- 1Center of Excellence in Soil and Fertilizer Research in Africa, College of Agriculture and Environmental Sciences, Mohammed VI Polytechnic University, Ben Guerir, Morocco
- 2Department of Soils and Agri-Food Engineering, Faculty of Agricultural Sciences and Food, Laval University, Quebec, QC, Canada
- 3Materials Science, Energy and Nanoengineering Department (MSN), Mohammed VI Polytechnic University, Ben Guerir, Morocco
Applying starter nitrogen (N) and potassium (K) fertilizers in a pop-up placement directly in the maize seed furrow is a delicate practice due to the direct contact between fertilizers and seeds. This proximity increases the risk of seed damage caused by the salinity of N and K fertilizers and the ammoniacal toxicity of nitrogen fertilizers. This study aims to determine the safe application rates of four commonly used starter fertilizers: monoammonium phosphate (MAP: NH4H2PO4), diammonium phosphate (DAP: (NH4)2HPO4), potassium chloride (KCl), and potassium sulfate (K2SO4) across three soil textures: fine (G1), medium (G2), and coarse (G3). A greenhouse experiment was conducted using a three-factor factorial design (four fertilizer sources, five application rates, and three soil textures) arranged in a randomized complete block design. ANOVA revealed significant effects of fertilizer source, application rate, soil texture, and significant two-way interactions between these factors. Polynomial contrasts of maize germination rates in response to increasing fertilizer doses allowed us to establish the maximum safe rates: i) DAP: 3 kg N ha-¹ in G1, 0.8 kg N ha-¹ in G2 and G3; ii) MAP: 5–7 kg N ha-¹ regardless of soil texture; iii) KCl: 10 kg K ha-¹ in G1, 14 kg K ha-¹ in G2 and G3; K2SO4: >16 kg K ha-¹ regardless of soil texture. The experiment also identified visual signs of toxicity, mainly associated with nitrogen fertilizers. These included delayed and reduced emergence, leaf chlorosis, necrotic roots and seeds, stunted and grooved coleoptiles, and, at high doses, seedling mortality. Other quantitative performance indicators, such as shoot and root biomass, chlorophyll readings, and early vigor, were strongly correlated with germination rates and supported the same conclusions regarding safe fertilizer rates. These findings provide practical recommendations for agronomists and farmers to optimize starter fertilizer management in maize by selecting appropriate application rates and fertilizer sources.
1 Introduction
Global maize production has surged in recent decades, propelled by the rising demand and technological advancements that enhance productivity (Erenstein et al., 2022). To promote rapid seedling establishment and reduce the need for multiple machinery passes, high rates of starter fertilizers are commonly applied at planting worldwide. However, this practice does not necessarily translate into higher yields (Chen et al., 2016; Vanlauwe et al., 2023). Under these conditions, direct contact between fertilizers and seeds can jeopardize germination and ultimately impact maize productivity.
The toxicity of fertilizers applied near seeds is primarily attributed to two mechanisms: high levels of ammonia (NH3) (Pan et al., 2016) or osmotic stress induced by fertilizer salinity (Laboski, 2008; Kaiser and Rubin, 2013a, b; Makaza and Khiari, 2023). NH3 toxicity occurs when this gas is released from N-based starter fertilizers passively diffuses into seedling cells (Vines and Wedding, 1960), disrupting intracellular pH regulation between the cytosol and vacuoles. These disturbances are strongly influenced by how fertilizers are placed near seeds in the furrow (Blandino et al., 2022). Pop-up placement of starter fertilizers is widely recognized as the most effective practice for many crops, including maize (Mullins and Burmester, 1997; Kaiser et al., 2005; Maeoka et al., 2020). Although the fertilizer salt index (FSI) may still be helpful, there is an opportunity to exploit ecotoxicological techniques and modernize fertilizer application guidelines, providing more suitable advice based on real-plant responses to starter fertilizer excess. Studies indicate that NH3 toxicity and fertilizer salinity can have lethal effects as early as seed germination and, in some cases, may also impact young seedlings (Wan et al., 2016; Pan et al., 2016).
For example, salt injury caused by applying 50 kg ha-¹ of potassium chloride (KCl) led to reduced germination rates and a yield loss of 1 t ha-¹ (Gordon and Pierzynski, 2006). Barker et al. (1970) soaked cucumber seeds in a 0.1 N potassium solution and observed severe germination damage. Wang et al. (2022) demonstrated that the higher a fertilizer’s salt index, the greater the inhibition of canola seed germination. This low water potential around the seed limits water uptake, ultimately compromising the germination process (Randall and Hoeft, 1988; Cassman et al., 1989; Mullins and Burmester, 1997).
NH3 toxicity is exacerbated when nitrogen-based starter fertilizers are broadcast or band-applied when a buffer zone is maintained between the fertilizer and the seeds (Kaiser et al., 2016; Galpottage Dona et al., 2020). In simultaneous applications, the more alkaline the ammoniacal fertilizer, the greater its toxicity to plants (Zhang and Rengel, 2002). For instance, urea is the most alkaline ammoniacal fertilizer. When applied near wheat seeds at 0.58 to 1.75 mg N g-¹ soil, it reduced germination by 51% to 95% and caused root and shoot growth arrest (Wan et al., 2016; Pan et al., 2016). Dowling (1993) also observed that coleoptile elongation in maize, wheat, and sorghum was particularly sensitive to NH3 when associated with the alkaline hydroxide anion.
From this perspective, farmers strive to achieve rapid and uniform seed germination and even crop emergence. Any delay in germination is irreversible, leading to reduced plant populations and lower yields, ultimately threatening food and nutritional security. Therefore, assessing the potential damage caused by starter fertilizer doses applied in direct contact with seeds is crucial to maximizing their benefits while minimizing risks. The key question is determining the maximum safe rate of starter fertilizers below which germination, root growth, and early seedling development remain unaffected to achieve modern site-and-crop-specific fertilizer applications. Exceeding this threshold increases the risk of seedling damage, primarily due to excessive salinity or NH3 toxicity. Establishing these safe maximum rates is both a theoretical and practical concern, requiring specific evaluations for each starter fertilizer used in major and sensitive crops, mainly maize.
Therefore, the primary aim of this study is to assess the impact of concurrently placed starter N and K fertilizers with maize seeds on seed emergence rates and the early development of maize seedlings, focusing on identifying toxicity symptoms in the roots, seeds, and above-ground parts. Through this analysis, we intend to determine the critical maximum application rates for commonly used ammoniacal and potassium fertilizers, to guide farmers in safely applying these nutrients alongside their maize seeds.
2 Materials and methods
2.1 The experimental site
This study was conducted under greenhouse conditions at the experimental farm of Mohammed VI Polytechnic University in Bengurier, Rehamna Province, central Morocco (32°13′08″N, 7°56′08″W, 449 m.a.s.l). Maize was grown in cylindrical pots with a capacity of 566.75 cm3, maintained at approximately 21°C. This experiment utilized three surface soil (0–20 cm) types representing a range of textures commonly found in smallholder farming systems (Table 1). Based on the historical discussions with local farmers, there was no record of herbicide application at these sites, eliminating concerns about herbicide residue carryover that could affect germination, emergence, or seedling establishment.
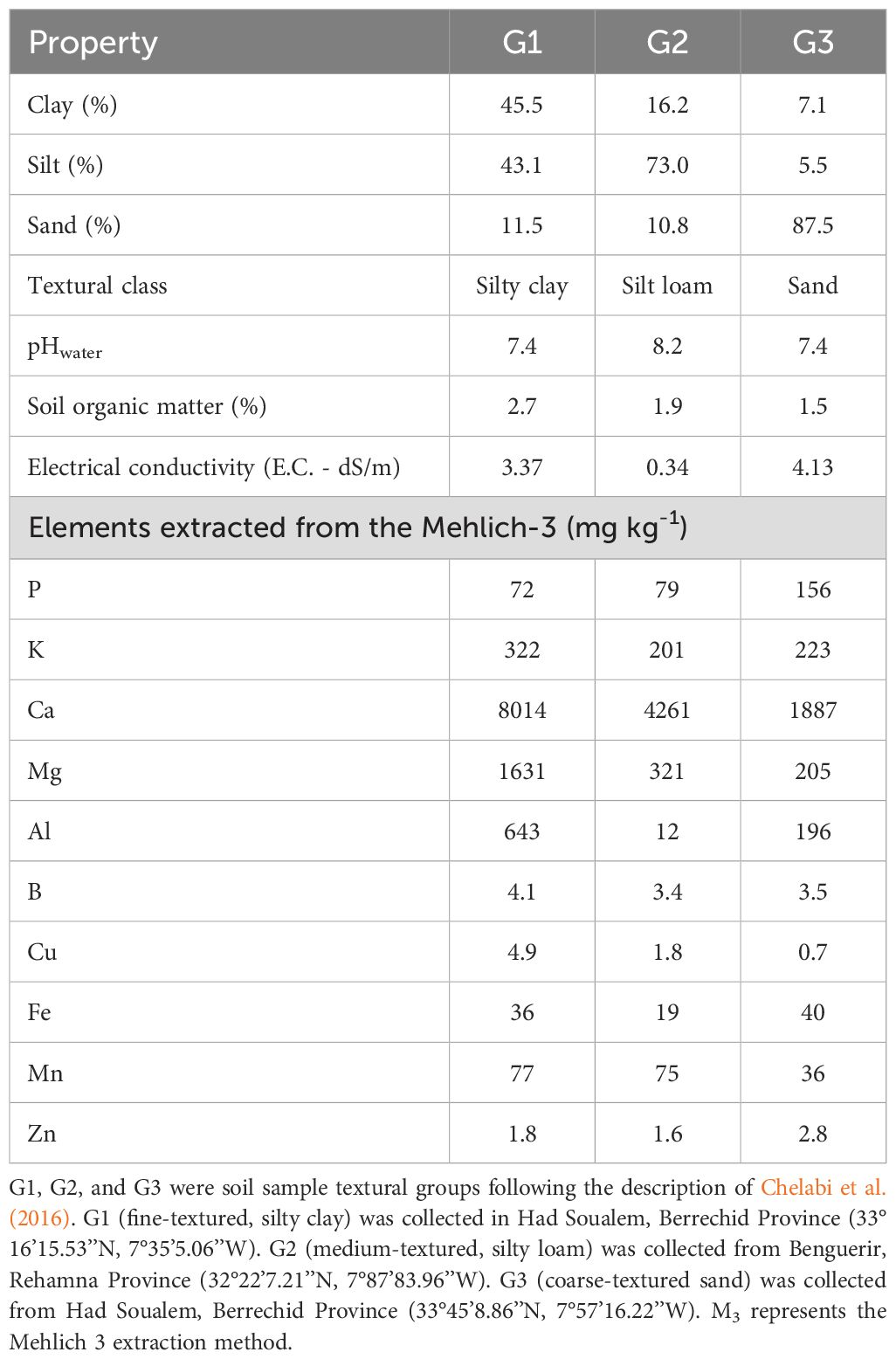
Table 1. Physicochemical characteristics of the three soil types (G1: fine texture, G2: medium texture, G3: coarse texture) used in the maize germination experiment.
