- School of Engineering and Architecture, Institute of Medical Engineering, Space Biology Group, Lucerne University of Applied Sciences and Arts, Hergiswil, Switzerland
With the onset of human space flight, the profound consequences of microgravity (or weightlessness) on living organisms became apparent. Subsequently, understanding the biological processes and developing effective countermeasures has moved into the research focus. Despite their small size, isolated cells also show many adaptations in microgravity, but many fundamental processes are not understood. Because the cytoskeleton largely determines cells’ mechanical properties and is thought to play an important role in cellular mechanosensing, cytoskeleton adaptation in microgravity have been the focus of many research studies. More than 35 years ago, microtubules assembled in a cell-free system were demonstrated to be gravity dependent. Since then, multiple studies have described cytoskeleton adaptations in varieties of cells exposed to short- or long-term microgravity. In this paper on cytoskeletons in microgravity research, I aimed to grasp the published results as a bigger picture and quantify the reported effects in a systematic and more objective manner. The paper focuses on mammalian cells exposed to real microgravity (free fall) and starts with a brief review on the mechanisms how cells can or could sense their physical environment and the role of the cytoskeleton in mechanobiology.
1 Introduction
Life has evolved under the constant influence of Earth’s gravity, and as a result, all organisms are well adapted to this constant force (Jamon, 2014; Najrana and Sanchez-Esteban, 2016; Vinogradova et al., 2021; Volkmann and Baluska, 2006). Many higher organisms, including plants and vertebrates, have gravity-sensitive structures for orientation. In the early days of space flight, there were major concerns if humans can survive in microgravity (or weightlessness). Pioneering test flights with dogs and monkeys, demonstrated that microgravity is not an acute lethal condition for mammals (Burgess and Dubbs, 2007). With the subsequent evolution of human space flight, the profound influence of microgravity on living organisms became apparent (Blaber et al., 2010; Clément, 2007; Demontis et al., 2017; Williams et al., 2009). Since then, understanding the fundamental biological processes of these adaptations and developing effective countermeasures has moved into the research focus.
Despite their small size, isolated cells in cell cultures also show many adaptations in a microgravity environment (Clément and Slenzka, 2006; Grimm et al., 2011; Lv et al., 2023; Nickerson et al., 2015; Wuest et al., 2018). However, to date, it is not clear how nonspecialized cells (without specific gravity sensing structures or organelles) can “sense” gravity or what fundamental mechanism triggers the adaptation processes in microgravity (Albrecht-Buehler, 1991; Hader et al., 2017; Ingber, 1999). Because the cytoskeleton largely determines cells’ mechanical properties (Fletcher and Mullins, 2010; Rajagopal et al., 2018) and is thought to play an important role in the cell’s ability to sense its mechanical environment (Eyckmans et al., 2011; Ingber, 2003a; 2003b; Jansen et al., 2015; Wang, 2017), cytoskeleton adaptation in microgravity have been the focus of many research studies. Indeed, more than 35 years ago (published in 1988), microtubules assembled in a cell-free system were shown to be gravity dependent (Moos et al., 1988). Later analysis showed that microtubule filaments align to gravity once they reach a critical length (Papaseit et al., 2000). This is surprising, as one might think that gravitational forces are too small at a molecular level to have an impact. Since then, multiple studies have described cytoskeleton adaptations in varieties of cells exposed to short- or long-term microgravity (Crawford-Young, 2006; Rudimov and Buravkova, 2016; Vorselen et al., 2014; Wu et al., 2022).
In this paper on cytoskeletons in microgravity research, I would like to take a step back and discuss the results from these studies from a wider perspective. This work focuses on microscopy images from mammalian cells exposed to real microgravity (free fall). Studies using simulated microgravity (Brungs et al., 2016; Herranz et al., 2013), such as clinostats (Briegleb, 1992; Klaus et al., 1998), rotating wall vessels (Ayyaswamy and Mukundakrishnan, 2007), random positioning machines (Wuest et al., 2015) or magnetic levitation (Qian et al., 2013) are not included. Also transcriptomics and proteomics studies are omitted and discussed in dedicated reviews (Abdelfattah et al., 2024; Corydon et al., 2023; Graf et al., 2024; Schulz et al., 2022; Strauch et al., 2019). But first, let’s briefly review some mechanisms on how cells can or could sense their physical environment and the role of cytoskeletons in mechanobiology.
1.1 Cytoskeletons and mechanomics
Cells are well known as “chemical machines” that are able to integrate, combine and respond to chemical cues from their environment. However, cells also sense and respond to their mechanical environment in an equally complex manner. Cells not only respond to a wide spectrum of mechanical stimulations, such as stretch, compression, vibrations and deformation, but also sense the physical properties of their environment, such as surface topography and substrate and matrix properties. Because cells incorporate, combine and respond to their complex mechanical environment, the terms “mechanomics” (van Loon, 2007; Wang et al., 2014) and “physicomics” (van Loon, 2009) were postulated. The mechanisms of mechanotransduction, which is the process of translating mechanical forces into a biological response (Cao et al., 2024; Huang et al., 2004; Hughes-Fulford and Boonstra, 2010; Humphrey et al., 2014; Janmey and McCulloch, 2007; Ogneva, 2013; Vogel, 2006; Wang et al., 2014; Wang and Thampatty, 2006), are not fully understood and are subjects of ongoing research. In the next section, the properties of cytoskeletal networks are briefly recapped, followed by a discussion on some of the current concepts related to mechanotransduction.
The cytoskeleton is a dense network of protein filaments that spans the entire cytosol (Zampieri et al., 2014). It consists of three major filament types: the rope-like actin filaments, the rod-like microtubules and the large protein family collectively named intermediate filaments (Schatten, 2015). Actin is the most abundant protein found in eukaryotic cells (Gardel et al., 2008). Its filaments are about 5–9 nm in diameter and are assembled from globular actin (G-actin) into a two-stranded helical polymer (F-actin) (Gardel et al., 2008). These flexible actin filaments interact with a large group of actin-binding proteins that help to organize and reorganize actin network architecture (Pollard, 2016). Broadly speaking, actin network architecture can be divided into gel-like networks, in which the filaments show a more random orientation, and bundles, in which the filaments are aligned in parallel. Cross-linked actin filament bundles are referred to as stress fibers (Tojkander et al., 2012), which can act as tension-bearing cables (Kumar et al., 2006). Myosin motor proteins are able to actively pull on the actin filaments and thereby help to organize and pre-stress the actin network (Vale and Milligan, 2000). In contrast, microtubules are long, hollow cylinders assembled from tubulin dimers. This hollow structure, with an outer diameter of 25 nm and an inner diameter of 17 nm, makes microtubules stiff polymers with a high bending rigidity (Gardel et al., 2008; Hawkins et al., 2010). Unlike actin and intermediate filaments, microtubules typically have a single microtubule organizing center (centrosome), from which the microtubules radiate to the cell periphery (Conduit et al., 2015). Microtubules are known to be highly dynamic, showing cycles of rapid growth and depolymerization, which is referred to as dynamic instability (Holy and Leibler, 1994; Mitchison and Kirschner, 1984). Similar to actin, microtubules interact with a large range of microtubule-associated proteins, of which some can cross-link and organize microtubules into bundles (Bodakuntla et al., 2019; Hawkins et al., 2010). Both actin and microtubules are polar molecules and act as intracellular “highways” along which motor proteins transport cargo, such as vesicles or ribonucleoprotein particles (Appert-Rolland et al., 2015; Mogre et al., 2020). Because microtubules are relatively straight, they are especially suitable for cellular trafficking (Barlan and Gelfand, 2017; Burute and Kapitein, 2019; Fletcher and Mullins, 2010; Gardel et al., 2008; Mogre et al., 2020). Finally, intermediate filaments are a heterogeneous protein family with more than 70 proteins in the human genome (Qin et al., 2010; Szeverenyi et al., 2008). They received their name because their filament size (ca. 10 nm) ranges between those of actin and microtubules (Ishikawa et al., 1968). The molecular building blocks of intermediate filaments are fibrous α-helical proteins that assemble into coiled-coil dimers. These dimers associate laterally and longitudinally to form rope-like filament bundles (Gardel et al., 2008). Unlike actin and microtubules, intermediate filaments are nonpolar (Gardel et al., 2008) and are the most flexible of all filaments (Charrier and Janmey, 2016; Fletcher and Mullins, 2010). In contrast to actin and microtubules, intermediate filaments and their networks can withstand large deformations to several times their original length before breaking (Guzmán et al., 2006; Janmey et al., 1991; Kreplak et al., 2005). However, intermediate filaments can also cross-link to each other as well as to microtubules and actin filaments (Wiche, 1998).
The cytoskeleton is a major contributor to cells’ mechanical properties (Fletcher and Mullins, 2010; Ingber, 2003a; 2003b; Rajagopal et al., 2018). Actin and the more elastic intermediate filaments are thought to mainly determine cell stiffness, whereas the stiff microtubules help cells to resist compressive loads (Ingber, 2008). In in vitro experiments, actin (Janmey et al., 1994; Storm et al., 2005; Xu et al., 2000) and intermediate filament (Janmey et al., 1991; Qin et al., 2010; Schopferer et al., 2009; Storm et al., 2005) networks become increasingly stiff with increasing extension under mechanical load (strain-stiffening behavior). However, apart from the individual components of the cytoskeleton, its architecture is a critical contributor to the cell’s mechanical properties (Fletcher and Mullins, 2010). The cytoskeletal network is highly cross-linked and prestressed by motor proteins (Ingber, 2003a; 2003b; Jensen et al., 2015). In contrast to the stress-stiffening behavior of cytoskeletal networks (Gardel et al., 2008), weakly cross-linked actin networks (Gardel et al., 2004; Xu et al., 1998) and pure microtubule networks (Lin et al., 2007) showed stress-softening behavior in in vitro experiments. Similarly, microtubules appear to help homogenize strain distribution in actin in in vitro networks (Gardel et al., 2008). This exemplifies the importance of cross-linking proteins in helping the cytoskeletal network resist large stresses and deformations without breaking (Gardel et al., 2008). Furthermore, cells prestress their cytoskeletal network using motor proteins, which is an important contributor to cells’ mechanical properties and morphology (Ingber, 2003a; 2003b; Jensen et al., 2015). Therefore, the tubulin network typically appears highly bent in cells (Gardel et al., 2008; Ingber, 2008). This prestressed interplay of flexible filaments (actin and intermediate filaments) with stiff microtubules eventually led to the formulation of the tensegrity model (Ingber, 1993; 2003a, 2003b). Apart from providing mechanical stability to the cells and allowing them to maintain their shape, the cytoskeleton also seems to stabilize intracellular components (Guo et al., 2013). Finally, the cytoskeleton is connected via specific junctions to the extracellular matrix (ECM) and other cells. Focal adhesions (FAs) (Burridge, 2017; Geiger et al., 2009; Wehrle-Haller, 2012) connect the actin cytoskeleton to the ECM, while hemidesmosomes (Borradori and Sonnenberg, 1999; Green and Jones, 1996; Walko et al., 2015) connect intermediate filaments to the cell substrate. Desmosomes (Desai et al., 2009; Green and Jones, 1996; Holthöfer et al., 2007) and adherens junctions (Dejana and Orsenigo, 2013; Niessen and Gottardi, 2008) connect the intermediate and actin networks of neighboring cells, respectively.
