- 1Shanghai Institute of Geriatrics and Gerontology, Shanghai Key Laboratory of Clinical Geriatrics, Huadong Hospital, and Research Center of Aging and Medicine, Shanghai Medical College, Fudan University, Shanghai, China
- 2Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Department of Otolaryngology, Huadong Hospital, Shanghai Medical College, Fudan University, Shanghai, China
Presbycusis (age-related hearing loss) is a potential risk factor for tinnitus and cognitive deterioration, which result in poor life quality. Presbycusis-related tinnitus with cognitive impairment is a common phenotype in the elderly population. In these individuals, the central auditory system shows similar pathophysiological alterations as those observed in Alzheimer’s disease (AD), including cholinergic hypofunction, epileptiform-like network synchronization, chronic inflammation, and reduced GABAergic inhibition and neural plasticity. Observations from experimental rodent models indicate that recovery of cholinergic function can improve memory and other cognitive functions via acetylcholine-mediated GABAergic inhibition enhancement, nicotinic acetylcholine receptor (nAChR)-mediated anti-inflammation, glial activation inhibition and neurovascular protection. The loss of cholinergic innervation of various brain structures may provide a common link between tinnitus seen in presbycusis-related tinnitus and age-related cognitive impairment. We hypothesize a key component of the condition is the withdrawal of cholinergic input to a subtype of GABAergic inhibitory interneuron, neuropeptide Y (NPY) neurogliaform cells. Cholinergic denervation might not only cause the degeneration of NPY neurogliaform cells, but may also result in decreased AChR activation in GABAergic inhibitory interneurons. This, in turn, would lead to reduced GABA release and inhibitory regulation of neural networks. Reduced nAChR-mediated anti-inflammation due to the loss of nicotinic innervation might lead to the transformation of glial cells and release of inflammatory mediators, lowering the buffering of extracellular potassium and glutamate metabolism. Further research will provide evidence for the recovery of cholinergic function with the use of cholinergic input enhancement alone or in combination with other rehabilitative interventions to reestablish inhibitory regulation mechanisms of involved neural networks for presbycusis-related tinnitus with cognitive impairment.
Introduction
Subjective tinnitus, mainly induced by hearing loss and emotional states, is heterogeneous, affecting the development of effective intervention strategies. Presbycusis, commonly referred to as age-related hearing impairment, is a potential risk factor for tinnitus (Shargorodsky et al., 2010; Knipper et al., 2013) and cognitive impairment, including Alzheimer’s disease (AD) and non-AD dementia (Lin et al., 2011, 2013; Bakhos et al., 2015; Panza et al., 2015a,b; Taljaard et al., 2016; Thomson et al., 2017). Thus, presbycusis-related tinnitus and cognitive impairment often appear stimulaneously within a subset of the elderly population.
Epidemiological studies have shown that the prevalence of both presbycusis and dementia increases with age. Approximately one-third of individuals over 65 years of age experience hearing loss greater than 40 dB (averaged across 0.5–4 kHz), more than 10% experience dementia, and more than 90% of individuals with dementia have hearing abnormalities (Marti et al., 2014). Presbycusis is associated with cognitive decline and late-life cognitive disorders due to peripheral hearing impairment (Gates and Mills, 2005; Wallhagen et al., 2008; Gallacher et al., 2012; Lin et al., 2013; Behrman et al., 2014; Deal et al., 2017; Loughrey et al., 2018) or central auditory processing dysfunction (Gennis et al., 1991; Gates et al., 2002, 2011). A prospective epidemiological cohort study showed that observed hearing loss was associated with a greater risk of incident dementia in a multiethnic population (n = 1881) followed up over a mean of 7.3 ± 4.4 years (Golub et al., 2017). Moreover, case-control and population-based studies have shown that patients with mild cognitive impairment (MCI), dementia, and AD also have central auditory processing dysfunction and topographically specific neurodegeneration resulting from amyloid senile plaques (SP) and neurofibrillary tangles (NFTs; Sinha et al., 1993; reviewed by Panza et al., 2015a,b).
It is difficult to establish a causal relationship between presbycusis and age-related cognitive decline. Nonetheless, hearing loss could be an early symptom of cognitive decline in elderly individuals, and therefore an appropriate component of screening tools for preclinical diagnosis (Wong et al., 2014). Presbycusis also could be seen as a modifiable factor for preventing cognitive impairment (Lin, 2011; Lin et al., 2011; Gurgel et al., 2014; Marti et al., 2014; Panza et al., 2015a,b). Indeed, timely hearing rehabilitation at the preclinical stage of cognitive decline, including hearing aids and/or cochlear implants, may act to suppress tinnitus and protect cognition by reducing social isolation and depression, reversing maladaptive neuronal plasticity, and improving neurotrophic support and working memory (Acar et al., 2011; Langguth et al., 2013; Marti et al., 2014; Panza et al., 2015a,b; Shore et al., 2016). A whole body of literature indicates that there is no causal relationship between hearing loss and general cognitive loss. Presentation of two age-related disorders together could purely reflect the fact that both conditions are more common in elderly individuals.
Epidemiological studies have also reported that the prevalence of tinnitus increases with age and is highest in elderly individuals aged 60 and 69 years (Adams et al., 1999; Ahmad and Seidman, 2004). The most common symptom of tinnitus is cognitive deficits (Andersson et al., 1999; Hallam et al., 2004; Andersson and McKenna, 2006; Pierce et al., 2012), including working memory and processing speeds on neurocognitive testing (Rossiter et al., 2006), cognitive efficiency (Hallam et al., 2004) and attention control (Stevens et al., 2007). The prevalence of cognitive deficits in patients with tinnitus is higher than would be expected by chance. Approximately 70% of patients with tinnitus had self-reported difficulty concentrating (Andersson et al., 1999). Compared with healthy controls and those with acquired hearing loss, patients with tinnitus also report a greater number of cognitive impairments (Hallam et al., 2004). However, individuals with normal-hearing and tinnitus report similar cognitive performance with individuals with normal hearing without tinnitus (Waechter and Brännström, 2015).
Presbycusis-related tinnitus and cognitive impairment are associated with aging. The former may reflect an independent pathological process that shares some etiologies and pathophysiological alterations with cognitive decline (Marti et al., 2014). The ApoE ε4 allele is a genetic risk factor for both age-related hearing loss (Kurniawan et al., 2012) and AD (Hollands et al., 2017). Cholinergic hypofunction, chronic inflammation and vascular factors are probably linked to the pathogenesis of both presbycusis-related tinnitus and age-related cognitive impairment (Benzing et al., 1993; Emre et al., 1993; Shulman et al., 2008; Daulatzai, 2010; Haase et al., 2011; Fortunato et al., 2016; Wu and Chiu, 2016; Panza et al., 2017). Particularly, cholinergic hypofunction related to aging can aggravate functional deficits of GABAergic interneurons, NFTs, chronic systemic inflammation, age-related blood-brain barrier dysfunction and maladaptive plasticity resulting in an increased spontaneous firing rate, synchronized epileptic-like neuronal activity and excitotoxicity (Knipper et al., 2013; Shore et al., 2016).
While the majority of studies that we refer to are based on animal models, age-related degeneration of synapses and neural anatomy in the peripheral and central nervous system (CNS) may represent a common neurophysiological basis of presbycusis-related tinnitus and age-related cognitive impairment. We hypothesize that age-related loss of cholinergic innervation of various brain structures may be a common link between tinnitus seen in presbycusis-related tinnitus and age-related cognitive impairment. Recovery of cholinergic function may be useful to treat presbycusis-related tinnitus with cognitive impairment by affecting multiple shared pathophysiological targets.
Declining Cholinergic Function in Humans With Presbycusis-Related Tinnitus and Age-Related Cognitive Impairment
Aging and neurodegenerative diseases are the major causes of declining cholinergic function. Aging leads to cholinergic hypofunction of the basal forebrain cholinergic complex, which is the main cholinergic projection to the cerebral cortex and hippocampus. Gradual age-related loss of cholinergic function results from decreased trophic support from nerve growth factor (NGF) and degeneration of dendritic, axonal and synaptic structures, which cause brain function decline, including cognitive impairment (Daulatzai, 2010; Schliebs and Arendt, 2011).
As in normal aging, patients with MCI and early-stage AD only exhibit declining cholinergic function without cholinergic neurodegeneration. Such changes include an imbalance in the expression of NGF, pro-NGF, the high NGF receptor, trkA and low NGF neurotrophin p75 receptor, as well as changes in acetylcholine release and choline uptake (Cohen et al., 1995; Schliebs and Arendt, 2011). The advanced stages of early-onset and late-onset AD and psychiatric disorders (e.g., Parkinson’s disease and Lewy body dementia) are characterized by a severe loss of NGF receptor positive cholinergic cells in the basal forebrain (Mufson and Kordower, 1989; Perry, 1990). NGF receptors play a role in cholinergic neuron death. Decreased expression of NGF receptors was also observed on among striatal cholinergic neurons in the AD brain (Boissière et al., 1996). Furthermore, encapsulated cell implants releasing NGF bilaterally to the basal forebrain of patients with AD across 12 months significantly enhanced cerebrospinal fluid levels of the cholinergic biomarker choline acetyltransferase (ChAT; Karami et al., 2015). Age-related loss of the calcium-binding protein, calbindin-D28K, in basal forebrain cholinergic neurons has been related to the full range of tau pathology of AD (Ahmadian et al., 2015).
Cholinergic hypofunction also involves changes in the presynaptic synthetic enzyme, ChAT and acetylcholine receptor (AChR) expression. In patients with AD compared with age-matched healthy controls, there is a 50%–90% decline in activity of presynaptic ChAT (Perry et al., 1978; Davies, 1979). Moreover, significant declines in enzyme activity that result in cholinergic dysfunction do not occur until a relatively late stage (Davies et al., 1999; Tiraboschi et al., 2000). In contrast, loss of ChAT activity in patients with Lewy bodies was present in the earliest stage (Tiraboschi et al., 2002). In the frontal cortex of individuals with AD, different alterations have been observed in muscarinic (M) subtypes, with diminished M1 and M2 but increased M4 immunoreactivity, and normal M1, decreased M2 and increased M4 numbers of binding sites (Flynn et al., 1995). Cholinergic deficits are associated with the loss or derangement of nicotinic acetylcholine receptors (nAChRs) in the brains of those with AD and Down syndrome (Engidawork et al., 2001), with significantly decreased alpha 7 and significantly increased alpha 3 receptors in the frontal cortex in AD. Autopsy brain tissue (Guan et al., 2000; Lee et al., 2000) and in vivo evaluations (Nordberg et al., 1997) of patients with AD have consistently shown decreased nAChR levels. Moreover, after blockade of muscarinic receptors with scopolamine, young healthy individuals have a similar pattern of memory and cognitive decline as aged individuals with cholinergic dysfunction (Drachman et al., 1980). Nicotinic cholinergic blockade with mecamylamine in elderly healthy individuals resulted in AD-like cognitive deficits and specific blood flow abnormalities in the parieto-temporal cortex (Gitelman and Prohovnik, 1992). Therefore, tacrine and nicotine, which stimulate the cholinergic system, could significantly improve attentional function associated with basal forebrain cholinergic innervation of the cortex and other brain regions in patients with AD (Lawrence and Sahakian, 1995).