2.2 Starter fertilizer materials collection, preparation, and characterization
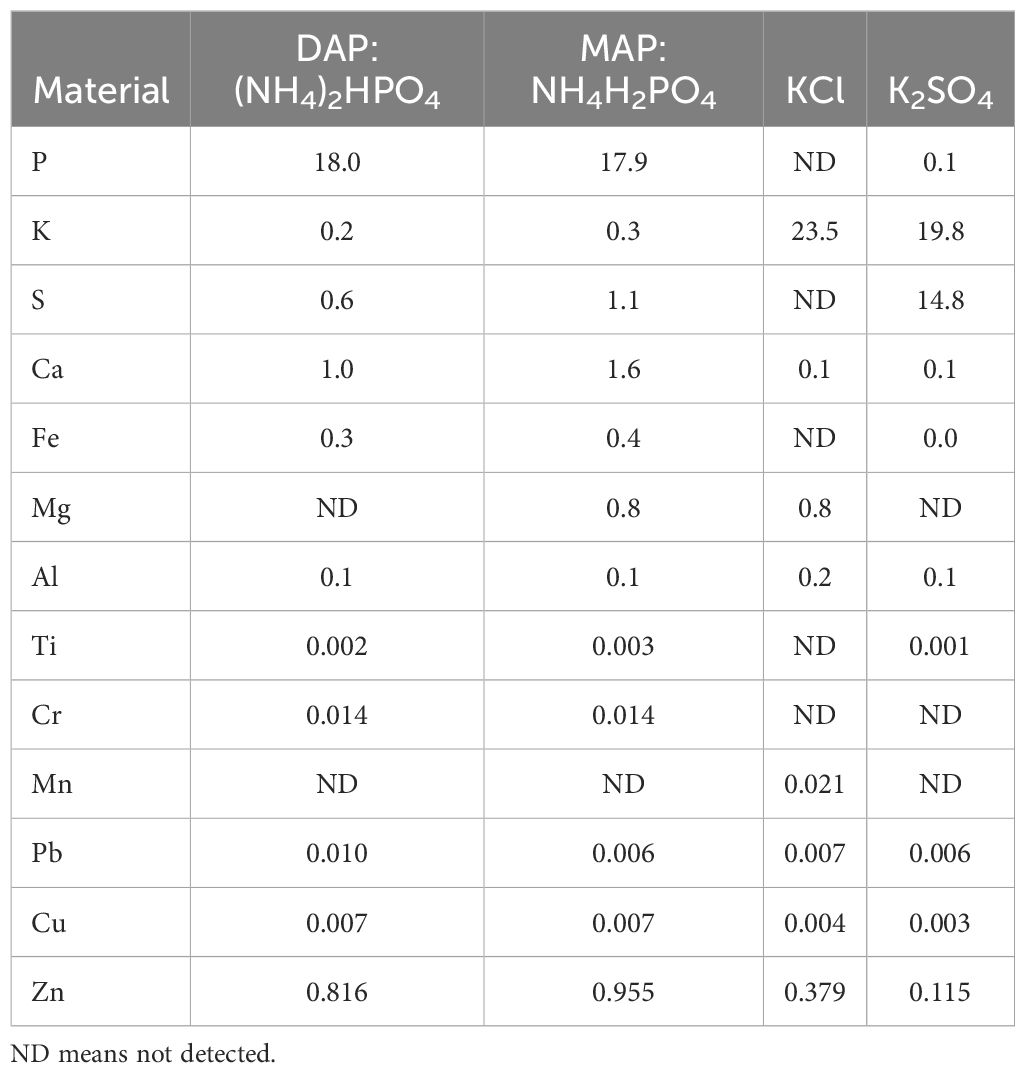
The Moroccan fertilizer industry provided starter ammoniacal-N and potassic (K) fertilizer samples for research. The study included four commercially available dry starter fertilizers: Ammoniacal-N fertilizers: diammonium phosphate (DAP: (NH4)2HPO4), and mono ammonium phosphate (MAP: NH4H2PO4), and potassium fertilizers: potassium sulfate (K2SO4) and KCl. The pH of the saturated fertilizer solution was measured (Lindsay et al., 1962) using Thermo Scientific™ Orion Star™ A211 Benchtop pH Meter, with values recorded as follows: DAP: 7.31, MAP: 4.28, KCl: 7.91, and K2SO4: 2.34.
The electrical conductivity (E.C. (dS/m)) of the saturated fertilizer solutions was determined using the Thermo Scientific Orion Lab Star EC112 Conductivity Bench Meter model, with values of 9.84 (DAP), 9.66 (MAP), 17.09 (KCl), and 10.97 (K2SO4). The fertilizers’ salt index for each material was calculated (Mortvedt, 2001; Murray and Clapp, 2004) using the new KCl-based method, which provides a more accurate estimation of salt effects compared to previous approaches. The FSI was determined using the following Equation 1:
The resulting FSI values were 58 (DAP), 57 (MAP), 65 (K2SO4), and 100 (KCl). Additionally, all fertilizers’ elemental composition was quantified and presented in Table 2.
2.3 Experimental conditions, plant material, and treatments
Soil samples were air-dried and passed through a 2-mm sieve. Soil texture was analyzed using the hydrometer method (Day, 1965). Soil pH was determined by a 1:1 (w/v) soil/water ratio, whilst organic matter content was determined by the modified Walkley-Black method (Allison, 1965; CEAEQ, 2003). The available nutrients were extracted using the Mehlich-3 procedure (Mehlich, 1984). The electrical conductivity of soil was measured using the Thermo Scientific Orion Lab Star EC112 Conductivity Bench Meter model.
The soil was homogenized and sieved through a 20-mm mesh to remove foreign material and gravel-sized particles before being placed in the pots. Starter fertilizer application rates were standardized based on total N content, with treatments set at 0, 5, 10, 15, and 20 kg N ha-1. Potassium fertilizer rates were similarly standardized at 0, 4, 8, 12, and 16 kg K ha-1.
Nitrogen and K are the two fertilizer elements most likely to cause seed and seedling toxicity (Kaiser and Rubin, 2013a; Abit et al., 2016). This study aimed to determine the maximum safe application rates of N and K. The experiment was conducted using a three-factor factorial design, with the following factors: i) Fertilizer source: Four starter fertilizers (MAP, DAP, KCl, and K2SO4), ii) Application rate: Five increasing levels of N (0, 5, 10, 15, and 20 kg N ha-¹) or K (0, 4, 8, 12, and 16 kg K ha-¹), iii) Soil texture: Three soil texture groups (G1, G2, and G3). In total, 60 treatments (4 fertilizer sources × 5 application rates × 3 soil textures) were tested, with three replications in a randomized complete block design (RCBD), resulting in 180 experimental units. Guard pots surrounded each block to minimize edge effects.
Maize seeds were surface-sterilized using a 1% sodium hypochlorite (NaClO) solution for 60 seconds to eliminate seed-borne pathogens. Fertilizer materials were precisely weighed and manually placed in each pot simultaneously with the seed. After fertilizer application, the upper soil layers were carefully added, leveled, and lightly compacted to ensure a consistent depth between the pot surface and the seed and fertilizer placement zone. The pots were irrigated on the planting day and daily thereafter to maintain soil moisture at approximately 80% of field capacity until the end of the experiment.
Maize was grown for 40 days after initial emergence. The number of emerging plants was recorded daily in each experimental unit until the end of the study. Emergence was expressed as the percentage of total seeds planted and the number of seeds that successfully emerged. The relative emergence percentage (EP) was calculated using the following Equation 2:
A critical EP threshold of 80% was applied to determine the maximum safe rate of starter fertilizers.
At the end of the experiment, the soil was carefully removed from each pot to separate the plant roots from the soil. Several emergence parameters were then calculated, including the mean emergence time (MET), mean emergence rate (MER), emergence speed (ES), uncertainty index (UNC), synchronization index (SYN), the index of emergence variance (EV), emergence standard deviation (SDE), and emergence coefficient of variation (CVE), following the methodology described by Makaza et al. (2024). MET represents the number of seeds that emerged to the number of seeds that emerged at the time of evaluation, whilst MER is the reciprocal of MET. The uncertainty index (UNC), an adaptation of the Shannon index, quantifies the variability in emergence frequency. Lower UNC values indicate higher emergence uniformity, as this index measures the degree of dispersion in emergence timing. The synchronization index (SYN) was initially developed to assess the overlap in flowering among individuals within a population. A SYN value of 1 indicates that seed emergence occurs simultaneously. In contrast, a SYN value close to 0 suggests that at least two seeds emerged at different times, reflecting greater variability in emergence timing.
The chlorophyll content index (CC) was measured on mature and fully expanded leaves 40 days after the first emergence for the maize biophysical data. The CC measurements were taken per treatment using a portable chlorophyll meter (SPAD 502, Spectrum Technologies; Inc. USA). Aboveground fresh biomass (AFB) and root fresh biomass (RFB) were separated at the approximate level of the soil and weighed, where the summation of AFB and RFB gives the total fresh biomass (TFB), which was later dried at 65°C. Salt injury and NH3 toxicity symptoms were assessed through visual observation. Root traits such as the maximum number of roots (MNR), the number of root tips (NRT), the total root length (TRL), average diameter (AD), perimeter (P), root volume (V), and root surface area (SA) were analyzed phenotypically using the RhizoVision Explorer tool (https://www.rhizovision.com/) with minor modifications based on the methodology of Seethepalli et al. (2021) and Makaza et al. (2024).
2.4 Data analyses
Statistical analyses were performed using the R software program (version 4.4.2) (Venables et al., 2024), assuming fixed effects for soil type, fertilizer source, fertilizer rate treatments, and random effects for replication. Statistical significance was set at P < 0.05 for all tests. Variance (ANOVA) was analyzed to assess treatment effects, and Duncan’s multiple range test was used to compare mean differences between treatments. The statistical model used in this study was as follows (Equation 3):
where Yijkm represents the overall maize response of the jth soil type, kth fertilizer type, and mth fertilizer rates within the ith replication, along with their interactive effects, and is the pooled error term. The GerminaR package is implemented to calculate nine (9) emergence indices described above (Lozano-Isla et al., 2019). The critical starter ammoniacal-N and K fertilizer rates for different soil fertilizer materials were determined for the emergence parameters, root traits, and biophysical data. Polynomial contrasts between the relative emergence percentage (EP) and N or K fertilizer rates were used to determine the maximum safe fertilizer dose, corresponding to a minimum emergence rate of 80% for maize seeds. Pearson correlations were performed to assess associations between different parameters using the prcomp function and the mixOmics package. Principal component analysis (PCA) was generated to determine traits that explain most of the variation as well as to determine treatment groups associated with major traits using ggplot2, factoextra, and FactoMineR packages in R Studio. Clustering was done to group each object into clusters using Ward’s method based on Euclidean distance over a complete linkage dissimilarity matrix.
