- 1Department of Psychiatry and Psychotherapy, Philipps-University Marburg, Marburg, Germany
- 2Department of Clinical Psychology and Psychotherapy, Philipps-University Marburg, Marburg, Germany
- 3Translational Clinical Psychology, Department of Psychology, Philipps-University Marburg, Marburg, Germany
Background: Ketamine and esketamine offer a novel approach in the pharmacological treatment of major depressive disorder (MDD). This meta-analysis aimed to investigate the placebo response in double-blind, randomized controlled studies (RCTs) on patients with MDD receiving ketamine or esketamine.
Methods: For this systematic review and meta-analysis Medline (PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), PsycInfo and Embase databases were systematically searched for citations published up to March 17, 2023. A total number of 5017 abstracts was identified. Quality of the included trials was assessed with the Cochrane risk-of-bias tool. The meta-analysis was performed using a restricted maximum likelihood model. This study is registered with PROSPERO, number CRD42022377591.
Results: A total number of 14 studies and 1100 participants (593 in the medication group and 507 in the placebo group) meeting the inclusion criteria were selected. We estimated the pooled effect sizes of the overall placebo (dpl = -1.85 [CI 95%: -2.9 to -0.79] and overall treatment (dtr = -2.57; [CI 95% -3.36 to -1.78]) response. The overall placebo response accounts for up to 72% of the overall treatment response. Furthermore, we performed subgroup analysis of 8 studies for the for the 7 days post-intervention timepoint. Seven days post-intervention the placebo response (dpl 7d = -1.98 [CI 95%: -3.26 to -0.69]) accounts for 66% of the treatment response (dtr 7d = - 3.01 [CI 95%, -4.28 to -1.74]).
Conclusion: Ketamine and esketamine show large antidepressant effects. However, our findings suggest that the placebo response plays a significant role in the antidepressant response and should be used for the benefit of the patients in clinical practice.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022377591.
Introduction
Placebo response is one of the mechanisms contributing to treatment response of antidepressant medication. Studies have estimated that placebo response may account for up to 62-82% of treatment response in randomized controlled trials (RCTs) of oral antidepressants (1–4). A recent meta-analysis suggests that only about 15% of participants in double-blind RCTs of antidepressants may benefit from antidepressants beyond the placebo response (5). Some authors argue there is an urgent need to develop new study designs controlling for the placebo effect (6).
Ketamine and esketamine offer a novel approach in the psychopharmacological treatment of major depressive disorder (MDD). A growing number of meta-analysis indicate that NMNDA-receptor-antagonists lead to a fast reduction of depressive symptoms within hours (7–9). The US Food and Drug Administration (FDA) (10) and European Medicines Agency (EMA) (11) have authorized the use of esketamine nasal spray for the treatment of depression. Although very promising, concerns have been raised about the long-term efficacy, safety and tolerability of esketamine (12–14).
MDD is a highly relevant disease worldwide. According to the World Health Organization (WHO) 300 million people globally are affected by depression (15). MDD is the third leading cause of years lost due to disability worldwide (16). Furthermore, the consumption of antidepressant medication reached a more than double fold increase in OECD countries between 2000 and 2019 (17). However, although pharmacological treatment is well established among treatment of MDD, there are indications that only one third of patients respond to first line treatment (18). New treatment approaches and further understanding of the mechanisms underlining the antidepressant treatment response are needed in order to provide improved healthcare to patients suffering from MDD.
The extent of the placebo response in the treatment of MDD with ketamine and esketamine has not been systematically studied yet. This meta-analysis aims to estimate the placebo response in double-blind, placebo-controlled, RCTs investigating the antidepressant pharmacological treatment with ketamine or esketamine in patients with MDD.
Methods
We conducted a systematic review and meta-analysis in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (19). The study protocol has been registered with PROSPERO number, CRD4202237759.
We included double-blinded, randomized, placebo-controlled trials with an inert placebo (e.g. saline or NaCL- infusions) as a comparator group investigating the intervention of ketamine or esketamine in a subanesthetic dose for the treatment of patients with MDD. We opted for inert placebos as a comparator group in order to avoid possible confounding pharmacological effects of active placebos (e.g. midazolam) and thus to calculate a representative placebo response in our analysis. Moreover, we excluded studies with cross-over design, active placebo, no placebo arm or no randomized controlled study design or including patients suffering of depressive symptoms due to another psychiatric condition than MDD.
We systematically searched the databases Embase, MEDLINE (PubMed), PsycINFO and the Cochrane Central Register of Controlled Trials (CENTRAL) for the keywords: placebo, esketamin* or ketamin* and depress*. A detailed overview of the search strategies for every database is provided in the supplement (Supplementary eTable 1). We performed the final systematic literature search on August 03, 2022. Additionally, a supplementary manual search using Google Scholar and the above-mentioned databases followed for articles published in 2023 on March 17, 2023 to identify newly published articles.
We exported results from the searches conducted at different databases and uploaded them in Rayyan, a web-based application for collaborative citation screening and full–text selection (20). After duplicate removal two independent reviewers (A.M. and L.N.) screened the abstracts and articles titles for eligibility. Following the completed eligibility check, the two reviewers screened the remaining studies for meeting the inclusion or exclusion criteria. Any discrepancies were resolved by a third reviewer (M.W.). Furthermore, we identified multiple reports of the same study and included only the report containing the information most relevant to answering the review question. Moreover, we identified study protocols of ongoing studies meeting the inclusion criteria and checked during the data extraction phase for study completion.
Data from identified reports was extracted independently by P.N. and C.T in separate uniform Microsoft Excel (21) spread sheets and compared after completion. We coded the extracted data in accordance to the guidelines provided by the Cochrane Handbook of Systematic Reviews of Interventions (22). Data extraction was supervised by A.M. We contacted authors in order to provide missing data and extracted additional data from figures using the WebPlotDigitizer tool (23) or from the study protocols of the included reports. Two independent reviewers (A.M. and L.N.) assessed study quality independently using the Cochrane risk-of-bias tool for randomized trials (RoB 2) (24). Discrepancies were resolved by a third reviewer (M.W.).
We performed the meta-analysis using the statistics software JASP (25). Missing standard deviations (SDs) were either calculated from other measures of variability (e.g. 95% confidence intervals) and test statistics or imputed from the SDs of the other similar studies (26). Placebo and treatment response were defined as the change of depressive symptoms in the placebo and medication groups respectively from baseline to the post-intervention time point. We used Cohen’s d effect size in a confidence interval of 95% as a measurement of effect to estimate the placebo and treatment response. A script using Pandas Python library (27) was developed in order to calculate automatically the effect sizes from the extracted data and increase precision.
