- Department of Ecological, Plant and Animal Sciences, La Trobe Institute for Sustainable Agriculture and Food (LISAF), AgriBio, La Trobe University, Melbourne, VIC, Australia
Gazania (Gazania spp.) is a highly invasive plant which is emerging as a difficult-to-control weed in grain production systems and grasslands in southern Australia. Different populations of gazania were compared for their seed morphology and germination response to various environmental factors including temperature, photoperiod, moisture, salinity and pH. Seeds of four populations of gazania were collected from contrasting geographic locations (South Australia and Victoria) and land-use scenarios [roadside (Pop-1), fence line (Pop-2), grain crop production field (Pop-3) and pasture field (Pop-4)] and compared for their seed morphology and germination response to different temperatures and photoperiods. The remaining germination experiments (osmotic potential, salinity and pH) were then conducted using two populations to compare two contrasting land use scenarios from the same location (Pop-1 and Pop-3) out of the original four. In addition, the effect of growing media and seed burial depth on seedling emergence of one population (Pop-1) was also observed. The results indicated that seeds of a population collected from a pasture field (Pop-4) were 75% heavier than the fence line population (Pop-2). Seed length did not vary across populations, but the seed width of a roadside population (Pop-1) was significantly less (37%) than a population from a cropping field (Pop-3). Germination response was same (>90%) in alternating light/dark (14/10 h) or complete dark (24 h) conditions. All populations had >78% germination across a wide range of alternating day/night temperatures (15/5, 25/15, and 35/25°C). Populations did not differ in their germination response to moisture stress. Seeds did not germinate beyond -1.2 MPa water potential, while 50% germination inhibition occurred at -0.67 MPa. Gazania seeds could germinate up to a high salinity level of 300 mM of sodium chloride. Population 1 was more tolerant to salinity than Pop-3 with a 50% reduction in germination occurring at 268 and 252 mM NaCl, respectively. The pH did not affect germination irrespective of the population. Less seeds could emerge from soil (32%) compared to sand (62%) and potting mix (69%). Seedling emergence and root length decreased with increasing burial depth and no emergence was observed at 6 cm depth. However, these results represent a single population tested in burial depth study. Despite some population variations, gazania has flexible germination requirements potentially enabling their invasion.
1 Introduction
Weeds and invasive plant species cause tremendous economic, environmental and social losses around the world with Australia being no exception. In terms of agricultural production losses and management interventions they cost over $5 billion to the Australian economy each year (Australian Agricultural Statistics, 2024). Australia is a hotspot for numerous invasive plant species that were either introduced as ornamentals or crop species or arrived as seed or food contaminants. The weedy species of genus Gazania are a prime example of such introduced, invasive plants (Shahzad et al., 2025).
The genus Gazania belongs to the Asteraceae (daisy) family which has sixteen herbaceous species all native to South Africa (Howis et al., 2009). These are perennial plant species commonly known for beautiful daisy flowers ranging in colors from red, purple, yellow and bright orange. Gazanias were brought to Australia as ornamental flowering plants and planted in lawns and nature strips in many regions (EPLB, 2023). Two species of this genus, Gazania linearis L. and Gazania rigens L., were introduced in Australia in the 1950s and 1970s, respectively (Weed Futures, 2024). While the two species can present some differences in their morphology and growth habit, it is not always easy to distinguish between them. For example, G. linearis mostly grows upward with dark green leaves, while G. rigens spreads horizontally forming mat like ground cover with hairy leaves (Wine Australia, 2016). G. linearis is thought to be more widely distributed across the south-eastern and western Australia, including many inland arid regions, while G. rigens is generally limited to coastal regions (Weeds of Australia, 2016). However, it is suspected that these two species freely hybridize, producing massive variation in growth habit and flowering color (Green Adelaide, 2023), which makes it difficult to classify them at species level. Therefore, they are commonly referred to as gazania (the term used hereafter). There is limited information available on their hybridization and its role in overall invasion success.
Historically, gazanias were grown in gardens and along fences as flowering plants (Groves et al., 2005). From there they ended up in garden waste as lawn clippings, seeds or other live plant material which helped them proliferate along roadsides and native vegetation (Green Adelaide, 2023). Over time they have become highly invasive and widespread across south Australia (Green Adelaide, 2023) and parts of Western Australia. Gazania has been declared as an environmental weed under the South Australia Act 2019 due to its increasing distribution and significant negative impacts on native vegetation (Speirs, 2021). It is also ranked as “very high-risk” category in the Advisory List of Environmental Weeds in Victoria with extensive potential for further spread (White et al., 2018).
Gazania is perennial in nature supported by shallow roots and woody rhizome system. It can easily outcompete the native vegetation by producing a monoculture groundcover depriving other plants of resources such as nutrients, moisture and light. The plants can grow up to an average height of 30 cm and produce flowers year around but mostly in summer and spring. A single flower can produce more than sixty hairy seeds with the ability to easily spread by wind up to one kilometer, while some seeds land on ground near the parent plant and germinate underneath to form dense vegetation (Green Adelaide, 2023).
Due to its flexible growth requirements, gazania is now widespread and naturalized in a variety of habitats including coastal sand dunes, stream banks, wastelands, open grasslands, along roadsides and on cultivated and irrigated sites (Speirs, 2021). While gazania has long been considered as an environmental weed in Australia, a trend of ‘jumping the fence’ has been observed in recent years (McCallum, 2024; Shahzad et al., 2025) infesting grain crop production fields in low-rainfall regions of South Australia. The presence of gazania in cropping fields is proving highly problematic, with farmers finding it difficult to control with common herbicides. Marginal sandy lands with heavy infestations of gazania are quickly becoming unproductive with crops quickly failing to compete with drought hardy and vigorous Gazania plants.
The gardening industry promotes gazania as a hardy ornamental, and it has been recommended as a successful flowering plant which can grow in conditions of low moisture, high temperatures and neutral pH (McIntosh, 2022). However, as an invasive weed there has been no research on the biology and ecology of these species. Better understanding of seed germination biology with respect to different environmental factors is vital for planning weed control. The germination of weed seeds and emergence from soil are critical factors defining the survival and establishment of weed species in an agroecosystem (Cochavi et al., 2018). Seed germination behavior of weeds is influenced by several environmental factors such as temperature, light (photoperiod, intensity and quality), soil pH, salinity, moisture availability, soil type and seed burial depth (Chauhan and Johnson, 2010; Bajwa et al., 2018; Hooda and Chauhan, 2024). Without understanding the impact of these factors on seed germination behavior of any weed species, it is difficult to understand its population dynamics and potential controls.
Temperature is a major factor influencing seed germination rate as it plays an important role in breaking seed dormancy (Baskin and Baskin, 1998; Geneve, 2003). Knowing optimal germination temperature could help predict the major emergence events in a particular season, which can be exploited for weed management. Similarly, moisture stress can reduce, delay, or prevent seed gemination (Javaid et al., 2022) but some weeds could germinate at very low moisture while others can stay dormant until the moisture increases. Australian soils are highly variable in moisture retention ability, pH, and salinity, which directly impacts weed seed germination dynamics and overall weed distribution (Chauhan, 2016; Bajwa et al., 2018; Loura et al., 2020; Hooda and Chauhan, 2024). Species specific information on germination behavior in relation to environmental and edaphic factors is vital for weed management. This information is currently lacking for gazania, which limits our understanding of its behavior across a wide range of climatic and soil conditions.
Weed seeds collected from different geographic locations and habitats can also display significant differences in their germination response (Singh et al., 2022). Such variations in germination ecology can have major influence on site-specific weed dynamics and ultimately weed management. As gazania enters crop production systems from non-cropped areas (roadsides or fence lines), its germination behavior can change due to variable soil and land-use conditions. However, there is no information available on seed biology or germination triggers, which are crucial to developing any management program. This understanding could provide crucial insights into gazania spread from environmental settings to cropping systems and be useful for future prevention and management programs.
Keeping in view the above knowledge gaps, a series of controlled environment experiments were carried out in this study to understand the germination ecology of gazania. The specific objectives were to:
1. investigate the germination response of gazania to different environmental factors, including alternating day/night temperatures, photoperiod, osmotic potential, salt and pH;
2. determine the effect of soil burial depth on seedling emergence of gazania; and
3. explore whether gazania populations collected from different geographic locations and land-use scenarios differ in their seed morphology and germination ability across various environmental conditions.
The knowledge gained from this research would help predict the key germination triggers for this species to enable timely planning of management interventions. This understanding will also help predict potential areas of its future spread in terms of climatic suitability which is important for prevention and proactive management.
2 Materials and methods
2.1 Seed collection and storage
Several thousand fully mature gazania seeds were collected in brown paper bags from different locations across South Australia and Victoria in November 2023. From this collection, four populations were selected that belonged to contrasting geographic locations and the land use types (Table 1).
