- 1Department of Microbiology, Immunology and Pathology, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO, United States
- 2Department of Pathobiology, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 3Antech Diagnostics, Fountain Valley, CA, United States
Camelid pathology submissions to veterinary diagnostic laboratories are on the rise given the increasing popularity and population of llamas and alpacas especially in the western United States. When compared to other animals, the field of camelid neoplasia has a relative paucity of cases reported in the literature. The Colorado State University Veterinary Diagnostic Laboratories (CSU-VDL) has had a steady increase in the numbers of camelid pathology submissions allowing for a robust review of diagnoses of neoplasia in new world camelids. Here we present a retrospective analysis of camelid neoplastic and proliferative lesions diagnosed at the CSU-VDL from 1995 to 2020, followed by an extensive literature review. Results show increasing incidence of camelid neoplasia reported in the literature, therefore becoming a common diagnosis in llamas and alpacas. Proliferative and neoplastic lesions were diagnosed in 8.8% of new world camelid submissions to CSU-VDL with the most common tumors being lymphomas, squamous cell carcinomas, fibromas, and adenocarcinomas. Risk factors are female sex and increased age except in the case of lymphoma, which tends to occur in younger camelids. Lymphomas, melanomas, and adenocarcinomas (especially of gastrointestinal tract) carry an increased risk of multiple-organ system involvement often with widespread metastases. Conditions described in camelids for the first time include osteosarcoma, cutaneous hemangiosarcoma, myxosarcoma, pilomatricoma, ovarian theca cell tumor, congenital nevus with malignant transformation, and various other neoplasia. This article will provide an operational guide for camelid neoplasia to further assist veterinary laboratory diagnosticians, researchers, and practicing veterinarians in the field of camelid medicine and pathology.
Introduction
The first report of neoplasia in new world camelids, published in 1974, described gastric squamous cell carcinoma in a guanaco (1). Since then, there has been increasing interest in neoplasia of llamas and alpacas especially as these animals have had increased popularity for their fiber and as companion and pack animals for integrated sheep protection. The majority of the earlier reports have been individual cases or brief communications with only very rare publications looking at overall prevalence of neoplasia in new world camelids (2, 3). Prevalence studies indicate that while common, neoplasia is not a primary cause of death and that llamas might have a slightly higher incidence of neoplasia than alpacas, although alpacas appear to have predisposition for developing neoplasia at a relatively younger age. Publications of neoplasia in camelids often report disseminated or metastatic disease at the time of diagnosis, making early diagnosis especially important in these animals.
Sample size in most of the reports in new world camelid literature is limited with only 6% of publications (5 of 83 publications) on neoplasia in camelids reporting numbers greater than five animals. Case reports are rather common and often report bizarre or highly aggressive neoplastic behavior discovered at the time of necropsy, leaving a gap in the current knowledge as to the outcome of these tumors. Pathologists, clinicians, and owners are often challenged with prognostication relating to cutaneous or visceral neoplasia that have been underreported or previously unreported. A guide to differentiate benign from malignant tumors is of crucial importance in those cases. A systematic review of neoplasia, to the authors' knowledge, has yet to be published on neoplasia in llamas and alpacas.
The current article presents a systematic review of neoplasia and proliferative diseases in new world camelids including cases from both the published literature and 201 case submissions to the Veterinary Diagnostic Laboratory at Colorado State University (CSU-VDL) from 1995 to 2020. The case series published here is the largest summary data ever reported on this subject and almost doubles the number of the known cases of neoplasia reported in South American camelids. This review will serve to broaden the knowledge base on camelid neoplasia and will highlight the most common neoplastic diseases and likelihood of malignancy and will also identify novel neoplastic/proliferative lesions in new world camelids.
