- University of KwaZulu-Natal, School of Agricultural, Earth and Environmental Sciences, Pietermaritzburg, South Africa
Crop residue incorporation into soil is one strategy for improving soil quality. While several studies have investigated the decomposition of different crop species, little is known about mineralization patterns amongst genotypes of the same crop. This study assessed the mineralization patterns of shoot (ST) and root (RT) residues from five wheat genotypes (LM70, LM75, BW140, BW152, and BW162), which were obtained from a drought-stress field trial. The ST/RT residue was mixed with 100 g of soil in airtight PVC containers and incubated for 120 days. Carbon dioxide (CO2) released during decomposition was trapped, whereas soil from each pot was analyzed for NH4+, NO3−, and extractable P throughout the incubation. The initial biochemical composition varied amongst genotypes and plant parts, with shoots exhibiting lower C:N and lignin content, and higher N and P concentrations, whereas the opposite was observed for roots. The STs also emitted higher net CO2 and mineralized higher net mineral N and P compared with RTs. LM70RT emitted the lowest CO2 (2.49 mg g−1 C), whereas BW140ST (11.3 mg g−1 C) and BW162ST (11.0 mg g−1 C) had the highest emissions. Net N mineralization initially increased before stabilizing by end of incubation, with BW152RT releasing the lowest (4.91 mg g−1 N), whereas BW162ST and BW140ST had the highest amounts (22.3 and 21.8 mg g−1, respectively). BW140RT also mineralized the lowest extractable P (0.98 mg g−1 P), whereas BW152ST had the highest (4.39 mg g−1 P). These results suggest that residue decomposition and nutrient release were influenced by the initial biochemical composition of wheat residues, which reflected the effects of drought stress during plant growth.
1 Introduction
Declining soil quality remains one of the key factors contributing to reduced crop yields globally (Agegnehu et al., 2014). Amongst various indicators of soil quality, soil organic carbon (SOC) is particularly important because it plays a central role in nutrient cycling, supports biological activity, and improves soil physicochemical properties (Lal et al., 2003). However, unsustainable agricultural practices such as continuous cultivation, intensive tillage, overgrazing, and frequent burning have led to the depletion of soil organic matter (SOM) (Mills and Fey, 2003), which is further exacerbated by the indiscriminate use of inorganic fertilizers (Feizienė and Kadžienė, 2008). This deterioration contributes not only to soil degradation but also to increased carbon dioxide (CO2) emissions from soil, thereby enhancing agriculture’s role in global greenhouse gas (GHG) emissions (Ussiri and Lal, 2008).
According to IPCC (2021), agricultural activities are estimated to contribute approximately 30%-40% of annual GHG emissions, making them one of the main drivers of global warming. Furthermore, Davis (2017) reported that atmospheric CO2 concentrations have been rising by 1–2 parts per million by volume (ppmv) annually, with 20% of this increase attributed to agriculture (Lal, 2001). Therefore, strong, rapid, and sustained reductions in emissions are needed to mitigate global warming (IPCC, 2022). Soils can act as either a source or sink of GHGs depending on their properties, land use, and management (Kopittke et al., 2024). As such, sustainable agricultural practices that minimize emissions while improving soil fertility through enhanced carbon sequestration are essential (Carlson et al., 2017). These practices include conservation tillage and the application of organic amendments such as manure, compost, and crop residues (Crystal-Ornelas et al., 2021). Amongst them, crop residues are particularly promising as they are locally available and can reduce the need for chemical fertilizers (Jat et al., 2018).
Globally, crop residues are produced in large quantities, with estimates reaching 3.8 billion tons annually, 74% of which comes from cereals (Lal, 2005). Their incorporation improves SOM content, nutrient cycling, and crop productivity (Sarkar et al., 2020). As residues decompose, they release essential nutrients including nitrogen (N), phosphorus (P), and sulfur (S), while simultaneously emitting CO2 depending on their biochemical composition (Li et al., 2024). The decomposition and nutrient release patterns of residues are mainly controlled by their quality, particularly C:N ratio and N and lignin content (Kaleeem-Abbasi et al., 2015; Ntonta et al., 2024) with the C:N ratio identified as a major determinant of mineralization dynamics. Residues with wide C:N ratios tend to immobilize nitrogen, whereas those with narrow C:N ratios decompose more rapidly, releasing nutrients (Lynch et al., 2016). However, residues with wider C:N ratios may reduce CO2 emissions, whereas narrow C:N ratios tend to promote greater CO2 release due to rapid microbial degradation (García-Ruiz et al., 2019).
While the importance of residue quality in decomposition and nutrient cycling has been studied, most previous studies have focused on comparing decomposition and nutrient cycling potential of crop residues from different crops such as wheat, maize, soybean, and rice (Hadas et al., 2004; Marín et al., 2011; Nicolardot et al., 2001; Sandhu et al., 2022). Fewer studies have investigated the variation in residue composition within a single crop species. Genotypes of the same crop, especially those bred under contrasting environmental conditions, can differ significantly in their tissue composition particularly in C:N ratios, lignin, and nutrient content (Ntonta et al., 2024; Petrović et al., 2024). These biochemical differences may influence residue decomposition rates and associated soil nutrient dynamics (Bhandari et al., 2023). As reported by García-Ruiz et al. (2019), varietal differences in tissue quality and nutrient content can affect soil carbon and nutrient cycling, even within the same crop species.
Wheat (Triticum aestivum L.) is a globally important cereal crop, used for food products, seed, and animal feed (DAFF (Department of Agriculture Forestry and Fisheries), 2015). However, in Sub-Saharan Africa (SSA), wheat yields remain low due to climate change-induced stresses such as rising temperatures and erratic rainfall (Amoak et al., 2023). In response, plant breeding programs have successfully developed drought-tolerant, high-yielding wheat genotypes (Anwaar et al., 2020; Mathew et al., 2019; Mzileni, 2023). These genotypes are adapted to a wide range of agroecological conditions (Goldringer et al., 2019) and differ in their biomass allocation patterns and residue chemistry, especially under drought stress (Xu et al., 2023). As a result, farmers now have access to wheat cultivars that not only withstand drought but also may vary in their potential to improve soil fertility or contribute to carbon sequestration when residues are incorporated into the soil.
Despite these developments, little is known about how residue quality amongst drought-tolerant wheat genotypes particularly between shoots and roots affects CO2 emissions and nutrient cycling. Understanding this variability can guide farmers in selecting genotypes that not only produce higher yields but also improve soil fertility and reduce reliance on mineral fertilizers. Some genotypes may release higher amounts of nutrients when decomposed, whereas others with recalcitrant biochemical properties may be more suitable for long-term carbon sequestration.
Therefore, this study aimed to assess the biochemical characteristics of shoot and root residues from five drought-tolerant wheat genotypes and evaluate their effects on CO2 emissions, nitrogen, and phosphorus mineralization during laboratory incubation. It was hypothesized that wheat genotypes differ significantly in their shoot and root biochemical composition particularly in C:N ratio and lignin and nutrient content and that these differences lead to distinct patterns of CO2 emissions and nutrient mineralization following residue incorporation into soil.
2 Materials and methods
A wheat residue incubation experiment was conducted in a laboratory at the University of Kwa-Zulu Natal (UKZN) (29.6263° S, 30.4034° E), Pietermaritzburg, South Africa, from May to September 2018.