To date, several possible mechanisms have been identified concerning how cells might sense their physical environment, including mechanical load (mechanotransduction). These pathways are not mutually exclusive and are likely overlapping, creating an actual mechanosensitive signaling network (Ingber, 1999; Wang et al., 2014). The first instance of mechanosensation is probably not within the cells but in the surrounding ECM. The ECM can act as a reservoir for many signaling molecules, which are released and presented to cells under mechanical load (Wang and Thampatty, 2006). For example, the transforming growth factor-β (TGF-β) can be activated and released from the ECM by contractile forces (Wipff et al., 2007). Furthermore, multiple proteins are known to unravel under mechanical load, exposing cryptic sites that are buried in a native unloaded state (Vogel, 2006). A prominent example is the ECM protein fibronectin, which has many recognition sites binding to other ECM proteins, serum proteins and cell adhesion proteins (Vogel, 2006; Vogel and Sheetz, 2009). Cells are attached via FAs to the ECM, and eventually, physical forces are passed through these protein complexes to the cells (Burridge, 2017; Eyckmans et al., 2011; Geiger et al., 2009; Hughes-Fulford and Boonstra, 2010; Wehrle-Haller, 2012). Members from the integrin protein family are a prominent example among the many proteins that cluster in FAs. Integrins are transmembrane glycoproteins that connect the actin cytoskeleton to ECM proteins (e.g., fibronectin and vitronectin) (Burridge, 2017; Geiger et al., 2009; Huang et al., 2004; Hughes-Fulford and Boonstra, 2010; Ogneva, 2013; Wehrle-Haller, 2012). Integrin binding to the ECM can activate transcription factors via the focal adhesion kinase (FAK), activated intermediate messengers and the MAP kinase (Hughes-Fulford and Boonstra, 2010). Not surprisingly, integrin is known to regulate various cellular functions, including cell attachment, proliferation, migration and differentiation (Coppolino and Dedhar, 2000). The formation and maturation of FAs requires the application of force (Eyckmans et al., 2011; Geiger and Bershadsky, 2001; Geiger et al., 2009; Hughes-Fulford and Boonstra, 2010). Correspondingly, inhibition of contractile forces leads to a disassembly of FAs (Chrzanowska-Wodnicka and Burridge, 1996; Hughes-Fulford and Boonstra, 2010). In a similar matter, cytoskeleton stability is likely dependent on acting forces. For instance, the pitch length of helical actin filaments increases under tensile force. This increases actin’s affinity to myosin II and reduces the affinity to cofilin, which is known to sever filamentous actin (Hayakawa et al., 2011; McGough et al., 1997).
Inside the nucleus, a dense network of lamins (members of intermediate filaments) and nuclear lamin-associated membrane proteins form the nuclear lamina (Gruenbaum and Foisner, 2015; Wang et al., 2009). The nuclear lamina is crucial for nuclear organization and involved in several cellular functions, including activation of transcription factors (Dorner et al., 2006; Ho et al., 2013; Ivorra et al., 2006; Osmanagic-Myers et al., 2015), regulation of chromatin epigenetic state (Harr et al., 2015; Solovei et al., 2013), chromosome tethering (Gruenbaum and Foisner, 2015) and cell polarization and migration (Davidson et al., 2014; Harada et al., 2014; Lee et al., 2007). The lamina is connected to the cytoskeletal network via the LINC complex (linker of nucleoskeleton and cytoskeleton) (Crisp et al., 2005; Sosa et al., 2013). It is therefore thought that forces transmitted via the cytoskeleton to the nucleus can lead to nuclear deformation, which modulates transcription and chromatin organization (Kirby and Lammerding, 2018; Uhler and Shivashankar, 2017; Wang et al., 2009).
Finally, the cell membrane and its associated proteins (Anishkin and Kung, 2013; Ingber, 2006), particularly the mechanosensitive ion channels (MSCs), have been proposed as mechanosensors as well. MSCs, also known as stretch-activated channels, are characterized by the ability to switch between closed and open states in response to mechanical load (Lecar and Morris, 1993; Sachs, 2010; Sachs and Morris, 1998). This conformational change allows specific ions (e.g., sodium, potassium and calcium) to cross the plasma membrane under local mechanical membrane tension (Wang and Thampatty, 2006). While it is not fully clear how these channels are coupled to mechanical forces, it is thought that some MSCs are linked directly or indirectly to the cytoskeleton or the ECM, and others interact only with the surrounding lipids (Anishkin et al., 2014; Hamill, 2006; Hayakawa et al., 2008; Sachs, 2010; Sachs and Morris, 1998). All membrane proteins, including MSCs, must match the hydrophobicity of the lipid bilayer. As mechanically stressed membranes become thinner under tension (Reddy et al., 2012), this could lead to hydrophobic mismatches at the protein–lipid interface, which could favor MSCs to undergo a conformational change (Anishkin et al., 2014).
In summary, the cytoskeleton plays a central role in numerous cellular functions. It largely determines cells’ mechanical stability and morphology. The cytoskeleton is also critically important for intracellular organization and molecular transport. It acts as a scaffold for many cellular organelles (Charrier and Janmey, 2016; Guo et al., 2013; Hughes-Fulford and Boonstra, 2010), and motor proteins transport their cargo along microtubules and actin filaments (Appert-Rolland et al., 2015; Mogre et al., 2020). Cells can selectively prestress or relax the cytoskeletal network via motor proteins and cytoskeleton remodeling (Ingber, 2003a; Jansen et al., 2015; Wang, 2017). Constant remodeling also allows cells to enforce or weaken the cytoskeleton to adapt to the governing forces (Kobayashi and Sokabe, 2010; Ohashi et al., 2017; Tojkander et al., 2012; Wang, 2017). In cellular mechanosensation, the cytoskeleton can dissipate mechanical forces (to reduce machanosensitivity) and channel mechanical forces to mechanosensitive structures, such as FAs, the nucleus or MSCs. These mechanical signals propagate through the prestressed cytoplasm much more quickly than diffusion-based chemical signals (Jaalouk and Lammerding, 2009).
2 Results
Due to the cytoskeleton’s central role in cellular mechanics and mechanosensation, the cytoskeleton was quickly thought to be gravity dependent. During the past three decades, numerous researchers have used isolated cell cultures to investigate cytoskeleton adaptations in (real) microgravity (Lewis, 2004; Rudimov and Buravkova, 2016; Vorselen et al., 2014; Wu et al., 2022), making use of parabolic flights (Karmali and Shelhamer, 2008; Pletser, 2016; Pletser et al., 2016), sounding rockets (Seibert, 2006) and orbiting spacecrafts (Duan and Long, 2019; NASA, 2010; Rai et al., 2016). The published data describe a large variety of cytoskeleton adaptations already within a minute after exposure to microgravity. Unfortunately, there is no clear pattern concerning how microgravity affects the cytoskeleton. Confounding factors could be the variety in study designs, types of hardware, cell sources and constraints of microgravity platforms. A minority of the authors reported not having detected any changes (negative finding; refer to Tables 1–3). However, the extent to which published data is biased toward positive findings is unclear.
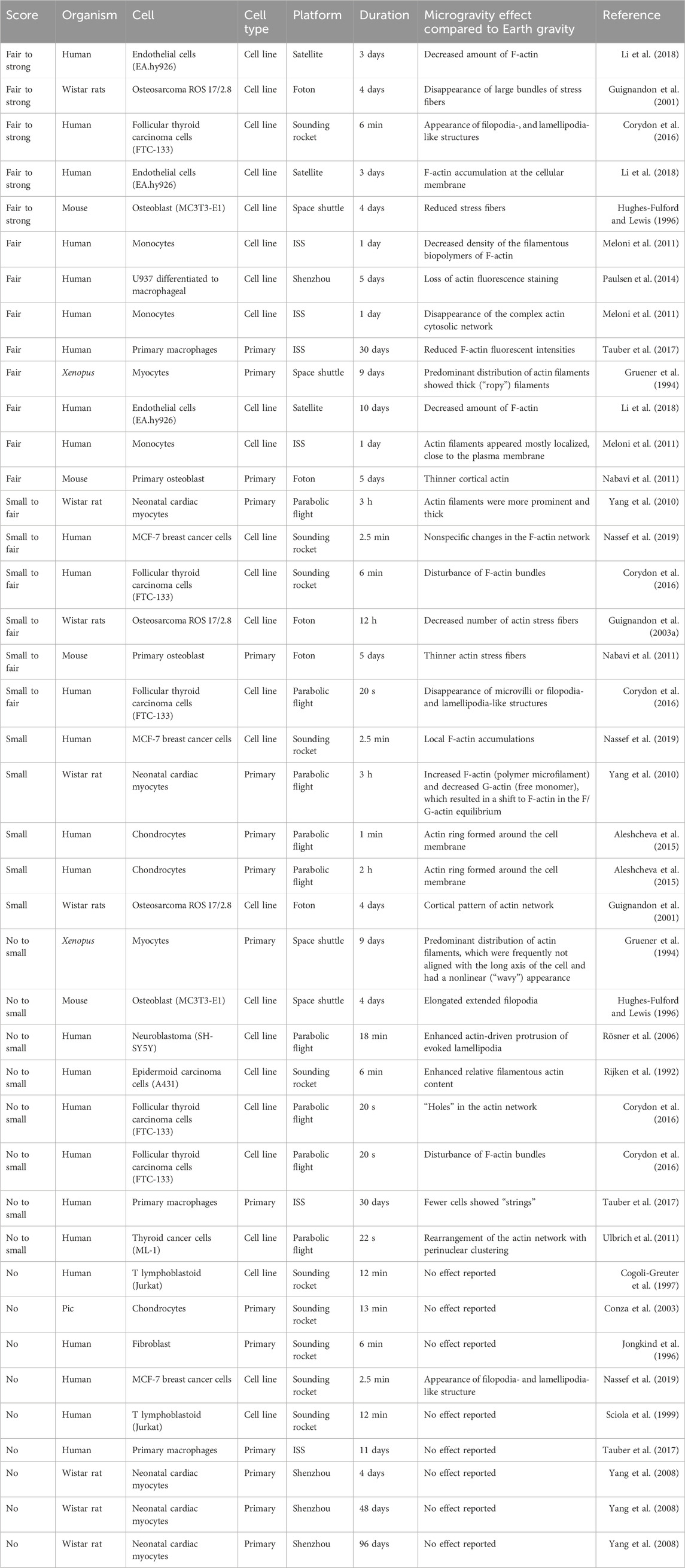
Table 1. Reported effects of actin adaptations in microgravity sorted from strong effect to no observable changes. Every line represents a reported claim in a publication. Therefore, publications with multiple claims and timepoints appear multiple times.
