- 1Agronomy Department, International Institute of Tropical Agriculture, Lilongwe, Malawi
- 2Agronomy Department, International Institute of Tropical Agriculture, Nampula, Mozambique
- 3Breeding Department, International Institute of Tropical Agriculture, Lusaka, Zambia
- 4Breeding Department, International Institute of Tropical Agriculture, Ibadan, Nigeria
- 5Administration Department, Fertilizer Association of Kenya, Nairobi, Kenya
Cassava is important as food, feed, and raw material, yet its root architecture is not fully understood and thoroughly studied, thereby limiting the realization of its full potential. Compounding this is the common practice of applying fertilizer to cassava at planting without extensive knowledge of the suitable growth stage or time for optimal nutrient uptake. Consequently, this study’s objective was to stagger fertilizer application in split doses to synchronize nutrient uptake and cassava growth and track the storage root bulking process. Nine treatments of normal and controlled-release fertilizers were tested in the field, each applied in four splits at 0, 2, 4, and 6 months after planting (MAPs) to the Binte Massude cassava variety. The results indicated that controlled-release fertilizers applied in two splits at planting and 6 MAPs led to 19.0% greater root yield, whereas normal fertilizers applied in four splits every 2 months led to 22.7% greater root yield compared to not applying fertilizer to cassava. Irrespective of the fertilizer form, when the number of roots increased, the root yield decreased gradually, while root diameter and root length had a direct relationship with storage root yield. Furthermore, the relationship between root number and either root diameter or root length was significantly inverse. This study has successfully indicated the root attributes that can be observed to predict storage root yield, and that staggering fertilizer application in targeted splits increases root yield, however, this must be synchronized with moisture availability for optimal benefit.
1 Introduction
Cassava (Manihot esculenta, Crantz), a root crop, is the third largest source of carbohydrates in the tropics and the sixth most important crop in the world (Lebot, 2008). In the tropics, it is a source of starch for over 500 million people (Balagopalan, 2002). Yet, its world production is only 262.6 million tons (FAO, 2017), of which the highest yield per hectare was achieved in India (36.4 t ha-1), while sub-Saharan Africa produced 6–8 t ha-1 (FAO, 2017). This yield gap per hectare between sub-Saharan African countries and India is attributed to several factors; however, the major factor is poor soils and minimal or lack of fertilizer application (Crawford et al., 2003). Interestingly, it has been observed that cassava root yield increases with fertilizer application even in sub-Saharan Africa (Fermont et al., 2009, 2007; Lawson et al., 2023; Mashuubu et al., 2024). Split application of fertilizer to cassava was reported to enhance the storage root yield (Katuromunda et al., 2021; Senkoro et al., 2018). Furthermore, Janat (2007) reported increased tuberous yield after fertigation of other root and tuber crops besides cassava. Recent studies on the effects of fertigation on growth and root yield of cassava indicated that leaf starch was more accurate in predicting storage root yield than leaf nitrogen (N) (Omondi et al., 2019b). Further, we showed that phosphorus (P) improved cassava storage root number (Omondi et al., 2019a), whereas potassium (K) led to a decrease in soluble carbohydrates and starch in the leaves; hence, we concluded that these sugars were translocated to the storage roots, thus improving bulking and development (Omondi et al., 2020a).
In another experiment, we established fertigation concentrations at which maximum storage root yields were achieved in the field for three cassava varieties (Mweru, Nalumino, and Kampolombo) (Omondi et al., 2018). This work laid the basis of fertigation in cassava and showed an increase in root yield using that method of fertilizer application. Yet, the application of fertilizer to cassava by most smallholder farmers is scarce (Howeler, 2002) and if done, it is usually at planting. Due to the growth habit of cassava, the applied fertilizer at planting could be lost either through leaching, surface run-off, or volatilization in the case of nitrogenous fertilizer before the critical stages of storage root bulking (Alves, 2002). Interestingly, the response of cassava and root crops to slow-release or controlled-release fertilizers compared to normal fertilizers is unknown. Controlled-release fertilizers gradually release nutrients into the soil over an extended period, matching plant nutrient uptake (Govil et al., 2024). The release is controlled by physical, chemical, or biological mechanisms, such as polymer, sulfur, and resin coats, reducing nutrient losses and improving efficiency (Govil et al., 2024; Jariwala et al., 2022). Since most cassava varieties’ maturity period is 8–18 or 24 months (Nzola et al., 2022), testing its response to fertilizers that match nutrient release with nutrient uptake over this long period is key. Consequently, tracking the bulking process will enable staggering of fertilizer application in split doses, synchronizing nutrient uptake and cassava growth. Thus, the objectives of this study were to track the storage root bulking of cassava through the growth period, determine the effects of split applications of fertilizers through banding on cassava growth and root yield, and evaluate the efficacy of controlled-release fertilizers in split applications on growth and root yield.
2 Materials and methods
2.1 Experimental sites
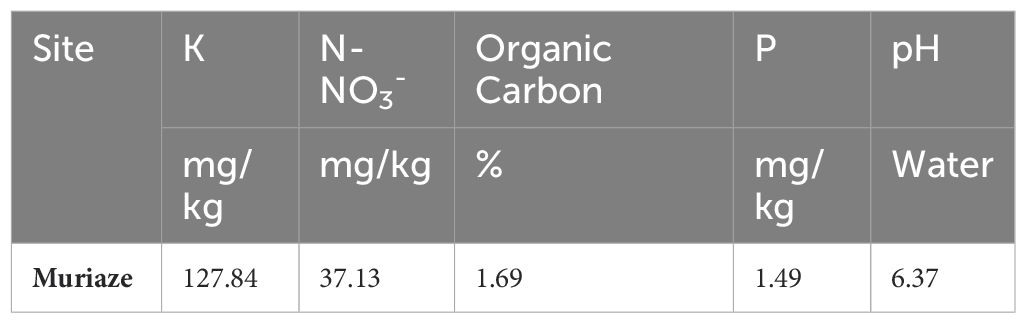
The study was conducted from 2021 to 2022 at Muriaze (15° 17’ S, 39° 19’ E) in Nampula province of Mozambique. Prior to the establishment of the experiment, initial random soil sampling in transects of 10 m by 10 m was conducted at 0–30 cm depth in a zigzag method (Maiti, 2013), mixed thoroughly to obtain a composite sample of 500 g, air-dried, and processed through a 2-mm sieve. These samples were analyzed for N-NO3-, P, K, Organic Carbon (OC), pH, and texture. N-NO3- was analyzed using the KCl extraction method (Bremner, 1960), and P and K using Mehlich 3 extraction (Mehlich, 1984). Soil pH was measured at a 1:2 soil:water ratio and texture using the hydrometer method. The results are presented in Table 1. The soil type was sandy clay. The rainfall amounts were 1,243.3 mm and 1,167.6 mm in 2021 and 2022, respectively, while the minimum and maximum atmospheric temperatures were 19.3°C and 30.7°C in 2021, and 19.7°C and 29.8°C in 2022 (Figure 1).
