- 1Department of Kinesiology, University of Maryland School of Public Health, College Park, Maryland, MD, United States
- 2Baltimore Veterans Affairs Geriatric Research, Education and Clinical Center, Baltimore, MD, United States
Introduction: Skeletal and cardiac muscle mitochondria exist in a dynamic reticulum that is maintained by a balance of mitochondrial biogenesis, fusion, fission, and mitophagy. This balance is crucial for adequate ATP production, and alterations in skeletal muscle mitochondria have been implicated in aging-associated declines in mitochondrial function.
Methods: We sought to determine whether age and biological sex affect mitochondrial content [Complex IV (CIV)], biogenesis (PGC-1ɑ), fusion (MFN2, OPA1), fission (DRP1, FIS1), and mitophagy (Parkin, Pink1) markers in skeletal and cardiac muscle by assessing protein expression in tibialis anterior (TA) and ventricular tissue from 16 young (≤6 months) and 16 old (≥20 months) male and female Sprague-Dawley rats.
Results: In the TA, CIV expression was 40% lower in old vs. young rats (p < 0.001), indicating lower mitochondrial content, and coincided with higher expression of Parkin (+4-fold, p < 0.001). Further, MFN2 expression was higher (+2-fold, p < 0.005) and DRP1 expression was lower (−40%, p = 0.014) in older rats. In cardiac muscle, mitochondrial content was maintained in old vs. young rats, and this occurred concomitantly with higher expression of both PGC-1ɑ and Parkin. MFN2 and OPA1 expression were also 1.2-5-fold higher in older rats (p < 0.05 for all). Largely, protein expression did not differ between male and female rats, with the exception of Pink1 and FIS1 expression in the TA.
Discussion: Collectively, older skeletal and cardiac muscle demonstrated higher expression of fusion and mitophagy proteins, which indicates age alters the balance of biogenesis, fission, fusion, and mitophagy. This may, in turn, affect the ability to provide ATP to these metabolically active tissues.
1 Introduction
Mitochondrial dysfunction is frequently observed in aging muscle, and previous studies have reported decreases in mitochondrial respiration (Gouspillou et al., 2014), reduced time to open the mitochondrial permeability transition pore (Gouspillou et al., 2014), and increased mitochondrial DNA damage and mutation frequency (Herbst et al., 2021; Short et al., 2005) in older skeletal and cardiac muscle. While not all studies show age-related reductions in mitochondrial function in muscle with aging (Tevald et al., 2014), changes that coincide with alterations in the structure of mitochondria in old vs. young skeletal and cardiac muscle may negatively impact the function of muscle mitochondria in aging.
In healthy skeletal and cardiac muscle, mitochondria are organized in a reticulum to efficiently produce and distribute ATP. This reticulum is maintained by multiple processes that are collectively referred to as mitochondrial quality control. Together, the processes of fusion, fission, mitophagy, and biogenesis ensure the mitochondrial reticulum is able to meet the energetic demands of the cell, while mitigating dysfunction. Constant cycles of fusion and fission ensure the mitochondrial reticulum functions optimally by redistributing matrix components and preserving membrane potential (Scorrano, 2013). Fusion creates a more expansive reticulum by joining adjacent mitochondria through outer (OMM) and inner mitochondrial membrane (IMM) proteins. OMM-bound proteins mitofusin 1 and 2 (MFN1 and MFN2) allow for OMM fusion, while IMM protein optic atrophy 1 (OPA1) fuses the IMM. Opposing fusion, is the process of fission which separates dysfunctional portions from the reticulum (Twig et al., 2008). OMM proteins such as fission 1 (FIS1) and mitochondrial fission factor (MFF) recruit dynamin-related protein 1 (DRP1) to the surface of the mitochondria (Loson et al., 2013). DRP1 physically cinches the mitochondrion causing it to cleave into two distinct sections of mitochondria (Twig et al., 2008).
Cleaved mitochondria can subsequently undergo selected degradation via mitophagy. The primary pathway for initiation of mitophagy is the phosphatase and tensin homologue-induced putative kinase 1 (Pink1) and Parkin pathway which occurs on the outer mitochondrial membrane (OMM). The accumulation of Pink1 on the OMM recruits and phosphorylates E3 ubiquitin ligase Parkin and causes ubiquitin chains to form (Marzetti et al., 2024). The ubiquitin chain serves as a binding site for a phagophore, which will engulf dysfunctional mitochondria before merging with a lysosome and degrading encapsulated mitochondria. Conversely, mitochondrial biogenesis is regulated by the transcriptional coactivator peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1α). PGC-1α translocates to the nucleus and activates several downstream transcription factors to promote the synthesis of new mitochondria (Halling et al., 2019). Carefully balanced mitophagy and biogenesis, along with fusion and fission, are therefore crucial for quality control and maintaining the mitochondrial reticulum.
Previous investigations have produced interesting, but at times disparate, data regarding how age affects mitochondrial quality control. Results from human vastus lateralis muscle are somewhat mixed, with some studies showing lower expression of markers of fusion (MFN2, OPA1) (Crane et al., 2010; Joseph et al., 2012) or fission (DRP1) (Crane et al., 2010), while other studies show no differences in these markers in young vs. older muscle (Balan et al., 2019; Distefano et al., 2017; Konopka et al., 2014). Interestingly, results of these human studies may differ based on whether only men are studied (Balan et al., 2019; Konopka et al., 2014), or whether men and women are combined for study (Crane et al., 2010; Joseph et al., 2012). In animals, some studies provide evidence of reduced fusion, with lower MFN1 and MFN2 protein content in old vs. young skeletal muscle (Capitanio et al., 2016; Faitg et al., 2019; Joseph et al., 2013; Koltai et al., 2012; O'Leary et al., 2013; Yeo et al., 2019), but OPA1 expression appears largely unchanged (Joseph et al., 2013; Leduc-Gaudet et al., 2015; Yeo et al., 2019). Fission and mitophagy may be increased in older age as FIS1 protein expression was shown to be higher in skeletal muscle of old animals (Faitg et al., 2019; Koltai et al., 2012; O'Leary et al., 2013; Yeo et al., 2019), DRP1 protein expression is the same (Joseph et al., 2013; Sebastian et al., 2016; Yeo et al., 2019) or higher (Faitg et al., 2019) in old vs. young, and the mitophagy-related protein Parkin protein expression increases with age (O'Leary et al., 2013; Triolo et al., 2022). Relatively few studies have addressed cardiac muscle, but MFN1 and MFN2 protein expression have been shown to be lower in old vs. young cardiac muscle (Sebastian et al., 2016; Zhao et al., 2014), while OPA1 protein expression may be increased (Ljubicic et al., 2010) and DRP1 protein expression does not appear to change (Zhao et al., 2014), highlighting some potential differences between skeletal and cardiac muscle.
While some patterns are emerging, the use of different muscles, animal models, and aging timepoints does not allow for a cohesive understanding of how age affects the expression of proteins controlling mitochondrial content (biogenesis and mitophagy) and morphology (fission and fusion). Further, limited data exist in aged cardiac tissue, likely stemming from the previously held notion that fusion and fission are unnecessary for normal heart function (Dorn, 2013), and differences that may exist due to biological sex remains understudied. Notably, only three of eleven aforementioned studies included females, and only one study included a comparison between non-transgenic male and female mice (Triolo et al., 2022). The study by Triolo et al. (2022) focused on and mitophagy and lysosomal pathways, finding higher Parkin protein expression in skeletal muscle from old vs. young mice, while also showing that Parkin expression was higher in the female compared with male mice. The expression of autophagy-related protein Beclin-1 was also higher in the old animals; however, when sexes were separated, only the old male mice had significantly higher Beclin-1 protein expression than the young group (Triolo et al., 2022), demonstrating the potential for sex-specific changes with age.
Based on these results, we hypothesized that age and sex may differentially affect markers of fusion, fission, and mitophagy in young and old skeletal muscle. Accordingly, the purpose of this study was to determine the effects of age and sex on mitochondrial content and the expression of mitochondrial quality control proteins regulating biogenesis, mitophagy, fusion and fission in both cardiac and skeletal muscle (tibialis anterior) of male and female rats.
2 Materials and methods
2.1 Animals
All animal research procedures were approved and conducted in accordance with the University of Maryland Institutional Animal Care and Use Committee (R-FEB-23–03). Sixteen young (2–5 months) and 16 old (21–34 months) adult Hilltop SD rats (Rattus norvegicus, Scottdale, PA, United States) were pair-housed fed ad-libitum with standard chow. Groups were further stratified by biological sex resulting in four groups with eight rats in each group. Rats were sacrificed at <6 months (young) or >20 months of age.
On the day of sacrifice, rats were massed and anaesthetized with 2%–4% isoflurane supplemented with 100% oxygen. Rats were placed supine on the surgical area and upon cessation of toe and tail-pinch reflexes, a thoracotomy was performed, and death was induced by exsanguination. Hearts were quickly removed and cannulated via the aorta and rinsed with phosphate buffer saline. Subsequently, the skin on the hindlimbs of the rats was removed, and the tibialis anterior (TA) from both legs were quickly excised. Heart and TA masses were determined before storing at −20°C. Hindlimb lengths (tibial, and knee-to-heel) were measured with digital calipers.