2.1 Soil sampling and preparation
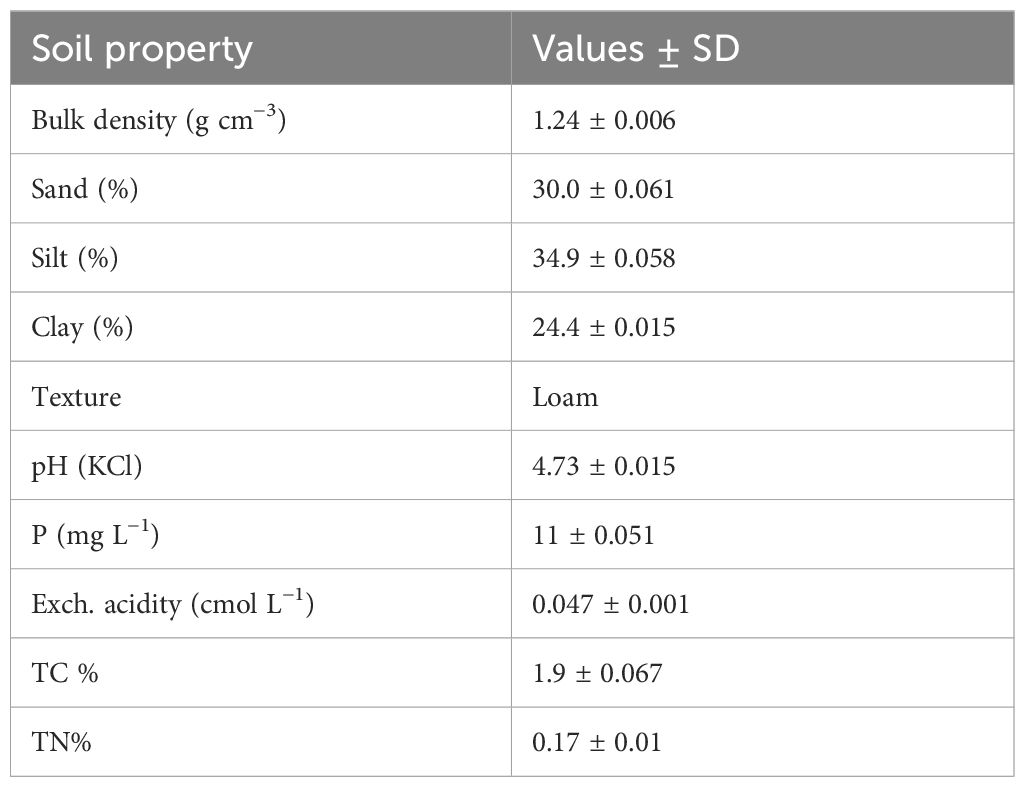
The soil used in the study was collected from an arable field at Ukulinga Research Farm of the UKZN, South Africa. The soil was loam and classified as Chromic Luvisols (IUSS Working Group WRB, 2015). Soil samples were collected at a 0-15-cm depth randomly using an auger and then mixed thoroughly to form a composite sample. This was then air dried, ground, and passed through a 2-mm sieve. A 0.5-kg subsample was then taken for determination of physicochemical properties shown in Table 1.
2.2 Wheat residues selection and biochemical characteristics
Shoot (ST) and root (RT) residues of five wheat (Triticum aestivum L.) genotypes were used in this study. The residues were obtained from a drought-stress field experiment conducted at the University of KwaZulu-Natal, Pietermaritzburg, South Africa, as described by Mathew et al. (2019). In that study, 100 wheat genotypes were grown under both well-watered and water-limited conditions to evaluate their performance under drought stress. Based on their adaptability and physiological performance under drought, 10 genotypes were selected for further evaluation. From these, five genotypes (BW140, BW152, BW162, LM70, and LM75) were selected for the current study due to their wide variation in residue biochemical composition, particularly in C:N ratio. These biochemical differences were as a result of the distinct genotypic responses to drought stress. Due to their genetic backgrounds, the selected genotypes exhibited varying abilities to cope with water limitation, which led to significant differences in biomass allocation and nutrient partitioning between shoot and root tissues. This in turn caused variation in biochemical composition of residues. Specifically, two genotypes (BW140 and BW162) exhibited low C:N ratios, two (BW152 and LM75) showed high C:N ratios, and one (LM70) had a moderate C:N ratio, making them ideal candidates for comparing decomposition and nutrient mineralization patterns.
After harvest, residues were separated into shoots and roots, oven-dried at 70°C for 48 h, and ground to pass through a 1-mm sieve. Subsamples were analyzed in triplicates for total carbon (TC), nitrogen (TN), phosphorus (TP), and lignin content. Total C and N were determined using a LECO TruMac CNS Auto Analyzer (LECO Corporation, 2012). Lignin content was measured using the Van Soest method (Van Soest et al., 1991), and total P was measured by acid digestion followed by ascorbic acid colorimetry (Okalebo et al., 2002).
Biochemical characteristics varied across the selected genotypes and residue types. Total C ranged from 23.9% to 36.2%, with lower values observed in LM70 roots. Total N ranged from 0.34% to 1.64%, with higher values found in shoot residues of BW140 and BW162. Phosphorus content ranged from 0.13% to 0.35%, and lignin content was generally higher in roots than in shoots. Calculated C:N ratios ranged from 19.7:1 to 89.4:1, C:P ratios from 98.6:1 to 217:1, and lignin:N ratios from 8.85:1 to 75.0:1. The full biochemical properties of the wheat residues are presented in Table 2.
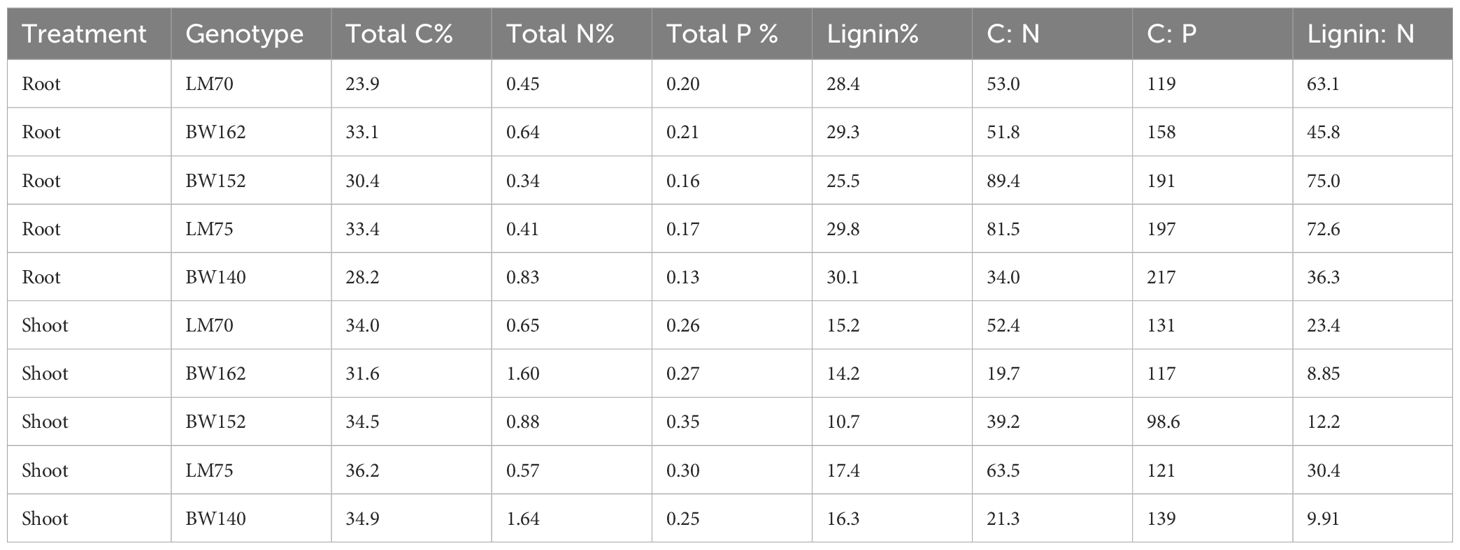
Table 2. Initial biochemical properties of shoot and root residues of five wheat genotypes used in the incubation study.
2.3 Incubation experiment
2.3.1 CO2-emission determination
The experiment was arranged in a completely randomized design with 11 treatments replicated three times. Treatments included a control (soil only), and either shoot (ST) or root (RT) residues of five wheat genotypes. Ground residues (0.25 g) were mixed with 100 g of soil in 100-mL plastic containers. Each container was placed into a 500-mL airtight plastic incubation jar with a vial containing 25 mL of 1 M NaOH to trap CO2. The soil was moistened to 50% of its water-holding capacity. The jars were sealed and incubated in the dark at 25°C in a constant-temperature laboratory room for 120 days. Separate jars were prepared for destructive sampling on each sampling day. This resulted in a total of 429 pots incubated with 33 pots being removed from the incubation room for each sampling day for analysis. The CO2-C emitted was measured at the 0, 7, 15, 23, 31, 39, 47, 55, 63, 77, 91, 105, and 120th days of incubation, where NaOH was titrated with 0.5 M HCl, using phenolphthalein as an indicator, after precipitating carbonates with BaCl2. The amount of CO2-C emitted was calculated from the volume of 1 M NaOH that reacted with CO2, determined by titration with 0.5 M HCl after BaCl2 precipitation (Anderson, 1982). CO2-C was calculated using Equation 1 below:
where VNaOH and VHCl are volumes (mL) of NaOH and HCl, respectively, N is the normality of the acid/base (mol L-¹), 12 is the molar mass of C (g mol-¹), and W is the weight of dry soil (kg).
