocysteine levels and gender, which showed that an interaction was present between homocysteine levels and gender with a p-interaction value of less than 0.05. This confirmed the statistical significance of the gender-based interaction analysis outlined in the article. Further, subgroup analyses substantiated these findings. The study indicated that female patients suffering from mental disorders with elevated levels of homocysteine faced a significantly high risk of developing venous thromboembolism (VTE). The OR value calculated in the model after adjusting for confounding factors was 3.34 (95%CI=1.68–6.65). There were some differences between this study and previous studies, the reasons may be as follows: 1. In the gender stratification analysis of hyperhomocysteinemia, the Norwegian study only explored the situation where the level of homocysteine was higher than 95 percentile (above 25.9 μ mol/l), which was different from the 15 μmol/L set in this study. 2. Different studies were aimed at different races and people, most of the previous research subjects are Caucasians, while the subjects of this study were Asians, and all of them were patients with mental illness. 3. There were certain selection bias and mixed bias in different studies.
Although a number of studies have shown that hyperhomocysteinemia is associated with the occurrence of VTE, clinical trials have found that reducing homocysteine therapy can not reduce the incidence of VTE in patients. The VITRO study (39) aimed to examine the effects of vitamin B supplementation on the reduction of DVT and PE by reducing homocysteine. The study recruited 701 patients with confirmed VTE (including 360 patients with hyperhomocysteinemia and 341 patients with normal homocysteine). Patients were randomized to the vitamin treatment and placebo treatment groups, and after 2.5 years of treatment and follow-up, vitamin treatment was found to reduce homocysteine levels in the hyperhomocysteine and normal homocysteine groups by 46% and 33%, but did not reduce the risk of recurrent venous thrombosis in the patients.
However, there are no randomized clinical trials on VTE in patients with mental disorders, and it is impossible to determine the correlation between lowering homocysteine levels with B vitamins and VTE in patients with mental disorders.
In this study, we plotted the ROC curve by combining DDI with homocysteine and prolactin for the first time. The value of DDI in the prediction of VTE had been unequivocally confirmed. A review of the previous literature found that a variety of antipsychotic drugs can lead to increased prolactin levels in patients (40, 41). It was found that the level of serum prolactin increased significantly in patients with mental illness treated with antipsychotics, and the increase of prolactin was positively correlated with the levels of DDI and fibrin/fibrinogen degradation products, which would increase the concentration of coagulation markers in patients (42). Some scholars had proposed that prolactin can bind to specific receptors on the surface of platelets to enhance platelet activity (43, 44). And prolactin can induce inflammatory response in the body, lead to the activation of a variety of inflammatory mediators and inflammatory cells, and affect endothelial function, thus directly or indirectly promote the formation of thrombus (45, 46). Therefore, we plotted the ROC curve combined with these three indicators for the first time. The results indicate that the combination of DDI, homocysteine and prolactin has a good predictive performance for the incidence of VTE in patients with mental illness.
Referring to the literature, this is the first clinical study to explore the relationship between hyperhomocysteinemia and VTE in patients with mental illness. as mentioned above, previous studies focused on the relationship between hyperhomocysteinemia and VTE in the general population, and the conclusions were different, which may be related to the selection bias of the included population, the different definition of hyperhomocysteinemia and the influence of confounding factors. Secondly, the blood samples in this study were collected before the VTE event, so it can further substantiate the causal relationship between hyperhomocysteinemia and VTE in patients with mental illness. This paper also performed a subgroup analysis according to the thrombus site, and further analyzed the interaction between sex and homocysteine, and then conducted stratified analysis by sex. Based on the study of this paper, we discovered that female mental patients with hyperhomocysteinemia were at high risk of VTE. This study is beneficial for clinicians to detect high-risk patients early and initiate thrombus prevention measures for high-risk patients as soon as possible, so as to effectively reduce poor prognosis.