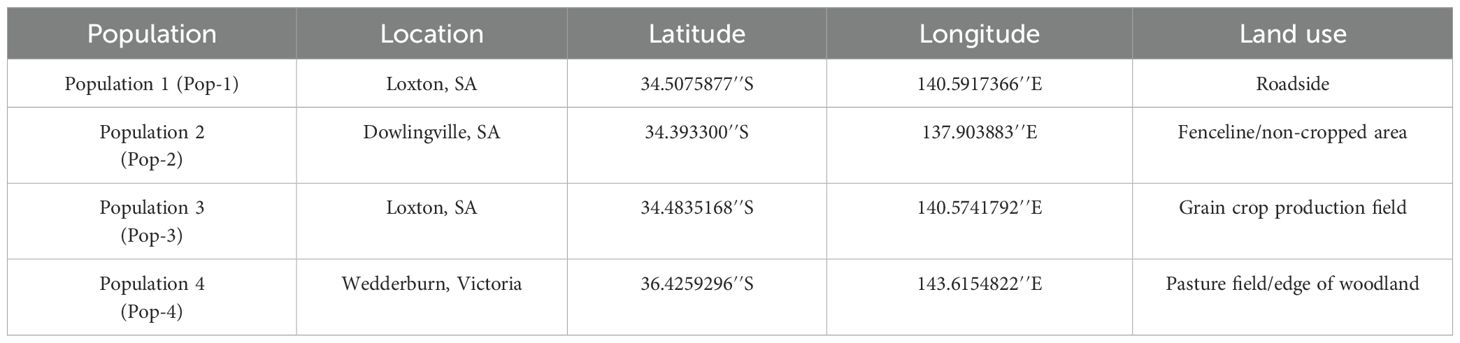
Table 1. The locations and land use situations for four gazania seed populations used in this study.
For each population, seeds were collected from several plants growing together in a thick dense stand and uniformly mixed in a single paper bag to represent the population. Seeds were cleaned by hand and examined physically by pressing and only mature, blackish seeds were stored for further studies while immature and empty seeds were discarded immediately. The fully matured filled seeds were then dried in the laboratory (20-25°C) for five days and placed in airtight plastic containers (separate for each population). Silica beads (Desicco Pty Ltd, Australia) wrapped in a perforated cloth envelops were placed at the bottom to absorb any remaining moisture in the seed.
A preliminary seed germination test was carried out for representative samples of each population at 25°C prior to the experiments which resulted in >80% germination across the board confirming there was no dormancy present. The seeds were stored in a cold room operating at 4°C with 15% relative humidity until the start of the experiments.
2.2 Experimental design
All the experiments described below were laid out in a completely randomized design with a factorial arrangement and three replicates for each experimental unit unless stated otherwise. All experiments were repeated once over time.
2.3 Seed morphology measurements
To assess the seed morphological characteristics, microscopic studies were conducted for seed size measurements. Five replicates (1 seed per replicate) from each population were randomly selected and imaged with microscope (Zeiss Ltd, Germany) equipped with a 1x PanNeoFluar lens and a Axiocam 305 color digital camera (Figure 1). The measurements of seed length and width were taken from the images by using the software ImageJ (Java-based). To evaluate the 100-seed weight (mg), three replicates with 100 seeds per replicate were randomly selected from each population and weighed using a precision weighing balance which can measure up to 4 decimal points (Ohaus Corporation, USA).

Figure 1. Seed images of different gazania populations used in this study taken with a camera microscope.
2.4 General protocol for seed germination experiments
A series of experiments were conducted to evaluate the impact of different environmental conditions, including alternating day/night temperatures, light (photoperiod), moisture availability (osmotic potential), salinity and pH on the seed germination of four gazania populations. These experiments were carried out during March to May 2024 at the Centre for AgriBioscience (AgriBio), La Trobe University, Melbourne, Victoria Australia (37. 7239232136′′S, 145. 0545055160′′E). Seed germination response was observed in purpose-built incubators (LABEC®, NSW, Australia) operating at chosen temperatures (described below) and 45% relative humidity. Before germination experiments, seeds were taken out of storage and sterilized by dipping and shaking in 10% sodium hypochlorite (NaClO) (Chem-Supply Pty Ltd®, Australia) for 15 minutes to avoid any fungal contamination. The seeds were thoroughly rinsed five times with reverse-osmosis (RO) water to avoid any residual effect of sodium hypochlorite. Seeds were then spread on a clean layer of paper towels before placement in Petri dishes.
A single experiment was conducted to evaluate the effect of three different temperatures and two photoperiods for all four gazania populations. The remaining experiments then only used two contrasting populations, Pop-1 and Pop-3. Thirty healthy looking, filled seeds were uniformly placed onto a double layer of Whatman 4 filter paper in a Petri dish (90 mm × 14mm, diameter × depth; Gamma Sterile, NSW, Australia) using laboratory forceps. The filter papers were moistened with 5 mL of RO water or selected treatment solution [polyethylene glycol (PEG), sodium chloride (NaCl) or pH solution] just before seed placement by using 5 mm micro pipette (Eppendorf®, Germany). Additional RO water or other treatment solutions were added in small amounts (1–2 mL) to the Petri dishes when needed throughout the experiment duration. The Petri dishes were wrapped with 5 cm laboratory parafilm (Bemis, USA) to reduce evaporation and ensure appropriate moisture throughout the experiment.
After examining the effects of temperature and photoperiod on seed germination in the first experiment, a day/night temperature of 15/5°C and a photoperiod of 14/10 h light/dark were selected as optimal germination conditions as they resulted in the highest germination percentage. All the rest of the experiments on seed germination were conducted under these conditions. Seed germination was recorded after every third day, and seeds with at least 2 mm long radicle were considered germinated and were removed after counting. Germination response was observed until 14 days after starting the experiment. Germination peaked 12 days after the start and no germination was observed beyond 14 days. All the germination data were recorded in numbers but reported as a percentage.
2.4.1 Effect of temperature and photoperiod
The effect of temperature and light on seed germination of four gazania populations was determined by incubating seeds at three different alternating day/night temperatures (15/5, 25/15, and 35/25°C) with two different photoperiods; 14/10-h light/dark and 24-h dark (complete dark). The alternating photoperiod was adjusted using the incubator settings while complete dark (24-h) conditions were imposed by wrapping the Petri dishes with two layers of the aluminum foil (Universal Choice, NSW, Australia). In completely dark treatment, Petri dishes were not opened at three-day interval like 14/10-h photoperiod and were only opened once at the time of final germination count after 14 days. The data presented for this experiment are based on 12 replicates, comprised of three pooled replicates each of light/dark and complete dark conditions over two runs.
2.4.2 Effect of moisture stress
Seven different levels of osmotic potential (−0.1, −0.2, −0.4, −0.6, −0.8, −1.0 and −1.2 MPa) were imposed to investigate the effect of moisture stress on seed germination of two gazania populations. The moisture stress levels were chosen based on previous weed seed germination studies (Bajwa et al., 2018; Tang et al., 2022). To prepare the aqueous solutions of the desired concentrations of osmotic potential at 15/5°C (optimal temperature determined in the first experiment), polyethylene glycol (PEG) 6000 (Merck, Germany) was dissolved in required quantities in RO water (Michel and Kaufmann, 1973). These concentrations were adjusted by converting the osmotic potential in bars into megapascals (MPa), and then calculations were done for 7.5°C which is the average of the optimal temperature regime i.e. 15/5°C. The data presented for this experiment are based on 12 replicates, comprised of three pooled replicates of both populations over two runs.
2.4.3 Effect of salt stress
To investigate the effect of salt stress on seed germination of gazania populations, seven salinity levels (25, 50, 100, 150, 200, 250 and 350 mM) were established. The aqueous solutions of desired concentrations were prepared using the laboratory grade NaCl. These levels were chosen based on the available literature and the fact that most plant species are unable to germinate beyond the highest level chosen (Bajwa et al., 2018; Loura et al., 2020; Hooda and Chauhan, 2024). The data presented for this experiment are based on six replicates, comprised of three replicates of each population pooled for two runs.
2.4.4 Effect of pH
To evaluate the effect of pH on seed germination, seven levels of pH (4, 5, 6, 7, 8, 9 and 10) were established. The buffer solutions of pH ranging between 4 to 10 were made following the protocol established by Chachalis and Reddy (2000). Briefly, a 2 mM MES solution [2-(N-morpholino) ethanesulfonic acid] was used to make solutions with pH 4, 5, and 6. The solutions of pH 7 and 8 were prepared by using 2 mM solution of HEPES [N-(2-hydroxymethyl) piperaziine-N’- (2-ethanesulfonic acid)], while 2 mM tricine [N-Tris (hydroxymethyl)] was used to prepare solutions of pH 9 and 10. All the solutions were then adjusted one by one and exact pH values were obtained by slowly adding sodium hydroxide (NaOH) or 0.1 N hydrogen chloride (HCl) as measured by a pH meter (Hanna, Romania). For control treatment RO water was used. The pH of RO water was 6, so it served as the control treatment as well as one of pH gradient levels. The data presented for this experiment are based on six replicates, comprised of three replicates of each population pooled for two runs.
