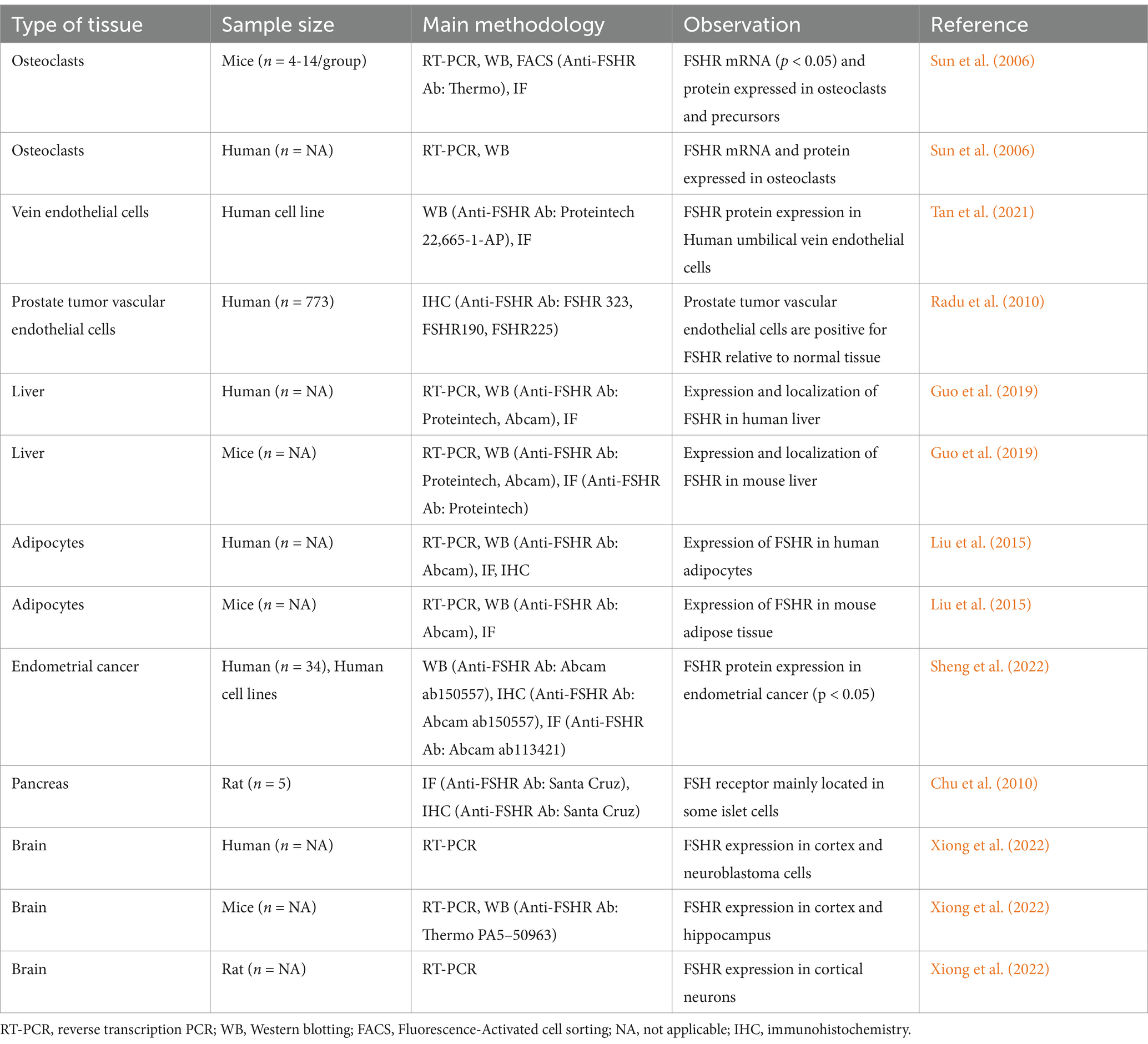
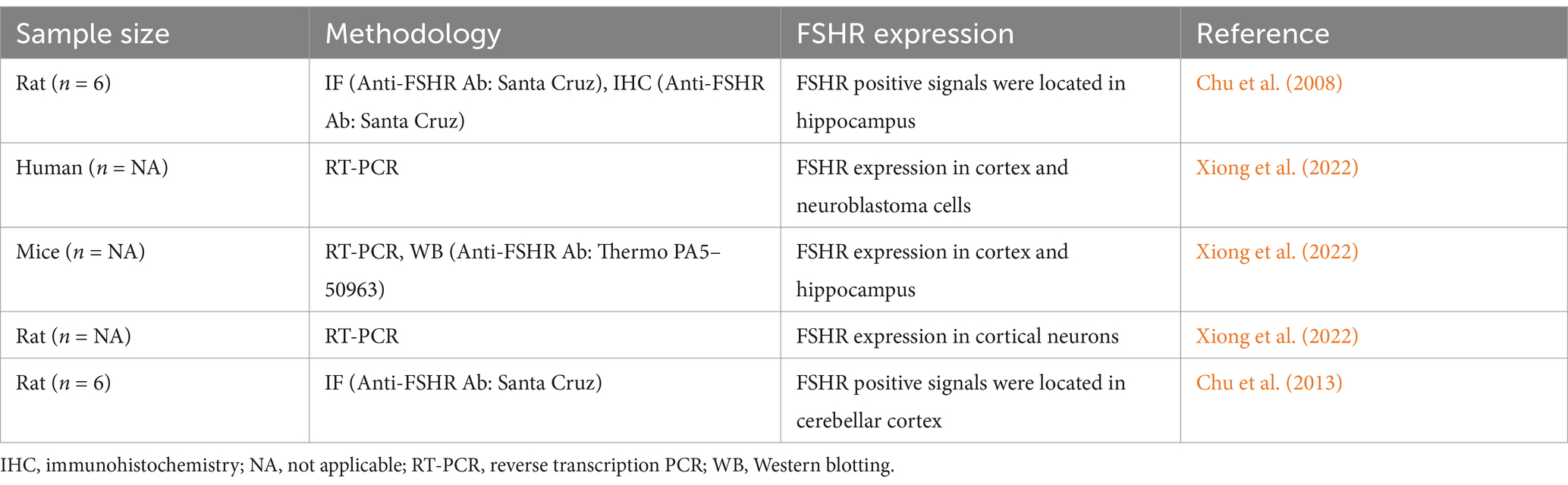
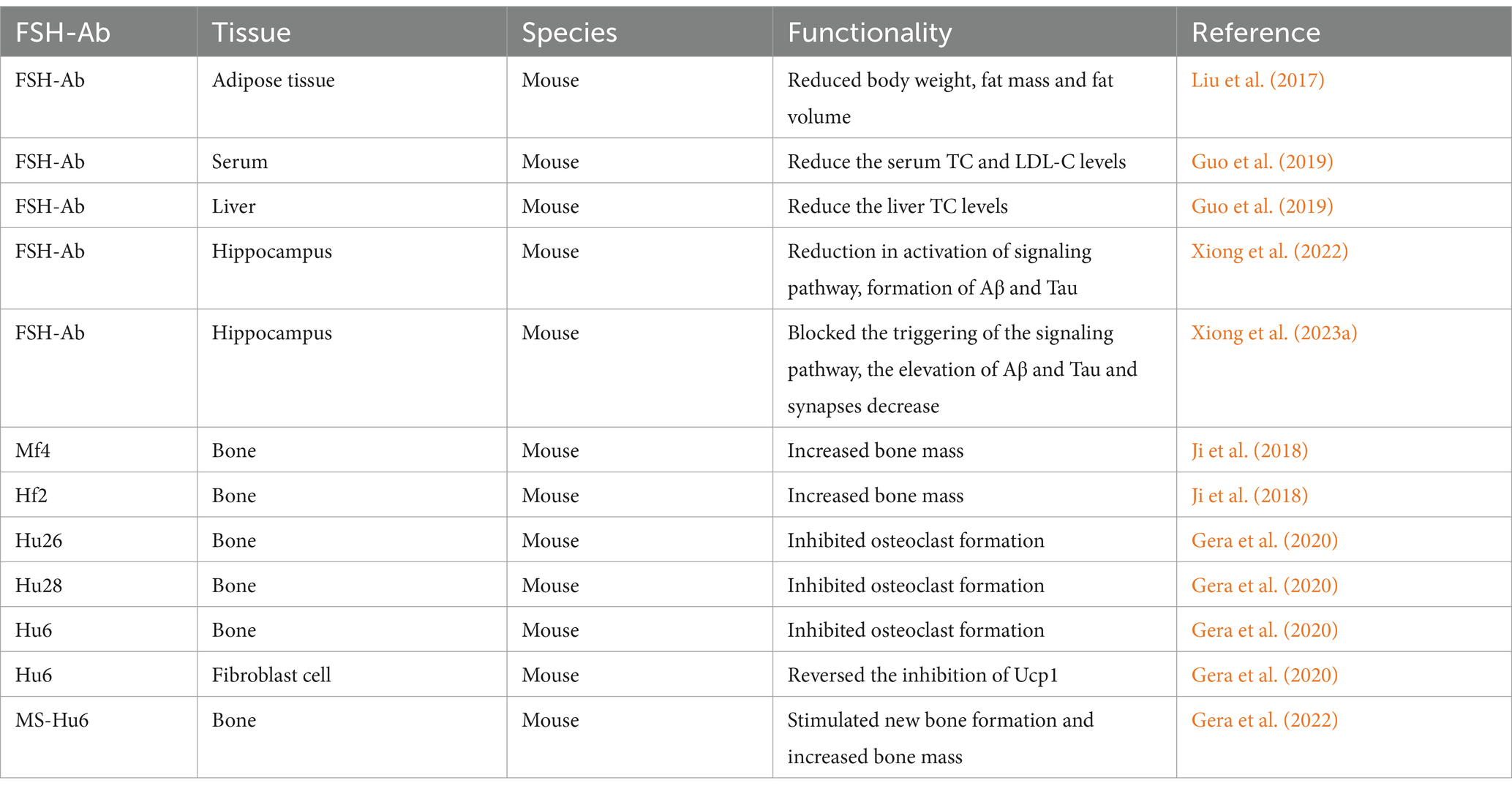
- Department of Obstetrics and Gynaecology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
The role and function of follicle-stimulating hormone in the gonads have been extremely studied. However, recent research has begun to explore the relationship between elevated follicle-stimulating hormone levels and the prevalence of extragonadal disorders, particularly in perimenopausal and postmenopausal women. These disorders include endometrial cancer, osteoporosis, obesity, and atherosclerosis. This review provides new insights into the relationship between follicle-stimulating hormone and the development of age-related diseases, with a focus on Alzheimer’s disease. Follicle-stimulating hormone does not act alone in promoting Alzheimer’s disease but often works in conjunction with inflammation, lipid accumulation, and vascular alterations. Furthermore, follicle-stimulating hormone synergizes with obesity, gut microbiota, autophagy, and aging, creating conditions that facilitate the onset and progression of Alzheimer’s disease. This review also summarizes the therapeutic potential of FSH-blocking antibodies in treating these diseases.
1 Introduction
Perimenopausal and postmenopausal women are at high risk for various age-related diseases, including cardiovascular diseases (Hodis and Mack, 2022), musculoskeletal symptoms (Wright et al., 2024) and cognitive dysfunction (Mosconi et al., 2021). Hormonal changes, particularly the decline in estrogen levels, are closely associated with these risks. As women transition from perimenopause to postmenopause, estrogen levels drop significantly and remain low. However, estrogen replacement therapy remains controversial, with some studies reporting no improvement or even worsening of cognitive function (Cheng et al., 2021). In contrast, follicle-stimulating hormone (FSH) levels begin to rise approximately 2 years before the final menstrual period (FMP) and stabilize 2 years after the FMP, often remaining elevated for decades (Wang et al., 2021). This trend parallels the onset of age-related diseases.
FSH is a gonadotropin secreted by the anterior pituitary gland that binds to FSH receptors (FSHRs), which belong to the class A/rhodopsin subfamily of G protein-coupled receptors. Traditionally, FSH was thought to act primarily on gonadal tissues, specifically sertoli cells in the testes and granulosa cells in the ovaries. However, recent evidence suggests that FSH also plays a role in extragonadal diseases (Table 1).