Degeneration of the basal forebrain cholinergic system due to aging and AD causes impairment of thalamo-cortical function, reduced connectivity between the thalamo-cortical system, hippocampus, and other key brain regions, and decreased cerebral blood flow (CBF), which has been associated with cognitive disturbances and age-related sensory loss (Daulatzai, 2010). The amygdala is a component of the limbic system involved in emotion, attention and memory. Differences have also been observed between the aging human brain and AD in the loss of cholinergic innervation of the amygdaloid complex (Benzing et al., 1993; Emre et al., 1993). Compared with middle-aged controls, no decline in cholinergic input of the amygdale was observed in immunohistological specimens from aging participants (Emre et al., 1993). Another study reported that individuals without dementia but with high rates of SP showed highly dystrophic neurites, but no significant loss of fiber innervations (Benzing et al., 1993). However, there does appear to be a severe and regionally selective loss of cholinergic innervations in the amygdaloid complex of patients with AD.
Cholinergic hypofunction results in impairments of the auditory pathway, as well as impaired cortico-cortical interactions between auditory and other sensory regions. In patients with mild to moderate AD, dysfunction is observed in the primary auditory pathway and ascending reticular activating system, which have cortical cholinergic innervation. Furthermore, significant delays in I~V interpeak latency of brain auditory evoked responses and dysfunction in the generation of primary auditory cortex evoked potentials, as well as reduced neuronal activity in the ascending reticular activating system are observed in AD (O’Mahony et al., 1994). There is a progressive decline in the attenuation of subsequent auditory evoked potentials by a visual stimulus from the young to the healthy elderly to individuals with MCI and AD (Golob et al., 2015). However, in the human cochlear nucleus, nAchR beta 2 immunostaining was unchanged from birth to 90 years (Sharma et al., 2014). Based on observations from human studies, the loss of cholinergic innervation to various brain structures may provide a link between tinnitus seen in presbycusis-related tinnitus and age-related cognitive impairment. Recovery of cholinergic function during an optimal time window before the loss of cholinergic neurons may therefore lead to better outcomes.
Declining Cholinergic Function May Contribute to the Accumulation of Beta-Amyloid Oligomers and NFTs in Age-Related Cognitive and Hearing Impairments
The neuropathological hallmarks of AD, including amyloid deposits and tau-immunoreactive NFTs, are also present in the healthy aging brain. An immunohistological study of serial sections from 105 autopsy brains of cognitively normal patients (age range: 40–104 years) showed that NFTs appear earlier than amyloid plaques during normal aging. All cases from people over 48 years old displayed at least a few NFTs (more frequently in the entorhinal than in the transentorhinal cortex), which was preceded by tau pathology in these areas rather than in the brainstem (Tsartsalis et al., 2018). In the auditory system of individuals with AD, the ventral nucleus of the medial geniculate body and central nucleus of the inferior colliculus show SP and NFT distributions with a topographically specific and consistent pattern of degeneration (Sinha et al., 1993). Significant age-related reductions in calcium binding proteins has been observed in later decades in the ventral cochlear nucleus, which is similar to results for cholinergic neurons of the basal forebrain in patients with AD, and might be related to tau pathology (Sharma et al., 2014; Ahmadian et al., 2015).
Noise exposure is a common cause of tinnitus and hearing impairment. Animal research shows that exposure to moderate intensity white noise (80 dB SPL, 2 h/day) can impair learning and memory in mice (Cheng et al., 2011). Moreover, it has been demonstrated that the hippocampus is more susceptible to noise than is the auditory cortex (Cheng et al., 2016). Indeed, significant increases in peroxidation and tau hyperphosphorylation in the hippocampus have been observed after a week of noise exposure, but there were no increases in the auditory cortex 3 weeks after exposure. Chronic white noise (100 dB SPL, 4 h/day × 14 day) persistently increased tau hyperphosphorylation at the same sites that are typically phosphorylated in the AD brain and glycogen synthase kinase 3β (GSK3β), as well as increased the formation of pathological NFT tau in the hippocampus and prefrontal cortex (Cui et al., 2012). Such changes in the frontal cortex also play an important role in the pathogenesis of frontal dementia, while changes in the frontal acoustic cortex are seen in the early onset of communication deficiency (Baloyannis et al., 2001).
Tau hyperphosphorylation sequesters normal tau and microtubule-associated proteins into insoluble NFTs and inhibits microtubule assembly (Iqbal et al., 2013). Tau reduction prevents cognitive decline, synaptic transmission and plasticity, and spontaneous epileptiform activity in AD model mice that overexpress Aβ, without changing the expression of Aβ (Ittner et al., 2010). Furthermore, tau-deficient AD models have demonstrated a reversal in the Aβ induced imbalance of excitation/inhibition, NMDA receptor dysfunction, and excitotoxicity in both transgenic and wild type mice (Roberson et al., 2007, 2011).
Loss of cholinergic innervations may play important roles in both AD and hearing impairment during aging. The AChE inhibitor donepezil can protect against Aβ induced neurotoxicity by enhancing protein phosphatase 2A (PP2A) activity and inhibiting GSK3β activity via the activation of nAChRs, which reduces tau-induced neuronal toxicity and neurodegeneration (Bitner et al., 2009; Noh et al., 2009). In the brain, mAChRs may mediate cognitive function and neuropsychiatric symptoms and they are also considered potential targets in AD and schizophrenia (Clader and Wang, 2005; Poulin et al., 2010; Foster et al., 2014). M1 type mAChRs, mainly present in the striatum, hippocampus and neocortex, are activated by M1 specific agonists doses without adverse effects. Such activation could improve learning, memory, synaptic plasticity, and cognitive functions via the activation of extracellular signal-regulated kinases (Berkeley et al., 2001; Ragozzino et al., 2012). In A7KO-APP AD transgenic mice, the absence of alpha-7 nAChRs leads to Aβ accumulation and oligomerization, exacerbating early-stage cognitive decline and septohippocampal pathology (Hernandez et al., 2010).
Cholinergic Denervation of NPY Neurogliaform Cells May Be Involved in Presbycusis-Related Tinnitus With Cognitive Impairment
Reduced functional connectivity in the brains of patients with AD or MCI, as well as the elderly with cognitive complaints or cognitively normal ApoEε4 carriers, reflects activity changes within the default-mode network, which is most active at rest and deactivated during cognitive tasks (Ruan et al., 2016). The loss of cholinergic innervations and reduced GABAergic inhibition might play important roles in such changes.
Distinct GABAergic cell types project to the surface of pyramidal cells in the cortex and hippocampus, forming neural circuits for inhibitory control of brain function and plasticity. Functional remodeling of GABAergic neurotransmission has been observed in the human brain with AD (Limon et al., 2012). Moreover, GABA currents in the temporal cortex of the AD brain show age-related reductions, which were associated with reduced mRNA and protein for the main GABA receptor subunits. In the ADbrain compared with controls, α1 and γ2 transcription shows down-regulation, while but α2, β1 and γ1 transcription shows up-regulation. In patients with AD and/or epilepsy, deficits of GABAergic interneurons are associated with aberrant network activity, including hyperexcitability, clusters of hyperactive and hypoactive neurons, and network/spontaneous epileptiform activity (Olney, 1995; Nägerl et al., 2000; Snider et al., 2005; Palop and Mucke, 2009).
Patients with tinnitus show alterations in global brain networks, including decreased default-mode network activity, and increased activation of the auditory cortex and amygdala (Schlee et al., 2009; Elgoyhen et al., 2015). These alterations may result from decreased fuctional connectivity from peripheral and other brain regions. Tinnitus may be a consequence of maladaptive plasticity-induced disturbances of excitation-inhibition homeostasis with net down-regulation of inhibitory neurotransmission in the central auditory pathway. Subsequently, the central auditory system compensates for decreased input by up-regulating network activity among central circuits (Salvi et al., 2000; Knipper et al., 2013; Shore et al., 2016). Decreased peripheral input induced by auditory trauma and aging leads to altered cortical activity patterns, including increased spontaneous firing rates, synchronized epileptic-like neuronal activity, and basal excitatory postsynaptic potentials (for a review, see Knipper et al., 2013). Plastic tinnitus-related changes include loss of glycinergic inhibition in the adult dorsal cochlear nucleus and/or loss of GABAergic inhibition in the inferior colliculus and higher centers, resulting in aberrant cortical activity patterns (Wang et al., 2011).
Although cholinergic drugs can temporarily suppress tinnitus in some patients, these interventions cannot eliminate the pathological neural activity. Mounting evidence from clinical trials suggests that vagus nerve stimulation (VNS)-based targeted plasticity therapies are effective in patients with neurological diseases (Hays, 2016). VNS in combination with auditory stimulation can reverse pathological neuroplastic changes of the auditory cortex toward physiological neural activity and synchronicity via M cholinergic neuromodulation (Engineer et al., 2013; Bojić et al., 2017; Tyler et al., 2017). Based on these studies in humans, GABAergic interneuron deficits in the auditory cortex and limbic system may play a key role in presbycusis-related tinnitus with cognitive impairment.
Loss of Cholinergic Innervation and Reduced Inhibition of NPY Neurogliaform Cells in Age-Related Cognitive Impairment
Animals studies have shown that GABAergic interneuron deficits result in aberrant excitatory neuronal activity in mouse AD models (Palop et al., 2007; Roberson et al., 2007, 2011; Verret et al., 2012; Iaccarino et al., 2016). Both nAChRs (Buhler and Dunwiddie, 2002; Maloku et al., 2011; Zappettini et al., 2011) and mAChRs (Pitler and Alger, 1992; Zhong et al., 2003; González et al., 2011; Yi et al., 2014) are expressed in GABAergic interneurons and mediate GABA release from these neurons. Neuropeptide Y (NPY)-neurogliaform (Faust et al., 2015), somatostatin (Faust et al., 2015; Muñoz et al., 2017) and parvalbumin (Yi et al., 2014) subtype interneurons express AChRs and receive cholinergic excitatory input. NPY-neurogliaform cells primarily reside within both the stratum radiatum and lacunosum-moleculare of the hippocampus, as well as the superficial and deep layers of the neocortex, which are significantly decreased in the hippocampus of animal models with AD or seizures (Mazarati and Wasterlain, 2002; Faust et al., 2015). However, optogenetic stimulation of cholinergic fibers in transgenic mice expressing the human ApoE ε4 allele has been shown to abolish partial neuronal loss in the entorhinal cortex induced by abnormal hyperactivity in dentate networks (Bott et al., 2016).
The activation of both the α(7) nAChR and α4β2 nAChR subtypes could enhance GABA release in hippocampal synaptosomes (Zappettini et al., 2011). Furthermore, α(4)β(2) nAChR agonists may control epigenetic alterations induced by glutamic acid decarboxylase 67 (GAD 67) increases in GABAergic neurons better in schizophrenia than do α(7) nAChR agonists (Maloku et al., 2011). M1 mAChRs in parvalbumin interneurons could improve GABAergic transmission in hippocampal and prefrontal cortical pyramidal neurons (Yi et al., 2014). Moreover, activation of M1–M5 mAChRs in rat hippocampal neurons in vitro increases GABAergic inhibitory transmission (González et al., 2011). Treatment with Huperzine A leads to robust and sustained seizure resistance in genetic epilepsy models with voltage-gated sodium channel mutation via the activation of mAChRs and GABAA receptors (Wong et al., 2014). However, nAChR-mediated GABAergic cortical inhibition in rats, related to increased high gamma frequency visible on electroencephalogram, might also be involved in the Huperzine A anticonvulsant mechanisms (Gersner et al., 2015). Thus, solely based on the animal models, the loss of cholinergic innervation of NPY-neurogliaform cells in various brain structures contributes to aberrant excitatory neuronal activity in age-related cognitive impairment.
Cholinergic Denervation of NPY Neurogliaform Cells in the Central Auditory System in Presbycusis With Tinnitus
In animal studies, changes in inhibitory properties that are induced by aging and acoustic trauma, similar to deafferentation plasticity changes in other mammalian sensory systems, have been observed from the cochlear nuclei to the auditory system. The cochlear nuclei of aged rats have lower glycine levels and altered glycine receptor subunit compositions compared with young rats (Banay-Schwartz et al., 1989). However, in the inferior colliculus of rats, age-related loss of GABAergic inhibition caused by the loss of the biosynthetic enzyme GAD, as well as reduced GABA levels and GABA release, may be involved in the abnormal perception of signals in noise and the deterioration of speech discrimination (Milbrandt et al., 1994, 2000; Raza et al., 1994).