3 Results
3.1 Effects of starter fertilizers on seed emergence parameters
The analysis of variance revealed the significant interaction between fertilizer type and the fertilizer rate (p<0.001), soil type and fertilizer rate (p<0.001), and soil type and fertilizer type (p<0.01) on emergence percentage under the influence of starter ammoniacal-N fertilizers (Table 3). Concurrently, increasing starter N (DAP and MAP) fertilizer rates have been shown to decrease the emergence percentage (EP) significantly. The cubic model shows that to ensure a minimum relative emergence rate of 80%, the maximum safe recommendation rate for DAP is 1 kg N per hectare (ha-1) (Figure 1; Supplementary Tables S1, S2). In contrast, MAP exhibits a significantly higher safe rate of 3.5 kg N ha–1 (Figure 1A). The maximum nitrogen rate favorable to ordinary maize emergence varies with soil texture. For G1 soil, the safe starter N-rate was 4.7 kg ha–1, while G2 and G3 recorded low safe rates of 1 and 2 kg N ha–1, respectively (Figure 1B). A comprehensive analysis of the cubic modeling results, aimed at identifying critical nitrogen thresholds for co-placement with maize seeds across the three soil types and for all ammonium-based fertilizers (Table 4), reveals that safe N-rates range from 1.4 to 7.8 kg ha–1 for MAP and from 0.8 to 2.8 kg ha-1 for DAP. However, no significant interactions were recorded between soil type, fertilizer type, and fertilizer rates on EP, MET, MER, ES, UNC, and SYN (Table 3A). This is contradictory to the influence of the main factors, which are soil type, fertilizer type, and fertilizer rate, that showed significant (p<0.05) differences in all emergence parameters (EP, MET, MER, ES, UNC, and SYN) (Table 3).
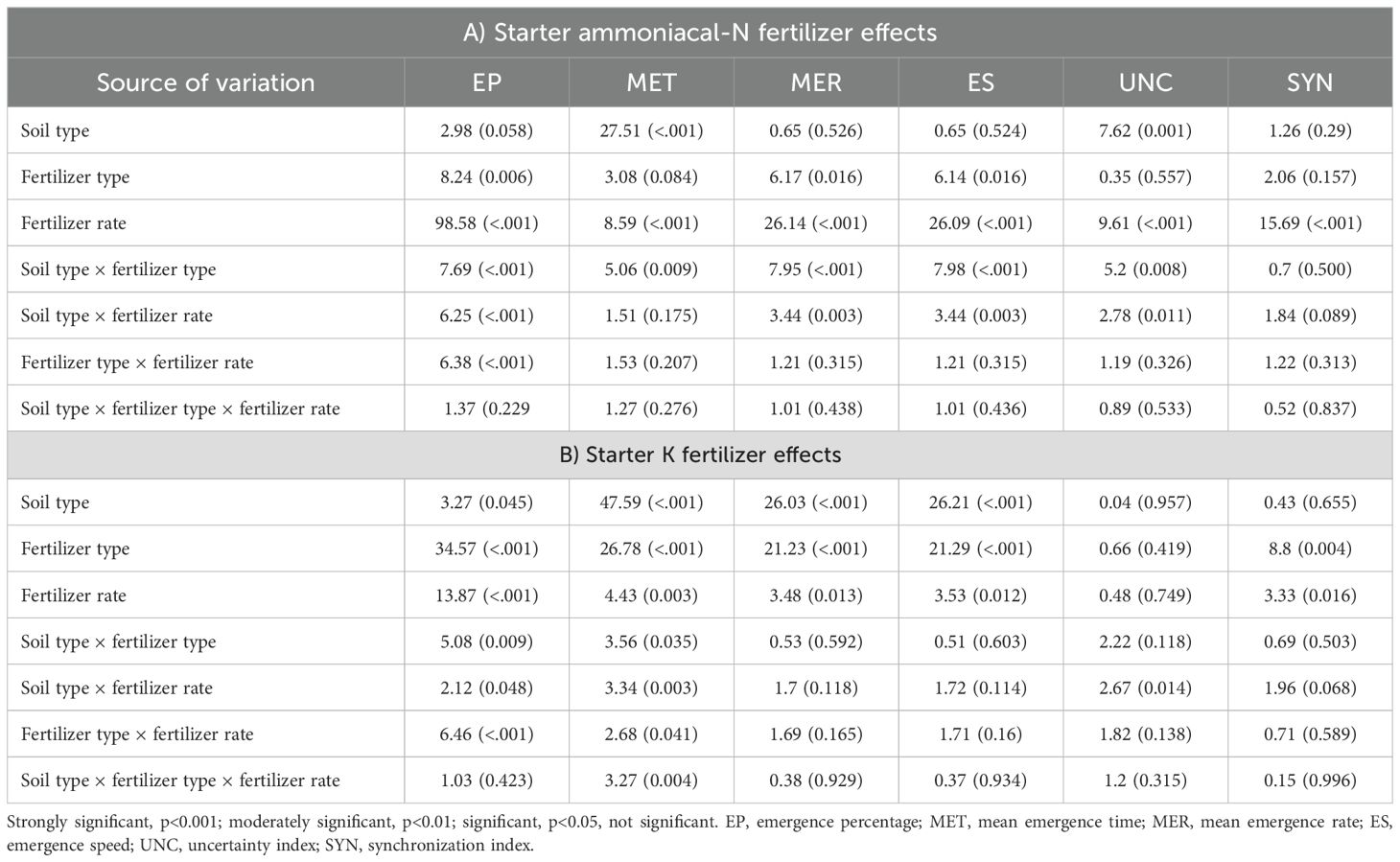
Table 3. The analysis of variance (ANOVA) for the maize seed emergence influenced by ammonia-nitrogen and potassium fertilizers applied in pop-up with seeds.
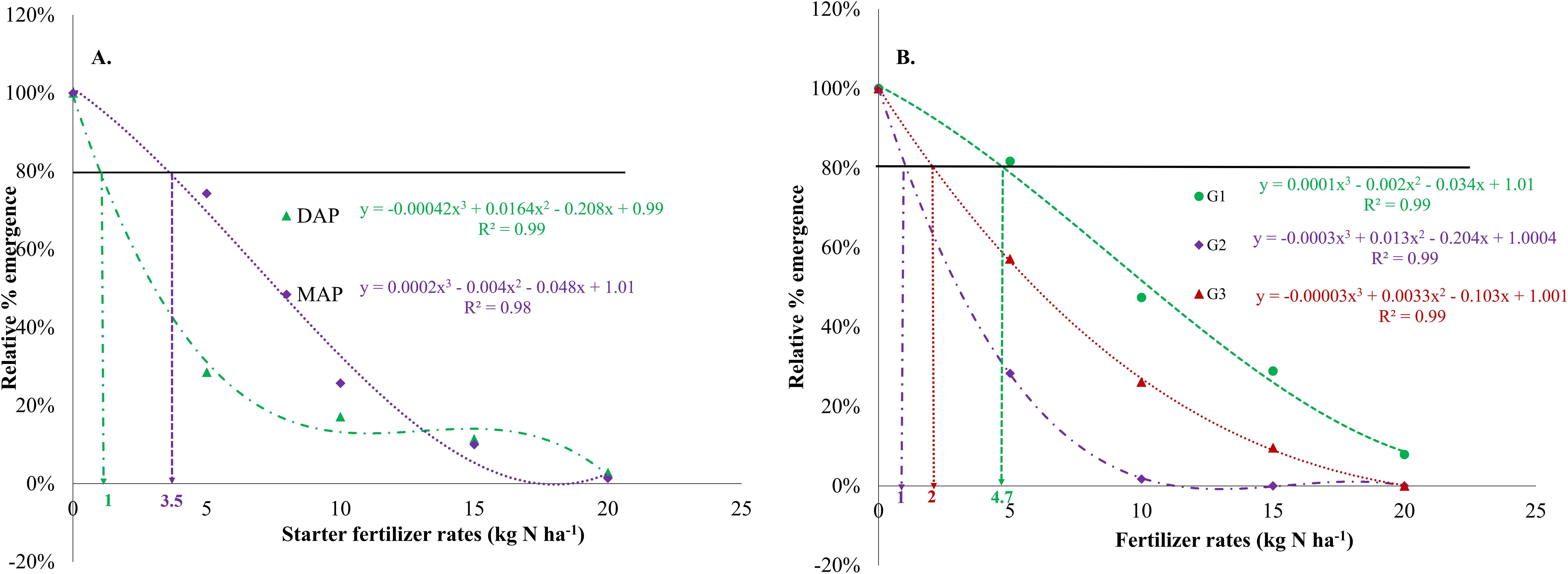
Figure 1. Significant relationship for maize emergence rate relative to: (A) Ammoniacal-N starter fertilizer type and nitrogen dosages (p<0.001) and (B) Soil type and initial ammoniacal-N fertilizer dosages. The dashed vertical line represents the best-fit curve based on the data.
