- 1Research Center for Estate Crops, National Research and Innovation Agency (BRIN), Cibinong, Indonesia
- 2Division of Collaboration and Dissemination of estate crops engineering and modernization, Indonesian Center for Estate Crops Engineering and Modernization, Indonesian Agency for Agricultural Engineering and Modernization, Ministry of Agriculture, Bogor, Indonesia
- 3Research Center for Horticulture, National Research and Innovation Agency (BRIN), Cibinong, Indonesia
Endophytic bacteria can be applied as biofertilizers and plant growth promoters due to their potential to release phytohormones and improve nutrient availability, thereby supporting plant growth and the biosynthesis of bioactive compounds. Effective practical cultivation of medicinal plants, including sambiloto (Andrographis paniculata), suggests using biofertilizer, in addition to inorganic fertilizer. Andrographolide, a key compound in A. paniculata, is recognized for its therapeutic properties, including anti-inflammatory and antiviral activities, making its enhanced biosynthesis significant in medicinal plant research. Phosphorus is required in andrographolide biosynthesis. The study aimed to determine the physiological and agronomic characteristics, as well as the bioactive compounds of A. paniculata, by applying endophytic bacteria and phosphate fertilizer. The trial was arranged in a completely randomized design with six treatments and three replications. The treatments consisted of the following: 1) no treatment (control), 2) 0.675 g P2O5 plant-1, 3) 1.35 g P2O5 plant-1, 4) endophytic bacteria Bacillus sp., 5) endophytic bacteria Bacillus sp. + 0.675 g P2O5 plant-1, and 6) endophytic bacteria Bacillus sp. + 1.35 g P2O5 plant-1. Endophytic bacteria Bacillus sp. and phosphate fertilizer significantly (P<0.05) influenced agronomic characteristics, including growth and fresh and dry biomass production, as well as physiological characteristics, including Net Assimilation Rate (NAR), Relative Growth Rate (RGR), Stem-to-Leaf Ratio (SLR), Leaf Area Index (LAI), and Leaf Area Ratio (LAR). The application of Bacillus sp. in combination with 0.675 g P2O5 plant-1 produced the highest herbage weight and secondary metabolites (andrographolide, 14-deoxy-11,12-didehydroandrographolide, and neo-andrographolide), and hence could be recommended in A. paniculata cultivation. Furthermore, the application of endophytic bacteria Bacillus sp. could reduce the use of chemical phosphate fertilizer by 50%, offering significant benefits for farmers and being more environmentally friendly to support sustainable agriculture.
1 Introduction
The king of bitter plant (Andrographis paniculata Nees.), or sambiloto in Indonesian, is one of the most widely used plants in pharmaceutical formulations (Irianti et al., 2022). Andrographolide is the primary bioactive compound found in A. paniculata and is responsible for the plant’s characteristic bitter taste. This diterpenoid lactone and its derivatives (neo-andrographolide, 14-deoxyandrographolide, and 14-deoxy-11,12-didehydro?>andrographolide) possess a wide range of pharmacological properties (Adiguna et al., 2021; Hao et al., 2020). These include anti-inflammatory, antipyretic, antihyperglycemic, antioxidant, antidiabetic (Islam et al., 2018; Liu et al., 2020), antiviral (Adiguna et al., 2021; Jiang et al., 2021; Shi et al., 2020), and gastrointestinal protective activities (Wang et al., 2021). Due to these properties, andrographolide and its biosynthesis have attracted considerable interest in pharmaceutical and agricultural research.
The concentration of andrographolide in A. paniculata varies among different plant tissues, with the highest levels found in the leaves (ranging from 2.5% to 4.8%), while only small amounts are detected in the roots, stems, and flowers (Fitriansyah et al., 2024). However, several studies have shown that andrographolide content is also influenced by the plant’s age and environmental conditions. For instance, Tajidin et al. (2019) reported that the highest concentration of andrographolide is present when the plants reached 18 WAS (week after sowing).
To improve the biosynthesis of andrographolide and support sustainable cultivation practices, biofertilizers such as endophytic bacteria have emerged as promising alternatives to chemical fertilizers. These bacteria play multiple roles in promoting plant health and productivity. Endophytic bacteria are a type of plant growth-promoting bacteria (PGPB) that can produce phytohormones likeindole acetic acid (IAA), gibberellin (GA3), abscisic acid (ABA), and cytokinin (Egamberdieva et al., 2017). In addition, it fixes nitrogen (N) from the atmosphere (Okamoto et al., 2021), solubilizes phosphate (Li et al., 2017; Alkahtani et al., 2020), and increases the organic matter level released from dead and decomposed bacterial biomass as a biofertilizer (Ali et al., 2022). The endophytic bacteria also generate siderophores (Chen et al., 2016; Maheshwari et al., 2019), which can chelate metals such as Hg, Fe, and Al (Ferreira et al., 2019). These metals, which can hamper plant growth and development, are commonly found in acid soils that dominate the land in Indonesia. Microbes that produce siderophores can also control pathogenic microbes (Kumari et al., 2022), protect plants from environmental stress (Ullah et al., 2019), increase crop productivity, and sustain soil health (Prasad et al., 2020). Endophytic bacteria offer an economical and environmentally friendly approach to increasing plant nutrient supply, reducing chemical fertilizer use, and protecting against abiotic and biotic stresses (Lachu et al., 2022; Prasad et al., 2020).
Recent studies have demonstrated that endophytic microbes can play a significant role in enhancing secondary metabolite production in medicinal plants. For example, Kumari et al. (2023) reported that the bacterial endophyte Micrococcus luteus (ASd6) significantly increased both biomass and andrographolide content in A. paniculata by 68.01% and 44.16%, respectively. Beyond bacterial endophytes, Nair and Sakuntala (2020) highlighted the role of the root-colonizing mutualistic fungus Piriformospora indica as a potent elicitor of andrographolide biosynthesis, achieving a marked increase in andrographolide content up to 58.2 mg/g dry weight compared to 19.5 mg/g in control plants. This improvement was mechanistically associated with the transcriptional upregulation of key genes involved in the mevalonate (MVA) and methylerythritol phosphate (MEP) pathways, including 3-hydroxy-3-methylglutaryl-CoA synthase (HMGS), 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR), 1-deoxy-D-xylulose 5-phosphate synthase (DXS), 1-Deoxy-D-xylulose 5-Phosphate reductoisomerase (DXR), geranylgeranyl pyrophosphate synthase (GGPS), and 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase (ISPH), all of which contribute to the biosynthesis of andrographolide precursors. These findings underscore the functional significance of plant and endophyte interactions in modulating secondary metabolism and offer a sustainable strategy to boost the production of pharmacologically important compounds.
One of the essential macronutrients for plant growth is phosphorus (P), which is involved in photosynthesis and primary metabolite processes, serving as an energy source to convert ADP into ATP. Glucose, produced from the primary metabolite process, is then used for secondary metabolite formation, including andrographolide, which is synthesized by A. paniculata. Andrographolide is a secondary metabolite classified under the diterpenoid group and synthesized through the mevalonate and non-mevalonate acid pathways (Bergman et al., 2019). Phosphorus is required in andrographolide biosynthesis for the production of isopentenyl pyrophosphate/diphosphate (IPP/IDP) and dimethylallyl pyrophosphate/diphosphate (DAMPP/DAMPP) (Bergman et al., 2019; Shailaja et al., 2021).
Given these facts, there is a strong need to explore the combined use of biofertilizers and phosphate fertilizer to maximize A. paniculata growth and andrographolide yield. Therefore, this study aimed to determine the most effective combination of endophytic bacteria and phosphate fertilizer dosage by evaluating physiological traits, agronomic performance, and secondary metabolite content. This work supports the application of sustainable cultivation technologies for medicinal plants, as promoted by the Indonesian Ministry of Agriculture.
2 Materials and methods
2.1 Treatments and plant cultivation
The study was conducted at the Cimanggu Research Installation, part of the Indonesian Spices and Medicinal Crops Research Institute. The experimental site was located at 0°41’38.17” N; 127°33’15.18” E, at an elevation of 250 meters above sea level (asl), with Latosol as the predominant soil type.
The field trial was arranged in a completely randomized block design with six treatments and three replications. The treatments were as follows: 1) no treatment (C), 2) 0.675 g P2O5 plant-1 (P1), 3) 1.35 g P2O5 plant-1 (P2), 4) Bacillus sp. (B1), 5) Bacillus sp. + 0.675 g P2O5 plant-1 (B1+P1), and 6) Bacillus sp. + 1.35 g P2O5 plant-1 (B1+P2). The 1.35 g P2O5 plant-1 dose represents the recommended phosphorus application for A. paniculata cultivation in Indonesia, while the 0.675 g P2O5 plant-1 dose corresponds to 50% of the recommended rate.
The A. paniculata cultivation procedures were as follows: seeds of the Sambina 1 variety were first germinated in a nursery. The germinated seedlings were then transplanted into small polybags filled with a soil-to-manure mixture at a 2:1 ratio and maintained for one month. The experimental site was subsequently cleared and divided into 27 plots, with a 1-meter spacing between blocks.
Each plot measured 2 × 4 m with a plant spacing of 40 × 60 cm. One week before planting, manure was applied at a rate of 250 g plant-1. Phosphorus fertilizer (0.675 and 1.35 g P2O5 plant-1) and potassium fertilizer (2.25 g K2O plant-1) were applied at the time of planting. Nitrogen fertilizer (2.30 g plant-1) was applied in two equal splits at 4 and 8 weeks after planting (WAP).
