- 1Department of Agronomy, Punjab Agricultural University, Ludhiana, India
- 2Livestock Research Station, Rajasthan University of Veterinary and Animal Sciences, Bikaner, Rajasthan, India
- 3Department of Soil Science, Punjab Agricultural University, Ludhiana, India
- 4Texas A&M University, Agrilife Research Center, Beaumont, TX, United States
- 5Department of Industrial Microbiology, Jacob Institute of Biotechnology and Bio-engineering, Sam Higginbottom University of Agriculture, Technology and Sciences (SHUATS), Prayagraj, Uttar Pradesh, India
Conservation agriculture (CA) practices have been widely promoted and recognized for their potential to enhance soil sustainability by improving soil properties. The purpose of the 2-year field experiment was to investigate the effect of diversified CA -based cropping systems on nutrient availability and soil characteristics. The study was conducted using a randomized complete block design (RCBD) with four replications at each site. Six cropping system (CS) scenarios were tested: S1—rice–wheat–mungbean (R-W-SM) under conventional tillage (CT) without residue retention (R0); S2—R-W-SM under CA with residue retention (R+); S3—maize–wheat–mungbean (M-W-SM) under CT (R0); S4—M-W-SM under permanent bed (PB) with R+; S5—soybean–wheat- mungbean (S-W-SM) under CT (R0); and S6—S-W-SM under PB with R +. Though each annual cropping cycle spanned 1 year, the inclusion of mungbean (summer mungbean) in the same year allowed the assessment of a three-crop rotation within each year. After two cropping years (effectively covering two complete crop rotation cycles), the results indicated that S6 significantly improved the soil properties: bulk density decreased by 4.4% and infiltration rate increased by 45.6% compared with S1. Soil organic carbon and macro- and micro-nutrient availability were notably higher under CA-based systems (S2, S4, and S6). The highest microbial biomass, enzymatic activity, and basal soil respiration (BSR) were recorded in S6. In both years, dehydrogenase activity (DHA) and BSR increased by 58.5%–64.6% under S6 compared with 40.7%–41.4% in S1. Micro-nutrients like Zn, Fe, Mn, and Cu were improved by 10%, 39%, 8%, and 63%, respectively, in S6 over S1. These findings suggest that CA-based soybean–wheat –mungbean systems (S6) can substantially enhance soil health and nutrient dynamics in a short-term rotation and may guide future sustainable agriculture.
1 Introduction
The continuous expansion of the rice–wheat (RW) cropping system in South Asia has raised concerns over the sustainability of intensive grain production. This results from the overuse of natural resources linked to certain farming methods (Tulu et al., 2023). As the world’s population steadily increases, there is a growing urgency to enhance agricultural production to meet the rising need for sustenance and agricultural commodities (Gudi et al., 2022; Singh et al., 2022). However, conventional agricultural practices have frequently engendered adverse consequences for soil health and environmental sustainability (Doran and Zeiss, 2000). Using sustainable intensification techniques in crop production, as a fundamental principle of conservation agriculture, offers promising solutions to address several challenges. These challenges include climatic anomalies, fluctuations in prices, ensuring a balanced food supply, preventing natural resource degradation, and reducing dependency on agro-chemicals (Bakala et al., 2020). Sandy loam soils dominate large areas in South west Asia and other regions across the globe. However, these soils face several production limitations, including high bulk density, low hydraulic conductivity, reduced water retention capacity, low soil organic carbon (SOC), and diminished biological activity (Kumari et al., 2018; Osunbitan et al., 2005; Singh et al., 2011). In intensified irrigated RW cropping systems, the low SOC content leads to unsustainable productivity and deteriorating soil health (Yadav M. et al., 2022). Factors driving the shift from rice–wheat rotations to maize/soybean or maize rotations include the adaptability of maize/soybean crops, increased maize demand in the livestock and fishery sectors, limited rice export opportunities, and higher yield potential of maize fodder (Congreves et al., 2015). The RW cropping system can negatively impact soil health through nutrient depletion, erosion, declining organic matter, and soil compaction (Bhatt et al., 2016). These interconnected issues pose serious threats to both ecosystem health and long-term agricultural sustainability (Bhuiyan et al., 2023). Incorporation of mungbean as a leguminous crop in the RW cropping system can mitigate these issues by enhancing soil fertility as well as soil health, reducing erosion, improving organic matter content, and alleviating compaction (Hazra et al., 2020a). This practice diversifies with maize/soybean cropping system and promotes sustainable nutrient cycling, leading to improved soil sustainability (Sharma et al., 2014).
Conservation agriculture (CA) has been implemented on a global scale, covering more than 125 million hectares of land (Kumar and Saini, 2022). This farming approach that focuses on minimizing soil disturbance through reduced or zero tillage (ZT), diversification of crops, and leftover of at least 30% crop residue on the soil surface (Dey et al., 2016; Ladha et al., 2004). ZT is a popular strategy among wheat farmers, as it allows for early planting, reduces production costs, and increases yield-attributing parameters, thus improving the overall sustainability, productivity, and profitability of the farmers (Singh et al., 2014). The development of the machine for zero-tilled wheat sowing named as “Happy Seeder “ has enabled farmers in South west Asia to retain the residue of crop and transition toward full CA-based systems (Sapkota et al., 2015). In addition to addressing water and labor shortages, the maize–wheat – mungbean and soybean–wheat –mungbean cropping systems are emerging as an alternative to conventional RW cropping systems due to the lower water and labor requirements of maize and soybean (Beare et al., 1994; Halvorson et al., 2002). A research study demonstrated that conservation tillage with crop management (CACM) produced the most favorable results, achieving the greatest economic yield for soybean production compared with other agricultural approaches including conventional tillage with chemical management (CTCM), conservation agriculture with organic management (CAOM), and conventional tillage with organic management (CTOM) (Meena et al., 2022a, b).
Numerous studies over the past decade have examined the importance of different tillage practices, residue management, and cropping sequences on various aspects of agricultural productivity, such as nutrient and water use efficiency, soil physical properties, greenhouse gas emissions, economic profitability, climate adaptation, and overall sustainability (Karlen et al., 2013; Sharma et al., 2022; Sharma and Singh, 2023). Research has indicated that CA practices can yield favorable results on soil health and also increase (50%–56%) the soil organic matter (Jat et al., 2021; Sharma et al., 2021), improve the soil structure through the preservation of soil aggregates (Srinivasarao et al., 2013), reduce the oxidation of organic matter, increase the soil enzymatic activity (Pankaj et al., 2023; Saikia et al., 2019; Sharma et al., 2022, 2025), and improve the soil micro-nutrient status (Sharma and Dhaliwal, 2021) compared with CT. Crop yields in agricultural systems are significantly influenced by several key factors, including tillage practices, nutrient management strategies, sowing density and timing, pest control measures, and the incorporation of leguminous crops into crop rotations (Meena et al., 2023a; Meena et. al., 2023b). Zero-till direct- seeded rice (DSR) and maize substitution offer water, energy, and labor savings compared with manual transplanting as well as improve soil health (Jat et al., 2018; Choudhary et al., 2018). Additionally, integrating mungbean into rice–wheat systems improves the soil carbon and nitrogen content, contributing to overall soil quality enhancement (Singh et al., 2015).
Although several studies, including meta-analyses, have evaluated the individual effects of tillage intensity, legume inclusion, and residue retention on soil biological activities, there remains limited information on their combined and interactive effects under diversified conservation agriculture (CA)-based cropping systems. Reduced tillage intensity, coupled with legume integration and residue management, may differentially influence soil physical and chemical properties as well as modulate soil microbial diversity and activity in response to changes in substrate availability.
We hypothesized that reduced tillage intensity, the inclusion of leguminous crops, and management of crop residues would collectively enhance nutrient availability and stimulate soil microbial activity. Furthermore, we proposed that prolonged implementation of these conservation agriculture practices could lead to shifts in the balance of soil ecological enzyme activities, ultimately influencing microbial processes and nutrient cycling under sustainable intensification systems. In addition, the study assessed the biochemical contributions of accumulated soil organic carbon by quantifying key soil enzyme activities under contrasting tillage and residue management regimes.
2 Materials and methods
2.1 Experiment site and weather conditions
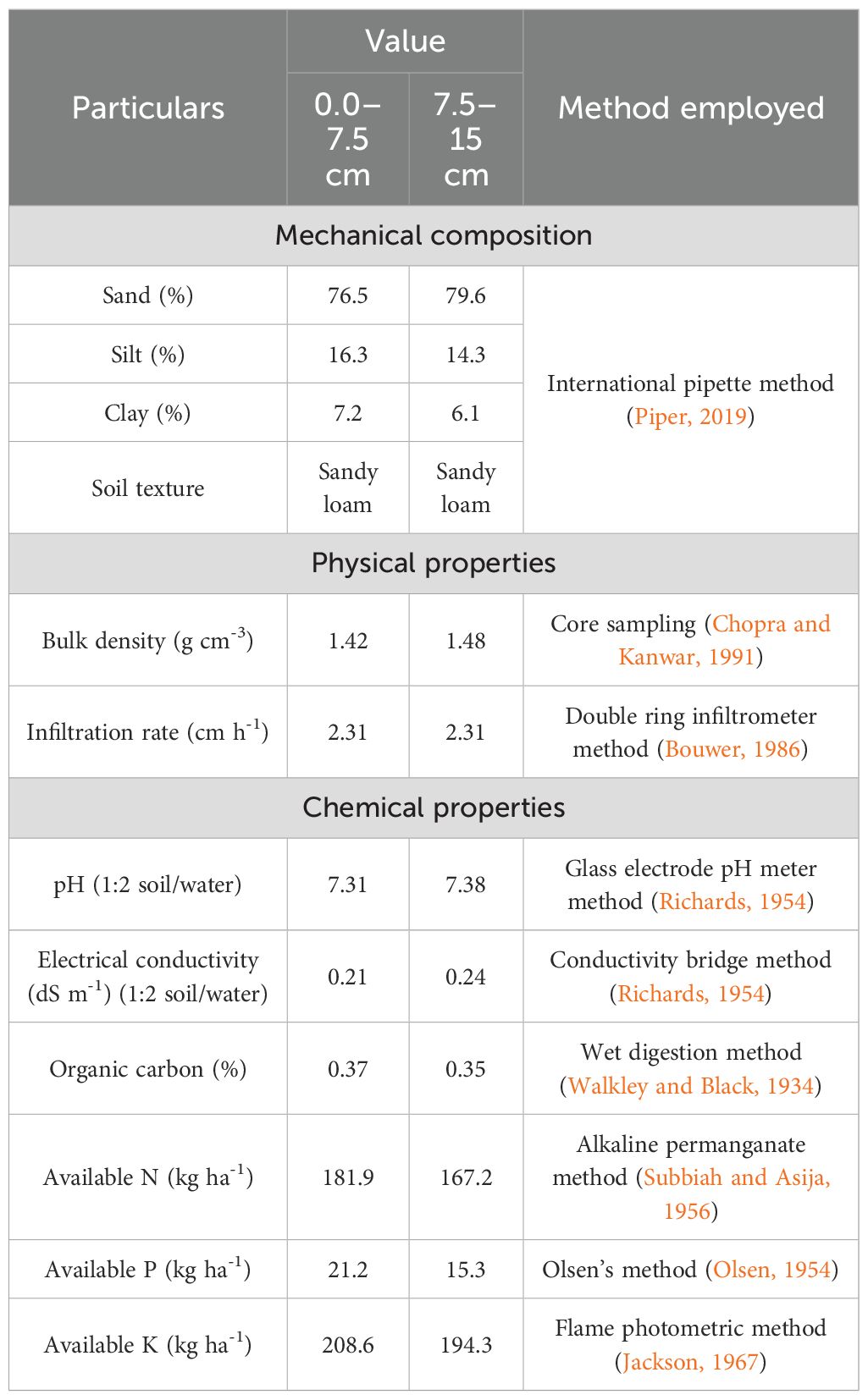
The present study was conducted at Agronomy Research Farm, Punjab Agricultural University (PAU), Ludhiana, India. The farm is located 247 m above mean sea level (MSL) at coordinates 30° 54′ N and 75° 48′ E. The location experiences semi-arid, sub-tropical climates and is classified under India’s Trans-Gangetic agroclimatic zone weather conditions. The maximum temperatures were 42.1°C and 40.0°C during 2019–2020 and 2020–2021. The mean relative humidity during the cropping season ranged from 30.50% to 86.92% and 34.00% to 85.14% during the crop season of 2019–2020– and 2020–2021, respectively. During summer season, a maximum temperature that ranged 32.9°C–42.1°C and a minimum temperature that ranged 11.1°C –27.6°C were recorded in the summer season of 2020, whereas during 2021 it was 23.4°C–39.4°C and 15.8°C– 26.6°C, respectively. Total rainfall received during the crop season was 72.4 mm and 126.8 mm during 2020 and 2021, respectively. The experimental soil was sandy loam in texture (76.5% sand, 16.3% silt, and 7.2% clay) and low in nitrogen (181.9 kg ha-1) and soil organic carbon (0.37%), medium in accessible potassium (208.6 kg ha-1) and phosphorus (21.2 kg ha-1), and neutral in reactions (pH 7.31).
