- 1Hawaii Institute of Marine Biology, University of Hawaii at Manoa, Kane'ohe, HI, United States
- 2International Business Services and Sales, Silver Spring, MD, United States
- 3Hawaiian Adventures Kona, Kailua-Kona, HI, United States
- 4Department of Land and Natural Resources' Division of Aquatic Resources, Protected Species Program (PSP), Honolulu, HI, United States
- 5Kaimana Ocean Safari, Kailua-Kona, HI, United States
Oceanic whitetip sharks, Carcharhinus longimanus, are known to be common scavengers; however, observations of C. longimanus scavenging events are extremely rare due to their classification as an oceanic pelagic species, typically solitary in nature. On April 9, 2024, over 8.5 h, at least nine C. longimanus were observed scavenging from a heavily degraded carcass off the coast of Kailua-Kona, Hawai‘i, USA. Five tiger sharks (Galeocerdo cuvier) were also observed scavenging on the same carcass. Simultaneous feeding within and between species occurred; however, no agonistic or aggressive interactions were observed. Although a small snapshot, this stochastic event sheds new light on trophic relationships and social interactions among aquatic apex predators that do not normally overlap in space and time.
1 Introduction
Apex predators such as carnivorous sharks occupy the top trophic level in a community and tend to have strong top-down effects on population demography, structure, and ecosystem productivity (1, 2). Many sharks, particularly pelagic sharks, are opportunistic predators and dietary generalists, and scavenging on carrion likely plays a significant role in supplementing their diet (3–11). For example, estimates suggest that a large (~4 m) white shark that consumes 30 kg of blubber can be sustained for up to 1.5 months without additional food (12). Scavenging can also facilitate both bottom-up and top-down regulation of populations through different trophic levels and represents an important energy transfer pathway in marine ecosystems (13–15).
Normally, cetacean carcasses provide the most substantial source of energy for scavengers, and some deep-sea communities appear to subsist exclusively from whale falls (16). Unsurprisingly, and given their stochastic nature, carcasses often attract large numbers of highly mobile, typically solitary predators that are usually sparsely distributed (10, 11, 14, 15, 17). Previous studies show large lamnid and carcharhinid sharks to be the most common surface scavengers. This includes white [Carcharodon carcharhinus; (5–8, 10)], tiger [Galeocerdo cuvier; (17, 18)], and bull sharks [C. leucas; (17)], although reports vary on the level of aggressive, competitive, or peaceful interactions displayed during a scavenging event. Scavenging events, therefore, provide a unique opportunity to examine inter- and intra-specific behaviors between species, often top predators, not normally encountered together.
Photo identification is a common technique used for individual identification, movements, and population estimates of many species of elasmobranchs globally [e.g., (19–22)]. The method allows individuals with distinctive body features, such as natural markings and pigmentations, to be uniquely identified and has been used to successfully document population demographics for white sharks (23, 24), whale sharks (25–27), tiger sharks [TIG; (18, 28)], and, more recently, oceanic whitetip sharks [OCS; (29)].
In this study, we used the opportunistic finding of a heavily degraded carcass to document, to our knowledge, one of the first observations of OCS and TIG scavenging concurrently. OCS are typically a solitary species with low population densities (30, 31). OCS are classified as “oceanic pelagic” (32), meaning that they can potentially complete their entire life cycle in the open ocean (32). These characteristics make OCS notoriously difficult to study. Here, we (1) document the novel observations of a feeding aggregation of OCS scavenging on a carcass, (2) describe the inter- and intra-specific behaviors of OCS and TIG and (3) use photo identification to determine the demographics and number of individuals present at the feeding aggregation.
2 Methods
2.1 Study site and observations
On April 9, 2024, at 10:30 a.m., a tourism operation (Hawaiian Adventures Kona and co-authors) sighted a heavily degraded carcass described as “a big chunk of flesh and blubber, rather than bones” (pers. comm. Olivia Miller, April 2024) ~3 m long × ~2 m wide × ~2 m high (Figure 1) and ~10.7 km off the west coast of Big Island, Hawai‘i (Figure 2). Although unconfirmed, the tourism operators suggested that the carcass may have been a sub-adult rorqual whale as ventral pleats were visible in a photograph exchange with a fisher 2 weeks earlier. On March 24, 2024, a fisher reported a larger chunk of whale between Ho‘okena and Black Pebble Beach (Figure 2). However, the tourism operators were unable to locate the carcass the following day (March 25). Whale carcasses are relatively uncommon in the area and two carcasses in two weeks is extremely rare (pers. comm. Olivia Miller, April 2024). We therefore assumed for this study that the carcass encountered on 9 April was the same piece observed two weeks earlier that had further degraded.

Figure 1. (A) The small and heavily degraded carcass ~3 m (L) × 2 m (W) × 2 m. (B) The carcass in relation to a tiger shark.
