Abstract
Background:
Vascular cognitive impairment (VCI) is a prevalent form of cognitive dysfunction. Resting-state functional magnetic resonance imaging (rs-fMRI) could serve as a potential biomarker for early detection. This study employed activation likelihood estimation (ALE) meta-analysis to investigate specific neural abnormalities in VCI patients.
Methods:
We systematically searched PubMed, Embase, and Web of Science for rs-fMRI studies on VCI that reported amplitude of low-frequency fluctuation (ALFF), regional homogeneity (ReHo), or functional connectivity (FC). Sixteen eligible fMRI studies were included in the ALE meta-analysis.
Results:
Compared to healthy controls (HCs), VCI patients exhibited the following rs-fMRI alterations. For ALFF, there was an increase in the left anterior cingulate (AC) and left inferior frontal gyrus, possibly a compensatory over - activation. Decreases were seen in regions like the bilateral precuneus and medial frontal gyri (mFG), linked to cognitive deficits. ReHo increased in the left claustrum and insula, suggesting enhanced local synchronization, but decreased in the right sub - gyral region and middle temporal gyru (MTG), which may relate to language issues. FC was enhanced in areas related to complex cognitive processes, yet reduced in regions crucial for memory.
Conclusion:
VCI patients exhibited distinct functional abnormalities in specific brain regions, reflecting their diverse cognitive impairments. These region-specific alterations may serve as potential biomarkers for early diagnosis and targeted intervention in VCI.
Introduction
Vascular cognitive impairment (VCI) encompasses a spectrum of cognitive deficits resulting from cerebrovascular diseases, ranging from mild vascular cognitive impairment (mVCI) and vascular dementia (VaD) (Skrobot et al., 2018; van der Flier et al., 2018). As the second most common cause of dementia, VCI accounts 20%–40% of all diagnoses (Rundek et al., 2022). Early identification and diagnosis of VCI are critically important and have garnered increasing attention. Early identification and diagnosis of VCI are critically important and have garnered increasing attention.
VCI involves complex mechanisms associated both macrostructural and microstructural levels (Badji et al., 2023). Cerebral small vessel disease (CSVD) is widely regarded as the primary driver of VCI pathogenesis, even in the absence of stroke (Inoue et al., 2023; Zanon Zotin et al., 2021). Neuroimaging markers of CSVD include small subcortical infarcts, lacunae, white matter hyperintensities (WMH), enlarged perivascular spaces (EPVS), microbleeds and brain atrophy (Litak et al., 2020). However, the underlying mechanisms linking VCI and CSVD remain highly intricate, posing challenges in identifying consistent pathological patterns across cases.
Disruptions in both structural and functional networks play a key mediating role in how vascular lesions affect cognitive function (Dichgans and Leys, 2017). Resting state functional magnetic resonance imaging (rs-fMRI) leverages spontaneous fluctuations of blood oxygen level dependent (BOLD) signal fluctuations to map functional brain activity, providing a highly reliable and reproducible method for investigating functional connectivity (FC) networks.
Amplitude of low-frequency fluctuation (ALFF), regional homogeneity (ReHo), and FC are widely used rs-fMRI metrics for whole-brain analysis. ALFF measures spontaneous regional neuronal activity, reflecting the brain’s physiological state, while ReHo assesses the synchronization of local neural activity (Jiang and Zuo, 2016; Xi et al., 2012). Higher ALFF values correlate with enhanced cognitive function, indicating increased neuronal excitability (Wang et al., 2020). Conversely, reduced ReHo suggests impaired local neural synchronization, implying abnormal activity in affected brain regions (Wang et al., 2020). FC quantifies temporal correlations in BOLD signals across distinct brain regions or voxels, mapping inter-regional communication (Lin et al., 2018). Together, these metrics provide valuable insights into functional brain variations.
The Vascular Impairment of Cognition Classification Consensus Study (VICCCS) established standardized diagnostic criteria and operational guidelines for VCI (Skrobot et al., 2017; Skrobot et al., 2018). However, the heterogeneous clinical manifestations of CSVD continue to pose significant diagnostic and management challenges (Badji et al., 2023).
Activation likelihood estimation (ALE) is a widely used coordinate-based meta-analysis method that models reported activation foci as spatial probability distributions centered at given coordinates. By computing the union of these probabilities for each voxel, ALE generates statistical maps (thresholded at p < 0.05) to identify consistent brain activation patterns across studies (Eickhoff et al., 2009; Turkeltaub et al., 2002). This approach has been extensively applied in rs-fMRI research and holds promise for identifying neuroimaging biomarkers (Xu et al., 2020).
Despite its utility, current VCI research faces several limitations, including small sample sizes, inconsistent inclusion criteria, conflicting findings, and ongoing debate regarding functional network alterations in VCI patients. Although ALE meta-analyses have been conducted in broader cognitive impairment populations, few studies have specifically focused on VCI. Moreover, existing ALE syntheses in this field have primarily examined isolated VCI subtypes (e.g., subcortical vascular cognitive impairment or vascular mild cognitive impairment), leaving a critical gap in comprehensive, spectrum-wide analyses (Xu et al., 2021; Zhang X. et al., 2021).
To address this, our study aimed to perform a systematic ALE meta-analysis of VCI, with three key objectives: (1) Identify rs-fMRI differences between VCI patients and healthy controls (HCs) to assess its diagnostic biomarker potential;(2) Investigate the relationship between altered brain regions and cognitive deficits in VCI;(3) Provide an integrative synthesis of functional network disruptions across the VCI spectrum.
Methods
The meta-analysis of neuroimaging studies was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and recorded using the PRISMA 2020 Checklist (Page et al., 2021).
Literature search and article selection
Database search
A systematic literature search was conducted in PubMed, Web of Science, and Embase (accessed on 13 September 2024) using the following key terms: VCI-related terms:(“vascular cognitive impairment” OR “vascular dementia” OR “vascular cognitive disorder” OR “VCI” OR “VD” OR “VaD”). Neuroimaging markers: AND (“amplitude of low-frequency fluctuation” OR “ALFF” OR “regional homogeneity” OR “ReHo” OR “functional connectivity” OR “FC”). Given the broad spectrum of VCI, additional terms were included to capture relevant subtypes and etiologies:(“small vessel disease” OR “vascular cognitive impairment-no dementia” OR “vascular cognitive impairment not dementia” OR “subcortical ischemic vascular disease” OR “recent small subcortical infarct” OR “white matter hyperintensity” OR “cerebral microbleed” OR “Leukoaraiosis” OR “leukodystrophy” OR “CADASIL”). Searches were limited to English-language publications.