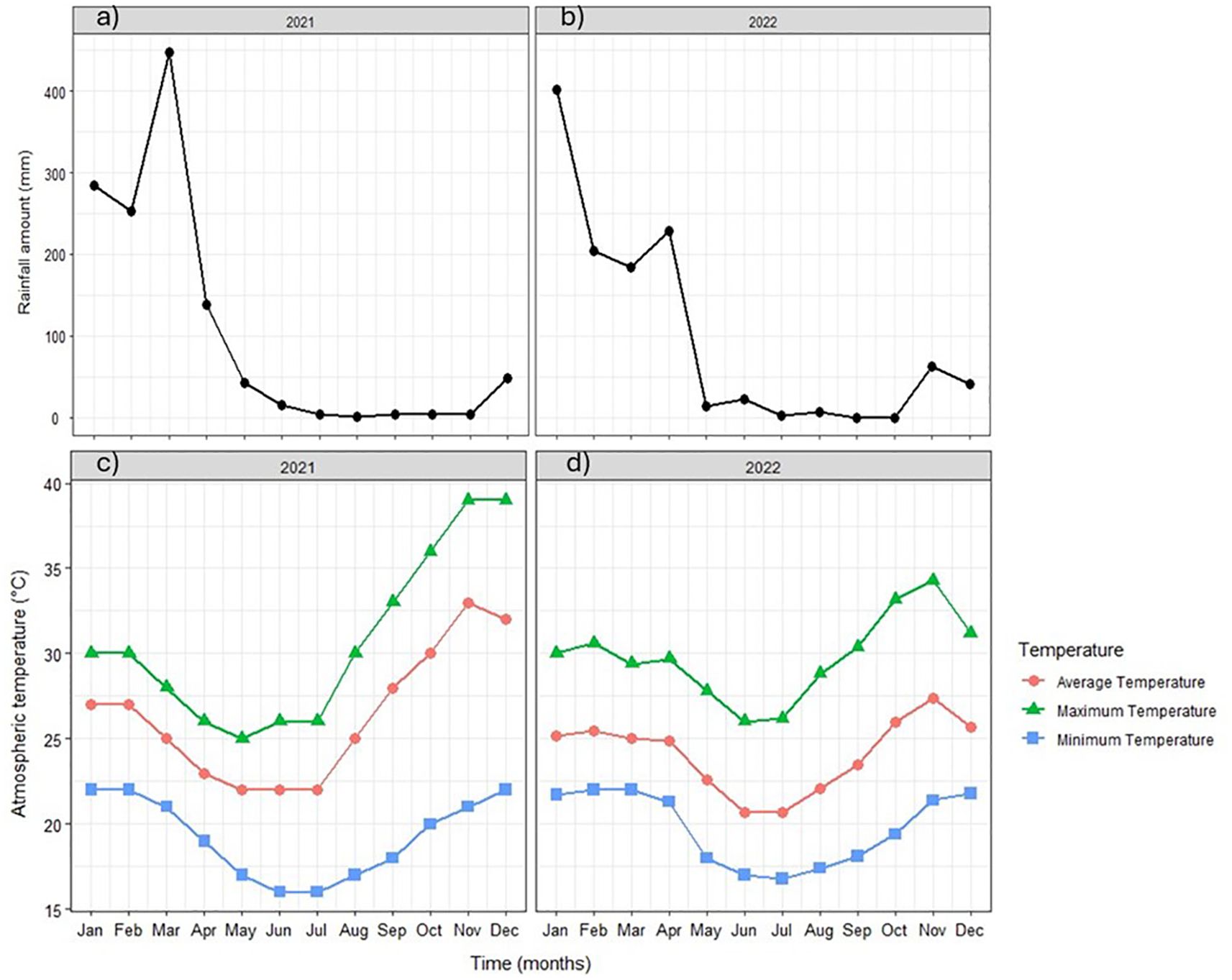
Figure 1. (a, b) Rainfall amount and (c, d) atmospheric temperature during the cassava growth period.
2.2 Experimental setup
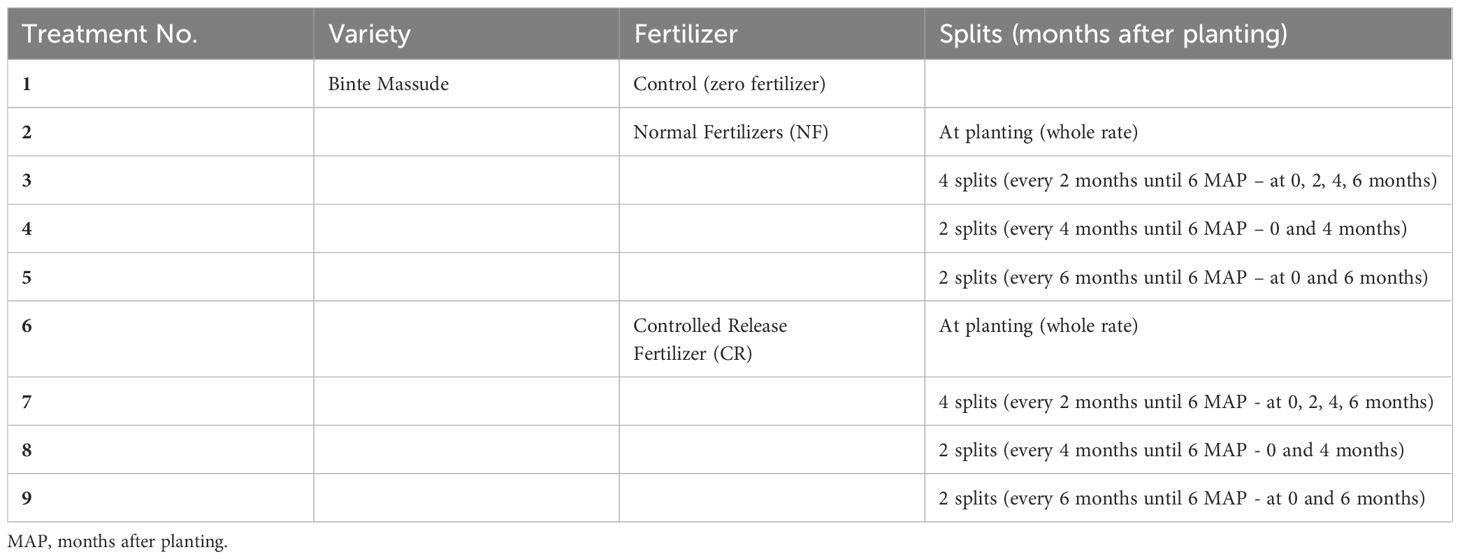
This experiment builds on other cassava trials that investigated the effect of fertigation on growth, root yield, and quality of cassava (Omondi et al., 2020b, 2018). This experiment consisted of split applications of nine treatments of fertilizers replicated four times in a randomized complete block design (Table 2). Normal fertilizers (NF) and controlled-release fertilizers (CR) were tested. A rate of 155, 23, 155 NP2O5K2O kg ha-1—the rate at which maximum root yield of Mweru was achieved in the previous experiment (Omondi et al., 2018)—was divided equally among the splits. The applied CR contained 16-10-16 + 2MgO+27SO3, and feeds the plant for 2–3 months.
Cuttings of Binte Massude measuring 30 cm long with a minimum of 5–6 nodes were planted in the field at a spacing of 1 m by 1m in plots measuring 8 m by 8m containing 64 plants. Fertilizers were applied until 6 months after planting (MAPs) because after 6 months, the rains had receded drastically (Figures 1a, b), hence it was assumed that fertilizer dissolution could have been lower, leading to minimum benefit. Sigtryggsson et al. (2020) observed that 2 mm of rain dissolves 50% of fertilizers, yet after 6 months, the amount of rain at the experimental site was lower than that.
2.3 Data collection and analysis
The chlorophyll content index was measured using a chlorophyll meter (SPAD 502 Plus chlorophyll meter) in the youngest fully expanded leaf (YFEL) of three plants within the net plot. This was done between 10:00 am and 13:00 pm at 6, 9, and 12 MAPs.
From each treatment in the field, the five YFELs of four plants within the net plot were sampled at 6 MAPs. The leaf blades were separated from the petioles, rinsed in deionized water for 15 seconds, dried using a paper towel, and oven-dried at 70°C to a constant weight. The dried leaf blades were ground in a stainless-steel coffee mill to 0.5 mm particle size. Total N concentration in the leaf blade was determined after digestion with sulfuric acid and peroxide using the Kjeldahl method (Bremner, 1960). Phosphorus was determined using an automated photometric analyzer (Thermo Scientific Gallery Plus), while K was analyzed using an atomic absorption spectrophotometer (Snell and Snell, 1949).