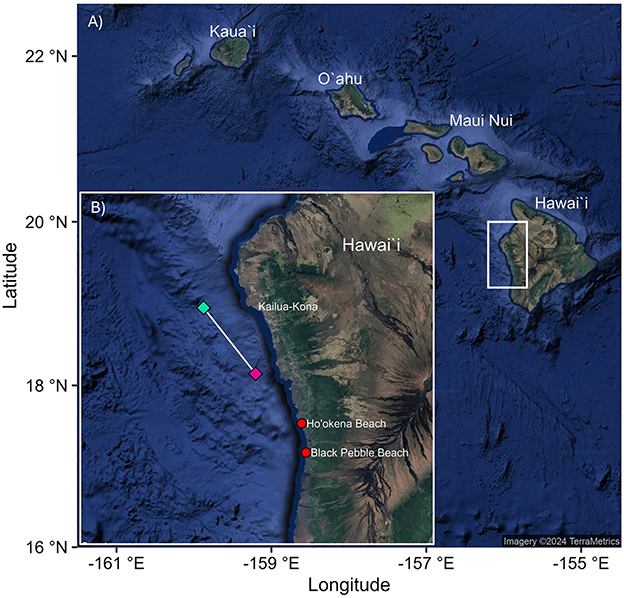
Figure 2. (A) Map of the main Hawaiian Islands with the white rectangle denoting the area of the carcass drift on April 9 2024 and (B) inset map of the drift (white line) of the carcass on April 9, 2024, between first sighting at 10:30 (pink diamond) and sunset 18:58 (green diamond) covering a distance of 21.2 km. Red circles denote Ho‘okena and Black Pebble Beach, the general area where a fisher first located the carcass on March 24, 2020.
Observers (and co-authors) drifted with the carcass and obtained 8.5 h of in-water video and photographic observations of the shark-feeding aggregation. On the day of the study, the predominant current was north–southeast; the water temperature was 25°C; seas were calm, with ~ >40 m vertical visibility; winds were between 1 and 4 knots; and the skies were clear. The observation's total drift time was from 10:30 to 18:58 and covered 21.2 km (Figure 2). Observers left the area just after sunset, so no data or observations were recorded at night.
High-quality (4K) in-water imagery of feeding behavior was collected using the following camera models: Canon R5s (Canon Inc., Ota City, Tokyo, Japan), Sony a6500 (Sony Corp., Minato City, Tokyo, Japan), all in Nauticam housings (Nauticam International Ltd. Fort Lauderdale, FL, USA), Gopro Hero 10 and 11 Black (Gopro Inc., San Mateo, CA, USA). Additional imagery was collected at the surface using a Nikon z6ii (Nikon Corp., Minato City, Tokyo, Japan), Canon 1Dxii (Canon Inc., Ota City, Tokyo, Japan), DJI Air 2s (SZ Dajiang Innovation Technology Co., Ltd, Shenzhen, China). The length of individual sharks were visually estimated (to the nearest 0.5 m) by one in-water photographer. For accuracy, the observer compared shark length with referenced objects, such as the carcass or the boat (18).
High-resolution (4K) aerial footage (28:29 min in total) recorded by drone DJI Air 2s (SZ Dajiang Innovation Technology Co., Ltd, Shenzhen, China) was used to document the demographics and behavior of sharks foraging on the carcass. The drone's observation height ranged from 3 m to 20 m.
2.2 Shark ethology assessment
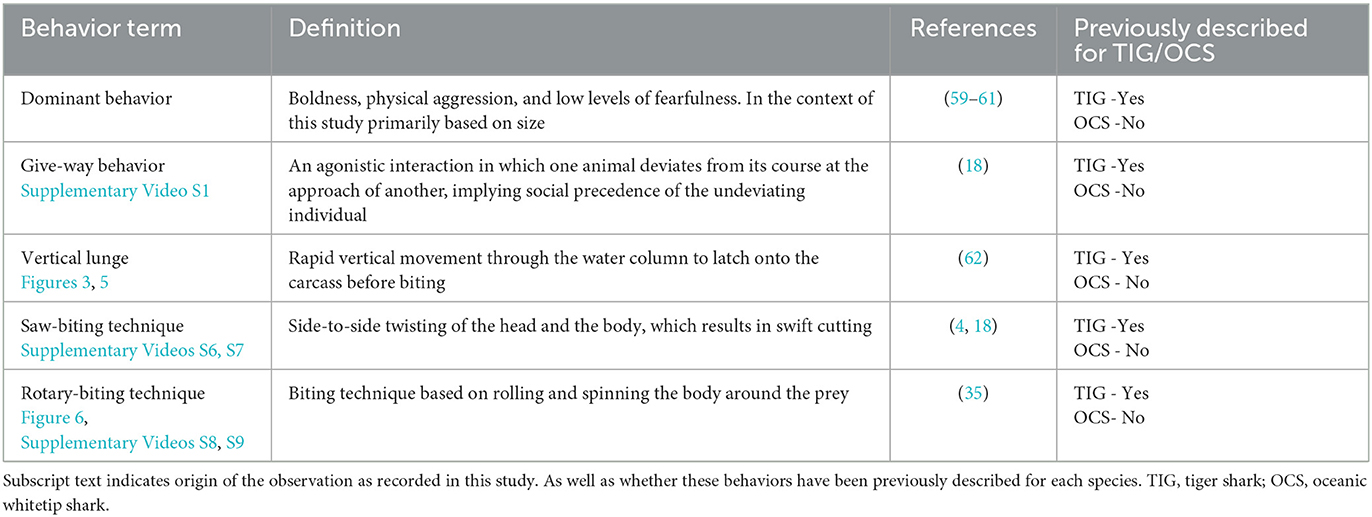
Shark inter- and intra-specific interactions and feeding behaviors around the carcass were categorized using a combination of in-water observations and previous studies and included dominant behavior, give-way behavior, vertical lunge, saw-biting, and rotary-biting. These are defined in Table 1.
