- 1Guangxi Key Laboratory of Arable Land Conservation, Guangxi Academy of Agricultural Sciences, Nanning, China
- 2Guangxi Tea Research Institute, Guilin, China
- 3Guilin Jifusi Monk Fruit Biotechnology Co. Ltd., Guilin, China
- 4Guangxi Vocational University of Agriculture, Nanning, China
Integrating high-value climbing fruit crops into tea (Camellia sinensis) systems offers potential to improve tea quality and diversify income, yet the density-dependent effects on both crops remain unquantified. This study evaluated tea intercropped with Siraitia grosvenorii (SG) and Passiflora edulis (PE) at three planting densities (low, medium, high), assessing tea leaf biochemical traits alongside fruit physical and intrinsic quality parameters. All intercropping treatments significantly increased tea leaf chlorophyll a, b, and total chlorophyll content compared with monoculture (CK), with maxima in PE-H (1.188, 0.447, and 1.635 mg/g) and SG-H (1.166, 0.425, and 1.591 mg/g), respectively. Tea polyphenol content decreased with increasing density, most notably in PE-H (−21.63% vs. CK), while free amino acids increased under SG-M (+20.50%) and PE-L (+19.10%). The polyphenol-to-amino acid ratio declined across treatments, with the largest reductions in PE-L (−24.36%) and SG-M (−19.62%). Water extract content rose in all intercropped systems, peaking in SG-H (+5.82%) and PE-H (+2.12%). For S. grosvenorii, SG-H achieved the highest single-fruit weight (94.37g), transverse diameter (54.56mm), proportion of medium/large fruits (94.58%), and sugar contents (reducing sugar 6.69%, total sugar 12.01%). For P. edulis, PE-H produced the highest soluble solids (17.82%), soluble sugars (12.27%), solid-acid ratio (6.72), and sugar-acid ratio (4.62), indicating superior flavor, whereas PE-M maximized titratable acids (2.74%) and peel thickness (5.16mm), and PE-L yielded the highest edible ratio (46.68%). This work provides density-resolved evidence that tea-climbing plant intercropping can simultaneously enhance tea leaf quality, and improve fruit sweetness and flavor profiles. Optimal densities vary by species, offering a dual-quality, agroecologically viable model for tea-based polyculture.
1 Introduction
Tea (Camellia sinensis) is among the most widely consumed non-alcoholic beverages worldwide, holding substantial economic, cultural, and nutritional significance, particularly in East Asia and increasingly across the globe (Brody, 2019; Liao et al., 2025). The quality of tea leaves, largely determined by their biochemical composition, primarily polyphenols, amino acids, and water-soluble extracts, directly influences the sensory attributes, market value, and health-promoting properties of tea products (Khan and Mukhtar, 2018; Ahammed and Li, 2022; Kochman et al., 2020). In response to the growing demand for high-quality, sustainably produced tea, there is increasing interest in innovative cultivation strategies that enhance tea quality while supporting ecological and economic sustainability (Ali Abaker Omer et al., 2025).
Although productive, traditional monoculture tea plantations are increasingly recognized for their ecological drawbacks, including soil degradation, biodiversity loss, and heightened susceptibility to pests and diseases (Yan et al., 2018, 2020; Gui et al., 2022). To address these challenges, intercropping, an agroecological practice involving the simultaneous cultivation of two or more crop species within the same field, has received increasing attention. Intercropping has been shown to improve resource use efficiency, suppress weeds, enhance soil fertility, and foster a more resilient agroecosystem (Brooker et al., 2015; Li et al., 2023; Wang et al., 2023a). In tea plantations, intercropping with trees, legumes, or medicinal plants has demonstrated improvements in microclimatic conditions, increased biodiversity, and enhanced tea leaf quality (Farooq et al., 2021; Wang et al., 2022; Pokharel et al., 2019; Wang et al., 2023b; Wang et al., 2025).
Among the diverse intercropping systems, integrating fruit trees with tea plantations offers a promising strategy for maximizing land productivity and diversifying farm income. Fruit trees can provide shade, modify soil nutrient dynamics, and create a more favorable microenvironment for tea cultivation (Bai et al., 2022; Duan et al., 2024). The effects of intercropping on tea quality are complex and can vary depending on the choice of companion species, planting density, and local environmental conditions (Duan et al., 2024; Wu et al., 2023). Despite their value, the use of climbing plants such as S. grosvenorii (luohan guo) and P. edulis (passion fruit), both important medicinal and nutritional crops, has received limited scientific attention in the context of tea intercropping systems.
S. grosvenorii, commonly known as luohan guo or monk fruit, is valued for its intensely sweet, non-caloric mogrosides and is widely used in traditional Chinese medicine and as a natural sweetener (Guo et al., 2024a, b). P. edulis, or passion fruit, is noted for its distinctive flavor, high nutritional value, and a range of health benefits, including antioxidant and anti-inflammatory properties (He et al., 2020; Zhang et al., 2023). Both species are climbing plants that can be cultivated on trellises or trees, making them suitable candidates for intercropping in tea plantations without directly competing for ground space. Their introduction into tea agroecosystems has the potential to improve land use efficiency, generate additional economic returns, and influence the growth and quality of both tea and fruit crops. However, the physiological trade-offs and quality interactions, particularly under varying planting densities, are not yet fully elucidated. Few studies have directly quantified the effects of such intercropping on key functional traits of tea leaves (e.g., chlorophyll, polyphenol, and amino acid contents) and assessed whether the benefits are reciprocal, that is, whether they also enhance fruit yield and quality.
Recent field studies increasingly highlight plant density as a crucial factor influencing intercropping outcomes, with optimal configurations often being specific to the crop species and the intercropping system (Postma et al., 2021; Li et al., 2024). Excessively high planting densities can intensify interspecific competition for light, water, and nutrients, while too low densities may result in suboptimal use of agroecological resources (Pelech et al., 2023; Zhang et al., 2025). In both tea and climbing plants, changes in the light environment caused by mutual shading can directly affect chlorophyll synthesis, secondary metabolite accumulation, and overall crop quality. Additionally, microclimatic shifts within intercropped systems may influence fruit set, sugar accumulation, and fruit physical characteristics, emphasizing the need for detailed, crop-specific studies.
Despite the potential benefits outlined above, there remains a notable lack of systematic experimental evidence evaluating the simultaneous effects of tea–climbing plant intercropping on the quality traits of both crops across different intercropping intensities. Most existing studies have primarily focused on yield or broad biophysical impacts of a single crop species (Peng et al., 2015; Zhang et al., 2021), with few examining quality indices for both tea and fruit concurrently. Consequently, the optimal balance for maximizing product quality remains unclear, particularly regarding the interactive effects on key metabolites, such as polyphenols, free amino acids, and water-soluble compounds in tea leaves, as well as the physical and intrinsic quality parameters of the fruit, which have yet to be comprehensively assessed.
Therefore, this study presents an integrative analysis of tea intercropped with two economically and nutritionally valuable climbing fruit species, S. grosvenorii and P. edulis, across varying intercropping densities. Specifically, we systematically quantified the effects of each intercropping system and density level on multiple tea leaf quality indicators, including chlorophyll a, chlorophyll b, total chlorophyll, polyphenol content, free amino acid content, water-extractable compounds, and the polyphenol-to-amino acid ratio. Concurrently, we assessed fruit yield, physical attributes (such as single-fruit weight, dimensions, peel thickness, edible ratio, and shape index), and intrinsic quality parameters (including soluble solids, soluble sugars, titratable acids, and solid–acid and sugar–acid ratios) in both S. grosvenorii and P. edulis. This study thus addresses critical knowledge gaps related to the dual objectives of enhancing tea leaf and fruit quality within agroecologically intensified production systems.
2 Materials and methods
2.1 Experimental site
The experiment was conducted at a tea plantation base located in Zhongdong Village, Weijiang Township, Longsheng County, Guilin City, Guangxi, China (110°4′10″E, 26°0′2″N; altitude: 805.2m). The region has a subtropical humid monsoon climate, characterized by an average annual temperature of 18.2 °C, average relative humidity of 80%, average annual precipitation of 1,544 mm, average annual sunshine duration of 1,247 hours, and an average frost-free period of approximately 314 days. The physicochemical properties of the tea plantation soil were as follows: total nitrogen, 2.78 g/kg; total phosphorus, 1.37 g/kg; total potassium, 24.53 g/kg; alkali-hydrolyzable nitrogen, 236.3 mg/kg; available phosphorus, 288.1 mg/kg; available potassium, 119.0 mg/kg; organic matter, 57.04 g/kg; and pH, 4.36.
