- 1School of Medicine, Texas Tech University Health Sciences Center, Lubbock, TX, United States
- 2Department of Orthopaedic Surgery, Texas Tech University Health Sciences Center, Lubbock, TX, United States
- 3Department of Orthopaedic Surgery, The Johns Hopkins Hospital, Baltimore, MD, United States
- 4Department of Health Sciences, College of Health Sciences, Rush University, Chicago, IL, United States
- 5Department of Hand and Microvascular Surgery, University Medical Center, Lubbock, TX, United States
Introduction: The rapid expansion of artificial intelligence (AI) in medicine has led to its increasing integration into upper extremity (UE) orthopedics. The purpose of this systematic review is to investigate the current landscape and impact of AI in the field of UE surgery.
Methods: Following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, a systematic search of PubMed was conducted to identify studies incorporating AI in UE surgery. Review articles, letters to the editor, and studies unrelated to AI applications in UE surgery were excluded.
Results: After applying inclusion/exclusion criteria, 118 articles were included. The publication years ranged from 2009 to 2024, with a median and mode of 2022 and 2023, respectively. The studies were categorized into six main applications: automated image analysis (36%), surgical outcome prediction (20%), measurement tools (14%), prosthetic limb applications (14%), intraoperative aid (10%), and clinical decision support tools (6%).
Discussion: AI is predominantly utilized in image analysis, including radiograph and MRI interpretation, often matching or surpassing clinician accuracy and efficiency. Additionally, AI-powered tools enhance the measurement of range of motion, critical shoulder angles, grip strength, and hand posture, aiding in patient assessment and treatment planning. Surgeons are increasingly leveraging AI for predictive analytics to estimate surgical outcomes, such as infection risk, postoperative function, and procedural costs. As AI continues to evolve, its role in UE surgery is expected to expand, improving decision-making, precision, and patient care.
Introduction
Artificial Intelligence (AI) refers to computational algorithms that model human intelligence in learning, decision-making, and problem-solving. In recent years, the application of AI in healthcare has exponentially increased, driven by advancements in machine learning models, increased computing power, and improved data availability. The development of sophisticated AI systems, such as ChatGPT and deep learning algorithms, has enhanced accessibility for healthcare professionals, patients, and researchers. Prior studies have shown the diverse applications of AI in medicine, including image recognition for fracture detection and classification, preoperative risk assessment, clinical decision support, and predictive modeling of treatment outcomes (Myers et al., 2020; Langerhuizen et al., 2019).
Due to the rapid expansion of AI implementation in medicine in recent years, AI is being used in more areas and more accurately than ever before, including in upper extremity (UE) orthopedics. A 2019 systematic review of 12 studies on AI-driven fracture detection in general orthopedics highlighted a promising performance with near-perfect prediction in five articles (AUC 0.95–1.0) (Langerhuizen et al., 2019). This near-perfect accuracy provided some insight into the capabilities of AI in advancing modern medicine and aiding clinicians in their work, especially as updated AI models continue to rise.
A scoping review by Keller et al. (2023) examined AI applications in hand surgery before April 2021, revealing limited utilization compared to other medical specialties). Given the rapid advancements since then, this systematic review aims to comprehensively assess the current landscape of AI in UE surgery. By analyzing the existing body of evidence, we seek to elucidate the potential clinical impacts of AI technologies and identify key areas for future research and development within this important field of UE orthopedics.
Materials and methods
Study search strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic and Meta-Analysis (PRISMA) (Tricco et al., 2018) guidelines, ensuring methodological transparency and accuracy. A comprehensive literature search was performed using the MEDLINE/PubMed database. The search focused on identifying relevant literature pertaining to the use of AI in UE surgery. The search strategy was designed to capture all relevant studies published between November 2009 and April 2024. The electronic search strategy used was: (Artificial Intelligence OR Machine Learning OR Deep Learning) AND (Diagnosis OR Detection) AND (Hand Surgery OR Arm Surgery OR Elbow Surgery OR Shoulder Surgery).
Inclusion and exclusion criteria
Studies were included if they evaluated AI applications in UE surgery and were original research articles. Excluded studies included those unrelated to AI in UE surgery, review articles, letters to the editor, conference abstracts, and articles not published in English.
Selection process
All database search results were imported into Rayyan, a systematic review management tool, where duplicates were automatically removed using a trained AI system, as described by Adu et al. (2024). Two independent reviewers then performed an initial screening of titles and abstracts to exclude studies that did not meet the eligibility criteria. Subsequently, full-text articles of potentially relevant studies were then reviewed independently by both reviewers. At any point, any disagreements regarding study inclusion were resolved through discussion, with the corresponding author serving as the final adjudicator in cases of unresolved discrepancies. Included studies were then sorted into categories based on the perceived primary focus of the paper. When study overlap between two categories occurred, discussion took place, and the studies were placed into their perceived primary category.
Results
The initial literature search generated 1,097 unique articles, of which 118 met the inclusion criteria after abstract review and application of the exclusion criteria. No sources were included from grey literature or non-PubMed sources.
These studies were categorized into six primary areas of AI implementation in upper extremity (UE) surgery: automated image analysis (36%), surgical outcome prediction (20%), measurement tools (14%), prosthetic limb applications (14%), intraoperative assistance (10%), and clinical decision support tools (6%) (Figures 1, 2).
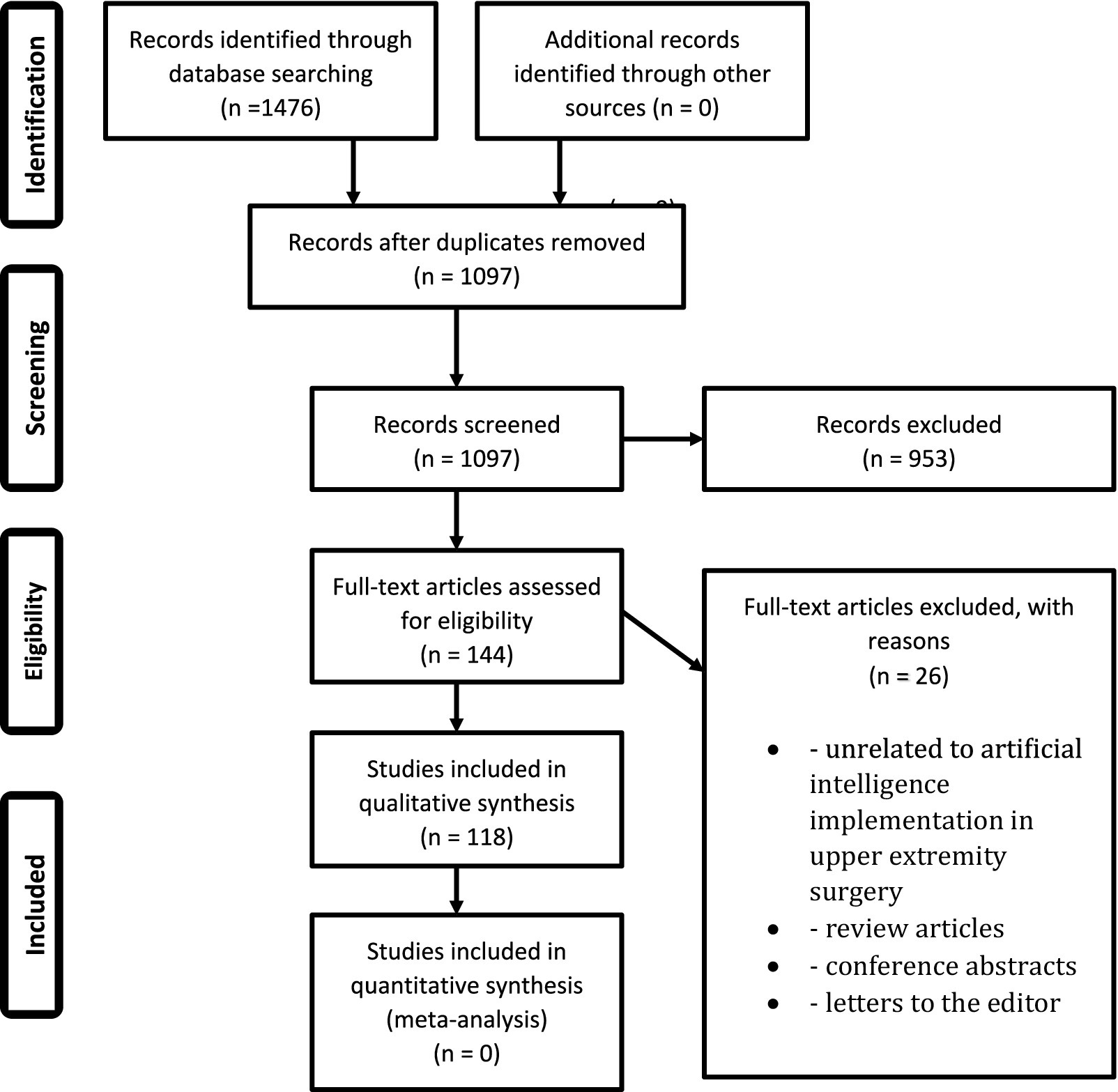
Figure 1. PRISMA flowchart. Represents the preferred reporting items for systematic and meta-analysis (PRISMA) flowchart for identification, screening, and eventual inclusion of articles in this study.
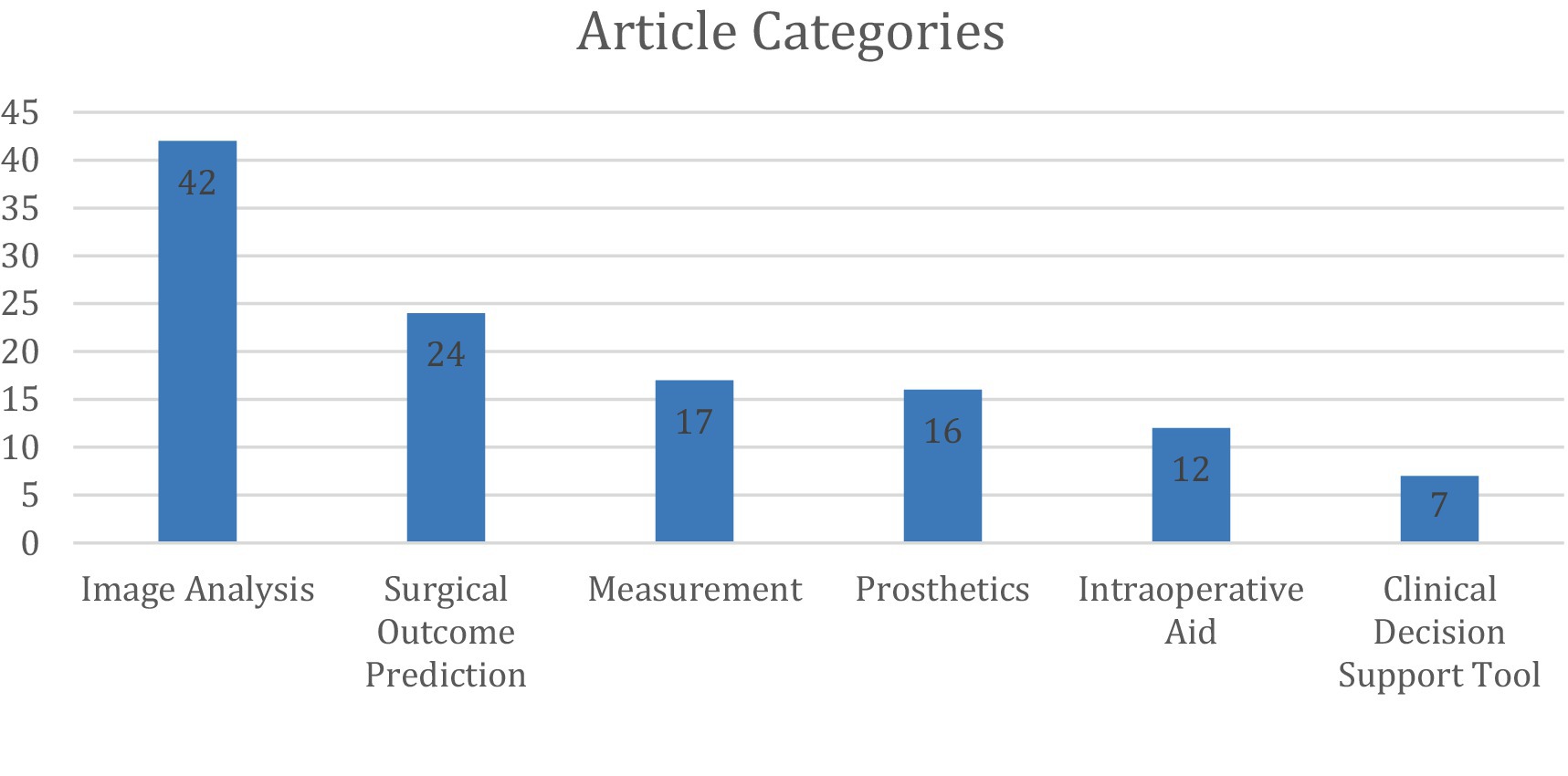
Figure 2. Distribution of 118 studies across 6 categories, with counts derived from non-overlapping classifications after consensus.
Study overlap
Following categorization, 11 of the 118 studies had overlap between two categories. Seven (Minelli et al., 2022; Ro et al., 2021; Alike et al., 2023; Lee et al., 2024; Gu et al., 2022; Kim et al., 2021; Ramkumar et al., 2018) of the studies overlapped between the Image Analysis and Measurement categories. Two (Kluck et al., 2023; Lu et al., 2021) of the studies overlapped between Image Analysis and Surgical Outcome Prediction. One study (Lee et al., 2018) overlapped between Image Analysis and Intraoperative Aid. One study (Cheng et al., 2023) overlapped between Intraoperative Aid and Clinical Decision Support Tool (Figures 3, 4).