Age-related decreases in GAD have been observed in the primary auditory cortex, parietal cortex and hippocampus, with more significant reductions observed in the auditory cortex of rats (Stanley and Shetty, 2004; Ling et al., 2005). Age-related alterations in GABA receptor subunit composition have also been observed in the inferior colliculus and primary auditory cortex of aged rats, such that there are changes to the wild-type receptor proportions (Caspary et al., 2013). These presynaptic and postsynaptic changes may contribute to increased spontaneous activity in neurons of the inferior colliculus and layer-specific increases in the spontaneous activity of the primary auditory cortex (Ling et al., 2005). Following sound exposure in rats with tinnitus, single units within the medial geniculate body of rats exhibited enhanced spontaneous firing, altered burst properties, and increased rate-level function slopes, which acts to alter sensory gating and enhance the gain of neuronal networks in the auditory cortex and limbic centers (Kalappa et al., 2014).
Inhibitory transmission and survival of NPY-neurogliaform cells in the hippocampus and prefrontal cortex is mainly under cholinergic regulation in experimental rodents (Mazarati and Wasterlain, 2002; Faust et al., 2015; Overstreet-Wadiche and McBain, 2015; Bott et al., 2016). Therefore, we hypothesized that withdrawal of nicotinic cholinergic input to NPY neurogliaform cells is a key component of the pathological mechanism underlying presbycusis with tinnitus and cognitive impairment, solely based on animal models. The enhancement of GABA release from NPY-neurogliaform cells and the reversal of the imbalance between excitation and inhibition in the central auditory system following the recovery of cholinergic function may provide an important target for interventions to treat presbycusis with tinnitus (Figure 1).
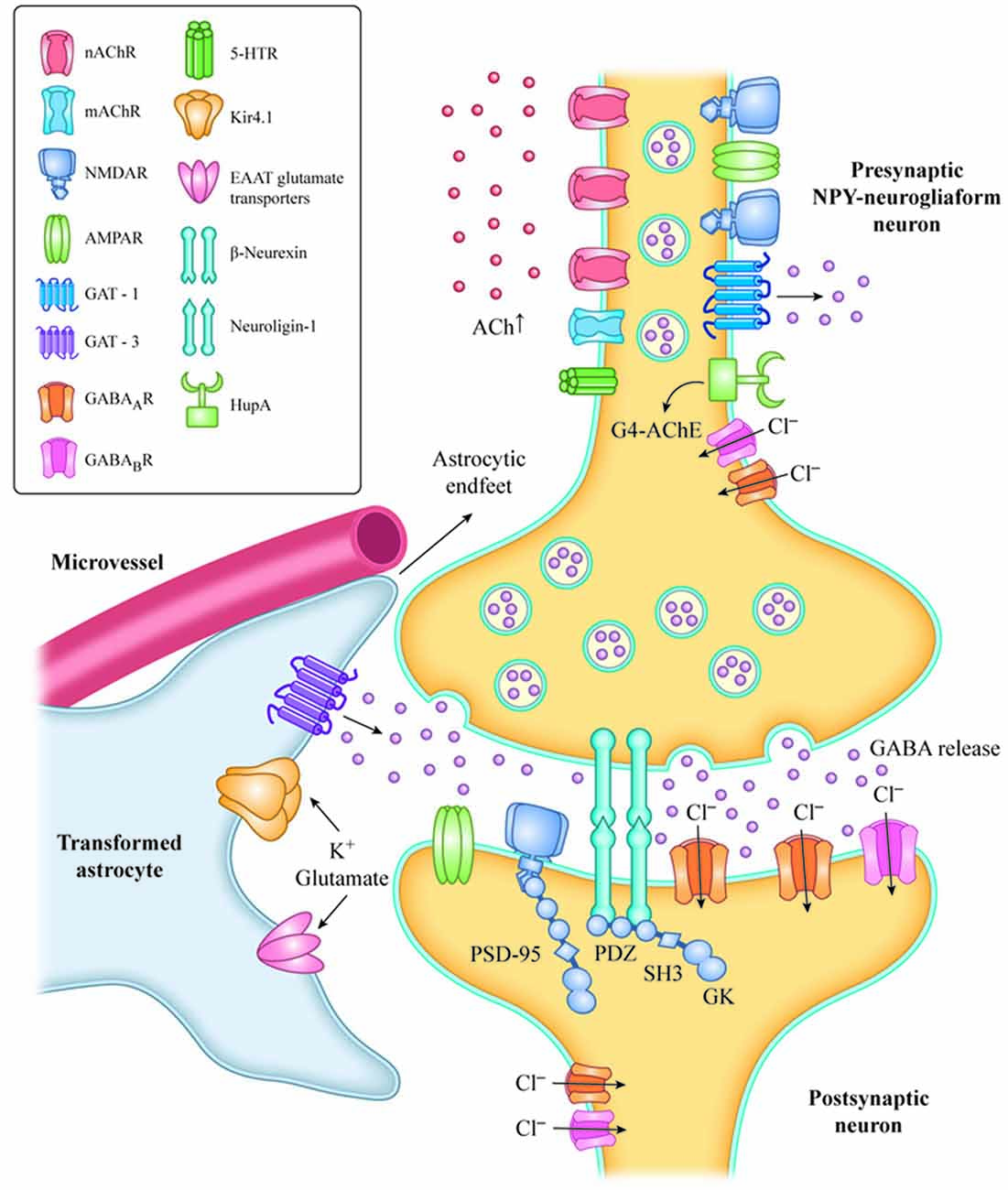
Figure 1. Increased level of Ach with HupA inhibition of AChE activation or VNS, resulting in activation of AChRs, in NPY-neurogliaform neurons.The increased GABAergic signaling may regulate inhibitory tone and network activity by phasic or synaptic transmission, tonic activation and volume transmission. The adhesion complex of Neuroligin-1 (NL1) and β-neurexin is involved in the maintenance of synapses. The C-terminal fragment of NL-1 and NMDA receptors interacts with the PDZ domains of PSD-95 in the postsynaptic region of neurons, and mediates excitatory synaptic efficacy and plasticity. Transformed astrocytes contribute to neuron hypersynchronicity and excitability, which mainly occurs by reduced expression of potassium inward-rectifying channels (Kir4.1), reduced gap junctions, impaired glutamate metabolism and increased release of inflammatory mediators. VNS, vagus nerve stimulation; NPY, neuropeptide Y; G4-ACh, tetrameric acetylcholinesterase; AChR, acetylcholine receptor; EAAT, glutamate transporter of astrocytes; and GAT, GABA transporter.
Beyond the central auditory system, axosomatic synapses between the medial olivocochlear efferent system and outer hair cells are cholinergic. A feedback system eliciting efferent suppression via alpha-9/alpha-10 nAChRs can improve the detection of signals in background noise, enable selective attention to particular signals, and protect the periphery from damage caused by overly loud sounds (Maison et al., 2002; Elgoyhen et al., 2009). Our previous animal studies have shown that aging and ototoxic drugs exacerbate the degeneration of the mouse medial olivocochlear efferent system (Ruan et al., 2014a,b,c). Furthermore, histopathological studies of the human cochlear have shown that those with presbycusis and tinnitus had a significantly greater loss of outer hair cells in the basal and upper middle turns, and greater atrophy of the stria vascularis in the basal turn compared with those with presbycusis without tinnitus (Terao et al., 2011). Therefore, nAChR activation in the peripheral medial olivocochlear efferent system may also play a role in the suppression of presbycusis with tinnitus.
Nicotinic Denervation Induced Immuno-Dysregulation May Involved in Presbycusis-Related Tinnitus With Cognitive Impairment
Observations from clinical studies indicate that, glial cell activation and chronic systemic inflammation during normal and pathologic brain aging are related to poor cognitive performance and a risk of cognitive decline in dementia, vascular dementia, and AD (Schmidt et al., 2002; Weaver et al., 2002; Engelhart et al., 2004; Yaffe et al., 2004). Inflammation plays a critical role in the fluctuation of non-cognitive neuropsychiatric symptoms (Kat et al., 2008; van Gool et al., 2010). Indeed, free radical-induced oxidative damage and chronic inflammation play important roles in the development of dysfunctional connections between the central cortex and the inner ear in hearing disorders (Haase et al., 2011).
Age-related increase in GFAP positive glial cells have been observed in the cochlear nucleus (Sharma et al., 2014). In a cross-sectional cohort of 360 community-dwelling individuals aged 60 years and over, increased inflammatory markers and white blood cell count were associated with worsening presbycusis, with the strongest positive correlation seen in those over 75 years (Verschuur et al., 2014). Furthermore, the inflammatory cytokine TNF-α (rs1800630) and the TNF receptor superfamily 1B (rs1061624) have been related to an increased risk of hearing damage in a population-based cohort study of elderly Japanese individuals (Uchida et al., 2014).
Chronic inflammation also leads to blood brain barrier (BBB) vulnerability and brain hypoperfusion. Increased release of neurotoxic and inflammatory mediators has been observed in the brain microvessels of patients with AD (Grammas, 2011). Further, chronic inflammation causes BBB dysfunction and increased vascular permeability during aging, as well as in AD and other neurodegenerative disorders (Farrall and Wardlaw, 2009; Erdő et al., 2017). Moreover, the loss of cholinergic innervation to the basal forebrain results in decreased CBF (Martin et al., 1991; Daulatzai, 2010). Compared with neurologically healthy individuals without the ApoE ε4 allele, those with the ApoE ε4 allele show greater regional CBF reductions in the brain, making it vulnerable to pathological alterations in AD (Thambisetty et al., 2010; Hollands et al., 2017) and presbycusis (Kurniawan et al., 2012).
These results suggest that chronic inflammation and hypoperfusion play important roles in the pathogenesis of presbucusis-related tinnitus with cognitive impairment. Recovery of cholinergic function with AChE inhibitors, including donepezil, tacrine, pyridostigmine, galantamine, rivastigmine and Huperzine A shows potential disease-modifying benefits in the treatment of neuropsychiatric symptoms in patients with AD (Linton, 2005; Rafii et al., 2011) and dementia (Freund-Levi et al., 2014), as well as for the musical hallucinations that occur with hearing loss (Ukai et al., 2007; Zilles et al., 2012; Blom et al., 2014, 2015) or hearing loss with tinnitus (Strauss and Gertz, 2009). However, there is no mechanistic explanation for the relationship between cholinergic hypofunction and chronic inflammation alterations in presbucusis-related tinnitus with cognitive impairment.
Loss of Cholinergic Innervation and Chronic Systemic Inflammation in Age-Related Cognitive Impairment
Observations from experimental rodent models indicate that anticholinergic activity might initiate and/or accelerate AD pathology in the tauopathy mouse model by enhancing neuroinflammation, including microglial activation. The recovery of lost cholinergic innervation or function by the cholinesterase inhibitor donepezil or Huperzine A could alleviate tau pathology as well as age- and AD-related chronic neuroinflammation (Yoshiyama et al., 2015), and D-galactose-induced neurovascular damage (Ruan et al., 2014d). Moreover, chronic inflammation induced cognitive decline in rats with cerebral hypoperfusion (Wang et al., 2010).
The mechanisms underlying cholinergic anti-inflammation were first observed in human immune cells (Wang et al., 2003). The observations suggested that nicotinic activation of α7nAChR in human macrophages or monocytes is necessary to attenuate the systemic inflammatory response and inhibit the production of proinflammatory mediators by suppression of I-κB phosphorylation and nuclear factor-κB transcriptional activity (Wang et al., 2003; Yoshikawa et al., 2006).