The net CO2-C emitted was obtained by calculating the differences in the values of the biomass treated soil and control, whereas cumulative mineralized CO2-C was calculated as the sum of all previous measurements. Carbon dioxide emitted was expressed per gram of C present in the residues to account for differences in C content amongst the wheat residues.
2.3.2 Mineral nitrogen and phosphorus determination
At each sampling interval, the soil from of each incubation pot was emptied and thoroughly homogenized using a sterile spatula. Subsamples of the moist soil were then immediately collected for nutrient analysis. Ammonium (NH4+-N) and nitrate (NO3−-N) were extracted using 2 M KCl. Specifically, 2.0 g of moist soil was suspended in 20 mL of 2 M KCl in a 100-mL extraction bottle and shaken at 180 cycles per minute for 30 min using a reciprocal shaker (Model E5850, Thomas Scientific, Swedesboro, NJ, USA). The mixture was filtered through Whatman No. 1 filter paper, and the filtrates were analyzed for NH4+-N and NO3−-N concentrations using a Thermo Scientific Gallery Discrete Autoanalyzer (Rayment and Lyons, 2011).
Extractable phosphorus was determined calorimetrically following the AMBIC-2 extraction method (Non-Affiliated Soil Analysis Work Committee, 1990). A 2.5-g sample of moist soil was placed in a 100-cm3 centrifuge tube and extracted with 25 mL of AMBIC-2 solution (containing 0.25 M NH4CO3, 0.01 M EDTA, 0.01 M NH4F, and 0.05 g L−1 SuperFLOC N100, adjusted to pH 8.0). The suspension was shaken at 180 cycles per minute for 30 min and then filtered through Whatman No. 41 filter paper. A 2-mL aliquot of the extract was diluted with 8 mL of distilled water, followed by the slow addition of 10 mL of color reagent (Murphy and Riley, 1962) with continuous mixing. The mixture was allowed to stand for 45 min before measuring absorbance at 670 nm using a UV/VIS spectrophotometer.
2.4 Statistical analysis
An analysis of variance (ANOVA) was conducted using GenStat (Payne et al., 2017) to test the effects of residue type (shoot, root), genotype, and incubation period on nitrogen and phosphorus mineralization, and CO2 emissions. The root treatments were analyzed separately from shoot treatments. Differences between treatment means were further separated by least significant differences (at p = 0.05). Pearson’s correlation analysis was also performed to assess relationships between CO2-C emissions, mineral N, extractable P, and the biochemical composition of the residues.
3 Results
3.1 Variation of CO2 emission amongst different wheat residues
The net CO2-C emission significantly differed amongst residue treatments and incubation times (p<0.001). It was higher for shoots compared with root residues throughout the incubation period (Figure 1; Tables 3A, B). It increased in the first two for shoots (Figure 1B) to three for roots (Figure 1A) weeks of incubation and decreased thereafter. Peaks were reached between days 7 and 23 for roots and between days 7 and 15 for shoot residues (Figure 1). Greater variation of net CO2-C emission amongst the shoot residues of different wheat genotypes was observed. The maximum net CO2-C emitted by shoot residues on day 15 were 15.54 = 15.14>12.53>10.61>8.87 mg CO2-C g−1 C added for shoot residues of BW140, BW162, BW152, LM70, and LM75, respectively, whereas the maximum net CO2-C emitted by root residues on day 23 were 6.52, 6.33, 5.73, 5.15, and 4.68 mg CO2-C g−1 C added for BW162, BW140, LM75, BW152, and LM70, respectively. Amongst the root treatments, LM70 emitted the lowest net CO2-C of 2.49 mg CO2-C g−1 C added, whereas the other four treatments did not significantly differ (Table 3A). In shoot-treated soils, genotypes LM70 and LM75 emitted lowest net CO2-C with average values of 7.13 and 6.82 mg CO2-C g−1 C, respectively, whereas BW162 and BW140 emitted highest net CO2-C of 11.05 and 11.26 mg CO2-C g−1 C, respectively (Table 3B).
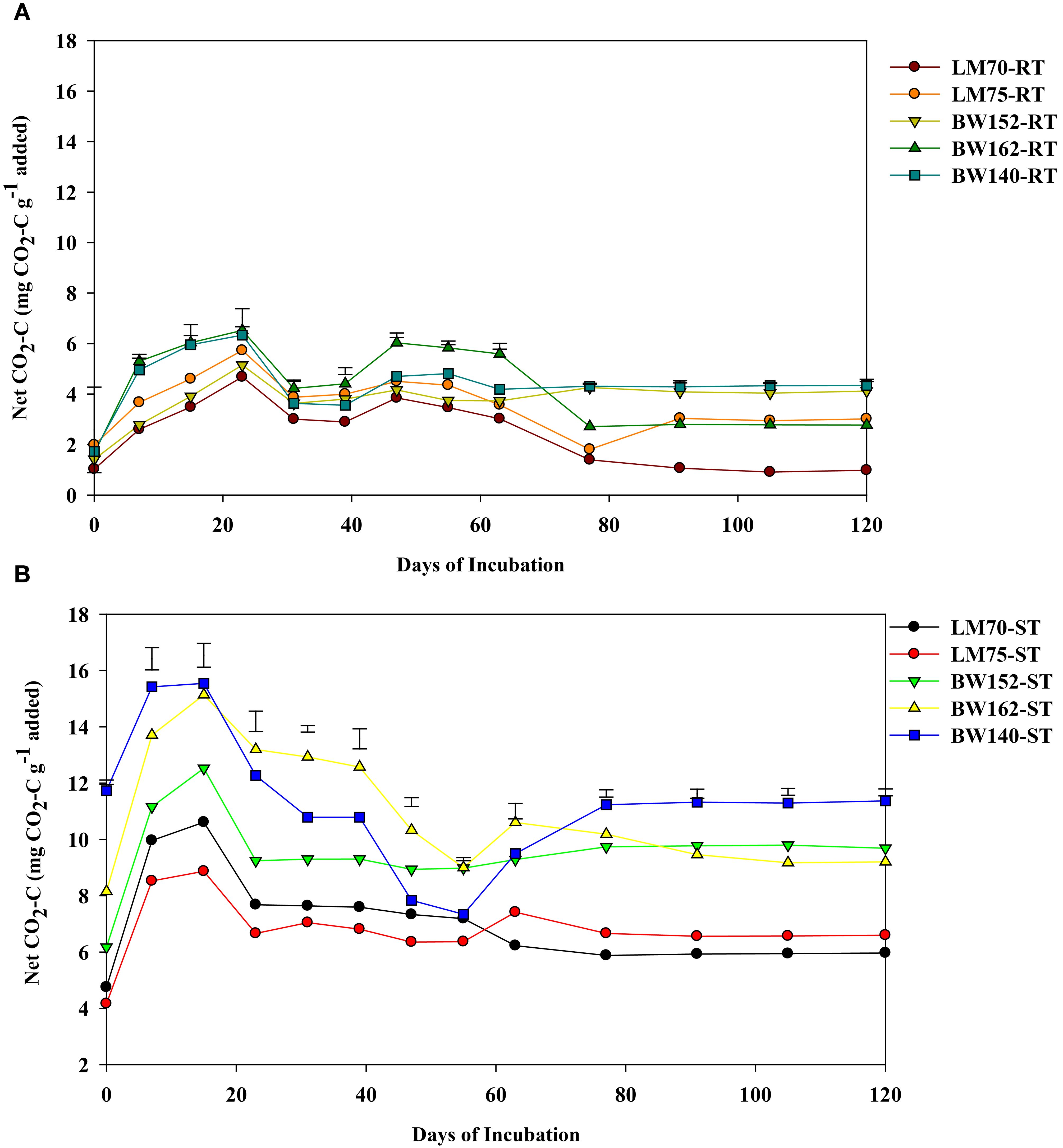
Figure 1. Net CO2-C emissions (mg CO2-C g−1 added) over 120 days from root (RT) (A) and shoot (ST) (B) residues of five wheat genotypes previously grown under drought stress conditions. Residues were incubated under controlled laboratory conditions. Vertical bars represent least significant difference (LSD) at p < 0.05.