2.2 Experimental design
The tea cultivar used in this study was Fuyun No. 6, a six-year-old plant. Fuyun No. 6 is a first-generation clonal variety developed by the Tea Research Institute, Fujian Academy of Agricultural Sciences. It was bred via interspecific hybridization, using the Yunnan large-leaf variety as the male parent and Fusheng Dabai tea as the female parent. This cultivar is known for its high yield and early budding. Tea was planted in double rows on terraced fields, with a row spacing of 3m. The S. grosvenorii (luohan guo) variety was Dadi No. 2, propagated via tissue culture seedlings, while the passion fruit (P. edulis) variety used was Tainong No. 1, established from cutting seedlings.
A randomized block design was employed, comprising seven treatments, including a control treatment of monoculture tea plantation without intercropping. Three intercropping densities, low, medium, and high, were established for both S. grosvenorii and passion fruit within the tea plantation, as detailed in Table 1. Each treatment plot measured 80 m² and was replicated three times.
2.3 Plant cultivation
The intercropping of S. grosvenorii and passion fruit within the tea plantation employed a box-frame root restriction method combined with a flat trellis system. For root restriction, devices measuring 30cm in height and 80cm in width were installed along the inner side of each tea plantation terrace row, according to the planting spacing requirements of each treatment. A cultivation substrate was prepared by mixing orchard soil collected from surrounding orchards with a nutrient substrate at a 6:4 volume ratio. This mixture was filled to two-thirds of the root restriction device volume, into which a single seedling was centrally planted. Subsequently, the device was filled with additional substrate, compacted, and thoroughly watered to ensure adequate moisture for seedling establishment. The flat trellis system consisted of 1.8m high structures covered with plastic mesh grids measuring 15cm × 15cm to support plant climbing and fruit load (Figure 1).
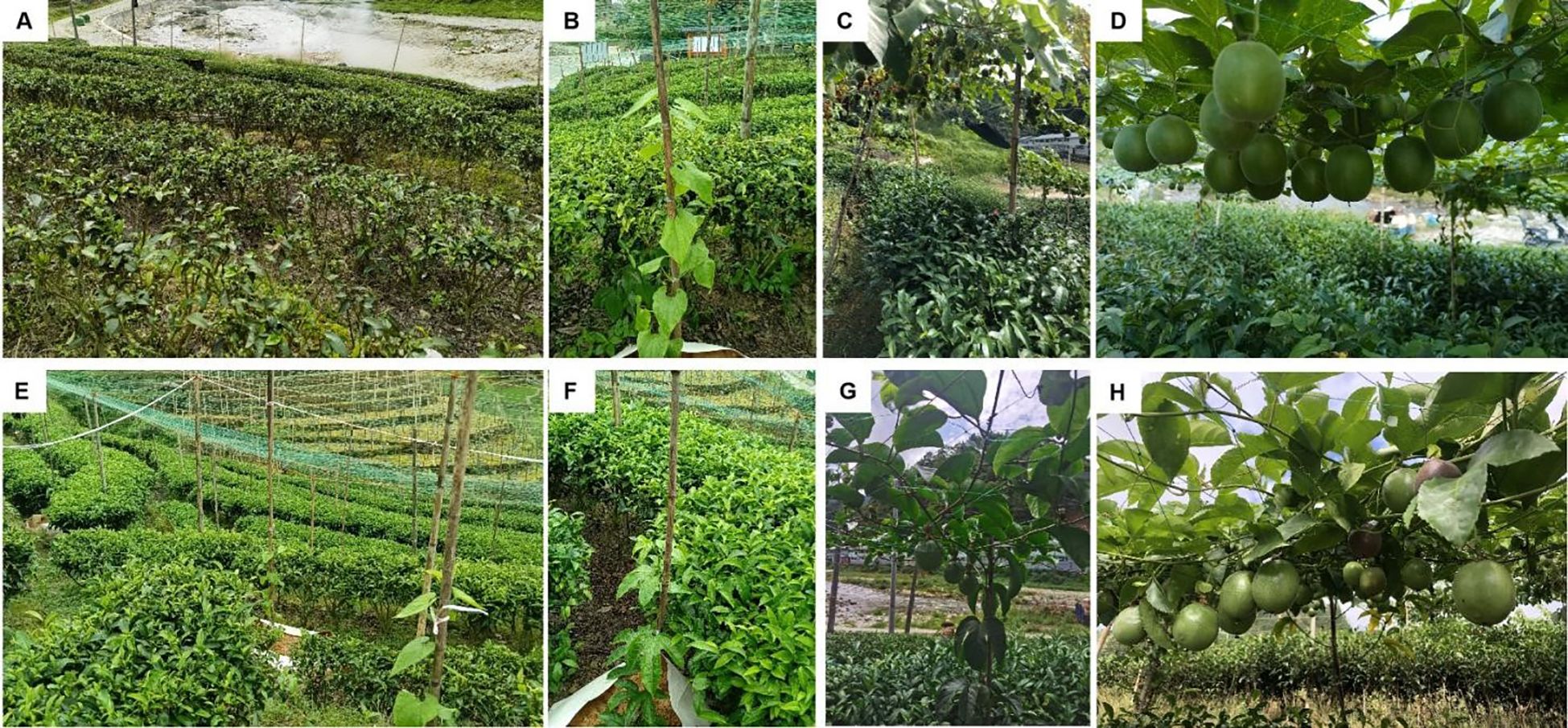
Figure 1. Cultivation stages of climbing plants. (A) monoculture control (CK). (B) S. grosvenorii at early growing stage. (C) S. grosvenorii at early fruiting stage. (D) S. grosvenorii at peak fruiting stage. (E) intercropping system. (F) P. edulis at early growing stage. (G) P. edulis at early fruiting stage. (H) P. edulis at peak fruiting stage.
The two climbing plants were initially grown in a greenhouse for approximately 40 days and reached a height of around 50cm. Then they were transplanted on May 21, 2024. About one month after transplantation, the plants were able to provide sufficient shade. Pest and disease management utilized biological pesticides, and all other cultural practices followed conventional standards.
Fertilization and management of tea plants were conducted following standard conventional practices.
2.4 Sample collection and measurement of tea
After 3 months, on August 14, 2024, fresh tea samples consisting of one bud and two leaves were collected. Chlorophyll content in the tea leaves was measured following the NY/T 3082–2017 standard, “Determination of Chlorophyll Content in Fruits, Vegetables and Their Products - Spectrophotometric Method”. Tea polyphenol content was determined according to GB/T 8313-2018, “Determination of Tea Polyphenols and Catechins in Te”. Free amino acid content was assessed using the GB/T 8314–2013 standard, “Determination of Total Free Amino Acids in Tea”. Water extract content was measured in accordance with GB/T 8305-2013, “Determination of Water Extract in Tea”.
2.5 Sample collection of climbing tree fruit
After 6 months, on November 21, 2024, fruits of S. grosvenorii and passion fruit were harvested. For each treatment replicate, fruits were collected separately, and 20 mature fruits were randomly selected from each plot for quality assessment.
2.6 Measurement of S. grosvenorii fruit
Quality indicators for S. grosvenorii fruit included single fruit weight, transverse diameter, reducing sugar content, total sugar content, and mogroside V content. Single fruit weight was measured using a 1% precision electronic balance, and transverse diameter was measured with a digital caliper. Fruits were categorized by transverse diameter as follows: extra-large (>57mm), large (52–56 mm), medium (48–51 mm), small (44–47 mm), and below standard (<43mm). The proportion of fruits classified as medium or larger was calculated. Reducing sugar, total sugar, and mogroside V contents were determined according to GB 5009.7-2016 “National Food Safety Standard - Determination of Reducing Sugar in Food” (Method 1), SB/T 10203-1994 “General Test Methods for Fruit Juice”, and high-performance liquid chromatography, respectively.