Heterogeneity of the included studies was assessed by performing an I²-test (28). The restricted maximum-likelihood (REML) model was implemented to estimate the pooled effect size. We opted for the REML model due to its favorable outcome in comparison with other heterogeneity variance estimators (29). An exploratory univariate meta-regression analyses was performed to estimate possible moderators of the placebo or treatment response and values were considered significant as p <.05. We estimated the placebo and treatment response by calculating the pooled effect size for the change in depression rating scores from baseline to post-intervention in the placebo and medication groups respectively.
In order to avoid an overestimation of the placebo response we preferred the 24-hour post-intervention time point for statistical synthesis, whenever data was available, because this time point is affiliated with the highest antidepressant treatment response in ketamine and esketamine studies (30). When data for the preferred time point was not available in the selected studies, the closest available time point to the 24-hours post-intervention time point was selected. A meta-regression analysis was performed to control for the different included time points in the pooled analysis. Subgroup analysis were performed to estimate the placebo and treatment response from available data for the 40-minute, 2-hour, 4-hours, 24-hours and 7-days post-intervention time points and separately for studies with ketamine and studies with esketamine.
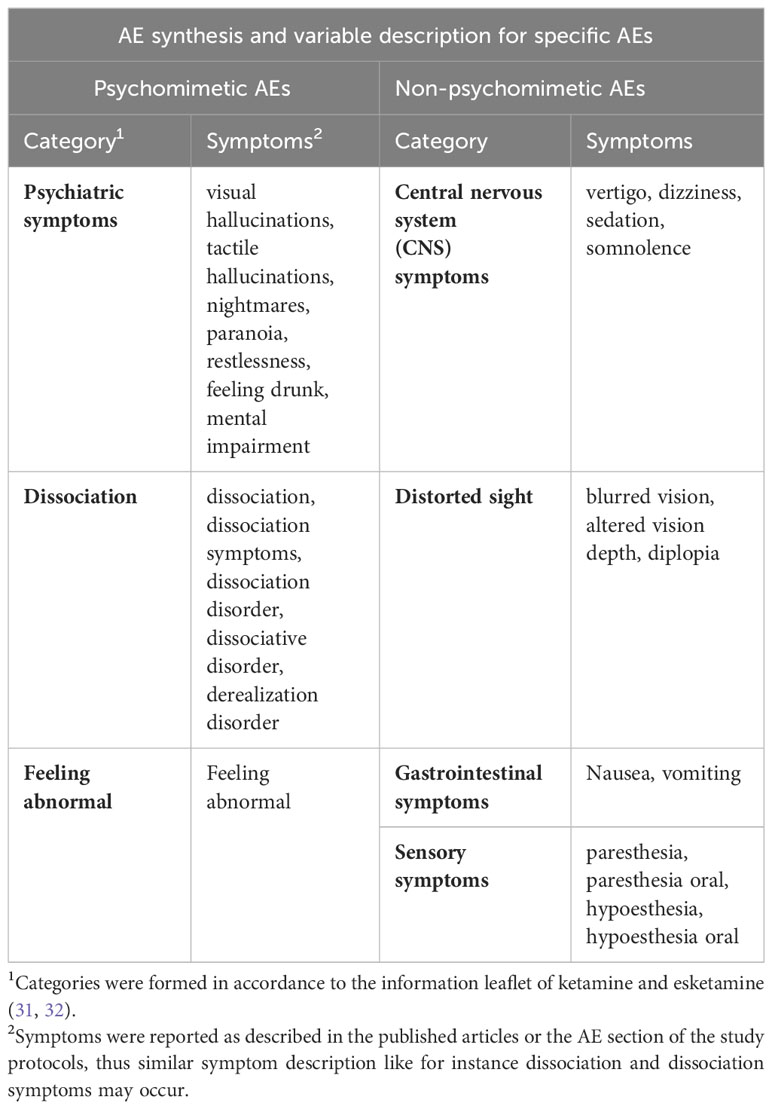
In order to quantify the effects of medication on study blinding we examined reported adverse events (AEs) of the included studies. We categorized reported AEs in specific and unspecific AEs. The specific AEs included medication-related AEs as described in the information leaflet for ketamine (31) and esketamine (32) and the rest of the AEs were categorized as unspecific. We formed the ratio between medication-related AEs and total AEs for each group and compared them via an unpaired t-Test to control for the validity of the categorization. Furthermore, we grouped together a subset of specific AEs containing distinct psychomimetic AEs attributed to ketamine and esketamine, like dissociation and hallucinations, that could lead to insufficient blinding. We formed the ratio between psychomimetic AEs and total AEs for each group and compared them via an unpaired t-Test to examine if they occur significantly more often in the medication arm and could be an indicator for potential bias due to insufficient blinding. More information about the categorization of the reported AEs is provided in (Table 1). Information about the summary of AEs in the placebo (Supplementary eTable 2) and medication (Supplementary eTable 3) group as well as a detailed report of the included non-psychomimetic and psychomimetic AEs for the placebo groups (Supplementary eTables 4, 5) and for the medication groups (Supplementary eTables 6, 7) is provided in the adverse events section of the supplement.
