- Department of Chemistry and Biochemistry, Worcester Polytechnic Institute, Worcester, MA, United States
Zn2+ transport across neuronal membranes relies on two classes of transition metal transporters: the ZnT (SLC30) and ZIP (SLC39) families. These proteins function to decrease and increase cytosolic Zn2+ levels, respectively. Dysfunction of ZnT and ZIP transporters can alter intracellular Zn2+ levels resulting in deleterious effects. In neurons, imbalances in Zn2+ levels have been implicated as risk factors in conditions such as Alzheimer’s disease and neurodegeneration, highlighting the pivotal role of Zn2+ homeostasis in neuropathologies. In addition, Zn2+ modulates the function of plasma membrane proteins, including ion channels and receptors. Changes in Zn2+ levels, on both sides of the plasma membrane, profoundly impact signaling pathways governing cell development, differentiation, and survival. This review is focused on recent developments of neuronal Zn2+ homeostasis, including the impact of Zn2+ dyshomeostasis in neurological disorders, therapeutic approaches, and the increasingly recognized role of Zn2+ as a neurotransmitter in the brain.
1 Introduction
Zinc (Zn2+) is one of the most abundant micronutrients in the human body and is essential for life (Maret, 2009). The human body contains between two and 3 g of Zn2+. Nearly sixty percent of total Zn2+ is found in skeletal muscle, thirty percent in bone, five percent in liver/skin, and the remaining Zn2+ is stored in other tissues.
Dietary intake is one of the most important factors that can affect the plasma Zn2+ pool (Taylor et al., 1991; Barnett et al., 2013). Zn2+ is first absorbed by the small intestine. Organisms dynamically regulate the uptake of Zn2+ in the gastrointestinal tract (Takagishi et al., 2017). Considering that Zn2+ is water-soluble, Zn2+ homeostasis is regulated by endogenous Zn2+ secretion rather than by dietary Zn2+ absorption (Krebs, 2013). When organisms are Zn2+- deficient, the body’s ability to absorb Zn2+ increases up to ninety percent. On the other hand, Zn2+ is secreted from the gastrointestinal tract or is disposed of through sloughing epithelial cells in the mucosa if Zn2+ levels are high (Taylor et al., 1991; Frederickson et al., 2000; Krebs, 2013). If excess Zn2+ is taken, for example, with supplements, abdominal cramps and vomiting may occur. These symptoms usually resolve within a few hours. Once absorbed by the gastrointestinal tract, Zn2+ is carried through blood by serum albumin which is the most abundant blood plasma protein. Around one percent of organismal Zn2+ is found in the blood plasma (Takagishi et al., 2017).
Once dispersed throughout the body, Zn2+ has a variety of essential roles, from brain development to apoptosis across different tissues (Figure 1). Zn2+ is essential for the structure, stability, and activity of hundreds of human proteins (Maret, 2009). In addition, Zn2+ plays an essential role in cellular signaling pathways as well as transcription factors (Hara et al., 2017). In mammalian cells, Zn2+ are either bound to proteins or “free.” In the vast majority of mammalian cell types, “free” Zn2+ is likely not truly free, but is bound by unknown (non-protein) ligands. The free Zn2+ concentration in mammalian cells, while in the picomolar range, likely represents a physiologically significant source of Zn2+, particularly regarding its role in signaling.
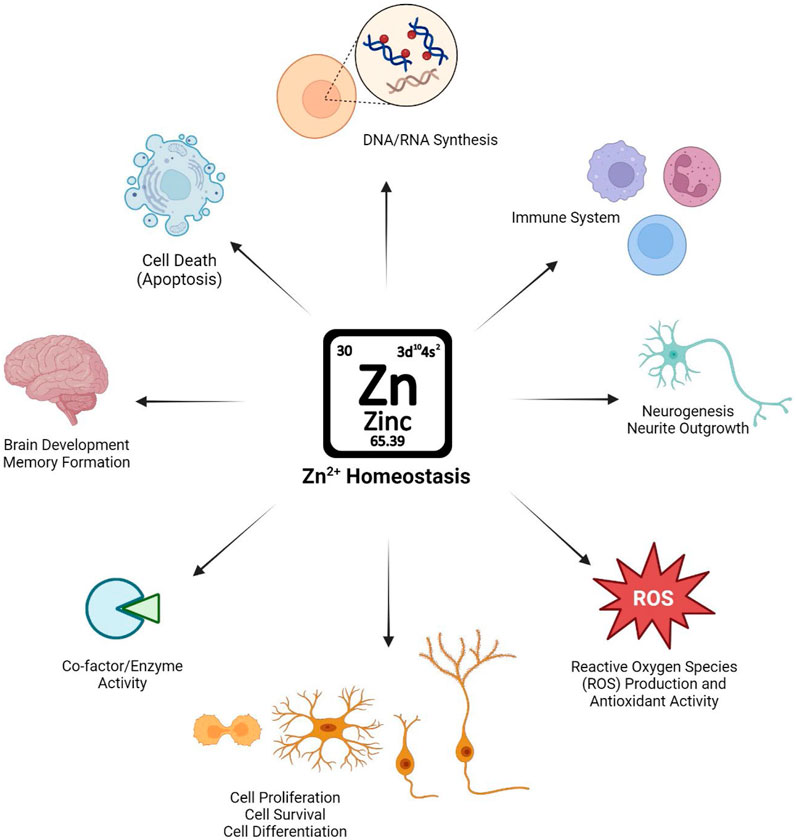
Figure 1. Schematic illustration of the importance of maintaining Zn2+ homeostasis in physiological conditions and Zn2+’s role in biological systems.
Neuronal cells are distinct from other cell types as they contain both “free” Zn2+ as well as Zn2+ which is truly free, i.e., not liganded to biomolecules. This free Zn2+ is localized in presynaptic vesicles, such as in the presynaptic vesicles of glutamatergic nerve terminals. These presynaptic vesicles fuse with the plasma membrane upon neuronal activation thereby releasing Zn2+ into the synaptic cleft. Release of Zn2+ into the synaptic cleft then regulates numerous physiological and pathophysiological functions of the brain, some of which will be described within this review.
2 Zn2+ homeostatic proteins in the brain
When compared to other organs, there is a high concentration of Zn2+ in the brain (∼150 μM) (Wang et al., 2020). Zn2+ is an essential micronutrient in the central nervous system (CNS) (Takeda, 2001). Differing levels of Zn2+ impacts learning, memory, information processing, synaptic plasticity, and regulation of neuronal development (Murakami and Hirano, 2008).
Zn2+ is transported through the bloodstream, often bound to carrier proteins like albumin and transferrin to the blood brain barrier (BBB). Once here, plasma membrane transporters facilitate the passage of Zn2+ across the blood-brain barrier. Zn2+ transport across neuronal membranes, including the BBB, is governed by two families within the solute carrier (SLC) superfamily of proteins. SLCs are one of two major membrane transport proteins superfamilies. SLCs include over 400 member proteins organized into 66 families (Colas et al., 2016). These proteins function to transport a diverse set of substrates including ions and micronutrients across biological membranes. When compared to the second major superfamily of membrane transport proteins [ATP-binding cassette (ABC) proteins], the physiological role of SLCs in human health and disease is not well understood. However, in recent years it has become increasingly recognized that SLCs have important roles in physiological processes. Dysfunction of SLCs can result in both rare and common diseases, thereby opening new opportunities for therapeutic targets (Lin et al., 2015).
Once inside neuronal cells Zn2+ is delivered to, or taken from, these transport proteins by a variety of chaperones. These chaperones transfer Zn2+ to other proteins including metalloenzymes and metalloproteins. Despite a long-standing search, the identify of these chaperones has long been elusive. However, and as will be described here, recent studies have identified the first intracellular Zn2+ chaperone. This provides an exciting opportunity to understand how this essential transition metal moves through cells.
2.1 ZnT family in the brain
In humans, SLC30 genes encode ten ZnT transporters (ZnT1-ZnT10) which function to decrease cytosolic Zn2+ levels either by transport out of the cell or into intracellular organelles (Hara et al., 2022). ZnTs are part of the larger Cation Diffusion Facilitator (CDF) protein family that contributes to transport of a variety of divalent ions, including Zn2+, Mn2+, and Fe2+ (Montanini et al., 2007). The expression of ZnTs is tightly and dynamically regulated, based on changes in cellular levels of Zn2+.
ZnT proteins are expressed dynamically and differentially in different neuronal cell types (see Table 1). ZnT’s in the brain have a critical role in Zn2+ homeostatic physiological and pathophysiological prospects. Dysfunction of these proteins have been correlated to numerous diseases. ZnT1 (SLC30A1) is the predominant surface expressed Zn2+-exporter in synaptic neurons and glia (Palmiter and Findley, 1995). ZnT1 has been shown to export Zn2+ to the extracellular space in the amygdala, hippocampus and parahippocampal gyrus, superior and middle temporal gyrus, inferior parietal lobule, and cerebellum. It is been shown that there is an increase in surface expression of ZnT1 in amygdala, hippocampus/parahippocampal and inferior parietal lobule of Alzheimer Disease (AD) patients (Lovell et al., 2005). In contrast, ZnT1 surface expression is repressed in superior and middle temporal gyrus. Therefore, there is a strong correlation between ZnT1 expression and senile plaques and neurofibrillary tangle levels in amygdala of AD conditions (Lovell et al., 2005). Interestingly, ZnT1 may play a protective role in glia when Zn2+ levels are high as pre-treatment with Zn2+ induced a four-fold increase in the expression of ZnT1 in astroglia (Nolte et al., 2004). In addition, increasing body mass index (BMI) is correlated with a significant reduction in ZnT1 expression in the brain suggesting a link between ZnT1 and obesity (Olesen et al., 2016).
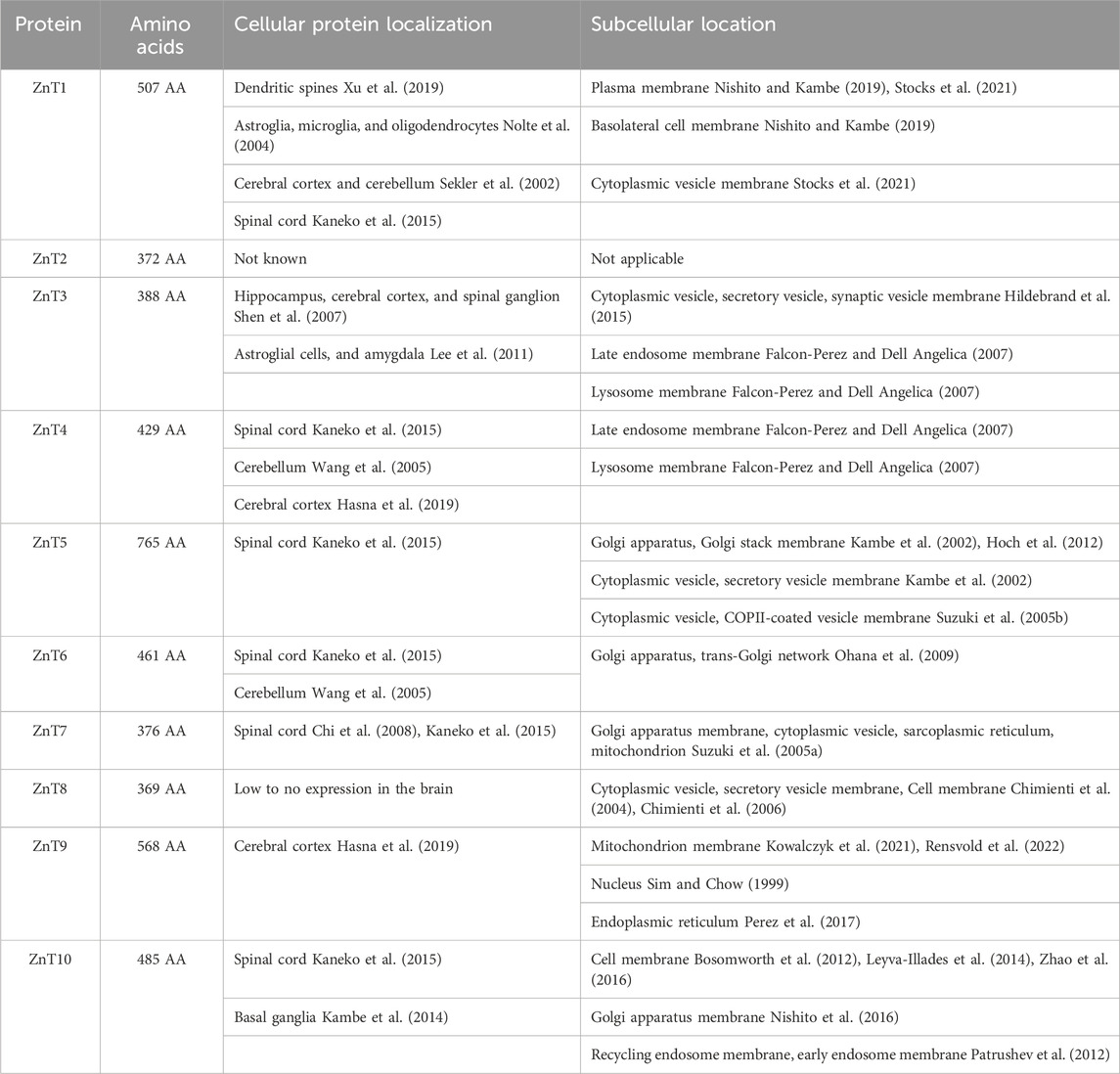
Table 1. List of ZnT proteins, including number of amino acids, major locations of expression in the brain as well as sub-cellular location.