Materials and Methods
Identification of Case Submissions
Query of camelid (alpaca, llama, or unspecified camelid) necropsy and biopsy cases submitted to the CSU-VDL from 1995 to 2020 identified 2,166 submissions. Review of these submissions identified 201 diagnoses (from 192 animals) of neoplasia or proliferative lesions. Results are summarized in Table 1. For retrospective evaluation of the types and frequencies of different camelid neoplasia, biopsy, and necropsy reports were reviewed, and in cases of previously undescribed proliferative or neoplastic disease the hematoxylin and eosin-stained (H&E) slides archived at CSU-VDL were pulled and reviewed to ensure accuracy of the diagnosis. In some of these cases, slides were reviewed by more than one pathologist.
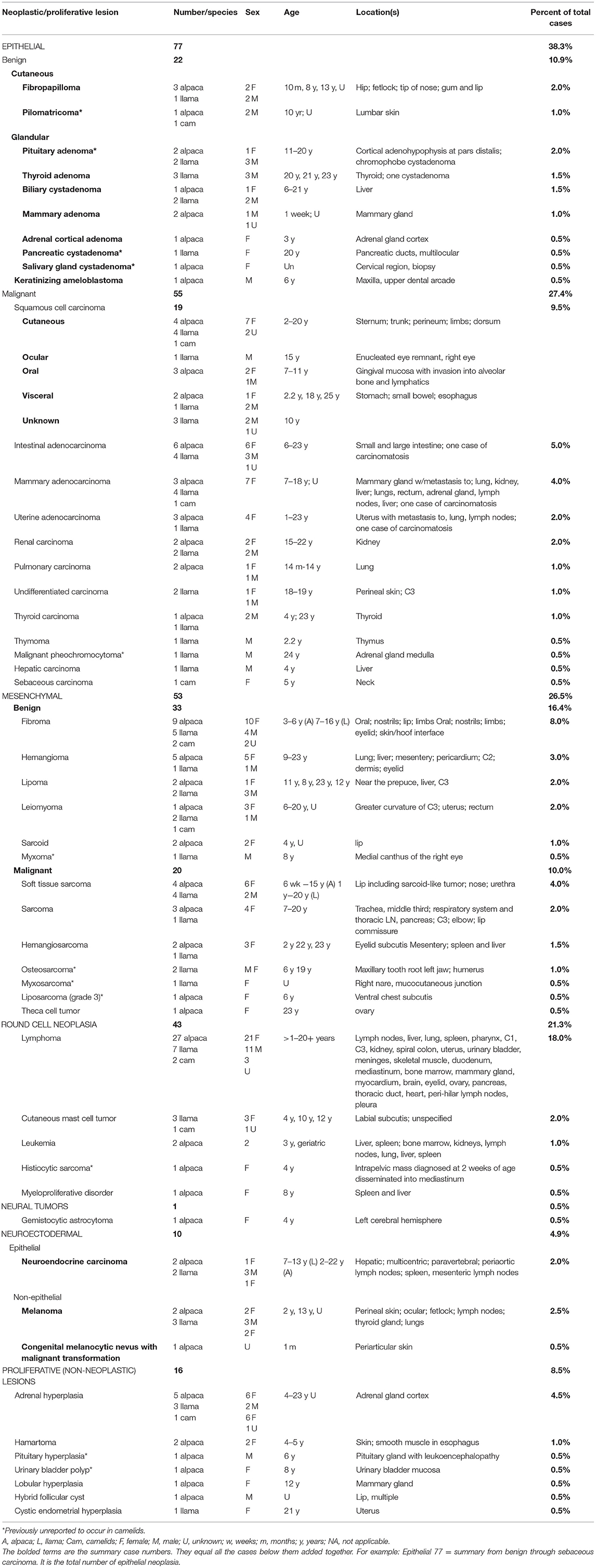
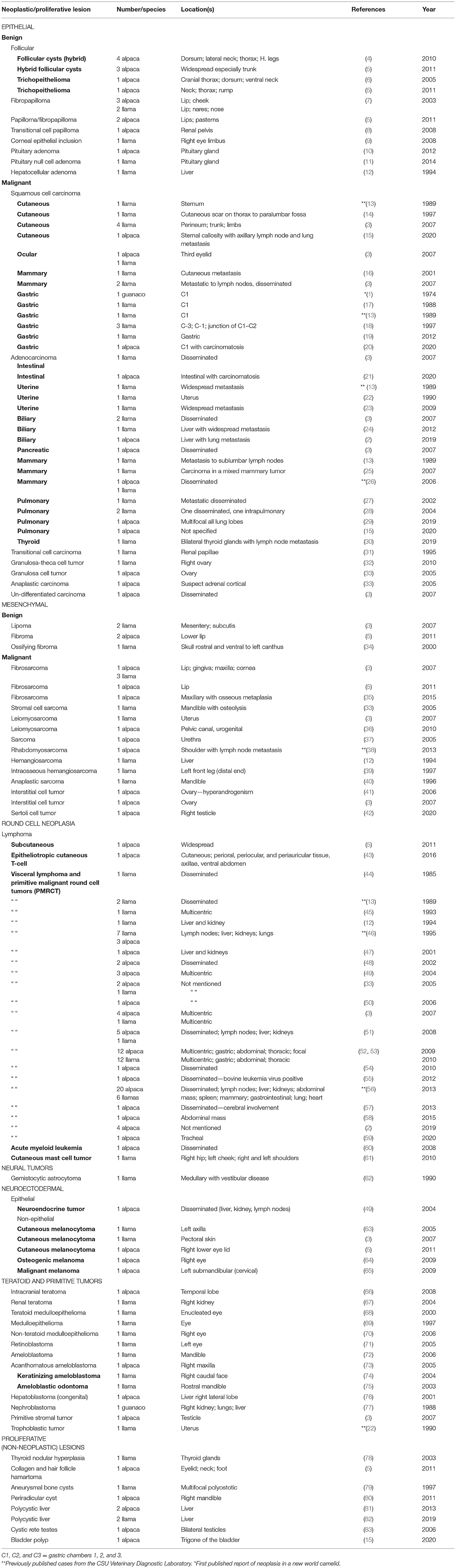
Table 1. Summary of 201 camelid neoplastic and proliferative lesions diagnosed at CSU-VDL (1995–2020).
Statistical Analysis
To assess if a specific diagnosis occurred more frequently in llamas vs. alpacas for CSU-VDL cases, a two-sided Fisher's exact t-test was conducted to compare categories of neoplastic lesions. A p < 0.05 was considered significant. If the t-test was significant, an odds ratio was calculated to compare the likelihood of neoplastic/proliferative lesions in alpacas compared to llamas.
Literature Review
For identification of literature reporting neoplasia in new world camelids, database search was performed, and 86 reports published between 1974 and 2020 were identified. These reports described proliferative and/or neoplastic lesion/s in llamas, alpacas, and/or guanaco. These reports are summarized in Table 2.
Results
Neoplasia Overview
CSU-VDL Cases
Two hundred and one diagnoses of proliferative lesions or neoplasia from 192 animals were identified from 2,166 total submissions to the CSU-VDL from 1995 to 2020 representing 8.8% of all camelid cases submitted for biopsy or necropsy. Sixteen cases were classified as proliferative (<1% of all submitted cases) and 185 cases diagnosed as benign or malignant neoplasia (8.5% of all submitted cases). Cases are summarized in Table 1. Of these cases, 57% were from alpacas and 36% were from llamas with the remaining cases only identified as camelid. Of the proliferative or neoplastic lesions, 59% occurred in females, 34% in males, and the remaining cases have an unreported sex. The percentage of lesions in animals <1 year in age was 3.5%, 1–4 years of age was 16%, 5–9 years of age was 21%, 10–14 years old was 14.5%, and >15 years of age was 25% (age was unknown in 18% of cases). The most common tumors diagnosed per age range are shown in Figure 1. Mesenchymal tumors were found to be more likely than epithelial tumors to be malignant (p = 0.0174).
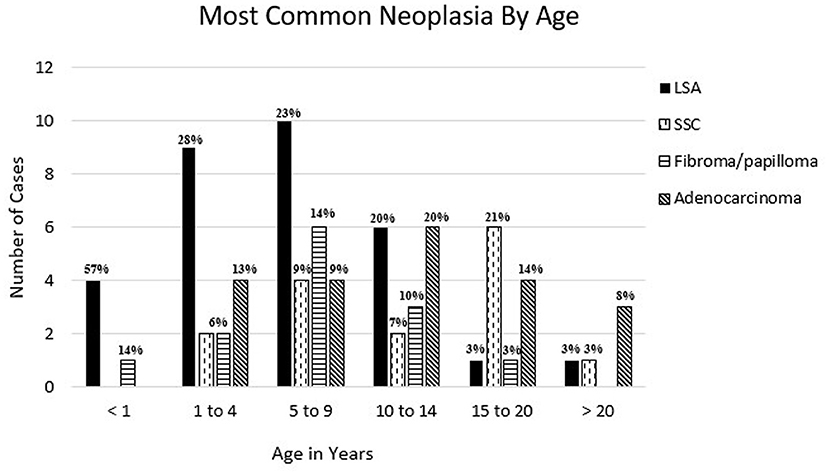
Figure 1. Most common reported neoplasia in llamas and alpacas by age at CSU-VDL from 1995 to 2020. Percentage of total cases per age is reported above bar. LSA, lymphoma; SCC, squamous cell carcinoma; adenocarcinoma = mammary, gastrointestinal, uterine, pulmonary, thyroid, and unspecified.
Literature Cases
Two-hundred and eighteen proliferative or neoplastic lesions have been reported in the literature since the first report of squamous cell carcinoma in a guanaco in 1974 (1). Of these cases, 54% are reported in alpacas, 45% in llamas, and 1% in guanaco. There has been one previous summary report of causes of mortality in 100 alpacas which reported 5% rate of neoplasia (four cases of lymphoma and one case of cholangiocellular carcinoma) (2). There is one review of 40 cases of neoplasia in alpacas and llamas that found a rate of 4.9% of neoplasia in alpacas and 11% of neoplasia in llamas (3).
Round Cell Neoplasia
Lymphoma
CSU-VDL Cases
From 1995 to 2020 CSU-VDL, a total of 36 cases were diagnosed as lymphoma or lymphoproliferative disease, more than any other single type of neoplasia. These cases represented 18% of all neoplastic and proliferative diseases diagnosed. Alpacas were significantly overrepresented with 27 cases vs. 7 in llamas (2 cases only identified as camelid) (p = 0.0192). The odds ratio of lymphoma compared to all other neoplastic/proliferative lesions in alpacas vs. llamas is 2.904 (95% CI: 1.213–7.555). Lymphoma was overrepresented in 21 (75%) females vs. 11 (31%) males with 3 cases being of unreported sex. Additionally, lymphoma was also the most common neoplasia to be diagnosed in animals under the age of 10 with it representing 57, 28, and 23% of diagnoses in animals <1, 1–4, and 5–9, respectively (Figure 1).