2.2 Protein expression and mitochondrial content
Mitochondrial quality control protein content was quantified using western blots. MFN2 and OPA1 were used as markers of mitochondrial fusion, while DRP1 and FIS1 were used as markers of mitochondrial fission. Specific to OPA1, the long (OPA1-L) and short (OPA1-S) isoforms were both assessed independently as they may reflect different physiological phenomena. For example, the disruption of membrane potential can cleave Opa1 into its short isoforms, which some have suggested inhibits inner mitochondrial membrane fusion and in turn, the sharing of membrane potential and matrix components (Baricault et al., 2007; Ehses et al., 2009; Head et al., 2009).
Parkin and Pink1 were used as markers of mitochondrial mitophagy, and mitochondrial biogenesis was measured by PGC-1α protein expression. Mitochondrial content in cardiac muscle and tibialis anterior muscle was quantified by CIV protein expression. Skeletal muscle mitochondrial content in skeletal muscle was subsequently confirmed by measuring citrate synthase activity in lateral gastrocnemius muscles.
Proteins were extracted from tissues in 1% Triton buffer with HALT protease and phosphatase inhibitor cocktails (Thermo Fisher Scientific 78,430 and 78,428, Waltman, MA, United States). Whole muscle homogenates were made for both the TA and heart using a hand-held homogenizer and subsequently centrifuged at 500 g for 5 min at 4°C. Supernatants were collected and protein concentration was quantified by Pierce bicinchoninic acid assay (Thermo Fisher Scientific 23,225, Waltham, MA, United States). Equal amounts of protein (30ug for TA; 60ug for heart) were separated by SDS-PAGE on 4%–15% Bio-Rad TGX stain free gels (4568085, Hercules, CA, United States) before photoactivation of the gels. Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad 1704156, Hercules, CA, United States). Protein transfer was confirmed by imaging total protein fluorescence. Membranes were blocked for 2 h at room temperature in TBS (Bio-Rad 1706435, Hercules, CA, United States) with 5% milk or BSA (Fisher Scientific BP1600-100, Hampton, NH, United States) and 0.1% Tween-20 (Bio-Rad 1610781, Hercules, CA, United States). Membranes were incubated overnight at 4°C with primary antibodies (Supplementary Table S1). A secondary antibody conjugated to horseradish peroxidase (Supplementary Table S1) was used to detect bands in conjunction with Clarity Western Enhanced Chemiluminescent substrate from Bio-Rad (Hercules, CA, United States). Band intensities were captured using a Bio-Rad ChemiDoc XRS and analyzed using Image Lab 8.0 (Bio-Rad, Hercules, CA, United States). Molecular weights of proteins were confirmed by comparing to a protein standard (Bio-Rad 1610373, Hercules, CA, United States). Band intensities were normalized to total protein in the corresponding lane. In secondary analyses to account for differences in mitochondrial content, MFN2, OPA1, FIS1, DRP1, Parkin, and Pink1 content were expressed relative to complex IV (CIV) content. Representative western blot images are shown in Supplementary Figures S1–S4.
For citrate synthase activity assays, lateral gastrocnemii were homogenized in 1% Trition X-100, followed by centrifugation at 500 g for 10 min. Supernatant was added to a cuvette containing 100 mM Tris pH 8.1, 100 mM 5,5 -dithiobis-(2-nitrobenzoic acid) (DTNB), 25 µM acetyl-CoA, and 0.1% Triton, and citrate synthase activity was initiated by addition of 500 µM oxaloacetate (final volume of 1.0 mL) (Kuzmiak et al., 2012). Absorbance was followed at 412 nm, and citrate synthase activity was calculated using a millimolar extinction coefficient of 13.6 for the mercaptide ion.
2.3 Statistical analysis
Statistical analyses were performed using GraphPad Prism 9 (GraphPad Software, Boston, MA). Mitochondrial quality control protein expression was analyzed separately for TA and cardiac muscle using two-way ANOVAs with age and sex as factors. Following significant interaction effects, post hoc comparisons were made using Fisher’s LSD. All tests were two-tailed with statistical significance set at P < 0.05. Data are presented as means ± standard error of the mean.
3 Results
3.1 Rat characteristics
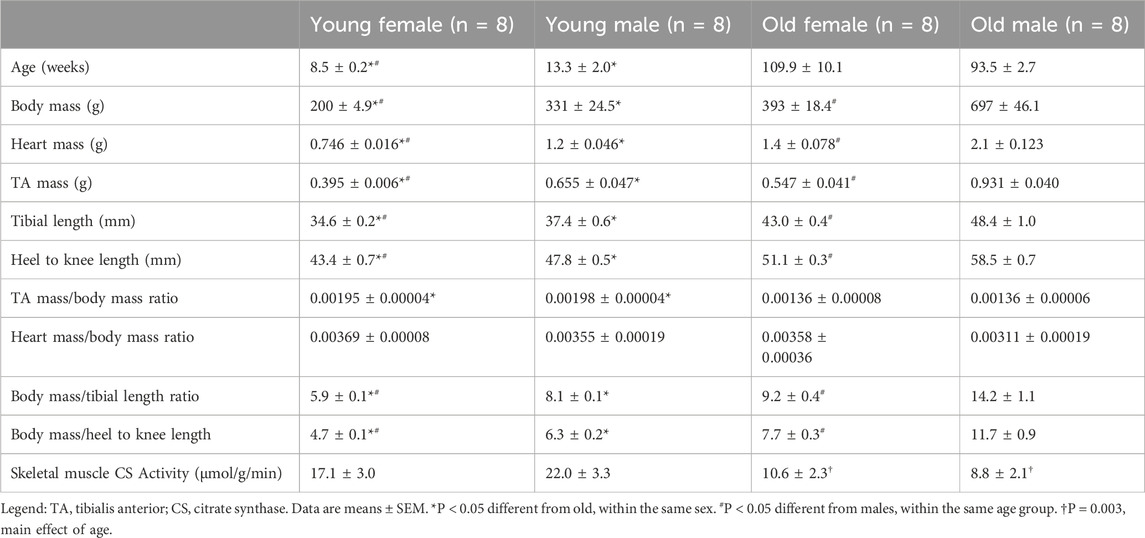
Rat characteristics are summarized in Table 1. Old rats were larger (P < 0.001) and had 83% larger hearts and 39% larger TA masses (P < 0.001) compared with young rats; however, old animals had lower skeletal muscle mass relative to body mass than young rats as evident by 31% lower TA to body mass ratio (P < 0.001). Body mass relative to tibial and heel to knee lengths were higher in old animals compared with young counterparts (P < 0.001), likely driven by higher fat mass in the older animals.
Overall, young and old female rats were smaller than their male counterparts. Compared with young males, young females had 66% lower body masses (P < 0.001), 54% lower heart masses (P < 0.001), and 66% lower TA masses (P < 0.001). Similarly, old females had 71% lower body masses (P < 0.001), 50% lower heart masses (P < 0.001), 70% lower TA masses (P < 0.001) than old males. Females also had lower body mass to tibial length ratio and body mass to heel to knee lengths when compared with male counterpart of similar age (P < 0.001). Neither young nor old females TA to body mass ratio differed from males, signifying relative skeletal muscle mass was similar between sexes (P = 0.60 and P = 0.99, respectively).
3.2 Skeletal muscle mitochondrial content, mitophagy, biogenesis, and quality control
Mitochondrial content: Skeletal muscle mitochondrial content was lower in old compared to young animals and was likely driven by an increase in mitophagy. Mitochondrial content, as measured by CIV protein expression, was 40% lower in the older TAs compared with the young counterparts (Figure 1A, main effect of age P < 0.001), with no effect of biological sex. Similarly, when measured by citrate synthase activity, mitochondrial content was ∼50% lower in older vs. young lateral gastrocnemius muscle (Table 1, main effect of age P = 0.003). While GAPDH was initially going to be used to normalize protein expression, GAPDH protein expression was 1.3-fold higher in the old TAs compared with the young (Figure 1B, main effect of age P < 0.005), making GAPDH inappropriate to use as a loading control. Thus, expression of specific proteins was normalized to total protein in each sample as acquired from stain-free imaging. Additionally, mitochondrial quality control protein expression in the TA muscle was also expressed relative to CIV expression to ensure changes in protein expression were not driven by changes in mitochondrial content.