In contrast to ZnT1, the remaining ZnTs found in the brain are expressed within the membranes of intracellular organelles. ZnT3 is predominantly expressed in the brain and has an outsized role in neurons as ZnT3 transports Zn2+ into synaptic vesicles for subsequent release into the synaptic cleft alongside neurotransmitters upon neuronal activation (Ohana et al., 2009; Sensi et al., 2009). Changes in the expression of ZnT3 have been linked to gender-specific susceptibility to AD (Lee et al., 2012). In addition, ZnT3 knockout mice show age-dependent deficits in learning and memory that are evident at 6 months, but not 3 months (Adlard et al., 2010). ZnT4 is expressed in the endo-lysosomal compartment of neurons. While dysfunction of ZnT4 is best known for resulting in Zn2+ deficiency in maternal milk, this protein has also been shown to be expressed in the prefrontal cortex and hippocampus of rats subjected to olfactory bulbectomy, a model of depression (McCormick et al., 2016; Rafalo et al., 2017). ZnT5 is localized to the Golgi apparatus and mediates Zn2+ transport that is essential for proper folding of Zn2+ binding proteins within this compartment (Suzuki et al., 2005a). ZnT5 is expressed in motor neurons (Pfaender et al., 2016). Zn2+ deficiency in motor neurons has been shown to lead to an increase in ZnT5 expression, presumably working towards transporting Zn2+ from the early secretory pathway to the cytosol of these neurons thereby counteracting Zn2+ deficiency conditions (Pfaender et al., 2016). ZnT6 is expressed in the membrane of the Golgi (Huang et al., 2002).
ZnT10 functions to decrease cytosolic Mn2+ levels. Hence, mutations in the ZnT10 gene and/or loss of ZnT10 expression can result in accumulation of Mn2+ in cells (Levy et al., 2019). In a novel mechanism, it has been shown that ZnT10 takes advantage of the Ca2+ gradient to move Mn2+ out of cells. ZnT10 is expressed in the brain and application of external Zn2+ downregulates the expression of this transporter (Bosomworth et al., 2012).
2.2 ZIP family in the brain
The first Zn2+ import transport protein, Zrt1 for Zn2+- regulated transporters, was identified in Saccharomyces cerevisiae in 1996 (Zhao and Eide, 1996). Shortly thereafter, two genes which encode Fe2+ transport proteins, Irt1 and Irt2, for Fe2+- regulated transporters, were identified in Arabidopsis thaliana (Eide et al., 1996). Following a rapid expansion of sequenced genomes, it was shown that there are hundreds of proteins which are homologous to the Zrt and Irt proteins throughout all kingdoms of life. Together, these proteins comprise the SLC39 group of proteins or ZIPs, for Zrt-, Irt-like Proteins. The ZIP family of proteins have been shown or are hypothesized to increase the cytosolic concentration of transition metals, most often Zn2+, but ZIPs can also transport cations including Fe2+, Cu2+, Ni2+, Cd2+, and Mn2+. These transition metals are transported into the cytosol across the plasma membrane or from intracellular organelles. Within humans, ZIPs have four subfamilies (ZIPI, ZIPII, gufA and LIV-1) and 14 members (ZIP1-ZIP14). LIV-1 is the most common subfamily with a predicted metalloprotease motif (domain V) and HSVFEGLAVGIQ conserved sequence in the fourth transmembrane domain. The conserved sequence is proposed as the main part of Zn2+ transport in LIV-1 subfamily members. The second most common subfamily is ZIPII with a conserved sequence (HSVXXGL) in their fourth TMD. Considering that Zn2+ transporters tightly regulate cellular Zn2+ accumulation, alterations in function of these proteins can result in Zn2+ dyshomeostasis and subsequent deleterious outcomes. Between TMDs four and five is a disordered cytoplasmic loop (Bafaro et al., 2015; Bafaro et al., 2019). It is been shown that Zn2+ coordination to this domain regulates ZIP surface expression.
ZIP transporters are expressed differentially across neuronal cells (Table 2). While ZIP1 transports Zn2+ into post synaptic neurons, ZIP3 transports Zn2+ from the synaptic cleft into dentate gyrus (DG) (Bogdanovic et al., 2022). Both ZIP1 and ZIP3 are expressed in hippocampal neurons and deliver approximately half of Zn2+ to these cells when Zn2+ concentration reaches the low micromolar range (Qian et al., 2011a). Interestingly, deletion of ZIP1 and ZIP3 reduces A1 pyramidal cell injury suggesting that reduced postsynaptic Zn2+ entry is a neuroprotectant in these cells.
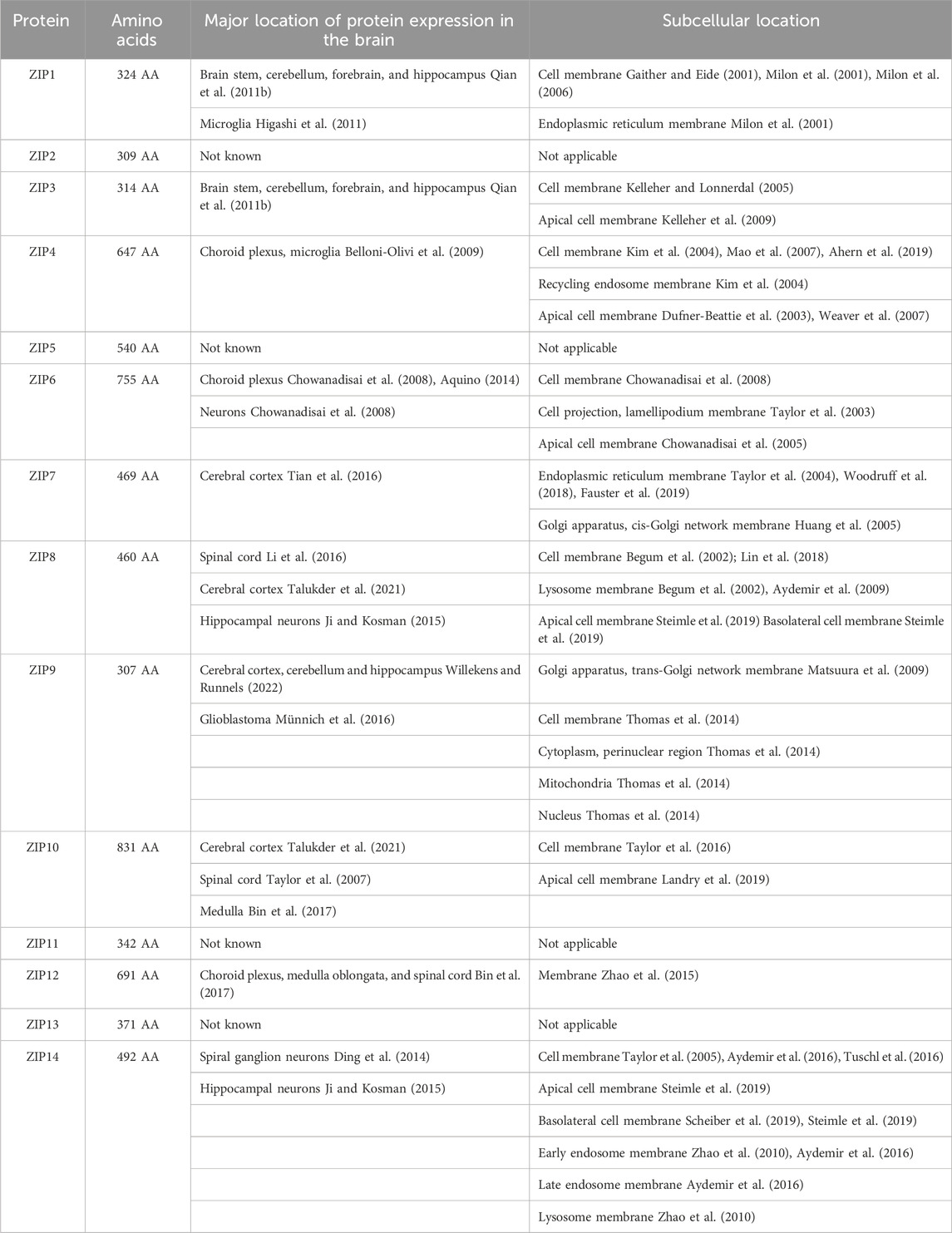
Table 2. List of ZIP transporters, including number of amino acids, major locations of expression in the brain as well as sub-cellular location.
ZIP4 is the most well understood ZIP transporter. ZIP4 was originally identified as it is expressed in the intestine, the main location of Zn2+ uptake. Mutations to this protein can lead to Acrodermatitis enteropathica, a Zn2+ deficiency disease. However, ZIP4 is also expressed in excitatory synapses where it associates in a complex with postsynaptic scaffold proteins (De Benedictis et al., 2021). Here, it has been suggested that ZIP4 is involved in regulating synaptic Zn2+ levels. ZIP6 has been observed both in rat neurons as well as in the human neuroblastoma cell model SH-SY5Y (Chowanadisai et al., 2008).
Neuronal Ceroid Lipofuscinoses (NCL) are fatal childhood neurodegenerative lysosomal diseases. NCL is most often associated with mutations within Ceroid Lipofuscinosis Neuronal (CLN) genes. CLN genes encode thirteen proteins that localize throughout the endomembrane system to regulate a variety of cellular processes. Mutations in CLN genes cause a devastating form of neurodegeneration commonly known as Batten disease (Huber, 2023). It was recently shown that the ER/Golgi-localized ZIP7 colocalizes with CLN6 (Grubman et al., 2014). Under these conditions, the expression level of ZIP7 decreases and it has been hypothesized that loss of ZIP7 may result in subcellular deregulation of biometal homeostasis in NCLs.
ZIP8 and ZIP14 are commonly mentioned together as they both transport Mn2+ across biological membranes. ZIP8 is a plasma membrane protein which mediates the uptake of Zn2+, Mn2+ and Fe2+ (Lin et al., 2017). ZIP14 transports Mn2+ as well as Zn2+into cells (Girijashanker et al., 2008; Fujishiro et al., 2014; Xin et al., 2017). Mn2+ is an essential micronutrient in human health as Mn2+ imbalances in the brain may cause parkinsonism–dystonia (Roth, 2014). Mutations in ZIP14 have been shown to disrupt Mn2+ homeostasis and cause childhood-onset parkinsonism–dystonia (Tuschl et al., 2016). In contrast to what one might expect from deletion of a transition metal importer, when ZIP14 is knocked out in mice, increased Mn2+ levels were observed in the brain as was diminished motor activity suggesting that ZIP14 function is an essential factor required to prevent Zn2+-linked neurodegeneration (Aydemir et al., 2017).
In contrast to ZIP8 and ZIP14, ZIP12 transports Zn2+ transporters and is expressed to a high level in the brain (Takagishi et al., 2017). ZIP12 is essential in the activation of cAMP response element binding protein (CREB) signaling for neuronal differentiation, neurite outgrowth, and tubulin polymerization (Chowanadisai et al., 2013). Although the association of ZIP12 in human diseases is not clear, ZIP12 mRNA is increased in brain regions of schizophrenic patients (Davis et al., 2021). ZIP12 is also thought to have a role in neural tube closure and embryonic development in Xenopus tropicalis (Davis et al., 2021).
While many studies in the literature have focused on expression patterns of single ZIP genes, in recent years, more systemic approaches have been utilized to quanitfy changes in expression profiles as undifferentiated cells were differentiated to neurons. For example, mouse fetal Neural Stem Progenitor Cells (NPSC) were cultured with 1.5 µM Zn2+ (Mori et al., 2024). Upon differentiation, ZIP1, ZIP4, ZIP12, and ZIP13 were expressed at a higher rate, while ZIP8 was downregulated. In addition, ZnT1, ZnT8 and ZnT10 were upregulated. This provides evidence that the expression profiles of multiple ZIPs are impacted upon neurodifferentiation.
2.3 Chaperones
Serum albumin is the major carrier for Zn2+ in plasma (Blindauer et al., 2009). Zn2+ coordinates to two binding sites within serum albumin, Site A and Site B. Site A of serum albumin has a moderate (micromolar) affinity for Zn2+ and therefore this labile pool of Zn2+ is responsible for the largest portion of exchangeable plasma Zn2+ pool (Kassaar et al., 2015). Recently, it has been suggested that serum albumin delivers Zn2+ to ZIPs and that allosteric inhibition of Zn2+-binding to albumin by free fatty acids increased Zn2+ influx (Coverdale et al., 2022). However, evidence directly linking transfer of Zn2+ from serum albumin to ZIPs remains unavailable.
It has been proposed that cellular Zn2+ resides in one of three pools: 1) free Zn2+ which is uncoordinated in solution, 2) loosely bound Zn2+ which can associate and dissociate from biomolecules such as metallothionein, and 3) tightly bound Zn2+ which cannot react with other biomolecules or be readily released (Krezel and Maret, 2006). Considering that uncoordinated Zn2+ is practically non-existent in most cell types, it has long been recognized that Zn2+ is largely bound to proteins and other biomolecules (Vallee, 1959). This requires that once Zn2+ is transported across the plasma membrane, there must be chaperones that move Zn2+ to its ultimate destination(s). However, identification of these chaperones is better understood in bacterial systems (Kandari et al., 2021).
There have been initial attempts to examine the proteins and mechanism that mediate Zn2+ intracellular movement. Zn2+ homeostasis in the brain, as in all cells, is tightly controlled by metallothionein’s (MTs). MTs are small, cysteine-rich proteins that play important roles in metal homeostasis and protect cells against heavy metal toxicity, DNA damage and oxidative stress. MTs have the potential to bind multiple transition metals including Zn2+ and Cu2+. MTs can also coordinate toxic metals such as Cd2+ and Hg2+. There are 4 MTs expressed in humans (MT1, MT2, MT3 and MT4). Up to seven Zn2+ ions can coordinate to MTs in a tetrahedral geometry. MT3 is expressed in astrocytes, cerebellar cortex and Zn2+ enriched neurons where it sequesters Zn2+ in synaptic vesicles (Masters et al., 1994).