However, this study also had some limitations, in the subgroup analysis according to the thrombus site, the number of cases in some subgroups was small, especially in the DVT+PE group, so the conclusion may be biased, resulting in underestimating the association between hyperhomocysteinemia and DVT+PE group. Secondly, most of the patients included in this study were over 50 years old, so the conclusions may not be applicable to young patients with mental illness. Additionally, this investigation was a single-center study involving Chinese patients with mental disorders, predominantly those suffering from depression, and owing to the lack of severity assessment in the initial case data, a classification of disease severity was not incorporated into the statistical model. Moreover, in the process of data collection, it was observed that antipsychotic medications varied significantly in their varieties and dosages, so they couldn’t be included in the statistical model. As a result, a binary classification approach was used without dosage information. Due to considerable variability in patient heterogeneity concerning the temporal relationship between fasting blood samples and thrombotic events, this section was not further analyzed in this paper. Finally, the predictors of this paper can not quantitatively evaluate the thrombus risk of patients with mental illness, and larger samples and further studies are needed in the future to develop a unique predictor of thrombus risk for patients with mental illness.
Patients with mental illness are at risk of VTE. Because of their diseases and the influence of taking antipsychotic drugs, they will increase the risk of metabolic syndrome. Therefore, the risk factors of VTE in patients with mental illness may be different from the general population. More studies are needed to explore the risk factors of VTE in patients with mental illness in the future. Further research will help to develop a unique thrombus risk assessment form for patients with mental illness in the future, so that high-risk patients can be identified early and accurate risk stratification can be carried out for patients. For high-risk patients, we should be vigilant, start physical prevention as early as possible (encourage more drinking water, more activities and the use of double lower extremity DVT pumps, etc.), and use drugs when necessary, so as to reduce the occurrence of VTE and improve the clinical prognosis of patients.
Conclusion
In patients with psychiatric disorders, a strong correlation was found between hyperhomocysteinemia and Pulmonary Embolism (PE). An interaction between homocysteine levels and gender was observed. Hyperhomocysteinemia significantly increases the risk of Venous Thromboembolism (VTE) in female psychiatric patients. Therefore, the conclusion of this article is that female psychiatric patients with hyperhomocysteinemia constitute a high-risk group for VTE. This discovery has important clinical implications, indicating that early preventive measures can be taken for this high-risk group. This will reduce the risk of thromboembolic events during hospitalization for patients with psychiatric disorders, thereby improving their clinical outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study passed ethical review by the Ethics Committee of Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University (Study Approval No.0433). The ethics committee waived the requirement of written informed consent for participation due to the retrospective design. The studies were conducted in accordance with the local legislation and institutional requirements.
Author contributions
JW: Data curation, Investigation, Methodology, Software, Writing – original draft. YZ: Data curation, Investigation, Methodology, Writing – review & editing. KR: Data curation, Investigation, Writing – review & editing. YL: Data curation, Methodology, Writing – review & editing. KY: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82170054), the Natural Science Foundation of Zhejiang Province (LY21H010003), the Medical Science and Technology Project of Zhejiang Province, China(2022RC037), the Medical and Health Science and Technology Program of Zhejiang Province, China(2019339118).