Alzheimer’s disease (AD) is a degenerative brain disorder and the leading cause of dementia worldwide. Perimenopausal and postmenopausal women are particularly susceptible to AD. According to the American Alzheimer’s Association in 2020, the prevalence of AD increases dramatically with age (Alzheimer's disease facts and figures, 2022), and women are disproportionately affected compared to men. While age is a significant factor, women live longer than men, they are more prone to developing brain lesions, thus increasing the prevalence of AD. The onset of AD is also strongly associated with elevated serum FSH levels. Studies have shown that postmenopausal women with AD have higher serum FSH concentrations than their healthy counterparts, independent of estrogen levels. Recent research has demonstrated that FSH affects neurons, establishing it as an AD-promoting hormone (Xiong et al., 2022). FSHR expression has been detected in the human cortex, neuroblastoma cells (SH-SY5Y), mouse cortex, hippocampus, and rat neurons (Table 2). Knockdown of hippocampal FSHR has been shown to improve AD neuropathology and spatial memory impairment (Xiong et al., 2022).
2 Role of FSH in Alzheimer’s disease
AD is pathologically characterized by amyloid plaques formed by amyloid-β (Aβ) peptides and neurofibrillary tangles (NFTs) composed of hyperphosphorylated Tau proteins. These pathological features lead to synaptic loss, neuronal degeneration, and the hallmark symptoms of AD: memory impairment, cognitive decline, and behavioral dysfunction (Fedele, 2023). The amyloid cascade hypothesis remains the dominant theory explaining AD pathogenesis. According to this hypothesis, Aβ peptides are produced through the cleavage of amyloid precursor protein (APP) by β-secretase and γ-secretase, resulting in various Aβ isoforms, including Aβ42, which is considered the primary driver of AD (Zhang Y. et al., 2023). Dysregulation of Aβ production or clearance leads to its accumulation and aggregation into soluble oligomers and insoluble fibrils. Tau, a protein predominantly found in neuronal axons, plays a critical role in neurite outgrowth, cell shape, and intracellular transport. Hyperphosphorylated Tau disrupts protein degradation systems, such as the ubiquitin-proteasome and phagosome-lysosome pathways, leading to the accumulation of waste proteins in neurons (Drummond et al., 2020).