Literature screening process
Two independent researchers conducted the literature search and screening. Discrepancies were resolved through discussion with a third reviewer. The screening process consisted of four sequential steps: (1) Title/Abstract Screening: Initial exclusion of irrelevant studies; (2) Full-Text Review: Further assessment of potentially eligible articles; (3) Final Eligibility Check: Detailed evaluation of remaining studies; (4) Cross-Verification: Ensured no relevant studies were omitted. After this rigorous selection process, 16 articles met the inclusion criteria. The PRISMA flow diagram (Figure 1) illustrates the search and screening process.
FIGURE 1

Flowchart shows study selection process.
Inclusion criteria
-
•
Studies were included if they met all of the following criteria:
-
•
Study Design: Resting-state fMRI (rs-fMRI) investigations of VCI.
-
•
Participants: Included both VCI patients and HCs, with baseline data comparisons between groups.
-
•
Analysis Method: Whole-brain analysis (not restricted to ROI-based approaches).
-
•
Reporting Standards: Provided Talairach or MNI coordinates for group-level comparisons (VCI vs. HCs).
-
•
Outcome Measures: Reported differences in ALFFs, ReHo, or FC.
Exclusion criteria
Studies were excluded if they met any of the following conditions:
-
•
Publication Type: Reviews, meta-analyses, case reports, animal studies, letters, protocols, theoretical models, conference abstracts, commentaries, or books.
-
•
Patient Population: Included individuals with Parkinson’s disease, Alzheimer’s disease (AD), frontotemporal dementia, psychiatric disorders, or acute cerebral infarction/intracerebral hemorrhage with a disease duration of less than 6 months.
-
•
Etiological Confounders: Enrolled patients with leukoencephalopathy due to immune, toxic, metabolic, or neoplastic causes.
-
•
Unclear Classification: Studies that could not be definitively classified as VCI-related research.
Quality assessment and data extraction
Given the lack of standardized tools for evaluating methodological quality in fMRI meta-analyses, we adapted the Newcastle-Ottawa Scale (mNOS) (Costa et al., 2021; Gentili et al., 2019; Wells et al., 2000) to assess study quality and risk of bias. The mNOS scoring system (range: 0–11) categorized studies as: High risk (0–3), Intermediate risk (4–7), and Low risk (8–11) (Costa et al., 2021). Two independent researchers performed the assessments, with inter-rater reliability evaluated using Cohen’s Kappa statistic. Discrepancies were resolved through consensus or consultation with a third reviewer.
Data extraction
The following variables were systematically extracted: (1) Demographics: Sample size, sex distribution, mean age; (2) Cognitive measures: Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA); (3) Imaging data: Group contrasts, peak coordinates (focus), significance thresholds (p-values); (4) Subgroup classification (if applicable).
Meta-analysis procedures
Spatial convergence analysis was performed using GingerALE 3.0.21 (Eickhoff et al., 2009; Eickhoff et al., 2012; Turkeltaub et al., 2012). All included studies reported coordinates in MNI space, eliminating the need for spatial normalization.
The ALE algorithm models each reported focus as a 3D Gaussian probability distribution (accounting for spatial uncertainty), then computes the union of these distributions across studies to identify statistically convergent activation patterns. This approach inherently controls for methodological heterogeneity (e.g., varying preprocessing pipelines, statistical thresholds, or cohort characteristics) by: (1) Weighting voxels based on cross-study consistency; (2) Applying cluster-level inference (primary threshold: p < 0.05, FWE-corrected via 1000 permutations. Resulting ALE maps were visualized on the MNI152 template using Mango V4.12, with anatomical labeling according to the AAL3 atlas.
Results
Research results
Following literature screening, 16 studies were included in the meta-analysis. VCI patients were categorized into the following subgroups based on diagnostic criteria: “VCI”, “vascular mild cognitive impairment (VaMCI)”, “vascular cognitive impairment, no dementia (VCIND)”, “CSVD with mild cognitive impairment (CSVD-M)”, “pontine stroke (PS)”, “subcortical ischemic vascular dementia (SIVD)”, “subcortical ischemic vascular disease with CI (SIVD-CI)”, “subcortical ischemic vascular disease with mild cognitive impairment (SIVD-MCI)”, “subcortical ischemic vascular disease with cognitive impairment (SIVD-CI)”, “CSVD with cognitive impairment (CSVD-CI)”, “white matter hyperintensities with CI (WMH-CI)”, “leukoaraiosis with vascular mild-cognitive impairment (LA-VaMCI)”, “leukoaraiosis with vascular-dementia (LA-VaD)”. For the ALFF analysis, 8 studies (involving 508 participants: 256 VCI patients and 252 HCs) revealed that VCI patients had increased ALFF in 20 foci and decreased ALFF in 20 foci compared to HCs. The ReHo analysis included 6 studies (316 participants: 149 VCI patients, 167 HCs), showing increased ReHo in 8 foci and decreased ReHo in 15 foci in VCI patients versus HCs. The FC analysis, comprising 4 studies (254 participants: 121 VCI patients, 133 HCs), demonstrated enhanced FC in 5 foci and reduced FC in 13 foci in VCI patients relative to HCs, with detailed data presented in Table 1.