Acknowledgments
We wish to thank all the study participants and colleagues who supported us.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. Lancet. (2021) 398:64–77. doi: 10.1016/S0140-6736(20)32658-1
2. Zhang Z, Lei J, Shao X, Dong F, Wang J, Wang D, et al. Trends in hospitalization and in-hospital mortality from VTE, 2007 to 2016, in China. Chest. (2019) 155:342–53. doi: 10.1016/j.chest.2018.10.040
3. Kowal C, Peyre H, Amad A, Pelissolo A, Leboyer M, Schürhoff F, et al. Psychotic, mood, and anxiety disorders and venous thromboembolism: A systematic review and meta-analysis. Psychosom Med. (2020) 82:838–49. doi: 10.1097/PSY.0000000000000863
4. Hoirisch-Clapauch S. Anxiety-related bleeding and thrombosis. Semin Thromb Hemost. (2018) 44:656–61. doi: 10.1055/s-0038-1639501
5. Takeshima M, Ishikawa H, Shimizu K, Kanbayashi T, Shimizu T. Incidence of venous thromboembolism in psychiatric inpatients: a chart review. Neuropsychiatr Dis Treat. (2018) 14:1363–70. doi: 10.2147/NDT
6. Wang Z, Yang Y, He X, Jiang X, Gao X, Liu P, et al. Incidence and clinical features of venous thromboembolism in inpatients with mental illness. Clin Appl Thromb Hemost. (2023) 29:10760296231160753. doi: 10.1177/10760296231160753
7. Fu Y, Wang X, Kong W. Hyperhomocysteinaemia and vascular injury: advances in mechanisms and drug targets. Br J Pharmacol. (2018) 175:1173–89. doi: 10.1111/bph.13988
8. Zhang N, Zhu L, Wu X, Yan R, Yang S, Jiang X, et al. The regulation of Ero1-alpha in homocysteine-induced macrophage apoptosis and vulnerable plaque formation in atherosclerosis. Atherosclerosis. (2021) 334:39–47. doi: 10.1016/j.atherosclerosis.2021.08.015
9. Škovierová H, Vidomanová E, Mahmood S, Sopková J, Drgová A, Červeňová T, et al. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci. (2016) 17(10):1733. doi: 10.3390/ijms17101733
10. Köktürk N, Kanbay A, Aydoğdu M, Özyılmaz E, Bukan N, Ekim N. Hyperhomocysteinemia prevalence among patients with venous thromboembolism. Clin Appl Thromb Hemost. (2011) 17:487–93. doi: 10.1177/1076029610378499
11. Den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost. (2005) 3:292–9. doi: 10.1111/j.1538-7836.2005.01141.x
12. Tsai AW, Cushman M, Tsai MY, Heckbert SR, Rosamond WD, Aleksic N, et al. Serum homocysteine, thermolabile variant of methylene tetrahydrofolate reductase (MTHFR), and venous thromboembolism: Longitudinal Investigation of Thromboembolism Etiology (LITE). Am J Hematol. (2003) 72:192–200. doi: 10.1002/ajh.10287
13. Yang Y, Wang J, Xiong Z, Yao X, Zhang Y, Ning X, et al. Prevalence and clinical demography of hyperhomocysteinemia in Han Chinese patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. (2021) 271:759–65. doi: 10.1007/s00406-020-01150-x
14. Smith AD, Refsum H. Homocysteine B. Vitamins, and cognitive impairment. Annu Rev Nutr. (2016) 36:211–39. doi: 10.1146/annurev-nutr-071715-050947
15. Obeid R, McCaddon A, Herrmann W. The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. Clin Chem Lab Med. (2007) 45:1590–606. doi: 10.1515/CCLM.2007.356
16. Kupfer DJ, Kuhl EA, Regier DA. DSM-5--the future arrived. JAMA (2013) 309(16):1691–2. doi: 10.1001/jama.2013.2298
17. Finkelstein JD, Martin JJ. Homocysteine. Int J Biochem Cell Biol. (2000) 32:385–9. doi: 10.1016/S1357-2725(99)00138-7
18. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:273–88. doi: 10.1210/jc.2010-1692
19. Rozemeijer K, le Cessie S, van Hylckama Vlieg A, Rosendaal FR, Vandenbroucke JP, Poole C, et al. Exposure opportunity: the advantages of including men in analyses of female-related risk factors. Am J Epidemiol. (2017) 185:965–73. doi: 10.1093/aje/kww193