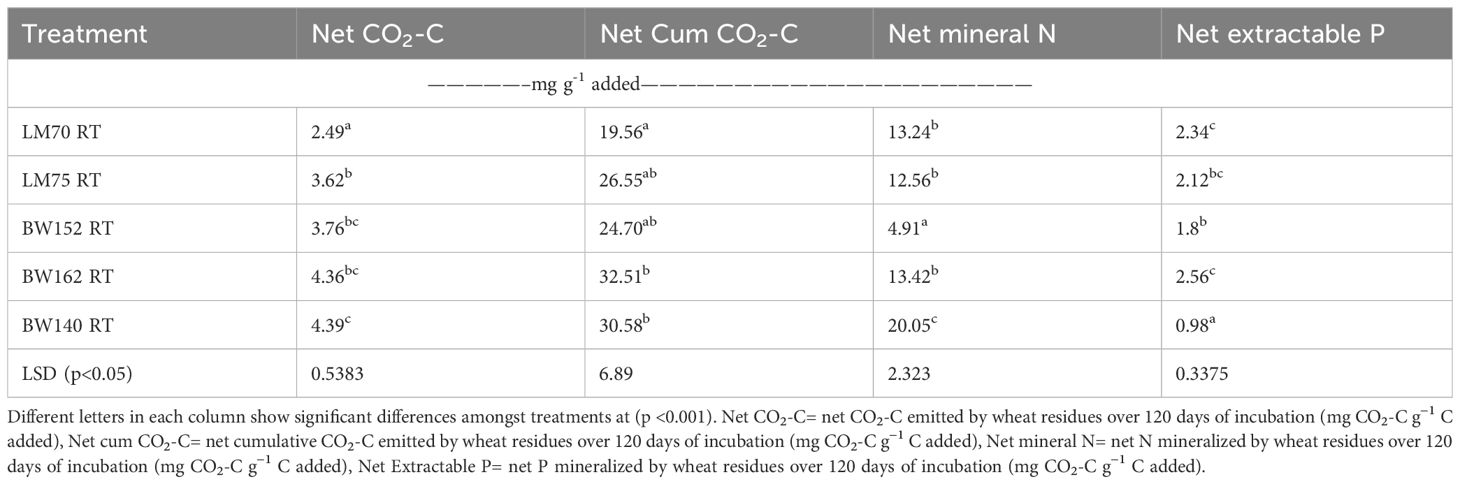
Table 3a. Average net CO2-C emissions (mg CO2-C g−1 C added) and mineral nitrogen (mg N g−1 N added) and phosphorus (mg P g−1 P added) released from root residues of different wheat genotypes incubated for 120 days.
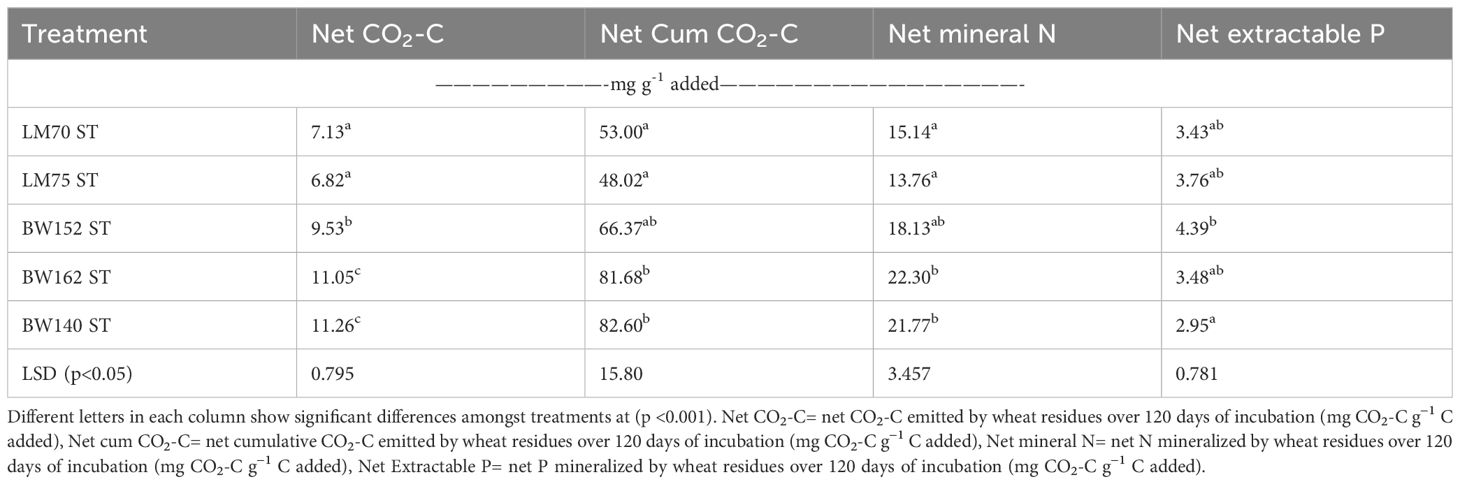
Table 3B. Average net CO2-C emissions (mg CO2-C g−1 C added) and mineral nitrogen (mg N g−1 N added) and phosphorus (mg P g−1 P added) released from shoot residues of different wheat genotypes incubated for 120 days.
Net cumulative CO2-C emission differed amongst all treatments (p<0.001), by increasing with incubation period, and was highest in shoot compared with root residues (Figure 2; Table 3). In root treatments, it was lowest in LM70 (19.6 mg CO2-C g−1 C added), which did not significantly differ from with BW152 and LM75 (Table 3A). Amongst shoots, it was highest in BW162 (81.7 mg CO2-C g−1 C added) and BW140 (82.6 mg CO2-C g−1 C added) (Table 3B), but lowest in LM75 (48.0 mg CO2-C g−1 C added) and LM70 (53.0 mg CO2-C g−1 C added), (Table 3B).