Results
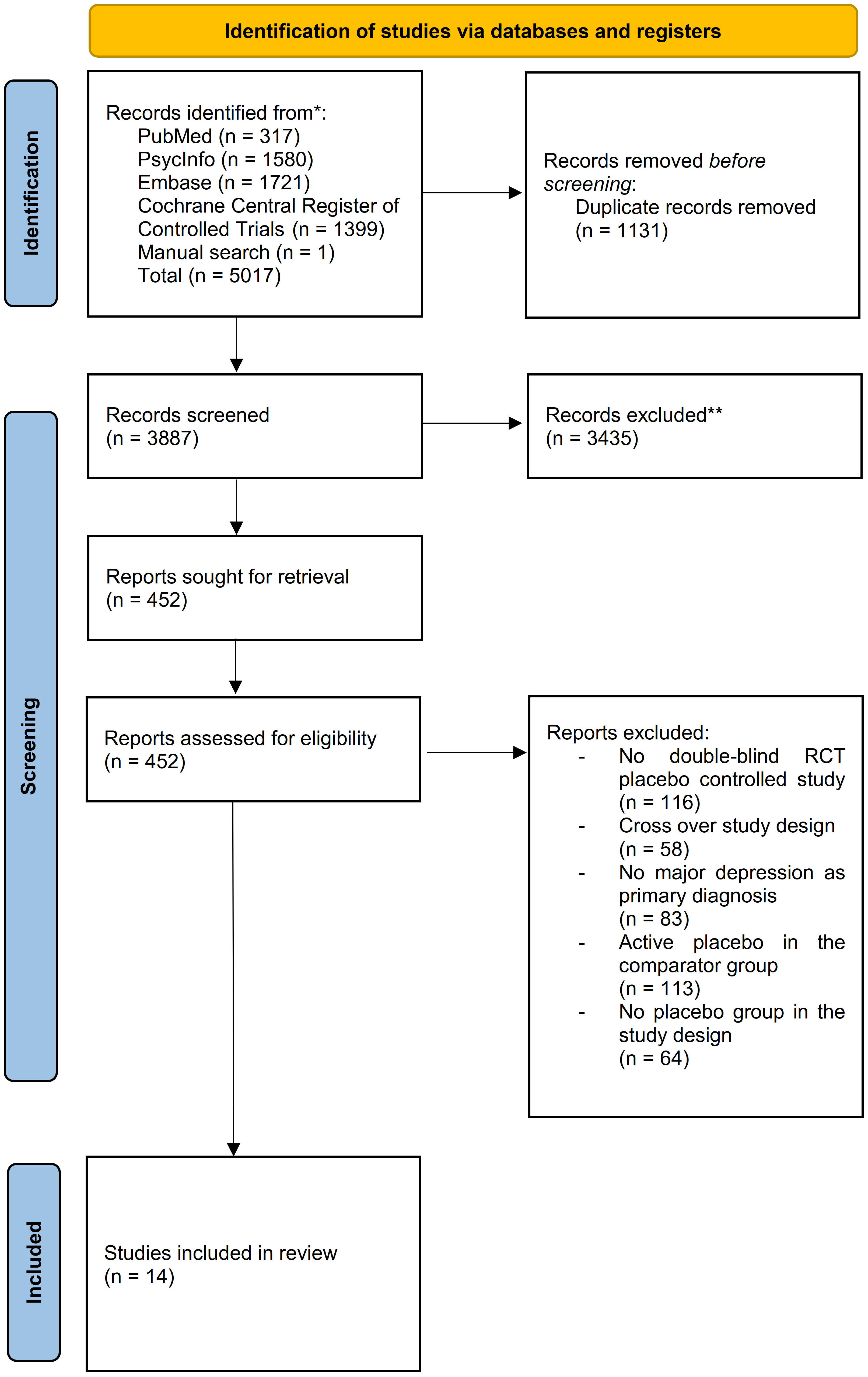
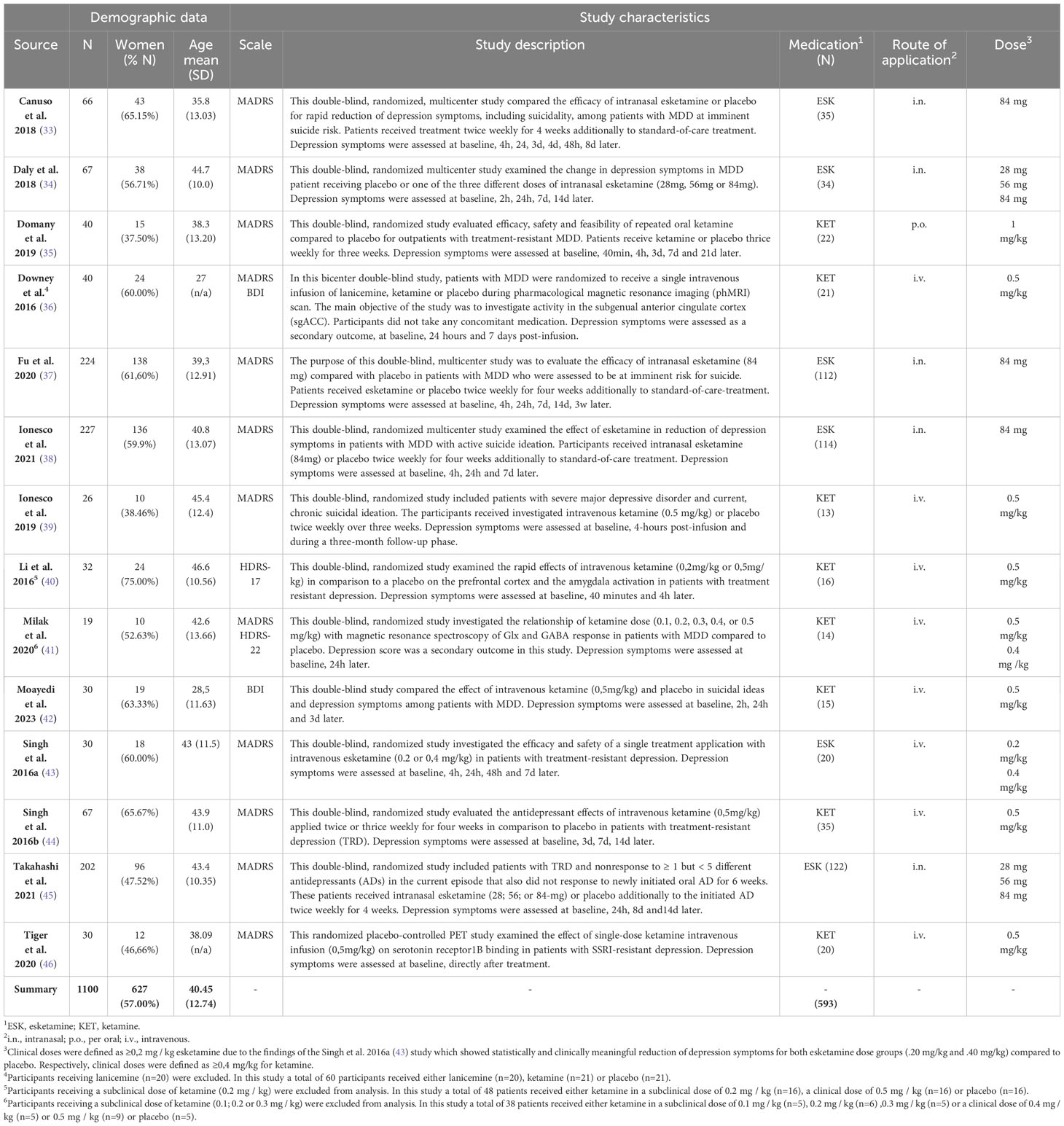
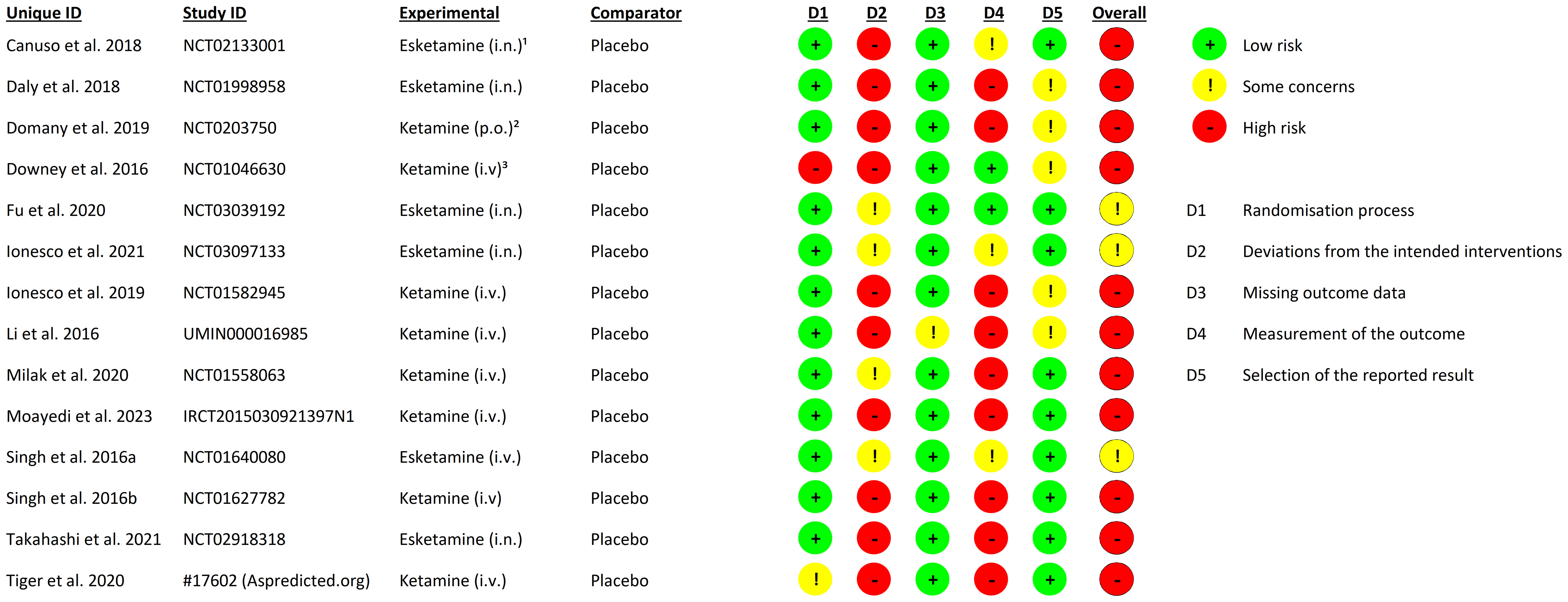
We screened 3887 abstracts that resulted from our search; assessed 452 reports for eligibility and included 14 studies (33–46) (N= 1100) meeting the inclusion criteria (Figure 1). The mean age of the participants was 40.09 years (SD = ± 12.75 years);57.00% were female; 593 were allocated in the medication group and 507 in the placebo group (Table 2). The risk of bias estimation showed 11 studies with a high risk of bias and 3 with some concerns about possible risk of bias (Figure 2).
The pooled effect size for the overall placebo response was: dpl = -1.85 (z = -3.42; p <.001; [CI 95%, -2.9 to -0.79]; I² = 86.22%) and for the overall treatment response: dtr = -2.57 (z =-6.36; p < 0.001; [CI 95% -3.36 to -1.78]; I² = 58.51%) (Figure 3). These results indicate that 71,43% of the treatment response is replicated in the placebo group. This analysis included the comparison of different post-intervention time points spanning from 4 hours to 72 hours post-intervention. No significant interaction between the pooled effect size and the different time points was found in both treatment and placebo groups.

Figure 3 Overall placebo and treatment response. (A) Forest plot overall placebo response dpl = -1.85; z = -3.42; p < 0.001; [CI 95%, -2.9 to -0.79]; I² = 86.22%. (1) ES = effect size (cohen’s d). (2) Singh et al., 2016b (44): pl1 = first placebo group pl2 = second placebo group. (B) Forest plot overall treatment response dtr = -2.57; z =-6.36; p < 0.001; [CI 95% -3.36 to -1.78]; I² = 58.51%. (1) ES = effect size (cohen’s d). (2) Takahashi et al. (45),: d1 = 28 mg esketamine; d2 = 56 mg esketamine; d3 = 84mg