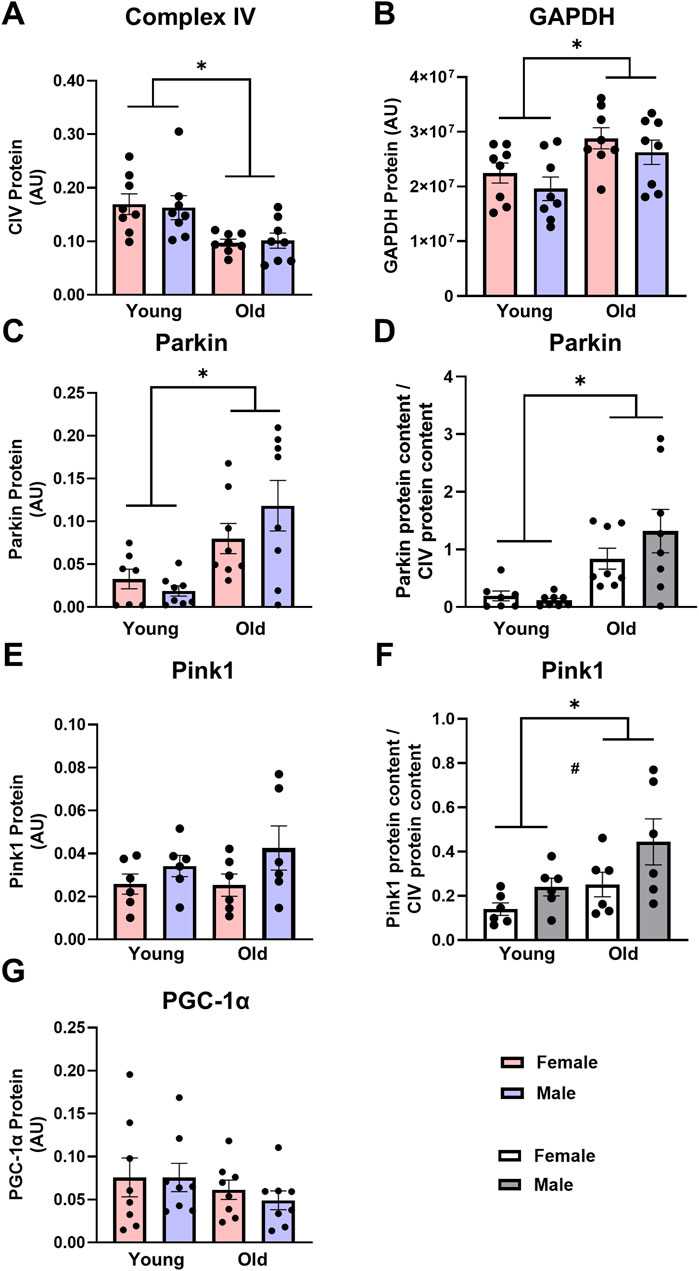
Figure 1. Complex IV [CIV, (A)] protein expression is lower, glyceraldehyde-3-phosphate dehydrogenase [GAPDH, (B)] and Parkin (C,D) protein expression are higher, and Pink1 (E,F) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha [PGC-1α, (G)] protein expression are not different in old vs. young tibialis anterior muscle. Data in (A–C,E,G) are normalized to total protein. Data in (D,F) are normalized to total protein and subsequently expressed relative to CIV expression. Legend = AU, arbitrary units; YF, young female; YM, young male; OF, old female; OM, old male. #Main effect of sex (P < 0.05), *Main effect of age (P < 0.05). Data are presented as means +SEM, n = 6-8 per group.
Mitophagy and biogenesis: Expression of markers of mitophagy was greater in old skeletal muscle. Parkin protein expression was almost 4-fold higher in old TAs compared with young counterparts (Figure 1C, main effect of age P < 0.001). Pink1 expression did not differ between young and old rat TAs (Figure 1E). When Parkin was expressed relative to CIV, the age differences were further exacerbated, with old rat TAs having 7-fold higher Parkin expression compared with young animals (Figure 1D, main effect of age P < 0.001). Pink1 expression was 83% higher in the old animals compared with young (Figure 1F, main effect of age P = 0.023), as well as 75% higher in males compared with to females when Pink1 was expressed relative to CIV (Figure 1F, main effect of sex P = 0.033). PGC-1α expression was measured as a marker of mitochondrial biogenesis, but this did not differ between young and old rats (Figure 1G, main effect of age P = 0.211), with no sex-specific changes in PGC-1α protein expression.
Fusion and fission: Mitochondrial fusion, but not fission, was higher in skeletal muscle from old compared with young animals. OMM fusion protein MFN2 and the long and short isoforms of IMM fusion protein OPA1 were analyzed. MFN2 protein expression was 2-fold higher in the TAs from the old animals compared with young animals (Figure 2A, main effect of age P < 0.005). Conversely, OPA1-Short and OPA1-Long protein content were unaffected by age or sex (Figures 2C,E, respectively). These results were consistent when MFN2, OPA1-Short, and OPA1-Long protein content were subsequently expressed relative to CIV content, with MFN2 protein levels 3.5-fold higher in old TAs compared with young when normalized to CIV expression (Figures 2B,D,F, main effect of age P < 0.001). No fusion proteins were significantly impacted by sex regardless of CIV normalization.
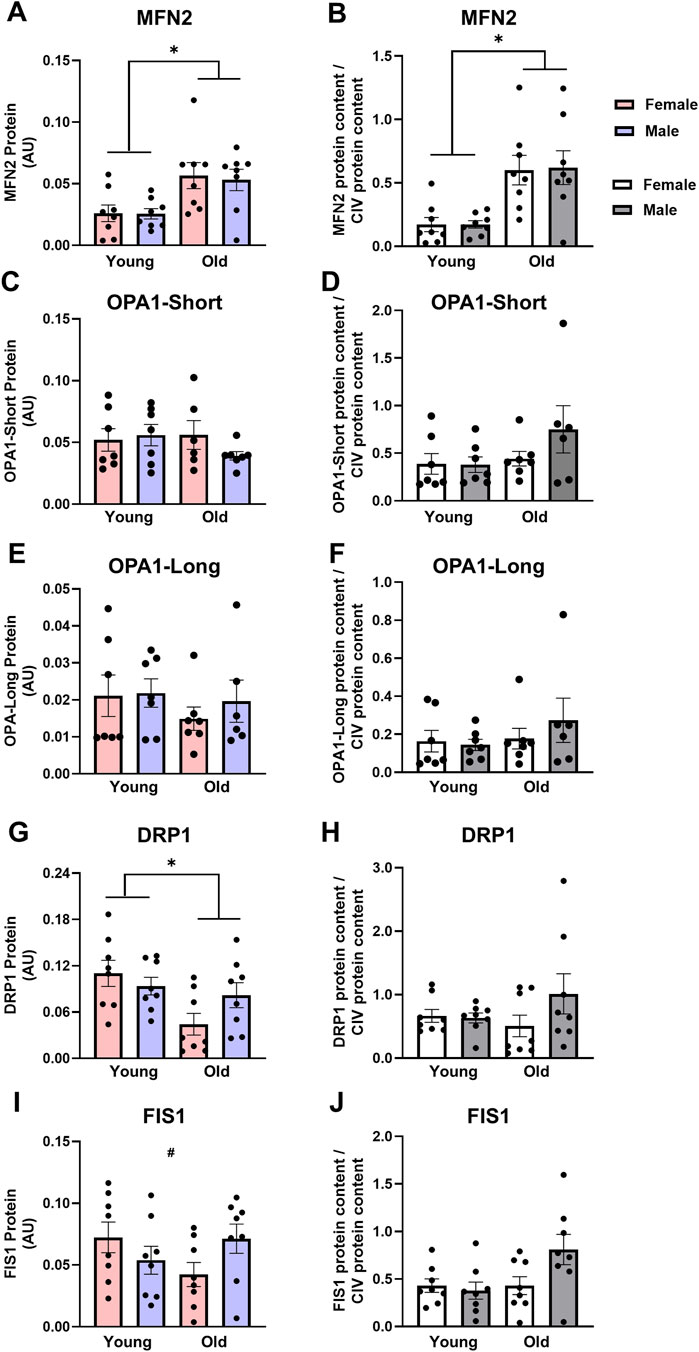
Figure 2. Mitofusin 2 [MFN2, (A,B)] protein expression is higher, optic atrophy 1-short [OPA1-Short, (C,D)] and OPA1-Long (E,F) protein expression are not different, dynamin-related protein 1 [DRP1, panels (G,H)] protein expression is lower in old vs. young tibialis anterior muscle, while there was an age*sex interaction for fission 1 [FIS1, (I,J)] protein expression. Data in (A,C,E,G,I) are normalized to total protein. Data in (B,D,F,H,J) are normalized to total protein and subsequently expressed relative to CIV expression. Legend = AU, arbitrary units; YF, young female; YM, young male; OF, old female; OM, old male. *Main effect of age (P < 0.05), #Interaction effect (P < 0.05). Data are presented as means +SEM, n = 7-8 per group.
DRP1 expression was 40% lower in the old rat TAs compared with young (Figure 2G, main effect of age P = 0.014), with no differences between sexes. There was a significant sex * age interaction effect for FIS1 protein expression (Figure 2I, interaction P = 0.047): While FIS1 expression was numerically lower in old vs. young females and numerically higher in old vs. young males, individual group differences did not reach statistical significance. The age and sex-related differences in DRP1 and FIS1 protein expression were likely due to differences in mitochondrial content as these differences were abolished when DRP1 and FIS1 were expressed relative to CIV content (Figures 2H,J, respectively).
3.3 Cardiac muscle mitochondrial content, mitophagy, biogenesis, and quality control
Mitochondrial content, mitophagy, and biogenesis: Unlike skeletal muscle, mitochondrial content was maintained in old cardiac muscle, but like skeletal muscle, old animals had higher expression of proteins associated with the initiation of mitophagy in the heart. CIV protein expression did not differ between young and old rat cardiac muscle (Figure 3A). Parkin protein expression was over 5-fold higher in the cardiac muscle from old rats compared with young counterparts (Figure 3C, main effect of age P = 0.019) and remained over 5-fold higher in old cardiac muscle when Parkin was expressed relative to CIV expression (Figure 3D, main effect of age P = 0.020). Contrary to findings in skeletal muscle, PGC-1α protein expression in old cardiac muscles was almost 1.5-fold higher than young cardiac muscles (Figure 3E, main effect of age P < 0.005), indicating a concomitant increase in biogenesis and mitophagy in old cardiac tissue. No sex-specific differences in markers of mitophagy or biogenesis were detected between young and old rat cardiac muscles.