2.3 Photo identification of individuals
Attempts were made to obtain high-resolution imagery of both right- and left-side dorsal fins and full-body shots from all sharks at the feeding event. These images were then cropped, scaled, and optimized in GNU Image Manipulation Program (GIMP™) to better visualize the dorsal patterns and body markings. Both OCS and TIG are uniquely identifiable from dorsal fin patterns, including dorsal notches and body markings [for OCS, (29), and TIG, (18, 28)], as well as the pattern of countershading along the side of the face for TIG (33). A photo database exists of OCS dorsal fin patterns from a citizen-science program operating around Kailua-Kona [Hawaii Community Tagging Program, https://www.sharktagger.org/; (29)], so dorsal fin clips for OCS were matched against the existing catalog to determine re-sighted individuals. Re-sighted individuals were confirmed by exact matches of dorsal fin patterns between a new and a previous submission. OCS have different right and left dorsal fin patterns (29); as such, OCS were only considered unique if there was clear imagery of the left-side dorsal. This method eliminates potential redundancies and avoids overestimations of the data (29, 34).
3 Results
3.1 Overview of the feeding event
Upon the observers' arrival at the carcass at 10:30 a.m., two OCS (one ~2.5 m; one ~1.9 m) were observed actively feeding at the surface. The larger OCS (~2.5 m, the largest OCS at the aggregation) was present throughout the entire 8.5-h observation. Between 10:35 and 10:45, two more OCS appeared, and all four individuals took turns feeding directly on the carcass. Over the next hour (10:45–11:45), two TIG arrived and two OCS left the area. Between 12:00 and 15:00, individuals from both species filtered in and out of the scene, intermittently feeding either directly on the carcass or on fallen scraps until 15:00 when the shark numbers became very consistent. From 15:00 until observers left the carcass at 18:58 (sunset), seven OCS and five TIG remained actively feeding for 4 h. Throughout this time, it did not appear that any individual reached a point of satiation and permanently left the area; rather, they stayed, loitering around the carcass and intermittently feeding. Observers made efforts to relocate the carcass the following morning based on drift and currents, but it could not be located.
3.2 Demographics of sharks at the carcass
From photo identification, nine unique OCS (n = 7 female, n = 1 male, n = 1 unknown) were recorded based on left-side dorsal images only. Three OCS had imagery of only the right-side dorsal, so they could not be considered unique individuals, and two OCS had images taken from the surface, so we were unable to identify their sex. The OCSs ranged in size from ~1.5 m to 2.5 m (TL, Supplementary Table S1). Five unique TIG (n = 1 female, n = 4 male) ranging in size from ~3 m to 4 m (TL) were also recorded (Supplementary Table S1). The maximum number of sharks observed at one time in the water was 12 (n = 7 OCS and n = 5 TIG). Despite the local abundance of other shark species, such as silky (C. falciformis), galapagos (C. galapagensis), oceanic blacktip (C. limbatus), sandbar (C. plumbeus), and, to a much lesser extent, white sharks (C. carcharhinus), none were observed at the carcass.
Using the existing OCS dorsal catalog, two female OCS (s298 and s389) were confirmed as re-sights (Supplementary Table S1). OCS s298 was re-sighted after 5 years. The female was first photographed on January 18, 2019, and this is the longest recorded re-sight to date in the catalog. s389, also a female, was re-sighted after a month. The first interaction was on March 9, 2024 (Supplementary Table S1).
3.3 Inter- and intra-species interactions
Overall, and despite a large range in body size (~1.5 m–~4 m) and a small carcass, no agonistic inter- or intra-species interactions between OCS and TIG were observed. Rather, interactions appeared relatively peaceful [Figures 3, 4], and all sharks swam and fed calmly with minimal signs of aggression [e.g., ramming, jaw gapping; (18)]. In fact, we observed instances of a “give-way” behavior, where if two sharks were approaching the carcass at the same time, the smaller shark would veer away and allow the larger shark to feed (Supplementary Video S1, Table 1). Similarly, if a larger shark approached the carcass while a smaller one was feeding, the smaller shark would leave the area immediately, allowing the larger shark to feed.

Figure 3. Concurrent feeding between an oceanic whitetip shark (right) and a tiger shark (left). The oceanic whitetip shark exhibits a vertical lunging motion.
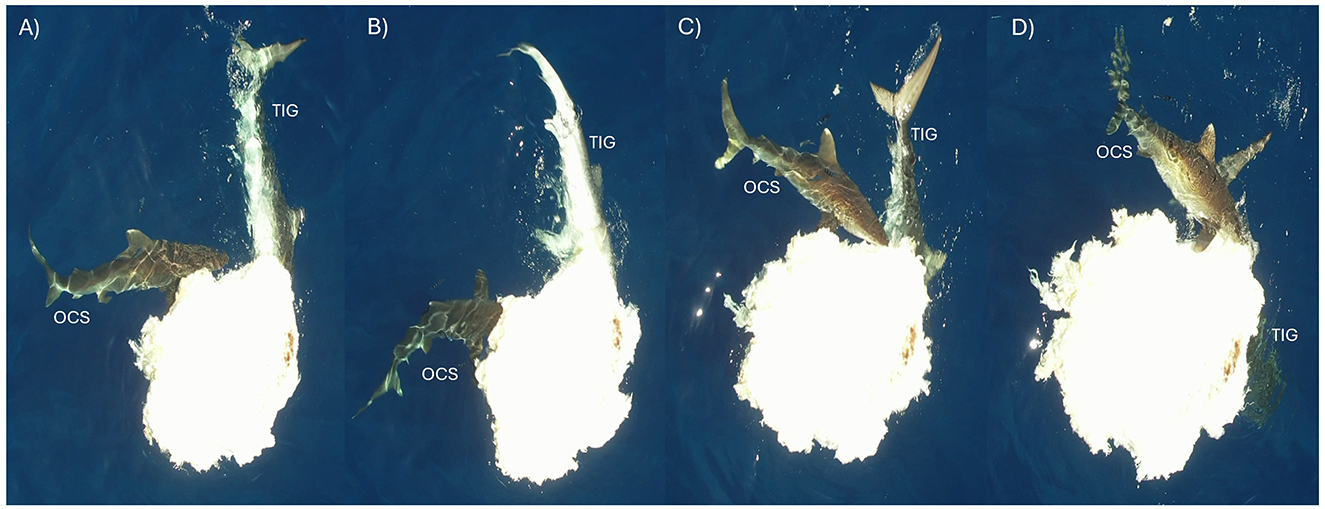
Figure 4. A series of stills (A–D) from drone footage of concurrent “ peaceful” feeding between an oceanic whitetip shark and a tiger shark, with both individuals feeding from the carcass.