20. Roach RE, Lijfering WM, Rosendaal FR, Cannegieter SC, le Cessie S. Sex difference in risk of second but not of first venous thrombosis: paradox explained. Circulation. (2014) 129:51–6. doi: 10.1161/CIRCULATIONAHA.113.004768
21. Arasteh O, Nomani H, Baharara H, Sadjadi SA, Mohammadpour AH, Ghavami V, et al. Antipsychotic drugs and risk of developing venous thromboembolism and pulmonary embolism: A systematic review and meta-analysis. Curr Vasc Pharmacol. (2020) 18:632–43. doi: 10.2174/1570161118666200211114656
22. Conti V, Venegoni M, Cocci A, Fortino I, Lora A, Barbui C. Antipsychotic drug exposure and risk of pulmonary embolism: a population-based, nested case-control study. BMC Psychiatry. (2015) 15:92. doi: 10.1186/s12888-015-0479-9
23. Barbui C, Conti V, Cipriani A. Antipsychotic drug exposure and risk of venous thromboembolism: a systematic review and meta-analysis of observational studies. Drug Saf. (2014) 37:79–90. doi: 10.1007/s40264-013-0127-6
24. Misiak B, Frydecka D, Slezak R, Piotrowski P, Kiejna A. Elevated homocysteine level in first-episode schizophrenia patients–the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. (2014) 29:661–70. doi: 10.1007/s11011-014-9534-3
25. Akanji AO, Ohaeri JU, Al-Shammri SA, Fatania HR. Associations of blood homocysteine concentrations in Arab schizophrenic patients. Clin Biochem. (2007) 40:1026–31. doi: 10.1016/j.clinbiochem.2007.06.001
26. Lehotsky J, Petras M, Kovalska M, Tothova B, Drgova A, Kaplan P. Mechanisms involved in the ischemic tolerance in brain: effect of the homocysteine. Cell Mol Neurobiol. (2015) 35:7–15. doi: 10.1007/s10571-014-0112-3
27. Lai WK, Kan MY. Homocysteine-induced endothelial dysfunction. Ann Nutr Metab. (2015) 67:1–12. doi: 10.1159/000437098
28. Litvinov RI, Peshkova AD, Le Minh G, Khaertdinov NN, Evtugina NG, Sitdikova GF, et al. Effects of hyperhomocysteinemia on the platelet-driven contraction of blood clots. Metabolites. (2021) 11(6):354. doi: 10.3390/metabo11060354
29. den Heijer M, Rosendaal FR, Blom HJ, Gerrits WB, Bos GM. Hyperhomocysteinemia and venous thrombosis: a meta-analysis. Thromb Haemost. (1998) 80:874–7.
30. Zaric BL, Obradovic M, Bajic V, Haidara MA, Jovanovic M, Isenovic ER. Homocysteine and hyperhomocysteinaemia. Curr Med Chem. (2019) 26:2948–61. doi: 10.2174/0929867325666180313105949
31. Aday AW, Duran EK, Van Denburgh M, Kim E, Christen WG, Manson JE, et al. Homocysteine is associated with future venous thromboembolism in 2 prospective cohorts of women. Arterioscler Thromb Vasc Biol. (2021) 41:2215–24. doi: 10.1161/ATVBAHA.121.316397
32. Ospina-Romero M, Cannegieter SC, den Heijer M, Doggen CJM, Rosendaal FR, Lijfering WM. Hyperhomocysteinemia and risk of first venous thrombosis: the influence of (Unmeasured) confounding factors. Am J Epidemiol. (2018) 187:1392–400. doi: 10.1093/aje/kwy004
33. Masopust J, Bazantova V, Kuca K, Klimova B, Valis M. Venous thromboembolism as an adverse effect during treatment with olanzapine: A case series. Front Psychiatry. (2019) 10:330doi: 10.3389/fpsyt.2019.00330
34. Stanger O, Fowler B, Piertzik K, Huemer M, Haschke-Becher E, Semmler A, et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev Neurother. (2009) 9:1393–412. doi: 10.1586/ern.09.75
35. Zhilyaeva TV, Kasyanov ED, Semennov IV, Rukavishnikov GV, Piatoikina AS, Kostina OV, et al. Tetrahydrobiopterin deficiency in schizophrenia: Biochemical and clinical aspects. J Psychiatr Res. (2022) 153:141–8. doi: 10.1016/j.jpsychires.2022.07.020