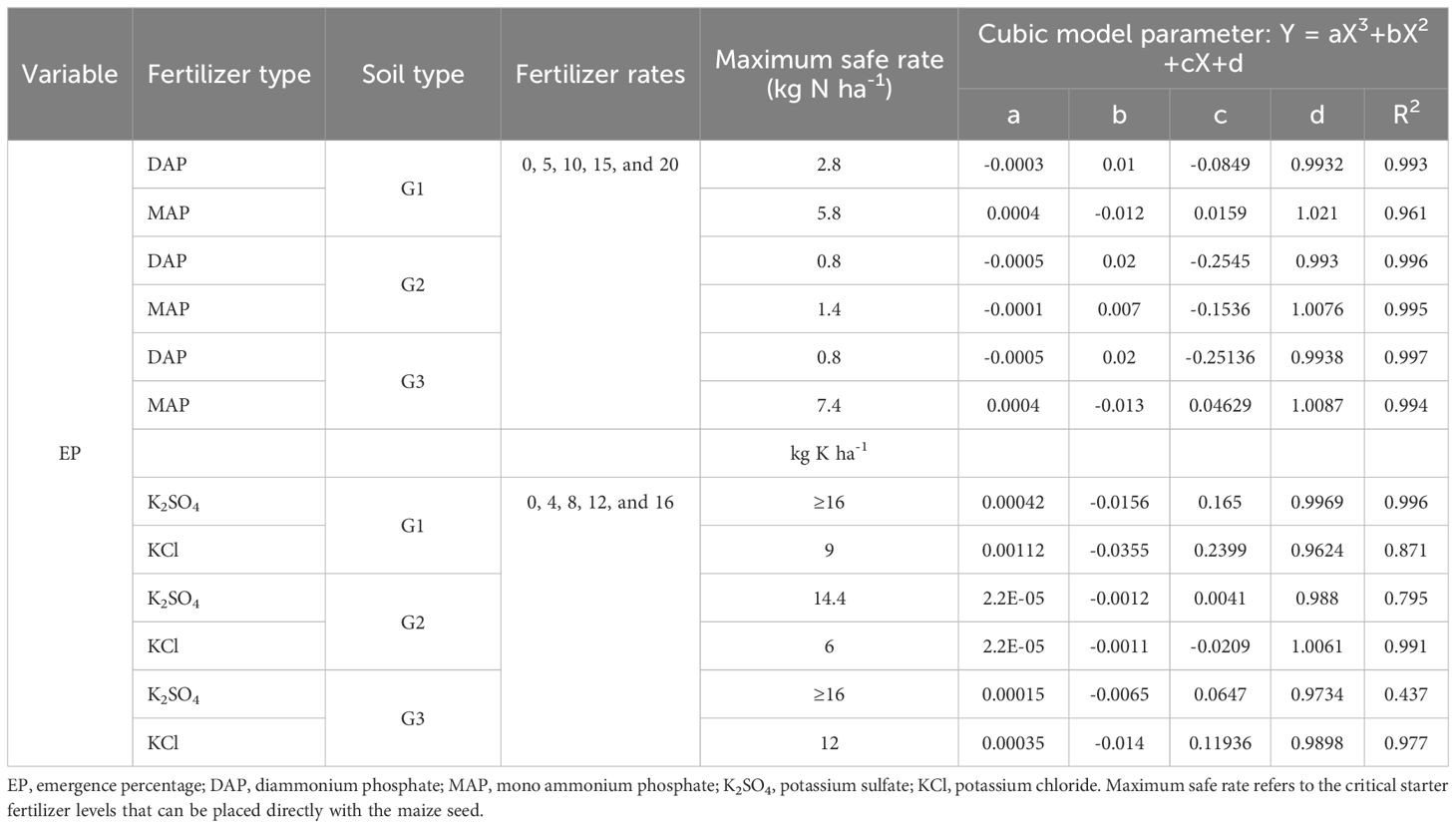
Table 4. Cubic modeling of emergence percentage displayed based on the interaction between soil type, fertilizer type, and fertilizer rates evaluated in young maize seedlings (Y) in response to ammonium and potassium fertilizer dosages applied in pop-up with seed (X).
Regarding the starter K fertilizers, significant interactions between soil type, fertilizer type, and fertilizer rate were observed only for MET (p<0.01). On the same note, the interactions between fertilizer type and the rate; soil type and fertilizer rate; and soil type and fertilizer type revealed significant interactions for EP and MET (Table 3B). Individual effects of soil type, fertilizer type, and fertilizer rate recorded significant differences among all the emergence parameters except for the uncertainty index (UNC, p>0.05), which describes the heterogeneity of the seed emergence process. The maximum permissible doses for mixing potassium fertilizers, K2SO4 or KCl, with maize seeds have been determined. For the G1 soil, 16 kg K ha-1 or higher doses are deemed safe. KCl is recommended at a maximum dose of only 8.8 kg K ha-1, as shown in Figure 2A and Supplementary Table S3). For G2 and G3 soils, the maximum safe rates were 8.7 kg K ha-1 and 14.4 kg K ha-1, respectively (Figure 2B). Moreover, the investigation into the interplay between fertilizer type and dosage reveals that K2SO4 is considerably safer for use at higher doses, specifically those ≥16 kg K ha-1. Upon reviewing the data for the three soil types and the two varieties of potassium fertilizer (as detailed in Table 4), it becomes clear that the critical dosage for K2SO4 in the pop-up is ≥14.4 kg K ha-1, while the KCl safe dosage range between 5.5 and 12 kg K ha-1. This has been strongly witnessed in the correlation matrix, where significant and relatively strong correlations were observed between emergence metrics when starter N and K fertilizers were applied (Supplementary Figure S1).
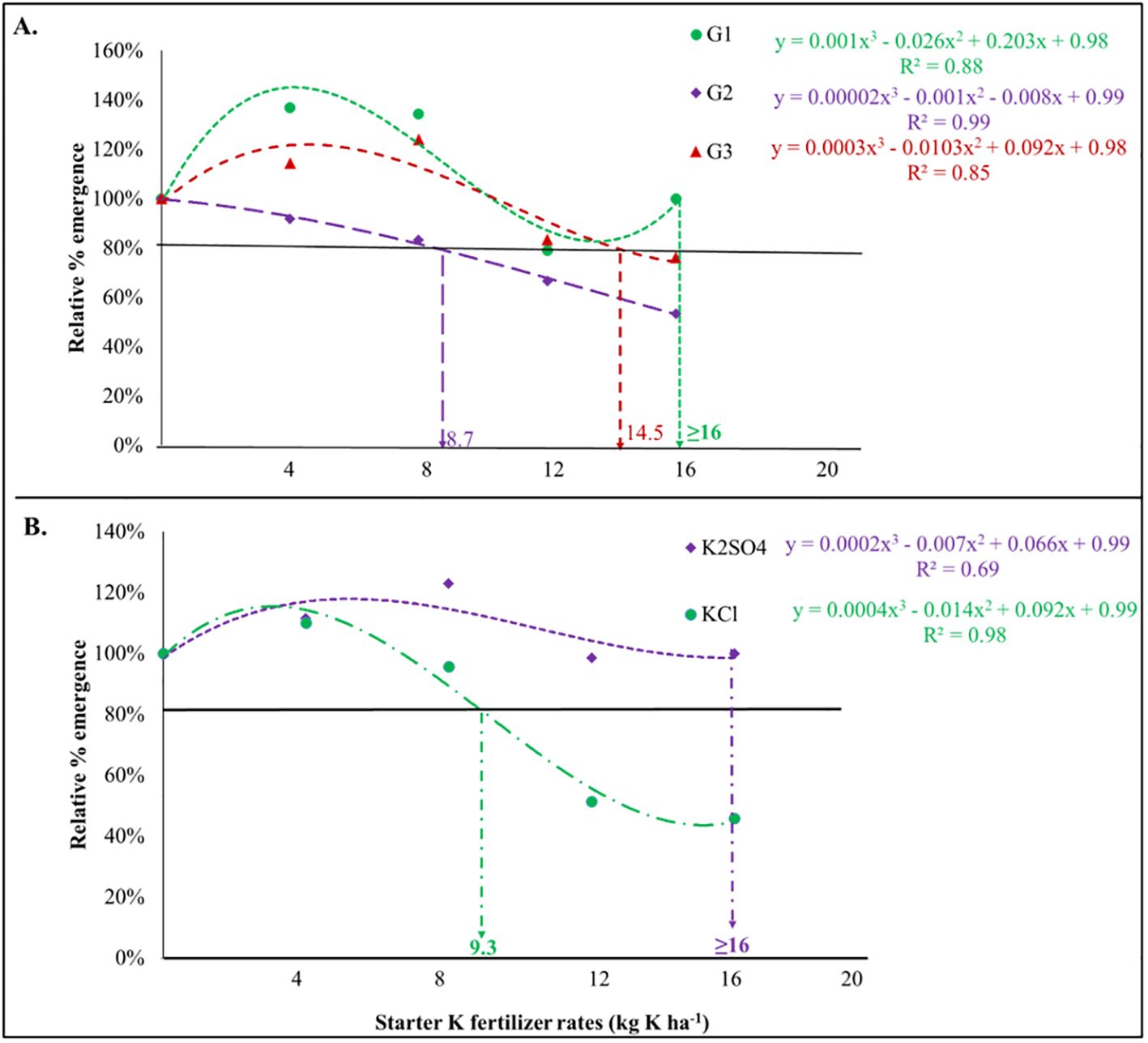
Figure 2. Significant correlations observed in maize emergence rate relative to: (A) Soil type and potassium dosages (p<0.001) and (B) Type of potassium fertilizer and potassium dosages (p<0.001). The dashed vertical line represents the best-fit curve based on the data.
3.2 Seed-placed starter fertilizers affect maize root-related components
Data illustrating the influence of soil type, starter fertilizer type, and fertilizer rate on maize root parameters are shown in Table 5. Starter ammoniacal-N fertilizers revealed significant interaction between soil type, fertilizer type, and fertilizer rate on MNR (p<0.001), NRT (p<0.01), TRL (p<0.001), AD (p<0.01), P (p<0.001), and SA (p<0.05) except for the root volume (V, p = 0.101) (Table 5A). At the same time, the interaction between soil type × fertilizer rate, and soil type × fertilizer type strongly influenced (p<0.001) all the recorded root parameters. On the other hand, fertilizer type × fertilizer rate was only significant (p<0.05) for MNR, NRT, and AD. The effect of fertilizer rate alone had highly influenced all recorded root traits (p<0.01) except for the root volume. Also, fertilizer-type effects were significant (p<0.05) for MNR, TRL, AD, P, and the V. Moreover, the influence of varying soil types had a strong significant (p<0.001) effect across all the measured root parameters. The ANOVA for starter K fertilizers’ interactive effects showed strong significant (p<0.01) interactions on soil type × fertilizer type × fertilizer rate, fertilizer type × fertilizer rate, soil type × fertilizer rate, and soil type × fertilizer type for all the root traits (MNR, NRT, TRL, AD, P, V, and SA) (Table 5B). The main effects: fertilizer rate, fertilizer type, and soil type, had significant (p<0.05) differences amongst all the root traits. In general, soil type, starter fertilizers, and fertilizer rate had strong positive correlations with the root parameters studied (Supplementary Figure S2).