2.7 Measurement of passion fruit
Quality indicators for passion fruit included single fruit weight, peel weight, longitudinal diameter, transverse diameter, peel thickness, soluble solids content, soluble sugar content, and titratable acid content. Single fruit weight and peel weight were measured using a 1% precision electronic balance, while longitudinal diameter, transverse diameter, and peel thickness were measured with a digital caliper. Edible rate and fruit shape index were calculated using the following formulas:
Soluble solids, soluble sugar, and titratable acid contents were determined according to NY/T 2637-2014 “Determination of Soluble Solids Content in Fruits and Vegetables”, NY/T 2742-2015 “Determination of Soluble Sugar in Fruits and Their Products - 3,5-Dinitrosalicylic Acid Colorimetric Method”, and GB 12456-2021 “National Food Safety Standard - Determination of Total Acid in Food” (Method 1), respectively.
The solid-acid ratio and sugar-acid ratio were calculated as follows:
Solid-acid ratio = soluble solids content/titratable acid content
Sugar-acid ratio = soluble sugar content/titratable acid content
2.8 Statistical analysis
Statistical analyses were carried out utilizing Microsoft Excel 2017 in conjunction with SPSS version 19.0. For comparative assessments, one-way analysis of variance (ANOVA) was implemented, followed by Duncan’s multiple range test. All experiments were conducted in triplicate, and the outcomes were presented as mean values accompanied by the standard error. Statistical significance was determined at P < 0.05.
3 Results
3.1 Influence of tea-climbing plant intercropping on the quality of tea leaves
3.1.1 Chlorophyll content
Chlorophyll a, chlorophyll b, and total chlorophyll contents were significantly higher in all intercropping treatments compared to CK (Figures 2A–C, Supplementary Table S1). The highest chlorophyll a and b content were observed in the PE-H (1.188 mg/g and 0.447 mg/g) and SG-H (1.166 mg/g and 0.425 mg/g) treatments, which were significantly greater than those in other treatments. Total chlorophyll content under PE-H (1.635 mg/g) and SG-H (1.591 mg/g) was also markedly higher than those in other treatments and the control (1.257 mg/g). Notably, the chlorophyll a/b ratio was highest in CK (2.930), while all intercropping treatments had significantly lower ratios (2.648-2.741), indicating a greater increase in chlorophyll b (Figure 2D). Overall, SG-H and PE-H significantly enhanced chlorophyll accumulation in tea leaves.
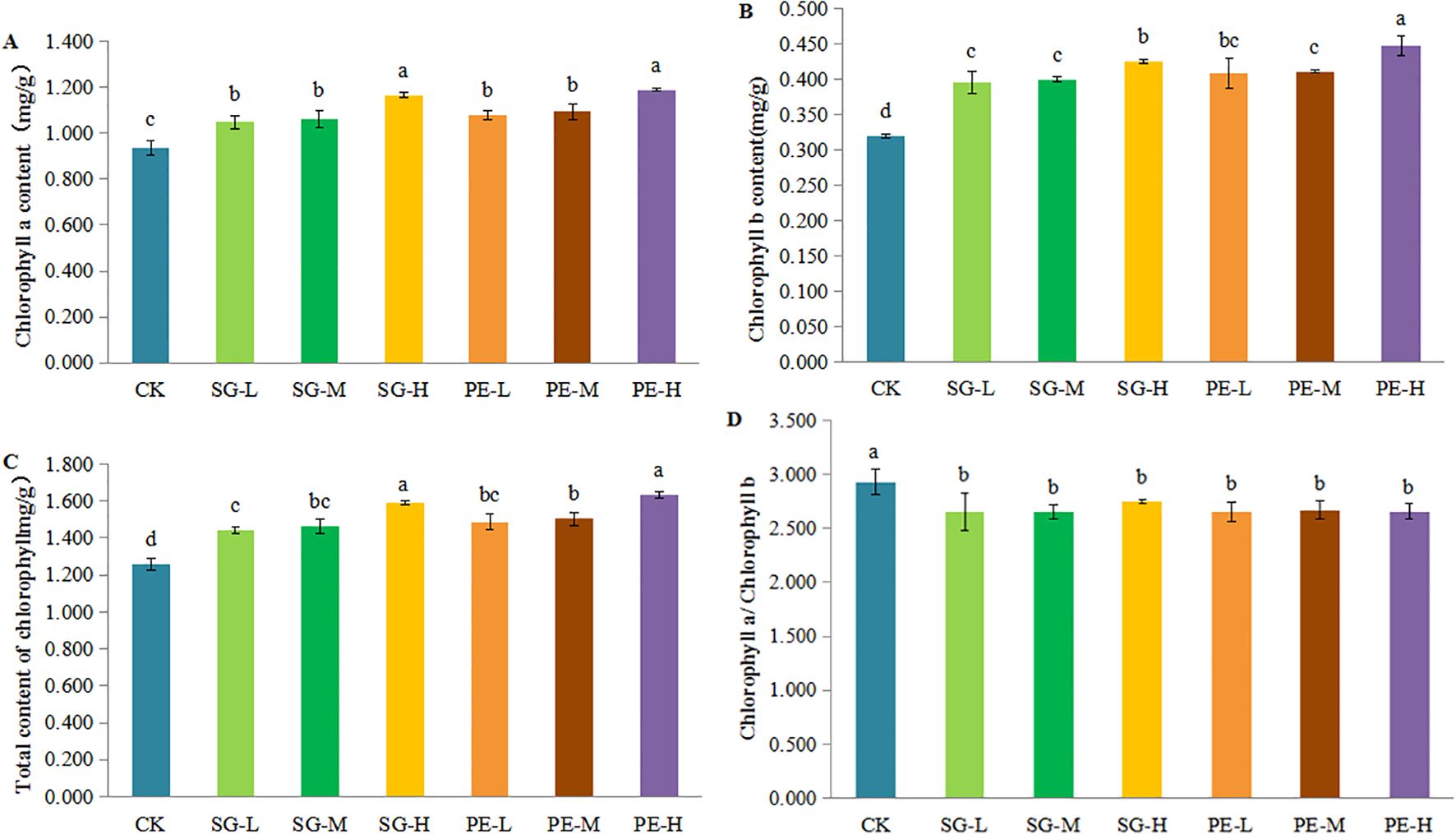
Figure 2. Effects of different intercropping systems on chlorophyll content in tea leaves. (A) chlorophyll a. (B) chlorophyll b. (C) total chlorophyll a. (D) chlorophyll a/chlorophyll b. Different lowercase letters indicate significant differences at P < 0.05.
3.1.2 Contents of tea leaf constituents (polyphenols, free amino acids, and water extracts)
Intercropping tea plantations with climbing fruit species significantly reduced tea polyphenol content, with greater decreases at higher intercropping densities (Figure 3A). In the S. grosvenorii treatments, SG-L resulted in a non-significant 0.68% reduction compared with CK, while SG-M and SG-H led to significant decreases of 3.10% and 6.63%, respectively, with SG-H significantly lower than SG-L and SG-M (Supplementary Table S2). P. edulis treatments showed marked reductions of 9.87%, 15.18%, and 21.63% for PE-L, PE-M, and PE-H treatments, respectively, compared to CK, with each increase in density leading to a significant further reduction (Supplementary Table S2). In both species, polyphenol content consistently decreased as intercropping density increased (Supplementary Table S2).
