- 1International Institute of Tropical Agriculture, Dar es Salaam, Tanzania
- 2Department of Zoology and Wildlife Conservation, University of Dar es Salaam, Dar es Salaam, Tanzania
- 3Warwick Crop Centre School of Life Sciences, University of Warwick Innovation Campus, Stratford-upon-Avon, United Kingdom
- 4Department of Agricultural Research Services, Tanzania Agricultural Research Institute, Ukiriguru, Tanzania
Introduction: The whitefly, Bemisia tabaci, is a serious pest of cassava that causes yield loss through physical damage and vectoring of viruses that cause devastating cassava mosaic (CMD) and cassava brown streak (CBSD) diseases. The objective of this study was to evaluate the efficacy of commercially available entomopathogenic fungi biopesticides (EPFs) and cutting dipping in insecticides alone and in combination with spraying EPFs against cassava B. tabaci.
Methods: Laboratory experiments were conducted to test three commercial EPFs against cassava whitefly at IITA Tanzania. Data were recorded for nymph mortality. Field experiments were conducted at three Tanzania Agricultural Research Institute (TARI) stations (Chambezi, Mkuranga, and Ukiruguru) to evaluate the efficacy of two EPFs, and the efficacy of cutting dipping in insecticides combined with spraying EPF against B. tabaci during the period of December 2021 to August 2023. The experimental design for field experiments was a randomized complete block design with four replicates. The data recorded for field experiments were on whitefly and nymph numbers, CMD and CBSD incidence and severity, and root yield.
Results and discussion: Mortality levels caused by EPFs under laboratory conditions were 35%–86% at 14 days after application. In field experiments, EPFs reduced the proportion of healthy nymphs by 64%–75% compared with the control, with no effect on adult whitefly numbers, CBSD, and root yield. For cutting dips in insecticides at Chambezi, MandiPlus, the most effective treatment, reduced adult whiteflies by 85% and nymphs by 88%, CMD incidence by 59%, and CBSD by 46% and increased stem number by 119% and root yield by 50%.
Conclusion: These findings confirm that the application of MandiPlus through cutting dips is effective at reducing whitefly populations on cassava in Tanzania, reducing virus incidence and increasing yield. Application of entomopathogenic fungi under field conditions for control of cassava whitefly does not confer any significant benefits in terms of disease reduction and yield gain. Cutting dipping in insecticides is recommended for adoption as a component in IPM strategy for managing whiteflies and the viruses that they transmit.
1 Introduction
The whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae), is a polyphagous and widely distributed pest on vegetables, field, and ornamental crops. Damage occurs when B. tabaci feeds on the leaves and secretes honeydew. This acts as a medium for sooty mold, which affects plant photosynthesis (Perring et al., 2018). In addition, this hemipteran is a key vector of economically important viruses causing cassava mosaic disease (CMD) and cassava brown streak disease (CBSD) in cassava (Dubern, 1994; Maruthi et al., 2005, 2017). These diseases together can severely reduce cassava productivity in sub-Saharan Africa, causing annual losses of up to US$1 billion to resource poor farmers (Legg et al., 2006, 2011, 2014). Since the outbreak of severe CMD in the 1980s and an expanding epidemic of CBSD from 2004 in Uganda, these diseases have been spreading rapidly. Among the factors responsible for the spread of these diseases was the rapid increase in the abundance of whiteflies (Legg et al., 2014).
Different methods have been used worldwide to control B. tabaci, for example, the use of insecticides and biocontrol using natural enemies such as parasitoids. The principal control method for B. tabaci is the use of broad-spectrum insecticides (Palumbo et al., 2001; Horowitz et al., 2020). The most widely used conventional insecticides are the neonicotinoids, which are utilized in more than 120 countries on 450 crops (Bakker et al., 2020). Neonicotinoids are applied through various application techniques (foliar, seed treatment, soil drench, and stem applications) and are effective in controlling sucking and biting insects such as whiteflies, aphids, thrips, leaf miners, beetles, and lepidopterans (Jeschke et al., 2011). Imidacloprid, the first neonicotinoid introduced in the early 1990s, and thiamethoxam introduced in 1998 are among the most used insecticides in prophylactic seed treatments and foliar application (Bakker et al., 2020; Goulson, 2013; Jeschke et al., 2011; Simon-Delso et al., 2015). Thiamethoxam exhibits exceptional systemic characteristics and is used for broad range control of crop pests such as whiteflies, aphids, jassids, thrips, rice hoppers, beetle, and wireworms, as well as some lepidopteran species. Thiamethoxam is developed both for foliar/soil applications and as a seed treatment for use in most agricultural crops all over the world (Maienfisch et al., 2001).
Conventional synthetic insecticides widely deployed in crop protection are harmful to humans, non-target insects, and aquatic invertebrates and cause environmental degradation and pollution of agroecosystems (Borsuah et al., 2020). Widespread indiscriminate use of these insecticides has triggered insect resistance development, rendering most of the available chemistries ineffective (Horowitz et al., 2020). This scenario has triggered increased damaging effects of pests, caused huge yield losses, increased production costs, and has compelled crop protection companies to invest in finding novel compounds with new modes of action (Sparks and Bryant, 2021; Sparks et al., 2021). There is a need to develop strategies that will include the judicious use of the current effective neonicotinoids, as the emergence of widespread insecticide resistance can pose threats to crop production and food security (Simon-Delso et al., 2015). Insecticide resistance in B. tabaci is widespread, and it has evolved to most of the insecticides used with approximately 650 reported cases detected to more than 60 active ingredients (Horowitz et al., 2020).
With the current stringent insecticide legislation, the exploitation of biocontrol agents and biopesticides has been widely considered as a suitable alternative to the use of chemical pesticides (Chandler et al., 2011; Singh et al., 2019). Mycoinsecticides including entomopathogenic fungi (EPF) play a vital role as an alternative to chemical pesticides in integrated pest management (IPM) and insecticide resistance management (IRM) programs in a more resilient and sustainable manner (Chandler et al., 2011; Li et al., 2024; Sain et al., 2021). Researchers in the past have demonstrated entomopathogenic fungi (EPF) as potential biopesticides. Approximately 700 species of EPF from approximately 90 genera are known to be pathogenic to arthropod pests (Sani et al., 2020). Studies of whitefly management using EPFs have focused on a few such as Beauveria bassiana (Bals-Criv.) Vuill., Cordyceps fumosorosea (Wize) Kepler, B. Shrestha and Spatafora [=Isaria fumosorosea (Wize) or Paecilomyces fumosoroseus (Wize) A.H.S. Brown and G. Smith], Metarhizium anisopliae (Metschn.) Sorok., and Akanthomyces lecanii (Zimm.) Spatafora, Kepler & B. Shrestha [=Lecanicillium lecanii (Zimm.) Zare & W. Gams], Akanthomyces muscarius (Petch) Spatafora, and Kepler & B. Shrestha [=Lecanicillium muscarium (Petch) Zare & W. Gams] (Chandler, 2017; Lacey et al., 2015; Li et al., 2024; Perring et al., 2018; Sain et al., 2021). Several studies have explored the efficiency of different EPF species to manage B. tabaci in both field and glasshouse settings (Aguilera Sammaritano et al., 2016; Garrido-Jurado et al., 2017; Li et al., 2024; Sain et al., 2021; Sani et al., 2020).
Cassava cuttings as seed material permit insecticide application with the soaking of the cuttings. This provides a protection for the initial growth phase. Studies conducted in Tanzania, Brazil, Uganda, and Malawi have shown positive impacts from treating planting materials with formulations of protective systemic compounds before planting (Bayiyana et al., 2023; Caspary et al., 2023; de Oliveira et al., 2020; Issa et al., 2022; Nwokoro et al., 2024; Omongo et al., 2022). The advantages of seed dressings/cutting dips are that they require no action from the farmer and prophylactically protect all parts of the crop for several months following sowing, and they are also regarded as providing better crop targeting than spray applications (Jeschke et al., 2011).
Recently, there has been a growing interest in the use of biopesticides as a part of IPM programs to reduce negative impacts of synthetic insecticides to the environment and non-target organisms. This could also mitigate the development of insecticide resistance, hence prolonging the efficacy and use of insecticides (Dimase et al., 2024; Li et al., 2024). Alternative control strategies are urgently needed for B. tabaci that contribute to economic gain through effective pest control and reducing resistance, and which are environmentally friendly. In this study, we evaluated the efficacy of three commercial entomopathogenic fungi (EPF) biopesticides, Lecatech (Akanthomyces lecanii), Mycotal (Akanthomyces muscarius), and Beauvitech (Beauveria bassiana), against cassava B. tabaci under laboratory conditions. The efficacy of the two most lethal EPFs—Mycotal and Lecatech—was then determined through field trials. The efficacy of cutting dipping in insecticides (MandiPlus) in combination with spraying the EPF Lecatech against B. tabaci was also tested in the field. The parameters measured were nymph mortality and emergence for the laboratory experiments, and adult whitefly and nymph numbers, cassava mosaic disease (CMD), and cassava brown streak disease (CBSD) incidence and yield for the field experiments. The overall aim of this study was to identify effective control measures for cassava B. tabaci, which would be of benefit to the millions of cassava seed and root producers in Tanzania, as well as cassava producers elsewhere.
2 Materials and methods
2.1 Study locations
The laboratory experiment of this study was conducted at the International Institute of Tropical Agriculture (IITA), Dar es Salaam, Tanzania (6.7566°S, 39.2350°E, ~19 m.a.s.l). The laboratory temperature range was at 25 ± 1°C and with a relative humidity of 65 ± 5%. The plants used for the laboratory study were partially held in the screen house with temperature of 25°C–35°C and 65%–75% RH. The field experiments to evaluate EPF biopesticides (Lecatech and Mycotal) were conducted in research plots at the Tanzania Agricultural Research Institute (TARI) Chambezi station in Bagamoyo District and the TARI Mkuranga station in Mkuranga District, Coast Region, Tanzania. The field experiments to evaluate cutting dipping in insecticides in combination with spraying Lecatech were conducted in research plots at the TARI Chambezi station and the TARI Ukiriguru station in Misungwi District (Mwanza Region, Tanzania). Chambezi (6.5164°S, 38.9154°E, ~34 m.a.s.l) has an annual average temperature range 26°C–28°C, relative humid 65%–90%, annual rainfall 1,000–1,300 mm, and sandy soils. Mkuranga (7.1180°S, 39.2078°E, ~50 m.a.s.l) has an annual average temperature range 26°C–28°C, relative humid 65%–95%, annual rainfall 800–1,000 mm, and sandy soils. Ukiriguru (2.7167°S, 33.0167°E, ~1,150 m.a.s.l) has an annual average temperature range 22°C–25°C, relative humid 50%–90%, annual rainfall 800–1,200 mm, and sandy loam soils. Chambezi and Mkuranga are hotspots for CMD and CBSD due to the presence of abundant whiteflies and high virus inoculum pressure. Ukiriguru has high whitefly numbers but low CMD and CBSD inoculum pressure.