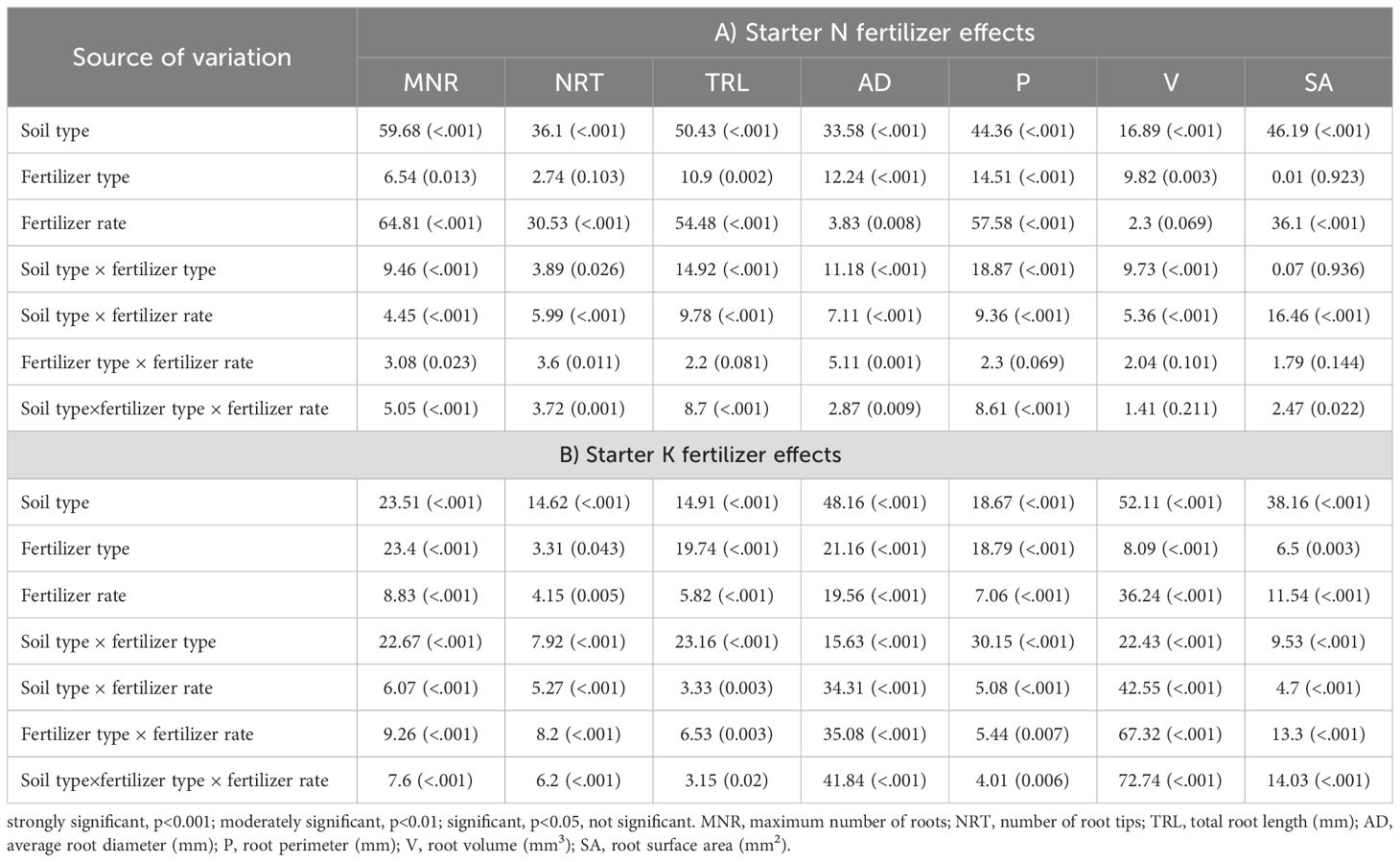
Table 5. The effect of soil type, fertilizer type, fertilizer rate, and their interactions, established on seed-placed root parameters of young maize seedlings, as assessed by three-way ANOVA (F values, and their probability).
3.3 Fertilizer salt effects and NH3 toxicity on the visual appearance of maize
The toxicity of starter N and K fertilizers was assessed qualitatively (Figure 3). The visual appearance of the plants was recorded from the day of the first emergence until harvesting. Since soils react to salts differently, depending on their chemistry and texture, starter ammoniacal-N fertilizers may result in physiological and morphological disorders that lead to reduced crop establishment, growth, and development. Although the salt index of DAP and MAP-containing treatments is less than that of K-containing treatments, starter N fertilizer effects may be attributed to NH3 toxicity. Significant and severe detrimental effects were recorded following starter ammoniacal-N treatments at emergence and seedling development. Symptoms of NH3 toxicity became apparent between 3 and 8 days after the first emergence, depending on the concentration of seed-placed starter ammoniacal-N placement. DAP was more lethal to maize emergence in its increasing order from 0 to 20 kg N ha-1 than MAP (Figure 3). NH3 toxicity was initially associated with interveinal chlorosis, which later extended to the older leaves. Simultaneously, shorter petioles of the younger leaves were observed. This was followed by die-back of leaf margins and upward leaf curling, which evolved with a strong fire tip burn and later necrotic appearance. Finally, the plants ceased to grow and continued to deteriorate. These emerged maize seedlings were globally stunted with very few numbers of leaves. In some cases, there was a severe “bloody yellowing” of the leaves from the tip into the interveinal plant parts, leaf wilting, desiccation, and global V-shaped sections.

Figure 3. Visual appearance and/or indicators of fertilizer salt injury and NH3 toxicity for maize grown under varying soil types, fertilizer types, and fertilizer rates. A) Aboveground and, (B) seeds.
Those seedlings that struggled to emerge fully had a canker and stubby coleoptile, which later became necrotic from the tip and dried out. Most plants, however, remained alive for quite some time despite physical stress conditions. For control treatments, maize plants grew much better than in seed-placed starter ammoniacal-N fertilizers (DAP and MAP). However, these seedlings eventually exhibited symptoms of N deficiency, which were quite dissimilar to the NH3 toxicity symptoms (Figure 3A). Non-emerged seeds under starter N treatments recorded a greater potential to draw water out of the roots, which can result in plant desiccation and shrinking seeds (Figure 3B). Moreover, severe darkening of the seed testa and discoloration were observed at varying N concentrations in all soils. The cause of the darkening of the testa and the discoloration of the residual moisture may be related to the accelerated leaching of electrolytes from the seed exposed to NH3 during imbibition. A recovery plan was employed to regerminate the seedlings under normal conditions, but none recorded any indication of radicle protrusion after 10 days of recuperation. This means that NH3 impacted the morphology of root development as no radicle growth was observed. In cases where germination happens, the radicle, or the first emerging root, was typically short, and the tip was brown or black in most soils under DAP and MAP influence. The radicles were killed in severe cases of damage.
In contrast, a high emergence rate was observed under seed-placed K fertilizers (K2SO4 >80% and KCl >60%). However, maize seedlings recorded general yellowing under starter K fertilizers. The leaves had V-shaped necrotic tips and yellow leaf margins compared to the control. The growth rate was relatively slow compared to the control. Few seeds failed to emerge under K fertilizers, with average stunting and stubby coleoptiles observed. Damaged plants may tend to recover by establishing and developing many roots in pop-up K fertilizers. An increasing rate of seed-placed K in G1 soils exhibited a low salinity effect compared to G3 and G2 soils, which have reduced fertilizer adsorption capacity and less water-holding capacity. The salt index of K2SO4 and KCl had no significant effect on maize seeds’ appearance as substantial growth and establishment can be visually witnessed but at varying growth rates and performances.
3.4 Starter fertilizer toxicity affects maize biophysical and yield-related components
The influence of seed-placed fertilizer toxicity on biophysical and yield-related traits was quantified (Table 6). The ANOVA indicated strong significant interactions between soil type × fertilizer type × fertilizer rate on the AFB (p<0.001). In contrast, the interaction effects of fertilizer type × fertilizer rate on CC and TFB, as well as soil type × fertilizer type on RFB, and TFB were insignificant (p>0.05) under the starter ammoniacal-N fertilizer treatments (Table 6A). The starter ammoniacal-N fertilizer rate had significantly influenced all biophysical and yield-related components, whilst the influence of fertilizer type was recorded only on the AFB (p<0.01). Also, soil type variability proved to have a strong significant (p<0.001) influence on AFB, RFB, TFB, and CC where G2 soil yielded the maximum biomass and G1 had the maximum CC (Table 6A). Concerning seed-placed K fertilizers, only CC confirmed a significant interaction between soil type × fertilizer type × fertilizer rate, and soil type × fertilizer rate. On the same note, no significant interactions were observed for soil type × fertilizer type on AFB, RFB, and TFB except for the CC (p<0.05). The results revealed that the main effects were predominant with soil type, starter K fertilizer type, and fertilizer rate exerting the most significant influence on the biophysical and yield-related components. The influence of starter fertilizers was evaluated using the Pearson correlation analysis, and all biophysical parameters had significantly high positive correlations. For example, EP and AFB (r = 0.76***), EP and TFB (r = 0.74***), CC and AFB (r = 0.88***) for starter N fertilizers, while EP and AFB (r = 0.55***) for starter K fertilizers (Figure 4).
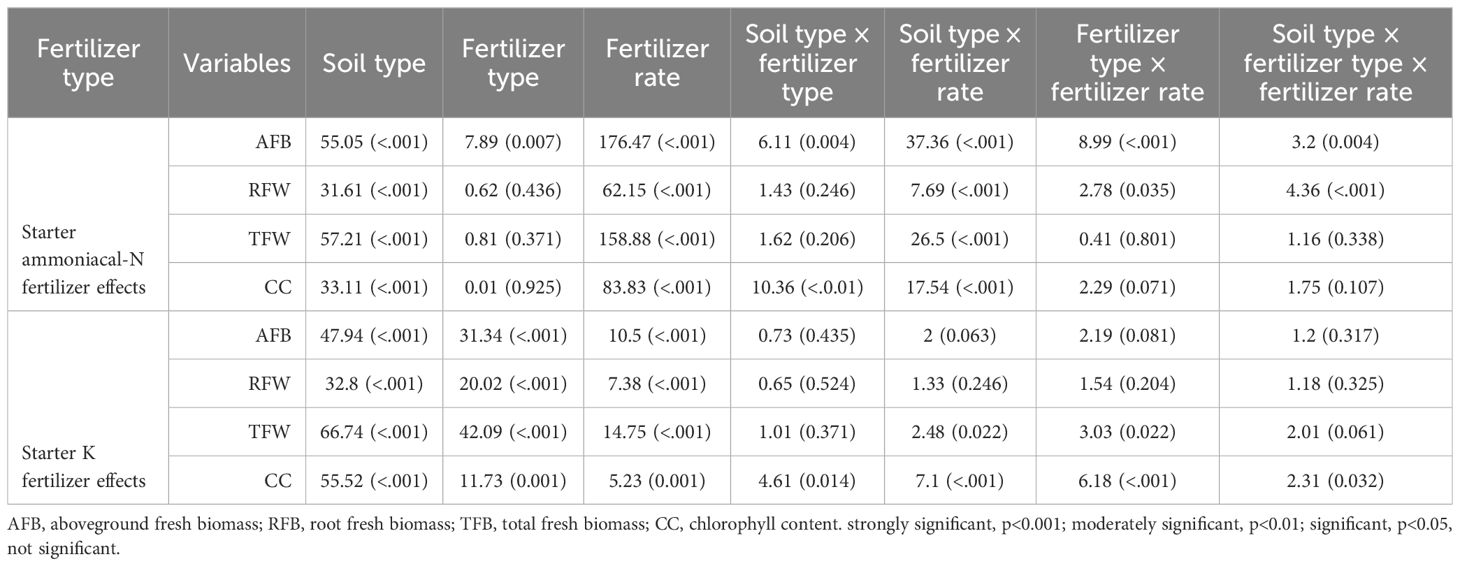
Table 6. Effect of soil type, fertilizer type, the fertilizer rate, and its interactive effects on physiological and yield-related traits of maize grown under greenhouse conditions.