TABLE 1
| Study | Group | N | Age | Sex (male/ female) |
MMSE | MocA | Group contrasts | Foci | Correction for multiple comparisons |
| ALFF | |||||||||
| Li et al., 2021b | VaMCI | 31 | 62.87 ± 7.07 | 20-Nov | – | 23 (20, 24) | – | – | – |
| HCs | 31 | 59.35 ± 8.15 | 14/17 | – | 28 (26, 30) | VaMCI < HC | 4 | p < 0.05 (cor) | |
| Ding et al., 2018 | VCIND | 14 | 67.9 ± 8.7 | 08-Jun | 26.87 ± 0.32 | 20.32 ± 3.72 | VCIND < HC | 2 | p < 0.05 (cor) |
| HCs | 15 | 65.8 ± 7.9 | 07-Aug | 28.51 ± 0.28 | 26.33 ± 2.98 | VCIND < HC | 7 | p < 0.05 (cor) | |
| Zhang et al., 2023 | CSVD-M | 19 | 67.89 ± 8.01 | 10-Sep | 26.63 ± 1.61 | 23.47 ± 2.01 | CSVD-M < HC | 1 | p < 0.05 (cor) |
| HCs | 18 | 61.67 ± 7.62 | 06-Dec | 27.71 ± 1.57 | 26.53 ± 0.62 | CSVD-M < HC | 2 | p < 0.05 (cor) | |
| Liu et al., 2014 | SIVD | 30 | 69.0 ± 7.8 | 19-Nov | 16.1 ± 5.1 | 9.4 ± 3.8 | SIVD < HC | 3 | p < 0.01 (cor) |
| HCs | 35 | 68.0 ± 5.8 | 22/13 | 28.4 ± 1.1 | 27.2 ± 1.5 | SIVD < HC | 1 | p < 0.01 (cor) | |
| Song Z. et al., 2023 | SIVD-CI | 32 | 75.09 ± 8.68 | 15/17 | 20.91 ± 4.19 | 17.91 ± 4.50 | SIVD-CI < HC | 1 | p < 0.05 (cor) |
| HCs | 32 | 73.36 ± 7.26 | 17/15 | 28.00 ± 2.32 | 27.61 ± 1.86 | SIVD-CI < HC | 1 | p < 0.05 (cor) | |
| Zhang X. et al., 2021 | VaMCI | 32 | 69.54 ± 7.23 | 18/14 | 24.11 ± 1.01 | 20.78 ± 1.52 | VaMCI < HC | 5 | p < 0.05 |
| HCs | 30 | 65.3 ± 9.38 | 17/13 | 27.46 ± 1.23 | 27.01 ± 1.12 | – | – | – | |
| Song J. et al., 2023 | CSVD-CI | 52 | 69.63 ± 5.75 | 31/21 | 22.58 ± 4.19 | – | CSVD-CI < HC | 7 | p < 0.001 (cor) |
| HCs | 63 | 67.62 ± 5.56 | 30/32 | 27.92 ± 1.56 | – | CSVD-CI < HC | 4 | p < 0.001 (cor) | |
| Wang et al., 2019 | LA-VaMCI | 28 | 59.28 ± 6.12 | 14/14 | 24.96 ± 1.48 | 21.68 ± 2.74 | LA-CI < HC | 1 | p < 0.05 (cor) |
| LA-VaD | 18 | 60.28 ± 11.65 | 08-Oct | 20.53 ± 1.77 | 17.17 ± 2.09 | ||||
| HCs | 28 | 58.35 ± 6.82 | 13/15 | 29.46 ± 1.07 | 28.64 ± 1.66 | LA-CI < HC | 1 | p < 0.05 (cor) | |
| ReHO | |||||||||
| Zuo et al., 2018 | VaMCI | 31 | 63.84 ± 14.1 | 18/13 | 26.32 ± 2.06 | 23.32 ± 1.33 | – | – | – |
| HCs | 32 | 62.72 ± 8.22 | 18/14 | 26.32 ± 2.06 | 27.75 ± 1.72 | VaMCI < HC | 2 | p < 0.05 (cor) | |
| Liu et al., 2021 | SIVD-MCI | 28 | 70.73 ± 5.58 | 16-Dec | 23.93 ± 1.90 | – | – | p < 0.05 (cor) | |
| HCs | 24 | 68.43 ± 8.02 | Oct-14 | 28.00 ± 1.06 | – | SIVD-MCI < HC | 3 | p < 0.05 (cor) | |
| Zhang X. et al., 2021 | VaMCI | 32 | 69.54 ± 7.23 | 18/14 | 24.11 ± 1.01 | 20.78 ± 1.52 | VaMCI < HC | 4 | p < 0.05 |
| HCs | 30 | 65.3 ± 9.38 | 17/13 | 27.46 ± 1.23 | 27.01 ± 1.12 | VaMCI < HC | 3 | p < 0.05 | |
| Tu et al., 2020 | SIVD | 20 | 75.8 ± 7.67 | 13-Jul | – | – | SIVD < HC | – | – |
| HCs | 23 | 65.1 ± 6.97 | 11-Dec | – | – | SIVD < HC | 1 | p < 0.001 | |
| Ye et al., 2019 | WMH-CI | 14 | 66.00 ± 5.13 | 07-Jul | 26.86 ± 2.66 | 20.43 ± 2.71 | WMH-CI < HC | 2 | p < 0.001 |
| HCs | 33 | 62.03 ± 7.53 | 16/17 | 28.47 ± 1.49 | 26.41 ± 2.30 | WMH-CI < HC | 1 | p < 0.001 | |
| Cai et al., 2023 | VCI | 24 | 63.75 ± 4.27 | Nov-13 | 21.12 ± 0.33 | 20.37 ± 1.24 | VCI < HC | 2 | p < 0.05 (cor) |
| HCs | 25 | 60.60 ± 3.95 | Dec-13 | 29.76 ± 0.43 | 29.00 ± 0.64 | VCI < HC | 5 | p < 0.05 (cor) | |
| FC | |||||||||
| Ding et al., 2018 | VCIND | 14 | 67.9 ± 8.7 | 08-Jun | 26.87 ± 0.32 | 20.32 ± 3.72 | VCIND < HC | 4 | p < 0.05 (cor) |
| HCs | 15 | 65.8 ± 7.9 | 07-Aug | 28.51 ± 0.28 | 26.33 ± 2.98 | VCIND < HC | 4 | p < 0.05 (cor) | |
| Wang et al., 2022 | PS | 47 | 57.75 ± 7.40 | 26/21 | – | – | – | – | – |
| HCs | 55 | 55.77 ± 8.03 | 33/23 | – | – | PS < HC | 8 | p < 0.01 | |
| Liu et al., 2019 | SVCI | 29 | 70.48 ± 5.76 | 16/13 | 24.00 ± 1.91 | – | SVCI < NC | 1 | p < 0.05 (cor) |
| HCs | 27 | 67.63 ± 8.19 | Oct-17 | 27.93 ± 1.03 | – | – | – | – | |
| Li et al., 2021a | VaMCI | 31 | 64.93 ± 10.11 | 18/13 | – | 23.32 ± 1.32 | – | – | |
| HCs | 36 | 64.22 ± 6.97 | 17/19 | – | 25.22 ± 2.89 | VaMCI < HC | 1 | p < 0.05 (cor) | |
Demographic data and clinical information.
VaMCI, vascular mild cognitive impairment; HCs, healthy controls; VCIND, vascular cognitive impairment, no dementia; CSVD-M, CSVD with mild cognitive impairment; SIVD, subcortical ischemic vascular dementia; SIVD-CI, subcortical ischemic vascular disease with cognitive impairment; CSVD-CI, CSVD with cognitive impairment; LA-VaMCI, leukoaraiosis with vascular mild-cognitive impairment; LA-VaD, leukoaraiosis with vascular-dementia; SIVD-MCI, subcortical ischemic vascular disease with mild cognitive impairment; WMH-CI, white matter hyperintensities with cognitive impairment; PS, pontine stroke.
