- 1Department of Geriatric Medicine, Marien Hospital Herne, Ruhr University Bochum, Herne, Germany
- 2Department of Neurology, University Hospital Düsseldorf, Düsseldorf, Germany
- 3Department of Neurology, University Hospital Münster, Münster, Germany
- 4Department of Neurology and Neurorehabilitation, Klinikum Osnabrück, Osnabrück, Germany
Objective: Diagnosing and treating dysphagia in patients with dementia is challenging and few studies have been performed to characterize dysphagia based on Flexible Endoscopic Evaluation of Swallowing (FEES). Therefore, we aimed to characterize and compare the dysphagia pathologies in various stages and types of dementia.
Methods: This is a retrospective study of 107 hospitalized geriatric patients with dysphagia and Alzheimer’s dementia, Alzheimer’s dementia with moderate to severe cerebral vasculopathy (mixed dementia), and patients with dementia associated with Parkinson’s syndrome who underwent FEES. A standardized FEES protocol was used to characterize the dysphagia pathologies, including premature bolus spillage, delayed swallowing reflex and bolus residue as well as penetration and aspiration and the white-out intensity. The distribution of different dysphagia pathologies was cross-tabulated with χ2 statistics across different types of dementia.
Results: A comparative analysis of dysphagia pathologies across the three dementia types revealed a relatively mixed picture of various dysphagia findings in all dementia types. However, a significantly higher prevalence of bolus penetration and complex dysphagia, which was defined as presence of at least two major findings simultaneously within a patient, was seen in patients with Parkinson’s-related dementia compared to other forms of dementia. In general, residue was the most frequent finding in all types of dementia (78%–100%). In contrast, aspiration was the least prevalent finding with no significant variation between dementia types.
Conclusion: Although participants with Parkinson’s-related dementia exhibited minor specific findings, our study revealed no distinct endoscopic dysphagia pathologies across various types of dementia.
Introduction
Oropharyngeal dysphagia (OD) is recognized as a critical geriatric syndrome that significantly diminishes the quality of life in older patients and is associated with several adverse outcomes, including hospitalizations, pneumonia, malnutrition, dehydration and increased mortality (Baijens et al., 2016; Banda et al., 2022).
The etiology of OD is complex and depends primarily on the presence of neurological diseases and age-related changes in multiple swallowing domains including sensory function and disease related changes of anatomical structures (Rajati et al., 2022; Abu-Ghanem et al., 2020). One of the neurological diseases that is frequently associated with dysphagia is dementia. Contrary to extensive research that has been conducted on dysphagia in stroke patients, where dysphagia may diminish over days to weeks, less is known about the dynamic in neurodegenerative diseases that typically show a progressive decline (Parlak et al., 2022; Sheikhany et al., 2019). As the condition advances in patients with dementia, there is often a notable decrease in pharyngeal clearance and opening of the upper esophageal sphincter, accompanied by frequent occurrences of penetration and/or aspiration (De Stefano et al., 2020; Mira et al., 2022). These changes exacerbate the risk of severe health consequences, underscoring the need for precise diagnostic and management strategies.
Flexible endoscopic examination of swallowing (FEES) is a well-established, reliable, bedside diagnostic tool for assessing swallowing disorders in geriatric patients (Dziewas et al., 2024). Recent advancements include FEES-based classification of dysphagia phenotypes (Warnecke et al., 2021). In recent studies, the use of FEES mainly focussed on geriatric patients with cognitive impairment resulting from cerebrovascular accidents and pneumonia (Benjapornlert et al., 2020), presbyphagia (Labeit et al., 2022) or rare neurodegenerative diseases such as amyotrophic lateral sclerosis (Tye et al., 2021). In particular, characteristic dysphagia patterns have been associated with Parkinson’s disease, stroke and neuromuscular diseases (Warnecke et al., 2021). Nevertheless, the specific swallowing pathologies associated with Alzheimer’s disease and other forms of dementia remain to be established, and the impact of dementia on dysphagia is still poorly understood. Accordingly, the objective of the present study was to provide a systematic analysis of the endoscopic pathologies associated with different types of dementia and concomitant dysphagia.
Methods
Subjects and methods
This retrospective analysis was conducted in a cohort of inpatients with dementia who underwent FEES between January 2019 and January 2024 on a geriatric ward. The most common reasons for performing FEES were weight loss of more than 3 kg within 3 months and/or a total score of ≤7 on the Mini Nutritional Assessment Short-Form (MNA-SF) (n = 47), indicating relevant malnutrition. Other reasons included abnormalities detected during speech therapist examinations (e.g., wet voice after swallowing water, dysarthria, and reduced cough force; n = 32), symptoms reported by caregivers (e.g., reduced appetite; n = 18), a history of dysphagia (n = 17), and self-reported swallowing difficulties (n = 13). In some cases, aspiration pneumonia was documented in the medical history (n = 11), and buccofacial apraxia was present (n = 5). Ten patients reported no prior symptoms. In patients with severe cognitive impairment, FEES was performed with the support of trained geriatric nurses and, when appropriate, in the presence of a familiar caregiver. The examination was carried out in a quiet setting with simplified instructions, and was immediately discontinued if distress occurred.
