- 1Oxitec Ltd, Abingdon, United Kingdom
- 2Oxitec do Brasil, São Paulo, Brazil
Introduction: The fall armyworm is one of the most globally significant agricultural pests, damaging corn, sorghum and other crops central to food production. It has developed resistance to several classes of chemical insecticides and, more recently, insect-resistant ‘Bt’ biotech corn varieties. As Bt varieties constitute the great majority of corn acreage in Brazil, proactive resistance management strategies are required to protect the durability of insecticidal efficacy of those cultivars. Previously, we reported on the development of a ‘self-limiting’ fall armyworm strain, called OX5382G, which – after release in the field – is engineered to suppress populations of fall armyworm and manage resistance to Bt crops in treated populations of this pest.
Methods: Here, we build on this work by carrying out contained studies to empirically assess the pest suppression and resistance management benefits of releasing OX5382G males. We also report on the first open field releases of the OX5382G self-limiting strain in Brazil. Following commercial biosafety approval of this strain by Brazilian government regulators, deployment-relevant OX5382G male performance was then assessed in larger, operational trials in Brazil.
Results: Pest suppression and resistance management benefits were demonstrated in contained studies. In the first open field releases, OX5382G males showed comparable performance with wild-type counterparts in terms of dispersal and mating ability. In the subsequent larger, farm-scale trials in Brazil, OX5382G mated effectively in the field and we demonstrated that relatively modest release rates can achieve over-flooding ratios expected to exert suppression and/or resistance management.
Discussion: All assessments to date suggest that self-limiting fall armyworm is a promising future tool for managing fall armyworm and extending the durability of Bt crops’ effectiveness against damaging lepidopteran pests.
Introduction
Crop pests and diseases cause an estimated 40% loss of agricultural food production (FAO, 2022), in large part caused by pest arthropods (Culliney, 2014). One of the most globally significant crop pests is the fall armyworm, Spodoptera frugiperda (J.E. Smith, 1797), the caterpillars of which feed on, and damage, corn, sorghum and many other economically important crops (Overton et al., 2021). Managing fall armyworm is challenging. It has developed resistance to several classes of chemical insecticides (Diez-Rodríguez and Omoto, 2001; Carvalho et al., 2013, 2018; Nascimento et al., 2016; Okuma et al., 2018). Over recent years, biotech corn varieties expressing insecticidal proteins – including Bacillus thuringiensis (Bt)-derived proteins – have proven to be a highly effective pest management approach for lepidopteran pests in many countries, including in Brazil, where the great majority of corn acreage is planted with Bt varieties (ISAAA, 2017). In Brazil, fall armyworm has undermined the durability of insecticidal corn traits by rapidly developing resistance to new varieties (Farias et al., 2014; Bernardi et al., 2015; Omoto et al., 2016). While a more recently developed protein, Vip3Aa20, has remained effective in Brazil, a Vip3Aa20 resistance allele has been isolated from the field (Bernardi et al., 2016; Amaral et al., 2020). The persistent erosion of new crop protection tools’ effectiveness by fall armyworm reinforces the need for proactive resistance management strategies and the development of alternative pest management approaches to enable a shift to more sustainable, long-term food production systems.
A new solution, the self-limiting fall armyworm, has been developed to manage fall armyworm and protect the efficacy of other management tools through proactive resistance management. The self-limiting fall armyworm is genetically engineered to exhibit tetracycline-repressible, female-specific mortality and a fluorescent protein marker – the DsRed2 protein – which emits visible fluorescence under appropriate excitation light and filters (Reavey et al., 2022). In the absence of dietary tetracycline (or suitable analogues), the self-limiting system – which comprises a ‘tetracycline off’ (tet-off) genetic system coupled to sex-alternate splicing components of the sex determination gene, doublesex (dsx) – prevents females from surviving to adulthood. This trait enables mass-production of male-only cohorts of self-limiting fall armyworm (and production of both sexes when sufficient tetracycline is provided in the larval feed). After release of self-limiting males, they find and mate with resident pest female counterparts. Female offspring from these matings cannot survive to the adult stage: with repeated releases of self-limiting males, the number of wild females in subsequent generations consequently declines and the pest population is reduced (Spinner et al., 2022). In addition, following the release of self-limiting males – which are susceptible to almost all Bt varieties and chemical insecticides – the survival of their male offspring leads to introgression of susceptibility genetics (at least for the majority of loci unlinked to the self-limiting gene insertion), leading to dilution of Bt and chemical insecticide resistance alleles in the pest population. Population modelling and empirical studies demonstrate that self-limiting Lepidoptera can provide highly effective pest suppression and management of resistance to insecticidal proteins (Alphey et al., 2009; Reavey et al., 2022). The species-specific effect of this strategy, coupled with a lack of toxicity to non-target organisms (for example, predators and parasitoids) of the proteins expressed from introduced genes (Nordin et al., 2013; Marubbi et al., 2017), is designed to deliver targeted pest management with a low ecological impact. The introduced female-specific trait is also self-limiting in the absence of a dietary antidote, declining to extinction in the generations after releases cease (Harvey-Samuel et al., 2014; Spinner et al., 2022).
We have previously reported on the development of a self-limiting strain of fall armyworm (OX5382G), which exhibited the target phenotype and performance parameters in the laboratory (Reavey et al., 2022). OX5382G showed highly penetrant female-specific mortality in the absence of tetracycline and males were sexually competitive against their wild-type comparators. The self-limiting nature of the strain’s transgene insertion was also confirmed in laboratory studies. Population modelling showed that deploying self-limiting fall armyworm males over corn-growing landscapes in Brazil has the potential to protect the durability of new Bt corn traits, and their consequent value to farmers, for many years beyond the current status quo (Reavey et al., 2022).
Here, we describe an empirical demonstration of the pest suppression and resistance management benefits of releasing OX5382G males, in contained studies, and the results of the first open field releases of OX5382G males in Brazil.
Materials and methods
Susceptibility of the background fall armyworm strain to biotech corn traits and chemical insecticides
The genetic background of the OX5382G strain was selected for its susceptibility to the most common biotech corn traits and insecticides. The OX5382G strain was created in 2018 as described previously (Reavey et al., 2022). The OX5382G transgene insertion was then introgressed into a Brazil-origin strain, by carrying out five generations of outcrosses to the Brazil-origin strain followed by making the strain homozygous for the transgene. The Brazil-origin strain has been held in culture in the laboratories of the agricultural research company, PROMIP, for more than 10 years. The colony exhibits a low level of resistance to Cry1F (far lower than most Brazilian field populations of fall armyworm), but otherwise exhibits very high susceptibility to biotech corn and insecticides. To validate this, the contract research organization, Pragas.com, measured the resistance profile of this colony, alongside two colonies field-collected in 2020, one from Bahia and one from Mato Grosso (in north-eastern and mid-western Brazil, respectively). These colonies were tested against the same panel of traits and insecticides before the farm-scale field trials were carried out in 2021.
Susceptibility of the background fall armyworm strain to biotech corn traits
Standard leaf disc assays were carried out against various traits expressed in commercial biotech corn varieties: Agrisure Viptera® (expressing Vip3Aa20), Powercore® (expressing Cry1A.105, Cry2Ab2 and Cry1F), VT PRO2® (expressing Cry1A.105 and Cry2Ab2) and Herculex® (expressing Cry1F), with 128 larvae assessed per corn variety per strain. Leaf discs were infested with larvae, incubated at 26°C, and assessed for damage after 5 days.
Susceptibility of the background fall armyworm strain to chemical insecticides
A panel of relevant insecticides representing the most commonly used modes of action were also assessed at the recommended LD99: Belt® (Bayer), flubendiamide, 2.84 µg i.a/cm2; Match® (Syngenta), lufenuron, 0.16 µg i.a/cm2; Karate® (Syngenta), lambda-cyhalothrin, 0.032 µg i.a/insect; Larvin® (Bayer), thiodicarb, 3.2 µg i.a/insect. For each colony a total of 288 larvae were assessed per chemistry, as follows: flubendiamide, diet overlay bioassay assessed at 5 days; lufenuron, diet overlay bioassay assessed at 5 days; lambda-cyhalothrin, topical bioassay assessed at 48 h; thiodicarb, topical bioassay assessed at 48 h.
Population suppression and resistance management through releases of self-limiting males
Modelling of population suppression and resistance management through releases of self-limiting males
A simplified version of a population model described previously (Reavey et al., 2022) and derived from models described by Alphey et al. (2007), was constructed in Excel (Microsoft Corp.) to inform the experimental design for an empirical proof-of-concept study of population suppression and resistance management effects through OX5382G male moth releases. This deterministic model simulated the release of OX5382G males into closed populations of wild-type fall armyworm, with one of the variables being the initial Bt trait resistance allele frequency in the treated population. The model assigned initial genotype frequencies for male and female insects using the defined resistance allele frequencies. In treated simulations of the model, it then simulated releases of OX5382G adult males into the population, re-calculating relative allele frequencies after these modelled releases. The proportion of the population with each genotype combination was then calculated for both males and females. The genotype frequencies of all possible gametes that the adult population can produce were then calculated, followed by the genotype frequencies of all potential zygotes that are subsequently produced by the combinations of sperm and egg. For each genotype, the frequency of this genotype was then multiplied by the number of offspring produced, and any fitness costs imposed by the self-limiting gene or Bt corn, where relevant, were applied. These insects formed the next generation of the model and the simulation repeated for four generations. The resistance status of the population, and survivorship on Bt corn, was calculated by applying defined fitness costs to the population, taking into account their genotypes.
The model assumes that: generations are discrete and do not overlap; OX5382G insects are released once per generation; in the absence of additional control measures (Bt corn or self-limiting releases) the population size remains at carrying capacity; fitness penalties imposed by the self-limiting gene or Bt corn are incurred during the larval stage; there is no fitness penalty associated with Bt resistance alleles; each insect with each genotype has an equal probability of mating; larvae do not move from the plant on which they were laid as eggs; it is a closed population with no immigration or emigration; the self-limiting gene is not linked to any resistance genes; and eggs have an equal chance of being male or female.
The following parameters were used in the model:
● The starting population contains 100 females and 100 males, and 200 offspring are collected per generation; i.e. the populations are capped at ~100 females;
● 100% of homozygote resistant larvae survive on Bt corn, 1% of homozygote susceptible larvae survive on Bt corn and 40% of heterozygote resistant larvae survive on Bt corn;
● There are no fitness costs to any genotype on conventional corn;
● Two scenarios were run in the model – 0.25 starting resistance allele frequency and 0.45 starting resistance allele frequency (i.e. initial survivorship was 21.25% and 40.05%, respectively);
● 100% of the crops were Bt corn in the Bt treatment groups;
● Males carrying the self-limiting gene all survive, females carrying a copy of the self-limiting gene do not survive;
● 100 OX5382G males are added to each generation and are similar in fitness to wild-type males.
Experimental demonstration of population suppression and resistance management through releases of self-limiting males
Experimental treatment groups
The effect of Bt corn and OX5382G releases, on the Bt resistance level (measured by survivorship on Cry1F corn) and population suppression (measured by the number of females in the population) was measured by conducting a multi-generational assessment of discrete populations (criteria outlined in Table 1). Cry1F was selected for this proof-of-concept study, but any insecticidal corn trait could have been used so long as resistance was detectable in the founding wild-type colony (i.e. the treated populations).
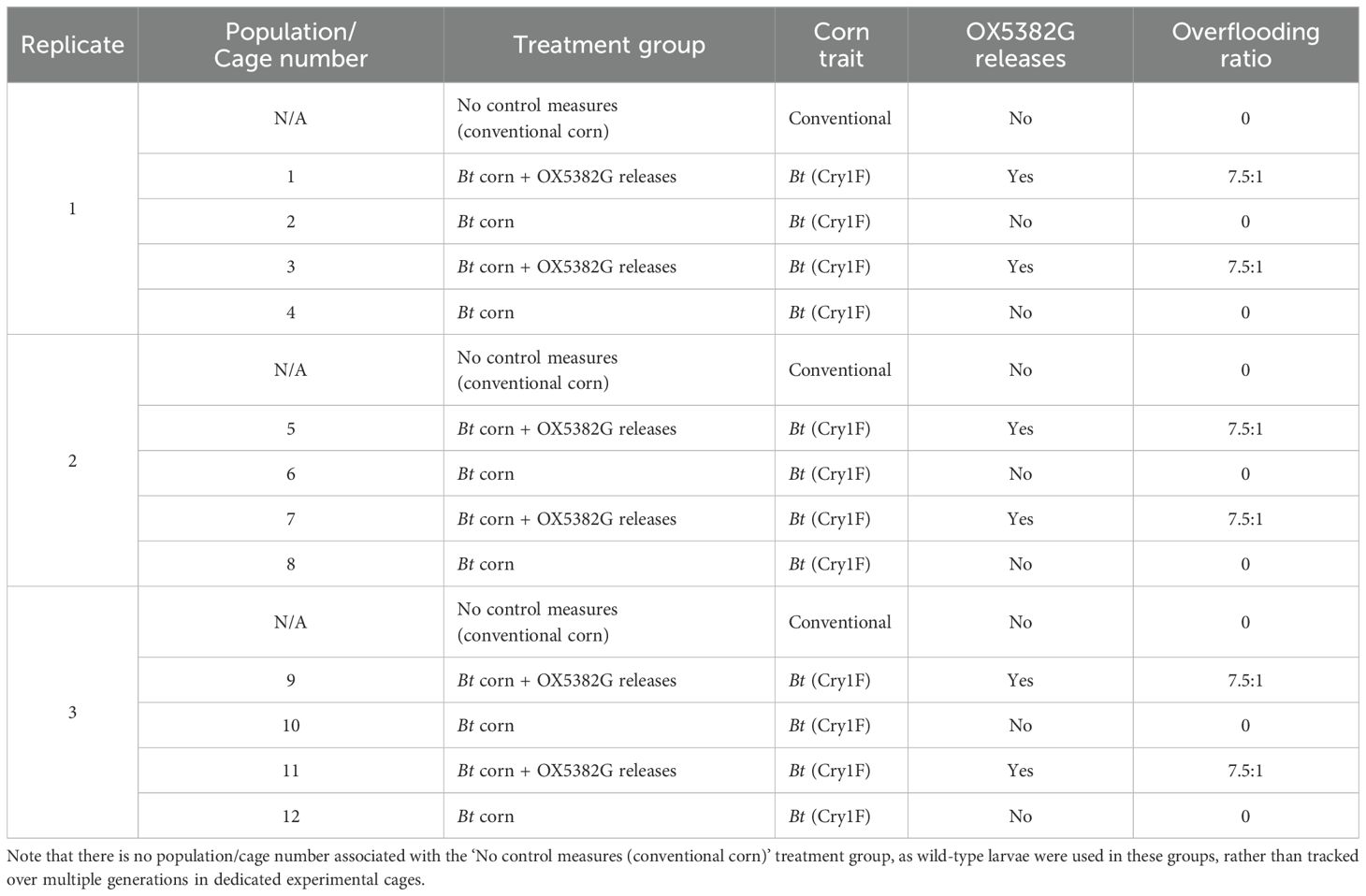
Table 1. Summary of the variables associated with each population (replicate, corn trait, presence/absence of OX5382G releases and the overflooding ratio) and the treatment group for statistical analysis.