Meta-analysis results
Altered ALFF in VCI patients
Compared to HCs, VCI patients demonstrated significant ALFF alterations, characterized by increased ALFF (4 clusters), left anterior cingulate cortex (ACC), left inferior frontal gyrus (IFG), decreased ALFF (11 clusters),left ACC (distinct subregion from increased cluster), bilateral medial frontal gyrus (mFG), bilateral precuneus, left middle temporal gyrus (MTG) and left superior occipital gyrus (SOG). Detailed spatial distributions and statistical thresholds are presented in Figure 2 and Table 1.
FIGURE 2
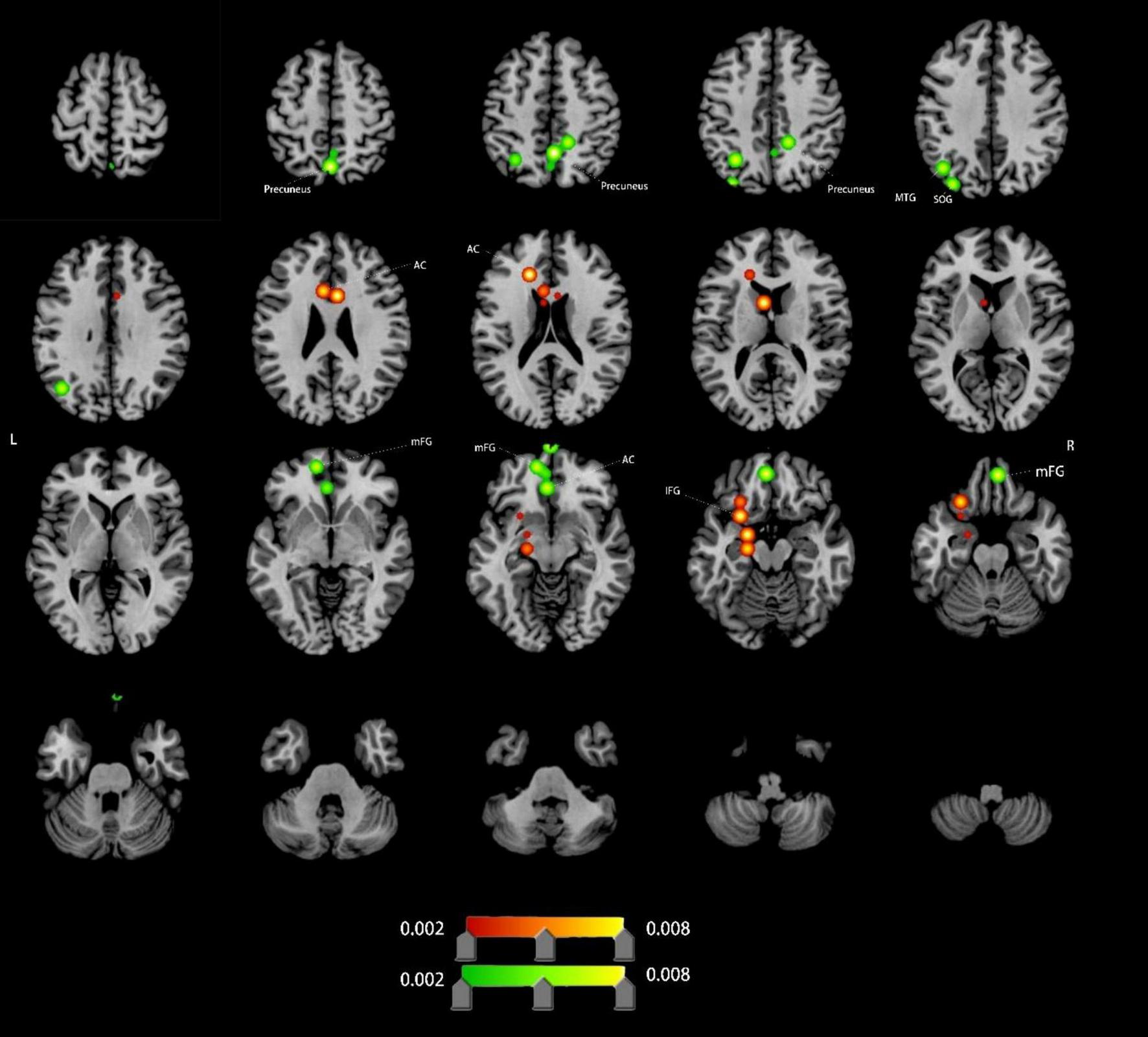
Brain regions showing increased/decreassed ALFF in VCI patients compared to HCs. Red indicates regions of increased ALFF values. Green indicates regions of decreased ALFF values. AC, anterior cingulate; IFG, inferior frontal gyrus; mFG, medial frontal gyrus; MTG, middle temporal gyrus; SOG, superior occipital gyrus.
Altered ReHo in VCI patients
Compared to HCs, VCI patients exhibited significant ReHo differences. Increased ReHo (8 clusters): left claustrum, left insula, left sub-gyral region, left mFG and left precuneus; Decreased ReHo (2 clusters): right sub-gyral and right MTG (Figure 3 and Table 1).
FIGURE 3

Brain regions showing increased/decreassed ReHo in VCI patients compared to HCs. Red indicates regions of increased ReHo values. Green indicates regions of decreased ReHo values. MTG, middle temporal gyrus; mFG, medial frontal gyrus;
Altered FC in VCI patients
Compared to HCs, VCI patients exhibited significant FC differences. Increased FC (4 clusters): right precentral gyrus (PreCG), left MTG, left precuneus, right superior temporal gyrus (STG); Decreased FC (9 clusters): left cingulate gyrus (CG), both posterior cingulate, right precuneus, right lingual gyrus (LING), right pyramis, right PreCG, and right MFG (Figure 4 and Table 1).