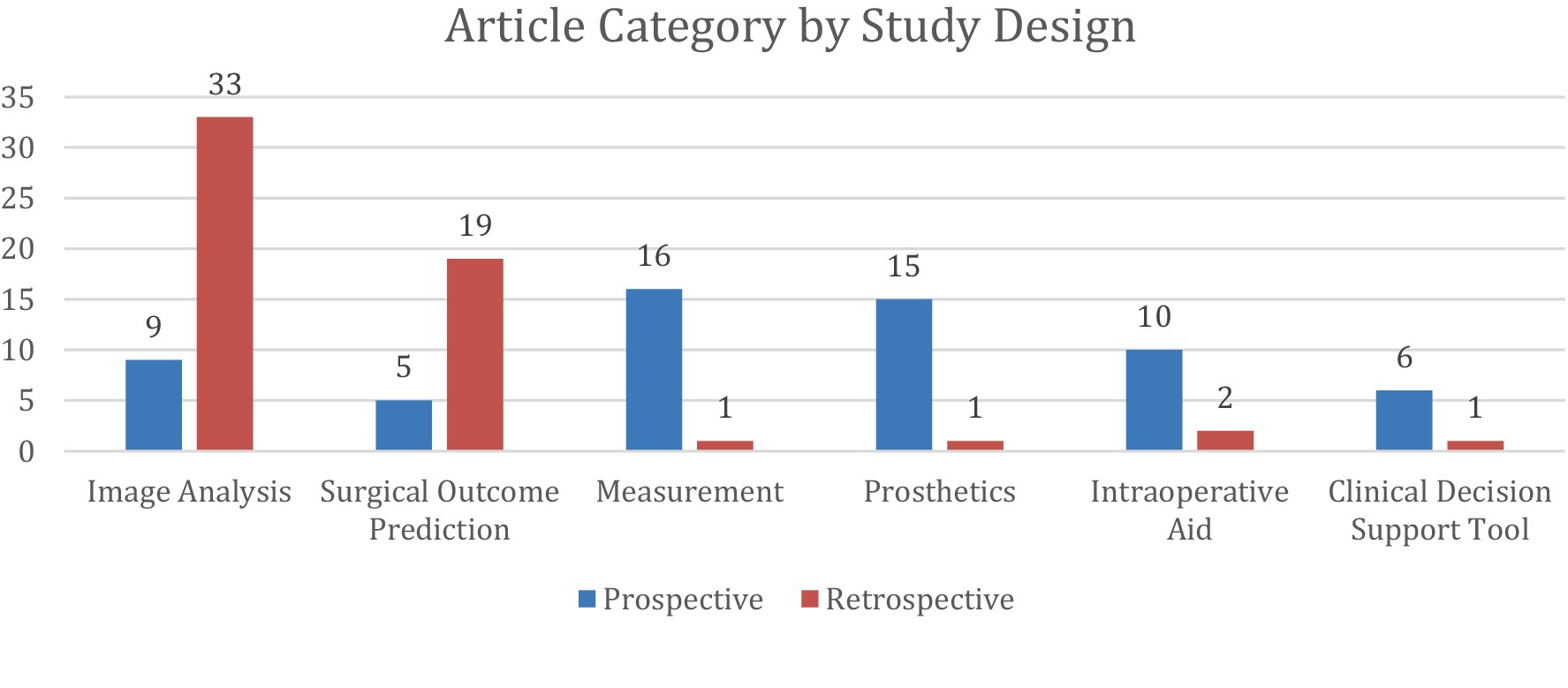
Figure 4. Distribution of 118 studies stratified into 6 categories and further divided by study design. Prospective studies were classified as those in which participants were followed forward in time from the point of the study’s initiation. Retrospective studies were classified as those in which researchers examined existing records of past events to find associations between exposures and outcomes. Any disagreements on study design were resolved through discussion, with the corresponding author serving as the final adjudicator in cases of unresolved discrepancies.
Automated image analysis
Similar to a prior review on hand surgery, the most common application of AI in UE surgery was automated image analysis (Keller et al., 2023), accounting for 42 articles (Anttila et al., 2023; Chung et al., 2018; Dipnall et al., 2022; Droppelmann et al., 2022; Guermazi et al., 2022; Guo et al., 2023; Hahn et al., 2022; Minelli et al., 2022; Ro et al., 2021; Yi et al., 2020; Anttila et al., 2022; Feuerriegel et al., 2023; Feuerriegel et al., 2024; Grauhan et al., 2022; Kang et al., 2021; Kim et al., 2022; Shinohara et al., 2023; Wei et al., 2022; Yang et al., 2024; Yoon et al., 2023; Zech et al., 2024; Zech et al., 2023; Alike et al., 2023; Alike et al., 2023; Benhenneda et al., 2023; Jopling et al., 2021; Keller et al., 2023; Kuok et al., 2020; Lee et al., 2024; Lee et al., 2023; Mert et al., 2024; Ni et al., 2024; Oeding et al., 2024; Shinohara et al., 2022; Suzuki et al., 2022; Anderson et al., 2023; Cirillo et al., 2019; Georgeanu et al., 2022; Jeon et al., 2023; Li and Ji, 2021; Cirillo et al., 2021; Yoon and Chung, 2021). These studies focused on AI-driven interpretation of radiographs, magnetic resonance imaging (MRI), ultrasound, and arthroscopic images, with radiographs being the most frequently analyzed modality.
Assessing the implementation of AI in examining radiographs accounted for 24 articles (Anttila et al., 2023; Chung et al., 2018; Dipnall et al., 2022; Guermazi et al., 2022; Minelli et al., 2022; Yi et al., 2020; Anttila et al., 2022; Grauhan et al., 2022; Kang et al., 2021; Wei et al., 2022; Yang et al., 2024; Yoon et al., 2023; Zech et al., 2024; Zech et al., 2023; Alike et al., 2023; Alike et al., 2023; Jopling et al., 2021; Keller et al., 2023; Lee et al., 2024; Mert et al., 2024; Suzuki et al., 2022; Anderson et al., 2023; Jeon et al., 2023; Yoon and Chung, 2021). AI models show promising capability by quickly and accurately detecting fractures (clavicle, arm, elbow, wrist, hand), measuring critical shoulder angle, identifying shoulder arthroplasty models, and detecting conditions such as enchondromas, joint dislocations, rotator cuff tendon tears, and scapholunate ligament ruptures.
Six studies (Chung et al., 2018; Guo et al., 2023; Zech et al., 2023; Mert et al., 2024; Ni et al., 2024; Suzuki et al., 2022) directly compared AI performance to human clinicians in image analysis, showing that AI matched or outperformed human readers in diagnostic accuracy and speed. One study demonstrated that an AI model achieved an accuracy of 99.3%, a sensitivity of 98.7%, and a specificity of 100% in detecting distal radius fractures, surpassing the performance of three hand orthopedic surgeons (Suzuki et al., 2022). In detecting proximal humerus fractures, AI also outperformed general physicians and non-specialized orthopedists, particularly in complex 3- and 4-part fractures (Chung et al., 2018). AI models integrating deep visual features with clinical data improved diagnostic accuracy for supraspinatus/infraspinatus tendon complex (SITC) injuries, significantly benefiting junior physicians with limited experience (Alike et al., 2023).
A separate study showed that the diagnostic accuracy of an AI algorithm on dorsopalmar radiography regarding scapholunate ligament integrity was close to that of the experienced human reader (e.g., differentiation of Geissler’s stages ≤ 2 versus > 2 with a sensitivity of 74% and a specificity of 78% compared to 77 and 80%) with a correlation coefficient of 0.81 (p < 0.01) (Keller et al., 2023). When AI and humans’ ability to analyze radiographs were directly compared to each other in terms of accuracy or speed, we did not identify any articles that showed humans significantly outperforming AI. Table 1 shows the results of each study that directly compared the image analysis performance between AI models and human readers.
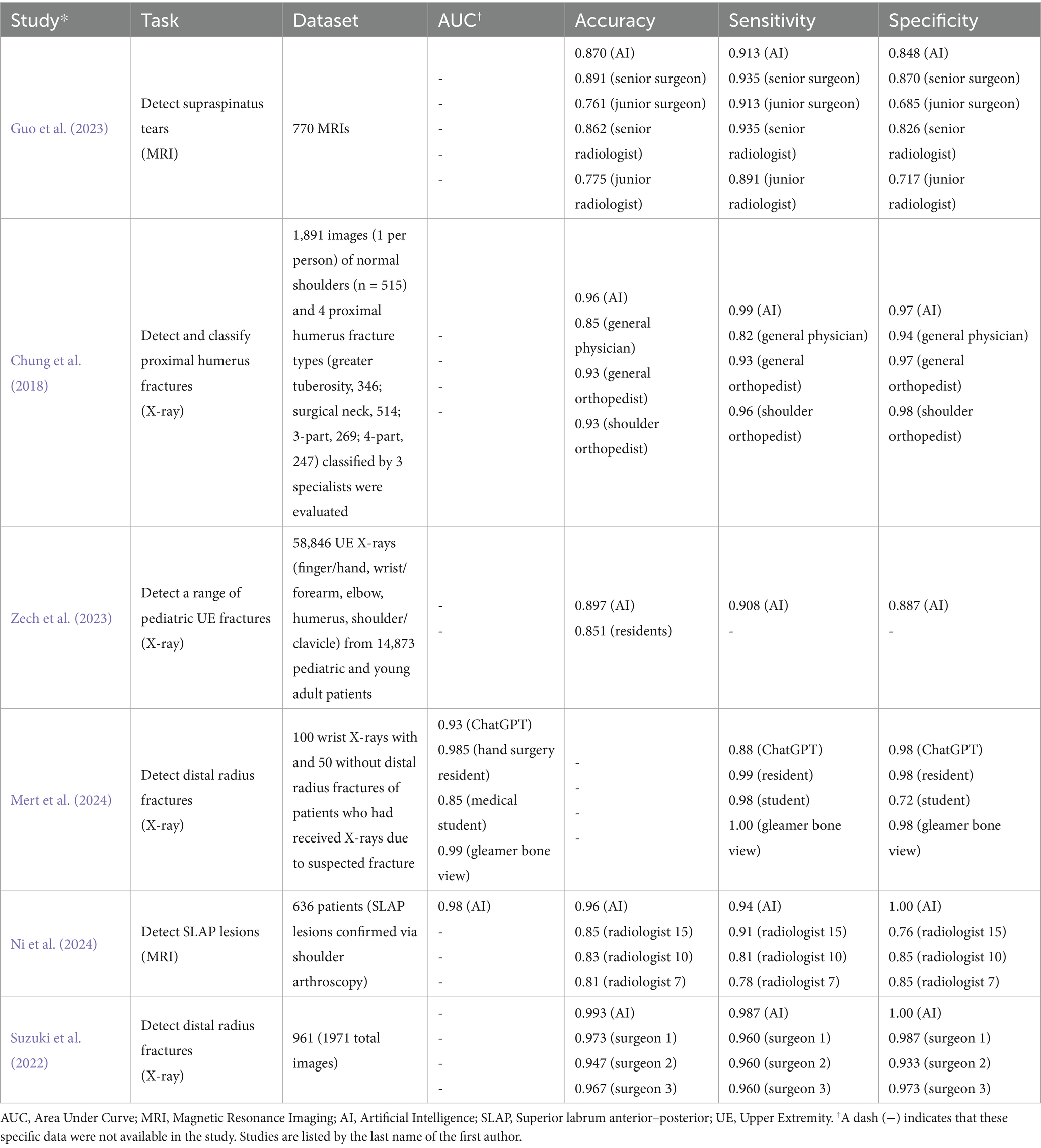
Table 1. The results of image analysis when various AI models were directly compared to human readers.
Additionally, several studies (Guermazi et al., 2022; Yoon et al., 2023; Zech et al., 2024; Alike et al., 2023; Anderson et al., 2023) evaluated AI-assisted human image analysis and found that AI augmentation improved clinician accuracy. In a retrospective study of fracture detection, AI-assisted readings increased sensitivity by 10.4% (75.2% vs. 64.8%), while maintaining specificity and reducing average reading time by 6.3 s per case (Guermazi et al., 2022). One study showed AI improves fracture detection among radiology and orthopedic residents in both pediatric and adult patients (Zech et al., 2024). Additionally, this study shows that AI enhances the specificity, sensitivity, and accuracy of physicians diagnosing supraspinatus/infraspinatus tendon complex injuries (Alike et al., 2023). Furthermore, AI assistance was shown to improve physician diagnostic sensitivity and specificity as well as interobserver agreement for the diagnosis of occult scaphoid fractures (Yoon et al., 2023). Similar findings were shown in several specialties, such as orthopedics, emergency medicine, radiology, and primary care, where the fracture miss rate was significantly reduced when aided by AI (Anderson et al., 2023).
Surgical outcome prediction
A total of 24 articles (Allen et al., 2024; Biron et al., 2020; Digumarthi et al., 2024; Giladi et al., 2023; Gowd et al., 2019; Gowd et al., 2022; Hoogendam et al., 2022; Karnuta et al., 2020; Kausch et al., 2020; King et al., 2023; Kluck et al., 2023; Kumar et al., 2021; Kumar et al., 2020; Kumar et al., 2022; Li et al., 2023; Lu et al., 2022; Lu et al., 2021; Mclendon, 2021; Oeding et al., 2023; Polce et al., 2021; Rajabzadeh-Oghaz et al., 2024; Roche et al., 2021; Shinohara et al., 2024; Simmons et al., 2023; Vassalou et al., 2022) investigated AI’s ability to predict surgical outcomes in UE surgery. These studies focused on rotator cuff arthropathy, carpal tunnel syndrome, and calcific tendonitis, with total shoulder arthroplasty (TSA) being the most frequently analyzed procedure. Among these, 10 studies specifically assessed AI’s ability to predict patient outcomes following anatomic (ASA) or reverse (RSA) total shoulder arthroplasty. All articles except one were retrospective and tested a variety of language learning models (LLMs) with different input variables.
AI models demonstrated high predictive accuracy in estimating postoperative outcomes, such as improvements in shoulder function, patient satisfaction, and complication risk. The predictive variables analyzed included patient history/demographics, pain and functionality scores, physical exam findings, imaging data (X-ray, CT), and laboratory values. Multiple studies showed that machine learning models could achieve AUC values between 0.71 and 0.94, effectively predicting postoperative range of motion (ROM), risk of infection, and the likelihood of requiring revision surgery. One study demonstrated 92.9% accuracy (AUC 0.875) in predicting multiple clinical outcomes after TSA using a limited set of 19 preoperative variables, minimizing the need for extensive data input (Kumar et al., 2020).
For each of the 10 studies involving total shoulder arthroplasty patients, Table 2 details the input variables used, data set size, predictive task, and predictive ability.