TIG were the dominant species, presumably because of their size. All TIG (except the smaller 3-m female) and the two largest OCS (>2 m) were observed feeding directly on the carcass, whereas the majority of the smaller OCS (individuals < 2 m) and the female TIG never fed directly on the carcass; rather, they stayed ~5 m beneath the surface and foraged on scraps that had drifted down from feeding events. This meant that the TIG were observed more often at the surface while the OCS tended to stay deeper.
Generally, and likely due to its size, one shark fed from the carcass at a time. Although the maximum number of sharks feeding on the carcass at any time was two (Figures 3, 4, Supplementary Videos S2, S3), it was more common to see two TIG feeding concurrently than OCS. Concurrent feeding between OCS and TIG was observed < 10 times (Figures 3, 4, Supplementary Videos S2, S3) and was only carried out by the larger OCS (2.5 m), but again, the interaction was considered relatively peaceful. Two OCS were observed feeding in tandem at the very beginning of the observation and before the arrival of the TIG but not again.
3.4 Feeding events and modes of feeding
We observed that size also determined the frequency and length of feeding events, with TIG and larger OCS feeding more frequently and for longer periods than the smaller individuals. The longest recorded feeding event for a TIG was 62 s; for an OCS, it was 47 s. From the 29 min of drone footage, we documented 10 OCS feeding events, 12 TIG feeds, and one concurrent OCS and TIG feeding event (Figure 4). We were unable to document feeding times in the water; however, we observed four different feeding modes exhibited by scavenging individuals (Table 1):
1. Vertical lunge up through the water column to get a sizable chunk of flesh. Oftentimes, their head would come out of the water (Figures 3 [OCS], 5 [TIG]).
2. A series of quick consecutive bites (Supplementary Videos S4, S5). On many occasions, this method was combined with one of the other feeding strategies.
3. “Saw-biting”, that is, lateral side-to-side movement of the head resulting in swift cutting of pieces of flesh [(4); Supplementary Videos S6, S7]. A notable difference in “saw-biting” was observed between the species, where OCS bit and shook the carcass at a much faster rate (Supplementary Video S6) than TIG (Supplementary Video S7).
4. “Rotary-biting,” where, after sticking their jaws deep in the flesh, the sharks use their body weight as leverage and spin around the jaw, to facilitate cutting of tissue (35) (Figure 6, Supplementary Videos S8, S9).

Figure 5. A tiger shark eating from the carcass after a vertical lunge with its head out of the water.

Figure 6. A sequence (A–E) of drone images of a tiger shark undertaking “rotary-biting” on the carcass.
After a feeding event, individuals from both species would sink beneath the surface and slowly swim away from the carcass. However, if no other sharks were in close proximity, individuals would return to the carcass within seconds to feed again. Occasionally, following a longer feeding event (i.e., >30 s) some TIG appeared to “overeat” and regurgitate (Supplementary Video S10). This would lead to a frenzy of smaller OCS under the surface rushing to consume the regurgitated scraps.
4 Discussion
Although scavenging by sharks is relatively common, and likely an important component of their feeding ecology (9–11), documentation of these stochastic events is rare. Studies of scavenging by sharks are often limited to coastal species such as white sharks [C. carcharhinus; (5–8, 10)], TIG [G. cuvier; (17, 18)], and bull sharks [C. leucas; (17)] presumably because these events occur closer to the coast and provide easier access for observers. To our knowledge, this study is the first to scientifically report a scavenging event of OCS concurrently feeding with TIG. Although both species are opportunistic predators and scavengers, documentation of OCS scavenging events is rare as OCS are typically solitary, highly migratory, and spend most of their time in the open ocean (32). However, the Big Island, Hawai‘i, is known to aggregate OCS seasonally, usually in the spring and summer (29), and hold TIG year-round (36, 37). Boat operators (and co-authors) working in Kona waters daily report sightings of OCS and TIG in close proximity as extremely rare, with only one observation of a TIG and a group of OCS following an injured pilot whale in 4 years (pers. comm. Jim Ward and Dylan Currier). This is presumably because the two species occupy vastly different habitats around the Hawaiian archipelago, where OCS are strictly oceanic pelagic and TIG exhibit sex-related variation in habitat preferences, with mature females predominantly using coastal habitats 0–200 m (36, 38, 39) and mature males occupying more offshore, open-ocean habitats (39, 40).
The feeding aggregation in this study was dominated by male TIG (n = 4 male, n = 1 female) and female OCS (n = 7 female, n = 1 male, n = 1 unknown); although these numbers are likely to be an underestimation of all individuals in the aggregation, it highlights how the temporary availability of a single carcass can promote opportunistic scavenging and support high abundances of marine predators (14, 15, 17, 41). Dominance of male TIG confirms their preference for offshore environments (36) and likely reflects TIG ability to adapt patterns of movement to local resource distribution (36). While dominance of female OCS aligns with Scott et al. (29) that shows a heavily skewed sex ratio of approximately ~2:1 female: male OCS around the Island of Hawai‘i, suggesting the west-coast of Hawai‘i Island, may be an area of biological importance for OCS in the Pacific.