2.5 Effect of burial depth on seedling emergence and vigor
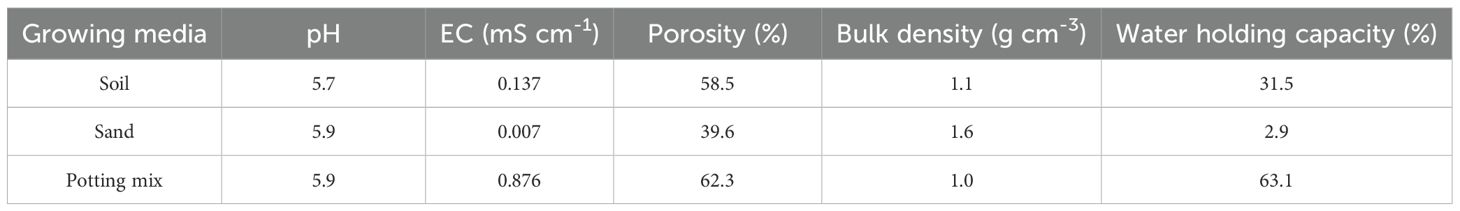
A separate experiment was conducted by using one population of gazania (Pop-1) and repeated once over time to evaluate the effect of different seed burial depths. We used a single population because weed populations have not been reported to differ much in terms of emergence patterns from different burial depths (Mutti et al., 2019; Singh et al., 2022). Seeds were buried at 0 (surface), 0.5, 1, 2, 4 and 6 cm depths in three growing media (soil, sand and potting mixture) in 85 mm square pots that were 90 mm deep. The physico-chemical properties of different growing media are presented in Table 2. The experiment was carried out in a glasshouse operating at day/night temperatures of 22/16°C at 16/8 h day/night photoperiod and 40% relative humidity. Twenty, healthy viable seeds of uniform size were placed at respective depths and covered with the respective medium. The pots were manually irrigated as needed in the form of mist using a handheld water sprayer to avoid seed disturbance. Seedling emergence was recorded at 3 days intervals for 21 days after sowing. After 21 days, five seedlings per replicate were randomly selected and carefully removed from the growing media and washed with tap water for shoot and root length measurements. The data presented for seedling emergence are based on six replicates, comprised of three replicates pooled for two runs. Seedling shoot and root length data are based on six replicates (with five seedlings measured per replicate), comprised of three replicates pooled for two runs.
2.6 Statistical analyses
The analysis of variance (ANOVA) was performed which showed there was no significant difference between the two experimental runs (Statistix 8.1). Therefore, data from both experimental runs were combined for all parameters before further analysis. In the case of temperature and photoperiod experiment, treatment means were separated using the least significant difference (LSD) test at 1% probability of error (P < 0.01). The treatment means were presented in bar charts with ± standard errors (SE) of the means.
For osmotic potential, salinity and burial depth experiments, data were analyzed using the relevant regression models through SigmaPlot software given the quantitative nature of the treatments (Version 15, Grafiti LLC, California, United States; Supplier: 4am Software). The coefficient of determination (R2) value was used to determine the model goodness of fit. Across different parameters, a three-parameter sigmoid (Equation 1) or a three-parameter gaussian regression model (Equation 2) was applied to the data:
In the equations above, G or E represent germination or emergence percentage, a represents maximum germination or emergence, b represents the slope and x0 is the level of treatment variable at which 50% inhibition of maximum germination or emergence occurred. The effect of different pH levels was non-significant, so regression analysis was not feasible.
3 Results
3.1 Seed morphology
Four populations of gazania differed significantly in terms of their seed weight and seed width (P < 0.01), but no difference was observed for seed length (P = 0.14; Figure 2). Population 4 had the highest seed weight (55 mg), while Pop-2 represented the lowest seed weight (31 mg). The seeds of Pop-3 were wider (2.6 mm) than those of Pop-1 (1.9 mm; Figure 2).
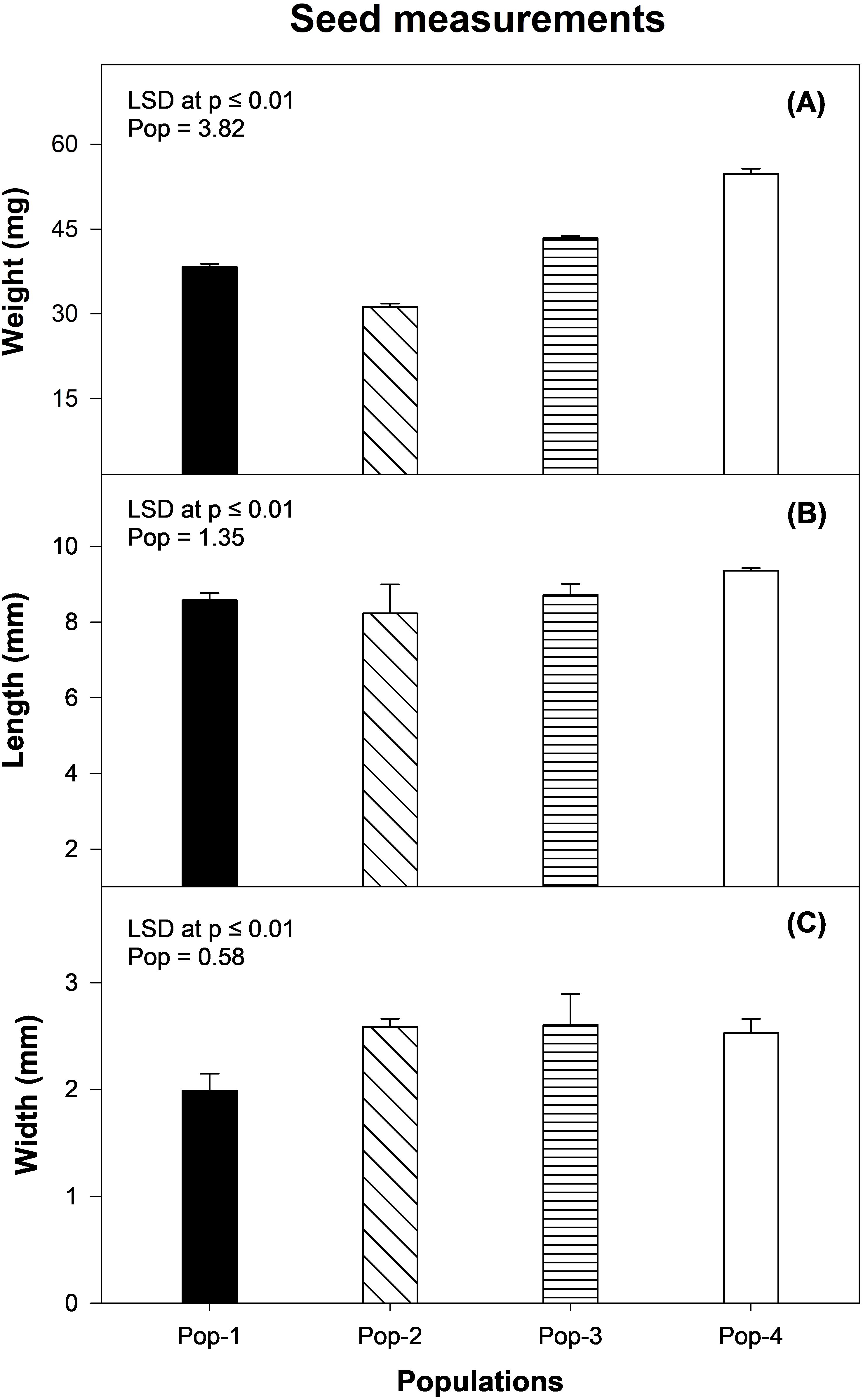
Figure 2. Seed morphology measurements, including (A) seed weight, (B) seed length and (C) seed width of four different populations of gazania. Data and error bars represent the means and ± SE of the means, respectively [number of replicates = 3 for seed weight (100 seeds per replicate); number of replicates = 5 for seed length and seed width (single seed per replicate)]. LSD is the least significant difference value for mean comparison at P ≤ 0.01.
3.2 Effect of temperature and photoperiod
Photoperiod did not affect the seed germination (P = 0.11), so data were pooled across temperature and photoperiod. Significant differences were observed among populations, temperature regimes and due to their interaction (Figure 3). Overall, all gazania populations germinated at all three alternating day/night temperature regimes (15/5, 25/15, and 35/25°C). The highest germination (98%) was observed for Pop-3 at 15/5°C, while the lowest germination (79%) was observed for Pop-2 at 25/15°C. Individually, 15/5°C day/night temperature regime resulted in maximum germination (95%), while the germination was lower at 25/15°C (90%) and 35/25°C (89%) (Figure 3). Populations 1, 3 and 4 all germinated equally well (all >90%) as compared to Pop-2 (83% germination).