Xiong et al. (2022) confirmed that FSHR is expressed in the cortex, hippocampus, and neuronal cells by end-point polymerase chain reaction (PCR) or immunofluorescence staining (IF). IF revealed that FSH triggers the expression of C/EBPβ, arginine endopeptidase (AEP), cleaved APP, Tau proteins, Aβ40, and Aβ42 in mice. Consistent with these molecular changes, Morris water maze testing demonstrated that FSH-injected mice exhibited spatial memory impairment, indicating FSH-induced cognitive decline. In ovariectomized mice, hippocampal FSHR knockdown reduced the expression of C/EBPβ, AEP, cleaved APP, and Tau, ameliorating AD neuropathology and spatial memory impairment, independent of estrogen. This was further supported by Golgi staining, transmission electron microscopy and Morris water maze testing. Additionally, FSHR activation in human SH-SY5Y cells and primary rat neuronal cells induced amyloidogenic protein accumulation and the release of inflammatory cytokines IL-6 and IL-1β. These findings confirm the direct role of FSH in AD through the C/EBPβ–δ-secretase pathway and provide a basis for targeting FSH in AD treatment (Figure 1).
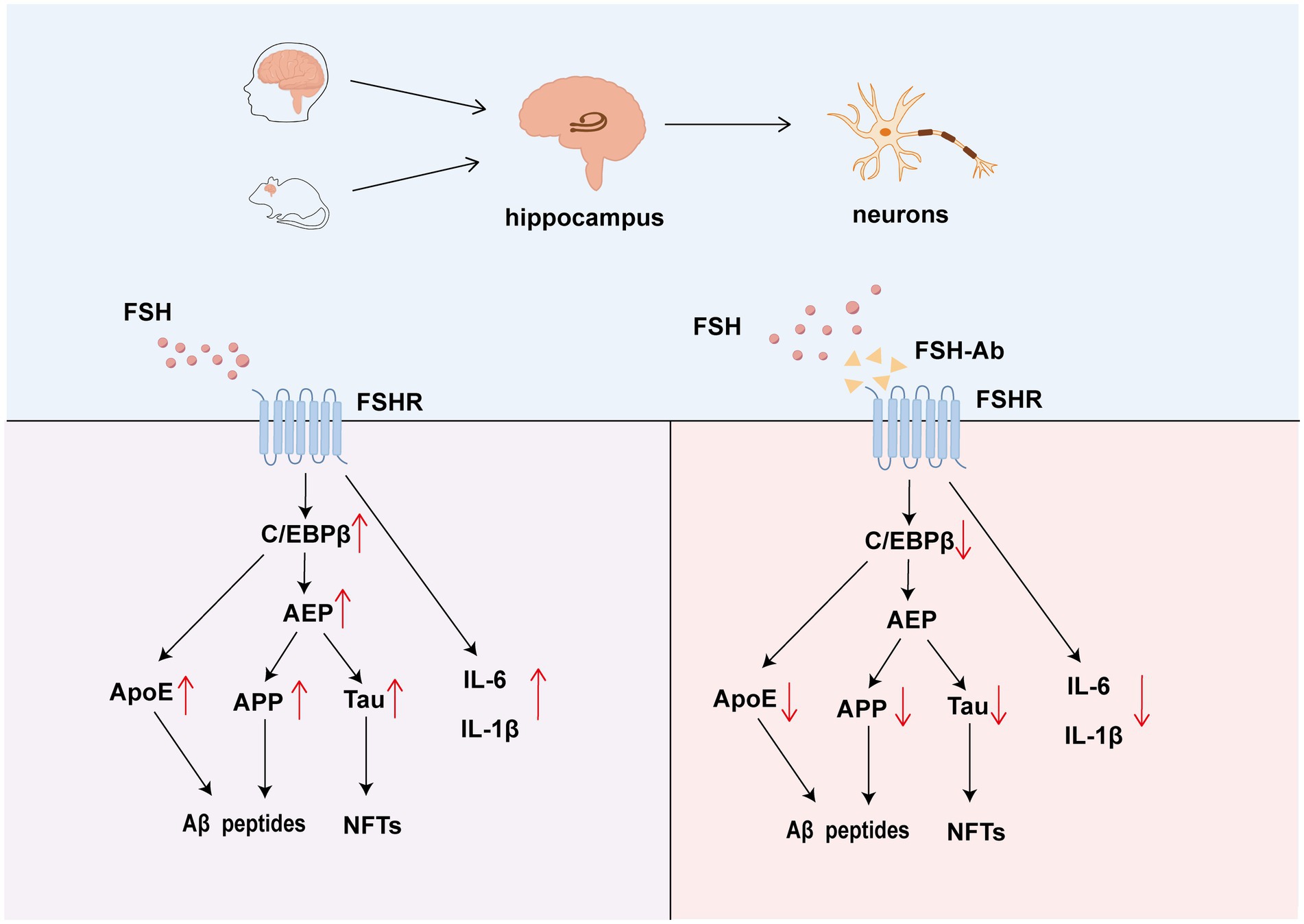
Figure 1. The role of FSH-FSHR signaling in neurons and hippocampus. FSH promotes the formation of amyloid plaques through Aβ peptide aggregation and the development of NFTs. Additionally, FSH triggers the release of ApoE via the C/EBPβ–δ-secretase pathway, which further elevates levels of IL-6 and IL-1β. The action of FSH is antagonized by FSH-Ab, which binds to FSHR and inhibits it’s signaling.
3 Potential mechanisms by which FSH increases risk of AD
FSH contributes to AD pathogenesis both directly and in tandem with other mechanisms, including inflammation, lipid accumulation, and vascular alterations.
3.1 Inflammation
Neuroinflammation is a key factor in AD pathogenesis. Chronic neuroinflammation, driven by glial overactivation, is considered the third core pathological feature of AD, alongside Aβ plaques and NFT (Leng and Edison, 2021). When the balance between pro-inflammatory and anti-inflammatory signals is disrupted, glial cells release interleukin (IL)-1β and tumor necrosis factor (TNF)-α, leading to neuronal damage through excessive phagocytosis (Twarowski and Herbet, 2023). Inflammatory cytokines also exacerbate Aβ accumulation and Tau propagation (Chen and Yu, 2023). Xiong et al. (2022) demonstrated that FSH increased the expression of IL-1β (1.4-fold, p < 0.001) and IL-6 (1.75-fold, p < 0.001) in human primary neuronal cells, along with elevated levels of APP and Tau. FSHR knockdown reduced the expression of these inflammatory markers, suggesting that FSH promotes neuroinflammation and exacerbates AD pathology (Xiong et al., 2022).
Systemic inflammation, triggered by external factors, also impacts neurodegenerative diseases. Inflammatory mediators can induce neuronal inflammation through neural and humoral pathways, leading to brain damage (Marizzoni et al., 2023). While peripheral lipopolysaccharide (LPS) and pro-inflammatory cytokines typically do not cause widespread neuronal damage, AD is an exception. The blood–brain barrier (BBB) becomes more permeable with age, particularly in the hippocampus, allowing peripheral inflammatory factors to activate microglia and increase central pro-inflammatory factors. In postmenopausal women, elevated systemic inflammatory factors such as TNF-α (95% CI 0.46 to 2.44, p = 0.005), IL-1β (95% CI 1.35 to 16.26, p = 0.02), and IL-6 (95% CI 0.06 to 1.98, p = 0.003) are strongly associated with increased FSH levels (Abildgaard et al., 2020). FSH induces the expression of these cytokines, contributing to both peripheral and central inflammation (Lai et al., 2022).