2.2 Experimental treatment details
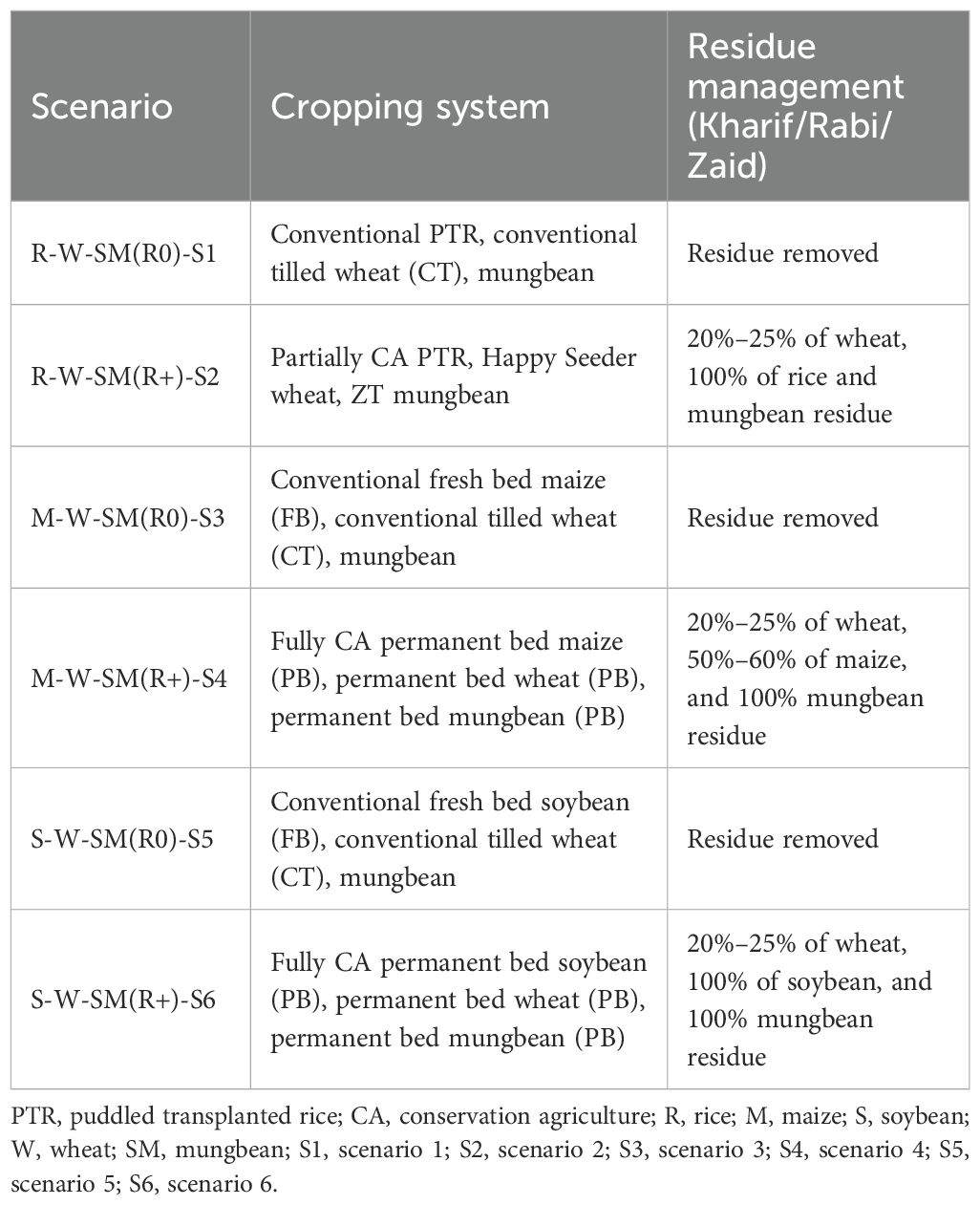
The field trials were established in a randomized complete block design (RCBD) with four replications of each of the six cropping system treatments, which varied in tillage intensity, cropping system, and residue management. Each experimental plot measured 18 m × 10.5 m (Table 1). The study was conducted over two cropping years from 2019–2020 to 2020–2021.
2.3 Crop residue management
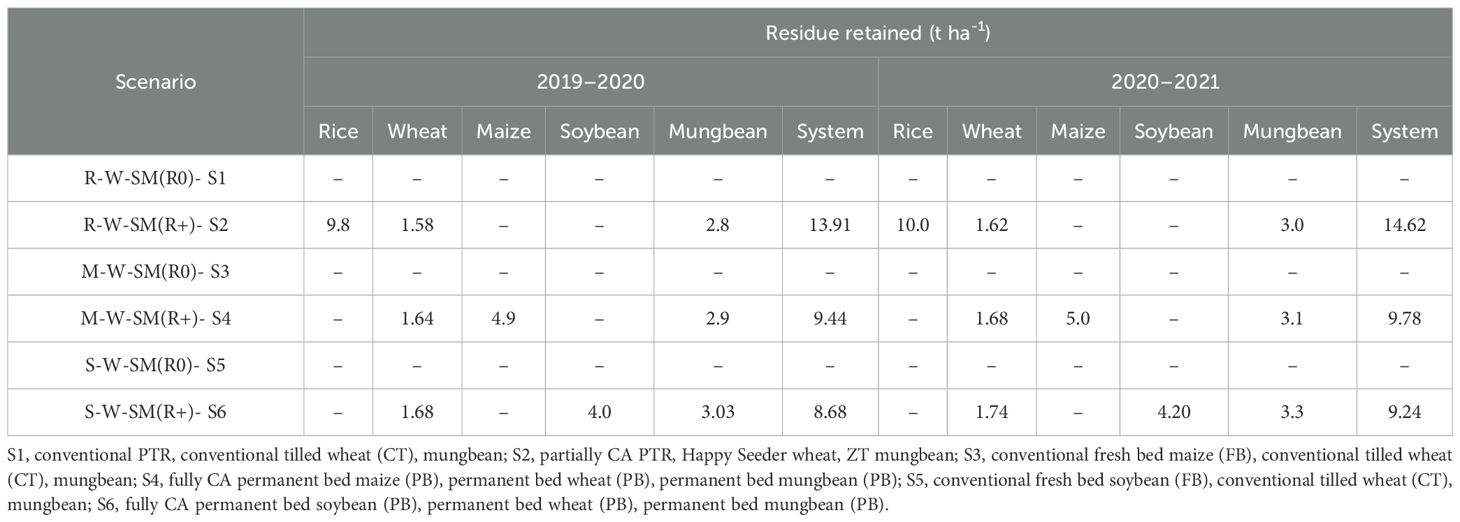
Crop residues of rice, maize, soybean, wheat, and mungbean were managed differently based on the treatments listed in Table 2. In treatments R-W-SM (R0)- S1, M-W-SM (R0)- S3, and S-W-SM (R0)- S5, the crop residues were removed, while in R-W-SM (R+)- S2, M-W-SM (R+)- S4, and S-W-SM (R+)- S6 the residues were retained. Harvesting operations for all crops in the experimental plots were conducted using a combine harvester integrated with the advanced Super SMS (straw management system) (Figure 2). This innovative technology incorporates a chopper and spreader, working in tandem to finely chop the straw and ensure its uniform distribution over a wider area, effectively acting as mulch. Harvesting was carried out at varying clearances from the ground level: 30–40 cm for rice, 10–15 cm for wheat, 125 cm for maize, 15–20 cm for soybean, and 20–25 cm for mungbean in treatments S2, S4, and S6. As per treatments, the crop residue load was calculated and mentioned in Table 2.
2.4 Crop management practices
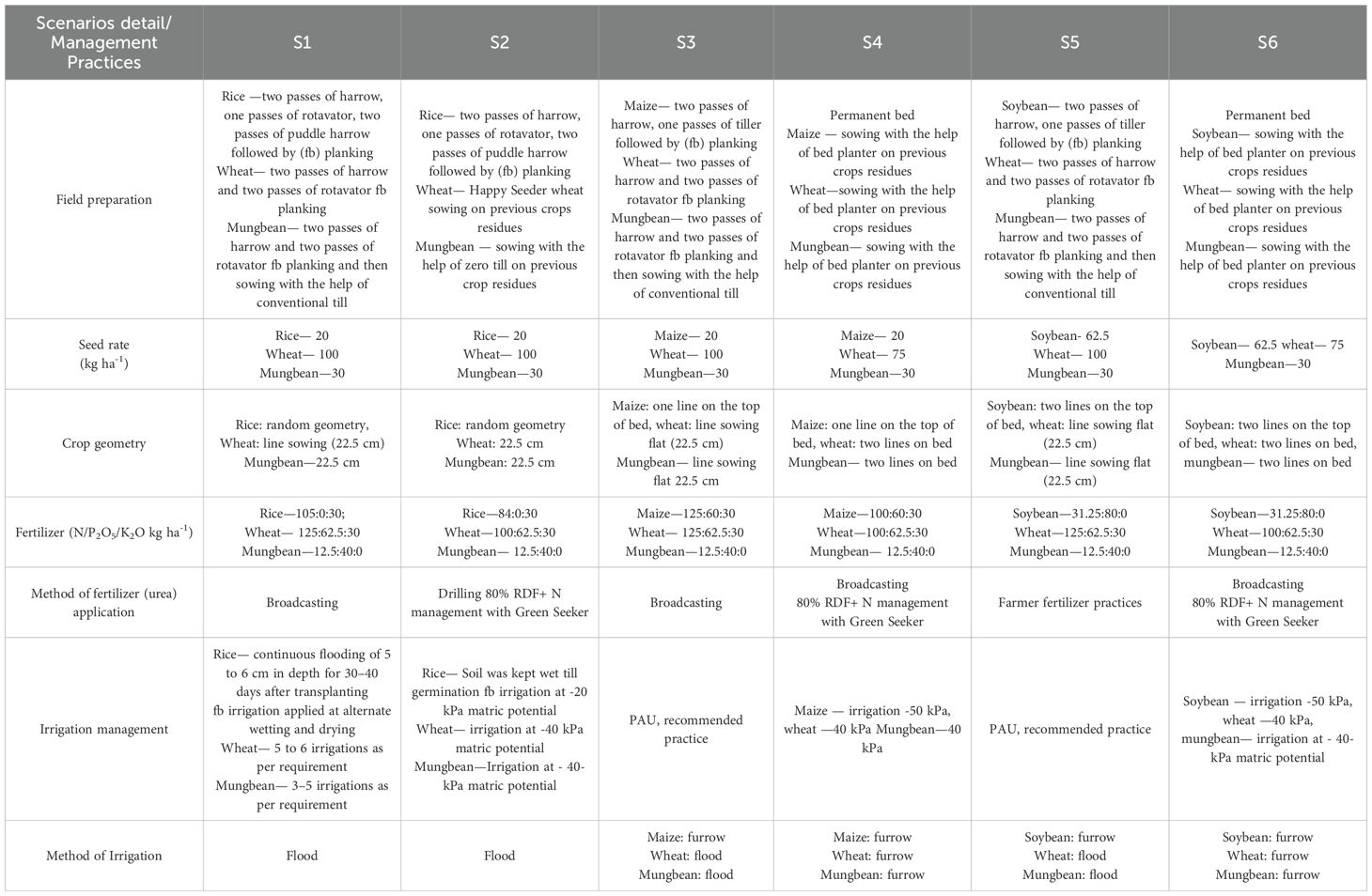
S1: Intensive tillage for rice–wheat–mung rotation with high NPK rates (105–125 N kg/ha), broadcast application, continuous flooding for rice, and flood irrigation for other crops. S2: Rice under intensive tillage, wheat with Happy Seeder, mung zero-tillage on residues. Reduced NPK (84–100 N kg/ha) with Green Seeker precision management and irrigation at -20 to -40 kPa matric potential. S3: Conventional tillage for maize–wheat–moong rotation with comprehensive NPK fertilization (125 N kg/ha for maize/wheat), broadcast application, and furrow/flood irrigation at -40 to -50 kPa. S4: Permanent bed system with residue retention for all crops. Reduced wheat seeding (75 kg/ha), lower NPK rates (100 N kg/ha), Green Seeker precision management, and furrow irrigation throughout, S5: Conventional tillage for soybean–wheat–moong rotation. Soybean at 62.5 kg/ha with reduced nitrogen (31.25 kg/ha) due to N-fixation, standard wheat fertilization, and mixed furrow/flood irrigation. S6: Permanent beds with full residue retention across soybean–wheat– mungbean rotation. Reduced wheat seeding (75 kg/ha), precision nitrogen management via Green Seeker, and consistent furrow irrigation for optimal water use efficiency (Table 3).
2.5 Soil analysis
Baseline soil samples were drawn from two soil depths of 0 to 7.5 and 7.5 to 15 cm using an auger of 5 cm in diameter prior to the initiation of the experiments. The soil bulk density was calculated as per standard protocol suggested by Chopra and Kanwar (1991). A double ring infiltrometer was used to measure the infiltration rate (Bouwer, 1986), which determines the rate at which water level recedes or the rate at which water is withdrawn from a supply source to maintain a constant head of water on the soil surface. The soil analysis for available NPK followed the standard method described in Jackson (1967) (Table 4). The determination of Fe, Mn, Zn, and Cu was carried out with DTPA (pH 7.3) extractant using an atomic absorption spectrophotometer (AAS Varian AAS-FS 240 model) (Arora, 2018).