Three plants were uprooted at 1, 3, 6, and 9 MAPs within the net plot at final harvesting (at 12 MAPs) for measurement of the primary root’s length using a ruler from the tip to the bottom and the diameter using a vernier callipers at the widest part, and the root density (number of roots per uprooted plant) and weight (fresh and dry).
At final harvesting, at 12 MAPs, five plants were uprooted using hand-hoes. The plants were separated into storage roots, stems, and leaves, and their fresh masses were recorded. A known fresh mass of the storage roots sub-samples was oven-dried at 70°C for 48 h, re-weighed until a constant weight was achieved, and then recorded.
Analysis of variance was conducted using R using the lmerTest package, and the means were compared by Fisher’s least significant difference (LSD) method, at a 95% confidence level (R Development Core Team, 2021). The site (location), the split fertilizer applications, and their interactions were fixed factors while the blocks were random factors. Regressions were done to determine the relationship between root yield, number of storage roots, root length, and root diameter.
3 Results
3.1 Chlorophyll content index and nutrient concentrations in leaves, petioles, and stems
Generally, the chlorophyll content of cassava was higher under controlled-release fertilizers, though significantly different among the split application treatments (Figure 2). As expected, the chlorophyll content increased with time of growth in all the split applications, and it was significantly lower when the entire amount of fertilizer was applied at planting, regardless of the fertilizer type (Figure 2). Applying controlled-release fertilizers in two splits, at planting and at 6 months after planting, led to more chlorophyll consistently from 8 to 12 months after planting, whereas, for normal fertilizers, this was achieved under four splits every 2 months (Figure 2).

Figure 2. Split application of: (a) controlled-released and (b) normal fertilizers’ effect on chlorophyll content index of cassava. The error bars are SE (standard error).
Similar to chlorophyll content, total N in the leaves of cassava was significantly higher under application of normal fertilizers in four splits, reaching 5.7% (Figure 3a). This was similar to that under controlled-release fertilizers applied in two splits, at planting and at 6 months after planting, which reached 5.6% N, while with no fertilizer application, N in the leaves was 4.8%. Total N in the petioles and stems followed a similar trend as in the leaves, though the content was lower (Figure 3a).
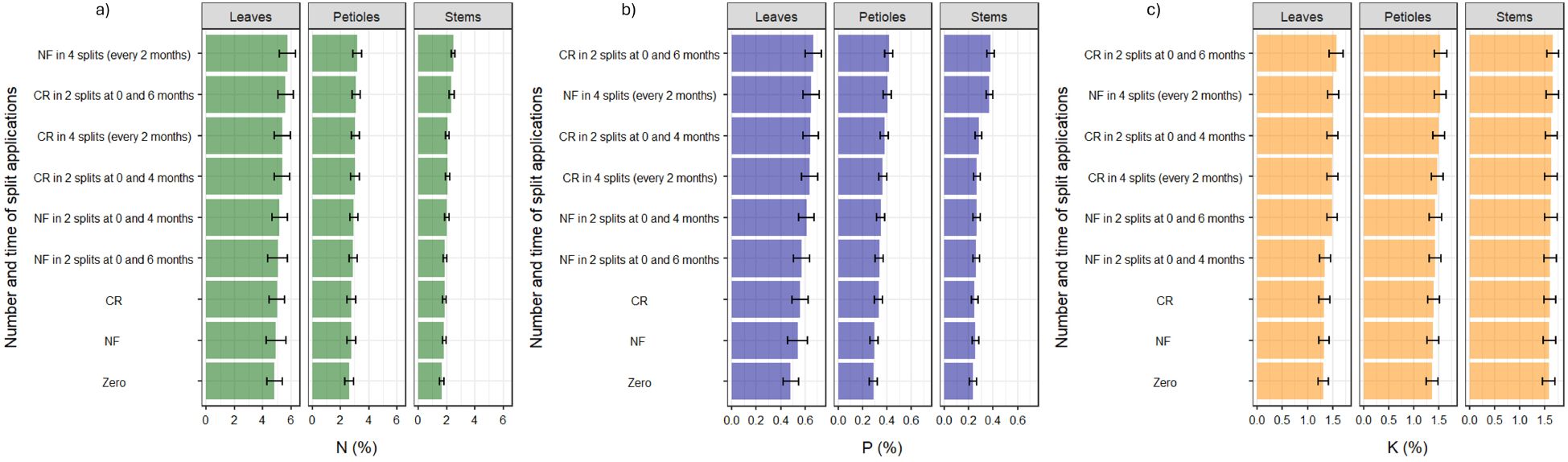
Figure 3. Effect of split application of different fertilizer forms on the concentration of: (a) N in the leaf blade (youngest fully expanded leaf–YFEL), petioles, and stems, (b) P in the leaf blade, petioles and stems, (c) K in the leaf blade, petioles and stems. The error bars are – SE (standard errors).
The concentration of P in the leaves of cassava under controlled-release fertilizers applied in two splits, at planting and at 6 months after planting, was 0.67%, while that under normal fertilizers in four splits was 0.65% (Figure 3b). These were the highest concentrations of P in the leaves. Likewise, in the petioles and stems, P was highest when controlled-release fertilizers were applied in two splits, at planting and at 6 months after planting, followed by when normal fertilizer was applied in four splits (Figure 3b).
Like the concentration of P, K in the leaves, petioles, and stems of cassava was highest under application of controlled-release fertilizers applied in two splits, at planting and at 6 months after planting, followed by normal fertilizer applied in four splits (Figure 3c). In general, total N and P were higher in the leaves, followed by the petioles and then the stems, whereas K was higher in the stems, followed by the petioles and the leaves (Figure 3).
3.2 Number of storage roots, diameter, and length
The number of roots declined significantly in all treatments, from over 35 at 1 month after planting to below 20 at 12 months after planting in both forms of fertilizers and across split applications of fertilizers (Figures 4a, b). Not applying fertilizers (control treatment) to cassava consistently led to fewer storage roots than in the other treatments throughout the growth period, while splitting the fertilizer amount four times—applying it every 2 months—produced more storage roots regardless of the fertilizer form (Figures 4a, b).