Subsequently, a similar anti-inflammatory mechanism was also observed in rat CNS. Increased brain ACh induced by Huperzine A activates cholinergic-mediated suppression of nuclear translocation of NF-κB, as well as inducing oxidative stress, glial cell activation, and neuroinflammation in rats with ischemia (Wang et al., 2008). Huperzine A combines tetrameric AChE (G4) and indirectly activates both muscarinic and nicotinic types of AChRs (Wang et al., 2010). Moreover, the obvious overlap of tetrameric AChE and α7nAChRs in the hypothalamus, hippocampus, amygdale, cerebral cortex and midbrain of humans and rats (reviewed by Damar et al., 2017) indicates that cholinergic anti-inflammatory effects occur mainly via α7nAChRs in glial and neuronal cells (Pavlov and Tracey, 2006; Wang et al., 2008).
The activation of α7nAChRs in neural cells suppresses central inflammatory responses in mice with Parkinson disease (Stuckenholz et al., 2013), stroke (Han et al., 2014), or traumatic brain injury (Kelso and Oestreich, 2012), and also suppresses glutamate-induced neurotoxicity in vitro (Shimohama et al., 1998; Iwamoto et al., 2013). Futhermore, the activation of α7nAChRs in astrocytes down-regulates Aβ1–42-induced increases in NF-κB in in vitro (Xie et al., 2016), and improves neurotrophic cytokine S100B secretion, which is decreased in the cerebrospinal fluid in rat models of dementia (Lunardi et al., 2013). Moreover, the upregulation of α7nAChR expression induced by neuregulin in microglial cells suppresses neuroinflammation in vitro (Mencel et al., 2013). Based on the above results from clinical and animal studies, loss of cholinergic innervations results in reduced cholinergic anti-inflammatory effects and glial activation, which further aggravates the loss of GABAergic interneurons. Therefore, we hypothesize that the withdrawal of nicotinic cholinergic input induces chronic inflammation, acting as another key step in the pathological mechanism underlying presbycusis with tinnitus and cognitive impairment.
Induction of Immuno-Dysregulation by Nicotinic Denervation in the Central Auditory System May Contribute to Presbycusis-Related Tinnitus With Cognitive Impairment
Animal research suggests that auditory cortical cholinergic inputs from the basal forebrain in adult ferrets contribute to cognitive functions related to the processing of auditory stimuli, including normal auditory perception and adaption to changes in spatial cues (Leach et al., 2013). Furthermore, the central auditory pathway, including the inferior colliculus and nuclei of the lateral lemniscus, but not the cochlear nucleus, show significantly reduced ChAT activity in aged Fischer-344 rats (Raza et al., 1994). A significant decrease in muscarinic receptors, but not ChAT activity, in the dorsal hippocampi of aged rats has also been observed (Lippa et al., 1980). Moreover, noise-induced hyperactivity in fusiform cells of the dorsal cochlear nucleus of adult male Syrian golden hamsters has been shown to be inhibited by the cholinergic agonist carbachol (Manzoor et al., 2013). There is also evidence in experimental animals that chronic inflammation contributes to the dysfunction of auditory pathways (Haase et al., 2011; Menardo et al., 2012; Tan et al., 2016). Acute and chronic noise exposure in C57BL/6 mice (Tan et al., 2016) and senescence-accelerated mouse prone 8 mice (Menardo et al., 2012) also results in increased inflammatory responses in the cochlea.
Chronic inflammation leads to BBB dysfunction and increased vascular permeability during aging, as well as in AD and other neurodegenerative disorders (Zlokovic, 2011; Takeda et al., 2014; Erdő et al., 2017). Increased vascular permeability facilitates the spread of peripheral inflammation into the brain and causes more severe non-cognitive symptoms in AD animal models (Takeda et al., 2013), as well as brain hypoperfusion (Zlokovic, 2011; Takeda et al., 2013). A prominent alteration following BBB breakdown is the decrease in the levels of tight junction proteins, which has been observed in an aging animal model and dementia-related diseases (Zlokovic, 2008; Kalaria, 2010; Ruan et al., 2014d).
Loss of cholinergic input during aging and neurodegenerative diseases causes decreased ACh release and brain hypoperfusion. Reduced sensory input can also lead to decreased ACh release in the neocortex and hippocampus (Penschuck et al., 2002), and decreased hippocampal blood flow (Cao et al., 1992). Hypoxia and ischemia clearly contribute to the pathogenesis of sensorineural tinnitus, and some agents can effectively suppress tinnitus by improving the blood supply and inhibiting chronic inflammatory damage in the acute stage (Mazurek et al., 2006). CBF reductions and hypoxia may not only result in the accumulation of hyperphosphorylated tau and filament formation in experimental animals (Gordon-Krajcer et al., 2007), but also cause increased β-secretase transcription (Zhang et al., 2007), decreased Aβ clearance due to loss or oxidization of lipoprotein receptors in endothelial cells and astrocytes (Bell et al., 2009; Owen et al., 2010), Reduced glutamate reuptake by astrocytes (Boycott et al., 2007), and the accumulation of oxidative damage in the vascular endothelium and high metabolic neurons (Fernández-Checa et al., 2010; Figure 1).
Based on animal research, we hypothesize that the cholinergic anti-inflammation mediated by α7nAChR may be one potential mechanism by which hearing loss occurs with tinnitus or cognitive impairment. AChE inhibitors might suppress presbycusis accompanied by tinnitus and may indirectly protect auditory and cognitive function by activating α7nAChR-mediated anti-inflammatory effects in various cells of the brain’s neural vascular unit. This might include the suppression of glial and endothelial activation, neuroinflammation, tau-induced neurotoxicity and decreased gap junctions, as well as improved glutamate and extracellular potassium reuptake by astrocytes. These effects inhibit network hyperexcitability and excitotoxicity in the auditory pathway (Figure 1).
Conclusion
Presbycusis is a risk factor for tinnitus and cognitive decline. Cholinergic hypofunction might be a major contributor to presbycusis-related tinnitus and age-related cognitive impairment. Cholinergic denervation in the CNS, might lead to the reduction of both inhibition by NPY neurogliaform cells and cholinergic anti-inflammatory effects on the neural vascular unit mediated by nAChRs, as well as suppression of GSK3β activity and tau-induced neurodegeneration.
Implementing VNS and AChE inhibitors alone or in combination with other hearing rehabilitative interventions during the optimal time window may lead to greater disease-modifying benefits in the treatment of presbycusis-related tinnitus with cognitive impairment. However, in the evidence reviewed here, data have mainly been obtained from animal experiments. Age-related hearing loss and AD in humans become apparent very slowly, and are associated with a long preclinical period. Therefore, animal models with a life expectancy of approximately 3 years are not really comparable to humans with these disorders. Further studies are required to elucidate the roles played by M or N cholinergic neuromodulation and distinct GABAergic cell types in the pathophysiological process. Furthermore, it must be investigated whether mechanisms underlying peripheral and central cholinergic regulation are the same.
The potential relationship between tinnitus and depressive systems or affective disorders, and the mechanisms underlying this, should also be investigated in rodents. In addition, dynamic changes in CNS-derived biomarkers of cholinergic hypofunction and neuronal impairment in peripheral body fluids should be investigated as possible screening tools for preclinical or early stage disease, predictors of diagnosis, predictors of intervention outcomes. Finally, innovative, specific and selective neuromodulatory methods and multi-center longitudinal cohort studies are also urgently needed.
Author Contributions
QR and ZY designed the study and analyzed the data. QR, ZY, WZ, JR, CL and RZ provided a consensus agreement on the final hypotheses and drafted the initial version of the manuscript. WZ, JR and QR collected the data. All authors contributed to the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the Shanghai Hospital Development Center (No. SHDC12014221), Shanghai Municipal Commission of Health and Family Planning, Key developing disciplines (2015ZB0501).
References
Acar, B., Yurekli, M. F., Babademez, M. A., Karabulut, H., and Karasen, R. M. (2011). Effects of hearing aids on cognitive functions and depressive signs in elderly people. Arch. Gerontol. Geriatr. 52, 250–252. doi: 10.1016/j.archger.2010.04.013
Adams, P. F., Hendershot, G. E., and Marano, M. A. (1999). Current estimates from the National Health Interview Survey, 1996. Vital Health Stat. 10, 1–203.