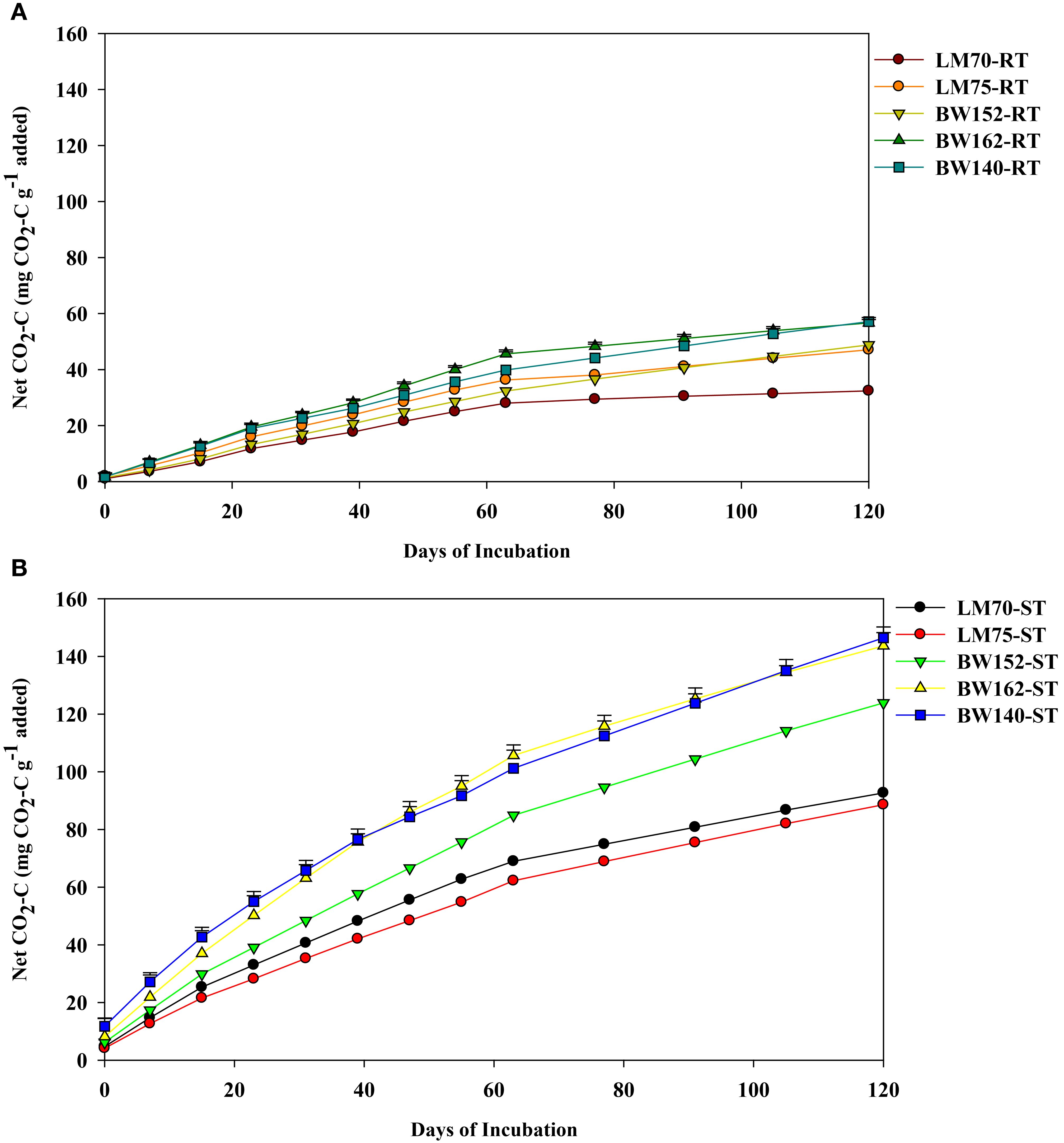
Figure 2. Net cumulative CO2-C emissions (mg CO2-C g−1 added) over 120 days from root (RT) (A) and shoot (ST) (B) residues of five wheat genotypes previously grown under drought stress conditions. Residues were incubated under controlled laboratory conditions. Vertical bars represent the least significant difference (LSD) at p < 0.05.
3.2 Variation of N mineralization amongst different wheat residues
Net mineral N (NH4+-N + NO3−-N) significantly differed amongst all treatments (p<0.001). It increased with increasing incubation period for most treatments and then showed a general steading after day 60 of incubation (Figure 3A). The peak net N was mineralized between days 39 and 63 for most treatments (Figure 3). Amongst the root residues, BW152 mineralized the lowest net N with an average value of 4.91 mg N g−1 N added, whereas roots of BW140 mineralized the highest net N of 20.1 mg N g−1 N added (Table 3A). In shoots, LM70 (15.1 mg N g−1 N added) and LM75 (13.8 mg N g−1 N added) mineralized lower net N whereas BW162 (22.3 mg N g−1 N added) and BW140 (21.8 mg N g−1 N added) mineralized highest net N (Table 3B) (Figure 3B).
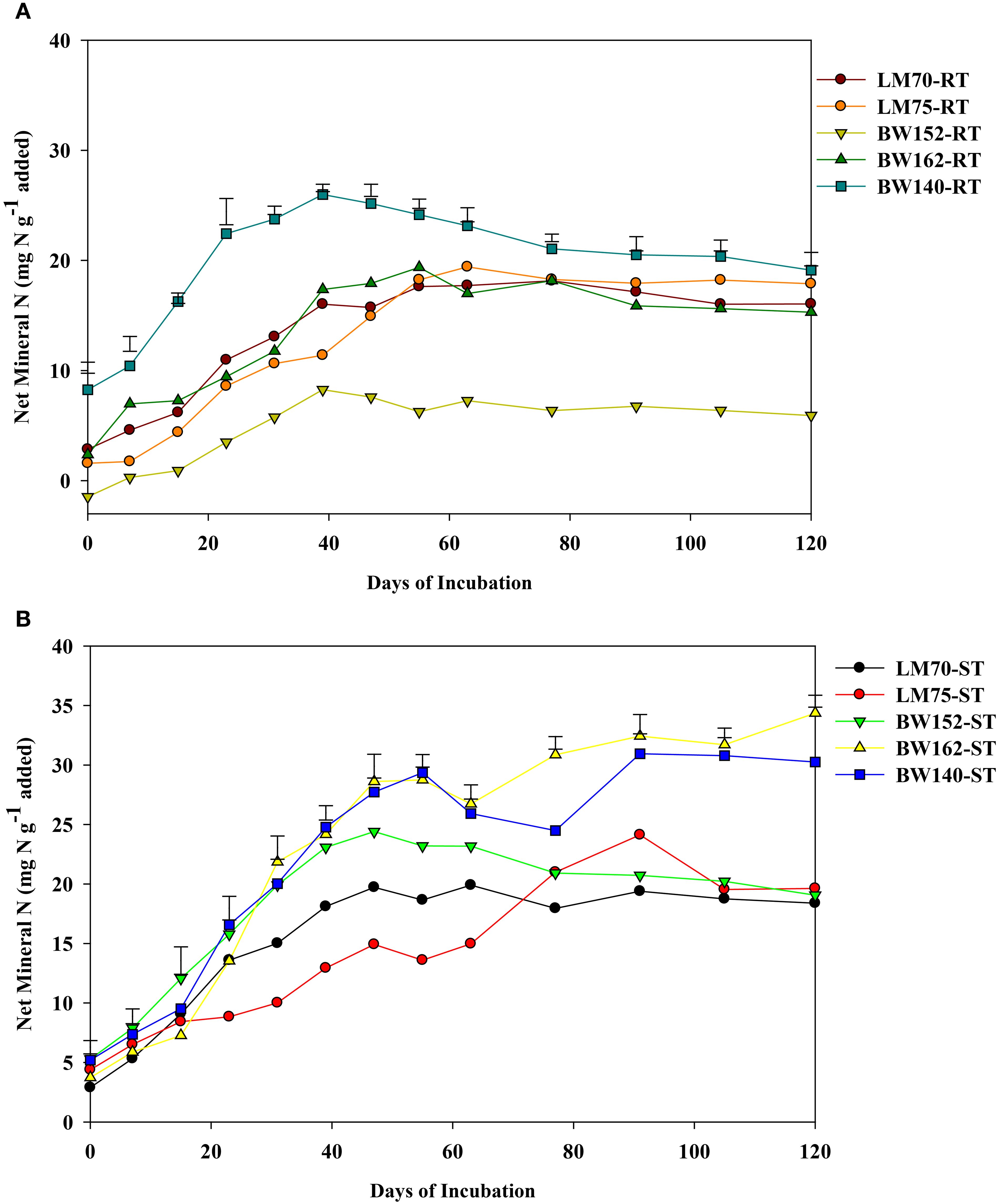
Figure 3. Net nitrogen (N) mineralized (sum of NH4+-N and NO3−-N) over 120 days from root (A) (RT) and shoot (ST) (B) residues of five wheat genotypes previously grown under drought stress conditions. Residues were incubated under controlled laboratory conditions. Vertical bars represent the least significant difference (LSD) at p < 0.05.
3.3 Variation of P mineralization amongst different wheat residues
Net extractable P mineralization significantly differed amongst all treatments at all incubation times (p<0.001). Root residues resulted in lower extractable P than shoots for each incubation period (Figure 4). They showed a slow increase in P up to day 31 with a rapid increase observed between days 47 and 55 and then no further increase thereafter (Figure 4A). In shoot treatments, rapid increases were observed between days 31 and 39 and also between days 47 and 55 becoming steady thereafter (Figure 4B). Amongst the roots, BW140 residues mineralized lowest net extractable P (average of 0.98 mg P g-1 P added), whereas the other four genotypes did not significantly differ (Table 3A). There were no significant differences in net extractable P in most shoot treatments (Table 3B).