Overall and similar to previous studies, we observed a size-dependent hierarchy of the feeding aggregation (10, 42). Therefore TIG, which ranged in size from ~3–4 m, were the dominant species. Surprisingly, given the variation in sex and size of the scavenging individuals and the small size of the carcass, we did not observe any aggressive interactions. There were some instances of smaller sharks giving way to larger sharks, implying social precedence (18). But overall, we observed peaceful concurrent feeding, with no inter- or intra- specific aggression. The absence of agonistic or competitive behaviors has been previously reported between TIG and white sharks (43, 44), TIG and crocodiles (15), 40 individual white sharks (10), and TIG, bull, and tawny nurse sharks (17) scavenging concurrently on carcasses, although agonistic behaviors are also commonly reported for scavengers [e.g., (18, 45, 46)]. It is not clear why TIG exhibit such contrasting social behaviors in different locations, but it could be related to differences in food availability, with sharks being less competitive if resources are abundant (17) and/or variation among individuals and personalities, which may trigger inter- or intra-specific risk-taking or risk-averse behaviors (47, 48).
In fact, we believe that the larger TIG may have facilitated scavenging by the smaller OCS that were only observed ~5 m below the surface feeding from scraps and/or regurgitations from the TIG and never feeding directly from the carcass. Around Hawai‘i, OCS are often observed following pilot whales (Globicephala spp.), most likely to forage on their scraps as pilot whales are efficient foragers and have similar prey preference to OCS [i.e., cephalopods; (49–52)]. TIG have also been documented facilitating scavenging for bull sharks on a whale carcass by exposing softer tissues for the bull sharks to forage on (17). Surprisingly, no other coastal or pelagic shark species, such as silky (C. falciformis), galapagos (C. galapagensis), oceanic blacktip (C. limbatus), sandbar (C. plumbeus), or white (C. carcharhinus), were observed at the feeding aggregation. This may be due to; (a) the smaller species avoiding the carcass to reduce the risk of predation from larger sharks (53); (b) the carcass being in water depths >5,000 ft, which is outside the preferred habitat and depth range of coastal species [i.e., galapagos, sandbars; (38, 54)] and/or; (c) variation in personalities between individuals and species (47, 48). For example, silky sharks are considered to be “shier” and less bold than OCS and TIG and therefore less likely to approach the carcass (pers. obs. Olivia Miller). The absence of white sharks around the carcass is not surprising. Although white shark movements to Hawai‘i from California increase during springtime (55), the species is not commonly encountered in the coastal waters off west Hawai‘i.
Although OCS and TIG are considered opportunistic predators and scavengers, marine mammals make up very small proportions of their overall diet. In general, OCS diet is primarily composed of cephalopods (44%) and teleosts (43%), with a smaller proportion (13%) being a mix of birds, mollusks, crustaceans, and mammals (56, 57), whereas, for large TIGs (>3 m), elasmobranchs (42%) and teleosts (40%) tend to be the most common prey items, followed by crustaceans (35%), birds (25%), land mammals (19%), turtles (15%), cephalopods (10%), and marine mammals [7%; (58)]. During the feeding event, both species used feeding techniques previously documented for sharks. This included “saw-biting”, referring to a “side-to-side” twisting of the head and body resulting in swift cutting (4, 18). We observed OCS moved their head more rapidly than TIG when undertaking “saw-biting.” Additionally, both species were observed “rotary-biting,” in which they rolled and spun their entire body around the carcass (Supplementary Videos S8, S9). Rotary-biting is most likely more energetically cost-effective in removing mouth-sized pieces of flesh (18).
The high-resolution footage obtained in this study made photo identification of sharks to an individual level possible based on features of their dorsal fins and body markings. However, the number of individuals at the carcass was likely underestimated. Specifically for OCS, as we were only able to obtain right side imagery for three OCS, where left side images are needed for unique identification because left and right side dorsal patterns are different (29). It is also possible that other individuals from both species were less bold and remained out of view of the observers capturing the footage. Regardless, this study confirms that photo identification is an extremely valuable tool for identification and abundance estimates for a small number of individuals. For OCS, in particular, seven new individuals were added to the existing Hawaii Community Tagging Program catalog, and two individuals (both female) were confirmed as re-sights, one after 5 years, which is currently the longest re-sighting in the database and suggests repeated visits by OCS to the west side of Hawai‘i island. These data are extremely important for collecting crucial baseline information on population demographics of OCS, a threatened species, around the Hawaiian Islands, and will increase the ability for re-sightings in the future.
Finally, we acknowledge that although novel, this study represents a very small snapshot of the scavenging behaviors of OCS and TIG. Nevertheless, these stochastic events serve as an important reference for investigating trophic relationships and social interactions among aquatic apex predators that do not normally overlap in space and time. Understanding and documenting the scavenging process is vital for comprehending the biology, evolution, and behavioral ecology of top predators [e.g., (13, 14)].
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by State of Hawaii Department of Land and Natural Resources Division of Boating and Ocean Recreation Commercial Operations. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MS: Investigation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. OM: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Writing – review & editing. DS: Data curation, Writing – review & editing. KG: Conceptualization, Investigation, Methodology, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Special thanks to Cameron Grant for operating the drone and contributing footage and Andrew Aggergard, who contributed in-water footage, as well as members of the Hawaii Community Tagging Program, who regularly contribute their oceanic whitetip shark dorsal fin imagery to the catalog and have amassed the largest oceanic whitetip shark dorsal fin catalog in the world!