Ahmad, N., and Seidman, M. (2004). Tinnitus in the older adult: epidemiology, pathophysiology and treatment options. Drugs Aging 21, 297–305. doi: 10.2165/00002512-200421050-00002
Ahmadian, S. S., Rezvanian, A., Peterson, M., Weintraub, S., Bigio, E. H., Mesulam, M. M., et al. (2015). Loss of calbindin-D28K is associated with the full range of tangle pathology within basal forebrain cholinergic neurons in Alzheimer’s disease. Neurobiol. Aging 36, 3163–3170. doi: 10.1016/j.neurobiolaging.2015.09.001
Andersson, G., Lyttkens, L., and Larsen, H. C. (1999). Distinguishing levels of tinnitus distress. Clin. Otolaryngol. Allied Sci. 24, 404–410. doi: 10.1046/j.1365-2273.1999.00278.x
Andersson, G., and McKenna, L. (2006). The role of cognition in tinnitus. Acta Otolaryngol. Suppl. 556, 39–43. doi: 10.1080/03655230600895226
Bakhos, D., Villeuneuve, A., Kim, S., Hammoudi, K., and Hommet, C. (2015). Hearing loss and Alzheimer’s disease. Geriatr. Psychol. Neuropsychiatr. Vieil. 13, 195–204. doi: 10.1684/pnv.2015.0539
Baloyannis, S. J., Manolidis, S. L., and Manolidis, L. S. (2001). The acoustic cortex in frontal dementia. Acta Otolaryngol. 121, 289–292. doi: 10.1080/000164801300043884
Banay-Schwartz, M., Lajtha, A., and Palkovits, M. (1989). Changes with aging in the levels of amino acids in rat CNS. structural elements. II. Taurine and small neutral amino acids. Neurochem. Res. 14, 563–570. doi: 10.1007/bf00964919
Behrman, S., Chouliaras, L., and Ebmeier, K. P. (2014). Considering the senses in the diagnosis and management of dementia. Maturitas 77, 305–310. doi: 10.1016/j.maturitas.2014.01.003
Bell, R. D., Deane, R., Chow, N., Long, X., Sagare, A., Singh, I., et al. (2009). SRF and myocardin regulate LRPmediated amyloidβ clearance in brain vascular cells. Nat. Cell Biol. 11, 143–153. doi: 10.1038/ncb1819
Benzing, W. C., Mufson, E. J., and Armstrong, D. M. (1993). Immunocytochemical distribution of peptidergic and cholinergic fibers in the human amygdala: their depletion in Alzheimer’s disease and morphologic alteration in non-demented elderly with numerous senile plaques. Brain Res. 625, 125–138. doi: 10.1016/0006-8993(93)90145-d
Berkeley, J. L., Gomeza, J., Wess, J., Hamilton, S. E., Nathanson, N. M., and Levey, A. I. (2001). M1 muscarinic acetylcholine receptors activate extracellular signal-regulated kinase in CA1 pyramidal neurons in mouse hippocampal slices. Mol. Cell. Neurosci. 18, 512–524. doi: 10.1006/mcne.2001.1042
Bitner, R. S., Nikkel, A. L., Markosyan, S., Otte, S., Puttfarken, P., and Gopalakrishnan, M. (2009). Selective α7 nicotinic acetylcholine receptor activation regulates glycogen synthase kinase3β and decreases tau phosphorylation in vivo. Brain Res. 1265, 65–74. doi: 10.1016/j.brainres.2009.01.069
Blom, J. D., Coebergh, J. A., Lauw, R., and Sommer, I. E. (2015). Musical hallucinations treated with acetylcholinesterase inhibitors. Front. Psychiatry 6:46. doi: 10.3389/fpsyt.2015.00046
Blom, J. D., Sommer, I. E., Koops, S., and Sacks, O. W. (2014). Prosopometamorphopsia and facial hallucinations. Lancet 384:1998. doi: 10.1016/s0140-6736(14)61690-1
Boissière, F., Lehéricy, S., Strada, O., Agid, Y., and Hirsch, E. C. (1996). Neurotrophin receptors and selective loss of cholinergic neurons in Alzheimer disease. Mol. Chem. Neuropathol. 28, 219–223. doi: 10.1007/bf02815225
Bojić, T., Perović, V. R., Senćanski, M., and Glišić, S. (2017). Identification of candidate allosteric modulators of the M1 muscarinic acetylcholine receptor which may improve vagus nerve stimulation in chronic tinnitus. Front. Neurosci. 11:636. doi: 10.3389/fnins.2017.00636
Bott, J. B., Héraud, C., Cosquer, B., Herbeaux, K., Aubert, J., Sartori, M., et al. (2016). Apoe-sensitive cholinergic sprouting compensates for hippocampal dysfunctions due to reduced entorhinal input. J. Neurosci. 36, 10472–10486. doi: 10.1523/JNEUROSCI.1174-16.2016
Boycott, H. E., Dallas, M., Boyle, J. P., Pearson, H. A., and Peers, C. (2007). Hypoxia suppresses astrocyte glutamate transport independently of amyloid formation. Biochem. Biophys. Res. Commun. 364, 100–104. doi: 10.1016/j.bbrc.2007.09.102
Buhler, A. V., and Dunwiddie, T. V. (2002). α7 nicotinic acetylcholine receptors on GABAergic interneurons evoke dendritic and somatic inhibition of hippocampal neurons. J. Neurophysiol. 87, 548–557. doi: 10.1152/jn.00316.2001
Cao, W. H., Sato, A., Sato, Y., and Zhou, W. (1992). Somatosensory regulation of regional hippocampal blood flow in anesthetized rats. Jap. J. Physiol. 42, 731–740. doi: 10.2170/jjphysiol.42.731
Caspary, D. M., Hughes, L. F., and Ling, L. L. (2013). Age-related GABAA receptor changes in rat auditory cortex. Neurobiol. Aging 34, 1486–1496. doi: 10.1016/j.neurobiolaging.2012.11.009
Cheng, L., Wang, S. H., Chen, Q. C., and Liao, X. M. (2011). Moderate noise induced cognition impairment of mice and its underlying mechanisms. Physiol. Behav. 104, 981–988. doi: 10.1016/j.physbeh.2011.06.018
Cheng, L., Wang, S. H., Huang, Y., and Liao, X. M. (2016). The hippocampus may be more susceptible to environmental noise than the auditory cortex. Hear. Res. 333, 93–97. doi: 10.1016/j.heares.2016.01.001
Clader, J. W., and Wang, Y. (2005). Muscarinic receptor agonists and antagonists in the treatment of Alzheimer’s disease. Curr. Pharm. Des. 11, 3353–3361. doi: 10.2174/138161205774370762
Cohen, B. M., Renshaw, P. F., Stoll, A. L., Wurtman, R. J., Yurgelun-Todd, D., and Babb, S. M. (1995). Decreased brain choline uptake in older adults. An in vitro proton magnetic resonance spectroscopy study. JAMA 274, 902–907. doi: 10.1001/jama.1995.03530110064037
Cui, B., Zhu, L., She, X., Wu, M., Ma, Q., Wang, T., et al. (2012). Chronic noise exposure causes persistence of tauhyperphosphorylation and formation of NFT tau in the rat hippocampus and prefrontal cortex. Exp. Neurol. 238, 122–129. doi: 10.1016/j.expneurol.2012.08.028
Damar, U., Gersner, R., Johnstone, J. T., Schachter, S., and Rotenberg, A. (2017). Huperzine A: a promising anticonvulsant, disease modifying, and memory enhancing treatment option in Alzheimer’s disease. Med. Hypotheses 99, 57–62. doi: 10.1016/j.mehy.2016.12.006
Daulatzai, M. A. (2010). Early stages of pathogenesis in memory impairment during normal senescence and Alzheimer’s Disease. J. Alzheimers Dis. 20, 355–367. doi: 10.3233/JAD-2010-1374
Davies, P. (1979). Neurotransmitter-related enzymes in senile dementia of the Alzheimer type. Brain Res. 171, 319–327. doi: 10.1016/0006-8993(79)90336-6
Davies, K. L., Mohs, R. C., Marin, D., Purohit, D. P., Perl, D. P., Lantz, M., et al. (1999). Cholinergic markers in the elderly patients with early signs of Alzheimer’s disease. JAMA 281, 1401–1406. doi: 10.1001/jama.281.15.1401
Deal, J. A., Betz, J., Yaffe, K., Harris, T., Purchase-Helzner, E., Satterfield, S., et al. (2017). Hearing impairment and incident dementia and cognitive decline in older adults: the health ABC study. J. Gerontol. A Biol. Sci. Med. Sci. 72, 703–709. doi: 10.1093/gerona/glw069
Drachman, D. A., Noffsinger, D., Sahakian, B. J., Kurdziel, S., and Fleming, P. (1980). Aging, memory, and the cholinergic system: a study of dichotic listening. Neurobiol. Aging 1, 39–43. doi: 10.1016/0197-4580(80)90022-6
Elgoyhen, A. B., Katz, E., and Fuchs, P. A. (2009). The nicotinic receptor of cochlear hair cells: a possible pharmacotherapeutic target? Biochem. Pharmacol. 78, 712–719. doi: 10.1016/j.bcp.2009.05.023
Elgoyhen, A. B., Langguth, B., De Ridder, D., and Vanneste, S. (2015). Tinnitus: perspectives from human neuroimaging. Nat. Rev. Neurosci. 16, 632–642. doi: 10.1038/nrn4003
Emre, M., Heckers, S., Mash, D. C., Geula, C., and Mesulam, M. M. (1993). Cholinergic innervation of the amygdaloid complex in the human brain and its alterations in old age and Alzheimer’s disease. J. Comp. Neurol. 336, 117–134. doi: 10.1002/cne.903360110
Engelhart, M. J., Geerlings, M. I., Meijer, J., Kiliaan, A., Ruitenberg, A., van Swieten, J. C., et al. (2004). Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch. Neurol. 61, 668–672. doi: 10.1001/archneur.61.5.668
Engidawork, E., Gulesserian, T., Balic, N., Cairns, N., and Lubec, G. (2001). Changes in nicotinic acetylcholine receptor subunits expression in brain of patients with down syndrome and Alzheimer’s disease. J. Neural. Transm. Suppl. 61, 211–222. doi: 10.1007/978-3-7091-6262-0_17
Engineer, N. D., Møller, A. R., and Kilgard, M. P. (2013). Directing neural plasticity to understand treat tinnitus. Hear. Res. 295, 58–66. doi: 10.1016/j.heares.2012.10.001
Erdő, F., Denes, L., and de Lange, E. (2017). Age-associated physiological and pathological changes at the blood-brain barrier: a review. J. Cereb. Blood Flow Metab. 37, 4–24. doi: 10.1177/0271678x16679420
Farrall, A. J., and Wardlaw, J. M. (2009). Blood-brain barrier: ageing and microvascular disease—systematic review and meta-analysis. Neurobiol. Aging 30, 337–352. doi: 10.1016/j.jns.2009.02.089
Faust, T. W., Assous, M., Shah, F., Tepper, J. M., and Koos, T. (2015). Novel fast adapting interneurons mediate cholinergicinduced fast GABAA inhibitory postsynaptic currents in striatal spiny neurons. Eur. J. Neurosci. 42, 1764–1774. doi: 10.1111/ejn.12915
Fernández-Checa, J. C., Fernández, A., Morales, A., Marí, M., García-Ruiz, C., and Colell, A. (2010). Oxidative stress and altered mitochondrial function in neurodegenerative diseases: lessons from mouse models. CNS Neurol. Disord. Drug Targets. 9, 439–454. doi: 10.2174/187152710791556113
Flynn, D. D., Ferrari-DiLeo, G., Levey, A. I., and Mash, D. C. (1995). Differential alterations in muscarinic receptor subtypes in Alzheimer’s disease: implications for cholinergic-based therapies. Life Sci. 56, 869–876. doi: 10.1016/0024-3205(95)00022-x
Fortunato, S., Forli, F., Guglielmi, V., De Corso, E., Paludetti, G., Berrettini, S., et al. (2016). A review of new insights on the association between hearing loss and cognitive decline in ageing. Acta Otorhinolaryngol. Ital. 36, 155–166. doi: 10.14639/0392-100X-993
Foster, D. J., Choi, D. L., Conn, P. J., and Rook, J. M. (2014). Activation of M1 and M4 muscarinic receptors as potential treatments for Alzheimer’s disease and schizophrenia. Neuropsychiatr. Dis. Treat. 10, 183–191. doi: 10.2147/NDT.s55104
Freund-Levi, Y., Jedenius, E., Tysen-Bäckström, A. C., Lärksäter, M., Wahlund, L. O., Eriksdotter, M., et al. (2014). Galantamine versus risperidone treatment of neuropsychiatric symptoms in patients with probable dementia: an open randomized trial. Am. J. Geriatr. Psychiatry 22, 341–348. doi: 10.1016/j.jagp.2013.05.005
Gallacher, J., Ilubaera, V., Ben-Shlomo, Y., Bayer, A., Fish, M., Babisch, W., et al. (2012). Auditory threshold, phonologic demand incident dementia. Neurology 79, 1583–1590. doi: 10.1212/WNL.0b013e31826e263d
Gates, G. A., Anderson, M. L., McCurry, S. M., Feeney, M. P., and Larson, E. B. (2011). Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch. Otolaryngol. Head Neck Surg. 137, 390–395. doi: 10.1001/archoto.2011.28
Gates, G. A., Beiser, A., Rees, T. S., D’Agostino, R. B., and Wolf, P. A. (2002). Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J. Am. Geriatr. Soc. 50, 482–488. doi: 10.1046/j.1532-5415.2002.50114.x
Gates, G. A., and Mills, J. H. (2005). Presbycusis. Lancet 366, 1111–1120. doi: 10.1016/S0140-6736(05)67423-5