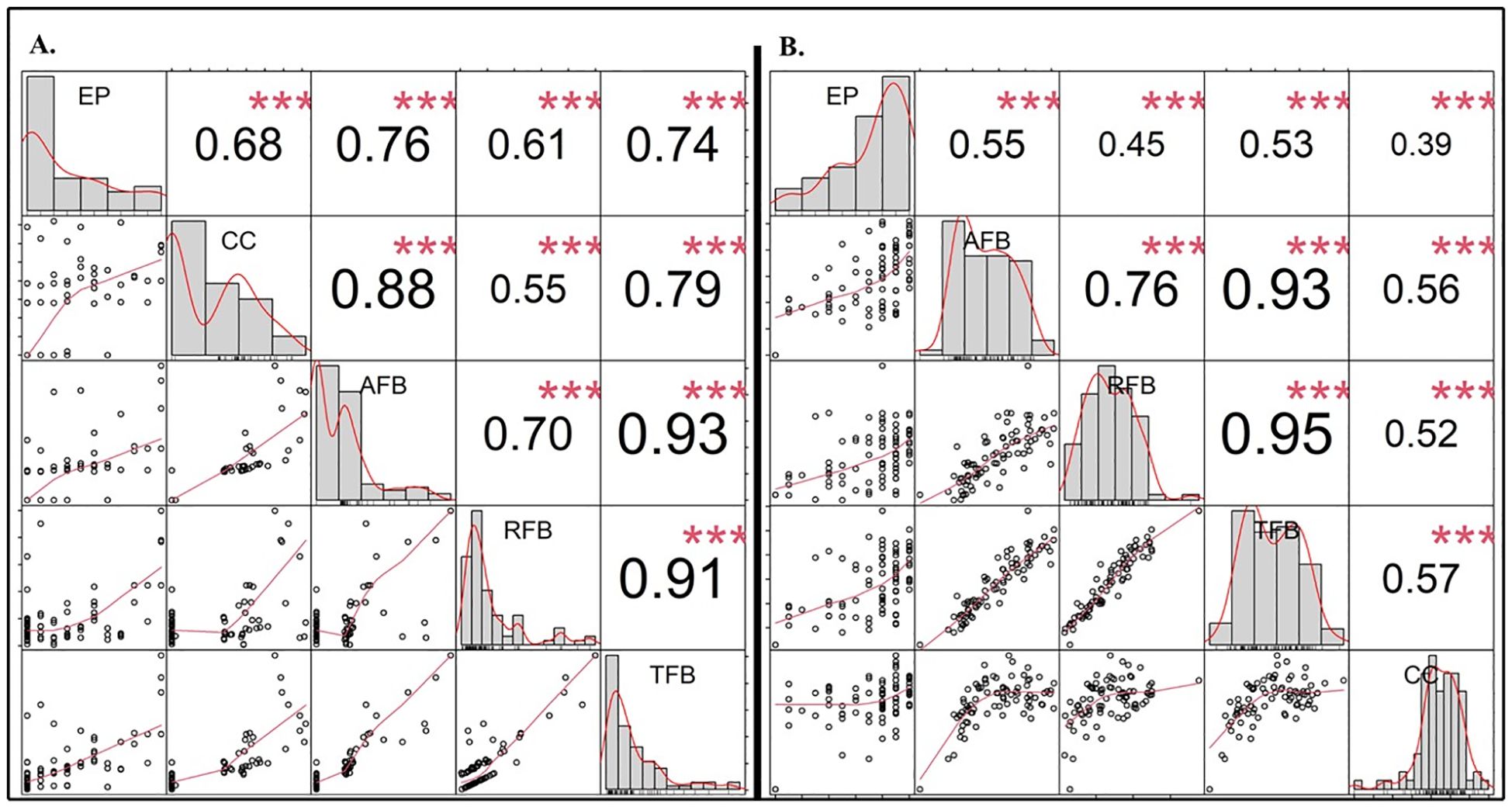
Figure 4. Pearson correlation analysis for the biophysical traits under the influence of (A) starter ammoniacal-N and, (B) starter K fertilizers, using different soil types and fertilizer placement rates. CC, chlorophyll content index; AFB, aboveground fresh biomass; RFB, root fresh biomass; TFB, total fresh biomass. Asterisks represent correlation significance: ***<0.001.
All the biophysical, root, and emergence traits measured were performed on PCA to identify the pattern between different treatment combinations and obtain clear information about maize behavior (Supplementary Figure S3). The results of PCA revealed two main components, which explain more than 70% of the original variability. PCA axis 1 (54.9%) was strongly determined and dominated by the emergence (EP, MET, MER, ES, UNC, and SYN), root (MNR, NRT, TRL, P, and SA), and biophysical (CC, AFB, RFB, and TRB) parameters. On the same note, PCA axis 2 (15.7%) was strongly determined by the AD (r = 0.469) and V (r = 0.519) (Supplementary Figure S3). The PCA biplot showed that all tested combinations could be grouped into three clusters. The first cluster consisted of treatment combinations that are negatively correlated with all maize emergence, root, and biophysical traits recorded. This cluster consists mainly of the treatment that contains DAP and MAP seed-placed starter N fertilizers applied at 5, 10, 15, and 20 kg N ha-1 for all the soil types (e.g., G1/DAP/20 combination). The second cluster contained treatment combinations of DAP and MAP at very low seed placement rates across 3 soil types (e.g., G1/DAP/5 or G3/MAP/0) and a few starter K treatment combinations (mainly the KCl combinations). The third cluster grouped soil type, fertilizer type, and fertilizer rate treatment combinations for which seed-placed starter K rates are dominant at all placement rates (e.g., G1/K2SO4/20, G2/K2SO4/5 treatments). On average, seed-placed K fertilizers were positively associated with emergence, root, and biophysical maize traits for all soils, whereas >5kg N ha-1 from DAP or MAP treatments were negatively correlated to all recorded traits with minor exceptions, thus indicating the variability in terms of the fertilizer salt injury and potential NH3 toxicity (Supplementary Figure S3).
4 Discussion
4.1 Seed-placed starter fertilizer toxicity impacted maize seed emergence parameters
Seed emergence, being a crucial step for establishing crops in the soil and ensuring uniformity of seedling emergence, is potentially threatened by seed-placed starter ammoniacal-N and K fertilizers, which interfere with plant growth and development. Our findings concerning the low emergence percentage for starter N (DAP and MAP) and delayed emergence for starter K (KCl and K2SO4) fertilizers complement and extend the elegant work by Court et al. (1964). They revealed that reduced seed emergence could be associated with excessive concentrations of fertilizer salts placed in contact with germinating seedlings to create an imbalance of ions. The fact that we found starter N fertilizers exhibiting detrimental effects in maize seeds and seedlings suggests the potential of the materials to liberate NH3, which can freely move within the soil solution and into plant cells, resulting in total desiccation of plant tissue (Qian et al., 2012; Pan et al., 2016). Similar findings were reported by Mahler et al. (1989) who postulated that N source, N application rate, and varied soil-water potential generally reduced winter wheat emergence by 5 to 26% when in-furrow placed with the seed. Given the implications of the difference between starter N fertilizers, DAP (pH: 7.31) releases NH4+ and volatile NH3 that is harmful to the radicle and plumule growth and development near the seed compared to MAP (pH: 4.28) fertilizer in calcareous soil (Colliver and Welch, 1970; Nadarajan and Sukumaran, 2021). These observations were comparable to the study on the effect of seed-placed anhydrous NH3, muriate of potash (MOP), ammonium nitrate, ammonium sulfate, 6-12–12 fertilizer, and sulfate of potash (SOP) where N and K fertilizers were highly toxic for maize emergence (Cummins and Parks, 1961; Sardi and Beres, 1996; Ritchie et al., 1998). Given the variability in soil textures, safe pop-up N and K fertilizer recommended rates and seed emergence were high in G1 compared to G2 and G3 soils. This agrees with Kaiser and Rubin (2013a), who observed higher emergence and seedling growth in fine-textured soils than in medium and coarse-textured soils.
The safe and maximum starter N and K fertilizer recommendation rates established in this study, notably <5kg N ha-1, and 8–16 kg K ha-1, could promote relatively uniform seed emergence, and shorten the emergence rate and time (Wan et al., 2016). These findings align with Hergert et al. (2012), who indicated that ammoniated zinc application in the seed furrow should be 5–7 kg N + K ha-1 on sandy soils to minimize seed damage from volatile NH3. Likewise, Kaiser and Rubin (2013b) predicted that 10.6 kg N + K ha-1 was ideal for direct placement with maize seeds for clay loam and silt loam soils, while 5.7 kg ha-1 for fine sand soils. In another study, starter K fertilizer (MOP and SOP) increased maize and soybeans establishment and productivity despite high levels of soil P and K, except where excessive rates were applied in-furrow (Gordon, 1999). Others reported a pronounced inhibitory effect with cations (K+), especially associated with an anion (Cl−) compared to SO42- (Makaza and Khiari, 2023). As for the lentils, peas, and chickpeas, they tolerated up to 10 kg N ha-1 rates, whilst soyabean and black beans could tolerate 10–20 kg N ha-1 of seed row-placed fertilizer for improved seed emergence (Galpottage Dona et al., 2020). In a similar pot experiment, seed-placed rates as low as 25 kg N ha−1 significantly increased seedling emergence of wild oats (Agenbag and De Villiers, 1989). This study clearly shows that EP, MET, and MER could be among the high emergence indicators of fertilizer ecotoxicological effects influenced by starter N and K seed placement as they describe the financial feedback in the yield acquisition.
4.2 NH3 toxicity and salt injury affect maize root architecture
Maintaining root system elongation and its development plays an essential role in alleviating fertilizer salt injury and NH3 toxicity (Chen et al., 2022). However, toxic concentrations of starter N and K fertilizers are perceived by the roots, followed by a series of adaptive responses at the physiological, cellular, and morphological levels (Zhao et al., 2016; Arif et al., 2019). Our findings showed that the safe pop-up fertilizer rates for N and K fertilizers significantly increased maize root architecture. This complements Schenk and Wehrmann (1979) who noticed the drastic reduction in the root morphology characterized by the root length, the number of tips, and the root surface area due to significant inhibition of root cells following NH3 exposure. Furthermore, we showed that starter K fertilizers had the highest root production compared to starter N fertilizers, which was supported by Zhao et al. (2016) and Tsialtas et al. (2016). They showed an improved root formation following K fertilizer application, which is an important organogenetic process that determines the establishment of root architecture, contributes to water-use efficiency, and improves the absorption of micro- and macro-nutrients from the soil. In an ex-ante study on canola (Starling et al., 1998; Qian et al., 2012) and barley (Makaza et al., 2024), a cessation of tap root elongation was observed, followed by progressive basal-directed necrosis, and shrinking of the root axis. Therefore, NH3 gas toxicity and root-specific root system architecture should be considered in N placement and source selection, while the fertilizer salt index should be our principal guide to minimize fertilizer toxicity.