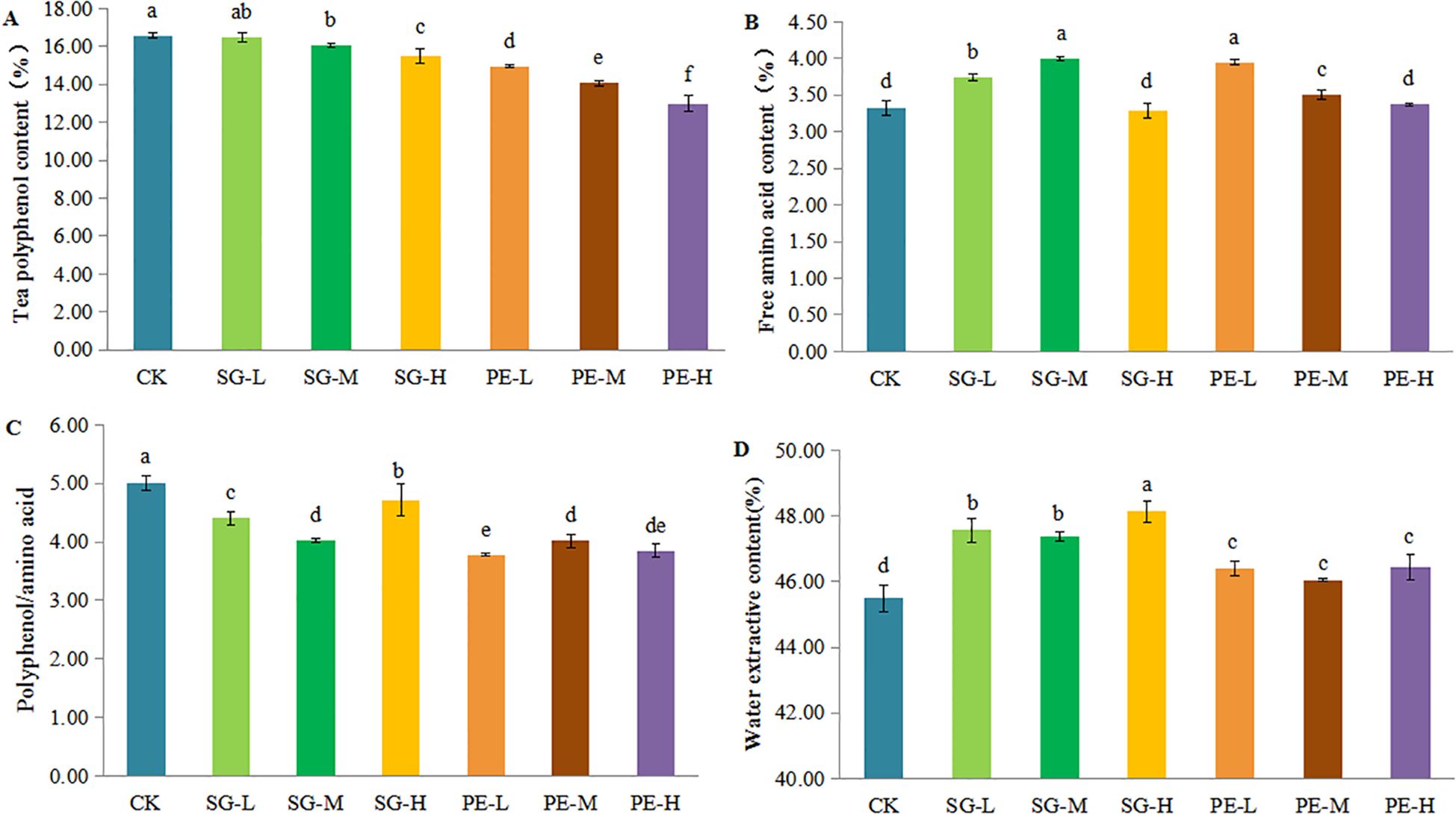
Figure 3. Effects of different intercropping systems on the constituents of tea leaves. (A) polyphenol. (B) free amino acid. (C) polyphenol/amino acid. (D) water extractive content. Different lowercase letters indicate significant differences at P < 0.05.
The free amino acid content in tea leaves varied considerably depending on the plant species and intercropping density (Figure 3B). For S. grosvenorii, free amino acid content was significantly elevated by 12.76% and 20.50% in SG-L and SG-M, respectively, compared to CK, while SG-H showed a non-significant 0.8% decrease. Free amino acid content enhanced and then declined with density, following the order SG-M > SG-L > SG-H, with all pairwise differences being highly significant. In P. edulis treatments, free amino acid increased by 19.10% and 5.63% in PE-L and PE-M, respectively, compared with CK, but PE-H showed a non-significant 1.71% increase. Here, free amino acid declined as density increased, following the order PE-L > PE-M > PE-H, with significant differences among all treatments (Supplementary Table S2).
Intercropping in tea plantations reduced the polyphenol-to-amino acid ratio in tea leaves, thereby improving tea quality, though the effect varied by plant species and density (Figure 3C, Supplementary Table S2). In S. grosvenorii treatments, the ratio decreased significantly by 11.95%, 19.62%, and 5.81% in SG-L, SG-M and SG-H, respectively, compared to CK, with a pattern of decrease then increase as density rose (SG-M < SG-L < SG-H), all significant. In P. edulis treatments, reductions were 24.36%, 19.71%, and 22.99% for PE-L, PE-M, and PE-H, respectively. The trend was an increase then decrease, following the order PE-L < PE-H < PE-M. PE-L was significantly lower than PE-M, while differences involving PE-H were not significant (Supplementary Table S2).
All intercropping treatments significantly increased water extract content in tea leaves (Figure 3D, Supplementary Table S2). In the S. grosvenorii treatments, water extract content increased significantly by 4.56%, 4.19%, and 5.82% in SG-L, SG-M, and SG-H, respectively, compared to CK. The trend showed an initial decrease and then an increase with higher density (SG-H > SG-L > SG-M), with SG-H significantly higher than SG-L and SG-M. In P. edulis treatments, water extract content significantly increased by 2.02%, 2.12%, and 1.22% in PE-L, PE-H, and PE-M, respectively, following a similar density trend, in the order PE-H > PE-L > PE-M. However, differences among P. edulis treatments were not statistically significant (Supplementary Table S2).
In summary, intercropping tea plantations with climbing plants can effectively reduce polyphenol content, increase free amino acid and water extract contents, lower the polyphenol-to-amino acid ratio, and thereby improve the biochemical quality of tea leaves.
3.2 Influence of tea-S. grosvenorii intercropping on the fruit quality of S. grosvenorii
The single fruit weight (Figure 4A) and fruit transverse diameter (Figure 4B) were significantly greater in the SG-H treatment (94.37 g and 54.56 mm, respectively) compared with SG-L and SG-M. The proportion of medium and large fruits (Figure 4C) was also highest in SG-H (94.58), followed by SG-M (90.32%) and SG-L (76.71%) (Supplementary Table S3).
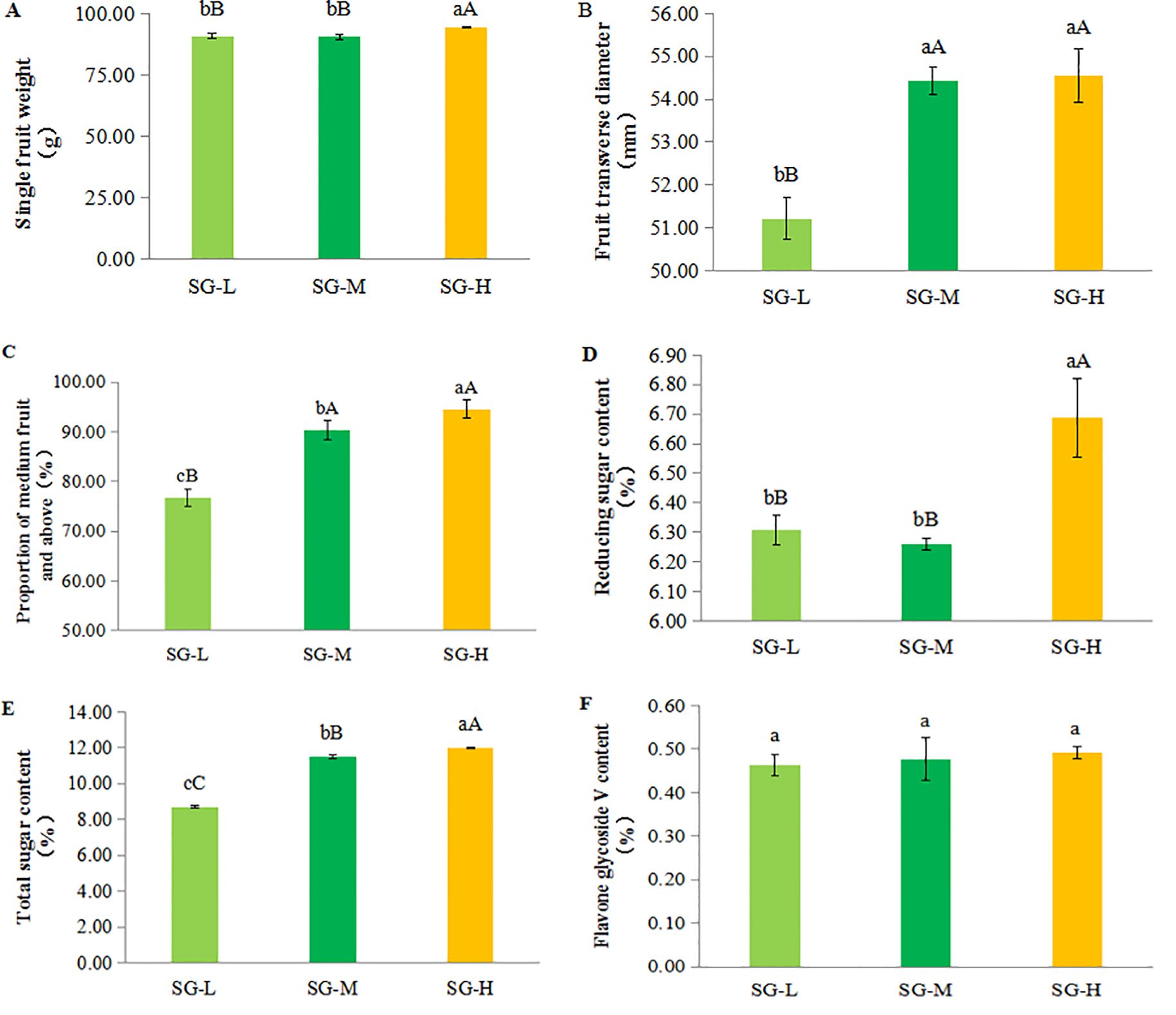
Figure 4. Effects of tea-S. grosvenorii intercropping on the quality of S. grosvenorii fruit. (A) Single fruit weight. (B) Fruit transverse diameter. (C) Proportion of medium fruit and above. (D) Reducing sugar. (E) Total sugar. (F) Flavone glycoside V. Different lowercase letters indicate significant differences at P < 0.05.