esketamine. (3) Singh et al., 2016b (44): f1 = 2 x week; f2 = 3 x week. (4) Singh et al., 2016a (43): d1 = 0.2 mg/kg esketamine; d2 = 0.4 mg/kg esketamine. (5) Milak et al. (41),: d1 = 0.4 mg/kg ketamine; d2 = 0.5 mg/kg ketamine. (6) Daly et al., 2018 (34): d1 = 28 mg esketamine; d2 = 56 mg esketamine; d3 = 84mg esketamine.
Subgroup analysis of 8 studies for the 7 days post-intervention time point resulted to the following pooled effect size for the placebo response: dpl 7d = -1.98 (z = -3.02; p = .003; [CI 95%, -3.26 to -0.69], I² = 86.07%) and the treatment response: dtr 7d = - 3.01 (z = -4.65; p <.001; [CI 95%, -4.28 to -1.74]; I² = 83,13%) was estimated. An overview is provided in the supplement (Supplementary eFigure 1). The placebo response accounts for 65,78% of the treatment effect. Subgroup analysis for the placebo response in studies with available data for the 40 minutes, 2 hours, 4 hours and 24 hours post-intervention time points respectively did not show any significant results. Subgroup analysis for studies with ketamine resulted to following placebo response dpl ket = -1.92 (z = -2.47; p = .0014; [CI 95% -3.44 to -0.37]; I² = 83,94%) and treatment response: dtr ket = -2.45 (z= -3.89; [CI 95% -3.69 to -1,21]; I² = 73,52%) (Supplementary eFigure 2). Subgroup analysis for studies with esketamine was also performed and resulted to following placebo response dpl esk = -1.72 (z = -1.72; p = .023; [CI 95% -3.2 to -0,24]; I² = 67,88%) and treatment response: dtr esk = -2.67 (z= -2,67; p <.001; [CI 95% - 3.70 to -1.649, I² = 33.80%) (Supplementary eFigure 3). According to this subgroup analysis the placebo response in studies with ketamine and in studies with esketamine accounts for 78% and 64% of the treatment response respectively.