Within each replicate there were two matched pairs – in total, six matched pairs – with each member of a given pair subjected to the same variables, with the only difference being whether OX5382G releases were applied, and this was incorporated into the statistical analysis. For practicality, the replicates were offset in time, with the experimental populations within each replicate running together at the same time under the same laboratory conditions. The founding generation was defined as ‘Generation 0’ and the experiment was carried out for four subsequent generations.
Each experimental population underwent the same laboratory rearing and testing per generation (Figure 1). From a given population, eggs were collected and, of the resulting larvae, some underwent Cry1F resistance assays, and others were transferred onto traited or untraited leaf discs, prior to transfer to artificial diet. Those larvae that survived to pupation were placed in cages to eclose to adulthood, to establish the next generation.
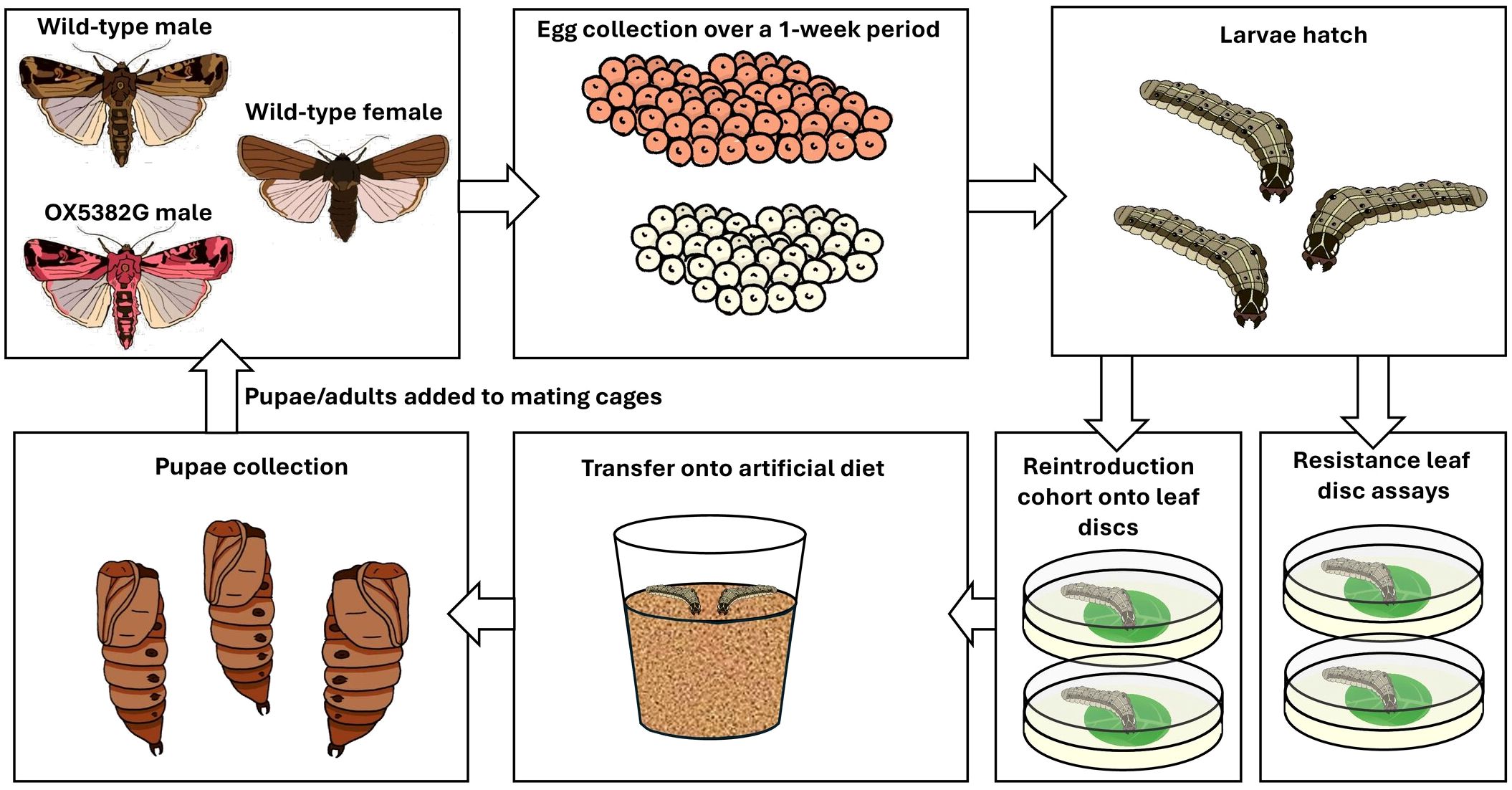
Figure 1. Schematic diagram showing the steps undertaken for each generation of the experimental populations.
Adult populations
The experiment was conducted using large cages (170 cm × 98 cm × 98 cm) for adult mating. Wild-type insects used to establish each caged population and subsequent reintroduction cohorts, as well as the associated OX5382G releases where required, were housed in smaller cages (20 cm height × 20 cm diameter) prior to release into the large cages. For each replicate, insects were separated in the small cages in accordance with their cage number, strain (wild-type or OX5382G) and sex.
To establish the starting populations, two wild-type colonies were used: one with a resistance allele frequency of ~0.015 (Colony A) and the other with a resistance allele frequency of ~0.94 (Colony B) (estimated by measuring survivorship on Cry1F corn and assuming a heterozygote survival of 40%). The overall ratio of founding insects from each colony throughout the trial ranged from ~1.2-2.3:1 (Colony A: Colony B), depending on insect availability and using a range rather than a set number so that the correct proportion of each colony could be used to obtain an appropriate starting resistance allele frequency (with equal numbers of males and females from each colony). 100 wild-type females and 100 wild-type males were used to establish each population. The measured starting resistance allele frequency in the populations was 0.11-0.62.
Large experimental cages were set up as soon as adult eclosion began in the small cages. All cages were set up to include sugar water sources ad libitum and ~5 corn plants to act as the primary oviposition surface. The insects introduced into the cages were a mixture of eclosed adults and pupae awaiting eclosion. For OX5382G-treated cages, 750 OX5382G males (mixture of eclosed males and pupae awaiting eclosion) were released per caged population. Experimental cohorts and the released OX5382G males were matched by their eclosion profile to achieve near-equivalence between competitor male types in their readiness to mate with the experimental females in the cages. Males of all strains were added to the cages first, followed by the addition of the wild-type females to complete cage initiation. The cages were maintained at 27°C (± 2°C).
Egg collections
Once oviposition commenced in each population, daily collections were made over a week (omitting Sunday collections). Eggs were collected from the corn plants, as well as the surface of the cages, and then transferred from each cage to the insectary, retaining each cage’s eggs as discrete collections. Each week’s collections were combined into three cohorts of eggs for each cage: (1) Saturday and Monday collection; (2) Tuesday and Wednesday collection; and (3) Thursday and Friday collection. For each cohort from each cage, the eggs were brushed into water, mixed and aliquoted onto filter paper before being placed in plastic boxes prior to hatching. After 1 week of egg collections were completed from a given cage, no further eggs were collected from that cage.
Larval rearing
Two larval cohorts (from discrete egg cohorts) were established from each caged population – one each from the first and second egg cohorts from each caged population (the third egg collection consisting of significantly fewer eggs)– to sample from as many mating events in each cage as possible. From each cohort, 400 larvae were used to generate the ‘reintroduction cohort’ and 256 larvae per population were used for the Cry1F resistance assay. For the reintroduction cohort, the 400 larvae were initially reared on corn leaf discs, with a single larva randomly infested per Cry1F leaf disc. The leaf disc plates were maintained at 25°C (± 2°C). At the same time, 128 larvae from either of the non-OX5382G release (wild-type) cages were placed on conventional leaf discs (again, one larva per leaf disc). This control group represented a population unexposed to Bt or OX5382G releases, enabling calibration against a baseline mortality rate.
After 5 days, the larvae forming the reintroduction cohort were transferred onto artificial diet in trays (one larva per cell). The number of larvae alive at the point of transfer to artificial diet was recorded. This was also completed for the conventional no-OX5382G-releases control.
To conduct the resistance assays for each population, 128 experimental larvae were infested on conventional leaf discs and 128 experimental larvae on Cry1F leaf discs (one larva per leaf disc). Alongside the experimental larvae in each replicate, control groups were set from Colony A (~0.015 resistance allele frequency) and Colony B (~0.94 resistance allele frequency). The resistance assays were carried out at 25°C (± 2°C). For the resistance assay plates, the survival rate was recorded 7 days post-infestation as a measure of resistance/susceptibility in the caged populations. Resistance levels were calculated by calibrating larval mortality on Cry1F corn to larval mortality on conventional corn.
Normalising the population size of the reintroduction cohort to account for natural mortality
All founding populations comprised 100 male and 100 female pupae. Theoretically, any changes in population size over subsequent generations would be driven by either the effect of the self-limiting gene or the effect of Bt. Practically, however, natural mortality rates vary for each cohort reared, even in controlled conditions. To normalise for the effect of mortality due to cohort rearing conditions, for the reintroduction cohorts double the eventual reintroduction number were set up (i.e. each with 400 larvae, rather than 200 larvae) and, where the control group (which was not exposed to Bt or OX5382G males) showed high mortality, the matched populations were then corrected to account for loss due to effects unrelated to the self-limiting gene or to Bt.
Pupae collection
Two pupae collections were completed from the reintroduction cohorts: (1) 2–3 days after the first pupation was observed and (2) 2–3 days after the first pupae collection. On the day of each collection, the pupae were scored for sex and the presence/absence of DsRed2 expression. The pupae from the conventional control group were collected in the same manner. Once the total pupation was recorded for each cohort, insects from the conventional control cohort were discarded.
Re-setting cages for the subsequent generation
For each experimental mating cage, the small eclosion cages for each sex and strain were again set up, prior to release. To mitigate the risk of population extinction and to simulate conditions in the wild, ‘immigrating pupae/moths’ were added conditionally if the number of experimental females fell below 15 females in any of the populations. ‘Immigrating’ females were added to return the female population to 15 individuals in total. The same number of ‘immigrating’ males were also added. If ‘immigrating pupae/moths’ were added to a cage, the same number of males and females would also be added to the paired cage from that replicate. These insects were reared from an intermated colony established at the start of the experiment from the two wild-type colonies, in the same ratio as that used to establish the experimental populations. The required small eclosion cages for the ‘immigrating pupae/moths’ were set up prior to release.
Once moths began eclosing in all groups, the experimental insects – and ‘immigrating’ insects where applicable – were released (eclosed moths and pupae awaiting eclosion) back into the cage from which they had been collected as eggs, ~4 weeks earlier. In OX5382G-treated cages, 750 OX5382G males were released (mixture of eclosed males and pupae awaiting eclosion). From this point onwards, this process was repeated for three further generations (four generations in total).
Statistical analysis
The effects of the different treatment groups on population size and resistance to Bt over four generations were tested using repeated-measures ANOVA. The effect of Bt corn and OX5382G releases and their interactions were considered with the data blocked by paired cages, and generation number included as a repeated measure. The data used for analysis did not include immigrating moths (either inclusion or exclusion of these moths is appropriate and generates the same outcome in terms of results). Cage populations that fell to extinction were excluded from the analysis (no measurement of resistance status was possible), along with their matched pair (cages 3, 4, 7 and 8), resulting in four matched pairs for comparison between no fall armyworm management measures (conventional corn), Bt corn and Bt corn plus OX5382G releases.
Regulated field trials in Brazil
Two seasons of field trials were carried out under an LPMA licence, issued by Brazilian government regulators (CTNBio Process 01250.062758/2018-90). The first releases were conducted in November 2019 (Season 1) – marking the first open-field release of a genetically engineered agricultural pest in Brazil – and the second commenced in January 2021 (Season 2). Both trials were undertaken at a field research station in Conchal, São Paulo state. The releases were carried out from the centre of a 15-acre corn field, planted for the purpose of the trial. In Season 1, the central plot was planted with 10 acres of Cry1F-traited corn, surrounded by a border of Vip3Aa20-traited corn and located within the designated “CQB area” (Biosafety Quality Certified, meaning the area permitted for research with genetically modified organisms) (Figure 2). In Season 2 (Figure 3), the entire 15-acre plot was planted with Agrisure Viptera 2® corn (Vip3Aa20 and Cry1Ab insecticidal proteins expressed in this variety). Insects for the trial were produced in Oxitec Ltd laboratory facilities in Campinas, São Paulo State. In both seasons, releases commenced on emergence of corn plants in the trial field.