Two groups recently described a family of COG0523 proteins, conserved from yeast to humans, whose members function as Zn2+ metallochaperones (Pasquini et al., 2022; Weiss et al., 2022). These proteins, named Zn2+-regulated GTPase metalloprotein activator 1 (ZNG1) have been shown to directly transfer Zn2+ to type 1 metallopeptidases. This finding provides an exciting starting point to better understand the molecular mechanism by which Zn2+ is moved through a cell. In addition, considering that these studies showed that disruption of ZNG1 metallochaperone activity results in decreased cellular proliferation and mitochondrial dysfunction, this is an important starting point to understand to decipher how Zn2+ dyshomeostasis can result in disease states.
3 Zn2+ as a neurotransmitter
Zn2+ serves as a crucial signaling molecule within the synaptic cleft, playing multifaceted roles in modulating neurotransmission and synaptic plasticity. Upon neuronal excitation, Zn2+ is released from synaptic vesicles alongside neurotransmitters such as glutamate and GABA. Within the synaptic cleft, Zn2+ directly activates or modulates a variety of receptors and ion channels, exerting both excitatory and inhibitory effects on synaptic transmission. Through its dynamic regulation of synaptic signaling pathways, Zn2+ contributes to the fine-tuning of synaptic activity, synaptic plasticity, and ultimately, neuronal communication within neural circuits. Interestingly, as we will describe below, that while there is recent evidence Zn2+ can directly gate a neuronal ion channel the role of this protein in synaptic signaling remains unclear.
3.1 Zinc Activated Channels
The Zn2+ Activated Channel (ZAC), is encoded by the ZACN gene. ZAC is a Cys-loop receptor (CLR) and comprises its own unique subfamily within the pentameric ligand-gated ion channels (LGIC) as ZAC diverged from neighboring proteins early in evolution. Genes for ZAC are present in humans, zebrafish, and dogs. However, the gene encoding ZAC is a non-functional pseudogene in mouse and rat genomes (Davies et al., 2003; Houtani et al., 2005). ZAC has been shown to be expressed in fetal and adult brain as well as the spinal cord. In addition, tissue-specific expression studies show that ZAC expression coincides with neuronal regions of high Zn2+ levels as human (h) ZAC mRNA is present in human hippocampal, striatum, amygdala, and thalamus tissues (Houtani et al., 2005).
The hZAC gene encodes a 411-residue protein with four TransMembrane Domains (TMD) and an extracellular N-terminus domain. This extracellular domain encodes the signature Cys-loop motif. ZAC is a non-selective monovalent cationic ion channel which can be activated by Zn2+ and Cu2+ (Trattnig et al., 2016). ZAC is the only known human ion channel to be directly activated by transition metals. To date, there is little information on the mechanism or physiological significance of ZAC.
3.2 Zinc modulates neurotransmitters
Synaptic vesicles of glutaminergic, glycinergic and GABAergic neurons in the hippocampus possess high concentration of Zn2+. In fact, fifteen percent of Zn2+ in the brain can be found in synaptic vesicles (Frederickson, 1989). Zn2+ is transported into these vesicles by ZnT3. When an action potential reaches the presynaptic terminal, it causes synaptic vesicles to fuse to the plasma membrane of the neuron and release Zn2+ as well as co-localized neurotransmitter(s) into the synaptic cleft.
The region most susceptible to Zn2+ deficiency in the brain is the hippocampus. Here, Zn2+ deficiency results in impaired neuronal proliferation, differentiation, and activation of apoptotic pathways, thus leading to unalterable impairment of learning and memory capacity during early development. In addition, if large quantities of Zn2+ are released from the presynaptic cleft to the postsynaptic neurons neurotoxicity can result. Neurotoxicity due to excess Zn2+ in the synaptic cleft can also be the result of traumatic injury (Morris and Levenson, 2017).
In the central nervous system, glutamate is the predominant excitatory neurotransmitter involved in numerous neural functions including learning and memory, long-term potentiation, and synaptic plasticity (Zhou and Danbolt, 2014). In the brain, glutamate binds several receptors. Glutamate receptors are classified into two main subgroups: ionotropic receptors and metabotropic receptors. Ionotropic receptors are transmembrane ligand-gated ion channels while metabotropic receptors act either directly or indirectly as signal transduction enzymes. There are three types of ionotropic glutamate receptors (Figure 2): N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methy-4-isoxazole propionic acid (AMPA) and kainite receptors. Zn2+ directly inhibits NMDA-sensitive glutamate-gated channels by two separate mechanisms: high-affinity binding to N-terminal domains of GluN2A subunits reduces channel open probability, and low-affinity voltage-dependent binding to pore-lining residues blocks the channel (Amico-Ruvio et al., 2011). Synaptically released Zn2+ modulates AMPA receptors and impacts fast excitatory neurotransmission and plasticity in glutamatergic synapses (Kalappa et al., 2015). Finally, it is been shown that synaptically released Zn2+ inhibits postsynaptic kainate receptors at mossy fiber synapses (Mott et al., 2008). Therefore, disruption of Zn2+ transport into synaptic vesicles can have broad impacts on neuronal development.
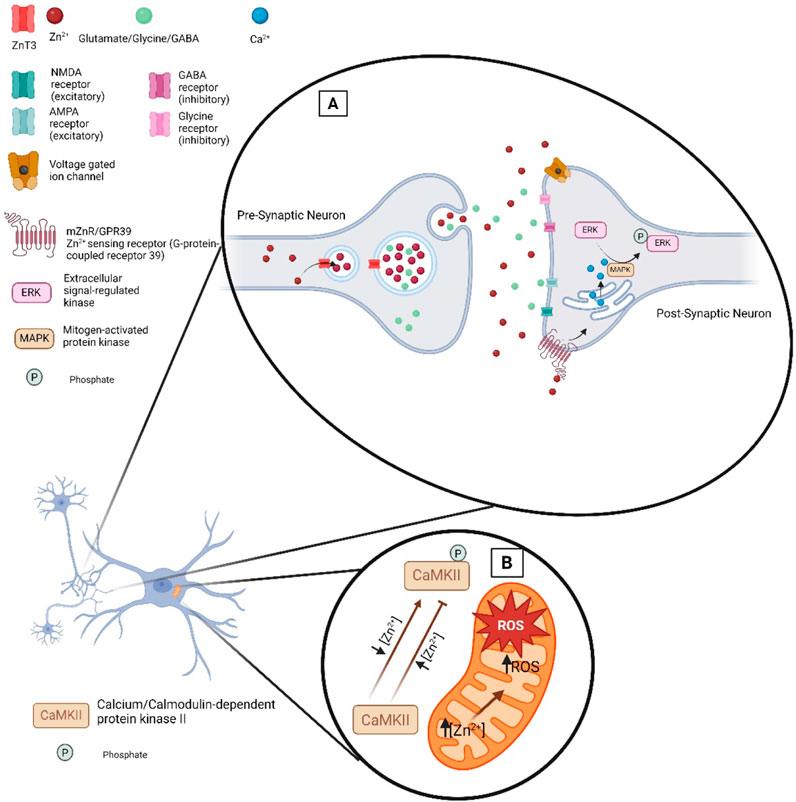
Figure 2. Zn2+ at the synaptic cleft. (A) Illustration of the proteins that regulate and are impacted by Zn2+ at the synaptic cleft. ZnT3 loads Zn2+ into presynaptic vesicles where upon neuronal activation it is released into the synaptic cleft alongside neurotransmitters. Zn2+ release in the synaptic cleft can modulate excitatory or inhibitory receptors. Transient increases in Zn2+ can modulate the function of voltage gated ion channels thereby impacting neuronal excitability. (B) Zn2+ signaling in ROS level regulation in mitochondria.
As a more specific physiological relevant example, it has been shown that Zn2+ has multiple roles in how neurons respond to sounds of different volumes (Anderson et al., 2017). First, the addition of Zn2+ causes excitatory neurons to increase responses to sounds. Second, addition of Zn2+ causes inhibitor neurons to decrease their responses to sounds. Taken together, it was suggested that Zn2+ enables the brain to process sounds when moving from one environment to another that has higher or lower sounds.
3.3 Zinc as a signaling molecule
Changes in extracellular Zn2+ levels impact a myriad of cellular signaling processes. For example, the plasma membrane G protein-coupled receptor 39 (mZnR/GPR39) senses changes in extracellular Zn2+ (Hershfinkel, 2018; Xia et al., 2022). Once Zn2+ binds to mZnR/GPR39 the ERK/MAPK and PI3K/AKT signaling pathways can be activated. Disruption of this signaling pathway can contribute to neurodegeneration (Abramovitch-Dahan et al., 2016; Khan, 2016; Rychlik and Mlyniec, 2019). Zn2+ has been shown to modulate the phosphorylation state of proteins including transcription factors by activating or inhibiting interactions of Zn2+ with several enzymes. Therefore, changes in Zn2+ levels regulate gene expression and biological outcomes. In addition, Zn2+ can function to suppress phosphatase activity and promote phosphorylation reactions. The activation of proteins and subsequent cell signaling pathways are modulated by changes in intracellular levels of Zn2+, including proteins such as MAPK, Ca2+/calmodulin-activated protein kinase-2 (CaMPK-2), protein kinase C (PKC), P70S6 kinase (P70S6K), cyclic nucleotide phosphodiesterases (PDE), and protein tyrosine phosphatases (PTP) (Figure 2) (Costa et al., 2023).
Changes in cellular Ca2+ levels can alter cellular Zn2+ levels and vice versa. Activation of the ZnR/GPR39 receptor leads to mobilization of Ca2+ in the cytosol and ER reservoirs (Sato et al., 2016). Increasing intracellular Ca2+ levels can trigger Zn2+ release, ROS production and Zn2+ waves which are generated from the endoplasmic reticulum (ER). Furthermore, Ca2+/calmodulin contributes Zn2+ regulation in cells under oxidative stress by NO signal generation. Under oxidative stress conditions, Zn2+ initiates antioxidant and repair response to restore cellular balance. Zn2+ can also modulate the activation of the major neuronal kinase, serine/threonine-specific kinase, CaMPK-2 (Figure 2) (Poddar et al., 2016). It has been reported that low levels of Zn2+ stimulate kinase activity, whereas high concentration of Zn2+ inhibits the binding of Ca2+/calmodulin and inactivate the substrate phosphorylation activity of CaMPK-2. Moreover, Ca2+ and Zn2+ shape each other’s intraneuronal dynamics (Dorward et al., 2023).
4 Zn2+’s role in neurodevelopment
Zn2+ plays a pivotal role in neurodevelopment, orchestrating a myriad of processes critical for the formation, maturation, and function of the nervous system. Zn2+ is intricately involved in various aspects of neuronal development, including neurogenesis, neuronal migration, synaptogenesis, and myelination. Zn2+ also serves as a cofactor for numerous enzymes and transcription factors involved in DNA synthesis, cell proliferation, and differentiation, thereby influencing the generation and organization of neural cells. Dysfunction in Zn2+ homeostasis during critical periods of neurodevelopment has been implicated in a range of neurological disorders, underscoring the significance of Zn2+ in shaping the structural and functional architecture of the developing brain.
4.1 Neurodifferentiation and Zn2+
Neurodifferentiation is a multi-stage process that involves morphological and functional changes of precursor cells into mature neurons (Martorana et al., 2018). Neurodifferentiation begins with the proliferation of neural progenitor cells, which subsequently undergo differentiation into various types of neurons and glial cells, each with distinct functions. This complex process involves a series of molecular signals and genetic cues that regulate cell fate determination, migration, and connectivity, ultimately sculpting the intricate circuitry of the brain and spinal cord. Neurodifferentiation plays a critical role in shaping the structure and function of the nervous system, enabling it to carry out a vast array of cognitive, sensory, and motor functions essential for human life. During neurodifferentiation, physical changes are accompanied by the expression of various membrane proteins and receptors (Ernfors et al., 1990).
Transcription factors influence the ability of precursor cells to differentiate into different neuronal cell types and thus impact the formation of different brain areas and sub-structures (Silbereis et al., 2016). Transcription factors are a family of protein molecules that drive gene transcription by binding directly and/or indirectly to upstream genome regulatory elements of protein-coding genes. Among these transcription factors, are Zn2+ finger proteins which participate in brain development (Grinberg et al., 2004; Nowick et al., 2009). Zn2+ coordinates to Zn2+ finger proteins, thereby regulating gene expression. Thus, it should come as no surprise that Zn2+ deficiency limits growth in children and can result in mental retardation and learning disabilities. Fortunately, it has been shown that supplemental Zn2+ can improve spatial memory, learning and exploratory activities (Piechal et al., 2016).
Among transcription factors, the C2H2-type Zn2+ finger proteins form the largest family in the animal kingdom (Nowick et al., 2010). The C2H2-type Zn2+ finger encodes a consensus sequence, CX2-4CX12HX2-8H where X is any amino acid. These proteins are small peptide domains, which upon Zn2+ coordination bind directly or indirectly to upstream DNA sequences to regulate gene transcription. Once Zn2+-containing C2H2-type Zn2+ finger proteins are bound to DNA, proteins including cofactors and RNA polymerase II are recruited to initiate and modulate transcription rates of downstream coding sequences (Urrutia, 2003).
Evidence of the importance of C2H2-type Zn2+ finger in neurodifferentiation includes studies showing that Zeb1, a C2H2-type Zn2+ promotes differentiation of radial glial progenitor cells (Yan et al., 2017). Zeb1 performs this function by acting as a transcriptional repressor, thereby regulating proliferation, migration and differentiation. The highest level of expression of Zeb1 are observed during neocortical development and then decreases following birth. Interestingly, genome wide association studies have linked Zeb1 with schizophrenia (Borglum et al., 2014).