2.2 Cassava plant materials, whitefly colonies, entomopathogenic fungi biopesticides, and insecticides
Virus-free planting materials of cassava variety ‘Albert’ was used for the EPF studies in the laboratory and research plots, whereas variety ‘Kiroba’ was used for cutting dipping in insecticides combined with spraying Lecatech. Clean stems for Albert were collected from the TARI Ilonga station, Morogoro. Albert is known to be whitefly-susceptible and CBSD-susceptible but tolerant to CMD. Kiroba was collected from TARI Ilonga and TARI Ukiriguru. Kiroba is known to host moderate numbers of whiteflies, is susceptible to CMD, and is tolerant to CBSD. The variety Albert was used in initial experiments in the laboratory and entomopathogenic fungi studies because it is used for research purposes but not widely cultivated by farmers due to its susceptibility to CBSD and susceptibility to whiteflies. In the subsequent experiments on cutting dipping in MandiPlus and application of EPF Lecatech, variety Kiroba was selected because it is one of the mostly widely cultivated improved varieties in Tanzania. The whitefly B. tabaci haplogroup for the laboratory studies was sub-Saharan Africa–East and Southern Africa (SSA-ESA—equivalent to mitotype SSA1-SG3 commonly found in the Coastal region of Tanzania) (Wosula et al., 2017). The whiteflies were collected from cassava plants at Chambezi in Bagamoyo District, Coast Region, Tanzania, in May 2019 and introduced to potted cassava plants placed in a 50 × 50 × 100 cm netted cages. The cassava plants were grown in 7.5-L pots containing a mixture of soil and farmyard manure at a 4:1 ratio, and the whitefly colonies were reared on the cassava plants in the screenhouse at 25°C–30°C and 65%–75% RH. Whiteflies were transferred to fresh 1-month-old cassava plants at intervals of 4–6 weeks to maintain the colonies. Three commercial entomopathogenic fungi (EPF) biopesticides tested were Beauvitech = Beauveria bassiana, Lecatech = Akanthomyces lecanii (Dudutech Ltd., Kenya), and Mycotal® WG = Akanthomyces muscarius (Koppert Biological Systems, Netherlands). These EPF strains are known to be infective to homopteran insects and so were considered suitable candidates for testing against cassava B. tabaci. The ‘MandiPlus’, a formulation prepared by mixing a systemic insecticide Cruiser® 350FS (thiamethoxam 350 g/L) and a systemic fungicide Maxim XL 035FS (fludioxonil 25 g/L and Metalaxyl-M [mefenoxam] 10 g/L) and white vinyl paint, was developed by the Syngenta Foundation for Sustainable Agriculture to boost cassava seed multiplication rate (de Oliveira et al., 2020). A MandiPlus solution was prepared by mixing Cruiser® 350FS and Maxim XL 035FS purchased from Syngenta East Africa Ltd. at the rate of 800 mL Cruiser + 1.6 L Maxim. To this was added 2 L of white vinyl paint in 100 L of water. Imidacloprid (Attakan 350SC, Arysta Life Science Tanzania Ltd) was included as a standard. The vinyl paint was added to act as a sticker.
2.3 Laboratory experiment on efficacy of entomopathogenic fungi biopesticides
Three-week-old plants of the variety Albert were used with five plants (replicates) per treatment. A total of 30 adult whiteflies which had emerged within 4 days from 6- to 8 week-old colonies were introduced to potted cassava plants. These plants had a single leaf (second from the top, all other leaves were removed from the plants 24 h before the experiment), and whiteflies were confined in transparent perforated bread bags covering each test plant. The whiteflies were removed after 48 h and plants were held in the screen house until nymphs developed to the second and third instar stage. The plants were then transferred to the laboratory and the total number of nymphs counted with the aid of a light microscope. The spore suspension for the three commercial EPF biopesticides (Beauvitech, Lecatech, Mycotal) were prepared according to the manufacturer’s recommendations (20 g/20 L of water) and were sprayed uniformly on nymph-bearing leaves just before runoff using a 100-mL finger-tip sprayer. Control (untreated) plants were sprayed with distilled water. The plants were allowed to stand on the bench for 10 min until the leaves dried. The plants were then covered with a misted polythene bag to maintain high humidity for 72 h, after which the polythene bags were removed, and plants were covered with bread bags (to confine emerging whiteflies) and held in the laboratory in an open area that allowed natural light to sustain survival of cassava plants for 2 weeks. The experimental design was a complete randomized design (CRD). Data were recorded at 5, 10, and 14 days for shriveled/dead and emerged nymphs. The experiment was repeated two times giving a total of 15 plants per treatment.
2.4 Field experiment on efficacy of entomopathogenic fungi biopesticides
Two entomopathogenic fungi (EPF) biopesticides—Lecatech and Mycotal—were evaluated against cassava whitefly under field conditions. The experiment was carried out at two locations—Chambezi and Mkuranga in the Coast Region of Tanzania. The cassava plants of variety Albert were established in December 2021 (short rainy season ‘Vuli’) and harvested in August 2022. The experimental design was a randomized complete block design (RCBD) with four replicates/blocks. The cuttings (~20 cm long, ~7–9 nodes) were prepared and planted in a slanted position directly into the ground by inserting approximately two thirds of its length into the soil at a spacing of 1 m × 1 m. A total of 60 cassava plants were planted in each of the four blocks that were divided into subplot of 20 plants each. The three subplots in each block were randomly allocated the Mycotal, Lecatech, and control (untreated). The EPF biopesticides were sprayed at a double rate as per the manufacturer’s recommendations (20 g/20 L of water) once every month (2 weeks prior to data collection) using a standard 20-L Knapsack sprayer. A total of 10 plants from each subplot were randomly selected and tagged for monthly data collection. The data were recorded monthly for 6 months after planting (MAP) for live adult whiteflies (top fully opened five leaves), healthy and dead nymphs (14th leaf from the top or the lowest leaf in young plants), and CMD and CBSD incidence (proportion of symptomatic plants). CMD severity was assessed using the 1–5 scoring scale according to Sseruwagi et al. (2004)—score 1 = cassava plant showing no leaf symptom, 2 = mild distortion and mild chlorosis on the leaves, 3 = significant distortion and chlorosis on one-third of most leaves, 4 = extreme distortion and presence of mosaic patterns on two-third of most leaves and general reduction of leaf size, and 5 = very severe mosaic symptoms on all leaves, appearance of distortion, twisting, mis-shapen, and severe leaf reduction of most leaves accompanied by severe stunting of plants. CBSD severity was assessed using a score of 1–5, where 1 = no apparent symptoms; 2 = slight foliar feathery chlorosis and no stem lesions; 3 = prominent foliar feathery chlorosis, mild stem lesions, and no dieback; 4 = severe foliar feathery chlorosis, severe stem lesions, and no dieback; and 5 = defoliation, severe stem lesions, and die-back (Gondwe et al., 2003). At 10 MAP, the 10 plants from which monthly data were collected per plot, giving total of 40 plants per treatment, were harvested to determine number of roots per plant and root weight. The CBSD root incidence was assessed on all harvested roots from a sampled plant, using the 1–5 scoring scale where 1 = no apparent necrosis, 2 is ≤5% of root necrosis, 3 is 6%–10% of root necrosis, 4 is 11%–25% of root necrosis and mild root constrictions, and 5 is 25% of root necrosis with severe root constriction (Gondwe et al., 2003).
2.5 Field experiment on efficacy of cutting dipping in insecticides combined with spraying entomopathogenic fungi biopesticides
This study was conducted at two locations, TARI Chambezi and TARI Ukiriguru. The cassava plants of variety Kiroba were established in December 2022 (short rainy season ‘Vuli’) and harvested in August 2023. Lecatech was selected because its efficacy under field conditions was not significantly different compared with Mycotal, and it is affordable and available in Tanzania. MandiPlus and imidacloprid were applied by cutting dips. The cuttings (~20 cm long, ~7–9 nodes) were prepared from cassava stems and placed in polythene bags and immersed in MandiPlus and imidacloprid solutions for 1 h. The 1-h duration was selected based on a previous study which recorded higher whitefly mortality compared with shorter durations of 15 and 30 min (Issa et al., 2022). The cuttings were removed and air dried for 20 min. Cuttings were then planted as described previously. Lecatech was applied monthly (2 weeks prior to data collection) for 6 months (six times). The experimental design was a randomized complete block design (RCBD) with four replicates per treatment with 30 plants per plot. Field assessments were conducted at 1MAP, 3MAP, and 6MAP. Data were collected on 12 plants per treatment per replicate to determine cutting sprouting percentage at 1MAP, abundance of adult whiteflies on the top fully opened five leaves and number of nymphs on the 14th leaf or lowest leaf (for young plants) of the tallest stem of each sampled plant, incidence of CMD and CBSD, and severity of CMD and CBSD using the standard 1–5 scoring at 1MAP, 3MAP, and 6MAP, as well as stem number (stems per plant), stem height (base to shoot apex of tallest stem) at 6MAP, and root number and yield at 10MAP. The cost–benefit analysis for MandiPlus use was estimated using yield and sales for the Chambezi location. The yield in kg/plant was used to estimate yield per hectare by multiplying by 10,000 plants and the percentage of sprouting. The total income was estimated by multiplying the yield per hectare by the cost per tonne estimated at 245 USD (IPPMedia, 2024). The cost of cutting treatment with MandiPlus (cost of pesticides and dipping labor) was deducted to determine the net profit. Other costs such as purchase and transport of cassava clean planting material and preparation of cuttings were not included because the comparison was for clean planting material vs. clean planting material + MandiPlus.
2.6 Statistical analysis
The data were subjected to a one-way analysis of variance (ANOVA) using the generalized linear mixed effects models in RStudio version 4.3.2 (R Core Team) with the Quasibinomial distribution for nymph mortality/emergence, the Poisson distribution for whitefly adults and nymph numbers, the Gaussian distribution on log-transformed data for percentage incidence of CMD and CBSD, and percent and Gaussian distribution for yield data. The fixed factor was treatment, and the random factors were block and replication. Treatment effects were considered significant at P = 0.05. The LSMEANS statement was used to obtain least squares means, and the Tukey–Kramer test was used for mean separation. The disease severity scores for CMD and CBSD were subjected to a non-parametric Kruskal–Wallis test, and Dunn’s test with Bonferroni was used for mean separation. Treatment means and standard errors were obtained using the means and standard error statements.
3 Results
3.1 Efficacy of EPF biopesticides on whitefly nymphs under laboratory conditions
Under laboratory conditions, EPF treatments caused moderate to high whitefly nymph mortality (Table 1). Among the EPFs evaluated, Mycotal exhibited the highest efficacy in inducing nymph mortality, with Lecatech demonstrating intermediate effectiveness, whereas Beauvitech displayed the least impact on nymph survival. Five days after application of EPFs, the percentage of dead nymphs was significantly higher (P < 0.0001) for Mycotal (57.5%), Lecatech (30.4%), and Beauvitech (16.5%) compared with the control (0.5%) (Table 1). At 10 days, the percentages of dead nymphs significantly increased (P < 0.0001) by 82.3%, 43.3%, and 31.7% on Mycotal, Lecatech, and Beauvitech, respectively, compared with control (1.1%). At 14 days, the percentages of dead nymphs were greater than at 5 days and differed for each of the treatments (P < 0.0001). Percent mortality was 86.0% for Mycotal, 48.2% for Lecatech, 34.6% for Beauvitech, and 2.0% for the control (Table 1). The nymph emergence was less than 2% and not significantly different at 5 days in treatments and control. At 10 days, Mycotal had the least emerged nymphs (6.0%), followed by Lecatech (24.3%) and Beauvitech (28.5%), which were all significantly different compared with the control (45.5%). At 14 days, Mycotal had the least emerged nymphs (13.7%), followed by Lecatech (49.6%) and Beauvitech (60.6%), which were all significantly different compared with the control (90.7%) (Table 1). All the three EPF treatments decreased survival rates of whitefly nymphs with Mycotal as the most lethal followed by Lecatech and Beauvitech.