Furthermore, we performed sensitivity analysis for the calculated pooled effect sizes by identifying outliers in the placebo groups and removing the respective studies from both groups before analysis. The estimated pooled effect size after sensitivity analysis for the overall placebo response was: d = -1.32 (z = -4.16; p <.001; [CI 95%, -1.94 to -0.70]; I² = 0%) and for the overall treatment response: d = -2.14 (z= -4.55; p <.001; [CI 95%, -3.07 to -1.22]; I² = 54.83%) (Supplementary eFigure 4). The overall placebo response accounts for 61.68% of the overall treatment response after sensitivity analysis. The estimated pooled effect size after sensitivity analysis for the placebo response 7 days post-intervention: d = -1.08 (z = -2.90; p = .004; [CI 95%, -1.80 to -0.35]; I² = 0%) and for the treatment response 7 days post-intervention: d = -2.48 (z = -3.08; p <.001; [CI 95% -4.06 to -0.90]; I² = 81.41%) (Supplementary eFigure 5). The placebo response accounts for 43.54% of the treatment response 7 days post-intervention.
Possible moderators of the placebo response such as sample size, age, sex, frequency of treatments, route of application, source of funding, country of study, year of publication, depressive symptoms at baseline, dosage and total number of the applications planned in the study, were investigated by performing meta-regression models. None of these above-mentioned variables were found to have a significant effect on the placebo response in the investigated studies. Adverse events were reported in 9 of the 14 included studies. A total of 1245 AEs (286 in the placebo and 959 in the medication group) were reported identified. A detailed overview of the reported AEs is provided in the supplement. We compared the rate of specific and psychomimetic AEs to the total AEs in the placebo and medication group. Medication specific AEs occur as expected significantly more often in the medication group t(14) = -2.67; p=.009 (Supplementary eFigure 6). Psychomimetic AEs are reported significantly more often in the medication group t(21) = -5.95; p <.001 (Figure 4).

Figure 4 Rate of psychometic AEs to total AEs. Raincloud plot visualizing the results of the unpaired T-Test for the rate of psychomimetic AEs to total AEs between the medication and placebo groups. The rate of psychomimetic AEs to total AEs is significantly higher than in the placebo group t(21)= -5.95; p <.001. (1) Psychomimetic adverse events (AEs) include distinct medication-specific side effects like dissociation and hallucinations. (2) Total AEs include the summary of all reported AEs. (3) Medication = ketamine and esketamine.
Discussion
This systematic review and meta-analysis included double-blind, randomized, placebo-controlled trials investigating the antidepressant effect of ketamine and esketamine in patients with MDD, with an overall placebo response of dpl = -1.98. Our findings suggest that the placebo response accounts for 72% of the overall treatment response (dtr = -2.57) reported in these studies.
Previous meta-analyses for placebo response in MDD only focused on oral antidepressants, our study however, is the first to address alternative routes of application and psychoactive antidepressants. Our findings are consistent with results from meta-analyses focused on oral antidepressants (1, 3). Moreover, our sensitivity analyses performed for the overall placebo and treatment response further highlight the robustness of our findings. Even though we opted for a conservative approach in our sensitivity analyses (avoiding overestimation of the placebo response by favoring the medication group in outlier removal) the placebo response it still accounts for 62% of the treatment response. The results of the placebo and the treatment response in studies with ketamine (Figure 2) and studies with esketamine (Figure 3) underline the results of our sensitivity analysis by showing that the placebo response accounts for 78% and 64% of the respective treatment response. These results also indicate a possible underestimation of the placebo response. The difference between ketamine and esketamine studies is possibly due to imbalance in residual heterogeneity in the esketamine studies treatment response (I²= 33%) and esketamine studies placebo response (I² = 68%) contrary to the balanced residual homogeneity in the placebo (84%) and treatment response (I² = 73%) of ketamine studies. We identified the small and differing sample sizes as a possible source of inhomogeneity.
In addition to sensitivity analysis, we also performed meta-regressions to investigate if the pooled effect sizes were influenced by differences in the study protocols (post-treatment time points, duration and number of treatments). Different intervention time points, frequency of treatments, route of application, dosage and total number of the applications planned did not interact significantly with the pooled effect size of the placebo and treatment response. In summary, our results indicate that 28-40% of the overall ketamine and esketamine treatment response can be attributed to factors other than the placebo response, like pharmacological effects. However, caution is advised in the interpretation of our findings in order to avoid an underestimation of psychopharmacological treatment effectivity since the drug–placebo differences in ketamine treatment for MDD are similar with the drug-placebo differences of general medicine drugs (47).
Seven days post-intervention the placebo response (dpl 7d = -1.98) accounts for 66% of the treatment response (dtr 7d = - 3.01), thus confirming the before mentioned results. After sensitivity analysis, the placebo response accounted for 43% of the treatment response. However, these results should be interpreted with caution due to the high imbalance in heterogeneity in the treatment (I² = 83%) and the placebo group (I² = 0%). A possible explanation for the high residual heterogeneity in the treatment group lies in the varying number of applied interventions per study spanning from 1 to 3 applications per week.
Our risk of bias calculation for the included studies indicates an overall high risk of bias in favor of the medication groups. These results suggest a possible underestimation of the placebo response. We identified insufficient blinding due to the distinct psychomimetic effects of ketamine and esketamine as the main source of possible assessor and participant bias. Some study designs reduced assessor bias by assigning different assessors for safety and efficacy rating. However, there is a high probability that the participants receiving medication were unblinded. This is also indicated by our analysis of the reported AEs; psychomimetic AEs show a significantly higher rate in the medication group t(21) = -5.95; p <.001 (Figure 4) than in the placebo group. Moreover, insufficient blinding has been addressed as a limitation in a number of double-blind trials investigating the effects of ketamine (48) and esketamine (49, 50) in patients with MDD.
The high placebo response in MDD treatment with NMDA receptor antagonists shown in our study is clinically relevant and should be considered in clinical practice to enhance patient treatment outcome. Additionally, from a clinical perspective it is important to take possible side-effects like psychomimetic AEs or other AEs like sedation or somnolence into consideration when discussing treatment options with patients. This is particularly relevant for patient groups, like elderly patients, whose tolerance for such symptoms could be compromised.