FIGURE 4
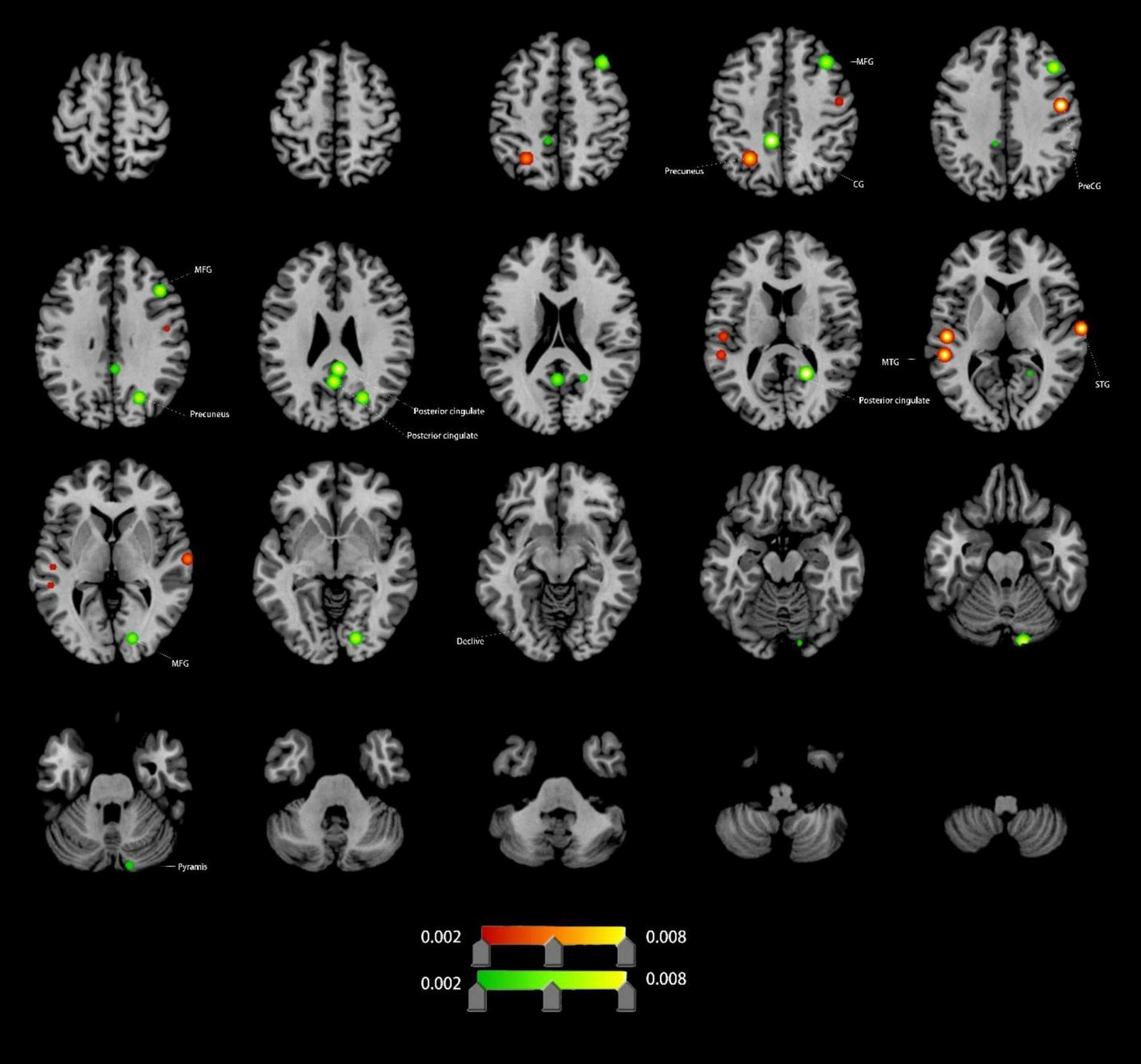
Brain regions showing increased/decreassed FC in VCI patients compared to HCs. Red indicates regions of increased FC values. Green indicates regions of decreased FC values. PreCG, precentral gyrus; MFG, middle frontal gyrus; MTG, middle temporal gyrus; STG, superior temporal gyrus; CG, cingulate gyrus; LING, lingual gyrus.
Discussion
This study investigated resting-state network alterations in VCI patients compared to HCs, focusing on amplitude of ALFF, ReHo, and FC. These metrics capture distinct yet complementary aspects of neural activity (ALFF), local synchronization (ReHo), and inter-regional communication (FC), offering a comprehensive view of VCI-related dysfunction. By identifying aberrant functional signatures, our findings may contribute to the development of probabilistic biomarkers for early VCI detection, facilitating timely and targeted interventions.
ALFF Abnormalities: ALFF alterations were predominantly localized in prefrontal, precuneus, and temporal regions. Increased ALFF in the left AC and IFG suggests enhanced neuronal excitability, potentially reflecting compensatory mechanisms for cognitive control, emotional processing, and multitasking in response to cerebral ischemia (Briggs et al., 2019; Kolling et al., 2016; Monosov et al., 2020; Sato et al., 2023; Shinozaki et al., 2016; Shu et al., 2022). Conversely, decreased ALFF in mFG, bilateral precuneus, and other default mode network (DMN) hubs indicates resting-state dysfunction, likely contributing to episodic memory decline, executive dysfunction, and emotional dysregulation in VCI (Dadario and Sughrue, 2023; Frascarelli et al., 2015; Myung et al., 2016).
ReHo Alterations: ReHo analysis revealed increased local synchronization in the left claustrum and mFG, suggesting adaptive changes in decision-making and executive control networks. In contrast, decreased ReHo in the right MTG implies disrupted language network coordination, aligning with semantic deficits in VCI (Briggs et al., 2021; Cui et al., 2020; Frascarelli et al., 2015; Jackson et al., 2020; Madden et al., 2022).
FC Changes: Enhanced FC in the right STG, MTG, and left precuneus may reflect enhancements in speech perception, auditory word comprehension, and language processing, as complex cognitive processes that require neural integration across multiple brain regions (Bhaya-Grossman and Chang, 2022; Dadario and Sughrue, 2023; Liu et al., 2023; Sugimoto et al., 2023). Conversely, reduced FC in the cingulate gyrus (CG), posterior cingulate, and precuneus reflects DMN disintegration, correlating with impaired self-referential processing and memory consolidation (Bubb et al., 2018; Dadario and Sughrue, 2023; Foster et al., 2023; Leech and Sharp, 2014).