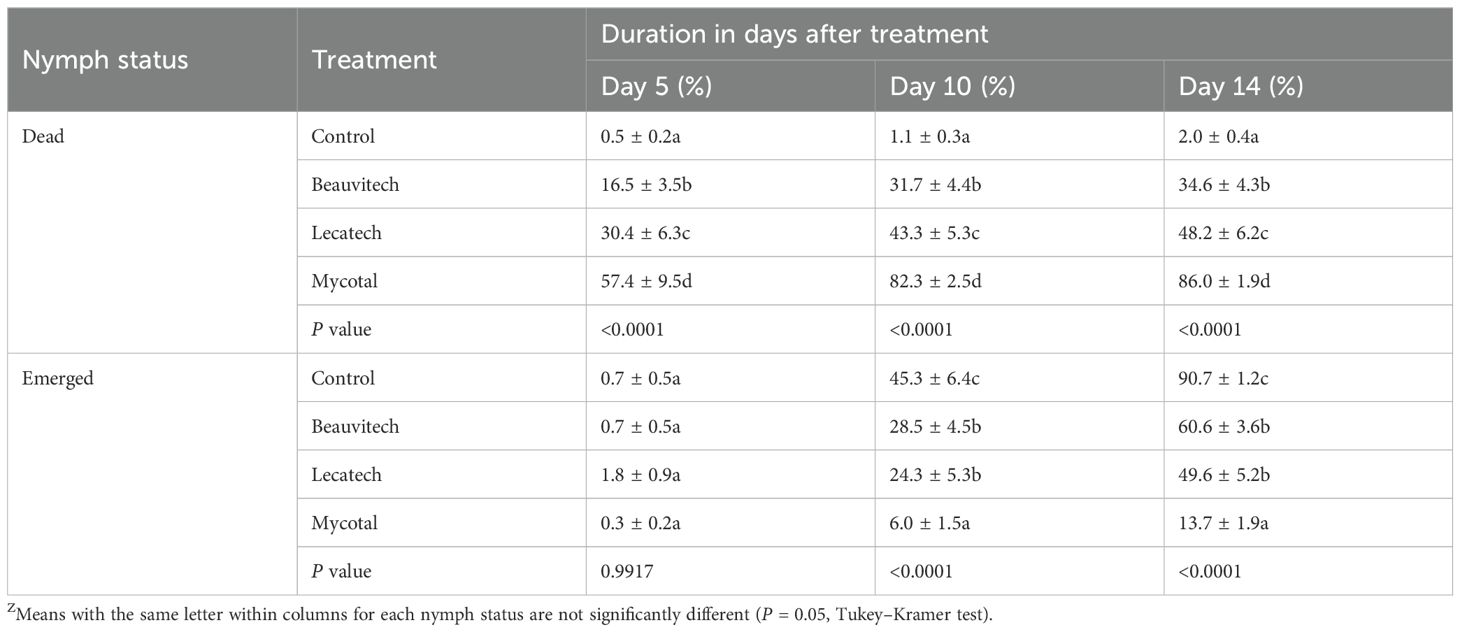
Table 1. The percentage of dead nymphs and emerged nymphs at 5, 10, and 14 days after application of EPF biopesticides under laboratory conditions (means ± SEM)Z.
3.2 Efficacy of EPF biopesticides on whitefly under field conditions
3.2.1 Whitefly abundance
The adult whitefly numbers on the top five leaves counted monthly for up to 6 months after planting were not significantly different in EPF treatments compared with the control except for Mycotal (P = 0.0023) 3 months after planting (3MAP) at Chambezi (Figures 1A, B). At Chambezi, cassava plants treated with Mycotal had 68% fewer (P < 0.05) healthy nymphs compared with the control, whereas for the Lecatech treatment, there were 64% fewer healthy nymphs than in the control (Figure 2A). The average number of dead nymphs on Mycotal-treated (38.9) and Lecatech-treated (39.0) plants were on average 28 times higher during the 6-month duration compared with the control (1.4) (Figure 2B). At Mkuranga, cassava plants treated with Mycotal had 75% fewer (P < 0.05) healthy nymphs compared with the control, whereas for Lecatech, there were 69% fewer healthy nymphs than in the control (Figure 2C). The average number of dead nymphs on Mycotal-treated (43.2) and Lecatech-treated (33.6) plants were on average 47 and 36 times higher, respectively, during the 6-month duration compared with the control (0.9) (Figure 2D).
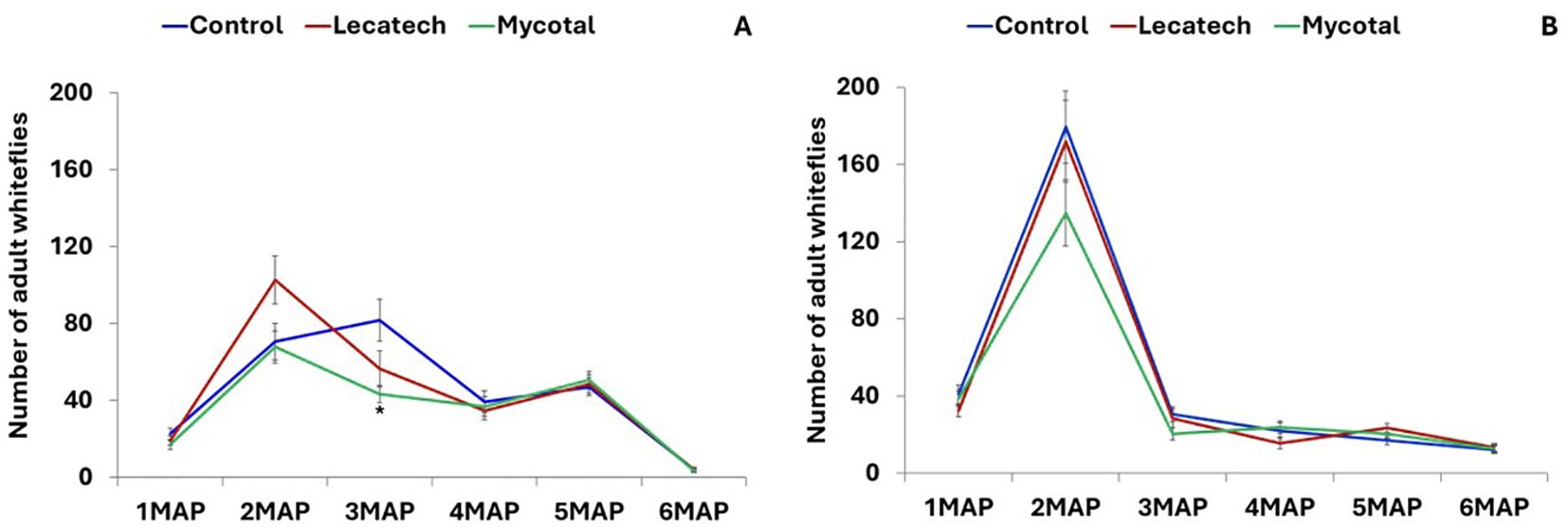
Figure 1. Adult whitefly numbers recorded from the top five leaves for a 6-month period at Chambezi (A) and Mkuranga (B). Field experiments on the application of EPF biopesticides. Treatment means denoted with an asterisk (*) within each MAP are significantly different from the control (P = 0.05, Tukey–Kramer test). MAP, months after planting.
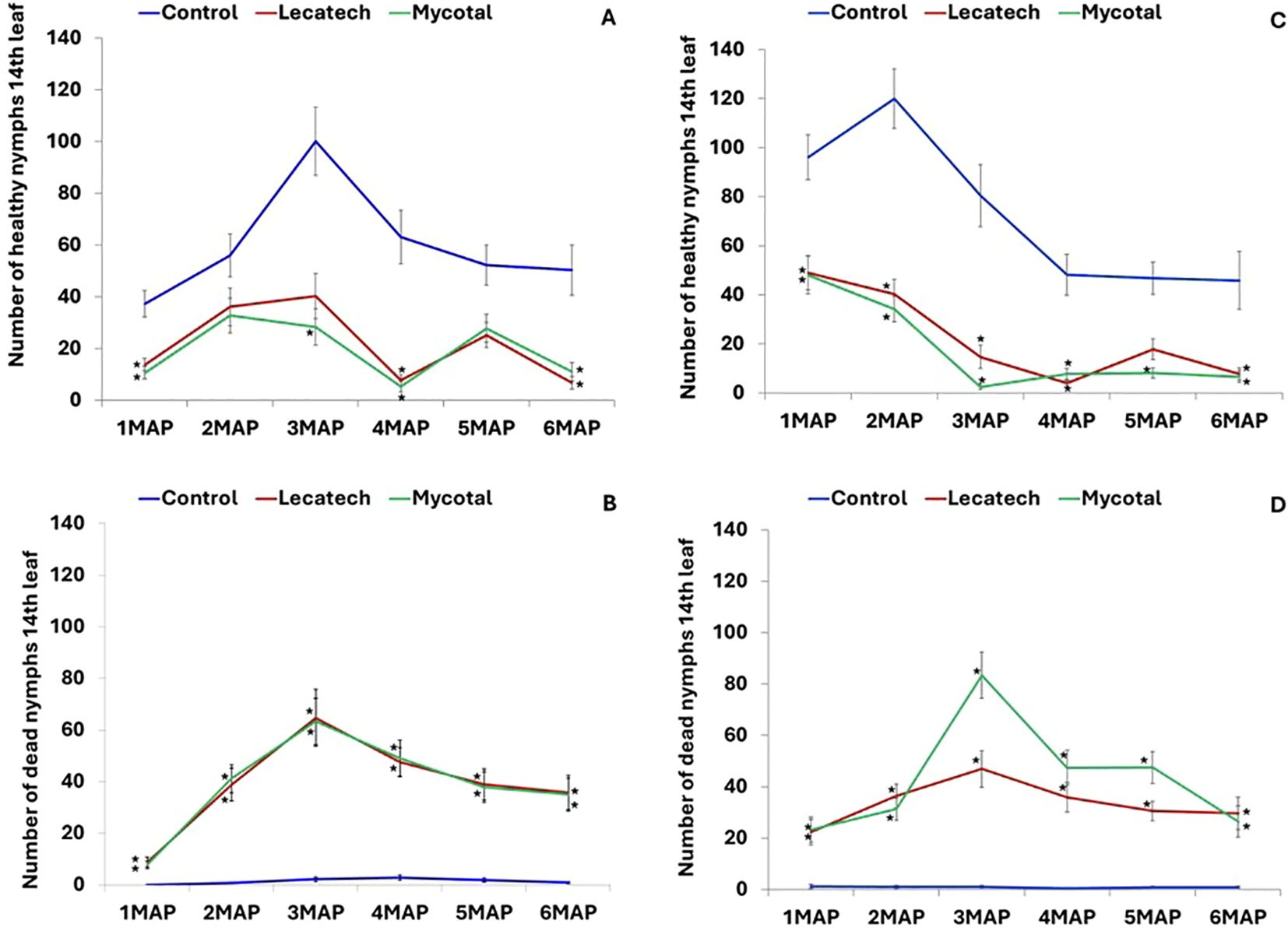
Figure 2. Whitefly nymph numbers on the 14th leaf for a 6-month period at Chambezi—healthy (A) and dead (B) and at Mkuranga—healthy (C) and dead (D). Field experiments on the application of EPF biopesticides. Treatment means denoted with an asterisk (*) within each MAP are significantly different from the control (P = 0.05, Tukey–Kramer test). MAP, months after planting.