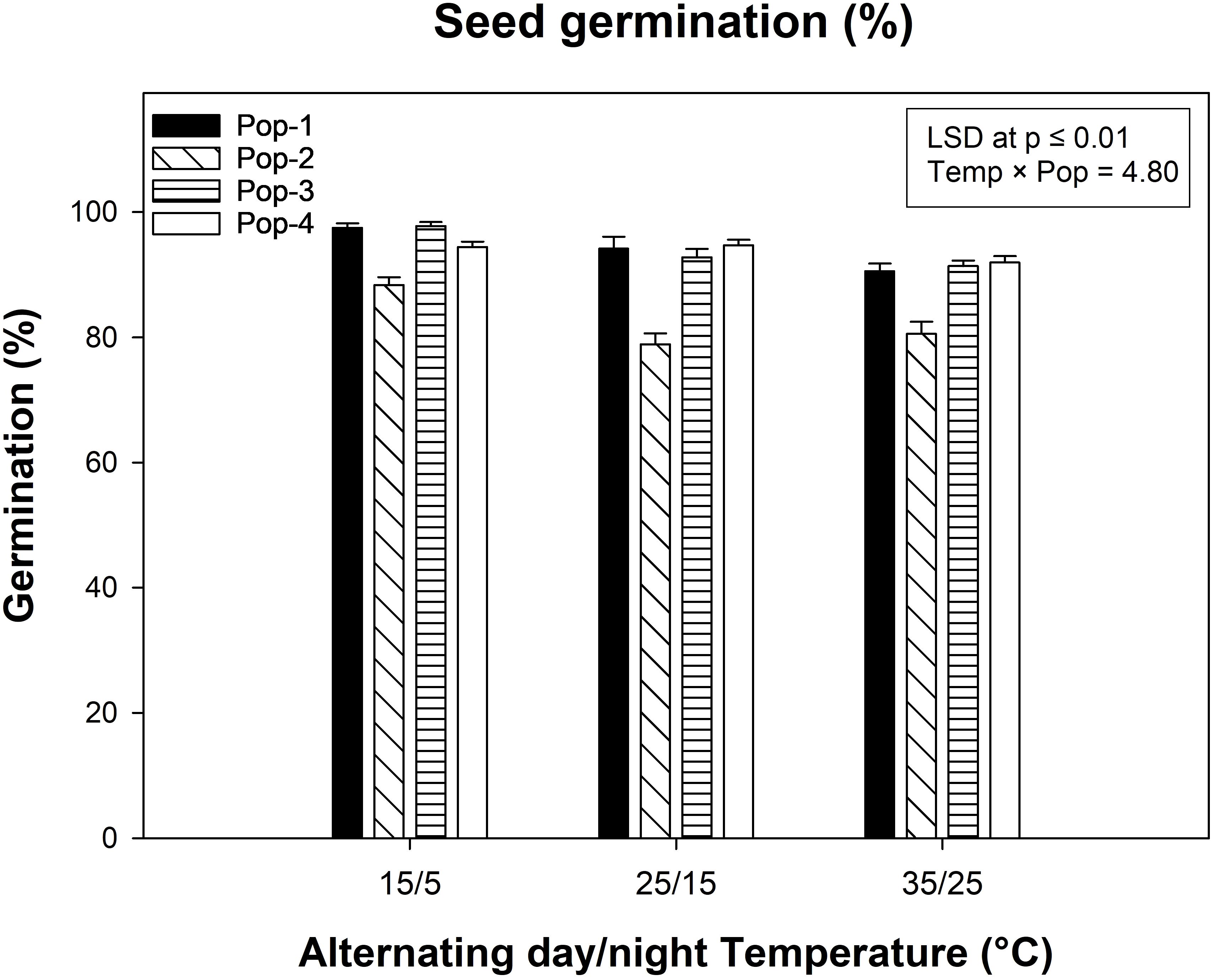
Figure 3. The seed germination percentage of four gazania populations at three alternating day/night temperature regimes (15/5, 25/15 and 35/25°C) after 14 d of incubation. Data and error bars represent the means and ± SE of the means, respectively (number of replicates = 12; pooled for three replicates each of light/dark and complete dark conditions over two runs; 30 seeds per replicate). LSD is the least significant difference value for mean comparison at P ≤ 0.01.
3.3 Effect of moisture stress
The two gazania populations studied (Pop-1 and Pop-3) did not differ in terms of their germination response to different osmotic potential levels (P = 0.21), so the data were pooled for both populations before regressing the effect of osmotic potential. Germination decreased in a non-linear, sigmoidal fashion as the osmotic potential reduced from 0 to -1.2 MPa (Figure 4). The highest germination (98%) was recorded at no water stress control and the lowest germination (<1%) was recorded at the highest moisture stress level (-1.2 MPa osmotic potential). Fifty percent inhibition of germination was predicted to occur at osmotic potential of -0.67 MPa (Figure 4).
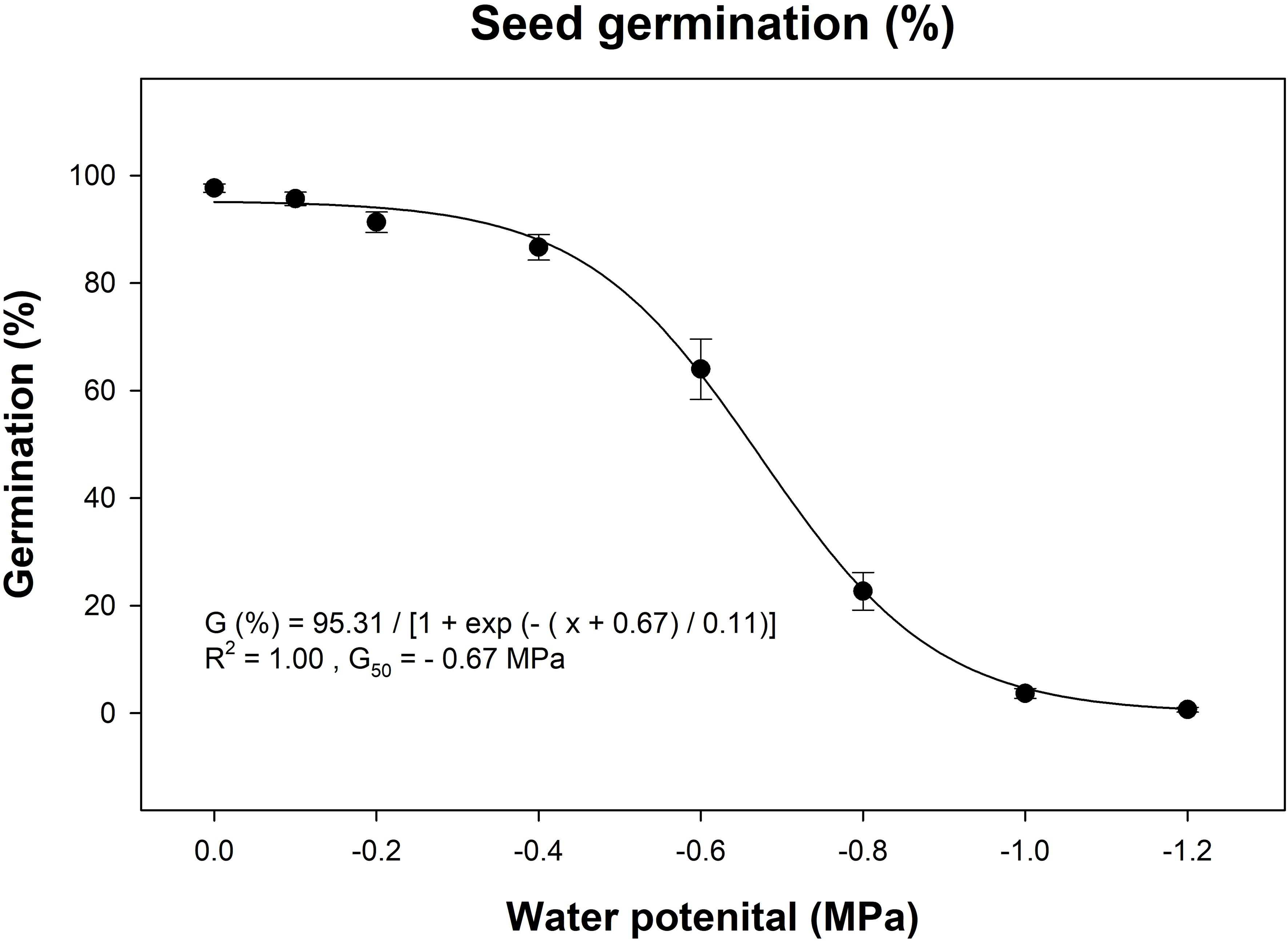
Figure 4. Effect of different water potential (MPa) levels on the seed germination of gazania after 14 d of incubation at alternating day/night temperatures of 15/5°C. Data are pooled for two populations (Pop-1 and Pop-3) due to non-significant difference between populations. The trend line represents a three-parameter sigmoidal, non-linear regression model fitted on the pooled data of two populations. The data points and error bars represent the means and ± SE of the means, respectively (number of replicates = 12; pooled for three replicates of both populations over two runs; 30 seeds per replicate).
3.4 Effect of salt stress
Salt stress significantly affected germination of gazania populations and there were significant interactions between salinity levels and populations (P < 0.01). Seeds of both populations germinated at up to 250 mM NaCl concentration (Figure 5). Population 3 was more affected by salinity as 50% of germination inhibition was observed at 252 mM NaCl concentration compared to 268 mM required for 50% germination inhibition of Pop-1 (Figure 5).