2.5.1 Soil biological properties
Total microbial count was counted on nutrient agar media using serial dilution technique plate technique (Arora, 2018). The alkaline phosphatase activity (APA) of soil was assessed using a standard method (Arora, 2018) and expressed as micrograms of p-nitrophenol formed per gram of oven- dried soil. Dehydrogenase activity (DHA) was estimated with the rate of triphenyl formazon (TPF) formation from triphenyl tetrazolium chloride (TTC) following the method of Arora (2018).
The basal soil respiration was estimated of the potential microbial activity which is determined by calculating the linear rate of respiration after a 7- day incubation period. The results were expressed as µg CO2-C per gram of soil per day. The detailed procedure involved taking a plastic bottle and adding 20 g of soil sample along with 5 mL of water. In a separate vial, 10 ml mL of standard NaOH solution was placed and suspended inside the capped plastic bottle. The bottle was then incubated for 7 days at a temperature of 30°C. After the incubation period, the vials containing NaOH solution were removed and titrated with 0.5 mL of HCl using an indicator called phenolphthalein.
2.6 Statistical analysis
The data from both experimental years were analyzed using two-way analysis of variance (ANOVA) in Statistical Analysis Software v9.4 (SAS Institute Inc. SAS/STAT® 9.4., 2013). Treatment effects on soil properties were evaluated through biplot and loading plot analyses using principal component analysis (PCA) with OriginPro software. A Pearson correlation matrix was constructed to assess the relationships between the measured soil variables. Tukey’s HSD test was used to compare the treatment means at 5% level of significance.
3 Results
3.1 Bulk density and infiltration rate
Bulk density (BD) was not significantly influenced by CA scenarios at 0–7.5 and 7.5–15 cm depth (Figure 1). In general, the BD of the upper 0–7.5 cm layer was lesser compared with the lower layer of soil (7.5–15 cm).
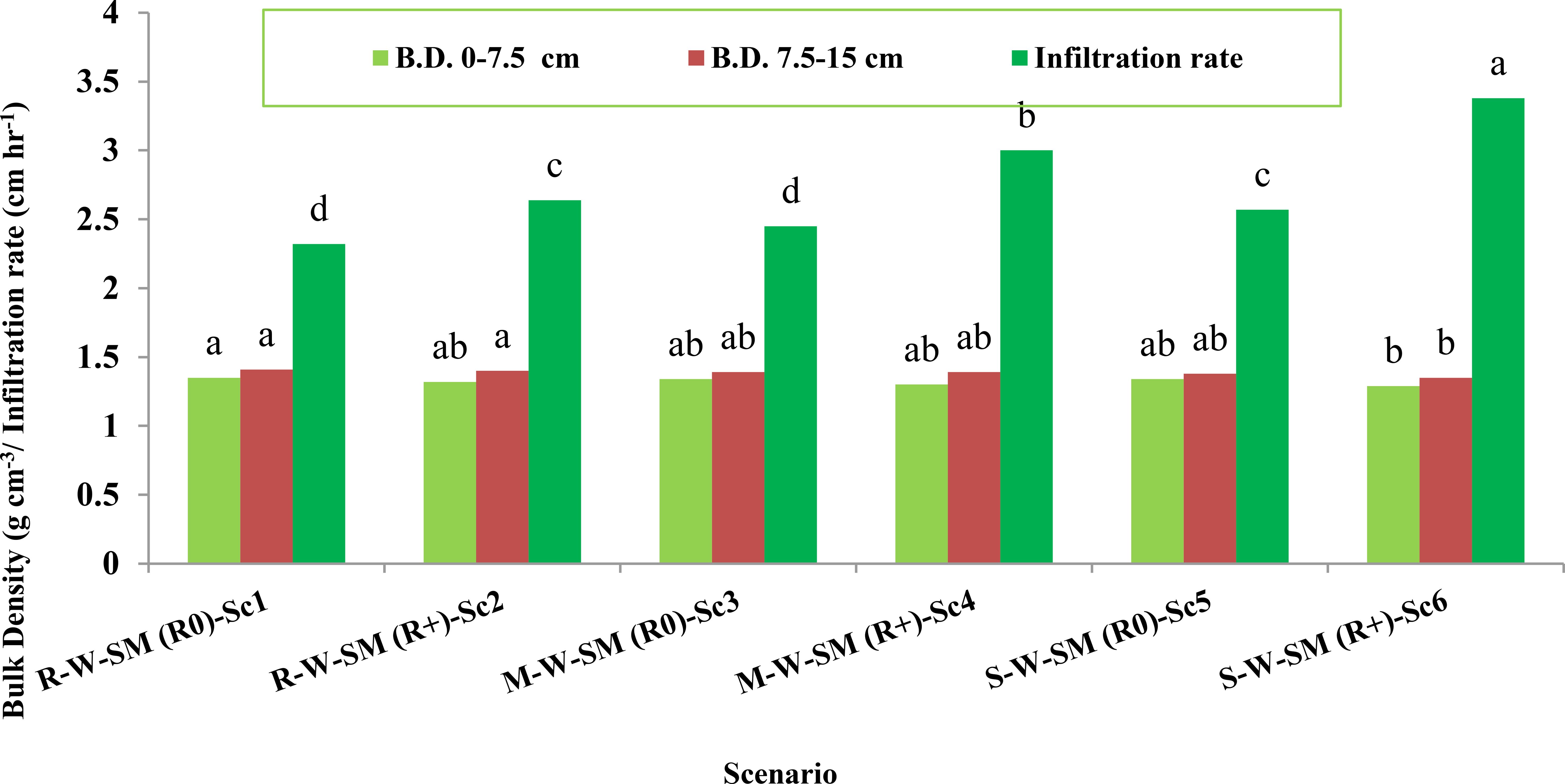
Figure 1. Effect of conservation agriculture-based cropping systems on the bulk density and infiltration rate of soil after 2 years. Similar letters with in a column indicate a non-significant difference at 0.05 level of probability using Tukey’s HSD test. S1, conventional PTR, conventional tilled wheat (CT), mungbean; S2, partially CA PTR, Happy Seeder wheat, ZT mungbean; S3, conventional fresh bed maize (FB), conventional tilled wheat (CT), mungbean; S4, fully CA permanent bed maize (PB), permanent bed wheat (PB), permanent bed mungbean (PB); S5,conventional fresh bed soybean (FB), conventional tilled wheat (CT), mungbean; S6, fully CA permanent bed soybean (PB), permanent bed wheat (PB), permanent bed mungbean (PB).
Puddled transplanted rice (PTR) recorded a higher BD compared with conservation wheat and maize. Scenarios S2, S4, and S6 recorded a lower BD by 2.22%, 3.70%, and 4.44%, respectively, in the 0–7. 5-cm soil layer and 0.70%, 1.41%, and 4.25% in the lower layer at 7.5–15-cm soil depth, respectively, compared with S1 (1.35 and 1.41 g cm-3). The infiltration rate was significantly higher by 45.68% under S6 over the S1 treatment. Moreover, residue retention significantly influenced the infiltration rate over no residue applied under the respective crop establishment techniques. The highest infiltration rate was noted under S6 (3.38 cm h-1) followed by S4 and S2 (3.00 and 2.64 cm h-1), whereas the lowest infiltration rate was noted under S1 and S3 (2.32 and 2.45 cm h-1, respectively).

Figure 2. Aerial view of the research experiment captured with the help of a drone. The left image shows six treatments with four blocks: in the first block from left to right were the first two types of rice (conventional puddled transplanted rice and conservation puddled transplanted rice), the third and fourth were maize (fresh bed maize and permanent bed maize), and the fifth and six were soybean (fresh bed soybean and permanent bed soybean). A similar trend is shown for the other three blocks and second aerial view (right image).
3.2 Soil pH, electrical conductivity, and soil organic carbon
The CA-based practices did not significantly influence soil pH and electrical conductivity at 0–7.5 and 7.5–15 cm of soil depth (Table 5). The maximum soil organic carbon (SOC) was recorded under S6 (0.50% to 0.52%), followed by S4 and S2 (0.49% to 0.51% and 0.48% to 0.50%, respectively), whereas minimum organic carbon was recorded under S1 (0.38% to 0.40%) at 0–7. 5-cm depth in both years. The top layer was found to have the highest SOC value (0–7.5 cm); following that, as soil depth increased, the SOC content dropped significantly across all scenarios (Table 5). After completion of the experiment, SOC was significantly higher by 31.5%, 28.9%, and 26.3% under S6, S4, and S2 compared with S1 (0.38%) at the upper most layers (0–7.5 cm) in the first year. Similarly, SOC was significantly higher by 30.0%, 27.5%, and 25.0% at 0–7. 5-cm depth under S6, S4, and S2 than S1 in the second year, but SOC remained unchanged at a – lower depth (7.5–15 cm) compared with S1.
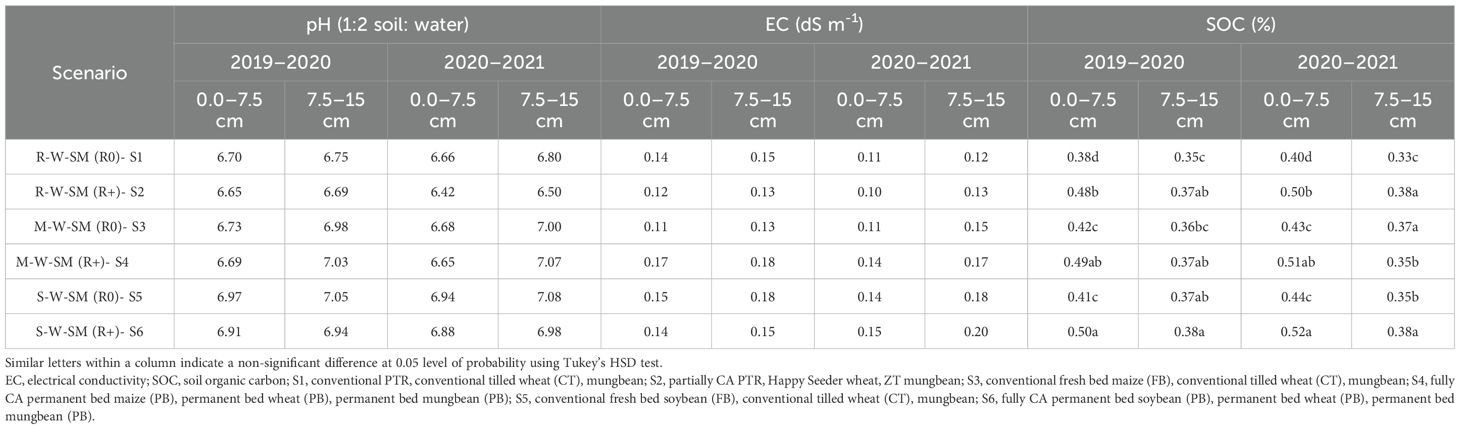
Table 5. Effect of conservation agriculture-based cropping systems on the chemical properties of soil after 2 years.
3.3 Available soil nitrogen, phosphorus, and potassium
The data depicted in Table 6 show that enhanced CA-based management techniques have a big impact on soil nutrient availability, specifically primary nutrients. Throughout the cropping cycles, conservation agriculture (CA) practices resulted in higher levels of available primary nutrients in the soil. The higher available N, P, and K was recorded under S6 (259.9, 30.50, and 230.1 kg ha-1), followed by S4 (250.2, 28.4, and 221.4), whereas minimum available N, P, and K was recorded under S1 (199.8, 23.30, and 215.4 kg ha-1) at 0–7. 5-cm depth. S6 and S4 resulted in a higher availability of N, P, and K in comparison with the rest of the scenarios. After 2 years of the study, the farmer’s practice (S1, S3, and S5) resulted into statistically less available N (199.8 kg ha-1), available P (23.30 kg ha-1), and available K (215.4 kg ha-1) content in S1 at 0–7. 5-cm depth in comparison with the rest of the scenarios (Table 6). The available N, P, and K were statistically higher by 30.0%, 30.9%, and 6.82% under S6 over the S1 treatment. Moreover, residue retention statistically affected the available primary nutrient over no residue applied under the respective crop establishment techniques.