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frish.2025.1520995/full#supplementary-material
References
1. Estes J, Terborgh J, Brashares J, Power M, Berger J, et al. Trophic downgrading of planet earth. Science. (2011) 33:301–6. doi: 10.1126/science.1205106
2. Ripple W, Estes J, Beschta R, Wilmers C, Ritchie E, Hebblewhite M, et al. Status and ecological effects of the world's largest carnivores. Science. (2014) 343:1241484. doi: 10.1126/science.1241484
3. McCosker J. White shark attack behavior: observations of and speculations about predator and prey strategies. Mem Southern Calif Acad Sci. (1985) 9:123–35.
4. Randall JE. Review of the biology of the tiger shark (Galeocerdo cuvier). Aus J Mar Fresh Res. (1992) 43:21–31. doi: 10.1071/MF9920021
5. Long D, Jones R. “White shark predation and scavenging on cetaceans in the eastern North Pacific Ocean.” In:Klimley AP, Ainley DG, , editors. Great White Sharks: The Biology of Carcharodon carcharias. San Diego, CA: Academic Press (1996). p. 293–307. doi: 10.1016/B978-012415031-7/50028-8
6. Kelly J, Klimley A. The occurrence of the white shark, Carcharodon carcharias, at the point reyes headlands, California. Calif Fish Game. (2003) 89:187–96.
7. Curtis T, Kelly J, Menard K, Laroche R, Jones R, Klimley A. Observations on the behaviour of white sharks scavenging from a whale carcass at point reyes, California. Calif Fish Game. (2006) 92:113–24.
8. Dicken M. First observations of young of the year and juvenile great white sharks (Carcharodon carcharias) scavenging from a whale carcass. Mar Freshwater Res. (2008) 59:596–602. doi: 10.1071/MF07223
9. Barnett A, Braccini J, Awruch C, Ebert D. An overview on the role of Hexanchiformes in marine ecosystems: biology, ecology and conservation status of a primitive order of modern sharks. J Fish Biol. (2012) 80:966–90. doi: 10.1111/j.1095-8649.2012.03242.x
10. Fallows C, Gallagher A, Hammerschlag N. White sharks (Carcharodon carcharias) scavenging on whales and its potential role in further shaping the ecology of an apex predator. PLoS ONE. (2013) 8:e60797. doi: 10.1371/journal.pone.0060797
11. Hammerschlag N, Bell I, Fitzpatrick R, Gallagher A, Hawkes L, Meekan M, et al. Behavioral evidence suggests facultative scavenging by a marine apex predator during a food pulse. Behav Ecol Sociobiol. (2016) 70:1777–88. doi: 10.1007/s00265-016-2183-2
12. Carey F, Kanwisher J, Brazier O, Gabrielson G, Casey J, Pratt H. Temperature and activities of a white shark, Carcharodon carcharias. Copeia. (1982) 1982:254–60. doi: 10.2307/1444603
13. DeVault T, Rhodes O, Shivik J. Scavenging by vertebrates: behavioral, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. Oikos. (2003) 102:225–34. doi: 10.1034/j.1600-0706.2003.12378.x
14. Wilson E, Wolkovich E. Scavenging: how carnivores and carrion structure communities. Trend Ecol Evo. (2011) 26:129–35. doi: 10.1016/j.tree.2010.12.011
15. Gallagher A, Papastamatiou Y, Barnett A. Apex predatory sharks and crocodiles simultaneously scavenge a whale carcass. J Ethol. (2018) 36:205–9. doi: 10.1007/s10164-018-0543-2
16. Smith C, Baco A. Ecology of whale falls at the deep-sea floor. Oceanog and Mar Biol: Ann Rev. (2003) 41:311–54.
17. Lea J, Daly R, Leon C, Daly K, Clarke C. Life after death: behaviour of multiple shark species scavenging a whale carcass. Mar Freshw Res. (2018) 70:302–6. doi: 10.1071/MF18157
18. Clua E, Chauvet C, Read T, Werry J, Lee S. Behavioural patterns of a tiger shark (Galeocerdo cuvier) feeding aggregation at a blue whale carcass in prony bay, New Caledonia. Mar Fresh Behav Physiol. (2013) 46:1–20. doi: 10.1080/10236244.2013.773127
19. Whitney N, Pyle R, Holland K, Barcz J. Movements, reproductive seasonality, and fisheries interactions in the whitetip reef shark (Triaenodon obesus) from community-contributed photographs. Environ Biol Fishes. (2012) 93:121–36. doi: 10.1007/s10641-011-9897-9
20. Germanov E, Marshall A. Running the gauntlet: regional movement patterns of Manta alfredi through a complex of parks and fisheries. PLoS ONE. (2014) 9:e110071. doi: 10.1371/journal.pone.0110071
21. Araujo G, Agustines A, Tracey B, Snow S, Labaja J, Ponzo A. Photo-ID and telemetry highlight a global whale shark hotspot in Palawan, Philippines. Sci Rep. (2019) 9:1. doi: 10.1038/s41598-019-53718-w
22. Diamant S, Pierce S, Rohner C, Graham R, Guillemain d'Echon A, Guillemain Echon T, et al. Population structure, residency, and abundance of whale sharks in the coastal waters off Nosy Be, north-western Madagascar. Aquat Conserv. (2021) 31:3492–506. doi: 10.1002/aqc.3743
23. Domeier M, Nasby-Lucas N. Annual re-sightings of photographically identified white sharks (Carcharodon carcharias) at an eastern Pacific aggregation site (Guadalupe Island, Mexico). Mar Biol. (2006) 150:977–84. doi: 10.1007/s00227-006-0380-7
24. Anderson S, Chapple T, Jorgensen S, Klimley A, Block B. Long-term individual identification and site fidelity of white sharks, Carcharodon carcharias, off California using dorsal fins. Mar Biol. (2011) 158:1233–7. doi: 10.1007/s00227-011-1643-5
25. Arzoumanian Z, Holmberg J, Norman B. An astronomical pattern-matching algorithm for computer-aided identification of whale sharks Rhincodon typus. J Appl Ecol. (2005) 42:999–1011. doi: 10.1111/j.1365-2664.2005.01117.x
26. Meekan M Bradshaw C Press M McLean C Richards A Quasnichka S . Population size and structure of whale sharks, Rhincodon typus, at Ningaloo Reef, Western Australia. Mar Ecol Prog Ser. (2006) 319:275–85. doi: 10.3354/meps319275
27. Marcoux T, Marcoux S, Harvey M, Araujo G. A first look at whale sharks in Hawaiian waters: using citizen science to study the world's largest fish, Rhincodon typus. Aquat Conserv. (2023) 33:264–75. doi: 10.1002/aqc.3915
28. Nakachi K. Heeding the History of Kahu Manō: Developing and Validating a Pono Photo- Identification Methodology for Tiger Sharks (Galeocerdo cuvier) in Hawai'i (Master's thesis). University of Hawai'i at Hilo, Hilo, HI (2021).