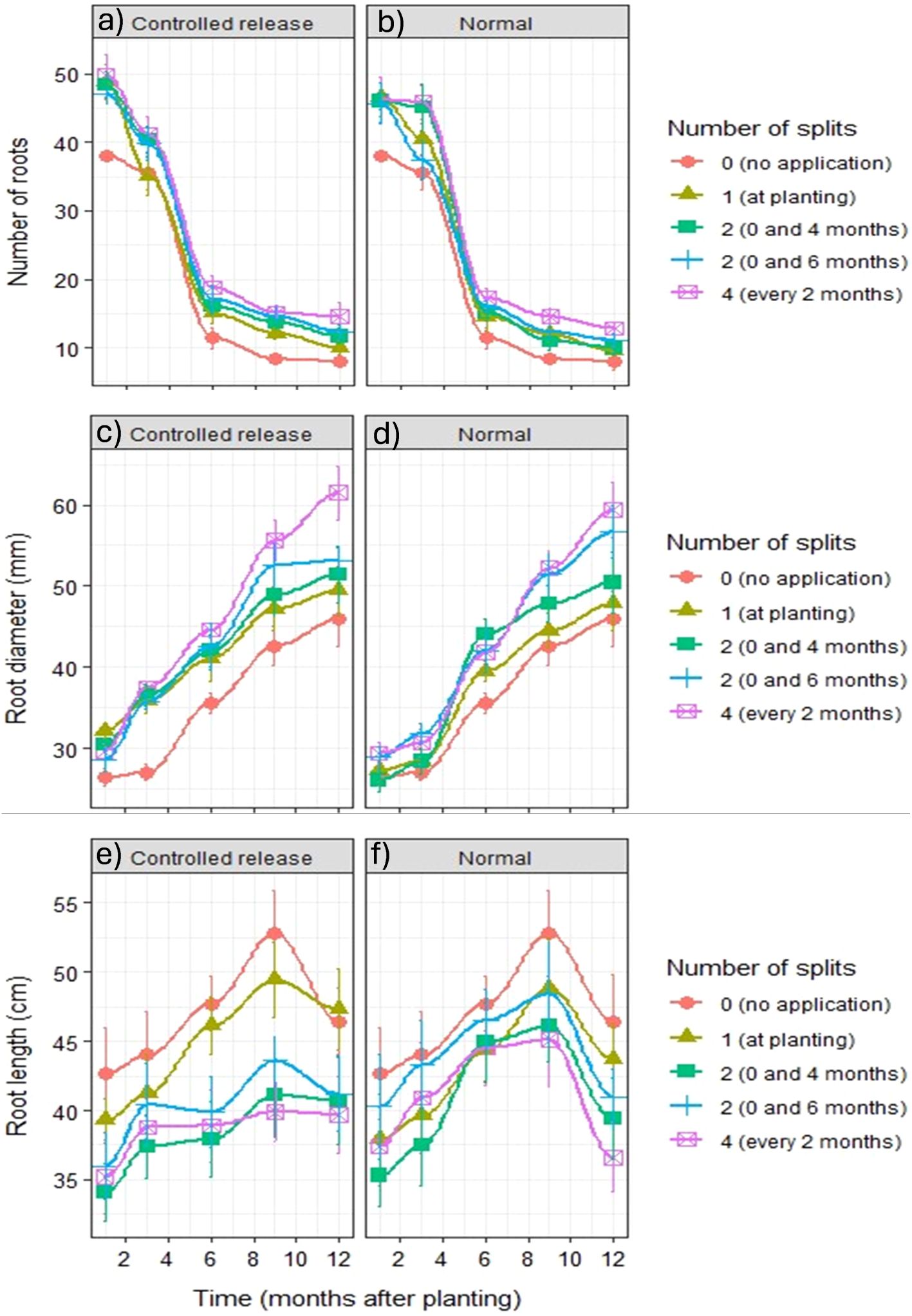
Figure 4. Number of cassava storage roots under (a) controlled-released and (b) normal fertilizers, their diameter on (c) controlled-released and (d) normal fertilizers, and the length under (e) controlled-released and (f) normal fertilizers applied at different times during the growth. The error bars are SE (standard error).
Conversely, root diameter significantly increased during the cassava growth period from 1 month after planting to 12 months after planting, and similar to the number of storage roots, the roots under no fertilizer application were the thinnest, whereas the cassava plants that received four splits of fertilizer every 2 months had the thickest storage roots (Figures 4c, d). Interestingly, for normal fertilizers, this was clearly observed from 9 to 12 months after planting, while for controlled-release fertilizers, it was from 3 to 12 months after planting (Figures 4c, d). At 12 months after planting, the size was above 60 mm, widening the gap between it and the second thickest, i.e., two split applications of controlled-release fertilizers at planting and at 6 months after planting (Figures 4c, d).
Contrary to the number of roots and root diameter, root length was significantly longer when no fertilizer was applied to cassava plants in both forms of fertilizers (Figures 4e, f). This significantly increased from 1 to 9 months after planting, then declined towards 12 months after planting in both forms of fertilizers (Figures 4e, f). Furthermore, from 9 to 12 months after planting, the split application where more and thicker storage roots were achieved was where the shortest roots were observed: with four splits of fertilizer application every 2 months (Figures 4e, f).
3.3 Root yield
Generally, regardless of fertilizer form or number of split applications, storage root yield significantly increased with time until 6 months after planting and then gradually dipped towards 9 months after planting, followed by a rise towards 12 months after planting (Figure 5). Under controlled-release fertilizers, in the first and third months after planting, root yield was highest when fertilizers were applied in two splits at planting, and at either 4 or 6 months after planting. In the successive months, 6 and 9 months after planting, the root yield was highest when fertilizer application was applied every 2 months in four splits. At harvesting, 12 months after planting, controlled-release fertilizers applied in two splits at planting and at 6 months after planting led to the highest root yield. However, this was not significantly different from root yields under two split applications at planting and at 4 months after planting. Instead, it was different from not applying fertilizer or applying controlled-release fertilizer only at planting, with a 19% and 12% difference, respectively (Figure 5a). Except for 1 and 3 months after planting, root yield under normal fertilizer was significantly high when the fertilizers were applied in four splits every 2 months, whereas not applying fertilizer to cassava led to the lowest root yields, which were 22% lower than under the four splits (Figure 5b).
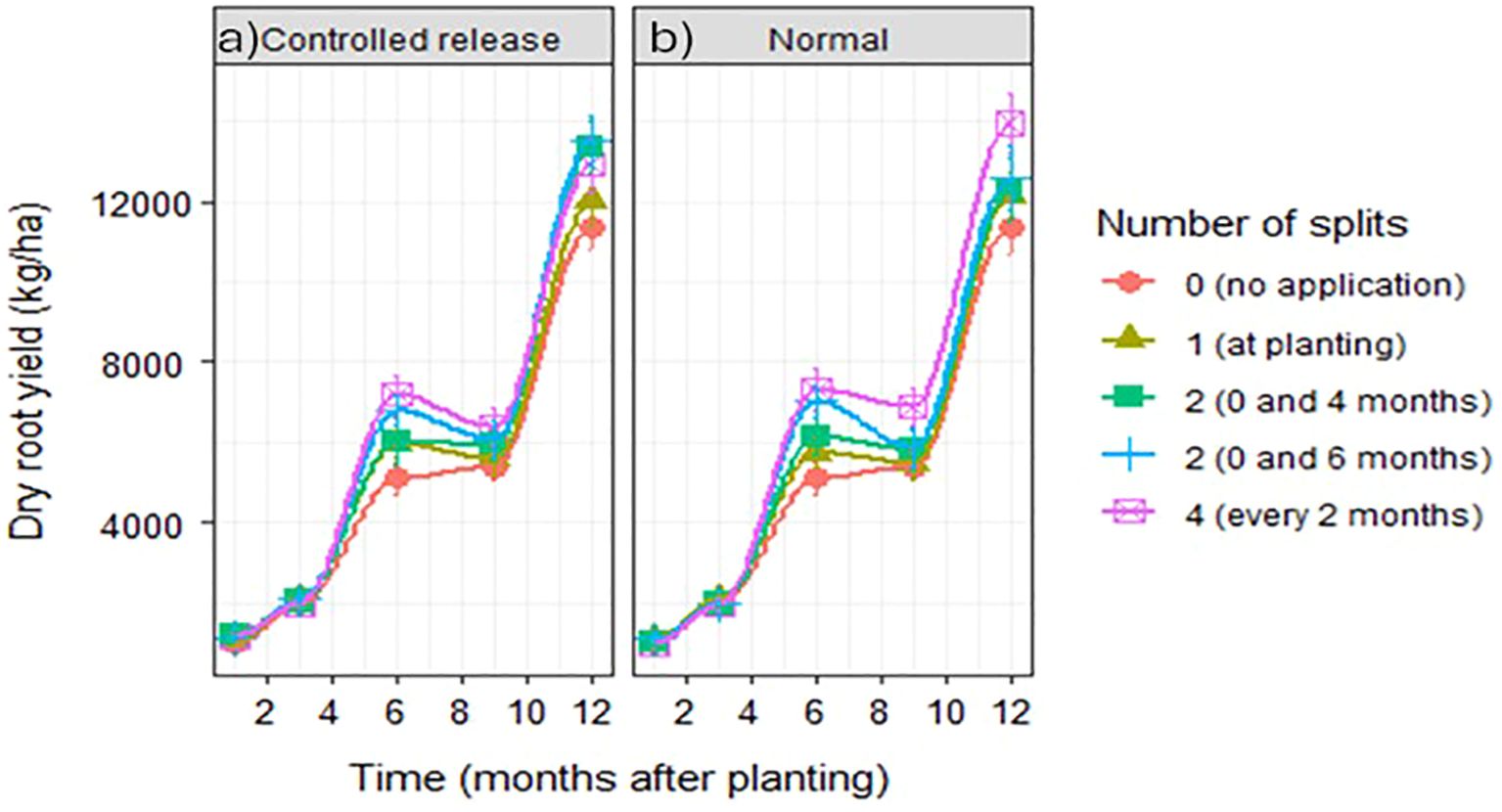
Figure 5. Dry storage root yield of cassava under: (a) controlled-released and (b) normal fertilizers applied in splits. The error bars are SE (standard error).