Figure 4. Net phosphorus (P) mineralized over 120 days from root (RT) (A) and shoot (ST) (B) residues of five wheat genotypes previously grown under drought stress conditions. Residues were incubated under controlled laboratory conditions. Vertical bars represent the least significant difference (LSD) at p < 0.05.
3.4 Relationship between CO2-C emission and N and P mineralization with residue quality
Table 4 shows that net CO2-C correlated positively (p < 0.05) with total C (r= 0.793) and negatively with C: P (r=−0.35) and lignin (r= −0.69) content. Net mineral N was positively correlated (p < 0.05) with total N (r=0.707), and negatively with C: N (r= −0.803), lignin (r= −0.684), and lignin: N (r= −0.588) (Table 4). Net extractable P also positively correlated with total P (r= 0.587 p<0.05) and negatively with C: P (r= −0.603, p<0.05).
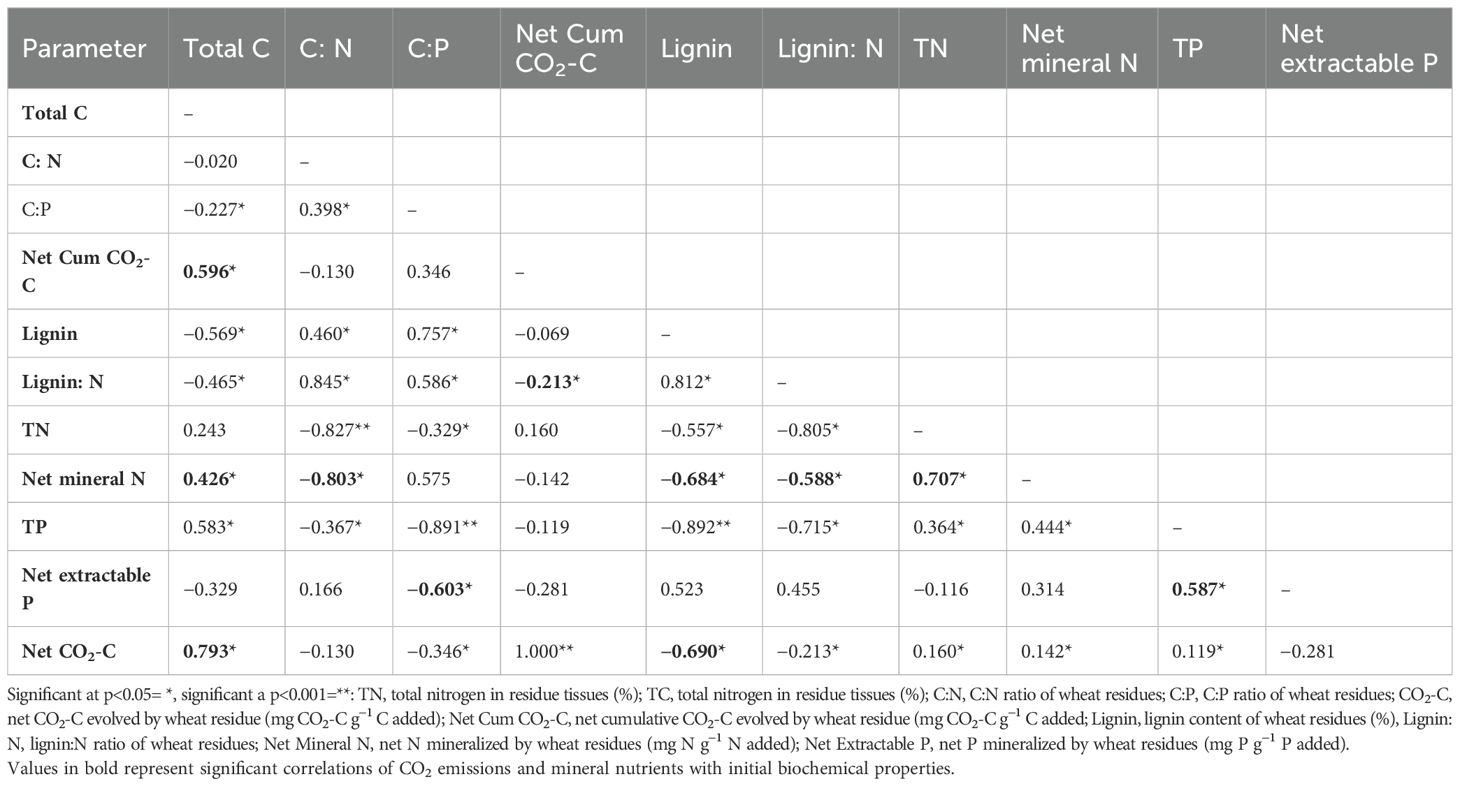
Table 4. Pearson’s correlation coefficients (r) between selected initial biochemical properties of wheat residues and different incubation variables after 120 days of incubation under controlled laboratory conditions.
4 Discussion
The incorporation of wheat residues significantly enhanced net CO2-C emissions compared with the control, due to the increased availability of labile carbon substrates. The initial peak in CO2-C emission during the early incubation phase likely reflects rapid microbial colonization and activity, stimulated by the readily available carbon under controlled moisture and temperature conditions. This response is consistent with observations by de Almeida et al. (2014) who noted that decomposition rates are higher in the beginning and decline over time due to the gradual depletion of labile compounds and accumulation of recalcitrant substances such as lignin.
While the findings of this study are consistent with observations by Patel et al. (2024) and Lenka et al. (2022), the magnitude and timing of CO2-C emissions were influenced by differences in residue biochemical composition amongst genotypes. These differences likely reflect both inherent genotypic variation and genotype-specific responses to drought stress during wheat growth, as previously reported by Mathew et al. (2019). Shoot residues from BW140 and BW162 exhibited markedly higher CO2-C emissions, likely due to their lower C:N and lignin:N ratios, which facilitated faster microbial decomposition. These biochemical traits enhance carbon availability and reduce physical barriers to microbial access, thereby accelerating respiration processes. In contrast, root residues, especially from LM70 and BW152, showed significantly lower CO2-C emissions. These genotypes had higher lignin content, and wider C:N and lignin:N ratios, which are known to inhibit microbial activity due to the recalcitrance of the material, and the need for microbes to scavenge nitrogen for biomass synthesis (Shaaban et al., 2016).
These findings not only reaffirm the role of residue quality in decomposition but also highlight that genotypic differences particularly under drought-induced physiological stress can lead to significant variations in decomposition dynamics even within a single crop species. Similar findings were reported by García-Ruiz et al. (2019) in their incubation study using residues from different wheat genotypes. The C:N ratio had a significant effect on CO2-C emissions amongst the wheat genotypes with the lowest CO2-C emissions found on wheat genotypes with a wide C:N ratio whereas those with a narrow C:N ratio emitted higher CO2-C emissions. This further confirms earlier findings by Macias-Corral et al. (2019), who noted that residues with low C:N ratios promote greater carbon mineralization and CO2-C release.
Although wheat is not typically bred for residue quality traits, our results show that genotypes responded differently to drought stress, leading to notable variations in residue composition that can significantly influence carbon turnover. Such differences, especially when residues are retained in conservation agriculture systems, could inform decisions about genotype use where long-term soil organic carbon dynamics are a concern (Stella et al., 2019). Nitrogen mineralization was also strongly influenced by genotype as BW140 ST and BW162 ST released more mineral N, attributed to their higher initial N content and narrower C:N and lignin:N ratios. These findings align with strong correlations between mineral N and total residue N (r= 0.707), and a negative relationship with C:N ratio (r= −0.803). Notably, BW152 RT, with the widest C:N ratio (89.4:1), immobilized the most nitrogen, reflecting microbial demand during decomposition consistent with the microbial N mineralization pattern (Jung et al., 2014; Kaleeem-Abbasi et al., 2015; Hou et al., 2020).