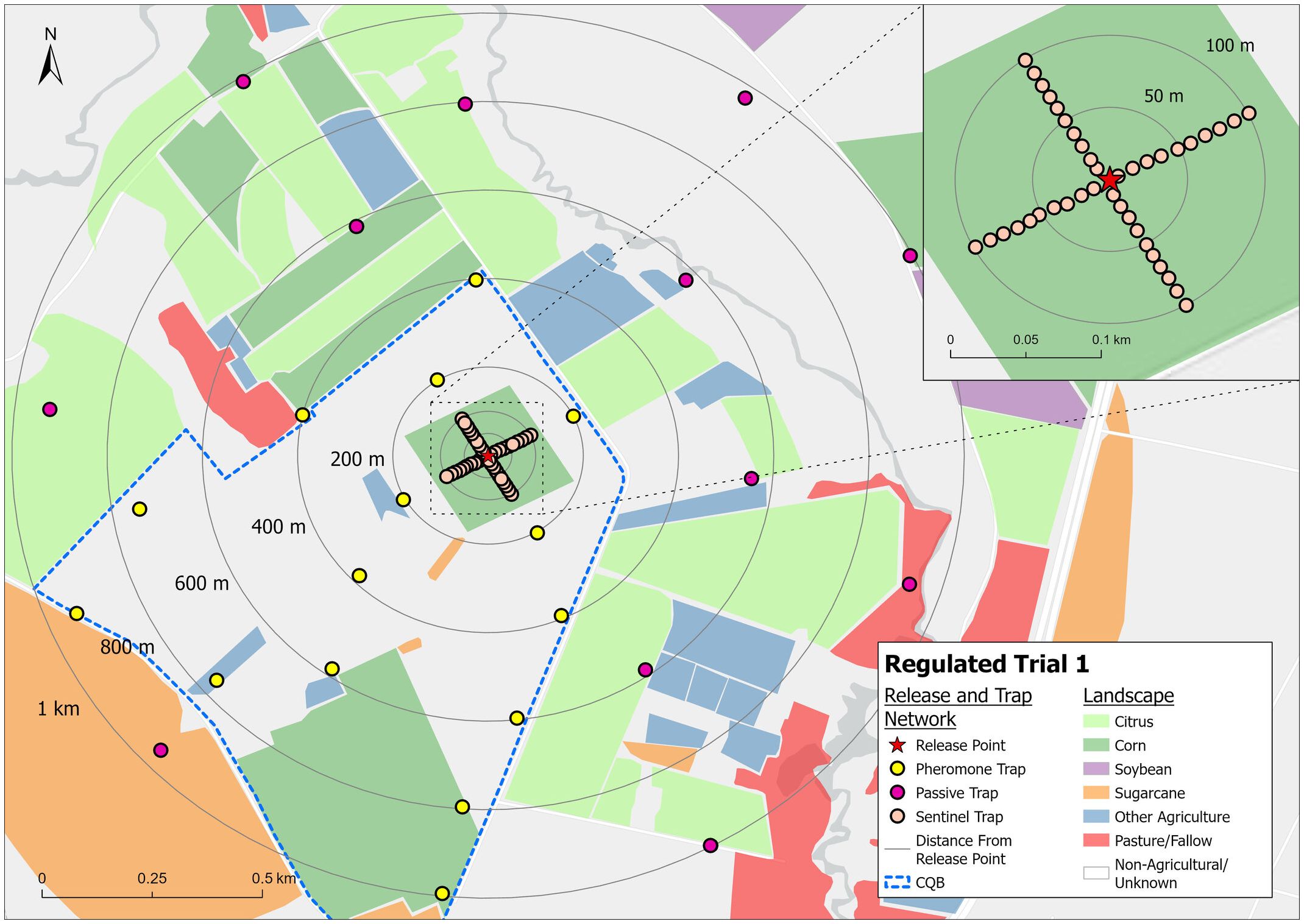
Figure 2. The 2019–2020 Season 1 trial area and trap layout. Within the CQB area (outlined in blue), pheromone traps were placed up to 1 km from the release point. Outside the CQB area, passive traps (containing no pheromone lure) were used as requested by the regulatory agency, CTNBio.
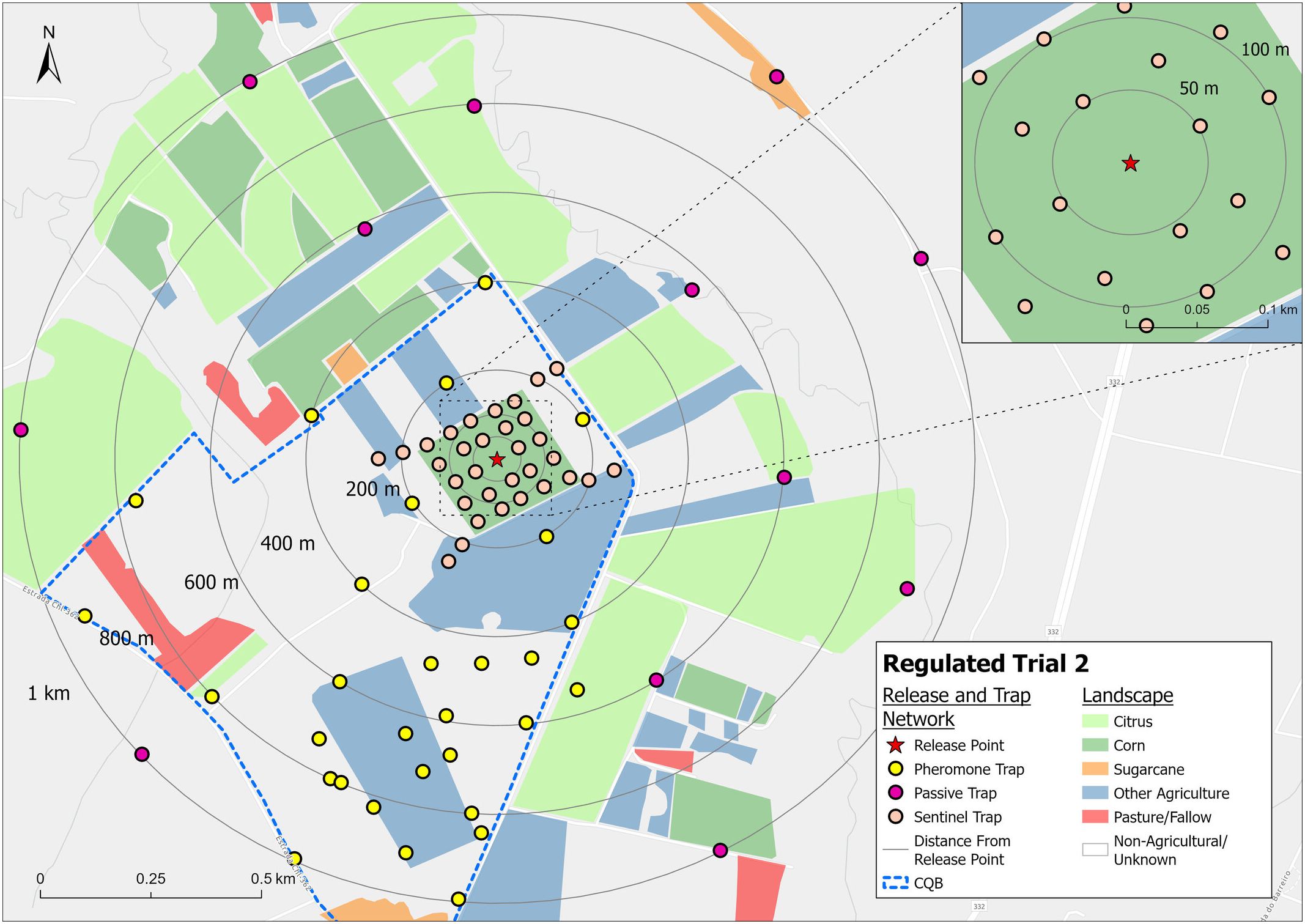
Figure 3. The 2021 Season 2 trial area and trap layout. Within the CQB area (outlined in blue), pheromone traps were placed up to 1 km from the release point. Outside the CQB area, passive traps (containing no pheromone lure) were used as requested by the regulatory agency, CTNBio.
During both seasons of field trials, the performance of the OX5382G strain was assessed relative to co-released wild-type males, using adult releases (direct releases of moths into the field). Prior to release as adults, pupae were held at 25°C (± 3°C) and resulting moths maintained in the laboratory for 1–3 days from first eclosion. The wild-type cohorts were sexed using standard microscopy methods as pupae but were otherwise reared and handled identically to the OX5382G males. Quality control analysis of a sub-set of 100 pupae from each release cohort individually assessed showed 100% male pupae. For both trials, adult releases took place at dusk from the pre-defined release point at the centre of the field, with the moths released from large cages. In Season 1, 11 releases of OX5382G males were carried out, as well as five co-releases of wild-type males (Brazil origin). A total of 20,589 OX5382G males and 5,353 wild-type males were released (fewer wild-type males were released due to the time-intensive manual sexing methods required for wild-type pupae). In Season 2, 18 releases of OX5382G males were carried out, as well as seven co-releases of wild-type males (Brazil origin). 36,855 OX5382G males and 7,510 wild-type males were released in total.
Each cohort of male moths was marked with a fluorescent powder (ECO Formaldehyde Free Pigments, DayGlo Color Corp.) and/or an internal dye (calco red dye, Royce Global), added to the larval rearing diet prior to release. No detectable fitness performance costs were observed in laboratory studies following the use of these marking agents. The powder colour was selected to enable unique identification of the strain and release date in recaptured moths collected from monitoring traps (Figure 4).
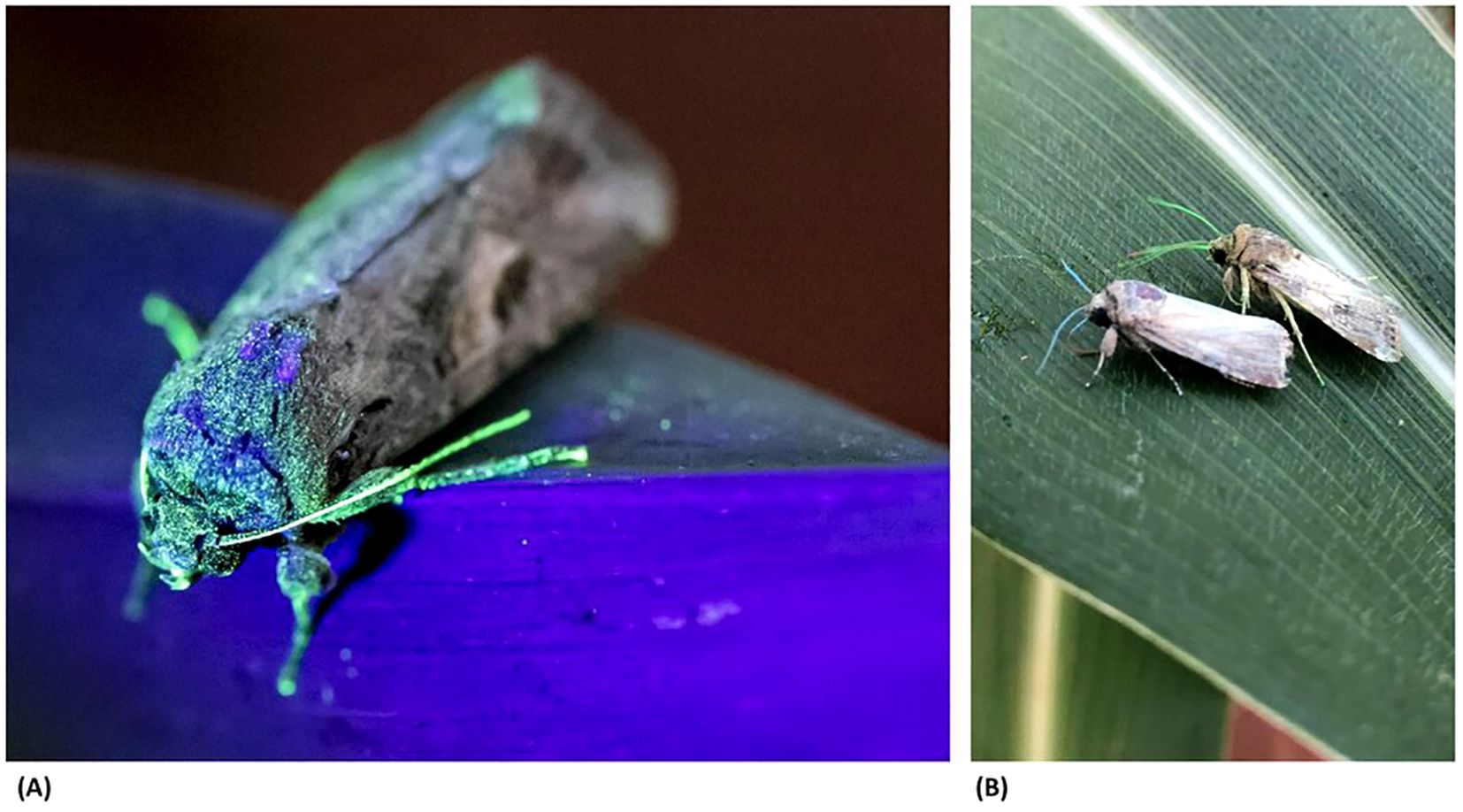
Figure 4. Photographs showing the powder marking of the released moths, with (A) a green powder-marked moth under ultraviolet light, and (B) a comparison of moths marked with green and blue powder under white light.
For both trials, the dispersal of adults was monitored using pheromone-baited traps [Delta trap with sticky floor and synthetic fall armyworm pheromone (Bio Spodoptera®, Biocontrole)], set in and around the trial corn field (Figures 2, 3). Within the area approved by regulators for releases and pheromone trapping, pheromone traps were placed at 200 m intervals, up to 1 km, from the release point. Informing this design was a mark-release-recapture study by Vilarinho et al. (2011), in which fall armyworm showed maximum recapture distances of 600–800 m; i.e. within the trapping range of this design. In accordance with the LPMA permit conditions, passive traps (containing no pheromone lure) were used outside the approved area. Pheromone traps were changed every 2–3 days to allow for accurate tracking of moth presence over time and to prevent trap saturation. Passive traps were changed weekly. Mean distance travelled was calculated using the method described by Morris et al. (1991).