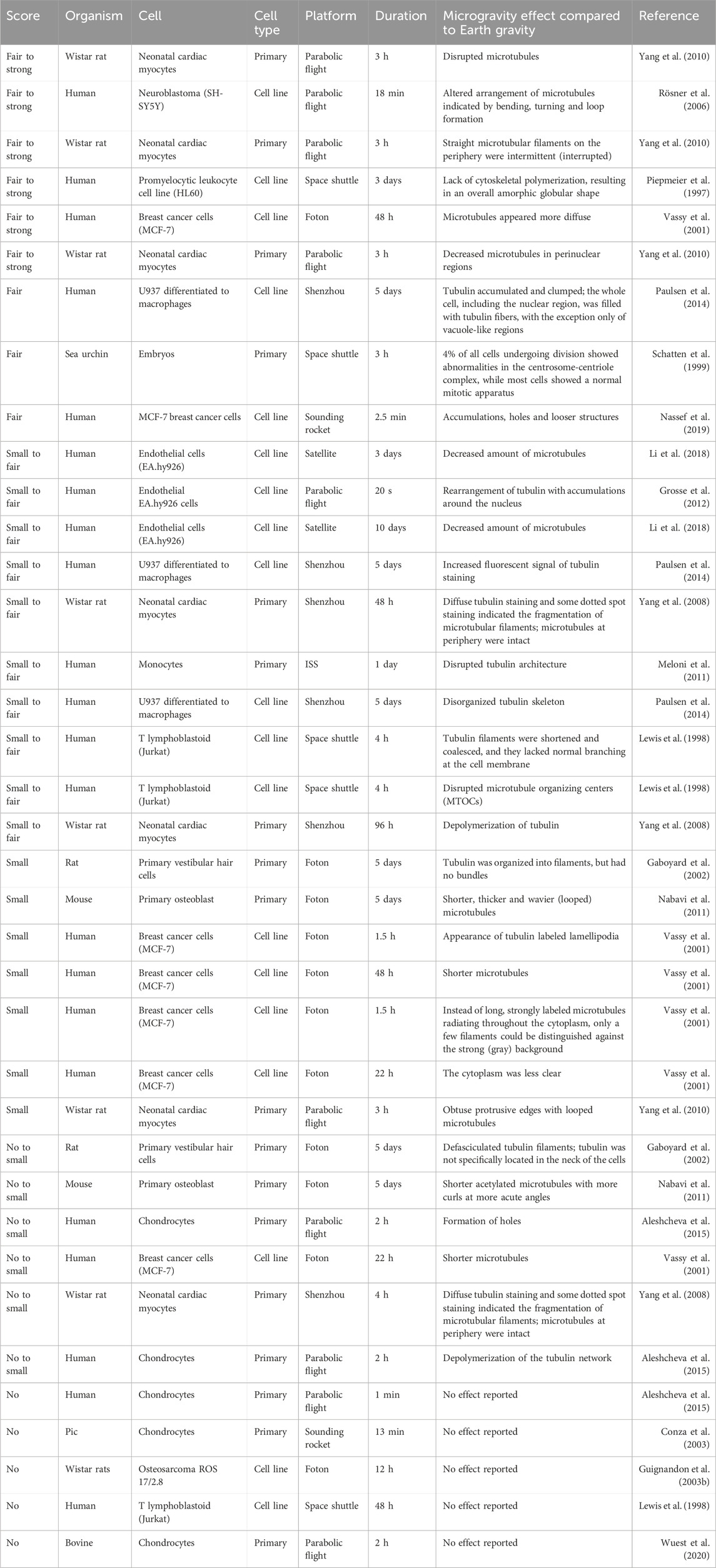
Table 2. Reported effects of microtubule adaptations in microgravity sorted from strong effect to no observable changes. Every line represents a reported claim in a publication. Therefore, publications with multiple claims and timepoints appear multiple times.
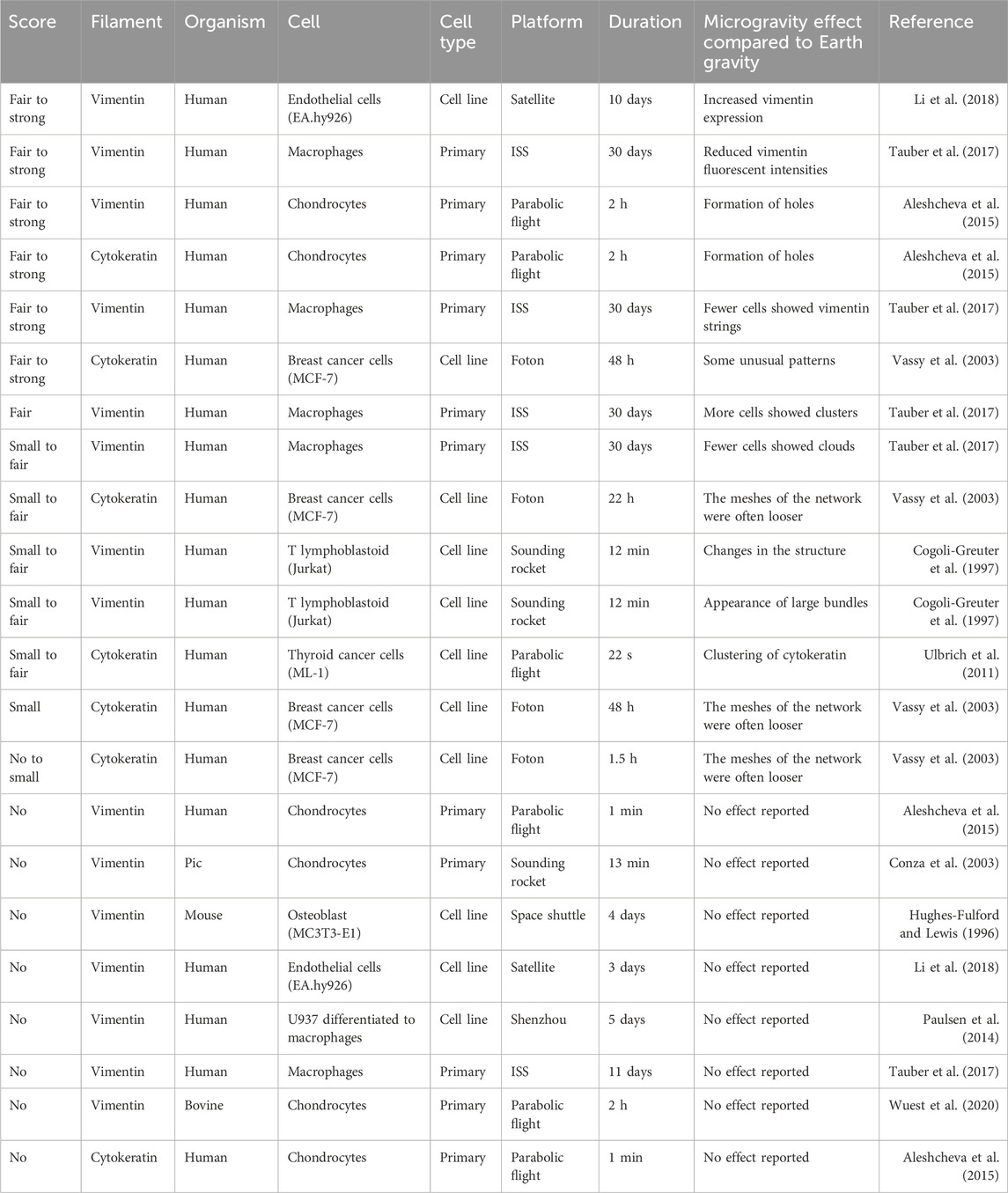
Table 3. Reported effects of intermediate filament adaptations in microgravity sorted from strong effect to no observable changes. Every line represents a reported claim in a publication. Therefore, publications with multiple claims and timepoints appear multiple times.
To get a more objective view, I have compiled the results of 30 publications and asked ten individuals who were familiar with cytoskeleton staining to score the authors’ claims. The allowed scores were “no” change, “small” (barely visible), “fair” (clearly visible) or “strong” (obvious) change. All information regarding the authors, research institutions, utilized microgravity platform or cell source was removed to avoid biasing the scoring. Additionally, the participating individuals were not familiar with the publications. Publications in which researchers investigated multiple proteins (e.g., actin and tubulin) or time points were split, and the proteins and timepoints were scored separately. Publications that reported no change were not displayed for scoring and directly scored as showing “no” change. Figures 1–3 highlight the diversity of the data set (Please refer to the Supplementary Material for a more detailed description of the data). Generally speaking, orbiting platforms tend to trigger larger cytoskeleton adaptations in microgravity, even though statistically not significant (Wilcoxon rank-sum test with an α of 5%, Supplementary Figure S1). However, time in microgravity seems to be the most dominant factor. (For publications that did not report a timeline, it was estimated from what could be expected from the respective microgravity platform). There is no clear detectable pattern of effect scores with regards to cell culture, cell types (primary or cell line) or cell sources.
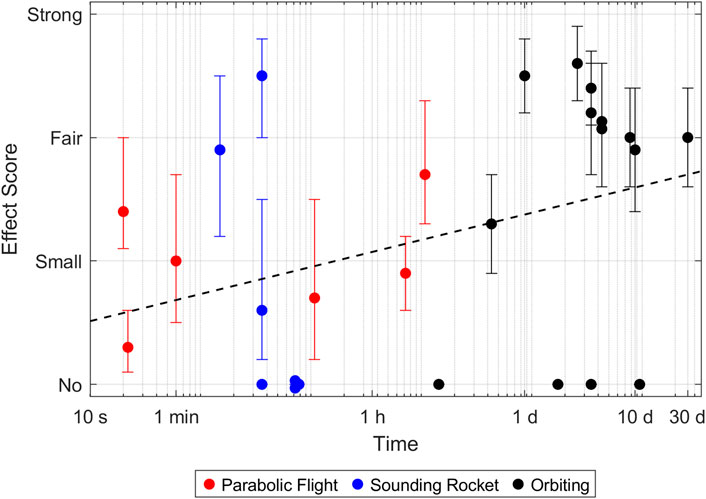
Figure 1. Effect size of actin adaptations in microgravity. Every point represents a reported timepoint of a publication. If the authors stated multiple claims, every claim was scored separately and the highest score of each claim was taken. The score represents the average over all participants. Error bars indicate the 95% confidence interval. A complete parabola during a parabolic flight lasts around 1 minute. Samples which were fixed later, therefore went through multiple parabolas and experienced multiple hyper- and microgravity phases.
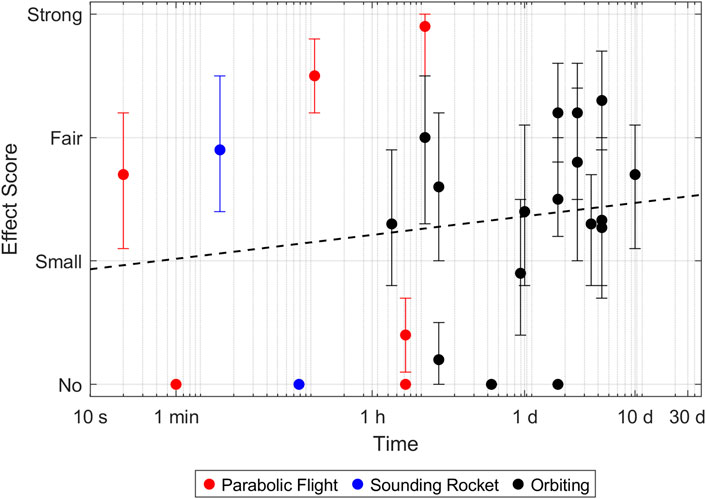
Figure 2. Effect size of microtubule adaptations in microgravity. Every point represents a reported timepoint of a publication. If the authors stated multiple claims, every claim was scored separately and the highest score of each claim was taken. The score represents the average over all participants. Error bars indicate the 95% confidence interval. A complete parabola during a parabolic flight lasts around 1 minute. Samples which were fixed later, therefore went through multiple parabolas and experienced multiple hyper- and microgravity phases.