3.4 Relationships among the root attributes
Irrespective of the fertilizer form, storage root yield was significantly inverse to the number of roots, when the number of roots increased (Figures 6a, b), the root yield decreased gradually, whereas root diameter and root length had a direct relationship with storage root yield (Figures 6c–f). However, the direct relationship between the root diameter and root yield was steeper and more significant (Figures 6c, d) than that between root length and root yield (Figures 6e, f).
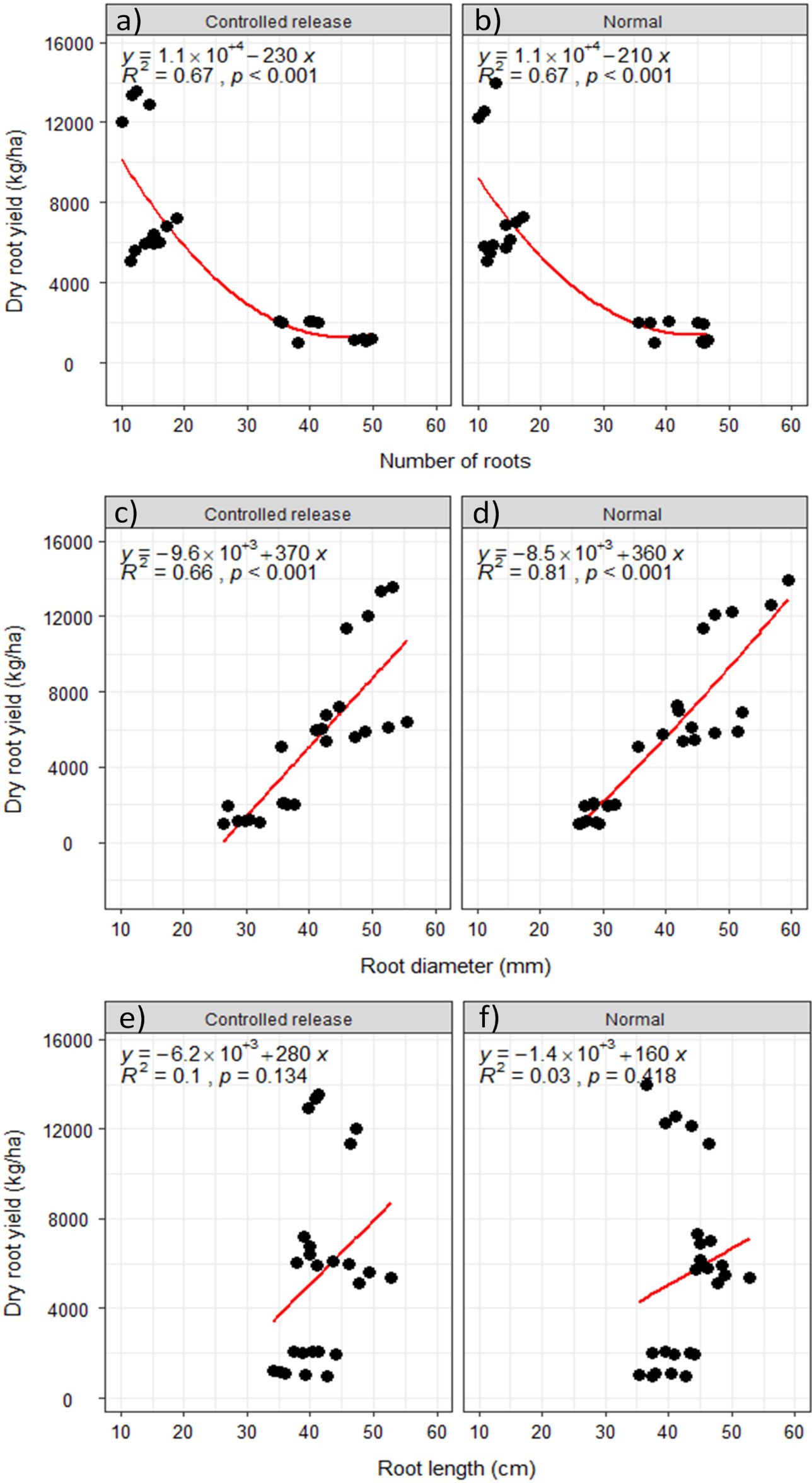
Figure 6. Relationship between root yield and (a, b) number of roots, (c, d) root diameter, and (e, f) root length.
Like the relationship between root yield and number of roots, the relationship between the number of roots and either root diameter or root length was significantly inverse (Figures 7a–d), even though it was steeper between the number of roots and root length (Figures 7c, d). The relationship between root diameter and root length was not significant, yet it was positively direct and gradual (Figures 7e, f).