ZNF536 is another C2H2-type Zn2+ transcription factor expressed in the brain, including the cerebral cortex, hippocampus, and hypothalamic area (Qin et al., 2009). As P19 cells are differentiated with retinoic acid, it was observed that ZNF536 expression increases. Furthermore, while overexpression of ZNF536 inhibits retinoic acid-induced differentiation, depletion of ZNF539 has the opposite effect. As a consequence of these experiments, it has been proposed that ZNF539 is involved in negative regulation of transcription by RNA polymerase II.
More recent efforts in understanding role of C2H2-type Zn2+ finger transcription factors have included high throughput methods. For example, leveraging the power of CRISPR-Cas in screening all ∼1900 human genome transcription factors, identified one C2H2-type Zn2+ transcription factor, ZBTB18, involved in neurodifferentiation (Lu et al., 2023). Loss of this protein resulted in cells which had cytoskeletal defects and stunted neurites/spines.
4.2 Neurite outgrowth
Newly generated neurons undergo neurite outgrowth to establish connections with other neurons and form neural circuits. In other words, neurite outgrowth refers to the extension of neurites (axons and dendrites) from neurons (Bostrom et al., 2010). Zn2+ is an essential participant in the neurogenesis process (Fidalgo et al., 2011). Neurite outgrowth occurs after neuronal differentiation from stem cell precursors and following the migration of immature neurons from their origin site in the embryo to their final positions. This is an essential step in nervous system development, as it produces new projections for the wiring of neurons.
Zn2+ has a direct impact on neurite outgrowth. Using adipose-derived mesenchymal stem cells, which can differentiate into neurons, it was shown that addition of Zn2+ promoted outgrowth, while addition of the chelator CaEDTA decreased the level of outgrowth (Moon et al., 2018). On the molecular level, Zn2+-enhanced neurite outgrowth was the result of inactivation of RhoA. The activated form of RhoA, V14RhoA, has been shown to inhibit the initiation of neuronal differentiation while the inactivated form of RhoA is necessary for neurite outgrowth (Sebok et al., 1999). Addition of Zn2+ promoted the expression of microtubule-associated protein 2 (MAP2) and nestin (NES), two neuronal markers (Moon et al., 2018). Correlated to these results is the observation that mouse neurons produce fewer and shorter neurites after the Zn2+ importer, ZIP12, is knocked down (Chowanadisai et al., 2013). Chelation of Zn2+ has the same impact as when ZIP12 is knocked down in neurons. In contrast, loading neurons with Zn2+ reduced the impact of ZIP12 knockdown neurons on neurite outgrowth.
A C2H2-type Zn2+-finger protein (DISC1- Zn2+ finger protein or DBZ) has been implicated in impacting neurite length in PC12 cells. DBZ is expressed solely in the brain of mice and is highly expressed in the cerebral cortex, hippocampus, olfactory tubercle, and striatum (Hattori et al., 2007). In PC12 cells, DBZ co-localizes with disrupted-in schizophrenia (DISC1). These two proteins were shown to interact with each other by immunoprecipitation. Furthermore, expression of DBZ led to a significant decrease of neurite length, implicating DBZ in neurite outgrowth.
5 Zn2+ as a function of age
Zn2+ levels change based on genetics, sex, nutritional intake, health status, physiological conditions, and age (Qu et al., 2020). Serum Zn2+ levels in newborn babies (70–150 μg/dL) decrease shortly after birth (60–120 μg/dL) (Hotz et al., 2003). Individuals, less than 10 years of age, with serum Zn2+ levels of less than 65 μg/dL are considered to be Zn2+ deficient. Above 10 years of age, Zn2+ levels are considered to be normal if they are at least 66 and 70 μg/dL for females and males, respectively. Recently, changes in Zn2+ levels as a function of aging have been quantified in the Japanese population (Yokokawa et al., 2024). Here it was shown that the proportion of patients with Zn2+ deficiency increased with age. Approximately 33% of patients 20–29 years old and 11% of patients in their 80s had a normal range of Zn2+ in their blood serum. Among the younger group of patients (20–29 years old), only 16% of them had Zn2+ deficiency (<60 μg/dL). However, Zn2+ deficiency increased to about 45% among those in their 80s (Yokokawa et al., 2024).
Zn2+ levels in the brain change during one’s lifespan (Santhakumar et al., 2018). Zn2+ levels remain relatively stable during childhood and adolescence in the brain (Black, 1998). In elderly people, changes in Zn2+ metabolism leads to dyshomeostasis of Zn2+ in certain brain regions including the olfactory bulb, cerebral cortex, and hippocampus (Sikora et al., 2022). In some regions of the brain (the olfactory bulb), Zn2+ levels decrease. While in other parts of the brain, Zn2+ levels increase (such as the hippocampus) (Sikora et al., 2022). Excessive Zn2+ accumulation in the hippocampus may contribute to synaptic dysfunction and cognitive decline associated with aging. Zn2+ dyshomeostasis is related to aging within the cerebral cortex, as well. As the cerebral cortex is involved in high levels of cognitive functions, alterations in Zn2+ levels within this area could impact cognitive performance in elderly individuals (Vogler et al., 2022). Fluctuations in Zn2+ distribution, transport, and signaling may contribute to age-related cognitive decline, neurodegenerative diseases, and other age-related neuropathological conditions and psychiatric disorders (Takeda et al., 2018; Marchetti et al., 2022). The level of Zn2+ in the brain is essential to support neuronal growth, synaptic plasticity, and cognitive development.
6 Zn2+ and neuronal disease
Considering that Zn2+ directly or indirectly impacts such a wide variety of cellular processes within the nervous system, Zn2+ dyshomeostasis exerts a profound impact on neuronal diseases including neurotransmission, synaptic plasticity, and oxidative stress responses. Dysregulation of Zn2+ homeostasis has been implicated in the pathophysiology of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS), where alterations in Zn2+ levels contribute to protein misfolding, aggregation, and neurotoxicity. Conversely, Zn2+ deficiency or excess has been associated with cognitive impairments, mood disorders, and epilepsy, highlighting the delicate balance required for optimal neuronal function. Understanding the intricate roles of Zn2+ in neuronal diseases offers promising avenues for therapeutic interventions aimed at restoring zinc homeostasis and ameliorating neurological dysfunction.
6.1 Zn2+ and Alzheimer’s disease
The β-amyloid precursor protein (APP) is a single-pass 695 residue transmembrane protein (Figure 3A). APP encodes a large extracellular region and a small intracellular domain. The extracellular domain encodes three distinct domains including E1, KPI and E2. E1 has also been proposed to be important for cell adhesion. The KPI domain is normally expressed in non-neuronal cells (Rohan de Silva et al., 1997). The E2 site can readily dimerize and has multiple metal binding sites (Dahms et al., 2012). The APP can be cleaved by multiple proteases including α-, β-, and γ-secretases. When α-secretase cleaves APP, the product is not amyloidogenic. In contrast when APP is sequentially cleaved by β- and γ-secretases, neurotoxic Aβ peptides are released into the extracellular space. Mutations within APP can result in a decreased rate of cleavage by α-secretase and a subsequent increase in proteolysis by β- γ-secretases. These Aβ peptides can form an oligomeric aggregate, which is the major protein component of amyloid plaques in Alzheimer’s disease (Masters et al., 1985).

Figure 3. Schematic demonstration of the (A) β-amyloid precursor protein (APP) and (B) residues which can coordinate Zn2+ leading to fibrilization.
Zn2+ coordinates with Aβ mainly through N-terminus histidine residues (H6, H13, H14) (Figure 3B) (Minicozzi et al., 2008; Nair et al., 2010; Rezaei-Ghaleh et al., 2011). Zn2+ can also coordinate by with Aβ aspartic acid (D1) and tyrosine (Y10) residues (Danielsson et al., 2007; Lee et al., 2018). Aβ self-assembly into an insoluble aggregated form is affected by metal binding to the Aβ peptide. For example, generation of a histidine- Zn2+-histidine inter-peptide bridge results in an insoluble aggregate (Miura et al., 2000). In addition, it has been shown that high concentrations of Zn2+ enhance Aβ oligomers’ stability thereby increasing cytotoxicity effect of Aβ aggregation. On the other hand, low Zn2+ levels may inhibit Aβ oligomerization aggregation.
6.2 Cerebral ischemia and hypoxia
When resting, the brain uses around twenty percent of the body’s metabolic energy. This includes about twenty percent of the body’s oxygen supply. Therefore, disrupting the blood supply to the brain can have serious health impacts. Ischemia occurs when blood supply to a specific organ tissue or muscle group is limited. This can lead to a lack of oxygen needed for cellular metabolism. Cerebral ischemia (or brain ischemia) can be a medical emergency that occurs when the brain does not receive enough blood flow to meet metabolic needs.
Cerebral ischemia and hypoxia lead to an abnormally high amount of synaptic Zn2+ to be released into the synaptic cleft (Ueba et al., 2018). This can lead to neuronal inflammation and in extreme cases, neuronal cell death. The molecular basis of Zn2+-induced neuronal inflammation/death is a complex process and damage can occur through multiple processes. For example, when Zn2+ levels are elevated in the synaptic cleft, Zn2+ can flow directly into postsynaptic neurons, inducing oxidative stress and resulting in neuronal cell death. In addition, Zn2+ can directly damage neurons by activating microglia to produce proinflammatory facts. Finally, increased levels of Zn2+ can result in the upregulation of inflammatory proteins which then are toxic to neurons. Interestingly, elevated levels of extracellular Zn2+ were reduced in mice with a neuronal-specific ZnT3 knockout (Qi et al., 2023). This suggests that ZnT3 could be a useful target to counteract the impact of elevated Zn2+ levels in ischemic neurons.
6.3 Kufor-Rakeb Syndrome
Kufor-Rakeb Syndrome is a very rare form of inherited juvenile-onset Parkinson’s Disease (PD). While PD usually affects individuals aged 60 and over, onset of KRS symptoms can be observed prior to age 20. Common symptoms of this disease include bradykinesia (slow movement), rigidity and tremors. Mutations in the ATP12A2 gene result in KRS. ATP13A2 is a P-type ATPase. P-type ATPases use the energy generated from ATP hydrolysis to pump cations and small molecules across biological membranes against their chemoelectrical gradient. ATP13A2 is expressed in intracellular vesicular compartments including lysosomes and early and late endosomes and functions to transport transition metals, including Mn2+, Fe2+ and Zn2+, into lysosomes (Ramirez et al., 2006). Therefore, ATP13A2 functions to prevent transition metal toxicity. Consequently, mutations in ATPA12A2 result in abnormally high cytosolic levels of transition metals leading to toxic effects.
7 Zn2+ based therapeutic targets
Considering the central role Zn2+ plays, either directly or indirectly, in initiating or progressing various disease states, regulating Zn2+ levels or the proteins with which Zn2+ coordinates in these pathologies may provide a novel avenue towards effective therapeutics. For example, reduced levels of serum Zn2+ have been observed in patients with Alzheimer’s disease when compared to healthy controls (Ventriglia et al., 2015). This suggests a linkage between Zn2+ dyshomeostasis and AD pathogenesis. This difference could be due to either changes in diet, metabolism or the expression/activities of proteins which regulate Zn2+ levels. In fact, there is accumulating evidence that AD coincides with changes in expression levels for proteins that regulate Zn2+ influx, efflux and homeostasis. It has been observed that protein levels of ZnT1 were altered in a variety of neuronal cell types in AD patients: Higher in amygdala, hippocampus/parahippocampal gyrus and inferior parietal lobule, while ZnT1 protein levels were lower in the superior and middle temporal gyrus (Lovell et al., 2005). In addition, the expression of ZnT4 and ZnT6 were shown to be higher in the hippocampus/parahippocampal gyrus of individuals with early AD and AD (Smith et al., 2006). ZnT6 is higher in the superior and middle temporal gyrus of AD patients (Smith et al., 2006). The expression of ZIP1 increases as a function of age in the human frontal cortex (Olesen et al., 2016). At the same time, higher mRNA levels of ZIP1 were observed in the cortex of AD patients (Beyer et al., 2012). Similar changes in expression levels of ZIP1 have been seen in a Drosophila model of AD leading these authors to suggest that manipulating Zn2+ transporters in AD brains could be a novel therapeutic strategy (Lang et al., 2012). Interestingly, MT-3 has been shown to be downregulated in patients with AD (Yu et al., 2001). In addition, MT-3 has been associated with neurodegenerative diseases including amyotrophic lateral sclerosis. Here, overexpression of MT-3 prevented neuronal death and prolonged the life span of mice modeling amyotrophic lateral sclerosis (Hashimoto et al., 2011). MTs have also been implicated in protecting neurons against Parkinson’s disease in mice (Miyazaki et al., 2007).
Considering these molecular changes, clinical trials which have focused on changing levels of Zn2+ carry a new level of importance. Considering that excess Zn2+ in the synaptic cleft can lead to neuroinflammation and/or death, application of zeolite-based nanomaterials which coordinate Zn2+ have been shown to counteract Zn2+-induced cerebral ischemia (Huang et al., 2022). Trials which included Zn2+ supplements resulted in individuals who had improved performance on cognition tests (Van Rhijn et al., 1990; Potocnik et al., 1997).
8 Perspective/conclusion
Zn2+ is an essential micronutrient used throughout the lifespan of the human brain. Zn2+ is a central participant in neuronal differentiation and as humans age, their levels of Zn2+ vary in a cell-type specific manner. These cell type specific changes are largely mediated by changes in ZIP and ZnT expression profiles. However, considering the central role of Zn2+-finger transcription factors in regulating cellular homeostasis, these changes in Zn2+ levels have an outsized impact on cellular homeostasis. At the same time, there is an increased awareness that changes in Zn2+ levels have been shown to be an important microenvironmental risk factor for the development of neurobiological pathologies. Recent studies suggest that there are important interactions between intracellular/extracellular Zn2+ levels and neuronal responses that may explain some elements of pathogenesis such as Alzheimer’s Disease. However, the long latency between Zn2+ dyshomeostasis and onset of neurological diseases outcome makes it challenging to study these correlations. Enhanced approaches, including new imaging approaches make it plausible to more accurately monitoring Zn2+ levels in a time-resolved manner. This provides hope that it may be possible to reduce the incidence of various neurobiological diseases. In addition therapeutics that specifically target Zn2+ levels could also lead to new avenues to treat neurological diseases. Thus, it is essential that as investigators learn more about the impact of Zn2+ on brain function, one eye is kept towards potential therapeutic approaches.