3.2.2 Cassava mosaic disease and cassava brown streak incidence
CMD symptoms were not observed in cassava plants at either Chambezi or Mkuranga during the 6-month data collection period. CBSD incidence at the end of the experimental period in Chambezi was 100% for both EPF treatments as well as the control (Figure 3A). The same situation was recorded at Mkuranga, where all treatments had 100% incidence by the end of the experiment (Figure 3B). CBSD leaf symptom severity was not significantly different in Mycotal and Lecatech treatments compared with the control at either of the two locations for any of the experimental records (1MAP-6MAP) (Table 2).
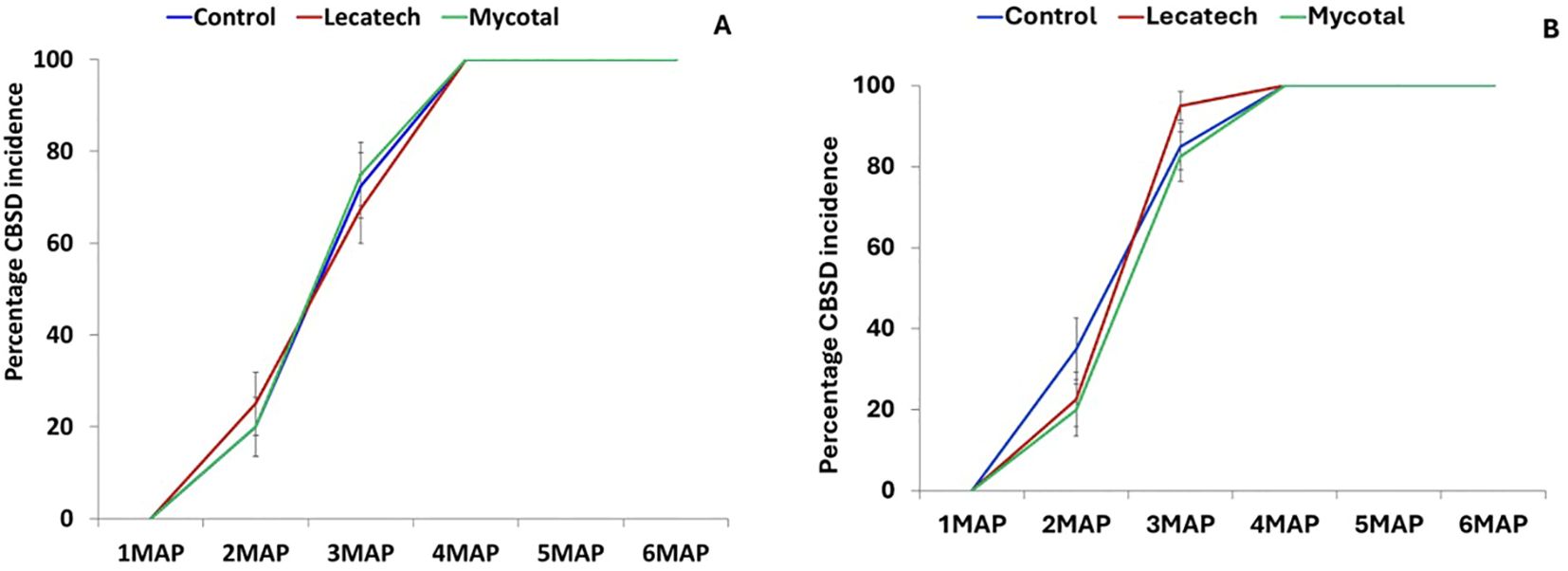
Figure 3. The percentage incidence of CBSD (A) at Chambezi and (B) at Mkuranga recorded for a 6-month period. Field experiments on the application of EPF biopesticides. Treatment means were not significantly different from the control (P = 0.05, Tukey–Kramer test). MAP, months after planting.
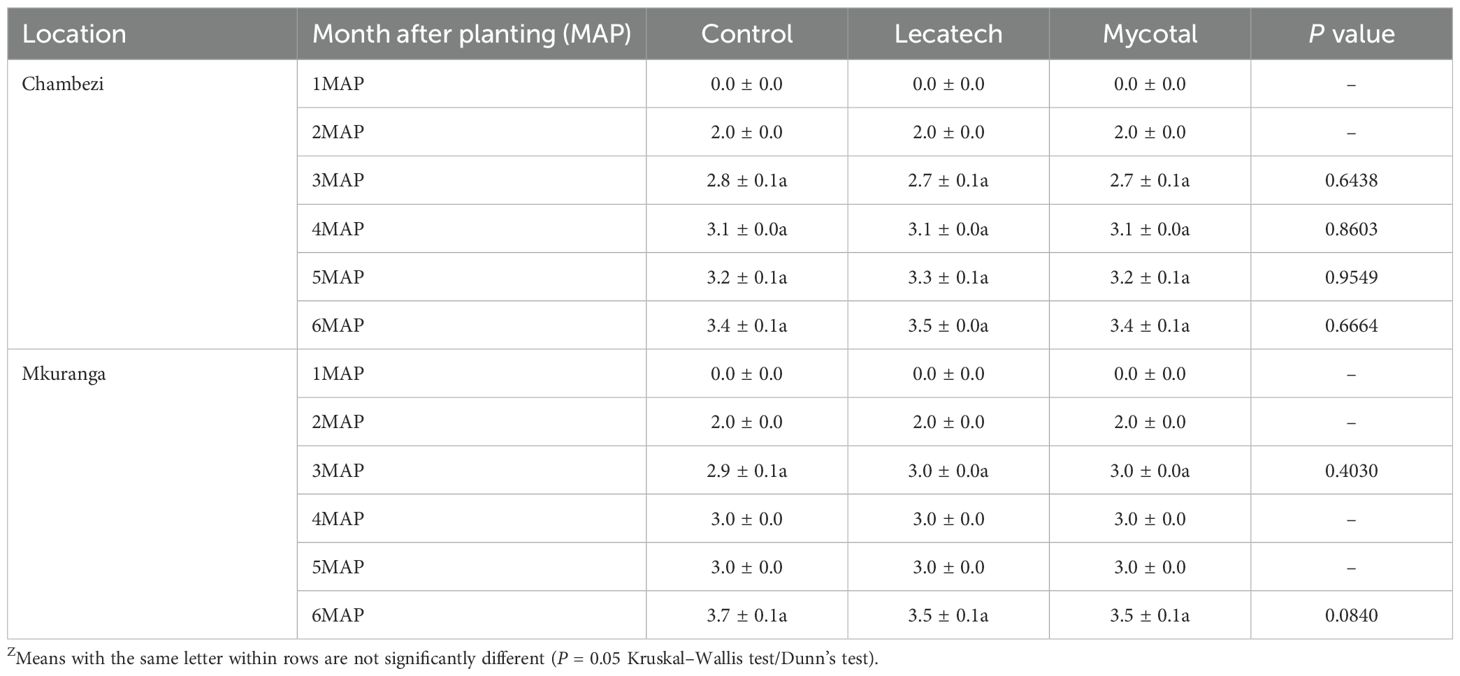
Table 2. The disease severity scores of CBSD in cassava plants sprayed with EPF biopesticides (means ± SEM)Z.
3.2.3 Yield parameters
Root CBSD incidence at Chambezi was not significantly different for Mycotal (93.3%) or Lecatech (92.8%) compared with the control (94.2%) (Table 3). Root CBSD severity values (3.1–3.3) also showed no significant difference between treatments. However, at Mkuranga, root CBSD incidence was significantly lower (P < 0.0001) for Mycotal (71.3%) and Lecatech (73.4%) compared with the control (93.4%). Furthermore, root CBSD severity was significantly lower (P = 0.0058) in Mycotal (2.8) compared with the control (3.3) (Table 3). The root number at Chambezi was not significantly different in treatments compared with the control, whereas at Mkuranga, Lecatech had significantly fewer roots (4.8) compared with the control (6.1). The root yield which ranged from 1.2 to 1.5kg/plant was not significantly different in EPF treatments compared with the control at both locations (Table 3).
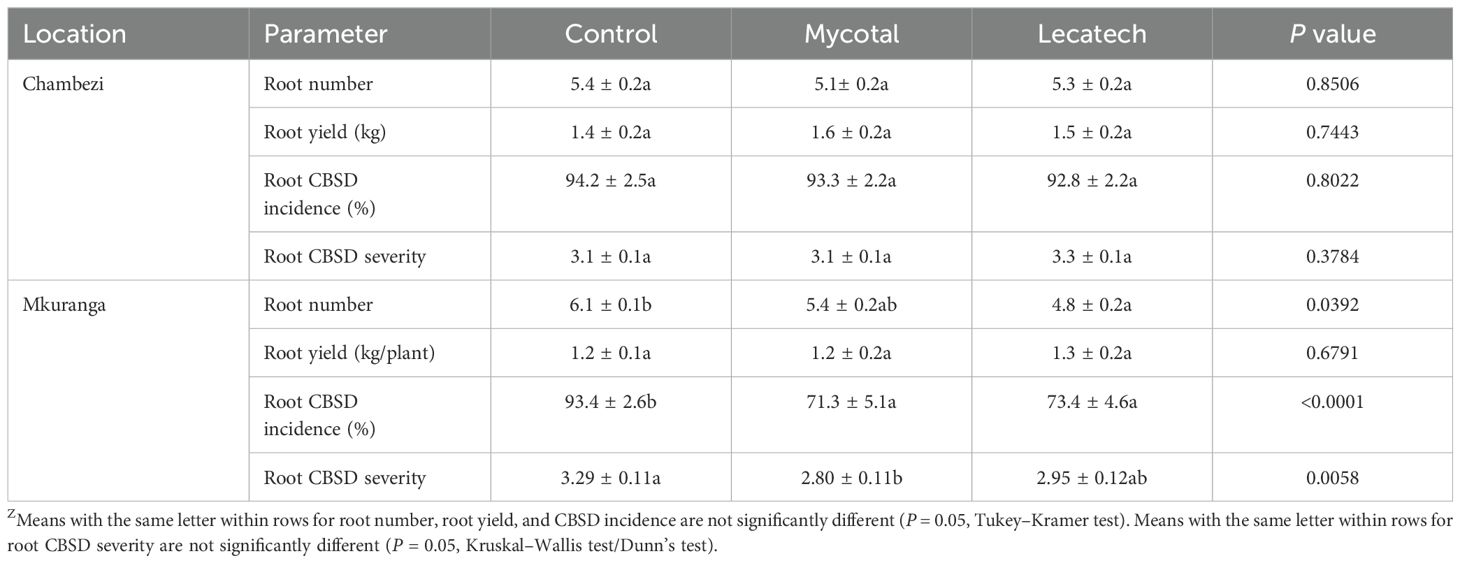
Table 3. Yield parameters and CBSD root incidence and severity of cassava plants sprayed with EPF biopesticides Mycotal and Lecatech (means ± SEM)Z.