The growing interest of the scientific community regarding psychedelic research (51), the increasing concerns about data quality of the studies (52), as well as the recent first worldwide authorization of psychedelic substances for the treatment of mental diseases from the Australian Therapeutic Goods Administration (TGA) (53) show the high relevance of our findings and highlight the need for further optimization of study designs as also described in the previous literature (54). Controlling for expectation with a 2x2 factorial design which systematically crosses the intervention (psychoactive drug, placebo) with the standardized induced expectation (high or low expectation to receive medication or placebo) (55) would enhance the quality of the results. To date we have identified only one and still ongoing study investigating the effects of esketamine in patients suffering from MDD with the above-mentioned study design (56) and no study with ketamine. Furthermore, data quality would benefit from interventions designed to neutralize subject expectations (57). For instance, a double-blind controlled study by Cohen et al. (58) including patients with MDD and schizophrenia showed that providing study participants information about factors contributing to the placebo response before each measurement of the primary outcome can significantly reduce the placebo response. Moreover, other suggestions include routinely measuring de-blinding and expectancy (59); implementation of independent raters for efficacy and safety assessments (60) and use of active placebos (61).
Limitations of this study include the high heterogeneity of the included studies in some outcomes. Causes of heterogeneity are (1) the differences in study protocols of the included studies, (2) the synthesis of similar but yet differing interventions (ketamine and esketamine), (3) the overall high risk of bias of the included studies and (4) that studies allowed concomitant antidepressant medication. The first cause could be mitigated by a future larger meta-analysis, the second by performing separate meta-analyses for each intervention and the third by optimizing future study designs of double-blind RCTs investigating the treatment response of psychoactive substances. Lastly, the forth cause can be alleviated by including naturalistic study arms. We excluded studies that experimentally evaluated the combined intervention of a particular antidepressant with ketamine or esketamine but included studies allowing parallel standard of care. An advantage of this approach is that it provides a more representative patient population sample by facilitating the inclusion of patients with treatment resistant depression (TRD) while minimizing noise from the concomitant medication. Moreover, this approach prevented an inflation of placebo response because although patients with TRD are associated with high placebo response rates (62) these are still lower in comparison to patients with non-TRD (63). Despite the reported study limitations, the robustness of our findings is supported by similar findings in the literature and by the results of the sensitivity and subgroup analysis.
This meta-analysis concludes that the placebo response accounts for 62-71% of the treatment response in the included double-blind RCTs examining the antidepressant effects of ketamine and esketamine in patients with MDD. Furthermore, insufficient blinding in the included studies pose an important source of bias. Optimization of future study designs in trials with psychoactive substances is urgently needed. The placebo response plays a significant role in the treatment of depression and should be used for the benefit of the patients in clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
AM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. MW: Conceptualization, Methodology, Writing – review & editing. LN: Data curation, Formal analysis, Investigation, Writing – review & editing. CY: Writing – review & editing. WR: Writing – review & editing. SH: Writing – review & editing. IF: Funding acquisition, Writing – review & editing. TK: Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - Project-ID 422744262 - TRR 289. Projects A08 and A16. Gefördert durch die Deutsche Forschungsgemeinschaft (DFG) – Projektnummer 422744262 – TRR 289. Teilprojekte A08 und A16.
Acknowledgments
We gratefully acknowledge Manolis Fragkiadakis (University of Leiden, Netherlands) for contributing in the development of the python code used to calculate the effect sizes and Laura Nerreter and Catarina Draguhn for contributing in the data extraction for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1346697/full#supplementary-material
References
1. Khan A, Warner Ha, Brown Wa. Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials: an analysis of the food and drug administration database. Arch Gen Psychiatry. (2000) 57:311–7. doi: 10.1001/Archpsyc.57.4.311
2. Kirsch I, Sapirstein G. Listening to prozac but hearing placebo: A meta-analysis of antidepressant medication. Prev Treat. (1998) 1. doi: 10.1037//1522-3736.1.0002a
3. Kirsch I, Moore Tj, Scoboria A, Nicholls Ss. The emperor’s new drugs: an analysis of antidepressant medication data submitted to the U.S. Food and drug administration. Prev Treat. (2002) 5. doi: 10.1037//1522-3736.5.0023a
4. Kirsch I, Deacon Bj, Huedo-Medina Tb, Scoboria A, Moore Tj, Johnson Bt. Initial severity and antidepressant benefits: A meta-analysis of data submitted to the food and drug administration. PloS Med. (2008) 5:E45. doi: 10.1371/Journal.Pmed.0050045
5. Stone Mb, Yaseen Zs, Miller Bj, Richardville K, Kalaria Sn, Kirsch I. Response to acute monotherapy for major depressive disorder in randomized, placebo controlled trials submitted to the us food and drug administration: individual participant data analysis. Bmj. (2022) 378:E067606. doi: 10.1136/Bmj-2021-067606
6. Fava M, Evins Ae, Dorer Dj, Schoenfeld Da. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and A novel study design approach. Psychother Psychosom. (2003) 72:115–27. doi: 10.1159/000069738
7. Mcgirr A, Berlim Mt, Bond Dj, Fleck Mp, Yatham Ln, Lam Rw. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. (2015) 45:693–704. doi: 10.1017/S0033291714001603
8. Alnefeesi Y, Chen-Li D, Krane E, Jawad My, Rodrigues Nb, Ceban F, et al. Real-world effectiveness of ketamine in treatment-resistant depression: A systematic review & Meta-analysis. J Psychiatr Res. (2022) 151:693–709. doi: 10.1016/J.Jpsychires.2022.04.037
9. Price Rb, Kissel N, Baumeister A, Rohac R, Woody Ml, Ballard Ed, et al. International pooled patient-level meta-analysis of ketamine infusion for depression: in search of clinical moderators. Mol Psychiatry. (2022) 27:5096–112. doi: 10.1038/S41380-022-01757-7
10. U.S. Food And Drug Administration. Fda approves new nasal spray medication for treatment-resistant depression; available only at A certified doctor’s office or clinic(2020). Available online at: https://www.Fda.Gov/News-Events/Press-Announcements/Fda-Approves-New-Nasal-Spray-Medication-Treatment-Resistant-Depression-Available-Only-Certified.
11. European Medicines Agency. Spravato(2023). Available online at: https://www.Ema.Europa.Eu/En/Medicines/Human/Epar/Spravato#Authorisation-Details-Section.