The findings strongly support CSVD as the principal pathological basis of VCI. CSVD-related structural damage—including WMH and lacunar infarcts—likely disrupts critical neural pathways, leading to widespread functional network dysfunction (Chen et al., 2018; Zanon Zotin et al., 2021). This disorganization particularly affects hub regions such as the precuneus, a key node in the default mode network (DMN) that is highly vulnerable to hypoperfusion in CSVD (Dadario and Sughrue, 2023; Love and Miners, 2016). In VCI patients, reduced ALFF and FC in the precuneus may underlie diverse cognitive deficits, particularly in spatial processing and navigation (Cavanna and Trimble, 2006), manifesting clinically as disorientation and impaired spatial cognition. Notably, our meta-analysis—encompassing multiple VCI subtypes (VaMCI, VaD, CSVD-related cognitive impairment) —revealed consistent functional abnormalities across the VCI spectrum, aligning with VICCCS guidelines that advocate for multidimensional neuroimaging markers in VCI diagnosis (Skrobot et al., 2017; Skrobot et al., 2018). By integrating ALFF, ReHo, and FC across the VCI continuum, this study provides preliminary evidence for their utility as complementary diagnostic biomarkers, potentially enhancing early detection and stratification of VCI.
Multimodal neuroimaging reveals a dynamic inter play in VCI, characterized by regional hyperactivity (↑ALFF/ReHo) alongside network disconnection (↓FC) —reflecting concurrent neuroplastic adaptation and pathological decompensation (Fornito et al., 2015). While the brain exhibits compensatory mechanisms to preserve homeostasis, these processes are complex and multifaceted, with VCI patients demonstrating more pronounced structural and functional alterations than typical aging (Jin et al., 2025).
Current limitations—such as heterogeneous datasets and inconsistent VCI subtyping—highlight the need for large-scale, prospective studies integrating multimodal neuroimaging (rs-fMRI, DTI, structural MRI) with detailed clinical profiles. Such efforts should: (1) Validate subtype-specific abnormalities across diverse VCI cohorts (e.g., VaMCI, VaD, CSVD-related cognitive impairment); (2) Clarify mechanistic links between functional disruptions (ALFF/ReHo/FC) and structural/metabolic changes; (3) Build upon this meta-analysis as an exploratory foundation for personalized diagnostic and therapeutic strategies.
This study has several limitations that warrant consideration. First, heterogeneity in meta-analysis, despite strict inclusion/exclusion criteria, variability in data sources, preprocessing methods, statistical thresholds, and imaging protocols was unavoidable, potentially influencing the results. Second, in whole-Brain Approach vs. Network-Specific Focus, the whole-brain ALE analysis—rather than targeting specific networks—limited the depth of investigation and may have excluded relevant studies. Third, subtype analysis challenges, the broad and heterogeneous nature of VCI, combined with the limited number of eligible studies, precluded meaningful subgroup analyses (e.g., VaMCI vs. VaD). Finally, in ALE methodological constraints, the ALE technique lacks significance testing for individual contributing studies, restricting quantitative interpretation of regional findings.
Conclusion
This ALE meta-analysis identified consistent rs-fMRI abnormalities (ALFF/ReHo/FC) in key cognitive hubs—including the cingulate gyrus (CG), precuneus, and anterior cingulate (AC)—providing mechanistic insights into VCI-related functional impairments. These disruptions may serve as early diagnostic biomarkers, enabling targeted interventions for at-risk patients before overt cognitive decline manifests.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
CZ: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review and editing. MX: Data curation, Writing – original draft, Writing – review and editing. HZ: Data curation, Investigation, Writing – review and editing. JL: Data curation, Investigation, Writing – review and editing. MH: Formal Analysis, Funding acquisition, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant no. 81970348 to Mingli He).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Badji A. Youwakim J. Cooper A. Westman E. Marseglia A. (2023). Vascular cognitive impairment - Past, present, and future challenges.Ageing Res. Rev.90:102042. 10.1016/j.arr.2023.102042
2
Bhaya-Grossman I. Chang E. (2022). Speech computations of the human superior temporal gyrus.Annu. Rev. Psychol.7379–102. 10.1146/annurev-psych-022321-035256
3
Briggs R. Chakraborty A. Anderson C. Abraham C. Palejwala A. Conner A. et al (2019). Anatomy and white matter connections of the inferior frontal gyrus.Clin. Anat.32546–556. 10.1002/ca.23349
4
Briggs R. Tanglay O. Dadario N. Young I. Fonseka R. Hormovas J. et al (2021). The unique fiber anatomy of middle temporal gyrus default mode connectivity.Oper. Neurosurg.21E8–E14. 10.1093/ons/opab109
5
Bubb E. Metzler-Baddeley C. Aggleton J. (2018). The cingulum bundle: Anatomy, function, and dysfunction.Neurosci. Biobehav. Rev.92104–127. 10.1016/j.neubiorev.2018.05.008
6
Cai L. Yue J. Cao D. Wang P. Zhang Q. Li A. et al (2023). Structural and functional activities of brain in patients with vascular cognitive impairment: A case-controlled magnetic resonance imaging study.Medicine (Baltimore)102:e33534. 10.1097/MD.0000000000033534
7
Cavanna A. Trimble M. (2006). The precuneus: A review of its functional anatomy and behavioural correlates.Brain129564–583. 10.1093/brain/awl004
8
Chen H. Gao Y. Che C. Lin H. Ruan X. (2018). Diffusion tensor imaging with tract-based spatial statistics reveals white matter abnormalities in patients with vascular cognitive impairment.Front. Neuroanat.12:53. 10.3389/fnana.2018.00053
9
Costa C. Cristea I. Dal Bò E. Melloni C. Gentili C. (2021). Brain activity during facial processing in autism spectrum disorder: An activation likelihood estimation (ALE) meta-analysis of neuroimaging studies.J. Child Psychol. Psychiatry621412–1424. 10.1111/jcpp.13412
10
Cui J. Li L. Li M. Siegler R. Zhou X. (2020). Middle temporal cortex is involved in processing fractions.Neurosci. Lett.725:134901. 10.1016/j.neulet.2020.134901
11
Dadario N. Sughrue M. (2023). The functional role of the precuneus.Brain1463598–3607. 10.1093/brain/awad181
12
Dichgans M. Leys D. (2017). Vascular cognitive impairment.Circ. Res.120573–591. 10.1161/CIRCRESAHA.116.308426
13
Ding H. Xu Y. Li X. Li D. Li E. Han Y. et al (2018). Aberrant default mode network in patients with vascular cognitive impairment, no dementia.Int. J. Clin. Exp. Med.111984–1993.