The findings were evaluated by two certified examiners with significant experience in the field of neurogenic dysphagia. The videos and findings were retrospectively reviewed by the first author and the findings were reevaluated. In case of deviations from the previous findings, a certified FEES specialist was consulted. The interrater reliability showed a strong agreement (kappa = 0.84). Patients were included if they were >65 years with either a pre-existing or newly diagnosed dementia and if a FEES-video for analysis was available. In the case of Parkinson’s-related dementia, Parkinson’s disease was diagnosed according to the Movement-Disorder-Society Criteria and graded according to the modified Hoehn and Yahr scale (Postuma et al., 2015; Goetz et al., 2004). The diagnosis of Alzheimer’s disease was made in accordance with the established diagnostic criteria outlined in the DSM-5 (Simpson, 2014). The diagnosis was based on a comprehensive clinical assessment, neuropsychological testing, and structural cerebral imaging (MRI or CT). When available, biomarker data (e.g., cerebrospinal fluid analysis) were incorporated. However, due to the retrospective and clinical nature of the study, the availability of biomarker testing was not uniform. Consequently, patients were also included if the diagnosis of Alzheimer’s disease was made based on the clinical syndrome, disease course, and supporting imaging findings. Clinical presentation and neurocognitive testing were used to classify the severity of dementia (Nasreddine et al., 2005; Williams et al., 2013). The diagnosis of “mixed dementia” was assigned to patients with Alzheimer’s dementia who exhibited cerebral microangiopathy with a Fazekas score of ≥2, as determined by MRI findings. In cases where MRI findings were not available, the diagnosis was based on CT (Scheltens et al., 1998). Patients with history of stroke other than lacunary infarction were excluded. Additional exclusion criteria included an incomplete data set and the presence of delirium as a potential cause of cognitive impairment. The study protocol was approved by the Ethics Committee of the Ruhr University Bochum (Reg.-Nr. 23–7,878). The study is registered at German Clinical trial register (DRKS-ID: DRKS00032433).
Geriatric assessment
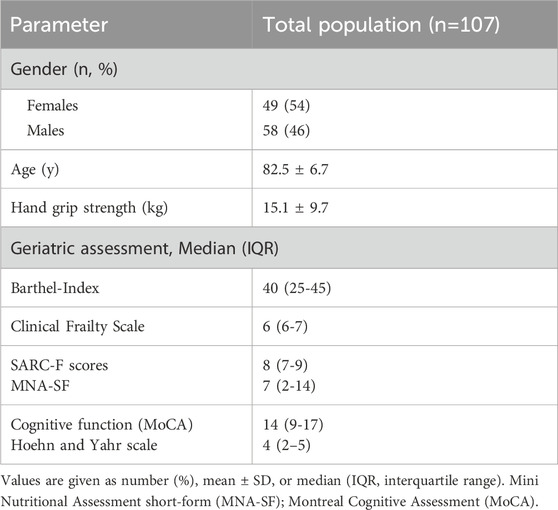
All study participants underwent a comprehensive geriatric assessment. Cognitive capabilities were evaluated using the Montreal Cognitive Assessment (MoCA) within the first 48 h of admission (Nasreddine et al., 2005). For patients with significant hearing impairment, the auditory subtests of the MoCA (digit span, sentence repetition and, if applicable, cued recall) were excluded. To ensure comparability with the standard 30-point scale, the maximum possible score was proportionally adjusted and the total score extrapolated. Similarly, patients with functional blindness received the MoCA-Blind version, which omits vision-dependent tasks such as trail-making, cube copying, clock drawing, and naming. This version has a maximum score of 22 points. Scores obtained were interpreted using established normative data for the MoCA-blind (Rossetti et al., 2011). Nutritional status was assessed using the MNA-SF (Kaiser et al., 2009), categorizing individuals into normal nutritional status (12–14 points), at risk of malnutrition (8–11 points), and malnourished (0–7 points). Performance of activities of daily living was assessed using the German version of the Barthel Index (Fi, 1965), where a scale from 0 to 100 reflects varying degrees of independence with higher scores denoting greater independence. Frailty was assessed with the Clinical Frailty Scale, which combines descriptive texts and pictograms to illustrate a patient’s functional and activity level, rated from 1 (very fit) to 9 (terminally ill) (Sternberg et al., 2011). Sarcopenia was assessed using the SARC-F questionnaire, with scores greater than 4 indicating probable sarcopenia (Drey et al., 2020). Handgrip strength was measured three times on the dominant or unaffected side, with the highest value recorded (Cruz-Jentoft et al., 2019).
Assessment of dysphagia
FEES and the subsequent evaluation of the video were performed collaboratively by a speech-and-language therapist and a physician, both of whom with extensive experience in the field of neurogenic dysphagia. The assessments utilized an ENF-VH2 laryngoscope provide by Olympus (Hamburg, Germany) and a video documentation system from Rheder/Partner (Hamburg, Germany) following a standardized protocol. In addition to assessing anatomical structures and evaluation of saliva management, different food consistencies were tested to examine swallowing function comprehensively in accordance with a well-established protocol (Dziewas et al., 2024) in the following order: Green coloured jelly (IDDSI-level 4), nectar-like thickened (IDDSI-level 2) and non-thickened green coloured water (IDDSI-level 0), and three trials of white solid bread measuring approximately 3 cm × 3 cm × 0.5 cm (Cichero et al., 2017). For each food consistency the occurrence of premature spillage, residue, penetration, aspiration was documented. The white-out, a crucial observational landmark where the pharyngeal wall undergoes maximal contraction resulting in white superimposition from light reflection of the endoscope, was specifically categorized as “complete” or “incomplete” at the first swallow of every bolus consistency based on a visual scale (Labeit et al., 2021a). Since the Penetration Aspiration Scale (PAS) (Rosenbek et al., 1996) very much focusses in airway safety and does not evaluate premature bolus spillage and residue, dysphagia severity was additionally categorized according to a FEES dysphagia severity score with four different levels of severity (Warnecke et al., 2016): Grade 0 denoted no clinically relevant neurogenic dysphagia, grade 1 represented the mildest form of neurogenic dysphagia (premature spillage and/or significant residue, but no penetration or aspiration), grade 2 indicated moderate neurogenic dysphagia with penetration or aspiration of one food consistency, and grade 3 indicated severe neurogenic dysphagia with penetration or aspiration of two or more food consistencies.