In terms of fruit nutritional quality, both reducing sugar (Figure 4D) and total sugar content (Figure 4E) increased with intensity. SG-H yielded the highest values (6.69% and 12.01%, respectively), which were significantly higher than SG-L and SG-M. No significant differences in flavone glycoside V content (Figure 4F) were detected among the treatments (Supplementary Table S3).
Overall, SG-H produced the most pronounced improvements in fruit weight, size, proportion of medium and large fruits, and sugar content, indicating a beneficial effect of higher intercropping intensity on S. grosvenorii fruit quality.
3.3 Influence of tea-P. edulis intercropping on the fruit quality of P. edulis
3.3.1 Physical characteristic
There were notable differences in fruit physical quality across treatments. Single fruit weight (75.42 to 78.55 g), longitudinal diameter (63.92 to 64.94 mm), and transverse diameter (59.56 to 61.36 mm) did not differ significantly among treatments (Supplementary Table S4, Figures 5A, D, E). PE-M exhibited the highest peel weight value (43.99 g), significantly greater than PE-L (39.99 g) and PE-H (43.21 g) (Figure 5B). The edible ratio (Figure 5C) recorded highest in PE-L (46.68%), significantly above PE-M (43.05%) and PE-H (43.96%). Fruit shape index (Figure 5F) was similar for PE-L and PE-M (both 1.08), significantly higher than PE-H (1.04). Peel thickness was greatest in PE-M (5.16 mm), significantly higher than PE-L (4.87mm) and PE-H (4.89mm) (Figure 5G).
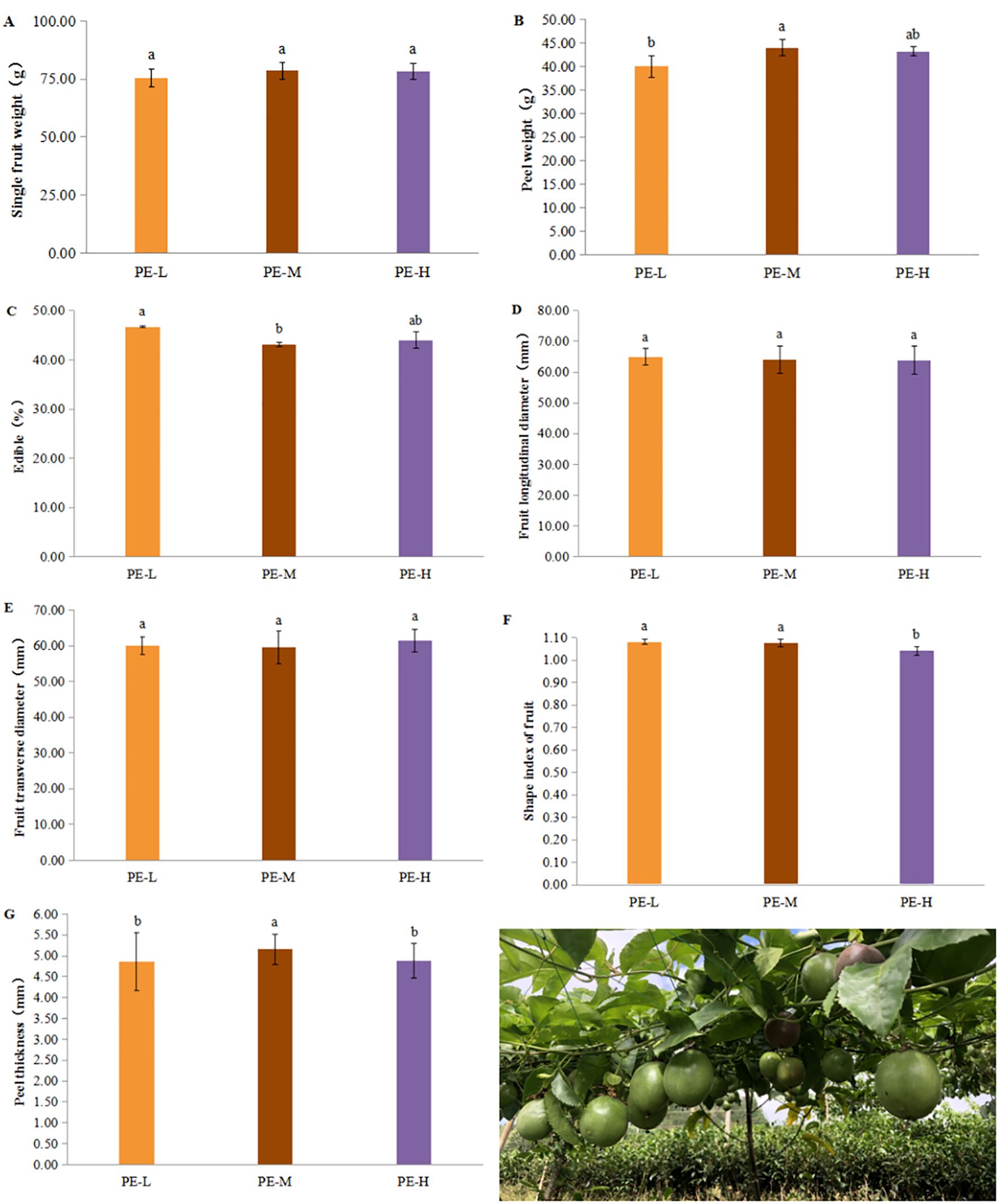
Figure 5. Effects of tea-P. edulis intercropping on the physical characteristic of P. edulis fruit. (A) Single fruit weight. (B) Peel weight. (C) Edible rate. (D) Fruit longitudinal diameter. (E) Fruit transverse diameter. (F) Fruit shape index. (G) Peel thickness. Different lowercase letters indicate significant differences at P < 0.05.
Overall, PE-M resulted in greater peel weight, fruit shape index, and peel thickness, but the lowest edible ratio, while PE-L produced a higher edible ratio. Different treatments exerted varying effects on multiple fruit trait indices (Supplementary Table S4).
3.3.2 Intrinsic quality
The soluble solids content was highest in PE-H (17.82%), which was significantly greater than PE-L (17.35%) and PE-M (16.42%) (Figure 6A). Similarly, the soluble sugar content was highest in PE-H (12.27%), followed by PE-M (11.18%), and lowest in PE-L (10.78%) (Figure 6B).