3.2 Lipid accumulation
Recent studies have highlighted the synergistic effects of FSH and apolipoprotein E4 (ApoE4) (Xiong et al., 2023a), the primary genetic risk factor for AD, in activating the C/EBPβ/δ-secretase pathway, which promotes AD-like pathologies (Figure 1). Ovariectomized mice mimicking a menopausal state developed AD-like pathologies, primarily driven by FSH rather than estrogen. ApoE4-knockin female mice also exhibited AD-like pathologies with increasing FSH levels, which were alleviated by anti-FSHβ antibody (FSH-Ab) treatment. Additionally, ApoE4-expressing mice showed impaired cerebrovascular integrity, elevated astrocyte hyperplasia, and disrupted BBB function, all of which accelerate AD pathogenesis (Liu C. C. et al., 2022).
Many of the proteins found in Alzheimer’s plaques have been hypothesized to be ligands for low-density lipoprotein receptors on neurons in the central nervous system (CNS), thus, FSH has been implicated in lipid deposition in neurons, leading to amyloid plaque formation (Bowen et al., 2000). A cross-sectional study found a correlation between FSH levels and cholesterol levels in women over 55 (Zhang W. et al., 2023). In mouse models, compared to the control group, high-dose FSH treatment increased serum levels of total cholesterol (TC) (1.25-fold, p < 0.01) and low-density lipoprotein cholesterol (LDL-C) (1.4-fold, p < 0.05) (Guo et al., 2019). Elevated LDL-C levels are associated with a higher incidence of AD (Saiz-Vazquez et al., 2020), with each 1 mmol/L increase in LDL-C linked to an approximately 17% increase in AD risk (Wee et al., 2023). High-density lipoprotein (HDL) facilitates Aβ transport and reduces Aβ accumulation in vascular tissue (Poliakova and Wellington, 2023). In conclusion, cholesterol may bind to lipoprotein receptor-related protein-1 (LRP1), promoting Aβ deposition and removal, vascular stiffness, arteriosclerosis, and cerebral amyloid angiopathy (CAA). Cholesterol also plays a structural role in cell membranes, which are major components of basic synaptic integrity and neurotransmission.
3.3 Vascular alterations
Cerebrovascular changes are another critical factor in AD. A large autopsy-based neuropathological study has revealed that 80% of AD patients without vascular dementia exhibit vascular lesions, including arteriosclerosis and CAA (Sweeney et al., 2019). Arterial stiffness is strongly associated with AD (Cortes-Canteli and Iadecola, 2020), as it reduces cerebral blood flow (CBF) and promotes Aβ deposition (Apatiga-Perez et al., 2022), involving in angiostarch deposition in early. Postmenopausal women show an inverse correlation between FSH levels and vascular compliance, with FSH contributing to vascular stiffness and endothelial damage (Laakkonen et al., 2021; Wenner et al., 2024). Notably, FSH levels were positively associated with the augmentation index (95% CI 0.68 to 1.09, p < 0.001) (Laakkonen et al., 2021). Path analysis further demonstrated that the effect of age on flow-mediated dilation (p = 0.01) was partially mediated by FSH (Wenner et al., 2024), suggesting a hormonal role in age-related vascular deterioration. FSH regulates the expression of vascular cell adhesion molecule 1 (VCAM-1) in endothelial cells through the FSHR/Gas/cAMP/PKA and PI3K/Akt/mTOR/NF-κB pathways, leading to vascular calcification and reduced elasticity (Piao et al., 2022). High FSH concentrations also disrupt the expression of V-cadherin and E-cadherin, increasing membrane permeability (Rocca et al., 2024). These vascular changes may contribute to the increased risk of AD in perimenopausal and postmenopausal women.
4 Blood–brain barrier breakdown
The BBB is composed of brain endothelial cells connected by tight junctions and brain perivascular cells, which include pericytes, astrocytes, microglia, and oligodendrocytes. The BBB can shield deleterious waste metabolites and remove Aβ, thereby maintaining brain homeostasis and protecting neurons (Takata et al., 2021).
Due to impairment of the BBB, there seems to be a close relationship between peripheral FSH, proinflammatory factors, lipids, vascular stiffness and the onset of AD. The two-hit hypothesis provides a compelling explanation for this phenomenon (Figure 2). The first hit involves BBB disruption and reduced CBF, leading to vascular-mediated neuronal dysfunction and the leakage of harmful metabolites, including FSH, lipids, and pro-inflammatory factors. The second hit involves the pathological accumulation of these risk factors, causing neurodegeneration (Eisenmenger et al., 2023). FSH may affect the cerebrovascular system on both sides of the BBB, highlighting the importance of the cerebrovascular system as a bridge between the brain and the body.
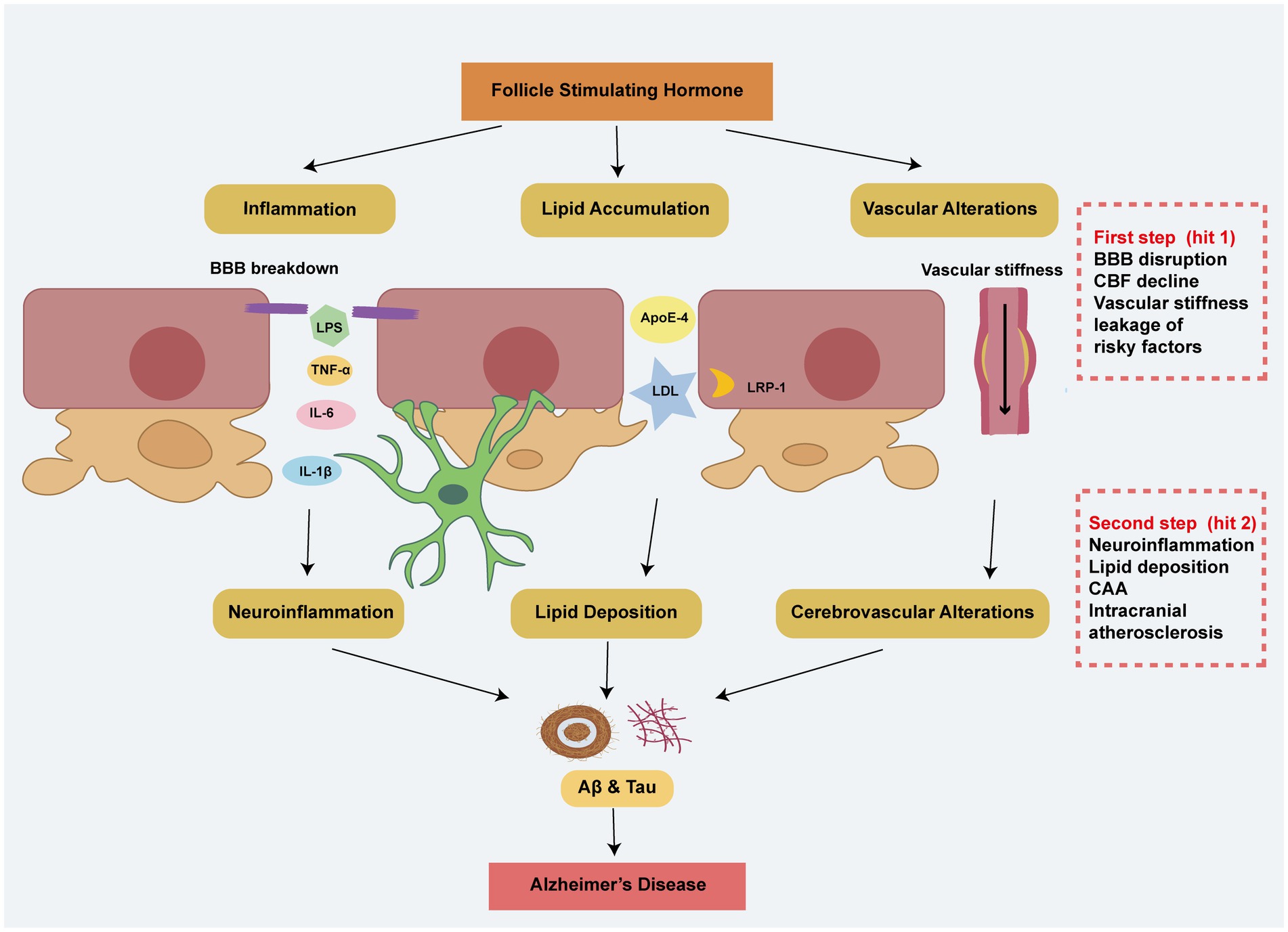
Figure 2. The two-hit hypothesis explains the effects of peripheral risky factors including FSH in AD. In the first step, FSH contributes to BBB breakdown, vascular stiffness, and a decline in CBF, leading to the leakage of risk factors through mechanisms involving inflammation, lipid accumulation, and vascular alterations. Building on the step 1, these peripheral risk factors exacerbate neuroinflammation, cerebral lipid deposition, CAA, and intracranial atherosclerosis, ultimately resulting in Alzheimer’s disease-like pathology.