We used the following parameters for the characterization of swallowing disorders:
• Premature bolus spillage: As in a previous study classifying different swallowing patterns in neurological disorders, premature spillage before triggering the swallowing reflex was defined as occurring at the level of the piriform sinus (Warnecke et al., 2021).
• Delayed swallowing reflex: As in previous studies, delayed swallowing was defined as a delay of at least 3 seconds after the bolus reached the vallecula without triggering a swallowing reflex (Warnecke et al., 2021; Labeit et al., 2021b).
• Residue in the valleculae and residue in the piriform sinus: All remaining except for coating after the first swallow was evaluated according to the Yale-Scale (Neubauer et al., 2015). Bolus residues were evaluated for each swallow and tested consistency. For the analysis, the worst residue finding across the three swallows for each consistency was used. The location of the residue (valleculae versus piriform sinus) was documented separately.
• Penetration and Aspiration were rated according to the PAS (Rosenbek et al., 1996).
• White-out was rated as “incomplete”, when the frame with the maximum white superimposition was not completely white (Labeit et al., 2021a).
• Complex swallowing disorder was rated if at least two of the abovementioned mechanisms occurred simultaneously within one patient. For better illustration, the localization of the residue depending on their localization in the vallecular space or in the piriform sinus is shown in Table 3. When defining a complex dysphagia pathology, the localization of the residues was not considered separately.
Statistical analysis
Statistical analyses were conducted using SPSS software (SPSS Statistics for Windows, Version 29.0, IBM Corp., Armonk, NY, USA). Continuous variables were reported as means ± standard deviations (SDs) for normally distributed data and medians with interquartile ranges (IQRs) for non-normally distributed data. Categorical variables were presented as counts and percentages. The distribution of dysphagia pathologies across different types of dementia was assessed using cross-tabulation, comparing observed counts against expected counts derived from χ2 statistics. For each type of dementia where n ≥ 5, a χ2 test of independence was utilized to examine the relationship between each dysphagia pathology and the type of dementia. When expected counts were less than 5, Fisher’s exact test was applied instead. The prevalence of dysphagia pathologies across different dementia types was also visualized in a bar chart. A regression analysis was performed to explore the independent effects of various risk factors—including age, gender, dementia severity, sarcopenia, handgrip strength, Barthel Index, and frailty—on the severity of dysphagia. Additionally, partial correlation analyses were conducted to assess the associations between dysphagia pathologies. A p-value of <0.05 was considered statistically significant.
Results
Baseline characteristics of study participants are presented in Table 1. Of the 313 geriatric inpatients with dementia who were screened between 2019 and 2024, 107 patients with endoscopically confirmed oropharyngeal dysphagia were included in the final analysis. Reasons for exclusion included missing or incomplete FEES (n = 87), predefined exclusion criteria such as delirium or infection (n = 60), and an absence of pathological FEES findings (n = 59). A detailed flowchart of patient selection is provided in Supplementary Figure S1. The age of the study population ranged between 65 and 96 years (82.5 ± 6.7 years), with 54% being females. Major reasons for hospital admission included falls and fractures, immobility after surgery cardiovascular diseases, malnutrition, heart failure and neurodegenerative diseases. The Barthel index recorded a median score of 40, indicating a moderate to severe dependency in activities of daily living among the participants. Furthermore, 80 patients (74.8%) were identified as having probable sarcopenia according to the Sarc-F score and frailty was observed in 97 patients (91.5%) based on Clinical Frailty Scale.
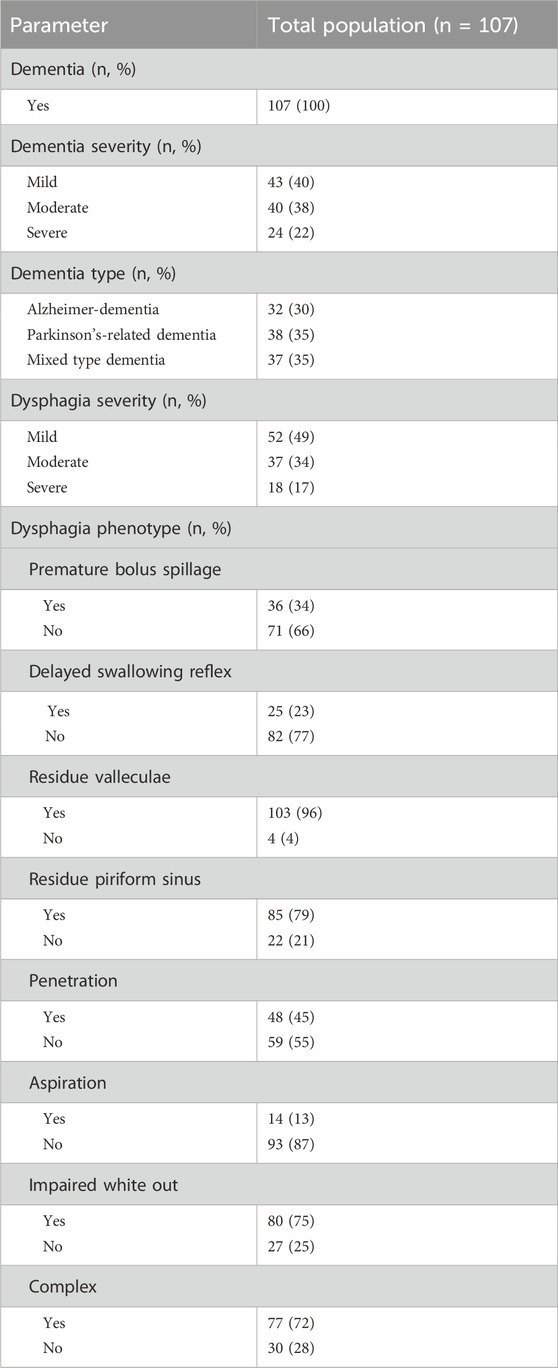
Table 2 summarizes the prevalence and severity of different dementia types and dysphagia pathologies as assessed by FEES. The cohort comprised 107 patients, with one-third of each dementia type including Alzheimer’s disease, mixed-type dementia, i.e., Alzheimer’s disease with vascular lesions and Parkinson’s-related, i.e., Parkinson’s disease with dementia and or possible diagnosis of Lewy body dementia (McKeith et al., 2017). Notable dysphagia pathologies included a high prevalence of residue in valleculae (96%), impaired white out (75%), and a complex dysphagia pathology (72%), with aspiration being the least common condition observed in 13%.