Sentinel female traps, funnel ball type (ISCA) – traps containing a single wild-type virgin female moth, colonized from multiple Brazilian States – were placed within the trial field (Figures 2, 3) to assess the mating ability of released male moths. Sentinel female traps are designed such that moths can enter but cannot escape (Supplementary Figure 1). These females ‘call’ (release sex pheromone) to attract males. In Season 1, the sentinel females were placed in the corn field in increments of 10 m up to 100 m from the release point on the same evening that releases were undertaken. In Season 2, sentinel traps were placed in the corn plot at dusk on four consecutive evenings each week throughout the trial, with some traps also placed beyond the corn plot. The traps on the corn plot were placed in lines across the corn plot at 50 m intervals. In both trials, the traps were monitored and trapped males removed to the laboratory after one night in the field, and their strain and release cohort identified. In Season 1, a sample of the sentinel females with OX5382G males present in the trap was maintained and their progeny assessed to validate successful mating and confirm paternity (through the status of the DsRed2 marker gene).
Following cessation of releases in both trials, a network of pheromone traps continued to be assessed to determine persistence of OX5382G moths and their offspring in the environment. Collected moths were scored for DsRed2 (using microscopy and PCR genotyping). Assessment continued until DsRed2-positive moths were not detected for at least a 2-week period. Trial crops were subsequently destroyed in alignment with permit requirements.
Farm-scale field trials
Two seasons of farm-scale trials were completed on commercial corn in Brazil following commercial approval by Brazilian government regulators at the 239th CTNBio meeting, held on 4th March 2021 (Technical Statement CTNBio 7.350/2021, published in the Official Gazette on 9th March 2021), working alongside host growers (Supplementary Figure 2). In the first farm-scale season (October 2021-January 2022) – referred to as Season 3 of the overall trials – two trial sites were selected in São Paulo State (Supplementary Figures 3, 4); in the second season of farm-scale trials (February-June 2022) – referred to as Season 4 of the overall trials – four trial sites were selected: two in São Paulo State and two in Mato Grosso State (Supplementary Figures 5–8).
Moths were released from 1–4 release points at each site. Trial sites representative of typical commercial corn-growing landscapes were selected: the landscape was a mixed agricultural landscape in São Paulo State and predominantly corn in Mato Grosso State. In total, across the two seasons of farm-scale trials, 533,442 OX5382G moths were released (Site 1, 185, 287 moths, Site 2, 49,801 moths, Site 3, 26,025 moths, Site 4, 38,308 moths, Site 5, 177,496 moths, Site 6, 56,525 moths). All trial sites had a more robust pheromone-baited trap network (higher trap density and more evenly spread around the radius of the release points) than that employed during the regulated trials, due to the necessary constraints in place prior to deregulation. In each season during the farm-scale trials, one of the sites was monitored with a more extensive trap network than the others, with pheromone-baited traps placed up to approximately 5 km from the release points. In Season 3 this site was in São Paulo State, and in Season 4 this site was in Mato Grosso State. The remaining sites had trap networks set up to approximately 1 km from the release point(s). The objective of trapping up to 5 km from the release point was to more accurately measure the dispersal of the released moths, given that moths were trapped up to 1 km in the earlier regulated trials. Nevertheless, dispersal data from the 1-km trap networks yields informative data in terms of relative dispersal of different release cohorts; within this distance, we would expect any severe fitness penalties to be apparent from dispersal metrics shown by different cohorts. Informative data on overflooding ratios (the ratio of released OX5382G males to wild males), a key metric in terms of deployment strategies, were obtained from all trap networks. The pheromone-baited traps were similar to those used during the regulated trials and were changed every 2–3 days to prevent trap saturation. Mean distance travelled was calculated using the method described by Morris et al. (1991).
Production and marking of insects were carried out using the same methodology as described for the regulated field trials. While the marking in the regulated trials allowed us to differentiate between strain and release date, the marking in the farm-scale trials allowed us to distinguish between OX5382G releases temporally and geographically (required due to the multiple release points). Releases took place from the start of each corn-growing season, on all trial sites. Prior to release as adults, pupae were held at 25°C (± 3°C) and the resulting moths maintained in the laboratory for 1–3 days from first eclosion. Concurrently with these releases, prototype pupae release devices were deployed to test form factors and materials. The comparatively small numbers of moths released from these devices are not expected to have contributed significantly to field metrics recorded here.
Egg collections were carried out in the field to assess field mating performance of the released OX5382G moths. Following corn planting and releases commencing, egg masses were collected for 5 weeks during Season 3 at Site 1, and 8 weeks during Season 4 at Site 5. Egg masses were collected from the field in the central part of the plot between the four release points and returned to the laboratory. The hatching larvae were then assessed for presence/absence of the DsRed2 marker gene to screen for OX5382G progeny in the trial plot, indicating successful mating between OX5382G males and wild females.
Results
Susceptibility of the background fall armyworm strain to biotech corn traits and chemical insecticides
To dilute resistance to Bt corn and/or chemical insecticides, and thereby deliver effective resistance management, the self-limiting fall armyworm must carry genetics conferring susceptibility to Bt corn and/or chemical insecticides. The susceptibility of the OX5382G background strain (i.e. the wild-type strain used to develop OX5382G) was therefore assessed against a panel of biotech corn and a range of commonly used insecticides. As a comparison, recently field-collected strains from Mato Grosso and Bahia States were also assessed.
Susceptibility of the background fall armyworm strain to biotech corn traits
The OX5382G background strain was completely susceptible to Agrisure Viptera® (Vip3Aa20), Powercore® (expressing Cry1A.105, Cry2Ab2 and Cry1F) and VT PRO2® (expressing Cry1A.105 and Cry2Ab2) (Figure 5). Some resistance to Herculex® was detected (Cry1F), with 9% larval survival. Both field-collected strains showed excellent susceptibility to Agrisure Viptera™, but considerable resistance to all other varieties tested (Figure 5).
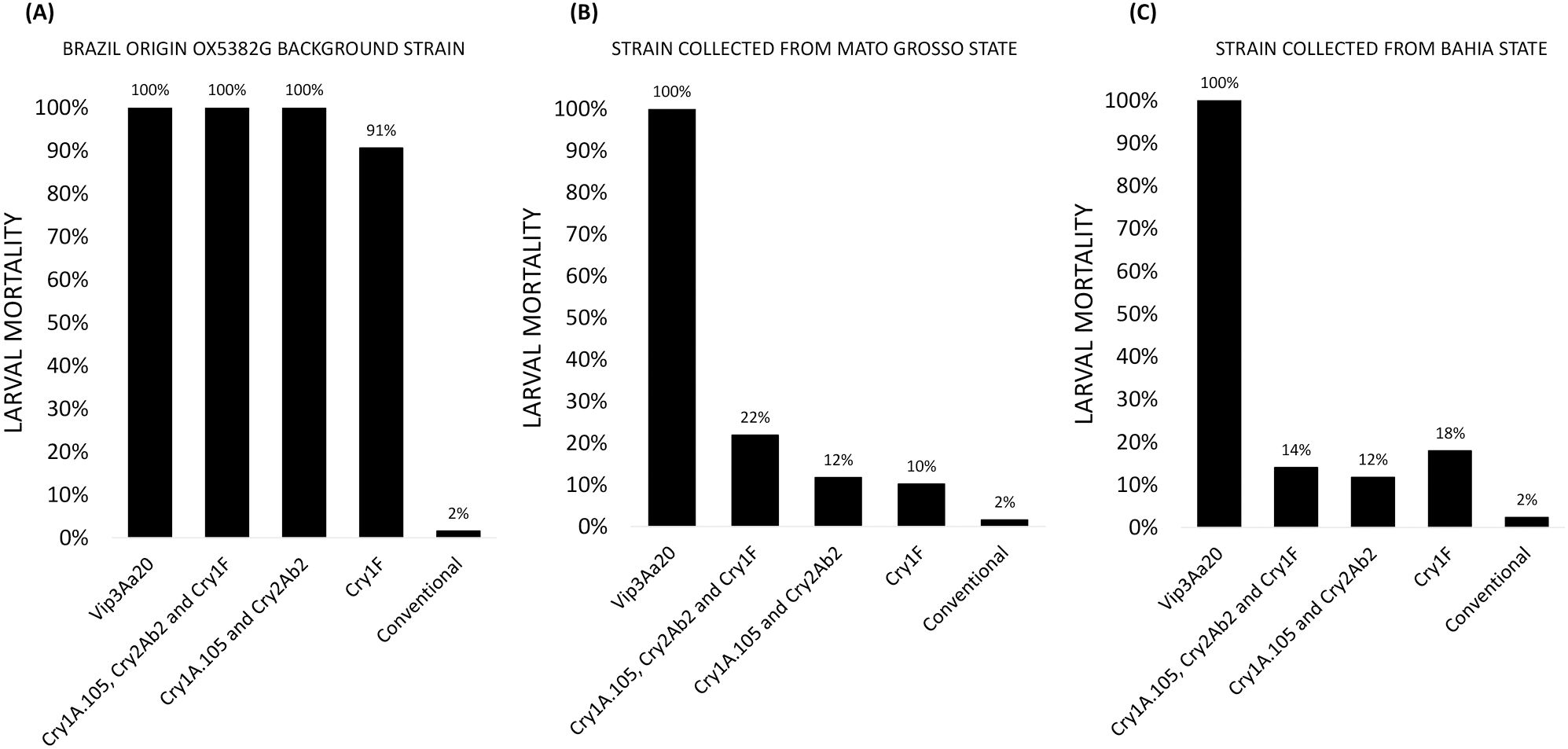
Figure 5. Mortality of fall armyworm larvae from three colonies, reared on a panel of corn varieties – Agrisure Viptera® (expressing Vip3Aa20), Powercore® (expressing Cry1A.105, Cry2Ab2 and Cry1F), VT PRO® (expressing Cry1A.105 and Cry2Ab2) and Herculex® (expressing Cry1F) – alongside a conventional control (no insecticidal traits): (A) the Brazil origin OX5382G background strain (wild-type); (B) a strain collected from Mato Grosso State; and (C) a strain collected from Bahia State. Technical replicates only were carried out, so no error bars are presented.
Susceptibility of the background fall armyworm strain to chemical insecticides
The insecticides selected for assessment represented the main modes of action currently in commercial use. The OX5382G background strain and the same Bahia and Mato Grosso field-collected colonies were tested against the LD99 of a range of insecticides (Figure 6), confirming that the background is more susceptible to commonly applied compounds than colonies collected from the field, in Mato Grosso and Bahia States.
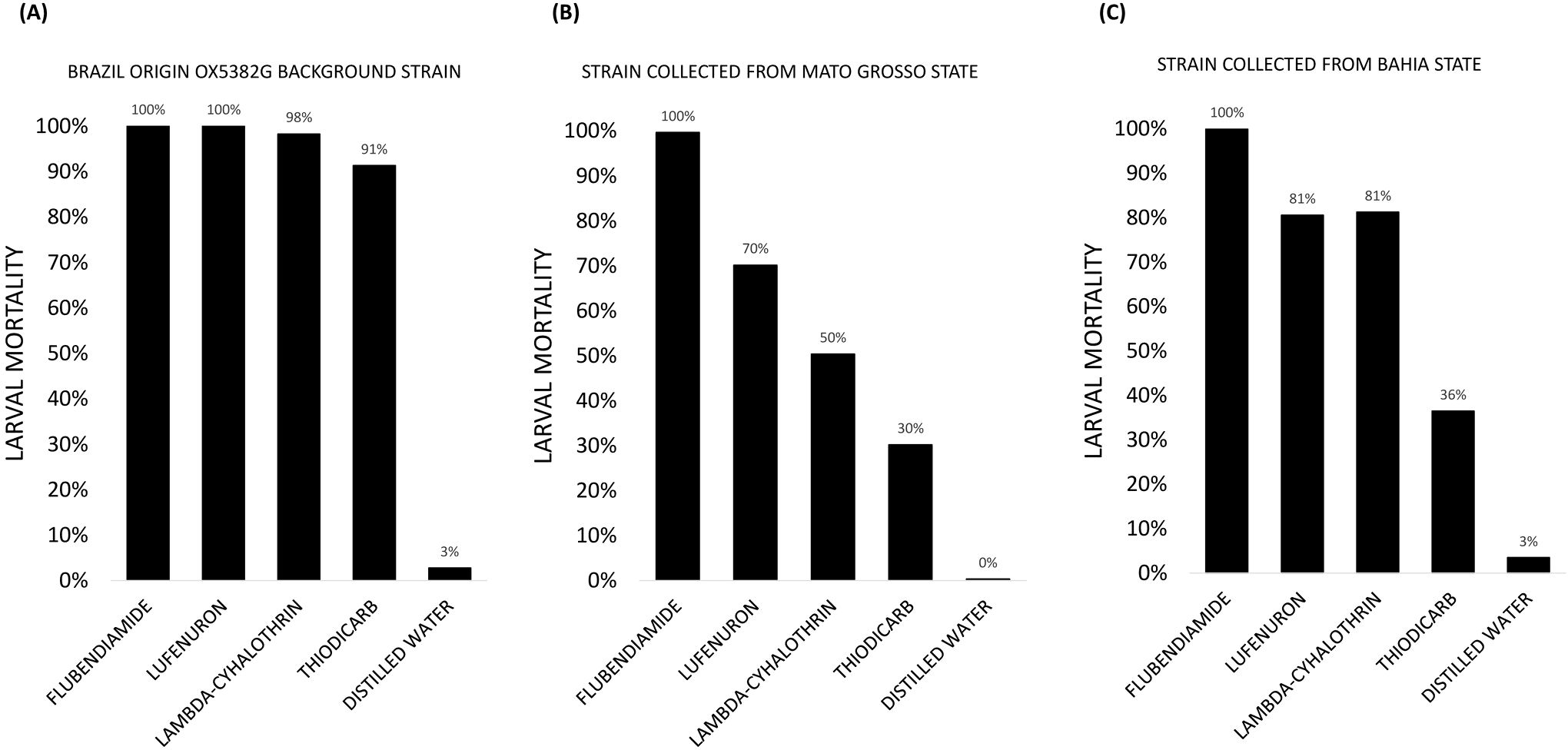
Figure 6. Mortality of fall armyworm larvae from three colonies exposed to a panel of chemical insecticides – Belt® (Flubendiamide), Match® (Lufenuron), Karate® (Lambda-Cyhalothrin), Larvin® (Thiodicarb) – alongside an untreated control (distilled water): (A) the Brazil origin OX5382G background strain (wild-type); (B) a strain collected from Mato Grosso State; and (C) a strain collected from Bahia State. Technical replicates only were carried out, so no error bars are presented.