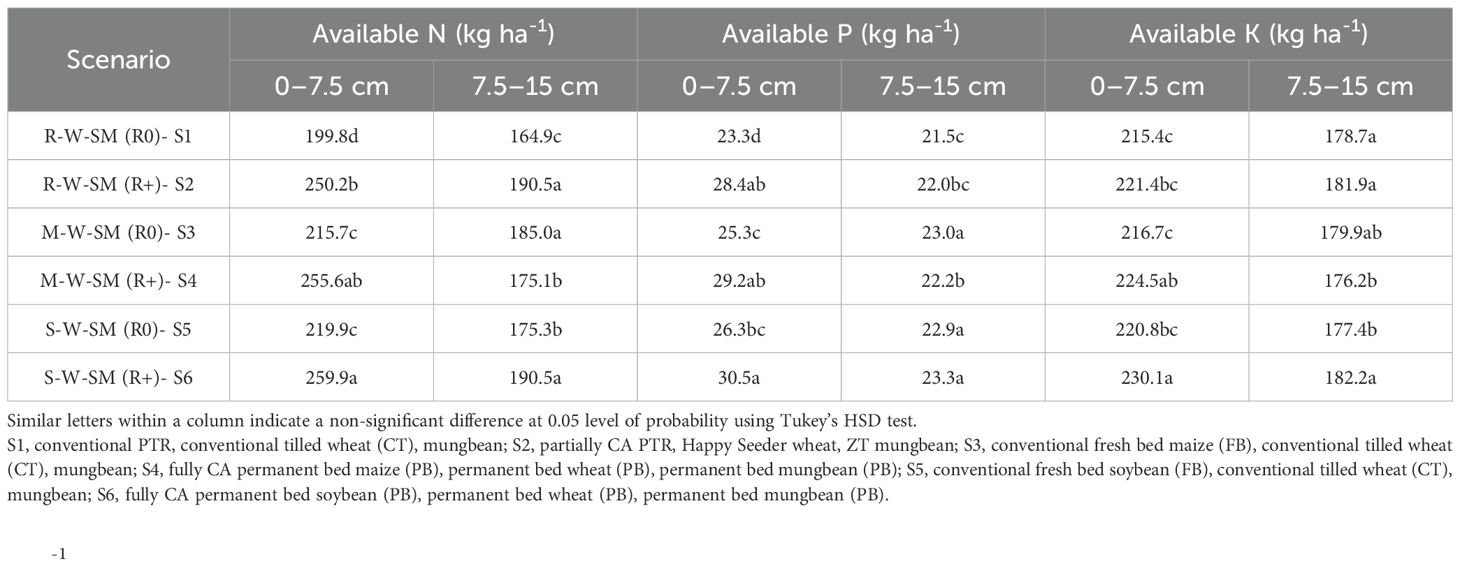
Table 6. Effect of conservation agriculture-based cropping systems on the available nitrogen, phosphorus, and potassium status of soil after 2 years.
3.4 DTPA soil micro-nutrients
The data shown in Table 7 reveal that improved management practices had a significant effect on the micro- nutrient content in the soil. Zn, Fe, Mn, and Cu with ranges of 1.90–2.10, 9.30–12.96, 8.40–9.10, and 0.43–0.70 mg/kg, respectively. The results show that S6 had the highest content of Zn, Fe, Mn, and Cu compared with the other treatments. S6 recorded the highest values of 2.10 mg kg-1 for Zn, 12.96 mg kg-1 for Fe, 9.10 mg kg-1 for Mn, and 0.70 mg kg-1 for Cu. This indicates that residue retention in S6 resulted in a higher micro-nutrient availability in the soil. Similarly, S4 also showed relatively higher micro-nutrient levels compared with S1, S2, S3, and S5. These results suggest that residue retention in the cropping systems contributes to improved soil micro-nutrient levels, particularly in the case of S6 and SS4.
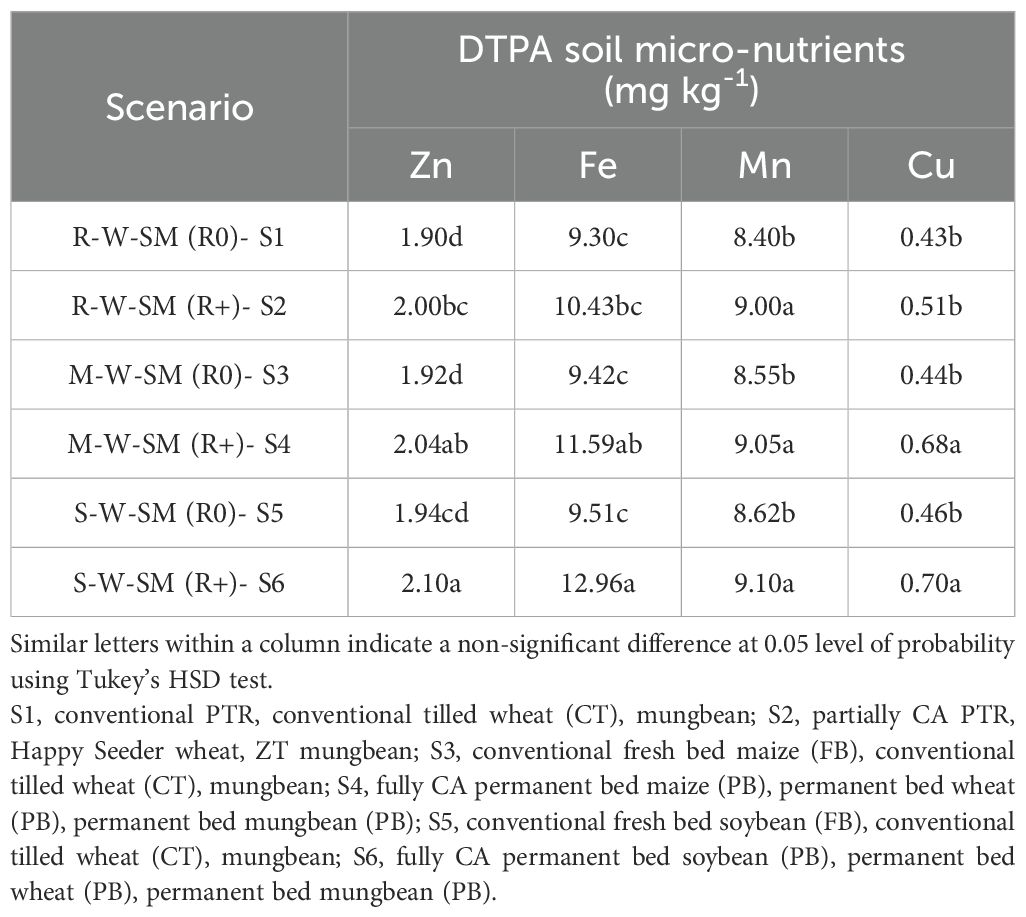
Table 7. Effect of conservation agriculture-based cropping systems on DTPA extractable soil micro-nutrients after 2 years.
3.5 Soil microbial properties
3.5.1 Soil enzymes
The data presented in Table 8 reveal that improved management practices had a significant effect on DHA, alkaline phosphatase enzyme (APA), total microbial population count, and BSR in soil.
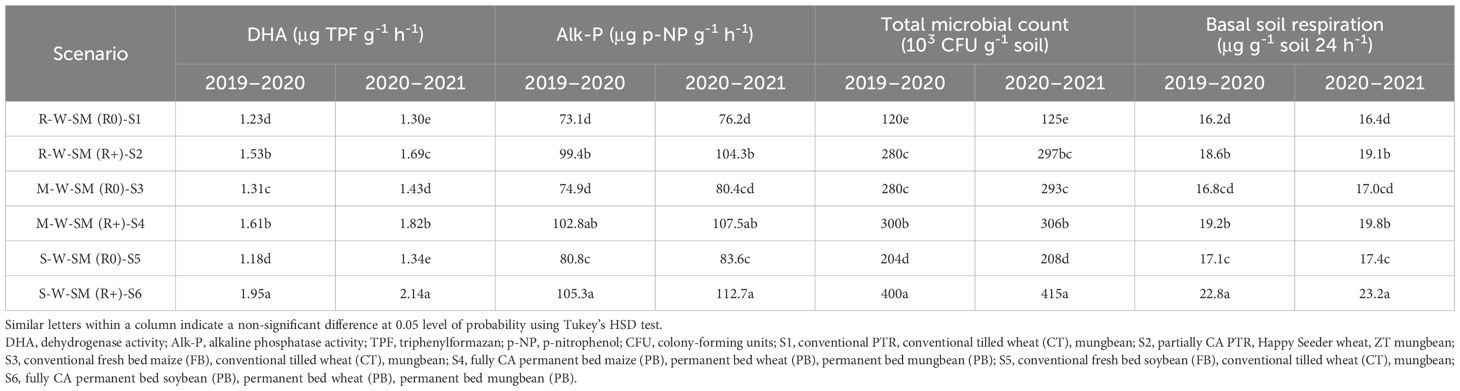
Table 8. Effect of conservation agriculture-based cropping systems on the soil microbiological properties of soil after 2 years.
The DHA content ranged from 1.23 to 2.14 μg TPF g−1 soil h−1; highest DHA was noted in S6 which was statistically higher with S4 and remained at par with S2. Compared to S1, DHA was 58.8% greater in S6 and 31.2% higher in S4. The APA values ranged between 73.1 and 112.7 μg p-NP g-1 h-1. The maximum APA was recorded under S6 (105.3 and 112.7 μg p-NP g-1 h-1), followed by S4 (102.8-107.5 μg p-NP g-1 h-1) and S2 (99.4-104.3 μg p-NP g-1 h-1), whereas minimum APA was recorded under S1 and S3 (73.1 and 74.9 μg p-NP g-1 h-1). The order of DHA and APA activity followed the pattern S6 > S4 > S2, with the lowest levels observed in S1.
3.5.2 Total microbial count
The total microbial population, including bacteria, fungi, and actinomycetes, varied across the different scenarios (Table 8). The higher total microbial populations were recorded in R-W-SM (R0)-S6 (4.00 to 4.15 × 105 CFU/g soil), followed by S4 and S2 (3.00 to 3.06 × 105 CFU/g soil and 2.80 to 2.97 × 105 CFU/g soil), whereas the lowest total microbial populations were recorded under S1 (1.20 to 1.25 × 105 CFU/g soil) in both years. During the first and second year, the total microbial population counts in S6 were 233.3% and 232% higher than those in Sc1, respectively. The increased microbial population may be attributed to the consistent food source provided by residue incorporation. The microbial count trend is likely similar in scenarios, subsequently resulting in the order S6 > S4 > S2 and >S1 (Table 8).
3.6 Basal soil respiration
The basal soil respiration varied from 16.2 to 23.2 μg g-1 soil 24 h-1. The maximum soil respiration was recorded under S6 (22.8 and 23.2 μg g-1 soil 24 h-1), followed by S4 and S2 (18.6 –19.1 μg g-1 soil 24 h-1), whereas minimum soil respiration was recorded under S1 and S3 (16.8 and 17.0 μg g-1 soil 24 h-1). The sequence of soil respiration of the different scenarios was S6 > S4 > S2, and the lowest was recorded in S1 (16.2 and 16.4 μg g-1 soil 24 h-1) and the highest was in S6 (Table 8).
3.7 Pearson’s correlation analysis and principal component analysis
Pearson’s correlation analysis was employed to assess the influence of CA-based cropping system on the properties of soil in reference with post-harvest soil fertility (Figure 3). A highly significant and positive relationship (R2 = 0.79) was found between soil pH and EC, whereas the EC of soil showed a high correlation with DTPA Cu (R2 = 0.64). Highly significant and maximum values of R2 were found for APA and DTPA Mn (0.99). Additionally, DTPA Zn, Fe, and Cu micro-nutrients were found to be more correlated with DHA activity in the soil.
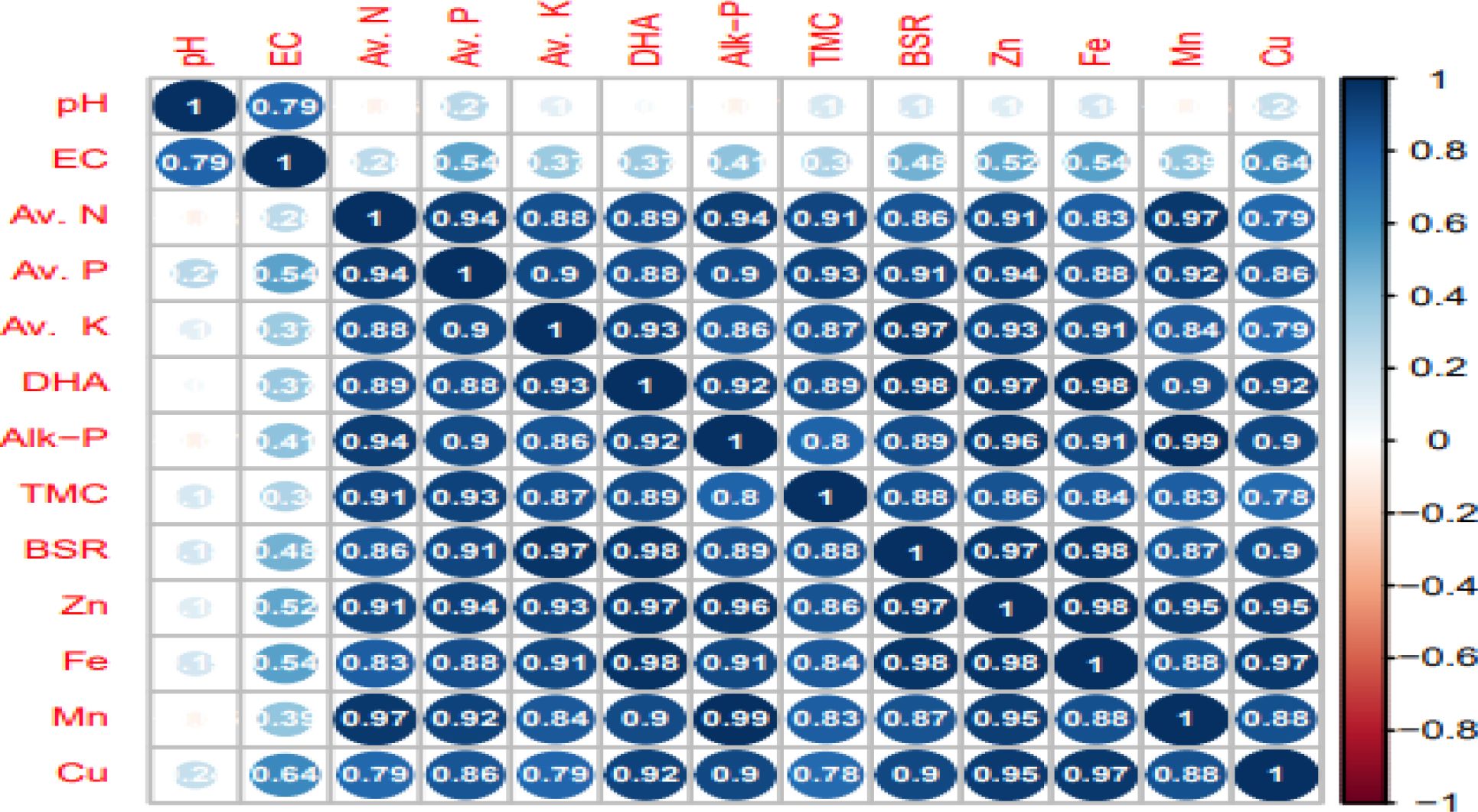
Figure 3. Correlation matrix between different soil properties under conservation agriculture practices. Av. N, available nitrogen; Av. K, available K; Av. P, available phosphorus; Cu, copper; Mn, manganese; Zn, zinc; Fe, iron; TMC, total microbial count; DHA, dehydrogenase activity; Alk. P, alkaline phosphate enzyme; BSR, basal soil respiration.