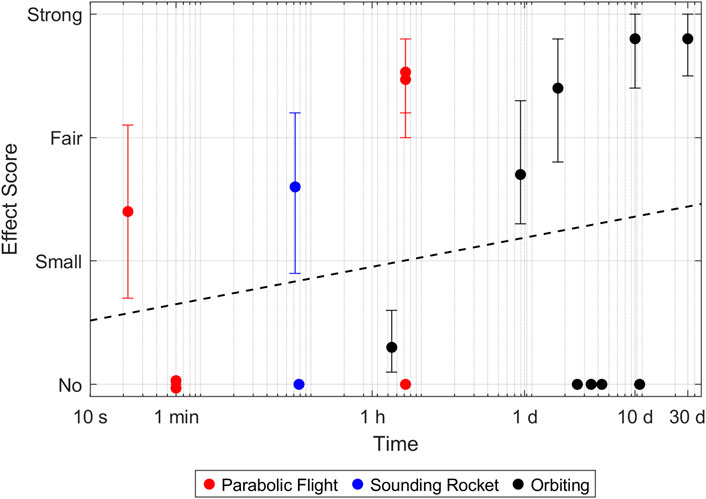
Figure 3. Effect size of intermediate filament adaptations in microgravity. Every point represents a reported timepoint of a publication. If the authors stated multiple claims, every claim was scored separately and the highest score of each claim was taken. The score represents the average over all participants. Error bars indicate the 95% confidence interval. A complete parabola during a parabolic flight lasts around 1 minute. Samples which were fixed later, therefore went through multiple parabolas and experienced multiple hyper- and microgravity phases.
The time-dependent effect is most striking for the actin network (The Pearson correlation coefficient of the log-transformed time versus the effect score was computed to be statistically significant with a p-value of 3.2%, Supplementary Table S1). Most experiments that reported “fair” to “strong” effects were done on orbiting platforms. They most often described a decrease or loss of F-actin (Table 1). Similarly, a reduction or loss of stress fibers was reported several times. A few papers reported rearrangements of F-actin such as atypical accumulation, appearance of filopodia- and lamellipodia-like structures (Corydon et al., 2016) or formation of actin filaments. However, effects that could be summarized as actin rearrangement were generally considered small and did not score higher than “fair”.
For the tubulin network, the results appear to be much more random and rather independent of time and employed microgravity platform. (Neither the Pearson correlation coefficient nor the Wilcoxon rang-sum test computed a statistically significant differences with p-values lower than 5%, Supplementary Table S1; Supplementary Figure S1). The most often reported effect (also in the “fair” to “strong” range) was a disrupted and more diffuse tubulin network (Table 2). Many papers also reported altered arrangements such as accumulations and holes. In a few experiments, shorter microtubules or filaments were observed, but overall, this seems to have been a rather minor effect.
Finally, a few experiments on the two intermediate filaments, vimentin and cytokeratin, were published. Overall, the effect of microgravity on intermediate filaments also seems to be time dependent at first. However, this claim could not be supported by Pearson correlation statistics (Supplementary Table S1) and the number of publications with positive and negative findings are more or less balanced for all platforms. Also, the findings are rather conflicting. For example, a reduction of vimentin was only reported in one experiment on human macrophages after 30 days in microgravity (Tauber et al., 2017). In contrast, an experiment with human endothelial cells reported an increase of vimentin expression after 10 days in microgravity (Li et al., 2018). Overall, most papers reported some sort of rearrangement, such as formation of holes, clusters, looser structures or formation of bundles (Table 3).
3 Discussion and conclusion
This work aimed at assessing whether the reported changes in the cytoskeleton in microgravity were substantial or relatively minor. The subjective scores of ten blinded individuals, confirmed the heterogeneous nature of previously published findings. Microgravity experiments are challenging in many aspects (Beysens and van Loon, 2015). All microgravity platforms, being parabolic flights, sounding rockets and orbiting space crafts, have a hypergravity period before the actual microgravity phase, which could influence the cellular response. (Due to the short microgravity period, none of the publications analyzed in this paper made use of a drop tower (Dittus, 1991).) For parabolic flights (typically around 1.5–2 g (Karmali and Shelhamer, 2008)) and large space crafts (typically around 3–5 g), the hypergravity level is rather modest. For sounding rockets, the launch conditions, typically lasting around 45 s, can be rather harsh, with peak linear accelerations of around 13 g accompanied by strong vibrations (Seibert, 2006). Furthermore, all vehicles show an inherent distinct pattern of rising and falling acceleration loads with different timings. For examples, the parabola of a parabolic flight typically last around 1 minute with two hypergravity periods of around 20 s before and after the microgravity phase (Karmali and Shelhamer, 2008). During one flight, often many parabolas are flown every few minutes. In contrast, a two-stage sounding rocket typically experiences about 8 g during the burn of the first stage, then a short microgravity phase (ca. 5 s) during stage separation and peak accelerations up to 13 g during the second stage burn. All microgravity platforms also have specific limitations in accessibility, available experiment space, timelines, environment stability and safety constraints. For orbiting space crafts, the up- and download conditions can be rather problematic. Launch scraps, operational constraints, reentry heat and long recoveries can interfere with the experiment timeline and environment conditions (e.g., optimal temperature range). The high costs related to space-flown experiments and the limited access to microgravity, lead to the fact that all experiments are unique “single-shot” experiments with different cell sources, platforms, hardware, timelines, microgravity qualities, environment conditions and data acquisition protocols. Finally, the 1 g control samples were often acquired under different conditions as well. While some experiments use an in-flight 1 g control, other studies used lab samples or hardware control samples, which were produced either in parallel or after the flight. All of these confounding factors can make data interpretation difficult. This is also mirrored by the very heterogeneous findings reported over the last decades. However, a real and global effect, which is present in many cell types, should still consistently appear in several studies.
This paper quantified whether the reported effects were clear and reasonable or rather small. One must keep in mind that researchers are inclined to look for positive findings and therefore tend to overrate their findings. Negative findings are also much more at risk of not being published (Baker, 2016; Editorial, 2019). The individuals who did the scoring were only shown the claims the authors published and were unaware of the study design, microgravity platform or timeline to prevent potential bias by this information. This resulted in a more generalized, quantified and objective picture than a list of qualitative findings. However, one limitation we faced was that the quality and presentation of the data were also very heterogeneous. The technological and methodological advances between the first experiments and the modern day were also striking. Furthermore, interpretation of cytoskeleton staining remains a qualitative task which is subjected to individual views, backgrounds and interpretations. Therefore, the raters showed strong agreement for some claims but also very diverse answers for other claims (Supplementary Tables S8, S12, S16). This is also reflected in the inter-rater reliability, which was computed to show a “slight agreement” by Fleiss’es κ (Cardillo, 2007). Finally, while great effort was invested in a thorough literature search, missed publications could bias the results.
For actin and tubulin cytoskeletons, the published data set is large enough to attempt a first quantitative summary. For the intermediate filaments, namely vimentin and cytokeratin, the number of publications is still rather small. Interestingly, the largest effects on actin were clearly observed on orbiting platforms. There is also a clear time dependency, with strong effects mainly reported after an experiment duration of around 1 day. Among them, the most frequent descriptions could be summarized as a decrease or loss of F-actin or F-actin structures (e.g., stress fibers). In contrast, the results for microtubules are rather random and independent of time and microgravity platform. Additionally, for this molecule, the most often reported findings were disruption and loss of microtubulin organization. Concerning the intermediate filaments, the small data set and incoherent reports do not really allow for a solid conclusion. However, network reorganization, rather than loss or disruption, seems to be the dominant effect.
Overall, this suggests that generally, the cytoskeleton is rather stable under short-term microgravity (on the order of minutes), as mostly small adaptations were reported. Large (obvious) changes were mostly reported after the cells had spent multiple hours or days in microgravity. This suggests that microgravity does not trigger an immediate and profound adaptation of the cytoskeleton. It is more likely that cells have trouble maintaining (or remodeling) the cytoskeleton while in microgravity.
The cytoskeleton could play an important role in acute cellular gravity sensing due to its central role in many cellular processes, including mechanotransduction. This view is also supported by the tensegrity model, which stresses the importance that the cytoskeleton needs to be in equilibrium to the governing forces (Wu et al., 2022). However, it is probably unlikely that exposure to microgravity directly and actively induces reorganization or disassembly of the cytoskeleton. It is more likely that maintenance of the cytoskeleton as part of the regular cell cycle is disturbed, particularly for the actin network. As a result, this could lead to altered cytoskeleton architecture, pre-stress and stability (Figure 4). Over a longer period, this may have serious consequences for cells or organisms, as the cytoskeleton is involved in many important cellular processes, such as cell migration and the cell cycle (Figure 4). Potentially the consequences could imply alterations in cellular organization, cell stiffness (tension & compression), attachment to the ECM, as well as dissipation and transmission of forces through the cells. As the cytoskeleton acts also as a scaffold, molecular transport and stability of intracellular organelles could be affected as well. Ultimately, the nucleus is also closely connected to the cytoskeleton, which could affect transcription, chromatin epigenetic, chromosome tethering and cell polarization. This could have potential implication for astronaut health, pathogen vitality and mutations, plant growth in space (space farming), and life science applications in microgravity, such as tissue engineering.
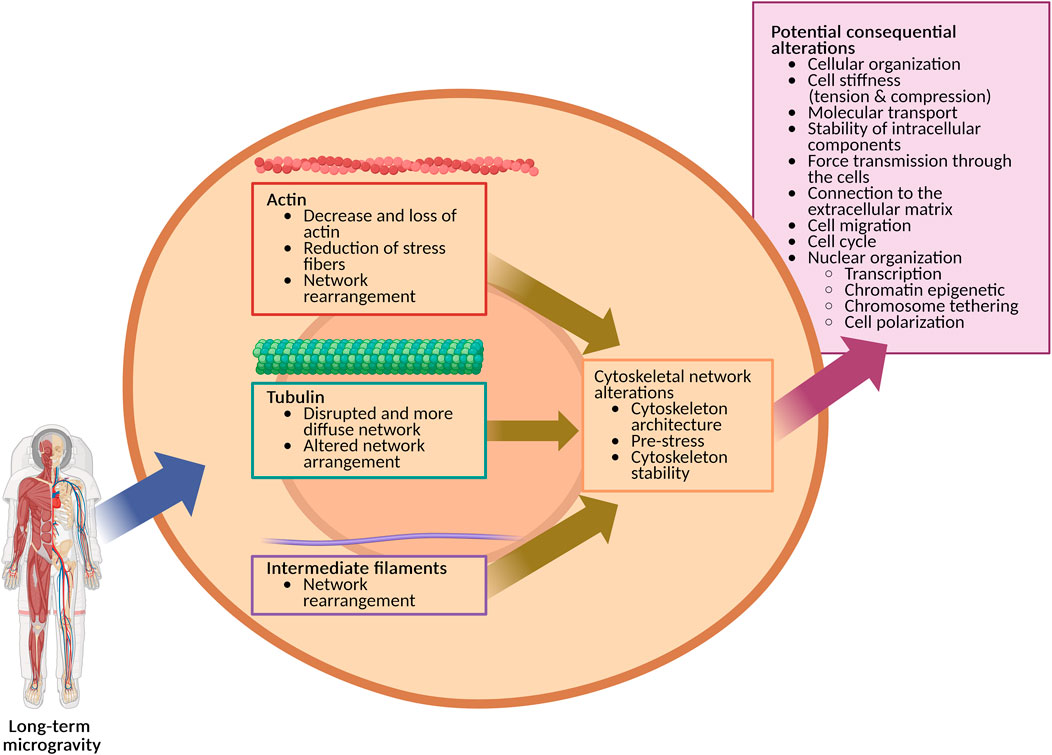
Figure 4. Long-term microgravity impacts the cytoskeleton’s organization, which could result in multiple subsequent cellular alterations (Created in BioRender. https://BioRender.com/gt4daok).