12. Freedman R, Brown As, Cannon Td, Druss Bg, Earls Fj, Escobar J, et al. Can A framework be established for the safe use of ketamine? Am J Psychiatry. (2018) 175:587–9. doi: 10.1176/Appi.Ajp.2018.18030290
13. Schatzberg Af. A word to the wise about intranasal esketamine. Am J Psychiatry. (2019) 176:422–4. doi: 10.1176/Appi.Ajp.2019.19040423
14. Turner Eh. Esketamine for treatment-resistant depression: seven concerns about efficacy and fda approval. Lancet Psychiatry. (2019) 6:977–9. doi: 10.1016/S2215-0366(19)30394-3
15. World Health Organization. Depression And Other Common Mental Disorders: Global Health Estimates. World Health Organization (2017). p. 24. Technical Documents.
16. Vos T, Allen C, Arora M, Barber Rm, Bhutta Za, Brown A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388:1545–602. doi: 10.1016/S0140-6736(16)31678-6
18. Gaynes Bn, Rush Aj, Trivedi Mh, Wisniewski, Spencer D, Fava M. The star*D study: treating depression in the real world. Cleve Clin J Med. (2008) 75:57–66. doi: 10.3949/Ccjm.75.1.57
19. Page Mj, Mckenzie Je, Bossuyt Pm, Boutron I, Hoffmann Tc, Mulrow Cd, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:N71. doi: 10.1136/Bmj.N71
20. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-A web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/S13643-016-0384-4
21. Microsoft Corporation. Microsoft excel(2023). Available online at: https://www.microsoft.com/excel.
22. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Mj P, et al eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023) (2023). Cochrane, 2023. Available online at: www.training.cochrane.org/handbook.
23. Rohatgi A. WebPlotDigitizer (Version 4.6) [Computer software] (2022). Available online at: https://automeris.io/WebPlotDigitizer
24. Sterne Ja, Savović J, Page Mj, Elbers Rg, Blencowe Ns, Boutron I, et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. Bmj. (2019) 366:L4898. doi: 10.1136/Bmj.L4898
25. Jasp Team. Jasp (Version 0.17.3)[Computer software](2023). Available online at: https://Jasp-Stats.Org/.
26. Furukawa Ta, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. (2006) 59:7–10. doi: 10.1016/J.Jclinepi.2005.06.006
27. Mckinney W. (2010). Data structures for statistical computing in python, in: Proceedings Of The 9th Python In Science Conference, . pp. 56–61. doi: 10.25080/Majora-92bf1922-00a
28. Higgins Jp, Thompson Sg, Deeks Jj. Altman dg. Measuring inconsistency in meta-analyses. Bmj. (2003) 327:557–60. doi: 10.1136/Bmj.327.7414.557
29. Langan D, Higgins Jp, Jackson D, Bowden J, Veroniki Aa, Kontopantelis E, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. (2019) 10:83–98. doi: 10.1002/Jrsm.1316
30. Mcintyre Rs, Carvalho Ip, Lui Lm, Majeed A, Masand Ps, Gill H, et al. The effect of intravenous, intranasal, and oral ketamine in mood disorders: A meta-analysis. J Affect Disord. (2020) 276:576–84. doi: 10.1016/J.Jad.2020.06.050
31. Pfizer Limited. Ketalar® 10 mg/ml, 50 mg/ml and 100 mg/ml injection. Ketamine Hydrochloride. (2016). Available online at: https://labeling.pfizer.com/ShowLabeling.aspx?id=13971.
32. Janssen Pharmaceuticals, Inc. Spravato (Esketamine) nasal spray. Highlights of prescribing information (2023). Available online at: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/SPRAVATO-pi.pdf.
33. Canuso Cm, Singh Jb, Fedgchin M, Alphs L, Lane R, Lim P, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of A double-blind, randomized, placebo-controlled study. Am J Psychiatry. (2018) 175:620–30. doi: 10.1176/Appi.Ajp.2018.17060720
34. Daly Ej, Singh Jb, Fedgchin M, Cooper K, Lim P, Shelton Rc, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry. (2018) 75:139–48. doi: 10.1001/Jamapsychiatry.2017.3739
35. Domany Y, Bleich-Cohen M, Tarrasch R, Meidan R, Litvak-Lazar O, Stoppleman N, et al. Repeated oral ketamine for out-patient treatment of resistant depression: randomised, double-blind, placebo-controlled, proof-of-concept study. Br J Psychiatry. (2019) 214:20–6. doi: 10.1192/Bjp.2018.196
36. Downey D, Dutta A, Mckie S, Dawson Gr, Dourish Ct, Craig K, et al. Comparing the actions of lanicemine and ketamine in depression: key role of the anterior cingulate. Eur Neuropsychopharmacol. (2016) 26:994–1003. doi: 10.1016/J.Euroneuro.2016.03.006
37. Fu D-J, Ionescu Df, Li X, Lane R, Lim P, Sanacora G, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (Aspire I). J Clin Psychiatry. (2020) 81. doi: 10.4088/Jcp.19m13191
38. Ionescu Df, Fu D-J, Qiu X, Lane R, Lim P, Kasper S, et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of A phase 3, double-blind, randomized study (Aspire ii). Int J Neuropsychopharmacol. (2021) 24:22–31. doi: 10.1093/Ijnp/Pyaa068
39. Ionescu Df, Bentley Kh, Eikermann M, Taylor N, Akeju O, Swee Mb, et al. Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: A randomized, double blind, placebo controlled trial. J Affect Disord. (2019) 243:516–24. doi: 10.1016/J.Jad.2018.09.037
40. Li C-T, Chen M-H, Lin W-C, Hong C-J, Yang B-H, Liu R-S, et al. The effects of low-dose ketamine on the prefrontal cortex and amygdala in treatment-resistant depression: A randomized controlled study. Hum Brain Mapp. (2016) 37:1080–90. doi: 10.1002/Hbm.23085
41. Milak Ms, Rashid R, Dong Z, Kegeles Ls, Grunebaum Mf, Ogden Rt, et al. Assessment of relationship of ketamine dose with magnetic resonance spectroscopy of glx and gaba responses in adults with major depression: A randomized clinical trial. JAMA Netw Open. (2020) 3:E2013211. doi: 10.1001/Jamanetworkopen.2020.13211
42. Moayedi F, Massoudifar A, Namazi S, Mirzaei Zadeh H, Seddigh Sh, Hosseini Teshnizi S. The effect of intravenous ketamine on suicidal ideation in depressed patients: A randomized clinical trial. Dis Diagn. (2023) 12:106–11. doi: 10.34172/Ddj.2023.437