Population suppression and resistance management through releases of self-limiting males
The effect of OX5382G releases on the development of Bt resistance was investigated by modelling and then tested empirically on contained populations of fall armyworm (Figure 7).
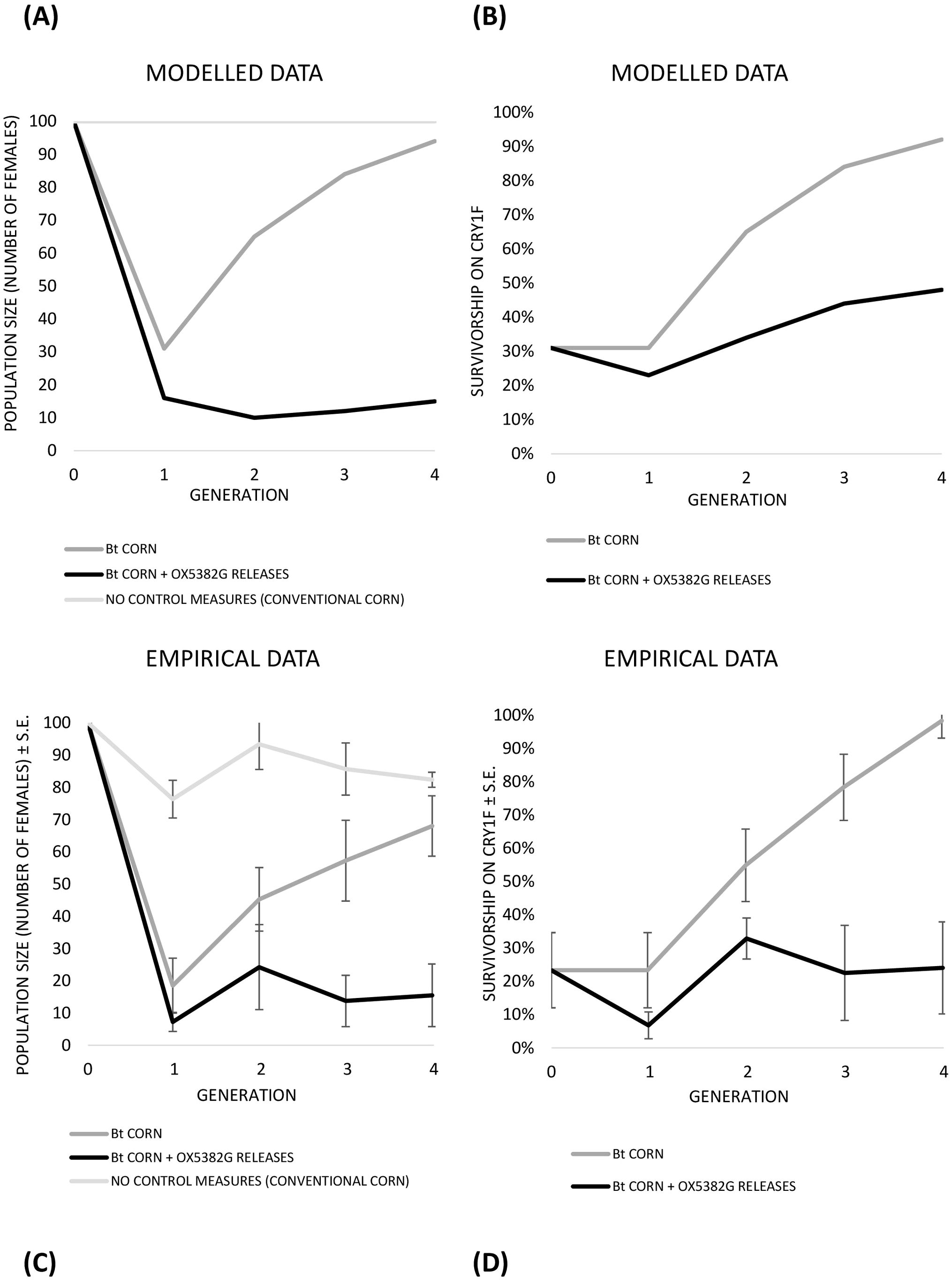
Figure 7. Modelled (A, B) and empirical (C, D) results showing the effects of Bt corn and/or OX5382G male releases on population size (A, C) and Cry1F resistance status (B, D) over the course of four generations. (A) Modelled results for population size (number of females); (B) Modelled results for survivorship on Cry1F (showing resistance/susceptibility to Bt); (C) Contained cage study results for population size (number of females); (D) Contained cage study results for survivorship on Cry1F (showing resistance/susceptibility to Bt). Note that the modelled results are based on the average of the two scenarios modelled: 0.25 and 0.45 starting resistance allele frequency.
Modelling showed that resistance to Bt corn will rapidly increase in frequency if Bt corn is used as a single control measure. However, an integrated approach using both Bt corn and OX5382G releases delayed the spread of resistance to Bt corn. In the absence of either Bt or OX5382G male releases the population size remained stable at a high level. On Bt corn, without OX5382G releases, the population size initially declined, before rising again as resistance to Bt increased. When populations were subjected to both Bt and OX5382G male releases, they were suppressed and remained so.
The results of the empirical demonstration closely resembled those of the modelled populations, demonstrating the population suppression and resistance management benefits of OX5382G releases on Bt corn (Figure 7; Supplementary Tables 1, 2).
Regulated field trials in Brazil
Dispersal
During the two trial seasons, the mean distance travelled by OX5382G males was not significantly different to the distance travelled by wild-type males; Season 1 (2-tailed t-test, t = 0.07, p = 0.94), Season 2 (2-tailed t-test, t = 0.12, p = 0.91) (Figure 8). OX5382G moths were recaptured up to 1 km from the release point – the upper limit of the trap network.
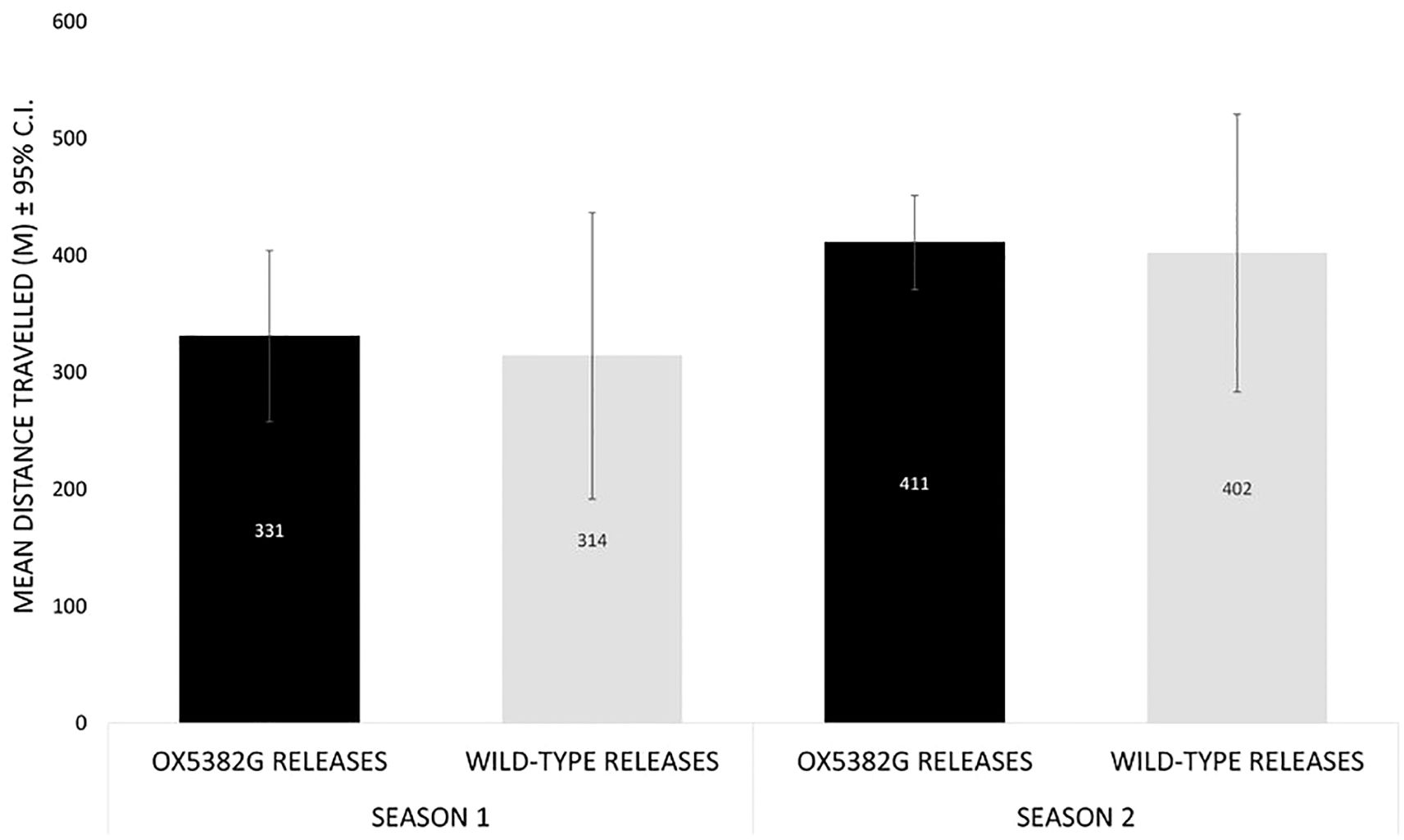
Figure 8. Dispersal from release points (mean distance travelled) of male moths: OX5382G releases (black) and wild-type releases (grey). Note: in Season 1, releases of cohorts of less than 700 males were omitted from this analysis due to the small sample size of recaptured moths. The OX5382G release on 12th December 2019 was omitted due to the cohort not meeting quality control targets.
Mating ability
In the two trial seasons, OX5382G males were frequently found in sentinel traps baited with wild-type females, indicating a strong pheromone response. OX5382G males were similar to wild-type males in their ability to find and mate with wild females. The recapture rates (Season 1: OX5382G, 1.12%, wild-type, 1.14%, Season 2: OX5382G, 0.76%, wild-type, 0.96%) were not significantly different between OX5382G and wild-type in either Season 1 (2-tailed t-test, t = -0.03, p = 0.98) or Season 2 (2-tailed t-test, t = -1.04, p = 0.32).
To validate successful mating by OX5382G males, in Season 1, a sample of sentinel females that had been located in sentinel traps with OX5382G males was taken to the laboratory to be maintained, and their progeny assessed to confirm paternity. Of the 52 females that laid hatching eggs, all had mated successfully with OX5382G males. 51 females produced 100% larvae positive for the DsRed2 marker, and one female produced mixed wild-type and DsRed2-positive progeny (this female was either not a virgin female as intended and/or was mated by a wild male or released wild-type male prior to escaping the sentinel trap).
Post-release monitoring and non-persistence of OX5382G in the environment
After the final release in both Seasons 1 and 2, recaptures of OX5382G ceased within four days, and no further OX5382G insects were found in the field following 25+ days of post-release monitoring (Supplementary Table 3).
Biosafety studies
Based on the data described in this manuscript, and others presented in the complete dossier, Brazil’s biosafety regulator CTNBio concluded that OX5382G fall armyworm presents no significant risk to the environment or to human or animal health (Technical Statement CTNBio 7.350/2021). As part of the risk assessment, CTNBio concluded that OX5382G fall armyworm would have no negative impact on predators (including endangered Brazilian species), parasitoids or ecosystem services such as decomposition or pollination. Additionally, safety studies (see Supplementary Material) demonstrated that the two inserted proteins, tTAV and DsRed2, are non-toxic (no acute toxic effects were observed in mammals) and non-allergenic, they are not expected to be concentrated along the food chain, they cause no adverse effects when fed to moth predators such as predatory birds or beetles, and they cause no effect on rates of parasitism by parasitoid wasps that target the eggs of fall armyworm. Commercial approval by Brazilian government regulators following submission of biosafety data (Technical Statement CTNBio 7.350/2021) enabled the operational deployment as outlined in the farm-scale studies.
Farm-scale field trials
Dispersal
Trapping networks in Seasons 3 and 4 were designed to capture two dispersal parameters: 1) The maximum dispersal capability of OX5382G and 2) Relative dispersal capacity between cohorts. Two trap networks covering distances up to 5 km from the release point [Site 1 in Season 3 (Supplementary Figure 3) and Site 5 in Season 4 (Supplementary Figure 7)] measured much more of the moths’ natural dispersal range, allowing for more accurate dispersal estimates. The mean distance travelled of released moths was 1394 m and 1922 m on Site 1 (Season 3) and Site 5 (Season 4), respectively (Figure 9), significantly further in Site 5 (two-tailed t-test t = 3.23, p = 0.003). Among the sites with traps set only up to 1 km (designed to compare relative dispersal distances between cohorts, rather than the more accurate dispersal measurements at the 5-km sites), there was a significant difference in mean distance travelled (ANOVA F = 5.0, p = 0.004). A post-hoc test determined that the mean distance travelled at Site 2 was significantly higher than at Site 4 and Site 6 (p = 0.012 and p = 0.006, respectively). Moths were recaptured up to the limit of the trap networks on all study sites.