Author contributions
SS: Writing–original draft, Writing–review and editing. MR: Writing–original draft, Writing–review and editing. RD: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Institutes of Health (R21 NS125242) to RD.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abramovitch-Dahan, C., Asraf, H., Bogdanovic, M., Sekler, I., Bush, A. I., and Hershfinkel, M. (2016). Amyloid β attenuates metabotropic zinc sensing receptor, mZnR/GPR39, dependent Ca2+, ERK1/2 and Clusterin signaling in neurons. J. Neurochem. 139 (2), 221–233. doi:10.1111/jnc.13760
Adlard, P. A., Parncutt, J. M., Finkelstein, D. I., and Bush, A. I. (2010). Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer's disease? J. Neurosci. 30 (5), 1631–1636. doi:10.1523/jneurosci.5255-09.2010
Ahern, M. E., Bafaro, E. M., Cowan, A., and Dempski, R. E. (2019). Quantifying the oligomeric state of hZIP4 on the surface of cells. Biochemistry 58 (13), 1705–1708. doi:10.1021/acs.biochem.9b00131
Amico-Ruvio, S. A., Murthy, S., Smith, T., and Popescu, G. (2011). Zinc effects on NMDA receptor gating kinetics. Biophysical J. 100 (8), 1910–1918. doi:10.1016/j.bpj.2011.02.042
Anderson, C. T., Kumar, M., Xiong, S., and Tzounopoulos, T. (2017). Cell-specific gain modulation by synaptically released zinc in cortical circuits of audition. Elife 6, e29893. doi:10.7554/elife.29893
Aydemir, T. B., Kim, M. H., Kim, J., Colon-Perez, L. M., Banan, G., Mareci, T. H., et al. (2017). Metal transporter Zip14 (Slc39a14) deletion in mice increases manganese deposition and produces neurotoxic signatures and diminished motor activity. J. Neurosci. 37 (25), 5996–6006. doi:10.1523/jneurosci.0285-17.2017
Aydemir, T. B., Liuzzi, J. P., McClellan, S., and Cousins, R. J. (2009). Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-γ expression in activated human T cells. J. Leukoc. Biol. 86 (2), 337–348. doi:10.1189/jlb.1208759
Aydemir, T. B., Troche, C., Kim, M. H., and Cousins, R. J. (2016). Hepatic ZIP14-mediated zinc transport contributes to endosomal insulin receptor trafficking and glucose metabolism. J. Biol. Chem. 291 (46), 23939–23951. doi:10.1074/jbc.m116.748632
Bafaro, E. M., Antala, S., Nguyen, T. V., Dzul, S. P., Doyon, B., Stemmler, T. L., et al. (2015). The large intracellular loop of hZIP4 is an intrinsically disordered zinc binding domain. Metallomics 7 (9), 1319–1330. doi:10.1039/c5mt00066a
Bafaro, E. M., Maciejewski, M. W., Hoch, J. C., and Dempski, R. E. (2019). Concomitant disorder and high-affinity zinc binding in the human zinc- and iron-regulated transport protein 4 intracellular loop. Protein Sci. 28 (5), 868–880. doi:10.1002/pro.3591
Barnett, J. P., Blindauer, C. A., Kassaar, O., Khazaipoul, S., Martin, E. M., Sadler, P. J., et al. (2013). Allosteric modulation of zinc speciation by fatty acids. Biochimica Biophysica Acta (BBA) - General Subj. 1830 (12), 5456–5464. doi:10.1016/j.bbagen.2013.05.028
Begum, N. A., Kobayashi, M., Moriwaki, Y., Matsumoto, M., Toyoshima, K., and Seya, T. (2002). Mycobacterium bovis BCG cell wall and lipopolysaccharide induce a novel gene, BIGM103, encoding a 7-TM protein: identification of a new protein family having Zn-transporter and Zn-metalloprotease signatures. Genomics 80 (6), 630–645. doi:10.1006/geno.2002.7000
Belloni-Olivi, L., Marshall, C., Laal, B., Andrews, G. K., and Bressler, J. (2009). Localization of zip1 and zip4 mRNA in the adult rat brain. J. Neurosci. Res. 87 (14), 3221–3230. doi:10.1002/jnr.22144
Beyer, N., Coulson, D. T., Heggarty, S., Ravid, R., Hellemans, J., Irvine, G. B., et al. (2012). Zinc transporter mRNA levels in Alzheimer's disease postmortem brain. J. Alzheimer's Dis. 29 (4), 863–873. doi:10.3233/jad-2012-112105
Bin, B.-H., Bhin, J., Takaishi, M., Toyoshima, Ke, Kawamata, S., Ito, K., et al. (2017). Requirement of zinc transporter ZIP10 for epidermal development: implication of the ZIP10–p63 axis in epithelial homeostasis. Proc. Natl. Acad. Sci. 114 (46), 12243–12248. doi:10.1073/pnas.1710726114
Black, M. M. (1998). Zinc deficiency and child development. Am. J. Clin. Nutr. 68 (2 Suppl. l), 464S–469S. doi:10.1093/ajcn/68.2.464s
Blindauer, C. A., Harvey, I., Bunyan, K. E., Stewart, A. J., Sleep, D., Harrison, D. J., et al. (2009). Structure, properties, and engineering of the major zinc binding site on human albumin. J. Biol. Chem. 284 (34), 23116–23124. doi:10.1074/jbc.m109.003459
Bogdanovic, M., Asraf, H., Gottesman, N., Sekler, I., Aizenman, E., and Hershfinkel, M. (2022). The ZIP3 zinc transporter is localized to mossy fiber terminals and is required for kainate-induced degeneration of CA3 neurons. J. Neurosci. 42 (13), 2824–2834. doi:10.1523/jneurosci.0908-21.2022
Borglum, A. D., Demontis, D., Grove, J., Pallesen, J., Hollegaard, M. V., Pedersen, C. B., et al. (2014). Genome-wide study of association and interaction with maternal cytomegalovirus infection suggests new schizophrenia loci. Mol. Psychiatry 19 (3), 325–333. doi:10.1038/mp.2013.2
Bosomworth, H. J., Thornton, J. K., Coneyworth, L. J., Ford, D., and Valentine, R. A. (2012). Efflux function, tissue-specific expression and intracellular trafficking of the Zn transporter ZnT10 indicate roles in adult Zn homeostasis. Metallomics 4 (8), 771–779. doi:10.1039/c2mt20088k
Bostrom, M., Khalifa, S., Boström, H., Liu, W., Friberg, U., and Rask-Andersen, H. (2010). Effects of neurotrophic factors on growth and glial cell alignment of cultured adult spiral ganglion cells. Audiology Neurotol. 15 (3), 175–186. doi:10.1159/000251915
Chi, Z. H., Ren, H., Wang, X., Rong, M., Huang, L., and Wang, Z. Y. (2008). The cellular and subcellular localization of zinc transporter 7 in the mouse spinal cord. Histol. Histopathol. 23 (7), 781–787. doi:10.14670/HH-23.781
Chimienti, F., Devergnas, S., Favier, A., and Seve, M. (2004). Identification and cloning of a β-cell–specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 53 (9), 2330–2337. doi:10.2337/diabetes.53.9.2330
Chimienti, F., Devergnas, S., Pattou, F., Schuit, F., Garcia-Cuenca, R., Vandewalle, B., et al. (2006). In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J. Cell Sci. 119 (Pt 20), 4199–4206. doi:10.1242/jcs.03164
Chowanadisai, W., Graham, D. M., Keen, C. L., Rucker, R. B., and Messerli, M. A. (2013). Neurulation and neurite extension require the zinc transporter ZIP12 (slc39a12). Proc. Natl. Acad. Sci. 110 (24), 9903–9908. doi:10.1073/pnas.1222142110
Chowanadisai, W., Kelleher, S. L., and Lönnerdal, B. (2005). Zinc deficiency is associated with increased brain zinc import and LIV-1 expression and decreased ZnT-1 expression in neonatal rats. J. Nutr. 135 (5), 1002–1007. doi:10.1093/jn/135.5.1002
Chowanadisai, W., Lonnerdal, B., and Kelleher, S. L. (2008). Zip6 (LIV-1) regulates zinc uptake in neuroblastoma cells under resting but not depolarizing conditions. Brain Res. 1199, 10–19. doi:10.1016/j.brainres.2008.01.015
Colas, C., Ung, P. M., and Schlessinger, A. (2016). SLC transporters: structure, function, and drug discovery. Medchemcomm 7 (6), 1069–1081. doi:10.1039/c6md00005c
Costa, M. I., Sarmento-Ribeiro, A. B., and Gonçalves, A. C. (2023). Zinc: from biological functions to therapeutic potential. Int. J. Mol. Sci. 24 (5), 4822. doi:10.3390/ijms24054822
Coverdale, J. P. C., van den Berg, H. A., Khazaipoul, S., Bridgewater, H. E., Stewart, A. J., and Blindauer, C. A. (2022). Albumin-mediated extracellular zinc speciation drives cellular zinc uptake. Chem. Commun. (Camb) 58 (53), 7384–7387. doi:10.1039/d2cc02278h
Dahms, S. O., Könnig, I., Roeser, D., Gührs, K. H., Mayer, M. C., Kaden, D., et al. (2012). Metal binding dictates conformation and function of the amyloid precursor protein (APP) E2 domain. J. Mol. Biol. 416 (3), 438–452. doi:10.1016/j.jmb.2011.12.057
Danielsson, J., Pierattelli, R., Banci, L., and Gräslund, A. (2007). High-resolution NMR studies of the zinc-binding site of the Alzheimer's amyloid β-peptide. FEBS J. 274 (1), 46–59. doi:10.1111/j.1742-4658.2006.05563.x
Davies, P. A., Wang, W., Hales, T. G., and Kirkness, E. F. (2003). A novel class of ligand-gated ion channel is activated by Zn2+. J. Biol. Chem. 278 (2), 712–717. doi:10.1074/jbc.m208814200
Davis, D. N., Strong, M. D., Chambers, E., Hart, M. D., Bettaieb, A., Clarke, S. L., et al. (2021). A role for zinc transporter gene SLC39A12 in the nervous system and beyond. Gene 799, 145824. doi:10.1016/j.gene.2021.145824
De Benedictis, C. A., Haffke, C., Hagmeyer, S., Sauer, A. K., and Grabrucker, A. M. (2021). Expression analysis of zinc transporters in nervous tissue cells reveals neuronal and synaptic localization of ZIP4. Int. J. Mol. Sci. 22 (9), 4511. doi:10.3390/ijms22094511
Ding, D., Salvi, R., and Roth, J. A. (2014). Cellular localization and developmental changes of Zip8, Zip14 and transferrin receptor 1 in the inner ear of rats. Biometals 27, 731–744. doi:10.1007/s10534-014-9765-0
Dorward, A. M., Stewart, A. J., and Pitt, S. J. (2023). The role of Zn2+ in shaping intracellular Ca2+ dynamics in the heart. J. general physiology 155 (7), e202213206. doi:10.1085/jgp.202213206
Dufner-Beattie, J., Wang, F., Kuo, Y. M., Gitschier, J., Eide, D., and Andrews, G. K. (2003). The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J. Biol. Chem. 278 (35), 33474–33481. doi:10.1074/jbc.m305000200
Eide, D., Broderius, M., Fett, J., and Guerinot, M. L. (1996). A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl. Acad. Sci. U. S. A. 93 (11), 5624–5628. doi:10.1073/pnas.93.11.5624
Ernfors, P., Ibáñez, C. F., Ebendal, T., Olson, L., and Persson, H. (1990). Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc. Natl. Acad. Sci. U. S. A. 87 (14), 5454–5458. doi:10.1073/pnas.87.14.5454
Falcon-Perez, J. M., and Dell Angelica, E. C. (2007). Zinc transporter 2 (SLC30A2) can suppress the vesicular zinc defect of adaptor protein 3-depleted fibroblasts by promoting zinc accumulation in lysosomes. Exp. Cell Res. 313 (7), 1473–1483. doi:10.1016/j.yexcr.2007.02.006
Fauster, A., Rebsamen, M., Willmann, K. L., César-Razquin, A., Girardi, E., Bigenzahn, J. W., et al. (2019). Systematic genetic mapping of necroptosis identifies SLC39A7 as modulator of death receptor trafficking. Cell Death Differ. 26 (6), 1138–1155. doi:10.1038/s41418-018-0192-6
Fidalgo, M., Shekar, P. C., Ang, Y. S., Fujiwara, Y., Orkin, S. H., and Wang, J. (2011). Zfp281 functions as a transcriptional repressor for pluripotency of mouse embryonic stem cells. Stem Cells 29 (11), 1705–1716. doi:10.1002/stem.736
Frederickson, C. J. (1989). Neurobiology of zinc and zinc-containing neurons. Int. Rev. Neurobiol. 31, 145–238. doi:10.1016/s0074-7742(08)60279-2
Frederickson, C. J., Suh, S. W., Silva, D., Frederickson, C. J., and Thompson, R. B. (2000). Importance of zinc in the central nervous system: the zinc-containing neuron. J. Nutr. 130 (5S Suppl. l), 1471S–83S. doi:10.1093/jn/130.5.1471s
Fujishiro, H., Yoshida, M., Nakano, Y., and Himeno, S. (2014). Interleukin-6 enhances manganese accumulation in SH-SY5Y cells: implications of the up-regulation of ZIP14 and the down-regulation of ZnT10. Metallomics 6 (4), 944–949. doi:10.1039/c3mt00362k
Gaither, L. A., and Eide, D. J. (2001). The human ZIP1 transporter mediates zinc uptake in human K562 erythroleukemia cells. J. Biol. Chem. 276 (25), 22258–22264. doi:10.1074/jbc.m101772200