Currently, while evidence regarding the direct effects of FSH on the blood–brain barrier (BBB) remains insufficient, several interconnected mechanisms may have affection. FSH regulates the expression of connexin 43 (Cx43) in postmenopausal women (Wilson et al., 2008), potentially disrupting gap junction communication critical for BBB maintenance. The glucose transporter GLUT1, highly expressed in brain microvascular endothelial cells forming the BBB, mediates glucose transport to maintain neuronal function and BBB integrity (Koepsell, 2020), whereas FSH regulates GULT1 expression via the HIF-1α-AMPK signaling pathway (Wu et al., 2022). Additionally, VCAM-1 expressed by cerebral microvascular endothelial cells promotes cerebral vascular inflammation and damages BBB (Salian et al., 2024). While FSHR expression has been detected in peripheral endothelial cells and pericytes but not yet confirmed in brain endothelial cells (Maclellan et al., 2018). Importantly, recent studies demonstrate that FSH stimulates VCAM-1 production in vascular endothelial cells via FSHR-dependent mechanisms, enhancing monocyte-endothelial adhesion (Li et al., 2017). These findings suggest that elevated FSH levels during menopause may accelerate AD pathogenesis through combined effects on endothelial metabolism, cell–cell communication, and neurovascular inflammation.
5 Potential synergistic effects of FSH with other risk factors
Other risk factors may synergize with FSH to create a permissive environment for the development of age-related diseases, especially AD (Figure 3).
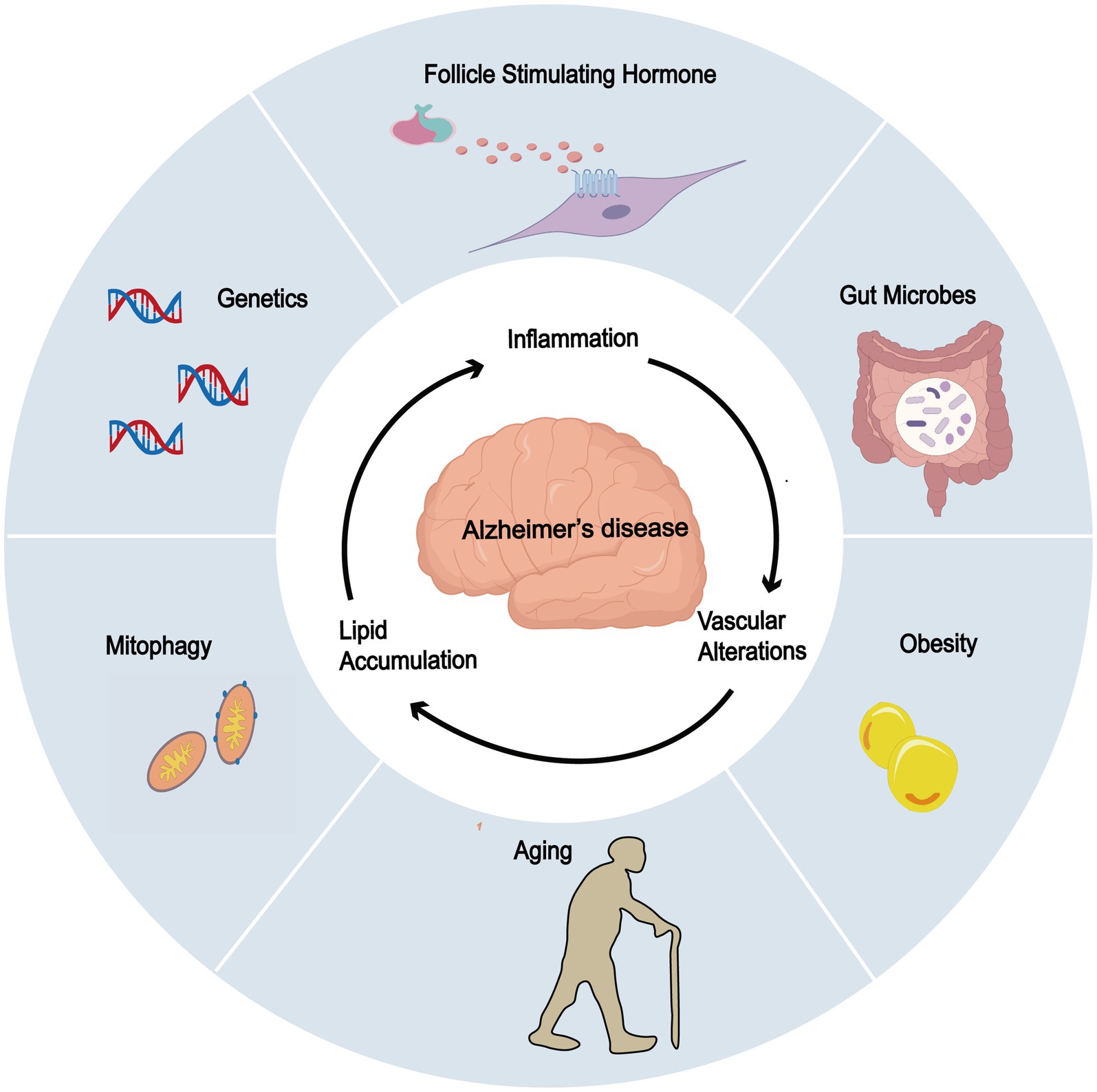
Figure 3. Potential association of FSH and other risk factors in AD. FSH exhibits potential synergistic effects with obesity, gut microbiota dysbiosis, impaired mitophagy, genetic factors (ApoE), and aging, all of which contribute to the development and progression of AD.
5.1 Obesity
Obesity is a significant risk factor for AD in perimenopausal and postmenopausal women. Obesity impairs endothelial function, disrupts the BBB, and induces adipose tissue inflammation, leading to elevated levels of adipokines and free fatty acids (FFAs) that promote amyloid and Tau aggregation (Kao et al., 2020). FSH levels are positively correlated with body mass index (BMI) and regulate fat accumulation through the Gαi/Ca2+/CREB pathway and Ucp1 expression in visceral adipose tissue (Xiong et al., 2023b).