Similarly, phosphorus mineralization was higher in shoot residues, particularly BW152 ST, which contained the highest total P and the lowest C:P ratio. Residues with P >0.2% and C:P <200:1 have been shown to favor net mineralization (Damon et al., 2014). Correlation analysis confirmed this, with extractable soil P positively related to residue total P (r= 0.587) and negatively related to C:P ratio (r = −0.603). The lowest P mineralization was observed in BW140 RT, which had a C:P ratio >200:1. This likely reflects P immobilization under conditions of microbial P demand and potential effects of localized low pH, which may limit P availability (Ara et al., 2018; Alamgir and Marschner, 2016). These findings demonstrate that, under drought stress, wheat genotypes exhibited substantial differences in both above- and belowground residue composition, which in turn influenced decomposition and nutrient cycling in soil.
Importantly, this work reveals that genotypes selected for agronomic performance under drought stress also differ in their potential to influence biogeochemical processes in the soil. By examining both shoot and root residues, this study provides a more comprehensive view of how whole-plant residue quality can shape soil carbon and nutrient dynamics during decomposition. These findings further confirm that CO2-C emissions (measured as an indicator of carbon mineralization), as well as nitrogen and phosphorus release, are closely linked to genotype-specific biochemical composition, highlighting the need to consider genotypic variation not only for yield but also for post-harvest soil impacts.
5 Conclusion
This study confirmed that wheat genotypes differ in the biochemical composition of their shoot and root residues induced by drought stress, and these differences significantly influence CO2 emissions, nitrogen, and phosphorus mineralization in soil. Genotypes with narrower C:N and lignin:N ratios promoted faster decomposition and nutrient release, whereas those with wider ratios decomposed more slowly, contributing to potential carbon stabilization in soil. These findings highlight that the interaction between genetic background and environmental conditions such as drought influence residue quality and its subsequent impact on soil nutrient cycling. While residue quality is not a typical breeding target, the variation observed in the present study suggests that wheat residues can be managed in ways that enhance their benefits. Instead of applying all residues uniformly, nutrient-rich shoot residues may be used to improve soil fertility, whereas carbon-rich roots can support long-term carbon sequestration. Although farmers primarily select varieties for their yield potential, considering residue characteristics in post-harvest practices could support soil fertility and climate resilience goals.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
NM: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. RZ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. PM: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Authors would like to appreciate financial support from the South African Water Research Commission (WRC) through project grant no. K5-2721-4, National Research Foundation (NRF) under Scarce Skills, and the University of KwaZulu-Natal, South Africa for the overall research support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agegnehu G., Vanbeek C., and Bird M. I. (2014). Influence of integrated soil fertility management in wheat and tef productivity and soil chemical properties in the highland tropical environment. J. Soil Sci. Plant Nutr. 14, 532–545. doi: 10.4067/S0718-95162014005000042
Alamgir M. and Marschner P. (2016). Changes in P pools over three months in two soils amended with legume residues. J. Soil Sci. Plant Nutr. 16, 76–87. doi: 10.4067/S0718-95162016005000006
Amoak D., Kwao B., Ishola T. O., and Mohammed K. (2023). Climate change induced ecological grief amongst smallholder farmers in semi-arid Ghana. SN Soc. Sci. 3, 131. doi: 10.1007/s43545-023-00721-8
Anderson J. P. E. (1982). “Soil Respiration,” in Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties. Ed. Page A. L. (Madison: Soil Science Soc. Am. Inc.), 831–871.
Anwaar H. A., Perveen R., Mansha M. Z., Abid M., Sarwar Z. M., Aatif H. M., et al. (2020). Assessment of grain yield indices in response to drought stress in wheat (Triticum aestivum L.). Saudi J. Biol. Sci. 27, 1818–1823. doi: 10.1016/j.sjbs.2019.12.009
Ara I., Islam M. S., Kashem M. A., and Osman K. T. (2018). A comparative study of phosphorus availability in an acidic soil and an alkaline soil amended with organic and inorganic phosphorus sources. J. Soil Sci. Plant Nutr. 18, 466–478. doi: 10.4067/S0718-95162018005001402
Bhandari U., Gajurel A., Khadka B., Thapa I., Chand I., Bhatta D., et al. (2023). Morpho-physiological and biochemical response of rice (Oryza sativa L.) to drought stress: A review. Heliyon 9 (3), e13744. doi: 10.1016/j.heliyon.2023.e13744
Carlson K. M., Gerber J. S., Mueller N. D., Herrero M., MacDonald G. K., Brauman K. A., et al. (2017). Greenhouse gas emissions intensity of global croplands. Nat. Climate Change 7, 63–68. doi: 10.1038/nclimate3158
Crystal-Ornelas R., Thapa R., and Tully K. L. (2021). Soil organic carbon is affected by organic amendments, conservation tillage, and cover cropping in organic farming systems: A meta-analysis. Agriculture Ecosyst. Environ. 312, 107356. doi: 10.1016/j.agee.2021.107356
DAFF (Department of Agriculture Forestry and Fisheries) (2015). A profile of the South African wheat market value chain (Directorate Marketing, Arcadia: Pretoria), 1–33
Damon P. M., Bowden B., Rose T., and Rengel Z. (2014). Crop residue contributions to phosphorus pools in agricultural soils: A review. Soil Biol. Biochem. 74, 127–137. doi: 10.1016/j.soilbio.2014.03.003
Davis W. J. (2017). The relationship between atmospheric carbon dioxide concentration and global temperature for the last 425 million years. Climate 5, 76. doi: 10.3390/cli5040076
de Almeida R. F., Silveira C. H., Mikhael J. E. R., Franco F. O., Ribeiro B. T., de Siqueira Ferreira A., et al. (2014). CO2 emissions from soil incubated with sugarcane straw and nitrogen fertilizer. Afr. J. Biotechnol. 13 (33), 3376–3384. doi: 10.1016/j.heliyon.2023.e13744
Feizienė D. and Kadžienė G. (2008). The influence of soil organic carbon, moisture and temperature on soil surface CO2 emission in the 10th year of different tillage-fertilisation management. Zemdirbyste-Agriculture 95, 29–45.
García-Ruiz R., Carranza-Gallego G., Aguilera E., González De Molina M., and Guzmán G. I. (2019). C and N mineralisation of straw of traditional and modern wheat varieties in soils of contrasting fertility. Nutrient Cycling Agroecosystems 113, 167–179. doi: 10.1007/s10705-019-09973-4
Goldringer I., van Frank G., Bouvier d’Yvoire C., Forst E., Galic N., Garnault M., et al. (2019). Agronomic evaluation of bread wheat varieties from participatory breeding: A combination of performance and robustness. Sustainability 12, 128. doi: 10.3390/su12010128
Hadas A., Kautsky L., Goek M., and Kara E. E. (2004). Rates of decomposition of plant residues and available nitrogen in soil, related to residue composition through simulation of carbon and nitrogen turnover. Soil Biol. Biochem. 36, 255–266. doi: 10.1016/j.soilbio.2003.09.012
Hou R., Li T., Fu Q., Liu D., Li M., Zhou Z., et al. (2020). The effect on soil nitrogen mineralization resulting from biochar and straw regulation in seasonally frozen agricultural ecosystem. J. Cleaner Production 255, 120302. doi: 10.1016/j.jclepro.2020.120302
IPCC (2021). Summary for Policymakers. In: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Eds. Masson-Delmotte V., Zhai P., Pirani A., S., Connors L., Péan C., Berger S., et al. Cambridge, United Kingdom and New York, USA: Cambridge University Press, 3−32. doi: 10.1017/9781009157896.001 (Accessed September 11, 2025).
IPCC (2022). Summary for Policymakers. In: Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Eds. Pörtner H. O., Roberts D. C., Tignor M., Poloczanska E. S., Mintenbeck K., Alegría A., et al. Cambridge, UK and New York, USA: Cambridge University Press, 3–33. doi: 10.1017/9781009325844.001 (Accessed September 11, 2025).
IUSS Working Group WRB (2015). World Reference Base for Soil Resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps (Rome: FAO).