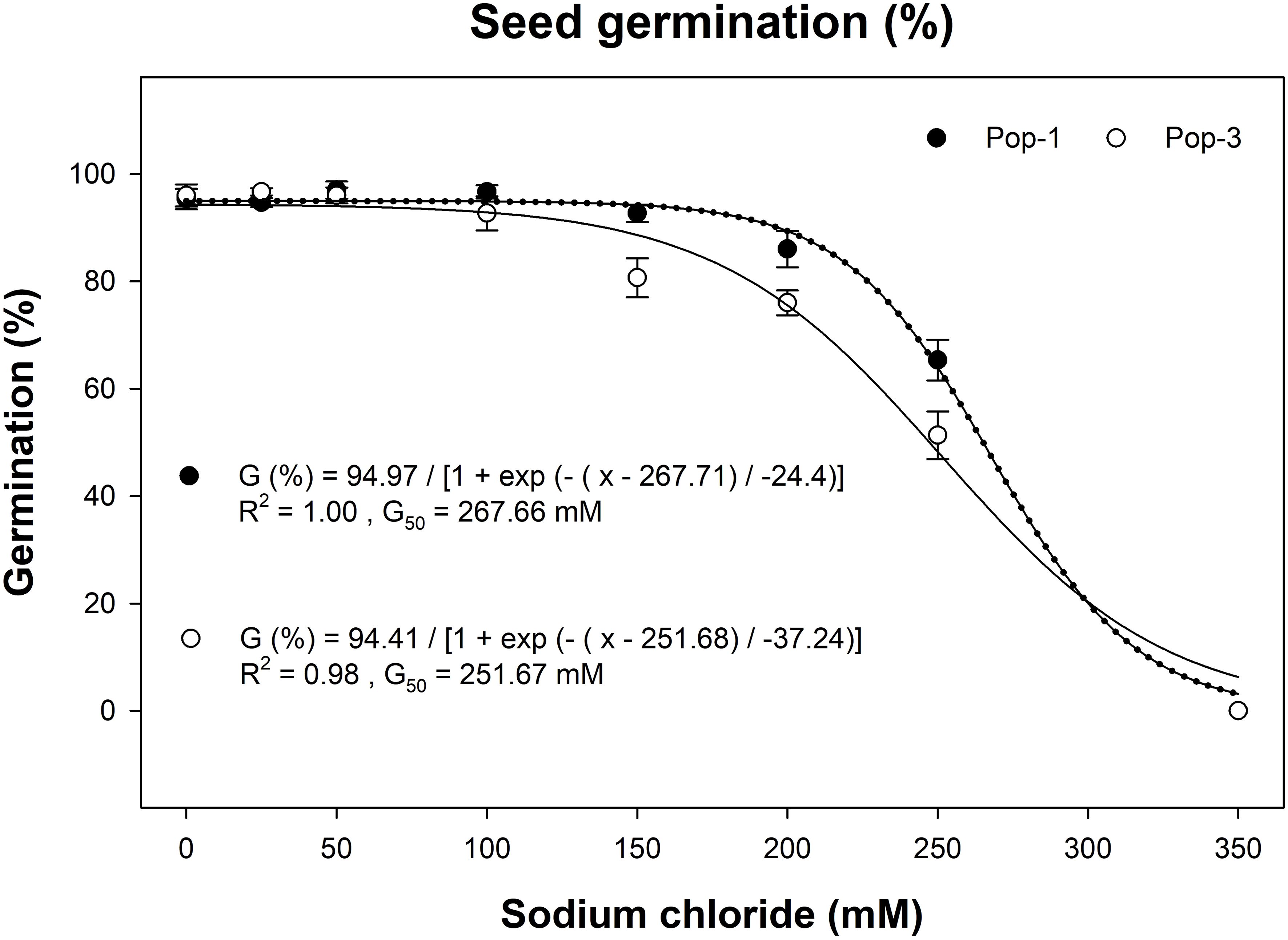
Figure 5. Effect of different sodium chloride concentrations (mM) on the germination of two gazania populations (Pop-1 and Pop-3) after 14 d of incubation at alternating day/night temperatures of 15/5°C. The trend lines represent three-parameter sigmoidal, non-linear regression model fitted separately on data of two populations. The data points and error bars represent the means and ± SE of the means, respectively (number of replicates = 6; three replicates of each population pooled for two runs; 30 seeds per replicate).
3.5 Effect of pH
There was no significant difference between gazania populations and also between pH levels studied (4 to 10; Figure 6). Overall, both populations germinated more than 85% across all the tested pH levels and germination ranged between 87 to 98%.
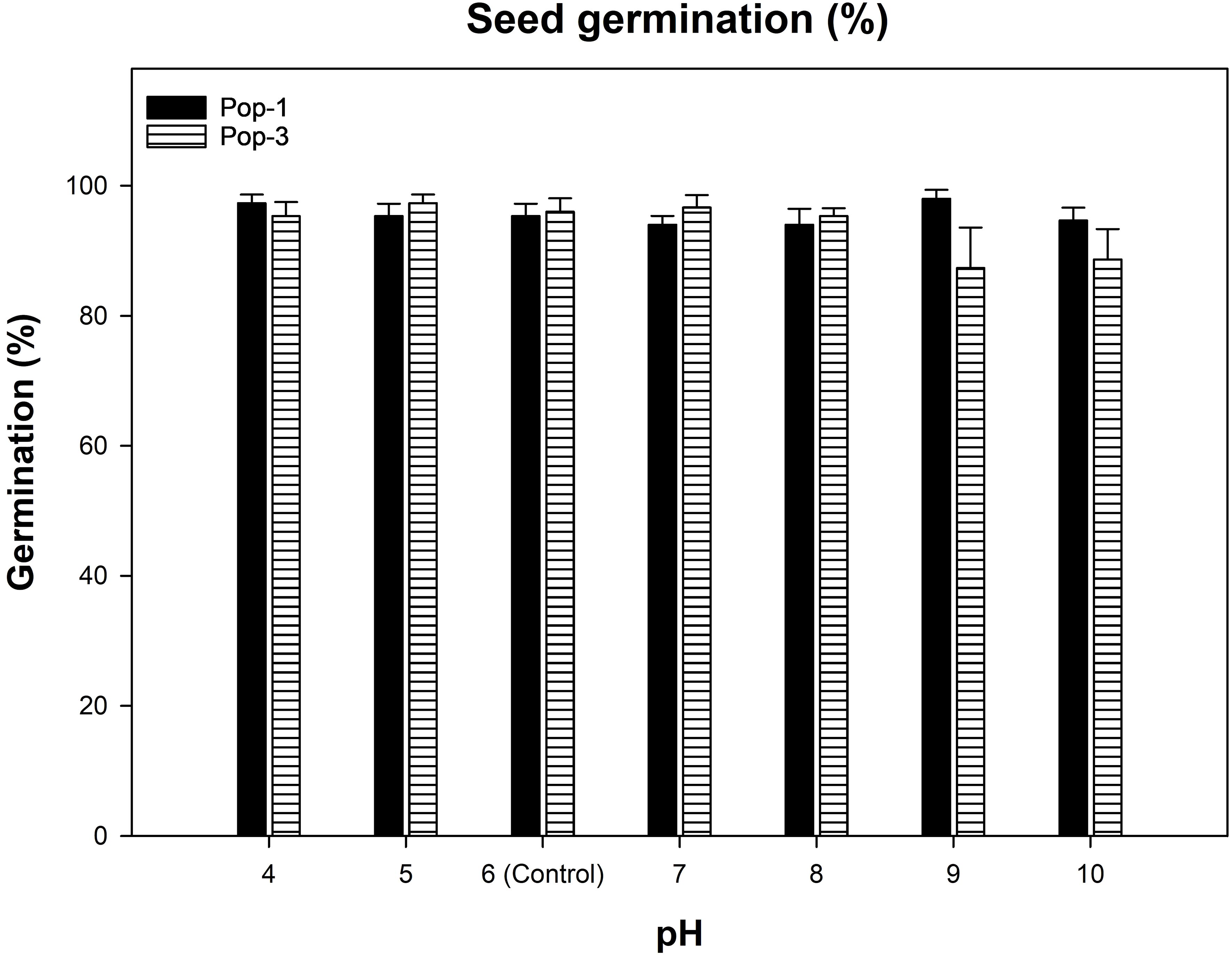
Figure 6. Effect of different pH levels on the germination of two gazania populations (Pop-1 and Pop-3) after 14 d of incubation at alternating day/night temperatures of 15/5°C. Data and error bars represent the means and ± SE of the means, respectively (number of replicates = 6; three replicates of each population pooled for two runs; 30 seeds per replicate). The effect of populations, pH levels or their interaction was non-significant.
3.6 Effect of burial depth on seedling emergence and vigor
The interaction of seed burial depth and growing media significantly affected the gazania seedling emergence (P < 0.01). Growing media also differed significantly in terms of seedling emergence with the highest emergence observed in sand, followed by potting mix and the lowest emergence in soil (Figure 7). Increasing burial depth caused a non-linear reduction in seedling emergence. A 50% reduction in seedling emergence was predicted to occur at 1.6, 2.0 and 3.9 cm seeding depth in soil, sand and potting mix, respectively (Figure 7).

Figure 7. Effect of seed burial depth on the emergence of gazania seeds in (A) potting mix, (B) sand and (C) soil as recorded 21 d after sowing. The trend lines represent three-parameter sigmoidal, non-linear regression model fitted. The data points and error bars represent the means and ± SE of the means, respectively (number of replicates = 6; three replicates pooled for two runs; 20 seeds per replicate).
Similar effect was observed for seedling vigor as measured in the form of shoot and root length (Figures 8, 9). The interaction of seed burial depth and growing media significantly affected the gazania shoot and root length (P < 0.01). In terms of growing media, maximum shoot length was observed in sand, then potting mix, and the lowest shoot length was observed in soil (Figures 8). On the other hand, root length was the highest in potting mix followed by soil and the lowest in sand (Figures 9). Seeds that emerged from surface had the longest roots (13 cm) compared to those that emerged from 4 cm burial depth (root length 3 cm).