The endophytic bacteria (Bacillus sp.) used in this study were obtained from previous research (Gusmaini et al., 2022) and cultured in Tryptic Soy Broth (TSB) media for 2 × 24 hours. The Bacillus sp. suspension, with a population density of 108 CFU mL-1, was applied at 3, 5, 7, and 9 WAP at a dose of 100 mL plant-1. The suspension was sprayed onto both the plants and the soil, while the control plants were sprayed with blank media (TSB suspension without bacteria).
2.2 Measurements
The agronomic characteristics, growth parameters including plant height and number of primary branches, were recorded every two weeks (2, 4, 6, 8, 10, 12, and 14 WAP). The yield parameters, such as the fresh and dry biomass weight, were measured at 8, 10, 12, and 14 WAP. The biomass was dried in an oven at 50–60°C to determine the dry weight. Data on physiological characteristics, including net assimilation rate (NAR), relative growth rate (RGR), stem-to-leaf ratio (SLR), and leaf area index (LAI), were also collected. The NAR (Equation 1) and RGR (Equation 2) were calculated according to the formulas below:
and
W1 and W2 represent the plant dry weights at times t1 and t2, respectively, while, A1 and A2 denote the corresponding leaf areas at times t1 and t2 (William and Joseph, 1976). The leaf aa index (LAI) was calculated by dividing the maximum leaf area by the land area occupied by the plants. The leaf area ratio (LAR) was determined as the total leaf area divided by the dry biomass weight (Morrison et al., 1999). Leaf area measurements were conducted using a leaf area meter (Li-3000).
Plant tissue nutrient analysis was conducted at 14 WAP. The 0.5 g samples of plant powder we digested using the wet digestion method with sulfuric acid (H2SO4) and hydrogen peroxide (H2O2). Phosphorus (P) and potassium (K) contents were measured using a spectrophotometer and atomic absorption spectroscopy (AAS), respectively, while nitrogen (N) content was determined using the kjeldahl method (Kjeldahl, 1883). The N, P, K content data are obtained using the formula below:
Nitrogen Content (Equation 3):
where V1 is sample titration volume, V2 is blanko titration volume, N is H2SO4 normality, 14.008 is nitrogen atomic weight, fp is dilution correction factor, fk is moisture content correction factor, and W is sample weight.
Phosphorus content (Equation 4):
Potassium content (Equation 5):
where fk is moisture correction factor, fp is dilution correction factor.
Andrographolide, neoandrographolide, and 14-deoxy-11,12-didehydroandrographolide were qntified using a TLC Scanner with a mobile phase n-hexane P-ethyl acetate P (2:8). The test solution was prepared by placing 500 mg of simplicia powder into a 10-mL volumetric flask, adding 10 mL of methanol P, and filtering the mixture. The standard solution consisted of 0.1% andrographolide in methanol P. A series of dilutions of the reference solution were prepared until the absorbance values closely matched those of the test solution. Absorbance was measured at the maximum absorption wavelength of 230 nm, and a calibration curve was established.
T andrographolide percentage in the simplicia powder was calculated using the standard curve or the following formula (Equation 3):
where C is the concentration of the reference solution, Au is the absorbance of the test solution, Ap is the absorbance of the reference solution, V is the volume of the test solution before dilution, fp is the dilution factor of the test solution, and W is the weight of the test material in dry form.
2.3 Data analysis
The effects of treatments on the observed variables were analyzed using analysis of variance. When significant differences were detected, Duncan’s Multiple Range Test (DMRT) was applied at a 5% significance level to determine differences among treatment means.
3 Results
3.1 Phosphate status in the field before trial
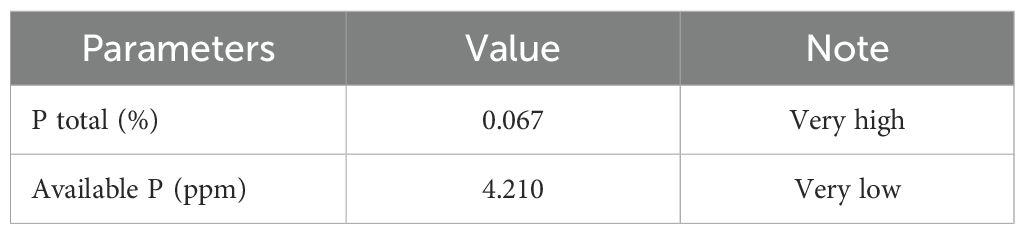
Information regarding the status and availability of nutrients in the soil was necessary before the application of fertilizers. The total phosphorus (P) content in the soil before the study was found to be very high (0.067%), while the available P was low (4.21 ppm) (Table 1). This result indicated that most of the P was unavailable for plant uptake. The enrichment of the soil with endophytic bacteria could potentially dissolve fixed P, converting it into available P, thereby enhancing the growth and development of A. paniculata.
3.2 Agronomic characteristics of A. paniculata
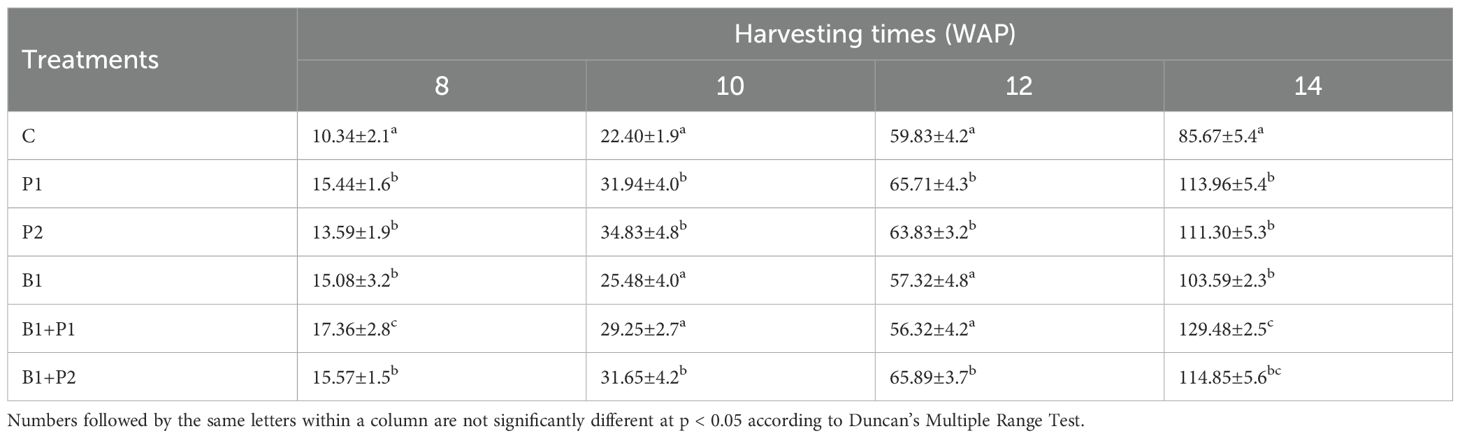
Plant height and the number of primary branches are important agronomic traits for the growth of A. paniculata. The growth pattern of A. paniculata at 2 to 14 WAP showed a steady increase. The application of endophytic bacteria and phosphate fertilizer resulted in a higher plant height compared to the control starting at 4 WAP. Additionally, the number of primary branches also increased from 10 to 14 WAP (Figure 1), and notably, it was significantly higher than the control at 14 WAP (Table 2).
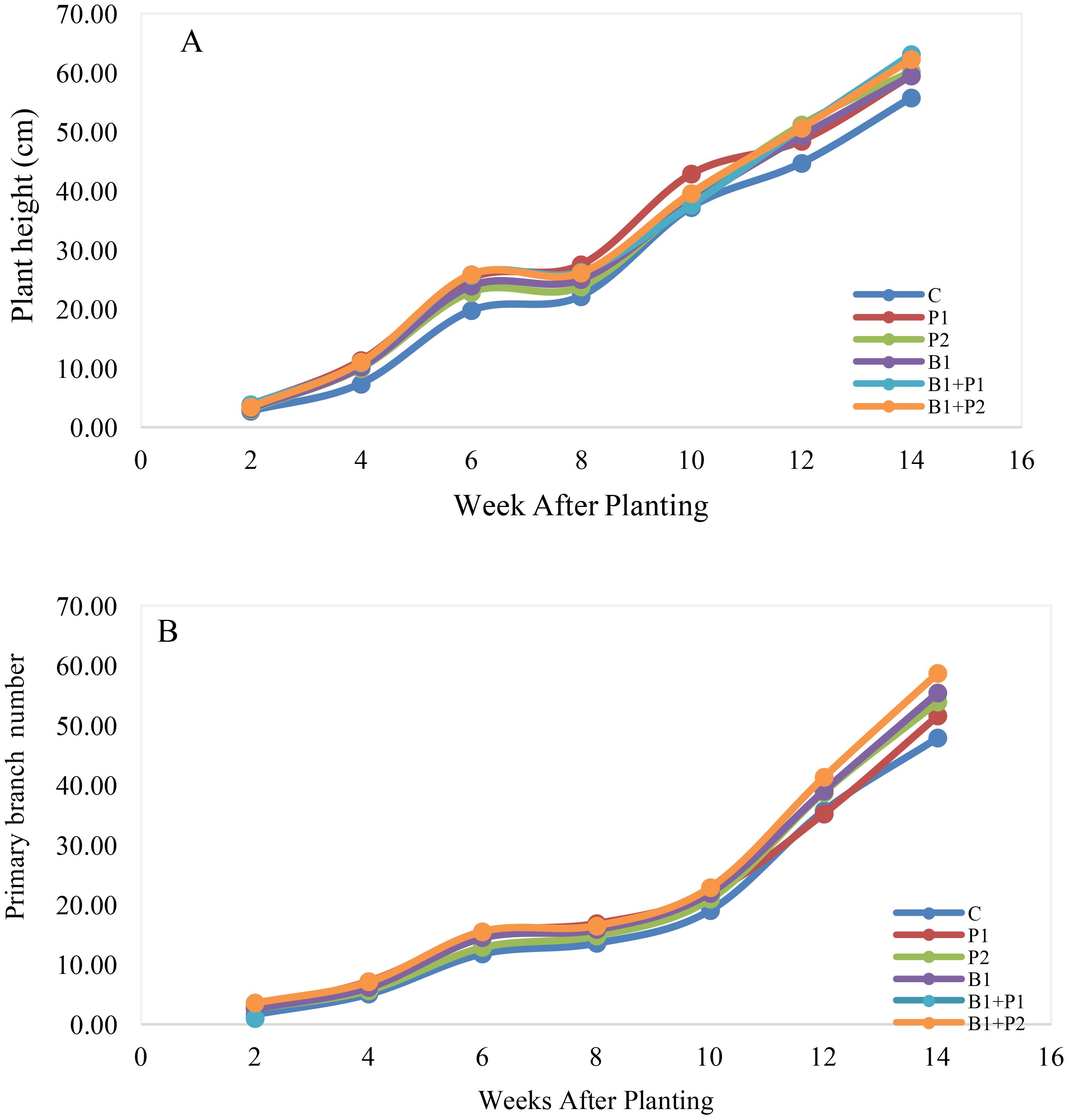
Figure 1. Effect of endophytic bacteria and phosphorus application on the growth pattern of A. paniculata from 2 to 14 WAP; (A) plant height and (B) number of primary branches.