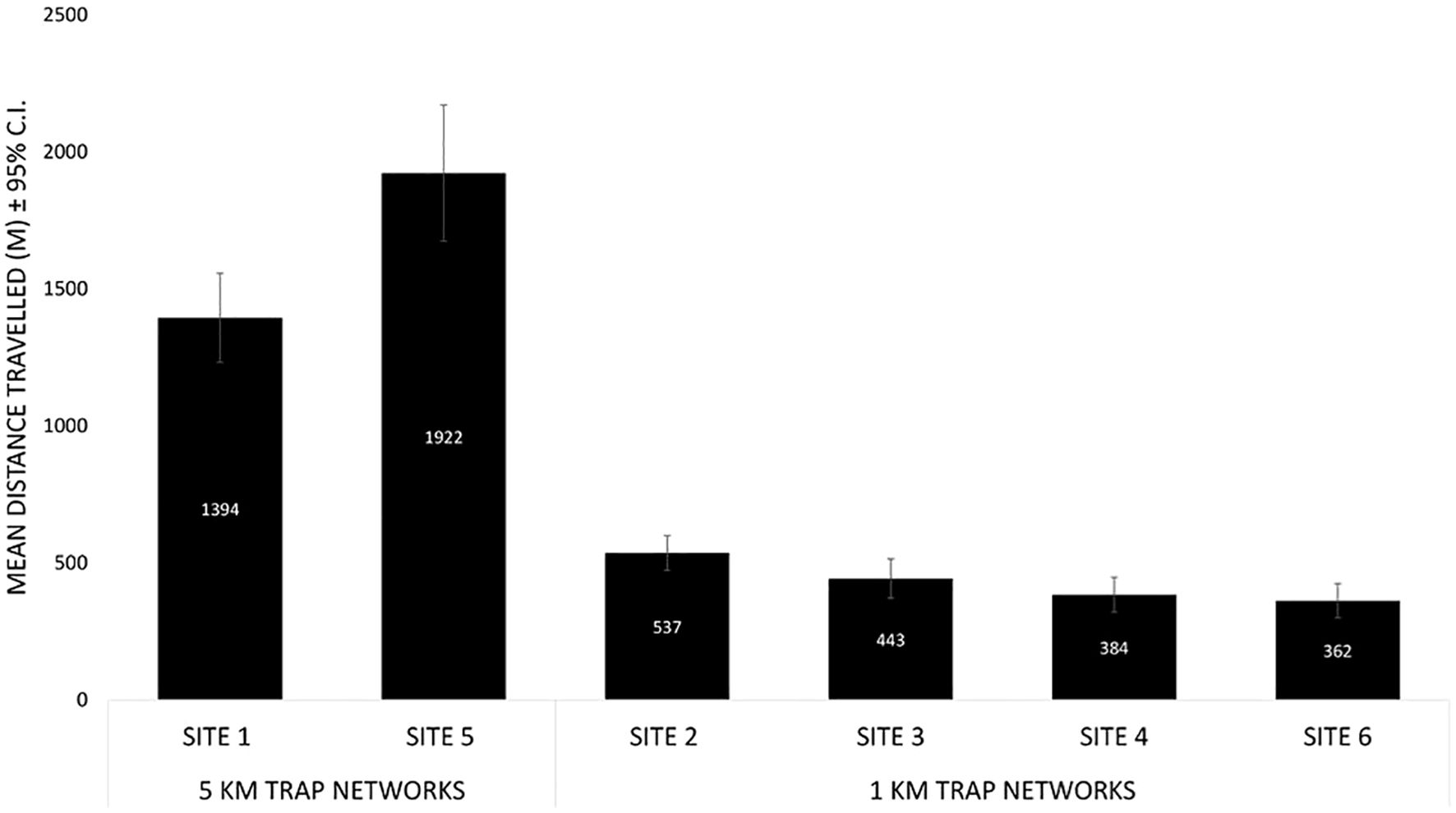
Figure 9. The mean distance travelled of OX5382G males released on the two study sites with traps set up to 5 km from the release point and the four study sites with traps set up to 1 km from the release point.
Overflooding ratio in relation to distance from single release points
The overflooding ratio – the relative number of OX5382G males to wild males – resulting from adult release cohorts was measured in all traps in the surrounding network of each study site. Figure 10 shows the overflooding ratio in relation to distance from a single release point (as averaged across the release points in all study sites). As distance from the release point increases, the density of OX5382G moths released from that point decreases in the landscape. To allow us to calculate both the overflooding ratio and release rate metrics associated with each release point (see later results sections), for these trials we have taken 500 m as an approximate ‘cut-off’ point (equivalent to approximately 200 acres) – beyond this point the overflooding ratio is much lower than the average within this area.
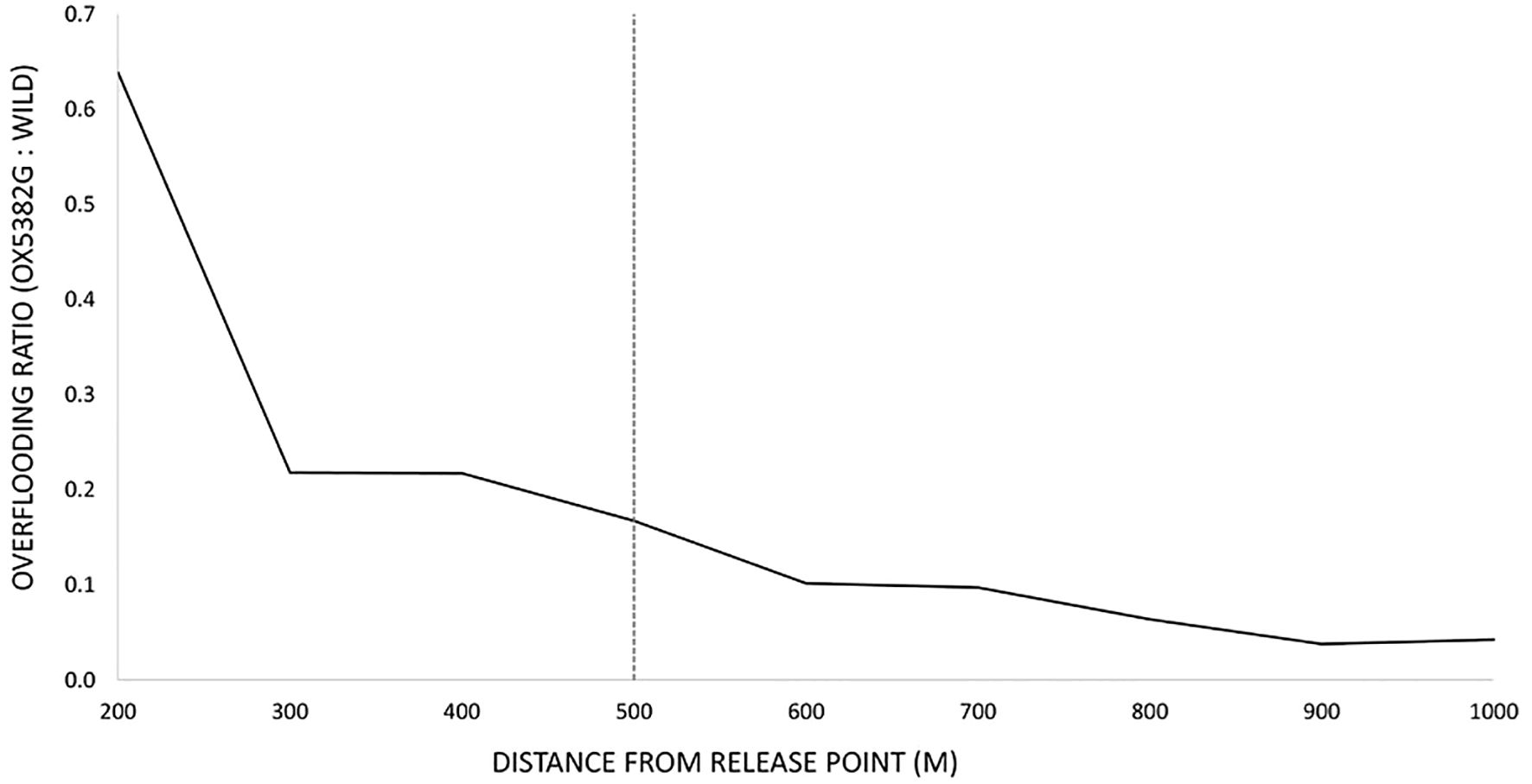
Figure 10. The overflooding ratio in relation to distance from release point (averaged across all release points in the six study sites). The 500 m point is marked with a dashed grey line.
Overflooding ratio in relation to distance on multiple release point sites
As discussed above, from a single release point, the overflooding ratio declines in relation to distance from the release point. With the ultimate aim of this crop protection tool being landscape treatment, deployment will focus on achieving a consistent overflooding ratio across the landscape. Consideration of the sites with four release points (Site 1 and Site 5) provides the first insight into how to optimize treatment at landscape scale. In Site 1, there was ~500 m between release points (Supplementary Figure 3). This array was selected due to the smaller contiguous corn acreage in São Paulo State. In Site 5, there was ~1 km between the release points (Supplementary Figure 7) – i.e. we were able to assess the ~200-acre treatment area in practice. As expected, Figure 11 shows that greater overflooding ratios are achieved when moths from all release points in the array are included – moths that disperse beyond a given treatment area will benefit the overflooding ratios in other treatment areas and vice versa. Although we have used a 200-acre treatment area for the calculations in this study, Figure 11 shows that as we move to multiple release points across larger areas, and consider all OX5382G moths in the landscape, overflooding ratios are maintained at similar levels for a greater distance from the release point (compared to consideration of moths from a single release point). Therefore, operationally, the eventual treatment area is expected to be greater than 200 acres.
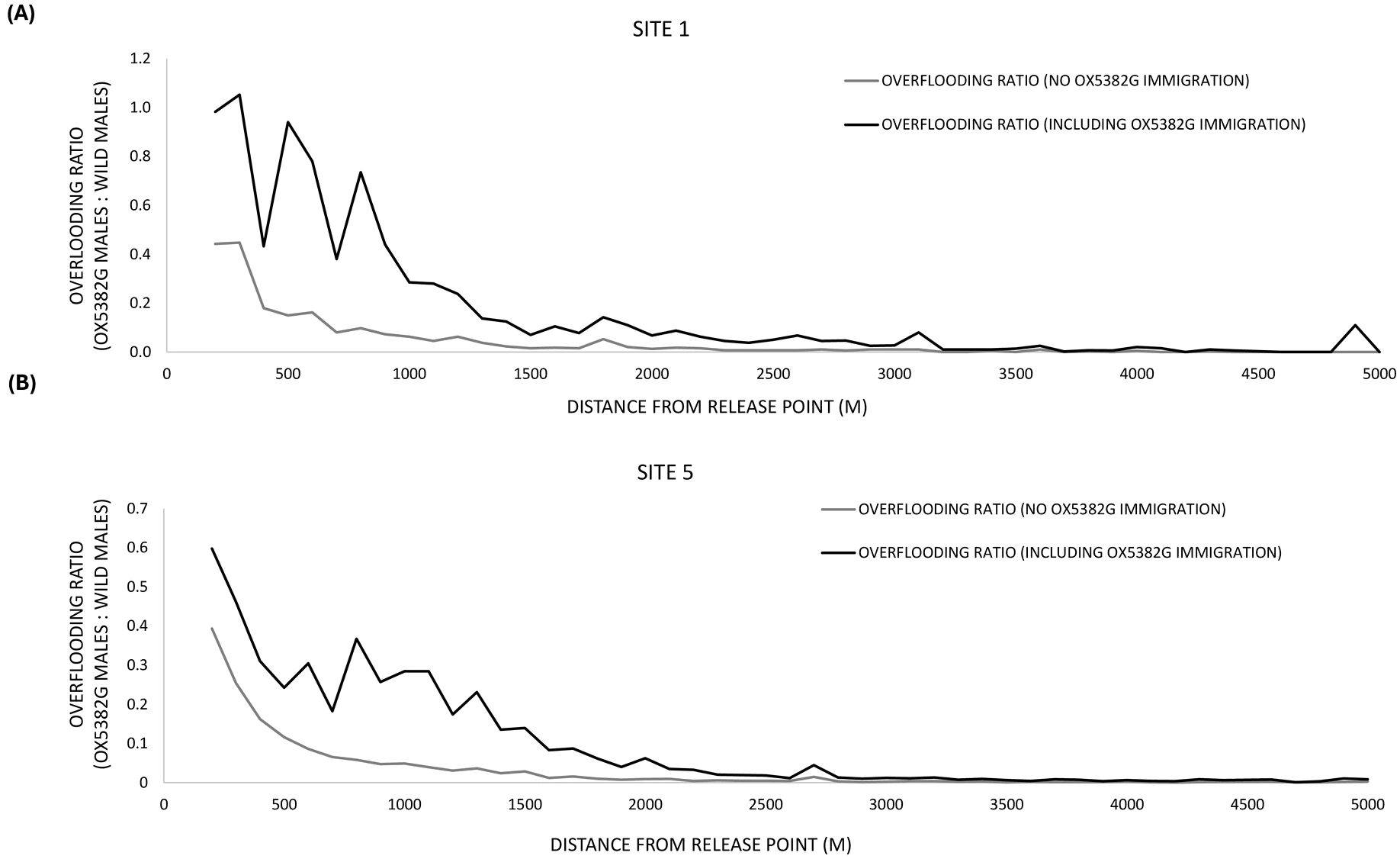
Figure 11. The overflooding ratio (OX5382G males: wild males) in relation to distance from release point (averaged across release points) in the sites with four release points and traps set up to 5 km from the release points – (A) Site 1 and (B) Site 5. The overflooding ratio is presented for both scenarios of including (black line) and excluding (grey line) OX5382G immigration.
Release rates
As OX5382G is eventually envisaged to be used as a farmer-deployed crop input, this study is the first effort to define future effective dosing. For these first trials, a treatment area is defined as a single release point and the surrounding 200 acres (approximately 500 m from the release point, as described above). Using the number of moths released from the central release point, we can then calculate release rates as per acre (Table 2). For the multiple release point sites, the values presented are an average across release points.