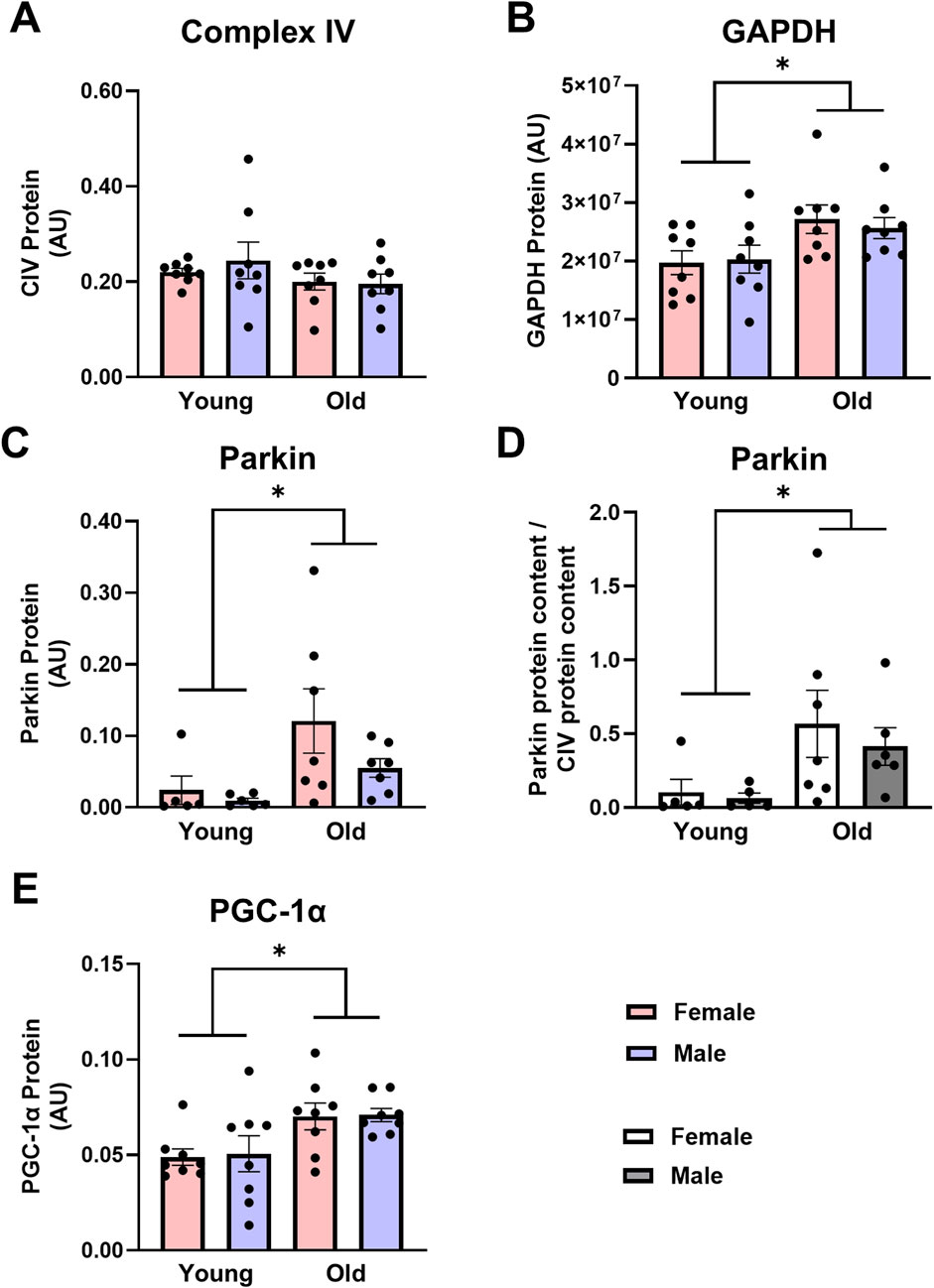
Figure 3. Complex IV [CIV, (A)] protein expression is not different, while glyceraldehyde-3-phosphate dehydrogenase [GAPDH, (B)] Parkin (C,D), and peroxisome proliferator-activated receptor gamma coactivator 1-alpha [PGC-1α, (E)] protein expression are higher in old vs. young cardiac muscle. Data in (A,B,C,E) are normalized to total protein. Data in (D) are normalized to total protein and subsequently expressed relative to CIV expression. Legend = AU, arbitrary units; YF, young female; YM, young male; OF, old female; OM, old male. *Main effect of age (P < 0.05). Data are presented as means ± SEM, n = 5-8 per group.
Fusion and fission: Age significantly impacted fusion protein expression in cardiac muscles, while sex did not. MFN2 was over 2-fold higher in old cardiac muscles compared with young (Figure 4A, main effect of age P = 0.018). Similarly, OPA1-Short and OPA1-Long protein expression were 1.2- and 1.8-fold higher in the cardiac muscle from old rats compared with young, respectively (Figures 4C,E, main effects of age P = 0.049 and P < 0.001, respectively). This pattern persisted when MFN2, OPA1-Short and OPA1-Long were expressed relative to CIV expression (Figures 4B,D,F, main effect of age P < 0.03 for all). There were no significant differences in cardiac muscle DRP1 or FIS1 protein expression between young or old, male or female animals, indicating no significant effect of age (Figures 4G,I, respectively). Expressing DRP1 and FIS1 protein expression relative to CIV expression did not alter these results (Figures 4H,J, respectively).
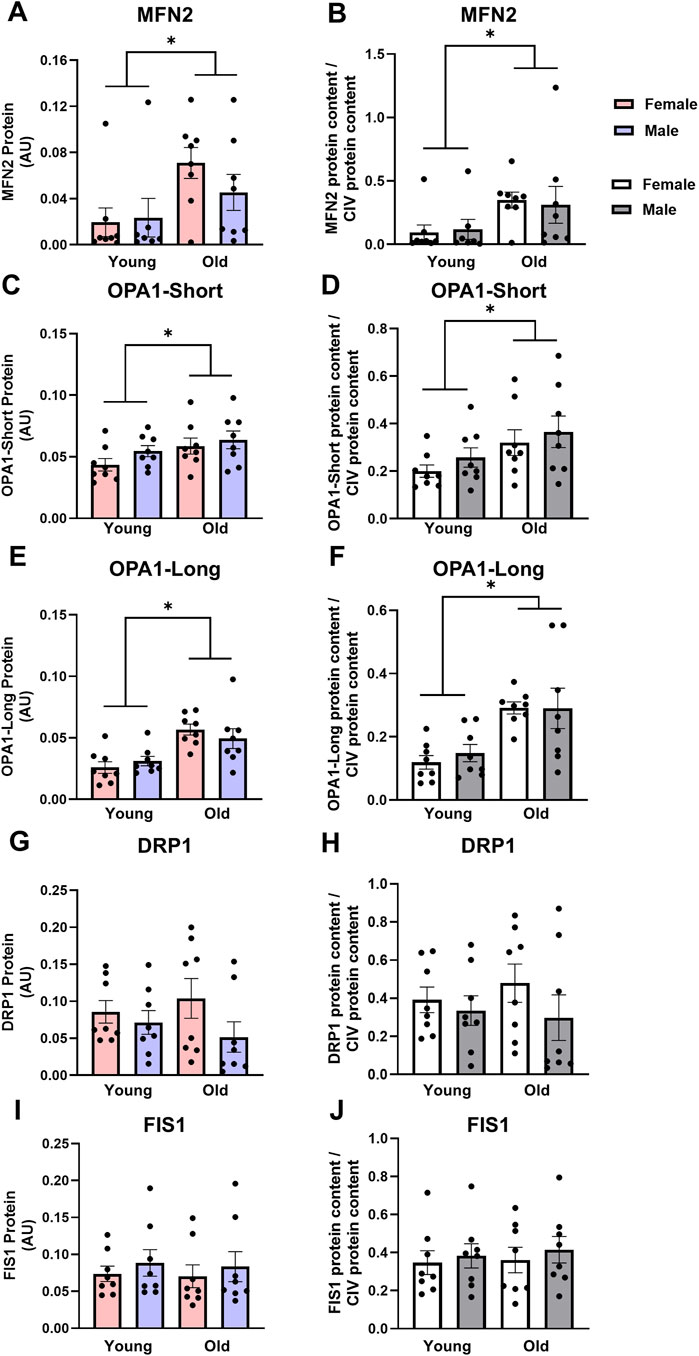
Figure 4. Mitofusin 2 [MFN2, (A,B)], optic atrophy 1-short [OPA1-Short, (C,D)] and OPA1-Long (E,F) protein expression are higher, while dynamin-related protein 1 [DRP1, (G,H)] and fission 1 [FIS1, (I,J)] protein expression are not different in old vs. young cardiac muscle. Data in (A,C,E,G,I) are normalized to total protein. Data in (B,D,F,H,J) are normalized to total protein and subsequently expressed relative to CIV expression. Legend = AU, arbitrary units; YF, young female; YM, young male; OF, old female; OM, old male. *Main effect of age (P < 0.05). Data are presented as means ± SEM, n = 7-8 per group.