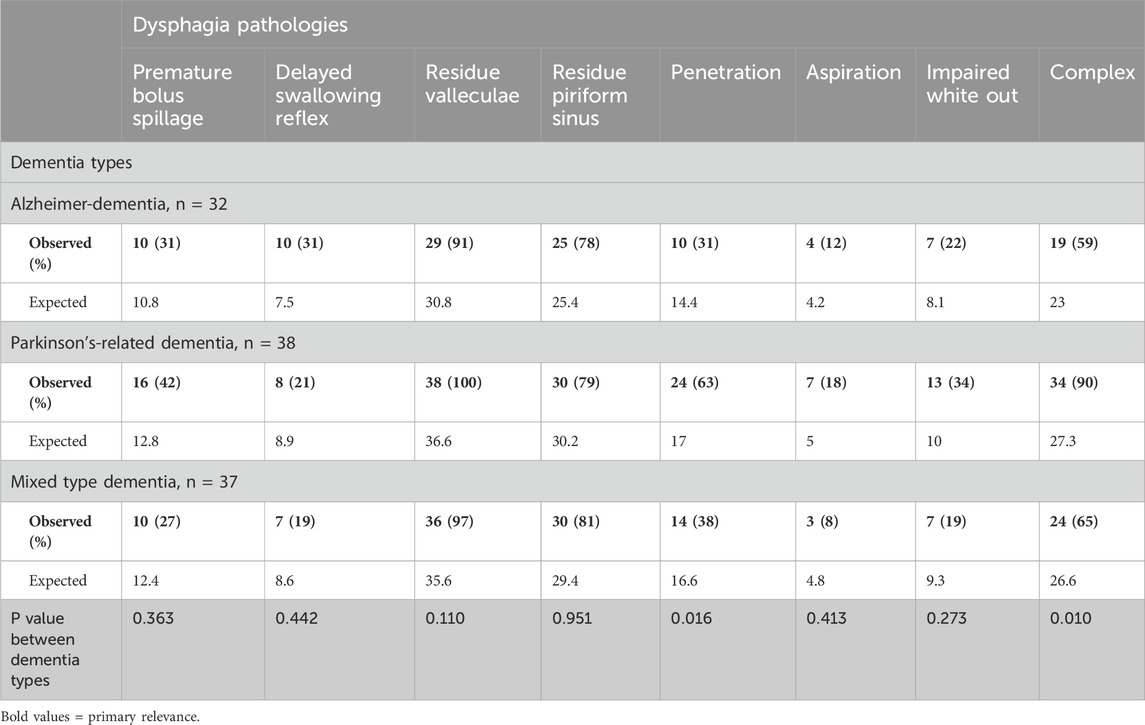
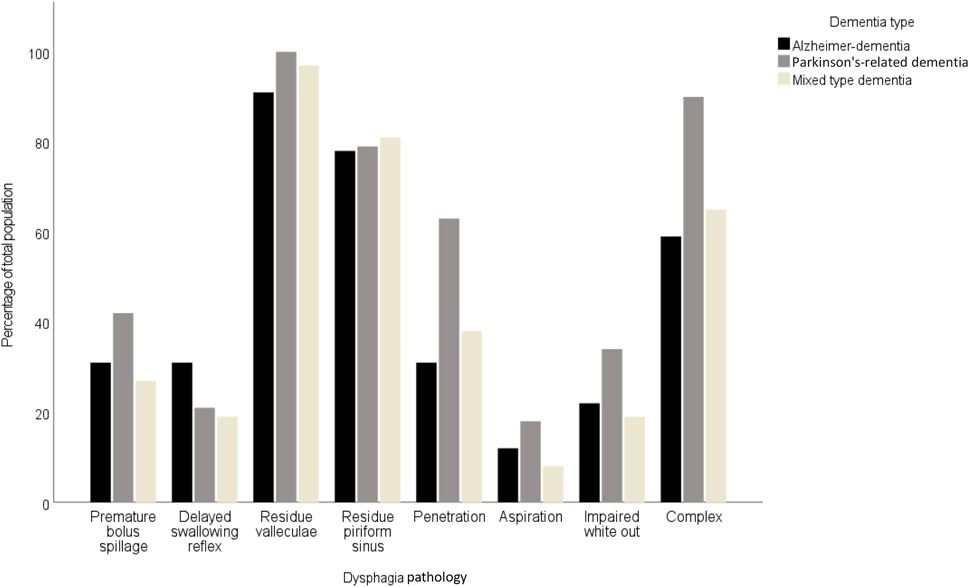
Table 3 provides a comparative analysis of dysphagia pathologies across three dementia types. Significant variation was noted in the prevalence of penetration in the complex dysphagia pathology. Penetration was significantly more frequent in Parkinson’s-related dementia patients (63%; p = 0.016), compared to Alzheimer’s disease (31%) and mixed-type dementia (38%). Complex dysphagia was also most prevalent in Parkinson’s-related dementia (90%; p < 0.010), and distinctly higher than in Alzheimer’s dementia (59%) and mixed-type dementia (65%). The most common pathology was residue in the valleculae with an equal distribution between dementia types. In contrast, aspiration was least prevalent, with a non-significant variation across types, indicating limited clinical utility for differentiating dementia types. These findings are illustrated by Figures 1, 2. Figure 1 shows the prevalence of the distinct endoscopic findings in the three dementia groups and Figure 2 the same from the perspective of the dementia type.