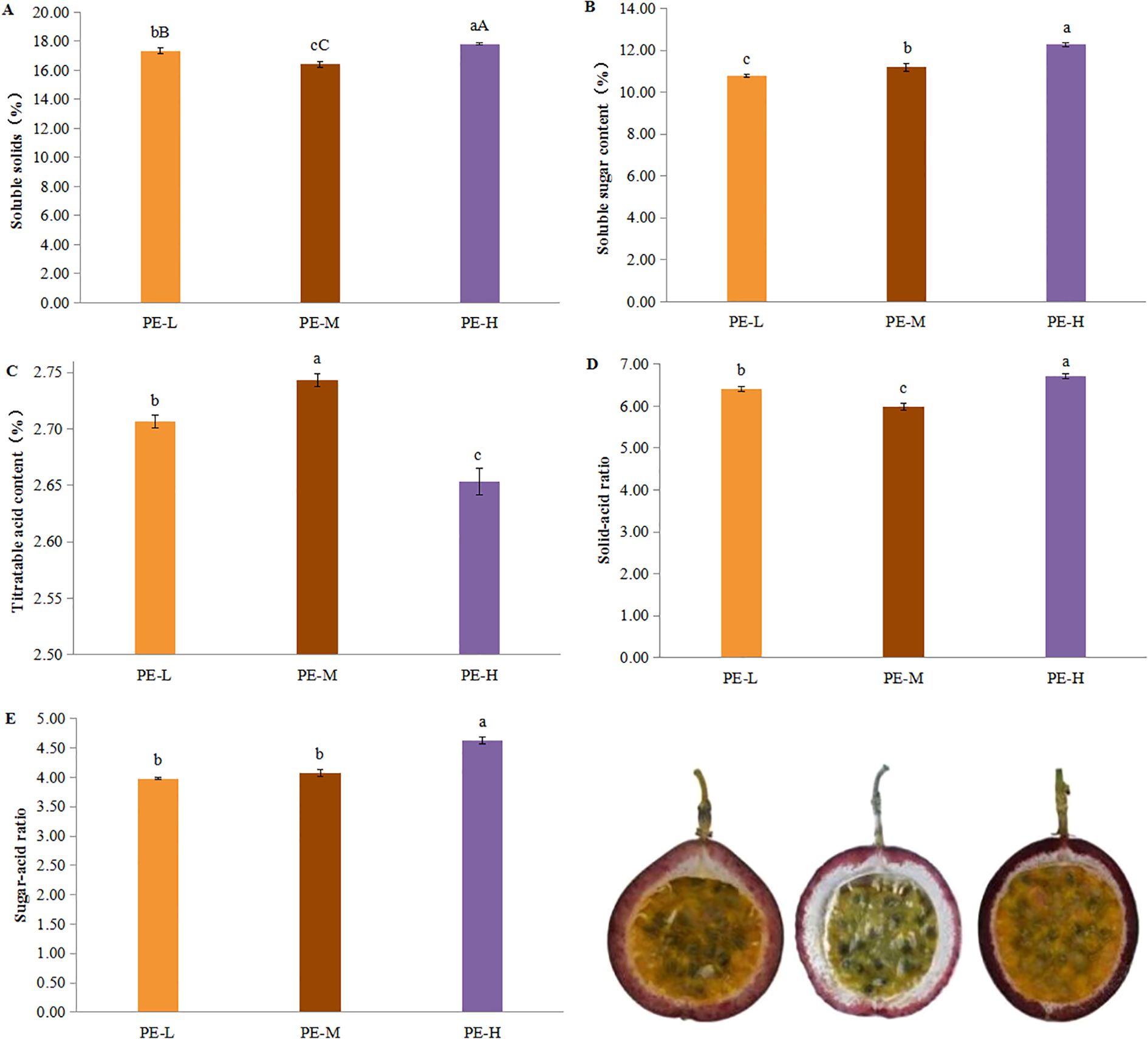
Figure 6. Effects of tea-P. edulis intercropping on the intrinsic quality of P. edulis fruit. (A), Soluble solids. (B) Soluble sugar. (C), Titratable acid. (D), Solid-acid ratio. (E), Sugar-acid ratio. Different lowercase letters indicate significant differences at P < 0.05.
With regard to titratable acid content, PE-M recorded the highest value (2.74%), followed closely by PE-L (2.71%), while PE-H had the lowest value (2.65%), with significant differences among the three treatments (Figure 6C). Both the solid-acid ratio and sugar-acid ratio were highest under PE-H treatment, at 6.72 and 4.62, respectively, and were significantly higher than those of PE-L and PE-M (Figures 6D, E).
In summary, PE-H resulted in the highest levels of soluble solids and soluble sugars, as well as the greatest solid-acid and sugar-acid ratios, indicating superior fruit flavor, whereas the PE-M yielded the highest titratable acid content. Overall, different treatments had significant effects on intrinsic fruit quality indices (Supplementary Table S5).
4 Discussion
This study systematically evaluated the bidirectional effects of intercropping tea (Camellia sinensis) with two climbing fruit species, Siraitia grosvenorii (luohan guo) and Passiflora edulis (passion fruit), at varying planting densities on the quality of tea leaves and companion fruits. Our findings demonstrate that such intercropping systems can significantly influence both the physiological and biochemical quality of tea, as well as the yield and quality attributes of S. grosvenorii and P. edulis fruits. These results offer valuable insights for the development of multifunctional and sustainable tea-based agroecosystems.
4.1 Effects of intercropping on tea leaf quality
A key finding of this study is the significant enhancement in tea leaf chlorophyll content under all intercropping treatments, with the most pronounced increases observed at high planting densities of both S. grosvenorii and P. edulis. Elevated chlorophyll content is typically associated with improved photosynthetic capacity and leaf vitality, which may contribute to higher tea yield and quality (Liu et al., 2019; Ali et al., 2024). The greater increase in chlorophyll b relative to chlorophyll a, reflected by a decreased chlorophyll a/b ratio, suggests that intercropping, particularly at higher densities, may induce adaptive modifications in the photosynthetic apparatus, potentially in response to altered light environments created by the climbing canopy (Brooker et al., 2015). Such shifts in pigment composition are consistent with previously reported shade-adaptation strategies of tea plants intercropped with taller shade trees (Qiao et al., 2019).
In addition to chlorophyll content, intercropping treatments led to significant changes in key biochemical constituents of tea leaves. Polyphenol content, a major determinant of tea astringency and antioxidant activity (Zhao et al., 2022), was significantly reduced across all intercropping treatments, with the most substantial decrease observed at higher planting densities. Notably, this reduction was more pronounced in the P. edulis intercropping system. In contrast, free amino acid content, which imparts umami flavor (Tang et al., 2019) and is positively correlated with tea quality, was significantly increased under low- and medium-density intercropping, particularly in the S. grosvenorii system, but declined under high-density conditions. The water extract content, a comprehensive indicator of soluble substances contributing to tea flavor and mouthfeel (Chen et al., 2023), was also significantly elevated in all intercropping treatments.
The polyphenol-to-amino acid ratio, a widely recognized indicator of tea quality, with lower values reflecting reduced astringency and improved taste (Chen et al., 2023), was decreased in all intercropping treatments, especially at low to medium densities. These findings suggest that intercropping, particularly when planting densities are suitable, can improve the sensory attributes of tea by enhancing umami and sweetness while reducing bitterness and astringency.
The mechanisms underlying these changes are likely multifactorial. First, the climbing canopy may help establish a more favorable microclimate (e.g., lower temperature, reduced light intensity, and increased humidity) beneath the fruit trees, conditions that promote amino acid accumulation and suppress polyphenol synthesis in tea leaves (Brooker et al., 2015). Second, intercropping can improve soil fertility through increased organic matter inputs and enhanced nutrient cycling, thereby supporting the biosynthesis of nitrogenous compounds such as amino acids (Brooker et al., 2015; Huang et al., 2022). Third, competition for soil nutrients and light at higher planting densities may impose mild abiotic stress on tea plants, potentially downregulating polyphenol biosynthetic pathways. The stronger effects observed in the P. edulis system may be attributed to its denser foliage and greater shading capacity compared to S. grosvenorii.