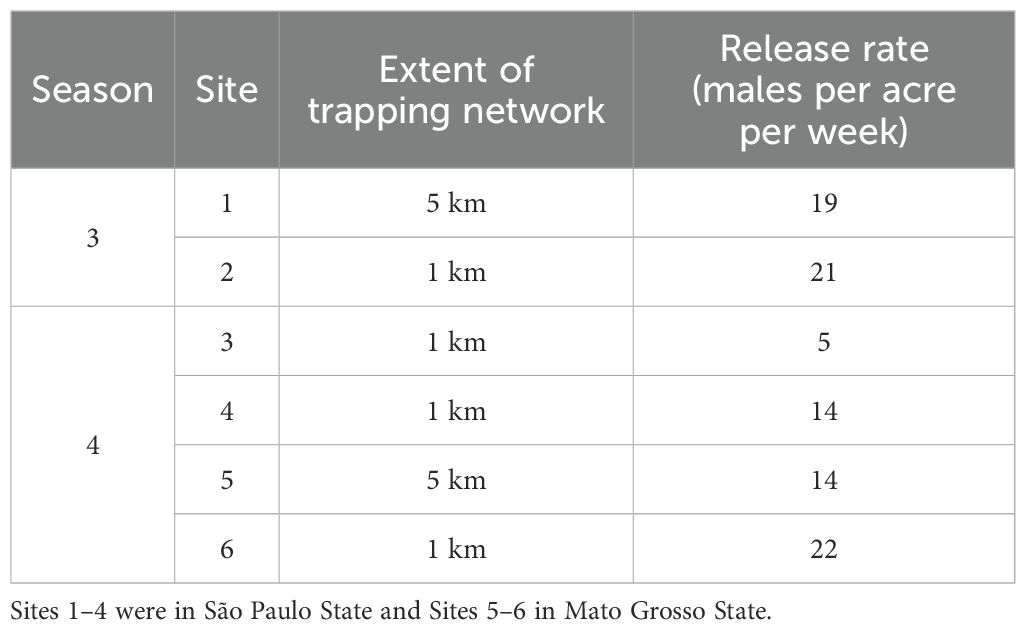
Table 2. Calculated release rate of OX5382G male moths across the seasons and sites of the farm-scale trials.
Overflooding ratio and projected release rates
The overflooding ratio (OX5382G males: wild males) resulting from adult release cohorts (Supplementary Figures 9–12) was measured in the 200-acre area around the release point, alongside the release rates. From this data, the release rate that would be required to achieve a 1:1 overflooding ratio was calculated (Table 3; Supplementary Figures 9–12). Without factoring in OX5382G males flying in from neighbouring release points, 27–200 males per acre per week were required to achieve 1:1 overflooding. In sites with multiple release points, this was reduced to 14–38 males per acre per week when incoming OX5382G males were accounted for: a more realistic scenario when considering the large size of corn fields cultivated in Brazil, often >1000 acres.
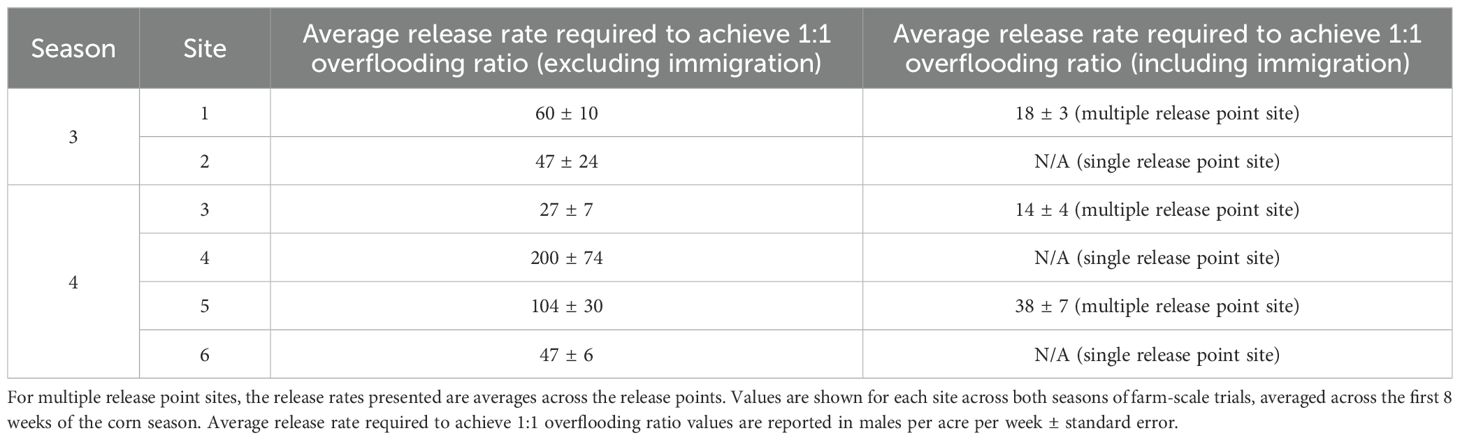
Table 3. The average release rate required to achieve 1:1 overflooding ratio is summarised for both scenarios of excluding (only considering moths from a single release point) and including immigrating OX5382G moths (relevant in multiple release point sites).
Field mating performance
In both seasons of the farm-scale trials, the field mating performance – quantified as the mating fraction, or proportion of OX5382G-carrying progeny in the field site – was assessed on Sites 1 and 5. The mating fraction was calculated, following screening of hatching progeny for the DsRed2 marker in the laboratory, in two ways: (a) excluding egg masses that were mixed status – comprising eggs that hatched into DsRed2-positive and -negative larvae – from the overall calculation (minimum mating fraction); and (b) including and assigning egg masses yielding both DsRed2-positive and -negative larvae to OX5382G paternity (maximum mating fraction). The average mating fraction across weeks for the minimum mating fraction was 4.7% and 24.7% for Seasons 3 and 4, respectively, and for the maximum mating fraction – 11.6% and 40.5% for Seasons 3 and 4, respectively (Table 4).
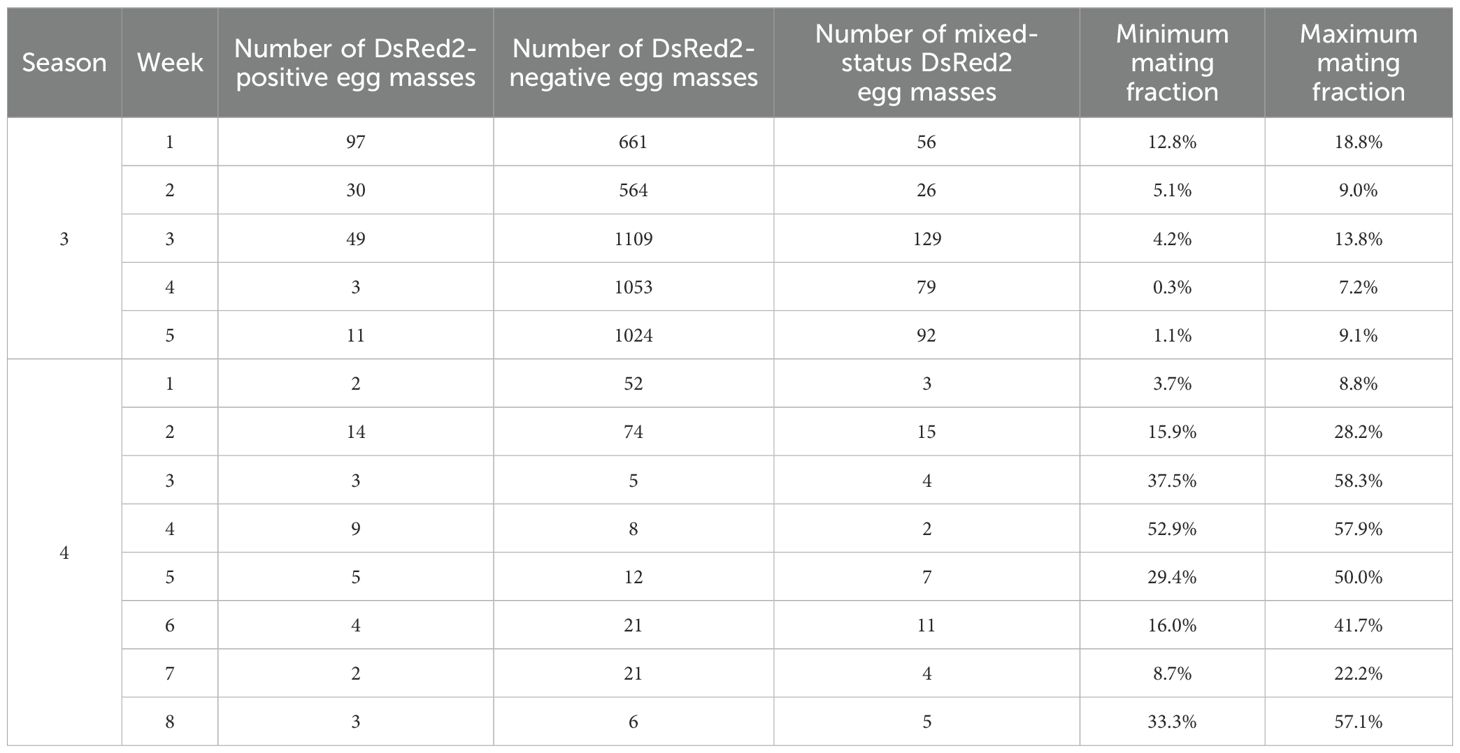
Table 4. The mating fraction – the proportion of OX5382G progeny in the field-sampled population – across trial weeks for each season of farm-scale trials on Site 1 (Season 3) and Site 5 (Season 4).
The number of egg masses collected was much lower in Season 4 compared to Season 3 despite similar collection efforts. Fall armyworm abundance was similar across the seasons/sites so the cause is unclear. Differences in agricultural landscape or grower management practices may have played a role.
Discussion
Previous work described the development of the OX5382G self-limiting fall armyworm and, through population modelling, validated the promise of this new approach for managing fall armyworm populations and delaying development of resistance to Bt corn varieties (Reavey et al., 2022). Here we describe the subsequent field trials carried out over a 3-year period (2019-2022), which validated the performance of the OX5382G strain in regulated trials (2019-2021) – leading to commercial biosafety approval of the strain in Brazil – and progressed our understanding of deployment-related criteria in larger-scale operational trials (2021-2022). All assessments to date suggest that self-limiting fall armyworm will provide an effective tool for controlling fall armyworm and extending the durability of Bt crops – and future such insecticidal traits – towards this highly damaging pest.
In two seasons of regulated trials, the OX5382G self-limiting fall armyworm strain showed similar performance to its wild-type counterpart in terms of dispersal and mating ability, showing no indication of a fitness penalty related to presence of the OX5382 transgene. This is promising for the efficacy and cost-effectiveness of future deployments for crop protection and resistance management.
The equivalence between OX5382G and wild-type insects also indicates no fitness advantage of the engineered strain relative to wild-type counterparts. Post-release monitoring demonstrated that OX5382G moths do not remain in the field beyond the usual lifespan of fall armyworm moths. After cessation of releases, the OX5382G self-limiting gene declined and was no longer present in the environment (Supplementary Table 3). In future applications on crops, with no apparent selective advantage (and a major selective disadvantage conferred by engineered female mortality), there is no evidence to suggest that the OX5382G trait could persist beyond a few generations in field populations of fall armyworm after OX5382G releases stop. Moreover, safety studies confirmed the lack of toxicity of the introduced proteins, underlining the robust biosafety of the OX5382G strain (Supplementary Material).
Alongside field trials, the background genetics of the strain were validated as susceptible to Bt corn traits and to chemical insecticides (Figures 5, 6), a prerequisite of the resistance management benefits following mating between OX5382G males and wild females. The contained studies conducted on Bt corn, alongside parallel population modelling, demonstrated that releases of self-limiting male moths lead to a decline of the target population of fall armyworm, and delay spread of resistance to a biotech corn trait. While only Cry1F was used in this proof-of-concept study, the same principles of delaying the spread of resistance could be applied to any insecticidal trait – susceptibility alleles for all technologies will be introgressed into the population. As previously discussed, the resistance management effect of releasing self-limiting OX5382G fall armyworm could help to mitigate the increased resistance risk associated with inadequate non-Bt refugia, with the promise of providing more sustainable protection of the durability of existing – and future – integrated pest management (IPM) strategies for fall armyworm.
The 2021–2022 farm-scale deployment-focused trials further advanced our knowledge of fall armyworm dispersal, with releases taking place on commercial corn with more extensive trap networks. OX5382G males showed a mean distance travelled of approximately 1–2 km (Figure 9). On the sites with trap networks extending to 5 km, low numbers of moths were detected at the limits of the trap networks – even with a relatively high trap density. We do not, therefore, expect that further increases in the spatial distribution of the trap network beyond 5 km would change the calculated mean distance travelled considerably. Even taking a conservative assumption that a single release point can treat 200–400 acres (508–717 m radius of the assumed treated circular area), deployment of self-limiting fall armyworm can be expected to be low effort for growers, with released males doing much of the ‘work’ in the field. Given that reasonable overflooding ratios are observed up to 1 km in the multiple release point trial sites and that, at operational scale, we expect to have many more release points across the landscape than the small arrays in these first pilot trials, it may be that a treatment area of > 400 acres per release point is achievable. This and future landscape-scale releases will ultimately define the optimal density of release points for cost-effective treatment, as well as frequency of release.
The farm-scale studies reported here provided a preliminary demonstration that the effective overflooding ratios estimated by modelling (Reavey et al., 2022) can be achieved by releasing reasonable numbers of adult male OX5382G (Table 3; Supplementary Figures 9–12). The overflooding ratio will depend on the OX5382G male release rate, the performance of the released OX5382G moths and the wild moth recruitment rate. These trials show that relatively modest release rates in multiple sites and geographies, working against typical wild fall armyworm abundance, result in what appears to be an operationally manageable overflooding ratio. Furthermore, for fall armyworm releases at landscape scale, much larger than the relatively small arrays of release points used in the multiple release point sites in these studies, we would anticipate that the required release rates will be lower than those observed in this preliminary demonstration (Table 3). The promising release rates in this pilot study, suggesting that < 50 males released per acre per week should be effective, compare favorably to lepidopteran sterile insect technique programs. For pink bollworm, Pectinophora gossypiella (W.W. Saunders, 1844), 400–800 males per acre per week were released in 2008 in Arizona as part of an area-wide eradication campaign (Simmons et al., 2011). Mating-based approaches to insect pest control have been used successfully for many decades (Klassen and Curtis, 2005), providing an operational foundation for the self-limiting approach, which – by avoiding the need to irradiate insects prior to release, and the ability to release male-only cohorts – can be expected to provide further benefits in terms of performance and scalability.