Figure 2. Prevalence of dysphagia pathologies across Alzheimer-dementia, Parkinson-dementia complex, and mixed type dementia.
Partial correlation analyses indicated a significant association between impaired white out and aspiration among various dysphagia pathologies (r = 0.283, p < 0.001). No other significant correlations were identified between different dysphagia pathologies. In addition, we conducted a regression analysis to investigate the independent effects of various factors, including age, gender, severity of dementia, sarcopenia, handgrip strength, Barthel Index, and frailty, on dysphagia severity. The analysis revealed that none of these risk factors demonstrated a significant association with the severity of dysphagia as evidenced by the following p-values: severity of dementia (p = 0.926), sarcopenia (p = 0.836), age (p = 0.267), gender (p = 0.304), handgrip strength (p = 0.174), Barthel Index (p = 0.609), and frailty (p = 0.737).
Discussion
The etiology of dysphagia is multifactorial, involving both cognitive impairments associated with dementia and behavioral disorders. These behavioral disorders may include buccofacial apraxia and refusal to eat, resulting in a prolonged oral phase and frontal bolus spillage. Additionally, inter- and post-deglutitive pathomechanistic changes can be observed using FEES. Only few studies have focused exclusively on employing FEES to explore dysphagia in patients with Alzheimer’s disease compared to various stages and forms of dementia (Parlak et al., 2022; Sheikhany et al., 2019; De Stefano et al., 2020; Shapira-Galitz et al., 2019; Suh et al., 2009). This study extends the current understanding by providing insights into the prevalence and characteristics of various dysphagia pathologies across different types of dementia using FEES. Bolus residuals in the vallecula and piriform sinus was detectable in almost all dementia patients (96%). This high prevalence exceeds rates reported in prospective age cohorts, underscoring that dementia-specific neurodegenerative changes significantly contribute to residual function in addition to age-related sensory loss (Labeit et al., 2022).
Our findings highlight significant variation in swallowing dysfunction and findings, with a notably higher prevalence of bolus penetration and complex dysphagia pathology observed in patients with Parkinson’s-related dementia. Our results are consistent with those of a previous study in a relatively large cohort of Parkinson’s disease patients studied with FEES. Similar to our cohort, bolus residues were observed in 93% and bolus penetration was observed in 55% (Pflug et al., 2018). Theories suggest that food residue accumulation, depending of their presence in the valleculae or the sinus piriformis, and bolus penetration could be due to reduced tongue base retraction, velopharyngeal closure, weaker pharyngeal muscles, and a decrease in upper esophageal sphincter opening (Taira et al., 2021; Higo et al., 2001).
Another notable finding of our study is that the most common dysphagia pathology observed across all types of dementia was the presence of residues in the vallecula. In line with our results, bolus residue in the vallecula and the sinus piriformis were also the most prevalent findings in another FEES-based study on Alzheimer’s disease patients (Parlak et al., 2022). Previous research has linked vallecular residues in dementia patients to weakened pharyngeal muscle strength and a delayed swallowing reflex, both of which are associated with an increased risk of aspiration (Shapira-Galitz et al., 2019; Molfenter and Steele, 2013). Contrary to expectations, our results did not indicate a significant association between the presence of residue and an increased rate of aspiration. However, we did observe a correlation between reduced pharyngeal muscle strength, quantified by the white-out phenomenon, and a higher frequency of aspiration in the comparative analysis across groups. The white-out phenomenon has been proposed as a semi-quantitative indicator of pharyngeal contractility (Labeit et al., 2021a) and pharyngeal contractility has been identified as a reliable predictor of aspiration in both FEES and videofluoroscopic swallowing studies (Miles and Hunting, 2023; Ku et al., 2021). This highlights the importance of measuring pharyngeal muscle dynamics as a component of comprehensive dysphagia assessment in dementia patients, providing critical insights into the mechanisms underlying swallowing disorders in this population.
The findings of our study indicate that there are no differences in dysphagia pathologies between patients with Alzheimer’s dementia and those with Alzheimer’s dementia and concomitant small vessel disease. Comparative analyses of these two forms of dementia are lacking in the literature, although an older study employed videofluoroscopy to examine the differences in dysphagia between patients with Alzheimer’s disease and those with vascular dementia (Suh et al., 2009). With regard to the characteristics of dysphagia, no relevant differences were observed in the prevalence of bolus residue in Alzheimer’s patients and patients with vascular dementia (80.0% vs 67.6%, p = 0.502), neither in the occurrence of penetration (73.3% vs. 55.8%, p = 0.248), which is in line with the data in our study. However, in comparison to our cohort, there was a prolongation of the oral phase in patients with Alzheimer’s disease (p = 0.008) and a higher incidence of aspiration in patients with vascular dementia (p = 0.024). It is important to note that the Alzheimer’s patients in the above-cited study showed significantly greater cognitive impairment than those in our cohort. Previous literature has indicated that as dementia progresses, the prevalence of behavioral eating disorders, such as buccofacial apraxia, tends to increase (Seçil et al., 2016). Due to this inter-individual variability of neuropsychiatric symptoms, the reported prevalence of dysphagia in Alzheimer’s disease spans from 2.4% to 100% (Mira et al., 2022).