The principal component analysis (PCA) reduced the six experimental treatments into two independent components (eigenvalues > 1), which together accounted for 92.8% of the total variation among the variables. The first principal component (PC1) explained 80.0% of the variation, with the highest significant contribution from DTPA-extractable Zn. The second principal component (PC2) accounted for 12.8% of the variation and had the greatest loadings for soil pH. A third component (PC3), contributing 3.43% of the variation, showed significant negative loadings for total microbial biomass carbon. The 3D PCA graphs for macro-nutrients, micro-nutrients, and biological properties depicted the relative positions of observations corresponding to each treatment, highlighting the interactions between the two main components (Figure 4).
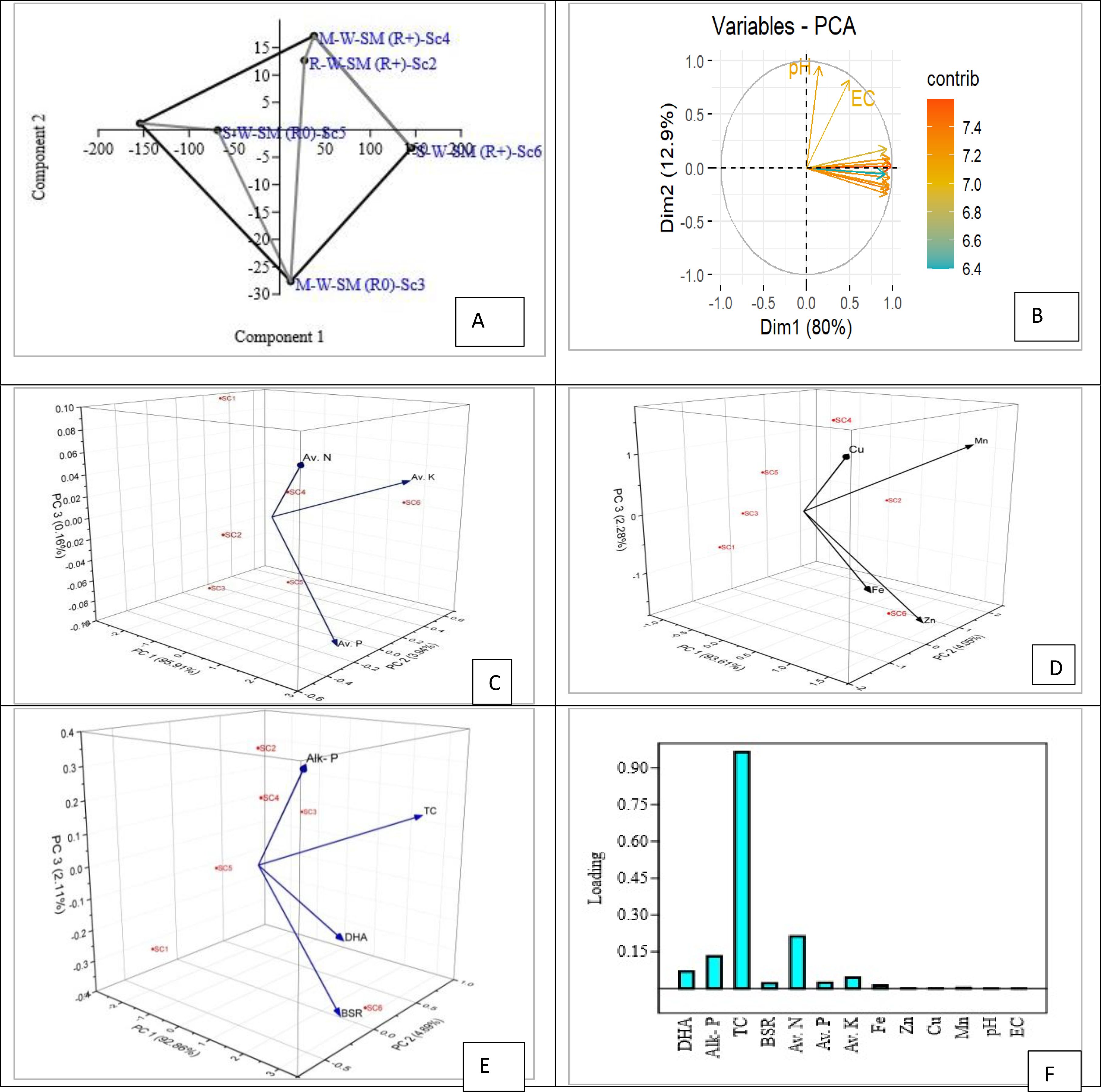
Figure 4. Principal component analysis of soil properties after 2years. (A, B) PCA of different treatments. (C) PCA of macro-nutrients (available N, P, and K). (D) PCA of micro-nutrients (Fe, Zn, Cu, and Mn). (E) PCA of biological properties. (F) Loading plot of PC1; Av. N, available nitrogen; Av. K, available K; Av. P, available phosphorus; Cu, copper; Mn, manganese; Zn, zinc; Fe, iron; TC, total count; DHA, dehydrogenase activity; Alk. P, alkaline phosphate enzyme; BSR, basal soil respiration.
4 Discussion
4.1 Bulk density
The bulk density decreased as a result of residue from crop retention with zero tillage and permanent bed; this effect was most apparent in the top soil layer. Among the scenarios studied, the combination of PTR followed by zero-till wheat, permanent bed maize, and permanent bed soybean (S2, S4, and S6) caused the bulk density of the soil to drop. Notably, the double legume-based cropping system with residue retention (S6) exhibited the lowest soil bulk density (Gogoi et al., 2018; Raj et al., 2023). This could be attributed to higher presence of organic C on the soil surface, resulting in improved soil aggregates and creation of more pore space, consequently leading to a lower bulk density in scenarios where crop residues were retained (Lynch et al., 2022; Musto et al., 2023; Yadvinder-Singh et al., 2022). As is frequently documented in the IGP region, the layering of various management approaches had a minor effect on soil bulk density, suggesting the presence of subsurface compaction in the rice–wheat–mungbean, maize–wheat–mungbean, and soybean–wheat–mungbean cropping systems (Chandra, 2011; Govaerts et al., 2006; Roldan et al., 2005).
4.2 Infiltration
Agronomic practices such as tillage, crop residue management, and changes in cropping systems can significantly impact soil infiltration rates (Dev et al., 2023; Indoria et al., 2020). Our study focused on intensifying cropping systems with mungbean under CA (S2, S4, and S6), which resulted in a substantial improvement in infiltration rates compared with CT. The higher infiltration observed in CA treatments can be attributed to three main factors. Firstly, the retention of crop residue protects the soil from the impact of raindrops, preventing displacement of surface aggregates and clogging of large pores (Fernández et al., 2017; Gómez-Paccard et al., 2015). Secondly, CA practices promote larger and more continuous pores through increased biological activity, creating root channels and macropore networks (de Moraes et al., 2016; Patra et al., 2023). Lastly, CA practices facilitate the accumulation of soil organic matter, contributing to macropore formation (Bhattacharyya et al., 2008; Gathala et al., 2011; Jat et al., 2013; Kahlon et al., 2013; Kumar et al., 2022). Similar results were found in northwest India, where CA-based systems like maize–wheat–mungbean and rice–wheat–mungbean demonstrated better infiltration rate and cumulative infiltration compared with the rice–wheat system under CT (Jat et al., 2018; Singh et al., 2014).
4.3 Soil organic carbon
After 2 years of field study assessment, SOC was significantly higher by 26%–31% under zero tillage and permanent bed CA treatments including S2, S4, and S6 because C tends to accumulate in less disturbed soils (Francaviglia et al., 2023; He et al., 2023). In Sc6, the SOC content increased by 31.6% due to retention of crop residue of zero cycle crops (wheat and mungbean), year-round soil cover with zero tillage, preventing direct sunlight exposure and oxidation of organic matter. Compared to rice residue retention (S2), the inclusion of double legume crops in S6 reduced the C/N ratio, leading to increased SOC content. In S2, the high rice residue load (14.2 t ha-1) and higher C/N ratio along with high silica and lignin content resulted in slower mineralization and subsequently low SOC (Choudhary et al., 2018; Das et al., 2013; Naorem et al., 2023; Qi et al., 2023; Balota et al., 2004). The decomposition of crop residues releases different organic compounds and increases microbial activity as binding agents, which cements the smaller particles into larger macro-aggregates (Sharma et al., 2025).
The incorporation of leguminous green manure has been reported to favor the net C buildup in soil, which is considered as an important indicator of C sequestration in soil (Six and Paustian, 2014; Sharma et al., 2019). The inclusion of legumes in rice–wheat systems releases root exudates, improves the soil biological activity, and thereby causes a higher C concentration in soil macro-aggregates (Sharma et al., 2021). Changes in SOC associated with different tillage practices can significantly affect the N content. Conventional tillage often leads to greater N losses due to repeated soil disturbance, enhanced leaching, and increased mineralization (Lal, 1997; Cui et al., 2023). The increase in SOC in the absence of tillage might be due to the deep penetration of wheat roots and the reduced oxidation of in situ organic matter (Modak et al., 2020). Crop residue retention or assimilation improved the productivity of intensified irrigated agriculture systems and increased the organic C (Modak et al., 2020; Sarkar and Kar, 2006). Reduced tillage and residue retention slow down the pace at which soil organic matter (SOM) breaks down, which causes SOC to rise over time (Gwenzi et al., 2009). It has been revealed that labile C derived from crop residues is first incorporated into labile C pools and subsequently accumulates and becomes stable or recalcitrant C in soils. Furthermore, the rice residue mulch may have improved the soil structure by protecting SOM through aggregation against microbial degradation and the reduced rate of SOC decomposition (Diekow et al., 2005; Gong et al., 2009). Moreover, an increase in SOC fractions with CA-based cropping systems caused slower SOC decomposition compared with CT and residue removal because of the reduction in soil disturbance and protection within aggregates and changes in the soil microbial environment under various tillage practices (Salve et al., 2012; Chen et al., 2009). In addition a large amount of rice residue addition provided C source which, upon decomposition, ultimately became part of SOC. These findings reinforce that changes in soil organic carbon (SOC) following tillage are largely attributed to active carbon pools, owing to their high turnover and sensitivity to disturbance (Culman et al., 2010). Additionally, the significant increase in SOC under scenario S6 might be due to the increase in annual C input and variations in organic matter quality, thus modifying the liability of C to change to an oxidized form (Ladha et al., 2004, 2003).
4.4 Available soil nutrients
The fertility of the soil and nutrient preservation are enhanced by conservation agriculture techniques like permanent bed (S4 and S6) and zero tillage (Anil et al., 2022; Chaudhary et al., 2019; Parihar et al., 2018; Palm et al., 2014). Zero tillage systems (S2, S4, and S6) slow down soil organic matter mineralization, increase soil N reserves, and enhance microbial activity compared with conventional tillage (S1, S3, and S5) (Acosta-Martínez et al., 2004; Thapa et al., 2023; Pisante et al., 2015). Double legume cropping systems (S6) contribute to a higher mineral N content due to the chelation of inorganic P and increased SOM (Jangir et al., 2021; Kumar et al., 2023; Mutuku et al., 2020). The higher N and phosphorus (P) availability is typically observed in the surface layers of soil under zero and minimum tillage systems than CT. The accumulation of available P is primarily due to its limited mobility within the soil profile, as previously documented (Nze Memiaghe et al., 2022). Similarly, the increased availability of available K and available P under conservation and organic management practices may be attributed to the reduced fixation and enhanced solubilization of fixed forms. This is often facilitated by the presence of organic acids and the mineralization of added organic manures, as reported in earlier studies (Elayarajan et al., 2015; Meena et al., 2019; Mahanta and Rai, 2008 et al., 2010). Moreover, CA-based practices also enhance the available K content by chelating nutrients with organic matter, resulting in higher soil nutrient availability (Jat et al., 2021; Lv et al., 2023).