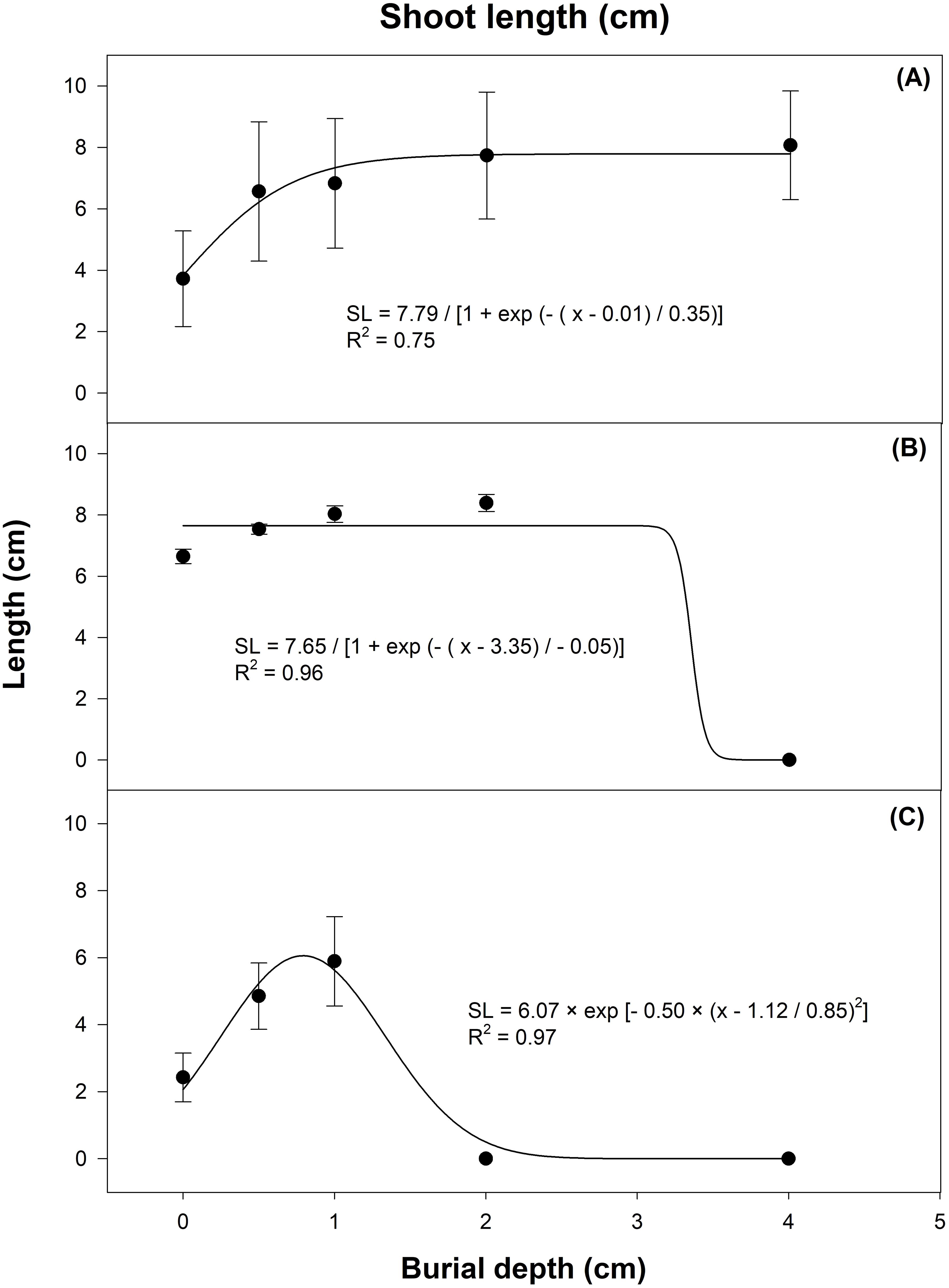
Figure 8. Effect of seed burial depth on shoot length of gazania seedlings emerged in (A) potting mix, (B) sand and (C) soil as recorded 21 d after sowing. The trend lines represent three-parameter sigmoidal (A, B) or gaussian (C), non-linear regression models fitted to the data. The data points and error bars represent the means and ± SE of the means, respectively (number of replicates = 6; three replicates pooled for two runs; 5 seedlings per replicate).
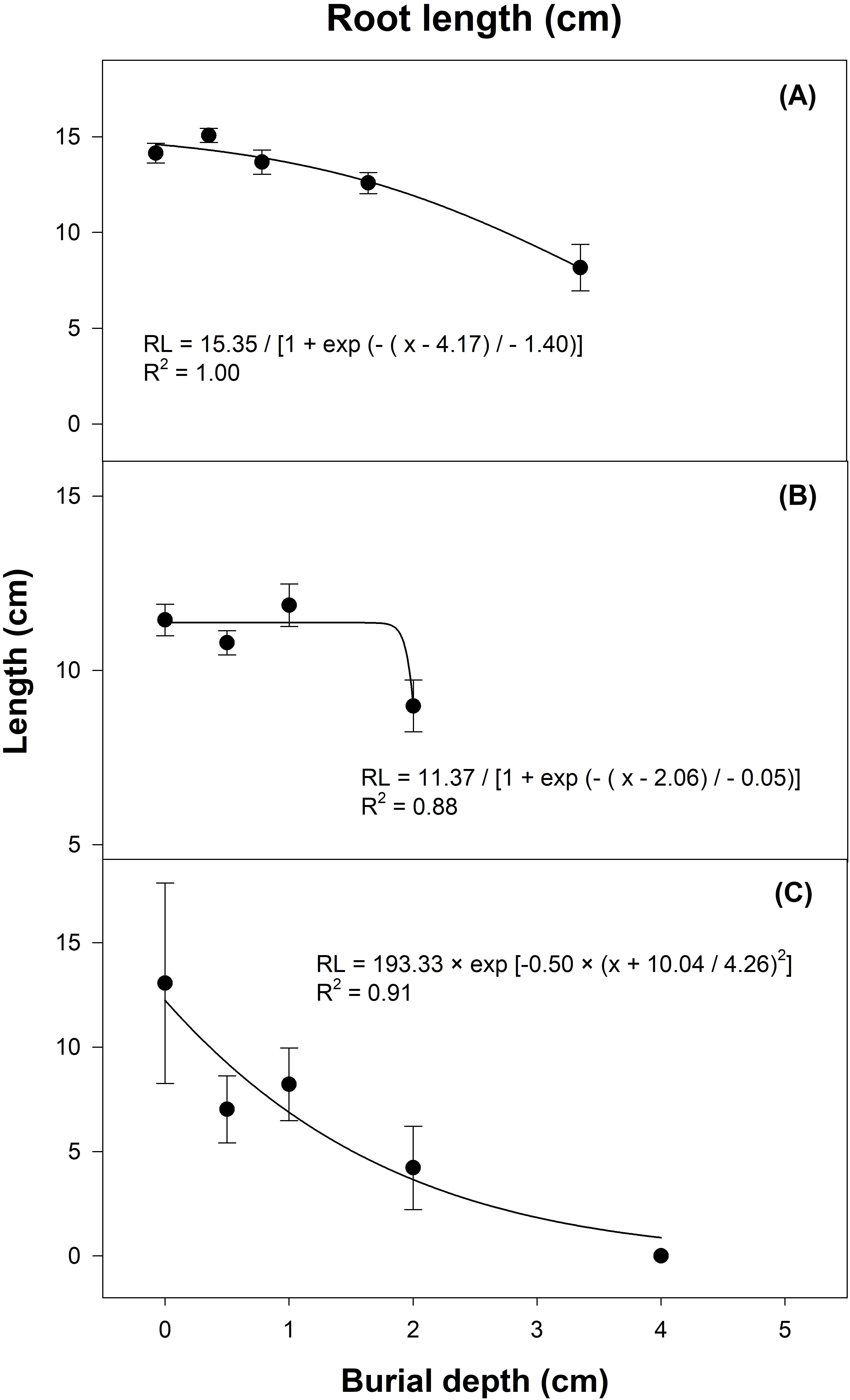
Figure 9. Effect of seed burial depth on root length of gazania seedlings emerged in (A) potting mix, (B) sand and (C) soil as recorded 21 d after sowing. The trend lines represent three-parameter sigmoidal (A, B) or gaussian (C), non-linear regression models fitted to the data. The data points and error bars represent the means and ± SE of the means, respectively (number of replicates = 6; three replicates pooled for two runs; 5 seedlings per replicate).
4 Discussion
This study clearly shows that gazania has a flexible germination across a wide range of climatic or edaphic conditions. Although a perennial with a tendency to actively grow during warmer months (spring to summer), the ability to germinate at relatively cold temperatures is the main reason for year-round seedling recruitment from seed banks. While different populations differed in some aspects of seed biology, overall, this weed demonstrated very high germination rates (>80%) across normal or minimal stress conditions. These findings illustrate why gazania is highly invasive and widespread in most parts of Australia.
4.1 Seed morphology
Intraspecific variation in morphological traits of weeds is a well-known phenomenon. Environmental factors and land management practices prevailing during plant growth and especially during seed production directly affect seed health (Fenner, 1991). Seeds of a population collected from a pasture field were heavier than a fence line population, probably due to additional nutrient availability and better growing environment during plant growth and seed development stages. On the other hand, plants growing along the fence line often experience hostile conditions with poor nutrient availability and strong competition from other weeds (Johnson et al., 2022). Phenotypic variation across populations may also be driven by genetic variation with gazania species prevailing in Australia likely to be genetically diverse and there are suggestions that at least two species hybridize.
4.2 Effect of photoperiod
Light is a crucial factor affecting seed germination and the seeds of many plant species require light to start their germination. However, some species do not have strong light requirements for germination (Baskin and Baskin, 1998). In our study, similar seed germination response in alternating light/dark and complete dark conditions suggests that photoperiod is not a limiting factor for gazania germination and it’s a non-photoblastic weed. A similar seed germination response to light and dark conditions has been reported for another Asteraceae weed, Centaurea balsamita L (Nosratti et al., 2017). The ability to germinate equally good under light or dark conditions means the seeds of this species have the potential to germinate under shade or when buried in soil as well as from the soil surface. Therefore, farming practices including mulching, or no till farming may have no significant influence in terms of its control.