4 Discussion
The data in this comprehensive analysis demonstrate that proteins regulating mitochondrial fusion, fission, mitophagy, and biogenesis are impacted by age in skeletal and cardiac muscle, with the effect of sex largely limited to differences in the expression of markers of fission (FIS1) and initiation of mitophagy (Pink1) in skeletal muscle. While patterns exist in how age affects mitochondrial dynamics in both skeletal and cardiac muscle, key differences may underlie how aging differentially affects these tissues. First, old skeletal and cardiac muscle both show markedly higher expression of proteins regulating mitophagy, indicating a need for a greater removal of damaged mitochondria in these tissues in older animals. Interestingly, in skeletal muscle, this increase in Pink1 protein expression is not accompanied by an increase in biogenesis proteins and is associated with lower mitochondrial content in old compared with young animals. In cardiac muscle from old animals, there is higher expression of biogenesis proteins in concert with higher expression of mitophagy proteins, and mitochondrial content is maintained. Second, both skeletal and cardiac muscle from old animals have higher expression of proteins regulating outer membrane fusion, while the expression of fission proteins isunchanged in cardiac muscle yet decreased in skeletal muscle in old animals. These indicate that aging has differential effects on proteins regulating mitochondrial quality control in skeletal vs. cardiac muscle. Finally, the present investigation shows GAPDH is an unreliable housekeeping/normalization protein for skeletal and cardiac muscle when age is a variable in the investigation. GAPDH expression in TA and cardiac muscle from old rats was 30%–40% higher than in young rats. Together, our results demonstrate alterations to expression of mitochondrial quality control proteins with age that differ by muscle type but are largely not different between males and females.
4.1 Skeletal muscle mitochondrial quality control
The skeletal muscle of old male and female rats have lower mitochondrial content, as measured by CIV protein expression in the TA muscles and citrate synthase activity in lateral gastrocnemius muscle, compared with their young counterparts. This is not surprising: studies in mice have report lower CIV protein expression, as well as citrate synthase activity, in old versus young counterparts (Koltai et al., 2012; Yeo et al., 2019), and some microscopy studies show mitochondrial volume density (mitochondria per um2) is lower in old compared to young skeletal muscle (Sebastian et al., 2016). Despite the lower mitochondrial content, the present study demonstrates PGC-1α protein expression is not different between young and old groups. This is somewhat surprising, as some studies indicate that consistent with lower mitochondrial content, PGC-1α protein expression is lower in old compared to young skeletal muscle (Chabi et al., 2008; Koltai et al., 2012), but differences among these and the present study could potentially explain different findings. Interestingly, in one study (Chabi et al., 2008) PGC-1α was lower in the soleus, but not the plantaris, of older rats, indicating these changes may be muscle- or fiber type-specific. Both investigations studied male rats that were ∼4–14 months older than the rats in the present study, suggesting that a decrease in PGC-1α expression may occur with more advanced age, but taken together with our data, this likely occurs after a decrease in mitochondrial content has already happened.
Consistent with both previous investigations and lower mitochondrial content in old skeletal muscle, mitophagy-related protein expression is higher in old rat skeletal muscle. Parkin protein expression, as well as Pink1 expression normalized to mitochondrial content (CIV), is higher in old rat TA. The accumulation of Pink1 on the OMM leads to the recruitment of Parkin and ultimately mitochondrial degradation, and higher Pink1 protein expression relative to mitochondrial content may indicate more Pink1 accumulation on the OMM due to mitochondrial dysfunction in the old compared with young TA. Indeed, Parkin expression is higher in old compared with young skeletal muscle, which would be consistent with increased mitochondrial degradation in the old animals, and is in agreement with previous studies in which Parkin expression is higher in old compared to young mouse quadriceps muscle (Triolo et al., 2022) and Parkin-independent mitophagy protein Bcl-2 19kD interacting protein (BNIP3) expression is higher in skeletal muscle of old compared with young mice (Triolo et al., 2022). Further, expression of Parkin and Parkin-independent mitophagy proteins, including BNIP3 and sequestosome 1 (p62), are higher in old male rat extensor digitorum longus and TA muscles (Carter et al., 2018; O'Leary et al., 2013). Taken together, these data indicate lower mitochondrial content in skeletal muscles of old animals is likely driven by an increase in mitophagy that is not matched with an increase in mitochondrial biogenesis.
Male and female rats did not differ in the majority of aforementioned proteins; however, Pink1 expression was higher in male compared with female skeletal muscle when normalized to CIV, which is consistent with a previous finding from Triolo et al. (2022) that autophagy protein Beclin-1 expression is higher in old male rats than in old females. Given mitophagy is mitochondrial-specific autophagy, it is not surprising that both autophagy- and mitophagy-related protein expression may be upregulated with age. It is unclear why these sex-specific differences are observed, but one hypothesis is that more mitophagy and autophagy in old male rat skeletal muscle might be a consequence of higher ROS production. A recent meta-analysis found ROS measures are higher in skeletal muscle from male subjects across a variety of experimental methods, and specifically, that older men have higher ROS production compared with older women (Junker et al., 2022). Elevated levels of ROS can activate both autophagy and mitophagy pathways (Barbieri and Sestili, 2012), therefore it is possible that higher markers of mitophagy in old male rats may be due, in part, to higher ROS.
Intriguingly, while expression of fission proteins DRP1 and FIS1 are not different between young male and young female skeletal muscle, DRP1 protein expression is lower in old rat TAs compared with young and there is a significant sex * age interaction effect for FIS1 protein expression: FIS1 is lower in old compared to young female skeletal muscle, but higher in old compared to young male skeletal muscle. These changes, however, appear to be driven by differences in mitochondrial content, as normalization to CIV protein expression abolished the significance of these effects. Our results differ from some previous studies that report DRP1 protein expression is the same in old compared with young skeletal muscle (Distefano et al., 2017; Joseph et al., 2012; Joseph et al., 2013; Leduc-Gaudet et al., 2015; Sebastian et al., 2016; Yeo et al., 2019). While the majority of these studies (Distefano et al., 2017; Joseph et al., 2012; Joseph et al., 2013; Sebastian et al., 2016; Yeo et al., 2019) used traditional normalization proteins as loading controls, Leduc-Gaudet et al. (2015) normalized protein expression to total protein via ponceau staining and found DRP1 protein expression is unaltered by age in male mice. Thus, we cannot rule out the possibility that the inclusion of female animals in the present study may drive the significant finding: DRP1 expression was ∼60% lower in old compared with young female skeletal muscle, while it was only ∼15% lower in old vs. young male skeletal muscle and may indicate important differences in the effect of age on proteins involved in mitochondrial fission. This finding of lower fission-associated protein DRP1 in old rats could seem discordant with the finding of higher expression mitophagy-associated proteins; however, it should be noted that fission and mitophagy are not inextricably linked. For example, when mitochondria experience stress, they can undergo mitochondrial remodeling, or rounding, (Glancy et al., 2017; Nemani et al., 2018; Tan et al., 2011), which initiates mitophagy independent of fission and not reliant on DRP1 (Anzell et al., 2021). While this remains a possibility, the precise mechanisms for this finding require future study.
The literature is divided on the effects of age on FIS1 expression, with studies reporting lower (Capitanio et al., 2016; Joseph et al., 2013; Sebastian et al., 2016), higher (Faitg et al., 2019; Yeo et al., 2019), or similar (Distefano et al., 2017; Joseph et al., 2012) FIS1 protein expression in old compared with young skeletal muscle. It is unclear why there are such varied results but methodological differences (e.g., animal age, muscle group, protein loading control) along with varied species used (mouse, rat, or human), may account for some of the discrepancies. Indeed, while we found the effect of age on FIS1 protein expression is sex-dependent, these data are no longer significant if normalized to CIV. These conflicting results highlight the need for future studies into sex-specific changes to mitochondrial related proteins with age.
In the present study, MFN2 protein expression is higher in old male and female TA compared with young animals, but neither OPA1-Short nor OPA1-Long was impacted by age or sex. This could possibly indicate greater OMM fusion without a concurrent increase in IMM fusion (Youle and van der Bliek, 2012). Indeed, OPA1 knockdown in young male mice produces enlarged mitochondria with disrupted cristae, demonstrating OMM are able to fuse normally without fully-functioning OPA1 (Caffin et al., 2013). Since fusion is normally regarded as a positive adaptation, higher MFN2 protein expression in aged skeletal muscle may be counterintuitive; but it has also been suggested that increased fusion with age may be a compensatory mechanism to combat mitochondrial dysfunction in skeletal muscle. When mitochondrial membrane potential is disrupted, mitochondria are selected for degradation via mitophagy. In some cases, these dysfunctional mitochondria can fuse with neighboring functional mitochondria as an attempt to rescue membrane potential (Youle and van der Bliek, 2012). The rescuing of mitochondria via fusion can also reduce the proportion of dysfunctional mitochondria (Youle and van der Bliek, 2012). Similarly, fusion allows for the mixing of matrix components, such as mitochondrial DNA (mtDNA), which is thought to reduce the likelihood of mutated mtDNA from reaching pathogenic threshold (Chen et al., 2010; Tezze et al., 2017). Diluting mutated mtDNA with normal mtDNA is important for maintaining mitochondrial function as mtDNA encodes for several subunits of the electron transport chain and damage to mtDNA can impact the production and in turn function of these proteins (Chen et al., 2010; Tezze et al., 2017). Thus, OMM fusion could be a compensatory mechanism to maintain membrane potential and dilute mutant mtDNA in skeletal muscle. Altogether, these findings suggest a pro-fusion shift in older skeletal muscle, which is consistent with previous studies showing higher expression of fusion relative to fission proteins in older animals (Capitanio et al., 2016; Joseph et al., 2013; Leduc-Gaudet et al., 2015). While the driver of an increase in fusion protein expression remains unclear, greater fusion in old skeletal muscle may indicate a larger need for compensatory fusion due to mitochondrial dysfunction associated with aging.