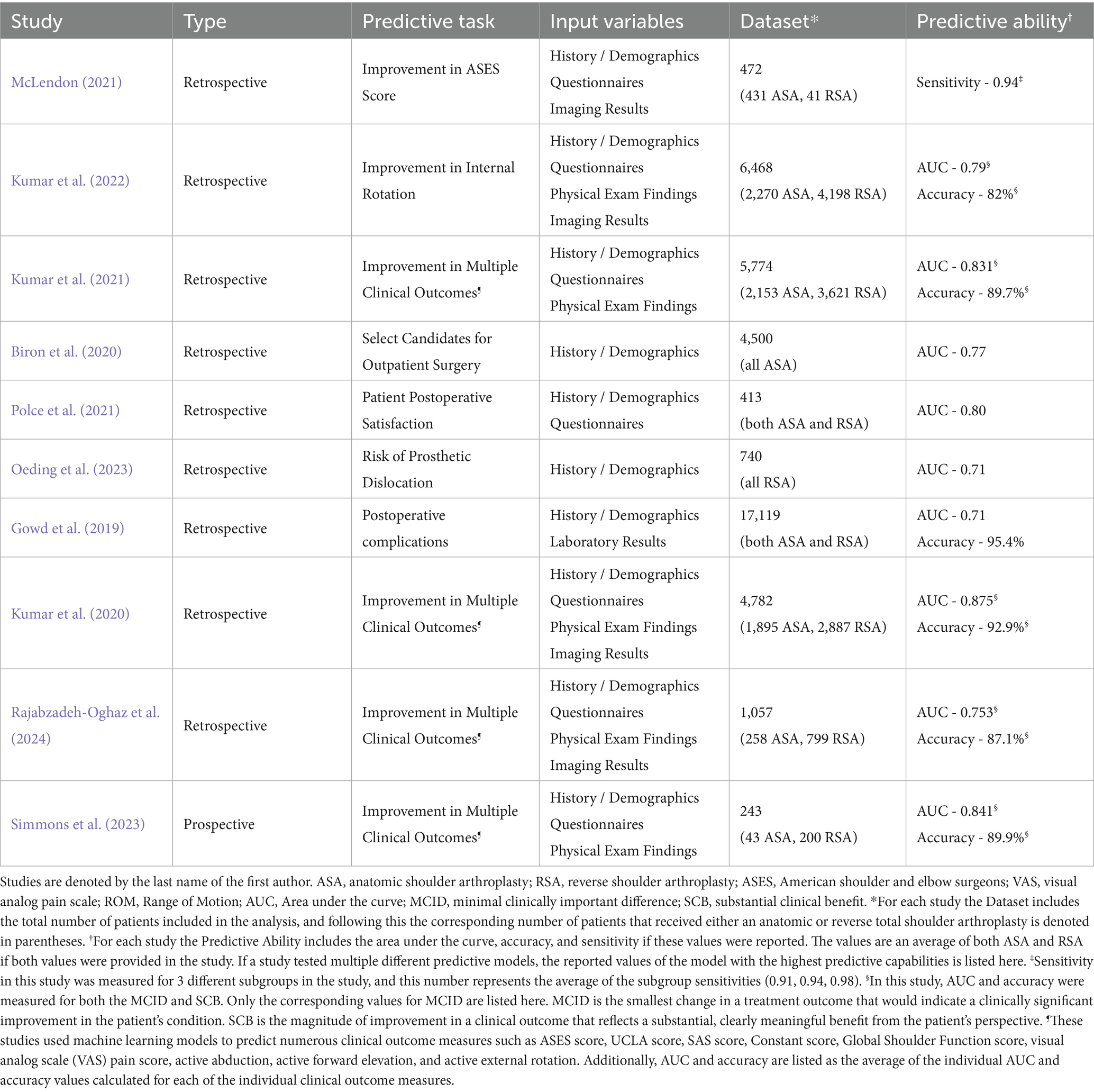
Table 2. The predictive task, utilized input variables, dataset, and predictive ability of the 10 studies involving total shoulder arthroplasty of the 24 that discussed the ability of AI to predict surgical outcomes.
Measurement tools
AI has also been applied to automated motion analysis and physical assessment in 16 articles (Burns et al., 2018; Darevsky et al., 2023; Darevsky, 2023; Dousty and Zariffa, 2021; Gauci et al., 2023; Gu et al., 2022; Ibara, 2023; Kim et al., 2021; Koyama et al., 2022; Koyama et al., 2021; Lee et al., 2016; Ramkumar et al., 2018; Rostamzadeh et al., 2024; Silver et al., 2006; Takigami et al., 2024; Tsukamoto et al., 2024; Tuan et al., 2022). These studies explored AI models designed to analyze videos or images of body movements including shoulder range of motion, hand gestures, grip strength, and thumb opposition.
Six studies (Darevsky et al., 2023; Gu et al., 2022; Koyama et al., 2021; Ramkumar et al., 2018; Takigami et al., 2024; Tsukamoto et al., 2024) utilized widely accessible devices, such as smartphones and smartwatches, to aid in automated physical examination. These AI models demonstrated high accuracy, exceeding 90% in classifying rotator cuff injuries and nerve dysfunction based on motion analysis. One study used AI-powered pose estimation to measure shoulder internal and external rotation, achieving a correlation coefficient of 0.971 and a mean absolute error of 5.778° compared to standard goniometric measurements (Takigami et al., 2024) (Table 3).
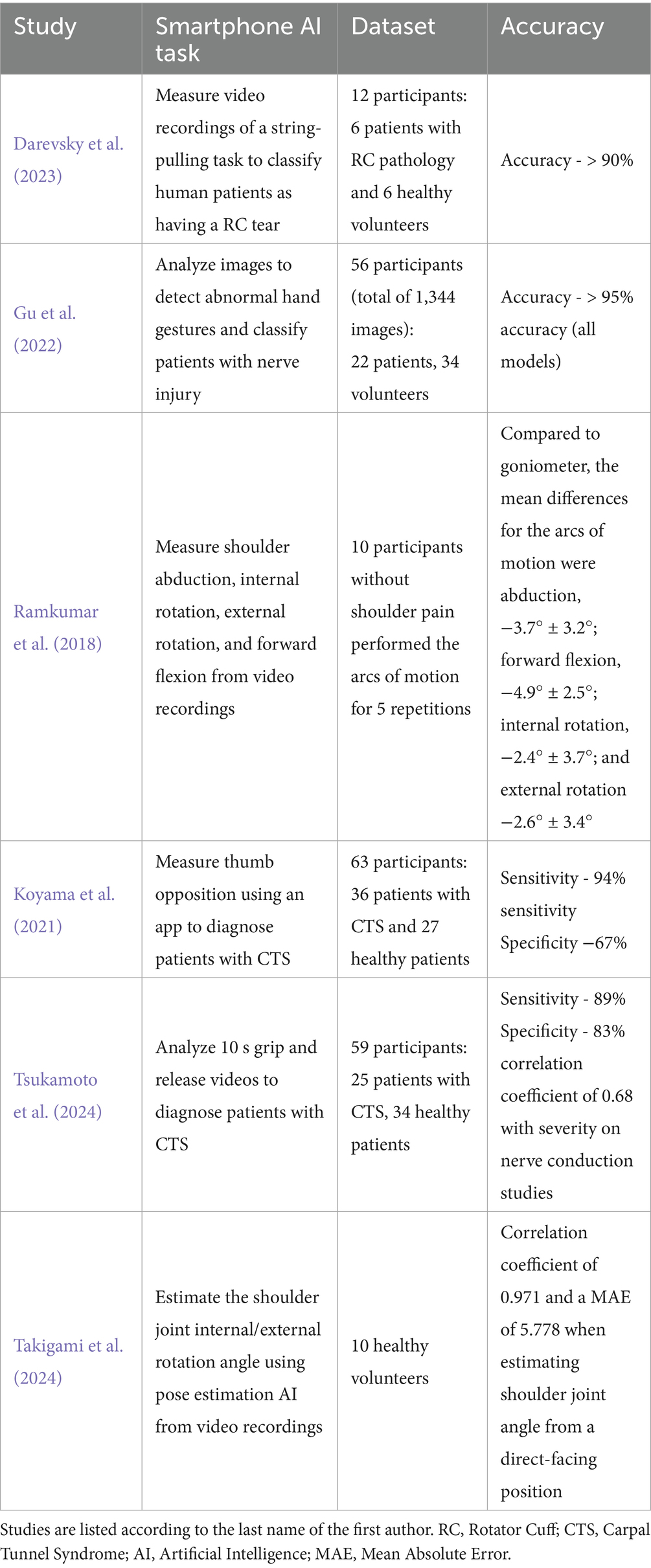
Table 3. The tasks and results from the six studies which analyzed AI models’ ability to perform measurements from easily accessible devices such as a smartphone or smart watch.
Prosthetic limb applications
UE orthopedics also includes prosthetic devices, which play a significant role for many amputee patients, and optimizing the function and utility of these devices with AI is an emerging topic of research. AI has played a key role in enhancing prosthetic limb control, particularly through surface electromyography (sEMG)-based myocontrol. Among the 16 studies (Atzori et al., 2014; Atzori et al., 2016; Castellini et al., 2009; Edwards et al., 2016; Hahne et al., 2017; Hwang et al., 2017; Jiang et al., 2020; Malešević et al., 2021; Mastinu et al., 2020; Nowak et al., 2023; Olsson et al., 2019; Osborn et al., 2021; Patel et al., 2017; Schmalfuss et al., 2018; Wang et al., 2022; Wang et al., 2020) in this category, many focused on improving real-time prosthesis functionality through AI-driven motor learning and predictive feedback systems (Table 4).
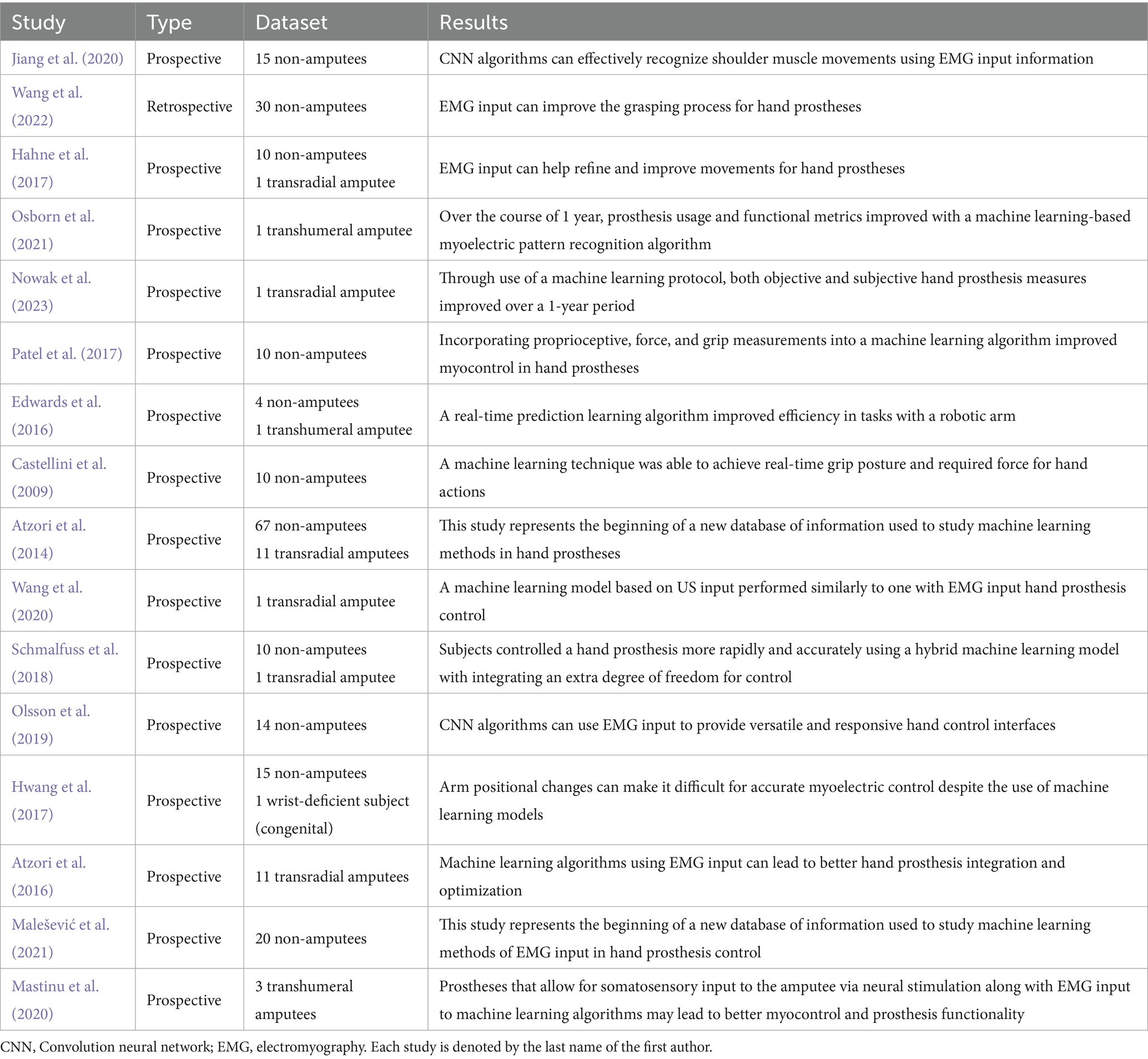
Table 4. Outlines for each of the studies relating to the use of prosthetics the study type (prospective/retrospective), the dataset (number of study participants, whether amputee or non-amputee), and the results of the study (short summary of study results).
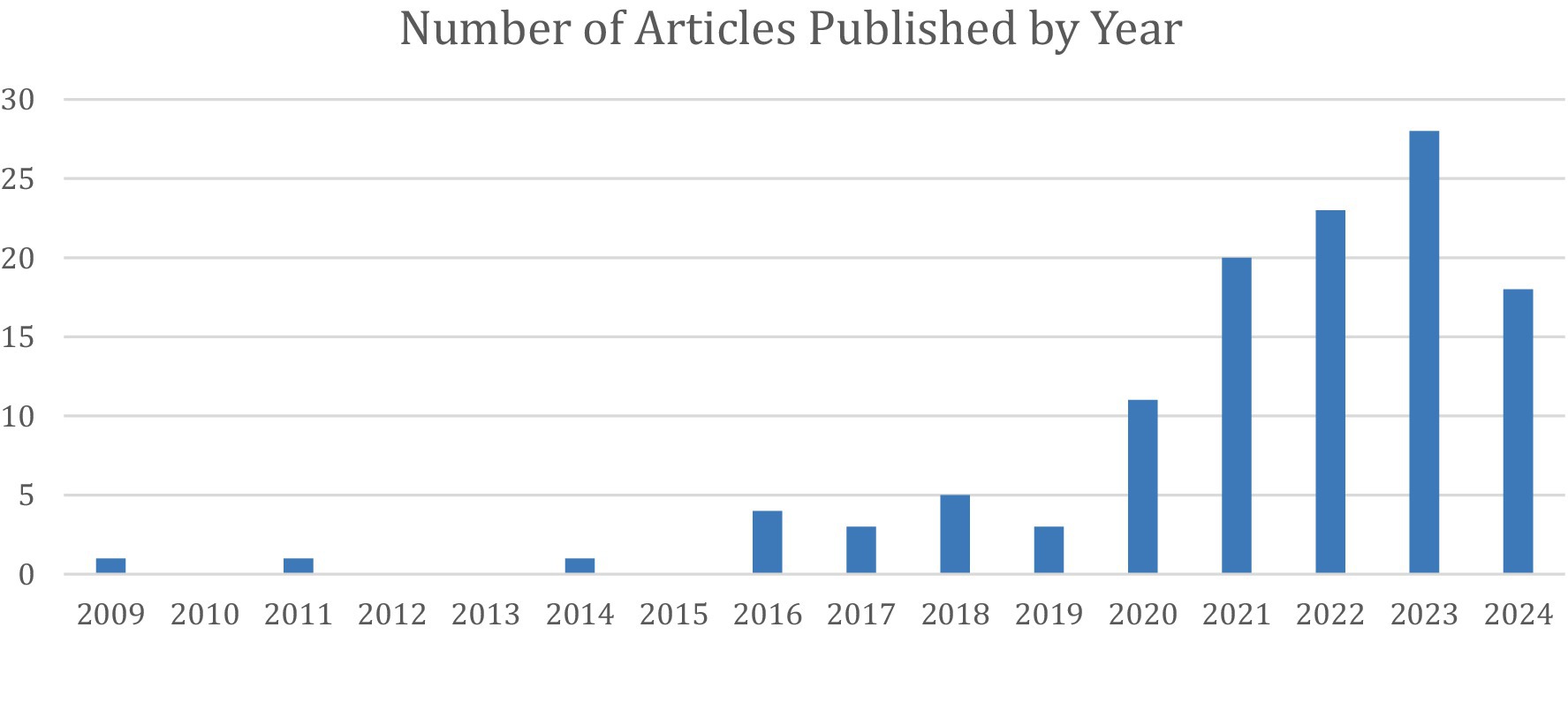
The first of these articles was published in 2009, and since then, interest in this field has increased significantly (Figure 5). In fact, this was the earliest article included in this review, showing that prosthetics was one of the first areas of interest to implement AI in the UE.