29. Scott M, Campbell A, Marcoux S, Marcoux T, Harvey M, Hutchinson M et al. Using photo identification to assess demographics and fishery interactions of oceanic whitetip sharks (Carcharhinus longimanus) in the main Hawaiian Islands. Mar Ecol Prog Ser. (2025). doi: 10.3354/meps14835
30. Young C, Carlson J. The biology and conservation status of the oceanic whitetip shark (Carcharhinus longimanus) and future directions for recovery. Rev Fish Biol Fish. (2020) 30:293–312. doi: 10.1007/s11160-020-09601-3
31. Pacoureau N, Rigby C, Kyne P, Sherley R, Winker H, Carlson J, et al. Half a century of global decline in oceanic sharks and rays. Nature. (2021) 7843:567–71. doi: 10.1038/s41586-020-03173-9
32. Dulvy NK, Baum JK, Clarke S, Compagno LJV, Cortés E, Domingo A, et al. You can swim but you can't hide: the global status and conservation of oceanic pelagic sharks and rays. Aquat Conserv. (2008) 18:459–82. doi: 10.1002/aqc.975
33. Vossgaetter L, Dudeck T, Crouch J, Cope M, Ivanova T, Siyan I, et al. Non-invasive methods characterise the world's largest tiger shark aggregation in Fuvahmulah, Maldives. Sci. Rep. (2024) 14:21998. doi: 10.1038/s41598-024-73079-3
34. McClintock B, Conn P, Alonso R, Crooks K. Integrated modeling of bilateral photo-identification data in mark–recapture analyses. Ecology. (2013) 94:1464–71. doi: 10.1890/12-1613.1
35. Motta P, Wilga C. Advances in the study of feeding behaviors, mechanisms, and mechanics of sharks. Environ Biol Fish. (2001) 60:131–56. doi: 10.1007/978-94-017-3245-1_10
36. Meyer C, Clark T, Papastamatiou Y, Whitney N, Holland K. Long-term movement patterns of tiger sharks, Galeocerdo cuvier in Hawaii. Mar Ecol Prog Ser. (2009) 381:223–35. doi: 10.3354/meps07951
37. Meyer C, Anderson J, Coffey D, Hutchinson M, Royer M, Holland K. Habitat geography around Hawaii's oceanic islands influences tiger shark (Galeocerdo cuvier) spatial behaviour and shark bite risk at ocean recreation sites. Sci Rep. (2018) 8:4945. doi: 10.1038/s41598-018-23006-0
38. Meyer C, Papastamatiou Y, Holland K. A multiple instrument approach to quantifying the movement patterns and habitat use of tiger (Galeocerdo cuvier) and galapagos sharks (Carcharhinus galapagensis) at French frigate shoals, Hawaii. Mar Biol. (2010) 157:1857–68. doi: 10.1007/s00227-010-1457-x
39. Papastamatiou Y, Meyer C, Carvalho F, Dale J, Hutchinson M, Holland K. Telemetry and random-walk models reveal complex patterns of partial migration in a large marine predator. Ecology. (2013) 94:2595–606. doi: 10.1890/12-2014.1
40. Meyer C, O'Malley J, Papastamatiou Y, Dale J, Hutchinson M, Anderson J et al. Growth and maximum size of tiger sharks (Galeocerdo cuvier) in Hawaii. PLoS ONE. (2014) 9:84799. doi: 10.1371/journal.pone.0084799
41. Pereira L, Owen N. Facultative predation and scavenging by mammalian carnivores: seasonal, regional and intra-guild comparisons. Mamm Rev. (2014) 44:44–55. doi: 10.1111/mam.12005
43. Dudley F, Anderson-Reade D, Thompson S, McMullen B. Concurrent scavenging off a whale carcass by great white sharks, Carcharodon carcharias, and tiger sharks, Galeocerdo cuvier. Fish Bull. (2000) 98:646–9.