4.5 DTPA soil micro-nutrients
In comparison with conventional tillage scenario (S1), the conservation agriculture scenario (S6) showed higher levels of DTPA Zn, Fe, Mn, and Cu by 10.5%, 39.5%, 8.3%, and 62.8%, respectively. The retention of crop residues on the soil surface in CA positively influences micro-nutrient availability, likely due to the mixing of previous crop residues (Mhlanga et al., 2022; Yadav et al., 2022), which increases the presence of labile C after the decomposition of residues from previous years. In order to preserve the availability of micro-nutrients in the soils of this area, conservation tillage in conjunction with organic nutrient management may be a viable strategy. Nevertheless, their phyto-availability in soils may be reduced in the future due to their removal from crop biomass with continued cropping. Thus, regular soil testing may aid in determining their depletion in soil so that suitable corrective action can be taken (Khoshgoftarmanesh et al., 2010). In a similar vein, Jayaraman et al. (2021) found that, in Central Indian vertisols, the available Fe content was comparatively greater under no-till scenarios than conventional tillage. Under conservation agriculture, the decomposition of fresh crop residues releases organic tissue-bound micro-nutrients and natural chelating agents like citric acid and humic acids, thereby enhancing micro-nutrient availability in the soil (Chaudhary et al., 2019). The lowest micro-nutrient levels were observed in the conventional tillage scenario (S1). Similar positive effects of conservation agriculture on micro-nutrient content have been reported by other researchers as well (Chaudhary et al., 2019; Das et al., 2018; Kharia et al., 2017; Yadav S. L. et al., 2022; Yadav M. et al., 2022).
4.6 Soil microbial properties
In the conservation agriculture scenario (S6), the total microbial population was 233% and 232% higher than CT (S1) in the first and second year, respectively, which is likely due to increased organic C addition and availability of food sources for microbial growth (Gupta et al., 2020; Yadav et al., 2023). Furthermore, in S6, a double legume crop was included in the cropping system, which resulted in the deposition of root exudates in soil, serving as a nutrient source for soil microbes (Hazra et al., 2020b). Conservation tillage techniques enhance fungal and bacterial populations, while the preservation of crop residues further stimulates microbial activity (Kumawat et al., 2022). The presence of cover crop residues as substrate enhances microbial diversity, C content, mineralization rate, soil respiration, and enzyme secretion (Choudhary et al., 2018; Dasila et al., 2023; Ghimire et al., 2014; Helgason et al., 2009). Additionally, minimum soil disturbance in conservation-based practices provides a suitable environment for microbes by moderating soil moisture and temperature than the CT practices (Choudhary et al., 2018; Saikia et al., 2019). An additional benefit for improved microbial growth was the retention of crop residue and the addition of organic manure and mineral fertilizers, which sped up nutrient mineralization and improved nutrient availability (Kiboi et al., 2021). The continued retention of crop waste above the soil surface may be caused by this impact since it increases the accessibility of labile carbon created by the breakdown of residues during the previous year (Piper, 2019). A high content of SOC is beneficial to the growth of microorganisms with active metabolic processes, which, in turn, leads to the accumulation of soil enzymes. In the present study, the increased availability of substrate (green manure and crop residues) and a favorable habitat for microbial communities seem to be responsible for higher enzyme activities. Furthermore, DHA and APA activities were linked to increased microbial activity, especially microbial biomass carbon and microbial biomass nitrogen through the release of organic compounds, contributing to a positive rhizosphere effect (Dasila et al., 2023; Roldán et al., 2005). In addition, DHA activity increased under CA-based cropping system, which supplied continuous substrates for microbial proliferation, along with improved SOC, which increased adsorption sites and supplied energy to micro-organisms throughout the decomposition process to improve enzyme activity (Sharma et al., 2025). Under CA-based practices, the increase in bacterial and fungal population was due to minimum soil disturbance, which plays a major role in the initial phases of decomposition of organic C compounds and degrades cellulolytic material through ligno-cellulytic enzyme activity (Sharma and Singh, 2023).
4.7 Principal component analysis and correlation matrix
The majority of the calculated variables were maximum with scenario S6, followed closely by S4 (Figure 4). The main influential variables for PC1 and PC2 were different available nitrogen, available K, available phosphorus, copper, manganese, zinc, iron, total microbial count, DHA enzyme, APA, and BSR with scenario S6, followed by S4 and S2 (Figure 4). The clustering of SOC with microbial activity under conservation agriculture (CA) creates synergistic effects that enhance both climate adaptation through improved water retention and soil structure stability and mitigation through increased carbon sequestration rates (Powlson et al., 2014). This implies that the continuous addition of C sources by previous crop residues raised more soil C pools and hydrolytic enzymatic activities, along with the activity of microbes, the accessibility of different communities of microbes in the soil, the availability of nutrients, and rhizodeposition. The majority of variables under study—microbial biomass, C respiration, and basal soil respiration—were more strongly conjugated in organically managed soils than in inorganically managed soils (Araújo et al., 2008).
5 Conclusion
The present study demonstrated the significant positive impact of conservation-diversified legume-based cropping system on nutrient availability and soil properties. The soybean–wheat –mungbean CA-based cropping system demonstrated significantly increased infiltration rate, SOC, DHA, and BSR by 45.6%, 31.5%, 58.8%, and 40.7%, respectively, compared with conventional practices. The results of the PCA indicated a robust association between SOC and biological properties, with scenario Sc6 surpassing the correlations observed in other cropping systems. CA, particularly soybean–wheat –mungbean cropping system with legume inclusion, has the potential to enhance soil properties and nutrient availability, contributing to improving long-term soil health and sustainability. The principal component analysis identified zinc, pH, and microbial biomass carbon as the most sensitive and influential variables to assess soil quality. Future studies should focus on the long-term monitoring of soil biological function across diverse agroecological zones to establish comprehensive sustainability of soil quality and crop production under climate-smart conservation agriculture systems.
6 Cautions and limitations
This study’s temporal scope may not capture the long-term cumulative effects of intensified conservation agriculture that typically manifest over decades. Site-specific conditions including soil type, climate, and topography may limit the transferability of results to other agroecological regions. The research assumes a consistent implementation of conservation practices, which may not reflect variable adoption rates in diverse farming systems. Seasonal weather variations during the experimental period could influence the outcomes and may not represent typical climatic patterns. Additionally, the focus on soil physico-chemical parameters may overlook broader ecosystem interactions and socioeconomic factors crucial for sustainable agriculture.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The allotment of land and, in this study, field research on plants or plant parts is conducted in accordance with the guidelines set forth by the Research Committee of PAU, Ludhiana.
Author contributions
AK: Conceptualization, Formal Analysis, Writing – original draft, Methodology, Validation, Writing – review & editing. KS: Supervision, Writing – original draft, Funding acquisition, Software, Formal Analysis, Writing – review & editing, Data curation, Resources, Methodology, Investigation, Visualization, Project administration, Conceptualization, Validation. SS: Writing – original draft, Supervision, Writing – review & editing, Formal Analysis, Software, Investigation, Data curation, Resources, Conceptualization, Methodology, Validation, Visualization. MY: Writing – original draft, Writing – review & editing, Data curation, Validation, Formal Analysis. GS: Writing – original draft, Formal Analysis, Validation, Data curation, Writing – review & editing. KD: Data curation, Validation, Formal Analysis, Writing – review & editing. KK: Writing – review & editing, Validation, Formal Analysis, Data curation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors acknowledge the valuable assistance provided by Punjab Agricultural University, located in Ludhiana, India, which made essential resources available for the project. Furthermore, the researchers thank the Department of Science and Technology (DST) in New Delhi, India, for providing them with access to vital facilities that allowed them to finish their research. The backing from these organizations was instrumental in the successful execution of the research work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acosta-Martínez V., Upchurch D. R., Schubert A. M., Porter D., and Wheeler T. (2004). Early impacts of cotton and peanut cropping systems on selected soil chemical, physical, microbiological and biochemical properties. Biol. Fertil. Soils 40, 44–54. doi: 10.1007/s00374-004-0745-3
Anil A. S., Sharma V. K., Jiménez-Ballesta R., Parihar C. M., Datta S. P., Barman M., et al. (2022). Impact of long-term conservation agriculture practices on phosphorus dynamics under maize-based cropping systems in a sub-tropical soil. Land 11, 1488. doi: 10.3390/land11091488
Araújo A. S. F., Santos V. B., and Monteiro R. T. R. (2008). Responses of soil microbial biomass and activity for practices of organic and conventional farming systems in Piauí State, Brazil. Eur. J. Soil Biol. 44, 225–3030. doi: 10.1016/j.ejsobi.2007.06.001
Arora N. K. (2018). Soil, Water and Plant Analysis Manual (Karnal: ICAR-Central Soil Salinity Research Institute).
Bakala H. S., Singh G., and Srivastava P. (2020). “Smart breeding for climate resilient agriculture,” in Plant breeding-current and future views.
Balota E. L., Kanashiro M., Colozzi Filho A., Andrade D. S., and Dick R. P. (2004). Soil enzyme activities under long-term tillage and crop rotation systems in subtropical agro-ecosystems. Braz. J. Microbiol. 35, 300–306. doi: 10.1590/S1517-83822004000300006
Beare M. H., Hendrix P. F., and Coleman D. C. (1994). Water-stable aggregates and organic matter fractions in conventional-and no-tillage soils. Soil Sci. Soc Am. J. 58, 777–786. doi: 10.2136/sssaj1994.03615995005800030020x
Bhatt R., Kukal S. S., Busari M. A., Arora S., and Yadav M. (2016). Sustainability issues on rice–wheat cropping system. ISWCR 4, 64–74. doi: 10.1016/j.iswcr.2015.12.001
Bhattacharyya R., Kundu S., Prakash V., and Gupta H. S. (2008). Sustainability under combined application of mineral and organic fertilizers in a rainfed soybean-wheat system of the Indian Himalayas. Eur. J. Agron. 28, 33–46. doi: 10.1016/j.eja.2007.04.006
Bhuiyan M. S. I., Rahman A., Loladze I., Das S., and Kim P. J. (2023). Subsurface fertilization boosts crop yields and lowers greenhouse gas emissions: A global meta-analysis. Sci. Total Environ. 876, 162712. doi: 10.1016/j.scitotenv.2023.162712, PMID: 36921862
Bouwer H. (1986). “Intake rate: cylinder infiltrometer,” in Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods, 825–844.
Chandra R. (2011). Effect of summer crops and their residue management on yield of succeeding wheat and soil properties. J. Ind. Soc. Soil Sci. 59, 37–42.
Chaudhary A., Meena M. C., Dwivedi B. S., Datta S. P., Parihar C. M., Dey A. B. I. R., et al. (2019). Effect of conservation agriculture on soil fertility in maize (Zea mays)-based systems. Ind. J. Agric. Sci. 89, 1654–1659. doi: 10.56093/ijas.v89i10.94599
Chen H., Hou R., Gong Y., Li H., Fan M., and Kuzyakov Y. (2009). Effects of 11 years of conservation tillage on soil organic matter fractions in wheat monoculture in Loess Plateau of China. Soil TillageRes. 106, 85–94. doi: 10.1016/j.still.2009.09.009
Chopra S. L. and Kanwar J. S. (1991). Analytical agriculture chemistry. Kalyani Publishers, 817096444X, 9788170964445.
Choudhary M., Datta A., Jat H. S., Yadav A. K., Gathala M. K., Sapkota T. B., et al. (2018). Changes in soil biology under conservation agriculture based sustainable intensification of cereal systems in Indo-Gangetic Plains. Geoderma 313, 193–204. doi: 10.1016/j.geoderma.2017.10.041
Congreves K. A., Hayes A., Verhallen E. A., and Van Eerd L. L. (2015). Long-term impact of tillage and crop rotation on soil health at four temperate agroecosystems. Soil Till Res. 152, 17–28. doi: 10.1016/j.still.2015.03.012
Cui H., Luo Y., Li C., Chang Y., Jin M., Li Y., et al. (2023). Improving soil fertility and wheat yield by tillage and nitrogen management in winter wheat–summer maize cropping system. Agronomy 13, 740. doi: 10.3390/agronomy13030740
Culman S. W., DuPont S. T., Glover J. D., Buckley D. H., Fick G. W., Ferris H., et al. (2010). Long-term impacts of high-input annual cropping and unfertilized perennial grass production on soil properties and belowground food webs in Kansas, USA. Agric. Ecosys. Environ. 137, 13–24. doi: 10.1016/j.agee.2009.11.008
Das T. K., Bhattacharyya R., Sharma A. R., Das S., Saad A. A., and Pathak H. (2013). Impacts of conservation agriculture on total soil organic carbon retention potential under an irrigated agro-ecosystem of the western Indo-Gangetic Plains. Eur. J. Agron. 51, 34–42. doi: 10.1016/j.eja.2013.07.003
Das T. K., Saharawat Y. S., Bhattacharyya R., Sudhishri S., Bandyopadhyay K. K., Sharma A. R., et al. (2018). Conservation agriculture effects on crop and water productivity, profitability and soil organic carbon accumulation under a maize-wheat cropping system in the North-western Indo-Gangetic Plains. Field Crops Res. 215, 222–231. doi: 10.1016/j.fcr.2017.10.021
Dasila H., Sah V. K., Jaggi V., Kumar A., Tewari L., Taj G., et al. (2023). Cold-tolerant phosphate-solubilizing Pseudomonas strains promote wheat growth and yield by improving soil phosphorous (P) nutrition status. Front. Microbiol. 14, 1135693. doi: 10.3389/fmicb.2023.1135693, PMID: 37025630
De Moraes M. T., Debiasi H., Carlesso R., Franchini J. C., da Silva V. R., and da Luz F. B. (2016). Soil physical quality on tillage and cropping systems after two decades in the subtropical region of Brazil. Soil Till Res. 155, 351–362. doi: 10.1016/j.still.2015.07.015
Dev P., Khandelwal S., Yadav S. C., Arya V., Mali H. R., and Yadav K. K. (2023). Conservation agriculture for sustainable agriculture. Int. J. Plant Soil Sci. 35, 11. doi: 10.9734/ijpss/2023/v35i52828
Dey A., Dwivedi B. S., Bhattacharyya R., Datta S. P., Meena M. C., Das T. K., et al. (2016). Conservation agriculture in a rice-wheat cropping system on an alluvial soil of north-western Indo-Gangetic plains: effect on soil carbon and nitrogen pools. J. Indian Soc. Soil Sci. 64, 246–254. doi: 10.5958/0974-0228.2016.00034.7
Diekow J., Mielniczuk J., Knicker H., Bayer C., and D.and Kogel-nabe I. (2005). Carbon and nitrogen stocks in physical fractions of a subtropical acrisol as influenced by long-term no-till cropping systems and N fertilization. Plant Soil 268, 319–328. doi: 10.1007/s11104-004-0330-4
Doran J. W. and Zeiss M. R. (2000). Soil health and sustainability: managing the biotic component of soil quality. Appl. Soil Ecol. 15, 3–11. doi: 10.1016/S0929-1393(00)00067-6
Elayarajan M., Sathya S., and Arulmozhiselvan K. (2015). Effect of inorganic fertilizers and organic manures on yield and nutrient uptake by maize hybrid under maize-sunflower cropping sequence in Typic Haplustalf. Karnataka J. Agric. Sci. 28, 29–33.