4.3 Effect of temperature
With above 75% germination across all temperature regimes tested in this study, gazania has the potential to germinate across all seasons in most parts of Australia. While the highest seed germination was observed at low temperature (15/5°C) which could be considered as optimum seed germination temperature for gazania. However, it is important to note that the germination at higher temperatures was also close to the low temperature. In the gardening sector, the ideal recommended temperature for gazania seed germination is 21-24°C (Smokeye’s Gardens, 2024; Shahzad et al., 2025). High seed germination across such a wide window means temperature is not a limiting factor either just like light conditions. This means germination could occur all year round, making its management more difficult.
This type of seed germination ability across a wide range of alternating day/night temperatures has been reported in some other Asteraceae weed species, including hairy fleabane (Conyza bonariensis L.) (Loura et al., 2020) and hairy beggarticks (Bidens pilosa L.) (Chauhan et al., 2019). While it is important to know that gazania seeds can germinate at a wide range of temperatures, it will be crucial to understand the growth and reproductive response of this species across a temperature gradient to understand the full invasion ecology. For example, growth and development response under high and low temperatures could reveal how gazania would behave and spread in a changing climate.
4.4 Effect of moisture stress
Moisture is often a significant factor affecting weed seed germination. Moisture stress can reduce, delay, or prevent seeds from gemination (Javaid et al., 2022). The results from our study indicate that gazania has great potential to germinate at high osmotic stress levels as 50% percent inhibition of germination was predicted to occur at osmotic potential of -0.67 MPa. However, osmotic stress levels higher than that had negative impact on germination, suggesting moisture is a key factor in gazania seed germination. This is similar to Lamsal et al. (2019) who found 50% inhibition of germination at the osmotic potential of -0.67 MPa in another Asteraceae weed, floss flower (Ageratum houstonianum L.).
Inhibition of seed germination at higher osmotic stress levels was likely due to low water availability to seeds for imbibition and activation of metabolic processes required to trigger germination. Only less than 1% seeds could germinate at the highest level of osmotic stress. This type of seed germination ability under low levels of moisture availability is not very common but has been observed in some invasive weeds, especially those from Asteraceae family, including C. balsamita, ragweed parthenium and Sumatran fleabane (Conyza sumatrensis L.) (Nosratti et al., 2017; Bajwa et al., 2018; Mahajan et al., 2021).
Our findings suggest that gazania has high potential to germinate with little available moisture and this attribute could help it to establish and outcompete other species in drought conditions. This would mean increased invasion into arid zones of Australia, which is probably well underway currently. It is important to understand if the seedlings can survive under such water-limited environments after the initial germination and establishment.
4.5 Effect of salt stress
Soil salinity is a major factor influencing agriculture crop production globally, including Australia (Rengasamy, 2010). According to Chauhan (2016), soil is considered to have high salt contents if it contains more than 100 mM NaCl. It is clear from our results that gazania populations were tolerant to fairly high levels of salt stress, but the two populations differed in their response. Higher salinity tolerance in the roadside population (Pop-1) could be because those areas are often exposed to more pollutants as compared to cropping situations. Our results are in line with the research findings of Loura et al. (2020), who also observed significant differences among hairy fleabane populations at different salinity levels. This type of seed germination ability at very high salinity levels has been reported in some other Asteraceae weed species such as tropic ageratum (Ageratum conyzoides L.) (Desai et al., 2024), hairy beggarticks (Chauhan et al., 2019) and ragweed parthenium (Bajwa et al., 2018). The complete inhibition of gazania germination happened only at the very high salinity level (350 mM), which is often due to ion toxicity causing disturbance in physiological and metabolic processes (Farooq et al., 2015).
High salt tolerance in gazania is worrying yet unsurprising as these species exist in coastal environments in their native range (South Africa) and therefore, have probably evolved to tolerate salt mist from sea. Unfortunately, widespread dryland salinity in many marginal agricultural lands of Australia (Rengasamy, 2010) could prove perfect habitat for gazania. Some inland incursions into dryland cropping systems could be the early signs of this trend. However, it will be important to study the growth response of gazania to salt stress to get a full picture of potential future spread into regions with salinity problem.
4.6 Effect of pH
Soil properties can impact weed seed germination. Most weeds have the ability to tolerate and germinate over a broad range of pH levels (Bajwa et al., 2018), but some weeds cannot tolerate highly acidic or basic conditions (Stokes et al., 2011). In our study, pH seemed to be not a limiting factor as gazania seeds successfully germinated at pH 4 to 10. Unfortunately, these pH levels cover most of the Australian soils (de Caritat et al., 2011). This attribute of seed germination across the board has been observed in hairy fleabane (Shrestha et al., 2022), ragweed parthenium (Bajwa et al., 2018), and C. balsamita (Nosratti et al., 2017). It is important to note that other soil properties could also play an important role in weed establishment and pH may not be the critical factor. However, in some areas of Australia where soil amelioration practices are undertaken in conservation tillage systems to fix soil constraints, the effect on germination ability of species like gazania should be studied.
4.7 Effect of burial depth on seedling emergence and vigor
The higher emergence of gazania in sand explains why this weed is more prevalent in marginal sandy soils in southern Australia. Reduction in emergence from deeper layers of soil was expected as most small-seeded Asteraceae weed species present this behavior. This might be due to low energy reserves in seed and lower gaseous diffusion and hypoxia at deeper depths. Decreased seedling emergence in response to increased burial depth has been observed in hairy beggarticks (Chauhan et al., 2019). The differential response was observed in shoot and root elongation of gazania in different growing media. This might be due to variations in physio-chemical properties of growing media such as pH, E.C, porosity, bulk density and water holding capacity.
These results suggest that gazania could become a highly challenging weed in conservation tillage systems widely adopted in grain production regions across Australia. However, it is important to note that our study only evaluated a single population. While there is little evidence of significant intraspecific variation in terms of seedling emergence from different depths in small-seeded weed species, potential differences among gazania populations cannot be ruled out. Strategic deep tillage can be used to bury the seeds at a depth of 6 cm or more from where they cannot emerge (Chauhan and Johnson, 2010). This could be a good management strategy in highly infested cropping fields. However, the tillage may increase gazania infestations by distributed underground rhizomes. Therefore, further research on the role of mechanical disturbance including tillage should be explored as part of integrated weed management (IWM) strategies.
5 Conclusion
Our results strongly suggest that gazania seeds have potential to germinate over a wide range of environmental conditions. The populations differed in terms of seed weight and seed width and their germination response to temperature and salt stress gradients. This could make management of different populations more complicated. Non-reliance on light conditions for germination suggests that this weed can emerge in land-use scenarios with different levels of residue management, mulching or shading. High seed germination across populations (>75%) over a wide range of alternating day/night temperatures (15/5, 25/15, and 35/25°C) suggests that gazania could become a problematic weed across all seasons in most parts of Australia while it has low dormancy levels which can lead to potential high infestation levels. Similarly, high tolerance to osmotic and salt stress and flexibility of germination across acidic to alkaline environments means this weed has features to establish in most parts and soil types across Australia. The seedling emergence of the tested population was high from the surface and shallow depth, but some seeds could emerge from up to 5 cm burial depth. While further populations should be compared for emergence response under field conditions, these preliminary findings suggest this weed has the potential to develop significant seedbank in Australian no-till grain production systems.
This knowledge on seed germination triggers and emergence dynamics should help to predict potential regions of spread of gazania and help develop and apply management strategies with respect to different environmental conditions. While this information is crucial for managing this highly problematic weed at the establishment stage (germination and emergence), further research is needed on growth and reproductive response of gazania to different environmental conditions. This will help understand the invasion ecology and develop long-term management strategies for different land-use scenarios for this weed, which is rapidly spreading across Australia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
MA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. BS: Methodology, Validation, Writing – original draft, Writing – review & editing, Data curation. MC: Methodology, Validation, Writing – original draft, Writing – review & editing, Supervision. AAB: Conceptualization, Resources, Methodology, Validation, Writing – original draft, Writing – review & editing, Project administration, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by La Trobe University (no specific funding number applicable).
Acknowledgments
The authors are thankful to Mr Chris Davey, Mr Robin Schaefer, Ms Kate Lee and Dr Fiona Murdoch for their help with seed collection of different populations. The authors acknowledge the La Trobe University Bioimaging Platform for the provision of instrumentation, training and technical support for microscopic imaging of seeds. The authors also acknowledge the La Trobe Institute for Sustainable Agriculture and Food (LISAF) and the School of Agriculture, Biomedicine and Environment (SABE) for the provision of research facilities and ongoing support for the research program. Muhammad Adnan is thankful to La Trobe University for the provision of a PhD scholarship. He is also thankful to Dr Arslan Peerzada for his assistance in data analysis.
Conflict of interest
The authors declare that they have no conflict of interest in relation to this study and publication.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2025.1596787/full#supplementary-material
References
Australian Agricultural Statistics (2024). Available online at: https://www.getonside.com/au/blog/Australian-agricultural-statistics (Accessed June 24, 2024).
Bajwa A. A., Chauhan B. S., Adkins S. W. (2018). Germination ecology of two Australian biotypes of ragweed parthenium (Parthenium hysterophorus L.) relates to their invasiveness. Weed Sci. 66, 62–70. doi: 10.1017/wsc.2017.61
Baskin C. C., Baskin J. M. (1998). Seeds: Ecology, biogeography, and evolution of dormancy and germination. 2nd ed (London: Academic Press).