In summary, no specific endoscopic pathologies of dysphagia could be detected in patients with dementia, except for minor specifics in the Parkinson’s group. The reasons for this may be found in the overlap of the cerebral swallowing network with the neuropathological spread of beta-amyloid pathology in Alzheimer’s disease and the pattern of cerebral microangiopathy. Cortical regions associated both with the swallowing network and the trajectory of neurodegeneration include the lower frontal gyrus, the anterior cingulate cortex, the orbitofrontal cortex, and the supramarginal gyrus (Cheng et al., 2022; Braak and Braak, 1991; Thal et al., 2006). A review of cerebral microangiopathy in patients with dysphagia revealed that white-matter lesions, particularly in the internal capsule, corona radiata, insula, and corpus callosum, were linked to swallowing disorders (Alvar et al., 2021). The lack of differences in the swallowing pathologies of patients in the FEES may be attributed to the fact that Alzheimer’s disease and cerebral microangiopathy progress along a continuum and overlap with areas of the cerebral swallowing network.
For clinical practice, our study shows that patients with dementia have impaired bolus clearance. The relatively rare occurrence of white-out cannot be identified as a reliable indicator of this due to the lack of standardization in recording, evaluation, and interpretation. Individualized dysphagia therapy should therefore continue to be decided on an individual basis for patients with dementia, for example, through meal assistance or careful adjustment of food consistency (Lueg et al., 2024). Our study has several limitations. Conducted as a retrospective analysis with a limited number of participants, generalizability of data is possibly constrained. In addition, the multimorbidity of the patients does not allow for a clear determination of causality between the pathophysiology of dementia and specific types of swallowing disorders. Furthermore, the clinical relevance of dysphagia, particularly in patients with Alzheimer’s disease, is likely influenced by kinematic aspects of swallowing alongside cognitive and behavioral symptoms - factors not fully captured due to the retrospective nature of our study. In future studies, a structured behavioral swallowing assessment, including validated screening for buccofacial apraxia and standardized dysphagia questionnaires administered by speech therapists, would be necessary to differentiate behavioral components of swallowing impairment in dementia. One strength of the study is the utilization of FEES in patients with dementia which is a challenge due to reduced cooperation of the participants. To gain further insight into the influence of dementia on swallowing disorders, it would be beneficial to conduct a comparative analysis with our collective and geriatric patients who have been diagnosed with mild cognitive impairment and Parkinson’s disease without concomitant dementia. Such studies will be important for developing and implementing effective therapies tailored to disease stages and individual dysphagia patterns, focused on enhancing pharyngeal muscle strength and optimizing nutritional interventions.
Conclusion
Despite small differences in participants with Parkinson’s-related dementia, our study did not identify a typical dysphagia pathology in different types of dementia. Therefore, the individual presentation of dysphagia must be determined in each patient to enable a tailored intervention.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the study protocol was approved by the Ethics Committee of the Ruhr University Bochum (Reg.-Nr. 23–7878). The study is registered at German Clinical trial register (DRKS-ID: DRKS00032433). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’; legal guardians/next of kin because the study is based on a retrospective analysis of routine medical data collected as part of the standard diagnostic procedures. No additional procedures or interventions were performed on the patients.
Author contributions
SP: Formal Analysis, Investigation, Writing – original draft. MP: Writing – review and editing, Data curation, Formal Analysis. BL: Methodology, Writing – review and editing. PM: Writing – review and editing. SS-K: Writing – review and editing. TW: Methodology, Writing – review and editing. RD: Writing – review and editing. UT: Writing – review and editing, Data curation. RWi: Conceptualization, Supervision, Writing – review and editing. GL: Conceptualization, Investigation, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
SS-K endowed professorship from the Else Kröner-Fresenius-Stiftung.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fragi.2025.1535137/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Flowchart of patient selection process for inclusion in the FEES-based dysphagia analysis.
References
Abu-Ghanem, S., Chen, S., and Amin, M. R. (2020). Oropharyngeal dysphagia in the elderly: evaluation and prevalence. Curr. Otorhinolaryngol. Rep. 8, 34–42. doi:10.1007/s40136-020-00258-x
Alvar, A., Hahn Arkenberg, R., McGowan, B., Cheng, H., and Malandraki, G. A. (2021). The role of white matter in the neural control of swallowing: a systematic review. Front. Hum. Neurosci. 15, 628424. doi:10.3389/fnhum.2021.628424