Fernández F. G., Sorensen B. A., and Villamil M. B. (2017). A comparison of soil properties after five years of no-till and strip-till. Agron. J. 107, 1339–1346.
Francaviglia R., Almagro M., and Vicente-Vicente J. L. (2023). Conservation agriculture and soil organic carbon: principles, processes, practices and policy options. Soil Syst. 7, 17. doi: 10.3390/soilsystems7010017
Gathala M. K., Ladha J. K., Kumar V., Saharawat Y. S., Kumar V., Sharma P. K., et al. (2011). Tillage and crop establishment affects sustainability of South Asian rice–wheat system. Agron. J. 103, 961–971. doi: 10.2134/agronj2010.0394
Ghimire R., Norton J. B., Stahl P. D., and Norton U. (2014). Soil microbial substrate properties and microbial community responses under irrigated organic and reduced-tillage crop and forage production systems. PloS One 9, e103901. doi: 10.1371/journal.pone.0103901, PMID: 25090235
Gogoi N., Baruah K. K., and Meena R. S. (2018). “Grain legumes: impact on soil health and agroecosystem,” in Legumes for soil health and sustainable management, 511–539.
Gómez-Paccard C., Hontoria C., Mariscal-Sancho I., Pérez J., León P., González P., et al. (2015). Soil–water relationships in the upper soil layer in a Mediterranean Palexerult as affected by no-tillage under excess water conditions–Influence on crop yield. Soil Till Resh 146, 303–312.
Gong W., Yan X. Y., Wang J. Y., Hu T. X., and Gong Y. B. (2009). Long-term manuring and fertilization effects on soil organic carbon pools under a wheat-maize cropping system in North China Plain. Plant Soil 314, 67–76. doi: 10.1007/s11104-008-9705-2
Govaerts B., Mezzalama M., Sayre K. D., Crossa J., Nicol J. M., and Deckers J. (2006). Long-term consequences of tillage, residue management, and crop rotation on maize/wheat root rot and nematode populations in subtropical highlands. Appl. Soil Ecol. 32, 305–315. doi: 10.1016/j.apsoil.2005.07.010
Gudi S., Saini D. K., Singh G., Halladakeri P., Kumar P., Shamshad M., et al. (2022). Unravelling consensus genomic regions associated with quality traits in wheat using meta-analysis of quantitative trait loci. Planta 255, 115. doi: 10.1007/s00425-022-03904-4, PMID: 35508739
Gupta V. V. S. R., Roper M. M., Thompson J., Pratley J. E., and Kirkegaard J. (2020). Harnessing the benefits of soil biology in conservation agriculture. Aust. J. Agric. Res., 237–253.
Gwenzi W., Gotosa J., Chakanetsa S., and Mutema Z. (2009). Effects of tillage systems on soil organic carbon dynamics, structural stability and crop yields in irrigated wheat (Triticum aestivum L.) - cotton (Gossypium hirsutum L.) rotation in semi-arid Zimbabwe. Nutr. Cycling Agroecosyst 83, 211–221. doi: 10.1007/s10705-008-9211-1
Halvorson A. D., Wienhold B. J., and Black A. L. (2002). Tillage, nitrogen, and cropping system effects on soil carbon sequestration. Soil Sci. Soc Am. J. 66, 906–912. doi: 10.2136/sssaj2002.9060
Hazra K. K., Nath C. P., Ghosh P. K., and Swain D. K. (2020a). “Inclusion of legumes in rice–wheat cropping system for enhancing carbon sequestration,” in Carbon management in tropical and sub-tropical terrestrial systems, 23–36.
Hazra K. K., Nath C. P., Ghosh P. K., and Swain D. K. (2020b). Inclusion of legumes in rice–wheat cropping system for enhancing carbon sequestration. Carbon Manage. Trop. Sub-Tropical Terrestrial Syst., 23–26.
He C., Chen Z., Qiu K. Y., Chen J. S., Bohoussou Y. N. D., Dang Y. P., et al. (2023). Effects of conservation agriculture on carbon mineralization: A global meta-analysis. SSoil Till Res. 229, 105685. doi: 10.1016/j.still.2023.105685
Helgason B. L., Walley F. L., and Germida J. J. (2009). Fungal and bacterial abundance in long-term no-till and intensive-till soils of the northern great plains. Soil Sci. Soc Am. J. 73, 120–127. doi: 10.2136/sssaj2007.0392
Indoria A. K., Sharma K. L., and Reddy K. S. (2020). “Hydraulic properties of soil under warming climate,” in Climate change and soil interactions (Elsevier), 473–508.
Jangir R., Maurya B., Yadav M., and Goswami S. P. (2021). Effect of potassium inoculants and black mica on rhizospheric soil properties of maize (Zea mays L.). Asian J. Microbiol. Biotechnol. Environ. Sci. 23, 372–380.
Jat H. S., Datta A., Sharma P. C., Kumar V., Yadav A. K., Choudhary M., et al. (2018). Assessing soil properties and nutrient availability under conservation agriculture practices in a reclaimed sodic soil in cereal-based systems of North-West India. Arch. Agron. Soil Sci. 64, 531–545. doi: 10.1080/03650340.2017.1359415, PMID: 30363929
Jat M. L., Gathala M. K., Saharawat Y. S., Tetarwal J. P., and Gupta R. (2013). Double no-till and permanent raised beds in maize–wheat rotation of north-western Indo-Gangetic plains of India: effects on crop yields, water productivity, profitability and soil physical properties. Field Crop Res. 149, 291–299. doi: 10.1016/j.fcr.2013.04.024
Jat R. A., Reddy K. K., Choudhary R. R., Rawal S., Thumber B., Misal N., et al. (2021). Effect of conservation agriculture practices on soil quality, productivity, and profitability of peanut-based system of Saurashtra, India. J. Agron. 113, 2102–2117. doi: 10.1002/agj2.20534
Jayaraman S., Sinha N. S., Mohanty M., Hati K. M., Chaudhary R. S., Shukla A. K., et al. (2021). Conservation tillage, residue management, and crop rotation effects on soil major and micro-nutrients in semi-arid Vertisols of India. J. Soil Sci. Plant Nutr. 21, 523–535. doi: 10.1007/s42729-020-00380-1
Kahlon M. S., Lal R., and Ann-Varughese M. (2013). Twenty two years of tillage and mulching impacts on soil physical characteristics and carbon sequestration in Central Ohio. Soil Till Res. 126, 15–158. doi: 10.1016/j.still.2012.08.001
Karlen D. L., Cambardella C. A., Kovar J. L., and Colvin T. S. (2013). Soil quality response to long-term tillage and crop rotation practices. Soil Till Res. 133, 54–64. doi: 10.1016/j.still.2013.05.013
Kharia S., Thind H., Sharma S., Sidhu H., Jat M., and Singh Y. (2017). Tillage and rice straw management affect soil enzyme activities and chemical properties after three years of conservation agriculture based rice-wheat system in north-western India. Int. J. Plant Soil Sci. 15, 1–13. doi: 10.9734/IJPSS/2017/33494
Khoshgoftarmanesh A. H., Schulin R., Chaney R. L., Daneshbakhsh B., and Afyuni M. (2010). Micronutrient-efficient genotypes for crop yield and nutritional quality in sustainable agriculture. A review. Agron. Sustain. Dev. 30, 83–107. doi: 10.1051/agro/2009017
Kiboi M. N., Ngetich F. K., Mucheru-Muna M. W., Diels J., and Mugendi D. N. (2021). Soil nutrients and crop yield response to conservation-effective management practices in the sub-humid highlands agro-ecologies of Kenya. Heliyon 7, e07156. doi: 10.1016/j.heliyon.2021.e07156, PMID: 34141923
Kumar A. and Saini K. S. (2022). Conservation agriculture effects on root traits, productivity and profitability of rice in Rice-Wheat-Summer moong cropping system. AMA-Agric. Mech. Asia Afr. Lat. Am. 53, 5245–5254.
Kumar A., Saini K. S., Dasila H., Kumar R., Devi K., Bisht Y. S., et al. (2023). Sustainable intensification of cropping systems under conservation agriculture practices: impact on yield, productivity and profitability of wheat. Sustainability 15, 7468. doi: 10.3390/su15097468
Kumar A., Saini K. S., Rolaniya L. K., Singh L. K., and Kaushik P. (2022). Root System Architecture and Symbiotic Parameters of Summer Mung Bean (Vigna radiata) under Different Conservation Agriculture Practices. Sustainability 14, 3901. doi: 10.3390/su14073901
Kumari K., Prasad J., Solanki I. S., and Chaudhary R. (2018). Long-term effect of crop residues incorporation on yield and soil physical properties under rice-wheat cropping system in calcareous soil. J. Soil Sci. Plant Nutr. 18, 27–40. doi: 10.4067/S0718-95162018005000103
Kumawat A., Vishwakarma A. K., Wanjari R. H., Sharma N. K., Yadav D., Kumar D., et al. (2022). Impact of levels of residue retention on soil properties under conservation agriculture in Vertisols of central India. Arch. Agron. Soil Sci. 68, 368–382. doi: 10.1080/03650340.2020.1836345
Ladha J. K., Dawas D., Pathak H., Padre A. T., Yadav R. L., and Singh B. (2003). How extensive are yield decline in long-term rice–wheat experiments in Asia. Field Crop Res. 81, 159–180. doi: 10.1016/S0378-4290(02)00219-8
Ladha J. K., Khind C. S., Khera T. S., and Bueno C. S. (2004). Effects of residue decomposition on productivity and soil fertility in rice–wheat rotation. Soil Sci. Soc Am. J. 68, 854–864. doi: 10.2136/sssaj2004.8540
Lal R. (1997). Long-term tillage and maize monoculture effects on a tropical alfisol in western Nigeria. II soil chemical properties. Soil Till Res. 42, 161–174. doi: 10.1016/S0167-1987(97)00007-X
Lv L., Gao Z., Liao K., Zhu Q., and Zhu J. (2023). Impact of conservation tillage on the distribution of soil nutrients with depth. Soil Till Res. 225, 105527. doi: 10.1016/j.still.2022.105527
Lynch J. P., Mooney S. J., Strock C. F., and Schneider H. M. (2022). Future roots for future soils. Plant Cell Environ. 45, 620–636. doi: 10.1111/pce.14213, PMID: 34725839
Mahanta D. and Rai R. K. (2008). Effects of sources of phosphorus and biofertilizers on productivity and profitability of soybean (Glycine max)-wheat (Triticum aestivum) system. Indian J. Agron. 53, 279–284. doi: 10.59797/ija.v53i4.4873
Meena B. P., Biswas A. K., Singh M., Chaudhary R. S., Singh A. B., Das H., et al. (2019). Long-term sustaining crop productivity and soil health in maize–chickpea system through integrated nutrient management practices in Vertisols of Central India. Field Crop Res. 232, 62–76. doi: 10.1016/j.fcr.2018.12.012
Meena S. N., Sharma S. K., Singh P., Jadon C. K., Jat M. L., Meena R. L., et al. (2022a). Crop-management practices infuence weed dynamics, yield and economics of soybean (Glycine max). Indian J. Agron. 67, 282–286. doi: 10.59797/ija.v67i3.30
Meena S. N., Sharma S. K., Singh P., Ram A., Meena B. P., Jain D., et al. (2023a). Tillage-based nutrient management practices for sustaining productivity and soil health in the soybean–wheat cropping system in Vertisols of the Indian semi-arid tropics. Front. Sustain. Food Syst. 7. doi: 10.3389/fsufs.2023.1234344
Meena S. N., Sharma S. K., Singh P., Ram A., Meena B. P., Prajapat K., et al. (2023b). Conservation and organic management practices infuenced wheat (Triticum aestivum) productivity, proftability and weed dynamics. Indian J. Agric. Sci. 93, 501–505. doi: 10.56093/ijas.v93i5.134868
Meena S. N., Sharma S. K., Singh P., Meena B. P., Jat M. L., Jadon C. K., et al. (2022b). Effect of various crop management practices on growth, yield and net return of soybean (Glycine max). Curr. Adv. Agric. Sci. 14, 34–38. doi: 10.5958/2394-4471.2022.00006.5
Mhlanga B., Pellegrino E., Thierfelder C., and Ercoli L. (2022). Conservation agriculture practices drive maize yield by regulating soil nutrient availability, arbuscular mycorrhizas, and plant nutrient uptake. Field Crop Res. 277, 108403. doi: 10.1016/j.fcr.2021.108403
Modak K., Biswas D. R., Ghosh A., Pramanik P., Das T. K., Das S., et al. (2020). Zero tillage and residue retention impact on soil aggregation and carbon stabilization within aggregates in subtropical India. Soil Till Res. 202, 104649. doi: 10.1016/j.still.2020.104649
Musto G. A., Swanepoel P. A., and Strauss J. A. (2023). Regenerative agriculture v. conservation agriculture: potential effects on soil quality, crop productivity and whole-farm economics in Mediterranean-climate regions. J. Agric. Sci., 1–11. doi: 10.1017/S0021859623000242
Mutuku E. A., Roobroeck D., Vanlauwe B., Boeckx P., and Cornelis W. M. (2020). Maize production under combined Conservation Agriculture and Integrated Soil Fertility Management in the sub-humid and semi-arid regions of Kenya. Field Crop Res. 254, 107833. doi: 10.1016/j.fcr.2020.107833
Naorem A., Jayaraman S., Sinha N. K., Mohanty M., Haudhary R. S., Hati K. M., et al. (2023). Eight-year impacts of conservation agriculture on soil quality, carbon storage, and carbon emission footprint. Soil Till Res. 232, 105748. doi: 10.1016/j.still.2023.105748
NzeMemiaghe J. D., Cambouris A. N., Ziadi N., and Karam A. (2022). Tillage management impacts on soil phosphorus variability under maize–soybean rotation in eastern Canada. Soil Syst. 6, 45. doi: 10.3390/soilsystems6020045
Olsen S. R. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate (Washington DC: US Department of Agriculture).