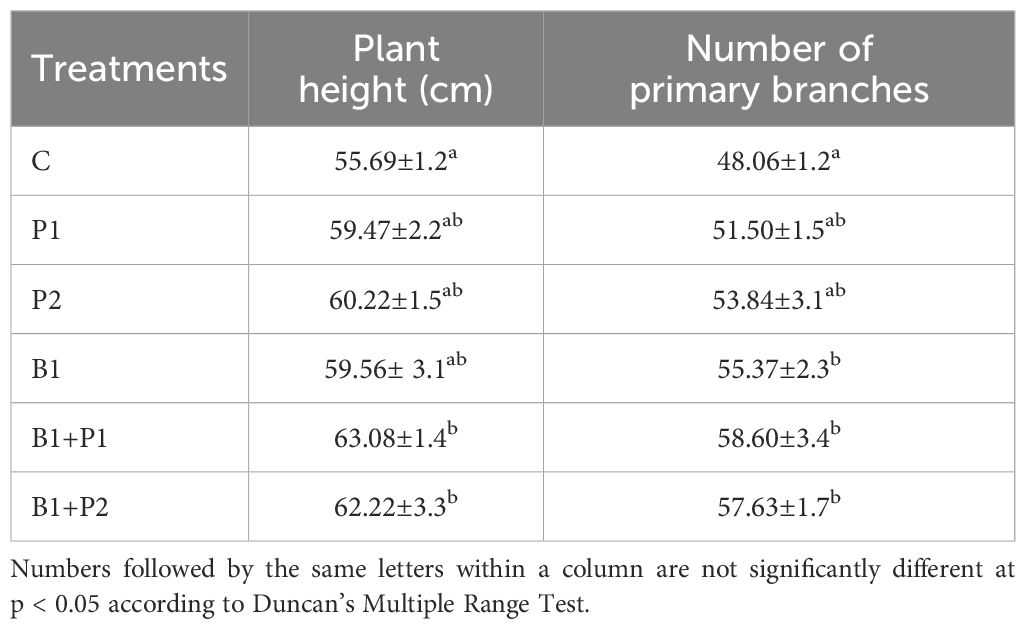
Table 2. Effect of endophytic bacteria and phosphorus application on the growth of A. paniculata at 14 weeks after planting (WAP).
Plant height increased by 6.79% to 13.27%, while the number of primary branches increased by 7.16% to 21.93%, following the application of endophytic bacteria and/or phosphate fertilizer at 14 WAP. The combination of endophytic bacteria Bacillus sp. and 0.675 g P2O5 plant-1 (B1+P1) resulted in the highest increases in both plant height (13.27%) and primary branch number (21.93%) (Table 2).
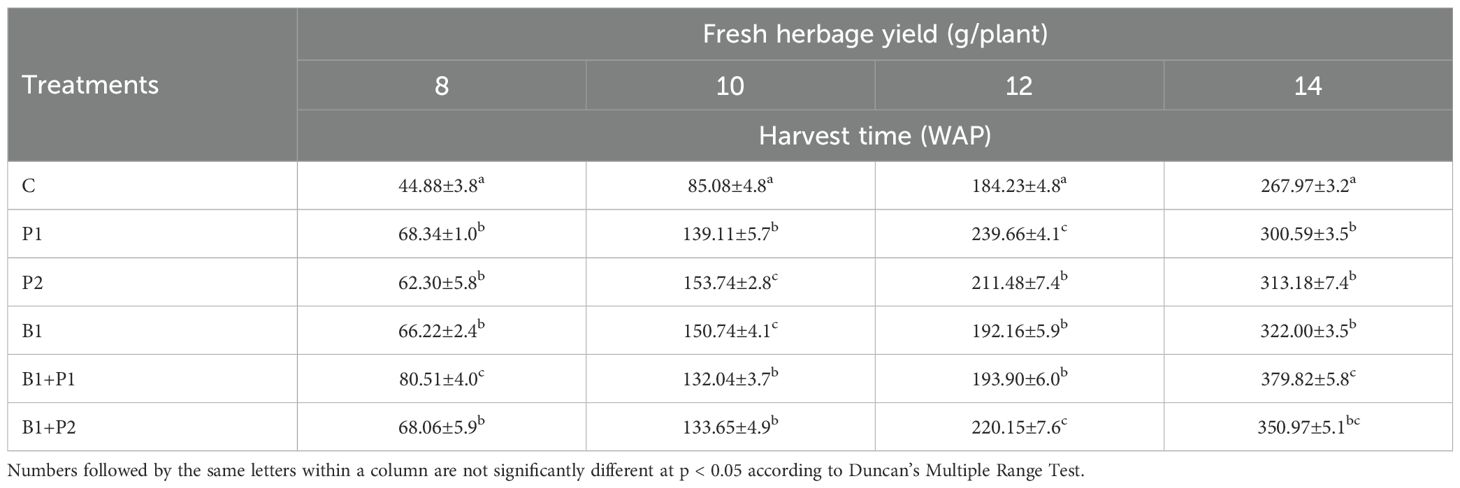
Fresh and dry herbage yields are key indicators of the success of A. paniculata cultivation. Consistent with its growth, the application of endophytic bacteria and phosphate fertilizer enhanced both fresh and dry weight yields from 8 to 14 WAP. The soil in the research site contained low levels of available P; therefore, applying even a small amount of P fertilizer significantly impacted growth and yield, as evidenced by the fresh and dry herbage yields.
The increase in fresh herb production ranged from 12.17-41.74%, while dry herb production ranged from 20.92% to 51.13% at 14 WAP with the application of endophytic bacteria and/or phosphate fertilizer. The combination of endophytic bacteria Bacillus sp. and 0.675 g P2O5 plant-1 (B1+P1) resulted in the highest increase in fresh (41.74%) and dry (51.13%) herb production compared to the control, with no significant difference when 1.35 g P2O5 was applied (Tables 3, 4).
Nutrient uptake represents the nutrients absorbed relative to the dry weight of plant biomass. At 14 WAP, N (2.56-2.85 g/plant), P (0.23-0.25 g/plant), and K (2.09-2.34 g/plant) uptake were higher in all treatments compared to the control (Figure 2). Nevertheless, with the application of 1.35 g P2O5 plant-1 (P2) resulting in the highest uptake of N and magnesium (Mg). Additionally, the combination of endophytic bacteria Bacillus sp. and 0.675 g P2O5 plant-1 (B1+P1) led to the highest uptake of phosphorus (P) and K. In contrast, the application of 0.675 g P2O5 plant-1 showed the most significant increase in calcium (Ca) nutrient uptake (Figure 2). The effect endophyte and P application on P uptake for all treatments there were no difference.