14
Eickhoff S. Bzdok D. Laird A. Kurth F. Fox P. (2012). Activation likelihood estimation meta-analysis revisited.Neuroimage592349–2361. 10.1016/j.neuroimage.2011.09.017
15
Eickhoff S. Laird A. Grefkes C. Wang L. Zilles K. Fox P. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty.Hum. Brain Mapp.302907–2926. 10.1002/hbm.20718
16
Fornito A. Zalesky A. Breakspear M. (2015). The connectomics of brain disorders.Nat. Rev. Neurosci.16159–172. 10.1038/nrn3901
17
Foster B. Koslov S. Aponik-Gremillion L. Monko M. Hayden B. Heilbronner S. R. (2023). A tripartite view of the posterior cingulate cortex.Nat. Rev. Neurosci.24173–189. 10.1038/s41583-022-00661-x
18
Frascarelli M. Tognin S. Mirigliani A. Parente F. Buzzanca A. Torti M. et al (2015). Medial frontal gyrus alterations in schizophrenia: Relationship with duration of illness and executive dysfunction.Psychiatry Res.231103–110. 10.1016/j.pscychresns.2014.10.017
19
Gentili C. Messerotti Benvenuti S. Lettieri G. Costa C. Cecchetti L. R. O. I. (2019). and phobias: The effect of ROI approach on an ALE meta-analysis of specific phobias.Hum. Brain Mapp.401814–1828. 10.1002/hbm.24492
20
Inoue Y. Shue F. Bu G. Kanekiyo T. (2023). Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease.Mol. Neurodegener.18:46. 10.1186/s13024-023-00640-5
21
Jackson J. Smith J. Lee A. (2020). The anatomy and physiology of claustrum-cortex interactions.Annu. Rev. Neurosci.43231–247. 10.1146/annurev-neuro-092519-101637
22
Jiang L. Zuo X. (2016). Regional homogeneity: A multimodal, multiscale neuroimaging marker of the human connectome.Neuroscientist22486–505. 10.1177/1073858415595004
23
Jin J. Ma J. Wu J. Lu J. Lu H. Zheng M. et al (2025). Neural correlates and adaptive mechanisms in vascular cognitive impairment: Exploration of a structure-function coupling network.CNS Neurosci. Ther.31:e70205. 10.1111/cns.70205
24
Kolling N. Behrens T. Wittmann M. Rushworth M. (2016). Multiple signals in anterior cingulate cortex.Curr. Opin. Neurobiol.3736–43. 10.1016/j.conb.2015.12.007
25
Leech R. Sharp D. (2014). The role of the posterior cingulate cortex in cognition and disease.Brain13712–32. 10.1093/brain/awt162
26
Li H. Gao S. Jia X. Jiang T. Li K. (2021a). Distinctive alterations of functional connectivity strength between vascular and amnestic mild cognitive impairment.Neural Plast.2021:8812490. 10.1155/2021/8812490
27
Li H. Jia X. Li Y. Jia X. Yang Q. (2021b). Aberrant amplitude of low-frequency fluctuation and degree centrality within the default mode network in patients with vascular mild cognitive impairment.Brain Sci.11:1534. 10.3390/brainsci11111534
28
Lin L. Xing G. Han Y. (2018). Advances in resting state neuroimaging of mild cognitive impairment.Front. Psychiatry9:671. 10.3389/fpsyt.2018.00671
29
Litak J. Mazurek M. Kulesza B. Szmygin P. Litak J. Kamieniak P. et al (2020). Cerebral small vessel disease.Int. J. Mol. Sci.21:9729. 10.3390/ijms21249729
30
Liu C. Li C. Yin X. Yang J. Zhou D. Gui L. et al (2014). Abnormal intrinsic brain activity patterns in patients with subcortical ischemic vascular dementia.PLoS One9:e87880. 10.1371/journal.pone.0087880
31
Liu L. Liu D. Guo T. Schwieter J. Liu H. (2023). The right superior temporal gyrus plays a role in semantic-rule learning: Evidence supporting a reinforcement learning model.Neuroimage282:120393. 10.1016/j.neuroimage.2023.120393
32
Liu X. Chen L. Cheng R. Luo T. Lv F. Fang W. et al (2019). Altered functional connectivity in patients with subcortical ischemic vascular disease: A resting-state fMRI study.Brain Res.1715126–133. 10.1016/j.brainres.2019.03.022
33
Liu X. Cheng R. Chen L. Gong J. Luo T. Lv F. (2021). Altered neurovascular coupling in subcortical ischemic vascular disease.Front. Aging Neurosci.13:598365. 10.3389/fnagi.2021.598365
34
Love S. Miners J. (2016). Cerebrovascular disease in ageing and Alzheimer’s disease.Acta Neuropathol.131645–658. 10.1007/s00401-015-1522-0
35
Madden M. Stewart B. White M. Krimmel S. Qadir H. Barrett F. et al (2022). A role for the claustrum in cognitive control.Trends Cogn. Sci.261133–1152. 10.1016/j.tics.2022.09.006
36
Monosov I. Haber S. Leuthardt E. Jezzini A. (2020). Anterior cingulate cortex and the control of dynamic behavior in primates.Curr. Biol.30R1442–R1454. 10.1016/j.cub.2020.10.009
37
Myung W. Na K. Ham B. Oh S. Ahn H. Jung H. (2016). Decreased medial frontal gyrus in patients with adjustment disorder.J. Affect. Disord.19136–40. 10.1016/j.jad.2015.11.028
38
Page M. McKenzie J. Bossuyt P. Boutron I. Hoffmann T. Mulrow C. et al (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews.BMJ372:n71. 10.1136/bmj.n71
39
Rundek T. Tolea M. Ariko T. Fagerli E. Camargo C. (2022). Vascular cognitive impairment (VCI).Neurotherapeutics1968–88. 10.1007/s13311-021-01170-y
40
Sato M. Nakai N. Fujima S. Choe K. Takumi T. (2023). Social circuits and their dysfunction in autism spectrum disorder.Mol. Psychiatry283194–3206. 10.1038/s41380-023-02201-0
41
Shinozaki R. Hojo Y. Mukai H. Hashizume M. Murakoshi T. (2016). Kainate-induced network activity in the anterior cingulate cortex.Neuroscience32520–29. 10.1016/j.neuroscience.2016.03.025
42
Shu Y. Wu G. Bi B. Liu J. Xiong J. Kuang L. (2022). Changes of functional connectivity of the subgenual anterior cingulate cortex and precuneus after cognitive behavioral therapy combined with fluoxetine in young depressed patients with suicide attempt.Behav. Brain Res.417:113612. 10.1016/j.bbr.2021.113612
43
Skrobot O. Black S. Chen C. DeCarli C. Erkinjuntti T. Ford G. et al (2018). Progress toward standardized diagnosis of vascular cognitive impairment: Guidelines from the vascular impairment of cognition classification consensus study.Alzheimers Dement.14280–292. 10.1016/j.jalz.2017.09.007