Literature Cases
Literature review identified 109 cases of lymphoma reported in llamas and alpacas with most of these cases occurring in alpacas (74/109; 68%). The report by Valentine et al. reports only 12.5% of all neoplastic cases being lymphoma (5 out of 40 cases), and it was the third most common tumor following fibromas/fibropapillomas/fibrosarcomas and squamous cell carcinomas (3). In the published cases, lymphoma generally presented widely disseminated with rare reports of cutaneous and solitary masses (5, 43, 59). Phenotyping by immunohistochemistry in 63 of these cases identified 24 B cell (38%), 28 T cell (44%), 1 mixed T and B cell, and 13 (20.6%) as non-B, non-T cell lymphoma (43, 48–52, 54–59).
Other
CSU-VDL Cases
Other round cell tumors were diagnosed much less frequently in CSU-VDL submissions. There were four mast cell tumors identified through the CSU-VDL submissions representing 2.0% of the total proliferative and neoplastic lesions. Metastasis was not found for any of these tumors. Additionally, there was a single diagnosis of disseminated histiocytic sarcoma (previously unreported in the literature).
Literature Cases
In the literature, there is only one report of multiple mast cell tumors in a llama and metastasis was not reported (61).
Epithelial Neoplasia
CSU-VDL Case Summary
Epithelial neoplasia represented 38.3% of cases with an average age of 13.4 years. There was approximately the same number of epithelial neoplasia diagnosed in llamas vs. alpacas (35 vs. 38 cases, respectively), and females were slightly overrepresented with 48% of all diagnoses vs. 42% males (in the remainder of cases, the sex was not reported).
Follicular and Benign Tumors
CSU-VDL Cases
Follicular cysts and tumors were only rarely diagnosed from CSU-VDL submissions. These lesions include follicular cysts and pilomatricoma. Both cases occurred in alpacas. This is the first report of a pilomatricoma in a new world camelid.
There were several proliferative lesions and cases of benign epithelial neoplasia diagnosed. The most common is adrenal cortical hyperplasia (nine cases) followed by fibropapillomas/papillomas (four cases) and pituitary adenomas (four cases) (Figure 2A) with one case of pituitary hyperplasia.
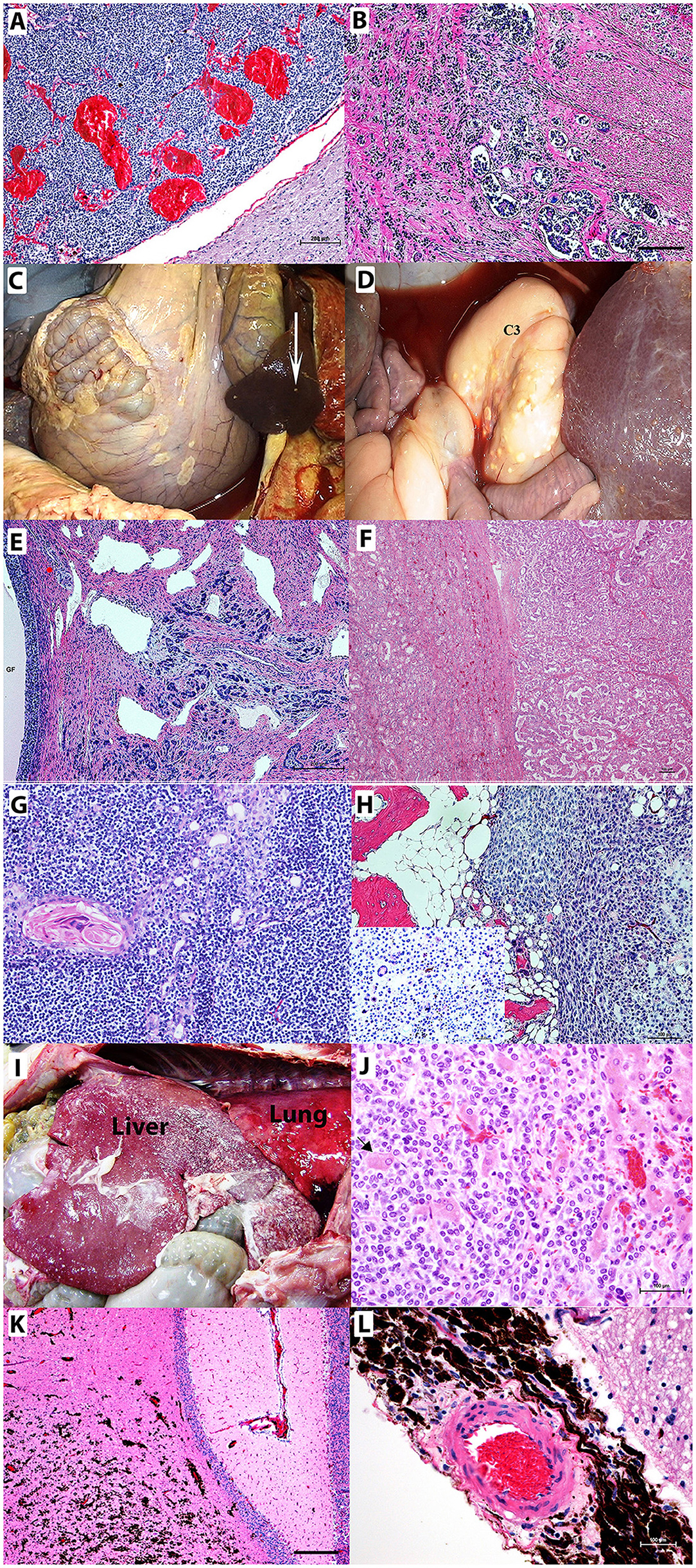
Figure 2. Neoplastic lesions in camelids. (A) Pituitary adenoma. Non-functional chromophobe pituitary adenoma replacing a large part of pars distalis composed of homogenous populations of small round basophilic cells in the midst of congested vascular sinuses. Bar = 200 μm (B) C3 adenocarcinoma with epithelial cells exhibiting invasive growth into the submucosa and muscularis with lymphatic tumor emboli. Bar = 200 μm (C,D) Abdominal carcinomatosis from C3 adenocarcinoma involving mesentery, intestines, spleen (arrow), liver, and third gastric chamber (C3) appears as plaques or nodules on surfaces of involved organs. (E) Uterine adenocarcinoma with carcinomatosis spreading to the ovary containing large graafian follicle (GF) Bar = 200 μm. (F) Renal carcinoma exhibiting a tubular pattern. (G) Lymphocyte-rich thymoma in a llama showing concentric clusters of epithelial cells with abundant eosinophilic cytoplasm, Hassall's corpuscles, and predominantly surrounding small lymphocytes. (H) Humeral osteosarcoma and giant cell osteosarcoma. Neoplastic cells surround a scant amount of eosinophilic osteoid. Inlay shows multinucleated giant cells. (I,J) Neuroendocrine carcinoma with invasive growth throughout the diaphragmatic surface of the liver. The liver also has multifocal accumulations of fibrin. (I) Multifocal hepatocytes are entrapped [(J) arrow] by the neoplastic cells. (K,L) Infiltrating the cerebellar neurophil (K) and surrounding the meningeal artery (L) are metastatic melanocytes in a newborn alpaca with a congenital nevus that has undergone malignant transformation. Hematoxylin and eosin staining. Bars = 100 μm unless otherwise noted.