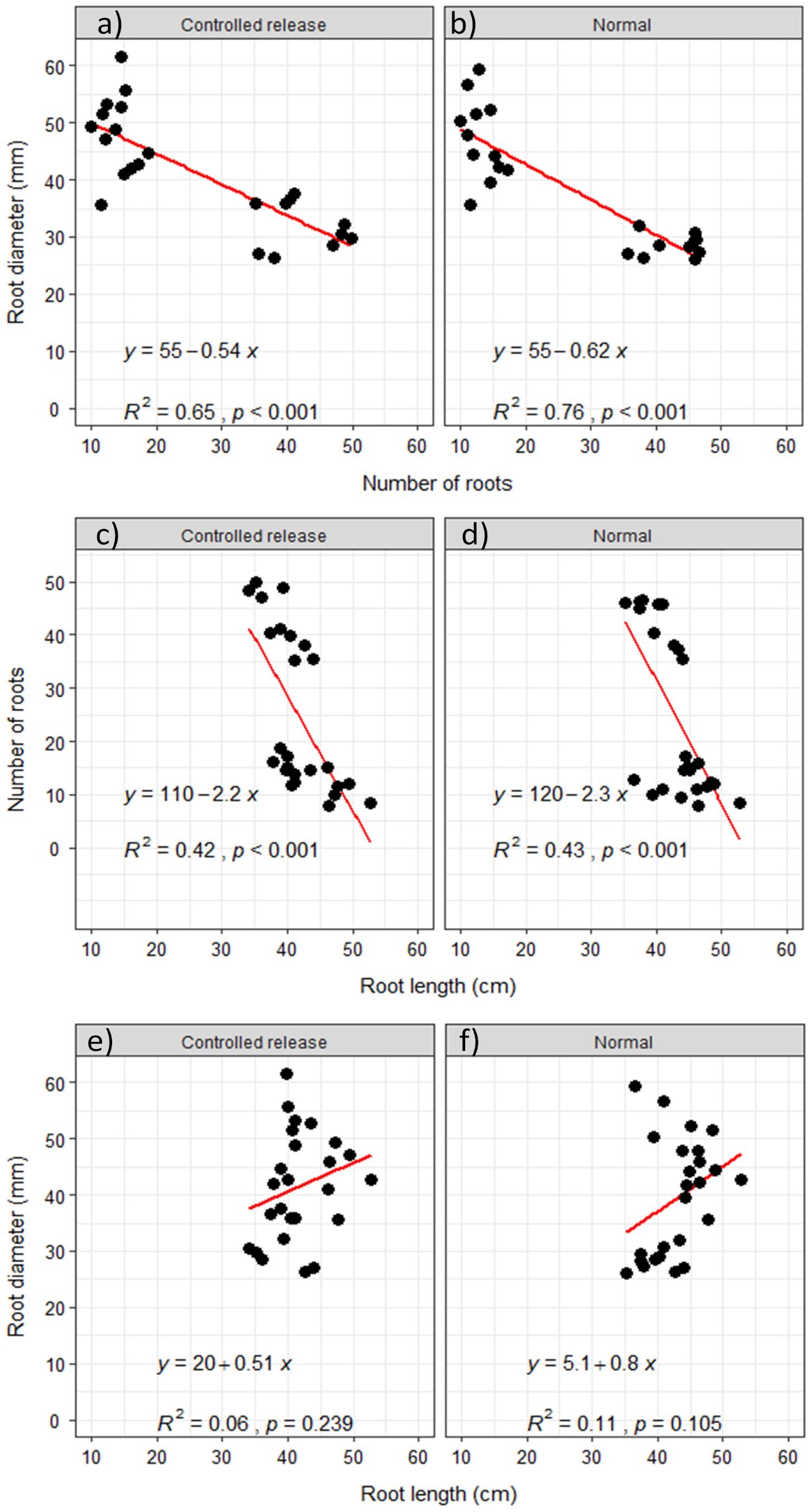
Figure 7. Relationships among root attributes: (a, b) between the number of roots and root diameter; (c, d) between root length and number of roots; (e, f) between root length and root diameter.
4 Discussion
To achieve the maximum from fertilizer application in terms of growth, yield, and quality, the “4Rs” of nutrient stewardship are supposed to be adhered to (Bruulsema et al., 2008), i.e., the right type of fertilizer, right placement, right timing of application, and right quantity. However, this is rarely the case for most smallholder farmers, regardless of the crop. In crops considered high value, there exists some effort to follow the 4R principles, although for those long categorized as “orphan” crops, these principles are hardly followed. Many studies have indicated the importance of fertilizer application to cassava, yet achieving synchrony between fertilizer application and nutrient uptake, plant growth, and development is elusive. This study has attempted to address this scarcity in knowledge by splitting fertilizer application to cassava throughout its growth period and tracking when the optimal storage root yields can be achieved. This is to enable identifying the best timing(s) for fertilizer application to cassava and which type of fertilizer, i.e., either controlled release or normal leading, should be used for an optimal yield.
The concentrations of N in the leaves of cassava grown without receiving fertilizers and those that received the full amount of normal fertilizers at planting were low, but not deficient. Indeed, the range of low N in the leaves was 4.8%–5.1% (Howeler, 2002). This affected the chlorophyll content and contributed to lower root yields. Conversely, N in the leaves of the cassava plants that received controlled-release fertilizers at planting and at 6 months and those that received normal fertilizers in four splits every 2 months, were within the sufficiency levels, i.e., 5.1%–5.8% (Howeler, 2002), consequently leading to higher chlorophyll content. Interestingly, K concentration in the plant tissues was the reverse of N and P’s trend, with more in the stems, followed by petioles and leaves, an indication of the importance of K in the translocation of sugars for bulking in the storage roots (Omondi et al., 2020a). Moreover, the highest root yields achieved were parallel to the K concentrations in various plant tissues.
Towards 9 months after planting, the root yields decreased, perhaps as a result of low atmospheric temperatures, which have been observed to lead to reduced shoots and stagnated growth (Brown et al., 2016; Omondi et al., 2020b). Consequently, this probably caused a reduction in net photosynthesis (Sagrilo et al., 2006), and thus decreased translocation of photo-assimilates to the sinks, i.e., the storage roots (Lemoine et al., 2013). Furthermore, production of new leaves during the low atmospheric temperature period possibly led to the remobilization of already stored starch in the roots (Hostettler, 2014; Lamm et al., 2023).
Under controlled-release fertilizers, the highest root yields were recorded when fertilizers were applied in two splits at planting and again at 6 months. However, in previous months, the highest yields were associated with four split applications every 2 months, similar to that of normal fertilizers. This pattern indicates that while initial growth may benefit from concentrated nutrient applications, sustained yields are better supported by regular nutrient availability (Umeh and Mbah, 2010). Further cementing this is the high chlorophyll content under four split applications of normal fertilizer, signaling probable high photosynthesis activity (Lahai et al., 2003; Yu et al., 2024) and consequently increased photosynthate translocation to the storage roots (Omondi et al., 2018). In fact, Omondi et al. (2019b) showed a positive relationship between root bulking and photosynthesis. Moreover, the number of roots and diameter strengthen this, as even though the root numbers decreased with the growing period, there were still more under the four-split applications. The relationship between storage roots and root diameter and root length was positively proportional compared to storage roots and the number of roots, which was inverse. This suggests that while having more roots may not directly translate to higher yields, the size and length of the roots are critical determinants of overall productivity (Kreye et al., 2020). Furthermore, Chen et al. (2017) observed that an increase in root diameter and their length per plant had a positive correlation with root yield.
The relationship between the number of roots and root diameter or length was significantly inverse, indicating that as the number of roots increases, the individual roots may become thinner or shorter. At the early stages of growth, the plants require nutrients for growth (Medina et al., 2007) before they begin to store starch in the roots, hence the need for many roots that are mostly fibrous (Siebers et al., 2017), and longer roots for nutrient exploration and absorption (Robin et al., 2021). Indeed, this study consistently indicated higher root length when no fertilizer was applied to cassava (Figures 4e, f). This observation showed that the cassava roots receiving no fertilizer were exploring the soils further to access nutrients as compared to when fertilizers were applied. This probably reallocates the plant’s energy to finding more nutrient resources rather than concentrating on building its sinks, i.e., the storage roots (Mehdi et al., 2019), as indicated by the lower storage root yield. As the plant grows and the roots are established, its attention is diverted to translocating photosynthates to the storage roots (Mehdi et al., 2019), leading to starch accumulation (Ma et al., 2024; Sheng et al., 2023; Zhang et al., 2024) and the thickening of the storage roots (Lamm et al., 2023; Siebers et al., 2017).
Since controlled-release fertilizers have a coating (Govil et al., 2024) that reduces nutrient losses either through leaching or runoff (Rajan et al., 2021), the number of split applications is reduced, an advantage that could reduce the cost of production incurred through many split applications in comparison to normal fertilizers. Moreover, early in the season, the number of split applications between controlled-release and normal fertilizers for higher root yields was similar, i.e., 4 splits, perhaps because of more soil moisture due to the rainfall amount (Figure 1). Sigtryggsson (2018) stated that different fertilizers have various dissolution rates that require soil moisture availability. Certainly, under four splits, the quantity of fertilizer for dissolution is less, and the plants could absorb it before it is leached, compared to large amounts in either one or two splits. Indeed, Toth et al. (2006) and Yao et al. (2021) reported that rainfall intensity and amounts can enhance leaching and surface run-off of the applied nutrients. The differences in yield outcomes between fertilizer types further underscore the importance of tailored fertilization strategies in optimizing cassava production (Islami et al., 2016).