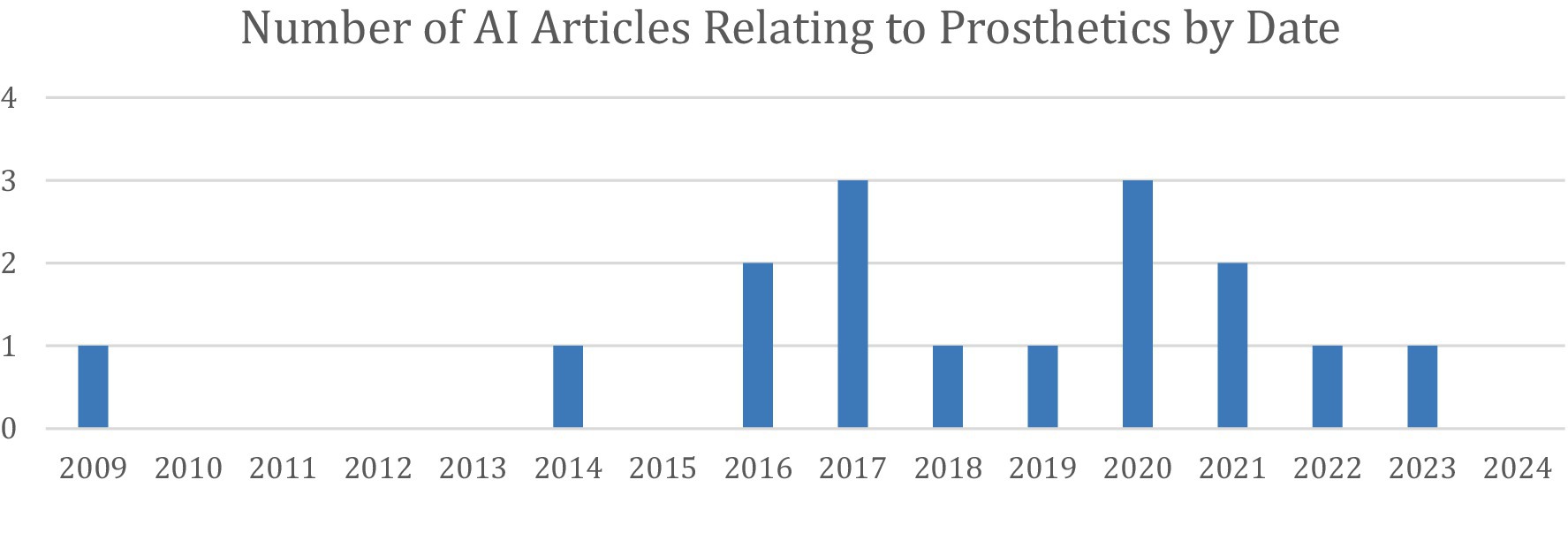
Figure 5. Distribution of the 16 articles under Prosthetic Limb Applications stratified by year of publication.
Movements performed by prostheses are performed in an “on/off” fashion, thus rendering coordinated movements with a set amount of force by particular muscles difficult. To overcome this, many prosthesis designs have aimed at incorporating electromyography (EMG) data to allow for more fine-tuned functionality. This is given further power when such input data is processed by way of a machine learning algorithm that can provide real-time feedback and updates as well as learn for future use. AI-driven pattern recognition algorithms have enabled fine-tuned, adaptive myoelectric control, allowing upper extremity amputees to achieve more coordinated, natural movement. Some studies incorporated real-time ultrasound feedback to improve AI-based prosthesis control, achieving accuracy comparable to electromyography-based models (Wang et al., 2020). Others demonstrated that machine learning-enhanced myoelectric control systems could significantly reduce reaction time and improve grip precision in prosthetic hand users (Nowak et al., 2023; Osborn et al., 2021; Patel et al., 2017).
Intraoperative AI applications
Twelve studies (Bernard et al., 2022; Bockhacker et al., 2020; Cheng et al., 2023; Eslamian et al., 2016; Eslamian et al., 2020; Hein et al., 2021; Kuthiala et al., 2022; Lee et al., 2018; Li et al., 2021; Shafiei et al., 2021; Sühn et al., 2023; Suh et al., 2011) investigated AI’s intraoperative applications, including robotic-assisted surgery, real-time bacterial identification, and automated instrument tracking. Most of these studies are lab-based, with no proof-of-concept in actual surgeries. One study showed that AI-based bacterial identification systems detected osteomyelitis-causing pathogens within five hours, significantly faster and in a less labor-intensive manner than traditional microbial cultures (Bernard et al., 2022). Similarly, another study demonstrated that AI-assisted intraoperative soft-tissue sarcoma classification achieved an accuracy above 85%, outperforming the traditional gold standard of H&E staining frozen sections, which often delays completion of the surgical procedure (Li et al., 2021).
AI-enhanced robotic surgery was explored in three studies (Eslamian et al., 2016; Eslamian et al., 2020; Sühn et al., 2023), showing that autonomous AI-controlled surgical cameras improved visualization, reduced unnecessary movements, and enhanced procedural efficiency and flow. This method was found to be superior to manual camera movement by the surgeon or a trained camera operator. Such technology additionally keeps the surgical instruments in view and avoids unnecessary movement of the camera, preventing inadequate visualization and distraction to the surgeon (Eslamian et al., 2016; Eslamian et al., 2020).
The direct tactile assessment of surface textures during palpation is an essential component of open surgery that is impeded in minimally invasive and robot-assisted surgery. A data generation framework proved accurate (>96%) in using vibro-acoustic sensing to differentiate materials during minimally invasive and robot-assisted surgery. This technology could provide valuable information during procedures such as a total joint replacement or arthroscopy, in which the osteoarthritic cartilage could be identified and graded to help the surgeon plan and make intraoperative decisions (Sühn et al., 2023).
Other intraoperative uses for AI included automated surgeon distraction monitoring (Shafiei et al., 2021), real-time detection of peripherally inserted central catheter (PICC) tips (Lee et al., 2018), segmenting arm venous images (Kuthiala et al., 2022), and gesture-controlled sterile navigation systems. One study evaluating AI-assisted touchless image viewing in the operating room, predicted the hand gestures of eight surgeons with an average of 6.5 years of experience, reaching a 98.94% accuracy in executing the correct task (Bockhacker et al., 2020).
Clinical decision support tool
AI was utilized as a clinical decision support tool (CDST) in six articles (Bulstra et al., 2022; Daher et al., 2023; Jagiella-Lodise et al., 2024; Rigamonti et al., 2021; Simmons et al., 2022; Yamamoto et al., 2024), meaning they were used in some degree to aid clinical decision-making but did not fall under any of the above categories. These studies focused on diagnostic guidance, treatment planning, and patient education.
Two studies evaluated ChatGPT’s diagnostic capabilities in UE conditions. One study found that ChatGPT correctly diagnosed and recommended appropriate management for 93 and 83% of shoulder and elbow cases, respectively (Daher et al., 2023). Another study assessed ChatGPT’s ability to answer common patient questions related to hand and wrist pathologies, with responses receiving an accuracy rating of 4.83 out of 6 (Jagiella-Lodise et al., 2024).
Another study tested the ability of an AI program to predict scaphoid fractures given elements of a patient’s demographics, history, and physical exam findings without being provided imaging (Bulstra et al., 2022). This machine learning algorithm achieved an area under the receiver operating characteristic curve of 0.77 when predicting the probability of a scaphoid fracture for a retrospective patient cohort. Although accurate, this performance does not exceed that of experienced physicians, who have shown a negative predictive value of up to 96% when predicting scaphoid fractures using a Clinical Scaphoid Score, without the aid of imaging (Pham, 2025). Additionally, this program was able to recommend advanced imaging for patients with a ≥ 10% risk of fracture, yielding 100% sensitivity, 38% specificity, and would have reduced the number of patients undergoing advanced imaging by 36% without missing a fracture.
Another study evaluated how a CDST would help surgeons plan preoperatively whether to perform an anatomic or reverse total shoulder arthroplasty for a patient with osteoarthritis. While this tool did not necessarily direct their decision, it improved their confidence in their own chosen decision (Simmons et al., 2022). Finally, one study discussed the ability of an AI model to analyze gait characteristics from in-shoe wearable monitors to predict distal radius fracture risks (Yamamoto et al., 2024).
These studies are outlined in Table 5.
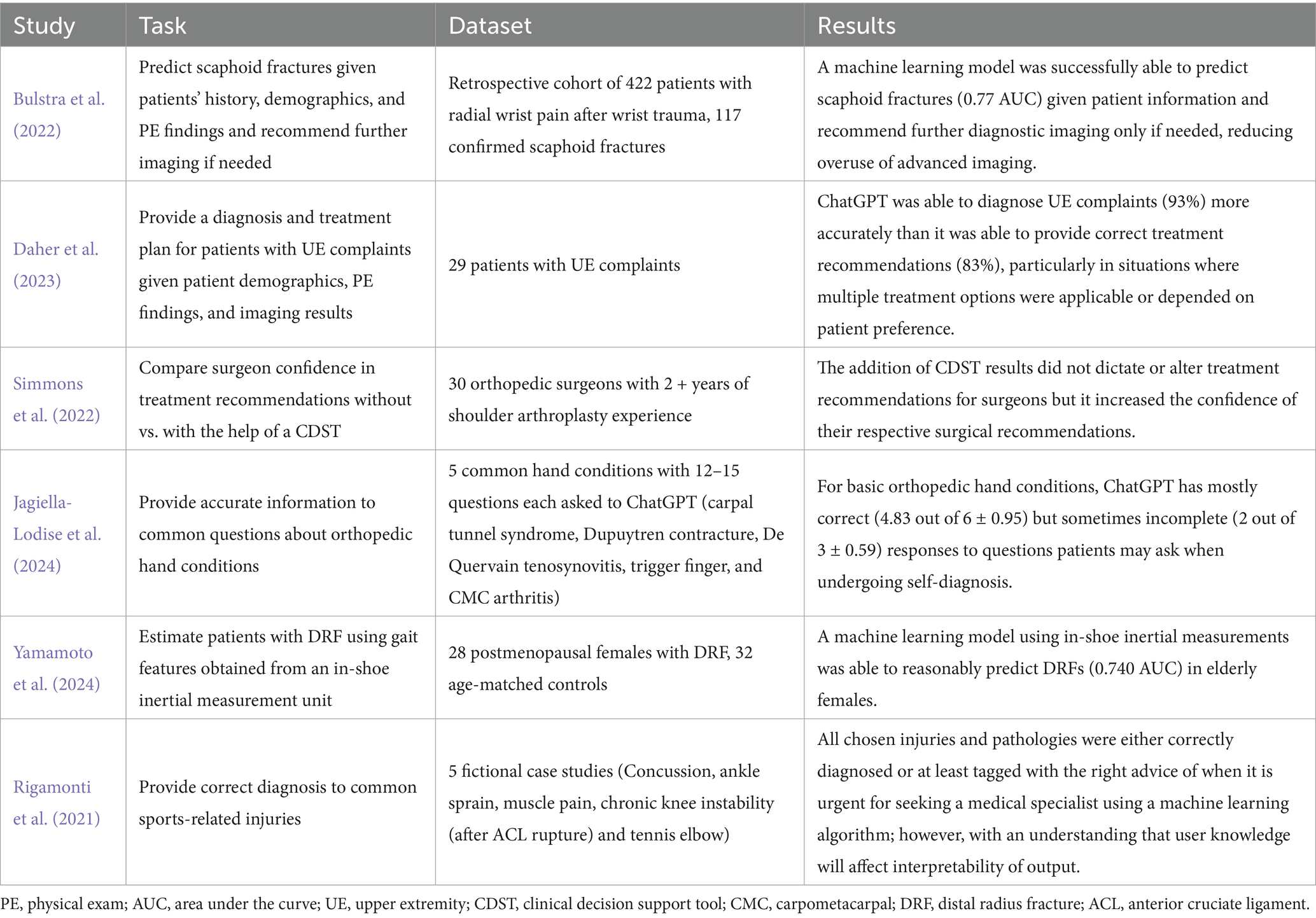
Table 5. Outlines for each of the studies relating to the clinical decision support tools the study task, the dataset (whether real patients, fictional case presentations, survey results, or algorithm responses to questions), and the results of the study (short summary of study results).
Risk of bias assessment
Risk of bias was assessed using the QUADAS-2 tool for diagnostic accuracy studies and the PROBAST tool for prediction model studies. Among the studies evaluated with QUADAS-2 (a total of 28), 15 were judged to have a high overall risk of bias, 10 had a low risk, and 3 had an unclear risk. For studies assessed with PROBAST (a total of 90), 53 demonstrated a high overall risk of bias and 37 had a low risk. No studies in the PROBAST group were rated as having an unclear risk of bias. These assessments provide insight into the methodological quality and potential limitations of the included studies. Table 6 and Figure 6 show the results of the QUADAS-2 analysis, and Table 7 and Figure 7 show the results of the PROBAST analysis.