3.3 Efficacy of cutting dipping in insecticides and application of EPF biopesticides on whitefly under field conditions
3.3.1 Whitefly abundance
This experiment combined dipping cuttings in insecticides once before planting and thereafter spraying of a commercial EPF (Lecatech) monthly for a duration of 6 months. At Chambezi, the average number of adult whiteflies on the top five leaves at 1MAP was significantly fewer than the control (P <0.0001) for all treatments, although all treatments including insecticides had fewer whitefly adults than Lecatech. At 3MAP, when whitefly numbers peaked, MandiPlus and MandiPlus + Lecatech had significantly fewer (P < 0.0001) adult whiteflies compared with the control. Imidacloprid and imidacloprid + Lecatech and Lecatech similarly had fewer whiteflies compared with the control. At 6MAP, MandiPlus and MandiPlus + Lecatech had significantly fewer whiteflies compared with other treatments, although the numbers were generally very low (Table 4). Nymphs were not present on the plants at 1MAP. At 3MAP MandiPlus, MandiPlus + Lecatech, imidacloprid, imidacloprid + Lecatech, and Lecatech had significantly fewer (P < 0.0001) nymphs compared with the control, although nymph numbers in all treatments that included insecticides were significantly less than those in the Lecatech only treatment. At 6MAP, all treatments had significantly fewer nymphs than the control, although reductions in nymph numbers were again greatest for the MandiPlus and MandiPlus + Lecatech treatments (Table 4). The percentage reduction of adult whiteflies in treatments at 3MAP was as follows: imidacloprid (57.4%), imidacloprid + Lecatech (65.4%), MandiPlus (83.3%), MandiPlus + Lecatech (79.6%), and Lecatech (35.8%) compared with the control. The percentage reduction of nymphs in treatments at 3MAP was as follows: imidacloprid (72.0%), imidacloprid + Lecatech (76.3%), MandiPlus (91.1%), MandiPlus + Lecatech (85.8%), and Lecatech (53.8%) compared with the control. At Ukiriguru, the average number of adult whiteflies on the top five leaves at 1MAP was significantly fewer in the imidacloprid, imidacloprid + Lecatech, MandiPlus, MandiPlus + Lecatech, and Lecatech treatments compared with the control, although all treatments with insecticides had fewer adult whiteflies than the Lecatech treatment (Table 5). At 3MAP, the percentage reduction in adult whitefly numbers for each of the treatments was as follows: imidacloprid (82.8%), imidacloprid + Lecatech (85.1%), MandiPlus (86.0%), MandiPlus + Lecatech (90.6%), and Lecatech (22.7%) compared with the control. At 6MAP, whitefly numbers were very low averaging less than one in treatments and the control (Table 5). There were no nymphs recorded in any of the treatments at 1MAP. At 3MAP, the percentage reduction in nymph numbers was as follows: imidacloprid (83.0%), imidacloprid + Lecatech (88.0%), MandiPlus (89.2%), MandiPlus + Lecatech (92.0%), and Lecatech (55.2%) compared with the control. At 6MAP, nymph numbers were very low averaging less than two in treatments and the control.
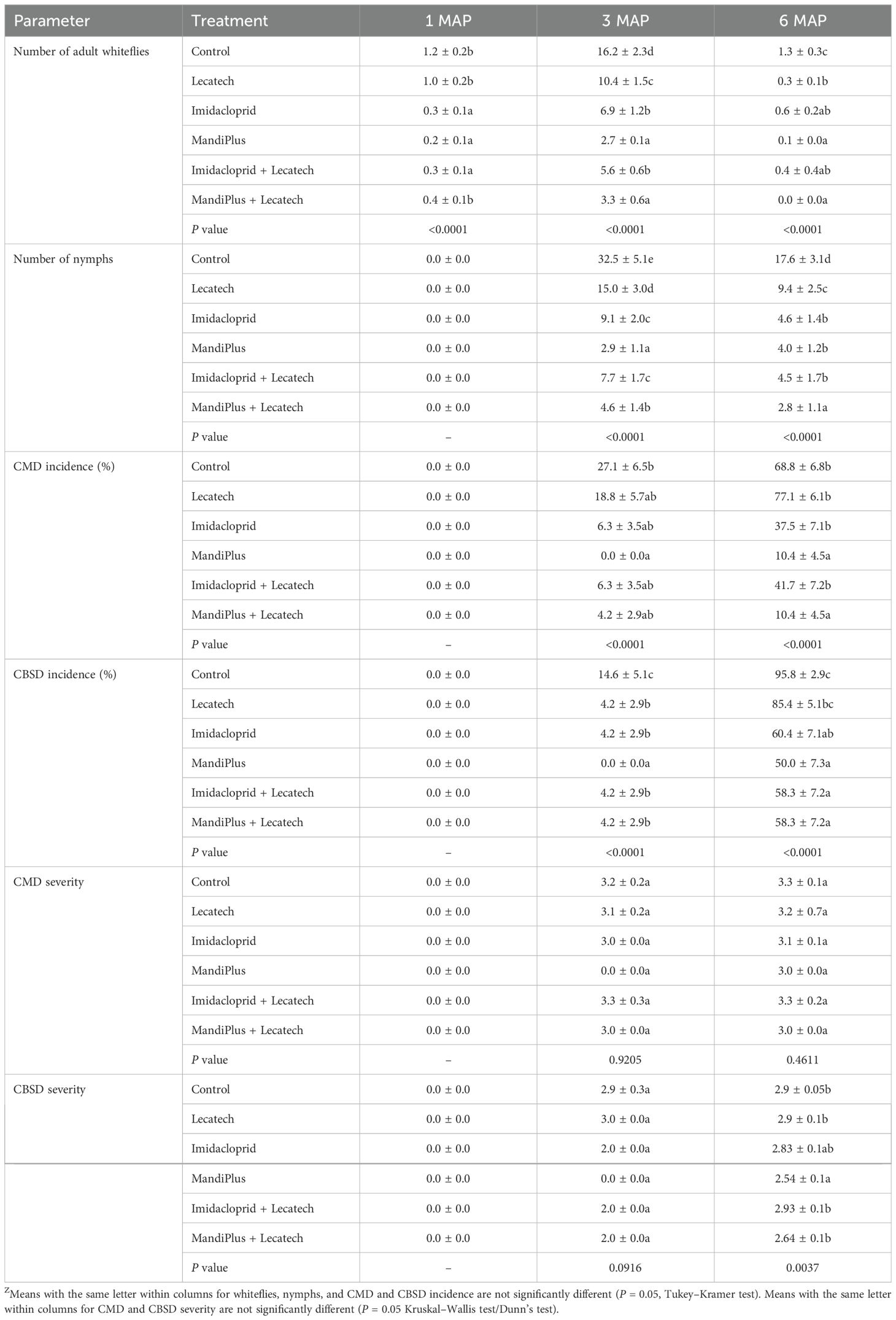
Table 4. Whitefly adult and nymph numbers, and CMD and CBSD incidence and severity at Chambezi for cutting dipping in insecticides and application of EPF Lecatech (means ± SEM)Z.
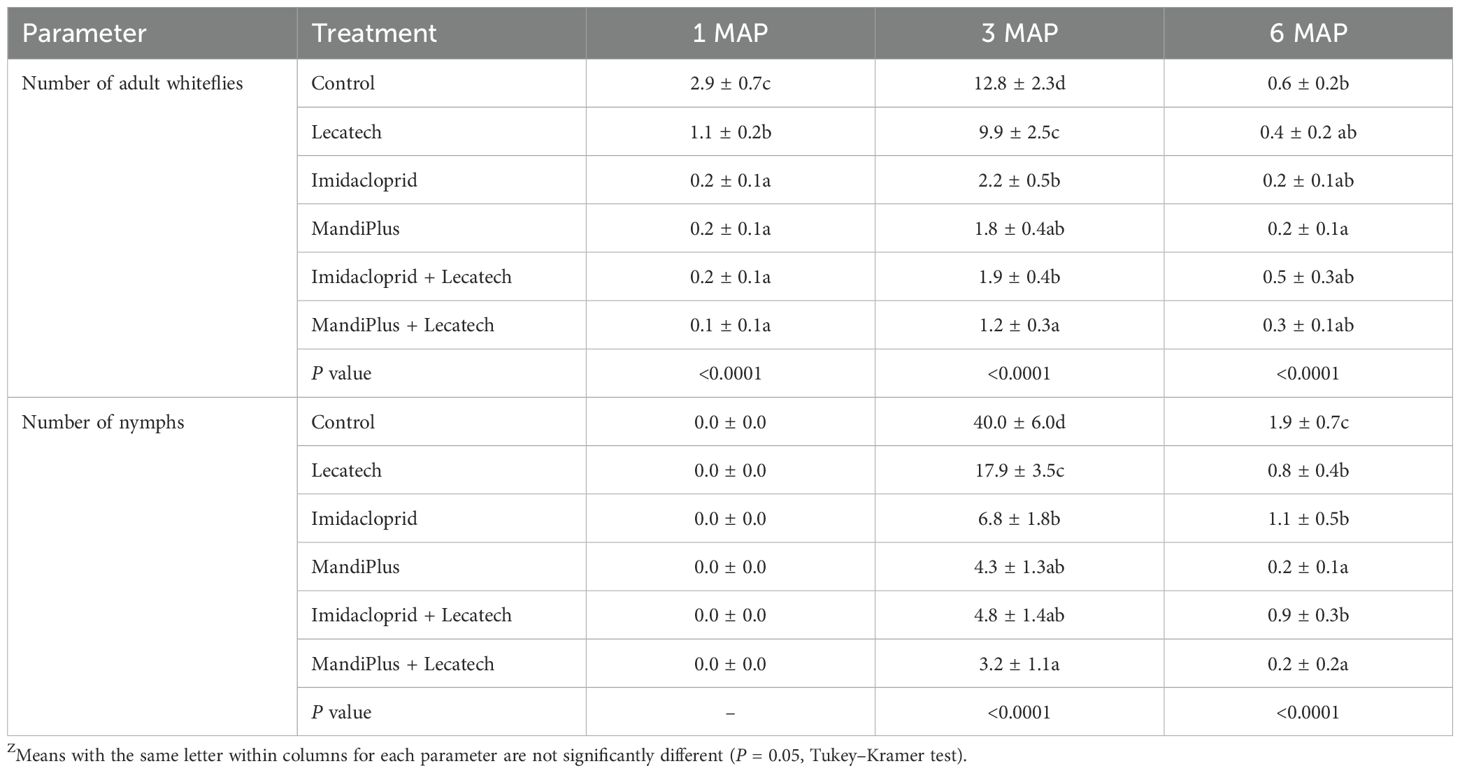
Table 5. Whitefly adult and nymph numbers at Ukiriguru for cutting dipping in insecticides and application for EPF Lecatech (means ± SEM)Z.
3.3.2 Cassava mosaic and cassava brown streak incidence
At Chambezi, CMD symptoms were not observed in plants at 1MAP. At 3MAP, no symptoms were observed in the MandiPlus treatment. MandiPlus + Lecatech, imidacloprid, and imidacloprid + Lecatech had lower CMD incidence 4.2%–6.3% compared with Lecatech (18.8%) and the control (27.1%), although these differences were not significant. At 6MAP, MandiPlus and MandiPlus + Lecatech had 10.4% CMD incidence, which was significantly lower than the 68.8% in the control. Imidacloprid, imidacloprid + Lecatech, and Lecatech had incidences of 37.5%, 41.7%, and 77.1%, respectively, which were not significantly different from the control (Table 4). CMD severity was not significantly different in treatments compared with the control with a score of ≥3 at 6MAP (Table 4). CBSD symptoms were not observed in plants at 1MAP. At 3MAP, no symptoms were observed in the MandiPlus treatment. MandiPlus + Lecatech, imidacloprid, imidacloprid + Lecatech, and Lecatech had low incidences of 4.2%, which were significantly less (P < 0.0001) than the control (14.6%). At 6MAP, MandiPlus and MandiPlus + Lecatech, imidacloprid, and imidacloprid + Lecatech had CBSD incidences ranging from 50.0% to 60.4%, which was significantly lower than the 95.8% in the control. Lecatech had an incidence of 85.4%, which was not significantly different compared with the control (Table 4). CBSD severity at 3MAP was not significantly different in treatments compared with the control, but at 6MAP, the score of 2.5 in MandiPlus was significantly lower (P = 0.0037) than the severity in all other treatments apart from that of imidacloprid (Table 4). At Ukiriguru, CMD symptoms were only observed in Lecatech <2%, whereas CBSD symptoms were not observed in any of the treatments or the control.