At this point, the underlying mechanisms leading to a disrupted cytoskeleton in microgravity remain speculative. It could be hypothesized that microgravity makes actin filaments more likely to be severed by cofilin (Hayakawa et al., 2011; McGough et al., 1997). However, it could also suggest that in the normal cell cycle, gravity could act as an “organizing force” supporting an efficient buildup and organization of the cytoskeleton. This perspective is backed by the early experiments which demonstrated that the self-assembly of microtubules in a cell-free system is indeed gravity dependent (Moos et al., 1988; Papaseit et al., 2000). As the entire evolution developed in the Earth’s constant gravity condition, even molecular processes are likely fine-tuned to work in a gravity field. Traditional “fix and rinse experiments” are relatively easy and cost-effective to implement but suffer from limited time points and large data variability (unpaired data). In contrast, life cell staining techniques (Corydon et al., 2016; Nassef et al., 2019) allow observing changes in real-time over extended periods with high special and temporal resolution. In combination with pharmaceutical blockers or genetic manipulations (e.g. knockout or knock-in) this technology could help to mechanistically dissect the underlying molecular pathways.
In conclusion, understanding and quantifying the effect of microgravity on the cytoskeleton and its potential implications is not trivial. The body of literature from the past decades does not always show consistent patterns and is sometimes even contradictory. Generally speaking, the time cells are exposed to microgravity seems to be the dominant contributor to trigger clearly observable modifications.
4 Methods
4.1 Score determination
First a thorough literature search was conducted, and the publications were manually screened. Scientific publications reporting cytoskeleton adaptions in real microgravity, using immunofluorescent imaging, were included. (A few potential hits could not be included, because the full text was not accessible.) The results of 30 included publications were subsequently compiled and rated by ten individuals. For each claim by the original author, the allowed scores were “no” change (0), “small” (barely visible; 1), “fair” (clearly visible; 2) or “strong” (obvious; 3) change. All information regarding the authors, research institutions, utilized microgravity platform or cell source was removed to avoid biasing the scoring. The raters were not familiar with the publications, and all had a master or PhD in the biomedical field and were familiar with cytoskeleton staining. Publications in which researchers investigated multiple proteins (e.g., actin and tubulin) or time points were split, and the proteins and timepoints were scored separately. Publications that reported no change were not displayed for scoring and directly scored as showing “no” change.
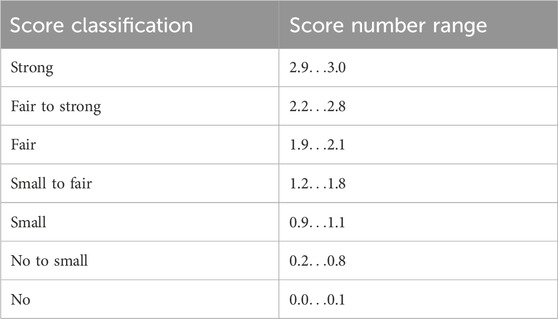
To compare the studies (Figures 1–3), for each protein and timepoint the largest score given by an individual rater was taken and then averaged over all ten raters for the final score. To assess the individually reported effects, for each author’s claim, the scores were averaged over the ten raters and ranked from “strong” effect to “no” effect. The resulting average scores were finally classified according to Table 4. The 95% confidence interval (CI) is the bootstrap CI of the mean value.
4.2 Time dependency
Time dependency was calculated by the single-tailed Pearson correlation coefficient of the log-transformed time versus the averaged score per publication and timepoint. Pearson’s ρ and p-values are reported in Supplementary Table S1.
Author contributions
SW: Investigation, Writing – review and editing, Conceptualization, Writing – original draft, Data curation, Visualization, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Open access funding by Lucerne University of Applied Sciences and Arts.
Acknowledgments
I thank Christina Giger-Lange, Cindy Follonier, Fabian Ille, Fabienne Wyss, Geraldine Cerretti, Jeannine Doswald-Winkler, Jennifer Polzer, Karin F. Rattenbacher-Kiser, Magdalena Herová, Martina Caliò and Samuel Tanner for supporting this paper. I also thank the Lucerne School of Engineering and Architecture (Switzerland) for the financial support.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frspt.2025.1677728/full#supplementary-material
References
Abdelfattah, F., Schulz, H., Wehland, M., Corydon, T. J., Sahana, J., Kraus, A., et al. (2024). Omics studies of specialized cells and stem cells under microgravity conditions. Int. J. Mol. Sci. 25 (18), 10014. doi:10.3390/ijms251810014
Albrecht-Buehler, G. (1991). Possible mechanisms of indirect gravity sensing by cells. ASGSB Bull. 4 (2), 25–34. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/11537179.
Aleshcheva, G., Wehland, M., Sahana, J., Bauer, J., Corydon, T. J., Hemmersbach, R., et al. (2015). Moderate alterations of the cytoskeleton in human chondrocytes after short-term microgravity produced by parabolic flight maneuvers could be prevented by up-regulation of BMP-2 and SOX-9. FASEB J. 29 (6), 2303–2314. doi:10.1096/fj.14-268151
Anishkin, A., and Kung, C. (2013). Stiffened lipid platforms at molecular force foci. Proc. Natl. Acad. Sci. 110 (13), 4886–4892. doi:10.1073/pnas.1302018110
Anishkin, A., Loukin, S. H., Teng, J., and Kung, C. (2014). Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc. Natl. Acad. Sci. U. S. A. 111 (22), 7898–7905. doi:10.1073/pnas.1313364111
Appert-Rolland, C., Ebbinghaus, M., and Santen, L. (2015). Intracellular transport driven by cytoskeletal motors: general mechanisms and defects. Phys. Rep. 593, 1–59. doi:10.1016/j.physrep.2015.07.001
Ayyaswamy, P. S., and Mukundakrishnan, K. (2007). Optimal conditions for simulating microgravity employing NASA designed rotating wall vessels. Acta Astronaut. 60 (4), 397–405. doi:10.1016/j.actaastro.2006.09.008
Baker, M. (2016). 1,500 scientists lift the lid on reproducibility. Nature 533 (7604), 452–454. doi:10.1038/533452a
Barlan, K., and Gelfand, V. I. (2017). Microtubule-based transport and the distribution, tethering, and organization of organelles. Cold Spring Harb. Perspect. Biol. 9 (5), a025817. doi:10.1101/cshperspect.a025817
Beysens, D. A., and van Loon, J. J. (2015). Generation and applications of extra-terrestrial environments on Earth. The Netherlands: Taylor & Francis.
Blaber, E., Marcal, H., and Burns, B. P. (2010). Bioastronautics: the influence of microgravity on astronaut health. Astrobiology 10 (5), 463–473. doi:10.1089/ast.2009.0415
Bodakuntla, S., Jijumon, A. S., Villablanca, C., Gonzalez-Billault, C., and Janke, C. (2019). Microtubule-associated proteins: structuring the cytoskeleton. Trends Cell Biol. 29 (10), 804–819. doi:10.1016/j.tcb.2019.07.004
Borradori, L., and Sonnenberg, A. (1999). Structure and function of hemidesmosomes: more than simple adhesion complexes. J. Investigative Dermatology 112 (4), 411–418. doi:10.1046/j.1523-1747.1999.00546.x
Briegleb, W. (1992). Some qualitative and quantitative aspects of the fast-rotating clinostat as a research tool. ASGSB Bull. Publ. Am. Soc. Gravitational Space Biol. 5 (2), 23–30.
Brungs, S., Egli, M., Wuest, S. L., M. Christianen, P. C., W. A. van Loon, J. J., Ngo Anh, T. J., et al. (2016). Facilities for simulation of microgravity in the ESA ground-based facility programme. Microgravity Sci. Technol. 28, 191–203. doi:10.1007/s12217-015-9471-8
Burridge, K. (2017). Focal adhesions: a personal perspective on a half century of progress. FEBS J. 284 (20), 3355–3361. doi:10.1111/febs.14195
Burute, M., and Kapitein, L. C. (2019). Cellular logistics: unraveling the interplay between microtubule organization and intracellular transport. Annu. Rev. Cell Dev. Biol. 35 (35), 29–54. doi:10.1146/annurev-cellbio-100818-125149
Cao, R., Tian, H., Tian, Y., and Fu, X. (2024). A hierarchical mechanotransduction system: from macro to micro. Adv. Sci. 11 (11), 2302327. doi:10.1002/advs.202302327
Cardillo, G. (2007). Fleiss'es kappa: compute the Fleiss'es kappa for multiple raters. Available online at: http://www.mathworks.com/matlabcentral/fileexchange/15426.
Charrier, E. E., and Janmey, P. A. (2016). Mechanical properties of intermediate filament proteins. Methods Enzymol. 568, 35–57). doi:10.1016/bs.mie.2015.09.009
Chrzanowska-Wodnicka, M., and Burridge, K. (1996). Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133 (6), 1403–1415. doi:10.1083/jcb.133.6.1403
Clément, G., and Slenzka, K. (2006). Fundamentals of space biology: research on cells, animals, and plants in space, 18. Springer Science & Business Media.
Cogoli-Greuter, M., Cogoli, A., Spano, A., Sciola, L., and Pippia, P. (1997). Influence of microgravity on mitogen binding and cytoskeleton in jurkat cells-experiment on MAXUS 2. Eur. Rocket Balloon Programmes Relat. Res.
Conduit, P. T., Wainman, A., and Raff, J. W. (2015). Centrosome function and assembly in animal cells. Nat. Rev. Mol. Cell Biol. 16 (10), 611–624. doi:10.1038/nrm4062
Conza, N., Cogoli, A., Dreier, R., Bruckner, P., Berardi-Vilei, S., Kraemer, J., et al. (2003). Test flight of a bioreactor module for cartilage tissue on MASER 9. Eur. Rocket Balloon Programmes Relat. Res.