A comprehensive analysis utilizing 3D microscopy characterized mitochondrial morphology in young and old human skeletal muscle (Scudese et al., 2025). While mitochondrial 3D area and perimeter do not differ between young and old, mitochondria from old skeletal muscle have a greater volume, are more spherical, and are less complex. These data are consistent with our findings of decreased DRP1 expression in old skeletal muscle: muscle-specific DRP1 knockout results in larger, but fewer, mitochondria (Favaro et al., 2019). Intriguingly, this may relate to altered mitophagy, as rounder mitochondria have a lower surface area to volume ratio for mitophagy to occur.
4.2 Cardiac muscle mitochondrial quality control
Mitochondrial content does not differ between young and old rat hearts in the present study, and is not surprising considering cardiac tissue relies on constant ATP production and losses in mitochondrial content could have severe consequences (Chaudhary et al., 2011). Similar to our findings, others report no changes in CIV protein expression or activity in old compared with young cardiac muscle (Ljubicic et al., 2010; Zhao et al., 2014). To account for any numerical, but non-statistical, differences in mitochondrial content, we also expressed mitochondrial quality control proteins in the heart relative to CIV protein. This secondary analysis did not impact any of the results, signifying the differences in mitochondrial quality control proteins were not attributable to differences in mitochondrial content.
Mitochondrial content was likely maintained in old cardiac tissue by higher mitochondrial biogenesis to match the higher mitophagy in old animals. PGC-1α protein expression is higher in old male and female rat hearts compared with young rats, indicating an upregulation of mitochondrial biogenesis. It has previously been reported that PGC-1α gene expression is higher in aged cardiac muscle, possibly as a compensatory mechanism to combat mitochondrial dysfunction (Dai and Rabinovitch, 2009). The production of new mitochondria likely preserves mitochondrial content in the presence of potentially greater mitochondrial dysfunction and subsequent degradation. Mitochondrial dysfunction in aged cardiac tissue stemming from mutated mtDNA, increased ROS production, and surplus calcium uptake can lead to poor cardiac function with age (Chaudhary et al., 2011; Dai and Rabinovitch, 2009). Mitophagy serves a crucial role in minimizing the negative effects of impaired mitochondrial function. Fittingly, the present study found Parkin expression is higher in old rat hearts compared with young. Limited investigations on mitophagy in aged cardiac muscle, demonstrate higher Parkin and p62 protein content, as well as higher autophagy proteins Beclin-2 and LC3-II in aged mice hearts (Boyle et al., 2011; Liang et al., 2020; Zhou et al., 2017). Together, this indicates that expression of proteins involde in mitochondria removal pathways is upregulated in aged cardiac muscle, but in the face of upregulated markers of biogenesis, this likely results in unchanged mitochondrial content.
Relatively few studies have assessed the effects of age on mitochondrial quality control in cardiac muscle; however, abnormal mitochondrial quality control is implicated in multiple cardiovascular adverse events including myocardial ischemia and reperfusion injury, atherosclerosis, and cardiac hypertrophy (Atici et al., 2023). This is likely due to the regulatory role mitochondrial structure imparts on function. Indeed, knockout models have demonstrated fusion, fission, and mitophagy proteins (MFN1, MFN2, OPA1, DRP1, Parkin) are necessary for appropriate mitochondrial structure as well as function (Papanicolaou et al., 2012; Song et al., 2017; Song et al., 2015). We found higher protein expression of all markers of fusion (MFN2, OPA1-Short, and OPA1-Long) in old compared with young cardiac muscle, though expression of fission proteins did not differ as a function of age. This shift in protein expression consistent with a pro-fusion state supports previous findings of enlarged mitochondria in old mouse hearts (Coleman et al., 1988), and 3D electron microscopy images reveal structural changes, but a maintenance of mitochondrial volume in cardiac muscle from old animals (Vue et al., 2023a). These findings have been corroborated by 3-D electron microscopy analysis (Vue et al., 2023b). Liang et al. (2020) isolated mitochondria from aged mouse hearts and separated them based on size by differential centrifugation. Large mitochondria had significantly lower Parkin and LC3-II protein expression compared with small mitochondrial from aged hearts (Liang et al., 2020). When both mitochondria were pooled together, Parkin and p62 were both elevated in the old hearts (Liang et al., 2020). Together, this suggests smaller mitochondria may be targeted for degradation leading to the accumulation of enlarged mitochondria, despite an apparent increase in the initiation of mitophagy. Such a pro-fusion shift in aged hearts may be an adaptive response to inadequate autophagy as an attempt to compensate for mitochondrial damage and increased ROS production (Sohal et al., 1994).
4.3 GAPDH
GAPDH is often used as a loading control in studies of protein expression in muscle. Interestingly, GAPDH expression was 30%–40% higher in both TA and cardiac muscle from old compared with young rats in this study. At minimum, this suggests that GAPDH should not be used as a loading control or normalization protein in studies including young and old TA and cardiac muscle. The complete physiological significance of higher GAPDH expression in older rat skeletal muscle is not currently clear, but this could signify an increased glycolytic capacity in muscle, potentially favoring anaerobic glycolysis or glycolytic pathways leading to oxidation of carbohydrate. The higher GAPDH protein expression in old heart muscle is consistent with extensive data demonstrating older hearts experience impaired metabolic flexibility, characterized by a reduced capacity for fatty acid oxidation and an increased reliance on glucose metabolism (Abu-Erreish et al., 1977; Kates et al., 2003; Lesnefsky et al., 2016; McMillin et al., 1993). Some studies report lower GAPDH expression in old compared to young skeletal muscle (Vigelso et al., 2015; Wyckelsma et al., 2016), this could be fiber type-dependent (Lowe et al., 2000). At the same time, GAPDH has roles in other cellular functions and pathways, so the higher expression in old muscle could represent effects ranging from autophagy to DNA repair, apoptosis and cellular dysfunction (Tristan et al., 2011).
The strengths of this study include the comprehensive nature of including multiple markers of fusion, fission, mitophagy and biogenesis, as well as the assessment of these markers in both cardiac and skeletal muscle of young and old, male and female rats to determine effects of age and sex. While both skeletal and cardiac muscle were assayed, we acknowledge that cardiac muscle was not assessed in a chamber- or region-specific manner, which represents a limitation of the current work. In addition, activation (i.e., phosphorylation) status of proteins was not assessed in this study. In the absence of such data, we are unable to make definitive conclusions regarding the regulation of the assessed proteins and our interpretation is limited to their expression. Finally, we acknowledge that tibial length in the young rats was lower than that in the old rats, indicating that young rats may not have reached full maturity. Nonetheless, data from these rats are likely representative of young, adult rats.