5.2 Gut microbiota
The brain-gut-microbiota axis has been increasingly studied in relation to AD. Dysbiosis of gut microbiota leads to an imbalance between pro-inflammatory and anti-inflammatory bacteria, contributing to peripheral inflammation in patients with cognitive dysfunction and cerebral amyloidosis. FSH levels are positively correlated with pro-inflammatory gut bacteria such as Escherichia/Shigella and negatively correlated with anti-inflammatory bacteria such as Eubacterium and Faecalibacterium (Kwon et al., 2023). Patients with AD-related cognitive impairment show higher levels of Firmicutes and Proteobacteria, consistent with FSH’s effects on gut microbiota (Liu Y. et al., 2022). Despite knowledge about the associations between FSH and gut microbiota, the exact mechanisms and their role in AD have not yet been fully elucidated.
5.3 Mitophagy
Mitophagy, the selective degradation of mitochondria, plays a crucial role in neurodegenerative diseases (Quinn et al., 2020). Increased PINK1-Parkin expression is associated with reduced Aβ levels and improved cognitive function in mice (Han et al., 2020). FSH inhibits oxidative stress in granulosa cells through the FSH-HIF-1α-PINK1-Parkin pathway (Li et al., 2020). mTOR, a downstream factor of FSH signaling, regulates autophagy. Inhibition of mTOR with rapamycin enhances autophagy, reduces Aβ levels, and alleviates AD progression.
5.4 Aging
In perimenopausal and postmenopausal women, aging is always the initial factor in the development of more common diseases. Chronic inflammation, dysbiosis, and impaired autophagy are emerging hallmarks of aging that interact synergistically to promote disease (Lopez-Otin et al., 2023). Moreover, during aging, the relationship between changes in hormone levels (including FSH) and pathological changes is not singular but rather involves multiple correlations. The aging of neuronal cells progressively worsens the intracranial environment, characterized by the accumulation of oxygen radicals, impaired BBB integrity, and the loss of functional synapses. These changes may drive or sustain brain inflammation by increasing the expression of inflammatory molecules, ultimately contributing to the development of AD (Guerrero et al., 2021).
6 Therapy and perspectives about FSH-blocking antibody
FSH has emerged as a promising therapeutic target for diseases in perimenopause and postmenopause. Antibodies targeting the β-subunit of FSH (FSH-Ab) have shown potential in alleviating disease progression (Table 3).
Early experiments demonstrated that FSH-Ab reduced fat mass and abdominal fat in mice without affecting total body weight (Liu et al., 2017). FSH-Ab also lowered serum TC and LDL-C levels in ovariectomized mice (Guo et al., 2019). In Alzheimer’s mice, FSH-Ab also inhibited the formation of plaques and neurofibrillary tangles and reversed cognitive decline (Xiong et al., 2022). Further studies revealed that FSH-Ab blocked the activation of the C/EBPβ/δ-secretase signaling pathway and reduced the levels of Aβ and Tau in ApoE4-TR mice. Additionally, FSH-Ab mitigated the extensive astrogliosis and microglia activation induced by ovariectomy, thereby rescuing impaired learning and memory (Xiong et al., 2023a). Monoclonal antibodies targeting FSHβ, such as Hf2 and Mf4, have been shown to increase bone mass by inhibiting osteoclast activity (Ji et al., 2018). Humanized antibodies (Hu6, Hu26, Hu28) bind FSH with high affinity and block FSHR activation, reducing osteoclast formation and promoting beige adipose tissue formation (Gera et al., 2020). New evidence also indicated that MS-Hu6 could stimulate new bone formation and increase bone mass (Gera et al., 2022). Additionally, formulated MS-Hu6 demonstrated improved stability and enhanced binding affinity to FSH at higher concentrations (Rojekar et al., 2023).
While FSH-blocking antibodies have shown promise in preclinical studies, they have yet to be applied in clinical practice.
7 Conclusion
This review summarizes the current understanding of the mechanisms and risk factors underlying AD in perimenopausal and postmenopausal women. FSH contributes to AD pathogenesis through neuronal signaling pathways, inflammation, lipid accumulation, and vascular alterations. The two-hit hypothesis provides a framework for understanding how peripheral risk factors, including FSH, influence AD development. Synergistic interactions between FSH and other risk factors, such as obesity, gut microbiota, autophagy, and aging, further exacerbate AD progression. A deeper understanding of the role of FSH in AD may lead to improved diagnostic methods and novel therapeutic strategies. FSH-blocking antibodies represent a promising avenue for AD treatment, though further research is needed to translate these findings into clinical applications.
Author contributions
YX: Writing – original draft, Writing – review & editing. SZ: Writing – review & editing. FW: Writing – review & editing. XQ: Funding acquisition, Investigation, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Financial support was prepared by the National Natural Science Foundation of China (Grant Number: 81902636), the Natural Science Foundation of Shandong Province (Grant Number: ZR2020MH066), and the China Postdoctoral Science Foundation (Grant Number: 2020M682219).
Acknowledgments
We would like to thank all the studies that have explored the mechanisms by which FSH promotes Alzheimer’s disease, as well as all the authors of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abildgaard, J., Tingstedt, J., Zhao, Y., Hartling, H. J., Pedersen, A. T., Lindegaard, B., et al. (2020). Increased systemic inflammation and altered distribution of T-cell subsets in postmenopausal women. PLoS One 15:e0235174. doi: 10.1371/journal.pone.0235174
Alzheimer's disease facts and figures (2022). 2022 Alzheimer's disease facts and figures. Alzheimers Dement. 18, 700–789. doi: 10.1002/alz.12638
Apatiga-Perez, R., Soto-Rojas, L. O., Campa-Cordoba, B. B., Luna-Viramontes, N. I., Cuevas, E., Villanueva-Fierro, I., et al. (2022). Neurovascular dysfunction and vascular amyloid accumulation as early events in Alzheimer's disease. Metab. Brain Dis. 37, 39–50. doi: 10.1007/s11011-021-00814-4
Bowen, R. L., Isley, J. P., and Atkinson, R. L. (2000). An association of elevated serum gonadotropin concentrations and Alzheimer disease? J. Neuroendocrinol. 12, 351–354. doi: 10.1046/j.1365-2826.2000.00461.x