Girijashanker, K., He, L., Soleimani, M., Reed, J. M., Li, H., Liu, Z., et al. (2008). Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol. Pharmacol. 73 (5), 1413–1423. doi:10.1124/mol.107.043588
Grinberg, I., Northrup, H., Ardinger, H., Prasad, C., Dobyns, W. B., and Millen, K. J. (2004). Heterozygous deletion of the linked genes ZIC1 and ZIC4 is involved in Dandy-Walker malformation. Nat. Genet. 36 (10), 1053–1055. doi:10.1038/ng1420
Grubman, A., Lidgerwood, G. E., Duncan, C., Bica, L., Tan, J. L., Parker, S. J., et al. (2014). Deregulation of subcellular biometal homeostasis through loss of the metal transporter, Zip7, in a childhood neurodegenerative disorder. Acta Neuropathol. Commun. 2, 25. doi:10.1186/2051-5960-2-25
Hara, T., Takeda, Ta, Takagishi, T., Fukue, K., Kambe, T., and Fukada, T. (2017). Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 67 (2), 283–301. doi:10.1007/s12576-017-0521-4
Hara, T., Yoshigai, E., Ohashi, T., and Fukada, T. (2022). Zinc transporters as potential therapeutic targets: an updated review. J. Pharmacol. Sci. 148 (2), 221–228. doi:10.1016/j.jphs.2021.11.007
Hashimoto, K., Hayashi, Y., Watabe, K., Inuzuka, T., and Hozumi, I. (2011). Metallothionein-III prevents neuronal death and prolongs life span in amyotrophic lateral sclerosis model mice. Neuroscience 189, 293–298. doi:10.1016/j.neuroscience.2011.05.034
Hasna, J., Bohic, S., Lemoine, S., Blugeon, C., and Bouron, A. (2019). Zinc uptake and storage during the formation of the cerebral cortex in mice. Mol. Neurobiol. 56 (10), 6928–6940. doi:10.1007/s12035-019-1581-7
Hattori, T., Baba, K., Matsuzaki, S., Honda, A., Miyoshi, K., Inoue, K., et al. (2007). A novel DISC1-interacting partner DISC1-Binding Zinc-finger protein: implication in the modulation of DISC1-dependent neurite outgrowth. Mol. Psychiatry 12 (4), 398–407. doi:10.1038/sj.mp.4001945
Hershfinkel, M. (2018). The zinc sensing receptor, ZnR/GPR39, in health and disease. Int. J. Mol. Sci. 19 (2), 439. doi:10.3390/ijms19020439
Higashi, Y., Segawa, S., Matsuo, T., Nakamura, S., Kikkawa, Y., Nishida, K., et al. (2011). Microglial zinc uptake via zinc transporters induces ATP release and the activation of microglia. Glia 59 (12), 1933–1945. doi:10.1002/glia.21235
Hildebrand, M. S., Phillips, A. M., Mullen, S. A., Adlard, P. A., Hardies, K., Damiano, J. A., et al. (2015). Loss of synaptic Zn2+ transporter function increases risk of febrile seizures. Sci. Rep. 5, 17816. doi:10.1038/srep17816
Hoch, E., Lin, W., Chai, J., Hershfinkel, M., Fu, D., and Sekler, I. (2012). Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc. Natl. Acad. Sci. U. S. A. 109 (19), 7202–7207. doi:10.1073/pnas.1200362109
Hotz, C., Peerson, J. M., and Brown, K. H. (2003). Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976-1980). Am. J. Clin. Nutr. 78 (4), 756–764. doi:10.1093/ajcn/78.4.756
Houtani, T., Munemoto, Y., Kase, M., Sakuma, S., Tsutsumi, T., and Sugimoto, T. (2005). Cloning and expression of ligand-gated ion-channel receptor L2 in central nervous system. Biochem. Biophysical Res. Commun. 335 (2), 277–285. doi:10.1016/j.bbrc.2005.07.079
Huang, L., Kirschke, C. P., and Gitschier, J. (2002). Functional characterization of a novel mammalian zinc transporter, ZnT6. J. Biol. Chem. 277 (29), 26389–26395. doi:10.1074/jbc.m200462200
Huang, L., Kirschke, C. P., Zhang, Y., and Yu, Y. Y. (2005). The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J. Biol. Chem. 280 (15), 15456–15463. doi:10.1074/jbc.m412188200
Huang, Z., Qian, K., Chen, J., Qi, Y., E, Y., Liang, J., et al. (2022). A biomimetic zeolite-based nanoenzyme contributes to neuroprotection in the neurovascular unit after ischaemic stroke via efficient removal of zinc and ROS. Acta Biomater. 144, 142–156. doi:10.1016/j.actbio.2022.03.018
Huber, R. J. (2023). Recent insights into the networking of CLN genes and proteins in mammalian cells. J. Neurochem. 165 (5), 643–659. doi:10.1111/jnc.15822
Ji, C., and Kosman, D. J. (2015). Molecular mechanisms of non-transferrin-bound and transferring-bound iron uptake in primary hippocampal neurons. J. Neurochem. 133 (5), 668–683. doi:10.1111/jnc.13040
Kalappa, B. I., Anderson, C. T., Goldberg, J. M., Lippard, S. J., and Tzounopoulos, T. (2015). AMPA receptor inhibition by synaptically released zinc. Proc. Natl. Acad. Sci. U. S. A. 112 (51), 15749–15754. doi:10.1073/pnas.1512296112
Kambe, T., Hashimoto, A., and Fujimoto, S. (2014). Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol. Life Sci. 71 (17), 3281–3295. doi:10.1007/s00018-014-1617-0
Kambe, T., Narita, H., Yamaguchi-Iwai, Y., Hirose, J., Amano, T., Sugiura, N., et al. (2002). Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic β cells. J. Biol. Chem. 277 (21), 19049–19055. doi:10.1074/jbc.m200910200
Kandari, D., Joshi, H., and Bhatnagar, R. (2021). Zur: zinc-sensing transcriptional regulator in a diverse set of bacterial species. Pathogens 10 (3), 344. doi:10.3390/pathogens10030344
Kaneko, M., Noguchi, T., Ikegami, S., Sakurai, T., Kakita, A., Toyoshima, Y., et al. (2015). Zinc transporters ZnT3 and ZnT6 are downregulated in the spinal cords of patients with sporadic amyotrophic lateral sclerosis. J. Neurosci. Res. 93 (2), 370–379. doi:10.1002/jnr.23491
Kassaar, O., Schwarz-Linek, U., Blindauer, C., and Stewart, A. (2015). Plasma free fatty acid levels influence Zn(2+) -dependent histidine-rich glycoprotein-heparin interactions via an allosteric switch on serum albumin. J. Thrombosis Haemostasis 13 (1), 101–110. doi:10.1111/jth.12771
Kelleher, S. L., and Lonnerdal, B. (2005). Zip3 plays a major role in zinc uptake into mammary epithelial cells and is regulated by prolactin. Am. J. Physiology-Cell Physiology 288 (5), C1042–C1047. doi:10.1152/ajpcell.00471.2004
Kelleher, S. L., Lopez, V., Lönnerdal, B., Dufner-Beattie, J., and Andrews, G. K. (2009). Zip3 (Slc39a3) functions in zinc reuptake from the alveolar lumen in lactating mammary gland. Am. J. Physiology-Regulatory, Integr. Comp. Physiology 297 (1), R194–R201. doi:10.1152/ajpregu.00162.2009
Khan, M. Z. (2016). A possible significant role of zinc and GPR39 zinc sensing receptor in Alzheimer disease and epilepsy. Biomed. Pharmacother. 79, 263–272. doi:10.1016/j.biopha.2016.02.026
Kim, B.-E., Wang, F., Dufner-Beattie, J., Andrews, G. K., Eide, D. J., and Petris, M. J. (2004). Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J. Biol. Chem. 279 (6), 4523–4530. doi:10.1074/jbc.m310799200
Kowalczyk, A., Gbadamosi, O., Kolor, K., Sosa, J., Andrzejczuk, L., Gibson, G., et al. (2021). Evolutionary rate covariation identifies SLC30A9 (ZnT9) as a mitochondrial zinc transporter. Biochem. J. 478 (17), 3205–3220. doi:10.1042/bcj20210342
Krebs, N. F. (2013). Update on zinc deficiency and excess in clinical pediatric practice. Ann. Nutr. Metab. 62 (Suppl. 1), 19–29. doi:10.1159/000348261
Krezel, A., and Maret, W. (2006). Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 11 (8), 1049–1062. doi:10.1007/s00775-006-0150-5
Landry, G. M., Furrow, E., Holmes, H. L., Hirata, T., Kato, A., Williams, P., et al. (2019). Cloning, function, and localization of human, canine, and Drosophila ZIP10 (SLC39A10), a Zn2+ transporter. Am. J. Physiology-Renal Physiology 316 (2), F263–F273. doi:10.1152/ajprenal.00573.2017
Lang, M., Wang, L., Fan, Q., Xiao, G., Wang, X., Zhong, Y., et al. (2012). Genetic inhibition of solute-linked carrier 39 family transporter 1 ameliorates Aβ pathology in a Drosophila model of alzheimer's disease. PLoS Genet. 8 (4), e1002683. doi:10.1371/journal.pgen.1002683
Lee, J. Y., Cho, E., Seo, J. W., Hwang, J. J., and Koh, J. Y. (2012). Alteration of the cerebral zinc pool in a mouse model of Alzheimer disease. J. Neuropathol. Exp. Neurol. 71 (3), 211–222. doi:10.1097/nen.0b013e3182417387
Lee, J. Y., Kim, J. S., Byun, H. R., Palmiter, R. D., and Koh, J. Y. (2011). Dependence of the histofluorescently reactive zinc pool on zinc transporter-3 in the normal brain. Brain Res. 1418, 12–22. doi:10.1016/j.brainres.2011.08.055
Lee, M.-C., Yu, W. C., Shih, Y. H., Chen, C. Y., Guo, Z. H., Huang, S. J., et al. (2018). Zinc ion rapidly induces toxic, off-pathway amyloid-β oligomers distinct from amyloid-β derived diffusible ligands in Alzheimer’s disease. Sci. Rep. 8 (1), 4772. doi:10.1038/s41598-018-23122-x
Levy, M., Elkoshi, N., Barber-Zucker, S., Hoch, E., Zarivach, R., Hershfinkel, M., et al. (2019). Zinc transporter 10 (ZnT10)-dependent extrusion of cellular Mn2+ is driven by an active Ca2+-coupled exchange. J. Biol. Chem. 294 (15), 5879–5889. doi:10.1074/jbc.ra118.006816
Leyva-Illades, D., Chen, P., Zogzas, C. E., Hutchens, S., Mercado, J. M., Swaim, C. D., et al. (2014). SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. J. Neurosci. 34 (42), 14079–14095. doi:10.1523/jneurosci.2329-14.2014
Li, D., Wang, G., Han, D., Bi, J., Li, C., Wang, H., et al. (2016). MP resulting in autophagic cell death of microglia through zinc changes against spinal cord injury. BioMed Res. Int. 2016, 1–14. doi:10.1155/2016/6090316
Lin, L., Yee, S. W., Kim, R. B., and Giacomini, K. M. (2015). SLC transporters as therapeutic targets: emerging opportunities. Nat. Rev. Drug Discov. 14 (8), 543–560. doi:10.1038/nrd4626
Lin, W., Li, D., Cheng, L., Li, L., Liu, F., Hand, N. J., et al. (2018). Zinc transporter Slc39a8 is essential for cardiac ventricular compaction. J. Clin. Investigation 128 (2), 826–833. doi:10.1172/jci96993
Lin, W., Vann, D. R., Doulias, P. T., Wang, T., Landesberg, G., Li, X., et al. (2017). Hepatic metal ion transporter ZIP8 regulates manganese homeostasis and manganese-dependent enzyme activity. J. Clin. Investigation 127 (6), 2407–2417. doi:10.1172/jci90896
Lovell, M. A., Smith, J. L., Xiong, S., and Markesbery, W. R. (2005). Alterations in zinc transporter protein-1 (ZnT-1) in the brain of subjects with mild cognitive impairment, early, and late-stage Alzheimer’s disease. Neurotox. Res. 7, 265–271. doi:10.1007/bf03033884
Lu, C., Garipler, G., Dai, C., Roush, T., Salome-Correa, J., Martin, A., et al. (2023). Essential transcription factors for induced neuron differentiation. Nat. Commun. 14 (1), 8362. doi:10.1038/s41467-023-43602-7
Mao, X., Kim, B. E., Wang, F., Eide, D. J., and Petris, M. J. (2007). A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J. Biol. Chem. 282 (10), 6992–7000. doi:10.1074/jbc.m610552200
Marchetti, M. F., Silva, G.Md, Freiria, C. N., Borim, F. S. A., Brito, T. R.Pd, Milanski, M., et al. (2022). Association between zinc deficiency and cognitive decline in community-dwelling older adults. Ciência Saúde Coletiva 27 (7), 2805–2816. doi:10.1590/1413-81232022277.19932021en
Maret, W. (2009). Molecular aspects of human cellular zinc homeostasis: redox control of zinc potentials and zinc signals. Biometals 22 (1), 149–157. doi:10.1007/s10534-008-9186-z
Martorana, F., Gaglio, D., Bianco, M. R., Aprea, F., Virtuoso, A., Bonanomi, M., et al. (2018). Differentiation by nerve growth factor (NGF) involves mechanisms of crosstalk between energy homeostasis and mitochondrial remodeling. Cell Death Dis. 9 (3), 391. doi:10.1038/s41419-018-0429-9
Masters, B. A., Quaife, C., Erickson, J., Kelly, E., Froelick, G., Zambrowicz, B., et al. (1994). Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J. Neurosci. 14 (10), 5844–5857. doi:10.1523/jneurosci.14-10-05844.1994