4.4 Conclusion
Collectively, our results indicate the expression of proteins associated with fission and mitophagy is higher in aged skeletal and cardiac muscle, while markers of fission are reduced in skeletal muscle of old rats. Similarly, we find that sex interacts with the effect of age on select fission and mitophagy proteins in skeletal, but not cardiac, muscle. These differences in protein expression may signal greater fusion and mitophagy, which may serve as a compensatory mechanism to mitigate mitochondrial dysfunction with age by restoring membrane potential via fusion and degrading unsalvageable mitochondria via mitophagy. In old skeletal muscle, evidence of higher mitophagy protein expression without a subsequent increase in the expression of biogenesis proteins occurs in tandem with lower mitochondrial content; however, in cardiac muscle the evidence of higher expression of proteins involved in initiation of mitophagy appears to be offset by protein markers of biogenesis, maintaining mitochondrial content. These findings underscore key similarities and differences in mitochondrial quality control between young and old skeletal and cardiac muscle that may contribute to differential regulation of mitochondrial morphology and function in different types of muscle with aging.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by University of Maryland Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CS-S: Writing – review and editing, Investigation, Writing – original draft, Conceptualization. OO: Investigation, Writing – review and editing. MC: Writing – review and editing, Investigation. AH: Investigation, Writing – review and editing. YL: Writing – review and editing, Investigation. SP: Investigation, Funding acquisition, Resources, Writing – original draft, Writing – review and editing, Supervision, Conceptualization. SK-G: Supervision, Writing – original draft, Investigation, Writing – review and editing, Resources, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Institute of Aging at the National Institutes of Health (R21AG064571 to SJP and R25AG045063 to James M. Hagberg) and the American Heart Association (grant number 16SDG30770015 to SKG).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fragi.2025.1606110/full#supplementary-material
References
Abu-Erreish, G. M., Neely, J. R., Whitmer, J. T., Whitman, V., and Sanadi, D. R. (1977). Fatty acid oxidation by isolated perfused working hearts of aged rats. Am. J. Physiol. 232 (3), E258–E262. doi:10.1152/ajpendo.1977.232.3.E258
Anzell, A. R., Fogo, G. M., Gurm, Z., Raghunayakula, S., Wider, J. M., Maheras, K. J., et al. (2021). Mitochondrial fission and mitophagy are independent mechanisms regulating ischemia/reperfusion injury in primary neurons. Cell Death and Dis. 12 (5), 475. doi:10.1038/s41419-021-03752-2
Atici, A. E., Crother, T. R., and Noval Rivas, M. (2023). Mitochondrial quality control in health and cardiovascular diseases. Front. Cell Dev. Biol. 11, 1290046. doi:10.3389/fcell.2023.1290046
Balan, E., Schwalm, C., Naslain, D., Nielens, H., Francaux, M., and Deldicque, L. (2019). Regular endurance exercise promotes fission, mitophagy, and oxidative phosphorylation in human skeletal muscle independently of age. Front. Physiol. 10, 1088. doi:10.3389/fphys.2019.01088
Barbieri, E., and Sestili, P. (2012). Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 982794. doi:10.1155/2012/982794
Baricault, L., Segui, B., Guegand, L., Olichon, A., Valette, A., Larminat, F., et al. (2007). OPA1 cleavage depends on decreased mitochondrial ATP level and bivalent metals. Exp. Cell Res. 313 (17), 3800–3808. doi:10.1016/j.yexcr.2007.08.008
Boyle, A. J., Shih, H., Hwang, J., Ye, J., Lee, B., Zhang, Y., et al. (2011). Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Exp. Gerontol. 46 (7), 549–559. doi:10.1016/j.exger.2011.02.010
Caffin, F., Prola, A., Piquereau, J., Novotova, M., David, D. J., Garnier, A., et al. (2013). Altered skeletal muscle mitochondrial biogenesis but improved endurance capacity in trained OPA1-deficient mice. J. Physiol. 591 (23), 6017–6037. doi:10.1113/jphysiol.2013.263079
Capitanio, D., Vasso, M., De Palma, S., Fania, C., Torretta, E., Cammarata, F. P., et al. (2016). Specific protein changes contribute to the differential muscle mass loss during ageing. Proteomics 16 (4), 645–656. doi:10.1002/pmic.201500395
Carter, H. N., Kim, Y., Erlich, A. T., Zarrin-Khat, D., and Hood, D. A. (2018). Autophagy and mitophagy flux in young and aged skeletal muscle following chronic contractile activity. J. Physiol. 596 (16), 3567–3584. doi:10.1113/JP275998
Chabi, B., Ljubicic, V., Menzies, K. J., Huang, J. H., Saleem, A., and Hood, D. A. (2008). Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 7 (1), 2–12. doi:10.1111/j.1474-9726.2007.00347.x
Chaudhary, K. R., El-Sikhry, H., and Seubert, J. M. (2011). Mitochondria and the aging heart. J. Geriatr. Cardiol. 8 (3), 159–167. doi:10.3724/SP.J.1263.2011.00159
Chen, H., Vermulst, M., Wang, Y. E., Chomyn, A., Prolla, T. A., McCaffery, J. M., et al. (2010). Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141 (2), 280–289. doi:10.1016/j.cell.2010.02.026
Coleman, R., Weiss, A., Finkelbrand, S., and Silbermann, M. (1988). Age and exercise-related changes in myocardial mitochondria in mice. Acta histochem. 83 (1), 81–90. doi:10.1016/S0065-1281(88)80075-8
Crane, J. D., Devries, M. C., Safdar, A., Hamadeh, M. J., and Tarnopolsky, M. A. (2010). The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J. Gerontol. A Biol. Sci. Med. Sci. 65 (2), 119–128. doi:10.1093/gerona/glp179
Dai, D. F., and Rabinovitch, P. S. (2009). Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc Med. 19 (7), 213–220. doi:10.1016/j.tcm.2009.12.004
Distefano, G., Standley, R. A., Dube, J. J., Carnero, E. A., Ritov, V. B., Stefanovic-Racic, M., et al. (2017). Chronological age does not influence ex-vivo mitochondrial respiration and quality control in skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 72 (4), 535–542. doi:10.1093/gerona/glw102
Dorn, G. W. (2013). Mitochondrial dynamics in heart disease. Biochim. Biophys. Acta 1833 (1), 233–241. doi:10.1016/j.bbamcr.2012.03.008
Ehses, S., Raschke, I., Mancuso, G., Bernacchia, A., Geimer, S., Tondera, D., et al. (2009). Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J. Cell Biol. 187 (7), 1023–1036. doi:10.1083/jcb.200906084
Faitg, J., Leduc-Gaudet, J. P., Reynaud, O., Ferland, G., Gaudreau, P., and Gouspillou, G. (2019). Effects of aging and caloric restriction on fiber type composition, mitochondrial morphology and dynamics in rat oxidative and glycolytic muscles. Front. Physiol. 10, 420. doi:10.3389/fphys.2019.00420
Favaro, G., Romanello, V., Varanita, T., Andrea Desbats, M., Morbidoni, V., Tezze, C., et al. (2019). DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nat. Commun. 10 (1), 2576. doi:10.1038/s41467-019-10226-9
Glancy, B., Hartnell, L. M., Combs, C. A., Femnou, A., Sun, J., Murphy, E., et al. (2017). Power grid protection of the muscle mitochondrial reticulum. Cell Rep. 19 (3), 487–496. doi:10.1016/j.celrep.2017.03.063
Gouspillou, G., Sgarioto, N., Kapchinsky, S., Purves-Smith, F., Norris, B., Pion, C. H., et al. (2014). Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J. 28 (4), 1621–1633. doi:10.1096/fj.13-242750
Halling, J. F., Jessen, H., Nohr-Meldgaard, J., Buch, B. T., Christensen, N. M., Gudiksen, A., et al. (2019). PGC-1α regulates mitochondrial properties beyond biogenesis with aging and exercise training. Am. J. Physiol. Endocrinol. Metab. 317 (3), E513–E525. doi:10.1152/ajpendo.00059.2019
Head, B., Griparic, L., Amiri, M., Gandre-Babbe, S., and van der Bliek, A. M. (2009). Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J. Cell Biol. 187 (7), 959–966. doi:10.1083/jcb.200906083
Herbst, A., Prior, S. J., Lee, C. C., Aiken, J. M., McKenzie, D., Hoang, A., et al. (2021). Skeletal muscle mitochondrial DNA copy number and mitochondrial DNA deletion mutation frequency as predictors of physical performance in older men and women. GeroScience 43 (3), 1253–1264. doi:10.1007/s11357-021-00351-z
Joseph, A. M., Adhihetty, P. J., Buford, T. W., Wohlgemuth, S. E., Lees, H. A., Nguyen, L. M., et al. (2012). The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 11 (5), 801–809. doi:10.1111/j.1474-9726.2012.00844.x
Joseph, A. M., Adhihetty, P. J., Wawrzyniak, N. R., Wohlgemuth, S. E., Picca, A., Kujoth, G. C., et al. (2013). Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging. PLoS One 8 (7), e69327. doi:10.1371/journal.pone.0069327
Junker, A., Wang, J., Gouspillou, G., Ehinger, J. K., Elmer, E., Sjovall, F., et al. (2022). Human studies of mitochondrial biology demonstrate an overall lack of binary sex differences: a multivariate meta-analysis. FASEB J. 36 (2), e22146. doi:10.1096/fj.202101628R