3.3.3 Yield parameters
At Chambezi, the sprouting percentage of cuttings at 1MAP (70.2–78.5%) in treatments was not significantly different compared with the control (73.2%) (Table 6). The number of stems in the MandiPlus treatment (4.6) was significantly higher (P = 0.0037) and more than twice the number of that in the control (2.1). Other treatments had 2.7 to 3.8 stems per cutting—values which were not significantly different from the control. The stem height for treatments ranged from 199 to 224 cm, with MandiPlus and MandiPlus + Lecatech having significantly longer (P < 0.0001) stems compared with control whereas other treatments were not significant (Table 6). The number of roots per plant was significantly higher (P = 0.0007) (41%–43%) in the imidacloprid + Lecatech, MandiPlus, and MandiPlus + Lecatech treatments compared with control, whereas other treatments did not differ from the control. Root yield was significantly higher (P < 0.0001) (45%–63%) in the imidacloprid + Lecatech, MandiPlus, and MandiPlus + Lecatech treatments compared with the control. Root yields for the imidacloprid and Lecatech treatments were not significantly different to the control. CBSD symptoms were not observed in harvested roots. At Ukiriguru, the sprouting percentage of cuttings at 1MAP (70.8%–75.5%) in treatments was not significantly different compared with the control (73.0%) (Table 6). Similarly, the number of stems per cutting in treatments (2.2–2.6) was not significantly different to the control (1.9). Stem height in treatments (105–139 cm) was significantly higher ((P < 0.0001)) for MandiPlus (34%) and MandiPlus + Lecatech (22%) compared with the control (104 cm). The number of roots was not significantly different in treatments compared with the control. Root yield was significantly higher (P < 0.0001) (82%) in the MandiPlus and MandiPlus + Lecatech treatments compared with the control. The yield in the imidacloprid + Lecatech treatment was also significantly greater (36%) than that of the control. Yields of the remaining treatments did not differ significantly from the control. CBSD symptoms were not observed in harvested roots for treatments and control.
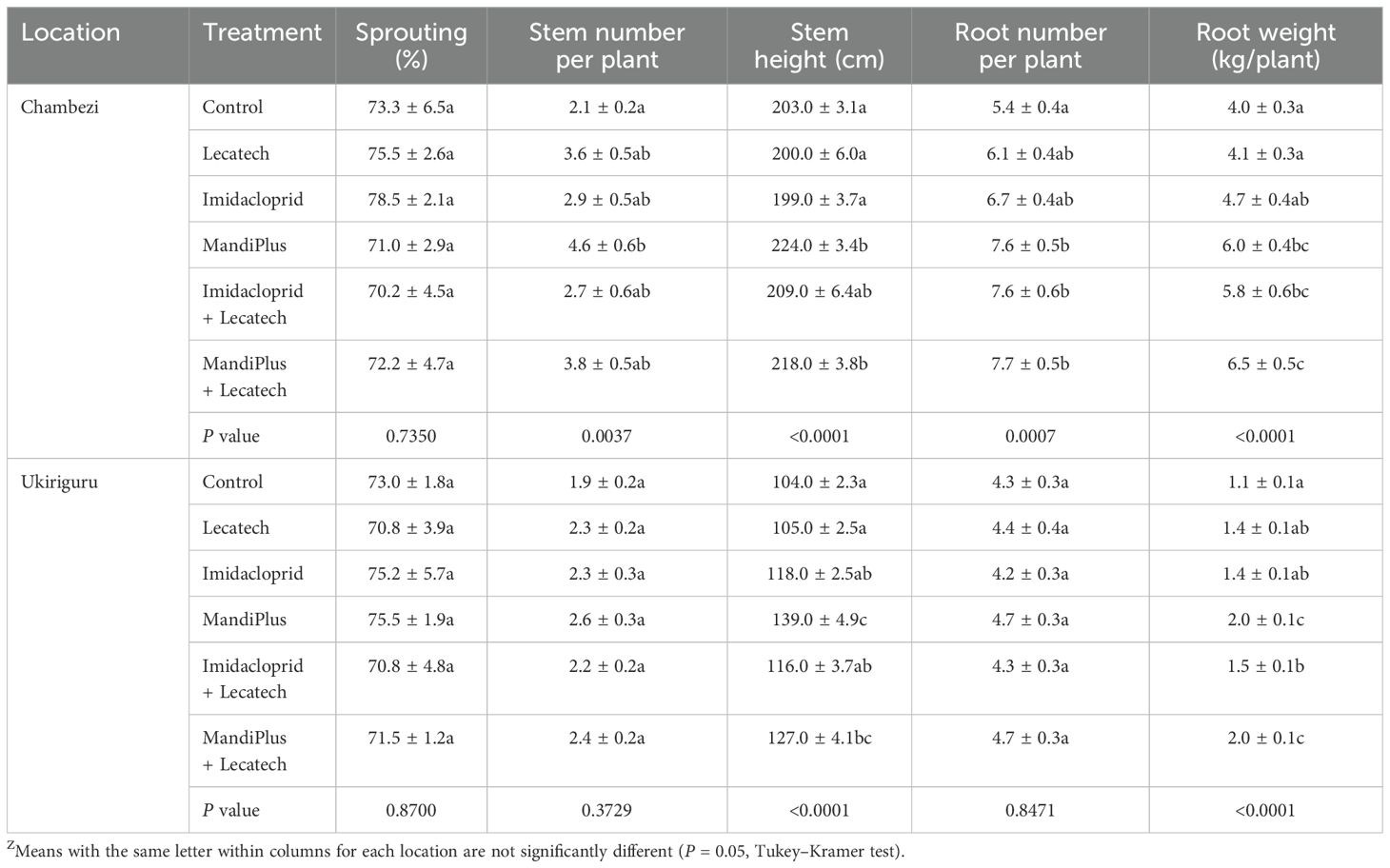
Table 6. Yield parameters and CBSD root incidence for cutting dipping in insecticides and application of EPF Lecatech (means ± SEM)Z.
4 Discussion
The current study sought to evaluate the effectiveness of EPFs in the control of B. tabaci, which is an important pest and vector of viruses of cassava. In addition, the study aimed to understand the effectiveness against cassava whiteflies of the MandiPlus insecticide/fungicide formulation when applied through cutting dips. The findings from this study reveal that EPF treatments caused medium to high mortality of whitefly nymph, with Mycotal being the most lethal under laboratory conditions. However, under field conditions, the efficacies of Mycotal and Lecatech were similar and both were relatively low, resulting in no significant yield benefits. Cutting dipping in MandiPlus was effective at reducing adult whitefly and nymph numbers, as well as incidence of cassava mosaic (CMD) and cassava brown streak (CBSD) diseases, and gave rise to significant yield increases. The application of Lecatech in combination with cutting dipping in MandiPlus did not confer any significant gains in terms of reduction of whitefly numbers, CMD and CBSD incidence, and increase in yield when compared with MandiPlus only.
Several studies have tested the efficacy of entomopathogenic biopesticides from the genus Beauveria and Akanthomyces against B. tabaci under laboratory, screen house, and field conditions. The whitefly mortality under laboratory conditions and field conditions for EPFs in this study is comparable with other studies that have shown that EPFs in the genera Beauveria and Akanthomyces spp. cause significant mortality in B. tabaci at all stages under laboratory, screen house, and field conditions (Assadi et al., 2021; Chouikhi et al., 2022; Ghongade and Sangha, 2021; Keerio et al., 2020; Sain et al., 2021; Wichienchote et al., 2024). In our study, however, application of EPFs under field conditions had no significant effect on CBSD incidence (leaf and root) and root yield. The high incidence of CBSD (100%) at 4MAP in treatments and control could be attributed to the susceptibility of variety Albert and the presence of inoculum from surrounding plots, combined with high whitefly abundances that averaged >100 per top five leaves at 2MAP in both locations. Other studies have reported similar findings, where the application of B. bassiana reduced whitefly numbers but had no effect on CMD incidence and cassava root yield (Wichienchote et al., 2024).
The combination of cutting dipping in MandiPlus and spraying of the EPF Lecatech shows that MandiPlus alone was very effective in reducing adult whitely numbers and CMD, as it substantially reduced CBSD and significantly increased stem and root yield. Lecatech applied in combination with MandiPlus did not yield significantly better results compared with MandiPlus alone, indicating that EPFs are less effective in controlling whitefly-transmitted viruses and increasing yield under field conditions. Cutting dips in imidacloprid produced greater yield benefits than EPFs although less than MandiPlus. Lecatech applied in combination with MandiPlus did not yield significantly better results compared with MandiPlus alone, indicating that Lecatech was not effective in controlling whitefly-transmitted viruses and increasing yield under the field conditions of this study. The low incidence of CMD in the MandiPlus treatment is notable considering that the variety Kiroba used in this study is known to be CMD-susceptible—readily expressing foliar symptoms (Shirima et al., 2019), although it is tolerant to CBSD in the sense that CBSD-infected plants rarely show symptoms of root necrosis in tuberous roots. In our field experiments, CBSD symptoms were observed in roots at harvest, and in the EPF treatment, the susceptible variety Albert had 93% CBSD root incidence. The very low incidence of CMD <2% only in Lecatech and no CBSD at Ukiriguru despite the presence of high whitefly populations could be attributed to a lack of virus inoculum in the surrounds of the experiment. Other studies have reported significant reduction in whiteflies, virus incidence, and increase in yield through cutting dipping in insecticides. An evaluation of MandiPlus in Malawi reported a significant reduction in adult whitefly numbers (87%), nymphs (70%), and CMD incidence (17% higher in untreated) but no significant effect on CBSD, and an increase of ~50% in stem and root yield (Nwokoro et al., 2024). A previous field study with cutting dipping in flupyradifurone reported reductions of 71% in adult whiteflies, 85% in nymphs, a 45% reduction in CMD, but no effect on CBSD which reached 100% in the susceptible variety Albert (Issa et al., 2022). In variety Mkuranga1, there was a reduction of 50% in adult whiteflies, 87% in nymphs, and 10% in CBSD. A farmer participatory field trial testing cutting dipping in flupyradifurone (variety Kiroba) demonstrated reductions of 41% in adult whiteflies, 65% in nymphs, and 14% in CMD, no effect on CBSD, an increased stem height of 46%, and root yield increases of 49% (Caspary et al., 2023). This 49% root yield increase, also recorded from the coastal district of Mkuranga, is closely comparable with the 50% increase derived from the MandiPlus treatment in our study at Chambezi. Root yields increased by 82% at Ukiriguru through the MandiPlus treatment, but the general yield was very low compared with Chambezi. This difference could be attributed to drought that had a general depressing effect on yields recorded at Ukiriguru. Imidacloprid, an insecticide that is readily available and affordable on the Tanzanian market, had significant effects in reducing whiteflies as well as incidences of CMD and CBSD, but there were no significant increases in root yield. A previous study on cassava tested combining cutting dipping and spraying of imidacloprid and reported reduced whitefly numbers and CMD and CBSD incidence and a yield gain of approximately 50% in treated compared with control plots (Omongo et al., 2022). In another study with research plots at multiple locations and several varieties, cutting dipping in imidacloprid alone with no subsequent spraying increased root yield by ca. 31% compared with the control (Bayiyana et al., 2023). The differences in performance of varieties emphasize the need to combine cutting dipping technology with clean planting materials of cassava varieties tolerant to CMD and CBSD.