Chachalis D., Reddy K. N. (2000). Factors affecting Campsis radicans seed germination and seedling emergence. Weed Sci. 48, 212–216. doi: 10.1614/0043-1745(2000)048[0212:FACRSG]2.0.CO;2
Chauhan B. S. (2016). Germination biology of Hibiscus tridactylites in Australia and the implications for weed management. Sci. Rep. 6, 1–6. doi: 10.1038/srep26006
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Chauhan B. S., Ali H. H., Florentine S. (2019). Seed germination ecology of Bidens pilosa and its implications for weed management. Sci. Rep. 9, 1–9. doi: 10.1038/s41598-019-52620-9
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Chauhan B. S., Johnson D. E. (2010). The role of seed ecology in improving weed management strategies in the tropics. Adv. Agron. 105, 221–262. doi: 10.1016/S00652113(10)05006-6
Cochavi A., Goldwasser Y., Horesh A., Igbariya K., Lati R. N. (2018). Impact of environmental factors on seed germination and emergence of wild poinsettia (Euphorbia geniculata Ortega). Crop Prot. 114, 68–75. doi: 10.1016/j.cropro.2018.08.019
de Caritat P., Cooper M., Wilford J. (2011). The pH of Australian soils: field results from a national survey. Soil Res. 49, 173–182. doi: 10.1071/SR10121
Desai D. H., Desai H. S., Chauhan B. S. (2024). Germination attributes of metsulfuron-resistant and metsulfuron-susceptible tropical ageratum (Ageratum conyzoides) populations under various environmental conditions. Weed Sci. 72, 1–8. doi: 10.1017/wsc.2024.31
EPLB (2023). Eyre Peninsula Landscape Board. Pest species regional management plan Gazania spp. Gazania. Available online at: https://cdn.environment.sa.gov.au/landscape/docs/ep/EPLB_Gazania_Plan.pdf (Accessed June 14, 2024).
Farooq M., Hussain M., Wakeel A., Siddique K. H. (2015). Salt stress in maize: effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 35, 461–481. doi: 10.1007/s13593-015-0287-0
Fenner M. (1991). The effects of the parent environment on seed germinability. Seed Sci. Res. 1, 75–84. doi: 10.1017/S0960258500000696
Geneve R. L. (2003). Impact of temperature on seed dormancy. HortScience 38, 336–341. doi: 10.21273/HORTSCI.38.3.336
Green Adelaide (2023). Gazania (Gazania spp.) fact sheet. Green Adelaide Government of South Australia. Available online at: https://cdn.environment.sa.gov.au/greenadelaide/images/GA-Pest-Plant-Fact-Sheet-gazania.pdf (Accessed June 26, 2024).
Groves R. H., Boden R., Lonsdale W. M. (2005). Jumping the garden fence. Invasive garden plants in Australia and their environmental and agricultural impacts (Sydney, Australia: CSIRO Rep), 1–173.
Hooda V. S., Chauhan B. S. (2024). Unraveling the influence of environmental factors on fireweed (Senecio Madagascariensis L.) germination and its management implications. Invasive Plant Sci. Manage. 17, 9–16. doi: 10.1017/inp.2024.8
Howis S., Barker N. P., Mucina L. (2009). Globally grown, but poorly known: species limits and biogeography of Gazania Gaertn. (Asteraceae) inferred from chloroplast and nuclear DNA sequence data. Taxon. 58, 871–882. doi: 10.1002/tax.583015
Javaid M. M., Mahmood A., Alshaya D. S., AlKahtani M. D., Waheed H., Wasaya A., et al. (2022). Influence of environmental factors on seed germination and seedling characteristics of perennial ryegrass (Lolium perenne L.). Sci. Rep. 12, 9522. doi: 10.1038/s41598-022-13416-6
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Johnson S. A., Janssen E., Glass N., Dickerson P., Whelan C. J., Molano-Flores B. (2022). The role of environmental stressors on reproduction, seed morphology, and germination: a case study of northern white cedar, Thuja occidentalis L. Bot. 100, 839–847. doi: 10.1139/cjb-2022-0007
Lamsal A., Devkota M. P., Shrestha D. S., Joshi S., Shrestha A. (2019). Seed germination ecology of Ageratum houstonianum: A major invasive weed in Nepal. PloS One 14, e0225430. doi: 10.1371/journal.pone.0225430
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Loura D., Florentine S., Chauhan B. S. (2020). Germination ecology of hairy fleabane (Conyza bonariensis) and its implications for weed management. Weed Sci. 68, 411–417. doi: 10.1017/wsc.2020.28
Mahajan G., Prasad A., Chauhan B. S. (2021). Seed germination ecology of Sumatran fleabane (Conyza sumatrensis) in relations to various environmental parameters. Weed Sci. 69, 687–694. doi: 10.1017/wsc.2021.56
McCallum Q. (2024). Effort underway to tackle South Australia’s growing gazania problem (Adelaide, Australia: Stock Journal). Available at: https://www.stockjournal.com.au/story/8527697/gazania-control-fight-just-beginning-in-south-Australia/.
McIntosh J. (2022). How to grow and care for gazania. Available online at: https://www.thespruce.com/gazania-flowers-1315701 (Accessed September 26, 2024).
Michel B. E., Kaufmann M. R. (1973). The osmotic potential of polyethylene glycol 6000. Plant Physiol. 51, 914–916. doi: 10.1104/pp.51.5.914
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Mutti N. K., Mahajan G., Chauhan B. S. (2019). Seed-germination ecology of glyphosate-resistant and glyphosate-susceptible biotypes of Echinochloa colona in Australia. Crop Pasture Sci. 70, 367–372. doi: 10.1071/CP18444
Nosratti I., Soltanabadi S., Honarmand S. J., Chauhan B. S. (2017). Environmental factors affect seed germination and seedling emergence of invasive Centaurea balsamita. Crop Pasture Sci. 68, 583–589. doi: 10.1071/CP17183
Rengasamy P. (2010). Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 37, 613–620. doi: 10.1071/FP09249
Shahzad B., Adnan M., Bajwa A. A. (2025). What’s wrong with Gazanias? A review of the biology and management of weedy Gazania species. Plants 14, 915. doi: 10.3390/plants14060915
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Shrestha A., Maier V., Steinhauer K., Frynzyan T., Navarro I. (2022). Hairy fleabane germination in response to temperature, osmotic potential, pH, salinity levels, and seed dormancy periods. Agron. J. 114, 2552–2561. doi: 10.1002/agj2.21145
Singh A., Mahajan G., Chauhan B. S. (2022). Germination ecology of wild mustard (Sinapis arvensis) and its implications for weed management. Weed Sci. 70, 103–111. doi: 10.1017/wsc.2021.66
Smokeye’s Gardens (2024).Gazania planting and growing instructions. Available online at: https://smokeysgardens.com/gazania-planting-growing-instructions/ (Accessed June 14, 2024).
Speirs D. (2021). Declared plant policy gazania (Gazania spp.) Government of South Australia. Available online at: https://pir.sa.gov.au/:data/assets/pdf_file/0016/234601/gazania_policy.pdf (Accessed June 24, 2024).
Stokes C. A., MacDonald G. E., Adams C. R., Langeland K. A., Miller D. L. (2011). Seed biology and ecology of natalgrass (Melinis repens). Weed Sci. 59, 527–532. doi: 10.1614/WS-D-11-00028.1
Tang W., Guo H., Yin J., Ding X., Xu X., Wang T., et al. (2022). Germination ecology of Chenopodium album L. and implications for weed management. PloS One 17, e0276176. doi: 10.1371/journal.pone.0276176
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Weed Futures (2024). Determining Current and Future Weed Threats in Australia. Available online at: https://weedfutures.net/species.php?id=140 (Accessed September 11, 2024).
Weeds of Australia (2016). Gazania Fact Sheet. Available online at: https://keyserver.lucidcentral.org/weeds/data/media/Html/gazania_linearis.htm:~:text=Gazania (Accessed November 02, 2024).
White M., Cheal D., Carr G. W., Adair R., Blood K., Meagher D. (2018). “Advisory list of environmental weeds in Victoria,” in Arthur Rylah Institute for Environmental Research Technical Report Series No. 287 (Department of Environment, Land, Water and Planning, Heidelberg, Victoria).
Wine Australia (2016). Controlling gazanias in the Riverland. Available online at: https://www.wineAustralia.com/growing-making/pest-and-disease-management/gazanias:~:text=They%20are%20best%20differentiated%20by,coloured%20silvery%20or%20hairy%20leaves (Accessed June 24, 2024).
Keywords: garden escaped, gazania weed, Gazania linearis, Gazania rigens, invasive plant
Citation: Adnan M, Shahzad B, Collins M and Bajwa AA (2025) Seeds of success: seed biology and germination response of Gazania weed in Australia. Front. Agron. 7:1596787. doi: 10.3389/fagro.2025.1596787
Received: 20 March 2025; Accepted: 28 April 2025;
Published: 14 May 2025.
Edited by:
Ricardo Alcántara-de la Cruz, Universidade Federal de Viçosa, BrazilReviewed by:
Carolina Zamorano-Montañez, University of Caldas, ColombiaDustin Moreno-Serrano, University of Panama, Panama
Copyright © 2025 Adnan, Shahzad, Collins and Bajwa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Ahsan Bajwa, QS5CYWp3YUBsYXRyb2JlLmVkdS5hdQ==
 Muhammad Adnan
Muhammad Adnan Babar Shahzad
Babar Shahzad Marisa Collins
Marisa Collins Ali Ahsan Bajwa
Ali Ahsan Bajwa