Coppolino, M. G., and Dedhar, S. (2000). Bi-directional signal transduction by integrin receptors. Int. J. Biochem. Cell Biol. 32 (2), 171–188. doi:10.1016/s1357-2725(99)00043-6
Corydon, T. J., Kopp, S., Wehland, M., Braun, M., Schutte, A., Mayer, T., et al. (2016). Alterations of the cytoskeleton in human cells in space proved by life-cell imaging. Sci. Rep. 6, 20043. doi:10.1038/srep20043
Corydon, T. J., Schulz, H., Richter, P., Strauch, S. M., Böhmer, M., Ricciardi, D. A., et al. (2023). Current knowledge about the impact of microgravity on gene regulation. Cells 12 (7), 1043. doi:10.3390/cells12071043
Crawford-Young, S. J. (2006). Effects of microgravity on cell cytoskeleton and embryogenesis. Int. J. Dev. Biol. 50 (2-3), 183–191. doi:10.1387/ijdb.052077sc
Crisp, M., Liu, Q., Roux, K., Rattner, J. B., Shanahan, C., Burke, B., et al. (2005). Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 172 (1), 41–53. doi:10.1083/jcb.200509124
Davidson, P. M., Denais, C., Bakshi, M. C., and Lammerding, J. (2014). Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments. Cell. Mol. Bioeng. 7 (3), 293–306. doi:10.1007/s12195-014-0342-y
Dejana, E., and Orsenigo, F. (2013). Endothelial adherens junctions at a glance. J. Cell Sci. 126 (12), 2545–2549. doi:10.1242/jcs.124529
Demontis, G. C., Germani, M. M., Caiani, E. G., Barravecchia, I., Passino, C., and Angeloni, D. (2017). Human pathophysiological adaptations to the space environment. Front. Physiol. 8, 547. doi:10.3389/fphys.2017.00547
Desai, B. V., Harmon, R. M., and Green, K. J. (2009). Desmosomes at a glance. J. Cell Sci. 122 (24), 4401–4407. doi:10.1242/jcs.037457
Dittus, H. (1991). Drop tower ‘bremen’: a weightlessness laboratory on Earth. Endeavour 15 (2), 72–78. doi:10.1016/S0160-9327(05)80008-0
Dorner, D., Vlcek, S., Foeger, N., Gajewski, A., Makolm, C., Gotzmann, J., et al. (2006). Lamina-associated polypeptide 2α regulates cell cycle progression and differentiation via the retinoblastoma–E2F pathway. J. Cell Biol. 173 (1), 83–93. doi:10.1083/jcb.200511149
Duan, E., and Long, M. (2019). Life science in space: experiments on board the SJ-10 recoverable satellite. Springer.
Editorial (2019). The importance of no evidence. Nat. Hum. Behav. 3 (3), 197. doi:10.1038/s41562-019-0569-7
Eyckmans, J., Boudou, T., Yu, X., and Chen, C. S. (2011). A Hitchhiker's guide to mechanobiology. Dev. Cell 21 (1), 35–47. doi:10.1016/j.devcel.2011.06.015
Fletcher, D. A., and Mullins, R. D. (2010). Cell mechanics and the cytoskeleton. Nature 463 (7280), 485–492. doi:10.1038/nature08908
Gaboyard, S., Blanchard, M.-P., Travo, C., Viso, M., Sans, A., and Lehouelleur, J. (2002). Weightlessness affects cytoskeleton of rat utricular hair cells during maturation in vitro. Neuroreport 13 (16), 2139–2142. doi:10.1097/00001756-200211150-00030
Gardel, M. L., Shin, J. H., MacKintosh, F. C., Mahadevan, L., Matsudaira, P., and Weitz, D. A. (2004). Elastic behavior of cross-linked and bundled actin networks. Science 304 (5675), 1301–1305. doi:10.1126/science.1095087
Gardel, M. L., Kasza, K. E., Brangwynne, C. P., Liu, J., and Weitz, D. A. (2008). Chapter 19 mechanical response of cytoskeletal networks. Methods Cell Biol. 89, 487–519. doi:10.1016/S0091-679X(08)00619-5
Geiger, B., and Bershadsky, A. (2001). Assembly and mechanosensory function of focal contacts. Curr. Opin. Cell Biol. 13 (5), 584–592. doi:10.1016/s0955-0674(00)00255-6
Geiger, B., Spatz, J. P., and Bershadsky, A. D. (2009). Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10 (1), 21–33. doi:10.1038/nrm2593
Graf, J., Schulz, H., Wehland, M., Corydon, T. J., Sahana, J., Abdelfattah, F., et al. (2024). Omics studies of tumor cells under microgravity conditions. Int. J. Mol. Sci. 25 (2), 926. doi:10.3390/ijms25020926
Green, K. J., and Jones, J. C. R. (1996). Desmosomes and hemidesmosomes: structure and function of molecular components. FASEB J. 10 (8), 871–881. doi:10.1096/fasebj.10.8.8666164
Grimm, D., Wise, P., Lebert, M., Richter, P., and Baatout, S. (2011). How and why does the proteome respond to microgravity? Expert Rev. Proteomics 8 (1), 13–27. doi:10.1586/epr.10.105
Grosse, J., Wehland, M., Pietsch, J., Ma, X., Ulbrich, C., Schulz, H., et al. (2012). Short-term weightlessness produced by parabolic flight maneuvers altered gene expression patterns in human endothelial cells. FASEB J. 26 (2), 639–655. doi:10.1096/fj.11-194886
Gruenbaum, Y., and Foisner, R. (2015). Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu. Rev. Biochem. 84 (84), 131–164. doi:10.1146/annurev-biochem-060614-034115
Gruener, R., Roberts, R., and Reitstetter, R. (1994). Reduced receptor aggragation and altered cytoskeleton in cultured myocytes after space-flight. Biol. Sci. Space 8 (2), 79–93. doi:10.2187/bss.8.79
Guignandon, A., Lafage-Proust, M.-H., Usson, Y., Laroche, N., Caillot-Augusseau, A., Alexandre, C., et al. (2001). Cell cycling determines integrin-mediated adhesion in osteoblastic ROS 17/2.8 cells exposed to space-related conditions. FASEB J. 15 (11), 2036–2038. doi:10.1096/fj.00-0837fje
Guignandon, A., Akhouayri, O., Usson, Y., Rattner, A., Laroche, N., Lafage-Proust, M.-H., et al. (2003a). Focal contact clustering in osteoblastic cells under mechanical stresses: microgravity and cyclic deformation. Cell Commun. & Adhesion 10 (2), 69–83. doi:10.1080/cac.10.2.69.83
Guignandon, A., Akhouayri, O., Laroche, N., Lafage-Proust, M.-H., Alexandre, C., and Vico, L. (2003b). Focal contacts organization in osteoblastic cells under microgravity and cyclic deformation conditions. Adv. Space Res. 32 (8), 1561–1567. doi:10.1016/S0273-1177(03)90396-6
Guo, M., Ehrlicher, A. J., Mahammad, S., Fabich, H., Jensen, M. H., Moore, J. R., et al. (2013). The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys. J. 105 (7), 1562–1568. doi:10.1016/j.bpj.2013.08.037
Guzmán, C., Jeney, S., Kreplak, L., Kasas, S., Kulik, A. J., Aebi, U., et al. (2006). Exploring the mechanical properties of single vimentin intermediate filaments by atomic force microscopy. J. Mol. Biol. 360 (3), 623–630. doi:10.1016/j.jmb.2006.05.030
Hader, D. P., Braun, M., Grimm, D., and Hemmersbach, R. (2017). Gravireceptors in eukaryotes-a comparison of case studies on the cellular level. NPJ Microgravity 3, 13. doi:10.1038/s41526-017-0018-8
Hamill, O. P. (2006). Twenty odd years of stretch-sensitive channels. Pflugers Arch. 453 (3), 333–351. doi:10.1007/s00424-006-0131-0
Harada, T., Swift, J., Irianto, J., Shin, J.-W., Spinler, K. R., Athirasala, A., et al. (2014). Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J. Cell Biol. 204 (5), 669–682. doi:10.1083/jcb.201308029
Harr, J. C., Luperchio, T. R., Wong, X., Cohen, E., Wheelan, S. J., and Reddy, K. L. (2015). Directed targeting of chromatin to the nuclear lamina is mediated by chromatin state and A-type lamins. J. Cell Biol. 208 (1), 33–52. doi:10.1083/jcb.201405110
Hawkins, T., Mirigian, M., Selcuk Yasar, M., and Ross, J. L. (2010). Mechanics of microtubules. J. Biomech. 43 (1), 23–30. doi:10.1016/j.jbiomech.2009.09.005
Hayakawa, K., Tatsumi, H., and Sokabe, M. (2008). Actin stress fibers transmit and focus force to activate mechanosensitive channels. J. Cell Sci. 121 (Pt 4), 496–503. doi:10.1242/jcs.022053
Hayakawa, K., Tatsumi, H., and Sokabe, M. (2011). Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J. Cell Biol. 195 (5), 721–727. doi:10.1083/jcb.201102039
Herranz, R., Anken, R., Boonstra, J., Braun, M., Christianen, P. C., de Geest, M., et al. (2013). Ground-based facilities for simulation of microgravity: organism-specific recommendations for their use, and recommended terminology. Astrobiology 13 (1), 1–17. doi:10.1089/ast.2012.0876
Ho, C. Y., Jaalouk, D. E., Vartiainen, M. K., and Lammerding, J. (2013). Lamin A/C and emerin regulate MKL1–SRF activity by modulating actin dynamics. Nature 497 (7450), 507–511. doi:10.1038/nature12105
Holthöfer, B., Windoffer, R., Troyanovsky, S., and Leube, R. E. (2007). Structure and function of desmosomes. Int. Rev. Cytol. 264, 65–163. doi:10.1016/S0074-7696(07)64003-0
Holy, T. E., and Leibler, S. (1994). Dynamic instability of microtubules as an efficient way to search in space. Proc. Natl. Acad. Sci. 91 (12), 5682–5685. doi:10.1073/pnas.91.12.5682
Huang, H., Kamm, R. D., and Lee, R. T. (2004). Cell mechanics and mechanotransduction: pathways, probes, and physiology. Am. J. Physiol. Cell Physiol. 287 (1), C1–C11. doi:10.1152/ajpcell.00559.2003
Hughes-Fulford, M., and Boonstra, J. (2010). Cell mechanotransduction: Cytoskeleton and related signalling pathways. Cell Mechanochemistry. Biol. Syst. Factors Inducing Mech. Stress, such as Light, Press. Gravity, 75–95.