Gennis, V., Garry, P. J., Haaland, K. Y., Yeo, R. A., and Goodwin, J. S. (1991). Hearing and cognition in the elderly. New findings and a review of the literature. Arch. Intern. Med. 151, 2259–2264. doi: 10.1001/archinte.151.11.2259
Gersner, R., Ekstein, D., Dhamne, S. C., Schachter, S. C., and Rotenberg, A. (2015). Huperzine a prophylaxis against pentylenetetrazole-induced seizures in rats is associated with increased cortical inhibition. Epilepsy Res. 117, 97–103. doi: 10.1016/j.eplepsyres.2015.08.012
Gitelman, D. R., and Prohovnik, I. (1992). Muscarinic and nicotinic contributions to cognitive function and cortical blood flow. Neurobiol. Aging 13, 313–318. doi: 10.1016/0197-4580(92)90044-x
Golob, E. J., Miranda, G. G., Johnson, J. K., and Starr, A. (2015). Sensory cortical interactions in aging, mild cognitive impairment, and Alzheimer’s disease. Neurobiol. Aging 22, 755–763. doi: 10.1016/s0197-4580(01)00244-5
Golub, J. S., Luchsinger, J. A., Manly, J. J., Stern, Y., Mayeux, R., and Schupf, N. (2017). Observed hearing loss and incident dementia in a multiethnic cohort. J. Am. Geriatr. Soc. 65, 1691–1697. doi: 10.1111/jgs.14848
González, J. C., Albiñana, E., Baldelli, P., García, A. G., and Hernández-Guijo, J. M. (2011). Presynaptic muscarinic receptor subtypes involved in the enhancement of spontaneous GABAergic postsynaptic currents in hippocampal neurons. Eur. J. Neurosci. 33, 69–81. doi: 10.1111/j.1460-9568.2010.07475.x
Gordon-Krajcer, W., Kozniewska, E., Lazarewicz, J. W., and Ksiezak-Reding, H. (2007). Differential changes in phosphorylation of tau at PHF1 and 12E8 epitopes during brain ischemia and reperfusion in gerbils. Neurochem. Res. 32, 729–737. doi: 10.1007/s11064-006-9199-3
Grammas, P. (2011). Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J. Neuroinflammation 8:26. doi: 10.1186/1742-2094-8-26
Guan, Z. Z., Zhang, X., Ravid, R., and Nordberg, A. (2000). Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer’s disease. J. Neurochem. 74, 237–243. doi: 10.1046/j.1471-4159.2000.0740237.x
Gurgel, R. K., Ward, P. D., Schwartz, S., Norton, M. C., Foster, N. L., and Tschanz, J. T. (2014). Relationship of hearing loss and dementia: a prospective, population-based study. Otol. Neurotol. 35, 775–781. doi: 10.1097/MAO.0000000000000313
Haase, G. M., Prasad, K. N., Cole, W. C., Baggett-Strehlau, J. M., and Wyatt, S. E. (2011). Antioxidant micronutrient impact on hearing disorders: concept, rationale, and evidence. Am. J. Otolaryngol. 32, 55–61. doi: 10.1016/j.amjoto.2009.09.002
Hallam, R. S., McKenna, L., and Shurlock, L. (2004). Tinnitus impairs cognitive efficiency. Int. J. Audiol. 43, 218–226. doi: 10.1080/14992020400050030
Han, Z., Li, L., Wang, L., Degos, V., Maze, M., and Su, H. (2014). α-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress, and brain injury in mice with ischemic stroke and bone fracture. J. Neurochem. 131, 498–508. doi: 10.1111/jnc.12817
Hays, S. A. (2016). Enhancing rehabilitative therapies with vagus nerve stimulation. Neurotherapeutics 13, 382–394. doi: 10.1007/s13311-015-0417-z
Hernandez, C. M., Kayed, R., Zheng, H., Sweatt, J. D., and Dineley, K. T. (2010). Loss of α7 nicotinic receptors enhances β-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septo-hippocampal pathology in a mouse model of Alzheimer’s disease. J. Neurosci. 30, 2442–2453. doi: 10.1523/JNEUROSCI.5038-09.2010
Hollands, S., Lim, Y. Y., Laws, S. M., Villemagne, V. L., Pietrzak, R. H., Harrington, K., et al. (2017). APOEε4 genotype, amyloid, and clinical disease progression in cognitively normal older adults. J. Alzheimers Dis. 57, 411–422. doi: 10.3233/JAD-161019
Iaccarino, H. F., Singer, A. C., Martorell, A. J., Rudenko, A., Gao, F., Gillingham, T. Z., et al. (2016). γ frequency entrainment attenuates amyloid load and modifies microglia. Nature 540, 230–235. doi: 10.1038/nature20587
Iqbal, K., Gong, C. X., and Liu, F. (2013). Hyperphosphorylation-induced tau oligomers. Front Neurol. 4:112. doi: 10.3389/fneur.2013.00112
Ittner, L. M., Ke, Y. D., Delerue, F., Bi, M., Gladbach, A., van Eersel, J., et al. (2010). Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell 142, 387–397. doi: 10.1016/j.cell.2010.06.036
Iwamoto, K., Mata, D., Linn, D. M., and Linn, C. L. (2013). Neuroprotection of rat retinal ganglion cells mediated through α7 nicotinic acetylcholine receptors. Neuroscience 237, 184–198. doi: 10.1016/j.neuroscience.2013.02.003
Kalappa, B. I., Brozoski, T. J., Turner, J. G., and Caspary, D. M. (2014). Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J. Physiol. 592, 5065–5078. doi: 10.1113/jphysiol.2014.278572
Kalaria, R. N. (2010). Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr. Rev. 68, S74–S87. doi: 10.1111/j.1753-4887.2010.00352.x
Karami, A., Eyjolfsdottir, H., Vijayaraghavan, S., Lind, G., Almqvist, P., Kadir, A., et al. (2015). Changes in CSF cholinergic biomarkers in response to cell therapy with ngf in patients with Alzheimer’s disease. Alzheimers Dement. 11, 1316–1328. doi: 10.1016/j.jalz.2014.11.008
Kat, M. G., Vreeswijik, R., de Jonghe, J. F., van der Ploeg, T., van Gool, W. A., Eikelenboom, P., et al. (2008). Long-term cognitive outcome of delirium in elderly hip surgery patients. A prospective matched controlled study over two and a half years. Dement. Geriatr. Cogn. Disord. 26, 1–8. doi: 10.1159/000140611
Kelso, M. L., and Oestreich, J. H. (2012). Traumatic brain injury: central and peripheral role of α7 nicotinic acetylcholine receptors. Curr. Drug Targets 13, 631–636. doi: 10.2174/138945012800398964
Knipper, M., Van Dijk, P., Nunes, I., Rüttiger, L., and Zimmermann, U. (2013). Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog. Neurobiol. 111, 17–33. doi: 10.1016/j.pneurobio.2013.08.002
Kurniawan, C., Westendorp, R. G., de Craen, A. J., Gussekloo, J., de Laat, J., and van Exel, E. (2012). Gene dose of apolipoprotein E and age-related hearing loss. Neurobiol. Aging 33, 2230.e7–2230.e12. doi: 10.1016/j.neurobiolaging.2012.04.001
Langguth, B., Kreuzer, P. M., Kleinjung, T., and De Ridder, D. (2013). Tinnitus: causes and clinical management. Lancet Neurol. 12, 920–930. doi: 10.1016/S1474-4422(13)70160-1
Lawrence, A. D., and Sahakian, B. J. (1995). Alzheimer disease, attention, and the cholinergic system. Alzheimer Dis. Assoc. Disord. 9, 43–49. doi: 10.1097/00002093-199501002-00008
Leach, N. D., Nodal, F. R., Cordery, P. M., King, A. J., and Bajo, V. M. (2013). Cortical cholinergic input is required for normal auditory perception and experience-dependent plasticity in adult ferrets. J. Neurosci. 33, 6659–6671. doi: 10.1523/JNEUROSCI.5039-12.2013
Lee, D. H., Dandrea, M. R., Plata-Salaman, C. R., and Wang, H. Y. (2000). Decreased a7 nicotinic acetylcholine receptor protein levels in sporadic Alzheimers disease hippocampus. Alzheimer Rep. 3, 217–220.
Limon, A., Reyes-Ruiz, J. M., and Miledi, R. (2012). Loss of functional GABAA receptors in the Alzheimer diseased brain. Proc. Natl. Acad. Sci. U S A 109, 10071–10076. doi: 10.1073/pnas.1204606109
Lin, F. R. (2011). Hearing loss and cognition among older adults in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 66, 1131–1136. doi: 10.1093/gerona/glr115
Lin, F. R., Thorpe, R., Gordonsalant, S., and Ferrucci, L. (2011). Hearing loss prevalence and risk factors among older adults in the united states. J. Gerontol. A Biol. Sci. Med. Sci. 66, 582–590. doi: 10.1093/gerona/glr002
Lin, F. R., Yaffe, K., Xia, J., Xue, Q. L., Harris, T. B., Purchase-Helzner, E., et al. (2013). Hearing loss and cognitive decline in older adults. JAMA Intern. Med. 173, 293–299. doi: 10.1001/jamainternmed.2013.1868
Ling, L. L., Hughes, L. F., and Caspary, D. M. (2005). Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience 132, 1103–1113. doi: 10.1016/j.neuroscience.2004.12.043
Linton, A. (2005). The benefits of cholinesterase inhibitors managing the behavioral and neuropsychiatric symptom of Alzheimer’s disease. J. Gerontol. Nurs. 31, 4–10. doi: 10.3928/0098-9134-20051201-04
Lippa, A. S., Pelham, R. W., Beer, B., Critchett, D. J., Dean, R. L., and Bartus, R. T. (1980). Brain cholinergic dysfunction and memory in aged rats. Neurobiol. Aging 1, 13–19. doi: 10.1016/0197-4580(80)90019-6
Loughrey, D. G., Kelly, M. E., Kelley, G. A., Brennan, S., and Lawlor, B. A. (2018). Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol. Head Neck Surg. 144, 115–126. doi: 10.1001/jamaoto.2017.2513
Lunardi, P., Nardin, P., Guerra, M. C., Abib, R., Leite, M. C., and Gonçalves, C. A. (2013). Huperzine A, but not tacrine, stimulates S100B secretion in astrocyte cultures. Life Sci. 92, 701–707. doi: 10.1016/j.lfs.2013.01.029
Maison, S. F., Luebke, A. E., Liberman, M. C., and Zuo, J. (2002). Efferent protection from acoustic injury is mediated via alpha9 nicotinic acetylcholine receptors on outer hair cells. J. Neurosci. 22, 10838–10846.
Maloku, E., Kadriu, B., Zhubi, A., Dong, E., Pibiri, F., Satta, R., et al. (2011). Selective α4β2 nicotinic acetylcholine receptor agonists target epigenetic mechanisms in cortical GABAergic neurons. Neuropsychopharmacology 36, 1366–1374. doi: 10.1038/npp.2011.21
Manzoor, N. F., Chen, G., and Kaltenbach, J. A. (2013). Suppression of noise-induced hyperactivity in the dorsal cochlear nucleus following application of the cholinergic agonist, carbachol. Brain Res. 1523, 28–36. doi: 10.1016/j.brainres.2013.05.025
Marti, A., Castiglione, A., Bovo, R., Vallesi, A., and Gabelli, C. (2014). Aging, cognitive load, dementia and hearing loss. Audiol. Neurotol. 19, 2–5. doi: 10.1159/000371593
Martin, A. J., Friston, K. J., Colebatch, J. G., and Frackowiak, R. S. (1991). Decreases in regional cerebral blood flow with normal aging. J. Cereb. Blood Flow Metab. 11, 684–689. doi: 10.1038/jcbfm.1991.121
Mazarati, A., and Wasterlain, C. G. (2002). Anticonvulsant effects of four neuropeptides in the rat hippocampus during self-sustaining status epilepticus. Neurosci. Lett. 331, 123–127. doi: 10.1016/s0304-3940(02)00847-9
Mazurek, B., Haupt, H., and Gross, J. (2006). Pharmacotherapy in acute tinnitis. The special role of hypoxia and ischemia in the pathogenesis of tinnitus. HNO 54, 9–15. doi: 10.1007/s00106-005-1292-4
Menardo, J., Tang, Y., Ladrech, S., Lenoir, M., Casas, F., Michel, C., et al. (2012). Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse Cochlea. Antioxid. Redox Signal. 16, 263–274. doi: 10.1089/ars.2011.4037
Mencel, M., Nash, M., and Jacobson, C. (2013). Neuregulin upregulates microglial α7 nicotinic acetylcholine receptor expression in immortalized cell lines: implications for regulating neuroinflammation. PLoS One 8:e70338. doi: 10.1371/journal.pone.0070338
Milbrandt, J. C., Albin, R. L., and Caspary, D. M. (1994). Age-related decrease in GABAB receptor binding in the Fischer 344 rat inferior colliculus. Neurobiol. Aging 15, 699–703. doi: 10.1016/0197-4580(94)90051-5
Milbrandt, J. C., Holder, T. M., Wilson, M. C., Salvi, R. J., and Caspary, D. M. (2000). GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear. Res. 147, 251–260. doi: 10.1016/s0378-5955(00)00135-0
Mufson, E. J., and Kordower, J. H. (1989). Nerve growth factor receptor expressing human basal forebrain neurons: pathologic alterations in Alzheimer’s and Parkinson’s disease. Prog. Clin. Biol. Res. 317, 401–414.