Literature Cases
Follicular cysts and tumors are reported more commonly in the literature than were diagnosed in the CSU-VDL submissions and occurred more often in alpacas (nine cases) than llamas (zero cases) (4–6). None of the follicular lesions showed any indication of malignancy or aggressive behavior.
There are two reports of pituitary adenomas with one case causing brain compression and neurologic signs (10, 11). Fibropapillomas have been identified as one of the most commonly reported lesions in new world camelids (3). (In one report, these tumors were combined with fibroma, making determination of exact numbers of fibropapillomas difficult to determine.) These lesions generally presented on the face with occasional development on the distal limbs.
Squamous Cell Carcinoma
CSU-VDL Cases
There were 19 (9.5% of all neoplastic and proliferative lesions) diagnoses of squamous cell carcinoma from CSU-VDL submissions, making them the second most common neoplasia behind lymphoma. The most common locations were cutaneous (nine cases) and oral (three cases) with single cases reported in the eye, esophagus, gastric compartment, and small intestine. Only two cases were associated with metastasis (22.2%).
Literature Cases
Squamous cell carcinoma was the first reported neoplasia in new world camelids, and it has been reported as one of the most common tumors to affect these animals (1, 3). The most common sites of occurrence were the gastric chamber one followed by skin, mammary gland, and third eyelid. Approximately a third of the squamous cell carcinomas were reported to have metastasized to regional lymph nodes and/or distant sites with one report of extensive carcinomatosis within the abdominal cavity (20).
Adenocarcinoma
CSU-VDL Cases
Adenocarcinomas were a commonly diagnosed neoplasia in both llamas and alpacas in the CSU-VDL submissions (22 cases, 11% of all neoplastic or proliferative lesions). The majority of these cases were associated with the gastrointestinal tract (10/22 cases, 45.5%), and all had metastasis at the time of diagnosis to the local lymph nodes, liver, spleen, and/or the lungs. There was one case of carcinomatosis associated with a gastrointestinal adenocarcinoma (Figures 2B–D). Female camelids were more likely to be affected by adenocarcinomas due to a high number of mammary (seven cases, 31.8%) and uterine tumors (four cases, 18.2%). Two of the six mammary carcinomas were found to have metastasized at the time of diagnosis along with two of the three uterine adenocarcinomas including one case of carcinomatosis to the ovaries and peritoneal cavity (Figure 2E).
Literature Cases
In the literature, adenocarcinomas were associated with an aggressive disease outcome as 18/20 adenocarcinomas had established disseminated or metastatic disease at the time of diagnosis. Additionally, there was one case of gastrointestinal adenocarcinoma associated with carcinomatosis reported in the literature (21).
Other
CSU-VDL Cases
There were epithelial tumors diagnosed at the CSU-VDL that have never or very rarely before been reported in camelids. These include renal carcinomas (four cases), thyroid carcinoma (2 cases), and thymoma (one case). Renal carcinomas occurred in older animals (average age 19), and all were tubular in pattern (Figure 2F). The thymoma was lymphocyte rich and occurred in a 2-year-old male llama (Figure 2G).
Mesenchymal Neoplasia
CSU-VDL Case Summary
Mesenchymal neoplasia represented 27% of cases with an average age of 12.2 years. Alpacas and females were overrepresented with 57 and 72% of mesenchymal neoplasia, respectively.
Benign
CSU-VDL Cases
From the CSU-VDL submissions, fibromas were the third most diagnosed tumor in camelids (16 cases, 8%). There were also many hemangiomas (6 cases). Hemangiomas tended to have visceral (liver, pericardium, lung) involvement and were diagnosed in older animals (average age 18 years) vs. fibromas which were cutaneous and generally occurred in younger animals (average age 8.5 years). Other reported benign mesenchymal tumors include leiomyoma (stomach chamber 3 [C3], uterus, and rectum), lipoma (subcutaneous, C3 causing obstruction, liver, and prepuce), and two cases of sarcoid. Additionally, there was one Sertoli cell tumor diagnosed at CSU-VDL in an alpaca.
Literature Cases
Valentine et al. reported fibromas/fibropapillomas as the most diagnosed lesion in new world camelids (3). Other benign mesenchymal tumors reported in the literature include two reports of ovarian interstitial cell tumors and one report of a Sertoli cell tumor in an alpaca (3, 41, 42).
Malignant
CSU-DVL Cases
Of the 53 mesenchymal tumors identified from CSU-VDL submissions, 19 were diagnosed as sarcomas (17% of mesenchymal tumors and 9.5% of all proliferative and neoplastic lesions). The average age of diagnosis was 10.3 years (range 6 weeks−23 years), 84% (16 of 19 cases) occurred in female animals, and sarcomas were almost evenly distributed between llamas and alpacas (9 cases vs. 12 cases). The most common diagnosed sarcomas were fibrosarcomas or soft tissue sarcomas (8/19) that typically occurred in the oral cavity. They were also reported to occur in the nose, trachea, subcutaneous adipose tissue, C3, and arising from the pancreas (reported to have metastasized to the local lymph nodes). There was one unusual case of a sarcoma arising from the urethra of a 6-week-old female alpaca. There were three diagnoses of hemangiosarcoma, two visceral (liver and spleen) and one cutaneous (eyelid), and two cases of osteosarcoma both arising from the jaw (one from a tooth root abscess and the other from a giant tumor of bone) (Figure 2H). Splenic, disseminated, and visceral hemangiosarcomas are herein reported for the first time.
Literature Cases
Fibrosarcomas are commonly reported in the literature and similar to the CSU-VDL cases most commonly reported in the oral cavity (3, 5, 33, 35). Two cases of hemangiosarcoma have been reported, one arising from the liver and one intraosseous hemangiosarcoma (12, 39).
Other
CSU-VDL Cases
There were four cases of neuroendocrine carcinomas and five melanocytic tumors identified through the CSU-VDL submissions representing 2.0 and 2.5% of total proliferative and neoplastic lesions, respectively. All neuroendocrine carcinomas were highly aggressive with metastasis at the time of diagnosis, making definitive identification of the origin difficult to determine (Figures 2I,J). Four of the five melanocytic tumors had metastases at the time of diagnosis with the most common sites of metastases being local lymph nodes and the lungs. There was a single case of a congenital melanocytic nevus with malignant transformation and metastasis to the brain and lungs in a 1-month-old alpaca, suggesting that it was congenital (Figures 2K,L).
Primitive and neuronal tumors were rare. There was one case of keratinizing ameloblastoma diagnosed and one case of gemistocytic astrocytoma diagnosed in a 4-year-old female alpaca in the CSU-VDL submissions.
Literature Cases
Neuroectodermal neoplasia are rarely reported with one case of neuroendocrine carcinoma (49) and five reports of melanoma (3, 5, 63–65). Melanomas have been reported to metastasize.
Primitive and neural tumors are rarely reported. Primitive tumors include teratomas (cerebellum and kidney), medulloepitheliomas and retinoblastoma of the eye, ameloblastoma of the mandible, hepatoblastoma, nephroblastoma, primitive stromal tumors of the testicle, and trophoblastic tumor of the uterus (3, 22, 66–74, 76, 77). The only primary neural neoplasm reported was a gemistocytic astrocytoma in a llama (62).