However, the density-dependent trends observed, particularly the decline in amino acid content at excessively high intercropping densities, underscore the importance of optimizing planting arrangements to maximize benefits to tea quality.
4.2 Reciprocal effects on companion fruit quality
A novel aspect of this study is the reciprocal assessment of how intercropping with tea influences the quality of the companion climbing fruit quality. For S. grosvenorii, high-density intercropping (SG-H) significantly increased single fruit weight, fruit size, marketable fruit proportion, and sugar content, without negatively affecting the content of flavone glycoside V. These enhancements in fruit quality may be attributed to the more favorable microclimate and improved soil conditions created by the tea canopy, which can mitigate heat stress, conserve soil moisture, and enhance nutrient availability (Bai et al., 2022; Duan et al., 2024). Increased sugar accumulation under shaded or moderately stressed environments has been documented in other fruit crops and is often associated with altered carbohydrate metabolism and reduced photorespiration (Guo et al., 2024a, b; Wu et al., 2022).
For P. edulis, the intercropping effects were more nuanced. While no significant differences were detected in basic physical traits such as fruit weight and diameter across treatments, the highest levels of soluble solids and sugar content, as well as the most favorable solid-acid and sugar-acid ratios (key determinants of fruit flavor), were observed in the high-density intercropping treatment (PE-H). These findings suggest that, similar to S. grosvenorii, moderate shading in tea intercropping systems may promote sugar accumulation and enhance flavor attributes. However, certain morphological traits such as edible ratio and fruit shape index were maximized at low or medium planting densities, indicating that excessively high intercropping density may adversely affect fruit morphology, likely due to intensified competition for light and soil nutrients (Brooker et al., 2015).
Interestingly, titratable acidity was highest in the medium-density treatment, suggesting that fruit acidity may respond to intercropping-induced microclimatic and physiological changes differently from sugar content. This result highlights the complexity of fruit quality responses under intercropping systems and underscores the need to balance planting density to improve both yield and quality traits in companion fruit crops.
4.3 Implications for sustainable agroecosystem design
The findings of this study offer valuable insights for the development of multifunctional and sustainable tea-based agroecosystems. First, the observed enhancements in tea quality, including elevated chlorophyll and free amino acid contents, reduced polyphenol-to-amino acid ratios, and increased water extract levels, indicate that intercropping with climbing fruit species can support the production of high-quality tea with improved sensory and health-related attributes. Second, the notable improvements in fruit quality and marketability of both S. grosvenorii and P. edulis provide economic incentives for growers by diversifying income sources and enhancing agroecosystem resilience.
Ecologically, such intercropping systems promote greater plant diversity, improve resource use efficiency, and reduce reliance on external inputs such as synthetic fertilizers and pesticides, thereby contributing to environmental sustainability. The use of climbing fruit species is particularly advantageous, as vertical stratification enables efficient spatial utilization without direct competition for ground-level resources with tea plants.
Nevertheless, the density-dependent responses observed in both tea and fruit crops underscore the importance of site-specific optimization of intercropping configurations. Excessively high planting densities may result in excessive shading and resource competition, offsetting the benefits to tea quality and certain fruit characteristics. Conversely, low densities may fail to fully capitalize on the advantages of intercropping. Future research should investigate the long-term performance of these systems, including their effects on soil health, pest and disease dynamics, and overall system productivity.
5 Conclusions
This study demonstrates that intercropping tea with the climbing plants S. grosvenorii and P. edulis can simultaneously enhance the biochemical quality of tea while improving fruit sweetness and flavor, with effects strongly modulated by planting density. Intercropping increased tea leaf chlorophyll a and b as well as total chlorophyll contents, elevated free amino acid levels at optimal densities, reduced polyphenol-to-amino acid ratios by up to 24.36%, and increased water-extractable compound content, collectively indicating enhanced sensory potential and market quality of tea leaves. Simultaneously, these intercropping systems improved the quality and marketability of companion climbing fruits, particularly under moderate to high planting densities. These findings underscore the potential of climbing plant–tea intercropping as a viable strategy for advancing high-quality, multifunctional, and environmentally sustainable tea-based agroecosystems. To fully realize the agronomic, economic, and ecological benefits of these innovative systems, continued research and adaptive management tailored to site-specific conditions are essential.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
QL: Conceptualization, Methodology, Writing – original draft, Formal analysis, Funding acquisition. YX: Formal analysis, Writing – original draft, Investigation. JC: Formal analysis, Data curation, Writing – original draft, Investigation. HM: Writing – original draft, Data curation, Investigation. QT: Data curation, Investigation, Formal analysis, Writing – original draft. LW: Investigation, Writing – original draft, Formal analysis. LG: Writing – original draft, Investigation, Data curation. PL: Data curation, Investigation, Writing – review & editing. ZL: Supervision, Writing – review & editing, Conceptualization, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (32160762), the Guangxi Key Research and Development Program (AB22080069, AB19245005), the Science and Technology Major Project of Guangxi (AA17202019).
Conflict of interest
Author LG was employed by company Guilin Jifusi Monk Fruit Biotechnology Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2025.1654806/full#supplementary-material
References
Ahammed G. J. and Li X. (2022). Hormonal regulation of health-promoting compounds in tea (Camellia sinensis L.). Plant Physiol. Biochem. 185, 390–400. doi: 10.1016/j.plaphy.2022.06.021, PMID: 35785551
Ali I., Hussain J., Yanwisetpakdee B., Iqbal I., and Chen X. (2024). The effects of monoculture and intercropping on photosynthesis performance correlated with growth of garlic and perennial ryegrass response to different heavy metals. BMC Plant Biol. 24, 659. doi: 10.1186/s12870-024-05371-3, PMID: 38987675
Ali Abaker Omer A., Zhang C. H., Liu J., and Shan Z. G. (2025). Comprehensive review of mapping climate change impacts on tea cultivation: bibliometric and content analysis of trends, influences, adaptation strategies, and future directions. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1542793, PMID: 39925372
Bai Y. C., Li B. X., Xu C. Y., Raza M., Wang Q., Wang Q. Z., et al. (2022). Intercropping walnut and tea: Effects on soil nutrients, enzyme activity, and microbial communities. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.852342, PMID: 35369467
Brooker R. W., Bennett A. E., Cong W. F., Daniell T. J., George T. S., Hallett P. D., et al. (2015). Improving intercropping: a synthesis of research in agronomy, plant physiology and ecology. New Phytol. 206, 107–117. doi: 10.1111/nph.13132, PMID: 25866856
Chen H., Yu F., Kang J., Li Q., Warusawitharana H. K., and Li B. (2023). Quality chemistry, physiological functions, and health benefits of organic acids from tea (Camellia sinensis). Molecules 28, 2339. doi: 10.3390/molecules28052339, PMID: 36903584