Kates, A. M., Herrero, P., Dence, C., Soto, P., Srinivasan, M., Delano, D. G., et al. (2003). Impact of aging on substrate metabolism by the human heart. J. Am. Coll. Cardiol. 41 (2), 293–299. doi:10.1016/s0735-1097(02)02714-6
Koltai, E., Hart, N., Taylor, A. W., Goto, S., Ngo, J. K., Davies, K. J., et al. (2012). Age-associated declines in mitochondrial biogenesis and protein quality control factors are minimized by exercise training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303 (2), R127–R134. doi:10.1152/ajpregu.00337.2011
Konopka, A. R., Suer, M. K., Wolff, C. A., and Harber, M. P. (2014). Markers of human skeletal muscle mitochondrial biogenesis and quality control: effects of age and aerobic exercise training. J. Gerontol. A Biol. Sci. Med. Sci. 69 (4), 371–378. doi:10.1093/gerona/glt107
Kuzmiak, S., Glancy, B., Sweazea, K. L., and Willis, W. T. (2012). Mitochondrial function in sparrow pectoralis muscle. J. Exp. Biol. 215 (Pt 12), 2039–2050. doi:10.1242/jeb.065094
Leduc-Gaudet, J. P., Picard, M., St-Jean Pelletier, F., Sgarioto, N., Auger, M. J., Vallee, J., et al. (2015). Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 6 (20), 17923–17937. doi:10.18632/oncotarget.4235
Lesnefsky, E. J., Chen, Q., and Hoppel, C. L. (2016). Mitochondrial metabolism in aging heart. Circ. Res. 118 (10), 1593–1611. doi:10.1161/CIRCRESAHA.116.307505
Liang, W., Moyzis, A. G., Lampert, M. A., Diao, R. Y., Najor, R. H., and Gustafsson, A. B. (2020). Aging is associated with a decline in Atg9b-mediated autophagosome formation and appearance of enlarged mitochondria in the heart. Aging Cell 19 (8), e13187. doi:10.1111/acel.13187
Ljubicic, V., Menzies, K. J., and Hood, D. A. (2010). Mitochondrial dysfunction is associated with a pro-apoptotic cellular environment in senescent cardiac muscle. Mech. Ageing Dev. 131 (2), 79–88. doi:10.1016/j.mad.2009.12.004
Loson, O. C., Song, Z., Chen, H., and Chan, D. C. (2013). Fis1, mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell 24 (5), 659–667. doi:10.1091/mbc.E12-10-0721
Lowe, D. A., Degens, H., Chen, K. D., and Alway, S. E. (2000). Glyceraldehyde-3-phosphate dehydrogenase varies with age in glycolytic muscles of rats. J. Gerontol. A Biol. Sci. Med. Sci. 55 (3), B160–B164. doi:10.1093/gerona/55.3.b160
Marzetti, E., Calvani, R., Coelho-Junior, H. J., Landi, F., and Picca, A. (2024). Mitochondrial quantity and quality in age-related sarcopenia. Int. J. Mol. Sci. 25 (4), 2052. doi:10.3390/ijms25042052
McMillin, J. B., Taffet, G. E., Taegtmeyer, H., Hudson, E. K., and Tate, C. A. (1993). Mitochondrial metabolism and substrate competition in the aging Fischer rat heart. Cardiovasc Res. 27 (12), 2222–2228. doi:10.1093/cvr/27.12.2222
Nemani, N., Carvalho, E., Tomar, D., Dong, Z., Ketschek, A., Breves, S. L., et al. (2018). MIRO-1 determines mitochondrial shape transition upon GPCR activation and Ca2+ stress. Cell Rep. 23 (4), 1005–1019. doi:10.1016/j.celrep.2018.03.098
O'Leary, M. F., Vainshtein, A., Iqbal, S., Ostojic, O., and Hood, D. A. (2013). Adaptive plasticity of autophagic proteins to denervation in aging skeletal muscle. Am. J. Physiol. Cell Physiol. 304 (5), C422–C430. doi:10.1152/ajpcell.00240.2012
Papanicolaou, K. N., Ngoh, G. A., Dabkowski, E. R., O'Connell, K. A., Ribeiro, R. F., Stanley, W. C., et al. (2012). Cardiomyocyte deletion of mitofusin-1 leads to mitochondrial fragmentation and improves tolerance to ROS-induced mitochondrial dysfunction and cell death. Am. J. Physiol. Heart Circ. Physiol. 302 (1), H167–H179. doi:10.1152/ajpheart.00833.2011
Scorrano, L. (2013). Keeping mitochondria in shape: a matter of life and death. Eur. J. Clin. Invest 43 (8), 886–893. doi:10.1111/eci.12135
Scudese, E., Marshall, A. G., Vue, Z., Exil, V., Rodriguez, B. I., Demirci, M., et al. (2025). 3D mitochondrial structure in aging human skeletal muscle: insights into MFN-2-mediated changes. Aging Cell, e70054. doi:10.1111/acel.70054
Sebastian, D., Sorianello, E., Segales, J., Irazoki, A., Ruiz-Bonilla, V., Sala, D., et al. (2016). Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 35 (15), 1677–1693. doi:10.15252/embj.201593084
Short, K. R., Bigelow, M. L., Kahl, J., Singh, R., Coenen-Schimke, J., Raghavakaimal, S., et al. (2005). Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. U. S. A. 102 (15), 5618–5623. doi:10.1073/pnas.0501559102
Sohal, R. S., Ku, H. H., Agarwal, S., Forster, M. J., and Lal, H. (1994). Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 74 (1-2), 121–133. doi:10.1016/0047-6374(94)90104-x
Song, M., Franco, A., Fleischer, J. A., Zhang, L., and Dorn, G. W. (2017). Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell Metab. 26 (6), 872–883.e5. doi:10.1016/j.cmet.2017.09.023
Song, M., Mihara, K., Chen, Y., Scorrano, L., and Dorn, G. W. (2015). Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 21 (2), 273–286. doi:10.1016/j.cmet.2014.12.011
Tan, A. R., Cai, A. Y., Deheshi, S., and Rintoul, G. L. (2011). Elevated intracellular calcium causes distinct mitochondrial remodelling and calcineurin-dependent fission in astrocytes. Cell Calcium 49 (2), 108–114. doi:10.1016/j.ceca.2010.12.002
Tevald, M. A., Foulis, S. A., and Kent, J. A. (2014). Effect of age on in vivo oxidative capacity in two locomotory muscles of the leg. Age 36 (5), 9713. doi:10.1007/s11357-014-9713-5
Tezze, C., Romanello, V., Desbats, M. A., Fadini, G. P., Albiero, M., Favaro, G., et al. (2017). Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab. 25 (6), 1374–1389.e6. doi:10.1016/j.cmet.2017.04.021
Triolo, M., Oliveira, A. N., Kumari, R., and Hood, D. A. (2022). The influence of age, sex, and exercise on autophagy, mitophagy, and lysosome biogenesis in skeletal muscle. Skelet. Muscle 12 (1), 13. doi:10.1186/s13395-022-00296-7
Tristan, C., Shahani, N., Sedlak, T. W., and Sawa, A. (2011). The diverse functions of GAPDH: views from different subcellular compartments. Cell Signal 23 (2), 317–323. doi:10.1016/j.cellsig.2010.08.003
Twig, G., Elorza, A., Molina, A. J., Mohamed, H., Wikstrom, J. D., Walzer, G., et al. (2008). Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27 (2), 433–446. doi:10.1038/sj.emboj.7601963
Vigelso, A., Dybboe, R., Hansen, C. N., Dela, F., Helge, J. W., and Guadalupe Grau, A. (2015). GAPDH and beta-actin protein decreases with aging, making Stain-Free technology a superior loading control in Western blotting of human skeletal muscle. J. Appl. Physiol. (1985) 118 (3), 386–394. doi:10.1152/japplphysiol.00840.2014
Vue, Z., Garza-Lopez, E., Neikirk, K., Katti, P., Vang, L., Beasley, H., et al. (2023a). 3D reconstruction of murine mitochondria reveals changes in structure during aging linked to the MICOS complex. Aging Cell 22 (12), e14009. doi:10.1111/acel.14009
Vue, Z., Neikirk, K., Vang, L., Garza-Lopez, E., Christensen, T. A., Shao, J., et al. (2023b). Three-dimensional mitochondria reconstructions of murine cardiac muscle changes in size across aging. Am. J. Physiology-Heart Circulatory Physiology 325 (5), H965–H982. doi:10.1152/ajpheart.00202.2023
Wyckelsma, V. L., McKenna, M. J., Levinger, I., Petersen, A. C., Lamboley, C. R., and Murphy, R. M. (2016). Cell specific differences in the protein abundances of GAPDH and Na(+),K(+)-ATPase in skeletal muscle from aged individuals. Exp. Gerontol. 75, 8–15. doi:10.1016/j.exger.2015.12.010
Yeo, D., Kang, C., Gomez-Cabrera, M. C., Vina, J., and Ji, L. L. (2019). Intensified mitophagy in skeletal muscle with aging is downregulated by PGC-1alpha overexpression in vivo. Free Radic. Biol. Med. 130, 361–368. doi:10.1016/j.freeradbiomed.2018.10.456
Youle, R. J., and van der Bliek, A. M. (2012). Mitochondrial fission, fusion, and stress. Science 337 (6098), 1062–1065. doi:10.1126/science.1219855
Zhao, L., Zou, X., Feng, Z., Luo, C., Liu, J., Li, H., et al. (2014). Evidence for association of mitochondrial metabolism alteration with lipid accumulation in aging rats. Exp. Gerontol. 56, 3–12. doi:10.1016/j.exger.2014.02.001
Keywords: fusion, fission, mitophagy, muscle health, biological sex
Citation: Springer-Sapp CB, Ogbara O, Canellas Da Silva M, Henderson A, Liu Y, Prior SJ and Kuzmiak-Glancy S (2025) Age and sex-specific changes in mitochondrial quality control in skeletal and cardiac muscle. Front. Aging 6:1606110. doi: 10.3389/fragi.2025.1606110
Received: 04 April 2025; Accepted: 17 June 2025;
Published: 25 June 2025.
Edited by:
Christa J. Nehs, Harvard Medical School, United StatesReviewed by:
Kimberly J. Dunham-Snary, Queen’s University, CanadaNeoma Tove Boardman, UiT The Arctic University of Norway, Norway
Copyright © 2025 Springer-Sapp, Ogbara, Canellas Da Silva, Henderson, Liu, Prior and Kuzmiak-Glancy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah Kuzmiak-Glancy, c2dsYW5jeUB1bWQuZWR1
 Catherine B. Springer-Sapp1
Catherine B. Springer-Sapp1 Steven J. Prior
Steven J. Prior