Other Non-neoplastic Proliferative Lesions
In addition to the benign and proliferative lesions previously discussed, there were a number of non-neoplastic proliferative lesions diagnosed in the CSU-VDL submissions and in the literature including polyps; hamartomas; bone cysts; polycystic liver; cystic rete testes; and thyroid, adrenocortical, lobular mammary, and endometrial hyperplasia (5, 15, 78–83).
Discussion and Conclusions
Examination of archived camelid submission to CSU-VDL in the period between April 20, 1995 to December 31, 2020, confirms that neoplasia in camelids is heterogeneous and affects both alpacas and llamas of wide age ranges. The prevalence of neoplasia and proliferative lesions in both species is 8.8% of the total camelid pathology submissions to CSU-VDL, which is higher than the prevalence of camelid neoplasia reported in the literature from other regions (5%) (2, 3). The considerably longer duration of the study period and the higher number of total camelid submissions including more alpacas than llamas might be among the reasons for this variation.
In the South American camelid species, there was a female predisposition for developing neoplasia with 113 female alpacas and llamas (57% of the total cases) developing one or more neoplastic/proliferative lesions compared to 64 male camelids (32%). The relative commonality of mammary and uterine neoplasia and the relative scarcity of testicular tumors, in part, explain why female camelids might be overrepresented in the current review. The predisposition of female alpacas to develop non-genital neoplasia was also observed especially in the case of lymphoma (58% of lymphoma cases were diagnosed in females). Females also had a much higher prevalence of sarcomas (84%) than males in both alpacas and llamas.
Round cell tumors were by far the most commonly diagnosed/reported neoplasia in camelids with lymphoma being the top tumor diagnosed from the CSU-VDL. Interestingly, lymphoma was diagnosed much more commonly in alpacas (75%) than llamas (19%) (p = 0.0192) and the most common tumor diagnosed in animals <5 years of age (Figure 1). Lymphoma was most likely to be disseminated at the time of diagnosis and was the most common tumor to affect the brain. The high prevalence of lymphoma in alpacas and in younger animals suggests a possible genetic link, as is observed in humans and dogs (84). B vs. T cell lymphomas were evenly distributed in cases that had been further immunophenotyped. Also, there is a single case report of bovine leukemia virus-associated lymphoma in an alpaca (55). Evaluation of CSU-VDL cases for bovine leukemia virus was not performed but could be an interesting area for further investigation.
Other round cell tumors included mast cell tumor and histiocytic sarcoma. The CSU-VDL submissions have increased numbers of these tumors when compared to the literature. Histiocytic sarcoma is reported here for the first time in the alpaca species. It occurred in a very young cria, suggesting that the tumor might have developed in utero as it was of considerable size at 2 weeks of age. None of the mast cell tumors found in the CSU-VDL submissions were found to have metastasized.
Malignant epithelial tumors accounted for 69% of camelid epithelial neoplasia. Squamous cell carcinomas were the most common malignant epithelial neoplasia with an overall prevalence of 9.5%, and intestinal, mammary, and uterine adenocarcinomas had an overall prevalence of 27%. Locations of the squamous cell carcinomas included cutaneous, oral, ocular, and visceral. To the authors' knowledge, oral squamous cell carcinomas are a novel category; however, gastric squamous cell carcinoma, ocular-third eyelid squamous cell carcinoma, and squamous cell carcinoma arising from a cutaneous scar have previously been reported in the literature (1, 3, 14, 17, 18). Although the exact etiopathogenesis of the squamous cell carcinomas encountered in camelids is largely unknown, genetic make-up, environmental conditions, and infectious agents are among the possibilities (18). In cases from the CSU-VDL database, only two squamous cell carcinomas were found to have metastasized at the time of the diagnosis, indicating that complete excision of these tumors could be potentially curative in affected animals. Reports from the literature find these tumors to be more aggressive than our database suggests, but this may represent a skewed dataset where only novel and aggressive lesions are published (3, 15, 16, 20).
Adenocarcinomas have a much more aggressive clinical course with most cases having had metastasized at the time of diagnosis. Gastrointestinal tumors were the most common category with all having metastasized at the time of diagnosis and one case having developed widespread carcinomatosis identified in CSU-VDL cases and one reported in the literature (21). It is unknown if these tumors metastasize early or if the frequent metastasis is due to the difficulty in detecting these tumors early in the course of the disease. Diagnosis of adenocarcinoma also had an increased female prevalence. This is likely due to the diagnoses of uterine and mammary carcinomas. Uterine and mammary adenocarcinomas were found to have metastasized in 66 and 33% of cases, respectively, indicating increased incidence of metastasis when compared to squamous cell carcinomas but less than gastrointestinal adenocarcinomas. Other organs that were affected by adenocarcinomas/carcinomas include thyroid, liver (biliary carcinomas), and kidney.
Fibropapilloma is the most prevalent benign epithelial neoplasm. The prevalence of fibropapilloma diagnosed at CSU was higher than in the Cornell study but lower than in the Oregon study, as the authors of the latter study combined both fibropapillomas and fibromas into one category (3, 5). Interestingly, there is a single report of papillomavirus isolated from a llama fibropapilloma which was 73% homologous with bovine papillomavirus type 1 (BPV1) (7). Evaluation for BPV1 in the fibropapillomas diagnosed at CSU was not performed but is of interest for future investigation.
Mesenchymal neoplasia occurred in 52 camelids with an overall prevalence of 26.5, 36% of which were identified as malignant. Fibroma was the most common benign mesenchymal neoplasm at 30.8% of mesenchymal neoplasia and overall prevalence of 8%. There was an even distribution between llamas and alpacas diagnosed with sarcomas and a higher number of females than males. Fibrosarcoma or soft tissue sarcoma, especially of the oral or nasal cavity, was the most common diagnosis. Only two of eight were found to have metastasized at the time of diagnosis. In the literature, there are no reports of metastasis of these tumors, suggesting good prognosis following complete surgical excision. Other less common diagnoses included osteosarcoma, liposarcoma, myxosarcoma, and hemangiosarcoma. Only the visceral sarcomas (affecting the liver and spleen) were found to have metastasized similar to the aggressive behavior of these tumors in other species (85).
Several neoplastic and proliferative lesions, never reported, were identified from the CSU-VDL submissions. These include osteosarcoma, liposarcoma, myxosarcoma, cutaneous hemangiosarcoma, gastric leiomyoma, thymoma, pilomatricoma, sarcoid, malignant giant nevus, pituitary hyperplasia, adrenal cortical hyperplasia, and malignant pheochromocytoma. There were additionally many non-neoplastic and proliferative lesions identified. While these lesions are unlikely to be of clinical relevance, they are important differentials as they could mimic more aggressive neoplastic lesions using imaging modalities including ultrasound or radiographs, or even upon gross exam or necropsy.
The previously unreported congenital giant nevus with malignant transformation in a newborn alpaca is an interesting case. In this mass, there was abnormal cutaneous proliferation of melanoblasts present at birth in the periauricular skin and choristomas discoloring the lungs, liver, and meninges. A similar condition has been described in humans, in which giant congenital melanocytic nevi underwent malignant transformation (86, 87).