5 Conclusion
This study has tracked the storage root bulking of cassava revealing that: 1) drastic atmospheric temperature decrease during cassava’s growth reduces root yield, and thereafter, an increase in temperature improves root yields; 2) irrespective of the fertilizer form, when the number of roots increases, the root yield decreases gradually, whereas root diameter and root length have a direct relationship with storage root yield; 3) a split application of fertilizer in either form is key to enhancing root yield of cassava, for example, controlled-release fertilizers applied in two splits at planting and 6 months after planting or four splits of normal fertilizer four times every 2 months. This indicates the importance of the choice of fertilizer form depending on the rainfall and thus soil moisture. Areas with higher rainfall amounts require controlled release fertilizers to minimize leaching and loss of nutrients while synchronizing normal fertilizers with the critical growth stages in moderate or low rainfall areas would be beneficial.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JO: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. S-KB: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. NdR: Data curation, Formal Analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing. NM: Data curation, Investigation, Writing – review & editing. PN: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. EP: Methodology, Resources, Writing – original draft, Writing – review & editing. HM: Conceptualization, Project administration, Funding acquisition, Resources, Writing – review & editing. LM: Conceptualization, Funding acquisition, Project administration, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the International Potash Institute for sponsoring this study and the International Institute of Tropical Agriculture (IITA).
Acknowledgments
The authors are grateful to Israel Chemical Limited through the International Potash Institute for sponsoring this study and the International Institute of Tropical Agriculture (IITA) for offering an environment for its successful implementation. The authors’ gratitude also goes to colleagues at IITA who aided in the implementation of the study, CGIAR Sustainable Farming and Breeding for Tomorrow Programs.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alves A. A. C. (2002). “Cassava botany and physiology,” in Cassava: Biology, Production and Utilization. Eds. Hillocks R. J., Thresh J. M., and Bellotti A. C. (CAB International 2002, Brazil), 67–89. doi: 10.1079/9780851995243.0000
Balagopalan C. (2002). “Cassava in Food, Feed and Industry, in Cassava: biology, production and utilization,” in Cassava: Biology, Production and Utilization. Eds. Hillocks R. J., Thresh J. M., and Bellotti A. C. (Kerala, India: CAB), 301–318. doi: 10.1079/9780851995243.0000
Bremner J. M. (1960). Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 55, 11–33. doi: 10.1017/S0021859600021572
Brown A. L., Cavagnaro T. R., Gleadow R., and Miller R. E. (2016). Interactive effects of temperature and drought on cassava growth and toxicity: implications for food security? Glob. Change Biol. 22, 3461–3473. doi: 10.1111/gcb.13380
Bruulsema T., Fixen P., and Olegario A. (2008). The ‘ 4R Nutrient Stewardship Framework ‘ links indicators of sustainability performance to policies and practices. Organisation for Economic Co-operation and Development (OECD), 1–8.
Chen X., Kou M., Tang Z., Zhang A., Li H., and Wei M. (2017). Responses of root physiological characteristics and yield of sweet potato to humic acid urea fertilizer. PloS One 12, 1–11. doi: 10.1371/journal.pone.0189715
Crawford E., Kelly V., Jayne T., and Howard J. (2003). Input use and market development in Sub-Saharan Africa: an overview. Food Policy 28, 277–292. doi: 10.1016/j.foodpol.2003.08.003
Fermont A. M., Obiero H. M., A V. A. P. J., Baguma Y., and Okwuosa E. (2007). “Improved cassava varieties increase the risk of soil nutrient mining: an ex-ante analysis for western Kenya and Uganda,” in Advances in Integrated Soil Fertility Management in Sub-Saharan Africa: Challenges and Opportunities. Ed. Bationo A. (Berlin, Germany: Springer), 511–519.
Fermont A. M., van Asten P. J. A., Tittonell P., van Wijk M. T., and Giller K. E. (2009). Closing the cassava yield gap: An analysis from smallholder farms in East Africa. F. Crop Res. 112, 24–36. doi: 10.1016/j.fcr.2009.01.009
Govil S., Van Duc Long N., Escribà-Gelonch M., and Hessel V. (2024). Controlled-release fertiliser: Recent developments and perspectives. Ind. Crops Prod. 219, 119160. doi: 10.1016/j.indcrop.2024.119160
Hostettler C. E. (2014). Investigation of starch metabolism in Cassava (Manihot esculenta Crantz). Ed. Zurich E. T. H. (Zurich, Switzerland: ETH Zurich).
Howeler R. H. (2002). “Cassava mineral nutrition and fertilization,” in Cassava: Biology, Production and Utilization. Eds. Hillocks R. J., Thresh J. M., and Belloti A. C. (Wallingford, Oxfordshire, United Kingdom: CAB International 2002), 115–147.
Islami T., Marjuki, and Howeler R. (2016). Cassava forages production for animal feeds in cassava based intercropping system. J. Adv. Agric. Technol. 3 (2), 1–5.
Janat M. (2007). Efficiency of nitrogen fertilizer for potato under fertigation utilizing a nitrogen tracer technique. Commun. Soil Sci. Plant Anal. 38, 2401–2422. doi: 10.1080/00103620701588775
Jariwala H., Santos R. M., Lauzon J. D., Dutta A., and Wai Chiang Y. (2022). Controlled release fertilizers (CRFs) for climate-smart agriculture practices: a comprehensive review on release mechanism, materials, methods of preparation, and effect on environmental parameters. Environ. Sci. Pollut. Res. 29, 53967–53995. doi: 10.1007/s11356-022-20890-y
Katuromunda S., Ekwaro B., and Wanaku B. (2021). Yield performance of newly developed cassava varieties in response to inorganic fertilizers. Mod. Appl. Sci. 15, 60. doi: 10.5539/mas.v15n4p60
Kreye C., Hauser S., Pypers P., and Vanlauwe B. (2020). Intensification options of small holders’ cassava production in South-west Nigeria. Agron. J. 112, 5312–5324. doi: 10.1002/agj2.20419
Lahai M. T., Ekanayake I. J., and George J. B. (2003). Leaf chlorophyll content and tuberous root yield of cassava in Inland Valley. Afr. Crop Sci. J. 11 (2), 107–117.
Lamm C. E., Rabbi I. Y., Medeiros D. B., Rosado-Souza L., Pommerrenig B., Dahmani I., et al. (2023). Efficient sugar utilization and transition from oxidative to substrate-level phosphorylation in high starch storage roots of African cassava genotypes. Plant J. 116, 38–57. doi: 10.1111/tpj.16357
Lawson T. S., Gbaraneh L. D., and Foby I. B. (2023). Effects of two compound fertilizer types on the growth and yield performance of three cassava varieties (Manihot esculenta crantz) in humid tropics Niger delta, Nigeria. Emerg. Issues Agric. Sci. 7 8, 17–30. doi: 10.9734/bpi/eias/v7/1005g
Lebot V. (2008). “Cassava: origin and history,” in Tropical root and Tuber Crops: Cassava, Sweet Potato, Yams and Aroids. Eds. Atherton J. and Rees A. (Wallingford, Oxfordshire, United Kingdom: CABI), 3–12.
Lemoine R., La Camera S., Atanassova R., Dédaldéchamp F., Allario T., Pourtau N., et al. (2013). Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 4. doi: 10.3389/fpls.2013.00272. Article 272.