Muñoz, W., Tremblay, R., Levenstein, D., and Rudy, B. (2017). Layer-specific modulation of neocortical dendritic inhibition during active wakefulness. Science 355, 954–959. doi: 10.1126/science.aag2599
Nägerl, U. V., Mody, I., Jeub, M., Lie, A. A., Elger, C. E., and Beck, H. (2000). Surviving granule cells of the sclerotic human hippocampus have reduced Ca2+ influx because of a loss of calbindin-D28K in temporal lobe epilepsy. J. Neurosci. 20, 1831–1836.
Noh, M. Y., Koh, S. H., Kim, Y., Kim, H. Y., Cho, G. W., and Kim, S. H. (2009). Neuroprotective effects of donepezil against Aβ42-induced neuronal toxicity are mediated through not only enhancing PP2A activity but also regulating GSK-3β and nAChRs activity. J. Neurochem. 108, 1116–1125. doi: 10.1111/j.1471-4159.2008.05837.x
Nordberg, A., Lundkvist, H., Hartvig, P., Andersson, J., Johansson, M., Hellstrom-Lindahl, E., et al. (1997). Imaging of nicotinic and muscarinic receptors in Alzheimer’s disease: effect of tacrine treatment. Dement. Geriatr. Cogn. Disord. 8, 78–84. doi: 10.1159/000106611
O’Mahony, D., Rowan, M., Feely, J., Walsh, J. B., and Coakley, D. (1994). Primary auditory pathway and reticular activating system dysfunction in Alzheimer’s disease. Neurology 44, 2089–2094. doi: 10.1212/WNL.44.11.2089
Olney, J. W. (1995). NMDA receptor hypofunction, excitotoxicity, and Alzheimer’s disease. Neurobiol. Aging 16, 459–461. doi: 10.1016/0197-4580(94)00185-4
Overstreet-Wadiche, L., and McBain, C. J. (2015). Neurogliaform cells in cortical circuits. Nat. Rev. Neurosci. 16, 458–468. doi: 10.1038/nrn3969
Owen, J. B., Sultana, R., Aluise, C. D., Erickson, M. A., Price, T. O., Bu, G., et al. (2010). Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Aβ accumulation in AD brain. Free Radic. Biol. Med. 49, 1798–1803. doi: 10.1016/j.freeradbiomed.2010.09.013
Palop, J. J., Chin, J., Roberson, E. D., Wang, J., Thwin, M. T., Bien-Ly, N., et al. (2007). Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55, 697–711. doi: 10.1016/j.neuron.2007.07.025
Palop, J. J., and Mucke, L. (2009). Epilepsy and cognitive impairments in Alzheimer disease. Arch. Neurol. 66, 435–440. doi: 10.1001/archneurol.2009.15
Panza, F., Solfrizzi, V., and Logroscino, G. (2015a). Age-related hearing impairment-a risk factor and frailty marker for dementia and AD. Nat. Rev. Neurol. 11, 166–175. doi: 10.1038/nrneurol.2015.12
Panza, F., Solfrizzi, V., Seripa, D., Imbimbo, B. P., Capozzo, R., Quaranta, N., et al. (2015b). Age-related hearing impairment and frailty in Alzheimer’s disease: interconnected associations and mechanisms. Front. Aging Neurosci. 7:113. doi: 10.3389/fnagi.2015.00113
Panza, F., Quaranta, N., and Logroscino, G. (2017). Sensory changes and the hearing loss-cognition link: the cognitive ear. JAMA Otolaryngol. Head Neck Surg. 144, 127–128. doi: 10.1001/jamaoto.2017.2514
Pavlov, V. A., and Tracey, K. J. (2006). Controlling inflammation: the cholinergic anti-inflammatory pathway. Biochem. Soc. Trans. 34, 1037–1040. doi: 10.1042/bst0341037
Penschuck, S., Chen-Bee, C. H., Prakash, N., and Frostig, R. D. (2002). In vivo modulation of a cortical functional sensory representation shortly after topical cholinergic agent application. J. Comp. Neurol. 452, 38–50. doi: 10.1002/cne.10361
Perry, E. K. (1990). Nerve growth factor and the basal forebrain cholinergic system: a link in the etiopathology of neurodegenerative dementias? Alzheimer Dis. Assoc. Disord. 4, 1–13. doi: 10.1097/00002093-199040100-00001
Perry, E. K., Tomlinson, B. E., Blessed, G., Bergman, K., Gibson, P. H., and Perry, R. H. (1978). Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br. Med. J. 2, 1457–1459. doi: 10.1136/bmj.2.6150.1457
Pierce, K. J., Kallogjeri, D., Piccirillo, J. F., Garcia, K. S., Nicklaus, J. E., and Burton, H. (2012). Effects of severe bothersome tinnitus on cognitive function measured with standardized tests. J. Clin. Exp. Neuropsychol. 34, 126–134. doi: 10.1080/13803395.2011.623120
Pitler, T. A., and Alger, B. E. (1992). Cholinergic excitation of GABAergic interneurons in the rat hippocampal slice. J. Physiol. 450, 127–142. doi: 10.1113/jphysiol.1992.sp019119
Poulin, B., Butcher, A., McWilliams, P., Bourgognon, J. M., Pawlak, R., Kong, K. C., et al. (2010). The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc. Natl. Acad. Sci. U S A 107, 9440–9445. doi: 10.1073/pnas.0914801107
Rafii, M. S., Walsh, S., Little, J. T., Behan, K., Reynolds, B., Ward, C., et al. (2011). A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology 76, 1389–1394. doi: 10.1212/WNL.0b013e318216eb7b
Ragozzino, M. E., Artis, S., Singh, A., Twose, T. M., Beck, J. E., and Messer, W. S. Jr. (2012). The selective M1 muscarinic cholinergic agonist CDD-0102A enhances working memory and cognitive flexibility. J. Pharmacol. Exp. Ther. 340, 588–594. doi: 10.1124/jpet.111.187625
Raza, A., Milbrandt, J. C., Arneric, S. P., and Caspary, D. M. (1994). Age-related changes in brainstem auditory neurotransmitters: measures of GABA and acetylcholine function. Hear. Res. 77, 221–230. doi: 10.1016/0378-5955(94)90270-4
Roberson, E. D., Halabisky, B., Yoo, J. W., Yao, J., Chin, J., Yan, F., et al. (2011). Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on Tau levels in multiple mouse models of Alzheimer’s disease. J. Neurosci. 31, 700–711. doi: 10.1523/JNEUROSCI.4152-10.2011
Roberson, E. D., Scearce-Levie, K., Palop, J. J., Yan, F., Cheng, I. H., Wu, T., et al. (2007). Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science 316, 750–754. doi: 10.1126/science.1141736
Rossiter, S., Stevens, C., and Walker, G. (2006). Tinnitus and its effect on working memory and attention. J. Speech Lang. Hear. Res. 49, 150–160. doi: 10.1044/1092-4388(2006/012)
Ruan, Q., Ao, H., He, J., Chen, Z., Yu, Z., Zhang, R., et al. (2014a). Topographic and quantitative evaluation of gentamicin-induced damage to peripheral innervation of mouse cochleae. Neurotoxicology 40, 86–96. doi: 10.1016/j.neuro.2013.11.002
Ruan, Q., Zeng, S., Liu, A., Chen, Z., Yu, Z., Zhang, R., et al. (2014b). Overexpression of X-Linked Inhibitor of Apoptotic Protein (XIAP) reduces age-related neuronal degeneration in the mouse cochlea. Gene Ther. 21, 967–974. doi: 10.1038/gt.2014.77
Ruan, Q., Ma, C., Zhang, R., and Yu, Z. (2014c). Current status of auditory aging and anti-aging research. Geriatr. Gerontol. Int. 14, 40–53. doi: 10.1111/ggi.12124
Ruan, Q., Hu, X., Ao, H., Ma, H., Gao, Z., Liu, F., et al. (2014d). The neurovascular protective effects of huperzine A on D-galactose-induced inflammatory damage in the rat hippocampus. Gerontology 60, 424–439. doi: 10.1159/000358235
Ruan, Q., D’Onofrio, G., Sancarlo, D., Bao, Z., Greco, A., and Yu, Z. (2016). Potential neuroimaging biomarkers of pathologic brain changes in Mild Cognitive Impairment and Alzheimer’s disease: a systematic review. BMC Geriatr. 16:104. doi: 10.1186/s12877-016-0281-7
Salvi, R. J., Wang, J., and Ding, D. (2000). Auditory plasticity and hyperactivity following cochlear damage. Hear. Res. 147, 261–274. doi: 10.1016/s0378-5955(00)00136-2
Schlee, W., Mueller, N., Hartmann, T., Keil, J., Lorenz, I., and Weisz, N. (2009). Mapping cortical hubs in tinnitus. BMC Biol. 7:80. doi: 10.1186/1741-7007-7-80
Schliebs, R., and Arendt, T. (2011). The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 221, 555–563. doi: 10.1016/j.bbr.2010.11.058
Schmidt, R., Schmidlt, H., Curb, J. D., Masaki, K., White, L. R., and Launer, L. J. (2002). Early inflammation and dementia: a 25-year follow-up of the Honolulu-asia aging study. Ann. Neurol. 52, 168–174. doi: 10.1002/ana.10265
Shargorodsky, J., Curhan, G. C., and Farwell, W. R. (2010). Prevalence and characteristics of tinnitus among US adults. Am. J. Med. 123, 711–718. doi: 10.1016/j.amjmed.2010.02.015
Sharma, S., Nag, T. C., Thakar, A., Bhardwaj, D. N., and Roy, T. S. (2014). The aging human cochlear nucleus: changes in the glial fibrillary acidic protein, intracellular calcium regulatory proteins, gaba neurotransmitter and cholinergic receptor. J. Chem. Neuroanat. 56, 1–12. doi: 10.1016/j.jchemneu.2013.12.001
Shimohama, S., Greenwald, D. L., Shafron, D. H., Akaika, A., Maeda, T., Kaneko, S., et al. (1998). Nicotinic α 7 receptors protect against glutamate neurotoxicity and neuronal ischemic damage. Brain Res. 779, 359–363. doi: 10.1016/s0006-8993(97)00194-7
Shore, S. E., Roberts, L. E., and Langguth, B. (2016). Maladaptive plasticity in tinnitus—triggers, mechanisms and treatment. Nat. Rev. Neurol. 12, 150–160. doi: 10.1038/nrneurol.2016.12
Shulman, A., Goldstein, B., and Strashun, A. M. (2008). Central nervous system neurodegeneration and tinnitus: a clinical experience. Part II: translational neurovascular theory of neurodegenerative CNS disease and tinnitus. Int. Tinnitus J. 14, 43–51.