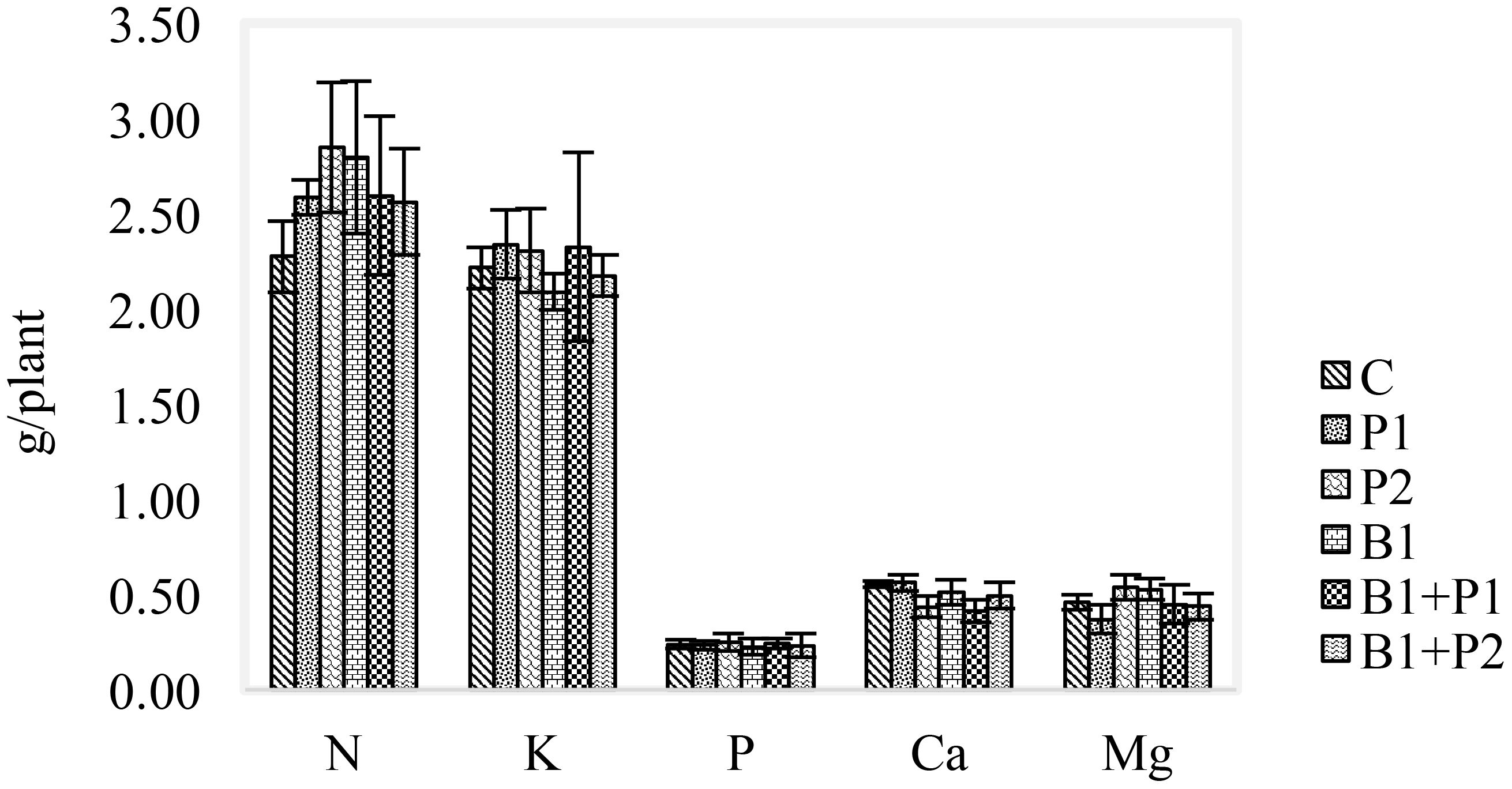
Figure 2. Effect of endophytic bacteria and phosphorus application on nutrient uptake of A. paniculata at 14 WAP.
3.3 Physiological characteristics of A. paniculata
A. paniculata fertilized with endophytic bacteria and phosphate showed varying dry-weight production depending on the harvesting time. As the plants aged, the yield increased. The combination of endophytic bacteria and 0.675 g P2O5 plant-1 (B1+P1) resulted in the highest net assimilate rate (NAR) and relative growth rate (RGR) at 12–14 WAP (Figure 3), consistent with the dry weight results (Table 4).
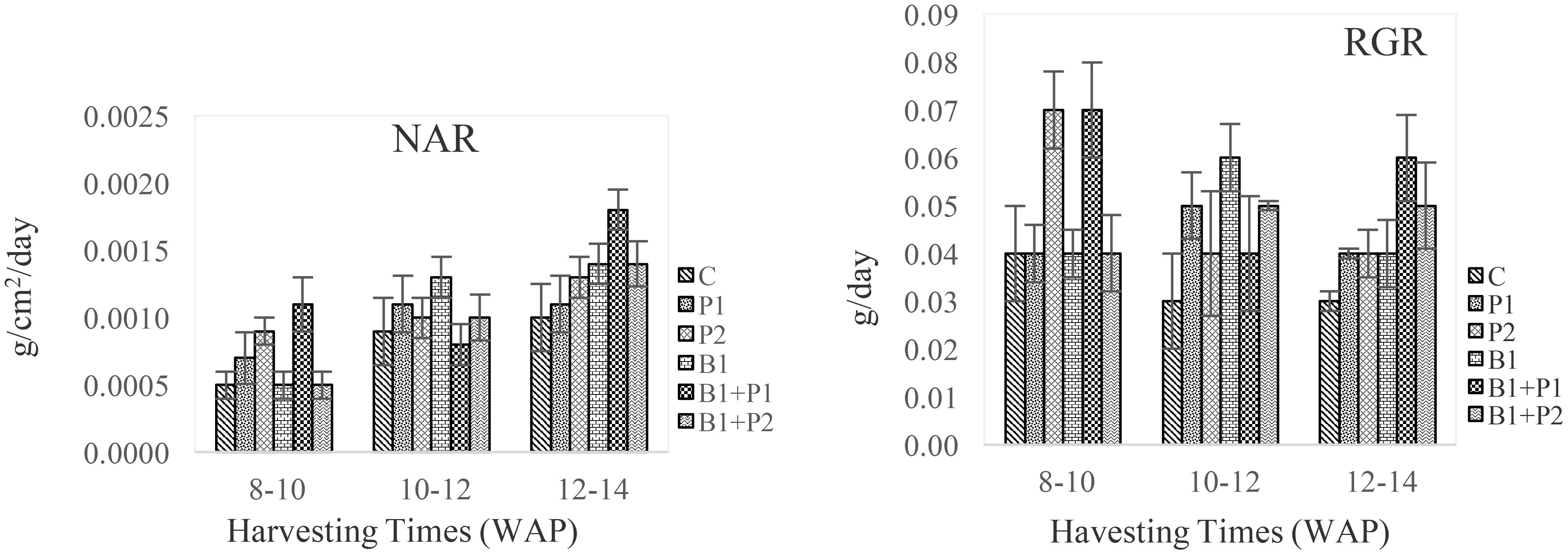
Figure 3. Effect of endophytic bacteria and phosphorus on the net assimilation rate (NAR) and relative growth rate (RGR) of A. paniculata from 8 to 14 WAP.
Leaf area index (LAI) and leaf area ratio (LAR) are used to assess plant productivity and calculate the amount of solar radiation absorbed by the leaves for photosynthesis, which directly influences plant biomass production. The LAI trend in A. paniculata showed that as the plant aged, the LAI value increased (Figure 4).
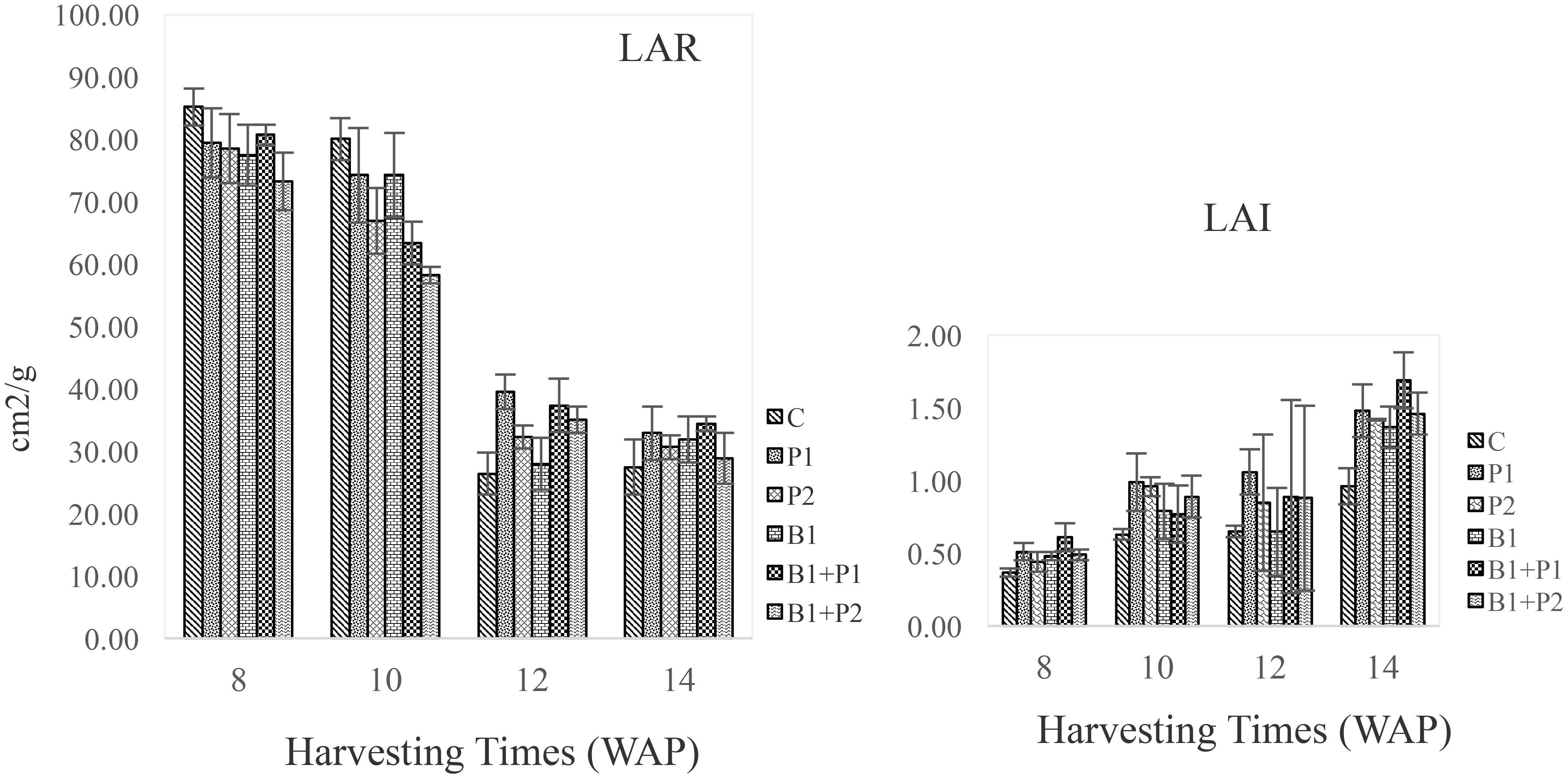
Figure 4. Effect of endophytic bacteria and phosphorus on leaf area ratio (LAR) and leaf area index (LAI) of A. paniculata from 8 to 14 WAP.