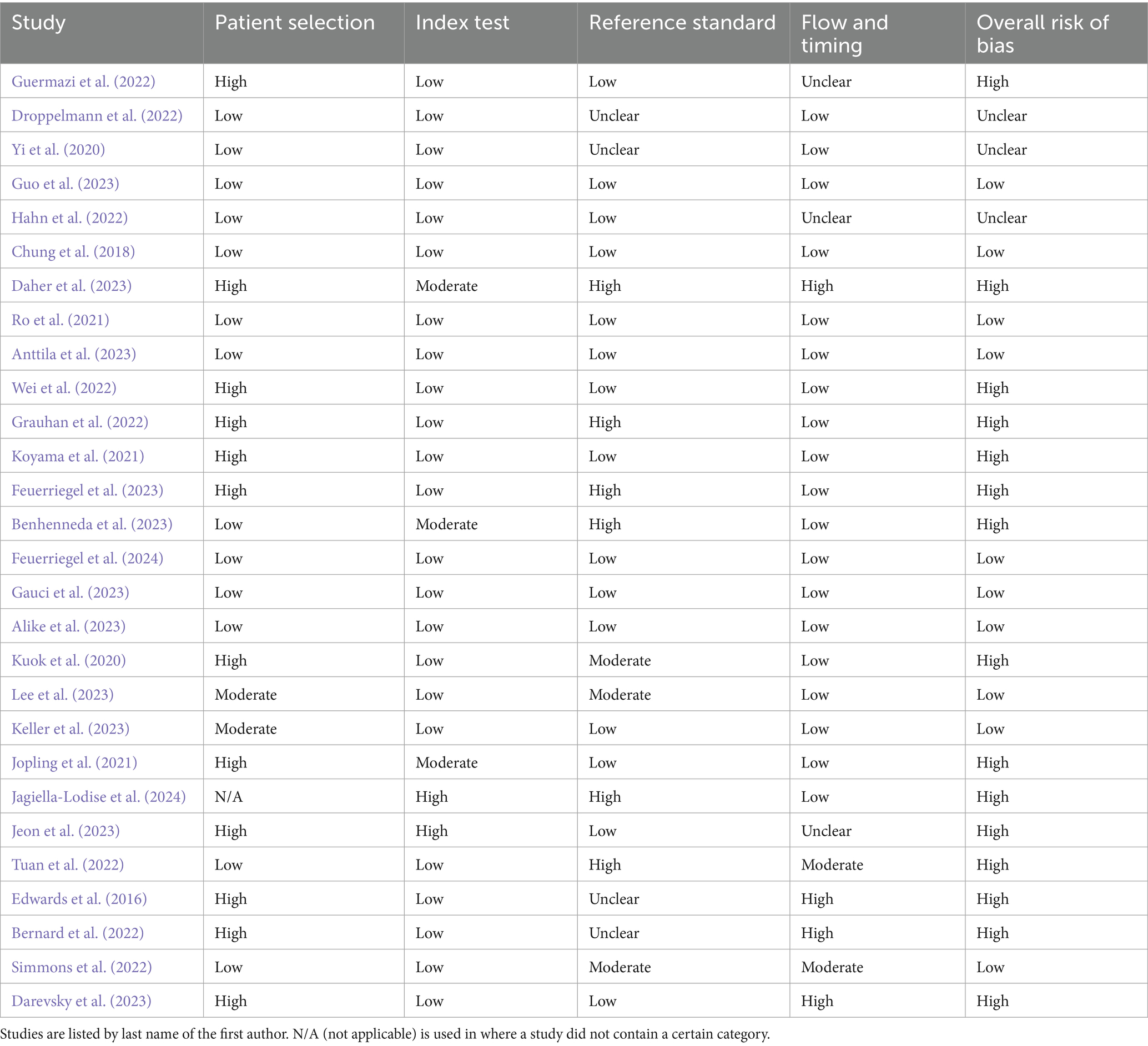
Table 6. The results of the QUADAS-2 bias analysis regarding whether included studies showed low, moderate, high, or unclear risk of bias in the categories of patient selection, index text, reference standard, flow and timing, as well as an overall risk of bias (QUADAS-2).
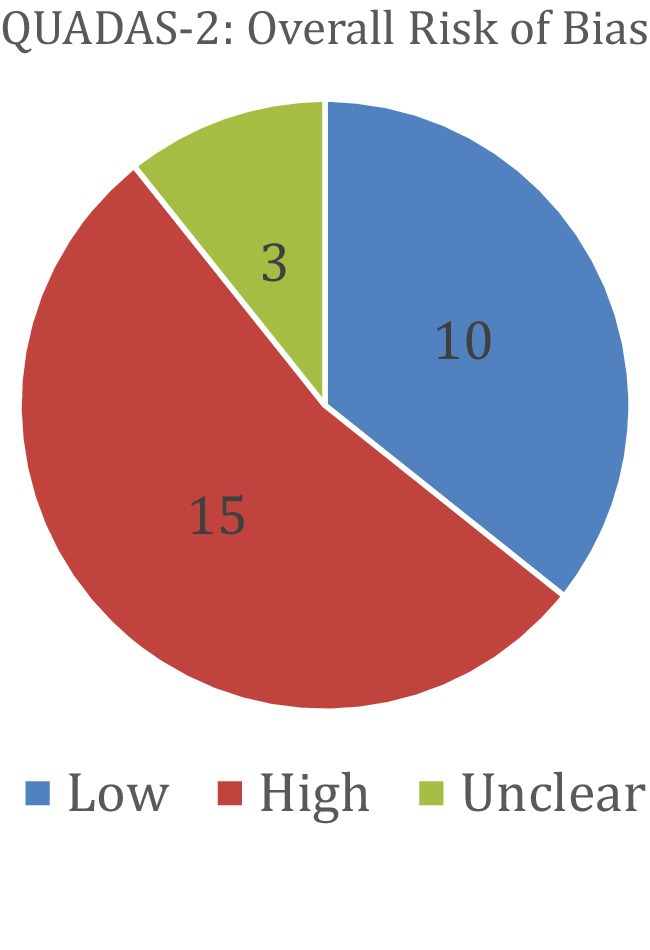
Figure 6. The results of the QUADAS-2 bias analysis regarding whether included studies showed low, high, or unclear overall risk of bias (QUADAS-2).
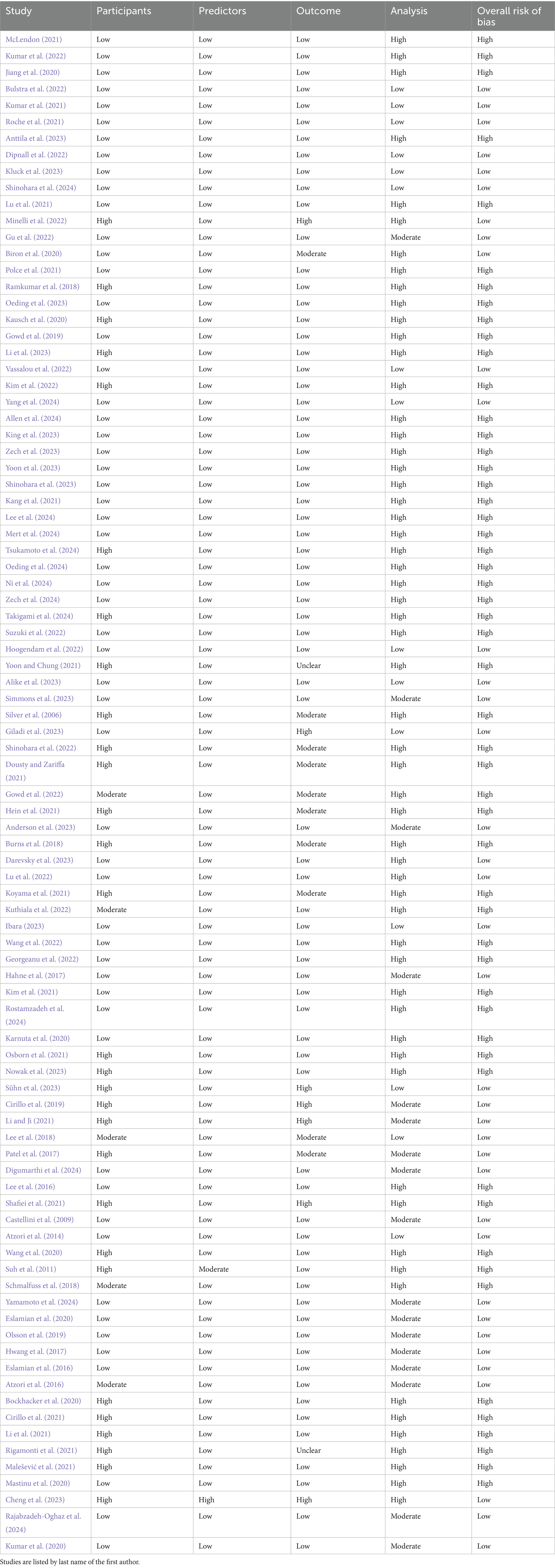
Table 7. The results of the PROBAST bias analysis regarding whether included studies showed low, moderate, or high risk of bias in the categories of participants, predictors, outcome, analysis, as well as an overall risk of bias.
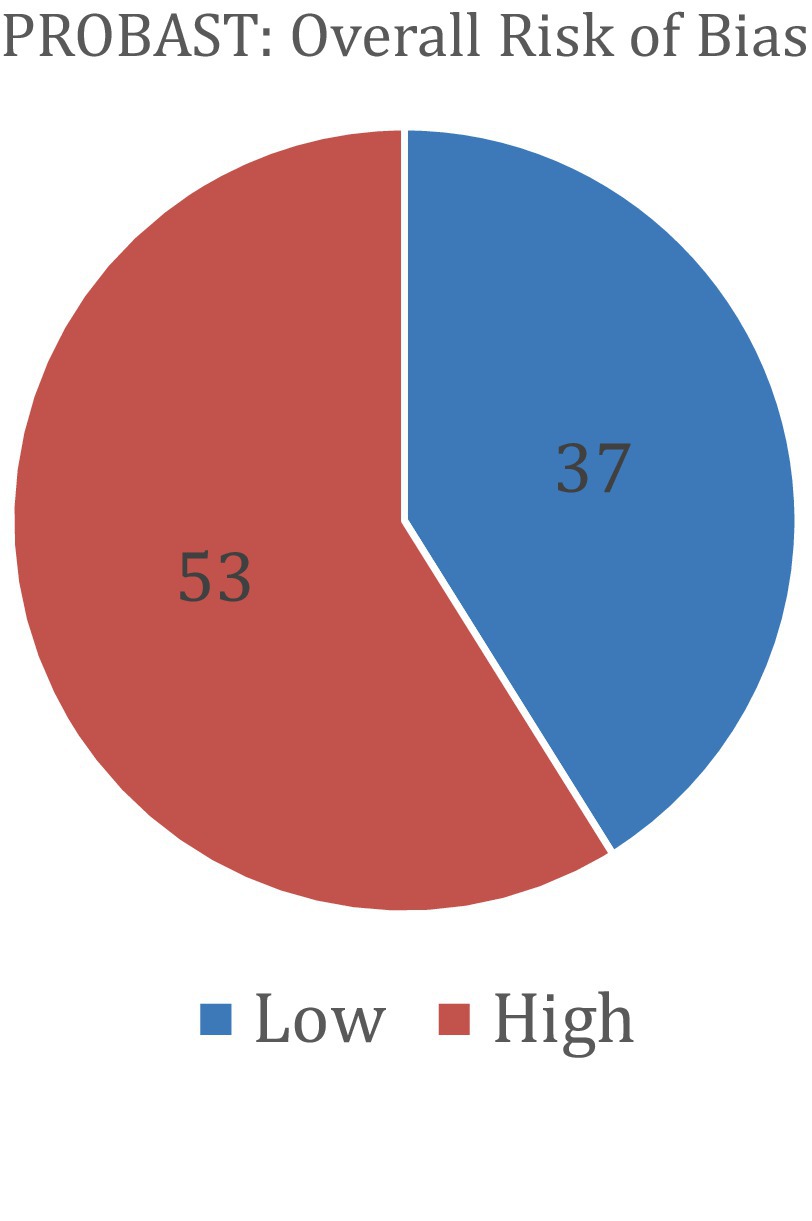
Figure 7. The results of the PROBAST bias analysis regarding whether included studies showed low or high overall risk of bias (PROBAST).
Discussion
The rapid evolution of AI has reshaped multiple domains of medicine, including orthopedics. While machine learning has been extensively used for over a decade in myoelectric control for upper limb amputees, the past 2 years have witnessed an unprecedented surge in AI applications across UE surgery. This growth reflects both the increasing sophistication of AI models and a growing recognition of their potential to enhance diagnostic precision, streamline surgical workflows, and improve patient outcomes. Our systematic review categorized AI applications into six primary domains: imaging analysis, surgical outcome prediction, intraoperative assistance, measurement tools, prosthetic limb control, and clinical decision support systems (CDSTs).
Among these, AI-driven imaging analysis has shown the most immediate and impactful benefits. AI models now routinely match or exceed human performance in detecting fractures (Chung et al., 2018; Zech et al., 2023; Mert et al., 2024; Suzuki et al., 2022), measuring critical anatomical angles (Minelli et al., 2022; Gu et al., 2022), and identifying soft tissue pathologies (Droppelmann et al., 2022; Guo et al., 2023; Hahn et al., 2022; Kang et al., 2021; Ni et al., 2024). Although few studies (Guo et al., 2023; Mert et al., 2024) showed surgeons capable of outperforming AI, deep learning algorithms have demonstrated higher sensitivity and specificity than experienced clinicians in certain diagnostic tasks, reinforcing their utility in radiographic interpretation. When AI and human performance are clinically integrated together, results improve. For example, Guermazi et al., demonstrated AI-assisted fracture readings increased sensitivity by 10% and reduced reading time (Guermazi et al., 2022). Such results emphasize that AI should not be replacing, rather enhancing clinician performance. AI-driven pre-screening of X-rays could improve radiology efficiency and speed by up to 16 s per image (Guermazi et al., 2022). AI-based measurement tools also provide precise quantifications of range of motion (ROM) (Li et al., 2023; Ramkumar et al., 2018), grip strength (Koyama et al., 2021), and hand posture (Gu et al., 2022) using accessible technologies like smartphones and smartwatches. These advancements offer a scalable, cost-effective means to enhance clinical assessments and facilitate remote patient monitoring.
Preoperatively, AI is increasingly utilized for surgical outcome prediction. Machine learning models can synthesize demographic, clinical, and imaging data to forecast postoperative ROM, complication risks, and patient satisfaction (Biron et al., 2020; Gowd et al., 2019; Kumar et al., 2021; Kumar et al., 2022; Mclendon, 2021; Oeding et al., 2023; Polce et al., 2021; Rajabzadeh-Oghaz et al., 2024; Simmons et al., 2023). Notably, some studies found that AI could achieve similar predictive accuracy using a reduced set of input variables, minimizing the burden of extensive data collection while still delivering actionable insights (Mclendon, 2021). This suggests that AI could streamline clinical workflows and assist in personalized treatment planning, optimizing decision-making without overwhelming surgeons with unnecessary data entry. Additionally, AI implementations continue to expand intraoperatively, with notable advancements in robotic-assisted surgery, real-time microbial identification, automated surgical instrument tracking, and vibro-acoustic sensing technologies capable of assessing cartilage integrity (Bernard et al., 2022; Hein et al., 2021; Sühn et al., 2023). For example, using AI to identify microbial infections could reduce waiting time on results from days to hours, allowing physicians a quicker response to identify and treat infections (Bernard et al., 2022). Such advancements could refine decision-making in joint preservation or arthroplasty procedures.