Baijens, L. W., Clavé, P., Cras, P., Ekberg, O., Forster, A., Kolb, G. F., et al. (2016). European society for swallowing disorders–European union geriatric medicine society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clin. interventions aging 11, 1403–1428. doi:10.2147/CIA.S107750
Banda, K. J., Chu, H., Chen, R., Kang, X. L., Jen, H.-J., Liu, D., et al. (2022). Prevalence of oropharyngeal dysphagia and risk of pneumonia, malnutrition, and mortality in adults aged 60 Years and older: a meta-analysis. a meta-analysis 68, 841–853. doi:10.1159/000520326
Benjapornlert, P., Kagaya, H., Shibata, S., Matsuo, K., Inamoto, Y., Kittipanya-ngam, P., et al. (2020). The prevalence and findings of fibre-optic endoscopic evaluation of swallowing in hospitalised patients with dysphagia. J. Oral Rehabilitation 47 (8), 983–988. doi:10.1111/joor.13026
Braak, H., and Braak, E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta neuropathol. 82 (4), 239–259. doi:10.1007/BF00308809
Cheng, I., Takahashi, K., Miller, A., and Hamdy, S. (2022). Cerebral control of swallowing: an update on neurobehavioral evidence. J. Neurological Sci. 442, 120434. doi:10.1016/j.jns.2022.120434
Cichero, J. A., Lam, P., Steele, C. M., Hanson, B., Chen, J., Dantas, R. O., et al. (2017). Development of international terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: the IDDSI framework. Dysphagia 32 (2), 293–314. doi:10.1007/s00455-016-9758-y
Cruz-Jentoft, A. J., Bahat, G., Bauer, J., Boirie, Y., Bruyère, O., Cederholm, T., et al. (2019). Sarcopenia: revised European consensus on definition and diagnosis. Age ageing 48 (1), 601–631. doi:10.1093/ageing/afz046
De Stefano, A., Di Giovanni, P., Kulamarva, G., Gennachi, S., Di Fonzo, F., Sallustio, V., et al. (2020). Oropharyngeal dysphagia in elderly population suffering from mild cognitive impairment and mild dementia: understanding the link. Am. J. Otolaryngology 41 (4), 102501. doi:10.1016/j.amjoto.2020.102501
Drey, M., Ferrari, U., Schraml, M., Kemmler, W., Schoene, D., Franke, A., et al. (2020). German version of SARC-F: translation, adaption, and validation. J. Am. Med. Dir. Assoc. 21 (6), 747–51. e1. doi:10.1016/j.jamda.2019.12.011
Dziewas, R., Warnecke, T., Labeit, B., Claus, I., Muhle, P., Oelenberg, S., et al. (2024). Systematic approach to contextualize findings of flexible endoscopic evaluation of swallowing in neurogenic dysphagia–towards an integrated FEES report. Neurological Res. Pract. 6 (1), 26. doi:10.1186/s42466-024-00321-8
Goetz, C. G., Poewe, W., Rascol, O., Sampaio, C., Stebbins, G. T., Counsell, C., et al. (2004). Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov. Disord. 19 (9), 1020–1028. doi:10.1002/mds.20213
Higo, R., Tayama, N., Watanabe, T., and Niimi, S. (2001). Abnormal elevation of resting pressure at the upper esophageal sphincter of Parkinson’s disease patients. Eur. archives oto-rhino-laryngology. 258, 552–556. doi:10.1007/s004050100401
Kaiser, M. J., Bauer, J. M., Ramsch, C., Uter, W., Guigoz, Y., Cederholm, T., et al. (2009). Validation of the Mini Nutritional Assessment Short-Form (MNA®-SF): a practical tool for identification of nutritional status. JNHA-The J. Nutr. Health Aging 13, 782–788. doi:10.1007/s12603-009-0214-7
Ku, P. K., Vlantis, A. C., Hui, T. S., Yeung, D. C., Lee, A. K., Law, T., et al. (2021). Assessment of pharyngeal motor function using a novel velopharyngeal squeeze maneuver and a novel endoscopic pharyngeal contraction grade scale in patients with dysphagia after radiotherapy for nasopharyngeal carcinoma. Head and Neck 43 (11), 3586–3597. doi:10.1002/hed.26871
Labeit, B., Claus, I., Muhle, P., Regner, L., Suntrup-Krueger, S., Dziewas, R., et al. (2021b). Effect of cognitive and motor dual-task on oropharyngeal swallowing in Parkinson's disease. Eur. J. neurology 28 (3), 754–762. doi:10.1111/ene.14603
Labeit, B., Muhle, P., von Itter, J., Slavik, J., Wollbrink, A., Sporns, P., et al. (2022). Clinical determinants and neural correlates of presbyphagia in community-dwelling older adults. Front. Aging Neurosci. 14, 912691. doi:10.3389/fnagi.2022.912691
Labeit, B., Perlova, K., Pawlitzki, M., Ruck, T., Muhle, P., Claus, I., et al. (2021a). Predictors, outcome and characteristics of oropharyngeal dysphagia in idiopathic inflammatory myopathy. Muscle and nerve 63 (6), 874–880. doi:10.1002/mus.27225
Lueg, G., Pourhassan, M., and Wirth, R. (2024). Progress in dysphagia management in older patients. Curr. Opin. Clin. Nutr. and Metabolic Care. doi:10.1097/mco.0000000000001086
McKeith, I. G., Boeve, B. F., Dickson, D. W., Halliday, G., Taylor, J.-P., Weintraub, D., et al. (2017). Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89 (1), 88–100. doi:10.1212/WNL.0000000000004058
Miles, A., and Hunting, A. (2023). Pharyngeal squeeze maneuver during endoscopy—what does it tell us? Laryngoscope 133 (12), 3429–3435. doi:10.1002/lary.30796
Mira, A., Gonçalves, R., and Rodrigues, I. T. (2022). Dysphagia in Alzheimer’s disease: a systematic review. Dementia and Neuropsychologia 16 (3), 261–269. doi:10.1590/1980-5764-DN-2021-0073
Molfenter, S. M., and Steele, C. M. (2013). The relationship between residue and aspiration on the subsequent swallow: an application of the normalized residue ratio scale. Dysphagia 28, 494–500. doi:10.1007/s00455-013-9459-8
Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatrics Soc. 53 (4), 695–699. doi:10.1111/j.1532-5415.2005.53221.x