This trend aligned with the SLR, which was higher during the vegetative phase than in the generative phase (Table 5). Conversely, the leaf area ratio (LAR) increased as the plant aged. Similar to the dry herb yield, the combination of Bacillus sp. and 0.675 g P2O5 plant-1 (B1+P1) resulted in the highest LAI and LAR at 8–14 WAP (Figure 4).
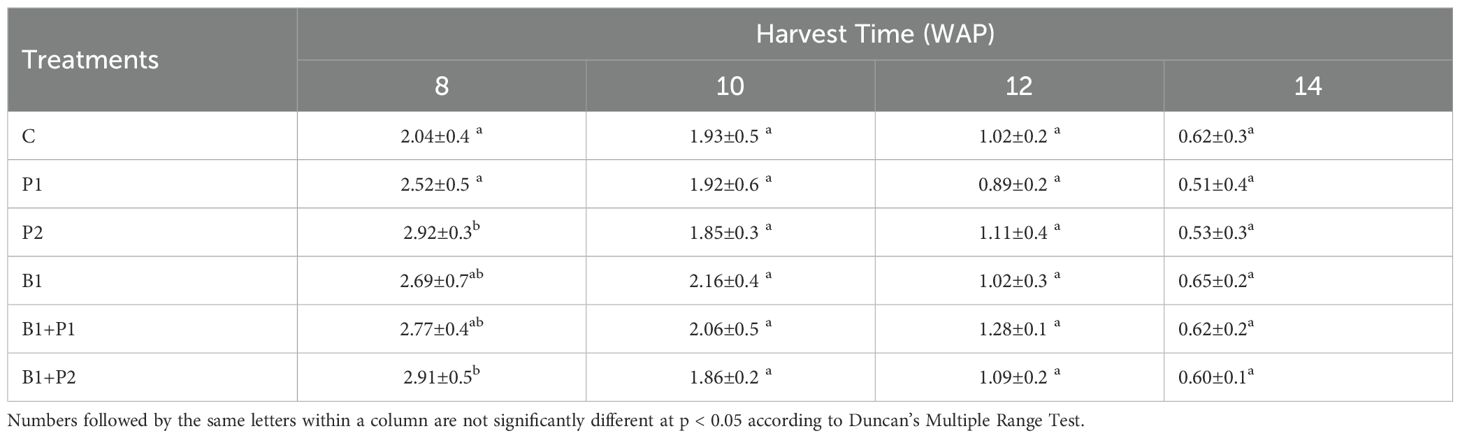
Table 5. Effect of endophytic bacteria and phosphorus on the dry stem-to-leaf ratio (SLR) of A. paniculata.
3.4 Andrographolide and its derivates of A. paniculata
Three bioactive compounds of A. paniculata measured at 8 and 14 WAP were andrographolide, 14-deoxy-11,12-didehydroandrographolide, and neoandrographolide. Generally, the levels of andrographolide and 14-deoxy11,12-didehydroandrographolide were lower in young plants during the vegetative phase (8 WAP) and increased in older plants during the generative phase (14 WAP) (Figures 5, 6). In contrast, the content of neoandrographolide decreased in older plants, except for the endophytic bacteria Bacillus sp. treatment, which showed a slight increase (Figure 5).
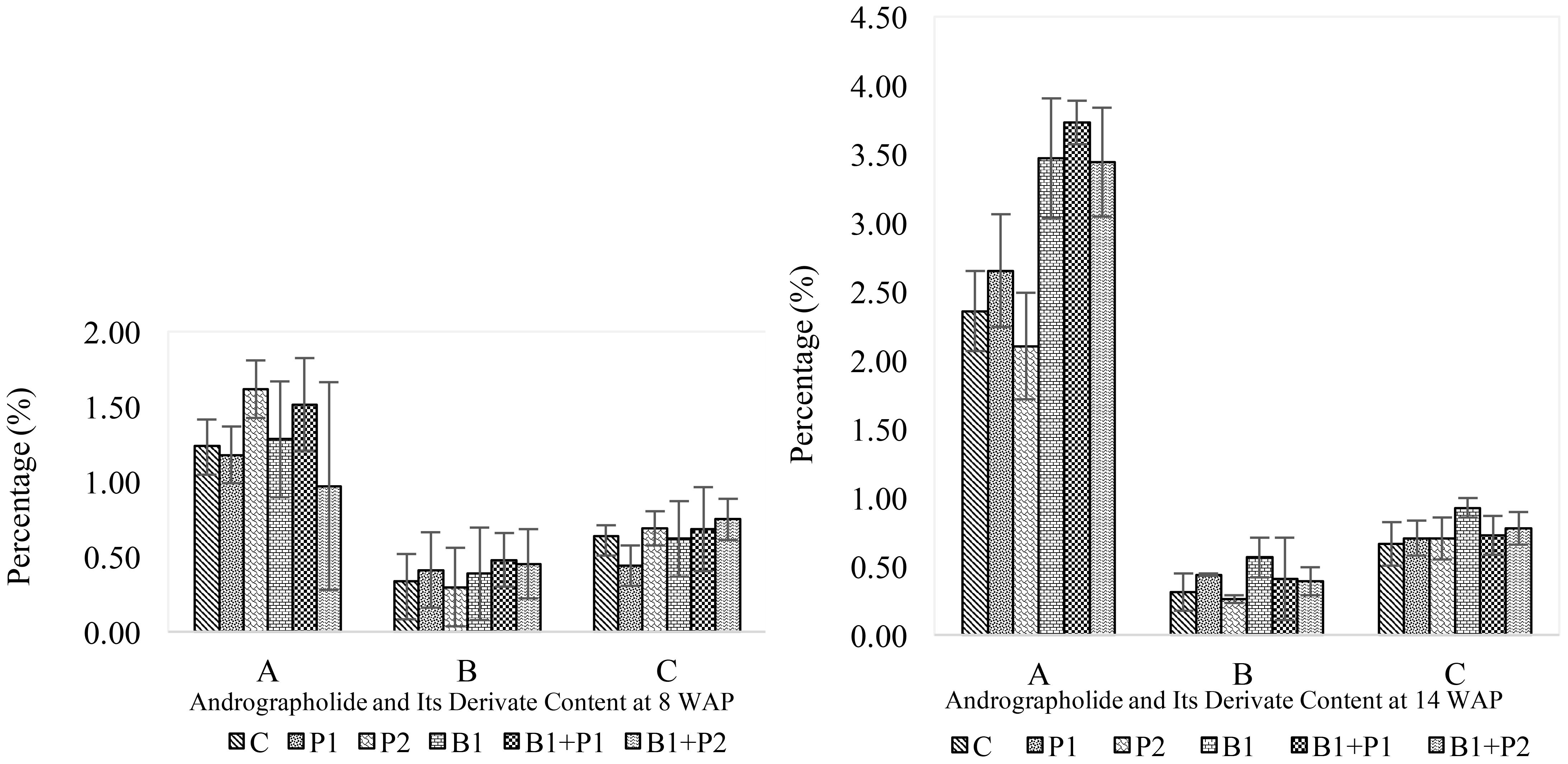
Figure 5. Effect of endophytic bacteria and phosphorus on andrographolide and its derivate content at 8 and 14 WAP. (A) andrographolide; (B) neoandrographolide; and (C) 14-deoxy-11,12-didehydroandrographolide.