Here we summarize and review neoplastic/proliferative lesions in llamas and alpacas. Overall, the behavior of these tumors is similar to the behavior reported in other species, indicating that complete surgical excision may be curative in many incidences of proliferative and neoplastic lesions. There is a substantial risk of aggressive disease outcome and metastasis with certain types of neoplasia and with tumors in particular locations, indicating that staging of some patients is prudent. The current article will likely help to provide relevant prognostication to guide owners and clinicians of the possible outcome and best treatment plan. Risk factors appear to be female sex and increased age except in the case of lymphoma, which tends to occur in younger camelids. Lymphoma, melanomas, and adenocarcinomas (especially of gastrointestinal tract) carry an increased risk of dissemination in a single-organ system or widespread metastases. Sarcomas and squamous cell carcinomas in contrast are less likely to metastasize, and resection of these tumors may be curative in many cases. An important and interesting follow-up would be evaluation of disease-free interval and survival following medical intervention in camelids affected by the most common malignant tumors. In conclusion, camelid neoplasia constitutes a reasonable proportion of pathology submissions to veterinary teaching hospitals and veterinary diagnostic laboratories and, therefore, merits further studies to establish standards for their classification and prognostication.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
AV: writing of manuscript, literature review, figures, and manuscript editing. MW: review and summary table Colorado State University cases. AH and JF: writing of manuscript and manuscript editing. TA: writing of manuscript, figures, and manuscript editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge Savannah Rocha Ph.D candidate at Colorado State University (Fort Collins, CO, USA) and Dr. Allison Ludwig Ph.D candidate at University of Pennsylvania (Philadelphia, PA, USA) for their assistance with statistical analysis.
References
1. Altman NH, Small JD, Squire RA. Squamous cell carcinoma of the rumen and thymic amyloidosis in a guanaco. J Am Vet Med Assoc. (1974) 165:820–2.
2. Bjorklund C, Bage R, Morrell J, de Verdier K, Nisu Hartzell L, Kjellinbro N, et al. Diseases and causes of death among alpacas in Sweden: a retrospective study. Vet Rec Open. (2019) 6:e000239. doi: 10.1136/vetreco-2017-000239
3. Valentine BA, Martin JM. Prevalence of neoplasia in llamas and alpacas (Oregon State University, 2001-2006). J Vet Diagn Invest. (2007) 19:202–4. doi: 10.1177/104063870701900213
4. Newkirk KM, Frank LA. Multiple follicular cysts in four alpacas (Vicugna pacos). Vet Dermatol. (2011) 22:275–8. doi: 10.1111/j.1365-3164.2010.00943.x
5. Scott DW, Vogel JW, Fleis RI, Miller WH Jr, Smith MC. Skin diseases in the alpaca (Vicugna pacos): a literature review and retrospective analysis of 68 cases (Cornell University 1997-2006). Vet Dermatol. (2011) 22:2–16. doi: 10.1111/j.1365-3164.2010.00918.x
6. Suedmeyer WK, Williams F III. Multiple trichoepitheliomas in an alpaca (Lama pacos). J Zoo Wildl Med. (2005) 36:706–8. doi: 10.1638/04115.1
7. Schulman FY, Krafft AE, Janczewski T, Reupert R, Jackson K, Garner MM. Camelid mucoutaneous fibropapillomas: clinicopathologic findings and association with papillomavirus. Vet Pathol. (2003) 40:103–7. doi: 10.1354/vp.40-1-103
8. Gerspach C, Hull BL, Rings DM, Chew DJ, Beamer GL, Hubbell JA, et al. Hematuria and transitional cell papilloma of the renal pelvis treated via unilateral nephrectomy in an alpaca. J Am Vet Med Assoc. (2008) 232:1206–9. doi: 10.2460/javma.232.8.1206
9. Pirie CG, Pizzirani S, Parry NM. Corneal epithelial inclusion cyst in a Llama. Vet Ophthalmol. (2008) 11:111–3. doi: 10.1111/j.1463-5224.2008.00608.x
10. Gilsenan WF, Habecker PL, Coyne TM, Johnson AL. Neurologic disease attributed to a pituitary adenoma in an alpaca. J Vet Intern Med. (2012) 26:1073–7. doi: 10.1111/j.1939-1676.2012.00948.x
11. Chalkley MD, Kiupel M, Draper AC. Pituitary null cell adenoma in a domestic llama (Lama glama). J Comp Pathol. (2014) 151:51–6. doi: 10.1016/j.jcpa.2014.02.006
13. Smith JA. Noninfectious diseases, metabolic diseases, toxicities, and neoplastic diseases of South American camelids. Vet Clin North Am Food Anim Pract. (1989) 5:101–43. doi: 10.1016/S0749-0720(15)31006-9
14. Rogers K, Barrington GM, Parish SM. Squamous cell carcinoma originating from a cutaneous scar in a llama. Can Vet J. (1997) 38:643–4.
15. Moittie S DP, Waine K, Ashfield S, Beiker K. Management of a geriatric alpaca with multiple neoplasms in a zoological setting. Vet Rec Case Rep. (2020) 8:e001065. doi: 10.1136/vetreccr-2019-001065
16. Leichner TL, Turner O, Mason GL, Barrington GM. Cutaneous metastases of a mammary carcinoma in a llama. Can Vet J. (2001) 42:204–6.
17. Cornick JL. Gastric squamous cell carcinoma and fascioliasis in a llama. Cornell Vet. (1988) 78:235–41.
18. Sartin EA, Waldridge BM, Carter DW, Herrera GA, Toivio-Kinnucan M, Lenz SD, et al. Gastric squamous cell carcinoma in three llamas. J Vet Diagn Invest. (1997) 9:103–6. doi: 10.1177/104063879700900123
19. Chu PY ZY, Wang FI, Jeng CR, Pang VF, Chang PH, Chin SC, et al. Spontaneous neoplasms in zoo mammals, birds, and reptiles in Taiwan – a 10-year survey. Anim Biol. (2012) 62:95–110. doi: 10.1163/157075611X616941
20. Rosiers K SM, Flahou T, Versnaeyen H, Ducatelle R, Roels S. Rare presentation of a squmaous cell carcinoma originating from hastrtic compartment 1 in an alpaca. Vlaams Diergeneeskundig Tijdschrift. (2020) 89:279–83. doi: 10.21825/vdt.v89i5.16955
21. Savage K, Sidor I, Mailhot N, Needle D. Pathology in practice. J Am Vet Med Assoc. (2020) 257:49–52. doi: 10.2460/javma.257.1.49
22. Powers BE, Johnson LW, Linton LB, Garry F, Smith J. Endometrial biopsy technique and uterine pathologic findings in llamas. J Am Vet Med Assoc. (1990) 197:1157–62.