Ma Y. Z., Pan N., Su W., Zhang F. J., Ye G. J., Pu X. Q., et al. (2024). Soil water stress effects on potato tuber starch quality formation. Potato. Res. 67, 1829–1848. doi: 10.1007/s11540-024-09720-5
Maiti S. K. (2013). Soil sampling techniques, in: Ecorestoration of the Coalmine Degraded Lands (Delhi, India: Springer India), 259–264. doi: 10.1007/978-81-322-0851-8
Mashuubu Y., Mourice S., Kudra A., and Baijukya F. (2024). Effect of fertilizer, planting density and variety on growth and yield of stem cuttings of cassava. J. Curr. Opin. Crop Sci. 5, 27–40. doi: 10.62773/jcocs.v5i1.226
Medina R. D., Faloci M. M., Gonzalez A. M., and Mroginski L. A. (2007). In vitro cultured primary roots derived from stem segments of cassava (Manihot esculenta) can behave like storage organs. Ann. Bot. 99, 409–423. doi: 10.1093/aob/mcl272
Mehdi R., Lamm C. E., Anjanappa R. B., Müdsam C., Saeed M., Klima J., et al. (2019). Symplasmic phloem unloading and radial post-phloem transport via vascular rays in tuberous roots of Manihot esculenta. J. Exp. Bot. 70, 5559–5573. doi: 10.1093/jxb/erz297
Mehlich A. (1984). Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 15, 1409–1416. doi: 10.1080/00103628409367568
Nzola M., Ndonda A., Bidiaka S., Gwanyebit Kehbila A., Tata-Hangy W., Tambu E., et al. (2022). Screening cassava for time of maturity to respond to various market needs: Case study in African sub-tropical zones. J. Sci. Food Agric. 102, 3169–3178. doi: 10.1002/jsfa.11660
Omondi J. O., Lazarovitch N., Rachmilevitch S., Boahen S., Ntawuruhunga P., Sokolowski E., et al. (2018). Nutrient use efficiency and harvest index of cassava decline as fertigation solution concentration increases. J. Plant Nutr. Soil Sci. 181, 644–654. doi: 10.1002/jpln.201700455
Omondi J. O., Lazarovitch N., Rachmilevitch S., Kukew T., Yermiyahu U., and Yasuor H. (2020a). Potassium and storage root development: focusing on photosynthesis, metabolites and soluble carbohydrates in cassava. Physiol. Plant 169, 169–178. doi: 10.1111/ppl.13060
Omondi J. O., Lazarovitch N., Rachmilevitch S., and Yermiyahu U. (2019a). Phosphorus affects storage root yield of cassava through root numbers. J. Plant Nutr. 42, 2070–2079. doi: 10.1080/01904167.2019.1655033
Omondi J. O., Lazarovitch N., Rachmilevitch S., Yermiyahu U., and Sperling O. (2019b). High nitrogen availability limits photosynthesis and compromises carbohydrate allocation to storage in roots of manihot esculenta crantz. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01041
Omondi J. O., Yermiyahu U., Rachmilevitch S., Boahen S., Ntawuruhunga P., Sokolowski E., et al. (2020b). Optimizing root yield of cassava under fertigation and the masked effect of atmospheric temperature. J. Sci. Food Agric. 100, 4592–4600. doi: 10.1002/jsfa.10519
Rajan M., Shahena S., Chandran V., and Mathew L. (2021). Controlled release of fertilizers—concept, reality, and mechanism, Controlled Release Fertilizers for Sustainable Agriculture (Amsterdam, Netherlands: Elsevier Inc). doi: 10.1016/b978-0-12-819555-0.00003-0
R Development Core Team (2021). R: A language and environment for statistical computing (Vienna, Austria: R Found. Stat. Comput).
Robin A. H. K., Irving L. J., Crush J., Schnyder H., Lattanzi F. A., and Matthew C. (2021). Time course of root axis elongation and lateral root formation in perennial ryegrass (Lolium perenne l.). Plants 10, 1677. doi: 10.3390/plants10081677
Sagrilo E., Vidigal Filho P. S., Pequeno M. G., Vidigal M. C. G., Scapim C. A., Kvitschal M. V., et al. (2006). Effect of harvest period on foliage production and dry matter distribution in five cassava cultivars during the second plant cycle. Braz. Arch. Biol. Technol. 49, 1007–1018. doi: 10.1590/s1516-89132006000700019
Senkoro C. J., Tetteh F. M., Kibunja C. N., Ndungu-Magiroi K. W., Quansah G. W., Marandu A. E., et al. (2018). Cassava yield and economic response to fertilizer in Tanzania, Kenya and Ghana. Agron. J. 110, 1600–1606. doi: 10.2134/agronj2018.01.0019
Sheng M., Xia H., Ding H., Pan D., He J., Li Z., et al. (2023). Long-term soil drought limits starch accumulation by altering sucrose transport and starch synthesis in sweet potato tuberous root. Int. J. Mol. Sci. 24, 3053. doi: 10.3390/ijms24033053
Siebers T., Catarino B., and Agusti J. (2017). Identification and expression analyses of new potential regulators of xylem development and cambium activity in cassava (Manihot esculenta). Planta 245, 539–548. doi: 10.1007/s00425-016-2623-2
Sigtryggsson C. (2018). Dissolution rates of mineral nitrogen fertilisers – Effects of moisture and precipitation (Uppsala, Sweden: Swedish University of Agricultural Sciences).
Sigtryggsson C., Hamnér K., and Kirchmann H. (2020). Laboratory studies on dissolution of nitrogen fertilizers by humidity and precipitation. Agric. Environ. Lett. 5, 1–5. doi: 10.1002/ael2.20016
Snell F. D. and Snell C. T. (1949). Calorimetric methods analysis including some turbidimetic and nephelometric methods Vol. 2 (Toronto, Canada: Inorganic. Van Nostrand Inc.).
Toth A., Jakab G., Madarasz B., Szalai Z., and Meszaros E. (2006). Migration of nutrients dissolved by precipitation and their role in soil erosion. Chin. J. Geochem. 25, 1–5. doi: 10.1007/bf02839740
Umeh S. I. and Mbah B. N. (2010). Intercrop performance of different varieties of soybean (Glycine Max. (L) Merril) in a cassava (Manihot esculenta Crantz) based cropping system within the derived savannah zone. Afr. J. Biotechnol. 9 (50), 8636–8642.
Yao Y., Dai Q., Gao R., Gan Y., and Yi X. (2021). Effects of rainfall intensity on runoff and nutrient loss of gently sloping farmland in a karst area of SW China. PloS One 16, 1–18. doi: 10.1371/journal.pone.0246505
Yu L., Luo X., Croft H., Rogers C. A., and Chen J. M. (2024). Seasonal variation in the relationship between leaf chlorophyll content and photosynthetic capacity. Plant Cell Environ. 10, 3953–3965. doi: 10.1111/pce.14997
Keywords: microdosing, root crops, controlled-release fertilizers, split application, targeted application, sub-Saharan Africa
Citation: Omondi JO, doRosario N, Manda N, Ntawuruhunga P, Parkes E, Mushoriwa H, Mbuthia LW and Kyei-Boahen S (2025) Tracking the storage root bulking of cassava under split application of fertilizer nutrients. Front. Agron. 7:1573919. doi: 10.3389/fagro.2025.1573919
Received: 10 February 2025; Accepted: 16 April 2025;
Published: 26 May 2025.
Edited by:
Sowbiya Muneer, VIT University, IndiaReviewed by:
Amit Anil Shahane, Central Agricultural University, IndiaPradeesh Kumar, VIT University, India
Copyright © 2025 Omondi, doRosario, Manda, Ntawuruhunga, Parkes, Mushoriwa, Mbuthia and Kyei-Boahen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John Okoth Omondi, b2tvdGgwNUBnbWFpbC5jb20=
 John Okoth Omondi
John Okoth Omondi Nelito doRosario2
Nelito doRosario2 Pheneas Ntawuruhunga
Pheneas Ntawuruhunga Elizabeth Parkes
Elizabeth Parkes Stephen Kyei-Boahen
Stephen Kyei-Boahen