4.3 Maize toxicity symptoms are elevated by fertilizer injury and NH3 toxicity
When the concentration of ions is greater in the soil than in the plant, the salt moves from the plant tissue to the soil, causing the plants’ lethality (Laboski, 2008). The details on the consequential effects of ammoniacal-N and/or salt toxicities on morpho-physiological and biochemical attributes of maize were reviewed. Our visual observations regarding stunted growth, chlorosis, necrotic leaves and roots, leaf curling, seed rotting, discoloration, and leakage of electrolytes concur with Makaza and Khiari (2023). Vines and Wedding (1960) showed that NH3, which is an effective inhibitor of respiration in plant tissues, results in poor seed viability and necrosis. Similar findings showed heavy chlorosis following NH3 treatment of 0.06 – 0.09 mM in cucumber (Schenk and Wehrmann, 1979). In addition, they noticed NH3 influenced the morphology of roots, for which the root length, tips, and area were drastically reduced due to significant inhibition of root cells. Moreover, seed-placed DAP revealed some root discoloration and root hair disfigurement, perhaps due to higher NH3 volatilization (Pan et al., 2016). Evidence supporting the involvement of NH3 toxicity concerning the leakage of electrolytes shows that its transport across the plasma membrane also comes from the N natural isotopic signature (δ15N) and discrimination (Δ15N) of NH4+-fed plants, which tend to be enriched in 14N (depleted in 15N) (Esteban et al., 2016). The various ammonium transport-related components, especially the non-electrogenic influx of NH3 and the electrogenic influx of NH4+, may contribute to ammonium accumulation, and therefore to NH3 toxicity. Furthermore, toxic rates of DAP and MAP can precipitate and inactivate Ca and Mg into relatively insoluble Ca-P and Mg-P complexes in crop seeds (Esteban et al., 2016; Makaza and Khiari, 2023). Nevertheless, starter K fertilizers placed with the seed had relative pH and electrical conductivity changes that pose general leaf yellowing and reduced growth when applied in excess (Schenk and Wehrmann, 1979). The influence of in-furrow-placed fertilizers at high rates could leave maize seedlings vulnerable to root disease infections, which could be exacerbated in fertilizer-injured roots (Pan et al., 2016).
4.4 Seed-placed starter fertilizers influence the maize yields
Starter fertilizer, regardless of placement, often increased early-season dry matter production and significantly increased grain yields (Battisti et al., 2022; Blandino et al., 2022). However, research indicated that ≥22 kg N ha−1 in direct seed contact did not increase yields and significantly reduced maize stands following seed-placed starter N fertilizers (Niehues et al., 2004). Similar results were recorded in this study when >5 kg N ha-1 DAP was applied in varying soils compared to K2SO4 and KCl. As opposed to the dribble over-the-row and subsurface placements, N rates between 11 and 34 kg ha-1 did not affect plant stands, albeit resulting in little added yield benefit (Niehues et al., 2004). This could be physio-metabolically associated with a reduction in the amount of carbon availability, damage to the chloroplast’s ultrastructure, an increase in proton efflux, ineffective transmembrane ammonium cycling, suppression of the enzyme GDP-mannose pyrophosphorylase, and oxidative stress (Shilpha et al., 2023). Our observations showed that established maximum N and K-placed fertilizer rates had the highest chlorophyll content compared to the toxic rates across all soil types. Shilpha et al. (2023) highlighted that fertilizer toxicity is always accompanied by lower levels of chlorophyll a and b and carotenoids, a decline in photosynthetic rates, and a rise in the production of ethylene. It is interesting to highlight that the higher the chlorophyll content index, the higher the crop biomass and vice versa, which could be a relative indicator of fertilizer toxicity that can be used as a decision support tool and may be used for improving the estimation of crop yield and biomass (Figures 3, 4; Supplementary Figure S3).
In this study, when >15 kg K ha-1 is seed-placed, it results in improved plant biomass. This often disagrees with Hergert et al. (2012) who reported that starter K fertilizer decreased the time to flowering compared with no starter but had little effect on the grain yield of no-till grain sorghum in eastern Nebraska. Mackenzie et al. (1988) postulated that even though the added KCl increased exchangeable and soluble K, probably due to reduced K fixation by illite, mica, and vermiculite minerals in the soil, high rates could reduce dry matter contents of silage maize on Ormstown silty clay loam. Starter K fertilizer placed in the furrow, for example, KCl could have the highest impact on seedling salinity injury (Kaiser et al., 2005; Lago et al., 2023). Therefore, ensuring and maintaining the osmotic potential of the in-furrow-placed starter N and K fertilizer is critical. This is because improper starter fertilizer placement injures crops by reducing the stand or retarding the development of established plants. In the context of the development of future cereal crop fertilization systems, it is vital to consider the introduction of environmentally friendly innovations in a sustainable intensification approach. This study has highlighted the importance of developing starter fertilizer recommendation models to guard against inaccurate and misleading fertilizer salt index characterized by the changes in the fertilizer industry, and this could promote high input use efficiency. In addition, this approach can promote plant vigor in the early vegetative stages and is a key factor that leads to significant and sustainable yield advantages. Here, the starter fertilization, with the application of N and K close to seed furrows, had the greatest effect, suggesting that simply reducing fertilizer inputs may represent a significant drawback for high N- and K-requiring crops, such as maize, and that it is necessary to re-design the fertilization strategies by above all enhancing the input use efficiency, focusing on the most critical growth stages for nutrient uptake.
5 Conclusion
As there are currently no established recommendations for the maximum safe rates of N and K fertilizers applied at maize planting, particularly when starter fertilizers are placed simultaneously with seeds, this study was designed to address this long-standing knowledge gap for practitioners. To achieve this, we examined three contrasting soil textures (G1, G2, and G3) and evaluated two nitrogen-based and two potassium-based starter fertilizers that are commonly applied in-furrow alongside maize seeds. The study assessed different fertilizer rates to determine their potential impact on seed and seedling viability. The findings indicate that K2SO4 posed the lowest risk, as even a 16 kg K ha-¹ application showed no signs of toxicity, regardless of soil texture. However, maximum safe application rates were identified for nitrogen fertilizers:
● DAP: 3 kg N ha-¹ in G1 and 0.8 kg N ha-¹ in G2 and G3.
● MAP: 5–7 kg N ha-¹, irrespective of soil texture.
● KCl, known for its high salt index, had a maximum safe rate of 10 kg K ha-¹ in G1 and 14 kg K ha-¹ in G2 and G3.
These safe application rates resulted in normal germination indicators (MET, MER, ES, UNC, and SYN) and healthy seedling development (MNR, NRT, TRL, AD, P, V, and SA), with no visual symptoms of toxicity observed in seeds, roots, or young seedlings. Future studies that benefit from examining the specific effects of each fertilizer salt to better understand distinct ionic, osmotic, and metabolic responses for the predicted recommendations could be explored. Overall, the insights from this study provide practitioners with science-based guidelines for optimizing starter fertilizer applications alongside maize seeds, ensuring effective nutrient management while minimizing the risk of seed and seedling damage.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
WM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. LK: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. MA: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was financially supported by the Office Chérifien des Phosphates (OCP) grant number AS No. 119/114DPPR012.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2025.1543564/full#supplementary-material
References
Abit M. J. M., Weathers K., and Arnall D. B. (2016). Evaluating the impact of starter fertilizer on winter canola grown in Oklahoma. Int. J. Agron. 2016, 7513486. doi: 10.1155/2016/7513486
Agenbag G. A. and De Villiers O. T. (1989). The effect of nitrogen fertilizers on the germination and seedling emergence of wild oat (A. fatua L.) seed in different soil types. Weed. Res. 29, 239–245. doi: 10.1111/j.1365-3180.1989.tb00908.x
Allison L. (1965). Organic carbon. Methods of soil analysis/Madison, Wisc. 1367–1376. doi: 10.2134/agronmonogr9.2.c39
Arif M. R., Islam M. T., and Robin A. H. K. (2019). Salinity stress alters root morphology and root hair traits in brassica napus. Plants (Basel). 8, 1–14. doi: 10.3390/plants8070192
Barker A. V., Maynard D. N., Mioduchowska B., and Buch A. (1970). Ammonium and salt inhibition of some physiological processes associated with seed germination. Physiol. Plant 23, 898–907. doi: 10.1111/j.1399-3054.1970.tb06487.x
Battisti M., Zavattaro L., Capo L., and Blandino M. (2022). Maize response to localized mineral or organic NP starter fertilization under different soil tillage methods. Eur. J. Agron. 138, 126534. doi: 10.1016/j.eja.2022.126534
Blandino M., Battisti M., Vanara F., and Reyneri A. (2022). The synergistic effect of nitrogen and phosphorus starter fertilization sub-surface banded at sowing on the early vigor, grain yield and quality of maize. Eur. J. Agron. 137, 126509. doi: 10.1016/j.eja.2022.126509
Cassman K. G., Kerby T. A., Roberts B. A., Bryant D. C., and Brouder S. M. (1989). Differential response of two cotton cultivars to fertilizer and soil potassium. J. Agron. 81, 870–876. doi: 10.2134/agronj1989.00021962008100060006x
CEAEQ (2003). Method of analysis. Determination of the lime requirement in agricultural soils by the SMP method (buffer pH). Center of expertise in environmental analysis of Quebec. MA. 1010–SMP 1.0.
Chelabi H., Khiari L., Gallichand J., and Joseph C. A. (2016). Soil sample preparation techniques on routine analyses in Quebec affect lime and fertilizer recommendations. Can. J. Soil Sci. 96, 244–255. doi: 10.1139/cjss-2015-0062
Chen W., Chen Y., Siddique K. H. M., and Li S. (2022). Root penetration ability and plant growth in agroecosystems. Plant Physiol. Biochem. 183, 160–168. doi: 10.1016/j.plaphy.2022.04.024
Chen Z., Wang H., Liu X., Liu Y., Gao S., and Zhou J. (2016). The effect of N fertilizer placement on the fate of urea-15N and yield of winter wheat in Southeast China. PloS One 11, e0153701. doi: 10.1371/journal.pone.0153701
Colliver G. W. and Welch L. F. (1970). Toxicity of preplant anhydrous ammonia to germination and early growth of corn: I. Field studies1. J. Agron. 62, 341–346. doi: 10.2134/agronj1970.00021962006200030009x
Court M. N., Stephen R. C., and Waid J. S. (1964). Toxicity as a cause of the inefficiency of urea as a fertilizer. I. Review. J. Soil Sci. 15, 42–48. doi: 10.1111/j.1365-2389.1964.tb00243.x
Cummins D. G. and Parks W. L. (1961). The germination of corn and wheat as affected by various fertilizer salts at different soil temperatures. Soil Sci. Soc Am. J. 25, 47–49. doi: 10.2136/sssaj1961.03615995002500010022x
Day P. R. (1965). “Particle fractionation and particle-size analysis,” in Methods of soil analysis. Part 1. Physical and mineralogical properties. Ed. Black C. A. (ASA, Madison, WI), 545–567.