23. Klopfleisch R, van der Grinten E, Gruber AD. Metastatic uterine adenocarcinoma and hepatic lipomatosis in a llama (Lama glama). J Vet Diagn Invest. (2009) 21:280–2. doi: 10.1177/104063870902100222
24. Taulescu MA, Bolfa PF, Buiga R, Gal AF, Sevastre B, Morar I, et al. Metastatic cholangiocarcinoma in a llama (Lama glama). J Vet Diagn Invest. (2012) 24:986–9. doi: 10.1177/1040638712452110
25. Bangari DS, Stevenson GW. Carcinoma in a mixed mammary tumor in a llama (Lama glama). J Vet Diagn Invest. (2007) 19:450–3. doi: 10.1177/104063870701900423
26. Gall DA, Zekas LJ, Van Metre D, Holt T. Imaging diagnosis–pulmonary metastases in New World camelids. Vet Radiol Ultrasound. (2006) 47:571–3. doi: 10.1111/j.1740-8261.2006.00187.x
27. Ramos-Vara JA, Miller MA. Metastatic pulmonary adenocarcinoma in a llama (Lama glama). J Vet Diagn Invest. (2002) 14:328–31. doi: 10.1177/104063870201400410
28. Ramos-Vara JA, Loiacono CM, Williams F 3rd, Pardo I, Lakritz J. Pulmonary neoplasia in two llamas (Lama glama). Vet Pathol. (2004) 41:520–3. doi: 10.1354/vp.41-5-520
29. Moser L, Kegler K, Precht C, Zanolari P. Bronchioalveolar carcinoma in an adult alpaca (Vicugna pacos). BMC Vet Res. (2019) 15:139. doi: 10.1186/s12917-019-1895-8
30. Carrasco RA, Verhoef J, Leonardi CEP, Lanigan EE, Adams GP. Bilateral thyroid follicular compact-cellular carcinoma in a llama. J Vet Diagn Invest. (2019) 31:913–6. doi: 10.1177/1040638719882734
31. Peauroi FMF JR, Fisher DJ, Misheff M, Grindley GJ, Campo MS. Anemia, hematuria, and multicentric urinary neoplasia in a llama (Lama glama) exposed to bracken fern. J Zoo Wildl Med. (1995) 26:315–20.
32. Anderson DE, Couto CG, Oglesbee M. Granulosa theca cell tumor with erythrocytosis in a llama. Can Vet J. (2010) 51:1157–60.
33. Shapiro JL, Watson P, McEwen B, Carman S. Highlights of camelid diagnoses from necropsy submissions to the Animal Health Laboratory, University of Guelph, from 1998 to 2004. Can Vet J. (2005) 46:317–8.
34. McCauley CT, Campbell GA, Cummings CA, Drost WT. Ossifying fibroma in a llama. J Vet Diagn Invest. (2000) 12:473–6. doi: 10.1177/104063870001200517
35. Higginbotham ML, McCaw DL, Anderson DE, Lattimer JC, Armbrust L, Andrews GA, et al. Treatment of a maxillary fibrosarcoma in an adult alpaca. J Am Vet Med Assoc. (2015) 246:674–80. doi: 10.2460/javma.246.6.674
36. Hardefeldt LY, Poulsen KP, McGuirk SM, Livesey MA, Koch C, Perrier MP, et al. Urogenital leiomyosarcoma in an alpaca. Can Vet J. (2010) 51:1387–90.
37. Gustafson NR, Severidt J, Van Metre DC, Schultheiss PC, Larue SM, Callan RJ. Radiation therapy for the treatment of urethral sarcoma in a cria. J Vet Intern Med. (2005) 19:271–4. doi: 10.1111/j.1939-1676.2005.tb02694.x
38. Goncarovs-Gran KO, Frank CB, Baird AN, Couetil LL, Ramos-Vara JA. Pathology in practice. embryonal rhabdomyosarcoma in an alpaca. J Am Vet Med Assoc. (2013) 243:1113–5. doi: 10.2460/javma.243.8.1113
39. Hamir AN, Pierce V, Richardson D. Intraosseous hemangiosarcoma with metastasis in a three-month-old llama. J Vet Diagn Invest. (1997) 9:210–3. doi: 10.1177/104063879700900221
40. Malone E, Roertgen K, Kobluk C. Anaplastic sarcoma of the mandible in a llama. Can Vet J. (1996) 37:426–8.
41. Gilbert R, Kutzler M, Valentine BA, Semevolos S. Hyperandrogenism from an ovarian interstitial-cell tumor in an alpaca. J Vet Diagn Invest. (2006) 18:605–7. doi: 10.1177/104063870601800616
42. Stewart JL, Brookhart ME, Clark SG, LeRoith T. Theriogenology question of the month. J Am Vet Med Assoc. (2020) 257:45–7. doi: 10.2460/javma.257.1.45
43. Hasbach AE, Stern AW. Pagetoid reticulosis (epitheliotropic cutaneous T-cell lymphoma) in an adult alpaca (Vicugna pacos). J Vet Diagn Invest. (2016) 28:469–72. doi: 10.1177/1040638716645833
44. Fowler ME, Gillespie D, Harkema J. Lymphosarcoma in a llama. J Am Vet Med Assoc. (1985) 187:1245–6.
45. Underwood WJ, Bell TG. Multicentric lymphosarcoma in a llama. J Vet Diagn Invest. (1993) 5:117–21. doi: 10.1177/104063879300500130
46. Cebra CK, Garry FB, Powers BE, Johnson LW. Lymphosarcoma in 10 New World Camelids. J Vet Intern Med. (1995) 9:381–5. doi: 10.1111/j.1939-1676.1995.tb03297.x
48. Hemsley S, Bailey G, Canfield P. Immunohistochemical characterization of lymphosarcoma in two alpacas (Lama pacos). J Comp Pathol. (2002) 127:69–71. doi: 10.1053/jcpa.2002.0555
49. Sartin EA, Crowe DR, Whitley EM, Treat RE Jr, Purdy SR, et al. Malignant neoplasia in four alpacas. J Vet Diagn Invest. (2004) 16:226–9. doi: 10.1177/104063870401600309
50. Pusterla N, Colegrove KM, Moore PF, Magdesian KG, Vernau W. Multicentric T-cell lymphosarcoma in an alpaca. Vet J. (2006) 171:181–5. doi: 10.1016/j.tvjl.2004.09.013
51. Twomey DF, Barlow AM, Hemsley S. Immunophenotyping of lymphosarcoma in South American camelids on six British premises. Vet J. (2008) 175:133–5. doi: 10.1016/j.tvjl.2006.12.008
52. Martin JM, Valentine BA, Cebra CK, Bildfell RJ, Lohr CV, Fischer KA. Malignant round cell neoplasia in llamas and alpacas. Vet Pathol. (2009) 46:288–98. doi: 10.1354/vp.46-2-288
53. Martin JM, Valentine BA, Cebra CK. Clinical, ultrasonographic, and laboratory findings in 12 llamas and 12 alpacas with malignant round cell tumors. Can Vet J. (2010) 51:1379–82.
54. Amory JT, Jones JC, Crisman MV, Zimmerman K, Tyson AR III, Larson MM, et al. Imaging diagnosis–Dorsal mediastinal T-cell lymphoma in an alpaca. Vet Radiol Ultrasound. (2010) 51:311–2. doi: 10.1111/j.1740-8261.2010.01666.x
55. Lee LC, Scarratt WK, Buehring GC, Saunders GK. Bovine leukemia virus infection in a juvenile alpaca with multicentric lymphoma. Can Vet J. (2012) 53:283–6.