44. Clua E, Séret B. New caledonia (South Pacific) as a potential tropical wintering ground for the white shark, Carcharodon carcharias. In:Domeier ML, , editor. Global Perspectives on the Biology and Life History of the White Shark. London: CRC Press, Taylor & Francis Group (2012). p. 343–53.
45. Ritter E. Food-related dominance between two Carcharhinid shark species, the Caribbean reef shark, Carcharhinus perezi, and the blacktip shark, Carcharhinus limbatus. Mar Fresh Behav Phys. (2001) 34:125–9. doi: 10.1080/10236240109379065
46. Gerry S, Scott A. Shark scavenging behavior in the presence of competition. Curr Zool. (2010) 561:100–8. doi: 10.1093/czoolo/56.1.100
47. Finger JS, Dhellemmes F, Guttridge TL. Personality in elasmobranchs with a focus on sharks: early evidence, challenges, and future directions. In:Vonk J, Weiss A, Kuczaj S, , editors. Personality in Nonhuman Animals. Cham: Springer (2017). p. 129–52. doi: 10.1007/978-3-319-59300-5_7
48. Vignaud TM, Meyer CG, Séguigne C, Bierwirth J, Clua EE. Examining individual behavioural variation in wild adult bull sharks (Carcharhinus leucas) suggests divergent personalities. Behaviour. (2023) 160:1283–301. doi: 10.1163/1568539X-bja10244
49. Reilly S, Shane S. “Pilot whale.” In:D. Haley, , editor. Marine Mammals of Eastern North Pacific and Arctic Waters. Pacific Search Press, Washington (1986). p. 133–9.
50. Connor R. “Group living in whales and dolphins.” In:Mann J, Connor RC, Tyack PL, and Whitehead H, , editors. Cetacean Societies: Field Studies of Dolphins and Whales. University of Chicago Press, Chicago, Illinois (2000). p. 197–208.
51. Migura K, Meadows D. Short-finned pilot whales (Globicephala macrorhynchus) interact with melon-headed whales (Peponocephala electra) in Hawaii. Aquat Mamm. (2002) 28:294–7.
52. Sinclair E. Stomach contents of four short-finned pilot whales (Globicephala macrorhynchus) from the Southern California bight. Mar Mamm Sci. (2006) 8:76–81. doi: 10.1111/j.1748-7692.1992.tb00127.x
53. Heithaus MR, Wirsing AJ, Burkholder D, Thomson J, Dill LM. Towards a predictive framework for predator risk effects: the interaction of landscape features and prey escape tactics. J Anim Ecol. (2009) 78:556–62. doi: 10.1111/j.1365-2656.2008.01512.x
54. McElroy WD, Wetherbee BM, Mostello CS, Lowe CG, Crow GL, Wass RC. Food habits and ontogenetic changes in the diet of the sandbar shark, Carcharhinus plumbeus, in Hawaii. Environ Biol Fishes. (2006) 76:81–92. doi: 10.1007/s10641-006-9010-y
55. Weng KC, Boustany AM, Pyle P, Anderson SD, Brown A, Block BA. Migration and habitat of white sharks (Carcharodon carcharias) in the eastern Pacific Ocean. Mar Biol. (2007) 152:877–94. doi: 10.1007/s00227-007-0739-4
56. Madigan D, Brooks E, Bond M, Gelsleichter J, Howey L, Abercrombie D, et al. Diet shift and site-fidelity of oceanic whitetip sharks, Carcharhinus longimanus, along the Great Bahama Bank. Mar Ecol Prog Ser. (2015) 8:185–97. doi: 10.3354/meps11302
57. Cortés E. Standardized diet compositions and trophic levels of sharks. ICES J Mar Sci. (1999) 56:707–17. doi: 10.1006/jmsc.1999.0489
58. Lowe C, Wetherbee B, Crow G, Tester A. Ontogenetic dietary shifts and feeding behavior of the tiger shark, Galeocerdo cuvier, in Hawaiian waters. Environ Biol Fishes. (1996) 47:203–11. doi: 10.1007/BF00005044
59. Gosling SD, John OP. Personality dimensions in nonhuman animals: a cross-species review. Curr Dir Psychol Sci. (1999) 8:69–75. doi: 10.1111/1467-8721.00017
60. Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biol Rev Camb Philos Soc. (2007) 82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x
61. Finkemeier MA, Langbein J, Puppe B. Personality research in mammalian farm animals: concepts, measures, and relationship to welfare. Front Vet Sci. (2018) 5:131. doi: 10.3389/fvets.2018.00131
Keywords: oceanic whitetip shark (Carcharhinus longimanus), tiger shark (Galeocerdo cuvier), scavenging, carcass, feeding aggregation
Citation: Scott M, Miller O, Stapleton D and Grant K (2025) Novel observations of an oceanic whitetip (Carcharhinus longimanus) and tiger shark (Galeocerdo cuvier) scavenging event. Front. Fish Sci. 3:1520995. doi: 10.3389/frish.2025.1520995
Received: 01 November 2024; Accepted: 02 April 2025;
Published: 29 May 2025.
Edited by:
Johann Mourier, Université de Montpellier, FranceReviewed by:
Maria Del Pilar Blanco Parra, National Council of Science and Technology (CONACYT), MexicoEric Emile Germain Clua, USR3278 Centre de Recherche Insulaire et Observatoire de L'environnement (CRIOBE), France
Copyright © 2025 Scott, Miller, Stapleton and Grant. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Molly Scott, bXNjb3R0MjNAaGF3YWlpLmVkdQ==
 Molly Scott
Molly Scott Olivia Miller
Olivia Miller Devon Stapleton4
Devon Stapleton4