Dowling C. W. (1993). “Tolerance of ten crop species to atmospheric ammonia during seed germination, radicle and coleoptile growth,” in Plant Nutrition — from Genetic Engineering to Field Practice: Proceedings of the Twelfth International Plant Nutrition Colloquium, 21–26 September 1993, Perth, Western Australia. Ed. Barrow N. J. (Springer Netherlands, Dordrecht).
Erenstein O., Jaleta M., Sonder K., Mottaleb K., and Prasanna B. M. (2022). Global maize production, consumption and trade: trends and R&D implications. Food Sec. 14, 1295–1319. doi: 10.1007/s12571-022-01288-7
Esteban R., Ariz I., Cruz C., and Moran J. F. (2016). Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 248, 92–101. doi: 10.1016/j.plantsci.2016.04.008
Galpottage Dona W. H., Schoenau J. J., and King T. (2020). Effect of starter fertilizer in seed-row on emergence, biomass and nutrient uptake by six pulse crops grown under controlled environment conditions. J. Plant Nutr. 43, 879–895. doi: 10.1080/01904167.2020.1711945
Gordon W. (1999). Starter fertilizers containing potassium for ridge-till corn and soybean production. Better. Crops. 83, 22–23. doi: 10.5555/19991909687
Gordon W. B. and Pierzynski G. M. (2006). Corn hybrid response to starter fertilizer combinations. J. Plant Nutr. 29, 1287–1299. doi: 10.1080/01904160600767591
Hergert G. W., Wortmann C. S., Ferguson R. B., Shapiro C. A., and Shaver T. M. (2012). Using starter fertilizers for corn, grain sorghum, and soybeans. NebGuide G361 (Lincoln: University of Nebraska).
Kaiser D. E., Coulter J. A., and Vetsch J. A. (2016). Corn hybrid response to in-furrow starter fertilizer as affected by planting date. J. Agron. 108, 2493–2501. doi: 10.2134/agronj2016.02.0124
Kaiser D. E., Mallarino A. P., and Bermudez M. (2005). Corn grain yield, early growth, and early nutrient uptake as affected by broadcast and in-furrow starter fertilization. J. Agron. 97, 620–626. doi: 10.2134/agronj2005.0620
Kaiser D. E. and Rubin J. C. (2013a). Maximum rates of seed placed fertilizer for corn for three soils. J. Agron. 105, 1211–1221. doi: 10.2134/agronj2013.0125
Kaiser D. E. and Rubin J. C. (2013b). Corn nutrient uptake as affected by in-furrow starter fertilizer for three soils. J. Agron. 105, 1199–1210. doi: 10.2134/agronj2013.0122
Laboski C. A. (2008). Understanding salt index of fertilizers. Proc. Wisconsin Fertilizer, Aglime ad Pest Management Conference Vol. 2008 (Madison, WI), 37–41.
Lago B. C., Favarin J. L., De Almeida R. E. M., Pierozan Junior C., De Oliveira S. M., Tezotto T., et al. (2023). Potassium application timing to improve corn K-fertilizer use in the oat-corn sequence: a tracer study for high yielding corn. J. Plant Nutr. 46, 618–629. doi: 10.1080/01904167.2022.2067054
Lindsay W. L., Frazier A. W., and Stephenson H. F. (1962). Identification of reaction products from phosphate fertilizers in soils. Soil Sci. Soc Am. Proc. 26, 446–451. doi: 10.2136/sssaj1962.03615995002600050013x
Lozano-Isla F., Benites-Alfaro O. E., and Pompelli M. F. (2019). GerminaR: An R package for germination analysis with the interactive web application “GerminaQuant for R” Vol. 34 (Hoboken, USA: John Wiley & Sons, Inc), 339–346.
Mackenzie A. F., Phillip L. E., and Kirby P. C. (1988). Effect of added urea and potassium chloride on yields of corn over four years and on soil potassium. J. Agron. 80, 773–777. doi: 10.2134/agronj1988.00021962008000050016x
Maeoka R. E., Sadras V. O., Ciampitti I. A., Diaz D. R., Fritz A. K., and Lollato R. P. (2020). Changes in the phenotype of winter wheat varieties released between 1920 and 2016 in response to in-furrow fertilizer: biomass allocation, yield, and grain protein concentration. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01786
Mahler R. L., Lutcher L. K., and Everson D. O. (1989). Evaluation of factors affecting emergence of winter wheat planted with seed-banded nitrogen fertilizers. Soil Sci. Soc Am. J. 53, 571–575. doi: 10.2136/sssaj1989.03615995005300020045x
Makaza W. and Khiari L. (2023). Too salty or toxic for use: A tale of starter fertilizers in agronomic cropping systems. Agronomy 13, 2690. doi: 10.3390/agronomy13112690
Makaza W., Khiari L., Lakrari Z., and El Achaby M. (2024). Optimizing co-application of starter ammoniacal-N and K-containing fertilizers for barley in the seed-furrow. J. Plant Nutr. 47, 1–23. doi: 10.1080/01904167.2024.2391544
Mehlich A. (1984). Mehlich–3 soil test extractant: A modification of Mehlich–2 extractant. Commun. Soil Sci. Plant Anal. 15, 1409–1416. doi: 10.1080/00103628409367568
Mullins G. L. and Burmester C. H. (1997). Starter fertilizer and the method and rate of potassium fertilizer effects on cotton grown on soils with and without winter grazing by cattle. Commun. Soil Sci. Plant Anal. 28, 739–746. doi: 10.1080/00103629709369826
Murray T. P. and Clapp J. G. (2004). Current fertilizer salt index tables are misleading. Commun. Soil Sci. Plant Anal. 35, 2867–2873. doi: 10.1081/CSS-200036474
Nadarajan S. and Sukumaran S. (2021). “Chapter 12 - Chemistry and toxicology behind chemical fertilizers,” in Controlled Release Fertilizers for Sustainable Agriculture. Eds. Lewu F. B., Volova T., Thomas S., and K.R R. (Academic Press).
Niehues B. J., Lamond R. E., Godsey C. B., and Olsen C. J. (2004). Starter Nitrogen fertilizer management for continuous no-till corn production. J. Agron. 96, 1412–1418. doi: 10.2134/agronj2004.1412
Pan W. L., Madsen I. J., Bolton R. P., Graves L., and Sistrunk T. (2016). Ammonia/ammonium toxicity root symptoms induced by inorganic and organic fertilizers and placement. J. Agron. 108, 2485–2492. doi: 10.2134/agronj2016.02.0122
Qian P., Urton R., Schoenau J., King T., Fatteicher C., and Grant C. (2012). Effect of seed-placed ammonium sulfate and monoammonium phosphate on germination, emergence and early plant biomass production of Brassicae oilseed crops. Oilseeds. 3, 53–62.
Randall G. W. and Hoeft R. G. (1988). Placement methods for improved efficiency of P and K fertilizers: A review. J. Prod. Agric. 1, 70–79. doi: 10.2134/jpa1988.0070
Ritchie J. T., Singh U., Godwin D. C., and Bowen W. T. (1998). “Cereal growth, development and yield,” in Understanding options for Agricultural Production. Eds. Tsuji G. Y., Hoohenboom G., and Thornton P. K. (Springer Netherlands, Dordrecht).
Sardi K. and Beres I. (1996). Effects of fertilizer salts on the germination of corn, winter wheat, and their common weed species. Commun. Soil Sci. Plant Anal. 27, 1227–1235. doi: 10.1080/00103629609369628
Schenk M. and Wehrmann J. (1979). The influence of ammonia in nutrient solution on growth and metabolism of cucumber plants. Plant Soil 52, 403–414. doi: 10.1007/BF02185583
Seethepalli A., Dhakal K., Griffiths M., Guo H., Freschet G. T., and York L. M. (2021). RhizoVision Explorer: open-source software for root image analysis and measurement standardization. AoB. Plants 13, 1–14. doi: 10.1093/aobpla/plab056
Shilpha J., Song J., and Jeong B. R. (2023). Ammonium phytotoxicity and tolerance: an insight into ammonium nutrition to improve crop productivity. Agronomy 13, 1487. doi: 10.3390/agronomy13061487
Starling M. E., Wood C. W., and Weaver D. B. (1998). Starter nitrogen and growth habit effects on late-planted soybean. J. Agron. 90, 658–662. doi: 10.2134/agronj1998.00021962009000050015x
Tsialtas I. T., Shabala S., Baxevanos D., and Matsi T. (2016). Effect of potassium fertilization on leaf physiology, fiber yield and quality in cotton (Gossypium hirsutum L.) under irrigated Mediterranean conditions. Field Crops Res. 193, 94–103. doi: 10.1016/j.fcr.2016.03.010
Vanlauwe B., Amede T., Bationo A., Bindraban P., Breman H., Cardinael R., et al. (2023). Fertilizer and soil health in Africa: The role of fertilizer in building soil health to sustain farming and address climate change.
Venables W. N., Smith D. M., and The R Core Team (2024). An Introduction to R. Notes on R: A Programming Environment for Data Analysis and Graphics Version 4.4.2.
Vines H. M. and Wedding R. T. (1960). Some effects of ammonia on plant metabolism and a possible mechanism for ammonia toxicity. Plant Physiol. 35, 820–825. doi: 10.1104/pp.35.6.820
Wan X., Wu W., Li C., Liu Y., Wen X., and Liao Y. (2016). Soil ammonia volatilization following urea application suppresses root hair formation and reduces seed germination in six wheat varieties. Environ. Exp. Bot. 132, 130–139. doi: 10.1016/j.envexpbot.2016.08.010
Wang W., Zhang F., Sun L., Yang L., Yang Y., Wang Y., et al. (2022). Alkaline salt inhibits seed germination and seedling growth of canola more than neutral salt. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.814755
Zhang X. K. and Rengel Z. (2002). Temporal dynamics of gradients of phosphorus, ammonium, pH, and electrical conductivity between a diammonium phosphate band and wheat roots. Aust. J. Agric. Res. 53, 985–992. doi: 10.1071/AR01149
Keywords: fertilizer toxicity, seed emergence, salt injury, NH3 toxicity, in-furrow placement, visual toxicity signs
Citation: Makaza W, Khiari L and El Achaby M (2025) Optimizing safe rates of pop-up inorganic starter nitrogen and potassium fertilizers for maize. Front. Agron. 7:1543564. doi: 10.3389/fagro.2025.1543564
Received: 11 December 2024; Accepted: 24 June 2025;
Published: 04 August 2025.
Edited by:
Khurram Shahzad, Lasbela University of Agriculture, Water and Marine Sciences, PakistanReviewed by:
Muhammad Tahir Shehzad, University of Agriculture, Faisalabad, PakistanManuel Ângelo Rodrigues, Centro de Investigação de Montanha (CIMO), Portugal
Copyright © 2025 Makaza, Khiari and El Achaby. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William Makaza, d2lsbGlhbS5tYWthemFAdW02cC5tYQ==
 William Makaza
William Makaza Lotfi Khiari
Lotfi Khiari Mounir El Achaby3
Mounir El Achaby3