43. Singh Jb, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, et al. Intravenous esketamine in adult treatment-resistant depression: A double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. (2016) 80:424–31. doi: 10.1016/J.Biopsych.2015.10.018
44. Singh Jb, Fedgchin M, Daly Ej, Boer P, Cooper K, Lim P, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. (2016) 173:816–26. doi: 10.1176/Appi.Ajp.2016.16010037
45. Takahashi N, Yamada A, Shiraishi A, Shimizu H, Goto R, Tominaga Y. Efficacy and safety of fixed doses of intranasal esketamine as an add-on therapy to oral antidepressants in Japanese patients with treatment-resistant depression: A phase 2b randomized clinical study. BMC Psychiatry. (2021) 21:526. doi: 10.1186/s12888-021-03538-y
46. Tiger M, Veldman Er, Ekman C-J, Halldin C, Svenningsson P, Lundberg J. A randomized placebo-controlled pet study of ketamine´S effect on serotonin1b receptor binding in patients with ssri-resistant depression. Transl Psychiatry. (2020) 10:159. doi: 10.1038/S41398-020-0844-4
47. Leucht S, Hierl S, Kissling W, Dold M, Davis Jm. Putting the efficacy of psychiatric and general medicine medication into perspective: review of meta-analyses. Br J Psychiatry. (2012) 200:97–106. doi: 10.1192/Bjp.Bp.111.096594
48. Fava M, Freeman Mp, Flynn M, Judge H, Hoeppner Bb, Cusin C, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (Trd). Mol Psychiatry. (2020) 25:1592–603. doi: 10.1038/S41380-018-0256-5
49. Popova V, Daly Ej, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with A newly initiated oral antidepressant in treatment-resistant depression: A randomized double-blind active-controlled study. Am J Psychiatry. (2019) 176:428–38. doi: 10.1176/Appi.Ajp.2019.19020172
50. Daly Ej, Trivedi Mh, Janik A, Li H, Zhang Y, Li X, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry. (2019) 76:893–903. doi: 10.1001/Jamapsychiatry.2019.1189
51. Kurtz Js, Patel Na, Gendreau Jl, Yang C, Brown N, Bui N, et al. The use of psychedelics in the treatment of medical conditions: an analysis of currently registered psychedelics studies in the American drug trial registry. Cureus. (2022) 14:E29167. doi: 10.7759/Cureus.29167
52. Hall Wd, Humphreys K. Is good science leading the way in the therapeutic use of psychedelic drugs? Psychol Med. (2022) 52:2849–51. doi: 10.1017/S0033291722003191
53. Therapeutic Goods Administration. Change to classification of psilocybin and mdma to enable prescribing by authorised psychiatrists(2023). Available online at: https://Www.Tga.Gov.Au/News/Media-Releases/Change-Classification-Psilocybin-And-Mdma-Enable-Prescribing-Authorised-Psychiatrists.
54. Aday Js, Heifets Bd, Pratscher Sd, Bradley E, Rosen R, Woolley Jd. Great expectations: recommendations for improving the methodological rigor of psychedelic clinical trials. Psychopharmacol (Berl). (2022) 239:1989–2010. doi: 10.1007/S00213-022-06123-7
55. Rohsenow Dj, Marlatt Ga. The balanced placebo design: methodological considerations. Addict Behav. (1981) 6:107–22. doi: 10.1016/0306-4603(81)90003-4
56. Falkenberg I, Bitsch F, Liu W, Matsingos A, Noor L, Vogelbacher C, et al. The effects of esketamine and treatment expectation in acute major depressive disorder (Expect): study protocol for A pharmacological fmri study using A balanced placebo design. Trials. (2023) 24:514. doi: 10.1186/s13063-023-07556-x
57. Evans K, Colloca L, Pecina M, Katz N. What can be done to control the placebo response in clinical trials? A narrative review. Contemp Clin Trials. (2021) 107:106503. doi: 10.1016/J.Cct.2021.106503
58. Cohen Ea, Hassman Hh, Ereshefsky L, Walling Dp, Grindell Vm, Keefe Rs, et al. Placebo response mitigation with A participant-focused psychoeducational procedure: A randomized, single-blind, all placebo study in major depressive and psychotic disorders. Neuropsychopharmacology. (2021) 46:844–50. doi: 10.1038/S41386-020-00911-5
59. Muthukumaraswamy Sd, Forsyth A, Lumley T. Blinding and expectancy confounds in psychedelic randomized controlled trials. Expert Rev Clin Pharmacol. (2021) 14:1133–52. doi: 10.1080/17512433.2021.1933434
60. Fava M. How should we design future mechanistic and/or efficacy clinical trials? Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. (2024) 49(1), 197–204. doi: 10.1038/S41386-023-01600-9
61. Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discovery. (2013) 12:191–204. doi: 10.1038/Nrd3923
62. Jones Bd, Razza Lb, Weissman Cr, Karbi J, Vine T, Mulsant Ls, et al. Magnitude of the placebo response across treatment modalities used for treatment-resistant depression in adults: A systematic review and meta-analysis. JAMA Netw Open. (2021) 4:E2125531. doi: 10.1001/Jamanetworkopen.2021.25531
Keywords: ketamine, esketamine, placebo, placebo response, psychoactive medication, depression (MDD), treatment expectation, NMDA-receptor antagonist
Citation: Matsingos A, Wilhelm M, Noor L, Yildiz C, Rief W, Hofmann SG, Falkenberg I and Kircher T (2024) Hype or hope? High placebo response in major depression treatment with ketamine and esketamine: a systematic review and meta-analysis. Front. Psychiatry 15:1346697. doi: 10.3389/fpsyt.2024.1346697
Received: 29 November 2023; Accepted: 13 February 2024;
Published: 08 March 2024.
Edited by:
Sherry-Anne Muscat, Alberta Hospital Edmonton, CanadaReviewed by:
Andy R. Eugene, Larned State Hospital, United StatesJian-Jun Yang, First Affiliated Hospital of Zhengzhou University, China
Copyright © 2024 Matsingos, Wilhelm, Noor, Yildiz, Rief, Hofmann, Falkenberg and Kircher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandros Matsingos, alexandros.matsingos@uni-marburg.de
 Alexandros Matsingos
Alexandros Matsingos Marcel Wilhelm2
Marcel Wilhelm2 Cüneyt Yildiz
Cüneyt Yildiz Winfried Rief
Winfried Rief Irina Falkenberg
Irina Falkenberg Tilo Kircher
Tilo Kircher