36. Geller V, Friger M, Sela BA, Levine J. Elevated homocysteine level in siblings of patients with schizophrenia. Psychiatry Res. (2013) 210:769–72. doi: 10.1016/j.psychres.2013.08.016
37. Tu W, Yan F, Chao B, Ji X, Wang L. Status of hyperhomocysteinemia in China: results from the China Stroke High-risk Population Screening Program, 2018. Front Med. (2021) 15:903–12. doi: 10.1007/s11684-021-0871-4
38. Naess IA, Christiansen SC, Romundstad PR, Cannegieter SC, Blom HJ, Rosendaal FR, et al. Prospective study of homocysteine and MTHFR 677TT genotype and risk for venous thrombosis in a general population–results from the HUNT 2 study. Br J Haematol. (2008) 141:529–35. doi: 10.1111/j.1365-2141.2008.07073.x
39. den Heijer M, Willems HP, Blom HJ, Gerrits WB, Cattaneo M, Eichinger S, et al. Homocysteine lowering by B vitamins and the secondary prevention of deep vein thrombosis and pulmonary embolism: A randomized, placebo-controlled, double-blind trial. Blood. (2007) 109:139–44. doi: 10.1182/blood-2006-04-014654
40. Krøigaard SM, Clemmensen L, Tarp S, Pagsberg AK. A meta-analysis of antipsychotic-induced hypo- and hyperprolactinemia in children and adolescents. J Child Adolesc Psychopharmacol. (2022) 32:374–89. doi: 10.1089/cap.2021.0140
41. Burghardt KJ, Mando W, Seyoum B, Yi Z, Burghardt PR. The effect of antipsychotic treatment on hormonal, inflammatory, and metabolic biomarkers in healthy volunteers: A systematic review and meta-analysis. Pharmacotherapy. (2022) 42:504–13. doi: 10.1002/phar.2689
42. Ishioka M, Yasui-Furukori N, Sugawara N, Furukori H, Kudo S, Nakamura K. Hyperprolactinemia during antipsychotics treatment increases the level of coagulation markers. Neuropsychiatr Dis Treat. (2015) 11:477–84. doi: 10.2147/NDT.S75176
43. Atmaca A, Gurlek A, Dagdelen S, Erarslan N, Buyukasik Y, Gultekin M, et al. Hyperprolactinemia of pregnancy is not associated with increased in vivo platelet activity and shortened in vitro bleeding times. Exp Clin Endocrinol Diabetes. (2006) 114:188–91. doi: 10.1055/s-2006-924064
44. Wallaschofski H, Kobsar A, Sokolova O, Siegemund A, Stepan H, Faber R, et al. Differences in platelet activation by prolactin and leptin. Horm Metab Res. (2004) 36:453–7. doi: 10.1055/s-2004-825727
45. van Zaane B, Squizzato A, Reuwer AQ, van Zanten AP, Twickler MT, Dekkers OM, et al. Prolactin and venous thrombosis: indications for a novel risk factor? Arterioscler Thromb Vasc Biol. (2011) 31:672–7. doi: 10.1161/ATVBAHA.110.209569
Keywords: mental illness, venous thromboembolism, hyperhomocysteinemia, hyperprolactinemia, pulmonary thromboembolism
Citation: Wang J, Zhang Y, Ren K, Li Y and Ying K (2024) Hyperhomocysteinemia is associated with the risk of venous thromboembolism in patients with mental illness: a case-control study. Front. Psychiatry 15:1340138. doi: 10.3389/fpsyt.2024.1340138
Received: 17 November 2023; Accepted: 29 April 2024;
Published: 17 May 2024.
Edited by:
Lisa Jones, University of Worcester, United KingdomReviewed by:
Takahiko Nagamine, Sunlight Brain Research Center, JapanMaría Hidalgo-Figueroa, University of Cádiz, Spain
Copyright © 2024 Wang, Zhang, Ren, Li and Ying. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kejing Ying, 3197061@zju.edu.cn
†These authors have contributed equally to this work
 Jiaoyan Wang
Jiaoyan Wang Yingchun Zhang3†
Yingchun Zhang3† Kejing Ying
Kejing Ying