Cassava whiteflies negatively impact cassava production through direct feeding damage and transmission of viruses that cause CMD and CBSD. The yield loss resulting from combined damage of whiteflies, CMD, and CBSD threaten the livelihoods of over 500 million people in sub-Saharan Africa. Breeding and phytosanitary strategies have focused more on CMD and CBSD with insignificant regard to the whitefly vector. The continued presence and abundance of these whiteflies threatens the sustainability of virus-resistant varieties as sustained virus pressure coupled with abundant whitefly vector populations could hasten resistance breakdown. Furthermore, efforts to implement sustainable cassava seed systems which include maximum allowable levels of virus infection (Legg et al., 2022) are also threatened by unmanaged whitefly populations. Results from the current study demonstrating significant reductions in virus incidence following single cutting dip treatments with MandiPlus show clearly that combining clean planting material and management of whiteflies through insecticide cutting dips could make it easier for both seed and root producers to achieve expected productivity targets. Based on the results from Chambezi, a farmer who plants clean cuttings treated with MandiPlus could harvest 43 t/ha compared with 29 t/ha obtained with clean planting material without MandiPlus treatment, and a cassava clean seed producer who uses MandiPlus treated cuttings could harvest 65,300 stems/ha compared with 30,800 stems/ha for untreated cuttings. An economic benefit analysis for cutting dipping in MandiPlus based on the farmgate cassava fresh root price in Tanzania of 245 USD/t (IPPMedia, 2024) shows that the yield/ha value for cassava of variety Kiroba produced with MandiPlus-treated cuttings (USD 10,535) is USD 3,430 greater than that for the yield produced by untreated cuttings (USD 7,105). The cost of MandiPlus (insecticide, fungicides, water, and dipping labor) was estimated at 600 USD/Ha. A farmer using MandiPlus will therefore make 2,830 USD more per hectare compared with one who plants only clean seed.
These findings show the importance of combining host plant resistance, clean planting material, and control of whiteflies to increase cassava production (Caspary et al., 2023; Issa et al., 2022; Shirima et al., 2020; Wamani et al., 2024; Yabeja et al., 2025). The impact of whiteflies is most significant during the early months of cassava crop production, when plants are most suitable for rapid population growth and rapid virus spread occurs (Caspary et al., 2023; Issa et al., 2022; Shirima et al., 2019; Yabeja et al., 2025). Cutting dipping using systemic insecticides has been shown to be highly effective in reducing whitefly numbers and transmission of viruses during the critical first 6 months of cassava crop growth (Caspary et al., 2023; this study). For this reason, coupled with the clear economic benefits that can be achieved, there is a strong justification to encourage cassava stem and root producers to adopt cutting dipping using MandiPlus. This strategy will be most effective in regions associated with abundant whiteflies. In this study, whiteflies occurred in high numbers in all the study field locations, although surveys of whitefly abundance have demonstrated variations between regions and agro-ecological zones (Jeremiah et al., 2015). A recent survey in western Kenya recorded super-abundant whitefly populations (>100 adults/5 top leaves) in two counties, and a positive relationship between whitefly numbers and the proportion of CMD attributed to whitefly transmission (Wosula et al., 2024). Although MandiPlus contains a neonicotinoid, laboratory studies in Tanzania (unpublished data) have shown that at 6MAP, cassava leaves of plants obtained from cuttings dipped in MandiPlus had thiamethoxam residues of 0.0036 mg/kg, whereas for imidacloprid, the residue level was 0.0023 mg/kg. These were both significantly below the recommended maximum residue level of 0.01 mg/kg in cassava leaves. These levels would probably drop further by the time of harvesting cassava at 12MAP, meaning that these products used as cutting dips in cassava represent an insignificant risk to human health. In addition, cassava rarely flowers, and typically toward the end of its cropping cycle, meaning that deleterious effects of neonicotinoids on bees reported elsewhere are likely to be minimal. The availability of new products, such as butenolides (flupyradifurone) (Nauen et al., 2014), promises to further improve both the efficacy and safety of cutting dips as a means of increasing cassava productivity in regions where yields are currently far below the potential as well as the global average. There is a need for availability and testing of diverse insecticides that would allow for alternation in application to mitigate whitefly resistance development. The environmental and health risk of cutting dipping in insecticides is the same as risks that are associated with the handling and application of insecticides. All insecticides must be used according to the manufacturer’s instructions on safe use and handling. The cutting dipping in insecticides based on leaf analysis 6 months after planting shows that the residual levels of thiamethoxam (0.0036 mg/kg) and imidacloprid (0.0023 mg/kg) are well below the recommended maximum residue limit of 0.01 mg/kg. Considering cassava roots are harvested starting at 9 months after planting, the level of the active ingredient would most likely drop even lower. This single-dip application only at the time of planting is more judicious compared with spray applications that could be more frequent and directly applied to plant foliage, hence posing a greater risk to human health, non-target organisms, pollution of the environment, and exposure to high residue levels if sprays are applied shortly before harvesting. The cutting dipping technology for management of whiteflies therefore has potential for scaling to cassava-growing regions where whiteflies are similarly abundant to those reported from both sites of the current study. Many countries in the East and Central African region have been affected by high whitefly populations and associated rapid spread of CMD and CBSD (Legg et al., 2006; 2014), meaning that this whitefly control approach is likely to be relevant in many of these countries, which represent some of the most important cassava-growing regions in the continent.
5 Conclusion
This study shows that treating cassava cuttings with the MandiPlus product, which includes the systemic insecticide thiamethoxam, effectively reduced whiteflies by 85%, CMD by 59%, CBSD by 46%, and increased stem number by 119% and root yield by 50% in coastal Tanzania. Although these benefits were replicated in north-western Tanzania, drought negatively impacted yield, making the results less clearcut. The application of EPFs under field conditions caused significant whitefly mortality but did not confer any significant benefits in terms of CMD and CBSD reduction or yield gain. The high degree of effectiveness of the MandiPlus product offers cassava farmers an option to manage whiteflies effectively through judicious application of insecticides through cutting dipping. This mode of application is recommended as it is less harmful to non-target organisms in cassava farms.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MA: Methodology, Data curation, Writing – original draft, Validation, Writing – review & editing, Investigation. KI: Investigation, Validation, Writing – review & editing, Data curation, Writing – original draft, Methodology. EW: Writing – original draft, Conceptualization, Funding acquisition, Validation, Investigation, Writing – review & editing, Project administration, Formal Analysis, Supervision, Methodology. MN: Methodology, Validation, Investigation, Writing – review & editing. FS: Writing – review & editing, Supervision, Validation, Conceptualization. DC: Conceptualization, Funding acquisition, Project administration, Methodology, Validation, Writing – review & editing. IN: Investigation, Validation, Writing – review & editing, Methodology. JL: Conceptualization, Funding acquisition, Writing – review & editing, Project administration, Supervision, Validation, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work received funding from BBSRC CONNECTED Network (BB/R005397/1) and the CGIAR Initiative on Plant Health and Rapid Response to Protect Food Security and Livelihoods (Plant Health Initiative). We would like to thank all funders who have supported this research through their contributions to the CGIAR Trust Fund: www.cgiar.org/funders/.
Acknowledgments
The authors wish to thank the Tanzania Agricultural Research Institute (TARI) for land used to conduct field experiments at Chambezi, Mkuranga, and Ukiriguru research stations. We thank Jonas Nickas who assisted with developing scripts for R Statistics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aguilera Sammaritano J. A., Lopez Lastra C. C., Leclerque A., Vazquez F., Toro M. E., D’Alessandro C. P., et al. (2016). Control of Bemisia tabaci by entomopathogenic fungi isolated from arid soils in Argentina. Biocontrol Sci. Technol. 26, 1668–1682. doi: 10.1080/09583157.2016.1231776
Assadi B. H., Chouikhi S., Ettaib R., M’hamdi N. B., and Belkadhi M. S. (2021). Effect of the native strain of the predator Nesidiocoris tenuis Reuter and the entomopathogenic fungi Beauveria bassiana and Lecanicillium muscarium against Bemisia tabaci (Genn.) under greenhouse conditions in Tunisia. Egypt. J. Biol. Pest Control 31, 1–11. doi: 10.1186/s41938-021-00395-5
Bakker L., van der Werf W., Tittonell P., Wyckhuys K. A., and Bianchi F. J. (2020). Neonicotinoids in global agriculture: evidence for a new pesticide treadmill? Ecol. Soc 25, 26. doi: 10.5751/es-11814-250326
Bayiyana I., Bua A., Ozimati A., Mugisha J., Colvin J., and Omongo C. A. (2023). Insecticide use by small-scale Ugandan cassava growers: An economic analysis. Agriculture 13, 1043. doi: 10.3390/agriculture13051043
Borsuah J. F., Messer T. L., Snow D. D., Comfort S. D., and Mittelstet A. R. (2020). Literature review: Global neonicotinoid insecticide occurrence in aquatic environments. Water. 12, 3388. doi: 10.3390/w12123388
Caspary R., Wosula E. N., Issa K. A., Amour M., and Legg J. P. (2023). Cutting dipping application of Flupyradifurone against cassava whiteflies Bemisia tabaci and impact on its parasitism in cassava. Insects 14, 796. doi: 10.3390/insects14100796
Chandler D. (2017). “Basic and applied research on entomopathogenic fungi,” in Microbial control of insect and mite pests from theory to practice. Ed. Lacey L. A. (London, UK: Academic Press, Elsevier), 69–89.
Chandler D., Bailey A. S., Tatchell G. M., Davidson G., Greaves J., and Grant W. P. (2011). The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc B Biol. Sci. 366, 1987–1998. doi: 10.1098/rstb.2010.0390
Chouikhi S., Assadi B. H., Lebdi K. G., and Belkadhi M. S. (2022). Efficacy of the entomopathogenic fungus, Beauveria bassiana and Lecanicillium muscarium against two main pests, Bemisia tabaci (Genn.) and Tetranychus urticae (Koch), under geothermal greenhouses of Southern Tunisia. Egypt. J. Biol. Pest Control 32, 125. doi: 10.1186/s41938-022-00627-2
de Oliveira E. J., de Oliveira S. A. S., Otto C., Alicai T., De Freitas J. P. X., Cortes D. F. M., et al. (2020). A novel seed treatment-based multiplication approach for cassava planting material. PloS One 15, e0229943. doi: 10.1371/journal.pone.0229943
Dimase M., Lahiri S., Beuzelin J., Hutton S., and Smith H. A. (2024). Evaluation of biopesticides for management of Bemisia tabaci Middle East-Asia Minor 1 (Hemiptera: Aleyrodidae) in Florida. Insects 15, 438. doi: 10.3390/insects15060438
Dubern J. (1994). Transmission of African cassavamosaic geminivirus by the whitefly (Bemisia tabaci). Trop. Sci. 34, 82–91.