The ethical implications surrounding AI integration in UE surgery demand consideration. One pressing concern is algorithmic bias: if training datasets lack sufficient representation of minority groups (e.g., racial or ethnic minorities), fracture-detection or surgical-planning algorithms may underperform for those populations, exacerbating existing health disparities. For instance, studies have documented that AI models trained on primarily White patient data perform less accurately on underrepresented groups, leading to potential misdiagnoses or treatment delays (Pham, 2025). Ethical best practices call for inclusive, diverse datasets, regular demographic performance audits, and adoption of fairness-aware algorithm design methods (e.g., reweighting, adversarial debiasing) to ensure equitable care across populations (Pham, 2025). Moreover, AI systems often function as “black boxes,” complicating informed consent and undermining the doctor-patient relationship if neither patient nor clinician can understand the rationale behind AI-driven recommendations (Kumar et al., 2025). Ensuring meaningful transparency, such as explainability reports and shared decision-making frameworks, is essential. Without these safeguards, AI risk reinforcing, rather than reducing, disparities in surgical care.
Integrating AI into UE surgery holds great promise, but significant implementation barriers remain. Regulatory delays, particularly lengthy FDA clearance processes, pose a major hurdle. Only about half of AI-assisted orthopedic devices have undergone dynamic clinical validation, and many remain untested in real-world surgical settings, slowing adoption (Kumar et al., 2025). Training needs represent another critical obstacle. Orthopedic surgeons often lack formal education in AI or data science; moreover, generational divides influence perceived ease of use, with senior surgeons reporting lower familiarity and higher learning effort requirements (Schmidt et al., 2024). Surveys highlight infrastructure limitations—such as lack of institutional support, AI courses, and interdisciplinary collaboration—as persistent constraints, despite growing interest and ethical concerns like explainability and accountability. Finally, there is the question of legal liability. When an AI-assisted diagnosis or treatment is incorrect and leads to an adverse medical outcome, there is debate whether liability should fall on the company that developed the algorithm, the physician who used the tool, or the regulatory agency that approved it (Cestonaro et al., 2023). These intertwined challenges, regulatory bottlenecks, educational gaps, and infrastructural barriers, need to be addressed systematically to enable safe, effective integration of AI into UE orthopedic practice.
The objective of this literature review was to identify the current applications of AI in UE surgery. In order to cover a broad spectrum to this robust topic and find studies which UE surgeons may find interesting, we selected general search keywords. In agreement with the objective of this review, to give the reader a meaningful overview of the broad topic, we conducted this systematic review with clustering of the articles into six groups of thematically related publications. One limitation to our study is publication bias as studies with successful or positive results are more likely to be published. In addition, most of the studies in prosthetics are characterized by small sample sizes, which may limit their clinical relevance. Another limitation is that some studies overlapped into multiple sections. For example, two studies (Minelli et al., 2022; Gu et al., 2022) tested an AI model’s ability to analyze radiographs and measure critical shoulder angles. One study segmented burn images, but also accurately predicted the length of recovery needed based on burn depth (Cirillo et al., 2021). Additionally, one study used AI as a CDST to effectively predict shoulder surgery outcomes (Simmons et al., 2023). To determine which section to label these “overlap” studies, discussion took place between the primary reviewers until a consensus was achieved. A numeric comparison (accuracy, AUC, dataset, sensitivity, etc.) between certain studies took place when feasible, and the results were listed in their respective tables; however, another limitation to our study is that the majority of our sections contained rather unclear boundaries in terms of association to “artificial intelligence” and “upper extremity surgery.” To address this limitation and achieve the objective of this systematic review, we decided to interpret these vague sections in a narrative and qualitative fashion with citation of comparable publications. Although the target audience of our study is primarily medical professionals, a limitation to this study is that our literature search was conducted using only the MEDLINE/PubMed database, which may introduce selection bias. Most of the studies in our review did not report AI tool type, future research could be directed toward investigating the differences between commercial and academic AI algorithms, particularly in terms of performance, scalability, and transparency. Incorporating Explainable AI techniques such as SHAP, LIME, and DeepSHap into future research and application could also be valuable in aiding physicians in their decision-making process.
Conclusion
In conclusion, AI is reshaping UE surgery by augmenting diagnostic accuracy, enhancing surgical precision, improving prosthetic control, and facilitating personalized predictive modeling. As AI becomes increasingly embedded in orthopedic practice, future efforts should focus on optimizing real-world applications, addressing ethical and regulatory considerations, and fostering AI literacy among both clinicians and patients. AI should complement, rather than replace, physician expertise, necessitating intuitive interfaces, targeted clinician training, and real-time interpretability to foster trust and adoption among orthopedic surgeons. With continued advancements, AI has the potential to revolutionize orthopedic surgery, driving improvements in patient care, surgical efficiency, and clinical decision-making for years to come.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DP: Writing – original draft, Writing – review & editing. BH: Writing – original draft, Writing – review & editing. PG: Writing – original draft, Writing – review & editing. DG: Writing – original draft, Writing – review & editing. EH: Writing – original draft, Writing – review & editing. AI: Writing – original draft, Writing – review & editing. TH: Writing – original draft, Writing – review & editing. BM: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adu, Y., Cox, C. T., Hernandez, E. J., Zhu, C., Trevino, Z., and MacKay, B. J. (2024). Psychology of nerve injury, repair, and recovery: a systematic review. Frontiers Rehabilitation Sci. 5:1421704. doi: 10.3389/fresc.2024.1421704
Alike, Y., Li, C., Hou, J., Long, Y., Zhang, Z., Ye, M., et al. (2023). Deep learning for automated measurement of CSA related acromion morphological parameters on anteroposterior radiographs. Eur. J. Radiol. 168:111083. doi: 10.1016/j.ejrad.2023.111083
Alike, Y., Li, C., Hou, J., Long, Y., Zhang, J., Zhou, C., et al. (2023). Enhancing prediction of supraspinatus/infraspinatus tendon complex injuries through integration of deep visual features and clinical information: a multicenter two-round assessment study. Insights Imaging 14:1551. doi: 10.1186/s13244-023-01551-1
Allen, C., Kumar, V., Elwell, J., Overman, S., Schoch, B. S., Aibinder, W., et al. (2024). Evaluating the fairness and accuracy of machine learning–based predictions of clinical outcomes after anatomic and reverse Total shoulder arthroplasty. J. Shoulder Elb. Surg. 33, 888–899. doi: 10.1016/j.jse.2023.08.005
Anderson, P. G., Baum, G. L., Keathley, N., Sicular, S., Venkatesh, S., Sharma, A., et al. (2023). Deep learning assistance closes the accuracy gap in fracture detection across clinician types. Clinical Orthopaedics Related Research 481, 580–588. doi: 10.1097/CORR.0000000000002385
Anttila, T. T., Aspinen, S., Pierides, G., Haapamäki, V., Laitinen, M. K., and Ryhänen, J. (2023). Enchondroma detection from hand radiographs with An interactive deep learning segmentation tool—a feasibility study. J. Clin. Med. 12:7129. doi: 10.3390/jcm12227129
Anttila, T. T., Karjalainen, T. V., Mäkelä, T. O., Waris, E. M., Lindfors, N. C., Leminen, M. M., et al. (2022). Detecting distal radius fractures using a segmentation-based deep learning model. J. Digit. Imaging 36, 679–687. doi: 10.1007/s10278-022-00741-5
Atzori, M., Gijsberts, A., Castellini, C., Caputo, B., Hager, A. G. M., Elsig, S., et al. (2014). Electromyography data for non-invasive naturally-controlled robotic hand prostheses. Scientific Data 1:140053. doi: 10.1038/sdata.2014.53
Atzori, M., Gijsberts, A., Castellini, C., Caputo, B., Hager, A. G. M., Elsig, S., et al. (2016). Effect of clinical parameters on the control of myoelectric robotic prosthetic hands. J. Rehabil. Res. Dev. 53, 345–358. doi: 10.1682/JRRD.2014.09.0218
Benhenneda, R., Brouard, T., Dordain, F., Gadéa, F., Charousset, C., and Berhouet, J. (2023). Can artificial intelligence help decision-making in arthroscopy? Part 1: use of a standardized analysis protocol improves inter-observer agreement of arthroscopic diagnostic assessments of the Long head of biceps tendon in small rotator cuff tears. Orthop. Traumatol. Surg. Res. 109:103648. doi: 10.1016/j.otsr.2023.103648
Bernard, E., Peyret, T., Plinet, M., Contie, Y., Cazaudarré, T., Rouquet, Y., et al. (2022). The Dendrischip® technology as a new, rapid and reliable molecular method for the diagnosis of Osteoarticular infections. Diagnostics 12:1353. doi: 10.3390/diagnostics12061353
Biron, D. R., Sinha, I., Kleiner, J. E., Aluthge, D. P., Goodman, A. D., Sarkar, I. N., et al. (2020). A novel machine learning model developed to assist in patient selection for outpatient Total shoulder arthroplasty. J. Am. Acad. Orthop. Surg. 28, E580–E585. doi: 10.5435/JAAOS-D-19-00395
Bockhacker, M., Syrek, H., Elstermann von Elster, M., Schmitt, S., and Roehl, H. (2020). Evaluating usability of a touchless image viewer in the operating room. Appl. Clin. Inform. 11, 088–094. doi: 10.1055/s-0039-1701003
Bulstra, A. E. J., Buijze, G. A., Bulstra, A. E. J., Cohen, A., Colaris, J. W., Court-Brown, C. M., et al. (2022). A machine learning algorithm to estimate the probability of a true scaphoid fracture after wrist trauma. J. Hand Surg. Am. 47, 709–718. doi: 10.1016/j.jhsa.2022.02.023
Burns, D. M., Leung, N., Hardisty, M., Whyne, C. M., Henry, P., and McLachlin, S. (2018). Shoulder physiotherapy exercise recognition: machine learning the inertial signals from a smartwatch. Physiol. Meas. 39:075007. doi: 10.1088/1361-6579/aacfd9
Castellini, C., Fiorilla, A. E., and Sandini, G. (2009). Multi-subject/daily-life activity EMG-based control of mechanical hands. J. Neuroeng. Rehabil. 6:41. doi: 10.1186/1743-0003-6-41
Cestonaro, C., Delicati, A., Marcante, B., Caenazzo, L., and Tozzo, P. (2023). Defining medical liability when artificial intelligence is applied on diagnostic algorithms: a systematic review. Front. Med. 10:1305756. doi: 10.3389/fmed.2023.1305756
Cheng, K., Li, Z., Li, C., Xie, R., Guo, Q., He, Y., et al. (2023). The potential of GPT-4 as An AI-powered virtual assistant for surgeons specialized in joint arthroplasty. Ann. Biomed. Eng. 51, 1366–1370. doi: 10.1007/s10439-023-03207-z
Chung, S. W., Han, S. S., Lee, J. W., Oh, K. S., Kim, N. R., Yoon, J. P., et al. (2018). Automated detection and classification of the proximal Humerus fracture by using deep learning algorithm. Acta Orthop. 89, 468–473. doi: 10.1080/17453674.2018.1453714
Cirillo, M. D., Mirdell, R., Sjöberg, F., and Pham, T. D. (2019). Time-independent prediction of burn depth using deep convolutional neural networks. J. Burn Care Res. 40, 857–863. doi: 10.1093/jbcr/irz103
Cirillo, M. D., Mirdell, R., Sjöberg, F., and Pham, T. D. (2021). Improving burn depth assessment for pediatric scalds by AI based on semantic segmentation of polarized light photography images. Burns 47, 1586–1593. doi: 10.1016/j.burns.2021.01.011
Daher, M., Koa, J., Boufadel, P., Singh, J., Fares, M. Y., and Abboud, J. A. (2023). Breaking barriers: can Chatgpt compete with a shoulder and elbow specialist in diagnosis and management? JSES Int. 7, 2534–2541. doi: 10.1016/j.jseint.2023.07.018
Darevsky, D. M. (2023). A tool for low-cost, quantitative assessment of shoulder function using machine learning. Laurel Hollow, NY: Cold Spring Harbor Laboratory.