Chen, Y., and Yu, Y. (2023). Tau and neuroinflammation in Alzheimer's disease: interplay mechanisms and clinical translation. J. Neuroinflammation 20:165. doi: 10.1186/s12974-023-02853-3
Cheng, Y. J., Lin, C. H., and Lane, H. Y. (2021). From menopause to neurodegeneration-molecular basis and potential therapy. Int. J. Mol. Sci. 22:8654. doi: 10.3390/ijms22168654
Chu, C., Gao, G., and Huang, W. (2008). A study on co-localization of FSH and its receptor in rat hippocampus. J. Mol. Histol. 39, 49–55. doi: 10.1007/s10735-007-9125-2
Chu, C., Xu, B., and Huang, W. (2010). A study on expression of FSH and its effects on the secretion of insulin and glucagon in rat pancreas. Tissue Cell 42, 370–375. doi: 10.1016/j.tice.2010.09.001
Chu, C., Zhou, J., Zhao, Y., Liu, C., Chang, P., Zhou, Q., et al. (2013). Expression of FSH and its co-localization with FSH receptor and GnRH receptor in rat cerebellar cortex. J. Mol. Histol. 44, 19–26. doi: 10.1007/s10735-012-9449-4
Cortes-Canteli, M., and Iadecola, C. (2020). Alzheimer's disease and vascular aging: JACC focus seminar. J. Am. Coll. Cardiol. 75, 942–951. doi: 10.1016/j.jacc.2019.10.062
Drummond, E., Pires, G., MacMurray, C., Askenazi, M., Nayak, S., Bourdon, M., et al. (2020). Phosphorylated tau interactome in the human Alzheimer's disease brain. Brain 143, 2803–2817. doi: 10.1093/brain/awaa223
Eisenmenger, L. B., Peret, A., Famakin, B. M., Spahic, A., Roberts, G. S., Bockholt, J. H., et al. (2023). Vascular contributions to Alzheimer's disease. Transl. Res. 254, 41–53. doi: 10.1016/j.trsl.2022.12.003
Fedele, E. (2023). Anti-amyloid therapies for Alzheimer's disease and the amyloid cascade hypothesis. Int. J. Mol. Sci. 24:14499. doi: 10.3390/ijms241914499
Gera, S., Kuo, T. C., Gumerova, A. A., Korkmaz, F., Sant, D., DeMambro, V., et al. (2022). FSH-blocking therapeutic for osteoporosis. eLife 11:11. doi: 10.7554/eLife.78022
Gera, S., Sant, D., Haider, S., Korkmaz, F., Kuo, T. C., Mathew, M., et al. (2020). First-in-class humanized FSH blocking antibody targets bone and fat. Proc. Natl. Acad. Sci. USA 117, 28971–28979. doi: 10.1073/pnas.2014588117
Guerrero, A., De Strooper, B., and Arancibia-Carcamo, I. L. (2021). Cellular senescence at the crossroads of inflammation and Alzheimer's disease. Trends Neurosci. 44, 714–727. doi: 10.1016/j.tins.2021.06.007
Guo, Y., Zhao, M., Bo, T., Ma, S., Yuan, Z., Chen, W., et al. (2019). Blocking FSH inhibits hepatic cholesterol biosynthesis and reduces serum cholesterol. Cell Res. 29, 151–166. doi: 10.1038/s41422-018-0123-6
Han, Y., Wang, N., Kang, J., and Fang, Y. (2020). β-Asarone improves learning and memory in Aβ(1-42)-induced Alzheimer's disease rats by regulating PINK1-Parkin-mediated mitophagy. Metab. Brain Dis. 35, 1109–1117. doi: 10.1007/s11011-020-00587-2
Hodis, H. N., and Mack, W. J. (2022). Menopausal hormone replacement therapy and reduction of all-cause mortality and cardiovascular disease: it is about time and timing. Cancer J. 28, 208–223. doi: 10.1097/PPO.0000000000000591
Ji, Y., Liu, P., Yuen, T., Haider, S., He, J., Romero, R., et al. (2018). Epitope-specific monoclonal antibodies to FSHbeta increase bone mass. Proc. Natl. Acad. Sci. USA 115, 2192–2197. doi: 10.1073/pnas.1718144115
Kao, Y. C., Ho, P. C., Tu, Y. K., Jou, I. M., and Tsai, K. J. (2020). Lipids and Alzheimer's disease. Int. J. Mol. Sci. 21:1505. doi: 10.3390/ijms21041505
Koepsell, H. (2020). Glucose transporters in brain in health and disease. Pflugers Arch. 472, 1299–1343. doi: 10.1007/s00424-020-02441-x
Kwon, Y. S., Ko, J. S., Oh, S. Y., Han, Y. T., and Jo, S. A. (2023). Oleracone F alleviates cognitive impairment and neuropathology in APPswe/PSEN1dE9 mice by reducing the expression of vascular cell adhesion molecule and leukocyte adhesion to brain vascular endothelial cells. Int. J. Mol. Sci. 24:2056. doi: 10.3390/ijms24032056
Laakkonen, E. K., Karppinen, J. E., Lehti, S., Lee, E., Pesonen, E., Juppi, H. K., et al. (2021). Associations of sex hormones and hormonal status with arterial stiffness in a female sample from reproductive years to menopause. Front. Endocrinol. 12:765916. doi: 10.3389/fendo.2021.765916
Lai, T. H., Chen, H. T., and Wu, W. B. (2022). Trophoblast coculture induces intercellular adhesion molecule-1 expression in uterine endometrial epithelial cells through TNF-alpha production: implication of role of FSH and ICAM-1 during embryo implantation. J. Reprod. Immunol. 152:103650. doi: 10.1016/j.jri.2022.103650
Leng, F., and Edison, P. (2021). Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 17, 157–172. doi: 10.1038/s41582-020-00435-y
Li, X., Chen, W., Li, P., Wei, J., Cheng, Y., Liu, P., et al. (2017). Follicular stimulating hormone accelerates Atherogenesis by increasing endothelial VCAM-1 expression. Theranostics 7, 4671–4688. doi: 10.7150/thno.21216
Li, C., Zhou, J., Liu, Z., Zhou, J., Yao, W., Tao, J., et al. (2020). FSH prevents porcine granulosa cells from hypoxia-induced apoptosis via activating mitophagy through the HIF-1α-PINK1-Parkin pathway. FASEB J. 34, 3631–3645. doi: 10.1096/fj.201901808RRR
Liu, X. M., Chan, H. C., Ding, G. L., Cai, J., Song, Y., Wang, T. T., et al. (2015). FSH regulates fat accumulation and redistribution in aging through the Gαi/ca(2+)/CREB pathway. Aging Cell 14, 409–420. doi: 10.1111/acel.12331
Liu, P., Ji, Y., Yuen, T., Rendina-Ruedy, E., DeMambro, V. E., Dhawan, S., et al. (2017). Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 546, 107–112. doi: 10.1038/nature22342
Liu, C. C., Zhao, J., Fu, Y., Inoue, Y., Ren, Y., Chen, Y., et al. (2022). Peripheral apoE4 enhances Alzheimer's pathology and impairs cognition by compromising cerebrovascular function. Nat. Neurosci. 25, 1020–1033. doi: 10.1038/s41593-022-01127-0
Liu, Y., Zhou, Y., Mao, T., Huang, Y., Liang, J., Zhu, M., et al. (2022). The relationship between menopausal syndrome and gut microbes. BMC Womens Health 22:437. doi: 10.1186/s12905-022-02029-w
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M., and Kroemer, G. (2023). Hallmarks of aging: an expanding universe. Cell 186, 243–278. doi: 10.1016/j.cell.2022.11.001
Maclellan, R. A., Konczyk, D. J., Goss, J. A., and Greene, A. K. (2018). Analysis of follicle-stimulating hormone receptor in infantile hemangioma. Ann. Plast. Surg. 80, S211–S213. doi: 10.1097/SAP.0000000000001438
Marizzoni, M., Mirabelli, P., Mombelli, E., Coppola, L., Festari, C., Lopizzo, N., et al. (2023). A peripheral signature of Alzheimer's disease featuring microbiota-gut-brain axis markers. Alzheimers Res. Ther. 15:101. doi: 10.1186/s13195-023-01218-5