Garrido-Jurado I., Resquín-Romero G., Amarilla S. P., Ríos-Moreno A., Carrasco L., and Quesada-Moraga E. (2017). Transient endophytic colonization of melon plants by entomopathogenic fungi after foliar application for the control of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). J. Pest Sci. 90, 319–330. doi: 10.1007/s10340-016-0767-2
Ghongade D. S. and Sangha K. S. (2021). Efficacy of biopesticides against the whitefly, Bemisia tabaci (Gennadius)(Hemiptera: Aleyrodidae), on parthenocarpic cucumber grown under protected environment in India. Egypt. J. Biol. Pest Control 31, 1–11. doi: 10.1186/s41938-021-00365-x
Gondwe F., Mahungu N., Hillocks R., Moyo C., Sok M., Chipungus F., et al. (2003). “Economic Losses Experienced by Small Scale Farmers in Malawi due to Cassava Brown Streak Virus Disease,” in Proceedings of an international workshop on cassava brown streak virus disease: past, present and future. Eds. Legg J. P. and Hillock R. J.(Mombasa, Kenya), 28–38. Available online at: https://assets.publishing.service.gov.uk/media/57a08cfded915d3cfd001752/R7563CBSDproceedings.pdf (Accessed July 8, 2025).
Goulson D. (2013). An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 50, 977–987. doi: 10.1111/1365-2664.12111
Horowitz A. R., Ghanim M., Roditakis E., Nauen R., and Ishaaya I. (2020). Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 93, 893–910. doi: 10.1007/s10340-020-01210-0
IPPMedia (2024). Guardian. Business media. April 23rd 2024. Available online at: https://www.ippmedia.com/the-guardian/business/read/hopes-for-farmers-as-cassava-prices-increase-in-tanga-2024-04-23-115602 (Accessed July 8, 2025).
Issa K. A., Wosula E. N., Stephano F., and Legg J. P. (2022). Evaluation of the Efficacy of Flupyradifurone against Bemisia tabaci on Cassava in Tanzania. Insects 13, 920. doi: 10.3390/insects13100920
Jeremiah S. C., Ndyetabula I. L., Mkamilo G. S., Haji S., Muhanna M. M., Chuwa C., et al. (2015). The dynamics and environmental influence on interactions between cassava brown streak virus disease and the whitefly, Bemisia tabaci. Phytopathol 105, 646–655. doi: 10.1094/PHYTO-05-14-0146-R
Jeschke P., Nauen R., Schindler M., and Elbert A. (2011). Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 59, 2897–2908. doi: 10.1021/jf101303g
Keerio A. U., Nazir T., Abdulle Y. A., Jatoi G. H., Gadhi M. A., Anwar T., et al. (2020). In vitro pathogenicity of the fungi Beauveria bassiana and Lecanicillium lecanii at different temperatures against the whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae). Egypt. J. Biol. Pest Control 30, 1–9. doi: 10.1186/s41938-020-00247-8
Lacey L. A., Grzywacz D., Shapiro-Ilan D. I., Frutos R., Brownbridge M., and Goettel M. S. (2015). Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 132, 1–41. doi: 10.1016/j.jip.2015.07.009
Legg J. P., Diebiru-Ojo E., Eagle D., Friedmann M., Kanju E., Kapinga R., et al. (2022). “Commercially sustainable cassava seed systems in africa,” in Root, tuber and banana food system innovations. Eds. Thiele G., Friedmann M., Campos H., Polar V., and Bentley J. W. (Gewerbestrasse, Cham, Switzerland, Nature Switzerland AG), 453–482.
Legg J. P., Jeremiah S. C., Obiero H. M., Maruthi M. N., Ndyetabula I., Okao-Okuja G., et al. (2011). Comparing the regional epidemiology of the cassava mosaic and cassava brown streak virus pandemics in Africa. Virus Res. 159, 161–170. doi: 10.1016/j.virusres.2011.04.018
Legg J. P., Owor B., Sseruwagi P., and Ndunguru J. (2006). Cassava mosaic virus disease in East and Central Africa: epidemiology and management of a regional pandemic. Adv. Virus Res. 67, 355–418. doi: 10.1016/S0065-3527(06)67010-3
Legg J. P., Shirima R., Tajebe L. S., Guastella D., Boniface S., Jeremiah S., et al. (2014). Biology and management of Bemisia whitefly vectors of cassava virus pandemics in Africa. Pest Manage. Sci. 70, 1446–1453. doi: 10.1002/ps.3793
Li Y., Mbata G. N., Simmons A. M., Shapiro-Ilan D. I., and Wu S. (2024). Management of Bemisia tabaci on vegetable crops using entomopathogens. Crop Prot. 180, 106638. doi: 10.1016/j.cropro.2024.106638
Maienfisch P., Angst M., Brandl F., Fischer W., Hofer D., Kayser H., et al. (2001). Chemistry and biology of thiamethoxam: a second generation neonicotinoid. Pest Manage. Sci. 57, 906–913. doi: 10.1002/ps.365
Maruthi M. N., Hillocks R. J., Mtunda K., Raya M. D., Muhanna M., Kiozia H., et al. (2005). Transmission of Cassava brown streak virus by Bemisia tabaci (Gennadius). J. Phytopathol. 153, 307–312. doi: 10.1111/j.1439-0434.2005.00974.x
Maruthi M. N., Jeremiah S. C., Mohammed I. U., and Legg J. P. (2017). The role of the whitefly, Bemisia tabaci (Gennadius), and farmer practices in the spread of cassava brown streak ipomoviruses. J. Phytopathol. 165, 707–717. doi: 10.1111/jph.12609
Nauen R., Jeschke P., Velten R., Beck M. E., Ebbinghaus-Kintscher U., Thielert W., et al. (2014). Flupyradifurone: A brief profile of a new butenolide insecticide. Pest Manage. Sci. 71, 850–862. doi: 10.1002/ps.3932
Nwokoro C. C., Kachigamba D., Chiipanthenga M., Klauser D., Robinson M., and Berlin R. (2024). Effects of seed treatment on cassava stake performance, whitefly population, disease incidence, and yield performance of cassava (Manihot esculenta Crantz) in Malawi. Front. Agron. 5. doi: 10.3389/fagro.2023.1303869
Omongo C. A., Opio S. M., Bayiyana I., Otim M. H., Omara T., Wamani S., et al. (2022). African cassava whitefly and viral disease management through timed application of imidacloprid. Crop Prot. 158, 106015. doi: 10.1016/j.cropro.2022.106015
Palumbo J. C., Horowitz A. R., and Prabhaker N. (2001). Insecticidal control and resistance management for Bemisia tabaci. Crop Prot 20, 739–765. doi: 10.1016/S0261-2194(01)00117-X
Perring T. M., Stansly P. A., Liu T. X., Smith H. A., and Andreason S. A. (2018). “Whiteflies: biology, ecology, and management,” in Sustainable management of arthropod pests of tomato. Eds. Wakil. W., Brust. G. E., and Perring T. M. (Academic Press, Elsevier, Cambridge, MA, USA).
Sain S. K., Monga D., Hiremani N. S., Nagrale D. T., Kranthi S., Kumar R., et al. (2021). Evaluation of bioefficacy potential of entomopathogenic fungi against the whitefly (Bemisia tabaci Genn.) on cotton under polyhouse and field conditions. J. Invertebr. Pathol. 183, 107618. doi: 10.1016/j.jip.2021.107618
Sani I., Ismail S. I., Abdullah S., Jalinas J., Jamian S., and Saad N. (2020). A review of the biology and control of whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), with special reference to biological control using entomopathogenic fungi. Insects 11, 619. doi: 10.3390/insects11090619
Shirima R. R., Legg J. P., Maeda D. G., Tumwegamire S., Mkamilo G., Mtunda K., et al. (2020). Genotype by environment cultivar evaluation for cassava brown streak disease resistance in Tanzania. Virus Res. 286, 198017. doi: 10.1016/j.virusres.2020.198017
Shirima R., Maeda D. G., Kanju E. E., Tumwegamire S., Ceasar G., Mushi E., et al. (2019). Assessing the degeneration of cassava under high virus inoculum conditions in Coastal Tanzania. Plant Dis. 103, 2652–2664. doi: 10.1094/PDIS-05-18-0750-RE
Simon-Delso N., Amaral-Rogers V., Belzunces L. P., Bonmatin J. M., Chagnon M., Downs C., et al. (2015). Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ. Sci. pollut. Res. 22, 5–34. doi: 10.1007/s11356-014-3470-y
Singh A., Bhardwaj R., and Singh I. K. (2019). “Biocontrol agents: potential of biopesticides for integrated pest management,” in Biofertilizers for sustainable agriculture and environment. Eds. Giri. B., Varma. R. P. A., and Wu Q. S. (Springer Nature, Switzerland AG), 413–433.
Sparks T. C. and Bryant R. J. (2021). Crop protection compounds–trends and perspective. Pest Manage. Sci. 77, 3608–3616. doi: 10.1002/ps.6293
Sparks T. C., Storer N., Porter A., Slater R., and Nauen R. (2021). Insecticide resistance management and industry: the origins and evolution of the I nsecticide R esistance A ction C ommittee (IRAC) and the mode of action classification scheme. Pest Manage. Sci. 77, 2609–2619. doi: 10.1002/ps.6254
Sseruwagi P., Sserubombwe W. S., Legg J. P., Ndunguru J., and Thresh J. M. (2004). Methods of surveying the incidence and severity of cassava mosaic disease and whitefly vector populations on cassava in Africa: a review. Virus Res. 100, 129–142. doi: 10.1016/j.virusres.2003.12.021
Wamani S., Opio S. M., Omara T., Ocitti P., Colvin J., and Omongo C. A. (2024). Resistance to cassava whitefly (Bemisia tabaci) among Eastern and Southern African elite cassava genotypes. Insects 15, 258. doi: 10.3390/insects15040258
Wichienchote N., Jaiyen S., Wasuwan R., Seepiban C., Charoenvilaisiri S., Tanticharoen M., et al. (2024). Beauveria bassiana biocontrol with neem oil adjuvant is effective for the management of the cassava mosaic virus vector Bemisia tabaci in field trials. BioControl 70, 217–228. doi: 10.1007/s10526-024-10301-1
Wosula E. N., Chen W., Fei Z., and Legg J. P. (2017). Unravelling the genetic diversity among cassava Bemisia tabaci whiteflies using NextRAD sequencing. Genome Biol. Evol. 9, 2958–2973. doi: 10.1093/gbe/evx219
Wosula E. N., Shirima R. R., Amour M., Woyengo V. W., Otunga B. M., and Legg J. P. (2024). Occurrence and distribution of major cassava pests and diseases in cultivated cassava varieties in western Kenya. Viruses 16, 1469. doi: 10.3390/v16091469
Keywords: cassava, cutting dipping, Bemisia tabaci, whitefly, entomopathogenic fungi, insecticides, cassava mosaic disease, cassava brown streak disease
Citation: Amour M, Issa KA, Wosula EN, Ndalahwa M, Stephano F, Chandler D, Ndyetabula I and Legg JP (2025) Stem-cutting dipping in insecticides and biopesticide application for the control of Bemisia tabaci whitefly in cassava. Front. Agron. 7:1623632. doi: 10.3389/fagro.2025.1623632
Received: 06 May 2025; Accepted: 25 June 2025;
Published: 14 July 2025.
Edited by:
Oscar Liburd, University of Florida, United StatesReviewed by:
Patrick Chiza Chikoti, Zambia Agriculture Research Institute (ZARI), ZambiaWenwu Zhou, Zhejiang University, China
Copyright © 2025 Amour, Issa, Wosula, Ndalahwa, Stephano, Chandler, Ndyetabula and Legg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Everlyne N. Wosula, ZS53b3N1bGFAY2dpYXIub3Jn
†These authors have contributed equally to this work
 Massoud Amour
Massoud Amour Khamis A. Issa1†
Khamis A. Issa1† Everlyne N. Wosula
Everlyne N. Wosula James P. Legg
James P. Legg