Darevsky, D. M., Hu, D. A., Gomez, F. A., Davies, M. R., Liu, X., and Feeley, B. T. (2023). Algorithmic assessment of shoulder function using smartphone video capture and machine learning. Sci. Rep. 13:19986. doi: 10.1038/s41598-023-46966-4
Digumarthi, V., Amin, T., Kanu, S., Mathew, J., Edwards, B., Peterson, L. A., et al. (2024). Preoperative prediction model for risk of readmission after Total joint replacement surgery: a random Forest approach leveraging NLP and unfairness mitigation for improved patient care and cost-effectiveness. J. Orthop. Surg. Res. 19:287. doi: 10.1186/s13018-024-04774-0
Dipnall, J. F., Lu, J., Gabbe, B. J., Cosic, F., Edwards, E., Page, R., et al. (2022). Comparison of state-of-the-art machine and deep learning algorithms to classify proximal humeral fractures using radiology text. Eur. J. Radiol. 153:110366. doi: 10.1016/j.ejrad.2022.110366
Dousty, M., and Zariffa, J. (2021). Tenodesis grasp detection in egocentric video. IEEE J. Biomed. Health Inform. 25, 1463–1470. doi: 10.1109/JBHI.2020.3003643
Droppelmann, G., Tello, M., García, N., Greene, C., Jorquera, C., and Feijoo, F. (2022). Lateral elbow tendinopathy and artificial intelligence: binary and multilabel findings detection using machine learning algorithms. Front. Med. 9:945698. doi: 10.3389/fmed.2022.945698
Edwards, A. L., Dawson, M. R., Hebert, J. S., Sherstan, C., Sutton, R. S., Chan, K. M., et al. (2016). Application of real-time machine learning to myoelectric prosthesis control. Prosthetics Orthotics Int. 40, 573–581. doi: 10.1177/0309364615605373
Eslamian, S., Reisner, L. A., King, B. W., and Pandya, A. K. (2016). Towards the implementation of An autonomous camera algorithm on the Da Vinci platform. Stud. Health Technol. Inform. 220, 118–123. doi: 10.13140/RG.2.2.25637.91364
Eslamian, S., Reisner, L. A., and Pandya, A. K. (2020). Development and evaluation of An autonomous camera control algorithm on the Da Vinci surgical system. Int. J. Med. Robotics Computer Assisted Surg. 16:2036. doi: 10.1002/rcs.2036
Feuerriegel, G. C., Weiss, K., Kronthaler, S., Leonhardt, Y., Neumann, J., Wurm, M., et al. (2023). Evaluation of a deep learning-based reconstruction method for Denoising and image enhancement of shoulder MRI in patients with shoulder pain. Eur. Radiol. 33, 4875–4884. doi: 10.1007/s00330-023-09472-9
Feuerriegel, G. C., Weiss, K., Tu van, A., Leonhardt, Y., Neumann, J., Gassert, F. T., et al. (2024). Deep-learning-based image quality enhancement of CT-like MR imaging in patients with suspected traumatic shoulder injury. Eur. J. Radiol. 170:111246. doi: 10.1016/j.ejrad.2023.111246
Gauci, M.-O., Olmos, M., Cointat, C., Chammas, P. E., Urvoy, M., Murienne, A., et al. (2023). Validation of the shoulder range of motion software for measurement of shoulder ranges of motion in consultation: coupling a red/green/blue-depth video camera to artificial intelligence. Int. Orthop. 47, 299–307. doi: 10.1007/s00264-022-05675-9
Georgeanu, V. A., Mămuleanu, M., Ghiea, S., and Selișteanu, D. (2022). Malignant bone tumors diagnosis using magnetic resonance imaging based on deep learning algorithms. Medicina 58:636. doi: 10.3390/medicina58050636
Giladi, A. M., Shipp, M. M., Sanghavi, K. K., Zhang, G., Gupta, S., Miller, K. E., et al. (2023). Patient-reported data augment health record data for prediction models of persistent opioid use after elective upper extremity surgery. Plastic Reconstructive Surg. 152, 358e–366e. doi: 10.1097/PRS.0000000000010297
Gowd, A. K., Agarwalla, A., Amin, N. H., Romeo, A. A., Nicholson, G. P., Verma, N. N., et al. (2019). Construct validation of machine learning in the prediction of short-term postoperative complications following Total shoulder arthroplasty. J. Shoulder Elb. Surg. 28, E410–E421. doi: 10.1016/j.jse.2019.05.017
Gowd, A. K., Agarwalla, A., Beck, E. C., Rosas, S., Waterman, B. R., Romeo, A. A., et al. (2022). Prediction of Total healthcare cost following Total shoulder arthroplasty utilizing machine learning. J. Shoulder Elb. Surg. 31, 2449–2456. doi: 10.1016/j.jse.2022.07.013
Grauhan, N. F., Niehues, S. M., Gaudin, R. A., Keller, S., Vahldiek, J. L., Adams, L. C., et al. (2022). Deep learning for accurately recognizing common causes of shoulder pain on radiographs. Skeletal Radiol. 51, 355–362. doi: 10.1007/s00256-021-03740-9
Gu, F., Fan, J., Cai, C., Wang, Z., Liu, X., Yang, J., et al. (2022). Automatic detection of abnormal hand gestures in patients with radial, ulnar, or median nerve injury using hand pose estimation. Front. Neurol. 13:1052505. doi: 10.3389/fneur.2022.1052505
Guermazi, A., Tannoury, C., Kompel, A. J., Murakami, A. M., Ducarouge, A., Gillibert, A., et al. (2022). Improving radiographic fracture recognition performance and efficiency using artificial intelligence. Radiology 302, 627–636. doi: 10.1148/radiol.210937
Guo, D., Liu, X., Wang, D., Tang, X., and Qin, Y. (2023). Development and clinical validation of deep learning for auto-diagnosis of supraspinatus tears. J. Orthop. Surg. Res. 18:3909. doi: 10.1186/s13018-023-03909-z
Hahn, S., Yi, J., Lee, H. J., Lee, Y., Lim, Y. J., Bang, J. Y., et al. (2022). Image quality and diagnostic performance of accelerated shoulder MRI with deep learning–based reconstruction. Am. J. Roentgenol. 218, 506–516. doi: 10.2214/AJR.21.26577
Hahne, J. M., Markovic, M., and Farina, D. (2017). User adaptation in myoelectric man-machine interfaces. Sci. Rep. 7:4437. doi: 10.1038/s41598-017-04255-x
Hein, J., Seibold, M., Bogo, F., Farshad, M., Pollefeys, M., Fürnstahl, P., et al. (2021). Towards Markerless surgical tool and hand pose estimation. Int. J. Comput. Assist. Radiol. Surg. 16, 799–808. doi: 10.1007/s11548-021-02369-2
Hoogendam, L., Bakx, J. A. C., Souer, J. S., Slijper, H. P., Andrinopoulou, E. R., and Selles, R. W. (2022). Predicting clinically relevant patient-reported symptom improvement after carpal tunnel release: a machine learning approach. Neurosurgery 90, 106–113. doi: 10.1227/NEU.0000000000001749
Hwang, H.-J., Hahne, J. M., and Müller, K.-R. (2017). Real-time robustness evaluation of regression based myoelectric control against arm position change and donning/doffing. PLoS One 12:E0186318. doi: 10.1371/journal.pone.0186318
Ibara, T. (2023). Screening for degenerative cervical myelopathy with the 10-second grip-and-release test using a smartphone and machine learning: a pilot study. Digital Health 9:205520762311790. doi: 10.1177/20552076231179030
Jagiella-Lodise, O., Suh, N., and Zelenski, N. A. (2024). Can patients rely on Chatgpt to answer hand pathology–related medical questions? Hand 20, 801–809. doi: 10.1177/15589447241247246
Jeon, Y.-D., Kang, M. J., Kuh, S. U., Cha, H. Y., Kim, M. S., You, J. Y., et al. (2023). Deep learning model based on You only look once algorithm for detection and visualization of fracture areas in three-dimensional skeletal images. Diagnostics 14:11. doi: 10.3390/diagnostics14010011
Jiang, Y., Chen, C., Zhang, X., Chen, C., Zhou, Y., Ni, G., et al. (2020). Shoulder muscle activation pattern recognition based on Semg and machine learning algorithms. Comput. Methods Prog. Biomed. 197:105721. doi: 10.1016/j.cmpb.2020.105721
Jopling, J. K., Pridgen, B. C., and Yeung, S. (2021). Deep convolutional neural networks as a diagnostic aid—a step toward minimizing undetected scaphoid fractures on initial hand radiographs. JAMA Netw. Open 4:E216393. doi: 10.1001/jamanetworkopen.2021.6393
Kang, Y., Choi, D., Lee, K. J., Oh, J. H., Kim, B. R., and Ahn, J. M. (2021). Evaluating subscapularis tendon tears on axillary lateral radiographs using deep learning. Eur. Radiol. 31, 9408–9417. doi: 10.1007/s00330-021-08034-1
Karnuta, J. M., Churchill, J. L., Haeberle, H. S., Nwachukwu, B. U., Taylor, S. A., Ricchetti, E. T., et al. (2020). The value of artificial neural networks for predicting length of stay, discharge disposition, and inpatient costs after anatomic and reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 29, 2385–2394. doi: 10.1016/j.jse.2020.04.009
Kausch, L., Thomas, S., Kunze, H., Privalov, M., Vetter, S., Franke, J., et al. (2020). Toward automatic C-arm positioning for standard projections in orthopedic surgery. Int. J. Comput. Assist. Radiol. Surg. 15, 1095–1105. doi: 10.1007/s11548-020-02204-0
Keller, M., Guebeli, A., Thieringer, F., and Honigmann, P. (2023). Artificial intelligence in patient-specific hand surgery: a scoping review of literature. Int. J. Comput. Assist. Radiol. Surg. 18, 1393–1403. doi: 10.1007/s11548-023-02831-3
Keller, G., Rachunek, K., Springer, F., and Kraus, M. (2023). Evaluation of a newly designed deep learning-based algorithm for automated assessment of Scapholunate distance in wrist radiography as a surrogate parameter for Scapholunate ligament rupture and the correlation with arthroscopy. Radiol. Med. 128, 1535–1541. doi: 10.1007/s11547-023-01720-8
Kim, D. W., Kim, J., Kim, T., Kim, T., Kim, Y. J., Song, I. S., et al. (2021). Prediction of hand-wrist maturation stages based on cervical vertebrae images using artificial intelligence. Orthod. Craniofac. Res. 24, 68–75. doi: 10.1111/ocr.12514
Kim, H., Shin, K., Kim, H., Lee, E. S., Chung, S. W., Koh, K. H., et al. (2022). Can deep learning reduce the time and effort required for manual segmentation in 3D reconstruction of MRI in rotator cuff tears? PLoS One 17:E0274075. doi: 10.1371/journal.pone.0274075
King, J. J., Wright, L., Hao, K. A., Roche, C., Wright, T. W., Vasilopoulos, T., et al. (2023). The shoulder arthroplasty smart score correlates well with legacy outcome scores without a ceiling effect. J. Am. Acad. Orthop. Surg. 31, 97–105. doi: 10.5435/JAAOS-D-22-00234
Kluck, D. G., Makarov, M. R., Kanaan, Y., Jo, C. H., and Birch, J. G. (2023). Comparison of “human” and artificial intelligence hand-and-wrist skeletal age estimation in An Epiphysiodesis cohort. J. Bone Joint Surg. 105, 202–206. doi: 10.2106/JBJS.22.00833
Koyama, T., Fujita, K., Watanabe, M., Kato, K., Sasaki, T., Yoshii, T., et al. (2022). Cervical myelopathy screening with machine learning algorithm focusing on finger motion using noncontact sensor. Spine 47, 163–171. doi: 10.1097/BRS.0000000000004243
Koyama, T., Sato, S., Toriumi, M., Watanabe, T., Nimura, A., Okawa, A., et al. (2021). A screening method using anomaly detection on a smartphone for patients with carpal tunnel syndrome: diagnostic case-control study. JMIR Mhealth Uhealth 9:E26320. doi: 10.2196/26320
Kumar, V., Roche, C., Overman, S., Simovitch, R., Flurin, P. H., Wright, T., et al. (2020). What is the accuracy of three different machine learning techniques to predict clinical outcomes after shoulder arthroplasty? Clinical Orthopaedics Related Research 478, 2351–2363. doi: 10.1097/CORR.0000000000001263
Kumar, V., Roche, C., Overman, S., Simovitch, R., Flurin, P. H., Wright, T., et al. (2021). Using machine learning to predict clinical outcomes after shoulder arthroplasty with a minimal feature set. J. Shoulder Elb. Surg. 30, E225–E236. doi: 10.1016/j.jse.2020.07.042
Kumar, V., Schoch, B. S., Allen, C., Overman, S., Teredesai, A., Aibinder, W., et al. (2022). Using machine learning to predict internal rotation after anatomic and reverse Total shoulder arthroplasty. J. Shoulder Elb. Surg. 31, E234–E245. doi: 10.1016/j.jse.2021.10.032
Kumar, R., Sporn, K., Ong, J., Waisberg, E., Paladugu, P., Vaja, S., et al. (2025). Integrating artificial intelligence in orthopedic care: advancements in bone care and future directions. Bioengineering 12:513. doi: 10.3390/bioengineering12050513
Kuok, C.-P., Yang, T. H., Tsai, B. S., Jou, I. M., Horng, M. H., Su, F. C., et al. (2020). Segmentation of finger tendon and synovial sheath in ultrasound image using deep convolutional neural network. Biomed. Eng. Online 19:24. doi: 10.1186/s12938-020-00768-1
Kuthiala, A., Tuli, N., Singh, H., Boyraz, O. F., Jindal, N., Mavuduru, R., et al. (2022). U-DAVIS-deep learning based arm venous image segmentation technique for venipuncture. Comput. Intell. Neurosci. 2022, 1–9. doi: 10.1155/2022/4559219
Langerhuizen, D. W. G., Janssen, S. J., Mallee, W. H., van den Bekerom, M. P. J., Ring, D., Kerkhoffs, G. M. M. J., et al. (2019). What are the applications and limitations of artificial intelligence for fracture detection and classification in Orthopaedic trauma imaging? A systematic review. Clinical Orthopaedics Related Research 477, 2482–2491. doi: 10.1097/CORR.0000000000000848
Lee, S. I., Huang, A., Mortazavi, B., Li, C., Hoffman, H. A., Garst, J., et al. (2016). Quantitative assessment of hand motor function in cervical spinal disorder patients using target tracking tests. J. Rehabil. Res. Dev. 53, 1007–1022. doi: 10.1682/JRRD.2014.12.0319
Lee, S., Kim, K. G., Kim, Y. J., Jeon, J. S., Lee, G. P., Kim, K. C., et al. (2024). Automatic segmentation and radiologic measurement of distal radius fractures using deep learning. Clin. Orthop. Surg. 16, 113–124. doi: 10.4055/cios23130
Lee, S. H., Lee, J. H., Oh, K. S., Yoon, J. P., Seo, A., Jeong, Y. J., et al. (2023). Automated 3-dimensional MRI segmentation for the Posterosuperior rotator cuff tear lesion using deep learning algorithm. PLoS One 18:E0284111. doi: 10.1371/journal.pone.0284111
Lee, H., Mansouri, M., Tajmir, S., Lev, M. H., and do, S. (2018). A deep-learning system for fully-automated peripherally inserted central catheter (PICC) tip detection. J. Digit. Imaging 31, 393–402. doi: 10.1007/s10278-017-0025-z
Li, C., Alike, Y., Hou, J., Long, Y., Zheng, Z., Meng, K., et al. (2023). Machine learning model successfully identifies important clinical features for predicting outpatients with rotator cuff tears. Knee Surg. Sports Traumatol. Arthrosc. 31, 2615–2623. doi: 10.1007/s00167-022-07298-4
Li, Z., and Ji, X. (2021). Ji, magnetic resonance imaging image segmentation under edge detection intelligent algorithm in diagnosis of surgical wrist joint injuries. Contrast Media Mol. Imaging 2021, 1–8. doi: 10.1155/2021/1667024