With the self-limiting approach relying on a mating-based mechanism, successful mate-seeking and copulation by OX5382G males under field conditions is key to effective pest management and resistance management. Mating in the field with captive wild-type sentinel females, as demonstrated in the regulated trials, provides promising data, but evidence of mating with free-flying wild females, as we show in the farm-scale trials, is the ultimate proof point. The field mating performance shown by OX5382G males, as measured by the mating fraction, is very encouraging, demonstrating that local releases are having a local effect on the next generation (Table 4). Furthermore, as these studies were affected by the immigration of wild male fall armyworm and emigration of OX5382G fall armyworm, as well as the movement of mated wild females (which likely contributes to the variation in mating fraction across seasons and weeks), we would expect an increase in the mating fraction with landscape scale releases. The small scale of the trials relative to ultimate deployment scale means that we cannot fully establish field competitiveness. To determine the relative fitness in terms of mating of OX5382G males and wild males, large-scale landscape treatment will be required, and mating fraction measured at this scale.
Beyond technical considerations, the fact that this work – the first releases of OX5382G fall armyworm – was conducted provides encouragement for other technology developers in this space that the regulatory landscape in Brazil and other needful markets are maturing and are ready to consider novel and urgently needed pest solutions. Earlier regulatory precedents for biotech crops and self-limiting mosquitoes, and their subsequent large-scale deployment, helped create the pathway for these approvals. This has demonstrated that Brazilian growers and communities are open to innovative new genetic solutions to significant pests in agriculture and public health. In parallel with the field studies described here, our engagements with growers – those hosting the pilots and the wider farming community – indicated general willingness to consider new pest management tools, particularly where they can deliver direct benefits for their livelihoods and for environmental sustainability.
All studies to date on self-limiting fall armyworm (OX5382G) – laboratory, modelling, contained studies and field assessment – suggest that OX5382G fall armyworm could provide a transformative management tool in terms of population control, resistance management and sustainable crop protection. Large-scale fall armyworm production methods are under development, and future field trials across multiple Brazilian States, and in countries beyond Brazil, will progress innovation focused on deployment methods and landscape-scale releases. Across the world, farmers are under increasing pressure in the face of climate change, pest resistance to crop protection tools, and many other challenges – the described self-limiting fall armyworm solution could deliver significant benefits for livelihoods, and also support the development of new IPM strategies for more sustainable food production.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript - with the exception of the supporting safety studies - presents research on animals that do not require ethical approval for their study. The safety studies were carried out at contract research organisations in line with the ethical guidelines for their species. The protein toxicity studies and the avian non-target feeding study were conducted in accordance with the applicable sections of the United Kingdom Animals (Scientific Procedures) Act, 1986, Amendment Regulations 2012 (the Act).
Author contributions
CR: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, Investigation, Visualization. FD: Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. KE: Data curation, Formal Analysis, Visualization, Writing – review & editing. RP: Data curation, Formal Analysis, Visualization, Writing – review & editing. NN: Conceptualization, Methodology, Supervision, Writing – review & editing. MY: Data curation, Formal Analysis, Visualization, Writing – review & editing, Supervision. TF: Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. KC: Data curation, Investigation, Methodology, Project administration, Resources, Writing – review & editing. ES: Investigation, Methodology, Project administration, Resources, Writing – review & editing, Data curation. AP-H: Methodology, Project administration, Resources, Supervision, Writing – review & editing. ME: Data curation, Investigation, Methodology, Resources, Writing – review & editing, Project administration. LM: Data curation, Investigation, Methodology, Project administration, Resources, Writing – review & editing. DA: Data curation, Investigation, Methodology, Project administration, Resources, Writing – review & editing. MD: Data curation, Investigation, Methodology, Project administration, Resources, Writing – review & editing. LS: Investigation, Methodology, Resources, Writing – review & editing. FF: Investigation, Methodology, Project administration, Resources, Writing – review & editing. JR: Data curation, Investigation, Methodology, Resources, Writing – review & editing. IO: Data curation, Investigation, Methodology, Resources, Writing – review & editing. TS: Data curation, Investigation, Methodology, Resources, Writing – review & editing. WM: Investigation, Methodology, Resources, Writing – review & editing, Data curation. TM: Investigation, Methodology, Resources, Writing – review & editing, Data curation. MP: Investigation, Methodology, Project administration, Resources, Writing – review & editing. BS: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. DT: Investigation, Methodology, Resources, Writing – review & editing. JM: Writing – original draft, Writing – review & editing. SB: Data curation, Formal Analysis, Visualization, Writing – review & editing. TA: Project administration, Writing – review & editing, Methodology, Resources. HC: Writing – review & editing, Methodology, Project administration, Supervision. NV: Methodology, Project administration, Supervision, Writing – review & editing. NR: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. GF: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. NM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. KM: Conceptualization, Funding acquisition, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study received funding from Bayer Crop Sciences (in a jointly funded collaboration between Oxitec Ltd and Bayer Crop Sciences).
Acknowledgments
We would like to acknowledge the contribution of PROMIP, Pragas.com and Covance Laboratories Ltd (now Labcorp Ltd). The regulated field trials were carried out at PROMIP’s research site in Conchal, São Paulo State, with Oxitec Ltd and PROMIP employees carrying out the operational work. The resistance characterization assays were completed by Pragas.com. The protein toxicity studies and non-target organism studies for birds and predator beetles were carried out by Covance Laboratories Ltd. We are also grateful to the contribution of Graham Head of Bayer Crop Science for valuable discussions on field trial design.
Conflict of interest
All authors are current or former employees of Oxitec Ltd.
The authors declare that this study received funding from Bayer Crop Sciences (in a jointly funded collaboration between Oxitec Ltd and Bayer Crop Sciences). Oxitec Ltd designed and carried out the studies presented in the manuscript, analysed and interpreted the data and wrote the article. Bayer Crop Sciences provided feedback on the field trial design.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2025.1613689/full#supplementary-material
References
Alphey N., Bonsall M. B., and Alphey L. (2009). Combining pest control and resistance management: synergy of engineered insects with Bt crops. J. Economic Entomology 102, 717–732. doi: 10.1603/029.102.0233
Alphey N., Coleman P. G., Donnelly C., and Alphey L. (2007). Managing insecticide resistance by mass release of engineered insects. J. Economic Entomology 100, 1642–1649. doi: 10.1093/jee/100.5.1642
Amaral F. S. A., Guidolin A. S., Salmeron E., Kanno R. H., Padovez F. E. O., Fatoretto J. C., et al. (2020). Geographical distribution of Vip3Aa20 resistance allele frequencies in Spodoptera frugiperda (Lepidoptera: Noctuidae) populations in Brazil. Pest Manage. Sci. 76, 169–178. doi: 10.1002/PS.5490
Bernardi O., Bernardi D., Horikoshi R. J., Okuma D. M., Miraldo L. L., Fatoretto J., et al. (2016). Selection and characterization of resistance to the Vip3Aa20 protein from Bacillus thuringiensis in Spodoptera frugiperda. Pest Manage. Sci. 72, 1794–1802. doi: 10.1002/PS.4223
Bernardi D., Salmeron E., Horikoshi R. J., Bernardi O., Dourado P. M., Carvalho R. A., et al. (2015). Cross-Resistance between Cry1 Proteins in fall armyworm (Spodoptera frugiperda) may affect the durability of current pyramided Bt maize hybrids in Brazil. PloS One 10, e0140130. doi: 10.1371/journal.pone.0140130
Carvalho I. F., Erdmann L. L., MaChado L. L., Paula A., Rosa S. A., Zotti M. J., et al. (2018). Metabolic resistance in the fall armyworm: an overview. J. Agric. Sci. 10, p426. doi: 10.5539/JAS.V10N12P426
Carvalho R. A., Omoto C., Field L. M., Williamson M. S., and Bass C. (2013). Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda. PloS One 8, e62268. doi: 10.1371/JOURNAL.PONE.0062268
Culliney T. (2014). “Crop losses to arthropods,” in Integrated pest management. Eds. Pimentel D. and Peshin R. (Springer, Dordrecht). doi: 10.1007/978-94-007-7796-5_8
Diez-Rodríguez G. I. and Omoto C. (2001). Herança da resistência de Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) a lambda-cialotrina. Neotropical Entomology 30, 311–316. doi: 10.1590/S1519-566X2001000200016
Farias J. R., Andow D. A., Horikoshi R. J., Sorgatto R. J., Fresia P., dos Santos A. C., et al. (2014). Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 64, 150–158. doi: 10.1016/j.cropro.2014.06.019
Harvey-Samuel T., Ant T., Gong H., Morrison N. I., and Alphey L. (2014). Population-level effects of fitness costs associated with repressible female-lethal transgene insertions in two pest insects. Evolutionary Appl. 7, 597–606. doi: 10.1111/eva.12159
ISAAA (2017). Global status of commercialized biotech/GM crops (ISAAA). Available online at: https://www.isaaa.org/resources/publications/briefs/53/.
Klassen W. and Curtis C. F. (2005). “History of the sterile insect technique,” in Sterile insect technique: principles and practice in area-wide integrated pest management (Berlin, Germany: Springer Nature), 3–36. doi: 10.1007/1-4020-4051-2_1/COVER
Marubbi T., Cassidy C., Miller E., Koukidou M., Martin-Rendon E., Warner S., et al. (2017). Exposure to genetically engineered olive fly (Bactrocera oleae) has no negative impact on three non-target organisms. Sci. Rep. 7, 11478. doi: 10.1038/s41598-017-11908-4
Morris C. D., Larson V., and Lounibos L. P. (1991). Measuring mosquito dispersal for control programs. J. Am. Mosq. Control Assoc. 7, 608–615.
Nascimento A. R. B. do, Farias J. R., Bernardi D., Horikoshi R. J., and Omoto C. (2016). Genetic basis of Spodoptera frugiperda (Lepidoptera: Noctuidae) resistance to the chitin synthesis inhibitor lufenuron. Pest Manage. Sci. 72, 810–815. doi: 10.1002/PS.4057/EPDF
Nordin O., Donald W., Ming W. H., Ney T. G., Mohamed K. A., Halim N. A. A., et al. (2013). Oral ingestion of transgenic RIDL Ae. aEgypti larvae has no negative effect on two predator Toxorhynchites species. MRID 50443507. PloS One 8, e58805. doi: 10.1371/journal.pone.0058805
Okuma D. M., Bernardi D., Horikoshi R. J., Bernardi O., Silva A. P., and Omoto C. (2018). Inheritance and fitness costs of Spodoptera frugiperda (Lepidoptera: Noctuidae) resistance to spinosad in Brazil. Pest Manage. Sci. 74, 1441–1448. doi: 10.1002/PS.4829
Omoto C., Bernardi O., Salmeron E., Sorgatto R. J., Dourado P. M., Crivellari A., et al. (2016). Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manage. Sci. 72, 1727–1736. doi: 10.1002/ps.4201
Overton K., Maino J. L., Day R., Umina P. A., Bett B., Carnovale D., et al. (2021). Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop Prot. 145, 105641. doi: 10.1016/J.CROPRO.2021.105641
Reavey C. E., Walker A. S., Joyce S. P., Broom L., Willse A., Ercit K., et al. (2022). Self-limiting fall armyworm: a new approach in development for sustainable crop protection and resistance management. BMC Biotechnol. 22, 5. doi: 10.1186/s12896-022-00735-9
Simmons G. S., McKemey A. R., Morrison N. I., O’Connell S., Tabashnik B. E., Claus J., et al. (2011). Field performance of a genetically engineered strain of pink bollworm. PloS One 6, e24110. doi: 10.1371/journal.pone.0024110
Spinner S. A. M., Barnes Z. H., Puinean A. M., Gray P., Dafa’alla T., Phillips C. E., et al. (2022). New self-sexing Aedes aEgypti strain eliminates barriers to scalable and sustainable vector control for governments and communities in dengue-prone environments. Front. Bioengineering Biotechnol. 10. doi: 10.3389/fbioe.2022.975786
Keywords: biological control, genetically engineered, fall armyworm, insect, lepidoptera, pest management, resistance management
Citation: Reavey CE, Domingues FA, Ercit K, Pinto RL, Naish N, Yadav M, Frazon T, Cabala K, Sulston E, Pickl-Herk A, Edwards M, Miraldo LL, Abbade Neto D, Darrington M, Silva L, Furquim FE, Rodrigues JG, de Oliveira Simoni I, Silva T, Magalhaes W, Marubbi T, Poletto M, Sperry BD, Treanor D, McAlinden J, Buckby S, de Andrade Bettoni T, Couto de Abreu HM, Verza NC, Rose NR, Frandsen GK, Morrison NI and Matzen KJ (2025) Field Performance of a Self-Limiting, Genetically Engineered Fall Armyworm for Biological Pest Management. Front. Agron. 7:1613689. doi: 10.3389/fagro.2025.1613689
Received: 23 April 2025; Accepted: 21 July 2025;
Published: 22 August 2025.
Edited by:
Oscar Liburd, University of Florida, United StatesReviewed by:
Dr. Ravendra Kumar, G. B. Pant University of Agriculture and Technology, IndiaYing Yan, University of Giessen, Germany
Robert Meagher, Agricultural Research Service (USDA), United States
Copyright © 2025 Reavey, Domingues, Ercit, Pinto, Naish, Yadav, Frazon, Cabala, Sulston, Pickl-Herk, Edwards, Miraldo, Abbade Neto, Darrington, Silva, Furquim, Rodrigues, de Oliveira Simoni, Silva, Magalhaes, Marubbi, Poletto, Sperry, Treanor, McAlinden, Buckby, de Andrade Bettoni, Couto de Abreu, Verza, Rose, Frandsen, Morrison and Matzen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Catherine E. Reavey, Y2F0aGVyaW5lLnJlYXZleUBveGl0ZWMuY29t; Kelly J. Matzen, a2VsbHkubWF0emVuQG94aXRlYy5jb20=
†These authors have contributed equally to this work and share first authorship
 Catherine E. Reavey
Catherine E. Reavey Felipe A. Domingues2†
Felipe A. Domingues2† Ricardo L. Pinto
Ricardo L. Pinto Edward Sulston
Edward Sulston Leonardo L. Miraldo
Leonardo L. Miraldo Natalia C. Verza
Natalia C. Verza Kelly J. Matzen
Kelly J. Matzen