Hughes-Fulford, M., and Lewis, M. L. (1996). Effects of microgravity on osteoblast growth activation. Exp. Cell Res. 224 (1), 103–109. doi:10.1006/excr.1996.0116
Humphrey, J. D., Dufresne, E. R., and Schwartz, M. A. (2014). Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15 (12), 802–812. doi:10.1038/nrm3896
Ingber, D. E. (1993). Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J. Cell Sci. 104 (Pt 3), 613–627. doi:10.1242/jcs.104.3.613
Ingber, D. (1999). How cells (might) sense microgravity. FASEB J. 13 (Suppl. l), S3–S15. doi:10.1096/fasebj.13.9001.s3
Ingber, D. E. (2003a). Tensegrity I. Cell structure and hierarchical systems biology. J. Cell Sci. 116 (Pt 7), 1157–1173. doi:10.1242/jcs.00359
Ingber, D. E. (2003b). Tensegrity II. How structural networks influence cellular information processing networks. J. Cell Sci. 116 (Pt 8), 1397–1408. doi:10.1242/jcs.00360
Ingber, D. E. (2006). Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20 (7), 811–827. doi:10.1096/fj.05-5424rev
Ingber, D. E. (2008). Tensegrity-based mechanosensing from macro to micro. Prog. Biophysics Mol. Biol. 97 (2), 163–179. doi:10.1016/j.pbiomolbio.2008.02.005
Ishikawa, H., Bischoff, R., and Holtzer, H. (1968). Mitosis and intermediate-sized filaments in developing skeletal muscle. J. Cell Biol. 38 (3), 538–555. doi:10.1083/jcb.38.3.538
Ivorra, C., Kubicek, M., González, J. M., Sanz-González, S. M., Álvarez-Barrientos, A., O'Connor, J.-E., et al. (2006). A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes & Dev. 20 (3), 307–320. doi:10.1101/gad.349506
Jaalouk, D. E., and Lammerding, J. (2009). Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 10 (1), 63–73. doi:10.1038/nrm2597
Jamon, M. (2014). The development of vestibular system and related functions in mammals: impact of gravity. Front. Integr. Neurosci. 8, 11. [Review]. doi:10.3389/fnint.2014.00011
Janmey, P. A., and McCulloch, C. A. (2007). Cell mechanics: integrating cell responses to mechanical stimuli. Annu. Rev. Biomed. Eng. 9, 1–34. doi:10.1146/annurev.bioeng.9.060906.151927
Janmey, P. A., Euteneuer, U., Traub, P., and Schliwa, M. (1991). Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J. Cell Biol. 113 (1), 155–160. doi:10.1083/jcb.113.1.155
Janmey, P. A., Hvidt, S., Käs, J., Lerche, D., Maggs, A., Sackmann, E., et al. (1994). The mechanical properties of actin gels. Elastic modulus and filament motions. J. Biol. Chem. 269 (51), 32503–32513. doi:10.1016/S0021-9258(18)31663-6
Jansen, K. A., Donato, D. M., Balcioglu, H. E., Schmidt, T., Danen, E. H. J., and Koenderink, G. H. (2015). A guide to mechanobiology: where biology and physics meet. Biochimica Biophysica Acta (BBA) - Mol. Cell Res. 1853 (11), 3043–3052. doi:10.1016/j.bbamcr.2015.05.007
Jensen, M. H., Morris, E. J., and Weitz, D. A. (2015). Mechanics and dynamics of reconstituted cytoskeletal systems. Biochimica Biophysica Acta (BBA) - Mol. Cell Res. 1853 (11, Part B), 3038–3042. doi:10.1016/j.bbamcr.2015.06.013
Jongkind, J. F., Visser, P., and Verkerk, A. (1996). Cell fusion in space: plasma membrane fusion in human fibroblasts during short term microgravity. Adv. Space Res. 17 (6), 21–25. doi:10.1016/0273-1177(95)00608-H
Karmali, F., and Shelhamer, M. (2008). The dynamics of parabolic flight: flight characteristics and passenger percepts. Acta Astronaut. 63 (5-6), 594–602. doi:10.1016/j.actaastro.2008.04.009
Kirby, T. J., and Lammerding, J. (2018). Emerging views of the nucleus as a cellular mechanosensor. Nat. Cell Biol. 20 (4), 373–381. doi:10.1038/s41556-018-0038-y
Klaus, D. M., Todd, P., and Schatz, A. (1998). Functional weightlessness during clinorotation of cell suspensions. Adv. Space Res. 21 (8-9), 1315–1318. doi:10.1016/s0273-1177(97)00404-3
Kobayashi, T., and Sokabe, M. (2010). Sensing substrate rigidity by mechanosensitive ion channels with stress fibers and focal adhesions. Curr. Opin. Cell Biol. 22 (5), 669–676. doi:10.1016/j.ceb.2010.08.023
Kreplak, L., Bär, H., Leterrier, J. F., Herrmann, H., and Aebi, U. (2005). Exploring the mechanical behavior of single intermediate filaments. J. Mol. Biol. 354 (3), 569–577. doi:10.1016/j.jmb.2005.09.092
Kumar, S., Maxwell, I. Z., Heisterkamp, A., Polte, T. R., Lele, T. P., Salanga, M., et al. (2006). Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 90 (10), 3762–3773. doi:10.1529/biophysj.105.071506
Lecar, H., and Morris, C. E. (1993). Biophysics of mechanotransduction. Mechanoreception by Vasc. Wall, 1–11.
Lee, J. S. H., Hale, C. M., Panorchan, P., Khatau, S. B., George, J. P., Tseng, Y., et al. (2007). Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys. J. 93 (7), 2542–2552. doi:10.1529/biophysj.106.102426
Lewis, M. L. (2004). The cytoskeleton in spaceflown cells: an overview. Gravitational Space Biol. 17, 1+. Available online at: https://link.gale.com/apps/doc/A176373076/AONE?u=anon∼1dce490a&sid=googleScholar&xid=da27caec.
Lewis, M. L., Reynolds, J. L., Cubano, L. A., Hatton, J. P., Lawless, B. D., and Piepmeier, E. H. (1998). Spaceflight alters microtubules and increases apoptosis in human lymphocytes (Jurkat). FASEB J. 12 (11), 1007–1018. doi:10.1096/fasebj.12.11.1007
Li, N., Wang, C., Sun, S., Zhang, C., Lü, D., Chen, Q., et al. (2018). Microgravity-induced alterations of inflammation-related mechanotransduction in endothelial cells on board SJ-10 satellite. Front. Physiol. 9, 1025. doi:10.3389/fphys.2018.01025
Lin, Y.-C., Koenderink, G. H., MacKintosh, F. C., and Weitz, D. A. (2007). Viscoelastic properties of microtubule networks. Macromolecules 40 (21), 7714–7720. doi:10.1021/ma070862l
Lv, H., Yang, H., Jiang, C., Shi, J., Chen, R. A., Huang, Q., et al. (2023). Microgravity and immune cells. J. R. Soc. Interface 20 (199), 20220869. doi:10.1098/rsif.2022.0869
McGough, A., Pope, B., Chiu, W., and Weeds, A. (1997). Cofilin changes the twist of F-Actin: implications for actin filament dynamics and cellular function. J. Cell Biol. 138 (4), 771–781. doi:10.1083/jcb.138.4.771
Meloni, M. A., Galleri, G., Pani, G., Saba, A., Pippia, P., and Cogoli-Greuter, M. (2011). Space flight affects motility and cytoskeletal structures in human monocyte cell line J-111. Cytoskelet. Hob. 68 (2), 125–137. doi:10.1002/cm.20499
Mitchison, T., and Kirschner, M. (1984). Dynamic instability of microtubule growth. Nature 312 (5991), 237–242. doi:10.1038/312237a0
Mogre, S. S., Brown, A. I., and Koslover, E. F. (2020). Getting around the cell: physical transport in the intracellular world. Phys. Biol. 17 (6), 061003. doi:10.1088/1478-3975/aba5e5
Moos, P., Graff, K., Edwards, M., Stodieck, L., Einhorn, R., and Luttges, M. (1988). Gravity-induced changes in microtubule formation. ASGSB Bull. 2, 55.
Nabavi, N., Khandani, A., Camirand, A., and Harrison, R. E. (2011). Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone 49 (5), 965–974. doi:10.1016/j.bone.2011.07.036
Najrana, T., and Sanchez-Esteban, J. (2016). Mechanotransduction as an adaptation to gravity. Front. Pediatr. 4, 140. doi:10.3389/fped.2016.00140
NASA (2010). Reference guide to the international space station. -- assembly complete edition. Washington, DC, USA: National Aeronautics and Space Administration NASA.
Nassef, M. Z., Kopp, S., Wehland, M., Melnik, D., Sahana, J., Kruger, M., et al. (2019). Real microgravity influences the cytoskeleton and focal adhesions in human breast cancer cells. Int. J. Mol. Sci. 20 (13), 3156. doi:10.3390/ijms20133156
Nickerson, C., Pellis, N. R., and Ott, C. M. (2015). Effect of spaceflight and spaceflight analogue culture on human and microbial cells. Springer.
Niessen, C. M., and Gottardi, C. J. (2008). Molecular components of the adherens junction. Biochimica Biophysica Acta (BBA) - Biomembr. 1778 (3), 562–571. doi:10.1016/j.bbamem.2007.12.015
Ogneva, I. V. (2013). Cell mechanosensitivity: mechanical properties and interaction with gravitational field. Biomed. Res. Int. 2013, 598461–17. doi:10.1155/2013/598461
Ohashi, K., Fujiwara, S., and Mizuno, K. (2017). Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J. Biochem. 161 (3), 245–254. doi:10.1093/jb/mvw082
Osmanagic-Myers, S., Dechat, T., and Foisner, R. (2015). Lamins at the crossroads of mechanosignaling. Genes & Dev. 29 (3), 225–237. doi:10.1101/gad.255968.114
Papaseit, C., Pochon, N., and Tabony, J. (2000). Microtubule self-organization is gravity-dependent. Proc. Natl. Acad. Sci. 97 (15), 8364–8368. doi:10.1073/pnas.140029597
Paulsen, K., Tauber, S., Goelz, N., Simmet, D. M., Engeli, S., Birlem, M., et al. (2014). Severe disruption of the cytoskeleton and immunologically relevant surface molecules in a human macrophageal cell line in microgravity—Results of an in vitro experiment on board of the Shenzhou-8 space mission. Acta Astronaut. 94 (1), 277–292. doi:10.1016/j.actaastro.2013.06.007
Piepmeier, E. H., Kalns, J. E., McIntyre, K. M., and Lewis, M. L. (1997). Prolonged weightlessness affects promyelocytic multidrug resistance. Exp. Cell Res. 237 (2), 410–418. doi:10.1006/excr.1997.3813
Pletser, V. (2016). European aircraft parabolic flights for microgravity research, applications and exploration: a review. REACH, 1, 11–19. doi:10.1016/j.reach.2016.05.002
Pletser, V., Rouquette, S., Friedrich, U., Clervoy, J.-F., Gharib, T., Gai, F., et al. (2016). The first European parabolic flight campaign with the airbus A310 ZERO-G. Microgravity Sci. Technol. 28 (6), 587–601. doi:10.1007/s12217-016-9515-8
Pollard, T. D. (2016). Actin and actin-binding proteins. Cold Spring Harb. Perspect. Biol. 8 (8), a018226. doi:10.1101/cshperspect.a018226
Qian, A. R., Yin, D. C., Yang, P. F., Lv, Y., Tian, Z. C., and Shang, P. (2013). Application of diamagnetic levitation technology in biological sciences research. IEEE Trans. Appl. Supercond. 23 (1), 3600305. doi:10.1109/TASC.2012.2232919
Qin, Z., Buehler, M. J., and Kreplak, L. (2010). A multi-scale approach to understand the mechanobiology of intermediate filaments. J. Biomech. 43 (1), 15–22. doi:10.1016/j.jbiomech.2009.09.004
Rai, A., Robinson, J. A., Tate-Brown, J., Buckley, N., Zell, M., Tasaki, K., et al. (2016). Expanded benefits for humanity from the international space station. Acta Astronaut. 126, 463–474. doi:10.1016/j.actaastro.2016.06.030
Rajagopal, V., Holmes, W. R., and Lee, P. V. S. (2018). Computational modeling of single-cell mechanics and cytoskeletal mechanobiology. Wiley Interdiscip. Rev. Syst. Biol. Med. 10 (2), e1407. doi:10.1002/wsbm.1407
Reddy, A. S., Warshaviak, D. T., and Chachisvilis, M. (2012). Effect of membrane tension on the physical properties of DOPC lipid bilayer membrane. Biochim. Biophys. Acta 1818 (9), 2271–2281. doi:10.1016/j.bbamem.2012.05.006
Rijken, P. J., de Groot, R. P., Kruijer, W., de Laat, S. W., Verkleij, A. J., and Boonstra, J. (1992). Identification of specific gravity sensitive signal transduction pathways in human A431 carcinoma cells. Adv. Space Res. 12 (1), 145–152. doi:10.1016/0273-1177(92)90277-5
Rösner, H., Wassermann, T., Möller, W., and Hanke, W. (2006). Effects of altered gravity on the actin and microtubule cytoskeleton of human SH-SY5Y neuroblastoma cells. Protoplasma 229 (2), 225–234. doi:10.1007/s00709-006-0202-2
Rudimov, E. G., and Buravkova, L. B. (2016). Endothelial gravisensitivity: the role of cytoskeleton and adhesion molecules. Fiziol. Cheloveka 42 (6), 116–123. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/29932534.