56. Aboellail TA. Pathologic and immunophenotypic characterization of 26 camelid malignant round cell tumors. J Vet Diagn Invest. (2013) 25:168–72. doi: 10.1177/1040638712471059
57. Rosa FB, Rissi DR. Pathology in practice. multicentric B-cell lymphoma. J Am Vet Med Assoc. (2013) 243:645–7. doi: 10.2460/javma.243.5.645
58. Sorensen NJ, Allison RW. What is your diagnosis? Abdominal fluid from an adult alpaca. Vet Clin Pathol. (2015) 44:459–60. doi: 10.1111/vcp.12260
59. Marchionatti E, Van der Vekens E, Peters LM, Kaiponen TS, Berenguer Veiga I, Zanolari P. Solitary tracheal B-cell lymphoma in an adult alpaca (Vicugna pacos). BMC Vet Res. (2020) 16:429. doi: 10.1186/s12917-020-02640-9
60. Steinberg JD, Olver CS, Davis WC, Arzt J, Johnson J, Callan R. Acute myeloid leukemia with multilineage dysplasia in an alpaca. Vet Clin Pathol. (2008) 37:289–97. doi: 10.1111/j.1939-165X.2008.00044.x
61. Lin TY, Hamberg A, Pentecost R, Wellman M, Stromberg P. Mast cell tumors in a llama (Lama glama). J Vet Diagn Invest. (2010) 22:808–11. doi: 10.1177/104063871002200531
62. Garlick DS, Doherty TJ, Paradis MR. Gemistocytic astrocytoma in a one-month-old llama. J Am Vet Med Assoc. (1990) 196:2009–10.
63. Radi ZA, Miller DL, Liggett AD. Cutaneous melanocytoma in a llama (Lama glama). Vet Res Commun. (2005) 29:137–40. doi: 10.1023/B:VERC.0000047491.52173.c3
64. Hill FI, Hughes SM. Osteogenic intraocular melanoma in an alpaca (Vicugna pacos). J Vet Diagn Invest. (2009) 21:171–3. doi: 10.1177/104063870902100132
65. Mollat WH, Gailbreath KL, Orbell GM. Metastatic malignant melanoma in an alpaca (Vicugna pacos). J Vet Diagn Invest. (2009) 21:141–4. doi: 10.1177/104063870902100124
66. Hill FI, Mirams CH. Intracranial teratoma in an alpaca (Vicugna pacos) in New Zealand. Vet Rec. (2008) 162:188–9. doi: 10.1136/vr.162.6.188
68. Hendrix DV, Bochsler PN, Saladino B, Cawrse MA, Thomas J. Malignant teratoid medulloepithelioma in a llama. Vet Pathol. (2000) 37:680–3. doi: 10.1354/vp.37-6-680
69. Gionfriddo JR, Gionfriddo JP, Krohne SG. Ocular diseases of llamas: 194 cases (1980-1993). J Am Vet Med Assoc. (1997) 210:1784–7.
70. Schoeniger S, Donner LR, Van Alstine WG. Malignant nonteratoid ocular medulloepithelioma in a llama (Llama glama). J Vet Diagn Invest. (2006) 18:499–503. doi: 10.1177/104063870601800517
71. Fugaro MN, Kiupel M, Montiani-Ferreira F, Hawkins JF, Janovitz EB. Retinoblastoma in the eye of a llama (Llama glama). Vet Ophthalmol. (2005) 8:287–90. doi: 10.1111/j.1463-5224.2005.00407.x
72. Ivester KM, Baird AN, Baird DK, Oertley-Pihera K American Veterinary Dental C. Diagnostic imaging in veterinary dental practice. Ameloblastoma. J Am Vet Med Assoc. (2006) 228:1343–4. doi: 10.2460/javma.228.9.1343
73. Britt LG, Middleton JR, Warhover TT, Kreeger JM, Branson KR. Acanthomatous ameloblastoma of the maxilla of an adult alpaca. Vet Radiol Ultrasound. (2005) 46:65–8. doi: 10.1111/j.1740-8261.2005.00013.x
74. Bird KE, Parker JE, Andreasen CB, Watrous BJ, Heidel JR. Keratinizing ameloblastoma in a 9-month-old llama (Lama glama). J Vet Diagn Invest. (2004) 16:89–92. doi: 10.1177/104063870401600117
75. Step DL, Ritchey JW, Drost WT, Bahr RJ. Ameloblastic odontoma in the mandible of a llama. Can Vet J. (2003) 44:824–7.
76. Watt BC, Cooley AJ, Darien BJ. Congenital hepatoblastoma in a neonatal alpaca cria. Can Vet J. (2001) 42:872–4.
77. Dietrich JG, Brewer JL. Nephroblastoma with pulmonary metastases in a guanaco. Vet Pathol. (1988) 25:329–30. doi: 10.1177/030098588802500417
78. Hamir AN, Timm KI. Nodular hyperplasia and cysts in thyroid glands of llamas (Lama glama) from north-west USA. Vet Rec. (2003) 152:507–8. doi: 10.1136/vr.152.16.507
79. Anderson DE, Midla LT, Scrivani PV, Rosario J, Leveille R, Long JF, et al. Multifocal polyostotic aneurysmal bone cysts in a llama. J Am Vet Med Assoc. (1997) 210:808–10.
80. Parker R, Hawkes C, Cox A, Barakzai SZ. Treatment of a periradicular lesion in an alpaca (Vicugna pacos). J Vet Dent. (2011) 28:22–4. doi: 10.1177/089875641102800104
81. Foster A, Duff P, Boufana B, Acaster E, Schock A. Adult polycystic liver disease in alpacas. Vet Rec. (2013) 172:666–7. doi: 10.1136/vr.f3968
82. Watanabe TTN, Chaigneau FRC, Adaska JM, Doncel-Diaz B, Uzal FA. Polycystic liver in two adult llamas. J Vet Diagn Invest. (2019) 31:280–3. doi: 10.1177/1040638718824736
83. Kutzler MA, Shoemaker M, Valentine BA, Bildfell RJ, Tornquist SJ. Bilateral cystic rete testis in an alpaca (Lama pacos). J Vet Diagn Invest. (2006) 18:303–6. doi: 10.1177/104063870601800315
84. Valli VE, Bienzle D, Meuten DJ. Tumors of the hemolymphatic system. In: Meuten DK, editor. Tumors in Domestic Animals. Ames, IA: John Wiley & Sons Inc. (2016). p. 203–321. doi: 10.1002/9781119181200.ch7
85. Cooper BJ, Valentine BA. Tumors of muscle. In: Meuten DK, editor. Tumors in Domestic Animals. Ames, IA: John Wiley & Sons Inc. (2016). p. 425–66. doi: 10.1002/9781119181200.ch11
86. Biswas J, Chandran TC, Natarajan N. Periorbital giant congenital melanocytic nevus with malignant changes. Orbit. (1996) 15:53–7. doi: 10.3109/01676839609150068
Keywords: new world camelid, alpaca, llama, neoplasia, proliferative lesions
Citation: Aboellail TA, Waugh M, Harvey A, Fisher J and Vilander AC (2021) Neoplasia and Proliferative Lesions of New World Camelids: A Systematic Literature Review and Retrospective Study of Cases Submitted to Colorado State University From 1995 to 2020. Front. Vet. Sci. 8:743498. doi: 10.3389/fvets.2021.743498
Received: 18 July 2021; Accepted: 13 September 2021;
Published: 22 October 2021.
Edited by:
Micaela Sgorbini, University of Pisa, ItalyReviewed by:
Joe S. Smith, Iowa State University, United StatesAlessandro Poli, University of Pisa, Italy
Copyright © 2021 Aboellail, Waugh, Harvey, Fisher and Vilander. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Allison C. Vilander, allison.vilander@colostate.edu
 Tawfik A. Aboellail
Tawfik A. Aboellail Max Waugh1
Max Waugh1 Alexandra Harvey
Alexandra Harvey Jade Fisher
Jade Fisher Allison C. Vilander
Allison C. Vilander