Mosconi, L., Berti, V., Dyke, J., Schelbaum, E., Jett, S., Loughlin, L., et al. (2021). Menopause impacts human brain structure, connectivity, energy metabolism, and amyloid-beta deposition. Sci. Rep. 11:10867. doi: 10.1038/s41598-021-90084-y
Piao, J., Yin, Y., Zhao, Y., Han, Y., Zhan, H., Luo, D., et al. (2022). Follicle-stimulating hormone accelerates atherosclerosis by activating PI3K/Akt/NF-κB pathway in mice with androgen deprivation. J. Vasc. Res. 59, 358–368. doi: 10.1159/000527239
Poliakova, T., and Wellington, C. L. (2023). Roles of peripheral lipoproteins and cholesteryl ester transfer protein in the vascular contributions to cognitive impairment and dementia. Mol. Neurodegener. 18:86. doi: 10.1186/s13024-023-00671-y
Quinn, P. M. J., Moreira, P. I., Ambrosio, A. F., and Alves, C. H. (2020). PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol. Commun. 8:189. doi: 10.1186/s40478-020-01062-w
Radu, A., Pichon, C., Camparo, P., Antoine, M., Allory, Y., Couvelard, A., et al. (2010). Expression of follicle-stimulating hormone receptor in tumor blood vessels. N. Engl. J. Med. 363, 1621–1630. doi: 10.1056/NEJMoa1001283
Rocca, M. S., Pannella, M., Bayraktar, E., Marino, S., Bortolozzi, M., Di Nisio, A., et al. (2024). Extragonadal function of follicle-stimulating hormone: evidence for a role in endothelial physiology and dysfunction. Mol. Cell. Endocrinol. 594:112378. doi: 10.1016/j.mce.2024.112378
Rojekar, S., Pallapati, A. R., Gimenez-Roig, J., Korkmaz, F., Sultana, F., Sant, D., et al. (2023). Development and biophysical characterization of a humanized FSH-blocking monoclonal antibody therapeutic formulated at an ultra-high concentration. eLife 12:e88898. doi: 10.7554/eLife.88898
Saiz-Vazquez, O., Puente-Martinez, A., Ubillos-Landa, S., Pacheco-Bonrostro, J., and Santabarbara, J. (2020). Cholesterol and Alzheimer's disease risk: a meta-meta-analysis. Brain Sci. 10:386. doi: 10.3390/brainsci10060386
Salian, V. S., Tang, X., Thompson, K. J., Curan, G. L., Lowe, V. J., Li, L., et al. (2024). Molecular mechanisms underlying amyloid Beta peptide mediated upregulation of vascular cell adhesion Molecule-1 in Alzheimer disease. J. Pharmacol. Exp. Ther. 391, 430–440. doi: 10.1124/jpet.124.002280
Sheng, S., Liu, W., Xue, Y., Pan, Z., Zhao, L., Wang, F., et al. (2022). Follicle-stimulating hormone promotes the development of endometrial Cancer in vitro and in vivo. Int. J. Environ. Res. Public Health 19:15344. doi: 10.3390/ijerph192215344
Sun, L., Peng, Y., Sharrow, A. C., Iqbal, J., Zhang, Z., Papachristou, D. J., et al. (2006). FSH directly regulates bone mass. Cell 125, 247–260. doi: 10.1016/j.cell.2006.01.051
Sweeney, M. D., Montagne, A., Sagare, A. P., Nation, D. A., Schneider, L. S., Chui, H. C., et al. (2019). Vascular dysfunction-the disregarded partner of Alzheimer's disease. Alzheimers Dement. 15, 158–167. doi: 10.1016/j.jalz.2018.07.222
Takata, F., Nakagawa, S., Matsumoto, J., and Dohgu, S. (2021). Blood-brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front. Cell. Neurosci. 15:661838. doi: 10.3389/fncel.2021.661838
Tan, D., Zhao, Y., Ma, D., and Tong, F. (2021). Role of FSH and FSH receptor on HUVECs migration. Gene Ther. 28, 155–161. doi: 10.1038/s41434-020-00195-w
Twarowski, B., and Herbet, M. (2023). Inflammatory processes in Alzheimer's disease-Pathomechanism, diagnosis and treatment: a review. Int. J. Mol. Sci. 24:6518. doi: 10.3390/ijms24076518
Wang, Y., Tang, R., Luo, M., Sun, X., Li, J., Yue, Y., et al. (2021). Follicle stimulating hormone and estradiol trajectories from menopausal transition to late postmenopause in indigenous Chinese women. Climacteric 24, 80–88. doi: 10.1080/13697137.2020.1775807
Wee, J., Sukudom, S., Bhat, S., Marklund, M., Peiris, N. J., Hoyos, C. M., et al. (2023). The relationship between midlife dyslipidemia and lifetime incidence of dementia: a systematic review and meta-analysis of cohort studies. Alzheimers Dement. 15:e12395. doi: 10.1002/dad2.12395
Wenner, M. M., Shenouda, N., Shoemaker, L., Kuczmarski, A., Haigh, K., Del Vecchio, A., et al. (2024). Characterizing vascular and hormonal changes in women across the life span: a cross-sectional analysis. Am. J. Physiol. Heart Circ. Physiol. 327, H1286–H1295. doi: 10.1152/ajpheart.00373.2024
Wilson, A. C., Clemente, L., Liu, T., Bowen, R. L., Meethal, S. V., and Atwood, C. S. (2008). Reproductive hormones regulate the selective permeability of the blood-brain barrier. Biochim. Biophys. Acta 1782, 401–407. doi: 10.1016/j.bbadis.2008.02.011
Wright, V. J., Schwartzman, J. D., Itinoche, R., and Wittstein, J. (2024). The musculoskeletal syndrome of menopause. Climacteric 27, 466–472. doi: 10.1080/13697137.2024.2380363
Wu, G., Li, C., Tao, J., Liu, Z., Li, X., Zang, Z., et al. (2022). FSH mediates estradiol synthesis in hypoxic granulosa cells by activating glycolytic metabolism through the HIF-1α-AMPK-GLUT1 signaling pathway. J. Biol. Chem. 298:101830. doi: 10.1016/j.jbc.2022.101830
Xiong, J., Kang, S. S., Wang, Z., Liu, X., Kuo, T. C., Korkmaz, F., et al. (2022). FSH blockade improves cognition in mice with Alzheimer's disease. Nature 603, 470–476. doi: 10.1038/s41586-022-04463-0
Xiong, J., Kang, S. S., Wang, M., Wang, Z., Xia, Y., Liao, J., et al. (2023a). FSH and ApoE4 contribute to Alzheimer's disease-like pathogenesis via C/EBPβ/δ-secretase in female mice. Nat. Commun. 14:6577. doi: 10.1038/s41467-023-42282-7
Xiong, J., Zhang, Z., and Ye, K. (2023b). C/EBPβ/AEP signaling drives Alzheimer's disease pathogenesis. Neurosci. Bull. 39, 1173–1185. doi: 10.1007/s12264-023-01025-w
Zhang, Y., Chen, H., Li, R., Sterling, K., and Song, W. (2023). Amyloid beta-based therapy for Alzheimer's disease: challenges, successes and future. Signal Transduct. Target. Ther. 8:248. doi: 10.1038/s41392-023-01484-7
Keywords: follicle-stimulating hormone, Alzheimer’s disease, aging, FSH-blocking antibodies, neuroinflammation, lipid accumulation
Citation: Xue Y, Zuo S, Wang F and Qi X (2025) From hormones to neurodegeneration: how FSH drives Alzheimer’s disease. Front. Aging Neurosci. 17:1578439. doi: 10.3389/fnagi.2025.1578439
Edited by:
Roberta Marongiu, Cornell University, United StatesReviewed by:
Sarah Holden, Oregon Health and Science University, United StatesDongwei Sun, University of California, Riverside, United States
Copyright © 2025 Xue, Zuo, Wang and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyi Qi, cWl4aWFveWlAc2RmbXUuZWR1LmNu
 Yafei Xue
Yafei Xue Xiaoyi Qi
Xiaoyi Qi