44
Skrobot O. O’Brien J. Black S. Chen C. DeCarli C. Erkinjuntti T. et al (2017). The vascular impairment of cognition classification consensus study.Alzheimers Dement.13624–633. 10.1016/j.jalz.2016.10.007
45
Song J. Lei T. Li Y. Zhou L. Yan W. Li H. et al (2023). Dynamic alterations in the amplitude of low-frequency fluctuation in patients with cerebral small vessel disease.Front. Mol. Neurosci.16:1200756. 10.3389/fnmol.2023.1200756
46
Song Z. Wu Z. Zhou Z. Feng M. Liu Y. Ma M. et al (2023). Altered static and dynamic indices of intrinsic brain activity in patients with subcortical ischemic vascular disease: A resting-state functional magnetic resonance imaging analysis.Neuroradiology65923–931. 10.1007/s00234-023-03135-8
47
Sugimoto H. Abe M. Otake-Matsuura M. (2023). Word-producing brain: Contribution of the left anterior middle temporal gyrus to word production patterns in spoken language.Brain Lang.238:105233. 10.1016/j.bandl.2023.105233
48
Tu M. Hsu Y. Yang J. Huang W. Deng J. Lin S. et al (2020). Attention and functional connectivity among patients with early-stage subcortical ischemic vascular disease and Alzheimer’s disease.Front. Aging Neurosci.12:239. 10.3389/fnagi.2020.00239
49
Turkeltaub P. Eden G. Jones K. Zeffiro T. (2002). Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation.Neuroimage16765–780. 10.1006/nimg.2002.1131
50
Turkeltaub P. Eickhoff S. Laird A. Fox M. Wiener M. Fox P. (2012). Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses.Hum. Brain Mapp.331–13. 10.1002/hbm.21186
51
van der Flier W. Skoog I. Schneider J. Pantoni L. Mok V. Chen C. et al (2018). Vascular cognitive impairment.Nat. Rev. Dis. Primers4:18003. 10.1038/nrdp.2018.3
52
Wang J. Chen H. Liang H. Wang W. Liang Y. Liang Y. et al (2019). Low-frequency fluctuations amplitude signals exhibit abnormalities of intrinsic brain activities and reflect cognitive impairment in leukoaraiosis patients.Med. Sci. Monit.255219–5228. 10.12659/MSM.915528
53
Wang R. Liu N. Tao Y. Gong X. Zheng J. Yang C. et al (2020). The application of rs-fMRI in vascular cognitive impairment.Front. Neurol.11:951. 10.3389/fneur.2020.00951
54
Wang Y. Wang C. Wei Y. Miao P. Liu J. Wu L. et al (2022). Abnormal functional connectivities patterns of multidomain cognitive impairments in pontine stroke patients.Hum. Brain Mapp.434676–4688. 10.1002/hbm.25982
55
Wells G. A. Shea B. O’Connell D. Peterson J. Welch P. Losos M. et al (2000). The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses.Ottawa: University of Ottawa.
56
Xi Q. Zhao X. Wang P. Guo Q. Jiang H. Cao X. et al (2012). Spontaneous brain activity in mild cognitive impairment revealed by amplitude of low-frequency fluctuation analysis: A resting-state fMRI study.Radiol. Med.117865–871. 10.1007/s11547-011-0780-8
57
Xu W. Chen S. Xue C. Hu G. Ma W. Qi W. et al (2020). Functional MRI-specific alterations in executive control network in mild cognitive impairment: An ALE meta-analysis.Front. Aging Neurosci.12:578863. 10.3389/fnagi.2020.578863
58
Xu W. Song Y. Chen S. Xue C. Hu G. Qi W. et al (2021). An ALE meta-analysis of specific functional MRI studies on subcortical vascular cognitive impairment.Front. Neurol.12:649233. 10.3389/fneur.2021.649233
59
Ye Q. Chen X. Qin R. Huang L. Yang D. Liu R. et al (2019). Enhanced regional homogeneity and functional connectivity in subjects with white matter hyperintensities and cognitive impairment.Front. Neurosci.13:695. 10.3389/fnins.2019.00695
60
Zanon Zotin M. Sveikata L. Viswanathan A. Yilmaz P. (2021). Cerebral small vessel disease and vascular cognitive impairment: From diagnosis to management.Curr. Opin. Neurol.34246–257. 10.1097/WCO.0000000000000913
61
Zhang L. Li Y. Bian L. Luo Q. Zhang X. Zhao B. (2021). Analysis of factors affecting cranial nerve function of patients with vascular mild cognitive impairment through functional magnetic resonance imaging under artificial intelligence environment.Front. Public Health9:803659. 10.3389/fpubh.2021.803659
62
Zhang X. Wang Z. Zheng D. Cao X. Qi W. Yuan Q. et al (2023). Aberrant spontaneous static and dynamic amplitude of low-frequency fluctuations in cerebral small vessel disease with or without mild cognitive impairment.Brain Behav.13:e3279. 10.1002/brb3.3279
63
Zhang X. Xue C. Cao X. Yuan Q. Qi W. Xu W. et al (2021). Altered patterns of amplitude of low-frequency fluctuations and fractional amplitude of low-frequency fluctuations between amnestic and vascular mild cognitive impairment: An ALE-based comparative meta-analysis.Front. Aging Neurosci.13:711023. 10.3389/fnagi.2021.711023
64
Zuo M. Xu Y. Zhang X. Li M. Jia X. Niu J. et al (2018). Aberrant brain regional homogeneity and functional connectivity of entorhinal cortex in vascular mild cognitive impairment: A resting-state functional MRI study.Front. Neurol.9:1177. 10.3389/fneur.2018.01177
Summary
Keywords
cognitive impairment, resting state functional magnetic resonance imaging, meta-analysis, amplitude of low-frequency fluctuation, regional homogeneity, functional connectivity
Citation
Zhang C, Xue M, Zhang H, Li J and He M (2025) Functional brain changes in vascular cognitive impairment: a whole brain ALE meta-analysis. Front. Aging Neurosci. 17:1521457. doi: 10.3389/fnagi.2025.1521457
Received
04 November 2024
Accepted
15 May 2025
Published
05 June 2025
Volume
17 - 2025
Edited by
Rebecca F. Gottesman, National Institute of Neurological Disorders and Stroke (NIH), United States
Reviewed by
Pei Shang, Mayo Clinic, United States
Uttam Khatri, The University of Texas at Austin, United States
Updates
Copyright
© 2025 Zhang, Xue, Zhang, Li and He.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingli He, lyghml@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.