Sachs, F. (2010). Stretch-activated ion channels: what are they? Physiol. (Bethesda) 25 (1), 50–56. doi:10.1152/physiol.00042.2009
Sachs, F., and Morris, C. E. (1998). Mechanosensitive ion channels in nonspecialized cells. Rev. Physiol. Biochem. Pharmacol. 132, 1–77. doi:10.1007/bfb0004985
Schatten, H. (2015). “Brief overview of the cytoskeleton,” in The cytoskeleton in health and disease. Editor H. Schatten (New York: Springer), 3–7. doi:10.1007/978-1-4939-2904-7_1
Schatten, H., Chakrabarti, A., Taylor, M., Sommer, L., Levine, H., Anderson, K., et al. (1999). Effects of spaceflight conditions on fertilization and embryogenesis in the sea urchin Lytechinus pictus. Cell Biol. Int. 23 (6), 407–415. doi:10.1006/cbir.1999.0371
Schopferer, M., Bär, H., Hochstein, B., Sharma, S., Mücke, N., Herrmann, H., et al. (2009). Desmin and vimentin intermediate filament networks: their viscoelastic properties investigated by mechanical rheometry. J. Mol. Biol. 388 (1), 133–143. doi:10.1016/j.jmb.2009.03.005
Schulz, H., Strauch, S. M., Richter, P., Wehland, M., Krüger, M., Sahana, J., et al. (2022). Latest knowledge about changes in the proteome in microgravity. Expert Rev. Proteomics 19 (1), 43–59. doi:10.1080/14789450.2022.2030711
Sciola, L., Cogoli-Greuter, M., Cogoli, A., Spano, A., and Pippia, P. (1999). Influence of microgravity on mitogen binding and cytoskeleton in jurkat cells. Adv. Space Res. 24 (6), 801–805. doi:10.1016/S0273-1177(99)00078-2
Seibert, G. (2006). in The history of sounding rockets and their contribution to European space research. Editor B. Battrick (2200AG Noordwijk, Netherlands: ESA Publications Division). ESTEC, PO Box 299.
Solovei, I., Wang, A. S., Thanisch, K., Schmidt, C. S., Krebs, S., Zwerger, M., et al. (2013). LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 152 (3), 584–598. doi:10.1016/j.cell.2013.01.009
Sosa, B. A., Kutay, U., and Schwartz, T. U. (2013). Structural insights into LINC complexes. Curr. Opin. Struct. Biol. 23 (2), 285–291. doi:10.1016/j.sbi.2013.03.005
Storm, C., Pastore, J. J., MacKintosh, F. C., Lubensky, T. C., and Janmey, P. A. (2005). Nonlinear elasticity in biological gels. Nature 435 (7039), 191–194. doi:10.1038/nature03521
Strauch, S. M., Grimm, D., Corydon, T. J., Krüger, M., Bauer, J., Lebert, M., et al. (2019). Current knowledge about the impact of microgravity on the proteome. Expert Rev. Proteomics 16 (1), 5–16. doi:10.1080/14789450.2019.1550362
Szeverenyi, I., Cassidy, A. J., Chung, C. W., Lee, B. T., Common, J. E., Ogg, S. C., et al. (2008). The human intermediate filament database: comprehensive information on a gene family involved in many human diseases. Hum. Mutat. 29 (3), 351–360. doi:10.1002/humu.20652
Tauber, S., Lauber, B. A., Paulsen, K., Layer, L. E., Lehmann, M., Hauschild, S., et al. (2017). Cytoskeletal stability and metabolic alterations in primary human macrophages in long-term microgravity. PLoS One 12 (4), e0175599. doi:10.1371/journal.pone.0175599
Tojkander, S., Gateva, G., and Lappalainen, P. (2012). Actin stress fibers – assembly, dynamics and biological roles. J. Cell Sci. 125 (8), 1855–1864. doi:10.1242/jcs.098087
Uhler, C., and Shivashankar, G. V. (2017). Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat. Rev. Mol. Cell Biol. 18 (12), 717–727. doi:10.1038/nrm.2017.101
Ulbrich, C., Pietsch, J., Grosse, J., Wehland, M., Schulz, H., Saar, K., et al. (2011). Differential gene regulation under altered gravity conditions in follicular thyroid cancer cells: relationship between the extracellular matrix and the cytoskeleton. Cell Physiol. Biochem. 28 (2), 185–198. doi:10.1159/000331730
Vale, R. D., and Milligan, R. A. (2000). The way things move: looking under the hood of molecular motor proteins. Science 288 (5463), 88–95. doi:10.1126/science.288.5463.88
van Loon, J. J. W. A. v. (2007). Micro-gravity and mechanomics. Gravitational Space Biol. 20 (2). [Review].
van Loon, J. J. W. A. (2009). Mechanomics and physicomics in gravisensing. Microgravity Sci. Technol. 21 (1), 159–167. doi:10.1007/s12217-008-9065-9
Vassy, J., Portet, S., Beil, M., Millot, G., Fauvel-Lafeve, F., Karniguian, A., et al. (2001). Effect of weightlessness on cytoskeleton architecture and proliferation of human breast cancer cell line MCF-7. FASEB J. 15 (6), 1104–1106. doi:10.1096/fsb2fj000527fje
Vassy, J., Portet, S., Beil, M., Millot, G., Fauvel-Lafève, F., Gasset, G., et al. (2003). Weightlessness acts on human breast cancer cell line MCF-7. Adv. Space Res. 32 (8), 1595–1603. doi:10.1016/S0273-1177(03)90400-5
Vinogradova, O. L., Tomilovskaya, E. S., and Kozlovskaya, I. B. (2021). Gravity as a factor in evolutionary adaptation of animals to living on the Earth. Hum. Physiol. 47 (7), 716–734. doi:10.1134/S0362119721070124
Vogel, V. (2006). Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu. Rev. Biophys. Biomol. Struct. 35, 459–488. doi:10.1146/annurev.biophys.35.040405.102013
Vogel, V., and Sheetz, M. P. (2009). Cell fate regulation by coupling mechanical cycles to biochemical signaling pathways. Curr. Opin. Cell Biol. 21 (1), 38–46. doi:10.1016/j.ceb.2009.01.002
Volkmann, D., and Baluska, F. (2006). Gravity: one of the driving forces for evolution. Protoplasma 229 (2-4), 143–148. doi:10.1007/s00709-006-0200-4
Vorselen, D., Roos, W. H., MacKintosh, F. C., Wuite, G. J., and van Loon, J. J. (2014). The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J. 28 (2), 536–547. doi:10.1096/fj.13-236356
Walko, G., Castañón, M. J., and Wiche, G. (2015). Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 360 (3), 529–544. doi:10.1007/s00441-015-2216-6
Wang, N. (2017). Review of cellular mechanotransduction. J. Phys. D. Appl. Phys. 50 (23), 233002. doi:10.1088/1361-6463/aa6e18
Wang, J. H., and Thampatty, B. P. (2006). An introductory review of cell mechanobiology. Biomech. Model Mechanobiol. 5 (1), 1–16. doi:10.1007/s10237-005-0012-z
Wang, N., Tytell, J. D., and Ingber, D. E. (2009). Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10 (1), 75–82. doi:10.1038/nrm2594
Wang, J., Lü, D., Mao, D., and Long, M. (2014). Mechanomics: an emerging field between biology and biomechanics. Protein & Cell 5, 518–531. doi:10.1007/s13238-014-0057-9
Wehrle-Haller, B. (2012). Structure and function of focal adhesions. Curr. Opin. Cell Biol. 24 (1), 116–124. doi:10.1016/j.ceb.2011.11.001
Wiche, G. (1998). Role of plectin in cytoskeleton organization and dynamics. J. Cell Sci. 111 (17), 2477–2486. doi:10.1242/jcs.111.17.2477
Williams, D., Kuipers, A., Mukai, C., and Thirsk, R. (2009). Acclimation during space flight: effects on human physiology. CMAJ 180 (13), 1317–1323. doi:10.1503/cmaj.090628
Wipff, P. J., Rifkin, D. B., Meister, J. J., and Hinz, B. (2007). Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol. 179 (6), 1311–1323. doi:10.1083/jcb.200704042
Wu, X. T., Yang, X., Tian, R., Li, Y. H., Wang, C. Y., Fan, Y. B., et al. (2022). Cells respond to space microgravity through cytoskeleton reorganization. FASEB J. 36 (2), e22114. doi:10.1096/fj.202101140R
Wuest, S. L., Richard, S., Kopp, S., Grimm, D., and Egli, M. (2015). Simulated microgravity: critical review on the use of random positioning machines for mammalian cell culture. Biomed. Res. Int. 2015, 1–8. Article 971474. doi:10.1155/2015/971474
Wuest, S. L., Gantenbein, B., Ille, F., and Egli, M. (2018). Electrophysiological experiments in microgravity: lessons learned and future challenges. NPJ Microgravity 4, 7. doi:10.1038/s41526-018-0042-3
Wuest, S. L., Arnold, J., Gander, S., Zumbühl, C., Jost, C., Giger-Lange, C., et al. (2020). Microtubules and vimentin fiber stability during parabolic flights. Microgravity Sci. Technol. 32 (5), 921–933. doi:10.1007/s12217-020-09818-8
Xu, J., Wirtz, D., and Pollard, T. D. (1998). Dynamic cross-linking by α-Actinin determines the mechanical properties of actin filament networks *. J. Biol. Chem. 273 (16), 9570–9576. doi:10.1074/jbc.273.16.9570
Xu, J., Tseng, Y., and Wirtz, D. (2000). Strain hardening of actin filament networks. J. Biol. Chem. 275 (46), 35886–35892. doi:10.1074/jbc.M002377200
Yang, F., Li, Y., Ding, B., Nie, J., Wang, H., Zhang, X., et al. (2008). Reduced function and disassembled microtubules of cultured cardiomyocytes in spaceflight. Chin. Sci. Bull. 53 (8), 1185–1192. doi:10.1007/s11434-008-0167-y
Yang, F., Dai, Z., Tan, Y., and Li, Y. (2010). Effects of altered gravity on the cytoskeleton of neonatal rat cardiocytes. Microgravity Sci. Technol. 22 (1), 45–52. [journal article]. doi:10.1007/s12217-008-9103-7
Keywords: cytoskeleton, actin, tubulin, intermediate filament, microgravity, space flight
Citation: Wuest SL (2025) Cytoskeleton changes of mammalian cells in microgravity: results from three decades of low-gravity research. Front. Space Technol. 6:1677728. doi: 10.3389/frspt.2025.1677728
Received: 01 August 2025; Accepted: 10 September 2025;
Published: 25 September 2025.
Edited by:
Timothy Grant Hammond, Duke University, United StatesReviewed by:
Rodolfo Negri, Sapienza University of Rome, ItalyNu Zhang, Northwestern Polytechnical University, China
Daan Van Den Nieuwenhof, Radboud University Medical Centre, Netherlands
Copyright © 2025 Wuest. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simon L. Wuest, c2ltb24ud3VlZXN0QGhzbHUuY2g=
†ORCID: Simon L. Wuest, orcid.org/0000-0002-5495-6951
 Simon L. Wuest
Simon L. Wuest