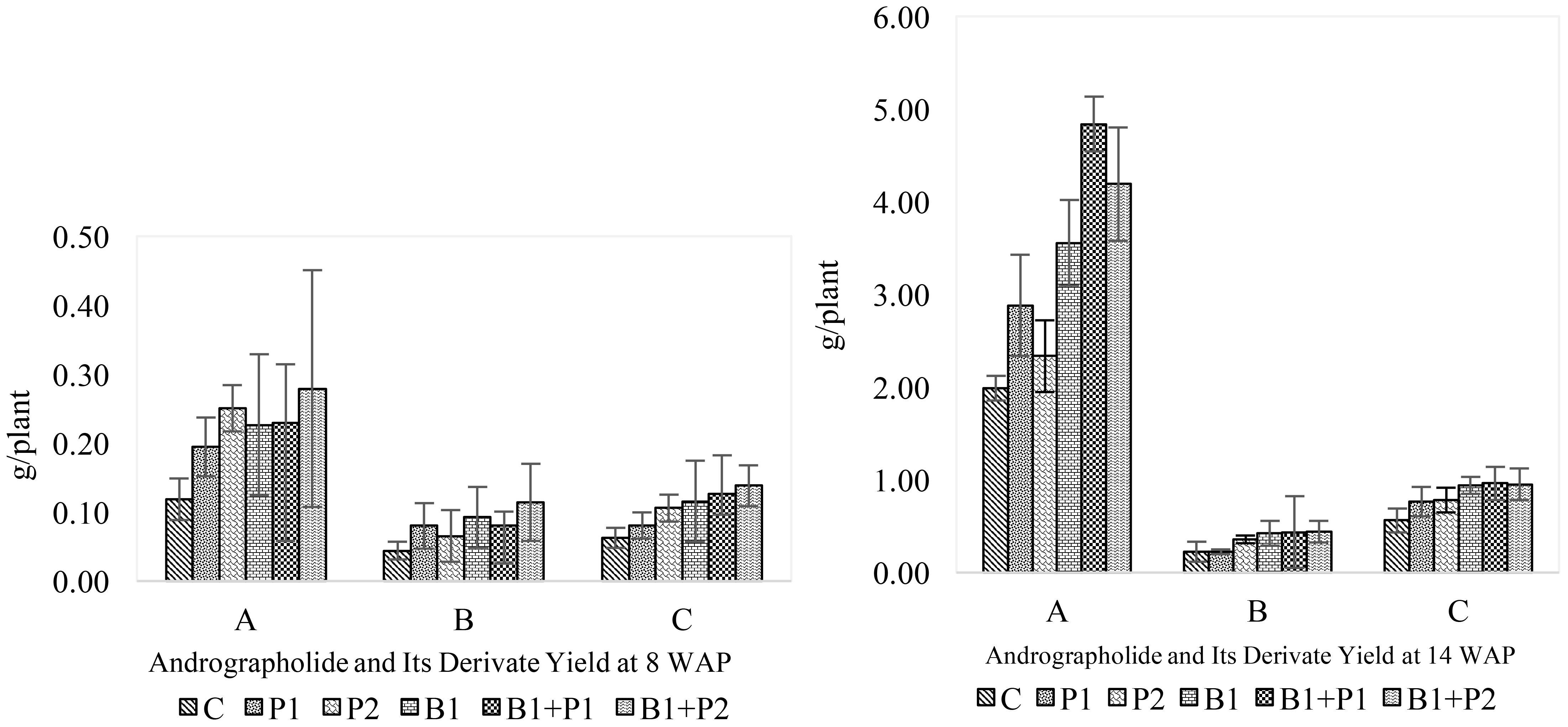
Figure 6. Effect of endophytic bacteria and phosphorus on andrographolide and its derivate yield at 8 and 14 WAP. (A) andrographolide; (B) neoandrographolide; and (C) 14-deoxy-11,12-didehydroandrographolide.
Andrographolide is the primary compound in A. paniculata that offers numerous benefits as a raw material for herbal medicine. In this study, andrographolide content increased linearly with the harvesting time. At 8 WAP, andrographolide (0.97–1.62%), neoandrographolide (0.30-0.48%), and 14-deoxy-11,12-didehydroandrographolide (0.44-0.75%) content. They did not show significant differences among treatments. However, at 14 WAP, the andrographolide, and 14-deoxy-11,12-didehydroandrographolide content increased to of range of 2.10–3.73% and 0.66-0.99% respectively, indicating significant differences among treatments, but neoandrographolide content (0.26-0.56%), lower than at 8 WAP (Figure 5).
The increase in andrographolide content was not directly proportional to its production, as the yield was also influenced by biomass production. As a result, andrographolide yield at 8 WAP (1.24-0.12-0.28 g plant-1) was lower than at 14 WAP (1.98–4.84 g plant-1) across all treatments. Likewise, neoandrograholide (0.04-0.11 to 0.22-0.44 g plant-1), and 14-deoxy-11,12-didehydroandrographolide (0.06-0.14 to 0.76-0.96 g plant-1) (Figure 6). Consequently, both the levels, content and production of total andrographolide at 14 WAP were higher than those at 8 WAP (Figures 5, 6). At 8 WAP, the treatment with Bacillus sp. + 1.35 g P2O5 plant-1 (B1+P2) resulted in higher levels of highest content and yield of andrographolide, neoandrographolide, and 14-deoxy-11,12-didehydroandrographolide (Figure 6). In contrast, at 14 weeks after planting (WAP), plants treated with the combination of Bacillus sp. + 0.675 g P2O5 plant-1 (B1+P1) showed the highest content of these compounds. Meanwhile, plants treated with Bacillus sp. (B1) exhibited the highest total andrographolide content (4.93%), while the combination of Bacillus sp. + 0.675 g P2O5 plant-1 (B1+P1) resulted in the highest total andrographolide yield (6.31 g plant-1) (Figure 6). Overall, the application of endophytic bacteria enhanced the levels of andrographolide and its derivatives in A. paniculata.
4 Discussions
The results indicate that the application of endophytic bacteria Bacillus sp. and phosphate fertilizer significantly improved plant growth, biomass production, and andrographolide accumulation, supporting the hypothesis that biofertilizers can reduce dependency on chemical inputs while sustaining or even improving yield and compound content. The improvement in agronomic traits such as plant height, branch number, and herbage yield is attributed to the role of Bacillus sp. in improving nutrient availability, particularly through phosphorus solubilization, as well as the production of plant growth-promoting phytohormones such as indole acetic acid (IAA) and gibberellic acid (GA3) (Adeleke et al., 2021). Similar findings have been reported in previous studies. For instance, Bacillus strains have been shown to positively influence maize growth and increase phosphorus (P) and nitrogen (N) content in the shoots (Alkahtani et al., 2020). Additionally, endophytic bacteria isolated from the medicinal plant Paris polyphylla var. yunnanensis have been found to enhance its growth (Tao et al., 2022). Likewise, the use of endophytic bacteria improved plant height in tomatoes (Abdallah et al., 2018) and peanuts (Lucero et al., 2021).
Endophytic bacteria not only release phytohormones but also play a crucial role in stimulating plant growth and improving overall plant performance. Previous studies have reported that bacterial endophytes can enhance tryptophan availability and produce substantial amounts of IAA, up to 60 µg mL-1 (Alkahtani et al., 2020), and 68 mg L-1 (Herlina et al., 2017). These phytohormones stimulated an increase in plant height and the number of primary branches in A. paniculata. IAA plays essential roles in regulating various plant cellular mechanisms, including cell orientation, division, organ development, differentiation, and elongation (Asgher et al., 2015; Labeeuw et al., 2016). Meanwhile, GA3 contributes significantly to the formation of lateral shoots (Cai et al., 2022), regulates stomatal behavior, supports metabolic activity under both normal and stress conditions (Zhou et al., 2024) and enhances root number and length (Choudhary et al., 2022).
Beyond promoting plant growth, endophytic bacteria also improve soil fertility by increasing nutrient availability. Previous studies have demonstrated that endophytic bacteria enhance pepper growth and soil fertility, leading to more efficient nutrient absorption (Gusmaini et al., 2020). In the present study, nutrient uptake patterns varied across treatments, reflecting the differential influence of Bacillus sp. and phosphate fertilizer on nutrient availability and transport. For example, the combination treatment (B1+P1) resulted in higher P and K uptake due to microbial phosphate solubilization and improved root architecture, which may enhance potassium mobility (Timofeeva et al., 2022). In contrast, 1.35 g P2O5 treatment (P2) led to the highest N and Mg uptake and the 0.675 g P2O5 treatment (P1) showed increased calcium uptake, likely because of the higher external availability of nutrients rather than microbial mediation.
These nutrient dynamics translated into higher biomass accumulation. At 14 weeks after planting (WAP), the application of endophytic Bacillus sp. and/or phosphate fertilizer led to increases in fresh herb production by 12.17–41.74% and in dry herb production by 20.92–51.13% at 14 WAP. The combined treatment of Bacillus sp. and 0.675 g P2O5 plant-1 (B1+P1) yielded the highest fresh (41.74%) and dry (51.13%) herb production compared to the control and showed no significant difference from the 1.35 g P2O5 treatment (Tables 3, 4). This indicates that microbial support may allow for reduced chemical input without compromising productivity. These results are consistent with previous findings where endophytic bacteria increased biomass production by up to 68.01% (Kumari et al., 2023).
In addition to producing phytohormones, Bacillus sp. also enhanced nutrient availability through nitrogen fixation and phosphorus solubilization (Lucero et al., 2021). These mechanisms allow nutrients to be utilized more efficiently by plants, thereby supporting their growth. Previous studies have also reported that endophytic bacteria from Bacillus strains improved dry biomass and phosphorus use efficiency in wheat cultivars (Wang et al., 2022), as well as enhanced phosphorus uptake in pearl millet under low-phosphorus conditions (Ribeiro et al., 2018).
Enhanced nutrient absorption positively influences plant growth and development, as demonstrated by the increased dry herb yield. The application of phosphate fertilizer, either alone or in combination with endophytic bacteria, significantly improved the uptake of N, P, K, Ca, and Mg, thereby boosting both fresh and dry herb yields (Tables 3, 4). These findings align with previous studies highlighting the role of phosphate-solubilizing endophytic bacteria in enhancing phosphorus uptake.
The net assimilation rate (NAR) describes the relationship between leaf area and dry matter accumulation over time, serving as an indicator of the plant’s efficiency in producing dry biomass per unit of leaf area per unit time. A reduction in leaf area adversely impacts dry biomass production. Similarly, the relative growth rate (RGR), which quantifies the rate of dry weight increase over time, is closely influenced by NAR (Lamont et al., 2023).
Endophytic bacteria significantly enhanced the accumulation of andrographolide and its derivatives in A. paniculata, with andrographolide content increasing by up to 44.16% following bacterial application (P. Kumari et al., 2023). This enhancement is attributed to the ability of Bacillus sp. to produce IAA and GA3, as well as to improve nitrogen and phosphorus acquisition. These factors collectively act as plant growth regulators, thereby promoting biomass production and stimulating the biosynthesis of secondary metabolite (Sales and Rigobelo, 2024).
In this study, the highest total andrographolide yield was observed at 14 weeks after planting (WAP). It is primarily due to two factors, i.e increased biomass and developmental stage. Plant age strongly influences secondary metabolite biosynthesis, with older plants often exhibiting higher metabolite accumulation due to metabolic shifts from growth to defence and storage (Nair and Sakuntala, 2020). Furthermore, enhanced nutrient availability at 14 WAP may have supported the mevalonate (MVA) and methylerythritol phosphate (MEP) pathways involved in andrographolide biosynthesis, especially under Bacillus sp. treatment (Kumari et al., 2023; Nair and Sakuntala, 2020).