Jat H. S., Datta A., Sharma P. C., Kumar V., Yadav A. K., Choudhary M., et al. (2018). Assessing soil properties and nutrient availability under conservation agriculture practices in a reclaimed sodic soil in cereal-based systems of North-West India. Arch. Agron. Soil Sci. 64, 531–545. doi: 10.1080/03650340.2017.1359415
Jung K., Duan M., House J., and Chang S. X. (2014). Textural interfaces affected the distribution of roots, water, and nutrients in some reconstructed forest soils in the Athabasca oil sands region. Ecol. Eng. 64, 240–249. doi: 10.1016/j.ecoleng.2013.12.037
Kaleeem-Abbasi M., Mahmood Tahir M., Sabir N., and Khurshid M. (2015). Impact of the addition of different plant residues on nitrogen mineralization–immobilization turnover and carbon content of a soil incubated under laboratory conditions. Solid Earth 6, 197–205. doi: 10.5194/se-6-197-2015
Kopittke P. M., Dalal R. C., McKenna B. A., Smith P., Wang P., Weng Z., et al. (2024). Soil is a major contributor to global greenhouse gas emissions and climate change. Soil 10, 873–885. doi: 10.5194/soil-10-873-2024
Lal R. (2001). Soil carbon sequestration and climate change (Washington, DC: Senate Hearing, Science and Technical Sub-Committee).
Lal R. (2005). World crop residues production and implications of its use as a biofuel. Environ. Int. 31, 575–584. doi: 10.1016/j.envint.2004.09.005
Lal R., Follett R. F., and Kimble J. M. (2003). Achieving soil carbon sequestration in the United States: A challenge to the policy makers. Soil Sci. 168, 827–845. doi: 10.1097/01.ss.0000106407.84926.6b
LECO Corporation (2012). Truspec CN Carbon /Nitrogen Determinator Instructions Manual (St Joseph, USA: LECO Corporation).
Lenka S., Choudhary R., Lenka N. K., Saha J. K., Amat D., Patra A. K., et al. (2022). Nutrient management drives the direction and magnitude of nitrous oxide flux in crop residue-returned soil under different soil moisture. Front. Environ. Sci. 10, 857233. doi: 10.3389/fenvs.2022.857233
Li H., Li J., Jiao X., Jiang H., Liu Y., Wang X., et al. (2024). The fate and challenges of the main nutrients in returned straw: A basic review. Agronomy 14, 698. doi: 10.3390/agronomy14040698
Lynch M. J., Mulvaney M. J., Hodges S. C., Thompson T. L., and Thomason W. E. (2016). Decomposition, nitrogen and carbon mineralization from food and cover crop residues in the central plateau of Haiti. SpringerPlus 5, 1–9. doi: 10.1186/s40064-016-2651-1
Macias-Corral M. A., Cueto-Wong J. A., Morán-Martínez J., and Reynoso-Cuevas L. (2019). Effect of different initial C/N ratio of cow manure and straw on microbial quality of compost. Int. J. Recycling Organic Waste Agric. 8, 357–365. doi: 10.1007/s40093-019-00308-5
Marín S. M. A., Spínola A. G., Cano A. F., Ortíz R. S., López Á.L., and Mendoza D. G. (2011). Decomposition rates of plant residues under different land uses. Afr. J. Agric. Res. 6, 2856–2860. doi: 10.5897/AJAR11.530
Mathew I., Shimelis H., Mutema M., Clulow A., Zengeni R., Mbava N., et al. (2019). Selection of wheat genotypes for biomass allocation to improve drought tolerance and carbon sequestration into soils. J. Agron. Crop Sci. 205, 385–400. doi: 10.1111/jac.2019.205.issue-4
Mills A. J. and Fey M. V. (2003). Declining soil quality in South Africa: effects of land use on soil organic matter and surface crusting. South Afr. J. Sci. 99, 429–436. doi: 10.1080/02571862.2004.10635071
Murphy J. and Riley J. P. (1962). A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27, 31–36. doi: 10.1016/S0003-2670(00)88444-5
Mzileni L. S. (2023). Investigation into response of wheat genotypes to drought and optimum conditions in the Eastern Cape Province, South Africa. MSc thesis. Alice, South Africa: University of Fort Hare.
Nicolardot B., Recous S., and Mary B. (2001). Simulation of C and N mineralisation during crop residue decomposition: A simple dynamic model based on the C: N ratio of the residues. Plant Soil 228, 83–103. doi: 10.1023/A:1004813801728
Non-Affiliated Soil Analysis Work Committee (1990). Handbook of standard soil testing methods for advisory purposes (Pretoria: Soil Science Society of South Africa).
Ntonta S., Zengeni R., Muchaonyerwa P., and Chaplot V. (2024). Variability in decomposition rate of sorghum cultivar residues linked to lignin content. Rhizosphere 29, 100850. doi: 10.1016/j.rhisph.2024.100850
Okalebo J. R., Gathua K. W., and Woomer P. L. (2002). Laboratory methods of soil and plant analysis: A working manual. Nairobi, Kenya: TSBF-CIAT and Sacred Africa. p 127.
Patel P. P., Singh S., Singh S., and Panda D. (2024). Release of carbon dioxide and water-soluble carbon amended with rice and wheat residues in an inceptisols of varanasi. Commun. Soil Sci. Plant Anal. 55, 1324–1334. doi: 10.1080/00103624.2024.2305839
Payne R. W., Murray D. A., and Harding S. A. (2017). An introduction to the GenStat command language (Hemel Hempstead, UK: VSN International).
Petrović S., Vila S., Grubišić Šestanj S., and Rebekić A. (2024). Variation in nutritional value of diverse wheat genotypes. Agronomy 14, 311. doi: 10.3390/agronomy14020311
Rayment G. E. and Lyons D. J. (2011). Soil chemical methods: Australasia (Collingwood VIC 3066 Australia: CSIRO publishing), 495.
Sandhu O. S., Jat M. L., Gupta R. K., Thind H. S., Sidhu H. S., and Singh Y. (2022). Influence of residue type and method of placement on dynamics of decomposition and nitrogen release in maize-wheat-mungbean cropping on permanent raised beds: A litterbag study. Sustainability 14, 864. doi: 10.3390/su14020864
Sarkar S., Skalicky M., Hossain A., Brestic M., Saha S., Garai S., et al. (2020). Management of crop residues for improving input use efficiency and agricultural sustainability. Sustainability 12, 9808. doi: 10.3390/su12239808
Shaaban M., Peng Q., Hu R., Lin S., and Zhao J. (2016). Soil nitrous oxide and carbon dioxide emissions following incorporation of above-and below-ground biomass of green bean. Int. J. Environ. Sci. Technol. 13, 179–186. doi: 10.1007/s13762-015-0843-9
Stella T., Mouratiadou I., Gaiser T., Berg-Mohnicke M., Wallor E., Ewert F., et al. (2019). Estimating the contribution of crop residues to soil organic carbon conservation. Environ. Res. Lett. 14, 094008. doi: 10.1088/1748-9326/ab395c
Ussiri D. A. and Lal R. (2008). Method for determining coal carbon in the reclaimed minesoils contaminated with coal. Soil Sci. Soc. America J. 72, 231–237. doi: 10.2136/sssaj2007.0047
Van Soest P. V., Robertson J. B., and Lewis B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Keywords: carbon sequestration, decomposition, nutrient mineralization, soil fertility, wheat residues
Citation: Mbava N, Zengeni R and Muchaonyerwa P (2025) Carbon dioxide emissions and nitrogen and phosphorus mineralization patterns from soil amended with shoot and root residues of different wheat genotypes. Front. Agron. 7:1576878. doi: 10.3389/fagro.2025.1576878
Received: 14 February 2025; Accepted: 01 September 2025;
Published: 17 September 2025.
Edited by:
Khurram Shahzad, Lasbela University of Agriculture, Water and Marine Sciences, PakistanReviewed by:
Hans-Werner Olfs, University of Applied Sciences Osnabrück, GermanyAndrew D. Cartmill, Massey University, New Zealand
Copyright © 2025 Mbava, Zengeni and Muchaonyerwa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nozibusiso Mbava, emVuZ2VuaUB1a3puLmFjLnph
†ORCID: Pardon Muchaonyerwa, orcid.org/0000-0001-5822-0290
 Nozibusiso Mbava*
Nozibusiso Mbava* Rebecca Zengeni
Rebecca Zengeni Pardon Muchaonyerwa
Pardon Muchaonyerwa