Masters, C. L., Simms, G., Weinman, N. A., Multhaup, G., McDonald, B. L., and Beyreuther, K. (1985). Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U. S. A. 82 (12), 4245–4249. doi:10.1073/pnas.82.12.4245
Matsuura, W., Yamazaki, T., Yamaguchi-Iwai, Y., Masuda, S., Nagao, M., Andrews, G. K., et al. (2009). SLC39A9 (ZIP9) regulates zinc homeostasis in the secretory pathway: characterization of the ZIP subfamily I protein in vertebrate cells. Biosci. Biotechnol. Biochem. 73 (5), 1142–1148. doi:10.1271/bbb.80910
McCormick, N. H., Lee, S., Hennigar, S. R., and Kelleher, S. L. (2016). ZnT4 (SLC30A4)-null ("lethal milk") mice have defects in mammary gland secretion and hallmarks of precocious involution during lactation. Am. J. Physiology-Regulatory, Integr. Comp. Physiology 310 (1), R33–R40. doi:10.1152/ajpregu.00315.2014
Milon, B., Dhermy, D., Pountney, D., Bourgeois, M., and Beaumont, C. (2001). Differential subcellular localization of hZip1 in adherent and non-adherent cells. FEBS Lett. 507 (3), 241–246. doi:10.1016/s0014-5793(01)02950-7
Milon, B., Wu, Q., Zou, J., Costello, L. C., and Franklin, R. B. (2006). Histidine residues in the region between transmembrane domains III and IV of hZip1 are required for zinc transport across the plasma membrane in PC-3 cells. Biochimica Biophysica Acta (BBA)-Biomembranes 1758 (10), 1696–1701. doi:10.1016/j.bbamem.2006.06.005
Minicozzi, V., Stellato, F., Comai, M., Serra, M. D., Potrich, C., Meyer-Klaucke, W., et al. (2008). Identifying the minimal copper- and zinc-binding site sequence in amyloid-β peptides. J. Biol. Chem. 283 (16), 10784–10792. doi:10.1074/jbc.m707109200
Miura, T., Suzuki, K., Kohata, N., and Takeuchi, H. (2000). Metal binding modes of alzheimer's amyloid β-peptide in insoluble aggregates and soluble complexes. Biochemistry 39 (23), 7024–7031. doi:10.1021/bi0002479
Miyazaki, I., Asanuma, M., Hozumi, H., Miyoshi, K., and Sogawa, N. (2007). Protective effects of metallothionein against dopamine quinone-induced dopaminergic neurotoxicity. FEBS Lett. 581 (25), 5003–5008. doi:10.1016/j.febslet.2007.09.046
Montanini, B., Blaudez, D., Jeandroz, S., Sanders, D., and Chalot, M. (2007). Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics 8, 107. doi:10.1186/1471-2164-8-107
Moon, M. Y., Kim, H. J., Choi, B. Y., Sohn, M., Chung, T. N., and Suh, S. W. (2018). Zinc promotes adipose-derived mesenchymal stem cell proliferation and differentiation towards a neuronal fate. Stem Cells Int. 2018, 1–9. doi:10.1155/2018/5736535
Mori, H., Goji, A., and Hara, M. (2024). Upregulation of intracellular zinc ion level after differentiation of the neural stem/progenitor cells in vitro with the changes in gene expression of zinc transporters. Biol. Trace Elem. Res. doi:10.1007/s12011-023-04033-z
Morris, D. R., and Levenson, C. W. (2017). Neurotoxicity of zinc. Adv. Neurobiol. 18, 303–312. doi:10.1007/978-3-319-60189-2_15
Mott, D. D., Benveniste, M., and Dingledine, R. J. (2008). pH-dependent inhibition of kainate receptors by zinc. J. Neurosci. 28 (7), 1659–1671. doi:10.1523/jneurosci.3567-07.2008
Münnich, N., Wernhart, S., Hogstrand, C., Schlomann, U., Nimsky, C., and Bartsch, J. W. (2016). Expression of the zinc importer protein ZIP9/SLC39A9 in glioblastoma cells affects phosphorylation states of p53 and GSK-3β and causes increased cell migration. Biometals 29, 995–1004. doi:10.1007/s10534-016-9971-z
Murakami, M., and Hirano, T. (2008). Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 99 (8), 1515–1522. doi:10.1111/j.1349-7006.2008.00854.x
Nair, N. G., Perry, G., Smith, M. A., and Reddy, V. P. (2010). NMR studies of zinc, copper, and iron binding to histidine, the principal metal ion complexing site of amyloid-β peptide. J. Alzheimer's Dis. 20 (1), 57–66. doi:10.3233/jad-2010-1346
Nishito, Y., and Kambe, T. (2019). Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels. J. Biol. Chem. 294 (43), 15686–15697. doi:10.1074/jbc.ra119.010227
Nishito, Y., Tsuji, N., Fujishiro, H., Takeda, Ta, Yamazaki, T., Teranishi, F., et al. (2016). Direct comparison of manganese detoxification/efflux proteins and molecular characterization of ZnT10 protein as a manganese transporter. J. Biol. Chem. 291 (28), 14773–14787. doi:10.1074/jbc.m116.728014
Nolte, C., Gore, A., Sekler, I., Kresse, W., Hershfinkel, M., Hoffmann, A., et al. (2004). ZnT-1 expression in astroglial cells protects against zinc toxicity and slows the accumulation of intracellular zinc. Glia 48 (2), 145–155. doi:10.1002/glia.20065
Nowick, K., Gernat, T., Almaas, E., and Stubbs, L. (2009). Differences in human and chimpanzee gene expression patterns define an evolving network of transcription factors in brain. Proc. Natl. Acad. Sci. U. S. A. 106 (52), 22358–22363. doi:10.1073/pnas.0911376106
Nowick, K., Hamilton, A. T., Zhang, H., and Stubbs, L. (2010). Rapid sequence and expression divergence suggest selection for novel function in primate-specific KRAB-ZNF genes. Mol. Biol. Evol. 27 (11), 2606–2617. doi:10.1093/molbev/msq157
Ohana, E., Hoch, E., Keasar, C., Kambe, T., Yifrach, O., Hershfinkel, M., et al. (2009). Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J. Biol. Chem. 284 (26), 17677–17686. doi:10.1074/jbc.m109.007203
Olesen, R. H., Hyde, T. M., Kleinman, J. E., Smidt, K., Rungby, J., and Larsen, A. (2016). Obesity and age-related alterations in the gene expression of zinc-transporter proteins in the human brain. Transl. Psychiatry 6 (6), e838. doi:10.1038/tp.2016.83
Palmiter, R. D., and Findley, S. D. (1995). Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 14 (4), 639–649. doi:10.1002/j.1460-2075.1995.tb07042.x
Pasquini, M., Grosjean, N., Hixson, K. K., Nicora, C. D., Yee, E. F., Lipton, M., et al. (2022). Zng1 is a GTP-dependent zinc transferase needed for activation of methionine aminopeptidase. Cell Rep. 39 (7), 110834. doi:10.1016/j.celrep.2022.110834
Patrushev, N., Seidel-Rogol, B., and Salazar, G. (2012). Angiotensin II requires zinc and downregulation of the zinc transporters ZnT3 and ZnT10 to induce senescence of vascular smooth muscle cells. PLoS One 7 (3), e33211. doi:10.1371/journal.pone.0033211
Perez, Y., Shorer, Z., Liani-Leibson, K., Chabosseau, P., Kadir, R., Volodarsky, M., et al. (2017). SLC30A9 mutation affecting intracellular zinc homeostasis causes a novel cerebro-renal syndrome. Brain 140 (4), 928–939. doi:10.1093/brain/awx013
Pfaender, S., Föhr, K., Lutz, A. K., Putz, S., Achberger, K., Linta, L., et al. (2016). Cellular zinc homeostasis contributes to neuronal differentiation in human induced pluripotent stem cells. Neural Plast. 2016, 1–15. doi:10.1155/2016/3760702
Piechal, A., Blecharz-Klin, K., Pyrzanowska, J., and Widy-Tyszkiewicz, E. (2016). Influence of long-term zinc administration on spatial learning and exploratory activity in rats. Biol. Trace Elem. Res. 172 (2), 408–418. doi:10.1007/s12011-015-0597-8
Poddar, R., Rajagopal, S., Shuttleworth, C. W., and Paul, S. (2016). Zn2+-dependent activation of the trk signaling pathway induces phosphorylation of the brain-enriched tyrosine phosphatase step: MOLECULAR basis for zn2+-INDUCED ERK MAPK activation. J. Biol. Chem. 291 (2), 813–825. doi:10.1074/jbc.m115.663468
Potocnik, F. C., van Rensburg, S. J., Park, C., Taljaard, J. J., and Emsley, R. A. (1997). Zinc and platelet membrane microviscosity in Alzheimer's disease. The in vivo effect of zinc on platelet membranes and cognition. S Afr. Med. J. 87 (9), 1116–1119.
Qi, Z., Zhou, X., Dong, W., Timmins, G. S., Pan, R., Shi, W., et al. (2023). Neuronal zinc transporter ZnT3 modulates cerebral ischemia-induced blood-brain barrier disruption. Aging Dis., 0. doi:10.14336/ad.2023.1011
Qian, J., Xu, K., Yoo, J., Chen, T. T., Andrews, G., and Noebels, J. L. (2011a). Knockout of Zn transporters Zip-1 and Zip-3 attenuates seizure-induced CA1 neurodegeneration. J. Neurosci. 31 (1), 97–104. doi:10.1523/jneurosci.5162-10.2011
Qian, J., Xu, K., Yoo, J., Chen, T. T., Andrews, G., and Noebels, J. L. (2011b). Knockout of Zn transporters Zip-1 and Zip-3 attenuates seizure-induced CA1 neurodegeneration. J. Neurosci. 31 (1), 97–104. doi:10.1523/jneurosci.5162-10.2011
Qin, Z., Ren, F., Xu, X., Ren, Y., Li, H., Wang, Y., et al. (2009). ZNF536, a novel zinc finger protein specifically expressed in the brain, negatively regulates neuron differentiation by repressing retinoic acid-induced gene transcription. Mol. Cell. Biol. 29 (13), 3633–3643. doi:10.1128/mcb.00362-09
Qu, X., Yang, H., Yu, Z., Jia, B., Qiao, H., Zheng, Y., et al. (2020). Serum zinc levels and multiple health outcomes: implications for zinc-based biomaterials. Bioact. Mater. 5 (2), 410–422. doi:10.1016/j.bioactmat.2020.03.006
Rafalo, A., Zadrozna, M., Nowak, B., Kotarska, K., Wiatrowska, K., Pochwat, B., et al. (2017). The level of the zinc homeostasis regulating proteins in the brain of rats subjected to olfactory bulbectomy model of depression. Prog. Neuro-Psychopharmacology Biol. Psychiatry 72, 36–48. doi:10.1016/j.pnpbp.2016.08.009
Ramirez, A., Heimbach, A., Gründemann, J., Stiller, B., Hampshire, D., Cid, L. P., et al. (2006). Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 38 (10), 1184–1191. doi:10.1038/ng1884
Rensvold, J. W., Shishkova, E., Sverchkov, Y., Miller, I. J., Cetinkaya, A., Pyle, A., et al. (2022). Defining mitochondrial protein functions through deep multiomic profiling. Nature 606 (7913), 382–388. doi:10.1038/s41586-022-04765-3
Rezaei-Ghaleh, N., Giller, K., Becker, S., and Zweckstetter, M. (2011). Effect of zinc binding on β-amyloid structure and dynamics: implications for Aβ aggregation. Biophysical J. 101 (5), 1202–1211. doi:10.1016/j.bpj.2011.06.062
Rohan de Silva, H. A., Jen, A., Wickenden, C., Jen, L. S., Wilkinson, S. L., and Patel, A. J. (1997). Cell-specific expression of β-amyloid precursor protein isoform mRNAs and proteins in neurons and astrocytes. Mol. Brain Res. 47 (1-2), 147–156. doi:10.1016/s0169-328x(97)00045-4
Roth, J. A. (2014). Correlation between the biochemical pathways altered by mutated Parkinson-related genes and chronic exposure to manganese. Neurotoxicology 44, 314–325. doi:10.1016/j.neuro.2014.08.006
Rychlik, M., and Mlyniec, K. (2019). Zinc-mediated neurotransmission in alzheimer's disease: a potential role of the gpr39 in dementia. Curr. Neuropharmacol. 18 (1), 2–13. doi:10.2174/1570159x17666190704153807
Santhakumar, H., Nair, R. V., Philips, D. S., Shenoy, S. J., Thekkuveettil, A., Ajayaghosh, A., et al. (2018). Real time imaging and dynamics of hippocampal Zn(2+) under epileptic condition using a ratiometric fluorescent probe. Sci. Rep. 8 (1), 9069. doi:10.1038/s41598-018-27029-5
Sato, S., Huang, X. P., Kroeze, W. K., and Roth, B. L. (2016). Discovery and characterization of novel GPR39 agonists allosterically modulated by zinc. Mol. Pharmacol. 90 (6), 726–737. doi:10.1124/mol.116.106112
Scheiber, I. F., Wu, Y., Morgan, S. E., and Zhao, N. (2019). The intestinal metal transporter ZIP14 maintains systemic manganese homeostasis. J. Biol. Chem. 294 (23), 9147–9160. doi:10.1074/jbc.ra119.008762
Sebok, A., Nusser, N., Debreceni, B., Guo, Z., Santos, M. F., Szeberenyi, J., et al. (1999). Different roles for RhoA during neurite initiation, elongation, and regeneration in PC12 cells. J. Neurochem. 73 (3), 949–960. doi:10.1046/j.1471-4159.1999.0730949.x