Additionally, Bacillus strains are known to produce a wide range of bioactive compounds, including aromatic compounds, lipopeptides, phytohormones, polysaccharides, and enzymes involved in phenylpropanoid metabolism (Ek-Ramos et al., 2019). These metabolites play critical roles in promoting plant growth and supporting effective crop management strategies. Importantly, the inoculation of endophytic bacteria can influence the biosynthetic pathways of host plants, modulating the production of pharmacologically active compounds while simultaneously supporting vegetative growth (Singh et al., 2017).
Reducing the use of chemical fertilizers is a key priority for sustainable agriculture, particularly in the cultivation of medicinal plants, where minimizing chemical residues is crucial. In tropical soils, chemical phosphorus fertilizers are often inefficient due to their strong fixation by soil particles, which limits their availability to plants. In contrast, microbial biofertilizers such as Bacillus sp. offer a more sustainable solution by mobilizing fixed soil nutrients, enhancing root development, and lowering the risk of environmental pollution (Ribeiro et al., 2018; Wang et al., 2022). In this study, Bacillus sp. alone or in combination with half-dose phosphate (0.675 g P2O5) achieved results comparable to full chemical application (1.35 g P2O5), indicating the potential to reduce chemical inputs by up to 50% without compromising plant growth or andrographolide production. These results reinforce the value of microbial biofertilizers as eco-friendly tools to enhance biomass and secondary metabolite production in A. paniculata, aligning with broader efforts to promote low input and sustainable medicinal plant cultivation.
5 Conclusions
The agronomic and physiological characteristics, as well as andrographolide yield of Andrographis paniculata were significantly improved by applying the endophytic bacteria Bacillus sp. and phosphorus fertilizer. The combination of Bacillus sp. and 0.675 g P2O5 plant-1 produced the best agronomic and physiological performance and andrographolide yield, with the best results observed at the flowering stage (14 weeks after planting). This suggests that using Bacillus sp. can reduce the use of chemical phosphorus fertilizer by up to 50%, benefiting farmers and contributing to more environmentally friendly practices in support of sustainable agriculture. These results also align with the Ministry of Agriculture’s regulation that encourages the use of organic fertilizers as part of Good Agricultural Practice (GAP) for medicinal plants.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
GG: Investigation, Writing – review & editing, Conceptualization, Writing – original draft. HN: Methodology, Data curation, Investigation, Writing – original draft. KK: Visualization, Writing – review & editing, Formal Analysis. SP: Writing – review & editing, Visualization, Formal Analysis. KS: Formal Analysis, Writing – review & editing, Visualization. WW: Writing – review & editing, Formal Analysis, Visualization. RR: Data curation, Methodology, Writing – original draft, Investigation. SS: Data curation, Methodology, Investigation, Writing – original draft. YF: Writing – original draft, Methodology, Investigation, Data curation. DP: Writing – review & editing, Visualization, Supervision. EW: Visualization, Supervision, Writing – review & editing. RY: Visualization, Supervision, Writing – review & editing. MS: Visualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank the Indonesian Agency for Agricultural Research and Development, Ministry of Agriculture supporting this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdallah R. A. B., Jabnoun-Khiareddine H., Nefzi A., and Daami-Remadi M. (2018). Evaluation of the growth-promoting potential of endophytic bacteria recovered from healthy tomato plants. J. Hortic. 05, 1–10. doi: 10.4172/2376-0354.1000234
Adeleke B. S., Babalola O. O., and Glick B. R. (2021). Plant growth-promoting root-colonizing bacterial endophytes. Rhizosphere 20, 100433. doi: 10.1016/j.rhisph.2021.100433
Adiguna S. P., Panggabean J. A., Atikana A., Untari F., Izzati F., Bayu A., et al. (2021). Antiviral activities of andrographolide and its derivatives: Mechanism of action and delivery system. Pharmaceuticals 14, 1–20. doi: 10.3390/ph14111102, PMID: 34832884
Ali B., Hafeez A., Ammar M., Siddique M., Ali H., Qayyum A., et al. (2022). Role of endophytic bacteria in salinity stress amelioration by physiological and molecular mechanisms of defense : A comprehensive review. S. Afr. J. Bot. 151, 33–46. doi: 10.1016/j.sajb.2022.09.036
Alkahtani M. D. F., Fouda A., Attia K. A., Al-Otaibi F., Eid A. M., El-Din Ewais E., et al. (2020). Isolation and characterization of plant growth promoting endophytic bacteria from desert plants and their application as bioinoculants for sustainable agriculture. Agronomy 10, 1–18. doi: 10.3390/agronomy10091325
Asgher M., Khan M. I. R., Anjum N. A., and Khan N. A. (2015). Minimising toxicity of cadmium in plants—role of plant growth regulators. Protoplasma 252, 399–413. doi: 10.1007/s00709-014-0710-4, PMID: 25303855
Bergman M. E., Davis B., and Phillips M. A. (2019). Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules 24, 1–23. doi: 10.3390/molecules24213961, PMID: 31683764
Cai B., Wang T., Sun H., Liu C., Chu J., Ren Z., et al. (2022). Gibberellins regulate lateral root development that is associated with auxin and cell wall metabolisms in cucumber. Plant Sci. 317, 1–14. doi: 10.1016/j.plantsci.2021.110995, PMID: 35193752
Chen C., Xin K., Li M., Li X., Cheng J., Zhang L., et al. (2016). Paenibacillus sinopodophylli sp. Nov., a siderophore-producing endophytic bacterium isolated from roots of Sinopodophyllum hexandrum (Royle) ying. Int. J. Sys. Evol. Microbiol. 66, 4993–4999. doi: 10.1099/ijsem.0.001458, PMID: 27565539
Choudhary R. C., Kanwar J., and Singh P. (2022). Effect of Gibberellic acid (GA 3) and growing media on seedling growth parameters of papaya (Carica papaya L.) cv. Pusa Nanha. Pharma Innovation J. 11, 247–251. doi: 10.1016/j.egg.2024.100301
Egamberdieva D., Wirth S. J., Alqarawi A. A., Abd-Allah E. F., and Hashem A. (2017). Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 8, 1–12. doi: 10.3389/fmicb.2017.02104, PMID: 29163398
Ek-Ramos M. J., Gomez-Flores R., Orozco-Flores A. A., Rodríguez-Padilla C., González-Ochoa G., and Tamez-Guerra P. (2019). Bioactive products from plant-endophytic Gram-positive bacteria. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.00463, PMID: 30984118
Ferreira C. M. H., Vilas-Boas Â., Sousa C. A., Soares H. M. V. M., and Soares E. V. (2019). Comparison of five bacterial strains producing siderophores with ability to chelate iron under alkaline conditions. AMB Express 9, 1–12. doi: 10.1186/s13568-019-0796-3, PMID: 31139942
Fitriansyah S. N., Ruslan K., Rizky D. N., Munthary D., and Riasari H. (2024). Optimization of Andrografolid extraction from the herb sambiloto (Andrographis paniculata (Burm. F.) Nees) using a combined maceration and refluction methode Using, F Nees Method, Refluction. Trop. J. Nat. Prod. Res. 8, 9529–9536. doi: 10.26538/tjnpr/v8i12.26
Gusmaini, Kartikawati A., Nurhayati H., R.A. P., and Syakir M. (2022). The utilization of plant growth-promoting bacteria to enhance stevia (Stevia rebaudiana) herb yield at low land. IOP Conf. Ser.: Earth Environ. Sci. 974, 1–7. doi: 10.1088/1755-1315/974/1/012028
Gusmaini, Kartikawati A., Nurhayati H., and Sarwendah M. (2020). Plant growth promoting effects of indigenous rhizosphere and endophytic bacteria on soil fertility and black pepper (Piper nigrum) yield at Bangka Belitung. IOP Conf. Series: Earth Environ. Sci. 418, 1–7. doi: 10.1088/1755-1315/418/1/012057
Hao M., Lv M., and Xu H. (2020). Andrographolide: Synthetic methods and biological activities. Mini Rev. Med. Chem. 20, 1633–1652. doi: 10.2174/1389557520666200429100326, PMID: 32348215
Herlina L., Pukan K. K., and Mustikaningtyas D. (2017). The endophytic bacteria producing IAA (Indole Acetic Acid) in Arachis hypogaea. Cell Biol. Dev. 1, 31–35. doi: 10.13057/cellbioldev/v010106
Irianti I. N., Wijayanti A. D., and Mulyani G. T. (2022). The anticoccidial property of Sambiloto (Andrographis paniculata) leaf extract. Indonesian J. Veterinary Sci. 3, 8–13. doi: 10.22146/ijvs.v3i1.85442
Islam M. T., Ali E. S., Jamal S., Islam A., Shaw S., Khan I. N., et al. (2018). Andrographolide, a diterpene lactone from Andrographis paniculata and its therapeutic promises in cancer Ana Am e. Cancer Lett. 420), 129–145. doi: 10.1016/j.canlet.2018.01.074, PMID: 29408515
Jiang M., Sheng F., Zhang Z., Ma X., Gao T., Fu C., et al. (2021). Andrographis paniculata (Burm.f.) Nees and its major constituent andrographolide as potential antiviral agents. J. Ethnopharmacol 272, 113954. doi: 10.1016/j.jep.2021.113954, PMID: 33610706
Kjeidahl J. (1883). Neue Methode zur Bestimmung des Stickstoffs in organischen Korpern (Carlsberg Laboratorium bei Kopenhagen, Zeitschrift F. Anal. Chemie) 22, 366–82.