Osunbitan J. A., Oyedele D. J., and Adekalu K. O. (2005). Tillage effects on bulk density, hydraulic conductivity and strength of a loamy sand soil in southwestern Nigeria. Soil Till Res. 82, 57–64. doi: 10.1016/j.still.2004.05.007
Palm C., Blanco-Canqui H., DeClerck F., Gatere L., and Grace P. (2014). Conservation agriculture and ecosystem services: An overview. Agric. Ecosyst. Environ. 187, 87–105. doi: 10.1016/j.agee.2013.10.010
Pankaj, Sharma S., Singh V., and Angmo P. (2023). Changes in soil biological and chemical environment during wheat growth stages under nitrogen and straw management practices in rice-wheat system. J. Plant Nutr., 1–18. doi: 10.1080/01904167.2023.2209112
Parihar C. M., Yadav M. R., Singh A. K., Kumar B., Pooniya V., Pradhan S., et al. (2018). Long-term conservation agriculture and intensified cropping systems: Effects on growth, yield, water, and energy-use efficiency of maize in northwestern India. Pedosphere 28, 952–963. doi: 10.1016/S1002-0160(17)60468-5
Patra S., Parihar C. M., Mahala D. M., Singh D., Nayak H. S., Patra K., et al. (2023). Influence of long-term tillage and diversified cropping systems on hydro-physical properties in a sandy loam soil of North-Western India. Soil Till Res. 229, 105655. doi: 10.1016/j.still.2023.105655
Pisante M., Stagnari F., Acutis M., Bindi M., Brilli L., Di Stefano V., et al. (2015). Conservation agriculture and climate change. Conserv. Agric., 579–620. doi: 10.1007/978-3-319-11620-4_22
Powlson D. S., Stirling C. M., Jat M. L., Gerard B. G., Palm C. A., Sanchez P. A., et al. (2014). Limited potential of no-till agriculture for climate change mitigation. Nat. Climate Change 4, 678–683. doi: 10.1038/nclimate2292
Qi J. Y., Yao X. B., Lu J., He L. X., Cao J. L., Kan Z. R., et al. (2023). A 40% paddy surface soil organic carbon increase after 5-year no-tillage is linked with shifts in soil bacterial composition and functions. Sci. Total Environ. 859, 160206. doi: 10.1016/j.scitotenv.2022.160206, PMID: 36400297
Raj R., Das T. K., Chakraborty D., Bhattacharyya R., Babu S., Govindasamy P., et al. (2023). Soil physical environment and active carbon pool in rice–wheat system of South Asia: Impact of long-term conservation agriculture practices. Environ. Technol. Innovation 29, 102966. doi: 10.1016/j.eti.2022.102966
Richards L. A. (1954). Diagnosis and improvement of saline and alkali soils (US Government Printing Office).
Roldán A., Salinas-García J. R., Alguacil M. M., Diaz E., and Caravaca F. (2005). Soil enzyme activities suggest advantages of conservation tillage practices in sorghum cultivation under subtropical conditions. Geoderma 129, 178–185. doi: 10.1016/j.geoderma.2004.12.042
Saikia R., Sharma S., Thind H. S., and Sidhu H. S. (2019). Temporal changes in biochemical indicators of soil quality in response to tillage, crop residue and green manure management in a rice-wheat system. Ecol. Indic. 103, 383–394. doi: 10.1016/j.ecolind.2019.04.035
Salve P. R., Lohkare H., Gobre T., Bodhe G., Krupadam R. J., Ramteke D. S., et al. (2012). Characterization of chromophoric dissolved organic matter (CDOM) in rainwater using fluorescence spectrophotometry. Bull. Environ. Contamination Toxicol. 88, 215–218. doi: 10.1007/s00128-011-0424-7, PMID: 22037660
Sapkota T. B., Jat M. L., Aryal J. P., Jat R. K., and Khatri-Chhetri A. (2015). Climate change adaptation, greenhouse gas mitigation and economic profitability of conservation agriculture: Some examples from cereal systems of Indo-Gangetic Plains. J. Integr. Agric. 14, 1524–1533. doi: 10.1016/S2095-3119(15)61093-0
Sarkar R. and Kar S. (2006). Evaluation of management strategies for sustainable rice–wheat cropping system, using DSSAT seasonal analysis. J. Agric. Sci.e 144, 421–434. doi: 10.1017/S0021859606006447
SAS (2013). SAS Institute Inc. SAS/STAT® 9.4 Interface to North Carolina State University-SFA-T (Cary, NC, USA: SAS Institute Inc).
Sharma S., Chander G., and Verma T. S. (2014). Copper dynamics in a typic hapludalf under rice-wheat cropping system after twelve years of annual Lantana camara L. residue incorporation. J. Plant Nutr. 37, 1093–1103. doi: 10.1080/01904167.2014.888738
Sharma S., Thind H. S., Singh Y., Sidhu H., Jat M. L., and Parihar C. M. (2019). Effects of crop residue retention on soil carbon pools after 6 years of rice–wheat cropping system. Environ. Earth Sci. 78(10). doi: 10.1007/s12665-019-8305-
Sharma S., Saikia R., Thind H. S., Singh Y., and Jat M. L. (2021). Tillage, green manure and residue management accelerate soil carbon pools and hydrolytic enzymatic activities for conservation agriculture based rice-wheat systems. Commun. Soil Sci. Plant Anals 52, 470–486. doi: 10.1080/00103624.2020.1862147
Sharma S. and Dhaliwal S. S. (2021). Conservation agriculture based practices enhanced micronutrients transformation in earthworm cast soil under rice-wheat cropping system. Ecological Engineering 163, 106195.
Sharma S. and Singh P. (2023). Tillage intensity and straw retention impacts on soil organic carbon, phosphorus and biological pools in soil aggregates under rice-wheat cropping system in Punjab, north-western India. Eur. J. Agron. 149, 126913. doi: 10.1016/j.eja.2023.126913
Sharma S., Singh P., and Singh Y. (2025). Soil enzymatic activity, bacterial diversity and organic carbon pool in response to residue management and intensive tillage in rice-wheat cropping. J. Soil Sci. Plant Nutr. 25, 3858–3880. doi: 10.1007/s42729-025-02371-6
Sharma S., Vashisht B. B., Singh P., and Singh Y. (2022). Changes in soil aggregate-associated organic carbon, enzymatic activity, and biological pools under conservation agriculture based practices in rice-wheat system. Biomass Conversion Biorefinery 13, 1–18. doi: 10.1007/s13399-021-02144-y
Singh B. P., Cowie A. L., and Chan K. Y. (2011). Soil health and climate change (Berlin Heidelberg: Springer).
Singh G., Kaur N., Khanna R., Kaur R., Gudi S., Kaur R., et al. (2022). 2Gs and plant architecture: breaking grain yield ceiling through breeding approaches for next wave of revolution in rice (Oryza sativa L.). Crit. Rev. Biotechnol., 1–24. doi: 10.1016/j.fcr.2018.03.025, PMID: 36176065
Singh G., Kumar D., and Sharma P. (2015). Effect of organics, biofertilizers and crop residue application on soil microbial activity in rice-wheat and rice-wheat mungbean cropping systems in the Indo-Gangetic plains. Cogent Geoscience 1, 1. doi: 10.1080/23312041.2015.1085296
Singh A., Phogat V. K., Dahiya R., and Batra S. D. (2014). Impact of long-term zero till wheat on soil physical properties and wheat productivity under rice–wheat cropping system. Soil Till Res. 140, 9–105. doi: 10.1016/j.still.2014.03.002
Six J. and Paustian K. (2014). Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 68, A4–A9. doi: 10.1016/j.soilbio.2013.06.014
Srinivasarao C., Venkateswarlu B., Lal R., Singh A. K., and Kundu S. (2013). Sustainable management of soils of dryland ecosystems of India for enhancing agronomic productivity and sequestering carbon. Adv. Agron. 121, 253–329. doi: 10.1016/B978-0-12-407685-3.00005-0
Subbiah B. V. and Asija G. L. (1956). A rapid procedure for the estimation of available nitrogen in soils. Curr. Sci. 25, 259–260.
Thapa V. R., Ghimire R., Paye W. S., and VanLeeuwen D. (2023). Soil organic carbon and nitrogen responses to occasional tillage in a continuous no-tillage system. Soil Till Res. 227, 105619. doi: 10.1016/j.still.2022.105619
Tulu D., Gadissa S., Hundessa F., and Kebede E. (2023). Contribution of climate-smart forage and fodder production for sustainable livestock production and environment: lessons and challenges from Ethiopia. Adv. Agric. 2023, 8067776. doi: 10.1155/2023/8067776
Walkley A. and Black I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
Yadav S. L., Birla D., Inwati D. K., Yadav M., Yadav I. R., Makwana S. N., et al. (2023). Impact of agrochemicals on soil biota and ways to mitigate it: a review. Int. J. Environ. Clim. Change 13, 366–375. doi: 10.9734/ijecc/2023/v13i51779
Yadav S. L., Chaudhary D., Kumar D., Jadav N. J., and Yadav M. (2022). Influence of Nutrient Management Practices on DTPA Soil Micronutrients and Its Relation with Soil pH and Cation Exchange Capacity in Pearlmillet (Pennisetum glaucum L.) Cultivated Soils of Western India. Int. J. Environ. Clim. Change, 105–111. doi: 10.9734/ijecc/2022/v12i230636
Yadav M., Vashisht B. B., Jalota S. K., Kumar A., and Kumar D. (2022). “Sustainable Water Management Practices for Intensified Agriculture,” in Soil-Water, Agriculture, and Climate Change (Springer International Publishing), 131–161. doi: 10.1007/978-3-031-12059-6_8
Keywords: cropping system, residue retention, soil organic carbon, basal soil respiration, summer mungbean
Citation: Kumar A, Saini KS, Sharma S, Yadav M, Singh G, Devi K and Kumawat KC (2025) Evaluating soil physico-chemical properties and nutrient availability through intensified conservation agriculture-based cropping systems. Front. Agron. 7:1612792. doi: 10.3389/fagro.2025.1612792
Received: 16 April 2025; Accepted: 19 August 2025;
Published: 12 September 2025.
Edited by:
Stéphane Cordeau, INRAE, FranceReviewed by:
Revappa Mohan Kumar, University of Agricultural Sciences, Bangalore, IndiaSatya Narayan Meena, Agriculture University (Kota), India
Raghavendra Kj, Indian Council of Agricultural Research (ICAR), India
Copyright © 2025 Kumar, Saini, Sharma, Yadav, Singh, Devi and Kumawat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kailash Chand Kumawat, a2FpbGFzaC5rdW1hd2F0QHNoaWF0cy5lZHUuaW4=; Arun Kumar, YXJ1bnNhbW90YTE5OTRAZ21haWwuY29t
†ORCID: Kailash Chand Kumawat, orcid.org/0000-0003-0177-772X
 Arun Kumar
Arun Kumar Kulvir Singh Saini1
Kulvir Singh Saini1 Kailash Chand Kumawat
Kailash Chand Kumawat