Neubauer, P. D., Rademaker, A. W., and Leder, S. B. (2015). The yale pharyngeal residue severity rating scale: an anatomically defined and image-based tool. Dysphagia 30, 521–528. doi:10.1007/s00455-015-9631-4
Parlak, M. M., Babademez, M. A., Alicura Tokgöz, S., Bizpınar, Ö., and Saylam, G. (2022). Evaluation of swallowing function according to the stage of Alzheimer’s disease. Folia Phoniatrica Logop. 74 (3), 186–194. doi:10.1159/000519263
Pflug, C., Bihler, M., Emich, K., Niessen, A., Nienstedt, J. C., Flügel, T., et al. (2018). Critical dysphagia is common in Parkinson disease and occurs even in early stages: a prospective cohort study. Dysphagia 33, 41–50. doi:10.1007/s00455-017-9831-1
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson's disease. Mov. Disord. 30 (12), 1591–1601. doi:10.1002/mds.26424
Rajati, F., Ahmadi, N., Naghibzadeh, Z. A.-S., and Kazeminia, M. (2022). The global prevalence of oropharyngeal dysphagia in different populations: a systematic review and meta-analysis. J. Transl. Med. 20 (1), 175. doi:10.1186/s12967-022-03380-0
Rosenbek, J. C., Robbins, J. A., Roecker, E. B., Coyle, J. L., and Wood, J. L. (1996). A penetration-aspiration scale. Dysphagia 11, 93–98. doi:10.1007/BF00417897
Rossetti, H. C., Lacritz, L. H., Cullum, C. M., and Weiner, M. F. (2011). Normative data for the Montreal cognitive assessment (MoCA) in a population-based sample. Neurology 77 (13), 1272–1275. doi:10.1212/WNL.0b013e318230208a
Scheltens, P., Erkinjunti, T., Leys, D., Wahlund, L.-O., Inzitari, D., del Ser, T., et al. (1998). White matter changes on CT and MRI: an overview of visual rating scales. European task force on age-related white matter changes. Eur. Neurol. 39 (2), 80–89. doi:10.1159/000007921
Seçil, Y., Arıcı, Ş., İncesu, T. K., Gürgör, N., Beckmann, Y., and Ertekin, C. (2016). Dysphagia in Alzheimer's disease. Neurophysiol. Clinique/Clinical Neurophysiol. 46 (3), 171–178. doi:10.1016/j.neucli.2015.12.007
Shapira-Galitz, Y., Shoffel-Havakuk, H., Halperin, D., and Lahav, Y. (2019). Correlation between pharyngeal residue and aspiration in fiber-optic endoscopic evaluation of swallowing: an observational study. Archives Phys. Med. rehabilitation 100 (3), 488–494. doi:10.1016/j.apmr.2018.05.028
Sheikhany, A. R., Hady, A. F. A., and Farag, S. (2019). Oropharyngeal dysphagia profile in early versus late stage dementia. Egypt. J. Otolaryngology 35, 103–109. doi:10.4103/ejo.ejo_98_18
Simpson, J. R. (2014). DSM-5 and neurocognitive disorders. J. Am. Acad. Psychiatry Law Online 42 (2), 159–164.
Sternberg, S. A., Schwartz, A. W., Karunananthan, S., Bergman, H., and Mark Clarfield, A. (2011). The identification of frailty: a systematic literature review. J. Am. Geriatrics Soc. 59 (11), 2129–2138. doi:10.1111/j.1532-5415.2011.03597.x
Suh, M. K., Kim, H., and Na, D. L. (2009). Dysphagia in patients with dementia: Alzheimer versus vascular. Alzheimer Dis. and Assoc. Disord. 23 (2), 178–184. doi:10.1097/WAD.0b013e318192a539
Taira, K., Fujiwara, K., Fukuhara, T., Koyama, S., Morisaki, T., and Takeuchi, H. (2021). Evaluation of the pharynx and upper esophageal sphincter motility using high-resolution pharyngeal manometry for Parkinson’s disease. Clin. Neurology Neurosurg. 201, 106447. doi:10.1016/j.clineuro.2020.106447
Thal, D. R., Capetillo-Zarate, E., Del Tredici, K., and Braak, H. (2006). The development of amyloid β protein deposits in the aged brain. Sci. aging Knowl. Environ. 2006 (6), re1–re. doi:10.1126/sageke.2006.6.re1
Tye, C. B., Gardner, P. A., Dion, G. R., Simpson, C. B., and Dominguez, L. M. (2021). Impact of fiberoptic endoscopic evaluation of swallowing outcomes and dysphagia management in neurodegenerative diseases. Laryngoscope 131 (4), 726–730. doi:10.1002/lary.28791
Warnecke, T., Labeit, B., Schroeder, J., Reckels, A., Ahring, S., Lapa, S., et al. (2021). Neurogenic dysphagia: systematic review and proposal of a classification system. Neurology 96 (6), e876–e889. doi:10.1212/WNL.0000000000011350
Warnecke, T., Suttrup, I., Schröder, J. B., Osada, N., Oelenberg, S., Hamacher, C., et al. (2016). Levodopa responsiveness of dysphagia in advanced Parkinson's disease and reliability testing of the FEES-Levodopa-test. Park. and Relat. Disord. 28, 100–106. doi:10.1016/j.parkreldis.2016.04.034
Keywords: dysphagia (swallowing disorder), dementia, alzheimer’s diaease, Parkinson’s disease dementia, fees, geriatric patients
Citation: Peranovic S, Pourhassan M, Labeit B, Muhle P, Suntrup-Krueger S, Warnecke T, Dziewas R, Trampisch U, Wirth R and Lueg G (2025) Endoscopic characterization of oropharyngeal dysphagia in patients with dementia. Front. Aging 6:1535137. doi: 10.3389/fragi.2025.1535137
Received: 27 February 2025; Accepted: 18 June 2025;
Published: 26 June 2025.
Edited by:
Maria Aparecida Bicalho, Federal University of Minas Gerais, BrazilReviewed by:
Laelia Vicente, Federal University of Minas Gerais, BrazilHans Heppner, University of Erlangen Nuremberg, Germany
Beth Solomon, Center for Infectious Disease Imaging, Radiology and Imaging Sciences, Clinical Center (NIH), United States
Copyright © 2025 Peranovic, Pourhassan, Labeit, Muhle, Suntrup-Krueger, Warnecke, Dziewas, Trampisch, Wirth and Lueg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gero Lueg, Z2Vyby5sdWVnQHJ1aHItdW5pLWJvY2h1bS5kZQ==, Z2Vyby5sdWVnQGVsaXNhYmV0aGdydXBwZS5kZQ==
 Sara Peranovic
Sara Peranovic Maryam Pourhassan1
Maryam Pourhassan1 Paul Muhle
Paul Muhle Tobias Warnecke
Tobias Warnecke Gero Lueg
Gero Lueg