Li, L., Mustahsan, V. M., He, G., Tavernier, F. B., Singh, G., Boyce, B. F., et al. (2021). Classification of soft tissue sarcoma specimens with Raman spectroscopy as smart sensing technology. Cyborg Bionic Systems 2021, 1–12. doi: 10.34133/2021/9816913
Lu, Y., Labott, J. R., Salmons IV, H. I., Gross, B. D., Barlow, J. D., Sanchez-Sotelo, J., et al. (2022). Identifying modifiable and nonmodifiable cost drivers of ambulatory rotator cuff repair: a machine learning analysis. J. Shoulder Elb. Surg. 31, 2262–2273. doi: 10.1016/j.jse.2022.04.008
Lu, Y., Pareek, A., Wilbur, R. R., Leland, D. P., Krych, A. J., and Camp, C. L. (2021). Understanding anterior shoulder instability through machine learning: new models that predict recurrence, progression to surgery, and development of arthritis. Orthop. J. Sports Med. 9:232596712110533. doi: 10.1177/23259671211053326
Malešević, N., Olsson, A., Sager, P., Andersson, E., Cipriani, C., Controzzi, M., et al. (2021). A database of high-density surface electromyogram signals comprising 65 isometric hand gestures. Sci. Data 8:843. doi: 10.1038/s41597-021-00843-9
Mastinu, E., Engels, L. F., Clemente, F., Dione, M., Sassu, P., Aszmann, O., et al. (2020). Neural feedback strategies to improve grasping coordination in Neuromusculoskeletal prostheses. Sci. Rep. 10:11793. doi: 10.1038/s41598-020-67985-5
Mclendon, P. B. (2021). Machine learning can predict level of improvement in shoulder arthroplasty. JBJS open. Access 6:128. doi: 10.2106/JBJS.OA.20.00128
Mert, S., Stoerzer, P., Brauer, J., Fuchs, B., Haas-Lützenberger, E. M., Demmer, W., et al. (2024). Retracted article: diagnostic power of Chatgpt 4 in distal radius fracture detection through wrist radiographs. Arch. Orthop. Trauma Surg. 144, 2461–2467. doi: 10.1007/s00402-024-05298-2
Minelli, M., Cina, A., Galbusera, F., Castagna, A., Savevski, V., and Sconfienza, L. M. (2022). Measuring the critical shoulder angle on radiographs: An accurate and repeatable deep learning model. Skeletal Radiol. 51, 1873–1878. doi: 10.1007/s00256-022-04041-5
Myers, T. G., Ramkumar, P. N., Ricciardi, B. F., Urish, K. L., Kipper, J., and Ketonis, C. (2020). Artificial intelligence and Orthopaedics. J. Bone Joint Surg. 102, 830–840. doi: 10.2106/JBJS.19.01128
Ni, M., Gao, L., Chen, W., Zhao, Q., Zhao, Y., Jiang, C., et al. (2024). Preliminary exploration of deep learning-assisted recognition of superior labrum anterior and posterior lesions in shoulder MR arthrography. Int. Orthop. 48, 183–191. doi: 10.1007/s00264-023-05987-4
Nowak, M., Bongers, R. M., van der Sluis, C. K., Albu-Schäffer, A., and Castellini, C. (2023). Simultaneous assessment and training of An upper-limb amputee using incremental machine-learning-based Myocontrol: a single-case experimental design. J. Neuroeng. Rehabil. 20:39. doi: 10.1186/s12984-023-01171-2
Oeding, J. F., Lu, Y., Pareek, A., Marigi, E. M., Okoroha, K. R., Barlow, J. D., et al. (2023). Understanding risk for early dislocation resulting in reoperation within 90 days of reverse Total shoulder arthroplasty: extreme rare event detection through cost-sensitive machine learning. J. Shoulder Elb. Surg. 32, E437–E450. doi: 10.1016/j.jse.2023.03.001
Oeding, J. F., Pareek, A., Nieboer, M. J., Rhodes, N. G., Tiegs-Heiden, C. A., Camp, C. L., et al. (2024). A machine learning model demonstrates excellent performance in predicting subscapularis tears based on pre-operative imaging parameters alone. Arthroscopy J. Arthroscopic Related Surgery 40, 1044–1055. doi: 10.1016/j.arthro.2023.08.084
Olsson, A. E., Sager, P., Andersson, E., Björkman, A., Malešević, N., and Antfolk, C. (2019). Extraction of multi-labelled movement information from the raw HD-Semg image with time-domain depth. Sci. Rep. 9:7244. doi: 10.1038/s41598-019-43676-8
Osborn, L. E., Moran, C. W., Johannes, M. S., Sutton, E. E., Wormley, J. M., Dohopolski, C., et al. (2021). Extended home use of An advanced Osseointegrated prosthetic arm improves function, performance, and control efficiency. J. Neural Eng. 18:026020. doi: 10.1088/1741-2552/abe20d
Patel, G. K., Hahne, J. M., Castellini, C., Farina, D., and Dosen, S. (2017). Context-dependent adaptation improves robustness of myoelectric control for upper-limb prostheses. J. Neural Eng. 14:056016. doi: 10.1088/1741-2552/aa7e82
Pham, T. (2025). Ethical and legal considerations in healthcare AI: innovation and policy for safe and fair use. R. Soc. Open Sci. 12:873. doi: 10.1098/rsos.241873
Polce, E. M., Kunze, K. N., Fu, M. C., Garrigues, G. E., Forsythe, B., Nicholson, G. P., et al. (2021). Development of supervised machine learning algorithms for prediction of satisfaction at 2 years following Total shoulder arthroplasty. J. Shoulder Elb. Surg. 30, E290–E299. doi: 10.1016/j.jse.2020.09.007
Rajabzadeh-Oghaz, H., Kumar, V., Berry, D. B., Singh, A., Schoch, B. S., Aibinder, W. R., et al. (2024). Impact of deltoid computer tomography image data on the accuracy of machine learning predictions of clinical outcomes after anatomic and reverse Total shoulder arthroplasty. J. Clin. Med. 13:1273. doi: 10.3390/jcm13051273
Ramkumar, P. N., Haeberle, H. S., Navarro, S. M., Sultan, A. A., Mont, M. A., Ricchetti, E. T., et al. (2018). Mobile technology and telemedicine for shoulder range of motion: validation of a motion-based machine-learning software development kit. J. Shoulder Elb. Surg. 27, 1198–1204. doi: 10.1016/j.jse.2018.01.013
Rigamonti, L., Estel, K., Gehlen, T., Wolfarth, B., Lawrence, J. B., and Back, D. A. (2021). Use of artificial intelligence in sports medicine: a report of 5 fictional cases. BMC Sports Sci. Med. Rehabil. 13:243. doi: 10.1186/s13102-021-00243-x
Ro, K., Kim, J. Y., Park, H., Cho, B. H., Kim, I. Y., Shim, S. B., et al. (2021). Deep-learning framework and computer assisted fatty infiltration analysis for the supraspinatus muscle in MRI. Sci. Rep. 11:15065. doi: 10.1038/s41598-021-93026-w
Roche, C., Kumar, V., Overman, S., Simovitch, R., Flurin, P. H., Wright, T., et al. (2021). Validation Of A Machine Learning–Derived Clinical Metric To Quantify Outcomes After Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 30, 2211–2224. doi: 10.1016/j.jse.2021.01.021
Rostamzadeh, S., Abouhossein, A., Alam, K., Vosoughi, S., and Sattari, S. S. (2024). Exploratory analysis using machine learning algorithms to predict pinch strength by anthropometric and socio-demographic features. Int. J. Occup. Saf. Ergon. 30, 518–531. doi: 10.1080/10803548.2024.2322888
Schmalfuss, L., Hahne, J., Farina, D., Hewitt, M., Kogut, A., Doneit, W., et al. (2018). A hybrid auricular control system: direct, simultaneous, and proportional myoelectric control of two degrees of freedom in prosthetic hands. J. Neural Eng. 15:056028. doi: 10.1088/1741-2552/aad727
Schmidt, M., Kafai, Y. B., Heinze, A., and Ghidinelli, M. (2024). Unravelling Orthopaedic surgeons’ perceptions and adoption of generative AI technologies. Journal of. CME 13:330. doi: 10.1080/28338073.2024.2437330
Shafiei, S. B., Iqbal, U., Hussein, A. A., and Guru, K. A. (2021). Utilizing deep neural networks and electroencephalogram for objective evaluation of surgeon’s distraction during robot-assisted surgery. Brain Res. 1769:147607. doi: 10.1016/j.brainres.2021.147607
Shinohara, I., Inui, A., Mifune, Y., Nishimoto, H., Yamaura, K., Mukohara, S., et al. (2022). Diagnosis of cubital tunnel syndrome using deep learning on Ultrasonographic images. Diagnostics 12:632. doi: 10.3390/diagnostics12030632
Shinohara, I., Mifune, Y., Inui, A., Nishimoto, H., Yoshikawa, T., Kato, T., et al. (2024). Re-tear after arthroscopic rotator cuff tear surgery: risk analysis using machine learning. J. Shoulder Elb. Surg. 33, 815–822. doi: 10.1016/j.jse.2023.07.017
Shinohara, I., Yoshikawa, T., Inui, A., Mifune, Y., Nishimoto, H., Mukohara, S., et al. (2023). Degree of accuracy with which deep learning for ultrasound images identifies osteochondritis Dissecans of the humeral Capitellum. Am. J. Sports Med. 51, 358–366. doi: 10.1177/03635465221142280
Silver, A. E., Lungren, M. P., Johnson, M. E., O’Driscoll, S. W., An, K. N., and Hughes, R. E. (2006). Using support vector machines to optimally classify rotator cuff strength data and quantify post-operative strength in rotator cuff tear patients. J. Biomech. 39, 973–979. doi: 10.1016/j.jbiomech.2005.01.011
Simmons, C., DeGrasse, J., Polakovic, S., Aibinder, W., Throckmorton, T., Noerdlinger, M., et al. (2023). Initial clinical experience with a predictive clinical decision support tool for anatomic and reverse Total shoulder arthroplasty. Eur. J. Orthop. Surg. Traumatol. 34, 1307–1318. doi: 10.1007/s00590-023-03796-4
Simmons, C. S., Roche, C., Schoch, B. S., Parsons, M., and Aibinder, W. R. (2022). Surgeon confidence in planning Total shoulder arthroplasty improves after consulting a clinical decision support tool. Eur. J. Orthop. Surg. Traumatol. 33, 2385–2391. doi: 10.1007/s00590-022-03446-1
Suh, I. H., Mukherjee, M., Schrack, R., Park, S. H., Chien, J. H., Oleynikov, D., et al. (2011). Electromyographic correlates of learning during robotic surgical training in virtual reality. Stud. Health Technol. Inform. 163, 630–634. doi: 10.3233/978-1-60750-706-2-630
Sühn, T., Esmaeili, N., Mattepu, S. Y., Spiller, M., Boese, A., Urrutia, R., et al. (2023). Vibro-acoustic sensing of instrument interactions as a potential source of texture-related information in robotic palpation. Sensors 23:3141. doi: 10.3390/s23063141
Suzuki, T., Maki, S., Yamazaki, T., Wakita, H., Toguchi, Y., Horii, M., et al. (2022). Detecting distal radial fractures from wrist radiographs using a deep convolutional neural network with An accuracy comparable to hand orthopedic surgeons. J. Digit. Imaging 35, 39–46. doi: 10.1007/s10278-021-00519-1
Takigami, S., Inui, A., Mifune, Y., Nishimoto, H., Yamaura, K., Kato, T., et al. (2024). Estimation of shoulder joint rotation angle using tablet device and pose estimation artificial intelligence model. Sensors 24:2912. doi: 10.3390/s24092912
Tricco, A. C., Lillie, E., Zarin, W., O'Brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA extension for scoping reviews (PRISMA-Scr): checklist and explanation. Ann. Intern. Med. 169, 467–473. doi: 10.7326/M18-0850
Tsukamoto, K., Matsui, R., Sugiura, Y., and Fujita, K. (2024). Diagnosis of carpal tunnel syndrome using a 10-S grip-and-release test with video and machine learning analysis. J. Hand Surgery 49, 634–636. doi: 10.1177/17531934231214661
Tuan, C.-C., Wu, Y. C., Yeh, W. L., Wang, C. C., Lu, C. H., Wang, S. W., et al. (2022). Development of joint activity angle measurement and cloud data storage system. Sensors 22:4684. doi: 10.3390/s22134684
Vassalou, E. E., Klontzas, M. E., Marias, K., and Karantanas, A. H. (2022). Predicting Long-term outcomes of ultrasound-guided percutaneous irrigation of calcific tendinopathy with the use of machine learning. Skeletal Radiol. 51, 417–422. doi: 10.1007/s00256-021-03893-7
Wang, Z., Fang, Y., Zhou, D., Li, K., Cointet, C., and Liu, H. (2020). Ultrasonography and electromyography based hand motion intention recognition for a trans-radial amputee: a case study. Med. Eng. Phys. 75, 45–48. doi: 10.1016/j.medengphy.2019.11.005
Wang, S., Zheng, J., Zheng, B., and Jiang, X. (2022). Phase-based grasp classification for prosthetic hand control using Semg. Biosensors 12:57. doi: 10.3390/bios12020057
Wei, J., Li, D., Sing, D. C., Beeram, I., Puvanesarajah, V., Tornetta, P. III, et al. (2022). Detecting upper extremity native joint dislocations using deep learning: a multicenter study. Clin. Imaging 92, 38–43. doi: 10.1016/j.clinimag.2022.09.005
Yamamoto, A., Fujita, K., Yamada, E., Ibara, T., Nihey, F., Inai, T., et al. (2024). Gait characteristics in patients with distal radius fracture using An in-shoe inertial measurement system at various gait speeds. Gait Posture 107, 317–323. doi: 10.1016/j.gaitpost.2023.10.023
Yang, L., Oeding, J. F., de Marinis, R., Marigi, E., and Sanchez-Sotelo, J. (2024). Deep learning to automatically classify very large sets of preoperative and postoperative shoulder arthroplasty radiographs. J. Shoulder Elb. Surg. 33, 773–780. doi: 10.1016/j.jse.2023.09.021
Yi, P. H., Kim, T. K., Wei, J., Li, X., Hager, G. D., Sair, H. I., et al. (2020). Automated detection and classification of shoulder arthroplasty models using deep learning. Skeletal Radiol. 49, 1623–1632. doi: 10.1007/s00256-020-03463-3
Yoon, A. P., and Chung, K. C. (2021). application of deep learning: detection of obsolete scaphoid fractures with artificial neural networks. J. Hand Surgery 46, 914–916. doi: 10.1177/17531934211026139
Yoon, A. P., Chung, W. T., Wang, C. W., Kuo, C. F., Lin, C., and Chung, K. C. (2023). Can a deep learning algorithm improve detection of occult scaphoid fractures in plain radiographs? A clinical validation study. Clinical Orthopaedics Related Research 481, 1828–1835. doi: 10.1097/CORR.0000000000002612
Zech, J. R., Ezuma, C. O., Patel, S., Edwards, C. R., Posner, R., Hannon, E., et al. (2024). Artificial intelligence improves resident detection of pediatric and young adult upper extremity fractures. Skeletal Radiol. 53, 2643–2651. doi: 10.1007/s00256-024-04698-0
Keywords: artificial intelligence, machine learning, orthopedics, surgery, upper extremity
Citation: Parry D, Henderson B, Gaschen P, Ghanem D, Hernandez E, Idicula A, Hanna T and MacKay B (2025) The implementation of artificial intelligence in upper extremity surgery: a systematic review. Front. Artif. Intell. 8:1621757. doi: 10.3389/frai.2025.1621757
Edited by:
Tuan D. Pham, Queen Mary University of London, United KingdomReviewed by:
TaChen Chen, Nihon Pharmaceutical University, JapanShanmugavalli Venkatachalam, Manipal Institute of Technology Bengaluru, India
Copyright © 2025 Parry, Henderson, Gaschen, Ghanem, Hernandez, Idicula, Hanna and MacKay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brendan MacKay, YnJlbmRhbi5qLm1hY2theUB0dHVoc2MuZWR1
 Dylan Parry
Dylan Parry Brennon Henderson1
Brennon Henderson1 Evan Hernandez
Evan Hernandez Brendan MacKay
Brendan MacKay