Duan Y., Wang G., Liang L., Wang M., Jiang J., Ma Y., et al. (2024). Intercropping fruit trees in tea plantation improves soil properties and the formation of tea quality components. Plant Physiol. Biochem. 210, 108574. doi: 10.1016/j.plaphy.2024.108574, PMID: 38564979
Farooq T. H., Kumar U., Mo J., Shakoor A., Wang J., Rashid M. H. U., et al. (2021). Intercropping of peanut-tea enhances soil enzymatic activity and soil nutrient status at different soil profiles in subtropical Southern China. Plants 10, 881. doi: 10.3390/plants10050881, PMID: 33925476
Gui H., Fan L., Wang D., Yan P., Li X., Pang Y., et al. (2022). Variations in soil nutrient dynamics and bacterial communities after the conversion of forests to long-term tea monoculture systems. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.896530, PMID: 35814650
Guo Y., Chen X., Gong P., Long H., Wang J., Yang W., et al. (2024b). Siraitia grosvenorii as a homologue of food and medicine: a review of biological activity, mechanisms of action, synthetic biology, and applications in future food. J. Agric. Food Chem. 72, 6850–6870. doi: 10.1021/acs.jafc.4c00018, PMID: 38513114
Guo Q., Shi M., Sarengaowa, Xiao Z., Xiao Y., and Feng K. (2024a). Recent advances in the distribution, chemical composition, health benefits, and application of the fruit of Siraitia grosvenorii. Foods 13, 2278. doi: 10.3390/foods13142278, PMID: 39063362
He X., Luan F., Yang Y., Wang Z., Zhao Z., Fang J., et al. (2020). Passiflora edulis: An insight into current researches on phytochemistry and pharmacology. Front. Pharmacol. 11. doi: 10.3389/fphar.2020.00617, PMID: 32508631
Huang Z., Cui C., Cao Y., Dai J., Cheng X., Hua S., et al. (2022). Tea plant-legume intercropping simultaneously improves soil fertility and tea quality by changing Bacillus species composition. Hortic. Res. 9, uhac046. doi: 10.1093/hr/uhac046, PMID: 35184199
Khan N. and Mukhtar H. (2018). Tea polyphenols in promotion of human health. Nutrients 11, 39. doi: 10.3390/nu11010039, PMID: 30585192
Kochman J., Jakubczyk K., Antoniewicz J., Mruk H., and Janda K. (2020). Health benefits and chemical composition of Matcha green tea: a review. Molecules 26, 85. doi: 10.3390/molecules26010085, PMID: 33375458
Li G., Liang Y., Liu Q., Zeng J., Ren Q., Guo J., et al. (2024). Enhancing production efficiency through optimizing plant density in maize-soybean strip intercropping. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1473786, PMID: 39464277
Li C., Stomph T. J., Makowski D., Li H., Zhang C., Zhang F., et al. (2023). The productive performance of intercropping. Proc. Natl. Acad. Sci. U.S.A. 120, e2201886120. doi: 10.1073/pnas.2201886120, PMID: 36595678
Liao Q., Liang P. X., Xing Y., Yao Z. F., Chen J. P., Pan L. P., et al. (2025). Optimizing selenium application for enhanced quality and nutritional value of spring tea (Camellia sinensis). Horticulturae 11, 423. doi: 10.3390/horticulturae11040423
Liu H., Gao Y., Gao C., Liu S., Zhang J., Chen G., et al. (2019). Study of the physiological mechanism of delaying cucumber senescence by wheat intercropping pattern. J. Plant Physiol. 234-235, 154–166. doi: 10.1016/j.jplph.2019.02.003, PMID: 30818185
Pelech E. A., Evers J. B., Pederson T. L., Drag D. W., Fu P., and Bernacchi C. J. (2023). Leaf, plant, to canopy: A mechanistic study on aboveground plasticity and plant density within a maize-soybean intercrop system for the Midwest, USA. Plant Cell Environ. 46, 405–421. doi: 10.1111/pce.14487, PMID: 36358006
Peng X., Thevathasan N. V., Gordon A. M., Mohammed I., and Gao P. (2015). Photosynthetic response of soybean to microclimate in 26-year-old tree-based intercropping systems in Southern Ontario, Canada. PloS One 10, e0129467. doi: 10.1371/journal.pone.0129467, PMID: 26053375
Pokharel S. S., Yu H., Fang W., Parajulee M. N., and Chen F. (2019). Intercropping cover crops for a vital ecosystem service: a review of the biocontrol of insect pests in tea agroecosystems. Plants 12, 2361. doi: 10.3390/plants12122361, PMID: 37375986
Postma J. A., Hecht V. L., Hikosaka K., Nord E. A., Pons T. L., and Poorter H. (2021). Dividing the pie: A quantitative review on plant density responses. Plant Cell Environ. 44, 1072–1094. doi: 10.1111/pce.13968, PMID: 33280135
Qiao X., Sai L., Chen X., Xue L., and Lei J. (2019). Impact of fruit-tree shade intensity on the growth, yield, and quality of intercropped wheat. PloS One 14, e0203238. doi: 10.1371/journal.pone.0203238, PMID: 30939172
Tang G. Y., Meng X., Gan R. Y., Zhao C. N., Liu Q., Feng Y. B., et al. (2019). Health functions and related molecular mechanisms of tea components: an update review. Int. J. Mol. Sci. 20, 6196. doi: 10.3390/ijms20246196, PMID: 31817990
Wang T., Duan Y., Liu G., Shang X., Liu L., Zhang K., et al. (2022). Tea plantation intercropping green manure enhances soil functional microbial abundance and multifunctionality resistance to drying-rewetting cycles. Sci. Total Environ. 810, 151282. doi: 10.1016/j.scitotenv.2021.151282, PMID: 34757096
Wang L., Geilfus C. M., Sun T., Zhao Z., Li W., Zhang X., et al. (2023a). Double gains: Boosting crop productivity and reducing carbon footprints through maize-legume intercropping in the Yellow River Delta, China. Chemosphere 344, 140328. doi: 10.1016/j.chemosphere.2023.140328, PMID: 37783359
Wang T., Mu X., Ni E., Wang Q., Li S., Mao J., et al. (2025). Belowground interaction in tea/soybean intercropping enhances tea quality by improving soil nutrient dynamics. Plants 14, 1691. doi: 10.3390/plants14111691, PMID: 40508364
Wang S., Zhang X., Li X., Shen J., Sun L., Zaman S., et al. (2023b). Different changes of bacterial diversity and soil metabolites in tea plants-legume intercropping systems. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1110623, PMID: 37008505
Wu J., Jian Y., Wang H., Huang H., Gong L., Liu G., et al. (2022). A review of the phytochemistry and pharmacology of the fruit of Siraitia grosvenorii (Swingle): a traditional Chinese medicinal food. Molecules 27, 6618. doi: 10.3390/molecules27196618, PMID: 36235155
Wu H., Long X., and Geng Y. (2023). Companion plants of tea: from ancient to terrace to forest. Plants 12, 3061. doi: 10.3390/plants12173061, PMID: 37687308
Yan P., Shen C., Fan L., Li X., Zhang L., Zhang L., et al. (2018). Tea planting affects soil acidification and nitrogen and phosphorus distribution in soil. Agric. Ecosyst. Environ. 254, 20–25. doi: 10.1016/j.agee.2017.11.015
Yan P., Wu L., Wang D., Fu J., Shen C., Li X., et al. (2020). Soil acidification in Chinese tea plantations. Sci. Total Environ. 715, 136963. doi: 10.1016/j.scitotenv.2020.136963, PMID: 32014781
Zhang W., Han S. A., Wang M., Alemujiang A., Pan M. Q., Aiermaike C. A., et al. (2021). Effects of fruit tree canopy shading on grain filling of intercropping winter wheat. Ying Yong Sheng Tai Xue Bao. 32, 2458–2468. doi: 10.13287/j.1001-9332.202107.028, PMID: 34313064
Zhang J., Tao S., Hou G., Zhao F., Meng Q., and Tan S. (2023). Phytochemistry, nutritional composition, health benefits and future prospects of Passiflora: a review. Food Chem. 428, 136825. doi: 10.1016/j.foodchem.2023.136825, PMID: 37441935
Zhang H., Tian L., Hao X., Li N., Shi X., Shi F., et al. (2025). Optimizing plant density to improve the soil microenvironment and enhance crop productivity in cotton/cumin intercropping systems. Front. Plant Sci. 16. doi: 10.3389/fpls.2025.1533211, PMID: 39996110
Keywords: Camellia sinensis, Siraitia grosvenorii (luohan guo), Passiflora edulis (passion fruit), planting density, chlorophyll
Citation: Liao Q, Xing Y, Chen J-P, Mou H-F, Tian Q-L, Wang L, Guo L-X, Liang P-X and Liu Z-S (2025) Improving tea quality and fruit yield through intercropping with climbing plants. Front. Agron. 7:1654806. doi: 10.3389/fagro.2025.1654806
Received: 27 June 2025; Accepted: 19 August 2025;
Published: 01 September 2025.
Edited by:
Qunfeng Zhang, Chinese Academy of Agricultural Sciences (CAAS), ChinaReviewed by:
Ali Raza, Chinese Academy of Sciences (CAS), ChinaSheng Tang, Anhui Agricultural University, China
Copyright © 2025 Liao, Xing, Chen, Mou, Tian, Wang, Guo, Liang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhu-Sheng Liu, Z2x4bGcwMzk3QDE2My5jb20=
 Qing Liao
Qing Liao Ying Xing1
Ying Xing1 Qing-Lan Tian
Qing-Lan Tian