Sekler, I., Moran, A., Hershfinkel, M., Dori, A., Margulis, A., Birenzweig, N., et al. (2002). Distribution of the zinc transporter ZnT-1 in comparison with chelatable zinc in the mouse brain. J. Comp. Neurology 447 (3), 201–209. doi:10.1002/cne.10224
Sensi, S. L., Paoletti, P., Bush, A. I., and Sekler, I. (2009). Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 10 (11), 780–791. doi:10.1038/nrn2734
Shen, H., Wang, F., Zhang, Y., Xu, J., Long, J., Qin, H., et al. (2007). Zinc distribution and expression pattern of ZnT3 in mouse brain. Biol. Trace Elem. Res. 119 (2), 166–174. doi:10.1007/s12011-007-0056-2
Sikora, J., Di Bisceglie Caballero, S., Reiss, D., Kieffer, B. L., Paoletti, P., Jacob, P. Y., et al. (2022). Zn(2+) inhibits spatial memory and hippocampal place cell representation through high-affinity binding to the NMDA receptor GluN2A subunit. iScience 25 (11), 105355. doi:10.1016/j.isci.2022.105355
Silbereis, J. C., Pochareddy, S., Zhu, Y., Li, M., and Sestan, N. (2016). The cellular and molecular landscapes of the developing human central nervous system. Neuron 89 (2), 248–268. doi:10.1016/j.neuron.2015.12.008
Sim, D. L., and Chow, V. T. (1999). The novel human HUEL (C4orf1) gene maps to chromosome 4p12-p13 and encodes a nuclear protein containing the nuclear receptor interaction motif. Genomics 59 (2), 224–233. doi:10.1006/geno.1999.5856
Smith, J. L., Xiong, S., Markesbery, W., and Lovell, M. (2006). Altered expression of zinc transporters-4 and -6 in mild cognitive impairment, early and late Alzheimer's disease brain. Neuroscience 140 (3), 879–888. doi:10.1016/j.neuroscience.2006.02.049
Steimle, B. L., Smith, F. M., and Kosman, D. J. (2019). The solute carriers ZIP8 and ZIP14 regulate manganese accumulation in brain microvascular endothelial cells and control brain manganese levels. J. Biol. Chem. 294 (50), 19197–19208. doi:10.1074/jbc.ra119.009371
Stocks, C. J., von Pein, J. B., Curson, J. E. B., Rae, J., Phan, M. D., Foo, D., et al. (2021). Frontline Science: LPS-inducible SLC30A1 drives human macrophage-mediated zinc toxicity against intracellular Escherichia coli. J. Leukoc. Biol. 109 (2), 287–297. doi:10.1002/jlb.2hi0420-160r
Suzuki, T., Ishihara, K., Migaki, H., Ishihara, K., Nagao, M., Yamaguchi-Iwai, Y., et al. (2005b). Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J. Biol. Chem. 280 (35), 30956–30962. doi:10.1074/jbc.m506902200
Suzuki, T., Ishihara, K., Migaki, H., Matsuura, W., Kohda, A., Okumura, K., et al. (2005a). Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J. Biol. Chem. 280 (1), 637–643. doi:10.1074/jbc.m411247200
Takagishi, T., Hara, T., and Fukada, T. (2017). Recent advances in the role of SLC39A/ZIP zinc transporters in vivo. Int. J. Mol. Sci. 18 (12), 2708. doi:10.3390/ijms18122708
Takeda, A. (2001). Zinc homeostasis and functions of zinc in the brain. Biometals 14 (3-4), 343–351. doi:10.1023/a:1012982123386
Takeda, A., Tamano, H., Hashimoto, W., Kobuchi, S., Suzuki, H., Murakami, T., et al. (2018). Novel defense by metallothionein induction against cognitive decline: from amyloid β1–42-induced excess Zn2+ to functional Zn2+ deficiency. Mol. Neurobiol. 55 (10), 7775–7788. doi:10.1007/s12035-018-0948-5
Talukder, M., Bi, S. S., Jin, H. T., Ge, J., Zhang, C., Lv, M. W., et al. (2021). Cadmium induced cerebral toxicity via modulating MTF1-MTs regulatory axis. Environ. Pollut. 285, 117083. doi:10.1016/j.envpol.2021.117083
Taylor, C. M., Bacon, J., Aggett, P., and Bremner, I. (1991). Homeostatic regulation of zinc absorption and endogenous losses in zinc-deprived men. Am. J. Clin. Nutr. 53 (3), 755–763. doi:10.1093/ajcn/53.3.755
Taylor, K., Morgan, H., Johnson, A., and Nicholson, R. (2005). Structure–function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 579 (2), 427–432. doi:10.1016/j.febslet.2004.12.006
Taylor, K. M., Morgan, H. E., Johnson, A., Hadley, L. J., and Nicholson, R. I. (2003). Structure–function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem. J. 375 (1), 51–59. doi:10.1042/bj20030478
Taylor, K. M., Morgan, H. E., Johnson, A., and Nicholson, R. I. (2004). Structure-function analysis of HKE4, a member of the new LIV-1 subfamily of zinc transporters. Biochem. J. 377 (1), 131–139. doi:10.1042/bj20031183
Taylor, K. M., Morgan, H. E., Smart, K., Zahari, N. M., Pumford, S., Ellis, I. O., et al. (2007). The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol. Med. 13, 396–406. doi:10.2119/2007-00040.taylor
Taylor, K. M., Muraina, I. A., Brethour, D., Schmitt-Ulms, G., Nimmanon, T., Ziliotto, S., et al. (2016). Zinc transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration. Biochem. J. 473 (16), 2531–2544. doi:10.1042/bcj20160388
Thomas, P., Pang, Y., Dong, J., and Berg, A. H. (2014). Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 155 (11), 4250–4265. doi:10.1210/en.2014-1201
Tian, T., Li, L. L., Zhang, S. Q., and Ni, H. (2016). Long-term effects of ketogenic diet on subsequent seizure-induced brain injury during early adulthood: relationship of seizure thresholds to zinc transporter-related gene expressions. Biol. trace Elem. Res. 174, 369–376. doi:10.1007/s12011-016-0730-3
Trattnig, S. M., Gasiorek, A., Deeb, T. Z., Ortiz, E. J. C., Moss, S. J., Jensen, A. A., et al. (2016). Copper and protons directly activate the zinc-activated channel. Biochem. Pharmacol. 103, 109–117. doi:10.1016/j.bcp.2016.02.004
Tuschl, K., Meyer, E., Valdivia, L. E., Zhao, N., Dadswell, C., Abdul-Sada, A., et al. (2016). Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism–dystonia. Nat. Commun. 7 (1), 11601. doi:10.1038/ncomms11601
Ueba, Y., Aratake, T., Onodera, Ki, Higashi, Y., Hamada, T., Shimizu, T., et al. (2018). Attenuation of zinc-enhanced inflammatory M1 phenotype of microglia by peridinin protects against short-term spatial-memory impairment following cerebral ischemia in mice. Biochem. Biophysical Res. Commun. 507 (1-4), 476–483. doi:10.1016/j.bbrc.2018.11.067
Urrutia, R. (2003). KRAB-containing zinc-finger repressor proteins. Genome Biol. 4 (10), 231. doi:10.1186/gb-2003-4-10-231
Vallee, B. L. (1959). Biochemistry, physiology and pathology of zinc. Physiol. Rev. 39 (3), 443–490. doi:10.1152/physrev.1959.39.3.443
Van Rhijn, A., Macintyre, F., Corrigan, F. M., Watt, C., Ijomah, G., and Skinner, E. R. (1990). Plasma lipoprotein profiles and the distribution of high-density lipoprotein subfractions in the elderly: the effect of Alzheimer's disease and multi-infarct dementia. Biochem. Soc. Trans. 18 (2), 324. doi:10.1042/bst0180324
Ventriglia, M., Brewer, G. J., Simonelli, I., Mariani, S., Siotto, M., Bucossi, S., et al. (2015). Zinc in alzheimer's disease: a meta-analysis of serum, plasma, and cerebrospinal fluid studies. J. Alzheimer's Dis. 46 (1), 75–87. doi:10.3233/jad-141296
Vogler, E. C., Mahavongtrakul, M., Sarkan, K., Bohannan, R. C., Catuara-Solarz, S., and Busciglio, J. (2022). Genetic removal of synaptic Zn(2+) impairs cognition, alters neurotrophic signaling and induces neuronal hyperactivity. Front. Neurol. 13, 882635. doi:10.3389/fneur.2022.882635
Wang, L., Yin, Y. L., Liu, X. Z., Shen, P., Zheng, Y. G., Lan, X. R., et al. (2020). Current understanding of metal ions in the pathogenesis of Alzheimer's disease. Transl. Neurodegener. 9, 10. doi:10.1186/s40035-020-00189-z
Wang, Z. Y., Stoltenberg, M., Huang, L., Danscher, G., Dahlström, A., Shi, Y., et al. (2005). Abundant expression of zinc transporters in Bergman glia of mouse cerebellum. Brain Res. Bull. 64 (5), 441–448. doi:10.1016/j.brainresbull.2004.10.001
Weaver, B. P., Dufner-Beattie, J., Kambe, T., and Andrews, G. K. (2007). Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). bchm 388, 1301–1312. doi:10.1515/bc.2007.149
Weiss, A., Murdoch, C. C., Edmonds, K. A., Jordan, M. R., Monteith, A. J., Perera, Y. R., et al. (2022). Zn-regulated GTPase metalloprotein activator 1 modulates vertebrate zinc homeostasis. Cell 185 (12), 2148–2163 e27. doi:10.1016/j.cell.2022.04.011
Willekens, J., and Runnels, L. W. (2022). Impact of zinc transport mechanisms on embryonic and brain development. Nutrients 14 (12), 2526. doi:10.3390/nu14122526
Woodruff, G., Bouwkamp, C. G., de Vrij, F. M., Lovenberg, T., Bonaventure, P., Kushner, S. A., et al. (2018). The zinc transporter SLC39A7 (ZIP7) is essential for regulation of cytosolic zinc levels. Mol. Pharmacol. 94 (3), 1092–1100. doi:10.1124/mol.118.112557
Xia, P., Yan, L., Ji, X., Wu, Y., Lian, S., and Zhu, G. (2022). Functions of the zinc-sensing receptor GPR39 in regulating intestinal health in animals. Int. J. Mol. Sci. 23 (20), 12133. doi:10.3390/ijms232012133
Xin, Y., Gao, H., Wang, J., Qiang, Y., Imam, M. U., Li, Y., et al. (2017). Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov. 3 (1), 17025–17113. doi:10.1038/celldisc.2017.25
Xu, Y., Xiao, G., Liu, L., and Lang, M. (2019). Zinc transporters in Alzheimer's disease. Mol. Brain 12 (1), 106. doi:10.1186/s13041-019-0528-2
Yan, L., Li, Y., Shi, Z., Lu, X., Ma, J., Hu, B., et al. (2017). The zinc finger E-box-binding homeobox 1 (Zeb1) promotes the conversion of mouse fibroblasts into functional neurons. J. Biol. Chem. 292 (31), 12959–12970. doi:10.1074/jbc.m116.771493
Yokokawa, H., Morita, Y., Hamada, I., Ohta, Y., Fukui, N., Makino, N., et al. (2024). Demographic and clinical characteristics of patients with zinc deficiency: analysis of a nationwide Japanese medical claims database. Sci. Rep. 14 (1), 2791. doi:10.1038/s41598-024-53202-0
Yu, W. H., Lukiw, W. J., Bergeron, C., Niznik, H. B., and Fraser, P. E. (2001). Metallothionein III is reduced in Alzheimer's disease. Brain Res. 894 (1), 37–45. doi:10.1016/s0006-8993(00)03196-6
Zhao, H., and Eide, D. (1996). The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc. Natl. Acad. Sci. U. S. A. 93 (6), 2454–2458. doi:10.1073/pnas.93.6.2454
Zhao, L., Oliver, E., Maratou, K., Atanur, S. S., Dubois, O. D., Cotroneo, E., et al. (2015). The zinc transporter ZIP12 regulates the pulmonary vascular response to chronic hypoxia. Nature 524 (7565), 356–360. doi:10.1038/nature14620
Zhao, N., Gao, J., Enns, C. A., and Knutson, M. D. (2010). ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J. Biol. Chem. 285 (42), 32141–32150. doi:10.1074/jbc.m110.143248
Zhao, Y., Feresin, R. G., Falcon-Perez, J. M., and Salazar, G. (2016). Differential targeting of slc30a10/ZnT10 heterodimers to endolysosomal compartments modulates EGF-induced MEK/ERK1/2 activity. Traffic 17 (3), 267–288. doi:10.1111/tra.12371
Keywords: zinc homeostasis, neurodegenerative diseases, zinc transporters, zinc homeostasis in brain, disease
Citation: Sabouri S, Rostamirad M and Dempski RE (2024) Unlocking the brain’s zinc code: implications for cognitive function and disease. Front. Biophys. 2:1406868. doi: 10.3389/frbis.2024.1406868
Received: 25 March 2024; Accepted: 15 May 2024;
Published: 12 June 2024.
Edited by:
Olga Vinogradova, University of Connecticut, United StatesReviewed by:
Jerry C. Chang, Columbia University, United StatesNicholas James, University of Hawaii at Manoa, United States
Copyright © 2024 Sabouri, Rostamirad and Dempski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert E. Dempski, cmRlbXBza2lAd3BpLmVkdQ==
†These authors have contributed equally to this work
 Soheila Sabouri
Soheila Sabouri Marzieh Rostamirad
Marzieh Rostamirad Robert E. Dempski
Robert E. Dempski