Kumari M., Qureshi K. A., Jaremko M., White J., Singh S. K., Sharma V. K., et al. (2022). Deciphering the role of endophytic microbiome in postharvest diseases management of fruits : Opportunity areas in commercial up-scale production. Front. Plant Sci. 13, 1026575. doi: 10.3389/fpls.2022.1026575, PMID: 36466226
Kumari P., Shanker K., and Singh A. (2023). Insight into Andrographis paniculata associated bacterial endomicrobiome and assessment of culturable bacterial endophytes for enhancement of industrially important andrographolide content. Ind. Crops Prod. 200, 1–15. doi: 10.1016/j.indcrop.2023.116840
Labeeuw L., Khey J., Bramucci A. R., Atwal H., de la Mata A. P., Harynuk J., et al. (2016). Indole-3-acetic acid is produced by Emiliania huxleyi coccolith-bearing cells and triggers a physiological response in bald cells. Front. Microbiol. 7, 1–16. doi: 10.3389/fmicb.2016.00828, PMID: 27375567
Lachu K., Kamle M., Borah R., Tiwari B., and Kumar P. (2022). “Endophytic bacteria: Application against biotic and abiotic stresses and plant health improvements for sustainable agriculture,” in Bacterial Endophytes for Sustainable Agriculture and Environmental Management. Eds. Singh P., Tripathi A. K., and Shukla Kumar V.A. K. (Singapore: Springer), 1–21. doi: 10.1007/978-981-16-4497-9_1
Lamont B. B., Williams M. R., and He T. (2023). Relative growth rate (RGR) and other confounded variables : mathematical problems and biological solutions. Ann. Bot. 131, 555–567. doi: 10.1093/aob/mcad031, PMID: 36794962
Li Y., Liu X., Hao T., and Chen S. (2017). Colonization and maize growth promotion induced by phosphate solubilizing bacterial isolates. Int. J. Mol. Sci. 18, 1–16. doi: 10.3390/ijms18071253, PMID: 28661431
Liu Y., Chen H., Lii C., Jhuang J., and Huang C. (2020). A Diterpenoid, 14-Deoxy-11, 12-Didehydroandrographolide, in Andrographis paniculata reduces steatohepatitis and liver injury in mice fed a high-fat and high-cholesterol diet. Nutrient 12, 1–17. doi: 10.3390/nu12020523, PMID: 32085637
Lucero C. T., Lorda G. S., Anzuay M. S., Ludueña L. M., and Taurian T. (2021). Peanut endophytic phosphate solubilizing bacteria increase growth and P content of soybean and maize plants. Curr. Microbiol. 78, 1961–1972. doi: 10.1007/s00284-021-02469-x, PMID: 33839883
Maheshwari R., Bhutani N., and Suneja P. (2019). Screening and characterization of siderophore producing endophytic bacteria from Cicer arietinum and Pisum sativum plants. J. Appl. Biol. Biotechnol. 7, 7–14. doi: 10.7324/JABB.2019.70502
Morrison M. J., Voldeng H. D., and Cober E. R. (1999). Physiological changes from 58 years of genetic improvement of short-season soybean cultivars in Canada. Agron. J. 91, 685–689. doi: 10.2134/agronj1999.914685x
Nair D. S. and Sakuntala M. (2020). Induction of root endosymbiosis as a highly sustainable and e ffi cient strategy for overproduction of the medicinally important diterpenoid lactone-andrographolide in Andrographis paniculata (Burm. F.) Wall. ex Nees. Ind. Crop Prod. 156, 112835. doi: 10.1016/j.indcrop.2020.112835
Okamoto T., Shinjo R., Nishihara A., Uesaka K., Tanaka A., Sugiura D., et al. (2021). Genotypic variation of endophytic nitrogen-fixing activity and bacterial flora in rice stem based on sugar content. Front. Plant Sci. 12, 1–21. doi: 10.3389/fpls.2021.719259, PMID: 34447404
Prasad M., Srinivasan R., Chaudhary M., Mahawer S. K., and Jat L. K. (2020). “Endophytic bacteria: Role in sustainable agriculture,” in Microbial Endophytes: Prospects for Sustainable Agriculture (Elsevier Inc). doi: 10.1016/B978-0-12-818734-0.00003-6
Ribeiro V. P., Marriel I. E., Sousa S. M., Lana U. G. P., Mattos B. B., Oliveira C. A., et al. (2018). Endophytic Bacillus strains enhance pearl millet growth and nutrient uptake under low-P. Braz. J. Microbiol. 49, 40–46. doi: 10.1016/j.bjm.2018.06.005, PMID: 30150087
Sales L. R. and Rigobelo E. C. (2024). The role of Bacillus sp. in reducing chemical inputs for sustainable crop production. Agron. J. 14, 1–16. doi: 10.3390/agronomy14112723
Shailaja A., Srinath M., Venkata B., and Bindu B. (2021). Isolation of 4-hydroxy 3-methyl 2-butenyl 4-diphosphate reductase (ApHDR) gene of methyl erythritol diphosphate (MEP) pathway, in silico analysis and differential tissue specific ApHDR expression in Andrographis paniculata (Burm. f) Nees. Physiol. Mol. Biol. Plants 27, 223–235. doi: 10.1007/s12298-021-00952-0, PMID: 33707865
Shi T. H., Huang Y. L., Chen C. C., Pi W. C., Hsu Y. L., Lo L. C., et al. (2020). Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage. Biochem. Biophys. Res. Commun. 533, 467–473. doi: 10.1016/j.bbrc.2020.08.086, PMID: 32977949
Singh M., Kumar A., Singh R., and Pandey K. D. (2017). Endophytic bacteria: a new source of bioactive compounds. 3 Biotech. 7, 1–14. doi: 10.1007/s13205-017-0942-z, PMID: 28955612
Tajidin N. E., Shaari K., Maulidiani M., and Salleh N. S. (2019). Metabolite profiling of Andrographis paniculata (Burm. f.) Nees. young and mature leaves at different harvest ages using 1 H NMR-based metabolomics approach. Sci. Rep. 9, 1–10. doi: 10.1038/s41598-019-52905-z, PMID: 31727911
Tao L., Qiuhong L., Fuqiang Y., Shuhui Z., Suohui T., and Linyuan F. (2022). Plant growth-promoting activities of bacterial endophytes isolated from the medicinal plant Pairs polyphylla var. yunnanensis. World J. Microbiol. Biotech. 38, 1–10. doi: 10.1007/s11274-021-03194-0, PMID: 34878606
Timofeeva A., Galyamova M., and Sedykh S. (2022). Prospects for using phosphate-solubilizing microorganisms as natural fertilizers in agriculture. Plants 11, 1–123. doi: 10.3390/plants11162119, PMID: 36015422
Ullah A., Nisar M., Ali H., Hazrat A., Hayat K., Keerio A. A., et al. (2019). Drought tolerance improvement in plants: an endophytic bacterial approach. Appl. Microbiol. Biotechnol. 103, 7385–7397. doi: 10.1007/s00253-019-10045-4, PMID: 31375881
Wang D. W., Xiang Y. J., Wei Z. L., Yao H., and Shen T. (2021). Andrographolide and its derivatives are effective compounds for gastrointestinal protection: A review. Eur. Rev. Med. Pharmacol. Sci. 25, 2367–2382. doi: 10.26355/eurrev_202103_25276, PMID: 33755974
Wang Z., Zhang H., Liu L., Li S., Xie J., Xue X., et al. (2022). Screening of phosphate-solubilizing bacteria and their abilities of phosphorus solubilization and wheat growth promotion. BMC Microbiol. 22, 1–15. doi: 10.1186/s12866-022-02715-7, PMID: 36494624
William C. N. and Joseph K. T. (1976). Climate, Soil and Crop Production in The Humid Tropics (Kuala Lumpur: Oxford Univ. Press).
Keywords: endophyte bacteria, bioactive compound, NAR, P fertilizer, productivity, RGR
Citation: Gusmaini G, Nurhayati H, Kartika K, Putra S, Sasmita KD, Rismayani R, Saefudin S, Wibawa W, Pranowo D, Wardiana E, Ferry Y, Yunita R and Syakir M (2025) Physio-agronomic characteristics and andrographolide yield of Andrographis paniculata in response to endophytic Bacillus sp and phosphorus. Front. Agron. 7:1642117. doi: 10.3389/fagro.2025.1642117
Received: 06 June 2025; Accepted: 14 July 2025;
Published: 30 July 2025.
Edited by:
Sowbiya Muneer, VIT University, IndiaReviewed by:
Ankita Rajendra Parab, University of Science Malaysia (USM), MalaysiaArabi Mohammed Saleh, VIT University, India
Copyright © 2025 Gusmaini, Nurhayati, Kartika, Putra, Sasmita, Rismayani, Saefudin, Wibawa, Pranowo, Wardiana, Ferry, Yunita and Syakir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gusmaini Gusmaini, Z3VzbWFpbmk2NzJAZ21haWwuY29t
 Gusmaini Gusmaini
Gusmaini Gusmaini Hera Nurhayati
Hera Nurhayati Kartika Kartika
Kartika Kartika Sunjaya Putra
Sunjaya Putra Kurnia Dewi Sasmita
Kurnia Dewi Sasmita Rismayani Rismayani
Rismayani Rismayani Saefudin Saefudin
Saefudin Saefudin Wahyu Wibawa
Wahyu Wibawa Dibyo Pranowo
Dibyo Pranowo Edi Wardiana
Edi Wardiana Yulius Ferry
Yulius Ferry Rossa Yunita
Rossa Yunita Muhammad Syakir
Muhammad Syakir