Sinha, U. K., Hollen, K. M., Rodriguez, R., and Miller, C. A. (1993). Auditory system degeneration in Alzheimer’s disease. Neurology 43, 779–785. doi: 10.1212/WNL.43.4.779
Snider, B. J., Norton, J., Coats, M. A., Chakraverty, S., Hou, C. E., Jervis, R., et al. (2005). Novel presenilin 1 mutation (S170F) causing Alzheimer disease with Lewy bodies in the third decade of life. Arch. Neurol. 62, 1821–1830. doi: 10.1001/archneur.62.12.1821
Stanley, D. P., and Shetty, A. K. (2004). Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase-67 positive interneurons but not interneuron degeneration. J. Neurochem. 89, 204–216. doi: 10.1111/j.1471-4159.2004.02318.x
Stevens, C., Walker, G., Boyer, M., and Gallagher, M. (2007). Severe tinnitus and its effect on selective and divided attention. Int. J. Audiol. 46, 208–216. doi: 10.1080/14992020601102329
Strauss, M., and Gertz, H. J. (2009). Treatment of musical hallucinations with acetylcholinesterase inhibitors. J. Neurol. Neurosurg. Psychiatry 80, 1298–1299. doi: 10.1136/jnnp.2008.160978
Stuckenholz, V., Bacher, M., Balzer-Geldsetzer, M., Alvarez-Fischer, D., Oertel, W. H., Dodel, R. C., et al. (2013). The α7 nAChR agonist PNU-282987 reduces inflammation and MPTP-induced nigral dopaminergic cell loss in mice. J. Parkinsons Dis. 3, 161–172. doi: 10.3233/JPD-120157
Takeda, S., Sato, N., Ikimura, K., Nishino, H., Rakugi, H., and Morishita, R. (2013). Increased blood-brain barrier vulnerability to systemic inflammation in an Alzheimer’s disease mouse model. Neurobiol. Aging 34, 2064–2070. doi: 10.1016/j.neurobiolaging.2013.02.010
Takeda, S., Sato, N., and Morishita, R. (2014). Systemic inflammation, blood-brain barrier vulnerability and congnitive/non-cognitive symptoms in Alzheimer’s disease: relevance to pathogenesis and therapy. Front. Aging Neurosci. 6:171. doi: 10.3389/fnagi.2014.00171
Taljaard, D. S., Olaithe, M., Brennan-Jones, C. G., Eikelboom, R. H., and Bucks, R. S. (2016). The relationship between hearing impairment and cognitive function: a meta-analysis in adults. Clin. Otolaryngol. 41, 718–729. doi: 10.1111/coa.12607
Tan, W. J. T., Thorne, P. R., and Vlajkovic, S. M. (2016). Characterisation of cochlear inflammation in mice following acute and chronic noise exposure. Histochem. Cell Biol. 146, 219–230. doi: 10.1007/s00418-016-1436-5
Terao, K., Cureoglu, S., Schachern, P. A., Morita, N., Nomiya, S., Deroee, A. F., et al. (2011). Cochlear changes in presbycusis with tinnitus. Am. J. Otolaryngol. 32, 215–220. doi: 10.1016/j.amjoto.2010.02.001
Thambisetty, M., Beason-Held, L., An, Y., Kraut, M. A., and Resnick, S. M. (2010). APOE ε4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch. Neurol. 67, 93–98. doi: 10.1001/archneurol.2009.913
Thomson, R. S., Auduong, P., Miller, A. T., and Gurgel, R. K. (2017). Hearing loss as a risk factor for dementia: a systematic review. Laryngoscope Investig. Otolaryngol. 2, 69–79. doi: 10.1002/lio2.65
Tiraboschi, P., Hansen, L. A., Alford, M., Masliah, E., Thal, L. J., and Corey-Bloom, J. (2000). The decline in synapses and cholinergic activity is asynchronous in Alzheimer’s disease. Neurology 55, 1278–1283. doi: 10.1212/WNL.55.9.1278
Tiraboschi, P., Hansen, L. A., Alford, M., Merdes, A., Masliah, E., Thal, L. J., et al. (2002). Early and widespread cholinergic losses differentiate dementia with lewy bodies from Alzheimer disease. Arch. Gen. Psychiatry 59, 946–951. doi: 10.1001/archpsyc.59.10.946
Tsartsalis, S., Xekardaki, A., Hof, P. R., Kövari, E., and Bouras, C. (2018). Early Alzheimer-type lesions in cognitively normal subjects. Neurobiol. Aging 62, 34–44. doi: 10.1016/j.neurobiolaging.2017.10.002
Tyler, R., Cacace, A., Stocking, C., Tarver, B., Engineer, N., Martin, J., et al. (2017). Vagus nerve stimulation paired with tones for the treatment of tinnitus: a prospectiverandomized double-blind controlled pilot study in humans. Sci. Rep. 7:11960. doi: 10.1038/s41598-017-12178-w
Uchida, Y., Sugiura, S., Ueda, H., Nakashima, T., Ando, F., and Shimokata, H. (2014). The association between hearing impairment and polymorphisms of genes encoding inflammatory mediators in Japanese aged population. Immun. Ageing 11:18. doi: 10.1186/s12979-014-0018-4
Ukai, S., Yamamoto, M., Tanaka, M., Shinosaki, K., and Takeda, M. (2007). Donezepil in the treatment of musical hallucinations. Psychiatry Clin. Neurosci. 61, 190–192. doi: 10.1111/j.1440-1819.2007.01636.x
van Gool, W. A., van de Beek, D., and Eikelenboom, P. (2010). Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 375, 773–775. doi: 10.1016/S0140-6736(09)61158-2
Verret, L., Mann, E. O., Hang, G. B., Barth, A. M., Cobos, I., Ho, K., et al. (2012). Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721. doi: 10.1016/j.cell.2012.02.046
Verschuur, C., Agyemangprempeh, A., and Newman, T. A. (2014). Inflammation is associated with a worsening of presbycusis: evidence from the MRC national study of hearing. Int. J. Audiol. 53, 469–475. doi: 10.3109/14992027.2014.891057
Waechter, S., and Brännström, K. J. (2015). The impact of tinnitus on cognitive performance in normal-hearing individuals. Int. J. Audiol. 54, 845–851. doi: 10.3109/14992027.2015.1055836
Wallhagen, M. I., Strawbridge, W. J., and Shema, S. J. (2008). The relationship between hearing impairment and cognitive function: a 5-year longitudinal study. Res. Gerontol. Nurs. 1, 80–86. doi: 10.3928/19404921-20080401-08
Wang, Z. F., Wang, J., Zhang, H. Y., and Tang, X. C. (2008). Huperzine A exhibits anti-inflammatory and neuroprotective effects in a rat model of transient focal cerebral ischemia. J. Neurochem. 106, 1594–1603. doi: 10.1111/j.1471-4159.2008.05504.x
Wang, Y., Wei, Y., Oguntayo, S., Jensen, N., Doctor, B. P., and Nambiar, M. P. (2011). [+]-Huperzine A protects against soman toxicity in guinea pigs. Neurochem. Res. 36, 2381–2390. doi: 10.1007/s11064-011-0564-5
Wang, H., Yu, M., Ochani, M., Amella, C. A., Tanovic, M., Susarla, S., et al. (2003). Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388. doi: 10.1038/nature01339
Wang, J., Zhang, H. Y., and Tang, X. C. (2010). Huperzine a improves chronic inflammation and cognitive decline in rats with cerebral hypoperfusion. J. Neurosci. Res. 88, 807–815. doi: 10.1002/jnr.22237
Weaver, J. D., Huang, M. H., Albert, M., Harris, T., Rowe, J. W., and Seeman, T. E. (2002). Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology 59, 371–378. doi: 10.1212/wnl.59.3.371
Wong, L. L. N., Yu, J. K. Y., Chan, S. S., and Tong, M. C. F. (2014). Screening of cognitive function and hearing impairment in older adults: a preliminary study. Biomed Res. Int. 2014:867852. doi: 10.1155/2014/867852
Wu, S. T., and Chiu, C. J. (2016). Age-related trajectories of memory function in middle-aged and older adults with and without hearing impairment. Neuroepidemiology 46, 282–289. doi: 10.1159/000445378
Xie, L., Jiang, C., Wang, Z., Yi, X., Gong, Y., Chen, Y., et al. (2016). Effect of Huperzine A on Aβ-induced p65 of astrocyte in vitro. Biosci. Biotechnol. Biochem. 80, 2334–2337. doi: 10.1080/09168451.2016.1222265
Yaffe, K., Kanaya, A., Lindquist, K., Simonsick, E. M., Harris, T., Shorr, R. I., et al. (2004). The metabolic syndrome, inflammation and risk of cognitive decline. JAMA 292, 2237–2242. doi: 10.1001/jama.292.18.2237
Yi, F., Ball, J., Stoll, K. E., Satpute, V. C., Mitchell, S. M., Pauli, J. L., et al. (2014). Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: roles in cellular excitability, inhibitory transmission and cognition. J. Physiol. 592, 3463–3494. doi: 10.1113/jphysiol.2014.275453
Yoshiyama, Y., Kojima, A., Itoh, K., Isose, S., Koide, M., Hori, K., et al. (2015). Does anticholinergic activity affect neuropathology implication of neuroinflammation in Alzheimer’s disease. Neurodegener. Dis. 15, 140–148. doi: 10.1159/000381484
Yoshikawa, H., Kurokawa, M., Ozaki, N., Nara, K., Atou, K., Takada, E., et al. (2006). Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of i-κb phosphorylation and nuclear factor-κb transcriptional activity through nicotinic acetylcholine receptor α7. Clin. Exp. Immunol. 146, 116–123. doi: 10.1111/j.1365-2249.2006.03169.x
Zappettini, S., Grilli, M., Lagomarsino, F., Cavallero, A., Fedele, E., and Marchi, M. (2011). Presynaptic nicotinic α7 and non-α7 receptors stimulate endogenous GABA release from rat hippocampal synaptosomes through two mechanisms of action. PLoS One 6:e16911. doi: 10.1371/journal.pone.0016911
Zhang, X., Zhou, K., Wang, R., Cui, J., Lipton, S. A., Liao, F. F., et al. (2007). Hypoxia-inducible factor 1α (HIF1α)-mediated hypoxia increases BACE1 expression and β-amyloid generation. J. Biol. Chem. 282, 10873–10880. doi: 10.1074/jbc.M608856200
Zhong, P., Gu, Z., Wang, X., Jiang, H., Feng, J., and Yan, Z. (2003). Impaired modulation of GABAergic transmission by muscarinic receptors in a mouse transgenic model of Alzheimer’s disease. J. Biol. Chem. 278, 26888–26896. doi: 10.1074/jbc.M302789200
Zilles, D., Zerr, I., and Wedekind, D. (2012). Successful treatment of musical hallucinations with the acetylcholinesterase inhibitor donezepil. J. Clin. Psychopharmacol. 32, 422–424. doi: 10.1097/JCP.0b013e318253a086
Zlokovic, B. V. (2008). The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201. doi: 10.1016/j.neuron.2008.01.003
Keywords: presbycusis, tinnitus, cognitive impairment, cholinergic hypofunction, glial cell, neurogliaform cell
Citation: Ruan Q, Yu Z, Zhang W, Ruan J, Liu C and Zhang R (2018) Cholinergic Hypofunction in Presbycusis-Related Tinnitus With Cognitive Function Impairment: Emerging Hypotheses. Front. Aging Neurosci. 10:98. doi: 10.3389/fnagi.2018.00098
Received: 24 April 2017; Accepted: 22 March 2018;
Published: 06 April 2018.
Edited by:
Berthold Langguth, University of Regensburg, GermanyReviewed by:
Veronica Fuentes, Universidad de Castilla-La Mancha, SpainMartin Meyer, Universität Zürich, Switzerland
Copyright © 2018 Ruan, Yu, Zhang, Ruan, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuowei Yu, aGR5dXpodW93ZWlAMTYzLmNvbQ==
 Qingwei Ruan
Qingwei Ruan Zhuowei Yu
Zhuowei Yu Weibin Zhang
Weibin Zhang Jian Ruan
Jian Ruan Chunhui Liu3
Chunhui Liu3